
Exhibit 99.2 COVALENT-111: Phase II First Data Readout of Initial Healthy Volunteer (HV) and Type 2 Diabetes Mellitus (T2DM) Cohorts March 28, 2023

COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Legal Disclaimer & Forward-Looking Statements Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future business and financial performance of Biomea Fusion, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any projections of financial information or profitability, the initiation, timing and results of pending or future preclinical studies and clinical trials, the actual or potential actions of the FDA, the status and timing of ongoing research, development and corporate partnering activities, any statements about historical results that may suggest trends for the Company's business; any statements of the plans, strategies, and objectives of management for future operations; any statements of expectation or belief regarding future events, potential markets or market size, or technology developments, unfavorable global economic conditions, including inflationary pressures, market volatility, acts of war and civil and political unrest, and other factors affecting the Company's financial condition or operations. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward- looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company's control. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled Risk Factors in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the Securities and Exchange Commission. The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Page 2

COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Agenda Introduction Ramses Erdtmann Chief Operating Officer & Co-Founder of Biomea Diabetes Background & Overview Dr. Juan Frias Medical Director & Principal Investigator of Velocity Clinical Research, Scientific Advisory Board Member of Biomea Diabetes & Beta Cell Function Dr. Rohit Kulkarni Senior Investigator and Professor of Medicine of Harvard Medical School, Faculty Member, Joslin Diabetes Center, Scientific Advisory Board Member of Biomea COVALENT-111 First Study Results Dr. Steve Morris Chief Medical Officer of Biomea Executive Summary & Outlook Thomas Butler Chief Executive Officer, Chairman of the Board & Co-Founder of Biomea Page 3
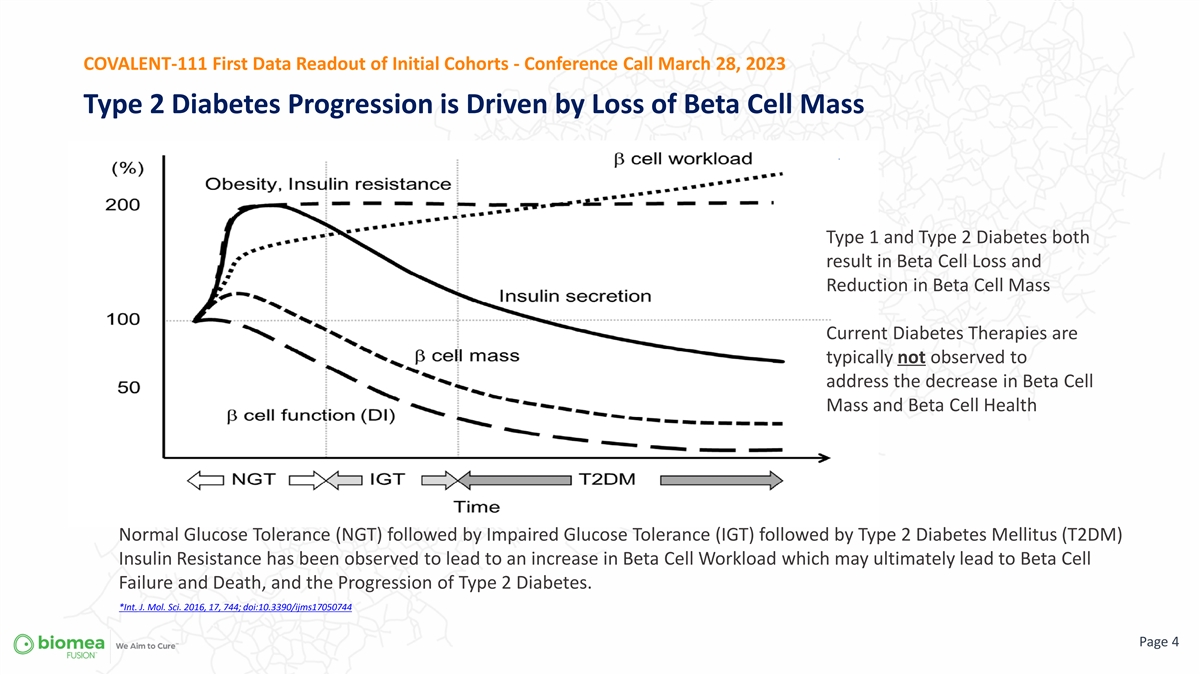
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Type 2 Diabetes Progression is Driven by Loss of Beta Cell Mass Type 1 and Type 2 Diabetes both result in Beta Cell Loss and Reduction in Beta Cell Mass Current Diabetes Therapies are typically not observed to address the decrease in Beta Cell Mass and Beta Cell Health Normal Glucose Tolerance (NGT) followed by Impaired Glucose Tolerance (IGT) followed by Type 2 Diabetes Mellitus (T2DM) Insulin Resistance has been observed to lead to an increase in Beta Cell Workload which may ultimately lead to Beta Cell Failure and Death, and the Progression of Type 2 Diabetes. *Int. J. Mol. Sci. 2016, 17, 744; doi:10.3390/ijms17050744 Page 4

COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Menin – Downregulated by Prolactin during Pregnancy Allowing for beta cell expansion and prevention of gestational diabetes • Stanford researchers have observed that during pregnancy, maternal pancreatic islets grow to match dynamic physiological demands. • Menin has been found to control islet growth in pregnant mice. Pregnancy stimulates proliferation of maternal pancreatic islet beta-cells, an effect accompanied by reduced beta-cell levels of menin and its targets. • Prolactin, a hormonal regulator of pregnancy, represses beta- cell menin levels and stimulates beta-cell proliferation. • Transgenic expression of menin in maternal beta-cells prevented islet expansion and led to hyperglycemia and impaired glucose tolerance, hallmark features of gestational diabetes. Karnik et al. Science, (2007), 801-806, 318(5851) Page 5 .
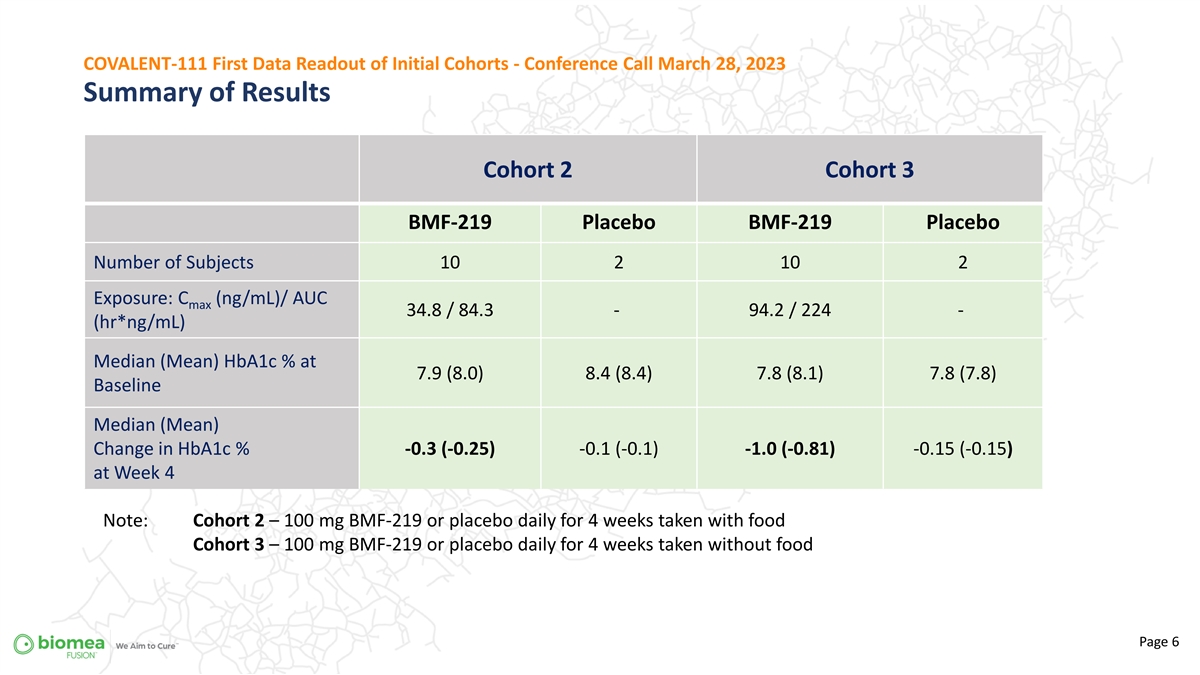
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Summary of Results Cohort 2 Cohort 3 BMF-219 Placebo BMF-219 Placebo Number of Subjects 10 2 10 2 400 mg Exposure: C (ng/mL)/ AUC max 34.8 / 84.3 - 94.2 / 224 - (hr*ng/mL) 200 mg Median (Mean) HbA1c % at 7.9 (8.0) 8.4 (8.4) 7.8 (8.1) 7.8 (7.8) Baseline Median (Mean) Change in HbA1c % -0.3 (-0.25) -0.1 (-0.1) -1.0 (-0.81) -0.15 (-0.15) at Week 4 Note: Cohort 2 – 100 mg BMF-219 or placebo daily for 4 weeks taken with food Cohort 3 – 100 mg BMF-219 or placebo daily for 4 weeks taken without food Page 6

COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 COVALENT-111: A Phase 1/2 Randomized, Double-Blind, Placebo-Controlled Single and Multiple Ascending Dose Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of BMF-219, an Oral Covalent Menin Inhibitor, in Healthy Adult Subjects and in Adult Subjects with Type 2 Diabetes Mellitus (NCT05731544) Study Treatment: BMF-219 SAD C1 to SAD C4 (HVs) A covalent small molecule menin inhibitor, administered orally daily in 28 day Total N=40 cycles Dose [100, 200, 400, and 600 mg] Primary Objective: MAD C1 (HVs) Evaluate safety and tolerability of BMF-219 Total N = 16 Secondary Objectives: Evaluate PK of BMF-219 MAD C2 to MAD C8 (T2DM) Evaluate the effect on BMF-219 on glycemic parameters (HbA1c, PG) Total N=108 and few additional parameters using OGTT, 7-day CGM Dose [100, 200, 300, 400, 600 mg] Evaluate the changes in beta cell function Evaluate impact on lipid parameters, body weight etc. Exploratory Objectives: In Phase 2, COVALENT-111 is enrolling subjects with an HbA1c of 7-10% despite being on standard of care (up to To assess the durability of response to glycemic parameters three T2DM agents). Page 7 Phase 2 Phase 1 (MAD) (SAD)
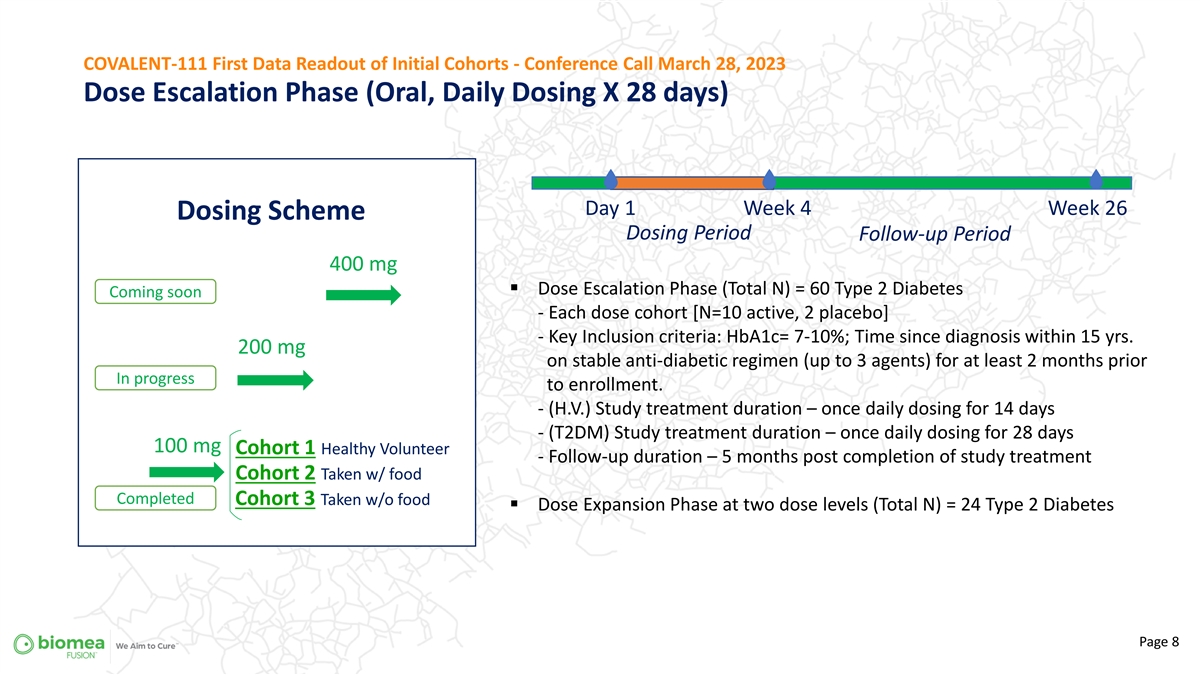
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Dose Escalation Phase (Oral, Daily Dosing X 28 days) Day 1 Week 4 Week 26 Dosing Scheme Dosing Period Follow-up Period 400 mg § Dose Escalation Phase (Total N) = 60 Type 2 Diabetes Coming soon - Each dose cohort [N=10 active, 2 placebo] - Key Inclusion criteria: HbA1c= 7-10%; Time since diagnosis within 15 yrs. 200 mg on stable anti-diabetic regimen (up to 3 agents) for at least 2 months prior In progress to enrollment. - (H.V.) Study treatment duration – once daily dosing for 14 days - (T2DM) Study treatment duration – once daily dosing for 28 days 100 mg Cohort 1 Healthy Volunteer - Follow-up duration – 5 months post completion of study treatment Cohort 2 Taken w/ food Completed Cohort 3 Taken w/o food § Dose Expansion Phase at two dose levels (Total N) = 24 Type 2 Diabetes Page 8

COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 COVALENT-111 Baseline Patient Characteristics Cohort 2 Cohort 3 BMF-219 Placebo BMF-219 Placebo Number of Subjects 10 2 10 2 Age (min, max) 35, 60 40, 53 38, 63 35, 61 Sex (M, F) 7, 3 2, 0 6, 4 2, 0 Time since T2DM 4 yrs, 14 yrs ~1 yrs, 5 yrs 6 mo, 9 yrs 9 mo, 9 yrs diagnosis (min, max) § Metformin (7/10) § Janumet (1/10)§ Metformin alone § Jardiance [Metformin + (1/2)§ Metformin alone (9/10) Concurrent Medications Empagliflozin] (1/10)§ Janumet § Janumet and Farxiga§ Metformin (2/2) for T2DM § Synjardy [Metformin + [Metformin + [Dapagliflozin] (1/10) Empagliflozin] (1/10) Sitagliptin] (1/2) Page 9
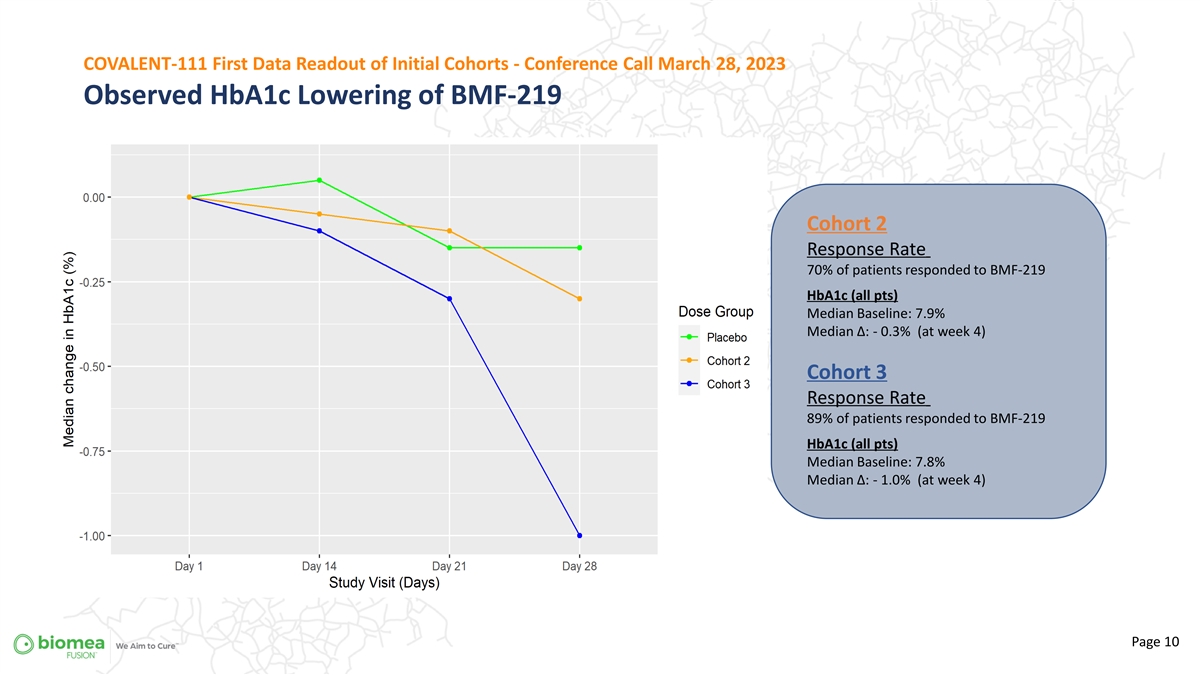
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Observed HbA1c Lowering of BMF-219 Cohort 2 Response Rate 70% of patients responded to BMF-219 HbA1c (all pts) Median Baseline: 7.9% Median ∆: - 0.3% (at week 4) Cohort 3 Response Rate 89% of patients responded to BMF-219 HbA1c (all pts) Median Baseline: 7.8% Median ∆: - 1.0% (at week 4) Page 10
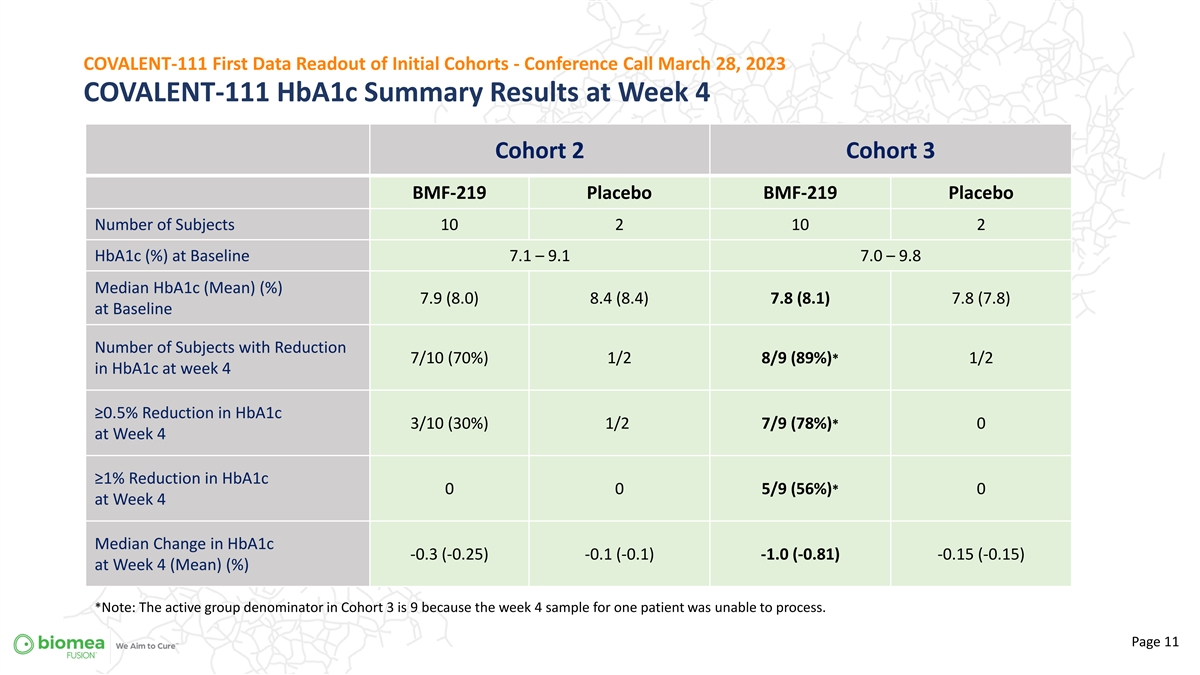
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 COVALENT-111 HbA1c Summary Results at Week 4 Cohort 2 Cohort 3 BMF-219 Placebo BMF-219 Placebo Number of Subjects 10 2 10 2 HbA1c (%) at Baseline 7.1 – 9.1 7.0 – 9.8 Median HbA1c (Mean) (%) 7.9 (8.0) 8.4 (8.4) 7.8 (8.1) 7.8 (7.8) at Baseline Number of Subjects with Reduction 7/10 (70%) 1/2 8/9 (89%)* 1/2 in HbA1c at week 4 ≥0.5% Reduction in HbA1c 3/10 (30%) 1/2 7/9 (78%)* 0 at Week 4 ≥1% Reduction in HbA1c * 0 0 0 5/9 (56%) at Week 4 Median Change in HbA1c -0.3 (-0.25) -0.1 (-0.1) -1.0 (-0.81) -0.15 (-0.15) at Week 4 (Mean) (%) *Note: The active group denominator in Cohort 3 is 9 because the week 4 sample for one patient was unable to process. Page 11
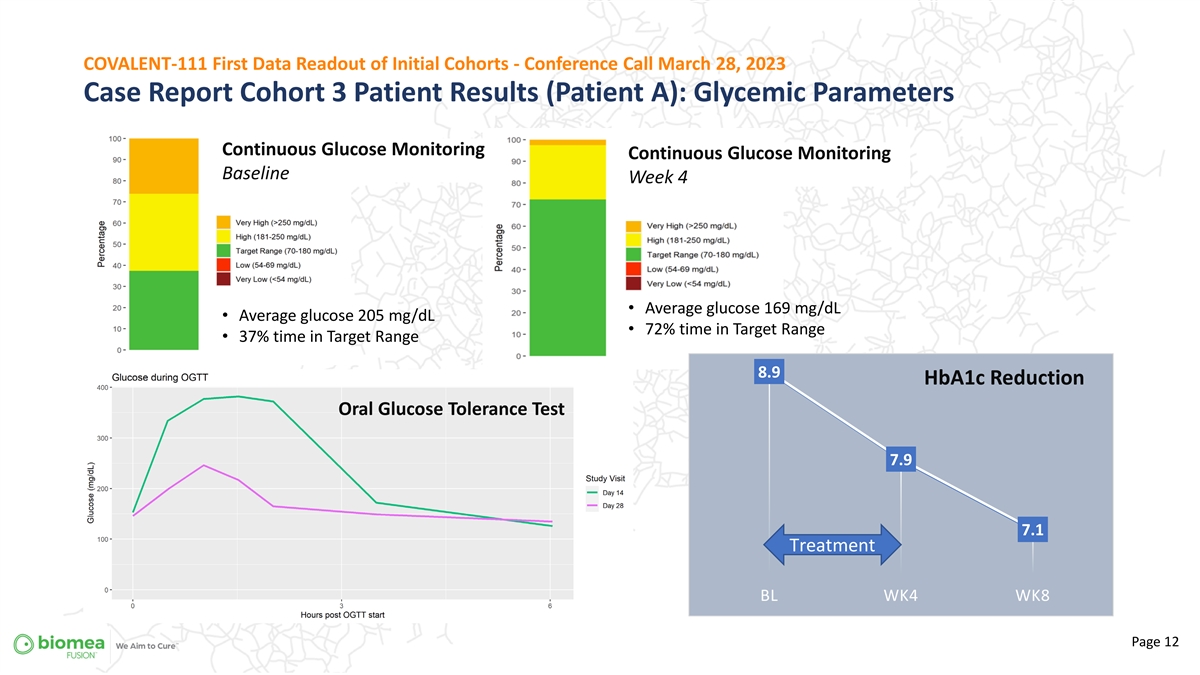
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Case Report Cohort 3 Patient Results (Patient A): Glycemic Parameters Continuous Glucose Monitoring Continuous Glucose Monitoring Baseline Week 4 + • Average glucose 169 mg/dL • Average glucose 205 mg/dL • 72% time in Target Range • 37% time in Target Range 8.9 HbA1c Reduction Oral Glucose Tolerance Test 7.9 Treatment 7.1 Treatment BL WK4 WK8 Page 12
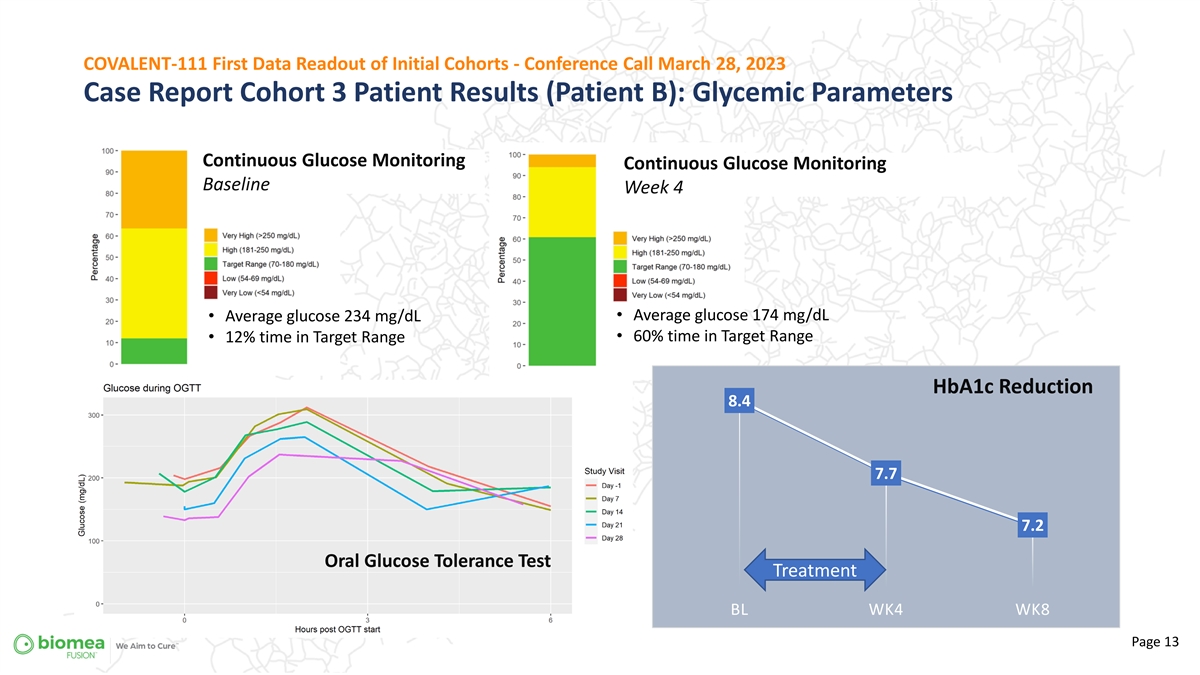
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Case Report Cohort 3 Patient Results (Patient B): Glycemic Parameters Continuous Glucose Monitoring Continuous Glucose Monitoring Baseline Week 4 + • Average glucose 174 mg/dL • Average glucose 234 mg/dL • 60% time in Target Range • 12% time in Target Range HbA1c Reduction 8.4 7.7 7.2 Oral Glucose Tolerance Test Treatment BL WK4 WK8 Page 13
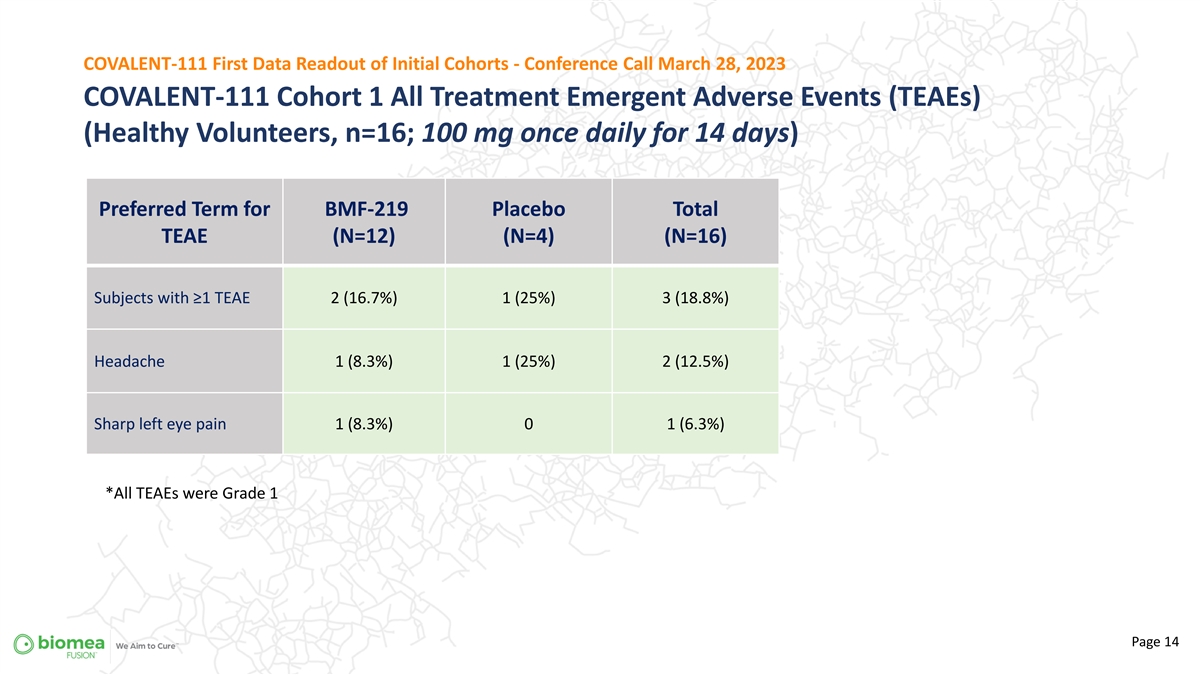
COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 COVALENT-111 Cohort 1 All Treatment Emergent Adverse Events (TEAEs) (Healthy Volunteers, n=16; 100 mg once daily for 14 days) Preferred Term for BMF-219 Placebo Total TEAE (N=12) (N=4) (N=16) Subjects with ≥1 TEAE 2 (16.7%) 1 (25%) 3 (18.8%) Headache 1 (8.3%) 1 (25%) 2 (12.5%) Sharp left eye pain 1 (8.3%) 0 1 (6.3%) *All TEAEs were Grade 1 Page 14

COVALENT-111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 COVALENT-111 Cohort 2 & 3 All TEAEs (Type 2 Diabetes n=24; 100 mg once daily dosing X 4 weeks) BMF-219 Placebo Total Preferred Term for TEAE (N=20) (N=4) (N=24) Subjects with ≥1 TEAE 7 (35%) 2 (50%) 9 (38%) Abdominal bloating 2 (10%) 0 2 (8.4%) Elevated pancreatic polypeptide in plasma 0 2 (50%) 2 (8.4%) Cough 1 (5%) 1 (25%) 2 (8.4%) Elevated GGT 1 (5%) 0 1 (4.2%) Elevated AST 1 (5%) 0 1 (4.2%) Elevated ALT 1 (5%) 0 1 (4.2%) Sore throat 1 (5%) 0 1 (4.2%) Non-specific GI symptoms 1 (5%) 0 1 (4.2%) Decreased interleukin-6 1 (5%) 0 1 (4.2%) Swollen lymph nodes 0 1 (25%) 1 (4.2%) Elevated lipase 1 (5%) 0 1 (4.2%) Intermittent headaches 1 (5%) 0 1 (4.2%) Contact dermatitis 1 (5%) 0 1 (4.2%) All TEAEs are Grade 1 except Elevated Lipase (Grade 2) Page 15
COVALENT 111 First Data Readout of Initial Cohorts - Conference Call March 28, 2023 Summary of Data Safety • BMF-219 demonstrated a well-tolerated safety profile • No dose discontinuations • All 20 subjects completed 4 weeks of treatment and continue in 5-month follow-up period • No severe or serious TEAEs were observed • No episodes of symptomatic hypoglycemia occurred in any patients Efficacy Cohort 3 highlights • 89% pts achieved a reduction in HbA1c • 78% pts achieved ≥ 0.5% reduction in HbA1c • 56% pts achieved ≥ 1% reduction in HbA1c • Positive trend in OGTT and CGM parameters Page 16

Q & A Biomea Fusion 900 Middlefield Road, 4th floor Redwood City, CA, 94063 biomeafusion.com Confidential 17

THANK YOU Biomea Fusion 900 Middlefield Road, 4th floor Redwood City, CA, 94063 biomeafusion.com Confidential 18

















