Exhibit 99.2

Next - Generation Life Saving Solutions Corporate Overview – September 2022

Direc t B iologics 2 Disclaimer This presentation (“ Presentation ”) is for informational purposes only to assist interested parties in making their own evaluation with respect to the propose d o ffering (the “ Offering ”) of Direct Biologics, LLC, a Wyoming limited liability company (“ Direct Biologics ”) of Class B Units. The offering is being made in connection with, but is not subject to or conditioned upon, the consummation of a propos ed business combination (the “ Business Combination ”) between Good Works II Acquisition Corp. (“ Good Works ”) and the Company (the “ Purpose ”). By accepting this Presentation, the recipient acknowledges and agrees that all of the information contained herein is confidenti al, that the recipient will distribute, disclose, and use such information only for such Purpose and that the recipient shall not d istribute, disclose or use such information in anyway detrimental to Direct Biologics or Good Works. The information contained herein does not purport to be all - inclusive and none of Good Works, Direct Biologics, and IB Capital, LLC (“ IBC ”) nor any of their respective affiliates nor any of its or their controlling persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to th e a ccuracy, completeness or reliability of the information contained in this Presentation. You should consult your own counsel a nd tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm tha t y ou are not relying upon the information contained herein to make any decision. Any securities to be offered in any transaction contemplated hereby have not been registered under the Securities Act of 1933 , a s amended (the “Securities Act”), or any applicable state or foreign securities law. Any securities to be offered in any tran sac tion contemplated hereby have not been approved or disapproved by the Securities and Exchange Commission (the “SEC”), any state securities comm iss ion or other United States or foreign regulatory authority, and will be offered and sold solely in reliance on an exemption f rom the registration requirements provided by the Securities Act and rules and regulations promulgated thereunder (including Regulation D or Regul ati on S under the Securities Act). This Presentation does not constitute, or form a part of, an offer to sell or the solicitatio n o f an offer to buy in any state or other jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such state or jurisdiction. Certain statements in this Presentation may be considered forward - looking statements. Forward - looking statements generally relat e to future events or Direct Biologics’ or Good Works’ future financial or operating performance. For example, statements con cer ning the following include forward - looking statements: Direct Biologics’ ability to identify, develop and commercialize product candidate s; the initiation, cost, timing, progress and results of research and development activities, preclinical or clinical trials wit h respect Direct Biologics’ drug candidates; future revenue, expenses, capital requirements and needs for additional financing; projections for market opportu nit y; the therapeutic potential of the Company's product candidate; financing and other business milestones and the potential ef fec ts of the proposed Business Combination on Good Works and Direct Biologics and related capital raising activities. In some cases, you can identi fy forward - looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believ e”, “predict”, “plan”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward - looking st atements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from th ose expressed or implied by such forward - looking statements. These forward - looking statements are based upon estimates and assumptions that, while considered rea sonable by Good Works and its management, and Direct Biologics and its management, as the case may be, are inherently uncerta in. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that m ay cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond D ire ct Biologics management’s control including general economic and geopolitical conditions as well as factors associated with companies, such as Direct B iol ogics, that are engaged in preclinical studies and other research and development activities in the biopharmaceutical industr y, including uncertainty in the timing or results of preclinical studies and clinical trials, the impact of competitive product candidates; ability to obtain su fficient supply of materials; product acceptance and/or receipt of regulatory approvals for product candidates, and including an y delays and other impacts from the Covid - 19 pandemic. Nothing in this Presentation should be regarded as a representation by any person that the forward - lookin g statements set forth herein will be achieved or that any of the contemplated results of such forward - looking statements will b e achieved. You should not place any reliance on forward - looking statements in this Presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Neither Good Works nor Direct Biologics underta ke s any duty to update these forward - looking statements. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and Direct Biolog ics ’ own internal estimates and research. In addition, all of the market data included in this Presentation involve a number of ass umptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while Direct Biolo gic s believes its internal research is reliable, such research has not been verified by any independent source. This Presentatio n a nd any information communicated at any meetings related to your evaluation of the Business Combination are strictly confidential and should not be discussed outside your organization. IBC, an affiliate of I - Bankers Securities, Inc., is acting as placement agent (together with its affiliates, partners, directors , agents, employees, representatives, and controlling persons, the “ Placement Agent ”) with respect to capital raising activities in connection with the Business Combination. The Placement Agent is acting solely as placement agent (and, for the avoidance of doubt, not as underw rit er, initial purchaser, dealer or any other principal capacity) for Direct Biologics in connection with a potential transactio n. The Placement Agent has not independently verified any of the information contained herein or any other information that has been or will be provided to you . Nothing contained herein or in any other oral or written information provided to you is, nor shall be relied upon as, a pro mis e or representation of any kind by the Placement Agent, Good Works or Direct Biologics, whether as to the past or the future. Without limitation of the foreg oin g, none of the Placement Agent, Good Works or Direct Biologics shall be liable to you or any prospective investor or any othe r p erson for any information contained herein or that otherwise has been or will be provided to you, or any action heretofore or hereafter taken or omitte d t o be taken, in connection with this potential transaction. This Presentation is being distributed solely for the consideratio n o f sophisticated prospective purchasers who are accredited investors, including institutional investors who are accredited investors, with sufficient know led ge and experience in investment, financial and business matters and the capability to conduct their own due diligence investi gat ion and evaluation in connection with the Purpose. This Presentation does not purport to summarize all of the conditions, risks and other attribute s o f an investment in Good Works or Direct Biologics. Information contained herein will be superseded by, and is qualified in it s e ntirety by reference to, any other information that is made available to you in connection with the Purpose, including your investigation of Good Works an d D irect Biologics. The offering of Direct Biologics' Class B Units is intended to be made in connection with, but is not subject to or condition ed upon, the consummation of the proposed Business Combination. In connection therewith, Direct Biologics and Good Works intend to enter into business combination or similar agreement and Good Works, or a to be formed entity, intends to file with the SEC a registrati on statement on Form S - 4 whereby, upon the closing of the transaction, each Class B Unit will be canceled and converted into a numb er of shares of capital stock of a newly organized corporation or other business entity listed on a national securities exchange. Participants in the Solicitation . Good Works and its directors and executive officers may be deemed participants in the solicitation of proxies from Good Wor ks’ shareholders with respect to the proposed Business Combination. A list of the names of those directors and executive officers and a description of their interests in Good Works is contained in Good Works’ final prospectus relating t o i ts initial public offering, July 13, 2021, which was filed with the SEC and is available free of charge at the SEC’s website at www.sec.gov. Additional information regarding the interests of such participants will be contained in the proxy statement/prospectus for the proposed Bu siness Combination when available.

Direc t B iologics 3 Offering Summary Private Placement Offering Summary Issuer • Direct Biologics, LLC Offering Size • Up to $100 million (including $5 million IB1 (1) investment) Pre - $ Valuation • $540 million (20% discount to anticipated public market valuation of $675 million) Potential Earnout • $325 million potential earnout contingent upon 2023 BLA approval or EUA of ExoFlo TM Security • Equity into the LLC (issued at 20% discount to $10.00 implied market price per share) Investment Details • LLC Units to be exchanged for registered shares (subject to lock - up period) following the closing of the proposed de - SPAC transaction • IB1 (1) has invested $5 million in the private placement Timing • Private placement closing on rolling basis and proposed de - SPAC transaction expected to close in Q1 2023 De - SPAC Transaction Overview • Direct Biologics has executed a letter of intent to enter into a business combination with Good Works II Acquisition Corporat ion (“Good Works II”) (Nasdaq:GWII) • Good Works II is a SPAC with $230 million cash in trust, which is subject to redemptions Minimum Cash Condition • The funds from Good Works II trust account (after giving effect to redemptions and after payment of all transaction fees and expenses), together with the net proceeds of the private placement, shall exceed $75 million Selected abbreviations: BLA = Biologics License Application; EUA = Emergency Use Authorization; IB1 = IB Investments 1 LLC. 1 IB1 (IB Investments 1 LLC) is an affiliate of I - Bankers Securities, Inc. and is owned by the CEO of IB Capital, LLC, the Placem ent Agent. No placement fees were paid on IB1's investment.

Direc t B iologics 4 Private Placement Offering - Detailed Overview Does not include $325 million potential earnout contingent upon 2023 BLA approval or EUA of ExoFlo TM . Private Placement Offering as Described in Direct Biologics Units Fully Diluted Direct Biologics Units (in millions) Price Per Unit for Private Placement (20% discount to de- SPAC transaction price) Value for Purposes of Private Placement ($ in millions) Pre-$ (Seller Equity) 113.2 $4.77 $540.0 Private Placement 21.0 4.77 100.0 Post-$ 134.1 $640.0 Conversion Ratio 0.597 Private Placement Offering as Described in Good Works II Shares Value for Purposes of De-SPAC Transaction Good Works II Shares (in millions) Price Per Share for Private Placement (20% discount to de- SPAC transaction price) Value for Purposes of Private Placement ($ in millions) Price Per Share for De-SPAC transaction Value for Purposes of De-SPAC Transaction ($ in millions) Pre-$ (Seller Equity) 67.5 $8.00 $540.0 $10.00 $675.0 Private Placement 12.5 8.00 100.0 10.00 125.0 Post-$ 80.0 $640.0 $800.0

Direc t B iologics 5 De - SPAC Transaction - Detailed Overview Assumes no redemption of $230 million cash in SPAC trust and no excise tax under the Inflation Reduction Act. Does not includ e $ 325 million potential earnout contingent upon 2023 BLA approval or EUA of ExoFlo TM . ($ in millions, except per share/unit data) Illustrative Pro Forma Valuation Pro Forma Good Works II Shares Outstanding (in millions) 109.4 Share Price $10.00 Pro Forma Equity Value $1,094.0 Less: Cash (305.0) Pro Forma Enterprise Value $789.0 Sources Seller Rollover Equity $675.0 67.2% Private Placement Proceeds 100.0 10.0% Cash from SPAC Trust 230.0 22.9% Total Sources $1,005.0 100.0% Uses Seller Rollover Equity $675.0 67.2% Cash to Company from Private Placement 100.0 10.0% Cash to Company from SPAC 205.0 20.4% Estimated Transaction Costs 25.0 2.5% Total Uses $1,005.0 100.0%

Company Overview

7 Direc t Biologics Overview Who We Are Our Mission Value Proposition L ate - stage biotechnology company focused on shepherding the next paradigm shift in medicine with its new platform of potential therapeutics called extracellular vesicles (EVs); the platform consists of a form of stem cell therapy without the cells, DNA or mitochondria To revolutionize the standard of care (SoC) for patients globally by harnessing the natural power of regenerative EV technology Development and commercialization of ExoFlo TM , the first MSC derived EV therapeutic candidate in Phase 3 clinical development, through expertise in manufacturing, R&D, quality assurance, clinical operations and regulatory affairs

Direc t B iologics 8 Sascha Qian Sengupta, M.D. Associate Chief Medical Officer Vikram Sengupta, M.D. Chief Medical Officer John Vacalis, J.D. Chief Legal Officer Executives Mark Adams Chief Executive Officer Co - Founder Joe Schmidt President Co - Founder Jeff Mims Chief Financial Officer Tim Moseley, Ph.D. Chief Science Officer
Direc t Biologics 9 Key Investment Highlights Proprietary extracellular vesicle (EV) platform technology derived from bone marrow mesenchymal stem cells (bmMSCs) ExoFlo TM is a therapeutic candidate isolated from human bmMSCs, containing growth factors and EVs, including exosomes Ongoing “EXTINGuish COVID - 19” Phase 3 clinical trial in acute respiratory distress syndrome (ARDS) induced by Covid - 19, with submission of a Biologics License Application (BLA) and Emergency Use Authorization (EUA) planned in 2023 Potential wide range of additional future opportunities for ExoFlo in other inflammation and tissue repair indications Additional near - term Investigational New Drug (IND) clinical trials planned in all - cause ARDS, mild to moderate Covid - 19, post - acute and chronic post - Covid - 19 syndrome, ulcerative colitis, Crohn’s disease and solid abdominal organ transplant

Direc t B iologics 10 ExoFlo TM ExoFlo is a first - in - class, next - generation biologic leveraging the Company’s proprietary EV platform technology Source: EVs isolated from human bmMSCs Components: Lipid nanovesicles containing 1,000+ regulatory chemokines and other natural signals including mRNA and miRNA Mechanism : Reduce inflammation, repair tissue by activating self - repair pathways and promote healing at the cellular level Selected abbreviations: bmMSC = bone marrow mesenchymal stem cell; EV = extracellular vesicle; miRNA = micro ribonucleic acid ; m RNA = messenger ribonucleic acid.

Direc t B iologics 11 ExoFlo TM ExoFlo is a potent acellular EV product candidate that is anti - inflammatory, non - immunogenic and designed to bypass the drawbacks of traditional stem cell therapies EVs bypass the drawbacks of traditional stem cell therapies • The secreted EVs of bmMSCs can reproduce similar biological activity to MSCs without requiring the transplantation of MSCs themselves • The small size of the EVs in ExoFlo will avoid capillary thrombosis, but are still powerful anti - inflammatory and non - immunogenic therapeutic signals • EVs have a wide array of regulatory proteins to stimulate many processes within the normal healing cascade EVs are derived from human bmMSCs and play a critical role in immune regulation and regeneration • Clinicians increasingly recognize that the mechanism of transplanted MSCs is not engraftment or differentiation in the target tissue, but rather cell - to - cell communication known as paracrine signaling • ExoFlo contains an array of over 1,000 growth factors and chemokines, including exosomes, which deliver mRNAs and miRNAs secreted from MSCs • EVs are paracrine mediators of anti - inflammatory response, immune regulation, tissue regeneration and repair EVs regulate the immune response and promote tissue repair • ExoFlo provides molecular and protein signals that have been shown to stimulate bioactivity and direct cellular communication • By activating and recruiting local cells, ExoFlo can reduce overactive inflammatory response and restore healing Selected abbreviations: bmMSC = bone marrow mesenchymal stem cell; EV = extracellular vesicle; miRNA = micro ribonucleic acid ; m RNA = messenger ribonucleic acid; MSC = mesenchymal stem cell.

Direc t B iologics 12 Virginia mother reunited with newborn after going into Covid - 19 induced coma (1) ExoFlo TM for Compassionate Use Selected abbreviations: EV = extracellular vesicle; FDA = Food and Drug Administration; MSC = mesenchymal stem cell. ExoFlo is an investigational product, the safety and efficacy of which continues to be evaluated in ongoing or planned clinic al trials. The experiences described above may not be representative of other experiences or future experiences with the investi gat ional product. ExoFlo has not been approved for any use in any jurisdiction and may not be in the future. 1 Please see https://www.fox5dc.com/news/virginia - mother - reunited - with - newborn - after - going - into - coma - due - to - covid - 19, which inclu des more information. 2 Please see https://my.clevelandclinic.org/patient - stories/581 - man - undergoes - first - in - world - multi - organ - transplant - to - treat - rare - appendix - cancer, which includes more information. Alma Zepeda Andy Voge Cleveland Clinic performs f irst - in - world f ull multi - organ t ransplant to treat r are a ppendix c ancer (2) “In a last - ditch effort, Zepeda was given ExoFlo … an investigational therapeutic available under compassionate emergency use by the FDA. ” “Andy underwent a procedure perfected and performed by Dr. Lightner [of Cleveland Clinic’s Center for Regenerative Medicine and Surgery]. She administered three doses of MSC derived [ EVs], a… novel treatment for solid organ transplants.”

Direc t B iologics 13 Clinical Pipeline ExoFlo TM Indication Discovery / Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestones (1) Moderate to Severe ARDS Induced by Covid - 19 Initiated Ph 3 in Q3 2022 Planned BLA and EUA Submission in 2023 Mild to Moderate Covid - 19 Initiate Ph 2 in 2023 All - Cause ARDS Initiate Ph 1/2 in Q1 2023 Post - Acute and Chronic Post - Covid - 19 Syndrome Initiate Ph 1/2 in 2023 Ulcerative Colitis (2) Initiate Ph 1 in Q4 2022 Crohn’s Disease (2) Initiate Ph 1 in Q4 2022 Solid Abdominal Organ Transplant (3) Initiate Ph 1 in Q1 2023 ARDS and/or Covid - 19 GI Other EXTINGuish COVID - 19 EXIT ARDS Selected abbreviations: ARDS = acute respiratory distress syndrome; BLA = Biologics License Application; EUA = Emergency Use Aut horization. 1 Estimated dates only; actual milestones may differ materially. 2 Medically refractory. 3 At risk of worsening allograft function with conventional immunosuppressive therapy alone.
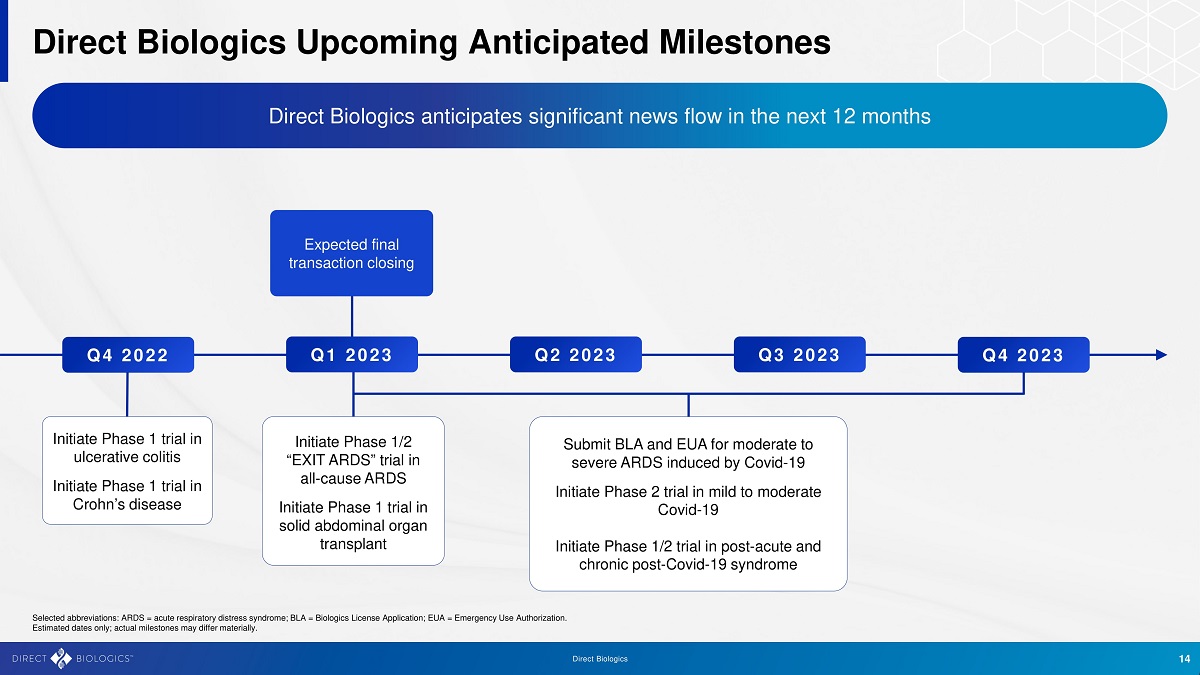
Direc t B iologics 14 Direct Biologics Upcoming Anticipated Milestones Direct Biologics anticipates significant news flow in the next 12 months Q4 2022 Q4 2023 Q1 2023 Submit BLA and EUA for moderate to severe ARDS induced by Covid - 19 Initiate Phase 2 trial in mild to moderate Covid - 19 Initiate Phase 1/2 trial in post - acute and chronic post - Covid - 19 syndrome Initiate Phase 1 trial in ulcerative colitis Initiate Phase 1 trial in Crohn’s disease Selected abbreviations: ARDS = acute respiratory distress syndrome; BLA = Biologics License Application; EUA = Emergency Use Aut horization. Estimated dates only; actual milestones may differ materially. Q2 2023 Q3 2023 Expected final transaction closing Initiate Phase 1/2 “EXIT ARDS” trial in all - cause ARDS Initiate Phase 1 trial in solid abdominal organ transplant

ExoFlo TM for ARDS induced by Covid - 19

Direc t B iologics 16 Acute Respiratory Distress Syndrome (ARDS) ARDS is an inflammatory lung injury that occurs when fluids build up in air sacs in the lungs and is often fatal; ARDS can be caused by many conditions, including Covid - 19, influenza, pneumonia and trauma No Approved Products in US Significant Unmet Need in US Direct Biologics’ Solution: ExoFlo TM • 40 - 50% mortality rate among patients with moderate to severe ARDS • $100,000 - 150,000 average cost of ICU stay for ARDS • Annual intensive care expenditure exceeding $80 billion with ARDS representing 15% of the cause for ICU admissions Compelling clinical data suggest the potential of ExoFlo to treat moderate to severe ARDS induced by Covid - 19 with a favorable tolerability profile ExoFlo has been shown to reduce inflammation and activate local cells to repair tissue damage; ExoFlo reduces neutrophils and increases CD4 & CD8 T Cells • There are currently no approved treatments for ARDS in the US despite decades of clinical trials • Current mainstay of management is low tidal volume mechanical ventilation Selected abbreviation: ARDS = acute respiratory distress syndrome. Moderate to severe ARDS as defined by classic Berlin criteria.

Direc t B iologics 17 Initial Focus on Moderate to Severe ARDS Induced by Covid - 19 Direct Biologics’ strategic initial focus on moderate to severe ARDS induced by Covid - 19 has enabled the Company to develop ExoFlo TM pursuant to an expedited regulatory path Treated Patients During Discretionary Period • In 2019 - 2021, during the FDA’s discretionary enforcement period for certain regenerative medicines, ExoFlo was launched as a tissue allograft for homologous use • ExoFlo has been used in multiple investigator - initiated trials for various indications with no reportable safety events Completed Successful Phase 1 and Phase 2 Trials • In April 2020, the first in - human study for ExoFlo demonstrated that 17 out of 24 hospitalized patients with ARDS induced by Covid - 19 experienced reversal of hypoxia following treatment with ExoFlo • The Phase 2 “EXIT COVID - 19” trial demonstrated safety, dosing regiment and efficacy Ongoing Phase 3 Clinical Trial Under RMAT Designation • In July 2022, the Company initiated its multicenter, double - blinded, placebo - controlled, randomized pivotal Phase 3 trial of ExoFlo in ARDS induced by Covid - 19 • ExoFlo also received RMAT designation, which provides opportunities to potentially expedite development and FDA review Planned Submission of BLA and EUA of ExoFlo in Moderate to Severe ARDS Induced by Covid - 19 in 2023 Selected abbreviations: ARDS = acute respiratory distress syndrome; BLA = Biologics License Application; EUA = Emergency Use Aut horization; FDA = Food and Drug Administration; RMAT = Regenerative Medicine Advanced Therapy. Moderate to severe ARDS as defined by classic Berlin criteria.

Direc t B iologics 18 Current US Standard of Care for Covid - 19 Small Molecule Drugs Vaccines, Monoclonal Antibodies MSC Cellular Therapies Direct Biologics’ Approach: ExoFlo TM Synthetic Synthetic Natural with synthetic adulterants Natural Reported allergic reactions, organ injury Reported allergic reactions, organ injury Reported pulmonary emboli (“lung clots”, allergic reaction) No reportable AEs observed in Phase 1 and 2 clinical trials Antiviral Antiviral Anti - inflammatory, regenerative properties Anti - inflammatory, regenerative properties Potential for variant independent response Waning efficacy response (2 monoclonal antibodies already lost EUA) Potential for variant independent response Potential for variant independent response No mortality reduction, only dexamethasone (steroid) helps No mortality reduction, not effective Potential limited by difficulty of redosing and scaling Potential for mortality reduction and global delivery Provenance Tolerability Mechanism of Action Response to SARS - CoV - 2 Variants Ability to treat ARDS Selected abbreviations: AE = adverse event; ARDS = acute respiratory distress syndrome; EUA = Emergency Use Authorization; EV = extracellular vesicle; MSC = mesenchymal stem cell. These data are derived from different clinical trials at different time points in time, with differences in trial design and pat ient populations. No head - to - head clinical trials have been conducted.

Direc t B iologics 19 ExoFlo TM Phase 3 Pivotal Trial Design and Path to Approval Selected abbreviations: ARDS = acute respiratory distress syndrome; BLA = Biologics License Application; mL = milliliters; SA E = serious adverse event; SoC = standard of care. Severe to critical Covid - 19 as defined by the Food and Drug Administration (FDA) and moderate to severe ARDS as defined by class ic Berlin criteria. EXTINGuish COVID - 19 Phase 3 Trial Design In July 2022, Direct Biologics initiated a multicenter, double - blinded, placebo - controlled, randomized clinical trial to evaluate the safety and efficacy of ExoFlo in treating patients with severe to critical Covid - 19, including those with moderate to severe ARDS caused by Covid - 19 Treatment Arm: SoC + Drug Up to 610 hospitalized, adult patients with severe to critical Covid - 19, including those with moderate to severe ARDS Dosing: ExoFlo - 15 mL diluted to 100 mL in normal saline infused intravenously over 1 hour; infusion repeated 72 hours later if patient has not recovered Placebo Arm: SoC + Placebo Dosing: equivalent volume (100 mL) of normal saline infused intravenously over 1 hour; infusion repeated 72 hours later if patient has not recovered • 60 - day mortality among patients with severe to critical Covid - 19 (full study population) • 60 - day mortality among a subset of patients with moderate to severe ARDS • Primary safety endpoint: incidence of SAEs • Direct Biologics plans to complete an interim efficacy analysis based on 50% enrollment (305 patients) • If the interim analysis reaches statistical significance, Direct Biologics plans to submit a BLA with the data and suspend enrollment in the trial • If additional data is required, full enrollment of 610 patients will be completed before BLA submission Planned BLA submission in 2023 Primary endpoints:

Direc t B iologics 20 Compelling Outcomes from Phase 2 EXIT COVID - 19 Trial Selected abbreviations: AE = adverse event; ARDS = acute respiratory distress syndrome; BiPAP = bilevel positive airway press ure ; CPAP = continuous positive airway pressure; HFNC = high - flow nasal cannula; IP = intraperitoneal; mL = milliliters; mmHg = mil limeters of mercury; NIH = National Institutes of Health; NR = not reached; PaO2/FiO2 = partial pressure of oxygen in arterial blood to fraction of inspired oxygen; SAE = seri ous adverse event; SoC = standard of care; VFDs = ventilator free days. Moderate to severe ARDS as defined by classic Berlin criteria. 1 The Low Dose treatment arm did not reach statistical significance compared to the placebo arm. 2 31 days for Placebo was estimated via nonparametric extrapolative methods by biostatisticians. Results (1) • For 60 - day mortality, the High Dose treatment arm demonstrated an absolute risk reduction of 17.7% compared to placebo plus SoC and a relative risk reduction of 37.6% • Subgroup analysis restricted to patients on HFNC, BiPAP and mechanical ventilation demonstrated an absolute risk reduction of 26 .1% with a relative risk reduction of 42.9% - The subgroup was not pre - specified in the Phase 2 trial design, but will be pre - specified for purposes of the pivotal Phase 3 • No AEs or SAEs were deemed attributable to ExoFlo 60 - day Overall Mortality Rate (%) 29.4% 47.1% Relative risk reduction in 60 - day mortality = 37.6% Absolute risk reduction in 60 - day mortality = 17.7% Median Time to Discharge (Days) 22 NR (31) (2) 15 mL dose IP reduced length of stay by approximately 9 days 7 - day Change in PaO2/FiO2 (mmHg) 56 49 15 mL dose IP showed higher 7 - day change in PaO2/FiO2 Ventilator Free Days (Days) 41 34 15 mL dose IP reduced mechanical ventilation time by 7.3 days SAEs 10 16 44% fewer SAEs in the High Dose treatment arm Safety and Efficacy Endpoints from Phase 2 EXIT COVID - 19 (N=102) Subgroup Analysis of Patients on HFNC, CPAP, BiPAP and Mechanical Ventilation Endpoint High Dose ExoFlo Placebo Notes Trial Design Double - blinded, placebo - controlled, randomized clinical trial of 102 adult Covid - 19 patients with moderate to severe ARDS • Randomized 1:1:1 into 3 treatment arms 1. Placebo + SoC 2. Low Dose ExoFlo - 10 mL + SoC 3. High Dose ExoFlo - 15 mL + SoC • Treatment consisted of one to two 60 - minute infusions of ExoFlo dosed 72 hours apart • Topline endpoints: 60 - day mortality, time to discharge, 7 - day change in PaO2/FiO2 ratio, VFDs and the incidence of SAEs • SoC was defined as per NIH guidelines and consisted of remdesivir, dexamethasone and other medications The trial yielded reduction in multiple clinically relevant endpoints in Covid - 19 patients with moderate to severe ARDS in the high dose ExoFlo TM treatment arm 60 - day Overall Mortality Rate (%) 34.8% 60.9% Relative risk reduction in 60 - day mortality = 42.9% Absolute risk reduction in 60 - day mortality = 26.1%

Direc t B iologics 21 EXIT COVID - 19: Phase 2 Trial Results Selected abbreviations: CI = confidence interval; IP = intraperitoneal; mL = milliliters; NR = not reached. Phase 2 EXIT COVID - 19: % Mortality Vs Time

Direc t B iologics 22 EXIT COVID - 19: Phase 2 Trial Results (continued) Selected abbreviations: CI = confidence interval; IP = intraperitoneal; mL = milliliters; NR = not reached. Time to discharge is the interval in days from randomization to discharge from the hospital. The interval is censored to 60 d ays if the subject did not discharge. Phase 2 EXIT COVID - 19: Time to Discharge
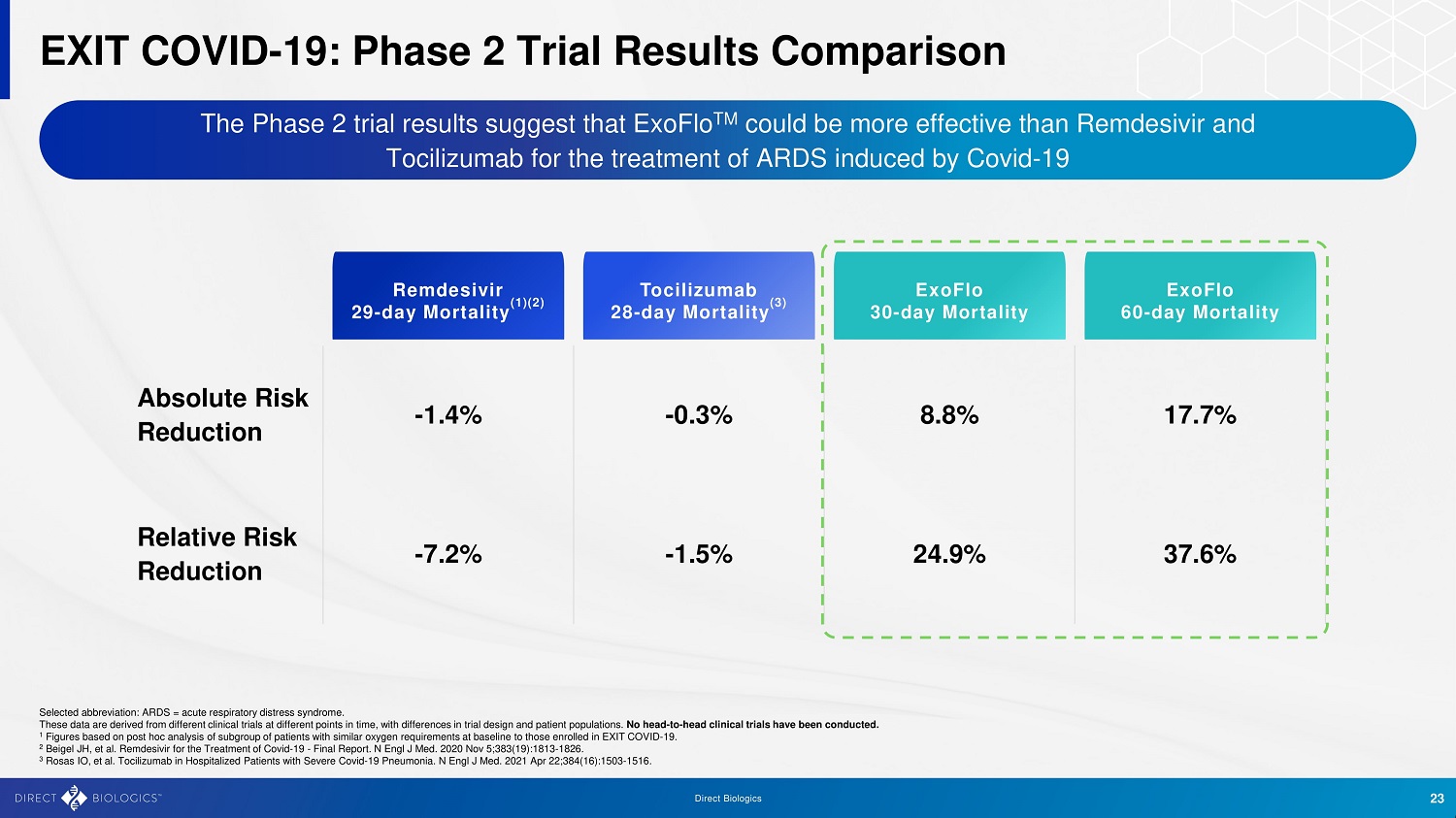
Direc t B iologics 23 EXIT COVID - 19: Phase 2 Trial Results Comparison The Phase 2 trial results suggest that ExoFlo TM could be more effective than Remdesivir and Tocilizumab for the treatment of ARDS induced by Covid - 19 Selected abbreviation: ARDS = acute respiratory distress syndrome. These data are derived from different clinical trials at different points in time, with differences in trial design and patie nt populations. No head - to - head clinical trials have been conducted. 1 Figures based on post hoc analysis of subgroup of patients with similar oxygen requirements at baseline to those enrolled in EXIT COV ID - 19. 2 Beigel JH, et al. Remdesivir for the Treatment of Covid - 19 - Final Report. N Engl J Med. 2020 Nov 5;383(19):1813 - 1826 . 3 Rosas IO, et al. Tocilizumab in Hospitalized Patients with Severe Covid - 19 Pneumonia. N Engl J Med. 2021 Apr 22;384(16):1503 - 15 16 . ExoFlo 30 - day Mortality Tocilizumab 28 - day Mortality (3) Remdesivir 29 - day Mortality (1)(2) ExoFlo 60 - day Mortality Absolute Risk Reduction - 1.4% - 0.3% 8.8% 17.7% Relative Risk Reduction - 7.2% - 1.5% 24.9% 37.6%

Direc t B iologics 24 Discharged within 5 - 6 days Patients 17/24 An independent pilot study of ExoFlo TM at the start of the pandemic demonstrated positive results in ARDS induced by Covid - 19 Phase 1 Clinical Trial Demonstrated Positive Results Selected abbreviations: ARDS = acute respiratory distress syndrome; IND = Investigational New Drug; IRB = Institutional Revie w B oard. Trial Origin • In April 2020, independent investigators performed an IRB - approved clinical trial using ExoFlo to treat 24 patients with moderate to severe ARDS due to Covid - 19 • The independent pilot study demonstrated reversal of hypoxia following treatment with ExoFlo • The results of this trial were used as Phase 1 data to support Direct Biologic’s IND submission allowing the Company to proceed directly into the Phase 2 “EXIT COVID - 19” trial Trial Outcome • After a single dose, 17 of 24 patients demonstrated reversal of hypoxia • Discharged from hospital within 5 - 6 days • 50% median time to recovery from a single dose compared to that observed over a 10 - day course of Remdesivir (11 days) Time to recovery 50% of Remdesivir

Direc t B iologics 25 ExoFlo TM Expanded Access Protocol for Intermediate Population • Safety analysis performed by the independent DSMB for the first 50 patients • No SAEs related to ExoFlo • Ad hoc efficacy analysis also performed which revealed the following outcomes for 26 patients • 30 - day mortality rate was 25.8%, outperforming historical SoC mortality rates of 45% to 55% or greater • 7 - day change in PaO2/FiO2 was + 132 mmHg, reflecting an improvement in oxygenation • Median TTD: 6.8 days • VFDs for non - intubated patients: 43.6 days • Proportion of non - intubated patients progressing to intubation: 8.3% Data Readout (1)(2) Positive Data to Date • The Company is actively enrolling the open - label clinical trial of ExoFlo in adult Covid - 19 patients with moderate to severe ARDS under an expanded access protocol authorized by the FDA • Single arm study, eligible patients receive up to 3 doses of High Dose intravenous ExoFlo, which is administered in 72 - hour intervals • Endpoints include 60 - day mortality, 7 - day change in PaO2/FiO2, TTD, VFDs and the incidence of SAEs • Safety assessed for every patient Background and Methods Open - label, compassionate use study demonstrated positive outcomes in patients with moderate to severe ARDS induced by Covid - 19 to date Selected abbreviations: ARDS = acute respiratory distress syndrome; DSMB = Data and Safety Monitoring Board; FDA = Food and D rug Administration; mmHg = millimeters of mercury; PaO2/FiO2 = partial pressure of oxygen in arterial blood to fraction of inspir ed oxygen; SAE = serious adverse event; SoC = standard of care; TTD = time to discharge; VFDs = ventilator free days. Moderate to severe ARDS as defined by classic Berlin criteria. 1 60 - day mortality rate not shown in data readout due to timing of efficacy analysis; 60 - day mortality is expected at a future dat e. 2 Eligibility for expanded access trial was partly determined based on patient exclusions from the Phase 2 trial due to high di sea se severity. Therefore, patients in expanded access have inherently greater disease severity.

Additional Planned ExoFlo TM Clinical Trials

Direc t B iologics 27 Upcoming Clinical Trial of ExoFlo TM in All - Cause ARDS Indication EXIT ARDS Phase 1/2 Trial Design In Q1 2023, Direct Biologics plans to initiate a placebo - controlled, randomized Phase 1/2 clinical trial to evaluate the safety and efficacy of ExoFlo in treating moderate to severe all - cause ARDS Treatment Arm 1: SoC + Drug ~81 adult patients with moderate to severe ARDS Dosing: ExoFlo - 10 mL diluted to 100 mL in normal saline infused intravenously over 1 hour; total of two infusions Placebo Arm: SoC + Placebo Dosing: equivalent volume (100 mL) of normal saline infused intravenously over 1 hour; total of two infusions Treatment Arm 2: SoC + Drug Dosing: ExoFlo - 15 mL diluted to 100 mL in normal saline infused intravenously over 1 hour; total of two infusions Secondary endpoints: • VFDs at 28 days • Change in PaO2/FiO2 ratio from pre - infusion baseline to day 7 – PaO2 may be calculated from ABG or imputed from the SpO2 daily Primary endpoints: • Number of patients with all - cause mortality at 28 days • Primary safety endpoint: incidence of SAEs Selected abbreviations: ABG = arterial blood gas; ARDS = acute respiratory distress syndrome; FiO2 = fraction of inspired oxy gen ; mL = milliliters; PaO2 = partial pressure of oxygen in arterial blood; SAE = serious adverse event; SpO2 = oxygen saturatio n; SoC = standard of care; VFDs = ventilator free days. Moderate to severe ARDS as defined by classic Berlin criteria. Trial structure may be subject to change based upon additional data gathered.

Direc t B iologics 28 Planned Clinical Trials of ExoFlo TM in Two New Covid - 19 Indications Direct Biologics plans to initiate two additional clinical trials in 2023 in Covid - 19 indications Phase 2: Mild to Moderate Covid - 19 • Estimated enrollment: 30 adult participants • Allocation: Randomized • Masking: Triple (Participant, Care Provider, Investigator) • Three arms: placebo arm, low dose treatment arm and high dose treatment arm Phase 1/2: Post - Acute and Chronic Post - Covid - 19 Syndrome • Estimated enrollment: 60 adult participants • Allocation: Randomized • Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) • Two arms: placebo arm and treatment arm Primary endpoints: • Increased distance on 6MWT at 61 days • Safety Endpoint: incidence of SAEs Secondary endpoints: • EQ - 5D test at 61 days • MRC Dyspnea Scale Primary endpoint: • Change in SARS - CoV - 2 log viral load from baseline to day 7 Secondary endpoints: • Change in viral load AUC from baseline to day 29 • Proportion of patients showing symptom improvement or resolution at 61 days • Proportion of patients who required Covid - 19 related hospitalization or EDV by day 29 Selected abbreviations: 6MWT = six - minute walk test; AUC = area under the curve; EDV = Emergency Department Visit; EQ - 5D = EuroQ ol five - dimension quality of life instrument test; MRC = Medical Research Council; SAE = serious adverse event; SoC = standard o f care. Mild to moderate Covid - 19 as defined by the Food and Drug Administration (FDA). Trial structures may be subject to change based upon additional data gathered.

Direc t B iologics 29 Three Additional Planned Clinical Trials of ExoFlo TM Direct Biologics plans to initiate three additional upcoming clinical trials in ulcerative colitis, Crohn’s disease and solid abdominal organ transplant Phase 1: Ulcerative Colitis An open - label Phase 1 trial of ExoFlo for the treatment of medically refractory UC • Estimated enrollment: 10 adult participants • Allocation: Non - randomized Expanded Access: Solid Abdominal Organ Transplant Expanded access, open - label trial for the use of ExoFlo in the treatment of solid abdominal organ transplant patients who are at risk of worsening allograft function with conventional immunosuppressive therapy alone • Estimated enrollment: 20 adult participants Phase 1: Crohn's Disease An open - label Phase 1 trial of ExoFlo for the treatment of medically refractory Crohn's disease • Estimated enrollment: 10 adult participants • Allocation: Non - randomized O utcome measurements: • Number of subjects who tolerate IV ExoFlo in those subjects with moderately to severely active UC who have failed or are intolerant to one or more mAbs over 70 weeks • Number of AEs in subjects who received IV ExoFlo in those subjects with moderately to severely active UC who have failed or are intolerant to one or more mAbs O utcome measurements: • To evaluate the feasibility of IV ExoFlo in subjects with moderately to severely active Crohn's disease who have failed or are intolerant to one or more mAbs over 70 weeks • To evaluate the safety of IV ExoFlo in subjects with moderately to severely active Crohn's disease who have failed or are intolerant to one or more mAbs over 70 weeks O utcome measurements: • Number of participants with AEs and SAEs Selected abbreviations: AE = adverse event; IV = intravenous; mAbs = monoclonal antibodies; SAE = serious adverse event; UC = ul cerative colitis. Trial structures may be subject to change based upon additional data gathered.

Manufacturing, IP & Future Opportunities

Direc t B iologics 31 Scalable Manufacturing Capabilities The Company’s proprietary EV technology is positioned to scale with global demand, including meeting rigorous FDA manufacturing requirements Direct Biologics already has substantial cGMP manufacturing capabilities and clinical experience with ExoFlo established during the FDA’s discretionary enforcement period for certain regenerative medicines Selected abbreviations: bmMSC = bone marrow mesenchymal stem cell; cGMP = current Good Manufacturing Practices; EV = extracel lul ar vesicle; FDA = Food and Drug Administration; GTP = Good Tissue Practice; MSC = mesenchymal stem cell; QC = quality control . Substantial progress in the development of proprietary technology using bioreactors capable of mass producing ExoFlo TM , significantly increases the yield of EV production while simultaneously reducing manufacturing costs cGMP manufacturing facilities across the US, including state - of - the - art R&D facilities at the Center for Novel Therapeutics at U C San Diego and QC lab in St. Louis Treatment with bmMSC derived EVs rather than transplantation of MSCs allows for minimal batch - to - batch variability as the produc t can be derived from a single donor rather than harvesting from multiple donors Key trade secrets and portfolio of patents protecting IP extensively in the US and in select jurisdictions throughout the wor ld Cell banks derived from healthy human donors are GTP compliant and have master files with the FDA

Direc t B iologics 32 Direct Biologics works to maintain its competitive position by utilizing a multi - layered approach that includes filing and prosecuting US and foreign patent applications and protecting proprietary know - how as trade secrets Robust Intellectual Property Selected abbreviations: bmMSC = bone marrow mesenchymal stem cell; EV = extracellular vesicle; MSC = mesenchymal stem cell; O US = outside the United States. Patent and Trade Secret Strategy • Focused on obtaining patents covering product technology, manufacturing and delivery processes, including the proprietary man ufa cturing process of refining EV therapeutics from bmMSCs and other regenerative cells • The EV technology platform is protected by a variety of patents pending in the US and OUS, as well as closely guarded trade s ecr ets • Key trade secrets serve to keep manufacturing processes undisclosed • Patents pending covering product technology, manufacturing and delivery processes, and release and stability assays • Additional patent applications in process to obtain a range of coverage for future opportunities Pending Patent Examples • Applications related to MSC secretomes, methods of making them and their use in treating and preventing a variety of conditio ns including wounds, skin disorders, orthopedic disorders/injuries and spinal disorders/injuries • Application related to a composition comprising a MSC secretome preparation and one or more biomolecules that selectively bin d t o one or more microbial immunogens, as well as a method of treating a microbial infection • Application related to a method of isolating platelet rich derived exosomes, platelet rich plasma derived exosome preparation an d a method of treating orthopedic or spinal pathology comprising administering a platelet rich plasma derived exosome • Application related to methods of treating certain hair and skin disorders • Application related to bacterial and viral infections and subsequent inflammation related disorders

Direc t B iologics 33 Multi - Billion Dollar Aggregate Market for Existing Clinical Programs Multiple Additional Potential Applications for ExoFlo TM Platform Long Term Opportunities Direct Biologics’ initial focus is on critical unmet needs with applications across inflammation and tissue repair MDI – ExoFlo formulation for outpatient pulmonary indications Lyophilized ExoFlo (powder formulation) – room temperature stable for ease of distribution (e.g., international markets) Product Extensions ALS Osteo - Arthritis Wound Care Hair Aesthetics Fertility Selected abbreviations: ARDS = acute respiratory distress syndrome; ALS = amyotrophic lateral sclerosis; GI = gastrointestina l; IBD = inflammatory bowel disease; MDI = Metered Dose Inhaler. ARDS Induced by Covid - 19 Post - Acute and Chronic Post - Covid - 19 Syndrome ARDS GI / IBD Transplant Rejection

Direc t B iologics 34 Direct Biologics Upcoming Anticipated Milestones Direct Biologics anticipates significant news flow in the next 12 months Q4 2022 Q4 2023 Q1 2023 Submit BLA and EUA for moderate to severe ARDS induced by Covid - 19 Initiate Phase 2 trial in mild to moderate Covid - 19 Initiate Phase 1/2 trial in post - acute and chronic post - Covid - 19 syndrome Initiate Phase 1 trial in ulcerative colitis Initiate Phase 1 trial in Crohn’s disease Selected abbreviations: ARDS = acute respiratory distress syndrome; BLA = Biologics License Application; EUA = Emergency Use Aut horization. Estimated dates only; actual milestones may differ materially. Q2 2023 Q3 2023 Expected final transaction closing Initiate Phase 1/2 “EXIT ARDS” trial in all - cause ARDS Initiate Phase 1 trial in solid abdominal organ transplant

Appendix

Direc t B iologics 36 AmnioWrap Although it is not the Company’s primary focus, Direct Biologics’ marketed product, AmnioWrap, is currently a revenue - generating product x Placental - based allografts that contain a collagen extracellular matrix and a wide array of regulatory proteins including growth factors, cytokines and chemokines x Addresses a wide variety of clinical needs for skin and dermal substitute applications by supporting the healing cascade in the treatment of acute and chronic wounds x Technology marketed under three brands 1. AmnioWrap – a single layer amnion allograft sized appropriately for ocular applications 2. AmnioWrap2 – a tri - layer amnion allograft ideal for acute and chronic wound application in the outpatient clinic setting (reimbursed with a respective Q code) 3. AmnioWrap3 – a tri - layer amnion allograft sized appropriately for surgical placement; targeting hospital and ASC surgical procedures x The AmnioWrap2 product is in the process of being sold (expected completion in Q4 2022) x AmnioWrap3 is being evaluated in an investigator - initiated trial for the prevention and healing of incisional hernias x Multi - billion dollar addressable market Selected abbreviation: ASC = ambulatory surgery center.