management and certain key members of our R&D team and other employees who have access to trade secrets or confidential information about our business. Our standard employment contract, which we use to employ each of our employees, contains an assignment clause, under which we own all the rights to all inventions, technology, know-how and trade secrets derived during the course of such employee’s work.
These agreements may not provide sufficient protection of our trade secrets and/or confidential information. These agreements may also be breached, resulting in the misappropriation of our trade secrets and/or confidential information, and we may not have an adequate remedy for any such breach. In addition, our trade secrets and/or confidential information may become known or be independently developed by a third party, or misused by any partners to whom we disclose such information. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to or successfully copy aspects of our products or to obtain or use information that we regard as proprietary without our consent. As a result, we may be unable to sufficiently protect our trade secrets and proprietary information.
We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Despite any measures taken to protect our data and intellectual property, unauthorized parties may attempt to or successfully gain access to and use information that we regard as proprietary. Please refer to the section entitled “Risk Factors—Risks Related to our Intellectual Property Rights” for a description of risks related to our intellectual property.
We conduct our business under the brand name of “Apollomics.” As of the date of this prospectus, we had primarily registered 14 trademarks/classes in China, 2 trademarks/classes in the United States, and 24 trademarks/classes in Hong Kong.
We enter into collaboration agreements and other relationships with pharmaceutical companies and other industry participants to leverage our intellectual property and gain access to the intellectual property of others. Please refer to “—Licensing and Collaboration Arrangements” above for further details.
As of the date of this prospectus, we were not involved in any proceedings in respect of, and we had not received notice of any claims of infringement of, any intellectual property rights that were threatened or pending, in which we were a claimant or a respondent.
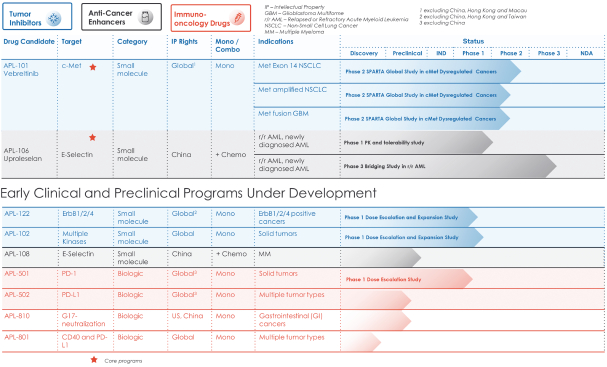
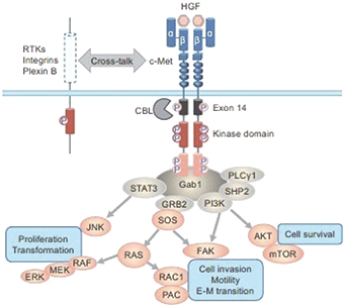
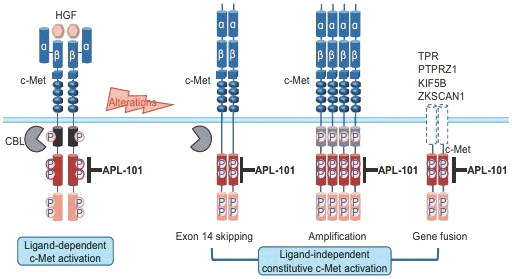
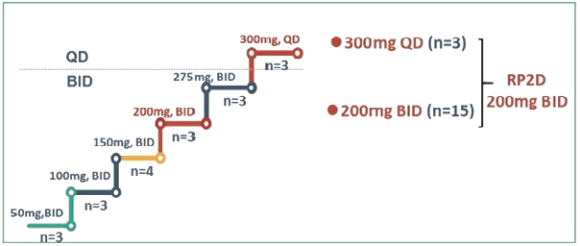
Our directors confirm that as of the date of this prospectus, there had been no instance in our R&D activities of drug candidates, including APL-101, that may give rise to a claim of infringement of intellectual property rights by any third party for injunctive relief or actual damages because the jurisdictions where we are conducting R&D of drug candidates exempt R&D activities from obtaining regulatory approvals for patent infringements. Such jurisdictions are Australia, Canada, China, Finland, France, Hungary, Italy, New Zealand, Russia, Singapore, Spain, Taiwan, the United Kingdom, Ukraine and the United States.
Government Regulations
Government authorities in the United States, at the federal, state and local level, in China, and in other countries and jurisdictions, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States, in China and in other foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
United States regulation of pharmaceutical product development and approval
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The FDC Act and other federal and state statutes and regulations, govern, among other things, the research, development,
176