UNITED STATES |
SECURITIES AND EXCHANGE COMMISSION |
Washington, D.C. 20549 |
|
SCHEDULE 14A |
|
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. ) |
|
Filed by the Registrant x |
|
Filed by a Party other than the Registrant o |
|
Check the appropriate box: |
o | Preliminary Proxy Statement |
o | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
o | Definitive Proxy Statement |
x | Definitive Additional Materials |
o | Soliciting Material Pursuant to §240.14a-12 |
|
GENZYME CORPORATION |
(Name of Registrant as Specified In Its Charter) |
|
N/A |
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
|
Payment of Filing Fee (Check the appropriate box): |
x | No fee required. |
o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies: |
| | |
| (2) | Aggregate number of securities to which transaction applies: |
| | |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| | |
| (4) | Proposed maximum aggregate value of transaction: |
| | |
| (5) | Total fee paid: |
| | |
o | Fee paid previously with preliminary materials. |
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount Previously Paid: |
| | |
| (2) | Form, Schedule or Registration Statement No.: |
| | |
| (3) | Filing Party: |
| | |
| (4) | Date Filed: |
| | |
| | | |

| May 25, 2010 RiskMetrics Group Presentation |
| Important Information On April 26, 2010, Genzyme filed a definitive proxy statement with the SEC in connection with the company’s 2010 annual meeting of shareholders. Genzyme shareholders are strongly advised to read carefully the company's definitive proxy statement and other proxy materials before making any voting or investment decision because the definitive proxy statement and other proxy materials contain important information. The company’s definitive proxy statement and any other reports filed by the company with the SEC can be obtained free of charge at the SEC’s web site at www.sec.gov or from Genzyme at www.genzyme.com. Copies of the company’s definitive proxy statement and other proxy materials are available for free by writing to Genzyme Corporation, 500 Kendall Street, Cambridge, MA 02142. In addition, copies of the proxy materials may be requested from our proxy solicitor, Innisfree M&A Incorporated, 501 Madison Avenue, 20th Floor, New York, NY 10022, toll free at: (888) 750-5835. This presentation contains forward-looking statements regarding Genzyme’s financial outlook and business plans including, without limitation, statements regarding: the sustainability of Genzyme’s financial performance; plans to establish operational excellence in manufacturing; its plan to address manufacturing challenges at the Allston facility, including the implementation of viral mitigation measures, and the expected timing thereof; plans to increase bulk manufacturing capacity for Cerezyme, Fabrazyme and Myzoyme and the expected timing thereof; plans to transfer and expand its fill/finish operations and the expected timing thereof; expectations regarding the terms of a consent decree being negotiated with the FDA; plans to pursue strategic alternatives for its Genetic Testing, Diagnostics and Pharmaceutical Intermediates businesses, and the impact on EPS and cash ROI of the potential divestitures; plans to create shareholder value by focusing on its core businesses; plans to capitalize on commercial opportunities for Myozyme, Synvisc-One and Mozobil; plans to launch alemtuzumab for MS, mipomersen and eliglustat by the end of 2013; its expectations on the timing of the availability of data from the alemtuzumab for MS, mipomersen and eliglustat trials, the timing of regulatory approvals for alemtuzumab for MS, mipomersen and eliglustat, and the potential for alemtuzumab for MS, mipomersen and eliglustat, including its assessment of efficacy and market positioning; plans to improve operating margins and generate cost savings; plans to optimize its capital structure by implementing a $2B stock buyback, the timing and funding thereof, including plans for incurring debt; its target cash and credit structure, including target credit ratings, cash levels, debt levels and ratios; business development criteria and plans; and plans to capitalize on near term revenue growth drivers and balance revenue and earnings growth with cash flow return on investment. These statements are subject to risks and uncertainties that may cause actual results to differ materially. These risks and uncertainties include, among others: that Genzyme is unable to meet its 2010 financial guidance for any reason; that Genzyme is unable to maintain regulatory approvals for its products and manufacturing facilities or obtain regulatory approvals for capacity expansions and proposed risk mitigation measures in the expected time frames; that Genzyme is unable to transition its fill/finish operations in the expected timeframes because of delays in regulatory approval or any other reason; that the final terms of the consent decree are different than anticipated; that production of products does not continue as planned due to any reason, including equipment failures, viral or bacterial contamination, cell growth at lower than expected levels, fill/finish issues, power outages, human error or regulatory issues; that Genzyme does not repurchase any or all of the $2B worth of Genzyme stock in the time frame indicated or at all; that Genzyme is unable to incur debt in the time frame indicated or in an amount anticipated, or at all; that Genzyme is unable to generate cash from strategic transactions involving its Genetic Testing, Diagnostics or Pharmaceutical Intermediates businesses; that the cash it does generate is less than expected, or that the timing of one or more of the strategic transactions is later than expected; that any or all of Genzyme’s five point plan to create shareholder value cannot be executed on or is otherwise ineffective; that Genzyme is not able to successfully complete clinical development and obtain regulatory approvals of its product candidates within anticipated timeframes and for anticipated indications, including eliglustat, alemtuzumab-MS and mipomersen, for any reason, including trial results that are not as favorable as expected and safety profiles that reduce the potential target population; that the estimates of the size and characteristics of the markets to be addressed by Genzyme’s products and services are not accurate; and the risks and uncertainties described in Genzyme's SEC reports filed under the Securities Exchange Act of 1934, including the factors referred to under the caption "Risk Factors" in Genzyme's Amended Quarterly Report on Form 10-Q/A for the quarter ended March 31, 2010. Genzyme cautions investors not to place substantial reliance on the forward-looking statements contained in this presentation. These statements speak only as of today’s date (other than 2010 revenue guidance ranges, which were last updated February 17, 2010) and Genzyme undertakes no obligation to update or revise these statements. |

| Robert Carpenter Lead Independent Director |

| Introductions Robert Carpenter, Lead Independent Director Ralph Whitworth, Director, Chairman, Strategic Planning & Capital Allocation Committee Robert Bertolini, Director, Chairman, Audit Committee Henri Termeer, Chairman and CEO Michael Wyzga, Chief Financial Officer Peter Wirth, Corporate Secretary, EVP Legal and Corporate Development Scott Canute, President Global Manufacturing and Corporate Operations Ron Branning, Sr. Vice President Global Product Quality Patrick Flanigan, Sr. Director, Investor Relations |
| A History of Leadership in Biotechnology Founded in 1981, Genzyme has grown into the fourth largest biotechnology company globally Pioneered the orphan disease drug market One of the highest revenue growth rates in the industry Consistent recognition of industry leadership National Medal of Technology James D. Watson Helix Award |

| THE RESULT: 12 Market Leading Products Delivering high value products #1 |

| THE RESULT: Sustainable Financial Performance ($ in millions) (1) Excluding charges for major acquisitions and FAS 123R. See GAAP to non-GAAP reconciliations in Appendix B to this presentation. Revenues Non-GAAP EPS(1) 23% CAGR 16% CAGR $4,516 $4,605 $3,814 $3,187 $2,735 $2,201 $1,575 $1,080 2002 2003 2004 2005 2006 2007 2008 2009 $2.27 $1.95 $2.60 $2.00 $1.74 $1.47 $1.12 $0.81 2002 2003 2004 2005 2006 2007 2008 2009 Revenues |
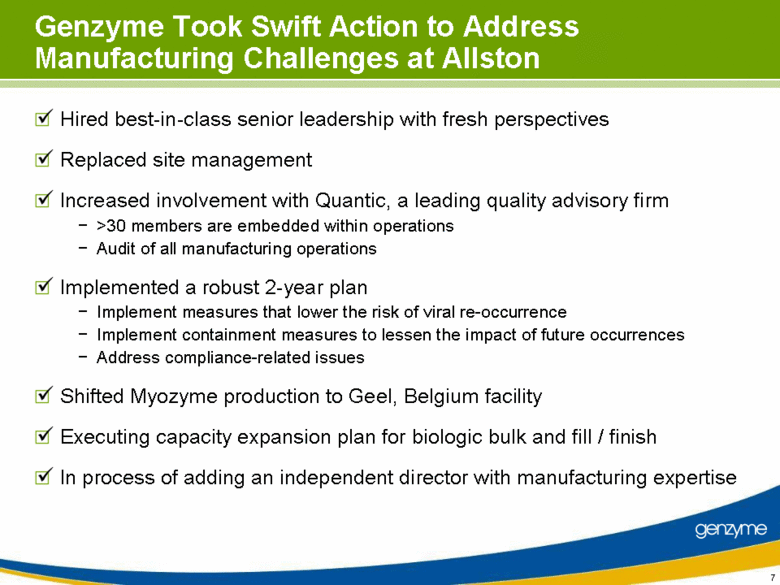
| Genzyme Took Swift Action to Address Manufacturing Challenges at Allston Hired best-in-class senior leadership with fresh perspectives Replaced site management Increased involvement with Quantic, a leading quality advisory firm >30 members are embedded within operations Audit of all manufacturing operations Implemented a robust 2-year plan Implement measures that lower the risk of viral re-occurrence Implement containment measures to lessen the impact of future occurrences Address compliance-related issues Shifted Myozyme production to Geel, Belgium facility Executing capacity expansion plan for biologic bulk and fill / finish In process of adding an independent director with manufacturing expertise |
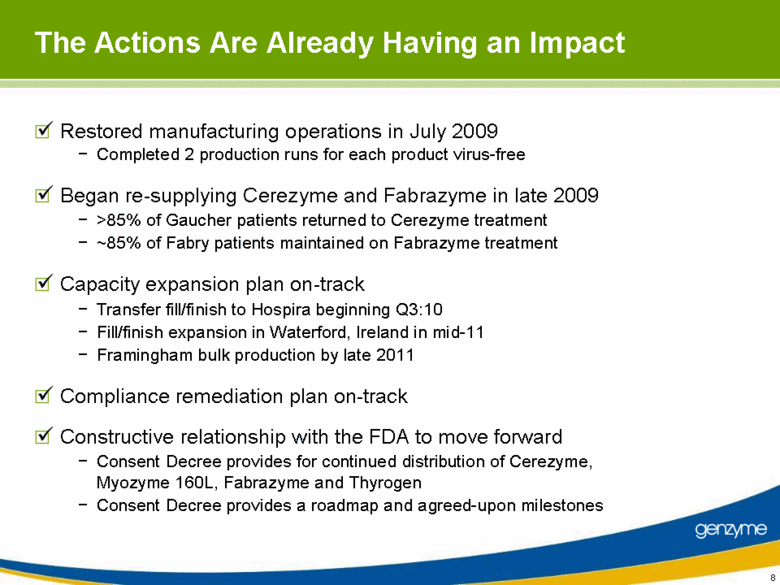
| The Actions Are Already Having an Impact Restored manufacturing operations in July 2009 Completed 2 production runs for each product virus-free Began re-supplying Cerezyme and Fabrazyme in late 2009 >85% of Gaucher patients returned to Cerezyme treatment ~85% of Fabry patients maintained on Fabrazyme treatment Capacity expansion plan on-track Transfer fill/finish to Hospira beginning Q3:10 Fill/finish expansion in Waterford, Ireland in mid-11 Framingham bulk production by late 2011 Compliance remediation plan on-track Constructive relationship with the FDA to move forward Consent Decree provides for continued distribution of Cerezyme, Myozyme 160L, Fabrazyme and Thyrogen Consent Decree provides a roadmap and agreed-upon milestones |

| Meeting Agenda Manufacturing Update (Scott Canute) Consent Decree Update (Ron Branning) Genzyme’s Board of Directors (Ralph Whitworth, Robert Bertolini) Capital Allocation Strategy (Michael Wyzga) Future Growth Drivers (Henri Termeer) Icahn’s Proxy Contest Nominees (Peter Wirth) Conclusion (Robert Carpenter) |

| Scott Canute President Global Manufacturing and Corporate Operations |

| Scott Canute: President of Global Manufacturing & Corporate Operations Bio Respected leader in the field with more than 25 years of experience Former President of Global Manufacturing Operations at Eli Lilly & Company Degree in chemical engineering from the University of Michigan MBA Harvard Business School Board Member of National Association of Manufacturers Left Eli Lilly in 2007 to pursue educational interests in business leadership and ethics Extensive experience successfully leading through similar situations Older biologics and parenteral manufacturing facilities Supplying medically necessary products Operating at full capacity Significant FDA inspection issues / Warning Letters Quality systems issues Inadequate validations Facilities and equipment out of date Aseptic processing concerns Implemented a voluntary Consent Decree including expert 3rd party oversight |
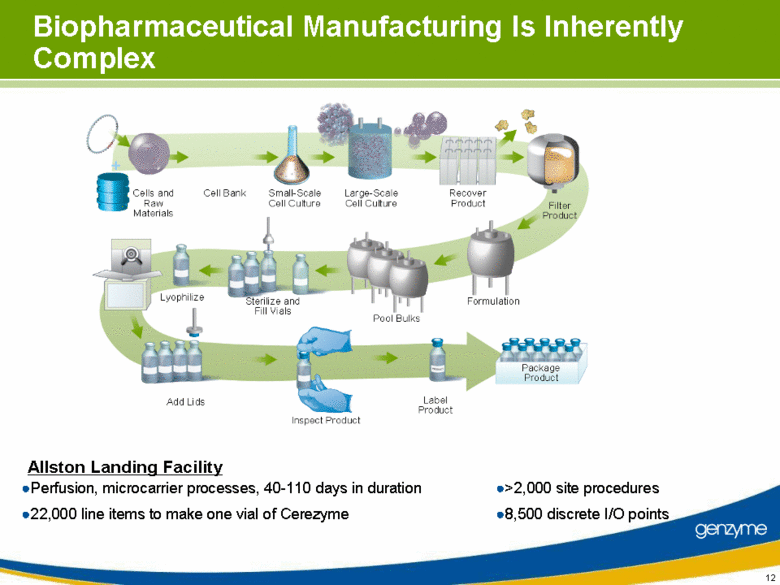
| Biopharmaceutical Manufacturing Is Inherently Complex 8,500 discrete I/O points 22,000 line items to make one vial of Cerezyme >2,000 site procedures Perfusion, microcarrier processes, 40-110 days in duration Allston Landing Facility |
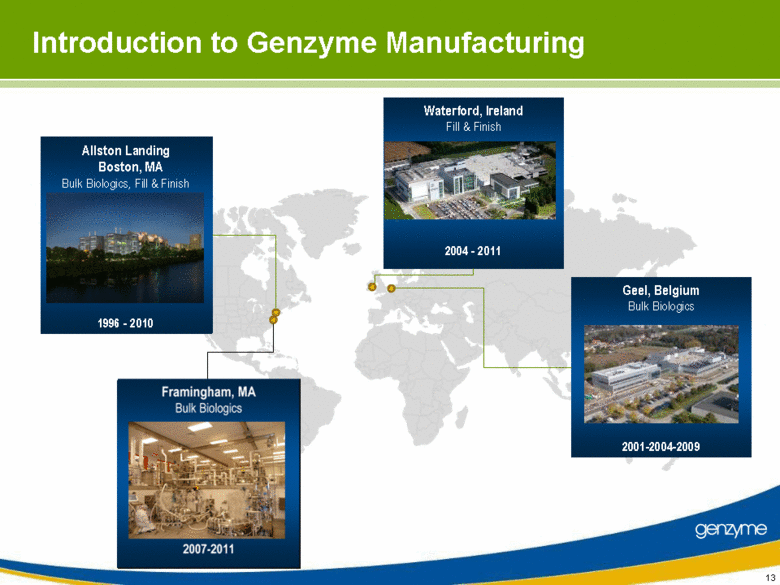
| Introduction to Genzyme Manufacturing Framingham, MA Bulk Biologics Geel, Belgium Bulk Biologics 2001-2004-2009 Waterford, Ireland Fill & Finish 2004 - 2011 Allston Landing Boston, MA Bulk Biologics, Fill & Finish 1996 - 2010 |
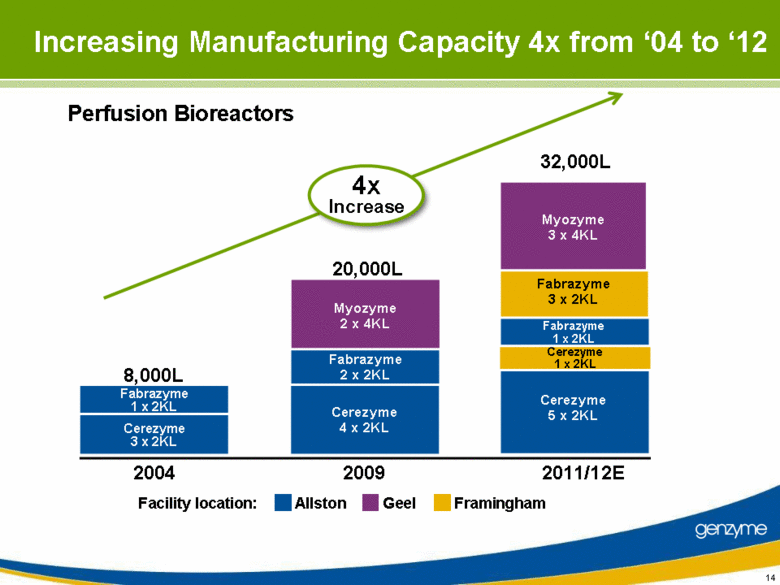
| Increasing Manufacturing Capacity 4x from ‘04 to ‘12 Perfusion Bioreactors 32,000L Allston Geel Framingham Facility location: 2011/12E 2009 2004 Cerezyme 4 x 2KL Fabrazyme 2 x 2KL Myozyme 2 x 4KL 20,000L 4x Increase Cerezyme 3 x 2KL Fabrazyme 1 x 2KL 8,000L Myozyme 3 x 4KL Fabrazyme 3 x 2KL Fabrazyme 1 x 2KL Cerezyme 1 x 2KL Cerezyme 5 x 2KL |
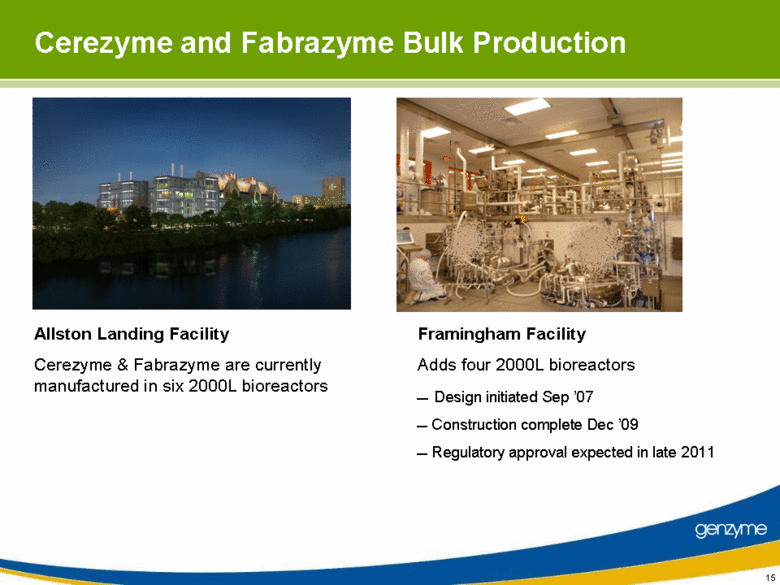
| Cerezyme and Fabrazyme Bulk Production Allston Landing Facility Cerezyme & Fabrazyme are currently manufactured in six 2000L bioreactors Framingham Facility Adds four 2000L bioreactors Design initiated Sep ’07 Construction complete Dec ’09 Regulatory approval expected in late 2011 |
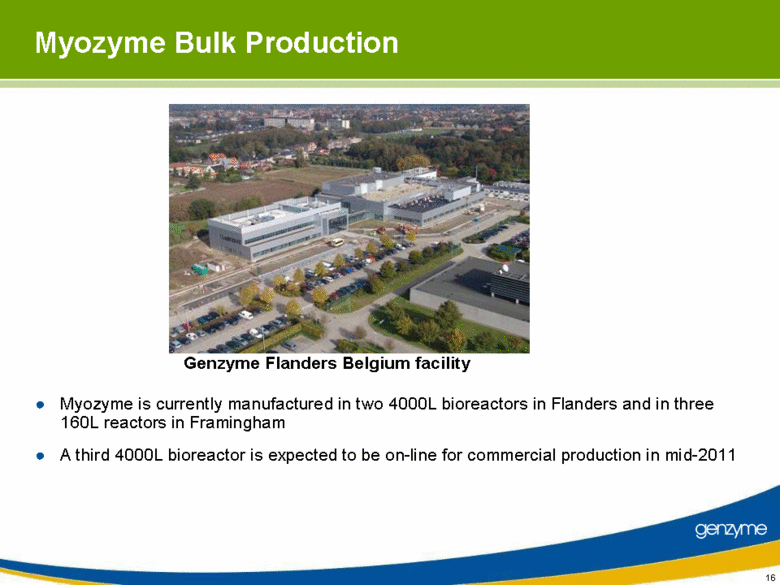
| Myozyme Bulk Production Myozyme is currently manufactured in two 4000L bioreactors in Flanders and in three 160L reactors in Framingham A third 4000L bioreactor is expected to be on-line for commercial production in mid-2011 Genzyme Flanders Belgium facility |

| Fill Finish Production Exit fill/finish operations at Allston Landing facility. Transferred Cerezyme 400U to Waterford Plan to transfer fill of remaining products to Hospira, KS beginning Q3:10 Fill/finish expansion to 4X in Waterford expected mid-2011 Waterford Ireland Isolated Filling Line |
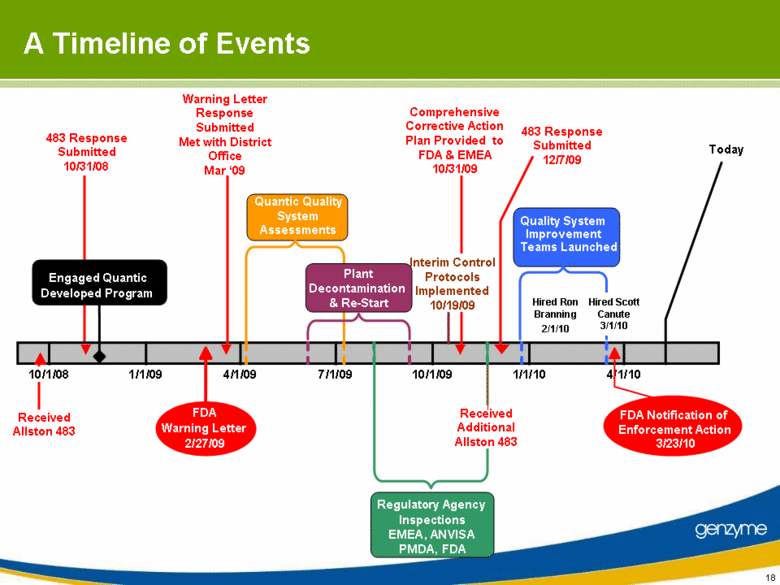
| A Timeline of Events 10/1/08 1/1/09 4/1/09 7/1/09 10/1/09 1/1/10 4/1/10 Quantic Quality System Assessments Regulatory Agency Inspections EMEA , ANVISA PMDA , FDA 2/27/09 FDA Warning Letter Quality System Improvement Teams Launched 483 Response Submitted 12/7/09 Comprehensive Corrective Action Plan Provided to FDA & EMEA 10/31/09 Plant Decontamination & Re - Start 3/23/10 FDA Notification of Enforcement Action Today Interim Control Protocols Implemented 10/19/09 Engaged Quantic Developed Program Received Allston 483 483 Response Submitted 10/31/08 Received Additional Allston 483 Warning Letter Response Submitted Met with District Office Mar ‘09 Hired Ron Branning 2/1/10 Hired Scott Canute 3/1/10 |
| Genzyme Took Swift Action to Address Manufacturing Challenges at Allston Made Significant Senior Leadership Changes and Strengthened Governance Replaced Allston site management Hired best-in-class senior leadership with fresh perspectives Established the Board Risk Oversight Committee to provide additional oversight of non financial risk, including supply chain and manufacturing issues In process to identify a new Board member with bio-manufacturing expertise Implemented Comprehensive Approach to Improve Quality and Compliance Developed Comprehensive Corrective Action Plan Engaged ~60 external consulting experts to supplement internal capabilities (> 30 from Quantic) Audited all manufacturing operations Conducted Quality Gap Assessment Launched Quality System Improvement Teams |

| Summary While much progress has been made there is clearly more for us to do Much of the work that remains is in the many “little” things that must be done right each and every day But we know what needs to be done and how to do it And most importantly, we (Ron and I) have done it before Overall, management is highly focused on manufacturing |

| May 2010 Ron Branning Senior Vice President Global Product Quality |

| Ron Branning: Senior Vice President of Global Product Quality Bio Over 40 years of experience in biopharmaceutical manufacturing quality and regulatory compliance Led quality and compliance functions at Gilead Sciences as Corporate Vice President of Quality and Compliance and Chief Compliance Officer Senior quality leadership positions at Genentech, Aventis Behring, and Somatogen (Baxter) Earlier in his career, he worked with Genetics Institute (Wyeth), Boehringer Ingelheim, GD Searle (Pfizer) and Johnson & Johnson Extensive experience successfully leading through similar situations Aventis Behring: Consent Decree to ‘in substantial compliance’ Genentech: Warning Letter to 5 new product approvals in 17 months Gilead: Proactive ICH Q-10 Pharmaceutical Quality Systems – basis for growth |
| What is a Consent Decree? The FDA has issued at least 20 consent decrees since 2000 List includes established and respected companies such as Wyeth, Schering-Plough, and GlaxoSmithKline Consent Decree can vary in scope, ex: Steris Laboratories: Facility shut-down, $6M penal bond Abbott: $100M profit disgorgement Wyeth: Seized inventory, $30M profit disgorgement Syntho: Facility shut-down, inventory destroyed Scientific Laboratories: Facility shut-down, recall, inventory destroyed Consent decrees can be issued when there are deviations from current good manufacturing practices (CGMP) in the manufacture of licensed therapeutic drug products, bulk drug substances, and drug components |
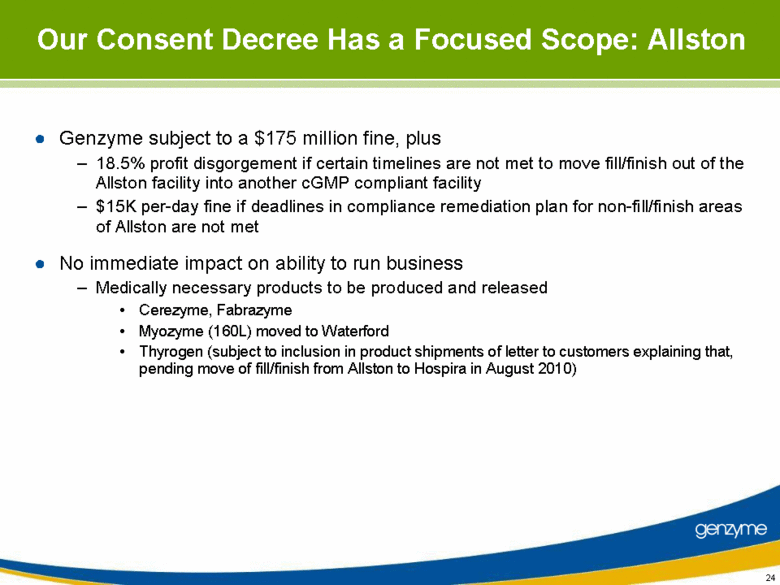
| Our Consent Decree Has a Focused Scope: Allston Genzyme subject to a $175 million fine, plus 18.5% profit disgorgement if certain timelines are not met to move fill/finish out of the Allston facility into another cGMP compliant facility $15K per-day fine if deadlines in compliance remediation plan for non-fill/finish areas of Allston are not met No immediate impact on ability to run business Medically necessary products to be produced and released Cerezyme, Fabrazyme Myozyme (160L) moved to Waterford Thyrogen (subject to inclusion in product shipments of letter to customers explaining that, pending move of fill/finish from Allston to Hospira in August 2010) |
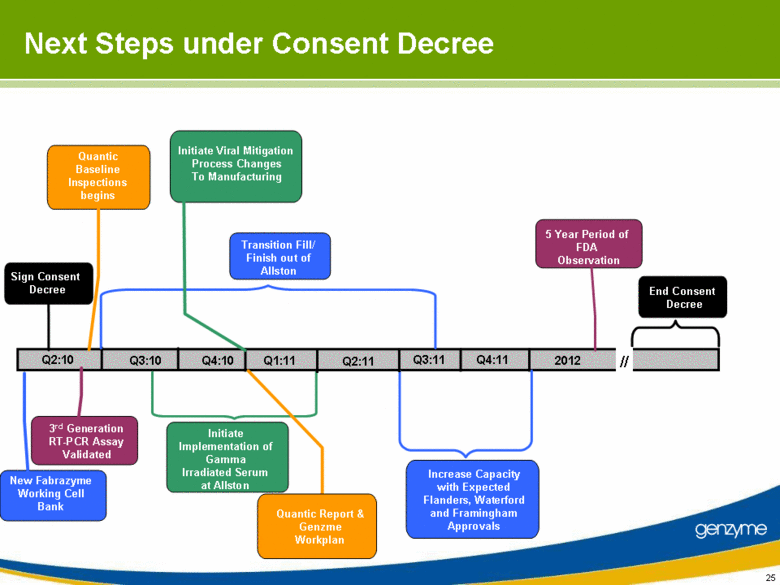
| Next Steps under Consent Decree Q2:10 Q3:10 Initiate Viral Mitigation Process Changes To Manufacturing Initiate Implementation of Gamma Irradiated Serum at Allston 3rd Generation RT-PCR Assay Validated - Sign Consent Decree New Fabrazyme Working Cell Bank Quantic Baseline Inspections begins Transition Fill/Finish out of Allston Q4:10 End Consent Decree // Quantic Report & Genzme Workplan Increase Capacity with Expected Flanders, Waterford and Framingham Approvals Q1:11 Q2:11 Q3:11 Q4:11 5 Year Period of FDA Observation 2012 |
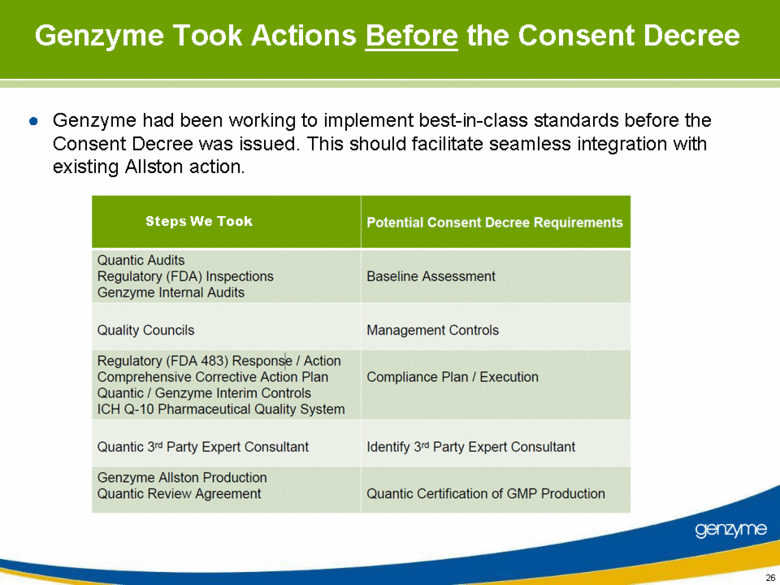
| Genzyme Took Actions Before the Consent Decree Genzyme had been working to implement best-in-class standards before the Consent Decree was issued. This should facilitate seamless integration with existing Allston action. Steps We Took Quantic Audits Regulatory (FDA) Inspections Genzyme Internal Audits Quality Councils Regulatory (FDA 483) Response / Action Comprehensive Corrective Action Plan Quantic / Genzyme Interim Controls ICH Q-10 Pharmaceutical Quality System Quantic 3rd Party Expert Consultant Genzyme Aliston Production Quantic Review Agreement Management Controls Compliance Plan / Execution Identify 3rd Party Expert Consultant Baseline Assessment Quantic Certification of GMP Production |
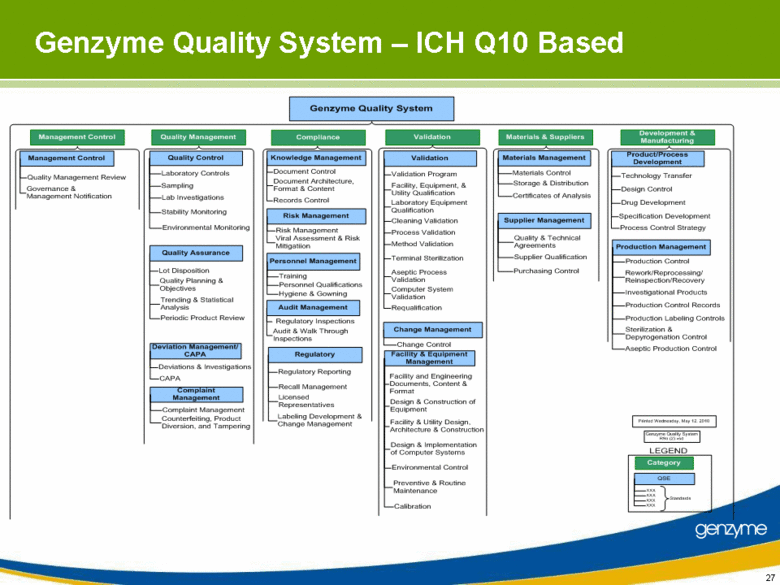
| Genzyme Quality System – ICH Q10 Based Genzyme Quality System Compliance Knowledge Management Document Control Document Architecture, Format & Content Records Control Risk Management Risk Management Viral Assessment & Risk Mitigation Personnel Management Training Personnel Qualifications Hygiene & Gowning Audit Management Regulatory Inspections Audit & Walk Through Inspections Regulatory Regulatory Reporting Recall Management Licensed Representatives Labeling Development & Change Management Validation Validation Validation Program Facility, Equipment, & Utility Qualification Laboratory Equipment Qualification Cleaning Validation Process Validation Method Validation Terminal Sterilization Aseptic Process Validation Computer System Validation Requalification Facility & Equipment Management Facility and Engineering Documents, Content & Format Design & Construction of Equipment Facility & Utility Design, Architecture & Construction Design & Implementation of Computer Systems Environmental Control Change Management Change Control Preventive & Routine Maintenance Calibration Materials & Suppliers Materials Control Storage & Distribution Certificates of Analysis Supplier Management Quality & Technical Agreements Supplier Qualification Purchasing Control Materials Management Development & Manufacturing Product/Process Development Technology Transfer Design Control Drug Development Specification Development Process Control Strategy Production Management Production Control Rework/Reprocessing/Reinspection/Recovery Investigational Products Production Control Records Production Labeling Controls Sterilization & Depyrogenation Control Aseptic Production Control Printed Wednesday, May 12, 2010 Genzyme Quality System R9a (2).vsd LEGEND Category QSE Standards Management Control Management Control Quality Management Review Governance & Management Notification Quality Assurance Lot Disposition Quality Planning & Objectives Trending & Statistical Analysis Periodic Product Review Deviation Management/CAPA Deviations & Investigations CAPA Quality Management Quality Control Laboratory Controls Sampling Lab Investigations Stability Monitoring Environmental Monitoring Complaint Management Complaint Management Counterfeiting, Product Diversion, and Tampering |

| Core Elements in Place to Achieve Success Most of our manufacturing network is high quality and state-of-the-art Significant upgrades to biologics capacity and infrastructure Exit Allston fill/finish Quality System remediation is well on its way Facilitates transition towards operating under a Consent Decree Significantly upgrading our leadership, organization and people capabilities Strong focus on our technical capabilities Viral risk mitigation Fabrazyme working cell bank Substantial changes have been implemented, a strong framework for continued progress has been put in place and we believe changing course at this point may negatively impact our success. |

| May 2010 Ralph Whitworth Director, Chairman, Strategic Planning & Capital Allocation Committee |

| May 2010 Robert Bertolini Director, Chairman, Audit Committee |
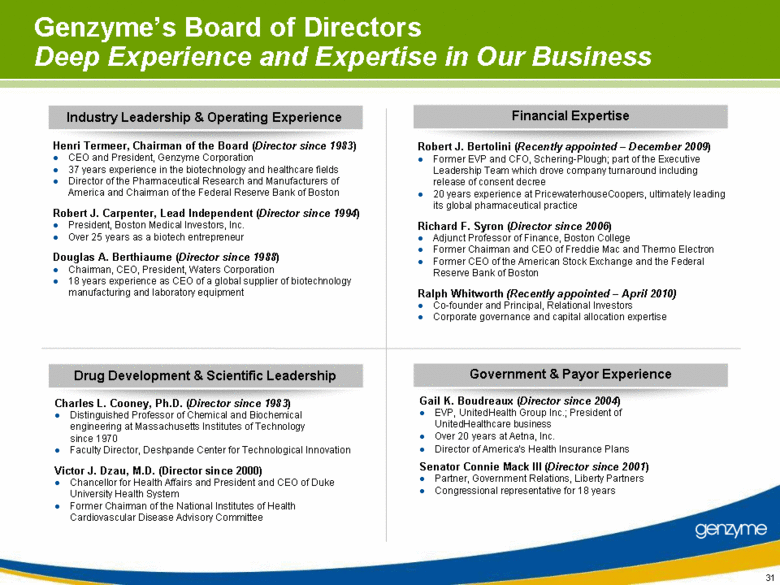
| Genzyme’s Board of Directors Deep Experience and Expertise in Our Business Henri Termeer, Chairman of the Board (Director since 1983) CEO and President, Genzyme Corporation 37 years experience in the biotechnology and healthcare fields Director of the Pharmaceutical Research and Manufacturers of America and Chairman of the Federal Reserve Bank of Boston Robert J. Carpenter, Lead Independent (Director since 1994) President, Boston Medical Investors, Inc. Over 25 years as a biotech entrepreneur Douglas A. Berthiaume (Director since 1988) Chairman, CEO, President, Waters Corporation 18 years experience as CEO of a global supplier of biotechnology manufacturing and laboratory equipment Robert J. Bertolini (Recently appointed – December 2009) Former EVP and CFO, Schering-Plough; part of the Executive Leadership Team which drove company turnaround including release of consent decree 20 years experience at PricewaterhouseCoopers, ultimately leading its global pharmaceutical practice Richard F. Syron (Director since 2006) Adjunct Professor of Finance, Boston College Former Chairman and CEO of Freddie Mac and Thermo Electron Former CEO of the American Stock Exchange and the Federal Reserve Bank of Boston Ralph Whitworth (Recently appointed – April 2010) Co-founder and Principal, Relational Investors Corporate governance and capital allocation expertise Charles L. Cooney, Ph.D. (Director since 1983) Distinguished Professor of Chemical and Biochemical engineering at Massachusetts Institutes of Technology since 1970 Faculty Director, Deshpande Center for Technological Innovation Victor J. Dzau, M.D. (Director since 2000) Chancellor for Health Affairs and President and CEO of Duke University Health System Former Chairman of the National Institutes of Health Cardiovascular Disease Advisory Committee Gail K. Boudreaux (Director since 2004) EVP, UnitedHealth Group Inc.; President of UnitedHealthcare business Over 20 years at Aetna, Inc. Director of America’s Health Insurance Plans Senator Connie Mack III (Director since 2001) Partner, Government Relations, Liberty Partners Congressional representative for 18 years Industry Leadership & Operating Experience Drug Development & Scientific Leadership Financial Expertise Government & Payor Experience |
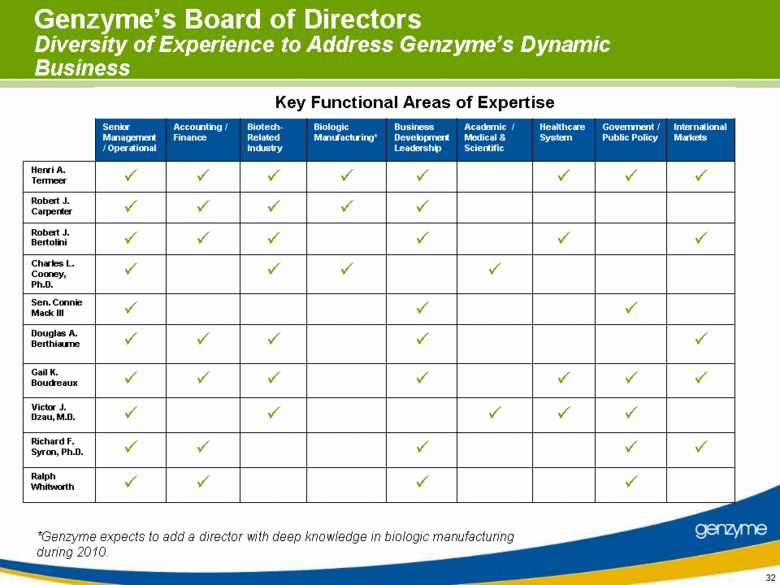
| Genzyme’s Board of Directors Diversity of Experience to Address Genzyme’s Dynamic Business Richard F. Syron, Ph.D. Key Functional Areas of Expertise Ralph Whitworth Victor J. Dzau, M.D. Gail K. Boudreaux Douglas A. Berthiaume Sen. Connie Mack III Charles L. Cooney, Ph.D. Robert J. Bertolini Robert J. Carpenter Henri A. Termeer International Markets Government / Public Policy Healthcare System Academic / Medical & Scientific Business Development Leadership Biologic Manufacturing* Biotech-Related Industry Accounting / Finance Senior Management / Operational *Genzyme expects to add a director with deep knowledge in biologic manufacturing during 2010. |

| Continuing to Enhance Corporate Governance Added Board members to enhance financial & manufacturing expertise Robert Bertolini, former CFO of Schering-Plough Ralph Whitworth, Principal, Relational Investors Process underway to add a member with manufacturing expertise Enhanced the role of the Lead Independent Director Restructured the short- and long-term executive compensation plan Focused on revenue growth, cash flow return on invested capital and relative stock performance Aligned with shareholder interests Created new Board committees to enhance Board oversight Risk Management (Gail Boudreaux, Chair) Strategic Planning and Capital Allocation (Ralph Whitworth, Chair) |
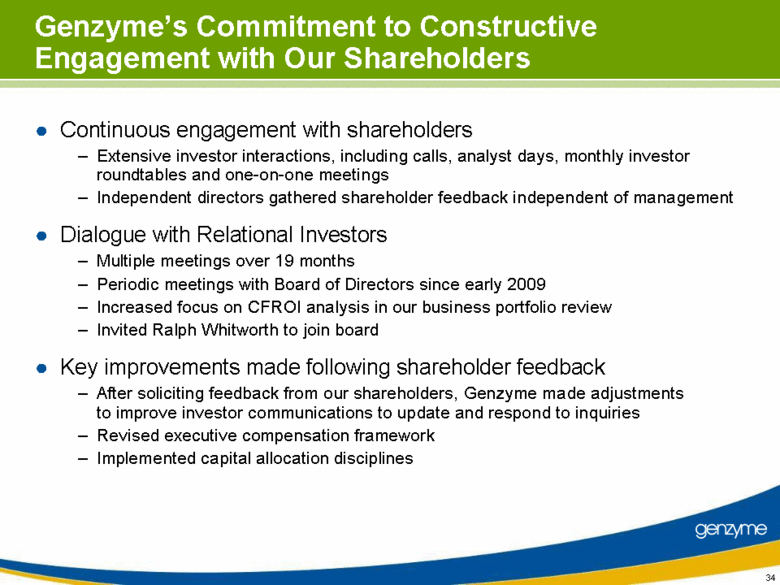
| Genzyme’s Commitment to Constructive Engagement with Our Shareholders Continuous engagement with shareholders Extensive investor interactions, including calls, analyst days, monthly investor roundtables and one-on-one meetings Independent directors gathered shareholder feedback independent of management Dialogue with Relational Investors Multiple meetings over 19 months Periodic meetings with Board of Directors since early 2009 Increased focus on CFROI analysis in our business portfolio review Invited Ralph Whitworth to join board Key improvements made following shareholder feedback After soliciting feedback from our shareholders, Genzyme made adjustments to improve investor communications to update and respond to inquiries Revised executive compensation framework Implemented capital allocation disciplines |

| Continually Improving Governance to Assure Independent Oversight & Responsiveness to Investors 2003 Established lead director position and enhanced the role in 2010 2006 Abolished staggered Board and fully implemented annual elections of all Directors in 2009 2007 Adopted majority voting 2007 Adopted policy limiting director service 2007 Adopted an Executive Severance Policy requiring shareholder approval for cash severance benefits exceeding 2.99 times base salary and bonus 2007 Amended Governance Guidelines to outline a process for board consideration of shareholder proposals that garner majority shareholder support 2009 Allowed our poison pill to lapse without renewal |
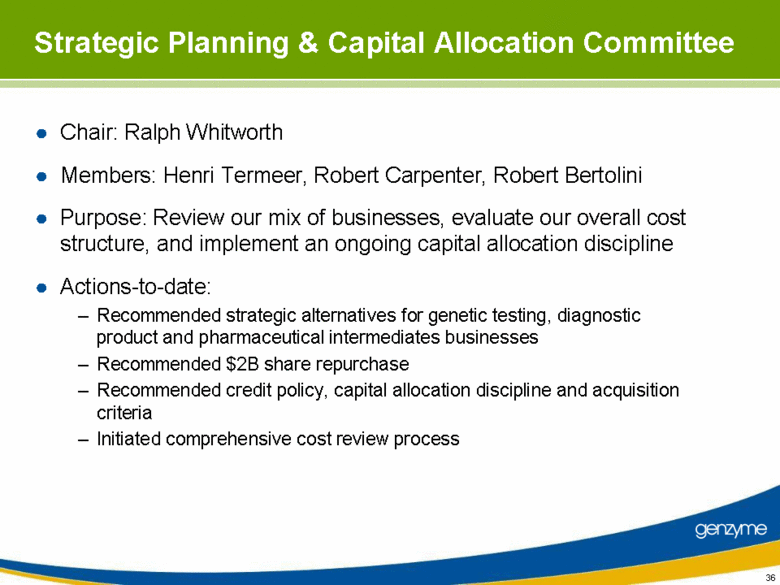
| Strategic Planning & Capital Allocation Committee Chair: Ralph Whitworth Members: Henri Termeer, Robert Carpenter, Robert Bertolini Purpose: Review our mix of businesses, evaluate our overall cost structure, and implement an ongoing capital allocation discipline Actions-to-date: Recommended strategic alternatives for genetic testing, diagnostic product and pharmaceutical intermediates businesses Recommended $2B share repurchase Recommended credit policy, capital allocation discipline and acquisition criteria Initiated comprehensive cost review process |

| Risk Oversight Committee Chair: Gail Bourdreaux Members: Robert Bertolini, Robert Carpenter, Victor Dzau Purpose: Oversee enterprise risk management outside of scope of Audit and Compensation committees including supply chain, manufacturing and distribution, product safety, business continuity and disaster recovery, regulatory compliance, and commercial compliance including financial interactions with health care professionals Actions-to-date: Reviewed internal risk assessments Adopted Risk Oversight Committee Charter Interviewed enterprise risk management consultants Discussed management of risk governance structure |

| Michael Wyzga Chief Financial Officer |
| Significant Value Creation Opportunities How Do We Analyze Our Business? Assessment based on growth and return benchmarks, including cash flow return on investment (CFROI) Review Assess Shareholder feedback External analysis Board and management continuously review business performance and capital allocation on a business-by-business basis We actively consider feedback from shareholders regarding the composition of our business portfolio and capital structure At the Board’s initiative, over the last year we received input and analyses from two outside financial advisors |
| 40 Components of Value Creation Seek Strong Earnings Growth while Maintaining Solid Return on Investment Maintain high return on invested capital Enforce strong financial criteria (focused revenue growth, improving margins and asset productivity) Invest only in the areas defined by our Core Business Criteria Benchmark all uses of capital against the highest risk adjusted returns available among the various alternatives Currently benchmarking all investments against share repurchases Core Business Criteria Strong Financial Criteria Capital Allocation Discipline |

| 41 Genzyme’s Key Criteria for Business Evaluation Commitment to the patient 1 Unmet medical need 2 Best in class, breakthrough therapies 3 Unique value to Genzyme and patients 4 Sustainable rates of growth with high financial returns 5 |
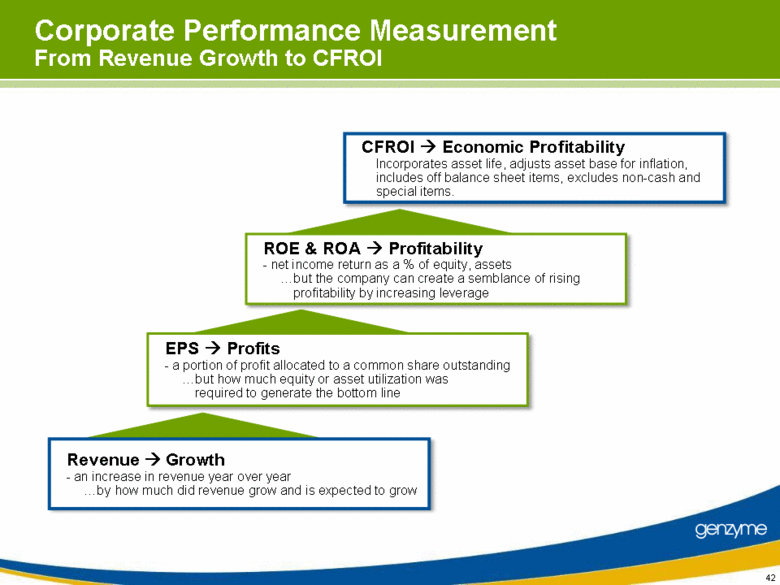
| 42 CFROI Economic Profitability Incorporates asset life, adjusts asset base for inflation, includes off balance sheet items, excludes non-cash and special items. Corporate Performance Measurement From Revenue Growth to CFROI ROE & ROA Profitability - net income return as a % of equity, assets but the company can create a semblance of rising profitability by increasing leverage EPS Profits - a portion of profit allocated to a common share outstanding but how much equity or asset utilization was required to generate the bottom line Revenue Growth - an increase in revenue year over year by how much did revenue grow and is expected to grow |
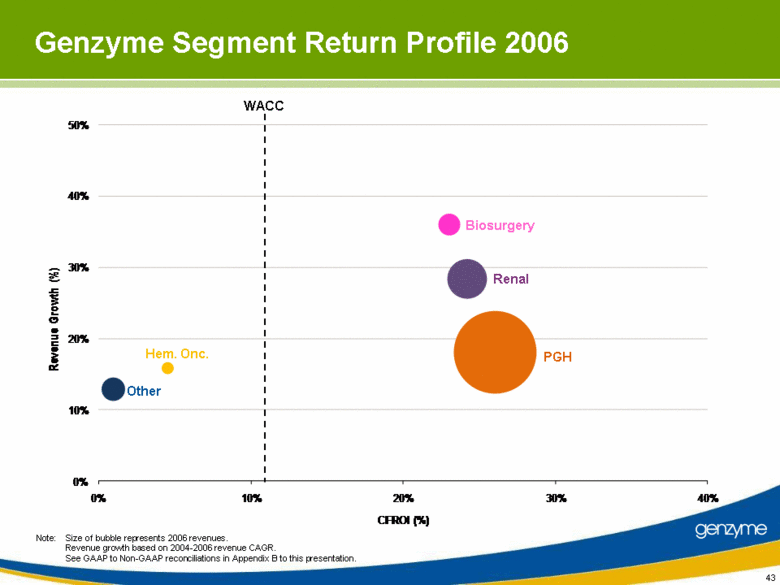
| 43 Note: Size of bubble represents 2006 revenues. Revenue growth based on 2004-2006 revenue CAGR. See GAAP to Non-GAAP reconciliations in Appendix B to this presentation. Other Renal Hem. Onc. Biosurgery PGH WACC Genzyme Segment Return Profile 2006 0% 10% 20% 30% 40% 50% 0% 10% 20% 30% 40% Revenue Growth (%) CFROI (%) |
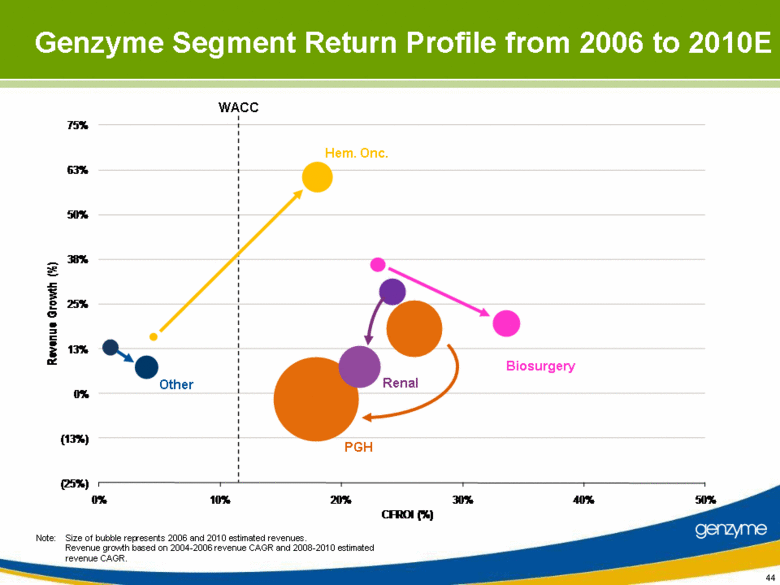
| 44 Note: Size of bubble represents 2006 and 2010 estimated revenues. Revenue growth based on 2004-2006 revenue CAGR and 2008-2010 estimated revenue CAGR. Other Renal Biosurgery PGH Hem. Onc. WACC Genzyme Segment Return Profile from 2006 to 2010E (25%) (13%) 0% 13% 25% 38% 50% 63% 75% 0% 10% 20% 30% 40% 50% Revenue Growth (%) CFROI (%) |
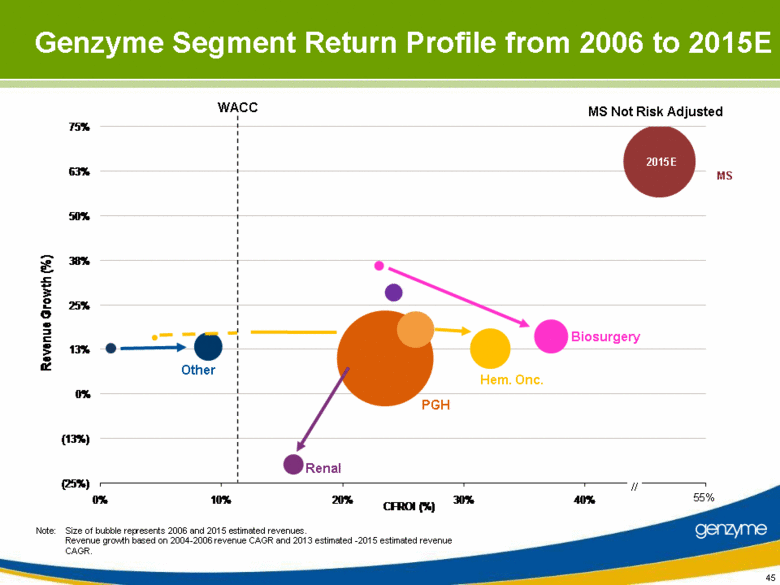
| 45 Note: Size of bubble represents 2006 and 2015 estimated revenues. Revenue growth based on 2004-2006 revenue CAGR and 2013 estimated -2015 estimated revenue CAGR. Genzyme Segment Return Profile from 2006 to 2015E WACC Other Renal Biosurgery Hem. Onc. PGH MS // 2015E 55% MS Not Risk Adjusted (25%) (13%) 0% 13% 25% 38% 50% 63% 75% 0% 10% 20% 30% 40% 50% Revenue Growth (%) CFROI (%) |

| Seeking Strategic Alternatives for Non-core Assets Board regularly reviews business portfolio and determined to explore strategic alternatives for three non-core assets: Genetic Testing Revenue: $371 million in 2009 Top 5 provider of reproductive and oncology testing in the US 9 laboratories and ~150 genetic counselors Diagnostics Revenue: $167 million in 2009 Leading provider of flu and LDL-c testing Pharmaceutical Intermediates Manufacturing facility located in Liestal, Switzerland New structure enables increased focus on therapeutics |
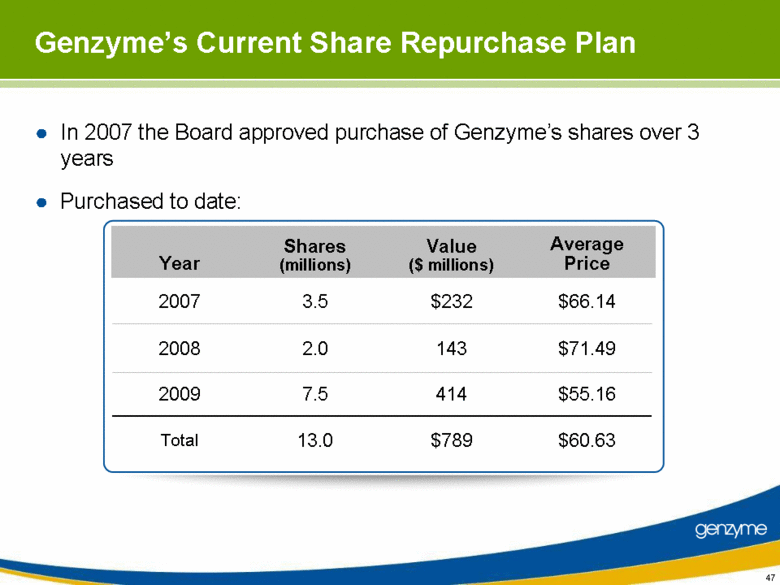
| In 2007 the Board approved purchase of Genzyme’s shares over 3 years Purchased to date: 47 Genzyme’s Current Share Repurchase Plan Year Shares (millions) Value ($ millions) Average Price 2007 3.5 $232 $66.14 2008 2.0 143 $71.49 2009 7.5 414 $55.16 Total 13.0 $789 $60.63 |

| 48 Genzyme’s Capital Structure Discipline Cash balance of $962 million in Q1 2010, with undrawn revolver Significant free cash flow generation with over $1 billion of cash flows annually by 2012 Limited current debt outstanding (Debt / 2009 EBITDA of 0.1x) Current S&P credit rating of A-, with significant debt capacity Goal is to create value for equity holders by optimizing the balance sheet Active Balance Sheet management to meet established credit objectives Maintain investment grade credit rating Total Debt/EBITDA goal not to exceed 1.5X Long term capital structure flexibility beyond 2010 Observations Credit Discipline |
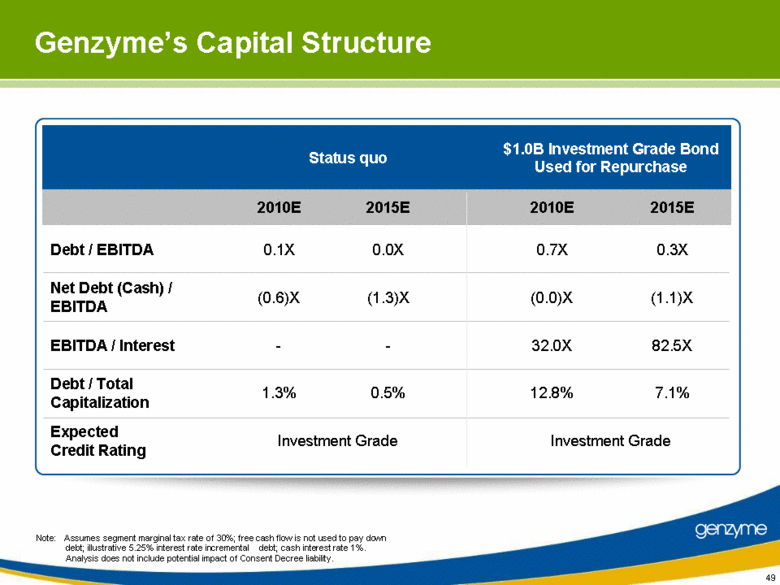
| 49 Genzyme’s Capital Structure Status quo $1.0B Investment Grade Bond Used for Repurchase 2010E 2015E 2010E 2015E Debt / EBITDA 0.1X 0.0X 0.7X 0.3X Net Debt (Cash) / EBITDA (0.6)X (1.3)X (0.0)X (1.1)X EBITDA / Interest - - 32.0X 82.5X Debt / Total Capitalization 1.3% 0.5% 12.8% 7.1% Expected Credit Rating Investment Grade Investment Grade Note: Assumes segment marginal tax rate of 30%; free cash flow is not used to pay down debt; illustrative 5.25% interest rate incremental debt; cash interest rate 1%. Analysis does not include potential impact of Consent Decree liability. |
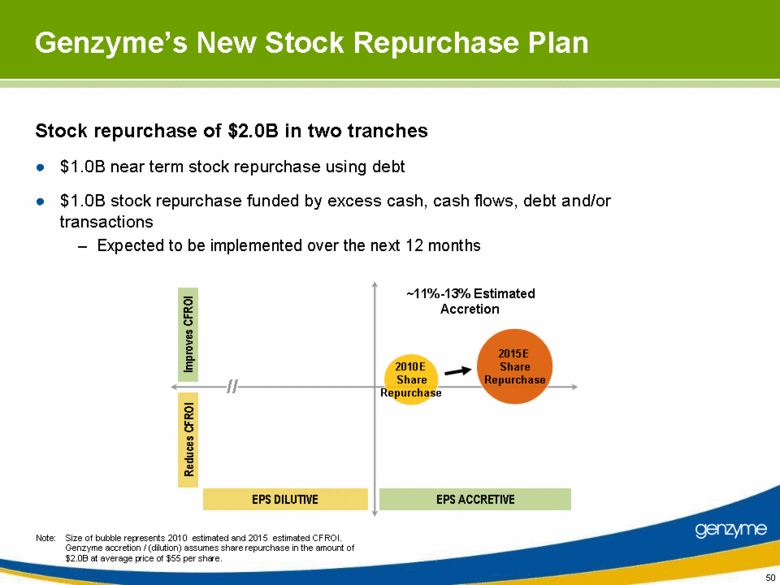
| Stock repurchase of $2.0B in two tranches $1.0B near term stock repurchase using debt $1.0B stock repurchase funded by excess cash, cash flows, debt and/or transactions Expected to be implemented over the next 12 months 50 Genzyme’s New Stock Repurchase Plan Note: Size of bubble represents 2010 estimated and 2015 estimated CFROI. Genzyme accretion / (dilution) assumes share repurchase in the amount of $2.0B at average price of $55 per share. 2010E Share Repurchase 2015E Share Repurchase Reduces CFROI Improves CFROI EPS DILUTIVE EPS ACCRETIVE ~11%-13% Estimated Accretion // |

| 51 Improve Operating Margins and Capital Expenses Genzyme has been evaluating its operating margins and expenses as part of its capital allocation review process Continuing oversight and direction by the Board and now by its Capital Allocation Committee Benchmarking against Peer group Outline of a detailed plan of our revised and reduced OPEX and CAPEX levels for our remaining businesses |
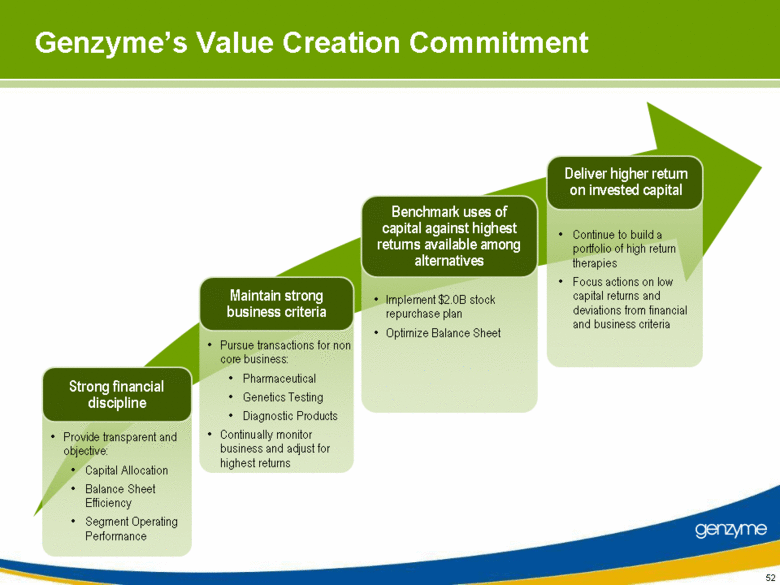
| 52 Genzyme’s Value Creation Commitment Provide transparent and objective: Capital Allocation Balance Sheet Efficiency Segment Operating Performance Pursue transactions for non core business: Pharmaceutical Genetics Testing Diagnostic Products Continually monitor business and adjust for highest returns Implement $2.0B stock repurchase plan Optimize Balance Sheet Continue to build a portfolio of high return therapies Focus actions on low capital returns and deviations from financial and business criteria Strong financial discipline Maintain strong business criteria Deliver higher return on invested capital Benchmark uses of capital against highest returns available among alternatives |

| Henri Termeer Chairman and CEO |
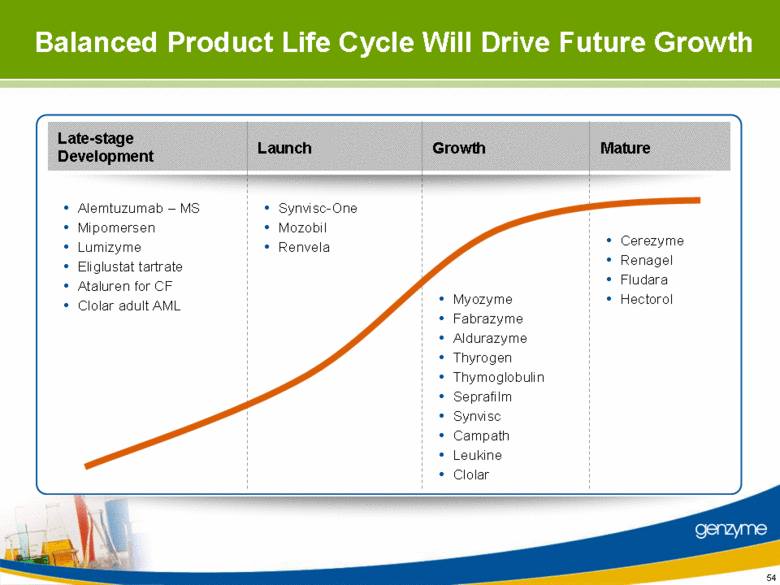
| Late-stage Development Growth Mature Launch Balanced Product Life Cycle Will Drive Future Growth Cerezyme Renagel Fludara Hectorol Synvisc-One Mozobil Renvela Myozyme Fabrazyme Aldurazyme Thyrogen Thymoglobulin Seprafilm Synvisc Campath Leukine Clolar Alemtuzumab – MS Mipomersen Lumizyme Eliglustat tartrate Ataluren for CF Clolar adult AML 54 |

| First in class therapy for Pompe disease with >$1B potential Approved in over 40 countries globally Opportunity: US FDA action date (PDUFA) on Lumizyme is June 17 Only single injection product approved in the US for osteoarthritic knee pain Market leading viscosupplement with >55% share Opportunity: less than 10% of market using a viscosupplement First-in-class stem cell mobilizer for bone marrow transplantation Significant benefits for the patient, provider, and payer Opportunity: chemosensitization trials underway in lymphoma Near-term opportunities 2010 Maximizing the Near-term Product Opportunities |
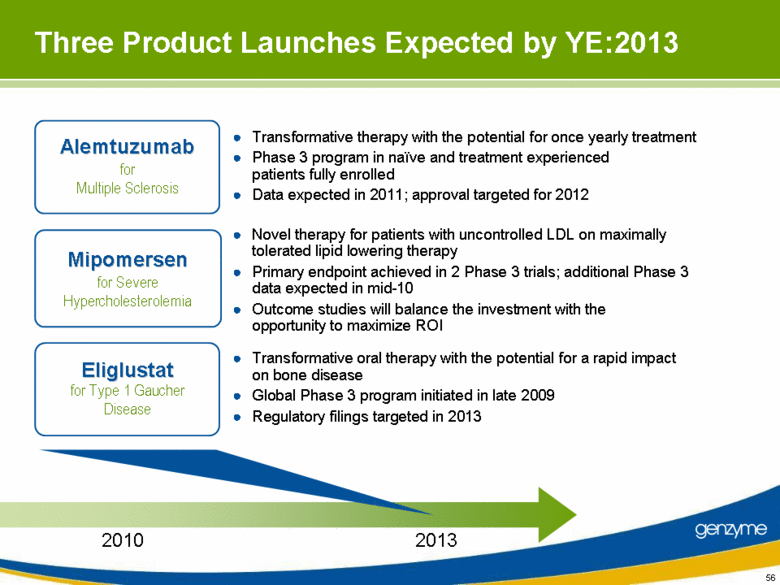
| Three Product Launches Expected by YE:2013 Novel therapy for patients with uncontrolled LDL on maximally tolerated lipid lowering therapy Primary endpoint achieved in 2 Phase 3 trials; additional Phase 3 data expected in mid-10 Outcome studies will balance the investment with the opportunity to maximize ROI Transformative oral therapy with the potential for a rapid impact on bone disease Global Phase 3 program initiated in late 2009 Regulatory filings targeted in 2013 Transformative therapy with the potential for once yearly treatment Phase 3 program in naïve and treatment experienced patients fully enrolled Data expected in 2011; approval targeted for 2012 2013 Alemtuzumab for Multiple Sclerosis Mipomersen for Severe Hypercholesterolemia Eliglustat for Type 1 Gaucher Disease 2010 |
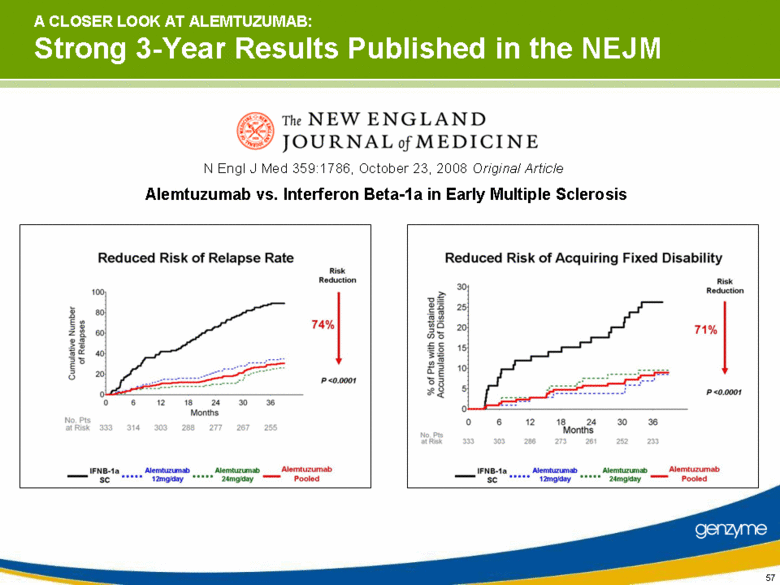
| Alemtuzumab vs. Interferon Beta-1a in Early Multiple Sclerosis N Engl J Med 359:1786, October 23, 2008 Original Article A CLOSER LOOK AT ALEMTUZUMAB: Strong 3-Year Results Published in the NEJM |
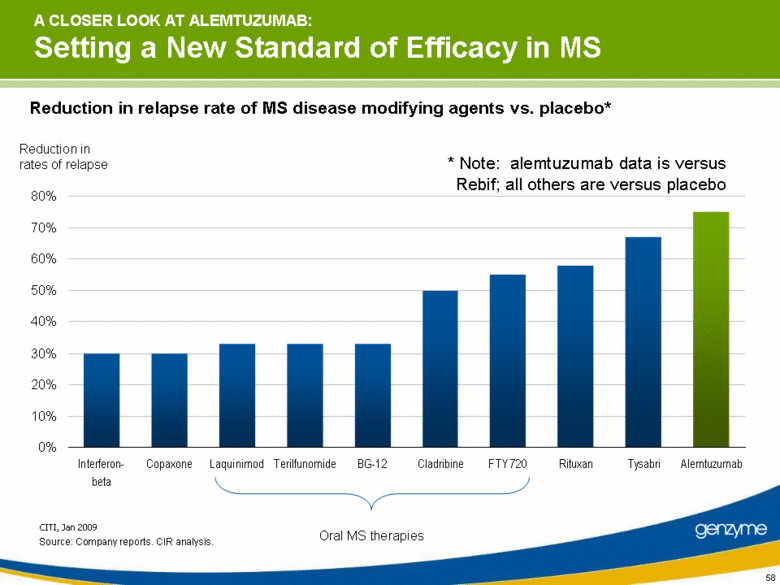
| A CLOSER LOOK AT ALEMTUZUMAB: Setting a New Standard of Efficacy in MS CITI, Jan 2009 Oral MS therapies Reduction in rates of relapse Reduction in relapse rate of MS disease modifying agents vs. placebo* Source: Company reports. CIR analysis. * Note: alemtuzumab data is versus Rebif; all others are versus placebo 0% 10% 20% 30% 40% 50% 60% 70% 80% Interferon-beta Copaxone Laquinimod Terilfunomide BG-12 Cladribine FTY 720 Rituxan Tysabri Alemtuzumab |
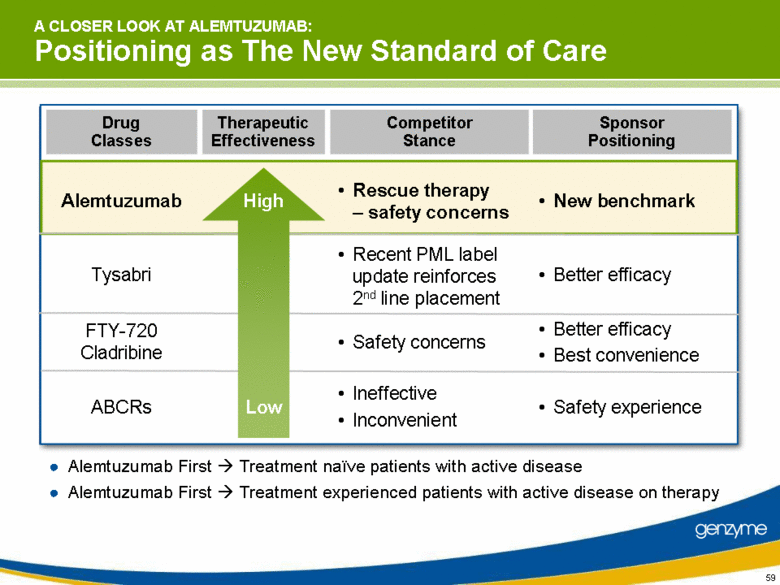
| 59 A CLOSER LOOK AT ALEMTUZUMAB: Positioning as The New Standard of Care Alemtuzumab First Treatment naïve patients with active disease Drug Classes Therapeutic Effectiveness Sponsor Positioning Competitor Stance Alemtuzumab Tysabri FTY-720 Cladribine ABCRs New benchmark Better efficacy Better efficacy Best convenience Safety experience High Low Rescue therapy – safety concerns Recent PML label update reinforces 2nd line placement Safety concerns Ineffective Inconvenient Alemtuzumab First Treatment experienced patients with active disease on therapy |
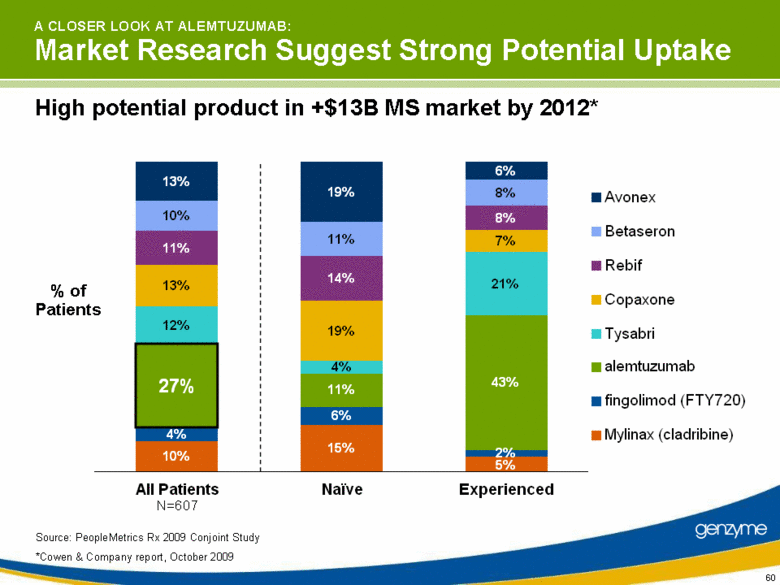
| N=607 A CLOSER LOOK AT ALEMTUZUMAB: Market Research Suggest Strong Potential Uptake Source: PeopleMetrics Rx 2009 Conjoint Study *Cowen & Company report, October 2009 % of Patients High potential product in +$13B MS market by 2012* 13% 10% 11% 13% 12% 27% 4% 10% 19% 11% 14% 19% 4% 11% 6% 15% 6% 8% 8% 7% 21% 43% 2% 5% All Patients Naive Experienced Avonex Betaseron Rebif Copaxone Tysabri alemtuzumab fingolimod (FTY720) Mylinax (cladribine) |

| Peter Wirth Corporate Secretary, EVP Legal and Corporate Development |

| Our Concerns with Icahn’s Proxy Contest Icahn provided no constructive input or perspective on the company prior to threatening a last minute proxy fight and still has not done so, despite our actively soliciting his views Contrasts with valuable input by Relational Investors LLC Icahn applies a “one size fits all” approach that is inappropriate for Genzyme’s current situation Unseating CEO risks serious business disruption The dissident slate lacks operational, manufacturing or regulatory experience Dissident slate poses serious antitrust problems and fundamental conflict of interests |

| Icahn’s “One Size Fits All” Approach Is Inappropriate for Genzyme Icahn’s stated thesis: All biotechnology companies should be owned by Big Pharma However, this approach has not led to a successful outcome at Biogen Idec Icahn’s attempt to oust the current team could impact Genzyme’s recovery that is underway Resolving manufacturing issues Stabilizing Cerezyme and Fabrazyme market share Maximizing alemtuzumab potential in multiple sclerosis We believe Icahn’s approach overlooks serious antitrust and conflicts issues |
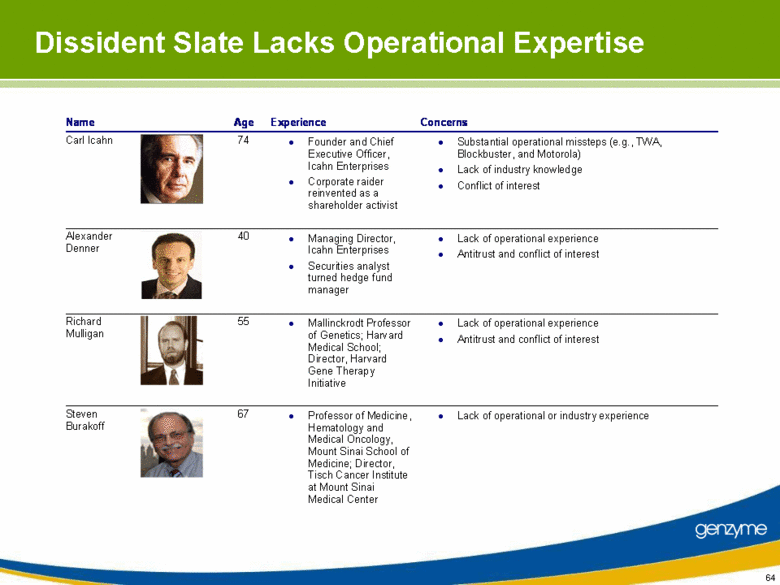
| Dissident Slate Lacks Operational Expertise Name Age Experience Concerns Carl Icahn 74 Founder and Chief Executive Officer, Icahn Enterprises Corporate raider reinvented as a shareholder activist Substantial operational missteps (e.g., TWA, Blockbuster, and Motorola) Lack of industry knowledge Conflict of interest Alexander Denner 40 Managing Director, Icahn Enterprises Securities analyst turned hedge fund manager Lack of operational experience Antitrust and conflict of interest Richard Mulligan 55 Mallinckrodt Professor of Genetics; Harvard Medical School; Director, Harvard Gene Therapy Initiative Lack of operational experience Antitrust and conflict of interest Steven Burakoff 67 Professor of Medicine, Hematology and Medical Oncology, Mount Sinai School of Medicine; Director, Tisch Cancer Institute at Mount Sinai Medical Center Lack of operational or industry experience |
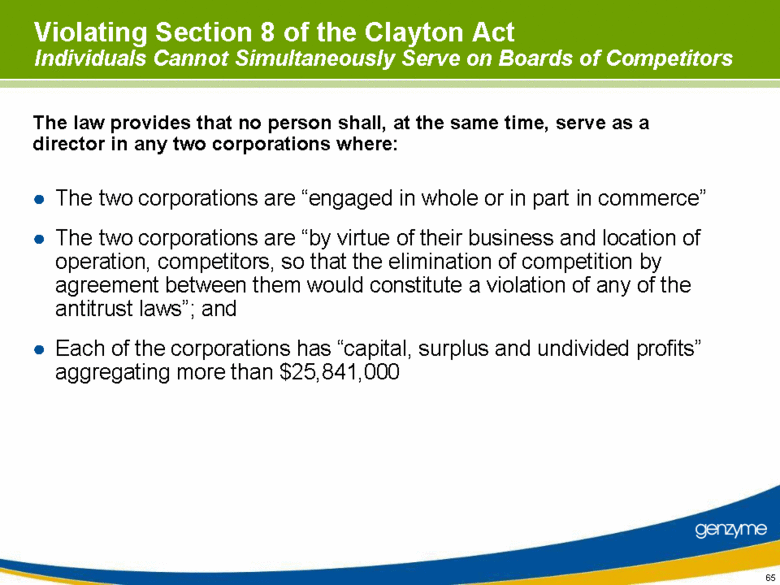
| Violating Section 8 of the Clayton Act Individuals Cannot Simultaneously Serve on Boards of Competitors The two corporations are “engaged in whole or in part in commerce” The two corporations are “by virtue of their business and location of operation, competitors, so that the elimination of competition by agreement between them would constitute a violation of any of the antitrust laws”; and Each of the corporations has “capital, surplus and undivided profits” aggregating more than $25,841,000 The law provides that no person shall, at the same time, serve as a director in any two corporations where: |

| Genzyme and Biogen Directly Compete in the Oncology Market Biogen’s billion dollar product, Rituxan, competes with the following Genzyme products in multiple disease areas: Solid Organ Transplant, Graft vs. Host Disease and Aplastic Anemia Thymoglobulin Acute Lymphoblastic Leukemia Clolar Non-Hodgkin’s Lymphoma Leukine Non-Hodgkin’s Lymphoma, Chronic Lymphocytic Leukemia Fludara Chronic Lymphocytic Leukemia, Non-Hodgkin’s Lymphoma and Solid Organ Transplant Campath Disease Area Product Additionally, Rituxan may also compete with Genzyme’s Campath in Hodgkin’s lymphoma, Clolar in acute myeloid leukemia, Leukine in acute myeloid leukemia and Mozobil in non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and multiple myeloma. |
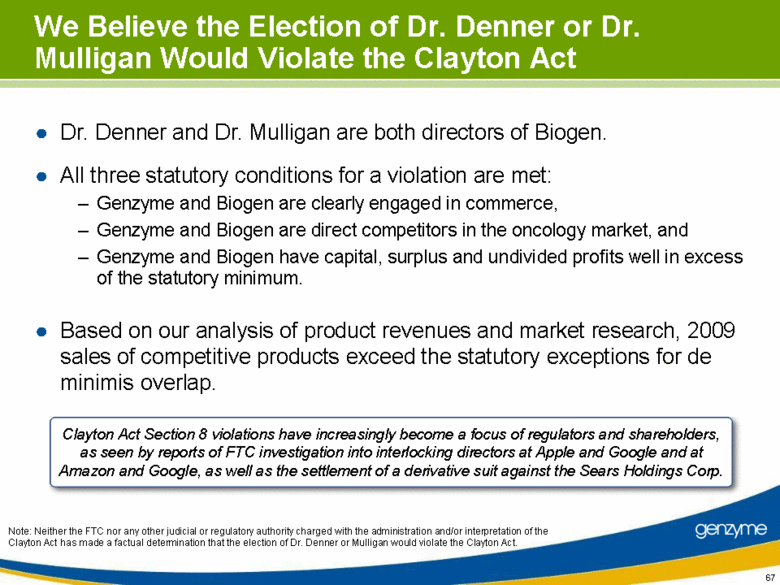
| We Believe the Election of Dr. Denner or Dr. Mulligan Would Violate the Clayton Act Dr. Denner and Dr. Mulligan are both directors of Biogen. All three statutory conditions for a violation are met: Genzyme and Biogen are clearly engaged in commerce, Genzyme and Biogen are direct competitors in the oncology market, and Genzyme and Biogen have capital, surplus and undivided profits well in excess of the statutory minimum. Based on our analysis of product revenues and market research, 2009 sales of competitive products exceed the statutory exceptions for de minimis overlap. Clayton Act Section 8 violations have increasingly become a focus of regulators and shareholders, as seen by reports of FTC investigation into interlocking directors at Apple and Google and at Amazon and Google, as well as the settlement of a derivative suit against the Sears Holdings Corp. Note: Neither the FTC nor any other judicial or regulatory authority charged with the administration and/or interpretation of the Clayton Act has made a factual determination that the election of Dr. Denner or Mulligan would violate the Clayton Act. |
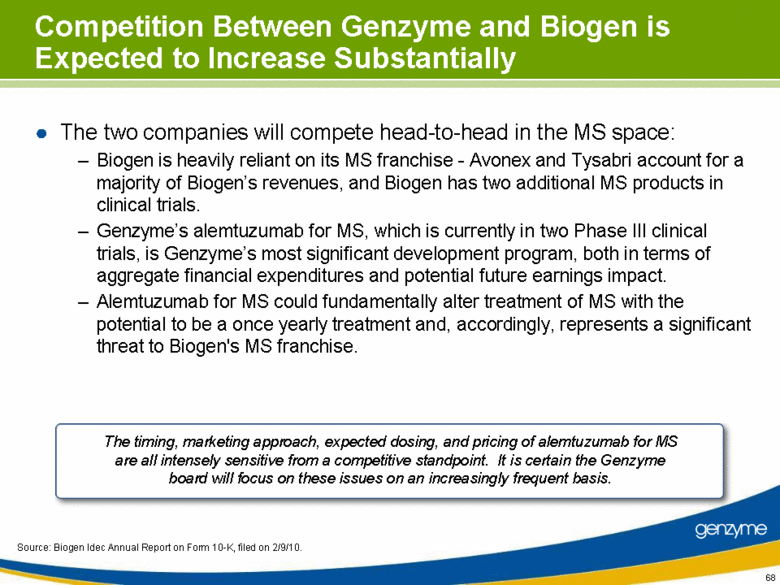
| Competition Between Genzyme and Biogen is Expected to Increase Substantially The two companies will compete head-to-head in the MS space: Biogen is heavily reliant on its MS franchise - Avonex and Tysabri account for a majority of Biogen’s revenues, and Biogen has two additional MS products in clinical trials. Genzyme’s alemtuzumab for MS, which is currently in two Phase III clinical trials, is Genzyme’s most significant development program, both in terms of aggregate financial expenditures and potential future earnings impact. Alemtuzumab for MS could fundamentally alter treatment of MS with the potential to be a once yearly treatment and, accordingly, represents a significant threat to Biogen's MS franchise. The timing, marketing approach, expected dosing, and pricing of alemtuzumab for MS are all intensely sensitive from a competitive standpoint. It is certain the Genzyme board will focus on these issues on an increasingly frequent basis. Source: Biogen Idec Annual Report on Form 10-K, filed on 2/9/10. |
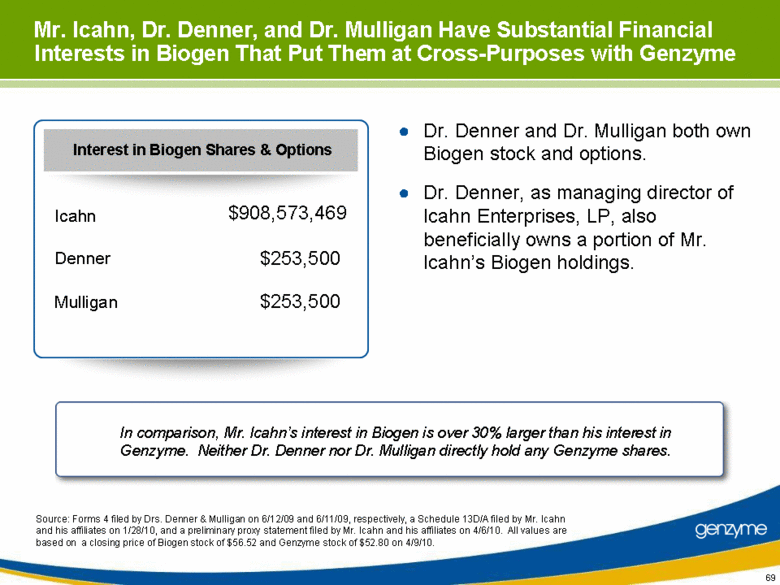
| Mr. Icahn, Dr. Denner, and Dr. Mulligan Have Substantial Financial Interests in Biogen That Put Them at Cross-Purposes with Genzyme Dr. Denner and Dr. Mulligan both own Biogen stock and options. Dr. Denner, as managing director of Icahn Enterprises, LP, also beneficially owns a portion of Mr. Icahn’s Biogen holdings. Source: Forms 4 filed by Drs. Denner & Mulligan on 6/12/09 and 6/11/09, respectively, a Schedule 13D/A filed by Mr. Icahn and his affiliates on 1/28/10, and a preliminary proxy statement filed by Mr. Icahn and his affiliates on 4/6/10. All values are based on a closing price of Biogen stock of $56.52 and Genzyme stock of $52.80 on 4/9/10. In comparison, Mr. Icahn’s interest in Biogen is over 30% larger than his interest in Genzyme. Neither Dr. Denner nor Dr. Mulligan directly hold any Genzyme shares. Interest in Biogen Shares & Options $253,500 Mulligan $253,500 Denner $908,573,469 Icahn |

| We Believe Icahn’s Biogen Conflicts and Problematic Incentives Are Immediate Concerns Genzyme anticipates a launch of alemtuzumab for MS in 2012 and is currently developing its overall launch plan strategy, including proprietary market research, compiling pharmacoeconomic data, planning launch sequencing, and evaluating pricing. These activities will continue during the next director term and will be discussed regularly at board meetings. How Genzyme invests in, prices, markets, and delivers alemtuzumab for MS will have a direct and substantial impact on Genzyme and Biogen. Mr. Icahn, Dr. Denner, and Dr. Mulligan have substantial financial incentives to allocate resources away from, and otherwise undermine, alemtuzumab for MS in order to protect the Biogen franchise. Even with the best intentions, it is inconceivable how the detailed information concerning Genzyme’s key future product will not affect how Mr. Icahn, Dr. Denner, and Dr. Mulligan influence Biogen in its approach to the MS market. |

| O U R P E R S P E C T I V E: The Biogen Conflict Prevents Mr. Icahn, Dr. Denner, and Dr. Mulligan From Serving as Effective Directors of Genzyme The importance of alemtuzumab for MS to Genzyme means that it will be discussed regularly by the board in contexts that are not separate and discrete – for instance, discussions on allocating capital to products will involve discussions of alemtuzumab for MS. Given the increasingly frequent and detailed discussions around alemtuzumab for MS, recusal from these decisions is not a realistic option and would compromise the value of Mr. Icahn, Dr. Denner, and Dr. Mulligan as Genzyme directors from the start. Genzyme shareholders should not be left to wonder if their directors are always acting in Genzyme’s best interests. Genzyme shareholders deserve directors who will participate fully in an informed manner in important decisions the Board expects to address during their term. |

| O U R C O N C L U S I O N: Icahn’s Nominees Are Tainted By Fundamental Conflicts of Interest We believe the election of Dr. Denner or Dr. Mulligan would produce a clear violation of the Clayton Act. Mr. Icahn, Dr. Denner, and Dr. Mulligan have substantial interests in Biogen that we believe fundamentally compromise their ability to serve as independent directors of Genzyme and act in the best interests of Genzyme shareholders. The development of alemtuzumab for MS, Genzyme’s most important pipeline product, is the clearest example of Mr. Icahn’s, Dr. Denner’s, and Dr. Mulligan’s conflicts of interest. Even with the best intentions, Mr. Icahn, Dr. Denner, and Dr. Mulligan may not be able to participate in important decisions the Genzyme board faces in the coming director term. We believe Genzyme shareholders cannot expect Dr. Denner, Dr. Mulligan, or Dr. Burakoff to act independently due to their relationships with Mr. Icahn. |

| Robert Carpenter Lead Independent Director |

| CONCLUSION Positioned for Renewed Value Creation Genzyme’s Board and management team are executing a proven strategy and are on track to restore Genzyme to profitable growth Executing With new directors and new world-class leaders at the operating level, the company is gaining momentum and building value for shareholders Building Value Genzyme shareholders are best served by re-electing the board’s nominees and enabling the company to continue on its current path On Track We are implementing new financial disciplines to allocate capital, improve operating margins and optimize our capital structure Exercising Discipline |

| Information Concerning Participants in the Company’s Solicitation of Proxies Scott Canute and Ron Branning may be considered to be “participants” in the company’s solicitation of proxies from its shareholders in connection with the company’s 2010 annual meeting of shareholders. Mr. Canute’s principal occupation is Genzyme’s President of Global Manufacturing and Corporate Operations and Mr. Branning’s principal occupation is Genzyme’s Senior Vice President of Global Product Quality. The business address and address of employment of the two participants is c/o Genzyme Corporation, 500 Kendall Street, Cambridge, MA 02142. As of April 9, 2010, neither Mr. Canute nor Mr. Branning was the beneficial owner of any shares of Genzyme common stock (“Shares”), including stock options and restricted stock units (“RSUs”) vesting within 60 days of April 9, 2010. Between April 9, 2008 and April 9, 2010, Mr. Canute acquired 14,250 Shares on March 1, 2010 though a grant of performance vesting RSUs and 25,000 Shares on March 15, 2010 though a grant of time vesting RSUs, and Mr. Branning acquired 5,000 Shares on February 16, 2010 through a grant of time vesting RSUs. Neither participant has disposed of any Shares between April 9, 2008 and April 9, 2010. Neither participant (i) beneficially owns (within the meaning of Rule 13d-3 under the Securities Exchange Act of 1934), directly or indirectly, any shares or other securities of Genzyme or any of its subsidiaries other than as set forth above, (ii) has purchased or sold any of such securities within the past two years, or (iii) is, or within the past year was, a party to any contract, arrangement or understanding with any person with respect to any such securities. Neither Participant has any substantial interests, direct or indirect, by security holding or otherwise, in any matter to be acted upon at the company’s 2010 annual meeting of shareholders or is, or has been within the past year, a party to any contract, arrangement or understanding with any person with respect to any Genzyme securities, including, but not limited to, joint ventures, loan or option agreements, puts or calls, guarantees against loss or guarantees of profit, division of losses or profits or the giving or withholding of proxies. Neither participants' associates beneficially owns, directly or indirectly, any Genzyme securities. Neither of the participants nor any of their associates (i) has had or will have a direct or indirect material interest in any transaction or series of similar transactions since the beginning of the company’s last fiscal year or any currently proposed transactions, or series of similar transactions, to which Genzyme or any of its subsidiaries was or is to be a party in which the amount involved exceeds $120,000 or (ii) has any arrangements or understandings with any person with respect to any future employment by Genzyme or its affiliates or with respect to any future transactions to which Genzyme or its affiliates will or may be a party. 75 |

| Appendix |
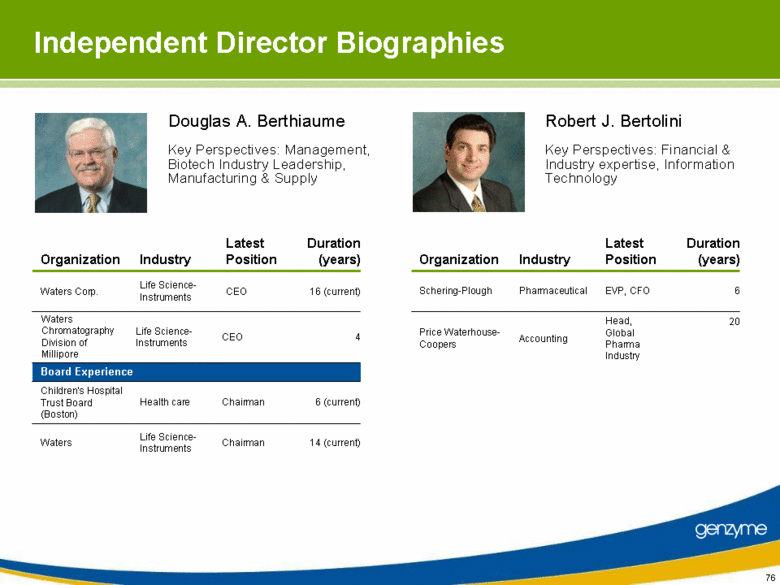
| Independent Director Biographies 6 (current) Chairman Health care Children’s Hospital Trust Board (Boston) 14 (current) Chairman Life Science-Instruments Waters CEO CEO Latest Position Life Science-Instruments Life Science-Instruments Industry Board Experience Waters Chromatography Division of Millipore Waters Corp. Organization 4 16 (current) Duration (years) Head, Global Pharma Industry EVP, CFO Latest Position Accounting Pharmaceutical Industry Price Waterhouse-Coopers Schering-Plough Organization 20 6 Duration (years) Douglas A. Berthiaume Key Perspectives: Financial & Industry expertise, Information Technology Robert J. Bertolini Key Perspectives: Management, Biotech Industry Leadership, Manufacturing & Supply |
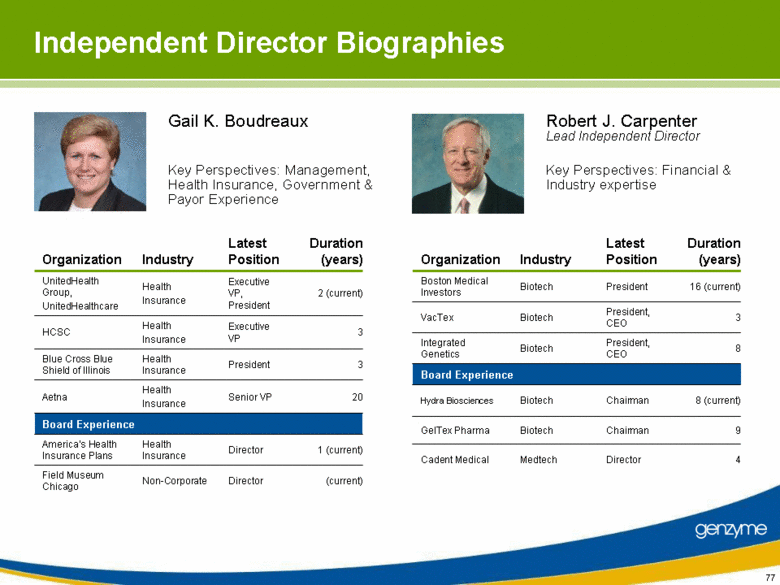
| Independent Director Biographies 3 President Health Insurance Blue Cross Blue Shield of Illinois 2 (current) Executive VP, President Health Insurance UnitedHealth Group, UnitedHealthcare 3 Executive VP Health Insurance HCSC 1 (current) Director Health Insurance America’s Health Insurance Plans (current) Director Non-Corporate Field Museum Chicago Senior VP Latest Position Health Insurance Industry Board Experience Aetna Organization 20 Duration (years) 3 President, CEO Biotech VacTex 9 Chairman Biotech GelTex Pharma 8 (current) Chairman Biotech Hydra Biosciences 4 Director Medtech Cadent Medical President, CEO President Latest Position Biotech Biotech Industry Board Experience Integrated Genetics Boston Medical Investors Organization 8 16 (current) Duration (years) Gail K. Boudreaux Key Perspectives: Financial & Industry expertise Robert J. Carpenter Lead Independent Director Key Perspectives: Management, Health Insurance, Government & Payor Experience |
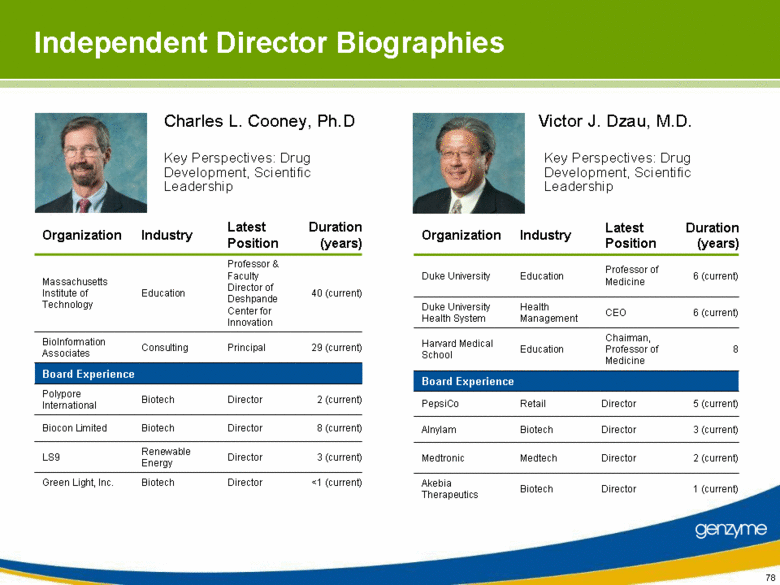
| Independent Director Biographies 2 (current) Director Biotech Polypore International 3 (current) Director Renewable Energy LS9 29 (current) Principal Consulting BioInformation Associates <1 (current) Director Biotech Green Light, Inc. 8 (current) Director Biotech Biocon Limited Professor & Faculty Director of Deshpande Center for Innovation Latest Position Education Industry Board Experience Massachusetts Institute of Technology Organization 40 (current) Duration (years) 1 (current) Director Biotech Akebia Therapeutics 6 (current) CEO Health Management Duke University Health System 3 (current) Director Biotech Alnylam 2 (current) Director Medtech Medtronic 5 (current) Director Retail PepsiCo Chairman, Professor of Medicine Professor of Medicine Latest Position Education Education Industry Board Experience Harvard Medical School Duke University Organization 8 6 (current) Duration (years) Charles L. Cooney, Ph.D Key Perspectives: Drug Development, Scientific Leadership Victor J. Dzau, M.D. Key Perspectives: Drug Development, Scientific Leadership |
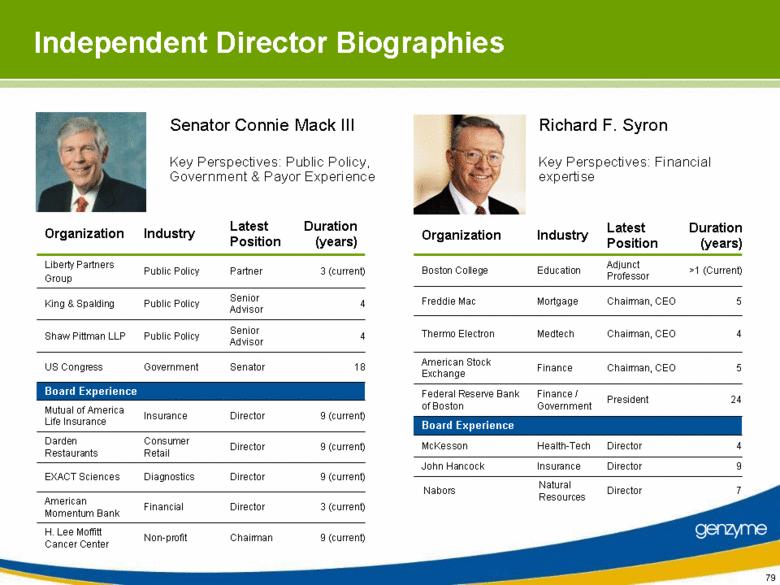
| Independent Director Biographies 4 Senior Advisor Public Policy King & Spalding 18 Senator Government US Congress 4 Senior Advisor Public Policy Shaw Pittman LLP 3 (current) Director Financial American Momentum Bank 9 (current) Director Diagnostics EXACT Sciences 9 (current) Chairman Non-profit H. Lee Moffitt Cancer Center 9 (current) Director Insurance Mutual of America Life Insurance 9 (current) Director Consumer Retail Darden Restaurants Partner Latest Position Public Policy Industry Board Experience Liberty Partners Group Organization 3 (current) Duration (years) 7 Director Natural Resources Nabors 5 Chairman, CEO Finance American Stock Exchange 9 Director Insurance John Hancock >1 (Current) Adjunct Professor Education Boston College 4 Chairman, CEO Medtech Thermo Electron 5 Chairman, CEO Mortgage Freddie Mac 4 Director Health-Tech McKesson President Latest Position Finance / Government Industry Board Experience Federal Reserve Bank of Boston Organization 24 Duration (years) Senator Connie Mack III Key Perspectives: Financial expertise Richard F. Syron Key Perspectives: Public Policy, Government & Payor Experience |
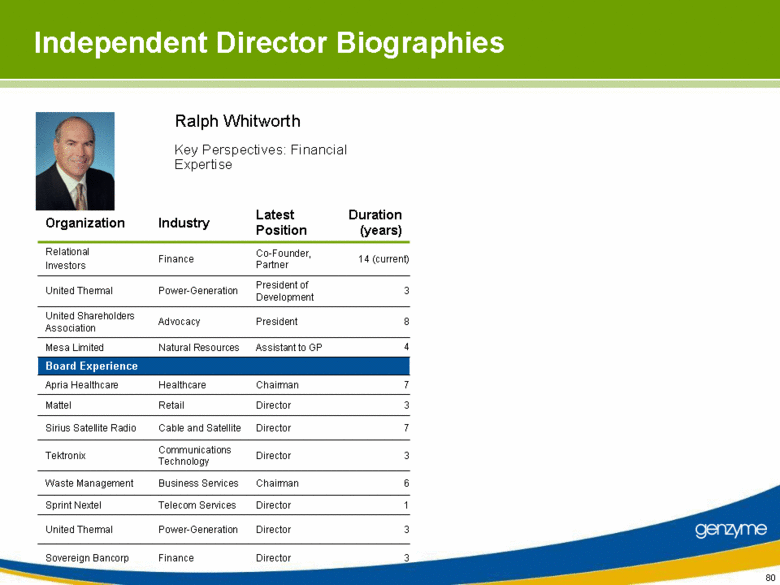
| Independent Director Biographies 3 Director Power-Generation United Thermal 3 Director Communications Technology Tektronix 6 Chairman Business Services Waste Management 8 President Advocacy United Shareholders Association 3 President of Development Power-Generation United Thermal 1 Director Telecom Services Sprint Nextel 7 Director Cable and Satellite Sirius Satellite Radio 3 Director Finance Sovereign Bancorp 7 Chairman Healthcare Apria Healthcare 3 Director Retail Mattel Assistant to GP Co-Founder, Partner Latest Position Natural Resources Finance Industry Board Experience Mesa Limited Relational Investors Organization 4 14 (current) Duration (years) Ralph Whitworth Key Perspectives: Financial Expertise [TBU] |
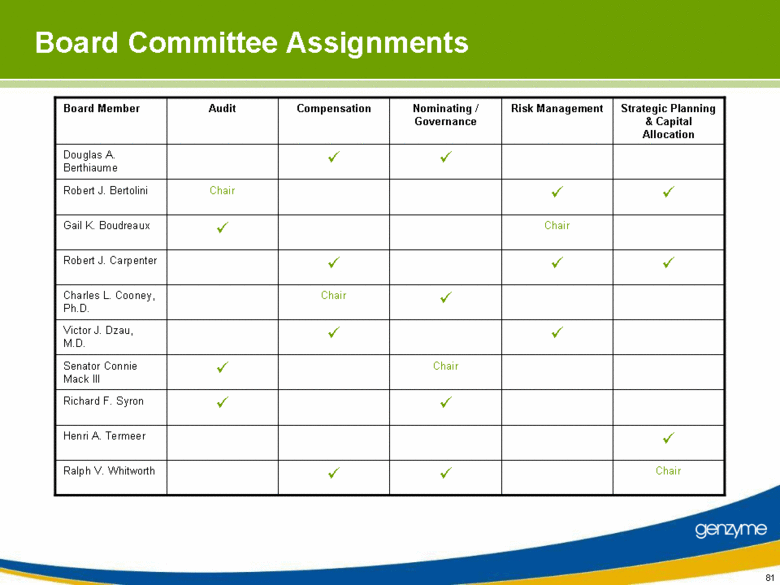
| Board Committee Assignments Chair Ralph V. Whitworth Henri A. Termeer Richard F. Syron Chair Senator Connie Mack III Victor J. Dzau, M.D. Chair Charles L. Cooney, Ph.D. Robert J. Carpenter Chair Gail K. Boudreaux Chair Robert J. Bertolini Douglas A. Berthiaume Strategic Planning & Capital Allocation Risk Management Nominating / Governance Compensation Audit Board Member |
Appendix B –
GAAP to Non-GAAP Reconciliations
GENZYME CORPORATION
CASH FLOW RETURN ON INVESTED CAPITAL CALCULATION
(in millions, except percentages)
The company uses Cash Flow Return on Tangible Invested Capital (CFROI) as a measure of the efficiency and effectiveness of its use of capital. CFROI is not a measure of financial performance under generally accepted accounting principles (GAAP) and may not be defined and calculated by other companies in the same manner. This non-GAAP financial measure is not intended to be considered in isolation or as a substitute for GAAP measures.
We define CFROI as follows:
| Total Adjusted Cash Profits | | | | |
Average Tangible Invested Capital | | | |
| | | |
We define Adjusted Cash Profits as follows: | | We define Tangible Invested Capital as follows: | |
GAAP net income (loss) | | Total assets | |
+ Operating lease expense | | - Non-interest bearing liabilities (d) | |
+ Depreciation expense | | - Deferred taxes | |
+ Amortization expense, net of tax | | + Capitalized research and development (e) | |
- Other income (expenses), net of tax | | + Capitalized operating leases (f) | |
+ Other non-operating items, net of tax | | + Accumulated depreciation | |
+ Acquisition related expense, net of tax | | - Goodwill and intangibles, net | |
+ Non-GAAP research and development expense | | - Excess cash (g) | |
= Total Adjusted Cash Profits | | - Long-term investments | |
| | =Total Tangible Invested Capital | |
| | | |
We define Average Tangible Invested Capital as follows: | | | |
Prior year tangible invested capital | | | |
+ Current year tangible invested capital
divided by 2 | | | |
= Average Tangible Invested Capital | | | |
Consolidated CFROI Calculation | | | | | |
| | Consolidated | |
| | 2006 | | 2009 | |
Adjusted Cash Profits | | | | | |
GAAP net income (loss) | | $ | (16.8 | ) | $ | 422.3 | |
Operating lease expense | | 60.9 | | 81.8 | |
Depreciation expense | | 122.0 | | 190.1 | |
Amortization expense, net of tax (a) | | 132.3 | | 185.0 | |
Other income (expenses), net of tax (a) | | (87.7 | ) | (27.7 | ) |
Other non-operating items, net of tax (b) | | 134.3 | | 19.2 | |
Acquisition related expense, net of tax | | 404.3 | | 48.5 | |
Non-GAAP research and development (c) | | 565.4 | | 803.9 | |
Total Adjusted Cash Profits | | $ | 1,314.7 | | $ | 1,723.1 | |
| | | | | |
Tangible Invested Capital | | | | | |
Total assets | | $ | 7,191.2 | | $ | 10,060.7 | |
Non-interesting bearing liabilities (d) | | (714.4 | ) | (1,237.2 | ) |
Deferred taxes | | (136.9 | ) | (555.2 | ) |
Capitalized research and development (e) | | 2,747.0 | | 3,910.0 | |
Capitalized operating leases (f) | | 730.8 | | 981.6 | |
Accumulated depreciation | | 695.4 | | 1,077.8 | |
Goodwill and intangibles, net | | (2,790.8 | ) | (3,716.6 | ) |
Excess cash (g) | | (252.5 | ) | (402.8 | ) |
Long-term investments | | (673.5 | ) | (143.8 | ) |
Total Tangible Invested Capital | | $ | 6,796.30 | | $ | 9,974.50 | |
| | | | | |
Average Tangible Invested Capital | | 6,466.0 | | 9,401.7 | |
| | | | | |
Cash Flow Return on Tangible Invested Capital | | 20.3 | % | 18.3 | % |
Business Unit CFROI Calculation
Solely for purposes of management reporting of CFROI by business unit, certain of Genzyme’s corporate expenses, income, assets and liabilities have been distributed to the business units. A large portion of Genzyme’s fixed assets are considered corporate assets because the assets support the manufacturing of multiple products. Corporate G&A is distributed amongst the business units based on revenue. In addition, cash, long-term investments and investments in equity securities are also considered corporate assets and are distributed to the business units based on net income(loss). Business units incurring net losses were charged with negative cash, long-term investments and investments in equity securities balances. Genzyme has treated the total of cash, long-term investments and investments in equity securities, if negative, as a loan and charges interest expense to the borrowing business units and credits interest income to the loaning business units. Genzyme provides CFROI by business unit data for its significant business units only. Therefore, the individual CFROI by business unit calculations are not intended to aggregate to Genzyme’s overall CFROI calculation.
| | 2006 | |
| | Personalized
Genetic Health | | Renal &
Endocrinology | | Biosurgery | | Hematology &
Oncology | | Other | |
Adjusted Cash Profits | | | | | | | | | | | |
Management reporting net income (loss) (h) | | $ | 459 | | $ | 124 | | $ | 10 | | $ | (443 | ) | $ | (176 | ) |
Operating lease expense | | 27 | | 13 | | 7 | | 4 | | 8 | |
Depreciation expense | | 54 | | 29 | | 9 | | 16 | | 14 | |
Amortization expense, net of tax | | 2 | | 52 | | 44 | | 27 | | 8 | |
Other income (expenses), net of tax | | — | | — | | — | | — | | — | |
Other non-operating items, net of tax | | (46 | ) | (24 | ) | (13 | ) | (7 | ) | 137 | |
Acquisition related expense, net of tax | | — | | — | | — | | 404 | | — | |
Non-GAAP research and development | | 301 | | 115 | | 54 | | 79 | | 15 | |
Inter-divisional interest | | 37 | | 5 | | (10 | ) | (30 | ) | (2 | ) |
Total Adjusted Cash Profits | | $ | 834 | | $ | 314 | | $ | 102 | | $ | 50 | | $ | 4 | |
| | | | | | | | | | | |
Tangible Invested Capital | | | | | | | | | | | |
Total assets | | $ | 4,405 | | $ | 1,812 | | $ | 568 | | $ | 1,237 | | $ | 513 | |
Non-interesting bearing liabilities (d) | | (256 | ) | (148 | ) | (109 | ) | (108 | ) | (137 | ) |
Deferred taxes | | (205 | ) | 226 | | 33 | | (115 | ) | (76 | ) |
Capitalized research and development (e) | | 1,047 | | 478 | | 211 | | 912 | | 100 | |
Capitalized operating leases (f) | | 336 | | 161 | | 89 | | 49 | | 95 | |
Accumulated depreciation | | 274 | | 162 | | 81 | | 40 | | 138 | |
Goodwill and intangibles, net | | (553 | ) | (985 | ) | (366 | ) | (750 | ) | (136 | ) |
Excess cash (g) | | (216 | ) | (43 | ) | — | | — | | 6 | |
Long-term investments | | (1,141 | ) | (189 | ) | — | | — | | — | |
Total Tangible Invested Capital | | $ | 3,691 | | $ | 1,474 | | $ | 507 | | $ | 1,265 | | $ | 503 | |
| | | | | | | | | | | |
Average Tangible Invested Capital | | 3,213 | | 1,296 | | 444 | | 1,113 | | 440 | |
| | | | | | | | | | | |
Cash Flow Return on Tangible Invested Capital | | 26.0 | % | 24.2 | % | 23.0 | % | 4.5 | % | 0.9 | % |
| | 2006 | | 2009 | | | | | | | | |
Notes: | | | | | | | | | | | | |
(a) Tax effect on: | | | | | | | | | | | | |
Other income (expenses) | | 50.2 | | 12.3 | | | | | | | | |
Amortization expense | | (77.0 | ) | (81.4 | ) | | | | | | | |
(b) Represents in 2009, gain on acquisition of business of $24.2 net of tax of ($6.6) and minority interest of $2.5 net of tax of ($0.9) and in 2006, minority interest of $10.4 net of tax of ($3.8), equity in income of equity method investments of $15.7 net of tax of ($5.7), impairment of goodwill of $219.2 net of tax of ($69.8) and settlement of tax audits of ($31.7).
(c) For 2006, Non-GAAP research and development expense excludes $19.3 million of expenses related to Genzyme’s joint venture with Dyax.
(d) Non-interest bearing liabilities equals total liabilities less debt and contingent consideration obligations.
(e) Represents approximate cumulative capitalized research and development, net of applicable depreciation. R&D is capitalized because it is considered an investment that should generate returns in the future.
(f) Represents operating lease expense multiplied by 12.
(g) Represents cash and short-term investments less 5% of total assets. Business units’ excess cash represents an allocation of corporate excess cash to cash positive businesses.
(h) Management reporting net income (loss) is derived solely for the purpose of calculating CFROI. It is not intended to represent GAAP net income (loss).
GENZYME CORPORATION
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2009
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | OTHER DISCRETE ITEMS | |
| | | | | | Bayer | | | | | | | | (included in GAAP and Non-GAAP results) | |
| | | | GAAP | | Acquisition | | FAS 123R | | | | | | Manufacturing | | Technology | | Q2 Inventory | |
| | | | As Reported | | Related (2) | | Expense | | | | NON-GAAP (1) | | Related | | Purchase | | Fair Value Step-up (2) | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 4,515,525 | | | | | | | | $ | 4,515,525 | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (1,386,076 | ) | $ | 36,822 | | $ | 32,314 | | | | $ | (1,316,940 | ) | $ | 76,398 | | | | $ | 6,639 | |
Gross margin | | 69 | % | $ | 3,129,449 | | $ | 36,822 | | $ | 32,314 | | 71 | % | $ | 3,198,585 | | $ | 76,398 | | | | $ | 6,639 | |
| | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (1,428,596 | ) | | | $ | 110,410 | | | | $ | (1,318,186 | ) | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (865,257 | ) | | | $ | 61,391 | | | | $ | (803,866 | ) | | | $ | 25,180 | | | |
| | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (266,305 | ) | | | | | | | $ | (266,305 | ) | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Contingent consideration expense | | | | $ | (65,584 | ) | $ | 65,584 | | | | | | $ | — | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Gains (losses) on investments in equity securities | | | | $ | (56 | ) | | | | | | | $ | (56 | ) | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Gain on acquisition of business | | | | $ | 24,159 | | $ | (24,159 | ) | | | | | $ | — | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Other | | | | $ | (1,719 | ) | | | | | | | $ | (1,719 | ) | $ | 1,484 | | | | | |
| | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 17,642 | | | | | | | | $ | 17,642 | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Interest expense | | | | $ | — | | | | | | | | $ | — | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | 543,733 | | $ | 78,247 | | $ | 204,115 | | | | $ | 826,095 | | $ | 77,882 | | $ | 25,180 | | $ | 6,639 | |
| | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 22 | % | $ | (121,433 | ) | $ | (29,739 | ) | $ | (53,434 | ) | 25 | % | $ | (204,606 | ) | $ | (17,956 | ) | $ | (8,118 | ) | $ | (1,302 | ) |
| | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | | $ | 422,300 | | $ | 48,508 | | $ | 150,681 | | | | $ | 621,489 | | $ | 59,926 | | $ | 17,062 | | $ | 5,337 | |
| | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | |
Basic | | | | $ | 1.57 | | $ | 0.18 | | $ | 0.56 | | | | $ | 2.31 | | $ | 0.22 | | $ | 0.06 | | $ | 0.02 | |
| | | | | | | | | | | | | | | | | | | |
Diluted | | | | $ | 1.54 | | $ | 0.18 | | $ | 0.55 | | | | $ | 2.27 | | $ | 0.22 | | $ | 0.06 | | $ | 0.02 | |
| | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | |
Basic | | | | 268,841 | | 268,841 | | 268,841 | | | | 268,841 | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Diluted | | | | 274,071 | | 274,071 | | 274,071 | | | | 274,071 | | | | | | | |
Notes:
(1) Represents the Non-GAAP results of operations for Genzyme Corporation for the year ended December 31, 2009. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP. Earnings per share are calculated as net income (loss) divided by weighted average shares outstanding. Therefore, earnings per share may not add across due to rounding.
(2) “Bayer Acquisition Related” includes the gain on acquisition, contingent consideration expense and, beginning with Q3 2009, the inventory fair value step-up associated with our acquisition from Bayer. The initial inventory fair value step-up in Q2 for the Bayer transaction was presented as “Other Discrete Items”.
GENZYME CORPORATION
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2008
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | Items Formerly Excluded from Non-GAAP [(Income)/Expense] | |
| | | | | | | | | | | | Dilution | | | | | | | | | |
| | | | | | | | | | | | Due to | | Validation/ | | | | | | | |
| | | | GAAP | | FAS 123R | | | | NON-GAAP | | Common Stock | | Inventory | | (Gain) Loss on | | License | | | |
| | | | As Reported | | Expense | | | | As Adjusted (1) | | Equivalents | | Write-offs | | Investments | | Fees | | Amortization | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 4,605,039 | | | | | | $ | 4,605,039 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (1,148,562 | ) | $ | 27,555 | | | | $ | (1,121,007 | ) | | | $ | 12,614 | | | | | | | |
Gross margin | | 75 | % | $ | 3,456,477 | | $ | 27,555 | | 76 | % | $ | 3,484,032 | | | | $ | 12,614 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (1,338,190 | ) | $ | 102,745 | | | | $ | (1,235,445 | ) | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (1,308,330 | ) | $ | 56,673 | | | | $ | (1,251,657 | ) | | | $ | 11,039 | | | | $ | 490,900 | | | |
| | | | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (226,442 | ) | | | | | $ | (226,442 | ) | | | | | | | | | $ | 226,442 | |
| | | | | | | | | | | | | | | | | | | | | |
Charge for impaired assets | | | | $ | (2,036 | ) | | | | | $ | (2,036 | ) | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Equity in income (loss) of equity method investments | | | | $ | 201 | | | | | | $ | 201 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Minority interest | | | | $ | 2,217 | | | | | | $ | 2,217 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Gains (losses) on investments in equity securities | | | | $ | (3,340 | ) | | | | | $ | (3,340 | ) | | | | | $ | 5,253 | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Other | | | | $ | (1,861 | ) | | | | | $ | (1,861 | ) | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 51,260 | | | | | | $ | 51,260 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Interest Expense | | | | $ | (4,418 | ) | | | | | $ | (4,418 | ) | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | 625,538 | | $ | 186,973 | | | | $ | 812,511 | | $ | — | | $ | 23,653 | | $ | 5,253 | | $ | 490,900 | | $ | 226,442 | |
| | | | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 33 | % | $ | (204,457 | ) | $ | (56,740 | ) | 32 | % | $ | (261,197 | ) | $ | — | | $ | (5,724 | ) | $ | (390 | ) | $ | (108,328 | ) | $ | (75,399 | ) |
| | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | | $ | 421,081 | | $ | 130,233 | | | | $ | 551,314 | | $ | — | | $ | 17,929 | | $ | 4,863 | | $ | 382,572 | | $ | 151,043 | |
| | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | $ | 1.57 | | $ | 0.50 | | | | $ | 2.07 | | $ | — | | $ | 0.07 | | $ | 0.02 | | $ | 1.42 | | $ | 0.56 | |
| | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | $ | 1.50 | | $ | 0.45 | | | | $ | 1.95 | | $ | 0.11 | | $ | 0.06 | | $ | 0.01 | | $ | 1.34 | | $ | 0.53 | |
| | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | 268,490 | | | | | | 268,490 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | 285,595 | | | | | | 285,595 | | | | | | | | | | | |
Notes:
(1) Represents the adjusted Non-GAAP results of operations and financial position for Genzyme Corporation for the year ended December 31, 2008. All other amounts presented herein represent previously reported amounts. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP.
(2) Diluted earnings per share and diluted weighted average shares outstanding reflect the adoption of EITF 04-8. In accordance with the provisions of EITF 04-8, interest and debt fees related to our 1.25% convertible senior notes of $6,915K, net of tax, have been added back to net income and approximately 8,851K shares have been added to diluted weighted average shares outstanding for purposes of computing GAAP and Non-GAAP diluted earnings per share.
GENZYME CORPORATION
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2007
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | Items Formerly Excluded From Non-GAAP [(Income)/Expense] | |
| | | | | | | | | | | | | | Dilution | | (Gain) on | | | | | | | | | | | |
| | | | | | | | | | | | | | Due to | | Investments | | | | | | | | | | | |
| | | | GAAP | | FAS 123R | | Acquisition | | | | NON-GAAP | | Common Stock | | in Equity | | Litigation | | Milestone | | Manufacturing | | | | Effect of | |
| | | | As Reported | | Expense | | Related | | | | As Adjusted (1) | | Equivalents | | Securities | | Settlement | | Payment | | Related | | Amortization | | FIN 46 | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 3,813,519 | | | | | | | | $ | 3,813,519 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (927,330 | ) | $ | 25,677 | | | | | | $ | (901,653 | ) | | | | | | | | | $ | 20,916 | | | | | |
Gross margin | | 76 | % | $ | 2,886,189 | | $ | 25,677 | | | | 76 | % | $ | 2,911,866 | | | | | | | | | | $ | 20,916 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (1,186,438 | ) | $ | 106,172 | | | | | | $ | (1,080,266 | ) | | | | | $ | 64,000 | | | | | | | | $ | 200 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (731,950 | ) | $ | 58,101 | | | | | | $ | (673,849 | ) | | | | | | | $ | 25,000 | | | | | | $ | 7,461 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (201,105 | ) | | | | | | | $ | (201,105 | ) | | | | | | | | | | | $ | 201,105 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Purchase of in-process research and development | | | | $ | (106,350 | ) | | | $ | 106,350 | | | | $ | — | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Equity in income (loss) of equity method investments | | | | $ | 7,398 | | | | $ | 19,150 | | | | $ | 26,548 | | | | | | | | | | | | $ | 830 | | $ | (3,830 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Minority interest | | | | $ | 3,932 | | | | | | | | $ | 3,932 | | | | | | | | | | | | | | $ | (3,831 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gains (losses) on investments in equity securities | | | | $ | 13,067 | | | | | | | | $ | 13,067 | | | | $ | (10,848 | ) | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other | | | | $ | (7,118 | ) | | | | | | | $ | (7,118 | ) | | | | | | | | | $ | 5,735 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 70,196 | | | | | | | | $ | 70,196 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest Expense | | | | $ | (12,147 | ) | | | | | | | $ | (12,147 | ) | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | 735,674 | | $ | 189,950 | | $ | 125,500 | | | | $ | 1,051,124 | | $ | — | | $ | (10,848 | ) | $ | 64,000 | | $ | 25,000 | | $ | 26,651 | | $ | 201,935 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 35 | % | $ | (255,481 | ) | $ | (58,148 | ) | (15,781 | ) | 31 | % | $ | (329,410 | ) | $ | — | | $ | 2,698 | | $ | — | | $ | (9,069 | ) | $ | (9,702 | ) | $ | (72,449 | ) | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | | $ | 480,193 | | $ | 131,802 | | $ | 109,719 | | | | $ | 721,714 | | $ | — | | $ | (8,150 | ) | $ | 64,000 | | $ | 15,931 | | $ | 16,949 | | $ | 129,486 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | $ | 1.82 | | $ | 0.50 | | $ | 0.41 | | | | $ | 2.73 | | $ | — | | $ | (0.03 | ) | $ | 0.24 | | $ | 0.06 | | $ | 0.06 | | $ | 0.49 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | $ | 1.74 | | $ | 0.47 | | $ | 0.39 | | | | $ | 2.60 | | $ | 0.09 | | $ | (0.03 | ) | $ | 0.23 | | $ | 0.06 | | $ | 0.06 | | $ | 0.46 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | 263,895 | | | | | | | | 263,895 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | 280,767 | | | | | | | | 280,767 | | | | | | | | | | | | | | | |
Notes:
(1) Represents the adjusted Non-GAAP results of operations and financial position for Genzyme Corporation for the year ended December 31, 2007. All other amounts presented herein represent previously reported amounts. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP.
(2) Diluted earnings per share and diluted weighted average shares outstanding reflect the adoption of EITF 04-8. In accordance with the provisions of EITF 04-8, interest and debt fees related to our 1.25% convertible senior notes of $7,543K, net of tax, have been added back to net income and approximately 9,686K shares have been added to diluted weighted average shares outstanding for purposes of computing GAAP and Non-GAAP diluted earnings per share.
GENZYME CORPORATION
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2006
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | Dilution | | | | | | | | | | | | | | | | | |
| | | | | | | | | | Due to | | | | | | Items Formerly Excluded From Non-GAAP [(Income)/Expense] | |
| | | | | | | | | | Transition | | | | | | | | (Gain)/Loss | | | | | | | | | |
| | | | | | | | | | from | | | | | | | | on Investments | | | | | | | | | |
| | | | GAAP | | FAS 123R | | | | (Net Loss) to | | | | NON-GAAP | | OFT | | in Equity | | Settlement of | | Impairment of | | | | Effect of | |
| | | | As Reported | | Expense | | IPR&D | | Net Income | | | | As Adjusted (1) | | Settlement | | Securities | | Tax Audits | | Goodwill | | Amortization | | FIN 46 | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 3,187,013 | | | | | | | | | | $ | 3,187,013 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (735,671 | ) | $ | 21,430 | | | | | | | | $ | (714,241 | ) | | | | | | | | | | | | |
Gross margin | | 77 | % | $ | 2,451,342 | | $ | 21,430 | | | | | | 78 | % | $ | 2,472,772 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (1,010,400 | ) | $ | 121,822 | | | | | | | | $ | (888,578 | ) | $ | 7,936 | | | | | | | | | | $ | 1,376 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (649,951 | ) | $ | 65,248 | | | | | | | | $ | (584,703 | ) | | | | | | | | | | | $ | 19,328 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (209,355 | ) | | | | | | | | | $ | (209,355 | ) | | | | | | | | | $ | 209,355 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Purchase of in-process research and development | | | | $ | (552,900 | ) | | | $ | 552,900 | | | | | | $ | — | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Charge for impaired goodwill | | | | $ | (219,245 | ) | | | | | | | | | $ | (219,245 | ) | | | | | | | $ | 219,245 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Equity in income (loss) of equity method investments | | | | $ | 15,705 | | | | | | | | | | $ | 15,705 | | | | | | | | | | | | $ | (10,348 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Minority interest | | | | $ | 10,418 | | | | | | | | | | $ | 10,418 | | | | | | | | | | | | $ | (10,352 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gains (losses) on investments in equity securities | | | | $ | 73,230 | | | | | | | | | | $ | 73,230 | | | | $ | (66,466 | ) | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other | | | | $ | (2,045 | ) | | | | | | | | | $ | (2,045 | ) | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 56,001 | | | | | | | | | | $ | 56,001 | | | | | | | | | | | | $ | (4 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest Expense | | | | $ | (15,478 | ) | | | | | | | | | $ | (15,478 | ) | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | (52,678 | ) | $ | 208,500 | | $ | 552,900 | | $ | — | | | | $ | 708,722 | | $ | 7,936 | | $ | (66,466 | ) | $ | — | | $ | 219,245 | | $ | 209,355 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 68 | % | $ | 35,881 | | $ | (66,331 | ) | $ | (148,565 | ) | $ | — | | 25 | % | $ | (179,015 | ) | $ | (2,920 | ) | $ | 24,459 | | $ | (31,748 | ) | $ | (69,823 | ) | $ | (77,043 | ) | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | | $ | (16,797 | ) | $ | 142,169 | | $ | 404,335 | | $ | — | | | | $ | 529,707 | | $ | 5,016 | | $ | (42,007 | ) | $ | (31,748 | ) | $ | 149,422 | | $ | 132,312 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | $ | (0.06 | ) | $ | 0.54 | | $ | 1.54 | | $ | — | | | | $ | 2.02 | | $ | 0.02 | | $ | (0.16 | ) | $ | (0.12 | ) | $ | 0.57 | | $ | 0.51 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | $ | (0.06 | ) | $ | 0.52 | | $ | 1.54 | | $ | — | | | | $ | 2.00 | | $ | 0.02 | | $ | (0.15 | ) | $ | (0.11 | ) | $ | 0.54 | | $ | 0.48 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | 261,124 | | | | | | | | | | 261,124 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | 261,124 | | | | | | 6,892 | | | | 268,016 | | | | | | | | | | | | | |
Notes:
(1) Represents the adjusted Non-GAAP results of operations and financial position for Genzyme Corporation for the year ended December 31, 2006. All other amounts presented herein represent previously reported amounts. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP.
(2) Common stock equivalents are included in the calculation of diluted earnings per share to the extent they are dilutive. Due to the significant IPR&D charge for AnorMED of $552,900K, Genzyme had a GAAP net loss for the year ended December 31, 2006 and, therefore, the effect of options, stock purchase rights and warrants to purchase shares of Genzyme Stock, which we refer to collectively as common stock equivalents, and the potentially dilutive effect of our 1.25% convertible notes are excluded from the calculation of GAAP diluted net loss per share because the effect would be anti-dilutive. Conversely, on a Non-GAAP basis, Genzyme produced a net profit and, therefore, the common stock equivalents are included in the calculation of Non-GAAP diluted net income per share because the effect is dilutive. Non-GAAP diluted earnings per share excludes the potentially dilutive effect of our 1.25% convertible notes.
GENZYME CORPORATION
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2005
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Items Formerly Excluded From Non-GAAP [(Income)/Expense] | |
| | | | | | | | | | | | Dilution | | | | | | | | | |
| | | | | | | | | | | | Due to | | Validation/ | | | | | | | |
| | | | GAAP | | | | | | NON-GAAP | | Common Stock | | Manufacturing | | Acquisition | | | | | |
| | | | As Reported | | IPR&D | | | | As Adjusted (1) | | Equivalents | | Related | | Related | | Amortization | | FIN 46 | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 2,734,842 | | | | | | $ | 2,734,842 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (632,652 | ) | | | | | $ | (632,652 | ) | | | $ | 16,912 | | $ | 15,214 | | | | | |
Gross margin | | 77 | % | $ | 2,102,190 | | | | 77 | % | $ | 2,102,190 | | | | $ | 16,912 | | $ | 15,214 | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (787,839 | ) | | | | | $ | (787,839 | ) | | | | | | | | | $ | 790 | �� |
| | | | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (502,657 | ) | | | | | $ | (502,657 | ) | | | | | | | | | $ | 23,112 | |
| | | | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (181,632 | ) | | | | | $ | (181,632 | ) | | | | | | | $ | 181,632 | | | |
| | | | | | | | | | | | | | | | $ | 7,000 | | | | | |
Purchase of in-process research and development | | | | $ | (29,200 | ) | $ | 22,200 | | | | $ | (7,000 | ) | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Equity in income (loss) of equity method investments | | | | $ | 151 | | | | | | $ | 151 | | | | | | | | | | $ | (11,948 | ) |
| | | | | | | | | | | | | | | | | | | | | |
Minority interest | | | | $ | 11,952 | | | | | | $ | 11,952 | | | | | | | | | | $ | (11,952 | ) |
| | | | | | | | | | | | | | | | | | | | | |
Other | | | | $ | 4,163 | | | | | | $ | 4,163 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 31,429 | | | | | | $ | 31,429 | | | | | | | | | | $ | (2 | ) |
| | | | | | | | | | | | | | | | | | | | | |
Interest Expense | | | | $ | (19,638 | ) | | | | | $ | (19,638 | ) | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | 628,919 | | $ | 22,200 | | | | $ | 651,119 | | $ | — | | $ | 16,912 | | $ | 22,214 | | $ | 181,632 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 30 | % | $ | (187,430 | ) | $ | — | | 29 | % | $ | (187,430 | ) | $ | — | | $ | (6,224 | ) | $ | (8,175 | ) | $ | (66,841 | ) | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | | $ | 441,489 | | $ | 22,200 | | | | $ | 463,689 | | $ | — | | $ | 10,688 | | $ | 14,039 | | $ | 114,791 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | $ | 1.73 | | $ | 0.09 | | | | $ | 1.82 | | $ | — | | $ | 0.04 | | $ | 0.05 | | $ | 0.45 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | $ | 1.65 | | $ | 0.09 | | | | $ | 1.74 | | $ | 0.03 | | $ | 0.04 | | $ | 0.05 | | $ | 0.42 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | 254,758 | | | | | | 254,758 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | 272,224 | | | | | | 272,224 | | | | | | | | | | | |
Notes:
(1) Represents the adjusted Non-GAAP results of operations and financial position for Genzyme Corporation for the year ended December 31, 2005. All other amounts presented herein represent previously reported amounts. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP.
(2) Diluted earnings per share and diluted weighted average shares outstanding reflect the adoption of EITF 04-8. In accordance with the provisions of EITF 04-8, interest and debt fees related to our 1.25% convertible senior notes of $7,496K, net of tax, have been added back to net income and approximately 9,686K shares have been added to diluted weighted average shares outstanding for purposes of computing GAAP and Non-GAAP diluted earnings per share.
GENZYME CORPORATION
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2004
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Items Formerly Excluded From Non-GAAP [(Income)/Expense] | |
| | | | | | | | | | | | SangStat | | | | | | Exit Costs | | | | | |
| | | | GAAP | | | | | | NON-GAAP | | Inventory | | Convert | | Convert | | Oklahoma | | Generic | | | | | |
| | | | As Reported | | IPR&D | | | | As Adjusted(1) | | Step Up | | Premium | | Fees | | City | | Cyclosporine | | Amortization | | FIN 46 | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 2,201,145 | | | | | | $ | 2,201,145 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (588,586 | ) | | | | | $ | (588,586 | ) | $ | 3,937 | | | | | | | | $ | 8,067 | | | | | |
Gross margin | | 73 | % | $ | 1,612,559 | | | | 73 | % | $ | 1,612,559 | | $ | 3,937 | | | | | | | | $ | 8,067 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (599,388 | ) | | | | | $ | (599,388 | ) | | | | | | | | | | | | | $ | 225 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (391,802 | ) | | | | | $ | (391,802 | ) | | | | | | | $ | 2,079 | | | | | | $ | 11,779 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (109,473 | ) | | | | | $ | (109,473 | ) | | | | | | | | | | | $ | 109,473 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Purchase of in-process research and development | | | | $ | (254,520 | ) | $ | 254,520 | | | | $ | — | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Charge for impaired assets | | | | $ | (4,463 | ) | | | | | $ | (4,463 | ) | | | | | | | $ | 4,463 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Equity in income (loss) of equity method investments | | | | $ | (15,624 | ) | | | | | $ | (15,624 | ) | | | | | | | | | | | | | $ | (5,997 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Minority interest | | | | $ | 5,999 | | | | | | $ | 5,999 | | | | | | | | | | | | | | $ | (5,999 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Gains (losses) on investments in equity securities | | | | $ | (1,252 | ) | | | | | $ | (1,252 | ) | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Other | | | | $ | (357 | ) | | | | | $ | (357 | ) | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 24,244 | | | | | | $ | 24,244 | | | | | | | | | | | | | | $ | (8 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Interest Expense | | | | $ | (38,227 | ) | | | | | $ | (38,227 | ) | | | $ | 4,313 | | $ | 5,329 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | 227,696 | | $ | 254,520 | | | | $ | 482,216 | | $ | 3,937 | | $ | 4,313 | | $ | 5,329 | | $ | 6,542 | | $ | 8,067 | | $ | 109,473 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 62 | % | $ | (141,169 | ) | $ | — | | 29 | % | $ | (141,169 | ) | $ | (1,449 | ) | $ | (1,587 | ) | $ | (1,961 | ) | $ | (2,407 | ) | $ | (2,969 | ) | $ | (40,286 | ) | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | | $ | 86,527 | | $ | 254,520 | | | | $ | 341,047 | | $ | 2,488 | | $ | 2,726 | | $ | 3,368 | | $ | 4,135 | | $ | 5,098 | | $ | 69,187 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | $ | 0.38 | | $ | 1.11 | | | | $ | 1.49 | | $ | 0.01 | | $ | 0.01 | | $ | 0.01 | | $ | 0.02 | | $ | 0.02 | | $ | 0.30 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | $ | 0.37 | | $ | 1.10 | | | | $ | 1.47 | | $ | 0.01 | | $ | 0.01 | | $ | 0.01 | | $ | 0.02 | | $ | 0.02 | | $ | 0.28 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | 228,175 | | | | | | 228,175 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | 234,318 | | | | | | 234,318 | | | | | | | | | | | | | | | |
Notes:
(1) Represents the adjusted Non-GAAP results of operations and financial position for Genzyme Corporation for the year ended December 31, 2004. All other amounts presented herein represent previously reported amounts. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP.
(2) Diluted earnings per share and diluted weighted average shares outstanding reflect the adoption of EITF 04-8. In accordance with the provisions of EITF 04-8, interest and debt fees related to our 1.25% convertible senior notes of$7,487K, net of tax, and approximately 9,686K shares have been excluded from the computation of GAAP and Non-GAAP diluted earnings per share and diluted weighted average shares outstanding because the effect of the assumed conversion of these notes would be anti-dilutive.
GENZYME CORPORATION
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2003
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Items Formerly Excluded From Non-GAAP [(Income)/Expense] | |
| | | | | | | | | | | | Tracking Stock | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Tax Benefit for | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Genzyme Biosurgery | | Impact of | | SangStat | | TKT | | Impaired | | | | | | | |
| | | | GAAP | | | | | | NON-GAAP | | Disposition of | | UK Judicial | | Acquisition- Related | | Settlement | | Equity | | Focal | | CT Devices | | | |
| | | | As Reported | | IPR&D | | | | As Adjusted (1) | | CT Business | | Decision | | Costs | | Costs | | Investment | | Restructuring | | Exit Costs | | Amortization | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 1,574,817 | | | | | | $ | 1,574,817 | | | | $ | 5,064 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (418,969 | ) | | | | | $ | (418,969 | ) | | | $ | — | | $ | 2,550 | | | | | | $ | 3,858 | | $ | 308 | | | |
Gross margin | | 73 | % | $ | 1,155,848 | | | | 73 | % | $ | 1,155,848 | | | | $ | 5,064 | | $ | 2,550 | | | | | | $ | 3,858 | | $ | 308 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (455,395 | ) | | | | | $ | (455,395 | ) | | | $ | 5,843 | | $ | 258 | | $ | 1,550 | | | | $ | 1,958 | | $ | 1,897 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (295,725 | ) | | | | | $ | (295,725 | ) | | | | | $ | 137 | | $ | 1,500 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (64,720 | ) | | | | | $ | (64,720 | ) | | | | | | | | | | | | | | | $ | 64,720 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Purchase of in-process research and development | | | | $ | (158,000 | ) | $ | 158,000 | | | | $ | — | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Charge for impaired assets | | | | $ | (7,996 | ) | | | | | $ | (7,996 | ) | | | | | | | | | | | $ | 7,996 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Equity in income (loss) of equity method investments | | | | $ | (16,743 | ) | | | | | $ | (16,743 | ) | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Minority interest | | | | $ | 2,232 | | | | | | $ | 2,232 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gains (losses) on investments in equity securities | | | | $ | (1,201 | ) | | | | | $ | (1,201 | ) | | | | | | | | | $ | 3,620 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other | | | | $ | 2,703 | | | | | | $ | 2,703 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 42,312 | | | | | | $ | 42,312 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest Expense | | | | $ | (22,380 | ) | | | | | $ | (22,380 | ) | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | 180,935 | | $ | 158,000 | | | | $ | 338,935 | | $ | — | | $ | 10,907 | | $ | 2,945 | | $ | 3,050 | | $ | 3,620 | | $ | 13,812 | | $ | 2,205 | | $ | 64,720 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 48 | % | $ | (86,652 | ) | $ | — | | 26 | % | $ | (86,652 | ) | $ | (4,032 | ) | $ | (1,863 | ) | $ | (1,084 | ) | $ | (1,122 | ) | $ | (1,332 | ) | $ | (5,083 | ) | $ | (812 | ) | $ | (23,817 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) (2) | | | | $ | 94,283 | | $ | 158,000 | | | | $ | 252,283 | | $ | (4,032 | ) | $ | 9,044 | | $ | 1,861 | | $ | 1,928 | | $ | 2,288 | | $ | 8,729 | | $ | 1,393 | | $ | 40,903 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | $ | 0.44 | | $ | 0.71 | | | | $ | 1.15 | | $ | (0.02 | ) | $ | 0.04 | | $ | 0.01 | | $ | 0.01 | | $ | 0.01 | | $ | 0.04 | | $ | 0.01 | | $ | 0.19 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2,3) | | | | $ | 0.42 | | $ | 0.70 | | | | $ | 1.12 | | $ | (0.02 | ) | $ | 0.04 | | $ | 0.01 | | $ | 0.01 | | $ | 0.01 | | $ | 0.04 | | $ | 0.01 | | $ | 0.18 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | 219,376 | | | | | | 219,376 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2,3) | | | | 225,976 | | | | | | 225,976 | | | | | | | | | | | | | | | | | |
Notes:
(1) Represents the adjusted Non-GAAP results of operations and financial position of Genzyme Corporation for the year ended December 31, 2003. All other amounts presented herein represent previously reported amounts. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP.
(2) Represents the operations of Genzyme General Division for January 1, 2003 through June 30, 2003 and the operations of Genzyme Corporation from July 1, 2003 through December 31, 2003.
(3) Diluted earnings per share and diluted weighted average shares outstanding reflect the adoption of EITF 04-8. In accordance with the provisions of EITF 04-8, interest and debt fees related to our 1.25% convertible senior notes of $497K, net of tax, have been added back to net income and approximately 557K shares have been added to diluted weighted average shares outstanding for purposes of computing GAAP and Non-GAAP diluted earnings per share.
GENZYME GENERAL
RECONCILIATION OF GAAP TO NON-GAAP EARNINGS
For the Year Ended December 31, 2002
(Amounts in thousands, except percentage and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | Items Formerly Excluded From Non-GAAP [(Income)/Expense] | |
| | | | | | | | | | | | | | | | | | Tax Benefit of | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | Recovery of | | Genzyme | | | | Failed | | | | | | | | | | Reverse Excess | | | | | |
| | | | | | | | | | | | | | Pompe | | Note Receivable | | Biosurgery | | | | Production | | Plant | | Damaged | | Impaired | | | | Future Funding | | Write Off | | | |
| | | | GAAP | | | | NON-GAAP | | Novazyme | | Diagnostic | | CHO/Synpac | | Previously | | Asset | | Argentina | | Runs by | | Shutdown | | In-Transit | | Equity | | | | Obligation for | | Engineering | | | |
| | | | As Reported | | | | As Adjusted (1) | | Restructuring | | Restructuring | | Program | | Written Off | | Impairment | | Bad Debt | | Joint Venture | | Maintenance | | Inventory | | Investment | | Severance | | Trans | | Costs | | Amortization | |
Income Statement Classification: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenues | | | | $ | 1,080,185 | | | | $ | 1,080,185 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of products and services sold | | | | $ | (265,818 | ) | | | $ | (265,818 | ) | | | $ | 2,856 | | | | | | | | | | | | $ | 2,832 | | $ | 2,214 | | | | | | | | | | | |
Gross margin | | 75 | % | $ | 814,367 | | 75 | % | $ | 814,367 | | | | $ | 2,856 | | | | | | | | | | | | $ | 2,832 | | $ | 2,214 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | | $ | (323,683 | ) | | | $ | (323,683 | ) | | | | | | | | | | | $ | 2,500 | | | | | | | | | | $ | 3,300 | | $ | (5,497 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | | $ | (230,043 | ) | | | $ | (230,043 | ) | $ | 1,968 | | | | $ | 8,786 | | | | | | | | | | | | | | | | $ | 927 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of intangibles | | | | $ | (38,998 | ) | | | $ | (38,998 | ) | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 38,998 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Charge for impaired assets | | | | $ | (13,986 | ) | | | $ | (13,986 | ) | | | | | | | | | | | | | | | | | | | | | | | | | $ | 13,986 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Equity in income (loss) of equity method investments | | | | $ | (16,858 | ) | | | $ | (16,858 | ) | | | | | | | | | | | | | $ | 3,604 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gains (losses) on investments in equity securities | | | | $ | (14,497 | ) | | | $ | (14,497 | ) | | | | | | | | | | | | | | | | | | | $ | 15,367 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other | | | | $ | (152 | ) | | | $ | (152 | ) | | | | | | | $ | (2,670 | ) | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Investment income | | | | $ | 48,944 | | | | $ | 48,944 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest Expense | | | | $ | (17,847 | ) | | | $ | (17,847 | ) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Summary: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | | $ | 207,247 | | | | $ | 207,247 | | $ | 1,968 | | $ | 2,856 | | $ | 8,786 | | $ | (2,670 | ) | $ | — | | $ | 2,500 | | $ | 3,604 | | $ | 2,832 | | $ | 2,214 | | $ | 15,367 | | $ | 4,227 | | $ | (5,497 | ) | $ | 13,986 | | $ | 38,998 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
(Provision for) benefit from income taxes | | 14 | % | $ | (28,721 | ) | 14 | % | $ | (28,721 | ) | $ | (724 | ) | $ | (1,051 | ) | $ | (3,233 | ) | $ | 983 | | $ | (3,297 | ) | $ | (920 | ) | $ | (1,326 | ) | $ | (1,042 | ) | $ | (815 | ) | $ | (5,655 | ) | $ | (1,556 | ) | $ | 2,023 | | $ | (5,147 | ) | $ | (14,351 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) (2) | | | | $ | 178,526 | | | | $ | 178,526 | | $ | 1,244 | | $ | 1,805 | | $ | 5,553 | | $ | (1,687 | ) | $ | (3,297 | ) | $ | 1,580 | | $ | 2,278 | | $ | 1,790 | | $ | 1,399 | | $ | 9,712 | | $ | 2,671 | | $ | (3,474 | ) | $ | 8,839 | | $ | 24,647 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic (2) | | | | $ | 0.83 | | | | $ | 0.83 | | $ | 0.006 | | $ | 0.008 | | $ | 0.026 | | $ | (0.008 | ) | $ | (0.015 | ) | $ | 0.007 | | $ | 0.011 | | $ | 0.008 | | $ | 0.007 | | $ | 0.045 | | $ | 0.012 | | $ | (0.016 | ) | $ | 0.041 | | $ | 0.115 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | $ | 0.81 | | | | $ | 0.81 | | $ | 0.006 | | $ | 0.008 | | $ | 0.025 | | $ | (0.008 | ) | $ | (0.015 | ) | $ | 0.007 | | $ | 0.010 | | $ | 0.008 | | $ | 0.006 | | $ | 0.044 | | $ | 0.012 | | $ | (0.016 | ) | $ | 0.040 | | $ | 0.112 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic (2) | | | | 214,038 | | | | 214,038 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted (2) | | | | 219,388 | | | | 219,388 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Notes:
(1) Represents the adjusted Non-GAAP results of operations and financial position of Genzyme General Division for the year ended December 31, 2002. All other amounts presented herein represent previously reported amounts. We believe that certain Non-GAAP financial measures, when considered together with the GAAP figures, can enhance the overall understanding of the company’s past financial performance and its prospects for the future. The Non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of the trends underlying our operating results and financial position and are among the primary indicators management uses for planning and forecasting purposes and measuring the company’s performance. Such Non-GAAP financial measures should not be considered in isolation or used as a substitute for GAAP.
(2) Represents the results of operations, net income per share and weighted average shares outstanding for Genzyme General Division.

















































































