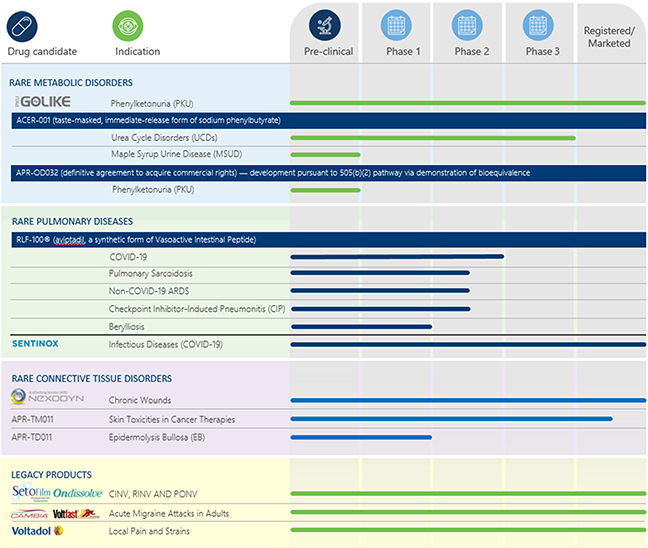
In July 2022 (post reporting period), the final agreement was executed. Pursuant to the final agreement, Meta shall transfer to Relief all data, know-how, as well as any intellectual property related to “APR-OD32”, as developed, or generated by Meta. Relief shall only be responsible for funding the remaining development activities as well as for filing and obtaining a new drug application in all countries worldwide except for the UK where Relief shall grant a license back to Meta, enabling Meta to market the product in that country. Other than the initial acquisition payment and low double-digit royalty payments on net profit of the product in the various countries, Relief shall be under no obligation to fund or pay any other amount to Meta.
RLF-100® (aviptadil)
RLF-100® (aviptadil), IV
In January 2022, the parent company, NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) (“NRx”), of Relief’s collaboration partner for RLF-100®, NeuroRx, Inc. (“NeuroRx”), announced that it had submitted an application to the FDA seeking Emergency Use Authorization (“EUA”) for the use of aviptadil to treat patients with critical COVID-19 who are at immediate risk of death from respiratory failure despite treatment with approved therapy including remdesivir and who are ineligible for enrollment into the ACTIV-3b NIH-sponsored trial.
In January 2022, NRx announced enhancements to its Expanded Access and Right to Try programs. NRx stated that these programs enable patients with respiratory failure from COVID-19, who have tried all approved medicines, including remdesivir, and who were not able to participate in a clinical study, to receive aviptadil upon a physician’s prescription. According to NRx, they would continue to provide aviptadil to hospitals enrolled in its Expanded Access Protocol under FDA guidelines. The press release also reported that NRx was making aviptadil available as an investigational medicine under the Federal Right to Try Act.
In January 2022, NRx announced receipt of a first safety report from a Southwestern hospital where physicians had administered aviptadil to patients with COVID-19 respiratory failure. According to NRx, the patients were treated under the Federal Right to Try Law that gives access to investigational medicines for patients who have been diagnosed with life-threatening diseases or conditions, who have tried all approved treatment options, and who are unable to participate in a clinical trial to access certain unapproved treatments. NRx stated that of the first 19 patients treated by December 31, 2021, three had died and 16 (84%) were reported to be alive by January 22, 2022. NRx also reported that this Right to Try use of aviptadil occurred during the Omicron surge, although patients were not necessarily tested for the specific COVID variant that caused their ICU admission. NRx noted that no serious adverse events were reported.
In February 2022, NRx announced results of a review conducted by the DSMB of the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH on February 14, 2022. According to NRx, the DSMB reviewed data on 448 ICU patients with Critical COVID-19 Respiratory Failure who were enrolled in the ACTIV-3b/TESICO trial. NRx reported that no new safety concerns were identified, and the study was cleared to continue enrollment to 640 patients. NRx also stated that the TESICO protocol was submitted by the NIH and cleared by the FDA as a phase 3 trial that, if positive, may be used in the submission of an NDA for aviptadil.
In February 2022, Relief announced that it had filed for a trademark application (U.S. Serial Number 90141290), for RLF-100® with the U.S. Patent and Trademark Office (“USPTO”). Subsequently, in March 2022, a certificate of registration was received. The trademark covers RLF-100® when used for pharmaceutical preparations and substances for the treatment of viral, metabolic, endocrine, musculoskeletal, cardiovascular, cardiopulmonary, genitourinary, sexual dysfunction, oncological, hepatological, ophthalmic, respiratory, neurological, gastrointestinal, hormonal, dermatological, psychiatric and immune system related diseases and disorders; pharmaceutical preparations for the treatment of viral diseases and; pharmaceutical preparations for the treatment of viral infections.
In April 2022, NRx filed a Breakthrough Therapy Designation (BTD) request for aviptadil with the FDA. NRx reported that the request was based on a post hoc analysis of Covid-19 patients that, in addition to aviptadil or placebo, were also treated with remdesivir and whose respiratory failure due to COVID-19 continued to progress. NRx also stated that its request included cumulative safety data on approximately 750 patients treated with RLF-100® IV for Critical COVID-19. Subsequently, in June 2022, Relief reported that NRx had announced an update on its BTD request and disclosed that the FDA had denied its BTD application for aviptadil.
In May 2022, Relief reported that NRx had announced results from NIH’s DSMB trial evaluating aviptadil for the treatment of COVID-19, noting discontinuation of the trial for futility.
In May 2022, Relief provided a corporate update noting that it intends to continue clinical assessment of both inhaled and IV formulations of RLF-100® for other indications, including (1) the continuation of the European study of inhaled RLF-100® for COVID-19-infected patients (the “Leuppi Study”) (2) the initiation of a clinical trial of RLF-100® in early 2023 in patients with sarcoidosis (3) the exploration of RLF-100® for checkpoint inhibitor-induced pneumonitis (4) testing of RLF-100® in the treatment of non-COVID-19-related acute respiratory distress syndrome (ARDS) and (5) conducting a European proof-of-concept of RLF-100® in the treatment of chronic berylliosis.