Patent Portfolio
As of March 11, 2025, we own or exclusively in-license 14 patent families that specifically cover our product candidates obexelimab (our CD19 x FcγRIIb antibody), ZB002 (our anti-TNFα antibody), and ZB004 (our CTLA-4-Ig fusion protein). These families include 14 issued U.S. patents, nine pending U.S. applications, 123 issued foreign patents, four pending PCT applications and 41 pending foreign patent applications in Australia, Brazil, Canada, China, Eurasia, Europe, Hong Kong, Israel, Japan, Malaysia, Mexico, New Zealand, Saudi Arabia, Singapore, South Africa, South Korea, Taiwan and the United Arab Emirates. In addition, we own or exclusively in-license eight U.S. provisional patent applications, which belong to four different patent families, within the priority year. Any U.S. or foreign patents issued from national stage filings of our owned, or exclusively in-licensed PCT patent applications, any U.S. patents issued from our exclusively in-licensed non-provisional applications, and any U.S. patents or foreign patents issued from non-provisional applications we may file in connection with our provisional patent applications would be scheduled to expire on various dates from 2027 through 2045.
All expected expiration dates provided herein are based on a 20-year term, without taking into account any possible PTA or PTE and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
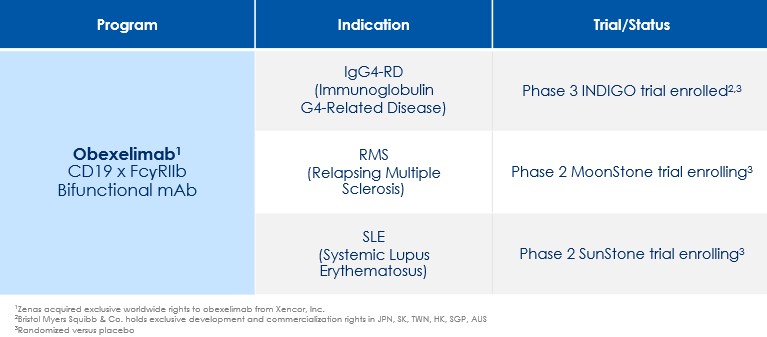
Obexelimab
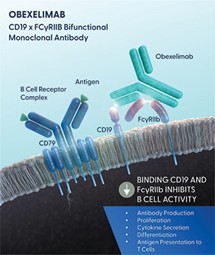
Obexelimab is a bifunctional, non-cytolytic, humanized monoclonal antibody that binds CD19 and FcγRIIb to inhibit B-lineage cell activity. As of March 11, 2025, we own or exclusively in-license from Xencor ten patent families that specifically cover the composition of matter, mechanism of action, manufacturing, biomarkers and the clinical uses of obexelimab for treating IgG4-RD, SLE, MS and wAIHA.
A first patent family specifically covers the composition of matter of obexelimab and includes six issued patents and two pending applications in the U.S., and 21 issued foreign patents in Australia, Hong Kong, India, Israel and various European countries including Belgium, Denmark, France, Germany, Ireland, Italy, Latvia, Lithuania, Luxembourg, Monaco, Netherlands, Spain, Sweden, Switzerland, and UK. The 20-year statutory term for the patents issued in this family expires in May 2028, excluding any extension of patent term that may be available.
A second patent family is directed to obexelimab’s mechanism of action and includes one issued patent and one pending application in the U.S., five pending applications in Canada, Europe, Hong Kong and Japan, and 20 issued patents in Japan and European countries including Austria, Belgium, Denmark, France, Finland, Germany, Hungary, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Spain, Switzerland, Sweden, Turkey and UK. The 20-year statutory term for any patents issued in this family expires in June 2037, excluding any extension of patent term that may be available.
A third patent family is directed to the use of biomarkers for treating autoimmune diseases (e.g., SLE) and includes one pending U.S. application, and 13 pending applications in Australia, Brazil, Canada, China, Eurasia, Europe, Hong Kong, Israel, Japan, South Korea, Mexico, South Africa and Taiwan. The 20-year statutory term for any patents issued in this family would expire in October 2041, excluding any extension of patent term that may be available.
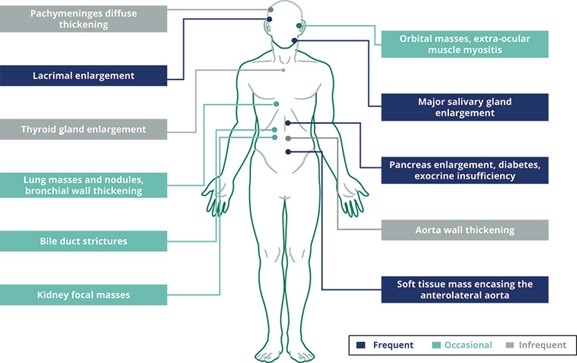
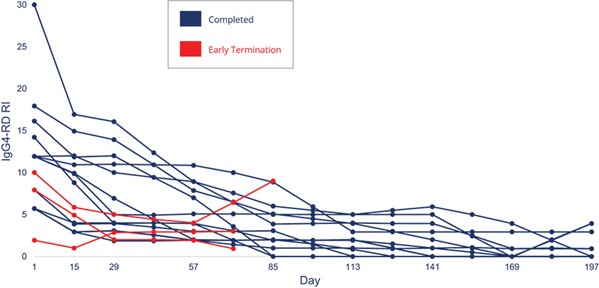
A fourth patent family is directed to the clinical use of obexelimab for treating IgG4-RD, currently including one pending U.S. Application, and 17 pending applications in Australia, Brazil, Canada, China, Eurasia, Europe, Isreal, Japan, Malaysia, Mexico, New Zealand, Saudi Arabia, Singapore, South Africa, South Korea, Taiwan and the United Arab Emirates. The 20-year statutory term for any patents issued in this family would expire in June 2043, excluding any extension of patent term that may be available.
A fifth patent family is directed to the clinical use of obexelimab for treating AIHA, including wAIHA, currently including a pending PCT application and a Taiwanese application. The 20-year statutory term for any patents issued in this family would expire in April 2044, excluding any extension of patent term that may be available.
A sixth patent family is directed to the clinical use and dosing regimen of obexelimab for treating forms of MS, including RMS, and currently including a pending PCT application and a pending U.S. application, and a pending Taiwanese application. The 20-year statutory term for any patents issued in this family would expire in October 2044, excluding any extension of patent term that may be available.