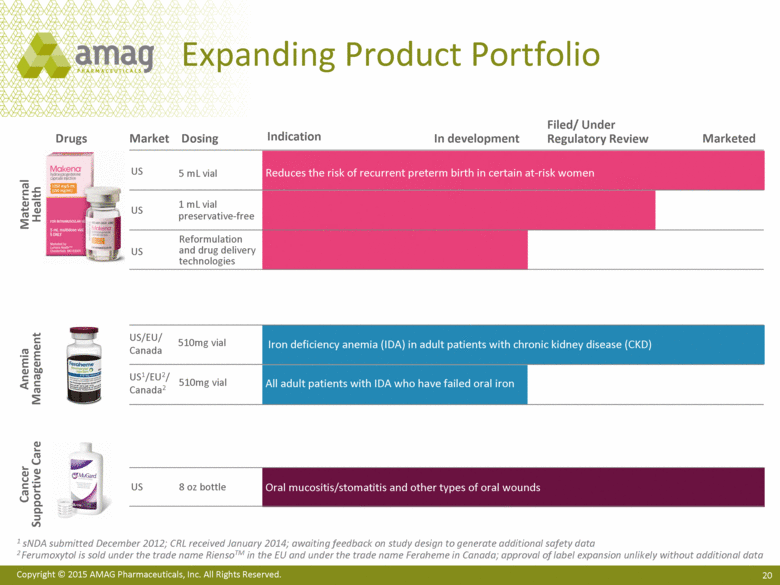
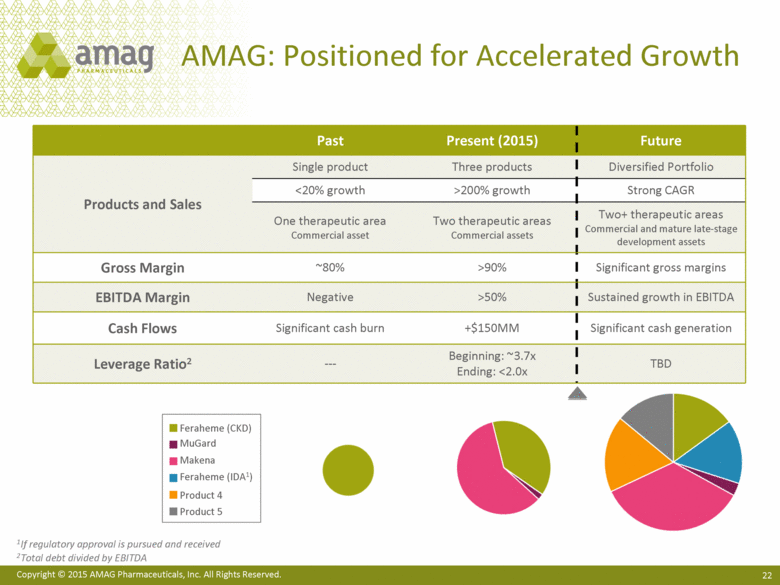
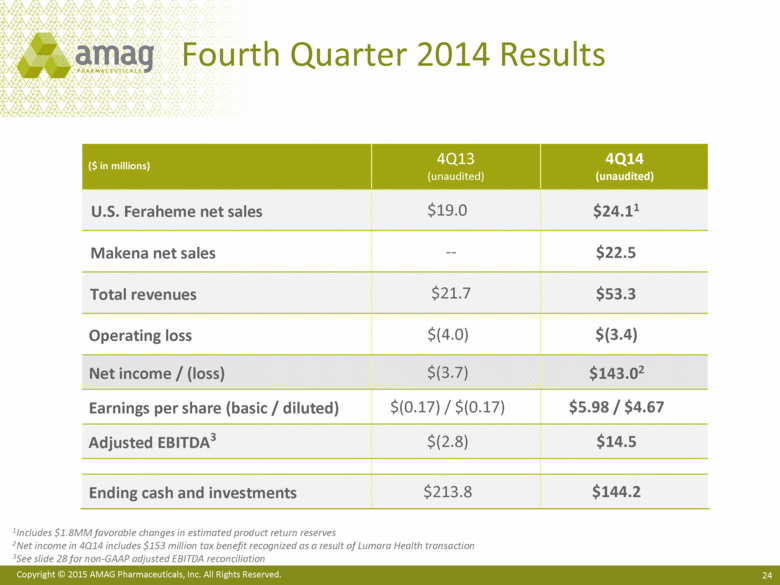
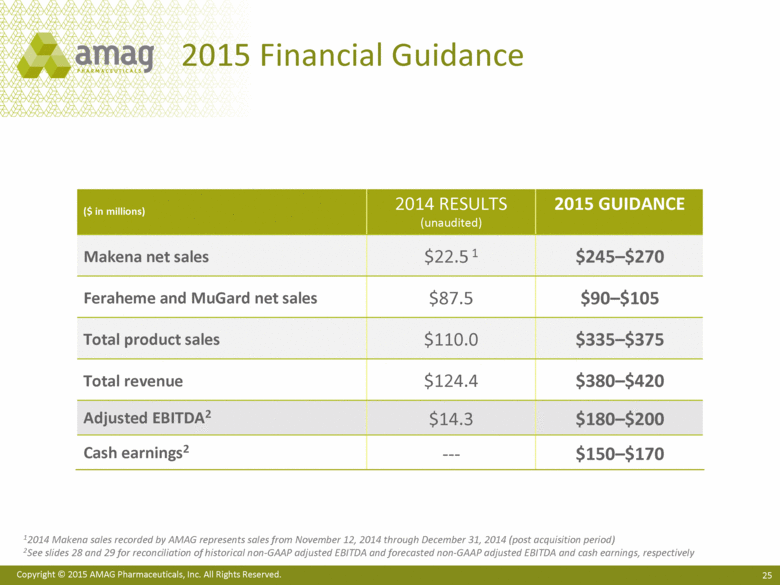
| This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including among others, statements regarding AMAG’s expected strong cash flow and earnings; beliefs about AMAG’s positioning for future acquisitions; expected annual synergy cost-savings with the Lumara Health transaction; the market opportunity for Makena®, including market dynamics and opportunities to increase market share and enhance patient compliance; Makena’s growth strategies for 2015; expected regulatory actions, and timing of such actions, for Feraheme® in the U.S. and abroad, including for the broad iron deficiency anemia (IDA) indication, the transfer of marketing authorizations from Takeda and potential U.S. label changes; Feraheme growth opportunities and Feraheme’s competitive landscape, including opportunities to increase market share in the chronic kidney disease (CKD) market and market opportunity growth if approval of the broader label is pursued and received; expected results for the quarter and year ended December 31, 2014, including net sales, operating loss, net income and adjusted EBITDA, as well as AMAG’s expected cash and investments balance; AMAG’s 2015 financial guidance, including net product sales, adjusted EBITDA and cash earnings; AMAG’s future expansion opportunities, including those related to Makena’s line extension and lifecycle management program and AMAG’s business development targeting strategy; and AMAG’s 2015 goals, including product growth across the portfolio, execution on Makena’s line extension/lifecycle management program, the path forward (if any) for the broad IDA indication in the U.S. and plans to further expand AMAG’s product portfolio are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: (1) demand for Feraheme and AMAG’s ability to successfully compete in the intravenous iron replacement market as a result of the FDA’s recommended label changes, including a boxed warning which would provide, among other things, (i) that Feraheme be administered only when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions, (ii) observation for signs or symptoms of hypersensitivity reactions during and for at least 30 minutes following infusion and (iii) that hypersensitivity reactions have occurred in patients in whom a previous Feraheme dose was tolerated; (2) the outcome and timing of the process in accordance with Section 505(o) of the Federal Food, Drug and Cosmetic Act whereby the FDA is authorized to require AMAG to make safety-related label changes, including prescribed periods for submitting proposed changes to the label recommended by the FDA; (3) the impact on sales if AMAG disseminates future Dear Healthcare Provider letters; (4) the ability of AMAG to invest in the development and commercialization of Feraheme/Rienso outside the U.S. (Rienso is the trade name for ferumoxytol outside of the U.S. and Canada) , and the level of commercial success of any of such efforts, given the December 2014 arrangement to terminate AMAG’s and Takeda’s license arrangement; (5) uncertainties regarding the likelihood and timing of potential approval of Feraheme/Rienso in the U.S., the EU and Canada in the broader IDA indication; (6) the possibility that following review of new safety information, the FDA or regulators in Europe and Canada will request additional technical or scientific information, new studies or reanalysis of existing data, on-label warnings, post-marketing requirements/commitments or risk evaluation and mitigation strategies (REMS) in the current CKD indication for Feraheme/Rienso, or cause Feraheme/Rienso to be withdrawn from the market, and the additional costs and expenses that will or may be incurred in connection with indication for Feraheme/Rienso, or cause Feraheme/Rienso to be withdrawn from the market, and the additional costs and expenses that will or may be incurred in connection with such activities; (7) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso or Makena and in turn affect sales or AMAG’s ability to market such product; (8) AMAG’s patents and proprietary rights; (9) maintaining the benefits associated with Makena’s orphan drug exclusivity status and the ability to successfully implement the lifecycle management program/line extensions; (10) the risk of an Abbreviated New Drug Application (ANDA) filing, especially (i) as to Feraheme following the FDA’s draft bioequivalence recommendation for ferumoxytol published in December 2012 and (ii) as to Makena given the history of the formerly FDA-approved drug Delalutin (the original version of 17-alpha-hydroxyprogesterone caproate) for conditions other than reducing the risk of preterm birth; (11) AMAG’s ability to execute on, or to realize the expected results from, its long-term strategic plan; (12) the possibility that AMAG will not realize expected synergies and other benefits from its acquisition of Lumara Health, as well as AMAG’s ability to pursue additional business development opportunities, especially in light of AMAG’s being highly leveraged; (13) the impact on sales of Makena from competitive, commercial payor, government (including federal and state Medicaid reimbursement policies), physician, patient or public responses with respect to product pricing, product access and sales and marketing initiatives, as well as patient compliance and the number of preterm birth risk pregnancies for which Makena may be prescribed; (14) the likelihood that labeling changes may be used to support product liability claims that the prior product labeling did not adequately disclose the risk of adverse events; (15) compliance with restrictive and affirmative covenants with respect to substantial indebtedness incurred to finance the acquisition of Lumara Health, including a requirement that AMAG reduce its leverage over time; (16) the possibility that AMAG will need to raise additional capital from the sale of its common stock, which will cause significant dilution to its stockholders, in order to satisfy its contractual obligations, including its debt service, milestone payments that may become payable to Lumara Health’s stockholders, or in order to pursue business development activities; (17) the availability and timing of tax net operating loss carryforwards; (18) the manufacture of AMAG’s products, including any significant interruption in the supply of raw materials or finished product and (19) other risks identified in AMAG’s filings with the U.S. Securities and Exchange Commission (SEC), including its Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and subsequent filings with the SEC. Any of the above risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. Use of the term “including” in the two paragraphs above shall mean in each case “including, but not limited to.” AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals® and Feraheme® are registered trademarks of AMAG Pharmaceuticals, Inc. Lumara Health™ is a trademark of Lumara Health Inc. Makena® is a registered trademark of Lumara Health Inc. MuGard® is a registered trademark of PlasmaTech Biopharmaceuticals, Inc. (formerly known as Access Pharmaceuticals, Inc.). Rienso™ is a trademark of Takeda Pharmaceutical Company Limited. Forward-Looking Statements |