| | |
 | | Exhibit 99.1 |
Vera Therapeutics Presents Positive 72-Week Data Showing eGFR Stabilization in the Phase 2b ORIGIN Clinical Trial OLE in IgA Nephropathy
– Participants treated with atacicept for 72 weeks showed consistent and sustained reductions in Gd-IgA1, hematuria, and UPCR, with stable eGFR over the duration of treatment
– Placebo cohort participants who crossed over to atacicept 150 mg in the OLE had similar outcomes at 72 weeks as atacicept cohort in the first 36 weeks of the trial
– Atacicept was generally well-tolerated in the OLE period of the trial, consistent with the randomized period
– 72-week data provide additional confidence in the ongoing pivotal Phase 3 ORIGIN 3 clinical trial
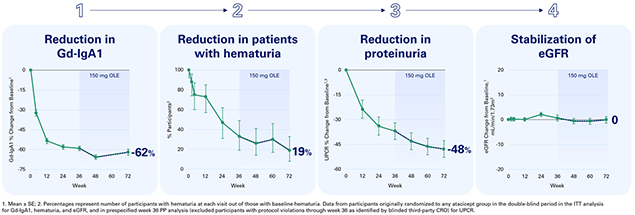
BRISBANE, Calif., January 25, 2024 — Vera Therapeutics, Inc. (Nasdaq: VERA), a late clinical-stage biotechnology company developing and commercializing transformative treatments for patients with serious immunologic diseases, today announced positive 72-week data from the open label extension (OLE) period of its Phase 2b ORIGIN clinical trial of atacicept in participants with IgA nephropathy (IgAN). In aggregate, the 72-week data with atacicept are consistent with a profile of true disease modification in IgAN.
“Data from the OLE show the consistent and sustained reduction of Gd-lgA1, hematuria, and UPCR, as well as the stability of eGFR over 72 weeks in participants with IgAN. The demonstration of stable eGFR well beyond a year in participants receiving atacicept represents an important potential advancement for IgAN patients and has potential implications for the future treatment paradigm in this disease. It is also exciting that the data from the OLE show that switching to atacicept halted the eGFR decline seen in participants initially treated with placebo, with similar reductions in Gd-IgA1, hematuria, and UPCR as shown in the active cohort in the first 36 weeks,” stated Richard Lafayette, M.D., F.A.C.P., Professor of Medicine, Nephrology and Director of the Stanford Glomerular Disease Center at Stanford University Medical Center.
“We are thrilled to present this package of positive new data from the OLE of the Phase 2b ORIGIN clinical trial during our R&D Day, which will be held today in New York. We believe these data further support our belief that atacicept has the disruptive potential to stand out as a disease-modifying treatment for patients with IgAN,” said Marshall Fordyce, M.D., Chief Executive Officer of Vera Therapeutics. “This is an exciting period for Vera as the integrated data package for atacicept continues to mature. Importantly, the ongoing pivotal ORIGIN 3 trial is well underway, with enrollment on track to be completed in the second half of this year.”
After completing the 36-week randomized, double-blind, placebo-controlled period of the Phase 2b ORIGIN trial, all participants were eligible to receive atacicept 150 mg in the OLE. Of the 116 randomized participants, 106 completed 72 weeks.