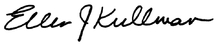2012
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K |
| | |
| (Mark One) |
| ý | | ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the fiscal year ended December 31, 2012 |
| OR |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
____________________________________________________________________________
Commission file number 1-815
E. I. DU PONT DE NEMOURS AND COMPANY
(Exact name of registrant as specified in its charter)
|
| | |
DELAWARE (State or Other Jurisdiction of Incorporation or Organization) | | 51-0014090 (I.R.S. Employer Identification No.) |
1007 Market Street
Wilmington, Delaware 19898
(Address of principal executive offices)
Registrant's telephone number, including area code: 302-774-1000
Securities registered pursuant to Section 12(b) of the Act
(Each class is registered on the New York Stock Exchange, Inc.):
Title of Each Class
__________________________________________________
Common Stock ($.30 par value)
Preferred Stock
(without par value-cumulative)
$4.50 Series
$3.50 Series
No securities are registered pursuant to Section 12(g) of the Act.
_____________________________________________________
Indicate by check mark whether the registrant is a well-known seasoned issuer (as defined in Rule 405 of the Securities Act).
Yes ý No o
Indicate by check mark whether the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of "accelerated filer and large accelerated filer" in Rule 12b-2 of the Exchange Act.
|
| | | |
Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
The aggregate market value of voting stock held by nonaffiliates of the registrant (excludes outstanding shares beneficially owned by directors and officers and treasury shares) as of June 30, 2012, was approximately $47.0 billion.
As of January 31, 2013, 934,288,000 shares (excludes 87,041,000 shares of treasury stock) of the company's common stock, $0.30 par value, were outstanding.
Documents Incorporated by Reference
(Specific pages incorporated are indicated under the applicable Item herein):
|
| | |
| | | Incorporated By Reference In Part No. |
| The company's Proxy Statement in connection with the Annual Meeting of Stockholders to be held on April 24, 2013. | | III |
E. I. du Pont de Nemours and Company
Form 10-K
Table of Contents
The terms "DuPont" or the "company" as used herein refer to E. I. du Pont de Nemours and Company and its consolidated subsidiaries, or to E. I. du Pont de Nemours and Company, as the context may indicate.
Note on Incorporation by Reference
Information pertaining to certain Items in Part III of this report is incorporated by reference to portions of the company's definitive 2013 Annual Meeting Proxy Statement to be filed within 120 days after the end of the year covered by this Annual Report on Form 10-K, pursuant to Regulation 14A (the Proxy).
ITEM 1. BUSINESS
DuPont was founded in 1802 and was incorporated in Delaware in 1915. DuPont brings world-class science and engineering to the global marketplace in the form of innovative products, materials and services. The company believes that by collaborating with customers, governments, non-governmental organizations and thought leaders it can help find solutions to such global challenges as providing enough healthy food for people everywhere, decreasing dependence on fossil fuels, and protecting life and the environment. Total worldwide employment at December 31, 2012, was about 70,000 people. The company has operations in more than 90 countries worldwide and about 60 percent of consolidated net sales are made to customers outside the United States of America (U.S.). See Note 21 to the Consolidated Financial Statements for additional details on the location of the company's sales and property.
Subsidiaries and affiliates of DuPont conduct manufacturing, seed production or selling activities and some are distributors of products manufactured by the company. As a science and technology based company, DuPont competes on a variety of factors such as product quality and performance or specifications, continuity of supply, price, customer service and breadth of product line, depending on the characteristics of the particular market involved and the product or service provided. Most products are marketed primarily through the company's sales force, although in some regions, more emphasis is placed on sales through distributors. The company utilizes numerous suppliers as well as internal sources to supply a wide range of raw materials, energy, supplies, services and equipment. To ensure availability, the company maintains multiple sources for fuels and many raw materials, including hydrocarbon feedstocks. Large volume purchases are generally procured under competitively priced supply contracts.
In third quarter 2012, the company entered into a definitive agreement to sell its Performance Coatings business (which represented a reportable segment) for approximately $4.9 billion in cash and the assumption of certain liabilities. In accordance with generally accepted accounting principles in the U.S. (GAAP), the results of Performance Coatings are presented as discontinued operations and, as such, have been excluded from continuing operations and segment results for all periods presented. On February 1, 2013, the sale of Performance Coatings was completed.
Business Segments
The company consists of 13 businesses which are aggregated into eight reportable segments based on similar economic characteristics, the nature of the products and production processes, end-use markets, channels of distribution and regulatory environment. The company's reportable segments are Agriculture, Electronics & Communications, Industrial Biosciences, Nutrition & Health, Performance Chemicals, Performance Materials, Safety & Protection and Pharmaceuticals. The company includes certain embryonic businesses not included in the reportable segments, such as pre-commercial programs, and nonaligned businesses in Other. Additional information with respect to business segment results is included in Item 7, Management's Discussion and Analysis of Financial Condition and Results of Operations, on page 22 of this report and Note 22 to the Consolidated Financial Statements.
Agriculture
Agriculture businesses, DuPont Pioneer and DuPont Crop Protection, leverage the company's technology, customer relationships and industry knowledge to improve the quantity, quality and safety of the global food supply and the global production agriculture industry. Land available for worldwide agricultural production is increasingly limited so production growth will need to be achieved principally through improving crop yields and productivity rather than through increases in planted area. The segment's businesses deliver a broad portfolio of products and services that are specifically targeted to achieve gains in crop yields and productivity, including Pioneer® brand seed products and well-established brands of insecticides, fungicides and herbicides. Research and development focuses on leveraging technology to increase grower productivity and enhance the value of grains and soy through improved seed traits, superior seed germplasm and effective use of insecticides, herbicides and fungicides. Agriculture accounted for approximately 50 percent of the company's total research and development expense in 2012.
Sales of the company's products in the segment are affected by the seasonality of global agriculture markets and weather patterns. Sales and earnings performance in the Agriculture segment are strongest in the first half of the year reflecting the northern hemisphere planting season. The segment generally operates at a loss during the third and fourth quarters of the year. As a result of the seasonal nature of its business, Agriculture's inventory is at its highest level at the end of the calendar year and is sold down in the first and second quarters. Trade receivables in the Agriculture segment are at a low point at year-end and increase through the selling season to peak at the end of the second quarter.
Pioneer is a world leader in developing, producing and marketing corn hybrid and soybean varieties which improve the productivity and profitability of its customers. Additionally, Pioneer develops, produces and markets canola, sunflower, sorghum, inoculants, wheat and rice. As the world's population grows and the middle class expands, the need for crops for animal feed, food, biofuels and industrial uses continues to increase. The business competes with other seed and plant biotechnology companies. Pioneer
Part I
ITEM 1. BUSINESS, continued
seed sales amounted to 21 percent, 19 percent and 19 percent of the company's total consolidated net sales for the years ended December 31, 2012, 2011 and 2010, respectively.
Pioneer's research and development focuses on integrating high yielding germplasm with value added proprietary and/or licensed native and biotechnology traits with local environment and service expertise. Pioneer uniquely develops integrated products for specific regional application based on local product advancement and testing of the product concepts. Research and development in this arena requires long-term commitment of resources, extensive regulatory efforts and collaborations, partnerships and business arrangements to successfully bring products to market. Pioneer licenses biotechnology traits from third parties as a normal course of business. To protect its investment, the business employs the use of patents covering germplasm and native and biotechnology traits in accordance with country laws.
Pioneer is actively pursuing the development of innovations for corn hybrid, soybean varieties, canola, sunflower, wheat and rice based on market assessments of the most valuable opportunities. In corn hybrids, programs include innovations for drought and nitrogen efficiency, insect protection and herbicide tolerance. In soybean varieties, programs include products with high oleic content, multiple herbicide tolerance and insect protection.
Pioneer has seed production facilities located throughout the world. Seed production is performed directly by the business or contracted with independent growers and conditioners. Pioneer's ability to produce seeds primarily depends upon weather conditions and availability of reliable contract growers.
Pioneer markets and sells seed product primarily under the Pioneer® brand but also sells and distributes products utilizing additional brand names. Pioneer promotes its products through multiple marketing channels around the world. In the corn and soybean markets of the U.S. Corn Belt, Pioneer® brand products are sold primarily through a specialized force of independent sales representatives. Outside of North America, Pioneer's products are marketed through a network of subsidiaries, joint ventures and independent producer-distributors.
DuPont Crop Protection serves the global production agriculture industry with crop protection products for field crops such as wheat, corn, soybean and rice; specialty crops such as fruit, nut, vine and vegetables; and non-crop segments, including forestry and land management. Principle crop protection products are weed control, disease control and insect control offerings. Crop Protection products are marketed and sold to growers and other end users through a network of wholesale distributors and crop input retailers. The sales growth of the business' insect control portfolio is led by DuPontTM Rynaxypyr® insecticide, a product registered for sale in 80 countries and sold under four key brands for use across a broad range of core agricultural crops.
The major commodities, raw materials and supplies for the Agriculture segment include: benzene and carbamic acid related intermediates, copper, corn and soybean seeds, insect control products, natural gas, seed treatments and sulfonamides.
Agriculture segment sales outside the U.S. accounted for 53 percent of the segment's total sales in 2012.
Electronics & Communications
Electronics & Communications (E&C) is a leading supplier of differentiated materials and systems for photovoltaics (PV), consumer electronics, displays and advanced printing that enable superior performance and lower total cost of ownership for customers. The segment leverages the company's strong materials and technology base to target attractive growth opportunities in PV materials, circuit and semiconductor fabrication and packaging materials, display materials, packaging graphics, and ink-jet printing. In the growing PV market, E&C continues to be an industry-leading innovator and supplier of metalization pastes and backsheet materials that improve the efficiency and lifetime of solar cells and modules. In 2012, the segment commercialized new DuPontTM Solamet® PV metallization paste to drive step-change gains in efficiency of solar cells. DuPont is a leading global supplier of materials to the PV industry.
In the displays market, E&C has developed solution-process technology, which it licenses, and a growing range of materials for active matrix organic light emitting diode (AMOLED) television displays. The segment has a broad portfolio of materials for semiconductor fabrication and packaging, as well as innovative materials for circuit applications, to address critical needs of electronic component and device manufacturers. In consumer electronics, E&C materials add value in the high growth hand-held markets of tablets and smart phones. In packaging graphics, E&C is a leading supplier of flexographic printing systems, including Cyrel® photopolymer plates and platemaking systems. The segment is investing in new products to strengthen its market leadership position in advanced printing markets. The segment is also expanding its leadership position in black-pigmented inks and developing new color-pigmented inks for network printing applications.
Part I
ITEM 1. BUSINESS, continued
The major commodities, raw materials and supplies for E&C include: block co-polymers, copper, difluoroethane, hydroxylamine, oxydianiline, polyester film, precious metals and pyromellitic dianhydride.
E&C segment sales outside the U.S. accounted for 84 percent of the segment's total sales in 2012.
Industrial Biosciences
Industrial Biosciences is a leader in developing and manufacturing a broad portfolio of enzymes. The segment's enzymes add value and functionality to a broad range of products and processes such as animal nutrition, detergents, food manufacturing, ethanol production and industrial applications resulting in cost and process benefits, better product performance and improved environmental outcomes. Industrial Biosciences also makes DuPontTM Sorona® PTT renewably sourced polymer for use in carpet and apparel fibers.
The segment includes a joint venture with Tate & Lyle PLC, DuPont Tate and Lyle Bio Products LLC, to produce BioPDOTM 1,3 propanediol using a proprietary fermentation and purification process. BioPDOTM is the key building block for DuPontTM Sorona® PTT polymer.
The major commodities, raw materials and supplies for the Industrial Biosciences segment include: glucoamylase, glycols, grain products, such as dextrose and glucose, and purified terephthalic acid.
Industrial Biosciences segment sales outside the U.S. accounted for 54 percent of the segment's total sales in 2012.
Nutrition & Health
Nutrition & Health is a premier provider of innovative solutions for specialty food ingredients, health and safety. The segment's products, which include cultures, emulsifiers, gums, natural sweeteners and soy-based food ingredients, hold leading market positions based on industry leading innovation, relevant product portfolio and close-partnering with the world's food manufacturers. Nutrition & Health serves various end markets within the food industry including meat, dairy, beverages and bakery segments. Nutrition & Health has research, production and distribution operations around the world.
Nutrition & Health products are marketed and sold under a variety of brand names and are distributed primarily through its direct route to market. The direct route to market focuses on strong customer collaborations and insights with multinational customers and regional customers alike.
The major commodities, raw materials and supplies for the Nutrition & Health segment include: acetyls, citrus peels, glycerin, grain products, locust bean gum, oils and fats, seaweed, soybean, soy flake, sugar and yeast.
Nutrition & Health segment sales outside the U.S. accounted for 69 percent of the segment's total sales in 2012.
Performance Chemicals
Performance Chemicals businesses, DuPont Titanium Technologies and DuPont Chemicals and Fluoroproducts, deliver customized solutions with a wide range of industrial and specialty chemical products for markets including plastics and coatings, textiles, mining, pulp and paper, water treatment and healthcare.
DuPont Titanium Technologies is the world's largest manufacturer of titanium dioxide, and is dedicated to creating greater value for the coatings, paper, plastics, specialties and minerals markets through service, brand and product. The business' main products include its broad line of DuPontTM Ti-Pure® titanium dioxide products. In 2011, the business announced a global expansion to support increased customer demand for titanium dioxide, including a $500 million investment in new production facilities at the company's Altamira, Mexico site scheduled for completion in 2015. In addition, the business continues to invest in facility upgrades to improve productivity at its other global manufacturing sites.
DuPont Chemicals and Fluoroproducts is a leading global manufacturer of industrial and specialty fluorochemicals, fluoropolymers and performance chemicals. The business' broad line of products that include refrigerants, lubricants, propellants, solvents, fire extinguishants and electronic gases, cover a wide range of industries and markets. Key brands include DuPontTM Teflon®, Capstone®, Dymel®, OpteonTM yf, Isceon®, Suva®, Vertrel®, Zyron®, Vazo® and Virkon®.
The major commodities, raw materials and supplies for the Performance Chemicals segment include: ammonia, benzene, chlorine, chloroform, fluorspar, hydrofluoric acid, industrial gases, methanol, natural gas, perchloroethylene, petroleum coke, sulfur and titanium ore.
Part I
ITEM 1. BUSINESS, continued
Performance Chemicals segment sales outside the U.S. accounted for 56 percent of the segment's total sales in 2012.
Performance Materials
Performance Materials businesses, Performance Polymers and Packaging & Industrial Polymers, provide productive, higher performance polymers, elastomers, films, parts, and systems and solutions which improve the uniqueness, functionality and profitability of its customers' offerings. The key markets served by the segment include the automotive original equipment manufacturers (OEMs) and associated after-market industries, as well as electrical, packaging, construction, oil, electronics, photovoltaics, aerospace, chemical processing and consumer durable goods. The segment has several large customers, primarily in the motor vehicle OEM industry supply chain. The company has long-standing relationships with these customers and they are considered to be important to the segment's operating results.
Performance Polymers delivers a broad range of polymer-based high performance materials in its product portfolio, including elastomers and thermoplastic and thermoset engineering polymers which are used by customers to fabricate components for mechanical, chemical and electrical systems. The main products include: DuPontTM Zytel® nylon resins, Delrin® acetal resins, Hytrel® polyester thermoplastic elastomer resins, Tynex® filaments, Vespel® parts and shapes, Vamac® ethylene acrylic elastomer, Kalrez® perfluoroelastomer and Viton® fluoroelastomers. Performance Polymers also includes the DuPont Teijin Films joint venture, whose primary products are Mylar® and Melinex® polyester films.
Packaging & Industrial Polymers specializes in resins and films used in packaging and industrial polymer applications, sealants and adhesives, sporting goods, and interlayers for laminated safety glass. Key brands include: DuPontTM Surlyn® ionomer resins, Bynel® coextrudable adhesive resins, Elvax® EVA resins, SentryGlas®, Butacite® laminate interlayers and Elvaloy® copolymer resins.
The major commodities, raw materials and supplies for the Performance Materials segment include: acrylic monomers, adipic acid, butadiene, butanediol, dimethyl terephthalate, ethane, fiberglass, hexamethylenediamine, methanol, natural gas and purified terephthalic acid.
Performance Materials segment sales outside the U.S. accounted for 69 percent of the segment's total sales in 2012.
Safety & Protection
Safety & Protection businesses, Protection Technologies, Sustainable Solutions and Building Innovations, satisfy the growing global needs of businesses, governments and consumers for solutions that make life safer, healthier and more secure. By uniting market-driven science with the strength of highly regarded brands, the segment delivers products and services to a large number of markets, including construction, transportation, communications, industrial chemicals, oil and gas, electric utilities, automotive, manufacturing, defense, homeland security and safety consulting.
Protection Technologies is focused on finding solutions to protect people and the environment. With products like DuPont™ Kevlar® high strength material, Nomex® thermal resistant material and Tyvek® protective material, the business continues to hold strong positions in life protection markets and meet the continued demand for body armor and personal protective gear for the military, law enforcement personnel, firefighters and other first responders, as well as for workers in the oil and gas industry around the world. In 2011, the business announced the start up of its $500 million Cooper River Kevlar® facility near Charleston, South Carolina. The Cooper River Kevlar® plant uses state-of-the-art technology that will allow the business to meet increased customer demand for advanced protective materials in emerging industries around the world by expanding its portfolio of science-based innovations and boosting productivity. Commercial supply began at the end of 2011.
Sustainable Solutions continues to help organizations worldwide reduce workplace injuries and fatalities while improving operating costs, productivity and quality. Sustainable Solutions is a leader in the safety consulting field, selling training products, as well as consulting services. Additionally, Sustainable Solutions is dedicated to clean air, clean fuel and clean water with offerings that help reduce sulfur and other emissions, formulate cleaner fuels, or dispose of liquid waste. Its goal is to help maintain business continuity and environmental compliance for companies in the refining and petrochemical industries, as well as for government entities. The business includes MECS, Inc. (MECS), which is a leading global provider of process technology, proprietary specialty equipment and technical services to the sulfuric acid industry.
Part I
ITEM 1. BUSINESS, continued
Building Innovations is committed to the building science behind increasing the performance of building systems, helping reduce operating costs and creating more sustainable structures. The business is a market leader of solid surfaces through its DuPontTM Corian® and Montelli® lines of products which offer durable and versatile materials for residential and commercial purposes. Other products such as DuPont™ Tyvek® and Typar® offer leading solutions for the protection and energy efficiency of buildings.
The major commodities, raw materials and supplies for the Safety & Protection segment include: alumina hydroxide, benzene, high density polyethylene, isophthaloyl chloride, metaphenylenediamine, methyl methacrylate, paraphenylenediamine, polyester fiber, terephthaloyl chloride and wood pulp.
Safety & Protection segment sales outside the U.S. accounted for 62 percent of the segment's total sales in 2012.
Pharmaceuticals
On October 1, 2001, DuPont Pharmaceuticals was sold to the Bristol-Myers Squibb Company. DuPont retained its interest in Cozaar® (losartan potassium) and Hyzaar® (losartan potassium with hydrochlorothiazide), which are used in the treatment of hypertension. DuPont has exclusively licensed worldwide marketing and manufacturing rights for Cozaar® and Hyzaar® to Merck & Co., Inc. (Merck).
Pharmaceuticals' Cozaar®/Hyzaar® income is the sum of two parts: income related to a share of the profits from North American sales and certain markets in Europe, and royalty income derived from worldwide contract net sales linked to the exclusivity term in a particular country. Patents and exclusivity started to expire in prior years and the U.S. exclusivity for Cozaar® ended in April 2010. The worldwide agreement with Merck expired December 31, 2012. The company expects 2013 earnings to be insignificant.
Backlog
In general, the company does not manufacture its products against a backlog of orders and does not consider backlog to be a significant indicator of the level of future sales activity. Production and inventory levels are based on the level of incoming orders as well as projections of future demand. Therefore, the company believes that backlog information is not material to understanding its overall business and should not be considered a reliable indicator of the company's ability to achieve any particular level of revenue or financial performance.
Intellectual Property
DuPont believes that its intellectual property estate provides it with an important competitive advantage. It has an established global network of attorneys, as well as branding, advertising and licensing professionals, to procure, maintain, protect, enhance and gain value from this estate.
The company has access to a large patent portfolio, both owned and licensed. These definite-lived patents cover many products, processes and product uses. These patents protect many aspects of the company's significant research programs and the goods and services it sells. The actual protection afforded by these patents varies from country to country and depends upon the scope of coverage of each individual patent as well as the availability of legal remedies in each country. DuPont owns about 25,635 worldwide patents and is awaiting action on about 20,925 worldwide patent applications. In 2012, the company was granted about 935 U.S. patents and about 2,910 international patents. DuPont's rights under its patents and licenses, as well as the products made and sold under them, are important to the company as a whole, and to varying degrees, important to each reportable segment.
Trade secrets are an important element of the company's intellectual property. Many of the processes used to make DuPont products are kept as trade secrets which, from time to time, may be licensed to third parties. DuPont vigilantly protects all of its intellectual property including its trade secrets. When the company discovers that its trade secrets have been unlawfully taken, it reports the matter to governmental authorities for investigation and potential criminal action, as appropriate. In addition, the company takes measures to mitigate any potential impact, which may include civil actions seeking redress, restitution and/or damages based on loss to the company and/or unjust enrichment.
Ownership of and access to intellectual property rights, particularly those relating to biotechnology and germplasm, will continue to be important to Pioneer and its competitors. The environment in which Pioneer competes is characterized by the use among competitors of intellectual property rights, including patent lawsuits, to gain advantage in commercial markets. In support of its business, Pioneer continues to build a large collection of intellectual property rights related to biotechnology and germplasm and to license technology from others, including competitors. Pioneer endeavors to obtain such licenses on commercially reasonable terms.
Part I
ITEM 1. BUSINESS, continued
The company has about 2,065 unique trademarks for its products and services and approximately 19,395 registrations for these trademarks worldwide. Ownership rights in trademarks do not expire if the trademarks are continued in use and properly protected. The company has many trademarks that have significant recognition at the consumer retail level and/or business to business level.
Research and Development
The company conducts research at either dedicated research facilities or manufacturing plants. There are eleven major research locations in the U.S. & Canada, with the highest concentration of facilities being located in the Wilmington, Delaware area. Reflecting the company's global interests, five major research locations are located in the Asia Pacific region, four major research locations are located in Europe, Middle East and Africa (EMEA) region and one major location is located in Latin America.
The objectives of the company's research and development programs are to create new technologies, processes and business opportunities in relevant fields, as well as to improve existing products and processes. Each segment of the company funds research and development activities that support its business mission. The company is expanding its offerings addressing safety, environment, energy and climate challenges in the global marketplace by developing and commercializing renewable, bio-based materials; advanced biofuels; energy-efficient technologies; enhanced safety and protection products; and alternative energy products and technologies. The goals are tied directly to business growth, including increasing food production, increasing renewable sources for energy and raw materials, and providing greater safety and protection for people and the environment. All research and development activities are administered by senior research and development management to ensure consistency with the business and corporate strategy. The future of the company is not dependent upon the outcome of any single research program.
Additional information with respect to research and development, including the amount incurred during each of the last three fiscal years, is included in Item 7, Management's Discussion and Analysis of Financial Condition and Results of Operations, on page 20 of this report.
Environmental Matters
Information related to environmental matters is included in several areas of this report: (1) Environmental Proceedings beginning on page 12, (2) Management's Discussion and Analysis of Financial Condition and Results of Operations beginning on pages 31, 35-37 and (3) Notes 1 and 16 to the Consolidated Financial Statements.
Available Information
The company is subject to the reporting requirements under the Securities Exchange Act of 1934. Consequently, the company is required to file reports and information with the Securities and Exchange Commission (SEC), including reports on the following forms: annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934.
The public may read and copy any materials the company files with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet site at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
The company's annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports are also accessible on the company's website at http://www.dupont.com by clicking on the section labeled "Investors", then on "Key Financials & Filings" and then on "SEC Filings." These reports are made available, without charge, as soon as is reasonably practicable after the company files or furnishes them electronically with the SEC.
Executive Officers of the Registrant
Information related to the company's Executive Officers is included in Item 10, Directors, Executive Officers and Corporate Governance, beginning on page 40 of this report.
Part I
ITEM 1A. RISK FACTORS
The company's operations could be affected by various risks, many of which are beyond its control. Based on current information, the company believes that the following identifies the most significant risk factors that could affect its businesses. Past financial performance may not be a reliable indicator of future performance and historical trends should not be used to anticipate results or trends in future periods.
Price increases for energy and raw materials could have a significant impact on the company's ability to sustain and grow earnings.
The company's manufacturing processes consume significant amounts of energy and raw materials, the costs of which are subject to worldwide supply and demand as well as other factors beyond the control of the company. Significant variations in the cost of energy, which primarily reflect market prices for oil, natural gas and raw materials, affect the company's operating results from period to period. Price increases for energy and raw materials were not significant to earnings in 2012 as compared to 2011. In 2011, price increases for energy and raw materials were about $2.0 billion as compared to 2010. Legislation to address climate change by reducing greenhouse gas emissions and establishing a price on carbon could create increases in energy costs and price volatility. When possible, the company purchases raw materials through negotiated long-term contracts to minimize the impact of price fluctuations. Additionally, the company enters into over-the-counter and exchange traded derivative commodity instruments to hedge its exposure to price fluctuations on certain raw material purchases. The company takes actions to offset the effects of higher energy and raw material costs through selling price increases, productivity improvements and cost reduction programs. Success in offsetting higher raw material costs with price increases is largely influenced by competitive and economic conditions and could vary significantly depending on the market served. If the company is not able to fully offset the effects of higher energy and raw material costs, it could have a significant impact on the company's financial results.
Failure to develop and market new products and manage product life cycles could impact the company's competitive position and have an adverse effect on the company's financial results.
Operating results are largely dependent on the company's assessment and management of its portfolio of current, new and developing products and services and its ability to bring those products and services to market. The company plans to grow earnings by focusing on solutions to meet increasing demand for food productivity, decrease dependency on fossil fuels, and protect people, assets and the environment. The company develops new products through multiple methods including, but not limited to, internal research, collaborations, acquisitions, joint ventures and licensing technology or know-how. Developing and marketing new products could be adversely affected by difficulties or delays in product development such as the inability to identify viable new products, successfully complete research and development, obtain and maintain relevant regulatory approvals, obtain and maintain intellectual property protection, obtain technology or gain market acceptance of new products and services. Because of the lengthy development process, technological challenges and intense competition, there can be no assurance that any of the products the company is currently developing, or in which it will invest to develop, will achieve substantial commercial success. Not currently matching investment choices in new products with market need and competitive offerings could adversely affect business performance. Sales of the company's new products could replace sales of some of its current products, offsetting the benefit of even a successful product introduction.
The company competes with major global companies that have strong intellectual property estates supporting the use of biotechnology to enhance products, particularly agricultural and bio-based products. Speed in discovering, developing and protecting new technologies and bringing related products to market is a significant competitive advantage. Failure to predict and respond effectively to this competition could cause the company's existing or candidate products to become less competitive, adversely affecting sales. Competitors are increasingly challenging intellectual property positions and the outcomes can be highly uncertain. If challenges are resolved adversely, it could negatively impact the company's ability to commercialize new products and generate sales from existing products.
The company's results of operations could be adversely affected by litigation and other commitments and contingencies.
The company faces risks arising from various unasserted and asserted litigation matters, including, but not limited to, product liability, patent infringement, antitrust claims, and claims for third party property damage or personal injury stemming from alleged environmental torts. The company has noted a nationwide trend in purported class actions against chemical manufacturers generally seeking relief such as medical monitoring, property damages, off-site remediation and punitive damages arising from alleged environmental torts without claiming present personal injuries. The company also has noted a trend in public and private nuisance suits being filed on behalf of states, counties, cities and utilities alleging harm to the general public. Various factors or developments can lead to changes in current estimates of liabilities such as a final adverse judgment, significant settlement or changes in applicable law. A future adverse ruling or unfavorable development could result in future charges that could have a material adverse effect on the company. An adverse outcome in any one or more of these matters could be material to the company's financial results.
Part I
ITEM 1A. RISK FACTORS, continued
In the ordinary course of business, the company may make certain commitments, including representations, warranties and indemnities relating to current and past operations, including those related to divested businesses and issue guarantees of third party obligations. If the company were required to make payments as a result, they could exceed the amounts accrued, thereby adversely affecting the company's results of operations.
The company's business, including its results of operations and reputation, could be adversely affected by process safety and product stewardship issues.
Failure to appropriately manage safety, human health, product liability and environmental risks associated with the company's products, product life cycles and production processes could adversely impact employees, communities, stakeholders, the environment, the company's reputation and its results of operations. Public perception of the risks associated with the company's products and production processes could impact product acceptance and influence the regulatory environment in which the company operates. While the company has procedures and controls to manage process safety risks, issues could be created by events outside of its control including natural disasters, severe weather events, acts of sabotage and substandard performance by the company's external partners.
As a result of the company's current and past operations, including operations related to divested businesses, the company could incur significant environmental liabilities.
The company is subject to various laws and regulations around the world governing the environment, including the discharge of pollutants and the management and disposal of hazardous substances. As a result of its operations, including its past operations and operations of divested businesses, the company could incur substantial costs, including remediation and restoration costs. The costs of complying with complex environmental laws and regulations, as well as internal voluntary programs, are significant and will continue to be so for the foreseeable future. The ultimate costs under environmental laws and the timing of these costs are difficult to predict. The company's accruals for such costs and liabilities may not be adequate because the estimates on which the accruals are based depend on a number of factors including the nature of the matter, the complexity of the site, site geology, the nature and extent of contamination, the type of remedy, the outcome of discussions with regulatory agencies and other Potentially Responsible Parties (PRPs) at multi-party sites and the number and financial viability of other PRPs.
Market acceptance, government policies, rules or regulations and competition could affect the company's ability to generate sales from products based on biotechnology.
The company is using biotechnology to create and improve products, particularly in its Agriculture and Industrial Biosciences segments. The use of biotechnology to characterize the genetic and performance characteristics of Pioneer seeds provides Pioneer with competitive advantages in the development of new products, and in the most effective placement of those products on customer planted area. Industrial Biosciences leverages the company's biotechnology capabilities to develop and manufacture a broad portfolio of enzymes and biomaterials. These products enable cost and process benefits, better product performance and improve environmental outcomes to a broad range of products and processes such as animal nutrition, detergents, food manufacturing, ethanol production and industrial applications. The company's ability to generate sales from such products could be impacted by market acceptance as well as governmental policies, laws and regulations that affect the development, manufacture and distribution of products, including the testing and planting of seeds containing biotechnology traits and the import of commodity grain grown from those seeds. The regulatory environment is lengthy and complex with requirements that can vary by industry and by country. The regulatory environment may be impacted by the activities of non-governmental organizations and special interest groups and stakeholder reaction to actual or perceived impacts of new technology on safety, health and the environment. Obtaining and maintaining regulatory approvals requires submitting a significant amount of information and data, which may require participation from technology providers. The ability to satisfy the requirements of regulatory agencies is essential to be able to continue to sell existing products or commercialize new products.
Changes in government policies and laws could adversely affect the company's financial results.
Sales to customers outside the U.S. constitute about 60 percent of the company's 2012 revenue. The company anticipates that international sales will continue to represent a substantial portion of its total sales and that continued growth and profitability will require further international expansion, particularly in developing markets. Sales from developing markets represent 34 percent of the company's revenue in 2012 and the company's growth plans include focusing on expanding its presence in developing markets. The company's financial results could be affected by changes in trade, monetary and fiscal policies, laws and regulations, or other activities of U.S. and non-U.S. governments, agencies and similar organizations. These conditions include, but are not limited to, changes in a country's or region's economic or political conditions, trade regulations affecting production, pricing and marketing of products, local labor conditions and regulations, reduced protection of intellectual property rights in some countries, changes in the regulatory or legal environment, restrictions on currency exchange activities, burdensome taxes and tariffs and
Part I
ITEM 1A. RISK FACTORS, continued
other trade barriers. International risks and uncertainties, including changing social and economic conditions as well as terrorism, political hostilities and war, could lead to reduced sales and profitability.
Economic factors, including inflation, deflation and fluctuations in currency exchange rates, interest rates and commodity prices could affect the company's financial results.
The company is exposed to fluctuations in currency exchange rates, interest rates and commodity prices. Because the company has significant international operations, there are a large number of currency transactions that result from international sales, purchases, investments and borrowings. The company actively manages currency exposures that are associated with net monetary asset positions, committed currency purchases and sales, foreign currency-denominated revenues and other assets and liabilities created in the normal course of business. Failure to successfully manage these risks could have an adverse impact on the company's financial position, results of operations and cash flows.
Conditions in the global economy and global capital markets may adversely affect the company's results of operations, financial condition, and cash flows.
The company's business and operating results may in the future be adversely affected by global economic conditions, including instability in credit markets, declining consumer and business confidence, fluctuating commodity prices, volatile exchange rates, and other challenges that could affect the global economy. The company's customers may experience deterioration of their businesses, cash flow shortages, and difficulty obtaining financing. As a result, existing or potential customers may delay or cancel plans to purchase products and may not be able to fulfill their obligations in a timely fashion. Further, suppliers could experience similar conditions, which could impact their ability to fulfill their obligations to the company. Adversity within capital markets may impact future return on pension assets, thus resulting in greater future pension costs that impact the company's results. Future weakness in the global economy could adversely affect the company's results of operations, financial condition and cash flows in future periods.
The company's results of operations and financial condition could be seriously impacted by business disruptions and security breaches, including cybersecurity incidents.
Business and/or supply chain disruptions, plant and/or power outages and information technology system and/or network disruptions, regardless of cause including acts of sabotage, employee error or other actions, geo-political activity, weather events and natural disasters could seriously harm the company's operations as well as the operations of its customers and suppliers. Failure to effectively prevent, detect and recover from security breaches, including attacks on information technology and infrastructure by hackers; viruses; breaches due to employee error or actions; or other disruptions could result in misuse of the company's assets, business disruptions, loss of property including trade secrets and confidential business information, legal claims or proceedings, reporting errors, processing inefficiencies, negative media attention, loss of sales and interference with regulatory compliance. Although management does not believe that the company has experienced any material losses to date related to security breaches, including cybersecurity incidents, there can be no assurance that it will not suffer such losses in the future. The company actively manages the risks within its control that could lead to business disruptions and security breaches. As these threats continue to evolve, particularly around cybersecurity, the company may be required to expend significant resources to enhance its control environment, processes, practices and other protective measures. Despite these efforts, such events could materially adversely affect the company's business, financial condition or results of operations.
Inability to protect and enforce the company's intellectual property rights could adversely affect the company's financial results.
Intellectual property rights, including patents, plant variety protection, trade secrets, confidential information, trademarks, tradenames and other forms of trade dress, are important to the company's business. The company endeavors to protect its intellectual property rights in jurisdictions in which its products are produced or used and in jurisdictions into which its products are imported. However, the company may be unable to obtain protection for its intellectual property in key jurisdictions. The company has designed and implemented internal controls to restrict access to and distribution of its intellectual property. Despite these precautions, the company's intellectual property is vulnerable to unauthorized access through employee error or actions, theft and cybersecurity incidents, and other security breaches. When unauthorized access and use or counterfeit products are discovered, the company reports such situations to governmental authorities for investigation, as appropriate, and takes measures to mitigate any potential impact.
Part I
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
The company's corporate headquarters are located in Wilmington, Delaware. The company's manufacturing, processing, marketing and research and development facilities, as well as regional purchasing offices and distribution centers are located throughout the world.
Information regarding research and development facilities is incorporated by reference to Item 1, Business-Research and Development. Additional information with respect to the company's property, plant and equipment and leases is contained in Notes 10, 16 and 21 to the Consolidated Financial Statements.
The company has investments in property, plant and equipment related to global manufacturing operations. Collectively there are over 300 principal sites in total. The number of sites used by their applicable segment(s) by major geographic area around the world is as follows:
|
| | | | | | | | | | | | | | | | |
| | Number of Sites |
| | Agriculture | Electronics & Communications | Industrial Biosciences | Nutrition & Health | Performance Chemicals | Performance Materials | Safety & Protection | Total 1 |
| Asia Pacific | 19 |
| 10 |
| 1 |
| 10 |
| 6 |
| 19 |
| 7 |
| 72 |
|
| EMEA | 17 |
| 3 |
| 7 |
| 20 |
| 4 |
| 11 |
| 5 |
| 67 |
|
| Latin America | 17 |
| — |
| 1 |
| 7 |
| 1 |
| 1 |
| — |
| 27 |
|
| U.S. & Canada | 56 |
| 18 |
| 6 |
| 12 |
| 29 |
| 19 |
| 11 |
| 151 |
|
| | 109 |
| 31 |
| 15 |
| 49 |
| 40 |
| 50 |
| 23 |
| 317 |
|
| |
1. | Sites that are used by multiple segments are included more than once in the figures above. |
The company's plants and equipment are well maintained and in good operating condition. The company believes it has sufficient capacity to meet demand in 2013. Properties are primarily owned by the company; however, certain properties are leased. No title examination of the properties has been made for the purpose of this report and certain properties are shared with other tenants under long-term leases.
DuPont recognizes that the security and safety of its operations are critical to its employees, community and to the future of the company. As such, the company has merged chemical site security into its safety core value where it serves as an integral part of its long standing safety culture. Physical security measures have been combined with process safety measures (including the use of inherently safer technology), administrative procedures and emergency response preparedness into an integrated security plan. The company has conducted vulnerability assessments at operating facilities in the U.S. and high priority sites worldwide and identified and implemented appropriate measures to protect these facilities from physical and cyber attacks. DuPont is partnering with carriers, including railroad, shipping and trucking companies, to secure chemicals in transit.
Part I
ITEM 3. LEGAL PROCEEDINGS
The company is subject to various litigation matters, including, but not limited to, product liability, patent infringement, antitrust claims, and claims for third party property damage or personal injury stemming from alleged environmental torts. Information regarding certain of these matters is set forth below and in Note 16 to the Consolidated Financial Statements.
Litigation
Imprelis® Herbicide Claims Process
Information related to this matter is included in Note 16 to the Consolidated Financial Statements under the heading Imprelis®.
PFOA: Environmental and Litigation Proceedings
For purposes of this report, the term PFOA means collectively perfluorooctanoic acid and its salts, including the ammonium salt and does not distinguish between the two forms. Information related to this matter is included in Note 16 to the Consolidated Financial Statements under the heading PFOA.
Monsanto Patent Dispute
Information related to this matter is included in Note 16 to the Consolidated Financial Statements under the heading Monsanto Patent Dispute.
Environmental Proceedings
Belle Plant, West Virginia
The U.S. Environmental Protection Agency (EPA) is investigating three chemical releases at DuPont's Belle facility in West Virginia which occurred in January 2010. One of the releases involved the death of a DuPont employee after exposure to phosgene.
Chambers Works Plant, Deepwater, New Jersey
In 2010, the government initiated an enforcement action alleging that the facility violated recordkeeping requirements of certain provisions of the Clean Air Act (CAA) and the Federal Clean Air Act Regulations (FCAR) governing Leak Detection and Reporting (LDAR) and that it failed to report emissions of a compound from Chambers Works' waste water treatment facility under the Emergency Planning and Community Right-to-Know Act. The alleged non-compliance was identified by EPA in 2007 and 2009 following separate environmental audits. DuPont is in settlement negotiations with EPA and the Department of Justice (DOJ).
LaPorte Plant, LaPorte, Texas
EPA conducted a multimedia inspection at the LaPorte facility in January 2008. DuPont, EPA and DOJ began discussions in the fall of 2011 relating primarily to the management of certain materials in the facility's wastewater treatment system. These negotiations continue.
Sabine Plant, Orange, Texas
In June 2012, DuPont received allegations related to a multimedia inspection that the EPA conducted at the Sabine facility in March 2009. DuPont, EPA and DOJ are in discussions relating to the management of materials in the facility's waste water treatment system, hazardous waste management and air emissions.
Yerkes Plant, Buffalo, New York
The government alleges that the facility violated recordkeeping requirements of certain provisions of the CAA and FCAR governing LDAR and that it failed to accurately report emissions under the Emergency Planning and Community Right-to-Know Act. The alleged non-compliance was identified by EPA in 2006 and 2010 following separate environmental audits. DuPont is in settlement negotiations with EPA and DOJ.
Federal Insecticide, Fungicide and Rodenticide Act (FIFRA)
In July 2012, DuPont received a “notice of noncompliance and show cause” letter from EPA Region III for alleged violations of FIFRA related to product labeling and adverse effects reporting for Imprelis®.
Washington Works Plant, West Virginia
In 2011, the U.S. government initiated an enforcement action alleging that the Washington Works plant violated certain regulatory provisions of the CAA governing LDAR. The alleged non-compliance was identified between 2007 and 2010, following an environmental audit conducted in 2007 and the submission of responses to an information request received in 2009. DuPont is in settlement negotiations with the EPA and DOJ.
Part I
ITEM 3. LEGAL PROCEEDINGS, continued
DuPont (Australia) Pty Limited
The New South Wales Environmental Protection Authority (NSWEPA) alleges that 2011 dust particulate emissions from a DuPont (Australia) Pty Limited facility caused damage to trees, shrubs, and garden plants. In April 2012, NSWEPA commenced proceedings against DuPont (Australia) Pty Limited and under applicable laws and regulations, fines of up to AUD 1,000,000 (approximately $1,000,000) can be imposed.
ITEM 4. MINE SAFETY DISCLOSURES
Information regarding mine safety and other regulatory actions at the company's surface mine in Starke, Florida is included in Exhibit 95 to this report.
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market for Registrant's Common Equity and Related Stockholder Matters
The company's common stock is listed on the New York Stock Exchange, Inc. (symbol DD) and certain non-U.S. exchanges. The number of record holders of common stock was approximately 73,000 at January 31, 2013.
Holders of the company's common stock are entitled to receive dividends when they are declared by the Board of Directors. While it is not a guarantee of future conduct, the company has continuously paid a quarterly dividend since the fourth quarter 1904. Dividends on common stock and preferred stock are usually declared in January, April, July and October. When dividends on common stock are declared, they are usually paid mid March, June, September and December. Preferred dividends are paid on or about the 25th of January, April, July and October. The Stock Transfer Agent and Registrar is Computershare Trust Company, N.A.
The company's quarterly high and low trading stock prices and dividends per common share for 2012 and 2011 are shown below.
|
| | | | | | | | | |
| | Market Prices | |
| 2012 | High | Low | Per Share Dividend Declared |
| Fourth Quarter | $ | 50.96 |
| $ | 41.67 |
| $ | 0.43 |
|
| Third Quarter | 52.33 |
| 46.15 |
| 0.43 |
|
| Second Quarter | 53.98 |
| 46.44 |
| 0.43 |
|
| First Quarter | 53.95 |
| 45.84 |
| 0.41 |
|
| | | | |
| 2011 | |
| |
| |
|
| Fourth Quarter | $ | 49.92 |
| $ | 37.10 |
| $ | 0.41 |
|
| Third Quarter | 56.20 |
| 39.94 |
| 0.41 |
|
| Second Quarter | 57.00 |
| 48.64 |
| 0.41 |
|
| First Quarter | 56.19 |
| 47.22 |
| 0.41 |
|
Issuer Purchases of Equity Securities
There were no purchases of the company's common stock during the three months ended December 31, 2012.
Part II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES, continued
Stock Performance Graph
The following graph presents the cumulative five-year total return for the company's common stock compared with the S&P 500 Stock Index and the Dow Jones Industrial Average.
|
| | | | | | | | | | | | | | | | | | |
| | 12/31/2007 | 12/31/2008 | 12/31/2009 | 12/31/2010 | 12/31/2011 | 12/31/2012 |
| DuPont | $ | 100 |
| $ | 60 |
| $ | 85 |
| $ | 131 |
| $ | 124 |
| $ | 126 |
|
| S&P 500 Index | 100 |
| 63 |
| 80 |
| 92 |
| 94 |
| 109 |
|
| Dow Jones Industrial Average | 100 |
| 68 |
| 84 |
| 95 |
| 103 |
| 114 |
|
The graph assumes that the values of DuPont common stock, the S&P 500 Stock Index and the Dow Jones Industrial Average were each $100 on December 31, 2007 and that all dividends were reinvested.
Part II
ITEM 6. SELECTED FINANCIAL DATA
|
| | | | | | | | | | | | | | | |
| (Dollars in millions, except per share) | 2012 | 2011 | 2010 | 2009 | 2008 |
Summary of operations1 | | | |
| |
| |
|
| Net sales | $ | 34,812 |
| $ | 33,681 |
| $ | 27,700 |
| $ | 22,681 |
| $ | 26,169 |
|
| Employee separation / asset related charges, net | $ | 493 |
| $ | 53 |
| $ | (40 | ) | $ | 195 |
| $ | 331 |
|
| Income from continuing operations before income taxes | $ | 3,115 |
| $ | 3,781 |
| $ | 3,260 |
| $ | 1,943 |
| $ | 2,388 |
|
| Provision for income taxes on continuing operations | $ | 622 |
| $ | 626 |
| $ | 515 |
| $ | 326 |
| $ | 368 |
|
| Net income attributable to DuPont | $ | 2,788 |
| $ | 3,474 |
| $ | 3,031 |
| $ | 1,755 |
| $ | 2,007 |
|
| Basic earnings per share of common stock from continuing operations | $ | 2.63 |
| $ | 3.35 |
| $ | 2.99 |
| $ | 1.76 |
| $ | 2.22 |
|
| Diluted earnings per share of common stock from continuing operations | $ | 2.61 |
| $ | 3.30 |
| $ | 2.94 |
| $ | 1.75 |
| $ | 2.21 |
|
| Financial position at year-end | | | |
| |
| |
|
Working capital2 | $ | 7,642 |
| $ | 6,873 |
| $ | 9,670 |
| $ | 7,898 |
| $ | 5,601 |
|
Total assets3 | $ | 49,736 |
| $ | 48,492 |
| $ | 40,410 |
| $ | 38,185 |
| $ | 36,209 |
|
| Borrowings and capital lease obligations | | | |
| |
| |
|
| Short-term | $ | 1,275 |
| $ | 817 |
| $ | 133 |
| $ | 1,506 |
| $ | 2,012 |
|
| Long-term | $ | 10,465 |
| $ | 11,736 |
| $ | 10,137 |
| $ | 9,528 |
| $ | 7,638 |
|
| Total equity | $ | 10,179 |
| $ | 9,062 |
| $ | 9,743 |
| $ | 7,651 |
| $ | 7,552 |
|
General1 | | | |
| |
| |
|
| For the year | | | |
| |
| |
|
Purchases of property, plant & equipment and investments in affiliates | $ | 1,890 |
| $ | 1,910 |
| $ | 1,608 |
| $ | 1,432 |
| $ | 2,033 |
|
| Depreciation | $ | 1,319 |
| $ | 1,199 |
| $ | 1,118 |
| $ | 1,144 |
| $ | 967 |
|
| Research and development expense | $ | 2,067 |
| $ | 1,910 |
| $ | 1,603 |
| $ | 1,324 |
| $ | 1,326 |
|
| Average number of common shares outstanding (millions) | | | |
| |
| |
|
| Basic | 933 |
| 928 |
| 909 |
| 904 |
| 902 |
|
| Diluted | 942 |
| 941 |
| 922 |
| 909 |
| 907 |
|
| Dividends per common share | $ | 1.70 |
| $ | 1.64 |
| $ | 1.64 |
| $ | 1.64 |
| $ | 1.64 |
|
| At year-end | | | |
| |
| |
|
| Employees (thousands) | 70 |
| 70 |
| 60 |
| 58 |
| 60 |
|
| Closing stock price | $ | 44.98 |
| $ | 45.78 |
| $ | 49.88 |
| $ | 33.67 |
| $ | 25.30 |
|
| Common stockholders of record (thousands) | 74 |
| 78 |
| 81 |
| 85 |
| 88 |
|
| |
1. | Information has been restated to reflect the impact of discontinued operations, as applicable. See Note 1, Basis of Presentation, to the Consolidated Financial Statements for further information. |
| |
2. | At December 31, 2012, working capital includes approximately $2.0 billion of net assets related to the Performance Coatings business, of which approximately $1.3 billion was previously considered to be noncurrent and is now classified as held for sale. See Note 2 to the Consolidated Financial Statements for further information. |
| |
3. | During 2011, the company acquired approximately $8.8 billion of assets in connection with the Danisco acquisition. See Note 4 to the Consolidated Financial Statements for further information. |
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
CAUTIONARY STATEMENTS ABOUT FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements which may be identified by their use of words like “plans,” “expects,” “will,” “anticipates,” “believes,” “intends,” “projects,” “estimates” or other words of similar meaning. All statements that address expectations or projections about the future, including statements about the company's strategy for growth, product development, regulatory approval, market position, anticipated benefits of recent acquisitions, outcome of contingencies, such as litigation and environmental matters, expenditures and financial results, are forward-looking statements.
Forward-looking statements are based on certain assumptions and expectations of future events which may not be accurate or realized. Forward-looking statements also involve risks and uncertainties, many of which are beyond the company's control. Some of the important factors that could cause the company's actual results to differ materially from those projected in any such forward-looking statements are:
| |
| • | Fluctuations in energy and raw material prices; |
| |
| • | Failure to develop and market new products and optimally manage product life cycles; |
| |
| • | Outcome of significant litigation and environmental matters, including those related to divested businesses; |
| |
| • | Failure to appropriately manage process safety and product stewardship issues; |
| |
| • | Effect of changes in tax, environmental and other laws and regulations or political conditions in the U.S. and other countries in which the company operates; |
| |
| • | Conditions in the global economy and global capital markets, including economic factors, such as inflation, deflation and fluctuations in currency exchange rates, interest rates and commodity prices, as well as regulatory requirements; |
| |
| • | Impact of business disruptions, including supply disruptions, and security threats, regardless of cause, including acts of sabotage, cyber-attacks, terrorism or war, weather events and natural disasters; |
| |
| • | Inability to protect and enforce the company's intellectual property rights; and |
| |
| • | Successful integration of acquired businesses and completion of divestitures of underperforming or non-strategic assets or businesses. |
For some of the important factors that could cause the company's actual results to differ materially from those projected in any such forward-looking statements, see the Risk Factors discussion set forth under Part I, Item 1A beginning on page 8.
Overview
Purpose DuPont is a science company. We work collaboratively to find sustainable, innovative, market-driven solutions to solve some of the world's biggest challenges, making lives better, safer, and healthier for people everywhere.
Strategy The company's strategy for growth is to apply its science and technology to address three challenges driven by global population growth: feeding the world, reducing our dependence on fossil fuels and keeping people and the environment safe. Critical areas for the company's growth are innovation, differential management and productivity. Applying science to deliver innovative solutions and new products in the marketplace generates shareholder value and profitable growth. Differential management is a disciplined process to prioritize and allocate resources across businesses and geographies aligned with growth opportunities. The company continues to achieve fixed cost, working capital and variable cost productivity through disciplined business processes called DuPont Integrated Business Management (DIBM) and DuPont Production System (DPS). DIBM focuses on the business supply chain to maximize efficiency and optimize working capital, while DPS focuses on productivity outcomes to eliminate operational inefficiencies and improve lead time, cycle time and quality. The company is committed to maintain a strong balance sheet and to return excess cash to shareholders unless there is a compelling opportunity to invest for growth.
Results In 2012, sales were up 3 percent as strong increases in the Agriculture, Nutrition & Health, and Industrial Biosciences segments were partly offset by lower sales for the remaining segments, particularly Performance Chemicals. The latter had financial results reflecting a declining business trend after reaching cyclical peaks for sales and earnings in 2011 and the first half of 2012. Total company sales grew 6 percent in developing markets, which include China, India, and the countries located in Latin America, Eastern and Central Europe, Middle East, Africa, and Southeast Asia. Sales of new products introduced in the last four years also contributed to sales growth. The company exceeded its three-year 2010-2012 plan of $1 billion fixed cost productivity actions and $1 billion working capital productivity.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
Analysis of Operations
Divestiture of Performance Coatings In third quarter 2012, the company entered into a definitive agreement with Flash Bermuda Co. Ltd., a Bermuda exempted limited liability company formed by affiliates of The Carlyle Group (collectively referred to as "Carlyle") in which Carlyle agreed to purchase certain subsidiaries and assets comprising the company's Performance Coatings business for approximately $4.9 billion in cash and the assumption of certain liabilities. On February 1, 2013, the sale of Performance Coatings was completed, resulting in about $4.0 billion in after-tax proceeds.
In accordance with GAAP, the results of Performance Coatings are presented as discontinued operations and, as such, have been excluded from continuing operations and segment results for all periods presented. See Note 2 to the Consolidated Financial Statements for additional information.
Acquisition of Danisco In 2011, the company acquired Danisco in a transaction valued at $6.4 billion, plus net debt assumed of $0.6 billion. As part of this acquisition, DuPont incurred $85 million in transaction related costs during 2011, which were recorded in costs of goods sold and other operating charges. In 2011, the businesses acquired from Danisco contributed net sales of $1.7 billion and net income attributable to DuPont of $(7) million, which excludes $30 million after-tax ($39 million pre-tax) of additional interest expense related to the debt issued to finance the acquisition. Danisco's contributions included a $125 million after-tax ($175 million pre-tax) charge related to the fair value step-up of inventories acquired and sold during 2011. See Note 4 to the Consolidated Financial Statements for additional information.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| NET SALES | $ | 34,812 |
| $ | 33,681 |
| $ | 27,700 |
|
2012 versus 2011 The table below shows a regional breakdown of 2012 consolidated net sales based on location of customers and percentage variances from prior year:
|
| | | | | | | | | | | | | |
| | Percent Change Due to: |
| (Dollars in billions) | 2012 Net Sales | Percent Change vs. 2011 | Local Price | Currency Effect | Volume | Portfolio / Other |
| Worldwide | $ | 34.8 |
| 3 |
| 4 |
| (2 | ) | (2 | ) | 3 |
|
| U.S. & Canada | 14.2 |
| 8 |
| 6 |
| — |
| — |
| 2 |
|
| EMEA | 8.1 |
| (1 | ) | 3 |
| (6 | ) | (4 | ) | 6 |
|
| Asia Pacific | 8.0 |
| (4 | ) | (1 | ) | (1 | ) | (5 | ) | 3 |
|
| Latin America | 4.5 |
| 11 |
| 9 |
| (5 | ) | 5 |
| 2 |
|
Sales increased 3 percent, reflecting a 3 percent net increase from portfolio changes, principally the Danisco acquisition, and 4 percent higher local prices, partly offset by 2 percent lower volume and a 2 percent negative currency impact. The 2 percent decline in worldwide sales volume principally reflects higher Agriculture, Nutrition & Health, and Industrial Biosciences volume, more than offset by lower volume for the other segments combined, particularly Performance Chemicals. Higher local prices were driven principally by increases for seeds, titanium dioxide, and specialty polymers. Currency effect primarily reflects the weaker Euro and Brazilian Real. Sales in developing markets of $11.9 billion improved 6 percent from 2011, and the percentage of total company sales in these markets increased to 34 percent from 33 percent in 2011.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
2011 versus 2010 The table below shows a regional breakdown of 2011 consolidated net sales based on location of customers and percentage variances from 2010:
|
| | | | | | | | | | | | | |
| | Percent Change Due to: |
| (Dollars in billions) | 2011 Net Sales | Percent Change vs. 2010 | Local Price | Currency Effect | Volume | Portfolio / Other |
| Worldwide | $ | 33.7 |
| 22 |
| 12 |
| 2 |
| 1 |
| 7 |
|
| U.S. & Canada | 13.1 |
| 16 |
| 9 |
| — |
| 1 |
| 6 |
|
| EMEA | 8.2 |
| 26 |
| 11 |
| 4 |
| — |
| 11 |
|
| Asia Pacific | 8.3 |
| 23 |
| 17 |
| 3 |
| (3 | ) | 6 |
|
| Latin America | 4.1 |
| 33 |
| 14 |
| 2 |
| 12 |
| 5 |
|
Sales increased 22 percent, principally reflecting higher local selling prices and the sales added from businesses acquired from Danisco. Local selling prices were significantly higher for titanium dioxide, seeds, fluoroproducts and electronic products, with the latter reflecting pass through pricing for higher precious metals costs. Worldwide sales volume increased 1 percent as strong volume growth in Agriculture was largely offset by declines in Electronic & Communications, Performance Chemicals and Performance Materials. The declines occurred primarily during the fourth quarter, resulting from destocking in photovoltaics, polymer and industrial supply chains, as well as weaker demand for company products supplying consumer electronics and construction. Volume growth in Latin America was driven by Agriculture and Safety & Protection. Sales in developing markets of $11.2 billion improved 29 percent from 2010, and the percentage of total company sales in these markets increased to 33 percent from 31 percent in 2010.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| OTHER INCOME, NET | $ | 498 |
| $ | 742 |
| $ | 1,199 |
|
2012 versus 2011 The $244 million decrease was largely attributable to a $228 million reduction of Cozaar®/Hyzaar® income, a decrease of $92 million in equity in earnings of affiliates, and an increase of $69 million in net pre-tax exchange losses, partially offset by a $122 million gain related to the sale of the company's interest in an equity method investment.
2011 versus 2010 The $457 million decrease was largely attributable to a $201 million reduction of Cozaar®/Hyzaar® income, an increase of $134 million in net pre-tax exchange losses, the absence of a benefit of $59 million recorded in 2010 related to accrued interest associated with settlements of income tax contingencies related to prior years, the absence of $41 million in insurance recoveries and a $32 million decrease in net gains on sales of assets.
Additional information related to the company's other income, net is included in Note 5 to the Consolidated Financial Statements.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| COST OF GOODS SOLD AND OTHER OPERATING CHARGES | $ | 25,604 |
| $ | 24,874 |
| $ | 20,574 |
|
| As a percent of net sales | 74 | % | 74 | % | 74 | % |
2012 versus 2011 Cost of goods sold and other operating charges (COGS) increased 3 percent to $25.6 billion. COGS as a percentage of net sales was 74 percent, unchanged from 2011. Increased charges of $537 million related to Imprelis® and other litigation matters were offset by higher selling prices. See Note 16 for additional information related to the Imprelis® matter.
2011 versus 2010 COGS of $24.9 billion increased 21 percent. COGS as a percentage of net sales was 74 percent, unchanged from prior year, as selling price increases offset inflation in raw material, energy and freight costs, and higher plant operating costs, including capacity expansions. 2011 COGS also included $175 million of additional costs related to the fair value step-up of inventory acquired from Danisco, $85 million of Danisco transaction related fees and $175 million for charges related to Imprelis® herbicide claims.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| SELLING, GENERAL AND ADMINISTRATIVE EXPENSES | $ | 3,567 |
| $ | 3,358 |
| $ | 2,912 |
|
| As a percent of net sales | 10 | % | 10 | % | 11 | % |
2012 versus 2011 The 2012 increase of $209 million was due to increased global commissions and selling and marketing investments, primarily in the Agriculture segment, and a full year of selling expense of acquired companies.
2011 versus 2010 The 2011 increase of $446 million was due to the additional selling expense of acquired companies and increased global commissions and selling and marketing investments, primarily in the Agriculture segment.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| RESEARCH AND DEVELOPMENT EXPENSE | $ | 2,067 |
| $ | 1,910 |
| $ | 1,603 |
|
| As a percent of net sales | 6 | % | 6 | % | 6 | % |
2012 versus 2011 The $157 million increase was primarily attributable to a full year of research and development expense from acquired companies and continued growth investments in the Agriculture segment offset by the absence of a $50 million charge for a payment related to a Pioneer licensing agreement in 2011.
2011 versus 2010 The $307 million increase was primarily attributable to research and development expense from acquired companies and continued growth investment in the Agriculture segment. Both periods include a $50 million charge for payments related to a Pioneer licensing agreement prior to the business receiving regulatory approval in the third quarter 2011.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| INTEREST EXPENSE | $ | 464 |
| $ | 447 |
| $ | 590 |
|
The $17 million increase in 2012 was due primarily to higher average borrowings and lower capitalized interest partially offset by a lower average borrowing rate. The $143 million decrease in 2011 was due primarily to the absence of a $179 million pre-tax charge on the early extinguishment of debt and lower interest rates, partially offset by higher average debt resulting from financing for the Danisco acquisition.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| EMPLOYEE SEPARATION/ASSET RELATED CHARGES, NET | $ | 493 |
| $ | 53 |
| $ | (40 | ) |
The $493 million in charges recorded during 2012 in employee separation / asset related charges, net consisted of $234 million in charges related to the 2012 restructuring program, a $16 million net reduction in the estimated costs associated with 2011 and prior years restructuring programs, and $275 million in asset impairment charges, as discussed below.
The net $53 million charge recorded during 2011 was primarily related to the 2011 restructuring program, as discussed below, and the $40 million credit recorded during 2010 was due to a net reduction in the estimated costs for prior years restructuring programs.
2012 Restructuring Program
In 2012, the company commenced a restructuring plan to increase productivity, enhance competitiveness and accelerate growth. The plan is designed to eliminate corporate costs previously allocated to the Performance Coatings business as well as utilize additional cost-cutting actions to improve competitiveness. As a result, pre-tax charges of $234 million were recorded in employee separation / asset related charges, net. The 2012 restructuring program charges consist of $157 million of employee separation costs, $8 million of other non-personnel charges, and $69 million of asset related charges, which includes $30 million of asset impairments and $39 million of asset shut downs. The company expects this plan and all related payments to be substantially complete by December 31, 2013. The actions related to this plan are expected to achieve pre-tax cost savings of approximately $300 million in 2013 increasing to $450 million per year in subsequent years.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
2011 Restructuring Program
In 2011, the company initiated a series of actions to achieve the expected cost synergies associated with the Danisco acquisition. As a result, the company recorded a $53 million charge in employee separation/asset related charges, net, primarily for employee separation costs in the U.S. and Europe.
In the fourth quarter 2012, the company recorded a net reduction of $15 million in the estimated costs associated with the 2011 restructuring program. This net reduction was primarily due to workforce reductions through non-severance programs and lower than estimated individual severance costs. The actions and payments related to the 2011 restructuring program were substantially complete as of December 31, 2012.
Asset Impairments
During 2012, the company recorded asset impairment charges of $275 million to write-down the carrying value of certain asset groups to fair value. These asset impairment charges resulted in a $150 million charge within the Electronics & Communications segment, a $92 million charge within the Performance Materials segment and a $33 million charge within the Performance Chemicals segment.
Additional details related to the restructuring programs and asset impairments discussed above can be found in Note 3 to the Consolidated Financial Statements.
Below is a summary of the net impact related to items recorded in employee separation / asset related charges, net:
|
| | | | | | | | | |
| (Dollars in millions) | 2012 (Charges) and Credits | 2011 (Charges) and Credits | 2010 (Charges) and Credits |
| Agriculture | $ | (11 | ) | $ | — |
| $ | — |
|
| Electronics & Communications | (159 | ) | — |
| 8 |
|
| Industrial Biosciences | (3 | ) | (9 | ) | — |
|
| Nutrition & Health | (49 | ) | (14 | ) | — |
|
| Performance Chemicals | (36 | ) | — |
| 10 |
|
| Performance Materials | (104 | ) | (2 | ) | 16 |
|
| Safety & Protection | (58 | ) | — |
| 5 |
|
| Other | 11 |
| (28 | ) | 1 |
|
| Corporate expenses | (84 | ) | — |
| — |
|
| Total (Charges) Credits | $ | (493 | ) | $ | (53 | ) | $ | 40 |
|
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| PROVISION FOR INCOME TAXES ON CONTINUING OPERATIONS | $ | 622 |
| $ | 626 |
| $ | 515 |
|
| Effective income tax rate | 20.0 | % | 16.6 | % | 15.8 | % |
In 2012, the company recorded a tax provision on continuing operations of $622 million, reflecting a marginal decrease from 2011. The increase in the 2012 effective tax rate compared to 2011 was primarily due to geographic mix of earnings, in addition to the absence of certain U.S. business tax provisions in 2012 (extended retroactively to 2012 in first quarter 2013).
In 2011, the company recorded a tax provision on continuing operations of $626 million, reflecting an increase from 2010 largely due to pre-tax earnings growth, which was partially offset by the impact associated with the company's policy of hedging the foreign currency-denominated monetary assets and liabilities of its operations.
See Note 6 to the Consolidated Financial Statements for additional details related to the provision for income taxes on continuing operations, as well as items that significantly impact the company's effective income tax rate.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| INCOME FROM CONTINUING OPERATIONS AFTER INCOME TAXES | $ | 2,493 |
| $ | 3,155 |
| $ | 2,745 |
|
Income from continuing operations after income taxes for 2012 was $2.5 billion compared to $3.2 billion in 2011 and $2.7 billion in 2010. The changes between periods were due to the reasons noted above.
Corporate Outlook
The company expects 2013 sales and earnings to benefit from modest growth in global gross domestic product and industrial production. The company’s market position and financial results will continue to be enhanced by market-driven innovation and productivity. Raw material, energy and freight costs are expected to increase, principally due to higher seed input costs and titanium ore. Currency impact for the full-year 2013 versus 2012 is expected to be flat. The company expects a dynamic market environment, including strong Agriculture demand and significant headwinds related to the cyclical nature of the titanium dioxide industry affecting the Performance Chemicals segment.
Segment Reviews
Segment sales include transfers to another business segment. Products are transferred between segments on a basis intended to reflect, as nearly as practicable, the market value of the products. Segment pre-tax operating income (loss) (PTOI) is defined as income (loss) from continuing operations before income taxes excluding exchange gains (losses), corporate expenses and interest. All references to prices are on a U.S. dollar (USD) basis, including the impact of currency. A reconciliation of segment sales to consolidated net sales and segment PTOI to income from continuing operations before income taxes for 2012, 2011 and 2010 is included in Note 22 to the Consolidated Financial Statements.
AGRICULTURE
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | 10,426 |
| $ | 9,166 |
| $ | 7,845 |
|
| PTOI | $ | 1,594 |
| $ | 1,527 |
| $ | 1,293 |
|
| PTOI margin | 15 | % | 17 | % | 16 | % |
|
| | | | |
| | 2012 | 2011 |
| Change in segment sales from prior period due to: | | |
| Price | 6 | % | 6 | % |
| Volume | 8 | % | 10 | % |
| Portfolio / Other | — | % | 1 | % |
| Total change | 14 | % | 17 | % |
2012 versus 2011 Pioneer seed sales reflect growth primarily in corn and soybean seeds. Volume increases in all regions reflect increased planted area. Global pricing gains reflect continued penetration of new genetics and trait packages, including the Optimum® AcreMax® Family of integrated and reduced refuge corn hybrids and Optimum® AQUAmaxTM products for improved drought tolerance. Crop Protection sales grew in all regions reflecting volume and price gains from herbicides, insect control products and fungicides, particularly continued strong demand for Rynaxypyr® insect control products.
2012 PTOI increased as strong sales and a $117 million gain on the sale of a business more than offset $575 million of charges related to Imprelis®, higher input costs in seeds, unfavorable currency and higher investments in commercial and R&D activities to support growth. 2012 PTOI margin decreased due to increased charges related to Imprelis®. See Note 16 to the Consolidated Financial Statements for more information related to the Imprelis® matter.
2011 versus 2010 Pioneer seed sales reflect growth primarily in corn and soybean seeds. Volume increases in all regions reflect increased planted area and market position. Pricing gains in all regions reflect the introduction and penetration of new products including Optimum® AcreMax® 1 into the North America corn lineup. Crop Protection sales growth reflects both volume and price gains with increases in insect control, weed control and fungicides product sales, particularly continued strong demand for
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
Rynaxypyr® insecticide and continued expansion of picoxystrobin fungicides. Sales grew in all regions, particularly Latin America and Europe.
2011 PTOI and PTOI margin increased on continued new product penetration and leverage on volume growth, partially offset by a $175 million charge related to Imprelis®. Additionally, aligned with the segment's long-term plan, research and development expense increased 15 percent to support continued growth in breeding, biotechnology and crop chemistry. 2011 and 2010 PTOI each included a licensing agreement charge of $50 million.
Outlook Pioneer anticipates continued strong demand in global corn and soybean markets, as well as pricing gains from new corn hybrids and soybean varieties and increased penetration of the Optimum® AcreMax® Family of integrated and reduced refuge corn hybrids and Optimum® AQUAmaxTM products for improved drought tolerance. Pioneer anticipates PTOI growth in 2013 reflecting higher sales, partially offset by higher seed input costs resulting from commodity price increases and the multi year weather related impact on production yields, as well as continued investment in commercial and R&D activities to support growth.
In the Crop Protection business, sales and earnings growth in 2013 is expected in all regions, and for all product groups, primarily in insect control products and fungicides. Sales and PTOI are expected to benefit from continued growth of Rynaxypyr®, broad registrations in new markets for CyazypyrTM insect control products, as well as the launch of picoxystrobin fungicides in the U.S. and continued expansion of penthiopyrad fungicide.
ELECTRONICS & COMMUNICATIONS
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | 2,701 |
| $ | 3,173 |
| $ | 2,764 |
|
| PTOI | $ | 135 |
| $ | 355 |
| $ | 445 |
|
| PTOI margin | 5 | % | 11 | % | 16 | % |
|
| | | | |
| | 2012 | 2011 |
| Change in segment sales from prior period due to: | | |
| Price | (4 | )% | 23 | % |
| Volume | (11 | )% | (8 | )% |
| Portfolio / Other | — | % | — | % |
| Total change | (15 | )% | 15 | % |
2012 versus 2011 Sales declined on lower volume in PV materials, partially offset by increased demand for smart phones and tablets. Lower price primarily reflects pass-through of lower metals prices.
2012 PTOI decreased on lower volume and a $150 million asset impairment charge noted above, partially offset by a $122 million gain related to the sale of an equity method investment. PTOI margin decreased primarily reflecting lower volume.
2011 versus 2010 Sales growth reflects higher selling prices, primarily pass-through of metals prices. Lower sales volume primarily reflects destocking in PV and softness in consumer electronics in the second half 2011, which more than offset strong demand in all market segments in the first half 2011.
2011 PTOI decreased primarily due to lower volume in the second half 2011. PTOI margin decreased primarily reflecting higher metal prices, as well as weaker product mix.
Outlook For 2013, sales are expected to increase slightly on higher volumes. PV installations are expected to be essentially flat versus 2012. Sales volumes are anticipated to improve as inventories across the value chain are at reasonable levels entering into 2013, offset in part by lower materials usage in modules. Sales into consumer electronics, driven by smart phones and tablets, are expected to be soft in the first half of the year with stronger demand in second half. Earnings are expected to increase reflecting the impact of higher volume.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
INDUSTRIAL BIOSCIENCES
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | 1,180 |
| $ | 705 |
| $ | — |
|
| PTOI | $ | 168 |
| $ | (1 | ) | $ | — |
|
| PTOI margin | 14 | % | — | % | — | % |
|
| | | | |
| | 2012 | 2011 |
| Change in segment sales from prior period due to: | | |
| Price | (4 | )% | — | % |
| Volume | 8 | % | — | % |
| Portfolio / Other | 63 | % | — | % |
| Total change | 67 | % | — | % |
2012 versus 2011 Sales were up primarily due to the Danisco enzyme business acquisition. Volume growth reflects strong sales of Sorona® polymer for carpeting, while lower price relates to unfavorable currency impact.
2012 PTOI and PTOI margin increased reflecting benefits of the acquisition and the absence of a $70 million charge recorded in the prior year for the fair value step-up of inventories acquired.
2011 versus 2010 Sales and PTOI primarily reflects the Danisco acquisition. 2011 PTOI included a $70 million charge for the fair value step-up of inventories that were acquired as part of the acquisition and a $9 million restructuring charge. PTOI also included $12 million of amortization expense associated with the fair value step-up of the acquired intangible assets.
Outlook Sales are expected to increase in 2013 as a result of continued strong demand for Sorona® polymer for carpeting and the introduction of new products in most enzyme markets. Demand for enzymes for ethanol production will remain soft until producer margins improve. Segment earnings are expected to increase consistent with volume growth, pricing gains and cost synergies derived from integration.
NUTRITION & HEALTH
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | 3,422 |
| $ | 2,460 |
| $ | 1,240 |
|
| PTOI | $ | 299 |
| $ | 44 |
| $ | 62 |
|
| PTOI margin | 9 | % | 2 | % | 5 | % |
|
| | | | |
| | 2012 | 2011 |
| Change in segment sales from prior period due to: | | |
| Price | 1 | % | 5 | % |
| Volume | 3 | % | 1 | % |
| Portfolio / Other | 35 | % | 92 | % |
| Total change | 39 | % | 98 | % |
2012 versus 2011 Sales were up primarily due to the Danisco specialty food ingredients business acquisition. Higher volume reflects strong demand for enablers, probiotics and cultures, particularly in North America. Higher local prices more than offset unfavorable currency impact.
2012 PTOI and PTOI margin increased reflecting benefits of the acquisition and the absence of a $112 million charge recorded in the prior year for transaction related costs and the fair value step-up of inventories acquired, partially offset by increased restructuring charges in 2012 as described above.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
2011 versus 2010 Sales were up primarily due to the Danisco acquisition. For Solae, higher selling prices and volume reflect strong demand for specialty soy products.
2011 PTOI and PTOI margin decreased as higher sales were more than offset by a $112 million charge for transaction related costs and the fair value step-up of inventories that were acquired and a $14 million restructuring charge. PTOI also included $49 million of amortization expense associated with the fair value step-up of the acquired intangible assets.
Outlook For 2013, sales are expected to increase driven by strong demand for enablers, probiotics and cultures. Growth is expected in all regions, particularly in Asia Pacific and Latin America, in the areas of nutrition solutions, improved health and food protection. Volume growth, mix enrichment, cost synergies derived from integration and productivity gains, partially offset by raw material increases, are expected to contribute to earnings and PTOI margin improvement.
PERFORMANCE CHEMICALS
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | 7,188 |
| $ | 7,794 |
| $ | 6,322 |
|
| PTOI | $ | 1,586 |
| $ | 1,923 |
| $ | 1,081 |
|
| PTOI margin | 22 | % | 25 | % | 17 | % |
|
| | | | |
| | 2012 | 2011 |
| Change in segment sales from prior period due to: | | |
| Price | 4 | % | 26 | % |
| Volume | (12 | )% | (3 | )% |
| Portfolio / Other | — | % | — | % |
| Total change | (8 | )% | 23 | % |
2012 versus 2011 Lower sales volume primarily reflects softness in titanium dioxide in all regions and weak demand in fluoropolymers. Higher local price primarily reflects favorable pricing for titanium dioxide in the first half 2012, which more than offset unfavorable currency impact.
2012 PTOI and PTOI margin decreased as higher local prices were more than offset by lower volume, lower plant utilization and a $33 million asset impairment charge noted above.
2011 versus 2010 Sales increased across all regions and market segments. The increase in sales reflects favorable pricing for titanium dioxide and fluoropolymers, as well as pass-through pricing of higher raw material costs for fluorochemicals and industrial chemicals.
2011 PTOI and PTOI margin improved driven by the higher selling prices and fixed cost productivity.
Outlook Sales are expected to decrease in 2013 reflecting weaker pricing for titanium dioxide and fluoropolymers, partially offset by strong demand for industrial chemicals. Segment earnings are also expected to decrease as productivity actions are more than offset by lower titanium dioxide prices. PTOI margins are expected to decline about 7 to 9 percentage points.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
PERFORMANCE MATERIALS
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | 6,447 |
| $ | 6,815 |
| $ | 6,287 |
|
| PTOI | $ | 1,013 |
| $ | 971 |
| $ | 994 |
|
| PTOI margin | 16 | % | 14 | % | 16 | % |
|
| | | | |
| | 2012 | 2011 |
| Change in segment sales from prior period due to: | | |
| Price | (2 | )% | 13 | % |
| Volume | — | % | (4 | )% |
| Portfolio / Other | (3 | )% | (1 | )% |
| Total change | (5 | )% | 8 | % |
2012 versus 2011 Lower sales reflect a 3 percent reduction from a portfolio change and lower prices due to unfavorable currency impact. Stable packaging markets and demand improvement in automotive were offset by continued softness in the industrial and electronics markets.
2012 PTOI and PTOI margin increased as lower feedstock costs more than offset a $92 million asset impairment charge noted above, unfavorable currency impact and the absence of a $49 million benefit from the gain on the sale of a business recorded in the prior year.
2011 versus 2010 Higher selling prices reflect pricing actions which offset higher feedstock costs. Lower sales volume reflect broad-based channel destocking with softening in consumer and industrial markets in the second half 2011, and production-related supply issues in ethylene-based polymers.
2011 PTOI was essentially flat. 2011 PTOI included a $49 million benefit from the gain on the sale of a business. 2010 PTOI included a combined $58 million gain on an asset purchase due to the acquisition and early termination of a supply agreement, a gain on the sale of a business and an insurance recovery. Lower PTOI margin primarily reflects feedstock costs increasing at a higher rate than selling prices.
Outlook 2013 sales are expected to grow due to anticipated increases in global motor vehicle OEM builds, particularly in China. The segment is also expected to benefit from volume growth in the consumer and industrial markets. PTOI is expected to improve due to the impact of higher volume and science-based innovations for products and processes, partially offset by higher feedstock costs.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
SAFETY & PROTECTION
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | 3,825 |
| $ | 3,934 |
| $ | 3,364 |
|
| PTOI | $ | 360 |
| $ | 500 |
| $ | 454 |
|
| PTOI margin | 9 | % | 13 | % | 13 | % |
|
| | | | |
| | 2012 | 2011 |
| Change in segment sales from prior period due to: | | |
| Price | — | % | 6 | % |
| Volume | (3 | )% | 4 | % |
| Portfolio / Other | — | % | 7 | % |
| Total change | (3 | )% | 17 | % |
2012 versus 2011 Lower U.S. public sector demand and softness in certain industrial markets, including stalled infrastructure projects in China, was partially offset by higher demand for Sustainable Solutions offerings. Higher local prices were offset by the impact of unfavorable currency.
2012 PTOI and PTOI margin decreased primarily due to a $58 million restructuring charge noted above, unfavorable currency and lower volume.
2011 versus 2010 Sales growth occurred in all regions. Sales growth primarily reflects the impact of the MECS acquisition and higher selling prices, including a favorable currency impact. Higher volume primarily reflects increased demand for aramid and nonwoven products primarily in the industrial markets in the first half 2011, with slower growth rates in the second half 2011.
2011 PTOI increased as the impact of the MECS acquisition and a favorable currency impact more than offset higher spending for growth initiatives and higher raw material costs. The Kevlar® expansion at Cooper River, South Carolina was completed and began commercial supply at the end of 2011.
Outlook For 2013, sales for Kevlar®, Nomex® and Tyvek® products are expected to benefit from improving industrial demand, including higher infrastructure spending in China. Additionally, Kevlar® and Nomex® sales are expected to improve as the year progresses from more consistent spending in the public sector. Higher demand for Sustainable Solutions offerings are expected, including growth in the areas of process safety management and sustainable operations. Building Innovations sales are expected to increase due to the continued recovery in the U.S. housing market, new product introductions and continued penetration in commercial construction applications. Earnings are expected to improve throughout the year from higher sales, reflecting innovative growth through products such as Kevlar® AP fiber, as well as continued productivity actions.
PHARMACEUTICALS
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Segment sales | $ | — |
| $ | — |
| $ | — |
|
| PTOI | $ | 62 |
| $ | 289 |
| $ | 489 |
|
Decreases in PTOI reflect the expiration of certain patents related to Cozaar®/Hyzaar®.
Outlook Earnings contributions to the company from the collaboration with Merck are expected to decline in 2013 to about $20 million.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
Liquidity & Capital Resources
|
| | | | | | |
| | December 31, |
| (Dollars in millions) | 2012 | 2011 |
| Cash, cash equivalents and marketable securities | $ | 4,407 |
| $ | 4,019 |
|
| Total debt | 11,740 |
| 12,553 |
|
The company believes its ability to generate cash from operations and access to capital markets will be adequate to meet anticipated cash requirements to fund working capital, capital spending, dividend payments, debt maturities and other cash needs. The company's liquidity needs can be met through a variety of sources, including: cash provided by operating activities, cash and cash equivalents, marketable securities, commercial paper, syndicated credit lines, bilateral credit lines, equity and long-term debt markets and asset sales. The company's current strong financial position, liquidity and credit ratings provide excellent access to the capital markets. In addition, spending and capital productivity actions have been implemented. The company will continue to monitor the financial markets in order to respond to changing conditions. Depending on these conditions, the proceeds of commercial paper may be invested in cash equivalents or marketable securities.
Pursuant to its cash discipline policy, the company seeks first to maintain a strong balance sheet and second, to return excess cash to shareholders unless the opportunity to invest for growth is compelling. Cash, cash equivalents and marketable securities provide primary liquidity to support all short-term debt obligations. A substantial majority of the company's cash, cash equivalents and marketable securities is held by foreign subsidiaries and is considered to be indefinitely reinvested and expected to be utilized to fund local operating activities and capital expenditure requirements. The company believes that it has sufficient sources of domestic liquidity to further support its assumption that undistributed earnings at December 31, 2012 can be considered reinvested indefinitely. The company has access to approximately $4.3 billion in unused credit lines with several major financial institutions, as additional support to meet short-term liquidity needs and general corporate purposes, including letters of credit.
The company continually reviews its debt portfolio and occasionally may rebalance it to ensure adequate liquidity and an optimum debt maturity schedule. In 2011, the company issued $2.0 billion in Senior Notes and $1.0 billion in commercial paper to finance the acquisition of Danisco. Additionally, the company assumed $0.7 billion in debt as part of the acquisition, which was refinanced through the issuance of commercial paper.
The company's credit ratings impact its access to the debt capital market and cost of capital. The company remains committed to a strong financial position and strong investment-grade rating. The company's long-term and short-term credit ratings are as follows:
|
| | | |
| | Long-term | Short-term | Outlook |
| Standard & Poor's | A | A-1 | Stable |
| Moody’s Investors Service | A2 | P-1 | Stable |
| Fitch Ratings | A | F1 | Stable |
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Cash provided by operating activities | $ | 4,849 |
| $ | 5,152 |
| $ | 4,559 |
|
Cash provided by operating activities decreased $303 million in 2012 compared to 2011 due mainly to lower earnings and a $500 million contribution to its principal U.S. pension plan, partially offset by less of an increase in operating assets and liabilities.
Cash provided by operating activities increased $593 million in 2011 compared to 2010. The increase was driven by higher earnings, lower contributions to pension plans and the weaker dollar, which was hedged with forward exchange contracts reflected in investing activities. These increases were partially offset by changes in operating assets and liabilities, mainly due to higher inventory.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Cash used for investing activities | $ | (1,346 | ) | $ | (6,238 | ) | $ | (2,439 | ) |
Cash used for investing activities decreased $4.9 billion in 2012 compared to 2011. The decrease was due mainly to the absence in 2012 of the company's Danisco acquisition in 2011.
The $3.8 billion increase in 2011 was mainly due to the payment for the Danisco acquisition, higher expenditures for the purchases of property, plant and equipment, and a net increase in payments for forward exchange contract settlements; partially offset by changes in investments in short-term financial instruments.
Purchases of property, plant and equipment totaled $1.8 billion in 2012 and 2011, and $1.5 billion in 2010. The company expects 2013 purchases of property, plant and equipment to be about $1.9 billion, an increase of $0.1 billion over 2012.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Cash (used for) provided by financing activities | $ | (2,697 | ) | $ | 403 |
| $ | (1,829 | ) |
The $3.1 billion increase in cash used for financing activities in 2012 was due mainly to a decrease in borrowings in 2012 versus an increase in 2011, less cash received from options exercised and the company's increased investment in Solae, LLC in 2012, partially offset by reduced purchases of common stock in 2012 versus 2011.
The $2.2 billion change in 2011 was primarily due to an increase in borrowings in 2011 to finance the Danisco acquisition as compared to a decrease in borrowings in 2010.
Dividends paid to common and preferred shareholders were $1.6 billion, $1.5 billion, and $1.5 billion in 2012, 2011, and 2010, respectively. Dividends per share of common stock were $1.70, $1.64, and $1.64 in 2012, 2011, and 2010, respectively. The common dividend declared in the first quarter 2013 was the company's 434th consecutive dividend since the company's first dividend in the fourth quarter 1904.
During 2012, the company purchased and retired 7.8 million shares at a total cost of $400 million. These purchases completed the 2001 $2 billion share buyback plan and began purchases under a $2 billion share buyback plan authorized by the company's Board of Directors in April 2011. Under the completed 2001 plan, the company purchased a total of 42.0 million shares. As of December 31, 2012, the company has purchased 5.5 million shares at a total cost of $284 million under the 2011 plan. There is no required completion date for the purchases under the 2011 plan.
In December 2012, the company's Board of Directors authorized a $1 billion share buyback plan, subject to receiving the proceeds from the Performance Coatings divestiture. On February 1, 2013, the sale of Performance Coatings was completed. The 2012 share buyback plan is expected to be completed in the first half 2013.
During 2011 and 2010, the company purchased and retired 13.8 million and 5.4 million shares at a total cost of $672 million and $250 million, respectively, under the 2001 plan.
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Cash provided by operating activities | $ | 4,849 |
| $ | 5,152 |
| $ | 4,559 |
|
| Purchases of property, plant and equipment | (1,793 | ) | (1,843 | ) | (1,508 | ) |
| Free cash flow | $ | 3,056 |
| $ | 3,309 |
| $ | 3,051 |
|
Free cash flow is a measurement not recognized in accordance with GAAP and should not be viewed as an alternative to GAAP measures of performance. All companies do not calculate non-GAAP financial measures in the same manner and, accordingly, the company's free cash flow definition may not be consistent with the methodologies used by other companies. The company defines free cash flow as cash provided by operating activities less purchases of property, plant and equipment, and therefore indicates operating cash flow available for payment of dividends, other investing activities and other financing activities. Free cash flow is useful to investors and management to evaluate the company's cash flow and financial performance, and is an integral financial measure used in the company's financial planning process.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
Critical Accounting Estimates
The company's significant accounting policies are more fully described in Note 1 to the Consolidated Financial Statements. Management believes that the application of these policies on a consistent basis enables the company to provide the users of the financial statements with useful and reliable information about the company's operating results and financial condition.
The preparation of the Consolidated Financial Statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts, including, but not limited to, receivable and inventory valuations, impairment of tangible and intangible assets, long-term employee benefit obligations, income taxes, restructuring liabilities, environmental matters and litigation. Management's estimates are based on historical experience, facts and circumstances available at the time and various other assumptions that are believed to be reasonable. The company reviews these matters and reflects changes in estimates as appropriate. Management believes that the following represents some of the more critical judgment areas in the application of the company's accounting policies which could have a material effect on the company's financial position, liquidity or results of operations.
Long-term Employee Benefits
Accounting for employee benefit plans involves numerous assumptions and estimates. Discount rate and expected return on plan assets are two critical assumptions in measuring the cost and benefit obligation of the company's pension and other long-term employee benefit plans. Management reviews these two key assumptions annually as of December 31st. These and other assumptions are updated periodically to reflect the actual experience and expectations on a plan specific basis as appropriate. As permitted by GAAP, actual results that differ from the assumptions are accumulated on a plan by plan basis and to the extent that such differences exceed 10 percent of the greater of the plan's benefit obligation or the applicable plan assets, the excess is amortized over the average remaining service period of active employees.
About 76 percent of the company's benefit obligation for pensions and essentially all of the company's other long-term employee benefit obligations are attributable to the benefit plans in the U.S. In the U.S. the discount rate is developed by matching the expected cash flow of the benefit plans to a yield curve constructed from a portfolio of high quality fixed-income instruments provided by the plan's actuary as of the measurement date. For non-U.S. benefit plans, the company utilizes prevailing long-term high quality corporate bond indices to determine the discount rate, applicable to each country, at the measurement date.
Within the U.S., the company establishes strategic asset allocation percentage targets and appropriate benchmarks for significant asset classes with the aim of achieving a prudent balance between return and risk. Strategic asset allocations in other countries are selected in accordance with the laws and practices of those countries. Where appropriate, asset-liability studies are also taken into consideration. The long-term expected return on plan assets in the U.S. is based upon historical real returns (net of inflation) for the asset classes covered by the investment policy, expected performance, and projections of inflation over the long-term period during which benefits are payable to plan participants. Consistent with prior years, the long-term expected return on plan assets in the U.S. reflects the asset allocation of the plan and the effect of the company's active management of the plans' assets.
In determining annual expense for the principal U.S. pension plan, the company uses a market-related value of assets rather than its fair value. The market-related value of assets is calculated by averaging market returns over 36 months. Accordingly, there may be a lag in recognition of changes in market valuation. As a result, changes in the fair value of assets are not immediately reflected in the company's calculation of net periodic pension cost. The following table shows the market-related value and fair value of plan assets for the principal U.S. pension plan:
|
| | | | | | | | | |
| (Dollars in billions) | 2012 | 2011 | 2010 |
| Market-related value of assets | $ | 14.8 |
| $ | 13.9 |
| $ | 13.9 |
|
| Fair value of plan assets | 15.1 |
| 13.9 |
| 14.8 |
|
For plans other than the principal U.S. pension plan, pension expense is typically determined using the fair value of assets.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
The following table highlights the potential impact on the company's pre-tax earnings due to changes in certain key assumptions with respect to the company's pension and other long-term employee benefit plans, based on assets and liabilities at December 31, 2012:
|
| | | | | | |
Pre-tax Earnings Benefit (Charge) (Dollars in millions) | 1/2 Percentage Point Increase | 1/2 Percentage Point Decrease |
| Discount rate | $ | 107 |
| $ | (114 | ) |
| Expected rate of return on plan assets | 92 |
| (92 | ) |
Additional information with respect to pension and other long-term employee benefits expenses, liabilities and assumptions is discussed under "Long-term Employee Benefits" beginning on page 34 and in Note 18 to the Consolidated Financial Statements.
Environmental Matters
DuPont accrues for remediation activities when it is probable that a liability has been incurred and a reasonable estimate of the liability can be made. The company has recorded a liability of $436 million as of December 31, 2012; these accrued liabilities exclude claims against third parties and are not discounted. As remediation activities vary substantially in duration and cost from site to site, it is difficult to develop precise estimates of future site remediation costs. The company's estimates are based on a number of factors, including the complexity of the geology, the nature and extent of contamination, the type of remedy, the outcome of discussions with regulatory agencies and other PRPs at multi-party sites and the number of and financial viability of other PRPs. Therefore, considerable uncertainty exists with respect to environmental remediation costs and, under adverse changes in circumstances, the potential liability may range up to three times the amount accrued.
Legal Contingencies
The company's results of operations could be affected by significant litigation adverse to the company, including product liability claims, patent infringement and antitrust claims, and claims for third party property damage or personal injury stemming from alleged environmental torts. The company records accruals for legal matters when the information available indicates that it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Management makes adjustments to these accruals to reflect the impact and status of negotiations, settlements, rulings, advice of counsel and other information and events that may pertain to a particular matter. Predicting the outcome of claims and lawsuits and estimating related costs and exposure involves substantial uncertainties that could cause actual costs to vary materially from estimates. In making determinations of likely outcomes of litigation matters, management considers many factors. These factors include, but are not limited to, the nature of specific claims including unasserted claims, the company's experience with similar types of claims, the jurisdiction in which the matter is filed, input from outside legal counsel, the likelihood of resolving the matter through alternative dispute resolution mechanisms and the matter's current status. Considerable judgment is required in determining whether to establish a litigation accrual when an adverse judgment is rendered against the company in a court proceeding. In such situations, the company will not recognize a loss if, based upon a thorough review of all relevant facts and information, management believes that it is probable that the pending judgment will be successfully overturned on appeal. A detailed discussion of significant litigation matters is contained in Note 16 to the Consolidated Financial Statements.
Income Taxes
The breadth of the company's operations and the global complexity of tax regulations require assessments of uncertainties and judgments in estimating taxes the company will ultimately pay. The final taxes paid are dependent upon many factors, including negotiations with taxing authorities in various jurisdictions, outcomes of tax litigation and resolution of disputes arising from federal, state and international tax audits in the normal course of business. The resolution of these uncertainties may result in adjustments to the company's tax assets and tax liabilities. It is reasonably possible that changes to the company's global unrecognized tax benefits could be significant, however, due to the uncertainty regarding the timing of completion of audits and possible outcomes, a current estimate of the range of increases or decreases that may occur within the next twelve months cannot be made.
Deferred income taxes result from differences between the financial and tax basis of the company's assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. Significant judgment is required in evaluating the need for and magnitude of appropriate valuation allowances against deferred tax assets. The realization of these assets is dependent on generating future taxable income, as well as successful implementation of various tax planning strategies. For example, changes in facts and circumstances that alter the probability that the company will realize deferred tax assets could result in recording a
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
valuation allowance, thereby reducing the deferred tax asset and generating a deferred tax expense in the relevant period. In some situations these changes could be material.
At December 31, 2012, the company had a deferred tax asset balance of $7.8 billion, net of valuation allowance of $1.9 billion. Realization of these assets is expected to occur over an extended period of time. As a result, changes in tax laws, assumptions with respect to future taxable income, and tax planning strategies could result in adjustments to these assets. See Note 6 to the Consolidated Financial Statements for additional details related to the deferred tax asset balance.
Valuation of Assets
The assets and liabilities of acquired businesses are measured at their estimated fair values at the dates of acquisition. The excess of the purchase price over the estimated fair value of the net assets acquired, including identified intangibles, is recorded as goodwill. The determination and allocation of fair value to the assets acquired and liabilities assumed is based on various assumptions and valuation methodologies requiring considerable management judgment, including estimates based on historical information, current market data and future expectations. The principal assumptions utilized in the company's valuation methodologies include revenue growth rates, operating margin estimates, royalty rates, and discount rates. Although the estimates were deemed reasonable by management based on information available at the dates of acquisition, those estimates are inherently uncertain.
Assessment of the potential impairment of property, plant and equipment, goodwill, other intangible assets and investments in affiliates is an integral part of the company's normal ongoing review of operations. Testing for potential impairment of these assets is significantly dependent on numerous assumptions and reflects management's best estimates at a particular point in time. The dynamic economic environments in which the company's diversified businesses operate, and key economic and business assumptions with respect to projected selling prices, market growth and inflation rates, can significantly affect the outcome of impairment tests. Estimates based on these assumptions may differ significantly from actual results. Changes in factors and assumptions used in assessing potential impairments can have a significant impact on the existence and magnitude of impairments, as well as the time in which such impairments are recognized. In addition, the company continually reviews its diverse portfolio of assets to ensure they are achieving their greatest potential and are aligned with the company's growth strategy. Strategic decisions involving a particular group of assets may trigger an assessment of the recoverability of the related assets. Such an assessment could result in impairment losses. During 2012, the company recorded asset impairments charges of $275 million to write-down the carrying value of certain asset groups to fair value. See Note 3 to the Consolidated Financial Statements for additional details related to these charges.
Based on the results of the company's annual goodwill impairment test in 2012, no impairments exist at this time. The company's methodology for estimating the fair value of its reporting units is using the income approach based on the present value of future cash flows. The income approach has been generally supported by additional market transaction analyses. There can be no assurance that the company's estimates and assumptions regarding forecasted cash flow and revenue and operating income growth rates made for purposes of the annual goodwill impairment test will prove to be accurate predictions of the future. The company believes the current assumptions and estimates utilized are both reasonable and appropriate.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
Off-Balance Sheet Arrangements
Certain Guarantee Contracts
Information with respect to the company's guarantees is included in Note 16 to the Consolidated Financial Statements. Historically, the company has not had to make significant payments to satisfy guarantee obligations; however, the company believes it has the financial resources to satisfy these guarantees.
Contractual Obligations
Information related to the company's significant contractual obligations is summarized in the following table:
|
| | | | | | | | | | | | | | | |
| | | Payments Due In |
| (Dollars in millions) | Total at December 31, 2012 | 2013 | 2014 – 2015 | 2016 – 2017 | 2018 and beyond |
Long-term debt obligations1 | $ | 11,693 |
| $ | 1,252 |
| $ | 3,124 |
| $ | 1,596 |
| $ | 5,721 |
|
Expected cumulative cash requirements for interest payments through maturity | 3,247 |
| 422 |
| 699 |
| 585 |
| 1,541 |
|
Capital leases1 | 27 |
| 3 |
| 7 |
| 4 |
| 13 |
|
| Operating leases | 1,414 |
| 320 |
| 473 |
| 331 |
| 290 |
|
Purchase obligations2 | |
| |
| |
| |
| |
|
Information technology infrastructure & services | 184 |
| 120 |
| 57 |
| 7 |
| — |
|
| Raw material obligations | 671 |
| 429 |
| 169 |
| 35 |
| 38 |
|
| Utility obligations | 230 |
| 71 |
| 71 |
| 41 |
| 47 |
|
INVISTA-related obligations3 | 1,293 |
| 165 |
| 328 |
| 328 |
| 472 |
|
| Human resource services | 83 |
| 28 |
| 55 |
| — |
| — |
|
| Other | 206 |
| 152 |
| 34 |
| 19 |
| 1 |
|
| Total purchase obligations | 2,667 |
| 965 |
| 714 |
| 430 |
| 558 |
|
Other liabilities1,4 | |
| |
| |
| |
| |
|
| Workers' compensation | 87 |
| 14 |
| 38 |
| 16 |
| 19 |
|
| Asset retirement obligations | 64 |
| 1 |
| 21 |
| 3 |
| 39 |
|
| Environmental remediation | 436 |
| 83 |
| 176 |
| 60 |
| 117 |
|
| Legal settlements | 20 |
| 7 |
| 5 |
| 4 |
| 4 |
|
License agreements5 | 551 |
| 153 |
| 259 |
| 139 |
| — |
|
Other6 | 199 |
| 73 |
| 33 |
| 18 |
| 75 |
|
| Total other long-term liabilities | 1,357 |
| 331 |
| 532 |
| 240 |
| 254 |
|
Total contractual obligations7,8 | $ | 20,405 |
| $ | 3,293 |
| $ | 5,549 |
| $ | 3,186 |
| $ | 8,377 |
|
| |
1. | Included in the Consolidated Financial Statements. |
| |
2. | Represents enforceable and legally binding agreements in excess of $1 million to purchase goods or services that specify fixed or minimum quantities; fixed, minimum or variable price provisions; and the approximate timing of the agreement. |
| |
3. | Primarily represents raw material supply obligations. |
| |
4. | Pension and other long-term employee benefit obligations have been excluded from the table as they are discussed below within Long-term Employee Benefits. |
| |
5. | Primarily represents remaining expected payments under Pioneer license agreements. |
| |
6. | Primarily represents employee-related benefits other than pensions and other long-term employee benefits. |
| |
7. | Due to uncertainty regarding the completion of tax audits and possible outcomes, the estimate of obligations related to unrecognized tax benefits cannot be made. See Note 6 to the Consolidated Financial Statements for additional detail. |
| |
8. | At December 31, 2012, approximately $140 million of the company's contractual obligations relate to the Performance Coatings business, which primarily represents operating leases. |
The company expects to meet its contractual obligations through its normal sources of liquidity and believes it has the financial resources to satisfy these contractual obligations.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
Long-term Employee Benefits
The company has various obligations to its employees and retirees. The company maintains retirement-related programs in many countries that have a long-term impact on the company's earnings and cash flows. These plans are typically defined benefit pension plans, as well as medical, dental and life insurance benefits for pensioners and survivors and disability and life insurance protection for employees (other long-term employee benefits). Approximately 76 percent of the company's worldwide benefit obligation for pensions and essentially all of the company's worldwide other long-term employee benefit obligations are attributable to the U.S. benefit plans. Pension coverage for employees of the company's non-U.S. consolidated subsidiaries is provided, to the extent deemed appropriate, through separate plans. The company regularly explores alternative solutions to meet its global pension obligations in the most cost effective manner possible as demographics, life expectancy and country-specific pension funding rules change. Where permitted by applicable law, the company reserves the right to change, modify or discontinue its plans that provide pension, medical, dental, life insurance and disability benefits.
The majority of employees hired in the U.S. on or after January 1, 2007 are not eligible to participate in the pension and post-retirement medical, dental and life insurance plans, but receive benefits in the defined contribution plans.
Benefits under defined benefit pension plans are based primarily on years of service and employees' pay near retirement. Pension benefits are paid primarily from trust funds established to comply with applicable laws and regulations. Unless required by law, the company does not make contributions that are in excess of tax deductible limits. The actuarial assumptions and procedures utilized are reviewed periodically by the plans' actuaries to provide reasonable assurance that there will be adequate funds for the payment of benefits. The company made a contribution of $500 million in 2010 to its principal U.S. pension plan and no contributions were made in 2011. In January 2012, the company contributed $500 million to its principal U.S. pension plan and anticipates no contributions will be made in 2013. The company expects to make contributions to its principal U.S. pension plan beyond 2013; however, the amount of any contributions is heavily dependent on the future economic environment and investment returns on pension trust assets. U.S. pension benefits that exceed federal limitations are covered by separate unfunded plans and these benefits are paid to pensioners and survivors from operating cash flows.
Funding for each pension plan is governed by the rules of the sovereign country in which it operates. Thus, there is not necessarily a direct correlation between pension funding and pension expense. In general, however, improvements in plans funded status tends to moderate subsequent funding needs. The company contributed $848 million to its pension plans in 2012 and anticipates that it will make approximately $340 million in contributions in 2013 to pension plans other than the principal U.S. pension plan.
The company's other long-term employee benefits are unfunded and the cost of the approved claims is paid from operating cash flows. Pre-tax cash requirements to cover actual net claims costs and related administrative expenses were $261 million, $312 million and $321 million for 2012, 2011 and 2010, respectively. This amount is expected to be about $260 million in 2013. Changes in cash requirements reflect the net impact of higher per capita health care costs, demographic changes, plan amendments and changes in participant premiums, co-pays and deductibles.
During the third quarter 2012, the company amended its U.S. parent company retiree medical and dental plans for Medicare-eligible pensioners and survivors. Beginning in 2013, the company is replacing the coverage for Medicare-eligible plan participants in the company sponsored plans with a new company-funded Health Reimbursement Arrangement (HRA). Medicare-eligible plan participants will enroll in individual health plans in the open market and the company will reimburse their health care expenses with an HRA based on the provisions of the amended plans. As a result of this change, the company's other long-term employee benefit expense was reduced by approximately $46 million in 2012. For 2013, the plan amendment is expected to result in a reduction in other long-term employee benefit expense by about $120 million. Additional information related to these changes in the plans noted above is included in Note 18 to the Consolidated Financial Statements.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
The company's income can be significantly affected by pension and defined contribution benefits as well as other long-term employee benefits. The following table summarizes the extent to which the company's income over each of the last 3 years was affected by pre-tax charges related to long-term employee benefits:
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
Long-term employee benefit plan charges 1 | $ | 1,321 |
| $ | 1,134 |
| $ | 1,030 |
|
| |
1. | The long-term employee benefit plan charges relating to discontinued operations was $74, $72 and $71 for 2012, 2011 and 2010, respectively. |
The above charges for pension and other long-term employee benefits are determined as of the beginning of each year. The increase in long-term employee benefit expense in 2012 is primarily related to the decrease in discount rates and lower return on plan assets, partially offset by plan amendments to the company's U.S. parent company retiree medical and dental plans. The increase in long-term employee benefit expense in 2011 is primarily related to the decrease in discount rates. See "Long-term Employee Benefits" under the Critical Accounting Estimates section beginning on page 30 of this report for additional information on determining annual expense for the principal U.S. pension plan.
The company's key assumptions used in calculating its pension and other long-term employee benefits are the expected return on plan assets, the rate of compensation increases and the discount rate (see Note 18 to the Consolidated Financial Statements). For 2013, long-term employee benefits expense from continuing operations is expected to decrease by about $30 million.
Environmental Matters
The company operates global manufacturing, product handling and distribution facilities that are subject to a broad array of environmental laws and regulations. Such rules are subject to change by the implementing governmental agency, and the company monitors these changes closely. Company policy requires that all operations fully meet or exceed legal and regulatory requirements. In addition, the company implements voluntary programs to reduce air emissions, minimize the generation of hazardous waste, decrease the volume of water use and discharges, increase the efficiency of energy use and reduce the generation of persistent, bioaccumulative and toxic materials. Management has noted a global upward trend in the amount and complexity of proposed chemicals regulation. The costs to comply with complex environmental laws and regulations, as well as internal voluntary programs and goals, are significant and will continue to be significant for the foreseeable future.
Pre-tax environmental expenses charged to current operations are summarized below:
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Environmental operating costs | $ | 595 |
| $ | 562 |
| $ | 521 |
|
| Increase in remediation accrual | 110 |
| 92 |
| 93 |
|
| | $ | 705 |
| $ | 654 |
| $ | 614 |
|
About 75 percent of total pre-tax environmental expenses charged to current operations in 2012 resulted from operations in the U.S. The increases in total pre-tax environmental expenses charged to operations were due primarily to increased environmental research activities and acquired businesses. Based on existing facts and circumstances, management does not believe that year over year changes, if any, in environmental expenses charged to current operations will have a material impact on the company's financial position, liquidity or results of operations.
Environmental Operating Costs
As a result of its operations, the company incurs costs for pollution abatement activities including waste collection and disposal, installation and maintenance of air pollution controls and wastewater treatment, emissions testing and monitoring, and obtaining permits. The company also incurs costs related to environmental related research and development activities including environmental field and treatment studies as well as toxicity and degradation testing to evaluate the environmental impact of products and raw materials.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
Remediation Accrual
Changes in the remediation accrual balance are summarized below:
|
| | | |
| (Dollars in millions) | |
| Balance at December 31, 2010 | $ | 407 |
|
| Remediation payments | (83 | ) |
| Increase in remediation accrual | 92 |
|
| Balance at December 31, 2011 | $ | 416 |
|
| Remediation payments | (90 | ) |
| Increase in remediation accrual | 110 |
|
| Balance at December 31, 2012 | $ | 436 |
|
Annual expenditures are expected to continue to increase in the near future; however, they are not expected to vary significantly from the range of such expenditures experienced in the past few years. Longer term, expenditures are subject to considerable uncertainty and may fluctuate significantly.
As of December 31, 2012, the company has been notified of potential liability under the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA or Superfund) or similar state laws at about 415 sites around the U.S., with active remediation under way at approximately 160 of these sites. In addition, the company has resolved its liability at approximately 175 sites, either by completing remedial actions with other PRPs or by participating in "de minimis buyouts" with other PRPs whose waste, like the company's, represented only a small fraction of the total waste present at a site. The company received notice of potential liability at five new sites during 2012 compared with six and ten similar notices in 2011 and 2010, respectively.
Considerable uncertainty exists with respect to environmental remediation costs, and, under adverse changes in circumstances, potential liability may range up to three times the amount accrued as of December 31, 2012. However, based on existing facts and circumstances, management does not believe that any loss, in excess of amounts accrued, related to remediation activities at any individual site will have a material impact on the financial position, liquidity or results of operations of the company.
Environmental Capital Expenditures
In 2012, the company spent approximately $65 million on environmental capital projects either required by law or necessary to meet the company's internal environmental goals. The company currently estimates expenditures for environmental-related capital projects to be approximately $85 million in 2013. In the U.S., additional capital expenditures are expected to be required over the next decade for treatment, storage and disposal facilities for solid and hazardous waste and for compliance with the CAA. Until all CAA regulatory requirements are established and known, considerable uncertainty will remain regarding estimates for future capital expenditures. However, management does not believe that the costs to comply with these requirements will have a material impact on the financial position or liquidity of the company.
Climate Change
The company believes that climate change is an important global issue that presents risks and opportunities. Expanding upon significant global greenhouse gas (GHG) emissions and other environmental footprint reductions made in the period 1990-2004, the company reduced its environmental footprint achieving in 2011 year-over-year reductions of eight percent in GHG emissions and nine percent in water consumption. In addition, the company achieved a year-over-year reduction of 2.4 percent in 2011 in energy use from non-renewable resources. The company continuously evaluates opportunities for existing and new product and service offerings in light of the anticipated demands of a low-carbon economy. About $1.9 billion of the company's 2012 revenue was generated from sales of products that help direct and downstream customers GHG emissions.
The company is actively engaged in the effort to develop constructive public policies to reduce GHG emissions and encourage lower carbon forms of energy. Legislative efforts to control or limit GHG emissions could affect the company's energy source and supply choices as well as increase the cost of energy and raw materials derived from fossil fuels. Such efforts are also anticipated to provide the business community with greater certainty for the regulatory future, help guide investment decisions, and drive growth in demand for low-carbon and energy-efficient products, technologies, and services.
Part II
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS, continued
At the national and regional level, there are existing efforts to address climate change. Several of the company's facilities in the European Union (EU) are regulated under the EU Emissions Trading Scheme. In other countries, including the U.S., policy debate continues. The current unsettled policy environment in the U.S. adds an element of uncertainty to business decisions particularly those relating to long-term capital investments. If in the absence of federal legislation, states were to implement programs mandating GHG emissions reductions, the company, its suppliers and customers could be competitively disadvantaged by the added administrative costs of complying with a variety of state-specific requirements.
In 2010, EPA launched a phased-in scheme to regulate GHG emissions first from large stationary sources under CAA permitting requirements administered by state and local authorities. As a result, large capital investments may be required to install Best Available Control Technology on major new or modified sources of GHG emissions. This type of GHG emissions regulation by EPA, in the absence of or in addition to federal legislation, could result in more costly, less efficient facility-by-facility controls versus a federal program that incorporates policies that provide an economic balance that does not severely distort markets. Differences in regional or national legislation could present challenges in a global marketplace highlighting the need for coordinated global policy action.
Registration
The European Union's regulatory framework concerning the Registration, Evaluation and Authorization of Chemicals (REACH) entered into force in 2007 and requires manufacturers and importers to gather and register information on the properties of their substances that meet certain volume or toxicological criteria. The company has successfully integrated REACH registration requirements into its safety, health & environment processes and timely met all such requirements to date. REACH also contains a mechanism for the progressive substitution of the most dangerous chemicals when suitable alternatives have been identified. Depending on which chemicals are identified, the requirement to use safer alternatives could necessitate changes in production processes.
PFOA
The Performance Chemicals segment uses a form of PFOA (collectively, perfluorooctanoic acid and its salts, including the ammonium salt) as a processing aid to manufacture some fluoropolymer resins. The Performance Materials segment uses PFOA in the manufacture of certain raw materials for perfluoroelastomer parts (and some fluoroelastomers). In the fall of 2002, DuPont began producing rather than purchasing PFOA to support these manufacturing processes. PFOA is not used in the manufacture of fluorotelomers; however, it is an unintended by-product present at trace levels in some fluorotelomer-based products.
PFOA is bio-persistent and has been detected at very low levels in the blood of the general population. As a result, EPA initiated a process to enhance its understanding of the sources of PFOA in the environment and the pathways through which human exposure to PFOA is occurring. Although EPA has stated that there remains considerable scientific uncertainty regarding potential risks associated with PFOA, it also stated that it does not believe that there is any reason for consumers to stop using any products because of concerns about PFOA.
DuPont respects EPA's position raising questions about exposure routes and the potential toxicity of PFOA. DuPont and other companies continue research, emission reduction and product stewardship activities to help address EPA's questions. In January 2006, DuPont pledged its commitment to EPA's 2010/15 PFOA Stewardship Program. The EPA program asks participants (1) to commit to achieve, no later than 2010, a 95 percent reduction in both facility emissions and product content levels of PFOA, PFOA precursors and related higher homologue chemicals and (2) to commit to working toward the elimination of PFOA, PFOA precursors and related higher homologue chemicals from emissions and products by no later than 2015. DuPont has exceeded the EPA's 2010 objective. In February 2007, DuPont announced its commitment to no longer make, use or buy PFOA by 2015, or sooner if possible. To achieve this goal, DuPont developed PFOA replacement technology and is converting customers to fluoropolymer resins and dispersions manufactured using the replacement technology. DuPont has been introducing its next generation fluorotelomers products and converting customers to their use.
For additional information regarding PFOA matters, see Note 16 to the Consolidated Financial Statements.
Part II
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Derivatives and Other Hedging Instruments
In the ordinary course of business, the company enters into contractual arrangements (derivatives) to hedge its exposure to foreign currency, interest rate and commodity price risks under established procedures and controls. For additional information on these derivatives and related exposures, see Note 20 to the Consolidated Financial Statements.
The following table summarizes the impacts of the company's foreign currency hedging program on the company's results of operations for the years ended December 31, 2012, 2011, and 2010, and includes the company's pro rata share of its equity affiliates' exchange gains and losses and corresponding gains and losses on foreign currency exchange contracts:
|
| | | | | | | | | |
| (Dollars in millions) | 2012 | 2011 | 2010 |
| Pre-tax exchange loss | $ | (215 | ) | $ | (146 | ) | $ | (11 | ) |
| Tax benefit (expense) | 73 |
| 81 |
| (69 | ) |
| After-tax exchange loss | $ | (142 | ) | $ | (65 | ) | $ | (80 | ) |
In addition to the contracts disclosed in Note 20 to the Consolidated Financial Statements, from time to time, the company will enter into foreign currency exchange contracts to establish with certainty the USD amount of future firm commitments denominated in a foreign currency. Decisions regarding whether or not to hedge a given commitment are made on a case-by-case basis, taking into consideration the amount and duration of the exposure, market volatility and economic trends. Foreign currency exchange contracts are also used, from time to time, to manage near-term foreign currency cash requirements.
Sensitivity Analysis
The following table illustrates the fair values of outstanding derivative contracts at December 31, 2012 and 2011, and the effect on fair values of a hypothetical adverse change in the market prices or rates that existed at December 31, 2012 and 2011. The sensitivity for interest rate swaps is based on a one percent change in the market interest rate. Foreign currency and commodity contracts sensitivities are based on a 10 percent change in market rates.
|
| | | | | | | | | | | | |
| | Fair Value Asset/(Liability) | Fair Value Sensitivity |
| (Dollars in millions) | 2012 | 2011 | 2012 | 2011 |
| Interest rate swaps | $ | 55 |
| $ | 66 |
| $ | (29 | ) | $ | (40 | ) |
| Foreign currency contracts | 9 |
| 154 |
| (659 | ) | (541 | ) |
| Commodity contracts | (1 | ) | (3 | ) | (3 | ) | (103 | ) |
Since the company's risk management programs are highly effective, the potential loss in value for each risk management portfolio described above would be largely offset by changes in the value of the underlying exposure.
Concentration of Credit Risk
The company maintains cash and cash equivalents, marketable securities, derivatives and certain other financial instruments with various financial institutions. These financial institutions are generally highly rated and geographically dispersed and the company has a policy to limit the dollar amount of credit exposure with any one institution.
As part of the company's financial risk management processes, it continuously evaluates the relative credit standing of all of the financial institutions that service DuPont and monitors actual exposures versus established limits. The company has not sustained credit losses from instruments held at financial institutions.
The company's sales are not materially dependent on any single customer. As of December 31, 2012, no one individual customer balance represented more than 5 percent of the company's total outstanding receivables balance. Credit risk associated with its receivables balance is representative of the geographic, industry and customer diversity associated with the company's global businesses.
The company also maintains strong credit controls in evaluating and granting customer credit. As a result, it may require that customers provide some type of financial guarantee in certain circumstances. Length of terms for customer credit varies by industry and region.
Part II
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and supplementary data required by this Item are included herein, commencing on page F-1 of this report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
The company maintains a system of disclosure controls and procedures to give reasonable assurance that information required to be disclosed in the company's reports filed or submitted under the Securities Exchange Act of 1934 (Exchange Act) is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. These controls and procedures also give reasonable assurance that information required to be disclosed in such reports is accumulated and communicated to management to allow timely decisions regarding required disclosures.
As of December 31, 2012, the company's Chief Executive Officer (CEO) and Chief Financial Officer (CFO), together with management, conducted an evaluation of the effectiveness of the company's disclosure controls and procedures pursuant to Rules 13a-15(e) and 15d-15(e) of the Exchange Act. Based on that evaluation, the CEO and CFO concluded that these disclosure controls and procedures are effective.
There has been no change in the company's internal control over financial reporting that occurred during the fourth quarter of 2012 that has materially affected, or is reasonably likely to materially affect, the company's internal control over financial reporting. The company has completed its evaluation of its internal controls and has concluded that the company's system of internal controls over financial reporting was effective as of December 31, 2012 (see page F-2).
ITEM 9B. OTHER INFORMATION
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information with respect to this Item is incorporated herein by reference to the Proxy. Information related to directors is included within the section entitled, "Election of Directors." The company has not made any material changes to the procedures by which security holders may recommend nominees to its Board of Directors since these procedures were communicated in the company's 2012 Proxy Statement for the Annual Meeting of Stockholders held on April 25, 2012. Information related to the Audit Committee is incorporated herein by reference to the Proxy and is included within the sections entitled "Committees of the Board" and "Committee Membership." Information regarding executive officers is contained in the Proxy section entitled "Section 16(a) Beneficial Ownership Reporting Compliance" and as set forth below.
The company has adopted a Code of Ethics for its CEO, CFO, and Controller that may be accessed from the company's website at www.dupont.com by clicking on "Investors" and then "Corporate Governance". Any amendments to, or waiver from, any provision of the code will be posted on the company's website at the above address.
Executive Officers of the Registrant
The following is a list, as of February 6, 2013, of the company's Executive Officers:
|
| | |
| | Age | Executive Officer Since |
| Chair of the Board of Directors and Chief Executive Officer: | | |
| Ellen J. Kullman | 57 | 2006 |
| Other Executive Officers: | | |
| James C. Borel | 57 | 2004 |
| Executive Vice President | | |
| Benito Cachinero-Sánchez | 54 | 2011 |
| Senior Vice President - Human Resources | | |
| Thomas M. Connelly, Jr. | 60 | 2000 |
| Executive Vice President and Chief Innovation Officer | | |
| Nicholas C. Fanandakis | 56 | 2009 |
| Executive Vice President and Chief Financial Officer | | |
| Thomas L. Sager | 62 | 2008 |
| Senior Vice President and General Counsel | | |
| Mark P. Vergnano | 55 | 2009 |
| Executive Vice President | | |
The company's Executive Officers are elected or appointed for the ensuing year or for an indefinite term and until their successors are elected or appointed.
Ellen J. Kullman joined DuPont in 1988 as marketing manager and progressed through various roles as global business director and was named Vice President and General Manager of White Pigment & Mineral Products in 1995. In 2000, Mrs. Kullman was named Group Vice President and General Manager of several businesses and new business development. She became Group Vice President-DuPont Safety & Protection in 2002. In June 2006, Mrs. Kullman was named Executive Vice President and assumed leadership of Marketing & Sales along with Safety and Sustainability. She was appointed President on October 1, 2008 and became Chief Executive Officer on January 1, 2009. On December 31, 2009, she became Chair of the Board of Directors.
James C. Borel joined DuPont in 1978, and held a variety of product and sales management positions for Agricultural Products. In 1993, he transferred to Tokyo, Japan with Agricultural Products as regional manager, North Asia and was appointed regional director, Asia Pacific in 1994. In 1997, he was appointed regional director, North America and was appointed Vice President and General Manager-DuPont Crop Protection later that year. In January 2004, he was named Senior Vice President-DuPont Global Human Resources. He became Group Vice President in 2008 and was named Executive Vice President with responsibility for DuPont Crop Protection and Pioneer in October 2009. In 2011, he assumed responsibility for DuPont Nutrition & Health.
Part III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE, continued
Benito Cachinero-Sánchez joined DuPont in April 2011 as Senior Vice President - Human Resources. Prior to joining DuPont, he was Corporate Vice President of Human Resources at Automatic Data Processing (ADP). Prior to ADP, he was Vice President, Human Resources for the Medical Devices & Diagnostics Group of Johnson & Johnson.
Thomas M. Connelly, Jr. joined DuPont in 1977 as a research engineer. Since then, Mr. Connelly has served in various research and plant technical leadership roles, as well as product management and business director roles. Mr. Connelly served as Vice President and General Manager-DuPont Fluoroproducts from 1999 until September 2000, when he was named Senior Vice President and Chief Science and Technology Officer. In June 2006, Mr. Connelly was named Executive Vice President and Chief Innovation Officer. In October 2009, responsibility for DuPont Performance Polymers, Packaging & Industrial Polymers as well as integrated operations was added. In 2011, he assumed responsibility for DuPont Industrial Biosciences and Performance Coatings.
Nicholas C. Fanandakis joined DuPont in 1979 as an accounting and business analyst. Since then, Mr. Fanandakis served in a variety of plant, marketing, and product management and business director roles. Mr. Fanandakis served as Vice President and General Manager—DuPont Chemical Solutions Enterprise from 2003 until February 2007 when he was named Vice President—Corporate Plans. In January 2008, Mr. Fanandakis was named Group Vice President—DuPont Applied BioSciences. In November 2009, he was named Senior Vice President and Chief Financial Officer. In August 2010, he was named Executive Vice President and Chief Financial Officer.
Thomas L. Sager joined DuPont in 1976 as an attorney in the labor and security group. In 1998, he was named Chief Litigation Counsel and assumed oversight responsibility for all company litigation matters. He was named Vice President and Assistant General Counsel in 1999. In July 2008, he was appointed Senior Vice President and General Counsel.
Mark P. Vergnano joined DuPont in 1980 as a process engineer. He has had several assignments in manufacturing, technology, marketing, sales and business strategy. He has held assignments in various DuPont locations including Geneva, Switzerland. In February 2003 he was named Vice President and General Manager—Nonwovens and Vice President and General Manager—Surfaces and Building Innovations in October 2005. In June 2006, he was named Group Vice President of DuPont Safety & Protection. In October 2009, Mr. Vergnano was appointed Executive Vice President with responsibility for DuPont Protection Technologies, Building Innovations, Sustainable Solutions, Chemicals & Fluoroproducts, Titanium Technologies and Electronics & Communications. He also leads the company's sustainability, safety, communications, and sales and marketing functions.
ITEM 11. EXECUTIVE COMPENSATION
Information with respect to this Item is incorporated herein by reference to the Proxy and is included in the sections "Compensation Discussion and Analysis," "2012 Summary Compensation Table," "2012 Grants of Plan-Based Awards," "Outstanding Equity Awards," "2012 Option Exercises and Stock Vested," "Pension Benefits," "Nonqualified Deferred Compensation," "Potential Payments Upon Termination or Change in Control," and "Directors' Compensation." Information related to the Compensation Committee is included within the sections entitled "Compensation Committee Interlocks and Insider Participation" and "Compensation Committee Report."
Part III
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Information with respect to Beneficial Owners is incorporated herein by reference to the Proxy and is included in the section entitled "Ownership of Company Stock."
Securities authorized for issuance under equity compensation plans as of December 31, 2012
(Shares in thousands, except per share)
|
| | | | | | | | | |
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights2 | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans3 | |
Equity compensation plans approved by security holders | 38,089 |
| 1 | $ | 39.70 |
| 59,953 |
| |
Equity compensation plans not approved by security holders | 87 |
| 4 | $ | 37.39 |
| — |
| 5 |
| Total | 38,176 |
| | $ | 39.70 |
| 59,953 |
| |
| |
1. | Includes stock-settled time-vested and performance-based restricted stock units granted and stock units deferred under the company's Equity and Incentive Plan, Stock Performance Plan, Variable Compensation Plan and the Stock Accumulation and Deferred Compensation Plan for Directors. Performance-based restricted stock units reflect the maximum number of shares to be awarded at the conclusion of the performance cycle (200 percent of the original grant). The actual award payouts can range from zero to 200 percent of the original grant. |
| |
2. | Represents the weighted-average exercise price of the outstanding stock options only; the outstanding stock-settled time-vested and performance-based restricted stock units and deferred stock units are not included in this calculation. |
| |
3. | Reflects shares available pursuant to the issuance of stock options, restricted stock, restricted stock units or other stock-based awards under the amended Equity and Incentive Plan approved by the shareholders in April 2011 (see Note 19 to the company's Consolidated Financial Statements). The maximum number of shares of stock reserved for the grant or settlement of awards under the Equity and Incentive Plan (Share Limit) shall be 110,000 and shall be subject to adjustment as provided therein; provided that each share in excess of 30,000 issued under the Equity and Incentive Plan pursuant to any award settled in stock, other than a stock option or stock appreciation right, shall be counted against the foregoing Share Limit as four and one-half shares for every one share actually issued in connection with such award. (For example, if 32,000 shares of restricted stock are granted under the Equity and Incentive Plan, 39,000 shall be charged against the Share Limit in connection with that award.) |
| |
4. | Includes 16 deferred stock units resulting from base salary and short-term incentive (STIP) deferrals under the Management Deferred Compensation Plan (MDCP). Under the MDCP, a select group of management or highly compensated employees can elect to defer the receipt of their base salary, STIP or Long Term Incentive (LTI) award. LTI deferrals are included in footnote 1 to the above chart. The company does not match deferrals under the MDCP. There are seven core investment options under the MDCP for base salary and STIP deferrals, including deferred stock units with dividend equivalents credited as additional stock units. In general, deferred stock units are distributed in the form of DuPont common stock and may be made in the form of lump sum at a specified future date prior to retirement or a lump sum or annual installments after separation from service. Shareholder approval of the MDCP was not required under the rules of the New York Stock Exchange. This column also includes 71 options from the conversion of DuPont Canada options to DuPont options in connection with the company's acquisition of the minority interest in DuPont Canada. |
| |
5. | There is no limit on the number of shares that can be issued under the MDCP and no further shares are available for issuance under the other equity compensation arrangements described in footnote 4 to the above chart. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information with respect to the company's policy and procedures for the review, approval or ratification of transactions with related persons is incorporated by reference herein to the Proxy and is included in the section entitled "Review and Approval of Transactions with Related Persons." Information with respect to director independence is incorporated by reference herein to the Proxy and is included in the sections entitled "DuPont Board of Directors—Corporate Governance Guidelines," "Guidelines for Determining the Independence of DuPont Directors," "Committees of the Board," "Committee Membership" and "Election of Directors".
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Information with respect to this Item is incorporated herein by reference to the Proxy and is included in the sections entitled "Ratification of Independent Registered Public Accounting Firm."
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| |
| (a) | Financial Statements, Financial Statement Schedules and Exhibits: |
| |
| 1. | Financial Statements (See the Index to the Consolidated Financial Statements on page F-1 of this report). |
| |
| 2. | Financial Statement Schedules |
Schedule II—Valuation and Qualifying Accounts
(Dollars in millions)
|
| | | | | | | | | |
| Year Ended December 31, | 2012 | 2011 | 2010 |
| Accounts Receivable—Allowance for Doubtful Receivables | |
| |
| |
|
| Balance at beginning of period | $ | 292 |
| $ | 326 |
| $ | 322 |
|
| Additions charged to cost and expenses | 33 |
| 73 |
| 75 |
|
| Deductions from reserves | (64 | ) | (107 | ) | (71 | ) |
| Amounts related to the Performance Coatings business | (18 | ) | — |
| — |
|
| Balance at end of period | $ | 243 |
| $ | 292 |
| $ | 326 |
|
| Deferred Tax Assets—Valuation Allowance | |
| |
| |
|
| Balance at beginning of period | $ | 1,971 |
| $ | 1,666 |
| $ | 1,759 |
|
| Net (benefits) charges to income tax expense | (77 | ) | 73 |
| (19 | ) |
| Additions charged to other comprehensive income (loss) | 10 |
| 236 |
| — |
|
| Currency translation | 10 |
| (4 | ) | (74 | ) |
| Balance at end of period | $ | 1,914 |
| $ | 1,971 |
| $ | 1,666 |
|
Financial Statement Schedules listed under SEC rules but not included in this report are omitted because they are not applicable or the required information is shown in the Consolidated Financial Statements or notes thereto incorporated by reference.
Part IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES, continued
The following list of exhibits includes both exhibits submitted with this Form 10-K as filed with the SEC and those incorporated by reference to other filings:
|
| | |
Exhibit Number | | Description |
| | | |
| 3.1 | | Company's Restated Certificate of Incorporation.
|
| | | |
| 3.2 | | Company’s Bylaws, as last amended effective November 1, 2009 (incorporated by reference to Exhibit 3.2 to the company’s Annual Report on Form 10-K for the year ended December 31, 2009). |
| | | |
| 4 | | The company agrees to provide the Commission, on request, copies of instruments defining the rights of holders of long-term debt of the company and its subsidiaries. |
| | | |
| 10.1* | | The DuPont Stock Accumulation and Deferred Compensation Plan for Directors, as last amended effective January 1, 2009 (incorporated by reference to Exhibit 10.1 to the company’s Annual Report on Form 10-K for the year ended December 31, 2008). |
| | | |
| 10.2* | | Company’s Supplemental Retirement Income Plan, as last amended effective June 4, 1996 (incorporated by reference to Exhibit 10.2 to the company's Annual Report on Form 10-K for the year ended December 31, 2011). |
| | | |
| 10.3* | | Company’s Pension Restoration Plan, as restated effective July 17, 2006 (incorporated by reference to Exhibit 10.3 to the company’s Quarterly Report on Form 10-Q for the period ended June 30, 2011). |
| | | |
| 10.4* | | Company’s Rules for Lump Sum Payments, as last amended effective December 20, 2007 (incorporated by reference to Exhibit 10.4 to the company’s Quarterly Report on Form 10-Q for the period ended June 30, 2011). |
| | | |
| 10.5* | | Company’s Stock Performance Plan, as last amended effective January 25, 2007 (incorporated by reference to Exhibit 10.5 to the company's Annual Report on Form 10-K for the year ended December 31, 2011). |
| | | |
| 10.6* | | Company’s Equity and Incentive Plan as amended and restated effective March 2, 2011 and approved by the company’s shareholders on April 27, 2011 (incorporated by reference to pages B1-B15 of the company’s Annual Meeting Proxy Statement dated March 18, 2011). |
| | | |
| 10.7* | | Form of Award Terms under the company’s Equity and Incentive Plan (incorporated by reference to Exhibit 10.8 to the company’s Quarterly Report on Form 10-Q for the period ended March 31, 2009). |
| | | |
| 10.8* | | Company’s Retirement Savings Restoration Plan, as last amended effective January 1, 2013. |
| | | |
| 10.9* | | Company’s Retirement Income Plan for Directors, as last amended January 2011 (incorporated by reference to Exhibit 10.9 to the company's Quarterly Report on Form 10-Q for the period ended March 31, 2012). |
| | | |
| 10.10* | | Company's Management Deferred Compensation Plan, adopted on May 2, 2008, as last amended May 12, 2010 (incorporated by reference to Exhibit 10.11 to the company's Quarterly Report on Form 10-Q for the period ended June 30, 2010). |
| | | |
| 10.11* | | Supplemental Deferral Terms for Deferred Long Term Incentive Awards and Deferred Variable Compensation Awards (incorporated by reference to Exhibit 10.15 to the company’s Annual Report on Form 10-K for the year ended December 31, 2008). |
| | | |
| 10.12* | | Purchase Agreement dated as of August 30 2012, by and between E. I. du Pont de Nemours and Company and Flash Bermuda Co. Ltd. (incorporated by reference to Exhibit 2.1 to the company's Current Report on Form 8-K filed on September 4, 2012)(the "Purchase Agreement"). The company agrees to furnish supplementally a copy of any omitted schedules to the Commission upon request. |
| | | |
| 10.13* | | Amendment to Purchase Agreement, dated as of January 31, 2013, by and between E. I. du Pont de Nemours and Company and Flash Bermuda Co. Ltd. |
| | | |
| 12 | | Computation of Ratio of Earnings to Fixed Charges. |
| | | |
| 21 | | Subsidiaries of the Registrant.
|
| | | |
| 23 | | Consent of Independent Registered Public Accounting Firm. |
Part IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES, continued
|
| | |
Exhibit Number | | Description |
| | | |
| 31.1 | | Rule 13a-14(a)/15d-14(a) Certification of the company’s Principal Executive Officer. |
| | | |
| 31.2 | | Rule 13a-14(a)/15d-14(a) Certification of the company’s Principal Financial Officer. |
| | | |
| 32.1 | | Section 1350 Certification of the company’s Principal Executive Officer. The information contained in this Exhibit shall not be deemed filed with the Securities and Exchange Commission nor incorporated by reference in any registration statement filed by the registrant under the Securities Act of 1933, as amended. |
| | | |
| 32.2 | | Section 1350 Certification of the company’s Principal Financial Officer. The information contained in this Exhibit shall not be deemed filed with the Securities and Exchange Commission nor incorporated by reference in any registration statement filed by the registrant under the Securities Act of 1933, as amended. |
| | | |
| 95 | | Mine Safety Disclosures. |
| | | |
| 101.INS | | XBRL Instance Document |
| | | |
| 101.SCH | | XBRL Taxonomy Extension Schema Document |
| | | |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document |
| | | |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document |
| | | |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document |
| | | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document |
|
| |
| * | Management contract or compensatory plan or arrangement required to be filed as an exhibit to this Form 10-K. |
Pursuant to the requirements of Section 13 of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | |
| February 6, 2013 | | |
| | E. I. DU PONT DE NEMOURS AND COMPANY |
| | By: | /s/ Nicholas C. Fanandakis |
| | | Nicholas C. Fanandakis Executive Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) |
_____________________________________________
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated:
|
| | | | |
| Signature | | Title(s) | | Date |
| | | | | |
| /s/ E.J. Kullman | | Chair of the Board of Directors and Chief Executive Officer and Director (Principal Executive Officer) | | February 6, 2013 |
| E. J. Kullman | | | |
| | | | | |
| /s/ L. Andreotti | | Director | | February 6, 2013 |
| L. Andreotti | | | | |
| | | | | |
| /s/ R.H. Brown | | Director | | February 6, 2013 |
| R. H. Brown | | | | |
| | | | | |
| /s/ R.A. Brown | | Director | | February 6, 2013 |
| R. A. Brown | | | | |
| | | | | |
| /s/ B.P. Collomb | | Director | | February 6, 2013 |
| B. P. Collomb | | | | |
| | | | | |
| /s/ C.J. Crawford | | Director | | February 6, 2013 |
| C. J. Crawford | | | | |
| | | | | |
| /s/ A.M. Cutler | | Director | | February 6, 2013 |
| A. M. Cutler | | | | |
| | | | | |
| /s/ E.I. du Pont, II | | Director | | February 6, 2013 |
| E. I. du Pont, II | | | | |
| | | | | |
| /s/ M.A. Hewson | | Director | | February 6, 2013 |
| M. A. Hewson | | | | |
| | | | | |
| /s/ L.D. Juliber | | Director | | February 6, 2013 |
| L. D. Juliber | | | | |
| | | | | |
| /s/ L.M. Thomas | | Director | | February 6, 2013 |
| L. M. Thomas | | | | |
E.I. du Pont de Nemours and Company
Index to the Consolidated Financial Statements
|
| |
| | Page(s) |
| Consolidated Financial Statements: | |
| |
| |
| |
| |
| |
| |
| |
| |
Management's Reports on Responsibility for Financial Statements and
Internal Control over Financial Reporting
Management's Report on Responsibility for Financial Statements
Management is responsible for the Consolidated Financial Statements and the other financial information contained in this Annual Report on Form 10-K. The financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (GAAP) and are considered by management to present fairly the company's financial position, results of operations and cash flows. The financial statements include some amounts that are based on management's best estimates and judgments. The financial statements have been audited by the company's independent registered public accounting firm, PricewaterhouseCoopers LLP. The purpose of their audit is to express an opinion as to whether the Consolidated Financial Statements included in this Annual Report on Form 10-K present fairly, in all material respects, the company's financial position, results of operations and cash flows in conformity with GAAP. Their report is presented on the following page.
Management's Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining an adequate system of internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. The company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. The company's internal control over financial reporting includes those policies and procedures that:
| |
| i. | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| |
| ii. | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of the company are being made only in accordance with authorization of management and directors of the company; and |
| |
| iii. | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisitions, use or disposition of the company's assets that could have a material effect on the financial statements. |
Internal control over financial reporting has certain inherent limitations which may not prevent or detect misstatements. In addition, changes in conditions and business practices may cause variation in the effectiveness of internal controls.
Management assessed the effectiveness of the company's internal control over financial reporting as of December 31, 2012, based on criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on its assessment and those criteria, management concluded that the company maintained effective internal control over financial reporting as of December 31, 2012.
PricewaterhouseCoopers LLP, an independent registered public accounting firm, has audited the effectiveness of the company's internal control over financial reporting as of December 31, 2012, as stated in their report, which is presented on the following page.
|
| | |
| | |
Ellen J. Kullman Chair of the Board and Chief Executive Officer | | Nicholas C. Fanandakis Executive Vice President and Chief Financial Officer |
February 6, 2013
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of
E. I. du Pont de Nemours and Company:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of income, comprehensive income, equity and cash flows present fairly, in all material respects, the financial position of E. I. du Pont de Nemours and Company and its subsidiaries at December 31, 2012 and 2011, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2012 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a) (2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2012, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in "Management's Report on Internal Control over Financial Reporting" appearing on page F-2. Our responsibility is to express opinions on these financial statements, on the financial statement schedule and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
PricewaterhouseCoopers LLP
Philadelphia, Pennsylvania
February 6, 2013
E. I. du Pont de Nemours and Company
Consolidated Financial Statements
CONSOLIDATED INCOME STATEMENTS
(Dollars in millions, except per share)
|
| | | | | | | | | |
| For the year ended December 31, | 2012 | 2011 | 2010 |
| Net sales | $ | 34,812 |
| $ | 33,681 |
| $ | 27,700 |
|
| Other income, net | 498 |
| 742 |
| 1,199 |
|
| Total | 35,310 |
| 34,423 |
| 28,899 |
|
| Cost of goods sold and other operating charges | 25,604 |
| 24,874 |
| 20,574 |
|
| Selling, general and administrative expenses | 3,567 |
| 3,358 |
| 2,912 |
|
| Research and development expense | 2,067 |
| 1,910 |
| 1,603 |
|
| Interest expense | 464 |
| 447 |
| 590 |
|
| Employee separation / asset related charges, net | 493 |
| 53 |
| (40 | ) |
| Total | 32,195 |
| 30,642 |
| 25,639 |
|
| Income from continuing operations before income taxes | 3,115 |
| 3,781 |
| 3,260 |
|
| Provision for income taxes on continuing operations | 622 |
| 626 |
| 515 |
|
| Income from continuing operations after income taxes | 2,493 |
| 3,155 |
| 2,745 |
|
| Income from discontinued operations after income taxes | 320 |
| 355 |
| 307 |
|
| Net income | 2,813 |
| 3,510 |
| 3,052 |
|
| Less: Net income attributable to noncontrolling interests | 25 |
| 36 |
| 21 |
|
| Net income attributable to DuPont | $ | 2,788 |
| $ | 3,474 |
| $ | 3,031 |
|
| Basic earnings per share of common stock: | | | |
| Basic earnings per share of common stock from continuing operations | $ | 2.63 |
| $ | 3.35 |
| $ | 2.99 |
|
| Basic earnings per share of common stock from discontinued operations | 0.34 |
| 0.38 |
| 0.34 |
|
| Basic earnings per share of common stock | $ | 2.98 |
| $ | 3.73 |
| $ | 3.32 |
|
| Diluted earnings per share of common stock: | | | |
| Diluted earnings per share of common stock from continuing operations | $ | 2.61 |
| $ | 3.30 |
| $ | 2.94 |
|
| Diluted earnings per share of common stock from discontinued operations | 0.34 |
| 0.38 |
| 0.33 |
|
| Diluted earnings per share of common stock | $ | 2.95 |
| $ | 3.68 |
| $ | 3.28 |
|
| Dividends per share of common stock | $ | 1.70 |
| $ | 1.64 |
| $ | 1.64 |
|
See Notes to the Consolidated Financial Statements beginning on page F-9.
E. I. du Pont de Nemours and Company
Consolidated Financial Statements
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(Dollars in millions, except per share)
|
| | | | | | | | | |
| For the year ended December 31, | 2012 | 2011 | 2010 |
| Net income | $ | 2,813 |
| $ | 3,510 |
| $ | 3,052 |
|
| Other comprehensive income (loss), before tax: | | | |
| Cumulative translation adjustment | 77 |
| (457 | ) | (6 | ) |
| Net revaluation and clearance of cash flow hedges to earnings: |
|
|
|
| Additions and revaluations of derivatives designated as cash flow hedges | 8 |
| 10 |
| (33 | ) |
| Clearance of hedge results to earnings | (65 | ) | 96 |
| 90 |
|
| Net revaluation and clearance of cash flow hedges to earnings | (57 | ) | 106 |
| 57 |
|
| Pension benefit plans: | | | |
| Net loss | (1,433 | ) | (4,069 | ) | (635 | ) |
| Prior service benefit (cost) | 22 |
| (2 | ) | — |
|
| Reclassifications to net income: |
|
|
|
|
|
|
| Amortization of prior service cost | 13 |
| 16 |
| 16 |
|
| Amortization of loss | 887 |
| 613 |
| 507 |
|
| Curtailment / settlement loss | 7 |
| — |
| — |
|
| Pension benefit plans, net | (504 | ) | (3,442 | ) | (112 | ) |
| Other benefit plans: | | | |
| Net loss | (60 | ) | (437 | ) | (94 | ) |
| Prior service benefit (cost) | 857 |
| (11 | ) | 189 |
|
| Reclassifications to net income: |
|
|
|
|
|
|
| Amortization of prior service benefit | (155 | ) | (121 | ) | (106 | ) |
| Amortization of loss | 94 |
| 60 |
| 58 |
|
| Curtailment loss | 3 |
| — |
| — |
|
| Other benefit plans, net | 739 |
| (509 | ) | 47 |
|
| Net unrealized (loss) gain on securities | (2 | ) | 2 |
| 2 |
|
| Other comprehensive income (loss), before tax | 253 |
| (4,300 | ) | (12 | ) |
| Income tax (expense) benefit related to items of other comprehensive income | (121 | ) | 1,322 |
| (5 | ) |
| Other comprehensive income (loss), net of tax | 132 |
| (2,978 | ) | (17 | ) |
| Comprehensive income | 2,945 |
| 532 |
| 3,035 |
|
| Less: Comprehensive income attributable to noncontrolling interests | 53 |
| 18 |
| 23 |
|
| Comprehensive income attributable to DuPont | $ | 2,892 |
| $ | 514 |
| $ | 3,012 |
|
See Notes to the Consolidated Financial Statements beginning on page F-9.
E. I. du Pont de Nemours and Company
Consolidated Financial Statements
CONSOLIDATED BALANCE SHEETS
(Dollars in millions, except per share)
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Assets | |
| |
|
| Current assets | |
| |
|
| Cash and cash equivalents | $ | 4,284 |
| $ | 3,586 |
|
| Marketable securities | 123 |
| 433 |
|
| Accounts and notes receivable, net | 5,452 |
| 6,022 |
|
| Inventories | 7,422 |
| 7,195 |
|
| Prepaid expenses | 204 |
| 151 |
|
| Deferred income taxes | 650 |
| 671 |
|
| Assets held for sale | 3,056 |
| — |
|
| Total current assets | 21,191 |
| 18,058 |
|
| Property, plant and equipment | 31,826 |
| 32,761 |
|
| Less: Accumulated depreciation | 19,085 |
| 19,349 |
|
| Net property, plant and equipment | 12,741 |
| 13,412 |
|
| Goodwill | 4,616 |
| 5,413 |
|
| Other intangible assets | 5,126 |
| 5,413 |
|
| Investment in affiliates | 1,163 |
| 1,117 |
|
| Deferred income taxes | 3,939 |
| 4,067 |
|
| Other assets | 960 |
| 1,012 |
|
| Total | $ | 49,736 |
| $ | 48,492 |
|
| Liabilities and Equity | |
| |
|
| Current liabilities | |
| |
|
| Accounts payable | $ | 4,853 |
| $ | 4,816 |
|
| Short-term borrowings and capital lease obligations | 1,275 |
| 817 |
|
| Income taxes | 340 |
| 255 |
|
| Other accrued liabilities | 5,997 |
| 5,297 |
|
| Liabilities related to assets held for sale | 1,084 |
| — |
|
| Total current liabilities | 13,549 |
| 11,185 |
|
| Long-term borrowings and capital lease obligations | 10,465 |
| 11,736 |
|
| Other liabilities | 14,687 |
| 15,508 |
|
| Deferred income taxes | 856 |
| 1,001 |
|
| Total liabilities | 39,557 |
| 39,430 |
|
| Commitments and contingent liabilities |
|
|
|
|
| Stockholders' Equity | |
| |
|
Preferred stock, without par value – cumulative; 23,000,000 shares authorized; issued at December 31, 2012 and 2011: | |
| |
|
| $4.50 Series – 1,673,000 shares (callable at $120) | 167 |
| 167 |
|
| $3.50 Series – 700,000 shares (callable at $102) | 70 |
| 70 |
|
Common stock, $.30 par value; 1,800,000,000 shares authorized; issued at December 31, 2012 – 1,020,057,000; 2011 – 1,013,164,000 | 306 |
| 304 |
|
| Additional paid-in capital | 10,632 |
| 10,107 |
|
| Reinvested earnings | 14,286 |
| 13,422 |
|
| Accumulated other comprehensive loss | (8,646 | ) | (8,750 | ) |
Common stock held in treasury, at cost (Shares: December 31, 2012 and 2011 – 87,041,000) | (6,727 | ) | (6,727 | ) |
| Total DuPont stockholders' equity | 10,088 |
| 8,593 |
|
| Noncontrolling interests | 91 |
| 469 |
|
| Total equity | 10,179 |
| 9,062 |
|
| Total | $ | 49,736 |
| $ | 48,492 |
|
See Notes to the Consolidated Financial Statements beginning on page F-9.
E. I. du Pont de Nemours and Company
Consolidated Financial Statements
CONSOLIDATED STATEMENTS OF EQUITY
(Dollars in millions, except per share) |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Preferred Stock | Common Stock | Additional Paid-in Capital | Reinvested Earnings | Accumulated Other Compre- hensive Loss | Treasury Stock | Non- controlling Interests | Total Equity |
| 2010 | |
| |
| |
| |
| |
| |
| |
| |
|
| Balance January 1, 2010 | $ | 237 |
| $ | 297 |
| $ | 8,469 |
| $ | 10,710 |
| $ | (5,771 | ) | $ | (6,727 | ) | $ | 436 |
| $ | 7,651 |
|
| Acquisition of a majority interest in a consolidated subsidiary | |
| |
| |
| |
| |
| |
| 9 |
| 9 |
|
| Net income | |
| |
| |
| 3,031 |
| |
| |
| 21 |
| 3,052 |
|
| Other comprehensive income (loss) | |
| |
| |
| |
| (19 | ) | |
| 2 |
| (17 | ) |
| Common dividends ($1.64 per share) | |
| |
| |
| (1,500 | ) | |
| |
| (3 | ) | (1,503 | ) |
| Preferred dividends | |
| |
| |
| (10 | ) | |
| |
| |
| (10 | ) |
| Common stock issued - compensation plans | |
| 6 |
| 805 |
| |
| |
| |
| |
| 811 |
|
| Common stock repurchased | |
| |
| |
| |
| |
| (250 | ) | |
| (250 | ) |
| Common stock retired | |
| (2 | ) | (47 | ) | (201 | ) | |
| 250 |
| |
| — |
|
| Balance December 31, 2010 | $ | 237 |
| $ | 301 |
| $ | 9,227 |
| $ | 12,030 |
| $ | (5,790 | ) | $ | (6,727 | ) | $ | 465 |
| $ | 9,743 |
|
| 2011 | |
| |
| |
| |
| |
| |
| |
| |
|
| Sale of a majority interest in a consolidated subsidiary | |
| |
| |
| |
| |
| |
| (3 | ) | (3 | ) |
| Net income | |
| |
| |
| 3,474 |
| |
| |
| 36 |
| 3,510 |
|
| Other comprehensive income (loss) | |
| |
| |
| |
| (2,960 | ) | |
| (18 | ) | (2,978 | ) |
| Common dividends ($1.64 per share) | |
| |
| |
| (1,531 | ) | |
| |
| (11 | ) | (1,542 | ) |
| Preferred dividends | |
| |
| |
| (10 | ) | |
| |
| |
| (10 | ) |
| Common stock issued - compensation plans | |
| 7 |
| 1,007 |
| |
| |
| |
| |
| 1,014 |
|
| Common stock repurchased | |
| |
| |
| |
| |
| (672 | ) | |
| (672 | ) |
| Common stock retired | |
| (4 | ) | (127 | ) | (541 | ) | |
| 672 |
| |
| — |
|
| Balance December 31, 2011 | $ | 237 |
| $ | 304 |
| $ | 10,107 |
| $ | 13,422 |
| $ | (8,750 | ) | $ | (6,727 | ) | $ | 469 |
| $ | 9,062 |
|
| 2012 | |
| |
| |
| |
| |
| |
| |
| |
|
| Acquisitions of a noncontrolling interest in consolidated subsidiaries | |
| |
| (25 | ) | |
| |
| |
| (370 | ) | (395 | ) |
| Net income | |
| |
| |
| 2,788 |
| |
| |
| 25 |
| 2,813 |
|
| Other comprehensive income (loss) | |
| |
| |
| |
| 104 |
| |
| 28 |
| 132 |
|
| Common dividends ($1.70 per share) | |
| |
| |
| (1,593 | ) | |
| |
| (61 | ) | (1,654 | ) |
| Preferred dividends | |
| |
| |
| (10 | ) | |
| |
| |
| (10 | ) |
| Common stock issued - compensation plans | |
| 4 |
| 627 |
| |
| |
| |
| |
| 631 |
|
| Common stock repurchased | |
| |
| |
| |
| |
| (400 | ) | |
| (400 | ) |
| Common stock retired | |
| (2 | ) | (77 | ) | (321 | ) | |
| 400 |
| |
| — |
|
| Balance December 31, 2012 | $ | 237 |
| $ | 306 |
| $ | 10,632 |
| $ | 14,286 |
| $ | (8,646 | ) | $ | (6,727 | ) | $ | 91 |
| $ | 10,179 |
|
See Notes to the Consolidated Financial Statements beginning on page F-9.
E. I. du Pont de Nemours and Company
Consolidated Financial Statements
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Dollars in millions)
|
| | | | | | | | | |
| For the year ended December 31, | 2012 | 2011 | 2010 |
| Operating activities | |
| |
| |
|
| Net income | $ | 2,813 |
| $ | 3,510 |
| $ | 3,052 |
|
| Adjustments to reconcile net income to cash provided by operating activities: | |
|
| |
|
| Depreciation | 1,376 |
| 1,283 |
| 1,204 |
|
| Amortization of intangible assets | 337 |
| 277 |
| 176 |
|
| Other operating charges and credits – net | 1,186 |
| 992 |
| 809 |
|
| Contributions to pension plans | (848 | ) | (341 | ) | (782 | ) |
| (Increase) decrease in operating assets: |
|
| |
| |
|
| Accounts and notes receivable | 114 |
| (360 | ) | (481 | ) |
| Inventories and other operating assets | (857 | ) | (902 | ) | (512 | ) |
| Increase (decrease) in operating liabilities: |
|
| |
| |
|
| Accounts payable and other operating liabilities | 1,037 |
| 526 |
| 1,010 |
|
| Accrued interest and income taxes | (309 | ) | 167 |
| 83 |
|
| Cash provided by operating activities | 4,849 |
| 5,152 |
| 4,559 |
|
| Investing activities | |
| |
| |
|
| Purchases of property, plant and equipment | (1,793 | ) | (1,843 | ) | (1,508 | ) |
| Investments in affiliates | (97 | ) | (67 | ) | (100 | ) |
| Payments for businesses – net of cash acquired | (18 | ) | (6,459 | ) | (637 | ) |
| Proceeds from sale of assets – net of cash sold | 302 |
| 214 |
| 195 |
|
| Net decrease (increase) in short-term financial instruments | 315 |
| 2,149 |
| (457 | ) |
| Forward exchange contract settlements | (40 | ) | (227 | ) | 176 |
|
| Other investing activities – net | (15 | ) | (5 | ) | (108 | ) |
| Cash used for investing activities | (1,346 | ) | (6,238 | ) | (2,439 | ) |
| Financing activities | |
| |
| |
|
| Dividends paid to stockholders | (1,594 | ) | (1,533 | ) | (1,501 | ) |
| Net (decrease) increase in short-term (less than 90 days) borrowings | (200 | ) | 185 |
| 20 |
|
| Long-term and other borrowings: | |
| |
| |
|
| Receipts | 323 |
| 2,539 |
| 2,061 |
|
| Payments | (916 | ) | (1,163 | ) | (2,859 | ) |
| Repurchase of common stock | (400 | ) | (672 | ) | (250 | ) |
| Proceeds from exercise of stock options | 550 |
| 952 |
| 708 |
|
| Payments for noncontrolling interest | (470 | ) | — |
| — |
|
| Other financing activities – net | 10 |
| 95 |
| (8 | ) |
| Cash (used for) provided by financing activities | (2,697 | ) | 403 |
| (1,829 | ) |
| Effect of exchange rate changes on cash | (13 | ) | 6 |
| (49 | ) |
| Cash classified as held for sale | (95 | ) | — |
| — |
|
| Increase (decrease) in cash and cash equivalents | 698 |
| (677 | ) | 242 |
|
| Cash and cash equivalents at beginning of year | 3,586 |
| 4,263 |
| 4,021 |
|
| Cash and cash equivalents at end of year | $ | 4,284 |
| $ | 3,586 |
| $ | 4,263 |
|
| Supplemental cash flow information: | |
| |
| |
|
| Cash paid during the year for | |
| |
| |
|
| Interest, net of amounts capitalized | $ | 501 |
| $ | 455 |
| $ | 623 |
|
| Income taxes | 1,054 |
| 527 |
| 416 |
|
See Notes to the Consolidated Financial Statements beginning on page F-9.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The company follows generally accepted accounting principles in the United States of America (GAAP). The significant accounting policies described below, together with the other notes that follow, are an integral part of the Consolidated Financial Statements.
Preparation of Financial Statements
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Basis of Consolidation
The Consolidated Financial Statements include the accounts of the company, subsidiaries in which a controlling interest is maintained and variable interest entities (VIEs) for which DuPont is the primary beneficiary. For those consolidated subsidiaries in which the company's ownership is less than 100 percent, the outside stockholders' interests are shown as noncontrolling interests. Investments in affiliates over which the company has significant influence but not a controlling interest are carried on the equity method. At December 31, 2012, the assets, liabilities and operations of VIEs for which DuPont is the primary beneficiary were not material to the Consolidated Financial Statements of the company.
The company is also involved with certain joint ventures accounted for under the equity method of accounting that are VIEs. The company is not the primary beneficiary, as the nature of the company's involvement with the VIEs does not provide it the power to direct the VIEs significant activities. Future events may require these VIEs to be consolidated if the company becomes the primary beneficiary. At December 31, 2012, the maximum exposure to loss related to the unconsolidated VIEs is not considered material to the Consolidated Financial Statements of the company.
Basis of Presentation
Certain reclassifications of prior year's data have been made to conform to current year's presentation. In the third quarter 2012, the company signed a definitive agreement to sell its Performance Coatings business (which represented a reportable segment). In accordance with GAAP, the results of Performance Coatings are presented as discontinued operations and, as such, have been excluded from continuing operations in the Consolidated Income Statements and from segment results for all periods presented. The sum of the individual earnings per share amounts from continuing and discontinued operations may not equal the total company earnings per share amounts due to rounding. The assets and liabilities of Performance Coatings at December 31, 2012 have been reclassified and segregated as held for sale in the Consolidated Balance Sheet while corresponding amounts at prior year-ends have not been reclassified and presented as such. The cash flows and comprehensive income related to Performance Coatings have not been segregated and are included in the Consolidated Statements of Cash Flows and Comprehensive Income, respectively, for all periods presented. Amounts related to Performance Coatings are consistently included in or excluded from the Notes to the Consolidated Financial Statements based on the financial statement line item and period of each disclosure. See Note 2 to the Consolidated Financial Statements for further information.
Revenue Recognition
The company recognizes revenue when the earnings process is complete. The company's revenues are from the sale of a wide range of products to a diversified base of customers around the world. Revenue for product sales is recognized upon delivery, when title and risk of loss have been transferred, collectability is reasonably assured and pricing is fixed or determinable. Substantially all product sales are sold FOB (free on board) shipping point or, with respect to non United States of America (U.S.) customers, an equivalent basis. Accruals are made for sales returns and other allowances based on the company's experience. The company accounts for cash sales incentives as a reduction in sales and noncash sales incentives as a charge to cost of goods sold or selling expense, depending on the nature of the incentive. Amounts billed to customers for shipping and handling fees are included in net sales and costs incurred by the company for the delivery of goods are classified as cost of goods sold and other operating charges in the Consolidated Income Statements. Taxes on revenue-producing transactions are excluded from net sales.
The company periodically enters into prepayment contracts with customers in the Agriculture segment and receives advance payments for product to be delivered in future periods. These advance payments are recorded as deferred revenue (classified as other accrued liabilities) or debt, depending on the nature of the program. Revenue associated with advance payments is recognized as shipments are made and title, ownership and risk of loss pass to the customer.
Licensing and royalty income is recognized in accordance with agreed upon terms, when performance obligations are satisfied, the amount is fixed or determinable and collectability is reasonably assured.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Cash and Cash Equivalents
Cash equivalents represent investments with maturities of three months or less from time of purchase. They are carried at cost plus accrued interest. The estimated fair value of the company's cash equivalents was determined using level 2 inputs within the fair value hierarchy, as described below. Based on current interest rates for similar investments with comparable credit risk and time to maturity, the fair value of the company's cash equivalents approximates its stated value of $2,026 and $1,932 as of December 31, 2012 and 2011, respectively.
Marketable Securities
Marketable securities represent investments in fixed and floating rate financial instruments with maturities greater than three months and up to twelve months at time of purchase. They are classified as held-to-maturity and recorded at amortized cost. The carrying value approximates fair value due to the short-term nature of the investments.
Fair Value Measurements
Under the accounting for fair value measurements and disclosures, a fair value hierarchy was established that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). A financial instrument's level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
The company uses the following valuation techniques to measure fair value for its assets and liabilities:
|
| | |
| Level 1 | – | Quoted market prices in active markets for identical assets or liabilities; |
| | | |
| Level 2 | – | Significant other observable inputs (e.g. quoted prices for similar items in active markets, quoted prices for identical or similar items in markets that are not active, inputs other than quoted prices that are observable such as interest rate and yield curves, and market-corroborated inputs); |
| | | |
| Level 3 | – | Unobservable inputs for the asset or liability, which are valued based on management's estimates of assumptions that market participants would use in pricing the asset or liability. |
Inventories
The majority of the company's inventories are valued at cost, as determined by the last-in, first-out (LIFO) method; in the aggregate, such valuations are not in excess of market. Seed, certain food-ingredient and enzyme inventories are valued at the lower of cost, as determined by the first-in, first-out (FIFO) method, or market.
Elements of cost in inventories include raw materials, direct labor and manufacturing overhead. Stores and supplies are valued at cost or market, whichever is lower; cost is generally determined by the average cost method.
Property, Plant and Equipment
Property, plant and equipment is carried at cost and is depreciated using the straight-line method. Property, plant and equipment placed in service prior to 1995 is depreciated under the sum-of-the-years' digits method or other substantially similar methods. Substantially all equipment and buildings are depreciated over useful lives ranging from 15 to 25 years. Capitalizable costs associated with computer software for internal use are amortized on a straight-line basis over 5 to 7 years. When assets are surrendered, retired, sold or otherwise disposed of, their gross carrying values and related accumulated depreciation are removed from the accounts and included in determining gain or loss on such disposals.
Maintenance and repairs are charged to operations; replacements and improvements are capitalized.
Goodwill and Other Intangible Assets
Goodwill represents the future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized. Goodwill and indefinite-lived intangible assets are tested for impairment at least annually; however, these tests are performed more frequently when events or changes in circumstances indicate that the asset may be impaired. Impairment exists when carrying value exceeds fair value. The company's fair value methodology is based on prices of similar assets or other valuation methodologies including discounted cash flow techniques. Impairment losses are included in cost of goods sold and other operating charges.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Definite-lived intangible assets, such as purchased and licensed technology, patents and customer lists are amortized over their estimated useful lives, generally for periods ranging from 1 to 20 years. The company continually evaluates the reasonableness of the useful lives of these assets. Once these assets are fully amortized, they are removed from the Consolidated Balance Sheets.
Impairment of Long-Lived Assets
The company evaluates the carrying value of long-lived assets to be held and used when events or changes in circumstances indicate the carrying value may not be recoverable. The carrying value of a long-lived asset is considered impaired when the total projected undiscounted cash flows from the asset are separately identifiable and are less than its carrying value. In that event, a loss is recognized based on the amount by which the carrying value exceeds the fair value of the long-lived asset. The company's fair value methodology is an estimate of fair market value which is made based on prices of similar assets or other valuation methodologies including present value techniques. Long-lived assets to be disposed of other than by sale are classified as held for use until their disposal. Long-lived assets to be disposed of by sale are classified as held for sale and are reported at the lower of carrying amount or fair market value less cost to sell. Depreciation is discontinued for long-lived assets classified as held for sale.
Research and Development
Research and development costs are expensed as incurred.
Environmental
Accruals for environmental matters are recorded in operating expenses when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. Accrued liabilities do not include claims against third parties and are not discounted.
Costs related to environmental remediation and restoration are charged to expense. Other environmental costs are also charged to expense unless they increase the value of the property or reduce or prevent contamination from future operations, in which case, they are capitalized.
Asset Retirement Obligations
The company records asset retirement obligations at fair value at the time the liability is incurred. Accretion expense is recognized as an operating expense using the credit-adjusted risk-free interest rate in effect when the liability was recognized. The associated asset retirement obligations are capitalized as part of the carrying amount of the long-lived asset and depreciated over the estimated remaining useful life of the asset, generally for periods ranging from 1 to 25 years.
Litigation
The company accrues for liabilities related to litigation matters when the information available indicates that it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. Legal costs such as outside counsel fees and expenses are charged to expense in the period incurred.
Insurance/Self-Insurance
The company self-insures certain risks where permitted by law or regulation, including workers' compensation, vehicle liability and employee related benefits. Liabilities associated with these risks are estimated in part by considering historical claims experience, demographic factors and other actuarial assumptions. For other risks, the company uses a combination of insurance and self-insurance, reflecting comprehensive reviews of relevant risks. A receivable for an insurance recovery is generally recognized when the loss has occurred and collection is considered probable.
Income Taxes
The provision for income taxes is determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. The provision for income taxes represents income taxes paid or payable for the current year plus the change in deferred taxes during the year. Deferred taxes result from differences between the financial and tax basis of the company's assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. Provision has been made for income taxes on unremitted earnings of subsidiaries and affiliates, except for subsidiaries in which earnings are deemed to be indefinitely invested. Investment tax credits or grants are accounted for in the period earned (the flow-through method). Interest accrued related to unrecognized tax benefits is included in miscellaneous income and expenses, net, under other income, net. Income tax related penalties are included in the provision for income taxes.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Foreign Currency Translation
The company's worldwide operations utilize the U.S. dollar or local currency as the functional currency, where applicable. For subsidiaries where the USD is the functional currency, all foreign currency asset and liability amounts are remeasured into USD at end-of-period exchange rates, except for inventories, prepaid expenses, property, plant and equipment, goodwill and other intangible assets, which are remeasured at historical rates. Foreign currency income and expenses are remeasured at average exchange rates in effect during the year, except for expenses related to balance sheet amounts remeasured at historical exchange rates. Exchange gains and losses arising from remeasurement of foreign currency-denominated monetary assets and liabilities are included in income in the period in which they occur.
For subsidiaries where the local currency is the functional currency, assets and liabilities denominated in local currencies are translated into USD at end-of-period exchange rates and the resultant translation adjustments are reported, net of their related tax effects, as a component of accumulated other comprehensive income (loss) in equity. Assets and liabilities denominated in other than the local currency are remeasured into the local currency prior to translation into USD and the resultant exchange gains or losses are included in income in the period in which they occur. Income and expenses are translated into USD at average exchange rates in effect during the period.
Hedging and Trading Activities
Derivative instruments are reported in the Consolidated Balance Sheets at their fair values. For derivative instruments designated as fair value hedges, changes in the fair values of the derivative instruments will generally be offset in the income statement by changes in the fair value of the hedged items. For derivative instruments designated as cash flow hedges, the effective portion of any hedge is reported in accumulated other comprehensive income (loss) until it is cleared to earnings during the same period in which the hedged item affects earnings. The ineffective portion of all hedges is recognized in current period earnings. Changes in the fair values of derivative instruments that are not designated as hedges are recorded in current period earnings.
In the event that a derivative designated as a hedge of a firm commitment or an anticipated transaction is terminated prior to the maturation of the hedged transaction, gains or losses realized at termination are deferred and included in the measurement of the hedged transaction. If a hedged transaction matures, or is sold, extinguished, or terminated prior to the maturity of a derivative designated as a hedge of such transaction, gains or losses associated with the derivative through the date the transaction matured are included in the measurement of the hedged transaction and the derivative is reclassified as for trading purposes. Derivatives designated as a hedge of an anticipated transaction are reclassified as for trading purposes if the anticipated transaction is no longer probable.
Cash flows from derivative instruments accounted for as either fair value hedges or cash flow hedges are reported in the same category as the cash flows from the items being hedged. Cash flows from all other derivative instruments are generally reported as investing activities in the Consolidated Statements of Cash Flows. See Note 20 for additional discussion regarding the company's objectives and strategies for derivative instruments.
2. DISCONTINUED OPERATIONS
On August 30, 2012, the company entered into a definitive agreement with Flash Bermuda Co. Ltd., a Bermuda exempted limited liability company formed by affiliates of The Carlyle Group (collectively referred to as "Carlyle") in which Carlyle agreed to purchase certain subsidiaries and assets comprising the company's Performance Coatings business for approximately $4,900 in cash and the assumption of certain liabilities. On February 1, 2013, the sale of Performance Coatings was completed, resulting in about $4,000 in after-tax proceeds.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The results of discontinued operations are summarized below:
|
| | | | | | | | | |
| For the year ended December 31, | 2012 | 2011 | 2010 |
| Net sales | $ | 4,218 |
| $ | 4,280 |
| $ | 3,805 |
|
| Income before income taxes | $ | 568 |
| $ | 501 |
| $ | 451 |
|
Provision for income taxes1 | 248 |
| 146 |
| 144 |
|
| Income from discontinued operations after income taxes | $ | 320 |
| $ | 355 |
| $ | 307 |
|
| |
1. | Full year 2012 includes expense of $70 to accrue taxes associated with earnings of certain Performance Coatings subsidiaries that were previously considered permanently reinvested as these entities have been reclassified as held for sale. |
The key components of the assets and liabilities classified as held for sale at December 31, 2012 related to Performance Coatings consisted of the following:
|
| | | |
| | December 31,
2012 |
| Cash and cash equivalents | $ | 95 |
|
| Accounts and notes receivable, net | 783 |
|
| Inventories | 468 |
|
| Prepaid expenses | 6 |
|
| Deferred income taxes - current | 32 |
|
| Property, plant and equipment, net of accumulated depreciation | 749 |
|
| Goodwill | 808 |
|
| Other intangible assets | 67 |
|
| Deferred income taxes - noncurrent | 14 |
|
| Other assets - noncurrent | 34 |
|
| Total assets held for sale | $ | 3,056 |
|
| Accounts payable | $ | 408 |
|
| Income taxes | 17 |
|
| Other accrued liabilities | 237 |
|
| Other liabilities - noncurrent | 388 |
|
| Deferred income taxes - noncurrent | 34 |
|
| Total liabilities related to assets held for sale | $ | 1,084 |
|
3. EMPLOYEE SEPARATION/ASSET RELATED CHARGES, NET
At December 31, 2012, total liabilities relating to restructuring activities were $180, primarily relating to the 2012 restructuring program.
2012 Restructuring Program
In 2012, the company commenced a restructuring plan to increase productivity, enhance competitiveness and accelerate growth. The plan is designed to eliminate corporate costs previously allocated to the Performance Coatings business as well as utilize additional cost-cutting actions to improve competitiveness. As a result, pre-tax charges of $234 were recorded in employee separation / asset related charges, net. The 2012 charges consist of $157 of employee separation costs, $8 of other non-personnel charges, and $69 of asset related charges, which includes $30 of asset impairments and $39 of asset shut downs.
The 2012 restructuring program charges impacted segment earnings as follows: Agriculture - $11, Electronics & Communications - $9, Industrial Biosciences - $3, Nutrition & Health - $53, Performance Chemicals - $3, Performance Materials - $13, and Safety & Protection - $58, as well as Corporate expenses - $84. The company expects this plan and all related payments to be substantially complete by December 31, 2013.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Account balances and activity for the 2012 restructuring program are summarized below:
|
| | | | | | | | | | | | |
| | Asset Related | Employee Separation Costs | Other Non-Personnel Charges1 | Total |
| Charges to income in 2012 | $ | 69 |
| $ | 157 |
| $ | 8 |
| $ | 234 |
|
| Charges to accounts: | | | | |
| Payments | — |
| (4 | ) | (1 | ) | (5 | ) |
| Net translation adjustment | — |
| 1 |
| — |
| 1 |
|
| Asset write-offs and adjustments | (69 | ) | — |
| — |
| (69 | ) |
| Balance as of December 31, 2012 | $ | — |
| $ | 154 |
| $ | 7 |
| $ | 161 |
|
1. Other non-personnel charges consist of contractual obligation costs.
2011 Restructuring Program
In 2011, the company initiated a series of actions to achieve the expected cost synergies associated with the Danisco acquisition. As a result, the company recorded a $53 charge in employee separation/asset related charges, net, primarily for employee separation costs in the U.S. and Europe which reduced segment earnings as follows: Industrial Biosciences - $9, Nutrition & Health - $14, and Other - $30.
In the fourth quarter 2012, the company recorded a net reduction of $15 in the estimated costs associated with the 2011 restructuring program. This net reduction was primarily due to lower than estimated individual severance costs and workforce reductions through non-severance programs. The net reduction impacted segment earnings for the year ended December 31, 2012 as follows: Nutrition & Health - $4 and Other - $11. There were $17 of employee separation cash payments related to the 2011 restructuring program during 2012. The actions and payments related to the 2011 restructuring program were substantially complete as of December 31, 2012.
Asset Impairments
During 2012, as a result of strategic decisions related to deteriorating conditions within a specific industrial chemicals market, the company determined that an impairment triggering event had occurred and that an assessment of the asset group related to this industrial chemical was warranted. This assessment determined that the carrying value of the asset group exceeded its fair value. As a result of the impairment test, a $33 pre-tax impairment charge was recorded during 2012 within the Performance Chemicals segment.
During 2012, as a result of conditions in the thin film photovoltaic market, the company determined that an impairment triggering event had occurred and that an assessment of the asset group related to its thin film photovoltaic modules and systems was warranted. This assessment determined that the carrying value of the asset group exceeded its fair value. As a result of the impairment test, a $150 pre-tax impairment charge was recorded during 2012 within the Electronics & Communications segment.
During 2012, as a result of deteriorating conditions in an industrial polymer market, the company determined that an impairment triggering event had occurred and that an assessment of the asset group related to this polymer product was warranted. This assessment determined that the carrying value of the asset group exceeded its fair value. As a result of the impairment test, a $92 pre-tax impairment charge was recorded during 2012 within the Performance Materials segment.
The bases of the fair value for the charges above were calculated utilizing a discounted cash flow approach which included assumptions concerning future operating performance and economic conditions that may differ from actual cash flows. In connection with the matters discussed above, as of December 31, 2012, the company had long-lived assets with a remaining net book value of approximately $150 accounted for at fair value on a nonrecurring basis after initial recognition. These nonrecurring fair value measurements were determined using level 3 inputs within the fair value hierarchy, as described in Note 1 to the Consolidated Financial Statements.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
4. DANISCO ACQUISITION
In January 2011, DuPont and its wholly owned subsidiary, DuPont Denmark Holding ApS (DDHA), entered into a definitive agreement with Danisco A/S (Danisco), a global enzyme and specialty food ingredients company, for DDHA to make a public tender offer for all of Danisco's outstanding shares at a price of 665 Danish Kroner (DKK) in cash per share. On April 29, 2011, DDHA increased the price of its tender offer to acquire all of the outstanding shares of Danisco to DKK 700 in cash per share.
On May 19, 2011, the company acquired approximately 92.2 percent of Danisco's outstanding shares, excluding treasury shares, pursuant to the previously announced tender offer. From May 19, 2011 to September 22, 2011, DuPont acquired all of Danisco's remaining outstanding shares. This acquisition has established DuPont as a leader in industrial biotechnology with science-intensive innovations that address global challenges in food production and reduced fossil fuel consumption. The Danisco acquisition was valued at $6,417, plus net debt assumed of $617.
As part of the Danisco acquisition, DuPont incurred $85 in transaction related costs during 2011, which were recorded in costs of goods sold and other operating charges. In 2011, Danisco contributed net sales of $1,713 and net income attributable to DuPont of $(7), which excludes $30 after-tax ($39 pre-tax) of additional interest expense related to the debt issued to finance the acquisition. Danisco's contributions included a $125 after-tax ($175 pre-tax) charge related to the fair value step-up of inventories acquired and sold during 2011.
The following table summarizes the fair value of the assets acquired and liabilities assumed as of the acquisition date:
|
| | | |
| Fair value of assets acquired | |
| Cash and cash equivalents | $ | 48 |
|
Accounts and notes receivable 1
| 522 |
|
Inventories 2 | 709 |
|
| Property, plant and equipment | 1,709 |
|
Goodwill 3 | 2,891 |
|
Other intangible assets 4 | 2,859 |
|
| Other current and non-current assets | 78 |
|
| Total assets acquired | $ | 8,816 |
|
| Fair value of liabilities assumed | |
Accounts payable and other accrued liabilities
| $ | 489 |
|
Short-term borrowings 5 | 342 |
|
Long-term borrowings 5 | 323 |
|
| Other liabilities | 219 |
|
Deferred income taxes 6 | 1,026 |
|
| Total liabilities assumed | $ | 2,399 |
|
| |
1. | The gross amount of accounts and notes receivable acquired was $531, of which $9 was expected to be uncollectible. |
| |
2. | The fair value of inventories acquired included a step-up in the value of $175, which was expensed to cost of goods sold and other operating charges in 2011. |
| |
3. | Goodwill will not be deductible for statutory tax purposes. Goodwill is attributable to Danisco's workforce and the synergies in technology, operations and market access that are expected from the acquisition. Approximately $900 and $2,000 of goodwill was allocated to the Industrial Biosciences and Nutrition & Health segments, respectively. |
| |
4. | Other intangible assets acquired of $1,002 are indefinite-lived (see Note 11). |
| |
5. | Debt assumed has been paid off as of December 31, 2011. |
| |
6. | The deferred income tax liabilities assumed represent the adjustments for the tax impact of fair value adjustments, primarily relating to definite-lived intangible assets. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
5. OTHER INCOME, NET
|
| | | | | | | | | |
| | 2012 | 2011 | 2010 |
Cozaar®/Hyzaar® income | $ | 54 |
| $ | 282 |
| $ | 483 |
|
| Royalty income | 177 |
| 189 |
| 140 |
|
| Interest income | 109 |
| 110 |
| 92 |
|
Equity in earnings of affiliates, excluding exchange gains/losses1 | 99 |
| 191 |
| 179 |
|
| Gain on sale of equity method investment | 122 |
| — |
| — |
|
| Net gains on sales of assets | 130 |
| 89 |
| 121 |
|
Net exchange losses1 | (215 | ) | (146 | ) | (11 | ) |
Miscellaneous income and expenses, net2 | 22 |
| 27 |
| 195 |
|
| | $ | 498 |
| $ | 742 |
| $ | 1,199 |
|
| |
1. | The company routinely uses foreign currency exchange contracts to offset its net exposures, by currency, related to the foreign currency-denominated monetary assets and liabilities. The objective of this program is to maintain an approximately balanced position in foreign currencies in order to minimize, on an after-tax basis, the effects of exchange rate changes on net monetary asset positions. The net pre-tax exchange gains and losses are recorded in other income, net and the related tax impact is recorded in provision for income taxes on continuing operations on the Consolidated Income Statements. Exchange gains (losses) related to earnings of affiliates was $3, $1 and $(2) for 2012, 2011 and 2010, respectively. |
| |
2. | Miscellaneous income and expenses, net, generally includes interest items, insurance recoveries, litigation settlements and other items. |
6. PROVISION FOR INCOME TAXES
|
| | | | | | | | | |
| | 2012 | 2011 | 2010 |
| Current tax expense (benefit) on continuing operations: | |
| |
| |
|
| U.S. federal | $ | 121 |
| $ | 353 |
| $ | (142 | ) |
| U.S. state and local | 16 |
| (20 | ) | (9 | ) |
| International | 663 |
| 482 |
| 401 |
|
| Total current tax expense on continuing operations | 800 |
| 815 |
| 250 |
|
| Deferred tax expense (benefit) on continuing operations: |
|
| |
| |
|
| U.S. federal | (103 | ) | (147 | ) | 240 |
|
| U.S. state and local | (46 | ) | (4 | ) | 22 |
|
| International | (29 | ) | (38 | ) | 3 |
|
| Total deferred tax (benefit) expense on continuing operations | (178 | ) | (189 | ) | 265 |
|
| Provision for income taxes on continuing operations | $ | 622 |
| $ | 626 |
| $ | 515 |
|
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The significant components of deferred tax assets and liabilities at December 31, 2012 and 2011, are as follows:
|
| | | | | | | | | | | | |
| | 2012 | 2011 |
| | Asset | Liability | Asset | Liability |
| Depreciation | $ | — |
| $ | 1,696 |
| $ | — |
| $ | 1,781 |
|
| Accrued employee benefits | 5,198 |
| 167 |
| 5,562 |
| 252 |
|
| Other accrued expenses | 1,157 |
| 499 |
| 1,020 |
| 354 |
|
| Inventories | 249 |
| 68 |
| 199 |
| 39 |
|
| Unrealized exchange gains/losses | — |
| 56 |
| — |
| 35 |
|
| Tax loss/tax credit carryforwards/backs | 2,733 |
| — |
| 2,854 |
| — |
|
| Investment in subsidiaries and affiliates | 81 |
| 92 |
| 46 |
| 259 |
|
| Amortization of intangibles | 58 |
| 1,335 |
| 69 |
| 1,399 |
|
| Other | 270 |
| 287 |
| 250 |
| 279 |
|
| Valuation allowance | (1,914 | ) | — |
| (1,971 | ) | — |
|
| | $ | 7,832 |
| $ | 4,200 |
| $ | 8,029 |
| $ | 4,398 |
|
| Net deferred tax asset | $ | 3,632 |
| |
| $ | 3,631 |
| |
|
An analysis of the company's effective income tax rate (EITR) on continuing operations is as follows:
|
| | | | | | |
| | 2012 | 2011 | 2010 |
| Statutory U.S. federal income tax rate | 35.0 | % | 35.0 | % | 35.0 | % |
Exchange gains/losses1 | 0.1 |
| (0.8 | ) | 2.2 |
|
Domestic operations2 | (2.3 | ) | (3.4 | ) | (3.3 | ) |
Lower effective tax rates on international operations-net2 | (10.8 | ) | (11.7 | ) | (16.0 | ) |
| Tax settlements | (2.0 | ) | (0.2 | ) | (2.1 | ) |
| Sale of a business | — |
| (2.3 | ) | — |
|
| | 20.0 | % | 16.6 | % | 15.8 | % |
| |
1. | Principally reflects the impact of non-taxable exchange gains and losses resulting from remeasurement of foreign currency-denominated monetary assets and liabilities. Further information about the company's foreign currency hedging program is included in Note 20 under the heading Foreign Currency Risk. |
| |
2. | On January 2, 2013, U.S. tax law was enacted which extends through 2013 several expired or expiring temporary business tax provisions. In accordance with GAAP, this extension will be taken into account in the quarter in which the legislation was enacted (i.e. first quarter 2013). The company is still quantifying the impact of this law change; however, it is expected that the retroactive 2012 benefit derived from these extenders will be approximately $70. |
Consolidated income from continuing operations before income taxes for U.S. and international operations was as follows:
|
| | | | | | | | | |
| | 2012 | 2011 | 2010 |
| U.S. (including exports) | $ | 652 |
| $ | 701 |
| $ | 793 |
|
| International | 2,463 |
| 3,080 |
| 2,467 |
|
| | $ | 3,115 |
| $ | 3,781 |
| $ | 3,260 |
|
The decrease in pre-tax earnings from continuing operations from 2011 to 2012 is primarily driven by pre-tax charges related to Imprelis® and employee separation/asset related charges in 2012, in addition to the results of the company's hedging program. See Note 3 and Note 16 for additional information. In 2012 and 2011, the U.S. recorded exchange losses associated with the hedging program of $157 and $133, respectively. While the taxation of the amounts reflected on the chart above does not correspond precisely to the jurisdiction of taxation (due to taxation in multiple countries, exchange gains/losses, etc.), it represents a reasonable approximation of the income before income taxes split between U.S. and international jurisdictions. See Note 20 for additional information regarding the company's hedging program.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Under the tax laws of various jurisdictions in which the company operates, deductions or credits that cannot be fully utilized for tax purposes during the current year may be carried forward or back, subject to statutory limitations, to reduce taxable income or taxes payable in future or prior years. At December 31, 2012, the tax effect of such carryforwards/backs, net of valuation allowance approximated $1,298. Of this amount, $1,074 has no expiration date, $42 expires after 2012 but before the end of 2017 and $182 expires after 2017.
At December 31, 2012, unremitted earnings of subsidiaries outside the U.S. totaling $13,179 were deemed to be indefinitely reinvested. No deferred tax liability has been recognized with regard to the remittance of such earnings. It is not practical to estimate the income tax liability that might be incurred if such earnings were remitted to the U.S.
Each year the company files hundreds of tax returns in the various national, state and local income taxing jurisdictions in which it operates. These tax returns are subject to examination and possible challenge by the taxing authorities. Positions challenged by the taxing authorities may be settled or appealed by the company. As a result, there is an uncertainty in income taxes recognized in the company's financial statements in accordance with accounting for income taxes and accounting for uncertainty in income taxes. It is reasonably possible that changes to the company's global unrecognized tax benefits could be significant, however, due to the uncertainty regarding the timing of completion of audits and possible outcomes, a current estimate of the range of increases or decreases that may occur within the next twelve months cannot be made.
The company and/or its subsidiaries files income tax returns in the U.S. federal jurisdiction, and various states and non-U.S. jurisdictions. With few exceptions, the company is no longer subject to U.S. federal, state and local, or non-U.S. income tax examinations by tax authorities for years before 1999. A reconciliation of the beginning and ending amounts of unrecognized tax benefits is as follows:
|
| | | | | | | | | |
| | 2012 | 2011 | 2010 |
| Total unrecognized tax benefits as of January 1 | $ | 800 |
| $ | 693 |
| $ | 739 |
|
Gross amounts of decreases in unrecognized tax benefits as a result of tax positions taken during the prior period | (94 | ) | (82 | ) | (155 | ) |
Gross amounts of increases in unrecognized tax benefits as a result of tax positions taken during the prior period | 73 |
| 170 |
| 169 |
|
Gross amounts of increases in unrecognized tax benefits as a result of tax positions taken during the current period | 78 |
| 79 |
| 51 |
|
Amount of decreases in the unrecognized tax benefits relating to settlements with taxing authorities | (29 | ) | (6 | ) | (90 | ) |
Reduction to unrecognized tax benefits as a result of a lapse of the applicable statute of limitations | (10 | ) | (32 | ) | (24 | ) |
| Exchange gain (loss) | (13 | ) | (22 | ) | 3 |
|
| Total unrecognized tax benefits as of December 31 | $ | 805 |
| $ | 800 |
| $ | 693 |
|
| Total unrecognized tax benefits that, if recognized, would impact the effective tax rate | $ | 693 |
| $ | 683 |
| $ | 545 |
|
| Total amount of interest and penalties recognized in the Consolidated Income Statements | $ | 4 |
| $ | 7 |
| $ | (70 | ) |
| Total amount of interest and penalties recognized in the Consolidated Balance Sheets | $ | 116 |
| $ | 113 |
| $ | 99 |
|
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
7. EARNINGS PER SHARE OF COMMON STOCK
Set forth below is a reconciliation of the numerator and denominator for basic and diluted earnings per share calculations for the periods indicated:
|
| | | | | | | | | |
| | 2012 | 2011 | 2010 |
| Numerator: | |
| |
| |
|
| Income from continuing operations after income taxes attributable to DuPont | $ | 2,468 |
| $ | 3,119 |
| $ | 2,724 |
|
| Preferred dividends | (10 | ) | (10 | ) | (10 | ) |
| Income from continuing operations after income taxes available to DuPont common stockholders | $ | 2,458 |
| $ | 3,109 |
| $ | 2,714 |
|
| |
|
|
|
|
|
|
| Income from discontinued operations after income taxes | $ | 320 |
| $ | 355 |
| $ | 307 |
|
| |
|
|
|
|
|
|
| Net income available to common stockholders | $ | 2,778 |
| $ | 3,464 |
| $ | 3,021 |
|
| |
|
|
|
|
|
|
| Denominator: |
|
| |
| |
|
| Weighted-average number of common shares outstanding – Basic | 933,275,000 |
| 928,417,000 |
| 908,860,000 |
|
| Dilutive effect of the company's employee compensation plans | 8,922,000 |
| 12,612,000 |
| 12,795,000 |
|
| Weighted average number of common shares outstanding – Diluted | 942,197,000 |
| 941,029,000 |
| 921,655,000 |
|
The weighted-average number of common shares outstanding in 2012 and 2011 increased as a result of the issuance of new shares from the company's equity compensation plans, partially offset by the company's repurchase and retirement of its common stock (see Note 17).
The following average number of stock options are antidilutive and therefore, are not included in the diluted earnings per share calculation:
|
| | | | | | |
| | 2012 | 2011 | 2010 |
| Average number of stock options | 12,158,000 |
| 4,361,000 |
| 45,508,000 |
|
The change in the average number of stock options that were antidilutive in 2012 and 2011 was primarily due to changes in the company's average stock price.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
8. ACCOUNTS AND NOTES RECEIVABLE, NET
|
| | | | | | |
| December 31, | 2012 | 2011 |
Accounts receivable – trade1 | $ | 4,069 |
| $ | 4,598 |
|
Notes receivable – trade1,2 | 131 |
| 207 |
|
Other3 | 1,252 |
| 1,217 |
|
| | $ | 5,452 |
| $ | 6,022 |
|
| |
1. | Accounts and notes receivable – trade are net of allowances of $243 in 2012 and $292 in 2011. Allowances are equal to the estimated uncollectible amounts. That estimate is based on historical collection experience, current economic and market conditions, and review of the current status of customers' accounts. |
| |
2. | Notes receivable – trade primarily consists of receivables within the Agriculture segment for deferred payment loan programs for the sale of seed products to customers. These loans have terms of one year or less and are primarily concentrated in North America. The company maintains a rigid pre-approval process for extending credit to customers in order to manage overall risk and exposure associated with credit losses. As of December 31, 2012 and 2011, there were no significant past due notes receivable, nor were there any significant impairments related to current loan agreements. |
| |
3. | Other includes receivables in relation to Cozaar®/Hyzaar® interests, fair value of derivative instruments, value added tax, general sales tax and other taxes. |
Accounts and notes receivable are carried at amounts that approximate fair value.
9. INVENTORIES
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Finished products | $ | 4,519 |
| $ | 4,541 |
|
| Semifinished products | 2,407 |
| 2,293 |
|
| Raw materials, stores and supplies | 1,332 |
| 1,262 |
|
| | 8,258 |
| 8,096 |
|
| Adjustment of inventories to a LIFO basis | (836 | ) | (901 | ) |
| | $ | 7,422 |
| $ | 7,195 |
|
Inventory values, before LIFO adjustment, are generally determined by the average cost method, which approximates current cost. Domestic and foreign inventories, excluding seeds, certain food-ingredients, enzymes, stores and supplies, valued under the LIFO method comprised 85 percent and 78 percent of consolidated inventories before LIFO adjustment as of December 31, 2012 and 2011, respectively. Seed, certain food-ingredient and enzyme inventories of $3,926 and $3,432 at December 31, 2012 and 2011, respectively, were valued under the FIFO method. Stores and supplies inventories of $263 and $258 at December 31, 2012 and 2011, respectively, were valued under the average cost method.
Effective January 1, 2013, the company changed its method of valuing inventory held at certain of its foreign and U.S. locations from the LIFO method to the average cost method. The company believes that the average cost method is preferable to the LIFO method as it more clearly aligns with how the company actually manages its inventory and will improve financial reporting by better matching revenues and expenses. In addition, the change from LIFO to average cost will enhance the comparability of our financial results with our peer companies. The impact of this change on income from continuing operations is $21, $(73), and $2 for 2012, 2011 and 2010, respectively. As described in the accounting guidance for accounting changes and error corrections, beginning with the first quarter 2013, the comparative Consolidated Financial Statements of prior periods will be adjusted to apply the new accounting method retrospectively.
10. PROPERTY, PLANT AND EQUIPMENT
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Buildings | $ | 5,490 |
| $ | 5,297 |
|
| Equipment | 24,090 |
| 25,338 |
|
| Land | 691 |
| 669 |
|
| Construction | 1,555 |
| 1,457 |
|
| | $ | 31,826 |
| $ | 32,761 |
|
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
11. GOODWILL AND OTHER INTANGIBLE ASSETS
Goodwill
The following table summarizes changes in the carrying amount of goodwill for the years ended December 31, 2012 and 2011, by reportable segment:
|
| | | | | | | | | | | | | | | |
| | Balance as of December 31, 2012 | Goodwill Adjustments and Acquisitions | Balance as of December 31, 2011 | Goodwill Adjustments and Acquisitions | Balance as of December 31, 2010 |
| Agriculture | $ | 231 |
| $ | (1 | ) | $ | 232 |
| $ | 4 |
| $ | 228 |
|
| Electronics & Communications | 149 |
| — |
| 149 |
| 32 |
| 117 |
|
| Industrial Biosciences | 890 |
| 24 |
| 866 |
| 866 |
| — |
|
| Nutrition & Health | 2,314 |
| (8 | ) | 2,322 |
| 1,898 |
| 424 |
|
| Performance Chemicals | 185 |
| — |
| 185 |
| — |
| 185 |
|
| Performance Coatings | — |
| (809 | ) | 809 |
| — |
| 809 |
|
| Performance Materials | 401 |
| (3 | ) | 404 |
| (6 | ) | 410 |
|
| Safety & Protection | 446 |
| — |
| 446 |
| 2 |
| 444 |
|
| Total | $ | 4,616 |
| $ | (797 | ) | $ | 5,413 |
| $ | 2,796 |
| $ | 2,617 |
|
Changes in goodwill in 2012 primarily relate to goodwill associated with the Performance Coatings business that has been reclassified as held for sale (see Note 2). Changes in goodwill in 2011 primarily relate to the goodwill associated with the Danisco acquisition (see Note 4). In 2012 and 2011, the company performed impairment tests for goodwill and determined that no goodwill impairments existed.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Other Intangible Assets
The following table summarizes the gross carrying amounts and accumulated amortization of other intangible assets by major class:
|
| | | | | | | | | | | | | | | | | | |
| | December 31, 2012 | December 31, 2011 |
| | Gross | Accumulated Amortization | Net | Gross | Accumulated Amortization | Net |
Intangible assets subject to amortization (Definite-lived) | | | | | | |
| Customer lists | $ | 1,847 |
| $ | (330 | ) | $ | 1,517 |
| $ | 1,841 |
| $ | (220 | ) | $ | 1,621 |
|
| Patents | 525 |
| (127 | ) | 398 |
| 518 |
| (77 | ) | 441 |
|
| Purchased and licensed technology | 1,929 |
| (1,016 | ) | 913 |
| 1,854 |
| (878 | ) | 976 |
|
| Trademarks | 57 |
| (29 | ) | 28 |
| 57 |
| (25 | ) | 32 |
|
Other1 | 206 |
| (98 | ) | 108 |
| 330 |
| (151 | ) | 179 |
|
| | 4,564 |
| (1,600 | ) | 2,964 |
| 4,600 |
| (1,351 | ) | 3,249 |
|
Intangible assets not subject to amortization (Indefinite-lived) | | | | | | |
| In-process research and development | 62 |
| — |
| 62 |
| 70 |
| — |
| 70 |
|
Microbial cell factories2 | 306 |
| — |
| 306 |
| 306 |
| — |
| 306 |
|
Pioneer germplasm3 | 975 |
| — |
| 975 |
| 975 |
| — |
| 975 |
|
| Trademarks/tradenames | 819 |
| — |
| 819 |
| 813 |
| — |
| 813 |
|
| | 2,162 |
| — |
| 2,162 |
| 2,164 |
| — |
| 2,164 |
|
| Total | $ | 6,726 |
| $ | (1,600 | ) | $ | 5,126 |
| $ | 6,764 |
| $ | (1,351 | ) | $ | 5,413 |
|
| |
1. | Primarily consists of sales and grower networks, marketing and manufacturing alliances and noncompetition agreements. |
| |
2. | Microbial cell factories, derived from natural microbes, are used to sustainably produce enzymes, peptides and chemicals using natural metabolic processes. The company recognized the microbial cell factories as an intangible asset upon the acquisition of Danisco. This intangible asset is expected to contribute to cash flows beyond the foreseeable future and there are no legal, regulatory, contractual, or other factors which limit its useful life. |
| |
3. | Pioneer germplasm is the pool of genetic source material and body of knowledge gained from the development and delivery stage of plant breeding. The company recognized germplasm as an intangible asset upon the acquisition of Pioneer. This intangible asset is expected to contribute to cash flows beyond the foreseeable future and there are no legal, regulatory, contractual, or other factors which limit its useful life. |
The aggregate pre-tax amortization expense from continuing operations for definite-lived intangible assets was $312, $253 and $155 for 2012, 2011 and 2010, respectively. The estimated aggregate pre-tax amortization expense from continuing operations for 2013, 2014, 2015, 2016 and 2017 is $302, $333, $345, $301 and $173, respectively, which are primarily reported in cost of goods sold and other operating charges.
12. SHORT-TERM BORROWINGS AND CAPITAL LEASE OBLIGATIONS
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Commercial paper | $ | — |
| $ | 390 |
|
| Other loans-various currencies | 20 |
| 15 |
|
| Long-term debt payable within one year | 1,252 |
| 410 |
|
| Capital lease obligations | 3 |
| 2 |
|
| | $ | 1,275 |
| $ | 817 |
|
The estimated fair value of the company's short-term borrowings, including interest rate financial instruments, was determined using level 2 inputs within the fair value hierarchy, as described in Note 1 to the Consolidated Financial Statements. Based on quoted market prices for the same or similar issues, or on current rates offered to the company for debt of the same remaining maturities, the fair value of the company's short-term borrowings was $1,300 and $830 at December 31, 2012 and 2011, respectively.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Unused bank credit lines were approximately $4,300 and $4,400 at December 31, 2012 and 2011, respectively. These lines are available to support short-term liquidity needs and general corporate purposes including letters of credit. Outstanding letters of credit were $503 and $354 at December 31, 2012 and 2011, respectively. These letters of credit support commitments made in the ordinary course of business.
The weighted-average interest rate on short-term borrowings outstanding at December 31, 2012 and 2011 was 4.8% and 2.6%, respectively. The increase in the interest rate for 2012 was primarily due to long-term debt maturing within one year.
13. OTHER ACCRUED LIABILITIES
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Compensation and other employee-related costs | $ | 1,092 |
| $ | 1,189 |
|
| Deferred revenue | 2,706 |
| 2,153 |
|
| Employee benefits (Note 18) | 367 |
| 423 |
|
| Discounts and rebates | 318 |
| 356 |
|
| Derivative instruments | 131 |
| 36 |
|
| Miscellaneous | 1,383 |
| 1,140 |
|
| | $ | 5,997 |
| $ | 5,297 |
|
Deferred revenue principally includes advance customer payments within the Agriculture segment. Miscellaneous other accrued liabilities principally includes accrued plant and operating expenses, accrued litigation costs, employee separation costs in connection with the company's restructuring programs, the estimated value of certain guarantees and accrued environmental remediation costs.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
14. LONG-TERM BORROWINGS AND CAPITAL LEASE OBLIGATIONS
|
| | | | | | |
| December 31, | 2012 | 2011 |
| U.S. dollar: | | |
Medium-term notes due 2013 – 20411,2 | $ | 374 |
| $ | 401 |
|
4.75% notes due 20122 | — |
| 400 |
|
5.00% notes due 20132 | 250 |
| 250 |
|
5.00% notes due 20132 | 749 |
| 747 |
|
| 5.875% notes due 2014 | 170 |
| 170 |
|
| 1.75% notes due 2014 | 400 |
| 400 |
|
Floating rate notes due 20143 | 600 |
| 600 |
|
| 4.875% notes due 2014 | 499 |
| 499 |
|
3.25% notes due 20154 | 1,054 |
| 1,065 |
|
| 4.75% notes due 2015 | 400 |
| 399 |
|
| 1.95% notes due 2016 | 497 |
| 496 |
|
| 2.75% notes due 2016 | 499 |
| 499 |
|
| 5.25% notes due 2016 | 599 |
| 599 |
|
6.00% notes due 20185 | 1,383 |
| 1,405 |
|
| 5.75% notes due 2019 | 499 |
| 499 |
|
| 4.625% notes due 2020 | 997 |
| 997 |
|
| 3.625% notes due 2021 | 999 |
| 999 |
|
| 4.25% notes due 2021 | 499 |
| 499 |
|
| 6.50% debentures due 2028 | 299 |
| 299 |
|
| 5.60% notes due 2036 | 395 |
| 395 |
|
| 4.90% notes due 2041 | 493 |
| 493 |
|
Other loans (average interest rate of 3.9 percent)2 | 36 |
| 8 |
|
Other loans-various currencies2 | 2 |
| 4 |
|
| | 11,693 |
| 12,123 |
|
| Less short-term portion of long-term debt | 1,252 |
| 410 |
|
| | 10,441 |
| 11,713 |
|
| Capital lease obligations | 24 |
| 23 |
|
| Total | $ | 10,465 |
| $ | 11,736 |
|
| |
1. | Average interest rates on medium-term notes at December 31, 2012 and 2011 were 4.0% and 3.7%, respectively. |
| |
2. | Includes long-term debt due within one year. |
| |
3. | Interest rate on floating rate notes at December 31, 2012 and 2011 was 0.7% and 1.0%, respectively. |
| |
4. | At December 31, 2012 and 2011, the company had outstanding interest rate swap agreements with gross notional amounts of $1,000. Over the remaining terms of the notes, the company will receive fixed payments equivalent to the underlying debt and pay floating payments based on USD LIBOR (London Interbank Offered Rate). The fair value of outstanding swaps was an asset of $55 and $66 at December 31, 2012 and 2011, respectively. |
| |
5. | During 2008, the interest rate swap agreement associated with these notes was terminated. The gain will be amortized over the remaining life of the bond, resulting in an effective yield of 3.85%. |
Maturities of long-term borrowings are $1,669, $1,455, $1,596 and $0 for the years 2014, 2015, 2016 and 2017, respectively, and $5,721 thereafter.
The estimated fair value of the company's long-term borrowings, including interest rate financial instruments, was determined using level 2 inputs within the fair value hierarchy, as described in Note 1 to the Consolidated Financial Statements. Based on quoted market prices for the same or similar issues, or on current rates offered to the company for debt of the same remaining maturities, the fair value of the company's long-term borrowings was $11,715 and $13,050 at December 31, 2012 and 2011, respectively.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
15. OTHER LIABILITIES
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Employee benefits: | |
| |
|
| Accrued other long-term benefit costs (Note 18) | $ | 3,271 |
| $ | 4,063 |
|
| Accrued pension benefit costs (Note 18) | 9,303 |
| 9,186 |
|
| Accrued environmental remediation costs | 353 |
| 316 |
|
| Miscellaneous | 1,760 |
| 1,943 |
|
| | $ | 14,687 |
| $ | 15,508 |
|
Miscellaneous includes asset retirement obligations, litigation accruals, tax contingencies, royalty payables and certain obligations related to divested businesses.
16. COMMITMENTS AND CONTINGENT LIABILITIES
Guarantees
Indemnifications
In connection with acquisitions and divestitures, the company has indemnified respective parties against certain liabilities that may arise in connection with these transactions and business activities prior to the completion of the transaction. The term of these indemnifications, which typically pertain to environmental, tax and product liabilities, is generally indefinite. In addition, the company indemnifies its duly elected or appointed directors and officers to the fullest extent permitted by Delaware law, against liabilities incurred as a result of their activities for the company, such as adverse judgments relating to litigation matters. If the indemnified party were to incur a liability or have a liability increase as a result of a successful claim, pursuant to the terms of the indemnification, the company would be required to reimburse the indemnified party. The maximum amount of potential future payments is generally unlimited. The carrying amounts recorded for all indemnifications as of December 31, 2012 and 2011 were $31 and $105, respectively. The decrease in the carrying amount at December 31, 2012 primarily relates to the payment associated with the settlement of the 2008 lawsuit filed by subsidiaries of Koch Industries, Inc. (INVISTA).
Obligations for Equity Affiliates & Others
The company has directly guaranteed various debt obligations under agreements with third parties related to equity affiliates, customers and suppliers. At December 31, 2012, the company had directly guaranteed $535 of such obligations. This amount represents the maximum potential amount of future (undiscounted) payments that the company could be required to make under the guarantees. The company would be required to perform on these guarantees in the event of default by the guaranteed party.
The company assesses the payment/performance risk by assigning default rates based on the duration of the guarantees. These default rates are assigned based on the external credit rating of the counterparty or through internal credit analysis and historical default history for counterparties that do not have published credit ratings. For counterparties without an external rating or available credit history, a cumulative average default rate is used.
In certain cases, the company has recourse to assets held as collateral, as well as personal guarantees from customers and suppliers. Assuming liquidation, these assets are estimated to cover approximately 50 percent of the $350 of guaranteed obligations of customers and suppliers. Set forth below are the company's guaranteed obligations at December 31, 2012:
|
| | | | | | | | | |
| | Short-Term | Long-Term | Total |
Obligations for customers and suppliers1: | |
| |
| |
|
| Bank borrowings (terms up to 5 years) | $ | 284 |
| $ | 64 |
| $ | 348 |
|
| Leases on equipment and facilities (terms up to 4 years) | — |
| 2 |
| 2 |
|
Obligations for equity affiliates2: | |
| |
| |
|
| Bank borrowings (terms up to 1 year) | 185 |
| — |
| 185 |
|
| Total | $ | 469 |
| $ | 66 |
| $ | 535 |
|
| |
1. | Existing guarantees for customers and suppliers arose as part of contractual agreements. As of December 31, 2012, approximately $14 of these guarantees relate to customers of the Performance Coatings business. |
| |
2. | Existing guarantees for equity affiliates arose for liquidity needs in normal operations. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Operating Leases
The company uses various leased facilities and equipment in its operations. The terms for these leased assets vary depending on the lease agreement.
Future minimum lease payments (including residual value guarantee amounts) under non-cancelable operating leases are $320, $260, $213, $178 and $153 for the years 2013, 2014, 2015, 2016 and 2017, respectively, and $290 for subsequent years and are not reduced by non-cancelable minimum sublease rentals due in the future in the amount of $3. Net rental expense under operating leases was $316, $268 and $229 in 2012, 2011 and 2010, respectively.
Asset Retirement Obligations
The company has recorded asset retirement obligations primarily associated with closure, reclamation and removal costs for mining operations related to the production of titanium dioxide in Performance Chemicals. The company's asset retirement obligation liabilities were $64 and $59 at December 31, 2012 and 2011.
Imprelis®
The company has received claims and been served with multiple lawsuits alleging that the use of Imprelis® herbicide caused damage to certain trees. The lawsuits seeking class action status have been consolidated in multidistrict litigation in federal court in Philadelphia, Pennsylvania. In addition, about 80 individual actions on behalf of approximately 180 plaintiffs have been filed in state court in various jurisdictions. DuPont has removed most of these cases to federal court in Philadelphia, Pennsylvania.
DuPont and lead and liaison counsel for plaintiffs in the multidistrict litigation sought preliminary approval from the court of a class action settlement; the court scheduled a hearing on February 5, 2013. The settlement would incorporate the company's existing claims process and provide certain additional relief. The proposed settlement class would include affected property owners and lawn care companies who do not "opt out" of the settlement. DuPont would have the ability to cancel the agreement if the number of “opt-outs” is unsatisfactory to DuPont. If the settlement is approved, DuPont would pay about $7 in plaintiffs' attorney fees and expenses and bear the costs of notifying potential class participants. In addition, DuPont would provide a warranty against new damage, if any, caused by the use of Imprelis® on class members' properties through May 2015.
In August 2011, the company suspended sales of Imprelis® and in September 2011 began a process to fairly resolve claims associated with the use of Imprelis®. The company believes that the number of unasserted claims is limited due to the fact that sales were suspended in August 2011 and the product was last applied during the 2011 spring application season.
The company has established review processes to verify and evaluate damage claims. There are several variables that impact the evaluation process including the number of trees on a property, the species of tree with reported damage, the height of the tree, the extent of damage and the possibility for trees to naturally recover over time. Upon receiving claims, DuPont verifies their accuracy and validity which often requires physical review of the property.
In 2012 and 2011, DuPont had recorded charges of $575 and $175, respectively, to resolve these claims, bringing the total charges to $750 at December 31, 2012. It is reasonably possible that additional charges could result related to this matter. While there is a high degree of uncertainty, total charges could range up to $900. Predicting the impact of Imprelis® on living organisms and how those organisms may react over time are significant factors driving the uncertainty of future charges. Imprelis® was applied throughout the United States and the ability of any particular species of tree to naturally recover over time may be different depending on the property's geography and associated climate. The company has an applicable insurance program with a deductible equal to the first $100 of costs and expenses. The insurance program limits are $725 for costs and expenses in excess of the $100. DuPont has submitted and will continue to submit requests for payment to its insurance carriers for costs associated with this matter.
Litigation
The company is subject to various legal proceedings arising out of the normal course of its business including product liability, intellectual property, commercial, environmental and antitrust lawsuits. It is not possible to predict the outcome of these various proceedings. Except as otherwise noted, management does not anticipate their resolution will have a materially adverse effect on the company's consolidated financial position or liquidity. However, the ultimate liabilities could be significant to results of operations in the period recognized.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
PFOA
DuPont uses PFOA (collectively, perfluorooctanoic acids and its salts, including the ammonium salt), as a processing aid to manufacture some fluoropolymer resins at various sites around the world including its Washington Works plant in West Virginia. At December 31, 2012, DuPont has accruals of $15 related to the PFOA matters discussed below.
The accrual includes charges related to DuPont's obligations under agreements with the U.S. Environmental Protection Agency and voluntary commitments to the New Jersey Department of Environmental Protection. These obligations include surveying, sampling and testing drinking water in and around certain company sites and offering treatment or an alternative supply of drinking water if tests indicate the presence of PFOA in drinking water at or greater than the national Provisional Health Advisory.
Drinking Water Actions
In August 2001, a class action, captioned Leach v DuPont, was filed in West Virginia state court alleging that residents living near the Washington Works facility had suffered, or may suffer, deleterious health effects from exposure to PFOA in drinking water.
DuPont and attorneys for the class reached a settlement in 2004 that binds about 80,000 residents. In 2005, DuPont paid the plaintiffs’ attorneys’ fees and expenses of $23 and made a payment of $70, which class counsel designated to fund a community health project. The company funded a series of health studies which were completed in October 2012 by an independent science panel of experts (the “C8 Science Panel”). The studies were conducted in communities exposed to PFOA to evaluate available scientific evidence on whether any probable link exists, as defined in the settlement agreement, between exposure to PFOA and human disease.
The C8 Science Panel found probable links, as defined in the settlement agreement, between exposure to PFOA and pregnancy-induced hypertension, including preeclampsia; kidney cancer; testicular cancer; thyroid disease; ulcerative colitis; and diagnosed higher cholesterol (hypocholesterolemia).
A panel of three medical experts will determine an appropriate medical monitoring protocol, if any, as a result of these findings. If a medical monitoring protocol for any of these diseases is defined, DuPont is required to fund a medical monitoring program to pay for such medical testing. Plaintiffs may pursue personal injury claims against DuPont only for those human diseases for which the C8 Science Panel determined a probable link exists. In January 2012, the company put $1 in an escrow account as required by the settlement agreement. Under the settlement agreement, the company's total obligation to pay for medical monitoring cannot exceed $235. In addition, the company must continue to provide water treatment designed to reduce the level of PFOA in water to six area water districts, including the Little Hocking Water Association (LHWA), and private well users.
An Ohio action brought by the LHWA is ongoing. In addition to general claims of PFOA contamination of drinking water, the action claims “imminent and substantial endangerment to health and or the environment” under the Resource Conservation and Recovery Act (RCRA). DuPont denies these claims and is defending itself vigorously.
At December 31, 2012, twenty-five lawsuits alleging personal injury and one lawsuit alleging wrongful death from exposure to PFOA in drinking water are pending in federal court in Ohio and West Virginia. DuPont denies the allegations in these lawsuits and is defending itself vigorously.
While DuPont believes that it is reasonably possible that it could incur losses related to PFOA matters in addition to those matters discussed above for which it has established accruals, a range of such losses, if any, cannot be reasonably estimated at this time.
Monsanto Patent Dispute
On August 1, 2012, a St. Louis, Missouri jury awarded $1,000 in damages to Monsanto on its claims that the company willfully infringed Monsanto's RE 39,247 patent directed to Roundup® Ready® soybean seed technology. On November 16, 2012, the court unsealed sanctions against the company for “fraud against the court,” which precluded the company from presenting a license defense at trial and ordered the company to pay Monsanto's attorneys' fees associated with the sanctions. The court has yet to decide several post-trial motions, including Monsanto's motion to enhance the damage award. The court has discretion to enhance damages for willful infringement by up to three times the jury verdict. The company intends to appeal this verdict, the sanctions ruling and damage enhancement, if any, when it is appropriate to do so. The company believes that it will prevail on appeal. Accordingly, as of December 31, 2012, no amounts have been accrued related to this matter.
Monsanto alleged that by combining Pioneer's Optimum® GAT® trait with Monsanto's patented Roundup® Ready® trait, Pioneer violated its 2002 Amended and Restated Roundup® Ready® Soybean License Agreement and, in doing so, infringed Monsanto's
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
RE 39,247 patent. The company has never sold soybeans containing a combination of the Optimum® GAT® and Roundup® Ready® traits and discontinued in 2011 its commercialization efforts for such soybeans.
Environmental
The company is also subject to contingencies pursuant to environmental laws and regulations that in the future may require the company to take further action to correct the effects on the environment of prior disposal practices or releases of chemical or petroleum substances by the company or other parties. The company accrues for environmental remediation activities consistent with the policy set forth in Note 1. Much of this liability results from the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA, often referred to as Superfund), RCRA and similar state and global laws. These laws require the company to undertake certain investigative, remediation and restoration activities at sites where the company conducts or once conducted operations or at sites where company-generated waste was disposed. The accrual also includes estimated costs related to a number of sites identified by the company for which it is probable that environmental remediation will be required, but which are not currently the subject of enforcement activities.
Remediation activities vary substantially in duration and cost from site to site. These activities, and their associated costs, depend on the mix of unique site characteristics, evolving remediation technologies, diverse regulatory agencies and enforcement policies, as well as the presence or absence of potentially responsible parties. At December 31, 2012, the Consolidated Balance Sheet included a liability of $436, relating to these matters and, in management's opinion, is appropriate based on existing facts and circumstances. The average time frame, over which the accrued or presently unrecognized amounts may be paid, based on past history, is estimated to be 15-20 years. Considerable uncertainty exists with respect to these costs and, under adverse changes in circumstances, potential liability may range up to three times the amount accrued as of December 31, 2012.
17. STOCKHOLDERS' EQUITY
Share Repurchase Program
During 2012, the company purchased and retired 7.8 million shares at a total cost of $400. These purchases completed the 2001 $2,000 share buyback plan and began purchases under a $2,000 share buyback plan authorized by the company's Board of Directors in April 2011. Under the completed 2001 plan, the company purchased a total of 42.0 million shares. As of December 31, 2012, the company has purchased 5.5 million shares at a total cost of $284 under the 2011 plan. There is no required completion date for the purchases under the 2011 plan.
In December 2012, the company's Board of Directors authorized a $1,000 share buyback plan, subject to receiving the proceeds from the Performance Coatings divestiture. On February 1, 2013, the sale of Performance Coating was completed. The 2012 share buyback plan is expected to be completed in the first half 2013.
Common stock held in treasury is recorded at cost. When retired, the excess of the cost of treasury stock over its par value is allocated between reinvested earnings and additional paid-in capital.
Set forth below is a reconciliation of common stock share activity for the years ended December 31, 2012, 2011 and 2010:
|
| | | | |
| Shares of common stock | Issued | Held In Treasury |
| Balance January 1, 2010 | 990,855,000 |
| (87,041,000 | ) |
| Issued | 18,891,000 |
| — |
|
| Repurchased | — |
| (5,395,000 | ) |
| Retired | (5,395,000 | ) | 5,395,000 |
|
| Balance December 31, 2010 | 1,004,351,000 |
| (87,041,000 | ) |
| Issued | 22,650,000 |
| — |
|
| Repurchased | — |
| (13,837,000 | ) |
| Retired | (13,837,000 | ) | 13,837,000 |
|
| Balance December 31, 2011 | 1,013,164,000 |
| (87,041,000 | ) |
| Issued | 14,671,000 |
| — |
|
| Repurchased | — |
| (7,778,000 | ) |
| Retired | (7,778,000 | ) | 7,778,000 |
|
| Balance December 31, 2012 | 1,020,057,000 |
| (87,041,000 | ) |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Noncontrolling Interest
In May 2012, the company completed the acquisition of the remaining 28 percent interest in the Solae, LLC joint venture from Bunge Limited for $447. As the purchase of the remaining interest did not result in a change of control, the difference between the carrying value of the noncontrolling interest of $362 and the consideration paid, net of taxes of $74, was recorded as an $11 reduction to additional paid-in capital.
Other Comprehensive Income
A summary of the pre-tax, tax, and after-tax effects of the components of other comprehensive income for the year ended December 31, 2012, 2011, and 2010 is provided as follows:
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the year ended December 31, | 2012 | 2011 | 2010 |
| | Pre-Tax | Tax | After-Tax | Pre-Tax | Tax | After-Tax | Pre-Tax | Tax | After-Tax |
| Cumulative translation adjustment | $ | 77 |
| $ | — |
| $ | 77 |
| $ | (457 | ) | $ | — |
| $ | (457 | ) | $ | (6 | ) | $ | — |
| $ | (6 | ) |
| Net revaluation and clearance of cash flow hedges to earnings: |
|
|
|
|
|
|
|
|
|
| Additions and revaluations of derivatives designated as cash flow hedges | 8 |
| (6 | ) | 2 |
| 10 |
| (5 | ) | 5 |
| (33 | ) | 14 |
| (19 | ) |
| Clearance of hedge results to earnings | (65 | ) | 28 |
| (37 | ) | 96 |
| (36 | ) | 60 |
| 90 |
| (34 | ) | 56 |
|
| Net revaluation and clearance of cash flow hedges to earnings | (57 | ) | 22 |
| (35 | ) | 106 |
| (41 | ) | 65 |
| 57 |
| (20 | ) | 37 |
|
| Pension benefit plans: |
|
|
|
|
|
|
|
|
|
| Net loss | (1,433 | ) | 437 |
| (996 | ) | (4,069 | ) | 1,402 |
| (2,667 | ) | (635 | ) | 224 |
| (411 | ) |
| Prior service benefit (cost) | 22 |
| (8 | ) | 14 |
| (2 | ) | — |
| (2 | ) | — |
| — |
| — |
|
| Amortization of prior service cost | 13 |
| (4 | ) | 9 |
| 16 |
| (5 | ) | 11 |
| 16 |
| (5 | ) | 11 |
|
| Amortization of loss | 887 |
| (305 | ) | 582 |
| 613 |
| (210 | ) | 403 |
| 507 |
| (173 | ) | 334 |
|
| Curtailment / settlement loss | 7 |
| (2 | ) | 5 |
| — |
| — |
| — |
| — |
| — |
| — |
|
| Pension benefit plans, net | (504 | ) | 118 |
| (386 | ) | (3,442 | ) | 1,187 |
| (2,255 | ) | (112 | ) | 46 |
| (66 | ) |
| Other benefit plans: |
|
|
|
|
|
|
|
|
|
| Net loss | (60 | ) | 17 |
| (43 | ) | (437 | ) | 151 |
| (286 | ) | (94 | ) | 31 |
| (63 | ) |
| Prior service benefit (cost) | 857 |
| (299 | ) | 558 |
| (11 | ) | 4 |
| (7 | ) | 189 |
| (77 | ) | 112 |
|
| Amortization of prior service benefit | (155 | ) | 54 |
| (101 | ) | (121 | ) | 43 |
| (78 | ) | (106 | ) | 37 |
| (69 | ) |
| Amortization of loss | 94 |
| (33 | ) | 61 |
| 60 |
| (21 | ) | 39 |
| 58 |
| (21 | ) | 37 |
|
| Curtailment loss | 3 |
| (1 | ) | 2 |
| — |
| — |
| — |
| — |
| — |
| — |
|
| Other benefit plans, net | 739 |
| (262 | ) | 477 |
| (509 | ) | 177 |
| (332 | ) | 47 |
| (30 | ) | 17 |
|
| Net unrealized gain (loss) on securities: |
|
|
|
|
|
|
|
|
|
| Unrealized (loss) gain on securities arising during the period | (5 | ) | 2 |
| (3 | ) | 2 |
| (1 | ) | 1 |
| (3 | ) | 1 |
| (2 | ) |
| Reclassification of loss realized in net income | 3 |
| (1 | ) | 2 |
| — |
| — |
| — |
| 5 |
| (2 | ) | 3 |
|
| Net unrealized (loss) gain on securities | (2 | ) | 1 |
| (1 | ) | 2 |
| (1 | ) | 1 |
| 2 |
| (1 | ) | 1 |
|
| Other comprehensive income (loss) | $ | 253 |
| $ | (121 | ) | $ | 132 |
| $ | (4,300 | ) | $ | 1,322 |
| $ | (2,978 | ) | $ | (12 | ) | $ | (5 | ) | $ | (17 | ) |
Tax (expense) benefit recorded in Stockholders' Equity was $(70), $1,365 and $12 for the years 2012, 2011 and 2010, respectively. Included in these amounts were tax benefits of $51, $43 and $17 for the years 2012, 2011 and 2010, respectively, associated with stock compensation programs. The remainder consists of amounts recorded within other comprehensive income (loss) as shown in the table above.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The changes and after-tax balances of components comprising accumulated other comprehensive income (loss) are summarized below:
|
| | | | | | | | | | | | | | | | | | |
| | Cumulative Translation Adjustment | Net Revaluation and Clearance of Cash Flow Hedges to Earnings | Pension Benefit Plans | Other Benefit Plans | Unrealized Gain (Loss) on Securities | Total |
| 2010 | |
| |
| |
| |
| |
| |
|
| Balance January 1, 2010 | $ | 219 |
| $ | (65 | ) | $ | (5,967 | ) | $ | 41 |
| $ | 1 |
| $ | (5,771 | ) |
| Other comprehensive income (loss) attributable to DuPont | (6 | ) | 34 |
| (65 | ) | 17 |
| 1 |
| (19 | ) |
| Balance December 31, 2010 | $ | 213 |
| $ | (31 | ) | $ | (6,032 | ) | $ | 58 |
| $ | 2 |
| $ | (5,790 | ) |
| 2011 | |
| |
| |
| |
| |
| |
|
| Other comprehensive income (loss) attributable to DuPont | (457 | ) | 72 |
| (2,244 | ) | (332 | ) | 1 |
| (2,960 | ) |
| Balance December 31, 2011 | $ | (244 | ) | $ | 41 |
| $ | (8,276 | ) | $ | (274 | ) | $ | 3 |
| $ | (8,750 | ) |
| 2012 | |
| |
| |
| |
| |
| |
|
| Other comprehensive income (loss) attributable to DuPont | 77 |
| (38 | ) | (410 | ) | 476 |
| (1 | ) | 104 |
|
| Balance December 31, 2012 | $ | (167 | ) | $ | 3 |
| $ | (8,686 | ) | $ | 202 |
| $ | 2 |
| $ | (8,646 | ) |
18. LONG-TERM EMPLOYEE BENEFITS
The company offers various long-term benefits to its employees. Where permitted by applicable law, the company reserves the right to change, modify or discontinue the plans.
Defined Benefit Pensions
The company has both funded and unfunded noncontributory defined benefit pension plans covering a majority of the U.S. employees hired prior to January 1, 2007. The benefits under these plans are based primarily on years of service and employees' pay near retirement. The company's funding policy is consistent with the funding requirements of federal laws and regulations. Pension coverage for employees of the company's non-U.S. consolidated subsidiaries is provided, to the extent deemed appropriate, through separate plans. Obligations under such plans are funded by depositing funds with trustees, covered by insurance contracts, or remain unfunded.
Other Long-term Employee Benefits
The parent company and certain subsidiaries provide medical, dental and life insurance benefits to pensioners and survivors, and disability and life insurance protection to employees. The associated plans for retiree benefits are unfunded and the cost of the approved claims is paid from company funds. Essentially all of the cost and liabilities for these retiree benefit plans are attributable to the U.S. parent company plans. The non-Medicare eligible retiree medical plan is contributory with pensioners and survivors' contributions adjusted annually to achieve a 50/50 target sharing of cost increases between the company and pensioners and survivors. In addition, limits are applied to the company's portion of the retiree medical cost coverage. Beginning in 2013, the company is providing the Medicare eligible pensioners and survivors with a company-funded Health Reimbursement Arrangement (HRA). The majority of U.S. employees hired on or after January 1, 2007 are not eligible to participate in the post retirement medical, dental and life insurance plans.
Employee life insurance and disability benefit plans are insured in many countries. However, primarily in the U.S., such plans are generally self-insured or are fully experience-rated. Obligations and expenses for self-insured and fully experience-rated plans are reflected in the figures below.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Summarized information on the company's pension and other long-term employee benefit plans is as follows:
|
| | | | | | | | | | | | | | | | |
| | Pension Benefits | Other Benefits |
| Obligations and Funded Status at December 31, | 2012 | 2011 | 2012 | 2011 |
| Change in benefit obligation | | | | | | | | |
| Benefit obligation at beginning of year | $ | 27,083 |
| | $ | 23,924 |
| | $ | 4,379 |
| | $ | 3,989 |
| |
| Service cost | 277 |
| | 249 |
| | 37 |
| | 33 |
| |
| Interest cost | 1,165 |
| | 1,253 |
| | 174 |
| | 212 |
| |
| Plan participants' contributions | 24 |
| | 21 |
| | 110 |
| | 112 |
| |
| Actuarial loss | 2,245 |
| | 3,062 |
| | 60 |
| | 441 |
| |
| Benefits paid | (1,593 | ) | | (1,610 | ) | | (371 | ) | | (424 | ) | |
| Amendments | (22 | ) | | 2 |
| | (857 | ) | 1 | 11 |
| |
| Net effects of acquisitions/divestitures | — |
| | 182 |
| | — |
| | 5 |
| |
| Benefit obligation at end of year | $ | 29,179 |
| | $ | 27,083 |
| | $ | 3,532 |
| | $ | 4,379 |
| |
| Change in plan assets | | | | | | | | |
| Fair value of plan assets at beginning of year | $ | 17,794 |
| | $ | 18,403 |
| | $ | — |
| | $ | — |
| |
| Actual gain on plan assets | 2,326 |
| | 471 |
| | — |
| | — |
| |
| Employer contributions | 848 |
| | 341 |
| | 261 |
| | 312 |
| |
| Plan participants' contributions | 24 |
| | 21 |
| | 110 |
| | 112 |
| |
| Benefits paid | (1,593 | ) | | (1,610 | ) | | (371 | ) | | (424 | ) | |
| Net effects of acquisitions/divestitures | — |
| | 168 |
| | — |
| | — |
| |
| Fair value of plan assets at end of year | $ | 19,399 |
| | $ | 17,794 |
| | $ | — |
| | $ | — |
| |
| Funded status | | | | | | | | |
| U.S. plans with plan assets | $ | (6,625 | ) | | $ | (6,894 | ) | | $ | — |
| | $ | — |
| |
| Non-U.S. plans with plan assets | (1,443 | ) | | (901 | ) | | — |
| | — |
| |
| All other plans | (1,712 | ) | 2 | (1,494 | ) | 2 | (3,532 | ) | | (4,379 | ) | |
| Total | $ | (9,780 | ) | | $ | (9,289 | ) | | $ | (3,532 | ) | | $ | (4,379 | ) | |
Amounts recognized in the Consolidated Balance Sheets consist of: | | | | | | | | |
| Other assets | $ | 5 |
| | $ | 4 |
| | $ | — |
| | $ | — |
| |
| Other accrued liabilities (Note 13) | (110 | ) | | (107 | ) | | (257 | ) | | (316 | ) | |
| Other liabilities (Note 15) | (9,303 | ) | | (9,186 | ) | | (3,271 | ) | | (4,063 | ) | |
| Liabilities related to assets held for sale | (372 | ) | | — |
| | (4 | ) | | — |
| |
| Net amount recognized | $ | (9,780 | ) | | $ | (9,289 | ) | | $ | (3,532 | ) | | $ | (4,379 | ) | |
| |
1. | Due to an amendment in 2012 to the company's U.S. parent company retiree medical and dental plans for Medicare eligible pensioners and survivors from the company sponsored group plans to a company-funded Health Reimbursement Arrangement (HRA), the benefit obligation decreased by $838 and prior service cost was credited by $838. |
| |
2. | Includes pension plans maintained around the world where funding is not customary. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The pre-tax amounts recognized in accumulated other comprehensive loss are summarized below:
|
| | | | | | | | | | | | |
| | Pension Benefits | Other Benefits |
| December 31, | 2012 | 2011 | 2012 | 2011 |
| Net loss | $ | (13,042 | ) | $ | (12,477 | ) | $ | (1,233 | ) | $ | (1,266 | ) |
| Prior service (cost) benefit | (62 | ) | (99 | ) | 1,567 |
| 862 |
|
| | $ | (13,104 | ) | $ | (12,576 | ) | $ | 334 |
| $ | (404 | ) |
The accumulated benefit obligation for all pension plans was $27,243 and $25,116 at December 31, 2012 and 2011, respectively.
|
| | | | | | |
| Information for pension plans with projected benefit obligation in excess of plan assets | 2012 | 2011 |
| Projected benefit obligation | $ | 29,043 |
| $ | 27,002 |
|
| Accumulated benefit obligation | 27,130 |
| 25,049 |
|
| Fair value of plan assets | 19,258 |
| 17,710 |
|
|
| | | | | | |
| Information for pension plans with accumulated benefit obligations in excess of plan assets | 2012 | 2011 |
| Projected benefit obligation | $ | 28,925 |
| $ | 25,810 |
|
| Accumulated benefit obligation | 27,064 |
| 23,974 |
|
| Fair value of plan assets | 19,179 |
| 16,576 |
|
|
| | | | | | | | | |
| | Pension Benefits |
Components of net periodic benefit cost (credit) and amounts recognized in other comprehensive income | 2012 | 2011 | 2010 |
| Net periodic benefit cost | | | |
| Service cost | $ | 277 |
| $ | 249 |
| $ | 207 |
|
| Interest cost | 1,165 |
| 1,253 |
| 1,262 |
|
| Expected return on plan assets | (1,517 | ) | (1,475 | ) | (1,435 | ) |
| Amortization of loss | 887 |
| 613 |
| 507 |
|
| Amortization of prior service cost | 13 |
| 16 |
| 16 |
|
| Curtailment / settlement loss | 7 |
| — |
| — |
|
Net periodic benefit cost1 | $ | 832 |
| $ | 656 |
| $ | 557 |
|
Changes in plan assets and benefit obligations recognized in other comprehensive income | | | |
| Net loss | $ | 1,433 |
| $ | 4,069 |
| $ | 635 |
|
| Amortization of loss | (887 | ) | (613 | ) | (507 | ) |
| Prior service (benefit) cost | (22 | ) | 2 |
| — |
|
| Amortization of prior service cost | (13 | ) | (16 | ) | (16 | ) |
| Curtailment / settlement loss | (7 | ) | — |
| — |
|
| Total loss recognized in other comprehensive income | $ | 504 |
| $ | 3,442 |
| $ | 112 |
|
| Noncontrolling interest | (1 | ) | (11 | ) | (1 | ) |
| Accumulated other comprehensive income assumed from purchase of noncontrolling interest | 25 |
| — |
| — |
|
| Total loss recognized in other comprehensive income, attributable to DuPont | $ | 528 |
| $ | 3,431 |
| $ | 111 |
|
| Total recognized in net periodic benefit cost and other comprehensive income | $ | 1,360 |
| $ | 4,087 |
| $ | 668 |
|
| |
1. | The above amounts include net periodic benefit cost relating to discontinued operations for 2012, 2011 and 2010 of $42, $41 and $38, respectively. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The estimated pre-tax net loss and prior service cost for the defined benefit pension plans that will be amortized from accumulated other comprehensive loss into net periodic benefit cost during 2013 are $972 and $12, respectively.
|
| | | | | | | | | |
| | Other Benefits |
Components of net periodic benefit cost (credit) and amounts recognized in other comprehensive income | 2012 | 2011 | 2010 |
| Net periodic benefit cost | | | |
| Service cost | $ | 37 |
| $ | 33 |
| $ | 29 |
|
| Interest cost | 174 |
| 212 |
| 238 |
|
| Amortization of loss | 94 |
| 60 |
| 58 |
|
| Amortization of prior service benefit | (155 | ) | (121 | ) | (106 | ) |
| Curtailment loss | 3 |
| — |
| — |
|
Net periodic benefit cost1 | $ | 153 |
| $ | 184 |
| $ | 219 |
|
Changes in plan assets and benefit obligations recognized in other comprehensive income | | | |
| Net loss | $ | 60 |
| $ | 437 |
| $ | 94 |
|
| Amortization of loss | (94 | ) | (60 | ) | (58 | ) |
| Prior service (benefit) cost | (857 | ) | 11 |
| (189 | ) |
| Amortization of prior service benefit | 155 |
| 121 |
| 106 |
|
| Curtailment loss | (3 | ) | — |
| — |
|
| Total (benefit) loss recognized in other comprehensive income | $ | (739 | ) | $ | 509 |
| $ | (47 | ) |
| Accumulated other comprehensive income assumed from purchase of noncontrolling interest | 1 |
| — |
| — |
|
| Total (benefit) loss recognized in other comprehensive income, attributable to DuPont | $ | (738 | ) | $ | 509 |
| $ | (47 | ) |
| Total recognized in net periodic benefit cost and other comprehensive income | $ | (585 | ) | $ | 693 |
| $ | 172 |
|
| |
1. | The above amounts include net periodic benefit cost relating to discontinued operations for 2012, 2011 and 2010 of $2, $2 and $1, respectively. |
The estimated pre-tax net loss and prior service credit for the other long-term employee benefit plans that will be amortized from accumulated other comprehensive loss into net periodic benefit cost during 2013 are $107 and $(187), respectively.
|
| | | | | | | | |
| | Pension Benefits | Other Benefits |
| Weighted-average assumptions used to determine benefit obligations at December 31, | 2012 | 2011 | 2012 | 2011 |
| Discount rate | 3.89 | % | 4.49 | % | 3.85 | % | 4.50 | % |
Rate of compensation increase1 | 4.13 | % | 4.18 | % | 4.40 | % | 4.40 | % |
| |
1. | The rate of compensation increase represents the single annual effective salary increase that an average plan participant would receive during the participant's entire career at the company. |
|
| | | | | | | | | | | | |
| | Pension Benefits | Other Benefits |
Weighted-average assumptions used to determine net periodic benefit cost for the years ended December 31, | 2012 | 2011 | 2010 | 2012 | 2011 | 2010 |
| Discount rate | 4.32 | % | 5.32 | % | 5.80 | % | 4.49 | % | 5.50 | % | 6.00 | % |
| Expected return on plan assets | 8.61 | % | 8.73 | % | 8.64 | % | — | % | — | % | — | % |
| Rate of compensation increase | 4.18 | % | 4.24 | % | 4.24 | % | 4.40 | % | 4.50 | % | 4.50 | % |
In connection with the planned sale of the Performance Coatings business (See Note 2), the company updated the discount rate and expected return on plan assets for the U.S. pension plans during 2012. For determining the U.S. pension plans' net periodic benefit costs, the weighted discount rate, weighted expected return on plan assets and the rate of compensation increase were 4.38 percent, 8.96 percent and 4.40 percent for 2012. With the continuing challenges in the global economy, the company lowered its long-term expected return on plan assets during 2012.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
For determining U.S. pension plans' net periodic benefit costs, the discount rate, expected return on plan assets and the rate of compensation increase were 5.50 percent, 9.00 percent and 4.50 percent for 2011 and 6.00 percent, 9.00 percent and 4.50 percent for 2010, respectively.
In the U.S., the discount rate is developed by matching the expected cash flow of the benefit plans to a yield curve constructed from a portfolio of high quality fixed-income instruments provided by the plan's actuary as of the measurement date. For non-U.S. benefit plans, the company utilizes prevailing long-term high quality corporate bond indices to determine the discount rate applicable to each country at the measurement date.
The long-term rate of return on assets in the U.S. was selected from within the reasonable range of rates determined by historical real returns (net of inflation) for the asset classes covered by the investment policy, expected performance, and projections of inflation over the long-term period during which benefits are payable to plan participants. Consistent with prior years, the long-term rate of return on plan assets in the U.S. reflects the asset allocation of the plan and the effect of the company's active management of the plans' assets. For non-U.S. plans, assumptions reflect economic assumptions applicable to each country.
|
| | | | |
| Assumed health care cost trend rates at December 31, | 2012 | 2011 |
| Health care cost trend rate assumed for next year | 8 | % | 8 | % |
| Rate to which the cost trend rate is assumed to decline (the ultimate trend rate) | 5 | % | 5 | % |
| Year that the rate reaches the ultimate trend rate | 2016 |
| 2015 |
|
Assumed health care cost trend rates have a modest effect on the amount reported for the health care plan. A one-percentage point change in assumed health care cost trend rates would have the following effects:
|
| | | | | | |
| | 1-Percentage Point Increase | 1-Percentage Point Decrease |
| Increase (decrease) on total of service and interest cost | $ | 5 |
| $ | (5 | ) |
| Increase (decrease) on post-retirement benefit obligation | 75 |
| (86 | ) |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Plan Assets
All pension plan assets in the U.S. are invested through a single master trust fund. The strategic asset allocation for this trust fund is selected by management, reflecting the results of comprehensive asset liability modeling. The general principles guiding U.S. pension asset investment policies are those embodied in the Employee Retirement Income Security Act of 1974 (ERISA). These principles include discharging the company's investment responsibilities for the exclusive benefit of plan participants and in accordance with the "prudent expert" standard and other ERISA rules and regulations. The company establishes strategic asset allocation percentage targets and appropriate benchmarks for significant asset classes with the aim of achieving a prudent balance between return and risk. Strategic asset allocations in other countries are selected in accordance with the laws and practices of those countries. Where appropriate, asset liability studies are utilized in this process. U.S. plan assets and a portion of non-U.S. plan assets are managed by investment professionals employed by the company. The remaining assets are managed by professional investment firms unrelated to the company. The company's pension investment professionals have discretion to manage the assets within established asset allocation ranges approved by senior management of the company. Additionally, pension trust funds are permitted to enter into certain contractual arrangements generally described as "derivatives." Derivatives are primarily used to reduce specific market risks, hedge currency and adjust portfolio duration and asset allocation in a cost-effective manner.
The weighted-average target allocation for plan assets of the company's U.S. and non-U.S. pension plan is summarized as follows:
|
| | | | |
| Target allocation for plan assets at December 31, | 2012 | 2011 |
| U.S. equity securities | 28 | % | 27 | % |
| Non-U.S. equity securities | 21 |
| 20 |
|
| Fixed income securities | 29 |
| 29 |
|
| Hedge funds | 2 |
| 2 |
|
| Private market securities | 13 |
| 14 |
|
| Real estate | 7 |
| 8 |
|
| Total | 100 | % | 100 | % |
Equity securities include varying market capitalization levels. U.S. equity investments are primarily large-cap companies. Fixed income investments include corporate-issued, government-issued and asset-backed securities. Corporate debt investments include a range of credit risk and industry diversification. U.S. fixed income investments are weighted heavier than non-U.S fixed income securities. Other investments include hedge funds, real estate and private market securities such as interests in private equity and venture capital partnerships.
Fair value calculations may not be indicative of net realizable value or reflective of future fair values. Furthermore, although the company believes its valuation methods are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different fair value measurement at the reporting date.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The tables below presents the fair values of the company's pension assets by level within the fair value hierarchy, as described in Note 1, as of December 31, 2012 and 2011, respectively.
|
| | | | | | | | | | | | |
| | Fair Value Measurements at December 31, 2012 |
| Asset Category | Total | Level 1 | Level 2 | Level 3 |
| Cash and cash equivalents | $ | 2,613 |
| $ | 2,584 |
| $ | 29 |
| $ | — |
|
U.S. equity securities1 | 3,647 |
| 3,604 |
| 25 |
| 18 |
|
| Non-U.S. equity securities | 3,928 |
| 3,842 |
| 86 |
| — |
|
| Debt – government-issued | 1,714 |
| 443 |
| 1,271 |
| — |
|
| Debt – corporate-issued | 2,236 |
| 378 |
| 1,831 |
| 27 |
|
| Debt – asset-backed | 1,059 |
| 40 |
| 1,017 |
| 2 |
|
| Hedge funds | 389 |
| — |
| 2 |
| 387 |
|
| Private market securities | 2,926 |
| — |
| 4 |
| 2,922 |
|
| Real estate | 1,236 |
| 82 |
| — |
| 1,154 |
|
| Derivatives – asset position | 129 |
| 6 |
| 123 |
| — |
|
| Derivatives – liability position | (80 | ) | (1 | ) | (79 | ) | — |
|
| | $ | 19,797 |
| $ | 10,978 |
| $ | 4,309 |
| $ | 4,510 |
|
Pension trust receivables2 | 312 |
| |
| |
| |
|
Pension trust payables3 | (710 | ) | |
| |
| |
|
| Total | $ | 19,399 |
| |
| |
| |
|
|
| | | | | | | | | | | | |
| | Fair Value Measurements at December 31, 2011 |
| Asset Category | Total | Level 1 | Level 2 | Level 3 |
| Cash and cash equivalents | $ | 2,085 |
| $ | 1,962 |
| $ | 123 |
| $ | — |
|
U.S. equity securities1 | 3,624 |
| 3,576 |
| 20 |
| 28 |
|
| Non-U.S. equity securities | 3,227 |
| 3,166 |
| 61 |
| — |
|
| Debt – government-issued | 1,596 |
| 391 |
| 1,205 |
| — |
|
| Debt – corporate-issued | 1,844 |
| 114 |
| 1,700 |
| 30 |
|
| Debt – asset-backed | 963 |
| 36 |
| 923 |
| 4 |
|
| Hedge funds | 396 |
| — |
| 4 |
| 392 |
|
| Private market securities | 2,959 |
| — |
| — |
| 2,959 |
|
| Real estate | 1,196 |
| 109 |
| — |
| 1,087 |
|
| Derivatives – asset position | 127 |
| 4 |
| 123 |
| — |
|
| Derivatives – liability position | (90 | ) | (2 | ) | (88 | ) | — |
|
| | $ | 17,927 |
| $ | 9,356 |
| $ | 4,071 |
| $ | 4,500 |
|
Pension trust receivables2 | 463 |
| |
| |
| |
|
Pension trust payables3 | (596 | ) | |
| |
| |
|
| Total | $ | 17,794 |
| |
| |
| |
|
| |
1. | The company's pension plans directly held $449 (2 percent of total plan assets) and $457 (3 percent of total plan assets) of DuPont common stock at December 31, 2012 and 2011, respectively. |
| |
2. | Primarily receivables for investment securities sold. |
| |
3. | Primarily payables for investment securities purchased. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The company's pension plans hold Level 3 assets which are primarily ownership interests in investment partnerships and trusts that own private market securities and real estate. Fair value is generally based on the company's units of ownership and net asset value of the investment entity or the company's share of the investment entity's total equity. The table below presents a rollforward of activity for these assets for the years ended December 31, 2012 and 2011:
|
| | | | | | | | | | | | | | | | | | | | | |
| | Level 3 Assets |
| | Total | U.S. Equity Securities | Debt- Corporate Issued | Debt- Asset- Backed | Hedge Funds | Private Market Securities | Real Estate |
| Beginning balance at December 31, 2010 | $ | 3,920 |
| $ | 20 |
| $ | 34 |
| $ | 4 |
| $ | — |
| $ | 2,931 |
| $ | 931 |
|
| Realized gain (loss) | 11 |
| — |
| (10 | ) | — |
| — |
| 21 |
| — |
|
| Change in unrealized gain (loss) | 201 |
| (3 | ) | 9 |
| — |
| (9 | ) | 124 |
| 80 |
|
| Purchases, sales and settlements | 375 |
| 10 |
| 5 |
| — |
| 401 |
| (117 | ) | 76 |
|
| Transfers (out) in of Level 3 | (7 | ) | 1 |
| (8 | ) | — |
| — |
| — |
| — |
|
| Ending balance at December 31, 2011 | $ | 4,500 |
| $ | 28 |
| $ | 30 |
| $ | 4 |
| $ | 392 |
| $ | 2,959 |
| $ | 1,087 |
|
| Realized gain (loss) | 14 |
| (3 | ) | — |
| — |
| (6 | ) | 23 |
| — |
|
| Change in unrealized gain (loss) | 253 |
| (8 | ) | (10 | ) | — |
| 17 |
| 179 |
| 75 |
|
| Purchases, sales and settlements | (134 | ) | (1 | ) | 7 |
| (2 | ) | (16 | ) | (114 | ) | (8 | ) |
| Transfers (out) in of Level 3 | (123 | ) | 2 |
| — |
| — |
| — |
| (125 | ) | — |
|
| Ending balance at December 31, 2012 | $ | 4,510 |
| $ | 18 |
| $ | 27 |
| $ | 2 |
| $ | 387 |
| $ | 2,922 |
| $ | 1,154 |
|
Cash Flow
Contributions
The company made a contribution of $500 to its principal U.S. pension plan in 2010 and made another $500 contribution in 2012. No contributions were required or made to the principal U.S. pension plan trust fund in 2011. No contributions are expected to be made to the principal U.S. pension plan in 2013. The company expects to contribute approximately $340 in 2013 to its pension plans other than the principal U.S. pension plan and also expects to make cash payments of approximately $260 in 2013 under its other long-term employee benefit plans.
Estimated Future Benefit Payments
The following benefit payments, which reflect future service, as appropriate, are expected to be paid:
|
| | | | | | |
| | Pension Benefits | Other Benefits |
| 2013 | $ | 1,629 |
| $ | 260 |
|
| 2014 | 1,604 |
| 254 |
|
| 2015 | 1,629 |
| 252 |
|
| 2016 | 1,637 |
| 250 |
|
| 2017 | 1,667 |
| 246 |
|
| Years 2018-2022 | 8,678 |
| 1,178 |
|
Defined Contribution Plan
The company sponsors several defined contribution plans, which cover substantially all U.S. employees. The most significant is the U.S. parent company's Retirement Savings Plan (the Plan), which reflects the 2009 merger of the Retirement Savings Plan and the Savings and Investment Plan. This Plan includes a non-leveraged Employee Stock Ownership Plan (ESOP). Employees are not required to participate in the ESOP and those who do are free to diversify out of the ESOP. The purpose of the Plan is to provide retirement savings benefits for employees and to provide employees an opportunity to become stockholders of the company. The Plan is a tax qualified contributory profit sharing plan, with cash or deferred arrangement and any eligible employee of the company may participate. The company contributes 100 percent of the first 6 percent of the employee's contribution election and also contributes 3 percent of each eligible employee's eligible compensation regardless of the employee's contribution.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
The company's contributions to the U.S. parent company's defined contribution plans were $212, $210 and $195 for the years ended December 31, 2012, 2011 and 2010, respectively. The company's matching contributions vest immediately upon contribution. The 3 percent nonmatching company contribution vests for employees with at least three years of service. In addition, the company made contributions to other defined contribution plans of $124, $84 and $59 for the years ended December 31, 2012, 2011 and 2010, respectively. Included in the company's contributions are amounts related to discontinued operations of $30, $29 and $32 for the years ended December 31, 2012, 2011 and 2010, respectively. The company expects to contribute about $320 to its defined contribution plans in 2013.
19. COMPENSATION PLANS
The total stock-based compensation cost included in the Consolidated Income Statements was $105, $113 and $108 for 2012, 2011 and 2010, respectively. The income tax benefits related to stock-based compensation arrangements were $35, $37 and $36 for 2012, 2011 and 2010, respectively.
In April 2011, the shareholders approved amendments to the DuPont Equity and Incentive Plan (EIP). The EIP provides for equity-based and cash incentive awards to certain employees, directors, and consultants. Under the amended EIP, the maximum number of shares reserved for the grant or settlement of awards is 110 million shares, provided that each share in excess of 30 million that is issued with respect to any award that is not an option or stock appreciation right will be counted against the 110 million share limit as four and one-half shares. At December 31, 2012, approximately 60 million shares were authorized for future grants under the company's EIP. The company satisfies stock option exercises and vesting of time-vested restricted stock units (RSUs) and performance-based restricted stock units (PSUs) with newly issued shares of DuPont common stock.
The company's Compensation Committee determines the long-term incentive mix, including stock options, RSUs and PSUs and may authorize new grants annually.
Stock Options
The exercise price of shares subject to option is equal to the market price of the company's stock on the date of grant. Options granted prior to 2004 expire 10 years from date of grant; options granted between 2004 and 2008 serially vested over a three-year period and carry a six-year option term. Stock option awards granted between 2009 and 2012 expire seven years after the grant date. The plan allows retirement eligible employees to retain any granted awards upon retirement provided the employee has rendered at least six months of service following grant date.
For purposes of determining the fair value of stock options awards, the company uses the Black-Scholes option pricing model and the assumptions set forth in the table below. The weighted-average grant-date fair value of options granted in 2012, 2011 and 2010 was $11.81, $12.32 and $6.44, respectively.
|
| | | | | | |
| | 2012 | 2011 | 2010 |
| Dividend yield | 3.2 | % | 3.2 | % | 4.9 | % |
| Volatility | 34.87 | % | 33.26 | % | 32.44 | % |
| Risk-free interest rate | 0.9 | % | 2.3 | % | 2.6 | % |
| Expected life (years) | 5.3 |
| 5.3 |
| 5.3 |
|
The company determines the dividend yield by dividing the current annual dividend on the company's stock by the option exercise price. A historical daily measurement of volatility is determined based on the expected life of the option granted. The risk-free interest rate is determined by reference to the yield on an outstanding U.S. Treasury note with a term equal to the expected life of the option granted. Expected life is determined by reference to the company's historical experience.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Stock option awards as of December 31, 2012, and changes during the year then ended were as follows:
|
| | | | | | | | | |
| | Number of Shares (in thousands) | Weighted Average Exercise Price (per share) | Weighted Average Remaining Contractual Term (years) | Aggregate Intrinsic Value (in thousands) |
| Outstanding, December 31, 2011 | 45,046 |
| $ | 38.40 |
| | |
|
| Granted | 4,728 |
| $ | 51.77 |
| | |
|
| Exercised | (12,995 | ) | $ | 38.21 |
| | |
|
| Forfeited | (135 | ) | $ | 46.92 |
| | |
|
| Cancelled | (3,285 | ) | $ | 44.57 |
| | |
|
| Outstanding, December 31, 2012 | 33,359 |
| $ | 39.70 |
| 3.03 | $ | 258,455 |
|
| Exercisable, December 31, 2012 | 24,254 |
| $ | 36.68 |
| 2.15 | $ | 235,063 |
|
The aggregate intrinsic values in the table above represent the total pre-tax intrinsic value (the difference between the company's closing stock price on the last trading day of 2012 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their in-the-money options at year end. The amount changes based on the fair market value of the company's stock. Total intrinsic value of options exercised for 2012, 2011 and 2010 were $147, $216 and $109, respectively. In 2012, the company realized a tax benefit of $49 from options exercised.
As of December 31, 2012, $29 of total unrecognized compensation cost related to stock options is expected to be recognized over a weighted-average period of 1.87 years.
RSUs and PSUs
The company issues RSUs that serially vest over a three-year period and, upon vesting, convert one-for-one to DuPont common stock. A retirement eligible employee retains any granted awards upon retirement provided the employee has rendered at least six months of service following the grant date. Additional RSUs are also granted periodically to key senior management employees. These RSUs generally vest over periods ranging from two to five years. The fair value of all stock-settled RSUs is based upon the market price of the underlying common stock as of the grant date.
The company also grants PSUs to senior leadership. In 2012, there were 233,422 PSUs granted. Vesting for PSUs granted in 2010, 2011 and 2012 is equally based upon corporate revenue growth relative to peer companies and total shareholder return (TSR) relative to peer companies. Performance and payouts are determined independently for each metric. The actual award, delivered as DuPont common stock, can range from zero percent to 200 percent of the original grant. The grant-date fair value of the PSUs granted in 2012, subject to the TSR metric, was $69.77, estimated using a Monte Carlo simulation. The grant-date fair value of the PSUs, subject to the revenue metric, was based upon the market price of the underlying common stock as of the grant date.
Non-vested awards of RSUs and PSUs as of December 31, 2012 and 2011 are shown below. The weighted-average grant-date fair value of RSUs and PSUs granted during 2012, 2011 and 2010 was $47.17, $53.19 and $34.60, respectively.
|
| | | | | |
| | Number of Shares (in thousands) | Weighted Average Grant Date Fair Value (per share) |
| Nonvested, December 31, 2011 | 3,581 |
| $ | 38.58 |
|
| Granted | 1,872 |
| $ | 47.17 |
|
| Vested | (2,240 | ) | $ | 30.42 |
|
| Forfeited | (93 | ) | $ | 43.07 |
|
| Nonvested, December 31, 2012 | 3,120 |
| $ | 49.42 |
|
As of December 31, 2012, there was $52 unrecognized stock-based compensation expense related to nonvested awards. That cost is expected to be recognized over a weighted-average period of 1.83 years. The total fair value of stock units vested during 2012, 2011 and 2010 was $68, $74 and $64, respectively.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Other Cash-based Awards
Cash awards under the EIP plan may be granted to employees who have contributed most to the company's success, with consideration being given to the ability to succeed to more important managerial responsibility. Such awards were $60, $85 and $112 for 2012, 2011 and 2010, respectively. The amounts of the awards are dependent on company earnings and are subject to maximum limits as defined under the governing plans.
In addition, the company has other variable compensation plans under which cash awards may be granted. These plans include the company's regional and local variable compensation plans and Pioneer's Annual Reward Program. Such awards were $379, $386 and $422 for 2012, 2011 and 2010, respectively.
20. DERIVATIVES AND OTHER HEDGING INSTRUMENTS
Objectives and Strategies for Holding Derivative Instruments
In the ordinary course of business, the company enters into contractual arrangements (derivatives) to reduce its exposure to foreign currency, interest rate and commodity price risks. The company has established a variety of derivative programs to be utilized for financial risk management. These programs reflect varying levels of exposure coverage and time horizons based on an assessment of risk.
Derivative programs have procedures and controls and are approved by the Corporate Financial Risk Management Committee, consistent with the company's financial risk management policies and guidelines. Derivative instruments used are forwards, options, futures and swaps. The company has not designated any nonderivatives as hedging instruments.
The company's financial risk management procedures also address counterparty credit approval, limits and routine exposure monitoring and reporting. The counterparties to these contractual arrangements are major financial institutions and major commodity exchanges. The company is exposed to credit loss in the event of nonperformance by these counterparties. The company utilizes collateral support annex agreements with certain counterparties to limit its exposure to credit losses. The company's derivative assets and liabilities are reported on a gross basis in the Consolidated Balance Sheets. The company anticipates performance by counterparties to these contracts and therefore no material loss is expected. Market and counterparty credit risks associated with these instruments are regularly reported to management.
The notional amounts of the company's derivative instruments were as follows:
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Derivatives designated as hedging instruments: | | |
| Interest rate swaps | $ | 1,000 |
| $ | 1,000 |
|
| Foreign currency contracts | 1,083 |
| 2,032 |
|
| Commodity contracts | 753 |
| 553 |
|
| Derivatives not designated as hedging instruments: | |
|
| Foreign currency contracts | 6,733 |
| 6,444 |
|
| Commodity contracts | 242 |
| 437 |
|
Foreign Currency Risk
The company's objective in managing exposure to foreign currency fluctuations is to reduce earnings and cash flow volatility associated with foreign currency rate changes. Accordingly, the company enters into various contracts that change in value as foreign exchange rates change to protect the value of its existing foreign currency-denominated assets, liabilities, commitments and cash flows.
The company routinely uses forward exchange contracts to offset its net exposures, by currency, related to the foreign currency-denominated monetary assets and liabilities of its operations. The primary business objective of this hedging program is to maintain an approximately balanced position in foreign currencies so that exchange gains and losses resulting from exchange rate changes, net of related tax effects, are minimized. The company also uses foreign currency exchange contracts to offset a portion of the company's exposure to certain foreign currency-denominated revenues so that gains and losses on these contracts offset changes in the USD value of the related foreign currency-denominated revenues. The objective of the hedge program is to reduce earnings and cash flow volatility related to changes in foreign currency exchange rates.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Interest Rate Risk
The company uses interest rate swaps to manage the interest rate mix of the total debt portfolio and related overall cost of borrowing.
Interest rate swaps involve the exchange of fixed for floating rate interest payments to effectively convert fixed rate debt into floating rate debt based on USD LIBOR. Interest rate swaps allow the company to achieve a target range of floating rate debt.
Commodity Price Risk
Commodity price risk management programs serve to reduce exposure to price fluctuations on purchases of inventory such as copper, corn, soybeans and soybean meal. The company enters into over-the-counter and exchange-traded derivative commodity instruments to hedge the commodity price risk associated with energy feedstock and agricultural commodity exposures.
Fair Value Hedges
Interest Rate Swaps
At December 31, 2012, the company maintained a number of interest rate swaps, which were implemented at the time debt instruments were issued. All interest rate swaps qualify for the shortcut method of hedge accounting, thus there is no ineffectiveness related to these hedges.
Cash Flow Hedges
Foreign Currency Contracts
The company uses foreign currency exchange instruments such as forwards and options to offset a portion of the company's exposure to certain foreign currency-denominated revenues so that gains and losses on these contracts offset changes in the USD value of the related foreign currency-denominated revenues.
Commodity Contracts
The company enters into over-the-counter and exchange-traded derivative commodity instruments, including options, futures and swaps, to hedge the commodity price risk associated with energy feedstock and agriculture commodity exposures.
Treasury Rate Contracts
During 2010, the company entered into treasury rate contracts to hedge the company's exposure to treasury rates on a portion of planned bond issuances. The contracts were terminated at the time the bonds were issued prior to year end.
While each risk management program has a different time maturity period, most programs currently do not extend beyond the next two-year period. Cash flow hedge results are reclassified into earnings during the same period in which the related exposure impacts earnings. Reclassifications are made sooner if it appears that a forecasted transaction will not materialize. The following table summarizes the after-tax effect of cash flow hedges on accumulated other comprehensive income (loss) for the years ended December 31, 2012 and 2011:
|
| | | | | | |
| December 31, | 2012 | 2011 |
| Beginning balance | $ | 41 |
| $ | (31 | ) |
| Additions and revaluations of derivatives designated as cash flow hedges | (1 | ) | 12 |
|
| Clearance of hedge results to earnings | (37 | ) | 60 |
|
| Ending balance | $ | 3 |
| $ | 41 |
|
During the next 12 months, the after-tax amount expected to be reclassified from accumulated other comprehensive income (loss) into earnings is $9.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Derivatives not Designated in Hedging Relationships
Foreign Currency Contracts
The company routinely uses forward exchange contracts to reduce its net exposure, by currency, related to foreign currency-denominated monetary assets and liabilities of its operations so that exchange gains and losses resulting from exchange rate changes are minimized. The netting of such exposures precludes the use of hedge accounting; however, the required revaluation of the forward contracts and the associated foreign currency-denominated monetary assets and liabilities intends to achieve a minimal earnings impact, after taxes. Additionally, the company has cross-currency swaps to hedge foreign currency fluctuations on long-term intercompany loans.
In 2012, the company initiated a program to utilize forward exchange contracts to reduce the net exposure related to foreign currency-denominated monetary assets and liabilities of its discontinued operations.
Commodity Contracts
The company utilizes options, futures and swaps that are not designated as hedging instruments to reduce exposure to commodity price fluctuations on purchases of inventory such as corn, soybeans and soybean meal.
Fair Values of Derivative Instruments
The table below presents the fair values of the company's derivative assets and liabilities within the fair value hierarchy, as described in Note 1, as of December 31, 2012 and 2011.
|
| | | | | | | |
| | | Fair Value at December 31 Using Level 2 Inputs |
| | Balance Sheet Location | 2012 | 2011 |
| Asset derivatives: | | | |
| Derivatives designated as hedging instruments: | | | |
Interest rate swaps1 | Other assets | $ | 55 |
| $ | 66 |
|
| Foreign currency contracts | Accounts and notes receivable, net | 7 |
| 44 |
|
| | | 62 |
| 110 |
|
| Derivatives not designated as hedging instruments: | | | |
Foreign currency contracts1 | Accounts and notes receivable, net | 88 |
| 100 |
|
| Foreign currency contracts | Other assets | — |
| 43 |
|
| | | 88 |
| 143 |
|
| Total asset derivatives | | $ | 150 |
| $ | 253 |
|
Cash collateral1 | Other accrued liabilities | $ | 44 |
| $ | — |
|
| | | | |
| Liability derivatives: | | | |
| Derivatives designated as hedging instruments: | | | |
| Foreign currency contracts | Other accrued liabilities | $ | 10 |
| $ | 12 |
|
| Commodity contracts | Other accrued liabilities | — |
| 1 |
|
| | | 10 |
| 13 |
|
| Derivatives not designated as hedging instruments: | | | |
| Foreign currency contracts | Other accrued liabilities | 76 |
| 21 |
|
| Commodity contracts | Other accrued liabilities | 1 |
| 2 |
|
| | | 77 |
| 23 |
|
| Total liability derivatives | | $ | 87 |
| $ | 36 |
|
| |
1. | Cash collateral held as of December 31, 2012 represents $13 related to interest rate swap derivatives designated as hedging instruments and $31 related to foreign currency derivatives not designated as hedging instruments. No cash collateral was held as of December 31, 2011. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Effect of Derivative Instruments
|
| | | | | | | | | | | | | | | | | | | |
| | Amount of Gain (Loss) Recognized in OCI1 (Effective Portion) | Amount of Gain (Loss) Recognized in Income2 | |
| | 2012 | 2011 | 2010 | 2012 | 2011 | 2010 | Income Statement Classification |
| Derivatives designated as hedging instruments: | | | | | | | |
| Fair value hedges: | | | | | | | |
| Interest rate swaps | $ | — |
| $ | — |
| $ | — |
| $ | (11 | ) | $ | 26 |
| $ | 40 |
| Interest expense3 |
| Cash flow hedges: |
|
| |
| |
| | |
| |
| |
| Foreign currency contracts | (2 | ) | (6 | ) | 2 |
| 21 |
| (15 | ) | (1 | ) | Net sales |
| Commodity contracts | 7 |
| 23 |
| (35 | ) | 44 |
| (81 | ) | (89 | ) | COGS4 |
| Treasury rate contracts | — |
| — |
| (3 | ) | — |
| — |
| — |
| |
| | 5 |
| 17 |
| (36 | ) | 54 |
| (70 | ) | (50 | ) | |
| Derivatives not designated as hedging instruments: | | |
| |
| | |
| |
| |
| Foreign currency contracts | — |
| — |
| — |
| (157 | ) | (133 | ) | 117 |
| Other income, net5 |
| Commodity contracts | — |
| — |
| — |
| (22 | ) | 3 |
| (18 | ) | COGS4 |
| Interest rate swaps | — |
| — |
| — |
| — |
| (1 | ) | — |
| COGS4 |
| | — |
| — |
| — |
| (179 | ) | (131 | ) | 99 |
| |
| Total derivatives | $ | 5 |
| $ | 17 |
| $ | (36 | ) | $ | (125 | ) | $ | (201 | ) | $ | 49 |
| |
| |
1. | OCI is defined as other comprehensive income (loss). |
| |
2. | For cash flow hedges, this represents the effective portion of the gain (loss) reclassified from accumulated OCI into income during the period. For the years ended December 31, 2012, 2011 and 2010, there was no material ineffectiveness with regard to the company's cash flow hedges. |
| |
3. | Gain (loss) recognized in income of derivative is offset to $0 by gain (loss) recognized in income of the hedged item. |
| |
4. | COGS is defined as costs of goods sold and other operating charges. |
| |
5. | Gain (loss) recognized in other income, net, was partially offset by the related gain (loss) on the foreign currency-denominated monetary assets and liabilities of the company's operations, which were $(58), $(13) and $(128) for 2012, 2011 and 2010, respectively. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
21. GEOGRAPHIC INFORMATION
|
| | | | | | | | | | | | | | | | | | |
| | 2012 | 2011 | 2010 |
| | Net Sales1 | Net Property2 | Net Sales1 | Net Property2 | Net Sales1 | Net Property2 |
| United States | $ | 13,279 |
| $ | 8,512 |
| $ | 12,234 |
| $ | 8,668 |
| $ | 10,539 |
| $ | 7,835 |
|
| Canada | $ | 921 |
| $ | 149 |
| $ | 880 |
| $ | 173 |
| $ | 795 |
| $ | 170 |
|
EMEA3 | | | | |
| | |
|
| Belgium | $ | 217 |
| $ | 133 |
| $ | 304 |
| $ | 190 |
| $ | 238 |
| $ | 139 |
|
| Denmark | 95 |
| 320 |
| 83 |
| 323 |
| 49 |
| — |
|
| Finland | 80 |
| 170 |
| 65 |
| 176 |
| 43 |
| 2 |
|
| France | 765 |
| 243 |
| 774 |
| 252 |
| 557 |
| 102 |
|
| Germany | 1,310 |
| 161 |
| 1,736 |
| 337 |
| 1,506 |
| 289 |
|
| Italy | 764 |
| 33 |
| 824 |
| 35 |
| 688 |
| 36 |
|
| Luxembourg | 77 |
| 252 |
| 74 |
| 250 |
| 65 |
| 244 |
|
| Russia | 376 |
| 7 |
| 357 |
| 8 |
| 234 |
| 7 |
|
| Spain | 326 |
| 269 |
| 390 |
| 266 |
| 320 |
| 259 |
|
| The Netherlands | 312 |
| 289 |
| 277 |
| 237 |
| 221 |
| 216 |
|
| United Kingdom | 545 |
| 96 |
| 493 |
| 110 |
| 415 |
| 116 |
|
| Other | 3,165 |
| 330 |
| 2,740 |
| 418 |
| 2,120 |
| 325 |
|
| Total EMEA | $ | 8,032 |
| $ | 2,303 |
| $ | 8,117 |
| $ | 2,602 |
| $ | 6,456 |
| $ | 1,735 |
|
| Asia Pacific | |
| | |
| |
| |
| |
|
| Australia | $ | 261 |
| $ | 20 |
| $ | 247 |
| $ | 19 |
| $ | 191 |
| $ | 9 |
|
| China/Hong Kong | 2,944 |
| 423 |
| 2,996 |
| 628 |
| 2,479 |
| 494 |
|
| India | 748 |
| 111 |
| 815 |
| 97 |
| 651 |
| 81 |
|
| Japan | 1,577 |
| 101 |
| 1,749 |
| 106 |
| 1,436 |
| 102 |
|
| Korea | 662 |
| 61 |
| 694 |
| 64 |
| 594 |
| 65 |
|
| Malaysia | 110 |
| 53 |
| 99 |
| 52 |
| 91 |
| 2 |
|
| Singapore | 147 |
| 55 |
| 186 |
| 42 |
| 176 |
| 31 |
|
| Taiwan | 594 |
| 135 |
| 654 |
| 133 |
| 522 |
| 129 |
|
| Thailand | 324 |
| 26 |
| 309 |
| 24 |
| 239 |
| 21 |
|
| Other | 666 |
| 62 |
| 599 |
| 63 |
| 432 |
| 49 |
|
| Total Asia Pacific | $ | 8,033 |
| $ | 1,047 |
| $ | 8,348 |
| $ | 1,228 |
| $ | 6,811 |
| $ | 983 |
|
| Latin America | |
| | |
| |
| |
| |
|
| Argentina | $ | 406 |
| $ | 43 |
| $ | 403 |
| $ | 40 |
| $ | 307 |
| $ | 26 |
|
| Brazil | 2,364 |
| 348 |
| 2,072 |
| 394 |
| 1,568 |
| 317 |
|
| Mexico | 1,046 |
| 307 |
| 972 |
| 276 |
| 731 |
| 215 |
|
| Other | 731 |
| 32 |
| 655 |
| 31 |
| 493 |
| 58 |
|
| Total Latin America | $ | 4,547 |
| $ | 730 |
| $ | 4,102 |
| $ | 741 |
| $ | 3,099 |
| $ | 616 |
|
| Total | $ | 34,812 |
| $ | 12,741 |
| $ | 33,681 |
| $ | 13,412 |
| $ | 27,700 |
| $ | 11,339 |
|
| |
1. | Net sales are attributed to countries based on the location of the customer. |
| |
2. | Includes property, plant and equipment less accumulated depreciation. |
| |
3. | Europe, Middle East, and Africa (EMEA). |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
22. SEGMENT INFORMATION
The company consists of 13 businesses which are aggregated into eight reportable segments based on similar economic characteristics, the nature of the products and production processes, end-use markets, channels of distribution and regulatory environment. The company's reportable segments are Agriculture, Electronics & Communications, Industrial Biosciences, Nutrition & Health, Performance Chemicals, Performance Materials, Safety & Protection and Pharmaceuticals. The company includes certain embryonic businesses not included in the reportable segments, such as pre-commercial programs, and nonaligned businesses in Other.
Major products by segment include: Agriculture (corn hybrids and soybean varieties, herbicides, fungicides and insecticides); Electronics & Communications (photopolymers and electronic materials); Industrial Biosciences (enzymes and bio-based materials); Nutrition & Health (cultures, emulsifiers, gums, natural sweeteners and soy-based food ingredients): Performance Chemicals (fluorochemicals, fluoropolymers, specialty and industrial chemicals, and white pigments); Performance Materials (engineering polymers, packaging and industrial polymers, films and elastomers); Safety & Protection (nonwovens, aramids and solid surfaces); and Pharmaceuticals (representing the company's interest in the collaboration relating to Cozaar®/Hyzaar® antihypertensive drugs, which is reported as other income). The company operates globally in substantially all of its product lines.
In general, the accounting policies of the segments are the same as those described in Note 1. Exceptions are noted as follows and are shown in the reconciliations below. Segment sales include transfers to another business segment. Products are transferred between segments on a basis intended to reflect, as nearly as practicable, the market value of the products. Segment pre-tax operating income (loss) (PTOI) is defined as income (loss) from continuing operations before income taxes excluding exchange gains (losses), corporate expenses and interest. Segment net assets includes net working capital, net property, plant and equipment and other noncurrent operating assets and liabilities of the segment. Affiliate net assets (pro rata share) excludes borrowing and other long-term liabilities. Depreciation and amortization includes depreciation on research and development facilities and amortization of other intangible assets, excluding write-down of assets. Prior years' data have been reclassified to reflect the current organizational structure.
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Agriculture | Electronics & Communications | Industrial Biosciences | Nutrition & Health | Performance Chemicals | Performance Materials | Safety & Protection | Pharma- ceuticals | Other | Total |
| 2012 | | | | | | | | | | |
| Segment sales | $ | 10,426 |
| $ | 2,701 |
| $ | 1,180 |
| $ | 3,422 |
| $ | 7,188 |
| $ | 6,447 |
| $ | 3,825 |
| $ | — |
| $ | 5 |
| $ | 35,194 |
|
| Less: Transfers | 5 |
| 17 |
| 11 |
| — |
| 247 |
| 91 |
| 11 |
| — |
| — |
| 382 |
|
| Net sales | 10,421 |
| 2,684 |
| 1,169 |
| 3,422 |
| 6,941 |
| 6,356 |
| 3,814 |
| — |
| 5 |
| 34,812 |
|
| PTOI | 1,594 |
| 135 |
| 168 |
| 299 |
| 1,586 |
| 1,013 |
| 360 |
| 62 |
| (385 | ) | 4,832 |
|
Depreciation and amortization | 337 |
| 113 |
| 79 |
| 288 |
| 245 |
| 182 |
| 197 |
| — |
| 1 |
| 1,442 |
|
Equity in earnings of affiliates | 30 |
| 19 |
| 1 |
| — |
| 28 |
| 42 |
| 32 |
| — |
| (53 | ) | 99 |
|
| Segment net assets | 4,539 |
| 1,534 |
| 2,571 |
| 6,515 |
| 3,663 |
| 3,537 |
| 2,970 |
| (18 | ) | 73 |
| 25,384 |
|
| Affiliate net assets | 389 |
| 151 |
| 53 |
| 8 |
| 180 |
| 567 |
| 106 |
| — |
| 14 |
| 1,468 |
|
Purchases of property, plant and equipment | 432 |
| 71 |
| 80 |
| 148 |
| 389 |
| 186 |
| 118 |
| — |
| 7 |
| 1,431 |
|
| 2011 | | | | | | | | | | |
| Segment sales | $ | 9,166 |
| $ | 3,173 |
| $ | 705 |
| $ | 2,460 |
| $ | 7,794 |
| $ | 6,815 |
| $ | 3,934 |
| $ | — |
| $ | 40 |
| $ | 34,087 |
|
| Less: Transfers | 1 |
| 19 |
| 7 |
| — |
| 257 |
| 109 |
| 13 |
| — |
| — |
| 406 |
|
| Net sales | 9,165 |
| 3,154 |
| 698 |
| 2,460 |
| 7,537 |
| 6,706 |
| 3,921 |
| — |
| 40 |
| 33,681 |
|
| PTOI | 1,527 |
| 355 |
| (1 | ) | 44 |
| 1,923 |
| 971 |
| 500 |
| 289 |
| (263 | ) | 5,345 |
|
Depreciation and amortization | 295 |
| 99 |
| 47 |
| 207 |
| 252 |
| 199 |
| 172 |
| — |
| 2 |
| 1,273 |
|
Equity in earnings of affiliates | 58 |
| 19 |
| (3 | ) | — |
| 43 |
| 74 |
| 47 |
| — |
| (47 | ) | 191 |
|
| Segment net assets | 4,754 |
| 1,867 |
| 2,542 |
| 6,225 |
| 3,536 |
| 3,468 |
| 3,055 |
| 35 |
| 69 |
| 25,551 |
|
| Affiliate net assets | 330 |
| 197 |
| 52 |
| 1 |
| 201 |
| 445 |
| 111 |
| — |
| 34 |
| 1,371 |
|
Purchases of property, plant and equipment | 420 |
| 198 |
| 61 |
| 115 |
| 326 |
| 197 |
| 208 |
| — |
| 5 |
| 1,530 |
|
| 2010 | | | | | | | | | | |
| Segment sales | $ | 7,845 |
| $ | 2,764 |
| $ | — |
| $ | 1,240 |
| $ | 6,322 |
| $ | 6,287 |
| $ | 3,364 |
| $ | — |
| $ | 194 |
| $ | 28,016 |
|
| Less: Transfers | 1 |
| 17 |
| — |
| — |
| 216 |
| 69 |
| 12 |
| — |
| 1 |
| 316 |
|
| Net sales | 7,844 |
| 2,747 |
| — |
| 1,240 |
| 6,106 |
| 6,218 |
| 3,352 |
| — |
| 193 |
| 27,700 |
|
| PTOI | 1,293 |
| 445 |
| — |
| 62 |
| 1,081 |
| 994 |
| 454 |
| 489 |
| (205 | ) | 4,613 |
|
Depreciation and amortization | 265 |
| 94 |
| — |
| 109 |
| 266 |
| 205 |
| 151 |
| — |
| 4 |
| 1,094 |
|
Equity in earnings of affiliates | 59 |
| 26 |
| — |
| — |
| 24 |
| 77 |
| 37 |
| — |
| (45 | ) | 178 |
|
| Segment net assets | 4,903 |
| 1,639 |
| — |
| 950 |
| 3,310 |
| 3,512 |
| 2,955 |
| 40 |
| 233 |
| 17,542 |
|
| Affiliate net assets | 289 |
| 195 |
| — |
| 2 |
| 184 |
| 485 |
| 103 |
| — |
| 90 |
| 1,348 |
|
Purchases of property, plant and equipment | 360 |
| 260 |
| — |
| 39 |
| 225 |
| 190 |
| 215 |
| — |
| 11 |
| 1,300 |
|
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Reconciliation to Consolidated Financial Statements
|
| | | | | | | | | |
| PTOI to income from continuing operations before income taxes | 2012 | 2011 | 2010 |
| Total segment PTOI | $ | 4,832 |
| $ | 5,345 |
| $ | 4,613 |
|
| Net exchange losses, including affiliates | (215 | ) | (146 | ) | (11 | ) |
| Corporate expenses and net interest | (1,502 | ) | (1,418 | ) | (1,342 | ) |
| Income from continuing operations before income taxes | $ | 3,115 |
| $ | 3,781 |
| $ | 3,260 |
|
|
| | | | | | | | | |
| Segment net assets to total assets at December 31, | 2012 | 2011 | 2010 |
| Total segment net assets | $ | 25,384 |
| $ | 25,551 |
| $ | 17,542 |
|
Corporate assets1 | 10,625 |
| 10,085 |
| 11,312 |
|
| Liabilities included in segment net assets | 10,671 |
| 9,882 |
| 8,524 |
|
Assets related to discontinued operations2 | 3,056 |
| 2,974 |
| 3,032 |
|
| Total assets | $ | 49,736 |
| $ | 48,492 |
| $ | 40,410 |
|
| |
1. | Pension assets are included in corporate assets. |
| |
2. | See Note 1 for additional information on the presentation of the Performance Coatings business which met the criteria for discontinued operations during 2012. |
|
| | | | | | | | | |
Other items1 | Segment Totals | Adjustments | Consolidated Totals |
| 2012 | |
| |
| |
|
| Depreciation and amortization | $ | 1,442 |
| $ | 271 |
| $ | 1,713 |
|
| Equity in earnings of affiliates | 99 |
| 3 |
| 102 |
|
| Affiliate net assets | 1,468 |
| (305 | ) | 1,163 |
|
| Purchases of property, plant and equipment | 1,431 |
| 362 |
| 1,793 |
|
| 2011 | |
| |
| |
|
| Depreciation and amortization | $ | 1,273 |
| $ | 287 |
| $ | 1,560 |
|
| Equity in earnings of affiliates | 191 |
| 1 |
| 192 |
|
| Affiliate net assets | 1,371 |
| (254 | ) | 1,117 |
|
| Purchases of property, plant and equipment | 1,530 |
| 313 |
| 1,843 |
|
| 2010 | |
| |
| |
|
| Depreciation and amortization | $ | 1,094 |
| $ | 286 |
| $ | 1,380 |
|
| Equity in earnings of affiliates | 178 |
| (1 | ) | 177 |
|
| Affiliate net assets | 1,348 |
| (307 | ) | 1,041 |
|
| Purchases of property, plant and equipment | 1,300 |
| 208 |
| 1,508 |
|
| |
1. | See Note 1 for additional information on the presentation of the Performance Coatings business which met the criteria for discontinued operations during 2012. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
Additional Segment Details
2012 included the following pre-tax benefits (charges):
|
| | | |
Agriculture1,2,3 | $ | (469 | ) |
Electronics & Communications3,4,5 | (37 | ) |
Industrial Biosciences3 | (3 | ) |
Nutrition & Health3 | (49 | ) |
Performance Chemicals3,5 | (36 | ) |
Performance Materials3,5 | (104 | ) |
Safety & Protection3 | (58 | ) |
Other3,6 | (126 | ) |
| | $ | (882 | ) |
| |
1. | Included a $(575) charge recorded in cost of goods sold and other operating charges associated with the company's process to fairly resolve claims related to the use of Imprelis®. See Note 16 for additional information. |
| |
2. | Included a $117 gain recorded in other income, net associated with the sale of a business. |
| |
3. | Included a $(134) restructuring charge recorded in employee separation/asset related charges, net primarily as a result of the company's plan to eliminate corporate costs previously allocated to Performance Coatings and cost-cutting actions to improve competitiveness, partially offset by a reversal of prior year restructuring accruals. Charges by segment were: Agriculture - $(11); Electronics & Communications - $(9); Industrial Biosciences - $(3); Nutrition & Health - $(49); Performance Chemicals - $(3); Performance Materials - $(12); Safety & Protection - $(58); and Other - $11. See Note 3 for additional information. |
| |
4. | Included a $122 gain recorded in other income, net associated with the sale of an equity method investment. |
| |
5. | Included a $(275) impairment charge recorded in employee separation/asset related charges, net related to asset groupings, which impacted the segments as follows: Electronics & Communications - $(150); Performance Chemicals - $(33); and Performance Materials - $(92). See Note 3 for additional information. |
| |
6. | Included a $(137) charge in cost of goods sold and other operating charges primarily related to the company's settlement of litigation with INVISTA. |
2011 included the following pre-tax benefits (charges):
|
| | | |
Agriculture1,2 | $ | (225 | ) |
Industrial Biosciences3,4 | (79 | ) |
Nutrition & Health3,4 | (126 | ) |
Performance Materials4,5 | 47 |
|
Other4 | (28 | ) |
| | $ | (411 | ) |
| |
1. | Included a $(50) charge recorded in research and development expense in connection with a milestone payment associated with a Pioneer licensing agreement. Since this milestone was reached before regulatory approval was secured by Pioneer, it was charged to research and development expense. |
| |
2. | Included a $(175) charge recorded in cost of goods sold and other operating charges associated with the company's process to fairly resolve claims associated with the use of Imprelis®. See Note 16 for additional information. |
| |
3. | Included a $(182) charge for transaction related costs and the fair value step-up of inventories that were acquired as part of the Danisco transaction, which impacted the segments as follows: Industrial Biosciences - $(70) and Nutrition & Health - $(112). |
| |
4. | Included a $(53) restructuring charge primarily related to severance and related benefit costs associated with the Danisco acquisition impacting the segments as follows: Industrial Biosciences - $(9); Nutrition & Health - $(14); Performance Materials - $(2); and Other - $(28). See Note 3 for additional information. |
| |
5. | Included a $49 benefit recorded in other income, net associated with the sale of a business. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
2010 included the following pre-tax benefits (charges):
|
| | | |
Agriculture1 | $ | (50 | ) |
Electronics & Communications2 | 8 |
|
Performance Chemicals2 | 10 |
|
Performance Materials2 | 16 |
|
Safety & Protection2 | 5 |
|
Other2 | 1 |
|
| | $ | (10 | ) |
| |
1. | Included a $(50) charge in research and development expense for an upfront payment related to a Pioneer licensing agreement. Since this payment was made before regulatory approval was secured by Pioneer, it was charged to research and development expense. |
| |
2. | Included a $40 reduction in estimated restructuring costs related to restructuring programs impacting the segments as follows: Electronics & Communications – $8; Performance Chemicals – $10; Performance Materials – $16; Safety & Protection – $5; and Other – $1. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
23. QUARTERLY FINANCIAL DATA
|
| | | | | | | | | | | | | | | | |
| Unaudited | For the quarter ended |
| | March 31, | June 30, | September 30, | December 31, |
| 2012 | |
| | |
| | |
| | |
| |
| Net sales | $ | 10,180 |
| | $ | 9,917 |
| | $ | 7,390 |
| | $ | 7,325 |
| |
Cost of goods sold and other expenses1 | 8,285 |
| 3 | 8,581 |
| 3,4 | 7,394 |
| 3,6,7 | 7,471 |
| 3,6,7 |
Income (loss) from continuing operations before income taxes | 1,795 |
| | 1,510 |
| 5 | (174 | ) | | (16 | ) | 8 |
| Net income | 1,500 |
| | 1,188 |
| | 13 |
| | 112 |
| |
Basic earnings (loss) per share of common stock from continuing operations2 | 1.49 |
| | 1.18 |
| | (0.05 | ) | | 0.02 |
| |
Diluted earnings (loss) per share of common stock from continuing operations2 | 1.47 |
| | 1.17 |
| | (0.05 | ) | | 0.02 |
| |
| 2011 | |
| | |
| | |
| | |
| |
| Net sales | $ | 9,041 |
| | $ | 9,159 |
| | $ | 8,138 |
| | $ | 7,343 |
| |
Cost of goods sold and other expenses1 | 7,382 |
| | 7,815 |
| 9 | 7,736 |
| 10,11,12 | 7,262 |
| 12,13 |
| Income from continuing operations before income taxes | 1,580 |
| | 1,447 |
| | 451 |
| | 303 |
| 14 |
| Net income | 1,444 |
| | 1,229 |
| | 460 |
| | 377 |
| 14 |
Basic earnings per share of common stock from continuing operations2 | 1.45 |
| | 1.20 |
| | 0.39 |
| | 0.31 |
| |
Diluted earnings per share of common stock from continuing operations2 | 1.43 |
| | 1.18 |
| | 0.39 |
| | 0.31 |
| |
| |
1. | Represents total expenses less interest expense. |
| |
2. | Earnings per share for the year may not equal the sum of quarterly earnings per share due to changes in average share calculations. |
| |
3. | First quarter, second quarter, third quarter, and fourth quarter 2012 included charges of $(50), $(265), $(125), and $(135), respectively, recorded in cost of goods sold and other operating charges associated with the company's process to fairly resolve claims related to the use of Imprelis®. See description in Note 16 for further details. |
| |
4. | Second quarter 2012 included a $(137) charge recorded in costs of goods sold and other operating charges primarily related to the company's settlement of litigation with INVISTA. See description in Note 16 for further details. |
| |
5. | Second quarter 2012 included a a pre-tax gain of $122 recorded in other income, net associated with the sale of an equity method investment in the Electronics & Communications segment. |
| |
6. | Third quarter 2012 included a $(152) restructuring charge recorded in employee separation / asset related charges, net related to the 2012 restructuring program. Fourth quarter 2012 included a net $(66) charge recorded in employee separation / asset related charges, net related to costs associated with the 2012 restructuring program partially offset by a reversal of prior years restructuring accruals. See description in Note 3 for further details. |
| |
7. | Third and fourth quarter 2012 included asset impairment charges of $(242) and $(33), respectively, recorded in employee separation / asset related charges, net related to certain asset groupings. See descriptions in Note 3 for further details. |
| |
8. | Fourth quarter 2012 included a pre-tax gain of $117 recorded in other income, net associated with the sale of a business within the Agriculture segment. |
| |
9. | Second quarter 2011 included charges related to the businesses acquired from Danisco of $(103), recorded in cost of goods sold and other operating charges, including $(60) of transaction costs and a $(43) charge related to the fair value step-up of inventories that were acquired from Danisco. |
| |
10. | Third quarter 2011 included charges related to the businesses acquired from Danisco of $(171). These charges included $(3) of transaction costs and a $(132) charge related to the fair value step-up of inventories that were acquired from Danisco, both recorded in cost of goods sold and other operating charges. These charges also included a $(36) restructuring charge recorded in employee separation / asset related charges, net related to severance and related benefit costs. |
| |
11. | Third quarter 2011 included a $(50) charge recorded in research and development expense in connection with a milestone payment associated with a Pioneer licensing agreement. |
| |
12. | Third and fourth quarter 2011 included charges of $(75) and $(100), respectively, recorded in costs of goods sold and other operating charges associated with the company's process to fairly resolve claims related to the use of Imprelis®. See description in Note 16 for further details. |
| |
13. | Fourth quarter 2011 included a $(17) restructuring charge recorded in employee separation / asset related charges, net related to severance and related benefit costs associated with the Danisco acquisition, partially offset by a reversal of prior year restructuring accruals. |
| |
14. | Fourth quarter 2011 included a pre-tax gain of $49 recorded in other income, net associated with the sale of a business in the Performance Materials segment and a related tax benefit of $73. |
E. I. du Pont de Nemours and Company
Notes to the Consolidated Financial Statements (continued)
(Dollars in millions, except per share)
24. SUBSEQUENT EVENTS
Divestiture of Performance Coatings
On February 1, 2013, the company completed its sale of the Performance Coatings business. See Note 2 for additional details.
American Taxpayer Relief Act of 2012
On January 2, 2013, U.S. tax law was enacted which extends, through 2013, several expired or expiring temporary business tax provisions. See Note 6 for additional details.
Inventory Valuation
Effective January 1, 2013, the company changed its method of valuing inventory held at certain of its foreign and U.S. locations from the LIFO method to the average cost method. See Note 9 for additional details.
|
| | |
| Information for Investors |
| | | |
Corporate Headquarters E. I. du Pont de Nemours and Company 1007 Market Street Wilmington, DE 19898 Telephone: 302 774-1000 E-mail: http://www.dupont.com (click on Contact)
2013 Annual Meeting The annual meeting of the shareholders will be held at 10:30 a.m., on Wednesday, April 24, in The DuPont Theatre in the DuPont Building, 1007 Market Street, Wilmington, Delaware.
Stock Exchange Listings DuPont common stock (Symbol DD) is listed on the New York Stock Exchange, Inc. (NYSE) and on certain foreign exchanges. Quarterly high and low market prices are shown in Item 5 of the Form 10-K. DuPont preferred stock is listed on the New York Stock Exchange, Inc. (Symbol DDPrA for $3.50 series and Symbol DDPrB for $4.50 series).
Dividends Holders of the company's common stock are entitled to receive dividends when they are declared by the Board of Directors. While it is not a guarantee of future conduct, the company has continuously paid a quarterly dividend since the fourth quarter 1904. Dividends on common stock and preferred stock are usually declared in January, April, July and October. When dividends on common stock are declared, they are usually paid mid March, June, September and December. Preferred dividends are paid on or about the 25th of January, April, July and October.
Shareholder Services Inquiries from shareholders about stock accounts, transfers, certificates, dividends (including direct deposit and reinvestment), name or address changes and electronic receipt of proxy materials may be directed to DuPont's stock transfer agent: Computershare Trust Company, N.A. P.O. Box 43078 Providence, RI 02940-3078 or call: in the United States and Canada 888 983-8766 (toll-free) other locations-781 575-2724 for the hearing impaired- TDD: 800 952-9245 (toll-free) or visit Computershare's home page at http://www.computershare.com | | Independent Registered Public Accounting Firm PricewaterhouseCoopers LLP Two Commerce Square, Suite 1700 2001 Market Street Philadelphia, PA 19103
Investor Relations Institutional investors and other representatives of financial institutions should contact: E. I. du Pont de Nemours and Company DuPont Investor Relations 1007 Market Street-D-11020 Wilmington, DE 19898 or call 302 774-4994
Bondholder Relations E. I. du Pont de Nemours and Company DuPont Finance 1007 Market Street-D-8028 Wilmington, DE 19898 or call 302 774-0564 or 302 774-8802
DuPont on the Internet Financial results, news and other information about DuPont can be accessed from the company's website at http://www.dupont.com. This site includes important information on products and services, financial reports, news releases, environmental information and career opportunities. The company's periodic and current reports filed with the SEC are available on its website, free of charge, as soon as reasonably practicable after being filed.
Product Information/Referral From the United States and Canada: 800 441-7515 (toll-free) From other locations: 302 774-1000 On the Internet: http://www.dupont.com (click on Contact)
Printed Reports Available to Shareholders The following company reports may be obtained, without charge: 1. 2012 Annual Report to the Securities and Exchange Commission, filed on Form 10-K; 2. Proxy Statement for 2013 Annual Meeting of Stockholders; and 3. Quarterly reports to the Securities and Exchange Commission, filed on Form 10-Q Requests should be addressed to: DuPont Inquiry Management Center CRP-735 (second floor) 974 Centre Road Wilmington, DE 19805 or call 302 774-1000 E-mail: http://www.dupont.com (click on Contact) |
| | | |
| Services for Shareholders |
Online Account Access Registered shareholders may access their accounts and obtain online answers to stock transfer questions by signing up for Internet access by visiting http://www.computershare.com.
Dividend Reinvestment Plan An automatic dividend reinvestment plan is available to all registered shareholders. Common or preferred dividends can be automatically reinvested in DuPont common stock. Participants also may add cash for the purchase of additional shares. A detailed account statement is mailed after each investment. Your account can also be viewed over the Internet if you have Online Account Access (see above). To enroll in the plan, please contact Computershare (listed above). | | Online Delivery of Proxy Materials Stockholders may request their proxy materials electronically in 2013 by visiting http://enroll.icsdelivery.com/dd.
Direct Deposit of Dividends Registered shareholders who would like their dividends directly deposited in a U.S. bank account should contact Computershare (listed above). |