| Item 7.01 | Regulation FD Disclosure. |
On January 25, 2024, Vera Therapeutics, Inc. (the “Company”) announced positive 72-week data from the open label extension (“OLE”) period of the Company’s Phase 2b ORIGIN clinical trial of atacicept in patients with immunoglobulin A nephropathy (“IgAN”). A copy of the press release is furnished as Exhibit 99.1. In connection with the data release, the Company compiled a presentation entitled “R&D Day” (the “R&D Day Presentation”) that includes the week 72 data from the Phase 2b ORIGIN clinical trial referenced above. A copy of the R&D Day Presentation is furnished as Exhibit 99.2. For important information about forward-looking statements, see the slide titled “Forward-Looking Statements” in Exhibit 99.2 attached hereto.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01, including Exhibits 99.1 and 99.2, shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission (“SEC”) made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
As noted in Item 7.01, on January 25, 2024, the Company announced positive 72-week data from the OLE period of the Company’s Phase 2b ORIGIN clinical trial of atacicept in patients with IgAN. Atacicept is the Company’s potential best-in-class, disease-modifying dual inhibitor of the cytokines B-cell activating factor and a proliferation-inducing ligand. ORIGIN is a multinational, randomized, double-blind, placebo-controlled clinical trial (n=116) evaluating the efficacy and safety of atacicept in patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite available ACE or ARB therapy.
After completing the 36-week randomized, double-blind, placebo-controlled period of the Phase 2b ORIGIN trial, all participants were eligible to receive atacicept 150 mg in the OLE. Of the 116 randomized participants, 106 completed 72 weeks.
Participants treated with atacicept for 72 weeks demonstrated a 62% reduction in Gd-IgA1, a reduction in the percentage of participants with hematuria to 19%, and a 48% reduction in urine protein to creatinine ratio (“UPCR”) in the per-protocol (“PP”) analysis. Importantly, participants had consistent and stable estimated glomerular filtration (“eGFR”) with 0 mL/min/1.73m2 change from baseline at 72 weeks. Of note, it has been shown that eGFR declines by approximately 1 mL/min/1.73m2 per year in the general population.
Atacicept 72-week data from the Phase 2b ORIGIN trial are consistent with a profile of true disease modification
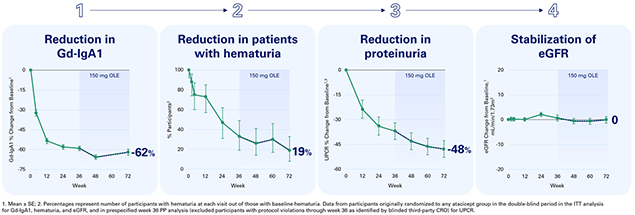
Participants who switched from placebo to atacicept demonstrated similar outcomes across each of the key indicators of IgAN as compared to participants originally randomized to atacicept during the first 36 weeks of the trial, including a 59% reduction in Gd-IgA1, a reduction in the percentage of participants with hematuria to 41%,
