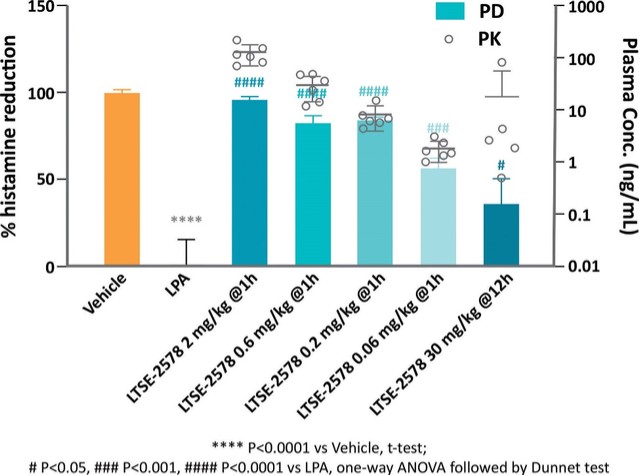
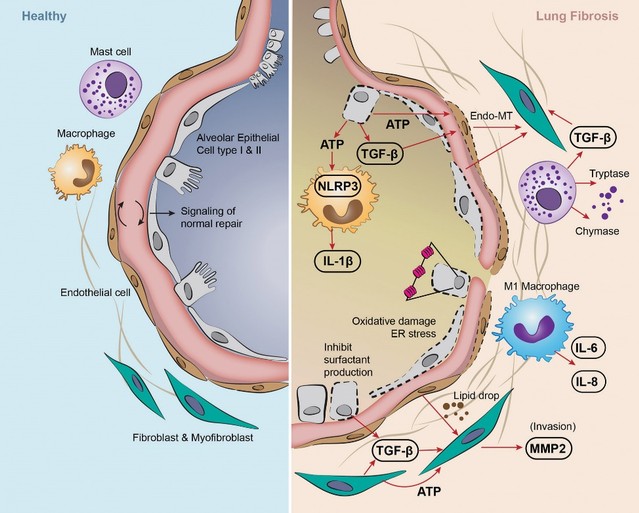
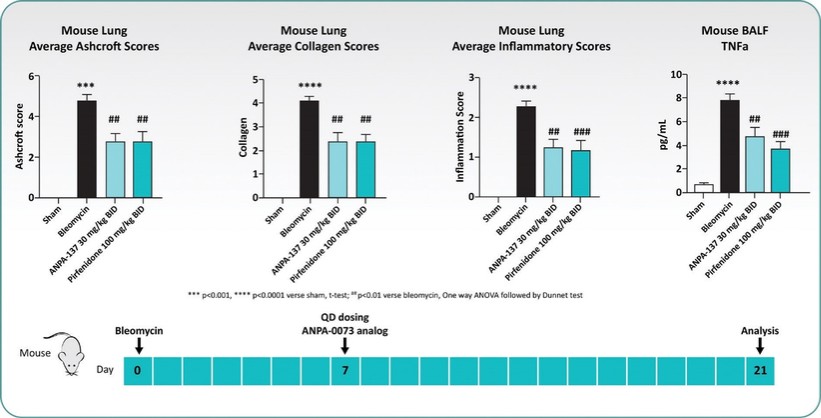
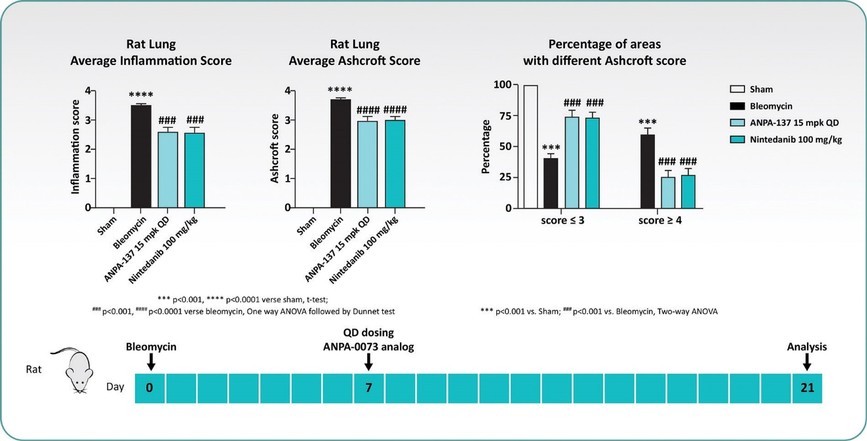
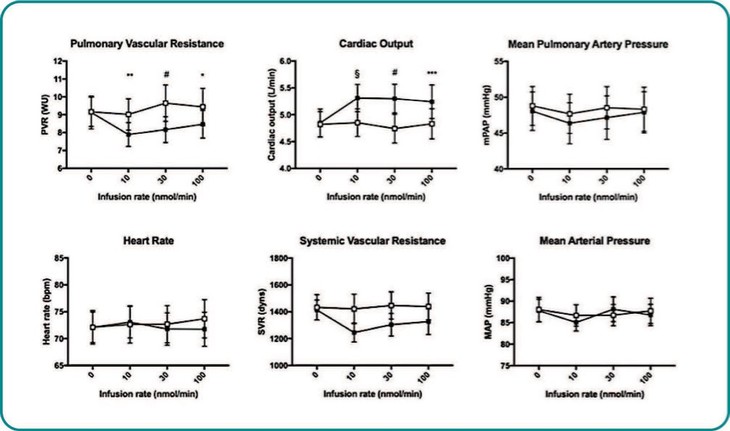
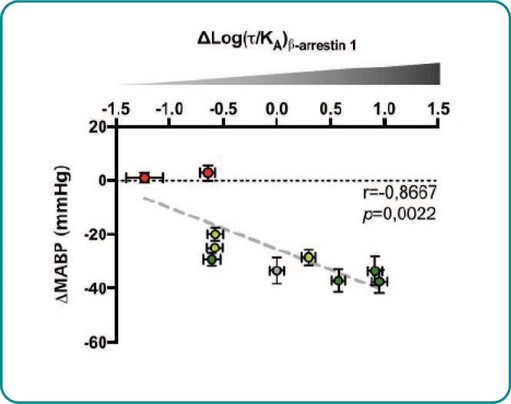
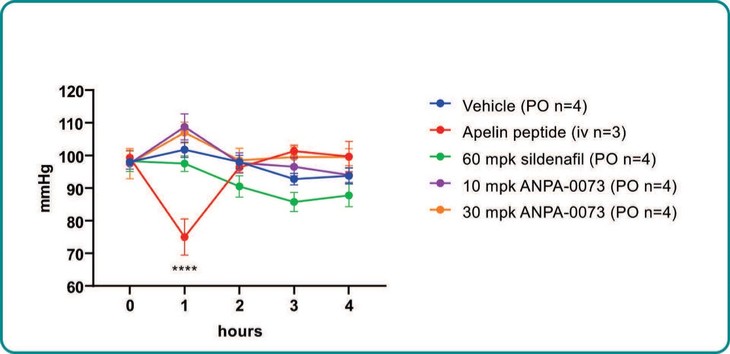
Treatment with sildenafil or an ANPA-0073 in an MCT rat model of PAH
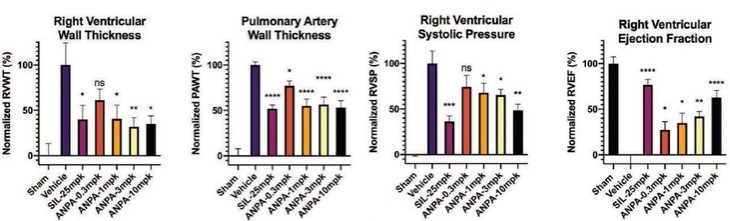
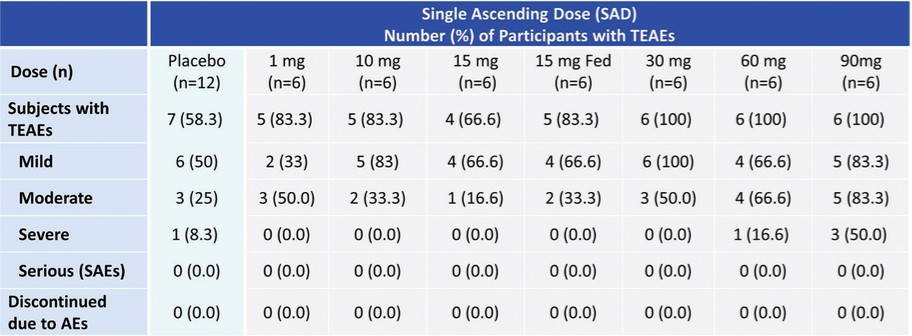
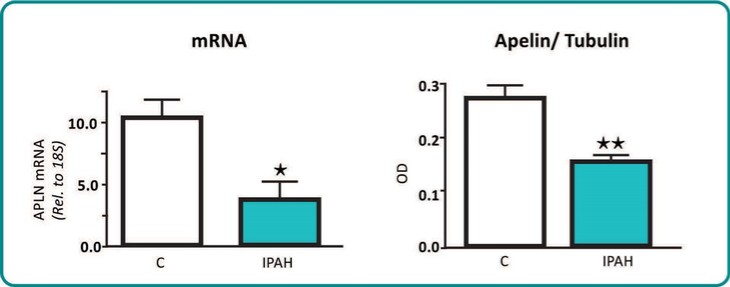
One way ANOVA analysis; *p<0.05, **p<0.01, **** p<0.0001 compared to vehicle
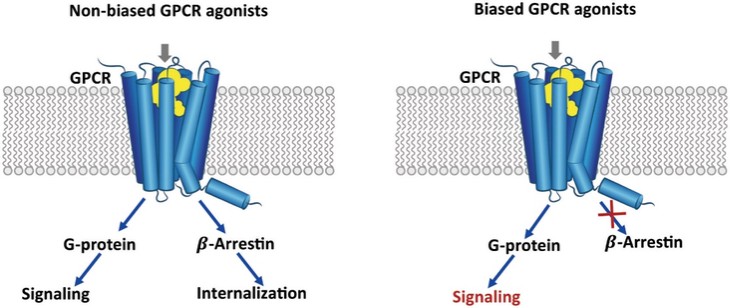
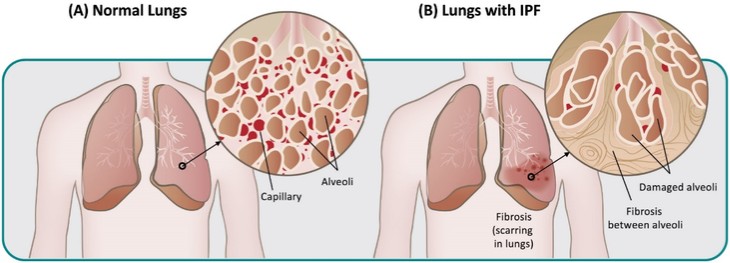
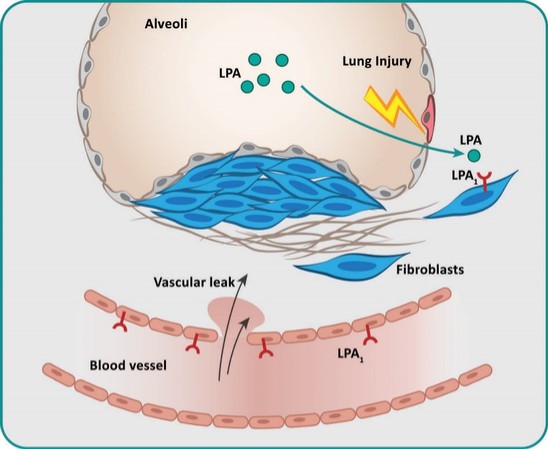
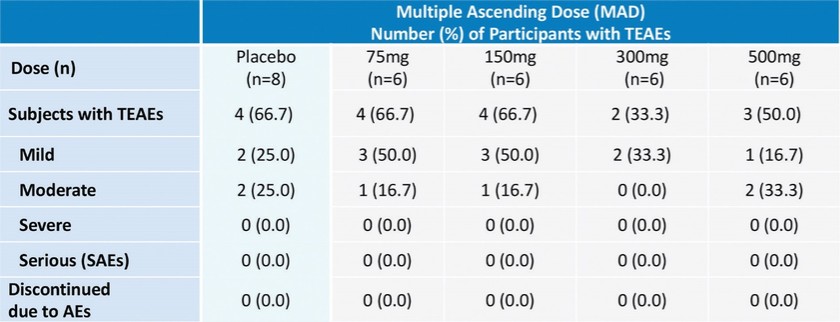
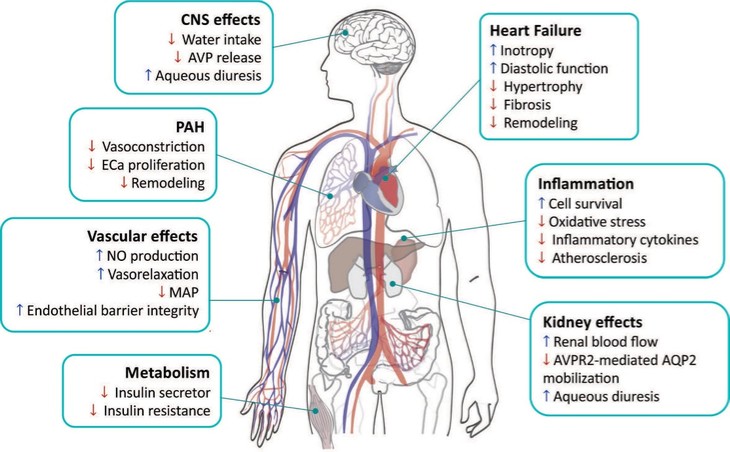
In summary, in a published third-party clinical proof-of-concept study, an intravenous infusion of apelin has demonstrated increased cardiac output, especially in combination with the PAH standard of care therapy, sildenafil. In the MCT rat model of PAH, our biased apelin agonists showed increased cardiac stroke volume and cardiac output without impacting heart rate, and also mitigation of PAH-induced vascular remodeling. Together, these data suggest that an orally-available, biased apelin agonist, such as ANPA-0073, may have potential as a treatment for PAH, providing benefits differentiated from current standard of care therapies.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates and other discoveries, inventions, trade secrets and know-how that are critical to our business operations. Our success also depends in part on our ability to operate without infringing the proprietary rights of others, and in part on our ability to prevent others from infringing our proprietary rights. A comprehensive discussion on risks relating to intellectual property is provided under Part I. Item 1A. “Risk Factors—Risks Related to Our Intellectual Property.”
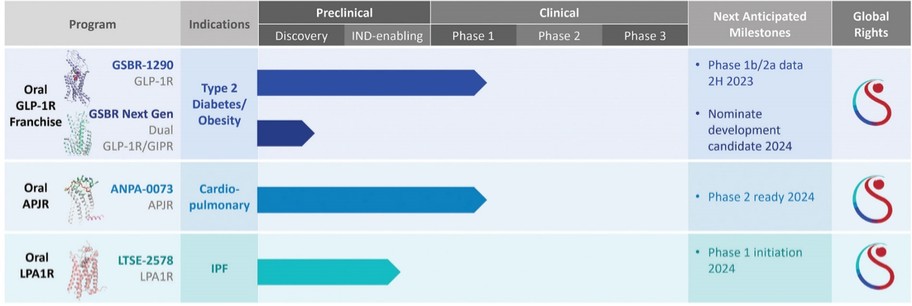
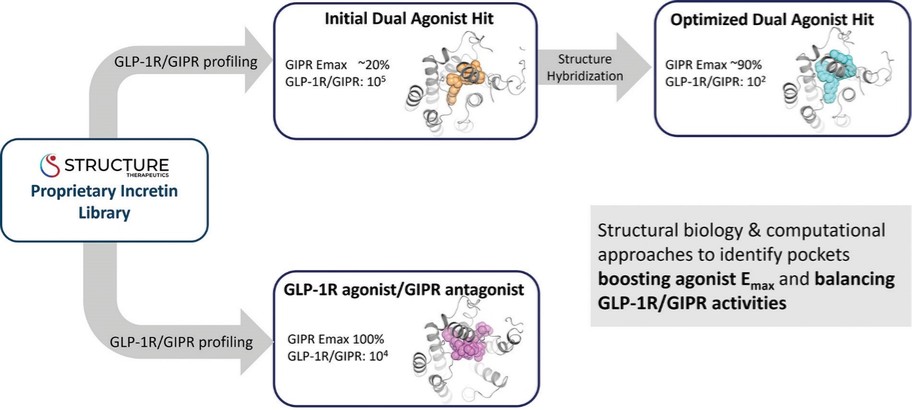
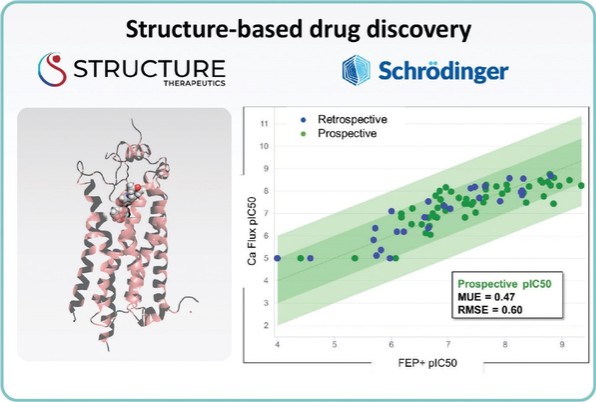
For our GLP-1R program, as of December 31, 2022, our wholly-owned subsidiary Gasherbrum Bio, Inc., is the sole owner of one granted U.S. patent and five pending U.S. patent applications, 16 Patent Cooperation Treaty (“PCT”) applications, and 42 pending foreign patent applications in Argentina, African Regional Intellectual Property Organization (“ARIPO”) Australia, Brazil, Canada, Chile, the People’s Republic of China (“PRC”), Colombia, Costa Rica, Dominican Republic, Egypt, Eurasian Patent Office (“EAPO”), European Patent Office (“EPO”), Guatemala, Indonesia, Israel, India, Japan, South Korea, Mexico, Malaysia, New Zealand, Panama, Peru, Philippines, Saudi Arabia, Singapore, Thailand, Taiwan, Ukraine, Vietnam, and South Africa. These patent applications, to the extent they issue (or in the case of priority applications, if issued from future non-provisional applications that we file), are expected to expire between 2041 and 2043, without accounting for potentially available patent term adjustments or extensions. These patent applications relate to compositions of matter of heterocyclic GLP-1 agonists, including GSBR-1290 and its analogs, solid forms and methods of treating conditions associated with GLP-1R activity. We intend to strengthen the patent protection of our product candidates and other discoveries, inventions, trade secrets and know-how that are critical to our business operations through additional patent application filings.
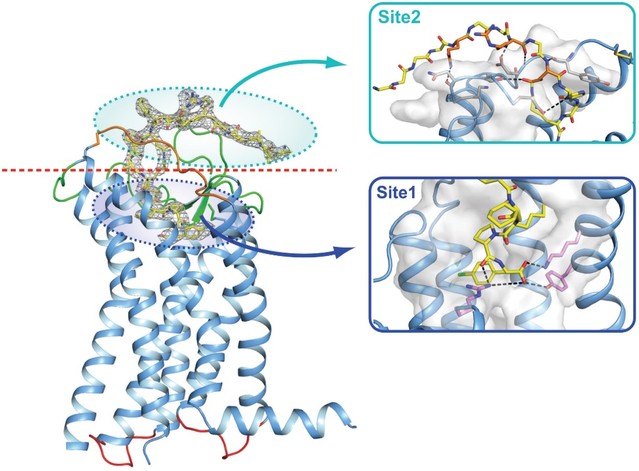
For our APJR program, as of December 31, 2022, our wholly-owned subsidiary Annapurna Bio, Inc. is the sole owner of one granted U.S. patent and two pending U.S. patent applications, one PCT, application, and 22 pending foreign patent applications in Argentina, Australia, Brazil, Canada, the PRC, EAPO, EPO, Hong Kong, Israel, India, Japan, South Korea, Mexico, New Zealand, Singapore, Taiwan and South Africa relating to compounds and compositions of matter for treating conditions associated with Apelin receptor activity, including ANPA-0073 and its analogs, solid forms and methods of treating conditions associated with Apelin receptor activity. Any patents issuing from these patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2039 and 2043, without accounting for potentially available patent term adjustments or extensions.