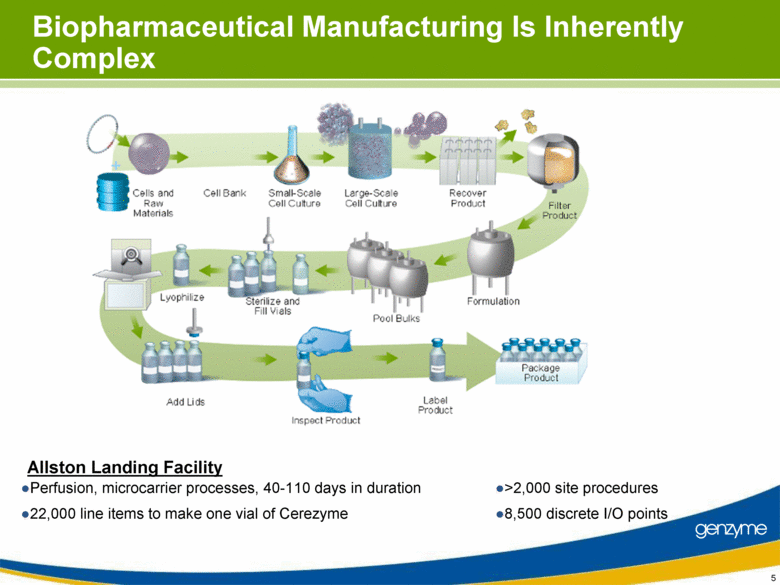
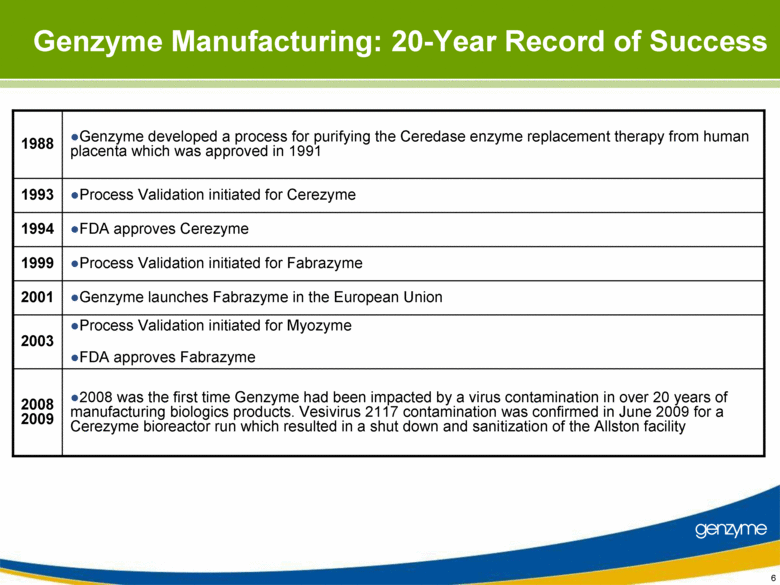
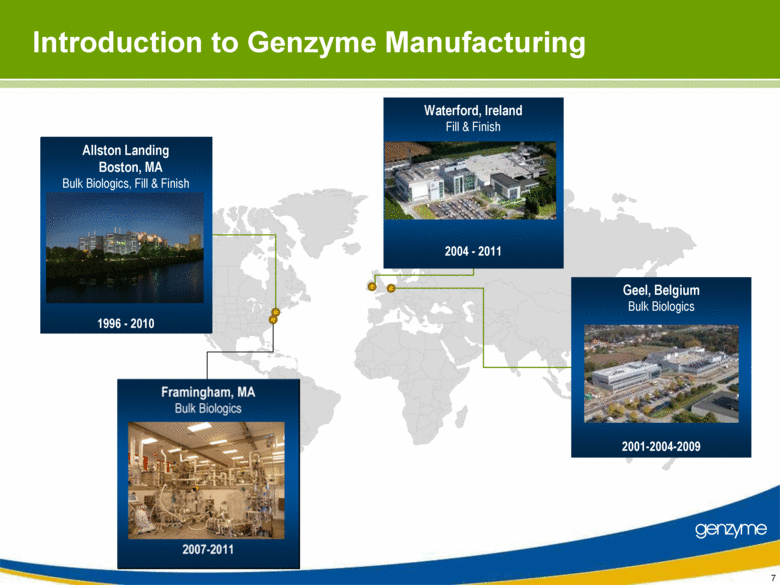
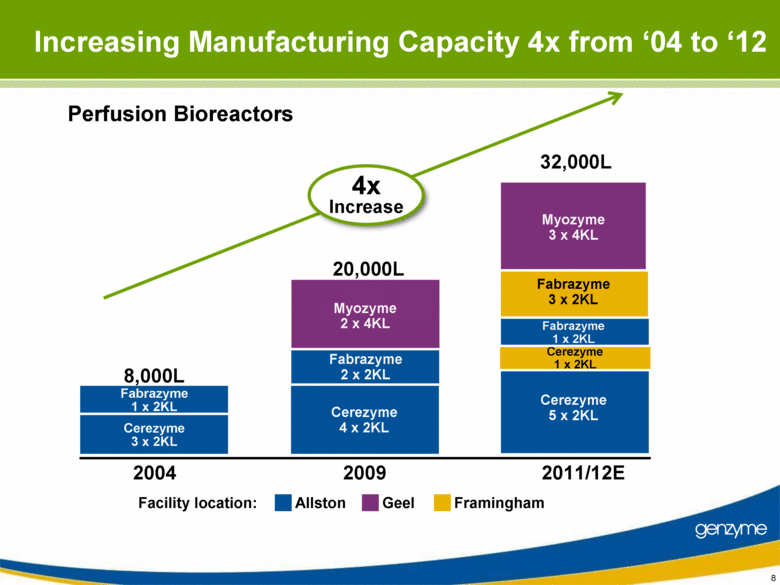
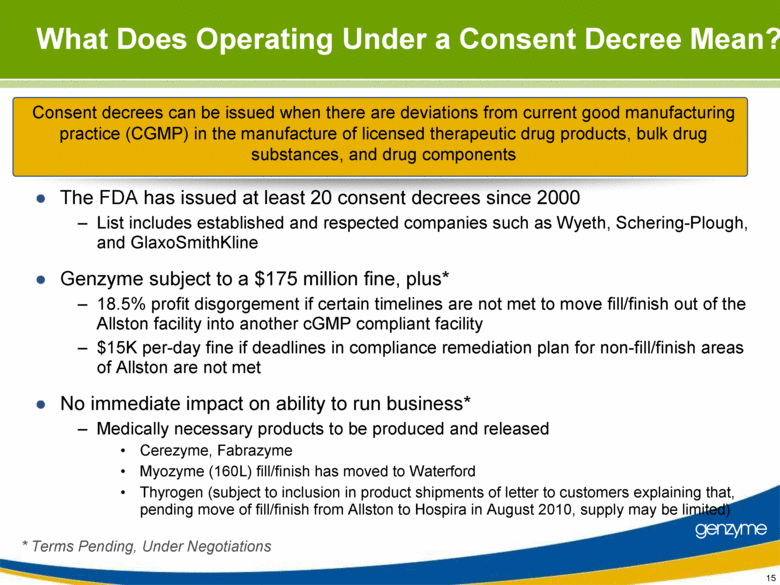
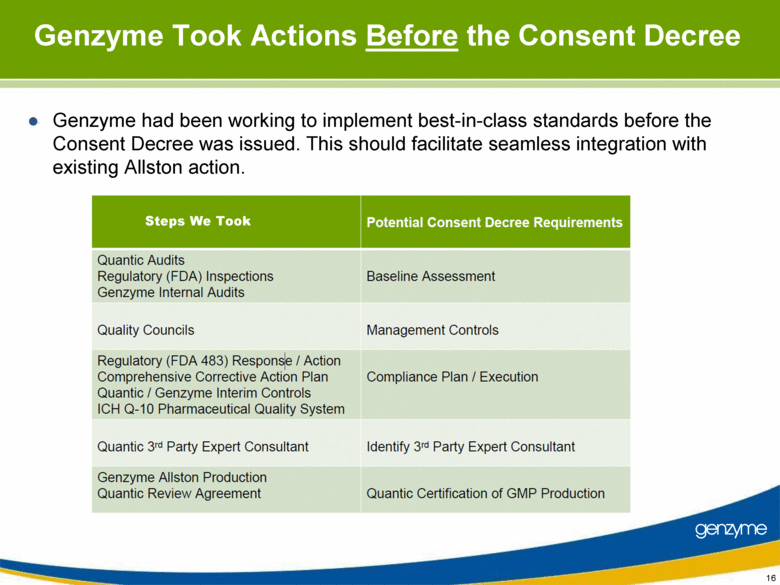
| Important Information On April 26, 2010, Genzyme filed a definitive proxy statement with the SEC in connection with the company’s 2010 annual meeting of shareholders. Genzyme shareholders are strongly advised to read carefully the company's definitive proxy statement and other proxy materials before making any voting or investment decision because the definitive proxy statement and other proxy materials contain important information. The company’s definitive proxy statement and any other reports filed by the company with the SEC can be obtained free of charge at the SEC’s web site at www.sec.gov or from Genzyme at www.genzyme.com. Copies of the company’s definitive proxy statement and other proxy materials are available for free by writing to Genzyme Corporation, 500 Kendall Street, Cambridge, MA 02142. In addition, copies of the proxy materials may be requested from our proxy solicitor, Innisfree M&A Incorporated, 501 Madison Avenue, 20th Floor, New York, NY 10022, toll free at: (888) 750-5835. This presentation contains forward-looking statements regarding Genzyme’s financial outlook and business plans including, without limitation, statements regarding its: two-year plan to address manufacturing challenges at the Allston facility; plans to increase bulk manufacturing capacity for Cerezyme, Fabrazyme and Myzoyme and the expected timing thereof; plans to transfer and expand its fill/finish operations and the expected timing thereof; and expectations regarding the terms of a consent decree being negotiated with the FDA. These statements are subject to risks and uncertainties that may cause actual results to differ materially. These risks and uncertainties include, among others: that Genzyme is unable to obtain regulatory approvals for capacity expansions and proposed manufacturing enhancements in the expected time frames; that Genzyme is unable to transition its fill/finish operations in the expected timeframes because of delays in regulatory approval or any other reason; that the final terms of the consent decree are different than anticipated; that production does not continue as planned due to any reason, including equipment failures, viral or bacterial contamination, cell growth at lower than expected levels, fill/finish issues, power outages, human error or regulatory issues; and the risks and uncertainties described in Genzyme's SEC reports filed under the Securities Exchange Act of 1934, including the factors referred to under the caption "Risk Factors" in Genzyme's Amended Quarterly Report on Form 10-Q/A for the quarter ended March 31, 2010. Genzyme cautions investors not to place substantial reliance on the forward-looking statements contained in this presentation. These statements speak only as of today’s date and Genzyme undertakes no obligation to update or revise these statements. |