SCHEDULE 14A INFORMATION
PROXY STATEMENT PURSUANT TO SECTION 14(a) OF THE
SECURITIES EXCHANGE ACT OF 1934
(AMENDMENT NO. )
Filed by the Registrant {X}
Filed by a Party other than the Registrant {_}
Check the appropriate box:
| | | |
| {_} | | Preliminary Proxy Statement |
| {_} | | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| {X} | | Definitive Proxy Statement |
| {_} | | Definitive Additional Materials |
| {_} | | Soliciting Material Pursuant to § 240.14a-12 |
| |
| GENENTECH, INC. |
|
| (Name of Registrant as Specified In Its Charter) |
| |
| |
|
| (Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
Payment of Filing Fee (Check the appropriate box):
| | | | | |
| {X} | | No fee required. |
| | | | | |
| {_} | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | | | | |
| | | (1) | | Title of each class of securities to which transaction applies: |
| | | | | |
| | | (2) | | Aggregate number of securities to which transaction applies: |
| | | | | |
| | | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): |
| | | | | |
| | | (4) | | Proposed maximum aggregate value of transaction: |
| | | | | |
| | | (5) | | Total fee paid: |
| | | | | |
| {_} | | Fee paid previously with preliminary materials. |
| | | | | |
| {_} | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | | | | |
| | | (1) | | Amount Previously Paid: |
| | | (2) | | Form, Schedule or Registration Statement No.: |
| | | (3) | | Filing Party: |
| | | (4) | | Date Filed: |
TABLE OF CONTENTS
1 DNA Way
South San Francisco, California 94080-4990
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
| | |
| DATE | | Wednesday, April 23, 2003 |
| |
| TIME | | 10:00 a.m., Pacific Daylight Time |
| |
| PLACE | | Westin Hotel
1 Old Bayshore Highway
Millbrae, California |
| |
| ITEMS OF BUSINESS | | 1. To elect six directors for one-year terms. |
| | 2. To approve the amended and restated 1999 Stock Plan. |
| |
| | 3. To approve an amendment to the amended 1991 Employee Stock Plan. |
| |
| | 4. To ratify Ernst & Young LLP as our independent auditors for 2003. |
| |
| | 5. To consider any other matter brought before the stockholders at the annual meeting. |
| |
| RECORD DATE | | You are entitled to vote at the annual meeting if you were a stockholder at the close of business on Monday, February 24, 2003. |
| |
| ADMISSION | | If you are a stockholder of record, you may be asked to present proof of identification for admission to the annual meeting. If your shares are held in the name of a broker, bank or other nominee, you may be requested to present proof of identification and a statement from your broker, bank or other nominee, reflecting your ownership of Genentech common stock as of February 24, 2003. Please be prepared to provide this documentation if requested. |
| |
| VOTING BY PROXY | | Please submit a proxy as soon as possible so that your shares can be voted at the annual meeting in accordance with your instructions. For specific instructions regarding voting, please refer to theQuestions and Answers beginning on page 1 of the Proxy Statement and the instructions on your proxy card. |
| |
| | By Order of the Board, |
| |
| | STEPHEN G. JUELSGAARD |
| | Executive Vice President, General |
| | Counsel and Secretary |
This Proxy Statement and accompanying proxy card are being
distributed on or about March 19, 2003.
ELECTRONIC DELIVERY OF STOCKHOLDER COMMUNICATIONS
Our annual meeting materials are available electronically. As an alternative to receiving printed copies of these materials in future years, you can elect to receive an e-mail which will provide an electronic link to these documents as well as allow you the opportunity to conduct your voting online. By registering for electronic delivery, you can conveniently receive stockholder communications as soon as they are available without waiting for them to arrive via postal mail. You can also reduce the number of documents in your personal files, eliminate duplicate mailings, conserve natural resources and help us reduce our printing and mailing expenses.
Stockholders of Record
| |
| | You are a stockholder of record if you hold your shares in certificate form. If you vote on the Internet atwww.eproxyvote.com/dna, simply follow the directions for enrolling in the electronic delivery service. You also may enroll in the electronic delivery service at any time in the future by going directly towww.econsent.com/dnaand following the instructions. |
Beneficial Stockholders
| |
| | You are a beneficial stockholder if your shares are held by a broker, bank or other nominee. Please check with your bank, broker or relevant nominee regarding the availability of this service. |
If you have any questions about electronic delivery, please contact Genentech’s Investor Relations Department by phone at (650) 225-1599 or by email atinvestor.relations@gene.com.
QUESTIONS AND ANSWERS ABOUT
THE PROXY MATERIALS AND THE ANNUAL MEETING
| |
| Q: | Why am I receiving these materials? |
| |
| A: | The Board of Directors of Genentech, Inc. (or the “Company”) is providing you with these proxy materials in connection with the annual meeting of stockholders to be held at 10:00 a.m. on April 23, 2003. As a stockholder, you are requested to vote on the proposals described in this Proxy Statement. |
Q: Who can vote at the annual meeting?
| |
| A: | Stockholders who owned our common stock on February 24, 2003 may vote at the annual meeting. Each share of common stock is entitled to one vote. There were 511,464,278 shares of our common stock outstanding on February 24, 2003. |
| |
| Q: | What is the proxy card? |
| |
| A: | The proxy card enables you to appoint Stephen G. Juelsgaard and Arthur D. Levinson as your representatives at the annual meeting. By completing and returning the proxy card, you are authorizing Mr. Juelsgaard and Dr. Levinson to vote your shares at the meeting as you have instructed them on the proxy card. This way, you can vote your shares whether or not you attend the meeting. |
| |
| Q: | What am I voting on? |
| |
| A: | We are asking you to vote on: |
| | |
| | • | the election of six directors for one-year terms, |
| |
| | • | a proposal to approve our amended and restated 1999 Stock Plan, |
| |
| | • | a proposal to amend our amended 1991 Employee Stock Plan, and |
| |
| | • | the ratification of Ernst & Young LLP as our independent auditors for 2003. |
Q: How do I vote?
| |
| A: | BY MAIL: Please complete and sign your proxy card and mail it in the enclosed pre-addressed envelope. If you mark your voting instructions on the proxy card, your shares will be voted as you instruct or in the best judgment of Mr. Juelsgaard and Dr. Levinson if a proposal comes up for a vote at the meeting that is not on the proxy card. |
If you do not mark your voting instructions on the proxy card, your shares will be voted as follows:
| | |
| | • | FOR the six named nominees as directors, |
| |
| | • | FOR approval of the amended and restated 1999 Stock Plan, |
| |
| | • | FOR approval of an amendment to the amended 1991 Employee Stock Plan, |
| |
| | • | FOR ratification of Ernst & Young as our independent auditors for 2003, or |
| |
| | • | according to the best judgment of Mr. Juelsgaard and Dr. Levinson if a proposal comes up for a vote at the meeting that is not on the proxy card. |
BY TELEPHONE: Please follow the “Vote by Telephone” instructions that accompanied your proxy card. If you vote by telephone, you do not have to mail in your proxy card.
BY INTERNET: Please follow the “Vote by Internet” instructions that accompanied your proxy card. If you vote by Internet, you do not have to mail in your proxy card.
IN PERSON: We will pass out written ballots to anyone who wants to vote in person at the meeting. However, if you hold your shares in street name, you must request a proxy card from your broker in order to vote at the meeting. Holding shares in “street name” means that you hold them through a brokerage firm, bank, or other nominee, and therefore, the
1
shares are not held in your individual name.
| |
| Q: | What does it mean if I receive more than one proxy card? |
| |
| A: | It means that you hold our shares in multiple accounts at the transfer agent or with brokers or other custodians of your shares. Please complete and return all the proxy cards you receive to ensure that all your shares are voted. |
Q: Can I change my vote?
| |
| A: | You may revoke your proxy and change your vote by: |
| | |
| | • | signing another proxy card with a later date and returning it before the polls close at the meeting, |
| |
| | • | voting by telephone or on the Internet before 10 p.m., Pacific Daylight Time, on April 22, 2003 (yourlatesttelephone or Internet vote is counted), or |
| |
| | • | voting at the meeting. |
| |
| Q: | How many shares must be present to hold the meeting? |
| |
| A: | To hold the meeting and conduct business, a majority of the Company’s outstanding shares as of February 24, 2003 must be present at the meeting. This is called a quorum. |
Shares are counted as present at the meeting if the stockholder either:
| | |
| | • | is present and votes in person at the meeting, or |
| |
| | • | has properly submitted a proxy (including voting by telephone or on the Internet). |
Both abstentions and broker non-votes are counted as present for the purposes of determining the presence of a quorum. Generally, broker non-votes occur when shares held by a stockholder in street name are not voted with respect to a proposal because the broker has not received voting instructions from the stockholder, and the broker lacks discretionary voting power to vote the shares.
| |
| Q: | How many votes must the nominees receive to be elected as directors? |
| |
| A: | Because six directors are to be elected at the annual meeting, the six individuals receiving the highest number of votes FOR election will be elected. |
| |
| Q: | How many votes must the amended and restated 1999 Stock Plan receive to be approved? |
| |
| A: | The amended and restated 1999 Stock Plan will be approved if a majority of the shares present at the meeting in person or by proxy vote FOR approval. |
| |
| Q: | How many votes must the amendment of the amended 1991 Employee Stock Plan receive to be approved? |
| |
| A: | The amendment to the 1991 Employee Stock Plan will be approved if a majority of the shares present at the meeting in person or by proxy vote FOR approval. |
| |
| Q: | How many votes must the ratification of Ernst & Young LLP as the Company’s independent auditors for 2003 receive to be approved? |
| |
| A: | The ratification of Ernst & Young LLP as our independent auditors for 2003 will be approved if a majority of the shares present at the meeting in person or by proxy vote FOR approval. |
| |
| Q: | How are votes counted? |
| |
| A: | You may vote either “FOR” each director nominee or withhold your vote from any one or more of the nominees. |
You may vote “FOR” or “AGAINST” or “ABSTAIN” from voting on each of the proposals to approve the amended and restated 1999 Stock Plan and an amendment to the amended 1991 Employee Stock Plan and to ratify Ernst & Young LLP as our independent auditors. If you abstain from voting on any of these
2
proposals, it will have the same effect as a vote “AGAINST” the proposal. Broker non-votes, if any, will not affect the outcome of the voting on any of the proposals described in this Proxy Statement.
Voting results are tabulated and certified by our transfer agent, EquiServe Trust Company, N.A.
| |
| Q: | Who will bear the cost of soliciting votes for the meeting? |
| |
| A: | We are paying for the distribution and solicitation of the proxies. As a part of this process, we reimburse brokers, nominees, fiduciaries and other custodians for reasonable fees and expenses in forwarding proxy materials to our stockholders. Our employees may also solicit proxies through mail, telephone, the Internet or other means, but they do not receive additional compensation for providing those services. |
3
RELATIONSHIP WITH ROCHE
History of Ownership
In June 1999, the Company redeemed all of our callable putable common stock, par value $.02 per share (or “special common stock”) held by stockholders other than Roche Holdings, Inc. (or “Roche”) in cash and retired all of the shares of special common stock including those held by Roche. As a result, Roche’s percentage ownership of our outstanding equity increased from approximately 65% to 100%.
Offerings of, and Notes Exchangeable for, our Common Stock
In July and October of 1999, Roche completed public offerings of 88 million and 80 million shares, respectively, of our common stock, par value $.02 per share (or “common stock”). Upon completion of the offering in October 1999, Roche’s percentage ownership of our outstanding common stock decreased to 66.4%. In January 2000, Roche completed an offering of zero-coupon notes which are exchangeable for an aggregate of 13,034,618 shares of our common stock held by Roche. In March 2000, Roche completed a public offering of 34.6 million shares of our common stock, decreasing its ownership of our outstanding common stock to 58.9%.
Arrangements between Genentech and Roche
As a result of the redemption of the special common stock, the governance agreement then existing between Genentech and Roche terminated, except for provisions relating to indemnification and stock options, warrants and convertible securities. Subsequently, we amended our Certificate of Incorporation and entered into an affiliation agreement with Roche that enabled our current management to conduct our business and operations as we had done in the past while at the same time reflecting Roche’s ownership in us. The affiliation agreement is for the exclusive benefit of Roche and can be amended at any time by Roche and us.
We also amended our bylaws to provide Roche with certain proportional representation rights with respect to membership on our Board of Directors and committees.
Our Amended and Restated Certificate of Incorporation provides that the provisions in our bylaws described below under:
| |
| • | “— Composition of Board of Directors,” |
| |
| • | “— Roche’s Right to Proportional Representation,” |
| |
| • | “— Membership of Committees,” |
| |
| • | “— Nomination of Directors,” |
may be repealed or amended only by a 60% vote of our stockholders. However, Roche’s right to nominate a number of directors proportional to Roche’s ownership interest rounded down to the next whole number until Roche’s ownership interest is less than 5%, may be repealed or amended only by a 90% vote of our stockholders.
The provisions of the affiliation agreement described below under “— Roche Approval Required for Certain Actions” and “— Licensing and Marketing Arrangements” will terminate if Roche owns less than 40% of our stock.
For purposes of the following provisions, an independent director is a director who is not:
| |
| • | one of our officers; or |
| |
| • | an employee, director, principal stockholder or partner of Roche or any Roche affiliate or an entity that depended on Roche for more than 10% of his, her or its revenues or earnings in its most recent fiscal year. |
Composition of Board of Directors
Our Board consists of six members: two nominees of Roche, one executive officer of Genentech and up to three independent directors. All of our directors are nominated by the Nominations Committee of the Board. Directors are elected to serve one-year terms or until their successors are elected and qualified. At all times our Board will include at least two independent directors and one executive officer of Genentech.
4
Roche’s Right to Proportional Representation
Roche is entitled to representation on our Board proportional to its ownership interest in our common stock. Roche will be entitled to have a number of directors equal to its percentage ownership of our common stock times the total number of directors, rounded up to the next whole number if Roche’s ownership interest is greater than 50% and rounded down if it is less than or equal to 50%. Upon Roche’s request, we will immediately take action to increase the size of our Board or to fill the vacancies by electing Roche nominees in order to achieve Roche’s proportional representation.
If Roche’s ownership interest of our common stock falls below 40%, the Roche directors will resign to the extent Roche’s representation exceeds its proportional ownership interest. The number of directors required to resign shall be rounded up to the next whole number. Roche shall thereafter be entitled to nominate a number of directors proportional to Roche’s ownership interest rounded down to the next whole number, until Roche’s ownership interest is less than 5%.
Membership of Committees
We have five committees of the Board:
| |
| • | an Audit Committee, |
| |
| • | a Compensation Committee, |
| |
| • | a Corporate Governance Committee, |
| |
| • | an Executive Committee, and |
| |
| • | a Nominations Committee |
and Roche is entitled to its proportional representation on each committee. However, New York Stock Exchange rules require that no Roche director be a member of the Audit Committee. Roche’s committee members may designate another Roche director to serve as their alternates on any committee.
The Nominations Committee shall at all times have three members. Any time that Roche’s ownership percentage of our stock is equal to or greater than 80%, the Nominations Committee shall be comprised of two Roche nominees and one independent director. Any time that Roche’s ownership percentage of our stock is less than 80%, the Nominations Committee shall be comprised of a number of Roche nominees equal to Roche’s ownership percentage times three, rounded up to the next whole number if Roche’s total voting power is greater than 50% and rounded down if Roche’s total voting power is less than or equal to 50%. However, Roche may not have more than two nominees at any time. Roche currently has two nominees on the Nominations Committee.
Nomination of Directors
A majority of the members of the Nominations Committee must approve the nomination of any person for director.
Roche Approval Required for Certain Actions
Without the prior approval of the Roche directors, we will not approve:
| |
| • | any acquisition constituting a substantial portion of our business or assets; |
| |
| • | any sale, lease, license, transfer or other disposal of all or a substantial portion of our business or assets not in the ordinary course of our business; |
| |
| • | any issuance of capital stock other than (1) issuances pursuant to employee incentive plans not exceeding 5% of our voting stock, (2) issuances upon the exercise, conversion or exchange of any of our outstanding capital stock, and (3) other issuances not exceeding 5% of our voting stock in any 24 month period; and |
| |
| • | any repurchase or redemption of our capital stock other than (1) a redemption required by the terms of a security and (2) purchases made at fair market value in connection with any of our deferred compensation plans. |
For purposes of the first and second bullet points of the previous paragraph, unless a majority of the Board of Directors have made a contrary determination in good faith, a “substantial portion of our business or assets” shall mean a portion of our business or assets accounting for 10% or more of our and our
5
consolidated subsidiaries’ consolidated total assets, contribution to net income or revenues.
If Roche makes a request for proportional representation on the Board, until the Roche designees take office as directors, we may not take any action not in the ordinary course of business without Roche’s consent.
Licensing and Marketing Arrangements
We have a licensing and marketing agreement with F. Hoffmann-La Roche Ltd (or “Hoffmann-La Roche”) and its affiliates, granting them an option to license to use and sell products in non-U.S. markets. The major provisions of that agreement include the following:
| |
| • | Hoffmann-La Roche may exercise its option to license our products upon the occurrence of any of the following: (1) our decision to file an Investigational New Drug application (or “IND”) for a product, (2) completion of a Phase II trial for a product or (3) if Hoffmann-La Roche previously paid us a fee of $10 million to extend its option on a product, completion of a Phase III trial for that product; |
| |
| • | if Hoffmann-La Roche exercises its option to license a product, it has agreed to reimburse Genentech for development costs as follows: (1) if exercise occurs at the time an IND is filed, Hoffmann-La Roche will pay 50% of development costs incurred prior to the filing and 50% of development costs subsequently incurred, (2) if exercise occurs at the completion of a Phase II trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, (3) if the exercise occurs at the completion of a Phase III trial, Hoffmann-La Roche will pay 50% of development costs incurred through completion of the trial and 75% of development costs subsequently incurred, and $5 million of the option extension fee paid by Hoffmann-La Roche to preserve its right to exercise its option at the completion of a Phase III trial will be credited against the total development costs payable to Genentech upon the exercise of the option; |
| |
| • | we agreed, in general, to manufacture for Hoffmann-La Roche its clinical requirements of our products at cost, and its commercial requirements at cost plus a margin of 20%; however, Hoffmann-La Roche will have the right to manufacture our products under certain circumstances; |
| |
| • | Hoffmann-La Roche has agreed to pay, for each product for which it exercises its option upon either a decision to file an IND with the U.S. Food and Drug Administration (or “FDA”) or completion of the Phase II trials, a royalty of 12.5% on the first $100 million on its aggregate sales of that product and thereafter a royalty of 15% on its aggregate sales of that product in excess of $100 million until the later in each country of the expiration of our last relevant patent or 25 years from the first commercial introduction of that product; |
| |
| • | for each product for which Hoffmann-La Roche exercises an option after completion of the Phase III trials, it will pay a royalty of 15% on its sales of that product in each country until the later of the expiration of our relevant patent or 25 years from the first commercial introduction of that product; however, $5 million of any option extension fee that Hoffmann-La Roche pays will be credited against royalties due to us in the first calendar year of sales by Hoffmann-La Roche in which aggregate sales of the product exceed $100 million; and |
| |
| • | Hoffmann-La Roche’s option expires in 2015. |
Registration Rights
We have agreed to use our best efforts to file one or more registration statements under the Securities Act of 1933 in order to permit Roche to offer and sell shares of our common stock.
Generally, we will pay all expenses incident to the performance of our obligations with respect to the registration of Roche’s shares of our common stock except that Roche has agreed to pay certain expenses to be directly incurred by Roche, including underwriting fees, discounts and commissions and counsel fees. In addition, we are only required to pay for two
6
registrations within a 12-month period. We and Roche have each agreed to customary indemnification and contribution provisions with respect to liability incurred in connection with these registrations.
Dispositions by Roche
If Roche and its affiliates sell their majority ownership in our common stock to a successor, Roche will cause the successor to purchase all shares of our common stock not held by Roche:
| |
| • | if the consideration is entirely in either cash or equity traded on a U.S. national securities exchange, with consideration in the same form and amounts per share as received by Roche and its affiliates; and |
| |
| • | in any other case, with consideration either in the same form and amounts per share as received by Roche and its affiliates or with consideration that has a value per share not less than the weighted average value per share received by Roche and its affiliates as determined by an investment bank of nationally recognized standing appointed by a committee of independent directors. |
| |
| • | Roche has agreed to cause the buyer to agree to be bound by the obligations described in the preceding paragraph as well as the obligations described under “— Business Combinations with Roche” and “— Compulsory Acquisitions” below. We have agreed that the buyer shall be entitled to succeed to Roche’s rights described under “— Roche’s Right to Proportional Representation.” |
Business Combinations with Roche
Roche has agreed that as a condition to any merger of Genentech with Roche or its affiliates or the sale of substantially all of our assets to Roche or its affiliates, that either:
| |
| • | the merger or sale must be authorized by the favorable vote at any meeting of a majority of the shares of common stock not owned by Roche, provided that no person or group shall be entitled to cast more than 5% of the votes cast at the meeting; or |
| |
| • | in the event a favorable vote is not obtained, the value of the consideration to be received by the holders of our common stock, other than Roche, shall be equal to or greater than the average of the means of the ranges of fair values for the common stock as determined by two investment banks of nationally recognized standing appointed by a committee of independent directors. |
Roche has agreed that it will not sell any shares of our common stock in the 90 days immediately preceding any proposal by Roche for a merger with us.
Roche also agreed that in the event of any merger of Genentech with Roche or its affiliates or sale of substantially all of our assets to Roche or its affiliates, each unvested option outstanding under our stock option plans will:
| |
| • | be accelerated and become exercisable immediately prior to the consummation of the transaction for the total number of shares of common stock covered by the option; |
| |
| • | become exchangeable upon the consummation of the transaction for deferred cash compensation, which vests on the same schedule as the shares of the common stock covered by the option, having a value equal to the product of (A) the number of shares covered by the option and (B) the amount which Roche, in its reasonable judgment, considers to be equivalent in value to the consideration per share received by common stock holders in the transaction other than Roche, minus the exercise price per share of the option; or |
| |
| • | be canceled in exchange for a replacement option to purchase stock of the surviving corporation in the transaction with the terms of the option to provide value equivalent, as determined by Roche in its reasonable discretion, to that of the canceled option. |
Compulsory Acquisitions
If Roche owns more than 90% of our common stock for more than two months, Roche has agreed to, as soon as reasonably practicable,
7
effect a merger of Genentech with Roche or an affiliate of Roche.
The merger shall be conditioned on the vote or the valuation described under the first two bullets of “— Business Combinations with Roche” above.
Roche’s Ability to Maintain its Percentage Ownership Interest in Our Stock
The affiliation agreement provides, among other things, that we will establish a stock repurchase program designed to maintain Roche’s percentage ownership interest in our common stock. The affiliation agreement provides that we will repurchase a sufficient number of shares pursuant to this program such that, with respect to any issuance of common stock by us in the future, the percentage of our common stock owned by Roche immediately after such issuance will be no lower than Roche’s lowest percentage ownership of our common stock at any time after the offering of common stock occurring in July 1999 but prior to the time of such issuance, except that we may issue shares up to an amount that would cause Roche’s lowest percentage ownership to be decreased up to 2% below the “Minimum Percentage.”
The Minimum Percentage equals a fraction (expressed as a percentage) where the numerator is the lowest number of shares of our common stock owned by Roche since the July 1999 offering (to be adjusted in the future for dispositions of shares of our common stock by Roche), and the denominator is 509,194,352 which is the number of shares of our common stock outstanding at the time of the July 1999 offering adjusted for the two-for-one splits of our common stock in November 1999 and October 2000. Each of the numerator and denominator are to be adjusted in the future for stock splits or stock combinations.
As long as Roche’s percentage ownership is greater than 50%, prior to issuing any shares, the affiliation agreement provides that we will repurchase a sufficient number of shares of our common stock such that, immediately after its issuance of shares, Roche’s percentage ownership will be greater than 50%. The affiliation agreement also provides that, upon Roche’s request, we will repurchase shares of our common stock to increase Roche’s ownership to the Minimum Percentage.
Roche owned approximately 59.8% of our common stock as of December 31, 2002. The obligations of this stock repurchase program terminate upon Roche owning less than 40% of our stock.
Furthermore, Roche has a continuing option (which is assignable by Roche to any of its affiliates) to (i) buy from us, prior to the occurrence of any event that could result in a decrease in the percentage of common stock owned by Roche and its affiliates, a sufficient amount of common stock to ensure that Roche and its affiliates maintain the percentage ownership of our common stock owned by them, and (ii) to buy from us 80% of any class of stock issued by us other than common stock, in each case with a price per share equal to either the average of the last sale price on each of the five immediately preceding trading days on a U.S. national securities exchange on which the shares are traded or, if the sale prices are unavailable, the value of the shares determined in accordance with procedures reasonably satisfactory to Roche and us.
Tax Sharing Agreement
We entered into a tax sharing agreement with Roche under which we and Roche make payments such that the net amount paid by us on account of consolidated or combined income taxes is determined as if we had filed separate, stand-alone federal, state and local income tax returns as the common parent of an affiliated group of corporations filing consolidated or combined federal, state and local returns. Our tax sharing agreement with Roche now pertains only to the state and local tax returns in which we will be consolidated or combined with Roche. We will continue to calculate our tax liability or refund with Roche for these state and local jurisdictions as if we were a stand-alone entity.
8
CORPORATE GOVERNANCE
The Board of Directors
Our Board of Directors is responsible for broad corporate policy and the overall performance of the Company. Board members remain informed of the Company’s business by various documents provided to them before each meeting and oral presentations made to them during these meetings by the Chairman and Chief Executive Officer and other members of management. They are advised of actions taken by the Audit, Compensation, Corporate Governance, Executive and Nominations Committees of the Board. In addition, the directors receive reports from management on recommendations for proposed Board actions. Directors have access to all books, records and reports, and members of management are available at all times to answer their questions.
Our Board of Directors is committed to sound and effective corporate governance practices. In this regard, our Board of Directors has formally adopted Principles of Corporate Governance that guide its actions with respect to the composition of the Board, how the Board will function, the Board’s standing committees and procedures for appointing committee members. The Principles of Corporate Governance are available for public review on our website atwww.gene.com/gene/ir/corp-gov.
Management Executive Committee
The management Executive Committee has responsibility for the overall direction, strategy and operations of the Company, including, among other things, corporate financial performance, commercial performance, research and development and product operations performance and employee development performance. All six members of the management Executive Committee are executive officers of Genentech, and one is also a director. Its members hold the following positions at the Company:
| |
| • | Chairman, President and Chief Executive Officer, |
| |
| • | Executive Vice President, Commercial Operations and Chief Operating Officer, |
| |
| • | Executive Vice President, Development and Product Operations and Chief Medical Officer, |
| |
| • | Executive Vice President and Chief Financial Officer, |
| |
| • | Executive Vice President, General Counsel and Secretary, and |
| |
| • | Senior Vice President, Research. |
PROPOSAL 1 — ELECTION OF DIRECTORS
NOMINEES FOR DIRECTOR
The Company’s Board of Directors is elected each year at the annual meeting of stockholders. The Company’s Board currently consists of six directors:
| |
| • | two Roche directors: Franz B. Humer and Jonathan K.C. Knowles, |
| |
| • | three independent directors: Herbert W. Boyer, Charles A. Sanders and Sir Mark Richmond, and |
| |
| • | one Genentech employee: Arthur D. Levinson, who is also the Chairman of the Board. |
All six directors are nominees for election, and if elected, will serve a one-year term until the next annual meeting in 2004 or until a successor is elected or appointed.
We expect each nominee for election as a director to be able to serve if elected. If a nominee is not able to serve, proxies will be voted in favor of the remainder of those nominated and may be voted for any other person the Board of Directors may select.
The persons named in the enclosed proxy card will vote your proxy for the election of each of the six nominees unless you indicate otherwise.
9
We show below the name and age of each director, his current principal occupation, any other position held with the Company, and the period during which he has served as a director of the Company.
| | | | | | | | | | | |
| | | | | | Served as |
| Name | | Age | | Principal Occupation/Position Held | | director since |
| |
| |
| |
|
| Herbert W. Boyer, Ph.D. | | | 66 | | | Director of Genentech | | | 1976 | |
| Franz B. Humer, Doctor of Law | | | 56 | | | Chairman and Chief Executive Officer,
The Roche Group | | | 1995 | |
| Jonathan K.C. Knowles, Ph.D. | | | 55 | | | Head of Global Pharmaceuticals Research,
The Roche Group | | | 1998 | |
| Arthur D. Levinson, Ph.D. | | | 53 | | | Chairman and Chief Executive Officer
of Genentech | | | 1995 | |
| Sir Mark Richmond, Ph.D. | | | 72 | | | Director of Genentech | | | 1999 | |
| Charles A. Sanders, M.D. | | | 71 | | | Lead Director* of Genentech | | | 1999 | |
| |
| * | Dr. Sanders was appointed lead director of the Board of Directors in February 2003. As the lead director, Dr. Sanders chairs sessions of the Board when the Chairman is not present and acts as a point of contact for stockholders of Genentech who wish to communicate with the Board of Directors other than through the Chairman of the Board. |
Dr. Boyer, a founder of Genentech, had been a director of Genentech since 1976 when he resigned from the Board in June 1999 in connection with the redemption of our special common stock. He was reelected to the Board in September 1999. He served as a Vice President of Genentech from 1976 to 1991. Dr. Boyer, a Professor of Biochemistry at the University of California at San Francisco from 1976 to 1991, demonstrated the usefulness of recombinant DNA technology to produce medicines economically, which laid the groundwork for Genentech’s development. Dr. Boyer has received numerous awards for his research, including the National Medal of Science from President Bush in 1990, the National Medal of Technology in 1989 and the Albert Lasker Basic Medical Research Award in 1980. He is an elected member of the National Academy of Sciences and a Fellow in the American Academy of Arts and Sciences. In 2001, Dr. Boyer was elected to the National Inventors Hall of Fame. In addition, Dr. Boyer serves as Vice-Chairman of the Board of Directors of Allergan, Inc. and as a member of the Boards of Directors of IDEC Pharmaceuticals Corporation and Sangamo BioSciences, Inc.
Dr. Humerwas elected a director of Genentech in the spring of 1995. He joined The Roche Group in 1995 as the Head of its Pharmaceuticals Division, and became Chief Executive Officer of The Roche Group in 1998 and Chairman of the Board of Directors of The Roche Group in April 2001. Prior to joining The Roche Group, Dr. Humer was an Executive Director and Chief Operating Officer of Glaxo Holdings, a United Kingdom public limited company. Pursuant to the affiliation agreement, Dr. Humer is a designee of Roche.
Dr. Knowleswas elected a director of Genentech in February 1998. He joined The Roche Group as Head of Global Pharmaceuticals Research in September 1997. In January 1998, he became a member of the Executive Committee of The Roche Group. Prior to joining The Roche Group, Dr. Knowles served as the Director of Research for Europe of Glaxo from 1995 to 1997 and served as the Director of the Geneva Institute of Glaxo from 1989 to 1995. Pursuant to the affiliation agreement, Dr. Knowles is a designee of Roche.
Dr. Levinsonwas appointed Chairman of the Board of Directors in September 1999 and was elected President and Chief Executive Officer and a director of the Company in July 1995. Since joining the Company in 1980, Dr. Levinson has been a Senior Scientist, Staff Scientist and the Director of the Company’s Cell Genetics Department. Dr. Levinson was appointed Vice President of Research Technology in April 1989, Vice President of Research in May 1990 and
10
Senior Vice President in January 1993. Dr. Levinson was formerly on the editorial boards of “Molecular Biology and Medicine” and “Molecular and Cellular Biology,” and is active in the American Society of Microbiology, the New York Academy of Sciences, the American Association for the Advancement of Science, and the American Society for Biochemistry and Molecular Biology. From 1977 to 1980, Dr. Levinson was a Postdoctoral Fellow in the Department of Microbiology at the University of California, San Francisco. In 1977, Dr. Levinson received his Ph.D. in Biochemistry from Princeton University. Dr. Levinson also serves as a member of the Board of Directors of Apple Computer, Inc.
Dr. Richmondwas elected a director of Genentech in August 1999. He was a senior research fellow at the School of Public Policy, University College London from February 1996 to February 2002. Previously, he held positions as science advisor at Glaxo Wellcome plc from 1995 to February 1996, as Group Head of Research at Glaxo plc from 1993 to 1995, as chairman of the Science and Engineering Council, London, from 1990 to 1993, as vice chancellor at the University of Manchester from 1981 to 1990, and professor and head of the Department of Bacteriology at the University of Bristol from 1968 to 1981. Dr. Richmond is currently a member of the Scientific Advisory Committee of the Institute for Biotechnology, ETH, Zurich and a member of the Scientific Advisory Board of the SPP-Biotechnology, Swiss National Foundation from 1996 to 2001. He is a member of the Boards of Directors of Targeted Genetics Corporation and OSI Pharmaceuticals, Inc.
Dr. Sanderswas elected a director of Genentech in August 1999 and the lead director of the Board in February 2003. He served as Chief Executive Officer of Glaxo Inc. from 1989 to 1994, and was the Chairman of the Board of Glaxo Inc. from 1992 to 1995. He also has served on the Board of Directors of Glaxo plc. Previously, he held a number of positions at Squibb Corporation, a multinational pharmaceutical corporation, including Vice Chairman, Chief Executive Officer of the Science and Technology Group and Chairman of the Science and Technology Committee on the Board. Dr. Sanders is a member of the Boards of Directors of Scios Inc., Genaera Corporation, Vertex Pharmaceuticals, Edgewater Technology, Inc., Trimeris, Inc., Biopure Corporation, Pharmacopeia, Inc. and Cephalon, Inc.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS
A VOTE FOR EACH NOMINEE.
11
BOARD COMMITTEES AND MEETINGS
During 2002, the Board of Directors held five meetings. Each director attended at least 75% of the aggregate number of meetings of the Board and the committees on which the directors serve. None of the members of the Audit, Compensation, Corporate Governance or Nominations Committees were an officer or employee of the Company.
We show below information on the membership, functions and number of meetings of each Board committee.
| | | | | | | |
| | | | Number of |
| | | | Meetings |
| Name of Committee | | | | Held in |
| and Members | | Functions of the Committee | | 2002 |
| |
| |
|
AUDIT
Herbert W. Boyer
Mark Richmond
Charles A. Sanders | | • Recommends the independent auditors to the Board.
• Meets with the Company’s independent auditors to review and discuss the Company’s financial statements and quarterly reporting process.
• Reviews and approves inventory and receivable reserve policies, tax compliance and strategy, investment policy, risk management programs, the scope of audit activities, the general audit program and non-audit services.
• Reviews the reports of the independent auditors and accompanying management letter on the scope and results of their work.
• Reviews the independent auditors’ recommendations concerning the Company’s financial practices and procedures. | | | 9 | |
| |
COMPENSATION
Herbert W. Boyer
Franz B. Humer
Jonathan K.C. Knowles
Mark Richmond
Charles A. Sanders | | • Administers the Company’s stock option plans, the stock purchase plan and other corporate benefits programs.
• Reviews and approves the Company’s annual bonus plan, annual stock option grants and officer compensation.
• Elects officers of the Company. | | | 5 | |
| |
CORPORATE GOVERNANCE
Jonathan K.C. Knowles
Mark Richmond | | • Reviews the Company’s policies relating to sales and marketing activities, investor relations, corporate communications, government relations, human resources, legal and regulatory affairs, compliance with laws and regulations, and Board of Directors and Board committee effectiveness. | | | 3 | |
| |
EXECUTIVE
Herbert W. Boyer
Franz B. Humer
Arthur D. Levinson | | • Established to act when the full Board of Directors is unavailable.
• Has the authority of the Board in the management of the business and affairs of the Company, except those powers that cannot be delegated by the Board of Directors by law. | | | 0 | |
| |
NOMINATIONS
Herbert W. Boyer
Franz B. Humer
Jonathan K.C. Knowles | | • Identifies, reviews and recommends potential nominees to the Board of Directors.
• Recommends to the Board candidates for election as executive officers of the Company. | | | 1 | |
12
The Nominations Committee will consider nominees to the Board of Directors recommended by Genentech stockholders. To be considered, stockholders who wish to nominate a person to the Board should send a letter to the Secretary of Genentech with the following information: (a) the name and address of the stockholder who intends to make the nomination and of the person(s) to be nominated, (b) a representation that the stockholder is a holder of record of stock of Genentech and entitled to vote at such meeting and intends to appear in person or by proxy at the meeting to nominate the person(s) specified in the notice, (c) a description of all arrangements or understandings between the stockholder and each nominee and any other person(s), (d) such other information regarding each nominee as would be required to be included in a proxy statement filed pursuant to the rules of the Securities and Exchange Commission had the nominee been nominated by the Board, and (e) the consent of each nominee to serve as a director of Genentech if so elected.
COMPENSATION OF DIRECTORS
Annual Cash Retainer Fee
Each non-employee director of Genentech receives an annual cash retainer fee of $30,000 per year.
Meeting Fees
Each non-employee director also receives a fee of $1,500 for each Board and committee meeting at which the director was present in person and $500 for each Board and committee meeting at which the director was present by telephone.
Equity Compensation
Under our amended and restated 1999 Stock Plan, each non-employee and non-Roche director receives a stock option to purchase 10,000 shares of our common stock upon each re-election to the Board. These options vest on a monthly basis in equal increments over a period of twelve months. The exercise price of the stock option is equal to the closing price of our common stock as reported in theWall Street Journalon the day of re-election. Upon re-election to the Board at the annual meeting of stockholders in 2002, Dr. Boyer, Dr. Richmond and Dr. Sanders each received an option to purchase 10,000 shares of common stock.
Any new Board member who is a non-employee and non-Roche director will receive a stock option to purchase 25,000 shares of our common stock upon first election to the Board. These options vest over four years, with the first 25% vesting one year from the grant date, and the remainder vesting monthly in equal increments during the 36-month period following the initial vesting date.
Reimbursement of Expenses
All directors are reimbursed for expenses incurred in connection with their service on the Board.
Consulting fees
Dr. Boyer also served as a consultant to the Company in 2002 and received compensation in the amount of $20,506 for his services. As of December 31, 2002, Dr. Boyer is no longer a consultant of Genentech.
13
PROPOSAL 2 — APPROVAL OF THE
AMENDED AND RESTATED 1999 STOCK PLAN
We are asking our stockholders to approve our amended and restated 1999 Stock Plan (the “1999 Plan”), a broad-based plan under which we grant options to our employees, directors and consultants. We want to increase the number of shares issuable under that plan by 25,000,000 shares so that we can continue to use that plan to achieve the Company’s goals of retaining, incenting and rewarding those individuals who provide significant services to us and to further our growth and profitability. Our Board of Directors approved the increase in the number of shares issuable under the 1999 Plan on February 13, 2003, subject to approval from our stockholders at the annual meeting. If the stockholders approve the 1999 Plan, it will replace the current version of the 1999 Plan.
A total of 63,000,000 shares of our common stock is currently reserved for issuance under the 1999 Plan. As of December 31, 2002, 52,570,440 shares were subject to outstanding awards granted under the 1999 Plan, and 4,048,713 shares remained available for any new awards to be granted in the future.
Approval of the 1999 Plan requires the vote of a majority of the shares that are present in person or by proxy and entitled to vote at the annual meeting.
Our top six mostly highly compensated executive officers, who constitute the members of the management Executive Committee (or the “Named Executive Officers”), and directors have an interest in this proposal.
Description of the 1999 Plan
The following paragraphs provide a summary of the principal features of the 1999 Plan and its operation.
The 1999 Plan permits the grant of stock options and stock purchase rights (each, an “Award”) to eligible employees, non-employee directors and consultants. The 1999 Plan is intended to increase incentives and to encourage share ownership on the part of those individuals who provide significant services to us.
Administration of the 1999 Plan
The 1999 Plan is administered by the Compensation Committee of our Board of Directors. Subject to the terms of the 1999 Plan, the Compensation Committee has the discretion to select the employees, non-employee directors and consultants who will receive Awards, determine the terms and conditions of Awards (for example, the exercise price and vesting schedule), and interpret the provisions of the 1999 Plan and outstanding Awards. The Compensation Committee may delegate any part of its authority and powers under the 1999 Plan to one or more directors and/or officers of the Company.
If an Award expires or is cancelled without having been fully exercised or vested, the unvested or cancelled shares generally will be returned to the available pool of shares reserved for issuance under the 1999 Plan. Also, if we experience a stock dividend, reorganization or other change in our capital structure, our Board of Directors has the discretion to adjust the number of shares available for issuance under the 1999 Plan, the outstanding Awards, and the per-person limits on options, all as appropriate to reflect the stock dividend or other change.
Eligibility to Receive Awards
The Compensation Committee selects the employees, non-employee directors and consultants who will be granted Awards under the 1999 Plan. The actual number of individuals who will receive an Award under the 1999 Plan cannot be determined in advance because the Committee has discretion to select the participants.
14
Stock Options
A stock option is the right to acquire shares of the Company’s common stock (“Shares”) at a fixed exercise price for a fixed period of time. Under the 1999 Plan, the Compensation Committee may grant nonqualified stock options and/or incentive stock options.
The number of Shares covered by each option will be determined by the Compensation Committee, but during any fiscal year of the Company, no participant may be granted options covering more than 2,000,000 Shares or 3,000,000 Shares in the year of first hire. The exercise price of the Shares subject to each option is set by the Committee but for incentive stock options and options intended to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code, the exercise price cannot be less than 100% of the fair market value on the date of grant of the Shares covered by the option. An exception may be made for any options that the Company grants in substitution for options held by employees of companies that the Company acquires (in which case the exercise price preserves the economic value of the employee’s cancelled option from his or her former employer).
The aggregate fair market value of the Shares (determined on the grant date) covered by incentive stock options which first become exercisable by any participant during any calendar year also may not exceed $100,000.
An option granted under the 1999 Plan cannot be exercised until it becomes vested unless otherwise approved by the Company. The Compensation Committee establishes the vesting schedule of each option at the time of grant. Options granted under the 1999 Plan expire at the times established by the Committee, but in no event later than 10 years after the grant date.
The exercise price of each option granted under the 1999 Plan must be paid in full in cash at the time of exercise. The Compensation Committee also may permit payment through the delivery of Shares that are already owned by the participant, or by any other means that the Compensation Committee determines to be consistent with the purpose of the 1999 Plan. The participant must pay any taxes required to be withheld at the time of exercise.
Stock Purchase Rights
Stock purchase rights generally are the same as options. Stock purchase rights may be granted alone or in combination with an option. Each right allows the participant to purchase Shares at a price determined by the Committee. Shares purchased may be subject to a vesting schedule or right of repurchase by the Company, all as determined by the Compensation Committee.
Awards to be Granted to Certain Individuals and Groups
The number of Awards that an employee, non-employee director or consultant may receive under the 1999 Plan is at the discretion of the Compensation Committee and therefore cannot be determined in advance. To date, only non-qualified stock options have been granted under the 1999 Plan.
15
Amended Plan Benefits
In 2002, we granted options to purchase approximately 13 million shares to approximately 4,900 employees representing 93% of our total employees. We show below the aggregate number of Shares subject to options granted under the 1999 Plan during 2002 to the individuals and groups identified below, the percent of the total options granted to those individuals and groups and the weighted average per Share exercise price of such options.
| | | | | | | | | | | | | |
| | | | Percent of Total | | Weighted |
| | Number of Shares | | Options Granted to | | Average Exercise |
| | Underlying Options | | Employees in | | or Base Price |
| Name | | Granted (#) | | Fiscal Year | | ($/Share) |
| |
| |
| |
|
Arthur D. Levinson | | | 450,000 | | | | 3.56 | % | | $ | 28.55 | |
| Chairman, President and Chief Executive Officer | | | | | | | | | | | | |
Myrtle S. Potter | | | 250,000 | | | | 1.98 | % | | $ | 28.55 | |
| Executive Vice President, Commercial Operations and Chief Operating Officer | | | | | | | | | | | | |
Susan D. Desmond-Hellmann | | | 250,000 | | | | 1.98 | % | | $ | 28.55 | |
| Executive Vice President, Development and Product Operations and Chief Medical Officer | | | | | | | | | | | | |
Louis J. Lavigne, Jr. | | | 175,000 | | | | 1.39 | % | | $ | 28.55 | |
| Executive Vice President and Chief Financial Officer | | | | | | | | | | | | |
Stephen G. Juelsgaard | | | 175,000 | | | | 1.39 | % | | $ | 28.55 | |
| Executive Vice President, General Counsel and Secretary | | | | | | | | | | | | |
Richard H. Scheller | | | 175,000 | | | | 1.39 | % | | $ | 28.55 | |
| Senior Vice President, Research | | | | | | | | | | | | |
| All executive officers, as a group | | | 1,695,000 | | | | 13.43 | % | | $ | 28.55 | |
| All employees who are not executive officers, as a group (approximately 4,900 employees) | | | 10,928,875 | | | | 86.57 | % | | $ | 29.02 | |
| All directors who are not executive officers, as a group | | | 30,000 | | | | — | | | $ | 37.75 | |
Limited Transferability of Awards
Awards granted under the 1999 Plan generally may not be sold, transferred, pledged, assigned, or otherwise alienated or hypothecated, other than by will or by the applicable laws of descent and distribution, unless the Compensation Committee determines otherwise.
Amendment and Termination of the 1999 Plan
The Board generally may amend or terminate the 1999 Plan at any time and for any reason.
Federal Tax Aspects
The following paragraphs are a summary of the general federal income tax consequences to the Company and U.S. taxpayers of Awards granted under the 1999 Plan. Tax consequences for any particular individual may be different.
Nonqualified Stock Options
No taxable income is reportable when a nonqualified stock option is granted to a participant. Upon exercise, the participant will recognize ordinary income in an amount equal to the excess of the sale price (or the closing market price of the previous day in the event of cash exercises) of the Shares purchased over the exercise price of the option.
Any additional gain or loss recognized upon any later disposition of the Shares acquired in a cash exercise will be capital gain or loss.
16
Incentive Stock Options
Taxable income is not generally recognized on the grant or exercise of an incentive stock option unless the alternative minimum tax rules apply. The difference between the sale price and the option price on the sale of Shares exercised and held for a period of at least two years after the grant date and one year after the issuance date will be long-term capital gain or loss. If the participant exercises the option and then later sells or otherwise disposes of the Shares before the end of the two or one-year holding periods described above, then the participant will generally have ordinary income at the time of the sale equal to the fair market value of the Shares on the exercise date (or the sale price, if less) minus the exercise price of the option.
Stock Purchase Rights
Stock purchase rights generally are taxed in the same manner as nonqualified stock options. However, when a stock purchase right is exercised, the participant typically receives “restricted” stock (that is, stock that is subject to a vesting schedule). At the time of purchase, restricted stock is subject to a “substantial risk of forfeiture” within the meaning of Section 83 of the Internal Revenue Code. As a result, the participant will not recognize ordinary income at the time of purchase. Instead, the participant will recognize ordinary income on the dates when the stock ceases to be subject to a substantial risk of forfeiture. The stock typically will cease to be subject to a substantial risk of forfeiture when it is no longer subject to the Company’s right to repurchase the stock upon the purchaser’s termination of employment with the Company.
The participant will recognize ordinary income measured as the difference between the purchase price and the fair market value of the stock on the date the stock is no longer subject to a substantial risk of forfeiture. The participant may accelerate to the date of purchase his or her recognition of ordinary income, if any, and the beginning of any capital gain holding period by timely filing an election pursuant to Section 83(b) of the Internal Revenue Code.
Tax Effect for the Company
The Company generally will be entitled to a tax deduction in connection with an Award under the 1999 Plan in an amount equal to the ordinary income realized by a participant and at the time the participant recognizes such income (for example, the exercise of a nonqualified stock option). Special rules limit the deductibility of compensation paid to our Chief Executive Officer and to each of our four most highly compensated executive officers. Under Section 162(m) of the Internal Revenue Code, the annual compensation paid to any of these specified executives will be deductible only to the extent that it does not exceed $1 million.
However, the Company can preserve the deductibility of certain compensation in excess of $1 million if the conditions of Section 162(m) are met. These conditions include stockholder approval of the 1999 Plan, setting limits on the number of Awards that any individual may receive and for Awards other than stock options, establishing performance criteria that must be met before the Award actually will vest or be paid.
The 1999 Plan has been designed to permit the Committee to grant options that qualify as performance-based for purposes of satisfying the conditions of Section 162(m), thereby permitting the Company to continue to receive a federal income tax deduction in connection with the exercise of those options.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS
A VOTE FOR APPROVAL OF PROPOSAL 2.
17
PROPOSAL 3 — AMENDMENT OF THE
AMENDED 1991 EMPLOYEE STOCK PLAN
We are asking our stockholders to approve an amendment to the amended 1991 Employee Stock Plan (the “1991 Plan”) so that we may continue to attract and retain talented employees necessary for the Company’s continued growth and success. We want to increase the number of shares issuable under that Plan by 2,000,000 shares.
The 1991 Plan provides eligible employees of the Company and its participating subsidiaries with the opportunity to purchase shares of our common stock through convenient payroll deductions.
On February 13, 2003, our Board of Directors approved an amendment to increase the number of shares of our common stock available for purchase under the 1991 Plan by 2,000,000, subject to approval from our stockholders at the annual meeting.
Amendment of the 1991 Plan requires the vote of a majority of the shares that are present in person or by proxy and entitled to vote at the annual meeting. Our Named Executive Officers have an interest in this proposal.
Description of the 1991 Plan
The following paragraphs provide a summary of the principal features of the 1991 Plan and its operation.
Eligibility to Participate
Most employees of the Company and its participating subsidiaries are eligible to participate in the 1991 Plan. However, an employee is not eligible if he or she has the right to acquire 5% or more of the voting stock of the Company or of any subsidiary of the Company or works less than or equal to twenty hours per week or five months per calendar year. Approximately 4,500 employees currently participate in the 1991 Plan.
Administration, Amendment and Termination
The Compensation Committee administers the 1991 Plan. The members of the Compensation Committee serve at the request of the Board. Subject to the terms of the 1991 Plan, the Compensation Committee has all discretion and authority necessary or appropriate to control and manage the operation and administration of the 1991 Plan. The Compensation Committee may make whatever rules, interpretations, and computations, and take any other actions to administer the 1991 Plan that it considers appropriate to promote the Company’s best interests, and to ensure that the 1991 Plan remains qualified under Section 423 of the Internal Revenue Code. The Compensation Committee may delegate one or more of ministerial duties in the administration of the 1991 Plan.
The Compensation Committee or the Company’s Board of Directors may amend or terminate the 1991 Plan at any time and for any reason. However, as required by Section 423 of the Internal Revenue Code, the Company’s stockholders must approve certain material amendments.
Number of Shares of Common Stock Available under the 1991 Plan
Subject to stockholder approval, a maximum of 3,832,987 shares of common stock will be available for issuance pursuant to the 1991 Plan, including the 2,000,000 that were added by the Board of Directors on February 13, 2003. Shares sold under the 1991 Plan may be newly issued shares or treasury shares. In the event of any stock split, stock dividend or other change in the capital structure of the Company, appropriate adjustments will be made in the number, kind and purchase price of the shares available for purchase under the 1991 Plan and the various limits on share purchases under the 1991 Plan.
18
Enrollment and Contributions
Eligible employees voluntarily elect whether or not to enroll in the 1991 Plan. Employees join for an enrollment period of fifteen months, and an employee may cancel his or her enrollment at any time (subject to Plan rules).
Employees contribute to the 1991 Plan through payroll deductions. Participating employees generally may contribute from 1% to 15% of their eligible compensation through after-tax payroll deductions. From time to time, the Compensation Committee may establish a maximum permitted contribution percentage, change the definition of eligible compensation, or change the length of the enrollment periods (but in no event may any enrollment period exceed 27 months). After an enrollment period has begun, an employee may increase or decrease his or her contribution percentage (subject to Plan rules).
Purchase of Shares
Within each fifteen-month enrollment period, there are five three-month purchase periods. On the last business day of each purchase period, the Company uses each participating employee’s payroll deductions to purchase shares of common stock for the employee. The price of the shares purchased will be 85% of the lower of (1) the stock’s market value on the first day of the enrollment period, or (2) the stock market’s value on the last day of the purchase period. Market value under the 1991 Plan means the closing price of the common stock on the New York Stock Exchange on the purchase date. However, in any single year, no employee may purchase more than the lesser of $25,000 of common stock (based on market value on the applicable enrollment date(s)) or 48,000 shares. In addition, on any purchase date no more than a total 720,000 shares may be purchased by all employees. (This number is increased to 800,000 if, during that purchase period, annual bonuses are paid to eligible employees.) The Committee also has discretion to set a different limit on the number of shares that may be purchased on any purchase date or to set a lower (but not higher) limit on the dollar value of shares that may be purchased.
Termination of Participation
Participation in the 1991 Plan terminates when a participating employee’s employment with the Company ceases for any reason, the employee withdraws from the 1991 Plan, or the Company terminates or amends the 1991 Plan such that the employee no longer is eligible to participate.
Number of Shares Purchased by Certain Individuals and Groups
Given that the number of shares that may be purchased under the 1991 Plan is determined, in part, on the stock’s market value on the first day of the enrollment period and last day of the purchase period and given that participation in the 1991 Plan is voluntary on the part of employees, the actual number of shares that may be purchased by any individual is not determinable.
19
Amended Plan Benefits
We show below the number of shares of our common stock purchased during 2002 under the 1991 Plan by the individuals and groups identified below and the weighted average purchase price of such shares.
| | | | | | | | | |
| | Number of Shares | | Weighted Average |
| Name | | Purchased (#) | | Purchase Price ($/share) |
| |
| |
|
Arthur D. Levinson | | | 550 | | | $ | 38.76 | |
| Chairman, President and Chief Executive Officer | | | | | | | | |
Myrtle S. Potter | | | 548 | | | $ | 38.76 | |
| Executive Vice President, Commercial Operations and Chief Operating Officer | | | | | | | | |
Susan D. Desmond-Hellmann | | | 837 | | | $ | 36.97 | |
| Executive Vice President, Development and Product Operations and Chief Medical Officer | | | | | | | | |
Louis J. Lavigne, Jr. | | | 548 | | | $ | 38.76 | |
| Executive Vice President and Chief Financial Officer | | | | | | | | |
Stephen G. Juelsgaard | | | 548 | | | $ | 38.76 | |
| Executive Vice President, General Counsel and Secretary | | | | | | | | |
Richard H. Scheller | | | 0 | | | | — | |
| Senior Vice President, Research | | | | | | | | |
| All executive officers, as a group | | | 4,677 | | | $ | 38.44 | |
All directors who are not executive officers, as a group(1) | | | — | | | | — | |
| All employees who are not executive officers, as a group | | | 1,062,260 | | | $ | 32.93 | |
| |
| (1) | Directors who are not employees of the Company may not purchase shares under the 1991 Plan. |
Tax Aspects
Based on management’s understanding of current federal income tax laws, the tax consequences of the purchase of shares of common stock under the 1991 Plan are described below.
An employee will not have taxable income when the shares of common stock are purchased for him or her, but the employee generally will have taxable income when the employee sells or otherwise disposes of stock purchased through the 1991 Plan.
For shares that the employee does not dispose of until more than 24 months after the applicable enrollment date and more than 12 months after the purchase date (the “holding period”), gain up to the amount of the discount (if any) from the market price of the stock on the enrollment date (or re-enrollment date) is taxed as ordinary income. Any additional gain above that amount is taxed at long-term capital gain rates. If, after the holding period, the employee sells the stock for less than the purchase price, the difference is a long-term capital loss. Shares sold within the holding period are taxed at ordinary income rates on the amount of discount received from the stock’s market price on the purchase date. Any additional gain (or loss) is taxed to the employee as long-term or short-term capital gain (or loss). The purchase date begins the period for determining whether the gain (or loss) is short-term or long-term.
The Company generally may deduct for federal income tax purposes an amount equal to the ordinary income an employee must recognize when he or she disposes of stock purchased under the 1991 Plan within the holding period. The Company may not deduct any amount for shares disposed of after the holding period.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS
A VOTE FOR APPROVAL OF PROPOSAL 3.
20
EQUITY COMPENSATION PLANS
We show below information as of December 31, 2002 on equity compensation plans under which our common stock is authorized for issuance.
| | | | | | | | | | | | | | |
| | | | | | Number of securities remaining |
| | Number of securities to be | | Weighted-average | | available for future issuance |
| | issued upon exercise of | | exercise price of | | (excluding securities reflected |
| Plan category | | outstanding options | | outstanding options | | in first column)(1) |
| |
| |
| |
|
Plans approved by stockholders | | | | | | | | | | | | |
| | 1999 Stock Plan | | | 55,419,415 | | | | $38.37 | | | | 4,048,713 | |
| | 1991 Employee Stock Plan | | | (2) | | | | (2) | | | | 1,832,987 | |
Plans not approved by stockholders | | | NONE | | | | N/A | | | | N/A | |
| |
| (1) | The numbers in this column for each equity compensation plan exclude the number of shares for which Genentech is requesting stockholder approval under this Proxy Statement. |
| |
| (2) | Under the 1991 Employee Stock Plan, participants are permitted to purchase our common stock on certain dates within a pre-determined purchase period. Accordingly, these numbers are not determinable. |
BENEFICIAL OWNERSHIP
OF PRINCIPAL STOCKHOLDERS, DIRECTORS AND MANAGEMENT
We show below the number of shares of our common stock beneficially owned as of December 31, 2002 by (a) Roche, (b) each of the directors, (c) each of the Named Executive Officers and (d) by the directors and executive officers as a group.
In general, “beneficial ownership” refers to shares that an individual or entity has the power to vote, or dispose of, and stock options that are currently exercisable or will become exercisable within 60 days of December 31, 2002. Unless otherwise indicated, each person named below holds sole investment and voting power, other than the powers that may be shared with the person’s spouse under applicable law.
| | | | | | | | | |
| | |
| | Genentech Common Stock |
| |
|
| Name of Beneficial Owner | | Number of Shares | | Percent of Class |
| |
| |
|
Roche Holdings, Inc.(1) | | | 306,594,352 | | | | 59.79 | % |
| Herbert W. Boyer | | | 46,833 | (2) | | | * | |
| Susan D. Desmond-Hellmann | | | 1,005,713 | (3) | | | * | |
| Franz B. Humer | | | 0 | (4) | | | * | |
| Stephen G. Juelsgaard | | | 675,195 | (5) | | | * | |
| Jonathan K. C. Knowles | | | 0 | (4) | | | * | |
| Louis J. Lavigne, Jr. | | | 905,115 | (6) | | | * | |
| Arthur D. Levinson | | | 2,280,495 | (7) | | | * | |
| Myrtle S. Potter | | | 262,563 | (8) | | | * | |
| Sir Mark Richmond | | | 37,333 | (9) | | | * | |
| Charles A. Sanders | | | 38,333 | (10) | | | * | |
| Richard H. Scheller | | | 85,520 | (11) | | | * | |
| All directors and executive officers as a group (14 persons) | | | 5,830,451 | (12) | | | 1.12 | % |
| | |
| | * | Less than 1% of the outstanding shares of our common stock. |
21
| |
| (1) | The address for Roche is One Commerce Center, Suite 1050, 1201 N. Orange Street, Wilmington, Delaware, 19801. |
| |
| (2) | Includes stock options to purchase 21,083 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (3) | Includes stock options to purchase 1,003,833 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (4) | Pursuant to the affiliation agreement, Roche has appointed Dr. Humer and Dr. Knowles as its representatives on our Board of Directors. |
| |
| (5) | Includes stock options to purchase 673,205 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (6) | Includes stock options to purchase 903,125 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (7) | Includes stock options to purchase 2,277,852 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (8) | Includes stock options to purchase 260,937 shares of common stock that were exercisable on or within 60 days of December 31, 2002. |
| |
| (9) | Includes stock options to purchase 37,333 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (10) | Includes stock options to purchase 37,333 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (11) | Includes stock options to purchase 85,520 shares that were exercisable on or within 60 days of December 31, 2002. |
| |
| (12) | Includes stock options, to purchase 488,011 shares, held by three other executive officers, which were exercisable on or within 60 days of December 31, 2002. |
22
COMPENSATION OF EXECUTIVE OFFICERS
SUMMARY OF COMPENSATION
We show below the compensation paid to the Named Executive Officers, including salary, bonuses, stock options and other compensation for the fiscal years ended December 31, 2002, 2001 and 2000:
SUMMARY COMPENSATION TABLE
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Long Term | | |
| | Annual Compensation | | Compensation | | |
| | | | Awards | | |
|
|
| | Securities | | |
| Name and | | Other Annual | | Underlying | | All Other |
| Principal Position | | Year | | Salary(1) | | Bonus | | Compensation(3) | | Options(8)(#) | | Compensation(9) |
|
|
Arthur D. Levinson, Ph.D. | | | 2002 | | | | $811,200 | | | | $1,135,000 | | | | | | | | 450,000 | | | | $77,848 | |
| Chairman, President and | | | 2001 | | | | $780,000 | | | | $1,135,000 | | | | | | | | 360,000 | | | | $70,600 | |
| Chief Executive Officer | | | 2000 | | | | $755,010 | | | | $985,000 | | | | | | | | 360,000 | | | | $69,400 | |
| |
| Myrtle S. Potter(10) | | | 2002 | | | | $543,656 | | | | $450,000 | | | | $762,536(4 | ) | | | 250,000 | | | | $38,736 | |
| Executive Vice President, | | | 2001 | | | | $520,256 | | | | $425,000 | | | | $1,207,608(5 | ) | | | 225,000 | | | | $30,800 | |
| Commercial Operations and | | | 2000 | | | | $315,169 | | | | $500,000 | (2) | | | $789,284(6 | ) | | | 300,000 | | | | $10,000 | |
| Chief Operating Officer | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Susan D. Desmond-Hellmann, | | | 2002 | | | | $530,462 | | | | $450,000 | | | | | | | | 250,000 | | | | $38,200 | |
| M.D., M.P.H. | | | 2001 | | | | $500,491 | | | | $425,000 | | | | | | | | 225,000 | | | | $37,000 | |
| Executive Vice President, | | | 2000 | | | | $452,918 | | | | $425,000 | | | | | | | | 250,000 | | | | $34,000 | |
| Development and Product Operations and Chief Medical Officer | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Louis J. Lavigne, Jr. | | | 2002 | | | | $386,864 | | | | $325,000 | | | | | | | | 175,000 | | | | $27,864 | |
| Executive Vice President and | | | 2001 | | | | $379,282 | | | | $310,000 | | | | | | | | 160,000 | | | | $27,560 | |
| Chief Financial Officer | | | 2000 | | | | $372,709 | | | | $310,000 | | | | | | | | 160,000 | | | | $27,100 | |
| |
| Stephen G. Juelsgaard | | | 2002 | | | | $341,800 | | | | $275,000 | | | | | | | | 175,000 | | | | $22,672 | |
| Executive Vice President, | | | 2001 | | | | $322,400 | | | | $225,000 | | | | | | | | 160,000 | | | | $21,896 | |
| General Counsel and Secretary | | | 2000 | | | | $314,528 | | | | $225,000 | | | | | | | | 160,000 | | | | $21,400 | |
| |
| Richard H. Scheller, Ph.D.(11) | | | 2002 | | | | $311,489 | | | | $250,000 | | | | $56,247(7 | ) | | | 175,000 | | | | $19,422 | |
| Senior Vice President, Research | | | 2001 | | | | $229,404 | | | | $175,000 | | | | | | | | 190,000 | | | | $8,250 | |
| | | | 2000 | | | | — | | | | — | | | | | | | | — | | | | — | |
| |
| (1) | Includes amounts earned but deferred at the election of the executive officer, such as salary deferrals under our 401(k) Plan established under Section 401(k) of the Internal Revenue Code. |
| |
| (2) | Includes payment of a sign-on bonus of $250,000. |
| |
| (3) | Under Securities and Exchange Commission rules, the payment of “perquisites” (such as imputed interest on loans at rates below market value) to a Named Executive Officer is not required to be disclosed where amounts paid do not exceed the lesser of (i) 10% of the sum of the amounts of Salary and Bonus for the Named Executive Officer, or (ii) $50,000. Other than the payment of “perquisites” to Ms. Potter as described in footnotes (4), (5), (6) and to Dr. Scheller as described in footnote (7) below, no other Named Executive Officer received the payment of any “perquisite” from the Company. |
| |
| (4) | Includes (a) payment of $14,979 for tax preparation fees incurred by Ms. Potter with KPMG (including a gross-up payment of $7,079 for the estimated income taxes attributable to the payment), (b) imputed interest of $118,353 on Ms. Potter’s relocation home loan and a gross-up payment of $120,021 for the estimated income taxes attributable to the imputed interest, (c) imputed interest of $53,341 for 2000 and 2001 on the repayable portion of Ms. Potter’s relocation home loan, as an adjustment for the interest required to be imputed in those years, and a gross-up payment of $53,022 for the estimated income taxes attributable to the imputed interest, and (d) loan forgiveness of $200,000 on Ms. Potter’s relocation home loan and a gross-up payment of $202,820 for the estimated income taxes attributable to the loan forgiveness. |
| |
| (5) | Includes (a) payment of $371,325 for the value of Bristol-Myers Squibb stock options stock surrendered by Ms. Potter upon her resignation of employment with Bristol-Myers Squibb, (b) a relocation benefit valued at $67,789 (including a gross-up payment of $34,132 for the estimated income taxes attributable to this relocation benefit), (c) an payment of $171,426 for an adjustment to the taxes payable by Ms. Potter for the relocation benefit she received from Genentech in 2000 (including a |
23
| |
| gross-up payment of $59,913 for the estimated income taxes attributable to the adjustment), (d) imputed interest of $194,248 on Ms. Potter’s relocation home loan (including a gross-up payment of $59,354 for the estimated income taxes attributable to the imputed interest), and (e) loan forgiveness of $200,000 on Ms. Potter’s relocation home loan and a gross-up payment of $202,820 for the estimated income taxes attributable to the loan forgiveness. |
| |
| (6) | Includes (a) a relocation benefit valued at $424,553 (including a gross-up payment of $147,885 for the estimated income taxes attributable to the relocation benefit), (b) imputed interest of $71,610 on Ms. Potter’s relocation home loan, (c) a gross-up payment of $33,008 for the estimated income taxes attributable to the imputed interest on the forgivable portion of the home loan, and (d) a gross-up payment of $260,113 for the estimated income taxes attributable to the sign-on bonus disclosed in footnote (2) above. |
| |
| (7) | Includes (a) imputed interest of $6,870 on Dr. Scheller’s mortgage assistance loan and a gross-up payment of $3,610 for the estimated income taxes attributable to the imputed interest and (b) loan forgiveness of $30,000 on Dr. Scheller’s mortgage assistance loan and a gross-up payment of $15,767 for the estimated income taxes attributable to the loan forgiveness. |
| |
| (8) | Genentech has awarded no stock appreciation rights (“SARs”). |
| |
| (9) | Consists of Genentech’s matching payments under its 401(k) Plan and Supplemental Plan for 2002, 2001 and 2000. Each of the Named Executive Officers received $8,000 in matching payments under the 401(k) Plan for 2002 and $6,800 for 2001 and each of the Named Executive Officers other than Ms. Potter and Dr. Scheller received $6,800 in matching payments under the 401(k) Plan for 2000. Under the Supplemental Plan, Dr. Levinson, Dr. Desmond-Hellmann, Mr. Lavigne, Ms. Potter, Mr. Juelsgaard and Dr. Scheller received matching payments of $69,848, $30,200, $19,864, $30,736, $14,672 and $11,422, respectively for 2002 and $63,800, $30,200, $20,760, $24,000, $15,096 and $8,250, respectively, for 2001. Dr. Levinson, Dr. Desmond-Hellmann, Mr. Lavigne, Ms. Potter and Mr. Juelsgaard received matching payments of $62,600, $27,200, $20,300, $10,000 and $14,600, respectively, for 2000. |
| |
| (10) | Ms. Potter began her employment with Genentech effective May 15, 2000. |
| |
| (11) | Dr. Scheller began his employment with Genentech effective March 1, 2001. |
24
STOCK OPTION GRANTS AND EXERCISES
OPTION GRANTS IN LAST FISCAL YEAR
We show below information on stock option grants made to the Named Executive Officers for the fiscal year ended December 31, 2002.
| | | | | | | | | | | | | | | | | | | | | |
| | |
| | Individual Grants |
| | |
| |
|
| | Number of | | Percent of Total | | | | Grant Date |
| | Securities | | Options | | | | Present |
| | Underlying | | Granted to | | Exercise or | | | | Value(4) |
| | Options Granted | | Employees in | | Base Price | | Expiration | | (in |
| Name | | (#)(1) | | Fiscal Year(2) | | ($/SH)(3) | | Date | | millions) |
|
|
Arthur D. Levinson | | | 450,000 | | | | 3.56 | % | | | $28.55 | | | | 09/12/12 | | | | $5.2 | |
Myrtle S. Potter | | | 250,000 | | | | 1.98 | % | | | $28.55 | | | | 09/12/12 | | | | $2.9 | |
Susan D. Desmond-Hellmann | | | 250,000 | | | | 1.98 | % | | | $28.55 | | | | 09/12/12 | | | | $2.9 | |
Louis J. Lavigne, Jr. | | | 175,000 | | | | 1.39 | % | | | $28.55 | | | | 09/12/12 | | | | $2.0 | |
Stephen G. Juelsgaard | | | 175,000 | | | | 1.39 | % | | | $28.55 | | | | 09/12/12 | | | | $2.0 | |
Richard H. Scheller | | | 175,000 | | | | 1.39 | % | | | $28.55 | | | | 09/12/12 | | | | $2.0 | |
| |
| (1) | The options were granted pursuant to the 1999 Plan and vest over four years, with the first 25% vesting one year from the grant date, and the remainder vesting on a monthly basis in equal increments during the 36-month period following the initial vesting date, assuming continued employment with the Company. |
| |
| (2) | Based on a total of approximately 13 million options granted in 2002 under our 1999 Plan to employees, including the Named Executive Officers. Approximately 88% of these options were granted to approximately 4,900 employees, other than the Named Executive Officers, representing more than 93% of the eligible employee population. |
| |
| (3) | Represented the fair market value of the underlying shares of common stock and based on the closing price of our common stock on the date of grant. |
| |
| (4) | Option value was determined using the Black-Scholes option pricing model based on the following assumptions: expected volatility of 43% based on historical volatility for the year and implied volatility from traded options and a risk free rate of 2.6% for the vesting term of the option. Each option is valued at its exercise price, which is assumed to be equivalent to the market price at the date of grant. This valuation model was not adjusted for the vesting restrictions or the risk of forfeiture of the options. Under SFAS 123, forfeitures may be estimated or assumed to be zero; in this model, the forfeiture rate was assumed to be zero. Our use of this model in accordance with rules adopted by the Securities and Exchange Commission does not constitute an endorsement of the model nor an acknowledgment that such model can accurately determine the value of options. The valuation calculations do not necessarily represent the fair market value of individual options, and are not intended to forecast possible future appreciation, if any, of the price of our common stock on the date of exercise as compared to the exercise price of the option. |
25
AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR
AND FY-END OPTION VALUES(1)
We show below information regarding stock options held by the Named Executive Officers as of December 31, 2002.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| | | | | | Number of Securities | | Value of Unexercised |
| | | | | | Underlying Unexercised | | in-the Money |
| | Shares | | | | Options at FY-end(#) | | Options at FY-end(2) |
| | Acquired on | | Value | | | | | | | | |
| Name | | Exercise(#) | | Realized | | Exercisable | | Unexercisable | | Exercisable | | Unexercisable |
|
|
Arthur D. Levinson | | | 0 | | | | $0 | | | | 2,247,852 | | | | 855,000 | | | | $17,221,711 | | | | $2,074,500 | |
Myrtle S. Potter | | | 0 | | | | $0 | | | | 239,062 | | | | 535,938 | | | | $0 | | | | $1,152,500 | |
Susan D. Desmond-Hellmann | | | 0 | | | | $0 | | | | 984,041 | | | | 514,063 | | | | $6,888,357 | | | | $1,152,500 | |
Louis J. Lavigne, Jr. | | | 0 | | | | $0 | | | | 889,792 | | | | 355,000 | | | | $6,680,647 | | | | $806,750 | |
Stephen G. Juelsgaard | | | 0 | | | | $0 | | | | 659,872 | | | | 355,000 | | | | $4,632,060 | | | | $806,750 | |
Richard H. Scheller | | | 0 | | | | $0 | | | | 77,604 | | | | 287,396 | | | | $0 | | | | $806,750 | |
| |
| (1) | Genentech has awarded no SARs. |
| |
| (2) | The value of the unexercised in-the-money options is based on the fair market value of our common stock ($33.16) at the close of business on December 31, 2002, minus the exercise price of the options. |
CHANGE IN CONTROL AGREEMENTS
Genentech entered into a Change of Control Agreement (the “Agreement”) on January 20, 2001 with Ms. Myrtle Potter, Executive Vice President, Commercial Operations and Chief Operating Officer. The Agreement is effective through the earlier of May 15, 2005 or 24 months following a Change of Control (as that term is defined in the Agreement). The Agreement generally provides that if Ms. Potter’s employment with Genentech is terminated following a Change of Control (i) by us, except for Cause or (ii) by Ms. Potter with Good Reason (as these terms are defined in the Agreement), we will pay Ms. Potter a lump sum severance payment equal to two times the sum of (i) Ms. Potter’s annual base salary and (ii) Ms. Potter’s average annual bonus and provide 24 months of health care and other insurance coverage. If the foregoing severance payments are subject to excise tax, then we will pay Ms. Potter an additional amount to cover the tax.
LOANS AND OTHER COMPENSATION
For purposes of the discussion below, applicable federal rate refers to the minimum interest rate required to be charged on a loan to avoid the imputation of interest income under the Internal Revenue Code, unless an exception applies. The Internal Revenue Service publishes the applicable federal rate on a monthly basis.
In 2000, Genentech lent $2,200,000 to Ms. Myrtle Potter for the purchase of a home in connection with her relocation to the San Francisco Bay Area. $1,000,000 of the loan is due and payable on the earlier of May 15, 2005 or within 30 days from the date of Ms. Potter’s termination of employment with Genentech. $1,000,000 of the loan will be forgiven in equal installments of $200,000 each on May 15, 2001 through 2005, if Ms. Potter is employed by Genentech on these dates. Genentech has agreed to pay to Ms. Potter the amount equal to the federal and state income taxes payable in connection with the forgiveness of the repayment of each installment. The remaining $200,000 of the loan shall be due and payable in four equal installments of $50,000 on each of the dates Ms. Potter receives her annual performance bonus from Genentech. The largest amount outstanding under this loan during 2002 was
26
$1,950,000. The amount of this loan outstanding as of December 31, 2002 was $1,700,000.
The loan is interest-free to Ms. Potter but interest is required to be imputed under the Internal Revenue Code. Imputed interest in the amount of $73,056 for the repayable portion of the loan and $45,297 for the forgivable portion of the loan were each calculated using the applicable federal rate of 6.51% and reported as taxable compensation to Ms. Potter. Additional taxable compensation attributable to imputed interest was grossed up for related taxes resulting in a tax payment by Genentech on behalf of Ms. Potter of $74,089 and $45,932 with respect to the repayable portion and forgivable portion, respectively, of the loan. In addition, imputed interest in the total amount of $53,341 attributable to the repayable portion of the loan for the years 2000 and 2001 were calculated using the applicable federal rate, reported as taxable compensation to Ms. Potter, and grossed up for the related taxes resulting in a tax payment by Genentech of $53,022 on behalf of Ms. Potter.
In 2001, Genentech lent $120,00 to Mr. David Ebersman, Senior Vice President, Product Operations, for the purchase of a home in connection with his relocation to San Mateo County. This loan is due and payable on the earlier of the fifth anniversary of the date of the loan, the date of termination of Mr. Ebersman’s employment with Genentech or sale of his residence. The largest amount outstanding under this loan during 2002, and the amount of this loan outstanding as of December 31, 2002, was $120,000. The loan is interest-free to Mr. Ebersman, but interest is required to be imputed under the Internal Revenue Code. Imputed interest in the amount of $6,026 was calculated based on the applicable federal rate of 4.96% and reported as taxable compensation to Mr. Ebersman.
In 2001, Genentech lent $150,000 to Dr. Richard Scheller, Senior Vice President, Research, as mortgage assistance. This loan is due and payable on the earlier of the fifth anniversary of the date of the loan, the date of termination of Dr. Scheller’s employment with Genentech or sale of his residence; provided, however, that the principal amount of the loan will be forgiven in five equal installments of $30,000 on each anniversary date of the loan if Dr. Scheller is employed by Genentech on such dates. The largest amount outstanding under this loan during 2002 was $150,000. The amount of this loan outstanding as of December 31, 2002 was $120,000. The loan is interest-free to Dr. Scheller, but interest is required to be imputed under the Internal Revenue Code. Imputed interest in the amount of $6,870 was calculated based on the applicable federal rate of 4.93% and, together with the forgiven principal amount of $30,000 on the first anniversary of the loan, reported as taxable compensation to Dr. Scheller. Additional taxable compensation attributable to the imputed interest and the forgiven principal amount was grossed up for related taxes resulting in a tax payment by Genentech of $3,610 and $15,767, respectively, on behalf of Dr. Scheller.
27
COMPENSATION COMMITTEE REPORT(1)
The Compensation Committee of the Board of Directors (the “Committee”) is comprised of all the directors, except Dr. Levinson. The Committee is responsible for establishing and administering the policies that govern executive officer salaries, bonuses and stock option programs. The Committee annually evaluates the performance, and determines the compensation of our executive officers based upon individual performance, comparisons with other pharmaceutical and biotechnology companies and the achievement of our corporate goals. In determining the compensation of our Chief Executive Officer (the “CEO”), the Committee uses the same criteria and procedures described below for determining the compensation of our other executive officers, unless otherwise noted.
Compensation Philosophy
The Committee’s philosophy with respect to our executive officers, including the CEO, is to provide compensation sufficient to attract, motivate and retain executives of outstanding ability and potential and to establish an appropriate relationship between executive compensation and the creation of stockholder value. To meet these goals, the Committee has adopted a mix among the compensation elements of base salary, bonus and stock options, with some bias toward stock options, to emphasize the link between executive incentives and the creation of stockholder value as measured by the equity markets.
Base Salary
The Committee determines executive officer base salaries after considering a survey of base salaries at comparable pharmaceutical and biotechnology companies, as well as companies in other industries when comparisons are appropriate to specific executive officer positions. The survey data is compiled by an independent compensation specialist who reviews base salaries at comparable pharmaceutical and biotechnology companies as well as those at any additional companies we select. The survey group of comparable companies is broader than the pharmaceutical and biotechnology companies included in the Standard & Poor’s 500 Pharmaceuticals Index and the Standard & Poor’s 500 Biotechnology Index shown on our Performance Graph. Based upon such surveys, the executive officers’ base salary is generally targeted to be competitive with the salaries of comparable executive officers at other comparable biotechnology and pharmaceutical companies.
Stock Option Grants
In awarding stock options, the Committee considers officer retention, individual performance, overall contribution to Genentech, the total number of stock options to be awarded and an analysis of stock option awards granted by a peer group of biotechnology and pharmaceutical companies. The peer group of biotechnology and pharmaceutical companies consists of 12 companies, half with a market capitalization above, and half with a market capitalization below, that of Genentech. A number of these companies are included in the Standard & Poor’s 500 Pharmaceuticals Index and the Standard & Poor’s 500 Biotechnology Index shown on our Performance Graph. The Committee uses a multi-step process to determine executive officer grants. It first calculates the number of shares in our overall option pool by analyzing the relationship between the number of shares in a peer group company’s stock option pool (as a percentage of outstanding shares) and the company’s market capitalization. Based on this analysis, the Committee derives an appropriate percentage of outstanding shares, based on our market capitalization for the overall option pool. The Committee then reviews the median and the average percentages of the stock option pool granted to comparable executive
| |
| (1) | The material in this report and under the caption “Performance Graph” are not “soliciting material,” are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference in any filing of the Company under the Securities Act of 1933 or the Securities Exchange Act of 1934, whether made before or after the date of this Proxy Statement and irrespective of any general incorporation language in those filings. |
28
officers at the peer group companies and chooses a mid-range of percentages for each of the executive officer positions, including that of the CEO, within that range. A given executive officer’s stock option award is then determined by the Committee, based on its subjective evaluation of factors such as the retention value of the options to be granted, the individual’s performance and the success of Genentech as measured by earnings per share, product sales revenues, product development progress, research prospects, and other critical success factors.
Bonuses
Bonuses are provided to our executive officers in connection with our company-wide bonus plan. Payment of bonuses is expressly linked to successful achievement of specified corporate goals that the Committee sets annually at its December meeting for the following fiscal year. Among other things, these goals determine if a bonus will be paid to all eligible employees and the amount of funds available for the bonus pool. For fiscal year 2002, the corporate performance goals, in order of importance, were as follows:
| |
| (i) | Business and financial performance, including increasing earnings per share, meeting specific productivity goals and managing operational budgets within specified targets; |
| |
| (ii) | Commercial performance, including increasing U.S. product sales revenues, increasing margins resulting from the sale of our products, forming strategic alliances by in-licensing products and establishing relationships/ partnerships with other companies; |
| |
| (iii) | Research and development and product manufacturing performance, including filing a BLA, initiating or completing specified clinical trials investigating the use of new products, selecting new products for development; and |
| |
| (iv) | Employee development performance, with a focus on enhancing career development and employee productivity. |
In setting these goals, the Committee is aware of the long development cycle for biotherapeutics. The Committee’s selection of corporate performance goals for bonuses seeks to balance the desire for immediate earnings and the longer term goal of enhancing stockholder value by bringing to market many of the potential therapies in our research and development pipeline.
In February 2003, the Committee reviewed the corporate performance goals for bonuses and determined that approximately $33 million under the 2002 Bonus Plan were achieved. The Committee allocated a bonus to our CEO based on the achievement of the requisite corporate goals discussed above and based on the Committee’s view that our CEO’s performance in achieving those goals had been excellent. The Committee set a range of 115-145% of salary for a bonus for the CEO and 70-85% of salary for bonuses for members of the Company’s management Executive Committee other than the CEO (Drs. Desmond-Hellmann and Scheller, Ms. Potter and Messrs. Juelsgaard and Lavigne). Within the applicable range, the Committee determined the bonus for each management Executive Committee member based on the Committee’s subjective evaluation of the individual’s performance and the recommendation of the CEO.
Deductibility of Executive Compensation
Section 162(m) of the Internal Revenue Code limits the federal income tax deductibility of compensation paid to Genentech’s five most highly compensated executive officers. Under Section 162(m), Genentech generally may deduct compensation paid to such an officer only to the extent that it does not exceed $1 million during any calendar year or is “performance-based” as defined in Section 162(m). Genentech expects that the deductibility limit of Section 162(m) currently will have an insignificant effect on Genentech. Current cash compensation paid to each of Genentech’s executives, with one exception, is less than $1 million per year and is thus generally fully deductible to the Company. In addition, in April 2002, Genentech obtained
29
stockholder approval of the 1999 Stock Plan for purposes of complying with Section 162(m) of the Internal Revenue Code in connection with the exercise of stock options by the five most highly compensated officers of the Company for compensation in an amount exceeding $1 million.
From the members of the Compensation Committee of Genentech:
| |
| | Herbert W. Boyer |
| | Franz B. Humer |
| | Jonathan K.C. Knowles |
| | Sir Mark Richmond |
| | Charles A. Sanders |
30
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
During 2002, Genentech’s Compensation Committee consisted of Drs. Boyer, Humer, Knowles, Richmond and Sanders.
Dr. Boyer, a founder of the Company, was a Vice President of Genentech from 1976 to 1991.
Dr. Humer joined The Roche Group in 1995 as the Head of its Pharmaceuticals Division and is currently the Chairman and Chief Executive Officer of The Roche Group. He is also Chairman of the Executive Committee of The Roche Group.
Dr. Knowles joined The Roche Group in 1997 as Head of Global Pharmaceuticals Research. He is a member of the Executive Committee of The Roche Group.
Pursuant to the terms of the affiliation agreement, Drs. Humer and Knowles are serving on Genentech’s Compensation Committee as designees of Roche. See “Relationship with Roche” above and “Certain Relationships and Related Transactions” below for a description of our relationship with Roche.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Herceptin Licensing Agreement
We have an agreement with Hoffmann-La Roche, providing them with exclusive marketing rights outside of the United States for Herceptin. Under the agreement, Hoffmann-La Roche paid us $40 million and has agreed to pay cash milestones tied to future product development activities, to contribute equally with us on global development costs up to a maximum of $40 million. Hoffmann-La Roche has also agreed to make royalty payments of 20% on aggregate net product sales outside the United States up to $500 million in each calendar year and 22.5% on such sales in excess of $500 million in each calendar year.
Amended and Restated Licensing Agreement
We have an amended and restated licensing agreement with Hoffmann-La Roche for licenses to use and sell some of our products in non-U.S. markets as described under the heading “— Licensing and Marketing Arrangements” under the section “Relationship with Roche.” See also “Relationship with Roche” above.
Contract revenue from Hoffmann-La Roche and their affiliates totaled $7.6 million in 2002. Other revenue, comprised principally of royalties on their sales of Genentech’s licensed products and sales of products outside the United States, from Roche, Hoffmann-La Roche and their affiliates, totaled $269.9 million in 2002.
31
PERFORMANCE GRAPH
We show below the cumulative total return to our stockholders during the period from July 20, 1999 through December 31, 2002 in comparison to the cumulative return on the Standard & Poor’s 500 Index, the Standard & Poor’s 500 Pharmaceuticals Index and the Standard & Poor’s 500 Biotechnology Index during that same period. The results assume that $100 was invested on July 20, 1999, that date when shares of our common stock were first offered to the public after all of our special common stock held by stockholders other than Roche was redeemed, and that dividends were reinvested.(1) See “Relationship with Roche” above for more information on the redemption and public offering of our common stock.
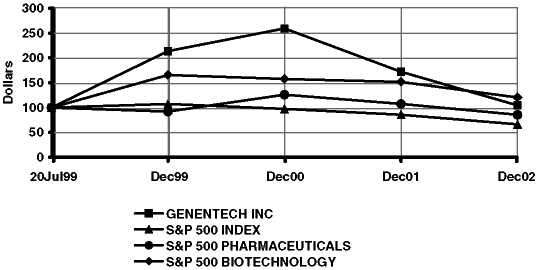
| | | | | | | | | | | | | | | | | | | | | |
|
|
| | Base Period | | December | | December | | December | | December |
| | 20 July 99 | | 99 | | 00 | | 01 | | 02 |
|
|
Genentech, Inc. | | | 100.00 | | | | 211.81 | | | | 256.69 | | | | 170.87 | | | | 104.44 | |
S&P 500 Index | | | 100.00 | | | | 107.27 | | | | 97.50 | | | | 85.92 | | | | 66.93 | |
S&P 500 Pharmaceuticals | | | 100.00 | | | | 92.15 | | | | 125.61 | | | | 107.35 | | | | 85.83 | |
S&P 500 Biotechnology | | | 100.00 | | | | 164.55 | | | | 156.68 | | | | 150.84 | | | | 120.04 | |
| |
| (1) | The total return on investment (change in year end stock price plus reinvested dividends) assumes $100 invested on July 20, 1999 in Genentech, the Standard & Poor’s 500 Index, the Standard & Poor’s 500 Pharmaceuticals Index and the Standard & Poor’s 500 Biotechnology Index. The Standard & Poor’s 500 Pharmaceuticals Index was comprised at December 31, 2002 of Abbott Laboratories, Allergan, Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, Forest Laboratories, Inc., Johnson & Johnson, King Pharmaceuticals, Inc., Merck & Co., Inc., Pfizer Inc., Pharmacia Corporation, Schering-Plough Corporation, Watson Pharmaceuticals, Inc. and WYETH. The Standard & Poor’s 500 Biotechnology Index was comprised at December 31, 2002 of Amgen Inc., Biogen, Inc., Chiron Corporation, Genzyme Corporation and MedImmune, Inc. |
32
AUDIT COMMITTEE REPORT
The Audit Committee of the Board of Directors currently consists of Drs. Boyer, Richmond and Sanders, and operates under a formal written charter. The Audit Committee meets regularly with management, the independent auditors and the internal auditors, both jointly and separately, recommends the independent auditors to the Board, and reviews our financial reporting process on behalf of the Board.
The Audit Committee has prepared the following report on its activities with respect to our audited financial statements for the year ended December 31, 2002.
Our management is responsible for the preparation, presentation and integrity of our financial statements. Management is also responsible for maintaining appropriate accounting and financial reporting practices and policies as well as internal controls and procedures designed to provide reasonable assurance that the Company is in compliance with accounting standards and applicable laws and regulations.
The independent auditors are responsible for planning and performing an independent audit of our financial statements in accordance with auditing standards generally accepted in the United States. The independent auditors are responsible for expressing an opinion on the conformity of those audited financial statements with accounting principles generally accepted in the United States.
In this context, the Audit Committee has reviewed and discussed the audited financial statements for the year ended December 31, 2002 with management and the independent auditors, Ernst & Young LLP. The Audit Committee has also discussed with the independent auditors the matters required to be discussed by Statement on Auditing Standards No. 61 (Communications with Audit Committees), as amended by Statement on Auditing Standards No. 90 (Audit Committee Communications).
Ernst & Young LLP has provided the Audit Committee with the written disclosures and letter required by Independence Standards Board Standard No. 1 (Independence Discussions with Audit Committees), and the Audit Committee discussed with the independent auditors that firm’s independence.
Based on the reviews and discussions referred to above, the Audit Committee recommended to the Board of Directors that the audited financial statements referred to above be included in Genentech’s Annual Report on Form 10-K for the year ended December 31, 2002.
Each member of the Audit Committee is independent as defined under the listing standards of the New York Stock Exchange.
From the members of the Audit Committee of Genentech:
Herbert W. Boyer
Sir Mark Richmond
Charles A. Sanders
33
PROPOSAL 4 — RATIFICATION OF SELECTION OF INDEPENDENT AUDITORS
The Board of Directors has selected Ernst & Young LLP as Genentech’s independent auditors for the year ending December 31, 2003 and has directed management to submit the selection of Ernst & Young LLP for ratification by the stockholders at the annual meeting.
Ernst & Young LLP has audited Genentech’s financial statements since Genentech’s inception in 1976. Representatives of Ernst & Young LLP will be present at the annual meeting, will have the opportunity to make a statement if they desire to do so and will be available to respond to questions from stockholders.
Stockholder ratification of Ernst & Young LLP as Genentech’s independent auditors is not required by the bylaws or otherwise. The Board of Directors is seeking such ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection of Ernst & Young LLP as independent auditors, the Board of Directors will consider whether to retain that firm for the year ended December 31, 2003.
A majority of the shares present in person or by proxy and entitled to vote at the annual meeting is required for approval of this proposal.
The aggregate fees billed by Ernst & Young LLP to Genentech for fiscal year 2002 for the professional services described below are as follows:
Audit Feesfor the audit of our annual consolidated financial statements for fiscal 2002 and review of the consolidated financial statements included in our Form 10-Q’s for fiscal 2002 were $648,861.
Audit-Related Feesfor services related to the performance of the year-end audit and quarterly review of the financial statements and the audit of our employee benefit plan were $268,044.
Financial Information Systems Design and Implementation Feeswere not incurred.
Tax Feesfor services relating to tax compliance, tax advice and tax planning were $238,826.
All Other Feesfor professional services rendered to us for audit-related consultations and internal audit services for special projects were $218,567.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS
A VOTE FOR APPROVAL OF PROPOSAL 4.
34
PROXY STATEMENT PROPOSALS
Stockholder proposals must be received by our Corporate Secretary no later than November 12, 2003 to be included in the 2004 annual meeting proxy materials.
SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities and Exchange Act requires our directors, officers and those persons owning more than 10% of our equity securities to file reports of holdings and transactions in our equity securities with the Securities and Exchange Commission. Copies of these reports are required to be furnished to us. We believe that all Forms 3, 4 and 5 required to be filed were filed on time during 2002.
OTHER MATTERS
The Board of Directors knows of no other business to be presented at the annual meeting, but if other matters do properly come before the annual meeting, it is intended that the persons named on the proxy card will vote on those matters in accordance with their best judgment.
35
0724-PS-03
DETACH HERE IF YOU ARE RETURNING YOUR PROXY CARD BY MAIL
PROXY
GENENTECH, INC.
PROXY SOLICITED BY THE BOARD OF DIRECTORS
FOR THE ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON APRIL 23, 2003
The undersigned appoints Stephen G. Juelsgaard and Arthur D. Levinson, and each of them, as proxies of the undersigned, each with full power of substitution, to vote all of the shares of Common Stock of Genentech, Inc. (“Genentech”) held of record by the undersigned as of February 24, 2003 at the Annual Meeting of Stockholders of Genentech to be held at the Westin Hotel, 1 Old Bayshore Highway, Millbrae, California on Wednesday, April 23, 2003, commencing at 10:00 a.m., local time, and at any adjournment or postponement of the Annual Meeting, with all powers that the undersigned would possess if personally present, upon and in respect of the following matters and in accordance with the following instructions.
FOR 401(K) PLAN PARTICIPANTS
THE SHARES CREDITED TO YOUR ACCOUNT WILL BE VOTED AS DIRECTED. IF NO DIRECTION IS MADE, IF THE CARD IS NOT SIGNED, OR IF THE CARD IS NOT RECEIVED BY APRIL 18, 2003, THE SHARES CREDITED TO YOUR ACCOUNT WILL NOT BE VOTED.
FOR REGISTERED STOCKHOLDERS
IF NO OTHER INDICATION IS MADE ON THE REVERSE SIDE OF THIS FORM, THIS PROXY WILL BE VOTED FOR ALL NOMINEES FOR DIRECTOR LISTED IN PROPOSAL 1 AND FOR PROPOSALS 2, 3 AND 4 AS MORE SPECIFICALLY DESCRIBED IN THE PROXY STATEMENT AND IN THE DISCRETION OF THE PERSONS NAMED ABOVE IN ANY OTHER MATTER WHICH MAY PROPERLY COME BEFORE THE ANNUAL MEETING. IF SPECIFIC INSTRUCTIONS ARE INDICATED, THIS PROXY WILL BE VOTED IN ACCORDANCE WITH THOSE INSTRUCTIONS.
YOU MAY REVOKE THIS PROXY AT ANY TIME PRIOR TO THE VOTE AT THE ANNUAL MEETING.
| | | | | |
SEE REVERSE
SIDE | | PLEASE COMPLETE, DATE AND SIGN THIS PROXY AND
RETURN IT IN THE ACCOMPANYING ENVELOPE. | | SEE REVERSE
SIDE |
GENENTECH, INC.
C/O EQUISERVE TRUST COMPANY N.A.
P.O. BOX 8694
EDISON, NJ 08818-8694
Your telephone or Internet vote authorizes the named proxies to vote your shares in the same manner as if you marked, signed and returned your proxy card by mail. Please note that all 401(k) Plan Participant votes cast via telephone or the Internet must be cast prior to 10:00 p.m., Pacific Daylight Time, on Friday, April 18, 2003. Please note that all registered stockholder votes cast via telephone or the Internet must be cast prior to 10:00 p.m., Pacific Daylight Time, on Tuesday, April 22, 2003. If you wish to change or revoke your vote you may re-vote via telephone or the Internet, or return your properly completed proxy card; your latest vote received prior to the deadline will override each of your previous votes. If you wish to change your address, please mark the box below and return your proxy card by mail.
Voter Control Number
Your vote is important. Please vote immediately.
| | | | | |
Vote-by-Internet 
1. Log on to the Internet and go to
http://www.eproxyvote.com/dna.
2. Enter your Voter Control Number listed
above and follow the easy steps outlined
on the secured website. | | OR | | Vote-by-Telephone 
1. Call toll-free 1-877-PRX-VOTE
(1-877-779-8683).
2. Enter your Voter Control Number listed
above and follow the easy recorded
instructions. |
If you vote over the Internet or by telephone, please do not mail your card.
DETACH HERE IF YOU ARE RETURNING YOUR PROXY CARD BY MAIL
| | | |
| x | | Please mark
votes as in
this example |
THE BOARD OF DIRECTORS OF GENENTECH, INC. RECOMMENDS A VOTE FOR THE NOMINEES FOR DIRECTOR LISTED BELOW AND A VOTE FOR PROPOSALS 2, 3 AND 4.
| | | 1. To elect six directors to hold office until the 2004 Annual Meeting of Stockholders. |
| |
| | Nominees:(01) Herbert W. Boyer, (02) Franz B. Humer,
(03) Jonathan K.C. Knowles, (04) Arthur D. Levinson,
(05) Mark Richmond and (06) Charles A. Sanders. |
| | | | | | | |
FOR
ALL
NOMINEES | | o | | o | | WITHHELD
FROM ALL
NOMINEES |
| | | |
| o | |
|
| | | For all nominees except as noted above |
| | | | | | | | | | | | | | | | | |
| | | | | | | FOR | | AGAINST | | ABSTAIN |
| 2 | | To approve the amended and restated 1999 Stock | | | o | | | | o | | | | o | |
| | | Plan. | | | | | | | | | | | | |
| 3 | | To approve an amendment to the amended 1991 | | | o | | | | o | | | | o | |
| | | Employee Stock Plan. | | | | | | | | | | | | |
| 4 | | To ratify the selection of Ernst & Young LLP as independent | | | | | | | | | | | | |
| | | auditors of Genentech for the year
ending December 31, 2003. | | | o | | | | o | | | | o | |
| | | | | | | | | | | | | | | |
| 5. | | By my signature below, I confer to the named proxies discretionary authority on any other business that may properly come before the Annual Meeting or any adjournment or postponement of the Annual Meeting. |
| | | |
| MARK HERE FOR ADDRESS CHANGE AND NOTE AT LEFT | | o |
| MARK HERE IF YOU PLAN TO ATTEND THE MEETING | | o |
Please sign exactly as name appears on this card. When signing as attorney, executor, administrator, trustee or guardian, please give full title. If more than one trustee, all should sign. All joint owners must sign.
| | | | | | | |
| Signature: _________________________ | | Date: _________________ | | Signature: _________________________ | | Date: _________________ |
We would like your opinion...
We would like your feedback on this year’s summary annual report.
Please take a moment to fill in the questionnaire below to help us plan for future reports.
Genentech 2002 Summary Annual Report Questionnaire:
| | | | | | | |
| 1. | | Does this report enhance your understanding or perception of Genentech? |
| | | | oYes oSomewhat oNo |
| 2. | | Did this report increase your understanding of the biotechnology industry? |
| | | | oYes oSomewhat oNo |
| 3. | | Did this report enhance your view of Genentech as an industry leader? |
| | | | oYes oSomewhat oNo |
| 4. | | How do you feel Genentech compares to other biotech companies after reading this report? |
| | | | oVery Favorably | | oFavorably | | oUnfavorably |
| 5. | | Is the financial information presented in a clear and understandable format? |
| | | | oYes oSomewhat oNo |
| |
| 6. | | Is there any additional financial information you would like included in the summary annual report? |
| | |
| |
|
| 7. | | Is this document useful to you as an investor? Please list any modifications that might increase its usefulness. |
| | |
| |
|
| 8. | | General Comments: |
| | |
| |
|
| | |
| |
|
| | |
| | SKU#0724-SC-03 Thank you for your assistance. |



