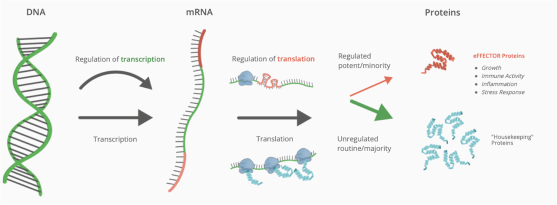
therapies was estimated to exceed $25 billion, of which more than $12 billion accounted for the treatment of patients with metastatic NSCLC. While checkpoint inhibitor treatment is very effective in patients for a variety of cancers, these agents are generally not curative, and a large majority of patients ultimately progress on their checkpoint inhibitor therapy, emphasizing the need for novel frontline combination treatments that may increase the proportion of patients that experience long term durable benefit, and delay progression in other patients. There are an estimated approximately 43,000 U.S. patients with metastatic NSCLC that have
PD-L1
expression
1-49%,
of which we estimate 80%, or 34,000 U.S. patients, continue on
maintenance therapy following chemotherapy and would therefore be potential candidates for tomivosertib in combination in the maintenance setting. We estimate that this segment represents a $5 billion total market opportunity in the United States. In addition, there are an estimated approximately 27,000 U.S. patients with metastatic NSCLC that have
PD-L1
expression ≥50%, which we estimate represents an additional $4 billion market opportunity.
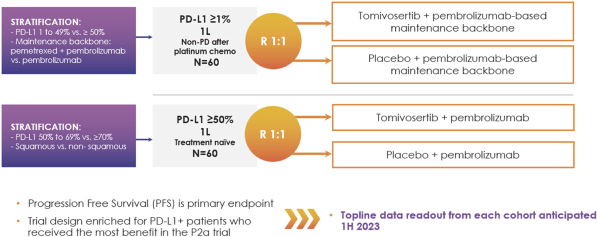
Based on the encouraging results in our Phase 2a clinical trial, in June 2021 we initiated patient dosing in KICKSTART, a double-blind, randomized, placebo-controlled Phase 2b trial of tomivosertib combined with pembrolizumab in patients with metastatic NSCLC, that now includes both
PD-L1≥50%
and
PD-L1≥1%
cohorts. Pembrolizumab is owned and marketed by Merck for frontline NSCLC and several other indications. We anticipate reporting topline data from both
PD-L1≥50%
and
PD-L1≥1%
cohorts in the first half of 2023.
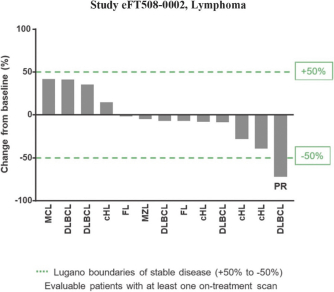
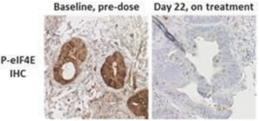
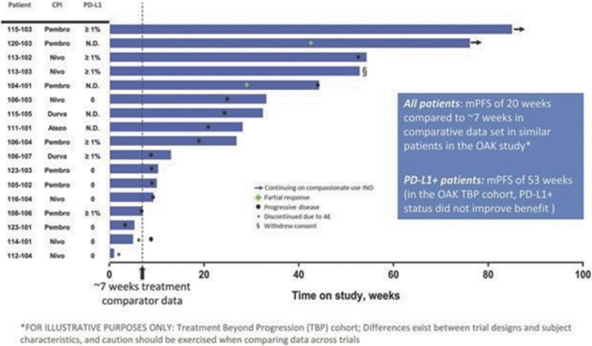
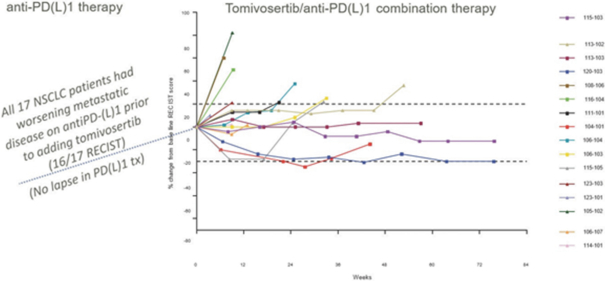
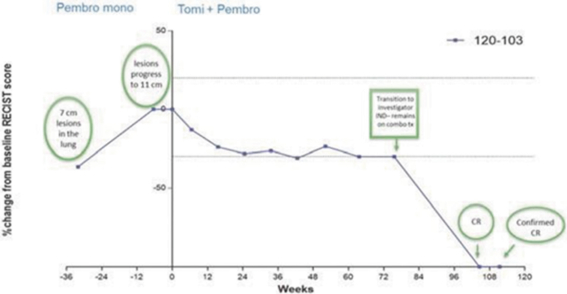
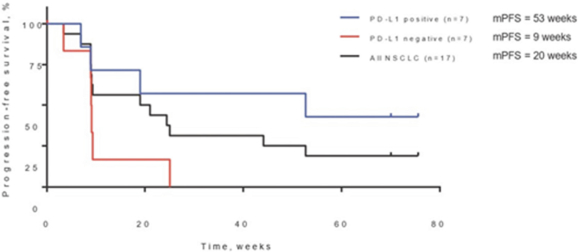
In our completed Phase 2a
CPI-A
clinical trial evaluating tomivosertib in combination with anti
PD-(L)1
therapy in 17 patients with metastatic NSCLC, tomivosertib substantially extended the median progression free survival (“mPFS”), the time duration during which patients remain alive and experience no disease progression, defined as an increase in their tumor assessment of greater than 20% or appearance of new lesions, in patients that were previously progressing on their anti
PD-(L)1
therapies. In addition, as of study completion in September 2020, two of those 17 patients (12%) had confirmed partial responses, or decreases in tumor assessments of greater than or equal to 30% from baseline(“PRs”) one of which went on to achieve a confirmed complete response, or no detectable tumor lesions(“CR”) with a third patient showing 28% tumor regression. Tomivosertib was generally well tolerated in this clinical trial. In these 17 patients, once tomivosertib was added without any change or break in the anti
PD-(L)1
therapy, there was a mPFS of 20 weeks. In this trial, patients with positive
PD-L1
expression, a biomarker of T cell infiltration into tumors, as determined by
post-hoc
analysis of available data from diagnostic assays conducted during their treatment history, had mPFS of 53 weeks relative to 9 weeks for
PD-L1
negative patients. Tomivosertib has been tested on over 200 patients through August 2021, including approximately 80 in combination with checkpoint inhibitors, and has demonstrated a favorable adverse event profile both as a single agent and in combination with checkpoint inhibitors.
We are enrolling NSCLC patients in KICKSTART in two cohorts. In our
PD-L1≥1%
cohort, we are enrolling patients with
PD-L1
≥1% NSCLC immediately after they complete the platinum chemotherapy phase
(4-6
cycles) of their frontline treatment without disease progression. Patients in this cohort will be randomized into two groups:
maintenance therapy plus tomivosertib in the treatment group versus
maintenance plus placebo in the control group.
maintenance therapy is defined as pembrolizumab + pemetrexed in
non-squamous
NSCLC and pembrolizumab in squamous NSCLC. Patients will continue treatment in this study until disease progression, and PFS is the primary endpoint of the study.
In our
PD-L1≥50%
cohort, we are enrolling NSCLC patients in KICKSTART with
PD-L1
biomarker expression≥50%. In this patient population, who are the most responsive to pembrolizumab monotherapy in the frontline setting, and for whom monotherapy is a
and pembrolizumab is the most widely used checkpoint treatment. Beyond this initial KICKSTART patient population, we plan to pursue additional clinical trials of tomivosertib in other indications where anti
PD-(L)1
therapy is the
including other cancers where
PD-(L)1
therapy is approved such as renal, bladder, triple negative breast cancer, or tumors with high microsatellite instability
(“MSI-H”).
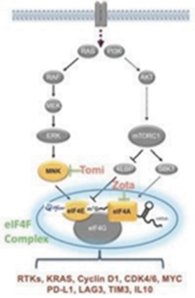
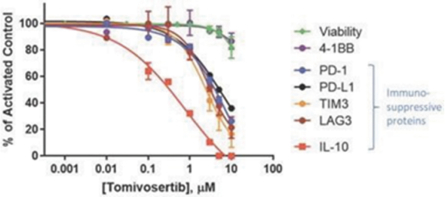
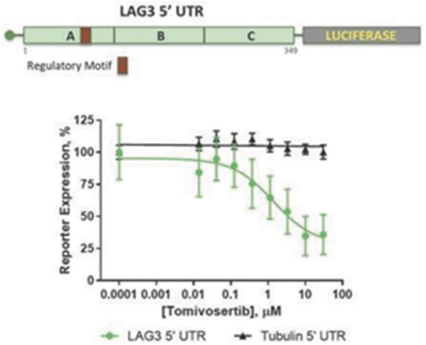
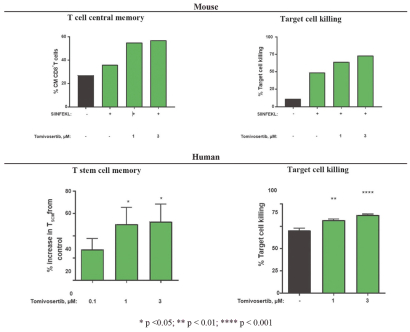
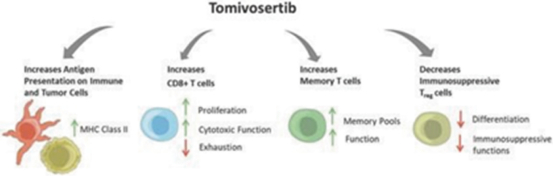
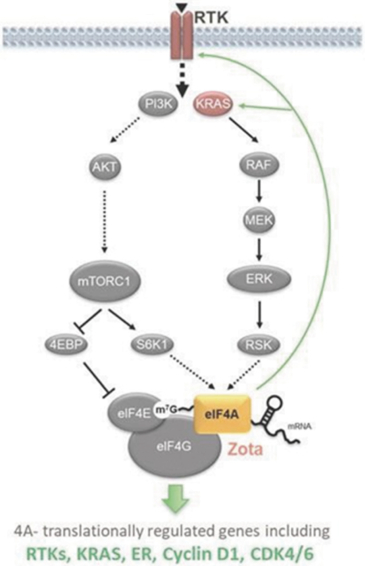
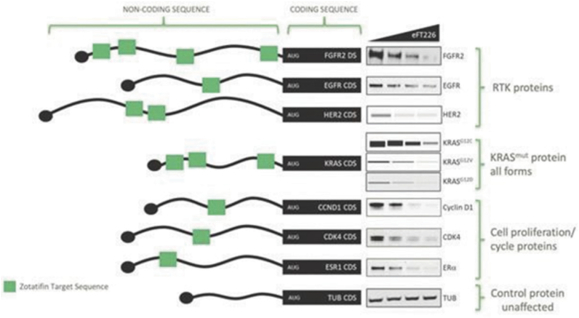
Our preclinical studies suggest combining tomivosertib with anti
PD-(L)1
treatments can overcome mechanisms of resistance to checkpoint inhibitors, resulting in enhanced and durable sensitivity. In addition, our