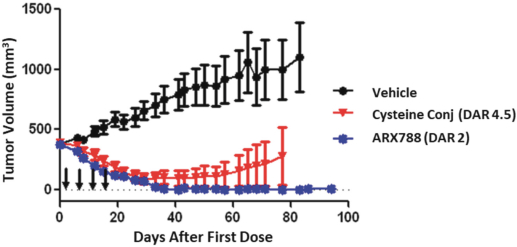
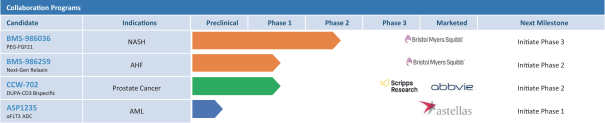
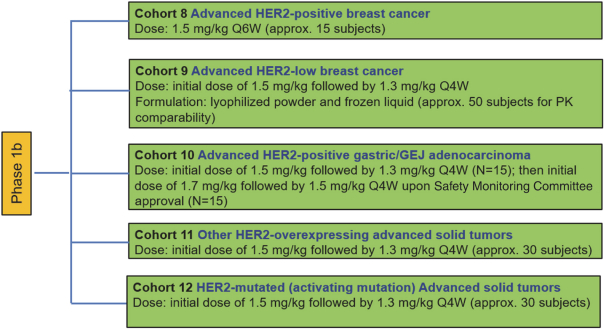
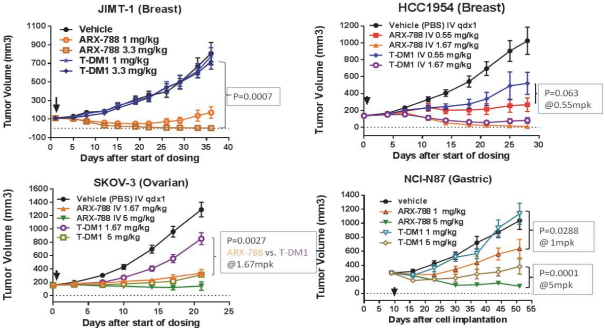
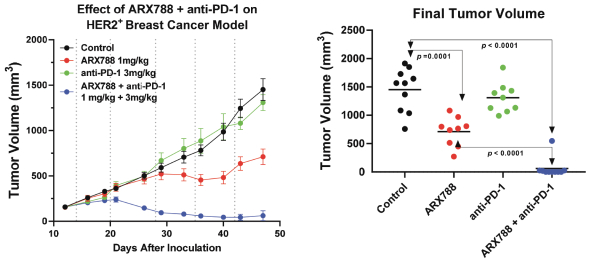
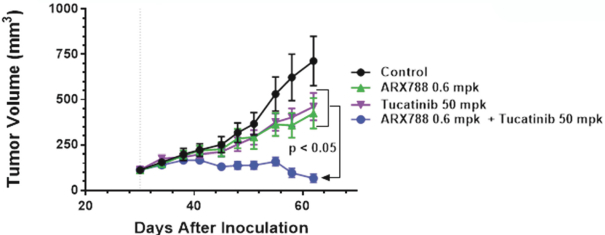
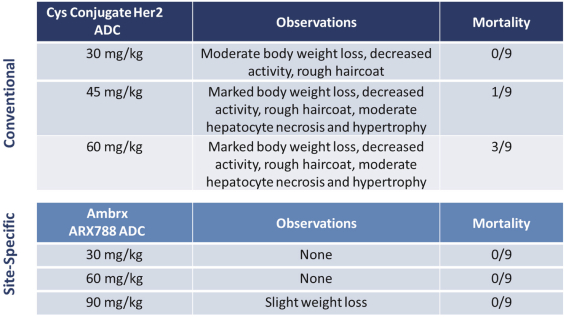
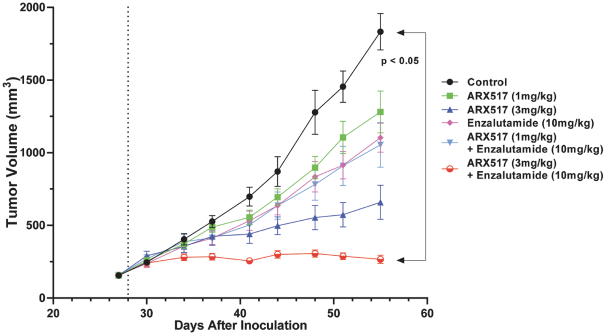
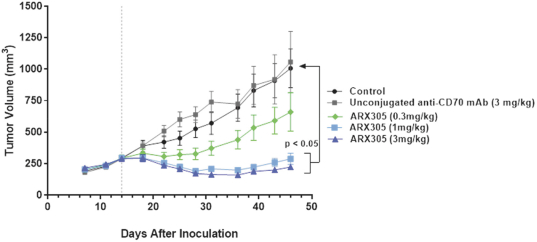
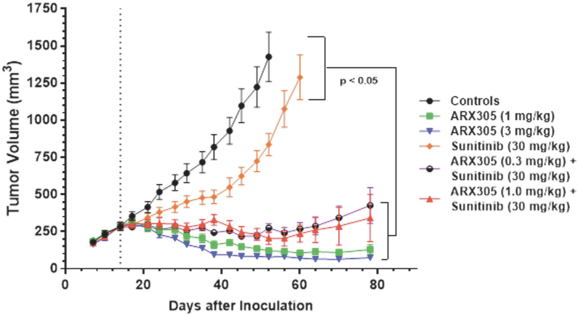
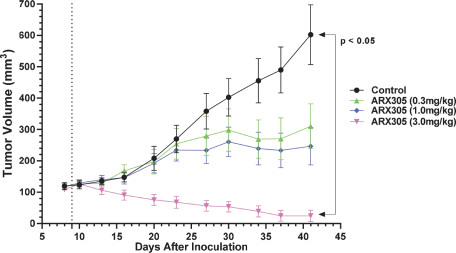
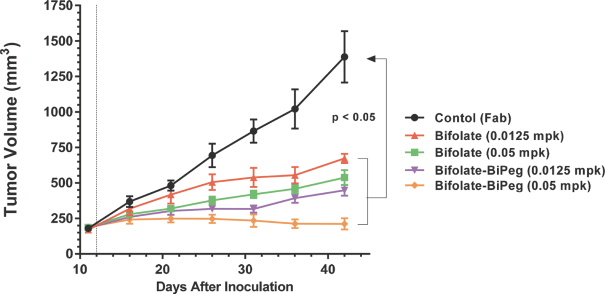
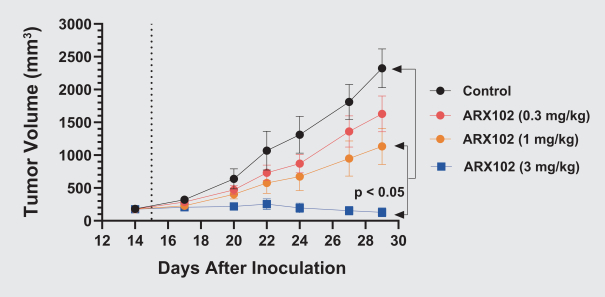
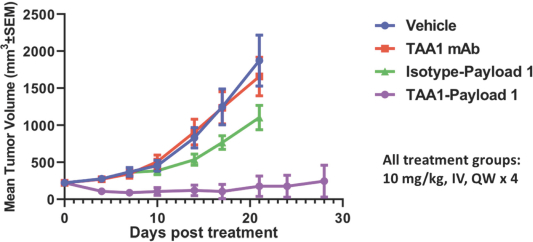
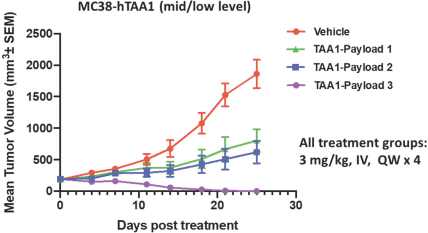
complex legal and factual questions. No consistent policy regarding the scope of claims allowable in patents in the field of antibody drug conjugates has emerged in the United States. The patent situation outside of the United States is even more uncertain. Changes in the patent laws and rules, either by legislation, judicial decisions, or regulatory interpretation in the United States and other countries, may diminish our ability to protect our inventions and enforce our intellectual property rights, and more generally could affect the value of our intellectual property. In particular, our ability to stop third parties from making, using, selling, offering to sell, importing, or otherwise commercializing any of our patented inventions, either directly or indirectly, will depend in part on our success in obtaining, defending, and enforcing patent claims that cover our technology, inventions, and improvements. With respect to both licensed and company-owned intellectual property, we cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our platform, product candidates, or the methods used to manufacture them. Moreover, our issued patents and those that may issue in the future may not guarantee us the right to practice our technology in relation to the commercialization of our platform’s product candidates. The area of patent and other intellectual property rights in biotechnology is an evolving one with many risks and uncertainties, and third parties may have blocking patents that could be used to prevent us from practicing our proprietary technology and commercializing our EuCODE and ReCODE technology platforms and product candidates. Our issued patents and those that may issue in the future may be challenged, narrowed, circumvented, or invalidated, which could limit our ability to stop competitors from marketing related platforms or product candidates or limit the length of the term of patent protection that we may have for our ADC and IOC product candidates. In addition, the rights granted under any issued patents may not provide us with protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies. For these reasons, we may have competition for our ADC and IOC product candidates. Moreover, because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that before any product candidate can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent for this and other risks related to our proprietary technology, inventions, improvements, platforms and product candidates. Please see “Risk Factors—Risks Related to Our Intellectual Property” for additional information on the risks associated with our intellectual property strategy and portfolio.
Government Regulation
Government authorities in the United States at the federal, state and local level and in other countries and jurisdictions, including the People’s Republic of China (PRC), extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, safety, efficacy, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug and biological products. Generally, before a new drug or biologic can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific for each regulatory authority, submitted for review and approved by the regulatory authority.
Regulatory Approval of Biologics in the United States
In the United States, biological products used for the prevention, treatment or cure of a disease or condition of a human being, such as our product candidates, are subject to regulation under the Federal Food, Drug, and Cosmetic Act (the FDCA), and the Public Health Service Act (the PHSA), their implementing regulations, and other federal, state, and local regulations that govern, among other things, the research, development, testing, manufacture, quality control, approval, safety, efficacy, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, import and export, post-approval monitoring and reporting, and marketing of biological products. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as clinical hold, FDA refusal to approve pending BLAs, warning or
186