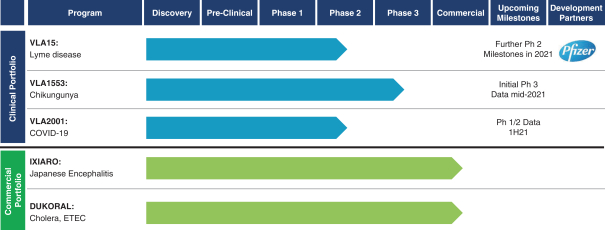
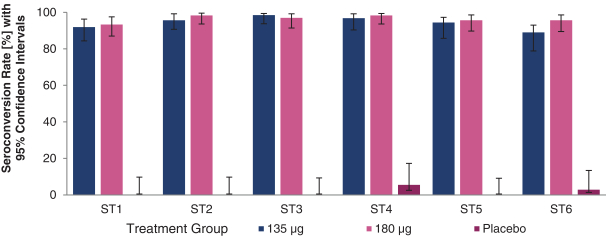
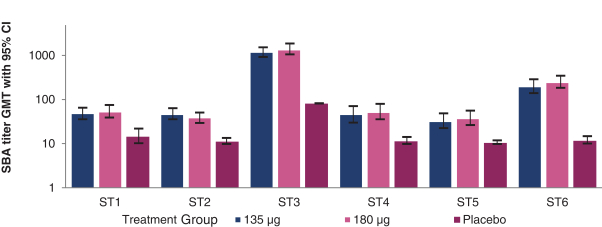
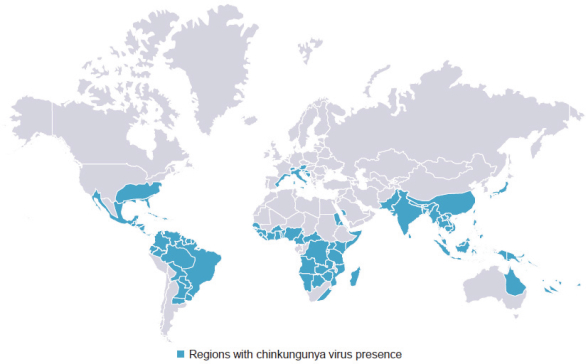
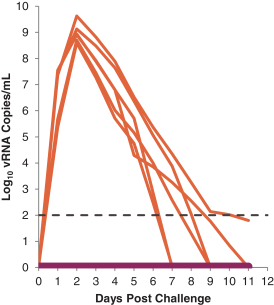
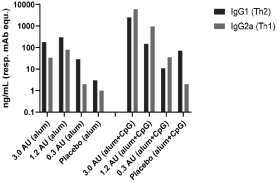
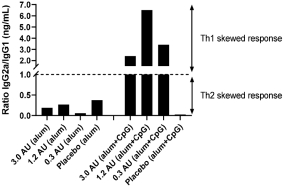
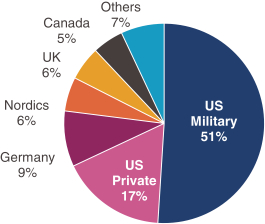
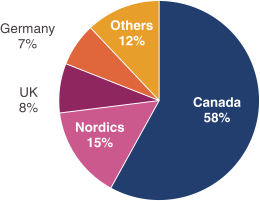
have a co-exclusive right to deliver, distribute, market, sell, promote, and import IXIARO in Germany solely with respect to certain non-profit organizations. Pursuant to the GSK Distribution Agreement, GSK is required to use reasonable commercial efforts to promote, sell and distribute IXIARO in Germany and is required to purchase an agreed upon minimum quantity of IXIARO doses during each year of the agreement. In connection with the GSK Distribution Agreement, we are obligated to supply (or designate a third-party entity to supply) GSK with all of its IXIARO supply requirements, subject to our reserved right to modify or discontinue manufacture and sale of IXIARO at our discretion. The GSK Distribution Agreement further provides that GSK must not manufacture, market, file applications for regulatory approval, distribute, sell or promote, in Germany manufacture, market, file applications for regulatory approval, distribute, sell or promote, in Germany a directly competing product that is a generic substitute for IXIARO.
The GSK Distribution Agreement shall continue until December 31, 2021. Either party may terminate the agreement upon (a) an uncured material breach of the agreement by, insolvency of, or change of control of the other party, or (b) withdrawal of marketing authorization for IXIARO in Germany. GSK may terminate this agreement if we fail to supply IXIARO under a firm purchase order for a specified period of time. In addition, we may terminate the agreement if GSK ceases to carry on business marketing pharmaceutical products in Germany, fails to comply with anti-corruption laws, does not achieve specified minimum purchase quantities, or breaches diligence obligations under that certain distribution agreement between the parties for the distribution of DUKORAL and we terminate such DUKORAL agreement for this same reason.
VaccGen Sublicense Agreement
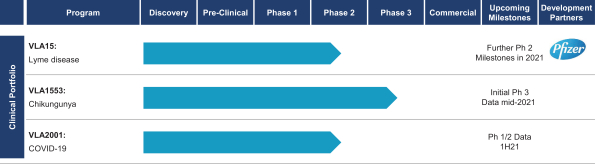
In April 2003, we (through our predecessor company Intercell Biomedical Ltd.) entered into a sublicense agreement, or the VaccGen Agreement, with VaccGen International, LLC, or VaccGen. We subsequently amended the VaccGen Agreement in October 2003, June 2004, March 2005, October 2005, April 2006, November 2006, December 2006, August 2007, and February 2010. Pursuant to this agreement, we obtained (a) an exclusive, worldwide (except the Caribbean), sublicensable sublicense under a prophylactic vaccine for Japanese encephalitis, the Vaccine, related patents and other intellectual property related to improvements made during the term of the agreement to develop, gain regulatory approval for, manufacture, have manufactured, distribute, use, offer for sale, import, sell, market, and otherwise commercially exploit the Vaccine and (b) an exclusive, worldwide (except for the Caribbean), royalty-free, transferable, sublicensable right and license under VaccGen’s interest in certain Vaccine information to use, reproduce, distribute, display, prepare derivative works of and otherwise modify, make, sell, offer to sell, import and otherwise use and exploit such information in connection with the foregoing license.
We are obligated to use commercially reasonable efforts to develop, manufacture, gain regulatory approval for and launch the Vaccine and to maximize net sales of the Vaccine worldwide (except the Caribbean). In connection with the VaccGen Agreement, we paid VaccGen an initial license fee of $350,000, a second license fee of $450,000, and $50,000 upon execution of the August 2007 amendment, pursuant to which the licensed territory was expanded to include the Republic of Korea. Additionally, we paid VaccGen $3.45 million in development and regulatory milestones and are obligated to pay VaccGen mid to high single-digit royalties on net sales of the Vaccine based on the entity making such sale, subject to specified reductions, and, in each case, subject to a minimum royalty payment ranging from mid six figures to low seven figures. Royalties on net sales of the Vaccine in specified countries are payable from January 1, 2010 until fourteen years thereafter or fourteen years from the date of regulatory approval in a specified country, based on the country of sale, marketing, or distribution. Royalties on other net sales of the Vaccine where the sale does not infringe, but for the sublicense granted to us under the VaccGen Agreement, a valid claim of the vaccine patents licensed to VaccGen issued in a country are payable to VaccGen until seven years from the first commercial sale of such Vaccine in such country. Royalties on other net sales of the Vaccine where the sale infringes a valid claim of the vaccine patents licensed to VaccGen issued in a country are payable to VaccGen beginning upon commercialization of such Vaccine and continue until the expiration or final determination of invalidity of the last such valid claim that would be infringed by such sale in such country. A further reduced royalty for a period of seven years from such expiration or final determination of invalidity of the last such valid claim that would be infringed by such sale in such
160