UNITED STATES |
SECURITIES AND EXCHANGE COMMISSION |
Washington, D.C. 20549 |
|
SCHEDULE 14A |
|
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. ) |
|
Filed by the Registrant x |
|
Filed by a Party other than the Registrant o |
|
Check the appropriate box: |
o | Preliminary Proxy Statement |
o | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
o | Definitive Proxy Statement |
x | Definitive Additional Materials |
o | Soliciting Material Pursuant to §240.14a-12 |
|
GENZYME CORPORATION |
(Name of Registrant as Specified In Its Charter) |
|
N/A |
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
|
Payment of Filing Fee (Check the appropriate box): |
x | No fee required. |
o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies: |
| | |
| (2) | Aggregate number of securities to which transaction applies: |
| | |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| | |
| (4) | Proposed maximum aggregate value of transaction: |
| | |
| (5) | Total fee paid: |
| | |
o | Fee paid previously with preliminary materials. |
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount Previously Paid: |
| | |
| (2) | Form, Schedule or Registration Statement No.: |
| | |
| (3) | Filing Party: |
| | |
| (4) | Date Filed: |
| | |
| | | |
Genzyme Corporation has revised the following slides from its Investor Presentation, which was previously filed with the Securities and Exchange Commission on May 7, 2010.
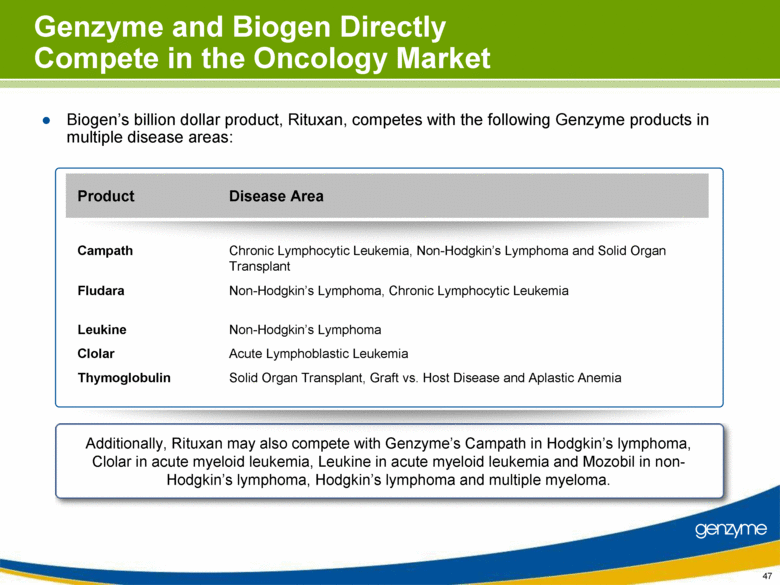
| Genzyme and Biogen Directly Compete in the Oncology Market Biogen’s billion dollar product, Rituxan, competes with the following Genzyme products in multiple disease areas: Solid Organ Transplant, Graft vs. Host Disease and Aplastic Anemia Thymoglobulin Acute Lymphoblastic Leukemia Clolar Non-Hodgkin’s Lymphoma Leukine Non-Hodgkin’s Lymphoma, Chronic Lymphocytic Leukemia Fludara Chronic Lymphocytic Leukemia, Non-Hodgkin’s Lymphoma and Solid Organ Transplant Campath Disease Area Product Additionally, Rituxan may also compete with Genzyme’s Campath in Hodgkin’s lymphoma, Clolar in acute myeloid leukemia, Leukine in acute myeloid leukemia and Mozobil in non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and multiple myeloma. 47 |
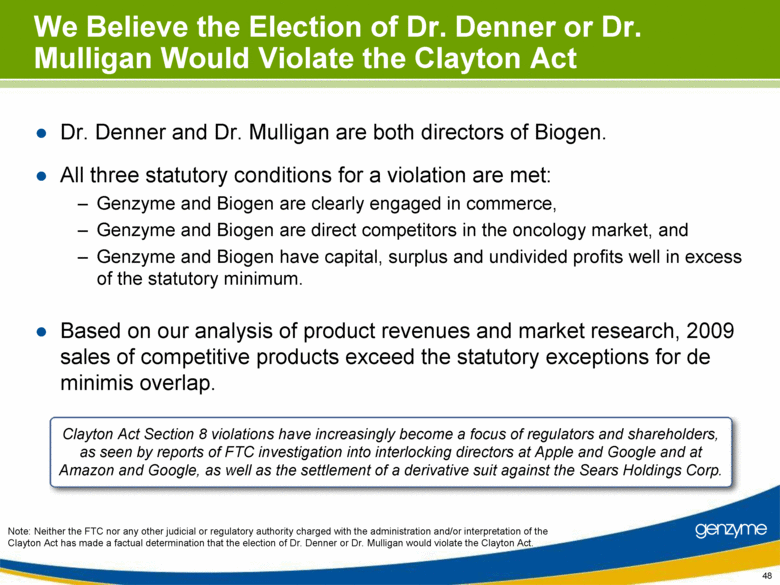
| We Believe the Election of Dr. Denner or Dr. Mulligan Would Violate the Clayton Act Dr. Denner and Dr. Mulligan are both directors of Biogen. All three statutory conditions for a violation are met: Genzyme and Biogen are clearly engaged in commerce, Genzyme and Biogen are direct competitors in the oncology market, and Genzyme and Biogen have capital, surplus and undivided profits well in excess of the statutory minimum. Based on our analysis of product revenues and market research, 2009 sales of competitive products exceed the statutory exceptions for de minimis overlap. Clayton Act Section 8 violations have increasingly become a focus of regulators and shareholders, as seen by reports of FTC investigation into interlocking directors at Apple and Google and at Amazon and Google, as well as the settlement of a derivative suit against the Sears Holdings Corp. Note: Neither the FTC nor any other judicial or regulatory authority charged with the administration and/or interpretation of the Clayton Act has made a factual determination that the election of Dr. Denner or Dr. Mulligan would violate the Clayton Act. 48 |
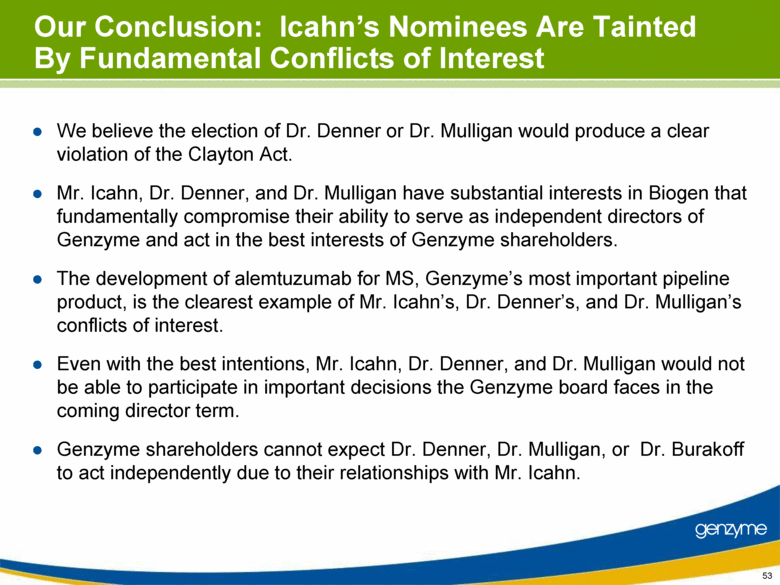
| Our Conclusion: Icahn’s Nominees Are Tainted By Fundamental Conflicts of Interest We believe the election of Dr. Denner or Dr. Mulligan would produce a clear violation of the Clayton Act. Mr. Icahn, Dr. Denner, and Dr. Mulligan have substantial interests in Biogen that fundamentally compromise their ability to serve as independent directors of Genzyme and act in the best interests of Genzyme shareholders. The development of alemtuzumab for MS, Genzyme’s most important pipeline product, is the clearest example of Mr. Icahn’s, Dr. Denner’s, and Dr. Mulligan’s conflicts of interest. Even with the best intentions, Mr. Icahn, Dr. Denner, and Dr. Mulligan would not be able to participate in important decisions the Genzyme board faces in the coming director term. Genzyme shareholders cannot expect Dr. Denner, Dr. Mulligan, or Dr. Burakoff to act independently due to their relationships with Mr. Icahn. 53 |
Important Information
On April 26, 2010, Genzyme filed a definitive proxy statement with the SEC in connection with the company’s 2010 annual meeting of shareholders. Genzyme shareholders are strongly advised to read carefully the company’s definitive proxy statement before making any voting or investment decision because the definitive proxy statement contains important information. The company’s definitive proxy statement and any other reports filed by the company with the SEC can be obtained free of charge at the SEC’s web site at www.sec.gov or from Genzyme at www.genzyme.com. A copy of the company’s definitive proxy statement is available for free by writing to Genzyme Corporation, 500 Kendall Street, Cambridge, MA 02142. In addition, copies of the proxy materials may be requested from our proxy solicitor, Innisfree M&A Incorporated, 501 Madison Avenue, 20th Floor, New York, NY 10022, toll free at: (888) 750-5835.


