EXHIBIT 10.14
| Corporation | TERMINATION, PURCHASE AND LICENSE AGREEMENT |
This Termination, Purchase and License Agreement ("Agreement") is made as of ____________________, 2008 ("Effective Date"), by and between Boston Scientific Corporation, One Boston Scientific Place, Natick, MA 01760 ("Seller"), and Bovie Medical Corporation, 7100 30th Avenue-North, St. Petersburg, FL 33710 ("Buyer") for the purpose of purchase and sale of certain rights and assets related to the Program (as defined in Section 1.1) and use of the rights and assets in the development, manufacture and sale of the Product (as defined in Section 1.2); assignments and licenses for certain intellectual property of the Parties; and termination of that certain Distribution Agreement between the Parties dated as of October 6, 2006, as amended on August 23, 2007 (the “Distribution Agreement”), all in accordance with this Agreement. Buyer and Seller are herein referred to collectively as “Parties” and individually as a “Party.”
| MAILING ADDRESSES AND FAX NUMBERS FOR NOTICES, ETC. UNDER AGREEMENT |
| | |
Buyer: Bovie Medical Corporation 7100 30th Avenue North St. Petersburg, FL 33710 Attn: Moshe Citronowicz, COO Fax: (727) 344-3876 | with copy to: Bovie Medical Corporation 7100 30th Avenue North St. Petersburg, FL 33710 Attn: General Counsel Fax: (727) 344-3876 |
Seller: Boston Scientific Corporation 100 Boston Scientific Way Marlborough, Massachusetts 01752 Attn: Michael Phalen, President Endoscopy Fax: (508) 683-5316 | with copy to: Boston Scientific Corporation One Boston Scientific Place Natick, Massachusetts 01760-1537 Attn: General Counsel Fax: (508) 650-8956 |
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereto agree as follows:
1. Purchase and Sale
1.1 Program. For purposes of this Agreement, “Program” means the SEER Sintered Tip Resection Device, BSC Project # M0380.
1.2 Product. For purposes of this Agreement, “Product” means any medical device having a sintered, conductive metal tip in accordance with the Product Specifications attached hereto as Exhibit A (Revisions A-1 & A-2) for use in the Field. Product specifically does not include any generator or generator accessories. “Field” means liver, pancreatic and kidney tumor, orthopedic, and blood vessel sealing, therapy by delivery of RF current and sterile saline for resection, hemostatic sealing and coagulation of soft tissue in open surgery and/or laparoscopic surgery.
1.3 Assets. At the closing, and upon the terms and conditions of this Agreement, Seller hereby sells, transfers, conveys, assigns and delivers to Buyer, and Buyer purchases from Seller, free and clear of any and all liabilities, obligations, liens and encumbrances, all right, title and interest in and to the following Program assets of Seller (collectively, the “Purchased Assets”), wherever located, :
(a) All equipment, machinery, prototypes, tooling, supplies, and other personal property of the Seller predominantly related to the Program and the design, development and manufacture of the Product, including all records related thereto, as set forth on Exhibit X hereto;
| BUYER: __________ | Page 1 of 8 | SELLER: __________ |
(b) all of Seller’s rights and interest in and under the contracts, licenses, leases and other agreements and instruments predominantly related to the Program and the design, development and manufacture of the Product, as set forth on Exhibit X hereto, together with true, complete and accurate copies of the same (the “Transferred Contracts”);
(c) to the extent assignable, approvals, clearances, authorizations, licenses and registrations required by any governmental authority, predominantly related to the Program and to permit the design, development, pre-clinical and clinical testing, manufacturing, labeling, sale, distribution, and promotion of the Product as set forth on Exhibit X hereto, together with true, complete and accurate copies of the same (the “Transferred Permits”);
(d) all scientific, clinical, technical, marketing, pre-sales and other data, predominantly related to the Program and the design, development and manufacture of the Product, including, without limitation, files, formulas, compositions, computer discs and tapes (and reasonable use of the means to access or convert them to a form useable by Buyer, if necessary), laboratory notebooks, design histories, operating manuals and procedures, instructions for use, device history records, bills of materials, and manufacturing, inspection and quality control records and procedures, as set forth on Exhibit X hereto;
(e) the Generated Product IP (as defined in Section 2.1), together with true, complete and accurate copies of the documents pertaining thereto including, without limitation, documents used by Seller (or its counsel) in the preparation and prosecution of the patents comprising the Generated Product IP, except as to the excluded document categories set forth on Exhibit X hereto;
(f) all guarantees, warranties, indemnities and similar rights in favor of Seller with respect to any Purchased Asset; and
(g) all rights to causes of action, lawsuits, judgments, claims and demands of any nature available to, or being pursued by, Seller, with respect to the Purchased Assets, whether arising by way of counterclaim or otherwise.
1.4 Delivery of Purchased Assets. No later than _____ (_) business days after the closing, Seller shall package and ship (or cause to be packaged and shipped) to Buyer all tangible personal property elements of the Purchased Assets not in Buyer’s possession and custody as of the closing, at Seller’s expense.
2. Assignment of Intellectual Property; Cross-Licenses.
2.1 Assignment of Generated Product IP. At the closing, and upon the terms and conditions of this Agreement, Seller hereby sells, transfers, conveys and assigns to Buyer, and Buyer purchases from Seller, free and clear of any and all liabilities, obligations, liens and encumbrances, all right, title and interest in and to any and all Intellectual Property Rights (as defined below) generated in the performance of the Program and as a result of development work on the Product by or on behalf of either Party (collectively, the “Generated Product IP”), including but not limited to:
(a) ** **;
(b) US2007/0156134, filed December 29, 2005, ** **;;
(c) U.S. Patent #7,282,051, filed February 4, 2004, issued October 16, 2007, and continuation 11/550,374 (US2007/0123848); and
(d) ** **;.
For purposes of this Agreement, “Intellectual Property Rights” means intellectual property or proprietary rights of any description worldwide including without limitation (i) rights in any patent, patent registration, patent application, copyrights, industrial designs, trademarks, (ii) trade secrets, moral rights, shop rights and publicity rights, (iii) inventions, discoveries, know-how, techniques, methodologies, designs or data, whether or not patented, patentable or copyrightable, (iv) rights to sue for and remedies against past, present and future infringements or misappropriations thereof, and rights of priority and protection of interests therein under the laws of any jurisdiction worldwide and all tangible embodiments thereof, and (v) goodwill related to any of the foregoing.
| BUYER: __________ | Page 2 of 8 | SELLER: __________ |
2.2 License to Generated Product IP. At the closing, and upon the terms and conditions of this Agreement, Buyer hereby grants to Seller a non-exclusive, fully paid up, royalty-free, worldwide, perpetual, irrevocable license to the Generated Product IP solely to make, have made, use, offer for sale, sell, have sold, import and export any Seller Products. For purposes of this Agreement, “Seller Products” means any product or device of Seller, provided however, such product or device shall be for use in applications outside of a Product for the Field. As of January 1, 2016, the license granted to Seller under this Section 2.2 shall cease to include the limitation in the previous sentence, i.e., “for use in applications outside of a Product for the Field.” The license granted to Seller hereunder is not sublicensable, but is transferable by Seller to a buyer only in connection with the sale of all or substantially all of the assets relating to any Seller Product.
2.3 License to Existing Product Patents. At the closing, and upon the terms and conditions of this Agreement, Seller hereby grants to Buyer a non-exclusive, fully paid up, royalty-free, worldwide, perpetual, irrevocable license to Existing Product Patents solely to make, have made, use, offer for sale, sell, have sold, import and export the Product for use in the Field. For purposes of this Agreement, “Existing Product Patents” shall include any of Seller’s invention disclosures, trade secrets and patent applications (but not to the extent of disclosing non-public details of such disclosures, trade secrets, or patent applications), and issued and issuing patents existing as of the Effective Date, including continuations and foreign counterparts thereof obtained thereafter, necessary for the non-infringing manufacture, use and sale of the Product. The foregoing license is not sublicensable but is transferable by Buyer to a buyer only in connection with the sale of all or substantially all of the assets relating to the Product.
3. Termination of Distribution Agreement and Releases.
3.1 Termination. The Distribution Agreement is hereby terminated as of the Effective Date.
3.2 Releases. Each Party, along with its employees, officers, directors, affiliates, subcontractors, agents, successors or assigns, hereby releases and discharges the other Party, along with its employees, officers, directors, affiliates, subcontractors, agents, successors or assigns, of any and all obligations, liabilities and claims, whether known or unknown, accrued or not accrued, contingent or otherwise, arising prior to the Effective Date, directly or indirectly, out of or related to, the Distribution Agreement, the performance of the Distribution Agreement or the termination of the Distribution Agreement.
4. Confidential Information
4.1 Confidential Information.
(a) "Confidential Information" means all proprietary information disclosed by or on behalf of either Party hereto (a “Disclosing Party”) to the other Party hereto (a “Receiving Party”), or any of the Disclosing Party’s or Receiving Party’s employees, officers, directors, affiliates, subcontractors, agents, successors or assigns (collectively “Representatives” and together with the Disclosing Party, the “Disclosing Group” or together with the Receiving Party, the “Receiving Group”), including information relating to the matters that are the subject of this Agreement, including the terms, existence and nature of this Agreement, or relating to the Disclosing Party’s other past, present or future research, technology, know-how, ideas, concepts, designs, products, markets, customer information, computer programs, prototypes, processes, machines, articles of manufacture, compositions of matter, business plans and operations, technical information, drawings, or specifications; except information which is: (i) at the time of disclosure, or thereafter becomes lawfully part of the public domain through no act or omission by the Receiving Party; (ii) lawfully in the possession of Receiving Party prior to disclosure by or on behalf of Disclosing Party, as shown by written records; (iii) lawfully disclosed to the Receiving Party by a third party which did not acquire the same under an obligation of confidentiality from or through the Disclosing Group; or (iv) independently developed by the Receiving Party without use of Confidential Information, as shown by written records. If a Receiving Party believes in good faith that it is required by law to disclose any Confidential Information, it shall provide notice to the Disclosing Party, prior to making such disclosure so as to allow Disclosing Party time to undertake legal or other action, to prevent such disclosure or otherwise obtain confidential treatment of such disclosure.
| BUYER: __________ | Page 3 of 8 | SELLER: __________ |
(b) A Receiving Party shall not, without the prior consent of the Disclosing Party, disclose any Confidential Information to anyone for any reason at any time or use any Confidential Information for any purpose except as requested by the Disclosing Party. The Receiving Party shall limit dissemination of Confidential Information to only those of the Receiving Group having a "need to know." A Receiving Party shall not, except as permitted under this Agreement: (i) appropriate or use a Disclosing Party’s Confidential Information in Receiving Party’s own manufacture of products for itself or for any third party or for any other purpose; or (ii) obtain any title to, or any interest or license in, any Confidential Information of a Disclosing Party.
(c) Neither Party shall issue a press release or other public announcement concerning this Agreement (or any term sheet, bids, negotiations or other related information), the transactions contemplated herein, or the relationship between the Parties without the prior written approval of an authorized representative of the other Party, which such approval the other Party shall not unreasonably withhold or delay, provided however, a Party may issue a press release, public announcement or disclosure of this Agreement if required by the regulations of a securities exchange on which such Party’s securities are listed if (i) it gives the other Party prompt notice of such requirement and (ii) it cooperates with the other Party with respect to reasonable requests for revisions to any announcement or reasonable requests for confidential treatment of certain sections of this Agreement.
(d) Neither Party shall: (i) disclose to the other Party any confidential or proprietary information belonging to any third party without the consent of such third party; nor (ii) represent as being unrestricted any designs, plans, models, samples, or other writings or products that the Disclosing Party knows or has reason to know are covered by Intellectual Property Rights of a third party.
5. Indemnification.
(a) Buyer shall indemnify, defend and hold harmless Seller and its affiliates and their respective directors, officers, employees and agents from and against any claim, action, suit, demand, damage, expense or losses (including reasonable attorneys’ fees) by a third party (collectively, "Claims") resulting from or to the extent relating to: (i) Buyer’s manufacture, use, sale and marketing of the Product, including but not limited to Claims relating to personal injury; or (ii) infringement or alleged infringement of any third party Intellectual Property Rights with respect to the sale of the Product.
(b) Seller shall indemnify, defend and hold harmless Buyer and its affiliates and their respective directors, officers, employees and agents from and against any Claims resulting from or to the extent relating to Seller’s material breach of this Agreement including, without limitation, any material inaccuracy of Seller’s representations or warranties, or material breach of Seller’s covenants, under Section 7 hereof.
(c) Either Party’s (the “indemnifying Party”) obligations to the other Party (the “indemnified Party”) under this Section 5 are conditioned upon the indemnified Party: (i) providing written notice to the indemnifying Party of any Claims promptly, but not later than fifteen (15) calendar days after the indemnified Party knows of such Claim; (ii) permitting the indemnifying Party to assume full responsibility for the defense of such Claim; (iii) assisting the indemnifying Party in defense of such Claim at the indemnifying Party’s expense; and (iv) not compromising or settling any such Claim without the indemnifying Party’s prior written consent. Indemnifying Party may not settle a Claim without the indemnified Party’s prior written consent, which consent shall not be unreasonably withheld or delayed, unless such settlement includes a full release of the indemnified Party from all liability and without any condition of future consideration. Notwithstanding the foregoing, the indemnified Party’s failure to give the notice specified in this Section or delay in giving such notice, shall not affect the indemnified Party’s right to indemnification under this Section except to the extent that indemnifying Party has been prejudiced by such failure or delay.
6. Remedies. Termination of this Agreement, or the exercise of any other remedy, shall not be deemed to be an exclusive remedy hereunder, and shall be in addition to any other remedies available at law or in equity (including a Party’s right to obtain specific performance and other equitable relief for other Party’s material breach hereof).
7. Representations and Warranties; Covenants. Each Party hereby represents and warrants to the other that: (a) the execution and delivery of and performance under this Agreement by such Party does not, and will not, conflict with or violate any other agreement or obligations with third parties or any restrictions of any kind or any law to which it is bound or subject; and (b) it has the unrestricted right to disclose any information it submits to the other Party, free of all claims of third parties, and that such disclosures do not breach or conflict with any confidentiality provisions of any agreement to which it is a party.
| BUYER: __________ | Page 4 of 8 | SELLER: __________ |
7.1 Purchased Assets. Seller hereby represents and warrants to Buyer that Seller has good title to the Purchased Assets, and that the Purchased Assets transferred or otherwise conveyed hereunder to Buyer are and shall be free and clear of any and all liabilities, obligations, liens and encumbrances.
7.2 Further Assurances. Following the closing, Seller shall, from time to time, execute and deliver such additional instruments, documents, conveyances or assurances, and take such other actions as shall be reasonably necessary, or otherwise reasonably requested by the Buyer, to confirm and assure the rights and obligations provided for in this Agreement and to render effective the consummation of the transactions contemplated hereby.
7.3 Authorization. Each Party has the corporate power and authority to execute and deliver this Agreement, to perform fully its obligations hereunder, and to consummate the transactions contemplated thereby. The execution and delivery by each Party of this Agreement, and the consummation of the transactions contemplated hereby, have been duly authorized by all requisite corporate action of each Party. This Agreement constitutes legal, valid and binding obligations of each Party, enforceable against it in accordance with the terms and conditions hereof.
7.4 Litigation. There is no action, claim, demand, suit, proceeding, arbitration, grievance, citation, summons, subpoena, inquiry or investigation of any nature, civil, criminal, regulatory or otherwise, in law or in equity, pending or threatened against Seller in connection with the Purchased Assets, and Seller does not know or have reason to be aware of any basis for the same.
7.5 Brokers, Finders. All negotiations relating to this Agreement have been carried on without the participation of any person acting on behalf of Seller or its affiliates in such manner as to give rise to any valid claim against the Buyer for any brokerage or finder's commission, fee or similar compensation, or for any bonus payable to any officer, director, employee, agent or sales representative of or consultant to Seller or their respective affiliates upon consummation of the transactions contemplated hereby.
7.6 Liability for Transfer Taxes. In the event any sales (including, without limitation, bulk sales), use, value-added, documentary, stamp, registration, transfer, conveyance, excise, recording, license and other similar taxes and fees (collectively, "Transfer Taxes"), arising out of or in connection with or attributable to the transactions effected pursuant to this Agreement are due, the Parties shall agree as to which Party shall prepare any required returns or notices and the Parties shall evenly split the cost of preparing such returns and the amount of any Transfer Taxes due.
8. Miscellaneous
8.1 Except as specifically set forth in Sections 2.2 and 2.3 neither Party shall assign this Agreement or its obligations hereunder, whether voluntarily or involuntarily, without the express prior written consent of the other Party.
8.2 This Agreement is fully binding upon the Parties’ successors and permitted assigns.
8.3 All requests, approvals, consents and notices must be in writing and will be effective as of the date actually received and, unless otherwise specified in this Agreement, shall be sent as follows: (i) certified mail - return receipt requested; (ii) a nationally recognized overnight delivery service that guarantees overnight delivery and requires the signature of recipient; or (iii) facsimile, transmission confirmed; to the addresses and fax numbers indicated on the first page of this Agreement; provided, however, that in the case of a facsimile transmission a copy is also sent one of the foregoing methods of subsection (i) or (ii), above.
8.4 This Agreement: (i) is governed by the laws of The State of New York, without reference to its internal principles of conflicts of laws, any disputes shall be brought exclusively in a federal or state court residing in New York, and the parties agree without objection to the jurisdiction and venue of such court; (ii) together with all Exhibits thereto (which are hereby incorporated into this Agreement) is the entire and exclusive set of terms and conditions and supersedes all prior agreements and understandings, both written and oral, between the Parties with respect to the subject matter of this Agreement and termination of the Distribution Agreement; and (iii) may only be modified by a writing signed by both Parties.
8.5 Headings of the articles, sections and subsections of this Agreement, and the name of this Agreement, are for reference purposes only and shall not limit or affect the meaning or construction of the terms and conditions hereof. Whenever the words “include”, “includes” or “including” are used in this Agreement, they shall be deemed in each instance to be followed by the words “without limitation.”
| BUYER: __________ | Page 5 of 8 | SELLER: __________ |
8.6 No failure of either Party to enforce any right under this Agreement shall be deemed a waiver thereof.
8.7 All obligations and rights which are by their nature continuing, including the obligations contained in Sections 2.2, 2.3, 4, 5, 7, and 8 shall survive the expiration or termination of this Agreement.
By signing below the undersigned acknowledge and accept all terms and conditions of this Agreement.
BUYER: BOVIE MEDICAL CORPORATION | | SELLER: BOSTON SCIENTIFIC CORPORATION | |
| | | | | | |
| By: | /S/ Moshe Citronowicz | | By: | /S/ Michael P. Phalen | |
| | (Signature) | | | (Signature) | |
| Print Name: | /S/ Moshe Citronowicz | | Print Name: | /S/ Michael P. Phalen | |
| Title: | Vice President / COO | | Title: | | |
| Date Signed: | 4-29-08 | | Date Signed: | 4-30-08 | |
| | | | | | |
| BUYER: __________ | Page 6 of 8 | SELLER: __________ |
Exhibit X: Purchased Assets
Sect 1.3a) Equipment, machinery, prototypes, tooling, supplies, and other personal property predominantly related to the Program
Prototypes
| 12 assembled prototypes in trays | (BSC) |
| 60 porous tips w/o powder coat | (BSC) |
| 20 short samples – blue powder coat | (BSC) |
| 20 laser and resistance welded samples | (BSC) |
| 1 laparoscopic prototype | (BSC) |
| 3 bipolar prototypes | (BSC) |
| Any prototype from ** ** | (Bovie) |
| Any prototype with ** ** | (Bovie) |
| Any packaging prototypes | (Bovie) |
PDM Project Files
See 1.3d, below
Equipment
None
Tooling and Molds
| All of BSC’s right, title and interest in component tooling with | ** ** |
| Handle Molds -9 tools | ** ** |
| Packaging Molds | ** ** |
Components and Test Fixtures
| Any components with ** ** | (Bovie) |
| Any components with ** ** | (Bovie) |
Sect. 1.3b) Seller’s right and interest in and under the contracts, licenses, leases and other agreements and instruments predominantly related to the Program
Contracts
| ** ** letter with terms and Conditions | (BSC) |
| PO’s to ** ** | (BSC) |
| ** ** quotes | (BSC) |
Section 1.3c) Approvals, clearances, authorizations, license and registrations required by any governmental authority, predominantly related to the Program
None
Section 1.3d) Clinical, technical, marketing, pre-sales and other data, predominantly related to the Program and the design, development and manufacture of the Product
| BUYER: __________ | Page 7 of 8 | SELLER: __________ |
Product and Marketing Specifications
| Product Spec attached as part of contract | (Bovie) |
| Drawings | (Bovie) |
| Customer Input | (BSC) |
| BSC PDM Files | (BSC) |
| Design History File | (Bovie) |
Technical Documents, Testing, Notebook Entries, Schedules and Activities
| BSC PDM Files | (BSC) |
| Personal Files | (BSC) |
| Network Drives | (BSC) |
| ** ** | (BSC) |
| ** ** Documents | (BSC) |
| ** ** Documents | (BSC) |
Meeting Minutes, Presentations, Trip Reports, Quality Documents, Design Control
| BSC PDM Files | (BSC) |
| Personal Files | (BSC) |
| Network Drives | (BSC) |
| ** ** Documents | (BSC) |
| ** ** Documents | (BSC) |
Animal and Bench Testing, Regulatory Documents, Physician Feedback
| Video and Photos | (BSC) |
| Includes ** ** demo | |
| BSC PDM Files | (BSC) |
| Personal Files | (BSC) |
| Network Drives | (BSC) |
Marketing Data, Customer Input
| Personal Files | (BSC) |
| Network Drives | (BSC) |
1.3e) Generated Product IP
Excluded Document Categories
Only documents or portions thereof, to the extent they incorporate attorney-client privileged legal analysis, prepared in response to BSC’s request to its internal or external counsel, related to the General Product IP. Nothwithstanding the foregoing, patent search results and drafts of unfiled patent applications shall not be excluded.
| BUYER: __________ | Page 8 of 8 | SELLER: __________ |
BOVIE –
Resection Device
Product Specifications
BOVIE MEDICAL CORPORATION
CONFIDENTIAL
FOR INTERNAL USE ONLY
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
Table of Contents
| 1 INTRODUCTION | 4 |
| 1.1 | Purpose | 4 |
| 1.2 | Scope | 4 |
| 2 APPLICABLE DOCUMENTS | 4 |
| 2.1 | Standards | 4 |
| 3.0 GENERAL DESCRIPTION | 4 |
| 3.1 | Concept Drawing | 5 |
| 3.2 | Application | 5 |
| 3.2.1 | Intended Use | 5 |
| 3.2.2 | Intended Users | 6 |
| 3.3 | Quality System Requirements | 6 |
| 3.3.1 | US Requirements | 6 |
| 3.3.2 | Canadian Requirements | 6 |
| 3.3.3 | European Classifications | 7 |
| 3.3.4 | Quality System Requirements | 7 |
| 4 PRODUCT DOCUMENT STRUCTURE | 7 |
| 4.1 | Project Documentation | 7 |
| 4.2 | Model Number Format | 7 |
| 5 PRODUCT REQUIREMENTS | 8 |
| 5.1 | Electrical and Mechanical | 8 |
| 5.1.1 | Insulated Handle | 8 |
| 5.1.2 | Shaft and Electrode Tip | 9 |
| 5.1.3 | Shipping and Handling | 10 |
| 5.1.4 | Sterilization | 10 |
| 6 Protection Against Hazards | 10 |
| 7 Packaging | 10 |
| 7.1 | Labeling | 10 |
| 7.2 | Packaging Configuration | 11 |
| 7.3 | Manufacturing | 11 |
| | | |
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
List of Tables
| Table One – Standards | Page 4 |
| Table Two – Device Classifications | Page 6 |
| Table Three – Model Number Format | Page 7 |
List of Figures
| Figure One – Concept Drawing | Page 5 |
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
This Product Specification defines preliminary product requirements and constitutes a part of the Design Inputs for the Resection Device Project for Boston Scientific Corporation (“BSC”).
This Product Specification sets forth the requirements, provided by BSC, for the Resection Device, (the “Device”).
The following is a list of all documents and other sources of information referenced in this Product Specification.
| Standard | Version/Date | Description |
| 21 CFR Part 820 | | Medical Devices, Current Good Manufacturing Practices, Final Rule, Quality System Regulation. |
| HF-18 | 2001 | AAMI/ANSI Electrosurgical Devices |
| 10993-1 | 3; Date 8-1-03 | AAMI/ANSI/ISO Biological evaluation of medical devices – Part 1: Evaluation and Testing |
| EN 980 | 2003 04/16/2003 | Graphical symbols for use in the labeling of medical devices |
| EN ISO 14971 | 2000 AMD 1 2003 03/01/2003 | Medical devices – Application of risk management to risk management to medical devices (ISO 14971:2000) |
| ISTA 2A 2006 | 2006 | Performance Test for Packaged-Products Weighing 150 lbs (68 kg) or Less |
Table 1 - Applicable Standards for Medical Devices (US)
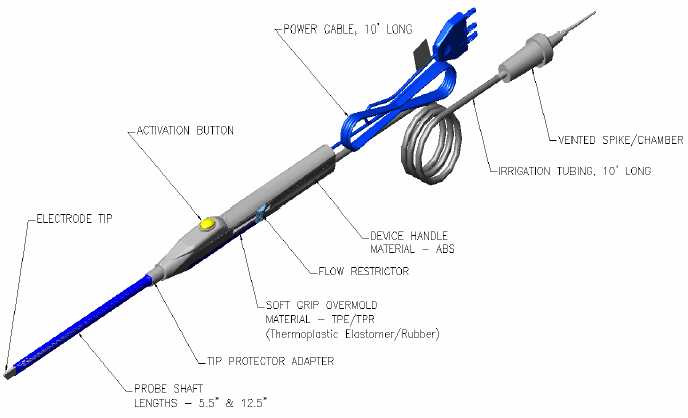
The Device is a sterile, single use electrosurgical device intended to be used in conjunction with an electrosurgical generator for the delivery of radiofrequency (“RF”) current and sterile saline for hemostatic sealing and coagulation of soft tissue in accordance with instructions and user procedures provided by BSC. The device is not intended for any other unspecified uses.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
Figure 1
The Device is a sterile, single use electrosurgical device intended to be used in conjunction with an electrosurgical generator for the delivery of radiofrequency current and sterile saline for hemostatic sealing and coagulation of the soft tissue in accordance with instructions and user procedures provided by BSC. The device is not intended for any other unspecified uses.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
Users of this Device will be healthcare professionals such as medical doctors and nurses, who are qualified and trained in electrosurgical procedures.
3.3 Quality System Requirements
The Device has the following classifications for FDA, CMDR, and MDD classification.
| Device | US Classification | Canada Classification | MDD Classification |
| Resection Device | II | III | IIb |
Table 2 - Device Classifications
To market medical products, the Food and Drug Administration (FDA) must determine that a medical device is substantially equivalent to similar marketed medical devices. The Bovie Regulatory department intends to use the dissecting sealer device manufactured by TissueLink as a predicate device.
The Device’s classification is Class II.
3.3.1.2 Safety and Effectiveness requirements
The Device will meet electrical safety requirements (ANSI/AAMI HF-18:2001).
3.3.1.3 Quality System Requirements
Quality system requirements are specified as part of 21 CFR Part 820, Quality System Regulation.
3.3.2 Canadian Requirements
To market medical products in Canada, a license application must be presented to Health Canada. Once the license application is approved, the medical product can be labeled and made available for sale in the Canadian provinces.
Class III
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
3.3.2.2 Safety and Effectiveness requirements
Safety and effectiveness requirements closely correspond to the essential requirements of the Medical Device Directive. A list of Health Canada recognized standards has been issued as of April 11, 2002 in Policy on Recognition and Use of Standards under Medical Device Regulations.
3.3.2.3 Quality System Requirements
Health Canada requires manufacturers of Class II, III and IV device to demonstrate that their devices are manufactured in accordance with internationally recognized standards. Demonstration of compliance with the quality system requirements will be required at the time an application is made for a medical device license.
3.3.3 European Classifications
The Device is classified IIb (Rule 9 ) in accordance with the Medical Devices Directive Annex IX.
(Reference: Guidelines for the Classification of Medical Devices – MEDDEV 2.4/1 Rev. 8, July 2001)
3.3.3.2 Conformance Assessment Route
The Device assessment route will be via Annex II (full quality assurance system).
3.3.4 Quality System Requirements
Bovie Medical Corporation (“Bovie”) has been certified to ISO13485
| 4 | PRODUCT DOCUMENT STRUCTURE |
Documents will be in standard Bovie document format.
The following table lists the Device’s model number and corresponding product / catalog code.
| Catalog # | Comments |
| TBD | Laparoscopic Monopolar Device |
| TBD | Open Abdominal Monopolar Device |
Table Three
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
The following section will describe requirements for the insulated handle and shaft with electrode tip.
| 5.1 | Electrical and Mechanical |
The Device hardware is comprised of two (2) major components: (i) an Insulated Handle and (ii) a Shaft with Electrode Tip. RF energy is passed through the Insulated Handle through the Shaft to the Electrode Tip by a powered lead from an electrosurgical generator.
5.1.1.1 Insulated Handle and Power Cord Insulation Resistance
The insulation of the Insulated Handle and Power Cord shall meet Requirements of Section 4.2.5.4 Dielectric withstands of accessories ANSI/AAMI HF-18:2001
5.1.1.2 Insulated Handle Ergonomics
The Insulated Handle shall be designed for comfortable and efficient hand operation. It will incorporate an over-mold to give the body an elegant feel and soft touch.
The Insulated Handle Electrical Cord shall be approximately ten (10) feet in length and incorporate a 3-prong electrical plug.
5.1.1.4 Insulated Handle Flow Control Mechanism
The Device will have a flow control mechanism, either on the tubing or in the handle itself so the flow can be regulated by the user within the sterile field.
The tubing length should be approximately seven (7) feet in length and incorporate an I.V. spike on the end to attach directly to a hanging IV bag.
5.1.1.6 Insulated Handle Tubing Material
The tubing material should be a material that will resist kinking but should not have so strong a memory as to pull the probe off of the sterile field.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
The Device should have a Cut and Coagulation Mode. “Cut”/”Coag” buttons must be easy to operate.
5.1.1.8 Insulated Handle Compatibility
| | · | The Insulated Handle should have compatibility with standard RF generators used for electrocautery and standard grounding pad. |
| | · | The Insulated Handle should have compatibility with standard 0.9% saline I.V. bag. |
The Shaft and Electrode Tip provide the actual working portion of this system.
5.1.2.1 Saline Flow Rate of Device
The Saline flow rate prior to use will be ** **
5.1.2.2 Device (Shaft) Length
The Device should be straight and have two (2) lengths: one for laparoscopic monopolar procedures (approximately thirty-two (32) cm) and one for open monopolar procedures (approximately fourteen (14) cm).
5.1.2.3 Shaft Configuration
The Shaft will be hollow to allow for fluid to flow to the sintered stainless steel tip. The inside diameter of the Shaft should be equal to the inside diameter of the tubing that feeds fluid to the Shaft to minimize any flow restriction in the fluid circuit.
5.1.2.4 Tip Material
The Tip shall be made of ** **
.
5.1.2.5 Tip Strength
The porous blade tip to shaft tensile bond strength (axial mode) must be greater than ** **
after being subjected to a full-power simulated use duty cycle.
The porous blade tip to shaft bond strength in an flexural mode must be greater than ** **
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
. after being subjected to a full-power simulated use duty cycle.
5.1.2.6 Tip performance
| | · | Bovie will exert its best efforts to meet the design goal that tip cutting performance be superior to competitive devices with respect to cutting speed. Tip will have the ability to coagulate soft tissue when performed in a suitable simulated use environment. |
4.1.3.1 Shipping Temperatures
Tropical (Wet and Dry) Conditions and Winter (Frozen) Conditions per ISTA 2A.
4.1.3.2 Transportation Testing
The Final Product Packaging Configuration for the Device shall meet the finished device requirements after being subjected to Simulated Transportation Conditioning per ISTA 2A.
5.1.4.1 Withstand 2x ETO Sterilization.
5.1.4.2 EN 550: 1994 – Sterilization of Medical Devices – Validation and routine control of ethylene oxide sterilization.
5.1.4.3 AAMI/ANSI/ISO 11135 -1994 Medical Devices – Validation and routine control of ethylene oxide sterilization.
| Protection Against Hazards |
A Hazard/Risk Analysis will be performed throughout the design process. The Hazard/Risk Analysis will be documented and filed as part of the design history file.
7.1.1 Package must be labeled in accordance with both BSC and Bovie Labeling Standards.
7.1.2 BSC and Bovie branding, including mutually approved trademarks, will be on the Handle and/or the shaft.
7.1.3 The Device is to have a three (3) year shelf life under normal storage conditions.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
| | · | The Tip and Shaft protector shall be included into the Design. |
| | · | The Scratch Pad shall be packaged with the Device. |
| | · | The Device will be packaged in a Sterile Pouch. |
Final assembly and packaging will be performed by Bovie.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
APPROVALS
| Author | | | |
| | Thomas Feldhaus | | Date |
| Sales & Marketing | | | |
| | Rick Pfahl | | Date |
| Regulatory | | | |
| | Rick Kozloff | | Date |
| Quality | | | |
| | John Woody | | Date |
| Manufacturing | | | |
| | Lillian Eshem | | Date |
| Engineering | | | |
| | Fred Baron | | Date |
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
EXHIBIT A (ver A-2)
BOVIE –
Resection Device
Product Specifications
BOVIE MEDICAL CORPORATION
CONFIDENTIAL
FOR INTERNAL USE ONLY
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
Table of Contents
| 1 INTRODUCTION | 4 |
| 1.1 | Purpose | 4 |
| 1.2 | Scope | 4 |
| 2 APPLICABLE DOCUMENTS | 4 |
| 2.1 | Standards | 4 |
| 3.0 GENERAL DESCRIPTION | 4 |
| 3.1 | Concept Drawing | 5 |
| 3.2 | Application | 5 |
| 3.2.1 | Intended Use | 5 |
| 3.2.2 | Intended Users | 5 |
| 3.3 | Quality System Requirements | 6 |
| 3.3.1 | US Requirements | 6 |
| 3.3.2 | Canadian Requirements | 6 |
| 3.3.3 | European Classifications | 7 |
| 3.3.4 | Quality System Requirements | 7 |
| 4 PRODUCT DOCUMENT STRUCTURE | 7 |
| 4.1 | Project Documentation | 7 |
| 4.2 | Model Number Format | 7 |
| 5 PRODUCT REQUIREMENTS | 7 |
| 5.1 | Electrical and Mechanical | 8 |
| 5.1.1 | Insulated Handle | 8 |
| 5.1.2 | Shaft and Electrode Tip | 9 |
| 5.1.3 | Shipping and Handling | 10 |
| 5.1.4 | Sterilization | 10 |
| 6 Protection Against Hazards | 10 |
| 7 Packaging | 10 |
| 7.1 | Labeling | 10 |
| 7.2 | Packaging Configuration | 10 |
| 7.3 | Manufacturing | 10 |
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
List of Tables
| Table One – Standards | Page 4 |
| Table Two – Device Classifications | Page 6 |
| Table Three – Model Number Format | Page 7 |
List of Figures
| Figure One – Concept Drawing | Page 5 |
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
This Product Specification defines preliminary product requirements and constitutes a part of the Design Inputs for the Resection Device Project for Boston Scientific Corporation (“BSC”).
This Product Specification sets forth the requirements, provided by BSC, for the Resection Device, (the “Device”).
The following is a list of all documents and other sources of information referenced in this Product Specification.
| Standard | Version/Date | Description |
| 21 CFR Part 820 | | Medical Devices, Current Good Manufacturing Practices, Final Rule, Quality System Regulation. |
| HF-18 | 2001 | AAMI/ANSI Electrosurgical Devices |
| 10993-1 | 3; Date 8-1-03 | AAMI/ANSI/ISO Biological evaluation of medical devices – Part 1: Evaluation and Testing |
| EN 980 | 2003 04/16/2003 | Graphical symbols for use in the labeling of medical devices |
| EN ISO 14971 | 2000 AMD 1 2003 03/01/2003 | Medical devices – Application of risk management to risk management to medical devices (ISO 14971:2000) |
| ISTA 2A 2006 | 2006 | Performance Test for Packaged-Products Weighing 150 lbs (68 kg) or Less |
Table 1 - Applicable Standards for Medical Devices (US)
The Device is a sterile, single use electrosurgical device intended to be used in conjunction with an electrosurgical generator for the delivery of radiofrequency (“RF”) current and sterile saline for hemostatic sealing and coagulation of soft tissue in accordance with instructions and user procedures provided by BSC. The device is not intended for any other unspecified uses.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
Figure 1
The Device is a sterile, single use electrosurgical device intended to be used in conjunction with an electrosurgical generator for the delivery of radiofrequency current and sterile saline for hemostatic sealing and coagulation of the soft tissue in accordance with instructions and user procedures provided by BSC. The device is not intended for any other unspecified uses.
Users of this Device will be healthcare professionals such as medical doctors and nurses, who are qualified and trained in electrosurgical procedures.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
3.3 Quality System Requirements
The Device has the following classifications for FDA, CMDR, and MDD classification.
| Device | US Classification | Canada Classification | MDD Classification |
| Resection Device | II | III | IIb |
Table 2 - Device Classifications
To market medical products, the Food and Drug Administration (FDA) must determine that a medical device is substantially equivalent to similar marketed medical devices. The Bovie Regulatory department intends to use the dissecting sealer device manufactured by TissueLink as a predicate device.
The Device’s classification is Class II.
3.3.1.2 Safety and Effectiveness requirements
The Device will meet electrical safety requirements (ANSI/AAMI HF-18:2001).
3.3.1.3 Quality System Requirements
Quality system requirements are specified as part of 21 CFR Part 820, Quality System Regulation.
3.3.2 Canadian Requirements
To market medical products in Canada, a license application must be presented to Health Canada. Once the license application is approved, the medical product can be labeled and made available for sale in the Canadian provinces.
Class III
3.3.2.2 Safety and Effectiveness requirements
Safety and effectiveness requirements closely correspond to the essential requirements of the Medical Device Directive. A list of Health Canada recognized standards has been issued as of April 11, 2002 in Policy on Recognition and Use of Standards under Medical Device Regulations.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
3.3.2.3 Quality System Requirements
Health Canada requires manufacturers of Class II, III and IV device to demonstrate that their devices are manufactured in accordance with internationally recognized standards. Demonstration of compliance with the quality system requirements will be required at the time an application is made for a medical device license.
3.3.3 European Classifications
The Device is classified IIb (Rule 9 ) in accordance with the Medical Devices Directive Annex IX.
(Reference: Guidelines for the Classification of Medical Devices – MEDDEV 2.4/1 Rev. 8, July 2001)
3.3.3.2 Conformance Assessment Route
The Device assessment route will be via Annex II (full quality assurance system).
3.3.4 Quality System Requirements
Bovie Medical Corporation (“Bovie”) has been certified to ISO13485
| 4 | PRODUCT DOCUMENT STRUCTURE |
Documents will be in standard Bovie document format.
The following table lists the Device’s model number and corresponding product / catalog code.
| Catalog # | Comments |
| TBD | Laparoscopic Monopolar Device |
| TBD | Open Abdominal Monopolar Device |
Table Three
The following section will describe requirements for the insulated handle and shaft with electrode tip.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
| 5.1 | Electrical and Mechanical |
The Device hardware is comprised of two (2) major components: (i) an Insulated Handle and (ii) a Shaft with Electrode Tip. RF energy is passed through the Insulated Handle through the Shaft to the Electrode Tip by a powered lead from an electrosurgical generator.
5.1.1.1 Insulated Handle and Power Cord Insulation Resistance
The insulation of the Insulated Handle and Power Cord shall meet Requirements of Section 4.2.5.4 Dielectric withstands of accessories ANSI/AAMI HF-18:2001
5.1.1.2 Insulated Handle Ergonomics
The Insulated Handle shall be designed for comfortable and efficient hand operation. It will incorporate an over-mold to give the body an elegant feel and soft touch.
The Insulated Handle Electrical Cord shall be approximately ten (10) feet in length and incorporate a 3-prong electrical plug.
5.1.1.4 Insulated Handle Flow Control Mechanism
The Device will have a flow control mechanism in the handle so the flow can be regulated by the user within the sterile field.
The tubing length should be approximately ten (10) feet in length and incorporate an I.V. spike on the end to attach directly to a hanging IV bag.
5.1.1.6 Insulated Handle Tubing Material
The tubing material should be a material that will resist kinking but should not have so strong a memory as to pull the probe off of the sterile field.
The Device should have an activation button. The activation button must be easy to operate.
5.1.1.8 Insulated Handle Compatibility
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
| | · | The Insulated Handle should have compatibility with standard RF generators used for electrocautery and standard grounding pad. |
| | · | The Insulated Handle should have compatibility with standard 0.9% saline I.V. bag. |
The Shaft and Electrode Tip provide the actual working portion of this system.
5.1.2.1 Saline Flow Rate of Device
The Saline flow rate prior to use will be ** **.
5.1.2.2 Device (Shaft) Length
The Device should be straight and have two (2) lengths: one for laparoscopic monopolar procedures (approximately thirty-two (32) cm) and one for open monopolar procedures (approximately fourteen (14) cm).
5.1.2.3 Shaft Configuration
The Shaft will be hollow to allow for fluid to flow to the sintered stainless steel tip. The inside diameter of the Shaft should be equal to the inside diameter of the tubing that feeds fluid to the Shaft to minimize any flow restriction in the fluid circuit.
5.1.2.4 Tip Material
The Tip shall be made of ** **.
5.1.2.5 Tip Strength
The porous blade tip to shaft tensile bond strength (axial mode) must be greater than ** ** after being subjected to a full-power simulated use duty cycle.
The porous blade tip to shaft bond strength in an flexural mode must be greater than ** ** after being subjected to a full-power simulated use duty cycle.
5.1.2.6 Tip performance
| | · | Bovie will exert its best efforts to meet the design goal that tip cutting performance be superior to competitive devices with respect to cutting speed. Tip will have the ability to coagulate soft tissue when performed in a suitable simulated use environment. |
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
4.1.3.1 Shipping Temperatures
Tropical (Wet and Dry) Conditions and Winter (Frozen) Conditions per ISTA 2A.
4.1.3.2 Transportation Testing
The Final Product Packaging Configuration for the Device shall meet the finished device requirements after being subjected to Simulated Transportation Conditioning per ISTA 2A.
5.1.4.1 Withstand 2x ETO Sterilization.
5.1.4.2 EN 550: 1994 – Sterilization of Medical Devices – Validation and routine control of ethylene oxide sterilization.
5.1.4.3 AAMI/ANSI/ISO 11135 -1994 Medical Devices – Validation and routine control of ethylene oxide sterilization.
| Protection Against Hazards |
A Hazard/Risk Analysis will be performed throughout the design process. The Hazard/Risk Analysis will be documented and filed as part of the design history file.
7.1.1 Package must be labeled in accordance with both BSC and Bovie Labeling Standards.
7.1.2 BSC and Bovie branding, including mutually approved trademarks, will be on the Handle and/or the shaft.
7.1.3 The Device is to have a three (3) year shelf life under normal storage conditions.
| | · | The Tip and Shaft protector shall be included into the Design. |
| | · | The Scratch Pad shall be packaged with the Device. |
| | · | The Device will be packaged in a Sterile Pouch. |
Final assembly and packaging will be performed by Bovie.
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.
APPROVALS
| Author | | | |
| | Thomas Feldhaus | | Date |
| Sales & Marketing | | | |
| | Rick Pfahl | | Date |
| Regulatory | | | |
| | Rick Kozloff | | Date |
| Quality | | | |
| | John Woody | | Date |
| Manufacturing | | | |
| | Lillian Eshem | | Date |
| Engineering | | | |
| | Fred Baron | | Date |
BOVIE MEDICAL CONFIDENTIAL
THIS DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE, USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT PROPER AUTHORIZATION.