SCHEDULE 14A INFORMATION
PROXY STATEMENT PURSUANT TO SECTION 14(a) OF THE
SECURITIES EXCHANGE ACT OF 1934
(AMENDMENT NO.___)
Filed by the Registrant [X]
Filed by a Party other than the Registrant [ ]
Check the appropriate box:
| | | |
| [ ] | | Preliminary Proxy Statement |
|
|
|
|
| [X] | | Definitive Proxy Statement |
|
|
|
|
| [ ] | | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
|
|
|
|
| [ ] | | Definitive Additional Materials |
|
|
|
|
| [ ] | | Soliciting Material Pursuant to sec. 240.14a-11(c) or sec. 240.14a-12 |
Genentech, Inc.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| | | | | |
| [X] | Fee not required. |
|
|
|
|
| [ ] | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
|
|
|
|
| | | (1) | | Title of each class of securities to which transaction applies:
|
|
|
|
|
| | | (2) | | Aggregate number of securities to which transaction applies:
|
|
|
|
|
| | | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
|
|
|
|
|
| | | (4) | | Proposed maximum aggregate value of transaction:
|
|
|
|
|
| | | (5) | | Total fee paid:
|
|
|
|
|
| [ ] | | Fee paid previously with preliminary materials. |
|
|
|
|
| [ ] | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
|
|
|
|
| | | (1) | | Amount Previously Paid:
|
|
|
|
|
| | | (2) | | Form, Schedule or Registration Statement No.:
|
|
|
|
|
| | | (3) | | Filing Party:
|
|
|
|
|
| | | (4) | | Date Filed:
|
TABLE OF CONTENTS
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
To Be Held on April 11, 2002
To the Stockholders of Genentech, Inc.:
The Annual Meeting of Stockholders of Genentech, Inc. will be held on Thursday, April 11, 2002, at 10:00 a.m. local time, at the Westin Hotel, 1 Old Bayshore Highway, Millbrae, California, for the following purposes, as more fully described in the Proxy Statement accompanying this Notice:
| |
| | 1. To elect all of the members of the Board of Directors for a one year term or until such directors’ successors are elected and qualified. |
| |
| | 2. To approve the amended and restated 1999 Stock Plan under which 63,000,000 shares have been reserved for issuance in order to qualify the plan as “performance-based” within the meaning of 162(m) of the Internal Revenue Code. |
| |
| | 3. To approve the ratification of Ernst & Young LLP as our independent auditors for the fiscal year ending December 31, 2002. |
| |
| | 4. To transact such other business as may properly come before the Annual Meeting or any adjournment or postponement of the Annual Meeting. |
Only holders of record of common stock of Genentech at the close of business on February 11, 2002 are entitled to notice of the Annual Meeting. This Notice and the accompanying proxy materials are sent to you by order of the Board of Directors.
| |
| | STEPHEN G. JUELSGAARD, |
| | Secretary |
South San Francisco, California
March 12, 2002
YOUR VOTE IS IMPORTANT. THERE ARE THREE WAYS TO VOTE:
| | | |
| | • | By Internet, | |
| | • | By telephone, or |
| | • | Sign, date and return your proxy card in the enclosed envelope as soon as possible. |
OUR ANNUAL MEETING WILL ALSO BE WEBCAST ATwww.gene.com
AT 10:00 A.M. PACIFIC DAYLIGHT TIME ON APRIL 11, 2002.
GENENTECH, INC.
PROXY STATEMENT
General
These proxy materials are being sent to you at the request of the Board of Directors of Genentech, Inc. (“Genentech,” “we,” “us,” “our” or the “Company”) to encourage you to vote your shares at the Annual Meeting of Stockholders to be held on April 11, 2002 or at any adjournment or postponement of the Annual Meeting. This Proxy Statement contains information on matters that will be presented at the Annual Meeting and is provided to assist you in voting your shares. We commenced mailing this Proxy Statement and the Proxy Card to the Company’s stockholders entitled to vote at the Annual Meeting on March 12, 2002.
Who Can Vote
Holders of Genentech common stock at the close of business on February 11, 2002 are entitled to vote at the Annual Meeting. As of that date, 526,300,038 shares of Genentech common stock were outstanding. Each share of common stock is entitled to one vote on each matter brought before the Annual Meeting. In this Proxy Statement, “common stock” refers to Genentech’s common stock, par value $.02 per share, and “special common stock” refers to Genentech’s callable putable common stock, par value $.02 per share.
How to Vote
Even if you plan to attend the Annual Meeting, you are encouraged to vote by proxy. You may vote by proxy in one of the following ways:
| | |
| | • | By Internet at the address listed on the Proxy Card. |
| |
| | • | By telephone using the toll-free number listed on the Proxy Card. |
| |
| | • | By returning the enclosed Proxy Card (signed and dated) in the envelope provided. |
When you vote by proxy, your shares will be voted according to your instructions. If you sign your Proxy Card but do not specify how you want your shares to be voted, they will be voted as the Board of Directors recommends. You can change or revoke your proxy by Internet or telephone at any time before the Annual Meeting in accordance with the instructions on the enclosed Proxy Card. You may also revoke it by attending the Annual Meeting and voting in person. If you would like to revoke your proxy by mail, please file a notice of revocation or an executed Proxy Card bearing a later date with the Secretary of Genentech, at the principal executive offices of Genentech, 1 DNA Way, South San Francisco, California 94080-4990.
Required Vote
In order to carry on business at the Annual Meeting, we must have a quorum. A quorum requires the presence, in person or by proxy, of the holders of a majority of the shares entitled to vote at the Annual Meeting. We will count abstentions and broker nonvotes as present for purposes of determining a quorum. A plurality of the votes cast is required for the election of the directors. The affirmative vote of holders of a majority of the shares present in person or by proxy at the Annual Meeting is required for the approval of the amended and restated 1999 Stock Plan and the ratification of Ernst & Young LLP as the Company’s independent auditors. We will count abstentions in the number of votes cast on the proposals presented to the stockholders, and they will have the same effect as negative votes, but broker nonvotes will not be counted for purposes of determining whether a proposal has been approved. All votes will be tabulated by the inspector of elections appointed for the Annual Meeting who will separately tabulate affirmative and negative votes, abstentions and broker nonvotes.
Solicitations of Proxies
We will pay all costs relating to the solicitations of proxies. We will also reimburse brokers, custodians, nominees, fiduciaries and other entities representing beneficial owners of common stock for reasonable expenses in forwarding proxy materials to the beneficial owners. Proxies may be solicited by directors, officers and other employees of the Company personally, by mail, telephone or other electronic means, but we will not pay those directors, officers or employees for those services.
Proxy Statement Proposals
At each Annual Meeting, stockholders will be asked to elect directors to serve on the Board of Directors and to ratify the appointment of our independent auditors for the year. Other proposals may be submitted by the Board of Directors or stockholders to be included in the proxy statement. To be considered for inclusion in the 2003 Annual Meeting proxy statement and the form of proxy card relating to that meeting, stockholder proposals must be received by the Secretary of the Company no later than November 12, 2002.
RELATIONSHIP WITH ROCHE
History of Ownership
On June 30, 1999, we redeemed all of our special common stock held by stockholders other than Roche Holdings, Inc. (“Roche”) at $20.625 per share in cash and retired all of the shares of special common stock including those held by Roche. As a result, Roche’s percentage ownership of our outstanding equity increased from approximately 65% to 100% and our then existing governance agreement terminated, except for provisions relating to indemnification and stock options, warrants and convertible securities.
Offerings of, and Notes Exchangeable for, our Common Stock
On July 23, 1999, Roche completed a public offering of 88 million shares of our common stock. In connection with that offering, we amended our Certificate of Incorporation and bylaws and entered into an affiliation agreement with Roche as described below. On October 26, 1999, Roche completed a public offering of 80 million shares of our common stock. Upon completion of that offering, Roche’s percentage ownership of our outstanding common stock decreased to 66.4%. On January 19, 2000, Roche completed an offering of zero-coupon notes which are exchangeable for an aggregate of 13,034,618 shares of our common stock held by Roche. On March 29, 2000, Roche completed a public offering of 34.6 million shares of our common stock, decreasing its ownership of our outstanding common stock to 58.9%.
Arrangements between Genentech and Roche
As a result of the redemption of the special common stock, the then existing governance agreement between Genentech and Roche terminated, except for provisions relating to indemnification and stock options, warrants and convertible securities. Subsequently, we entered into an affiliation agreement with Roche that enabled our current management to conduct our business and operations as we had done in the past while at the same time reflecting Roche’s ownership in us. The affiliation agreement is for the exclusive benefit of Roche and can be amended at any time by Roche and us. We have amended our bylaws in order to maintain certain proportional representation rights of Roche under the bylaws with respect to membership on our Board of Directors and Board committees to the extent that we do not make repurchases of our common stock as provided by the affiliation agreement.
Our Amended and Restated Certificate of Incorporation provides that the provisions in our bylaws described below under “— Composition of Board of Directors,” “— Roche’s Right to Proportional Representation,” “— Membership of Committees” and “— Nomination of Directors” may be repealed or amended only by a 60% vote of our stockholders, except for Roche’s right to nominate a number of directors proportional to Roche’s ownership interest rounded down to the next whole number until Roche’s ownership interest is less than 5%, which may be repealed or amended only by a 90% vote of our stockholders. The
2
provisions of the affiliation agreement described below under “— Roche Approval Required for Certain Actions” and “— Licensing and Marketing Arrangements” terminate upon Roche owning less than 40% of our stock.
For purposes of the following provisions, an independent director is a director who is not:
| | |
| | • | one of our officers; or |
| |
| | • | an employee, director, principal stockholder or partner of Roche or any affiliate of Roche or an entity that was dependent upon Roche for more than 10% of its revenues or earnings in its most recent fiscal year. |
| |
| | Composition of Board of Directors |
Our Board consists of six members, all of whom are nominated by the Nominations Committee of the Board: two nominees of Roche, one executive officer of Genentech and up to three independent directors. Directors are elected to serve one year terms or until their successors are elected and qualified. At all times our Board will include at least two independent directors and one executive officer of Genentech.
| |
| | Roche’s Right to Proportional Representation |
We have agreed that upon Roche’s request, Roche will be entitled to representation on our Board proportional to its ownership interest in our common stock. Roche will be entitled to have the number of Roche designated directors equal to the percentage of our common stock owned by Roche times the total number of directors, rounded up to the next whole number if Roche’s ownership interest is greater than 50% and rounded down if Roche’s ownership percentage is less than or equal to 50%. Upon Roche’s request, we will immediately take action to cause the size of our Board to be increased and to cause our Board to fill the vacancies by electing Roche nominees in order to achieve Roche’s proportional representation. If Roche’s ownership interest of common stock drops below 40%, Roche will cause its directors to resign to the extent its representation is in excess of its proportional ownership interest. The number of directors who are required to resign upon such event shall be rounded up to the next whole number. Roche shall thereafter be entitled to nominate a number of directors which is proportional to Roche’s ownership interest rounded down to the next whole number, until Roche’s ownership interest is less than 5%.
We have five standing committees of the Board: an Audit Committee, a Compensation Committee, a Corporate Governance Committee, an Executive Committee and a Nominations Committee and Roche is entitled upon request to its proportional representation on each committee. However, New York Stock Exchange rules require that no Roche director be a member of the Audit Committee. Roche’s committee members may designate another Roche director to serve as their alternates on any committee.
The Nominations Committee shall at all times have three members. At anytime that Roche owns 80% or more of the total voting power of our stock, the Nominations Committee shall include two nominees of Roche and one of the independent directors. At any time that Roche owns less than 80% of the total voting power of our stock, the Nominations Committee shall include a number of nominees of Roche that is equal to the percentage owned by Roche of the total voting power of our common stock times three, rounded up to the next whole number if Roche’s total voting power is greater than 50% and rounded down to the next whole number if Roche’s total voting power is less than or equal to 50%,provided that Roche shall at no time have more than two nominees.
The nomination of any person for director requires the approval of a majority of the members of the Nominations Committee.
3
| |
| | Roche Approval Required for Certain Actions |
Without the prior approval of the Roche directors, we have agreed not to approve:
| | |
| | • | any acquisition that would constitute a substantial portion of our business or assets; |
| |
| | • | any sale, lease, license, transfer or other disposal of all or a substantial portion of our business or assets other than in the ordinary course of our business; |
| |
| | • | any issuance of capital stock except (1) issuances of capital stock pursuant to employee incentive plans not exceeding 5% of our voting stock, (2) issuances of capital stock upon the exercise, conversion or exchange of any of our outstanding capital stock, and (3) other issuances of capital stock not exceeding 5% of our voting stock in any 24 month period; and |
| |
| | • | any repurchase or redemption of our capital stock other than redemption required by the terms of any security and purchases made at fair market value in connection with any of our deferred compensation plans. |
For purposes of the first and second bullet points in this paragraph, unless a majority of the Board of Directors have made a contrary determination in good faith, a “substantial portion of our business or assets” shall mean a portion of our business or assets accounting for 10% or more of our and our consolidated subsidiaries’ consolidated total assets, contribution to net income or revenues.
Following a request by Roche for proportional representation on the Board, until the Roche designees take office as directors we may not take any action other than in the ordinary course of business without the consent of Roche.
| |
| | Licensing and Marketing Arrangements |
We have a licensing and marketing agreement with F. Hoffmann-La Roche Ltd (“Hoffmann-La Roche”) and its affiliates granting it an option to license to use and sell products in non-U.S. markets. The major provisions of that agreement include the following:
| | |
| | • | Hoffmann-La Roche’s option expires in 2015; |
| |
| | • | Hoffmann-La Roche may exercise its option to license our products upon the occurrence of any of the following: (1) our decision to file an Investigational New Drug application, or IND, for a product, (2) completion of a Phase II trial for a product or (3) if Hoffmann-La Roche previously paid us a fee of $10 million to extend its option on a product, completion of a Phase III trial for that product; |
| |
| | • | if Hoffmann-La Roche exercises its option to license a product, it has agreed to reimburse Genentech for development costs as follows: (1) if exercise occurs at the time an IND is filed, Hoffmann-La Roche will pay 50% of development costs incurred prior to the filing and 50% of development costs subsequently incurred, (2) if exercise occurs at the completion of a Phase II trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, (3) if the exercise occurs at the completion of a Phase III trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, and $5 million of the option extension fee paid by Hoffmann-La Roche to preserve its right to exercise its option at the completion of a Phase III trial will be credited against the total development costs payable to Genentech upon the exercise of the option; |
| |
| | • | we agreed, in general, to manufacture for and supply to Hoffmann-La Roche its clinical requirements of our products at cost, and its commercial requirements at cost plus a margin of 20%; however, Hoffmann-La Roche will have the right to manufacture our products under certain circumstances; |
| |
| | • | Hoffmann-La Roche has agreed to pay, for each product for which Hoffmann-La Roche exercises its option upon either a decision to file an IND with the U.S. Food and Drug Administration, or FDA, or completion of the Phase II trials, a royalty of 12.5% on the first $100 million on its aggregate sales of |
4
| | |
| | | that product and thereafter a royalty of 15% on its aggregate sales of that product in excess of $100 million until the later in each country of the expiration of our last relevant patent or 25 years from the first commercial introduction of that product; and |
| |
| | • | Hoffmann-La Roche will pay, for each product for which Hoffmann-La Roche exercises its option after completion of the Phase III trials, a royalty of 15% on its sales of that product until the later in each country of the expiration of our relevant patent or 25 years from the first commercial introduction of that product; however, $5 million of any option extension fee paid by Hoffmann-La Roche will be credited against royalties payable to us in the first calendar year of sales by Hoffmann-La Roche in which aggregate sales of the product exceed $100 million. |
We have agreed that, upon Roche’s request, we will file one or more registration statements under the Securities Act of 1933 in order to permit Roche to offer and sell shares of our common stock. We have agreed to use our best efforts to facilitate the registration and offering of those shares designated for sale by Roche.
We have the right to postpone the filing or effectiveness of a registration statement for a period of up to 60 days in any 12-month period if:
| | |
| | • | in the reasonable good faith judgment of our Board, fulfillment of our obligations would require us to make disclosures that would be detrimental to Genentech and premature; or |
| |
| | • | we have filed a registration statement with respect to securities to be distributed in an underwritten public offering and we have been advised by the lead or managing underwriter that an offering by Roche would materially and adversely affect the distribution of our securities. |
Generally, we will pay all expenses incident to the performance of our obligations with respect to the registration of Roche’s shares of our common stock except that Roche has agreed to pay certain expenses to be directly incurred by Roche, including underwriting fees, discounts and commissions and counsel fees. In addition, we are only required to pay for two registrations within a 12-month period. We and Roche each have agreed to customary indemnification and contribution provisions with respect to liability incurred in connection with these registrations.
If Roche and its affiliates sell their majority ownership of shares of our common stock to a successor, Roche has agreed that it will cause the successor to purchase all shares of our common stock not held by Roche:
| | |
| | • | if the consideration is composed entirely of either cash or equity traded on a U.S. national securities exchange, with consideration in the same form and amounts per share as received by Roche and its affiliates; and |
| |
| | • | in any other case, with consideration either in the same form and amounts per share as received by Roche and its affiliates or with consideration that has a value per share not less than the weighted average value per share received by Roche and its affiliates as determined by an investment bank of nationally recognized standing appointed by a committee of independent directors. |
Roche has agreed to cause the buyer to agree to be bound by the obligations described in the preceding paragraph as well as the obligations described under “— Business Combinations with Roche” and “— Compulsory Acquisitions” below. We have agreed that the buyer shall be entitled to succeed to Roche’s rights described under “— Roche’s Right to Proportional Representation.”
5
| |
| | Business Combinations with Roche |
Roche has agreed that as a condition to any merger of Genentech with Roche or its affiliates or the sale of substantially all of our assets to Roche or its affiliates, that either:
| | |
| | • | the merger or sale must be authorized by the favorable vote at any meeting of a majority of the shares of common stock not owned by Roche, provided that no person or group shall be entitled to cast more than 5% of the votes cast at the meeting; or |
| |
| | • | in the event such a favorable vote is not obtained, the value of the consideration to be received by the holders of our common stock, other than Roche, shall be equal to or greater than the average of the means of the ranges of fair values for the common stock as determined by two investment banks of nationally recognized standing appointed by a committee of independent directors. |
Roche has agreed that it will not sell any shares of our common stock in the 90 days immediately preceding any proposal by Roche for a merger with us.
Roche also agreed that in the event of any merger of Genentech with Roche or its affiliates or sale of substantially all of our assets to Roche or its affiliates, each unvested option then outstanding under our stock option plan will:
| | |
| | • | be accelerated so that each option shall become exercisable immediately prior to the consummation of the transaction for the full number of shares of common stock covered by the option; |
| |
| | • | become exchangeable upon the consummation of the transaction for deferred cash compensation, which vests on the same schedule as the shares of the common stock covered by the option, having a value equal to the product of (A) the number of shares covered by the option and (B) the amount which Roche, in its reasonable judgment, considers to be equivalent in value to the consideration per share received by holders of shares of common stock other than Roche in the transaction, minus the exercise price per share under the option; or |
| |
| | • | be canceled in exchange for a replacement option to purchase stock of the surviving corporation in the transaction with the terms of the option to provide value equivalent, as determined by Roche in its reasonable discretion, to that of the canceled option. |
If Roche owns more than 90% of our common stock for more than two months, Roche has agreed to, as soon as reasonably practicable, effect a merger of Genentech with Roche or an affiliate of Roche.
The merger shall be conditioned on the vote or the valuation described under the first two bullets of “— Business Combinations with Roche” above.
Roche’s Ability to Maintain its Percentage Ownership Interest in Our Stock
The affiliation agreement provides, among other things, that we will establish a stock repurchase program designed to maintain Roche’s percentage ownership interest in our common stock. The affiliation agreement provides that we will repurchase a sufficient number of shares pursuant to this program such that, with respect to any issuance of common stock by us in the future, the percentage of our common stock owned by Roche immediately after such issuance will be no lower than Roche’s lowest percentage ownership of our common stock at any time after the offering of common stock occurring in July 1999 but prior to the time of such issuance, except that we may issue shares up to an amount that would cause Roche’s lowest percentage ownership to be decreased up to 2% below the “Minimum Percentage.” The Minimum Percentage equals a fraction (expressed as a percentage) where the numerator is the lowest number of shares of our common stock owned by Roche since the July 1999 offering (to be adjusted in the future for dispositions of shares of our common stock by Roche), and the denominator is 509,194,352 which is the number of shares of our common stock outstanding at the time of the July 1999 offering adjusted for the two-for-one splits of our common stock in November 1999 and October 2000. Each of the numerator and denominator are to be adjusted in the future
6
for stock splits or stock combinations. As long as Roche’s percentage ownership is greater than 50%, prior to issuing any shares, the affiliation agreement provides that we will repurchase a sufficient number of shares of our common stock such that, immediately after its issuance of shares, Roche’s percentage ownership will be greater than 50%. The affiliation agreement also provides that, upon Roche’s request, we will repurchase shares of our common stock to increase Roche’s ownership to the Minimum Percentage. Roche currently owns approximately 58% of our common stock. The obligations of this stock repurchase program terminate upon Roche owning less than 40% of our stock.
Furthermore, Roche has (i) a continuing option (which is assignable by Roche to any of its affiliates) to buy from us, prior to the occurrence of any event that could result in a decrease in the percentage of common stock owned by Roche and its affiliates, a sufficient amount of common stock to ensure that Roche and its affiliates maintain the percentage ownership of our common stock owned by them, and (ii) a continuing option (which is assignable by Roche to any of its affiliates) to buy from us 80% of any class of stock issued by us other than common stock, in each case with a price per share equal to either the average of the last sale price on each of the five immediately preceding trading days on a U.S. national securities exchange on which the shares are traded or, if the sale prices are unavailable, the value of the shares determined in accordance with procedures reasonably satisfactory to Roche and us.
Tax Sharing Agreement
From the redemption of our special common stock in June 1999 until Roche completed its public offering of our common stock in October 1999, we were included in Roche’s U.S. consolidated federal income tax group and included with Roche and/or one or more Roche subsidiaries in consolidated or combined income tax groups for certain state and local tax jurisdictions. Accordingly, we entered into a tax sharing agreement with Roche. Pursuant to the tax sharing agreement, we and Roche are to make payments such that the net amount paid by us on account of consolidated or combined income taxes is determined as if we had filed separate, stand-alone federal, state and local income tax returns as the common parent of an affiliated group of corporations filing consolidated or combined federal, state and local returns.
Effective upon the consummation of the public offering in October 1999, we ceased to be a member of the consolidated federal income tax group (and certain consolidated or combined state or local income tax groups) of which Roche is the common parent. Accordingly, our tax sharing agreement with Roche now pertains only to the state and local tax returns in which we will be consolidated or combined with Roche. We will continue to calculate our tax liability or refund with Roche for these state and local jurisdictions as if we were a stand-alone entity.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
The table below shows, as of December 31, 2001, information relating to stockholders known by us to be the beneficial owners of more than five percent of any class of Genentech’s voting securities, based on Securities and Exchange Commission filings made pursuant to Section 13(d) and Section 13(g) of the Securities Exchange Act of 1934:
| | | | | | | | | | | | | | |
| | Number | | | | Percent |
| Name and Address of Beneficial Owner | | of Shares | | Class | | of Class |
| |
| |
| |
|
| Roche Holdings, Inc. | | | 306,594,352 | | | | Common Stock | | | | 58.03 | % |
| | One Commerce Center, Suite 1050 | | | | | | | | | | | | |
| | 1201 N. Orange Street | | | | | | | | | | | | |
| | Wilmington, DE 19801 | | | | | | | | | | | | |
7
PROPOSAL 1 — ELECTION OF DIRECTORS
NOMINEES FOR DIRECTOR
The Company’s Board currently consists of two Roche directors, Franz B. Humer and Jonathan K.C. Knowles, three independent directors, Herbert W. Boyer, Charles A. Sanders and Sir Mark Richmond, and one Genentech employee, Arthur D. Levinson, who is also the chairman of the Board. The Board has proposed the election of each of these individuals at the Annual Meeting for a one year term expiring on the date of the Annual Meeting in 2003 or until a director’s successor has been elected or appointed. The persons named in the enclosed Proxy Card intend to vote the proxy for the election of each of the six nominees unless you indicate otherwise.
We expect each nominee for election as a director to be able to serve if elected. If any nominee is not able to serve, proxies will be voted in favor of the remainder of those nominated and may be voted for any other person the Board of Directors may select.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS
A VOTE FOR EACH NOMINEE
The table below shows the name and age (as of the date of the Annual Meeting) of each of the directors, their current principal occupations, any positions and offices held by each with Genentech in addition to their position as a director, and the period during which each has served as a director of Genentech.
| | | | | | | | | | | |
| | | | | | Served as |
| Name | | Age | | Principal Occupation/Position Held | | Director Since |
| |
| |
| |
|
| Herbert W. Boyer, Ph.D. | | | 65 | | | Director of Genentech | | | 1976 | |
| Franz B. Humer | | | 55 | | | Chairman and Chief Executive Officer, The Roche Group | | | 1995 | |
| Jonathan K.C. Knowles, Ph.D. | | | 54 | | | Head of Global Pharmaceutical Research, The Roche Group | | | 1998 | |
| Arthur D. Levinson, Ph.D. | | | 52 | | | Chairman, President and Chief Executive Officer of Genentech | | | 1995 | |
| Sir Mark Richmond, Ph.D. | | | 71 | | | Director of Genentech | | | 1999 | |
| Charles A. Sanders, M.D. | | | 70 | | | Director of Genentech | | | 1999 | |
Dr. Boyer, a founder of Genentech, had been a director of Genentech since 1976 when he resigned from the Board in June 1999 in connection with the redemption of our special common stock. He was reelected to the Board in September 1999. Dr. Boyer is a consultant to Genentech. He served as a Vice President of Genentech from 1976 to 1991. Dr. Boyer, a Professor of Biochemistry at the University of California at San Francisco from 1976 to 1991, demonstrated the usefulness of recombinant DNA technology to produce medicines economically, which laid the groundwork for Genentech’s development. Dr. Boyer has received numerous awards for his research, including the National Medal of Science from President Bush in 1990, the National Medal of Technology in 1989 and the Albert Lasker Basic Medical Research Award in 1980. He is an elected member of the National Academy of Sciences and a Fellow in the American Academy of Arts and Sciences. In 2001, Dr. Boyer was elected to the National Inventors Hall of Fame. In addition, Dr. Boyer serves as Chairman of the Board of Directors of Allergan, Inc. and a member of the Boards of Directors of IDEC Pharmaceuticals Corporation and Sangamo BioSciences, Inc.
Dr. Humer was elected a director of Genentech in the spring of 1995. He joined The Roche Group in 1995 as the Head of its Pharmaceuticals Division, and became Chief Executive Officer of The Roche Group in 1998 and Chairman of the Board of Directors of The Roche Group in April 2001. Prior to joining The Roche Group, Dr. Humer was an Executive Director and Chief Operating Officer of Glaxo Holdings, a United Kingdom public limited company. Pursuant to the affiliation agreement, Dr. Humer is a designee of Roche.
Dr. Knowles was elected a director of Genentech in February 1998. He joined The Roche Group as Head of Global Pharmaceutical Research in September 1997. In January 1998, he became a member of the
8
Executive Committee of The Roche Group. Prior to joining The Roche Group, Dr. Knowles served as the Director of Research for Europe of Glaxo from 1995 to 1997 and served as the Director of the Geneva Institute of Glaxo from 1989 to 1995. Pursuant to the affiliation agreement, Dr. Knowles is a designee of Roche.
Dr. Levinson was appointed Chairman of the Board of Directors in September 1999 and was elected President and Chief Executive Officer and a director of the Company in July 1995. Since joining the Company in 1980, Dr. Levinson has been a Senior Scientist, Staff Scientist and the Director of the Company’s Cell Genetics Department. Dr. Levinson was appointed Vice President of Research Technology in April 1989, Vice President of Research in May 1990 and Senior Vice President in January 1993. Dr. Levinson was formerly on the editorial boards of “Molecular Biology and Medicine” and “Molecular and Cellular Biology,” and is active in the American Society of Microbiology, the New York Academy of Sciences, the American Association for the Advancement of Science, and the American Society for Biochemistry and Molecular Biology. From 1977 to 1980, Dr. Levinson was a Postdoctoral Fellow in the Department of Microbiology at the University of California, San Francisco. In 1977, Dr. Levinson received his Ph.D. in Biochemistry from Princeton University. Dr. Levinson also serves as a member of the Board of Directors of Apple Computer, Inc.
Dr. Richmond was elected a director of Genentech in August 1999. He was a senior research fellow at the School of Public Policy, University College London from February 1996 to February 2002. Previously, he held positions as science advisor at Glaxo Wellcome plc from 1995 to February 1996, as Group Head of Research at Glaxo plc from 1993 to 1995, as chairman of the Science and Engineering Council, London, from 1990 to 1993, as vice chancellor at the University of Manchester from 1981 to 1990, and professor and head of the Department of Bacteriology at the University of Bristol from 1968 to 1981. Dr. Richmond is currently a member of the Scientific Advisory Committee of the Institute for Biotechnology, ETH, Zurich, a member of the Scientific Advisory Board of the SPP-Biotechnology, Swiss National Foundation and a member of the Boards of Directors of Targeted Genetics Corporation and OSI Pharmaceuticals, Inc.
Dr. Sanders was elected a director of Genentech in August 1999. He served as Chief Executive Officer of Glaxo Inc. from 1989 to 1994, and was the Chairman of the Board of Glaxo Inc. from 1992 to 1995. He also has served on the Board of Directors of Glaxo plc. Previously, he held a number of positions at Squibb Corporation, a multinational pharmaceutical corporation, including Vice Chairman, Chief Executive Officer of the Science and Technology Group and Chairman of the Science and Technology Committee on the Board. Dr. Sanders is a member of the Boards of Directors of Scios Inc., Genaera Corporation, Vertex Pharmaceuticals, Edgewater, Inc., Trimeris, Inc., Biopure Corporation, Pharmacopeia, Inc. and Cephalon, Inc.
COMMITTEES AND MEETINGS
During 2001, the Board of Directors held four meetings, the Audit Committee held seven meetings, the Compensation Committee held four meetings, the Corporate Governance Committee held three meetings, the Executive Committee did not meet and the Nominations Committee held one meeting. All directors who served on the Board throughout the year attended at least 75% of the aggregate number of meetings of the Board and of the committees on which the directors serve. None of the members of the Audit, Compensation, Corporate Governance or Nominations Committees were an officer or employee of the Company. We summarize below the functions of the various committees and identify the directors who served on each committee.
9
| | | |
| Name of Committee | | |
| and Members | | Functions of the Committee |
| |
|
Audit Committee
Herbert W. Boyer
Mark Richmond
Charles A. Sanders | | • Recommends the independent auditors to the Board
• Meets with the Company’s independent auditors to review and discuss the Company’s financial statements, quarterly reporting process, inventory and receivable reserve policies, tax compliance and strategy, investment policy and risk management programs, and scope of audit activities
• Reviews the reports of the independent auditors and accompanying management letter on the scope and results of their work
• Reviews the independent auditors’ recommendations concerning the Company’s financial practices and procedures |
| |
Compensation Committee
Herbert W. Boyer
Franz B. Humer
Jonathan K.C. Knowles
Mark Richmond
Charles A. Sanders | | • Administers the Company’s stock option plans, the stock purchase plan and other corporate benefits programs
• Reviews and approves the Company’s annual bonus plan, annual stock option grants, corporate benefits strategy, compensation philosophy and executive officer compensation
• Recommends to the Board candidates for election as executive officers of the Company |
| |
Corporate Governance
Committee
Jonathan K.C. Knowles
Mark Richmond | | • Reviews the Company’s policies relating to sales and marketing activities, investor relations, corporate communications, government relations, human resources, legal and regulatory affairs, compliance with laws and regulations, and Board of Directors and Board committee effectiveness |
| |
Executive Committee
Herbert W. Boyer
Franz B. Humer
Arthur D. Levinson | | • Established to act when the full Board of Directors is unavailable
• Has the authority of the Board in the management of the business and affairs of the Company, except those powers that cannot be delegated by the Board of Directors by law |
| |
Nominations Committee
Herbert W. Boyer
Franz B. Humer
Jonathan K.C. Knowles | | • Identifies, reviews and recommends potential nominees to the Board of Directors
• Recommends to the Board candidates for election as executive officers of the Company |
PROPOSAL 2 — APPROVAL OF AMENDED AND RESTATED
1999 STOCK PLAN FOR CERTAIN CIRCUMSTANCES
We are asking our stockholders to approve the amended and restated 1999 Stock Plan (the “Plan”), under which a total of 63,000,000 shares of common stock have been reserved for issuance, so that the Company can receive a federal income tax deduction under the Plan in connection with options exercised by the Company’s five most highly compensated executive officers after stockholder approval. If the stockholders approve the amended and restated Plan, it will permit the Company to receive a federal income tax deduction for compensation paid under the Plan when those options are exercised by those individuals. Our Board of Directors previously approved the Plan. The affirmative vote of holders of a majority of the shares present in person or by proxy at the Annual Meeting is required for the approval of this proposal. Our five most highly compensated executive officers and directors have an interest in this proposal.
As of December 31, 2001, approximately 43,377,723 shares were subject to outstanding awards granted under the Plan, and approximately 14,500,000 shares remained available for any new awards to be granted in the future. The Board of Directors believes that the approval of the Plan solely for the purpose stated above provides the Company with an important benefit of being eligible to receive a federal income tax deduction in connection with options exercised by the Company’s five most highly compensated executive officers.
10
Description of the Amended and Restated 1999 Stock Plan
The following paragraphs provide a summary of the principal features of the Plan and its operation. The Plan is attached as Appendix A to this Proxy Statement, and the following summary is qualified in its entirety by reference to Appendix A.
The Plan permits the grant of stock options and stock purchase rights (each, an “Award”). The Plan is intended to increase incentives and to encourage share ownership on the part of eligible employees, non-employee directors and consultants who provide significant services to us. The Plan also is intended to further our growth and profitability.
| |
| | Administration of the Plan |
The Plan is administered by the Compensation Committee of our Board of Directors (the “Committee”). Subject to the terms of the Plan, the Committee has the sole discretion to select the employees, non-employee directors and consultants who will receive Awards, determine the terms and conditions of Awards (for example, the exercise price and vesting schedule), and interpret the provisions of the Plan and outstanding Awards. The Committee may delegate any part of its authority and powers under the Plan to one or more directors and/or officers of the Company.
If an Award expires or is cancelled without having been fully exercised or vested, the unvested or cancelled Shares generally will be returned to the available pool of Shares reserved for issuance under the Plan. Also, if we experience a stock dividend, reorganization or other change in our capital structure, our Board of Directors has discretion to adjust the number of Shares available for issuance under the Plan, the outstanding Awards, and the per-person limits on options, all as appropriate to reflect the stock dividend or other change.
| |
| Eligibility to Receive Awards |
The Committee selects the employees, non-employee directors and consultants who will be granted Awards under the Plan. The actual number of individuals who will receive an Award under the Plan cannot be determined in advance because the Committee has discretion to select the participants.
A stock option is the right to acquire shares of the Company’s common stock (“Shares”) at a fixed exercise price for a fixed period of time. Under the Plan, the Committee may grant nonqualified stock options and/or incentive stock options. The number of Shares covered by each option will be determined by the Committee, but during any fiscal year of the Company, no participant may be granted options covering more than 2,000,000 Shares or 3,000,000 Shares in the year of first hire. The exercise price of the Shares subject to each option is set by the Committee but for incentive stock options and options intended to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code, the exercise price cannot be less than 100% of the fair market value (on the date of grant) of the Shares covered by the option. An exception may be made for any options that the Company grants in substitution for options held by employees of companies that the Company acquires (in which case the exercise price preserves the economic value of the employee’s cancelled option from his or her former employer). The aggregate fair market value of the Shares (determined on the grant date) covered by incentive stock options which first become exercisable by any participant during any calendar year also may not exceed $100,000.
An option granted under the Plan cannot be exercised until it becomes vested unless otherwise approved by the Company. The Committee establishes the vesting schedule of each option at the time of grant. Options granted under the Plan expire at the times established by the Committee, but in no event later than 10 years after the grant date.
The exercise price of each option granted under the Plan must be paid in full in cash at the time of exercise. The Committee also may permit payment through the tender of Shares that are already owned by
11
the participant, or by any other means that the Committee determines to be consistent with the purpose of the Plan. Any taxes required to be withheld must be paid by the participant at the time of exercise.
Stock purchase rights generally are the same as options. Stock purchase rights may be granted alone or in combination with an option. Each right allows the participant to purchase shares at a price determined by the Committee. Shares purchased may be subject to a vesting schedule or right of repurchase by the Company, all as determined by the Committee.
| |
| Awards to be Granted to Certain Individuals and Groups |
The number of Awards that an employee, non-employee director or consultant may receive under the Plan is in the discretion of the Committee and therefore cannot be determined in advance. To date, only stock options have been granted under the Plan. The table below shows the aggregate number of Shares subject to options granted under the Plan during the last fiscal year and the average per Share exercise price of such options as follows:
| | | | | | | | | |
| | Number of | | Average Per Share |
| Name of Individual or Group | | Options Granted | | Exercise Price |
| |
| |
|
| Arthur D. Levinson | | | 360,000 | | | $ | 41.80 | |
| Myrtle S. Potter | | | 225,000 | (1) | | $ | 43.70 | (1) |
| Susan D. Desmond-Hellmann | | | 225,000 | | | $ | 41.80 | |
| Louis J. Lavigne, Jr | | | 160,000 | | | $ | 41.80 | |
| Stephen G. Juelsgaard | | | 160,000 | | | $ | 41.80 | |
| All executive officers, as a group | | | 2,256,600 | | | $ | 42.53 | |
| All directors who are not executive officers, as a group | | | 30,000 | | | $ | 52.50 | |
| All employees who are not executive officers, as a group | | | 8,454,089 | | | $ | 42.56 | |
| |
| (1) | 150,000 of the options have an exercise price of $41.80 and 75,000 of the options have an exercise price of $47.50. The exercise price of $43.70 in theAverage Per Share Exercise Price column above was calculated as a weighted average. |
| |
| Limited Transferability of Awards |
Awards granted under the Plan generally may not be sold, transferred, pledged, assigned, or otherwise alienated or hypothecated, other than by will or by the applicable laws of descent and distribution, unless the Committee determines otherwise.
| |
| Amendment and Termination of the Plan |
The Board generally may amend or terminate the Plan at any time and for any reason.
Federal Tax Aspects
The following paragraphs are a summary of the general federal income tax consequences to U.S. taxpayers and the Company of Awards granted under the Plan. Tax consequences for any particular individual may be different.
| |
| Nonqualified Stock Options |
No taxable income is reportable when a nonqualified stock option is granted to a participant. Upon exercise, the participant will recognize ordinary income in an amount equal to the excess of the fair market value (on the exercise date) of the Shares purchased over the exercise price of the option. Any additional gain or loss recognized upon any later disposition of the Shares will be capital gain or loss.
12
Taxable income is not generally recognized on the grant or exercise of an incentive stock option (unless the alternative minimum tax rules apply). The difference between the sale price and the option price on the sale of Shares exercised and held for a period of at least two years after the grant date and one year after the issuance date will be long-term capital gain or loss. If the participant exercises the option and then later sells or otherwise disposes of the Shares before the end of the two or one-year holding periods described above, then the participant will generally have ordinary income at the time of the sale equal to the fair market value of the Shares on the exercise date (or the sale price, if less) minus the exercise price of the option.
Stock purchase rights generally are taxed in the same manner as nonqualified stock options. However, when a stock purchase right is exercised, the participant typically receives “restricted” stock (that is, stock that is subject to a vesting schedule). At the time of purchase, restricted stock is subject to a “substantial risk of forfeiture” within the meaning of Section 83 of the Internal Revenue Code. As a result, the participant will not recognize ordinary income at the time of purchase. Instead, the participant will recognize ordinary income on the dates when the stock ceases to be subject to a substantial risk of forfeiture. The stock typically will cease to be subject to a substantial risk of forfeiture when it is no longer subject to the Company’s right to repurchase the stock upon the purchaser’s termination of employment with the Company. The participant will recognize ordinary income measured as the difference between the purchase price and the fair market value of the stock on the date the stock is no longer subject to a substantial risk of forfeiture. The participant may accelerate to the date of purchase his or her recognition of ordinary income, if any, and the beginning of any capital gain holding period by timely filing an election pursuant to Section 83(b) of the Internal Revenue Code.
Tax Effect for the Company
The Company generally will be entitled to a tax deduction in connection with an Award under the Plan in an amount equal to the ordinary income realized by a participant and at the time the participant recognizes such income (for example, the exercise of a nonqualified stock option). Special rules limit the deductibility of compensation paid to our Chief Executive Officer and to each of our four most highly compensated executive officers. Under Section 162(m) of the Internal Revenue Code, the annual compensation paid to any of these specified executives will be deductible only to the extent that it does not exceed $1 million. However, the Company can preserve the deductibility of certain compensation in excess of $1 million if the conditions of Section 162(m) are met. These conditions include stockholder approval of the Plan, setting limits on the number of Awards that any individual may receive and for Awards other than stock options, establishing performance criteria that must be met before the Award actually will vest or be paid. The Plan has been designed to permit the Committee to grant options that qualify as performance-based for purposes of satisfying the conditions of Section 162(m), thereby permitting the Company to continue to receive a federal income tax deduction in connection with the exercise of those options.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS
A VOTE FOR APPROVAL OF PROPOSAL 2
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act requires the Company’s directors, officers and persons owning more than 10% of any class of the Company’s equity securities (collectively, “Reporting Persons”) to file reports of holdings and transactions in the Company’s equity securities with the Securities and Exchange Commission and the New York Stock Exchange. The Reporting Persons are required to furnish the Company with copies of all Section 16(a) reports they file. We believe that all Forms 3, 4 and 5 required to be filed by its Reporting Persons were filed on time during 2001.
13
SECURITY OWNERSHIP OF MANAGEMENT
The table below shows the beneficial ownership of shares of common stock of the Company as of December 31, 2001, unless otherwise noted, held by (i) each of Genentech’s directors, (ii) Genentech’s Chief Executive Officer, (iii) each of Genentech’s four other most highly compensated executive officers (together with Genentech’s Chief Executive Officer, the “Named Executive Officers”), and (iv) all of Genentech’s directors and executive officers as a group. Unless otherwise indicated, each person has sole voting and investment power, other than the powers that may be shared with the person’s spouse under applicable law.
AMOUNT AND NATURE OF BENEFICIAL OWNERSHIP
| | | | | | | | | |
| | |
| | Genentech Common Stock |
| |
|
| | | | Percent of |
| Name of Beneficial Owner | | Shares | | Class |
| |
| |
|
| Herbert W. Boyer | | | 30,050(1 | ) | | | * | |
| Susan D. Desmond-Hellmann | | | 733,091(2 | ) | | | * | |
| Franz B. Humer | | | 0(3 | ) | | | * | |
| Stephen G. Juelsgaard | | | 505,776(4 | ) | | | * | |
| Jonathan K. C. Knowles | | | 0(3 | ) | | | * | |
| Louis J. Lavigne, Jr. | | | 691,262(5 | ) | | | * | |
| Arthur D. Levinson | | | 1,793,992(6 | ) | | | * | |
| Myrtle S. Potter | | | 88,211(7 | ) | | | * | |
| Sir Mark Richmond | | | 20,500(8 | ) | | | * | |
| Charles A. Sanders | | | 21,500(9 | ) | | | * | |
| All Directors and Executive Officers as a Group (33 persons) | | | 5,886,119(10 | ) | | | 1.11 | % |
Asterisk (*) indicates that the amount beneficially owned is less than one percent (1%) of the outstanding shares of common stock of the Company.
| |
| (1) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 9,250 shares of common stock. |
| |
| (2) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 732,048 shares of common stock. |
| |
| (3) | As of December 31, 2001, Roche owned 306,594,352 shares of common stock, representing 58.03% of the class. Pursuant to the affiliation agreement, Roche has appointed Drs. Humer and Knowles as its representatives on Genentech’s Board of Directors. |
| |
| (4) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 504,334 shares of common stock. |
| |
| (5) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 689,820 shares of common stock. |
| |
| (6) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 1,791,899 shares of common stock. |
| |
| (7) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 87,500 shares of common stock. |
| |
| (8) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 20,500 shares of common stock. |
| |
| (9) | Includes stock options exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 20,500 shares of common stock. |
| |
| (10) | Includes stock options held by 23 other current executive officers, exercisable on December 31, 2001 or exercisable by March 1, 2002 to purchase 1,968,027 shares of common stock. This amount excludes the shares of an officer who resigned from the Company in January 2002 and includes the shares of an individual who was appointed an officer in February 2002. |
14
EXECUTIVE COMPENSATION
COMPENSATION OF DIRECTORS
Each of the non-employee directors of Genentech is paid an annual retainer fee of $30,000. In addition, each of the non-employee directors receives a total of $1,500 for each Board meeting at which the director was present in person and a total of $500 for each Board meeting at which the director was present by telephone. As an employee of Genentech, Dr. Levinson is not paid for his services as a director. All directors are reimbursed for expenses incurred in connection with their service on the Board. Dr. Boyer also serves as a consultant to Genentech and received compensation in the amount of $20,000 for his services as a consultant in 2001.
Under the Plan, each non-Roche and non-employee Board member (an “Independent Director”) is granted a stock option to purchase 25,000 shares of common stock upon first election to the Board. These options vest over four years, with the first 25% vesting one year from the grant date, and the remainder vesting monthly in equal increments during the 36-month period following the initial vesting date. In addition, each Independent Director is granted a stock option to purchase 10,000 shares of common stock of the Company upon a reelection to the Board. These options vest on a monthly basis in equal increments over a period of twelve months. The exercise price of any option granted to an Independent Director will be equal to the closing price of the common stock as reported in theWall Street Journalon the day of election or reelection, as applicable. Drs. Boyer, Richmond and Sanders, as Independent Directors, each received an option to purchase 10,000 shares of common stock upon reelection to the Board in 2001.
COMPENSATION OF EXECUTIVE OFFICERS
SUMMARY OF COMPENSATION
The table below shows the compensation paid by Genentech to the Named Executive Officers, including salary, bonuses, stock options and certain other compensation for the fiscal years ended December 31, 2001, 2000 and 1999:
SUMMARY COMPENSATION TABLE
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | Long Term | | |
| | | | Annual Compensation | | Compensation | | |
| | | |
| | Awards/Securities | | All Other |
| | | | | | Other Annual | | Underlying | | Compensation |
| Name and Principal Position | | Year | | Salary(1) | | Bonus | | Compensation (4) | | Options(7)(#) | | (9) |
| |
| |
| |
| |
| |
| |
|
| | | | | | | | | | | | | | |
| Arthur D. Levinson, Ph.D. | | | 2001 | | | $ | 780,000 | | | $ | 985,000 | | | | — | | | | 360,000 | | | | — | | | $ | 70,600 | |
| | Chairman, President and Chief | | | 2001 | | | | — | | | $ | 150,000 | (2) | | | — | | | | — | | | | — | | | | — | |
| | Executive Officer | | | 2000 | | | $ | 755,010 | | | $ | 985,000 | | | | — | | | | 360,000 | | | | — | | | $ | 69,400 | |
| | | | 1999 | | | $ | 750,000 | | | $ | 985,000 | | | | — | | | | 1,932,852 | | | | (1,450,000 | )(8) | | $ | 68,000 | |
| |
| Myrtle S. Potter(10) | | | 2001 | | | $ | 520,256 | | | $ | 425,000 | | | $ | 1,207,608 | (5) | | | 225,000 | | | | — | | | $ | 30,800 | |
| | Executive Vice President, | | | 2000 | | | $ | 315,169 | | | $ | 500,000 | (3) | | $ | 789,284 | (6) | | | 300,000 | | | | — | | | $ | 10,000 | |
| | Commercial Operations and | | | 1999 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | Chief Operating Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Susan D. Desmond-Hellmann, M.D., M.P.H | | | 2001 | | | $ | 500,491 | | | $ | 425,000 | | | | — | | | | 225,000 | | | | — | | | $ | 37,000 | |
| | Executive Vice President, | | | 2000 | | | $ | 452,918 | | | $ | 425,000 | | | | — | | | | 250,000 | | | | — | | | $ | 34,000 | |
| | Development and Product | | | 1999 | | | $ | 375,208 | | | $ | 400,000 | | | | — | | | | 933,104 | | | | (700,000 | )(8) | | $ | 27,400 | |
| | Operations and Chief Medical | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Louis J. Lavigne, Jr. | | | 2001 | | | $ | 379,282 | | | $ | 310,000 | | | | — | | | | 160,000 | | | | — | | | $ | 27,560 | |
| | Executive Vice President and | | | 2000 | | | $ | 372,709 | | | $ | 310,000 | | | | — | | | | 160,000 | | | | — | | | $ | 27,100 | |
| | Chief Financial Officer | | | 1999 | | | $ | 367,500 | | | $ | 310,000 | | | | — | | | | 839,792 | | | | (630,000 | )(8) | | $ | 27,100 | |
| |
| Stephen G. Juelsgaard | | | 2001 | | | $ | 322,400 | | | $ | 225,000 | | | | — | | | | 160,000 | | | | — | | | $ | 21,896 | |
| | Senior Vice President, General | | | 2000 | | | $ | 314,528 | | | $ | 225,000 | | | | — | | | | 160,000 | | | | — | | | $ | 21,400 | |
| | Counsel and Secretary | | | 1999 | | | $ | 295,000 | | | $ | 239,939 | | | | — | | | | 519,872 | | | | (390,000 | )(8) | | $ | 20,000 | |
| |
| (1) | Includes amounts earned but deferred at the election of the executive, such as salary deferrals under Genentech’s Tax Reduction Investment Plan (the “401(k) Plan”) established under Section 401(k) of the Internal Revenue Code. |
15
| |
| (2) | In addition to his regular bonus of $985,000, Dr. Levinson was granted an additional bonus of $150,000 to reflect both the significant success and achievements of Genentech in 2001 and the fact that Dr. Levinson’s bonus in 2000 was, at his request, the same as that awarded in 1999 even though the Company’s performance was significantly better in 2000 than in 1999, and that bonuses of Chief Executive Officers in comparable companies increased substantially in the same period. |
| |
| (3) | The amount disclosed for Ms. Potter includes payment by Genentech of a sign-on hiring bonus of $250,000. |
| |
| (4) | Under the rules promulgated by the Securities and Exchange Commission, the payment of “perquisites” (such as imputed interest on loans at below market value rates) to a Named Executive Officer is not required to be disclosed where such amounts paid do not exceed the lesser of (i) 10% of the sum of the amounts of Salary and Bonus for the Named Executive Officer, or (ii) $50,000. Other than the payment of “perquisites” to Ms. Potter as described in footnotes (5) and (6) below, no other Named Executive Officer received the payment of any “perquisite” from the Company. |
| |
| (5) | The amount disclosed for Ms. Potter for 2001 includes payment by Genentech of (a) $371,325 for the value of options to purchase stock of Bristol-Myers Squibb surrendered by Ms. Potter upon her resignation of employment with Bristol-Myers Squibb, (b) a relocation benefit valued at $67,789 (including a gross-up payment of $34,132 included therein to reimburse Ms. Potter for the estimated income taxes attributable to the payment of the relocation benefit), (c) an adjustment in the amount of $171,426 for taxes payable by Ms. Potter for the relocation benefit paid by Genentech in 2000 (such adjustment amount includes a gross-up payment of $59,913 to reimburse Ms. Potter for the estimated income taxes attributable to the adjustment), (d) imputed interest of $194,248 on Ms. Potter’s relocation home loan (including a gross-up payment of $59,354 included therein to reimburse Ms. Potter for the estimated income taxes attributable to the imputed interest), and (e) compensation in the amount of $402,820 (including forgiveness of $200,000 on Ms. Potter’s relocation home loan and a gross-up payment of $202,820 to reimburse Ms. Potter for the estimated income taxes attributable to the loan forgiveness). |
| |
| (6) | The amount disclosed for Ms. Potter for 2000 includes payment by Genentech of (a) a relocation benefit valued at $424,553 (including a gross-up payment of $147,885 included therein to reimburse Ms. Potter for the estimated income taxes attributable to the relocation benefit), (b) imputed interest of $71,610 on Ms. Potter’s relocation home loan, (c) a gross-up payment of $33,008 to reimburse Ms. Potter for the estimated income taxes attributable to the imputed interest on the forgivable portion of the home loan, and (d) a gross-up payment of $260,113 to reimburse Ms. Potter for the estimated income taxes attributable to the sign-on bonus disclosed in footnote (3) above. |
| |
| (7) | Genentech has awarded no stock appreciation rights (“SARs”). |
| |
| (8) | The options granted in 1999 were replacements for the unvested portion of options granted in prior years that were canceled in connection with Genentech’s redemption of its special common stock in June of 1999. In replacement of these canceled unvested options, Genentech granted replacement options in connection with Roche’s public offering of Genentech common stock in July of 1999. These replacement options were granted at a higher exercise price and for a longer vesting period than the canceled unvested options they replaced. The number of replacement options granted equaled the number of canceled options multiplied by 1.333. No new options other than these replacement options were granted to the Named Executive Officers in 1999. For each Named Executive Officer, the first number shown is the number of replacement options granted in 1999 and the second number shown in parenthesis is the portion of unvested options granted in prior years that were canceled prior to the time of the replacement grant. |
| |
| (9) | Consists of Genentech’s matching payments under its 401(k) Plan and Supplemental Plan for 2001, 2000 and 1999. Each of the Named Executive Officers received $6,800 in matching payments under the 401(k) Plan for 2001 and each of the Named Executive Officers other than Ms. Potter received $6,800 in matching payments under the 401(k) Plan for 2000 and $6,400 in matching payments under the 401(k) Plan for 1999. Under the Supplemental Plan, Dr. Levinson, Dr. Desmond-Hellmann, Mr. Lavigne, Ms. Potter and Mr. Juelsgaard received matching payments of $63,800, $30,200, $20,760, $24,000 and $15,096, respectively, for 2001 and $62,600, $27,200, $20,300, $10,000 and $14,600, respectively, for 2000. Dr. Levinson, Dr. Desmond-Hellmann and Messrs. Lavigne and Juelsgaard received matching payments of $61,600, $21,000, $20,700 and $13,600, respectively, for 1999. |
| |
| (10) | Ms. Potter began her employment with Genentech effective May 15, 2000. |
16
STOCK OPTION GRANTS AND EXERCISES
OPTION GRANTS IN LAST FISCAL YEAR
| | | | | | | | | | | | | | | | | | | | | |
| | |
| | Individual Grants |
| |
|
| | | | Percent of | | |
| | | | Total Options | | | | Grant Date |
| | Number of Securities | | Granted to | | Exercise or | | | | Present |
| | Underlying Options | | Employees in | | Base Price | | Expiration | | Value(5) |
| Name | | Granted (#)(1) | | Fiscal Year(2) | | ($/SH)(3) | | Date(4) | | (in millions) |
| |
| |
| |
| |
| |
|
| Arthur D. Levinson | | | 360,000 | | | | 3.36 | % | | $ | 41.80 | | | | 09/26/11 | | | $ | 8.5 | |
| Myrtle S. Potter | | | 225,000 | (6) | | | 2.10 | % | | $ | 43.70 | (6) | | | (6) | | | $ | 5.6 | (6) |
| Susan D. Desmond-Hellmann | | | 225,000 | | | | 2.10 | % | | $ | 41.80 | | | | 09/26/11 | | | $ | 5.3 | |
| Louis J. Lavigne, Jr. | | | 160,000 | | | | 1.49 | % | | $ | 41.80 | | | | 09/26/11 | | | $ | 3.8 | |
| Stephen G. Juelsgaard | | | 160,000 | | | | 1.49 | % | | $ | 41.80 | | | | 09/26/11 | | | $ | 3.8 | |
| |
| (1) | The options were granted pursuant to the Plan and vest over four years, with the first 25% vesting one year from the grant date, and the remainder vesting monthly during the 36-month period following the initial vesting date, unless otherwise indicated. |
| |
| (2) | Based on a total of approximately 11 million options granted in 2001 under our Plan to employees, including the Named Executive Officers. Approximately 89% of these options were granted to more than 4,600 employees, other than the Named Executive Officers, representing more than 95% of the eligible employee population. |
| |
| (3) | The exercise price of options granted represented the fair market value of the underlying shares of common stock as based on the closing price of the Company’s common stock on the date of grant. |
| |
| (4) | These options have a term of ten years subject to earlier termination upon the occurrence of certain events related to termination of employment with the Company. |
| |
| (5) | Option value was determined using the Black-Scholes option pricing model based on the following assumptions: expected volatility of 63% based on historical volatility for the year and implied volatility from traded options and a risk free rate of 3.99% for the vesting term of the option. Each option is valued at its exercise price, which is assumed to be equivalent to the market price at the date of grant. This valuation model was not adjusted for the vesting restrictions or the risk of forfeiture of the options. Under SFAS 123, forfeitures may be estimated or assumed to be zero; in this model, the forfeiture rate was assumed to be zero. Our use of this model in accordance with rules adopted by the Securities and Exchange Commission does not constitute an endorsement of the model nor an acknowledgment that such model can accurately determine the value of options. The valuation calculations do not necessarily represent the fair market value of individual options, and are not intended to forecast possible future appreciation, if any, of the price of our common stock on the date of exercise as compared to the exercise price of the option. |
| |
| (6) | All of these options were granted pursuant to the Plan and vest over four years, with the first 25% vesting as of September 26, 2002 and the remainder vesting monthly during the 36-month period following the initial vesting date. 150,000 of the options have an exercise price of $41.80 and an expiration date of September 26, 2011 and 75,000 of the options have an exercise price of $47.50 and an expiration date of October 23, 2011. The exercise price of $43.70 in the Exercise or Base Price column above and the present value of the options in the Grant Date Present Value column above were calculated as weighted averages. |
The table below shows for the fiscal year ended December 31, 2001 certain information regarding options exercised by, and held at year end by, the Named Executive Officers:
AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR
AND FY-END OPTION VALUES(1)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
| | | | | | Number of Securities | | |
| | | | | | Underlying Unexercised | | Value of Unexercised in-the- |
| | Shares | | | | Options at FY-end(#) | | Money Options at FY-end(3) |
| | Acquired on | | Value | |
| |
|
| Name | | Exercise(#) | | Realized(2) | | Exercisable | | Unexercisable | | Exercisable | | Unexercisable |
| |
| |
| |
| |
| |
| |
|
| Arthur D. Levinson | | | 10,000 | | | $ | 359,263 | | | | 1,669,518 | | | | 983,334 | | | $ | 46,710,552 | | | $ | 15,757,008 | |
| Myrtle S. Potter | | | 0 | | | $ | 0 | | | | 79,166 | | | | 445,834 | | | $ | 0 | | | $ | 2,373,750 | |
| Susan D. Desmond-Hellmann | | | 50,000 | | | $ | 1,499,750 | | | | 669,793 | | | | 578,311 | | | $ | 17,750,040 | | | $ | 8,244,330 | |
| Louis J. Lavigne, Jr. | | | 0 | | | $ | 0 | | | | 636,498 | | | | 433,294 | | | $ | 17,594,952 | | | $ | 6,890,808 | |
| Stephen G. Juelsgaard | | | 0 | | | $ | 0 | | | | 468,786 | | | | 371,086 | | | $ | 12,563,592 | | | $ | 5,024,568 | |
| |
| (1) | Genentech has awarded no SARs. |
| |
| (2) | Sale price of Genentech’s common stock on the date of exercise minus the exercise price. |
| |
| (3) | The value of the unexercised in-the-money options is based on the fair market value of Genentech’s common stock, $54.25, at the close of business on December 31, 2001, the last business day of 2001, minus the exercise price of the options. |
17
CHANGE IN CONTROL AGREEMENTS
Genentech and Ms. Potter, Executive Vice President, Commercial Operations and Chief Operating Officer, entered into a Change of Control Agreement on January 20, 2001 (the “Agreement”). The Agreement is effective through the earlier of May 15, 2005 or 24 months following a Change of Control (as that term is defined in the Agreement). The Agreement generally provides that, in the event Ms. Potter’s employment with Genentech is terminated following a Change of Control (i) by Genentech, except for Cause or (ii) by Ms. Potter with Good Reason (as these terms are defined in the Agreement), Genentech will pay Ms. Potter a lump sum severance payment equal to two times the sum of (i) Ms. Potter’s annual base salary and (ii) Ms. Potter’s average annual bonus and provide 24 months of health care and other insurance coverage. If the foregoing severance payments are subject to excise tax, then Genentech will pay Ms. Potter an additional amount to cover such tax.
LOANS AND OTHER COMPENSATION
For purposes of the discussion below, applicable federal rate refers to the minimum interest rate required to be charged on a loan to avoid the imputation of interest income under the Internal Revenue Code, unless an exception applies. The Internal Revenue Service publishes the applicable federal rate on a monthly basis.
In 1999, Genentech lent $250,000 to Dr. Stephen Dilly, Vice President, Medical Affairs, for the purchase of a home in connection with his relocation to the San Francisco Bay Area. $175,000 of this loan is due and payable in equal installments of $58,333 each on the fifth, sixth and seventh anniversary of the date of the loan or, if earlier, the remaining balance is due on the date of termination of Dr. Dilly’s employment with Genentech. The remaining $75,000 will be forgiven on April 12, 2004, if Dr. Dilly remains employed by Genentech. The largest amount outstanding under this loan during 2001 was $250,000. The amount of this loan outstanding as of December 31, 2001 was $250,000. The loan is interest-free to Dr. Dilly, and imputed interest on this loan was not required under the Internal Revenue Code.
In 1999, Genentech made two loans to Ms. Diane Parks, Vice President, Cardiovascular and Specialty Therapeutics, one in the amount of $250,000 and one in the amount of $200,000 for a total of $450,000 for the purchase of a home in connection with her relocation to the San Francisco Bay Area. In July 1999, Genentech extended to Ms. Parks the first loan for $250,000. $150,000 of this loan is due and payable on the earlier of the fifth anniversary of the date of the loan or the date of termination of Ms. Parks’ employment with Genentech. The remaining $100,000 will be forgiven in five equal installments of $20,000 on the anniversary date of the loan, provided that Ms. Parks is employed by Genentech on the forgiveness date. In November 1999, Genentech extended to Ms. Parks the second loan for $200,000, of which $175,000 was repaid in November 1999 and the remaining $25,000 was repaid in April 2001. The largest amount outstanding under all loans during 2001 was $255,000. The amount of the loans outstanding as of December 31, 2001 was $210,000. The loan is interest-free to Ms. Parks, but interest is required to be imputed under the Internal Revenue Code. Imputed interest in the amount of $17,643 was calculated using the applicable federal rate of 5.74% and reported as taxable compensation to Ms. Parks.
In 2000, Genentech lent $2,200,000 to Ms. Myrtle Potter, Executive Vice President, Commercial Operations and Chief Operating Officer, for the purchase of a home in connection with her relocation to the San Francisco Bay Area. $1,000,000 of the loan is due and payable on the earlier of May 15, 2005 or within 30 days from the date of termination of Ms. Potter’s employment with Genentech. $1,000,000 of the loan will be forgiven in equal installments of $200,000 each on May 15, 2001 through 2005, provided that Ms. Potter is employed by Genentech on these dates. Genentech has agreed to pay to Ms. Potter the amount equal to the federal and state income taxes payable in connection with the forgiveness of the repayment of each installment. The remaining $200,000 of the loan shall be due and payable in four equal installments of $50,000 each on the dates Ms. Potter receives her annual performance bonus from Genentech. The largest amount outstanding under this loan during 2001 was $2,200,000. The amount of this loan outstanding as of December 31, 2001 was $1,950,000. The loan is interest-free to Ms. Potter but interest is required to be imputed under the Internal Revenue Code. Imputed interest in the amount of $76,364 for the repayable portion of the loan and $117,884 for the forgivable portion of the loan were each calculated using the
18
applicable federal rate of 6.51% and reported as taxable compensation to Ms. Potter. Additional taxable compensation attributable to imputed interest was grossed up for related taxes resulting in a tax payment by Genentech of $59,354 on behalf of Ms. Potter with respect to the forgivable portion of the loan.
In 2001, Genentech lent $120,000 to Mr. David Ebersman, Senior Vice President, Product Operations, for the purchase of a home in connection with his relocation to San Mateo County. This loan is due and payable on the earlier of the fifth anniversary of the date of the loan, the date of termination of Mr. Ebersman’s employment with Genentech or sale of his residence. The largest amount outstanding under this loan during 2001 was $120,000, and the amount of this loan outstanding as of December 31, 2001 was $120,000. The loan is interest-free to Mr. Ebersman, but interest is required to be imputed under the Internal Revenue Code. Imputed interest in the amount of $3,484 was calculated based on the applicable federal rate of 4.96% and reported as taxable compensation to Mr. Ebersman.
In 2001, Genentech lent $150,000 to Dr. Richard Scheller, Senior Vice President, Research, as mortgage assistance. This loan is due and payable on the earlier of the fifth anniversary of the date of the loan, the date of termination of Dr. Scheller’s employment with Genentech or sale of his residence; provided, however, that the principal amount of the loan will be forgiven in five equal installments of $30,000 on each anniversary date of the loan if Dr. Scheller is employed by Genentech on such dates. The largest amount outstanding under this loan during 2001 was $150,000. The amount of this loan outstanding as of December 31, 2001 was $150,000. The loan is interest-free to Dr. Scheller, but interest is required to be imputed under the Internal Revenue Code. Imputed interest in the amount of $4,773 was calculated based on the applicable federal rate of 4.93% and reported as taxable compensation to Dr. Scheller. Additional taxable compensation attributable to imputed interest was grossed up for related taxes resulting in a tax payment by Genentech of $1,692 on behalf of Dr. Scheller.
In 2001, Genentech made loans under the Company’s Relocation Loan Program in the principal amounts of $15,003 and $880,000, respectively, to Mr. Ronald Branning, Vice President, Global Quality, for the purchase of a home in connection with his relocation to the San Francisco Bay Area. The loan in the principal amount of $15,003 is due and payable on the earlier of the date of the sale of Mr. Branning’s former home in the state where he resided prior to his relocation or the termination of Mr. Branning’s employment with Genentech. The loan is interest-free to Mr. Branning. Imputed interest on the loan may or may not be required under the Internal Revenue Code. If required, imputed interest will be based on the applicable federal rate and reported as taxable compensation to Mr. Branning. The loan in the principal amount of $880,000 is due and payable on the earlier of five years from the date of the loan, the date of termination of Mr. Branning’s employment with Genentech, the sale of his new home or upon the occurrence of any other event specified in the promissory note representing the loan. The loan bears interest at the applicable federal rate of 4.09%, compounded semi-annually, subject to adjustment as specified in the promissory note. On the fifth anniversary of the loan, if Mr. Branning is still employed by Genentech, one half of the accrued interest will be forgiven by Genentech and reported as compensation to Mr. Branning, if required under the Internal Revenue Code and all vested stock options for Genentech stock held by Mr. Branning will be exercised, the underlying shares sold, and the proceeds of such sale applied to repay the loan. In the event the stock option proceeds are not sufficient to repay the outstanding amounts due under the promissory note, the remaining balance will be forgiven in equal increments over a five year period with additional interest accruing at the applicable federal rate and forgiven annually with all forgiven amounts reported as compensation to Mr. Branning, if required under the Internal Revenue Code. The proceeds from any stock option exercise prior to the fifth anniversary of the loan will be applied to repayment of the outstanding loan. The largest amount outstanding under the loans made during 2001 was $895,003.
In 2001, Genentech made loans under the Company’s Relocation Loan Program in the principal amounts of $400,000, $313,542 and $645,000, respectively, to Mr. Mark J. Ahn, Vice President, Hematology, Marketing and Sales, for the purchase of a home in connection with his relocation to the San Francisco Bay Area. The loans in the principal amounts of $400,000 and $313,542 were due and payable on the earlier of the date of the sale of Mr. Ahn’s former home in the state where he resided prior to his relocation or the termination of Mr. Ahn’s employment with Genentech. These loans were interest-free to Mr. Ahn, and imputed interest on the loans may or may not be required under the Internal Revenue Code. If required,
19
imputed interest will be based on the applicable federal rate and reported as taxable compensation to Mr. Ahn. These loans were repaid in February 2002. The loan in the principal amount of $645,000 is due and payable on the earlier of five years from the date of the loan, the date of termination of Mr. Ahn’s employment with Genentech, the sale of his new home or upon the occurrence of any other event specified in the promissory note representing the loan. The loan will bear interest at the applicable federal rate of 3.93%, compounded semi-annually, subject to adjustment as specified in the promissory note. On the fifth anniversary of the loan, if Mr. Ahn is still employed by Genentech, one half of the accrued interest will be forgiven by Genentech and reported as compensation to Mr. Ahn, if required under the Internal Revenue Code and all vested stock options for Genentech stock held by Mr. Ahn will be exercised, the underlying shares sold, and the proceeds of such sale applied to repay the loan. In the event the stock option proceeds are not sufficient to repay the outstanding amounts due under the promissory note, the remaining balance will be forgiven in equal increments over a five year period with additional interest accruing at the applicable federal rate and forgiven annually with all forgiven amounts reported as compensation to Mr. Ahn, if required under the Internal Revenue Code. The proceeds from any stock option exercise prior to the fifth anniversary of the loan will be applied to repayment of the outstanding loan. The largest amount outstanding under the loans made during 2001 was $1,358,542.
COMPENSATION COMMITTEE REPORT(1)
The Compensation Committee of the Board of Directors (the “Committee”) is currently composed of all the directors, except Dr. Levinson. The Committee is responsible for setting and administering the policies that govern annual executive salaries, bonuses (if any) and stock ownership programs. The Committee annually evaluates the performance, and determines the compensation of, the Chief Executive Officer (“CEO”) and the other executive officers of Genentech based upon a mix of the achievement of corporate goals, individual performance and comparisons with other pharmaceutical and biotechnology companies. The CEO is not present during the discussion of his compensation.
The policies of the Committee with respect to executive officers, including the CEO, are to provide compensation sufficient to attract, motivate and retain executives of outstanding ability and potential and to establish an appropriate relationship between executive compensation and the creation of shareholder value. To meet these goals, the Committee has adopted a mix among the compensation elements of salary, bonus and stock options, with some bias toward stock options, to emphasize the link between executive incentives and the creation of shareholder value as measured by the equity markets. In general, the salaries and stock option awards of executive officers are not determined by the Company’s achievement of specific corporate performance criteria. Instead, the Committee determines the salaries for executive officers based upon a review of salary surveys of other pharmaceutical and biotechnology companies, as well as companies in other industries when comparisons are appropriate to specific officer positions. To provide the Committee with more information for making salary and bonus compensation comparisons, Genentech surveys a broader group of pharmaceutical and biotechnology companies other than those companies included in the Standard & Poors 500 Healthcare (Drugs — Major Pharmaceuticals) Index and the Standard & Poors 500 Biotechnology Index shown on Genentech’s Performance Graph. Based upon such surveys, the executive officers’ total direct cash compensation is generally targeted at or near the fiftieth (50th) percentile of the salaries of comparable executive officers as found in the surveys, making officers’ salaries and bonuses, together with all other benefits, competitive with other biotechnology companies. Stock options are set in the mid-range compared to a comparable group, based on market capitalization, of biotechnology and pharmaceutical companies.
In awarding stock options, the Committee considers individual performance, overall contribution to Genentech, officer retention, the total number of stock options to be awarded and an analysis of stock option awards granted by a comparable group of companies, based on market capitalization, of biotechnology and
1 The material in this report and under the caption “Performance Graph” are not “soliciting material,” are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference in any filing of the Company under the Securities Act of 1933 or the Securities Exchange Act of 1934, whether made before or after the date of this Proxy Statement and irrespective of any general incorporation language in those filings.
20
pharmaceutical companies and, to a significantly lesser extent, by a comparable group of companies, in terms of market capitalization and growth, of high technology companies in the San Francisco Bay Area. In determining where a given officer’s total compensation, including the CEO’s, is set within the ranges and in light of the considerations described above, the Committee subjectively evaluates such factors as the individual’s performance and the success of Genentech as measured by earnings per share, product sales revenues, product development progress, research prospects, and other critical success factors.
Bonuses are provided to executive officers in connection with Genentech’s company-wide bonus plan. Payment of bonuses is expressly linked to the attainment of specified corporate goals that the Committee sets at each year’s December meeting for the next year. Among other things, these goals determine whether a bonus will be paid to all eligible employees and the amount of funding available for the bonus pool. For the bonus for services rendered in 2001, the corporate performance goals, in order of importance, related to: (i) business and financial performance, including but not limited to increasing earnings per share, meeting specific productivity goals and managing operational budgets within specified targets; (ii) commercial performance, including increasing U.S. product sales revenues, increasing margins resulting from the sale of Genentech’s products and forming strategic alliances by in-licensing products and establishing relationships with other companies; (iii) accelerating and expanding product development by filing Investigational New Drug Applications, initiating or completing specified clinical trials investigating the use of new products, selecting new products for development and filing New Drug Applications or Product License Applications for new indications of marketed products and increasing efficiencies in the cost of producing Genentech’s products; and (iv) enhancing employee development. In setting these goals, the Committee is cognizant of the long development cycle for human pharmaceuticals. The corporate performance goals for bonuses selected by the Committee seek to balance the desire for immediate earnings and the longer term goal of enhancing shareholder value by bringing to market many of the potential therapies in Genentech’s research and development pipeline. In February 2002, the Committee reviewed the corporate performance goals for bonuses and determined that approximately $26.275 million under the 2001 Bonus Plan were achieved. The Committee allocated a bonus to the current CEO based on the achievement of the requisite corporate goals, as discussed above, and based on the Committee’s view that the CEO’s performance in achieving those goals had been excellent. The Committee set a range of 63.6% to 85.0% of salary for bonuses for members of the Company’s Executive Committee other than the CEO (Drs. Desmond-Hellmann and Scheller, Ms. Potter and Messrs. Juelsgaard and Lavigne). Within the applicable range, the Committee set the bonus for each Executive Committee member based on the Committee’s subjective evaluation of the individual’s performance and the recommendation of the CEO.
The Committee uses the same procedures described above for the other executive officers in setting the annual salary, bonus and stock option awards for the CEO. The CEO’s salary is determined based on comparisons with pharmaceutical and biotechnology companies as described above. In awarding stock options, the Committee considers the CEO’s performance, overall contribution to Genentech, retention, the total number of options to be awarded and a review of stock options awarded to CEOs by a comparable group of companies, based on market capitalization, of biotechnology and pharmaceutical companies and, to a significantly lesser extent, by a comparable group of companies, in terms of market capitalization and growth, of high technology companies in the San Francisco Bay Area. The CEO’s bonus is dependent on Genentech achieving the performance goals outlined above and the Committee’s subjective evaluation of the CEO’s performance. As described above, in determining where the CEO’s total compensation is set within the ranges and in light of the considerations described above, the Committee subjectively evaluates such factors as the individual’s performance and the success of Genentech as measured by the factors outlined above. Compared to other companies surveyed by Genentech, the current CEO’s salary, bonus and stock options are competitive.
Section 162(m) of the Code limits the federal income tax deductibility of compensation paid to Genentech’s CEO and to each of the other Named Executive Officers. Under Section 162(m), Genentech generally may deduct compensation paid to such an officer only to the extent that it does not exceed $1 million during any calendar year or is “performance-based” as defined in Section 162(m). Genentech expects that the deductibility limit of Section 162(m) currently will have an insignificant effect on Genentech. Current cash
21
compensation paid to each of Genentech��s executives, with one exception, is less than $1 million per year and is thus generally fully deductible to the Company. In addition, Genentech is seeking stockholder approval of the Plan for purposes of complying with Section 162(m) of the Code in connection with the exercise of stock options by the five most highly compensated officers of the Company for compensation in an amount exceeding $1 million.
From the members of the Compensation Committee of Genentech:
| |
| | Herbert W. Boyer |
| | Franz B. Humer |
| | Jonathan K.C. Knowles |
| | Sir Mark Richmond |
| | Charles A. Sanders |
AUDIT COMMITTEE REPORT
The Audit Committee of the Board of Directors currently consists of Drs. Boyer, Richmond and Sanders, and operates under a formal written charter attached as Appendix B to this Proxy Statement. The Audit Committee meets regularly with management, the independent auditors and the internal auditors, both jointly and separately, recommends the independent auditors to the Board, and reviews the Company’s financial reporting process on behalf of the Board. The Audit Committee has prepared the following report on its activities with respect to the Company’s audited financial statements for the year ended December 31, 2001.
Genentech’s management is responsible for the preparation, presentation and integrity of the Company’s financial statements. Management is also responsible for maintaining appropriate accounting and financial reporting practices and policies as well as internal controls and procedures designed to provide reasonable assurance that Genentech is in compliance with accounting standards and applicable laws and regulations. The independent auditors are responsible for planning and performing an independent audit of the Company’s financial statements in accordance with auditing standards generally accepted in the United States. The independent auditors are responsible for expressing an opinion on the conformity of those audited financial statements with accounting principles generally accepted in the United States.
In this context, the Audit Committee has reviewed and discussed the audited financial statements for the year ended December 31, 2001 with management and the independent auditors, Ernst & Young LLP. The Audit Committee has also discussed with the independent auditors the matters required to be discussed by Statement on Auditing Standards No. 61 (Communications with Audit Committees), as amended by Statement on Auditing Standards No. 90 (Audit Committee Communications).
Ernst & Young LLP has provided the Audit Committee with the written disclosures and letter required by Independence Standards Board Standard No. 1 (Independence Discussions with Audit Committees), and the Audit Committee discussed with the independent auditors that firm’s independence.
Based on the reviews and discussions referred to above, the Audit Committee recommended to the Board of Directors that the audited financial statements referred to above be included in Genentech’s Annual Report on Form 10-K for the year ended December 31, 2001.
Each of the members of the Audit Committee is independent as defined under the listing standards of the New York Stock Exchange.
From the members of the Audit Committee of Genentech:
| |
| | Herbert W. Boyer |
| | Sir Mark Richmond |
| | Charles A. Sanders |
22
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
During 2001, Genentech’s Compensation Committee consisted of Drs. Boyer, Humer, Knowles, Richmond and Sanders. Dr. Boyer, a founder of the Company, was a vice president of Genentech from 1976 to 1991 and is currently a consultant to the Company. Dr. Humer joined The Roche Group in 1995 as the Head of its Pharmaceuticals Division and is currently Chairman and Chief Executive Officer of The Roche Group. He is also Chairman of the Executive Committee of The Roche Group. Dr. Knowles joined The Roche Group in 1997 as Head of Global Pharmaceutical Research. He is a member of the Executive Committee of The Roche Group. Pursuant to the terms of the affiliation agreement, Drs. Humer and Knowles are serving on Genentech’s Compensation Committee as designees of Roche. See “Relationship with Roche” above and “Certain Relationships and Related Transactions” below for a description of Genentech’s relationship with Roche.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
| |
| Herceptin Licensing Agreement |
We have an agreement with Hoffmann-La Roche, providing them with exclusive marketing rights outside of the United States for Herceptin. Under the agreement, Hoffmann-La Roche paid us $40 million and has agreed to pay cash milestones tied to future product development activities, to contribute equally with us on global development costs up to a maximum of $40 million and to make royalty payments of 20% on aggregate net product sales outside the United States up to $500 million in each calendar year and 22.5% on such sales in excess of $500 million in each calendar year.
| |
| Amended and Restated Licensing Agreement |
We have an amended and restated licensing agreement with Hoffmann-La Roche for licenses to use and sell some of our products in non-U.S. markets as described under the heading “— Licensing and Marketing Arrangements” on page 4 under the section “Relationship with Roche.” See also “Relationship with Roche” starting on page 2.
Contract revenue from Hoffmann-La Roche and their affiliates totaled $5.8 million in 2001. Other revenue, comprised of royalties and product sales, from Roche, Hoffmann-La Roche and their affiliates, totaled $164.1 million in 2001.
23
PERFORMANCE GRAPH
The chart below shows a comparison of five-year cumulative total stockholder return as of December 31 among Genentech, the Standard & Poors 500 Index, the Standard & Poors 500 Healthcare (Drugs — Major Pharmaceuticals) Index and the Standard & Poors 500 Biotechnology Index:(1)
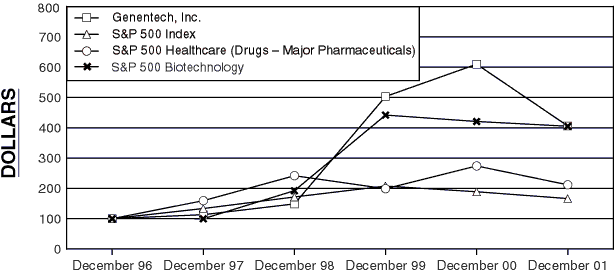
| | | | | | | | | | | | | | | | | | | | | | | | | |
|
|
| | December 96 | | December 97 | | December 98 | | December 99 | | December 00 | | December 01 |
|
|
| Genentech, Inc. | | | 100.00 | | | | 113.05 | | | | 148.60 | | | | 503.16 | | | | 609.77 | | | | 405.89 | |
| S&P 500 Index | | | 100.00 | | | | 133.36 | | | | 171.48 | | | | 207.56 | | | | 188.66 | | | | 166.24 | |
| S&P 500 Healthcare | | | 100.00 | | | | 159.27 | | | | 241.63 | | | | 198.72 | | | | 273.93 | | | | 211.54 | |
| S&P 500 Biotechnology | | | 100.00 | | | | 99.54 | | | | 192.30 | | | | 441.84 | | | | 420.70 | | | | 405.01 | |
| |
| (1) | The total return on investment (change in year end stock price plus reinvested dividends) assumes $100 invested on December 31, 1996, in Genentech, the Standard & Poors 500 Index, the Standard & Poors 500 Healthcare (Drugs — Major Pharmaceuticals) Index comprised at December 31, 2001 of Allergan, Inc., Forest Laboratories, Inc., Eli Lilly and Company, Merck & Co., Inc., Pfizer Inc., Pharmacia Corporation and Schering-Plough Corporation, and the Standard & Poors 500 Biotechnology Index comprised at December 31, 2001 of Amgen Inc., Biogen, Inc., Chiron Corporation, Genzyme Corporation, Immunex Corporation and MedImmune, Inc. |
PROPOSAL 3 — RATIFICATION OF SELECTION OF INDEPENDENT AUDITORS
The Board of Directors has selected Ernst & Young LLP as Genentech’s independent auditors for the year ending December 31, 2002, and has directed management to submit the selection of independent auditors for ratification by the stockholders at the Annual Meeting. Ernst & Young LLP has audited Genentech’s financial statements since its inception in 1976. Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting, will have an opportunity to make a statement if they desire to do so and will be available to respond to appropriate questions.
Stockholder ratification of the selection of Ernst & Young LLP as Genentech’s independent auditors is not required by the bylaws or otherwise. The Board of Directors has elected to seek such ratification as a matter of good corporate practice. Should the stockholders fail to ratify the selection of Ernst & Young LLP as independent auditors, the Board of Directors will consider whether to retain that firm for the year ended December 31, 2002.
24
The table below shows the aggregate fees billed by Ernst & Young LLP to Genentech for:
| |
| | (i) Audit Feesin connection with the audit of Genentech’s 2001 annual financial statements and the review of financial statements in Genentech’s quarterly reports on Form 10-Q filed in 2001; |
| |
| | (ii) Financial Information Systems Design and Implementation Feesfor the year 2001; and |
| |
| | (iii) All Other Feesfor the year 2001 other than for services covered in (i) or (ii).All Other Feesis primarily comprised of fees relating to internal audit services for special projects, tax services, and other audit related services, including the delivery of opinions and consents in connection with Genentech’s filings with the Securities and Exchange Commission. |
| | | | | |
| Audit Fees | | $ | 595,000 | |
| Financial Information Systems Design and Implementation Fees | | $ | 0 | |
| All Other Fees | | $ | 693,000 | |
The affirmative vote of holders of a majority of the shares present in person or by proxy at the Annual Meeting is required for approval of this proposal.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS
A VOTE FOR SUCH RATIFICATION
OTHER MATTERS
The Board of Directors knows of no other business to be presented at the Annual Meeting, but if other matters do properly come before the Annual Meeting, it is intended that the persons named on the Proxy Card will vote on those matters in accordance with their best judgment.
FINANCIAL MATTERS
Attached as Appendix C is financial information of the Company that was originally filed with the Securities and Exchange Commission on March 4, 2002 as part of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2001. The Company has not undertaken any updates or revisions to the financial information since the date it was originally filed except as required under applicable law. Accordingly, you are encouraged to review this financial information together with any information subsequently filed by the Company with the Securities and Exchange Commission and other publicly available information.
25
APPENDIX A
GENENTECH, INC.
1999 STOCK PLAN
AMENDED AND RESTATED AS OF 12/08/00
1. Purposes of the Plan. The purposes of this 1999 Stock Plan are:
| | |
| | • | to attract and retain the best available personnel for positions of substantial responsibility, |
| |
| | • | to provide additional incentive to Employees and Consultants, and |
| |
| | • | to promote the success of the Company’s business. |
Options granted under the Plan may be Incentive Stock Options or Nonstatutory Stock Options, as determined by the Administrator at the time of grant. Stock Purchase Rights may also be granted under the Plan.
2. Definitions. As used herein, the following definitions shall apply:
| |
| | (a) “Administrator” means the Board or any of its Committees as shall be administering the Plan, in accordance with Section 4 of the Plan. |
| |
| | (b) “Applicable Laws” means the requirements relating to the administration of stock option plans under U. S. state corporate laws, U.S. federal and state securities laws, the Code, any stock exchange or quotation system on which the Common Stock is listed or quoted and the applicable laws of any foreign country or jurisdiction where Options or Stock Purchase Rights are, or will be, granted under the Plan. |
| |
| | (c) “Board” means the Board of Directors of the Company. |
| |
| | (d) “Code” means the Internal Revenue Code of 1986, as amended. |
| |
| | (e) “Committee” means a committee of Board members appointed by the Board in accordance with Section 4 of the Plan. |
| |
| | (f) “Common Stock” means the common stock of the Company. |
| |
| | (g) “Company” means Genentech, Inc., a Delaware corporation. |
| |
| | (h) “Director” means a member of the Board. |
| |
| | (i) “Consultant” means any person, including an advisor, engaged by the Company or Subsidiary to render services to such entity but shall not include an Employee. |
| |
| | (j) “Disability” means total and permanent disability as defined in Section 22(e)(3) of the Code. |
| |
| | (k) “Employee” means any person, including Officers and Directors, employed by the Company or Subsidiary of the Company. An individual shall not cease to be an Employee in the case of (i) any leave of absence approved by the Company or (ii) transfers between locations of the Company or between the Company, any Subsidiary, or any successor. For purposes of Incentive Stock Options, no such leave may exceed ninety days, unless reemployment upon expiration of such leave is guaranteed by statute or contract. If reemployment upon expiration of a leave of absence approved by the Company is not so guaranteed, then three (3) months following the 91st day of such leave any Incentive Stock Option held by the Optionee shall cease to be treated as an Incentive Stock Option and shall be treated for tax purposes as a Nonstatutory Stock Option. Neither service as a Director nor payment of a director’s fee by the Company shall be sufficient to constitute “employment” by the Company. |
| |
| | (l) “Exchange Act” means the Securities Exchange Act of 1934, as amended. |
A-1
| |
| | (m) “Fair Market Value” means, as of any date, the value of Common Stock determined as follows: |
| |
| | (i) If the Common Stock is listed on any established stock exchange or a national market system, including without limitation the Nasdaq National Market or The Nasdaq SmallCap Market of The Nasdaq Stock Market, its Fair Market Value shall be the closing sales price for such stock (or the closing bid, if no sales were reported) as quoted on such exchange or system on the day of determination, as reported inThe Wall Street Journal or such other source as the Administrator deems reliable; |
| |
| | (ii) If the Common Stock is regularly quoted by a recognized securities dealer but selling prices are not reported, the Fair Market Value of a Share of Common Stock shall be the mean between the high bid and low asked prices for the Common Stock on the day of determination, as reported inThe Wall Street Journalor such other source as the Administrator deems reliable; or |
| |
| | (iii) In the absence of an established market for the Common Stock, the Fair Market Value shall be determined in good faith by the Administrator. |
| |
| | (n) “Incentive Stock Option” means an Option intended to qualify as an incentive stock option within the meaning of Section 422 of the Code and the regulations promulgated thereunder. |
| |
| | (o) “Nonstatutory Stock Option” means an Option not intended to qualify as an Incentive Stock Option. |
| |
| | (p) “Notice of Grant” means a written or electronic notice evidencing certain terms and conditions of an individual Option or Stock Purchase Right grant. The Notice of Grant is part of the Option Agreement. |
| |
| | (q) “Officer” means a person who is an officer of the Company within the meaning of Section 16 of the Exchange Act and the rules and regulations promulgated thereunder. |
| |
| | (r) “Option” means a stock option granted pursuant to the Plan. |
| |
| | (s) “Option Agreement” means an agreement between the Company and an Optionee evidencing the terms and conditions of an individual Option grant. The Option Agreement is subject to the terms and conditions of the Plan. |
| |
| | (t) “Optioned Stock” means the Common Stock subject to an Option or Stock Purchase Right. |
| |
| | (u) “Optionee” means the holder of an outstanding Option or Stock Purchase Right granted under the Plan. |
| |
| | (v) “Parent” means a “parent corporation,” whether now or hereafter existing, as defined in Section 424(e) of the Code. |
| |
| | (w) “Plan” means this 1999 Stock Plan, as amended and restated. |
| |
| | (x) “Restricted Stock” means shares of Common Stock acquired pursuant to a grant of Stock Purchase Rights under Section 11 of the Plan. |
| |
| | (y) “Restricted Stock Purchase Agreement” means a written agreement between the Company and the Optionee evidencing the terms and restrictions applying to stock purchased under a Stock Purchase Right. The Restricted Stock Purchase Agreement is subject to the terms and conditions of the Plan and the Notice of Grant. |
| |
| | (z) “Retirement” means a Service Provider who leaves the employment of the Company after reaching age sixty-five (65). |
| |
| | (aa) “Rule 16b-3” means Rule 16b-3 of the Exchange Act or any successor to Rule 16b-3, as in effect when discretion is being exercised with respect to the Plan. |
| |
| | (bb) “Section 16(b)” means Section 16(b) of the Exchange Act. |
A-2
| |
| | (cc) “Service Provider” means an Employee, Director or Consultant. |
| |
| | (dd) “Share” means a share of the Common Stock, as adjusted in accordance with Section 13 of the Plan. |
| |
| | (ee) “Stock Purchase Right” means the right to purchase Common Stock pursuant to Section 11 of the Plan, as evidenced by a Notice of Grant. |
| |
| | (ff) “Subsidiary” means a “subsidiary corporation”, whether now or hereafter existing, as defined in Section 424(f) of the Code. |
3. Stock Subject to the Plan. Subject to the provisions of Section 13 of the Plan, the maximum aggregate number of Shares that may be optioned and sold under the Plan is 63,000,000 Shares. The Shares may be authorized, but unissued, or reacquired Common Stock.
If an Option or Stock Purchase Right expires or becomes unexercisable without having been exercised in full, the unpurchased Shares which were subject thereto shall become available for future grant or sale under the Plan (unless the Plan has terminated); provided, however, that Shares that have actually been issued under the Plan, whether upon exercise of an Option or Right, shall not be returned to the Plan and shall not become available for future distribution under the Plan, except that if Shares of Restricted Stock are repurchased by the Company at their original purchase price, such Shares shall become available for future grant under the Plan.
4. Administration of the Plan.
(a) Procedure.
| |
| | (i) Multiple Administrative Bodies. The Plan may be administered by different Committees with respect to different groups of Service Providers. |
| |
| | (ii) Section 162(m). To the extent that the Administrator determines it to be desirable to qualify Options granted hereunder as “performance-based compensation” within the meaning of Section 162(m) of the Code, the Plan shall be administered by a Committee of two or more “outside directors” within the meaning of Section 162(m) of the Code. |
| |
| | (iii) Rule 16b-3. To the extent desirable to qualify transactions hereunder as exempt under Rule 16b-3, the transactions contemplated hereunder shall be structured to satisfy the requirements for exemption under Rule 16b-3. |
| |
| | (iv) Other Administration. Other than as provided above, the Plan shall be administered by (A) the Board or (B) a Committee, which committee shall be constituted to satisfy Applicable Laws. |
(b) Powers of the Administrator. Subject to the provisions of the Plan, and in the case of a Committee, subject to the specific duties delegated by the Board to such Committee, the Administrator shall have the authority, in its discretion:
| |
| | (i) to determine the Fair Market Value; |
| |
| | (ii) to select the Service Providers to whom Options and Stock Purchase Rights may be granted hereunder; |
| |
| | (iii) to determine the number of shares of Common Stock to be covered by each Option and Stock Purchase Right granted hereunder; |
| |
| | (iv) to approve forms of agreement for use under the Plan; |
| |
| | (v) to determine the terms and conditions, not inconsistent with the terms of the Plan, of any Option or Stock Purchase Right granted hereunder. Such terms and conditions include, but are not limited to, the exercise price, the time or times when Options or Stock Purchase Rights may be exercised (which may be based on performance criteria), any vesting acceleration or waiver of forfeiture restrictions, and any restriction or limitation regarding any Option or Stock Purchase Right or the shares |
A-3
| |
| | of Common Stock relating thereto, based in each case on such factors as the Administrator, in its sole discretion, shall determine; |
| |
| | (vi) to construe and interpret the terms of the Plan and awards granted pursuant to the Plan; |
| |
| | (vii) to prescribe, amend and rescind rules and regulations relating to the Plan, including rules and regulations relating to sub-plans established for the purpose of qualifying for preferred treatment under foreign laws; |
| |
| | (viii) to modify or amend each Option or Stock Purchase Right (subject to Section 15(c) of the Plan), including the discretionary authority to extend the post-termination exercisability period of Options longer than is otherwise provided for in the Plan; |
| |
| | (ix) to allow Optionees to satisfy withholding tax obligations by electing to have the Company withhold from the Shares to be issued upon exercise of an Option or Stock Purchase Right that number of Shares having a Fair Market Value equal to (or less than) the minimum amount required to be withheld. The Fair Market Value of the Shares to be withheld shall be determined on the date that the amount of tax to be withheld is to be determined. All elections by an Optionee to have Shares withheld for this purpose shall be made in such form and under such conditions as the Administrator may deem necessary or advisable; |
| |
| | (x) to authorize any person to execute on behalf of the Company any instrument required to effect the grant of an Option or Stock Purchase Right previously granted by the Administrator; |
| |
| | (xi) to make all other determinations deemed necessary or advisable for administering the Plan. |
(c) Effect of Administrator’s Decision. The Administrator’s decisions, determinations and interpretations shall be final and binding on all Optionees and any other holders of Options or Stock Purchase Rights.
5. Eligibility. Nonstatutory Stock Options and Stock Purchase Rights may be granted to Service Providers. Incentive Stock Options may be granted only to Employees.
6. Limitations.
(a) Each Option shall be designated in the Option Agreement as either an Incentive Stock Option or a Nonstatutory Stock Option. However, notwithstanding such designation, to the extent that the aggregate Fair Market Value of the Shares with respect to which Incentive Stock Options are exercisable for the first time by the Optionee during any calendar year (under all plans of the Company and any Parent or Subsidiary) exceeds $100,000, such Options shall be treated as Nonstatutory Stock Options. For purposes of this Section 6(a), Incentive Stock Options shall be taken into account in the order in which they were granted. The Fair Market Value of the Shares shall be determined as of the time the Option with respect to such Shares is granted.
(b) Neither the Plan nor any Option or Stock Purchase Right shall confer upon an Optionee any right with respect to continuing the Optionee’s relationship as a Service Provider with the Company, nor shall they interfere in any way with the Optionee’s right or the Company’s right to terminate such relationship at any time, with or without cause.
(c) The following limitations shall apply to grants of Options:
| |
| | (i) No Service Provider shall be granted, in any fiscal year of the Company, Options to purchase more than 2,000,000 Shares. |
| |
| | (ii) In connection with his or her initial service, a Service Provider may be granted Options to purchase up to an additional 1,000,000 Shares which shall not count against the limit set forth in subsection (i) above. |
| |
| | (iii) The foregoing limitations shall be adjusted proportionately in connection with any change in the Company’s capitalization as described in Section 13. |
A-4
7. Term of Plan. The Plan shall become effective upon its adoption by the Board. It shall continue in effect for a term of ten (10) years unless terminated earlier under Section 15 of the Plan.
8. Term of Option. The term of each Option shall be stated in the Option Agreement. In the case of an Incentive Stock Option, the term shall be ten (10) years from the date of grant or such shorter term as may be provided in the Option Agreement. Moreover, in the case of an Incentive Stock Option granted to an Optionee who, at the time the Incentive Stock Option is granted, owns stock representing more than ten percent (10%) of the total combined voting power of all classes of stock of the Company or any Parent or Subsidiary, the term of the Incentive Stock Option shall be five (5) years from the date of grant or such shorter term as may be provided in the Option Agreement.
9. Option Exercise Price and Consideration.
(a) Exercise Price. The per share exercise price for the Shares to be issued pursuant to exercise of an Option shall be determined by the Administrator, subject to the following:
| |
| | (i) In the case of an Incentive Stock Option, the per Share exercise price shall be no less than 100% of the Fair Market Value per Share on the date of grant. |
| |
| | (ii) In the case of a Nonstatutory Stock Option, the per Share exercise price shall be determined by the Administrator. In the case of a Nonstatutory Stock Option intended to qualify as “performance-based compensation” within the meaning of Section 162(m) of the Code, the per Share exercise price shall be no less than 100% of the Fair Market Value per Share on the date of grant. |
| |
| | (iii) Notwithstanding the foregoing, Options may be granted with a per Share exercise price of less than 100% of the Fair Market Value per Share on the date of grant pursuant to a merger or other corporate transaction. |
(b) Waiting Period and Exercise Dates. At the time an Option is granted, the Administrator shall fix the period within which the Option may be exercised and shall determine any conditions that must be satisfied before the Option may be exercised.
(c) Form of Consideration. The Administrator shall determine the acceptable form of consideration for exercising an Option, including the method of payment. In the case of an Incentive Stock Option, the Administrator shall determine the acceptable form of consideration at the time of grant. Such consideration may consist entirely of:
| |
| | (i) cash; |
| |
| | (ii) check; |
| |
| | (iii) promissory note; |
| |
| | (iv) other Shares which (A) in the case of Shares acquired upon exercise of an option, have been owned by the Optionee for more than six months on the date of surrender, and (B) have a Fair Market Value on the date of surrender equal to the aggregate exercise price of the Shares as to which said Option shall be exercised; |
| |
| | (v) consideration received by the Company under a cashless exercise program implemented by the Company in connection with the Plan; |
| |
| | (vi) a reduction in the amount of any Company liability to the Optionee, including any liability attributable to the Optionee’s participation in any Company-sponsored deferred compensation program or arrangement; |
| |
| | (vii) any combination of the foregoing methods of payment; or |
| |
| | (viii) such other consideration and method of payment for the issuance of Shares to the extent permitted by Applicable Laws. |
A-5
10. Exercise of Option.
(a) Procedure for Exercise; Rights as a Shareholder. Any Option granted hereunder shall be exercisable according to the terms of the Plan and at such times and under such conditions as determined by the Administrator and set forth in the Option Agreement. Unless the Administrator provides in writing otherwise, vesting of Options granted hereunder shall be suspended during any unpaid leave of absence. An Option may not be exercised for a fraction of a Share.
An Option shall be deemed exercised when the Company receives: (i) written or electronic notice of exercise (in accordance with the Option Agreement) from the person entitled to exercise the Option, and (ii) full payment for the Shares with respect to which the Option is exercised. Full payment may consist of any consideration and method of payment authorized by the Administrator and permitted by the Option Agreement and the Plan. Shares issued upon exercise of an Option shall be issued in the name of the Optionee or, if requested by the Optionee, in the name of the Optionee, his or her spouse or in any other name or names designated by Optionee. Until the Shares are issued (as evidenced by the appropriate entry on the books of the Company or of a duly authorized transfer agent of the Company), no right to vote or receive dividends or any other rights as a shareholder shall exist with respect to the Optioned Stock, notwithstanding the exercise of the Option. The Company shall issue (or cause to be issued) such Shares promptly after the Option is exercised. No adjustment will be made for a dividend or other right for which the record date is prior to the date the Shares are issued, except as provided in Section 13 of the Plan.
Exercising an Option in any manner shall decrease the number of Shares thereafter available, both for purposes of the Plan and for sale under the Option, by the number of Shares as to which the Option is exercised.
(b) Termination of Relationship as a Service Provider. If an Optionee ceases to be a Service Provider, other than upon the Optionee’s death, Disability, or Retirement, the Optionee may exercise his or her Option within such period of time as is specified in the Option Agreement to the extent that the Option is vested on the date of termination (but in no event later than the expiration of the term of such Option as set forth in the Option Agreement). In the absence of a specified time in the Option Agreement, the Option shall remain exercisable for three (3) months following the Optionee’s termination. If, on the date of termination, the Optionee is not vested as to his or her entire Option, the Shares covered by the unvested portion of the Option shall revert to the Plan. If, after termination, the Optionee does not exercise his or her Option within the time specified by the Administrator, the Option shall terminate, and the Shares covered by such Option shall revert to the Plan.
(c) Disability of Optionee. If an Optionee ceases to be a Service Provider as a result of the Optionee’s Disability, the Optionee may exercise his or her Option within such period of time as is specified in the Option Agreement to the extent the Option is vested on the date Optionee ceases to be a Service Provider (but in no event later than the expiration of the term of such Option as set forth in the Option Agreement). In the absence of a specified time in the Option Agreement, the Option shall remain exercisable for twelve (12) months following the date the Optionee ceases to be a Service Provider. If, on the date Optionee ceases to be a Service Provider, the Optionee is not vested as to his or her entire Option, the Shares covered by the unvested portion of the Option shall revert to the Plan. If, after Optionee ceases to be a Service Provider, the Optionee does not exercise his or her Option within the time specified herein, the Option shall terminate, and the Shares covered by such Option shall revert to the Plan.
(d) Death of Optionee. Unless otherwise provided by the Administrator or in any Option Agreement, immediately upon Optionee’s death, the Option shall automatically accelerate and become fully-vested for all of the Shares covered by such Option, and the expiration date of the Option shall automatically be extended to the expiration date of the Option term specified in the Option Agreement. If an Optionee dies while a Service Provider, the Option may be exercised by the Optionee’s designated beneficiary, provided such beneficiary has been designated prior to Optionee’s death in a form acceptable by the Administrator. If no such beneficiary has been designated by the Optionee, then such Option may be exercised by the personal representative of the Optionee’s estate or by the person or persons to whom the Option is transferred pursuant to the Optionee’s will or in accordance with the laws of descent and distribution. If the Option is not exercised following Optionee’s
A-6
death within the time specified herein, the Option shall terminate, and the Shares covered by such Option shall revert to the Plan.
(e) Retirement of Optionee. Unless otherwise provided by the Administrator or in any Option Agreement, immediately upon Optionee’s Retirement, the Option shall automatically accelerate and become fully-vested for that number of Shares covered by such Option which would otherwise vest during the twelve (12) month period immediately following the date of Retirement had the Optionee remained a Service Provider during that period, and the expiration date of the Option shall automatically be extended to the expiration date of the Option term specified in the Option Agreement. In the event Optionee ceases to be a Service Provider by reason of Retirement, the Option may continue to be exercised by Optionee during the terms of the Option. If the Option is not exercised following Optionee’s Retirement within the time specified, the Option shall terminate, and the Shares covered by such Option shall revert to the Plan.
(f) Buyout Provisions. The Administrator may at any time offer to buy out for a payment in cash or Shares an Option previously granted based on such terms and conditions as the Administrator shall establish and communicate to the Optionee at the time that such offer is made.
11. Stock Purchase Rights.
(a) Rights to Purchase. Stock Purchase Rights may be issued either alone, in addition to, or in tandem with other awards granted under the Plan and/or cash awards made outside of the Plan. After the Administrator determines that it will offer Stock Purchase Rights under the Plan, it shall advise the offeree in writing or electronically, by means of a Notice of Grant, of the terms, conditions and restrictions related to the offer, including the number of Shares that the offeree shall be entitled to purchase, the price to be paid, and the time within which the offeree must accept such offer. The offer shall be accepted by execution of a Restricted Stock Purchase Agreement in the form determined by the Administrator.
(b) Repurchase Option. Unless the Administrator determines otherwise, the Restricted Stock Purchase Agreement shall grant the Company a repurchase option exercisable upon the voluntary or involuntary termination of the purchaser’s service with the Company for any reason (including death or Disability). Unless the Administrator determines otherwise, the purchase price for Shares repurchased pursuant to the Restricted Stock Purchase Agreement shall be the original price paid by the purchaser and may be paid by cancellation of any indebtedness of the purchaser to the Company. The repurchase option shall lapse at a rate or under such conditions as shall be determined by the Administrator and set forth in the Restricted Stock Purchase Agreement.
(c) Other Provisions. The Restricted Stock Purchase Agreement shall contain such other terms, provisions and conditions not inconsistent with the Plan as may be determined by the Administrator in its sole discretion.
(d) Rights as a Shareholder. Once the Stock Purchase Right is exercised, the purchaser shall have the rights equivalent to those of a shareholder, and shall be a shareholder when his or her purchase is entered upon the records of the duly authorized transfer agent of the Company. No adjustment will be made for a dividend or other right for which the record date is prior to the date the Stock Purchase Right is exercised, except as provided in Section 13 of the Plan.
12. Transferability of Options and Stock Purchase Rights.Unless determined otherwise by the Administrator, an Option or Stock Purchase Right may not be sold, pledged, assigned, hypothecated, transferred, or disposed of in any manner other than by will or by the laws of descent or distribution and may be exercised, during the lifetime of the Optionee, only by the Optionee. If the Administrator makes an Option or Stock Purchase Right transferable, such Option or Stock Purchase Right shall contain such additional terms and conditions as the Administrator deems appropriate.
13. Adjustments Upon Changes in Capitalization, Dissolution, Merger or Asset Sale.
(a) Changes in Capitalization.Subject to any required action by the shareholders of the Company, the number of shares of Common Stock covered by each outstanding Option and Stock Purchase Right, and the number of shares of Common Stock which have been authorized for issuance under the Plan but as to which
A-7
no Options or Stock Purchase Rights have yet been granted or which have been returned to the Plan upon cancellation or expiration of an Option or Stock Purchase Right, as well as the price per share of Common Stock covered by each such outstanding Option or Stock Purchase Right, shall be proportionately adjusted for any increase or decrease in the number of issued shares of Common Stock resulting from a stock split, reverse stock split, stock dividend, combination or reclassification of the Common Stock, or any other increase or decrease in the number of issued shares of Common Stock effected without receipt of consideration by the Company; provided, however, that conversion of any convertible securities of the Company shall not be deemed to have been “effected without receipt of consideration.” Such adjustment shall be made by the Board, whose determination in that respect shall be final, binding and conclusive. Except as expressly provided herein, no issuance by the Company of shares of stock of any class, or securities convertible into shares of stock of any class, shall affect, and no adjustment by reason thereof shall be made with respect to, the number or price of shares of Common Stock subject to an Option or Stock Purchase Right.
(b) Dissolution or Liquidation.In the event of the proposed dissolution or liquidation of the Company, the Administrator shall notify each Optionee as soon as practicable prior to the effective date of such proposed transaction. The Administrator in its discretion may provide for an Optionee to have the right to exercise his or her Option until ten (10) days prior to such transaction as to all of the Optioned Stock covered thereby, including Shares as to which the Option would not otherwise be exercisable. In addition, the Administrator may provide that any Company repurchase option applicable to any Shares purchased upon exercise of an Option or Stock Purchase Right shall lapse as to all such Shares, provided the proposed dissolution or liquidation takes place at the time and in the manner contemplated. To the extent it has not been previously exercised, an Option or Stock Purchase Right will terminate immediately prior to the consummation of such proposed action.
(c) Merger or Asset Sale.In the event of a merger of the Company with or into another corporation, or the sale of substantially all of the assets of the Company, each outstanding Option and Stock Purchase Right shall be assumed or an equivalent option or right substituted by the successor corporation or a Parent or Subsidiary of the successor corporation. In the event that the successor corporation refuses to assume or substitute for the Option or Stock Purchase Right, the Optionee shall fully vest in and have the right to exercise the Option or Stock Purchase Right as to all of the Optioned Stock, including Shares as to which it would not otherwise be vested or exercisable. If an Option or Stock Purchase Right becomes fully vested and exercisable in lieu of assumption or substitution in the event of a merger or sale of assets, the Administrator shall notify the Optionee in writing or electronically that the Option or Stock Purchase Right shall be fully vested and exercisable for a period of fifteen (15) days from the date of such notice, and the Option or Stock Purchase Right shall terminate upon the expiration of such period. For the purposes of this paragraph, the Option or Stock Purchase Right shall be considered assumed if, following the merger or sale of assets, the option or right confers the right to purchase or receive, for each Share of Optioned Stock subject to the Option or Stock Purchase Right immediately prior to the merger or sale of assets, the consideration (whether stock, cash, or other securities or property) received in the merger or sale of assets by holders of Common Stock for each Share held on the effective date of the transaction (and if holders were offered a choice of consideration, the type of consideration chosen by the holders of a majority of the outstanding Shares); provided, however, that if such consideration received in the merger or sale of assets is not solely common stock of the successor corporation or its Parent, the Administrator may, with the consent of the successor corporation, provide for the consideration to be received upon the exercise of the Option or Stock Purchase Right, for each Share of Optioned Stock subject to the Option or Stock Purchase Right, to be solely common stock of the successor corporation or its Parent equal in fair market value to the per share consideration received by holders of Common Stock in the merger or sale of assets.
14. Date of Grant.The date of grant of an Option or Stock Purchase Right shall be, for all purposes, the date on which the Administrator makes the determination granting such Option or Stock Purchase Right, or such other later date as is determined by the Administrator. Notice of the determination shall be provided to each Optionee within a reasonable time after the date of such grant.
A-8
15. Amendment and Termination of the Plan.
(a) Amendment and Termination.The Board may at any time amend, alter, suspend or terminate the Plan.
(b) Shareholder Approval.The Company shall obtain shareholder approval of any Plan amendment to the extent necessary and desirable to comply with Applicable Laws.
(c) Effect of Amendment or Termination.No amendment, alteration, suspension or termination of the Plan shall impair the rights of any Optionee, unless mutually agreed otherwise between the Optionee and the Administrator, which agreement must be in writing and signed by the Optionee and the Company. Termination of the Plan shall not affect the Administrator’s ability to exercise the powers granted to it hereunder with respect to Options granted under the Plan prior to the date of such termination.
16. Conditions Upon Issuance of Shares.
(a) Legal Compliance.Shares shall not be issued pursuant to the exercise of an Option or Stock Purchase Right unless the exercise of such Option or Stock Purchase Right and the issuance and delivery of such Shares shall comply with Applicable Laws and shall be further subject to the approval of counsel for the Company with respect to such compliance.
(b) Investment Representations.As a condition to the exercise of an Option or Stock Purchase Right, the Company may require the person exercising such Option or Stock Purchase Right to represent and warrant at the time of any such exercise that the Shares are being purchased only for investment and without any present intention to sell or distribute such Shares if, in the opinion of counsel for the Company, such a representation is required.
17. Inability to Obtain Authority.The inability of the Company to obtain authority from any regulatory body having jurisdiction, which authority is deemed by the Company’s counsel to be necessary to the lawful issuance and sale of any Shares hereunder, shall relieve the Company of any liability in respect of the failure to issue or sell such Shares as to which such requisite authority shall not have been obtained.
18. Reservation of Shares.The Company, during the term of this Plan, will at all times reserve and keep available such number of Shares as shall be sufficient to satisfy the requirements of the Plan.
A-9
APPENDIX B
GENENTECH, INC.
AUDIT COMMITTEE CHARTER AND ANNUAL AGENDA
Purpose of Committee
Genentech’s financial reporting is the responsibility of senior management, but is overseen by the Board of Directors. It is the charter of the Genentech Audit Committee to assist the Board as follows:
| | |
| | • | Carry out the oversight responsibility for monitoring the integrity of Genentech’s financial reporting process. |
| |
| | • | Review management’s programs to: |
| | |
| | 1. | Maintain adequate systems of internal controls regarding finance and accounting and related legal compliance matters, |
2. Safeguard Company assets,
3. Provide appropriate reserves for any legal or regulatory issues and
4. Assess and manage risk.
| | |
| | • | Monitor the independence and performance of Genentech’s independent auditors, including annual financial audit, quarterly reviews, general audit and non-audit services. The independent auditor is ultimately accountable to the Board of Directors and the Audit Committee, and the Audit Committee is responsible for recommending to the Board of Directors the selection, evaluation and replacement, where appropriate, of the auditors. |
| |
| | • | Provide an avenue of communication among the independent auditors, management and the Board of Directors. |
| |
| | • | Assure that the Company has provided the NYSE with annual written reaffirmation that the Board of Directors has reviewed, on an annual basis, this charter for adequacy. |
| |
| | • | Assure that the Company has disclosed this charter in an appendix to Genentech’s proxy statement at least once every three years. |
| |
| | • | Discuss, with the independent auditors, the matters required to be discussed by Statement on Auditing Standards No. 61(1), and as amended by Statement on Auditing Standards No. 90(2) |
| |
| | • | Review the report of the Audit Committee as required by the rules of the Securities and Exchange Commission to be included in the Company’s annual proxy statement, in accordance with the required frequency. |
| |
| | • | Request the review of significant changes or new events in the Company or significant developments in accounting rules which have significant financial implications or risks or will likely require additional reporting or changes in accounting or operating practices. |
To carry out these responsibilities, the Audit Committee shall meet regularly and report its activities to the full Board after such meetings.
While the Audit Committee has the responsibilities and powers set forth in this Charter, it is not the duty of the Audit Committee to plan or conduct audits or to determine that the Company’s financial statements are
(1) SAS No. 61 requires an independent auditor to communicate to the Audit Committee matters related to the conduct of the audit such as the selection of and changes in significant accounting policies, the methods used to account for significant unusual transactions, the effect of significant accounting policies in controversial or emerging areas, the process used by management in formulating particularly sensitive estimates and the basis for the auditor’s conclusions regarding the reasonableness of those estimates, significant adjustments arising from the audit, and disagreements with management over the application of accounting principles, the basis for management’s accounting estimates and the disclosures in the financial statements.
(2) SAS No. 90 states that discussions on the quality of an entity’s accounting principles should include such matters as consistency of accounting policies and their application, and the clarity and completeness of financial statements, which include related disclosures.
B-1
complete and accurate and are in accordance with generally accepted accounting principles. This is the responsibility of management and the independent auditor. Nor is it the duty of the Audit Committee to conduct investigations, to resolve disagreements, if any, between management and the independent auditor or to assure compliance with laws and regulations and the Company’s Good Operating Practices.
Term and Membership
The Audit Committee shall be appointed by the Board of Directors and shall consist of three independent directors. Audit Committee membership will conform to the following independence limitations:
| | |
| | • | An employee of the company or its affiliates may not serve on the Audit Committee until three years following termination of such employment, subject to the Board’s override |
| |
| | • | A director who (1) is a partner, controlling shareholder or executive officer of an organization that has a business relationship with the company or (2) has a direct business relationship with the company (e.g., a consultant), cannot serve on the Audit Committee until three years after the termination of such relationship, unless the Company’s Board determines in its business judgement that the relationship does not interfere with the director’s exercise of independent judgement, taking into account among other things, the materiality of the relationship to the company, the director or such organization |
| |
| | • | A director who is employed as an executive of another company where any of the Company’s executives serves on that corporation’s Compensation Committee may not serve on the Audit Committee |
| |
| | • | A director who is an immediate family member of an individual who is an executive officer of the Company or any of its affiliates cannot serve on the Audit Committee until three years following the termination of such employment relationship, subject to the Board’s override |
Each Audit Committee member shall serve until resignation from the Committee or replacement by the Board. Each Audit Committee member shall be “financially literate,” as defined by the Board, or attain such status within a reasonable period after appointment. At least one member shall have “accounting or related financial expertise” as defined by the Board.
Responsibilities — Meeting Frequency
In general, it is expected that the Audit Committee be vigilant and effective overseers of the financial reporting process and the Company’s internal financial controls. In so doing, it is intended that the following standing annual agenda of the Committee represents the Board’s expectations of the Committee’s activities. Four meetings and four teleconferences of the Committee have been established to provide sufficient time for discussion of the agenda topics.
In addition, any other business which either the Audit Committee, the independent auditors, or management feels is appropriate will be added to the agenda along with review of any significant financing transactions. Also, the Audit Committee shall have the authority to retain special legal, accounting or other consultants to advise the Committee. The Audit Committee may request any officer or employee of the company or the company’s outside counsel or independent auditor to attend a meeting of the Committee or to meet with any members of, or consultants to, the Committee.
Date Agenda
| | | |
| Each Meeting | • | Provide time for the Committee to meet with management as well as separately with the independent auditors (representing financial audit and general audit) and for the review of significant general audit reports. Review and approve the Minutes of the prior meeting. |
| |
| • | Review current developments in accounting and reporting. Discuss impact, if any, on Genentech. |
B-2
| | | |
| • | Review all analyst reports and any press stories about Genentech’s accounting and disclosure. Management and the independent auditors should be available to explain comments. |
| |
Quarterly
| • | Conduct a quarterly teleconference to review with management, and the independent auditors, the company’s quarterly (or annual) financial results prior to the release of quarterly (or annual) results. |
| |
February
| • | Review and discuss audited financial statements with management and discuss audit and other matters as required by SAS No. 61, and as amended by SAS No. 90, with independent auditors. |
| |
| • | Review and discuss audited financial statements with management and discuss audit and other matters as required by SAS No. 61, and as amended by SAS No. 90, with independent auditors. |
| |
| • | Review independent auditor’s management letter and management’s responses. |
| |
| • | Independent auditors review GAAP methods of accounting and Genentech’s methodology. |
| |
| • | Review the report of the Audit Committee as required by the rules of the Securities and Exchange Commission to be included in the Company’s annual proxy statement, in accordance with the required frequency. |
| |
| • | Review and reassess the adequacy of this Charter and recommend any proposed changes to the Board for approval. |
| |
| • | Review the statement of affirmation for the NYSE related to the adequacy of the charter. |
| |
May
| • | Review quarterly reporting process and managements’ control thereof. |
| |
| • | Review tax compliance program and plan. |
| |
September
| • | Review scope of annual financial audit, quarterly review and general audit activities for coming year. |
| |
| • | Review independence of independent auditors and their staffing, fees and nature of annual financial audit, quarterly reviews, and general audit and non-audit services. |
| |
| • | Review: 1) resumes of the independent auditor partners and managers, 2) a description of the quality control procedures the independent auditor firm has established and 3) a report from the independent auditor firm describing any material issues raised by the most recent quality control review of the firm and describing the steps the firm has taken to deal with any reported problems. |
| |
| • | Review Company’s investment policy, interest rate management program and foreign exchange hedging program. |
| |
December
| • | Review Company’s insurance coverage and other risk management programs. |
| |
| • | Review with management, including General Counsel or his/ her designee, any legal matters that could have a significant impact on the Company’s financial statements. Discuss the relevant reserves with management. |
| |
| • | Review performance of independent auditors, for their annual financial audit, quarterly reviews, general audit and non-audit services, with management and recommend independent auditors for upcoming year for Board of Director approval. |
B-3
APPENDIX C
FINANCIAL MATTERS
In this document, “Genentech,” “we,” “us” and “our” refer to Genentech, Inc. “Common Stock” refers to Genentech’s common stock, par value $0.02 per share, “Special Common Stock” refers to Genentech’s callable putable common stock, par value $0.02 per share and “Redeemable Common Stock” refers to Genentech’s redeemable common stock, par value $0.02 per share.
Business Overview
Genentech is a leading biotechnology company using human genetic information to discover, develop, manufacture and market human pharmaceuticals that address significant unmet medical needs. Fifteen of the approved products of biotechnology stem from or are based on our science. We manufacture and market ten protein-based pharmaceuticals and license several additional products to other companies. See the “Products” section below for further information.
Redemption of Our Special Common Stock
On June 30, 1999, we redeemed all of our outstanding Special Common Stock held by stockholders other than Roche Holdings, Inc. (or Roche) at a price of $20.63 per share in cash with funds deposited by Roche for that purpose. We refer to this event as the “Redemption.” As a result, on that date, Roche’s percentage ownership of our outstanding Common Stock increased from 65% to 100%. Consequently, under U.S. generally accepted accounting principles, we were required to use push-down accounting to reflect in our financial statements the amounts paid for our stock in excess of our net book value. Push-down accounting required us to record $1,685.7 million of goodwill and $1,499.0 million of other intangible assets onto our balance sheet on June 30, 1999. Also, as a result of push-down accounting, we recorded special charges of $1,207.7 million related to the Redemption on June 30, 1999. For more information about special charges and push-down accounting, you should read “Special Charges” and the “Redemption of Our Special Common Stock” note in the Notes to Consolidated Financial Statements below. Roche subsequently completed public offerings of our Common Stock as described below.
Public Offerings
On July 23, 1999, October 26, 1999, and March 29, 2000, Roche completed public offerings of our Common Stock. We did not receive any of the net proceeds from these offerings. On January 19, 2000, Roche completed an offering of zero-coupon notes that are exchangeable for an aggregate of approximately 13.0 million shares of our Common Stock held by Roche. Roche’s percentage ownership of our outstanding Common Stock was 58.0% at December 31, 2001.
As a result of the Redemption and the subsequent public offerings, changes occurred with respect to our stock options as discussed in “Stock Options Changes” below. In addition, we amended our certificate of incorporation and bylaws, amended our licensing and marketing agreement with F. Hoffmann-La Roche Ltd (or Hoffmann-La Roche), an affiliate of Roche, and entered into or amended certain agreements with Roche, which are discussed in “Relationship With Roche” below.
Products
We manufacture and market ten protein-based pharmaceuticals listed below and license several additional products to other companies.
| | |
| | • | Herceptin antibody for the treatment of certain patients with metastatic breast cancer whose tumors overexpress the Human Epidermal growth factor Receptor type 2, or HER2, protein; |
C-1
| | |
| | • | Rituxan antibody which we market together with IDEC Pharmaceuticals Corporation (or IDEC) for the treatment of patients with relapsed or refractory low-grade or follicular, CD20-positive B-cell non-Hodgkin’s lymphoma; |
| |
| | • | TNKase single-bolus thrombolytic agent for the treatment of acute myocardial infarction (heart attack); |
| |
| | • | Activase tissue plasminogen activator (or t-PA) for the treatment of acute myocardial infarction, acute ischemic stroke (brain attack) within three hours of the onset of symptoms and acute massive pulmonary embolism (blood clots in the lungs); |
| |
| | • | Cathflo Activase tissue plasminogen activator approved for the restoration of function to central venous access devices that have become occluded due to a blood clot; |
| |
| | • | Nutropin Depot long-acting growth hormone for the treatment of growth failure associated with pediatric growth hormone deficiency; |
| |
| | • | Nutropin AQ liquid formulation growth hormone for the same indications as Nutropin; |
| |
| | • | Nutropin human growth hormone for the treatment of growth hormone deficiency in children and adults, growth failure associated with chronic renal insufficiency prior to kidney transplantation and short stature associated with Turner syndrome; |
| |
| | • | Protropin growth hormone for the treatment of inadequate endogenous growth hormone secretion, or growth hormone deficiency, in children; and |
| |
| | • | Pulmozyme inhalation solution for the treatment of cystic fibrosis. |
We receive royalties on sales of rituximab, Pulmozyme and Herceptin outside of the United States and on sales of human growth hormone, Rituxan, Pulmozyme, Activase and TNKase in Canada from Hoffmann-La Roche. We receive royalties on sales of growth hormone products within the United States and outside of the United States and t-PA outside of the United States and Canada, and on sales of tenecteplase outside of the United States, Canada and Japan. We also receive worldwide royalties on additional licensed products that are marketed by other companies. A number of these products originated from our technology.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Critical Accounting Policies and the Use of Estimates
The preparation of our financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. Actual results could differ materially from those estimates. The items in our financial statements requiring significant estimates and judgements are as follows:
| | |
| | • | Our inventories are stated at the lower of cost or market. Cost is determined using a weighted-average approach which approximates the first-in first-out method. If the cost of the inventories exceeds their expected market value, provisions are recorded currently for the difference between the cost and the market value. These provisions are determined based on significant estimates. Inventories consist of currently marketed products and pre-launch product candidates, which we expect to commercialize in the near term. |
| |
| | • | Nonmarketable equity securities and convertible debt are carried at cost. We periodically monitor the liquidity progress and financing activities of these entities to determine if impairment write downs are required. |
| |
| | • | We lease various real properties under operating leases that generally require us to pay taxes, insurance and maintenance. Five of our operating leases are commonly referred to as “synthetic leases.” A synthetic lease is a form of off-balance sheet financing under which an unrelated third party funds |
C-2
| | |
| | | 100% of the costs for the acquisition and/or construction of the property and leases the asset to a lessee, and at least 3% of the third party funds represent at risk equity. Our synthetic leases are treated as operating leases for accounting purposes and financing leases for tax purposes. We periodically review the fair values of the properties leased in order to determine potential accounting ramifications. Adverse changes in the fair value of the properties leased, or changes of the equity participation of the third parties could affect the classification of this lease from operating to financing. |
| |
| | • | We are currently involved in certain legal proceedings as discussed in the “Leases, Commitments and Contingencies” note in the Notes to Consolidated Financial Statements. We do not believe these legal proceedings will have a material adverse effect on our consolidated financial position, results of operations or cash flows. However, were an unfavorable ruling to occur in any quarterly period, there exists the possibility of a material impact on the operating results of that period. |
| |
| | • | We recognize revenue from product sales when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable, and collectibility is reasonably assured. Allowances are established for estimated uncollectible amounts, product returns and discounts. |
| |
| | • | We receive royalties from licensees, which are based on third party sales of licensed products or technologies. Royalties are recorded as earned in accordance with the contract terms when third party results are reliably measured and collectibility is reasonably assured. Royalty estimates are made in advance of amounts collected using historical and forecasted trends. |
| |
| | • | Contract revenue for research and development (or R&D) is recorded as earned based on the performance requirements of the contract. Non-refundable contract fees for which no further performance obligations exist, and there is no continuing involvement by Genentech, are recognized on the earlier of when the payments are received or when collection is assured. |
| |
| | Revenue from non-refundable upfront license fees and certain guaranteed payments where we continue involvement through development collaboration or an obligation to supply product is recognized ratably over the development period when, at the execution of the agreement, the development period involves significant risk due to the incomplete stage of the product’s development, or over the period of the manufacturing obligation, when, at the execution of the agreement, the product is approved for marketing, or nearly approvable, and development risk has been substantially eliminated. Deferred revenues related to manufacturing obligations are recognized on a straight-line basis over the longer of the contractual term of the manufacturing obligation or the expected period over which we will supply the product. |
| |
| | Revenue associated with performance milestones is recognized based upon the achievement of the milestones, as defined in the respective agreements. Revenue under R&D cost reimbursement contracts is recognized as the related costs are incurred. |
| |
| | Advance payments received in excess of amounts earned are classified as deferred revenue until earned. |
| | |
| | • | Research and development (or R&D) expenses include related salaries, contractor fees, building costs, utilities, administrative expenses and allocations of corporate costs. R&D expenses consist of independent R&D costs and costs associated with collaborative R&D and in-licensing arrangements. In addition, we fund R&D at other companies and research institutions under agreements, which are generally cancelable. R&D expenses also include activities such as product registries and investigator sponsored trials. All such costs are charged to R&D expense as incurred. |
Results of Operations
(dollars in millions, except per share amounts)
This discussion of our Results of Operations contains forward-looking statements regarding sales of Rituxan and expenses attributable to Research and Development (or R&D), Marketing, General and Administrative (or MG&A) and collaboration profit sharing. Actual sales or expenses could differ materially. For a discussion of the risks and uncertainties associated with sales of Rituxan, see “The Successful
C-3
Development of Pharmaceutical Products is Highly Uncertain,” “We May Be Unable to Retain Skilled Personnel and Maintain Key Relationships,” “We Face Growing and New Competition,” “Other Competitive Factors Could Affect Our Product Sales,” “Protecting Our Proprietary Rights Is Difficult and Costly,” “We May Be Unable to Obtain Regulatory Approvals for Our Products,” and “Difficulties or Delays in Product Manufacturing Could Harm Our Business” sections of “Forward-Looking Information and Cautionary Factors That May Affect Future Results” below; for R&D, MG&A and collaboration profit sharing expenses, see all of the foregoing and “We May Incur Material Litigation Costs” and “We May Incur Material Product Liability Costs” sections of “Forward-Looking Information and Cautionary Factors That May Affect Future Results” below.
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| | | | | | | | Annual Percent Change |
| | | | | | | |
|
| | 2001 | | 2000 | | 1999 | | 01/00 | | 00/99 |
| |
| |
| |
| |
| |
|
| Revenues | | $ | 2,212.3 | | | $ | 1,736.4 | | | $ | 1,401.0 | | | | 27 | % | | | 24 | % |
Total revenues for 2001 reached $2,212.3 million, a 27% increase from 2000 primarily due to higher product sales, royalties and interest income. These increases were partially offset by lower contract and other revenues. Revenues for 2000 increased 24% from 1999 primarily due to higher product sales and higher contract and other revenues. These revenue changes are further discussed below.
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| | | | | | | | Annual Percent Change |
| | | | | | | |
|
| Product Sales | | 2001 | | 2000 | | 1999 | | 01/00 | | 00/99 |
| |
| |
| |
| |
| |
|
| Herceptin | | $ | 346.6 | | | $ | 275.9 | | | $ | 188.4 | | | | 26 | % | | | 46 | % |
| Rituxan | | | 818.7 | | | | 444.1 | | | | 279.4 | | | | 84 | | | | 59 | |
| Activase/ TNKase/ Cathflo Activase | | | 197.1 | | | | 206.2 | | | | 236.0 | | | | (4 | ) | | | (13 | ) |
| Growth Hormone | | | 250.2 | | | | 226.6 | | | | 221.2 | | | | 10 | | | | 2 | |
| Pulmozyme | | | 122.9 | | | | 121.8 | | | | 111.4 | | | | 1 | | | | 9 | |
| Actimmune | | | 7.4 | | | | 3.7 | | | | 2.7 | | | | 100 | | | | 37 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Total product sales | | $ | 1,742.9 | | | $ | 1,278.3 | | | $ | 1,039.1 | | | | 36 | % | | | 23 | % |
| Percent of total revenues | | | 79 | % | | | 74 | % | | | 74 | % | | | | | | | | |
Total net product sales were $1,742.9 million in 2001, an increase of 36% from 2000 primarily as a result of higher sales of our bio-oncology products, Rituxan and Herceptin, and higher sales of our growth hormone products. Total net product sales were $1,278.3 million in 2000, an increase of 23% from 1999 reflecting the effect of increased Rituxan and Herceptin sales. Product sales in connection with our licensing agreement with F. Hoffmann-La Roche (or Hoffmann-La Roche) were $76.3 million in 2001, $67.4 million in 2000 and $41.3 million in 1999. See “Relationship With Roche” below for further information about our licensing agreement with Hoffmann-La Roche. See below for further information.
Net sales of Herceptin were $346.6 million in 2001, a 26% increase from 2000, and $275.9 million in 2000, a 46% increase from 1999. The year to year increases continue to be driven primarily by increased penetration in the metastatic breast cancer market. In addition, the increase in 2001 included approximately $19.5 million related to a change in our distribution process for Herceptin. During the fourth quarter of 2001, we began shipping Herceptin to drug wholesaler distributors rather than direct shipment to customers. As is typical with this process, Herceptin was purchased by the wholesalers in order to stock sufficient inventory to assume product distribution. The initial stocking orders resulted in unusually higher sales in the fourth quarter of 2001 that may not be experienced in future periods.
C-4
We have granted Hoffmann-La Roche exclusive marketing rights to Herceptin outside of the United States. Hoffmann-La Roche markets Herceptin for the treatment of HER2-positive metastatic breast cancer in Europe. We receive royalties from Hoffmann-La Roche for these European Herceptin product sales.
Net sales of Rituxan were $818.7 million in 2001, an 84% increase from 2000, and $444.1 million in 2000, a 59% increase from 1999. The year to year increases were primarily due to increased market penetration for the treatment of B-cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. In addition, sales of Rituxan increased in 2001 and in the last quarter of 2000 due to the announcement at the American Society of Hematology of the results of a study conducted by the Groupe d’Etude des Lymphomes de l’Adulte (or GELA) reporting on the benefits of using Rituxan, combined with standard chemotherapy, for treating aggressive non-Hodgkin’s lymphoma. We expect these factors to continue to positively impact Rituxan sales in 2002, however, the rate of sales growth is expected to be more modest than that seen in 2001.
We co-developed Rituxan with IDEC Pharmaceuticals Corporation (or IDEC) from which we license Rituxan. IDEC and Genentech jointly promote Rituxan in the United States. Hoffmann-La Roche markets rituximab under the tradename MabThera® in the European Union. Hoffmann-La Roche holds marketing rights for Rituxan in Canada and for MabThera outside of the U.S., excluding Japan, and has agreed to pay us royalties and cost plus a mark-up on the product we supply them. We receive net sales of MabThera from Zenyaku Kogyo Co., LTD., a distribution company that markets MabThera in Japan.
| |
| Activase, TNKase and Cathflo Activase |
Net sales of our three cardiovascular products, Activase, TNKase and Cathflo Activase, were $197.1 million, a decrease of 4% from sales of Activase and TNKase in 2000. Cathflo Activase received FDA approval in early September 2001 and was launched in late September 2001. In 2000, net sales of our two cardiovascular products, Activase and TNKase, were $206.2 million, a decrease of 13% from 1999. The year to year decreases continue to be driven by modest loss of market share resulting from aggressive price discounting by one of our competitors. The year to year decreases were also attributable to the decline in the overall size of the thrombolytic market as a result of increasing use of mechanical reperfusion as well as early intervention with other therapies in the treatment of acute myocardial infarction. These factors are expected to continue to impact sales of our cardiovascular products in 2002.
Net sales of our four growth hormone products, Nutropin Depot, Nutropin AQ, Nutropin and Protropin, were $250.2 million in 2001, an increase of 10% from 2000. This net sales growth primarily reflects an increase in adult new patient starts, patients staying on the product longer, and to a lesser extent, the effects of a price increase in January 2001 for these products and an increase in sales of Nutropin Depot. Net sales of our growth hormone products increased slightly in 2000 compared to 1999. This increase was largely due to fluctuations in customer ordering patterns and the introduction of Nutropin Depot. Nutropin Depot is a long-acting dosage form of recombinant growth hormone approved for pediatric growth hormone deficiency.
Net Pulmozyme sales were $122.9 million in 2001, a slight increase over 2000 and primarily reflects fluctuations in distributor ordering patterns. Net Pulmozyme sales were $121.8 million in 2000, a 9% increase from 1999. This increase was attributable to increased market penetration in the early and mild patient populations for the treatment of cystic fibrosis.
Net sales of Actimmune were $7.4 million in 2001, $3.7 million in 2000 and $2.7 million in 1999. In the second quarter of 1998, in return for a royalty on net sales, we licensed U.S. marketing and development rights to interferon gamma, including Actimmune, to Connetics Corporation. Thereafter, Connetics sublicensed all
C-5
of its rights to InterMune Pharmaceuticals, Inc. (or InterMune). As of January 1999, we no longer sell Actimmune directly in the U.S. We agreed to sell packaged drug product to InterMune at cost plus a mark-up through December 31, 2001. As of January 1, 2002, we no longer manufacture, use or sell Actimmune to InterMune.
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| | | | | | | | Annual Percent |
| | | | | | | | Change |
| | | | | | | |
|
| Royalties, Contract and Other, and Interest Income | | 2001 | | 2000 | | 1999 | | 01/00 | | 00/99 |
| |
| |
| |
| |
| |
|
| Royalties | | $ | 264.5 | | | $ | 207.2 | | | $ | 189.3 | | | | 28 | % | | | 9 | % |
| Contract and other | | | 74.4 | | | | 160.4 | | | | 83.2 | | | | (54 | ) | | | 93 | |
| Interest income | | | 130.5 | | | | 90.4 | | | | 89.4 | | | | 44 | | | | 1 | |
Royalty income was $264.5 million in 2001, an increase of 28% from 2000. This increase was primarily due to higher third-party sales by Hoffmann-La Roche and various licensees, offset in part by lower sales by several licensees including one that has been addressing manufacturing issues which has temporarily impacted their ability to manufacture product for sale. Royalty income was $207.2 million in 2000, an increase of 9% from 1999. This increase was due to higher third-party sales by various licensees. Royalty income from Hoffmann-La Roche totaled $87.9 million in 2001, $46.8 million in 2000 and $42.5 million in 1999.
Cash flows from royalty income include revenues denominated in foreign currencies. We currently purchase simple foreign currency put option contracts (or options) to hedge these foreign royalty cash flows. The term of these options is generally one to three years. See “Forward-Looking Information and Cautionary Factors That May Affect Future Results” below for a discussion of market risks related to these financial instruments.
| |
| Contract and Other Revenues |
Contract and other revenues were $74.4 million, a decrease of 54% from 2000. This decrease was primarily due to lower gains from the sale of biotechnology equity securities, partially offset by higher contract revenues, and the recognition of $10.0 million in gains related to the change in the time value of certain hedging instruments in the first quarter of 2001. (See the “Derivative Financial Instruments” note of the Notes to Consolidated Financial Statements below for more information on our derivative and hedging activities.) The increase in the contract revenue component of this line in 2001 was due to the recognition of $21.2 million of revenues from third-party collaborators that were previously recognized then deferred under the Securities and Exchange Commission’s Staff Accounting Bulletin No. 101 (or SAB 101), offset in part by lower contract revenues from third-party collaborators. Contract and other revenues were $160.4 million in 2000, an increase of 93% over 1999. This increase was primarily due to higher gains from the sale of biotechnology equity securities in 2000 and the recognition of $8.6 million of deferred revenues related to SAB 101, offset in part by lower contract revenues from third-party collaborators. (See “Changes in Accounting Principles” below for further discussion of SAB 101.)
Contract revenues from Hoffmann-La Roche, including reimbursement for ongoing development expenses after the option exercise date, totaled $5.8 million in 2001, $3.5 million in 2000 and $17.2 million in 1999.
We expect quarterly fluctuations in contract and other revenues depending on milestone payments, the number of new contract arrangements and Hoffmann-La Roche’s potential opt-ins for products.
Interest income was $130.5 million in 2001, a 44% increase from 2000. This increase was primarily due to higher average portfolio balances. Interest income was $90.4 million in 2000, which was comparable to 1999. Our fixed income portfolio includes cash and cash equivalents, short-term and long-term investments, excluding marketable equity securities. Interest income will depend on fluctuations of interest rates, our use of
C-6
cash for Genentech common stock repurchases, the payment of our maturing convertible subordinated debentures and possible acquisitions in 2002.
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| | | | | | | | Annual Percent |
| | | | | | | | Change |
| | | | | | | |
|
| Cost and Expenses | | 2001 | | 2000 | | 1999 | | 01/00 | | 00/99 |
| |
| |
| |
| |
| |
|
| Cost of sales | | $ | 354.5 | | | $ | 364.9 | | | $ | 285.6 | | | | (3 | )% | | | 28 | % |
| Research and development | | | 526.2 | | | | 489.9 | | | | 367.3 | | | | 7 | | | | 33 | |
| Marketing, general and administrative | | | 474.4 | | | | 368.2 | | | | 393.6 | | | | 29 | | | | (6 | ) |
| Collaboration profit sharing | | | 246.7 | | | | 128.8 | | | | 74.3 | | | | 92 | | | | 73 | |
| Special charges: | | | | | | | | | | | | | | | | | | | | |
| | Related to redemption | | | — | | | | — | | | | 1,207.7 | | | | — | | | | — | |
| | Legal settlements | | | — | | | | — | | | | 230.0 | | | | — | | | | — | |
| Recurring charges related to redemption | | | 321.8 | | | | 375.3 | | | | 197.7 | | | | (14 | ) | | | 90 | |
| Interest expense | | | 5.7 | | | | 5.3 | | | | 5.4 | | | | 8 | | | | (2 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total costs and expenses | | $ | 1,929.3 | | | $ | 1,732.4 | | | $ | 2,761.6 | | | | 11 | % | | | (37 | )% |
| Percent of total revenues | | | 87 | % | | | 100 | % | | | 197 | % | | | | | | | | |
| COS as a % of product sales | | | 20 | | | | 29 | | | | 27 | | | | | | | | | |
| R&D as % of total revenues | | | 24 | | | | 28 | | | | 26 | | | | | | | | | |
| MG&A as % of total revenues | | | 21 | | | | 21 | | | | 28 | | | | | | | | | |
Cost of sales (or COS) was $354.5 in 2001, a decrease of 3% from 2000. COS as a percentage of product sales was 20%, a decrease from 29% in 2000. The decrease primarily reflects a decline in the costs recognized on the sale of inventory that was written up at the Redemption due to push-down accounting, lower provisions for nonuseable inventory, a change in the product mix and lower overall costs due to manufacturing efficiencies. The inventory written up at the Redemption was sold by December 31, 2000. COS was $364.9 million in 2000, an increase of 28% from 1999. COS as a percentage of product sales was 29% in 2000, an increase from 27% in 1999. This increase primarily reflects the effect of push-down accounting, a change in the product mix, an increase in provisions established for nonuseable inventory and higher product sales to Hoffmann-La Roche. As a result of push-down accounting, we recognized additional expense of $92.8 million in 2000 and $93.4 million in 1999 related to the sale of inventory that was written up as a result of the Redemption.
COS for products sold to Hoffmann-La Roche totaled $63.8 million in 2001, $56.7 million in 2000 and $36.3 million in 1999.
Research and development expenses in 2001 were $526.2 million, an increase of 7% from 2000. The increase was primarily due to higher expenses related to late-stage clinical trials, higher repairs and maintenance expenses, higher provisions for pre-launch commercial inventory, offset in part by lower in-licensing expenses. R&D expenses in 2000 were $489.9 million, up 33% from 1999. This increase was due to higher costs related to late-stage clinical trials and higher in-licensing and collaboration expenses.
C-7
The major components of R&D expenses for 2001, 2000 and 1999 were as follows (in millions):
| | | | | | | | | | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| Research | | $ | 122.5 | | | $ | 118.4 | | | $ | 100.3 | |
| Development | | | 362.9 | | | | 309.6 | | | | 253.7 | |
| In-licensing | | | 40.8 | | | | 61.9 | | | | 13.3 | |
| | | |
| | | |
| | | |
| |
| | Total | | $ | 526.2 | | | $ | 489.9 | | | $ | 367.3 | |
| | | |
| | | |
| | | |
| |
R&D is expected to trend higher in 2002 due to the number of products in late-stage clinical development and higher costs related to potential regulatory filings.
In-licensing expenses in 2001 included a $15.0 million upfront payment to OSI Pharmaceuticals, Inc. (or OSI) for the purchase of in-process research and development (or IPR&D) under an agreement with us, OSI and Hoffmann-La Roche for the global co-development and commercialization of Tarceva for the potential treatment of solid tumor cancers. One of the members of the Board of Directors of OSI is also a member of the Board of Directors of Genentech.
In-licensing expenses in 2000 included a $25.0 million upfront payment to Actelion Ltd., for the purchase of IPR&D under an agreement with Actelion to develop and co-promote Tracleer in the U.S. for the potential treatment of acute and chronic heart failure. Actelion led the development efforts for Tracleer. In February 2002, Genentech and Actelion announced that the Phase III clinical trial of Tracleer did not meet its primary objective of significantly improving symptoms associated with chronic heart failure. In-licensing expenses in 2000 also included a $15.0 million payment for the purchase of IPR&D under an agreement with Actelion for the rights to develop and co-promote Veletri in the U.S. for the potential treatment of acute heart failure. In April 2001, Genentech and Actelion announced that the second pivotal Phase III clinical trial of Veletri did not meet its primary objective of significantly improving symptoms associated with acute heart failure. Actelion is planning an additional Phase III trial of Veletri.
We determined that the above acquired IPR&D was not yet technologically feasible and that the acquired technology had no future alternative uses.
Biopharmaceutical products that we develop internally generally take 10 to 15 years (an average of 12 years) to research, develop and bring to market a new prescription medicine in the United States. Drug development in the U.S. is a process that includes several steps defined by the FDA. The process begins with the filing of an Initial Drug Application (or IND) which, if successful, allows opportunity for clinical study of the potential new medicine. Clinical development typically involves three phases of study: Phase I, II, and III, and we have found that it accounts for an average of seven years of a drug’s total development time. The most significant costs associated with clinical development are the Phase III trials as they tend to be the longest and largest studies conducted during the drug development process. We currently have approximately 10 potential products in development that are in Phase III or are preparing for Phase III studies. The successful development of our products is highly uncertain. An estimation of product completion dates and completion costs can vary significantly for each product and are difficult to predict. Various statutes and regulations also govern or influence the manufacturing, safety, labeling, storage, record keeping and marketing of each product. The lengthy process of seeking these approvals, and the subsequent compliance with applicable statutes and regulations, require the expenditure of substantial resources. Any failure by us to obtain, or any delay in obtaining, regulatory approvals could materially adversely affect our business. In responding to a New Drug Application (or NDA) or a Biologic License Application (or BLA), the FDA may grant marketing approval, request additional information or deny the application if it determines that the application does not provide an adequate basis for approval. We can not assure you that any approval required by the FDA will be obtained on a timely basis, if at all. For additional discussion of the risks and uncertainties associated with completing development of potential products, see “The Successful Development of Pharmaceutical Products is Highly Uncertain” section of our “Forward-Looking Information and Cautionary Factors That May Affect Future Results” below.
C-8
Below is a summary of products and the related stages of development for each product in clinical development. The information in the column labeled “Estimate of Completion of Phase” contains forward-looking statements regarding timing of completion of product development phases. The actual timing of completion of those phases could differ materially from the estimates provided in the table. For a discussion of the risks and uncertainties associated with the timing of completing a product development phase, see “The Successful Development of Pharmaceutical Products is Highly Uncertain,” “We May Be Unable to Retain Skilled Personnel and Maintain Key Relationships,” “Protecting Our Proprietary Rights Is Difficult and Costly” and “We May Be Unable to Obtain Regulatory Approvals for Our Products” sections of “Forward-Looking Information and Cautionary Factors That May Affect Future Results” below.
| | | | | | | | | | | | |
| | | | | | | | Estimate of |
| | | | Phase of | | | | Completion of |
| Product | | Description/Indication | | Development | | Collaborator | | Phase* |
| |
| |
| |
| |
|
| Xolair (Anti-IgE | | | | | | | | | | |
| | antibody) | | allergic asthma | | Awaiting regulatory approval | | Novartis Pharmaceuticals Corporation | | | 2003 | |
| Nutropin AQ Pen | | liquid formulation growth hormone | | Awaiting regulatory approval | | | | | 2002 | |
| Xanelim (Anti-CD11a | | | | | | | | | | |
| | antibody) | | psoriasis | | Phase III | | XOMA Ltd. | | | 2002 | |
| Rituxan antibody | | intermediate- and high-grade non-Hodgkin’s lymphoma | | Phase III | | IDEC Pharmaceuticals | | | 2002 | |
| Avastin (Anti-VEGF | | | | | | | | | | |
| | antibody) | | colorectal cancer; second/third line metastatic breast cancer; non-small cell lung cancer; first line metastatic breast cancer | | Phase III | | | | | 2002-2005 | |
| Herceptin antibody | | adjuvant early-stage breast cancer | | Phase III | | F. Hoffmann- La Roche and U.S. national cooperative groups | | | 2006 | |
| Tarceva | | solid tumor cancers, pancreatic cancer; non-small cell lung cancer | | Phase III | | OSI Pharmaceuticals and F. Hoffmann-La Roche | | | 2003 | |
| Nutropin Depot | | adults with growth hormone deficiency | | Phase III | | Alkermes, Inc. | | | 2003 | |
| Cathflo Activase t-PA | | treatment of hemodialysis catheters experiencing sluggish flow | | Preparing for Phase III | | | | | 2002 | |
| Rituxan | | ideopathic thrombocytopenic purpura | | Preparing for Phase III | | IDEC Pharmaceuticals | | | 2002 | |
| LDP-02 | | inflammatory bowel diseases | | Phase II | | Millennium Pharmaceuticals, Inc. | | | 2003 | |
| Avastin (Anti-VEGF | | | | | | | | | | |
| | antibody) | | renal cell carcinoma | | Phase II | | | | | 2002 | |
C-9
| | | | | | | | | | | | |
| | | | | | | | Estimate of |
| | | | Phase of | | | | Completion of |
| Product | | Description/Indication | | Development | | Collaborator | | Phase* |
| |
| |
| |
| |
|
| Efalizumab (Anti- | | | | | | | | | | |
| | CD11a antibody) | | rheumatoid arthritis | | Preparing for Phase II | | XOMA Ltd. | | | 2002 | |
| RhuFab V2 AMD | | age-related macular degeneration | | Phase I | | | | | 2002 | |
| Efalizumab (Anti- | | | | | | | | | | |
| | CD11a antibody) | | treatment to prevent solid organ transplant rejection | | Phase I | | XOMA Ltd. | | | 2002 | |
| 2C4 | | cancer | | Phase I | | F. Hoffmann-La Roche | | | 2002 | |
| PRO64553 (Anti- | | | | | | | | | | |
| | CD40 antibody) | | hematologic malignancies | | Preparing for Phase I | | | | | 2002 | |
| Trastuzumab-DM1 | | human epidermal growth factor receptor-type 2 | | Preparing for Phase I | | | | | 2002 | |
| Anti-Tissue Factor | | | | | | | | | | |
| | antibody | | acute coronary syndrome | | Preparing for Phase I | | | | | 2002 | |
| |
| * | Note: For those projects preparing for a Phase, the estimated date of completion refers to the date the project enters the Phase. |
We establish strategic alliances with various companies to gain additional access to potential new products and technologies, and to utilize companies to help develop potential new products. These companies are developing technologies that may fall outside our research focus and through technology exchanges and investments with these companies, we may have the potential to generate new products. As part of these strategic alliances, we have acquired equity and convertible debt securities of such companies. We have also entered into product-specific collaborations to acquire development and marketing rights for potential products as discussed below.
We entered into a research collaboration agreement with CuraGen Corporation in November 1997, as amended and restated in March 2000, and agreed to provide a convertible equity loan to CuraGen of up to $21.0 million. In October 1999, CuraGen exercised its right to borrow $16.0 million. Simultaneously, with this draw down, CuraGen repaid the loan by issuing common shares of CuraGen stock valued at $16.0 million. Our remaining commitment to CuraGen on the convertible equity loan is $5.0 million. At December 31, 2001, there were no outstanding loans to CuraGen.
In December 1997, we entered into a research collaboration agreement with Millennium to develop and commercialize Millennium’s LDP-02. Under the terms of the agreement, we have agreed to provide a convertible equity loan for approximately $15.0 million to fund Phase II development costs. Upon successful completion of Phase II, if Millennium agrees to fund 25% of Phase III development costs, we have agreed to provide a second loan to Millennium for such funding. As of December 31, 2001, there were no outstanding loans to Millennium.
In April 1996, we entered into a research collaboration agreement with XOMA to develop and commercialize Xanelim. Under the terms of the agreement, we have agreed to provide a convertible equity loan to XOMA of up to $60.0 million to fund XOMA’s share of development costs for Xanelim until the completion of Phase III clinical trials. There is no revenue impact on our statements of operations as it relates to this loan. As of December 31, 2001, XOMA had an outstanding loan balance of approximately $51.0 million.
C-10
| |
| Marketing, General and Administrative |
Marketing, general and administrative (or MG&A) expenses in 2001 increased 29% from 2000. The general and administrative component of this line was higher by $65.9 million in 2001 due to the write-down of certain biotechnology equity investments as a result of other than temporary impairment, higher royalty expenses, and higher legal and other corporate expenses. The marketing and sales component of this line was higher by $40.3 million in 2001 due to the continued growth of our bio-oncology products, higher expenses related to the commercial development of pipeline products, new information technology, and additional programs and increased headcount to support all products. MG&A expenses in 2000 decreased 6% from 1999 due to lower general and administrative expenses while marketing and sales expenses were higher. The general and administrative component of this line was lower by $57.9 million in 2000 primarily due to the write-down of certain biotechnology investments as a result of other than temporary impairment and higher legal expenses in 1999. The marketing and sales component of this line was higher by $32.5 million in 2000 driven by the continued growth of our bio-oncology products, the launch of TNKase, and the prelaunch support of Xolair for the potential treatment of allergic asthma.
MG&A expenses are expected to continue to trend higher in 2002 with the increases driven by the marketing and sales component of this line as we prepare for the potential product launches in 2003.
| |
| Collaboration Profit Sharing |
Collaboration profit sharing consists primarily of the net operating profit sharing with IDEC on Rituxan sales and, to a much lesser extent the sharing of costs with collaborators related to the commercialization of future products. Collaboration profit sharing expenses increased to $246.7 million in 2001, a 92% increase from 2000. Collaboration profit sharing expenses were $128.8 million in 2000, a 73% increase from 1999. These increases were primarily due to increased Rituxan profit sharing with IDEC due to higher Rituxan sales.
Collaboration profit sharing expense is expected to increase in 2002 consistent with our expectations of higher Rituxan sales.
During 1999, we had special charges of $1,437.7 million related to the Redemption and the application of push-down accounting, and legal settlements. The Redemption related charge of $1,207.7 million primarily included: (1) a non-cash charge of $752.5 million for IPR&D, (2) $284.5 million of compensation expense related to early cash settlement of certain employee stock options and (3) an aggregate of approximately $160.1 million as a non-cash charge for the remeasurement of the value of continuing employee stock options. See “In-Process Research and Development” below and the “Redemption of Our Special Common Stock” note in the Notes to Consolidated Financial Statements below for further information regarding these special charges.
The legal settlements charge included: (1) a $50.0 million settlement related to a federal investigation of our past clinical, sales and marketing activities associated with human growth hormone; and (2) a $180.0 million charge for the settlement of the patent infringement lawsuits brought by the University of California relating to our human growth hormone products. See the “Leases, Commitments and Contingencies” note in the Notes to Consolidated Financial Statements below for further information regarding these special charges.
| |
| Recurring Charges Related to Redemption |
We began recording recurring charges related to the Redemption and push-down accounting in the third quarter of 1999. These charges were $321.8 million in 2001, $375.3 million in 2000 and $197.7 million in 1999. These charges were comprised of $317.6 million in 2001, $364.2 million in 2000 and $190.4 million in 1999 related to the amortization of other intangible assets and goodwill, and $4.2 million in 2001, $11.1 million in 2000 and $7.3 million in 1999 of compensation expense related to alternative arrangements provided at the time of the Redemption for certain holders of some of the unvested options.
C-11
We adopted the new Statement of Financial Accounting Standards (or FAS) on accounting for goodwill and other intangible assets (or FAS 141 and 142) on January 1, 2002. As a result, we will no longer amortize goodwill and our trained and assembled workforce intangible asset, which we estimate will increase reported net income by approximately $150.0 million (or $0.28 per share) in 2002 (net of related taxes). See also “New Accounting Pronouncements Will Impact Our Financial Position and Results of Operations” below in the “Forward-Looking Information and Cautionary Factors That May Affect Future Results.”
Interest expense has fluctuated depending on the amounts invested and the level of interest capitalized on construction projects. Interest expense, net of amounts capitalized, relates to interest on our 5% convertible subordinated debentures. Interest expense in 2002 is expected to decline as a result of the repayment of our debentures, which mature on March 27, 2002. See the “Debt Obligations” note in the Notes to Consolidated Financial Statements below for further information regarding these debentures.
| | | | | | | | | | | | | |
| Income (Loss) Before Taxes and Cumulative Effect of Accounting | | | | | | |
| Change, Income Taxes and Cumulative Effect of Accounting Change | | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| Income (loss) before taxes and cumulative effect of accounting change | | $ | 283.0 | | | $ | 4.0 | | | $ | (1,360.6 | ) |
| Income tax provision (benefit) | | | 127.1 | | | | 20.4 | | | | (203.1 | ) |
| Income (loss) before cumulative effect of accounting change | | | 155.9 | | | | (16.4 | ) | | | (1,157.5 | ) |
| Cumulative effect of accounting change, net of tax | | | (5.6 | ) | | | (57.8 | ) | | | — | |
| |
| Changes in Accounting Principles |
We adopted FAS 133, “Accounting for Derivatives and Hedging Activities,” on January 1, 2001. Upon adoption, we recorded a $5.6 million charge, net of tax, ($0.01 per share), as a cumulative effect of a change in accounting principle and an increase of $5.0 million, net of tax, in other comprehensive income related to recording derivative instruments at fair value. See the “Description of Business and Summary of Significant Accounting Policies” note in the Notes to Consolidated Financial Statements below for further information on our adoption of FAS 133.
We adopted SAB 101 on January 1, 2000, and recorded a $57.8 million charge (net of tax) as a cumulative effect of a change in accounting principle related to contract revenues recognized in prior periods. The related deferred revenue is being recognized over the appropriate terms in each of the effected agreements. For the year ended December 31, 2000, the impact of the change in accounting principle was to increase net loss by $52.6 million (or $0.10 per share) comprised of $57.8 million cumulative effect of an accounting change, net of tax, (or $0.11 per share) net of $5.2 million of the related deferred revenue, net of tax, (or $0.01 per share) that was recognized as revenue during the year ended December 31, 2000.
The tax provision of $127.1 million in 2001 increased over the tax provision of $20.4 million in 2000 primarily due to increased pretax income before non-deductible goodwill amortization related to the Redemption. The 2001 tax provision reflects decreased benefit of R&D tax credits which was offset by prior years items. Prior years items relate principally to changes in estimate resulting from events in 2001 that provided greater certainty as to the expected outcome of prior matters. The tax provision of $20.4 million for 2000 increased over the 1999 tax benefit of $203.1 million primarily due to increased pretax income before non-deductible goodwill amortization related to the Redemption and non-deductible IPR&D charges in 1999. The increase was partially offset by the increased benefit of R&D tax credits in 2000.
The elimination of the amortization of goodwill pursuant to the adoption of FAS 141 and 142 will have a favorable impact on our effective tax rate in 2002. See also “New Accounting Pronouncements Will Impact Our Financial Position and Results of Operations” below in the “Forward-Looking Information and Cautionary Factors That May Affect Future Results.” Other factors may have favorable or unfavorable effects
C-12
upon our effective tax rate in 2002 and subsequent years. These factors include, but are not limited to, interpretations of existing tax laws, changes in tax laws and rates, future levels of R&D spending, future levels of capital expenditures, and our success in R&D and commercializing products.
| | | | | | | | | | | | | | | |
| Net Income (Loss) | | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| Net income (loss) | | $ | 150.3 | | | $ | (74.2 | ) | | $ | (1,157.5 | ) |
| Earnings (loss) per share: | | | | | | | | | | | | |
| | Basic: | | | | | | | | | | | | |
| | | Earnings (loss) before cumulative effect of accounting change | | $ | 0.30 | | | $ | (0.03 | ) | | $ | (2.26 | ) |
| | | Cumulative effect of accounting change, net of tax | | | (0.01 | ) | | | (0.11 | ) | | | — | |
| | | |
| | | |
| | | |
| |
| Net earnings (loss) per share | | $ | 0.29 | | | $ | (0.14 | ) | | $ | (2.26 | ) |
| | | |
| | | |
| | | |
| |
| | Diluted: | | | | | | | | | | | | |
| | | Earnings (loss) before cumulative effect of accounting change | | $ | 0.29 | | | $ | (0.03 | ) | | $ | (2.26 | ) |
| | | Cumulative effect of accounting change, net of tax | | | (0.01 | ) | | | (0.11 | ) | | | — | |
| | | |
| | | |
| | | |
| |
| Net earnings (loss) per share | | $ | 0.28 | | | $ | (0.14 | ) | | $ | (2.26 | ) |
| | | |
| | | |
| | | |
| |
Net income increased in 2001 to $150.3 million, or $0.28 per diluted share, from a net loss of $(74.2) million in 2000, or $(0.14) per diluted share. The increase from last year primarily reflects higher revenues largely from increased product sales, a decrease in costs related to the sale of inventory written up at the Redemption, a decrease in recurring charges related to the Redemption, and the cumulative effect of an accounting change impact in 2001 related to the adoption of FAS 133 as compared to the adoption of SAB 101 in 2000. These favorable variances were offset in part by increased collaboration profit sharing expenses, higher MG&A, R&D and income tax expenses and a decrease in contract and other revenues.
The net loss of $(74.2) million, or $(0.14) per diluted share in 2000, primarily reflects a full year of recurring charges for the amortization of goodwill and other intangible assets related to the Redemption and push-down accounting, costs related to the sale of inventory that was written up at the Redemption and the cumulative effect of an accounting change related to our adoption of SAB 101. The net loss in 1999 of $(1,157.5) million, or $(2.26) per diluted share, is attributable to the Redemption and related push-down accounting, and legal settlements, net of their related tax effects.
| |
| In-Process Research and Development |
At June 30, 1999, the Redemption date, we determined that the acquired in-process technology was not technologically feasible and that the in-process technology had no future alternative uses. In 1990 and 1991 through 1997, Roche Holdings, Inc. (or Roche) purchased 60% and 5%, respectively, of our outstanding common stock. The push-down effect of Roche’s aggregate purchase price is allocated based on Roche’s ownership percentages as if the purchases had occurred at the original purchase dates for the 1990 and 1991 through 1997 purchases. Therefore, 65% of the purchase price allocated to IPR&D as of September 7, 1990, or 65% of $770.0 million ($500.5 million) was recorded as an adjustment to additional paid-in capital related to the 1990-1997 acquisitions. The remaining 35% of our outstanding common stock not owned by Roche was purchased in 1999. Accordingly, 35% of $2,150.0 million of total fair value at the Redemption date, or $752.5 million, was expensed on June 30, 1999.
The amounts of IPR&D were determined based on an analysis using the risk-adjusted cash flows expected to be generated by the products that result from the in-process projects. The analysis included forecasted future cash flows that were expected to result from the progress made on each of the in-process projects prior to the purchase dates. These cash flows were estimated by first forecasting, on a product-by-product basis, total revenues expected from sales of the first generation of each in-process product. A portion
C-13
of the gross in-process product revenues was then removed to account for the contribution provided by any core technology, which was considered to benefit the in-process products. The net in-process revenue was then multiplied by the project’s estimated percentage of completion as of the purchase dates to determine a forecast of net IPR&D revenues attributable to projects completed prior to the purchase dates. Appropriate operating expenses, cash flow adjustments and contributory asset returns were deducted from the forecast to establish a forecast of net returns on the completed portion of the in-process technology. Finally, these net returns were discounted to a present value at discount rates that incorporate both the weighted-average cost of capital (relative to the biotech industry and us) as well as the product-specific risk associated with the purchased IPR&D products. The product-specific risk factors included each product in each phase of development, type of molecule under development, likelihood of regulatory approval, manufacturing process capability, scientific rationale, pre-clinical safety and efficacy data, target product profile and development plan. The discount rates ranged from 16% to 19% for the 1999 valuation and 20% to 28% for the 1990 purchase valuation, all of which represent a significant risk premium to our weighted-average cost of capital.
The forecast data in the analysis was based on internal product level forecast information maintained by our management in the ordinary course of managing the business. The inputs used by us in analyzing IPR&D were based on assumptions, which we believed to be reasonable but which were inherently uncertain and unpredictable. These assumptions may be incomplete or inaccurate, and no assurance can be given that unanticipated events and circumstances will not occur.
A brief description of projects that were included in the IPR&D charge is set forth below, including an estimated percentage of completion as of the Redemption date. Projects subsequently added to the research and development pipeline are not included. Except as otherwise noted below, since the Redemption date there have been no significant changes to the phase of development for the projects listed. We do not track all costs associated with research and development on a project-by-project basis. Therefore, we believe a calculation of cost incurred as a percentage of total incurred project cost as of FDA approval is not possible. We estimated, however, that the R&D expenditures that will be required to complete the in-process projects will total at least $550.0 million as of December 31, 2001, as compared to $700.0 million as of the Redemption date. This estimate reflects costs incurred since the Redemption date, discontinued projects, and decreases in cost to complete estimates for other projects, partially offset by an increase in certain cost estimates related to early stage projects and changes in expected completion dates.
The foregoing discussion of our IPR&D projects, and in particular the following table and subsequent paragraphs regarding the future of these projects, our additional product programs and our process technology program include forward-looking statements that involve risks and uncertainties, and actual results may vary materially. For a discussion of risk factors that may affect projected completion dates and the progress of research and development, see the “Forward-Looking Information and Cautionary Factors That May Affect Future Results” below.
At the Redemption date, we estimated percentage complete data for each project based on weighing of three indicators, as follows:
PTS: Probability of technical success, or PTS, is a project level statistic maintained by us on an ongoing basis, which is intended to represent the current likelihood of project success, i.e., FDA approval. This is a quantitative calculation based on the stage of development and the complexity of the project, and it is highly correlated with the project’s phase of development. PTS is periodically adjusted to reflect actual experiences over a reasonable period of time.
Status Compared to Baseline Model:We developed a baseline model which allocated percentages of a standard development project to each major phase of the project based on our experience. We then overlaid the time-based status of each project to this baseline model, in order to calculate a percentage complete for each project.
C-14
Management’s Estimate of Percentage Complete:Below is a list of the projects and their estimated percentage complete included in the IPR&D charge related to the Redemption:
| | | | | | | | | | | | | |
| | | | |
| | | | As of the Redemption Date, June 30, 1999 |
| | | |
|
| | | | | | Substantial | | |
| | | | Phase of | | Completion | | |
| Product | | Description/Indication | | Development | | Date | | % Complete |
| |
| |
| |
| |
|
| Nutropin Depot | | long-acting dosage form of recombinant growth hormone | | Awaiting Regulatory Approval | | | 2000 | | | | 85% | |
| TNKase, second generation t-PA | | acute myocardial infarction | | Awaiting Regulatory Approval | | | 2000 | | | | 90% | |
| Anti-IgE antibody | | allergic asthma, seasonal allergic rhinitis | | Phase III | | | 2001 | | | | 75% | |
| Pulmozyme | | early-stage cystic fibrosis | | Phase III | | | 2003 | | | | 75% | |
Dornase alfa AERxTMDelivery System | | cystic fibrosis | | Preparing for Clinical Testing | | | 2003 | | | | 45% | |
| Rituxan antibody | | intermediate- and high- grade non-Hodgkin’s lymphoma | | Phase III | | | 2004 | | | | 60% | |
| Xubix (sibrafiban) oral IIb/IIIa antagonist | | orally administered inhibitor of platelet aggregation | | Phase III | | | 2000 | | | | 65% | |
| Activase t-PA | | intravenous catheter clearance | | Preparing for Phase III | | | 1999 | | | | 90% | |
| Anti-CD11a antibody (hull24) | | psoriasis | | Preparing for Phase III | | | 2003 | | | | 50% | |
| Herceptin antibody | | adjuvant therapy for breast cancer | | Preparing for Phase III | | | 2007 | | | | 45% | |
| Thrombopoietin (TPO) | | thrombocytopenia related to cancer treatment | | Preparing for Phase III | | | 2002 | | | | 55% | |
| Anti-CD18 antibody | | acute myocardial infarction | | Phase II | | | 2004 | | | | 55% | |
| Anti-VEGF antibody | | colorectal and lung cancer | | Phase II | | | 2003 | | | | 35-40% | |
| Herceptin antibody | | other tumors | | Phase II | | | 2004 | | | | 40-45% | |
| AMD Fab | | age-related macular degeneration | | Preparing for Phase I | | | 2004 | | | | 20% | |
| LDP-02 | | inflammatory bowel disease | | Phase Ib/ IIa | | | 2005 | | | | 30% | |
We also identified five additional product programs that were at different stages of IPR&D. As of June 30, 1999, the Redemption date, we estimated that these projects would be substantially complete in years 1999 through 2004. The percent completion for each of these additional programs ranged from an estimated 35% to 90%. These projects did not receive material allocations of the purchase price.
In addition, our IPR&D at the Redemption date included a process technology program. The process technology program included the R&D of ideas and techniques that could improve the bulk production of antibodies, including cell culture productivity, and streamlined and improved recovery processes, and improvements in various areas of pharmaceutical manufacturing. We estimated that the process technology program was approximately 50% complete at the Redemption date. Material cash inflows from significant projects are generally expected to commence within one to two years after the substantial completion date has been reached.
C-15
The significant changes to the projects in the IPR&D charge since the Redemption date include:
| | |
| | • | Nutropin Depot long-acting growth hormone — project received FDA approval in December 1999. |
| |
| | • | TNKase second generation t-PA — project received FDA approval in June 2000. |
| |
| | • | Anti-IgE antibody — FDA complete response letter received. We are preparing an amendment to the BLA. |
| |
| | • | Pulmozyme — Phase III trial in early stage cystic fibrosis has been completed and we plan to publish these results. |
| |
| | • | Dornase alfa AERx — project has been discontinued. |
| |
| | • | Xubix (sibrafiban) oral IIb/IIIa antagonist — project has been discontinued. |
| |
| | • | Activase t-PA for intravenous catheter clearance — project received FDA approval in September 2001. |
| |
| | • | Anti-CD11a antibody — Phase III studies ongoing. An additional pharmacokinetic comparability study is currently underway. |
| |
| | • | Herceptin antibody for adjuvant therapy for breast cancer — project has moved to Phase III. |
| |
| | • | Thrombopoietin (or TPO) — we are waiting for confirmation from Pharmacia on whether Pharmacia plans to continue development of this project. |
| |
| | • | Anti-CD18 antibody — project has been discontinued. |
| |
| | • | Anti-VEGF antibody — project has moved to Phase III studies. |
| |
| | • | Herceptin antibody for non-small cell lung cancer (or NSCLC) — project has been discontinued for this indication. |
| |
| | • | AMD Fab — project has moved to Phase I trials. |
| |
| | • | LDP-02 — project has moved to Phase II studies. |
Stock Options Changes
In connection with the Redemption of our Special Common Stock, the following changes occurred with respect to our stock options that were outstanding as of June 30, 1999:
| | |
| | • | Options for the purchase of approximately 27.2 million shares of Special Common Stock were canceled in accordance with the terms of the applicable stock option plans, and the holders received cash payments in the amount of $20.63 per share, less the exercise price; |
| |
| | • | Options for the purchase of approximately 16.0 million shares of Special Common Stock were converted into options to purchase a like number of shares of Common Stock at the same exercise price; and |
| |
| | • | Options for the purchase of approximately 19.6 million shares of Special Common Stock were canceled in accordance with the terms of our 1996 Stock Option/ Stock Incentive Plan, or the 1996 Plan. With certain exceptions, we granted new options for the purchase of 1.333 times the number of shares under the previous options with an exercise price of $24.25 per share, which was the July 23, 1999, public offering price of our Common Stock. The number of shares that were the subject of these new options, which were issued under our 1999 Stock Plan, or the 1999 Plan, was approximately 20.0 million. Alternative arrangements were provided for certain holders of some of the unvested options under the 1996 Plan. |
Of the approximately 16.0 million shares of converted options, options with respect to approximately 3.3 million shares were outstanding at December 31, 2001, all of which are currently exercisable except for
C-16
options with respect to approximately 93,373 shares. These outstanding options are held by 1,202 employees; no non-employee directors hold these options.
Our board of directors and Roche, then our sole stockholder, approved the 1999 Plan on July 16, 1999. Under the 1999 Plan, we granted new options to purchase approximately 26.0 million shares (including the 20.0 million shares referred to above) of Common Stock to approximately 2,400 employees at an exercise price of $24.25 per share, with the grant of such options made effective as of July 16, 1999. Of the options to purchase these 26.0 million shares, options to purchase approximately 17.2 million shares were outstanding at December 31, 2001, of which options to purchase approximately 12.5 million shares are currently exercisable.
In connection with these stock option transactions, we recorded:
| | |
| | • | (1) cash compensation expense of approximately $284.5 million associated with the cash-out of such stock options and (2) non-cash compensation expense of approximately $160.1 million associated with the remeasurement, for accounting purposes, of the converted options, which non-cash amount represents the difference between each applicable option exercise price and the redemption price of the Special Common Stock; and |
| |
| | • | Over a two-year period beginning July 1, 1999, an aggregate of approximately $27.4 million of deferred cash compensation available to be earned by a limited number of employees who elected the alternative arrangements described above. We recorded $4.2 million in 2001, $11.1 million in 2000 and $7.3 million in 1999 of compensation expense related to these alternative arrangements. |
Relationship with Roche
As a result of the Redemption of our Special Common Stock, the then-existing governance agreement between us and Roche terminated, except for provisions relating to indemnification and stock options, warrants and convertible securities. In July 1999, we entered into certain affiliation arrangements with Roche, amended our licensing and marketing agreement with Hoffmann-La Roche, and entered into a tax sharing agreement with Roche as follows:
Our board of directors consists of two Roche directors, three independent directors nominated by a nominating committee currently controlled by Roche, and one Genentech employee. However, under the affiliation agreement, Roche has the right to obtain proportional representation on our board at any time. Roche intends to continue to allow our current management to conduct our business and operations as we have done in the past. However, we cannot ensure that Roche will not implement a new business plan in the future.
Except as follows, the affiliation arrangements do not limit Roche’s ability to buy or sell our Common Stock. If Roche and its affiliates sell their majority ownership of shares of our Common Stock to a successor, Roche has agreed that it will cause the successor to agree to purchase all shares of our Common Stock not held by Roche as follows:
| | |
| | • | with consideration, if that consideration is composed entirely of either cash or equity traded on a U.S. national securities exchange, in the same form and amounts per share as received by Roche and its affiliates; and |
| |
| | • | in all other cases, with consideration that has a value per share not less than the weighted-average value per share received by Roche and its affiliates as determined by a nationally recognized investment bank. |
If Roche owns more than 90% of our Common Stock for more than two months, Roche has agreed that it will, as soon as reasonably practicable, effect a merger of Genentech with Roche or an affiliate of Roche.
C-17
Roche has agreed, as a condition to any merger of Genentech with Roche or the sale of our assets to Roche, that either:
| | |
| | • | the merger or sale must be authorized by the favorable vote of a majority of non-Roche stockholders, provided no person will be entitled to cast more than 5% of the votes at the meeting; or |
| |
| | • | in the event such a favorable vote is not obtained, the value of the consideration to be received by non-Roche stockholders would be equal to or greater than the average of the means of the ranges of fair values for the Common Stock as determined by two nationally recognized investment banks. |
We have agreed not to approve, without the prior approval of the directors designated by Roche:
| | |
| | • | any acquisition, sale or other disposal of all or a portion of our business representing 10% or more of our assets, net income or revenues; |
| |
| | • | any issuance of capital stock except under certain circumstances; or |
| |
| | • | any repurchase or redemption of our capital stock other than a redemption required by the terms of any security and purchases made at fair market value in connection with any of our deferred compensation plans. |
We have a licensing and marketing agreement with Hoffmann-La Roche and its affiliates granting an option to license, use and sell our products in non-U.S. markets. The major provisions of that agreement include the following:
| | |
| | • | Hoffmann-La Roche’s option expires in 2015; |
| |
| | • | Hoffmann-La Roche may exercise its option to license our products upon the occurrence of any of the following: (1) our decision to file an Investigational New Drug application (or IND) for a product, (2) completion of a Phase II trial for a product or (3) if Hoffmann-La Roche previously paid us a fee of $10.0 million to extend its option on a product, completion of a Phase III trial for that product; |
| |
| | • | if Hoffmann-La Roche exercises its option to license a product, it has agreed to reimburse Genentech for development costs as follows: (1) if exercise occurs at the time an IND is filed, Hoffmann-La Roche will pay 50% of development costs incurred prior to the filing and 50% of development costs subsequently incurred, (2) if exercise occurs at the completion of a Phase II trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, (3) if the exercise occurs at the completion of a Phase III trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, and $5.0 million of the option extension fee paid by Hoffmann-La Roche to preserve its right to exercise its option at the completion of a Phase III trial will be credited against the total development costs payable to Genentech upon the exercise of the option; |
| |
| | • | we agreed, in general, to manufacture for and supply to Hoffmann-La Roche its clinical requirements of our products at cost, and its commercial requirements at cost plus a margin of 20%; however, Hoffmann-La Roche will have the right to manufacture our products under certain circumstances; |
| |
| | • | Hoffmann-La Roche has agreed to pay, for each product for which Hoffmann-La Roche exercises its option upon either a decision to file an IND with the FDA or completion of the Phase II trials, a royalty of 12.5% on the first $100.0 million on its aggregate sales of that product and thereafter a royalty of 15% on its aggregate sales of that product in excess of $100.0 million until the later in each country of the expiration of our last relevant patent or 25 years from the first commercial introduction of that product; and |
C-18
| | |
| | • | Hoffmann-La Roche will pay, for each product for which Hoffmann-La Roche exercises its option after completion of the Phase III trials, a royalty of 15% on its sales of that product until the later in each country of the expiration of our relevant patent or 25 years from the first commercial introduction of that product; however, $5.0 million of any option extension fee paid by Hoffmann-La Roche will be credited against royalties payable to us in the first calendar year of sales by Hoffmann-La Roche in which aggregate sales of that product exceed $100.0 million. |
Since the redemption of our Special Common Stock, and until Roche completed its second public offering of our Common Stock in October 1999, we were included in Roche’s U.S. federal consolidated income tax group. Accordingly, we entered into a tax sharing agreement with Roche. Pursuant to the tax sharing agreement, we and Roche were to make payments such that the net amount paid by us on account of consolidated or combined income taxes was determined as if we had filed separate, stand-alone federal, state and local income tax returns as the common parent of an affiliated group of corporations filing consolidated or combined federal, state and local returns.
Effective with the consummation of the second public offering on October 26, 1999, we ceased to be a member of the consolidated federal income tax group (and certain consolidated or combined state and local income tax groups) of which Roche is the common parent. Accordingly, our tax sharing agreement with Roche now pertains only to the state and local tax returns in which we are consolidated or combined with Roche. We will continue to calculate our tax liability or refund with Roche for these state and local jurisdictions as if we were a stand-alone entity.
| |
| Roche’s Ability to Maintain Its Percentage Ownership Interest in Our Stock |
We expect from time to time to issue additional shares of common stock in connection with our stock option and stock purchase plans, and we may issue additional shares for other purposes. Our affiliation agreement with Roche provides, among other things, that we establish a stock repurchase program designed to maintain Roche’s percentage ownership interest in our common stock. The affiliation agreement provides that we will repurchase a sufficient number of shares pursuant to this program such that, with respect to any issuance of common stock by Genentech in the future, the percentage of Genentech common stock owned by Roche immediately after such issuance will be no lower than Roche’s lowest percentage ownership of Genentech common stock at any time after the offering of common stock occurring in July 1999 and prior to the time of such issuance, except that Genentech may issue shares up to an amount that would cause Roche’s lowest percentage ownership to be no more than 2% below the “Minimum Percentage.” The Minimum Percentage equals the lowest number of shares of Genentech common stock owned by Roche since the July 1999 offering (to be adjusted in the future for dispositions of shares of Genentech common stock by Roche as well as for stock splits or stock combinations) divided by 509,194,352 (to be adjusted in the future for stock splits or stock combinations), which is the number of shares of Genentech common stock outstanding at the time of the July 1999 offering, as adjusted for the two-for-one splits of Genentech common stock in November 1999 and October 2000. As long as Roche’s percentage ownership is greater than 50%, prior to issuing any shares, the affiliation agreement provides that we will repurchase a sufficient number of shares of our common stock such that, immediately after our issuance of shares, Roche’s percentage ownership will be greater than 50%. The affiliation agreement also provides that, upon Roche’s request, we will repurchase shares of our common stock to increase Roche’s ownership to the Minimum Percentage. In addition, Roche will have a continuing option to buy stock from us at prevailing market prices to maintain its percentage ownership interest. On December 31, 2001, Roche’s percentage ownership of our common stock was 58.0%, which was 2.2% below the Minimum Percentage.
Related Party Transactions
We enter into transactions with Roche, Hoffmann-La Roche and its affiliates in the ordinary course of business. In July 1998, we entered into an agreement with Hoffmann-La Roche to provide them with exclusive marketing rights outside of the U.S. for Herceptin. Under the agreement, Hoffmann-La Roche paid
C-19
us $40.0 million and has agreed to pay us cash milestones tied to future product development activities, to share equally global development costs up to a maximum of $40.0 million and to make royalty payments on product sales. In 1999, Hoffmann-La Roche paid an additional $10.0 million toward global development costs. Contract revenue from Hoffmann-La Roche, including reimbursement for ongoing development expenses after the option exercise date, totaled $5.8 million in 2001, $3.5 million in 2000, and $17.2 million in 1999. All other revenue from Roche, Hoffmann-La Roche and their affiliates, principally royalties and product sales, totaled $164.1 million in 2001, $114.2 million in 2000, and $83.9 million in 1999.
During 2001, Novartis AG (Novartis) acquired 20% of the outstanding voting stock of Roche Holding, Ltd. As a result of this investment, Novartis is deemed to have an indirect beneficial ownership interest under FAS 57 “Related Party Disclosures” of more than 10% of Genentech’s voting stock. During 2000, we entered into an arrangement with our collaboration partner, Novartis, whereby Novartis is required to fund a portion of the cost of our Xolair inventory until the product is approved for marketing by the FDA. Through December 31, 2001, Novartis has paid $38.4 million of our Xolair inventory costs (no amounts were funded through December 31, 2000). This amount is required to be returned to Novartis upon the earlier of regulatory approval of Xolair in the U.S. or the European Union, and has been recorded in accrued liabilities in our financial statements.
Liquidity and Capital Resources
| | | | | | | | | | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
December 31: | | | | | | | | | | | | |
| Cash, cash equivalents, short-term investments and long-term marketable debt and equity securities | | $ | 2,816.5 | | | $ | 2,459.4 | | | $ | 1,957.4 | |
| Working capital | | | 1,557.6 | | | | 1,340.1 | | | | 849.1 | |
| Current ratio | | | 3.4:1 | | | | 4.0:1 | | | | 2.8:1 | |
Year Ended December 31: | | | | | | | | | | | | |
| Cash provided by (used in): | | | | | | | | | | | | |
| | Operating activities | | | 480.6 | | | | 193.5 | | | | (7.4 | ) |
| | Investing activities | | | (704.0 | ) | | | (160.2 | ) | | | (96.2 | ) |
| | Financing activities | | | 67.2 | | | | 180.4 | | | | 160.2 | |
| Capital expenditures (included in investing activities above) | | | (213.4 | ) | | | (112.7 | ) | | | (95.0 | ) |
We used cash generated from operations, income from investments and proceeds from stock issuances to fund operations, purchase marketable securities and make capital and equity investments during 2001 and 2000, and to also fund stock repurchases in 2001. In 1999, income from investments and proceeds from stock issuances were used to fund operations, pay for the cash-out of stock options related to the Redemption in 1999, to purchase marketable securities and to make capital and equity investments.
We repurchased a total of 800,000 shares of our common stock through October 30, 2001 at a cost of $34.0 million. On October 31, 2001, our Board of Directors authorized a stock repurchase program to repurchase up to $625.0 million of our common stock over the next 12 months. Purchases may be made in the open market or in privately negotiated transactions from time to time at management’s discretion. We may also engage in transactions in other Genentech securities in conjunction with the repurchase program, including derivative securities. Under the program approved by our Board of Directors on October 31, 2001, we repurchased 100,000 shares of our common stock at a cost of $5.7 million.
Capital expenditures in 2001 primarily consisted of improvements to existing manufacturing and service facilities, land and equipment purchases. Capital expenditures in 2000 and 1999 primarily consisted of equipment purchases and improvements to existing manufacturing and service facilities.
We believe that our cash, cash equivalents and short-term investments, together with funds provided by operations and leasing arrangements, will be sufficient to meet our foreseeable operating cash requirements including any cash utilized under our stock repurchase program. In addition, we believe we could access
C-20
additional funds from the debt and, under certain circumstances, capital markets. See also “Our Affiliation Agreement With Roche Could Aversely Affect Our Cash Position” below for factors that could negatively affect our cash position.
Our short-term debt consists of $149.7 million of convertible subordinated debentures, with interest payable at 5%, maturing on March 27, 2002. As a result of the redemption of our Special Common Stock in 1999, upon conversion, the holder will receive, for each $74 in principal amount of debenture converted, $59.25 in cash, of which $18 will be reimbursed to us by Roche. Generally, we may redeem the debentures until maturity. We expect to redeem the debentures in cash in 2002.
We lease various real properties under operating leases that generally require us to pay taxes, insurance and maintenance. Five of our operating leases are commonly referred to as synthetic leases. A synthetic lease represents a form of off-balance sheet financing under which an unrelated third party funds 100% of the costs of the acquisition and/or construction of the property and leases the asset to a lessee, and at least 3% of the third party funds represent at risk equity. Our synthetic leases are treated as operating leases for accounting purposes and as financing leases for tax purposes. Under our synthetic lease structures, upon termination or expiration, at our option, we must either purchase the property from the lessor at a predetermined amount that does not constitute a bargain purchase, sell the real property to a third party, or renew the lease arrangement. If the property is sold to a third party at an amount less than the amount financed by the lessor, we have agreed under residual value guarantees to pay the lessor up to an agreed upon percentage of the amount financed by the lessor.
Four of our synthetic leases were entered into with BNP Paribas Leasing Corporation, who leases directly to us various buildings that we occupy in South San Francisco, California. Under certain of these leases, we are required to maintain cash collateral of $56.6 million, which we have included in other long-term assets on our balance sheet as restricted cash.
The most significant of our synthetic leases relates to our manufacturing facility located in Vacaville, California. In November 2001, we completed a synthetic lease transaction for this facility, which had previously been leased by us under a predecessor synthetic lease. This new synthetic lease is structured differently from our other synthetic leases. We are leasing the property from an unrelated special purpose trust (owner/lessor) under an operating lease agreement for five years ending November 2006. Third party financing is provided in the form of a 3% at risk equity participation from investors and 97% debt commitment. Investors’ equity contributions were equal to or greater than 3% of the fair value of the property at the lease’s inception and are required to remain so for the term of the lease. A bankruptcy remote, special purpose corporation (the SPC) was formed to fund the debt portion through the issuance of commercial paper notes. The SPC lends the proceeds from the commercial paper to the owner/lessor, who issues promissory notes to the SPC. The SPC Loans mature in 5 years (November 2006). The SPC promissory notes are supported by a credit facility provided by financing institutions and draws are generally available under that credit facility to repay the SPC’s commercial paper. The collateral for the SPC Loans includes the leased property, and an interest in the residual value guarantee provided by us. At any time during the lease term, we have the option to purchase the property at an amount that does not constitute a bargain purchase. Our off-balance sheet contingent liability under the residual value guarantees is summarized in the table below.
Under all of our synthetic leases, we are also required to maintain certain pre-defined financial ratios and are limited to the amount of additional debt we can assume. In addition, no Genentech officers or employees have any financial interest with regards to these synthetic lease arrangements or with any of the special purpose entities used in these arrangements. In the event of a default, the maximum amount payable under the residual value guarantee would equal 100% of the amount financed by the lessor, and our obligation to purchase the leased properties or pay the related residual value guarantees could be accelerated. We believe at the lease’s inception and continue to believe that the occurrence of any event of default that could trigger our purchase obligation is remote.
C-21
Future minimum lease payments under operating leases, exclusive of the residual value guarantees, executory costs and sublease income, at December 31, 2001, are as follows (in millions). These minimum lease payments were computed based on current interest rates, which are subject to fluctuations in certain market-based interest rates:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | | Thereafter | | Total |
| |
| |
| |
| |
| |
| |
| |
|
| Synthetic leases | | $ | 12.9 | | | $ | 13.8 | | | $ | 12.8 | | | $ | 12.0 | | | $ | 11.3 | | | $ | 1.6 | | | $ | 64.4 | |
| Other operating leases | | | 4.8 | | | | 3.0 | | | | 1.7 | | | | 1.6 | | | | 1.6 | | | | 4.3 | | | | 17.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Total | | $ | 17.7 | | | $ | 16.8 | | | $ | 14.5 | | | $ | 13.6 | | | $ | 12.9 | | | $ | 5.9 | | | $ | 81.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The following summarizes the residual value guarantee amounts for our synthetic leases (in millions):
| | | | | | | | | | | | | | |
| | Approximate | | | | Residual |
| | Fair Value of | | Lease | | Value |
| | Leased Property | | Expiration | | Guarantee |
| |
| |
| |
|
| South San Francisco Lease 1 | | $ | 56.6 | | | | 07/2004 | | | $ | 48.1 | |
| South San Francisco Lease 2 | | | 133.2 | | | | 06/2007 | | | | 113.2 | |
| South San Francisco Lease 3 | | | 25.0 | | | | 01/2004 | | | | 21.3 | |
| South San Francisco Lease 4 | | | 22.5 | | | | 01/2004 | | | | 19.1 | |
| Vacaville Lease | | | 425.0 | | | | 11/2006 | | | | 371.5 | |
| | | |
| | | | | | | |
| |
| | Total | | $ | 662.3 | | | | | | | $ | 573.2 | |
| | | |
| | | | | | | |
| |
There are no impairments in the fair value or use of the properties that we lease under synthetic leases wherein we believe that we would be required to pay amounts under any of the residual value guarantees. We will continue to assess the fair values of the underlying properties and the use of the properties for impairment on an annual basis.
FORWARD-LOOKING INFORMATION AND CAUTIONARY FACTORS
THAT MAY AFFECT FUTURE RESULTS
The following section contains forward-looking information based on our current expectations. Because our actual results may differ materially from any forward-looking statements made by or on behalf of Genentech, this section includes a discussion of important factors that could affect our actual future results, including, but not limited to, our product sales, royalties, contract revenues, expenses and net income.
Fluctuations in Our Operating Results Could Affect the Price of Our Common Stock
Our operating results may vary from period to period for several reasons including:
| | |
| | • | The overall competitive environment for our products. |
| |
| | | For example, sales of our Activase product decreased in 2001 primarily due to aggressive price discounting by competitors and to a decreasing size of the thrombolytic marketplace as other forms of acute myocardial infarction treatment gain acceptance. |
| |
| | |
| | • | The amount and timing of sales to customers in the United States. |
| |
| | For example, sales of our growth hormone products increased in 2001 from 2000 due to fluctuations in distributor ordering patterns. |
| | |
| | • | The amount and timing of our sales to F. Hoffmann-La Roche (or Hoffmann-La Roche) of products for sale outside of the United States and the amount and timing of its sales to its customers, which directly impact both our product sales and royalty revenues. |
| |
| | | For example, sales of Pulmozyme to Hoffmann-La Roche decreased in 2001 compared to 2000. |
| | |
| | • | The timing and volume of bulk shipments to licensees. |
C-22
| | |
| | • | The availability of third-party reimbursements for the cost of therapy. |
| |
| | • | The extent of product discounts extended to customers. |
| |
| | • | The effectiveness and safety of our various products as determined both in clinical testing and by the accumulation of additional information on each product after it is approved by the U.S. Food and Drug Administration (or FDA) for sale. |
| |
| | • | The rate of adoption and use of our products for approved indications and additional indications. |
| |
| | | For example, sales of Rituxan increased in 2001 due to the announcement at the American Society of Hematology of the results of a study conducted by the Groupe d’Etude des Lymphomes de l’Adulte, or GELA, reporting on the benefits of using Rituxan, combined with standard chemotherapy, for treating aggressive non-Hodgkin’s lymphoma. |
| |
| | |
| | • | The potential introduction of new products and additional indications for existing products in 2002 and beyond. |
| |
| | • | The ability to successfully manufacture sufficient quantities of any particular marketed product. |
| |
| | • | The number and size of any product price increases we may issue. |
The Successful Development of Pharmaceutical Products is Highly Uncertain
Successful pharmaceutical product development is highly uncertain and is dependent on numerous factors, many of which are beyond our control. Products that appear promising in the early phases of development may fail to reach the market for several reasons including:
| | |
| | • | Preclinical and clinical trial results that may show the product to be less effective than desired or to have harmful problematic side effects. |
| |
| | | For example, in April 2001, we announced that a Phase III clinical trial of Veletri — an intravenous dual endothelin receptor antagonist for the treatment of symptoms (dyspnea, or shortness of breath) associated with acute heart failure (or AHF) — did not meet its primary objectives. |
| |
| | |
| | • | Failure to receive the necessary regulatory approvals or delay in receiving such approvals. |
| |
| | | For example, in July 2001, we received a Complete Response letter from the FDA for the license application for Xolair, requesting additional preclinical and clinical data, as well as pharmacokinetic information. With the requirement of additional data, FDA approval of Xolair has been delayed beyond 2001. It is also anticipated that the initial proposed label claim will likely be for only adult allergic asthma. The exact timing of resubmission to the FDA will be dependent on the scope of the discussions with the FDA but is expected to occur in 2002 or early 2003. |
| |
| | | For example, in October 2001, the FDA requested inclusion of an additional pharmacokinetics study in the potential Biologic License Application (or BLA) submission for Xanelim which will result in the filing date occurring later than originally estimated. |
| |
| | • | Manufacturing costs or other factors that make the product uneconomical. |
| |
| | • | The proprietary rights of others and their competing products and technologies that may prevent the product from being commercialized. |
Success in preclinical and early clinical trials does not ensure that large-scale clinical trials will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical trials and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly and may be difficult to predict.
C-23
Factors affecting our research and development (or R&D) expenses include, but are not limited to:
| | |
| | • | The number of and the outcome of clinical trials currently being conducted by us and/or our collaborators. |
| |
| | | For example, we have experienced an increase in R&D expenses in 2001 compared to 2000 due to the number of late-stage clinical trials being conducted by us and/or our collaborators. |
| |
| | • | The number of products entering into development from late-stage research. |
| |
| | | For example, there is no guarantee that internal research efforts will succeed in generating sufficient data for us to make a positive development decision or that an external candidate will be available on terms acceptable to us. In the past, promising candidates have not yielded sufficiently positive preclinical results to meet our stringent development criteria. |
| |
| | • | Hoffmann-La Roche’s decisions whether to exercise its options to develop and sell our future products in non-U.S. markets and the timing and amount of any related development cost reimbursements. |
| |
| | • | In-licensing activities, including the timing and amount of related development funding or milestone payments. |
| |
| | | For example, in January 2001, we entered into an agreement with OSI Pharmaceuticals, Inc. (or OSI) for the global co-development and commercialization of an anti-cancer drug, Tarceva, and paid OSI an upfront fee of $15.0 million for the purchase of in-process research and development (or IPR&D) which was recorded as R&D expense. |
| |
| | • | As part of our strategy, we invest in R&D. R&D as a percent of revenues can fluctuate with the changes in future levels of revenue. Lower revenues can lead to more disciplined spending of R&D efforts. |
| |
| | • | Future levels of revenue. |
Roche Holdings, Inc., Our Controlling Stockholder, May Have Interests That Are Adverse to Other Stockholders
Roche as our majority stockholder, controls the outcome of actions requiring the approval of our stockholders. Our bylaws provide, among other things, that the composition of our board of directors shall consist of two Roche directors, three independent directors nominated by a nominating committee and one Genentech employee nominated by the nominating committee. As long as Roche owns in excess of 50% of our common stock, Roche directors will comprise two of the three members of the nominating committee. However, at any time until Roche owns less than 5% of our stock, Roche will have the right to obtain proportional representation on our board. Roche intends to continue to allow our current management to conduct our business and operations as we have done in the past. However, we cannot assure stockholders that Roche will not institute a new business plan in the future. Roche’s interests may conflict with minority shareholder interests.
Our Affiliation Agreement With Roche Could Limit Our Ability to Make Acquisitions and Could Have a Material Negative Impact on Our Liquidity
The affiliation agreement between us and Roche contains provisions that:
| | |
| | • | Require the approval of the directors designated by Roche to make any acquisition or any sale or disposal of all or a portion of our business representing 10% or more of our assets, net income or revenues. |
| |
| | • | Enable Roche to maintain its percentage ownership interest in our common stock. |
| |
| | • | Call for us to establish a stock repurchase program designed to maintain Roche’s percentage ownership interest in our common stock based on an established Minimum Percentage. For information regarding Minimum Percentage, see the “Relationship with Roche — Roche’s Ability to Maintain Its Percent- |
C-24
| | |
| | | age Ownership Interest in Our Stock” note in the Notes to Consolidated Financial Statements below. For more information on our stock repurchase program, see the “Capital Stock” note in the Notes to Consolidated Financial Statements below. |
These provisions may have the effect of limiting our ability to make acquisitions and while the dollar amounts associated with the stock repurchase program cannot currently be estimated, these stock repurchases could have a material adverse impact on our liquidity, credit rating and ability to access additional capital in the financial markets.
Our Stockholders May Be Unable to Prevent Transactions That Are Favorable to Roche but Adverse to Us
Our certificate of incorporation includes provisions relating to:
| | |
| | • | Competition by Roche with us. |
| |
| | • | Offering of corporate opportunities. |
| |
| | • | Transactions with interested parties. |
| |
| | • | Intercompany agreements. |
| |
| | • | Provisions limiting the liability of specified employees. |
Our certificate of incorporation provides that any person purchasing or acquiring an interest in shares of our capital stock shall be deemed to have consented to the provisions in the certificate of incorporation relating to competition with Roche, conflicts of interest with Roche, the offer of corporate opportunities to Roche and intercompany agreements with Roche. This deemed consent may restrict your ability to challenge transactions carried out in compliance with these provisions.
Potential Conflicts of Interest Could Limit Our Ability to Act on Opportunities That Are Adverse to Roche
Persons who are directors and/or officers of Genentech and who are also directors and/or officers of Roche may decline to take action in a manner that might be favorable to us but adverse to Roche. Two of our directors, Dr. Franz B. Humer and Dr. Jonathan K.C. Knowles, currently serve as directors, officers and employees of Roche Holding Ltd and its affiliates.
We May Be Unable to Retain Skilled Personnel and Maintain Key Relationships
The success of our business depends, in large part, on our continued ability to attract and retain highly qualified management, scientific, manufacturing and sales and marketing personnel, and on our ability to develop and maintain important relationships with leading research institutions and key distributors. Competition for these types of personnel and relationships is intense.
Roche has the right to maintain its percentage ownership interest in our common stock. Our affiliation agreement with Roche provides that, among other things, we will establish a stock repurchase program designed to maintain Roche’s percentage ownership in our common stock if we issue or sell any shares. This could have an effect on the number of shares we are able to grant under our stock option plans. We therefore cannot assure you that we will be able to attract or retain skilled personnel or maintain key relationships.
We Face Growing and New Competition
We face growing competition in two of our therapeutic markets and expect new competition in a third market. First, in the thrombolytic market, Activase has lost market share and could lose additional market share to Centocor’s RetavaseTM either alone or in combination with the use of another Centocor product, ReoPro® (abciximab) and to the use of mechanical reperfusion therapies to treat acute myocardial infarction; the resulting adverse effect on sales has been and could continue to be material. Retavase received approval from the FDA in October 1996 for the treatment of acute myocardial infarction. We expect that the use of mechanical reperfusion in lieu of thrombolytic therapy for the treatment of acute myocardial infarction will continue to grow.
C-25
Second, in the growth hormone market, we continue to face increased competition from at least four other companies currently selling growth hormone. As a result of that competition, we have experienced a loss in market share. Four competitors have also received approval to market their existing human growth hormone products for additional indications. As a result of this competition, sales of our growth hormone products may decline, perhaps significantly.
Third, in the non-Hodgkin’s lymphoma market, Corixa Corporation has filed a revised BLA, for BexxarTM (tositumomab and iodine I 131 tositumomab), which may potentially compete with our product Rituxan, and IDEC has received an approval letter from the FDA for ZevalinTM (ibritumomab tiuxetan), a product which could also potentially compete with Rituxan. Both Bexxar and Zevalin are radiolabeled molecules while Rituxan is not. We are also aware of other potentially competitive biologic therapies for non-Hodgkin’s lymphoma in development.
Other Competitive Factors Could Affect Our Product Sales
Other competitive factors that could affect our product sales include, but are not limited to:
| | |
| | • | The timing of FDA approval, if any, of competitive products. |
| |
| | • | Our pricing decisions and the pricing decisions of our competitors. |
| |
| |
| | For example, we raised the prices of Herceptin by 3% and growth hormone product by 5% in January 2001. |
| |
| | |
| | • | The degree of patent protection afforded our products by patents granted to us and by the outcome of litigation involving our patents. |
| |
| | • | The outcome of litigation involving patents of other companies concerning our products or processes related to production and formulation of those products or uses of those products. |
| |
| | | For example, as described in the “Leases, Commitments and Contingencies” note in the Notes to Consolidated Financial Statements below, several companies have filed patent infringement lawsuits against us alleging that the manufacture, use and sale of certain of our products infringe their patents. |
| |
| | |
| | • | The increasing use and development of alternate therapies. |
| |
| | | For example, the overall size of the market for thrombolytic therapies, such as our Activase product, continues to decline as a result of the increasing use of mechanical reperfusion. |
| |
| | |
| | • | The rate of market penetration by competing products. |
| |
| | | For example, we have lost market share to new competitors in the thrombolytic and, in the past, growth hormone markets. |
In Connection With the Redemption of Our Special Common Stock, We Recorded Substantial Goodwill and Other Intangibles, the Amortization or Impairment of Which May Adversely Affect Our Earnings
As a result of the redemption of our Special Common Stock, Roche owned all of our outstanding common stock. Consequently, push-down accounting under generally accepted accounting principles in the U.S. was required. Push-down accounting required us to establish a new accounting basis for our assets and liabilities, based on Roche’s cost in acquiring all of our stock. In other words, Roche’s cost of acquiring Genentech was “pushed down” to us and reflected on our financial statements. Push-down accounting required us to record goodwill of approximately $1,685.7 million and other intangible assets of $1,499.0 million on June 30, 1999. The other intangible assets are being amortized over their estimated useful lives ranging from 5 to 15 years. See the “Redemption of Our Special Common Stock” note in the Notes to Consolidated Financial Statements of below, for further information on the useful lives of these intangible assets.
Effective for fiscal years beginning after December 15, 2001, the adoption of Statement of Financial Accounting Standards (or FAS) No. 142 will require that goodwill not be amortized, but rather be subject to an impairment test at least annually. Separately identified and recognized intangible assets resulting from
C-26
business combinations completed before July 1, 2001, that do not meet the new criteria under FAS 141 for separate recognition of intangible assets will be reclassified into goodwill upon adoption. In addition, the useful lives of recognized intangible assets acquired in transactions completed before July 1, 2001 will be reassessed and the remaining amortization periods adjusted accordingly. We will annually evaluate whether events and circumstances have occurred that indicate the remaining balance of goodwill and other intangible assets may not be recoverable. If our evaluation of the assets results in a possible impairment, we may have to reduce the carrying value of our intangible assets. This could have a material adverse effect on our financial condition and results of operations during the periods in which we recognize a reduction. We may have to write down intangible assets in future periods. For more information about push-down accounting, see the “Redemption of Our Special Common Stock” note in the Notes to Consolidated Financial Statements below. For more information regarding FAS 142 and 141, see “New Accounting Pronouncements Will Impact Our Financial Position and Results of Operations” below.
Our Royalty and Contract Revenues Could Decline
Royalty and contract revenues in future periods could vary significantly. Major factors affecting these revenues include, but are not limited to:
| | |
| | • | Hoffmann-La Roche’s decisions whether to exercise its options and option extensions to develop and sell our future products in non-U.S. markets and the timing and amount of any related development cost reimbursements. |
| |
| | • | Variations in Hoffmann-La Roche’s sales and other licensees’ sales of licensed products. |
| |
| | • | The conclusion of existing arrangements with other companies and Hoffmann-La Roche. |
| |
| | For example, in the second quarter of 2001, we reacquired from Schwarz Pharma AG the exclusive development and marketing rights for Nutropin AQ and Nutropin Depot in Europe and other countries outside the United States, Canada, China and Japan. |
| | |
| | • | The timing of non-U.S. approvals, if any, for products licensed to Hoffmann-La Roche and to other licensees. |
| |
| | • | Fluctuations in foreign currency exchange rates. |
| |
| | • | The initiation of new contractual arrangements with other companies. |
| |
| | • | Whether and when contract benchmarks are achieved. |
| |
| | • | The failure of or refusal of a licensee to pay royalties. |
| |
| | • | The expiration or invalidation of patents or licensed intellectual property. |
| |
| | • | Decreases in licensees’ sales of product due to competition, manufacturing difficulties or other factors that affect sales of product. |
Protecting Our Proprietary Rights Is Difficult and Costly
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions. Accordingly, we cannot predict the breadth of claims allowed in these companies’ patents. Patent disputes are frequent and can preclude the commercialization of products. We have in the past been, are currently, and may in the future be, involved in material patent litigation. Our current patent litigation matters are discussed in the “Leases, Commitments and Contingencies” note in the Notes to Consolidated Financial Statements below. Patent litigation is costly in its own right and could subject us to significant liabilities to third parties. In addition, an adverse decision could force us to either obtain third-party licenses at a material cost or cease using the technology or product in dispute.
The presence of patents or other proprietary rights belonging to other parties may lead to our termination of the R&D of a particular product.
C-27
We believe that we have strong patent protection or the potential for strong patent protection for a number of our products that generate sales and royalty revenue or that we are developing. However, the courts will determine the ultimate strength of patent protection of our products and those on which we earn royalties.
We May Incur Material Litigation Costs
Litigation to which we are currently or have been subjected relates to, among other things, our patent and intellectual property rights, licensing arrangements with other persons, product liability and financing activities. We cannot predict with certainty the eventual outcome of pending litigation, and we might have to incur substantial expense in defending these lawsuits.
We May Incur Material Product Liability Costs
The testing and marketing of medical products entail an inherent risk of product liability. Pharmaceutical product liability exposures could be extremely large and pose a material risk. Our business may be materially and adversely affected by a successful product liability claim in excess of any insurance coverage that we may have.
We May Be Unable to Obtain Regulatory Approvals for Our Products
The pharmaceutical industry is subject to stringent regulation with respect to product safety and efficacy by various federal, state and local authorities. Of particular significance are the FDA’s requirements covering R&D, testing, manufacturing, quality control, labeling and promotion of drugs for human use. A pharmaceutical product cannot be marketed in the United States until it has been approved by the FDA, and then can only be marketed for the indications and claims approved by the FDA. As a result of these requirements, the length of time, the level of expenditures and the laboratory and clinical information required for approval of a New Drug Application, or NDA, or a BLA, are substantial and can require a number of years. In addition, after any of our products receive regulatory approval, they remain subject to ongoing FDA regulation, including, for example, changes to their label, written advisements to physicians and product recall.
We cannot be sure that we can obtain necessary regulatory approvals on a timely basis, if at all, for any of the products we are developing or that we can maintain necessary regulatory approvals for our existing products, and all of the following could have a material adverse effect on our business:
| | |
| | • | Significant delays in obtaining or failing to obtain required approvals. |
| |
| | For example, see “The Successful Development of Pharmaceutical Products is Highly Uncertain” above for a description of the delay in receipt of FDA approval for Xolair. |
| | |
| | • | Loss of, or changes to, previously obtained approvals. |
| |
| | • | Failure to comply with existing or future regulatory requirements. |
Moreover, it is possible that the current regulatory framework could change or additional regulations could arise at any stage during our product development, which may affect our ability to obtain approval of our products.
Difficulties or Delays in Product Manufacturing Could Harm Our Business
We currently produce all of our products at our manufacturing facilities located in South San Francisco, California and Vacaville, California or through various contract manufacturing arrangements. Problems with any of our or our contractors’ manufacturing processes could result in product defects, which could require us to delay shipment of products, recall products previously shipped or be unable to supply products at all.
In April 2001, we issued an important drug notification regarding a separate defect in the manufacture of a Pulmozyme product lot which was causing a small puncture in a small number of ampules of the product. We suspended shipping the product upon discovery of this defect and recalled the few cases of the product lot
C-28
that had been distributed. In July 2001, we passed a full inspection by the FDA Team Biologics confirming that Genentech is in a full state of manufacturing compliance.
In addition, any prolonged interruption in the operations of our or our contractors’ manufacturing facilities could result in cancellations of shipments or loss of product in the process of being manufactured. A number of factors could cause interruptions, including equipment malfunctions or failures, or damage to a facility due to natural disasters, including earthquakes as our South San Francisco facilities are located in an area where earthquakes could occur, rolling blackouts imposed by a utility or otherwise. Because our manufacturing processes and those of our contractors are highly complex and are subject to a lengthy FDA approval process, alternative qualified production capacity may not be available on a timely basis or at all. Difficulties or delays in our and our contractors’ manufacturing of existing or new products could increase our costs, cause us to lose revenue or market share and damage our reputation.
Future Stock Repurchases Could Adversely Affect Our Cash Position
In November 2001, our Board of Directors authorized a stock repurchase program to repurchase up to $625.0 million of our common stock over the next 12 months. Purchases may be made in the open market or in privately negotiated transactions from time to time at management’s discretion. We may also engage in transactions in other Genentech securities in conjunction with the repurchase program, including derivative securities.
While the dollar amounts associated with these future stock repurchases cannot currently be estimated, these stock repurchases could have a material adverse effect on our cash position, credit rating and ability to access capital in the financial markets, and could limit our ability to use our capital stock as consideration for acquisitions.
Our Stock Price, Like That of Many Biotechnology Companies, Is Highly Volatile
The market prices for securities of biotechnology companies in general have been highly volatile and may continue to be highly volatile in the future. In addition, due to the absence of the put and call that were associated with our Special Common Stock, the market price of our common stock has been and may continue to be more volatile than our Special Common Stock was in the past. For example, our common stock reached a high of $122.50 per share in March 2000 and decreased, as the biotech sector and stock market in general decreased, to $38.65 per share in July 2001.
In addition, the following factors may have a significant impact on the market price of our common stock:
| | |
| | • | Announcements of technological innovations or new commercial products by us or our competitors. |
| |
| | • | Developments concerning proprietary rights, including patents. |
| |
| | • | Publicity regarding actual or potential medical results relating to products under development or being commercialized by us or our competitors. |
| |
| | • | Regulatory developments concerning our products in the United States and foreign countries. |
| |
| | • | Issues concerning the safety of our products or of biotechnology products generally. |
| |
| | • | Economic and other external factors or a disaster or crisis. |
| |
| | • | Period-to-period fluctuations in financial results. |
Our Affiliation Agreement With Roche Could Adversely Affect Our Cash Position
Our affiliation agreement with Roche provides that we will establish a stock repurchase program designed to maintain Roche’s percentage ownership interest in our common stock based on an established Minimum Percentage. For more information on our stock repurchase program, see the “Capital Stock” note in the Notes to Consolidated Financial Statements below. See the “Relationship with Roche — Roche’s Ability to
C-29
Maintain Its Percentage Ownership Interest in Our Stock” note in the Notes to Consolidated Financial Statements below, for information regarding the Minimum Percentage.
While the dollar amounts associated with these future stock repurchases cannot currently be estimated, these stock repurchases could have a material adverse effect on our cash position, and may have the effect of limiting our ability to use our capital stock as consideration for acquisitions.
Future Sales of Our Common Stock by Roche Could Cause the Price of Our Common Stock to Decline
As of December 31, 2001, Roche owned 306,594,352 shares of our common stock or 58.0% of our outstanding shares. All of our shares owned by Roche are eligible for sale in the public market subject to compliance with the applicable securities laws. We have agreed that, upon Roche’s request, we will file one or more registration statements under the Securities Act in order to permit Roche to offer and sell shares of our common stock. Sales of a substantial number of shares of our common stock by Roche in the public market could adversely affect the market price of our common stock.
Other Risks
We generally deal with some hazardous materials in connection with our research and manufacturing activities. In the event such hazardous materials are stored, handled or released into the environment in violation of law or any permit, we could be subject to loss of our permits, government fines or penalties and/or other adverse governmental action. The levy of a substantial fine or penalty, the payment of significant environmental remediation costs or the loss of a permit or other authorization to operate or engage in our ordinary course of business could materially adversely affect our business.
We Are Exposed to Market Risk
We are exposed to market risk, including changes to interest rates, foreign currency exchange rates and equity investment prices. To reduce the volatility relating to these exposures, we enter into various derivative hedging transactions pursuant to our investment and risk management policies and procedures. We do not use derivatives for speculative purposes.
We maintain risk management control systems to monitor the risks associated with interest rates, foreign currency exchange rates and equity investment price changes, and our derivative and financial instrument positions. The risk management control systems use analytical techniques, including sensitivity analysis and market values. Though we intend for our risk management control systems to be comprehensive, there are inherent risks that may only be partially offset by our hedging programs should there be unfavorable movements in interest rates, foreign currency exchange rates or equity investment prices.
The estimated exposures discussed below are intended to measure the maximum amount we could lose from adverse market movements in interest rates, foreign currency exchange rates and equity investment prices, given a specified confidence level, over a given period of time. Loss is defined in the value at risk estimation as fair market value loss. The exposures to interest rate, foreign currency exchange rate and equity investment price changes are calculated based on proprietary modeling techniques from a Monte Carlo simulation value at risk model using a 30-day holding period and a 95% confidence level. The value at risk model assumes non-linear financial returns and generates potential paths various market prices could take and tracks the hypothetical performance of a portfolio under each scenario to approximate its financial return. The value at risk model takes into account correlations and diversification across market factors, including interest rates, foreign currencies and equity prices. Market volatilities and correlations are based on J.P. Morgan RiskmetricsTM dataset as of December 31, 2001.
| |
| Our Interest Income is Subject to Fluctuations in Interest Rates |
Our material interest-bearing assets, or interest-bearing portfolio, consisted of cash, cash equivalents, restricted cash, short-term investments and long-term investments. The balance of our interest bearing portfolio was $2,337.6 million or 33% of total assets at December 31, 2001. Interest income related to this
C-30
portfolio was $130.5 million or 6% of total revenues. Our interest income is sensitive to changes in the general level of interest rates, primarily U.S. interest rates. In this regard, changes in U.S. interest rates affect the interest bearing portfolio. To mitigate the impact of fluctuations in U.S. interest rates, for a portion of our portfolio, we have entered into swap transactions which involve the receipt of fixed rate interest and the payment of floating rate interest without the exchange of the underlying principal.
Based on our overall interest rate exposure at December 31, 2001, including derivative and other interest rate sensitive instruments, a near-term change in interest rates, within a 95% confidence level based on historical interest rate movements could result in a potential loss in fair value of our interest rate sensitive instruments of $32.2 million. At December 31, 2000 and at December 31, 1999, we estimated that the potential losses in fair value of our interest rate sensitive instruments were not material.
| |
| We Are Exposed to Risks Relating to Foreign Currency Exchange Rates and Foreign Economic Conditions |
We receive royalty revenues from licensees selling products in countries throughout the world. As a result, our financial results could be significantly affected by factors such as changes in foreign currency exchange rates or weak economic conditions in the foreign markets in which our licensed products are sold. We are exposed to changes in exchange rates in Europe, Asia (primarily Japan) and Canada. Our exposure to foreign exchange rates primarily exists with the Swiss franc. When the dollar strengthens against the currencies in these countries, the dollar value of non-dollar-based revenue decreases; when the dollar weakens, the dollar value of the non-dollar-based revenues increases. Accordingly, changes in exchange rates, and in particular a strengthening of the dollar, may adversely affect our royalty revenues as expressed in dollars. Exchange rate exposures on these royalties are being offset by expenses arising from our foreign manufacturing facility as well as non-dollar expenses incurred in our collaborations. Currently, our foreign royalty revenues exceed our expenses. In addition, as part of our overall investment strategy, a portion of our portfolio is primarily in non-dollar denominated investments. As a result, we are exposed to changes in the exchange rates of the countries in which these non-dollar denominated investments are made.
To mitigate our net foreign exchange exposure, our policy allows us to hedge certain of our anticipated royalty revenues by purchasing option contracts with expiration dates and amounts of currency that are based on 25% to 90% of probable future revenues so that the potential adverse impact of movements in currency exchange rates on the non-dollar denominated revenues will be at least partly offset by an associated increase in the value of the option. Currently, the term of these options is generally one to three years. To hedge the non-dollar expenses arising from our foreign manufacturing facility, we may enter into forward contracts to lock in the dollar value of a portion of these anticipated expenses.
Based on our overall currency rate exposure at December 31, 2001, 2000 and 1999, including derivative and other foreign currency sensitive instruments, a near-term change in currency rates within a 95% confidence level based on historical currency rate movements would not materially affect the fair value of our foreign currency sensitive instruments.
| |
| Our Investments in Equity Securities Are Subject to Market Risks |
As part of our strategic alliance efforts, we invest in equity instruments of biotechnology companies. Our biotechnology equity investment portfolio totaled $583.9 million or 8% of total assets at December 31, 2001. These investments are subject to fluctuations from market value changes in stock prices. For example, in the first quarter of 2001, we recorded a significant charge on the write down of an equity security investment that had an other than temporary impairment.
To mitigate the risk of market value fluctuation, certain equity securities are hedged with zero-cost collars and forward contracts. A zero-cost collar is a purchased put option and a written call option in which the cost of the purchased put and the proceeds of the written call offset each other; therefore, there is no initial cost or cash outflow for these instruments at the time of purchase. The purchased put protects us from a decline in the market value of the security below a certain minimum level (the put “strike” level), while the call effectively limits our potential to benefit from an increase in the market value of the security above a
C-31
certain maximum level (the call “strike” level). A forward contract is a derivative instrument where we lock-in the termination price we receive from the sale of stock based on a pre-determined spot price. The forward contract protects us from a decline in the market value of the security below the spot price and limits our potential benefit from an increase in the market value of the security above the spot price. Throughout the life of the contract, we receive interest income based on the notional amount and a floating-rate index. In addition, as part of our strategic alliance efforts, we hold dividend-bearing convertible preferred stock and have made interest-bearing loans that are convertible into the equity securities of the debtor.
Based on our overall exposure to fluctuations from market value changes in marketable equity prices at December 31, 2001, a near-term change in equity prices within a 95% confidence level based on historic volatilities could result in a potential loss in fair value of our equity securities portfolio of $22.7 million. We estimated that the potential loss in fair value of our equity securities portfolio was $94.0 million at December 31, 2000 and $43.2 million at December 31, 1999.
| |
| We Are Exposed to Credit Risk of Counterparties |
We could be exposed to losses related to the financial instruments described above should one of our counterparties default. We attempt to mitigate this risk through credit monitoring procedures.
New Accounting Pronouncements Will Impact Our Financial Position and Results of Operations
In June 2001, the Financial Accounting Standards Board (or FASB) issued FAS 141 on Business Combinations and FAS 142 on Goodwill and Other Intangible Assets, effective for fiscal years beginning after December 15, 2001. FAS 141 requires that the purchase method of accounting be used for all business combinations initiated after June 30, 2001 and also specifies the criteria for the recognition of intangible assets separately from goodwill. Under the new rules, goodwill will no longer be amortized but will be subject to an impairment test at least annually. Separately identified and recognized intangible assets resulting from business combinations completed before July 1, 2001 that do not meet the new criteria for separate recognition of intangible assets will be subsumed in goodwill upon adoption. FAS 141 specifically identified assembled workforce as an intangible asset that is not to be recognized apart from goodwill. Other intangible assets that meet the new criteria will continue to be amortized over their useful lives.
We will apply the new rules on accounting for goodwill and other intangible assets on January 1, 2002. The adoption of FAS 141 and 142 is not expected to have a significant impact on our financial position at transition. We expect that the cessation of goodwill amortization and the amortization of our trained and assembled workforce intangible asset (net of related taxes) will increase reported net income by approximately $150.0 million (or $0.28 per share) in 2002. We performed an impairment test of goodwill as of January 1, 2002 and will not record an impairment charge at transition. We will continue to monitor the fair value of our goodwill through the annual impairment tests.
There may be potential new accounting pronouncements or regulatory rulings which may have an impact on our future results of operations and financial position.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Refer to the section labeled “Forward-Looking Information and Cautionary Factors That May Affect Future Results — We Are Exposed to Market Risk” above.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
C-32
REPORT OF ERNST & YOUNG LLP, INDEPENDENT AUDITORS
The Board of Directors and Stockholders of Genentech, Inc.
We have audited the accompanying consolidated balance sheets of Genentech, Inc. as of December 31, 2001 and 2000, and the related consolidated statements of operations, stockholders’ equity and cash flows for the years ended December 31, 2001 and 2000, and for the period from June 30, 1999 to December 31, 1999 (all “New Basis”). We have also audited the related consolidated statements of operations, stockholders’ equity and cash flows for the period from January 1, 1999 to June 30, 1999, (“Old Basis”). These financial statements are the responsibility of Genentech, Inc.’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Genentech, Inc. at December 31, 2001 and 2000, and the consolidated results of its operations and its cash flows for the years ended December 31, 2001 and 2000, the period from June 30, 1999 to December 31, 1999 and the period from January 1, 1999 to June 30, 1999, in conformity with accounting principles generally accepted in the United States.
As discussed in the notes to the consolidated financial statements, in 2001 the Company changed its method of accounting for derivative instruments and hedging activities, and in 2000 changed its method of accounting for revenue recognition.
| |
| |  |
| | ERNST & YOUNG LLP |
Palo Alto, California
January 15, 2002,
except for the note titled
Subsequent Event, as
to which the date is
February 26, 2002
C-33
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| | | | | | | | | | | | | | | | | | | |
| | |
| | Year Ended December 31, |
| |
|
| | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| | | | | | New Basis | | Old Basis |
| | | | | | (June 30 to | | (January 1 to |
| | | | | | December 31)(1) | | June 30)(1) |
| | | | | |
| |
|
Revenues | | | | | | | | | | | | | | | | |
| | Product sales (including amounts from related party:
2001-$76,290; 2000-$67,392; 1999-$41,324) | | $ | 1,742,897 | | | $ | 1,278,344 | | | $ | 535,671 | | | $ | 503,424 | |
| | Royalties (including amounts from related party:
2001-$87,854; 2000-$46,795; 1999-$42,528) | | | 264,475 | | | | 207,241 | | | | 96,666 | | | | 92,604 | |
| | Contract and other (including amounts from related party:
2001-$5,754; 2000-$3,506; 1999-$17,170) | | | 74,361 | | | | 160,363 | | | | 26,398 | | | | 56,844 | |
| | Interest income | | | 130,544 | | | | 90,408 | | | | 45,049 | | | | 44,385 | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total revenues | | | 2,212,277 | | | | 1,736,356 | | | | 703,784 | | | | 697,257 | |
Cost and expenses | | | | | | | | | | | | | | | | |
| | Cost of sales (including amounts from related party:
2001-$63,761; 2000-$56,674; 1999-$36,267) | | | 354,442 | | | | 364,892 | | | | 187,145 | | | | 98,404 | |
| | Research and development (including contract related:
2001-$9,434; 2000-$25,709; 1999-$18,366) | | | 526,230 | | | | 489,879 | | | | 182,387 | | | | 184,951 | |
| | Marketing, general and administrative | | | 474,410 | | | | 368,224 | | | | 210,548 | | | | 183,109 | |
| | Collaboration profit sharing | | | 246,657 | | | | 128,812 | | | | 42,808 | | | | 31,464 | |
| | Special charges: | | | | | | | | | | | | | | | | |
| | | Related to redemption | | | — | | | | — | | | | 1,207,700 | | | | — | |
| | | Legal settlements | | | — | | | | — | | | | 180,008 | | | | 50,000 | |
| | Recurring charges related to redemption | | | 321,816 | | | | 375,300 | | | | 197,742 | | | | — | |
| | Interest expense | | | 5,736 | | | | 5,276 | | | | 2,641 | | | | 2,719 | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total costs and expenses | | | 1,929,291 | | | | 1,732,383 | | | | 2,210,979 | | | | 550,647 | |
| Income (loss) before taxes and cumulative effect of accounting change | | | 282,986 | | | | 3,973 | | | | (1,507,195 | ) | | | 146,610 | |
| Income tax provision (benefit) | | | 127,112 | | | | 20,414 | | | | (262,083 | ) | | | 58,974 | |
| | | |
| | | |
| | | |
| | | |
| |
| Income (loss) before cumulative effect of accounting change | | | 155,874 | | | | (16,441 | ) | | | (1,245,112 | ) | | | 87,636 | |
| Cumulative effect of accounting change, net of tax | | | (5,638 | ) | | | (57,800 | ) | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
Net income (loss) | | $ | 150,236 | | | $ | (74,241 | ) | | $ | (1,245,112 | ) | | $ | 87,636 | |
| | | |
| | | |
| | | |
| | | |
| |
Earnings (loss) per share: | | | | | | | | | | | | | | | | |
| | Basic: | | | | | | | | | | | | | | | | |
| | | Earnings (loss) before cumulative effect of accounting change | | $ | 0.30 | | | $ | (0.03 | ) | | $ | (2.43 | ) | | $ | 0.17 | |
| | | Cumulative effect of accounting change, net of tax | | | (0.01 | ) | | | (0.11 | ) | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Net earnings (loss) per share | | $ | 0.29 | | | $ | (0.14 | ) | | $ | (2.43 | ) | | $ | 0.17 | |
| | | |
| | | |
| | | |
| | | |
| |
| | Diluted: | | | | | | | | | | | | | | | | |
| | | Earnings (loss) before cumulative effect of accounting change | | $ | 0.29 | | | $ | (0.03 | ) | | $ | (2.43 | ) | | $ | 0.16 | |
| | | Cumulative effect of accounting change, net of tax | | | (0.01 | ) | | | (0.11 | ) | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Net earnings (loss) per share | | $ | 0.28 | | | $ | (0.14 | ) | | $ | (2.43 | ) | | $ | 0.16 | |
| | | |
| | | |
| | | |
| | | |
| |
| Weighted-average shares used to compute basic earnings (loss) per share: | | | 527,022 | | | | 522,179 | | | | 513,352 | | | | 512,368 | |
| | | |
| | | |
| | | |
| | | |
| |
| Weighted-average shares used to compute diluted earnings (loss) per share: | | | 535,291 | | | | 522,179 | | | | 513,352 | | | | 531,868 | |
| | | |
| | | |
| | | |
| | | |
| |
Pro forma amounts assuming the new revenue recognition policy was applied retroactively (unaudited): | | | | | | | | | | | | | | | | |
| | Net income (loss) | | | — | | | $ | (16,441 | ) | | $ | (1,248,632 | ) | | $ | 79,916 | |
| | | |
| | | |
| | | |
| | | |
| |
| |
| (1) | All amounts related to the Redemption of our Special Common Stock transaction are reflected in the New Basis presentation. |
See notes to consolidated financial statements.
C-34
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | | | | | | | | |
| | |
| | Increase (Decrease) in Cash and Cash Equivalents |
| |
|
| | |
| | Year Ended December 31, |
| |
|
| | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| | | | | | | | |
| | | | | | New Basis | | Old Basis |
| | | | | | (June 30 to | | (January 1 to |
| | | | | | December 31)(1) | | June 30)(1) |
| | | | | |
| |
|
Cash flows from operating activities: | | | | | | | | | | | | | | | | |
| | Net income (loss) | | $ | 150,236 | | | $ | (74,241 | ) | | $ | (1,245,112 | ) | | $ | 87,636 | |
| | Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | | | | | | | | | | | | | | | |
| | | Depreciation and amortization | | | 428,091 | | | | 463,004 | | | | 236,365 | | | | 44,317 | |
| | | In-process research and development | | | — | | | | — | | | | 752,500 | | | | — | |
| | | Non-cash compensation related to stock options, net of tax | | | — | | | | — | | | | 119,153 | | | | — | |
| | | Deferred income taxes | | | 12,853 | | | | (235,315 | ) | | | (143,371 | ) | | | (924 | ) |
| | | Gain on sales of securities available-for-sale | | | (30,001 | ) | | | (132,307 | ) | | | (7,092 | ) | | | (12,283 | ) |
| | | Loss on sales of securities available-for-sale | | | 2,011 | | | | 3,957 | | | | 884 | | | | 921 | |
| | | Write-down of securities available-for-sale | | | 27,504 | | | | 4,800 | | | | 4,955 | | | | 8,467 | |
| | | Write-down of nonmarketable securities | | | — | | | | — | | | | — | | | | 432 | |
| | | Loss (gain) on fixed asset dispositions | | | 4,211 | | | | 1,123 | | | | 902 | | | | (16 | ) |
| | Changes in assets and liabilities: | | | | | | | | | | | | | | | | |
| | | Investments in trading securities | | | (85,712 | ) | | | (20,963 | ) | | | (5,215 | ) | | | (4,944 | ) |
| | | Receivables and other current assets | | | (43,008 | ) | | | (65,330 | ) | | | (32,350 | ) | | | (38,644 | ) |
| | | Inventories, including inventory write-up effect | | | (91,116 | ) | | | 9,415 | | | | 49,228 | | | | 10,333 | |
| | | Accounts payable, other current liabilities and other long-term liabilities | | | 105,558 | | | | 239,388 | | | | 138,135 | | | | 28,277 | |
| | | |
| | | |
| | | |
| | | |
| |
| | Net cash provided by (used in) operating activities | | | 480,627 | | | | 193,531 | | | | (131,018 | ) | | | 123,572 | |
Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| | Purchases of securities held-to-maturity | | | — | | | | — | | | | — | | | | (186,612 | ) |
| | Proceeds from maturities of securities held-to-maturity | | | — | | | | — | | | | 136,140 | | | | 150,357 | |
| | Purchases of securities available-for-sale | | | (1,559,230 | ) | | | (560,405 | ) | | | (294,814 | ) | | | (300,254 | ) |
| | Proceeds from sales of securities available-for-sale | | | 1,084,546 | | | | 574,145 | | | | 369,311 | | | | 257,752 | |
| | Purchases of nonmarketable equity securities | | | (5,830 | ) | | | (5,663 | ) | | | (13,781 | ) | | | (39,177 | ) |
| | Capital expenditures | | | (213,351 | ) | | | (112,681 | ) | | | (53,495 | ) | | | (41,513 | ) |
| | Change in other assets | | | (10,105 | ) | | | (55,604 | ) | | | (62,430 | ) | | | 38,879 | |
| | Transfer to restricted cash included in other long-term assets | | | — | | | | — | | | | — | | | | (56,600 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| | Net cash (used in) provided by investing activities | | | (703,970 | ) | | | (160,208 | ) | | | 80,931 | | | | (177,168 | ) |
Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| | Stock issuances | | | 106,866 | | | | 180,379 | | | | 95,912 | | | | 64,291 | |
| | Stock repurchases | | | (39,704 | ) | | | — | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| | Net cash provided by financing activities | | | 67,162 | | | | 180,379 | | | | 95,912 | | | | 64,291 | |
| | | |
| | | |
| | | |
| | | |
| |
| Net (decrease) increase in cash and cash equivalents | | | (156,181 | ) | | | 213,702 | | | | 45,825 | | | | 10,695 | |
| Cash and cash equivalents at beginning of period | | | 551,384 | | | | 337,682 | | | | 291,857 | | | | 281,162 | |
| | | |
| | | |
| | | |
| | | |
| |
Cash and cash equivalents at end of period | | $ | 395,203 | | | $ | 551,384 | | | $ | 337,682 | | | $ | 291,857 | |
| | | |
| | | |
| | | |
| | | |
| |
Supplemental cash flow data: | | | | | | | | | | | | | | | | |
| | Cash paid during the year for: | | | | | | | | | | | | | | | | |
| | | Interest | | $ | 7,493 | | | $ | 7,493 | | | $ | — | | | $ | 7,500 | |
| | | Income taxes paid (received) | | | 36,450 | | | | (5,005 | ) | | | 2,842 | | | | 15,898 | |
| | Stock received as consideration for outstanding loans | | | 6,490 | | | | 5,000 | | | | 16,000 | | | | — | |
| |
| (1) | All amounts related to the Redemption of our Special Common Stock transaction are reflected in the New Basis presentation. |
See notes to consolidated financial statements.
C-35
CONSOLIDATED BALANCE SHEETS
(Dollars in thousands, except par value)
| | | | | | | | | | | |
| | |
| | December 31, |
| |
|
| | 2001 | | 2000 |
| |
| |
|
Assets: | | | | | | | | |
| Current assets: | | | | | | | | |
| | Cash and cash equivalents | | $ | 395,203 | | | $ | 551,384 | |
| | Short-term investments | | | 952,875 | | | | 642,475 | |
| | Accounts receivable — trade (net of allowances of: 2001-$17,337; 2000-$14,126) | | | 193,203 | | | | 162,121 | |
| | Accounts receivable — other (net of allowances of: 2001-$5,005; 2000-$3,184) | | | 55,270 | | | | 63,262 | |
| | Accounts receivable — related party | | | 54,825 | | | | 36,299 | |
| | Inventories | | | 356,946 | | | | 265,830 | |
| | Deferred tax assets | | | 139,567 | | | | 40,619 | |
| | Prepaid expenses and other current assets | | | 61,463 | | | | 31,432 | |
| | | |
| | | |
| |
| | | Total current assets | | | 2,209,352 | | | | 1,793,422 | |
| Long-term marketable securities | | | 1,468,450 | | | | 1,265,515 | |
| Property, plant and equipment, net | | | 865,668 | | | | 752,892 | |
| Goodwill (net of accumulated amortization of: 2001-$996,779; 2000-$843,494) | | | 1,302,493 | | | | 1,455,778 | |
| Other intangible assets (net of accumulated amortization of: 2001-$1,459,285; 2000-$1,282,090) | | | 1,113,299 | | | | 1,280,359 | |
| Other long-term assets | | | 175,585 | | | | 168,458 | |
| | | |
| | | |
| |
Total assets | | $ | 7,134,847 | | | $ | 6,716,424 | |
| | | |
| | | |
| |
Liabilities and stockholders’ equity: | | | | | | | | |
| Current liabilities: | | | | | | | | |
| | Short-term debt | | $ | 149,692 | | | $ | — | |
| | Accounts payable | | | 33,348 | | | | 39,114 | |
| | Accrued liabilities — related parties | | | 45,259 | | | | 12,265 | |
| | Deferred revenue | | | 19,543 | | | | 15,433 | |
| | Other accrued liabilities | | | 403,913 | | | | 386,480 | |
| | | |
| | | |
| |
| | | Total current liabilities | | | 651,755 | | | | 453,292 | |
| Long-term debt | | | — | | | | 149,692 | |
| Deferred tax liabilities | | | 447,809 | | | | 349,848 | |
| Deferred revenue | | | 68,033 | | | | 87,600 | |
| Other long-term liabilities | | | 47,431 | | | | 1,789 | |
| | | |
| | | |
| |
| | | Total liabilities | | | 1,215,028 | | | | 1,042,221 | |
| Commitments and contingencies | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| | Preferred stock, $0.02 par value; authorized: 100,000,000 shares; none issued | | | — | | | | — | |
| | Common stock, $0.02 par value; authorized: 1,200,000,000 shares; outstanding: 2001-528,313,286 and 2000-525,476,771 | | | 10,566 | | | | 10,510 | |
| | Additional paid-in capital | | | 6,794,831 | | | | 6,651,428 | |
| | Accumulated deficit, since June 30, 1999 | | | (1,197,300 | ) | | | (1,319,353 | ) |
| | Accumulated other comprehensive income | | | 311,722 | | | | 331,618 | |
| | | |
| | | |
| |
| | | Total stockholders’ equity | | | 5,919,819 | | | | 5,674,203 | |
| | | |
| | | |
| |
Total liabilities and stockholders’ equity | | $ | 7,134,847 | | | $ | 6,716,424 | |
| | | |
| | | |
| |
See notes to consolidated financial statements.
C-36
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | Shares | | | | | | | | | | | | |
| |
| | | | | | | | Retained | | Accumulated | | |
| | Special | | | | Special | | | | Additional | | Earnings | | Other | | |
| | Common | | Common | | Common | | Common | | Paid-in | | (Accumulated | | Comprehensive | | |
| Old Basis(1) | | Stock | | Stock | | Stock | | Stock | | Capital | | Deficit) | | Income | | Total |
| |
| |
| |
| |
| |
| |
| |
| |
|
Balance December 31, 1998 | | | 201,975 | | | | 306,484 | | | $ | 4,040 | | | $ | 6,130 | | | $ | 1,581,362 | | | $ | 693,050 | | | $ | 59,263 | | | $ | 2,343,845 | |
| Period from January 1 to June 30, 1999: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Net income | | | | | | | | | | | | | | | | | | | | | | | 87,636 | | | | | | | | 87,636 | |
| | Changes in unrealized (loss) on securities available-for-sale, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,158 | ) | | | (1,158 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 86,478 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Issuance of stock upon exercise of options | | | 5,085 | | | | | | | | 102 | | | | | | | | 51,613 | | | | | | | | | | | | 51,715 | |
| Issuance of stock under employee stock plan | | | 1,014 | | | | | | | | 20 | | | | | | | | 12,557 | | | | | | | | | | | | 12,577 | |
| Income tax benefits realized from employee stock option exercises | | | | | | | | | | | | | | | | | | | 6,162 | | | | | | | | | | | | 6,162 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance June 30, 1999 | | | 208,074 | | | | 306,484 | | | $ | 4,162 | | | $ | 6,130 | | | $ | 1,651,694 | | | $ | 780,686 | | | $ | 58,105 | | | $ | 2,500,777 | |
|
New Basis(Effective June 30, 1999)(1) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Period from June 30 to December 31, 1999: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Push-down accounting: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Redemption of Special Common Stock and related issuance of Common Stock | | | (208,074 | ) | | | 202,710 | | | $ | (4,162 | ) | | $ | 4,054 | | | $ | 5,361,972 | | | $ | — | | | $ | (20,337 | ) | | $ | 5,341,527 | |
| | Eliminate Retained earnings (Old Basis) | | | | | | | | | | | | | | | | | | | 780,686 | | | | (780,686 | ) | | | | | | | — | |
| | | Adjustments related to the 1990 through 1997 purchase period: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | In-process research and development | | | | | | | | | | | | | | | | | | | (500,500 | ) | | | | | | | | | | | (500,500 | ) |
| | | | Amortization of goodwill, intangibles and fair value adjustment to inventories, net of tax | | | | | | | | | | | | | | | | | | | (1,221,644 | ) | | | | | | | | | | | (1,221,644 | ) |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | | | | | | | | | (1,245,112 | ) | | | | | | | (1,245,112 | ) |
| | Changes in unrealized gain on securities available-for-sale, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | 221,731 | | | | 221,731 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,023,381 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Issuance of stock upon exercise of options | | | | | | | 6,551 | | | | | | | | 131 | | | | 90,056 | | | | | | | | | | | | 90,187 | |
| Issuance of stock under employee stock plan | | | | | | | 476 | | | | | | | | 9 | | | | 6,057 | | | | | | | | | | | | 6,066 | |
| Income tax benefits realized from employee stock option exercises | | | | | | | | | | | | | | | | | | | 76,825 | | | | | | | | | | | | 76,825 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance December 31, 1999 | | | — | | | | 516,221 | | | $ | — | | | $ | 10,324 | | | $ | 6,245,146 | | | $ | (1,245,112 | ) | | $ | 259,499 | | | $ | 5,269,857 | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | | | | | | | | | (74,241 | ) | | | | | | | (74,241 | ) |
| | Changes in unrealized gain on securities available-for-sale, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | 72,119 | | | | 72,119 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (2,122 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Issuance of stock upon exercise of options | | | | | | | 8,259 | | | | | | | | 166 | | | | 148,241 | | | | | | | | | | | | 148,407 | |
| Issuance of stock under employee stock plan | | | | | | | 997 | | | | | | | | 20 | | | | 31,968 | | | | | | | | | | | | 31,988 | |
| Income tax benefits realized from employee stock option exercises | | | | | | | | | | | | | | | | | | | 226,073 | | | | | | | | | | | | 226,073 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance December 31, 2000 | | | — | | | | 525,477 | | | $ | — | | | $ | 10,510 | | | $ | 6,651,428 | | | $ | (1,319,353 | ) | | $ | 331,618 | | | $ | 5,674,203 | |
C-37
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY — (Continued)
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | Shares | | | | | | | | | | | | |
| |
| | | | | | | | Retained | | Accumulated | | |
| | Special | | | | Special | | | | Additional | | Earnings | | Other | | |
| | Common | | Common | | Common | | Common | | Paid-in | | (Accumulated | | Comprehensive | | |
| | Stock | | Stock | | Stock | | Stock | | Capital | | Deficit) | | Income | | Total |
| |
| |
| |
| |
| |
| |
| |
| |
|
Balance December 31, 2000 | | | — | | | | 525,477 | | | $ | — | | | $ | 10,510 | | | $ | 6,651,428 | | | $ | (1,319,353 | ) | | $ | 331,618 | | | $ | 5,674,203 | |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Net income | | | | | | | | | | | | | | | | | | | | | | | 150,236 | | | | | | | | 150,236 | |
| | Changes in unrealized (loss) on securities available-for-sale, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | (27,741 | ) | | | (27,741 | ) |
| | Cumulative effect of adopting FAS 133, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,020 | | | | | |
| | Changes in fair value of derivatives, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,757 | | | | | |
| | Derivative gains reclassified from other comprehensive income, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | (2,932 | ) | | | 7,845 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 130,340 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Issuance of stock upon exercise of options | | | | | | | 2,898 | | | | | | | | 57 | | | | 71,538 | | | | | | | | | | | | 71,595 | |
| Issuance of stock under employee stock plan | | | | | | | 838 | | | | | | | | 17 | | | | 35,254 | | | | | | | | | | | | 35,271 | |
| Repurchase of common stock | | | | | | | (900 | ) | | | | | | | (18 | ) | | | (11,503 | ) | | | (28,183 | ) | | | | | | | (39,704 | ) |
| Income tax benefits realized from employee stock option exercises | | | | | | | | | | | | | | | | | | | 48,114 | | | | | | | | | | | | 48,114 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance December 31, 2001 | | | — | | | | 528,313 | | | $ | — | | | $ | 10,566 | | | $ | 6,794,831 | | | $ | (1,197,300 | ) | | $ | 311,722 | | | $ | 5,919,819 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| |
| (1) | All amounts related to the Redemption of our Special Common Stock transaction are reflected in the New Basis presentation. |
See notes to consolidated financial statements.
C-38
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Description of Business and Summary of Significant Accounting Policies
Genentech is a leading biotechnology company using human genetic information to discover, develop, manufacture and market human pharmaceuticals that address significant unmet medical needs. Fifteen of the approved products of biotechnology stem from or are based on our science. We manufacture and market ten protein-based pharmaceuticals, and license several additional products to other companies.
On June 30, 1999, we redeemed all of our outstanding Special Common Stock held by stockholders other than Roche Holdings, Inc. (or Roche) with funds deposited by Roche for that purpose. This event, referred to as the “Redemption” in this report, caused Roche to own 100% of the outstanding common stock of Genentech on that date. The Redemption of our Special Common Stock on June 30, 1999 was reflected as a purchase of a business which, under U.S. generally accepted accounting principles, required push-down accounting to reflect in our financial statements the amounts paid for our stock in excess of our net book value. The Redemption created our New Basis of accounting. The Redemption was effective as of June 30, 1999, however, the transaction was reflected as of the end of the day on June 30, 1999 in the financial statements. As such, the “Old Basis” and “New Basis” are presented separately in the consolidated financial statements and notes to consolidated financial statements where applicable. Accordingly, the Old Basis reflects the period January 1 through June 30, 1999, and the New Basis reflects the period from June 30 through December 31, 1999, and all subsequent periods.
On July 23, 1999, October 26, 1999, and March 29, 2000, Roche completed public offerings of our Common Stock. We did not receive any of the net proceeds from these offerings. On January 19, 2000, Roche completed an offering of zero-coupon notes that are exchangeable for an aggregate of approximately 13.0 million shares of our Common Stock held by Roche. Roche’s percentage ownership of our outstanding Common Stock was 58.0% at December 31, 2001.
| |
| Principles of Consolidation |
The consolidated financial statements include the accounts of Genentech and all subsidiaries. Material intercompany balances and transactions are eliminated.
The preparation of our financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. Actual results could differ from those estimates.
| |
| Change in Accounting Principles |
On January 1, 2001, we adopted statement of Financial Accounting Standards (or FAS) No. 133, “Accounting for Derivative Instruments and Hedging Activities” as amended by FAS 138, “Accounting for Certain Derivative Instruments and Certain Hedging Activities.” FAS 133 requires us to recognize all derivatives on the balance sheet at fair value. Derivatives that are not designated as hedges must be adjusted to fair value through earnings. If the derivative is designated and qualifies as a hedge, depending on the nature of the hedge, changes in the fair value of the derivative are either offset against the change in fair value of assets, liabilities, or firm commitments through earnings or recognized in other comprehensive income until the hedged item is recognized in earnings. The ineffective portion of a derivative’s change in fair value will be immediately recognized in earnings. The adoption of FAS 133 resulted in a $5.6 million charge, net of tax, ($0.01 per share) as a cumulative effect of an accounting change and the recognition of $6.0 million in gains, net of tax, ($0.01 per share) related to the change in the time value of certain hedging instruments in the statement of operations, and an increase of $5.0 million, net of tax, in other comprehensive income.
C-39
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
We previously recognized non-refundable, upfront product license fees as revenue when the technology was transferred and when all of our significant contractual obligations relating to the fees had been fulfilled. Effective January 1, 2000, we changed our method of accounting for non-refundable upfront product license fees and certain guaranteed payments to recognize such fees over the term of the related development collaboration when, at the execution of the agreement, the development period involves significant risk due to the incomplete stage of the product’s development, or over the period of manufacturing obligation when, at the execution of the agreement, the product is approved for marketing, or nearly approvable, and development risk has been substantially eliminated. Deferred revenue related to manufacturing obligations will be recognized on a straight-line basis over the longer of the contractual term of the manufacturing obligation or the expected period over which we will supply the product. We believe the change in accounting principle is preferable based on guidance provided in the Securities and Exchange Commission’s, or SEC, Staff Accounting Bulletin No. 101, “Revenue Recognition in Financial Statements.”
The cumulative effect of the change in accounting principle was reported as a charge in the year ended December 31, 2000. The cumulative effect was initially recorded as deferred revenue that will be recognized as revenue over the remaining term of the research and development collaboration or distribution agreements, as appropriate. For the year ended December 31, 2000, the impact of the change in accounting was to increase net loss by $52.6 million, or $0.10 per share, comprised of the $57.8 million cumulative effect of the change (net of tax impact) as described above ($0.11 per share), net of $5.2 million of the related deferred revenue (less related tax impact of $3.4 million) that was recognized as revenue during the year ($0.01 per share). The remainder of the related deferred revenue of $90.7 million will be recognized in 2001 through 2019. Pro forma amounts of net income (loss) and related per share amounts, assuming retroactive application of the accounting change for all periods presented, are as follows (in thousands, except per share amounts):
| | | | | | | | | | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| As Reported: | | | | | | | | | | | | |
| | Net income (loss) | | | — | | | $ | (74,241 | ) | | $ | (1,157,476 | ) |
| | Net income (loss) per share — diluted | | | — | | | $ | (0.14 | ) | | $ | (2.26 | ) |
| Pro forma amounts with the change in accounting principle related to revenue recognition applied retroactively (unaudited): | | | | | | | | | | | | |
| | Net income (loss) | | | — | | | $ | (16,441 | ) | | $ | (1,168,716 | ) |
| | Net income (loss) per share — diluted | | | — | | | $ | (0.03 | ) | | $ | (2.28 | ) |
| |
| Cash and Cash Equivalents |
We consider all highly liquid debt instruments purchased with an original maturity of three months or less to be cash equivalents.
| |
| Short-Term Investments and Long-Term Marketable Securities |
We invest our excess cash balances in short-term and long-term marketable securities, primarily corporate notes, certificates of deposit, preferred stock, asset-backed securities and municipal bonds. As part of our strategic alliance efforts, we may also invest in equity securities, dividend bearing convertible preferred stock and interest bearing convertible debt of other biotechnology companies. All of our equity investments represent less than a 20% ownership position. Marketable equity securities are accounted for as available-for-sale investment securities as described below. Nonmarketable equity securities and convertible debt are carried at cost. We periodically monitor the liquidity and financing activities of these entities to determine if impairment write downs are required. We had investments of $48.4 million at December 31, 2001, and $48.5 million at December 31, 2000, in convertible debt of various biotechnology companies.
Investment securities are classified into one of three categories: held-to-maturity, available-for-sale or trading. Securities are considered held-to-maturity when we have the positive intent and ability to hold the securities to maturity. Held-to-maturity securities are stated at amortized cost, including adjustments for
C-40
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
amortization of premiums and accretion of discounts. Securities are considered trading when bought principally for the purpose of selling in the near term. These securities are recorded as short-term investments and are carried at market value. Unrealized holding gains and losses on trading securities are included in interest income. Securities not classified as held-to-maturity or as trading are considered available-for-sale. These securities are recorded as either short-term investments or long-term marketable securities and are carried at market value with unrealized gains and losses included in accumulated other comprehensive income in stockholders’ equity. If a decline in the fair value of a marketable equity security is below its cost for two consecutive quarters or if the decline is due to a significant adverse event, it is considered to be an other than temporary decline. Accordingly, the marketable equity security is written down to estimated fair value with a charge to marketing, general and administrative expenses. Other than temporary declines in fair value on short-term and long-term investments are charged against interest income. The cost of all securities sold is based on the specific identification method. We recognized expense of $27.5 million in 2001, $4.8 million in 2000 and $13.4 million in 1999 as a result of charges related to other than temporary declines in the fair values of certain of our investments.
We use derivatives to partially offset our market exposure to fluctuations in foreign currencies, U.S. interest rates and marketable equity investments. We record all derivatives on the balance sheet at fair value. For derivative instruments that are designated and qualify as a fair value hedge (i.e., hedging the exposure to changes in the fair value of an asset or a liability or an identified portion thereof that is attributable to a particular risk), the gain or loss on the derivative instrument, as well as the offsetting loss or gain on the hedged item attributable to the hedged risk, is recognized in current earnings during the period of the change in fair values. For derivative instruments that are designated and qualify as a cash flow hedge (i.e., hedging the exposure to variability in expected future cash flows that is attributable to a particular risk), the effective portion of the gain or loss on the derivative instrument is reported as a component of other comprehensive income and reclassified into earnings in the same period or periods during which the hedged transaction affects earnings. Any gain or loss on the derivative instrument in excess of the cumulative change in the present value of future cash flows of the hedged transaction, if any, is recognized in current earnings during the period of change. For derivative instruments not designated as hedging instruments, the gain or loss is recognized in current earnings during the period of change. See the “Derivative Financial Instruments” note below for further information on our accounting for derivatives.
Inventories are stated at the lower of cost or market. Cost is determined using a weighted-average approach which approximates the first-in first-out method. If the cost of the inventories exceeds their expected market value, provisions are recorded currently for the difference between the cost and the market value. These provisions are determined based on significant estimates. Inventories consist of currently marketed products and pre-launch product candidates, which we expect to commercialize in the near term.
Inventories increased in 2001 primarily due to higher pre-launch inventories of Xolair and Xanelim and higher Herceptin inventories. Inventories in 2000 decreased from 1999 primarily due to the effect of the Redemption and push-down accounting, offset by increases in inventory production. As a result of push-down accounting, we recorded $186.2 million related to the write up of inventory to its then fair value, of which we recognized in cost of sales $92.8 million in 2000 and $93.4 million in 1999 upon the sale of inventory. In anticipation of the launch of Xolair, we have produced approximately $77.2 million of Xolair inventory, of which $42.3 million has been paid by our collaborator, Novartis Pharmaceuticals Corporation, or covered by inventory provisions. Due to the launch delay of Xolair, we will continually assess the realizability of our
C-41
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Xolair inventory based on an expected U.S. Food and Drug Administration (or FDA) approval date and forecasted sales. Inventories at December 31, 2001 and 2000 are summarized below (in thousands):
| | | | | | | | | | |
| | 2001 | | 2000 |
| |
| |
|
| Raw materials and supplies | | $ | 23,633 | | | $ | 17,621 | |
| Work in process | | | 299,717 | | | | 233,121 | |
| Finished goods | | | 33,596 | | | | 15,088 | |
| | | |
| | | |
| |
| | Total | | $ | 356,946 | | | $ | 265,830 | |
| | | |
| | | |
| |
| |
| Property, Plant and Equipment |
The costs of buildings and equipment are depreciated using the straight-line method over the following estimated useful lives of the assets:
| | | |
| | Useful Lives |
| |
|
| Buildings | | 25 years |
| Certain manufacturing equipment | | 15 years |
| Other equipment | | 4 or 8 years |
| Leasehold improvements | | length of applicable lease |
The costs of repairs and maintenance are expensed as incurred. Capitalized interest on construction-in-progress is included in property, plant and equipment. The repairs and maintenance expenses and capitalized interest were as follows (in millions):
| | | | | | | | | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| Repairs and maintenance expenses | | $ | 52.8 | | | $ | 42.1 | | | $ | 39.9 | |
| Capitalized interest | | | 1.8 | | | | 2.2 | | | | 2.1 | |
Property, plant and equipment balances at December 31, 2001 and 2000 are summarized below (in thousands):
| | | | | | | | | | |
| | 2001 | | 2000 |
| |
| |
|
| At cost: | | | | | | | | |
| | Land | | $ | 125,029 | | | $ | 90,274 | |
| | Buildings | | | 402,473 | | | | 392,119 | |
| | Equipment | | | 788,198 | | | | 761,696 | |
| | Leasehold improvements | | | 30,632 | | | | 18,456 | |
| | Construction-in-progress | | | 155,563 | | | | 94,679 | |
| | | |
| | | |
| |
| | | | 1,501,895 | | | | 1,357,224 | |
| Less: accumulated depreciation and amortization | | | 636,227 | | | | 604,332 | |
| | | |
| | | |
| |
| Net property, plant and equipment | | $ | 865,668 | | | $ | 752,892 | |
| | | |
| | | |
| |
Depreciation expense was $96.3 million in 2001, $88.8 million in 2000 and $80.9 million in 1999.
FDA validation costs are capitalized as part of the effort required to acquire and construct long-lived assets, including readying them for their intended use, and are amortized over the estimated useful life of the asset or the term of the lease, whichever is shorter.
C-42
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Goodwill represents the difference between the purchase price and the fair value of the net assets when accounted for by the purchase method of accounting arising from Roche’s purchases of our Special Common Stock and push-down accounting. Goodwill is amortized on a straight-line basis over 15 years. See also the “New Accounting Pronouncements” section below.
Other intangible assets arising from Roche’s purchases of our Special Common Stock and push-down accounting are amortized over their estimated useful lives ranging from five to 15 years. Costs of patents and patent applications related to products and processes of significant importance to us are capitalized and amortized on a straight-line basis over their estimated useful lives of approximately 12 years. Other intangible assets are generally amortized on a straight-line basis over their estimated useful lives.
Under certain lease agreements, we may be required from time to time to set aside cash as collateral. At December 31, 2001 and 2000, other long-term assets included $56.6 million of restricted cash related to such lease agreements.
| |
| Impairment of Long-Lived Assets |
Long-lived assets and certain identifiable intangible assets to be held and used are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values. Long-lived assets and certain identifiable intangible assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. See “New Accounting Pronouncements” below.
We recognize revenue from product sales when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed, and determinable and collectibility is reasonably assured. Allowances are established for estimated uncollectible amounts, product returns and discounts.
Royalties from licensees are based on third-party sales of licensed products or technologies and recorded as earned in accordance with contract terms when third-party results are reliably measured and collectibility is reasonably assured. Royalty estimates are made in advance of amounts collected using historical and forecasted trends.
We receive royalties on sales of rituximab, outside of the U.S. (excluding Japan), on sales of Pulmozyme and Herceptin outside of the U.S. and on sales of certain of our products in Canada from F. Hoffmann-La Roche Ltd, a subsidiary of Roche (or Hoffmann-La Roche). See “Relationship With Roche” note below for further discussion.
We receive royalties on sales of growth hormone, tissue-plasminogen activator and tenecteplase products outside of the U.S. and Canada through other licensees. We also receive worldwide royalties on additional licensed products that are marketed by other companies.
C-43
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Contract revenue for research and development (or R&D) is recorded as earned based on the performance requirements of the contract. Non-refundable contract fees for which no further performance obligations exist, and there is no continuing involvement by Genentech, are recognized on the earlier of when the payments are received or when collection is assured.
Revenue from non-refundable upfront license fees and certain guaranteed payments where we continue involvement through development collaboration or an obligation to supply product is recognized ratably over the development period when, at the execution of the agreement, the development period involves significant risk due to the incomplete stage of the product’s development, or over the period of the manufacturing obligation, when, at the execution of the agreement, the product is approved for marketing, or nearly approvable, and development risk has been substantially eliminated. Deferred revenues related to manufacturing obligations are recognized on a straight-line basis over the longer of the contractual term of the manufacturing obligation or the expected period over which we will supply the product.
Revenue associated with performance milestones is recognized based upon the achievement of the milestones, as defined in the respective agreements. Revenue under R&D cost reimbursement contracts is recognized as the related costs are incurred.
Advance payments received in excess of amounts earned are classified as deferred revenue until earned.
| |
| Research and Development Expenses |
Research and development (or R&D) expenses include related salaries, contractor fees, building costs, utilities, administrative expenses and allocations of corporate costs. R&D expenses consist of independent R&D costs and costs associated with collaborative R&D and in-licensing arrangements. In addition, we fund R&D at other companies and research institutions under agreements, which are generally cancelable. R&D expenses also include activities such as product registries and investigator sponsored trials. All such costs are charged to R&D expense as incurred.
| |
| Collaboration Profit Sharing |
Collaboration profit sharing includes primarily the net operating profit sharing with IDEC Pharmaceuticals Corporation on Rituxan sales, and the sharing of costs with collaborators related to the commercialization of future products.
Royalty expenses directly related to product sales are classified in cost of sales. Other royalty expenses, relating to royalty revenue, totaled $59.5 million in 2001, $34.4 million in 2000, and $39.0 million in 1999 and are classified in marketing, general and administrative expenses.
We expense the costs of advertising, which also includes promotional expenses, as incurred. Advertising expenses were $91.9 million in 2001, $86.5 million in 2000, and $80.0 million in 1999.
Our 401(k) Plan, or the Plan, covers substantially all of our employees. Under the Plan, eligible employees may contribute up to 15% of their eligible compensation, subject to certain Internal Revenue Service restrictions. We match a portion of employee contributions, up to a maximum of 4% of each employee’s eligible compensation. The match is effective December 31 of each year and is fully vested when made. We provided $11.9 million in 2001, $10.1 million in 2000, and $8.5 million in 1999, for our match under the Plan.
C-44
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Income tax expense is based on pretax financial accounting income under the liability method. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse.
| |
| Earnings (Loss) Per Share |
Basic earnings (loss) per share is computed based on the weighted-average number of shares of our Common Stock and Special Common Stock outstanding. Diluted earnings (loss) per share is computed based on the weighted-average number of shares of our Common Stock, Special Common Stock and other dilutive securities. See also “Earnings (Loss) Per Share” note below. All numbers relating to the number of shares, price per share and per share amounts of Common Stock, Special Common Stock and Redeemable Common Stock give effect to the two-for-one splits of our Common Stock that were effected on October 24, 2000 and November 2, 1999.
Comprehensive income is comprised of net income (loss) and other comprehensive income. Other comprehensive income includes certain changes in stockholders’ equity that are excluded from net income. Other comprehensive income includes changes in fair value of derivatives designated as and effective as cash flow hedges and unrealized gains and losses on our available-for-sale securities. Comprehensive income for years ended December 31, 2001, 2000 and 1999 has been reflected in the Consolidated Statements of Stockholders’ Equity.
| |
| New Accounting Pronouncements |
In June 2001, the Financial Accounting Standards Board (or FASB) issued FAS 141 on Business Combinations and FAS 142 on Goodwill and Other Intangible Assets, effective for fiscal years beginning after December 15, 2001. FAS 141 requires that the purchase method of accounting be used for all business combinations initiated after June 30, 2001 and also specifies the criteria for the recognition of intangible assets separately from goodwill. Under the new rules, goodwill will no longer be amortized but will be subject to an impairment test at least annually. Separately identified and recognized intangible assets resulting from business combinations completed before July 1, 2001 that do not meet the new criteria for separate recognition of intangible assets will be subsumed in goodwill upon adoption. FAS 141 specifically identified assembled workforce as an intangible asset that is not to be recognized apart from goodwill. Other intangible assets that meet the new criteria will continue to be amortized over their useful lives.
We will apply the new rules on accounting for goodwill and other intangible assets on January 1, 2002. The adoption of FAS 141 and 142 is not expected to have a significant impact on our financial position at transition. We expect that the cessation of goodwill amortization and the amortization of our trained and assembled workforce intangible asset (net of related taxes) will increase reported net income by approximately $150.0 million (or $0.28 per share) in 2002. At December 31, 2001, the carrying value of our goodwill was $1,302.5 million and our trained and assembled workforce intangible asset was $31.7 million. We performed an impairment test of goodwill as of January 1, 2002 and will not record an impairment charge at transition. We will continue to monitor the fair value of our goodwill through the annual impairment tests.
In October 2001, the FASB issued FAS 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.” FAS 144 supersedes FAS 121, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to Be Disposed Of.” The primary objectives of FAS 144 are to develop one accounting model based on the framework established in FAS 121 for long-lived assets to be disposed of by sale, and to address significant implementation issues. Our adoption of FAS 144 on January 1, 2002 is not expected to have a material impact on our financial position and results of operations.
C-45
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Certain reclassifications of prior year amounts have been made to conform with the current year presentation.
Redemption of Our Special Common Stock
Roche accounted for the Redemption as a purchase of a business. As a result, we were required to push down the effect of the Redemption and Roche’s 1990 through 1997 purchases of our Common and Special Common Stock into our consolidated financial statements at the date of the Redemption, which results in our New Basis presentation. Under this method of accounting, our assets and liabilities, including other intangible assets, were recorded at their fair values not to exceed the aggregate purchase price plus Roche’s transaction costs at June 30, 1999. In 1990 and 1991 through 1997 Roche purchased 60% and 5%, respectively, of the outstanding stock of Genentech. In June 1999, we redeemed all of our Special Common Stock held by stockholders other than Roche resulting in Roche owning 100% of our Common Stock. The push-down effect of Roche’s aggregate purchase price and the Redemption price in our consolidated balance sheet as of June 30, 1999 was allocated based on Roche’s ownership percentages as if the purchases occurred at the original purchase dates for the 1990 and 1991 through 1997 purchases, and at June 30, 1999 for the Redemption. Management of Genentech determined the values of tangible and intangible assets, including in-process research and development (or IPR&D) used in allocating the purchase prices. The aggregate purchase prices for the acquisition of all of Genentech’s outstanding shares, including Roche’s estimated transaction costs of $10.0 million, was $6,604.9 million, consisting of approximately $2,843.5 million for the 1990 and 1991 through 1997 purchases and approximately $3,761.4 million for the Redemption.
The following table shows details of the excess of purchase price over net book value (in millions):
| | | | | | | | | | | | | | |
| | | | |
| | Purchase Period | | |
| |
| | |
| | 1990-1997 | | 1999 | | Total |
| |
| |
| |
|
| Total purchase price | | $ | 2,843.5 | | | $ | 3,761.4 | | | $ | 6,604.9 | |
| | Less portion of net book value purchased | | | 566.6 | | | | 836.4 | | | | 1,403.0 | |
| | | |
| | | |
| | | |
| |
| Excess of purchase price over net book value | | $ | 2,276.9 | | | $ | 2,925.0 | | | $ | 5,201.9 | |
| | | |
| | | |
| | | |
| |
The following table shows the allocation of the excess of the purchase price over net book value (in millions):
| | | | | | | | | | | | | | |
| | | | |
| | Purchase Period | | |
| |
| | |
| | 1990-1997 | | 1999 | | Total |
| |
| |
| |
|
| Inventories | | $ | 102.0 | | | $ | 186.2 | | | $ | 288.2 | |
| Land | | | — | | | | 16.6 | | | | 16.6 | |
| In-process research and development | | | 500.5 | | | | 752.5 | | | | 1,253.0 | |
| Developed product technology | | | 429.0 | | | | 765.0 | | | | 1,194.0 | |
| Core technology | | | 240.5 | | | | 203.0 | | | | 443.5 | |
| Developed license technology | | | 292.5 | | | | 175.0 | | | | 467.5 | |
| Trained and assembled workforce | | | 32.5 | | | | 49.0 | | | | 81.5 | |
| Tradenames | | | 39.0 | | | | 105.0 | | | | 144.0 | |
| Key distributor relationships | | | 6.5 | | | | 73.5 | | | | 80.0 | |
| Goodwill | | | 1,091.2 | | | | 1,208.1 | | | | 2,299.3 | |
| Deferred tax liability | | | (456.8 | ) | | | (629.2 | ) | | | (1,086.0 | ) |
| Write up of valuation allowance related to marketable securities | | | — | | | | 20.3 | | | | 20.3 | |
| | | |
| | | |
| | | |
| |
| | Total | | $ | 2,276.9 | | | $ | 2,925.0 | | | $ | 5,201.9 | |
| | | |
| | | |
| | | |
| |
C-46
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
| |
| Push-Down Accounting Adjustments |
The following is a description of accounting adjustments and related useful lives that reflect push-down accounting in our financial statements. These adjustments were based on management’s estimates of the value of the tangible and intangible assets acquired:
| | |
| | • | We recorded charges of $1,207.7 million in 1999. These charges primarily included: a non-cash charge of $752.5 million for IPR&D; $284.5 million of compensation expense related to early cash settlement of certain employee stock options; and an aggregate of approximately $160.1 million of non-cash compensation expense in connection with the modification and remeasurement, for accounting purposes, of continuing employee stock options, which represents the difference between each applicable option exercise price and the redemption price of the Special Common Stock. (Please refer to the “Capital Stock” note below for further information on these charges.) |
| |
| | • | We recorded an income tax benefit of $177.8 million related to the above early cash settlement and non-cash compensation related to certain employee stock options. The income tax benefit reduced the current tax payable in other accrued liabilities by $56.9 million and reduced long-term deferred income taxes by $120.9 million. |
| |
| | • | The estimated useful life of the inventory adjustment to fair value resulting from the Redemption was approximately one year based upon the expected time to sell inventories on hand at June 30, 1999. As the fair-valued inventory is sold, the related write up amount is charged to cost of sales. In 2000, we recognized $92.8 million of expense related to the inventory write up adjustment. In 1999, we recognized $93.4 million of expense related to the inventory write up adjustment. All inventory written up as a result of the Redemption was sold as of December 31, 2000. The entire inventory adjustment related to Roche’s 1990 through 1997 purchases was reflected as an adjustment to additional paid-in capital. |
| |
| | • | An adjustment was made to record the fair value of land as a result of the Redemption. There were no such adjustments for the purchase periods from 1990 through 1997. |
| |
| | • | Recorded $1,091.2 million of goodwill, which reflects Roche’s 1990 through 1997 purchases, net of related accumulated amortization of $613.6 million through June 30, 1999. The accumulated amortization was recorded as an adjustment to additional paid-in capital at June 30, 1999. Included in goodwill was $456.8 million related to the recording of deferred tax liabilities. Deferred taxes were recorded for the adjustment to fair value for other intangible assets and inventories as a result of Roche’s 1990 through 1997 purchases. The deferred tax liability was calculated based on a marginal tax rate of 40%. The goodwill related to the 1990 through 1997 purchases was amortized over 15 years. |
| |
| | • | $1,208.1 million of goodwill was recorded as a result of the Redemption. Included in goodwill was $629.2 million related to the recording of deferred tax liabilities. Deferred taxes were recorded for the adjustment to fair value for other intangible assets, inventories and land. The deferred tax liability was calculated based on a marginal tax rate of 40% and was allocated between short- and long-term classifications to match the asset classifications. The goodwill related to the Redemption is being amortized over 15 years. |
| |
| | • | We recorded a write up of our valuation allowance related to marketable securities of $20.3 million related to Roche’s percentage ownership purchased, at the time of Redemption, of the net unrealized gains on investments. |
| |
| | • | In 2001, we recorded amortization expense of $153.3 million related to goodwill and $164.3 million related to other intangible assets. In 2000, we recorded amortization expense of $153.3 million related to goodwill and $211.0 million related to other intangible assets. In 1999, we recorded amortization expense of $76.6 million related to goodwill and $113.8 million related to other intangible assets. |
C-47
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
| | |
| | • | The existing deferred tax asset valuation allowance of $62.8 million related to the tax benefits of stock option deductions which have been realized and credited to paid-in capital as a result of establishing deferred tax liabilities under push-down accounting was eliminated at June 30, 1999. |
| |
| | • | The redemption of our Special Common Stock and the issuance of new shares of Common Stock to Roche resulted in substantially the same number of total shares outstanding as prior to the Redemption. |
| |
| | • | The balances of our Common Stock and additional paid-in capital at the Redemption include Roche’s cost of acquiring our shares in 1990 and the cost of subsequent equity purchases, net of the amortization of the goodwill, IPR&D and other prior period charges related to the 1990 through 1997 purchases. The excess of purchase price over net book value of $2,276.9 million for 1990 through 1997 and $2,925.0 million in 1999, and $160.1 million for the remeasurement of continuing employee stock options at the remeasurement date was recorded in additional paid-in capital. |
| |
| | In addition, the following adjustments were made to additional paid-in capital for the 1990 through 1997 purchase period (in millions): |
| | | | | | |
| | 1990-1997 |
| | Purchases |
| |
|
| In-process research and development | | $ | (500.5 | ) |
| Amortization of goodwill, intangibles and fair value adjustment to inventories, net of tax | | | (1,221.6 | ) |
| | | |
| |
| | Total adjustment to additional paid-in capital | | $ | (1,722.1 | ) |
| | | |
| |
| | |
| | • | Our retained earnings prior to the Redemption was not carried forward. This resulted in an adjustment of $780.7 million to increase additional paid-in capital and eliminate the retained earnings balance immediately prior to the Redemption. |
| |
| | • | The tax provision benefit of $203.1 million for 1999 consists of tax expense of $114.8 million on pretax income excluding the income and deductions attributable to push-down accounting and legal settlements, and tax benefits of $317.9 million for 1999 related to the income and deductions attributable to push-down accounting and legal settlements. |
| |
| | • | Recorded $1,040.0 million of other intangible assets, which reflects Roche’s 1990 through 1997 purchases, net of related accumulated amortization of $911.5 million of those assets through June 30, 1999. The accumulated amortization was recorded as an adjustment to additional paid-in capital at June 30, 1999. The components of other intangible assets related to these purchases and their estimated lives are as follows (in millions): |
| | | | | | | | | | | | | | |
| | Fair | | Accumulated | | Estimated |
| | Value | | Amortization | | Life |
| |
| |
| |
|
| Developed product technology | | $ | 429.0 | | | $ | 361.8 | | | | 10 | |
| Core technology | | | 240.5 | | | | 202.9 | | | | 10 | |
| Developed license technology | | | 292.5 | | | | 286.9 | | | | 6 | |
| Trained and assembled workforce | | | 32.5 | | | | 31.6 | | | | 7 | |
| Tradenames | | | 39.0 | | | | 21.9 | | | | 15 | |
| Key distributor relationships | | | 6.5 | | | | 6.4 | | | | 5 | |
| | | |
| | | |
| | | | | |
| | Total | | $ | 1,040.0 | | | $ | 911.5 | | | | | |
| | | |
| | | |
| | | | | |
| | |
| | • | $1,370.5 million of other intangible assets was recorded as a result of the Redemption. The components of other intangible assets related to the Redemption and their estimated lives are as follows (in millions): |
C-48
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
| | | | | | | | | | |
| | Fair | | Estimated |
| | Value | | Life |
| |
| |
|
| Developed product technology | | $ | 765.0 | | | | 10 | |
| Core technology | | | 203.0 | | | | 10 | |
| Developed license technology | | | 175.0 | | | | 6 | |
| Trained and assembled workforce | | | 49.0 | | | | 7 | |
| Tradenames | | | 105.0 | | | | 15 | |
| Key distributor relationships | | | 73.5 | | | | 5 | |
| | | |
| | | | | |
| | Total | | $ | 1,370.5 | | | | | |
| | | |
| | | | | |
| | |
| | • | $500.5 million and $752.5 million of IPR&D was recorded as a result of Roche’s 1990 through 1997 purchases and the Redemption, respectively. At the date of each purchase, Genentech concluded that technological feasibility of the acquired in-process technology was not established and that the in-process technology had no future alternative uses. The amount related to the 1990 through 1997 purchases was recorded as an adjustment to additional paid-in capital at June 30, 1999. The amount related to the Redemption was charged to operations at June 30, 1999. |
| |
| | The amounts of IPR&D were determined based on an analysis using the risk-adjusted cash flows expected to be generated by the products that result from the in-process projects. The analysis included forecasting future cash flows that were expected to result from the progress made on each of the in-process projects prior to the purchase dates. These cash flows were estimated by first forecasting, on a product-by-product basis, total revenues expected from sales of the first generation of each in-process product. A portion of the gross in-process product revenues was then removed to account for the contribution provided by any core technology, which was considered to benefit the in-process products. The net in-process revenue was then multiplied by the project’s estimated percentage of completion as of the purchase dates to determine a forecast of net IPR&D revenues attributable to projects completed prior to the purchase dates. Appropriate operating expenses, cash flow adjustments and contributory asset returns were deducted from the forecast to establish a forecast of net returns on the completed portion of the in-process technology. Finally, these net returns were discounted to a present value at discount rates that incorporate both the weighted-average cost of capital (relative to the biotech industry and us) as well as the product-specific risk associated with the purchased IPR&D products. The product specific risk factors included each phase of development, type of molecule under development, likelihood of regulatory approval, manufacturing process capability, scientific rationale, preclinical safety and efficacy data, target product profile and development plan. The discount rates ranged from 16% to 19% for the 1999 valuation and 20% to 28% for the 1990 purchase valuation, all of which represent a significant risk premium to our weighted-average cost of capital. |
| |
| | The forecast data employed in the analysis was based on internal product level forecast information maintained by our management in the ordinary course of managing the business. The inputs used by us in analyzing IPR&D were based on assumptions, which we believed to be reasonable but which are inherently uncertain and unpredictable. These assumptions may be incomplete or inaccurate, and no assurance can be given that unanticipated events and circumstances will not occur. |
C-49
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Segment, Significant Customer and Geographic Information
Our operations are treated as one operating segment as we only report profit and loss information on an aggregate basis to our chief operating decision-makers. Information about our product sales, major customers and material foreign source of revenues is as follows (in millions):
| | | | | | | | | | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| Herceptin | | $ | 346.6 | | | $ | 275.9 | | | $ | 188.4 | |
| Rituxan | | | 818.7 | | | | 444.1 | | | | 279.4 | |
| Activase/ TNKase/ Cathflo Activase | | | 197.1 | | | | 206.2 | | | | 236.0 | |
| Growth hormone (Nutropin Depot, Nutropin AQ, Nutropin and Protropin) | | | 250.2 | | | | 226.6 | | | | 221.2 | |
| Pulmozyme | | | 122.9 | | | | 121.8 | | | | 111.4 | |
| Actimmune | | | 7.4 | | | | 3.7 | | | | 2.7 | |
| | | |
| | | |
| | | |
| |
| | Total product sales | | $ | 1,742.9 | | | $ | 1,278.3 | | | $ | 1,039.1 | |
| | | |
| | | |
| | | |
| |
Three major customers, Amerisource/ Bergen, Corp., Cardinal Health, Inc. and McKesson, Inc. each contributed 10% or more of our total revenues in at least one of the last three years. Amerisource/ Bergen, formerly Amerisource and Bergen Brunswig (merger effective August 2001), a national wholesale distributor of all of our products, contributed 21% in 2001, 20% in 2000 and 21% in 1999 of our total revenues. Cardinal Health, a national wholesaler distributor of all our products, contributed 18% in 2001, 15% in 2000 and 13% in 1999 of our total revenues. McKesson, a national wholesale distributor of all of our products, contributed 14% in 2001 and less than 10% in 2000 and 1999 of our total revenues.
Net foreign revenues were $230.0 million in 2001, $164.2 million in 2000 and $155.0 million in 1999. Material foreign revenues by country were as follows (in millions):
| | | | | | | | | | | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| Europe: | | | | | | | | | | | | |
| | Switzerland | | $ | 74.9 | | | $ | 72.6 | | | $ | 61.5 | |
| | Germany | | | 39.2 | | | | 22.5 | | | | 39.6 | |
| | Italy | | | 18.0 | | | | 10.4 | | | | 14.6 | |
| | Great Britain | | | 24.5 | | | | 9.6 | | | | 6.0 | |
| | Others | | | 25.5 | | | | 14.7 | | | | 11.9 | |
| Canada | | | 24.0 | | | | 19.8 | | | | 11.8 | |
| Japan | | | 23.9 | | | | 14.6 | | | | 9.6 | |
| | | |
| | | |
| | | |
| |
| | | Total | | $ | 230.0 | | | $ | 164.2 | | | $ | 155.0 | |
| | | |
| | | |
| | | |
| |
We currently sell primarily to distributors and health care companies throughout the U.S., perform ongoing credit evaluations of our customers’ financial condition and extend credit, generally without collateral. In 2001, 2000 and 1999, we did not record any material additions to, or losses against, our provision for doubtful accounts.
Research and Development Arrangements
To gain access to potential new products and technologies and to utilize other companies to help develop our potential new products, we establish strategic alliances with various companies. These strategic alliances include the acquisition of marketable and nonmarketable equity investments and convertible debt of companies developing technologies that fall outside our research focus and include companies having the
C-50
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
potential to generate new products through technology exchanges and investments. Potential future payments may be due to certain collaborative partners achieving certain benchmarks as defined in the collaborative agreements. We also entered into product-specific collaborations to acquire development and marketing rights for products.
Income Taxes
The income tax provision (benefit) consists of the following amounts (in thousands):
| | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| | | | | | New Basis | | Old Basis |
| | | | | |
| |
|
| Current: | | | | | | | | | | | | | | | | |
| | Federal | | $ | 72,731 | | | $ | 191,334 | | | $ | (110,991 | ) | | $ | 76,819 | |
| | State | | | 25,024 | | | | 25,862 | | | | (6,165 | ) | | | 1,366 | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total current | | | 97,755 | | | | 217,196 | | | | (117,156 | ) | | | 78,185 | |
| | | |
| | | |
| | | |
| | | |
| |
| Deferred: | | | | | | | | | | | | | | | | |
| | Federal | | | 47,043 | | | | (151,817 | ) | | | (119,624 | ) | | | (16,397 | ) |
| | State | | | (17,686 | ) | | | (44,965 | ) | | | (25,303 | ) | | | (2,814 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total deferred | | | 29,357 | | | | (196,782 | ) | | | (144,927 | ) | | | (19,211 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| Total income tax provision (benefit) | | $ | 127,112 | | | $ | 20,414 | | | $ | (262,083 | ) | | $ | 58,974 | |
| | | |
| | | |
| | | |
| | | |
| |
Tax benefits of $48.1 million in 2001, $226.1 million in 2000 and $83.0 million in 1999 related to employee stock options and stock purchase plans were credited to stockholders’ equity, and reduced the amount of taxes currently payable and deferred income taxes.
A reconciliation between our income tax provision and the U.S. statutory rate follows (in thousands):
| | | | | | | | | | | | | | | | | |
| | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| | | | | | | | |
| | | | | | New Basis | | Old Basis |
| | | | | |
| |
|
| Tax at U.S. statutory rate of 35% | | $ | 99,045 | | | $ | 1,391 | | | $ | (527,518 | ) | | $ | 51,313 | |
| Research credits | | | (24,114 | ) | | | (32,092 | ) | | | (5,803 | ) | | | (5,802 | ) |
| Prior years items | | | (14,000 | ) | | | — | | | | — | | | | — | |
| Tax benefit of certain realized gains on securities available-for-sale | | | (396 | ) | | | (6,604 | ) | | | (617 | ) | | | (2,388 | ) |
| Foreign losses realized | | | — | | | | — | | | | (1,363 | ) | | | (1,364 | ) |
| State taxes | | | 16,219 | | | | 959 | | | | (22,924 | ) | | | 5,371 | |
| Goodwill amortization | | | 53,649 | | | | 53,649 | | | | 26,825 | | | | — | |
| Legal settlements | | | — | | | | — | | | | — | | | | 12,250 | |
| IPR&D | | | — | | | | — | | | | 263,375 | | | | — | |
| Other | | | (3,291 | ) | | | 3,111 | | | | 5,942 | | | | (406 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| Income tax provision (benefit) | | $ | 127,112 | | | $ | 20,414 | | | $ | (262,083 | ) | | $ | 58,974 | |
| | | |
| | | |
| | | |
| | | |
| |
Prior years items relate principally to changes in estimate resulting from events in 2001 that provided greater certainty as to the expected outcome of prior matters.
C-51
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The components of deferred taxes consist of the following at December 31 (in thousands):
| | | | | | | | | | | |
| | 2001 | | 2000 |
| |
| |
|
| Deferred tax liabilities: | | | | | | | | |
| | Depreciation | | $ | (179,930 | ) | | $ | (130,892 | ) |
| | Unrealized gain on securities available-for-sale | | | (211,695 | ) | | | (229,294 | ) |
| | Adjustment to fair value of intangibles | | | (410,579 | ) | | | (476,313 | ) |
| | Other | | | (17,654 | ) | | | (18,999 | ) |
| | | |
| | | |
| |
| | | Total deferred tax liabilities | | | (819,858 | ) | | | (855,498 | ) |
| Deferred tax assets: | | | | | | | | |
| | Capitalized R&D costs | | | 66,527 | | | | 58,333 | |
| | Federal credit carryforwards | | | 101,052 | | | | 109,917 | |
| | Expenses not currently deductible | | | 268,222 | | | | 150,638 | |
| | State credit carryforwards | | | 74,149 | | | | 73,827 | |
| | Net operating losses | | | — | | | | 153,097 | |
| | Other | | | 1,666 | | | | 457 | |
| | | |
| | | |
| |
| | | Total deferred tax assets | | | 511,616 | | | | 546,269 | |
| | | |
| | | |
| |
| Total net deferred taxes | | $ | (308,242 | ) | | $ | (309,229 | ) |
| | | |
| | | |
| |
Total tax credit carryforwards of $175.2 million expire in the years 2006 through 2018, except for $103.2 million of California R&D credits and $44.9 million of alternative minimum tax credits which have no expiration date.
Net operating loss carryfowards of $459.5 million were fully utilized in 2001.
Earnings (Loss) per Share
The following is a reconciliation of the numerator and denominators of the basic and diluted earnings (loss) per share computations for the years ended December 31, 2001, 2000 and 1999 (in thousands):
| | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| | | | | | | | |
| | | | | | New Basis | | Old Basis |
| | | | | |
| |
|
Numerator: | | | | | | | | | | | | | | | | |
| | Net income (loss) — numerator for basic and diluted earnings (loss) per share | | $ | 150,236 | | | $ | (74,241 | ) | | $ | (1,245,112 | ) | | $ | 87,636 | |
Denominator: | | | | | | | | | | | | | | | | |
| | Denominator for basic earnings (loss) per share — weighted-average shares | | | 527,022 | | | | 522,179 | | | | 513,352 | | | | 512,368 | |
| | Effect of dilutive securities: | | | | | | | | | | | | | | | | |
| | | Stock options | | | 8,269 | | | | — | | | | — | | | | 19,500 | |
| | Denominator for diluted earnings (loss) per share — adjusted weighted-average shares and assumed conversions | | | 535,291 | | | | 522,179 | | | | 513,352 | | | | 531,868 | |
Options to purchase 9.7 million shares of our Common Stock with exercise prices ranging from $52.00 to $95.66 per share were outstanding during 2001, but were excluded from the computation of diluted earnings per share. The option exercise prices were greater than the average market price of the Common Stock and therefore, the effect would have been antidilutive. Options to purchase 40.9 million shares of our Common Stock during 2000 and 41.6 million shares of our Common Stock and Special Common Stock during 1999
C-52
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
were excluded from the computation of diluted loss per share as the effect would have been antidilutive. See “Capital Stock” note for information on option expiration dates.
Fair Values of Investment Securities and Financial Instruments
Securities classified as trading and available-for-sale at December 31, 2001 and 2000 are summarized below. Estimated fair value is based on quoted market prices for these or similar investments.
| | | | | | | | | | | | | | | | | | | |
| | | | Gross | | Gross | | Estimated |
| | Amortized | | Unrealized | | Unrealized | | Fair |
| December 31, 2001 | | Cost | | Gains | | Losses | | Value |
| |
| |
| |
| |
|
| | | | | | |
| | | | (In thousands) | | |
Total Trading Securities | | | | | | | | | | | | | | | | |
| | (carried at estimated fair value) | | $ | 365,618 | | | $ | 2,478 | | | $ | (4,557 | ) | | $ | 363,539 | |
| | | |
| | | |
| | | |
| | | |
| |
Securities Available-for-Sale | | | | | | | | | | | | | | | | |
| | (carried at estimated fair value): | | | | | | | | | | | | | | | | |
| Equity securities | | $ | 86,257 | | | $ | 498,200 | | | $ | (539 | ) | | $ | 583,918 | |
| Preferred stock | | | 148,107 | | | | 4,280 | | | | (989 | ) | | | 151,398 | |
| U.S. Treasury securities and obligations of other U.S. government agencies maturing: | | | | | | | | | | | | | | | | |
| | | between 1-5 years | | | 50,052 | | | | 1,007 | | | | (190 | ) | | | 50,869 | |
| | | between 5-10 years | | | 118,214 | | | | 5,573 | | | | — | | | | 123,787 | |
| Corporate debt securities maturing: | | | | | | | | | | | | | | | | |
| | | within 1 year | | | 432,688 | | | | 486 | | | | (144 | ) | | | 433,030 | |
| | | between 1-5 years | | | 405,505 | | | | 8,324 | | | | (492 | ) | | | 413,337 | |
| | | between 5-10 years | | | 203,592 | | | | 2,724 | | | | (1,712 | ) | | | 204,604 | |
| Other debt securities maturing: | | | | | | | | | | | | | | | | |
| | | within 1 year | | | 4,980 | | | | — | | | | (72 | ) | | | 4,908 | |
| | | between 1-5 years | | | 58,149 | | | | 326 | | | | (445 | ) | | | 58,030 | |
| | | between 5-10 years | | | 33,576 | | | | 530 | | | | (201 | ) | | | 33,905 | |
| | | |
| | | |
| | | |
| | | |
| |
Total Available-for-Sale | | $ | 1,541,120 | | | $ | 521,450 | | | $ | (4,784 | ) | | $ | 2,057,786 | |
| | | |
| | | |
| | | |
| | | |
| |
C-53
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
| | | | | | | | | | | | | | | | | | |
| | | | Gross | | Gross | | |
| | Amortized | | Unrealized | | Unrealized | | Estimated |
| December 31, 2000 | | Cost | | Gains | | Losses | | Fair Value |
| |
| |
| |
| |
|
| | |
| | (In thousands) |
Total Trading Securities
(carried at estimated fair value) | | $ | 273,348 | | | $ | 1,827 | | | $ | (4,152 | ) | | $ | 271,023 | |
| | | |
| | | |
| | | |
| | | |
| |
Securities Available-for-Sale
(carried at estimated fair value): | | | | | | | | | | | | | | | | |
| Equity securities | | $ | 120,416 | | | $ | 585,961 | | | $ | (21,546 | ) | | $ | 684,831 | |
| Preferred stock | | | 88,517 | | | | 4,335 | | | | (20 | ) | | | 92,832 | |
| U.S. Treasury securities and obligations of other U.S. government agencies maturing: | | | | | | | | | | | | | | | | |
| | between 5-10 years | | | 84,796 | | | | 2,497 | | | | (242 | ) | | | 87,051 | |
| Corporate debt securities maturing: | | | | | | | | | | | | | | | | |
| | within 1 year | | | 169,569 | | | | 2,079 | | | | (2,248 | ) | | | 169,400 | |
| | between 1-5 years | | | 217,838 | | | | 1,865 | | | | (1,463 | ) | | | 218,240 | |
| | between 5-10 years | | | 103,309 | | | | 935 | | | | (1,572 | ) | | | 102,672 | |
| Other debt securities maturing: | | | | | | | | | | | | | | | | |
| | within 1 year | | | 109,132 | | | | 211 | | | | (123 | ) | | | 109,220 | |
| | between 1-5 years | | | 138,854 | | | | 284 | | | | (1,541 | ) | | | 137,597 | |
| | between 5-10 years | | | 34,911 | | | | 492 | | | | (279 | ) | | | 35,124 | |
| | | |
| | | |
| | | |
| | | |
| |
Total Available-for-Sale | | $ | 1,067,342 | | | $ | 598,659 | | | $ | (29,034 | ) | | $ | 1,636,967 | |
| | | |
| | | |
| | | |
| | | |
| |
The carrying value of all investment securities held at December 31, 2001 and 2000 is summarized below (in thousands):
| | | | | | | | | | |
| Security | | 2001 | | 2000 |
| |
| |
|
| Trading securities | | $ | 363,539 | | | $ | 271,023 | |
| Securities available-for-sale maturing within one year | | | 437,938 | | | | 278,620 | |
| Preferred stock | | | 151,398 | | | | 92,832 | |
| | | |
| | | |
| |
| | Total short-term investments | | $ | 952,875 | | | $ | 642,475 | |
| | | |
| | | |
| |
| Securities available-for-sale maturing between 1-10 years, including equity securities | | $ | 1,468,450 | | | $ | 1,265,515 | |
| | | |
| | | |
| |
| | Total long-term marketable securities | | $ | 1,468,450 | | | $ | 1,265,515 | |
| | | |
| | | |
| |
In 2001, proceeds from the sales of available-for-sale securities totaled $1,084.5 million; gross realized gains totaled $30.0 million and gross realized losses totaled $2.0 million. In 2000, proceeds from the sales of available-for-sale securities totaled $574.1 million; gross realized gains totaled $132.3 million and gross realized losses totaled $4.0 million. We recorded charges of $27.5 million in 2001, $0.8 million in 2000 and $13.4 million in 1999, to write down certain available-for-sale biotechnology equity securities for which the decline in fair value below carrying value was other than temporary.
Net change in unrealized holding gains (losses) on trading securities included in net income (loss) totaled $0.2 million in 2001, $0.2 million in 2000 and ($6.1) million in 1999.
The marketable debt securities we hold are issued by a diversified selection of corporate and financial institutions with strong credit ratings. Our investment policy limits the amount of credit exposure with any one institution. Other than asset-backed and mortgage-backed securities, these debt securities are generally not collateralized. In 2001, we did not have charges for credit impairment on marketable debt securities. In 2000,
C-54
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
we recorded a charge of $4.0 million for credit impairment on marketable debt securities and in 1999, no material charges were recorded.
The fair value of the foreign exchange put options was based on the forward exchange rates as of December 31, 2001 and 2000. The fair value of the interest rate swaps was obtained from Bloomberg and represents the estimated amount that we would receive or pay to terminate the agreements. The fair value of the equity forwards and collars was determined based on the closing market prices of the underlying securities at year-end. The fair value of the long-term debt was estimated based on the quoted market price at year end. The table below summarizes the carrying value and fair value at December 31, 2001 and 2000, of our financial instruments (in thousands):
| | | | | | | | | | | | | | | | | | |
| | | | |
| | 2001 | | 2000 |
| |
| |
|
| | Carrying | | Fair | | Carrying | | Fair |
| Financial Instrument | | Value | | Value | | Value | | Value |
| |
| |
| |
| |
|
| Assets: | | | | | | | | | | | | | | | | |
| | Investment securities (including accrued interest, outstanding interest rate swaps and forward foreign exchange contracts) | | $ | 2,421,325 | | | $ | 2,421,325 | | | $ | 1,907,990 | | | $ | 1,907,990 | |
| | Convertible equity loans | | | 48,363 | | | | 48,363 | | | | 48,492 | | | | 48,492 | |
| | Purchased foreign exchange put options and forward contracts | | | 2,326 | | | | 2,326 | | | | 384 | | | | 3,342 | |
| | Equity forwards | | | — | | | | — | | | | 7,372 | | | | 7,372 | |
| | Outstanding interest rate swaps | | | 15,935 | | | | 15,935 | | | | 2,519 | | | | 8,228 | |
| Liabilities: | | | | | | | | | | | | | | | | |
| | Current portion of long-term debt | | | 149,692 | | | | 155,500 | | | | — | | | | — | |
| | Long-term debt | | | — | | | | — | | | | 149,692 | | | | 151,438 | |
| | Equity collars | | | 6,990 | | | | 6,990 | | | | 32,172 | | | | 41,569 | |
| | Equity forwards | | | 8,148 | | | | 8,148 | | | | — | | | | — | |
| | Forward foreign exchange contracts | | | — | | | | — | | | | 5,839 | | | | 6,139 | |
The financial instruments we hold are entered into with a diversified selection of institutions with strong credit ratings which minimizes the risk of loss due to nonpayment from the counterparty. Credit exposure is limited to the unrealized gains on our contracts. We have not experienced any material losses due to credit impairment of our foreign currency or equity financial instruments.
Derivative Financial Instruments
| |
| Foreign Currency Instruments |
To protect against currency exchange risks on forecasted foreign currency cash flows from royalties to be received from licensees’ foreign product sales over the next one to three years and expenses related to our foreign facility and our collaboration development expenses denominated in foreign currencies, we have instituted a foreign currency cash flow hedging program. We hedge portions of our forecasted foreign currency revenues with option contracts and we hedge our foreign currency expenses from our foreign facility with forward contracts. When the dollar strengthens significantly against the foreign currencies, the decline in value of future foreign currency revenues or expenses is offset by gains or losses, respectively, in the value of the option or forward contracts designated as hedges. Conversely, when the dollar weakens, the increase in the value of future foreign currency expenses is offset by gains in the value of the forward contracts. In accordance with FAS 133, hedges related to anticipated transactions are designated and documented at the hedge’s inception as cash flow hedges and evaluated for hedge effectiveness at least quarterly.
C-55
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
During the year ended December 31, 2001, the ineffective portion of our foreign currency hedging instruments was not material. Gains and losses related to option and forward contracts that hedge future cash flows are recorded against the hedged revenues or expenses in the statement of operations.
We enter into interest-rate swap agreements to limit our exposure to fluctuations in U.S. interest rates. Our material interest bearing assets, or interest-bearing portfolio, consisted of cash, cash equivalents, restricted cash, short-term investments, convertible preferred stock investments, convertible loans and long-term investments as of December 31, 2001 and 2000. Our interest-rate swap agreements effectively convert a portion of our short-term investments in our interest-bearing portfolio to a fixed-rate basis over the next two years, thus reducing the impact of interest rate changes on future interest income. Our interest rate swap agreements are designated as cash flow hedges and the future interest receipts on approximately $200.0 million of our interest-bearing portfolio was designated as the hedged transaction at December 31, 2001. No ineffectiveness was required to be recognized in earnings related to our swap agreements during 2001.
Our marketable equity securities portfolio consists primarily of investments in biotechnology companies whose risk of market fluctuations is greater than the stock market in general. To manage a portion of this risk, we enter into derivative instruments such as zero-cost collar instruments and equity forward contracts to hedge equity securities against changes in market value. During 2001, we entered into zero-cost collars that expire in 2005 through 2007 and will require settlement in equity securities. A zero-cost collar is a purchased put option and a written call option on a specific equity security such that the cost of the purchased put and the proceeds of the written call offset each other; therefore, there is no initial cost or cash outflow for these instruments. At December 31, 2001, our zero-cost collars were designated and qualify as cash flow hedges.
As part of our fair value hedging strategy, we have also entered into equity forwards that mature in 2002 through 2004. An equity forward is a derivative instrument where we pay the counterparty the total return of the security above the current spot price and receive interest income on the notional amount for the term of the equity forward. A forward contract is a derivative instrument where we lock-in the termination price we receive from the sale of stock based on a pre-determined spot price. The forward contract protects us from a decline in the market value of the security below the spot price and limits our potential benefit from an increase in the market value of the security above the spot price. Throughout the life of the contract, we receive interest income based on the notional amount and a floating-rate index.
In the year ended December 31, 2001, we recognized a net gain of $10.0 million related to certain derivative instruments as a result of FAS 133. We record gains in contract and other revenues, and losses in marketing, general and administrative expenses in the statement of operations.
At December 31, 2001, net gains on derivative instruments expected to be reclassified from accumulated other comprehensive income to earnings during the next twelve months due to the receipt of the related net revenues denominated in foreign currencies are not material.
C-56
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Other Accrued Liabilities
Other accrued liabilities at December 31 are as follows (in thousands):
| | | | | | | | | | |
| | 2001 | | 2000 |
| |
| |
|
| Accrued compensation | | $ | 63,103 | | | $ | 56,028 | |
| Accrued royalties | | | 69,660 | | | | 34,811 | |
| Hedge payable | | | — | | | | 32,172 | |
| Accrued clinical and other studies | | | 42,434 | | | | 35,626 | |
| Accrued marketing and promotion costs | | | 28,395 | | | | 21,229 | |
| Taxes payable | | | 52,185 | | | | 29,022 | |
| Accrued collaborations | | | 71,046 | | | | 111,254 | |
| Other | | | 77,090 | | | | 66,338 | |
| | | |
| | | |
| |
| | Total other accrued liabilities | | $ | 403,913 | | | $ | 386,480 | |
| | | |
| | | |
| |
Debt Obligations
Our short-term debt consists of $149.7 million of convertible subordinated debentures, with interest payable at 5%, due in March 2002. As a result of the redemption of our Special Common Stock in 1999, upon conversion, the holder will receive, for each $74 in principal amount of debenture converted, $59.25 in cash, of which $18 will be reimbursed to us by Roche. Generally, we may redeem the debentures until maturity.
Leases, Commitments and Contingencies
We lease various real property under operating leases that generally require us to pay taxes, insurance and maintenance. Rent expense was approximately $14.4 million in 2001, $17.5 million in 2000 and $13.9 million in 1999. Sublease income was not material in any of the three years presented.
Five of our operating leases are commonly referred to as synthetic leases. A synthetic lease represents a form of off-balance sheet financing under which an unrelated third party funds 100% of the costs of the acquisition and/or construction of the property and leases the asset to a lessee, and at least 3% of the third party funds represent at risk equity. Our synthetic leases are treated as operating leases for accounting purposes and as financing leases for tax purposes. Under our synthetic lease structures, upon termination or expiration, at our option, we must either purchase the property from the lessor at a predetermined amount that does not constitute a bargain purchase, sell the real property to a third party, or renew the lease arrangement. If the property is sold to a third party at an amount less than the amount financed by the lessor, we have agreed under residual value guarantees to pay the lessor up to an agreed upon percentage of the amount financed by the lessor.
Four of our synthetic leases were entered into with BNP Paribas Leasing Corporation, who leases directly to us various buildings that we occupy in South San Francisco, California. Under certain of these leases, we are required to maintain cash collateral of $56.6 million, which we have included in other long-term assets on our balance sheet as restricted cash.
The most significant of our synthetic leases relates to our manufacturing facility located in Vacaville, California. In November 2001, we completed a synthetic lease transaction for this facility, which had previously been leased by us under a predecessor synthetic lease. This new synthetic lease is structured differently from our other synthetic leases. We are leasing the property from an unrelated special purpose trust (owner/lessor) under an operating lease agreement for five years ending November 2006. Third party financing is provided in the form of a 3% at risk equity participation from investors and 97% debt commitment. Investors’ equity contributions were equal to or greater than 3% of the fair value of the property at the lease’s
C-57
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
inception and are required to remain so for the term of the lease. A bankruptcy remote, special purpose corporation (the SPC) was formed to fund the debt portion through the issuance of commercial paper notes. The SPC lends the proceeds from the commercial paper to the owner/lessor, who issues promissory notes to the SPC. The SPC Loans mature in 5 years (November 2006). The SPC promissory notes are supported by a credit facility provided by financing institutions and draws are generally available under that credit facility to repay the SPC’s commercial paper. The collateral for the SPC Loans includes the leased property, and an interest in the residual value guarantee provided by us. At any time during the lease term, we have the option to purchase the property at an amount that does not constitute a bargain purchase. Our off-balance sheet contingent liability under the residual value guarantees is summarized in the table below.
Under all of our synthetic leases, we are also required to maintain certain pre-defined financial ratios and are limited to the amount of additional debt we can assume. In addition, no Genentech officers or employees have any financial interest with regards to these synthetic lease arrangements or with any of the special purpose entities used in these arrangements. In the event of a default, the maximum amount payable under the residual value guarantee would equal 100% of the amount financed by the lessor, and our obligation to purchase the leased properties or pay the related residual value guarantees could be accelerated. We believe at the lease’s inception and continue to believe that the occurrence of any event of default that could trigger our purchase obligation is remote.
Future minimum lease payments under operating leases, exclusive of the residual value guarantees, executory costs and sublease income, at December 31, 2001, are as follows (in millions). These minimum lease payments were computed based on current interest rates, which are subject to fluctuations in certain market-based interest rates:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | | Thereafter | | Total |
| |
| |
| |
| |
| |
| |
| |
|
| Synthetic leases | | $ | 12.9 | | | $ | 13.8 | | | $ | 12.8 | | | $ | 12.0 | | | $ | 11.3 | | | $ | 1.6 | | | $ | 64.4 | |
| Other operating leases | | | 4.8 | | | | 3.0 | | | | 1.7 | | | | 1.6 | | | | 1.6 | | | | 4.3 | | | | 17.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Total | | $ | 17.7 | | | $ | 16.8 | | | $ | 14.5 | | | $ | 13.6 | | | $ | 12.9 | | | $ | 5.9 | | | $ | 81.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The following summarizes the residual value guarantee amounts for our synthetic leases (in millions):
| | | | | | | | | | | | | | |
| | Approximate | | | | Residual |
| | Fair Value of | | Lease | | Value |
| | Leased Property | | Expiration | | Guarantee |
| |
| |
| |
|
| South San Francisco Lease 1 | | $ | 56.6 | | | | 07/2004 | | | $ | 48.1 | |
| South San Francisco Lease 2 | | | 133.2 | | | | 06/2007 | | | | 113.2 | |
| South San Francisco Lease 3 | | | 25.0 | | | | 01/2004 | | | | 21.3 | |
| South San Francisco Lease 4 | | | 22.5 | | | | 01/2004 | | | | 19.1 | |
| Vacaville Lease | | | 425.0 | | | | 11/2006 | | | | 371.5 | |
| | | |
| | | | | | | |
| |
| | Total | | $ | 662.3 | | | | | | | $ | 573.2 | |
| | | |
| | | | | | | |
| |
There are no impairments in the fair value or use of the properties that we lease under synthetic leases wherein we believe that we would be required to pay amounts under any of the residual value guarantees. We will continue to assess the fair values of the underlying properties and the use of the properties for impairment on an annual basis.
We entered into a research collaboration agreement with CuraGen Corporation in November 1997, as amended and restated in March 2000, and agreed to provide a convertible equity loan to CuraGen of up to $21.0 million. In October 1999, CuraGen exercised its right to borrow $16.0 million. Simultaneously, with this draw down, CuraGen repaid the loan by issuing common shares of CuraGen stock valued at $16.0 million.
C-58
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Our remaining commitment to CuraGen on the convertible equity loan is $5.0 million. At December 31, 2001, there were no outstanding loans to CuraGen.
In December 1997, we entered into a research collaboration agreement with Millennium Pharmaceuticals, Inc. (or Millennium) to develop and commercialize Millennium’s LDP-02. Under the terms of the agreement, we have agreed to provide a convertible equity loan for approximately $15.0 million to fund Phase II development costs. Upon successful completion of Phase II, if Millennium agrees to fund 25% of Phase III development costs, we have agreed to provide a second loan to Millennium for such funding. As of December 31, 2001, there were no outstanding loans to Millennium.
In April 1996, we entered into a research collaboration agreement with XOMA Ltd. to develop and commercialize Xanelim. Under the terms of the agreement, we have agreed to provide a convertible equity loan to XOMA of up to $60.0 million to fund XOMA’s share of development costs for Xanelim until the completion of Phase III clinical trials. There is no revenue impact on our statements of operations as it relates to this loan. As of December 31, 2001, we had an outstanding loan of approximately $51.0 million to XOMA.
In addition, we entered into research collaborations with companies whereby potential future payments may be due to selective collaboration partners achieving certain benchmarks as defined in the collaboration agreements. We may also, from time to time, lend additional funds to these companies, subject to our approval.
We are a limited partner in the Vector Later-Stage Equity Fund II, L.P., which is referred to as the Vector Fund. The General Partner is Vector Fund Management II, L.L.C., a Delaware limited liability company. The purpose of the Vector Fund is to invest in biotech equity and equity-related securities. Under the terms of the Vector Fund agreement, we contribute to the capital of the Vector Fund through installments in cash as called by the General Partner. Our total commitment to the Vector Fund through September 2003 is $25.0 million, of which $18.1 million was contributed through December 31, 2001. The Vector Fund will terminate and be dissolved in September 2007.
We are a party to various legal proceedings, including patent infringement litigation relating to our antibody products, and one of our thrombolytic products, securities litigation, and licensing and contract disputes, and other matters.
On May 28, 1999, GlaxoSmithKline plc (or Glaxo) filed a patent infringement lawsuit against us in the U.S. District Court in Delaware. The suit asserted that we infringe four U.S. patents owned by Glaxo. Two of the patents relate to the use of specific kinds of antibodies for the treatment of human disease, including cancer. The other two patents asserted against us relate to preparations of specific kinds of antibodies which are made more stable and the methods by which such preparations are made. After a trial, the jury hearing the lawsuit unanimously found that our Herceptin and Rituxan antibody products do not infringe the patents and therefore that Genentech is not required to pay royalties to Glaxo. The jury also unanimously found that all of the patent claims that Glaxo asserted against Genentech were invalid. Glaxo filed a notice of appeal of the jury’s verdict with the U.S. Court of Appeals for the Federal Circuit. The oral argument of the appeal took place on February 6, 2002. Proceedings in connection with Genentech’s claim against Glaxo for inequitable conduct and other related issues are still pending before the district court.
On September 14, 2000, Glaxo filed another patent infringement lawsuit against us in the U.S. District Court in Delaware, alleging that we are infringing U.S. Patent No. 5,633,162 owned by Glaxo. The patent relates to specific methods for culturing Chinese Hamster Ovary cells. The complaint fails to specify which of our products or methods of manufacture are allegedly infringing that patent. However, the complaint makes a general reference to Genentech’s making, using, and selling “monoclonal antibodies,” and so we believe that the suit relates to our Herceptin and Rituxan antibody products. We have filed our answer to Glaxo’s complaint, and in our answer we also stated counterclaims against Glaxo. The trial of this suit has been
C-59
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
rescheduled to begin on April 14, 2003. This lawsuit is separate from and in addition to the Glaxo suit mentioned above.
We and the City of Hope Medical Center are parties to a 1976 agreement relating to work conducted by two City of Hope employees, Arthur Riggs and Keiichi Itakura, and patents that resulted from that work, which are referred to as the “Riggs/ Itakura Patents.” Since that time, Genentech has entered into license agreements with various companies to make, use and sell the products covered by the Riggs/ Itakura Patents. On August 13, 1999 the City of Hope filed a complaint against us in the Superior Court in Los Angeles County, California, alleging that we owe royalties to the City of Hope in connection with these license agreements, as well as product license agreements that involve the grant of licenses under the Riggs/ Itakura Patents. The complaint states claims for declaratory relief, breach of contract, breach of implied covenant of good faith and fair dealing, and breach of fiduciary duty. On December 15, 1999, we filed our answer to the City of Hope’s complaint. The trial of this suit began on August 28, 2001, in which City of Hope was seeking compensatory damages in the amount of approximately $445.0 million (including interest) and special damages. On October 24, 2001, the jury hearing the lawsuit announced that it was unable to reach a verdict and on that basis the Court declared a mistrial. City of Hope requested a retrial, and the retrial is scheduled to begin on March 4, 2002.
On June 7, 2000, Chiron Corporation filed a patent infringement suit against us in the U.S. District Court in the Eastern District of California (Sacramento), alleging that the manufacture, use, sale and offer for sale of our Herceptin antibody product infringes Chiron’s U.S. Patent No. 6,054,561. This patent relates to certain antibodies that bind to breast cancer cells and/or other cells. Chiron is seeking compensatory damages for the alleged infringement, special damages, and attorneys fees and costs. We have filed our answer to Chiron’s complaint, and in our answer we also stated counterclaims against Chiron. The trial of this suit has been rescheduled to begin on August 6, 2002.
On March 13, 2001, Chiron filed another patent infringement lawsuit against us in the U.S. District Court in the Eastern District of California, alleging that the manufacture, use, sale, and/or offer for sale of our Herceptin antibody product infringes Chiron’s U.S. Patent No. 4,753,894. Chiron is seeking compensatory damages for the alleged infringement, special damages, and attorneys fees and costs. Genentech filed a motion to dismiss this second lawsuit, which was denied. The judge has scheduled the trial of this suit to begin on March 24, 2003. This lawsuit is separate from and in addition to the Chiron suit mentioned above.
We and Pharmacia AB are parties to a 1978 agreement relating to Genentech’s development of recombinant human growth hormone products, under which Pharmacia is obligated to pay Genentech royalties on sales of Pharmacia’s growth hormone products throughout the world. Pharmacia filed a Request for Arbitration with the International Chamber of Commerce (or ICC) to resolve several disputed issues between Genentech and Pharmacia under the 1978 agreement. One of the claims made by Pharmacia is for a refund of some of the royalties previously paid to Genentech for sales of Pharmacia’s growth hormone products in certain countries. On February 14, 2002, the ICC issued a decision in Genentech’s favor on that claim, ruling that no refund of royalties is due to Pharmacia.
On March 13, 2001, Genentech filed a complaint in the United States District Court in Delaware against Genzyme Corporation seeking a declaratory judgment that Genentech does not infringe Genzyme’s U.S. Patent No. 5,344,773 and that Genentech has not breached a 1992 Patent License and Interference Settlement Agreement between Genentech and Genzyme relating to that patent. Genentech is seeking a declaration that Genzyme’s patent is not infringed by any Genentech product, that the patent is invalid, that Genzyme be enjoined from further legal action against Genentech regarding the patent, and that Genentech has not breached the 1992 Agreement. Genzyme has filed its answer to our complaint.
On or about April 6, 2001, Genzyme filed a complaint in the same court against Genentech alleging that our TNKase product infringes the Genzyme patent and that Genentech is in breach of the 1992 Agreement referred to above. Genzyme’s complaint also alleges willful infringement and reckless breach of contract by Genentech. Genzyme is seeking to enjoin Genentech from infringing the patent, and is also seeking
C-60
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
compensatory damages for the alleged infringement and breach of contract, special damages, and attorneys fees and costs. We have filed our answer to Genzyme’s complaint. The court has consolidated this lawsuit and the declaratory judgement lawsuit suit referred to above for further proceedings. The trial of this consolidated lawsuit is scheduled to begin on January 21, 2003.
On November 15, 2001, a shareholder of XOMA Ltd. filed a class action lawsuit against XOMA, Genentech, and certain officers of each of the two companies in the United States District Court for the Northern District of California. The complaint was filed on behalf of all persons who purchased XOMA common stock during the period May 24, 2001 through October 4, 2001. The complaint alleges that XOMA and Genentech made misleading statements and failed to disclose material facts about the timing of the filing of a U.S. Food and Drug Administration application for Xanelim, the potential psoriasis drug that XOMA is co-developing with Genentech. The plaintiff(s) seek to recover as damages the losses suffered by the plaintiff(s) as a result of the alleged federal securities law violations. Based on a stipulation filed with the court, the defendants have no obligation to respond to the complaint until the court appoints a lead plaintiff, which has not yet occurred.
Based upon the nature of the claims made and the information available to date to us and our counsel through investigations and otherwise, we believe the outcome of these actions is not likely to have a material adverse effect on our financial position, result of operations or cash flows. However, were an unfavorable ruling to occur in any quarterly period, there exists the possibility of a material impact on the operating results of that period.
In addition to the above, in 1990 and 1997, the Regents of the University of California, or UC, filed patent infringement lawsuits against Genentech, alleging that the manufacture, use and sale of our Protropin and Nutropin human growth hormone products infringe a patent known as the “Goodman patent” that is owned by UC. On November 19, 1999, we and UC announced a proposed settlement of those lawsuits, and on or about December 17, 1999, the parties entered into a definitive written agreement on the terms of the settlement. Under the terms of the settlement, Genentech agreed to pay UC $150.0 million and agreed to make a contribution in the amount of $50.0 million toward construction of the first biological sciences research building at the University of California, San Francisco Mission Bay campus, and Genentech and UC granted certain releases to one another and dismissed with prejudice the 1990 and 1997 patent infringement lawsuits and related appeals. Such amounts were included in other accrued liabilities at December 31, 1999 and paid in January 2000. The settlement resolves all outstanding litigation between Genentech and UC relating to our growth hormone products.
In April 1999, we paid $50.0 million to settle a federal investigation relating to our past clinical, sales and marketing activities associated with human growth hormone.
Relationship with Roche
On June 30, 1999, Roche exercised its option to cause us to redeem all of our Special Common Stock held by stockholders other than Roche, at a price of $20.63 per share in cash with funds deposited by Roche for such purpose and we retired all of the shares of Special Common Stock including those held by Roche. As a result of the Redemption, on that date, Roche owned 100% of our outstanding Common Stock. On July 23, 1999, Roche completed a public offering of 88.0 million shares of our Common Stock. On October 26, 1999, Roche completed a public offering of 80.0 million shares of our Common Stock. On January 19, 2000, Roche completed an offering of zero-coupon notes that are exchangeable for an aggregate of approximately 13.0 million shares of our Common Stock held by Roche. On March 29, 2000, Roche completed a public offering of 34.6 million shares of our Common Stock. Roche’s percentage ownership of our Common Stock was 58.0% at December 31, 2001.
In July 1999, we entered into certain affiliation arrangements with Roche, amended our licensing and marketing agreement with F. Hoffmann-La Roche Ltd, an affiliate of Roche commonly known as Hoffmann-La Roche, and entered into a tax sharing agreement with Roche.
C-61
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In July 1999, we amended our certificate of incorporation and bylaws and entered into an affiliation agreement with Roche. As a result, our board changed to consist of two Roche directors, three independent directors nominated by a nominating committee currently controlled by Roche, and one Genentech employee. However, under the affiliation agreement, Roche has the right to obtain proportional representation on our board at any time. Roche intends to continue to allow our current management to conduct our business and operations as we have done in the past. However, we cannot ensure that Roche will not implement a new business plan in the future.
We have a licensing and marketing agreement with Hoffmann-La Roche and its affiliates granting it a ten-year option to license to use and sell our products in non-U.S. markets. The major provisions of that agreement include:
| | |
| | • | Hoffmann-La Roche’s option expires in 2015; |
| |
| | • | Hoffmann-La Roche may exercise its option to license our products upon the occurrence of any of the following: (1) our decision to file an Investigational New Drug application (or IND) for a product, (2) completion of a Phase II trial for a product or (3) if Hoffmann-La Roche previously paid us a fee of $10.0 million to extend its option on a product, completion of a Phase III trial for that product; |
| |
| | • | if Hoffmann-La Roche exercises its option to license a product, it has agreed to reimburse Genentech for development costs as follows: (1) if exercise occurs at the time an IND is filed, Hoffmann-La Roche will pay 50% of development costs incurred prior to the filing and 50% of development costs subsequently incurred, (2) if exercise occurs at the completion of a Phase II trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, (3) if the exercise occurs at the completion of a Phase III trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, and $5.0 million of the option extension fee paid by Hoffmann-La Roche to preserve its right to exercise its option at the completion of a Phase III trial will be credited against the total development costs payable to Genentech upon the exercise of the option; |
| |
| | • | we agreed, in general, to manufacture for and supply to Hoffmann-La Roche its clinical requirements of our products at cost, and its commercial requirements at cost plus a margin of 20%; however, Hoffmann-La Roche will have the right to manufacture our products under certain circumstances; |
| |
| | • | Hoffmann-La Roche has agreed to pay, for each product for which Hoffmann-La Roche exercises its option upon either a decision to file an IND with the FDA or completion of the Phase II trials, a royalty of 12.5% on the first $100.0 million on its aggregate sales of that product and thereafter a royalty of 15% on its aggregate sales of that product in excess of $100.0 million until the later in each country of the expiration of our last relevant patent or 25 years from the first commercial introduction of that product; and |
| |
| | • | Hoffmann-La Roche will pay, for each product for which Hoffmann-La Roche exercises its option after completion of the Phase III trials, a royalty of 15% on its sales of that product until the later in each country of the expiration of our relevant patent or 25 years from the first commercial introduction of that product; however, $5.0 million of any option extension fee paid by Hoffmann-La Roche will be credited against royalties payable to us in the first calendar year of sales by Hoffmann-La Roche in which aggregate sales of that product exceed $100.0 million. |
C-62
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Since the redemption of our Special Common Stock, and until Roche completed its second public offering of our Common Stock in October 1999, we were included in Roche’s U.S. federal consolidated income tax group. Accordingly, we entered into a tax sharing agreement with Roche. Pursuant to the tax sharing agreement, we and Roche were to make payments such that the net amount paid by us on account of consolidated or combined income taxes is determined as if we had filed separate, stand-alone federal, state and local income tax returns as the common parent of an affiliated group of corporations filing consolidated or combined federal, state and local returns.
Effective with the consummation of the second public offering on October 26, 1999, Genentech ceased to be a member of the consolidated federal income tax group (and certain consolidated or combined state and local income tax groups) of which Roche is the common parent. Accordingly, our tax sharing agreement with Roche now pertains only to the state and local tax returns in which we are consolidated or combined with Roche. We will continue to calculate our tax liability or refund with Roche for these state and local jurisdictions as if we were a stand-alone entity.
| |
| Roche’s Ability to Maintain Its Percentage Ownership Interest in Our Stock |
We expect from time to time to issue additional shares of common stock in connection with our stock option and stock purchase plans, and we may issue additional shares for other purposes. Our affiliation agreement with Roche provides, among other things, that we will establish a stock repurchase program designed to maintain Roche’s percentage ownership interest in our common stock. The affiliation agreement provides that we will repurchase a sufficient number of shares pursuant to this program such that, with respect to any issuance of common stock by Genentech in the future, the percentage of Genentech common stock owned by Roche immediately after such issuance will be no lower than Roche’s lowest percentage ownership of Genentech common stock at any time after the offering of common stock occurring in July 1999 and prior to the time of such issuance, except that Genentech may issue shares up to an amount that would cause Roche’s lowest percentage ownership to be no more than 2% below the “Minimum Percentage.” The Minimum Percentage equals the lowest number of shares of Genentech common stock owned by Roche since the July 1999 offering (to be adjusted in the future for dispositions of shares of Genentech common stock by Roche as well as for stock splits or stock combinations) divided by 509,194,352 (to be adjusted in the future for stock splits or stock combinations), which is the number of shares of Genentech common stock outstanding at the time of the July 1999 offering, as adjusted for the two-for-one splits of Genentech common stock in November 1999 and October 2000. As long as Roche’s percentage ownership is greater than 50%, prior to issuing any shares, the affiliation agreement provides that we will repurchase a sufficient number of shares of our common stock such that, immediately after our issuance of shares, Roche’s percentage ownership will be greater than 50%. The affiliation agreement also provides that, upon Roche’s request, we will repurchase shares of our common stock to increase Roche’s ownership to the Minimum Percentage. In addition, Roche will have a continuing option to buy stock from us at prevailing market prices to maintain its percentage ownership interest. On December 31, 2001, Roche’s percentage ownership of our common stock was 58.0%, which was 2.2% below the Minimum Percentage.
Related Party Transactions
We enter into transactions with Roche, Hoffmann-La Roche and its affiliates in the ordinary course of business. We recorded contract revenues from Hoffmann-La Roche of $40.0 million for Herceptin, marketing rights outside of the U.S. in 1998 (see below). Contract revenue from Hoffmann-La Roche, including reimbursement for ongoing development expenses after the option exercise date, totaled $5.8 million in 2001, $3.5 million in 2000, and $17.2 million in 1999. All other revenue from Roche, Hoffmann-La Roche and their affiliates, principally royalties and product sales, totaled $164.1 million in 2001, $114.2 million in 2000, and $83.9 million in 1999.
C-63
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In the second quarter of 1999, we entered into a license agreement with Immunex Corporation that grants rights under our immunoadhesin patent portfolio to Immunex for its product Enbrel® (etanercept) biologic response modifier. In exchange for a worldwide, co-exclusive license covering fusion proteins such as Enbrel, Immunex paid us an initial non-refundable license fee which was recorded in contract revenues net of a portion paid to Hoffmann-La Roche pursuant to an agreement between Hoffmann-La Roche and us.
In July 1998, we entered into an agreement with Hoffmann-La Roche to provide them with exclusive marketing rights outside of the U.S. for Herceptin. Under the agreement, Hoffmann-La Roche paid us $40.0 million and has agreed to pay us cash milestones tied to future product development activities, to share equally global development costs up to a maximum of $40.0 million and to make royalty payments on product sales. In 1999, Hoffmann-La Roche paid an additional $10.0 million toward global development costs.
During 2001, Novartis AG (Novartis) acquired 20% of the outstanding voting stock of Roche Holding, Ltd. As a result of this investment, Novartis is deemed to have an indirect beneficial ownership interest under FAS 57 “Related Party Disclosures” of more than 10% of Genentech’s voting stock. During 2000, we entered into an arrangement with our collaboration partner, Novartis, whereby Novartis is required to fund a portion of the cost of our Xolair inventory until the product is approved for marketing by the FDA. Through December 31, 2001, Novartis has paid $38.4 million of our Xolair inventory costs (no amounts were funded through December 31, 2000). This amount is required to be returned to Novartis upon FDA approval of Xolair, and has been recorded in accrued liabilities in our financial statements.
Capital Stock
| |
| Common Stock and Special Common Stock |
On June 30, 1999, we redeemed all of our outstanding Special Common Stock held by stockholders other than Roche. Subsequently, in July and October 1999, and March 2000, Roche consummated public offerings of our Common Stock. On January 19, 2000, Roche completed an offering of zero-coupon notes that are exchangeable for an aggregate of approximately 13.0 million shares of our Common Stock held by Roche. See “Redemption of Our Special Common Stock” and “Relationship With Roche” notes above for a discussion of these transactions.
On October 24, 2000, we effected a two-for-one stock split of our Common Stock in the form of a dividend of one share of Genentech Common Stock of each share held at the close of business on October 17, 2000. Our stock began trading on a split-adjusted basis on October 25, 2000. On November 2, 1999, we effected a two-for-one stock split of our Common Stock in the form of a dividend of one share of Genentech Common Stock for each share held at the close of business on October 29, 1999. Our stock began trading on a split-adjusted basis on November 3, 1999.
We repurchased a total of 800,000 shares of our common stock through October 30, 2001 at a cost of $34.0 million. On October 31, 2001, our Board of Directors authorized a stock repurchase program to repurchase up to $625.0 million of our common stock over the next 12 months. Purchases may be made in the open market or in privately negotiated transactions from time to time at management’s discretion. We may also engage in transactions in other Genentech securities in conjunction with the repurchase program, including derivative securities. Under the program approved by our Board of Directors on October 31, 2001, we repurchased 100,000 shares of our common stock at a cost of $5.7 million.
The par value method of accounting is used for common stock repurchases. The excess of the cost of shares acquired over their par value is allocated to additional paid-in capital with the excess charged to retained earnings.
C-64
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In connection with the redemption of our Special Common Stock, the following changes occurred with respect to our stock options that were outstanding as of June 30, 1999:
| | |
| | • | Options for the purchase of approximately 27.2 million shares of Special Common Stock were canceled in accordance with the terms of the applicable stock option plans, and the holders received cash payments in the amount of $20.63 per share, less the exercise price; |
| |
| | • | Options for the purchase of approximately 16.0 million shares of Special Common Stock were converted into options to purchase a like number of shares of Common Stock at the same exercise price; and |
| |
| | • | Options for the purchase of approximately 19.6 million shares of Special Common Stock were canceled, in accordance with the terms of our 1996 Stock Option/ Stock Incentive Plan, or the 1996 Plan. With certain exceptions, we granted new options for the purchase of 1.333 times the number of shares under the previous options with an exercise price of $24.25 per share, which was the public offering price of the Common Stock. The number of shares that were the subject of these new options, which were issued under our 1999 Stock Plan, or the 1999 Plan, was approximately 20.0 million. Certain key employees who held unvested options under the 1996 Plan were provided the opportunity to participate in a cash basis long-term incentive plan in lieu of their options. |
Of the approximately 16.0 million shares of converted options, options with respect to approximately 3.3 million shares were outstanding at December 31, 2001, all of which are currently exercisable except for options with respect to approximately 93,373 shares. These outstanding options are held by 1,202 employees; no non-employee directors hold these options.
Our board of directors and Roche, then our sole stockholder, approved the 1999 Plan on July 16, 1999. Under the 1999 Plan, we granted new options to purchase approximately 26.0 million shares (including the 20.0 million shares referred to above) of Common Stock to approximately 2,400 employees at an exercise price of $24.25 per share. The grant date of such options was July 16, 1999. Of the options to purchase these 26.0 million shares, options to purchase approximately 17.2 million shares were outstanding at December 31, 2001, of which options to purchase approximately 12.5 million shares are currently exercisable.
In connection with these stock option transactions, we recorded:
| | |
| | • | (1) cash compensation expense of approximately $284.5 million associated with the cash-out of such stock options and (2) non-cash compensation expense of approximately $160.1 million associated with the remeasurement, for accounting purposes, of the converted options, which non-cash amount represents the difference between each applicable option exercise price and the redemption price of the Special Common Stock; and |
| |
| | • | Over a two-year period beginning July 1, 1999, an aggregate of approximately $27.4 million of deferred cash compensation available to be earned by a limited number of employees who elected the alternative arrangements described above. As of December 31, 2001, 2000 and 1999, $4.2 million, $11.1 million and $7.3 million, respectively, of compensation expense has been recorded related to these alternative arrangements. |
We have a stock option plan adopted in 1999, and amended in 2000, which variously allows for the granting of non-qualified stock options, stock awards and stock appreciation rights to employees, directors and consultants of Genentech. Incentive stock options may only be granted to employees under this plan. Generally, non-qualified options have a maximum term of 10 years. Incentive options have a maximum term of 10 years. In general, options vest in increments over four years from the date of grant, although we may grant options with different vesting terms from time to time. No stock appreciation rights have been granted to date.
C-65
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
We adopted the 1991 Employee Stock Plan, or the 1991 Plan, on December 4, 1990, and amended it during 1993, 1995, 1997 and 1999. The 1991 Plan allows eligible employees to purchase Common Stock at 85% of the lower of the fair market value of the Common Stock on the grant date or the fair market value on the first business day of each calendar quarter. Purchases are limited to 15% of each employee’s eligible compensation. All full-time employees of Genentech are eligible to participate in the 1991 Plan. Of the 21.2 million shares of Common Stock reserved for issuance under the 1991 Plan, 18.3 million shares have been issued as of December 31, 2001. During 2001, 4,382 of the eligible employees participated in the 1991 Plan.
We have elected to continue to follow Accounting Principles Board (or APB 25) to account for employee stock options because the alternative fair value method of accounting prescribed by FAS 123, “Accounting for Stock-Based Compensation,” requires the use of option valuation models that were not developed for use in valuing employee stock options. Under APB 25, “Accounting for Stock Issued to Employees,” no compensation expense is recognized because the exercise price of our employee stock options equals the market price of the underlying stock on the date of grant.
The information regarding net income (loss) and earnings (loss) per share with FAS 123 has been determined as if we had accounted for our employee stock options and employee stock plan under the fair value method prescribed by FAS 123 and the earnings (loss) per share method under FAS 128. The resulting effect on net income (loss) and earnings (loss) per share with FAS 123 disclosed is not likely to be representative of the effects on net income (loss) and earnings (loss) per share with FAS 123 in future years, due to subsequent years including additional grants and years of vesting. The fair value of options was estimated at the date of grant using a Black-Scholes option valuation model with the following weighted-average assumptions for 2001, 2000 and 1999, respectively: risk-free interest rates of 3.9%, 5.3% and 5.8%; dividend yields of 0%; volatility factors of the expected market price of our Common Stock of 63.0%, 75.0% and 45.0%, and a weighted-average expected life of the option of five years.
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected stock price volatility. Because our employee stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion the existing models do not necessarily provide a reliable single measure of the fair value of its employee stock options.
For purposes of disclosures with FAS 123, the estimated fair value of options is amortized to expense over the options’ vesting period. Information with FAS 123 for the periods presented (in thousands, except per share amounts):
| | | | | | | | | | | | | | | | | | |
| | | | | | |
| | 2001 | | 2000 | | 1999 |
| |
| |
| |
|
| | | | | | New Basis | | Old Basis |
| | | | | |
| |
|
| Net income (loss) — as reported | | $ | 150,236 | | | $ | (74,241 | ) | | $ | (1,245,112 | ) | | $ | 87,636 | |
| Net income (loss) — with FAS 123 | | | (2,563 | ) | | | (159,067 | ) | | | (1,275,577 | ) | | | 57,105 | |
| Earnings (loss) per share — as reported: | | | | | | | | | | | | | | | | |
| | Basic | | | 0.29 | | | | (0.14 | ) | | | (2.43 | ) | | | 0.17 | |
| | Diluted | | | 0.28 | | | | (0.14 | ) | | | (2.43 | ) | | | 0.16 | |
| Earnings (loss) per share — with FAS 123: | | | | | | | | | | | | | | | | |
| | Basic | | | 0.00 | | | | (0.31 | ) | | | (2.49 | ) | | | 0.11 | |
| | Diluted | | | 0.00 | | | | (0.31 | ) | | | (2.49 | ) | | | 0.11 | |
C-66
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
A summary of our stock option activity and related information is as follows:
| | | | | | | | | | |
| | | | Weighted-Average |
| | Shares | | Exercise Price |
| |
| |
|
| Options outstanding at December 31, 1998 | | | 68,502,424 | | | $ | 12.82 | |
| | Grants | | | 34,092,336 | | | | 28.54 | |
| | Exercises | | | (11,638,378 | ) | | | 12.19 | |
| | Cancellations | | | (49,404,778 | ) | | | 13.03 | |
| | | |
| | | | | |
| Options outstanding at December 31, 1999 | | | 41,551,604 | | | $ | 25.65 | |
| | Grants | | | 9,986,353 | | | | 78.70 | |
| | Exercises | | | (8,258,743 | ) | | | 17.96 | |
| | Cancellations | | | (2,334,352 | ) | | | 30.82 | |
| | | |
| | | | | |
| Options outstanding at December 31, 2000 | | | 40,944,862 | | | $ | 39.84 | |
| | Grants | | | 10,740,689 | | | | 42.58 | |
| | Exercises | | | (2,899,135 | ) | | | 24.69 | |
| | Cancellations | | | (2,146,446 | ) | | | 45.84 | |
| | | |
| | | | | |
| Options outstanding at December 31, 2001 | | | 46,639,970 | | | $ | 41.06 | |
The following table summarizes information concerning currently outstanding and exercisable options:
| | | | | | | | | | | | | | | | | | | | | |
| | |
| | As of December 31, 2001 |
| |
|
| | | | |
| | Options Outstanding | | |
| |
| | Options Exercisable |
| | | | Weighted- | | | |
|
| | | | Average Years | | Weighted- | | | | Weighted- |
| | | | Remaining | | Average | | | | Average |
| Range of | | Number | | Contractual | | Exercise | | Number | | Exercise |
| Exercise Prices | | Outstanding | | Life | | Price | | Exercisable | | Price |
| |
| |
| |
| |
| |
|
| $12.531-$17.781 | | | 3,131,219 | | | | 7.83 | | | $ | 15.00 | | | | 3,081,516 | | | $ | 14.96 | |
| $20.000-$24.250 | | | 17,359,226 | | | | 7.62 | | | | 24.23 | | | | 12,560,979 | | | | 24.23 | |
| $32.094-$47.500 | | | 16,439,927 | | | | 9.05 | | | | 42.29 | | | | 3,243,858 | | | | 42.84 | |
| $50.550-$74.844 | | | 1,328,898 | | | | 8.77 | | | | 62.46 | | | | 333,673 | | | | 66.01 | |
| $76.440-$95.655 | | | 8,380,700 | | | | 8.89 | | | | 79.88 | | | | 2,234,836 | | | | 80.09 | |
| | | |
| | | | | | | | | | | |
| | | | | |
| | | | 46,639,970 | | | | | | | | | | | | 21,454,862 | | | | | |
Using the Black-Scholes option valuation model, the weighted-average fair value of options granted was $24.00 in 2001, $51.05 in 2000 and $13.66 in 1999. Shares of Common Stock available for future grants under all stock option plans were 14,509,668 at December 31, 2001.
Subsequent Event
Under our stock repurchase program approved by our Board of Directors on October 31, 2001, we have repurchased approximately 3.3 million shares of our common stock at a cost of approximately $160.1 million since December 31, 2001. For more information on our stock repurchase program, see the “Capital Stock” note above.
C-67
QUARTERLY FINANCIAL DATA (Unaudited)
(In thousands, except per share amounts)
| | | | | | | | | | | | | | | | | | |
| | |
| | 2001 Quarter Ended |
| |
|
| | December 31 | | September 30 | | June 30 | | March 31 |
| |
| |
| |
| |
|
| Total revenues | | $ | 600,156 | | | $ | 556,165 | | | $ | 515,874 | | | $ | 540,082 | |
| Product sales | | | 492,036 | | | | 448,700 | | | | 410,258 | | | | 391,904 | |
| Gross margin from product sales | | | 393,608 | | | | 352,670 | | | | 334,070 | | | | 308,108 | |
| Income before cumulative effect of accounting change(1) | | | 42,097 | | | | 42,741 | | | | 38,648 | | | | 32,388 | |
| Cumulative effect of accounting change, net of tax(2) | | | — | | | | — | | | | — | | | | 5,638 | |
| Net income | | | 42,097 | | | | 42,741 | | | | 38,648 | | | | 26,750 | |
| Earnings per share: | | | | | | | | | | | | | | | | |
| | Basic | | | 0.08 | | | | 0.08 | | | | 0.07 | | | | 0.05 | |
| | Diluted | | | 0.08 | | | | 0.08 | | | | 0.07 | | | | 0.05 | |
| | | | | | | | | | | | | | | | | | |
| | |
| | 2000 Quarter Ended |
| |
|
| | December 31 | | September 30 | | June 30 | | March 31 |
| |
| |
| |
| |
|
| Total revenues | | $ | 485,340 | | | $ | 447,340 | | | $ | 415,826 | | | $ | 387,850 | |
| Product sales | | | 351,579 | | | | 334,173 | | | | 309,414 | | | | 283,178 | |
| Gross margin from product sales(3) | | | 281,835 | | | | 242,817 | | | | 211,757 | | | | 177,043 | |
| Income (loss) before cumulative effect of accounting change(4) | | | 15,274 | | | | 5,760 | | | | (12,865 | ) | | | (24,610 | ) |
| Cumulative effect of accounting change, net of tax(6) | | | — | | | | — | | | | — | | | | (57,800 | ) |
| Net income (loss) | | | 15,274 | | | | 5,760 | | | | (12,865 | ) | | | (82,410 | ) |
| Earnings (loss) per share(5): | | | | | | | | | | | | | | | | |
| | Basic | | | 0.03 | | | | 0.01 | | | | (0.02 | ) | | | (0.16 | ) |
| | Diluted | | | 0.03 | | | | 0.01 | | | | (0.02 | ) | | | (0.16 | ) |
| |
| Increase (decrease)(6): | | | | | | | | | | | | | | | | |
| | Revenues | | | — | | | $ | 2,158 | | | $ | 2,158 | | | $ | 2,158 | |
| | Net income (loss) | | | — | | | | 1,295 | | | | 1,295 | | | | (56,505 | ) |
| | Earnings (loss) per share — diluted | | | — | | | | 0.00 | | | | 0.00 | | | | (0.11 | ) |
| |
| (1) | Includes recurring charges related to the Redemption, primarily the amortization of goodwill and other intangible assets of $79.4 million in the fourth, third, second and first quarters of 2001. |
| |
| (2) | We adopted the Statement of Financial Accounting Standards No. 133, “Accounting for Derivatives and Hedging Activities,” on January 1, 2001. Upon adoption, we recorded a $5.6 million charge, net of tax, as a cumulative effect of a change in accounting principle and an increase of $5.0 million, net of tax, in other comprehensive income related to recording derivative instruments at fair value. |
| |
| (3) | Reflects expense of $2.3 million, $15.8 million, $31.4 million and $43.3 million in the fourth, third, second and first quarters of 2000, respectively, related to the sale of inventory that was written up to fair value as a result of the Redemption on June 30, 1999, and related push-down accounting. |
| |
| (4) | Primarily reflects the impact of the Redemption and push-down accounting, including: the sale of inventory that was written up to fair value, see note (3) above; the amortization of goodwill and other intangible assets of $78.6 million, $95.2 million, $95.2 million and $95.2 million in the fourth, third, second and first quarters of 2000, respectively. |
| |
| (5) | Reflects the two-for-one stock split in October of 2000. |
| |
| (6) | We adopted the Securities and Exchange Commission’s Staff Accounting Bulletin No. 101 on revenue recognition effective January 1, 2000, and recorded a $57.8 million charge, net of tax, as a cumulative effect of a change in accounting principle related to contract revenues recognized in prior periods. The related deferred revenue is being recognized over the term of the agreements. The increase (decrease) in revenues, net income (loss) and earnings (loss) per diluted shares reflect the impact of this adoption. |
C-68
PROXY
GENENTECH, INC.
PROXY SOLICITED BY THE BOARD OF DIRECTORS
FOR THE ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON APRIL 11, 2002
The undersigned appoints Stephen G. Juelsgaard and Arthur D. Levinson, and each of them, as proxies of the undersigned, each with full power of substitution, to vote all of the shares of Common Stock of Genentech, Inc. (“Genentech”) held of record by the undersigned as of February 11, 2002 at the Annual Meeting of Stockholders of Genentech to be held at the Westin Hotel, 1 Old Bayshore Highway, Millbrae, California on Thursday, April 11, 2002, commencing at 10:00 a.m., local time, and at any adjournment or postponement of the Annual Meeting, with all powers that the undersigned would possess if personally present, upon and in respect of the following matters and in accordance with the following instructions.
IF NO OTHER INDICATION IS MADE ON THE REVERSE SIDE OF THIS FORM, THIS PROXY WILL BE VOTED FOR ALL NOMINEES FOR DIRECTOR LISTED IN PROPOSAL 1 AND FOR PROPOSALS 2 AND 3 AS MORE SPECIFICALLY DESCRIBED IN THE PROXY STATEMENT AND IN THE DISCRETION OF THE PERSONS NAMED ABOVE IN ANY OTHER MATTER WHICH MAY PROPERLY COME BEFORE THE ANNUAL MEETING. IF SPECIFIC INSTRUCTIONS ARE INDICATED, THIS PROXY WILL BE VOTED IN ACCORDANCE WITH THOSE INSTRUCTIONS.
YOU MAY REVOKE THIS PROXY AT ANY TIME PRIOR TO THE VOTE AT THE ANNUAL MEETING.
| | | | | |
| | | |
|
SEE REVERSE
SIDE | | PLEASE COMPLETE, DATE AND SIGN THIS PROXY AND
RETURN IT IN THE ACCOMPANYING ENVELOPE | | SEE REVERSE
SIDE |
| | | |
|
GENENTECH, INC.
C/O EQUISERVE
P.O. BOX 43068
PROVIDENCE, RI 02940
| | | | | | | |
| Vote by Telephone | | Vote by Internet |
It’s fast, convenient, and immediate!
Call Toll-Free on a Touch-Tone Phone | | It’s fast, convenient, and your vote is immediately confirmed and posted. |
| 1-877-PRX-VOTE (1-877-779-8683) | | |
| (Telephone Voting is available only in the United States and Canada | | | | |
| |
| Follow these four easy steps: | | Follow these four easy steps: |
| |
| 1. | | Read the accompanying Proxy Statement and Proxy Card | | 1. | | Read the accompanying Proxy Statement and Proxy Card |
| |
| 2. | | Call the toll-free number
1-877-PRX-VOTE (1-877-779-8683) | | 2. | | Go to the Website
http://www.eproxyvote.com/dna |
| |
| 3. | | Enter your Voter Control Number located on
your Proxy Card above your name | | 3. | | Enter your Voter Control Number located on
your Proxy Card above your name |
| |
| 4. | | Follow the recorded instructions | | 4. | | Follow the instructions provided |
| |
| Your vote is important! | | Your vote is important! |
| Call1-877-PRX-VOTEanytime! | | Go tohttp://www.eproxyvote.com/dna anytime! |
Do not return your Proxy Card if you are voting by Telephone or Internet
Your telephone or Internet vote authorizes the named proxies to vote your shares in the same manner as if you marked, signed and returned your proxy card by mail. Please note that all votes cast via telephone or the Internet must be cast prior to 11:59 p.m., eastern time, on Wednesday, April 10, 2002. If you wish to change or revoke your vote you may re-vote via telephone or the Internet, or return your properly completed proxy card; your latest vote received prior to the deadline will override each of your previous votes. If you wish to change your address, please mark the box below and return your proxy card by mail.
| | | | | |
| [X] | | Please mark
votes as in
this example | | |
THE BOARD OF DIRECTORS OF GENENTECH, INC. RECOMMENDS A VOTE FOR THE NOMINEES FOR DIRECTOR LISTED BELOW AND A VOTE FOR PROPOSALS 2 AND 3.
| | | | | | | | | | |
| 1. | | To elect six directors to hold office until the 2003 Annual Meeting of Stockholders |
| |
| | | Nominees: | | (01) Herbert W. Boyer, (02) Franz B. Humer,
(03) Jonathan K.C. Knowles, (04) Arthur D. Levinson,
(05) Mark Richmond and (06) Charles A. Sanders. |
| |
| | | | | FOR
ALL
NOMINEES | [ ] | [ ] | WITHHELD
FROM ALL
NOMINEES |
| |
| | | | | [ ] |
For all nominees except as noted above |
| | | | | | | | | |
| 2. | | To approve the amended and restated 1999 Stock Plan for certain circumstances. | | FOR
[ ] | | AGAINST
[ ] | | ABSTAIN
[ ] |
| |
| 3. | | To ratify the selection of Ernst & Young LLP as independent public accountants of Genentech for the year ending December 31, 2002. | | [ ] | | [ ] | | [ ] |
| |
| 4. | | By my signature below, I confer to the named proxies discretionary authority on any other business that may properly come before the Annual Meeting or any adjournment or postponement of the Annual Meeting. |
| |
| | | MARK HERE FOR ADDRESS CHANGE AND NOTE AT LEFT | | | | | | [ ] |
| |
| | | MARK HERE IF YOU PLAN TO ATTEND THE MEETING | | | | | | [ ] |
Please sign exactly as name appears on this card. When signing as attorney, executor, administrator, trustee or guardian, please give full title. If more than one trustee, all should sign. All joint owners must sign.
| | | | | | | | | | | | | | | |
| Signature: | |
| | Date: | |
| | Signature: | |
| | Date: | |
|