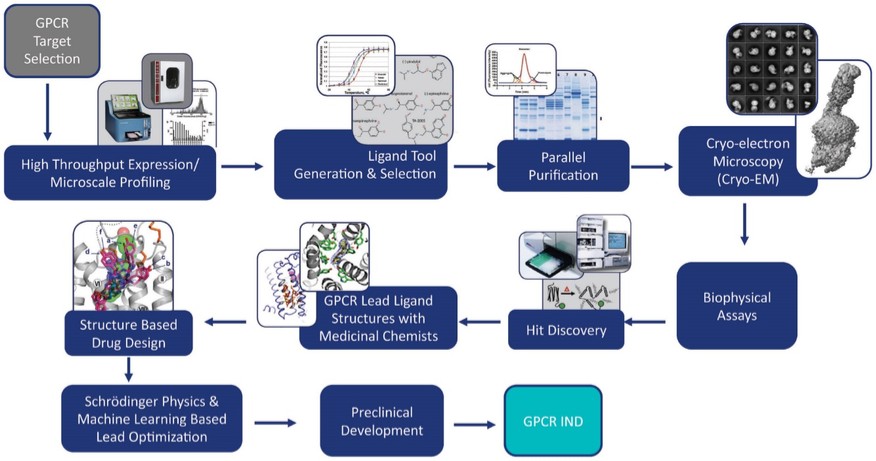
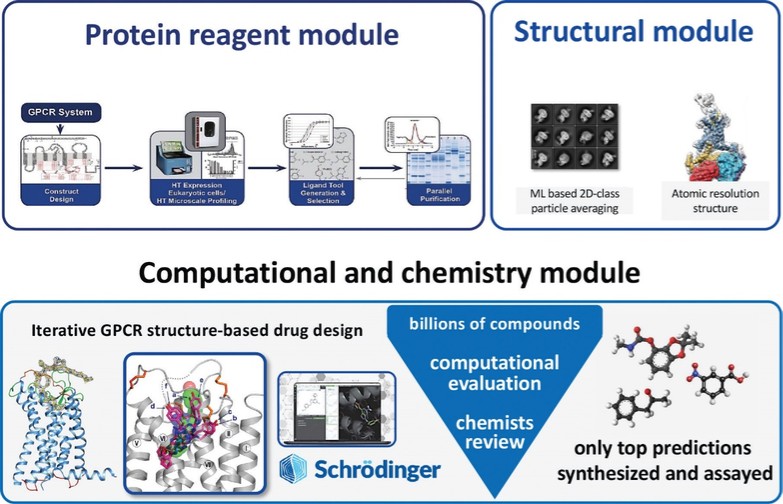
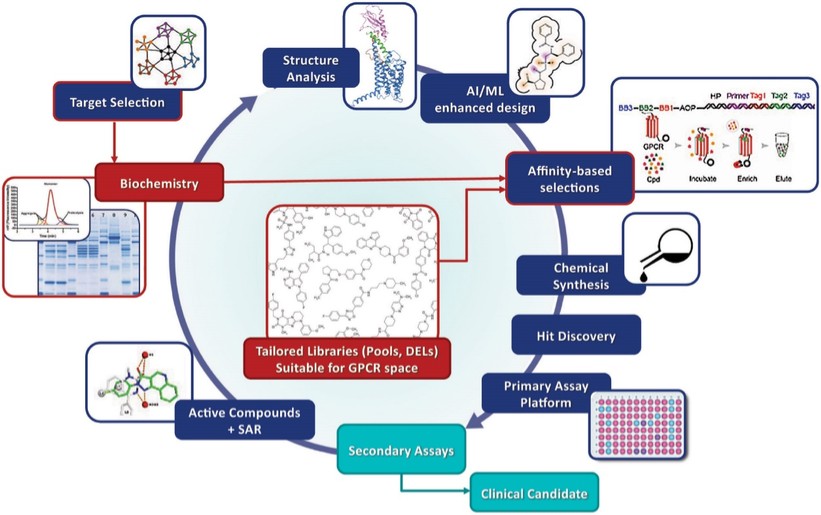
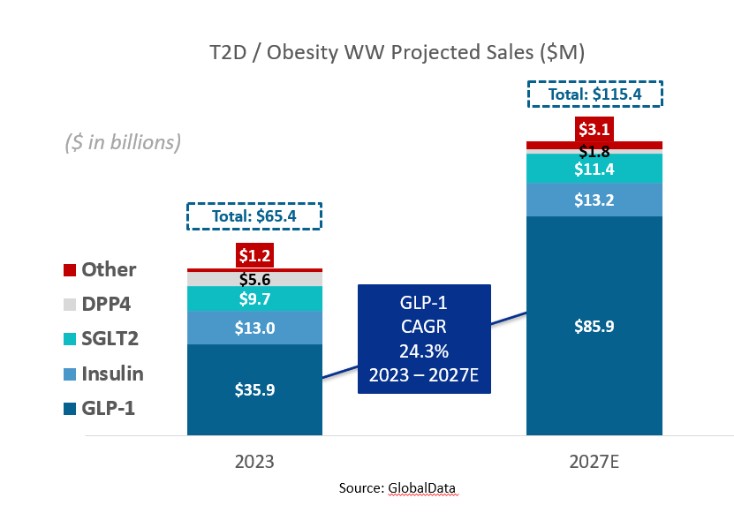
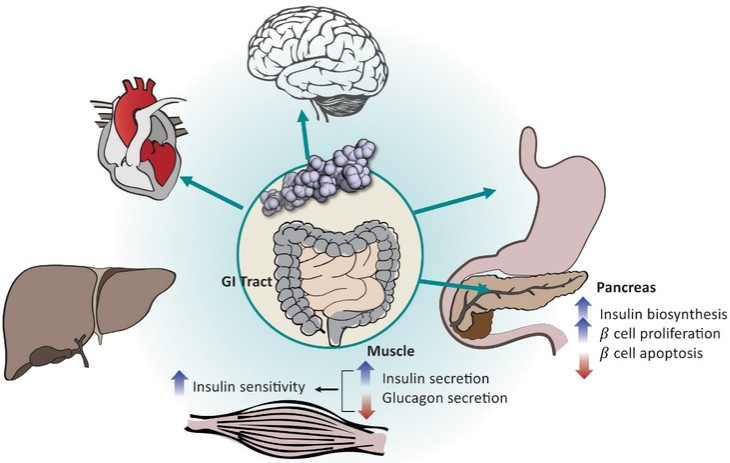
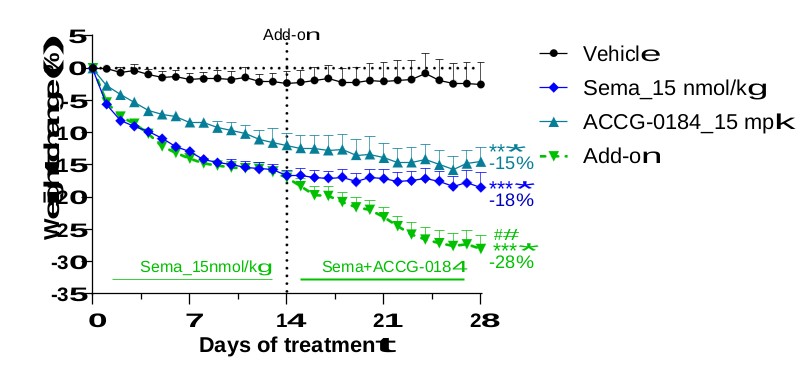
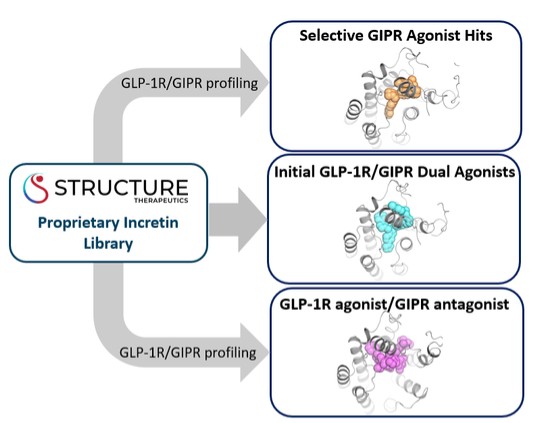
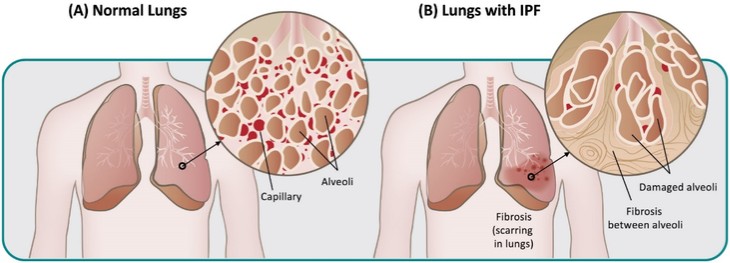
discussion on risks relating to intellectual property is provided under Part I. Item 1A. “Risk Factors—Risks Related to Our Intellectual Property.”
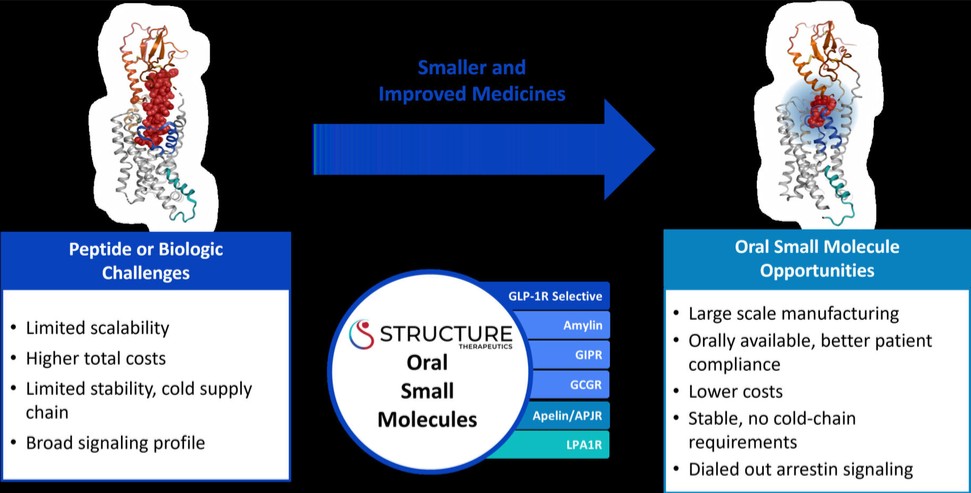
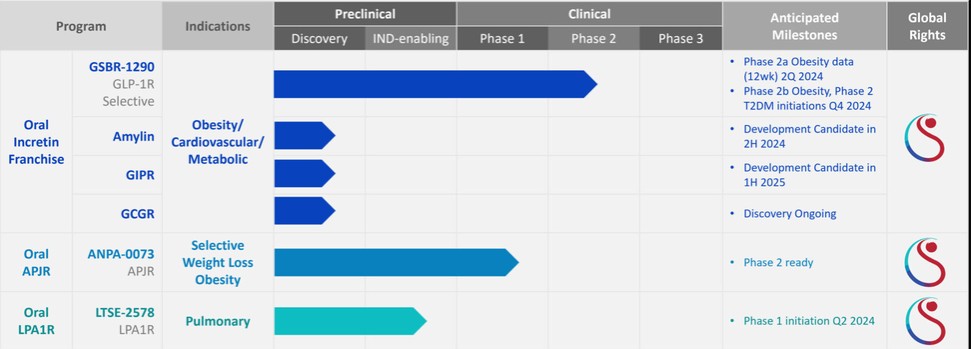
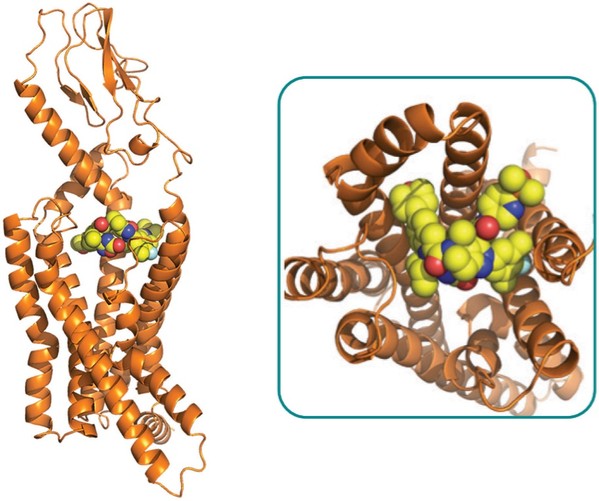
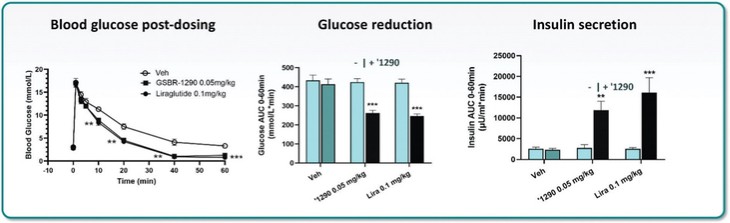
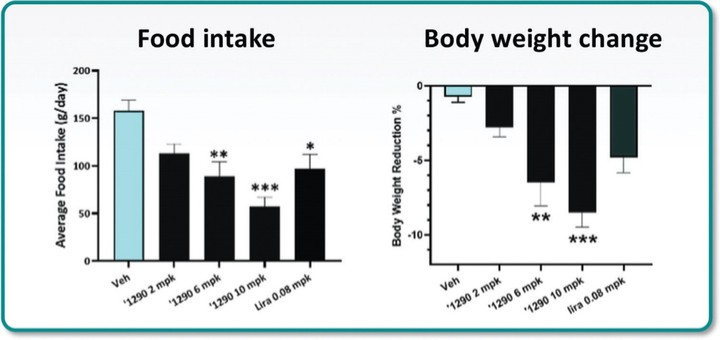
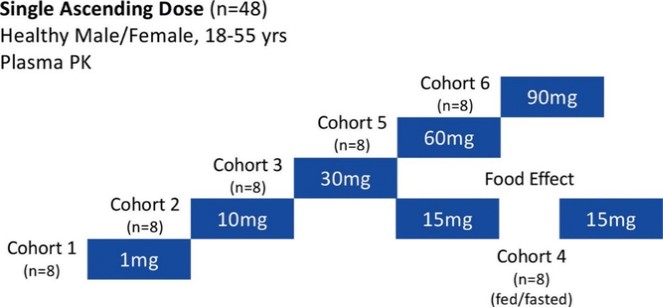
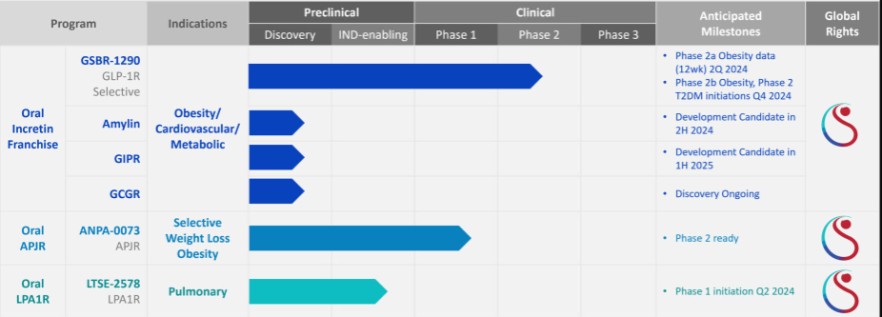
For our GLP-1R program, as of December 31, 2023, our wholly-owned subsidiary Gasherbrum Bio, Inc., is the sole owner of one granted U.S. patent and 11 pending U.S. patent applications, 14 Patent Cooperation Treaty (“PCT”), applications, and 94 pending foreign patent applications in Argentina, the African Regional Intellectual Property Organization (“ARIPO”), Australia, Brazil, Canada, Chile, the People’s Republic of China (“PRC”), Colombia, Costa Rica, Dominican Republic, Egypt, the Eurasian Patent Office (the “EAPO”), the European Patent Office (the “EPO”), Guatemala, Hong Kong, Indonesia, Israel, India, Japan, South Korea, Mexico, Malaysia, New Zealand, Panama, Peru, Philippines, Saudi Arabia, Singapore, Thailand, Taiwan, Ukraine, Vietnam, and South Africa. These patent applications, to the extent they issue (or in the case of priority applications, if issued from future non-provisional applications that we file), are expected to expire between 2041 and 2044, without accounting for potentially available patent term adjustments or extensions. These patent applications relate to compositions of matter of heterocyclic GLP-1 agonists, including GSBR-1290 and its analogs, solid forms and methods of treating conditions associated with GLP-1R activity. We intend to strengthen the patent protection of our product candidates and other discoveries, inventions, trade secrets and know-how that are critical to our business operations through additional patent application filings.
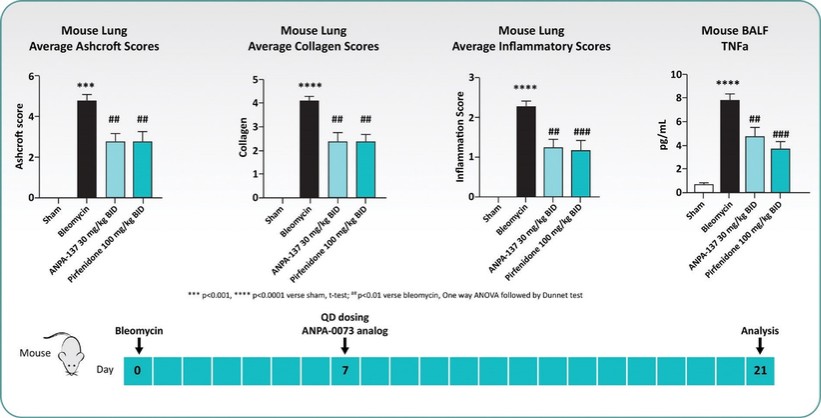
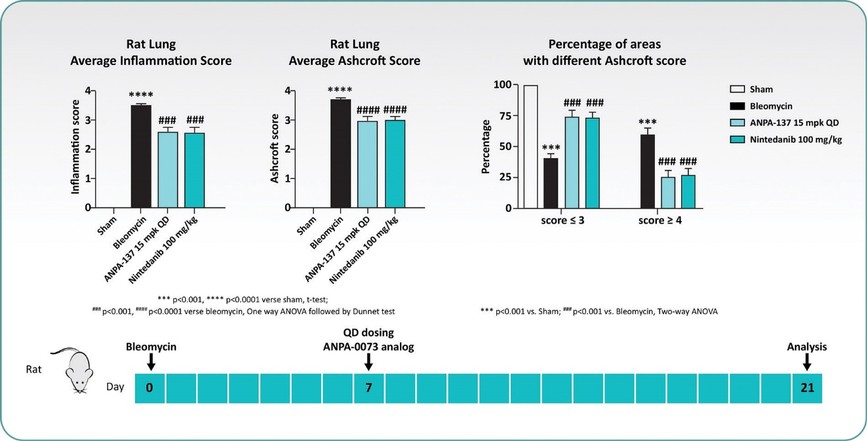
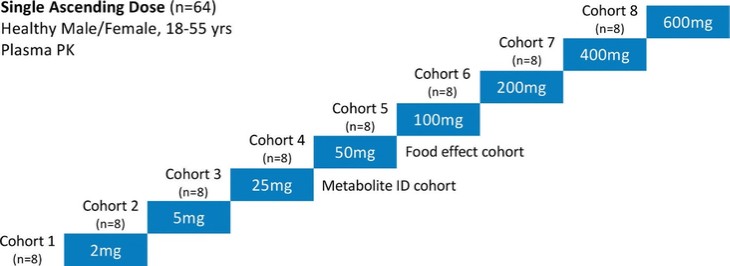
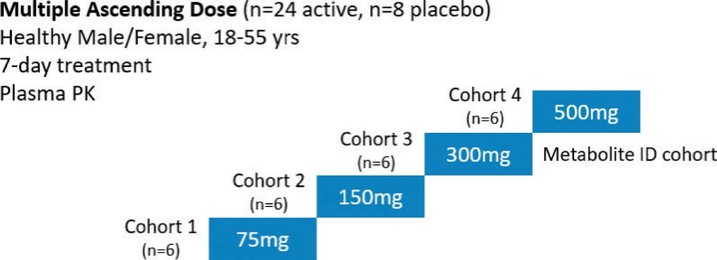
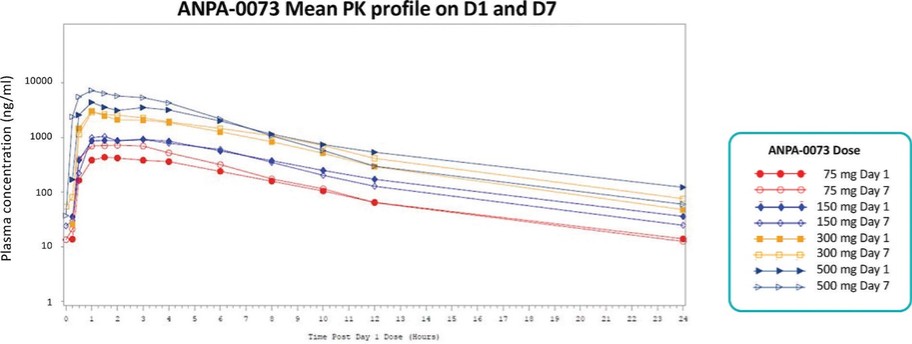
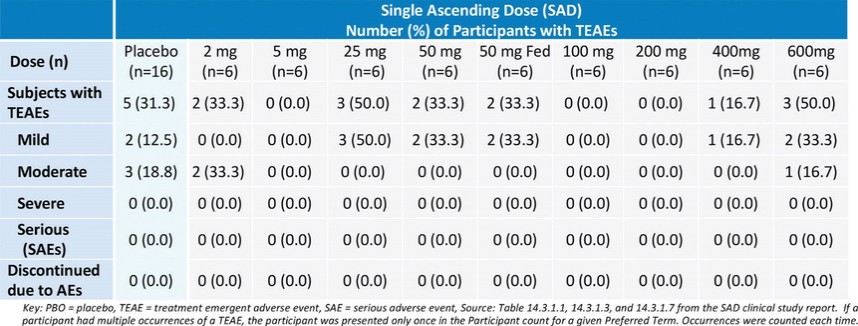
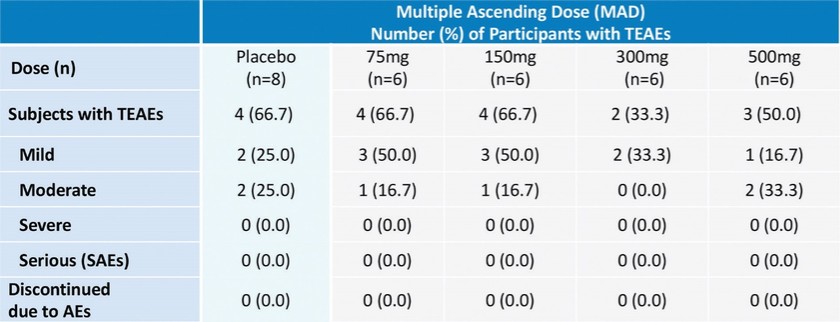
For our oral small molecule APJR program, as of December 31, 2023, our wholly-owned subsidiary Annapurna Bio, Inc. is the sole owner of two granted U.S. patents and three pending U.S. patent applications, one PCT application, one granted European patent and 24 pending foreign patent applications in Argentina, Australia, Brazil, Canada, the PRC, the EAPO, the EPO, Hong Kong, Israel, India, Japan, South Korea, Mexico, New Zealand, Singapore, Taiwan, and South Africa relating to compounds and compositions of matter for treating conditions associated with Apelin receptor activity, including ANPA-0073 and its analogs, solid forms and methods of treating conditions associated with Apelin receptor activity. Any patents issuing from these patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2039 and 2043, without accounting for potentially available patent term adjustments or extensions.
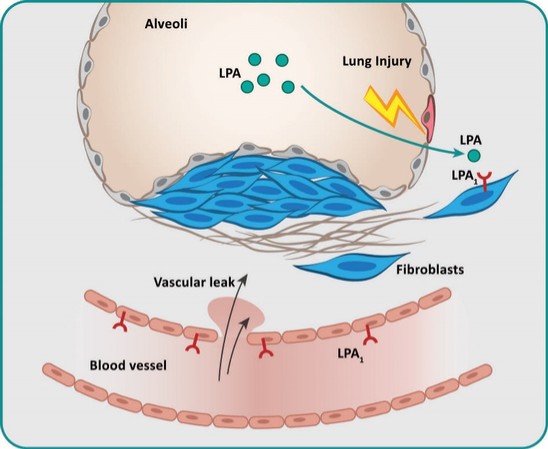
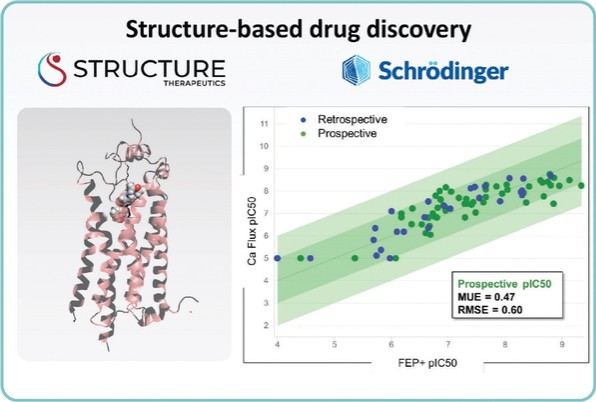
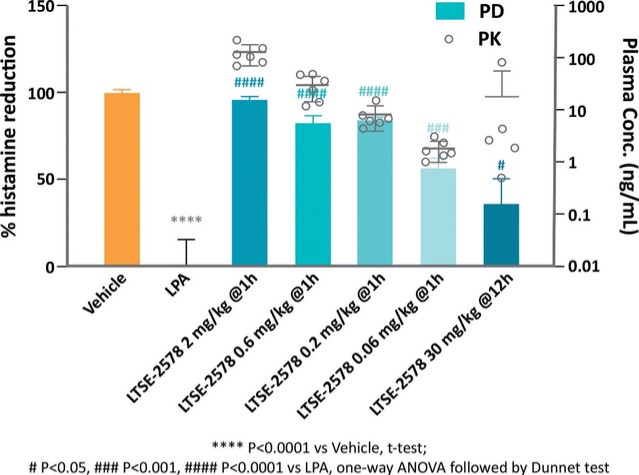
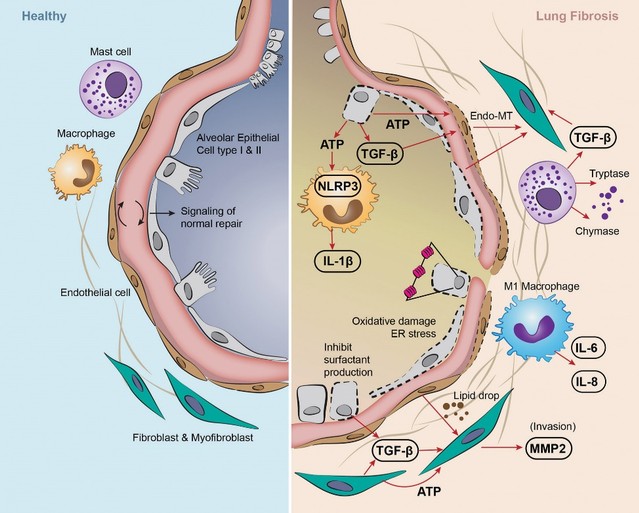
For our LPA1R program, as of December 31, 2023, our wholly-owned subsidiary Lhotse Bio, Inc. (“Lhotse”) is the sole owner of three pending U.S. patent applications, four PCT applications and seven pending foreign patent applications in Argentina, the PRC, the EPO, Japan, and Taiwan relating to compounds and compositions of matter for treating conditions associated with LPA receptor activity, including LTSE-2578 and its analogs, and methods of treating conditions associated with LPA receptor activity. Any patents issuing from these patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2041 and 2044, without accounting for potentially available patent term adjustments or extensions.
For our oral small molecule Amylin program, as of December 31, 2023, our wholly-owned subsidiary Aconcagua Bio, Inc. (“Aconcagua”) is the sole owner of two PCT applications relating to compounds and compositions of matter for treating conditions associated with Amylin receptor activity and methods of treating conditions associated with Amylin receptor activity. Any patents issuing from these patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire in 2044, without accounting for potentially available patent term adjustments or extensions.
In addition to patent protection, we also rely on trade secrets, know-how, trademarks, other proprietary information and continuing technological innovation to develop and maintain our competitive position. We seek to protect and maintain the confidentiality of proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Although we take steps to protect our proprietary information and trade secrets, including through contractual means with our employees and consultants, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. Thus, we may not be able to meaningfully protect our trade secrets. It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us.