
1 Corporate Presentation NASDAQ: NBS Transforming Personalized Medicine David J. Mazzo, PhD Chief Executive Officer March 2015

Forward-looking statements 2 This presentation contains “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995, as well as historical information. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements, or industry results, to be materially different from anticipated results, performance or achievements expressed or implied by such forward-looking statements. When used in this presentation, statements that are not statements of current or historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words “plan,” “intend,” “may,” “will,” “expect,” “believe,” “could,” “anticipate,” “estimate,” or “continue” or similar expressions or other variations or comparable terminology are intended to identify such forward-looking statements, although some forward-looking statements are expressed differently. We remind readers that forward- looking statements are merely predictions and therefore inherently subject to uncertainties and other factors and involve known and unknown risks that could cause the actual results, performance, levels of activity or our achievements or industry results, to be materially different from any future results, performance levels of activity or our achievements or industry results expressed or implied by such forward-looking statements. Such forward looking statements appear in this presentation. Factors that could cause our actual results to differ materially from anticipated results expressed or implied by forward-looking statements include, among others: • our ability to obtain sufficient capital or strategic business arrangements to fund our operations and expansion plans, including meeting our financial obligations under various licensing and other strategic arrangements, the funding of our clinical trials for product candidates in our development programs for our Targeted Cancer Immunotherapy Program, our Ischemic Repair Program and our Immune Modulation Program, and the commercialization of the relevant technology; • our ability to build and maintain the management and human resources infrastructure necessary to support the growth of our business; • our ability to integrate our acquired businesses successfully and grow such acquired businesses as anticipated, including expanding our PCT business internationally; • whether a large global market is established for our cellular-based products and services and our ability to capture a meaningful share of this market; • scientific and medical developments beyond our control; • our ability to obtain and maintain, as applicable, appropriate governmental licenses, accreditations or certifications or comply with healthcare laws and regulations or any other adverse effect or limitations caused by government regulation of our business; • whether any of our current or future patent applications result in issued patents, the scope of those patents and our ability to obtain and maintain other rights to technology required or desirable for the conduct of our business; our ability to commercialize products without infringing the claims of third party patents; • whether any potential strategic or financial benefits of various licensing agreements will be realized; • the results of our development activities, especially: • the results of our planned Intus Phase 3 clinical trial of NBS20 being developed to treat metastatic melanoma; • the results of our PreSERVE Phase 2 clinical trial of NBS10 being developed to treat acute myocardial infarction for which we released initial data on November 17, 2014 and for which all 6 and 12 month data has been collected; however it is subject to ongoing analysis, and currently reported results, although promising, are preliminary and there can be no assurance that further analysis may not reveal negative, or less promising, results; • our ability to complete our other planned clinical trials (or initiate other trials) in accordance with our estimated timelines due to delays associated with enrolling patients due to the novelty of the treatment, the size of the patient population and the need of patients to meet the inclusion criteria of the trial or otherwise; and • the other factors discussed in “Risk Factors” in our Form 10-K filed with the Securities and Exchange Commission (“the SEC”) on March 2, 2015, and elsewhere in the Annual Report on Form 10-K. The factors discussed herein, including those risks described in Item 1A. “Risk Factors” in the Company's Annual Report on Form 10-K filed with the SEC on March 2, 2015 and in the Company's other periodic filings with the Securities and Exchange Commission (the “SEC”) which are available for review at www.sec.gov under “Search for Company Filings” could cause actual results and developments to be materially different from those expressed or implied by such statements. All forward-looking statements attributable to us are expressly qualified in their entirety by these and other factors. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the Company undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
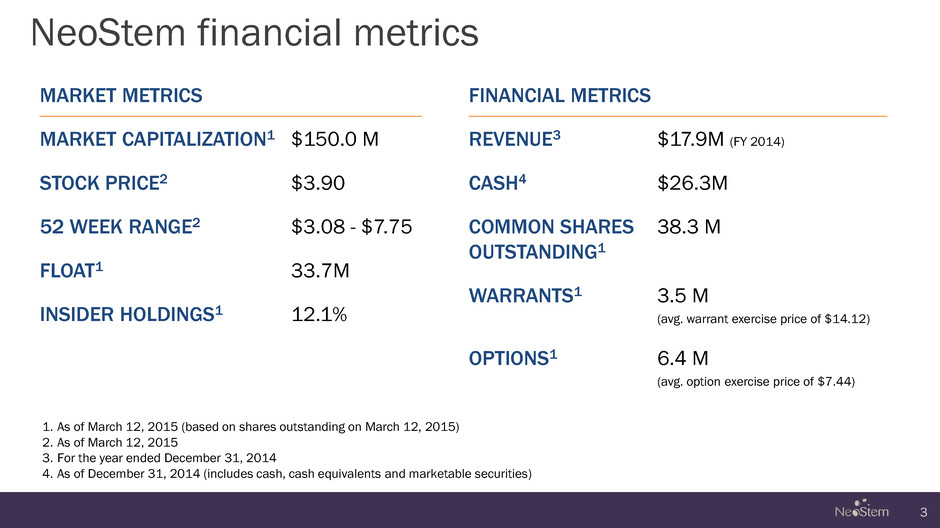
NeoStem financial metrics 3 MARKET METRICS MARKET CAPITALIZATION1 $150.0 M STOCK PRICE2 $3.90 52 WEEK RANGE2 $3.08 - $7.75 FLOAT1 33.7M INSIDER HOLDINGS1 12.1% FINANCIAL METRICS REVENUE3 $17.9M (FY 2014) CASH4 $26.3M COMMON SHARES OUTSTANDING1 38.3 M WARRANTS1 3.5 M (avg. warrant exercise price of $14.12) OPTIONS1 6.4 M (avg. option exercise price of $7.44) 1. As of March 12, 2015 (based on shares outstanding on March 12, 2015) 2. As of March 12, 2015 3. For the year ended December 31, 2014 4. As of December 31, 2014 (includes cash, cash equivalents and marketable securities)
Transforming cells into therapies 4
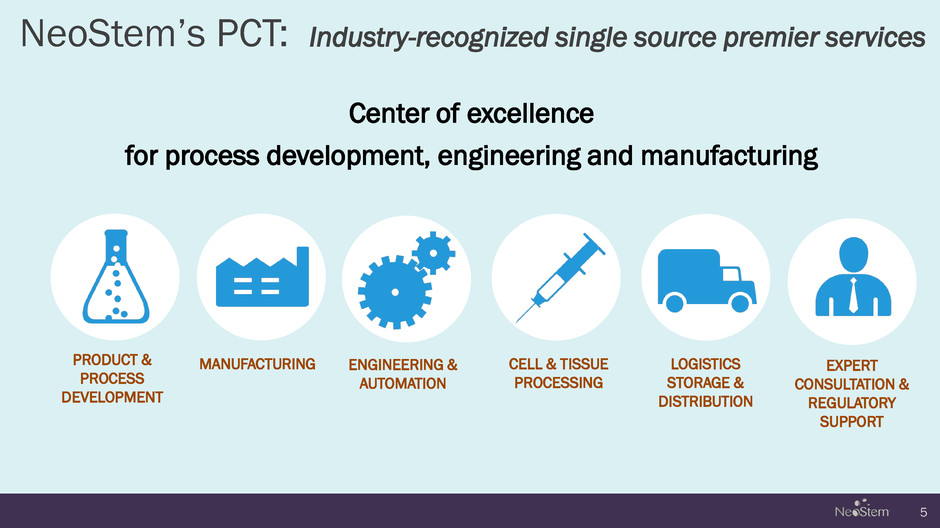
NeoStem’s PCT: Industry-recognized single source premier services Center of excellence for process development, engineering and manufacturing 5 PRODUCT & PROCESS DEVELOPMENT MANUFACTURING CELL & TISSUE PROCESSING LOGISTICS STORAGE & DISTRIBUTION EXPERT CONSULTATION & REGULATORY SUPPORT ENGINEERING & AUTOMATION
At a glance 6 Highly experienced management and scientific team Proprietary platform technologies yielding a diversified, balanced pipeline targeting critical unmet needs in large global markets and near-term development milestones Immunotherapy platform applicable across multiple solid tumors with Fast Track and Orphan Drug Designations and a SPA for a phase 3 study in metastatic melanoma Cell therapy platform applicable in multiple cardiovascular indications with ongoing Phase 2 study for acute myocardial infarction – compelling and consistent interim results Immunomodulation therapy platform targeting autoimmune disorders with FDA cleared phase 2 study in adolescents with type I diabetes Internal center of excellence (PCT) with bicoastal facilities and proven capabilities innovating discovery, development, manufacturing and delivery of cell-based therapies

Experienced executive team 7 David J. Mazzo, PhD Chief Executive Officer Over 30 years experience in all aspects of large and emerging global biotech/biopharma company operations and successful international drug development Robert S. Vaters, MBA President and Chief Financial Officer Over 25 years financial and management experience in a variety of healthcare, biotechnology, biologics, medical device and pharmaceutical companies Douglas W. Losordo, MD Chief Medical Officer A leader in cell therapy research and development and renowned cardiologist with noteworthy academic and industry credentials Robert A. Preti, PhD President of NeoStem’s PCT A leading authority on cell-based therapy engineering with unique development and commercialization experience Robin L. Smith, MD, MBA Executive Chairman Extensive background in healthcare, business development and management; led NeoStem 2006-2014
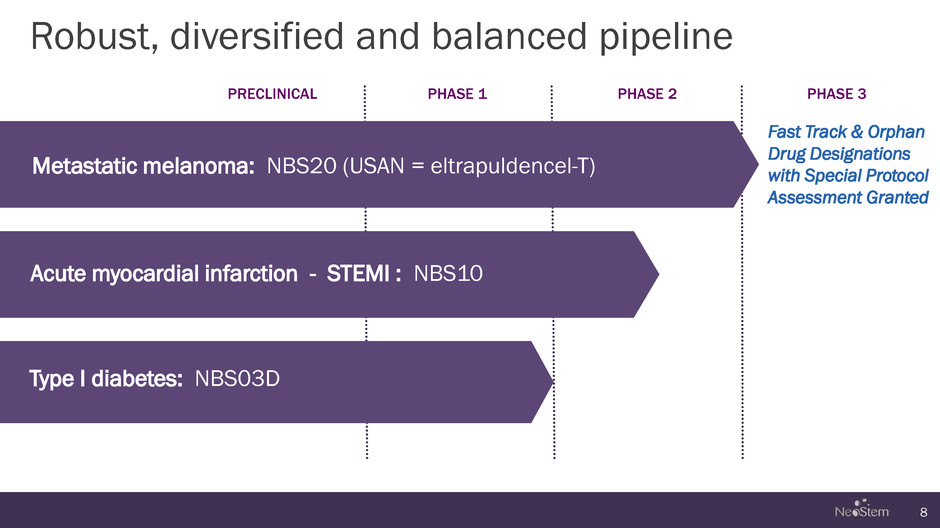
Robust, diversified and balanced pipeline 8 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Acute myocardial infarction - STEMI : NBS10 Type I diabetes: NBS03D Metastatic melanoma: NBS20 (USAN = eltrapuldencel-T) Fast Track & Orphan Drug Designations with Special Protocol Assessment Granted
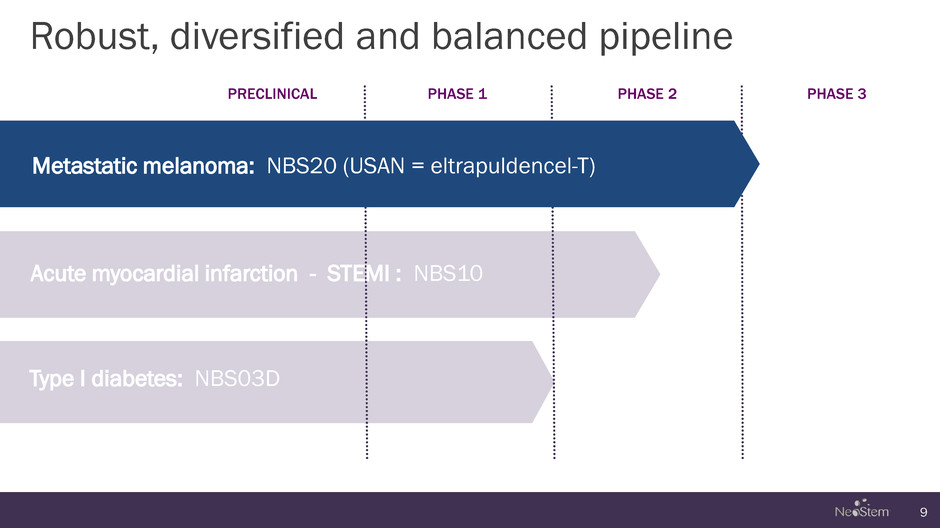
Robust, diversified and balanced pipeline 9 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Metastatic melanoma: NBS20 (USAN = eltrapuldencel-T) Acute myocardial infarction - STEMI : NBS10 Type I diabetes: NBS03D

10 Fast Track designation Orphan Drug designation Special Protocol Assessment NBS20: (USAN = eltrapuldencel-T) Metastatic melanoma
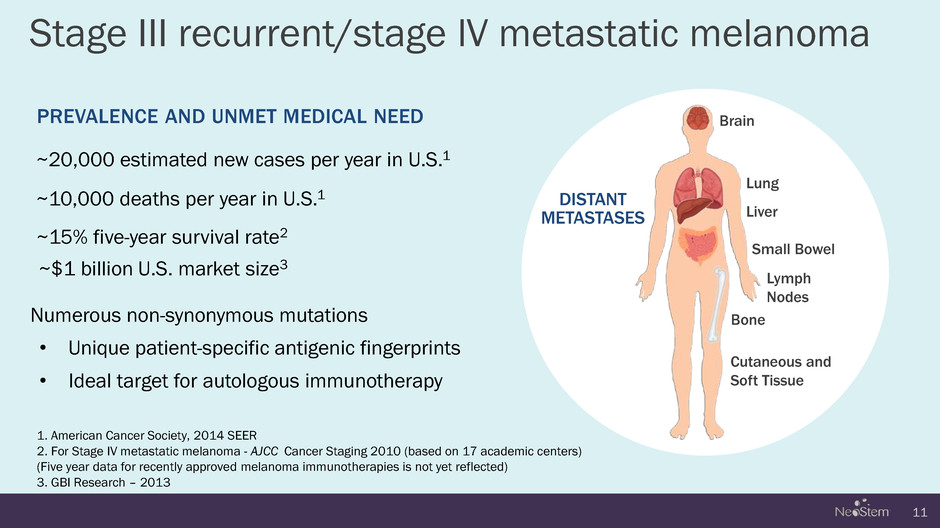
Numerous non-synonymous mutations • Unique patient-specific antigenic fingerprints • Ideal target for autologous immunotherapy Stage III recurrent/stage lV metastatic melanoma 11 Brain Lung Bone Liver 1. American Cancer Society, 2014 SEER 2. For Stage IV metastatic melanoma - AJCC Cancer Staging 2010 (based on 17 academic centers) (Five year data for recently approved melanoma immunotherapies is not yet reflected) 3. GBI Research – 2013 PREVALENCE AND UNMET MEDICAL NEED ~20,000 estimated new cases per year in U.S.1 DISTANT METASTASES ~10,000 deaths per year in U.S.1 ~15% five-year survival rate2 ~$1 billion U.S. market size3 Small Bowel Cutaneous and Soft Tissue Lymph Nodes
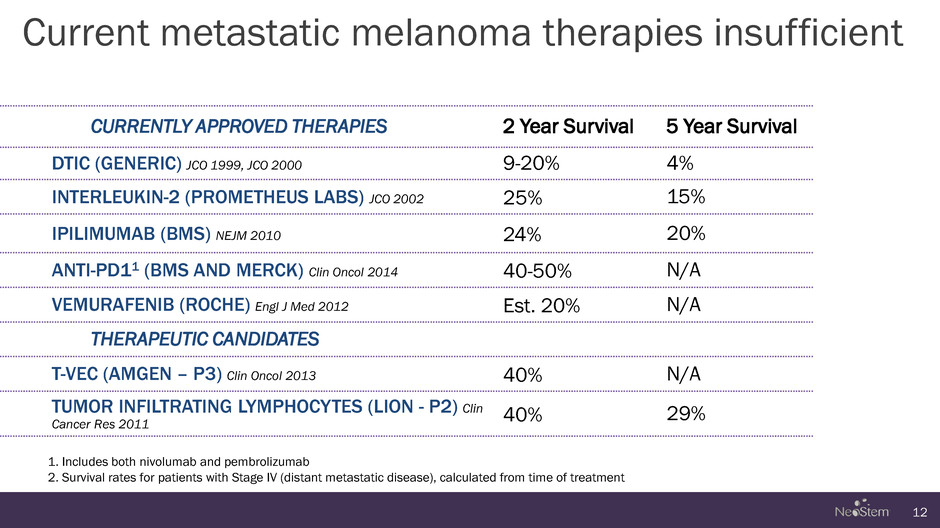
Current metastatic melanoma therapies insufficient 12 CURRENTLY APPROVED THERAPIES 2 Year Survival 5 Year Survival DTIC (GENERIC) JCO 1999, JCO 2000 9-20% 4% INTERLEUKIN-2 (PROMETHEUS LABS) JCO 2002 25% 15% IPILIMUMAB (BMS) NEJM 2010 24% 20% ANTI-PD11 (BMS AND MERCK) Clin Oncol 2014 40-50% N/A VEMURAFENIB (ROCHE) Engl J Med 2012 Est. 20% N/A THERAPEUTIC CANDIDATES T-VEC (AMGEN – P3) Clin Oncol 2013 40% N/A TUMOR INFILTRATING LYMPHOCYTES (LION - P2) Clin Cancer Res 2011 40% 29% 1. Includes both nivolumab and pembrolizumab 2. Survival rates for patients with Stage IV (distant metastatic disease), calculated from time of treatment
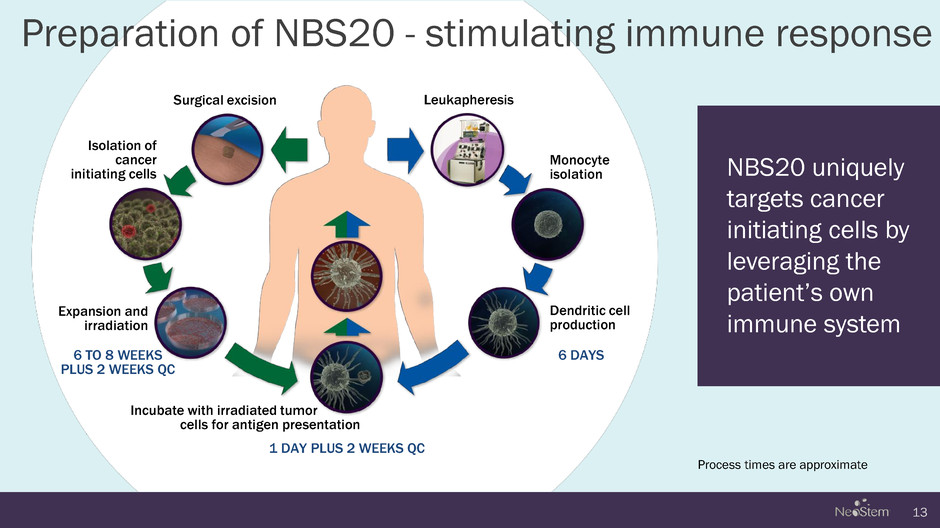
13 Process times are approximate Isolation of cancer initiating cells Expansion and irradiation Incubate with irradiated tumor _ cells for antigen presentation Dendritic cell production Leukapheresis Monocyte isolation Surgical excision NBS20 uniquely targets cancer initiating cells by leveraging the patient’s own immune system Preparation of NBS20 - stimulating immune response 6 TO 8 WEEKS PLUS 2 WEEKS QC 1 DAY PLUS 2 WEEKS QC 6 DAYS

0% 20% 40% 60% 80% 100% 0 10 20 30 40 50 60 P e rc ent S u rv iv in g Months Phase 2: multiple trials; consistent, compelling data 14 Dillman, et al. Journal Immunotherapy 2012 Historical control for distant metastases 0% 20% 40% 60% 80% Control Group Irradiated Tumor Cells Treatment Group 2-YEAR OVERALL SURVIVAL 42 patient randomized P2 trial p = 0.007; Hazard ratio = 0.27 Treatment considered safe and generally well tolerated ― Minor local injection site reactions 72% 31% N=24 N=18 50% observed 5-year survival rate Treatment considered safe and generally well tolerated ― Minor local injection site reactions Dillman, et al. Cancer Biother Radiopharm 2009 Historical control for distant metastases: Balch J Clin Oncol 2009 N=54 5-YEAR OVERALL SURVIVAL 54 patient single arm P2 trial 2 Y ea rs 73% Treatment 10% 25%
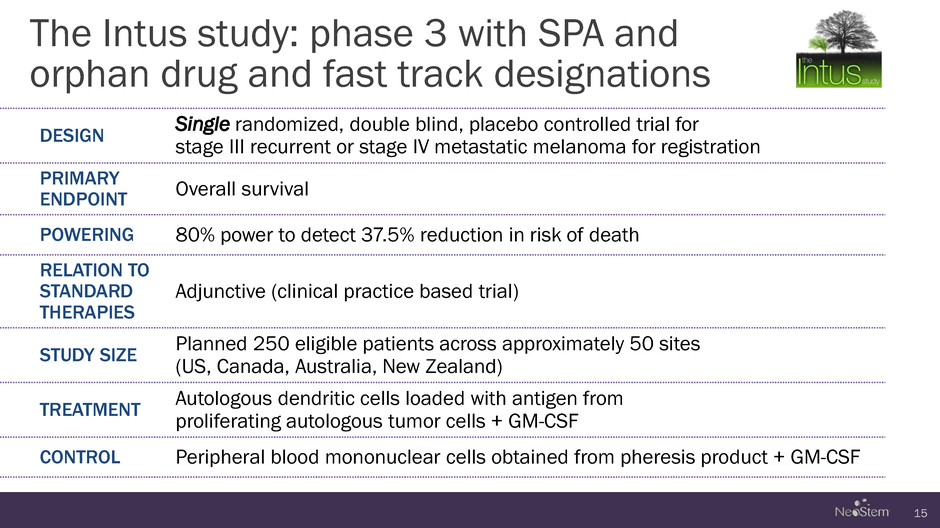
The Intus study: phase 3 with SPA and orphan drug and fast track designations 15 DESIGN Single randomized, double blind, placebo controlled trial for stage III recurrent or stage IV metastatic melanoma for registration PRIMARY ENDPOINT Overall survival POWERING 80% power to detect 37.5% reduction in risk of death RELATION TO STANDARD THERAPIES Adjunctive (clinical practice based trial) STUDY SIZE Planned 250 eligible patients across approximately 50 sites (US, Canada, Australia, New Zealand) TREATMENT Autologous dendritic cells loaded with antigen from proliferating autologous tumor cells + GM-CSF CONTROL Peripheral blood mononuclear cells obtained from pheresis product + GM-CSF
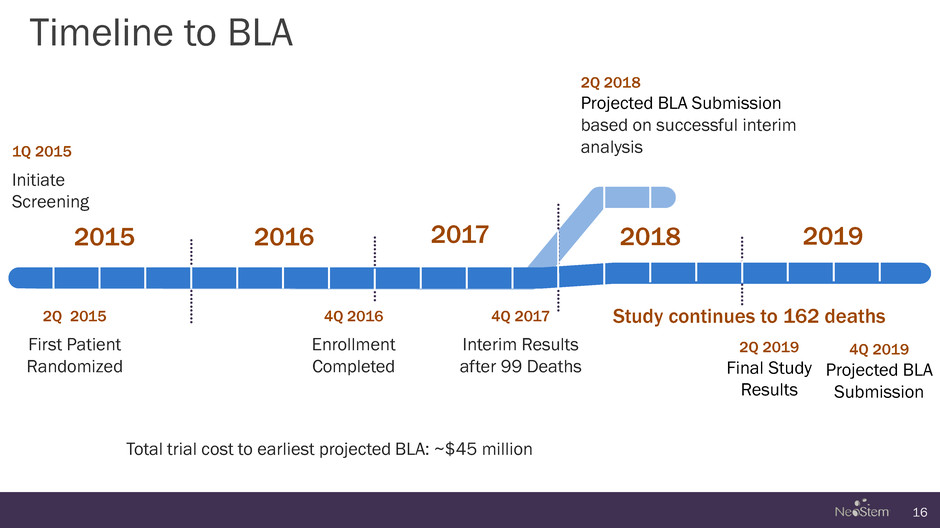
Timeline to BLA 16 1Q 2015 Initiate Screening 4Q 2017 Interim Results after 99 Deaths 2Q 2015 First Patient Randomized 2Q 2018 Projected BLA Submission based on successful interim analysis 2Q 2019 Final Study Results 4Q 2019 Projected BLA Submission 4Q 2016 Enrollment Completed 2015 2016 2017 2018 2019 Study continues to 162 deaths Total trial cost to earliest projected BLA: ~$45 million
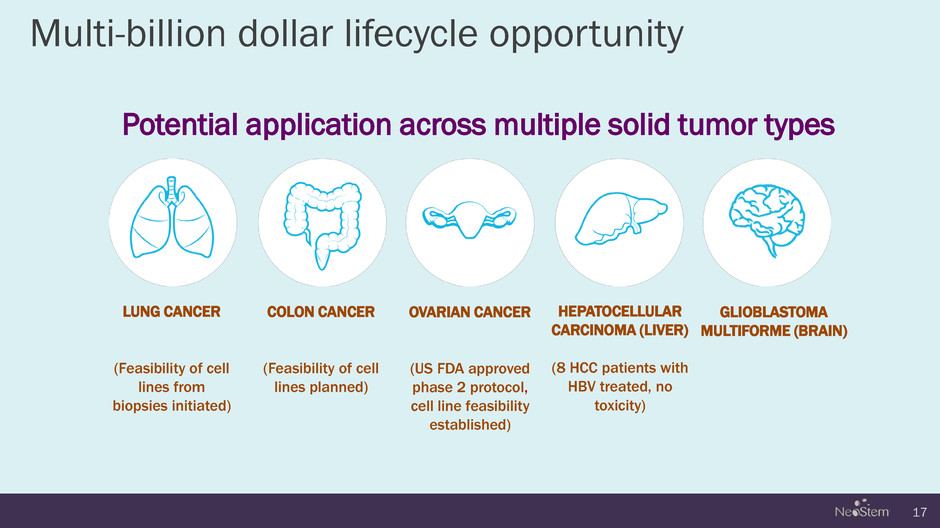
Multi-billion dollar lifecycle opportunity Potential application across multiple solid tumor types 17 LUNG CANCER (Feasibility of cell lines from biopsies initiated) COLON CANCER (Feasibility of cell lines planned) OVARIAN CANCER (US FDA approved phase 2 protocol, cell line feasibility established) HEPATOCELLULAR CARCINOMA (LIVER) (8 HCC patients with HBV treated, no toxicity) GLIOBLASTOMA MULTIFORME (BRAIN)
Robust, diversified and balanced pipeline 18 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Acute myocardial infarction - STEMI : NBS10 Type 1 diabetes: NBS03D Metastatic melanoma: NBS20 (USAN = eltrapuldencel-T)

19 NBS10: ST segment elevation myocardial infarction (STEMI)
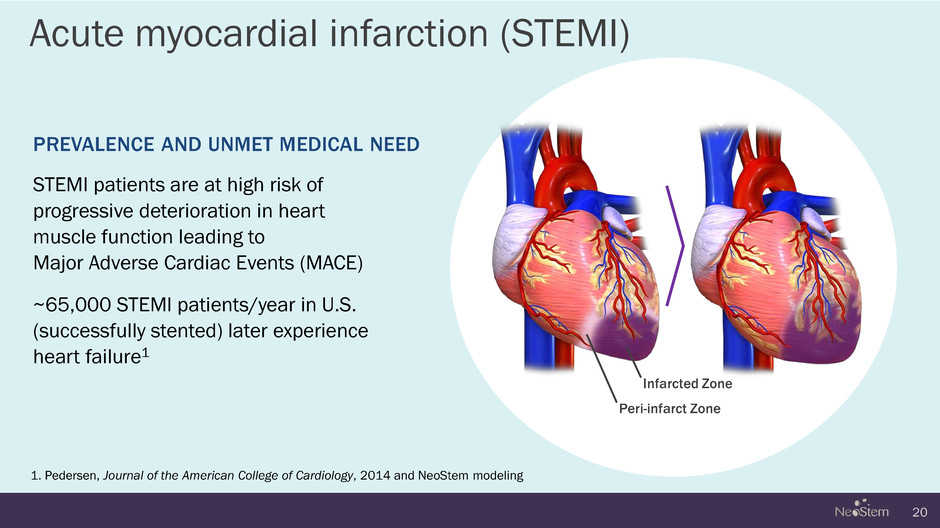
Acute myocardial infarction (STEMI) 20 1. Pedersen, Journal of the American College of Cardiology, 2014 and NeoStem modeling PREVALENCE AND UNMET MEDICAL NEED STEMI patients are at high risk of progressive deterioration in heart muscle function leading to Major Adverse Cardiac Events (MACE) ~65,000 STEMI patients/year in U.S. (successfully stented) later experience heart failure1 Infarcted Zone Peri-infarct Zone
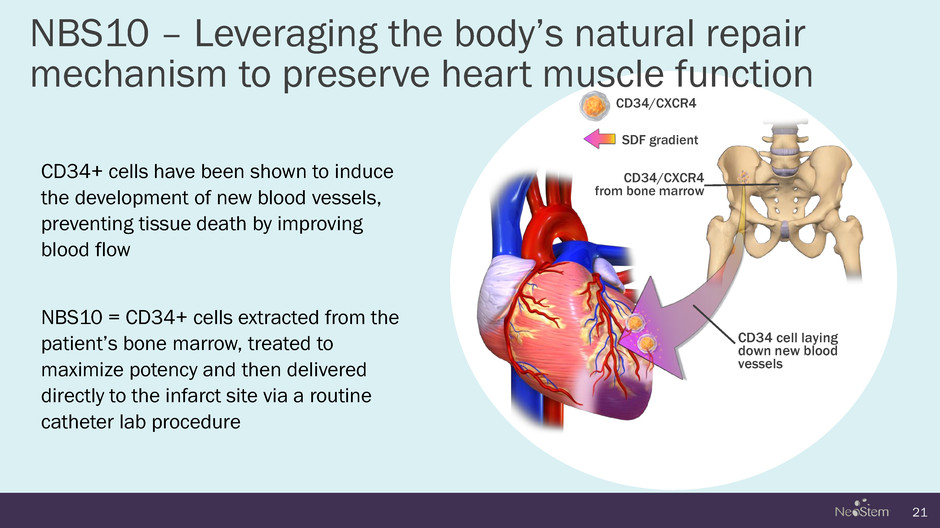
NBS10 – Leveraging the body’s natural repair mechanism to preserve heart muscle function 21 CD34/CXCR4 from bone marrow CD34 cell laying down new blood vessels CD34/CXCR4 SDF gradient CD34+ cells have been shown to induce the development of new blood vessels, preventing tissue death by improving blood flow NBS10 = CD34+ cells extracted from the patient’s bone marrow, treated to maximize potency and then delivered directly to the infarct site via a routine catheter lab procedure
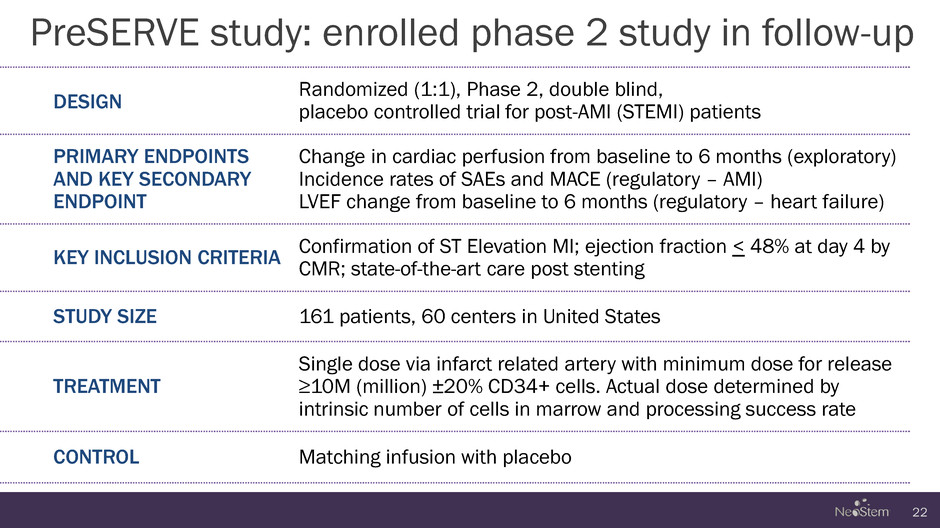
PreSERVE study: enrolled phase 2 study in follow-up 22 DESIGN Randomized (1:1), Phase 2, double blind, placebo controlled trial for post-AMI (STEMI) patients PRIMARY ENDPOINTS AND KEY SECONDARY ENDPOINT Change in cardiac perfusion from baseline to 6 months (exploratory) Incidence rates of SAEs and MACE (regulatory – AMI) LVEF change from baseline to 6 months (regulatory – heart failure) KEY INCLUSION CRITERIA Confirmation of ST Elevation MI; ejection fraction < 48% at day 4 by CMR; state-of-the-art care post stenting STUDY SIZE 161 patients, 60 centers in United States TREATMENT Single dose via infarct related artery with minimum dose for release ≥10M (million) ±20% CD34+ cells. Actual dose determined by intrinsic number of cells in marrow and processing success rate CONTROL Matching infusion with placebo
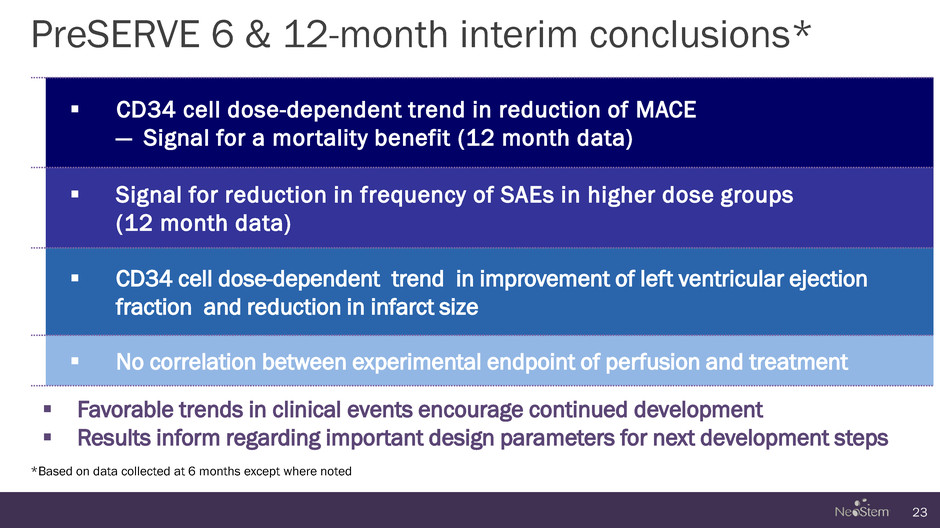
PreSERVE 6 & 12-month interim conclusions* 23 CD34 cell dose-dependent trend in reduction of MACE — Signal for a mortality benefit (12 month data) Signal for reduction in frequency of SAEs in higher dose groups (12 month data) CD34 cell dose-dependent trend in improvement of left ventricular ejection fraction and reduction in infarct size No correlation between experimental endpoint of perfusion and treatment Favorable trends in clinical events encourage continued development Results inform regarding important design parameters for next development steps *Based on data collected at 6 months except where noted
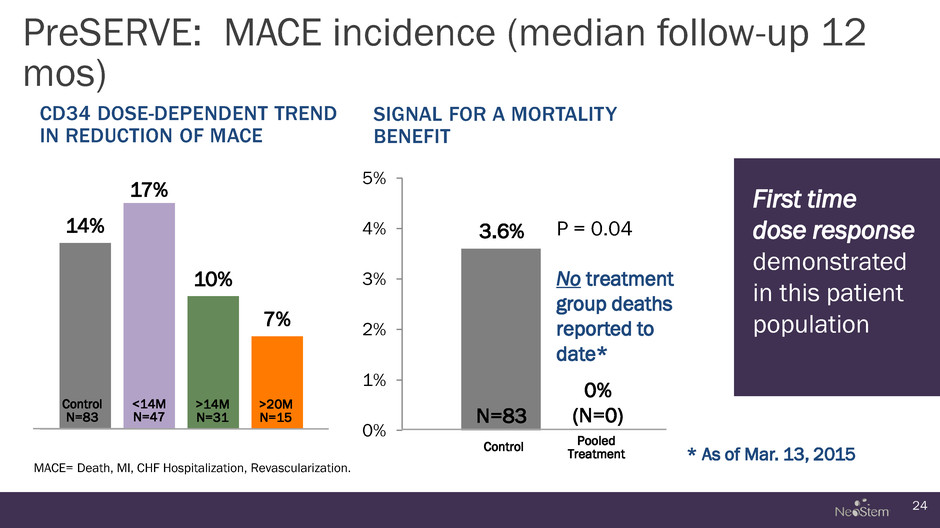
PreSERVE: MACE incidence (median follow-up 12 mos) 24 CD34 DOSE-DEPENDENT TREND IN REDUCTION OF MACE 14% 17% 10% 7% SIGNAL FOR A MORTALITY BENEFIT MACE= Death, MI, CHF Hospitalization, Revascularization. Control N=83 <14M N=47 >14M N=31 >20M N=15 0% 1% 2% 3% 4% 5% N=83 3.6% 0% (N=0) Pooled Treatment P = 0.04 No treatment group deaths reported to date* First time dose response demonstrated in this patient population Control * As of Mar. 13, 2015
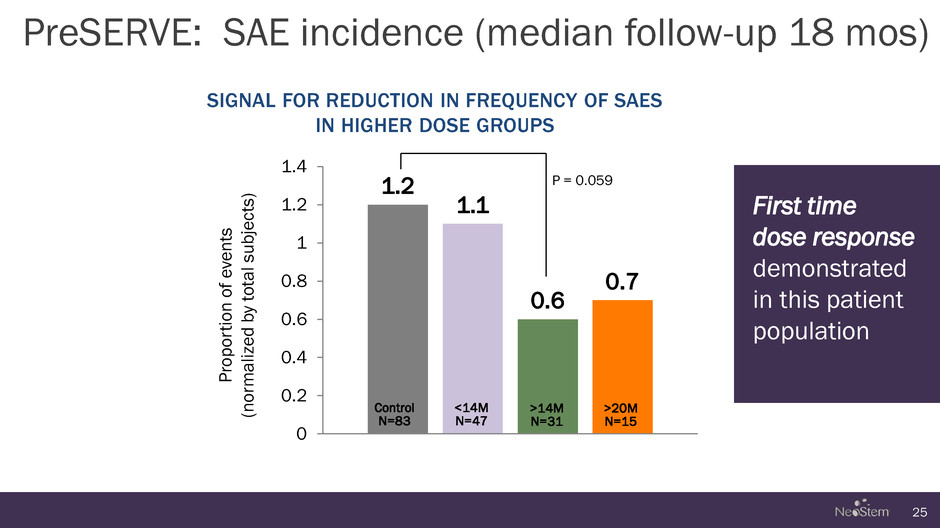
1.2 1.1 0.6 0.7 0 0.2 0.4 0.6 0.8 1 1.2 1.4 PreSERVE: SAE incidence (median follow-up 18 mos) 25 SIGNAL FOR REDUCTION IN FREQUENCY OF SAES IN HIGHER DOSE GROUPS P ropo rtion of e ve n ts (n o rmali zed b y to tal s u b je cts ) P = 0.059 First time dose response demonstrated in this patient population Control N=83 <14M N=47 >14M N=31 >20M N=15
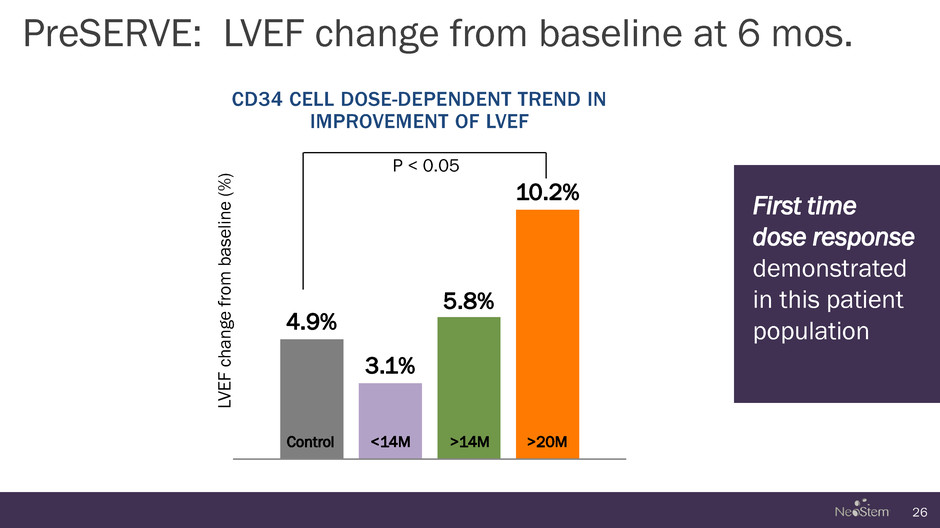
PreSERVE: LVEF change from baseline at 6 mos. 26 4.9% 3.1% 5.8% 10.2% Control <14M >14M >20M CD34 CELL DOSE-DEPENDENT TREND IN IMPROVEMENT OF LVEF LVE F chan g e f rom baseline ( % ) P < 0.05 First time dose response demonstrated in this patient population
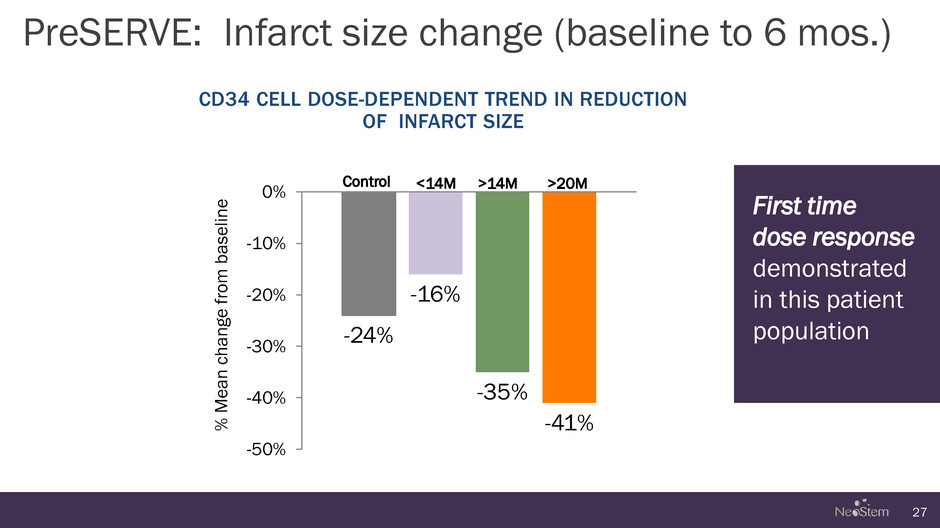
-24% -16% -35% -41% -50% -40% -30% -20% -10% 0% PreSERVE: Infarct size change (baseline to 6 mos.) 27 Control <14M >14M >20M CD34 CELL DOSE-DEPENDENT TREND IN REDUCTION OF INFARCT SIZE % Me a n c ha n g e f rom baseli n e First time dose response demonstrated in this patient population
Next steps for ischemic repair program 28 NEXT POTENTIAL DEVELOPMENT STEPS IN OTHER INDICATIONS • Phase 2 in chronic heart failure • Phase 2 in critical limb ischemia STEMI NEXT DEVELOPMENT STEPS • March 2015: • 2H 2015: • March 2016: • March 2017: One-year data Determine next development steps based on PreSERVE interim results and consultation with medical advisors (potentially Phase 2B/3 trial) Two-year data Three-year data, end of study
Multi-billion dollar lifecycle opportunity Potential application across several cardiovascular indications 29 CHRONIC HEART FAILURE CRITICAL LIMB ISCHEMIA
Robust, diversified and balanced pipeline 30 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Acute myocardial infarction - STEMI : NBS10 Type I diabetes: NBS03D Metastatic melanoma: NBS20 (USAN = eltrapuldencel-T)

31 NBS03D: Diabetes Mellitus Type-1 (T1D)
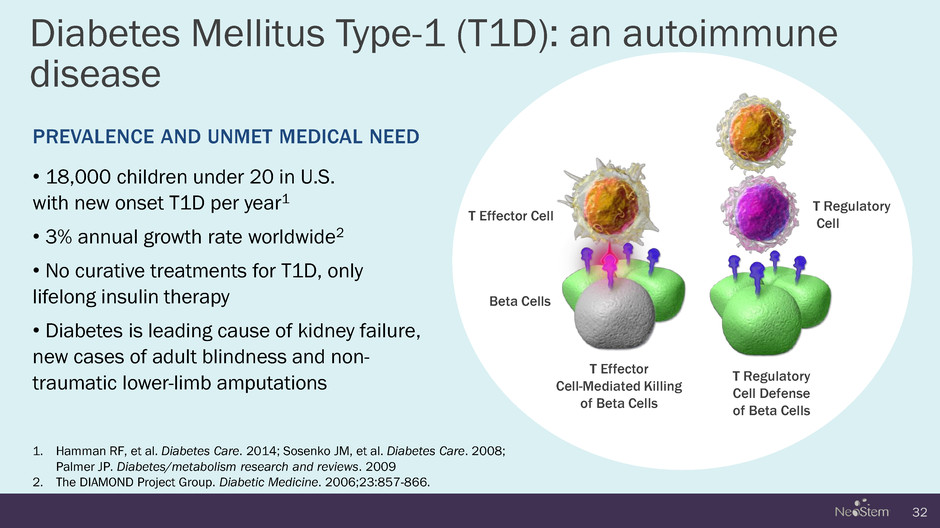
Diabetes Mellitus Type-1 (T1D): an autoimmune disease 32 1. Hamman RF, et al. Diabetes Care. 2014; Sosenko JM, et al. Diabetes Care. 2008; Palmer JP. Diabetes/metabolism research and reviews. 2009 2. The DIAMOND Project Group. Diabetic Medicine. 2006;23:857-866. PREVALENCE AND UNMET MEDICAL NEED • 18,000 children under 20 in U.S. with new onset T1D per year1 • 3% annual growth rate worldwide2 • No curative treatments for T1D, only lifelong insulin therapy • Diabetes is leading cause of kidney failure, new cases of adult blindness and non- traumatic lower-limb amputations Beta Cells T Regulatory Cell T Effector Cell-Mediated Killing of Beta Cells T Effector Cell T Regulatory Cell Defense of Beta Cells
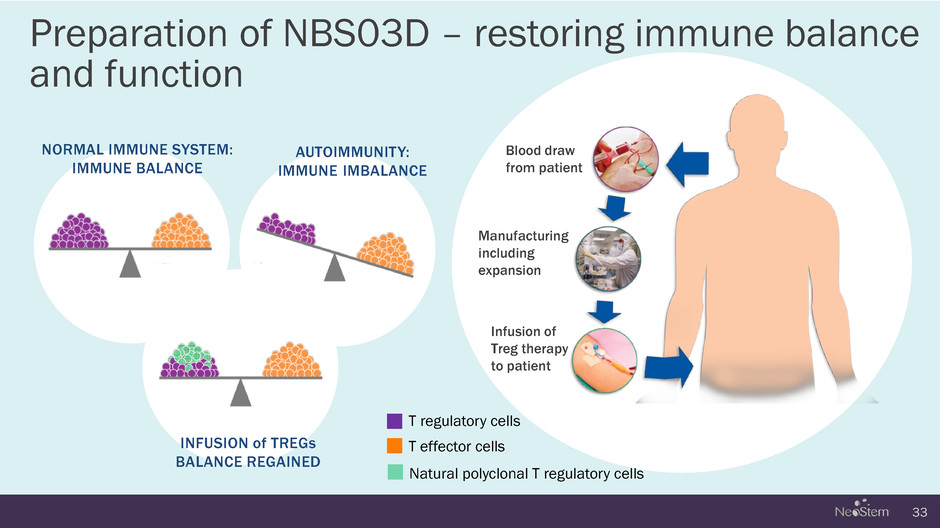
33 NORMAL IMMUNE SYSTEM: IMMUNE BALANCE TREG INFUSION T regulatory cells T effector cells Blood draw from patient Manufacturing including expansion Infusion of Treg therapy to patient Preparation of NBS03D – restoring immune balance and function INFUSION of TREGs BALANCE REGAINED AUTOIMMUNITY: IMMUNE IMBALANCE Natural polyclonal T regulatory cells
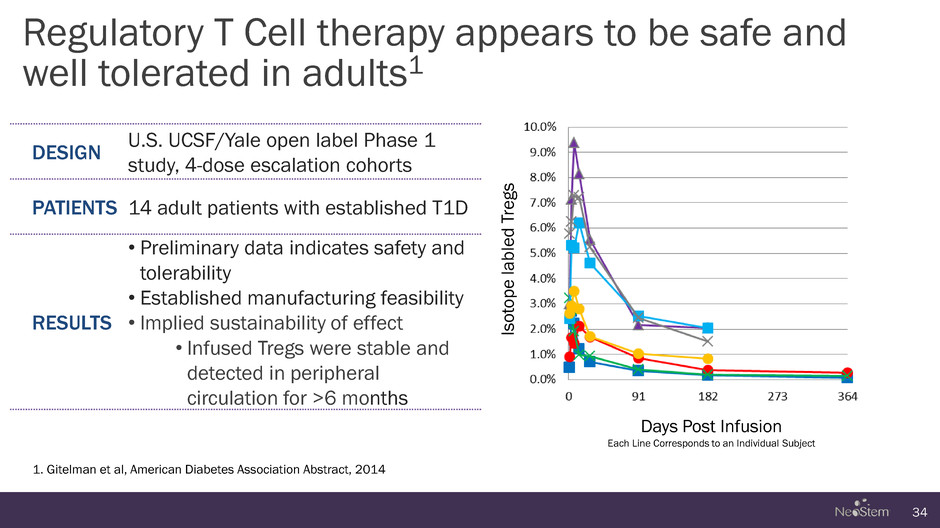
Regulatory T Cell therapy appears to be safe and well tolerated in adults1 34 1. Gitelman et al, American Diabetes Association Abstract, 2014 DESIGN U.S. UCSF/Yale open label Phase 1 study, 4-dose escalation cohorts PATIENTS 14 adult patients with established T1D RESULTS • Preliminary data indicates safety and tolerability • Established manufacturing feasibility • Implied sustainability of effect • Infused Tregs were stable and detected in peripheral circulation for >6 months Is o tope la b le d T re g s Days Post Infusion Each Line Corresponds to an Individual Subject
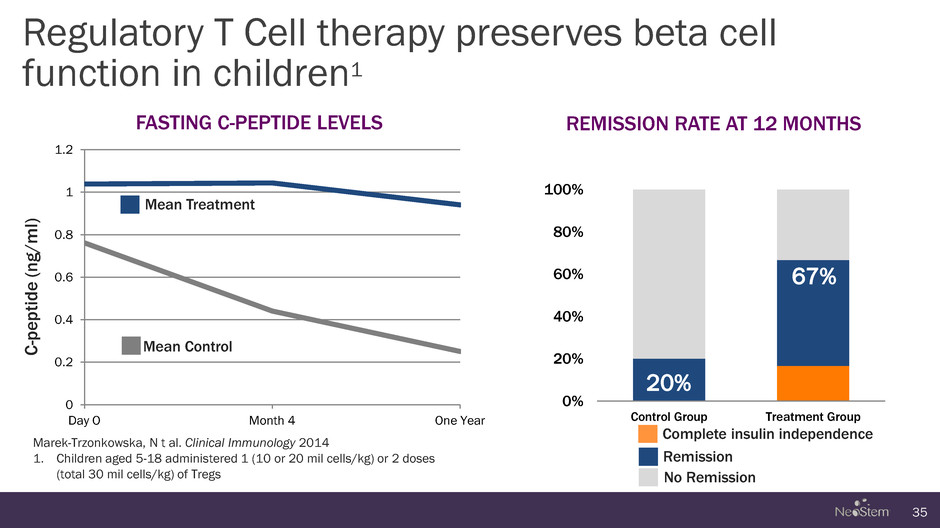
0 0.2 0.4 0.6 0.8 1 1.2 Day O Month 4 One Year 0% 20% 40% 60% 80% 100% Control Group Treatment Group Regulatory T Cell therapy preserves beta cell function in children1 35 Marek-Trzonkowska, N t al. Clinical Immunology 2014 1. Children aged 5-18 administered 1 (10 or 20 mil cells/kg) or 2 doses (total 30 mil cells/kg) of Tregs REMISSION RATE AT 12 MONTHS FASTING C-PEPTIDE LEVELS C -p e pt id e ( n g /m l) 67% 20% Mean Treatment Mean Control Complete insulin independence Remission No Remission
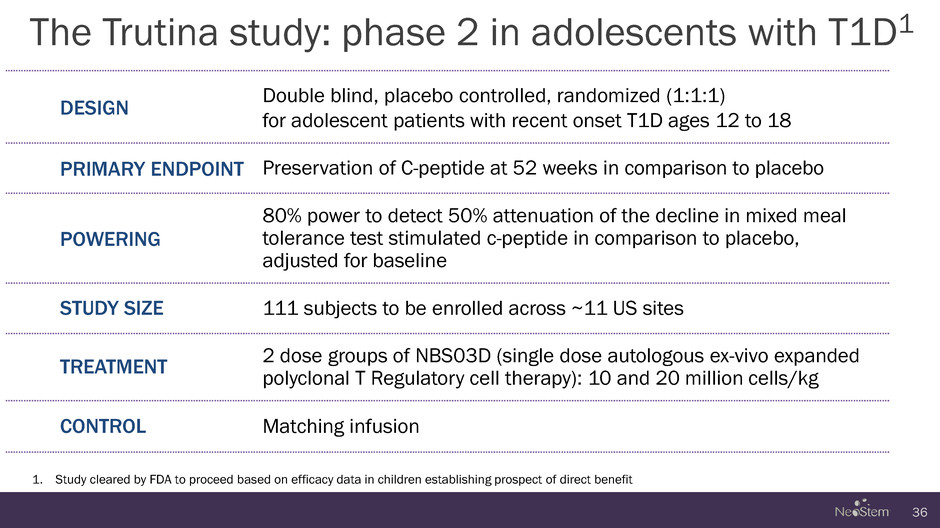
The Trutina study: phase 2 in adolescents with T1D1 36 DESIGN Double blind, placebo controlled, randomized (1:1:1) for adolescent patients with recent onset T1D ages 12 to 18 PRIMARY ENDPOINT Preservation of C-peptide at 52 weeks in comparison to placebo POWERING 80% power to detect 50% attenuation of the decline in mixed meal tolerance test stimulated c-peptide in comparison to placebo, adjusted for baseline STUDY SIZE 111 subjects to be enrolled across ~11 US sites TREATMENT 2 dose groups of NBS03D (single dose autologous ex-vivo expanded polyclonal T Regulatory cell therapy): 10 and 20 million cells/kg CONTROL Matching infusion 1. Study cleared by FDA to proceed based on efficacy data in children establishing prospect of direct benefit
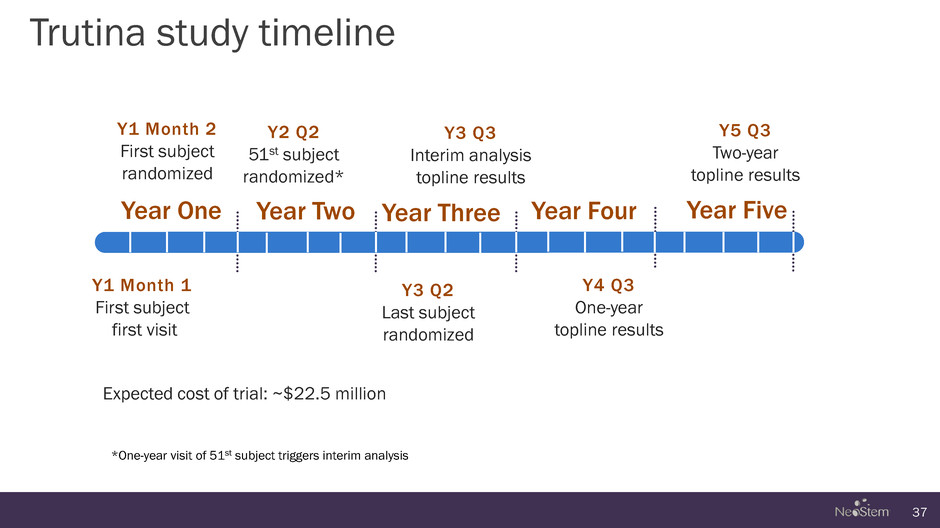
Trutina study timeline 37 Y1 Month 1 First subject first visit Year One Year Two Year Three Year Four Year Five Y1 Month 2 First subject randomized Y2 Q2 51st subject randomized* Y3 Q2 Last subject randomized Y3 Q3 Interim analysis topline results Y4 Q3 One-year topline results Y5 Q3 Two-year topline results *One-year visit of 51st subject triggers interim analysis Expected cost of trial: ~$22.5 million
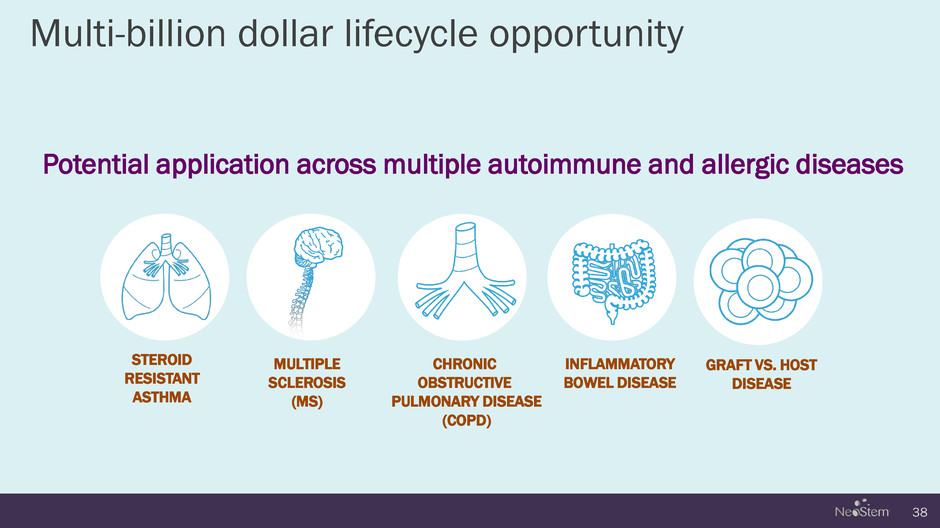
Multi-billion dollar lifecycle opportunity Potential application across multiple autoimmune and allergic diseases 38 STEROID RESISTANT ASTHMA MULTIPLE SCLEROSIS (MS) CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) INFLAMMATORY BOWEL DISEASE GRAFT VS. HOST DISEASE
Investment summary 39 Highly experienced management and scientific team Proprietary platform technologies yielding a diversified, balanced pipeline targeting critical unmet needs in large global markets and near-term development milestones Immunotherapy platform applicable across multiple solid tumors with Fast Track and Orphan Drug designations and a SPA for a phase 3 study in metastatic melanoma Cell therapy platform applicable in multiple cardiovascular indications with ongoing Phase 2 study for acute myocardial infarction – compelling and consistent interim results Immunomodulation therapy platform targeting autoimmune disorders with FDA cleared phase 2 study in adolescents with type I diabetes Internal center of excellence (PCT) with bicoastal facilities and proven capabilities innovating discovery, development, manufacturing and delivery of cell-based therapies

Corporate Presentation NASDAQ: NBS March 2015 Transforming Personalized Medicine Contact: Eric Powers, Manager of Communications & Marketing Phone: 212.584.4173 Email: epowers@neostem.com Web: www.neostem.com







































