
BIOMEA cONFERENCE Call Preclinical Data Combining icovamenib with a GLP-1-Based Therapy & Biomea’s Oral GLP-1 RA Candidate BMF-650 October 30, 2024 Exhibit 99.1

Legal Disclaimer & Forward-Looking Statements Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future business and financial performance of Biomea Fusion, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any projections of financial information or profitability, the initiation, timing and results of pending or future preclinical studies and clinical trials, the actual or potential actions of the FDA, the status and timing of ongoing research, development and corporate partnering activities, any statements about historical results that may suggest trends for the Company's business; any statements of the plans, strategies, and objectives of management for future operations; any statements of expectation or belief regarding future events, potential markets or market size, or technology developments, unfavorable global economic conditions, including inflationary pressures, market volatility, acts of war and civil and political unrest, and other factors affecting the Company's financial condition or operations. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company's control. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent periodic report filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the Securities and Exchange Commission. The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Page Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib

Agenda Page Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Ramses Erdtmann Chief Operating Officer & Co-Founder of Biomea Thomas Butler Chief Executive Officer, Chairman of the Board & Co-Founder of Biomea Juan Frias, M.D. Chief Medical Director of Biomea Mini Balakrishnan, Ph.D. Executive Director of Biology, Biomea Thorsten Kirschberg, Ph.D. Executive Vice President of Chemistry, Biomea Thomas Butler Chief Executive Officer, Chairman of the Board & Co-Founder of Biomea Introduction Executive Summary GLP-1 Based Therapy Background & Overview Icovamenib in combination with GLP-1 Based Therapy BMF-650 Preclinical Summary Closing Remarks
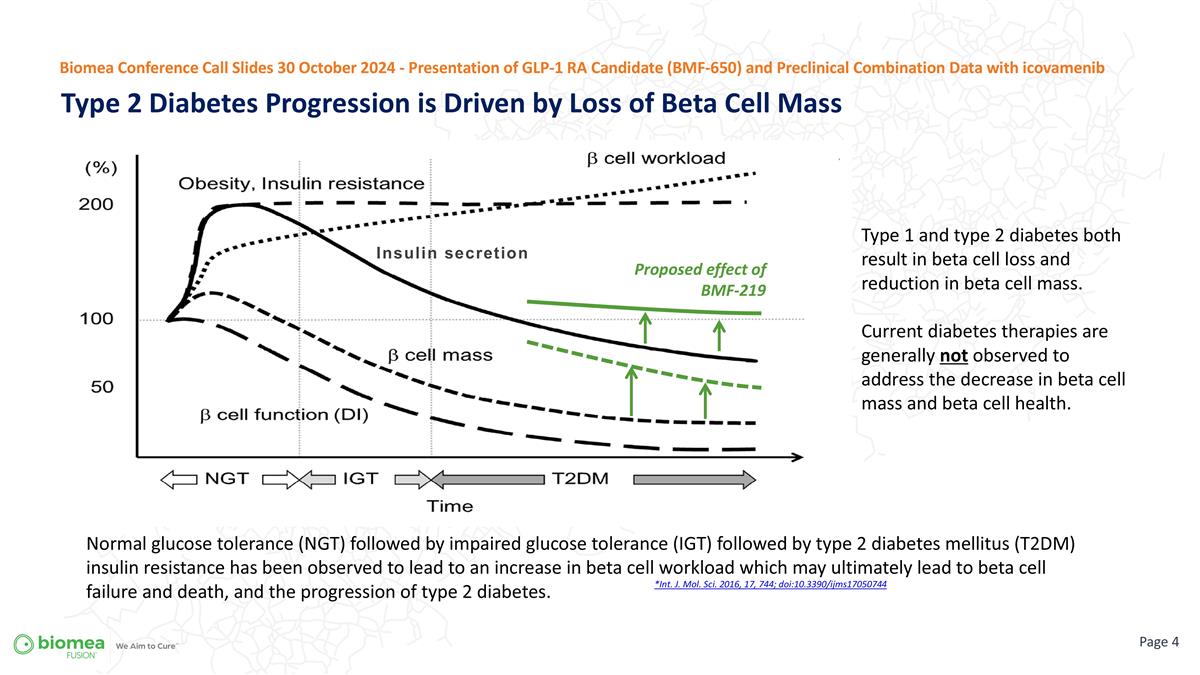
*Int. J. Mol. Sci. 2016, 17, 744; doi:10.3390/ijms17050744 Type 1 and type 2 diabetes both result in beta cell loss and reduction in beta cell mass. Current diabetes therapies are generally not observed to address the decrease in beta cell mass and beta cell health. Normal glucose tolerance (NGT) followed by impaired glucose tolerance (IGT) followed by type 2 diabetes mellitus (T2DM) insulin resistance has been observed to lead to an increase in beta cell workload which may ultimately lead to beta cell failure and death, and the progression of type 2 diabetes. Type 2 Diabetes Progression is Driven by Loss of Beta Cell Mass Page Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Proposed effect of BMF-219 Insulin secretion
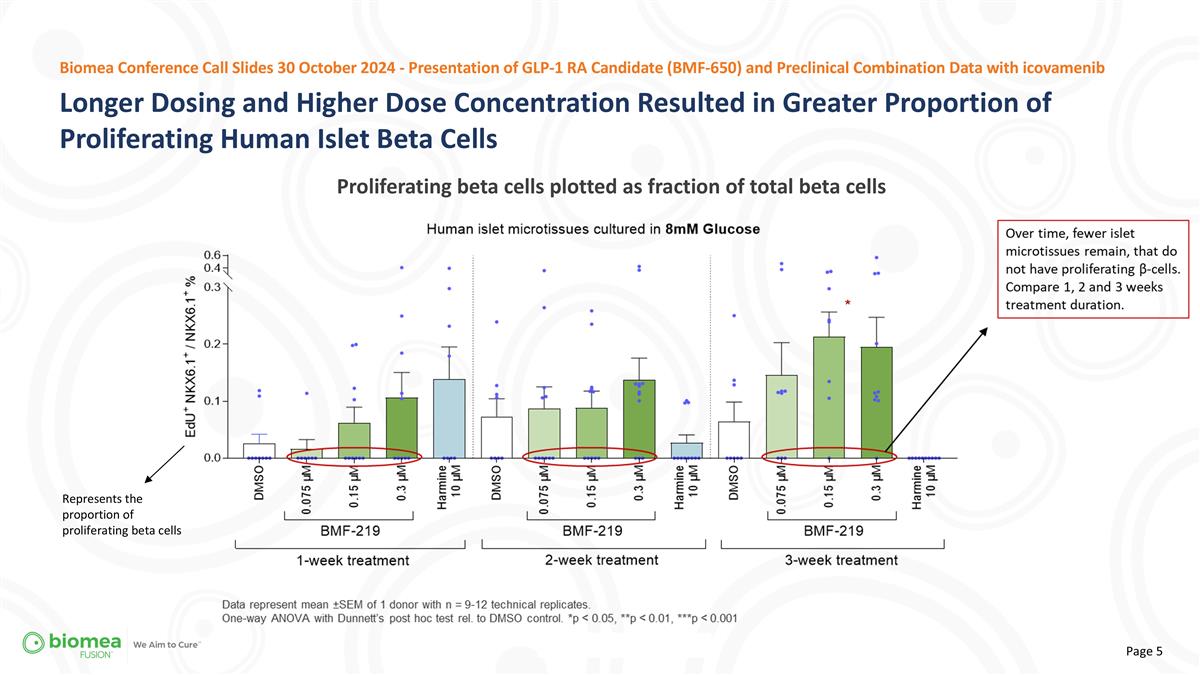
Longer Dosing and Higher Dose Concentration Resulted in Greater Proportion of Proliferating Human Islet Beta Cells Proliferating beta cells plotted as fraction of total beta cells Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Represents the proportion of proliferating beta cells
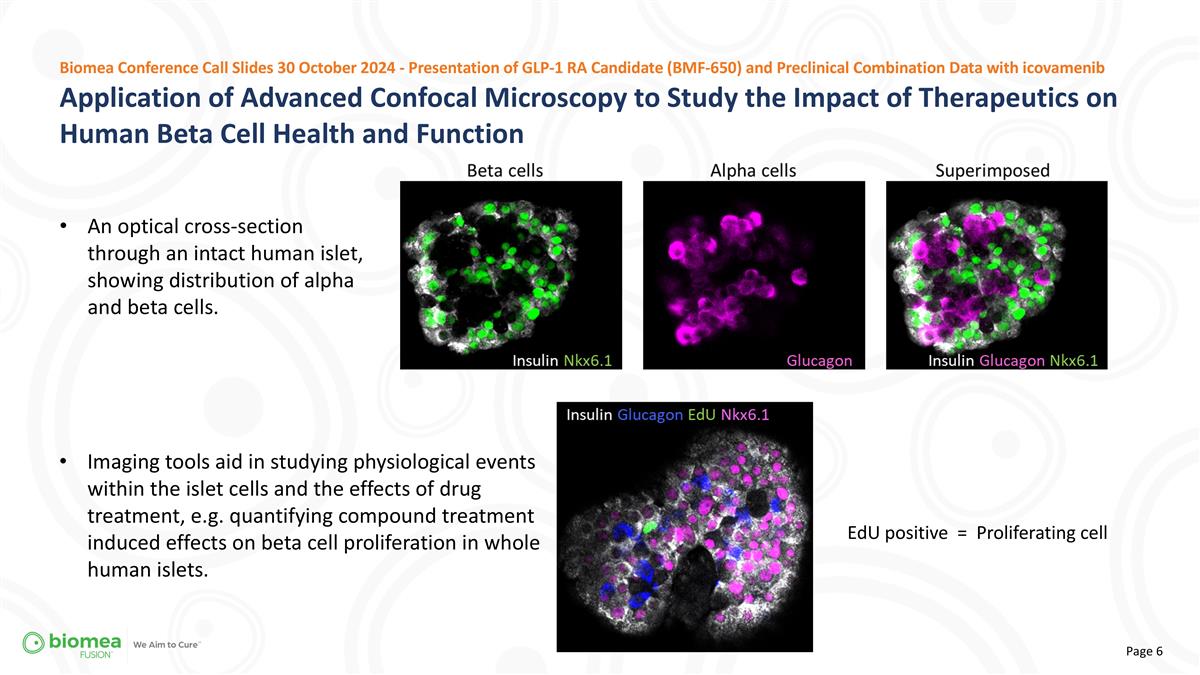
Application of Advanced Confocal Microscopy to Study the Impact of Therapeutics on Human Beta Cell Health and Function Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib An optical cross-section through an intact human islet, showing distribution of alpha and beta cells. Imaging tools aid in studying physiological events within the islet cells and the effects of drug treatment, e.g. quantifying compound treatment induced effects on beta cell proliferation in whole human islets. EdU positive = Proliferating cell

Icovamenib (BMF-219) in Combination with a GLP-1 Based Therapy – An Overview of Pre-Clinical Findings
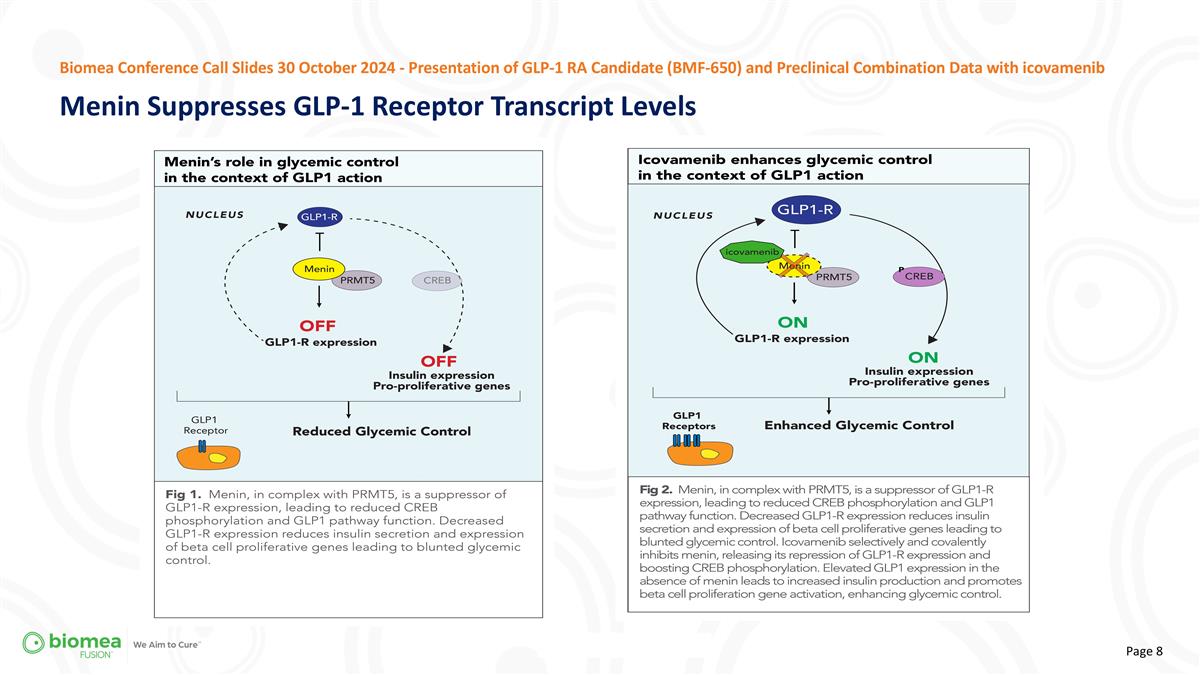
Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Menin Suppresses GLP-1 Receptor Transcript Levels
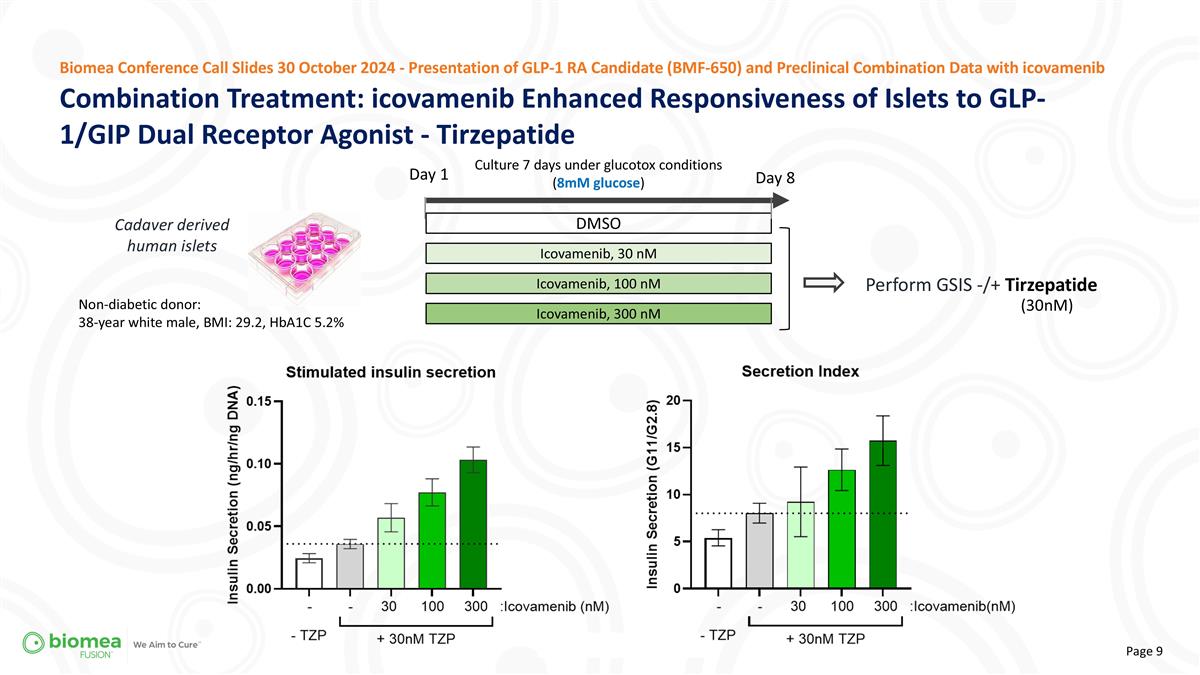
Cadaver derived human islets Combination Treatment: icovamenib Enhanced Responsiveness of Islets to GLP-1/GIP Dual Receptor Agonist - Tirzepatide Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Day 1 Perform GSIS -/+ Tirzepatide Culture 7 days under glucotox conditions (8mM glucose) DMSO Icovamenib, 30 nM Icovamenib, 100 nM Day 8 Icovamenib, 300 nM Non-diabetic donor: 38-year white male, BMI: 29.2, HbA1C 5.2% (30nM)
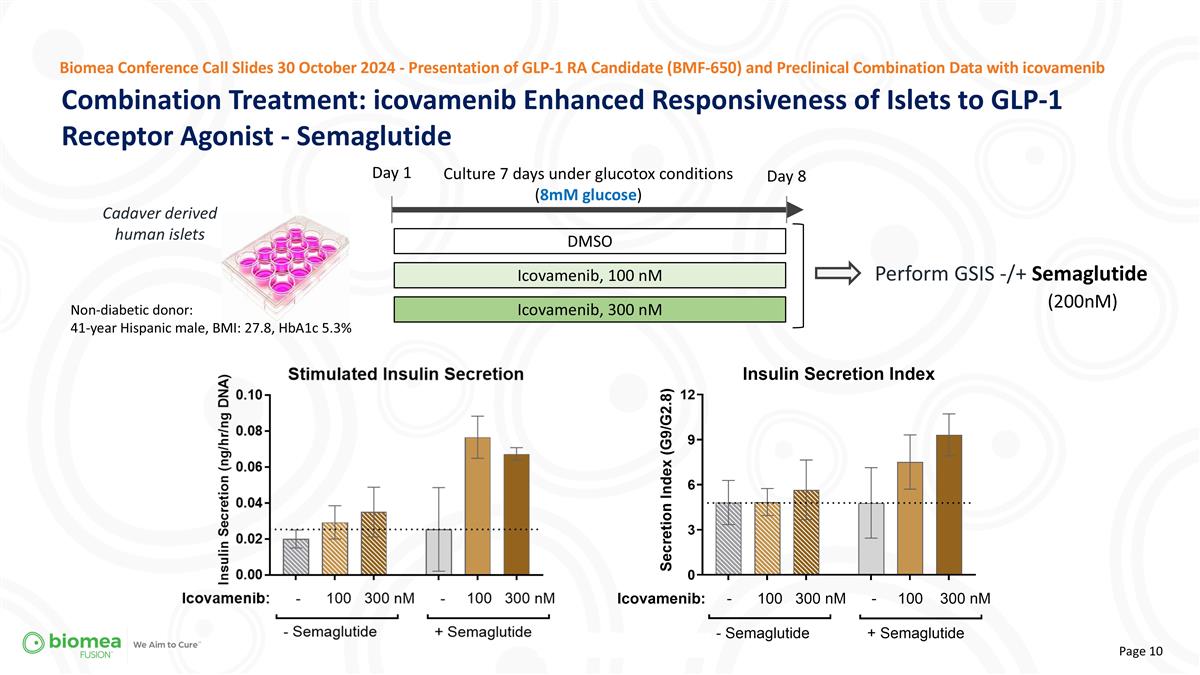
Combination Treatment: icovamenib Enhanced Responsiveness of Islets to GLP-1 Receptor Agonist - Semaglutide Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Day 1 Perform GSIS -/+ Semaglutide (200nM) Culture 7 days under glucotox conditions (8mM glucose) DMSO Icovamenib, 100 nM Icovamenib, 300 nM Day 8 Non-diabetic donor: 41-year Hispanic male, BMI: 27.8, HbA1c 5.3% Cadaver derived human islets
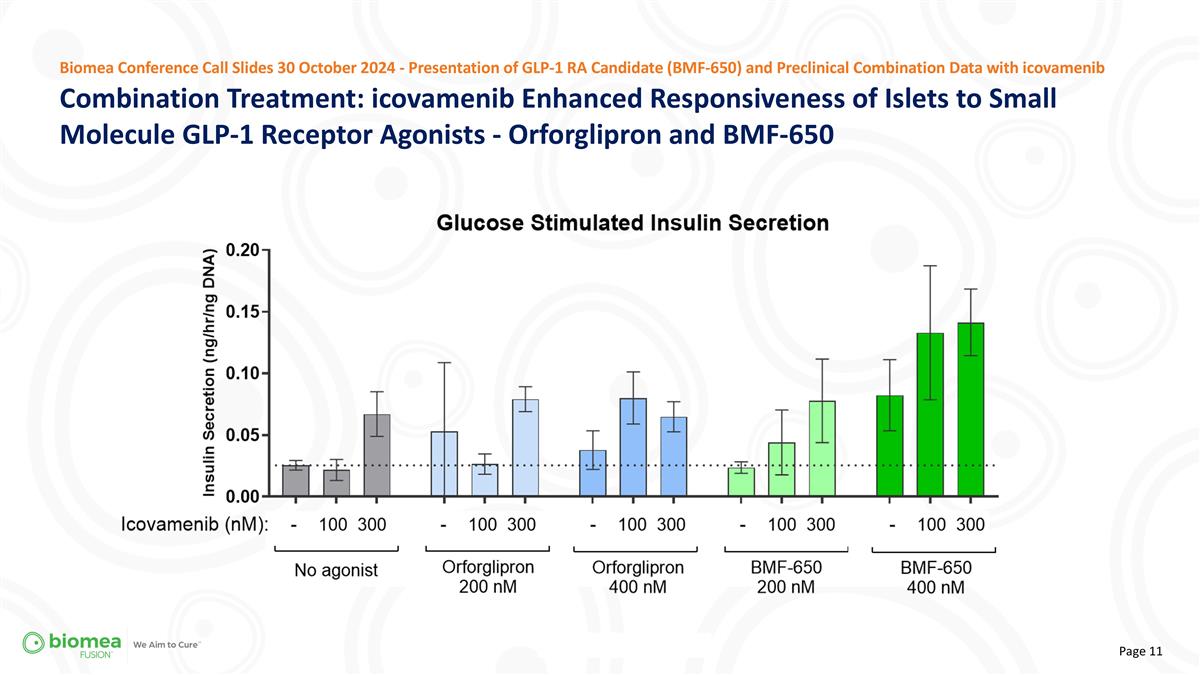
Combination Treatment: icovamenib Enhanced Responsiveness of Islets to Small Molecule GLP-1 Receptor Agonists - Orforglipron and BMF-650 Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib

Potential benefits of using icovamenib together with approved GLP-1 based therapeutics: Lower dosing requirements of existing GLP-1 based therapy Improved tolerability Improved adherence Improved therapeutic window Improved initial responsiveness - Greater patient persistence and treatment results with GLP-1 based therapeutics Next steps in Biomea’s clinical development: - COVALENT-211 (icovamenib in combination with GLP-1 based therapeutics) Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib New Treatment Potential in Diabetes and for Obesity Combining Covalent Binding Menin Inhibitor icovamenib with GLP-1 Based Therapy

BMF-650 – An Investigational, Next-Generation, Oral Small Molecule GLP-1 Receptor Agonist

Attributes of Biomea’s GLP-1 Receptor Agonist Development Candidate: Less PK variability Greater bioavailability Greater protein binding Less side effects Why a greater “Therapeutic Window”? Only 3 of 10 patients in the real-world setting are staying on a GLP-1 based therapy after 12 months Drive for a Greater “Therapeutic Window” with our Next-Generation GLP-1 Receptor Agonist – BMF-650 Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib

GLP-1 human EC50 b-arrestin1 EC50 b-arrestin2 EC50 BMF-650 < 5 nM > 10 mM > 10 mM orforglipron < 5 nM > 10 mM > 10 mM Good potency on-target No off-target concerns from counter-screening assays BMF-650 Showed Favorable In Vitro On-Target Activity and Off-Target Selectivity Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib
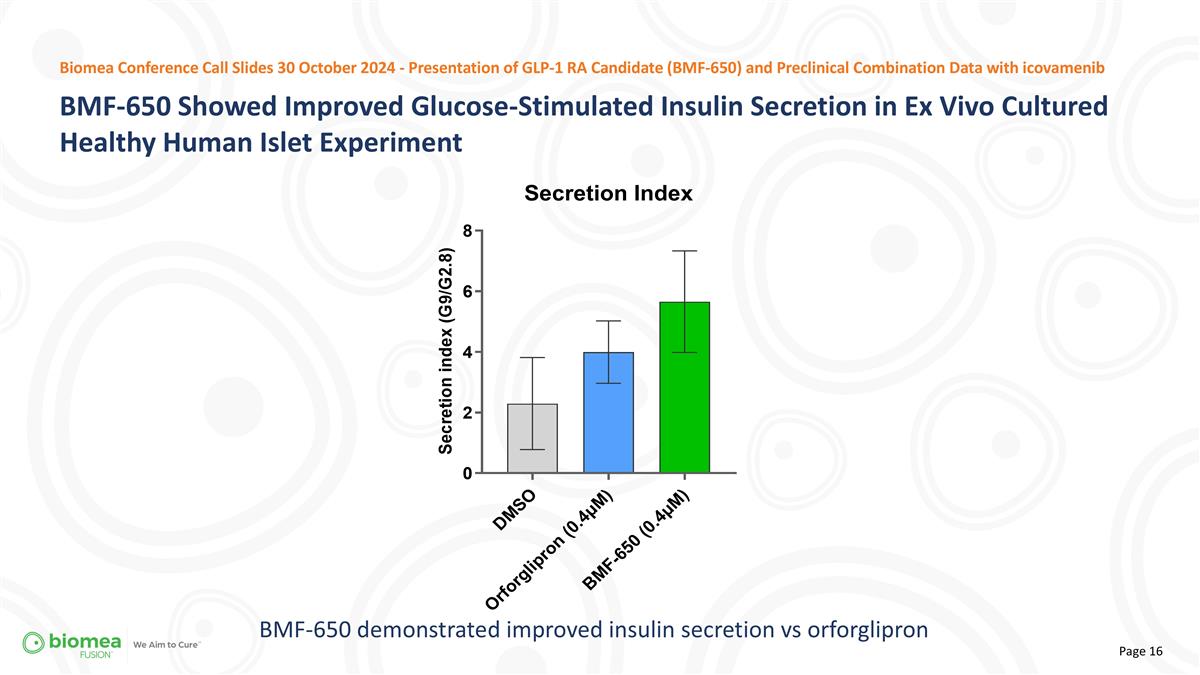
BMF-650 demonstrated improved insulin secretion vs orforglipron BMF-650 Showed Improved Glucose-Stimulated Insulin Secretion in Ex Vivo Cultured Healthy Human Islet Experiment Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib
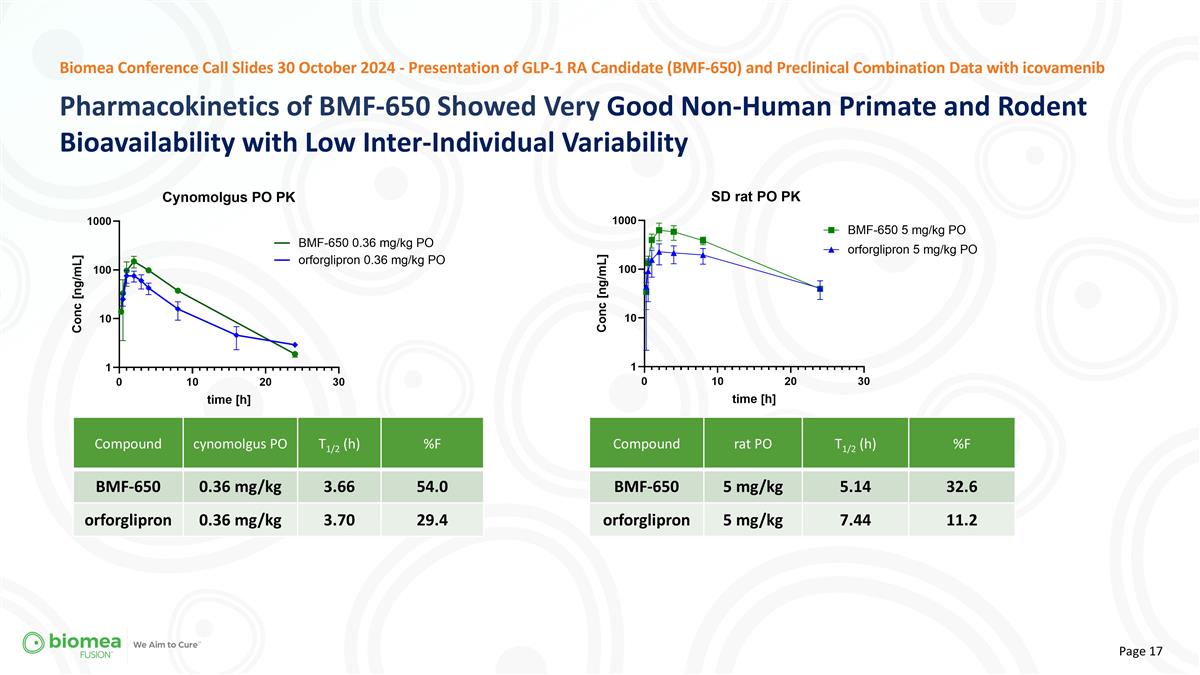
Pharmacokinetics of BMF-650 Showed Very Good Non-Human Primate and Rodent Bioavailability with Low Inter-Individual Variability Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Compound cynomolgus PO T1/2 (h) %F BMF-650 0.36 mg/kg 3.66 54.0 orforglipron 0.36 mg/kg 3.70 29.4 Compound rat PO T1/2 (h) %F BMF-650 5 mg/kg 5.14 32.6 orforglipron 5 mg/kg 7.44 11.2
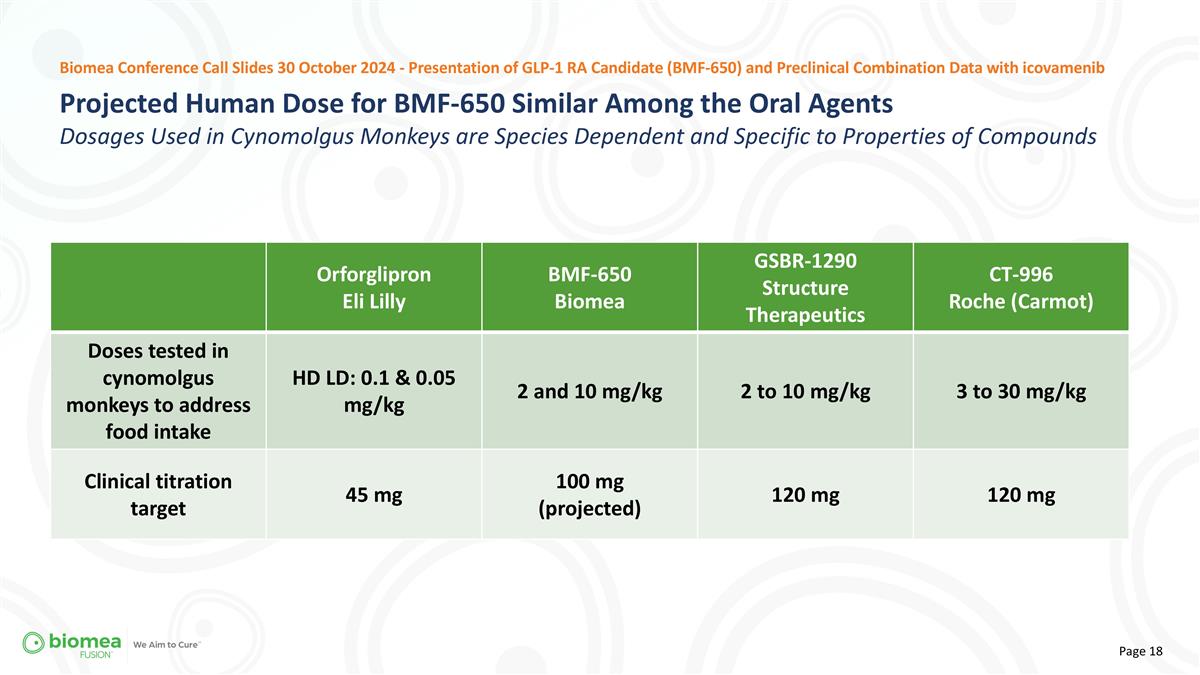
Projected Human Dose for BMF-650 Similar Among the Oral Agents Dosages Used in Cynomolgus Monkeys are Species Dependent and Specific to Properties of Compounds Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Orforglipron Eli Lilly BMF-650 Biomea GSBR-1290 Structure Therapeutics CT-996 Roche (Carmot) Doses tested in cynomolgus monkeys to address food intake HD LD: 0.1 & 0.05 mg/kg 2 and 10 mg/kg 2 to 10 mg/kg 3 to 30 mg/kg Clinical titration target 45 mg 100 mg (projected) 120 mg 120 mg

Period of Anesthesia of Cynomolgus Monkeys Compound: Bolus and continuous infusion to steady-state level Measure glucose and Insulin for 40 min Glucose IV Cynomolgus monkeys (n=4) were anesthetized. Compounds were administered via IV route. Glucose was infused. Blood Glucose and Insulin levels were measured during the following 40 minutes window. Study designed to capture both glucose lowering and insulin release properties Primary Evaluation of Preclinical Activity Set up of Intravenous Glucose Tolerance Test in Cynomolgus Monkeys Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib
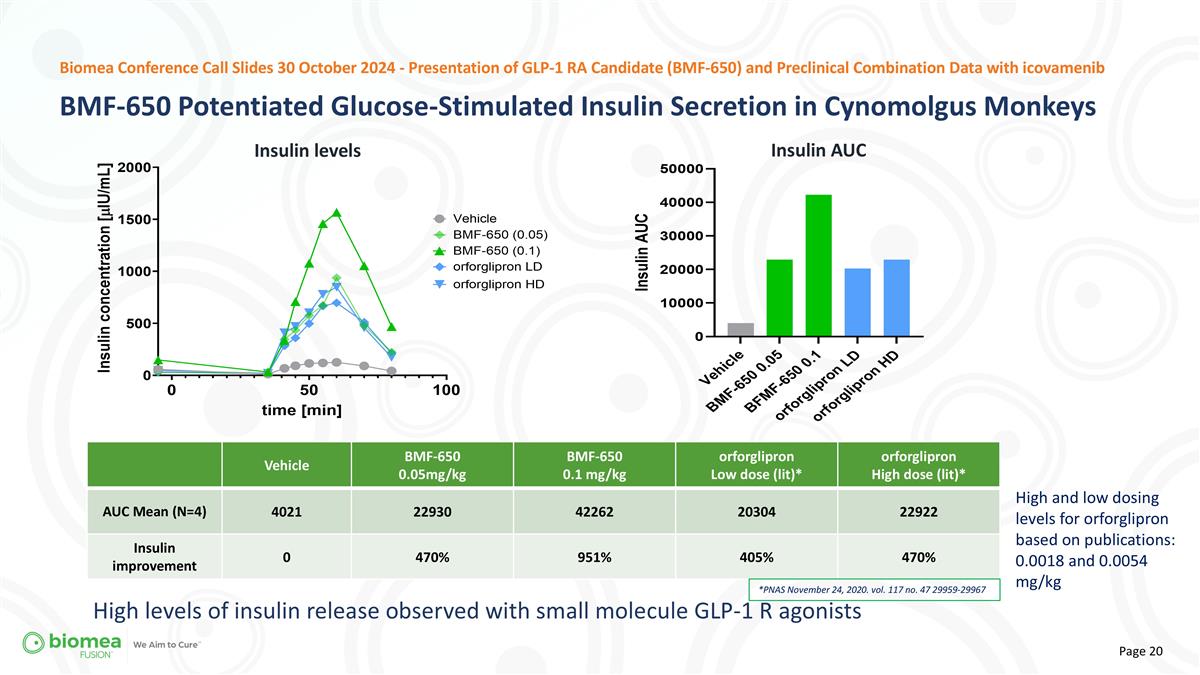
Vehicle BMF-650 0.05mg/kg BMF-650 0.1 mg/kg orforglipron Low dose (lit)* orforglipron High dose (lit)* AUC Mean (N=4) 4021 22930 42262 20304 22922 Insulin improvement 0 470% 951% 405% 470% *PNAS November 24, 2020. vol. 117 no. 47 29959-29967 High and low dosing levels for orforglipron based on publications: 0.0018 and 0.0054 mg/kg High levels of insulin release observed with small molecule GLP-1 R agonists BMF-650 Potentiated Glucose-Stimulated Insulin Secretion in Cynomolgus Monkeys Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Insulin AUC Insulin levels
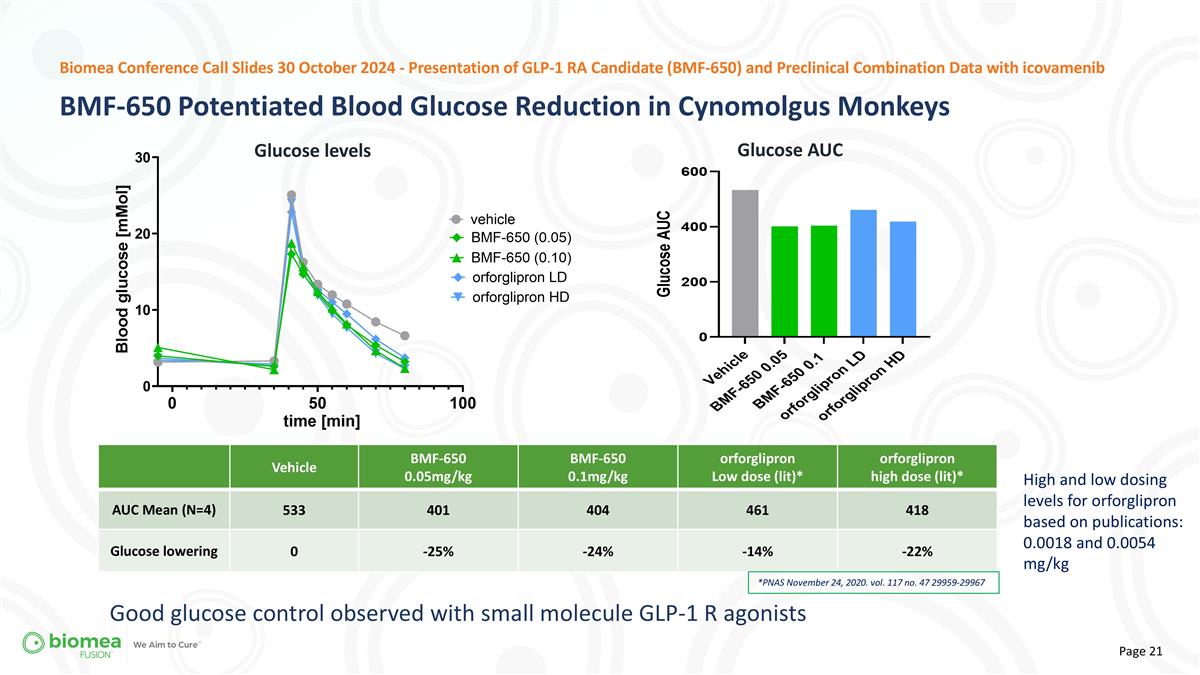
Vehicle BMF-650 0.05mg/kg BMF-650 0.1mg/kg orforglipron Low dose (lit)* orforglipron high dose (lit)* AUC Mean (N=4) 533 401 404 461 418 Glucose lowering 0 -25% -24% -14% -22% *PNAS November 24, 2020. vol. 117 no. 47 29959-29967 High and low dosing levels for orforglipron based on publications: 0.0018 and 0.0054 mg/kg Good glucose control observed with small molecule GLP-1 R agonists BMF-650 Potentiated Blood Glucose Reduction in Cynomolgus Monkeys Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Glucose levels Glucose AUC
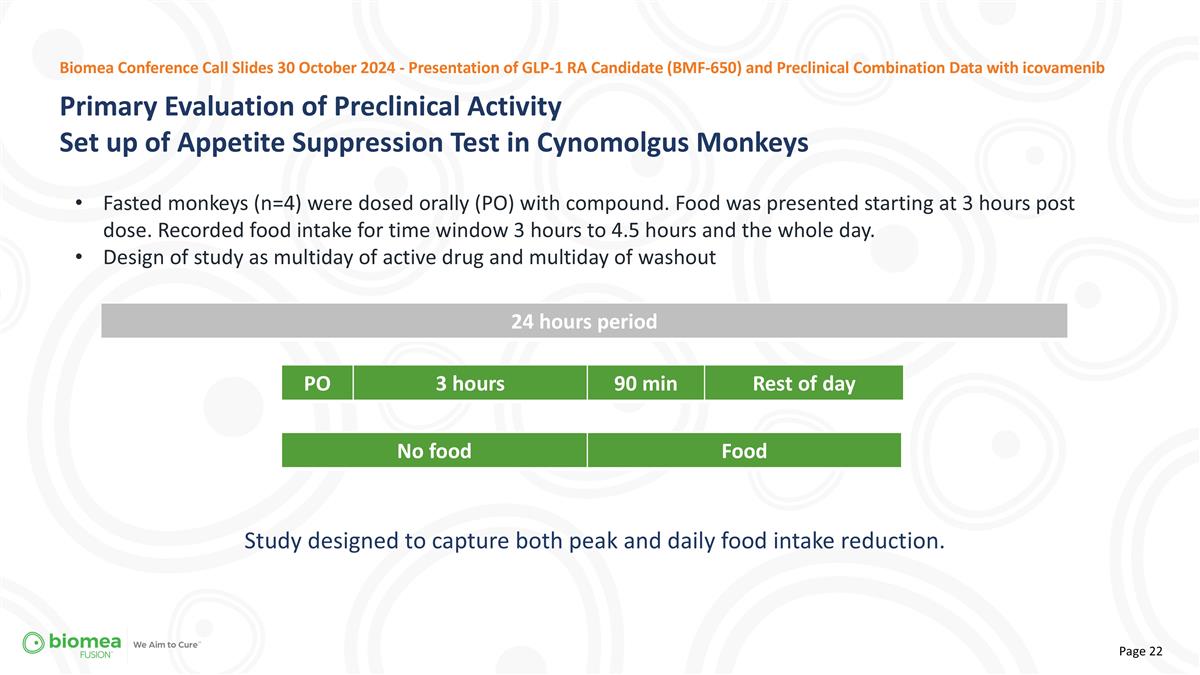
24 hours period PO 3 hours 90 min Rest of day No food Food Fasted monkeys (n=4) were dosed orally (PO) with compound. Food was presented starting at 3 hours post dose. Recorded food intake for time window 3 hours to 4.5 hours and the whole day. Design of study as multiday of active drug and multiday of washout Primary Evaluation of Preclinical Activity Set up of Appetite Suppression Test in Cynomolgus Monkeys Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Study designed to capture both peak and daily food intake reduction.
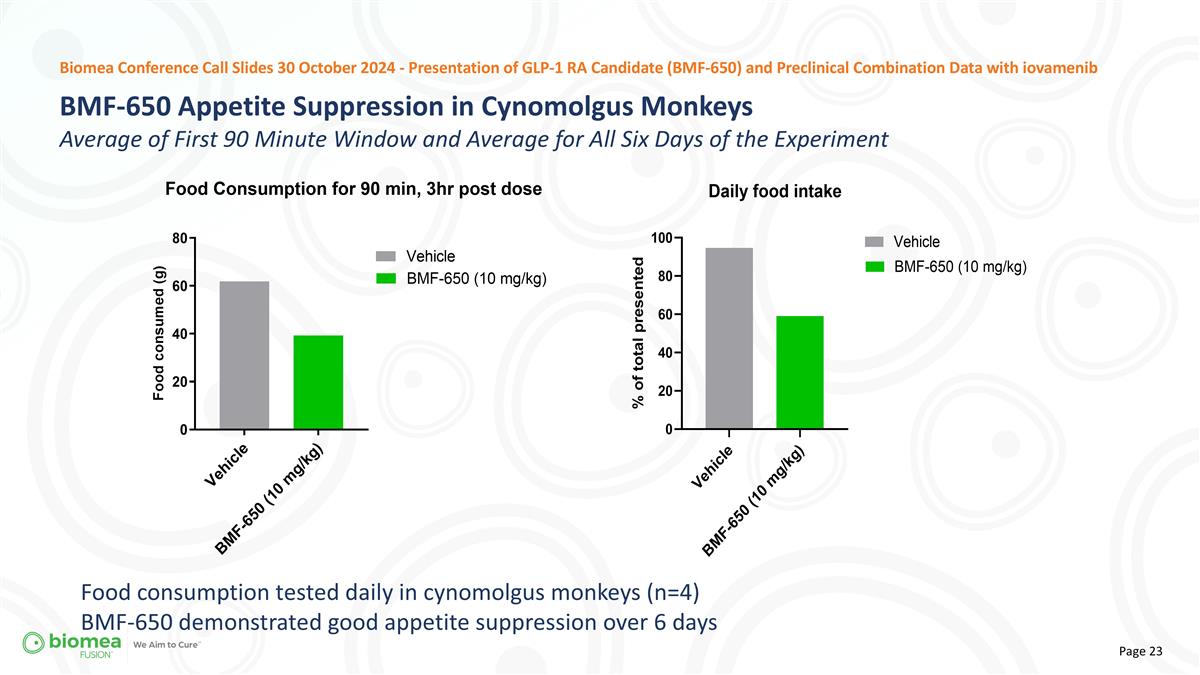
Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with iovamenib Food consumption tested daily in cynomolgus monkeys (n=4) BMF-650 demonstrated good appetite suppression over 6 days BMF-650 Appetite Suppression in Cynomolgus Monkeys Average of First 90 Minute Window and Average for All Six Days of the Experiment
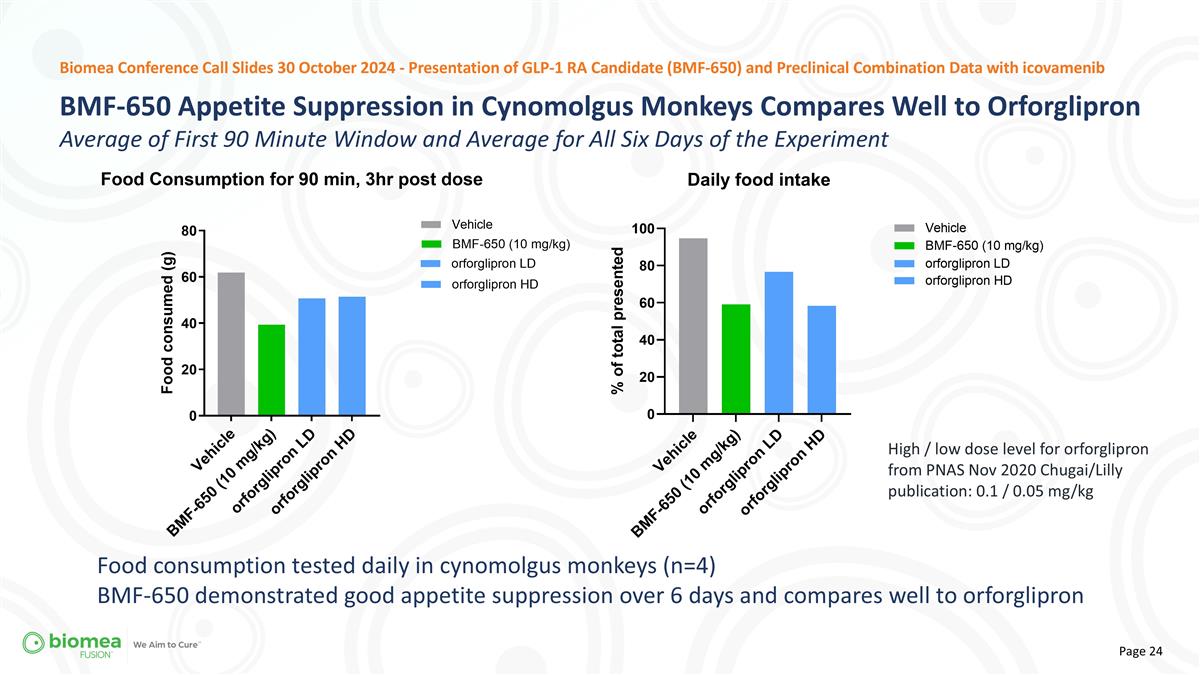
High / low dose level for orforglipron from PNAS Nov 2020 Chugai/Lilly publication: 0.1 / 0.05 mg/kg Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib Food consumption tested daily in cynomolgus monkeys (n=4) BMF-650 demonstrated good appetite suppression over 6 days and compares well to orforglipron BMF-650 Appetite Suppression in Cynomolgus Monkeys Compares Well to Orforglipron Average of First 90 Minute Window and Average for All Six Days of the Experiment
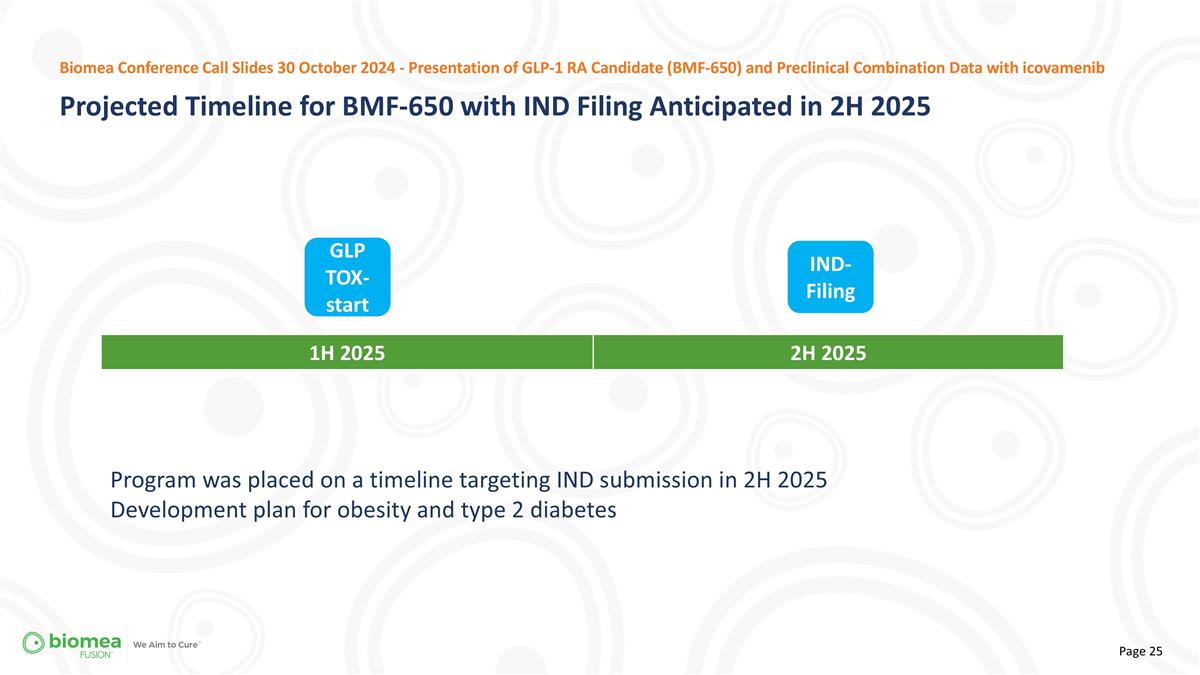
Program was placed on a timeline targeting IND submission in 2H 2025 Development plan for obesity and type 2 diabetes 1H 2025 2H 2025 IND-Filing GLP TOX-start Projected Timeline for BMF-650 with IND Filing Anticipated in 2H 2025 Biomea Conference Call Slides 30 October 2024 - Presentation of GLP-1 RA Candidate (BMF-650) and Preclinical Combination Data with icovamenib

THANK YOU Biomea Fusion 900 Middlefield Road, 4th floor Redwood City, CA, 94063 biomeafusion.com

























