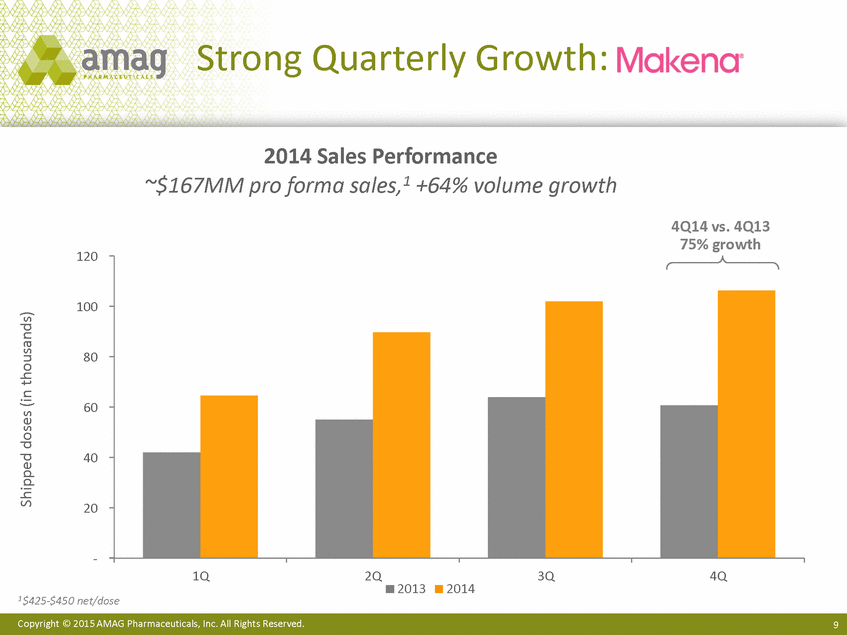
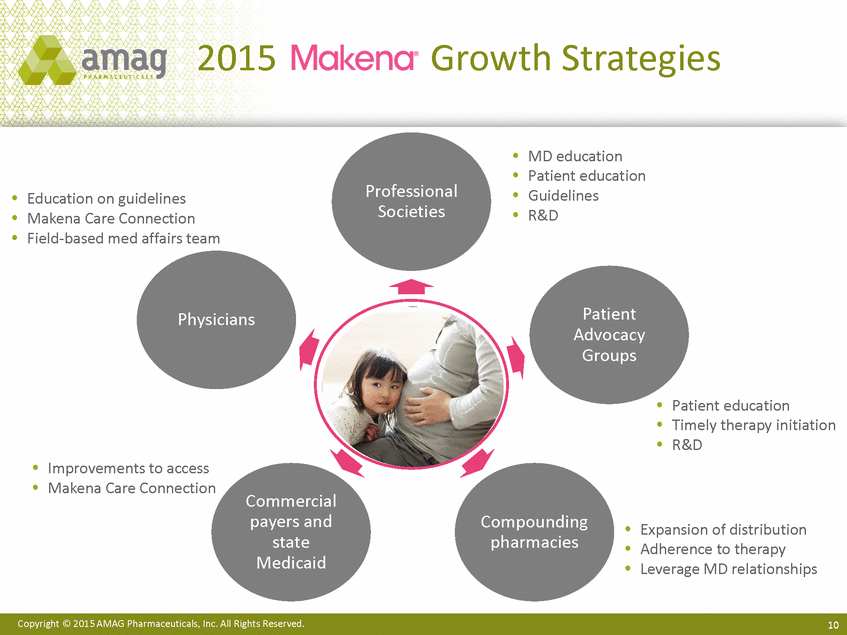
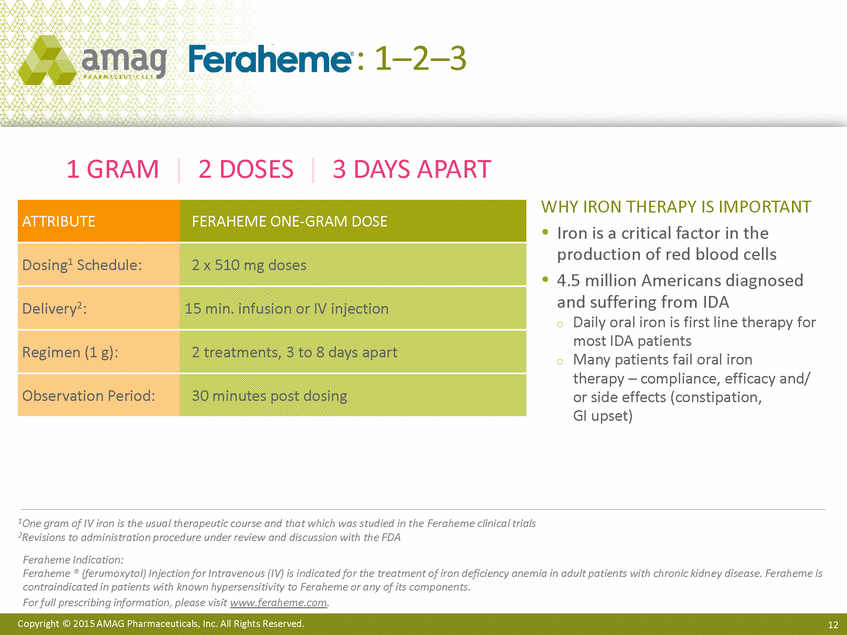
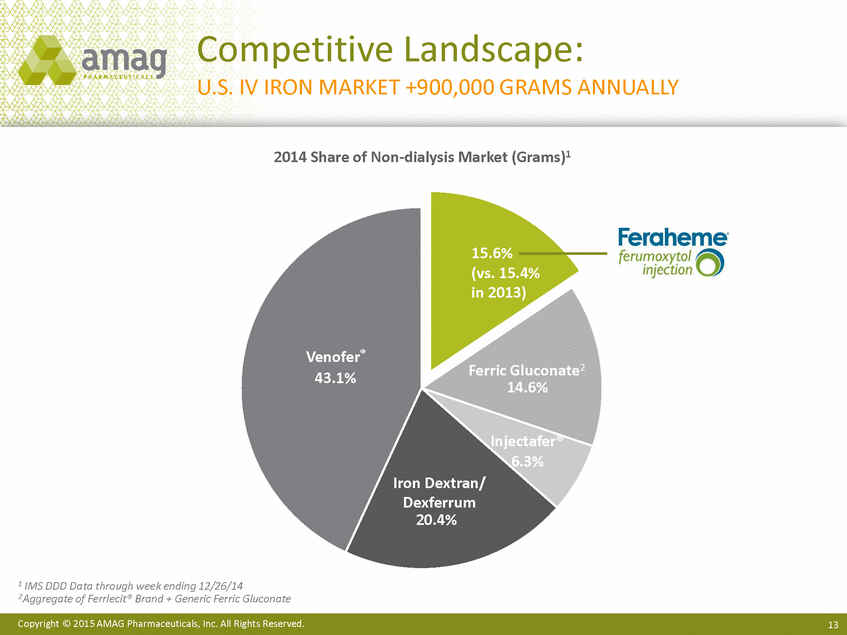
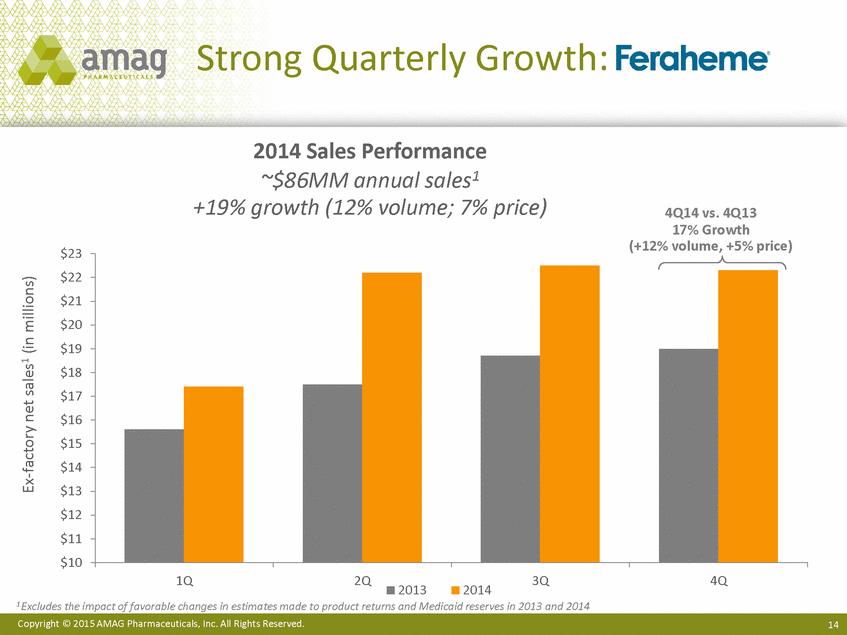
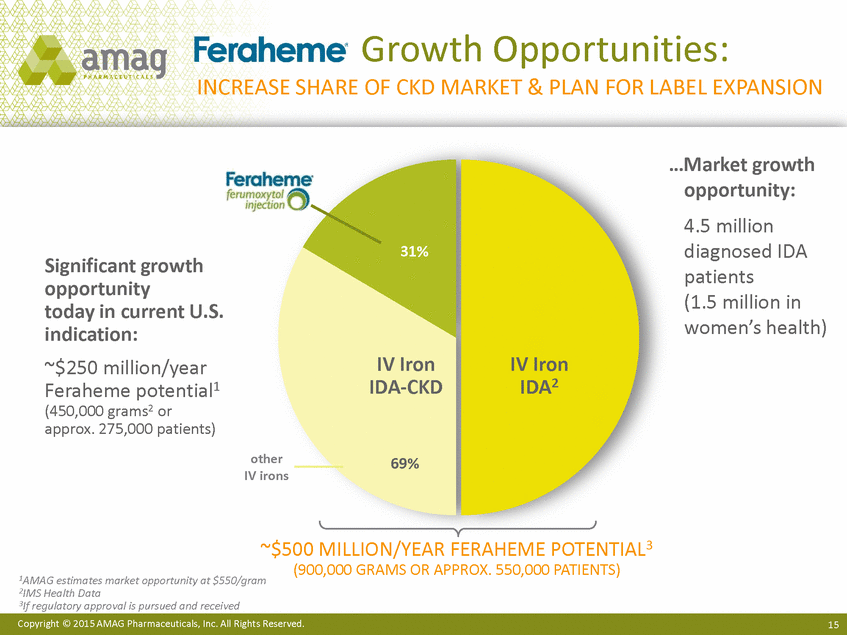
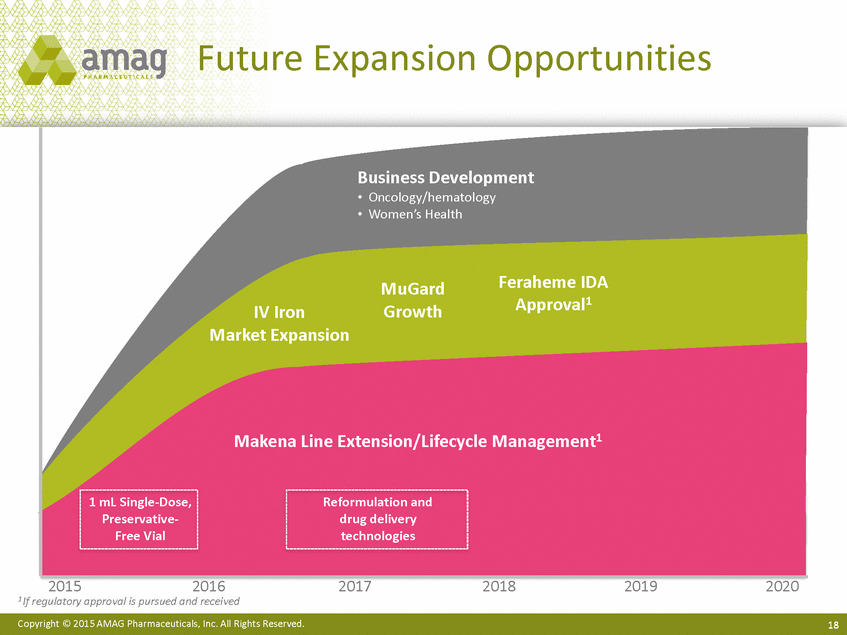
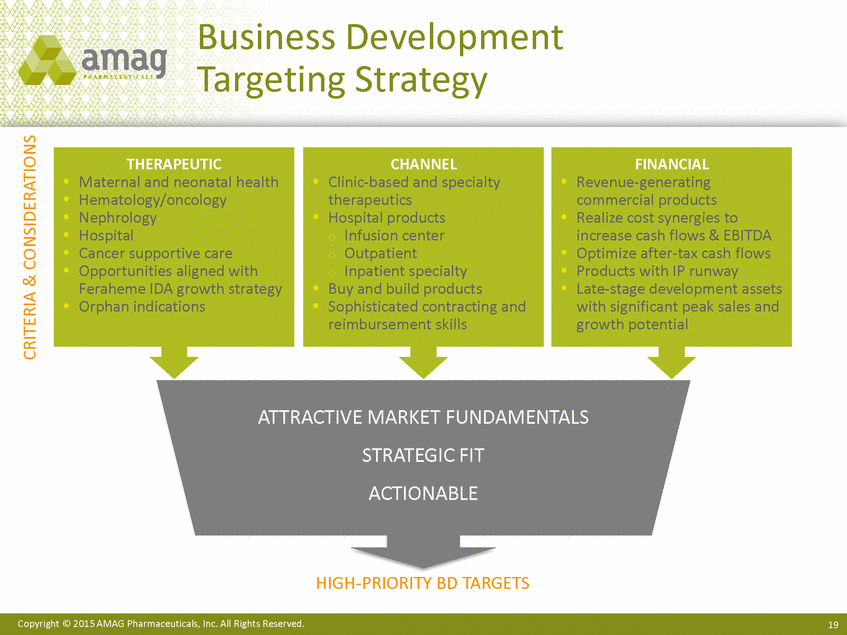
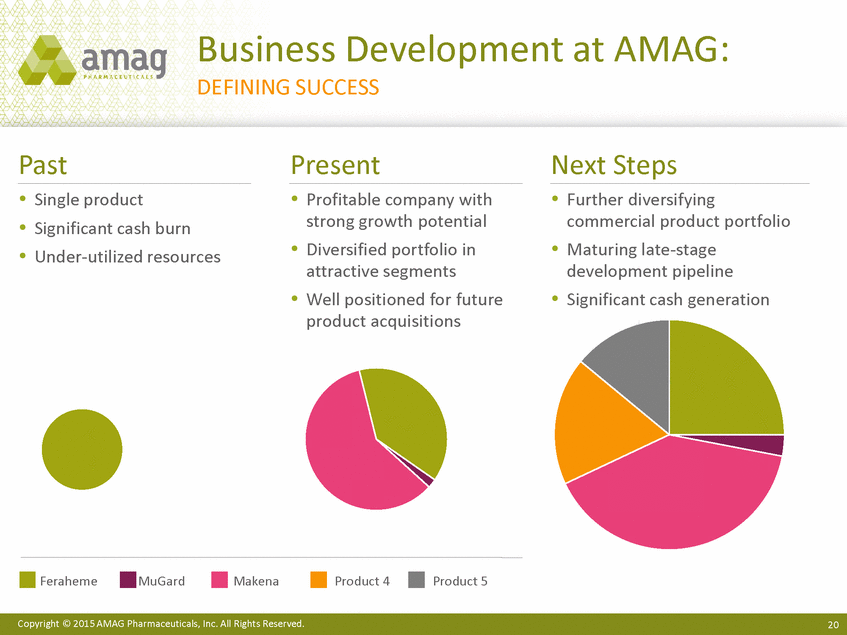
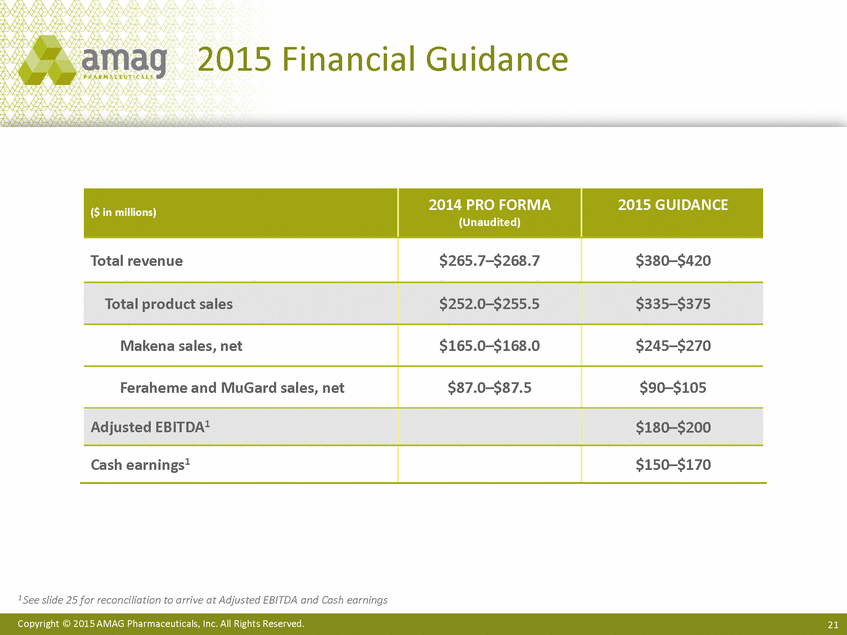
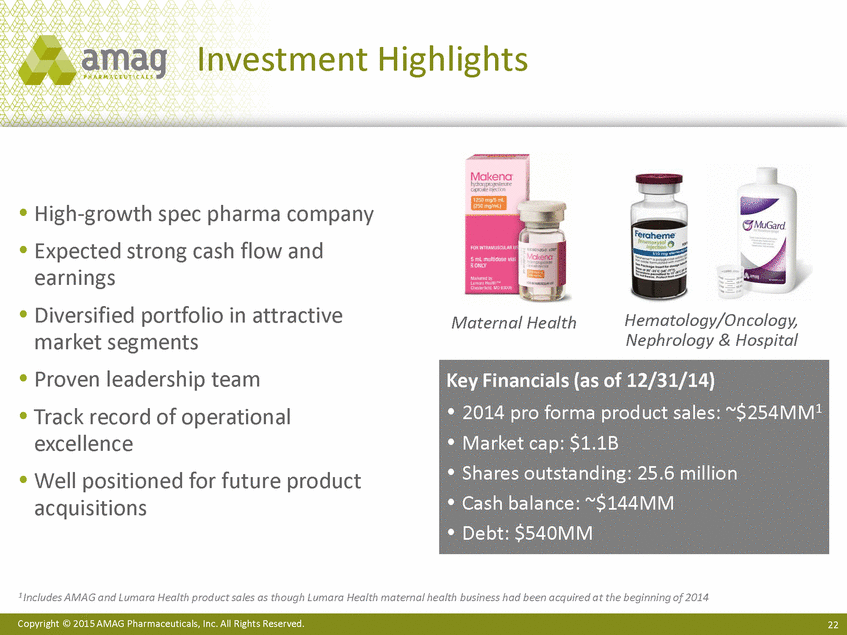
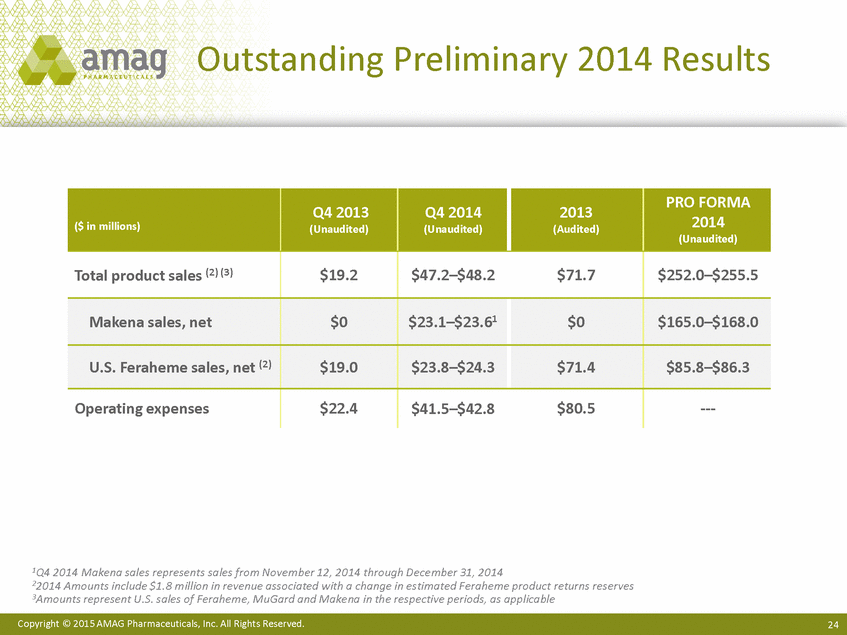
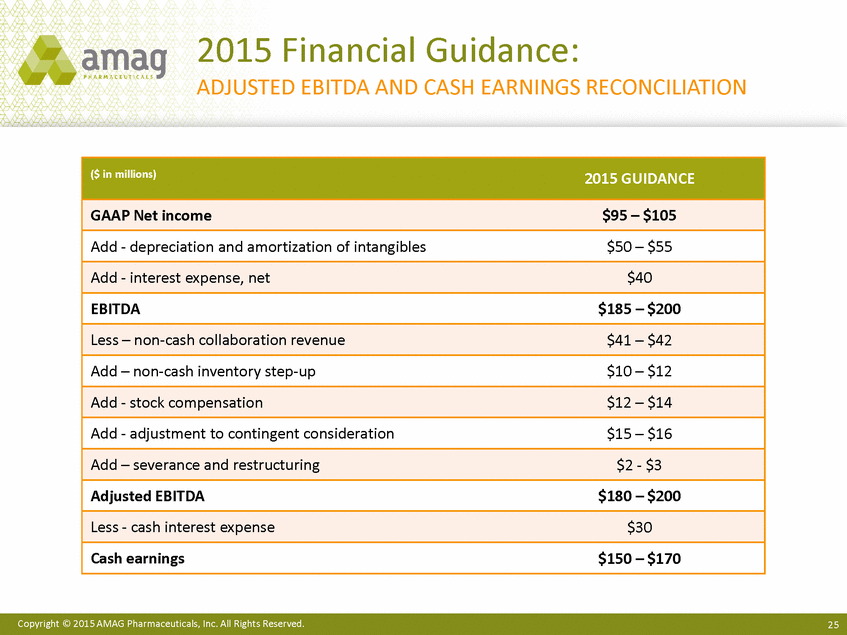
| Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including among others, statements regarding beliefs about AMAG’s cash flow, earnings, market segments, position for portfolio expansion and future effective tax rate; the potential timing of regulatory approval for the Makena 1mL preservative-free vial; the Makena market opportunity; beliefs about market dynamics and reimbursement trends for Makena; Makena’s patient-centric approach; the competitive landscape of Feraheme; Feraheme growth opportunities, including for the current indication and if a broader indication is pursued and obtained, as well as potential future call points; expectations for MuGard; AMAG’s future expansion opportunities, including business development, product growth and Makena line extension/lifecycle management; AMAG’s business development targeting strategy, including its criteria and considerations; plans to further diversify AMAG’s product portfolio, opportunities with maturing late-stage development pipeline and expected cash generation; expected 2014 preliminary results, including revenues and operating expenses; 2015 financial guidance, including expected Makena sales, Feraheme sales (which projected range takes into account expected label changes), adjusted EBITDA and cash earnings; and AMAG’s being well-positioned for success in 2015 and beyond are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: (1) demand for Feraheme and AMAG’s ability to successfully compete in the intravenous iron replacement market as a result of the FDA’s recommended label changes, including a boxed warning which would provide, among other things, (i) that Feraheme be administered only when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions, (ii) observation for signs or symptoms of hypersensitivity reactions during and for at least 30 minutes following infusion and (iii) that hypersensitivity reactions have occurred in patients in whom a previous Feraheme dose was tolerated; (2) the outcome and timing of the process in accordance with Section 505(o) of the Federal Food, Drug and Cosmetic Act whereby the FDA is authorized to require AMAG to make safety-related label changes, including prescribed periods for submitting proposed changes to the label recommended by the FDA; (3) the impact on sales if AMAG disseminates future Dear Healthcare Provider letters; (4) the ability of AMAG to invest in the development and commercialization of Feraheme/Rienso outside the U.S., and the level of commercial success of any of such efforts, given the December 2014 arrangement to terminate AMAG’s and Takeda’s license arrangement; (5) uncertainties regarding the likelihood and timing of potential approval of Feraheme/Rienso in the U.S., the EU and Canada in the broader IDA indication; (6) the possibility that following review of new safety information, the FDA or regulators in Europe and Canada will request additional technical or scientific information, new studies or reanalysis of existing data, on-label warnings, post-marketing requirements/commitments or risk evaluation and mitigation strategies (REMS) in the current CKD indication for Feraheme/Rienso, or cause Feraheme/Rienso to be withdrawn from the market, and the additional costs and expenses that will or may be incurred in connection with such activities; (7) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso or Makena and in turn affect sales or AMAG’s ability to market such product; (8) AMAG’s patents and proprietary rights; (9) maintaining the benefits associated with Makena’s orphan drug exclusivity status; (10) the risk of an Abbreviated New Drug Application (ANDA) filing, especially (i) as to Feraheme following the FDA’s draft bioequivalence recommendation for ferumoxytol published in December 2012 and (ii) as to Makena given the history of the formerly FDA-approved drug Delalutin (the original version of 17-alpha-hydroxyprogesterone caproate) for conditions other than reducing the risk of preterm birth; (11) AMAG’s ability to execute on, or to realize the expected results from, its long-term strategic plan; (12) the possibility that AMAG will not realize expected synergies and other benefits from its acquisition of Lumara Health, as well as AMAG’s ability to pursue additional business development opportunities, especially in light of AMAG’s being highly leveraged; (13) the impact on sales of Makena from competitive, commercial payor, government (including federal and state Medicaid reimbursement policies), physician, patient or public responses with respect to product pricing, product access and sales and marketing initiatives, as well as patient compliance and the number of preterm birth risk pregnancies for which Makena may be prescribed; (14) the likelihood that labeling changes may be used to support product liability claims that the prior product labeling did not adequately disclose the risk of adverse events; (15) compliance with restrictive and affirmative covenants with respect to substantial indebtedness incurred to finance the acquisition of Lumara Health, including a requirement that AMAG reduce its leverage over time; (16) the possibility that AMAG will need to raise additional capital from the sale of its common stock, which will cause significant dilution to its stockholders, in order to satisfy its contractual obligations, including its debt service, milestone payments that may become payable to Lumara Health’s stockholders, or in order to pursue business development activities; (17) the availability and timing of tax net operating loss carryforwards; (18) the manufacture of AMAG’s products, including any significant interruption in the supply of raw materials or finished product and (19) other risks identified in AMAG’s filings with the U.S. Securities and Exchange Commission (SEC), including its Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and subsequent filings with the SEC. Any of the above risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. Use of the term “including” in the two paragraphs above shall mean in each case “including, but not limited to.” AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals® and Feraheme® are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard® is a registered trademark of PlasmaTech Biopharmaceuticals, Inc. (formerly known as Access Pharmaceuticals, Inc.). Rienso™ is a trademark of Takeda Pharmaceutical Company Limited. Lumara Health™ is a trademark of Lumara Health Inc. Makena® is a registered trademark of Lumara Health Inc. Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. |