Exhibit 99.1

ADAGIO MEDICAL Investor Presentation | June 2024 1

DISCLAIMER This investor presentation (together with the oral statements made in connection herewith, this “Presentation”) is for informational purposes only to assist interested parties in making their own evaluation with respect to the proposed business combination and any related transaction, including with the PIPE financing described herein (collectively, the “Business Combination”), by and among ARYA Sciences Acquisition Corp IV (NASDAQ: ARYD) (“ARYA”), Adagio Medical, Inc. (the “Company”) and Aja Holdco, Inc., of which the Company will become a subsidiary following the consummation of the Business Combination (“ListCo”), and for no other purpose. The information contained herein is subject to change, and any such change could be material, and does not purport to be all-inclusive and none of ARYA, the Company, ListCo, Jefferies LLC (“Jefferies”) or Chardan Capital Markets, LLC (“Chardan”) or any of their respective affiliates (including, without limitation, control persons, directors, officers, employees, shareholders, representatives, legal counsel or advisors) makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation or any other written or oral communication communicated to the recipient in the course of the recipient’s evaluation of ARYA, the Company and ListCo. Please refer to the definitive merger agreement and other related transaction documents for the full terms of the Business Combination. This Presentation does not constitute (i) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Business Combination or (ii) an offer to sell, a solicitation of an offer to buy, or a recommendation to purchase any security of ARYA, the Company, ListCo or any of their respective affiliates. You should not construe the contents of this Presentation as legal, tax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any investment decision. The reader shall not rely upon any statement, representation or warranty made by any other person, firm or corporation (including, without limitation, Jefferies, Chardan and any of their affiliates or control persons, officers, directors and employees) in making its investment or decision to invest in ARYA, the Company or ListCo. To the fullest extent permitted by law, none of ARYA, the Company, ListCo, Jefferies and Chardan nor any of their respective affiliates nor any of its or their control persons, officers, directors, employees or representatives, shall be responsible or liable to the reader for any information set forth herein or any action taken or not taken by any reader, including any investment in ARYA, the Company or ListCo. The distribution of this Presentation may also be restricted by law and persons into whose possession this Presentation comes should inform themselves about and observe any such restrictions. The recipient acknowledges that it is (a) aware that the United States securities laws prohibit any person who has material, non-public information concerning a company from purchasing or selling securities of such company or from communicating such information to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities, and (b) familiar with the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder (collectively, the “Exchange Act”), and that the recipient will neither use, nor cause any third party to use, this Presentation or any information contained herein in contravention of the Exchange Act, including, without limitation, Rule 10b-5 thereunder. This Presentation and information contained herein constitutes confidential information and is provided to you on the condition that you agree that you will hold it in strict confidence and not use, discuss, reproduce, disclose, forward or distribute it in whole or in part without the prior written consent of ARYA, the Company and ListCo and is intended for the recipient hereof only. No securities commission or securities regulatory authority in the United States or any other jurisdiction has in any way passed upon the merits of the Business Combination or the accuracy or adequacy of this Presentation. Certain monetary amounts, percentages and other figures included in this Presentation have been subject to rounding adjustments. Certain amounts that appear in this Presentation may not sum due to rounding. Management's Estimates The Company has based its estimates of the total addressable market and growth forecasts on a number of internal and third-party estimates and resources, including, without limitation, third party reports and the experience of the management team across the industries. While the Company believes its assumptions and the data underlying its estimates are reasonable, these assumptions and estimates may not be correct and the conditions supporting such assumptions or estimates may change at any time, thereby reducing the predictive accuracy of these underlying factors. In addition, the novelty of the markets for the Company’s products may make its assumptions and estimates more uncertain. As a result, the Company's estimates of the total addressable market and growth forecasts for its products are subject to significant uncertainty and may prove to be incorrect. If third-party or internally generated data prove to be inaccurate or the Company makes errors in its assumptions based on that data, the total addressable market for the Company's products may be smaller than it has estimated, its future growth opportunities and sales growth may be impaired, any of which could have a material adverse effect on the Company's business, financial condition and results of operations. Forward-Looking Statements Certain statements in this Presentation may be considered “forward-looking statements” within the meaning of the “safe harbor” provisions of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally relate to future events or ARYA’s, the Company’s or ListCo’s future financial or operating performance. For example, any statements that refer to expectations, projections or other characterizations of future events or circumstances, including post-Business Combination fully diluted equity value, the anticipated enterprise value of ListCo, expected ownership in ListCo, projections of market opportunity and market share, the capability of the Company’s or ListCo’s business plans including its plans to expand, the sources and uses of cash from the Business Combination, any benefits of the Company’s partnerships, strategies or plans as they relate to the Business Combination, anticipated benefits of the Business Combination and expectations related to the terms and timing of the Business Combination, the Company’s expected pro forma cash, the Company’s or ListCo’s expected cash runway through 2025 or statements related to the Company’s or ListCo’s funding gap, funded business plan or use of proceeds, or other metrics or statements derived therefrom, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “future,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “propose,” “seek,” “should,” “strive,” “will,” or “would” or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which may be beyond the control of ARYA, the Company or ListCo and could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by ARYA and its management, the Company and its management and ListCo and its management, as the case may be, are inherently uncertain. Each of ARYA, the Company and ListCo caution you that these statements are based on a combination of facts and factors currently known and projections of the future, which are inherently uncertain. There will be risks and uncertainties described in the proxy statement/prospectus included in the registration statement on Form S-4 (the “Registration Statement”) relating to the Business Combination, which is expected to be filed by ListCo with the U.S. Securities and Exchange Commission (the “SEC”), and described in other documents filed by ARYA or ListCo from time to time with the SEC. These filings may identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Neither ARYA nor the Company can assure you that the forward-looking statements in this presentation will prove to be accurate. In addition, new risks and uncertainties may emerge from time to time, and it may not be possible to identify and accurately predict the potential impacts of any such risks and uncertainties that may arise in the future. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: (1) the occurrence of any event, change or other circumstances that could give rise to the termination of negotiations and any subsequent definitive agreements with respect to the Business Combination; (2) the outcome of any potential litigation, government or regulatory proceedings that may be instituted against ARYA, the Company, ListCo or others; (3) the inability to complete the Business Combination due to the failure to obtain approval of the shareholders of ARYA, to obtain financing to complete the Business Combination or to satisfy other conditions to closing; (4) the amount of redemption requests made by ARYA’s public shareholders; (5) changes to the proposed structure of the Business Combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining regulatory approval of the Business 2 PRIVATE AND CONFIDENTIAL

DISCLAIMER (CONT.) Combination; (6) delays in obtaining, adverse conditions in, or the inability to obtain regulatory approvals, or delays in completing regulatory reviews, required to complete the Business Combination; (7) the ability to meet stock exchange listing standards prior to or following the consummation of the Business Combination; (8) the risk that the Business Combination disrupts current plans and operations of the Company or ListCo as a result of the announcement and consummation of the Business Combination; (9) Adagio’s ability to remain compliant with the covenants of its existing debt, including any convertible or bridge financing notes; (10) ListCo’s ability to remain compliant with the covenants of the senior secured convertible notes that will be issued in connection with the closing of the Business Combination; (11) the ability to recognize the anticipated benefits of the Business Combination, which may be affected by, among other things, competition, the ability of ListCo to grow and manage growth profitably, maintain relationships with customers and suppliers and retain its management and key employees; (12) costs related to the Business Combination; (13) risks associated with changes in applicable laws or regulations and the Company’s or ListCo’s international operations and operations in a regulated industry; (14) the possibility that the Company or ListCo may be adversely affected by other economic, business, and/or competitive factors; (15) the Company’s or ListCo’s use of proceeds, post-Business Combination fully diluted equity value or fully diluted enterprise value, expected pro forma cash, expected cash runway or funding gap, estimates of expenses and profitability; and (16) the risks described in the “Risk Factor Summary” included in this Presentation, and other risks and uncertainties set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in ARYA’s Annual Report on Form 10-K for the year ended December 31, 2023, its Quarterly Reports on Form 10-Q, and other documents filed, or to be filed, with the SEC. There may be additional risks that ARYA, the Company or ListCo do not presently know or that ARYA, the Company or ListCo currently believe are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. Actual events and circumstances are difficult or impossible to predict and may materially differ from assumptions. Many actual events and circumstances are beyond the control of ARYA, the Company and ListCo. Nothing in this Presentation should be regarded as a representation or warranty by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward- looking statements will be achieved in any specified time frame or at all. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made in this Presentation. Subsequent events and developments may cause those views to change. Neither ARYA, the Company nor ListCo undertakes any duty to update these forward-looking statements. Use of Projections This Presentation contains forecasts with respect to the Company’s minimum total pro forma cash after expenses at announcement of the Business Combination and ListCo’s expected cash runway through 2025, based on current plans and estimates of the Company. Neither ARYA’s, the Company’s nor ListCo’s independent auditors have audited, reviewed, compiled or performed any procedures with respect to such projected or forecasted information included in this Presentation and, accordingly, they did not express an opinion or provide any other form of assurance with respect thereto for the purpose of this Presentation. The inclusion of the forecasted information should not be relied upon as being necessarily indicative of future results and should not be regarded as an indication that ARYA, the Company, ListCo or any other person considered, or now considers, the projections to be a reliable prediction of future events, and does not constitute an admission or representation by any person that the expectations, beliefs, opinions, and assumptions that underlie such forecasts remain the same following the date of this Presentation, and readers are cautioned not to place undue reliance on any prospective information. The assumptions and estimates underlying the prospective financial information are inherently uncertain and are subject to a wide variety of significant business, economic and competitive risks and uncertainties that could cause actual cash or cash needs to differ materially from those contained in the prospective financial information. Accordingly, there can be no assurance that the prospective cash or cash needs are indicative of the future performance of the Company or ListCo or that actual cash or cash needs will not differ materially from those presented in the prospective financial information. ARYA, the Company and ListCo do not assume any obligation to update the projected information or any other information in this Presentation, and do not expect to continue to disclose detailed prospective financial information going forward. Actual cash or cash needs may differ as a result of the completion of the Company’s or ListCo’s applicable financial reporting period closing procedures, review adjustments and other developments that may arise between now and the time such financial information for the presented or projected periods is finalized. As a result, these estimates are preliminary, may change and constitute forward-looking information, and are subject to significant risks and uncertainties. See “Forward-Looking Statements” above. Any such forecasted information presented herein was not prepared with a view towards compliance with the published guidelines of the SEC, Regulation S-X promulgated under the Securities Act of 1933, as amended (the “Securities Act”) or any guidelines established by the American Institute of Certified Public Accountants for the presentation and preparation of “prospective financial information.” Accordingly, the information and data presented in this Presentation may not be included, may be adjusted, or may be presented differently, in any proxy statement or registration statement that may be filed in connection with a Business Combination. Industry and Market Data; Trademarks Certain information contained in the Presentation relates to or is based on studies, publications, statistics and surveys from third-party sources, and on ARYA’s and the Company’s own internal estimates and research. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. While ARYA and the Company believe that the third-party sources and ARYA’s and the Company’s internal research are reliable, such sources and research have not been verified by any independent source. Any data on past performance or modeling contained herein is not an indication as to future performance. This information involves many assumptions and limitations, and you are cautioned not to give undue weight to such industry and market data. The information contained in the third party citations referenced in this presentation is not incorporated by reference into this presentation. This Presentation may include trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. The inclusion of particular trademarks, service marks, trade names and copyrights of other companies is not intended to, and does not, imply a relationship with ARYA, the Company or ListCo or an endorsement or sponsorship by or of ARYA, the Company or ListCo. ARYA, the Company and ListCo own or have rights to various trademarks, service marks, trade names and copyrights in connection with the operation of their respective businesses which are also included in this Presentation. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the , ℠, ©, or ® symbols, but the Company, ARYA and ListCo will assert, to the fullest extent under applicable law, the right of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights. Additional Information In connection with the Business Combination, ListCo filed with the SEC a registration statement on Form S-4 containing a preliminary proxy statement of ARYA and a preliminary prospectus of ListCo, and after the Registration Statement is declared effective, ARYA expects to mail a definitive proxy statement/prospectus related to the Business Combination to its shareholders. The proxy statement/prospectus contains important information about the Business Combination and the other matters to be voted upon at ARYA’s shareholder meeting to be held to approve the Business Combination. ARYA and ListCo may also file other documents with the SEC regarding the Business Combination. This Presentation does not contain all the information that should be considered concerning the Business Combination and is not intended to form the basis of any investment decision or any other decision in respect of the Business Combination. Before making any voting or other investment decisions, shareholders of ARYA and other interested persons are advised to read the preliminary proxy statement/prospectus and any amendments thereto, the definitive proxy statement/prospectus and other documents filed in connection with the Business Combination, as these materials contain important information about ARYA, the Company and the Business Combination. After the Registration Statement becomes effective, the definitive proxy statement/prospectus and other relevant materials for the Business Combination will be mailed to shareholders of ARYA as of a record date to be established for voting on the Business Combination. Shareholders will also be able to obtain copies of the definitive proxy statement/prospectus and other documents filed with the SEC, without charge, once available, at the SEC’s website at www.sec.gov, or by directing a request to: ARYA Sciences Acquisition Corp IV, 51 Astor Place, 10th Floor, New York, New York, Attention: Secretary, ARYA4@perceptivelife.com. INVESTMENT IN ANY SECURITIES DESCRIBED HEREIN HAS NOT BEEN APPROVED OR DISAPPROVED BY THE SEC OR ANY OTHER REGULATORY AUTHORITY NOR HAS ANY AUTHORITY PASSED UPON OR ENDORSED THE MERITS OF THE OFFERING OR THE ACCURACY OR ADEQUACY OF THE INFORMATION CONTAINED HEREIN. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE. 3 PRIVATE AND CONFIDENTIAL

DISCLAIMER (CONT.) Participants in the Solicitation ARYA, the Company, ListCo and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from ARYA’s shareholders with respect to the Business Combination. A list of the names of ARYA’s directors and executive officers and a description of their interests in ARYA is contained in ARYA’s Annual Report on Form 10-K, which was filed with the SEC and is available free of charge at the SEC’s web site at www.sec.gov, or by directing a request to ARYA Sciences Acquisition Corp IV, 51 Astor Place, 10th Floor, New York, New York, Attention: Secretary, ARYA4@perceptivelife.com. Additional information regarding the interests of such participants is contained in the proxy statement/prospectus for the Business Combination. Investors, security holders and other interested persons of ARYA, the Company and ListCo are urged to carefully read in their entirety the proxy statement/prospectus and other relevant documents that have been filed or will be filed with the SEC because they contain important information about the Business Combination. Also see above under the heading “Additional Information.” The Company and ListCo, and its directors and executive officers may also be deemed to be participants in the solicitation of proxies from the shareholders of ARYA in connection with the proposed Business Combination. A list of the names of such directors and executive officers and information regarding their interests in the proposed Business Combination is included in the proxy statement/prospectus for the proposed Business Combination. No Offer and Non-Solicitation This Presentation does not constitute (i) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Business Combination or (ii) an offer to sell, a solicitation of an offer to buy, or a recommendation to purchase any security of ARYA, the Company, ListCo or any of their respective affiliates. No such offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act, or an exemption therefrom. The offering and resale of the securities issuable in connection with the PIPE financing described herein has not been and will not be registered under the Securities Act or any applicable state securities laws. If the proposed Business Combination is entered into, the PIPE financing will be offered and sold only to “qualified institutional buyers” (as defined in Rule 144A under the Securities Act) and institutional “accredited investors” (as defined in Rule 501(a)(1), (2), (3) or (7) promulgated under the Securities Act) upon the consummation of the proposed Business Combination. Notice to investors in the European Economic Area / Prohibition of sales to EEA retail investors In member states of the European Economic Area (the “EEA”), this Presentation and any offer if made subsequently is directed exclusively at persons who are “qualified investors” within the meaning of Article 2(e) of Regulation (EU) 2017/1129 (the “Prospectus Regulation”). The securities are not intended to be offered, sold or otherwise made available to and should not be offered, sold or otherwise made available to any retail investor in the EEA. For these purposes, a retail investor means a person who is one (or more) of: (i) a retail client as defined in point (11) of Article 4(1) of Directive 2014/65/EU (as amended, “MiFID II”); (ii) a customer within the meaning of Directive 2002/92/EC (as amended, the “Insurance Mediation Directive”), where that customer would not qualify as a professional client as defined in point (10) of Article 4(1) of MiFID II; or (iii) not a qualified investor as defined in the Prospectus Regulation. Consequently, no key information document required by Regulation (EU) No 1286/2014 (as amended the “PRIIPs Regulation”) for offering or selling the securities or otherwise making them available to retail investors in the EEA has been prepared and therefore offering or selling the securities or otherwise making them available to any retail investor in the EEA may be unlawful under the PRIIPs Regulation. Notice to investors in the UK / Prohibition of sales to UK retail investors In the United Kingdom (“UK”), any offer of the securities will be made pursuant to an exemption under Regulation (EU) 2017/1129 as it forms part of domestic law by virtue of the European Union (Withdrawal) Act 2018 (the “EUWA”) (the “UK Prospectus Regulation”) from a requirement to publish a prospectus for offers of securities. This Presentation is for distribution in the UK only to (i) investment professionals falling within article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”); or (ii) high net worth entities and other persons to whom it may lawfully be communicated, falling within article 49(2)(a) to (d) of the Order; and (iii) “qualified investors” within the meaning of article 2(e) of the UK Prospectus Regulation. The securities are not intended to be offered, sold or otherwise made available to and should not be offered, sold or otherwise made available to any retail investor in the UK. For these purposes, a “retail investor” means a person who is one (or more) of: (i) a retail client, as defined in Directive (EU) 2014/65/EU on markets in financial instruments (as amended) and implemented in the UK as it forms part of the domestic law of the United Kingdom by virtue of the EUWA (“UK MIFID II”); (ii) a customer within the meaning of Directive (EU) 2016/97 (as amended) as it forms part of the domestic law of the UK by virtue of the EUWA, where that customer would not qualify as a professional client as defined in UK MIFID II; or (iii) not a “qualified investor” as defined in Article 2(e) of the UK Prospectus Regulation. Consequently, no key information document required by Regulation (EU) No 1286/2014 as it forms part of the domestic law of the UK by virtue of the EUWA (the “UK PRIIPs Regulation”) for offering or selling the securities or otherwise making them available to retail investors in the UK has been prepared and, therefore, offering or selling the securities or otherwise making them available to any retail investor in the UK may be unlawful under the UK PRIIPs Regulation. 4 PRIVATE AND CONFIDENTIAL

TODAY’S PRESENTERS Olav Bergheim CEO & President 30+ years of experience in life sciences Adagio at a Glance… ~90 Employees Founded in 2011 Headquartered in Laguna Hills, California Founder of Innovative cardiac ablation medical technology company John Dahldorf Chief Financial Officer 20+ years of corporate finance experience Focused on large, underserved market – cardiac arrythmia addressing atrial fibrillation and ventricular tachycardia Unique portfolio that works – supported by compelling clinical data Poised to disrupt the market with unique technologies with commercial approvals in progress and pivotal data readouts Note: Olav Bergheim serves as the CEO of the Company pursuant to the terms of a Facilities and Shared Services Agreement between the Company and Fjord Ventures, LLC. Based on such agreement, Mr. Bergheim is compensated for serving in such position by Fjord Ventures, LLC. Two funds managed by Mr. Bergheim, one of which is affiliated with Fjord Ventures, LLC, have invested an aggregate of approximately $10M among the approximately $100M investment in aggregate that the Company has received so far. 5 PRIVATE AND CONFIDENTIAL
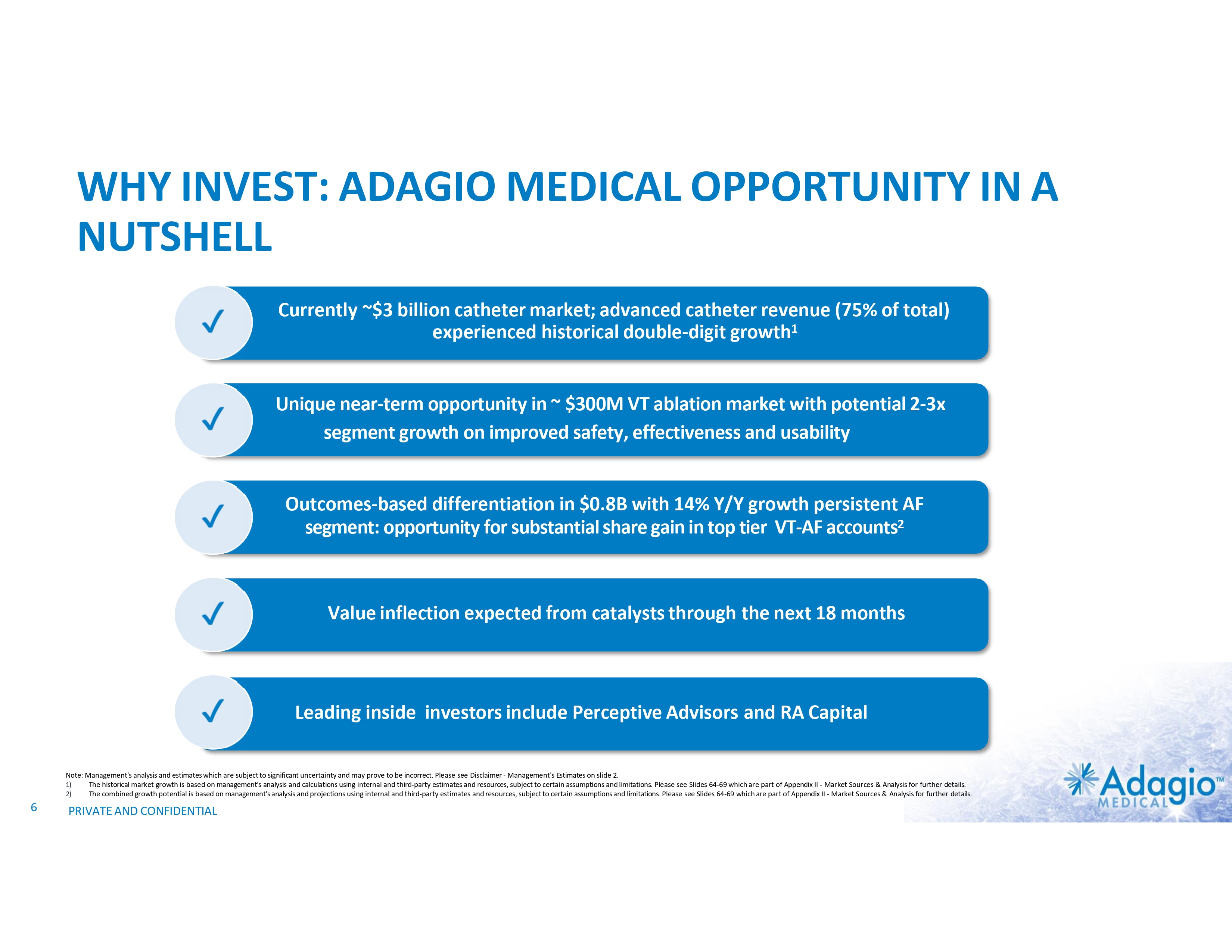
WHY INVEST: ADAGIO MEDICAL OPPORTUNITY IN A NUTSHELL Currently ~$3 billion catheter market; advanced catheter revenue (75% of total) experienced historical double-digit growth1 Unique near-term opportunity in ~ $300M VT ablation market with potential 2-3x segment growth on improved safety, effectiveness and usability Outcomes-based differentiation in $0.8B with 14% Y/Y growth persistent AF segment: opportunity for substantial share gain in top tier VT-AF accounts2 Value inflection expected from catalysts through the next 18 months Leading inside investors include Perceptive Advisors and RA Capital Note: Management's analysis and estimates which are subject to significant uncertainty and may prove to be incorrect. Please see Disclaimer - Management's Estimates on slide 2. 1) The historical market growth is based on management's analysis and calculations using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 64-69 which are part of Appendix II - Market Sources & Analysis for further details. 2) The combined growth potential is based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 64-69 which are part of Appendix II - Market Sources & Analysis for further details. 6 PRIVATE AND CONFIDENTIAL
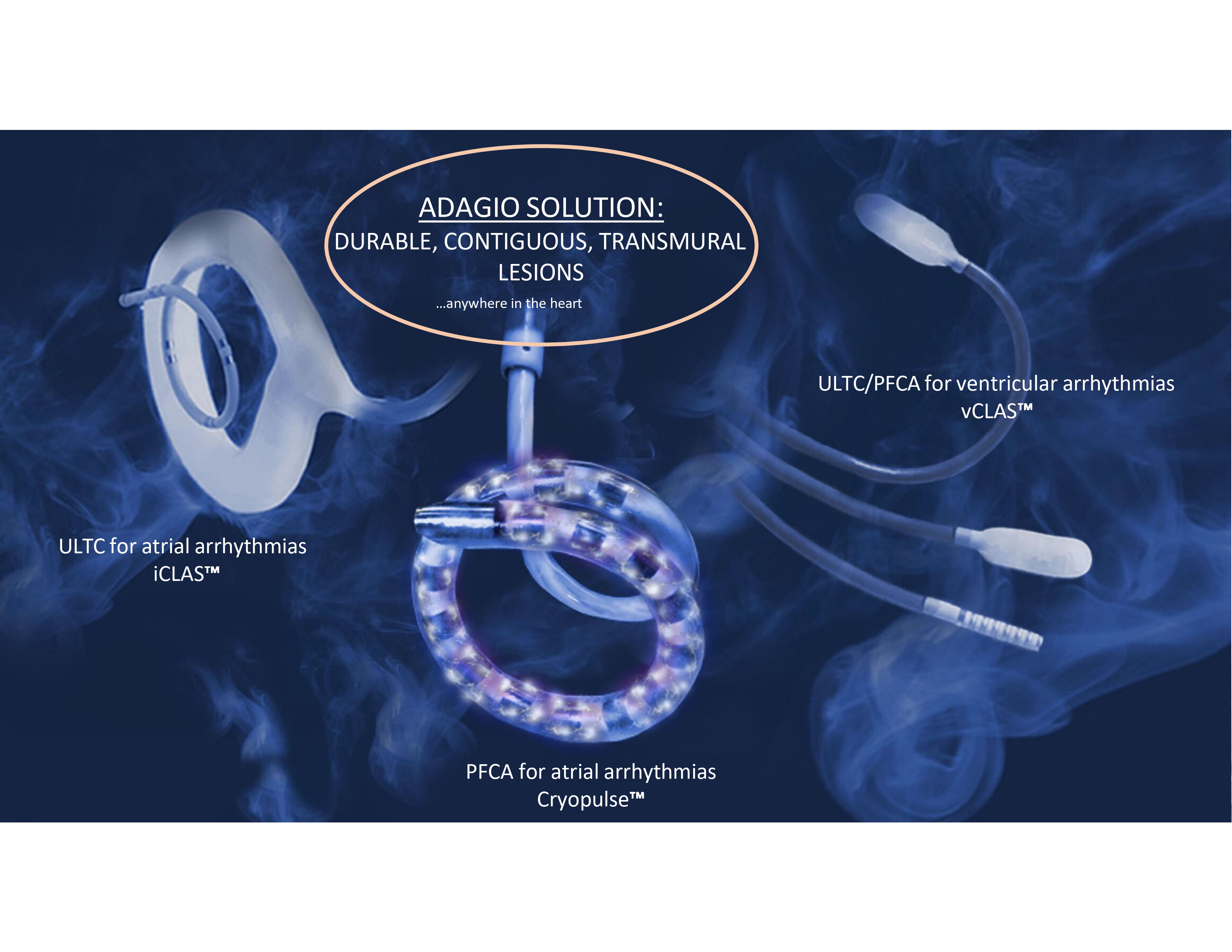
ADAGIO SOLUTION: DURABLE, CONTIGUOUS, TRANSMURAL LESIONS …anywhere in the heart ULTC/PFCA for ventricular arrhythmias vCLAS ULTC for atrial arrhythmias iCLAS PRIVATE AND CONFIDENTIAL PFCA for atrial arrhythmias Cryopulse

ULTC TEMPERATURES AND LESIONS https://vimeo.com/936025754/f221e1388a?share=copy 8 PRIVATE AND CONFIDENTIAL
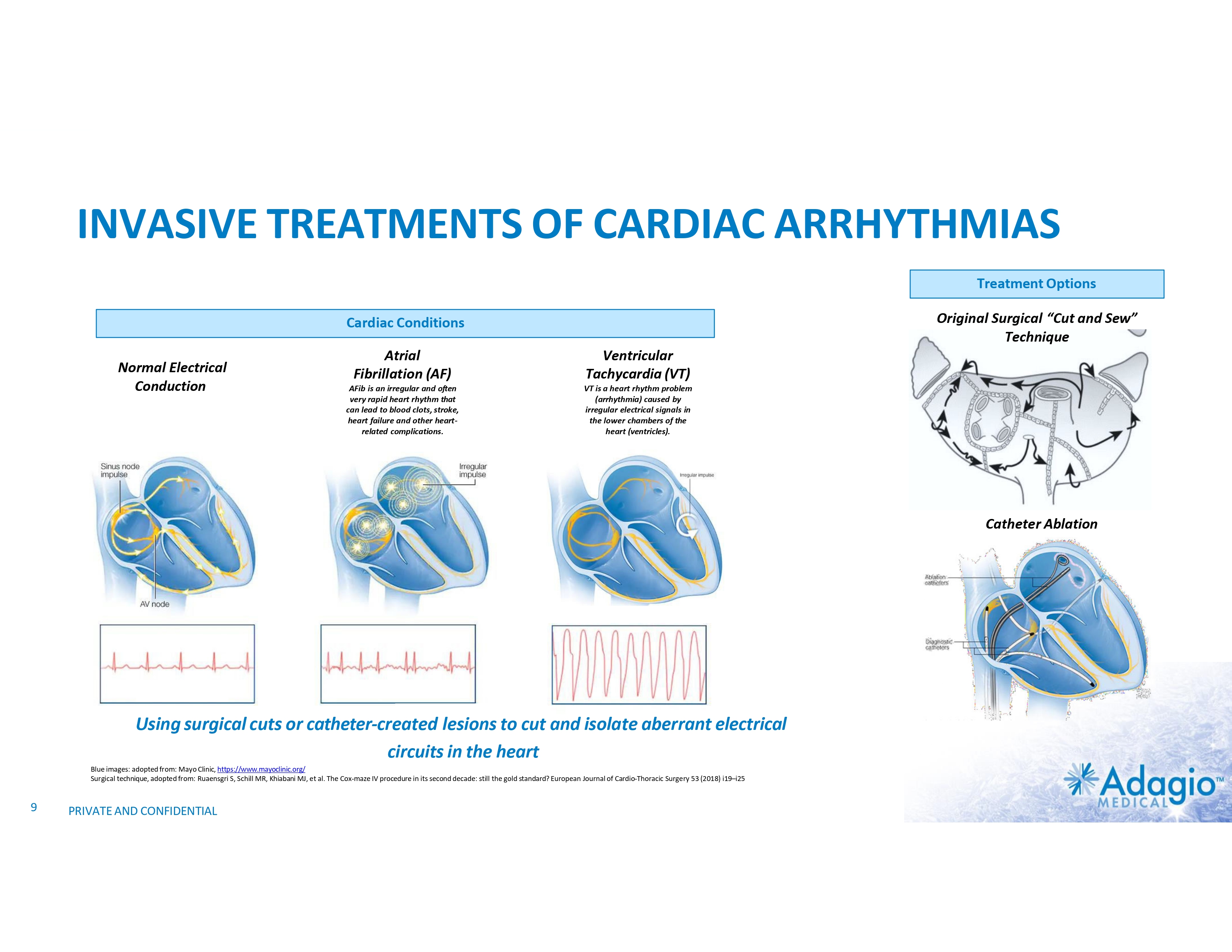
INVASIVE TREATMENTS OF CARDIAC ARRHYTHMIAS Treatment Options Original Surgical “Cut and Sew” Technique Cardiac Conditions Normal Electrical Conduction Atrial Fibrillation (AF) Ventricular Tachycardia (VT) AFib is an irregular and often very rapid heart rhythm that can lead to blood clots, stroke, heart failure and other heart- related complications. VT is a heart rhythm problem (arrhythmia) caused by irregular electrical signals in the lower chambers of the heart (ventricles). Catheter Ablation Using surgical cuts or catheter-created lesions to cut and isolate aberrant electrical circuits in the heart Blue images: adopted from: Mayo Clinic, https://www.mayoclinic.org/ Surgical technique, adopted from: Ruaensgri S, Schill MR, Khiabani MJ, et al. The Cox-maze IV procedure in its second decade: still the gold standard? European Journal of Cardio-Thoracic Surgery 53 (2018) i19–i25 9 PRIVATE AND CONFIDENTIAL

VENTRICULAR TACHYCARDIA ABLATIONS MARKET: OPPORTUNITY, DRIVERS AND INHIBITORS
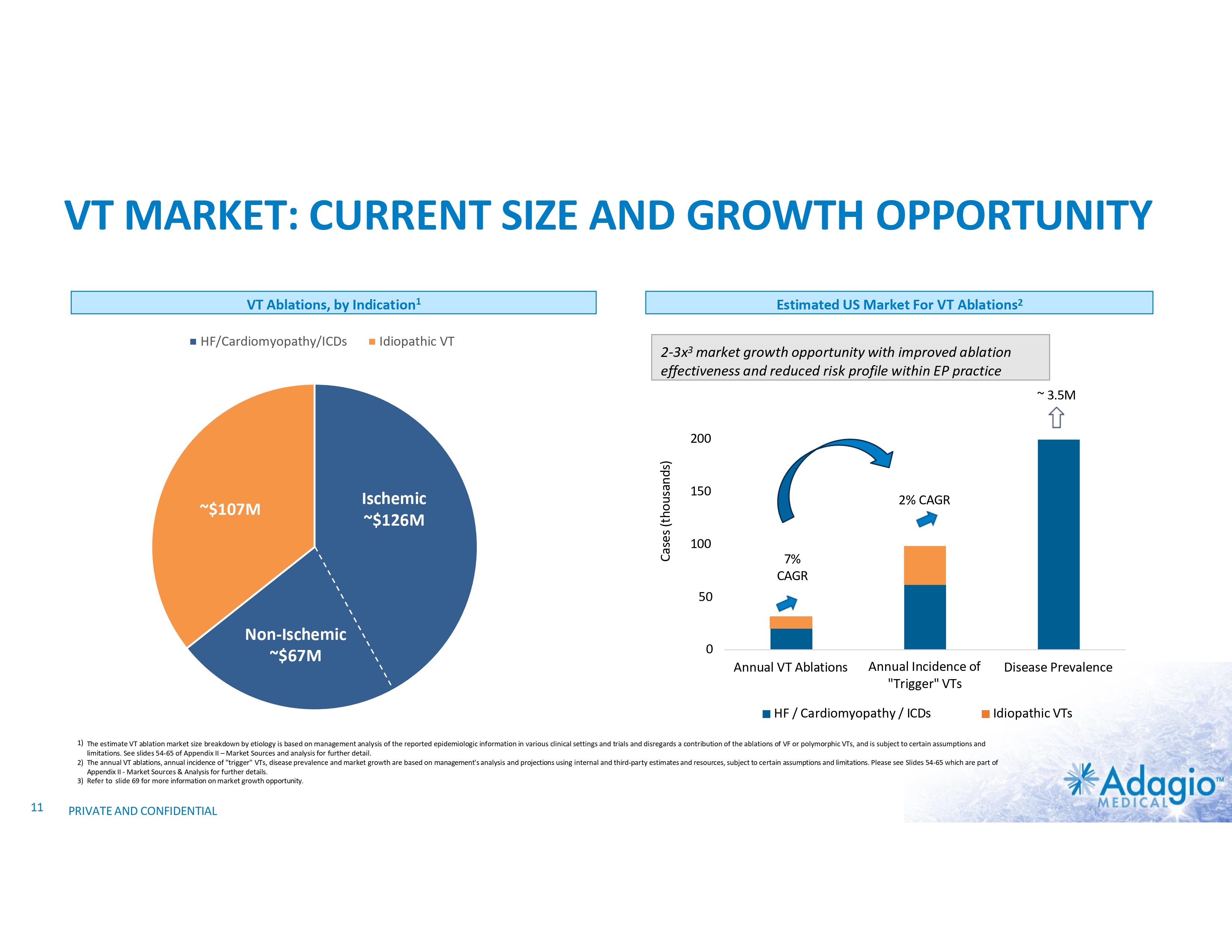
VT MARKET: CURRENT SIZE AND GROWTH OPPORTUNITY VT Ablations, by Indication1 HF/Cardiomyopathy/ICDs Idiopathic VT Estimated US Market For VT Ablations2 2-3x3 market growth opportunity with improved ablation effectiveness and reduced risk profile within EP practice ~ 3.5M ~$107M Ischemic ~$126M Cases (thousands) 200 150 2% CAGR 100 7% CAGR 50 Non-Ischemic ~$67M 0 Annual VT Ablations Annual Incidence of "Trigger" VTs HF / Cardiomyopathy / ICDs Disease Prevalence Idiopathic VTs 1) The estimate VT ablation market size breakdown by etiology is based on management analysis of the reported epidemiologic information in various clinical settings and trials and disregards a contribution of the ablations of VF or polymorphic VTs, and is subject to certain assumptions and limitations. See slides 54-65 of Appendix II – Market Sources and analysis for further detail. 2) The annual VT ablations, annual incidence of "trigger" VTs, disease prevalence and market growth are based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 54-65 which are part of Appendix II - Market Sources & Analysis for further details. 3) Refer to slide 69 for more information on market growth opportunity. 11 PRIVATE AND CONFIDENTIAL
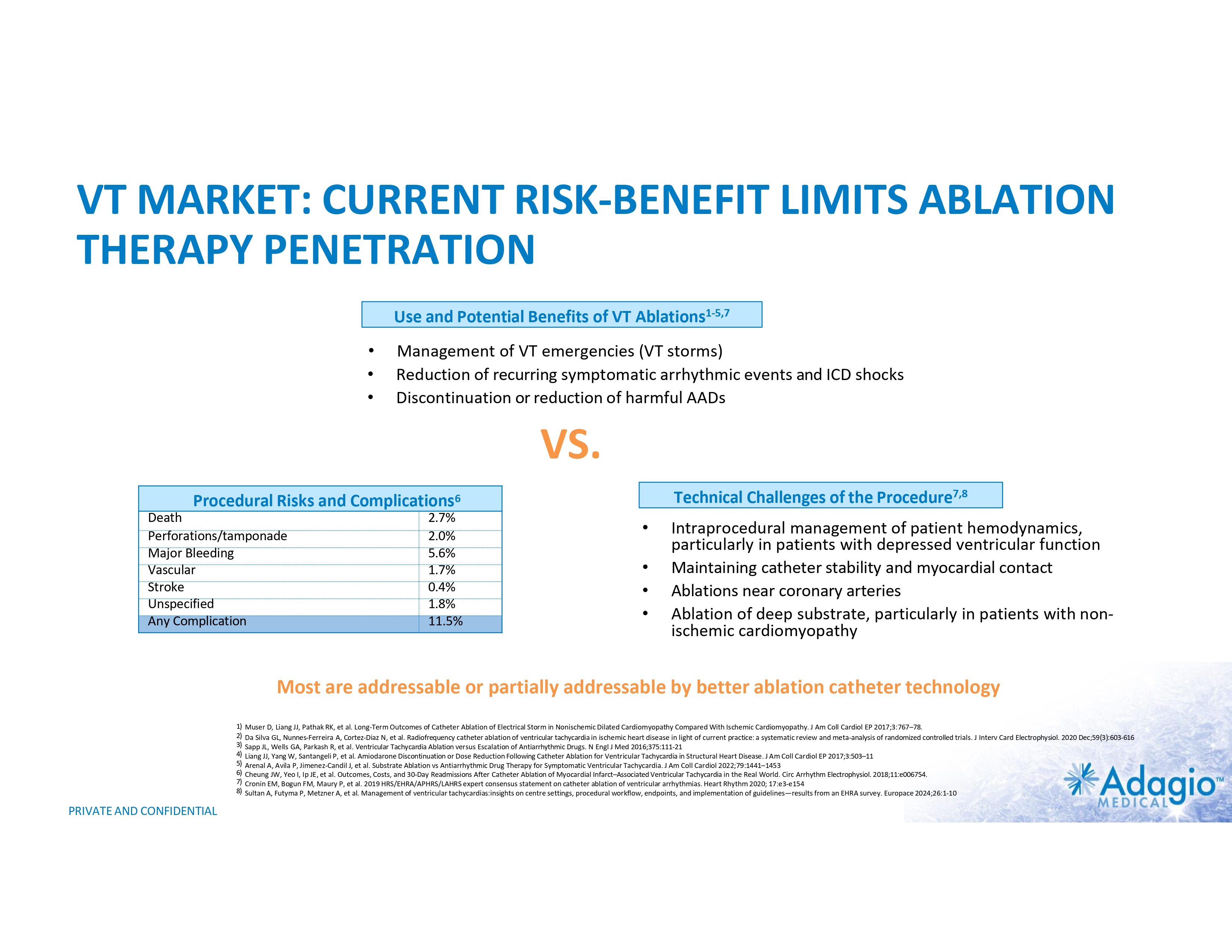
VT MARKET: CURRENT RISK-BENEFIT LIMITS ABLATION THERAPY PENETRATION Use and Potential Benefits of VT Ablations1-5,7 • • • Management of VT emergencies (VT storms) Reduction of recurring symptomatic arrhythmic events and ICD shocks Discontinuation or reduction of harmful AADs VS. Technical Challenges of the Procedure7,8 Procedural Risks and Complications6 Death Perforations/tamponade Major Bleeding Vascular Stroke Unspecified Any Complication 2.7% 2.0% 5.6% 1.7% 0.4% 1.8% 11.5% • • • • Intraprocedural management of patient hemodynamics, particularly in patients with depressed ventricular function Maintaining catheter stability and myocardial contact Ablations near coronary arteries Ablation of deep substrate, particularly in patients with non- ischemic cardiomyopathy Most are addressable or partially addressable by better ablation catheter technology 1) 2) 3) 4) 5) 6) 7) 8) PRIVATE AND CONFIDENTIAL Muser D, Liang JJ, Pathak RK, et al. Long-Term Outcomes of Catheter Ablation of Electrical Storm in Nonischemic Dilated Cardiomyopathy Compared With Ischemic Cardiomyopathy. J Am Coll Cardiol EP 2017;3:767–78. Da Silva GL, Nunnes-Ferreira A, Cortez-Diaz N, et al. Radiofrequency catheter ablation of ventricular tachycardia in ischemic heart disease in light of current practice: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2020 Dec;59(3):603-616 Sapp JL, Wells GA, Parkash R, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 2016;375:111-21 Liang JJ, Yang W, Santangeli P, et al. Amiodarone Discontinuation or Dose Reduction Following Catheter Ablation for Ventricular Tachycardia in Structural Heart Disease. J Am Coll Cardiol EP 2017;3:503–11 Arenal A, Avila P, Jimenez-Candil J, et al. Substrate Ablation vs Antiarrhythmic Drug Therapy for Symptomatic Ventricular Tachycardia. J Am Coll Cardiol 2022;79:1441–1453 Cheung JW, Yeo I, Ip JE, et al. Outcomes, Costs, and 30-Day Readmissions After Catheter Ablation of Myocardial Infarct–Associated Ventricular Tachycardia in the Real World. Circ Arrhythm Electrophysiol. 2018;11:e006754. Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020; 17:e3-e154 Sultan A, Futyma P, Metzner A, et al. Management of ventricular tachycardias:insights on centre settings, procedural workflow, endpoints, and implementation of guidelines—results from an EHRA survey. Europace 2024;26:1-10
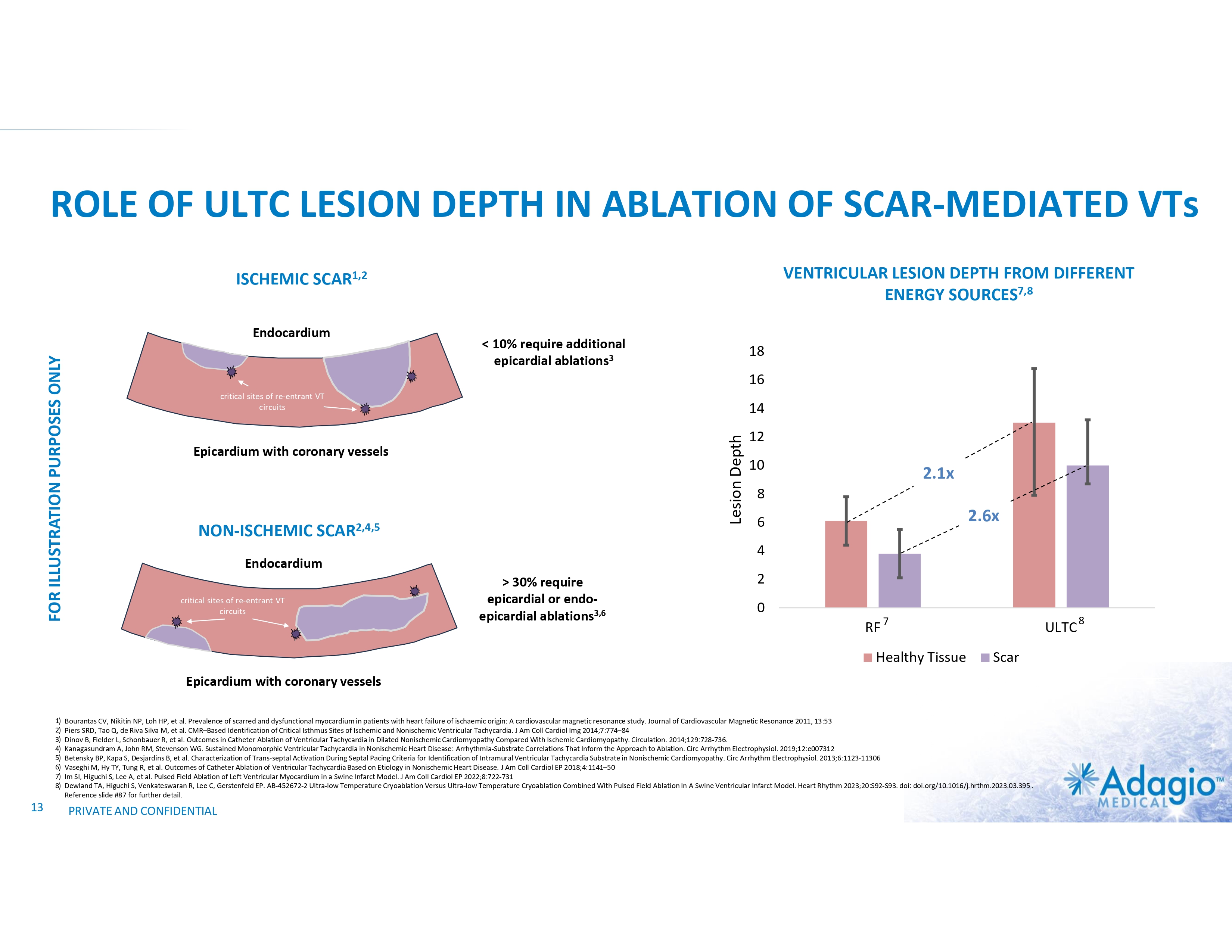
ROLE OF ULTC LESION DEPTH IN ABLATION OF SCAR-MEDIATED VTs VENTRICULAR LESION DEPTH FROM DIFFERENT ENERGY SOURCES7,8 ISCHEMIC SCAR1,2 < 10% require additional epicardial ablations3 18 16 critical sites of re-entrant VT circuits 14 Lesion Depth FOR ILLUSTRATION PURPOSES ONLY Endocardium Epicardium with coronary vessels NON-ISCHEMIC SCAR2,4,5 10 2.1x 8 2.6x 6 4 Endocardium critical sites of re-entrant VT circuits 12 > 30% require epicardial or endo- epicardial ablations3,6 2 0 RF 7 Healthy Tissue ULTC Scar Epicardium with coronary vessels 1) 2) 3) 4) 5) 6) 7) 8) 13 Bourantas CV, Nikitin NP, Loh HP, et al. Prevalence of scarred and dysfunctional myocardium in patients with heart failure of ischaemic origin: A cardiovascular magnetic resonance study. Journal of Cardiovascular Magnetic Resonance 2011, 13:53 Piers SRD, Tao Q, de Riva Silva M, et al. CMR–Based Identification of Critical Isthmus Sites of Ischemic and Nonischemic Ventricular Tachycardia. J Am Coll Cardiol Img 2014;7:774–84 Dinov B, Fielder L, Schonbauer R, et al. Outcomes in Catheter Ablation of Ventricular Tachycardia in Dilated Nonischemic Cardiomyopathy Compared With Ischemic Cardiomyopathy. Circulation. 2014;129:728-736. Kanagasundram A, John RM, Stevenson WG. Sustained Monomorphic Ventricular Tachycardia in Nonischemic Heart Disease: Arrhythmia-Substrate Correlations That Inform the Approach to Ablation. Circ Arrhythm Electrophysiol. 2019;12:e007312 Betensky BP, Kapa S, Desjardins B, et al. Characterization of Trans-septal Activation During Septal Pacing Criteria for Identification of Intramural Ventricular Tachycardia Substrate in Nonischemic Cardiomyopathy. Circ Arrhythm Electrophysiol. 2013;6:1123-11306 Vaseghi M, Hy TY, Tung R, et al. Outcomes of Catheter Ablation of Ventricular Tachycardia Based on Etiology in Nonischemic Heart Disease. J Am Coll Cardiol EP 2018;4:1141–50 Im SI, Higuchi S, Lee A, et al. Pulsed Field Ablation of Left Ventricular Myocardium in a Swine Infarct Model. J Am Coll Cardiol EP 2022;8:722-731 Dewland TA, Higuchi S, Venkateswaran R, Lee C, Gerstenfeld EP. AB-452672-2 Ultra-low Temperature Cryoablation Versus Ultra-low Temperature Cryoablation Combined With Pulsed Field Ablation In A Swine Ventricular Infarct Model. Heart Rhythm 2023;20:S92-S93. doi: doi.org/10.1016/j.hrthm.2023.03.395 . Reference slide #87 for further detail. PRIVATE AND CONFIDENTIAL 8
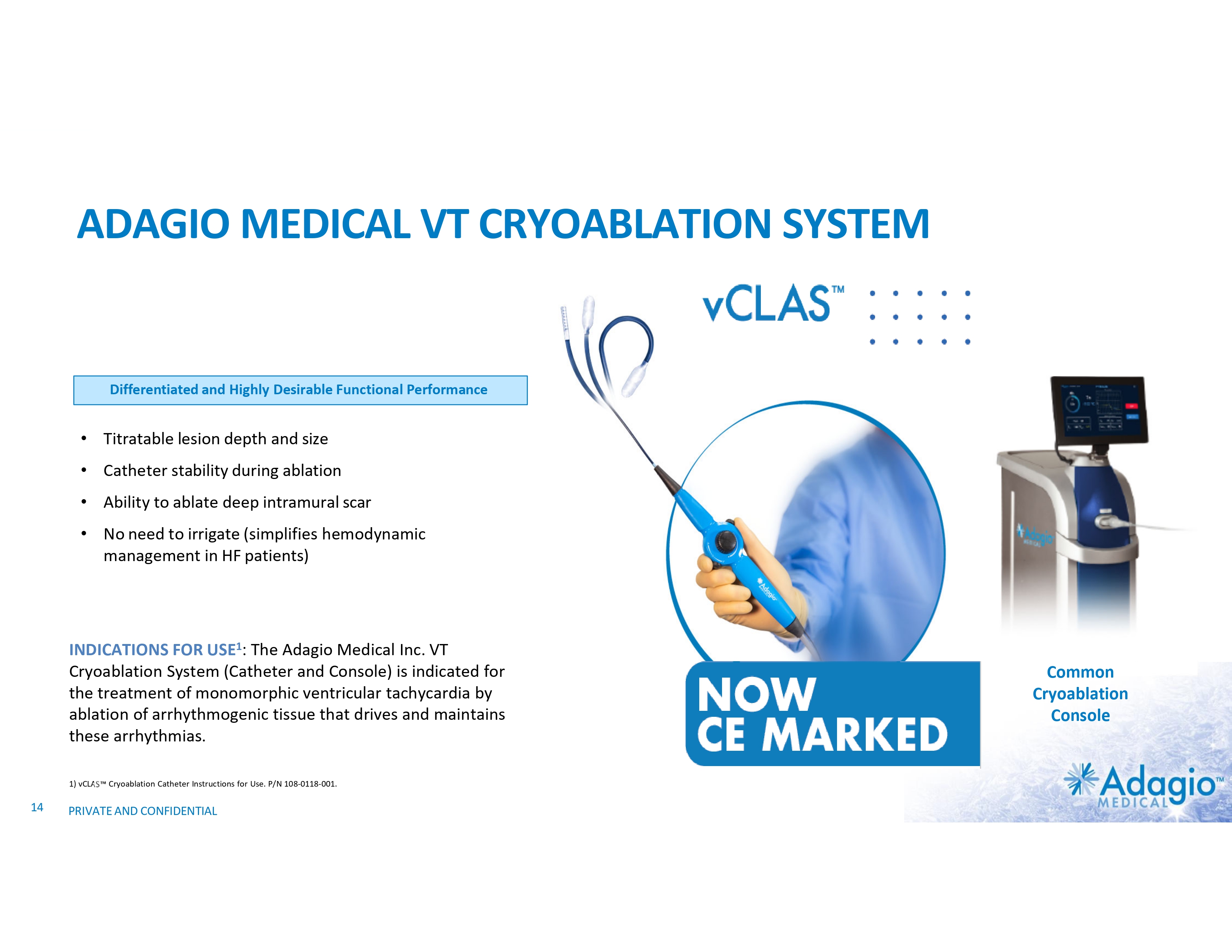
ADAGIO MEDICAL VT CRYOABLATION SYSTEM A New Benchmark in VT Ablations Differentiated and Highly Desirable Functional Performance • Titratable lesion depth and size • Catheter stability during ablation • Ability to ablate deep intramural scar • No need to irrigate (simplifies hemodynamic management in HF patients) INDICATIONS FOR USE1: The Adagio Medical Inc. VT Cryoablation System (Catheter and Console) is indicated for the treatment of monomorphic ventricular tachycardia by ablation of arrhythmogenic tissue that drives and maintains these arrhythmias. 1) vCLAS Cryoablation Catheter Instructions for Use. P/N 108-0118-001. 14 PRIVATE AND CONFIDENTIAL Common Cryoablation Console

CRYOCURE-VT STUDY (NCT # 04893317) Patients 64 patients, Rx-refractory persistent recurring monomorphic VT of both ischemic and non-ischemic etiology Endpoints Procedural safety, acute and chronic effectiveness Sites 9 centers Data Readout Late-Breaking Clinical Trial Presentation at EHRA 2024 CE-Mark Received on March 15, 2024 Next Steps Commercial launch in Q1 2024 / Results to be presented at EHRA 2024 / Initiation of post-market studies Initial Results First-in-human experience with ultra-low temperature cryoablation for monomorphic ventricular tachycardia1 Note: Expectations are preliminary and subject to change. Please see Disclaimer Forward Looking Statements on slide 2. (1) De Potter T, Balt J, Boersma L, et al. First-in-Human Experience With Ultra-Low Temperature Cryoablation for Monomorphic Ventricular Tachycardia Open Access. J Am Coll Cardiol EP. 2023 May, 9 (5) 686–691 15 PRIVATE AND CONFIDENTIAL
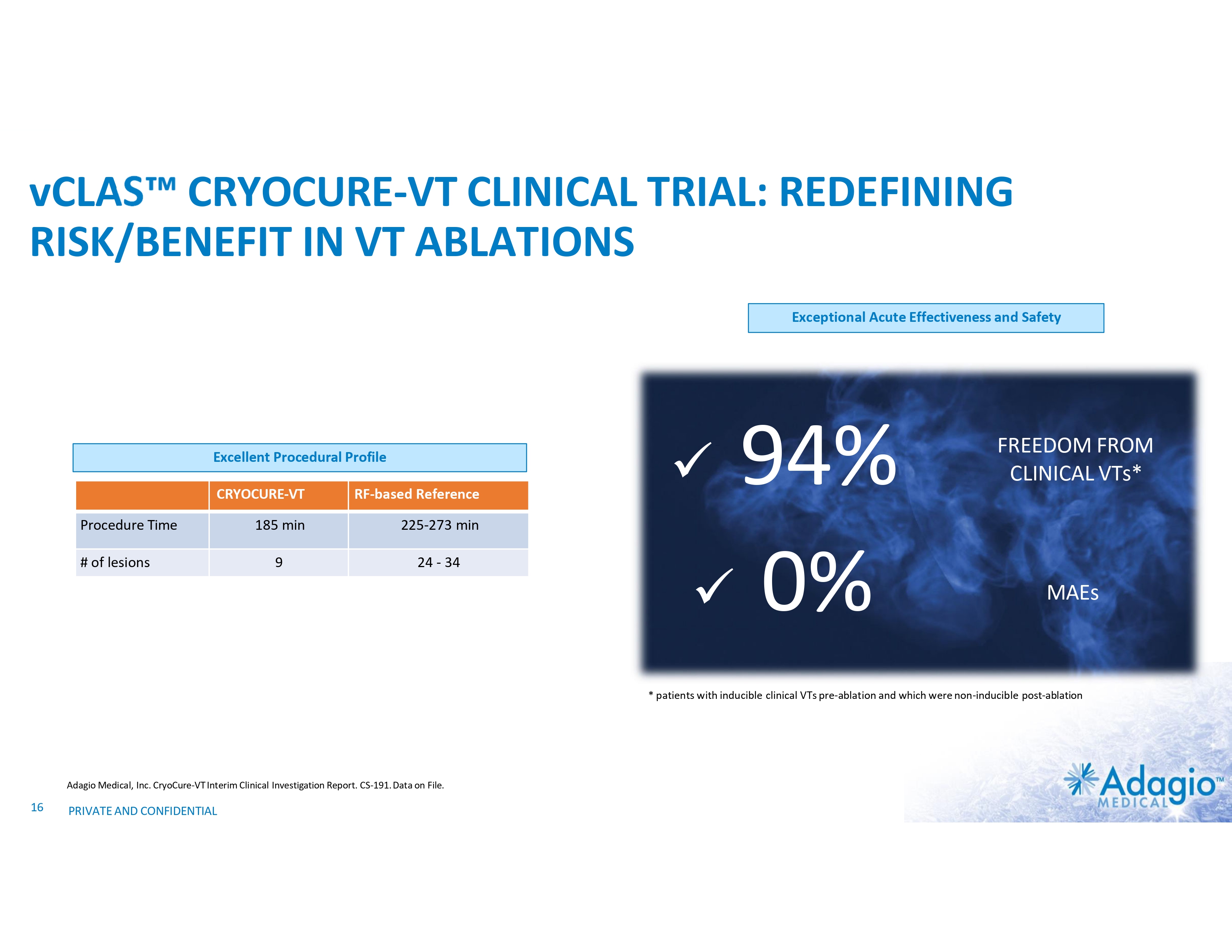
vCLAS CRYOCURE-VT CLINICAL TRIAL: REDEFINING RISK/BENEFIT IN VT ABLATIONS Exceptional Acute Effectiveness and Safety Excellent Procedural Profile CRYOCURE-VT RF-based Reference 185 min 225-273 min 9 24 - 34 Procedure Time # of lesions 94% x 0% x FREEDOM FROM CLINICAL VTs* MAEs * patients with inducible clinical VTs pre-ablation and which were non-inducible post-ablation Adagio Medical, Inc. CryoCure-VT Interim Clinical Investigation Report. CS-191. Data on File. 16 PRIVATE AND CONFIDENTIAL
FULCRUM-VT PIVOTAL STUDY (NCT # 05675865) Patients 206 patients, inclusive of patients enrolled in Early Feasibility Study, Rx-refractory persistent recurring monomorphic VT of both ischemic and non-ischemic etiology Endpoints Procedural safety, acute and chronic effectiveness Sites Up to 20 centers Data Readout Expected in Q3 2025 https://clinicaltrials.gov/study/NCT05675865 FULCRUM-VT Early Feasibility IDE Study Vanderbilt University Medical Center Banner University Health Center Mount Sinai Hospital UC San Francisco 17 PRIVATE AND CONFIDENTIAL • 20 patients enrolled • On April 26, 2024 US FDA approved FULCRUM-VT Pivotal IDE Supplement, increasing the number of study sites to 20 and number of subjects to 206.
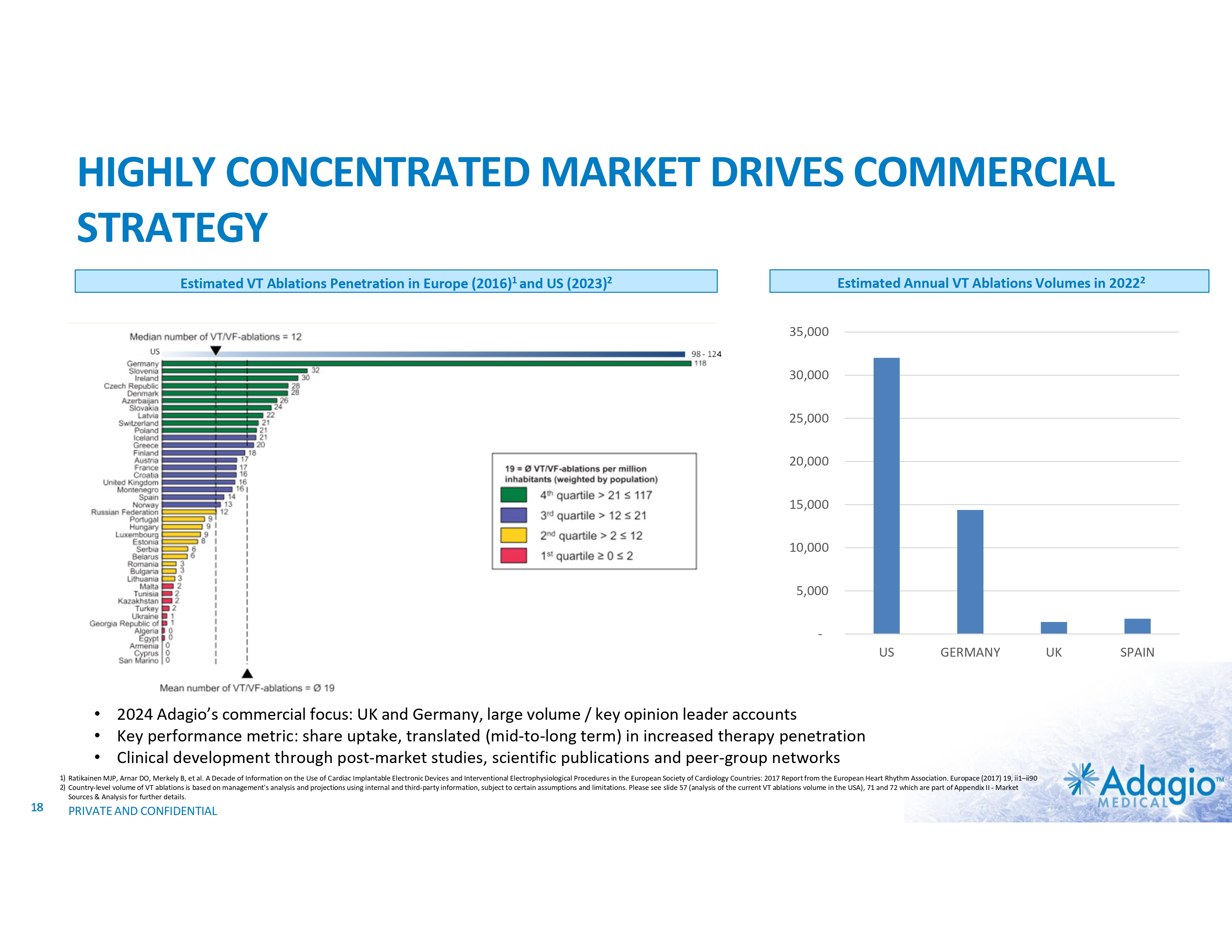
HIGHLY CONCENTRATED MARKET DRIVES COMMERCIAL STRATEGY Estimated Annual VT Ablations Volumes in 20222 Estimated VT Ablations Penetration in Europe (2016)1 and US (2023)2 35,000 US 98 - 124 30,000 25,000 20,000 15,000 10,000 5,000 - US GERMANY • 2024 Adagio’s commercial focus: UK and Germany, large volume / key opinion leader accounts • Key performance metric: share uptake, translated (mid-to-long term) in increased therapy penetration • Clinical development through post-market studies, scientific publications and peer-group networks 1) Ratikainen MJP, Arnar DO, Merkely B, et al. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace (2017) 19, ii1–ii90 2) Country-level volume of VT ablations is based on management's analysis and projections using internal and third-party information, subject to certain assumptions and limitations. Please see slide 57 (analysis of the current VT ablations volume in the USA), 71 and 72 which are part of Appendix II - Market Sources & Analysis for further details. 18 PRIVATE AND CONFIDENTIAL UK SPAIN
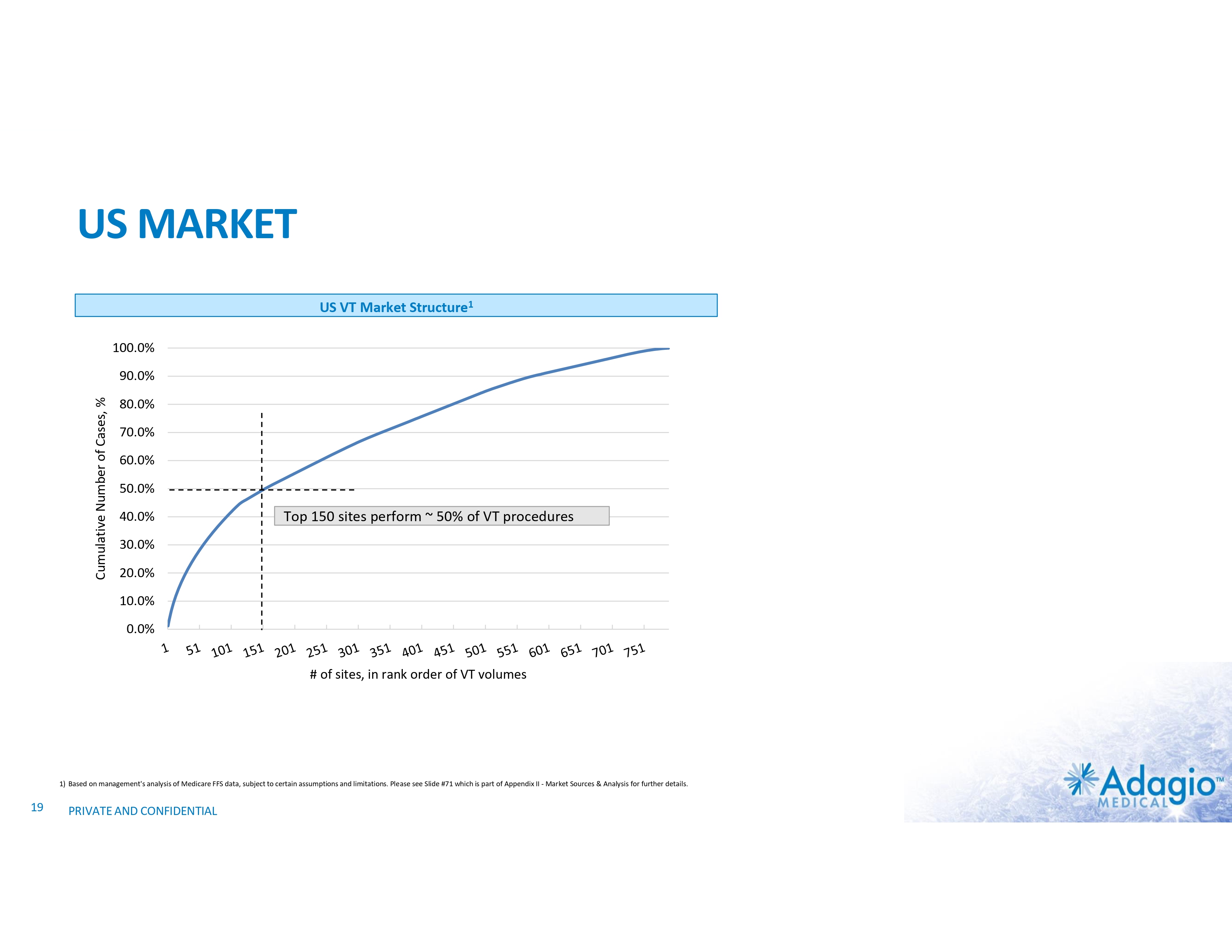
US MARKET US VT Market Structure1 100.0% Cumulative Number of Cases, % 90.0% 80.0% 70.0% 60.0% 50.0% 40.0% Top 150 sites perform ~ 50% of VT procedures 30.0% 20.0% 10.0% 0.0% # of sites, in rank order of VT volumes 1) Based on management's analysis of Medicare FFS data, subject to certain assumptions and limitations. Please see Slide #71 which is part of Appendix II - Market Sources & Analysis for further details. 19 PRIVATE AND CONFIDENTIAL
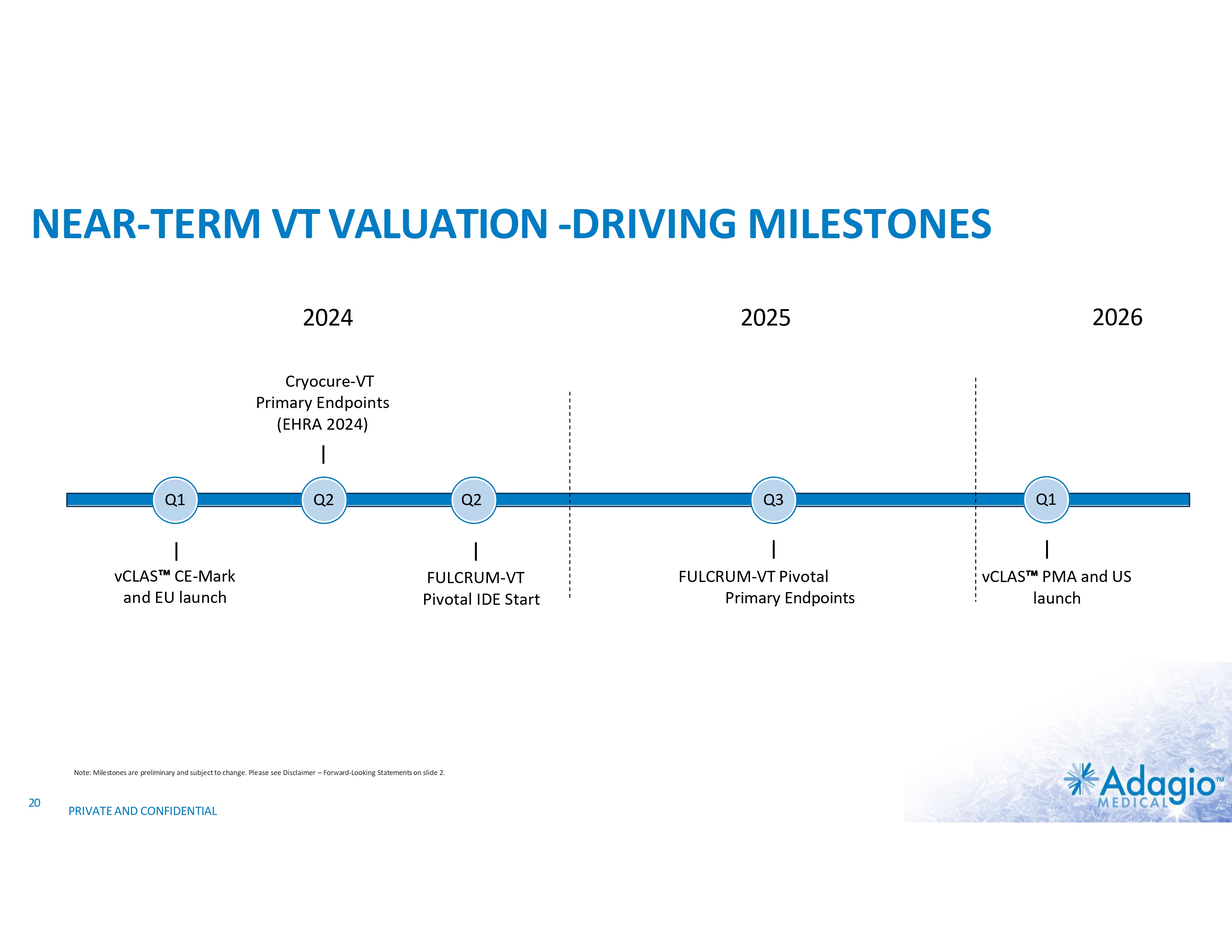
NEAR-TERM VT VALUATION -DRIVING MILESTONES 2024 2026 2025 Cryocure-VT Primary Endpoints (EHRA 2024) Q1 vCLAS CE-Mark and EU launch Q2 Q2 FULCRUM-VT Pivotal IDE Start Note: Milestones are preliminary and subject to change. Please see Disclaimer – Forward-Looking Statements on slide 2. 20 PRIVATE AND CONFIDENTIAL Q3 FULCRUM-VT Pivotal Primary Endpoints Q1 vCLAS PMA and US launch

ATRIAL FIBRILLATION MARKET: OPPORTUNITY, DRIVERS AND INHIBITORS
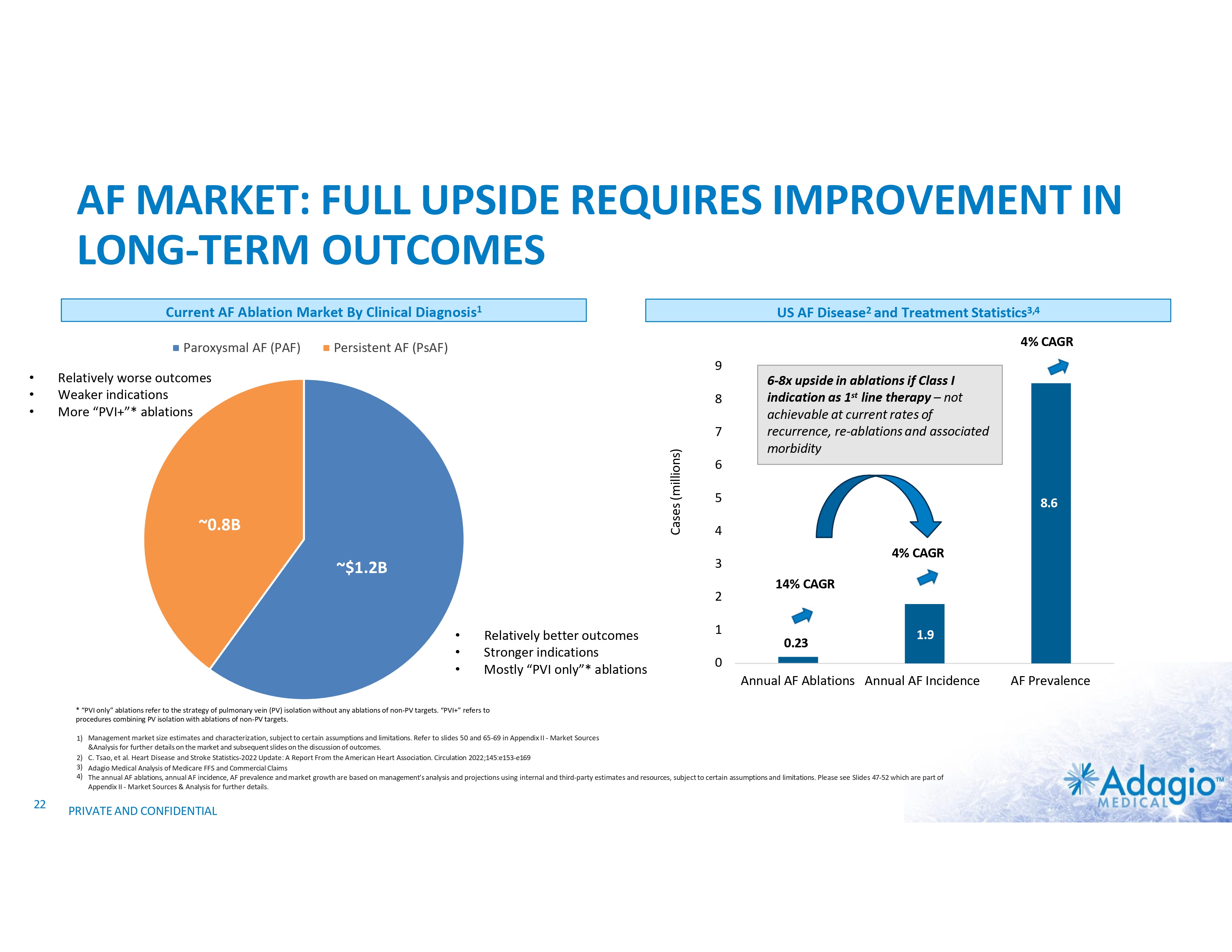
AF MARKET: FULL UPSIDE REQUIRES IMPROVEMENT IN LONG-TERM OUTCOMES Current AF Ablation Market By Clinical Diagnosis1 Paroxysmal AF (PAF) • • • US AF Disease2 and Treatment Statistics3,4 4% CAGR Persistent AF (PsAF) 9 Relatively worse outcomes Weaker indications More “PVI+”* ablations 8 Cases (millions) 7 ~0.8B 6-8x upside in ablations if Class I indication as 1st line therapy – not achievable at current rates of recurrence, re-ablations and associated morbidity 6 5 8.6 4 4% CAGR 3 ~$1.2B 2 • • • Relatively better outcomes Stronger indications Mostly “PVI only”* ablations 1 14% CAGR 0.23 1.9 0 Annual AF Ablations Annual AF Incidence * “PVI only” ablations refer to the strategy of pulmonary vein (PV) isolation without any ablations of non-PV targets. “PVI+” refers to procedures combining PV isolation with ablations of non-PV targets. 1) Management market size estimates and characterization, subject to certain assumptions and limitations. Refer to slides 50 and 65-69 in Appendix II - Market Sources &Analysis for further details on the market and subsequent slides on the discussion of outcomes. 2) C. Tsao, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153-e169 3) Adagio Medical Analysis of Medicare FFS and Commercial Claims 4) The annual AF ablations, annual AF incidence, AF prevalence and market growth are based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 47-52 which are part of Appendix II - Market Sources & Analysis for further details. 22 PRIVATE AND CONFIDENTIAL AF Prevalence

AF ABLATION MODALITIES: EQUIVALENT SAFETY AND EFFICACY OUTCOMES Freedom From AF/AT After Single Procedure In Paroxysmal AF Patients CIRCA-DOSE TRIAL (2019)1 ADVENT TRIAL (2023)2 - 73.1% • 55% RF • 45% Cryoballoon . 1) 2) 23 Andrade JG, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: A randomized clinical trial. Circulation 2019;140:1779–1788 Reddy VY, Gerstenfeld W, Natale A, et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med 2023; 389:1660-1671 PRIVATE AND CONFIDENTIAL - 71.3%
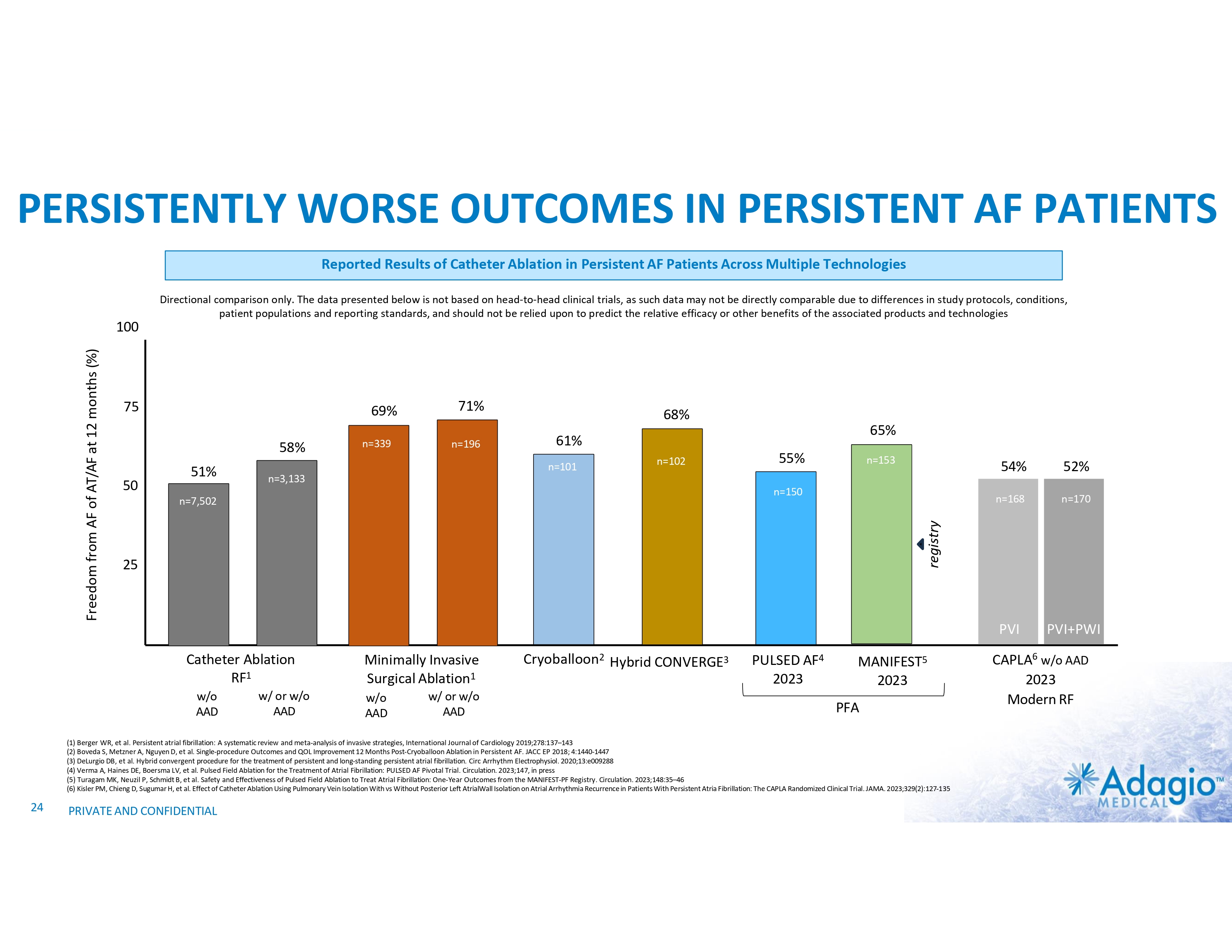
PERSISTENTLY WORSE OUTCOMES IN PERSISTENT AF PATIENTS Reported Results of Catheter Ablation in Persistent AF Patients Across Multiple Technologies 75 69% 58% 50 51% n=339 71% n=196 68% 65% 61% n=101 n=102 55% n=153 n=3,133 n=150 n=7,502 25 Catheter Ablation RF1 w/o AAD w/ or w/o AAD Minimally Invasive Surgical Ablation1 w/o AAD w/ or w/o AAD Cryoballoon2 Hybrid CONVERGE3 PULSED AF4 2023 MANIFEST5 2023 PFA (1) Berger WR, et al. Persistent atrial fibrillation: A systematic review and meta-analysis of invasive strategies, International Journal of Cardiology 2019;278:137–143 (2) Boveda S, Metzner A, Nguyen D, et al. Single-procedure Outcomes and QOL Improvement 12 Months Post-Cryoballoon Ablation in Persistent AF. JACC EP 2018; 4:1440-1447 (3) DeLurgio DB, et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e009288 (4) Verma A, Haines DE, Boersma LV, et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation. 2023;147, in press (5) Turagam MK, Neuzil P, Schmidt B, et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes from the MANIFEST-PF Registry. Circulation. 2023;148:35–46 (6) Kisler PM, Chieng D, Sugumar H, et al. Effect of Catheter Ablation Using Pulmonary Vein Isolation With vs Without Posterior Left AtrialWall Isolation on Atrial Arrhythmia Recurrence in Patients With Persistent Atria Fibrillation: The CAPLA Randomized Clinical Trial. JAMA. 2023;329(2):127-135 24 54% 52% n=168 n=170 PVI PVI+PWI registry Freedom from AF of AT/AF at 12 months (%) 100 Directional comparison only. The data presented below is not based on head-to-head clinical trials, as such data may not be directly comparable due to differences in study protocols, conditions, patient populations and reporting standards, and should not be relied upon to predict the relative efficacy or other benefits of the associated products and technologies PRIVATE AND CONFIDENTIAL CAPLA6 w/o AAD 2023 Modern RF
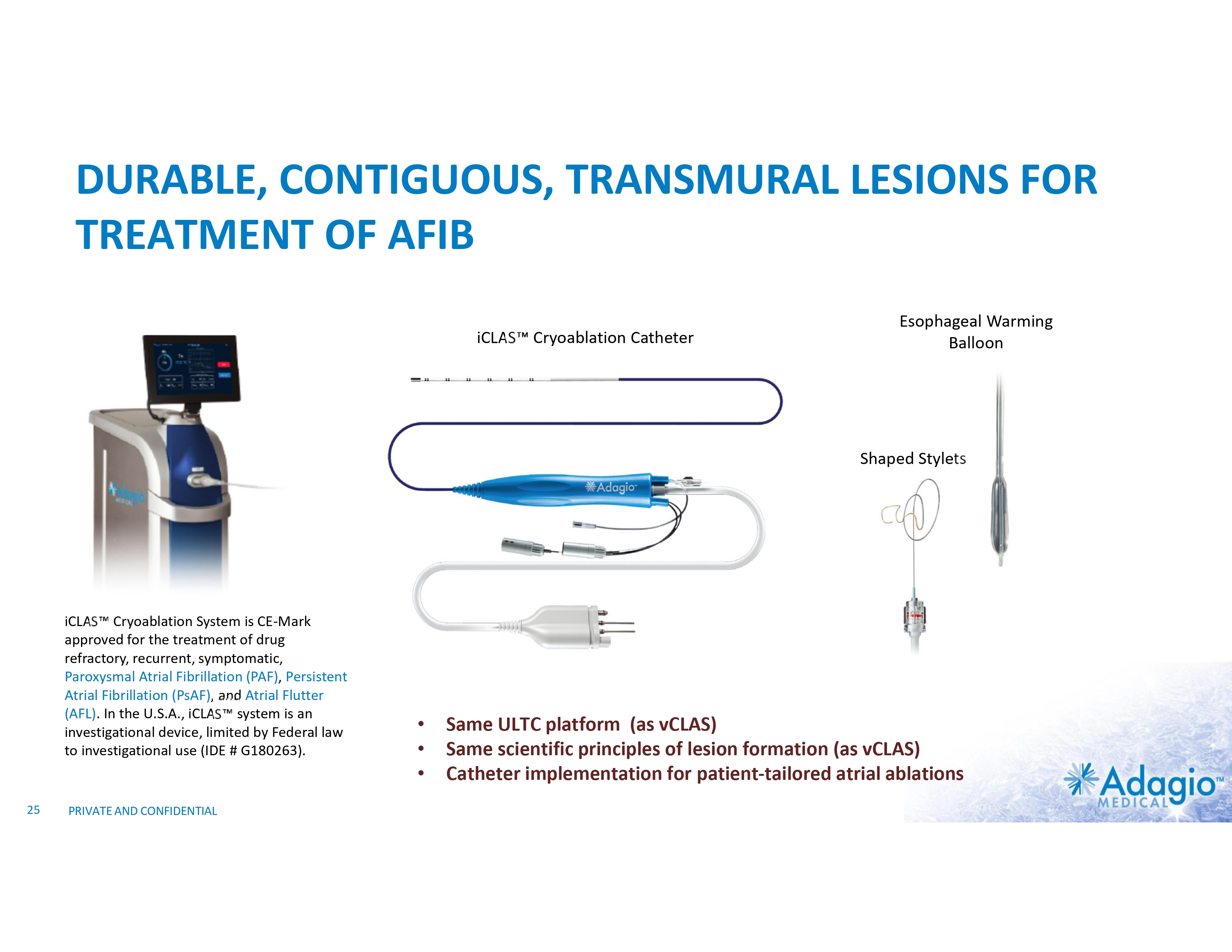
DURABLE, CONTIGUOUS, TRANSMURAL LESIONS FOR TREATMENT OF AFIB iCLAS Cryoablation Catheter Esophageal Warming Balloon Shaped Stylets iCLAS Cryoablation System is CE-Mark approved for the treatment of drug refractory, recurrent, symptomatic, Paroxysmal Atrial Fibrillation (PAF), Persistent Atrial Fibrillation (PsAF), and Atrial Flutter (AFL). In the U.S.A., iCLAS system is an investigational device, limited by Federal law to investigational use (IDE # G180263). 25 PRIVATE AND CONFIDENTIAL • • • Same ULTC platform (as vCLAS) Same scientific principles of lesion formation (as vCLAS) Catheter implementation for patient-tailored atrial ablations
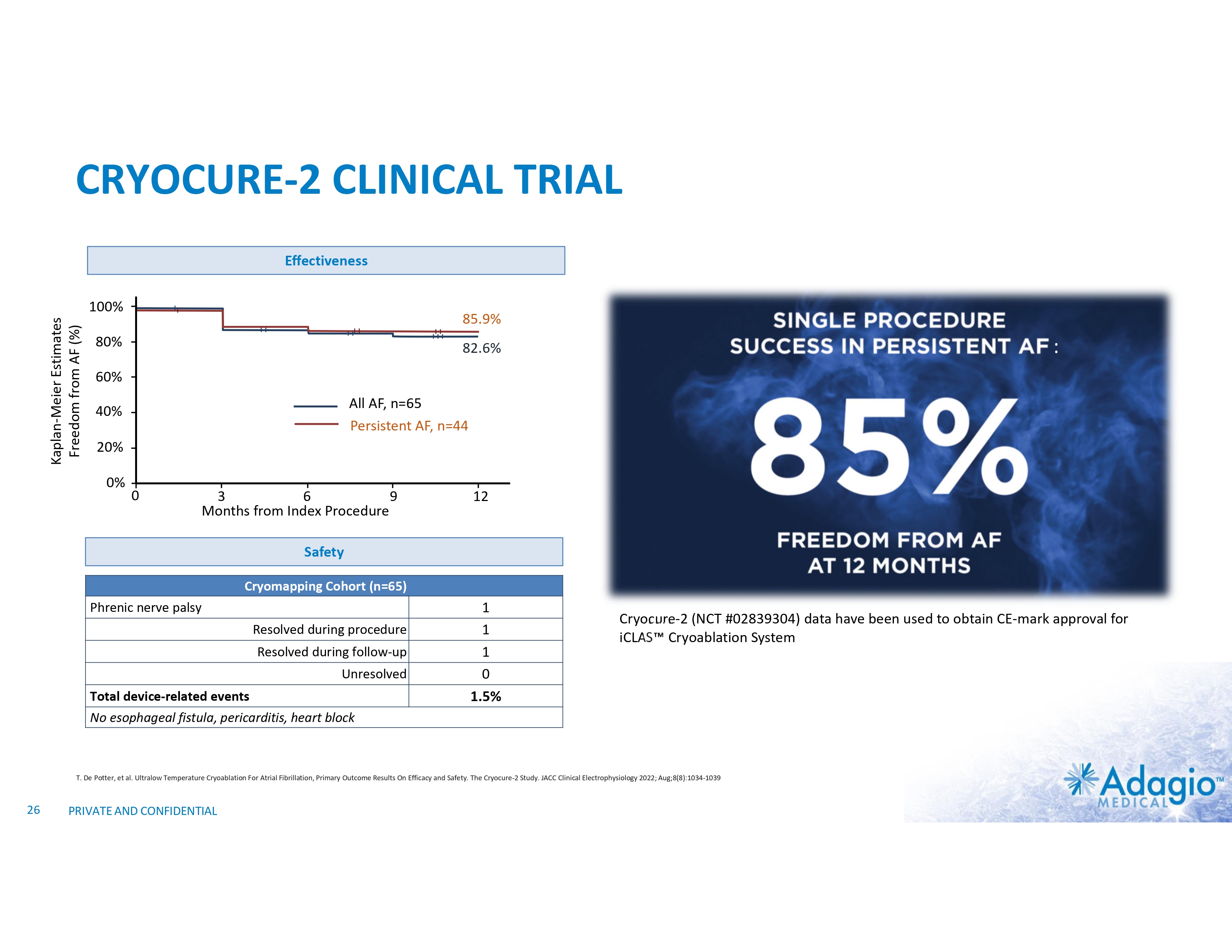
CRYOCURE-2 CLINICAL TRIAL Effectiveness Kaplan-Meier Estimates Freedom from AF (%) 100% 85.9% 80% : 82.6% 60% All AF, n=65 Persistent AF, n=44 40% CRYOCURE-2 20% 0% 0 3 6 9 Months from Index Procedure NCT #02839304 12 Safety Cryomapping Cohort (n=65) Phrenic nerve palsy Resolved during procedure Resolved during follow-up Unresolved Total device-related events 1 1 1 0 1.5% Cryocure-2 (NCT #02839304) data have been used to obtain CE-mark approval for iCLAS Cryoablation System No esophageal fistula, pericarditis, heart block T. De Potter, et al. Ultralow Temperature Cryoablation For Atrial Fibrillation, Primary Outcome Results On Efficacy and Safety. The Cryocure-2 Study. JACC Clinical Electrophysiology 2022; Aug;8(8):1034-1039 26 PRIVATE AND CONFIDENTIAL
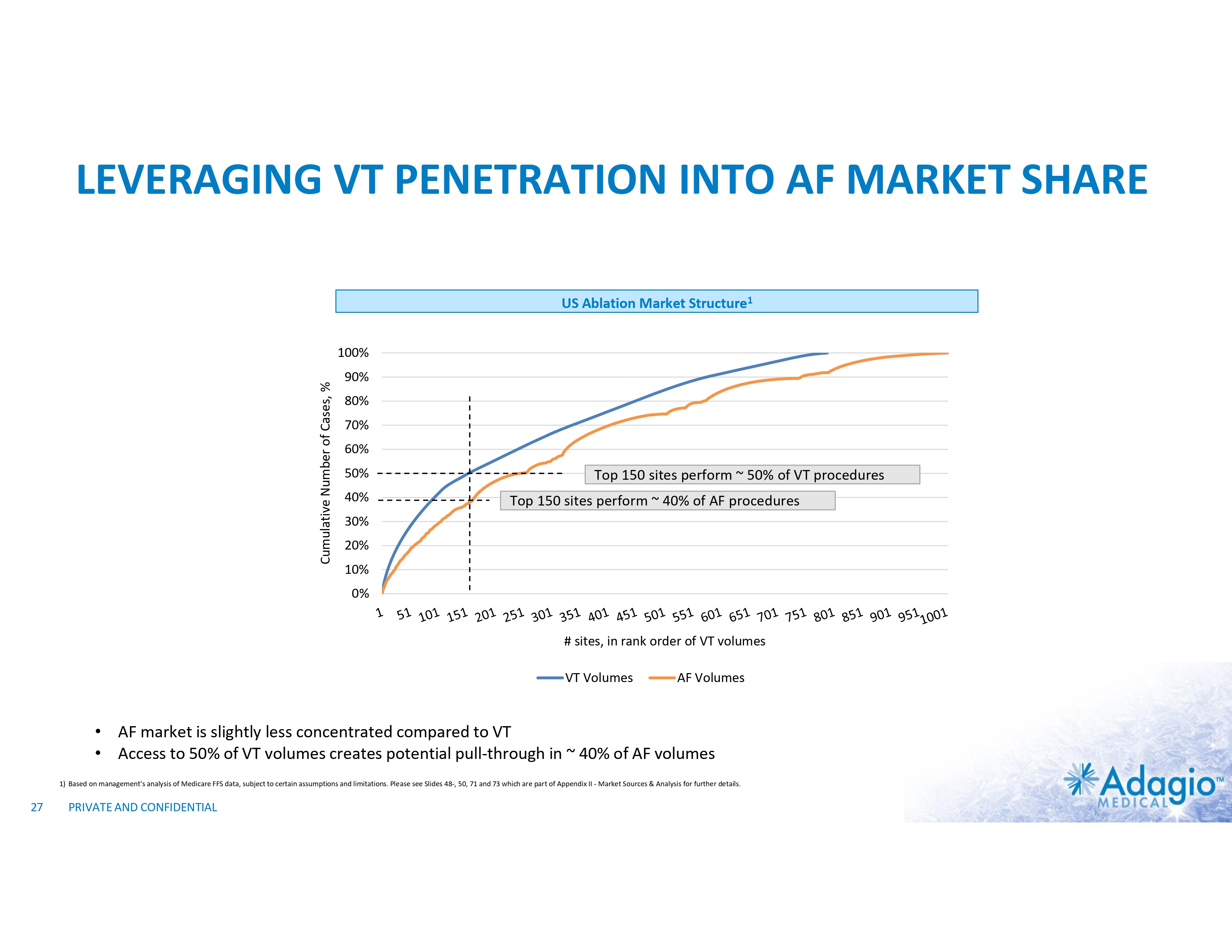
LEVERAGING VT PENETRATION INTO AF MARKET SHARE US Ablation Market Structure1 Cumulative Number of Cases, % 100% 90% 80% 70% 60% 50% 40% Top 150 sites perform ~ 50% of VT procedures Top 150 sites perform ~ 40% of AF procedures 30% 20% 10% 0% # sites, in rank order of VT volumes VT Volumes AF Volumes • AF market is slightly less concentrated compared to VT • Access to 50% of VT volumes creates potential pull-through in ~ 40% of AF volumes 1) Based on management's analysis of Medicare FFS data, subject to certain assumptions and limitations. Please see Slides 48-, 50, 71 and 73 which are part of Appendix II - Market Sources & Analysis for further details. 27 PRIVATE AND CONFIDENTIAL

WHAT IS PULSED FIELD CRYOABLATION?
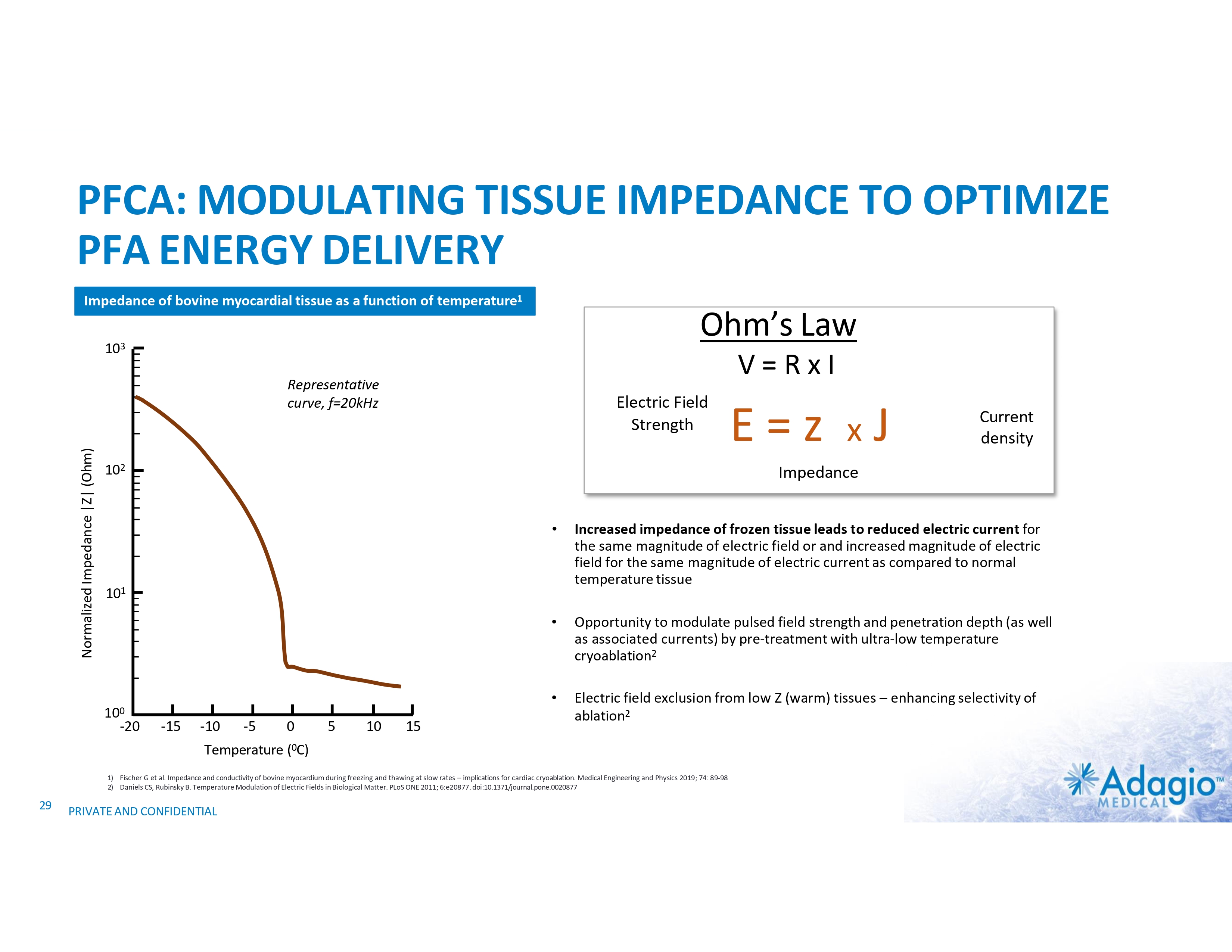
PFCA: MODULATING TISSUE IMPEDANCE TO OPTIMIZE PFA ENERGY DELIVERY Impedance of bovine myocardial tissue as a function of temperature1 Ohm’s Law 103 V=RxI Normalized Impedance |Z| (Ohm) Representative curve, f=20kHz Electric Field Strength 102 -15 -10 -5 Temperature • Increased impedance of frozen tissue leads to reduced electric current for the same magnitude of electric field or and increased magnitude of electric field for the same magnitude of electric current as compared to normal temperature tissue • Opportunity to modulate pulsed field strength and penetration depth (as well as associated currents) by pre-treatment with ultra-low temperature cryoablation2 • Electric field exclusion from low Z (warm) tissues – enhancing selectivity of ablation2 0 5 10 15 (0C) 1) Fischer G et al. Impedance and conductivity of bovine myocardium during freezing and thawing at slow rates – implications for cardiac cryoablation. Medical Engineering and Physics 2019; 74: 89-98 2) Daniels CS, Rubinsky B. Temperature Modulation of Electric Fields in Biological Matter. PLoS ONE 2011; 6:e20877. doi:10.1371/journal.pone.0020877 29 Current density Impedance 101 100 -20 E=z xJ PRIVATE AND CONFIDENTIAL

PFCA: COMBINING THE BENEFITS OF ULTC AND MINIMIZING THE LIMITATIONS OF PFA Single catheter with ablation element capable of both ultra-low temperature cryoablation and PFA PFA Console Cryoablation Console 30 PRIVATE AND CONFIDENTIAL Connected to standalone or integrated cryoablation and PFA consoles 1 2 Lesion Formation: Short duration ultra-low temperature cryoablation Immediately followed by PFA
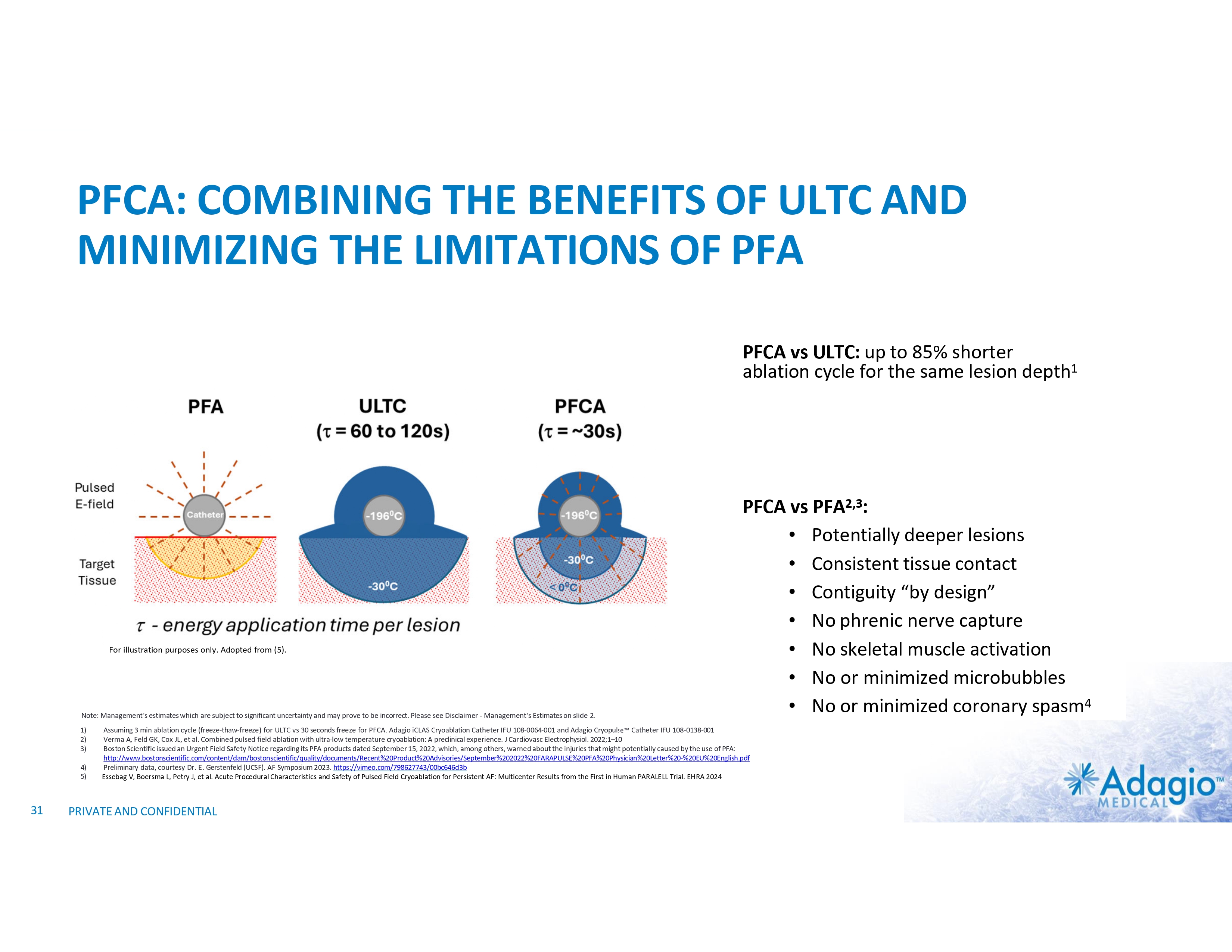
PFCA: COMBINING THE BENEFITS OF ULTC AND MINIMIZING THE LIMITATIONS OF PFA PFCA vs ULTC: up to 85% shorter ablation cycle for the same lesion depth1 For illustration purposes only. Adopted from (5). Note: Management's estimates which are subject to significant uncertainty and may prove to be incorrect. Please see Disclaimer - Management's Estimates on slide 2. 1) 2) 3) 4) 5) 31 PFCA vs PFA2,3: • Potentially deeper lesions • Consistent tissue contact • Contiguity “by design” • No phrenic nerve capture • No skeletal muscle activation • No or minimized microbubbles • No or minimized coronary spasm4 Assuming 3 min ablation cycle (freeze-thaw-freeze) for ULTC vs 30 seconds freeze for PFCA. Adagio iCLAS Cryoablation Catheter IFU 108-0064-001 and Adagio Cryopulse Catheter IFU 108-0138-001 Verma A, Feld GK, Cox JL, et al. Combined pulsed field ablation with ultra-low temperature cryoablation: A preclinical experience. J Cardiovasc Electrophysiol. 2022;1–10 Boston Scientific issued an Urgent Field Safety Notice regarding its PFA products dated September 15, 2022, which, among others, warned about the injuries that might potentially caused by the use of PFA: http://www.bostonscientific.com/content/dam/bostonscientific/quality/documents/Recent%20Product%20Advisories/September%202022%20FARAPULSE%20PFA%20Physician%20Letter%20-%20EU%20English.pdf Preliminary data, courtesy Dr. E. Gerstenfeld (UCSF). AF Symposium 2023. https://vimeo.com/798627743/00bc646d3b Essebag V, Boersma L, Petry J, et al. Acute Procedural Characteristics and Safety of Pulsed Field Cryoablation for Persistent AF: Multicenter Results from the First in Human PARALELL Trial. EHRA 2024 PRIVATE AND CONFIDENTIAL
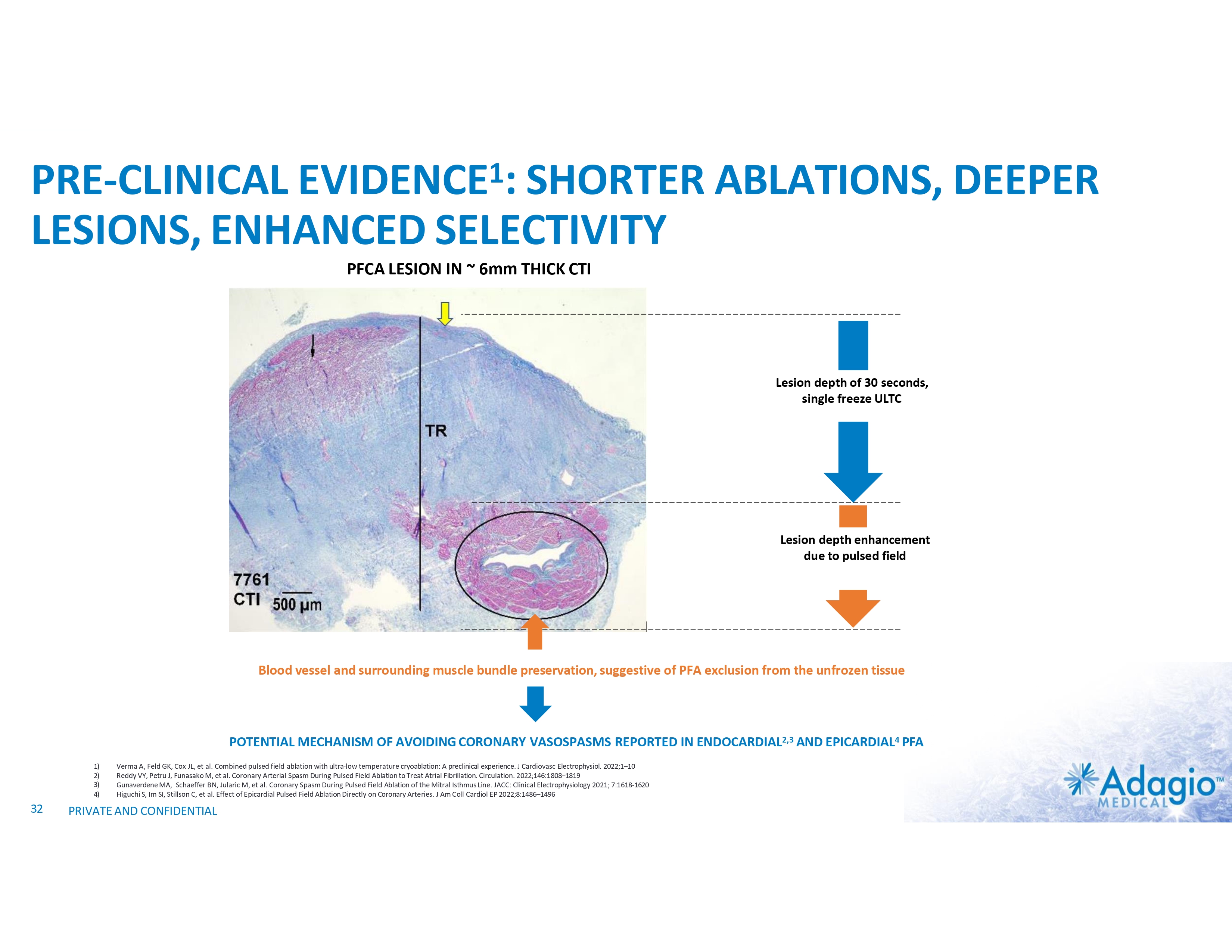
PRE-CLINICAL EVIDENCE1: SHORTER ABLATIONS, DEEPER LESIONS, ENHANCED SELECTIVITY PFCA LESION IN ~ 6mm THICK CTI Lesion depth of 30 seconds, single freeze ULTC Lesion depth enhancement due to pulsed field Blood vessel and surrounding muscle bundle preservation, suggestive of PFA exclusion from the unfrozen tissue POTENTIAL MECHANISM OF AVOIDING CORONARY VASOSPASMS REPORTED IN ENDOCARDIAL2,3 AND EPICARDIAL4 PFA 1) 2) 3) 4) 32 Verma A, Feld GK, Cox JL, et al. Combined pulsed field ablation with ultra-low temperature cryoablation: A preclinical experience. J Cardiovasc Electrophysiol. 2022;1–10 Reddy VY, Petru J, Funasako M, et al. Coronary Arterial Spasm During Pulsed Field Ablation to Treat Atrial Fibrillation. Circulation. 2022;146:1808–1819 Gunaverdene MA, Schaeffer BN, Jularic M, et al. Coronary Spasm During Pulsed Field Ablation of the Mitral Isthmus Line. JACC: Clinical Electrophysiology 2021; 7:1618-1620 Higuchi S, Im SI, Stillson C, et al. Effect of Epicardial Pulsed Field Ablation Directly on Coronary Arteries. J Am Coll Cardiol EP 2022;8:1486–1496 PRIVATE AND CONFIDENTIAL
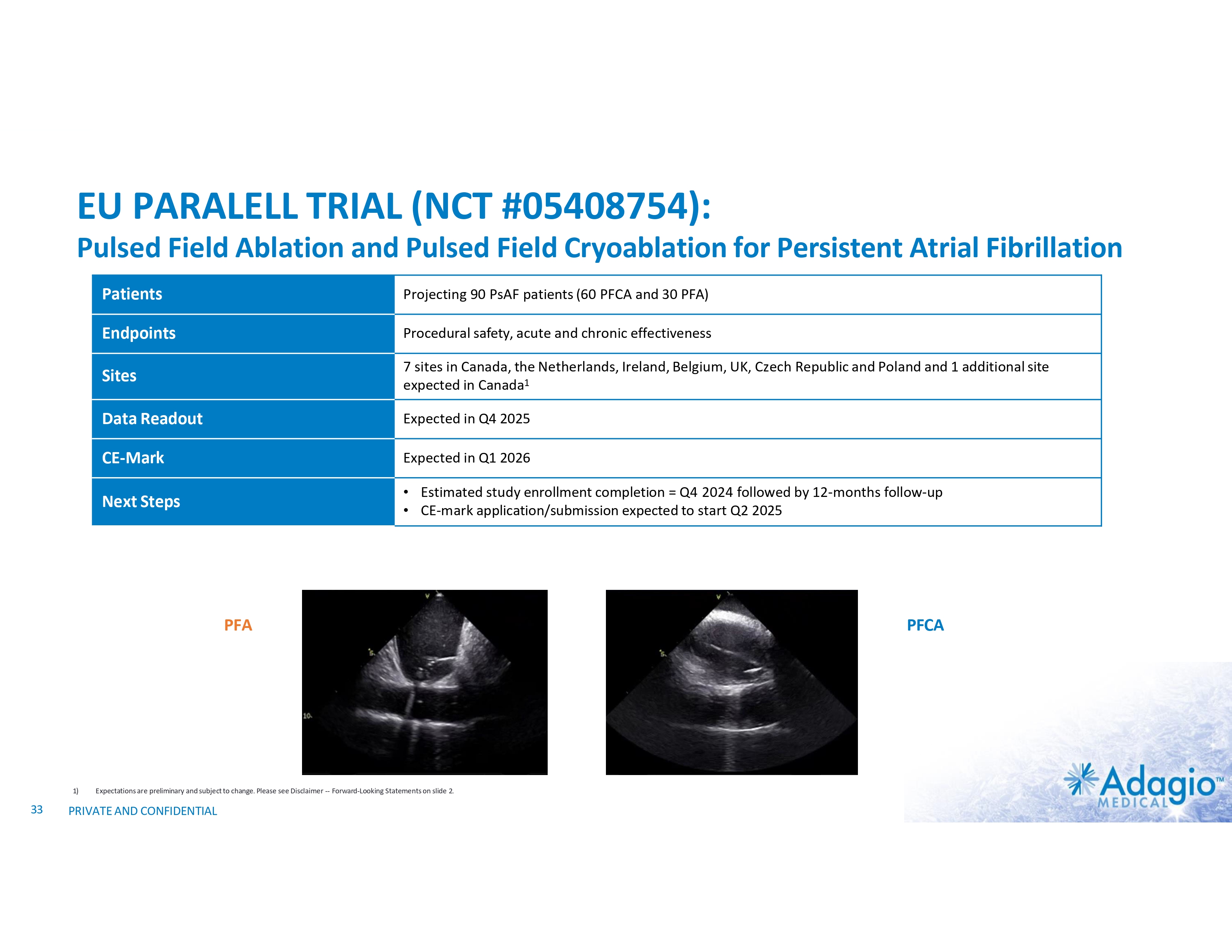
EU PARALELL TRIAL (NCT #05408754): Pulsed Field Ablation and Pulsed Field Cryoablation for Persistent Atrial Fibrillation Patients Projecting 90 PsAF patients (60 PFCA and 30 PFA) Endpoints Procedural safety, acute and chronic effectiveness Sites 7 sites in Canada, the Netherlands, Ireland, Belgium, UK, Czech Republic and Poland and 1 additional site expected in Canada1 Data Readout Expected in Q4 2025 CE-Mark Expected in Q1 2026 Next Steps • Estimated study enrollment completion = Q4 2024 followed by 12-months follow-up • CE-mark application/submission expected to start Q2 2025 PFA 1) 33 Expectations are preliminary and subject to change. Please see Disclaimer -- Forward-Looking Statements on slide 2. PRIVATE AND CONFIDENTIAL PFCA
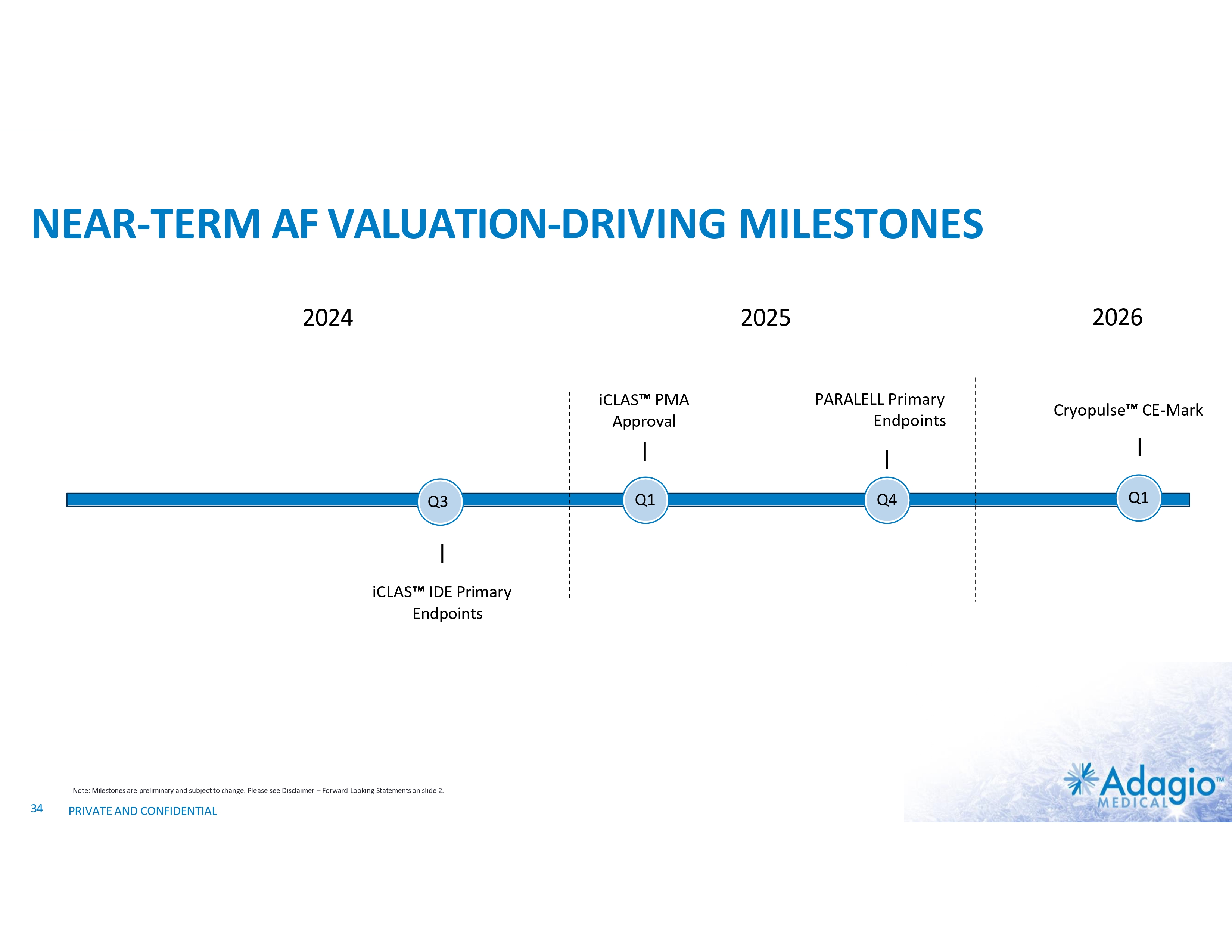
NEAR-TERM AF VALUATION-DRIVING MILESTONES 2024 iCLAS PMA Approval Q3 iCLAS IDE Primary Endpoints Note: Milestones are preliminary and subject to change. Please see Disclaimer – Forward-Looking Statements on slide 2. 34 PRIVATE AND CONFIDENTIAL 2026 2025 Q1 PARALELL Primary Endpoints Q4 Cryopulse CE-Mark Q1

THE COOLEST CATHETER ABLATION TECHNOLOGY THANK YOU 35 © COPYRIGHT 2023 ADAGIO MEDICAL, INC. COMPANY CONFIDENTIAL

APPENDIX I 36

HIGHLY ACCOMPLISHED EXECUTIVE TEAM Olav Bergheim CEO & President 30+ years of experience in life sciences Hakon Bergheim Chief Operating Officer 10+ years of experience in medical devices John Dahldorf Chief Financial Officer Nabil Jubran Chief Compliance Officer 20+ years of corporate finance experience 20+ years of medical device experience Tim Glynn VP of Global Sales Ilya Grigorov Vice President, Global Marketing and Product Management 25+ years of medical experience 1 Founder of 3F Therapeutics Prelude Corporation 1 20+ years of medical device experience 1 Note: Olav Bergheim serves as the CEO of the Company pursuant to the terms of a Facilities and Shared Services Agreement between the Company and Fjord Ventures, LLC. Based on such agreement, Mr. Bergheim is compensated for serving in such position by Fjord Ventures, LLC. Two funds managed by Mr. Bergheim, one of which is affiliated with Fjord Ventures, LLC, have invested an aggregate of approximately $10M among the approximately $100M investment in aggregate that the Company has received so far. 1) Volcano Corporation was acquired by Royal Philips in 2015. 37 Doug Kurschinski Vice President of Clinical Affairs 30+ years of medical device experience

SELECTED RISK FACTORS No representation or warranty (whether express or implied) has been made by ARYA, the Company, ListCo or any of their respective directors, officers, employees, affiliates, agents, advisors or representatives with respect to the proposed PIPE financing or Business Combination or the manner in which the proposed PIPE financing or Business Combination is conducted, and the recipient hereby disclaims any such representation or warranty. The recipient of this Presentation acknowledges that ARYA, the Company, ListCo and their respective directors, officers, employees, affiliates, agents, advisors or representatives are under no obligation to accept any offer or proposal by any person or entity regarding the PIPE financing and the Business Combination. None of ARYA, the Company, ListCo or any of their respective directors, officers, employees, affiliates, agents, advisors or representatives has any legal, fiduciary or other duty to any recipient of this Presentation with respect to the manner in which the proposed PIPE financing or Business Combination is conducted. Unless the context otherwise requires, all reference in this subsection to the “Company,” “Adagio,” “we,” “us” or “our” refer to Adagio Medical, Inc. and its subsidiaries, prior to, or following, the consummation of the Business Combination, as the context requires. The risks presented below are some of the general risks to the business and operations of Adagio, ARYA Sciences Acquisition Corp IV (“ARYA”) and Aja Holdco, Inc., of which Adagio will become a subsidiary following the consummation of the Business Combination (the “Post-Combination Company”), and such risks are not exhaustive. The list below is qualified in its entirety by disclosures that will be contained in the future filings by ARYA and the Post-Combination Company, or of each of their respective affiliates or by third parties with the U.S. Securities and Exchange Commission (the “SEC”), including any documents filed in connection with the proposed transaction. The risks presented in such filings may differ significantly from and may be more extensive than those presented below. The list below is not exhaustive, and you are encouraged to perform your own investigation with respect to the business, financial condition and prospects of Adagio or the Post-Combination Company. You should carefully consider the following risk factors in addition to the information included in this presentation. Adagio or the Post-Combination Company may face additional risks and uncertainties that are not presently known to it or that it currently deems immaterial, which may also impair Adagio’s or the Post-Combination Company’s business or its financial condition. These risks speak only as of the date of this presentation, and neither the Company, ARYA nor the Post-Combination Company undertake any obligation to update the disclosure contained herein. In making any investment decision, you should rely solely upon independent investigation made by you. You acknowledge that you are not relying upon, and have not relied upon, any of the summary of risks or any other statement, representation or warranty made by any person or entity other than the statements, representations and warranties of the Company, ARYA or the Post-Combination Company explicitly contained in any definitive agreement you enter into. You acknowledge that you have such knowledge and experience in financial and business matters as to be capable of evaluating the merits and risks of an investment in the Company or the Post-Combination Company and you have sought such accounting, legal and tax advice from your own advisors as you have considered necessary to make an informed decision. 38 PRIVATE AND CONFIDENTIAL

SELECTED RISK FACTORS (CONT.) 39 The consummation of the Business Combination is subject to a number of conditions, and if those conditions are not satisfied or waived, the Business Combination may not be completed; Some of ARYA’s, the Company’s or the Post-Combination Company’s officers and directors may have conflicts of interest that may influence them to approve the Business Combination without regard to your interests; ARYA’s directors and officers may have interests in the Business Combination different from the interests of ARYA, the Company, the Post-Combination Company or their respective shareholders; If ARYA is unable to close certain financing transactions and sufficient shareholders exercise their redemption rights in connection with the Business Combination such that there is less than $60 million of cash proceeds available from ARYA’s trust account and the financing transactions, then ARYA may lack sufficient funds to consummate the Business Combination; A portion of the total outstanding shares of the Post-Business Combination Company is expected to be restricted from immediate resale but may be sold into the market in the near future; Sales of a substantial number of shares of the Post-Business Combination Company’s common stock in the public market by existing stockholders could cause the Post-Business Combination Company’s share price to decline, even if our business is doing well; ARYA’s shareholders will experience dilution due to (i) the issuance to existing Company security holders and investors in the financing transactions in connection with the Business Combination of securities, and (ii) additional sources of dilution upon exercise or conversion of securities that will be issued in connection with or following the Business Combination (for instance, any earn-out shares, the PIPE Warrants, the Convert Warrants, the New Adagio Convertible Notes, securities issued in connection with the post-Business Combination Company equity plan or employee share purchase plan), in each case potentially entitling recipients of such securities to a significant voting stake in the Post-Business Combination Company; If ARYA does not consummated an initial business combination within the required time period, as may be extended at the option of ARYA Sciences Holdings IV (the “Sponsor”)), its public shareholders may receive only their pro rata portion of the funds in the ARYA’s trust account that are available for distribution its public shareholders; There are no assurances that ARYA will be able to complete the Business Combination prior to its expiration date or that the Sponsor will continue to exercise its monthly options to extend the time period ARYA has in order to consummate an initial business combination; The Company or Post-Business Combination Company stockholders cannot be certain of the value of the merger consideration they will receive until the closing of the Business Combination; Because there are no current plans to pay cash dividends on the common stock of the Post-Business Combination Company for the foreseeable future, you may not receive any return on investment unless you sell your ARYA ordinary shares or the Post-Business Combination Company common stock at a price greater than what you paid for it; ARYA, the Company and the Post-Business Combination Company expect to incur substantial transaction fees and costs in connection with the Business Combination and the integration of their businesses; The costs related to the Business Combination could be significantly higher than currently anticipated; ARYA’s, the Company’s or the Post-Business Combination Company’s business and operations could be negatively affected, or the Business Combination may be delayed or prevented from being completed, if they become subject to any securities litigation or shareholder activism; In connection with the Business Combination, the Sponsor and ARYA’s directors, executive officers, advisors and their affiliates may elect to purchase Class A ordinary shares of ARYA from public shareholders, which may reduce the public “float” of ARYA’s Class A ordinary shares; PRIVATE AND CONFIDENTIAL

SELECTED RISK FACTORS (CONT.) 40 The proceeds held in ARYA’s trust account could be reduced and the per-share redemption amount received by ARYA shareholders may be less than $10.00 per share; The Nasdaq Stock Market LLC may delist ARYA’s Class A ordinary shares from its exchange prior to the closing of the Business Combination or Nasdaq may not list the Post-Business Combination Company’s securities on its exchange, which could limit investors’ ability to make transactions in the Post-Business Combination Company’s securities and subject the Post-Business Combination Company to additional trading restrictions Following the closing of the Business Combination, an active trading market for the Post-Business Combination Company’s common stock may not be available on a consistent basis to provide stockholders with adequate liquidity. The share price may be extremely volatile and shareholders could lose a significant part of their investment; If, following the Business Combination, securities or industry analysts do not publish or cease publishing reports about the Post-Business Combination Company, its business, or its market, or if they change their recommendations regarding the Post-Business Combination Company’s securities adversely, the price and trading volume of the securities of the Post-Business Combination Company could decline; The benefits of the Business Combination may not be realized to the extent currently anticipated by ARYA, the Company and the Post-Business Combination Company, or at all. The ability to recognize any such benefits may be affected by, among other things, competition, the ability of the Post-Business Combination Company to grow and manage growth profitably, maintain relationships with customers, landlords and suppliers and retain its management and key employees. If the Business Combination’s benefits do not meet the expectations of investors, shareholders or financial analysts, the market price of ARYA’s or the Post-Business Combination Company’s securities may decline; The potential business combination will result in changes to the composition of the board of directors of the Company and the composition of the board of directors of the Post-Business Combination Company which may affect the strategy of the Post-Business Combination Company; The ability of ARYA, the Company and the Post-Business Combination Company to successfully effect the Business Combination and to be successful thereafter will be dependent upon the efforts of certain key personnel, including the Company’s key personnel. The loss of key personnel could negatively impact the operations and profitability of the Post-Business Combination Company and its financial condition could suffer as a result; The Post-Business Combination Company does not have experience operating as a public company subject to U.S. federal securities laws and may not be able to adequately develop and implement the governance, compliance, risk management and control infrastructure and culture required for a public company, including compliance with the Sarbanes Oxley Act; The requirements of being a public company may strain the Post-Business Combination Company’s resources, incur increased costs and distract its management, which could make it difficult to manage its business, particularly after the Post- Business Combination Company is no longer an emerging growth company; Subsequent to the completion of the Business Combination, the post-Business Combination company may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on its financial condition, results of operations and stock price, which could cause you to lose some or all of your investment; As a private company, the Company has not been required to document and test its internal controls over financial reporting nor has management been required to certify the effectiveness of its internal controls and its auditors have not been required to opine on the effectiveness of its internal control over financial reporting. As such, material weaknesses may be identified in the Company’s or the Post-Business Combination Company’s internal control over financial reporting that could lead to errors in the Post-Business Combination Company’s financial reporting, which could adversely affect the Post-Business Combination Company’s business and the market price of its securities; If the Post-Business Combination Company fails to maintain an effective system of disclosure controls and internal controls over financial reporting, its ability to produce timely and accurate financial statements or comply with applicable regulations could be impaired; If the Post-Business Combination Company’s estimates or judgments relating to its critical accounting standards prove to be incorrect, or such standards change over time, its results of operations could be adversely affected; Because the Post-Business Combination Company will become a publicly traded company by virtue of mergers in connection with the Business Combination as opposed to an underwritten initial public offering, there are no underwriters involved in the process, which could result in less diligence being conducted on the Company or the Post-Business Combination Company than in an underwritten initial public offering; The ability of ARYA’s public shareholders to exercise redemption rights with respect to a large number of ARYA’s public shares may not allow the Post-Business Combination Company to complete the most desirable business combination, fully fund the Company’s business plan, or changes thereto, or optimize the capital structure of the Post-Business Combination Company; Past performance by ARYA’s management team or their affiliates, including Perceptive Advisors, ARYA Sciences Acquisition Corp., ARYA Sciences Acquisition Corp II, ARYA Sciences Acquisition Corp III, or their respective business combination targets, may not be indicative of future performance of an investment in ARYA or the Post-Business Combination Company; The Post-Business Combination Company’s governing documents may include provisions that may discourage takeover attempts; The Company’s operating and financial results, which were presented to the ARYA board of directors, may not prove accurate; Activities taken by existing ARYA shareholders to increase the likelihood of approval of the Business Combination proposal and the other proposals to be described in the proxy statement/prospectus that will be filed in connection with the Business Combination could have a depressive effect on ARYA’s share price; PRIVATE AND CONFIDENTIAL

SELECTED RISK FACTORS (CONT.) Upon executing a definitive agreement with respect to the Business Combination by and among the Company, ARYA and the Post-Business Combination Company, ARYA may be prohibited from entering into certain transactions that might otherwise be beneficial to it or its shareholders; The Business Combination may be completed even though material adverse effects may result from the announcement of the Business Combination, industry wide changes, and other causes; Delays in completing the Business Combination may substantially reduce the expected benefits of the Business Combination; Adagio has, and the Post-Business Combination Company will have, broad discretion in the use of cash on hand and may not use it effectively. There is no guarantee that Adagio or the Post-Business Combination Company will have sufficient capital to fund the Post-Business Combination Company business plan through 2025 and Adagio’s or the Post-Business Combination Company’s anticipated cash runway through 2025 may be shorter than expected; The Post-Business Combination Company may not be able to remain compliant with the covenants of, and other obligations under, the senior secured convertible notes that will be issued in connection with the closing of the Business Combination. Risks Related to Adagio’s Business Adagio is a medical device company that has incurred net losses in every period to date and expects to continue to incur significant losses as it develops its business. Adagio’s growth prospects partially depend on its ability to accelerate the commercialization of its products and to capitalize on market opportunities. Adagio is dependent on the success of its pipeline portfolio, which remains in the development stage and subject to on-going scientific and technical validation. Even if Adagio is able to launch its pipeline portfolio successfully, it may experience material delays in its commercialization program relative to its current expectations. The commercialization of Adagio’s products will require Adagio to establish relationships and successfully collaborate with leading life science companies and research institutions. The life sciences technology market is highly competitive. Competitors include new entrants and established companies, many of which have significantly greater resources than Adagio. If Adagio fails to compete effectively, its business and results of operation will suffer. If Adagio is unable to establish manufacturing capacity by itself or with third-party partners in a timely and cost-effective manner, commercialization of its products would be delayed, which would result in lost revenue and harm its business. 41 If Adagio is unable to establish an effective network for commercialization, including effective distribution channels and sales and marketing functions, it may adversely affect its business, results of operations, financial condition and prospects. Adagio’s operating results may fluctuate significantly in the future, which makes its future operating results difficult to predict and could cause its operating results to fall below expectations or any guidance Adagio may provide. There is no assurance that Adagio will be able to execute on its business model, including achieving market acceptance of its products. Adoption of Adagio’s products depends upon appropriate physician training, practice and patient selection. Even if Adagio’s products are commercialized and achieve broad scientific and market acceptance, if Adagio fails to improve them or introduce compelling new products, its revenue and its prospects could be harmed. Adagio may need to raise additional capital to fund its development and commercialization plans. The size of the markets for Adagio’s products may be smaller than estimated, limiting Adagio’s ability to successfully sell its products. Adagio is dependent on limited third-party suppliers and manufacturers for some of the components and materials used in its products, and the loss of any of these suppliers and manufacturers, or any difficulties encountered by these suppliers and manufacturers in the production of Adagio’s products, could harm its business. If Adagio experiences a significant disruption in its information technology systems or security incidents, its business could be adversely affected, including its ability to operate, the loss of confidential and proprietary information, increased remediation costs, and reputational damage. Adagio may be unable to manage its anticipated growth effectively. Adagio may acquire other companies or technologies, or form strategic partnership with other companies, which could divert its management’s attention, increase its capital requirements, and otherwise disrupt its operations, subject it to other risks and harm its operating results. If Adagio is unable to recruit and retain key executives and scientists, it may be unable to achieve its goals. Adagio’s products could have unknown defects or errors, which may give rise to claims against it and adversely affect market adoption of its products. Consolidation in the medical device industry could have an adverse effect on Adagio’s revenue and results of operations. Since Adagio commercializes its products outside of the United States, its international business could expose it to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States. Unfavorable U.S. or global economic conditions as a result of the COVID-19 pandemic, political instability, natural disasters, or otherwise, could adversely affect Adagio’s ability to raise capital and its business, results of operations and financial condition. If Adagio fails to maintain an effective system of internal control over financial reporting, the Post-Combination Company may not be able to accurately report its financial results in a timely manner or prevent fraud, which would harm its business. PRIVATE AND CONFIDENTIAL

SELECTED RISK FACTORS (CONT.) If the Post-Combination Company estimates or judgments relating to its critical accounting policies are based on assumptions that change or prove to be incorrect, its results of operation could fall below its publicly announced guidance or the expectations of securities analysts and investors, resulting in a decline in the market price of its common stock. If Adagio’s facilities become unavailable or inoperable, Adagio’s research and development program and commercialization launch plan could be adversely affected, which could materially and adversely impact Adagio’s business and operations. Adagio uses hazardous chemicals and biological materials in its business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly. Risks Related to Adagio’s Intellectual Property If Adagio is unable to obtain and maintain sufficient intellectual property protection for its products and technology, or if the scope of the intellectual property protection obtained is not sufficiently broad, competitors could develop and commercialize products similar or identical to Adagio’s products, and Adagio’s ability to successfully commercialize its products may be impaired. The U.S. law relating to the patentability of certain inventions in the life sciences technology industry is uncertain and rapidly changing, which may adversely impact Adagio’s existing patents or its ability to obtain patents in the future. Adagio may not be able to protect its intellectual property rights throughout the world. Adagio may become involved in lawsuits to defend against third-party claims of infringement, misappropriation or other violations of intellectual property or to protect or enforce Adagio’s intellectual property, any of which could be expensive, time consuming and unsuccessful, and may prevent or delay its development and commercialization efforts. Issued patents covering Adagio’s products could be found invalid or unenforceable if challenged. If Adagio is unable to protect the confidentiality of its trade secrets, the value of its technology could be materially adversely affected and its business could be harmed. Adagio may not be able to protect and enforce its trademarks and trade names, or build name recognition in its markets of interest thereby harming its competitive position. Patent terms may be inadequate to protect Adagio’s competitive position of their products for an adequate amount of time. Obtaining and maintaining Adagio’s patent protection depends on compliance with various required procedures, document submissions, fee payments and other requirements imposed by governmental patent agencies, and Adagio’s patent protection could be reduced or eliminated for non-compliance with these requirements. Adagio may be subject to claims that its employees, consultants, independent contractors or any third parties that have access to Adagio’s confidential information or trade secrets have wrongfully used or disclosed confidential information of third parties or that its employees have wrongfully used or disclosed trade secrets of their former employers. If Adagio cannot license rights to use technologies on reasonable terms, it may not be able to commercialize new products in the future. Adagio’s use of open source software and failure to comply with the terms of the underlying open source software licenses could impose limitations on its ability to commercialize its products and provide third parties access to its proprietary software. Intellectual property rights do not necessarily address all potential threats. Risks Related to Regulatory and Legal Compliance Matters 42 Adagio expects to incur substantial expenses in its pursuit of regulatory clearances and approvals for its products in the United States and can provide no assurances that it will obtain the necessary approvals from the FDA to market its products in the United States. Adverse findings in post-marketing vigilance or regulatory audits could subject Adagio to suspension or withdrawal of its certificates of conformity, mandatory product recalls and significant legal liability, which could materially and adversely affect its business, financial condition and results of operations. Adagio may be subject to enforcement action if Adagio engages in marketing of its products pursuant to improper regulatory classifications in the EU, including suspension or withdrawal of its certificates of conformity, mandatory product recalls and significant legal liability, fines, penalties, and injunctions, which could materially and adversely affect its business, financial condition and results of operations. PRIVATE AND CONFIDENTIAL

SELECTED RISK FACTORS (CONT.) Adagio is subject to regulation by the FDA or other regulatory authorities in the future and would be required to obtain prior approval or clearance by the FDA or other regulatory authorities, which could take significant time and expense and could fail to result in FDA clearance or approval for the intended uses Adagio believes are commercially attractive. Adagio’s products are subject to government regulation as medical devices by the FDA and other regulatory agencies and the regulatory clearance or approval and the maintenance of continued and post-market regulatory compliance for such products will be expensive, time-consuming, and uncertain both in timing and in outcome. Adagio is currently subject to, and may in the future become subject to additional, U.S. federal and state laws and regulations imposing obligations on how Adagio collects, stores and processes personal information. Adagio’s actual or perceived failure to comply with such obligations could harm its business. Ensuring compliance with such laws could also impair Adagio’s efforts to maintain and expand its future customer base, and thereby decrease its revenue. If Adagio expands its development and commercialization activities outside of the United States, it will be subject to an increased risk of inadvertently conducting activities in a manner that violates the U.S. Foreign Corrupt Practices Act and similar laws. If that occurs, Adagio may be subject to civil or criminal penalties which could have a material adverse effect on its business, financial condition, results of operations and growth prospects. Adagio’s employees, independent contractors, consultants, commercial partners, distributors and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements. Adagio is or will be subject to anti-corruption and anti-bribery and anti-money laundering and similar laws, and non-compliance with such laws can subject it to administrative, civil and criminal fines and penalties, collateral consequences, remedial measures and legal expenses, all of which could adversely affect its reputation, business and results of operations. Risks Related to Litigation and Regulation Adagio is subject to evolving laws and regulations that could impose substantial costs, legal prohibitions or unfavorable changes upon its operations, and any failure to comply with these laws and regulations, including as they evolve, could result in litigation and substantially harm Adagio’s business and results of operations. Adagio is subject to risks relating to disputes and other legal proceedings, product liability lawsuits, that may be time consuming and costly. Adagio’s lack of a trade compliance program leaves certain regulatory trade risk inherent in international business unmitigated. If Adagio fails to comply with applicable international trade and sanctions regulations, Adagio may become subject to regulatory investigations, penalties, and fines. A trade compliance program including a screening process for customers, independent contractors, and other third parties would help avoid violations, and if a violation occurred, having a trade compliance program is often a mitigating factor in determining penalties. Risks Related to Financing Transactions ARYA and Adagio will incur significant transaction and transition costs in connection with the Business Combination. Whether or not the Business Combination is completed, the incurrence of these costs will reduce the amount of cash available to the Post-Business Combination Company for other corporate purposes. Adagio or the Post-Business Combination Company is subject to financing risks. There are no guarantees that Adagio or the Post-Business Combination Company can meet its financing needs for its operations and future investments at a reasonable cost or at all. Adagio or the Post-Business Combination Company is subject to risks relating to increased interest rates and any adverse developments in the credit markets. There are risks associated with the senior secured convertible notes that will be issued by the Post-Business Combination Company in connection with closing and the Post-Business Combination Company may be unable to remain in compliance with covenants or other obligations and restrictions under such convertible notes, which could materially impact the Post-Business Combination Company’s business, prospectus and plans or result in the Post-Business Combination Company’s bankruptcy or insolvency. Risks Related to Tax Unanticipated tax laws or any changes in tax rates or in the application of the existing tax laws to Adagio may adversely impact its results of operations. 43 PRIVATE AND CONFIDENTIAL

APPENDIX II – MARKET SOURCES & ANALYSES

ATRIAL FIBRILLATION
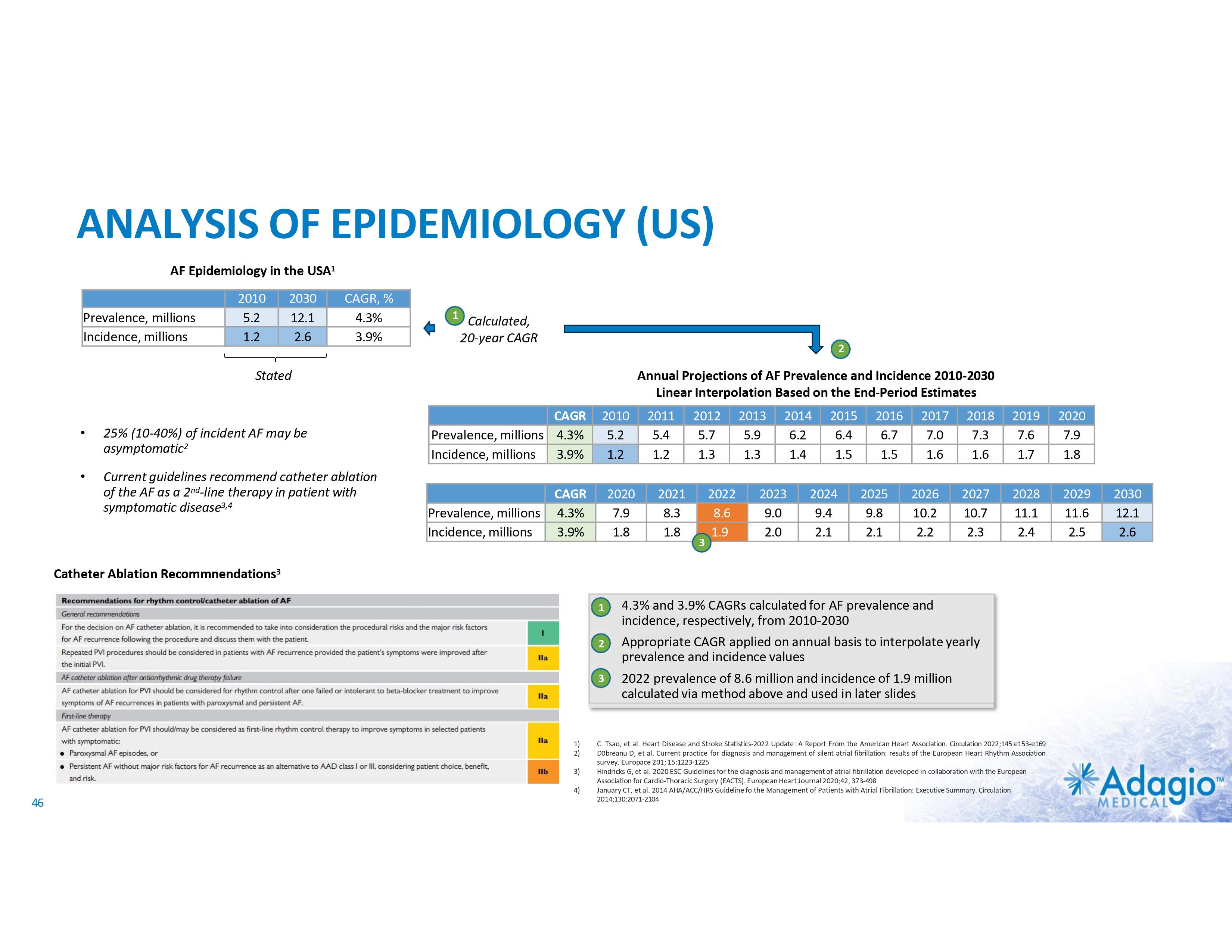
ANALYSIS OF EPIDEMIOLOGY (US) AF Epidemiology in the USA1 Prevalence, millions Incidence, millions 2010 5.2 1.2 2030 12.1 2.6 CAGR, % 4.3% 3.9% 1 Calculated, 20-year CAGR 2 Stated • 25% (10-40%) of incident AF may be asymptomatic2 • Current guidelines recommend catheter ablation of the AF as a 2nd-line therapy in patient with symptomatic disease3,4 Annual Projections of AF Prevalence and Incidence 2010-2030 Linear Interpolation Based on the End-Period Estimates CAGR Prevalence, millions 4.3% Incidence, millions 3.9% Prevalence, millions Incidence, millions 2010 5.2 1.2 CAGR 4.3% 3.9% 2020 7.9 1.8 2011 5.4 1.2 2021 8.3 1.8 2012 5.7 1.3 3 2022 8.6 1.9 2013 5.9 1.3 2014 6.2 1.4 2023 9.0 2.0 2015 6.4 1.5 2024 9.4 2.1 2016 6.7 1.5 2025 9.8 2.1 2017 7.0 1.6 2026 10.2 2.2 2018 7.3 1.6 2027 10.7 2.3 2019 7.6 1.7 2028 11.1 2.4 Catheter Ablation Recommnendations3 1 2 3 1) 2) 3) 4) 46 4.3% and 3.9% CAGRs calculated for AF prevalence and incidence, respectively, from 2010-2030 Appropriate CAGR applied on annual basis to interpolate yearly prevalence and incidence values 2022 prevalence of 8.6 million and incidence of 1.9 million calculated via method above and used in later slides C. Tsao, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153-e169 D0breanu D, et al. Current practice for diagnosis and management of silent atrial fibrillation: results of the European Heart Rhythm Association survey. Europace 201; 15:1223-1225 Hindricks G, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal 2020;42, 373-498 January CT, et al. 2014 AHA/ACC/HRS Guideline fo the Management of Patients with Atrial Fibrillation: Executive Summary. Circulation 2014;130:2071-2104 2020 7.9 1.8 2029 11.6 2.5 2030 12.1 2.6
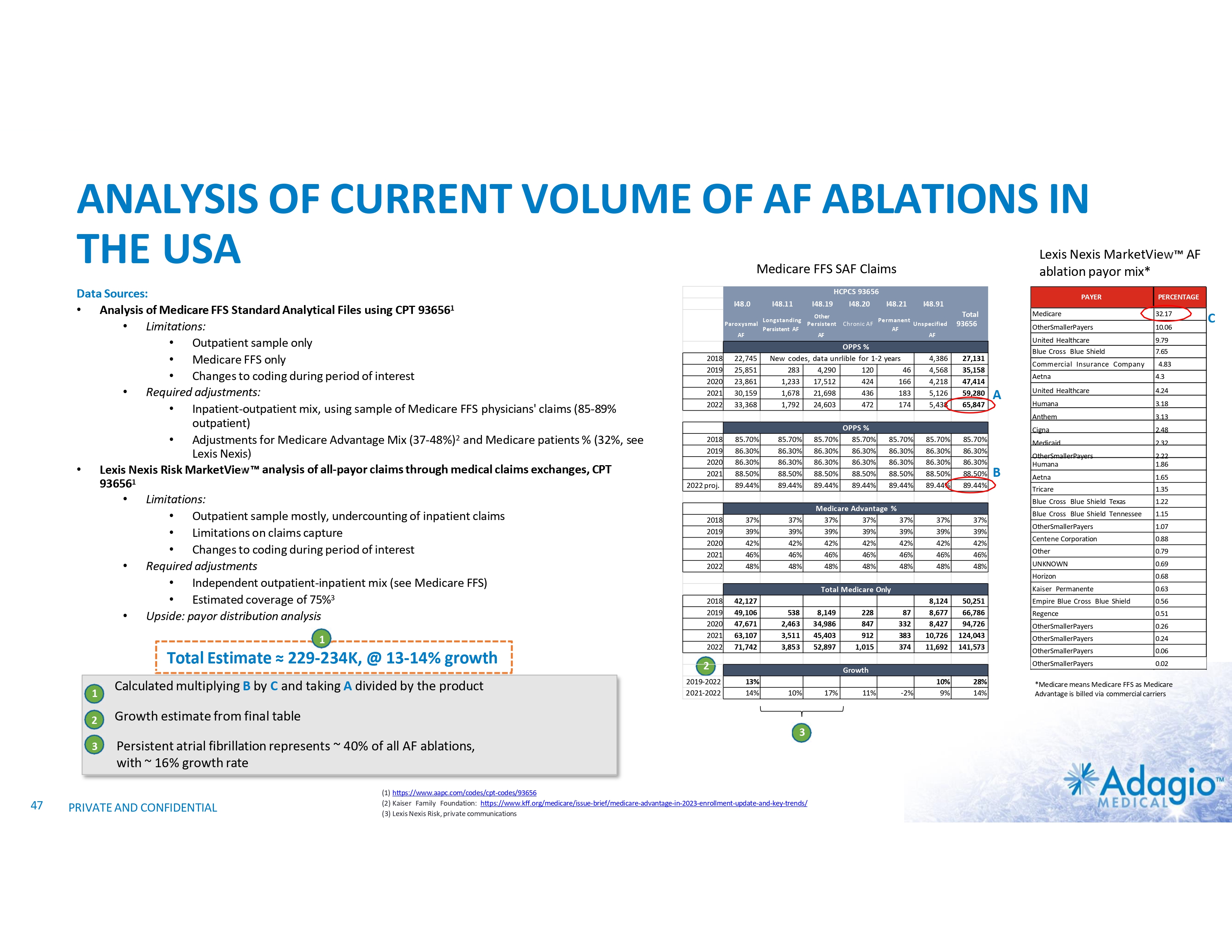
ANALYSIS OF CURRENT VOLUME OF AF ABLATIONS IN THE USA Lexis Nexis MarketView AF ablation payor mix* Medicare FFS SAF Claims Data Sources: • Analysis of Medicare FFS Standard Analytical Files using CPT 936561 • Limitations: • Outpatient sample only • Medicare FFS only • Changes to coding during period of interest • Required adjustments: • Inpatient-outpatient mix, using sample of Medicare FFS physicians' claims (85-89% outpatient) • Adjustments for Medicare Advantage Mix (37-48%)2 and Medicare patients % (32%, see Lexis Nexis) • Lexis Nexis Risk MarketView analysis of all-payor claims through medical claims exchanges, CPT 936561 • Limitations: • Outpatient sample mostly, undercounting of inpatient claims • Limitations on claims capture • Changes to coding during period of interest • Required adjustments • Independent outpatient-inpatient mix (see Medicare FFS) • Estimated coverage of 75%3 • Upside: payor distribution analysis 1 Total Estimate ≈ 229-234K, @ 13-14% growth •1 Calculated multiplying B by C and taking A divided by the product 2 Growth estimate from final table 3 Persistent atrial fibrillation represents ~ 40% of all AF ablations, with ~ 16% growth rate I48.0 I48.11 I48.19 Other Longstanding Paroxysmal Persistent Persistent AF AF AF 2018 2019 2020 2021 2022 2018 2019 2020 2021 2022 proj. 2018 2019 2020 2021 2022 22,745 25,851 23,861 30,159 33,368 85.70% 86.30% 86.30% 88.50% 89.44% 37% 39% 42% 46% 48% OPPS % data unrlible for 1-2 years 4,290 120 46 17,512 424 166 21,698 436 183 24,603 472 174 85.70% 86.30% 86.30% 88.50% 89.44% OPPS % 85.70% 86.30% 86.30% 88.50% 89.44% 37% 39% 42% 46% 48% PRIVATE AND CONFIDENTIAL 85.70% 86.30% 86.30% 88.50% 89.44% 85.70% 86.30% 86.30% 88.50% 89.44% Medicare Advantage % 37% 37% 37% 39% 39% 39% 42% 42% 42% 46% 46% 46% 48% 48% 48% PAYER I48.91 Permanent Chronic AF Unspecified AF AF New codes, 283 1,233 1,678 1,792 4,386 4,568 4,218 5,126 5,438 85.70% 86.30% 86.30% 88.50% 89.44% 37% 39% 42% 46% 48% Total 93656 27,131 35,158 47,414 59,280 65,847 85.70% 86.30% 86.30% 88.50% 89.44% 37% 39% 42% 46% 48% Total Medicare Only 2018 2019 2020 2021 2022 42,127 49,106 47,671 63,107 71,742 538 2,463 3,511 3,853 8,149 34,986 45,403 52,897 2 2019-2022 2021-2022 228 847 912 1,015 87 332 383 374 8,124 8,677 8,427 10,726 11,692 50,251 66,786 94,726 124,043 141,573 Growth 13% 14% 10% 3 47 HCPCS 93656 I48.20 I48.21 (1) https://www.aapc.com/codes/cpt-codes/93656 (2) Kaiser Family Foundation: https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2023-enrollment-update-and-key-trends/ (3) Lexis Nexis Risk, private communications 17% 11% -2% 10% 9% 28% 14% 32.17 OtherSmallerPayers 10.06 United Healthcare Blue Cross Blue Shield 9.79 7.65 Commercial Insurance Company Aetna A B PERCENTAGE Medicare 4.83 4.3 United Healthcare 4.24 Humana 3.18 Anthem 3.13 Cigna 2.48 Medicaid 2.32 OtherSmallerPayers Humana 2.22 1.86 Aetna 1.65 Tricare 1.35 Blue Cross Blue Shield Texas 1.22 Blue Cross Blue Shield Tennessee 1.15 OtherSmallerPayers 1.07 Centene Corporation 0.88 Other 0.79 UNKNOWN 0.69 Horizon 0.68 Kaiser Permanente 0.63 Empire Blue Cross Blue Shield 0.56 Regence 0.51 OtherSmallerPayers 0.26 OtherSmallerPayers 0.24 OtherSmallerPayers 0.06 OtherSmallerPayers 0.02 *Medicare means Medicare FFS as Medicare Advantage is billed via commercial carriers C
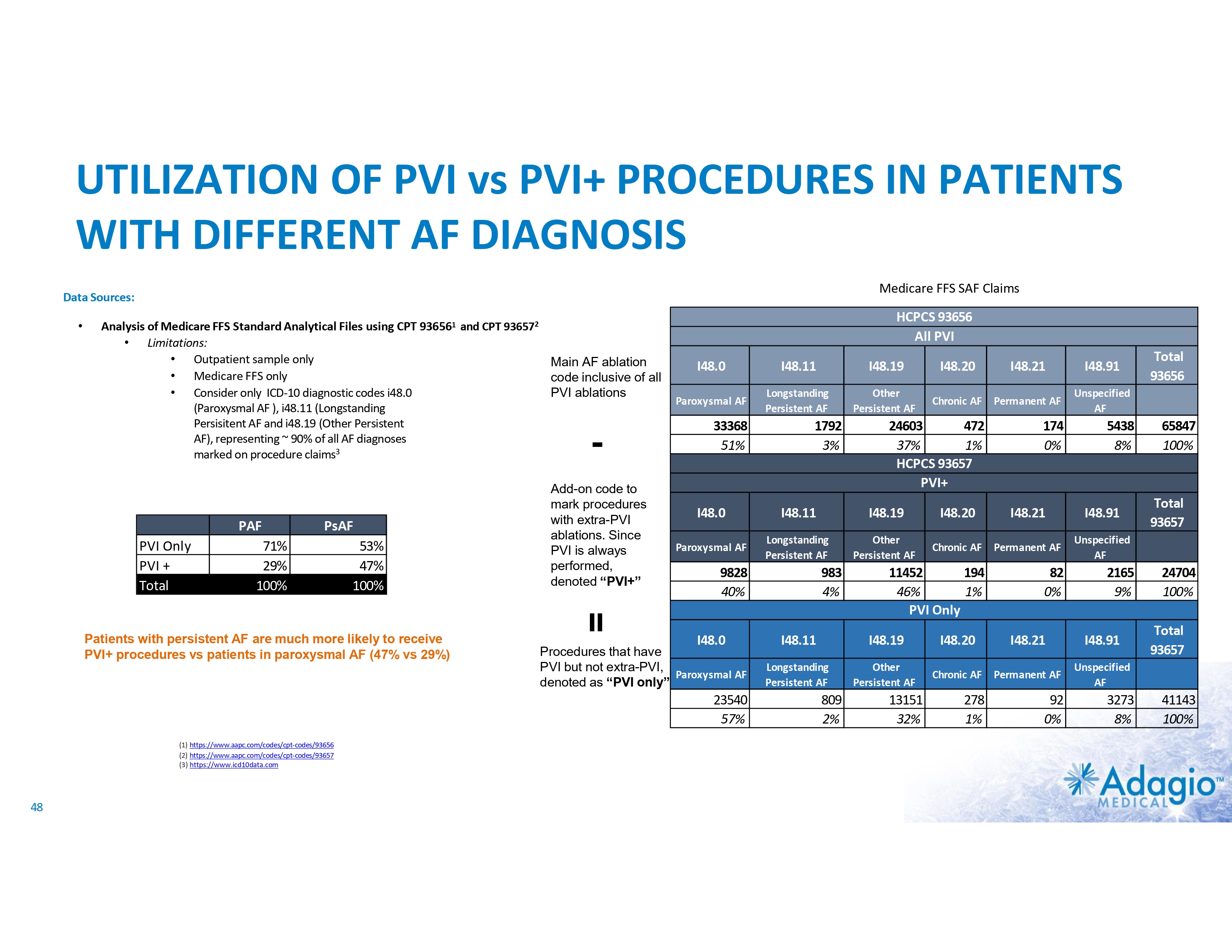
UTILIZATION OF PVI vs PVI+ PROCEDURES IN PATIENTS WITH DIFFERENT AF DIAGNOSIS Medicare FFS SAF Claims Data Sources: • Analysis of Medicare FFS Standard Analytical Files using CPT 936561 and CPT 936572 • Limitations: • Outpatient sample only Main AF ablation • Medicare FFS only code inclusive of all • Consider only ICD-10 diagnostic codes i48.0 PVI ablations (Paroxysmal AF ), i48.11 (Longstanding Persisitent AF and i48.19 (Other Persistent AF), representing ~ 90% of all AF diagnoses marked on procedure claims3 - PAF PVI Only PVI + Total PsAF 71% 29% 100% 53% 47% 100% I48.0 I48.11 I48.19 I48.20 I48.21 I48.91 Paroxysmal AF Longstanding Persistent AF Other Persistent AF Chronic AF Permanent AF Unspecified AF 24603 472 37% 1% HCPCS 93657 PVI+ 174 0% 33368 51% 48 5438 8% I48.0 I48.11 I48.19 I48.20 I48.21 I48.91 Paroxysmal AF Longstanding Persistent AF Other Persistent AF Chronic AF Permanent AF Unspecified AF 11452 194 46% 1% PVI Only 82 0% 9828 40% I48.0 Procedures that have PVI but not extra-PVI, Paroxysmal AF denoted as “PVI only” 23540 57% (1) https://www.aapc.com/codes/cpt-codes/93656 (2) https://www.aapc.com/codes/cpt-codes/93657 (3) https://www.icd10data.com 1792 3% 983 4% = Patients with persistent AF are much more likely to receive PVI+ procedures vs patients in paroxysmal AF (47% vs 29%) Add-on code to mark procedures with extra-PVI ablations. Since PVI is always performed, denoted “PVI+” HCPCS 93656 All PVI 2165 9% I48.11 I48.19 I48.20 I48.21 I48.91 Longstanding Persistent AF Other Persistent AF Chronic AF Permanent AF Unspecified AF 278 1% 92 0% 809 2% 13151 32% 3273 8% Total 93656 65847 100% Total 93657 24704 100% Total 93657 41143 100%

MARKET OPPORTUNITY ASSESSMENT Assumptions: • Outcomes with Adagio technology are sufficient to establish ablation as a 1st line therapy for symptomatic patients • Calculation to be made on incidence basis, larger prevalence representing additional upside Opportunity in 2022-2023: • Total incidence / current ablation volume: 8.3x(1) • Discounting for lower number (75%)(2) of symptomatic patients: 6.2x Total Market Growth Opportunity: 6-8x (1) (2) 49 Calculated as 1.9 million incidence divided by 0.23 million current ablation volume. Management estimate based on Gibbs H, et al. Clinical Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation Presentations in GARFIELD-AF: Implications for AF Screening. The American Journal of Medicine 2021;134:893−901
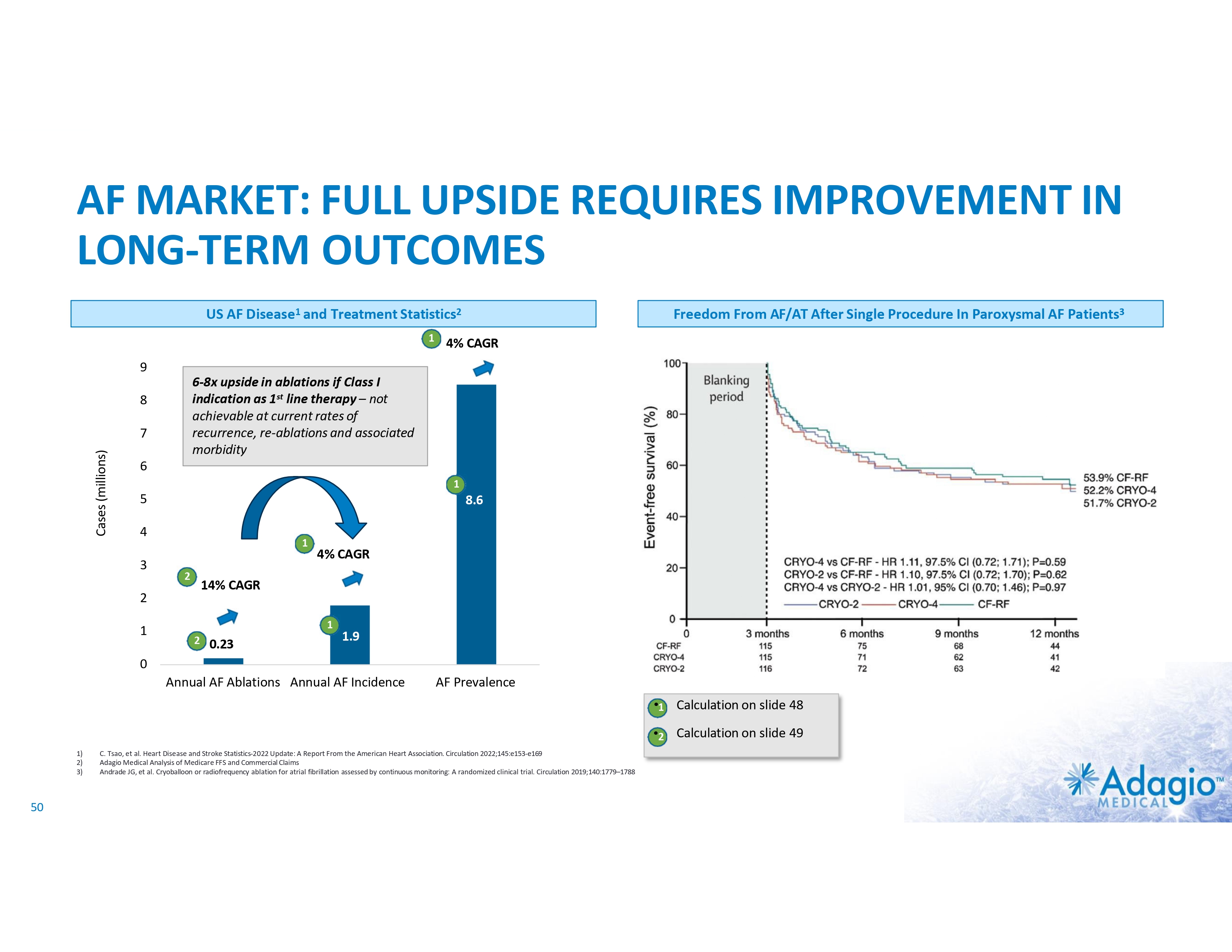
AF MARKET: FULL UPSIDE REQUIRES IMPROVEMENT IN LONG-TERM OUTCOMES US AF Disease1 and Treatment Statistics2 1 Freedom From AF/AT After Single Procedure In Paroxysmal AF Patients3 4% CAGR 9 6-8x upside in ablations if Class I indication as 1st line therapy – not achievable at current rates of recurrence, re-ablations and associated morbidity 8 Cases (millions) 7 6 1 5 8.6 4 3 1 2 14% CAGR 2 1 4% CAGR 1 2 0.23 1.9 0 Annual AF Ablations Annual AF Incidence AF Prevalence •1 Calculation on slide 48 •2 Calculation on slide 49 1) 2) 3) 50 C. Tsao, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153-e169 Adagio Medical Analysis of Medicare FFS and Commercial Claims Andrade JG, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: A randomized clinical trial. Circulation 2019;140:1779–1788

MONOMORPHIC VENTRICULAR TACHYCARDIA 51
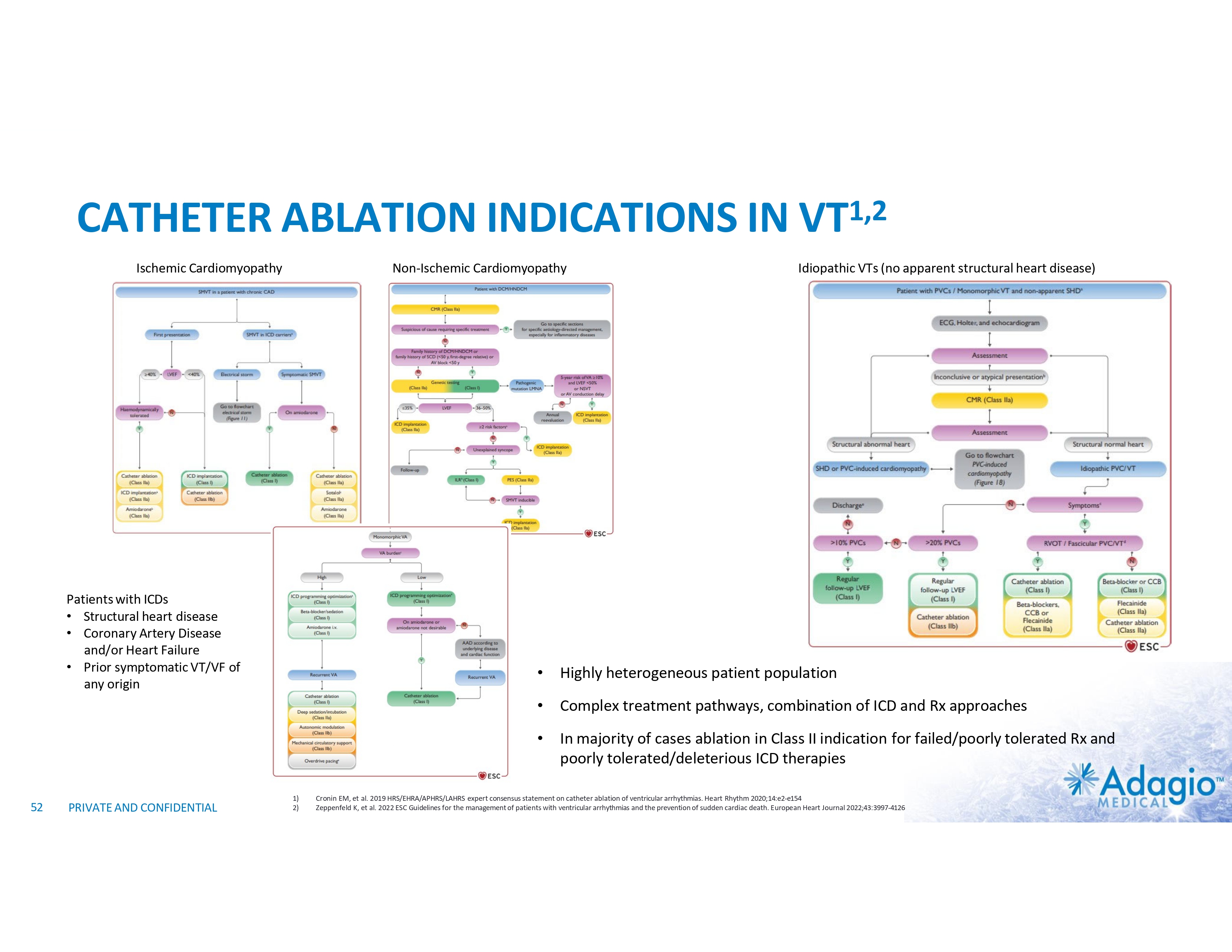
CATHETER ABLATION INDICATIONS IN VT1,2 Ischemic Cardiomyopathy Non-Ischemic Cardiomyopathy Patients with ICDs • Structural heart disease • Coronary Artery Disease and/or Heart Failure • Prior symptomatic VT/VF of any origin 52 PRIVATE AND CONFIDENTIAL 1) 2) Idiopathic VTs (no apparent structural heart disease) • Highly heterogeneous patient population • Complex treatment pathways, combination of ICD and Rx approaches • In majority of cases ablation in Class II indication for failed/poorly tolerated Rx and poorly tolerated/deleterious ICD therapies Cronin EM, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020;14:e2-e154 Zeppenfeld K, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. European Heart Journal 2022;43:3997-4126
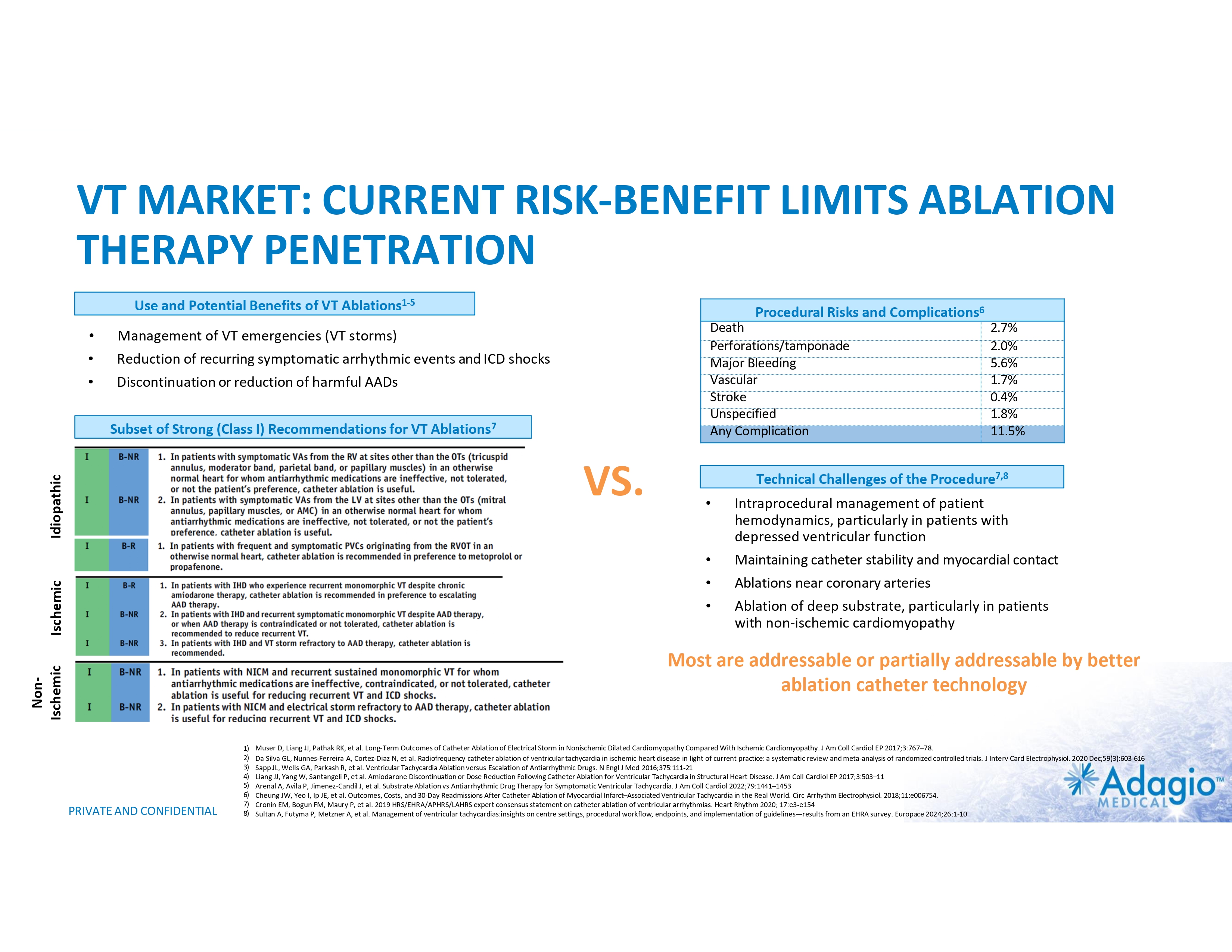
VT MARKET: CURRENT RISK-BENEFIT LIMITS ABLATION THERAPY PENETRATION Use and Potential Benefits of VT Ablations1-5 • Management of VT emergencies (VT storms) • • Reduction of recurring symptomatic arrhythmic events and ICD shocks Discontinuation or reduction of harmful AADs Procedural Risks and Complications6 Death Perforations/tamponade Major Bleeding Vascular Stroke Unspecified Any Complication Subset of Strong (Class I) Recommendations for VT Ablations7 Idiopathic VS. Technical Challenges of the Procedure7,8 • • • • Ischemic 2.7% 2.0% 5.6% 1.7% 0.4% 1.8% 11.5% Intraprocedural management of patient hemodynamics, particularly in patients with depressed ventricular function Maintaining catheter stability and myocardial contact Ablations near coronary arteries Ablation of deep substrate, particularly in patients with non-ischemic cardiomyopathy Non- Ischemic Most are addressable or partially addressable by better ablation catheter technology PRIVATE AND CONFIDENTIAL 1) 2) 3) 4) 5) 6) 7) 8) Muser D, Liang JJ, Pathak RK, et al. Long-Term Outcomes of Catheter Ablation of Electrical Storm in Nonischemic Dilated Cardiomyopathy Compared With Ischemic Cardiomyopathy. J Am Coll Cardiol EP 2017;3:767–78. Da Silva GL, Nunnes-Ferreira A, Cortez-Diaz N, et al. Radiofrequency catheter ablation of ventricular tachycardia in ischemic heart disease in light of current practice: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2020 Dec;59(3):603-616 Sapp JL, Wells GA, Parkash R, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 2016;375:111-21 Liang JJ, Yang W, Santangeli P, et al. Amiodarone Discontinuation or Dose Reduction Following Catheter Ablation for Ventricular Tachycardia in Structural Heart Disease. J Am Coll Cardiol EP 2017;3:503–11 Arenal A, Avila P, Jimenez-Candil J, et al. Substrate Ablation vs Antiarrhythmic Drug Therapy for Symptomatic Ventricular Tachycardia. J Am Coll Cardiol 2022;79:1441–1453 Cheung JW, Yeo I, Ip JE, et al. Outcomes, Costs, and 30-Day Readmissions After Catheter Ablation of Myocardial Infarct–Associated Ventricular Tachycardia in the Real World. Circ Arrhythm Electrophysiol. 2018;11:e006754. Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020; 17:e3-e154 Sultan A, Futyma P, Metzner A, et al. Management of ventricular tachycardias:insights on centre settings, procedural workflow, endpoints, and implementation of guidelines—results from an EHRA survey. Europace 2024;26:1-10
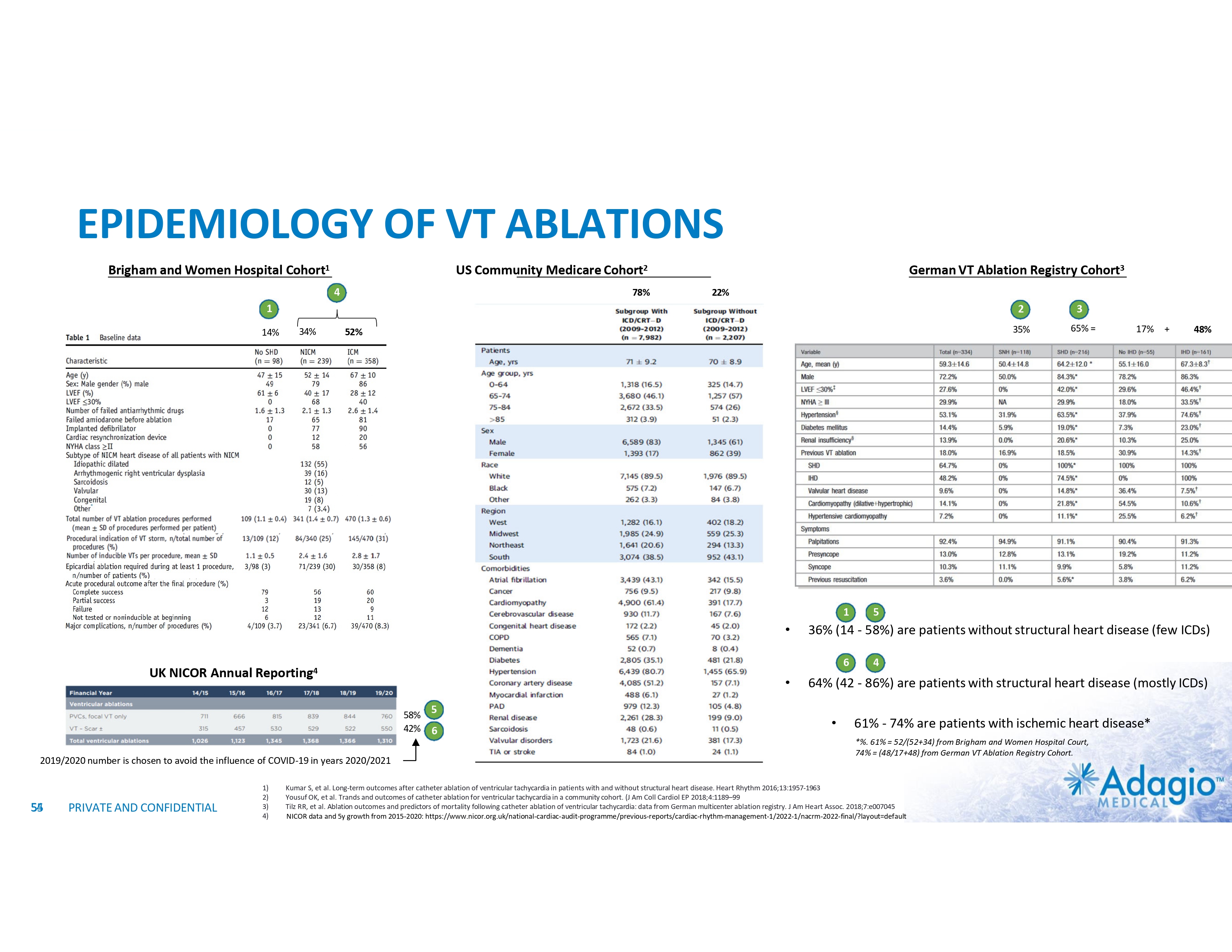
EPIDEMIOLOGY OF VT ABLATIONS Brigham and Women Hospital Cohort1 US Community Medicare Cohort2 4 78% German VT Ablation Registry Cohort3 22% 1 14% 2 34% 35% 52% 1 58% 42% 2019/2020 number is chosen to avoid the influence of COVID-19 in years 2020/2021 54 55 PRIVATE AND CONFIDENTIAL 1) 2) 3) 4) 5 6 17% + 48% 5 • 36% (14 - 58%) are patients without structural heart disease (few ICDs) • 64% (42 - 86%) are patients with structural heart disease (mostly ICDs) 6 UK NICOR Annual Reporting4 3 65% = • 4 61% - 74% are patients with ischemic heart disease* *%. 61% = 52/(52+34) from Brigham and Women Hospital Court, 74% = (48/17+48) from German VT Ablation Registry Cohort. Kumar S, et al. Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm 2016;13:1957-1963 Yousuf OK, et al. Trands and outcomes of catheter ablation for ventricular tachycardia in a community cohort. (J Am Coll Cardiol EP 2018;4:1189–99 Tilz RR, et al. Ablation outcomes and predictors of mortality following catheter ablation of ventricular tachycardia: data from German multicenter ablation registry. J Am Heart Assoc. 2018;7:e007045 NICOR data and 5y growth from 2015-2020: https://www.nicor.org.uk/national-cardiac-audit-programme/previous-reports/cardiac-rhythm-management-1/2022-1/nacrm-2022-final/?layout=default
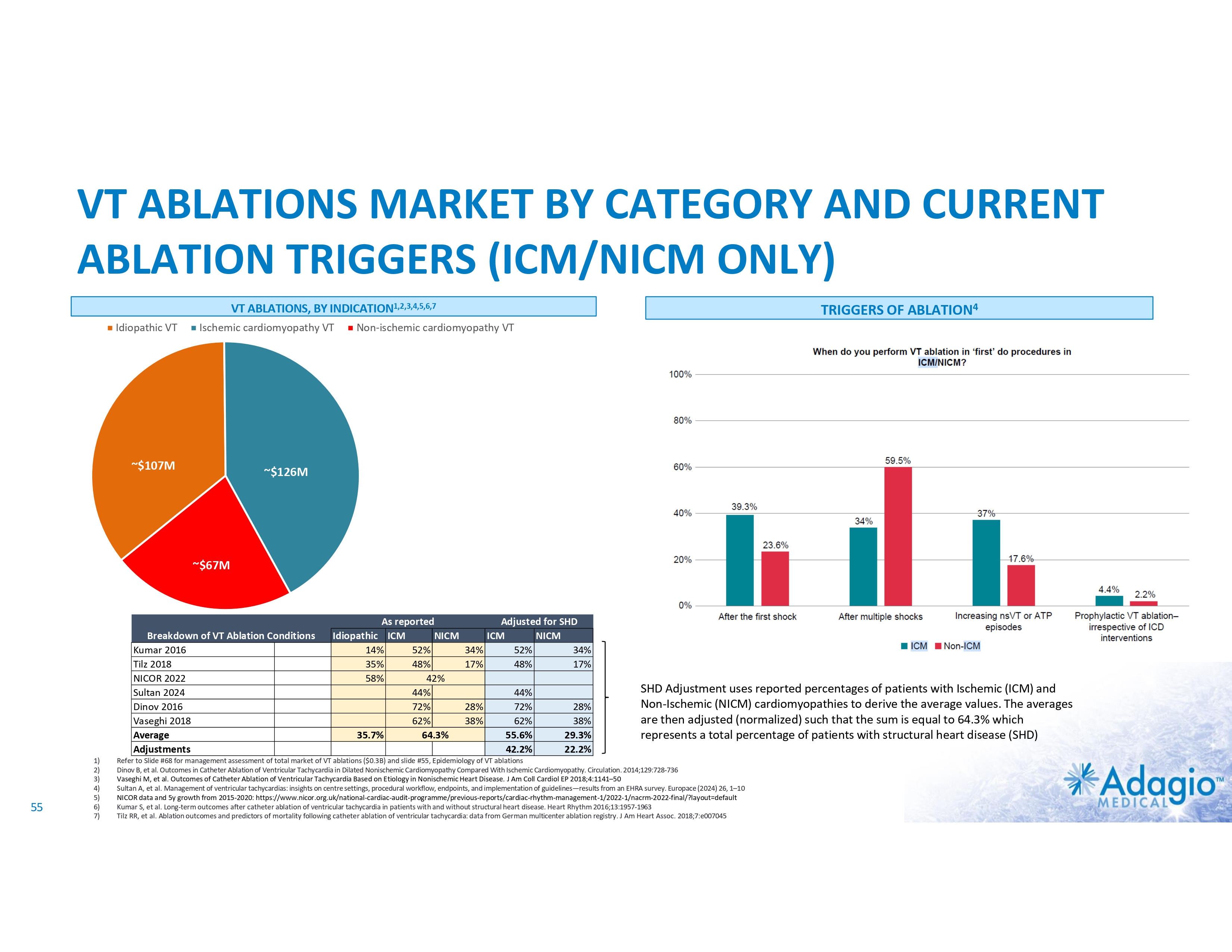
VT ABLATIONS MARKET BY CATEGORY AND CURRENT ABLATION TRIGGERS (ICM/NICM ONLY) VT ABLATIONS, BY INDICATION1,2,3,4,5,6,7 Idiopathic VT ~$107M Ischemic cardiomyopathy VT TRIGGERS OF ABLATION4 Non-ischemic cardiomyopathy VT $150M ~$126M ~$67M ~$75M Breakdown of VT Ablation Conditions Kumar 2016 Tilz 2018 NICOR 2022 Sultan 2024 Dinov 2016 Vaseghi 2018 Average Adjustments 55 1) 2) 3) 4) 5) 6) 7) As reported Idiopathic ICM NICM 14% 52% 35% 48% 58% 42% 44% 72% 62% 35.7% 64.3% Adjusted for SHD ICM NICM 34% 52% 34% 17% 48% 17% 28% 38% 44% 72% 62% 55.6% 42.2% 28% 38% 29.3% 22.2% SHD Adjustment uses reported percentages of patients with Ischemic (ICM) and Non-Ischemic (NICM) cardiomyopathies to derive the average values. The averages are then adjusted (normalized) such that the sum is equal to 64.3% which represents a total percentage of patients with structural heart disease (SHD) Refer to Slide #68 for management assessment of total market of VT ablations ($0.3B) and slide #55, Epidemiology of VT ablations Dinov B, et al. Outcomes in Catheter Ablation of Ventricular Tachycardia in Dilated Nonischemic Cardiomyopathy Compared With Ischemic Cardiomyopathy. Circulation. 2014;129:728-736 Vaseghi M, et al. Outcomes of Catheter Ablation of Ventricular Tachycardia Based on Etiology in Nonischemic Heart Disease. J Am Coll Cardiol EP 2018;4:1141–50 Sultan A, et al. Management of ventricular tachycardias: insights on centre settings, procedural workflow, endpoints, and implementation of guidelines—results from an EHRA survey. Europace (2024) 26, 1–10 NICOR data and 5y growth from 2015-2020: https://www.nicor.org.uk/national-cardiac-audit-programme/previous-reports/cardiac-rhythm-management-1/2022-1/nacrm-2022-final/?layout=default Kumar S, et al. Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm 2016;13:1957-1963 Tilz RR, et al. Ablation outcomes and predictors of mortality following catheter ablation of ventricular tachycardia: data from German multicenter ablation registry. J Am Heart Assoc. 2018;7:e007045
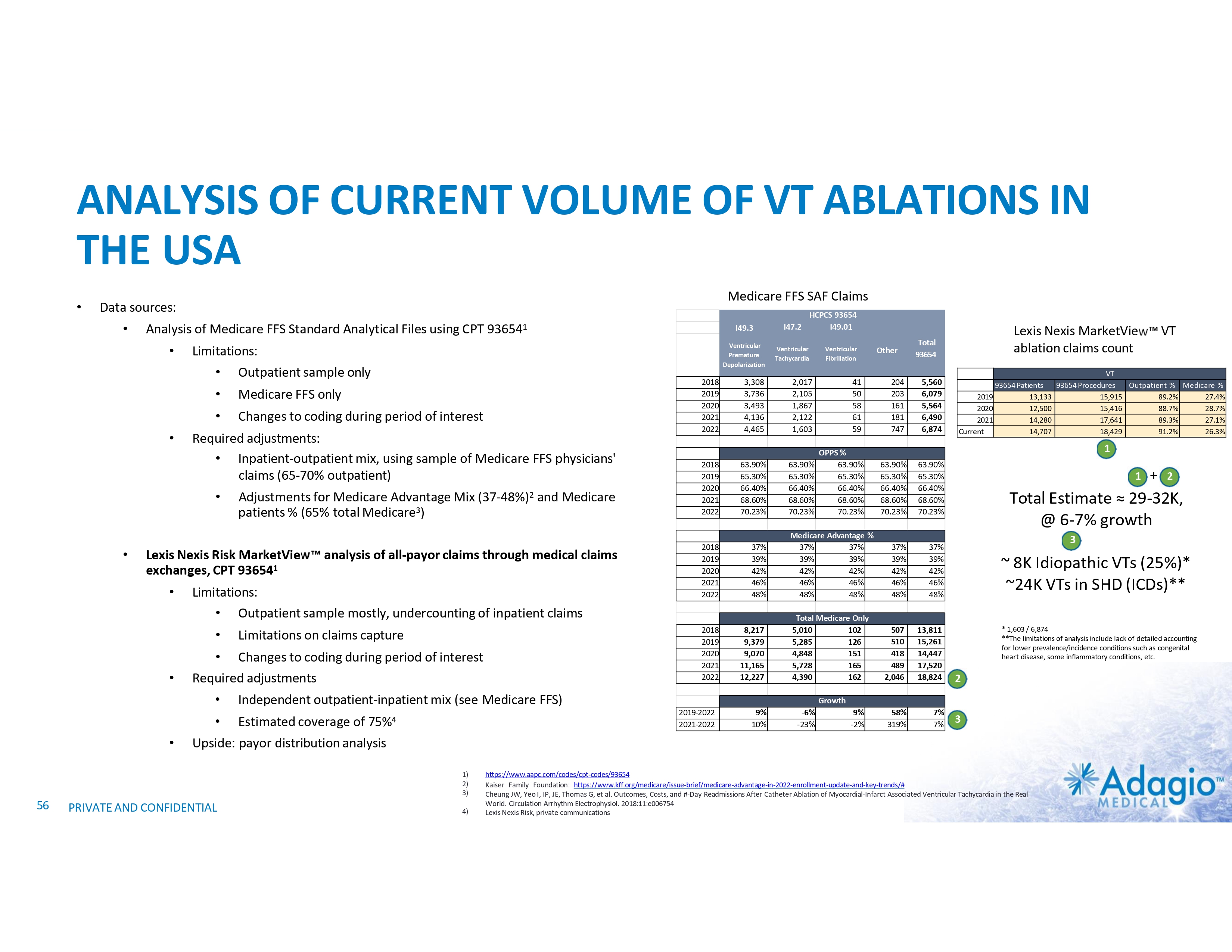
ANALYSIS OF CURRENT VOLUME OF VT ABLATIONS IN THE USA • Medicare FFS SAF Claims Data sources: • Analysis of Medicare FFS Standard Analytical Files using CPT 936541 • • Limitations: • Outpatient sample only • Medicare FFS only • Changes to coding during period of interest Required adjustments: • Inpatient-outpatient mix, using sample of Medicare FFS physicians' claims (65-70% outpatient) • • Adjustments for Medicare Advantage Mix (37-48%)2 and Medicare patients % (65% total Medicare3) Lexis Nexis Risk MarketView analysis of all-payor claims through medical claims exchanges, CPT 936541 • Limitations: • Outpatient sample mostly, undercounting of inpatient claims • • • Limitations on claims capture • Changes to coding during period of interest Required adjustments • Independent outpatient-inpatient mix (see Medicare FFS) • Estimated coverage of 75%4 I47.2 Ventricular Premature Depolarization Ventricular Tachycardia 2018 2019 2020 2021 2022 3,308 3,736 3,493 4,136 4,465 2,017 2,105 1,867 2,122 1,603 2018 2019 2020 2021 2022 63.90% 65.30% 66.40% 68.60% 70.23% 63.90% 65.30% 66.40% 68.60% 70.23% 2018 2019 2020 2021 2022 37% 39% 42% 46% 48% 2018 2019 2020 2021 2022 8,217 9,379 9,070 11,165 12,227 Ventricular Fibrillation 41 50 58 61 59 OPPS % 63.90% 65.30% 66.40% 68.60% 70.23% Medicare Advantage % 37% 37% 39% 39% 42% 42% 46% 46% 48% 48% Total Medicare Only 5,010 102 5,285 126 4,848 151 5,728 165 4,390 162 Other Lexis Nexis MarketView VT ablation claims count Total 93654 VT 93654 Patients 93654 Procedures Outpatient % Medicare % 2019 13,133 15,915 89.2% 27.4% 2020 12,500 15,416 88.7% 28.7% 2021 14,280 17,641 89.3% 27.1% Current 14,707 18,429 91.2% 26.3% 204 203 161 181 747 5,560 6,079 5,564 6,490 6,874 63.90% 65.30% 66.40% 68.60% 70.23% 63.90% 65.30% 66.40% 68.60% 70.23% 37% 39% 42% 46% 48% 37% 39% 42% 46% 48% 507 510 418 489 2,046 13,811 15,261 14,447 17,520 18,824 2 58% 319% 7% 7% 3 1 1 9% 10% -6% -23% 9% -2% 4) 2 3 ~ 8K Idiopathic VTs (25%)* ~24K VTs in SHD (ICDs)** * 1,603 / 6,874 **The limitations of analysis include lack of detailed accounting for lower prevalence/incidence conditions such as congenital heart disease, some inflammatory conditions, etc. Upside: payor distribution analysis PRIVATE AND CONFIDENTIAL + Total Estimate ≈ 29-32K, @ 6-7% growth Growth 2019-2022 2021-2022 1) 2) 3) 56 HCPCS 93654 I49.01 I49.3 https://www.aapc.com/codes/cpt-codes/93654 Kaiser Family Foundation: https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2022-enrollment-update-and-key-trends/# Cheung JW, Yeo I, IP, JE, Thomas G, et al. Outcomes, Costs, and #-Day Readmissions After Catheter Ablation of Myocardial-Infarct Associated Ventricular Tachycardia in the Real World. Circulation Arrhythm Electrophysiol. 2018:11:e006754 Lexis Nexis Risk, private communications
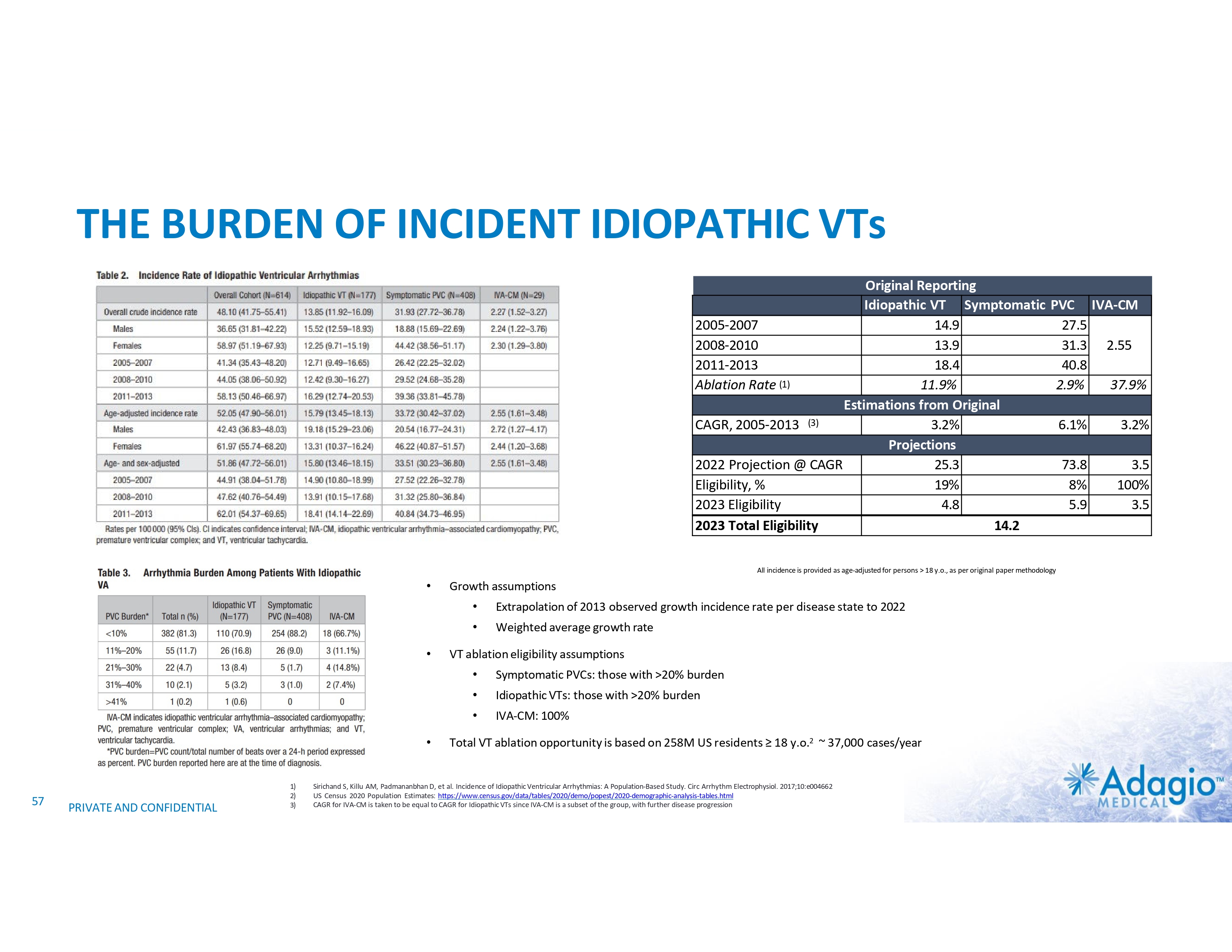
THE BURDEN OF INCIDENT IDIOPATHIC VTs Original Reporting Idiopathic VT Symptomatic PVC IVA-CM 2005-2007 14.9 27.5 2008-2010 13.9 31.3 2.55 2011-2013 18.4 40.8 Ablation Rate (1) 11.9% 2.9% 37.9% Estimations from Original CAGR, 2005-2013 (3) 3.2% 6.1% 3.2% Projections 2022 Projection @ CAGR 25.3 73.8 3.5 Eligibility, % 19% 8% 100% 2023 Eligibility 4.8 5.9 3.5 2023 Total Eligibility 14.2 All incidence is provided as age-adjusted for persons > 18 y.o., as per original paper methodology • • • 57 PRIVATE AND CONFIDENTIAL 1) 2) 3) Growth assumptions • Extrapolation of 2013 observed growth incidence rate per disease state to 2022 • Weighted average growth rate VT ablation eligibility assumptions • Symptomatic PVCs: those with >20% burden • Idiopathic VTs: those with >20% burden • IVA-CM: 100% Total VT ablation opportunity is based on 258M US residents ≥ 18 y.o.2 ~ 37,000 cases/year Sirichand S, Killu AM, Padmananbhan D, et al. Incidence of Idiopathic Ventricular Arrhythmias: A Population-Based Study. Circ Arrhythm Electrophysiol. 2017;10:e004662 US Census 2020 Population Estimates: https://www.census.gov/data/tables/2020/demo/popest/2020-demographic-analysis-tables.html CAGR for IVA-CM is taken to be equal to CAGR for Idiopathic VTs since IVA-CM is a subset of the group, with further disease progression
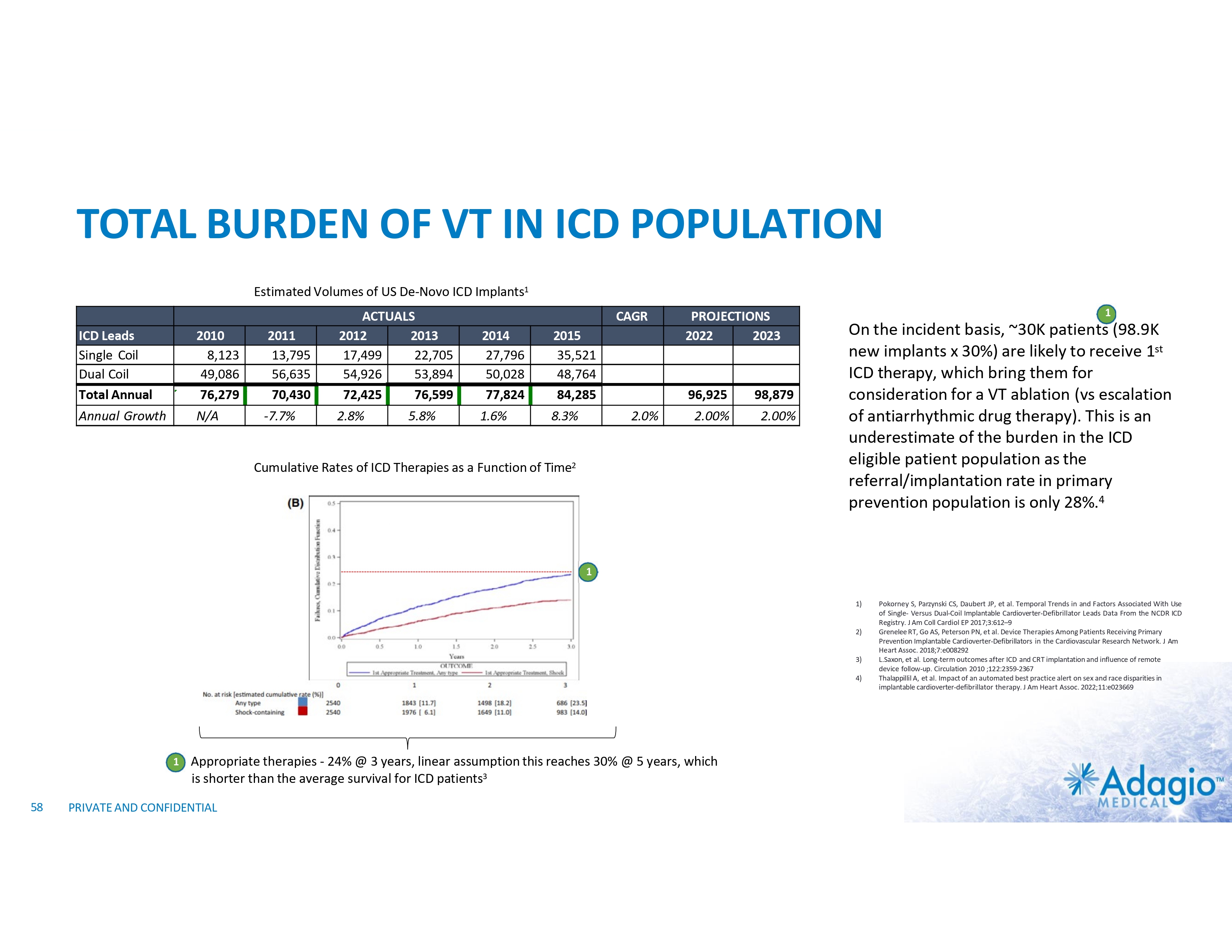
TOTAL BURDEN OF VT IN ICD POPULATION Estimated Volumes of US De-Novo ICD Implants1 ICD Leads Single Coil Dual Coil Total Annual 2010 8,123 49,086 76,279 2011 13,795 56,635 70,430 ACTUALS 2012 2013 17,499 22,705 54,926 53,894 72,425 76,599 Annual Growth N/A -7.7% 2.8% 5.8% CAGR 2014 27,796 50,028 77,824 2015 35,521 48,764 84,285 1.6% 8.3% 2.0% PROJECTIONS 2022 2023 96,925 98,879 2.00% 2.00% Cumulative Rates of ICD Therapies as a Function of Time2 1 On the incident basis, ~30K patients (98.9K new implants x 30%) are likely to receive 1st ICD therapy, which bring them for consideration for a VT ablation (vs escalation of antiarrhythmic drug therapy). This is an underestimate of the burden in the ICD eligible patient population as the referral/implantation rate in primary prevention population is only 28%.4 1 1) 2) 3) 4) 1 58 Appropriate therapies - 24% @ 3 years, linear assumption this reaches 30% @ 5 years, which is shorter than the average survival for ICD patients3 PRIVATE AND CONFIDENTIAL Pokorney S, Parzynski CS, Daubert JP, et al. Temporal Trends in and Factors Associated With Use of Single- Versus Dual-Coil Implantable Cardioverter-Defibrillator Leads Data From the NCDR ICD Registry. J Am Coll Cardiol EP 2017;3:612–9 Grenelee RT, Go AS, Peterson PN, et al. Device Therapies Among Patients Receiving Primary Prevention Implantable Cardioverter-Defibrillators in the Cardiovascular Research Network. J Am Heart Assoc. 2018;7:e008292 L.Saxon, et al. Long-term outcomes after ICD and CRT implantation and influence of remote device follow-up. Circulation 2010 ;122:2359-2367 Thalappillil A, et al. Impact of an automated best practice alert on sex and race disparities in implantable cardioverter-defibrillator therapy. J Am Heart Assoc. 2022;11:e023669
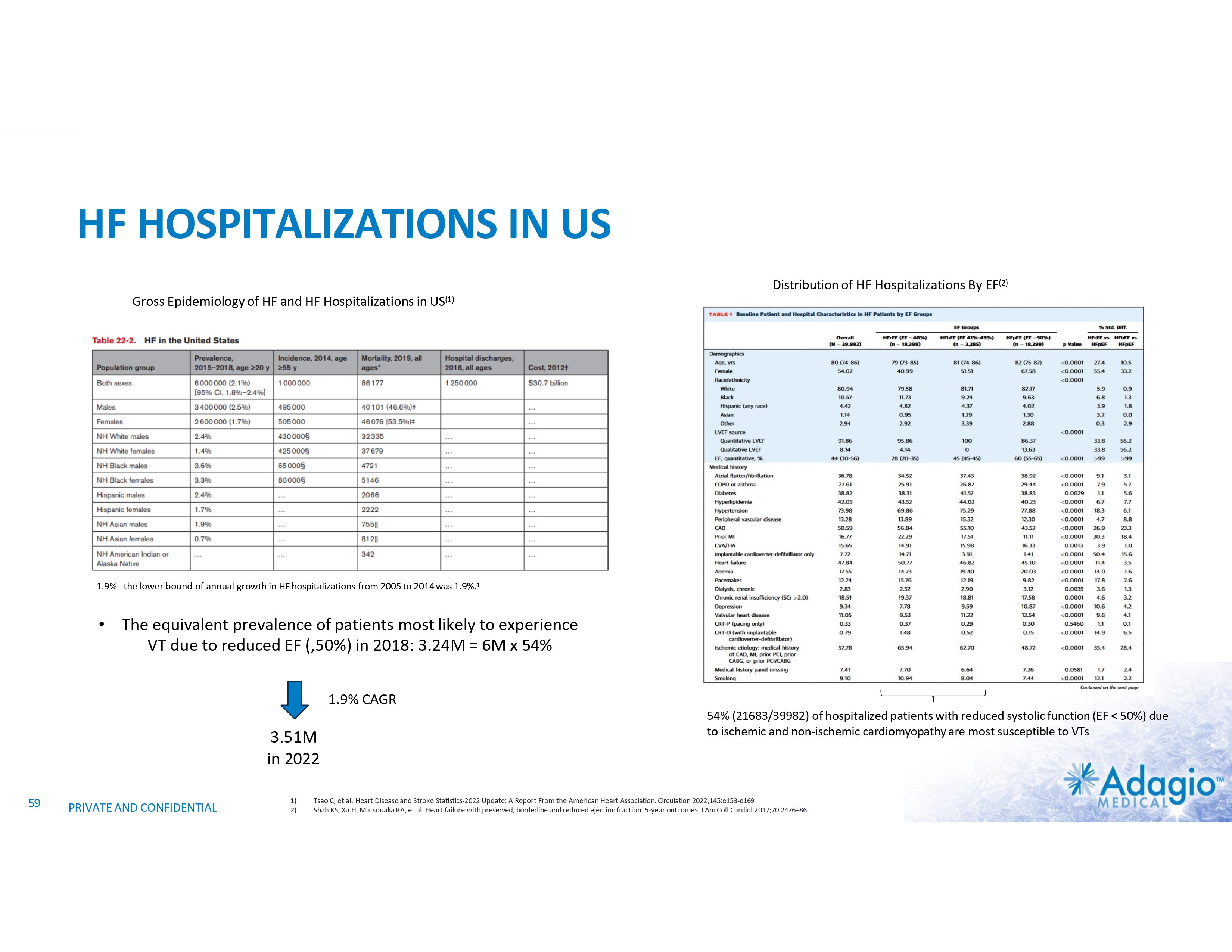
HF HOSPITALIZATIONS IN US Distribution of HF Hospitalizations By EF(2) Gross Epidemiology of HF and HF Hospitalizations in US(1) 1.9% - the lower bound of annual growth in HF hospitalizations from 2005 to 2014 was 1.9%.1 • The equivalent prevalence of patients most likely to experience VT due to reduced EF (,50%) in 2018: 3.24M = 6M x 54% 1.9% CAGR 3.51M in 2022 59 PRIVATE AND CONFIDENTIAL 1) 2) 54% (21683/39982) of hospitalized patients with reduced systolic function (EF < 50%) due to ischemic and non-ischemic cardiomyopathy are most susceptible to VTs Tsao C, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153-e169 Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol 2017;70:2476–86
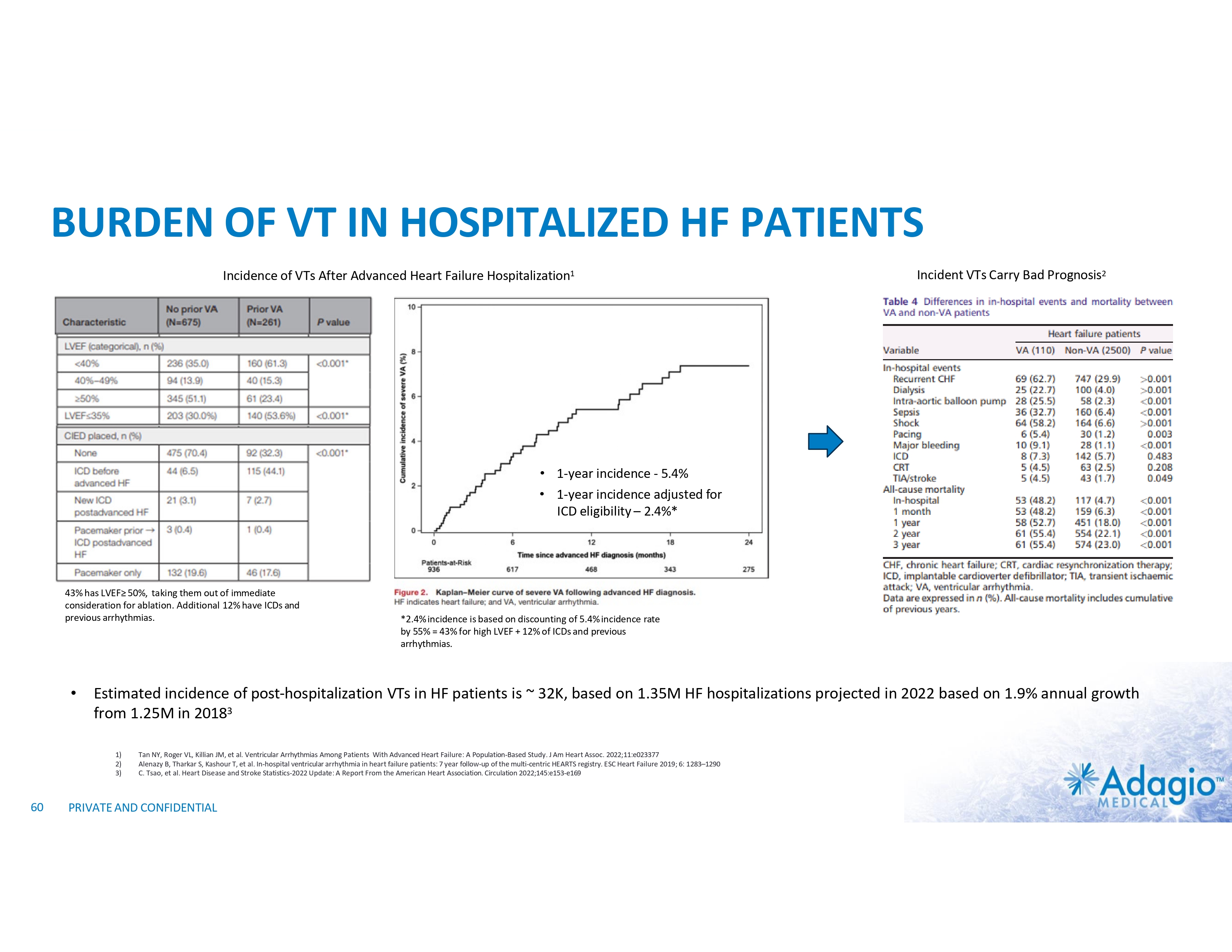
BURDEN OF VT IN HOSPITALIZED HF PATIENTS Incidence of VTs After Advanced Heart Failure Hospitalization1 Incident VTs Carry Bad Prognosis2 • 1-year incidence - 5.4% • 1-year incidence adjusted for ICD eligibility – 2.4%* 43% has LVEF≥ 50%, taking them out of immediate consideration for ablation. Additional 12% have ICDs and previous arrhythmias. • Estimated incidence of post-hospitalization VTs in HF patients is ~ 32K, based on 1.35M HF hospitalizations projected in 2022 based on 1.9% annual growth from 1.25M in 20183 1) 2) 3) 60 *2.4% incidence is based on discounting of 5.4% incidence rate by 55% = 43% for high LVEF + 12% of ICDs and previous arrhythmias. Tan NY, Roger VL, Killian JM, et al. Ventricular Arrhythmias Among Patients With Advanced Heart Failure: A Population-Based Study. J Am Heart Assoc. 2022;11:e023377 Alenazy B, Tharkar S, Kashour T, et al. In-hospital ventricular arrhythmia in heart failure patients: 7 year follow-up of the multi-centric HEARTS registry. ESC Heart Failure 2019; 6: 1283–1290 C. Tsao, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153-e169 PRIVATE AND CONFIDENTIAL
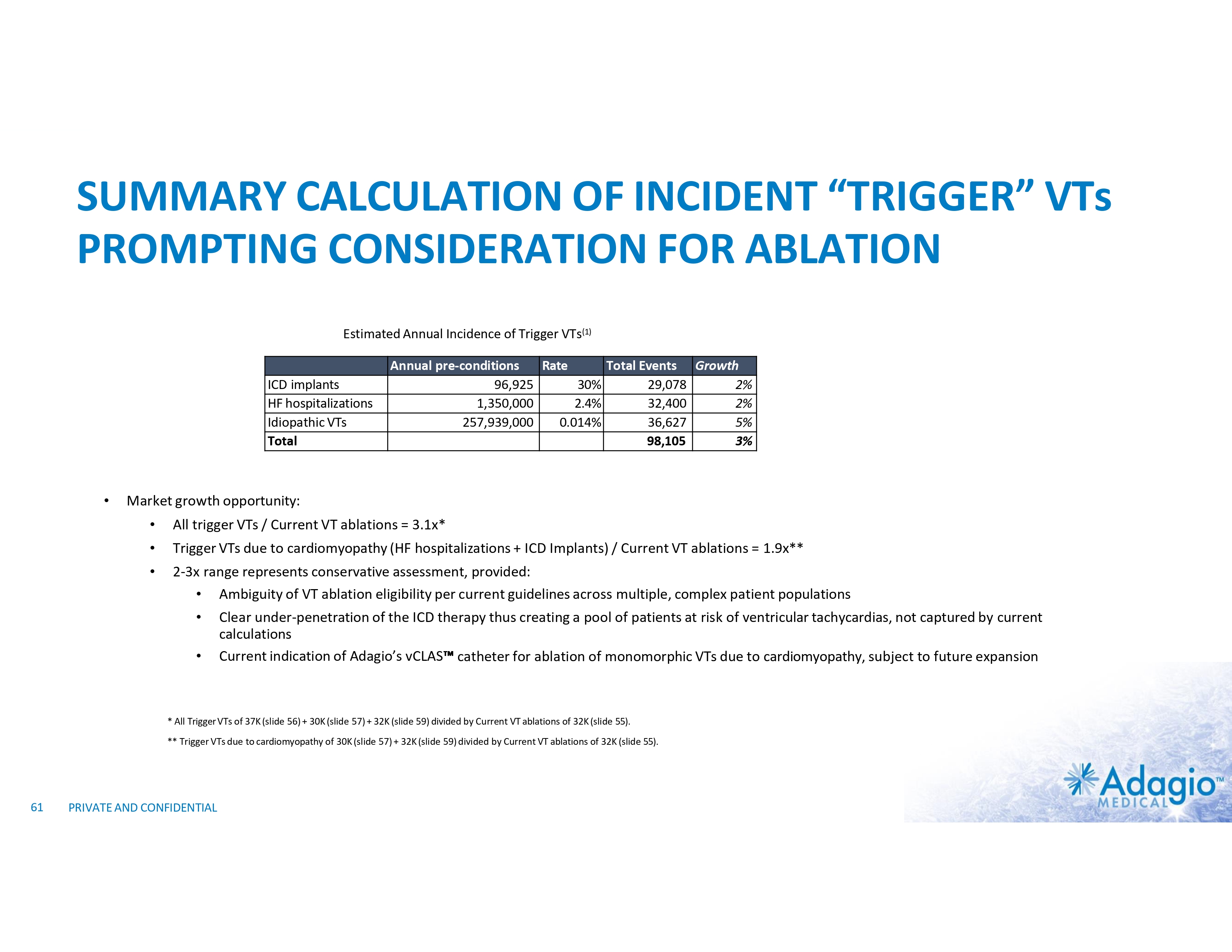
SUMMARY CALCULATION OF INCIDENT “TRIGGER” VTs PROMPTING CONSIDERATION FOR ABLATION Estimated Annual Incidence of Trigger VTs(1) ICD implants HF hospitalizations Idiopathic VTs Total • Annual pre-conditions Rate Total Events Growth 96,925 30% 29,078 2% 1,350,000 2.4% 32,400 2% 257,939,000 0.014% 36,627 5% 98,105 3% Market growth opportunity: • All trigger VTs / Current VT ablations = 3.1x* • Trigger VTs due to cardiomyopathy (HF hospitalizations + ICD Implants) / Current VT ablations = 1.9x** • 2-3x range represents conservative assessment, provided: • Ambiguity of VT ablation eligibility per current guidelines across multiple, complex patient populations • Clear under-penetration of the ICD therapy thus creating a pool of patients at risk of ventricular tachycardias, not captured by current calculations • Current indication of Adagio’s vCLAS catheter for ablation of monomorphic VTs due to cardiomyopathy, subject to future expansion * All Trigger VTs of 37K (slide 56) + 30K (slide 57) + 32K (slide 59) divided by Current VT ablations of 32K (slide 55). ** Trigger VTs due to cardiomyopathy of 30K (slide 57) + 32K (slide 59) divided by Current VT ablations of 32K (slide 55). 61 PRIVATE AND CONFIDENTIAL
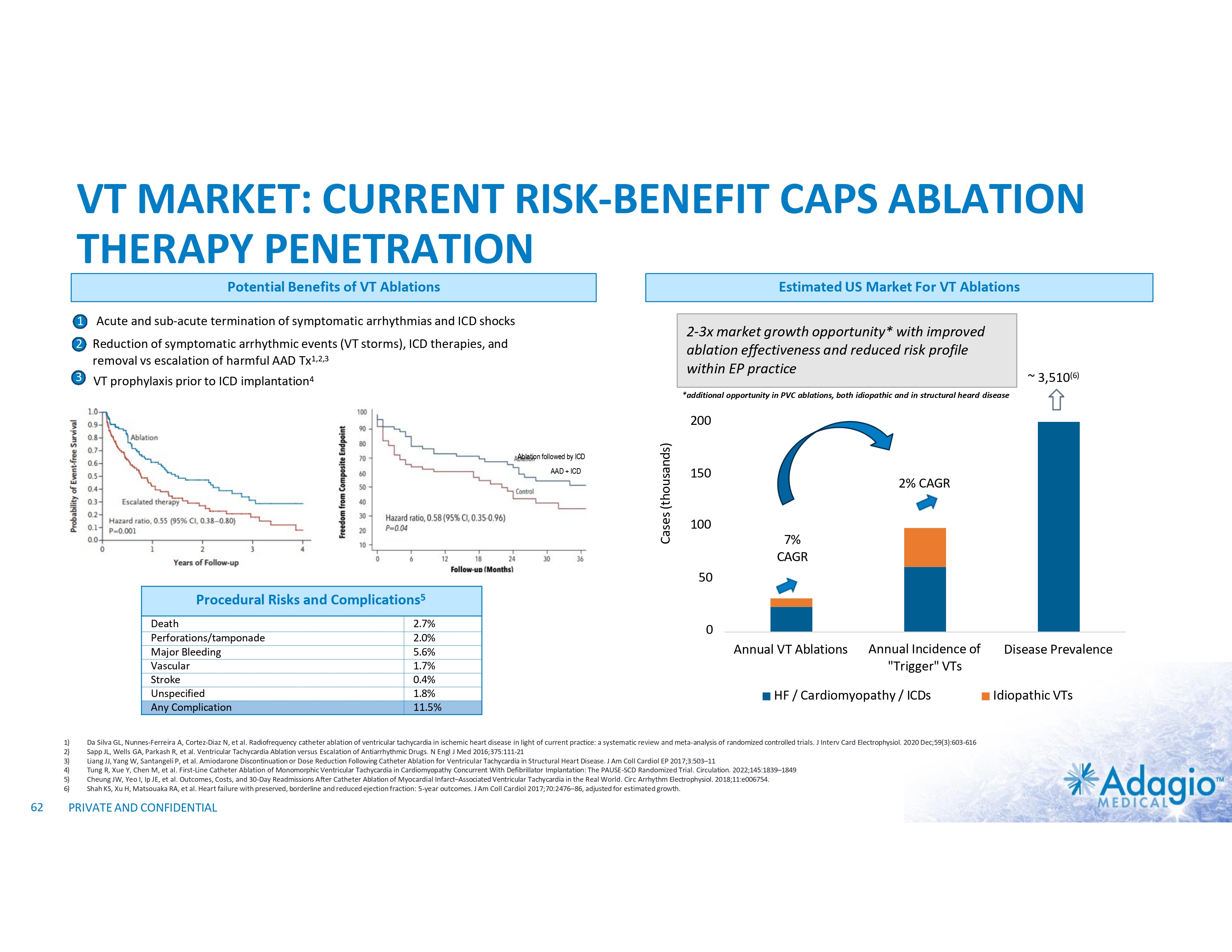
VT MARKET: CURRENT RISK-BENEFIT CAPS ABLATION THERAPY PENETRATION Potential Benefits of VT Ablations Estimated US Market For VT Ablations 1 Acute and sub-acute termination of symptomatic arrhythmias and ICD shocks 2-3x market growth opportunity* with improved ablation effectiveness and reduced risk profile within EP practice 2 Reduction of symptomatic arrhythmic events (VT storms), ICD therapies, and removal vs escalation of harmful AAD Tx1,2,3 3 VT prophylaxis prior to ICD implantation4 ~ 3,510(6) *additional opportunity in PVC ablations, both idiopathic and in structural heard disease Ablation followed by ICD AAD + ICD Cases (thousands) 200 150 2% CAGR 100 7% CAGR 50 Procedural Risks and Death Perforations/tamponade Major Bleeding Vascular Stroke Unspecified Any Complication 1) 2) 3) 4) 5) 6) 62 Complications5 2.7% 2.0% 5.6% 1.7% 0.4% 1.8% 11.5% 0 Annual VT Ablations Annual Incidence of "Trigger" VTs HF / Cardiomyopathy / ICDs Da Silva GL, Nunnes-Ferreira A, Cortez-Diaz N, et al. Radiofrequency catheter ablation of ventricular tachycardia in ischemic heart disease in light of current practice: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2020 Dec;59(3):603-616 Sapp JL, Wells GA, Parkash R, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 2016;375:111-21 Liang JJ, Yang W, Santangeli P, et al. Amiodarone Discontinuation or Dose Reduction Following Catheter Ablation for Ventricular Tachycardia in Structural Heart Disease. J Am Coll Cardiol EP 2017;3:503–11 Tung R, Xue Y, Chen M, et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent With Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation. 2022;145:1839–1849 Cheung JW, Yeo I, Ip JE, et al. Outcomes, Costs, and 30-Day Readmissions After Catheter Ablation of Myocardial Infarct–Associated Ventricular Tachycardia in the Real World. Circ Arrhythm Electrophysiol. 2018;11:e006754. Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol 2017;70:2476–86, adjusted for estimated growth. PRIVATE AND CONFIDENTIAL Disease Prevalence Idiopathic VTs

OVERALL MARKET SIZING AND GROWTH
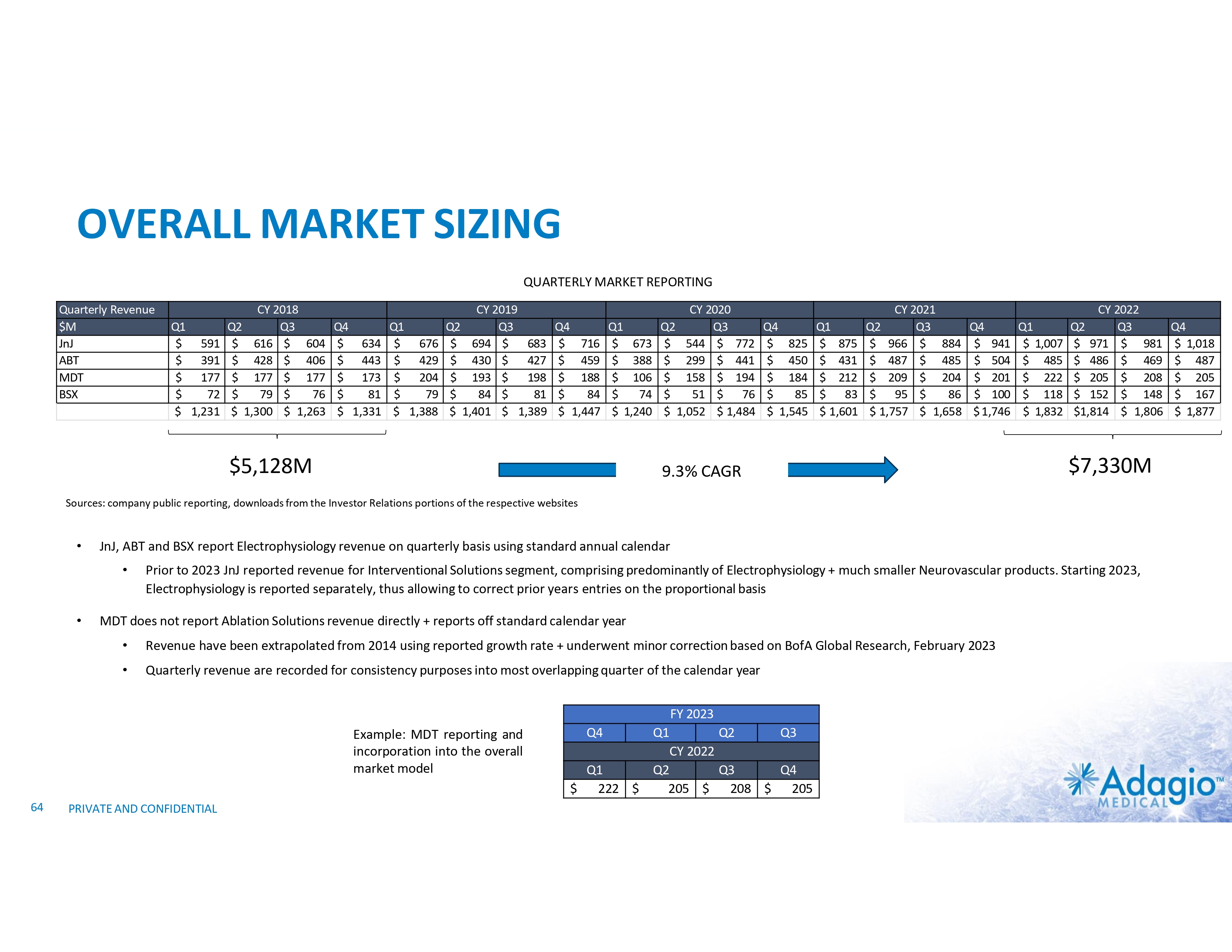
OVERALL MARKET SIZING QUARTERLY MARKET REPORTING Quarterly Revenue $M JnJ ABT MDT BSX Q1 $ 591 $ 391 $ 177 $ 72 $ 1,231 CY 2018 Q2 Q3 $ 616 $ 604 $ 428 $ 406 $ 177 $ 177 $ 79 $ 76 $ 1,300 $ 1,263 Q4 $ 634 $ 443 $ 173 $ 81 $ 1,331 Q1 $ 676 $ 429 $ 204 $ 79 $ 1,388 CY 2019 Q2 Q3 $ 694 $ 683 $ 430 $ 427 $ 193 $ 198 $ 84 $ 81 $ 1,401 $ 1,389 Q4 $ 716 $ 459 $ 188 $ 84 $ 1,447 Q1 $ 673 $ 388 $ 106 $ 74 $ 1,240 $5,128M CY 2020 Q2 Q3 $ 544 $ 772 $ 299 $ 441 $ 158 $ 194 $ 51 $ 76 $ 1,052 $ 1,484 Q4 $ 825 $ 450 $ 184 $ 85 $ 1,545 Q1 $ 875 $ 431 $ 212 $ 83 $ 1,601 CY 2021 Q2 Q3 $ 966 $ 884 $ 487 $ 485 $ 209 $ 204 $ 95 $ 86 $ 1,757 $ 1,658 Q4 $ 941 $ 504 $ 201 $ 100 $ 1,746 Q1 $ 1,007 $ 485 $ 222 $ 118 $ 1,832 CY 2022 Q2 Q3 $ 971 $ 981 $ 486 $ 469 $ 205 $ 208 $ 152 $ 148 $1,814 $ 1,806 $7,330M 9.3% CAGR Sources: company public reporting, downloads from the Investor Relations portions of the respective websites • JnJ, ABT and BSX report Electrophysiology revenue on quarterly basis using standard annual calendar • • Prior to 2023 JnJ reported revenue for Interventional Solutions segment, comprising predominantly of Electrophysiology + much smaller Neurovascular products. Starting 2023, Electrophysiology is reported separately, thus allowing to correct prior years entries on the proportional basis MDT does not report Ablation Solutions revenue directly + reports off standard calendar year • Revenue have been extrapolated from 2014 using reported growth rate + underwent minor correction based on BofA Global Research, February 2023 • Quarterly revenue are recorded for consistency purposes into most overlapping quarter of the calendar year FY 2023 Q4 Example: MDT reporting and incorporation into the overall market model PRIVATE AND CONFIDENTIAL Q2 Q3 CY 2022 $ 64 Q1 Q1 Q2 Q3 Q4 222 $ 205 $ 208 $ 205 Q4 $ 1,018 $ 487 $ 205 $ 167 $ 1,877
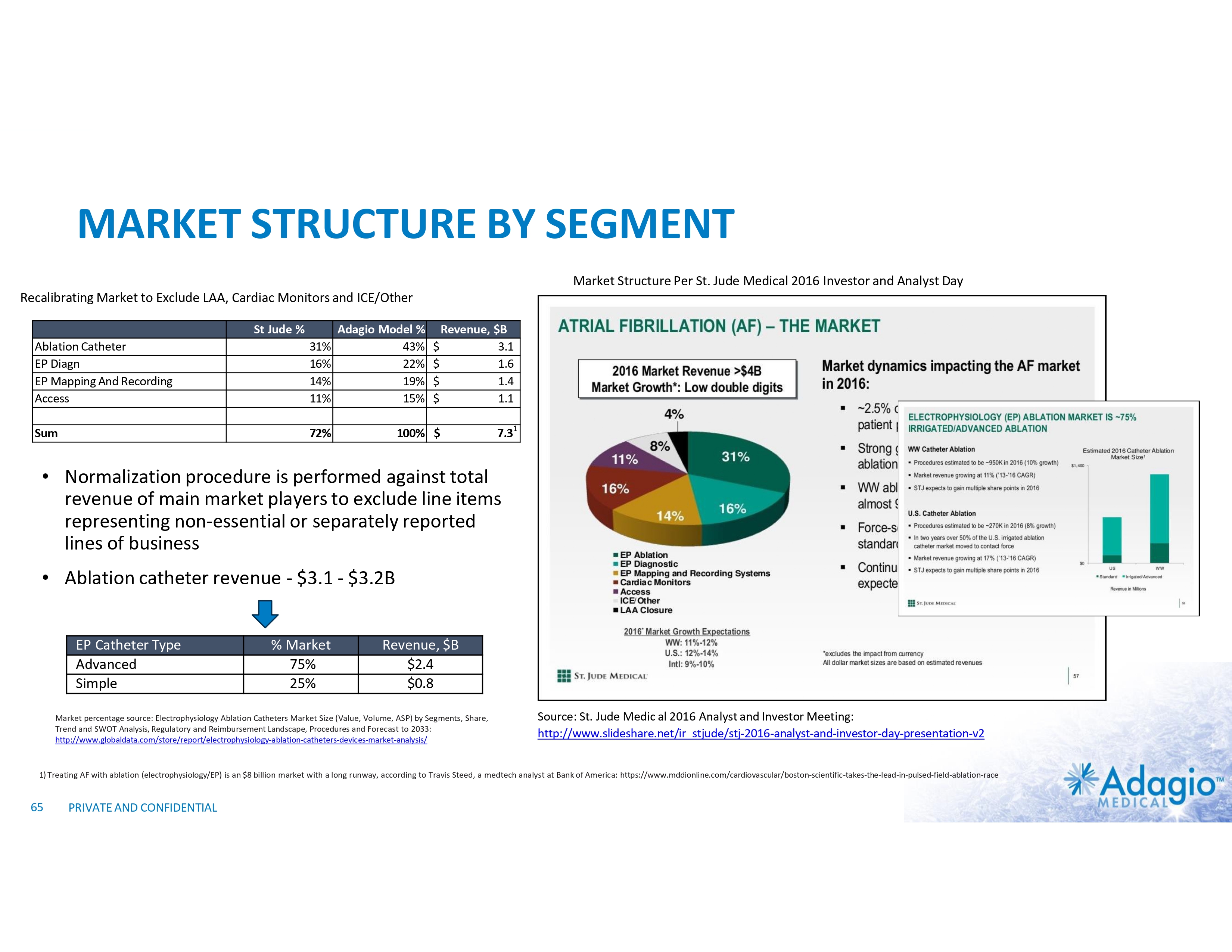
MARKET STRUCTURE BY SEGMENT Market Structure Per St. Jude Medical 2016 Investor and Analyst Day Recalibrating Market to Exclude LAA, Cardiac Monitors and ICE/Other St Jude % Ablation Catheter EP Diagn EP Mapping And Recording Access 31% 16% 14% 11% Sum 72% Adagio Model % 43% 22% 19% 15% $ $ $ $ Revenue, $B 3.1 1.6 1.4 1.1 100% $ 7.31 • Normalization procedure is performed against total revenue of main market players to exclude line items representing non-essential or separately reported lines of business • Ablation catheter revenue - $3.1 - $3.2B EP Catheter Type Advanced Simple % Market 75% 25% Revenue, $B $2.4 $0.8 Market percentage source: Electrophysiology Ablation Catheters Market Size (Value, Volume, ASP) by Segments, Share, Trend and SWOT Analysis, Regulatory and Reimbursement Landscape, Procedures and Forecast to 2033: http://www.globaldata.com/store/report/electrophysiology-ablation-catheters-devices-market-analysis/ Source: St. Jude Medic al 2016 Analyst and Investor Meeting: http://www.slideshare.net/ir_stjude/stj-2016-analyst-and-investor-day-presentation-v2 1) Treating AF with ablation (electrophysiology/EP) is an $8 billion market with a long runway, according to Travis Steed, a medtech analyst at Bank of America: https://www.mddionline.com/cardiovascular/boston-scientific-takes-the-lead-in-pulsed-field-ablation-race 65 PRIVATE AND CONFIDENTIAL
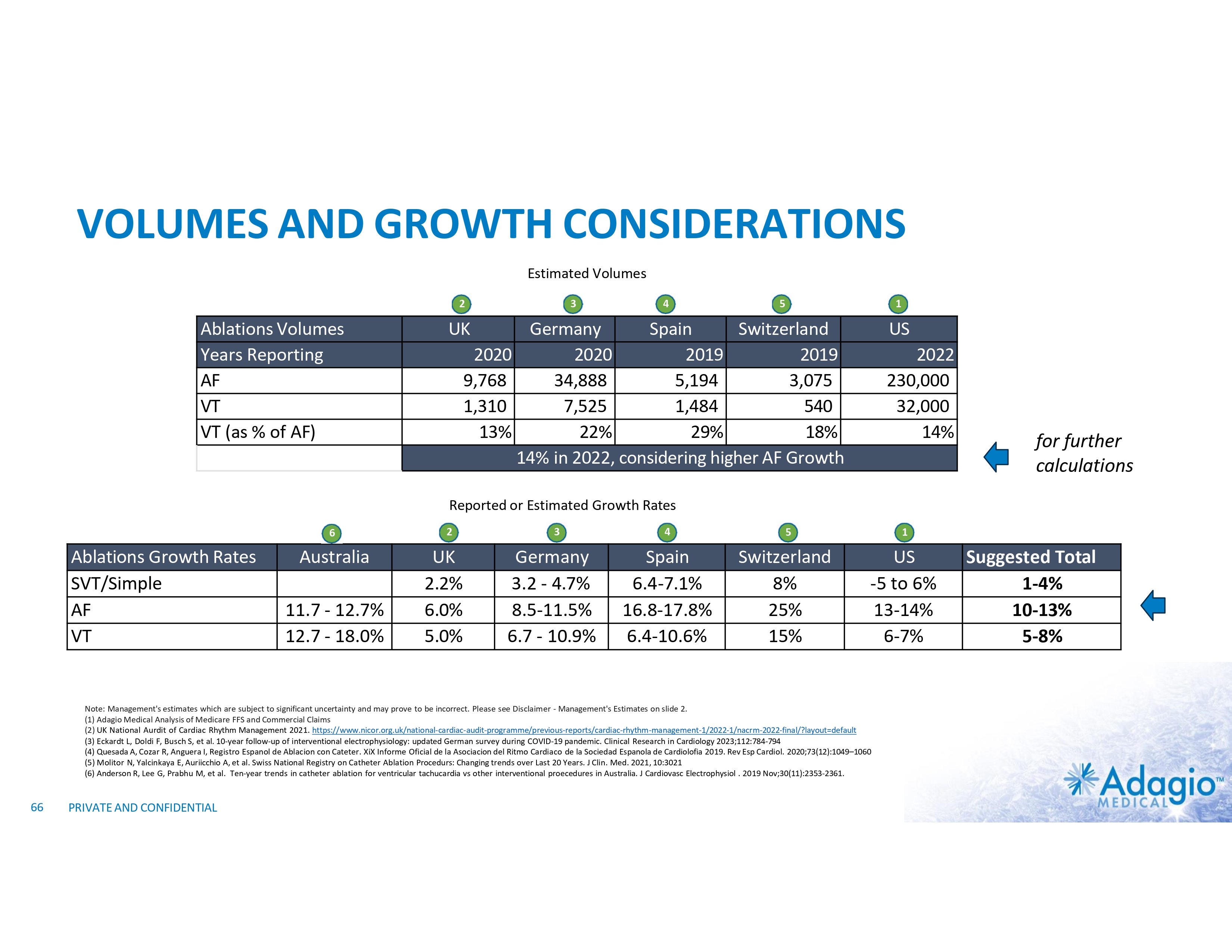
VOLUMES AND GROWTH CONSIDERATIONS Estimated Volumes 2 Ablations Volumes Years Reporting AF VT VT (as % of AF) 3 4 5 1 UK Germany Spain Switzerland 2020 2020 2019 2019 9,768 34,888 5,194 3,075 1,310 7,525 1,484 540 13% 22% 29% 18% 14% in 2022, considering higher AF Growth US 2022 230,000 32,000 14% for further calculations Reported or Estimated Growth Rates 6 Ablations Growth Rates SVT/Simple AF VT Australia 11.7 - 12.7% 12.7 - 18.0% 2 UK 2.2% 6.0% 5.0% 3 Germany 3.2 - 4.7% 8.5-11.5% 6.7 - 10.9% 4 Spain 6.4-7.1% 16.8-17.8% 6.4-10.6% 5 Switzerland 8% 25% 15% 1 US -5 to 6% 13-14% 6-7% Note: Management's estimates which are subject to significant uncertainty and may prove to be incorrect. Please see Disclaimer - Management's Estimates on slide 2. (1) Adagio Medical Analysis of Medicare FFS and Commercial Claims (2) UK National Aurdit of Cardiac Rhythm Management 2021. https://www.nicor.org.uk/national-cardiac-audit-programme/previous-reports/cardiac-rhythm-management-1/2022-1/nacrm-2022-final/?layout=default (3) Eckardt L, Doldi F, Busch S, et al. 10-year follow-up of interventional electrophysiology: updated German survey during COVID-19 pandemic. Clinical Research in Cardiology 2023;112:784-794 (4) Quesada A, Cozar R, Anguera I, Registro Espanol de Ablacion con Cateter. XiX Informe Oficial de la Asociacion del Ritmo Cardiaco de la Sociedad Espanola de Cardiolofia 2019. Rev Esp Cardiol. 2020;73(12):1049–1060 (5) Molitor N, Yalcinkaya E, Auriicchio A, et al. Swiss National Registry on Catheter Ablation Procedurs: Changing trends over Last 20 Years. J Clin. Med. 2021, 10:3021 (6) Anderson R, Lee G, Prabhu M, et al. Ten-year trends in catheter ablation for ventricular tachucardia vs other interventional proecedures in Australia. J Cardiovasc Electrophysiol . 2019 Nov;30(11):2353-2361. 66 PRIVATE AND CONFIDENTIAL Suggested Total 1-4% 10-13% 5-8%
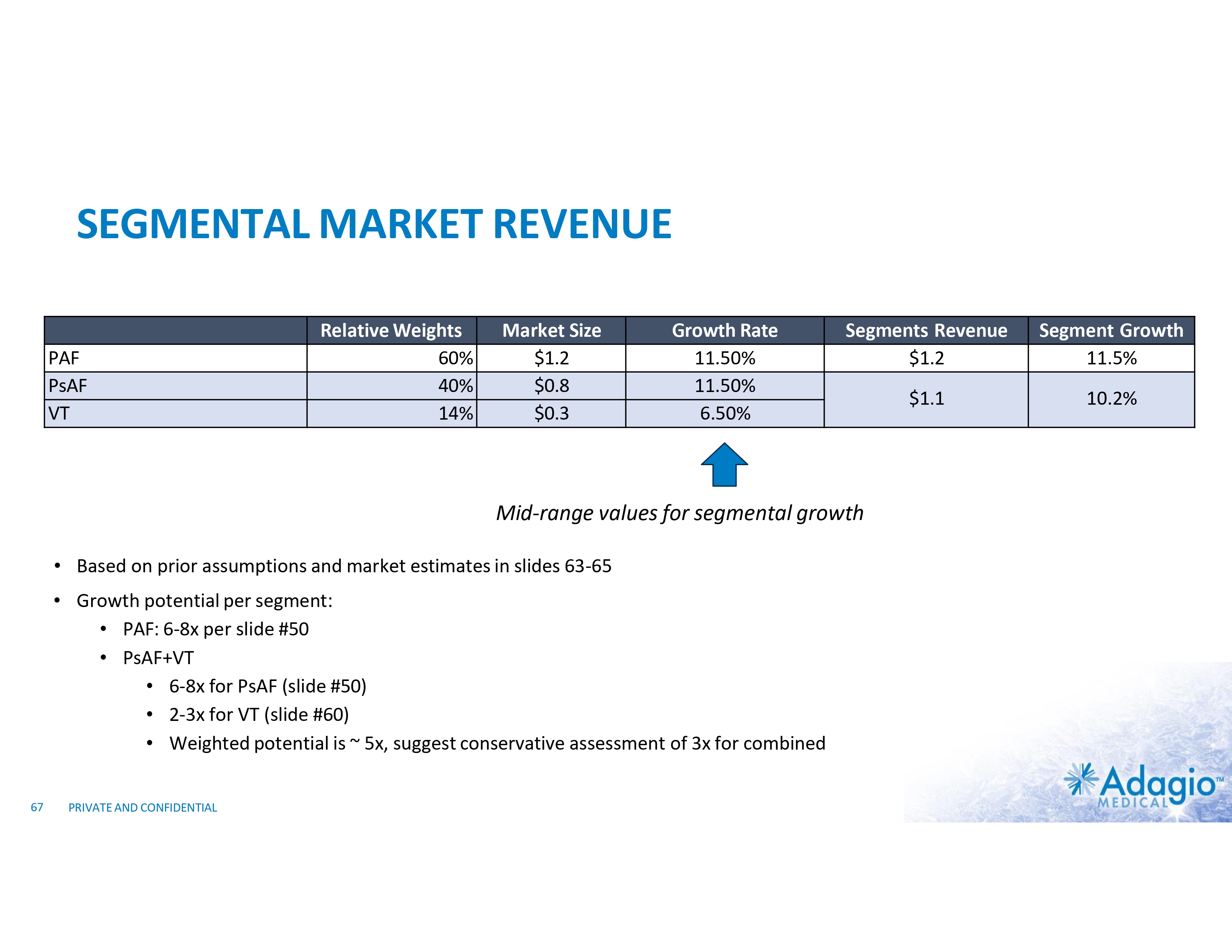
SEGMENTAL MARKET REVENUE PAF PsAF VT Relative Weights 60% 40% 14% Market Size $1.2 $0.8 $0.3 Growth Rate 11.50% 11.50% 6.50% Segments Revenue $1.2 Segment Growth 11.5% $1.1 10.2% Mid-range values for segmental growth • Based on prior assumptions and market estimates in slides 63-65 • Growth potential per segment: • PAF: 6-8x per slide #50 • PsAF+VT • 6-8x for PsAF (slide #50) • 2-3x for VT (slide #60) • Weighted potential is ~ 5x, suggest conservative assessment of 3x for combined 67 PRIVATE AND CONFIDENTIAL
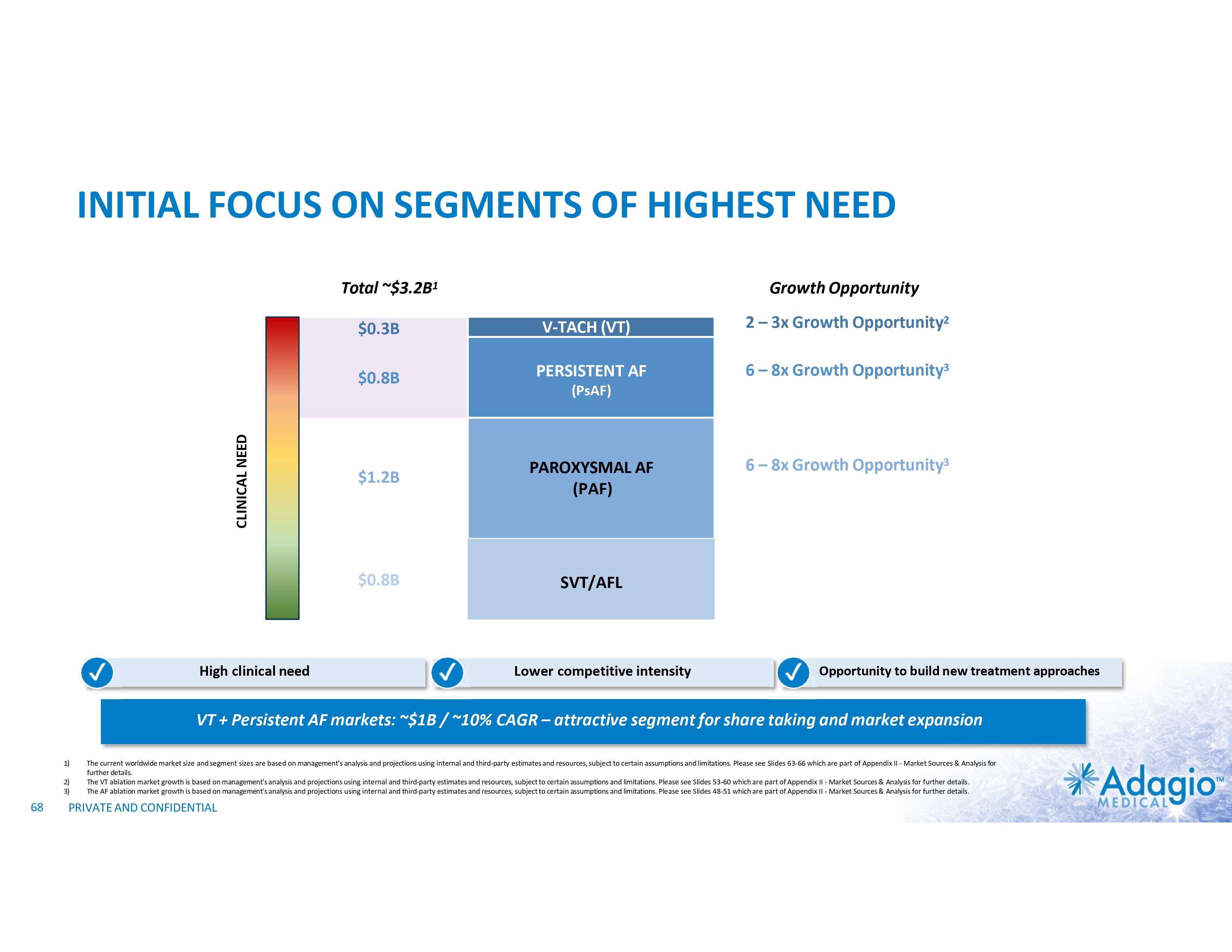
INITIAL FOCUS ON SEGMENTS OF HIGHEST NEED CLINICAL NEED Total ~$3.2B1 High clinical need Growth Opportunity $0.3B V-TACH (VT) 2 – 3x Growth Opportunity2 $0.8B PERSISTENT AF 6 – 8x Growth Opportunity3 $1.2B PAROXYSMAL AF (PAF) $0.8B SVT/AFL (PsAF) Lower competitive intensity 6 – 8x Growth Opportunity3 Opportunity to build new treatment approaches VT + Persistent AF markets: ~$1B / ~10% CAGR – attractive segment for share taking and market expansion 1) 2) 3) 68 The current worldwide market size and segment sizes are based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 63-66 which are part of Appendix II - Market Sources & Analysis for further details. The VT ablation market growth is based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 53-60 which are part of Appendix II - Market Sources & Analysis for further details. The AF ablation market growth is based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 48-51 which are part of Appendix II - Market Sources & Analysis for further details. PRIVATE AND CONFIDENTIAL

GO-TO-MARKET ASSESSMENTS
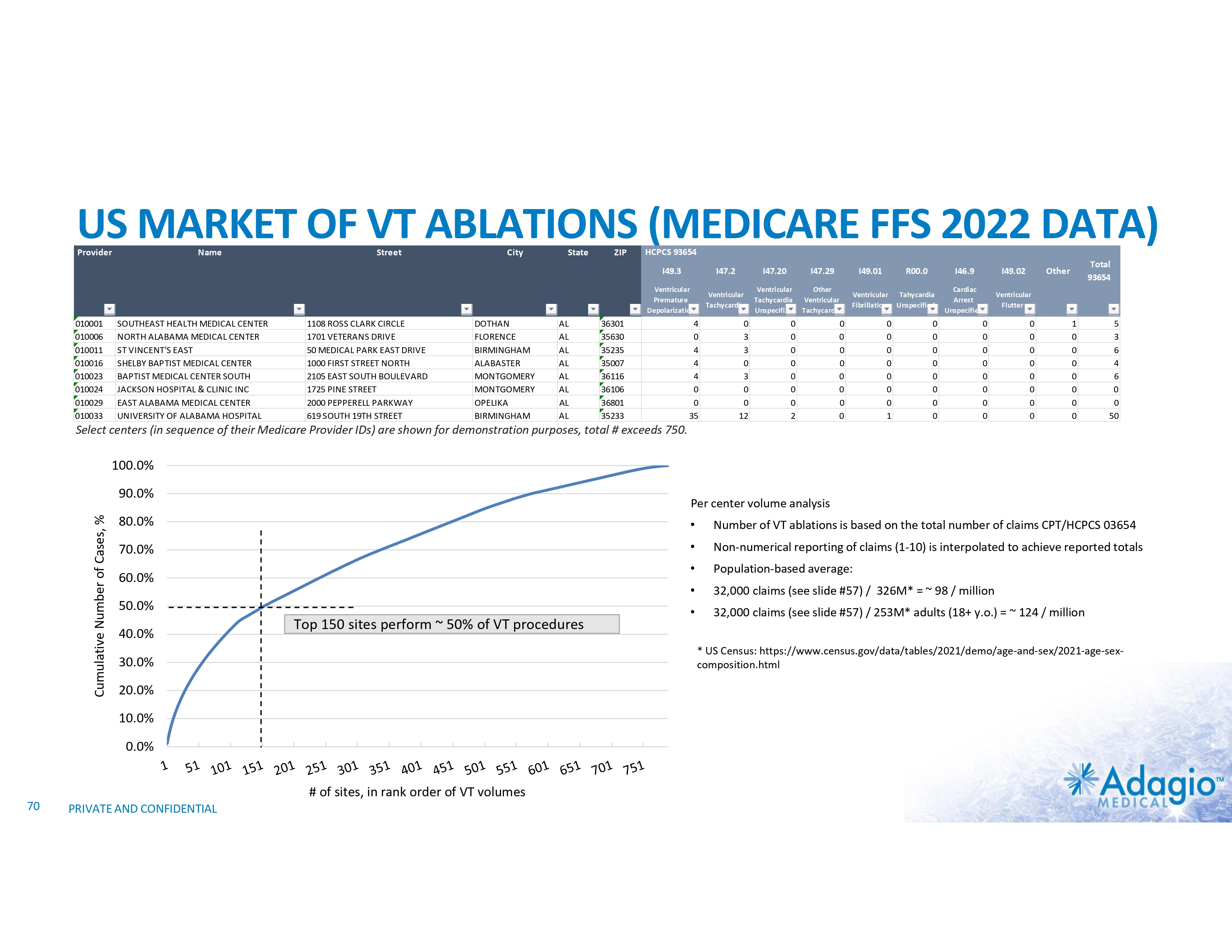
US MARKET OF VT ABLATIONS (MEDICARE FFS 2022 DATA) Provider 010001 010006 010011 010016 010023 010024 010029 010033 Name SOUTHEAST HEALTH MEDICAL CENTER NORTH ALABAMA MEDICAL CENTER ST VINCENT'S EAST SHELBY BAPTIST MEDICAL CENTER BAPTIST MEDICAL CENTER SOUTH JACKSON HOSPITAL & CLINIC INC EAST ALABAMA MEDICAL CENTER UNIVERSITY OF ALABAMA HOSPITAL Street 1108 ROSS CLARK CIRCLE 1701 VETERANS DRIVE 50 MEDICAL PARK EAST DRIVE 1000 FIRST STREET NORTH 2105 EAST SOUTH BOULEVARD 1725 PINE STREET 2000 PEPPERELL PARKWAY 619 SOUTH 19TH STREET City DOTHAN FLORENCE BIRMINGHAM ALABASTER MONTGOMERY MONTGOMERY OPELIKA BIRMINGHAM State AL AL AL AL AL AL AL AL ZIP HCPCS 93654 I49.3 I47.2 I47.20 I47.29 I49.01 R00.0 I46.9 I49.02 Ventricular Premature Depolarization Ventricular Tachycardia Ventricular Tachycardia Unspecified Other Ventricular Tachycardia Ventricular Fibrillation Tahycardia Unspecified Cardiac Arrest Unspecified Ventricular Flutter 36301 35630 35235 35007 36116 36106 36801 35233 4 0 4 4 4 0 0 35 0 3 3 0 3 0 0 12 0 0 0 0 0 0 0 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Total 93654 Other 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 5 3 6 4 6 0 0 50 Select centers (in sequence of their Medicare Provider IDs) are shown for demonstration purposes, total # exceeds 750. 100.0% Cumulative Number of Cases, % 90.0% Per center volume analysis 80.0% • Number of VT ablations is based on the total number of claims CPT/HCPCS 03654 70.0% • Non-numerical reporting of claims (1-10) is interpolated to achieve reported totals • Population-based average: • 32,000 claims (see slide #57) / 326M* = ~ 98 / million • 32,000 claims (see slide #57) / 253M* adults (18+ y.o.) = ~ 124 / million 60.0% 50.0% 40.0% Top 150 sites perform ~ 50% of VT procedures * US Census: https://www.census.gov/data/tables/2021/demo/age-and-sex/2021-age-sex- composition.html 30.0% 20.0% 10.0% 0.0% # of sites, in rank order of VT volumes 70 PRIVATE AND CONFIDENTIAL
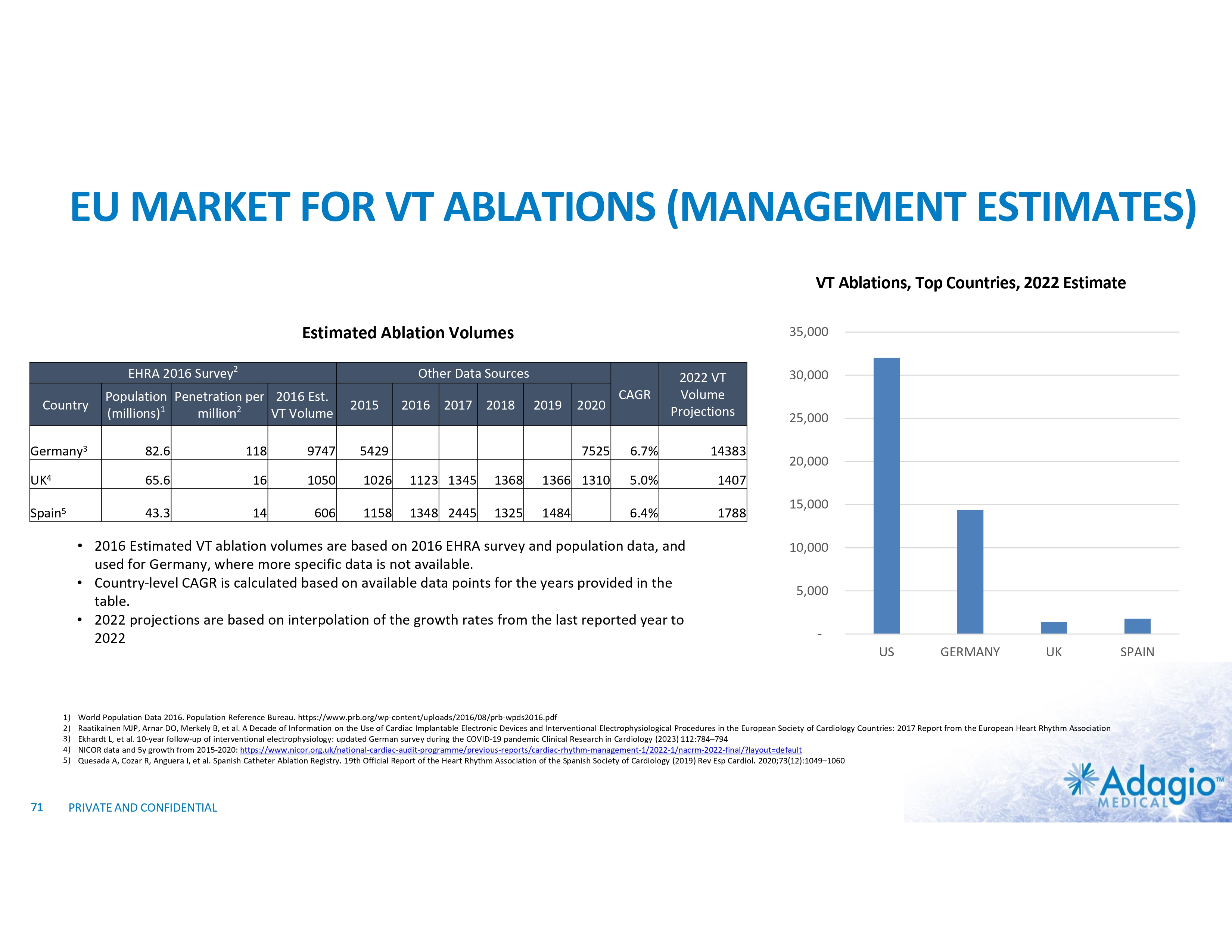
EU MARKET FOR VT ABLATIONS (MANAGEMENT ESTIMATES) VT Ablations, Top Countries, 2022 Estimate Estimated Ablation Volumes EHRA 2016 Survey2 Country 35,000 Other Data Sources Population Penetration per 2016 Est. (millions)1 million2 VT Volume 2015 2016 2017 2018 Germany3 82.6 118 9747 5429 UK4 65.6 16 1050 1026 1123 1345 Spain5 43.3 14 606 1158 1348 2445 2019 2020 CAGR 2022 VT Volume Projections 7525 6.7% 14383 1368 1366 1310 5.0% 1407 1325 1484 6.4% 1788 • 2016 Estimated VT ablation volumes are based on 2016 EHRA survey and population data, and used for Germany, where more specific data is not available. • Country-level CAGR is calculated based on available data points for the years provided in the table. • 2022 projections are based on interpolation of the growth rates from the last reported year to 2022 1) 2) 3) 4) 5) 71 30,000 25,000 20,000 15,000 10,000 5,000 - US GERMANY UK World Population Data 2016. Population Reference Bureau. https://www.prb.org/wp-content/uploads/2016/08/prb-wpds2016.pdf Raatikainen MJP, Arnar DO, Merkely B, et al. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association Ekhardt L, et al. 10-year follow-up of interventional electrophysiology: updated German survey during the COVID-19 pandemic Clinical Research in Cardiology (2023) 112:784–794 NICOR data and 5y growth from 2015-2020: https://www.nicor.org.uk/national-cardiac-audit-programme/previous-reports/cardiac-rhythm-management-1/2022-1/nacrm-2022-final/?layout=default Quesada A, Cozar R, Anguera I, et al. Spanish Catheter Ablation Registry. 19th Official Report of the Heart Rhythm Association of the Spanish Society of Cardiology (2019) Rev Esp Cardiol. 2020;73(12):1049–1060 PRIVATE AND CONFIDENTIAL SPAIN
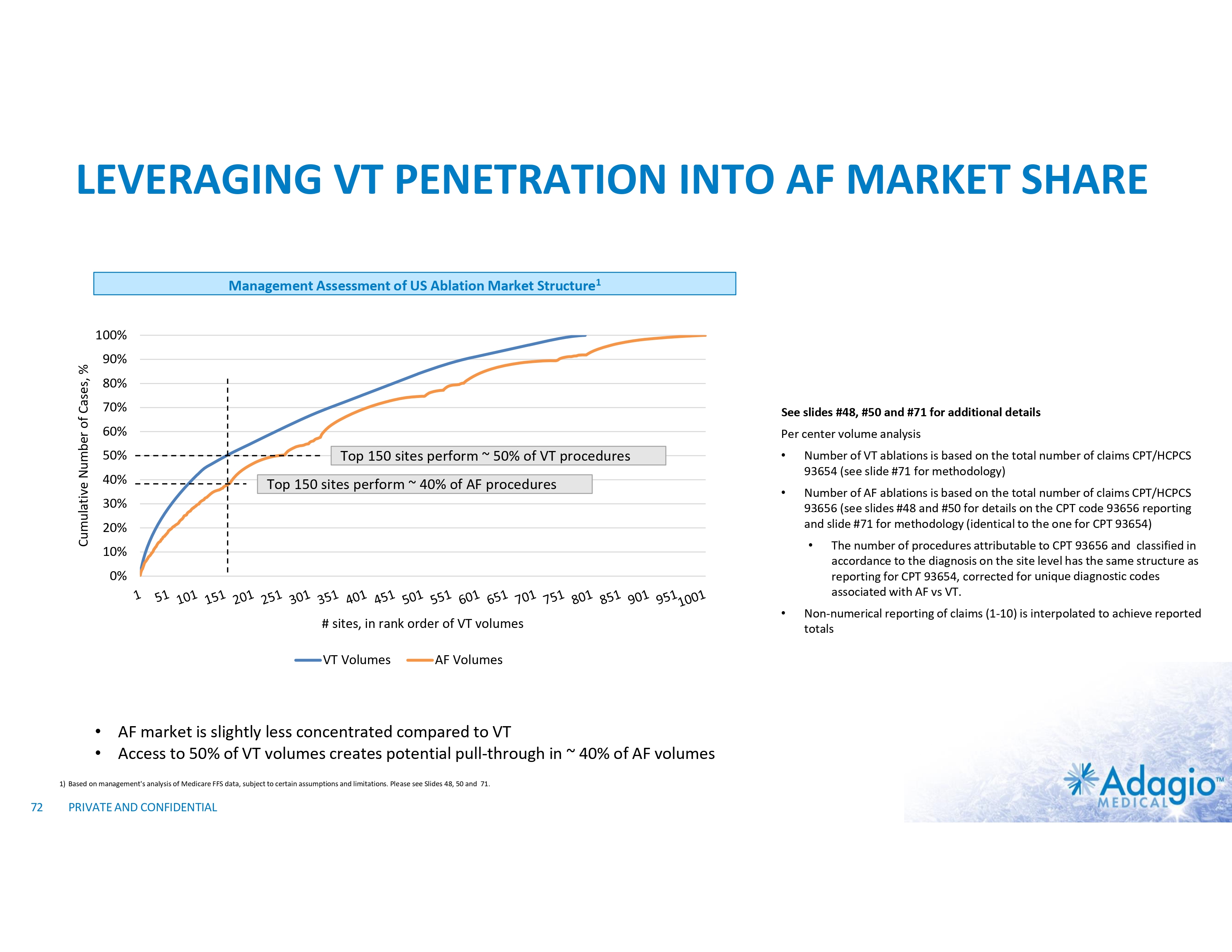
LEVERAGING VT PENETRATION INTO AF MARKET SHARE Management Assessment of US Ablation Market Structure1 Cumulative Number of Cases, % 100% 90% 80% 70% See slides #48, #50 and #71 for additional details 60% Per center volume analysis 50% • Number of VT ablations is based on the total number of claims CPT/HCPCS 93654 (see slide #71 for methodology) • Number of AF ablations is based on the total number of claims CPT/HCPCS 93656 (see slides #48 and #50 for details on the CPT code 93656 reporting and slide #71 for methodology (identical to the one for CPT 93654) 40% Top 150 sites perform ~ 50% of VT procedures Top 150 sites perform ~ 40% of AF procedures 30% 20% • 10% 0% # sites, in rank order of VT volumes VT Volumes AF Volumes • AF market is slightly less concentrated compared to VT • Access to 50% of VT volumes creates potential pull-through in ~ 40% of AF volumes 1) Based on management's analysis of Medicare FFS data, subject to certain assumptions and limitations. Please see Slides 48, 50 and 71. 72 PRIVATE AND CONFIDENTIAL • The number of procedures attributable to CPT 93656 and classified in accordance to the diagnosis on the site level has the same structure as reporting for CPT 93654, corrected for unique diagnostic codes associated with AF vs VT. Non-numerical reporting of claims (1-10) is interpolated to achieve reported totals

COMPETITIVE DEVICES AND INDICATIONS
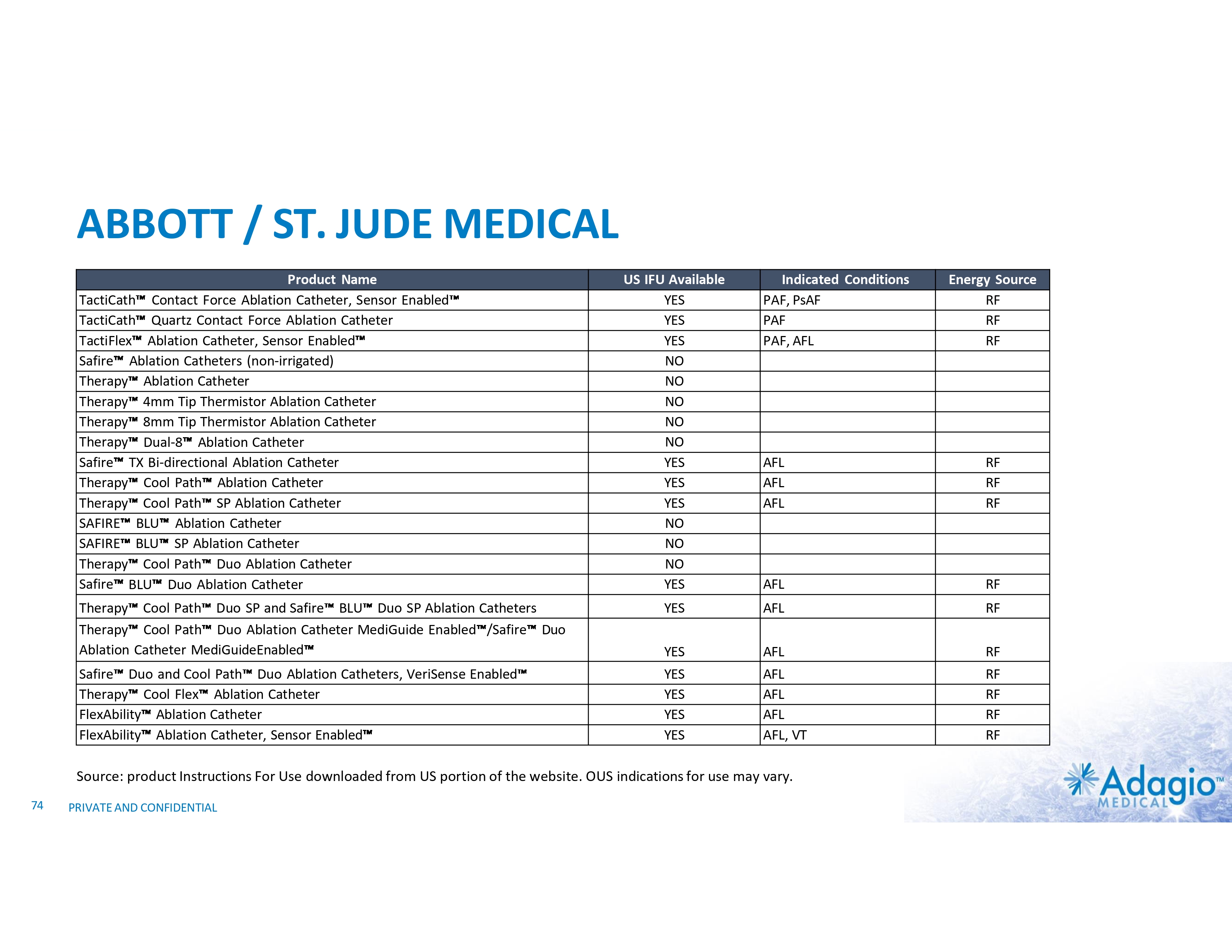
ABBOTT / ST. JUDE MEDICAL Product Name TactiCath Contact Force Ablation Catheter, Sensor Enabled TactiCath Quartz Contact Force Ablation Catheter TactiFlex Ablation Catheter, Sensor Enabled Safire Ablation Catheters (non-irrigated) Therapy Ablation Catheter Therapy 4mm Tip Thermistor Ablation Catheter Therapy 8mm Tip Thermistor Ablation Catheter Therapy Dual-8 Ablation Catheter Safire TX Bi-directional Ablation Catheter Therapy Cool Path Ablation Catheter Therapy Cool Path SP Ablation Catheter SAFIRE BLU Ablation Catheter SAFIRE BLU SP Ablation Catheter Therapy Cool Path Duo Ablation Catheter Safire BLU Duo Ablation Catheter US IFU Available YES YES YES NO NO NO NO NO YES YES YES NO NO NO YES Indicated Conditions PAF, PsAF PAF PAF, AFL AFL AFL AFL RF RF RF AFL RF Therapy Cool Path Duo SP and Safire BLU Duo SP Ablation Catheters Therapy Cool Path Duo Ablation Catheter MediGuide Enabled /Safire Duo Ablation Catheter MediGuideEnabled YES AFL RF YES AFL RF Safire Duo and Cool Path Duo Ablation Catheters, VeriSense Enabled Therapy Cool Flex Ablation Catheter FlexAbility Ablation Catheter FlexAbility Ablation Catheter, Sensor Enabled YES YES YES YES AFL AFL AFL AFL, VT RF RF RF RF Source: product Instructions For Use downloaded from US portion of the website. OUS indications for use may vary. 74 Energy Source RF RF RF PRIVATE AND CONFIDENTIAL
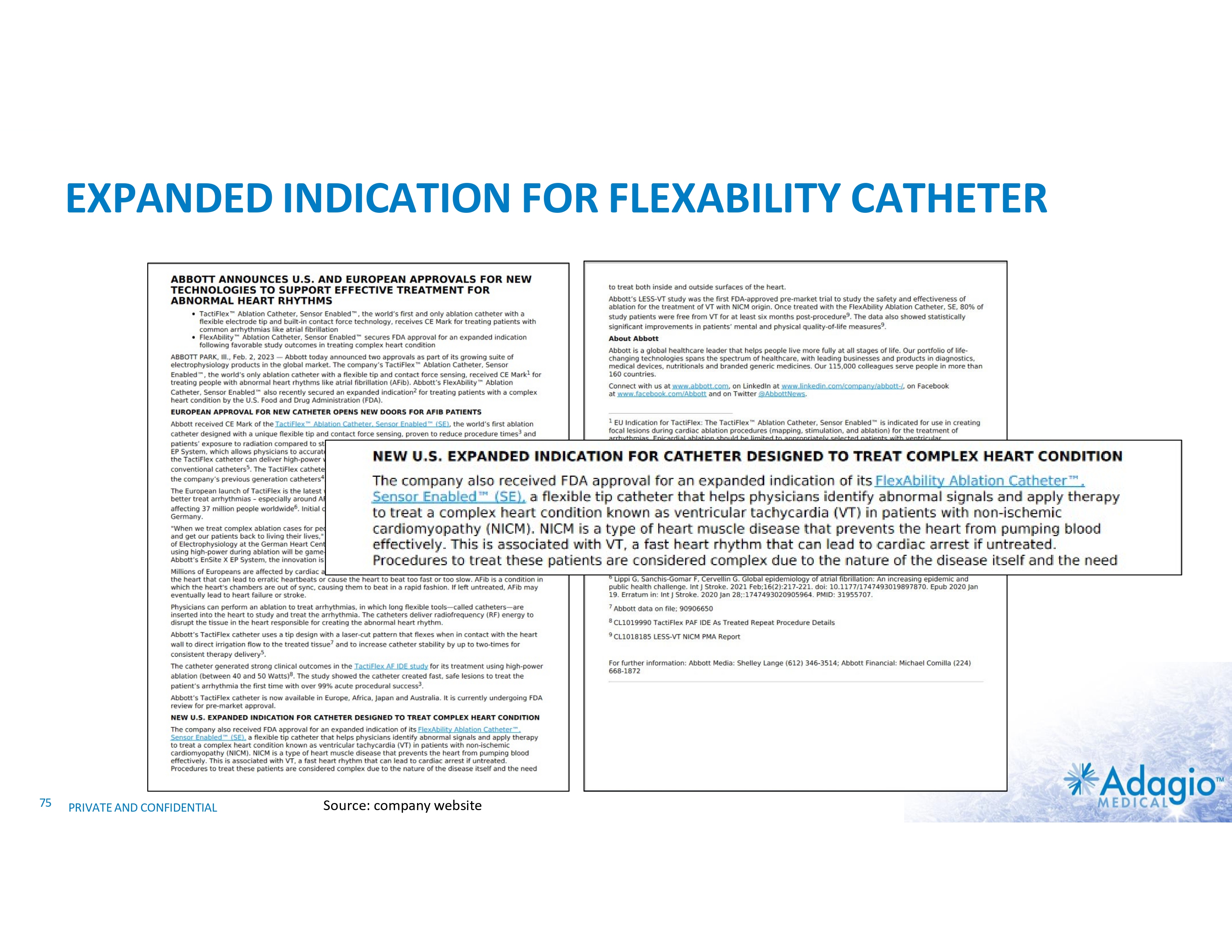
EXPANDED INDICATION FOR FLEXABILITY CATHETER 75 PRIVATE AND CONFIDENTIAL Source: company website
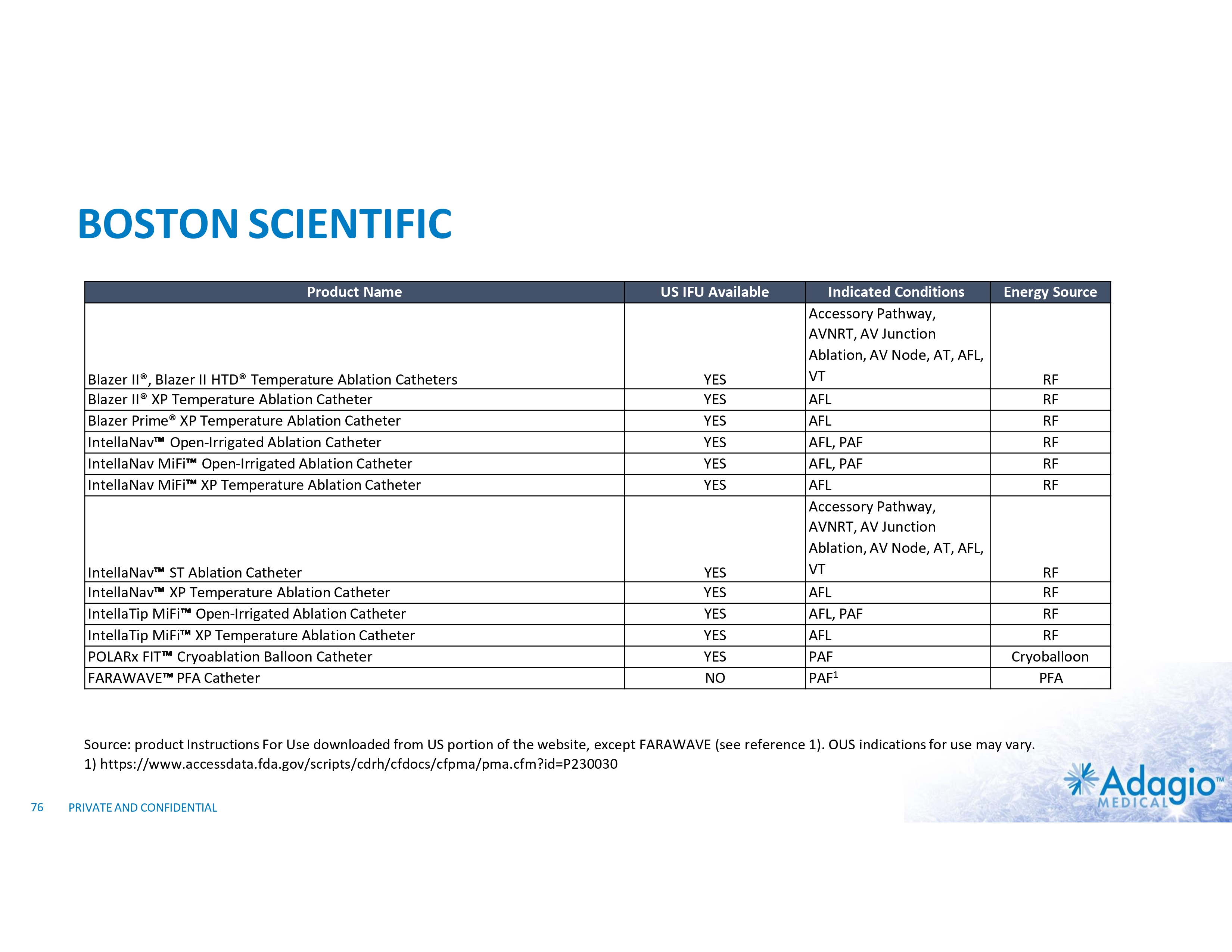
BOSTON SCIENTIFIC Product Name US IFU Available Blazer II®, Blazer II HTD® Temperature Ablation Catheters Blazer II® XP Temperature Ablation Catheter Blazer Prime® XP Temperature Ablation Catheter IntellaNav Open-Irrigated Ablation Catheter IntellaNav MiFi Open-Irrigated Ablation Catheter IntellaNav MiFi XP Temperature Ablation Catheter YES YES YES YES YES YES IntellaNav ST Ablation Catheter IntellaNav XP Temperature Ablation Catheter IntellaTip MiFi Open-Irrigated Ablation Catheter IntellaTip MiFi XP Temperature Ablation Catheter POLARx FIT Cryoablation Balloon Catheter FARAWAVE PFA Catheter YES YES YES YES YES NO Indicated Conditions Accessory Pathway, AVNRT, AV Junction Ablation, AV Node, AT, AFL, VT AFL AFL AFL, PAF AFL, PAF AFL Accessory Pathway, AVNRT, AV Junction Ablation, AV Node, AT, AFL, VT AFL AFL, PAF AFL PAF PAF1 Energy Source RF RF RF RF RF RF RF RF RF RF Cryoballoon PFA Source: product Instructions For Use downloaded from US portion of the website, except FARAWAVE (see reference 1). OUS indications for use may vary. 1) https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P230030 76 PRIVATE AND CONFIDENTIAL
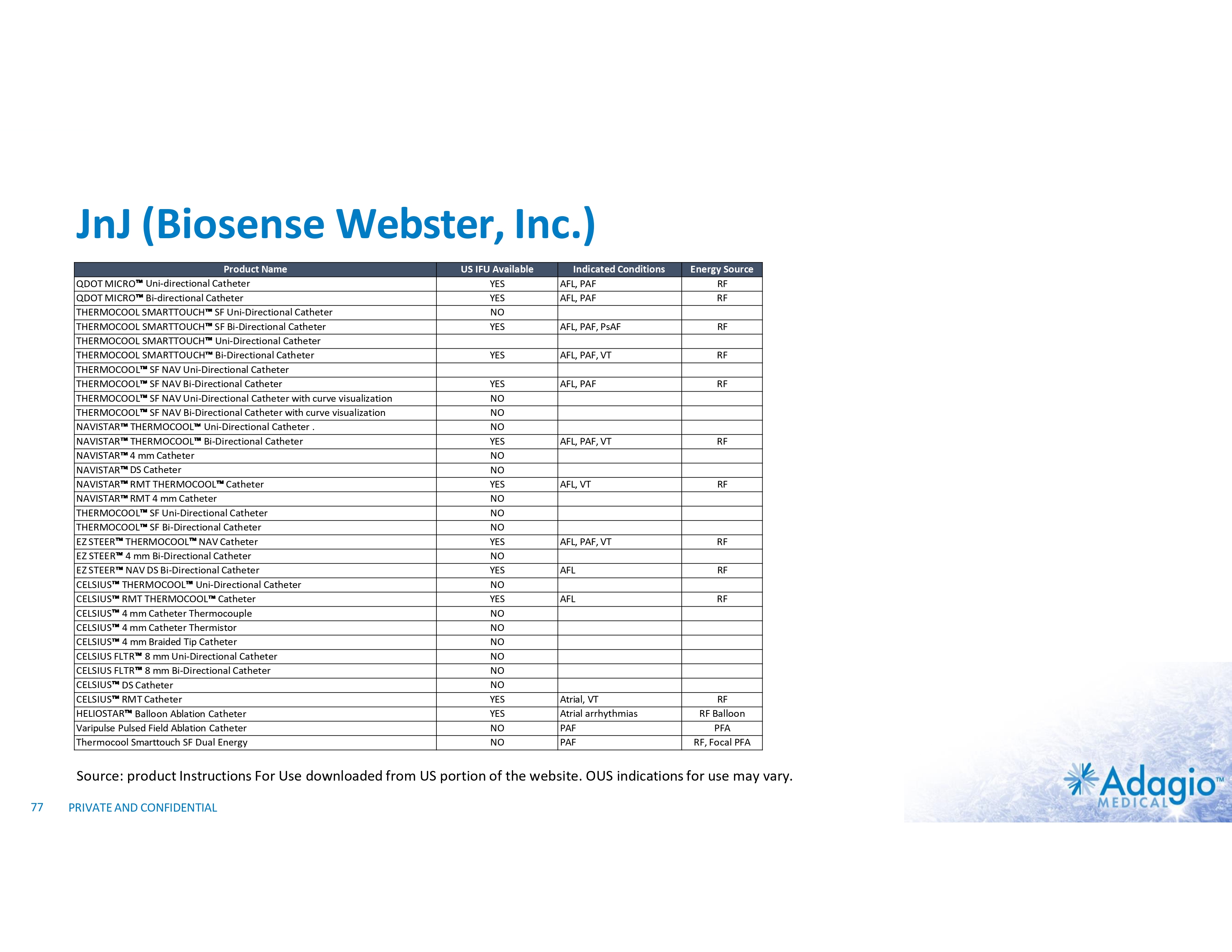
JnJ (Biosense Webster, Inc.) Product Name QDOT MICRO Uni-directional Catheter QDOT MICRO Bi-directional Catheter THERMOCOOL SMARTTOUCH SF Uni-Directional Catheter THERMOCOOL SMARTTOUCH SF Bi-Directional Catheter THERMOCOOL SMARTTOUCH Uni-Directional Catheter THERMOCOOL SMARTTOUCH Bi-Directional Catheter THERMOCOOL SF NAV Uni-Directional Catheter THERMOCOOL SF NAV Bi-Directional Catheter THERMOCOOL SF NAV Uni-Directional Catheter with curve visualization THERMOCOOL SF NAV Bi-Directional Catheter with curve visualization NAVISTAR THERMOCOOL Uni-Directional Catheter . NAVISTAR THERMOCOOL Bi-Directional Catheter NAVISTAR 4 mm Catheter NAVISTAR DS Catheter NAVISTAR RMT THERMOCOOL Catheter NAVISTAR RMT 4 mm Catheter THERMOCOOL SF Uni-Directional Catheter THERMOCOOL SF Bi-Directional Catheter EZ STEER THERMOCOOL NAV Catheter EZ STEER 4 mm Bi-Directional Catheter EZ STEER NAV DS Bi-Directional Catheter CELSIUS THERMOCOOL Uni-Directional Catheter CELSIUS RMT THERMOCOOL Catheter CELSIUS 4 mm Catheter Thermocouple CELSIUS 4 mm Catheter Thermistor CELSIUS 4 mm Braided Tip Catheter CELSIUS FLTR 8 mm Uni-Directional Catheter CELSIUS FLTR 8 mm Bi-Directional Catheter CELSIUS DS Catheter CELSIUS RMT Catheter HELIOSTAR Balloon Ablation Catheter Varipulse Pulsed Field Ablation Catheter Thermocool Smarttouch SF Dual Energy US IFU Available YES YES NO YES Indicated Conditions AFL, PAF AFL, PAF Energy Source RF RF AFL, PAF, PsAF RF YES AFL, PAF, VT RF YES NO NO NO YES NO NO YES NO NO NO YES NO YES NO YES NO NO NO NO NO NO YES YES NO NO AFL, PAF RF AFL, PAF, VT RF AFL, VT RF AFL, PAF, VT RF AFL RF AFL RF Atrial, VT Atrial arrhythmias PAF PAF RF RF Balloon PFA RF, Focal PFA Source: product Instructions For Use downloaded from US portion of the website. OUS indications for use may vary. 77 PRIVATE AND CONFIDENTIAL
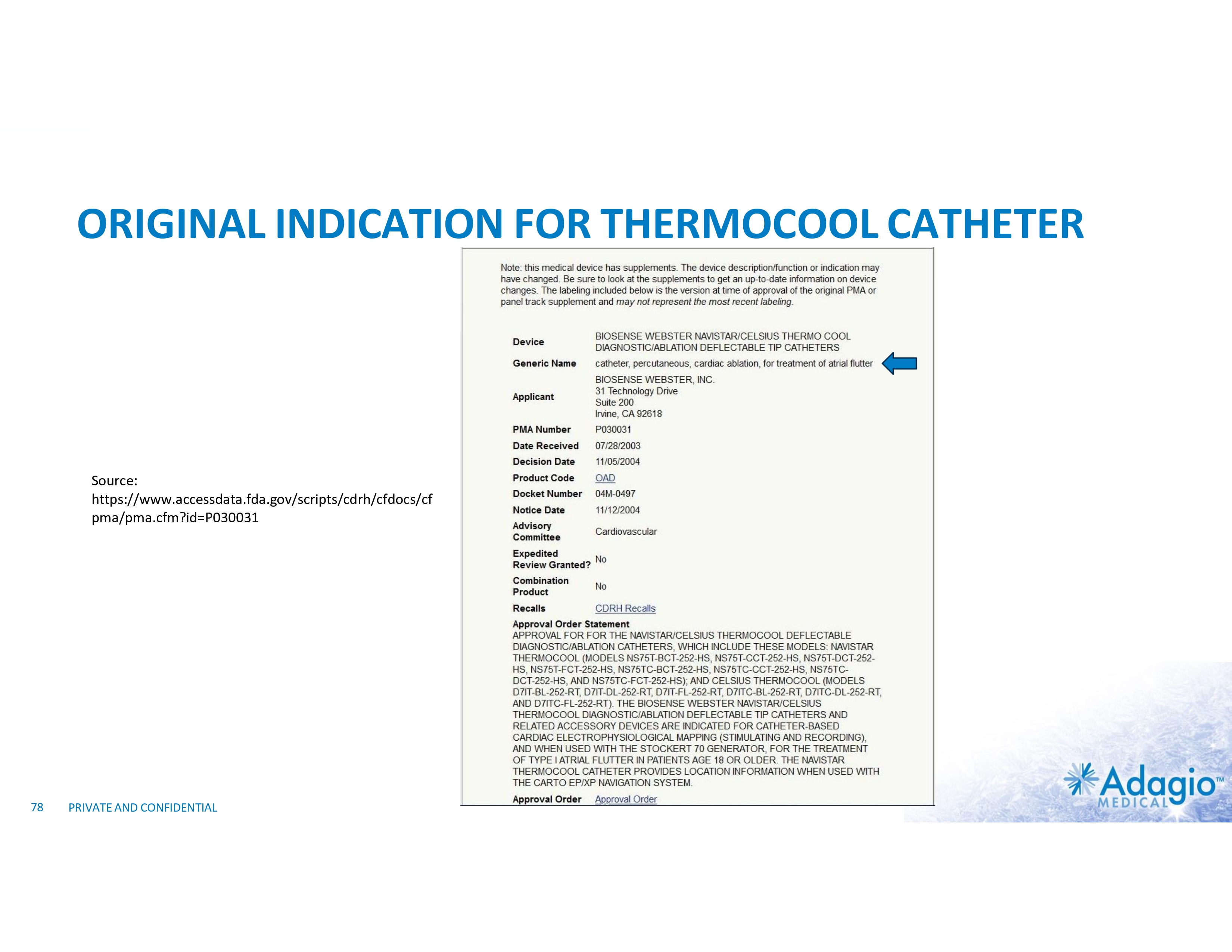
ORIGINAL INDICATION FOR THERMOCOOL CATHETER Source: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cf pma/pma.cfm?id=P030031 78 PRIVATE AND CONFIDENTIAL
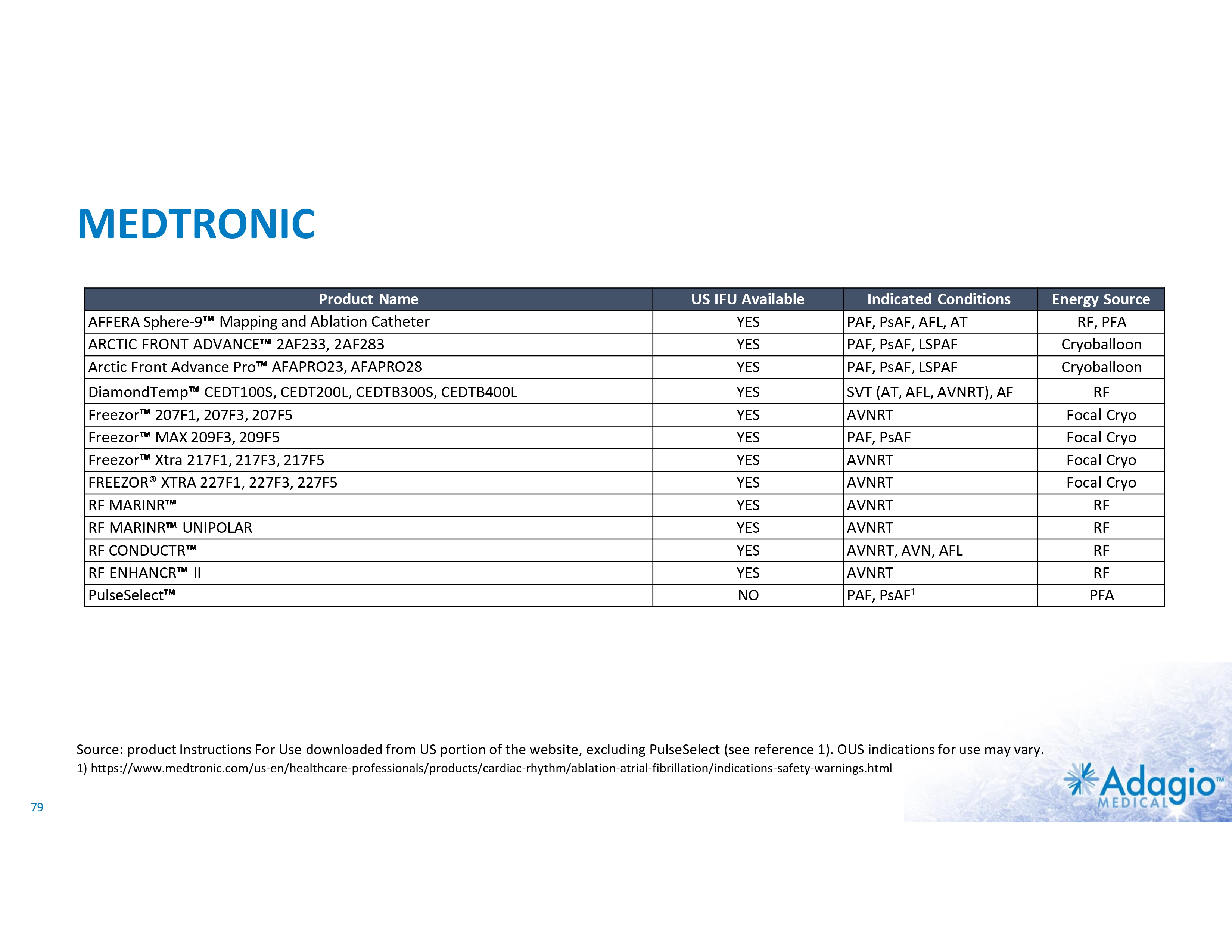
MEDTRONIC Product Name AFFERA Sphere-9 Mapping and Ablation Catheter ARCTIC FRONT ADVANCE 2AF233, 2AF283 Arctic Front Advance Pro AFAPRO23, AFAPRO28 DiamondTemp CEDT100S, CEDT200L, CEDTB300S, CEDTB400L Freezor 207F1, 207F3, 207F5 Freezor MAX 209F3, 209F5 Freezor Xtra 217F1, 217F3, 217F5 FREEZOR® XTRA 227F1, 227F3, 227F5 RF MARINR RF MARINR UNIPOLAR RF CONDUCTR RF ENHANCR II PulseSelect US IFU Available YES YES YES Indicated Conditions PAF, PsAF, AFL, AT PAF, PsAF, LSPAF PAF, PsAF, LSPAF Energy Source RF, PFA Cryoballoon Cryoballoon YES YES YES YES YES YES YES YES YES NO SVT (AT, AFL, AVNRT), AF AVNRT PAF, PsAF AVNRT AVNRT AVNRT AVNRT AVNRT, AVN, AFL AVNRT PAF, PsAF1 RF Focal Cryo Focal Cryo Focal Cryo Focal Cryo RF RF RF RF PFA Source: product Instructions For Use downloaded from US portion of the website, excluding PulseSelect (see reference 1). OUS indications for use may vary. 1) https://www.medtronic.com/us-en/healthcare-professionals/products/cardiac-rhythm/ablation-atrial-fibrillation/indications-safety-warnings.html 79

ABLATION MODALITIES COMPARISON
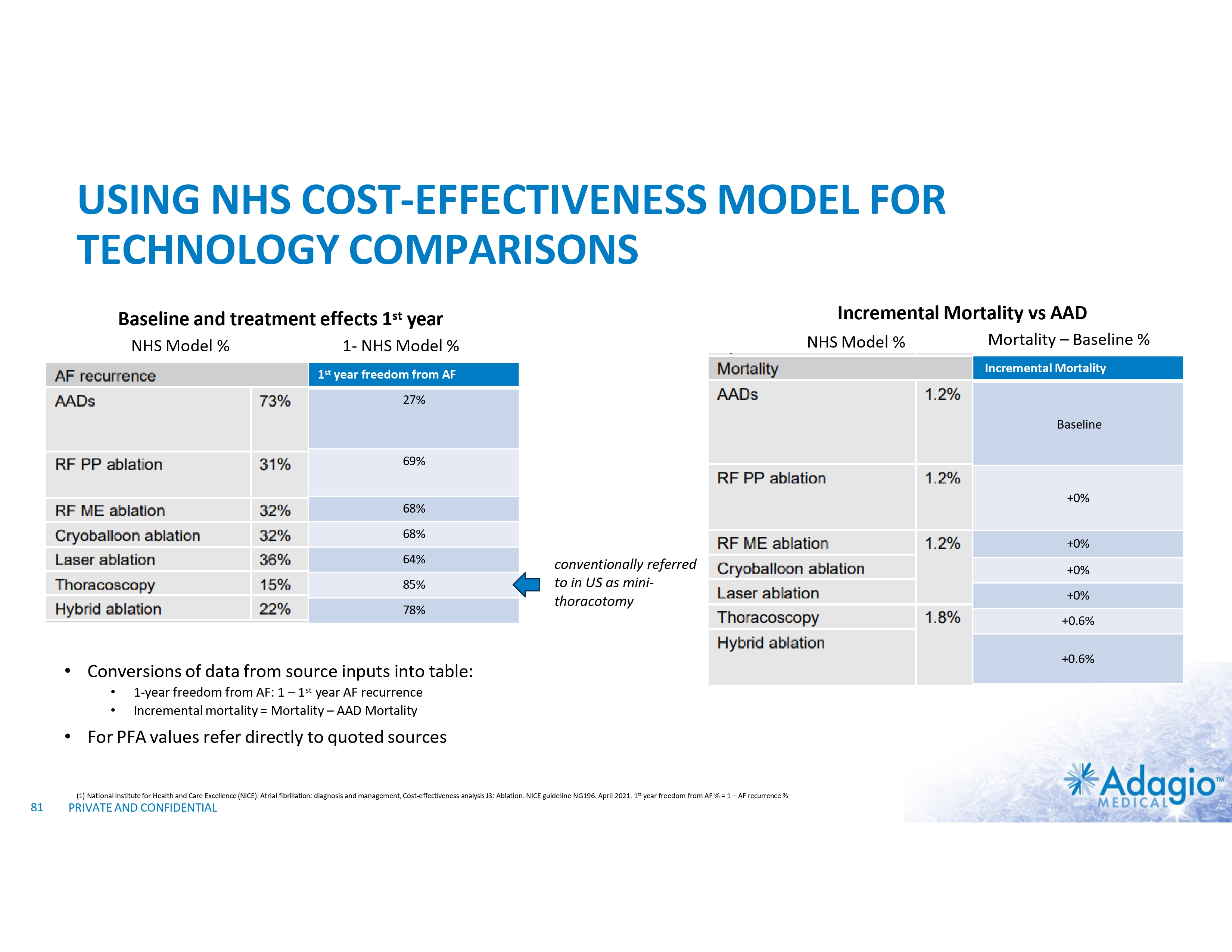
USING NHS COST-EFFECTIVENESS MODEL FOR TECHNOLOGY COMPARISONS Incremental Mortality vs AAD Baseline and treatment effects 1st year NHS Model % NHS Model % 1- NHS Model % Mortality – Baseline % Incremental Mortality 1st year freedom from AF 27% Baseline 69% +0% 68% 68% 64% 85% 78% +0% conventionally referred to in US as mini- thoracotomy • Conversions of data from source inputs into table: • • 1-year freedom from AF: 1 – 1st year AF recurrence Incremental mortality = Mortality – AAD Mortality • For PFA values refer directly to quoted sources (1) National Institute for Health and Care Excellence (NICE). Atrial fibrillation: diagnosis and management, Cost-effectiveness analysis J3: Ablation. NICE guideline NG196. April 2021. 1st year freedom from AF % = 1 – AF recurrence % 81 PRIVATE AND CONFIDENTIAL +0% +0% +0.6% +0.6%
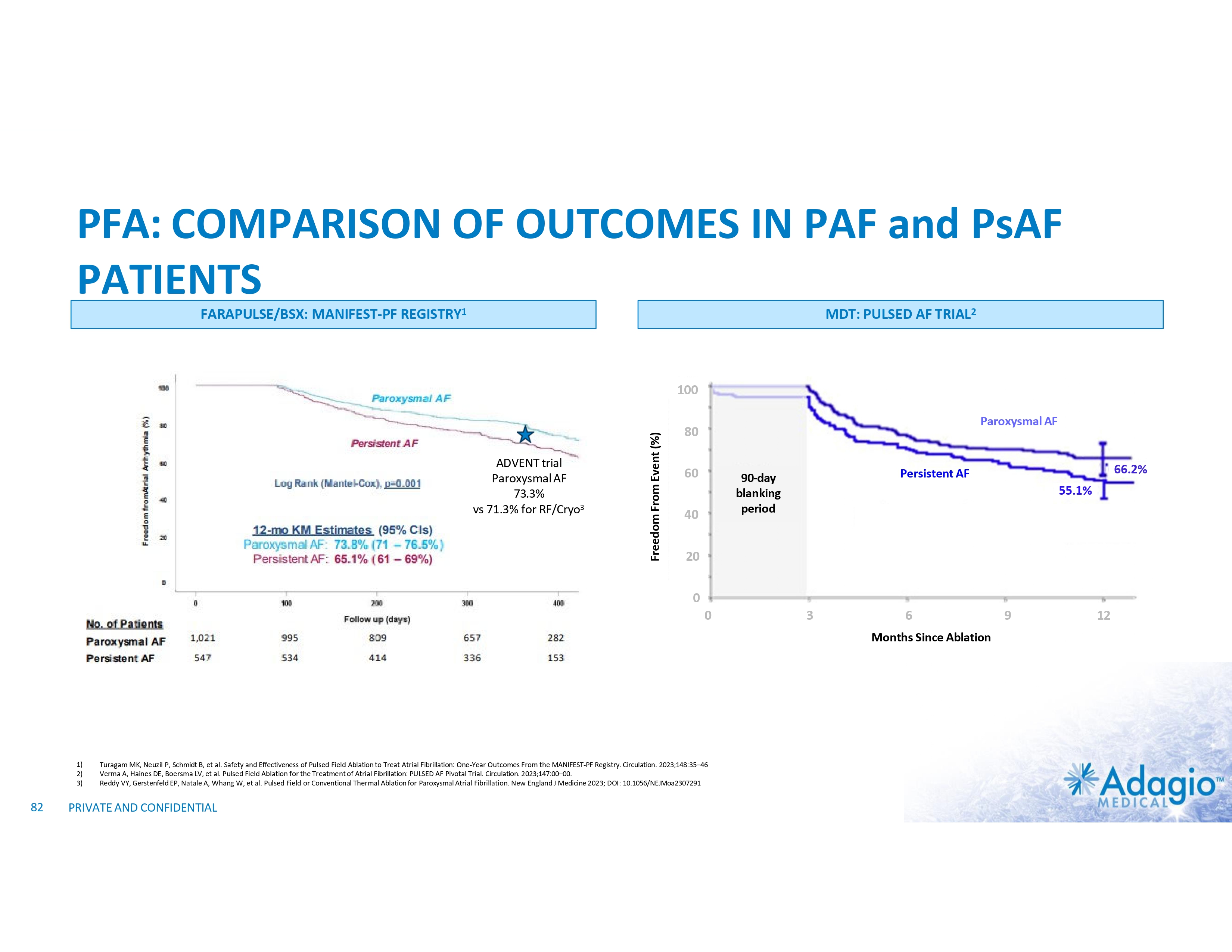
PFA: COMPARISON OF OUTCOMES IN PAF and PsAF PATIENTS FARAPULSE/BSX: MANIFEST-PF REGISTRY1 MDT: PULSED AF TRIAL2 ADVENT trial Paroxysmal AF 73.3% vs 71.3% for RF/Cryo3 Freedom From Event (%) 100 Paroxysmal AF 80 60 40 66.2% Persistent AF 90-day blanking period 55.1% 20 0 0 3 6 Months Since Ablation 1) 2) 3) 82 Turagam MK, Neuzil P, Schmidt B, et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes From the MANIFEST-PF Registry. Circulation. 2023;148:35–46 Verma A, Haines DE, Boersma LV, et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation. 2023;147:00–00. Reddy VY, Gerstenfeld EP, Natale A, Whang W, et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. New England J Medicine 2023; DOI: 10.1056/NEJMoa2307291 PRIVATE AND CONFIDENTIAL 9 12
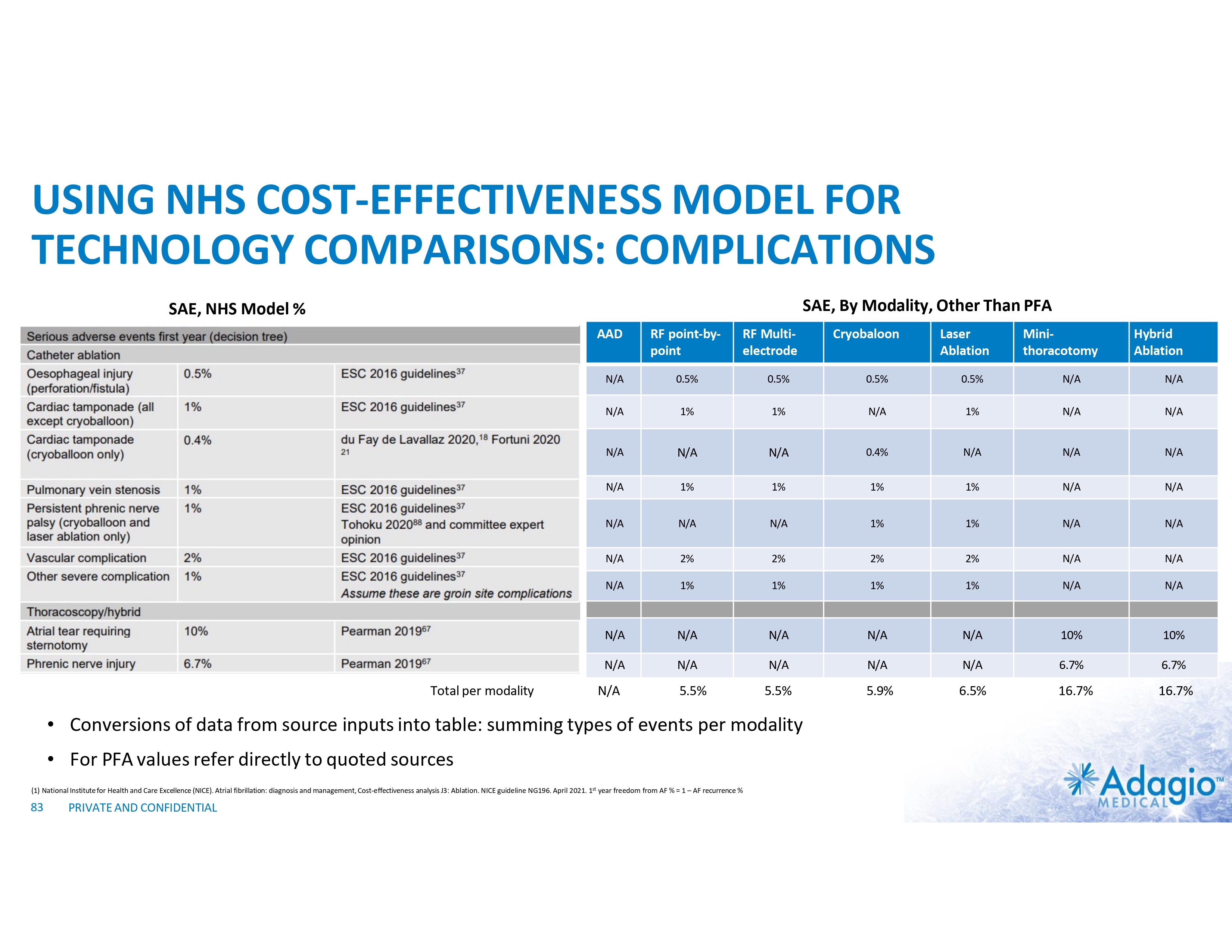
USING NHS COST-EFFECTIVENESS MODEL FOR TECHNOLOGY COMPARISONS: COMPLICATIONS SAE, By Modality, Other Than PFA SAE, NHS Model % AAD Total per modality RF point-by- point RF Multi- electrode Mini- thoracotomy Hybrid Ablation 0.5% 0.5% 0.5% 0.5% N/A N/A N/A 1% 1% N/A 1% N/A N/A N/A N/A N/A 0.4% N/A N/A N/A N/A 1% 1% 1% 1% N/A N/A N/A N/A N/A 1% 1% N/A N/A N/A 2% 2% 2% 2% N/A N/A N/A 1% 1% 1% 1% N/A N/A N/A N/A N/A N/A N/A 10% 10% N/A N/A N/A N/A N/A 6.7% 6.7% 5.5% 5.9% 6.5% 16.7% 16.7% N/A 5.5% • For PFA values refer directly to quoted sources (1) National Institute for Health and Care Excellence (NICE). Atrial fibrillation: diagnosis and management, Cost-effectiveness analysis J3: Ablation. NICE guideline NG196. April 2021. 1st year freedom from AF % = 1 – AF recurrence % PRIVATE AND CONFIDENTIAL Laser Ablation N/A • Conversions of data from source inputs into table: summing types of events per modality 83 Cryobaloon
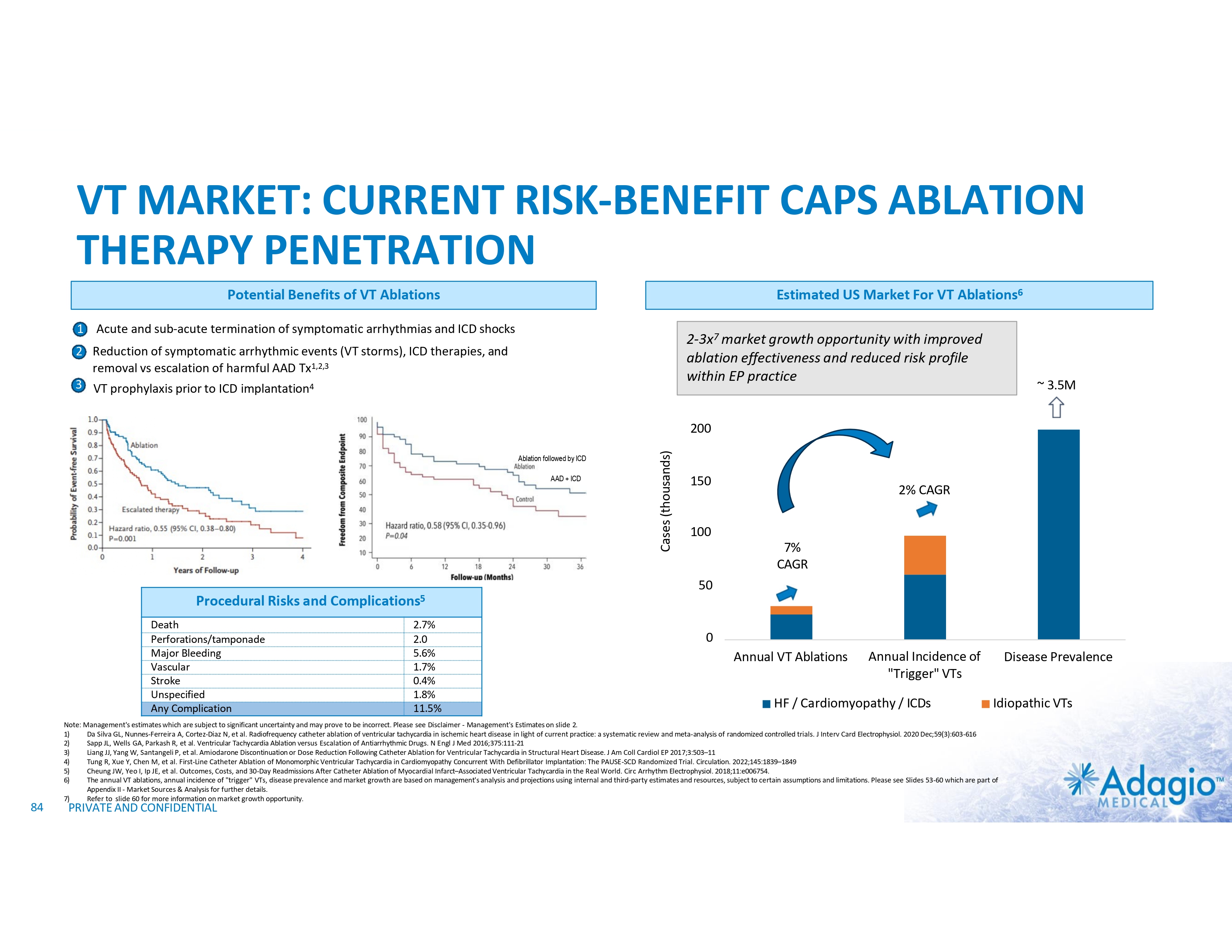
VT MARKET: CURRENT RISK-BENEFIT CAPS ABLATION THERAPY PENETRATION Potential Benefits of VT Ablations Estimated US Market For VT Ablations6 1 Acute and sub-acute termination of symptomatic arrhythmias and ICD shocks 2-3x7 market growth opportunity with improved ablation effectiveness and reduced risk profile within EP practice 2 Reduction of symptomatic arrhythmic events (VT storms), ICD therapies, and removal vs escalation of harmful AAD Tx1,2,3 3 VT prophylaxis prior to ICD implantation4 ~ 3.5M Ablation followed by ICD AAD + ICD Cases (thousands) 200 150 2% CAGR 100 7% CAGR 50 Procedural Risks and Complications5 Death Perforations/tamponade Major Bleeding Vascular Stroke Unspecified Any Complication 84 2.7% 2.0 5.6% 1.7% 0.4% 1.8% 11.5% 0 Annual VT Ablations Annual Incidence of "Trigger" VTs HF / Cardiomyopathy / ICDs Disease Prevalence Idiopathic VTs Note: Management's estimates which are subject to significant uncertainty and may prove to be incorrect. Please see Disclaimer - Management's Estimates on slide 2. 1) Da Silva GL, Nunnes-Ferreira A, Cortez-Diaz N, et al. Radiofrequency catheter ablation of ventricular tachycardia in ischemic heart disease in light of current practice: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2020 Dec;59(3):603-616 2) Sapp JL, Wells GA, Parkash R, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 2016;375:111-21 3) Liang JJ, Yang W, Santangeli P, et al. Amiodarone Discontinuation or Dose Reduction Following Catheter Ablation for Ventricular Tachycardia in Structural Heart Disease. J Am Coll Cardiol EP 2017;3:503–11 4) Tung R, Xue Y, Chen M, et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent With Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation. 2022;145:1839–1849 5) Cheung JW, Yeo I, Ip JE, et al. Outcomes, Costs, and 30-Day Readmissions After Catheter Ablation of Myocardial Infarct–Associated Ventricular Tachycardia in the Real World. Circ Arrhythm Electrophysiol. 2018;11:e006754. 6) The annual VT ablations, annual incidence of "trigger" VTs, disease prevalence and market growth are based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 53-60 which are part of Appendix II - Market Sources & Analysis for further details. 7) Refer to slide 60 for more information on market growth opportunity. PRIVATE AND CONFIDENTIAL

Appendix III – TECHNICAL SUPPLEMENT 85
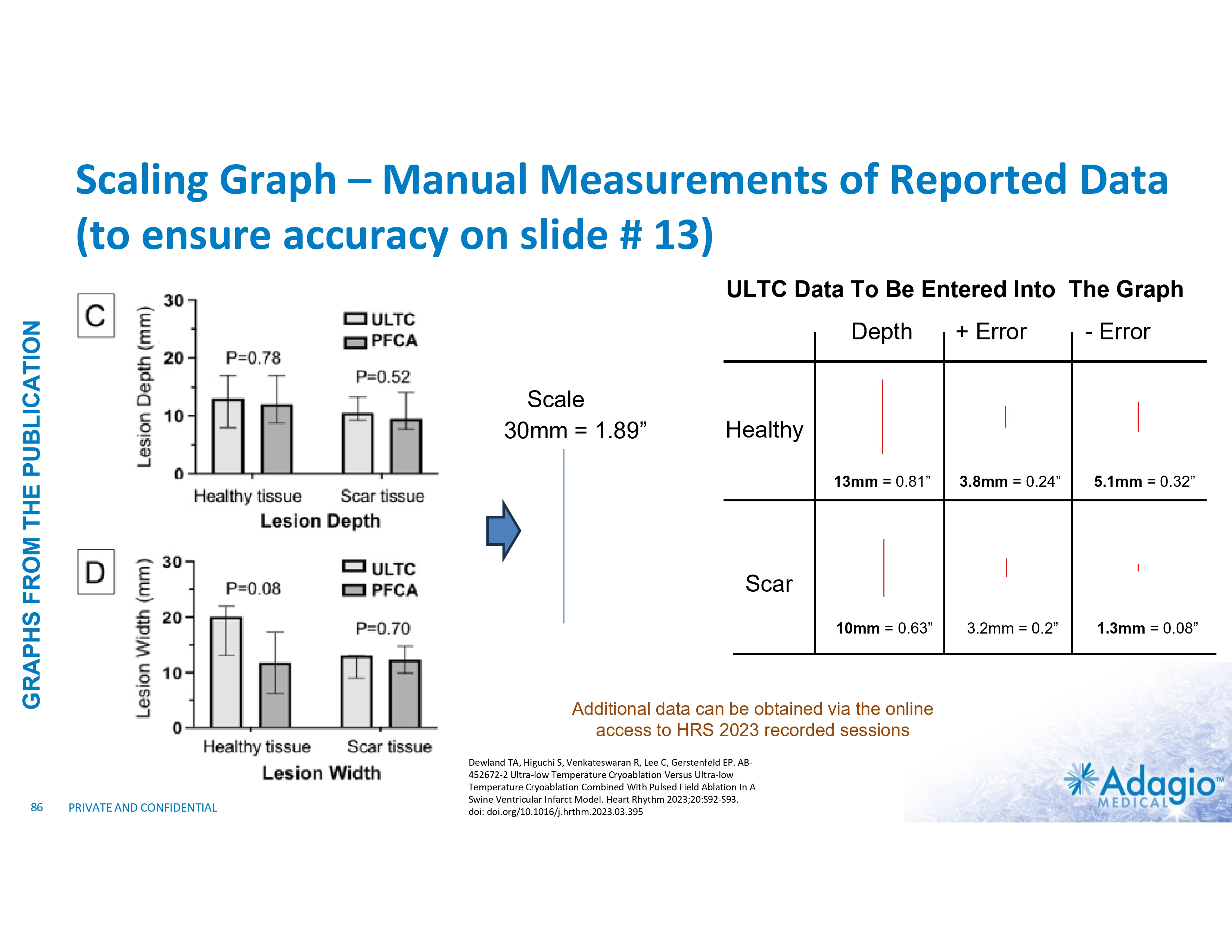
Scaling Graph – Manual Measurements of Reported Data (to ensure accuracy on slide # 13) ULTC Data To Be Entered Into The Graph GRAPHS FROM THE PUBLICATION Depth 86 Scale 30mm = 1.89” + Error - Error Healthy 13mm = 0.81” 3.8mm = 0.24” 5.1mm = 0.32” 10mm = 0.63” 3.2mm = 0.2” 1.3mm = 0.08” Scar Additional data can be obtained via the online access to HRS 2023 recorded sessions PRIVATE AND CONFIDENTIAL Dewland TA, Higuchi S, Venkateswaran R, Lee C, Gerstenfeld EP. AB- 452672-2 Ultra-low Temperature Cryoablation Versus Ultra-low Temperature Cryoablation Combined With Pulsed Field Ablation In A Swine Ventricular Infarct Model. Heart Rhythm 2023;20:S92-S93. doi: doi.org/10.1016/j.hrthm.2023.03.395

Appendix IV - OLDER SLIDES 87
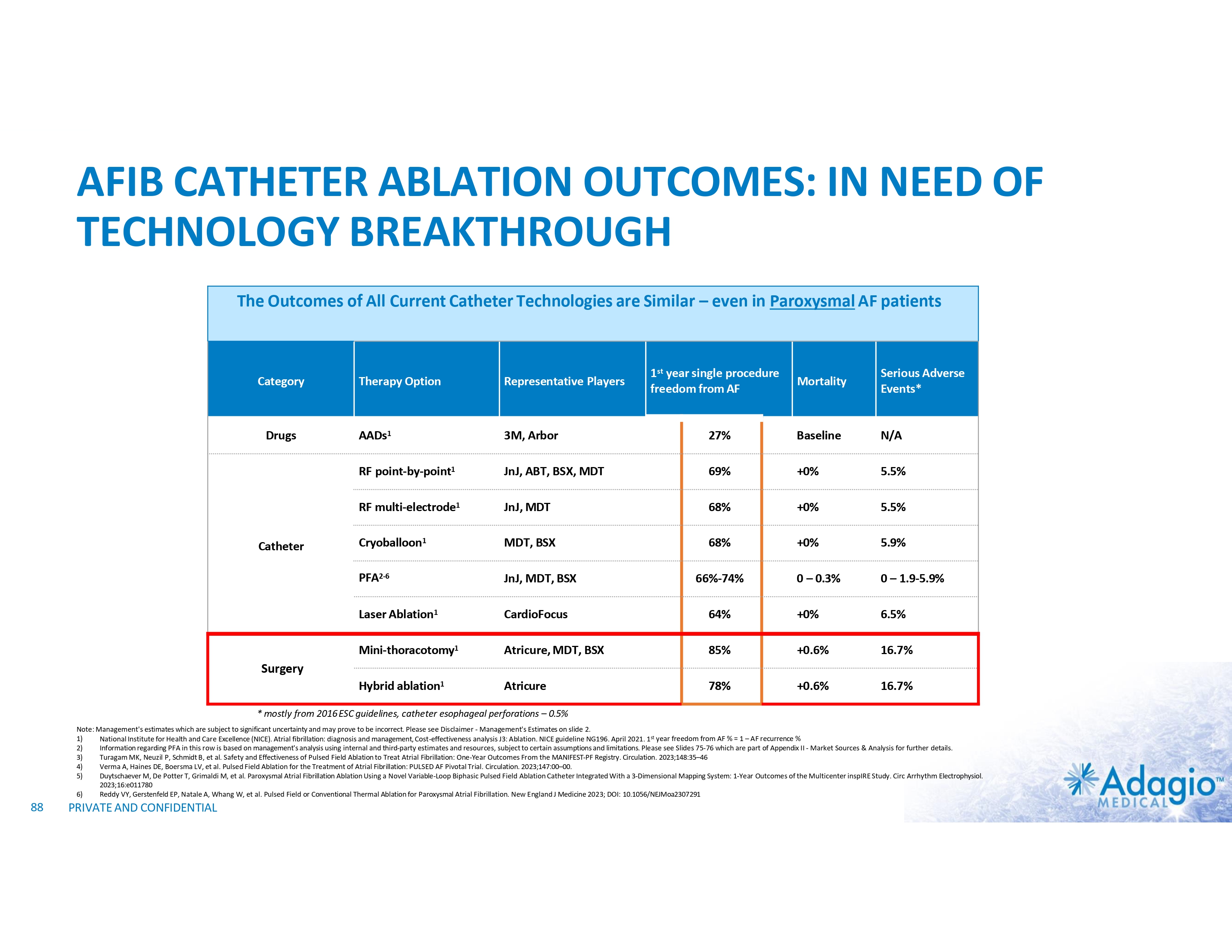
AFIB CATHETER ABLATION OUTCOMES: IN NEED OF TECHNOLOGY BREAKTHROUGH The Outcomes of All Current Catheter Technologies are Similar – even in Paroxysmal AF patients Category Drugs Catheter Mortality Serious Adverse Events* 27% Baseline N/A JnJ, ABT, BSX, MDT 69% +0% 5.5% RF multi-electrode1 JnJ, MDT 68% +0% 5.5% Cryoballoon1 MDT, BSX 68% +0% 5.9% PFA2-6 JnJ, MDT, BSX 0 – 0.3% 0 – 1.9-5.9% Laser Ablation1 CardioFocus 64% +0% 6.5% Mini-thoracotomy1 Atricure, MDT, BSX 85% +0.6% 16.7% Hybrid ablation1 Atricure 78% +0.6% 16.7% Therapy Option Representative Players AADs1 3M, Arbor RF point-by-point1 1st year single procedure freedom from AF 66%-74% Surgery * mostly from 2016 ESC guidelines, catheter esophageal perforations – 0.5% Note: Management's estimates which are subject to significant uncertainty and may prove to be incorrect. Please see Disclaimer - Management's Estimates on slide 2. 1) National Institute for Health and Care Excellence (NICE). Atrial fibrillation: diagnosis and management, Cost-effectiveness analysis J3: Ablation. NICE guideline NG196. April 2021. 1st year freedom from AF % = 1 – AF recurrence % 2) Information regarding PFA in this row is based on management's analysis using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 75-76 which are part of Appendix II - Market Sources & Analysis for further details. 3) Turagam MK, Neuzil P, Schmidt B, et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes From the MANIFEST-PF Registry. Circulation. 2023;148:35–46 4) Verma A, Haines DE, Boersma LV, et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation. 2023;147:00–00. 5) Duytschaever M, De Potter T, Grimaldi M, et al. Paroxysmal Atrial Fibrillation Ablation Using a Novel Variable-Loop Biphasic Pulsed Field Ablation Catheter Integrated With a 3-Dimensional Mapping System: 1-Year Outcomes of the Multicenter inspIRE Study. Circ Arrhythm Electrophysiol. 2023;16:e011780 6) Reddy VY, Gerstenfeld EP, Natale A, Whang W, et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. New England J Medicine 2023; DOI: 10.1056/NEJMoa2307291 88 PRIVATE AND CONFIDENTIAL

ACQUISITIONS HAVE DRIVEN CONSOLIDATION AMONG COMPETITORS / Year Purchase Price ($mm) Year Purchase Price ($mm) Year Purchase Price ($mm) Target Year Purchase Price ($mm) 2008 388 2001 115 2004 47 Irvine Biomedical 1997 400 2009 225 2012 265 2005 237 2019 316 2013 275 2008 92 2022 925 2018 202 2008 250 2021 450 2013 331 2022 1,750 2014 250 Target $1.8bn+ worth of acquisitions Target $3.0bn+ worth of acquisitions Source: Press releases 89 PRIVATE AND CONFIDENTIAL $1.2bn+ worth of acquisitions Target
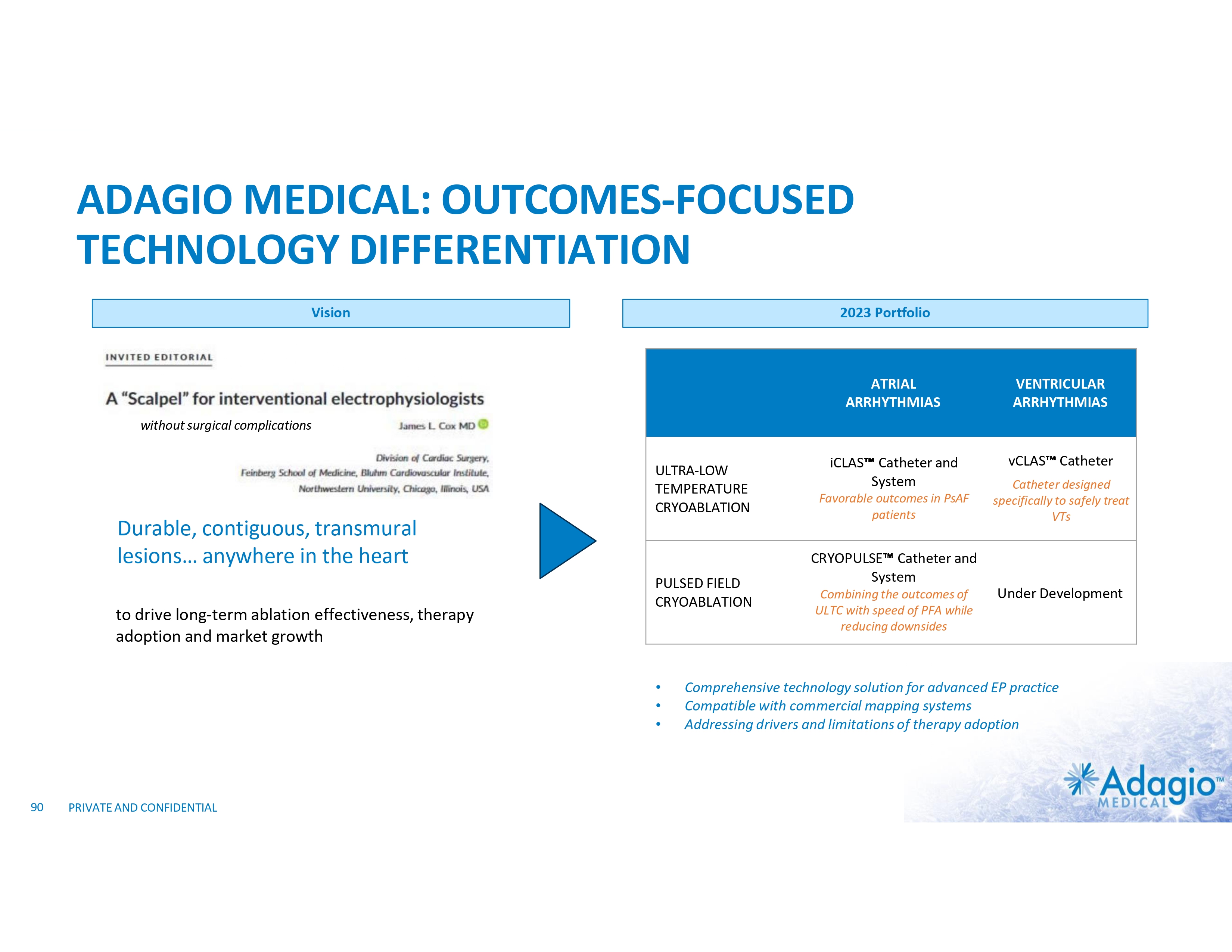
ADAGIO MEDICAL: OUTCOMES-FOCUSED TECHNOLOGY DIFFERENTIATION Vision 2023 Portfolio ATRIAL ARRHYTHMIAS VENTRICULAR ARRHYTHMIAS iCLAS Catheter and System vCLAS Catheter without surgical complications ULTRA-LOW TEMPERATURE CRYOABLATION Durable, contiguous, transmural lesions… anywhere in the heart to drive long-term ablation effectiveness, therapy adoption and market growth PULSED FIELD CRYOABLATION • • • 90 PRIVATE AND CONFIDENTIAL Favorable outcomes in PsAF patients Catheter designed specifically to safely treat VTs CRYOPULSE Catheter and System Combining the outcomes of ULTC with speed of PFA while reducing downsides Under Development Comprehensive technology solution for advanced EP practice Compatible with commercial mapping systems Addressing drivers and limitations of therapy adoption
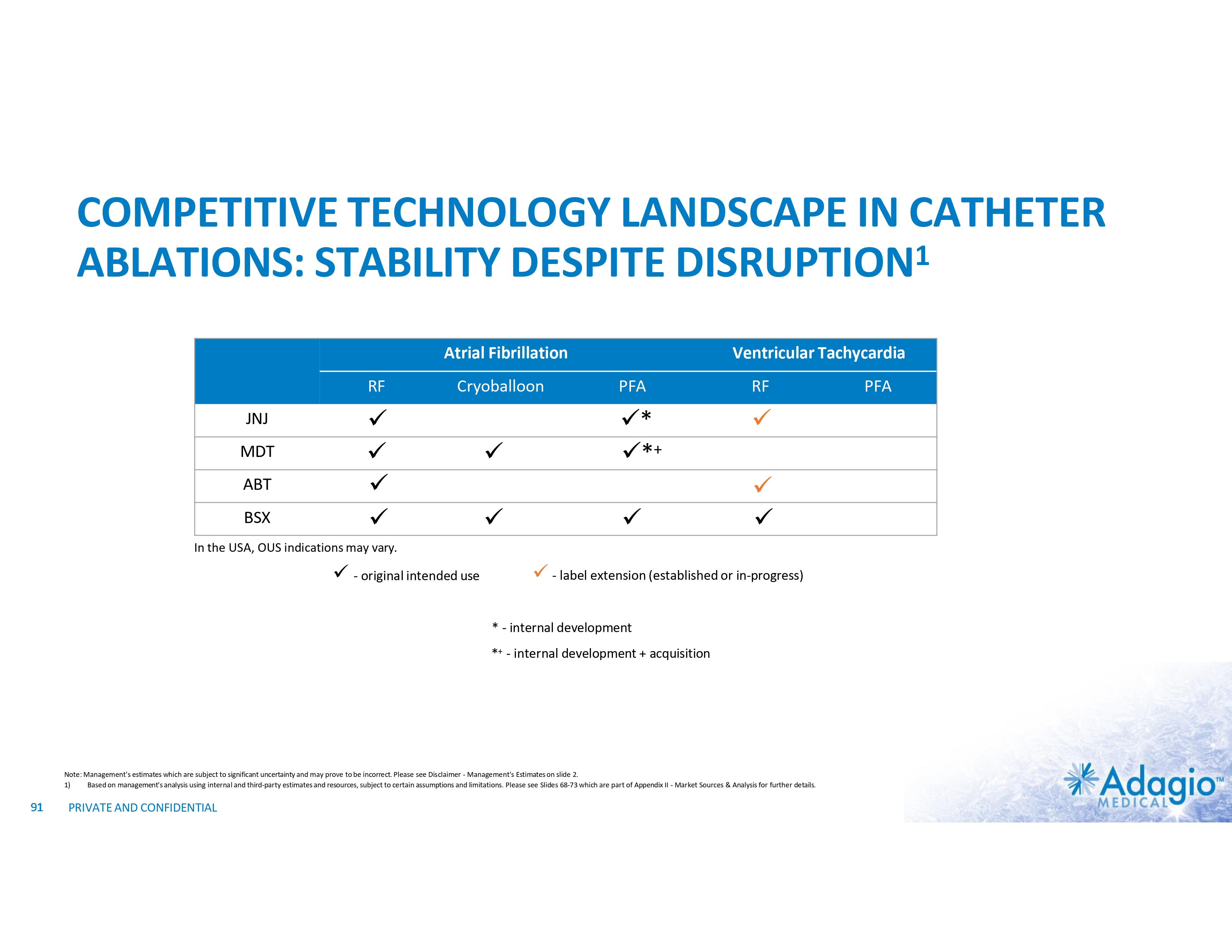
COMPETITIVE TECHNOLOGY LANDSCAPE IN CATHETER ABLATIONS: STABILITY DESPITE DISRUPTION1 Atrial Fibrillation RF JNJ MDT ABT BSX Cryoballoon x x x x x x Ventricular Tachycardia PFA RF x* x*+ x x x x In the USA, OUS indications may vary. x - original intended use x - label extension (established or in-progress) * - internal development *+ - internal development + acquisition Note: Management's estimates which are subject to significant uncertainty and may prove to be incorrect. Please see Disclaimer - Management's Estimates on slide 2. 1) Based on management's analysis using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 68-73 which are part of Appendix II - Market Sources & Analysis for further details. 91 PRIVATE AND CONFIDENTIAL PFA
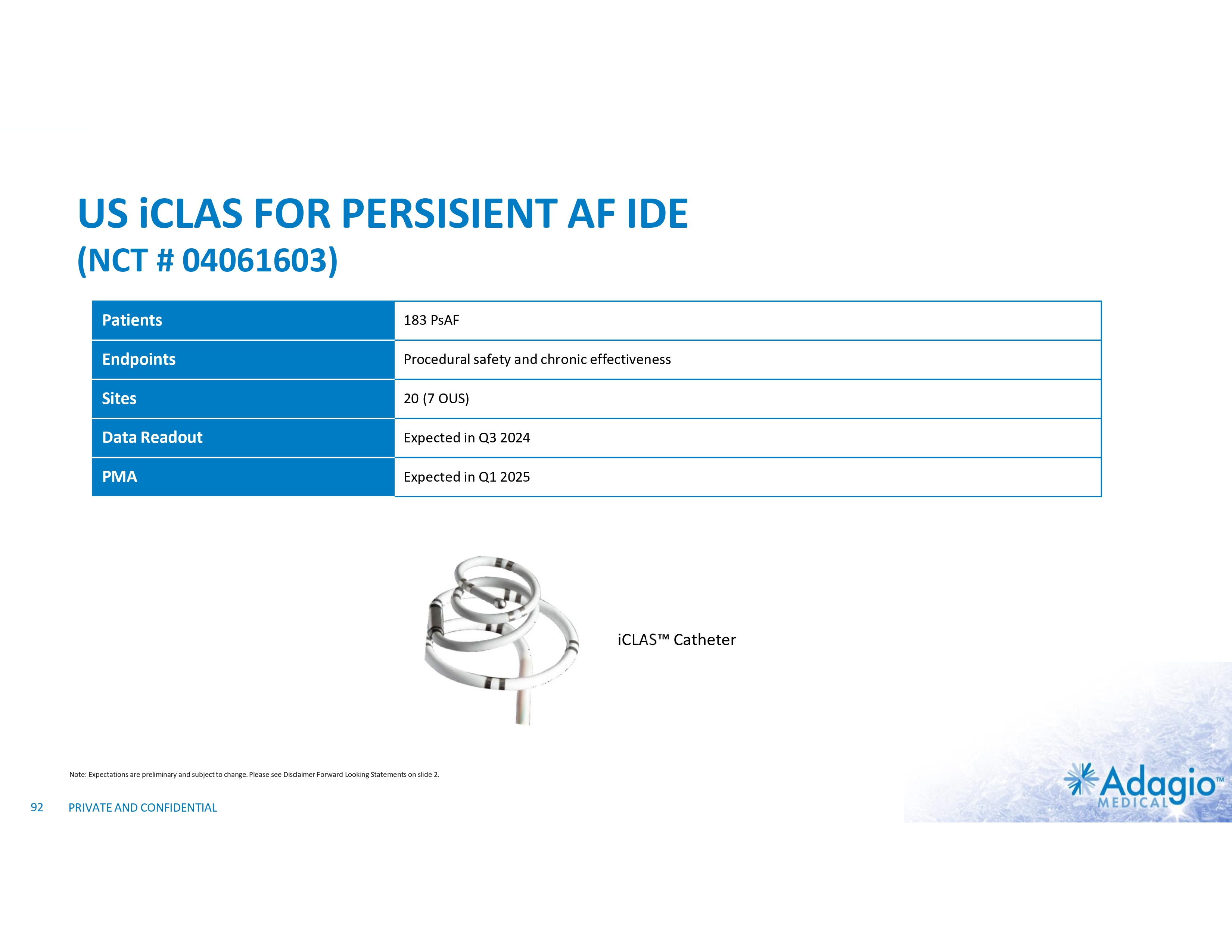
US iCLAS FOR PERSISIENT AF IDE (NCT # 04061603) Patients 183 PsAF Endpoints Procedural safety and chronic effectiveness Sites 20 (7 OUS) Data Readout Expected in Q3 2024 PMA Expected in Q1 2025 iCLAS Catheter Note: Expectations are preliminary and subject to change. Please see Disclaimer Forward Looking Statements on slide 2. 92 PRIVATE AND CONFIDENTIAL
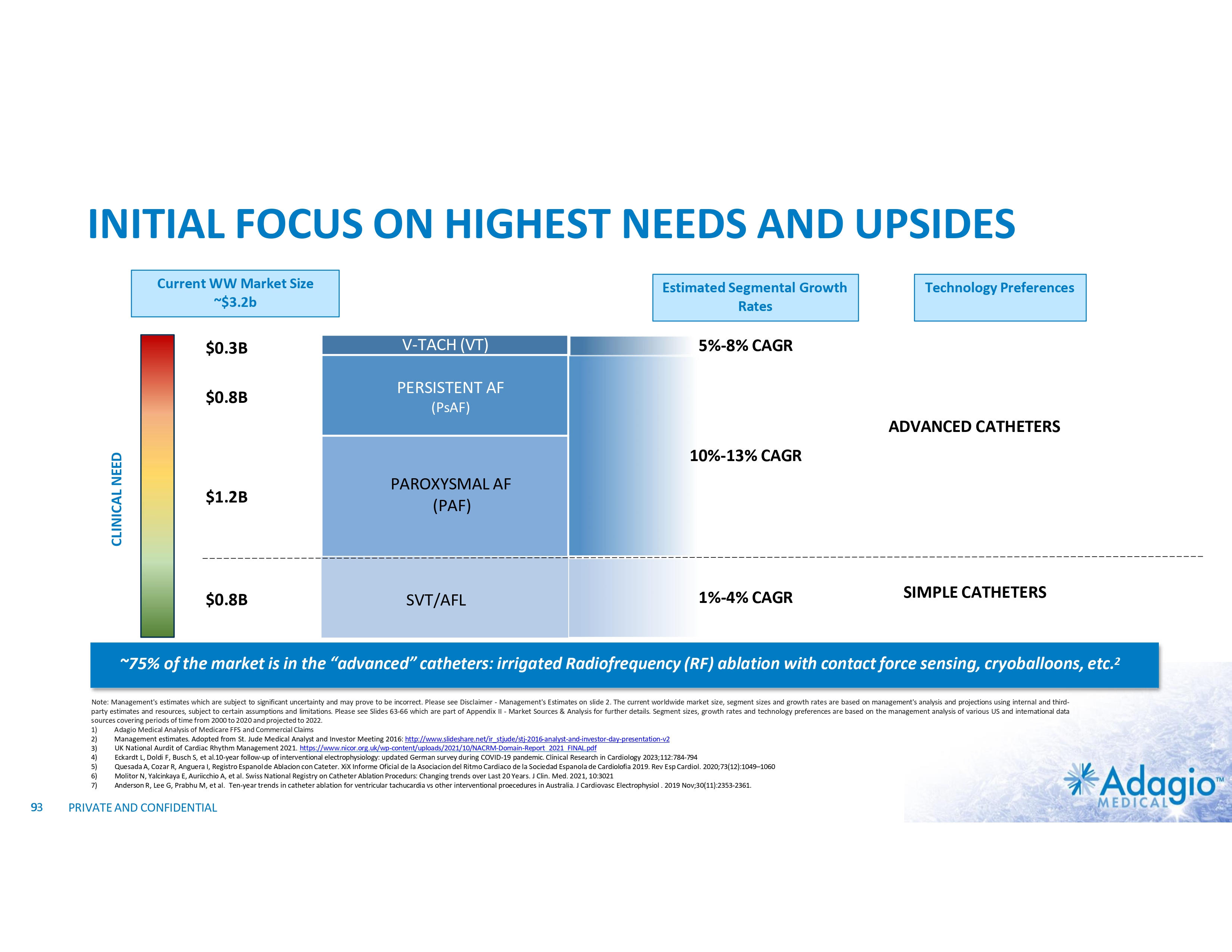
INITIAL FOCUS ON HIGHEST NEEDS AND UPSIDES Current WW Market Size ~$3.2b $0.3B $0.8B Estimated Segmental Growth Rates V-TACH (VT) Technology Preferences 5%-8% CAGR PERSISTENT AF (PsAF) CLINICAL NEED ADVANCED CATHETERS 10%-13% CAGR $1.2B $0.8B PAROXYSMAL AF (PAF) SVT/AFL 1%-4% CAGR SIMPLE CATHETERS ~75% of the market is in the “advanced” catheters: irrigated Radiofrequency (RF) ablation with contact force sensing, cryoballoons, etc.2 Note: Management's estimates which are subject to significant uncertainty and may prove to be incorrect. Please see Disclaimer - Management's Estimates on slide 2. The current worldwide market size, segment sizes and growth rates are based on management's analysis and projections using internal and third- party estimates and resources, subject to certain assumptions and limitations. Please see Slides 63-66 which are part of Appendix II - Market Sources & Analysis for further details. Segment sizes, growth rates and technology preferences are based on the management analysis of various US and international data sources covering periods of time from 2000 to 2020 and projected to 2022. 1) Adagio Medical Analysis of Medicare FFS and Commercial Claims 2) Management estimates. Adopted from St. Jude Medical Analyst and Investor Meeting 2016: http://www.slideshare.net/ir_stjude/stj-2016-analyst-and-investor-day-presentation-v2 UK National Aurdit of Cardiac Rhythm Management 2021. https://www.nicor.org.uk/wp-content/uploads/2021/10/NACRM-Domain-Report_2021_FINAL.pdf 3) Eckardt L, Doldi F, Busch S, et al.10-year follow-up of interventional electrophysiology: updated German survey during COVID-19 pandemic. Clinical Research in Cardiology 2023;112:784-794 4) Quesada A, Cozar R, Anguera I, Registro Espanol de Ablacion con Cateter. XiX Informe Oficial de la Asociacion del Ritmo Cardiaco de la Sociedad Espanola de Cardiolofia 2019. Rev Esp Cardiol. 2020;73(12):1049–1060 5) Molitor N, Yalcinkaya E, Auriicchio A, et al. Swiss National Registry on Catheter Ablation Procedurs: Changing trends over Last 20 Years. J Clin. Med. 2021, 10:3021 6) Anderson R, Lee G, Prabhu M, et al. Ten-year trends in catheter ablation for ventricular tachucardia vs other interventional proecedures in Australia. J Cardiovasc Electrophysiol . 2019 Nov;30(11):2353-2361. 7) 93 9 PRIVATE AND CONFIDENTIAL
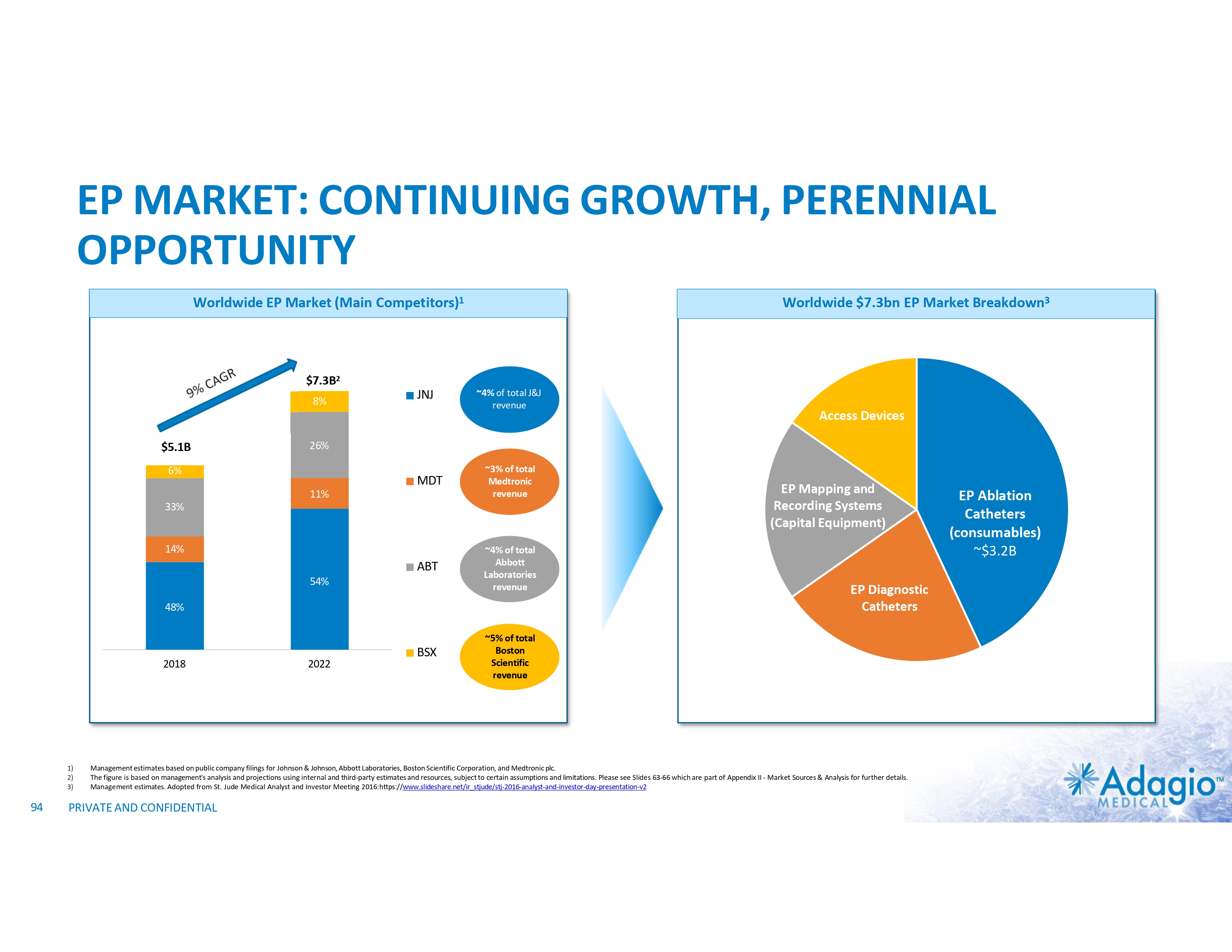
EP MARKET: CONTINUING GROWTH, PERENNIAL OPPORTUNITY Worldwide EP Market (Main Competitors)1 Worldwide $7.3bn EP Market Breakdown3 $7.3B2 8% $5.1B JNJ ~4% of total J&J revenue 26% 6% MDT 11% ~3% of total Medtronic revenue 33% 14% ABT 54% ~4% of total Abbott Laboratories revenue 48% 2018 1) 2) 3) 94 Access Devices 2022 BSX EP Mapping and Recording Systems (Capital Equipment) EP Diagnostic Catheters ~5% of total Boston Scientific revenue Management estimates based on public company filings for Johnson & Johnson, Abbott Laboratories, Boston Scientific Corporation, and Medtronic plc. The figure is based on management's analysis and projections using internal and third-party estimates and resources, subject to certain assumptions and limitations. Please see Slides 63-66 which are part of Appendix II - Market Sources & Analysis for further details. Management estimates. Adopted from St. Jude Medical Analyst and Investor Meeting 2016:https://www.slideshare.net/ir_stjude/stj-2016-analyst-and-investor-day-presentation-v2 PRIVATE AND CONFIDENTIAL EP Ablation Catheters (consumables) ~$3.2B





























































































