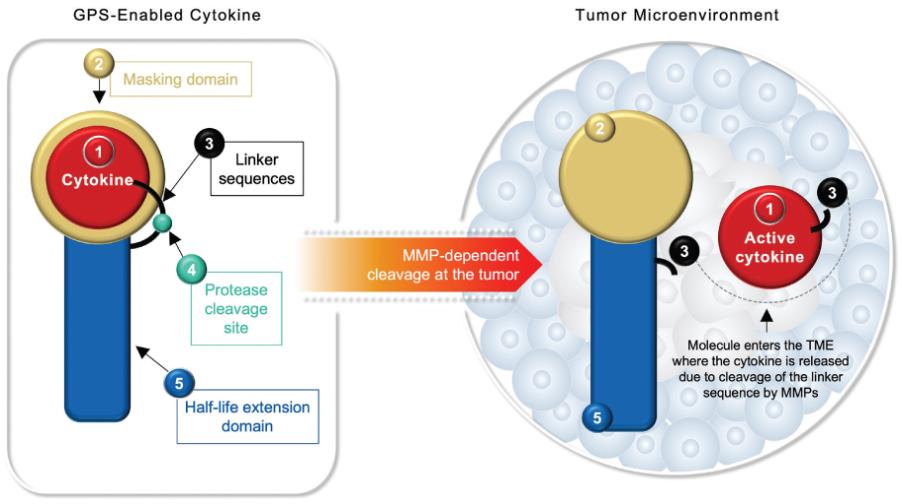
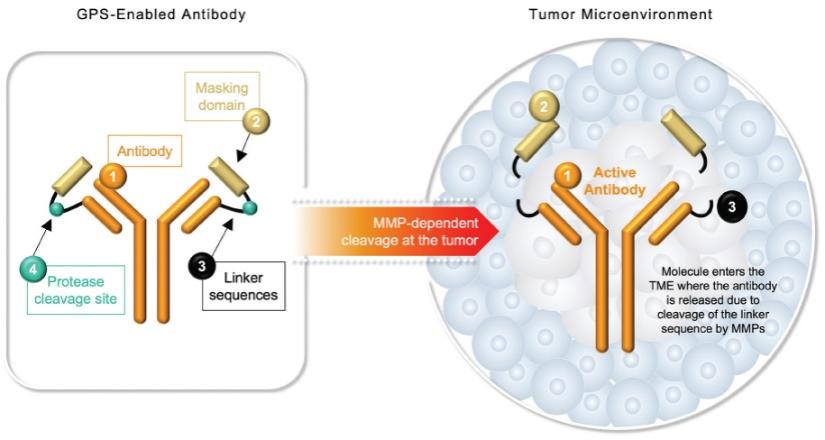
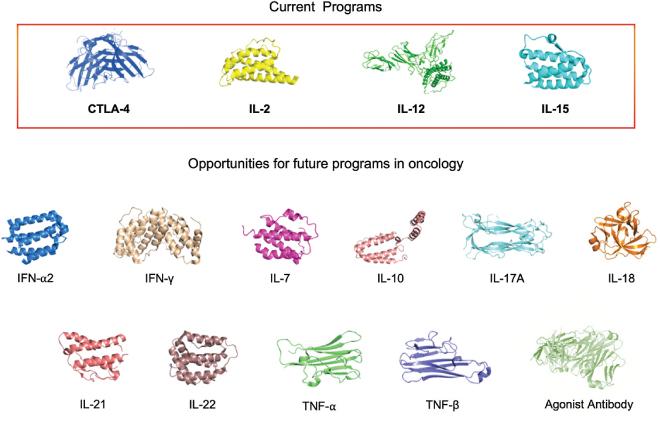
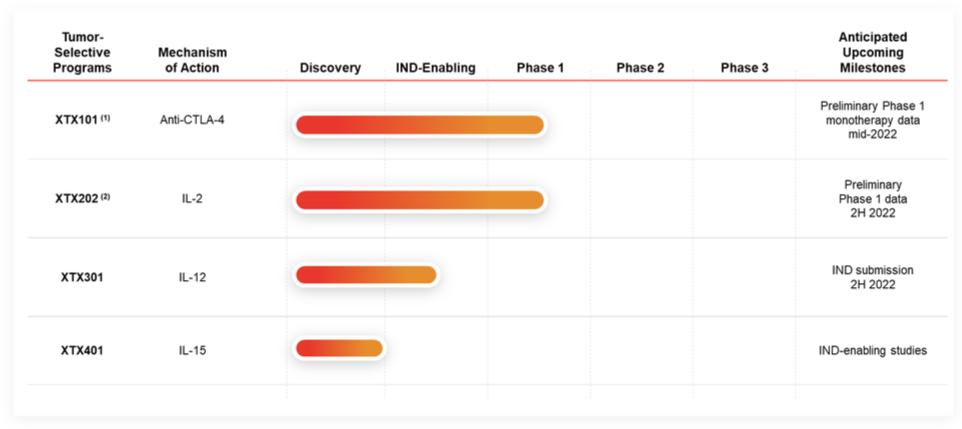
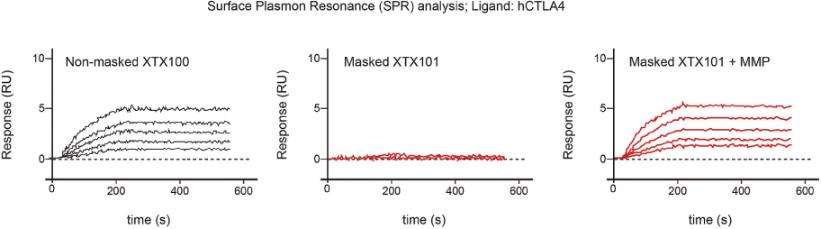
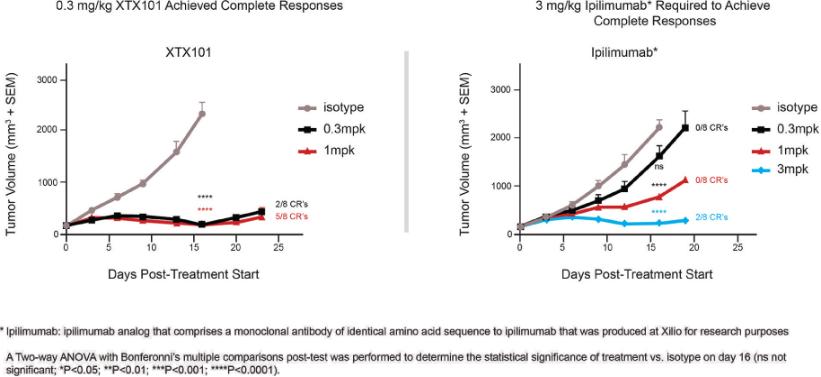
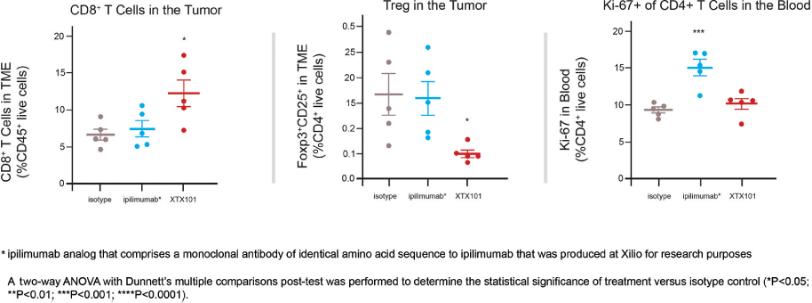
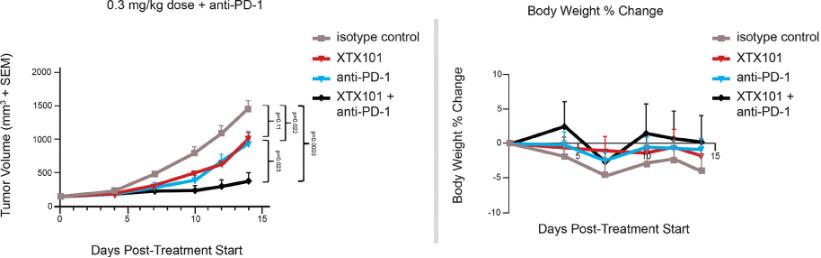
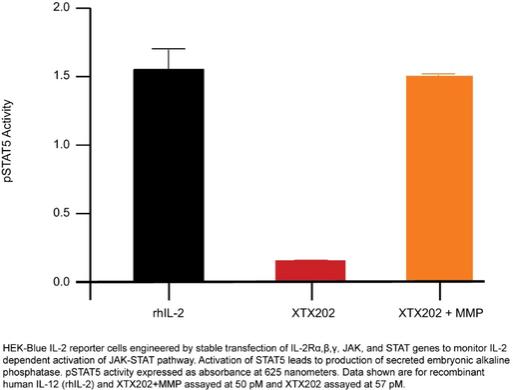
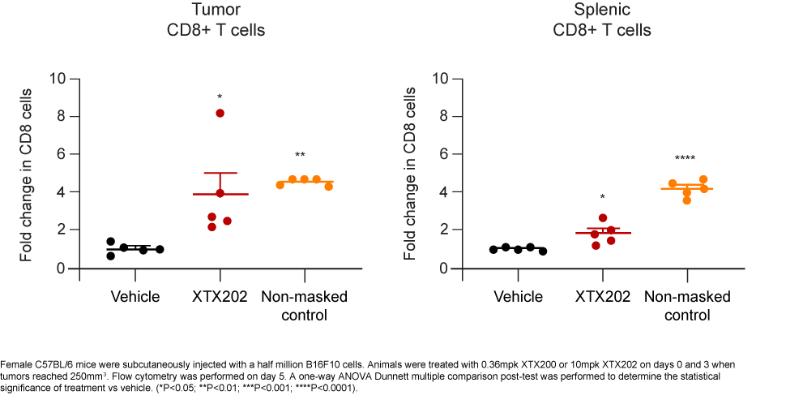
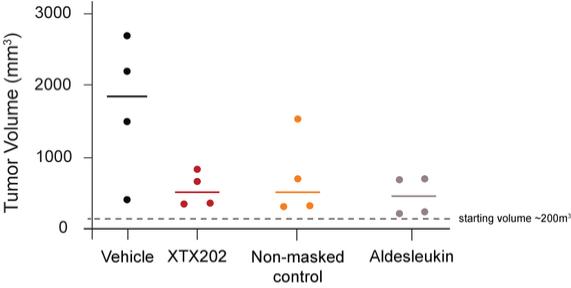
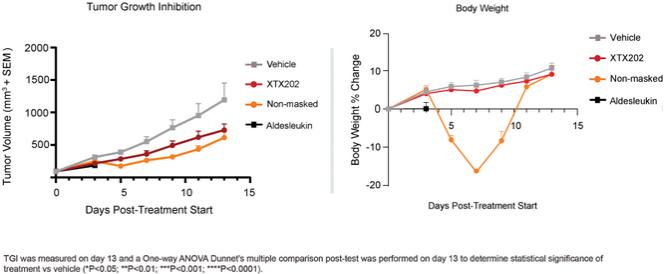
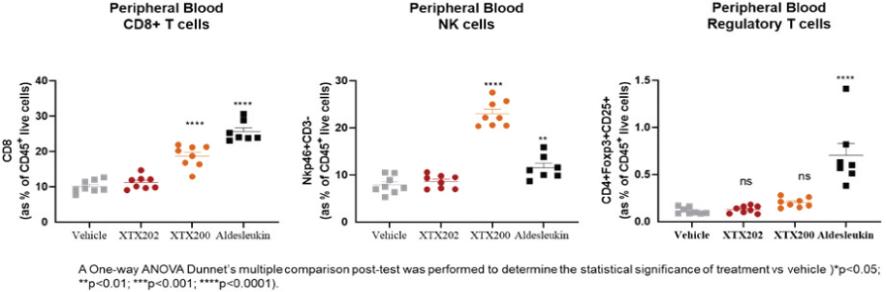
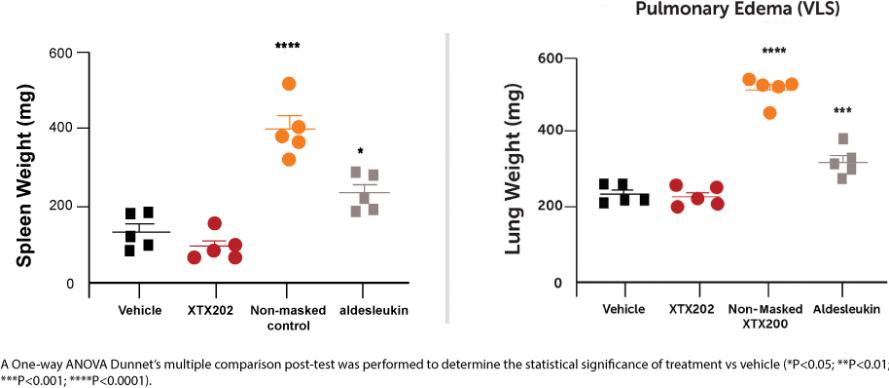
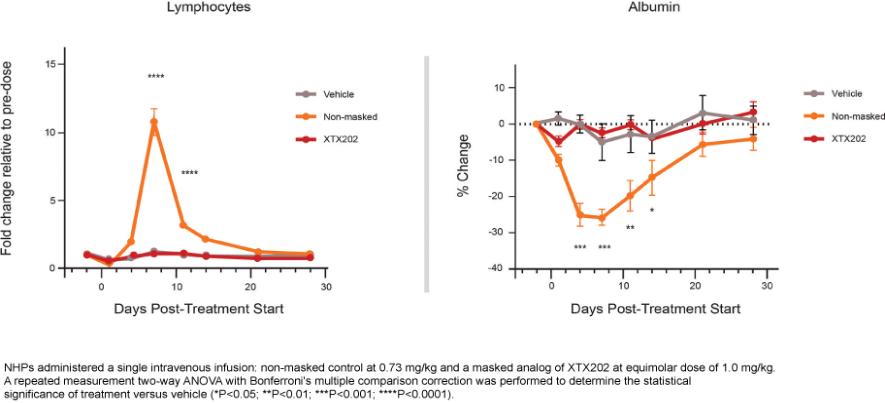
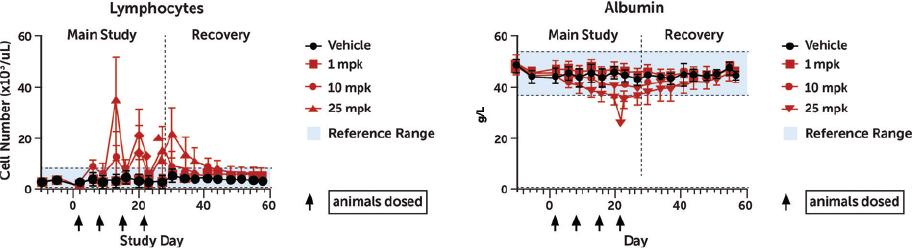
Checkpoint Inhibitor Program
We own, co-own or exclusively in-license three patent families relating to masked anti-CTLA-4 antibody constructs and sequences, including XTX101, with composition of matter and methods of use claims. A first patent family is exclusively in-licensed from WuXi Biologics (Shanghai) Co., Ltd. and directed to anti-CTLA-4 antibodies. This family includes one pending U.S. application and one issued U.S. patent covering certain complementarity-determining regions and variable region sequences of anti-CTLA4 antibodies, including XTX101. Corresponding foreign applications are pending in Taiwan, Australia, Brazil, Canada, China, Eurasian Patent Organization, European Patent Office, Hong Kong, India, Indonesia, Israeli, Japan, Republic of Korea, Mexico, Malaysia, New Zealand, Philippines, Saudi Arabia, Singapore, and South Africa. A second patent family is owned and directed to anti-CTLA-4 antibodies with modifications that improve antibody-dependent cellular cytotoxicity and includes one pending U.S. application. Corresponding foreign applications are pending in Australia, Brazil, Canada, China, Eurasian Patent Organization, European Patent Office, India, Indonesia, Israeli, Japan, Republic of Korea, Mexico, Malaysia, New Zealand, Philippines, Saudi Arabia, Singapore, and South Africa. A third patent family is co-owned and directed to masked anti-CTLA-4 antibodies, which includes one pending U.S. application. Corresponding foreign applications are pending in Australia, Brazil, Canada, Eurasian Patent Organization, European Patent Office, India, Indonesia, Israeli, Japan, Republic of Korea, Mexico, Malaysia, New Zealand, Philippines, Saudi Arabia, Singapore, South Africa and Taiwan. In addition, we own two U.S. provisional applications directed to combination therapies using masked or unmasked anti-CTLA-4 antibodies, including XTX101 and PD-1/PD-L1 antibodies. The statutory expiration date for these owned, co-owned, and licensed families ranges from 2037 to 2042, excluding any extension of patent term that may be available.
Trademark portfolio
As of February 15, 2022, we own two federal trademark registrations for XILIO and XILIO THERAPEUTICS in the United States, and we have received notices of allowance for two additional federal trademark applications for XILIO and XILIO THERAPEUTICS in the United States.
Patent prosecution
A PCT patent application is not eligible to become an issued patent until, among other things, we file one or more national stage patent applications within 30 months, 31 months or 32 months of the PCT application’s priority date, depending on the jurisdiction, in the countries in which we seek patent protection. If we do not timely file any national stage patent applications, we may lose our priority date with respect to our PCT patent application and any potential patent protection on the inventions disclosed in such PCT patent application. Moreover, a provisional patent application is not eligible to become an issued patent. A provisional patent application may serve as a priority filing for a non-provisional patent application, we file within 12 months of such provisional patent application. If we do not timely file non-provisional patent applications, we may lose our priority date with respect to our existing provisional patent applications and any potential patent protection on the inventions disclosed in our provisional patent applications.
While we intend to timely file additional provisional patent applications and national stage and non-provisional patent applications relating to our PCT patent applications, we cannot predict whether any of our patent applications will result in the issuance of patents. If we do not successfully obtain patent protection, or if the scope of the patent protection we or our licensors obtain with respect to our product candidates or technology, including our GPS, cytokine and antibody technologies is not sufficiently broad, we will be unable to prevent others from using our technology or from developing or commercializing technology and products similar or identical to ours or other similar competing products and technologies. Our ability to stop third parties from making, using, selling, offering to sell, importing or otherwise commercializing any of our technology, inventions and improvements, either directly or indirectly, will depend in part on our success in obtaining, maintaining, defending and enforcing patent claims that cover our technology, inventions and improvements.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. The protection afforded by a patent varies on a product-by-product basis, from jurisdiction-to-jurisdiction, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of patent term adjustments and regulatory-related patent term extensions, the availability of legal remedies in a particular jurisdiction and the validity and