us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials and acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
In addition to competitors specifically targeting anti-CTLA-4, IL-12 and IL-2, we also face competition more broadly across the oncology market. The most common methods of treating patients with cancer are surgery, radiation and drug therapy, including chemotherapy, hormone therapy, biologic therapy, such as monoclonal and bispecific antibodies, immunotherapy, cell-based therapy and targeted therapy, or a combination of any such treatments. Beyond these treatments, we may also be subject to competition from additional modalities, including oncolytic viruses and cancer vaccines.
Our commercial opportunity could be substantially limited if our competitors develop and commercialize products that are more effective, safer, less toxic, more convenient, or less expensive than products we may develop. In geographies that are critical to our commercial success, competitors may also obtain regulatory approvals before us, resulting in our competitors building a strong market position in advance of the entry of our products. In addition, our ability to compete may be affected in many cases by insurers or other third-party payers seeking to encourage the use of other drugs. The key competitive factors affecting the success of any products we may develop are likely to be their efficacy, safety, convenience, price and availability of reimbursement.
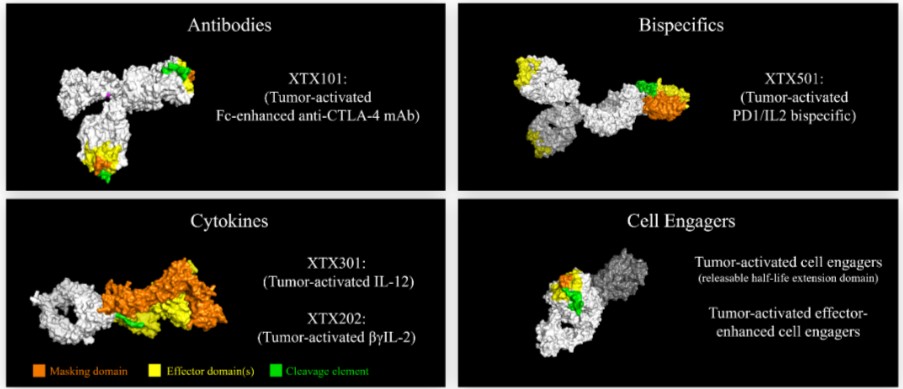
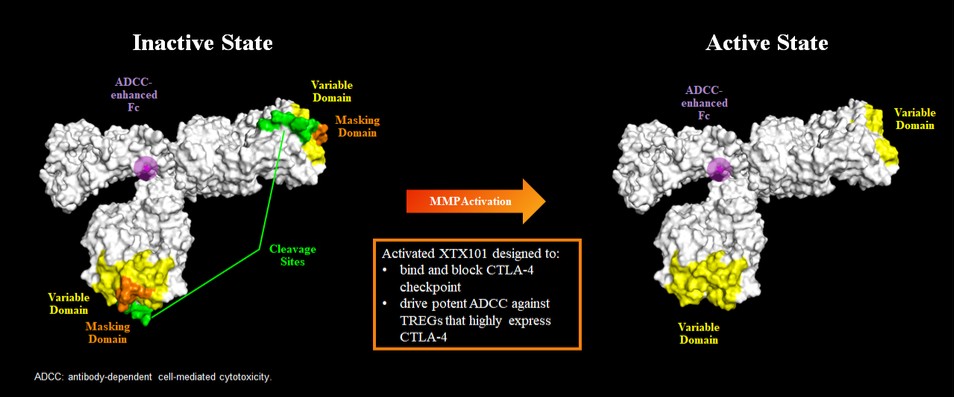
Tumor-Activated Anti-CTLA-4 Program
We are aware of a number of companies that are developing anti-CTLA-4 therapies as immunotherapies. With respect to XTX101, if approved, we may face competition from other anti-CTLA-4 based therapies. For example, Yervoy (ipilimumab), an anti-CTLA-4, is approved to treat melanoma, RCC, NSCLC and certain cancers of the large intestine, and Imjudo (tremelimumab) is approved as a combination therapy to treat unresectable hepatocellular carcinoma. In addition, we are aware that several companies have anti-CTLA-4 programs in development, including Adagene, Inc., Agenus Inc., AstraZeneca plc, BioAtla, Inc., CytomX Therapeutics, Inc., MacroGenics, Inc. and OncoC4, Inc.
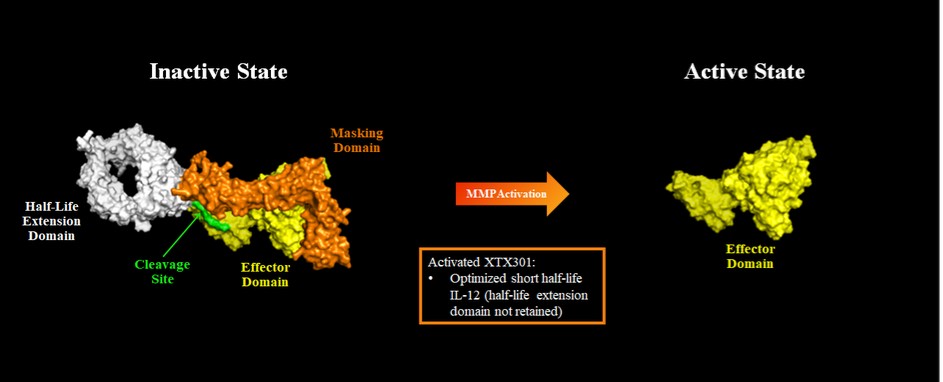
Tumor Activated Cytokine Programs
With respect to XTX301, there are no approved IL-12 therapies currently on the market for the treatment of cancer; however, we are aware of several other companies that have modified IL-12 or intra-tumoral IL-12 delivery programs for the treatment of cancer in development, including Amunix Pharmaceuticals, Inc., AstraZeneca plc / Moderna, Inc., Cullinan Management Inc., Dragonfly Therapeutics, Inc., ImmunityBio, Inc., PDS Biotechnology Corporation, Philogen S.p.A., Sonnet BioTherapeutics, Werewolf Therapeutics, Inc., Xencor Inc. and Zymeworks Inc.
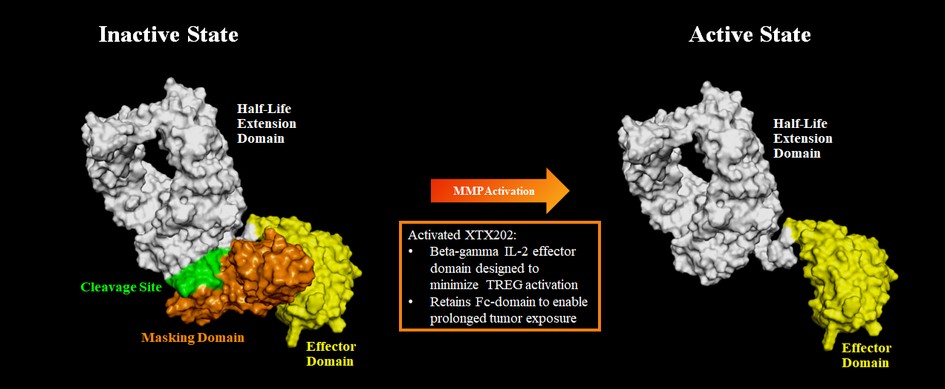
With respect to XTX202, if approved, it may face competition from other IL-2 based cancer therapies. For example, Proleukin (aldesleukin), a human recombinant interleukin-2 product, is approved and marketed for the treatment of metastatic RCC and melanoma. In addition, we are aware that a number of other companies have modified or low-dose IL-2 programs in development for the treatment of cancer, including Alkermes plc, Anaveon AG, Ascendis Pharma A/S, Asher Biotherapeutics, Inc., Aulos Bioscience, Inc., Bright Peak Therapeutics, Cue Biopharma, Inc., Cugene Inc., Cullinan Management Inc., Egle Therapeutics SAS, GI Innovation, Iovance Biotherapeutics, Inc., Kymab Ltd., Medicenna Therapeutics Corp., Medikine, Inc., Modulate Therapeutics, Inc., Neoleukin Therapeutics, Inc., Philogen S.p.A., Proviva Therapeutics, Inc., Roche AG, Sanofi, Selecxine, Synthekine, Inc., Trutino Biosciences Inc., Werewolf Therapeutics, Inc., XOMA Corporation and Zydus Cadila.
Intellectual Property
We strive to protect our proprietary technology, inventions, improvements, and platforms, including composition of matter for product candidates, methods of use and processes for their manufacture that we believe are important to our business, including by obtaining, maintaining, defending and enforcing patent and other intellectual property rights for the foregoing in the United States and in certain foreign jurisdictions. We also rely on trade secrets and confidentiality agreements to