
PreSERVE-AMI: A Randomized, Double-Blind, Placebo Controlled Clinical Trial of Intracoronary Infusion of Autologous CD34+ Cells (NBS10) in Patients with Left Ventricular Dysfunction Post STEMI Arshed Quyyumi1, Dean Kereiakes2, David Shavelle3, Timothy Henry4, Ali Denktas5, Ahmed Abdel-Latif6, Catalin Toma7, Gregory Barsness8, Stephen Frohwein9, Richard Schatz10, Martin Cohen11, Charles Davidson12, Nabil Dib13, Marc Klapholz14, Gary Schaer15, Alejandro Vasquez16, Andrew Pecora17, Thomas Moss17, Pamela Hyde17, Anna Maria Kanakaraj17, Le Dich17, Vitaly Druker17, Candice Junge17, Robert Preti17, Douglas Losordo17 1Emory Clinical Cardiovascular Research Institute, Cardiology Division, Emory University School of Medicine, Atlanta, GA 2The Christ Hospital Heart and Vascular Center, Cincinnati, OH 3University of Southern California, Los Angeles, CA 4Cedars-Sinai Heart Institute, Los Angeles, CA 5Baylor College of Medicine/Michael E Debakey VA Medical Center, Houston, TX 6University of Kentucky, Lexington, KY 7University of Pittsburgh Medical Center, Pittsburgh, PA 8University of Pittsburgh Medical Center, Pittsburgh, PA 9Emory St. Josephs Hospital, Atlanta, GA 10Scripps Health, La Jolla, CA 11Westchester Medical Center, Valhalla, NY 12Bluhm Cardiovascular Institute Northwestern Memorial Hospital, Chicago, IL 13Heart Sciences Center, Gilbert, AZ 14Rutgers University, New Jersey Medical School, Newark, NJ 15Rush University Medical Center, Chicago, IL 16Huntsville Hospital, Huntsville, AL 17Neostem Inc., New York, NY www.clinicaltrials.gov Identifier: NCT01495364

The PreSERVE AMI Study: Funding Sources & Disclosures Conflict of Interest Disclosures • Quyyumi: NeoStem Advisory Board member Funding Source • Study funded by NeoStem, Inc. 2

Background • Recent meta-analysis of unselected bone marrow mononuclear cell therapy after STEMI suggest that there may be no impact on LVEF changes and MACE1 • Clinical studies evaluating the therapeutic potential of cells selected for CD34 expression have demonstrated a consistent favorable impact on outcomes2-5 • Phase 1 study of autologous CD34+ cells (NBS10) provided initial evidence of feasibility and safety and suggested a threshold dose of 10 million CD34 cells for bioactivity6 3 1. Gyongyosi M et al. Circ Res 2015: CIRCRESAHA.114.304346. 2. Vrtovec B et al Circ Res. 2013 Jan 4;112(1):165-73. 3. Lezaic L et al. J Card Fail 2015; 21: 145-152. 3. Losordo DW et al. Circ Res. 2011 Aug 5;109(4):428-36. 4. Losordo DW et al. Circ Cardiovasc Interv. 2012 Dec;5(6):821-30.5. Poglajen G et al. Circ Cardiovasc Interv. 2014 Aug;7(4):552-9. 6. Quyyumi AA et al; Am Heart J. 2011 Jan;161(1):98-105.
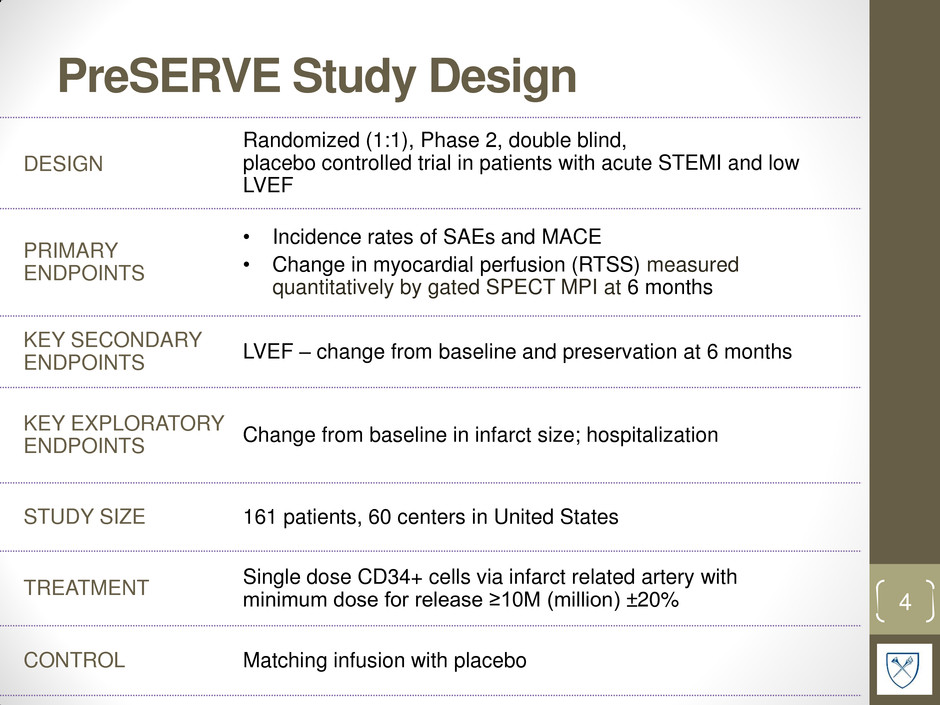
PreSERVE Study Design 4 DESIGN Randomized (1:1), Phase 2, double blind, placebo controlled trial in patients with acute STEMI and low LVEF PRIMARY ENDPOINTS • Incidence rates of SAEs and MACE • Change in myocardial perfusion (RTSS) measured quantitatively by gated SPECT MPI at 6 months KEY SECONDARY ENDPOINTS LVEF – change from baseline and preservation at 6 months KEY EXPLORATORY ENDPOINTS Change from baseline in infarct size; hospitalization STUDY SIZE 161 patients, 60 centers in United States TREATMENT Single dose CD34+ cells via infarct related artery with minimum dose for release ≥10M (million) ±20% CONTROL Matching infusion with placebo

PreSERVE: Eligibility INCLUSION CRITERIA • Acute ST elevation myocardial infarction. • Stenting within 3 days of chest pain • LVEF ≤48% by CMR or ≤45% by SPECT after 4 days • Wall motion abnormality associated with the target lesion • NYHA heart failure class I, II or III. EXCLUSION CRITERIA • STEMI > 4 days before stenting. • Cardiogenic shock • Severe aortic stenosis. • Re-occlusion of the infarct related artery (IRA) prior to the infusion. • Planned revascularization during the next 6 months. 5

PreSERVE Sites 6 Investigator Name Site Arshed Quyyumi Emory Alejandro Vasquez Heart Center Research Dean Kereiakes The Christ Hospital Marc Klapholz UMDNJ-Newark Kenichi Fujise Univ. Texas - Galveston Nandish Thukral Methodist San Antonio Gary Schaer Rush University Medical Center Robert Iwaoka Presbyterian CVI Research Ahmed Abdel-Latif U. of Kentucky, Gill Heart Institute Vijaykumar Kasi Orlando Health Vernon Anderson U. of Texas HSC - Houston Roger Gammon Austin Heart PLLC Stephen Frohwein St Joseph's Research Institute Tim Henry Minneapolis Heart Richard Schatz Scripps-La Jolla Tanvir Bajwa Aurora Health Nabil Dib Mercy Gilbert Medical Center Catalin Toma UPMC Presbyterian Michael Tamberella CaroMont Heart Pradyumna Tummala Northeast Georgia Heart Center Charles Davidson Northwestern University Gregory Barsness Mayo Clinic - Minnesota Virender Sethi Hackensack University Tarek Helmy University of Cincinnati David Shavelle Keck School of Medicine-USC Fadi El-Ahdab CV Group Central Lynchburg Martin Cohen Westchester Medical Center Gerald Koenig Henry Ford Carl Pepine University of Florida-Gainesville Vincent Pompili Ohio State University Robert Frankel Maimonides Medical Center- Brooklyn Mark Vesely University of Maryland Investigator Name Site Theodore Schreiber Detroit Receiving/Harper Hospital Mazen Abu-Fadel U. of Oklahoma HSC Emerson Perin Texas Heart Institute David Fortuin Mayo Clinic - Arizona Luis Gruberg Stony Brook University Hospital Charles Lambert Florida Hospital Massoud Leesar University of Alabama- Birmingham Joseph Wu Stanford University Howard Eisen Drexel University Lawrence Barr Advocate Health Elm Hurst Buddhadeb Dawn Kansas U. Medical Center Amit Patel University of Utah Christopher Gange MetroWest Medical Center Paul Gordon Miriam Hospital Richard Rothschild St. John's Regional Hospital Peter Kerwin Advocate Health Oakbrook Hitinder Gurm U. Michigan Michael Imburgia Louisville Cardiology Kimberly Skelding Geisinger Medical Center Vijay Iyer Buffalo General Hospital Frank McGrew Stern Cardiovascular Foundation/Baptist Hospital Zachary Hodes St. Vincent Medical Group Augusto Prichard Medstar Heart Institute Michael Ragosta UVA Health System Cardiology Research Barry Bertolet Cardiology Associates Research LLC Majid Qazi Detroit Clinical Reseach Center PC Paul Huang Swedish Medical Center
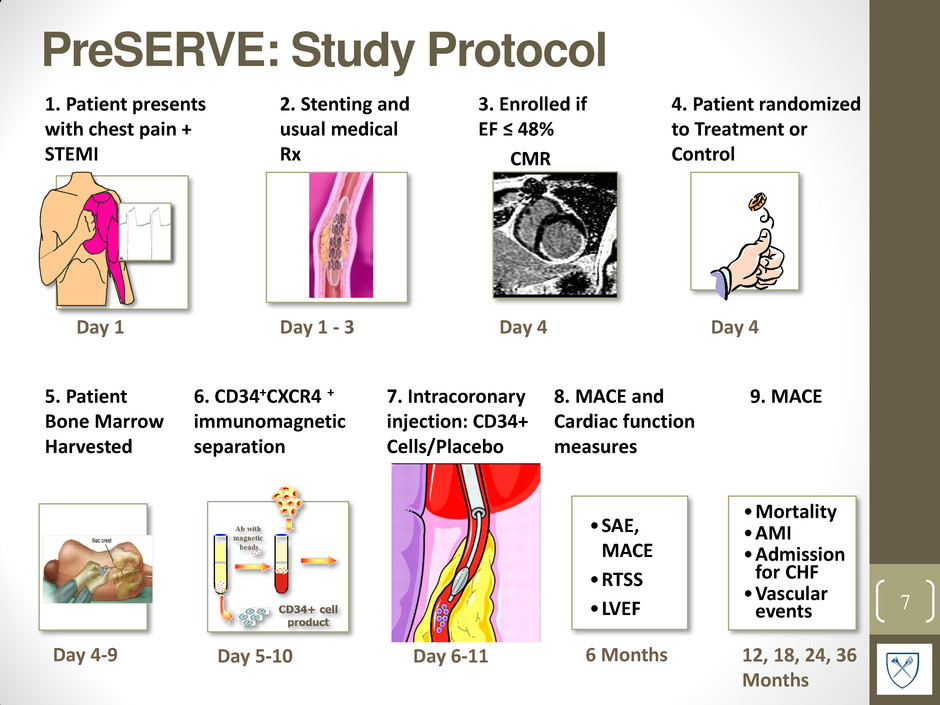
PreSERVE: Study Protocol 7 1. Patient presents with chest pain + STEMI Day 1 2. Stenting and usual medical Rx Day 1 - 3 5. Patient Bone Marrow Harvested Day 4-9 6. CD34+CXCR4 + immunomagnetic separation Day 5-10 Day 6-11 7. Intracoronary injection: CD34+ Cells/Placebo 3. Enrolled if EF ≤ 48% CMR Day 4 8. MACE and Cardiac function measures 6 Months 4. Patient randomized to Treatment or Control •SAE, MACE •RTSS •LVEF 9. MACE •Mortality •AMI •Admission for CHF •Vascular events 12, 18, 24, 36 Months Day 4 Copyright ©2009 BMJ Publishing Group Ltd. Mollmann, H. et al. Heart 2009;95:508-514 Figure 2 Application of stem cells into infarcted tissue by intracoronary transplantation. Cells are delivered over the lumen of an inflated over-the-wire balloon catheter placed in the reopened infarct artery. MI, infarcted myocardium.

PreSERVE-AMI Screened (N = 281) • Patients with STEMI and successful stent placement • LVEF ≤ 48% by CMR or ≤ 45% by SPECT measured ≥ 4 days after stent Underwent BM Harvest (n = 96) CD34+ cells infusion (n = 78) CD34+ cells (NBS10; n = 100) Underwent BM harvest (n = 88) Placebo infusion (n = 83) Placebo (n= 95) Allocation Bone Marrow Harvest and Infusion Randomized (n=195) Enrollment 8 Follow-up Efficacy and Safety Analysis Intent-to-Treat (n=100) Modified Intent-to-Treat (n=78) Per Protocol (n=75) Intent-to-Treat (n=95) Modified Intent-to-Treat (n=83) Per Protocol (n=81) Screen Failure (n = 86) Did not undergo BM harvest (n=11) • Death (n=2); • Withdrawal (n=6); • Screen Failure (n=2); • AE (n=1) Post-harvest, no infusion (n=23) • Cell product did not meet release criteria (n=16); • AE (n=6); • Withdrawal (n=1)

Baseline Characteristics 9 Treated NBS10 (N=78) Placebo (N=83) P-value* Demographics Age; mean ± SD 57.1 ± 10.1 56.4 ± 10.1 0.65 Female; n (%) 12 (15%) 17 (20%) 0.4 Race; White, n (%) 56 (72%) 62 (75%) 0.87 CV Risk Factors Hypertension (%) 53 (68%) 56 (67%) 0.80 Diabetes (%) 27 (35%) 19 (23%) 0.1 Hyperlipidemia (%) 13 (17%) 17 (20%) 0.82 NYHA Class*; mean ± SD 1.8 ± 0.6 1.9 ± 0.7 0.59 CV Medical History Prior CABG; n(%) 2 (3%) 2 (2%) 0.95 Prior PCI; n(%) 15 (19%) 15 (18%) 0.85 Prior CHF; n(%) 11 (14%) 11 (13%) 0.88 Prior MI; n(%) 13 (17%) 15 (18%) 0.34 Index AMI/PCI Infarct size (grams); mean ± SD 33.8 ± 17.4 38.6 ± 19.5 0.16 Pre-discharge LVEF (%); mean ± SD 34.3 ± 7.3 34.1 ± 8.4 0.90 LVEDV index; mean ± SD 98.0 ± 25.6 91.9 ± 20.8 0.12 LVESV index; mean ± SD 61.2 ± 23.6 58.5 ± 19.9 0.46 Time from symptoms to stent (min); mean ± SD 931 ± 1277 569 ± 864 0.041 Time from stent to infusion (days); mean ± SD 9.3 ± 1.23 9.4 ± 1.43 0.60 *P-values for quantitative characteristics are based on a t-test. P-values for categorical characteristics are based on a Chi-square test.
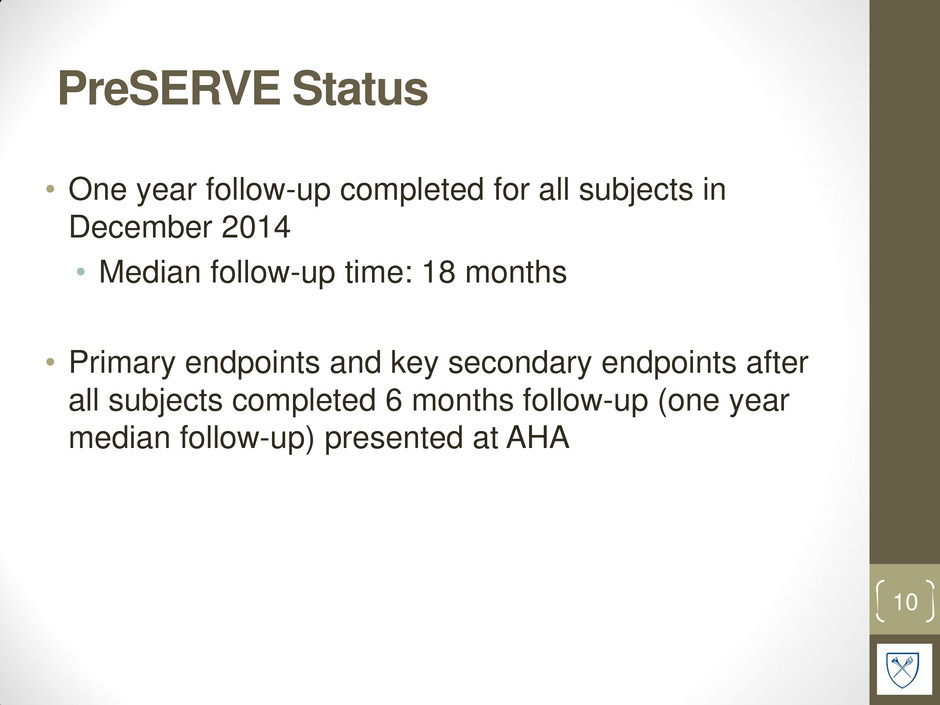
PreSERVE Status • One year follow-up completed for all subjects in December 2014 • Median follow-up time: 18 months • Primary endpoints and key secondary endpoints after all subjects completed 6 months follow-up (one year median follow-up) presented at AHA 10

Preserve: Cardiac Death 11 P ro b abili ty o f sur v iv a l MEDIAN FOLLOW-UP: 18 MONTHS No treatment group deaths to date* mITT Population **P-value calculated using a z-test. ‡P-value calculated using log rank test * As of March 13, 2015 0% 1% 2% 3% 4% 5% 3.6% (N=3) 0% (N=0) Treatment N=78 Control N=83 P=0.04** Days P=0.055‡ Control Treatment 200 400 600 800 1000 1200
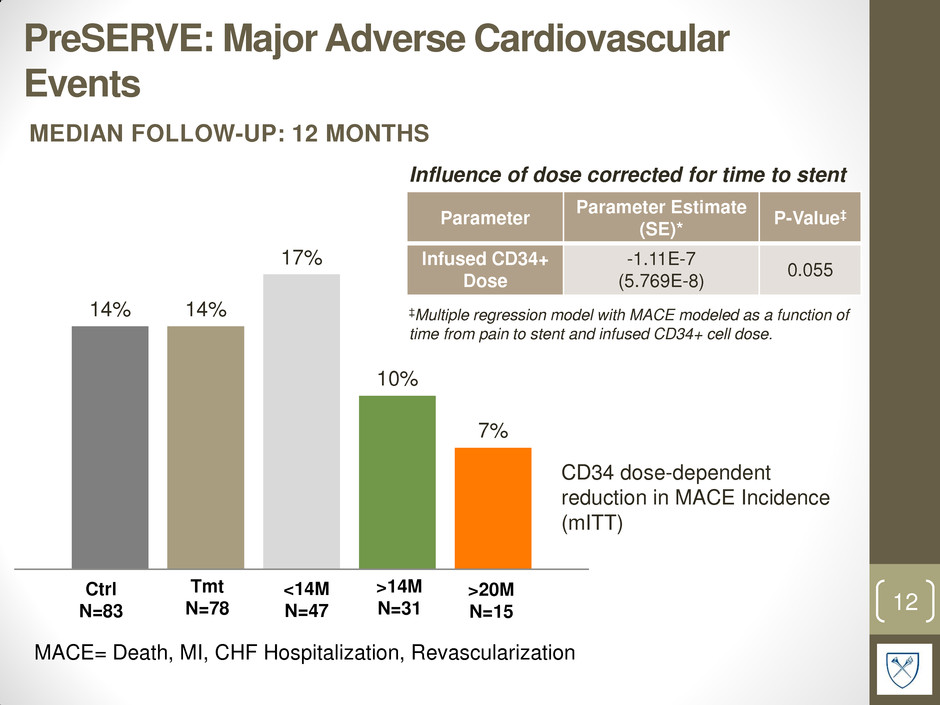
PreSERVE: Major Adverse Cardiovascular Events 12 MACE= Death, MI, CHF Hospitalization, Revascularization Ctrl N=83 <14M N=47 >14M N=31 >20M N=15 Parameter Parameter Estimate (SE)* P-Value‡ Infused CD34+ Dose -1.11E-7 (5.769E-8) 0.055 ‡Multiple regression model with MACE modeled as a function of time from pain to stent and infused CD34+ cell dose. Influence of dose corrected for time to stent 14% 14% 17% 10% 7% Tmt N=78 MEDIAN FOLLOW-UP: 12 MONTHS CD34 dose-dependent reduction in MACE Incidence (mITT)

PreSERVE: Serious Adverse Events 1.2 0.9 1.1 0.6 0.7 0 0.2 0.4 0.6 0.8 1 1.2 1.4 Control <14M >14M >20M P ro p o rt io n o f e v e n ts (n o rma li z e d b y t o ta l s u b jects ) Tmt SAE frequency normalized by total subjects per group Control (N=83) Treatment (N=78) <14M (N=47) >14M (N=31) >20M (N=15) Any SAE 99 71 53 18 11 P=0.059 *P values based on two sample t-test using Satterthwaite method. P=NS for tmt, <14M and >20M vs control. SAE = any untoward medical occurrence that results in death or is life-threatening MEDIAN FOLLOW-UP: 18 MONTHS (mITT)

PreSERVE: Hospitalization during Follow- up 14 160.5 165.6 164.4 167.5 167.6 Control N=83 <14M N=46 >14M N=29 >20M N=14 P = 0.04* P = 0.04* *P values based on two sample t- test using Satterthwaite method Tmt N=75 T im e ( d a y s ) A li v e an d O u t o f Hos p ita l Parameter Parameter Estimate (SE)* P-Value* CD34+ Cell Dose 1.6E-7(8.0E-7) 0.053 Time to Stent 1.3E-4(5.2E-4) 0.8 ANCOVA with time alive and out of hospital as outcome, cell dose, and time to stent as covariate MEDIAN FOLLOW-UP: 12 MONTHS

PreSERVE: Myocardial Perfusion • Improvement in clinically relevant endpoints not mirrored by change in SPECT perfusion • Results indicate that SPECT myocardial perfusion is not a suitable surrogate 15 SPECT Resting Total Severity Score (RTSS) Change from Baseline to 6 months (mITT) Placebo CD34 P-value RTSS Mean Change from Baseline (±SD) -149.6 ± 221.1 -142.7 ± 257.8 NS

4.9 4.1 3.1 5.8 10.2 PreSERVE: Left Ventricular Ejection Fraction Change from Baseline to 6 months (mITT) CMR (SPECT when unavailable) 16 Control (N=80) <14M (N=45) >14M (N=15) >20M (N=13) L V E F c h an g e fro m b a seli n e ( % ) P < 0.05* Tmt (N=73) Parameter Parameter Estimate (SE) P-Value CD34+ Cell Dose 2.21 (1.084) 0.045† CD34+ CXCR4+ cell Dose 4.8E-7 (2.1E-7) 0.062‡ Multiple regression† and ANOVA‡ models *P-values are based on a t-test of the means Influence of dose corrected for time to stent
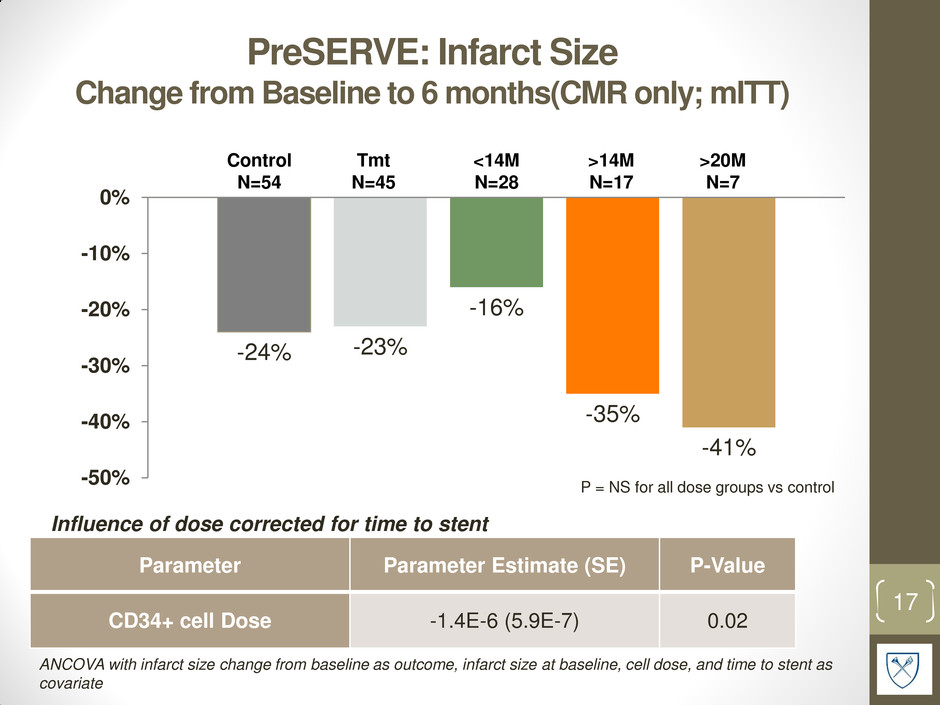
PreSERVE: Infarct Size Change from Baseline to 6 months(CMR only; mITT) -24% -23% -16% -35% -41% -50% -40% -30% -20% -10% 0% 17 Control N=54 <14M N=28 >14M N=17 >20M N=7 P = NS for all dose groups vs control Influence of dose corrected for time to stent Parameter Parameter Estimate (SE) P-Value CD34+ cell Dose -1.4E-6 (5.9E-7) 0.02 ANCOVA with infarct size change from baseline as outcome, infarct size at baseline, cell dose, and time to stent as covariate Tmt N=45
PreSERVE Sites 18 Investigator Name Site Arshed Quyyumi Emory Alejandro Vasquez Heart Center Research Dean Kereiakes The Christ Hospital Marc Klapholz UMDNJ-Newark Kenichi Fujise Univ. Texas - Galveston Nandish Thukral Methodist San Antonio Gary Schaer Rush University Medical Center Robert Iwaoka Presbyterian CVI Research Ahmed Abdel-Latif U. of Kentucky, Gill Heart Institute Vijaykumar Kasi Orlando Health Vernon Anderson U. of Texas HSC - Houston Roger Gammon Austin Heart PLLC Stephen Frohwein St Joseph's Research Institute Tim Henry Minneapolis Heart Richard Schatz Scripps-La Jolla Tanvir Bajwa Aurora Health Nabil Dib Mercy Gilbert Medical Center Catalin Toma UPMC Presbyterian Michael Tamberella CaroMont Heart Pradyumna Tummala Northeast Georgia Heart Center Charles Davidson Northwestern University Gregory Barsness Mayo Clinic - Minnesota Virender Sethi Hackensack University Tarek Helmy University of Cincinnati David Shavelle Keck School of Medicine-USC Fadi El-Ahdab CV Group Central Lynchburg Martin Cohen Westchester Medical Center Gerald Koenig Henry Ford Carl Pepine University of Florida-Gainesville Vincent Pompili Ohio State University Robert Frankel Maimonides Medical Center- Brooklyn Mark Vesely University of Maryland Investigator Name Site Theodore Schreiber Detroit Receiving/Harper Hospital Mazen Abu-Fadel U. of Oklahoma HSC Emerson Perin Texas Heart Institute David Fortuin Mayo Clinic - Arizona Luis Gruberg Stony Brook University Hospital Charles Lambert Florida Hospital Massoud Leesar University of Alabama- Birmingham Joseph Wu Stanford University Howard Eisen Drexel University Lawrence Barr Advocate Health Elm Hurst Buddhadeb Dawn Kansas U. Medical Center Amit Patel University of Utah Christopher Gange MetroWest Medical Center Paul Gordon Miriam Hospital Richard Rothschild St. John's Regional Hospital Peter Kerwin Advocate Health Oakbrook Hitinder Gurm U. Michigan Michael Imburgia Louisville Cardiology Kimberly Skelding Geisinger Medical Center Vijay Iyer Buffalo General Hospital Frank McGrew Stern Cardiovascular Foundation/Baptist Hospital Zachary Hodes St. Vincent Medical Group Augusto Prichard Medstar Heart Institute Michael Ragosta UVA Health System Cardiology Research Barry Bertolet Cardiology Associates Research LLC Majid Qazi Detroit Clinical Reseach Center PC Paul Huang Swedish Medical Center

Thank you for your attention 19

BACK UP SLIDES 20

Time to Cardiac Death (ITT Population) 21 MEDIAN FOLLOW-UP: 18 MONTHS Pro b a b ilit y of surv iva l Days since informed consent 200 400 600 800 1000 1200 P=0.008‡ ‡P-value calculated using log rank test ‡ P-value calculated using log rank test

Time to First Hospitalization (mITT) 22 MEDIAN FOLLOW-UP: 12 MONTHS Pro b a b ilit y of b e in g a liv e a n d o u t of h o sp ita l >20M >14M Control





















