
Exhibit 99.2 COVALENT-111 Topline Results December 17, 2024

COVALENT-111 Topline Results - Conference Call December 17, 2024 Agenda Thomas Butler Introduction & Chief Executive Officer, Chairman of the Board & Co-Founder of Biomea Fusion Executive Summary COVALENT-111 Dr. Juan Pablo Frias Chief Medical Officer of Biomea Fusion Topline Results Dr. Alex Abitbol Key Opinion LMC Healthcare, Endocrinologist, Scientific Advisory Board Member of Biomea Fusion Leader Insights Question & Answer Session

COVALENT-111 Topline Results - Conference Call December 17, 2024 Legal Disclaimer & Forward-Looking Statements Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future business and financial performance of Biomea Fusion, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any projections of financial information or profitability, the initiation, timing and results of pending or future preclinical studies and clinical trials, the actual or potential actions of the FDA, the status and timing of ongoing research, development and corporate partnering activities, any statements about historical results that may suggest trends for the Company's business; any statements of the plans, strategies, and objectives of management for future operations; any statements of expectation or belief regarding future events, potential markets or market size, or technology developments, unfavorable global economic conditions, including inflationary pressures, market volatility, acts of war and civil and political unrest, and other factors affecting the Company's financial condition or operations. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward- looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company's control. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled Risk Factors in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the Securities and Exchange Commission. The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Slide 3

Introduction & Executive Summary COVALENT-111 Topline Results December 17, 2024 Thomas Butler Chief Executive Officer, Chairman of the Board & Co-Founder of Biomea Fusion
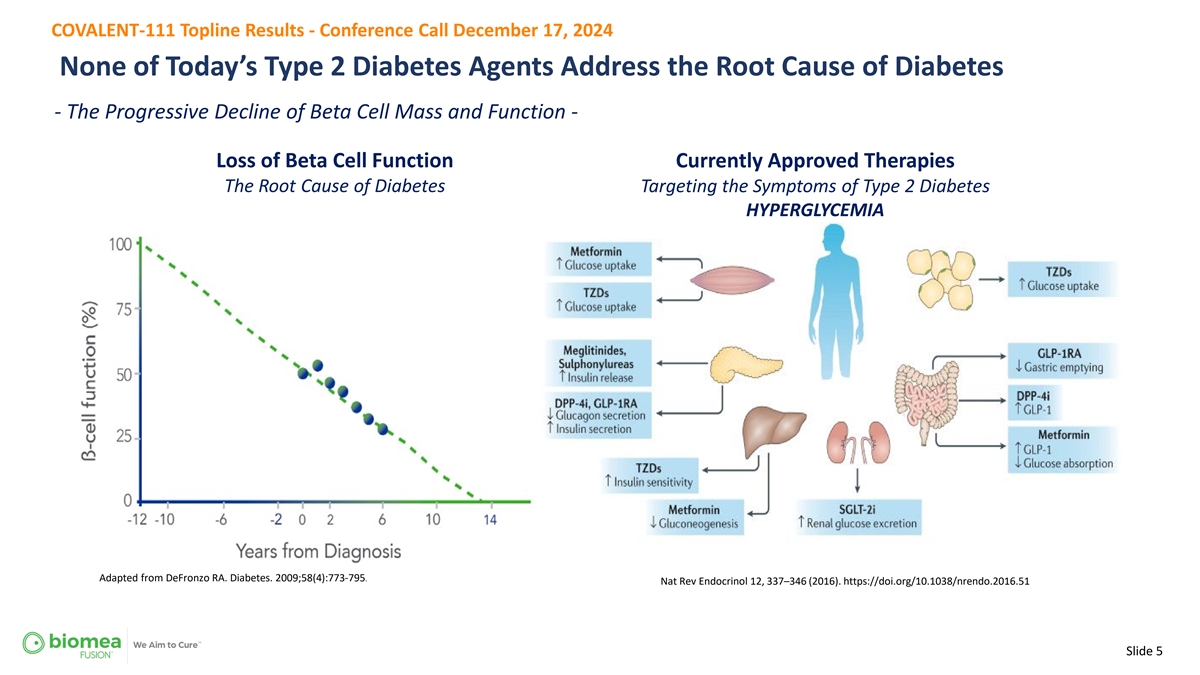
COVALENT-111 Topline Results - Conference Call December 17, 2024 None of Today’s Type 2 Diabetes Agents Address the Root Cause of Diabetes - The Progressive Decline of Beta Cell Mass and Function - Loss of Beta Cell Function Currently Approved Therapies The Root Cause of Diabetes Targeting the Symptoms of Type 2 Diabetes HYPERGLYCEMIA Adapted from DeFronzo RA. Diabetes. 2009;58(4):773-795. Nat Rev Endocrinol 12, 337–346 (2016). https://doi.org/10.1038/nrendo.2016.51 Slide 5

COVALENT-111 Top-Line Results COVALENT-111 Topline Results December 17, 2024 Dr. Juan Pablo Frias Chief Medical Officer of Biomea Fusion
COVALENT-111 Topline Results - Conference Call December 17, 2024 Heterogeneity in Type 2 Diabetes Slide 7
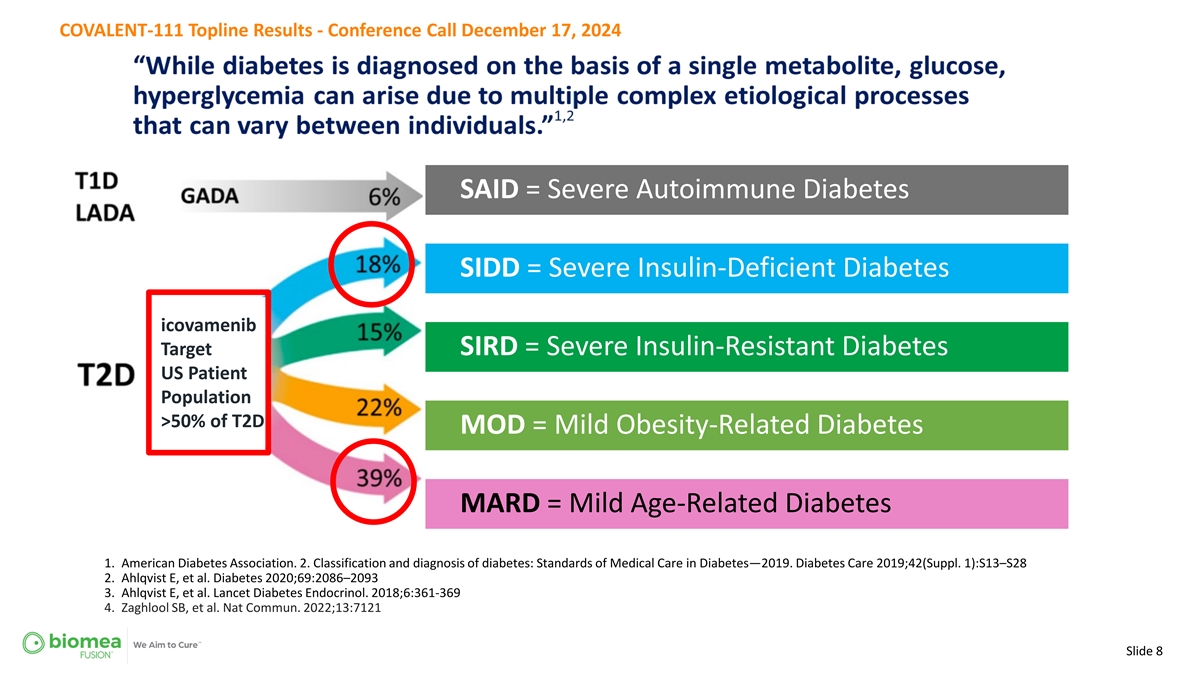
COVALENT-111 Topline Results - Conference Call December 17, 2024 SAID = Severe Autoimmune Diabetes SIDD = Severe Insulin-Deficient Diabetes icovamenib Target SIRD = Severe Insulin-Resistant Diabetes US Patient Population >50% of T2D MOD = Mild Obesity-Related Diabetes MARD = Mild Age-Related Diabetes 1. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S13–S28 2. Ahlqvist E, et al. Diabetes 2020;69:2086–2093 3. Ahlqvist E, et al. Lancet Diabetes Endocrinol. 2018;6:361-369 4. Zaghlool SB, et al. Nat Commun. 2022;13:7121 Slide 8
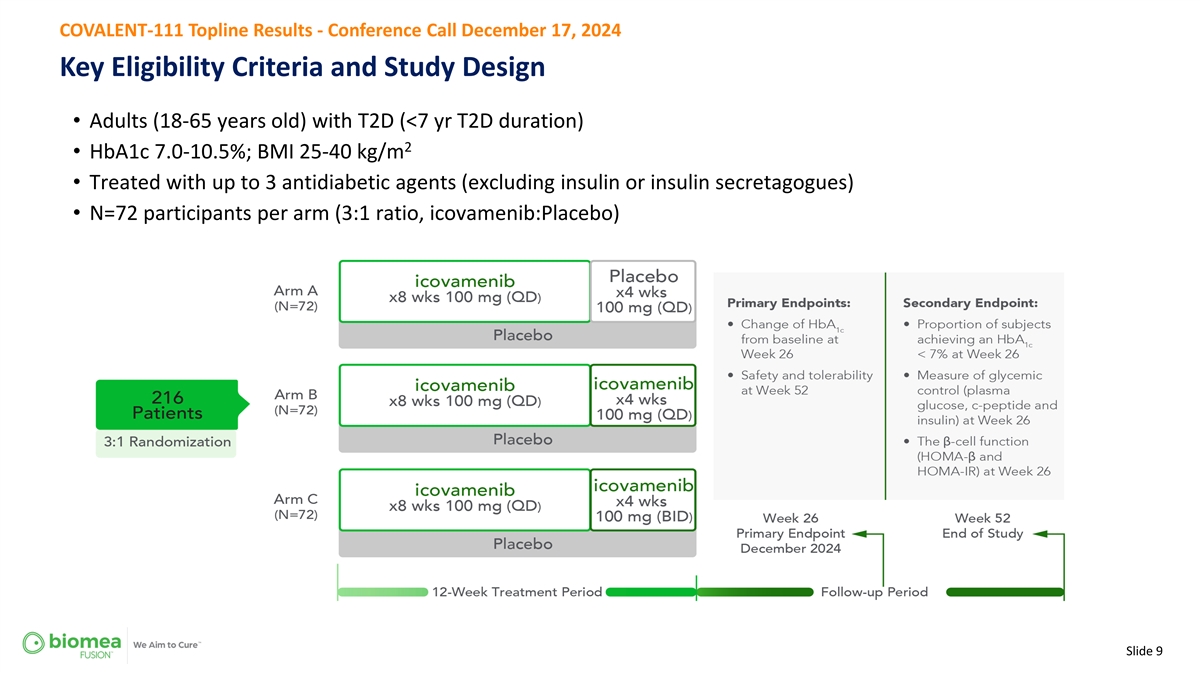
COVALENT-111 Topline Results - Conference Call December 17, 2024 Key Eligibility Criteria and Study Design • Adults (18-65 years old) with T2D (<7 yr T2D duration) 2 • HbA1c 7.0-10.5%; BMI 25-40 kg/m • Treated with up to 3 antidiabetic agents (excluding insulin or insulin secretagogues) • N=72 participants per arm (3:1 ratio, icovamenib:Placebo) Slide 9
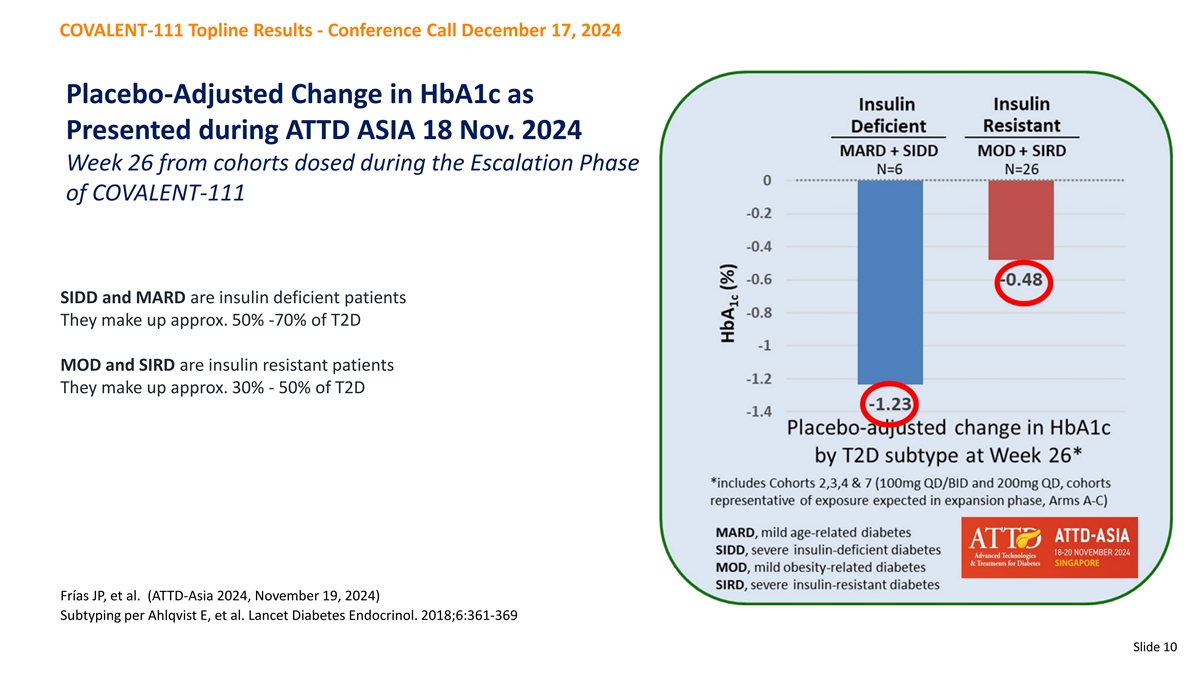
COVALENT-111 Topline Results - Conference Call December 17, 2024 Placebo-Adjusted Change in HbA1c as Presented during ATTD ASIA 18 Nov. 2024 Week 26 from cohorts dosed during the Escalation Phase of COVALENT-111 SIDD and MARD are insulin deficient patients They make up approx. 50% -70% of T2D MOD and SIRD are insulin resistant patients They make up approx. 30% - 50% of T2D Frías JP, et al. (ATTD-Asia 2024, November 19, 2024) Subtyping per Ahlqvist E, et al. Lancet Diabetes Endocrinol. 2018;6:361-369 Slide 10

COVALENT-111 Topline Results - Conference Call December 17, 2024 Baseline Patient Population • mITT population: Defined as all randomized participants who took at least one dose of study drug (N=225) • The efficacy analysis focuses on those patients that completed the dosing period prior to the clinical hold and were “uncontrolled” on at least one anti-diabetic medication at baseline (N=168) Per Protocol Patients = 113 Placebo patients = 55 • 10% of all patients enrolled were on no background therapy 69% were on metformin alone 15% were on two therapies 5% were on three therapies Slide 11
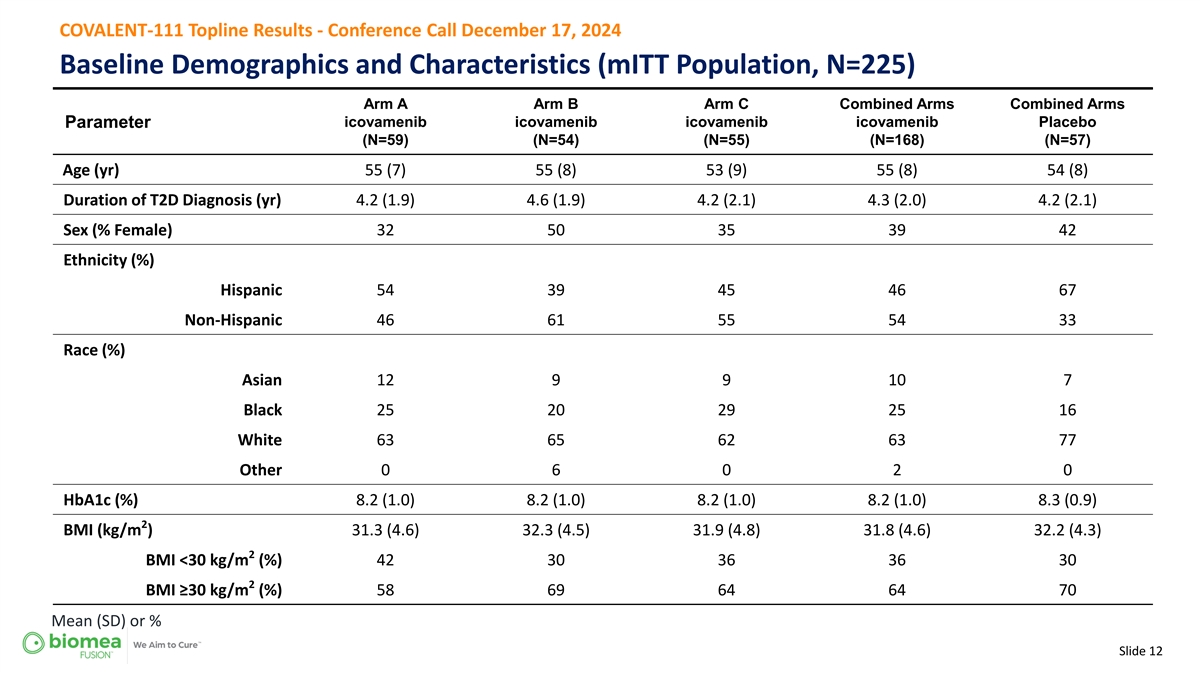
COVALENT-111 Topline Results - Conference Call December 17, 2024 Baseline Demographics and Characteristics (mITT Population, N=225) Arm A Arm B Arm C Combined Arms Combined Arms Parameter icovamenib icovamenib icovamenib icovamenib Placebo (N=59) (N=54) (N=55) (N=168) (N=57) Age (yr) 55 (7) 55 (8) 53 (9) 55 (8) 54 (8) Duration of T2D Diagnosis (yr) 4.2 (1.9) 4.6 (1.9) 4.2 (2.1) 4.3 (2.0) 4.2 (2.1) Sex (% Female) 32 50 35 39 42 Ethnicity (%) Hispanic 54 39 45 46 67 Non-Hispanic 46 61 55 54 33 Race (%) Asian 12 9 9 10 7 Black 25 20 29 25 16 White 63 65 62 63 77 Other 0 6 0 2 0 HbA1c (%) 8.2 (1.0) 8.2 (1.0) 8.2 (1.0) 8.2 (1.0) 8.3 (0.9) 2 BMI (kg/m ) 31.3 (4.6) 32.3 (4.5) 31.9 (4.8) 31.8 (4.6) 32.2 (4.3) 2 BMI <30 kg/m (%) 42 30 36 36 30 2 BMI ≥30 kg/m (%) 58 69 64 64 70 Mean (SD) or % Slide 12
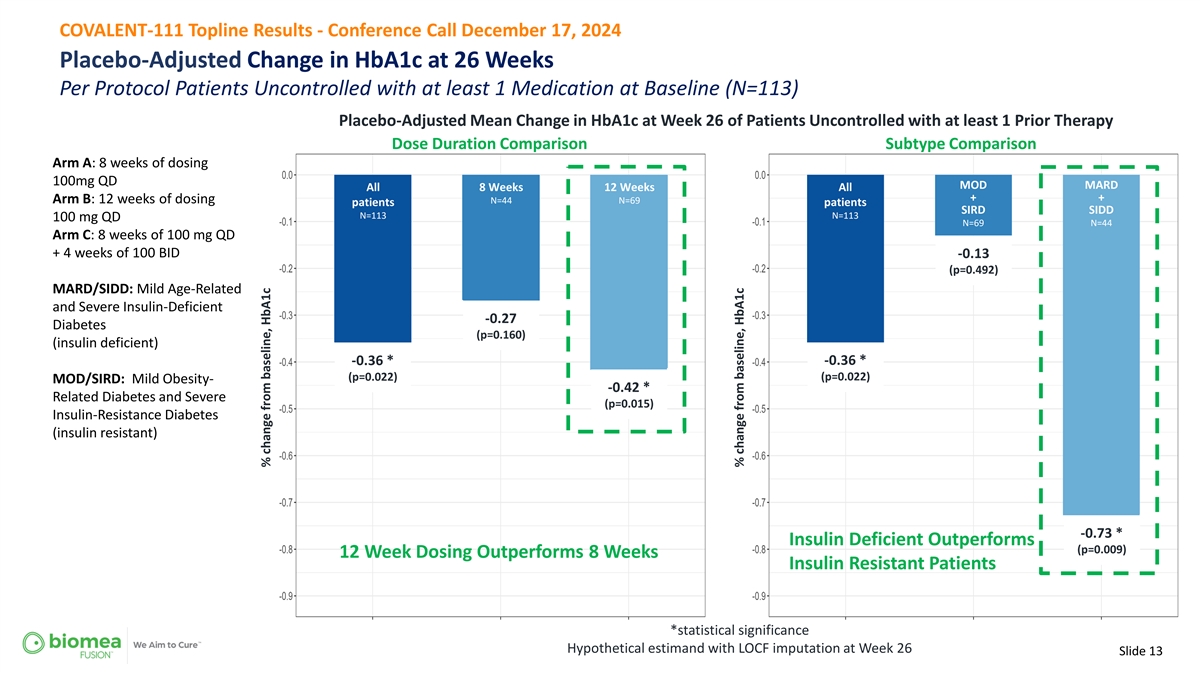
COVALENT-111 Topline Results - Conference Call December 17, 2024 Placebo-Adjusted Change in HbA1c at 26 Weeks Per Protocol Patients Uncontrolled with at least 1 Medication at Baseline (N=113) Placebo-Adjusted Mean Change in HbA1c at Week 26 of Patients Uncontrolled with at least 1 Prior Therapy Dose Duration Comparison Subtype Comparison Arm A: 8 weeks of dosing 100mg QD MOD MARD All 8 Weeks 12 Weeks All + + Arm B: 12 weeks of dosing N=44 N=69 patients patients SIRD SIDD N=113 N=113 100 mg QD N=69 N=44 Arm C: 8 weeks of 100 mg QD + 4 weeks of 100 BID -0.13 (p=0.492) MARD/SIDD: Mild Age-Related and Severe Insulin-Deficient -0.27 Diabetes (p=0.160) (insulin deficient) -0.36 * -0.36 * (p=0.022) (p=0.022) MOD/SIRD: Mild Obesity- -0.42 * Related Diabetes and Severe (p=0.015) Insulin-Resistance Diabetes (insulin resistant) -0.73 * Insulin Deficient Outperforms (p=0.009) 12 Week Dosing Outperforms 8 Weeks Insulin Resistant Patients *statistical significance Hypothetical estimand with LOCF imputation at Week 26 Slide 13 % change from baseline, HbA1c % change from baseline, HbA1c
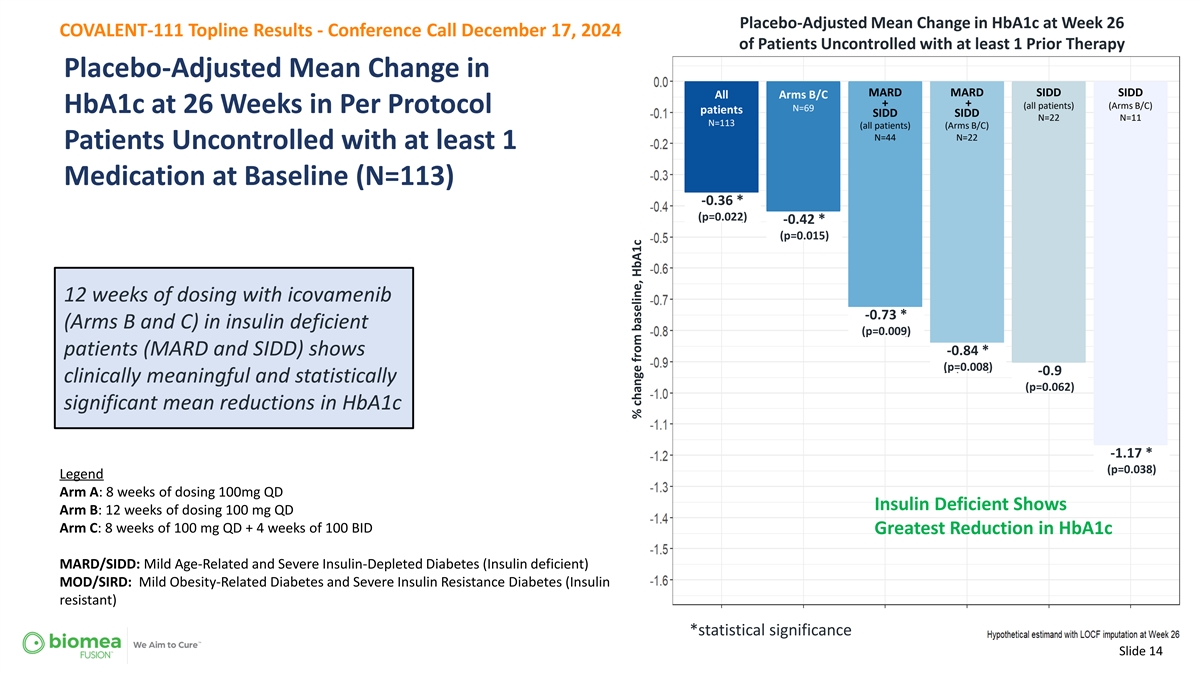
Placebo-Adjusted Mean Change in HbA1c at Week 26 COVALENT-111 Topline Results - Conference Call December 17, 2024 of Patients Uncontrolled with at least 1 Prior Therapy Placebo-Adjusted Mean Change in MARD MARD SIDD SIDD All Arms B/C + + (all patients) (Arms B/C) N=69 HbA1c at 26 Weeks in Per Protocol patients SIDD SIDD N=22 N=11 N=113 (all patients) (Arms B/C) N=44 N=22 Patients Uncontrolled with at least 1 Medication at Baseline (N=113) -0.36 * (p=0.022) -0.42 * (p=0.015) 12 weeks of dosing with icovamenib -0.73 * (Arms B and C) in insulin deficient (p=0.009) -0.84 * patients (MARD and SIDD) shows (p=0.008) -0.9 clinically meaningful and statistically (p=0.062) significant mean reductions in HbA1c -1.17 * (p=0.038) Legend Arm A: 8 weeks of dosing 100mg QD Insulin Deficient Shows Arm B: 12 weeks of dosing 100 mg QD Arm C: 8 weeks of 100 mg QD + 4 weeks of 100 BID Greatest Reduction in HbA1c MARD/SIDD: Mild Age-Related and Severe Insulin-Depleted Diabetes (Insulin deficient) MOD/SIRD: Mild Obesity-Related Diabetes and Severe Insulin Resistance Diabetes (Insulin resistant) *statistical significance Slide 14 % change from baseline, HbA1c
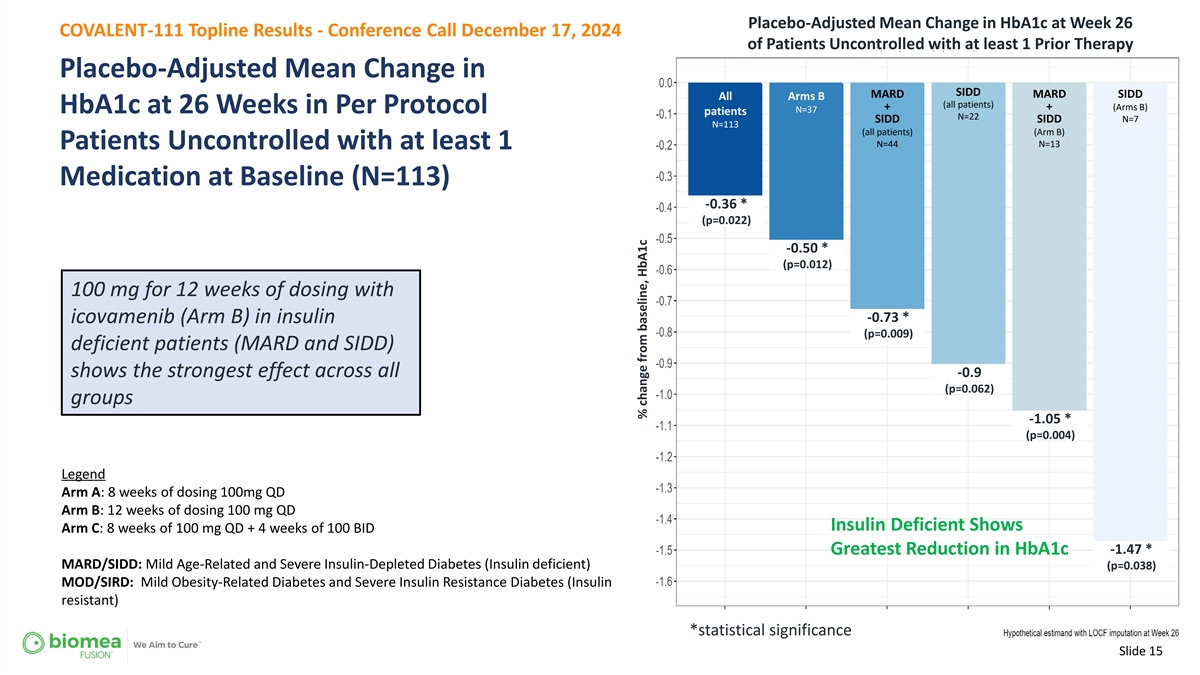
Placebo-Adjusted Mean Change in HbA1c at Week 26 COVALENT-111 Topline Results - Conference Call December 17, 2024 of Patients Uncontrolled with at least 1 Prior Therapy Placebo-Adjusted Mean Change in SIDD MARD MARD SIDD All Arms B (all patients) + + (Arms B) N=37 HbA1c at 26 Weeks in Per Protocol patients N=22 SIDD SIDD N=7 N=113 (all patients) (Arm B) N=44 N=13 Patients Uncontrolled with at least 1 Medication at Baseline (N=113) -0.36 * (p=0.022) -0.50 * (p=0.012) 100 mg for 12 weeks of dosing with -0.73 * icovamenib (Arm B) in insulin (p=0.009) deficient patients (MARD and SIDD) shows the strongest effect across all -0.9 (p=0.062) groups -1.05 * (p=0.004) Legend Arm A: 8 weeks of dosing 100mg QD Arm B: 12 weeks of dosing 100 mg QD Insulin Deficient Shows Arm C: 8 weeks of 100 mg QD + 4 weeks of 100 BID -1.47 * Greatest Reduction in HbA1c MARD/SIDD: Mild Age-Related and Severe Insulin-Depleted Diabetes (Insulin deficient) (p=0.038) MOD/SIRD: Mild Obesity-Related Diabetes and Severe Insulin Resistance Diabetes (Insulin resistant) *statistical significance Slide 15 % change from baseline, HbA1c
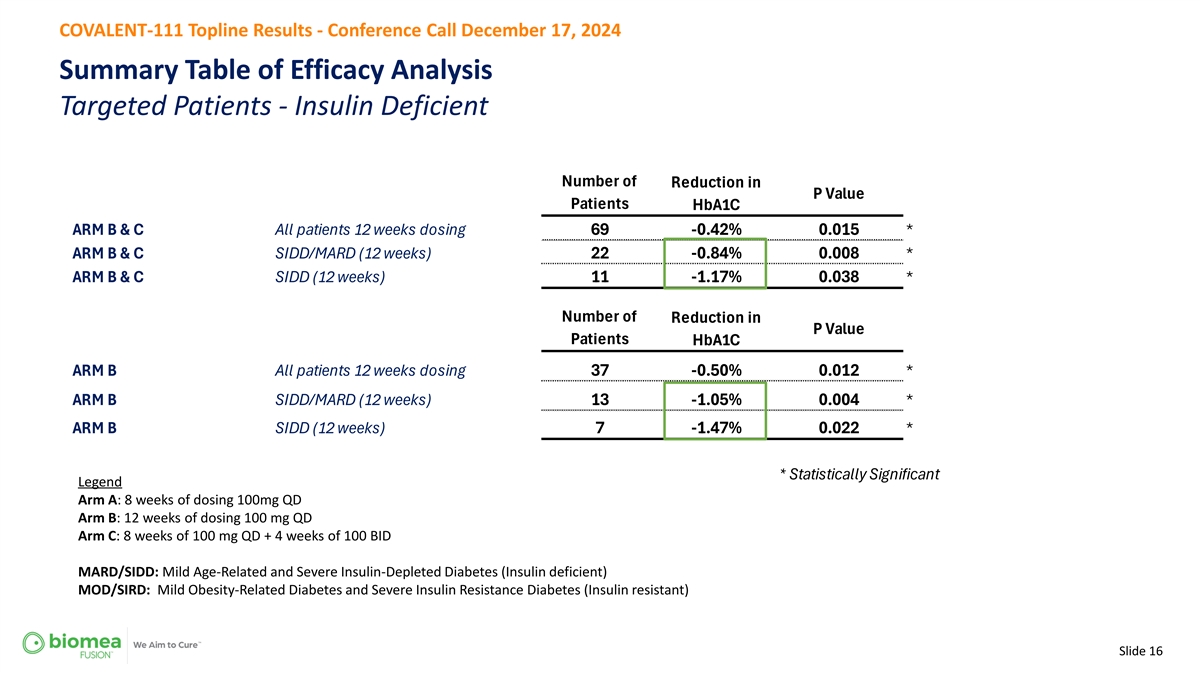
COVALENT-111 Topline Results - Conference Call December 17, 2024 Summary Table of Efficacy Analysis Targeted Patients - Insulin Deficient Number of Reduction in P Value Patients HbA1C ARM B & C comb All patients 12 weeks dosing 69 -0.42% 0.015 * ARM B & C comb SIDD/MARD (12 weeks) 22 -0.84% 0.008 * ARM B & C comb SIDD (12 weeks) 11 -1.17% 0.038 * Number of Reduction in P Value Patients HbA1C ARM B All patients 12 weeks dosing 37 -0.50% 0.012 * ARM B SIDD/MARD (12 weeks) 13 -1.05% 0.004 * ARM B SIDD (12 weeks) 7 -1.47% 0.022 * * Statistically Significant Legend Arm A: 8 weeks of dosing 100mg QD Arm B: 12 weeks of dosing 100 mg QD Arm C: 8 weeks of 100 mg QD + 4 weeks of 100 BID MARD/SIDD: Mild Age-Related and Severe Insulin-Depleted Diabetes (Insulin deficient) MOD/SIRD: Mild Obesity-Related Diabetes and Severe Insulin Resistance Diabetes (Insulin resistant) Slide 16
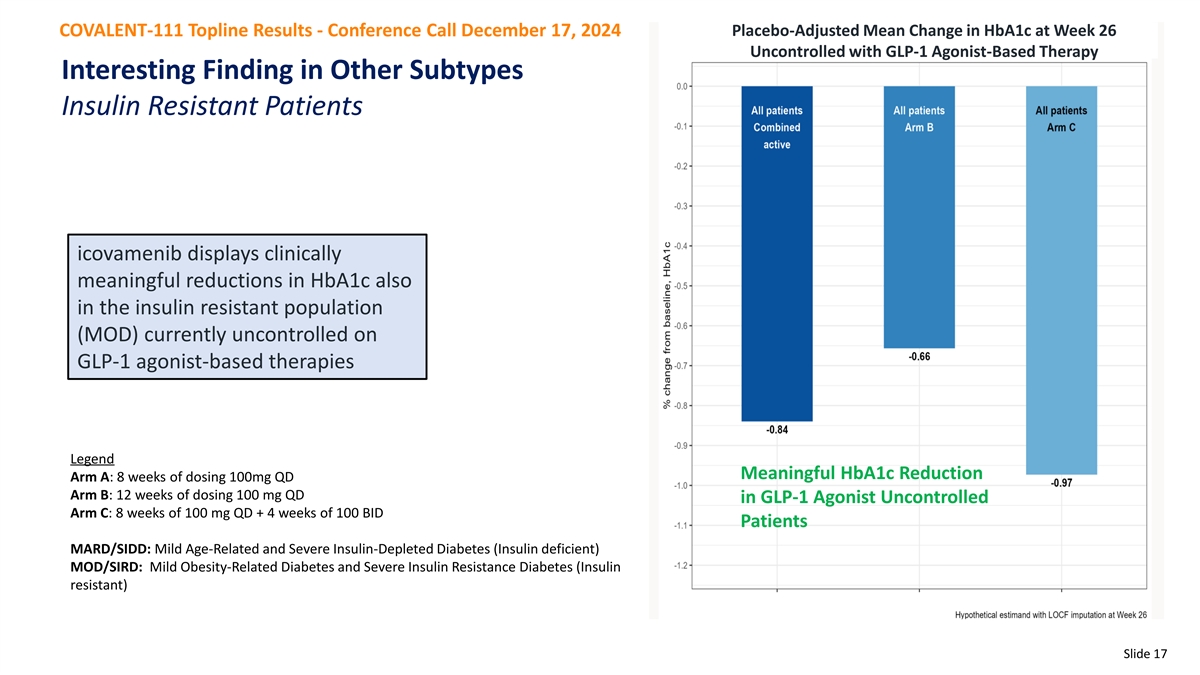
COVALENT-111 Topline Results - Conference Call December 17, 2024 Placebo-Adjusted Mean Change in HbA1c at Week 26 Uncontrolled with GLP-1 Agonist-Based Therapy Interesting Finding in Other Subtypes Insulin Resistant Patients icovamenib displays clinically meaningful reductions in HbA1c also in the insulin resistant population (MOD) currently uncontrolled on GLP-1 agonist-based therapies Legend Meaningful HbA1c Reduction Arm A: 8 weeks of dosing 100mg QD Arm B: 12 weeks of dosing 100 mg QD in GLP-1 Agonist Uncontrolled Arm C: 8 weeks of 100 mg QD + 4 weeks of 100 BID Patients MARD/SIDD: Mild Age-Related and Severe Insulin-Depleted Diabetes (Insulin deficient) MOD/SIRD: Mild Obesity-Related Diabetes and Severe Insulin Resistance Diabetes (Insulin resistant) Slide 17
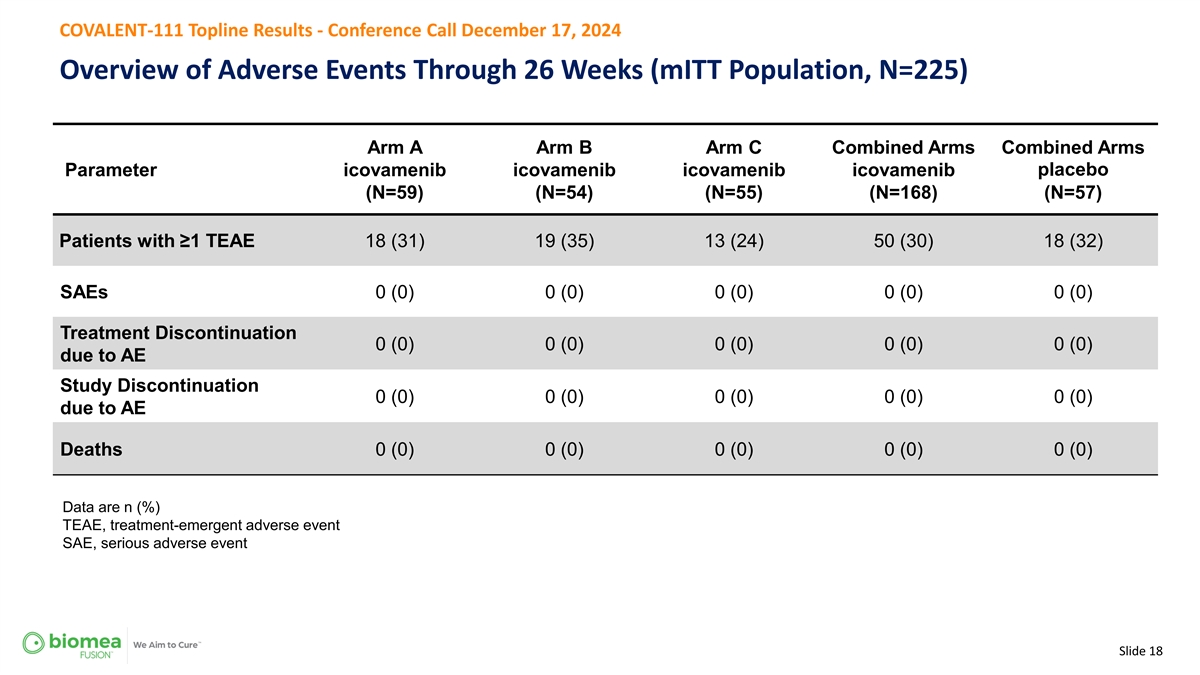
COVALENT-111 Topline Results - Conference Call December 17, 2024 Overview of Adverse Events Through 26 Weeks (mITT Population, N=225) Arm A Arm B Arm C Combined Arms Combined Arms Parameter icovamenib icovamenib icovamenib icovamenib placebo (N=59) (N=54) (N=55) (N=168) (N=57) Patients with ≥1 TEAE 18 (31) 19 (35) 13 (24) 50 (30) 18 (32) SAEs 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) Treatment Discontinuation 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) due to AE Study Discontinuation 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) due to AE Deaths 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) Data are n (%) TEAE, treatment-emergent adverse event SAE, serious adverse event Slide 18

COVALENT-111 Topline Results - Conference Call December 17, 2024 Safety and Tolerability (mITT Population, N=225) Arm A Arm B Arm C Combined Arms Combined Arms Parameter icovamenib icovamenib icovamenib icovamenib placebo (N=59) (N=54) (N=55) (N=168) (N=57) Diarrhea 4 (7) 2 (4) 1 (2) 7 (4) 0 (0) Nausea 2 (3) 3 (6) 2 (4) 7 (4) 1 (2) Hyperglycemia 1 (2) 4 (7) 1 (2) 6 (4) 3 (5) Headache 0 3 (6) 1 (2) 4 (2) 3 (5) ALT increase 2 (3) 0 2 (4) 4 (2) 0 AST increase 2 (3) 0 1 (2) 3 (2) 0 Data are n (%) of TEAE with ≥5% frequency in any arm and ALT or AST increase irrespective of incidence; mITT population (safety analysis set) TEAE, treatment-emergent adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase Diarrhea: In icovamenib arms, all 7 events were Grade 1. Nausea: In icovamenib arms, 6 of 7 events were Grade 1 and 1 event was Grade 2 (Arm B). In placebo arm, the 1 event was Grade 1. Hyperglycemia: In icovamenib and placebo arms, all events were Grade 2. Headache: In icovamenib arms, 3 of the 4 events were Grade 1 and 1 event was Grade 2 (Arm B). In the placebo arm, 3 of the 4 events were Grade 1 and 1 event was Grade 2. ALT increase: In the icovamenib arms, 3 of the 4 events were Grade 1 and 1 event was Grade 2 (Arm A). AST increase: In the icovamenib arms, all 4 events were Grade 1. Slide 19
COVALENT-111 Topline Results - Conference Call December 17, 2024 Efficacy and Safety Analysis of Per Protocol Patient Population • Icovamenib met the primary endpoint, displaying a clinically meaningful and statistically significant placebo-corrected reduction in HbA1c in the prespecified Per Protocol Patient population • Icovamenib was well tolerated with no study drug discontinuations due to TEAEs • Icovamenib displayed the greatest statistically significant mean HbA1c reduction in Patients dosed for 12 weeks and in the T2D subtypes characterized by insulin-deficiency. o SIDD (Arms B and C combined) mean HbA1c reduction = 1.17% o SIDD (Arm B) mean HbA1 reduction = 1.47% • Icovamenib demonstrated also a clinically meaningful reduction in HbA1c in the T2D subtype characterized by insulin resistance (MOD), in study participants uncontrolled on a GLP-1 RA-based therapy at baseline Slide 20

Key Opinion Leader INSIGHTS COVALENT-111 Topline Results December 17, 2024 Dr. Alex Abitbol Endocrinologist, Scientific Advisory Board Member of Biomea Fusion
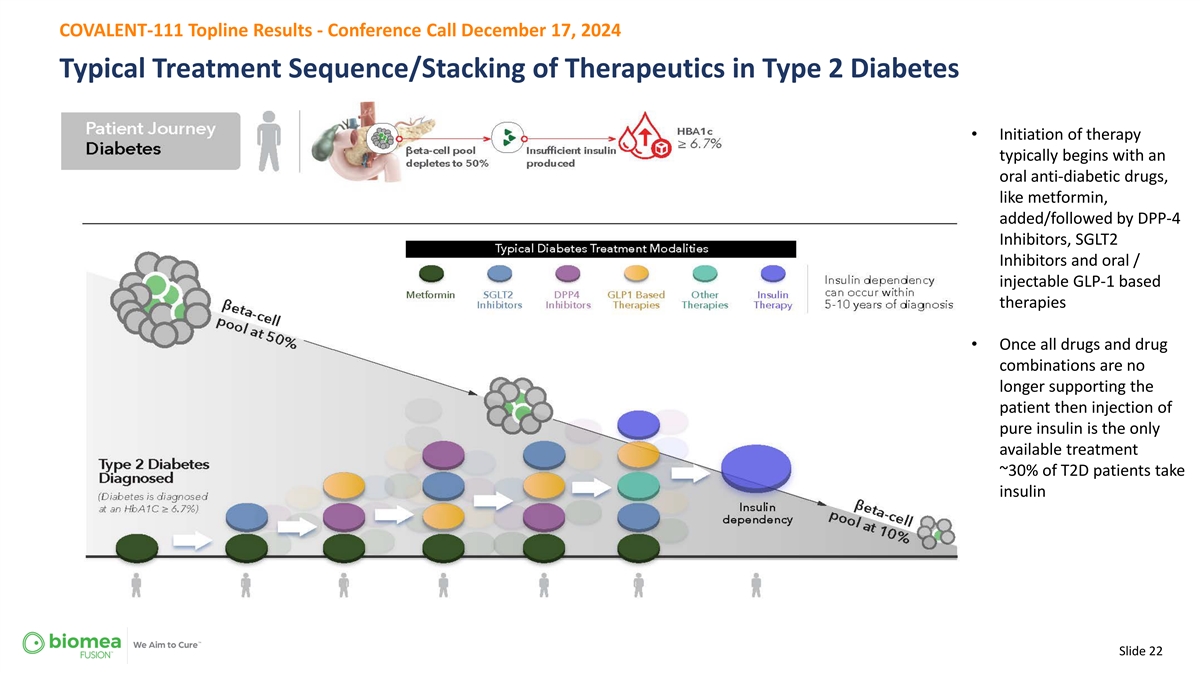
COVALENT-111 Topline Results - Conference Call December 17, 2024 Typical Treatment Sequence/Stacking of Therapeutics in Type 2 Diabetes • Initiation of therapy typically begins with an oral anti-diabetic drugs, like metformin, added/followed by DPP-4 Inhibitors, SGLT2 Inhibitors and oral / injectable GLP-1 based therapies • Once all drugs and drug combinations are no longer supporting the patient then injection of pure insulin is the only available treatment ~30% of T2D patients take insulin Slide 22
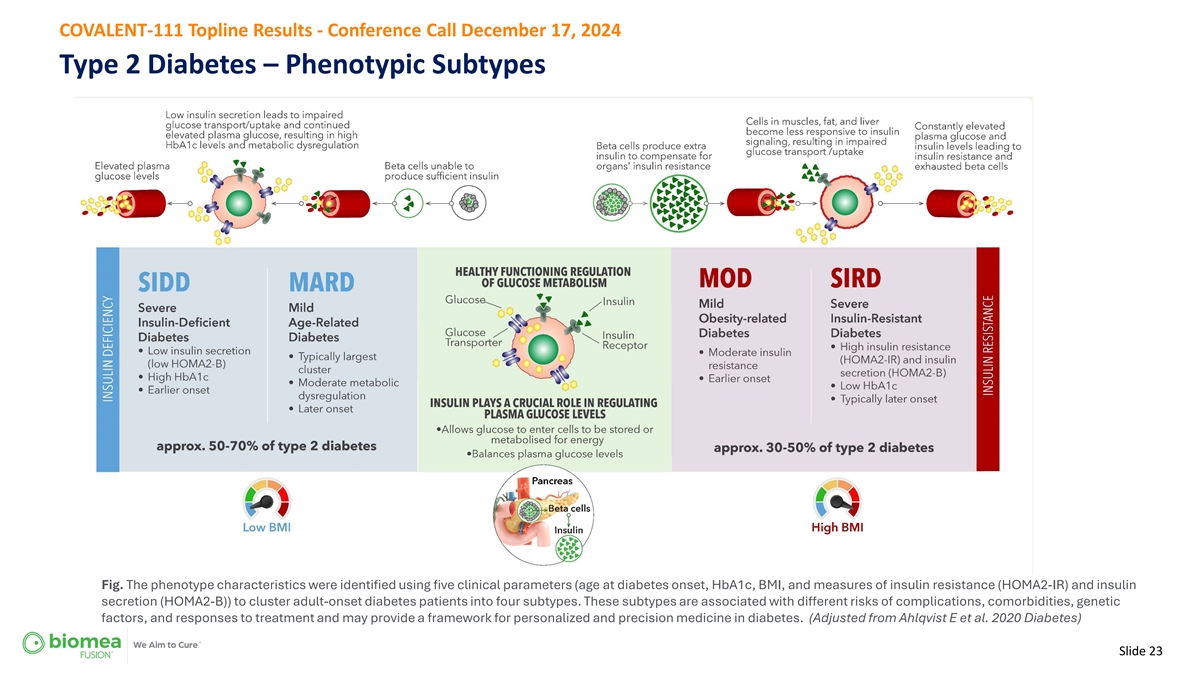
COVALENT-111 Topline Results - Conference Call December 17, 2024 Type 2 Diabetes – Phenotypic Subtypes Fig. The phenotype characteristics were identified using five clinical parameters (age at diabetes onset, HbA1c, BMI, and measures of insulin resistance (HOMA2-IR) and insulin secretion (HOMA2-B)) to cluster adult-onset diabetes patients into four subtypes. These subtypes are associated with different risks of complications, comorbidities, genetic factors, and responses to treatment and may provide a framework for personalized and precision medicine in diabetes. (Adjusted from Ahlqvist E et al. 2020 Diabetes) Slide 23
Q & A Confidential 24 Slide 24

THANK YOU Slide 25
























