SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934
Filed by the Registrant þ
Filed by a Party other than the Registrant o
Check the appropriate box:
| | | |
| o Preliminary Proxy Statement | | |
| o Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| þ Definitive Proxy Statement |
| o Definitive Additional Materials |
| o Soliciting Material under §240.14a-12 |
Epimmune Inc.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box)
| |
| o | No fee required. |
| |
| þ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11 |
| |
| (1) | Title of each class of securities to which transaction applies: class A ordinary shares and class B ordinary shares of IDM S.A. |
| |
| (2) | Aggregate number of securities to which transaction applies: 21,004,573 class A and class B ordinary shares of IDM S.A., which includes the anticipated issuance of 1,156,997 shares of class A ordinary shares pursuant to the IDM S.A. 1998 Stock Option Plan and the IDM S.A. 2000 Stock Option Plan prior to the closing of the transaction and 4,153,130 class A and class B ordinary shares issuable upon exercise of certain warrants. |
| |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): per unit price of $3.24 calculated by multiplying the average of the high and low sales price of the registrant’s common stock on April 19, 2005 as reported on the Nasdaq National Market by 3.771865, the exchange ratio applicable to the transaction described in this proxy statement. In accordance with Section 14(g) of the Securities Exchange Act of 1934, as amended, the filing fee was determined by multiplying $0.0001177 by the sum of the preceding sentence. |
| |
| (4) | Proposed maximum aggregate value of transaction: $68,054,816.00 |
| |
| (5) | Total fee paid: $8,010.05 |
| |
| þ | Fee paid previously with preliminary materials. |
| |
| o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| |
| (1) | Amount Previously Paid: |
| |
| (2) | Form, Schedule or Registration Statement No.: |
EPIMMUNE INC.
5820 Nancy Ridge Drive
San Diego, California 92121
(858) 860-2500
July 8, 2005
Dear Stockholder:
We invite you to attend the annual meeting of stockholders of Epimmune Inc. to be held at our offices at 5820 Nancy Ridge Drive, San Diego, California, at 11:00 a.m., local time, on August 11, 2005. Holders of record of Epimmune common stock at the close of business on June 21, 2005 will be entitled to vote at the annual meeting or any adjournment of the annual meeting.
At the annual meeting, we will ask you to consider and approve the issuance of shares of our common stock pursuant to a Share Exchange Agreement, dated March 15, 2005, as amended, between Epimmune and certain shareholders of IDM S.A. and the issuance of shares of Epimmune common stock in related transactions. As a result of the transactions contemplated by the Share Exchange Agreement, IDM will become a subsidiary of Epimmune and the shareholders of IDM will become stockholders of Epimmune. At the annual meeting, we will ask you to approve matters relating to the transactions, including amending our Amended and Restated Certificate of Incorporation to change our corporate name to IDM, Inc., effecting a reverse stock split of our outstanding common stock, increasing the authorized shares of capital stock, amending our equity plans and adopting a new equity plan. We will also ask you to elect six directors and ratify the selection of Ernst & Young LLP, Independent Registered Public Accounting Firm, as our independent auditors.
We will not be able to complete the transactions unless all of the conditions to closing contemplated by the Share Exchange Agreement are satisfied, including the approval of the issuance of shares of our common stock by holders of a majority of the outstanding shares of our common stock.
Our Board of Directors unanimously determined that the transactions contemplated by the Share Exchange Agreement are advisable and that the issuance of shares of our common stock pursuant to the transactions contemplated by the Share Exchange Agreement and related agreements is fair to and in the best interests of our stockholders. Our Board unanimously recommends that our stockholders vote “FOR” the transactions under the Share Exchange Agreement and related matters.
In arriving at its recommendation, our Board carefully considered a number of factors described in the accompanying proxy statement. One of the factors considered was the written opinion of Jefferies & Company, Inc., which acted as our financial advisor in connection with the transactions, to the effect that as of March 15, 2005, and based upon the qualifications, limitations and assumptions set forth in the opinion, the consideration to be paid by Epimmune to the IDM shareholders in connection with the transactions was fair, from a financial point of view, to Epimmune. The full text of this opinion is attached asAnnex Bto the accompanying proxy statement, and the opinion should be carefully read in its entirety. Jefferies provided its opinion solely for the information and assistance of our Board in connection with its consideration of the transactions under the Share Exchange Agreement. Jefferies’ opinion is not a recommendation as to how any holder of our common stock or any other person should vote or act with respect to the transactions under the Share Exchange Agreement.
The proxy statement attached to this letter provides you with information about the proposed transactions under the Share Exchange Agreement and other actions to be taken at the annual meeting of Epimmune’s stockholders. Before voting, we urge you to read the entire proxy statement carefully, including the section entitled“Risk Factors.” We have also enclosed a proxy card; our annual report to stockholders for the year ended December 31, 2004, which contains audited consolidated financial statements and other information of interest to our stockholders; and our quarterly report on Form 10-Q/ A for the quarter ended March 31, 2005, filed with the Securities and Exchange Commission on June 23, 2005. You may also obtain more information about Epimmune from other documents we have filed with the Securities and Exchange Commission.
The ability to have your vote counted at the meeting is an important stockholder right. Regardless of the number of shares you hold, and whether or not you plan to attend the meeting, your vote is very important and we hope that you will cast your vote. If you are a stockholder of record, you may vote in person at the annual meeting or by proxy by mailing the enclosed proxy card in the envelope provided or appointing a proxy over the Internet or by telephone as instructed in these materials. You will find voting instructions in the proxy statement and on the enclosed proxy card. If your shares are held in “street name” — that is, held for your account by a broker or other nominee — you will receive instructions from the holder of record that you must follow for your shares to be voted.
On behalf of our Board, thank you for your ongoing support and continued interest in Epimmune.
| |
| | Sincerely, |
| |
| |  |
| | Emile Loria, M.D. |
| | President and Chief Executive Officer |
5820 Nancy Ridge Drive
San Diego, California 92121
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
To Be Held August 11, 2005
Dear Stockholder:
You are cordially invited to attend the annual meeting of stockholders of Epimmune Inc., a Delaware corporation. The meeting will be held on August 11, 2005 at 11:00 a.m. local time at our offices located at 5820 Nancy Ridge Drive, San Diego, California 92121 for the following purposes:
| | |
| | 1. | To approve the issuance of shares of Epimmune’s common stock in accordance with the terms of the Share Exchange Agreement, dated March 15, 2005, as amended, between Epimmune and certain shareholders of IDM S.A., referred to as IDM, the Put/ Call Agreements to be entered into between Epimmune and certain shareholders of IDM, the Option Liquidity Agreements to be entered into between Epimmune and certain optionholders of IDM and the Amended and Restated Preferred Exchange Agreement, dated April 12, 2005, between Epimmune and G.D. Searle LLC. |
| |
| | 2. | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to change the corporate name of Epimmune to “IDM, Inc.” |
| |
| | 3. | To approve a series of amendments of Epimmune’s Amended and Restated Certificate of Incorporation as provided below, the specific level of reverse stock split to be determined by Epimmune’s Board of Directors: |
| | |
| | A. | To effect a one-for-four reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | B. | To effect a one-for-five reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | C. | To effect a one-for-six reverse stock split of the outstanding shares of Epimmune’s common stock. |
| |
| | D. | To effect a one-for-seven reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | E. | To effect a one-for-eight reverse stock split of the outstanding shares of Epimmune’s common stock. |
| |
| | F. | To effect a one-for-nine reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | G. | To effect a one-for-ten reverse stock split of the outstanding shares of Epimmune’s common stock |
| | |
| | 4. | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to increase Epimmune’s authorized capital stock to a total of 65,000,000 shares, consisting of 10,000,000 shares of authorized preferred stock and 55,000,000 shares of authorized common stock after giving effect to the reverse stock split. |
| |
| | 5. | To approve an amendment of the Epimmune 2000 Stock Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 8,800,000 shares (on a pre-reverse stock split basis). |
| | |
| | 6. | To approve an amendment of the Epimmune 2001 Employee Stock Purchase Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 185,000 shares (on a pre-reverse stock split basis). |
| |
| | 7. | To approve the adoption of the Epimmune Employee Stock Purchase Plan for IDM employees with 215,000 shares (on a pre-reverse stock split basis) of Epimmune’s common stock available for issuance under the plan. |
| |
| | 8. | To elect six directors to serve for the ensuing year or until successors are elected. |
| |
| | 9. | To ratify the selection by the Board of Directors of Ernst & Young LLP, Independent Registered Public Accounting Firm, as the independent auditors of Epimmune for its fiscal year ending December 31, 2005. |
| | |
| | 10. | To act on such other matters as the Chief Executive Officer or Chief Financial Officer of Epimmune may determine. |
These items of business are more fully described in the proxy statement accompanying this notice. We encourage you to read the proxy statement and its annexes in their entirety before voting.
Under the terms of the Share Exchange Agreement, approval by Epimmune’s stockholders of proposals 1, 3 and 4 is a condition to closing the transactions under the Share Exchange Agreement. Accordingly, in the event that any of proposals 1, 3 or 4 do not receive the required vote by our stockholders, the transactions under the Share Exchange Agreement will not close unless the IDM shareholders who are parties to the Share Exchange Agreement waive the corresponding condition. We are also seeking approval by Epimmune’s stockholders of proposal 1 in order to comply with the rules of the Nasdaq Stock Market regarding the issuance of shares of our common stock and of a reverse stock split of our common stock under proposal 3 in order to satisfy the minimum bid price per share under the Nasdaq National Market’s listing requirements. Accordingly, even if the IDM shareholders waive the requirement of Epimmune stockholder approval of these matters under the Share Exchange Agreement, we would face the delisting of our common stock from the Nasdaq Stock Market if we issue the shares as provided in proposal 1 without stockholder approval or if we do not obtain stockholder approval of the proposed reverse stock split of our common stock under proposal 3.
The record date for the annual meeting is June 21, 2005. Only stockholders of record at the close of business on that date may vote at the meeting or any adjournment thereof.
| |
| | By Order of the Board of Directors |
| |
| |  |
| | Robert J. De Vaere |
| | Vice President, Finance and Administration, |
| | Chief Financial Officer and Secretary |
San Diego, California
July 8, 2005
You are cordially invited to attend the meeting in person. Whether or not you expect to attend the meeting, please complete, date, sign and return the enclosed proxy as promptly as possible in order to ensure your representation at the meeting. A return envelope (which is postage prepaid if mailed in the United States) is enclosed for your convenience. You also have the option of voting by telephone or by using the Internet as instructed in these materials. Your vote by telephone or using the Internet must be received by 11:59 P.M., Eastern Daylight Time on August 10, 2005 to be counted. Even if you have voted by proxy, you may still vote in person if you attend the meeting. Please note, however, that if your shares are held of record by a broker, bank or other nominee and you wish to vote at the meeting, you must obtain a proxy issued in your name from that record holder.
TABLE OF CONTENTS
| | | | | | |
| | | 1 | |
| | | 1 | |
| | | 6 | |
| | | 12 | |
| | | | | 12 | |
| | | | | 13 | |
| | | | | 13 | |
| | | | | 14 | |
| | | | | 14 | |
| | | | | 14 | |
| | | | | 14 | |
| | | | | 15 | |
| | | | | 15 | |
| | | | | 15 | |
| | | | | 15 | |
| | | | | 15 | |
| | | | | 15 | |
| | | | | 15 | |
| | | | | 16 | |
| | | | | 16 | |
| | | 17 | |
| | | | | 17 | |
| | | | | 18 | |
| | | | | 20 | |
| | | | | 21 | |
| | | 22 | |
| | | | | 22 | |
| | | | | 24 | |
| | | | | 39 | |
| | | 41 | |
| | | 41 | |
| | | 41 | |
| | | | | 41 | |
| | | | | 49 | |
| | | | | 52 | |
| | | | | 57 | |
| | | | | 59 | |
| | | | | 59 | |
| | | | | 60 | |
| | | | | 60 | |
| | | | | 60 | |
i
| | | | | | |
| | | | | 61 | |
| | | | | 62 | |
| | | | | 62 | |
| | | 65 | |
| | | | | 65 | |
| | | | | 65 | |
| | | | | 66 | |
| | | | | 66 | |
| | | | | 67 | |
| | | | | 67 | |
| | | | | 67 | |
| | | | | 68 | |
| | | | | 68 | |
| | | | | 69 | |
| | | | | 70 | |
| | | | | 71 | |
| | | | | 72 | |
| | | | | 72 | |
| | | | | 73 | |
| | | | | 74 | |
| | | | | 76 | |
| | | | | 76 | |
| | | | | 77 | |
| | | | | 78 | |
| | | 78 | |
| | | | | 78 | |
| | | | | 78 | |
| | | | | 79 | |
| | | | | 80 | |
| | | | | 80 | |
| | | | | 81 | |
| | | | | 81 | |
| | | 83 | |
| | | | | 83 | |
| | | | | 86 | |
| | | | | 89 | |
| | | | | 101 | |
| | | 102 | |
| | | | | 102 | |
| | | | | 102 | |
| | | | | 102 | |
| | | | | 102 | |
| | | | | 102 | |
| | | 104 | |
| | | 105 | |
| | | 118 | |
| | | 118 | |
ii
| | | | | | |
| | | 119 | |
| | | 125 | |
| | | 125 | |
| | | 126 | |
| | | 126 | |
| | | 127 | |
| | | 127 | |
| | | 128 | |
| | | 129 | |
| | | 131 | |
| | | 140 | |
| | | 145 | |
| | | 151 | |
| | | | | 151 | |
| | | | | 152 | |
| | | | | 152 | |
| | | | | 153 | |
| | | | | 154 | |
| | | | | 154 | |
| | | | | 154 | |
| | | 156 | |
| | | | | 156 | |
| | | | | 156 | |
| | | 157 | |
| | | | | 157 | |
| | | | | 157 | |
| | | | | 159 | |
| | | | | 159 | |
iii
| | | | | | |
| | | | | 160 | |
| | | | | 160 | |
| | | | | 161 | |
| | | | | 162 | |
| | | | | 163 | |
| | | | | 163 | |
| | | | | 165 | |
| | | | | 167 | |
| | | | | 168 | |
| | | 168 | |
| | | 169 | |
| | | 170 | |
| | | 170 | |
| | | 171 | |
| | | F-1 | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
iv
CHAPTER ONE — OVERVIEW
QUESTIONS AND ANSWERS ABOUT THE EXCHANGE
Throughout this proxy statement, when we use the term “Epimmune,” the “Company,” “we,” “us” or “our,” we are referring to Epimmune Inc.; when we use the term “IDM,” we are referring to IDM S.A.; and when we use the term “Share Exchange Agreement,” we are referring to the Share Exchange Agreement, dated March 15, 2005, by and between Epimmune and certain shareholders of IDM, as amended by Amendment No. 1 to the Share Exchange Agreement, dated March 15, 2005, Amendment No. 2 to the Share Exchange Agreement, dated April 21, 2005, Amendment No. 3 to the Share Exchange Agreement, dated May 31, 2005, and Amendment No. 4 to the Share Exchange Agreement, dated June 30, 2005, all of which are attached to this proxy statement asAnnex Aand each of which is incorporated by reference into this proxy statement. Unless expressly specified otherwise, all of the numbers of shares of our common stock referred to in this proxy statement are calculated without giving effect to any adjustments that will result from the reverse stock split of our common stock if it is approved by our stockholders. For details of the reverse stock split proposal, see the section entitled“Proposal 3 —Approval of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation to Effect Reverse Stock Split.”
Additionally, when we use the terms “Exchange,” “transaction,” “transactions contemplated by the Share Exchange Agreement” or “transactions under the Share Exchange Agreement,” we are referring to:
| | |
| | • | the offer to acquire all of the issued and outstanding class A ordinary shares, nominal value€0.10 per share, of IDM and all of the issued and outstanding class B ordinary shares, nominal value€0.10 per share, of IDM and all warrants to acquire shares of IDM, other than shares of IDM held inplan d’épargne en actions, referred to as a PEA, in exchange for shares of our common stock, par value $0.01 per share, at an exchange ratio of 3.771865 shares of our common stock for each share of IDM, as adjusted pursuant to the terms of the Share Exchange Agreement, referred to as the exchange ratio, and upon the terms and subject to the conditions of the Share Exchange Agreement whereby IDM will become our subsidiary; |
| |
| | • | the execution of a put/call agreement with certain IDM shareholders, referred to as Put/ Call Agreements, under which we will have the right to acquire each share of IDM held in a PEA for cash, using the proceeds from the sale of our common stock in a financing transaction, or a number of shares of our common stock equal to the exchange ratio if the financing transaction does not occur within two years following the closing of the Exchange; |
| |
| | • | the offer to holders of options to acquire shares of IDM and who do not reside in France of substitute options to acquire shares of our common stock under our 2000 Stock Plan, referred to as the 2000 Plan, for their IDM options and, simultaneously, cancel their IDM options in connection with the closing of the Exchange; |
| |
| | • | the offer to holders of options to acquire shares of IDM and who reside in France to retain their outstanding IDM options and enter into an option liquidity agreement, referred to as an Option Liquidity Agreement, pursuant to which each share of IDM issued upon exercise of such IDM options will be exchanged at a future date for a number of shares of our common stock equal to the exchange ratio; and |
| |
| | • | the exchange of all outstanding shares of our Series S preferred stock and Series S-1 preferred stock for an aggregate of 1,949,278 shares of our common stock, subject to adjustment for our reverse stock split described in proposal 3 or any other stock split, combination or stock dividend, pursuant to the Preferred Exchange Agreement with G.D. Searle LLC, a subsidiary of Pfizer Inc., referred to as G.D. Searle, dated March 15, 2005, as amended in its entirety by the Amended and Restated Preferred Exchange Agreement with G.D. Searle, dated April 12, 2005, referred to as the Amended and Restated Preferred Exchange Agreement. |
1
| |
| Q: | Why am I receiving this proxy statement? |
| |
| A: | We and IDM have agreed to a combination under the terms of the Share Exchange Agreement. In connection with the transaction, we have agreed to make an offer to acquire all of the outstanding shares of IDM and all of the outstanding IDM options on the terms and subject to the conditions set forth in the Share Exchange Agreement, which terms and conditions are described in this proxy statement. |
| |
| | In order to complete the transactions under the Share Exchange Agreement, our stockholders must vote to approve the issuance of shares of our common stock in the Exchange. We are sending this proxy statement and the enclosed proxy card to our stockholders because our Board of Directors, referred to as the Board, is soliciting their proxy to vote on this matter and various other matters set forth in this proxy statement at the 2005 annual meeting of our stockholders, referred to as the annual meeting. |
| |
|
| | In order to complete the transactions under the Share Exchange Agreement, IDM shareholders holding at least 95% of the outstanding class A and class B ordinary shares of IDM, including the shares issuable upon exercise of warrants, referred to collectively as the IDM shares, must accept the offer. As of June 29, 2005, IDM shareholders collectively owning approximately 98% of the IDM shares have thus far entered into the Share Exchange Agreement. |
|
| |
| | This proxy statement contains important information about the Exchange and the other proposals to be presented at the annual meeting, including a change in our corporate name, a reverse stock split, an increase in authorized shares, an increase in the number of shares reserved for issuance under our 2000 Plan, an increase in the number of shares reserved for issuance under our 2001 Employee Stock Purchase Plan, referred to as the Purchase Plan, adoption of the Epimmune Employee Stock Purchase Plan for IDM employees, referred to as the French Purchase Plan, the election of six directors, and the ratification of the selection of our independent auditors. Please read it carefully. |
| |
| | You are invited to attend the annual meeting, and we request that you vote on the proposals described in this proxy statement. However, you do not need to attend the meeting to vote your shares. Instead, you may simply complete, sign and return the enclosed proxy card. You also have the option of voting by telephone or by using the Internet as instructed in these materials. |
| |
| | We intend to mail this proxy statement and accompanying proxy card on or about July 8, 2005 to all stockholders of record entitled to vote at the annual meeting. |
| |
| Q: | Why is it important for Epimmune’s stockholders to vote? |
| |
| A: | Under the rules of the Nasdaq Stock Market, referred to as Nasdaq, we are required to obtain stockholder approval for proposal 1 (issuance of shares of Epimmune’s common stock). In addition, under the terms of the Share Exchange Agreement, unless IDM shareholders who are parties to the Share Exchange Agreement, referred to as principal company shareholders, waive the relevant condition, we cannot complete the Exchange without, in the case of proposal 1, the affirmative vote of a majority of shares of our common stock entitled to vote and present at the annual meeting either in person or by proxy and, in the case of proposal 3 (amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a reverse stock split of the outstanding shares of our common stock at the ratios of one-for-four (under subproposal A), one-for-five (under subproposal B), one-for-six (under subproposal C), one-for-seven (under subproposal D), one-for-eight (under subproposal E), one-for-nine (under subproposal F) and one-for-ten (under subproposal G)) and proposal 4 (amendment of Epimmune’s Amended and Restated Certificate of Incorporation to increase Epimmune’s authorized capital stock), the affirmative vote of a majority of the shares of our common stock outstanding and entitled to vote as of the record date. For more information on the votes required to approve each proposal, see the section entitled“Questions and Answers about the Annual Meeting of Epimmune’s Stockholders.” |
| |
| Q: | Why is Epimmune proposing the transaction? |
| |
| A: | We believe that the proposed Exchange will provide strategic and financial benefits to both companies. The technologies, products, and indications that we and IDM are pursuing are highly complementary. By combining IDM’s delivery and production expertise with our epitope target identification capabilities, we |
2
| |
| believe that we will improve the opportunities for success of each party’s technology and, most importantly, expand the prospects for the many patients who could benefit from our combined expertise in developing innovative immunotherapeutics. In addition, we believe our prospects of raising additional capital in the future will improve as a combined company. For details of the reasons for the transaction, see the section entitled“The Exchange —Epimmune’s Reasons for the Exchange.” |
| |
| Q: | What will happen in the Exchange? |
| |
| A: | In accordance with the provisions of the Share Exchange Agreement, each outstanding share of IDM held by the principal company shareholders, except for shares of IDM held in a PEA, will be exchanged for shares of our common stock. In addition, we will reserve shares of our common stock for issuance pursuant to the Put/ Call Agreements, the Option Liquidity Agreements and options to acquire our common stock substituted for certain options to acquire IDM shares. Following the closing of the transactions under the Share Exchange Agreement, IDM shareholders will become holders of our common stock and IDM will become a subsidiary of Epimmune. |
| |
| | Upon satisfaction of the conditions under a shareholders agreement dated as of December 20, 1996, as amended, to which all IDM shareholders are parties, referred to as the IDM shareholders agreement, any IDM shareholders, other than the principal company shareholders, will be required to accept shares of our common stock in the Exchange at the closing of the Share Exchange Agreement pursuant to a drag-along right under the IDM shareholders agreement. For a description of the drag-along right, see the section entitled“The Exchange — Description of the Drag-along Right under the IDM Shareholders Agreement.” |
| |
|
| | Based on the number of shares of our common stock outstanding as of June 29, 2005 and the shares of our common stock to be issued or reserved for issuance, following the closing of the transactions under the Share Exchange Agreement, the current shareholders and optionholders of IDM will, upon such issuance, own approximately 78% of our outstanding common stock on a fully diluted basis, and the current shareholders and optionholders of Epimmune will, upon such issuance, own approximately 22% of our outstanding common stock on a fully diluted basis. |
|
| |
| Q: | Does Epimmune’s Board recommend voting in favor of the proposals related to the Exchange? |
| |
| A: | Yes. After careful consideration, our Board determined that the Exchange is fair to, and in the best interests of, Epimmune and its stockholders. Our Board recommends that our stockholders vote “For” proposals 1, 3 and 4, each of which is required to close the Exchange. |
| |
| | For a description of the factors considered by our Board in making its determination, Epimmune stockholders should read the section entitled“The Exchange —Epimmune’s Reasons for the Exchange.” |
| |
| Q: | Have the IDM shareholders agreed to the transaction? |
| |
| A: | Following the execution of the Share Exchange Agreement, the principal company shareholders sent an offer notice dated March 19, 2005 to the other IDM shareholders disclosing the principal terms of the Exchange in order to exercise their drag-along right under the terms of the IDM shareholders agreement. Upon receipt of the offer notice, the other shareholders of IDM, in compliance with the terms of the IDM shareholders agreement, were given the option to either submit a counterbid before April 29, 2005 or execute a joinder agreement pursuant to which they would become parties to the Share Exchange Agreement and be deemed to be a principal company shareholder for all purposes under this agreement. No counterbid was submitted by April 29, 2005, the deadline under the IDM shareholders agreement, and, as of June 29, 2005, IDM shareholders holding approximately 98% of the outstanding share capital and voting rights of IDM, including IDM shares issuable upon exercise of outstanding warrants to purchase IDM shares, have agreed to the Exchange by executing either the Share Exchange Agreement or a joinder agreement. In order for the principal company shareholders to exercise their drag-along right with respect to the shareholders of IDM holding the remaining 2% of the outstanding share capital and voting rights of IDM, the closing of the Exchange must occur on or before July 13, 2005. Because the Exchange will not occur by July 13, 2005, then the IDM shareholders who have not become principal |
3
| |
| company shareholders or who do not voluntarily exchange their shares will remain shareholders of IDM, which will be a majority controlled subsidiary of Epimmune. |
| |
| Q: | Have any Epimmune stockholders committed to vote in favor of the transaction? |
| |
| A: | Yes. The executive officers and directors of Epimmune have entered into agreements with IDM agreeing to vote the shares of our common stock they hold in favor of the approval of the transactions under the Share Exchange Agreement. As of June 21, 2005, the record date, these executive officers and directors own 294,446 shares of our common stock, or 1.7% of our common stock outstanding on an as-converted to common stock basis, and beneficially own 1,843,133 shares of our common stock. |
| |
| Q: | When do you expect the closing of the transactions under the Share Exchange Agreement to occur? |
| |
| A: | We and IDM are working to complete the Exchange as quickly as possible and expect to complete the transaction shortly after obtaining the requisite stockholder approval at our annual meeting. We would expect this to occur in the third quarter of 2005. However, we cannot predict the exact timing of the closing of the transaction because the transaction is subject to several conditions. For a description of the conditions to the closing of the transactions under the Share Exchange Agreement, see the section entitled“The Share Exchange Agreement —Conditions to Completion of the Exchange.” |
| |
| Q: | What do I need to do now? |
| |
| A: | You should carefully read and consider the information contained in this proxy statement, including the annexes, our Annual Report on Form 10-K/A for the year ended December 31, 2004 and our Quarterly Report on Form 10-Q/A for the quarter ended March 31, 2005, enclosed herewith, and consider how the transaction will affect you as a stockholder of Epimmune. |
| |
| | You should complete and return the enclosed proxy card as soon as possible in accordance with the instructions provided in this proxy statement and on the enclosed proxy card. |
| |
| Q: | Are there risks associated with this proposed transaction? |
| |
| A: | Yes. We and IDM have each incurred losses since inception and such losses may continue for the next several years as together we proceed with the development and commercialization of our products. Additionally, we expect the combined company will require additional financial resources to fund its operations in the future. The combined company may not achieve the expected benefits of this transaction because of the risks and uncertainties discussed in the section entitled“Risk Factors.” In deciding whether to approve, in connection with the Exchange, the issuance of shares of Epimmune’s common stock, the reverse stock split and the increase in Epimmune’s authorized capital stock, we urge you to carefully read and consider the risk factors contained in the section entitled“Risk Factors.” |
| |
| Q: | Whom should I call with questions? |
| |
| A: | If you have any questions about the transaction or if you need additional copies of this proxy statement or the enclosed proxy card, you should contact: |
| |
| | Epimmune Inc. |
| | 5820 Nancy Ridge Drive |
| | San Diego, California 92121 |
| | (858) 860-2500 |
| | Attention: Corporate Secretary |
| |
| Q: | What percentage of the common stock of Epimmune after the proposed transaction will be owned by former affiliates and non-affiliates of Epimmune? |
| |
| A: | Our current stockholders who are affiliates will own approximately 2.36% of the outstanding common stock of Epimmune and our current stockholders who are not affiliates of Epimmune will own approximately 15.15% of the outstanding common stock of Epimmune, on a fully diluted basis, after the closing of the proposed transaction. |
4
| |
| Q: | Will current holders of outstanding options and warrants to purchase common stock of Epimmune be able to vote the shares of common stock underlying their options and warrants for purposes of approving the proposed transaction? |
| |
| A: | No, holders of outstanding options and warrants to purchase common stock of Epimmune are not able to vote the shares of common stock underlying their options and warrants for purposes of approving the proposed transaction with IDM or for any other proposal submitted for stockholder approval. If the holder of an option or warrant purchased Epimmune common stock upon the exercise of his option or warrant prior to the record date and holds those shares as of the record date of the annual meeting, then he may vote those shares on the matters submitted for stockholder approval at the annual meeting. |
| |
| Q: | Will current holders of outstanding options and warrants to purchase common stock of Epimmune be able to exercise their options and warrants prior to the annual meeting, and, if so, what are the mechanics to exercise the options and warrants? |
| |
| A: | Whether a current holder of an outstanding option or warrant to purchase common stock of Epimmune is able to exercise his option or warrant depends upon whether the terms of the option or warrant permit the option or warrant to be exercised in whole or in part prior to the record date for the annual meeting. To the extent that the terms of the option or warrant permit its exercise prior to the record date for the annual meeting, the holder may exercise the option or warrant by complying with the procedures described in the option or warrant and any related plan or agreement pursuant to which the option or warrant was granted, as applicable. Each holder of an option or warrant should review his option or warrant and any related plan or agreement pursuant to which the option or warrant was granted to determine whether the option or warrant may be exercised and, if so, the procedures for exercising the option or warrant. |
You may also obtain additional information about us from documents filed with or furnished to the United States Securities and Exchange Commission, referred to as the SEC, by following the instructions in the section entitled“Where You Can Find More Information.”
5
QUESTIONS AND ANSWERS ABOUT THE
ANNUAL MEETING OF EPIMMUNE’S STOCKHOLDERS
When and where is the annual meeting?
The annual meeting will be held on August 11, 2005 at 11:00 a.m. local time at Epimmune’s offices located at 5820 Nancy Ridge Drive, San Diego, California 92121.
Who can vote at the annual meeting?
Only stockholders of record at the close of business on June 21, 2005 will be entitled to vote at the annual meeting. On this record date, there were 16,023,786 shares of common stock outstanding and 1,787,572 shares of preferred stock outstanding, on an as-converted to common stock basis, and entitled to vote.
| |
| Stockholder of Record: Shares Registered in Your Name |
If on June 21, 2005, your shares were registered directly in your name with Epimmune’s transfer agent, American Stock Transfer and Trust Company, then you are a stockholder of record. As a stockholder of record, you may vote in person at the meeting or vote by proxy. Whether or not you plan to attend the meeting, we urge you to fill out and return the enclosed proxy card to ensure your vote is counted. You also have the option of voting by telephone or by using the Internet as instructed in these materials.
| |
| Beneficial Owner: Shares Registered in the Name of a Broker or Bank |
If on June 21, 2005, your shares were held in an account at a brokerage firm, bank, dealer, or other similar organization, then you are the beneficial owner of shares held in “street name,” and these proxy materials are being forwarded to you by that organization. The organization holding your account is considered the stockholder of record for purposes of voting at the annual meeting. As a beneficial owner, you have the right to direct your broker or other agent on how to vote the shares in your account. You are also invited to attend the annual meeting. However, because you are not the stockholder of record, you may not vote your shares in person at the meeting unless you request and obtain a valid proxy from your broker or other agent.
What am I voting on?
The following matters are scheduled for a vote:
| | |
| | • | Approval of the issuance of shares of Epimmune’s common stock in accordance with the terms of the Share Exchange Agreement, the Put/ Call Agreements, the Option Liquidity Agreements and the Amended and Restated Preferred Exchange Agreement. |
| |
| | • | Approval of an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to change the corporate name of Epimmune to “IDM, Inc.” |
| |
| | • | Approval of a series of amendments of Epimmune’s Amended and Restated Certificate of Incorporation as provided below, the specific level of reverse stock split to be determined by the Board: |
| | |
| | A. | To effect a one-for-four reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | B. | To effect a one-for-five reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | C. | To effect a one-for-six reverse stock split of the outstanding shares of Epimmune’s common stock. |
| |
| | D. | To effect a one-for-seven reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | E. | To effect a one-for-eight reverse stock split of the outstanding shares of Epimmune’s common stock. |
| |
| | F. | To effect a one-for-nine reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | |
| | G. | To effect a one-for-ten reverse stock split of the outstanding shares of Epimmune’s common stock. |
6
| | |
| | • | Approval of an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to increase Epimmune’s authorized capital stock to a total of 65,000,000 shares, consisting of 10,000,000 shares of authorized preferred stock and 55,000,000 shares of authorized common stock after giving effect to the reverse stock split. |
| |
| | • | Approval of an amendment of the 2000 Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 8,800,000 shares. |
| |
| | • | Approval of an amendment of the Purchase Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 185,000 shares. |
| |
| | • | Approval of the adoption of the French Purchase Plan with 215,000 shares of Epimmune’s common stock available for issuance under the plan. |
| |
| | • | Election of six directors to serve for the ensuing year or until successors are elected. |
| |
| | • | Ratification of the selection by the Board of Ernst & Young LLP as independent registered public accounting firm of Epimmune for its fiscal year ending December 31, 2005. |
If any other matter is properly presented at the meeting, your proxy (one of the individuals named on your proxy card) will vote your shares using his best judgment.
How do I vote?
You may either vote “For” all the nominees to the Board or you may “Withhold” your vote for any nominee you specify. For each of the other matters to be voted on, you may vote “For” or “Against” or abstain from voting. The procedures for voting are fairly simple:
| |
| Stockholder of Record: Shares Registered in Your Name |
If you are a stockholder of record, you may vote in one of four ways:
| | |
| | 1. | By voting in person. Come to the annual meeting and we will give you a ballot when you arrive. |
| |
| | 2. | By completing, signing, dating and promptly returning the enclosed proxy card in the envelope provided. If you return your signed proxy card to us before the annual meeting, we will vote your shares as you direct. |
| |
| | 3. | By calling toll-free (in the United States), on a touch-tone phone, the 800 number printed on your proxy card, which is available 24 hours a day. Your vote must be received by 11:59 P.M., Eastern Daylight Time on August 10, 2005 to be counted. Have your proxy card in hand when you call, then follow the recorded instructions. |
| |
| | 4. | By visiting the Internet site at www.proxyvote.com. Have your proxy card in hand when you go online, then follow the instructions. Your vote must be received by 11:59 P.M., Eastern Daylight Time on August 10, 2005 to be counted. Have the proxy card in hand when you access the website and follow the instructions to create an electronic voting instruction form. |
Whether or not you plan to attend the meeting, we urge you to vote by proxy to ensure your vote is counted. You may still attend the meeting and vote in person if you have already voted by proxy.
| |
| Beneficial Owner: Shares Registered in the Name of Broker or Bank |
If you are a beneficial owner of shares registered in the name of your broker, bank, or other agent, you should have received a proxy card and voting instructions with these proxy materials from that organization rather than from Epimmune. Simply complete and mail the proxy card to ensure that your vote is counted. Some banks and brokers may offer telephone and Internet voting. To vote in person at the annual meeting, you must obtain a valid proxy from your broker, bank or other agent. Follow the instructions from your broker or bank included with these proxy materials, or contact your broker or bank to request a proxy form.
7
We provide Internet proxy voting to allow you to vote your shares on-line, with procedures designed to ensure the authenticity and correctness of your proxy vote instructions. However, please be aware that you must bear any costs associated with your Internet access, such as usage charges from Internet access providers and telephone companies.
How many votes do I have?
On each matter to be voted upon, you have one vote for each share of common stock that you own as of June 21, 2005. At the close of business on June 21, 2005, Epimmune had outstanding 859,666 shares of Series S preferred stock and 549,622 shares of Series S-1 preferred stock held by G.D. Searle. In addition to any vote to which G.D. Searle is entitled by virtue of its ownership of shares of our common stock, G.D. Searle will be entitled to vote its shares of Series S preferred stock and Series S-1 preferred stock held on all matters to be voted upon at the annual meeting on an as-converted to common stock basis which will entitle G.D. Searle to 1,787,572 votes (in addition to any votes to which it may be entitled by virtue of its ownership of shares of our common stock).
What if I return a proxy card but do not make specific choices?
If you return a signed and dated proxy card without marking any voting selections, your shares will be voted as follows:
| | |
| | • | “For” the approval of the issuance of shares of Epimmune’s common stock in accordance with the terms of the Share Exchange Agreement, the Put/ Call Agreements, the Option Liquidity Agreements and the Amended and Restated Preferred Exchange Agreement; |
| |
| | • | “For” the approval of an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to change the corporate name of Epimmune to “IDM, Inc.”; |
| |
| | • | “For” the approval of a series of amendments of Epimmune’s Amended and Restated Certificate of Incorporation as provided below, the specific level of reverse stock split to be determined by the Board: |
| | |
| | A. | to effect a one-for-four reverse stock split of the outstanding shares of Epimmune’s common stock, |
| | |
| | B. | to effect a one-for-five reverse stock split of the outstanding shares of Epimmune’s common stock, |
| | |
| | C. | to effect a one-for-six reverse stock split of the outstanding shares of Epimmune’s common stock, |
| |
| | D. | to effect a one-for-seven reverse stock split of the outstanding shares of Epimmune’s common stock, |
| | |
| | E. | to effect a one-for-eight reverse stock split of the outstanding shares of Epimmune’s common stock, |
| |
| | F. | to effect a one-for-nine reverse stock split of the outstanding shares of Epimmune’s common stock, and |
| | |
| | G. | to effect a one-for-ten reverse stock split of the outstanding shares of Epimmune’s common stock; |
| | |
| | • | “For” the approval of an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to increase Epimmune’s authorized capital stock to a total of 65,000,000 shares, consisting of 10,000,000 shares of authorized preferred stock and 55,000,000 shares of authorized common stock after giving effect to the reverse stock split; |
| |
| | • | “For” the approval of an amendment of the 2000 Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 8,800,000 shares; |
| |
| | • | “For” the approval of an amendment of the Purchase Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 185,000 shares; |
| |
| | • | “For” the approval of the adoption of the French Purchase Plan with 215,000 shares of Epimmune’s common stock available for issuance under the plan; |
8
| | |
| | • | “For” the election of all six nominees for director; and |
| |
| | • | “For” the ratification of the selection by the Board of Ernst & Young LLP as independent auditors of Epimmune for its fiscal year ending December 31, 2005. |
If any other matter is properly presented at the meeting, your proxy (one of the individuals named on your proxy card) will vote your shares using his best judgment.
Who is paying for this proxy solicitation?
We will pay for the entire cost of soliciting proxies. In addition to these mailed proxy materials, our directors and employees and Georgeson Shareholder Communications Inc. may also solicit proxies in person, by telephone, or by other means of communication. Directors and employees will not be paid any additional compensation for soliciting proxies, but Georgeson Shareholder Communications Inc. will be paid its customary fee of approximately $7,500 plus out-of-pocket expenses if it solicits proxies. We may also reimburse brokerage firms, banks and other agents for the cost of forwarding proxy materials to beneficial owners.
What does it mean if I receive more than one proxy card?
If you receive more than one proxy card, your shares are registered in more than one name or are registered in different accounts. Please complete, sign and return each proxy card to ensure that all of your shares are voted.
Can I change my vote after submitting my proxy?
Yes. You can revoke your proxy at any time before the final vote at the meeting. You may revoke your proxy in any one of three ways:
| | |
| | • | You may submit another properly completed proxy card with a later date. |
| |
| | • | You may send a written notice that you are revoking your proxy to Epimmune’s Corporate Secretary at Epimmune Inc., 5820 Nancy Ridge Drive, San Diego, California 92121. |
| |
| | • | You may attend the annual meeting and vote in person. Simply attending the meeting will not, by itself, revoke your proxy. |
If your shares are held by your broker or bank as a nominee or agent, you should follow the instructions provided by your broker or bank.
When are stockholder proposals due for next year’s annual meeting?
To be considered for inclusion in next year’s proxy materials, your proposal must be submitted in writing by March 10, 2006 to Robert J. De Vaere; Epimmune Inc., 5820 Nancy Ridge Drive, San Diego, California 92121. Under our bylaws, if you wish to submit a proposal or nominate a director to be voted at the 2006 annual meeting, you must do so by May 24, 2006.
How are votes counted?
Votes will be counted by the inspector of election appointed for the meeting, who will separately count “For” and “Withhold,” and with respect to proposals other than the election of directors, “Against” votes, abstentions and broker non-votes. “Broker non-votes” occur when a nominee holding shares for a beneficial owner does not vote on a particular proposal because the nominee does not have discretionary voting power with respect to that proposal and has not received instructions with respect to that proposal from the beneficial owner (despite voting on at least one other proposal for which is does have discretionary authority or for which it has received instructions). Abstentions will be counted towards the vote total for each proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the
9
vote total for any proposal except proposals 2, 3 and 4. For proposals 2, 3 and 4, broker non-votes will not be counted as being voted but will have the same effect as “Against” votes.
If your shares are held by your broker as your nominee (that is, in “street name”), you will need to obtain a proxy form from the institution that holds your shares and follow the instructions included on that form regarding how to instruct your broker to vote your shares. If you do not give instructions to your broker, your broker can vote your shares with respect to “discretionary” items, but not with respect to “non-discretionary” items. Discretionary items are proposals considered routine under the rules of the New York Stock Exchange on which your broker may vote shares held in street name in the absence of your voting instructions. On non-discretionary items for which you do not give your broker instructions, the shares will be treated as broker non-votes. The matters described in proposals 8 and 9 will be considered discretionary items, and the matters described in proposals 1, 2, 3, 4, 5, 6 and 7 will be considered non-discretionary items.
How many votes are needed to approve each proposal?
| | |
| | • | To be approved, proposal 1, approval of the issuance of shares of Epimmune’s common stock in accordance with the terms of the Share Exchange Agreement, the Put/ Call Agreements, the Option Liquidity Agreements and the Amended and Restated Preferred Exchange Agreement, must receive a “For” vote from the majority of shares entitled to vote and present either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have no effect. |
| |
| | • | To be approved, proposal 2, approval of an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to change the corporate name of Epimmune to “IDM, Inc.,” must receive a “For” vote from the majority of the outstanding shares either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have the same effect as an “Against” vote. |
| |
| | • | To be approved, proposal 3, approval of an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-four (under subproposal A), one-for-five (under subproposal B), one-for-six (under subproposal C), one-for-seven (under subproposal D) one-for-eight (under subproposal E), one-for-nine (under subproposal F) and one-for-ten (under subproposal G) reverse stock split of the outstanding shares of Epimmune’s common stock, must receive a “For” vote from the majority of the outstanding shares either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have the same effect as an “Against” vote. |
| |
| | • | To be approved, proposal 4, approval of an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to increase Epimmune’s authorized capital stock to a total of 65,000,000 shares, consisting of 10,000,000 shares of authorized preferred stock and 55,000,000 shares of authorized common stock after giving effect to the reverse stock split, must receive a “For” vote from the majority of the outstanding shares either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have the same effect as an “Against” vote. |
| |
| | • | To be approved, proposal 5, approval of an amendment of the 2000 Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 8,800,000 shares, must receive a “For” vote from the majority of shares entitled to vote and present either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have no effect. |
| |
| | • | To be approved, proposal 6, approval of an amendment of the Purchase Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 185,000 shares, must receive a “For” vote from the majority of shares entitled to vote and present either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have no effect. |
10
| | |
| | • | To be approved, proposal 7, approval of the adoption of the French Purchase Plan with 215,000 shares of Epimmune’s common stock available for issuance under the plan, must receive a “For” vote from the majority of shares entitled to vote, and present either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have no effect. |
| |
| | • | To be approved, proposal 8, the election of directors, the six nominees receiving the most “For” votes (among votes properly cast in person or by proxy) will be elected. Broker non-votes will have no effect. |
| |
| | • | To be approved, proposal 9, ratification of the selection of Ernst & Young LLP as independent auditors of Epimmune for its fiscal year ending December 31, 2005, must receive a “For” vote from the majority of shares entitled to vote and present either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have no effect. |
Shares of our Series S preferred stock and Series S-1 preferred stock outstanding as of the record date may be voted with respect to each proposal listed above together with shares of our common stock, as a single class, on an as-if-converted to common stock basis. As of the record date, there were 859,666 shares of Series S preferred stock and 549,622 shares of Series S preferred stock outstanding and convertible into an aggregate of 1,787,572 shares of common stock.
What is the quorum requirement?
A quorum of stockholders is necessary to hold a valid meeting. A quorum will be present if at least a majority of the outstanding shares are represented by votes at the meeting or by proxy. On the record date, there were 17,811,358 shares outstanding on an as-converted to common stock basis and entitled to vote. Thus 8,905,680 shares must be represented in person or by proxy at the annual meeting to have a quorum.
Your shares will be counted toward the quorum only if you submit a valid proxy (or one is submitted on your behalf by your broker, bank or other nominee) or if you vote in person at the meeting. Abstentions and broker non-votes will be counted towards the quorum requirement. If there is no quorum, the chairman of the meeting or a majority of the votes present at the meeting may adjourn the meeting to another date.
How can I find out the results of the voting at the annual meeting?
Preliminary voting results will be announced at the annual meeting. Final voting results will be published in our quarterly report on Form 10-Q for the second quarter of 2005.
11
SUMMARY OF THE EXCHANGE (PROPOSAL 1)
This summary highlights selected information from this proxy statement relating to the proposed Exchange and related transactions (Proposal 1) and does not contain all of the information that is important to you. To better understand the Exchange and related transactions, you should read this entire document carefully, including the Share Exchange Agreement attached asAnnex A and incorporated by reference into this proxy statement, the opinion of Jefferies & Company, Inc. attached asAnnex Band the other documents to which we refer. In addition, we incorporate important business and financial information about Epimmune by reference to our Annual Report on Form 10-K/A for the year ended December 31, 2004 and our Quarterly Report on Form 10-Q/A for the quarter ended March 31, 2005, copies of which have been provided to you with this proxy statement. You may obtain additional copies of the information incorporated by reference into this proxy statement without charge by following the instructions in the section entitled“Where You Can Find More Information.” We have included page references parenthetically to direct you to a more complete description of the topics presented in this summary.
The Companies
| |
| | Epimmune Inc. |
| | 5820 Nancy Ridge Drive |
| | San Diego, California 92121 |
| | (858) 860-2553 |
Epimmune develops therapeutic vaccines that use multiple epitopes, or protein fragments, to specifically activate the body’s immune system for the more effective management of infectious diseases and cancer. Epimmune is currently developing vaccines to treat HIV and lung cancer, and is conducting research and preclinical development of vaccines to treat breast, prostate and other cancers and vaccines for the prevention of HIV and malaria. Epimmune has entered into research and development collaborations with multiple pharmaceutical and biotechnology companies to develop and commercialize these therapeutic and prophylactic vaccines.
Epimmune is a publicly traded corporation, incorporated under Delaware law on July 10, 1987. Epimmune as it is currently constituted was formed by the merger of a majority owned Epimmune subsidiary into Epimmune on July 1, 1999. Epimmune’s common stock is listed and traded on the Nasdaq National Market under the symbol “EPMN.” For more information, visit Epimmune’s website located atwww.epimmune.com; however, the information contained in Epimmune’s website is not a part of this proxy statement.
| |
| | IDM S.A. |
| | 172, rue de Charonne |
| | 75011 Paris, France |
| | +33(0) 1 40 09 04 11 |
IDM is a biopharmaceutical company focused on the development of innovative products to treat and control cancer while maintaining the patient’s quality of life. IDM is currently developing two lines of products in the area of cancer therapy designed to improve the patient’s immune response. The first line of product candidates consists of products to destroy residual cancer cells remaining after traditional therapies and is based on the activation of certain immune cells called macrophages. IDM’s lead product candidate, Mepact, activates macrophagesin vivo, or inside the body, in order to increase their capacity to destroy cancer cells. IDM is developing Mepact for the treatment of osteosarcoma, a type of bone cancer. IDM’s second line of product candidates, designed to prevent tumor recurrence by stimulating an immune response, is based on dendritophages, which are specialized immune cells derived from the patient’s own white blood cells. Dendritophages are exposed to tumor cell antigens and reinjected into the patient in order to stimulate the immune system to recognize and kill tumor cells which display specific antigens on their surface. IDM has entered into a number of collaborations with academic and non-academic institutions and pharmaceutical companies.
12
IDM is a privately owned société anonyme, organized under the laws of France in December 1993. IDM’s wholly owned subsidiary, IDM, Inc., a Delaware corporation, maintains offices at 9 Parker, Irvine, CA 92618. For more information, visit IDM’s website located atwww.idm-biotech.com; however, the information contained in IDM’s website is not a part of this proxy statement.
The Exchange (see Page 41)
We have agreed with the principal company shareholders of IDM to exchange 3.771865 shares of our common stock for each outstanding class A and class B ordinary share of IDM in the Exchange. This exchange ratio is subject to adjustment as described in the section entitled“The Share Exchange Agreement — Exchange Ratio” beginning on page 66. Other than certain IDM shares and warrants held in a PEA, all outstanding IDM shares will be exchanged for shares of our common stock in accordance with the terms of the Share Exchange Agreement. Additional terms and conditions of the Exchange are described in the section entitled“The Share Exchange Agreement” beginning on page 65.
As of June 29, 2005, IDM shareholders holding approximately 98% of the IDM ordinary shares issuable as of the closing of the Exchange are principal company shareholders. IDM shareholders are principal company shareholders if they either signed the Share Exchange Agreement or subsequently signed a joinder agreement to become a party to the Share Exchange Agreement. Under the terms of the IDM shareholders agreement, the principal company shareholders have a right, referred to as a drag-along right, to cause the other IDM shareholders to participate in the Exchange on the same terms and conditions as the principal company shareholders so long as they comply with the terms of the IDM shareholders agreement. The drag-along right and the IDM shareholders agreement are described in more detail in the section entitled“The Exchange — Description of Drag-Along Right under the IDM Shareholders Agreement” beginning on page 59.
We will also offer each holder of options to purchase IDM shares, referred to as IDM options, who resides outside of France substitute options issued under our 2000 Plan. The substitute options will be subject to the same vesting and exercise provisions as the original IDM options. We will offer each holder of IDM options who resides in France the right to enter into an Option Liquidity Agreement with us. Pursuant to the terms of the Option Liquidity Agreement, upon exercise of an IDM option, the French optionholder will exchange with us each IDM share acquired upon the option exercise for shares of our common stock equal to the exchange ratio. The treatment of IDM options in the Exchange is described in the section entitled“The Share Exchange Agreement —Treatment of IDM Options” beginning on page 66.
Based on the exchange ratio and the number of IDM shares and options to purchase IDM shares outstanding as of June 29, 2005, a total of approximately 74,860,000 shares of our common stock and options to purchase approximately 4,365,000 shares of our common stock will be issued in the Exchange, including shares of our common stock to be issued pursuant to the Put/ Call Agreements and the Option Liquidity Agreements. Immediately following the closing of the Exchange, IDM shareholders will own shares of our common stock representing in the aggregate approximately 78% of our outstanding common stock, on a fully diluted basis.
Recommendation of the Board of Directors of Epimmune and Its Reasons for the Exchange (see Page 52)
Our Board unanimously approved the Share Exchange Agreement and related agreements, the issuance of our common stock in accordance with the Share Exchange Agreement, the Put/Call Agreements, the Option Liquidity Agreements and the Amended and Restated Preferred Exchange Agreement and unanimously recommends that our stockholders vote “FOR” each of the proposals in this proxy statement.
Our Board considered many factors in making the determination that the combination with IDM through the Exchange is fair to our stockholders and in their best interests, which are discussed in the section entitled“The Exchange — Epimmune’s Reasons for the Exchange”beginning on page 49.
13
Opinion of Epimmune’s Financial Advisor to the Board of Directors (see Page 52)
In connection with the transaction, our financial advisor, Jefferies & Company, Inc., referred to as Jefferies, rendered its opinion to our Board to the effect that as of March 15, 2005, and based upon the qualifications, limitations and assumptions set forth in the opinion, the consideration to be paid by us to the IDM shareholders in connection with the transaction was fair, from a financial point of view, to Epimmune. A copy of Jefferies opinion is attached asAnnex Bto this proxy statement. We urge you to read carefully this opinion in its entirety for information with respect to the assumptions made, matters considered and limits of review in connection with the opinion.
Directors and Management of Epimmune Following the Exchange (see Page 62)
Effective as of the closing of the Exchange, our Board will be expanded to nine members, three of our current directors will resign and we will name three current directors of IDM, the Managing General Partner of one of IDM’s shareholders and two other persons designated by IDM to our Board.
Effective as of the closing of the Exchange, Jean-Loup Romet-Lemonne, M.D., the current Chief Executive Officer of IDM, will be appointed our Chairman of the Board and Chief Executive Officer, Emile Loria, M.D., our current President and Chief Executive Officer, will be appointed our President and Chief Business Officer, and Robert J. De Vaere, our current Vice President, Finance and Administration and Chief Financial Officer, will be appointed our Chief Financial Officer.
Amended and Restated Preferred Exchange Agreement (see Page 60)
On March 15, 2005, we entered into a Preferred Exchange Agreement with G.D. Searle, the holder of all of the outstanding shares of our preferred stock. On April 12, 2005, we entered into an Amended and Restated Preferred Exchange Agreement with G.D. Searle. Pursuant to this agreement, effective immediately prior to the closing of the Exchange, G.D. Searle will exchange 859,666 shares of our Series S preferred stock and 549,622 shares of our Series S-1 preferred stock for an aggregate of 1,949,278 shares of our common stock, subject to adjustment for our reverse stock split described in proposal 3 or any other stock split, combination or stock dividend.
Interests of Directors, Officers and Affiliates (see Page 57)
Some of our directors and executive officers have agreements or arrangements that provide them with interests in the combination with IDM that are different from, or in addition to, your interests.
Effective as of the closing of the Exchange, Dr. Loria, our President and Chief Executive Officer, Mark Newman, our Vice President, Research and Development, and Mr. De Vaere, our Vice President, Finance and Administration and Chief Financial Officer will (i) receive cash bonuses in the amount of $375,000, $117,500 and $117,500, respectively, and (ii) receive base salary increases retroactive to January 1, 2005 in the amount of $25,000, $10,000 and $20,000, respectively. In addition, as of the closing of the Exchange the vesting of certain options held by Dr. Loria, Dr. Newman and Mr. De Vaere will be accelerated and the options will vest in full, and new employment agreements that we have entered into with each of them will become effective. As of June 29, 2005, the vesting of 4,856, 48,564 and 3,399 options held by Mr. De Vaere, Dr. Loria and Dr. Newman, respectively, would accelerate.
Effective as of the closing of the Exchange, options to purchase shares of our common stock granted to Howard E. (“Ted”) Greene, Jr., Georges Hibon and William T. Comer, Ph.D., each of whom is a member of the Board and will resign as of the closing of the Exchange, will be amended to remain exercisable until the date that the option would have originally expired but for the resignation of the optionholder from service as a director. As of June 29, 2005, 34,167 47,856 and 49,284 options held by Mr. Hibon, Mr. Greene and Dr. Comer, respectively, would remain exercisable. In addition, for six years after the closing we will maintain an extension of coverage of our directors and officers insurance for the benefit of those of our directors and officers who are covered by those policies immediately prior to the closing, with respect to matters occurring prior to the closing.
14
Risk Factors (see Page 22)
Ownership of our common stock, the Exchange and the business to be conducted by the combined company following the Exchange involve risks.
No Appraisal Rights (see Page 124)
Epimmune stockholders and IDM shareholders will not be entitled to demand appraisal of, or receive any appraisal or similar payments for, their shares in connection with the combination of Epimmune and IDM.
U.S. Federal Income Tax Consequences to Epimmune and Epimmune Stockholders
Because the consideration issued by Epimmune to IDM shareholders in exchange for their IDM class A and class B ordinary shares consists solely of Epimmune common stock, and because the Exchange does not involve an exchange of shares or securities by Epimmune stockholders (as determined immediately before the Exchange), the closing of the Exchange and related transactions under the Share Exchange Agreement do not have material U.S. federal income tax consequences to the holders of Epimmune common stock. The Exchange may, when considered with other transactions occurring before and after the Exchange, result in an “ownership change” of Epimmune as that term is described under Section 382 of the Internal Revenue Code of 1986, as amended. If an ownership change occurs, Epimmune’s ability to use its net operating losses from pre-ownership change periods to reduce taxable income in post-ownership change periods would be subject to an annual limitation. In general, the annual limitation would equal the value of Epimmune immediately before the ownership change multiplied by the federal long-term tax-exempt interest rate.
Listing of Epimmune’s Common Stock on the Nasdaq National Market (see Page 61)
The continued listing of our common stock on the Nasdaq National Market is one of the conditions to closing the Exchange. Under the Nasdaq rules, the Exchange is expected to be viewed as a “reverse merger” which requires that we satisfy all the requirements for initial listing on the Nasdaq National Market, including a $5.00 per share minimum bid price for the 90 trading days preceding the closing. We do not currently satisfy the minimum bid price requirement and the reverse stock splits proposed in proposals 3-A, 3-B, 3-C, 3-D, 3-E, 3-F and 3-G are intended to enable us to meet the minimum bid price requirement.
Date, Time and Location of the Annual Meeting of Epimmune’s Stockholders (see Page 6)
The annual meeting will be held at Epimmune’s offices at 5820 Nancy Ridge Drive, San Diego, California, at 11:00 a.m., local time, on August 11, 2005.
Record Date and Voting Rights for the Annual Meeting of Epimmune’s Stockholders (see Page 6)
Holders of record of our common stock at the close of business on June 21, 2005 will be entitled to vote at the annual meeting or any adjournment of the annual meeting. On the record date, 16,023,786 shares of our common stock and 1,787,572 shares of our preferred stock, on an as-converted to common stock basis, were outstanding and entitled to vote at the annual meeting and at any adjournments thereof. Each share of our common stock is entitled to one vote on each matter to be voted upon at the annual meeting. G.D. Searle, the sole holder of our preferred stock, will be entitled to vote its shares of preferred stock on an as-converted to common stock basis which will entitle G.D. Searle to 1,787,572 votes (in addition to any votes to which it may be entitled by virtue of its ownership of shares of our common stock).
Vote Required by Epimmune’s Stockholders to Approve the Proposals
| | |
| | • | To be approved, each of proposals 1, 5, 6, 7 and 9 must receive a “For” vote from the majority of shares entitled to vote and present either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have no effect. Proposals 1 (page 41), 5 (page 131), 6 (page 140), proposal 7 (page 145) and 9 (page 156) are discussed in more detail beginning on the indicated pages. |
15
| | |
|
| | • | To be approved, each of proposals 2, 3 and 4 must receive a “For” vote from the majority of the outstanding shares either in person or by proxy. If you “Abstain” from voting, it will have the same effect as an “Against” vote. Broker non-votes will have the same effect as an “Against” vote. Proposals 2 (page 118), 3 (page 119) and 4 (page 129) are discussed in more detail beginning on the indicated pages. |
|
| |
|
| | • | For the election of the directors, which is proposal 8, the six nominees receiving the most “For” votes (among votes properly cast in person or by proxy) will be elected. Broker non-votes will have no effect. Proposal 8 is discussed in more detail beginning on page 151. |
|
Epimmune and the Principal Company Shareholders May Amend or Terminate the Share Exchange Agreement (see Page 78)
We and the principal company shareholders may amend the Share Exchange Agreement under certain conditions. If the Share Exchange Agreement is terminated, the agreement provides, in specified circumstances, that we would pay IDM a termination fee of $1,334,600. If the principal company shareholders terminate the Share Exchange Agreement as permitted under the agreement no termination fee would be payable to us.
Market Price and Dividend Data
Our common stock is traded publicly on the Nasdaq National Market under the trading symbol “EPMN.” On March 14, 2005, the last full trading day prior to the public announcement of the Share Exchange Agreement, our common stock closed at $1.31 per share. On June 29, 2005, our common stock closed at $0.72 per share. Currently, there is no public trading market for IDM shares.
The following table presents quarterly information on the price range of our common stock. This information indicates the high and low sale prices reported by the Nasdaq National Market System. These prices do not include retail markups, markdowns or commissions.
| | | | | | | | | | |
| | | High | | | Low | |
| | | | | | | |
2003 | | | | | | | | |
| | Second Quarter | | $ | 2.07 | | | $ | 0.76 | |
| | Third Quarter | | $ | 4.29 | | | $ | 1.01 | |
| | Fourth Quarter | | $ | 3.21 | | | $ | 1.50 | |
2004 | | | | | | | | |
| | First Quarter | | $ | 2.99 | | | $ | 1.76 | |
| | Second Quarter | | $ | 2.47 | | | $ | 1.60 | |
| | Third Quarter | | $ | 1.94 | | | $ | 1.10 | |
| | Fourth Quarter | | $ | 1.99 | | | $ | 1.15 | |
2005 | | | | | | | | |
| | First Quarter | | $ | 1.73 | | | $ | 1.04 | |
| | Second Quarter (through June 29) | | $ | 1.18 | | | $ | 0.14 | |
As of June 29, 2005, there were approximately 255 stockholders of record of our common stock. We have never declared or paid dividends on our common stock and do not anticipate the payment of dividends in the foreseeable future.
16
SELECTED SUMMARY HISTORICAL AND PRO FORMA COMBINED FINANCIAL DATA
The following tables present summary historical financial data, summary unaudited pro forma condensed combined financial data, exchange rate data and comparative per share data.
Selected Summary Historical Financial Data of Epimmune
The following table sets forth selected summary historical financial data of Epimmune. The information presented below is derived from Epimmune’s audited financial statements as of December 31, 2000, 2001, 2002, 2003 and 2004 and the unaudited financial statements as of March 31, 2004 and 2005. This information is only a summary. You should read it together with Epimmune’s historical financial statements and accompanying notes incorporated by reference into this proxy statement. Historical results are not necessarily indicative of future results.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Three Months Ended | |
| | | Year Ended December 31, | | | March 31, | |
| | | | | | | |
| | | 2000 | | | 2001 | | | 2002 | | | 2003 | | | 2004 | | | 2004 | | | 2005 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | (In thousands, except per share data) | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| License fees and milestone revenue | | $ | — | | | $ | 3,750 | | | $ | 487 | | | $ | 1,118 | | | $ | 712 | | | $ | 97 | | | $ | 114 | |
| Research grants and contract revenue | | | 1,603 | | | | 1,831 | | | | 1,899 | | | | 2,521 | | | | 7,909 | | | | 1,454 | | | | 1,719 | |
| Related party revenue | | | — | | | | 2,585 | | | | 4,684 | | | | 3,519 | | | | 1,026 | | | | 1,026 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | |
| | Total Revenues | | | 1,603 | | | | 8,166 | | | | 7,070 | | | | 7,158 | | | | 9,647 | | | | 2,577 | | | | 1,833 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Costs and Expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 6,285 | | | | 7,870 | | | | 11,257 | | | | 10,495 | | | | 10,895 | | | | 2,407 | | | | 2,686 | |
| General and administrative | | | 2,305 | | | | 3,363 | | | | 2,887 | | | | 3,567 | | | | 2,273 | | | | 658 | | | | 457 | |
| Transaction costs for business combination | | | — | | | | — | | | | — | | | | — | | | | 443 | | | | — | | | | 1,089 | |
| Restructuring costs | | | (100 | ) | | | — | | | | — | | | | 336 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | |
| | Total costs and expenses | | | 8,490 | | | | 11,233 | | | | 14,144 | | | | 14,398 | | | | 13,611 | | | | 3,065 | | | | 4,242 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (6,887 | ) | | | (3,067 | ) | | | (7,074 | ) | | | (7,240 | ) | | | (3,964 | ) | | | (488 | ) | | | (2,409 | ) |
| Interest income, net | | | 564 | | | | 424 | | | | 587 | | | | 191 | | | | 89 | | | | 4 | | | | 36 | |
| Other income (expense), net | | | 1,581 | | | | (1 | ) | | | (13 | ) | | | (7 | ) | | | (7 | ) | | | (1 | ) | | | (5 | ) |
| Gain on sales and disposal of assets | | | 5 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | (4,737 | ) | | | (2,644 | ) | | | (6,500 | ) | | | (7,056 | ) | | | (3,882 | ) | | | (485 | ) | | | (2,378 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| Net loss per share — basic and diluted | | $ | (0.68 | ) | | $ | (0.31 | ) | | $ | (0.57 | ) | | $ | (0.58 | ) | | $ | (0.25 | ) | | $ | (0.04 | ) | | $ | (0.15 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| Shares used in computing net loss per share — basic and diluted | | | 6,971 | | | | 8,534 | | | | 11,446 | | | | 12,239 | | | | 15,305 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, | | | |
| | | | | | March 31, | |
| | | 2000 | | | 2001 | | | 2002 | | | 2003 | | | 2004 | | | 2005 | |
| | | | | | | | | | | | | | | | | | | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash, cash equivalents and short-term investments | | $ | 9,593 | | | $ | 19,036 | | | $ | 9,745 | | | $ | 6,416 | | | $ | 7,006 | | | $ | 5,673 | |
| Total assets | | | 14,486 | | | | 23,910 | | | | 15,528 | | | | 12,693 | | | | 14,807 | | | | 11,880 | |
| Long-term obligations | | | 389 | | | | 43 | | | | — | | | | — | | | | — | | | | — | |
| Stockholders’ equity | | | 12,091 | | | | 19,351 | | | | 12,586 | | | | 9,711 | | | | 11,116 | | | | 8,766 | |
17
Selected Summary Historical Financial Data of IDM
The information below and IDM’s financial statements are reported in euros. The selected summary historical data for the years ended December 31, 2004, 2003, 2002 and 2001 have been derived from IDM’s consolidated financial statements for the same periods, which have been prepared in accordance with generally accepted accounting principles in the United States of America, referred to as U.S. GAAP. These financial statements were audited by Ernst & Young Audit, IDM’s independent auditors, whose report is included elsewhere in this proxy statement. The selected summary historical financial data of IDM for the year ended December 31, 2000 and the three months ended March 31, 2004 and 2005 have been derived from unaudited financial statements, which have been prepared in accordance with U.S. GAAP on the basis of audited financial statements prepared in accordance with generally accepted accounting principles in France, referred to as French GAAP.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | | |
| | | 2000 | | | 2001 | | | 2002 | | | 2003 | | | 2004 | | | 2004(1) | |
| | | | | | | | | | | | | | | | | | | |
| | | (Unaudited) | | | (In thousands, except per share data) | | | |
Statements of operations data: | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development revenues | | € | 0 | | | € | 17 | | | € | 3,571 | | | € | 5,325 | | | € | 4,652 | | | $ | 5,890 | |
| | | | | | | | | | | | | | | | | | | |
| Research and development expenses and impairment costs | | | (26,169 | ) | | | (13,166 | ) | | | (12,301 | ) | | | (16,601 | ) | | | (22,374 | ) | | | (28,328 | ) |
| Selling and marketing expenses | | | (184 | ) | | | (622 | ) | | | (1,602 | ) | | | (1,456 | ) | | | (944 | ) | | | (1,195 | ) |
| General and administrative expenses | | | (8,961 | ) | | | (3,751 | ) | | | (4,796 | ) | | | (4,686 | ) | | | (7,675 | ) | | | (9,717 | ) |
| | | | | | | | | | | | | | | | | | | |
| | Total operating expenses | | | (35,314 | ) | | | (17,539 | ) | | | (18,699 | ) | | | (22,743 | ) | | | (30,993 | ) | | | (39,240 | ) |
| | | | | | | | | | | | | | | | | | | |
| | Loss from operations | | | (35,314 | ) | | | (17,522 | ) | | | (15,128 | ) | | | (17,418 | ) | | | (26,341 | ) | | | (33,350 | ) |
| | | | | | | | | | | | | | | | | | | |
| Interest income net | | | 764 | | | | 1,865 | | | | 1,284 | | | | 961 | | | | 561 | | | | 710 | |
| Other income | | | 35 | | | | 4 | | | | 241 | | | | — | | | | — | | | | — | |
| Foreign exchange gain (loss) | | | 26 | | | | (15 | ) | | | 16 | | | | 29 | | | | (17 | ) | | | (21 | ) |
| | | | | | | | | | | | | | | | | | | |
| | Loss before income tax benefit | | | (34,489 | ) | | | (15,668 | ) | | | (13,587 | ) | | | (16,428 | ) | | | (25,797 | ) | | | (32,661 | ) |
| | | | | | | | | | | | | | | | | | | |
| Income tax benefit | | | 465 | | | | 463 | | | | 640 | | | | 208 | | | | 281 | | | | 356 | |
| | | | | | | | | | | | | | | | | | | |
| | Net loss | | | (34,024 | ) | | | (15,205 | ) | | | (12,947 | ) | | | (16,220 | ) | | | (25,516 | ) | | | (32,305 | ) |
| | | | | | | | | | | | | | | | | | | |
| Weighted average number of shares | | | 8,323 | | | | 11,633 | | | | 12,063 | | | | 13,516 | | | | 13,649 | | | | 13,649 | |
| Basic and diluted loss per share | | € | (4.09 | ) | | € | (1.31 | ) | | € | (1.07 | ) | | € | (1.20 | ) | | € | (1.87 | ) | | $ | (2.37 | ) |
| | | | | | | | | | | | | | | | | | | |
Balance sheet data (at the end of period): | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | € | 48,430 | | | € | 38,240 | | | € | 49,290 | | | € | 33,326 | | | € | 30,859 | | | $ | 41,777 | |
| Total assets | | | 67,067 | | | | 55,626 | | | | 64,730 | | | | 52,271 | | | | 40,840 | | | | 55,289 | |
| Total current liabilities | | | 2,370 | | | | 4,179 | | | | 4,977 | | | | 4,970 | | | | 6,355 | | | | 8,603 | |
| Total shareholders’ equity | | | 64,099 | | | | 49,616 | | | | 56,805 | | | | 43,745 | | | | 31,394 | | | | 42,501 | |
| |
| (1) | For your convenience, U.S. dollar amounts have been presented for the year ended December 31, 2004, calculated at the exchange rate of €1.00 = $1.2661 for the statement of operations data and €1.00 = $1.3538 for the balance sheet data. The balance sheet rate was the Federal Reserve Bank of New York’s noon buying rate in New York as of December 31, 2004 and the statement of operations rate is an average exchange rate for the year ended December 31, 2004. |
18
| | | | | | | | | | | | | | |
| | | Three Months Ended March 31, | |
| | | | |
| | | 2004 | | | 2005 | | | 2005(1) | |
| | | | | | | | | | |
| | | (Unaudited, in thousands, except | |
| | | per share data) | |
Statements of operations data: | | | | | | | | | | | | |
| Research and development revenues | | € | 1,213 | | | € | 1,200 | | | $ | 1,573 | |
| | | | | | | | | | |
| Research and development expenses and impairment costs | | | (3,851 | ) | | | (3,902 | ) | | | (5,117 | ) |
| Selling and marketing expenses | | | (256 | ) | | | (197 | ) | | | (258 | ) |
| General and administrative expenses | | | (1,046 | ) | | | (1,068 | ) | | | (1,400 | ) |
| | | | | | | | | | |
| Other operating expenses | | | — | | | | (1,157 | ) | | | (1,517 | ) |
| | | | | | | | | | |
| | Total operating expenses | | | (5,153 | ) | | | (6,324 | ) | | | (8,292 | ) |
| | | | | | | | | | |
| | Loss from operations | | | (3,940 | ) | | | (5,124 | ) | | | (6,719 | ) |
| | | | | | | | | | |
| Interest income net | | | 169 | | | | 142 | | | | 186 | |
| Other income | | | — | | | | — | | | | — | |
| Foreign exchange gain (loss) | | | 20 | | | | 3 | | | | 4 | |
| | | | | | | | | | |
| | Loss before income tax benefit | | | (3,751 | ) | | | (4,979 | ) | | | (6,529 | ) |
| | | | | | | | | | |
| Income tax benefit | | | 6 | | | | 336 | | | | 441 | |
| | | | | | | | | | |
| | Net loss | | | (3,745 | ) | | | (4,643 | ) | | | (6,088 | ) |
| | | | | | | | | | |
| Weighted average number of shares | | | 13,604 | | | | 15,694 | | | | 15,694 | |
| Basic and diluted loss per share | | € | (0.28 | ) | | € | (0.28 | ) | | $ | (0.39 | ) |
| | | | | | | | | | |
Balance sheet data (at the end of period): | | | | | | | | | | | | |
| Cash and cash equivalents | | | | | | € | 26,765 | | | $ | 34,711 | |
| Total assets | | | | | | | 36,840 | | | | 47,778 | |
| Total current liabilities | | | | | | | 7,110 | | | | 9,221 | |
| Total shareholders’ equity | | | | | | | 26,781 | | | | 34,732 | |
| |
| (1) | For your convenience, U.S. dollar amounts have been presented for the three months ended March 31, 2005, calculated at the exchange rate of€1.00 = $1.3112 for the statement of operations and€1.00 = $1.2969 for the balance sheet. The balance sheet rate was the Federal Reserve Bank of New York’s noon buying rate in New York as of March 31, 2005 and the statement of operations rate is an average exchange rate for the three months ended March 31, 2005. |
19
Selected Unaudited Pro Forma Condensed Combined Financial Data of Epimmune and IDM
The following selected unaudited pro forma condensed combined financial data was prepared using the purchase method of accounting. For accounting purposes, IDM is considered to be acquiring Epimmune in this transaction. The unaudited pro forma condensed combined statements of operations data combines the historical statements of operations of Epimmune and IDM for the year ended December 31, 2004 and for the three months ended March 31, 2005, giving effect to the proposed transaction as if it had occurred on January 1, 2004. The unaudited pro forma condensed combined balance sheet data combines the historical balance sheets of Epimmune and IDM as of March 31, 2005, giving effect to the proposed transaction as if it had occurred as of March 31, 2005.
The selected unaudited pro forma condensed combined financial data is based on estimates and assumptions that are preliminary. The data is presented for informational purposes only and is not intended to represent or be indicative of the consolidated results of operations or financial condition of Epimmune that would have been reported had the proposed transaction been completed as of the dates presented, and should not be taken as representative of future consolidated results of operations or financial condition of Epimmune. Please also read the section in this proxy statement entitled“Forward Looking Statements”for more information on the statements made in this section.
This selected unaudited pro forma condensed combined financial data should be read in conjunction with the selected summary historical financial data, the unaudited pro forma condensed combined financial statements and accompanying notes, and IDM’s historical financial statements and accompanying notes contained elsewhere in this proxy statement, and Epimmune’s historical financial statements and accompanying notes incorporated by reference into this proxy statement. See the section entitled“Where You Can Find More Information.”
| | | | | | | | | |
| | | Year Ended | | | Three Months Ended | |
| Pro Forma Condensed Combined Statements of Operations Data | | December 31, 2004 | | | March 31, 2005 | |
| | | | | | | |
| | | (In thousands, except per share data) | |
| Total revenues | | $ | 15,218 | | | $ | 3,360 | |
| Loss from operations | | | (37,314 | ) | | | (9,104 | ) |
| Net loss | | | (36,187 | ) | | | (8,442 | ) |
| Basic and diluted net loss per share | | | (0.39 | ) | | | (0.09 | ) |
| Shares used in computation of basic and diluted net loss per share(1) | | | 92,117 | | | | 92,827 | |
| | | | | |
| | | As of | |
| Pro Forma Condensed Combined Balance Sheet Data | | March 31, 2005 | |
| | | | |
| | | (In thousands) | |
| Cash and cash equivalents | | $ | 35,716 | |
| Total assets | | | 64,136 | |
| Long-term obligations | | | 3,825 | |
| Total stockholders’ equity | | | 48,519 | |
| |
| (1) | Assumes 74,862,378 new shares of Epimmune common stock issued to IDM shareholders. |
20
Comparative Historical and Pro Forma Combined Per Share Data
The following table presents historical per share data regarding the net loss and book value of each of Epimmune and IDM and unaudited combined pro forma per share data after giving effect to the transaction as a purchase of Epimmune by IDM. The pro forma net loss per share information gives effect to the proposed transaction for the year ended December 31, 2004 and for the three months ended March 31, 2005 as if it had occurred on January 1, 2004. The pro forma book value per share information gives effect to the proposed transaction as if it had occurred on December 31, 2004 and March 31, 2005, respectively. The pro forma combined shares consist of 74,862,378 new shares Epimmune common stock to be issued to IDM shareholders (excluding the 4,364,036 Epimmune share equivalents to be issued to IDM optionholders) and 1,949,278 new shares of common shares to be issued to the holder of shares of Epimmune’s preferred stock upon conversion of the preferred to common stock in connection with the transaction, added to the shares used in computation of Epimmune’s historical basic and diluted net loss per share or historical book value for the period. The IDM equivalent shares assumes 15,694,446 IDM ordinary shares, 4,153,130 IDM ordinary shares to be issued upon exercise of warrants, and an exchange ratio of 3.771865 shares of Epimmune common stock to be issued in the transaction for each IDM ordinary share. Neither Epimmune nor IDM has paid any cash dividends during the period presented.
The data has been derived from and should be read in conjunction with IDM’s historical financial statements and accompanying notes and the unaudited pro forma condensed combined financial statements contained elsewhere in this proxy statement, and Epimmune’s historical consolidated financial statements and the accompanying notes incorporated by reference into this proxy statement. The unaudited pro forma per share data is presented for informational purposes only and is not intended to represent or be indicative of the consolidated results of operations or financial condition of Epimmune that would have been reported had the transaction been completed as of the dates presented, and should not be taken as representative of future consolidated results of operations or financial condition of the combined company.
The IDM equivalent pro forma per share data is calculated by multiplying the pro forma combined per share amounts by the exchange ratio of 3.771865 shares of Epimmune common stock for each ordinary share of IDM.
| | | | | | | | | | | | | | | | | | |
| | | | | | | Pro Forma | |
| | | | | | |
| | | Historical | | | Epimmune | | | |
| | | | | | and IDM | | | IDM | |
| | | Epimmune | | | IDM | | | Combined | | | Equivalent | |
| | | | | | | | | | | | | |
| | | | | (Shares in thousands) | | | |
| Basic and diluted net loss per common share: | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2004 | | $ | (0.25 | ) | | $ | (2.37 | ) | | $ | (0.39 | ) | | $ | (1.47 | ) |
| | Three months ended March 31, 2005 | | $ | (0.15 | ) | | $ | (0.39 | ) | | $ | (0.09 | ) | | $ | (0.34 | ) |
| Shares used in computation of basic and diluted net loss per common share: | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2004 | | | 15,304 | | | | 13,649 | | | | 92,117 | | | | | |
| | Three months ended March 31, 2005 | | | 16,015 | | | | 15,694 | | | | 92,827 | | | | | |
| Book value per share as of: | | | | | | | | | | | | | | | | |
| | December 31, 2004 | | $ | 0.69 | | | $ | 2.71 | | | $ | 0.61 | | | $ | 2.30 | |
| | March 31, 2005 | | $ | 0.55 | | | $ | 2.21 | | | $ | 0.52 | | | $ | 1.96 | |
| Shares used in computation of book value per share: | | | | | | | | | | | | | | | | |
| | December 31, 2004 | | | 16,012 | | | | 15,694 | | | | 92,823 | | | | | |
| | March 31, 2005 | | | 16,024 | | | | 15,694 | | | | 92,835 | | | | | |
21
RISK FACTORS
The business in which we and IDM are currently engaged, and in which the combined company will be engaged following the Exchange, is rapidly changing and involves a high degree of risk. The combination of Epimmune and IDM also involves risks relating to the integration of the two companies as a multi-national biopharmaceutical company focused on research, clinical development, manufacturing and product development for cancer immunotherapeutics. We urge you to consider carefully the following risks before deciding whether to approve the proposals to be voted upon by our stockholders at the annual meeting. These factors should be considered in conjunction with the other information included in or incorporated by reference into this proxy statement, including the risks discussed in our Form 10-K/A for the year ended December 31, 2004.
Risks Related to the Exchange
We currently do not meet the Nasdaq National Market’s continued listing requirements and the combined company may be delisted after the consummation of the Exchange, which could reduce the liquidity of our common stock and adversely affect the combined company’s ability to raise additional necessary capital. In order to continue the trading of our common stock on the Nasdaq National Market following the Exchange, the combined company must meet the initial listing requirements of the Nasdaq National Market. To meet these requirements, the combined company must have, among other things, a minimum stockholders’ equity of $30 million and a minimum closing bid price of $5.00 per share. As of the date of this proxy statement, Epimmune alone would not meet these standards because of the market price of our common stock. In proposal 3, we are seeking stockholder approval to effect a one-for-four, one-for-five, one-for-six, one-for-seven, one-for-eight, one-for-nine or one-for-ten reverse stock split in connection with the closing of the Exchange. The intended purpose of the reverse stock split is to increase the market price of our common stock so that we will be able to satisfy the initial listing requirements of the Nasdaq National Market. The Exchange is conditioned upon, among other things, the receipt of Nasdaq approval of the quotation on the Nasdaq National Market of the shares of our common stock to be issued in the Exchange.
The combined company will have to meet the Nasdaq National Market’s continued listing requirements after the closing of the transactions under the Share Exchange Agreement to maintain the quotation of our common stock on the Nasdaq National Market. We and IDM cannot be certain that the combined company will be able to continue to qualify for listing on the Nasdaq National Market following its initial qualification. If the combined company fails to satisfy the Nasdaq National Market’s continued listing requirements at the time of our next quarterly report on Form 10-Q or at any other time in the future, our common stock may be delisted from the Nasdaq National Market.
On May 23, 2005, we filed a Current Report on Form 8-K reporting that we received a letter from the Nasdaq Stock Market which indicated that, based on the stockholders’ equity disclosed in our Form 10-Q/A for the period ended March 31, 2005, we do not comply with the minimum stockholders’ equity requirement for continued listing on the Nasdaq National Market. Pursuant to the Nasdaq staff’s request, we submitted to The Nasdaq National Market a specific plan to effect the Exchange as the way to achieve and sustain compliance with the minimum stockholders’ equity requirement. On June 20, 2005, we received a letter from the Nasdaq Stock Market indicating that our plan to achieve compliance with the minimum stockholders’ equity requirement was not accepted by the Nasdaq staff because, among other things, we will be unable to demonstrate compliance until the Exchange is complete and the Exchange is subject to stockholder approval and therefore is not definitive. On June 27, 2005, we filed a request for an appeal with the Nasdaq Listing Qualifications Panel and an oral hearing date has been set for July 21, 2005. During the appeal process and until a final determination is made with respect to our continued listing, our common stock will continue to trade on The Nasdaq National Market. As stated in our Form 10-Q/A for the period ended March 31, 2005, we believe there is a significant risk that we will not meet the Nasdaq stockholders’ equity requirement in the future if we do not complete the Exchange.
In addition, on May 26, 2005, we received notice from Nasdaq that, based on the closing bid price of our common stock for the 30 consecutive business days preceding the date of the letter, we did not comply with
22
the minimum $1.00 per share requirement for continued listing on the Nasdaq National Market. If the Exchange is consummated, we believe the combined company will meet the closing minimum bid per share requirement.
The delisting of our common stock may result in the trading of the stock on the Nasdaq SmallCap Market or the OTC Bulletin Board. Consequently, a delisting of the shares of our common stock from the Nasdaq National Market may reduce the liquidity of the combined company and adversely affect its ability to raise additional necessary capital.
Some of the directors and executive officers of Epimmune and IDM may have interests in the Exchange that are different from or in conflict with those of the shareholders of IDM and/or stockholders of Epimmune that may influence such directors and executive officers to support the approval of the Exchange. Some of the directors and officers of Epimmune and IDM participate in arrangements that provide them with interests in the Exchange that are different from those of the stockholders of Epimmune and/or the shareholders of IDM. These interests, which may influence these individuals to support the Exchange and the other transactions under the Share Exchange Agreement, include the following:
| | |
| | • | at the closing of the Exchange, certain executive officers and directors of IDM who are residents of the United States will receive substitute options exercisable to acquire an aggregate of approximately 593,700 shares of our common stock, based on the IDM stock options held by such executive officers and directors as of March 15, 2005; |
| |
|
| | • | at the closing of the Exchange, options granted in connection with a reduction in force of Epimmune in September 2003 to Emile Loria, our President and Chief Executive Officer, Robert De Vaere, our Vice President, Finance and Administration and Chief Financial Officer, and Mark Newman, our Vice President, Research and Development, will immediately vest in full, to the extent not already vested. As of June 29, 2005, the vesting of 48,564, 4,856 and 3,399 options held by Dr. Loria, Mr. DeVaere and Dr. Newman, respectively, would accelerate; |
|
| |
| | • | at the closing of the Exchange, Jean-Loup Romet-Lemonne, the President and Chief Executive Officer of IDM, Donald Drakeman and David Haselkorn, each a current director of IDM, Jean Deleage, Managing Director of Alta Partners, a shareholder of IDM, and Sylvie Grégoire and Robert Beck, two other individuals designated by IDM, will become members of the board of directors of the combined company, and Drs. Romet-Lemonne and Loria, and Mr. De Vaere, will be named executive officers of the combined company; |
| |
| | • | at the closing of the Exchange, Dr. Loria will receive a cash bonus equal to 12 months of his salary ($375,000) and each of Mr. De Vaere and Dr. Newman will receive a cash bonus equal to six months of their respective salaries ($117,500); |
| |
|
| | • | at the closing of the Exchange, Epimmune will extend the term of certain options granted to Howard E. (“Ted”) Greene, Jr., William Comer and Georges Hibon, each a current director of Epimmune who will resign from the Board as of the closing. As of June 29, 2005, 47,856, 49,284 and 34,167 options held by Mr. Greene, Dr. Comer and Mr. Hibon, respectively, would remain exercisable; |
|
| |
| | • | at the closing of the Exchange, Drs. Loria and Newman, and Mr. De Vaere will each receive an increase in annual base salary, retroactive to January 1, 2005, equal to $25,000, $10,000 and $20,000, respectively; |
| |
| | • | Epimmune has entered into new employment agreements with Dr. Romet-Lemonne and Dr. Loria, Mr. De Vaere and Dr. Newman, pursuant to which the employees will serve as our Chief Executive Officer, President and Chief Business Officer, Chief Financial Officer, and Vice President, Infectious Diseases, respectively, following the closing of the transactions under the Share Exchange Agreement. These employment agreements will become effective at the closing of the Exchange and will supersede in their entirety at such time the existing employment agreements and other compensatory arrangements with these employees. Please see“Agreements Related to the Exchange —Employment Agreements”for a description of the material terms and conditions of these employment agreements. |
23
There will be challenges involved in the integration of Epimmune and IDM and, as a result, the combined company may not realize the expected benefits of the Exchange. If the stockholders of the combined company are to realize the anticipated benefits of the Exchange, the operations of Epimmune and IDM must be integrated and combined efficiently. Neither we nor IDM can assure you that the integration will be successful or that the anticipated benefits of the Exchange will be fully realized. Similarly, neither we nor IDM can guarantee that the IDM shareholders will achieve greater value through their ownership of our common stock than they would have achieved as shareholders of IDM as a separate entity. The dedication of the combined company’s management resources to integration activities relating to the Exchange may detract attention from the day-to-day business of the combined company. The difficulties of combining the operations of both companies include, among others:
| | |
| | • | consolidating research and development operations; |
| |
| | • | retaining key personnel; |
| |
| | • | preserving the licensing, research and development, manufacturing, supply, collaboration and other important relationships of Epimmune and IDM; |
| |
| | • | motivating employees in light of organizational changes resulting from the Exchange; |
| |
| | • | combining corporate cultures and coordinating multi-national operations; and |
| |
| | • | minimizing the diversion of management’s attention from ongoing business concerns. |
It is possible that the combined company will be unable to integrate the two businesses so as to realize all of the benefits that each expects to result from the Exchange. Integration of operations may be difficult and may have unintended consequences. The diversion of attention of management from its current operations to integration efforts and any difficulties encountered in combining the operations could adversely affect the combined company’s ability to execute its strategy of commercializing its lead drug candidate and advancing the clinical development of its pipeline of immunotherapeutic products.
Risks Related to the Business of the Combined Company
IDM’s lead product candidate, Mepact, may never obtain regulatory approval. The results of a Phase III clinical trial for IDM’s lead product candidate, Mepact, for the treatment of osteosarcoma have been analyzed and were submitted to the U.S. Food and Drug Administration, referred to as the FDA, in 2004. This trial was conducted by Children’s Oncology Group under an Investigational New Drug Application, referred to as an IND, held by the National Cancer Institute, prior to IDM’s purchase of Mepact from Jenner Biotherapies, Inc. in 2003. IDM is in discussion with the FDA regarding the most expedient pathway for potential approval of Mepact. The combined company plans to request fast track designation and, possibly, accelerated approval for Mepact. IDM has also initiated a Protocol Assistance Request Process to facilitate feedback from the European Agency for the Evaluation of Medicinal Products, referred to as the EMEA, on the most expedient pathway for potential approval of Mepact in the European Union. However, the combined company may not receive necessary approvals from the FDA, the EMEA or similar drug regulatory agencies, collectively referred to as the regulatory agencies, for the marketing and commercialization of Mepact and/or may be required to conduct additional clinical trials. Even assuming that the combined company receives regulatory approval for Mepact, IDM does not expect such regulatory approval to occur before 2007 at the earliest. The FDA may not agree to grant fast track designation, which may delay the submission or approval process in the United States.
Currently, IDM does not have Mepact materials in its inventory that could be used in human trials or for sales (assuming receipt of the regulatory agencies’ approval of Mepact for commercialization, which may not occur). IDM has resumed manufacturing Mepact components by third-party suppliers based on the specifications and processes established during the Phase III trial. If IDM or the combined company fails to demonstrate, through extensive analytical testing and appropriate preclinical studies, that the new Mepact material produced by subcontractors is comparable to the materials used in the Phase III clinical trial,
24
additional clinical studies may also be required by the regulatory agencies in order to complete the comparability analysis.
The development of Mepact, the preparation of IDM’s or the combined company’s marketing approval applications to the FDA and the EMEA and stringent manufacturing requirements have required and will continue to require significant investments of IDM’s and the combined company’s time and money, as well as the focus and attention of its key personnel. As a result, if the combined company fails to receive regulatory approval for Mepact, its financial condition and results of operations will be significantly and adversely affected.
Even if the combined company receives regulatory approval for Mepact, it may not be able to market it successfully. The combined company expects that it will depend in the medium term on the commercialization of Mepact for the majority of its revenues, assuming that Mepact receives regulatory approval. Mepact is the only product candidate for which a biologic license application is being prepared. Any revenues generated will be limited by the number of patients with osteosarcoma, the combined company’s ability to obtain appropriate pricing and reimbursement for Mepact, and the effects of competition.
In particular, the combined company will face competition from existing therapies and, potentially, competition from any new future treatments. Mepact has received orphan drug designation in the United States and in Europe, which will provide the combined company with a seven-year period of exclusive marketing in the United States commencing on the date of FDA approval and a 10-year period of exclusive marketing in Europe commencing on the date of EMEA approval. This will apply only to osteosarcoma, the indication for which Mepact has been designated as an orphan product. However, the combined company may lose this marketing exclusivity should a new treatment be developed which is proven to be more effective than Mepact. In addition, although IDM’s and the combined company’s patents will protect the liposomal formulation of Mepact until 2005 in Europe and 2007 in the United States, with a possible extension until 2010 in Europe and 2012 in the United States, certain other patents covering the active ingredient in Mepact expired at the end of 2003. As a result, if a competitor develops a new formulation for Mepact, the combined company may face generic competition following the expiration of market exclusivity under the orphan drug designation, which the combined company expects to occur in 2014 with respect to the United States and 2017 with respect to Europe.
If the combined company is not able to successfully commercialize Mepact, it may not bring to market its other product candidates for several years, if ever, and its prospects will be harmed as a result.
The process of developing immunotherapeutic products requires significant research and development, preclinical testing and clinical trials, all of which are extremely expensive and time-consuming and may not result in a commercial product. Epimmune’s and IDM’s product candidates (other than Mepact) are at early stages of development and the combined company may fail to develop and successfully commercialize safe and effective treatments based on these products or other technology. For each product candidate, the combined company must demonstrate safety and efficacy in humans through extensive clinical testing, which is very expensive, can take many years, and has an uncertain outcome. The combined company may experience numerous unforeseen events during or as a result of the testing process that could delay or prevent testing or commercialization of the combined company’s products, including the following:
| | |
| | • | the results of preclinical studies may be inconclusive, or they may not be indicative of results that will be obtained in human clinical trials; |
| |
| | • | after reviewing test results, the combined company or its collaborators may abandon projects that the combined company might previously have believed to be promising; for example, in November 2000, Pharmacia Corporation terminated its research and collaboration with Epimmune for therapeutic vaccines based on Epimmune’s cancer epitopes, and Epimmune has borne the operating expenses and capital requirements of continued development of its therapeutic cancer vaccines; |
| |
| | • | the combined company, its collaborators or government regulators may suspend or terminate clinical trials if the participating subjects or patients are being exposed to unacceptable health risks; |
25
| | |
| | • | the combined company may have to delay clinical trials as a result of scheduling conflicts with participating clinicians and clinical institutions, or difficulties in identifying and enrolling patients who meet trial eligibility criteria; |
| |
| | • | safety and efficacy results attained in early human clinical trials may not be indicative of results that are obtained in later clinical trials; |
| |
| | • | the effects of the combined company’s immunotherapeutic product candidates may not be the desired effects or may include undesirable side effects or other characteristics that preclude regulatory approval or limit their commercial use, if ever approved; |
| |
| | • | enrollment in clinical trials for the combined company product candidates may be slower than anticipated, resulting in significant delays; and |
| |
| | • | the effects of the combined company’s product candidates on patients may not have the desired effects or may include undesirable side effects or other characteristics that may delay or preclude regulatory approval or limit their commercial use, if approved. |
The data collected from clinical trials may not be sufficient to support regulatory approval of any of the combined company’s products, and the regulatory agencies may not ultimately approve any of the combined company’s products for commercial sale, which will adversely affect the combined company’s business and prospects. If the combined company fails to commence or complete, or experiences delays in, any of its planned clinical trials, the combined company’s operating income, stock price and ability to conduct business as currently planned could be materially and adversely affected.
Some of our research and development programs are funded by the U.S. government and the U.S. government may not allocate funds for these programs in future fiscal years. Certain research and development related to our HIV, cancer and malaria programs are funded pursuant to multi-year grants and contracts from the U.S. government. Our prophylactic HIV vaccine program, malaria vaccine program and three cancer programs, which are aimed primarily at epitope identification and vaccine immunogenicity analysis, are exclusively funded by the U.S. government. During 2004, on a pro forma combined basis, these programs represented $5.7 million in revenue or approximately 38% of the total pro forma combined revenue. The U.S. government is under no obligation to, and may not, fund these programs over their full term, which would have a significant impact on the combined company’s ability to continue development of its HIV, cancer and malaria programs.
IDM’s principal source of revenues and cash receipts is a collaboration agreement under which its partner has limited obligations. The principal source of revenues and cash receipts for IDM is the July 2001 collaboration agreement between IDM and Sanofi-Aventis S.A., a Frenchsociété anonyme, referred to as Sanofi-Aventis. Payments from Sanofi-Aventis under this agreement represented 94%, 100% and 100% of IDM’s total revenues in 2002, 2003 and 2004, respectively. For 2004, on a pro forma combined basis, Sanofi-Aventis represented approximately 39% of the revenue of the combined company. Although Sanofi-Aventis has the option to jointly develop and commercialize up to 20 of IDM’s therapeutic products derived from the patient’s own white blood cells, referred to as cell drugs, over a 10-year period, to date, Sanofi-Aventis has exercised an option for only one product candidate, Uvidem. Under the collaboration agreement, Sanofi-Aventis has no obligation to participate in the development of additional cell drugs. If the combined company is not successful in developing commercially viable product candidates, Sanofi-Aventis may not elect to exercise additional options. If the combined company fails to meet further milestones in the clinical development of Uvidem, Sanofi-Aventis has no further milestone obligations with respect to Uvidem. Additionally, Sanofi-Aventis may terminate its participation in any given development program at any time without penalty and without affecting its unexercised options for other product candidates. If Sanofi-Aventis does not exercise additional options, or if the combined company is not successful in achieving additional development milestones for Uvidem, the combined company will not receive additional payments from Sanofi-Aventis and its prospects, revenues and operating cash flows will be significantly and negatively affected.
26
The revenues and operating results of the combined company are likely to fluctuate. IDM’s and Epimmune’s revenues and operating results have fluctuated in the past, and the revenues and operating results of the combined company are likely to continue to do so in the future. This is due to the non-recurring nature of these revenues, which are derived principally, with respect to IDM, from payments made under IDM’s collaboration agreement with Sanofi-Aventis and, with respect to Epimmune, from government grants and contracts. For example, IDM had only €21,000 in revenues in 2001 (of which €17,000 was from its collaboration agreement with Sanofi-Aventis), while IDM had €3.6 million in revenues in 2002 and €5.3 million in revenues in 2003 which were derived exclusively from the Sanofi-Aventis collaboration agreement. The combined company expects that its only sources of revenues until commercialization of its first immunotherapy product will be:
| | |
| | • | any payments from Sanofi-Aventis and any other collaborative partners; |
| |
| | • | any payments from future collaborative partners; |
| |
| | • | any government and European Union grants and contracts; and |
| |
| | • | investment income. |
These revenues have varied considerably from one period to another and may continue to do so, since they depend on the terms of the particular agreement or grant, or the performance of the particular investment. In addition, termination of any of these arrangements would have a significant impact on the combined company’s prospects, revenues and results of operations. As a result, IDM and Epimmune believe that revenues in any period may not be a reliable indicator of the combined company’s future performance. Deviations in the combined company’s results of operations from those expected by securities analysts or investors also could have a material adverse effect on the market price of our common stock.
Epimmune’s and IDM’s history of operating losses and the combined company’s expectation of continuing losses may hurt the combined company’s ability to reach profitability or continue operations. We and IDM have each experienced significant operating losses since its inception. Our cumulative net loss since inception, as of March 31, 2005 is $164.2 million. In 2004, 2003 and 2002, our net loss was $3.9 million, $7.1 million and $6.5 million, respectively. IDM’s cumulative net loss since inception, as of March 31, 2005, is€116.7 million. In 2004, 2003 and 2002, IDM’s net loss was€25.5 million,€16.2 million and€12.9 million, respectively. It is likely that the combined company will continue to incur substantial operating expenses and net operating losses for the foreseeable future, which may hurt its ability to continue operations. Neither we nor IDM has generated revenues from the commercialization of any product. All of our and IDM’s revenues to date have consisted of contract research and development revenues, license and milestone payments, research grants, certain asset divestitures and interest income. Substantially all of the revenues of the combined company for the foreseeable future are expected to result from similar sources. To achieve profitable operations, the combined company, alone or with collaborators, must successfully identify, develop, register and market proprietary products. The combined company is not expected to generate revenues from the commercialization of any product for at least two years. This assumes one or more regulatory agencies approve Mepact’s commercialization, which may not occur. The combined company may not be able to generate sufficient product revenue to become profitable. Even if it does achieve profitability, the combined company may not be able to sustain or increase its profitability on a quarterly or yearly basis.
The combined company’s substantial additional capital requirements and potentially limited access to financing may adversely affect its ability to develop products and fund its operations. The combined company will continue to spend substantial amounts on research and development, including amounts spent for manufacturing clinical supplies, conducting clinical trials for its product candidates, advancing development of certain sponsored and partnered programs and commercialization efforts for its Phase III product candidate, Mepact. Therefore, the combined company will need to raise additional funding. The combined company will not have committed external sources of funding and may not be able to obtain any additional funding, especially if volatile market conditions persist for biotechnology companies. If the combined company is unable to obtain additional funding, the combined company may be required to delay, reduce the scope of or eliminate one or more of the combined company’s research and development projects, sell certain of its assets
27
(including one or more of its drug programs or technologies), sell the combined company, or dissolve and liquidate all of its assets. The combined company’s future operational and capital requirements will depend on many factors, including:
| | |
| | • | whether the combined company is able to secure additional financing on favorable terms, or at all; |
| |
| | • | the costs associated with, and the success of, obtaining marketing approval for Mepact for the treatment of osteosarcoma in the United States, Europe and other jurisdictions and the timing of any such approval; |
| |
| | • | the success of the product launch and commercialization of Mepact, if ever; |
| |
| | • | the costs associated with the launch and the commercialization of Mepact in the United States, Europe and other jurisdictions upon obtaining marketing approval; |
| |
| | • | the costs associated with the combined company’s clinical trials for its product candidates, including IDM’s cell drugs and Epimmune’s HIV and lung cancer vaccine candidates; |
| |
| | • | progress with other preclinical testing and clinical trials in the future; |
| |
| | • | costs associated with integrating Epimmune and IDM, especially given the multi-national nature of the combined company; |
| |
| | • | the combined company’s ability to establish and maintain collaboration and license agreements and any government contracts and grants; |
| |
| | • | the actual revenue the combined company receives under its collaboration and license agreements; |
| |
| | • | the actual costs the combined company incurs under its collaboration agreement; |
| |
| | • | the time and costs involved in obtaining regulatory approvals for the combined company’s products; |
| |
| | • | the costs involved in filing, prosecuting, enforcing and defending patent claims and any other proprietary rights; |
| |
| | • | competing technological and market developments; and |
| |
| | • | the magnitude of the combined company’s immunotherapeutic product discovery and development programs. |
The combined company will likely seek additional funding through collaboration and license agreements, government research grants and/or equity or debt financings. In the event the combined company is able to obtain financing, it may not be on favorable terms. In addition, the combined company may not be able to enter into additional collaborations to reduce its funding requirements. If the combined company acquires funds by issuing securities, dilution to existing stockholders will result. If the combined company raises funds through additional collaborations and license agreements, it will likely have to relinquish some or all of the rights to its product candidates or technologies that it may have otherwise developed itself. If the combined company is unable to obtain funding, it may be required to engage in restructuring activities, cease development of some product candidates, delay or reduce the scope of its operations, sell the combined company or certain of its assets or technologies or cease operations.
If the combined company loses its key scientific and management personnel or is unable to attract and retain qualified personnel, it could delay or hurt the combined company’s research and product development efforts. The combined company will be highly dependent on the principal members of its scientific and management staff, including Dr. Jean-Loup Romet-Lemonne, CEO, Dr. Emile Loria, President and Chief Business Officer, Mr. Robert De Vaere, Chief Financial Officer, Dr. Mark Newman, Vice President, Infectious Diseases, Dr. Bonnie Mills, Vice President Regulatory and Irvine Site Manager, Mr. Guy Charles Fanneau de la Horie, Paris Site Manager, and Mr. Herve Duchesne de Lamotte, Vice President Finance, Europe. We have previously entered into employment contracts with Dr. Romet-Lemonne, Dr. Loria, Mr. De Vaere and Mr. Newman, which will become effective on the closing of the Exchange and which we believe provide them an incentive to remain as employees for at least twelve months following the closing
28
although there can be no assurance they will. We also expect to enter into employment agreements with Dr. Mills and Messrs. Fanneau de la Horie and Duchesne de Lamotte although there can be no assurance we will be successful and that even if we are, that such employment agreements will provide an adequate incentive for them to stay with the combined company. We are aware that Dr. Jean-Pierre Abastado, currently Chief Scientific Officer of IDM, will be leaving IDM prior to the closing of the Exchange to return to a research position with the French government following the expiration of his leave of absence from the French government. IDM is conducting a search for a replacement for Dr. Abastado. We do not believe that Dr. Abastado’s departure will have a significant impact on the combined company in the near-term as we expect our focus to be on clinical and product development which are not directed by Dr. Abastado. Neither Epimmune nor IDM has reason to believe that other key employees would leave either company as a result of the proposed transaction, independent of the proposed transaction or through retirement. Neither Epimmune nor IDM maintains key person life insurance on the life of any employee. The combined company’s ability to develop immunotherapeutic products and vaccines, identify epitopes, and achieve its other business objectives also will depend in part on the continued service of its key scientific and management personnel and its ability to identify, hire and retain additional qualified personnel. The combined company does not have employment agreements with the non-management scientific personnel. There is intense competition for qualified personnel in biochemistry, molecular biology, immunology and other areas of the combined company’s proposed activities, and the combined company may not be able to continue to attract and retain such personnel necessary for the development of its business. Because of the intense competition for qualified personnel among technology-based businesses, particularly in the Southern California area, the combined company may not be successful in adding technical personnel as needed to meet the staffing requirements of additional collaborative relationships. The combined company’s failure to attract and retain key personnel could delay or be significantly detrimental to its product development programs and could cause its stock price to decline.
The combined company may experience difficulties managing its growth, which could adversely affect its results of operations. It is expected that the combined company will grow in certain areas of its operations as it develops and, assuming receipt of the necessary regulatory approvals, markets its products. In particular, the combined company will need to expand its sales and marketing capabilities to support its plans to market Mepact. The combined company will therefore need to recruit personnel, particularly sales and marketing personnel, and expand its capabilities, which may strain its managerial, operational, financial and other resources. To compete effectively and manage its growth, the combined company must:
| | |
| | • | train, manage, motivate and retain a growing employee base, particularly given the combined company’s operations in both California and France; |
| |
| | • | accurately forecast demand for, and revenues from, its product candidates, particularly Mepact; and |
| |
| | • | expand existing operational, financial and management information systems to support its development and planned commercialization activities and the multiple locations of its offices. |
The combined company’s failure to manage these challenges effectively could harm its business.
Unexpected or undesirable side effects or other characteristics of the combined company’s business and technology may delay or otherwise hurt the development of the combined company’s product candidates, or may expose the combined company to significant liability that could cause it to incur significant costs. Certain immunotherapy products may produce serious side effects. Many antibody-based therapies have shown toxicity in clinical trials. If the combined company’s immunotherapy product candidates prove to be ineffective, or if they result in unacceptable side effects, the combined company will not be able to successfully commercialize them and its prospects will be significantly and adversely affected. In addition, there may be side effects in our or IDM’s current clinical trials or the combined company’s future clinical trials that may be discovered only after long-term exposure, even though our, IDM’s or the combined company’s safety tests may indicate favorable results. The combined company may also encounter technological challenges relating to these technologies and applications in its research and development programs that it may not be able to resolve. Any such unexpected side effects or technological challenges may delay or otherwise adversely affect the development, regulatory approval or commercialization of the combined company’s drug candidates.
29
The combined company’s business will expose it to potential product liability risks that are inherent in the testing, manufacturing and marketing of human therapeutic products. While both Epimmune and IDM currently have product liability insurance for early stage clinical trials, neither Epimmune nor IDM can be sure that the combined company will be able to maintain such insurance on acceptable terms or obtain acceptable insurance as such products progress through product development and commercialization, or that the combined company’s insurance will provide adequate coverage against potential liabilities, either in human clinical trials or following commercialization of any products it may develop.
Epimmune has not yet sought commitments for product liability insurance for the combined company. Epimmune believes that the product liability insurance currently maintained by Epimmune and IDM separately will remain in force after the combination and Epimmune and IDM expect to consolidate the insurance programs of the separate companies as part of the ongoing integration planning and efforts.
Adverse publicity regarding the safety or side effects of the technology approach or products of others could negatively impact the combined company and cause the price of our common stock to fall. Despite any favorable safety tests that may be completed with respect to the combined company’s product candidates, adverse publicity regarding immunotherapeutic products or other products being developed or marketed by others could negatively affect the combined company. If other researchers’ studies raise or substantiate concerns over the safety or side effects of the combined company’s technology approach or product development efforts generally, the combined company’s reputation and public support for its clinical trials or products could be harmed, which would adversely impact its business and could cause the price of our common stock to fall.
The combined company’s treatment approach may not prove effective. IDM’s immunotherapeutic treatment approach, which the combined company is expected to continue as well, is largely untested. To date, only a limited number of immunotherapeutic antibody-based and vaccine-based products designed to fight cancer have been approved for commercialization, and for only a few specific types of cancer. The basis for most immunotherapeutic treatment approaches being developed for the treatment of cancer is the discovery that cancer cells express more of certain proteins (known as antigens) on their surfaces, which may allow them to be distinguished from normal cells. Immunotherapy is designed either to manipulate the body’s immune cells to target antigens and destroy the cancer cells that overexpress them or to activate the body’s immune system generally. However, immunotherapy has failed in the past for a number of reasons, including:
| | |
| | • | the targeted antigens are not sufficiently different from those normal cells to cause an immune reaction; |
| |
| | • | the tumor cells do not express the targeted antigen at all or in sufficient quantities to be recognized by immune system cells, such as T cells or macrophages; |
| |
| | • | the immune response provoked by the immunotherapeutic agent is not strong enough to destroy the cancer; or |
| |
| | • | cancer cells may, through various biochemical mechanisms, escape an immune response. |
Our strategy, which the combined company is expected to continue, involves identifying multiple epitopes in order to create our vaccines. If the combined company is unable to identify the correct epitopes, or to combine them in the correct manner, to stimulate desired immune responses, it may never develop a vaccine that is safe or effective in any of the indications that it is pursuing.
If the combined company cannot enter into and maintain strategic collaborations on acceptable terms in the future, it may not be able to develop products in markets where it would be too costly or complex to do so on its own. The combined company will need to enter into and maintain collaborative arrangements with pharmaceutical and biotechnology companies or other strategic partners both for development and for commercialization of potential products in markets where it would be too costly or complex to do so on its own. Currently, our only collaborations are with Innogenetics and Bavarian Nordic. IDM’s principal source of revenues and operating cash flows is its July 2001 collaboration agreement with Sanofi-Aventis. If the combined company is not able to maintain existing strategic collaborations and enter into and maintain additional research and development collaborations or other collaborations in the future on acceptable terms,
30
it may be forced to abandon development and commercialization of some product candidates and its business will be harmed.
If the combined company’s collaboration or license arrangements are unsuccessful, the combined company’s revenues and product development may be limited. The combined company’s collaborations and license arrangements generally pose the following risks:
| | |
| | • | collaborators and licensees may not pursue further development and commercialization of potential products resulting from the combined company’s collaborations or may elect not to renew research and development programs; |
| |
| | • | collaborators and licensees may delay clinical trials, underfund a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require new formulation of a product candidate for clinical testing; |
| |
| | • | expected revenue might not be generated because milestones may not be achieved and product candidates may not be developed; |
| |
| | • | collaborators and licensees could independently develop, or develop with third parties, products that could compete with the combined company’s future products; |
| |
| | • | the terms of the combined company’s contracts with current or future collaborators and licensees may not be favorable to the combined company in the future; |
| |
| | • | a collaborator or licensee with marketing and distribution rights to one or more of combined company’s products may not commit enough resources to the marketing and distribution of the combined company’s products, limiting the combined company’s potential revenues from the commercialization of a product; |
| |
| | • | disputes may arise delaying or terminating the research, development or commercialization of the combined company’s product candidates, or result in significant and costly litigation or arbitration; and |
| |
| | • | collaborations and licensee arrangements may be terminated and the combined company will experience increased operating expenses and capital requirements if it elects to pursue further development of the product candidate. For example, in March 1999, Epimmune halted clinical trials of Cylexin, a cell adhesion inhibitor then being developed by the company, based on results in a Phase II/ III clinical trial indicating that the drug was not effective. |
The combined company may not be able to obtain licenses to technology that is necessary to develop products. The combined company may be required to enter into licenses or other collaborations with third parties in order to access technology that is necessary to successfully develop certain of the combined company’s products. The combined company may not successfully negotiate acceptable licenses or other collaborative arrangements that will allow it to access such technologies. In addition, certain license agreements to which IDM is a party may not be transferred to the combined company without the licensor’s consent and the combined company may not be able to obtain such consent. If the combined company cannot obtain and maintain license rights on acceptable terms to access necessary technologies, it may be prevented from developing some product candidates. In addition, any technologies accessed through such licenses or other collaborations may not help the combined company achieve its product development goals.
The combined company’s supplies of certain materials necessary to its business may be limited and key raw materials may be scarce. IDM and Epimmune have entered into several licensing and collaboration arrangements for the supply of various materials, chemical compounds, antibodies and antigens that are necessary to manufacture its product candidates. For example, IDM relies on external suppliers for the production of IL-13, which is used in the manufacturing of its dendritophage product candidates. IL-13 is an inherently scarce raw material. IDM believes that it currently possesses enough IL-13 for its short- to medium-term needs. However, once IDM’s dendritophage product candidates enter into Phase III clinical trials, IDM will require a supply of IL-13 that conforms to Good Manufacturing Practices, or clinical grade IL-13. In 2003, IDM entered into an IL-13 Development and Manufacturing Agreement with
31
Biotecnol, S.A., a Portuguese company, aimed at developing a clinical grade IL-13 manufacturing process. Under the agreement, Biotecnol has agreed to complete development of clinical grade IL-13 according to a program of GMP manufacturing, control, testing and release, as defined with advice from Sanofi-Aventis, and IDM has agreed to provide financial support payable upon the occurrence of certain milestone events and based on the decisions of the parties to continue development. Once development of the IL-13 production process is completed, Biotecnol will oversee the ongoing management of the outsourcing of manufacturing and release of the finished product for a renewable five-year period beginning with the release of the first finished product batch. Either party may terminate the IL-13 Development and Manufacturing Agreement on the basis of a recommendation from a joint management committee if certain program specifications and targets are not met and/or before manufacturing of the first product batch is initiated. IDM is also entitled to terminate the IL-13 Development and Manufacturing Agreement at any time during the manufacturing period if the finished product stability does not reach two years. Biotecnol is entitled to terminate the process performance at any time by providing 18 months’ prior notice. In addition, either IDM or Biotecnol may terminate the agreement with immediate effect upon written notice on or at any time after the occurrence of certain events (e.g., breach of contract, liquidation, etc.). There are no assurances that Biotecnol will successfully manufacture clinical grade IL-13, or that it will be able to produce sufficient quantities of clinical grade IL-13 if it is successful. Without a sufficient supply of clinical grade IL-13, IDM would not be able to conduct Phase III clinical trials of its dendritophage product candidates.
With respect to our current business only, Epimmune has one sole source supplier for a component of its EP-2101 NSCLC vaccine. This material is not supplied under a long-term contract but Epimmune has not had difficulties obtaining the material in a timely manner in the past. The supplier also provides the same material to other customers and Epimmune does not believe it is at risk of losing this supplier. Epimmune has several other suppliers which are currently its sole sources for the materials they supply, though Epimmune believes alternate suppliers could be developed in a reasonable period of time. Epimmune is not aware of any scarcity of raw materials used in any of its products.
Supply of any of these products could be limited, interrupted or restricted in certain geographic regions. In such a case, the combined company may not be able to obtain from other manufacturers alternative materials, chemical compounds, components, antibodies or antigens of acceptable quality, in commercial quantities and at an acceptable cost. If the key suppliers or manufacturers of the combined company fail to perform, or if the supply of products or materials is limited or interrupted, the combined company may not be able to produce or market its products on a timely and competitive basis.
If the combined company and/or its collaborators cannot cost-effectively manufacture its immunotherapeutic product candidates in commercial quantities or for clinical trials in compliance with regulatory requirements, the combined company and/or its collaborators may not be able to successfully commercialize the products. Neither Epimmune nor IDM has commercialized any products, and the combined company will not have the experience, resources or facilities to manufacture therapeutic vaccines and other products on a commercial scale. The combined company will not be able to commercialize any products and earn product revenues unless the combined company or its collaborators demonstrate the ability to manufacture commercial quantities in accordance with regulatory requirements. Among the other requirements for regulatory approval is the requirement that prospective manufacturers conform to the GMP requirements of the respective regulatory agencies specifically for biological drugs, as well as for other drugs. In complying with GMP requirements, manufacturers must continue to expend time, money and effort in production, record keeping and quality control to assure that the product meets applicable specifications and other requirements.
IDM is currently dependent on third parties for the production and testing of its lead product candidate, Mepact and Mepact components. The combined company may not be able to enter into future subcontracting agreements for the commercial supply of Mepact or any of its other products, or to do so on terms that are acceptable to it. If the combined company is unable to enter into acceptable subcontracting agreements, it will not be able to successfully commercialize Mepact or any of its other products. In addition, reliance on third-
32
party manufacturers poses additional risks which the combined company would not face if it produced Mepact or any of its other products itself, including:
| | |
| | • | non-compliance by these third parties with regulatory and quality control standards; |
| |
| | • | breach by these third parties of their agreements with the combined company; and |
| |
| | • | termination or nonrenewal of these agreements for reasons beyond the combined company’s control. |
If products manufactured by third-party suppliers fail to comply with regulatory standards, sanctions could be imposed on the combined company. These sanctions could include fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of the combined company’s product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of its products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect the combined company’s business. If the combined company changes manufacturers for Mepact, it will be required to undergo revalidation of the manufacturing process and procedures in accordance with GMP. This revalidation could be costly and time-consuming and require the attention of the key personnel of the combined company. If revalidation is not successful, the combined company may be forced to look for an alternative supplier, which could delay the marketing of Mepact or increase its manufacturing costs. The combined company will also need to demonstrate through preclinical studies that Mepact as produced by the new manufacturers is comparable to the materials used in the Phase III clinical trial. New clinical studies may also be required if comparability cannot be fully demonstrated by preclinical studies.
IDM prepares its cell drugs in its own facilities for purposes of its research and development programs, preclinical testing and clinical trials. IDM currently has one clinical scale facility for cell drug manufacturing in Paris, France and a second one in Irvine, California. However, it lacks experience in manufacturing its cell drugs on a large scale. It is expected that the combined company will construct commercial scale manufacturing plants in Europe and the United States in the future, but it may not be able to successfully carry out such construction. As a result, the combined company may not be able to manufacture its cell drugs on acceptable economic terms or on a sufficient scale for its needs.
The combined company cannot be sure that it can manufacture, either on its own or through contracts with outside parties, its immunotherapeutic product candidates at a cost or in quantities that are commercially viable.
The combined company will be subject to extensive and uncertain government regulation and may not be able to obtain necessary regulatory approvals. To date, none of the potential products of Epimmune or IDM has been approved for marketing by any regulatory agencies. The combined company cannot be sure that it will receive the regulatory approvals necessary to commercialize any of its potential products. The combined company’s product candidates will be subject to extensive and rigorous governmental regulation, and the applicable regulatory requirements are uncertain and subject to change. The FDA and the EMEA maintain rigorous requirements for, among other things, the research and development, preclinical testing and clinical trials, manufacture, safety, efficacy, record keeping, labeling, marketing, sale and distribution of therapeutic products. In particular, the United States is the world’s largest pharmaceutical market. Without FDA approval, the combined company would be unable to access the U.S. market.
The regulatory process for approval of new therapeutic products will require the combined company to submit extensive product characterization, manufacturing and control, and preclinical and clinical data and supportive information for each indication in order to establish the potential product’s safety and effectiveness. It may also involve ongoing requirements for post-marketing studies, as well as manufacturing and quality control requirements on a continuous basis. For example, IDM modified the manufacturing process and characteristics of Osidem and developed its second-generation product candidate, Osidem-2, in order to comply with regulatory requirements for manufacturing.
To market any drug products outside of the United States and the European Union, the combined company and its collaborators will also be subject to numerous and varying foreign regulatory requirements, implemented by foreign health authorities, governing the design and conduct of human clinical trials and
33
marketing approval for biologics or other drug products. The approval procedure varies among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA or EMEA approval. The foreign regulatory approval processes usually include all of the risks associated with obtaining FDA or EMEA approval, and approval by the FDA does not ensure approval by the health authorities of any other country, nor does the approval by the EMEA or the foreign health authorities ensure approval by the FDA.
The lengthy approval process and uncertainty of U.S. and European government regulatory requirements may impair the combined company’s ability to develop, manufacture and sell any immunotherapeutic products in the United States or the European Union. The combined company and its collaborators will not be able to commercialize the combined company’s immunotherapeutic products in the United States or the European Union if the combined company does not receive FDA and EMEA approval, respectively, to market the combined company’s products. The regulatory process for new drug products, including the required preclinical studies and clinical testing, is lengthy, uncertain and expensive. The combined company and its collaborators may not receive necessary FDA or EMEA clearances for any of the combined company’s immunotherapeutic products in a timely manner, or at all. Once the products are approved, the combined company will be subject to the continuing requirements of the FDA and/or the EMEA. Noncompliance with initial or continuing requirements can result in, among other things:
| | |
| | • | fines and penalties; |
| |
| | • | injunctions; |
| |
| | • | seizure of products; |
| |
| | • | total or partial suspension of product marketing; |
| |
| | • | failure of a regulatory agency to grant a new drug application; |
| |
| | • | withdrawal of marketing approvals; and |
| |
| | • | criminal prosecution. |
The length of the clinical trial process and the number of patients the FDA or the EMEA will require to be enrolled in clinical trials in order to establish the safety and efficacy of the combined company’s products are uncertain. In addition, the combined company’s clinical studies may not provide the FDA or EMEA with sufficient clinical data to permit approval of a marketing approval application, even though the combined company or its collaborators believe it is conducting the right studies based on the protocol. The regulatory agencies or the combined company and its collaborators may decide to discontinue or suspend clinical trials at any time if the subjects or patients who are participating in such trials are being exposed to unacceptable health risks or if the results show no or limited benefit in patients treated with the immunotherapeutic product compared to patients in the control group.
Regulatory requirements are evolving and uncertain. Future U.S. or European laws and regulations could also prevent or delay regulatory approval of the combined company’s products. Even if the combined company obtains commercial regulatory approvals, the approvals may significantly limit the indicated uses for which the combined company may market its products.
Even if the combined company obtains regulatory approval for its products, it may be required to perform additional clinical trials or change the labeling of its products if the combined company or others identify side effects after its products are on the market, which could harm sales of the affected products. If the combined company or others identify adverse side effects after any of the combined company’s products are on the market, or if manufacturing problems occur:
| | |
| | • | regulatory approval may be withdrawn; |
| |
| | • | reformulation of the combined company’s products, additional clinical trials, changes in labeling of the combined company’s products or changes to or re-approvals of the combined company’s manufacturing facilities may be required; |
34
| | |
| | • | sales of the affected products may drop significantly; |
| |
| | • | the combined company’s reputation in the marketplace may suffer; and |
| |
| | • | lawsuits, including costly and lengthy class action suits, may be brought against the combined company. |
Any of the above occurrences could halt or reduce sales of the affected products or could increase the costs and expenses of commercializing and marketing these products, which would materially and adversely affect the combined company’s business, operations, financial results and prospects.
The combined company may not be able to commercialize products under development by Epimmune or IDM if those products infringe claims in existing patents or patents that have not yet issued, and this would materially harm the combined company’s ability to operate. As is typical in the biotechnology industry, the combined company’s commercial success will depend in part on its ability to avoid infringing patents issued to others or breaching the technology licenses upon which it might base its products. Epimmune and IDM are aware of patents issued to others that contain claims that may cover certain aspects of Epimmune’s or IDM’s technologies or those of their collaborators, including, with respect to Epimmune, cancer vaccine epitopes, HIV vaccine epitopes, and methods for delivering DNA vaccines to patients and, with respect to both Epimmune and IDM, peptide vaccines. Epimmune and IDM believe that licenses to these patents are not necessary or could be obtained in order to pursue the development or commercialization of its respective product candidates. If the combined company is required to obtain a license under one or more of these patents to practice certain aspects of its immunotherapy technologies in Europe and in the United States, such a license may not be available on commercially reasonable terms, if at all. If the combined company fails to obtain a license on acceptable terms to any technology that it needs in order to develop or commercialize its products, or to develop an alternative product that does not infringe on the patent rights of others, the combined company would be prevented from commercializing its products and its business and prospects would be harmed.
The combined company’s failure to obtain issued patents and, consequently, to protect its proprietary technology, could hurt its competitive position. The combined company’s success will depend in part on its ability to obtain and enforce claims in its patents directed to the combined company’s products, technologies and processes, both in the United States and in other countries. Although each of Epimmune and IDM has issued patents and has filed various patent applications, each company’s patent position is highly uncertain and involves complex legal and factual questions. Legal standards relating to patentability, validity and scope of patent claims in epitope identification, immunotherapy and other aspects of the combined company’s technology field are still evolving. Patents issued, or which may be issued, to Epimmune, IDM or the combined company may not be sufficiently broad to protect the combined company’s immunotherapy technologies and processes and patents may issue from any of Epimmune’s, IDM’s or the combined company’s patent applications. For example, even though Epimmune’s patent portfolio includes patent applications with claims directed to peptide epitopes and methods of utilizing sequence motifs to identify peptide epitopes and IDM’s portfolio includes patent applications with claims directed to vaccines derived from blood monocytes, neither IDM nor Epimmune can assure you of the breadth of claims that will be allowed or that may issue in future patents. Other risks and uncertainties that the combined company will face with respect to its patents and patent applications include the following:
| | |
| | • | the pending patent applications Epimmune or IDM has filed or to which they have exclusive rights may not result in issued patents or may take longer than the combined company expects to result in issued patents; |
| |
| | • | the allowed claims of any patents that issue may not provide meaningful protection; |
| |
| | • | the combined company may be unable to develop additional proprietary technologies that are patentable; |
| |
| | • | the patents licensed or issued to Epimmune, IDM or the combined company may not provide a competitive advantage; |
35
| | |
| | • | other companies may challenge patents licensed or issued to Epimmune, IDM or the combined company; |
| |
| | • | disputes may arise regarding inventions and corresponding ownership rights in inventions and know-how resulting from the joint creation or use of intellectual property by Epimmune, IDM or the combined company and their respective licensors or collaborators; and |
| |
| | • | other companies may design around the technologies patented by Epimmune, IDM or the combined company. |
If the combined company is unable to compete effectively in the highly competitive biotechnology industry, its business will fail. The market for cancer therapeutics is characterized by rapidly evolving technology, an emphasis on proprietary products and intense competition. Many entities, including pharmaceutical and biotechnology companies, academic institutions and other research organizations, are actively engaged in the discovery, research and development of immunotherapy and other products for the treatment of cancer and other diseases. Should any of the combined company’s product candidates be approved for marketing and launched, they would compete against a range of established therapies.
Our vaccines under development address a range of cancer and infectious disease markets. The competition in these markets is extremely formidable. There are 27 drugs currently approved in the United States for HIV and, according to a PhRMA 2003 report on pharmaceutical drug development, there were 83 new product candidates in clinical development for HIV and related conditions, including 15 HIV vaccines. Epimmune’s potential products would also compete with a range of novel therapies either under development or recently introduced onto the market, including monoclonal antibodies, cancer vaccines and cell therapy, gene therapy, angiogenesis inhibitors and signal transduction inhibitors. The strongest competition is likely to come from other immunotherapies (such as monoclonal antibodies) and, to a lesser extent, from chemotherapeutic agents and hormonal therapy.
In addition, according to the PhRMA 2003 report, there were 395 new product candidates in clinical development for the treatment of cancer, and at least 30 companies were developing more than 50 vaccines against various cancers. An important factor in competition may be the timing of market introduction of the combined company’s vaccines and competitive products. Accordingly, the relative speed with which the combined company can develop vaccines, complete the clinical trials and approval processes and supply commercial quantities of the vaccines to the market is expected to be an important competitive factor. The combined company expects that competition among products approved for sale will be based, among other things, on product effectiveness, safety, reliability, availability, price and patent position.
Many of the companies developing competing technologies and products have significantly greater financial resources and expertise in research and development, manufacturing, preclinical and clinical development, obtaining regulatory approvals and marketing than the combined company will, and the combined company may not be able to compete effectively against them. Large pharmaceutical companies in particular, such as Bristol-Myers Squibb, Roche, Novartis and AstraZeneca, have substantially more extensive experience in clinical testing and in obtaining regulatory approvals than the combined company. Smaller or early-stage companies, most importantly those in the immunotherapy field such as Dendreon or CancerVax, may also prove to be significant competitors. These companies may become even stronger competitors through collaborative arrangements with large companies. All of these companies may compete with the combined company to acquire rights to promising antibodies, antigens and other complementary technologies.
Litigation regarding intellectual property rights owned or used by Epimmune, IDM or the combined company may be costly and time-consuming. Litigation may be necessary to enforce the claims in any patents issued to Epimmune, IDM or the combined company or to defend against any claims of infringement of patents owned by third parties that are asserted against Epimmune, IDM or the combined company. In addition, the combined company may have to participate in one or more interference proceedings declared by the United States Patent and Trademark Office or other foreign patent governing authorities, which could result in substantial costs to determine the priority of inventions.
36
If the combined company becomes involved in litigation or interference proceedings, it may incur substantial expense, and the proceedings may divert the attention of the combined company’s technical and management personnel, even if the combined company ultimately prevails. An adverse determination in proceedings of this type could subject the combined company to significant liabilities, allow the combined company’s competitors to market competitive products without obtaining a license from the combined company, prohibit the combined company from marketing its products or require the combined company to seek licenses from third parties that may not be available on commercially reasonable terms, if at all. If the combined company cannot obtain such licenses, it may be restricted or prevented from developing and commercializing its product candidates.
The enforcement, defense and prosecution of intellectual property rights, including the United States Patent and Trademark Office’s and related foreign patent offices’ interference proceedings, and related legal and administrative proceedings in the United States and elsewhere involve complex legal and factual questions. As a result, these proceedings are costly and time-consuming, and their outcome is uncertain. Litigation may be necessary to:
| | |
| | • | assert against others or defend the combined company against claims of infringement; |
| |
| | • | enforce patents owned by, or licensed to, Epimmune, IDM or the combined company from another party; |
| |
| | • | protect Epimmune’s, IDM’s or the combined company’s trade secrets or know-how; or |
| |
| | • | determine the enforceability, scope and validity of the proprietary rights of Epimmune, IDM, the combined company or others. |
If the combined company is unable to protect its trade secrets, it may be unable to protect from competitors its interests in proprietary know-how that is not patentable or for which it has elected not to seek patent protection. The combined company’s competitive position will depend in part on its ability to protect trade secrets that are not patentable or for which it has elected not to seek patent protection. To protect its trade secrets, Epimmune and IDM rely primarily on confidentiality agreements with their collaborative partners, employees and consultants, and the combined company will continue to rely on such agreements. Nevertheless, the combined company’s collaborative partners, employees and consultants may breach these agreements and the combined company may be unable to enforce these agreements. In addition, other companies may develop similar or alternative technologies, methods or products or duplicate the combined company’s technologies, methods, vaccines or immunotherapy products that are not protected by the combined company’s patents or otherwise obtain and use information that the combined company regards as proprietary, and the combined company may not have adequate remedies in such event. Any material leak of the combined company’s confidential information into the public domain or to third parties could harm its competitive position.
Both Epimmune and IDM out-license technology outside of their core area of focus, and these licensees may not develop any products using such technology, which may limit the combined company’s revenue. Both Epimmune and IDM have licensed to third parties some of their respective technology in markets that they are not pursuing themselves or with their respective collaborators. If these licensees are not successful in developing and commercializing products using its technology, the combined company’s revenues would be limited. The combined company’s licensees may pursue alternative technologies or develop alternative products either on their own or in collaboration with others in competition with products developed under licenses or collaborations with the combined company.
Some of the combined company’s programs will be funded by the U.S. government and, therefore, the U.S. government may have rights to certain of the combined company’s technology and could require the combined company to grant licenses of its inventions to third parties. Epimmune currently funds, and the combined company expects to fund, certain of its research and development related to its HIV, cancer and malaria programs pursuant to grants and contracts from the U.S. government. As a result of these grants and contracts, the U.S. government has certain rights in the inventions, including a non-inclusive, non-transferable, irrevocable license to practice the invention throughout the world. Our failure to disclose, file,
37
prosecute patent applications or elect to retain title to such inventions may result in conveyance of title to the United States. In addition, the U.S. government may require the combined company to grant to a third party an exclusive license to any inventions resulting from the grant if the U.S. government determines that the combined company has not taken adequate steps to commercialize inventions, or for public health or safety needs.
Successful commercialization of future products of the combined company will depend on its ability to gain acceptance by the medical community. If the combined company succeeds in receiving regulatory approval and launching its product candidates based on its immunotherapeutic technology, it will take time to gain market acceptance with the medical community, including health care providers, patients and third-party payers. The degree of market acceptance will depend on several factors, including:
| | |
| | • | the extent to which its therapeutic product candidates are demonstrated to be safe and effective in clinical trials; |
| |
| | • | the existence of adverse side effects; |
| |
| | • | convenience and ease of administration; |
| |
| | • | the success of sales, marketing and public relations efforts; |
| |
| | • | the availability of alternative treatments; |
| |
| | • | competitive pricing; |
| |
| | • | the reimbursement policies of governments and other third parties; |
| |
| | • | effective implementation of a publications strategy; and |
| |
| | • | garnering support from well-respected external advocates. |
If the combined company’s products are not accepted by the market or only receive limited market acceptance, the combined company’s business and prospects will be adversely affected.
The combined company’s use of hazardous materials could expose it to significant costs. The combined company’s research and development processes will involve the controlled storage, use and disposal of hazardous materials, chemicals and radioactive compounds. The combined company will be subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and some waste products. The risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of an accident, the combined company could be held liable for any damages that result, and any liability could exceed its resources. Compliance with environmental laws and regulations in the future may entail significant costs and the combined company’s ability to conduct research and development activities may be harmed by current or future environmental laws or regulations. The combined company anticipates carrying liability insurance for contamination or injury resulting from the use of hazardous materials.
Examples of hazardous materials Epimmune currently uses in its business include flammable liquids and solids, Iodine 125 which is a radioactive material, carcinogens and reproductive toxins such as Chloroform and Formaldehyde and biological products and waste such as blood products from clinical samples. Personal injury resulting from the use of hazardous materials is covered up to the limit of Epimmune’s workers’ compensation insurance which is presently one million dollars. Contamination clean-up resulting from an accident involving hazardous materials would be covered to the limit of Epimmune’s property insurance, with certain exclusions, which is presently $250,000.
IDM currently uses minimal amounts of flammable liquids and solids, as well as Chromium-51, a radioactive material, in a very small quantity. IDM generates waste such as blood products from clinical samples. All samples are prescreened prior to arrival and usage at the facility. Personal injury resulting from the use of hazardous materials is covered up to the limit of IDM’s workers’ compensation insurance, which is one million dollars. IDM has insurance in the amount of $250,000 for property damage and/or business interruption due to contamination; $25,000 for an offsite contamination problem; $50,000 for clean up as a
38
result of contamination; and $25,000 for business interruption, all of which are included in the general liability policy. The liability of the combined company for personal injury or hazardous waste clean up and remediation may not be covered by these insurance policies or the costs may exceed policy limits.
The combined company’s financial results may be adversely affected by fluctuations in foreign currency exchange rates. The combined company will be exposed to currency exchange risk with respect to the U.S. dollar in relation to the euro, because a portion of its operating expenses will be incurred in euros. This exposure may increase if the combined company expands its operations in Europe. Neither Epimmune nor IDM has entered into any hedging arrangements to protect its business against certain currency fluctuations. The combined company will monitor changes in its exposure to exchange rate risk that result from changes in its situation. If the combined company does not enter into effective hedging arrangements in the future, its results of operations and prospects could be materially and adversely affected.
Risks Related to Epimmune’s Common Stock
The volatility of the price of our common stock before and after the closing of the transactions under the Share Exchange Agreement may adversely affect stockholders. The market prices for securities of biotechnology companies, including our common stock, have historically been highly volatile, and the market from time to time has experienced significant price and volume fluctuations that are not necessarily related to the operating performance of such companies. Neither we nor IDM can predict how the market will react to the proposed Exchange and how the Exchange and exchange ratio may impact the market prices of our common stock before or after the closing of the transactions under the Share Exchange Agreement. From January 1, 2004 through June 29, 2005, the closing stock price of our common stock has ranged from $0.66 to $2.66 and has been and will continue to be influenced by general market and industry conditions. In addition, the following factors may have a significant effect on the market price of our common stock:
| | |
| | • | whether the combined company is able to secure additional financing on favorable terms, or at all; |
| |
| | • | announcements of technological innovations or new commercial immunotherapeutic products by the combined company or others; |
| |
| | • | governmental regulation that affects the biotechnology and pharmaceutical industries in general or the combined company in particular; |
| |
| | • | developments in patent or other proprietary rights by the combined company; |
| |
| | • | receipt of funding by the combined company under collaboration and license agreements and government grants; |
| |
| | • | developments in, or termination of, the combined company’s relationships with its collaborators and licensees; |
| |
| | • | public concern as to the clinical results and/or the safety of drugs developed by the combined company or others; and |
| |
| | • | announcements related to the sale of our common stock or other securities of the combined company. |
Fluctuations in the combined company’s financial performance from period to period also may have a significant impact on the market price of our common stock.
IDM’s principal shareholders, executive officers and directors will own a significant percentage of shares of our common stock following the closing of the Exchange and, as a result, the trading price for shares of our common stock may be depressed. These shareholders may make decisions that may be adverse to your interests. IDM’s executive officers and directors (excluding, with respect to Dr. Drakeman, the shares owned by Medarex, Inc., a New Jersey corporation, referred to as Medarex) will, in the aggregate, beneficially own approximately 5.0% of the shares of our common stock following the Exchange. Moreover, in connection with the exercise of the warrants held by Medarex and the warrants held by Sanofi-Aventis and their participation in the Exchange, Medarex and Sanofi-Aventis will own approximately 20% and approximately 15%, respectively, of the total shares of our common stock outstanding at the closing of the transactions under the
39
Share Exchange Agreement. As a result, Sanofi-Aventis, Medarex and the combined company’s other principal shareholders, executive officers and directors, should they decide to act together, will have the ability to exert substantial influence over all matters requiring approval by the stockholders of the combined company, including the election and removal of directors, distribution of dividends, changes to its bylaws and other important decisions, such as future equity issuances. Sanofi-Aventis and Medarex have not entered into any voting agreements or formed a group as defined under the Securities Exchange Act of 1934, as amended, referred to as the Exchange Act.
This significant concentration of share ownership in a limited number of investors may adversely affect the trading price for the shares of our common stock because investors often perceive such a concentration as a disadvantage. It could also have the effect of delaying, deferring or preventing a change in control, or impeding a merger or consolidation, takeover or other transactions that could be otherwise favorable to you.
Future sales of shares of our common stock may cause the market price of your shares to decline. The sale of a large number of shares of our common stock, including through the exercise of outstanding warrants and stock options, following the Exchange, or the perception that such sales could occur, could adversely affect the market price of our common stock. In connection with the Exchange, pursuant to the terms of the Share Exchange Agreement and the Voting Agreement each of the principal company shareholders and certain executive officers and directors of Epimmune, respectively, who will own in the aggregate approximately 75% of the outstanding shares of our common stock following the closing of the transactions under the Share Exchange Agreement, will agree to restrictions on their ability to dispose of their shares of our common stock and related securities for a period of six months following the Exchange. Following these lock-up periods, the principal company shareholders and such executive officers and directors of Epimmune will be free to sell their shares of our common stock (subject to applicable U.S. securities laws), which could cause the market price of such shares of our common stock to decline. Subject to volume restrictions for a further six-month period, 81,328,064 shares (prior to any contemplated reverse stock split), including underlying derivative securities, will be eligible for sale in the public markets following the lock-up periods.
Because an active trading market for shares of our common stock may not develop after the Exchange, it may be difficult for you to sell your shares of our common stock. The average weekly trading volume of our common stock over the past three months ending June 29, 2005 has been approximately 155,000. While approximately 74,860,000 shares of our common stock and options to purchase approximately 4,365,000 shares of our common stock will be issued in the Exchange, no assurance can be made that a more active trading market for the shares of our common stock will develop following the Exchange or how liquid that market might be. If an active and liquid trading market for shares does not develop, investors may have difficulty selling their shares of our common stock in a timely manner, if at all.
40
FORWARD LOOKING STATEMENTS
This proxy statement and the documents incorporated by reference into this proxy statement contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 with respect to Epimmune’s and IDM’s financial condition, results of operations and businesses and the expected impact of the proposed transaction with IDM on Epimmune’s financial performance and the combined company’s selected financial projections. Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “could,” “would,” “will,” “may,” “can,” “continue,” “potential,” “should,” and the negative of these terms or other comparable terminology often identify forward-looking statements. Statements in this proxy statement and the other documents incorporated by reference that are not historical facts are “forward-looking statements” for the purpose of the safe harbor provided by Section 21E of the Exchange Act, and Section 27A of the Securities Act of 1933, as amended, referred to as the Securities Act. These forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materially from the results contemplated by the forward-looking statements, including the risks discussed in this proxy statement, in Epimmune’s Annual Report on Form 10-K/A for the fiscal year ended December 31, 2004, and the risks detailed from time to time in Epimmune’s future reports filed with the SEC. Many of the important factors that will determine these results are beyond Epimmune’s and IDM’s ability to control or predict. Epimmune’s stockholders are cautioned not to put undue reliance on any forward-looking statements, which speak only as of the date of the proxy statement or, in the case of documents incorporated by reference, as of the date of such documents. Except as otherwise required by law, Epimmune does not assume any obligation to publicly update or release any revisions to these forward-looking statements to reflect events or circumstances after the date of this proxy statement or to reflect the occurrence of unanticipated events.
CHAPTER TWO — PROPOSAL 1 —
APPROVAL OF ISSUANCE OF EPIMMUNE COMMON STOCK
IN ACCORDANCE WITH THE SHARE EXCHANGE AGREEMENT,
THE PUT/ CALL AGREEMENTS,
THE AMENDED AND RESTATED PREFERRED EXCHANGE AGREEMENT AND
THE OPTION LIQUIDITY AGREEMENTS
THE EXCHANGE
Background of the Exchange
Since 1997, we have devoted substantially all of our financial resources to the discovery and development of potential therapeutic and prophylactic vaccine products. We have funded our research and development primarily through equity-derived working capital, strategic alliances and collaborations with other companies and government research funding. Because of our market capitalization and low trading volumes, the stage of our clinical and pre-clinical pipeline and other factors, we have faced and continue to face significant challenges in accessing capital markets to fund our ongoing development efforts. As a consequence, we have explored strategic transactions that could enhance our stockholders’ ability to realize greater value from our technology and our clinical and preclinical pipeline of vaccine candidates. In February 2003, we preliminarily agreed with Anosys, Inc. to create a combined company focused in the field of immunotherapeutics and products for the treatment of cancer and infectious diseases. In August 2003, we announced that the merger agreement with Anosys was terminated. Subsequently, we raised money through two private placements of our equity securities and continued to seek strategic transactions as part of our business development.
As part of our overall strategy, we have licensed our technology in areas that are outside our focus of clinical development. Our first contact with IDM was in connection with a license option agreement we entered into with IDM in October 2002. Under the agreement, we granted IDM a non-exclusive option to license certain patented and non-patented rights to our universal cancer epitope packages for use in connection with IDM’s Dentritophageex vivo technology. IDM exercised its option to license in July 2003.
41
On August 24, 2004, Jean-Loup Romet-Lemonne, M.D., the Chief Executive Officer of IDM, contacted Emile Loria, M.D., our Chief Executive Officer, to propose a potential combination of IDM and Epimmune. Dr. Romet-Lemonne subsequently sent Dr. Loria a copy of IDM’s global offering memorandum for its proposed but not completed initial public offering and analyst reports on IDM.
On September 10, 2004, Dr. Romet-Lemonne and Dr. Loria held a meeting at our offices to discuss the potential benefits of a combination of the companies.
On September 14, 2004, our Board held a regular meeting attended by Robert De Vaere, our Vice President, Finance and Administration, Chief Financial Officer and Secretary, Mark Newman, Ph.D., our Vice President, Research and Development, Tereasa Spehar, Ph.D., J.D., our in-house patent counsel and a representative of Cooley Godward LLP, our outside counsel. During the meeting, management informed our Board of the preliminary discussions with IDM. Following a discussion, our Board authorized management to pursue an evaluation of the potential combination.
Our management met with representatives of Jefferies & Company, Inc., financial advisor to Epimmune, on September 16, 2004 in order to obtain investment banking advice with respect to the proposed combination with IDM.
We and IDM entered into a mutual nondisclosure agreement on September 28, 2004 so we could share nonpublic information and engage in nonpublic discussions.
On September 29, 2004, Dr. Loria and Mr. De Vaere met with Dr. Romet-Lemonne in New York, New York in conjunction with an analyst conference and held further discussions about the terms of the proposed combination. Later that day, they met with representatives of Jefferies and UBS Investment Bank, financial advisor to IDM, in Jefferies’ offices in New York to discuss the proposed combined company’s ability to access capital markets to fund its working capital.
On October 4, 2004, Dr. Romet-Lemonne met with Dr. Loria and Mr. De Vaere in order to prepare a presentation on IDM and the proposed combined company for our Board.
On October 6, 2004, Dr. Loria and Mr. De Vaere met with representatives of IDM, including Dr. Romet-Lemonne, Herve Duchesne de Lamotte, the Chief Financial Officer of IDM, Bonnie Mills, M.D., the Vice President, Regulatory Affairs and Irvine site manager of IDM at IDM’s Irvine, California manufacturing facility to discuss the terms of the proposed combination.
On October 8, 2004, we provided to IDM a draft of a term sheet for a proposed combination. During the period of October 8, 2004 through December 3, 2004, we and IDM continued to negotiate the terms outlined in the term sheet, including the structure of the transaction, relative valuations and the formula for determining the exchange rate, pre-signing financing requirements, board and management issues, lock-ups, the treatment of IDM options, indemnification and escrow provisions and conditions to closing.
The parties discussed the need to structure a transaction that would ensure that the combined company would have 18 to 24 months of its projected cash needs as of the closing of the transaction. The parties considered a structure that would condition the proposed combination on completing a financing concurrent with the closing. Because IDM had an existing commitment from Sanofi-Aventis to make additional investments in IDM under certain circumstances, the parties decided that the better alternative was to have IDM raise a minimum of $15 million before Epimmune committed to proceeding with the proposed combination.
The parties also negotiated the exchange ratio during this two month period. IDM initially sought to value IDM at approximately $200 million and sought an exchange ratio that would provide aggregate consideration to the IDM shareholders equal to 4 times the number of shares of Epimmune outstanding, before giving effect to the additional Epimmune shares to be issued as a result of the IDM financing. Epimmune sought to value IDM at approximately $150 million based upon IDM’s valuation in its last private financing and to value Epimmune at approximately $50 million based upon its market capitalization and its value as a public company to IDM. Accordingly, Epimmune sought an exchange ratio that would provide aggregate consideration to the IDM shareholders equal to 3 times the number of shares of Epimmune
42
outstanding, before giving effect to the additional shares to be issued as a result of the IDM financing. During the course of these negotiations, Epimmune offered to include in the transaction a contingent payment right that would require Epimmune to later issue shares to IDM shareholders, if IDM obtained market registration of Mepact by certain deadlines in the United States and Europe. The parties ultimately negotiated an exchange ratio determined by dividing 3.6 times the Epimmune shares outstanding using the treasury method on the date of signing the definitive share exchange agreement by the number of IDM shares outstanding using the treasury method on the same date. In addition, 7,886,109 shares of Epimmune would be issued in connection with the approximately $17.8 million raised by IDM on December 23, 2004. Together these issuances were combined into the exchange ratio of 3.771865 shares of Epimmune common stock for each share of IDM.
On October 8, 2004, Dr. Loria met with Dr. Romet-Lemonne and Laurent Ganem, a member of the board of directors of IDM in London, England in connection with an industry conference. During the meeting, the parties further discussed the potential strategic benefits of the proposed combination.
On October 12, 2004, Dr. Loria met with Guy-Charles Fanneau de la Horie, the Paris site manager for IDM in IDM’s offices in Paris, France to discuss in detail the clinical development and commercialization plan for IDM’s lead drug candidate, Mepact, and its projected sales revenues.
On October 26, 2004, our Board held a regular meeting attended by Mr. De Vaere, Dr. Newman, representatives of Cooley Godward and representatives of Jefferies. At this meeting:
| | |
| | • | Dr. Loria updated our Board on the status of the evaluation of the proposed combination, including our diligence review, the ongoing valuation discussions with IDM and the proposed $15 million to $20 million financing of IDM that would close before the definitive agreement for the proposed combination was signed. The Board discussed and endorsed management’s strategy of proposing to IDM a contingent stock issuance as a way to resolve the parties’ differences as to the appropriate exchange ratio. The Board also made it clear that it would not approve the proposed transaction if IDM were not successful in closing the proposed $15 million to $20 million financing. |
| |
| | • | Representatives of Jefferies gave an oral presentation summarizing the financial terms for the proposed combination as well as certain preliminary financial metrics regarding IDM. |
| |
| | • | A representative of Cooley Godward discussed the fiduciary duties of Epimmune directors in connection with a potential combination with IDM. |
| |
| | • | The Board again reviewed the potential strategic benefits and risks of the potential combination and our alternatives. Our Board considered the diversification of the product pipeline, the technology synergies, including the opportunities under the IDM collaboration agreement with Sanofi-Aventis to use Epimmune’s epitopes to generate drug candidates, and the combined company’s potential ability to access capital markets as strategic benefits of the potential transaction. The Board also considered Epimmune’s alternative of continuing to access capital markets to fund its clinical development efforts, the company’s experience raising $5.5 million through a private placement earlier in 2004 and the Board’s assessment of the continuing difficulties Epimmune could face in accessing capital markets considering its market capitalization, stage of product development and other factors. The representatives of Jefferies concurred with the Board’s assessment of Epimmune’s continuing challenges in accessing capital markets as a standalone public company. |
Following a discussion, the Board authorized management to continue its evaluation of the proposed combination and the negotiations of the term sheet.
On November 5, 2004, our Board held a special meeting attended by Mr. De Vaere and Dr. Newman and representatives of Cooley Godward and by Dr. Romet-Lemonne and Mr. Duchesne de Lamotte for a portion of the meeting. At this meeting:
| | |
| | • | Dr. Loria led a discussion on the updated valuation analyses of IDM prepared by Jefferies and supplemented by internal analyses that circulated in advance of the meeting. |
43
| | |
| | • | Dr. Romet-Lemonne and Mr. Duchesne de Lamotte then joined the meeting by telephone and updated the Board on the status and timing of IDM’s proposed financing, including the valuation of IDM to be used in the financing and the identity of the existing IDM investors participating in the financing. The IDM representatives then left the meeting. |
| |
| | • | Our Board, with the assistance of outside counsel, reviewed in detail the current version of the term sheet and our proposed response to the open issues. Our Board discussed, among other terms, the proposed formula for determining the exchange ratio; the composition of the board of directors of the combined company; the name of the combined company, which the Board believed should not be Immuno Design Molecules because use of a descriptive name could impose artificial limitations on the combined company’s activities; the stockholders that would be subject to the post closing-lockup; the indemnification and escrow provisions and whether Epimmune would sign a binding no-shop agreement prior to the execution of the definitive share exchange agreement. The Board determined that Epimmune would not sign a binding no-shop agreement, but that IDM should be expected to sign one that would be binding while it pursued the $15 million equity financing that was a precondition to signing a definitive share exchange agreement. |
| |
| | • | Our Board reviewed the proposed engagement of Jefferies to serve as our financial advisor for the proposed combination, including the proposed fee arrangement and proposed ongoing obligations for other investment banking engagements. Following a discussion, our Board authorized the engagement of Jefferies subject to changes in the fee arrangement as discussed during the meeting. |
On November 12, 2004, management of both Epimmune and IDM, along with representatives of Cooley Godward and Shearman & Sterling LLP, counsel to IDM, and representatives of Jefferies and UBS, held a teleconference to commence the diligence process.
Following this meeting, our management retained outside consultants to assist us with scientific, clinical, regulatory and manufacturing diligence focused primarily on IDM’s lead product, Mepact, patent diligence and legal and financial diligence. We retained French counsel to conduct legal diligence on IDM in France and to advise us on issues of French law relevant to the transaction. Through to the execution of the definitive Share Exchange Agreement, we and IDM exchanged information and engaged in a diligence review of each other.
On November 17 through November 19, 2004, IDM and we held diligence meetings on Epimmune at Cooley Godward’s offices in San Diego, California. These meetings were attended by Dr. Loria, Mr. De Vaere, Dr. Romet-Lemonne, Mr. Duchesne de Lamotte, representatives of Ernst & Young, on behalf of IDM, representatives of UBS, representatives of Shearman & Sterling and for portions of the meetings, representatives of Cooley Godward.
On November 24, 2004, our Board held a special telephonic meeting attended by Mr. De Vaere and a representative of Cooley Godward. At this meeting:
| | |
| | • | Mr. De Vaere presented the open issues with respect to the engagement of Jefferies as our financial advisor. Following a discussion, our Board again authorized the engagement of Jefferies, subject to the satisfactory resolution of the open issues. |
| |
| | • | Mr. De Vaere also led a discussion regarding our plans to approach G.D. Searle, a subsidiary of Pfizer Inc., to request that G.D. Searle exchange all of our preferred stock it holds for our common stock in connection with the proposed combination with IDM. Various Board members provided guidance on negotiating this issue with Pfizer. |
| |
| | • | Dr. Loria including the proposed resolution of the formula for determining the share exchange ratio updated our Board on the changes in the terms of the proposed combination since the last meeting and the ongoing negotiations to finalize the term sheet. |
| |
| | • | Management also updated our Board on the status of its ongoing diligence of IDM. |
44
Following these discussions, our Board authorized our continuing negotiations with IDM consistent with the discussions during the meeting.
On November 29, 2004, IDM and we entered into an agreement pursuant to which IDM agreed that until the earlier of January 5, 2005 or our advising IDM that we were terminating our negotiations, IDM will not, and will not permit its representatives, to solicit or knowingly encourage the submission of any proposals, participate in any discussions or negotiations, provide any non-public information or entertain, consider or accept any proposal or offer from any person relating to a possible acquisition of IDM.
On November 30, 2004, we entered into an engagement letter pursuant to which we retained Jefferies as our financial advisor for the proposed combination.
Also, on November 30, 2004, we entered into a nondisclosure agreement with Pfizer Inc. and began discussions relating to our proposal to have G.D. Searle exchange or convert our preferred stock that G.D. Searle holds into our common stock in connection with the proposed combination.
On December 7, 2004, Shearman & Sterling circulated an initial draft of the Share Exchange Agreement. We responded to the draft with proposed changes and IDM’s and our outside counsel held follow-up telephone conferences identifying business and legal issues to be negotiated. The parties exchanged subsequent drafts of the Share Exchange Agreement and drafts of other ancillary agreements prior to our face to face negotiating sessions.
On December 7, 2004, our Board held a regular meeting attended by Mr. De Vaere, Dr. Newman and representatives of Cooley Godward. At this meeting:
| | |
| | • | Dr. Loria led a discussion about the status of the discussions with IDM, including developments since the last meeting. |
| |
| | • | Mr. De Vaere, with the assistance of outside counsel, reviewed the final term sheet, including the formula for determining the exchange rate, the indemnification and escrow provisions and other key terms. |
| |
| | • | Dr. Loria and Dr. Newman then gave reports on the results of the scientific, clinical, regulatory and manufacturing diligence. Mr. De Vaere reported on the financial and tax diligence. The representatives of Cooley Godward gave a report on the ongoing legal diligence. Our Board discussed in detail the diligence reports and requested a follow up diligence discussion with our outside consultants concerning IDM’s lead drug, Mepact. |
On December 10, 2004, some of the members of our Board, Dr. Loria, and Mr. De Vaere held a teleconference with our outside consultants to review in more detail the results of clinical, regulatory and manufacturing diligence and allow our Board members to ask the consultants questions. Later in the meeting, Dr. Romet-Lemonne and Dr. Mills joined the teleconference to answer additional questions about the manufacturing and regulatory plans for Mepact.
On December 12, 2004, Dr. Loria met with Dr. Romet-Lemonne, Dr. Mills and Messrs. Duchesne de Lamotte and Fanneau de la Horie as well as Henri Lipanowicz and Marc Guyader, both of whom are members of the board of directors of IDM, in IDM’s offices in Paris, France for the purpose of discussing the Mepact strategy and preparing for a presentation to the board of directors of IDM concerning the proposed combination.
On December 23, 2004, IDM closed the sale of its ordinary shares for total gross proceeds of €13.2 million or approximately $17.8 million based upon the conversion rate on December 31, 2004.
Beginning on January 12, 2005, we and IDM along with our respective outside counsel held negotiating sessions at the offices of UBS in San Francisco, California, on the Share Exchange Agreement. A representative of UBS attended a portion of the meeting. We continued the negotiations on January 13th at the offices of Shearman & Sterling in San Francisco. The following week, we continued negotiations on January 17th in Cooley Godward’s offices in San Diego, California. Negotiations of the Share Exchange Agreement and each of the related ancillary agreements continued until February 24, 2005.
45
On January 17, 2005, IDM sent a nondisclosure agreement to be entered into between IDM and certain of its shareholders and an investor questionnaire to certain IDM shareholders believed to be accredited investors or non-U.S. persons as defined under the Securities Act in order to allow communications with the IDM shareholders concerning the Share Exchange Agreement prior to our Board’s approval of the proposed combination, and our public announcement of the proposed combination and in order to allow us to determine whether the proposed combination could be made under applicable exemptions from registration under the Securities Act.
On January 24, 2005, our Board held a special telephonic meeting attended by Mr. De Vaere and a representative of Cooley Godward. One of our directors, Mr. Hibon, was not present at this meeting. At this meeting:
| | |
| | • | Dr. Loria led a discussion, with the assistance of outside counsel, updating our Board as to the status of the negotiations of the Share Exchange Agreement, including the proposed termination provisions and termination fee payable by us in certain events and the fact that no termination fee is payable by IDM or its shareholders if the IDM shareholders terminate the Share Exchange Agreement due to a counterbid made pursuant to IDM shareholders agreement. Our Board discussed the other terms that were negotiated to reduce the risk of a counterbid. |
| |
| | • | Our Board also discussed the terms of employment agreements to be negotiated and entered into with Dr. Romet-Lemonne, Dr. Loria, Mr. De Vaere, Mr. Newman and certain other officers of IDM. Following a discussion, our Board authorized the hiring of a compensation consultant to assist in developing the financial terms of these agreements. |
On January 31, 2005, our Board held a telephonic special meeting attended by Mr. De Vaere and a representative of Cooley Godward. At this meeting:
| | |
| | • | Dr. Loria and Mr. De Vaere, with the assistance of outside counsel, led a discussion on the status of the proposed combination, including the remaining issues to be negotiated and the expected timing for final approval and execution by us of the Share Exchange Agreement. |
| |
| | • | Our Board discussed the report of the compensation consultant, distributed in advance of the meeting, concerning the proposed terms of incentive compensation packages for our officers to remain with the combined company following the completion of the transaction. |
| |
| | • | Our Board evaluated and discussed the projected operating statements for the combined company. |
| |
| | • | Mr. De Vaere led a discussion regarding a proposed reverse stock split to be effected in connection with the proposed combination in order to meet the minimum stock price for listing on the Nasdaq National Market, including the advice provided by Jefferies with respect to the size of the reverse stock split. |
| |
| | • | Our Board also discussed management’s recommendations as to the increase to the available shares in the 2000 Plan and the Purchase Plan and a proposed increase in the authorized capital to be effected in connection with the completion of the proposed combination. |
On February 2, 2005, we reached agreement with G.D. Searle on the terms of the Preferred Exchange Agreement pursuant to which G.D. Searle will exchange our preferred stock it holds for our common stock.
On February 4, 2005, our Board held a special meeting attended by Mr. De Vaere and a representative of Cooley Godward LLP and Praveen Tyle, one of our consultants, and by Dr. Romet-Lemonne and Dr. Mills for a portion of the meeting. One of our directors, John P. McKearn, Ph.D. was not present at this meeting. At this meeting:
| | |
| | • | Mr. Tyle updated our Board on the results of the diligence review of the clinical development activities of IDM, particularly focusing on Mepact. |
| |
| | • | Dr. Mills joined the meeting by telephone to provide information regarding IDM’s discussions with the FDA regarding Mepact and IDM’s plans for further development and manufacture of Mepact. After answering our Board’s questions, Dr. Mills and Mr. Tyle left the meeting. |
46
| | |
| | • | Dr. Romet-Lemonne joined the meeting and Dr. Loria and Mr. De Vaere left the meeting so that Dr. Romet-Lemonne could discuss with our Board the proposed employment agreements with our officers and bonuses that would be payable upon closing of the proposed combination. After Dr. Romet-Lemonne left the meeting, our Board discussed the employment matters in detail. Dr. Loria and Mr. De Vaere then rejoined the meeting. |
| |
| | • | Dr. Romet-Lemonne then rejoined the meeting and led our Board in a discussion of the proposed operating plan for the combined company. |
Following the Board meeting, negotiations of the employment agreements continued.
On February 24, 2005, IDM mailed to all of its shareholders who signed nondisclosure agreements an information statement dated February 24, 2005, copies of our periodic reports filed with the SEC and the Share Exchange Agreement to be executed by each such shareholder. During the period from February 24, 2005 through March 11, 2005, IDM’s management answered shareholder questions and solicited and obtained signatures for the Share Exchange Agreement from shareholders holding more than 85% of the outstanding ordinary shares of IDM.
On March 11, 2005, IDM held a meeting with Sanofi-Aventis in order to solicit Sanofi-Aventis’ execution of the Share Exchange Agreement. Following this meeting, we and IDM negotiated some minor changes to the Share Exchange Agreement to respond to Sanofi-Aventis’s comments. These changes were documented through an Amendment No. 1 to the Share Exchange Agreement.
On March 15, 2005, our Board held a special meeting attended by Mr. De Vaere, representatives of Cooley Godward and representatives of Jefferies. At this meeting:
| | |
| | • | Dr. Loria updated our Board on developments since the Board meeting on February 4, 2005, including the fact that holders of more than 85% of the IDM ordinary shares had already executed the Share Exchange Agreement, and the fact that we had agreed to an amendment to the Share Exchange Agreement to reflect minor changes to the agreement requested by Sanofi-Aventis. |
| |
| | • | Representatives of Jefferies presented a financial analysis pertaining to certain aspects of the transaction and delivered their oral opinion (later confirmed by delivery of a written opinion) to the effect that as of March 15, 2005, and based upon the qualifications, limitations and assumptions set forth in the opinion, the consideration to be paid by Epimmune to the IDM shareholders in connection with the transaction was fair, from a financial point of view, to Epimmune. Please see the section entitled“The Exchange — Opinion of Epimmune’s Financial Advisor to the Board of Directors” for additional information. |
| |
| | • | Representatives of Cooley Godward reviewed legal matters, including issues resolved since the last Board meeting, and reviewed the proposed Board resolutions circulated in advance of the meeting. |
| |
| | • | Our Board, with the assistance of the representatives of Cooley Godward, discussed the draft press release announcing the combination, the preparation for the conference call and the legal requirements relating to these communications. |
Following a discussion of all these matters, our Board unanimously approved and determined advisable the Share Exchange Agreement and the issuance of our shares of common stock pursuant to the Share Exchange Agreement, the Put/ Call Agreements, the Option Liquidity Agreements and the Preferred Exchange Agreement. In addition, our Board unanimously approved, subject to receipt of stockholder approval, the amendment of our Amended and Restated Certificate of Incorporation to change our corporate name to IDM, Inc., to effect a reverse stock split and to increase our authorized capital stock, approved an amendment of our 2000 Plan to increase the shares available for issuance under the plan, and approved an amendment of our Purchase Plan to increase the number of shares available for issuance under the plan. Further, our Board unanimously approved the nomination of six directors to be submitted for election by the stockholders at the annual meeting, selected Ernst & Young as our independent auditors for the fiscal year ending December 31, 2005, approved the new employment agreements with our officers, approved the payment of bonuses to officers following the closing of the combination, approved amendments to options held
47
by directors expected to resign from our Board at the closing and approved a new severance plan for our employees. Our Board unanimously resolved to recommend that our stockholders vote in favor of all of the proposals to be submitted to the stockholders for approval pursuant to the Share Exchange Agreement and otherwise as part of the annual meeting.
On March 15, 2005, the parties executed the Share Exchange Agreement, Amendment No. 1 to the Share Exchange Agreement, the letter agreement between us and IDM pursuant to which IDM agreed not to solicit a competing transaction and not to provide access to information to any potentially competing bidder and stockholder voting agreements with directors and officers of Epimmune. The same day we and G.D. Searle executed the Preferred Exchange Agreement.
On March 16, 2005, we and IDM issued a joint press release announcing the execution of the Share Exchange Agreement and held a conference call to discuss the transaction.
On March 17, 2005, we executed new employment agreements with Dr. Loria, Mr. De Vaere and Dr. Newman.
In a teleconference on March 24, 2005, our management discussed with representatives of Cooley Godward and Jefferies a proposed change in the reverse stock split of our common stock from a reverse stock split in which each four shares of our common stock would be combined into one share of our common stock, as previously approved by the Board, to a reverse stock split in which each four, five or six outstanding shares of our common stock would be combined into one share of our common stock, with the specific ratio to be determined by the Board prior to the closing, in order to provide for greater flexibility to enable Epimmune to satisfy the listing requirements of the Nasdaq National Market.
By action by unanimous written consent dated March 29, 2005, taking into consideration market conditions, the price of our common stock and the desire for flexibility in enabling Epimmune to satisfy the listing requirements of the Nasdaq National Market, our Board approved, subject to receipt of stockholder approval, the amendment of our Amended and Restated Certificate of Incorporation to effect a reverse stock split in which each four, five or six outstanding shares of our common stock would be combined into one share of our common stock, with the specific ratio to be determined by the Board prior to the closing of the Exchange from among the ratios approved by the stockholders, in lieu of the four-for-one reverse stock split previously approved by the Board. In the action by unanimous written consent, our Board also approved the amendment and restatement of the Preferred Exchange Agreement to clarify that the number of shares of our common stock for which the outstanding preferred stock will be exchanged is subject to adjustment to reflect the effect of any reverse stock split or similar change in our common stock that occurs prior to the Exchange.
On April 12, 2005, we entered into the Amended and Restated Preferred Exchange Agreement with G.D. Searle.
By action by unanimous written consent dated April 21, 2005, our Board approved Amendment No. 2 to the Share Exchange Agreement, changing the reference to the proposed reverse stock split to a reverse stock split in which each four, five or six outstanding shares of our common stock would be combined into one share of our common stock upon the closing of the Exchange and providing that an employee stock purchase plan for employees who are residents of France would be submitted to stockholders at the annual meeting upon satisfaction of certain conditions or mutual agreement of Epimmune and IDM. In the action by unanimous written consent, our Board also approved employment agreements with Dr. Romet-Lemonne and Dr. Mills, which agreements will be effective upon the closing of the Exchange.
On April 21, 2005, we and the principal company shareholders, represented by the shareholder representative, executed Amendment No. 2 to the Share Exchange Agreement.
On April 21, 2005, we executed an employment agreement with Dr. Romet-Lemonne.
By action by unanimous written consent dated May 31, 2005, taking into consideration market conditions, the price of our common stock and the desire for flexibility in enabling Epimmune to satisfy the listing requirements of the Nasdaq National Market, our Board approved, subject to receipt of stockholder approval, the amendment of our Amended and Restated Certificate of Incorporation to effect a reverse stock split in
48
which each four, five, six, seven, eight, nine or ten outstanding shares of our common stock would be combined into one share of our common stock, with the specific ratio to be determined by the Board prior to the closing of the Exchange from among the ratios approved by the stockholders, in lieu of the one-for-four, one-for-five and one-for-six reverse stock split previously approved by the Board. In the action by unanimous written consent, our Board also approved Amendment No. 3 to the Share Exchange Agreement, changing the reference to the proposed reverse stock split to a reverse stock split in which each four, five, six, seven, eight, nine or ten outstanding shares of our common stock would be combined into one share of our common stock upon the closing of the Exchange. In the action by unanimous written consent, our Board also approved the adoption of the French Purchase Plan.
On May 31, 2005, we and the principal company shareholders represented by the shareholder representative executed Amendment No. 3 to the Share Exchange Agreement.
By action by unanimous written consent dated June 29, 2005, our Board approved Amendment No. 4 to the Share Exchange Agreement, changing the end date from July 31, 2005 to August 26, 2005. On June 30, 2005, we and the principal company shareholders, represented by the shareholder representative, executed Amendment No. 4 to the Share Exchange Agreement.
Epimmune’s Reasons for the Exchange
Our Board believes that the combination with IDM will provide substantial benefits to the stockholders of Epimmune. The combination will create a company with a late stage clinical drug candidate; more than seven drug or vaccine candidates in clinical development for cancer and infectious diseases; the potential for greater revenue diversity and earlier realization of commercial revenue; a stronger number of alliance partners; and working capital that we believe will be sufficient to fund the operations of the combined company for 18 to 24 months from January 1, 2005. Our Board believes that the combination reduces the inherent risks of our earlier stage clinical pipeline, increases our financial strength in ways that we could not achieve on our own and enhances our ability to execute on our strategy of developing commercial drugs. At its meeting on March 15, 2005, our Board unanimously adopted the Share Exchange Agreement and resolved to recommend that the Epimmune stockholders vote “For” the approval of the Exchange and related proposals.
In making its determination to approve the Share Exchange Agreement, our Board consulted with our officers regarding the strategic and operational aspects of the combination and the results of our diligence review of IDM. In addition, our Board consulted with representatives of Jefferies regarding financial matters and with representatives of Cooley Godward regarding legal matters (other than intellectual property diligence and issues of French law, which were provided by other outside counsel). In the course of reaching its determination, our Board considered a variety of factors, including the following factors:
| | |
| | • | Strategic Benefits of the Combination. Our Board considered the strategic benefits of the proposed combination, including the following benefits: |
| | |
| | – | Greater Product Diversification. The combined company will have a significantly larger pipeline of clinical drug candidates under development, will have a Phase III drug candidate with a prospect for commercialization by 2007, and will have greater diversification of development risk than Epimmune has on a standalone basis. The clinical pipeline will include Mepact, a drug candidate for the treatment of osteosarcoma that has completed Phase III trials, Bexidem a Phase II/ III drug candidate for the treatment of bladder cancer, Uvidem, a Phase II drug candidate for the treatment of melanoma that is partnered with Sanofi-Aventis, Collidem a Phase I/ II drug candidate for the treatment of colorectal cancer, EP-2101, a Phase II vaccine candidate for the treatment of non-small cell lung cancer, EP HIV-1090, a Phase I/ II vaccine candidate for the treatment of HIV and EP-HBS, a Phase I vaccine candidate for the treatment of hepatitis B that is partnered with Innogenetics. The combined company will also have a pipeline of preclinical vaccines and other drug candidates for the treatment of certain cancers and infectious diseases and the prevention of certain infectious diseases. |
| |
| | – | Enhanced Research and Development Capabilities. Our Board believes that the transactions combines two technology platforms that may be essential to the successful development of |
49
| | |
| | | advanced vaccines: antigen targeting technology is provided through our proprietary epitope discovery and design technology and vaccine delivery technology is provided through IDM’s proprietary Dendritophages, which are epitope-stimulated dendritic cells designed to stimulate anti-tumor immune responses. |
| | |
| | – | Stronger Balance Sheet of the Combined Company. Our Board believes that the balance sheet of the combined company should improve our ability to advance our most promising drug candidates through the clinic while continuing research and development on promising preclinical drug programs. Further, our Board believes that these financial resources will enable our stockholders to realize technology value from our proprietary epitope discovery and design technology. The stronger balance sheet also should enable the combined company to commercialize Mepact and seek the best partnering opportunities for the combined company’s clinical drug candidates. Finally, our Board believes that the stronger balance sheet will enhance the combined company’s ability to access capital markets to fund the ongoing advancement of its product candidates and its evolution into a commercial stage biopharmaceutical company. |
| | |
| | • | Attractive Financial Terms. Our Board believes that the exchange ratio which gives IDM shareholders approximately 78% and Epimmune stockholders approximately 22% of the combined company on a fully diluted basis, values IDM at an attractive level considering the pre-announcement market value of the Epimmune common stock to be issued in the Exchange, and the range of values of IDM on a standalone basis. In reaching this conclusion, our Board considered various factors and analyses with respect to the financial terms of the proposed merger including the following: |
| | |
| | – | information concerning the financial condition, burn rate, business and prospects of Epimmune and IDM, as well as conditions in the biotechnology industry generally. |
| |
| | – | information concerning the recent and historical stock price performance of Epimmune common stock and the trading volume and volatility of Epimmune common stock and the impact of these fundamentals on our ability to attract institutional investors and access capital markets. |
| |
| | – | information concerning forecasted revenues, expenses and cash flows from IDM’s lead drug, Mepact, as well as the risks associated with obtaining marketing approval for this drug candidate and commercialization related risks. |
| |
| | – | the fixed nature of the exchange ratio for the Exchange so that any increase or decrease in the market value of Epimmune common stock following the date of announcement through the closing will not increase or decrease the percentage of the combined company owned by the Epimmune stockholders. |
| | |
| | • | Opinion of Financial Advisor. Our Board reviewed the financial presentations prepared by Jefferies and the Jefferies opinion (including the qualifications, limitations, assumptions and methodologies underlying the analyses in connection therewith) to the effect that as of March 15, 2005, the consideration to be paid by Epimmune to the IDM shareholders in connection with the transaction, was fair, from a financial point of view, to Epimmune. Please see the section entitled“The Exchange — Opinion of Epimmune’s Financial Advisor to the Board of Directors” for further information. Jefferies provided its opinion solely for the information and assistance of our Board in connection with its consideration of the Exchange. Jefferies’ opinion is not a recommendation as to how any holder of our common stock or any other person should vote or act with respect to the Exchange or any related transactions. |
| |
| | • | Terms of the Share Exchange Agreement. Our Board, with the assistance of outside counsel, considered the terms and conditions of the Exchange, including: |
| | |
| | – | restrictions on the conduct of business by Epimmune and IDM between the signing of the Share Exchange Agreement and the closing of the Exchange. |
| |
| | – | the potential effect of the Share Exchange Agreement on the potential for a third party to make a proposal to acquire Epimmune, including the right of our Board to provide information in response |
50
| | |
| | | to an unsolicited superior proposal and to withdraw its recommendation of the Exchange following the receipt of a superior proposal. |
| | |
| | – | the fact that the stockholders of Epimmune can vote against the Exchange and other proposals and that no termination fee is payable if the Share Exchange Agreement is terminated due to a negative vote by the stockholders of Epimmune unless a superior proposal to acquire Epimmune was announced prior to the annual meeting and Epimmune enters into an agreement for a third party acquisition of Epimmune within nine months following the termination. |
| |
| | – | the fact that certain IDM shareholders holding more than 85% of the IDM ordinary shares executed the Share Exchange Agreement prior to its approval by our Board and our execution of the agreement, together with the existence of the drag-along right under the IDM shareholders agreement that should enable us to acquire any shares held by IDM shareholders who do not subsequently become parties to the Share Exchange Agreement. |
| |
| | – | the conditions to the closing of the Exchange. |
| |
| | – | the terms of the lock-up for resales of the combined company’s common stock following the closing of the Exchange. |
| | |
| | • | Board of Directors and Employee Matters. Our Board considered the terms of the Share Exchange Agreement with respect to the composition of the Board of the combined company, the designation of executive officers of the combined company and the arrangements with respect to the Board, executives and employees intended to integrate the combined company and preserve our value prior to and following the combination, including: |
| | |
| | – | We will designate three of the nine members to the combined company’s board of directors to fill terms until the next annual election of directors. |
| |
| | – | Our existing executives, Dr. Loria, Mr. De Vaere and Dr. Newman have entered into new one year employment agreements and are expected to remain with the combined company for at least an integration period. |
| |
| | – | Our existing employees are expected to remain employees of Epimmune (which will be renamed IDM, Inc.). |
In its review of the proposed combination, our Board identified and considered a variety of potentially negative factors, including:
| | |
| | • | The risks described under the section entitled“Risk Factors — Risks Related to the Exchange.” |
| |
| | • | The risk of failure to obtain marketing approval for IDM’s lead drug candidate, Mepact, and the impact of such a failure on the stock price of the combined company and its prospects. |
| |
| | • | The possibility that the Epimmune’s stock price could decline following the announcement of the proposed combination, and the impact that a falling stock price could have on our stockholders’ support for the Exchange. |
| |
| | • | The risk that sales of substantial amounts of the combined company’s common stock in the public market after the closing of the proposed combination could materially adversely affect the market price of such common stock. |
| |
| | • | The risk that the proposed combination could not be completed as expected because there could be no assurance that the stockholders of Epimmune would approve the transaction or that other conditions to the parties’ obligations to close the combination would be satisfied even if the stockholders approved the transaction. |
| |
| | • | The possibility of disruption in our operations or those of IDM and a loss of key employees of either company because of the proposed combination. |
| |
| | • | The possibility that the benefits anticipated in connection with the proposed combination might not be realized by the combined company. |
51
In addition to the factors considered that are described above, our Board also was aware of the interests that some executive officers and directors of Epimmune may have with respect to the proposed combination in addition to their interests as stockholders of Epimmune generally. Please see the section entitled“The Exchange — Interests of Directors, Officers and Affiliates” for further details.
In analyzing the proposed combination, our Board did not view any of the factors listed above as determinative or find it practical to quantify or otherwise attempt to assign any rank or assign relative weights to any of the foregoing factors. Our Board conducted an overall analysis of the factors described above, and overall considered the factors to be favorable and to support its determination. The individual members of our Board may have given different weight to different factors in considering the factors.
The Board unanimously recommends that the Epimmune stockholders vote “FOR” approval of the Exchange and the related proposals.
Opinion of Epimmune’s Financial Advisor to the Board of Directors
Pursuant to an engagement letter dated November 30, 2004, Epimmune engaged Jefferies to act as financial advisor to the Board. Epimmune selected Jefferies based on Jefferies’ reputation and experience in the biotechnology industry. In particular, Jefferies’ dedicated healthcare group focuses on providing investment banking services, including merger and acquisition advisory services, to healthcare, life science and biotechnology companies. In this capacity, Jefferies is continually engaged in valuing these businesses. On March 15, 2005, Jefferies rendered its opinion to Epimmune’s Board to the effect that, as of such date and based upon and subject to the assumptions, qualifications, limitations and factors described in such opinion, the Epimmune common stock to be issued to IDM shareholders as consideration for the acquisition of IDM, referred to as the Consideration, was fair from a financial point of view, to Epimmune.
For purposes of its opinion, and notwithstanding any terms in the Share Exchange Agreement to the contrary, Jefferies assumed that (i) the aggregate number of our common stock constituting the Consideration is equal to 79,226,275, (ii) upon the closing of the transaction, the IDM shareholders (including optionholders and warrant holders) will own 77.8% of the outstanding shares of Epimmune, on a fully diluted basis, (iii) upon the closing of the transaction, Epimmune will own 100% of the issued and outstanding stock of IDM (including without limitation, any shares held in a PEA that are subject to a Put/ Call Agreement and any shares that are held by IDM shareholders who do not become principal company shareholders, and will acquire such shares simultaneously and (iv) no warrants or options or similar agreements to purchase shares of IDM stock will remain outstanding or allow for the exercise into or purchase of shares of our common stock after the closing of the transaction, and that all such options and warrants were exchanged for consideration as if exercised on the date of the closing of the transaction.
Jefferies’ opinion, which describes the assumptions made, matters considered and limitations on the review undertaken by Jefferies, is attached asAnnex Bto this proxy statement. Holders of our common stock are urged to, and should, read the Jefferies opinion carefully and in its entirety. The Jefferies opinion was provided solely to our Board and addresses only the fairness of the Consideration from a financial point of view to Epimmune as of the date of the opinion. The Jefferies opinion does not address any other aspect of the transaction. The summary of the Jefferies opinion set forth in this proxy statement is qualified in its entirety by reference to the full text of such opinion.
Jefferies’ opinion does not address the underlying business decision to enter into the transaction, nor does it evaluate alternative transaction structures or other financial or strategic alternatives or business strategies.
In reading the discussion of the fairness opinion set forth below, you should be aware that Jefferies’ opinion does not constitute a recommendation to any Epimmune stockholder or any other person as to how they should vote or act with respect to the transaction or any other matter.
In reading the discussion of the fairness opinion set forth below, you should be aware that Jefferies:
| | |
| | • | reviewed a draft of the Share Exchange Agreement circulated on March 14, 2005, as amended by the accompanying Amendment No. 1 to the Share Exchange Agreement also circulated on such date; |
52
| | |
| | • | reviewed the Annual Reports on Form 10-K and related publicly-available financial information for the three fiscal years ended December 31, 2001, 2002, 2003 and the Form 10-Q and the related unaudited financial information for the nine months ended September 30, 2004; |
| |
| | • | reviewed certain financial projections prepared by Epimmune and IDM management; |
| |
| | • | conducted discussions with members of senior management of Epimmune and IDM concerning the respective operations, financial conditions and prospects of Epimmune and IDM; |
| |
| | • | reviewed the historical market prices and trading activity for the shares of the common stock of Epimmune and compared them with those of certain publicly-traded companies which Jefferies deemed to be reasonably similar to Epimmune and IDM; |
| |
| | • | analyzed the industry, Epimmune’s key competitors and trends in the industry in which Epimmune and IDM operate; |
| |
| | • | analyzed financial information of key competitors, similar publicly-traded companies and/or similar precedent transactions to determine appropriate valuation multiples or enterprise values; |
| |
| | • | analyzed the performance and market position of IDM relative to its key competitors and/or similar traded companies; |
| |
| | • | performed a relative revenue contribution analysis with respect to the transaction; |
| |
| | • | compared the proposed financial terms of Epimmune and the transaction with the financial terms of certain other mergers and acquisitions which it deemed to be relevant; and |
| |
| | • | reviewed such other financial studies, performed such other analyses and investigations and took into account such other matters as Jefferies deemed appropriate. |
In rendering its opinion, Jefferies relied, without independent verification, on the accuracy and completeness of all the financial and other information (including without limitation the representations and warranties contained in the Share Exchange Agreement) that was publicly available or furnished to Jefferies by us or our advisors and by IDM and its advisors. With respect to the financial projections examined by Jefferies, Jefferies assumed that they were reasonably prepared and reflected the best available estimates and good faith judgments of our management as to the future performance of Epimmune and IDM. Jefferies also assumed, that in the course of obtaining the necessary regulatory and third party approvals, consents and releases for the transaction, no modification, delay, limitation, restriction or condition would be imposed that would have a material adverse effect on the transaction and that the transaction would be consummated in accordance with applicable laws and regulations and the terms of the Share Exchange Agreement as amended by the accompanying Amendment No. 1 to the Share Exchange Agreement as set forth in the March 14, 2005 draft thereof, without waiver, amendment or modification of any material term, condition or agreement. Jefferies has not made or taken into account any independent appraisal or valuation of any assets or liabilities of Epimmune or IDM. Jefferies expresses no view as to the federal, state or local tax consequences of the transaction.
Jefferies did not make or assume any responsibility for making an independent valuation or appraisal of the assets or liabilities of Epimmune or IDM, nor was Jefferies furnished with any such appraisals, nor did Jefferies evaluate the solvency or fair value of Epimmune or IDM under any state or federal securities laws relating to bankruptcy, insolvency or similar matters.
For purposes of its opinion, Jefferies assumed that Epimmune and IDM were not currently involved in any material transaction other than the transaction, other publicly announced transactions and those activities undertaken in the ordinary course of conducting its business. Jefferies’ opinion was necessarily based upon market, economic, financial and other conditions as they existed and could be evaluated as of the date of the opinion. It should be understood that, although subsequent developments may affect its opinion, Jefferies has no obligation to update, revise or reaffirm its opinion.
53
The following is a summary explanation of various sources of information and valuation methodologies employed by Jefferies in rendering its opinion. Jefferies did not explicitly assign any relative weights to the various factors or analyses considered. This summary of financial analyses includes information presented in tabular format. In order to fully understand the financial analyses used by Jefferies, the tables must be read together with the text of each summary. Considering the tables alone could create a misleading or incomplete view of Jefferies’ financial analyses.
Jefferies performed an analysis of the transaction value of the Consideration to be paid to the IDM shareholders defined as the shares of Epimmune common stock to be issued times the Epimmune share price, less the cash IDM had on its balance sheet as of December 31, 2004. As of March 14, 2005, the transaction value of the Consideration was approximately $61.8 million based upon the pre-announcement closing market price of our common stock on the Nasdaq National Market on March 14, 2005. Such valuation does not reflect additional value with regard to any imputed control premium that might result from the issuance to IDM shareholders of a majority of the outstanding shares of Epimmune’s common stock.
Comparable Company Analysis. Jefferies considered enterprise values of comparable public companies in order to derive the implied enterprise values placed on IDM in its particular market segment. In order to perform this analysis, Jefferies compared financial information of IDM with publicly available information for comparable cancer vaccine and oncology companies. Jefferies selected companies competing in the cancer vaccine sector and, more generally, in the oncology sector with a market capitalization up to $500 million and at least one Phase III product in clinical trials for this analysis. Jefferies further refined its criteria by excluding oncology companies that were not primarily focused in oncology, had a significantly larger product pipeline than IDM or had substantially different therapeutic/technology platforms from IDM. The comparable companies consist of the following:
| | | | | | | | | | | |
| Cancer Vaccine Comparable Companies | | Enterprise Value | | | Oncology Comparable Companies | | Enterprise Value | |
| | | | | | | | | |
| Biomira, Inc. | | | $125,764 | | | DUSA Pharmaceuticals | | | $132,322 | |
| Cancervax Corporation | | | 129,250 | | | GTx, Inc. | | | 191,203 | |
| Dendreon Corporation | | | 166,497 | | | NeoPharm, Inc. | | | 134,661 | |
| Favrille Inc. | | | 148,814 | | | Threshold Pharmaceuticals | | | 121,002 | |
| Genitope Corporation | | | 240,024 | | | Vion Pharmaceuticals Inc. | | | 132,109 | |
For this analysis, as well as other analyses, Jefferies examined publicly available information. The following table presents, as of March 14, 2005, the median enterprise value and range of enterprise values for the cancer vaccine and oncology comparable companies. For purposes of this comparable company analysis, Jefferies defines enterprise value as market capitalization plus the book value of total debt plus the liquidation value of any outstanding preferred stock less cash and cash equivalents. With respect to IDM, because IDM is not a public company with a market capitalization, Jefferies considered the median enterprise value of cancer vaccine and oncology comparable companies described below to be a more relevant reflection of the enterprise value of IDM.
| | | | | | | | | |
| | | Enterprise Value | |
| | | | |
| | | Median | | | Range | |
| | | | | | | |
| | | (Dollars in thousands) | |
| Cancer Vaccine Comparable Companies | | $ | 148,814 | | | $ | 125,764 - $240,024 | |
| Oncology Comparable Companies | | | 132,322 | | | | 121,002 - 191,203 | |
No cancer vaccine or oncology company listed above is identical to IDM. In evaluating such comparables, Jefferies made assumptions with respect to the biotechnology industry’s performance and general economic conditions, many of which are beyond the control of Epimmune. Such assumptions include the assumption that the current trading levels of companies within Jefferies’ comparable company analysis and biotechnology companies in general are currently trading at reasonable levels and the assumption that the stock price of each such comparable company does not reflect a control premium. Mathematical analysis,
54
such as determining the median or range, is not in itself a meaningful method of using comparable company data.
Comparable M&A Transaction Analysis. Jefferies considered transaction value, adjusted for the seller’s cash and debt when appropriate, to indicate enterprise values strategic and financial acquirers have been willing to pay for companies in a particular market segment, in order to analyze the Consideration to be paid by Epimmune to acquire IDM. In performing this analysis, Jefferies reviewed a number of transactions that it considered similar to the transaction. Jefferies selected these transactions from transactions occurring since January 1, 2002 involving sellers in the biotechnology industry with a transaction value of up to $500 million and with at least one product in clinical trials. For purposes of this M&A transaction analysis, Jefferies defines transaction value as the value of the consideration paid to the target stockholders for equity including the liquidation value of any preferred stock, plus the book value of total debt of the target less cash and cash equivalents held by the target. For this analysis, as well as other analyses, Jefferies examined publicly available information. These transactions consisted of the acquisition of:
| | | | | |
| Transaction | | Transaction Value | |
| | | | |
| • Xcel Pharmaceuticals by Valeant Pharmaceuticals | | $ | 280,000 | |
| • Corvas International, Inc. by Dendreon Corporation | | | 19,190 | |
| • Cell Pathways, Inc. by OSI Pharmaceuticals, Inc. | | | 25,178 | |
| • Eos Biotechnology by Protein Designs Lab | | | 38,679 | |
| • Triangle Pharmaceuticals by Gilead Sciences | | | 465,020 | |
| • Synaptic Pharmaceutical Corporation by H Lundbeck A/ S | | | 94,847 | |
| • OraPharma Inc. by Johnson & Johnson Inc. | | | 85,000 | |
| • Matrix Pharmaceuticals by Chiron Corporation | | | 41,972 | |
The following table presents, as of March 14, 2005, the median transaction value and the range of transaction values for the transactions listed above:
| | | | | | | | | |
| | | Comparable M&A Transaction | |
| | | Analysis | |
| | | | |
| | | Median | | | Range | |
| | | | | | | |
| | | (Dollars in thousands) | |
| Transaction Value | | $ | 63,486 | | | $ | 19,190 - $465,020 | |
No transaction utilized as a comparable in the transaction comparables analysis is identical to the transaction. For example, no such transaction resulted in the target’s shareholders owning more than 50% of the acquiror’s outstanding shares. In evaluating the comparables, Jefferies made assumptions with respect to the biotechnology industry’s performance and general economic conditions, many of which are beyond the control of Epimmune. Such assumptions include the assumption that all transactions reflected a control premium and represented the prices fully informed third party buyers would be willing to pay to third party sellers. Mathematical analysis, such as determining the median or range, is not in itself a meaningful method of using comparable transaction data.
Discounted Cash Flow Analysis. Jefferies considered a discounted cash flow analysis of IDM to calculate the present value of the stand-alone unlevered free cash flows that IDM would generate from January 1, 2005 through December 31, 2014, assuming that IDM’s operating performance would be reflective of the projections of IDM’s management as to the potential future performance of IDM. Using a perpetual growth rate of 3.0% and a discount rate range of 25.0% to 45.0%, Jefferies derived a range of implied enterprise values. These discount rates are based on the indicative weighted average cost of capital calculated by analyzing the companies Jefferies included in the comparable public company analysis, which is 25.7%. In order to reflect certain inherent risks associated with IDM’s business, Jefferies applied a range of risk premiums to the calculated weighed average cost of capital, as reflected in the 25.0% to 45.0% discount rate range utilized in the discounted cash flow analysis.
55
In its discounted cash flow analysis, Jefferies made the following adjustments to the projections provided by IDM: (i) conversion of projections provided in Euros to U.S. Dollars using a rate of 1 Euro to 1.3346 U.S. Dollars, the spot exchange rate as of March 14, 2005; (ii) exclusion of approximately $2.3 million of non-recurring transaction costs in the 2005 period; and (iii) assumption of 3% perpetual growth rate for terminal period projections.
The following table summarizes, as of March 14, 2005, the implied enterprise values of IDM resulting from the discounted cash flow analysis: The table does not reflect additional value with regard to any imputed control premium that might result from the issuance to IDM shareholders of a majority of the outstanding shares of Epimmune’s common stock.
| | | | | |
| | | Implied Total Enterprise Value | |
| | | Based on 3% Terminal | |
| Discount Rate | | Growth Rate | |
| | | | |
| | | (Dollars in thousands) | |
| 25% | | $ | 283,875 | |
| 30% | | | 168,226 | |
| 35% | | | 102,014 | |
| 40% | | | 61,928 | |
| 45% | | | 36,662 | |
All projected data regarding the future performance of IDM were prepared and furnished by IDM management. All assumptions derived from such projections by Jefferies in performing the discounted cash flow analysis were reviewed by Epimmune’s management, who agreed they were reasonable in light of IDM’s historical operations and forecasted financial performance.
The merger and acquisition transaction environment varies over time because of macroeconomic factors such as interest rate and equity market fluctuations and microeconomic factors such as industry results and growth expectations. No company or transaction reviewed was identical to the proposed transaction and, accordingly, the foregoing analyses involve complex considerations and judgments concerning differences in financial and operating characteristics and other factors that would affect the acquisition values in the comparable transactions, including the size and demographic and economic characteristics of the markets of each company and the competitive environment in which it operates.
The preparation of a fairness opinion is a complex process involving determinations as to the most appropriate and relevant methods of financial analysis and the application of these methods to the particular circumstances and, therefore, is not necessarily susceptible to partial analysis or summary description. Selecting portions of the analysis or the summary set forth above, without considering the analysis as a whole, could create an incomplete view of the processes underlying the opinion of Jefferies. In arriving at its fairness determination, Jefferies considered the results of all these constituent analyses and did not attribute any particular weight to any particular factor or analysis considered by it; rather, Jefferies made its determination as to fairness on the basis of its experience and professional judgment after considering the results of all such analyses. Certain Jefferies analyses are based upon forecasts of future results and are not necessarily indicative of actual future results, which may be significantly more or less favorable than suggested by such analyses. The foregoing summary does not purport to be a complete description of the analyses performed by Jefferies. Additionally, analyses relating to the value of businesses or securities are not appraisals. Accordingly, such analyses and estimates are inherently subject to substantial uncertainty.
As described above, Jefferies’ opinion to the Board was among many factors taken into consideration by the Board in making its determination to approve the transaction. Such decisions were solely those of our Board. The opinion of Jefferies was provided solely to our Board and does not constitute a recommendation to any person, including the holders of our common stock, as to how such person should vote or act on any matter related to the transaction.
56
Based upon and subject to the foregoing qualifications, limitations, factors and assumptions and those set forth in the opinion, Jefferies was of the opinion that, as of March 15, 2005, the Consideration was fair from a financial point of view, to Epimmune.
Pursuant to the terms of the Jefferies engagement letter, Epimmune paid Jefferies an engagement fee of $100,000 upon execution of its engagement letter, a fee of $250,000 upon delivery of Jefferies’ opinion and a fee of $150,000 upon the 15th day after the date of delivery of Jefferies’ opinion and has agreed to pay Jefferies a transaction fee equal to $500,000, which is only payable upon consummation of the transaction. Jefferies will also be reimbursed for its reasonable and customary expenses, and it and related parties will be indemnified against certain liabilities, including liabilities under the federal securities laws, in connection therewith. No limitations were imposed on Jefferies by Epimmune with respect to the investigations made or procedures followed by it in rendering its opinion.
Jefferies and its affiliates in the past have provided investment banking, financial and advisory services to Epimmune for which services they have received compensation, including separate engagements in 2003 and 2004 relating to private placements of our equity securities. In connection with such engagements, Jefferies received two separate warrants to purchase shares of our common stock, the first for 250,000 shares with an exercise price of $2.33406 per share which expires on September 17, 2006 and the second for 250,000 shares with an exercise price of $2.655 per share which expires on April 12, 2007. In connection with the latter engagement, Jefferies was afforded the right to provide certain financial advisory services to Epimmune, including certain of the services it provided in connection with this transaction. Jefferies and its affiliates currently are providing, and in the future may provide investment banking, financial and advisory services to Epimmune for which services they have received, or expect to receive, compensation.
In the ordinary course of business Jefferies and its affiliates, including Jefferies Group, Inc., Jefferies’ parent company, may publish research reports on the securities of Epimmune, IDM or their affiliates and may actively trade or hold the securities of Epimmune, IDM or their affiliates for their own accounts and for the accounts of customers and, accordingly, may at any time hold long or short positions in those securities.
Interests of Directors, Officers and Affiliates
In considering the recommendation of the Board to vote in favor of the issuance of our common stock in connection with the Exchange and related transactions, stockholders should be aware that some of our executive officers and directors may have interests in the transaction that may be different from, or in addition to, their interests as stockholders. The Board was aware of these interests and considered them, among other things, in making its recommendations. These interests include the following:
Executive options. In September 2003, at the time of our reduction in force, we granted stock options with two-year vesting to all of our employees, including 48,564 shares with an exercise price of $1.54 and a value of $74,789 to Dr. Loria, our President and Chief Executive Officer, 3,399 shares with an exercise price of $1.54 and a value of $5,234 to Dr. Newman, our Vice President, Research and Development, and 4,856 shares with an exercise price of $1.54 and a value of $7,478 to Mr. De Vaere, our Vice President, Finance and Administration and Chief Financial Officer as incentives. Effective as of the closing under the Share Exchange Agreement, these options will immediately vest in full to the extent not already vested.
Executive bonuses. Effective as of the closing under the Share Exchange Agreement, Dr. Loria will be entitled to receive a $375,000 cash bonus and Mr. De Vaere and Dr. Newman will each be entitled to receive a $117,500 cash bonus.
Executive salaries. Effective as of the closing under the Share Exchange Agreement, Drs. Loria and Newman, and Mr. De Vaere will each receive an increase in annual base salary, to account for inflation, of $25,000, $10,000 and $20,000, respectively, retroactive to January 1, 2005.
Executive employment agreements. We have entered into new employment agreements with our executive officers, pursuant to which Dr. Loria will serve as our President and Chief Business Officer, Mr. De Vaere will serve as our Chief Financial Officer, and Dr. Newman will serve as our Vice President, Infectious Diseases, respectively. These employment agreements will become effective as of the closing under the Share
57
Exchange Agreement and will supersede the existing employment agreements and other compensatory arrangements with these executive officers. Please see the section entitled“Agreements Related to the Exchange —Employment Agreements”for a description of the material terms and conditions of these employment agreements.
Options held by certain directors. Effective as of the closing under the Share Exchange Agreement, options to purchase shares of our common stock granted to Howard E. (“Ted”) Greene, Jr., Georges Hibon and William T. Comer, Ph.D., each of whom is a member of the Board and will resign as of the closing, will be amended to remain exercisable until the date that the option would have originally expired but for the resignation of the optionholder from service as a director. Any of such options that have an exercise price as of the closing that is less than the fair market value of our common stock as of March 15, 2005 (the date on which the Board approved the amendment of the options), will remain exercisable until the earlier of (i) the date on which the options would have originally expired but for the resignation of the optionholder from service as a director or (ii) the latest date on which the option can expire without the option being treated as deferred compensation under Section 409A of the Internal Revenue Code of 1986, as amended, referred to as the Code, and the treasury regulations thereunder and subject to the additional tax under Section 409A (which under current guidance would be March 15, 2006 but could be extended).
As of June 29, 2005, the following options to purchase shares of our common stock held by Mr. Greene, Mr. Hibon and Mr. Comer were outstanding:
| | | | | | | | | | | | | |
| | | Number of Shares | | | | | |
| | | Underlying Options | | | | | |
| | | Granted | | | Exercise Price | | | Expiration Date | |
| | | | | | | | | | |
| Howard E. (“Ted”) Greene, Jr. | | | 5,000 | | | $ | 1.92 | | | | 6/14/14 | |
| | | | 5,000 | | | $ | 1.70 | | | | 7/14/13 | |
| | | | 5,000 | | | $ | 1.80 | | | | 6/17/12 | |
| | | | 10,000 | | | $ | 2.85 | | | | 6/17/11 | |
| | | | 20,000 | | | $ | 3.75 | | | | 7/12/09 | |
| | | | 714 | | | $ | 2.25 | | | | 1/01/09 | |
| | | | 714 | | | $ | 10.50 | | | | 1/01/08 | |
| | | | 714 | | | $ | 24.066 | | | | 1/01/07 | |
| | | | 714 | | | $ | 42.875 | | | | 1/01/06 | |
| Georges Hibon | | | 5,000 | | | $ | 1.92 | | | | 6/14/14 | |
| | | | 5,000 | | | $ | 1.70 | | | | 7/14/13 | |
| | | | 5,000 | | | $ | 1.80 | | | | 6/17/12 | |
| | | | 20,000 | | | $ | 2.85 | | | | 8/16/11 | |
| William T. Comer, Ph.D. | | | 5,000 | | | $ | 1.92 | | | | 6/14/14 | |
| | | | 5,000 | | | $ | 1.70 | | | | 7/14/13 | |
| | | | 5,000 | | | $ | 1.80 | | | | 6/17/12 | |
| | | | 10,000 | | | $ | 2.85 | | | | 6/17/11 | |
| | | | 20,000 | | | $ | 3.75 | | | | 7/12/09 | |
| | | | 714 | | | $ | 2.25 | | | | 1/01/09 | |
| | | | 714 | | | $ | 10.50 | | | | 1/01/08 | |
| | | | 714 | | | $ | 24.066 | | | | 1/01/07 | |
| | | | 714 | | | $ | 42.875 | | | | 1/01/06 | |
| | | | 1,428 | | | $ | 33.25 | | | | 12/18/05 | |
Director and officer insurance coverage. We will maintain an extension of coverage of our directors and officers insurance with effect from the closing under the Share Exchange Agreement, for the benefit of those of our directors and officers who are covered by those policies immediately prior to the closing with respect to matters occurring prior to the closing, for six years after the closing. We will not be required to maintain such
58
liability insurance policy to the extent that the annual cost of maintaining such policy exceeds 200% of the premium we currently pay for that insurance.
In considering the recommendation of the Board to vote in favor of the issuance of our common stock in connection with the Exchange and related transactions, stockholders should be aware that some of the IDM executive officers and directors may have interests in the transaction that may be different from, or in addition to, their interests as IDM shareholders. The Board was aware of these interests and considered them among other things in making its recommendations. Please see the section entitled“Risk Factors —Risk Related to the Exchange” for additional information.
Description of Drag-Along Right under the IDM Shareholders Agreement
All of the shareholders of IDM are party to the IDM shareholders agreement. The principal company shareholders who executed the Share Exchange Agreement held more than 85% of the outstanding share capital and voting rights of IDM, including IDM shares issuable upon exercise of outstanding warrants to purchase IDM shares. As a result, under the IDM shareholders agreement, the principal company shareholders had a drag-along right to require each other IDM shareholder to tender its IDM shares in the Exchange pursuant to the Share Exchange Agreement.
In order to exercise the drag-along right, the principal company shareholders were required to send an offer notice to the other IDM shareholders disclosing the principal terms of the Exchange within four business days of the execution of the Share Exchange Agreement. The offer notice was sent to the other IDM shareholders on March 19, 2005.
Upon receipt of the offer notice, each IDM shareholder not a party to the Share Exchange Agreement had the option to either submit a counterbid in accordance with the terms of the IDM shareholders agreement by April 29, 2005 or execute a joinder agreement making such shareholder party to the Share Exchange Agreement. No counterbid was submitted by April 29, 2005 and, as of June 29, 2005, IDM shareholders holding approximately 98% of the outstanding share capital and voting rights of IDM, including IDM shares issuable upon the exercise of outstanding warrants to purchase IDM shares, were either party to the Share Exchange Agreement or had executed a joinder agreement.
Under the IDM shareholders agreement, the closing of the Exchange must occur by July 13, 2005, the date 120 days following the execution of the Share Exchange Agreement. Each principal company shareholder has waived the 120-day deadline by executing the Share Exchange Agreement or a joinder agreement. Because the closing will not occur by July 13, 2005, the IDM shareholders who are not parties to the Share Exchange Agreement will not be required to participate in the Exchange and may remain shareholders of IDM following the Exchange unless they agree to exchange their shares. If the exchange is closed, IDM would become a majority controlled subsidiary of Epimmune.
As of June 29, 2005, the IDM shareholders not parties to the Share Exchange Agreement represented approximately 2% of the outstanding share capital and voting rights of IDM.
Reverse Stock Split; Increase of Authorized Capital Stock
In the Share Exchange Agreement, we agreed to take all action necessary prior to the closing of the transactions under the Share Exchange Agreement to effect a reverse stock split of our outstanding shares of common stock. The ratio of the reverse stock split will be determined prior to the closing so that every four, five, six, seven, eight, nine or ten shares of common stock will be combined into one share. We also agreed to cause our authorized capital stock after giving effect to the reverse stock split to be increased to a total of 65,000,000 shares, consisting of 55,000,000 shares of common stock and 10,000,000 shares of preferred stock. The reverse stock split and the increase in authorized shares are subject to stockholder approval, as described in more detail in this proxy statement in the sections entitled“Proposal 3 —Approval of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation to Effect a Reverse Stock Split”and“Proposal 4 —Approval of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation to Effect an Increase in Authorized Shares.”
59
Accounting Treatment
Because IDM’s shareholders and optionholders will own approximately 78%, on fully diluted basis, of the shares of our common stock after the acquisition, IDM’s designees to our Board will represent a majority of the directors and IDM’s senior management will represent a majority of our senior management, IDM is deemed to be the acquiring company for accounting purposes and the transaction will be accounted for as a reverse acquisition under the purchase method of accounting for business combinations in accordance with accounting principles generally accepted in the United States. Accordingly, the assets and liabilities of Epimmune will be recorded, as of the completion of Exchange, at their respective fair values and added to those of IDM. Our reported results of operations after completion of the transaction will reflect those of IDM, to which the operations of Epimmune will be added from the date of the completion of the transaction. Our operating results will reflect purchase accounting adjustments, including increased amortization and depreciation expense for acquired assets. Additionally, historical financial condition and results of operations shown for comparative purposes in periodic filings subsequent to the completion of the transaction will reflect those of IDM. Furthermore, pursuant to Statements of Financial Accounting Standards No. 141, “Business Combinations,” and No. 142, “Goodwill and other Intangible Assets,” goodwill arising from the transaction will be subject to at least an annual assessment for impairment. Identified intangible assets with finite lives will be amortized over those lives. A final determination of the intangible asset values and required purchase accounting adjustments, including the allocation of the purchase price to the assets acquired and liabilities assumed based on their respective fair values, has not been made. However, for purposes of disclosing unaudited pro forma information in this proxy statement, a preliminary determination has been made of the purchase price allocation, based upon current estimates and assumptions, which is subject to revision upon completion of the transaction.
Amended and Restated Preferred Exchange Agreement
It is a condition to the closing under the Share Exchange Agreement that all of our outstanding preferred stock, consisting of 859,666 shares of our Series S preferred stock and 549,622 shares of our Series S-1 preferred stock, be exchanged for shares of our common stock prior to the closing of the Exchange. On March 15, 2005, we entered into a Preferred Exchange Agreement with G.D. Searle, the holder of all of the outstanding shares of our preferred stock, which was amended in its entirety in the Amended and Restated Preferred Exchange Agreement with G.D. Searle, dated April 12, 2005. Pursuant to this agreement, effective immediately prior to the closing of the Exchange, all outstanding shares of our preferred stock will be exchanged for an aggregate of 1,949,278 shares of our common stock, subject to adjustment for our reverse stock split described in proposal 3 or any other stock split, combination or stock dividend occurring after March 15, 2005. We have agreed to include the shares of our common stock issued in exchange for the outstanding preferred stock in the registration statement described in the following section.
Restrictions on Ability to Sell Epimmune Common Stock; Resale Registration Statement
The shares of our common stock issued in the connection with the transactions contemplated by the Share Exchange Agreement will be issued in reliance on one or more exemptions from the registration requirements of federal and state securities laws. As a result, IDM shareholders may not sell any of the shares of our common stock they receive in the Exchange except pursuant to an effective registration statement under the Securities Act covering the resale of those shares or an applicable exemption under the Securities Act.
Principal Company Shareholders. All principal company shareholders agreed not to transfer any of the shares of our common stock they receive in the Exchange for a period of six months after the closing. On the six-month anniversary of the closing, the restrictions on transfer will be released with respect to 50% of the shares they hold and the remaining 50% will be released on the one-year anniversary of the closing. Each principal company shareholder further agreed to limit daily sales of our common stock during the second six-month period to a maximum number of shares equal to 15% of the average daily trading volume of our common stock as reported by the Nasdaq National Market during the five trading days prior to such date.
60
Transfers of shares of our common stock to family members or affiliates of such principal company shareholder that are not made in open market transactions will not be prohibited or restricted, if the transferee agrees in writing to be bound by such restrictions on transfer or if we consent to such transfer. Notwithstanding the foregoing, these restrictions on sales of our common stock will cease to apply to a principal company shareholder who was an employee of us or IDM as of the closing: (i) if the principal company shareholder is a party to an employment agreement with us or IDM, upon the termination of such principal company shareholder’s employment by us or IDM, as the case may be, without cause, or such principal company shareholder’s resignation for good reason (as “cause” and “good reason” are defined in his employment agreement with us or IDM, as the case may be) or death, or (ii) if the principal company shareholder is not a party to an employment agreement with us or IDM, upon the termination of such principal company shareholder’s employment by us or IDM, his resignation from such employment or death.
Resale Registration Statement. We have agreed to file a registration statement with the SEC as promptly as practicable after the closing of the Exchange, and in any event within 60 days after the closing, providing for an offering to be made on a continuous basis of all of the shares of our common stock issued pursuant to the Share Exchange Agreement and the Amended and Restated Preferred Exchange Agreement. After it has been filed with the SEC, we will use our best efforts to cause the resale registration statement to be declared effective as soon as practicable and keep the resale registration statement continuously effective under the Securities Act for one year after the effective date, or such earlier date when all shares of common stock covered by the resale registration statement have been sold. Under certain circumstances, we will be entitled to postpone or suspend the filing, effectiveness or use of the resale registration statement.
We have agreed to pay all expenses incurred in connection with the resale registration statement, excluding underwriters’ discounts and commissions and any stamp or transfer tax or duty. The Share Exchange Agreement provides for us to provide customary indemnification to the principal company shareholders, and for the principal company shareholders to provide customary indemnification to us, severally but not jointly.
Epimmune Voting Agreement. At the time of execution of the Share Exchange Agreement, our directors and officers entered into a voting agreement, pursuant to which they agreed, among other things, (i) to vote the shares of our common stock that they hold in favor of the Exchange and the other transactions contemplated by the Share Exchange Agreement that will be submitted for approval to our stockholders, and (ii) not to sell, transfer or assign shares of our common stock held by them during the period between the closing of the transactions under the Share Exchange Agreement and the date six months after the closing. On the sixth-month anniversary of the closing, the restrictions on transfer will be released with respect to 50% of the shares they hold and the remaining 50% will be released on the one-year anniversary of the closing. Each stockholder agreed to limit daily sales of shares of our common stock during the second six-month period to a maximum number of shares equal to 15% of the average daily trading volume of shares of our common stock on the Nasdaq National Market. Transfers of shares of our common stock to family members or affiliates of such stockholder that are not made in open market transactions will not be prohibited or restricted, if the transferee agrees in writing to be bound by such restrictions on transfer or if we consent to such transfer. In addition, if the stockholder is an employee of us or IDM upon the consummation of the Exchange and related transactions, upon the subsequent termination of his employment by us or IDM, as the case may be, without cause, or his resignation for good reason (as “cause” and “good reason” are defined in his employment agreement with us or IDM, as the case may be) or death, the restrictions on transfer will cease to apply to him effective as of the date of his employment termination, resignation or death, as applicable.
A more detailed description of the voting agreement can be found in the section entitled“Agreements Related to the Exchange —Voting Agreement.”
Listing of Epimmune Common Stock
The Exchange is conditioned upon, among other things, the receipt of Nasdaq approval of the quotation on the Nasdaq National Market of the shares of our common stock to be issued in the Exchange. In addition, under Nasdaq Marketplace Rule 4330(f), the Nasdaq staff has indicated to us that they will view the Exchange as a “reverse merger.” As a result, we have filed an application with Nasdaq for initial inclusion of our common
61
stock for quotation on the Nasdaq National Market following the closing of the transactions under the Share Exchange Agreement. We will be required to satisfy all the requirements for initial listing on the Nasdaq National Market, including a $5.00 per share minimum bid price for the 90 trading days preceding the closing. We do not currently satisfy the minimum bid price requirement and plan to amend our Amended and Restated Certificate of Incorporation to effect a reverse stock split of our outstanding shares of common stock in order to meet the minimum bid price requirement. The proposed amendment of our Amended and Restated Certificate of Incorporation regarding the reverse stock split can be found in the section entitled“Proposal 3 — Approval of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation to Effect a Reverse Stock Split.”We cannot assure you that Nasdaq will approve the inclusion of our common stock for quotation on the Nasdaq National Market upon the closing of the transactions under the Share Exchange Agreement.
Regulatory and Other Matters
Under French law and under Delaware law, no holder of ordinary shares of IDM or of Epimmune common stock will be entitled to demand appraisal of, or to receive payment for, their shares in connection with the Exchange.
The Exchange is not presently believed to be subject to the requirements of the Hart-Scott-Rodino Antitrust Improvements Act of 1976, referred to as the HSR Act, which prevents transactions meeting certain size tests, and not otherwise exempt, from being completed until required information and materials are furnished to the Antitrust Division of the United States Department of Justice, referred to as the DOJ, and the Federal Trade Commission, referred to as the FTC, and the related waiting period expires or is terminated early. Also, the Exchange is not believed to be subject to approval under the competition laws of the European Union.
Although it is not anticipated that circumstances will change in such a way that prior to the closing HSR Act filings would be required, it is possible that a change could occur and thereby trigger filing requirements. If that were to occur, the parties would, at that time, be required to file notifications with the DOJ and the FTC and wait for the termination or expiration of the waiting period before closing the transaction. The initial waiting period under the HSR Act is 30 days, beginning on the date that both parties complete their filings. The waiting period can be terminated early by action of both the Antitrust Division of the DOJ and by the FTC. Either agency can extend the waiting period by issuing a request for additional information or second request. Such a request extends the waiting period until 30 days after each of the parties has substantially complied with the second request.
Whether or not the parties are subject to the notice and waiting period requirements of the HSR Act, and if so, even if the waiting period has been terminated or expired, the DOJ or the FTC, as well as a foreign regulatory agency or government, state or private person, may challenge the transaction at any time before or after its completion. We cannot assure you that the DOJ or the FTC will not try to prevent the transaction or seek to impose restrictions or conditions on us as a condition of not challenging the transaction. Depending on the nature of any restrictions or conditions, these restrictions or conditions may jeopardize or delay completion of the transaction, or lessen the anticipated benefits of the transaction.
Directors and Management of Epimmune Following the Exchange
Effective as of the closing of the Exchange, the number of directors comprising our Board will be increased to nine and Messrs. Greene and Hibon and Dr. Comer will resign as directors. The Share Exchange
62
Agreement provides that immediately following the closing of the Exchange, our Board will consist of the following nine individuals:
| | | | | | | |
| Name | | Age | | | Current Position Held with Epimmune or IDM |
| | | | | | |
| Michael G. Grey | | | 52 | | | Director of Epimmune |
| Emile Loria, M.D. | | | 55 | | | President, Chief Executive Officer and Director of Epimmune |
| John P. McKearn, Ph.D. | | | 51 | | | Director of Epimmune |
| Jean-Loup Romet-Lemonne, M.D. | | | 55 | | | President and Chief Executive Officer of IDM |
| Donald Drakeman, Ph.D. | | | 52 | | | Director of IDM |
| David Haselkorn, Ph.D. | | | 61 | | | Director of IDM |
| Jean Deleage, Ph.D. | | | 64 | | | Managing General Partner of Alta Partners, an IDM shareholder |
| Sylvie Grégoire, Pharm.D. | | | 43 | | | None |
| Robert Beck, M.D. | | | 51 | | | None |
Mr. Greyhas served as our director since July 1999. Since January 1, 2005, he has served as President and Chief Executive Officer of Structural GenomiX, Inc., a privately held biotechnology company, where he previously served as President from June 2003 to January 1, 2005 and as Chief Business Officer from April 1, 2001 until June 2003. In addition, Mr. Grey has been a member of the board of directors of Structural GenomiX since September 2001. Between January 1999 and September 2001, he served as President and Chief Executive Officer of Trega Biosciences, Inc., a biotechnology company. Prior to joining Trega, Mr. Grey served as President of BioChem Therapeutics, Inc., a division of BioChem Pharma, Inc., a pharmaceutical company, from November 1994 to August 1998. During 1994, Mr. Grey served as President and Chief Operating Officer of Ansan, Inc., a biopharmaceutical company. From 1974 to 1993, Mr. Grey served in various roles with Glaxo, Inc. and Glaxo Holdings, plc, a pharmaceutical company, culminating in his position as Vice President, Corporate Development. Mr. Grey serves on the board of directors of Achillion Pharmaceuticals, Inc.
Dr. Loriahas served as our director since January 2001. He joined us as President and Chief Executive Officer in June 2001. From 1995 to 2000, he served as President and Chief Executive Officer of Biovector Therapeutics, a vaccine company. Prior to his appointment as Chief Executive Officer, he served as Senior Vice President, Business Development at Biovector from 1994 to 1995. From 1986 to 1993, he was founder and Managing Director of MS Medical Synergy, a company specialized in drug delivery. From 1978 to 1985, Dr. Loria held various positions with the pharmaceutical companies Hoffman La Roche-Kontron, Ciba-Geigy and Sanofi Pharma.
Dr. McKearnhas served as our director since April 2000. Since March 2005, he has served as Chief Executive Officer of Kalypsys Inc., a privately held biotechnology company, where he also served as President and Chief Scientific Officer from August 2004 to March 2005 and Chief Scientific Officer from July 2003 to August 2004. In addition, Dr. McKearn has been a member of the board of directors of Kalypsys since July 2003. Prior to that, he was with Pharmacia Corporation, formerly G.D. Searle and Co., a pharmaceutical company, since 1987. From August 2000 until June 2003, he served as Senior Vice President, Pharmacia Discovery Research, responsible for research activities in cardiovascular diseases, arthritis and oncology. Prior to that he served as Vice President, Searle Discovery Research from 1999 to 2000, Executive Director of Oncology from 1995 to 1999, and directed all arthritis, inflammation and oncology research from 1987 to 1995. Dr. McKearn was a Senior Scientist at E.I. DuPont de Nemours and Company, a pharmaceutical company, from 1985 to 1987 and a member of the Basel Institute for Immunology from 1982 to 1985.
Dr. Romet-Lemonne has served as President and Chief Executive Officer of IDM since he founded the company in December 1993. From September 2000 to April 2005, Dr. Romet-Lemonne served as Vice President of France-Biotech, a biotechnology association. Since April 2001, Dr. Romet-Lemonne has served as a director of Natural Implant, a biotechnology company. From May 1988 to July 1991, he served as General Manager and Scientific Director of Transfusion Merieux Innovation, a biotechnology company. Prior to his position with Transfusion Merieux Innovation, Dr. Romet-Lemonne spent twelve years working in the
63
French university hospital system as a medical researcher and assistant professor and four years at Harvard University’s Department of Cancer Biology. He has authored more than 50 international scientific articles. Dr. Romet-Lemonne received his medical degree from the University of Tours.
Dr. Drakemanhas served as President, Chief Executive Officer and a Director of Medarex, a biopharmaceutical company, since its inception in 1987. Since October 2004, Dr. Drakeman has served as Chairman of the New Jersey Commission on Science and Technology. Dr. Drakeman received a bachelor’s degree from Dartmouth College, a J.D. from Columbia University, where he was a Harlan Fiske Stone scholar, and a Ph.D. in the humanities from Princeton University.
Dr. Haselkornis the Chief Executive Officer of 5-5 Technologies, a privately held consulting biotechnology company, the Chairman of Tel Aviv University’s TauTec venture fund scientific advisory board and a director of IDEA AG. From 1998 to 2003, Dr. Haselkorn held the position of Chief Executive Officer of Clal Biotechnology Industries, which was the investment arm of Clal, one of Israel’s largest industrial and financial groups. Dr. Haselkorn represented Clal Biotechnology Industries on IDM’s board of directors from November 2000 through July 2003. From May 1987 to May 1998, Dr. Haselkorn served as the Chief Operating Officer, Managing Director and Senior Vice President for Bio-Technology General Corporation, a biotechnology company. Dr. Haselkorn has served as a director for several biopharmaceutical companies, including D-Pharm Ltd, a pharmaceutical company, from 1995 to 2002, Compugen Ltd, a bioinformatics company, from 1998 to 2004 and Protalix Biotherapeutics Ltd, a biopharmaceutical company, from 1998 to 2004. Dr. Haselkorn received a degree in chemistry and a Masters Degree in biochemistry from The Hebrew University of Jerusalem and received a Ph.D. in chemical immunology from the Weizmann Institute of Science.
Dr. Deleagehas been the managing general partner of Alta Partners, a venture capital partnership investing in information technologies and life science companies, since founding the company in February 1996. From 1979 to 1996, Dr. Deleage served as a managing partner of Burr, Egan, Deleage & Co., a venture capital firm of which he was a founder. In 1971, Dr. Deleage founded Sofinnova, a venture capital firm in France, and in 1976 he founded Sofinnova, Inc., the U.S. subsidiary of Sofinnova. Dr. Deleage currently serves as a director of Kosan Biosciences Incorporated, a biotechnology company, Rigel Pharmaceuticals, Inc., a biopharmaceuticals company, Xcyte Therapies, Inc., a biotechnology company, and several privately held companies. Dr. Deleage received a Baccalaureate in France, a masters degree in electrical engineering from the Ecole superieure d’Electricite, and a Ph.D. in economics from the Sorbonne.
Dr. Gregoirehas been the Chief Executive Officer of GlycoFi, Inc., a biotechnology company, since October 2004. Prior to that, Dr. Gregoire was with Biogen (now Biogen Idec) since 1995, where she led the European development and approval of AVONEX and served as Vice President, Regulatory Affairs from January 1999 to October 2000. She subsequently served as Biogen’s Vice President of Manufacturing from October 2000 to August 2001 and as Executive Vice President of Technical Operations from August 2001 to December 2003. Dr. Gregoire served as a consultant to the biopharmaceutical industry from December 2003 to September 2004. Dr. Gregoire is a non-executive director of Caprion Pharmaceuticals, a privately held proteomics and product company. Dr. Gregoire received a college degree in Sciences from the Seminaire de Sherbrooke, a pharmacy graduate degree from the Universite Laval and a Pharm.D from the State University of New York at Buffalo.
Dr. Beckhas been the Vice President and Chief Information Officer of the Fox Chase Cancer Center since September 2001 and a senior faculty member in the Population Science department since July 2003. From October 1992 to August 2001 he served as a director for the Houston Academy of Medicine — Texas Medical Center Library, where he was the Chair from July 1998 to August 1999 and Interim Executive Director from August 1999 to August 2001. Since March 2000, Dr. Beck has served as a director for RosettaMed, a start-up company based in Houston developing automated patient data entry forms and devices. From July 1997 to June 2000, Dr. Beck served as a director for VidiMedix Corporation, a start-up telemedicine company that was acquired by e-MedSoft.com in June 2000. From August 1992 to September 2001, Dr. Beck served as a Professor of Pathology with the Baylor College of Medicine, where he also served as a Professor of Family and Community Medicine from July 1997 to September 2001, Vice President for Information Research and Planning from July 2000 to September 2001 and Vice President for Information
64
Technology from August 1992 to June 2000. Since July 1999, Dr. Beck has served as an Adjunct Professor of Health Informatics with the University of Texas — Houston Health Science Center.
THE SHARE EXCHANGE AGREEMENT
The following is a description of the material terms of the Share Exchange Agreement. Although we believe that the following description includes the material terms of the agreement, the description may not contain all of the information that is important to you. We encourage you to read carefully this entire proxy statement, including the Share Exchange Agreement and Amendments No. 1, 2, 3 and 4 thereto, attached to this proxy statement as Annex A, for a more complete understanding of the transaction. The following description is subject to, and is qualified in its entirety by reference to, the Share Exchange Agreement.
General
We entered into the Share Exchange Agreement with the principal company shareholders as of March 15, 2005. The closing of the Exchange is expected to occur following:
| | |
| | • | the approval of the issuance of shares of our common stock in the Exchange and the other matters related to the Exchange, each as described in proposals 1, 2, 3 and 4 by our stockholders; |
| |
| | • | the satisfaction or waiver of the terms and conditions set forth in the IDM shareholders agreement, including the exercise of the drag-along right by the principal company shareholders, if required; and |
| |
| | • | the satisfaction or waiver of the other conditions to the Exchange. |
We expect that the closing of the transactions contemplated by the Share Exchange Agreement will occur as soon as possible after receipt of the requisite approvals from our stockholders.
The Exchange
In accordance with the Share Exchange Agreement, all outstanding IDM shares, except as discussed in the following paragraph, will be exchanged for shares of our common stock. As of June 29, 2005, principal company shareholders who own in the aggregate approximately 98% of the shares of capital stock and voting rights of IDM, including IDM shares issuable upon exercise of outstanding warrants, were parties to the Share Exchange Agreement as of the date it was signed or had entered into a joinder agreement to become a party to the Share Exchange Agreement. Following the execution of the Share Exchange Agreement, the principal company shareholders notified the other IDM shareholders of their intent to exercise their right under the IDM shareholders agreement to require all other IDM shareholders to participate in the Exchange on the same terms and conditions as the principal company shareholders. For additional information regarding this right and the IDM shareholders agreement, please see the section entitled“The Exchange — Description of Drag-Along Right under the IDM Shareholders Agreement.”
Certain IDM shares and warrants are held in PEAs and are referred to as PEA shares and PEA warrants, respectively. A PEA is a tax efficient vehicle under French law whereby a holder of securities may receive preferential tax treatment provided that, among other things, the securities are held in a separate account for a certain period of time. As of June 29, 2005, the PEA shares and the PEA warrants represented approximately 1.2% of the total number of IDM shares outstanding on a fully diluted basis. The treatment of the PEA shares and the PEA warrants in the Exchange is described below in the section entitled“The Share Exchange Agreement — Treatment of IDM Shares and IDM Warrants Held in a PEA.”
Based on the exchange ratio, as explained in more detail below, and the number of IDM shares and options to purchase IDM shares outstanding as of June 29, 2005, a total of approximately 74,860,000 shares of our common stock and options to purchase approximately 4,365,000 shares of our common stock will be issued in the Exchange, including shares of our common stock to be issued pursuant to the Put/ Call Agreements and the Option Liquidity Agreements. Immediately following the closing of the Exchange, IDM shareholders and optionholders will own shares of our common stock representing in the aggregate approximately 78% of our outstanding common stock, on a fully diluted basis.
65
Exchange Ratio
In the Exchange, each outstanding IDM share, except for PEA shares, will be exchanged for 3.771865 shares of our common stock. This exchange ratio will be adjusted to reflect the effect of our reverse stock split and any other stock split, reverse stock split, stock dividend (including any dividend or distribution of securities convertible into our common stock or IDM shares), extraordinary cash dividends, reorganization, recapitalization, reclassification, combination, exchange of shares or other like change with respect to our common stock or IDM shares occurring on or after the date of the Share Exchange Agreement and prior to the closing of the Exchange. Our reverse stock split is subject to stockholder approval, as described in more detail in this proxy statement in the section entitled“Proposal 3 — Approval of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation to Effect a Reverse Stock Split.”
At the closing, we will deliver to each IDM shareholder the number of shares of our common stock equal to the exchange ratio in exchange for each outstanding IDM share held by such shareholder, less the sum of (A) 10% of such number of shares, which represent shares of our common stock to be held in escrow pursuant to the terms of the Share Exchange Agreement and the Indemnity Escrow Agreement (as defined below), and (B) 1.2% of such number of shares, which represent shares of our common stock to be held in escrow pursuant to the terms of the Share Exchange Agreement and the Expense Escrow Agreement (as defined below).
No fractional shares of our common stock will be issued in the Exchange. Instead, each holder of a fractional share interest will receive cash, without interest, in an amount equal to the holder’s fractional share interest multiplied by the average of the closing prices on the Nasdaq National Market of shares of our common stock during the five consecutive trading days ending on and including the trading day immediately preceding the closing of the Exchange.
Treatment of IDM Options
All IDM options granted under the IDM 1998 Stock Option Plan or the IDM S.A. 2000 Stock Option Plan will remain outstanding in accordance with their existing terms following the closing of the transactions under the Share Exchange Agreement, except as otherwise described below.
Prior to and effective as of the closing of the Exchange, we will offer each holder of IDM options who resides in France, referred to as a French optionholder, the right to enter into an Option Liquidity Agreement with us. Pursuant to the terms of the Option Liquidity Agreement, each French optionholder who enters into an Option Liquidity Agreement with us and subsequently exercises its IDM option by satisfying the conditions for exercise, including paying any requisite exercise price, will eventually transfer to us each IDM share acquired upon exercise of the IDM option in exchange for the number of shares of our common stock equal to the exchange ratio. The Option Liquidity Agreements are described in more detail in this proposal 1 in the section entitled“Agreements Related to the Exchange — Option Liquidity Agreements.” The form of Option Liquidity Agreement is attached to this proxy statement asAnnex D.
Prior to the closing of the transactions under the Share Exchange Agreement and effective as of the closing, we will offer each holder of IDM options who resides outside of France, referred to as a non-French optionholder, the right to waive such holder’s rights in his or her IDM options in exchange for substitute options issued under our 2000 Plan. The substitute option will be vested to the same extent as the IDM option for which the substitute option was exchanged as of the date the substitute option is granted, and will continue to vest in accordance with the same vesting schedule applicable to the related IDM option. In addition, the substitute option will be exercisable for the remaining period that the IDM option for which it was exchanged is exercisable. However, if the amendment to increase the shares available for issuance under our 2000 Plan described in proposal 5 is not approved by our stockholders, we will not be required to offer the substitute options and the non-French optionholders will instead have the same right as the French optionholders to enter into an Option Liquidity Agreement as described above. Each substitute option will entitle its holder to acquire the number of shares of our common stock equal to the number of IDM shares that were issuable upon exercise of the related IDM option immediately prior to closing multiplied by the exchange ratio. The per share exercise price of each substitute option will be equal to the per share exercise price for each related IDM option (as converted from Euros to U.S. dollars) divided by the exchange ratio.
66
Up to 4,365,000 shares of our common stock, subject to adjustment for stock splits, combinations and the like, may be issued in exchange of IDM shares acquired upon exercise of IDM options or pursuant to the exercise of substitute options for the IDM options.
Treatment of IDM Warrants
Each warrant to purchase IDM shares with an exercise price immediately prior to the closing of less than€6.32, referred to as an IDM warrant, other than IDM warrants held in a PEA, will be exchanged for shares of our common stock. Each IDM warrant will be exchanged for the number of shares of our common stock (rounded to the nearest whole number of shares) equal to (A) the difference between (1) the number of IDM shares issuable upon exercise of the related IDM warrant immediately prior to the closing of the Exchange and (2) the result of the aggregate exercise price payable upon the exercise of the related IDM warrant divided by€6.32, multiplied by (B) the exchange ratio. The holder of any IDM warrant with an exercise price immediately prior to the closing of at least€6.32, effective as of the closing, will irrevocably waive all of its rights in such warrant.
Medarex holds warrants issued by IDM in July 2000, referred to as the Medarex warrants, to subscribe for convertible redeemable bonds and has agreed to exercise the Medarex warrants in full immediately prior to the closing, convert the convertible redeemable bonds issued upon exercise of the Medarex warrants into IDM shares and exchange such IDM shares for shares of our common stock in the Exchange.
Sanofi-Aventis holds warrants issued by IDM in July 1999, referred to as the Sanofi warrants, to purchase IDM shares and has agreed to exercise the Sanofi warrants in full immediately prior to the closing for IDM shares and exchange such IDM shares for shares of our common stock in the Exchange.
Treatment of IDM Shares and IDM Warrants Held in a PEA
Holders of PEA shares will have the option either (i) to exchange their PEA shares for shares of our common stock in the Exchange or (ii) to enter into a Put/ Call Agreement with us. Pursuant to the terms of the Put/ Call Agreement, holders of PEA shares will have the right to require us to purchase, and we will have the right to require such holders to sell, the PEA shares for a period of 30 days after the closing of our first offering of equity securities completed after the closing of the transactions under the Share Exchange Agreement with net aggregate proceeds of at least 10 times the U.S. dollar amount payable to the holders of all PEA shares, referred to as the first equity financing, excluding any issuance of equity securities in a strategic partnering, licensing, merger or acquisition transaction. The aggregate purchase price for PEA shares, payable in cash, will be equal to the (A) the number of PEA shares multiplied by the exchange ratio, multiplied by (B) the price per share of our common stock sold in the first equity financing, less underwriters’ discounts or commissions. If the first equity financing does not close within two years following the closing of the Exchange, each PEA share subject to a Put/ Call Agreement will be exchanged for a number of shares of our common stock equal to the exchange ratio. The form of Put/ Call Agreement is attached to this proxy statement asAnnex C.
Each PEA warrant held immediately prior to the closing will be exchanged immediately after the closing for the number of IDM shares issuable to the holder upon exercise of the PEA warrant, less the result of the aggregate exercise price payable upon the exercise of such PEA warrant divided by 6.32. Each holder of a PEA warrant will inform us whether it will elect to either (i) exchange the PEA shares received in exchange for its PEA warrant for shares of our common stock pursuant to the Exchange at the closing or (ii) enter into a Put/ Call Agreement with respect to the PEA shares received in exchange for its PEA warrant.
Indemnity Escrow
At or prior to the closing of the Exchange, we and the shareholder representative will enter into an escrow agreement, referred to as the Indemnity Escrow Agreement, with an escrow agent reasonably acceptable to the shareholder representative and us, referred to as the indemnity escrow agent. At the closing, we will deliver to the indemnity escrow agent stock certificates issued in the name of each principal company shareholder representing the number of shares of our common stock equal to 10% of the number of shares of our common
67
stock otherwise issuable to the principal company shareholder in the Exchange, referred to as the indemnity escrow shares. The indemnity escrow shares will secure the indemnification obligations of the principal company shareholders under the Share Exchange Agreement and described below in the section entitled“The Share Exchange Agreement — Indemnification Provisions.”
Expense Escrow
At or prior to the closing of the Exchange, the principal company shareholders and the shareholder representative will enter into an escrow agreement, referred to as the Expense Escrow Agreement, with an escrow agent reasonably acceptable to the principal company shareholders and the shareholder representative, referred to as the expense escrow agent. At the closing, we will deliver to the expense escrow agent stock certificates issued in the name of each principal company shareholder representing the number of shares of our common stock equal to 1.2% of the number of shares of our common stock otherwise issuable to such principal company shareholder in the Exchange, referred to as the expense escrow shares. The expense escrow shares will secure the indemnification and reimbursement obligations of the principal company shareholders to the shareholder representative under the Share Exchange Agreement and/or the Indemnity Escrow Agreement.
Representations and Warranties
We and the principal company shareholders each made a number of representations and warranties in the Share Exchange Agreement regarding aspects of Epimmune’s and IDM’s respective businesses, financial condition, structure and other facts pertinent to the Exchange. In addition, the Share Exchange Agreement included representations and warranties of the principal company shareholders with regard to their ownership of IDM shares.
We made representations and warranties, and the Share Exchange Agreement included representations and warranties regarding IDM, as to:
| | |
| | • | corporate organization and qualification to conduct business; |
| |
| | • | subsidiaries; |
| |
| | • | certificate of incorporation, bylaws and minute books or their equivalents; |
| |
| | • | capitalization; |
| |
| | • | authorization of the Exchange by the respective companies and stockholders’ vote required to approve the Exchange and related agreements; |
| |
| | • | the effect of the Exchange on obligations of the respective companies under applicable laws and existing contractual arrangements; |
| |
| | • | regulatory approvals required to complete the Exchange; |
| |
| | • | permits required to conduct business and compliance with those permits; |
| |
| | • | financial statements; |
| |
| | • | changes in the respective companies’ business since September 30, 2004; |
| |
| | • | litigation; |
| |
| | • | employee benefit plans; |
| |
| | • | labor and employment matters; |
| |
| | • | properties owned or leased; |
| |
| | • | intellectual property; |
| |
| | • | taxes; |
| |
| | • | applicable environmental laws; |
| |
| | • | material agreements, contracts and commitments; |
| |
| | • | insurance; |
| |
| | • | approval by the respective companies’ boards of directors; |
68
| | |
| | • | certain unlawful business practices; |
| |
| | • | interested party transactions; and |
| |
| | • | payments, if any, required to be made to brokers and agents on account of the Exchange. |
In addition, we made representations and warranties as to:
| | |
| | • | the conversion price of our preferred stock; |
| |
| | • | the expiration of the Rights Agreement between American Stock Transfer & Trust Company and us; |
| |
| | • | filings and reports with applicable securities regulators; |
| |
| | • | correspondence from or to the SEC and the Nasdaq National Market; |
| |
| | • | the opinion of our financial advisor; and |
| |
| | • | the termination of the agreement and plan of merger with Anosys, Inc. |
The principal company shareholders made representations and warranties as to:
| | |
| | • | corporate organization and qualification or legal capacity, as the case may be, to enter into the Share Exchange Agreement; |
| |
| | • | authorization of the Share Exchange Agreement by the principal company shareholders; |
| |
| | • | the effect of the Exchange on obligations of the principal company shareholders under applicable laws and existing contractual arrangements; |
| |
| | • | regulatory approvals required to complete the Exchange; |
| |
| | • | title to the shares held by the principal company shareholders; and |
| |
| | • | investment experience and status. |
All representations and warranties of the principal company shareholders, and the representations and warranties regarding IDM, will survive the closing of the Exchange for a period of six months. All of our representations and warranties terminate at the closing of the Exchange.
The representations and warranties included in the Share Exchange Agreement are complicated and not easily summarized. We urge you to carefully read the articles of the Share Exchange Agreement entitled “Representations and Warranties Regarding the Company” relating to IDM, “Representations and Warranties Regarding the Issuer” relating to us, and “Representations and Warranties Regarding the Principal Company Shareholders” relating to the principal company shareholders.
The representations and warranties contained in the Share Exchange Agreement are made for the purposes of allocation of risk and as conditions to closing, may be modified, qualified and subject to exceptions in the disclosure schedules provided in accordance with the Share Exchange Agreement. Accordingly, you should not rely on the representations and warranties as characterizations of the actual state of facts, since they are modified in important part by the underlying disclosure schedules. Moreover, information concerning the subject matter of the representations and warranties may have changed since the date of the Share Exchange Agreement.
Conduct of Business Prior to the Exchange
We and the principal company shareholders agreed that unless the Share Exchange Agreement states otherwise, until the earlier of the completion of the Exchange or unless we or IDM consents in writing, each company will:
| | |
| | • | conduct its business in the ordinary course of business and in a manner consistent with past practice and each company’s respective 2005 budget; |
| |
| | • | use its reasonable best efforts to preserve substantially intact its present business organization; |
| |
| | • | use its reasonable best efforts to keep available the services of its present officers, employees and consultants; and |
| |
| | • | use its reasonable best efforts to preserve its relationships with customers, suppliers and others with which it has significant business relations. |
69
We and the principal company shareholders also agreed that, unless the Share Exchange Agreement states otherwise, until the earlier of the completion of the Exchange or unless we or IDM consents in writing, each company will conduct its business in compliance with certain specific restrictions related to the following:
| | |
| | • | the amendment to its organizational documents; |
| |
| | • | the issuance, encumbrance, purchase, grant or other disposition of its securities or assets; |
| |
| | • | the issuance of dividends or other distributions; |
| |
| | • | the reclassification, combination, split, subdivision, redemption or acquisition of its securities; |
| |
| | • | the acquisition of any stock or assets of another company; |
| |
| | • | the incurrence of indebtedness; |
| |
| | • | the entering into of any agreements other than agreements entered into in the ordinary course of business and consistent with past practice; |
| |
| | • | the commitment to any single capital expenditure over $100,000, or capital expenditures in aggregate in excess of $500,000; |
| |
| | • | the adoption, amendment or increase of director or employee compensation, benefit plans, policies or arrangements; |
| |
| | • | changing of accounting policies and procedures; |
| |
| | • | making of tax elections; |
| |
| | • | the payment or settlement of liabilities; |
| |
| | • | the modification, amendment or termination of material contracts; |
| |
| | • | the commencement or settlement of a litigation or other proceeding; |
| |
| | • | the maintenance, sale, assignment and license of intellectual property; or |
| |
| | • | the announcement of an intention, entering into an agreement, or making a commitment, to do any of the foregoing. |
In addition, we will conduct our business in compliance with specific restrictions relating to the timely filing of reports with the SEC.
The agreements related to the conduct of IDM’s business and our business in the Share Exchange Agreement are complicated and not easily summarized. We urge you to carefully read the section of the Share Exchange Agreement entitled “Conduct of Business Pending the Closing” relating to both IDM and us.
No Solicitation
We and the principal company shareholders further agreed not to engage in any and all activities, discussions or negotiations with any parties with respect to any competing transactions. A “competing transaction” as defined in the Share Exchange Agreement is a transaction, other than the Exchange, involving any of the following:
| | |
| | • | any merger, consolidation, share exchange, business combination, recapitalization, liquidation, dissolution or other similar transaction involving IDM or us; |
| |
| | • | any sale, lease, exchange, transfer or other disposition of all or a substantial part of the assets of IDM or us; |
| |
| | • | any sale, exchange, transfer or other disposition of IDM securities representing 15% or more of the voting power of or equity interest in IDM or us; |
| |
| | • | any tender offer or exchange offer that, if consummated, would result in any person beneficially owning IDM securities representing 15% or more of the voting power of or equity interest in IDM or us; |
| |
| | • | in our case, any solicitation in opposition to approval of any transactions related to the Share Exchange Agreement; or |
70
| | |
| | • | any other transaction the consummation of which would reasonably be expected to impede, interfere with, prevent or materially delay any of the transactions contemplated under the Share Exchange Agreement and related agreements. |
Until the Exchange is completed or the Share Exchange Agreement is terminated, we and the principal company shareholders agreed not to, and the principal company shareholders will not authorize and will use their reasonable best efforts not to permit any of IDM or its directors, officers, employees, agents, advisors or other representatives to, directly or indirectly, take any of the following actions:
| | |
| | • | solicit, initiate, or knowingly facilitate or encourage, or take any other action knowingly to facilitate any inquiries or the making of a proposal or offer that constitutes, or may be reasonably expected to lead to, a competing transaction; |
| |
| | • | participate in discussions or negotiations with a third party in furtherance of any inquiries or attempts to obtain a competing transaction; or |
| |
| | • | agree to or endorse a competing transaction. |
We and the principal company shareholders agreed to inform each other promptly if any proposal or offer regarding a competing transaction is made, if any inquiry or contact with the party making the proposal regarding a competing transaction is made, or if any meeting of the board of directors of either company is held in which the board of directors is reasonably expected to consider any competing transaction.
Our Board may, without breaching the Share Exchange Agreement, respond to an unsolicited, bona fide, written proposal or offer regarding a competing transaction by discussing the proposal with the party making the proposal and by furnishing information to the party making the proposal if all of the following conditions are met:
| | |
| | • | our Board determines in good faith, after having consulted with its current financial advisor and outside counsel, that the proposal constitutes, or would reasonably be expected to lead to, a superior proposal (as defined below); |
| |
| | • | our Board determines in good faith, after having consulted with its outside counsel, that its fiduciary obligations require it to do so; |
| |
| | • | we provide prior written notice to IDM and the shareholder representative of our intent to enter into discussions with or furnish information to the party making the proposal; and |
| |
| | • | we receive from the party making the proposal an executed confidentiality agreement. |
A “superior proposal” is an unsolicited written bona fide offer made by a third party to consummate any of the following transactions:
| | |
| | • | a merger, consolidation, share exchange, business combination or other similar transaction involving us pursuant to which the stockholders of such party immediately preceding such transaction would hold less than 50% of the equity interest in the surviving or resulting entity; or |
| |
| | • | the acquisition by any person or group (including by means of a tender offer, exchange offer or two-step transaction involving a tender offer followed by a cash-out merger) of ownership of 100% of the outstanding shares of our stock; and |
where our Board determines in good faith, after having consulted with its current financial advisor and outside counsel, the transaction is more favorable to our stockholders from a financial point of view than the Exchange and is reasonably capable of being completed on the proposed terms.
Side Letter between IDM and Epimmune
IDM entered into a side letter agreement with us pursuant to which IDM agreed to ensure the access to officers, employees, agents, properties, offices and other facilities of IDM and its subsidiaries and to the books and records thereof; and the delivery of information concerning the business, properties, contracts, assets,
71
liabilities, personnel and other aspects of IDM as we or our representatives may reasonably request. IDM also agreed not to solicit or encourage another merger, acquisition or other transaction with a third party that would compete with the Exchange. The rights and obligations of IDM under the side letter are similar in all respects to our corresponding rights and obligations under the Share Exchange Agreement.
Corporate Governance Matters
We agreed to take all actions, as of the closing of the Exchange, as may be necessary to cause:
| | |
| | • | the number of directors comprising the Board to be increased to nine; |
| |
| | • | the resignation of each of Messrs. Greene and Hibon and Dr. Comer as members of our Board; |
| |
| | • | the appointment of Drs. Romet-Lemonne, Deleage, Drakeman, Haselkorn and Beck and Ms. Grégoire to our Board; |
| |
| | • | the appointment of Dr. Romet-Lemonne as the chairman of our Board; |
| |
| | • | the maintenance of our headquarters at our current headquarters in San Diego, California; |
| |
| | • | the change of our corporate name to IDM, Inc. or such other name as we and IDM may mutually agree upon, subject to the receipt of the approval of our stockholders at our annual meeting; and |
| |
| | • | the appointment of Dr. Romet-Lemonne as our Chief Executive Officer, Dr. Loria as our President and Chief Business Officer and Mr. De Vaere as our Chief Financial Officer. |
In addition, immediately prior to the closing of the Exchange, we agreed to take all actions as may be necessary to:
| | |
| | • | effect a reverse stock split of our common stock pursuant to which four, five, six, seven, eight, nine, or ten shares of our common stock will be consolidated into one share of our common stock; and |
| |
| | • | cause our authorized stock to be increased to a total of 65,000,000 shares, consisting of 55,000,000 shares of common stock and 10,000,000 shares of preferred stock, after giving effect to our reverse stock split. |
Other Agreements
Under the Share Exchange Agreement, we and the principal company shareholders have made additional agreements as follows:
| | |
| | • | The principal company shareholders have agreed not to transfer any of the shares of our common stock they receive in the Exchange for six months after closing and to additional restrictions on transfer of the shares of our common stock they receive in the Exchange prior to the one-year anniversary of the closing. For additional information regarding the restrictions on transfer, please see the section entitled“The Exchange — Restrictions on Ability to Sell Epimmune Common Stock; Resale Registration Statement.” |
| |
| | • | We have agreed to file a registration statement with the SEC within 60 days after the closing of the Exchange providing for an offering to be made on a continuous basis of all of the shares of our common stock issued pursuant to the Share Exchange Agreement and the Amended and Restated Preferred Exchange Agreement. For additional information regarding the filing of the registration statement, please see the section entitled“The Exchange — Restrictions on Ability to Sell Epimmune Common Stock; Resale Registration Statement.” |
| |
| | • | We have agreed to maintain an extension of coverage under our directors and officers insurance policy for six years after the closing of the Exchange for those of our directors and officers who are covered by those policies immediately prior to the closing with respect to matters occurring prior to the closing. We have also agreed to maintain an extension of coverage of IDM’s directors and officers insurance policy for six years after the closing of the Exchange for those of directors and officers of IDM who are |
72
| | |
| | | covered by those policies immediately prior to the closing with respect to matters occurring prior to the closing. We will not be required to maintain such liability insurance policy to the extent that the annual cost of maintaining such policy exceeds 200% of the premium we or IDM currently pay for that insurance. |
| |
| | • | We have agreed to purchase directors and officers liability insurance policies with an effective date as of the closing on commercially available terms and conditions. |
Conditions to Completion of the Exchange
Our obligation and the obligation of the principal company shareholders to complete the Exchange are subject to the satisfaction or waiver of the following conditions, among others:
| | |
| | • | all transactions related to the Share Exchange Agreement must have been approved by our stockholders in accordance with the regulations of the Nasdaq National Market, the Delaware General Corporate Law, referred to as the DGCL, and our certificate of incorporation; |
| |
| | • | no law, rule, regulation, judgment, decree, executive order or award has been enacted or entered into which has the effect of making the Exchange illegal or otherwise prohibiting completion of the Exchange; |
| |
| | • | the shares of our common stock to be issued in connection with the Exchange must have been authorized for quotation on the Nasdaq National Market; |
| |
| | • | all of the outstanding shares of our preferred stock must have been exchanged for shares of our common stock in accordance with the terms of the Amended and Restated Preferred Exchange Agreement; |
| |
| | • | either all IDM shareholders have waived their rights under Section 6 of the IDM shareholders agreement, or the principal company shareholders have complied with the requirements of Section 6 of the IDM shareholders agreement and are permitted thereunder to require the other IDM shareholders who have not executed a joinder agreement to exchange their IDM shares for shares of our common stock pursuant to the Exchange; |
| |
| | • | we must have effected the reverse stock split and an increase in our authorized stock as contemplated in the Share Exchange Agreement and described in proposals 3 and 4 herein; and |
| |
| | • | the principal company shareholders own in the aggregate at least 95% of IDM shares outstanding as of the closing of the Exchange (including IDM shares issuable upon exercise of IDM warrants outstanding as of the closing of the Exchange) and no other IDM shareholder owns 5% or more of the outstanding IDM shares as of the closing of the Exchange. |
Our obligation to complete the Exchange is subject to the satisfaction or waiver of the following additional conditions, among others:
| | |
| | • | the representations and warranties of IDM and the principal company shareholders must be true and correct as of the closing, as though made on and as of the closing, except where the failure of such representations to be so true and correct would not, individually or in the aggregate, have a Company Material Adverse Effect (as defined below); |
| |
| | • | the principal company shareholders must in all material respects perform or comply with all their respective agreements and covenants required by the Share Exchange Agreement to be performed or complied with on or prior to the closing; |
| |
| | • | no Company Material Adverse Effect can have occurred since the execution of the Share Exchange Agreement; and |
| |
| | • | the shareholder representative must have entered into the Indemnity Escrow Agreement and the Indemnity Escrow Agreement shall be in full force and effect at the closing. |
73
The principal company shareholders’ obligation to complete the Exchange is subject to the satisfaction or waiver of the following additional conditions:
| | |
| | • | our representations and warranties must be true and correct as of the closing, as though made on and as of the closing, except where the failure of such representations to be so true and correct would not, individually or in the aggregate, have an Issuer Material Adverse Effect (as defined below); |
| |
| | • | we must in all material respects perform or comply with all our agreements and covenants required by the Share Exchange Agreement to be performed or complied with on or prior to the closing; |
| |
| | • | no Issuer Material Adverse Effect can have occurred since the signing of the Share Exchange Agreement; |
| |
| | • | we must have entered into the Indemnity Escrow Agreement and the Indemnity Escrow Agreement must be in full force and effect at the closing; |
| |
| | • | we must have complied with our obligations related to IDM options and must have entered into Option Liquidity Agreements with each holder of IDM options who has tendered a signed Option Liquidity Agreement to us; and |
| |
| | • | we must have entered into Put/ Call Agreements with each holder of PEA shares as of the date of the Share Exchange Agreement who has tendered a signed Put/ Call Agreement to us. |
Definition of Material Adverse Effect. Under the Share Exchange Agreement, either an “Issuer Material Adverse Effect” or a “Company Material Adverse Effect” is defined to mean any event, circumstance, change or effect that, either individually or in the aggregate with all other events, circumstances, changes and effects, is or would reasonably be likely to be materially adverse to the business, condition (financial or otherwise), assets, liabilities or results of operations of the applicable company and its subsidiaries taken as a whole.
In addition, a “Company Material Adverse Effect” is defined as any event, circumstance, change or effect that, individually or in the aggregate with all other events, circumstances, changes and effects, results in the receipt by IDM of a notice from any drug regulatory agency, whether in writing or based on minutes of a call or meeting with such drug regulatory agency, which is or would be likely to be materially adverse to the manufacturing, sale, marketing and distribution of Mepact.
However, under the terms of the Share Exchange Agreement, none of the following will be taken into account in determining whether there has been or will be an Issuer Material Adverse Effect or a Company Material Adverse Effect:
| | |
| | • | changes in general economic conditions in the United States or the European Union or changes in U.S. securities markets in general; |
| |
| | • | general changes in the industry in which we and IDM or its subsidiaries operate, except those events, circumstances, changes or effects that adversely affect us or IDM or its subsidiaries to a materially greater extent than they affect other entities operating in the same industry; |
| |
| | • | the public announcement of or pendency of the Exchange and related transactions; or |
| |
| | • | changes in accounting requirements or applicable laws, rules or regulations after the date of the Share Exchange Agreement. |
Termination of the Share Exchange Agreement
The Share Exchange Agreement may be terminated and the Exchange and related transactions may be abandoned at any time prior to the closing as follows:
| | |
| | • | by mutual written consent of the shareholder representative on behalf of the principal company shareholders and us; |
74
| | |
|
| | • | by either the shareholder representative on behalf of the principal company shareholders or us if the closing has not occurred before August 26, 2005, referred to as the end date, provided that the right to terminate the Share Exchange Agreement will not be available to any party whose failure to fulfill any obligation under the Share Exchange Agreement was the cause of, or resulted in, the failure of the closing to occur on or before such date; |
|
| |
| | • | by either the shareholder representative on behalf of the principal company shareholders or us if any governmental authority has taken action which has become final and nonappealable and has the effect of making completion of the Exchange illegal or otherwise preventing or prohibiting completion of the Exchange; |
| |
| | • | by the shareholder representative on behalf of the principal company shareholders (at any time prior to the approval of the transactions related to the Exchange by the required vote of our stockholders) if any of the following events, each referred to as a triggering event, has occurred: |
| | |
| | - | our Board withdraws, modifies or changes its recommendation of the Share Exchange Agreement and related transactions in a manner adverse to the principal company shareholders; |
| |
| | - | our Board recommends to our stockholders a competing transaction or enters into any letter of intent or similar document or any agreement, contract or commitment accepting any competing transaction; or |
| |
| | - | a tender offer or exchange offer for 15% or more of our outstanding stock is commenced and our Board recommends in favor of such tender offer or exchange offer or fails to recommend against acceptance of such tender offer or exchange offer by our stockholders. |
| | |
| | • | by either the shareholder representative on behalf of the principal company shareholders or us if any of the proposals 1, 3 or 4 in this proxy statement has failed to receive the requisite votes for approval at the annual meeting; |
| |
| | • | by us upon a breach of any representation, warranty, covenant or agreement regarding or on the part of IDM or the principal company shareholders set forth in the Share Exchange Agreement, or if any representation or warranty regarding or on the part of IDM or the principal company shareholders has become untrue, in either case such that the conditions, related to the truth of their representations and warranties and their compliance with all agreements and covenants required by the Share Exchange Agreement as set forth in the Share Exchange Agreement in relation to our obligation to complete the Exchange, would not be satisfied, except that if any such breach is curable by IDM or the principal company shareholders, we cannot terminate the Share Exchange Agreement for so long as IDM or the principal company shareholders continue to exercise their reasonable best efforts to cure such breach, unless such breach is not cured within 30 days after notice of such breach is provided by us to the shareholder representative; |
| |
| | • | by the shareholder representative on behalf of the principal company shareholders upon a breach of any of our representations, warranties, covenants or agreements set forth in the Share Exchange Agreement, or if any of our representations or warranties has become untrue, in either case such that the conditions, related to the truth of our representations and warranties and our compliance with all agreements and covenants required by the Share Exchange Agreement as set forth in the Share Exchange Agreement in relation to the principal company shareholders’ obligation to complete the Exchange, would not be satisfied, except that if any such breach is curable by us, the shareholder representative cannot terminate the Share Exchange Agreement for so long as we continue to exercise our reasonable best efforts to cure such breach, unless such breach is not cured within 30 days after notice of such breach is provided by the shareholder representative to us; or |
| |
| | • | by us, if, prior to the annual meeting we receive a superior proposal, our Board resolves to accept such superior proposal (after our Board has determined that such acceptance is required to comply with its fiduciary duties to our stockholders under applicable law) and we provide the shareholder representa- |
75
| | |
| | | tive with four business days’ prior written notice of our intention to terminate the Share Exchange Agreement. |
Payment of Fees and Expenses
Expenses. All fees and expenses incurred in connection with the Share Exchange Agreement and any related transactions will be paid by the party incurring such expenses, whether or not the Exchange or any other transaction is completed.
Termination Fee. We will promptly, but no later than one business day after the first of such events has occurred, pay to IDM a termination fee of $1,334,600 if any of the following conditions occur:
| | |
| | • | the shareholder representative terminates the Share Exchange Agreement due to the occurrence of a triggering event; |
| |
| | • | (a) we or the shareholder representative terminate the Share Exchange Agreement pursuant to the failure of the Exchange to close on or before the end date or the failure of the Exchange, the issuance of our common stock pursuant to the Option Liquidity Agreements, the increase of our authorized capital stock or the reverse stock split to receive the requisite vote for approval at the annual meeting, (b) prior to the time of such termination, a competing transaction has been publicly announced, and (c) we enter into an agreement providing for a third party acquisition (as defined below) within nine months after the date of such termination or a third party acquisition is completed within nine months after the date of such termination; or |
| |
| | • | we terminate the Share Exchange Agreement after having received a superior proposal and after our Board has determined that acceptance of such superior proposal is required to comply with the Board’s fiduciary duties to our stockholders under applicable law. |
A “third party acquisition” means any of the following transactions:
| | |
| | • | a merger, consolidation, business combination, recapitalization, liquidation, dissolution or similar transaction involving us pursuant to which our stockholders immediately preceding such transaction hold less than 50% of the aggregate equity interests in the surviving or resulting entity of such transaction or of any direct or indirect parent thereof; |
| |
| | • | a sale or other disposition by us of assets representing in excess of 50% of the aggregate fair market value of our business immediately prior to such sale or other disposition; |
| |
| | • | an acquisition by any person or group (including by way of a tender offer or an exchange offer) or an issuance of capital stock by us, directly or indirectly, of beneficial ownership of 50% or more of the voting power of the then outstanding shares of our stock; |
| |
| | • | our adoption of a plan of liquidation or declaration of payment of an extraordinary dividend; or |
| |
| | • | our repurchase of 50% or more of the outstanding shares of our stock. |
Failure to Pay. In the event that we fail to pay the termination fee when due, we will also be obligated to pay the costs and expenses actually incurred or accrued by IDM (including, without limitation, fees and expenses of counsel) in connection with the collection under and enforcement of the termination fee provision of the Share Exchange Agreement, together with interest on such unpaid fees, commencing on the date that the termination fee became due, at a rate equal to that publicly announced by Citibank in New York City, at the bank’s prime rate plus 1.00%.
Indemnification Provisions
Indemnification Obligations. After the closing of the Exchange, Epimmune and its affiliates, officers, directors, employees, agents, successors and assigns, each referred to as an issuer indemnified party, will be indemnified and held harmless by the principal company shareholders, severally and not jointly in the case of losses arising out of or resulting from the matters set forth in clause (i)(y) below, and jointly and severally in
76
the case of losses arising out of or resulting from the other matters set forth below, from (i) (x) the breach of any statement regarding IDM in Article II of the Share Exchange Agreement or (y) the breach of any representation or warranty made by such principal company shareholder in Article III of the Share Exchange Agreement, and (ii) the breach of any covenant or agreement regarding IDM or by the principal company shareholders contained in Article V or Article VI of the Share Exchange Agreement.
Indemnification Threshold and Cap. The indemnity escrow shares will be the sole and exclusive remedy for any losses arising out of any and all claims by an issuer indemnified party relating to the subject matter of the Share Exchange Agreement, and the maximum amount that may be recovered from any principal company shareholder will be limited to such person’s pro rata share of the indemnity escrow shares.
In addition, no issuer indemnified party will be held harmless pursuant to the Share Exchange Agreement unless and until the aggregate amount of such party’s losses equals or exceeds $500,000, after which time the principal company shareholders will be liable only for those losses in excess of $500,000. Neither we nor any other party may seek recourse against IDM, either before or after the closing, for any breach of representation, warranty, covenant or other agreement of or regarding IDM or the principal company shareholders contained in the Share Exchange Agreement or any other matter related to the Share Exchange Agreement. No principal company shareholder will be liable to indemnify or hold harmless any issuer indemnified party for any losses to the extent arising out of or resulting from a breach of representation or warranty made by any other principal company shareholder contained in Article III of the Share Exchange Agreement.
Survival of Indemnification Obligations. The statements regarding IDM contained in Article II of the Share Exchange Agreement and the representations and warranties of the principal company shareholders contained in Article III of the Share Exchange Agreement will terminate at 11:59 p.m. California time, six months following the closing of the Exchange, except that, if written notice of a claim has been given prior to the expiration of the applicable representations and warranties by a party to the Share Exchange Agreement to another party thereto, then the relevant representations and warranties will survive as to such claim until such claim has been fully resolved.
Shareholder Representative and Power of Attorney
The shareholder representative, Hélène Ploix, is authorized to act on behalf of the principal company shareholders and to take any and all actions required or permitted to be taken by the shareholder representative under the Share Exchange Agreement or the Indemnity Escrow Agreement, with respect to any claims made by an issuer indemnified party for indemnification or to be held harmless pursuant to the Share Exchange Agreement and with respect to any actions to be taken by the shareholder representative pursuant to the terms of the Indemnity Escrow Agreement.
Each principal company shareholder severally will indemnify and reimburse the shareholder representative for its ratable share of any liabilities, losses or expenses incurred by the shareholder representative arising out of or resulting from any action taken by the shareholder representative under the Share Exchange Agreement or the Indemnity Escrow Agreement, other than liabilities, losses or expenses incurred because of the shareholder representative’s gross negligence, bad faith or willful misconduct.
If, at any time prior to the termination of the Expense Escrow Agreement, the shareholder representative suffers or incurs a loss for which the shareholder representative is indemnified or reimbursed pursuant to the Share Exchange Agreement, then the shareholder representative may make claims first against the expense escrow shares, and second, against any remaining indemnity escrow shares not subject to any claim for indemnity under the Indemnity Escrow Agreement after the date six months following the closing, for any and all such losses. In the event that the expense escrow shares and any available indemnity escrow shares are not, or the shareholder representative reasonably believes will not be, sufficient to indemnify the shareholder representative pursuant to the terms of the Share Exchange Agreement, the shareholder representative may make a written request that additional funds be deposited by the principal company shareholders with the expense escrow agent, pursuant to the terms of the Expense Escrow Agreement. If the holders of a majority of the outstanding IDM shares held by the principal company shareholders, as of the effective date of the Share Exchange Agreement, agree to the shareholder representative’s request for additional funds, each principal
77
company shareholder will deliver to the expense escrow agent either cash in an amount equal to such principal company shareholder’s pro rata amount or the number of shares of our common stock having a value equal to such principal company shareholder’s pro rata amount, determined based on the average of the closing prices on the Nasdaq National Market of shares of our common stock during the five consecutive trading days ending on and including the trading day immediately preceding the date of delivery of such shares to the expense escrow agent.
Each principal company shareholder delivered to the shareholder representative a power of attorney appointing the shareholder representative as its attorney in fact to perform any act required under the Share Exchange Agreement and the Indemnity Escrow Agreement and to execute the Expense Escrow Agreement.
Amendment, Extension and Waiver of the Share Exchange Agreement
We and the holders of a majority of the outstanding IDM shares held by the principal company shareholders as of the date the Share Exchange Agreement was originally signed may amend the Share Exchange Agreement only by mutual written consent. In addition, at any time prior to the closing of the Exchange, either we or the shareholder representative may, through an instrument in writing signed by the party or parties to be bound, extend the time for the performance of any obligation or other act of the other party, waive any inaccuracy in the representations and warranties of the other party, and waive compliance with any agreement of the other party or any condition to its own obligations contained in the Share Exchange Agreement.
AGREEMENTS RELATED TO THE EXCHANGE
Put/ Call Agreements
At the closing, IDM shareholders who hold IDM shares in a PEA will have the option either (i) to exchange their IDM shares for shares of our common stock in the Exchange or (ii) to enter into a Put/ Call Agreement with us. Pursuant to the terms of the Put/ Call Agreement, each IDM shareholder who holds shares in a PEA, including each holder of an IDM warrant held in a PEA with respect to the IDM shares received in exchange for its IDM warrant, who elects to enter into a Put/ Call Agreement with us will have the right to require us to purchase, and we will have the right to require such holders to sell, the IDM shares held in a PEA for a period of 30 days after the closing of our first equity financing. The aggregate purchase price for IDM shares held in a PEA, payable in cash, will be equal to the (A) the number of IDM shares held in the PEA multiplied by the exchange ratio, multiplied by (B) the price per share of our common stock sold in the first equity financing, less underwriters’ discounts or commissions. If the first equity financing does not close within two years following the closing of the Exchange, each IDM share subject to a Put/ Call Agreement will be exchanged for a number of shares of our common stock equal to the exchange ratio. The issuance of our common stock pursuant to the Put/ Call Agreements is subject to stockholder approval, as described in more detail in this proxy statement in the section entitled“Proposal 1 —Approval of Issuance of Epimmune Common Stock in Accordance with the Share Exchange Agreement, the Put/ Call Agreements, the Amended and Restated Preferred Exchange Agreement and the Option Liquidity Agreements.” The form of Put/ Call Agreement is attached to this proxy statement asAnnex C.
Option Liquidity Agreements
Prior to and effective as of the closing of the Exchange, we will offer each French optionholder and, in the event that the amendment to increase the number of shares available for issuance under our 2000 Plan as described in proposal 5 is not approved by our stockholders, each non-French optionholder the right to enter into an Option Liquidity Agreement with us. Each optionholder who elects to enter into an Option Liquidity Agreement with us and who subsequently exercises its IDM option by satisfying the conditions for exercise, including paying any requisite exercise price, will eventually acquire shares of our common stock in exchange for the IDM shares. Pursuant to the terms of the Option Liquidity Agreement, the optionholder will transfer to us each IDM share acquired upon exercise of the IDM option in exchange for the number of shares of our
78
common stock equal to the exchange ratio. Such share exchange shall generally take place within six business days from the date on which the IDM shares are registered in the name of the optionholder following the exercise of the IDM option. However, pursuant to the terms of the Option Liquidity Agreement, the IDM shares may be held by the optionholder for a specified period following the exercise of the IDM option and prior to exchanging the IDM shares for shares of our common stock. This specified holding period is for the purpose of preserving eligibility for reduced capital gains tax rates under French law and compliance with the terms of the IDM options. In the event that the specified holding period applies to the IDM shares, the exchange of IDM shares for shares of our common stock will generally take place within six business days following the expiration of the specified holding period.
The Option Liquidity Agreements permit the French optionholders, many of whom are expected to remain employees of IDM after it becomes a subsidiary of Epimmune as a result of the Exchange, to retain the tax advantages they currently enjoy under French law related to their existing IDM options. These tax advantages might be lost if the IDM options were cancelled, exchanged for options to purchase our common stock, or exercised in anticipation of the Exchange, These tax advantages might also be lost if the IDM shares acquired upon exercise of the IDM options are exchanged for shares of our common stock within specified holding periods following exercise of the IDM options. The Option Liquidity Agreements provide assurance that we will eventually acquire the IDM shares issued upon the future exercise of the IDM options in exchange for the same number of shares of our common stock as would have been issued if the IDM options were exercised prior to the Exchange, or for such other number of shares of our common stock as would result from any adjustment made from time to time by Epimmune to reflect appropriately the effect of any changes affecting our shares of common stock between the closing of the Exchange and the date on which the IDM shares are transferred to Epimmune.
Both Epimmune and IDM rely significantly on equity incentives in the form of stock option grants and other stock awards to attract and retain key employees, and we believe that such equity incentives are necessary for the proposed combined company to remain competitive in the marketplace for executive talent and other key employees. The French optionholders shall continue to vest in the IDM options following the Exchange in accordance with the existing vesting schedule for the IDM options and all other terms of the IDM options will remain effective following execution of the Option Liquidity Agreements and the closing of the Exchange. While our shares of common stock are freely tradable, there is no public market for the IDM shares. Therefore, the incentive effect of the IDM options to the French optionholders may be enhanced under the Option Liquidity Agreements because following the exercise of the IDM options, the French optionholders will be able to eventually receive shares of our common stock in exchange for the IDM shares.
The issuance of our common stock pursuant to the Option Liquidity Agreements is subject to stockholder approval, as described in more detail in this proxy statement in the section entitled“Proposal 1 —Approval of Issuance of Epimmune Common Stock in Accordance with the Share Exchange Agreement, the Put/ Call Agreements, the Amended and Restated Preferred Exchange Agreement, and the Option Liquidity Agreements.” The form of Option Liquidity Agreement is attached to this proxy statement asAnnex D.
Amended and Restated Preferred Exchange Agreement
On March 15, 2005, we entered into a Preferred Exchange Agreement with G.D. Searle, the holder of all of the outstanding shares of our preferred stock. We entered into an Amended and Restated Preferred Exchange Agreement with G.D. Searle on April 12, 2005. Pursuant to this agreement, effective immediately prior to the closing under the Share Exchange Agreement, 859,666 shares of our Series S preferred stock and 549,622 shares of our Series S-1 preferred stock will be exchanged for an aggregate of 1,949,278 shares of our common stock, subject to adjustment for our reverse stock split described in proposal 3 or any other stock split, combination or stock dividend. We have agreed to include the shares of common stock issued in exchange for the outstanding preferred stock in the registration statement to be filed with the SEC providing for an offering to be made on a continuous basis of all of the shares of our common stock issued pursuant to the Share Exchange Agreement. A copy of the Amended and Restated Preferred Exchange Agreement is attached to this proxy statement asAnnex E.
79
Indemnity Escrow Agreement
Under the Share Exchange Agreement, we agreed to enter into an Indemnity Escrow Agreement with the shareholder representative and a mutually agreeable escrow agent prior to the closing. At the closing, we will deposit into escrow certificates representing the indemnity escrow shares, which will equal an aggregate of 10% of the shares of our common stock issued in the Exchange, to secure our right to indemnification under the Share Exchange Agreement. Pursuant to the terms of the Indemnity Escrow Agreement, the indemnity escrow shares will secure the indemnification obligations of the principal company shareholders relating to any damages suffered by an issuer indemnified party as a result of the breach of any statement regarding IDM contained in the Share Exchange Agreement, or the breach of any representation or warranty in the Share Exchange Agreement made by the principal company shareholders. The indemnity escrow shares will serve as our sole recourse for breach of any such statements or representations following the closing. To the extent that any indemnity escrow shares are available after the payment of any and all amounts due to an issuer indemnified party, such indemnity escrow shares will be available to secure indemnification and reimbursement obligations of the principal company shareholders relating to any losses suffered by the shareholder representative in connection with her obligations as provided in the Share Exchange Agreement.
Under the Indemnity Escrow Agreement and during the escrow period, each principal company shareholder will have all rights of ownership of those indemnity escrow shares attributable to such principal company shareholder, except for the right to possession or the right to sell or assign such indemnity escrow shares. In particular, the principal company shareholders will have the right to exercise voting rights and will receive any cash dividends or cash distributions in respect of the indemnity escrow shares.
The Indemnity Escrow Agreement will terminate on the earlier of (i) the date on which there are no funds, shares of our common stock or other property remaining in the escrow fund, or (ii) 10 business days following the date on which all claims delivered to the escrow agent prior to the date six months after the closing of the Exchange have been resolved.
Voting Agreement
On March 15, 2005, we entered into a voting agreement with our directors and executive officers pursuant to which they agreed, among other things, to vote the shares of our common stock that they hold in favor of all matters to be submitted to stockholder approval in connection with the Share Exchange Agreement, the Exchange and the transactions related to the Exchange, and against any action or agreement that could reasonably be expected to adversely affect the Share Exchange Agreement or result in any of the conditions to the obligations of the parties under the Share Exchange Agreement not being fulfilled. In addition, each director and executive officer agreed that during the period between the closing and the date six months after the closing, he will not sell, transfer or assign his shares of our common stock. On the sixth month anniversary of the closing, the restrictions on transfer on shares held by each director and executive officer will be released with respect to 50% of such shares, with the restrictions on transfer for the remaining 50% being released on the one-year anniversary of the closing. Each director and executive officer also agreed to limit daily sales of shares of our common stock during the second six-month period to a maximum number of shares equal to 15% of the average daily trading volume of shares of our common stock on the Nasdaq National Market. Transfers of shares of our common stock to family members or affiliates of a director or executive officer that are not made in open market transactions will be permitted if the transferee agrees in writing to be bound by such restrictions on transfer or if we consent to the transfer. In addition, if the director or executive officer is an employee of Epimmune or IDM upon the closing of the Exchange, upon the subsequent termination of his employment by Epimmune or IDM, as the case may be, without cause, his resignation for good reason (as “cause” and “good reason” are defined in such director or executive officer’s employment agreement with Epimmune or IDM, as the case may be) or his death, the restrictions on transfer will cease to apply to such director or executive officer effective as of the date of his employment termination, resignation or death, as applicable.
80
Expense Escrow Agreement
Pursuant to the Share Exchange Agreement, at the closing we will deposit the expense escrow shares, representing in the aggregate 1.2% shares of our common stock issued in the Exchange, into escrow to secure the indemnification and reimbursement obligations of the principal company shareholders to the shareholder representative for any losses suffered by the shareholder representative arising out of any action taken by the shareholder representative under the Share Exchange Agreement or the Indemnity Escrow Agreement.
During the period when any expense escrow shares are held in escrow, each principal company shareholder will have all rights with respect to those expense escrow shares attributable to such principal company shareholder, except the right of possession or the right to sell or assign such shares, including the right to vote and the right to receive any cash dividends or other cash distributions.
The Expense Escrow Agreement will terminate on the earlier of (i) the date on which there are no funds, shares of our common stock or other property remaining in the escrow fund, or (ii) 20 business days following the termination of the Indemnity Escrow Agreement.
Employment Agreements
On March 17, 2005, we entered into employment agreements having a term of one year with each of Emile Loria, Mark Newman, and Robert De Vaere, our President and Chief Executive Officer, Vice President, Research and Development, and Vice President, Finance and Administration and Chief Financial Officer, respectively. The employment agreements, which do not become effective until the consummation of the Exchange provide that Drs. Loria and Newman, and Mr. De Vaere will serve as President and Chief Business Officer, Vice President, Infectious Diseases, and Chief Financial Officer, respectively, of the combined company. In addition to a minimum annual salary of $375,000 for Dr. Loria and $235,000 for each of Mr. De Vaere and Dr. Newman, each agreement grants the employee the right to receive a restricted stock grant. Pursuant to the terms of the restricted stock grants, Drs. Loria and Newman, and Mr. De Vaere are eligible to receive up to 370,700 shares, 128,300 shares, and 127,200 shares, respectively. The restricted stock grants are subject to the following terms:
| | |
| | • | the restricted stock vests in one or more installments, subject to continuous employment with us through the applicable installment date; |
| |
| | • | the restricted stock is subject to accelerated vesting upon the closing of a transaction providing a specified level of financing to the combined company, or the closing of a transaction providing a specified level of funding to the combined company’s infectious disease business, or both, depending on the employee; and |
| |
| | • | shares subject to the restricted stock grant that become vested will be issued to the employee on the earlier of (i) the employee’s termination, or (ii) 36 months from the date of the agreement. |
Each agreement provides for continued exercisability of outstanding options granted to the employee prior to the effective date of the agreement, to the extent the options were not in the money on the effective date of the agreement, generally until the later of (i) three months after employee’s termination, or (ii) December 31, 2007.
The agreements with Dr. Newman and Mr. De Vaere provide for the grant of retention bonuses, as follows:
| | |
| | • | Dr. Newman will be eligible for up to two retention bonuses at six and 12 months after the date of his agreement equal, in total, to 50% of his annual salary if he has been employed by the combined company through the applicable bonus date; upon closing of a transaction providing a specified level of funding for Epimmune’s infectious disease business, any such retention bonuses not previously earned will be paid immediately; and |
81
| | |
| | • | Mr. De Vaere will be eligible for up to three retention bonuses at six, nine, and 12 months after the date of his agreement, equal, in total, to 100% of his annual salary if he has been employed by the combined company through the applicable bonus date. |
In case of a termination of the employee’s employment due to death or disability during the term of his agreement, the employee will be entitled to full acceleration of vesting and exercisability of any outstanding options granted before the effective date of the agreement. In the event that the combined company terminates such employee’s employment without cause (as “without cause” is defined in the employment agreement), or such employee terminates his employment with good reason (as “good reason” is defined in the employment agreement), in each case during the term of his agreement, or upon the expiration of the term of his agreement, the employee will be entitled to the following, subject to the execution by the employee of an effective waiver and release of claims against the combined company:
| | |
| | • | severance payments, consisting of such employee’s base salary in effect at the time of termination, paid for a period of 12 months (or, at the employee’s option, payment in a lump sum of the employee’s base salary) in the case of termination without cause, and, in the case of termination by such employee with good reason or upon the expiration of the agreement for a period of the shorter of 12 months or until the date such employee begins full time employment with another entity; |
| |
| | • | reimbursement for a portion of COBRA health insurance premiums for a period of up to 12 months; |
| |
| | • | full acceleration, as of the date of termination, of vesting and exercisability of any outstanding options granted before the effective date of the agreement; and |
| |
| | • | full acceleration of vesting and exercisability of any unvested restricted stock granted pursuant the agreement. |
On April 21, 2005, we entered into an employment agreement with Jean-Loup Romet-Lemonne, the President and Chief Executive Officer of IDM. The employment agreement, which does not become effective until the consummation of the Exchange, provides that Dr. Romet-Lemonne will serve as Chief Executive Officer of the combined company. The agreement provides for a minimum annual salary of $385,000, as well as an annual performance-based bonus in a target amount of 35% of base salary. In addition, the agreement grants Dr. Romet-Lemonne the right to receive a restricted stock grant of up to 179,700 shares. The restricted stock grant is subject to the following terms:
| | |
| | • | the restricted stock vests as to 100% of the underlying shares on the date 42 months after the effective date of the employment agreement; |
| |
| | • | the restricted stock is subject to accelerated vesting upon the closing of one or more transactions providing a specified level of financing to the combined company, the timely filing of a marketing approval application with the appropriate agency with respect to the combined company’s Mepact product, the regulatory approval in the United States or Europe of the Mepact product and the closing of a transaction providing a specified level of funding to the combined company’s infections disease business; and |
| |
| | • | shares subject to the restricted stock grant that become vested will be issued to the executive on the earlier of (i) Dr. Romet-Lemmone’s termination, or (ii) 48 months from the effective date of the agreement. |
The agreement also provides for the grant of an option to purchase up to 544,300 shares of our common stock. The option grant shall vest over a four-year period with 25% of the underlying shares vesting on the first anniversary of the grant date and the balance vesting ratably on a daily basis thereafter, subject to Dr. Romet-Lemmone’s continuous employment with the combined company through the applicable vesting date.
The agreement provides for continued exercisability of outstanding options to purchase ordinary shares of IDM granted to Dr. Romet-Lemonne prior to the effective date of the agreement which will be replaced with substitute options to purchase our common stock in connection with the Exchange, to the extent the current market price of the shares underlying the options is less than the exercise price of the options on the effective
82
date of the agreement, generally until the later of (i) three months after employee’s employment termination, or (ii) December 31, 2007.
In case of termination of Dr. Romet-Lemmone’s employment due to death or disability, he will be entitled to full acceleration of vesting and exercisability of any outstanding options granted before the effective date of the agreement. In the event that the combined company terminates his employment without cause (as “cause” is defined in the employment agreement), or Dr. Romet-Lemonne terminates his employment with good reason (as “good reason” is defined in the employment agreement), Dr. Romet-Lemonne will be entitled to, subject to the execution by him of an effective waiver and release of claims against the combined company:
| | |
| | • | severance payments, consisting of his base salary in effect at the time of termination, paid for a period of 24 months (or, at his option, payment in a lump sum of such amount), in the case of termination without cause, and, in the case of termination by Dr. Romet-Lemonne with good reason, such severance shall be paid from the date of termination until the earlier of twelve months or until the date he begins full time employment with another entity; |
| |
| | • | reimbursement for a portion of COBRA heath insurance premiums, for up to 12 months in the case of termination with good reason, and in the case of termination without cause, reimbursement shall continue for up to 24 months; |
| |
| | • | full acceleration, as of the date of termination, of vesting and exercisability of any outstanding options granted before the effective date of the agreement; and |
| |
| | • | full acceleration of vesting and exercisability of any unvested restricted stock or options granted pursuant to the agreement. |
Stockholders are requested in this proposal 1 to approve the issuance of Epimmune common stock in accordance with the Share Exchange Agreement, the Put/ Call Agreements, the Amended and Restated Preferred Exchange Agreement and the Option Liquidity Agreements. The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the annual meeting will be required to approve this proposal 1. Abstentions will be counted toward the tabulation of votes cast on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether this proposal has been approved.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 1.
INFORMATION ABOUT IDM
IDM’s Business
IDM is a biopharmaceutical company focused on developing innovative products to treat and control cancer while maintaining the patient’s quality of life. IDM is currently developing two lines of products in the area of cancer therapy:
| | |
| | • | products to destroy cancer cells remaining after traditional therapies; and |
| |
| | • | products to prevent tumor recurrence by triggering an immune response. |
Macrophages. IDM’s first line of product candidates, which are designed to destroy residual cancer cells, are based on eitherin vivo, inside the body, orex vivo, outside the body, activation of certain immune cells called macrophages. IDM’s lead product candidate, Mepact, is part of this family of immunotherapeutic agents that activate the body’s natural defenses. Mepact activates macrophagesin vivoin order to enhance their ability to destroy cancer cells. IDM is developing Mepact for the treatment of osteosarcoma, a rare, aggressive bone tumor that principally affects adolescents. Mepact has received orphan drug designation in the United States and the European Union for this indication. A Phase III clinical trial conducted by the Children’s Oncology Group was completed for Mepact, involving almost 800 patients over a six-year period. Statistical analyses indicate that the use of Mepact prolongs disease-free and overall survival of osteosarcoma
83
patients. An analysis conducted by IDM, the sponsor of the trial, showed that both disease-free survival (i.e. the absence of any relapse or death) and overall survival were significantly improved for those patients who received Mepact compared to those patients who did not receive Mepact (p-value of 0.030 and 0.039 respectively), but were not improved by the addition of ifosfamide to standard chemotherapy (p-value of 0.934 and 0.992 respectively). For purposes of this analysis, the reference to p-value means the probability of being wrong when asserting that a true difference exists between the results for the patients treated with Mepact and treated without Mepact. For example, a p-value of 0.030 indicates that there is less than 30 in a thousand chance that results observed in the group treated with Mepact and the results observed without Mepact are not really different. This corresponds to a relative reduction in the risk of recurrence of 25% and a relative reduction in the risk of death of 30% by the addition of Mepact.
The Children’s Oncology Group performed a different analysis of the same study, based on a slightly different subset of patients. In this analysis, it concluded that there was an interaction between the addition of ifosfamide and the addition of Mepact to the standard chemotherapy. Patients given standard chemotherapy had a 3-year event free survival (i.e. the absence of any relapse, second malignancy or death), referred to as EFS, of 71%. The 3-year EFS was 68% after addition of Mepact, 61% after addition of ifosfamide and 78% after the addition of both agents. Based on this analysis, the addition of Mepact to standard chemotherapy that includes ifosfamide may improve EFS in these patients. IDM is currently in discussion with the FDA and the EMEA regarding the appropriate pathway for regulatory approval of Mepact. IDM has exclusive worldwide sales and marketing rights for Mepact.
The other product candidates that IDM is developing to destroy residual cancer cells involve Monocyte-derived Activated Killer cells, or MAK cells. IDM produces MAK cells from the patient’s own white blood cells by activating these cellsex vivoto stimulate them to recognize and destroy diseased cells. IDM’s most advanced MAK cell product candidate is Bexidem, which is in Phase II/ III clinical development. Bexidem is designed to treat bladder cancer, a large patient market with limited treatment options. In a pilot Phase I/ II study, IDM evaluated the ability of Bexidem to reduce tumor recurrence in superficial bladder cancer. No serious side effects were observed in the patients tested and the study demonstrated the potential clinical efficacy of Bexidem and that it was well tolerated. The study included 17 patients with superficial bladder cancer with a high probability of recurrence. Patients received six weekly local injections of Bexidem into the bladder. Five patients also received maintenance therapy at three-month intervals. All patients were followed for two years or more. A total of 112 injections were performed with no serious side effects observed. The most frequent associated adverse effects were urinary tract disorders (in six patients) and prostatic disorders (in two patients). The total number of tumor occurrences experienced by the 17 patients decreased from 34 during the year prior to the six-week treatment to 8 during the first year after treatment, a statistically significant decrease (p-value of 0.0005). For purposes of this study, the reference to p-value means the probability of being wrong when asserting that a true difference exists between the results for patients prior to treatment and after treatment. In the second year following treatment, the same 17 patients experienced a total of 10 recurrences, suggesting the continuing effects of the MAK treatment. This proof of concept demonstrating potential clinical efficacy provided the basis for IDM’s Phase II/ III study of Bexidem in patients with superficial bladder cancer at high risk of recurrence. IDM has exclusive worldwide sales and marketing rights for Bexidem.
Dendritophages. IDM’s second line of product candidates is dendritophage-based vaccines, which are designed to prevent tumor recurrence. Dendritophages are IDM’s engineered dendritic cells, or specialized immune cells, that IDM derives from the patient’s own white blood cells by exposing them to interleukin-13, or “IL-13,” a biological compound that contributes to the transformation of white blood cells into dendritophages. These dendritophages are loaded with cancer antigens and injected into the patient in order to stimulate the immune system to recognize and kill cancer cells that display those specific antigens on their surface. IDM has three dendritophage-based products, Uvidem, Collidem and Eladem, in clinical development, two of which are currently in clinical trials. Similar products are being developed to treat prostate and ovarian cancers and are currently in preclinical development.
Uvidem, a therapeutic vaccine developed by IDM in partnership with Sanofi-Aventis, is in Phase II clinical development for the treatment of melanoma. IDM recently completed a Phase II trial, which included 60 patients with stage IV melanoma and was conducted at 12 clinical centers in France and Australia. The
84
primary objective of the trial was to assess the safety of the vaccine and the patient’s immune response following the treatment. The results of this trial demonstrated that Uvidem was well tolerated: most side effects observed were mild or moderate and without any direct link to the treatment. In 77% of the 13 patients who received the full treatment (six injections), an immune response against tumor-associated antigens was detected after vaccination. Although there were no partial or complete responses, stabilization of the disease was reported in 10 patients out of 13. Based on these results, IDM has initiated another Phase II trial of Uvidem in the United States, which will include 37 patients with stage III or IV melanoma.
Collidem is in Phase I/ II clinical development for the treatment of advanced colorectal cancer. A Phase I/ II clinical study is on-going in the United States at the University of California at San Francisco, the University of Pittsburgh and the City of Hope National Medical Center to evaluate the safety and activity of Collidem in 37 patients with metastatic colorectal cancer. Both immune and clinical responses will be measured in addition to any side effects.
IDM has also completed a Phase I/ II trial for Eladem for the treatment of prostate cancer. The study was conducted on 24 patients with prostate cancer and whose prostate specific antigen, referred to as PSA, levels had increased following surgical removal of their prostates, indicating disease progression. Each patient received nine doses of Eladem over a 21-week period. PSA levels in the blood were monitored as the indicator of a drug effect. No major side effects were observed after a total of 204 administered doses, and there were no clinical recurrences during treatment. Eleven of the 24 patients showed either a stabilization or decrease in the level of PSA in their blood. PSA levels decrease correlated with low Gleason scores at the beginning of the study (p-value of 0.016) and positive skin tests (p-value of 0.04). For purposes of this study, the reference to p-value means the probability of being wrong when asserting that a true difference exists between the results for the patients prior to treatment and after treatment. A Gleason score is a system of grading prostate cancer, where a low score corresponds to a less aggressive tumor. In addition, all six patients identified as having circulating tumor cells in their blood prior to treatment showed a complete disappearance of these cells for at least six months after the treatment, and the cells remained undetectable 12 months after the treatment.
MAK cells and dendritophages are types of “Cell Drugs,” a term IDM uses to refer to therapeutic products derived from the patient’s own white blood cells.
85
Products in Development. IDM has completed a Phase III clinical trial for one product (Mepact). IDM currently has five product candidates in clinical development and five product candidates in preclinical development:
| | | | | |
| Product Candidate | | Primary Indication(s) | | Status |
| | | | | |
| Product Candidates to Destroy Residual Cancer Cells | | | | |
| Mepact | | Osteosarcoma | | Phase III trial completed |
| Bexidem | | Bladder cancer | | Phase II/III initiated in March 2004; trial on- going |
| IDM-4 | | Chronic lymphocytic leukemia | | Phase I/II trial completed |
| Osidem-2 | | Ovarian cancer | | Preclinical |
| Jenact | | Lung or liver metastases in | | Preclinical |
| | | relevant cancers | | |
| Product Candidates to Stimulate an Immune Response and Prevent Tumor Recurrence | | | | |
| Uvidem | | Melanoma | | Phase II initiated in September 2004; trial on-going |
| Collidem | | Colorectal cancer | | Phase I/II initiated in September 2003; trial on-going |
| Eladem | | Prostate cancer | | Phase I/II trial completed |
| Eladem-2 | | Prostate cancer | | Late preclinical |
| IDD-6 | | Ovarian cancer | | Late preclinical |
| Liposomal KSA | | Breast, colon, lung and | | Preclinical |
| | | prostate cancers | | |
* * *
IDM’s principal executive offices are located at 172, rue de Charonne, 75011 Paris, France and IDM’s telephone number is +33 (0)1 40 09 04 11. The address of IDM’s Irvine, California office is 9 Parker, Irvine, CA 92618-1605, United States and its telephone number at this address is +1 (949) 470-4751. The address of IDM’s web site iswww.idm-biotech.com. The information on IDM’s web site is not incorporated in this proxy statement.
Selected Historical Financial Data of IDM
You should read this data together with the section of this proxy statement entitled“Information About IDM — Operating and Financial Review and Prospects” and IDM’s financial statements and notes included elsewhere in this proxy statement. The following tables set forth selected financial data for IDM for the years ended December 31, 2004, 2003, 2002, 2001 and 2000 and for the three months ended March 31, 2004 and 2005.
The selected historical financial data for the years ended December 31, 2004, 2003, 2002 and 2001 have been derived from IDM’s consolidated financial statements for the same periods, which have been prepared in accordance with U.S. GAAP. These financial statements were audited by Ernst & Young Audit, IDM’s independent auditors, whose report is included elsewhere in this proxy statement. The selected historical financial data for the year ended December 31, 2000 and the three months ended March 31, 2004 and 2005 have been derived from unaudited financial statements, which have been prepared in accordance with U.S. GAAP on the basis of audited financial statements prepared in accordance with French GAAP.
86
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | | |
| | | 2000 | | | 2001 | | | 2002 | | | 2003 | | | 2004 | | | 2004($)(1) | |
| | | | | | | | | | | | | | | | | | | |
| | | (Unaudited) | | | |
| | | | | (In thousands, except per share data) | |
Statements of operations data: | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development revenues | | € | 0 | | | € | 17 | | | € | 3,571 | | | € | 5,325 | | | € | 4,652 | | | $ | 5,890 | |
| Other revenues | | | 35 | | | | 4 | | | | 241 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | |
| | Total revenues | | | 35 | | | | 21 | | | | 3,812 | | | | 5,325 | | | | 4,652 | | | | 5,890 | |
| | | | | | | | | | | | | | | | | | | |
| Research and development expenses and impairment costs | | | (26,169 | ) | | | (13,166 | ) | | | (12,301 | ) | | | (16,601 | ) | | | (22,374 | ) | | | (28,328 | ) |
| Selling and marketing expenses | | | (184 | ) | | | (622 | ) | | | (1,602 | ) | | | (1,456 | ) | | | (944 | ) | | | (1,195 | ) |
| General and administrative expenses | | | (8,961 | ) | | | (3,751 | ) | | | (4,796 | ) | | | (4,686 | ) | | | (7,675 | ) | | | (9,717 | ) |
| | | | | | | | | | | | | | | | | | | |
| | Total operating expenses | | | (35,314 | ) | | | (17,539 | ) | | | (18,699 | ) | | | (22,743 | ) | | | (30,993 | ) | | | (39,240 | ) |
| | | | | | | | | | | | | | | | | | | |
| | Loss from operations | | | (35,279 | ) | | | (17,518 | ) | | | (14,887 | ) | | | (17,418 | ) | | | (26,341 | ) | | | (33,350 | ) |
| | | | | | | | | | | | | | | | | | | |
| Interest income net | | | 764 | | | | 1,865 | | | | 1,284 | | | | 961 | | | | 561 | | | | 710 | |
| Foreign exchange gain (loss) | | | 26 | | | | (15 | ) | | | 16 | | | | 29 | | | | (17 | ) | | | (21 | ) |
| | | | | | | | | | | | | | | | | | | |
| | Loss before income tax benefit | | | (34,489 | ) | | | (15,668 | ) | | | (13,587 | ) | | | (16,428 | ) | | | (25,797 | ) | | | (32,661 | ) |
| | | | | | | | | | | | | | | | | | | |
| Income tax benefit | | | 465 | | | | 463 | | | | 640 | | | | 208 | | | | 281 | | | | 356 | |
| | | | | | | | | | | | | | | | | | | |
| | Net loss | | | (34,024 | ) | | | (15,205 | ) | | | (12,947 | ) | | | (16,220 | ) | | | (25,516 | ) | | | (32,305 | ) |
| | | | | | | | | | | | | | | | | | | |
| Weighted average number of shares | | | 8,323 | | | | 11,633 | | | | 12,063 | | | | 13,516 | | | | 13,649 | | | | 13,649 | |
| Basic and diluted loss per share | | € | (4.09 | ) | | € | (1.31 | ) | | € | (1.07 | ) | | € | (1.20 | ) | | € | (1.87 | ) | | $ | (2.37 | ) |
| | | | | | | | | | | | | | | | | | | |
Balance sheet data (at the end of period): | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | € | 48,430 | | | € | 38,240 | | | € | 49,290 | | | € | 33,326 | | | € | 30,859 | | | $ | 41,777 | |
| Total assets | | | 67,067 | | | | 55,626 | | | | 64,730 | | | | 52,271 | | | | 40,840 | | | | 55,289 | |
| Total current liabilities | | | 2,370 | | | | 4,179 | | | | 4,977 | | | | 4,970 | | | | 6,355 | | | | 8,603 | |
| Total shareholders’ equity | | | 64,099 | | | | 49,616 | | | | 56,805 | | | | 43,745 | | | | 31,394 | | | | 42,501 | |
| |
| (1) | For your convenience, U.S. dollar amounts have been presented for the year ended December 31, 2004, calculated at the exchange rate of€1.00 = $1.2661 for the statement of operations data and€1.00 = $1.3538 for the balance sheet data. The balance sheet rate was the Federal Reserve Bank of New York’s noon buying rate in New York as of December 31, 2004 and the statement of operations rate is an average exchange rate for the year ended December 31, 2004. |
87
| | | | | | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31, | |
| | | | |
| | | 2004 | | | 2005 | | | 2005(1) | |
| | | | | | | | | | |
| | | (Unaudited, in thousands, | |
| | | except per share data) | |
Statements of operations data: | | | | | | | | | | | | |
| Research and development revenues | | € | 1,213 | | | € | 1,200 | | | $ | 1,573 | |
| Other revenues | | | — | | | | — | | | | — | |
| | Total revenues | | € | 1,213 | | | € | 1,200 | | | $ | 1,573 | |
| Research and development expenses and impairment costs | | | (3,851 | ) | | | (3,902 | ) | | | (5,117 | ) |
| Selling and marketing expenses | | | (256 | ) | | | (197 | ) | | | (258 | ) |
| General and administrative expenses | | | (1,046 | ) | | | (1,068 | ) | | | (1,400 | ) |
| | | | | | | | | | |
| Other operating expenses | | | — | | | | (1,157 | ) | | | (1,517 | ) |
| | | | | | | | | | |
| | Total operating expenses | | | (5,153 | ) | | | (6,324 | ) | | | (8,292 | ) |
| | | | | | | | | | |
| | Loss from operations | | | (3,940 | ) | | | (5,124 | ) | | | (6,719 | ) |
| | | | | | | | | | |
| Interest income net | | | 169 | | | | 142 | | | | 186 | |
| Foreign exchange gain (loss) | | | 20 | | | | 3 | | | | 4 | |
| | | | | | | | | | |
| | Loss before income tax benefit | | | (3,751 | ) | | | (4,979 | ) | | | (6,529 | ) |
| | | | | | | | | | |
| Income tax benefit | | | 6 | | | | 336 | | | | 441 | |
| | | | | | | | | | |
| | Net loss | | | (3,745 | ) | | | (4,643 | ) | | | (6,088 | ) |
| | | | | | | | | | |
| Weighted average number of shares | | | 13,604 | | | | 15,694 | | | | 15,694 | |
| Basic and diluted loss per share | | € | (0.28 | ) | | € | (0.28 | ) | | $ | (0.39 | ) |
| | | | | | | | | | |
Balance sheet data (at the end of period): | | | | | | | | | | | | |
| Cash and cash equivalents | | € | 30,859 | | | € | 26,765 | | | $ | 34,711 | |
| Total assets | | | 40,840 | | | | 36,840 | | | | 47,778 | |
| Total current liabilities | | | 6,355 | | | | 7,110 | | | | 9,221 | |
| Total shareholders’ equity | | | 31,394 | | | | 26,781 | | | | 34,732 | |
| |
| (1) | For your convenience, U.S. dollar amounts have been presented for the three months ended March 31, 2005, calculated at the exchange rate of€1.00 = $1.3112 for the statement of operations and€1.00 = $1.2969 for the balance sheet. The balance sheet rate was the Federal Reserve Bank of New York’s noon buying rate in New York as of March 31, 2005 and the statement of operations rate is an average exchange rate for the three months ended March 31, 2005. |
Exchange Rates. In order that you may ascertain how the trends in IDM’s financial results might have appeared had they been expressed in U.S. dollars, the following table shows the euro/ U.S. dollar exchange rate for the periods 2000 through 2004 based on the noon buying rate expressed in dollars per€1.00.
The noon buying rate is the exchange rate in New York City for cable transfers in foreign currencies as certified for customs purposes by the Federal Reserve Bank of New York. This does not mean that IDM actually converted any amounts into U.S. dollars at that rate, and you should not assume that any amounts could have been converted at that or any other rate. On June 29, 2005, the noon buying rate for euros to U.S. dollars was€1.00 = $1.2101.
88
These rates have been provided solely for your convenience and, unless IDM indicates otherwise, IDM has not used these rates in the preparation of its financial statements included in this proxy statement.
| | | | | | | | | | | | | | | | | |
| | | Year-End | | | Average | | | | | |
| Year | | Rate | | | Rate(1) | | | High | | | Low | |
| | | | | | | | | | | | | |
Euro/Dollar | | | | | | | | | | | | | | | | |
| 2004 | | | 1.36 | | | | 1.24 | | | | 1.36 | | | | 1.18 | |
| 2003 | | | 1.26 | | | | 1.14 | | | | 1.26 | | | | 1.04 | |
| 2002 | | | 1.05 | | | | 0.95 | | | | 1.05 | | | | 0.86 | |
| 2001 | | | 0.88 | | | | 0.90 | | | | 0.95 | | | | 0.84 | |
| 2000 | | | 0.93 | | | | 0.92 | | | | 1.03 | | | | 0.83 | |
| | | | | | | | | | | | | | | | | |
| | | Period-End | | | Average | | | | | |
| Most Recent Six Months | | Rate | | | Rate(2) | | | High | | | Low | |
| | | | | | | | | | | | | |
Euro/Dollar | | | | | | | | | | | | | | | | |
| June 2005 (through June 29) | | | 1.21 | | | | 1.22 | | | | 1.23 | | | | 1.20 | |
| May 2005 | | | 1.23 | | | | 1.27 | | | | 1.29 | | | | 1.23 | |
| April 2005 | | | 1.29 | | | | 1.29 | | | | 1.31 | | | | 1.28 | |
| March 2005 | | | 1.33 | | | | 1.33 | | | | 1.35 | | | | 1.31 | |
| February 2005 | | | 1.33 | | | | 1.30 | | | | 1.33 | | | | 1.28 | |
| January 2005 | | | 1.30 | | | | 1.31 | | | | 1.35 | | | | 1.30 | |
| December 2004 | | | 1.36 | | | | 1.34 | | | | 1.36 | | | | 1.32 | |
| |
| (1) | The average of the noon buying rates for euros on the last business day of each month during the relevant year. |
| |
| (2) | The average of the noon buying rates for euros on the business days occurring during such month (or partial month). |
Operating and Financial Review and Prospects
You should read the following discussion of IDM’s financial condition and results of operations in conjunction with IDM’s consolidated financial statements and the notes to those statements included elsewhere in this proxy statement. All figures for the three months ended March 31, 2005 are unaudited. This discussion contains forward-looking statements, including, without limitation, certain statements made in the section below entitled “Liquidity and Capital Resources.” These forward-looking statements are subject to numerous risks and uncertainties. As a result of many factors, IDM’s actual results or performance may differ materially from those anticipated in these forward-looking statements.
IDM is a biopharmaceutical company focused on the development of innovative products to treat and control cancer. IDM is currently developing two lines of products in the area of cancer therapy: products to destroy residual cancer cells and products to prevent tumor recurrence. IDM’s lead product candidate, Mepact, is one of its family of immunotherapeutic agents that activate the body’s natural defenses in order to enhance their ability to destroy residual cancer cells. A Phase III clinical trial was completed for Mepact, which has received orphan drug designation for the treatment of osteosarcoma. Another type of product that IDM is developing to destroy cancer cells is MAK cells. IDM’s MAK cell product candidate in the most advanced stage of clinical testing is Bexidem, which is in Phase II/ III clinical development for bladder cancer. IDM’s second line of products, which includes IDM’s dendritophages, is designed to prevent tumor recurrence. IDM has two dendritophage-based product candidates currently in clinical trials, Uvidem, in Phase II for melanoma and Collidem, in Phase I/ II for colorectal cancer. IDM is jointly developing Uvidem with Sanofi-Aventis.
89
IDM has incurred significant net losses and has generated limited revenues since inception. As of December 31, 2004, IDM’s accumulated deficit was€112.1 million and IDM’s revenues for the fiscal year ended December 31, 2004 were€4.7 million. IDM’s historical financial results reflect increasing research and development and general administrative expenses related to expanding its product development programs. The table below presents IDM’s income statement figures for the years ended December 31, 2004, 2003 and 2002 and for the three months ended March 31, 2004 and 2005.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Three Months Ended | |
| | | Year Ended December 31, | | | March 31, | |
| | | | | | | |
| | | 2002 | | | 2003 | | | 2004 | | | 2004(1) | | | 2004 | | | 2005 | | | 2005(2) | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | (In thousands) | | | (Unaudited, in thousands, | |
| | | | | except per share data) | |
Statements of operations data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development revenues | | € | 3,571 | | | € | 5,325 | | | € | 4,652 | | | $ | 5,890 | | | € | 1,213 | | | € | 1,200 | | | $ | 1,573 | |
| Research and development expenses and impairment costs | | | (12,301 | ) | | | (16,601 | ) | | | (22,374 | ) | | | (28,328 | ) | | | (3,851 | ) | | | (3,902 | ) | | | (5,117 | ) |
| Selling and marketing expenses | | | (1,602 | ) | | | (1,456 | ) | | | (944 | ) | | | (1,195 | ) | | | (256 | ) | | | (197 | ) | | | (258 | ) |
| General and administrative expenses and other operating expenses | | | (4,796 | ) | | | (4,686 | ) | | | (7,675 | ) | | | (9,717 | ) | | | (1,046 | ) | | | (2,225 | ) | | | (2,917 | ) |
| | Total operating expenses | | | (18,699 | ) | | | (22,743 | ) | | | (30,993 | ) | | | (39,240 | ) | | | (5,153 | ) | | | (6,324 | ) | | | (8,292 | ) |
| | Loss from operations | | | (15,128 | ) | | | (17,418 | ) | | | (26,341 | ) | | | (33,350 | ) | | | (3,940 | ) | | | (5,124 | ) | | | (6,719 | ) |
| Interest income net | | | 1,284 | | | | 961 | | | | 561 | | | | 710 | | | | 169 | | | | 142 | | | | 186 | |
| Other Income | | | 241 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Foreign exchange gain (loss) | | | 16 | | | | 29 | | | | (17 | ) | | | (21 | ) | | | 20 | | | | 3 | | | | 4 | |
| | Loss before income tax benefit | | | (13,587 | ) | | | (16,428 | ) | | | (25,797 | ) | | | (32,661 | ) | | | (3,751 | ) | | | (4,979 | ) | | | (6,529 | ) |
| Income tax benefit | | | 640 | | | | 208 | | | | 281 | | | | 356 | | | | 6 | | | | 336 | | | | 441 | |
| | Net loss | | € | (12,947 | ) | | € | (16,220 | ) | | € | (25,516 | ) | | $ | (32,305 | ) | | € | (3,745 | ) | | € | (4,643 | ) | | $ | (6,088 | ) |
| |
| (1) | For your convenience, U.S. dollar amounts have been presented for the year ended December 31, 2004, calculated at the exchange rate of€1.00 = $1.2661 for the statement of operations. The statement of operations rate is an average exchange rate of the Federal Reserve Bank of New York’s noon buying rate in New York for the year ended December 31, 2004. |
| |
| (2) | For your convenience, U.S. dollar amounts have been presented for the three months ended March 31, 2005, calculated at the exchange rate of€1.00 = $1.3112 for the statement of operations. The statement of operations rate is an average exchange rate of the Federal Reserve Bank of New York’s noon buying rate in New York for the three months ended March 31, 2005. |
IDM’s research and development expenses mainly include costs associated with preclinical development and clinical trials of its product candidates, salaries and other expenses for personnel, laboratory supplies and materials, consulting and contract research costs, facility costs, amortization of intangible assets such as patents and licenses, and depreciation of laboratory and office equipment. From inception through December 31, 2004, IDM has incurred costs of approximately€102 million associated with research and development in all program areas, while IDM has recorded only€15 million in research and development revenues (of which€13.5 million has been recorded since 2001). Following IDM’s acquisition of Mepact and certain other assets, valued at€2.9 million, from Jenner Biotherapies in early 2003, IDM’s research and development expenses related to Mepact have amounted to approximately€2.3 million, consisting mainly of external consultant fees, manufacturing and employment costs. IDM charges all research and development expenses to operations as they are incurred. Since 2001, IDM’s research and development expenses have represented approximately 61% of total operating expenses. IDM expects its research and development expenses to
90
increase significantly over the next several years, primarily due to costs related to clinical trials and the regulatory approval process.
IDM expects that research and development expenses will continue to increase in the future as it advances its product candidates through development. Clinical development timelines, likelihood of success and total costs vary widely; therefore IDM currently does not have estimates of total costs or time to reach the market for any of its particular product candidates. IDM’s potential product candidates are subject to a lengthy and uncertain regulatory process that may not result in the necessary regulatory approvals, which could adversely affect its ability to commercialize the product candidates. In addition, clinical trials of IDM’s potential product candidates may fail to demonstrate safety and/or efficacy, which could prevent or significantly delay regulatory approval. IDM anticipates that it will make determinations as to which research and development projects to pursue and how much funding to direct to each project on an on-going basis in response to the scientific and clinical success of each product candidate.
Product candidate completion dates and completion costs vary significantly for each product candidate and are difficult to estimate. The lengthy process of seeking regulatory approvals, and the subsequent compliance with applicable regulations, require the expenditure of substantial resources. Any failure by IDM to obtain, or any delay in obtaining, regulatory approvals could cause its research and development expenditures to increase and, in turn, have a material adverse effect on its results of operations. IDM cannot be certain when any net cash inflow from Mepact or any of IDM’s other development projects will commence.
As IDM’s expenses increase, IDM expects to continue to incur net losses for the next several years while it pursues its strategy of advancing the development of certain products to commercialization, broadening its development pipeline and in-licensing of new biological compounds and complementary technologies. Moreover, the amount of future net losses and the time IDM will require to reach profitability, if at all, are highly uncertain.
IDM’s historical revenues have principally been derived from up-front fees, milestone payments and reimbursement of expenses under its collaboration agreement with Sanofi-Aventis, as well as certain government grants. Since these revenues fluctuate significantly, IDM’s financial results for any single period may not be directly comparable to those for any other period. In addition, results in any one period may not be an indication of future results.
In addition to the revenues described above, IDM’s financial requirements have been met to date through private placements of equity securities. IDM has received a total of€102 million in gross proceeds from private placements of equity securities, including€20 million from Sanofi-Aventis in 2002 and€10 million in 2004, as well as€8.1 million from Medarex in 2000.
IDM has entered into a number of collaborations with academic and non-academic institutions and pharmaceutical companies. In July 2001, IDM entered into a significant collaboration agreement with Sanofi-Aventis under which IDM has generated revenue. IDM expects one of its principal sources of revenues over the next several years to be additional up-front payments, milestone payments and reimbursement of research and development expenses from its collaboration with Sanofi-Aventis, although these payments are contingent upon meeting certain development goals. IDM is also seeking to enter into other collaborative agreements for certain products with other partners, which may provide additional sources of revenues. Consequently, IDM’s financial statements have been prepared as if it were an operating company.
| |
| Critical Accounting Policies and Estimates |
IDM’s discussion and analysis of its operating and financial results and trends are based on its consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires IDM to make estimates and judgments in certain circumstances that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. IDM’s management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances. IDM reviews its estimates on an on-going basis. Actual results may differ from these estimates under different assumptions or conditions. While
91
IDM’s significant accounting policies are described in more detail in Note 1 to its consolidated financial statements, included in this proxy statement, IDM believes that the policies described below involve the most significant judgments and estimates used in the preparation of its consolidated financial statements.
Revenue recognition. IDM generates revenues from a collaborative agreement with Sanofi-Aventis. These revenues consist of up-front fees, milestone payments and ongoing research and development funding.
Non-refundable up-front payments that IDM receives in connection with collaborative research and development agreements are deferred and recognized on a straight-line basis over the research term. When the research term cannot be specifically identified from the agreement, IDM estimates it based upon its current development plan for the product. These estimates are continually reviewed and could result in a change in the deferral period, such as, for example, when the estimated development period for a product changes. As a result, the timing and amount of revenue recognized may change. For example, IDM’s current estimated development period for Uvidem, which is the only product candidate for which IDM currently receives revenues, is nine years. If this estimated development period is extended or shortened, the amount of revenues recognized per period would decrease or increase correspondingly.
Revenues from milestone payments for products selected by collaborative partners are recognized in full upon achievement of the relevant milestone when a fair value can be ascribed. During the development phase of a collaborative research and development agreement, IDM considers that no fair value can be ascribed to the upfront fee and the milestone payments, given the inherent uncertainty of the technological outcome at this stage of the research and development process, which does not enable IDM to make a reliable, verifiable and objective determination of the fair value of each payment. As no fair value can reasonably be ascribed, such payments are recorded as deferred revenue and recognized over the remaining development term on a straight-line basis.
Reimbursement of ongoing research and development expenses for products selected by collaborative partners are recognized as revenues when the services have been performed and the payment is assured. Reimbursement of research and development expenses incurred prior to selection of a product by a collaborative partner are considered as additional up-front payments and are recorded as deferred revenue and are recognized on a straight-line basis over the research term. IDM believes that the value assigned to the funding of research and development costs incurred prior to the selection of a product by a collaborative partner cannot be deemed to be representative of the fair value of the corresponding research and development costs incurred prior to such product selection given the uncertainty of the technological outcome in the development stage.
Research and development expenses and related tax credit. Research and development expenses consist primarily of costs associated with the clinical trials of IDM’s products, compensation and other expenses for research and development personnel, supplies and development materials, costs for consultants and related contract research, facility costs and amortization and depreciation of patents and licenses. These costs are expensed as incurred.
Research and development expenses incurred in France and Quebec, relating to the activities of IDM’s wholly-owned Canadian subsidiary, IDM-Biotech Ltd., which closed its office in August 2003, form the basis for a tax credit, which is recorded as a current income tax benefit in the period in which the expenses are incurred and the credit is claimed. The credit is recoverable in cash if not used to offset taxes payable in the fourth year following its generation after a governmental evaluation in France, and in the year following its generation in Quebec. The research and development tax credit is recorded as a current asset if payable within one year, or as a long-term asset if payable beyond one year.
Intangible assets. Intangible assets principally include patent registration costs and acquisition of licenses. Patent registration costs are amortized on a straight-line basis over their useful life, which is estimated to be ten years and corresponds to the average biotechnology product life. Costs associated with licenses acquired in order to be able to use products from third parties prior to receipt of regulatory approval to market IDM’s products are capitalized if (i) the licenses are to be used in the scope of a research and development program in Phase III clinical development at the time the license is acquired, at which stage the
92
absence of toxicity has been assessed and IDM has a reasonable expectation to achieve marketing approval for the program, or (ii) the licenses can be used in other specifically identified research and development programs to be begun after the date of acquisition. Costs of acquisition of licenses are capitalized and amortized on a straight-line basis over the useful life of the license, which IDM considers to begin on the date of acquisition of the license and continue through the end of the estimated term during which the technology is expected to generate substantial revenues. In the case of the licenses or assets acquired from Medarex and Jenner Biotherapies, IDM estimated their useful lives to be ten years from the date of acquisition.
Impairment of long lived assets. In accordance with Statement of Financial Accounting Standards No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets,” IDM periodically evaluates the value reflected on its balance sheet of long-lived assets, such as patents and licenses, when events and circumstances indicate that the carrying amount of an asset may not be recovered. Such events and circumstances include the use of the asset in current research and development projects, any potential alternative uses of the asset in other research and development projects in the short to medium term, clinical trial results and research and development portfolio management options.
Results of Operations for the Three Months Ended March 31, 2005 and 2004
Revenues. IDM’s total revenues for the three months ended March 31, 2005 remained stable at€1.2 million, compared to total revenues for the first three months of 2004. All of its revenues for the periods ended March 31, 2005 and 2004 were generated from its research and development activities in France and derived entirely from reimbursement of current and past research and development expenses and up-front fees and milestone payments received from Sanofi-Aventis under the terms of its collaboration agreement, as described below.
On December 21, 2001, Sanofi-Aventis exercised its first option to initiate product development on the on-going melanoma development program for Uvidem. Between January and June 2002, Sanofi-Aventis paid us a total of€5.9 million in relation to Uvidem as a combination of up-front fees, milestone payments and reimbursement of expenses IDM had incurred in prior years while developing Uvidem. The revenue corresponding to these payments is being recognized on a straight-line basis over the development period for Uvidem, which was changed to nine years in December 2003, as described below. Accordingly, IDM recognized€138,000 in revenues in the first three months of 2004 and 2005 from these payments. Additional milestone payments by Sanofi-Aventis under IDM’s collaboration agreement are contingent upon the success of several on-going Phase II clinical trials.
IDM recorded€1.1 million in revenues from Sanofi-Aventis in the first three months of 2004 and 2005 for reimbursement of current expenses related to the development of Uvidem.
For the periods ended March 31, 2005 and 2004, IDM’s other revenues were not significant.
Research and Development Expenses and Impairment of Patents and Licenses. Total research and development expenses and impairment of patents and licenses were stable at€3.9 million for the three months ended March 31, 2005 and 2004.
IDM regularly undertakes detailed reviews of its patents and licenses in order to check the development stage and the viability of associated products. When certain product development projects remain at an early stage or are abandoned, IDM writes down in full the remaining value of licenses, patents or trademarks associated with those projects, if they are found to have no alternative future use. During the first quarter of 2005, this review led IDM to write down certain patents and licenses for a total impairment charge of€206,000, compared to no impairment charge for the first quarter of 2004.
For the first three months of 2005 there was no amortization of Medarex’s licences since they had no remaining value, compared to an amortization charge of€242,000 for the first quarter of 2004.
Globally, direct research and development expenses related to IDM’s product candidates to destroy residual cancer cells were approximately€1.2 million and€0.5 million for the three months ended March 31, 2005 and 2004, respectively, or€9.4 million for the period from January 1, 2001, the earliest date for which
93
relevant cumulated cost information is available, to March 31, 2005. Direct research and development expenses related to IDM’s product candidates to stimulate an immune response and prevent tumor recurrence were approximately€1.0 million for each of the three months ended March 31, 2005 and 2004, or€12.7 million for the period from January 1, 2001, the earliest date for which relevant cumulated cost information is available, to March 31, 2005.
Selling and Marketing Expense. Selling and marketing expenses were€0.2 million for the three months ended March 31, 2005, a decrease of approximately 23% compared to€0.3 million for the three months ended March 31, 2004. These expenses consisted primarily of costs related to IDM’s participation in trade conferences and to the employment costs of its business development and communications employees. The decrease reflects lower salary charges related to a lower number of employees.
General and Administrative Expenses and Other Operating Expenses. General and administrative expenses remained stable at€1.0 million for the three months ended March 31, 2005 and 2004.
Other operating expenses totaled€1.2 million during the first three months of 2005 and correspond to legal and accounting fees paid in relation to the combination between IDM and Epimmune Inc., including the negotiation of the Share Exchange Agreement and its signature by IDM shareholders, and the contribution of IDM information to the proxy statement filed by Epimmune.
Interest Income. Net interest income was€142,000 for the three months ended March 31, 2005, a decrease of approximately 16% compared to€169,000 for the quarter ended March 31, 2004, reflecting the effect of lower cash reserves.
Income Tax Benefit. Despite the stability in IDM’s research and development expenses, IDM recorded an increased research tax credit in the amount of€0.3 million for the first three months of 2005 compared to a non-significant value in the first quarter of 2004. This increase resulted primarily from the fact that the method of calculation of the research tax credit in France was recently modified to be more advantageous.
As of March 31, 2005, IDM had accounts receivable of€1.8 million, mainly representing an account receivable from the French government, of which€0.5 million is recoverable in 2005.
Net Loss. IDM’s net loss increased to€4.6 million for the three months ended March 31, 2005, compared to€3.7 million for the three months ended March 31, 2004, as a result of the factors described above.
| |
| Results of Operations for the Years Ended December 31, 2004 and 2003 |
Revenues. IDM’s total revenues for the year ended December 31, 2004 were€4.7 million, a decrease of approximately 11% compared to total revenues of€5.3 million for the year ended December 31, 2003. All of IDM’s revenues for the periods ended December 31, 2004 and 2003 were generated from its research and development activities in France.
On December 21, 2001, Sanofi-Aventis exercised its first option to initiate product development on the on-going melanoma development program for Uvidem. Between January and June 2002, Sanofi-Aventis paid IDM a total of€5.9 million in relation to Uvidem as a combination of up-front fees, milestone payments and reimbursement of expenses IDM had incurred in prior years while developing Uvidem. The revenue corresponding to these payments is being recognized on a straight-line basis over the development period for Uvidem, which was changed to nine years in December 2003, as described below. Accordingly, IDM recognized€0.6 million in revenues in 2003 and 2004 from these payments.
In June 2003, the joint development committee for the Uvidem project, which includes representatives from IDM and Sanofi-Aventis, recommended an enhanced development program for Uvidem, including several new Phase II clinical trials. This plan, which increased the program development period for Uvidem from 3.5 to nine years, was finalized and approved in December 2003. Additional milestone payments by Sanofi-Aventis under IDM’s collaboration agreement are contingent upon the success of these clinical trials.
94
IDM recorded€4.1 million in revenues from Sanofi-Aventis in 2004 for reimbursement of current expenses related to the development of Uvidem, compared to€4.8 million for 2003.
For the periods ended December 31, 2004 and 2003, IDM’s other revenues were not significant.
Research and Development Expenses and Impairment of Patents and Licenses. Total research and development expenses and impairment of patents and licenses were€22.4 million for the year ended December 31, 2004, an increase of approximately 35% compared to€16.6 million for the year ended December 31, 2003. The increase was mainly due to impairment charges related to a Medarex license for€5.5 million, and impairment of certain patents for€0.7 million. IDM regularly undertakes detailed reviews of its patents and licenses in order to check the development stage and the viability of associated products. When certain product development projects remain at an early stage or are abandoned, IDM writes down in full the remaining value of licenses, patents or trademarks associated with those projects, if they are found to have no alternative future use. IDM’s research and development expenses increased by€0.5 million in the year ended December 31, 2004, resulting primarily from an increase in research and development costs incurred by IDM, Inc., IDM’s wholly owned U.S. subsidiary. IDM, Inc.’s research and development expenses were€4.5 million for the year ended December 31, 2004, an increase of approximately 22% compared to€3.7 million for the year ended December 31, 2003, primarily reflecting increases in supplier and employment costs. In particular, the increase in supplier expenses of IDM, Inc. reflected the expansion of preclinical, clinical and regulatory activities related to Collidem, which is currently undergoing a Phase I/ II trial in the United States.
Globally, direct research and development expenses related to IDM’s product candidates to destroy residual cancer cells were approximately€3.9 million and€1.9 million 2004 and 2003 respectively, or€8.2 million for the period from January 1, 2001, the earliest date for which relevant cumulated cost information is available, to December 31, 2004. Direct research and development expenses related to IDM’s product candidates to stimulate an immune response and prevent tumor recurrence were approximately€3.8 million and€4.5 million in 2004 and 2003 respectively, or€11.7 million for the period from January 1, 2001, the earliest date for which relevant cumulated cost information is available, to December 31, 2004.
Selling and Marketing Expenses. Selling and marketing expenses were€0.9 million for the year ended December 31, 2004, a decrease of approximately 40% compared to€1.5 million for the year ended December 31, 2003. These expenses consisted primarily of costs related to IDM’s participation in trade conferences and to the employment costs of its business development and communications employees.
General and Administrative Expenses and Other Operating Expenses. General and administrative expenses were€5.3 million for the year ended December 31, 2004, an increase of approximately 13% compared to€4.7 million for the year ended December 31, 2003. The higher level of general and administrative expenses in 2004 resulted primarily from legal fees incurred in relation to strategic transactions, including the negotiation of the Share Exchange Agreement.
Other operating expenses totaled€2.4 million in 2004 and correspond to legal, accounting and communications fees paid for IDM’s attempted initial public offering in June 2004.
Interest Income. Net interest income was€0.6 million for the year ended December 31, 2004, a decrease of approximately 40% compared to€1.0 million for the year ended December 31, 2003, reflecting the combined effect of lower cash reserves and lower interest rates.
Income Tax Benefit. As a result of the increase in IDM’s research and development expenses, IDM recorded a research tax credit in the amount of€0.2 million for the year ended December 31, 2003, a decrease of approximately 33% compared to€0.3 million for the year ended December 31, 2004. This decrease resulted primarily from the fact that the method of calculation of the research tax credit was modified in France to be more advantageous, even though research and development expenses that are reimbursed by Sanofi-Aventis under the collaboration agreement are not eligible for such research tax credit.
As of December 31, 2004, IDM had accounts receivable of€1.4 million, mainly representing an account receivable from the French government, of which€0.5 million is recoverable in 2005.
95
Net Loss. IDM’s net loss increased to€25.5 million for the year ended December 31, 2004, compared to€16.2 million for the year ended December 31, 2003, as a result of the factors described above.
| |
| Results of Operations for the Years Ended December 31, 2003 and 2002 |
Revenues. IDM’s total revenues for the year ended December 31, 2003 were€5.3 million, an increase of approximately 47% compared to total revenues of€3.6 million for 2002. All of IDM’s revenues for the periods ended December 31, 2003 and 2002 were generated from its activities in France.
The increase in research and development revenues was derived entirely from reimbursement of current and past research and development expenses and up-front fees and milestone payments received from Sanofi-Aventis under the terms of the collaboration agreement.
Accordingly, IDM recorded€4.8 million in revenues from Sanofi-Aventis in 2003 for reimbursement of current expenses related to the development of Uvidem, an increase of approximately 129% compared to€2.1 million in 2002. In 2002 and 2003, IDM also recognized€1.4 million and€0.6 million, respectively, in revenues corresponding to the up-front fee, milestone payments and reimbursement of expenses IDM had incurred in prior years, which are all being recognized on a straight-line basis over the development of Uvidem, which was changed to nine years in December 2003.
Research and Development Expenses and Impairment of Patents and Licenses. Total research and development expenses and impairment of patents and licenses were€16.6 million for the year ended December 31, 2003 an increase of approximately 35% compared to€12.3 million for the year ended December 31, 2002. Of this increase, approximately 60% was linked to the growth in IDM’s activities in the United States through its subsidiary, IDM, Inc. In June 2002, IDM, Inc. expanded its operations in California by opening a new facility and recruiting 15 employees, including ten employees focused on research and development. IDM, Inc.’s research and development expenses increased to€3.7 million for 2003, an increase of approximately 164% compared to€1.4 million for 2002, primarily reflecting the expansion of preclinical, clinical and regulatory activities related to Collidem, which is currently undergoing a Phase I/ II trial in the United States, combined with increases in supplier and employment costs.
Another 25% of this increase between 2003 and 2002 was linked to the amortization of IDM’s licenses, patents and trademarks, as well as the impairment of certain patents which amounted to€1 million in 2003, compared to 2002 in which there was negligible cost associated with impairment of patents.
Globally, direct research and development expenses related to IDM’s product candidates to destroy residual cancer cells were approximately€1.9 million and€1.6 million in 2003 and 2002 respectively, or€4.3 million for the period from January 1, 2001, the earliest date for which relevant cumulated cost information is available, to December 31, 2003. Direct research and development expenses related to IDM’s product candidates to stimulate an immune response and prevent tumor recurrence were approximately€4.5 million and€1.8 million in 2003 and 2002 respectively, or€7.9 million for the period from January 1, 2001, the earliest date for which relevant cumulated cost information is available, to December 31, 2003.
Selling and Marketing Expenses. Selling and marketing expenses were€1.5 million for the year ended December 31, 2003 a decrease of approximately 6% compared to€1.6 million for the year ended December 31, 2002. These expenses consisted primarily of costs related to IDM’s participation in trade conferences and to its business development and communications employees.
General and Administrative Expenses. General and administrative expenses remained stable at€4.7 million for the year ended December 31, 2003, as compared to€4.8 million for the year ended December 31, 2002.
Interest Income. Net interest income was€1.0 million for the year ended December 31, 2003, a decrease of approximately 23% compared to€1.3 million for the year ended December 31, 2002. This decrease in interest income reflects the combined effect of lower cash reserves and lower interest rates.
Other Income. Other income decreased from€0.2 million for the year ended December 31, 2002 to a negligible amount for 2003. In November 2000, IDM received an interest-free conditionally reimbursable loan
96
from Anvar, a French governmental agency, in an amount of€305,000, in connection with IDM’s then planned initial public offering. In accordance with the terms of the loan, IDM repaid€64,000 of the principal balance in 2001. After receiving approval from Anvar in 2002, the outstanding obligation of€241,000 was forgiven and was recognized as other income in 2002.
Income Tax Benefit. Despite the increase in IDM’s research and development expenses, IDM recorded a research tax credit of€0.2 million for the year ended December 31, 2003, a decrease of approximately 67%, compared to€0.6 million for the year ended December 31, 2002. This was primarily due to the fact that IDM’s research and development expenses increased in the United States, where there is no applicable research tax credit. In addition, IDM is not eligible in France for a research tax credit for research and development expenses that are reimbursed by Sanofi-Aventis under the collaboration agreement.
As of December 31, 2003, IDM had accounts receivable of€1.6 million, mainly representing an account receivable from the French government, of which€0.5 million was recoverable in 2004.
Net Loss. IDM’s net loss was€16.2 million for the year ended December 31, 2003, an increase of approximately 26% compared to a net loss of€12.9 million for the year ended December 31, 2002, as a result of the factors described above.
| |
| Liquidity and Capital Resources |
As of March 31, 2005, IDM’s cash and cash equivalents totalled€26.8 million, compared to€30.9 million as of December 31, 2004. Cash and cash equivalents include principally cash, money-market funds and short-term certificates of deposit and are denominated primarily in euros. IDM uses its cash and cash equivalents to cover research and development expenses and corporate expenses related to selling and marketing and general and administrative activities. If IDM enters into partnerships for certain of its products, IDM expects that its partners would assume most, if not all, of the costs of further product development. Unless IDM finds a partner for a product, IDM bears all costs related to its development. As IDM pursues its strategy of bringing certain of its products further into clinical development and possible commercialization on its own, while also seeking partners for the development and commercialization of other products, IDM expects its expenses to increase.
Net cash used in operating activities increased to€3.9 million for the three months ended March 31, 2005, compared to€2.2 million for the first quarter of 2004. This increase was principally due to higher other operating expenses corresponding to legal and accounting fees paid in relation to the combination between IDM and Epimmune Inc., as well as a smaller decrease in accounts receivable from Sanofi-Aventis during the first quarter of 2005 compared to the decrease that occurred during the first three months of 2004. Net cash used in investing activities decreased during the first three months of 2005 to€179,000, compared to€448,000 for the first quarter of 2004. Cash used in investing activities includes purchase of property and equipment and payment of patent development costs and acquisition of other intangibles.
Net cash used in operating activities remained stable at€14.1 million for the year ended December 31, 2004, compared to€14.0 million for the year ended December 31, 2003. Net cash used in investing activities decreased in the year ended December 31, 2004 to€0.9 million, compared to€1.7 million for the year ended December 31, 2003. Net cash from financing activities increased from negative€0.09 million in 2003 to€12.6 million in 2004. The increase reflects gross proceeds of€12.5 million received by IDM from a private placement of IDM ordinary shares to Sanofi-Aventis and other investors.
As of March 31, 2005, IDM did not have short-term debt and its current liabilities amounted to€7.1 million. IDM’s current liabilities included€4.7 million in accounts payable to suppliers,€0.6 million in the current portion of deferred revenues from the collaboration agreement with Sanofi-Aventis, which are recognized as revenue on a straight-line basis over the remaining term of the agreement, and€1.2 million in accrued compensation for employees. IDM’s long-term liabilities as of March 31, 2005 were composed primarily of€2.6 million in deferred revenues from Sanofi-Aventis and an interest-free loan of€0.2 million from the French government that provides support to French companies for research and development. IDM must repay the principal amount of this loan in two equal installments of€0.1 million each in September 2007 and September 2010.
97
IDM’s financial requirements have been met to date through private placements of equity securities, payments received under IDM’s 2001 agreement with Sanofi-Aventis and IDM’s 1996 agreement with Medarex, together with grants received from governmental agencies. IDM has received a total of€99.2 million in gross proceeds from private placements of equity securities since its inception: in 1996,€3.1 million, including€0.3 million from the conversion of convertible bonds; in 1998,€18.4 million, including€2.8 million from the conversion of convertible bonds; in 2000,€44.5 million; in 2002,€20 million; and in 2004,€12.5 million.
IDM expects its principal sources of revenues to be up-front fees, milestone payments and reimbursements of research and development expenses under the collaboration agreement with Sanofi-Aventis, until such time as it successfully develops one or more products for sale outside the Sanofi-Aventis agreement. However, if IDM does not meet further development milestones with respect to Uvidem, or if Sanofi-Aventis does not elect to develop additional product candidates, IDM will not receive additional payments. In addition, IDM expects to receive revenues from sales of its lead product candidate, Mepact, assuming that IDM receives regulatory approval and chooses to market Mepact itself. However, IDM may not receive regulatory approval and, even if it does, any efforts by IDM or any future partners to commercialize Mepact may not be successful. In keeping with IDM’s overall strategy, IDM is seeking to enter into collaboration agreements for certain products with other partners, which may provide additional sources of revenues, including other milestone payments. However, IDM cannot be certain that it will enter into such agreements. In addition, the timing of IDM’s milestone payments cannot be predicted with certainty, and IDM may not receive payments if development targets are not achieved. Also, it is unlikely that milestone payments, even if received when expected, would fully cover IDM’s total research and development expenses for all of its projects. Royalties, if any, on commercial sales of products under development with partners will not be received until at least such time as such products receive the required regulatory approvals and are launched on the market. IDM does not expect any of its products to receive regulatory approval until at least 2007, and IDM cannot be sure of the timing of any such approval or successful commercialization following such approval.
IDM’s capital expenditures include purchase of property and equipment, including research and development laboratory equipment and product manufacturing facilities. Capital expenditures also include purchase of intangible assets, including payment of patent development costs, acquisition of third party licenses (such as from Medarex and Jenner Biotherapies) and acquisition of other intangibles. Capital expenditures amounted to€0.2 million for the three months ended March 31, 2005, and to€0.5 million for the same period in 2004. Capital expenditures amounted to€0.9 million for the year ended December 31, 2004, and€4.6 million (including€2.9 million related to the non-cash acquisition of certain assets from Jenner Biotherapies) for the year ended December 31, 2003.
IDM’s major outstanding contractual obligations relate to its long-term debt, operating lease obligations, and obligations under a number of its collaboration, licensing and consulting agreements. At December 31, 2004, IDM had no outstanding capital lease obligations. The following table summarizes IDM’s long-term debt, operating lease obligations and fixed mandatory payments under its collaboration, licensing and consulting agreements as of December 31, 2004.
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
| | | | |
| | | | | Less Than | | | Between 1 | | | Between 3 | | | More Than | |
| | | Total | | | 1 Year | | | and 3 Years | | | and 5 Years | | | 5 Years | |
| | | | | | | | | | | | | | | | |
| | | (In thousands of€) | |
Contractual Obligations | | | | | | | | | | | | | | | | | | | | |
| Long-Term Debt | | | 234 | | | | 0 | | | | 117 | | | | 0 | | | | 117 | |
| Operating Lease Obligations | | | 3,074 | | | | 729 | | | | 1,390 | | | | 955 | | | | 0 | |
| Fixed Mandatory Payments under Collaboration, Licensing and Consulting Agreements | | | 3,782 | | | | 3,216 | | | | 213 | | | | 197 | | | | 156 | |
| | | |
| Total | | | 7,090 | | | | 3,945 | | | | 1,720 | | | | 1,152 | | | | 273 | |
| | | |
98
Under certain of IDM’s collaboration and licensing agreements, such as its agreements with Epimmune and Institut de Recherche Pierre Fabre, IDM is obligated to make specified payments upon achieving certain milestones relating to the development and approval of its products, or on the basis of net sales of its products. In addition, under certain of its agreements with clinical sites for the conduct of its clinical trials, IDM makes payments based on the number of patients enrolled. Due to the variability associated with these agreements, these contingent payment obligations are not included in the table above. Globally, IDM expects that these contingent payment obligations could range from€6 million to€11 million in total over the next five years. However, such amounts are based on a variety of estimates and assumptions, including future sales volumes and timing of clinical trials and regulatory processes, which may not be accurate, may not be realized, and are inherently subject to various risks and uncertainties that are difficult to predict and are beyond the control of IDM. The table above discloses only future payments that can be determined with a reasonable level of certainty, which includes payments to external consultants, suppliers and subcontractors, principally for the recruitment of patients and monitoring of clinical centers (Accovion) and for the manufacturing of compounds required for IDM’s product candidates (Biotecnol).
IDM expects to incur approximately $4.7 million in costs related to the Exchange, consisting primarily of legal and accounting fees, and fees paid to its financial advisor in connection with the transaction.
IDM believes that its existing cash resources are sufficient to meet its cash requirements for the next 21 months. IDM’s future capital requirements, the timing and amount of expenditures and the adequacy of available capital will depend upon a number of factors. These factors include the scope and progress of IDM’s research and development programs, IDM’s ability to sign new collaboration agreements and maintain its current collaboration agreement with Sanofi-Aventis and whether Sanofi-Aventis elects to develop additional product candidates, IDM’s progress in developing and commercializing new products resulting from its development programs and collaborations including the achievement of milestones, IDM’s plans to expand or construct manufacturing or other facilities, technological developments, IDM’s preparation and filing of patent applications, IDM’s securing and maintaining patents and other intellectual property rights and IDM’s dealings with the regulatory process. See the section entitled“Trends” below.
| |
| Off-Balance Sheet Arrangements |
As of March 31, 2004, IDM was not a party to any transactions, agreements or contractual arrangements to which an entity that is not consolidated with IDM was a party, under which IDM had:
| | |
| | • | any obligation under a guarantee contract; |
| |
| | • | a retained or contingent interest in assets transferred to an unconsolidated entity or similar arrangement that serves as credit, liquidity or market risk support for such assets; |
| |
| | • | any obligation under a derivative instrument that is both indexed to IDM’s stock and classified in shareholders’ equity, or not reflected, in IDM’s statement of financial position; or |
| |
| | • | any obligation, including a contingent obligation, arising out of a variable interest, in an unconsolidated entity that is held by, and material to, IDM, where such entity provides financing, liquidity, market risk or credit risk support to IDM, or engages in leasing, hedging or research and development services with IDM. |
The level of IDM’s research and development spending will depend on numerous factors including the number of products in development, the number of products partnered, the results and progress of preclinical and clinical testing, IDM’s financial condition and general market conditions.
IDM expects its research and development expenses to increase due to the maturation of the development stage for certain of its products, since clinical trial expenses increase significantly when moving from Phase I to Phase II and from Phase II to Phase III. Furthermore, IDM expects that several new product candidates from its research portfolio, such as Jenact or Liposomal KSA, will enter into clinical development.
99
As products successfully mature, IDM also expects to pay filing fees in connection with the regulatory submission process and incur expenses related to the maintenance and potential expansion of its product manufacturing facilities. IDM’s strategy is to pursue the development of certain of its existing products on its own until commercialization, including completion of Phase II and III clinical trials, and its portfolio of products in development will be prioritized in order to maintain research and development expenses in line with available financial resources.
If IDM succeeds in gaining regulatory approval for Mepact, IDM expects its selling and marketing expenses to increase correspondingly with its activities to commercialize Mepact. In addition, IDM would expect to incur significant costs related to manufacturing Mepact, which would be recorded as cost of goods sold under IDM’s total revenues. Furthermore, IDM would expect to owe milestone payments upon final completion of Phase III trials and submission of a BLA for Mepact, as well as royalties in the event of its commercialization, under the licensing agreement for a component of Mepact. However, actual milestone and royalty payments are subject to reaching profitability within the Mepact product line.
For each product, IDM also expects its general and administrative expenses to increase in order to support its expanding clinical development and regulatory activities. Other factors that will contribute to the increase in these expenses include the expansion of IDM’s operations in the United States through internal growth or acquisition of existing facilities, the establishment of IDM’s manufacturing facilities and the build-up of a sales force as IDM approaches product commercialization, as well as potential acquisitions and in-licensing of complementary technologies, compounds and products.
| |
| Recent Accounting Pronouncements |
In January 2003, the FASB issued FASB Interpretation No. 46,“Consolidation of Variable Interest Entities an interpretation of ARB No. 51,” referred to as FIN 46. FIN 46 requires certain variable interest entities to be consolidated by the primary beneficiary of the entity if the equity investors in the entity do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. The disclosure provisions of FIN 46 are effective for financial statements initially issued after January 31, 2003. Public entities with a variable interest in a variable interest entity created before February 1, 2003 shall apply the consolidation requirements of FIN 46 to that entity no later than the beginning of the first annual reporting period beginning after June 15, 2003. FIN 46 is effective for all new variable interest entities created or acquired after January 31, 2003. Adoption of FIN 46 did not have a significant impact on IDM’s financial statements.
In November 2000, the EITF reached a consensus on EITF 00-21, “Revenue Arrangements with Multiple Deliverables,” referred to as EITF 00-21. EITF 00-21 provides guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets. The provisions of EITF 00-21 will apply to revenue arrangements entered into in fiscal periods beginning after June 15, 2003. The adoption of EITF 00-21 did not have a significant effect on IDM’s financial statements.
In May 2003, the FASB issued Statement of Financial Accounting Standard No. 150, “Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity,” referred to as SFAS 150. SFAS 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. It requires that an issuer classify a financial instrument that is within its scope as a liability (or an asset in some circumstances). SFAS 150 affects the issuer’s accounting for three types of freestanding financial instruments:
| |
| | (1) mandatorily redeemable shares, which embodies an unconditional obligation requiring the issuing company to redeem in exchange for cash or other assets at a specified or determinable date(s) or upon an event that is certain to occur; |
| |
| | (2) instruments that require or may require the issuer to repurchase some of its shares in exchange for cash or other assets, which includes put options and forward purchase contracts; and |
100
| |
| | (3) obligations that must or may be settled by issuing a variable number of equity shares, the monetary value of which is fixed, tied solely or predominantly to a variable such as a market index, or varies inversely with the value of the issuer’s shares. |
SFAS 150 does not apply to features embedded in a financial instrument that is not a derivative in its entirety. Most of the guidance in SFAS 150 is effective for all financial instruments entered into or modified after May 31, 2003, and otherwise is effective for the fiscal years ending after June 15, 2003.
The adoption of SFAS 150 did not have a significant impact on IDM’s results of operations, financial position or cash flows.
On December 16, 2004, the Financial Accounting Standards Board (FASB) issued FASB Statement No. 123 (revised 2004), Share-Based Payment, which is a revision of FASB Statement No. 123, Accounting for Stock-Based Compensation. Statement 123(R) supersedes APB Opinion No. 25, Accounting for Stock Issued to Employees, and amends FASB Statement No. 95, Statement of Cash Flows. Generally, the approach in Statement 123(R) is similar to the approach described in Statement 123. However, Statement 123(R) requires all share-based payments to employees, including grants of employee stock options and warrants, to be recognized in the income statement based on their fair values. Pro forma disclosure is no longer an alternative.
Statement 123(R) is effective for non-public companies in the fiscal years beginning after December 15, 2005. Currently, as permitted by Statement 123, IDM accounts for share-based payments to employees using Opinion No. 25’s intrinsic value method and, as such, no compensation expense is recognized for stock options and warrants with an exercise price equal to or greater than the fair market value of the underlying shares.
Note 1 to IDM’s consolidated financial statements describes the impact on the pro forma net loss and loss per share using the fair values at the date of grant based on the fair value method, excluding the impact of stock volatility.
Under Statement 123(R), non-public entities that used the minimum value method to measure compensation costs for pro forma disclosure purposes, as in IDM’s case, will be required to use the prospective method. Under the prospective method, non-public entities would continue to account for non-vested awards outstanding at the date of the adoption of Statement 123(R)in the same manner s they had been accounted for prior to adoption for financial recognition purposes. All awards granted, modified or settled after the date of adoption will be accounted for using the measurement, recognition and attribution provisions of Statement 123(R).
Disclosure about Market Risk
IDM’s is exposed to limited market risk through its investment of cash in high grade securities, generally with maturities of less than three months. The securities contained in IDM’s cash and cash equivalents are typically debt instruments purchased at inception and held until maturity. Due to their very short-term nature, such securities are subject to minimal interest rate risk. IDM currently does not hedge interest rate exposure. IDM also does not hedge currency exchange rate exposure (including against the U.S. dollar). Historically, IDM’s revenues, long-term commitments and receivables have principally been denominated in euros, while IDM’s expenses are denominated in euros and U.S. dollars. Historically, foreign currency exchange rate risk has not been material to IDM. However, this situation may be subject to change, for example, if IDM pursues a strategy of expanding its operations in the United States.
101
DESCRIPTION OF EPIMMUNE CAPITAL STOCK
The following is a summary of the terms of our capital stock immediately prior to the closing of the Share Exchange Agreement. This summary is qualified in its entirety by the provisions of our Amended and Restated Certificate of Incorporation and Bylaws and by the applicable provisions of Delaware law.
Common Stock
We are authorized to issue 40,000,000 shares of our common stock, par value $0.01 per share. As of June 29, 2005, there were 16,023,786 shares of our common stock outstanding. Holders of shares of our common stock are entitled to one vote per share. Subject to the right of the holders of any class of our preferred stock, holders of shares of our common stock are entitled to receive dividends that may be declared by our Board out of legally available funds. Dividends may be declared at any regular or special meeting of the Board and paid in cash, property or shares of capital stock. We have never declared or paid any cash dividends on our capital stock.
Preferred Stock
We are authorized to issue 10,000,000 shares of preferred stock, par value $0.01 per share, issuable in any series or number of series as our Board shall determine, of which 250,000 shares of Series A Junior Participating preferred stock, 859,666 shares of Series S preferred stock and 549,622 shares of Series S-1 preferred stock have been authorized. As of June 29, 2005, there were 1,409,288 total shares of preferred stock outstanding, consisting of 859,666 shares of Series S preferred stock and 549,622 shares of Series S-1 preferred stock. G.D. Searle, the holder of all outstanding shares of Series S preferred stock and Series S-1 preferred stock, will be entitled to vote such shares on all matters to be voted upon at the annual meeting on an as-converted to common stock basis.
Concurrently with the execution of the Share Exchange Agreement, we entered into a Preferred Exchange Agreement with G.D. Searle. On April 12, 2005, we entered into an Amended and Restated Preferred Exchange Agreement with G.D. Searle. Under this agreement, it agreed to exchange all of the outstanding shares of Series S preferred stock and S-1 preferred stock it holds for 1,949,278 shares of our common stock, subject to adjustment pursuant to the reverse stock split described in proposal 3 or any other stock split, combination or stick dividend, immediately prior to the closing of the Share Exchange Agreement.
Reverse Stock Split and Increase of Authorized Shares
Prior to the closing of the Share Exchange Agreement, we agreed to take all action necessary to effect a reverse stock split and to cause our authorized capital stock to be increased to a total of 65,000,000 shares, consisting of 55,000,000 shares of common stock and 10,000,000 shares of preferred stock after giving effect to the reverse stock split. For more information on the reverse stock split and the capital increase, please see the sections entitled“Proposal 3 — Approval of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation to Effect a Reverse Stock Split” and“Proposal 4 — Approval of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation to Effect an Increase in Authorized Shares.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer and Trust Company.
Listing
Our common stock is currently quoted on the Nasdaq National Market under the symbol “EPMN.” On May 17, 2005, we received a letter from Nasdaq informing us that we currently fail to meet the minimum stockholders’ equity requirement of $10 million for continued listing on the Nasdaq National Market. In
102
addition, on June 20, 2005, we received a letter from the Nasdaq Stock Market indicating that our plan to achieve compliance with the minimum stockholders’ equity requirement was not accepted by the Nasdaq staff because, among other things, we will be unable to demonstrate compliance until the Exchange is complete and the Exchange is subject to stockholder approval and therefore is not definitive. On June 27, 2005, we filed a request for an appeal with the Nasdaq Listing Qualifications Panel and an oral hearing date has been set for July 21, 2005. During the appeal process and until a final determination is made with respect to our continued listing, our common stock will continue to trade on The Nasdaq National Market. In addition, on May 26, 2005, we received a letter from Nasdaq informing us that we currently fail to meet the $1.00 per share minimum closing bid price requirement for continued listing on the Nasdaq National Market. For further information regarding the quotation of our common stock upon the completion of the Exchange, see the section entitled“The Exchange — Listing of Epimmune Common Stock.”
103
SELECTED FINANCIAL PROJECTIONS
Neither Epimmune nor IDM, as a matter of course, make public forecasts or projections as to future financial performance. In January 2005, after IDM and Epimmune had negotiated the exchange ratio and other material terms of the Share Exchange Agreement, management of Epimmune and IDM prepared certain financial projections for the combined company, including those summarized below. The information was provided to our Board as part of our confirmatory financial diligence, including the Board’s evaluation of the capital budget requirements for the combined company and its assessment of the likelihood of obtaining adequate levels of financing. The information was also provided to our financial advisor. The financial projections were not prepared with a view towards public disclosure and were not provided to the IDM shareholders in connection with the solicitation of their approval and execution of the Share Exchange Agreement. The projections do not purport to present the combined company’s operations in accordance with generally accepted accounting principles and our independent auditors have not examined or compiled the projections and accordingly assume no responsibility for them.
The financial projections of the combined company constitute “forward looking” statements. The financial projections were prepared by the management of Epimmune and IDM based on numerous estimates and assumptions with respect to clinical and other product development results, revenue under collaboration and other agreements, product revenue and costs, and competitive, general business, economic, market and other conditions, all of which are difficult to predict and are subject to risks and uncertainties. These risks and uncertainties include those described under the heading “Risk Factors” beginning on page 22 of this proxy statement. Many of the factors impacting the assumptions are beyond the control of Epimmune and IDM. The actual results are likely to materially differ from the results predicted in the financial projections. Accordingly, there can be no assurance that the financial projections will be indicative of the future performance of the combined company or that the financial projections will prove accurate. See “Forward Looking Statements” beginning on page 41 of this proxy statement. The inclusion of these projections should not be regarded as an indication that IDM or Epimmune, or their respective affiliates or representatives, considered or consider such data to be a reliable prediction of future events, especially in later years, and such data should not be relied upon as such. None of IDM, Epimmune or any of their respective affiliates or representatives has made or makes any representations to any person regarding the ultimate performance of IDM, Epimmune or the combined company compared to the information contained in the projections, and none of them intends to provide any update or revision of the projections, except as required by applicable law.
The assumptions made in developing the projections in the following table include the assumption that the most advanced clinical drug candidates in the combined company’s pipeline, including Mepact, Uvidem, Bexidem, Collidem, EP-HIV 1090 and EP-2101 will either continue to advance in clinical development independently or be partnered to limit the financial exposure of the combined company, and that Mepact will be successfully commercialized with a product launch in 2007 and that Bexidem will be successfully commercialized with a product launch in 2009. All products in development were assumed to proceed successfully to commercialization or to be partnered. Also included are many assumptions about manufacturing costs, research and development expense, sales and marketing expense and general and administrative expense that could prove to be materially inaccurate, especially in later years. There were no assumptions included regarding new partners or collaborators which could bring upfront or milestone payments, nor was there any assumption regarding additional financing for the combined company. Management cannot predict the integration costs or the savings, if any, that will be achieved when the Epimmune and IDM organizations are combined. Drug development is inherently risky and any one or more of the drug candidates under development could fail for lack of efficacy or unknown safety issues. Further there is no assurance that any drug candidate will obtain required regulatory approvals or that any drug that does receive marketing approvals will be successful as a commercial drug or will achieve projected sales levels. Any delays in obtaining marketing approvals or other commercial launch delays would materially affect the accuracy of the financial projections. Finally, management of Epimmune and IDM prepared these financial projections for internal use and they remain subject to change based upon the new Board’s and management’s decisions about strategy and the operations of the combined company.
104
Combined Projected Statements of Operations and Cash Balances
(US$ in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| $ | | 2005 | | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | 2013 | | | 2014 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net Revenues | | | 17,346 | | | | 25,584 | | | | 33,076 | | | | 70,628 | | | | 151,543 | | | | 212,230 | | | | 384,840 | | | | 559,400 | | | | 655,793 | | | | 810,375 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Gross Margin | | | 17,346 | | | | 25,584 | | | | 31,508 | | | | 61,856 | | | | 122,642 | | | | 162,647 | | | | 287,025 | | | | 412,152 | | | | 477,712 | | | | 590,493 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and Development | | | 33,227 | | | | 37,289 | | | | 41,235 | | | | 55,618 | | | | 64,549 | | | | 59,190 | | | | 52,775 | | | | 44,801 | | | | 36,403 | | | | 12,568 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sales and Marketing | | | 520 | | | | 845 | | | | 4,206 | | | | 8,522 | | | | 11,668 | | | | 9,744 | | | | 11,928 | | | | 8,834 | | | | 13,600 | | | | 16,146 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| General and Administrative | | | 9,176 | | | | 7,726 | | | | 8,544 | | | | 9,442 | | | | 10,222 | | | | 10,151 | | | | 10,580 | | | | 10,349 | | | | 10,575 | | | | 10,828 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Acquisition Costs | | | 4,840 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Loss)/Income from Operations | | | (30,417 | ) | | | (20,276 | ) | | | (22,477 | ) | | | (11,726 | ) | | | 36,203 | | | | 83,562 | | | | 211,742 | | | | 348,168 | | | | 417,134 | | | | 550,951 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Interest Income | | | 586 | | | | 212 | | | | 0 | | | | 0 | | | | 0 | | | | 920 | | | | 2,772 | | | | 5,946 | | | | 10,673 | | | | 16,972 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net (Loss)/Income | | | (29,831 | ) | | | (20,064 | ) | | | (22,477 | ) | | | (11,726 | ) | | | 36,203 | | | | 84,482 | | | | 214,514 | | | | 354,114 | | | | 427,807 | | | | 567,923 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash at End of Period | | | 15,638 | | | | (4,425 | ) | | | (28,062 | ) | | | (43,428 | ) | | | (21,062 | ) | | | 50,971 | | | | 236,927 | | | | 559,627 | | | | 965,625 | | | | 1,495,864 | |
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
The following unaudited pro forma condensed combined financial statements give effect to the proposed transaction between Epimmune and IDM. Pursuant to the Share Exchange Agreement, Epimmune will acquire all of the outstanding share capital of IDM, with certain exceptions related to PEA shares and PEA warrants, in exchange for Epimmune common stock. Following the transaction, the former shareholders and optionholders of IDM will hold approximately 78% of the Epimmune outstanding common stock, on a fully diluted basis. Because IDM’s shareholders will own a majority of the shares of Epimmune common stock after the transaction, IDM’s designees to the combined company’s board of directors will represent a majority of the combined company’s directors and IDM’s senior management will represent a majority of the senior management of the combined company, IDM is deemed to be the acquiring company for accounting purposes. Accordingly, the purchase price is allocated among the fair values of the assets and liabilities of Epimmune, while the historical results of IDM are reflected in the results of the combined company. The transaction will be accounted for under the purchase method of accounting in accordance with Statement of Financial Accounting Standards, referred to as SFAS, No. 141, “Business Combinations.” Under the purchase method of accounting, the total estimated purchase price, calculated as described in Note 1 to these unaudited pro forma condensed combined financial statements, is allocated to the net tangible and intangible assets acquired and liabilities assumed in connection with the transaction, based on their estimated fair values as of the completion of the transaction.
For a summary of the Exchange, see the section entitled“The Exchange.”
The unaudited pro forma condensed combined financial statements presented below are based upon the historical financial statements of Epimmune and IDM, adjusted to give effect to the acquisition of Epimmune by IDM. The pro forma adjustments are described in the accompanying notes presented on the following pages.
The unaudited pro forma condensed combined balance sheet as of March 31, 2005 gives effect to the proposed transaction as if it occurred on March 31, 2005 and combines the historical balance sheets of IDM and Epimmune at March 31, 2005. The IDM balance sheet information was derived from its unaudited March 31, 2005 balance sheet included herein. The Epimmune balance sheet information was derived from its unaudited March 31, 2005 balance sheet included in its Quarterly Report on Form 10-Q/A for the quarter ended March 31, 2005, incorporated herein by reference.
The unaudited pro forma condensed combined statement of operations for the year ended December 31, 2004 is presented as if the transaction was consummated on January 1, 2004 and combines the historical results of IDM and Epimmune for the year ended December 31, 2004. The historical results of IDM were derived from its audited December 31, 2004 statement of operations included herein. The historical results of
105
Epimmune were derived from its statement of operations included in its Annual Report on Form 10-K/A for its fiscal year ended December 31, 2004 incorporated herein by reference.
The unaudited pro forma condensed combined statement of operations for the three months ended March 31, 2005 is presented as if the transaction was consummated on January 1, 2005 and combines the historical results of IDM and Epimmune for the three months ended March 31, 2005. The historical results of IDM were derived from its unaudited March 31, 2005 statement of operations included herein. The historical results of Epimmune for the three months ended March 31, 2005 were derived from its statement of operations included in its Quarterly Report on Form 10-Q/A for the quarter ended March 31, 2005, incorporated herein by reference.
The unaudited pro forma condensed combined financial statements reflect the preliminary estimates of the fair market value of the assets acquired and liabilities assumed of Epimmune, including preliminary valuations associated with intangible assets, certain contracts, and property, plant, and equipment. The final determination of the fair market value of assets acquired and liabilities assumed of Epimmune, as well as the useful lives of the assets acquired, and the resulting effect on income from operations may differ significantly from the pro forma amounts included herein, and will be done with the assistance of an independent valuation firm. There can be no assurance that such adjustments will not be material.
All intercompany balances and transactions between IDM and Epimmune, consisting primarily of a license agreement from Epimmune to IDM, as of the dates and for the periods of these unaudited pro forma condensed combined financial statements have been eliminated in the unaudited pro forma condensed combined financial statements. Certain reclassification adjustments have been made to conform Epimmune’s historical reported balances to IDM’s financial statement basis of presentation.
The unaudited pro forma condensed combined financial statements are provided for illustrative purposes only and do not purport to represent the financial condition or results of operations had the acquisition been completed as of the dates indicated, nor are they necessarily indicative of future consolidated results of operations or financial position.
The unaudited pro forma condensed combined financial statements do not include the realization of cost saving from operating efficiencies, synergies, or other restructurings resulting from the transaction.
The unaudited pro forma condensed combined financial statements should be read in conjunction with the historical financial statements and accompanying notes of Epimmune, which are incorporated by reference in this proxy statement, and the historical consolidated financial statements and accompanying notes of IDM, which are included elsewhere in this proxy statement.
106
Unaudited Pro Forma Condensed Combined Balance Sheet
As of March 31, 2005
| | | | | | | | | | | | | | | | | | | | | | |
| | | Historical | | | Historical | | | Pro Forma | | | | | Pro Forma | |
| | | IDM | | | Epimmune | | | Adjustments | | | | | Combined | |
| | | $US(1) | | | $US | | | $US(2) | | | Footnote | | | $US | |
| | | | | | | | | | | | | | | | |
| | | U.S. GAAP (In thousands) | |
| Current Assets: | | | | | | | | | | | | | | | | | | | | |
| | Cash and cash equivalents | | | 34,711 | | | | 5,673 | | | | (4,668 | ) | | | (3) | | | | 35,716 | |
| | Accounts Receivable | | | 1,646 | | | | 1,110 | | | | | | | | | | | | 2,756 | |
| | Research and development tax credit | | | 695 | | | | | | | | | | | | | | | | 695 | |
| | Prepaid expenses and other current assets | | | 1,616 | | | | 334 | | | | | | | | | | | | 1,950 | |
| | | | | | | | | | | | | | | | |
| Total current assets | | | 38,668 | | | | 7,117 | | | | (4,668 | ) | | | | | | | 41,117 | |
| Restricted cash | | | | | | | 354 | | | | | | | | | | | | 354 | |
| Property and equipment, net | | | 2,755 | | | | 962 | | | | | | | | | | | | 3,717 | |
| Intangibles other than goodwill, net | | | 4,693 | | | | 3,447 | | | | (1,947 | ) | | | (4) | | | | 6,193 | |
| Goodwill | | | | | | | | | | | 11,093 | | | | (6) | | | | 11,093 | |
| Research and development tax credit | | | 1,578 | | | | | | | | | | | | | | | | 1,578 | |
| Other noncurrent assets | | | 84 | | | | | | | | | | | | | | | | 84 | |
| | | | | | | | | | | | | | | | |
| Total Assets | | | 47,778 | | | | 11,880 | | | | 4,478 | | | | | | | | 64,136 | |
| Current Liabilities: | | | | | | | | | | | | | | | | | | | | |
| | Accounts payable and accrued liabilities | | | 6,146 | | | | 2,221 | | | | | | | | | | | | 8,367 | |
| | Deferred contract revenues | | | 716 | | | | 493 | | | | (335 | ) | | | (7) | | | | 874 | |
| | Accrued payroll | | | 1,560 | | | | 192 | | | | | | | | | | | | 1,752 | |
| | Other current liabilities | | | 799 | | | | | | | | | | | | | | | | 799 | |
| | | | | | | | | | | | | | | | |
| Total current liabilities | | | 9,221 | | | | 2,906 | | | | (335 | ) | | | | | | | 11,792 | |
| Deferred contract revenues | | | 3,403 | | | | | | | | | | | | | | | | 3,403 | |
| Deferred Rent | | | | | | | 208 | | | | (208 | ) | | | (4) | | | | — | |
| Long term debt, less current portion | | | 303 | | | | | | | | | | | | | | | | 303 | |
| Other long term liabilities | | | 119 | | | | | | | | | | | | | | | | 119 | |
| Stockholders’ Equity: | | | | | | | | | | | | | | | | | | | | |
| | Preferred stock | | | | | | | 14 | | | | (14 | ) | | | (8) | | | | — | |
| | Common stock | | | 2,035 | | | | 160 | | | | (1,443 | ) | | | (10) | | | | 928 | |
| | | | | | | | | | | | 19 | | | | (8) | | | | | |
| | | | | | | | | | | | 15 | | | | (9) | | | | | |
| | | | | | | | | | | | 138 | | | | (12) | | | | | |
| | | | | | | | | | | | 4 | | | | (11) | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | (1,267 | ) | | | | | | | | |
| | Additional paid-in capital | | | 184,374 | | | | 172,942 | | | | (172,942 | ) | | | (16) | | | | 214,755 | |
| | | | | | | | | | | | (138 | ) | | | (12) | | | | | |
| | | | | | | | | | | | (4 | ) | | | (11) | | | | | |
| | | | | | | | | | | | 2,831 | | | | (17) | | | | | |
| | | | | | | | | | | | 1,443 | | | | (10) | | | | | |
| | | | | | | | | | | | 2,015 | | | | (9) | | | | | |
| | | | | | | | | | | | 23,677 | | | | (14) | | | | | |
| | | | | | | | | | | | 557 | | | | (15) | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | (142,561 | ) | | | | | | | | |
| | Deferred compensation | | | (25 | ) | | | (165 | ) | | | 165 | | | | (17) | | | | (582 | ) |
| | | | | | | | | | | | (557 | ) | | | (15) | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | (392 | ) | | | | | | | | |
| | Accumulated other comprehensive loss | | | (287 | ) | | | | | | | | | | | | | | | (287 | ) |
| | Accumulated Deficit | | | (151,365 | ) | | | (164,185 | ) | | | (2,030 | ) | | | (9) | | | | (166,295 | ) |
| | | | | | | | | | | | 164,185 | | | | (16) | | | | | |
| | | | | | | | | | | | (12,900 | ) | | | (5) | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | 149,255 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Total stockholders’ equity | | | 34,732 | | | | 8,766 | | | | 5,021 | | | | | | | | 48,519 | |
| | | | | | | | | | | | | | | | |
| Total liabilities and stockholders’ equity | | | 47,778 | | | | 11,880 | | | | 4,478 | | | | | | | | 64,136 | |
See accompanying Notes to Unaudited Pro Forma Consolidated Condensed Financial Information.
107
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Year Ended December 31, 2004
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | Historical | | | Pro Forma | | | | | Pro Forma | |
| | | Historical IDM | | | Epimmune | | | Adjustments | | | | | Combined | |
| | | $US (1) | | | $US | | | $US (2) | | | Footnote | | | $US | |
| | | | | | | | | | | | | | | | |
| | | U.S. GAAP (In thousands, except per share data) | |
| Revenues: | | | | | | | | | | | | | | | | | | | | |
| | Research grants and contract revenue | | | | | | | 7,909 | | | | | | | | | | | | 7,909 | |
| | License fees and milestones | | | | | | | 712 | | | | (319 | ) | | | (7) | | | | 393 | |
| | Related party revenue | | | 5,890 | | | | 1,026 | | | | | | | | | | | | 6,916 | |
| | | | | | | | | | | | | | | | |
| Total revenues | | | 5,890 | | | | 9,647 | | | | (319 | ) | | | | | | | 15,218 | |
| Costs and expenses: | | | | | | | | | | | | | | | | | | | | |
| | Research and development | | | 20,368 | | | | 10,895 | | | | (301 | ) | | | (4) | | | | 30,962 | |
| | General and administrative | | | 6,685 | | | | 2,716 | | | | (2 | ) | | | (4) | | | | 9,383 | |
| | | | | | | | | | | | (71 | ) | | | (17) | | | | | |
| | | | | | | | | | | | 55 | | | | (18) | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | (18 | ) | | | | | | | | |
| | Impairment of patents & licenses | | | 7,960 | | | | | | | | | | | | | | | | 7,960 | |
| | Selling & Marketing | | | 1,195 | | | | | | | | | | | | | | | | 1,195 | |
| | Other operating expenses | | | 3,032 | | | | | | | | | | | | | | | | 3,032 | |
| | | | | | | | | | | | | | | | |
| Total costs and expenses | | | 39,240 | | | | 13,611 | | | | (319 | ) | | | | | | | 52,532 | |
| | | | | | | | | | | | | | | | |
| Loss from operations | | | (33,350 | ) | | | (3,964 | ) | | | — | | | | | | | | (37,314 | ) |
| Interest income, net | | | 710 | | | | 89 | | | | | | | | | | | | 799 | |
| Other (expense) income, net | | | (21 | ) | | | (7 | ) | | | | | | | | | | | (28 | ) |
| | | | | | | | | | | | | | | | |
| Loss before income tax benefit | | | (32,661 | ) | | | (3,882 | ) | | | — | | | | | | | | (36,543 | ) |
| Income tax benefit | | | 356 | | | | | | | | | | | | | | | | 356 | |
| | | | | | | | | | | | | | | | |
| Net loss | | | (32,305 | ) | | | (3,882 | ) | | | — | | | | | | | | (36,187 | ) |
| | | | | | | | | | | | | | | | |
| Weighted average shares, basic and diluted: | | | 13,649 | | | | 15,304 | | | | | | | | | | | | 92,117 | (13) |
| Net loss per share, basic and diluted: | | | (2.37 | ) | | | (0.25 | ) | | | | | | | | | | | (0.39 | )(13) |
See accompanying Notes to Unaudited Pro Forma Consolidated Condensed Financial Information.
108
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Quarter Ended March 31, 2005
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | Historical | | | Pro Forma | | | | | Pro Forma | |
| | | Historical IDM | | | Epimmune | | | Adjustments | | | | | Combined | |
| | | $US (1) | | | $US | | | $US (2) | | | Footnote | | | $US | |
| | | | | | | | | | | | | | | | |
| | | U.S. GAAP (In thousands, except per share data) | |
| Revenues: | | | | | | | | | | | | | | | | | | | | |
| | Research grants and contract revenue | | | | | | | 1,719 | | | | | | | | | | | | 1,719 | |
| | License fees and milestones | | | | | | | 114 | | | | (46 | ) | | | (7) | | | | 68 | |
| | Related party revenue | | | 1,573 | | | | — | | | | | | | | | | | | 1,573 | |
| | | | | | | | | | | | | | | | |
| Total revenues | | | 1,573 | | | | 1,833 | | | | (46 | ) | | | | | | | 3,360 | |
| Costs and expenses: | | | | | | | | | | | | | | | | | | | | |
| | Research and development | | | 4,847 | | | | 2,686 | | | | (67 | ) | | | (4) | | | | 7,466 | |
| | General and administrative | | | 1,400 | | | | 467 | | | | (2 | ) | | | (4) | | | | 1,864 | |
| | | | | | | | | | | | (15 | ) | | | (17) | | | | | |
| | | | | | | | | | | | 14 | | | | (18) | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | (3 | ) | | | | | | | | |
| | Impairment of patents & licenses | | | 270 | | | | | | | | | | | | | | | | 270 | |
| | Selling & Marketing | | | 258 | | | | | | | | | | | | | | | | 258 | |
| | Transaction Costs | | | 1,517 | | | | 1,089 | | | | | | | | | | | | 2,606 | |
| | | | | | | | | | | | | | | | |
| Total costs and expenses | | | 8,292 | | | | 4,242 | | | | (70 | ) | | | | | | | 12,464 | |
| | | | | | | | | | | | | | | | |
| Loss from operations | | | (6,719 | ) | | | (2,409 | ) | | | 24 | | | | | | | | (9,104 | ) |
| Interest income, net | | | 186 | | | | 36 | | | | | | | | | | | | 222 | |
| Other (expense) income, net | | | 4 | | | | (5 | ) | | | | | | | | | | | (1 | ) |
| | | | | | | | | | | | | | | | |
| Loss before income tax benefit | | | (6,529 | ) | | | (2,378 | ) | | | 24 | | | | | | | | (8,883 | ) |
| Income tax benefit | | | 441 | | | | | | | | | | | | | | | | 441 | |
| | | | | | | | | | | | | | | | |
| Net loss | | | (6,088 | ) | | | (2,378 | ) | | | 24 | | | | | | | | (8,442 | ) |
| | | | | | | | | | | | | | | | |
| Weighted average shares, basic and diluted: | | | 15,694 | | | | 16,015 | | | | | | | | | | | | 92,827 | (13) |
| Net loss per share, basic and diluted: | | | (0.39 | ) | | | (0.15 | ) | | | | | | | | | | | (0.09 | )(13) |
See accompanying Notes to Unaudited Pro Forma Consolidated Condensed Financial Information.
109
NOTES TO UNAUDITED PRO FORMA CONDENSED
COMBINED FINANCIAL STATEMENTS
On March 16, 2005, Epimmune and IDM announced they had entered into a Share Exchange Agreement pursuant to which Epimmune will issue approximately 74.9 million shares of its common stock in exchange for all of IDM’s outstanding common stock, except for the PEA shares, and may issue options to purchase approximately 4.3 million shares of Epimmune common stock in exchange for all outstanding IDM share options, for a total of approximately 79.2 million shares of Epimmune common stock. Because IDM shareholders and optionholders will own approximately 78% of the shares of Epimmune after the transaction, on a fully-diluted basis, IDM’s designees to the combined company’s board of directors will represent a majority of the combined company’s board of directors and IDM’s senior management will represent a majority of the senior management of the combined company, IDM is deemed to be the acquiring company for accounting purposes and the transaction will be accounted for as a reverse acquisition under the purchase method of accounting for business combinations in accordance with accounting principles generally accepted in the United States.
As of March 16, 2005, Epimmune had 17,963,847 shares of common stock outstanding including 1,949,278 shares on an as-converted to common stock basis, after giving effect to the conversion of the preferred stock pursuant to the terms of the Amended and Restated Preferred Exchange Agreement. Based on the average of the closing prices for a range of trading days (March 14, 2005 through March 18, 2005, inclusive) around and including the announcement date of the transaction, the fair value of the outstanding shares of Epimmune common stock is $1.33 per share or approximately $23.9 million, on an as-converted to common stock basis. The total preliminary estimated purchase price of approximately $31.4 million includes the estimated fair value of the Epimmune common stock, on an as-converted basis, of approximately $23.9 million, the estimated fair value of Epimmune outstanding stock options and warrants of approximately $2.8 million and the estimated IDM direct transaction costs of approximately $4.7 million. The assumptions used to calculate the estimated fair value of the outstanding Epimmune stock options and warrants were as follows: risk-free interest rate of 4%, dividend yield of 0%, stock volatility factor of ..947, stock price of $1.33, and a weighted average expected life of 2.9 years.
The allocation of the estimated purchase price discussed below is preliminary because the transaction has not yet been completed. The final allocation of the purchase price will be based on Epimmune’s assets and liabilities as of the closing of the Exchange.
The preliminary estimated total purchase price of the proposed transaction is as follows (in thousands):
| | | | | |
| Epimmune common stock | | $ | 21,268 | |
| Epimmune preferred stock, as-converted to common | | | 2,588 | |
| Estimated fair value of options assumed | | | 2,831 | |
| Estimated IDM direct transaction costs | | | 4,668 | |
| | | | |
| Total preliminary estimated purchase price | | $ | 31,355 | |
| | | | |
Under the purchase method of accounting, the total preliminary estimated purchase price as shown in the table above is allocated to the Epimmune net tangible and identifiable intangible assets acquired and liabilities assumed based on their estimated fair values as of the date of the completion of the transaction. The preliminary estimated purchase price has been allocated based on estimates taking into account various factors as described in the introduction to these unaudited pro forma condensed combined financial statements.
110
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL STATEMENTS — (Continued)
The allocation of the preliminary estimated purchase price, the estimated useful lives and related first-year amortization associated with certain assets is as follows (in thousands):
| | | | | | | | | | | | | | |
| | | | | First Year | | | Estimated Useful | |
| | | Amount | | | Amortization | | | Life (Years) | |
| | | | | | | | | | |
| Preliminary estimated purchase price allocation: | | | | | | | | | | | | |
| | Net tangible assets (net of liabilities) | | $ | 5,862 | | | $ | — | | | | — | |
| | Licensing and milestone agreements | | | 1,500 | | | | 300 | | | | 5 years | |
| | In-process research and development (“IPR&D”) | | | 12,900 | | | | — | | | | — | |
| | Goodwill | | | 11,093 | | | | — | | | | — | |
| | | | | | | | | | |
| | Total preliminary estimated purchase price | | $ | 31,355 | | | $ | 300 | | | | | |
| | | | | | | | | | |
In management’s opinion, the preliminary purchase price allocation is not expected to materially differ from the final purchase price allocation.
Management evaluated Epimmune’s projects currently under development and determined that $12.9 million was attributable to in-process research and development. The amounts allocated to IPR&D were determined through established valuation techniques used in the high technology industry and were expensed upon acquisition as it was determined that the underlying projects had not reached technological feasibility and no alternative future uses existed. In accordance with SFAS No. 2,Accounting for Research and Development Costs, as clarified by FIN No. 4,Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method, an Interpretation of FASB Statement No. 2, amounts assigned to IPR&D meeting the above-stated criteria will be charged to expense as part of the allocation of the purchase price.
Epimmune has two products in various states of clinical trials as of the valuation date; EP HIV-1090, a therapeutic vaccine for HIV in Phase I clinical trials, and EP-2101, a therapeutic vaccine for non-small cell lung cancer which entered Phase II clinical trials in December 2004. The fair value of the IPR&D was determined using the income approach. Under the income approach, the expected future cash flows for each product under development are estimated and discounted to their net present value at an appropriate risk-adjusted rate of return. Significant factors considered in the calculation of the rate of return are the weighted-average cost of capital and return on assets, as well as the risks inherent in the development process. For purposes of the analysis, EP HIV-1090 was projected to generate material revenue and cash flows beginning in 2013 and EP-2101 was projected to generate material revenue and cash flows beginning in 2014. Remaining research and development expenses for both EP HIV-1090 and EP-2101 are based on management’s best estimates to bring the drugs candidates to market. A 25% risk adjusted discount rate was applied to the cash flow projected for EPHIV-1090 and a discount rate of 29% was applied to the EP-2101 projected cash flow. The application of this methodology resulted in a fair value of $8.8 million being assigned to EP HIV-1090 and $4.1 million being assigned to EP-2101. Licensing and milestone agreements represents a combination of Epimmune patents, trade secrets, core technology and services that Epimmune has developed through years of work in the field of epitope identification. This proprietary knowledge base has been leveraged by Epimmune to enter into agreements with licensing and milestone opportunities. Epimmune expects to amortize the licensing and milestone agreements on a straight-line basis over an estimated life of five years.
In accordance with SFAS No. 142, “Goodwill and Other Intangible Assets,” goodwill will not be amortized but instead will be tested for impairment at least annually (more frequently if certain indicators are present). In the event that management determines that the value of goodwill has become impaired, Epimmune will incur an accounting charge for the amount of impairment during the fiscal quarter in which the determination is made.
111
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL STATEMENTS — (Continued)
Pro forma adjustments are necessary to reflect the estimated purchase price, to adjust amounts related to Epimmune’s net tangible and identifiable intangible assets to a preliminary estimate of their fair values, to reflect the amortization expense related to the estimated amortizable intangible assets and deferred stock compensation, to reflect the exchange of shares of Epimmune’s preferred stock for shares of Epimmune common stock in connection with the transaction, and to reflect the exercise of certain warrants by IDM warrant holders in connection with the transaction.
Certain reclassifications have been made to conform IDM’s historical reported balances to Epimmune’s financial statement basis of presentation.
The unaudited pro forma condensed combined financial statements do not include any adjustments for liabilities resulting from integration planning, as management of IDM and Epimmune are in the process of making these assessments, and estimates of these costs are not currently known. Management currently does not expect to record any significant liabilities related to integration planning as a result of the proposed transaction.
In addition, management currently does not expect to incur significant restructuring charges upon completion of the proposed transaction for costs associated with exiting activities of IDM.
The pro forma adjustments included in the unaudited pro forma condensed financial statements are as follows:
| |
| | (1) The historical financial statements of IDM have been translated into US dollars, using an exchange rate of 1 Euro to 1.2969 US dollars for the balance sheet as of March 31, 2005, and 1 Euro to 1.2661 US dollars and 1 Euro to 1.3112 US dollars for the statement of operations for the year ended December 31, 2004 and the quarter ended March 31, 2005, respectively. The balance sheet rate is the exchange rate as of March 31, 2005. The statement of operations rates are an average exchange rate for the year ended December 31, 2004 and the quarter ended March 31, 2005. |
| |
| | (2) There are no pro forma tax adjustments as the effective tax rate for the combined entity is expected to be 0% as both IDM and Epimmune have historically incurred losses. |
| |
| | (3) Adjustment to reflect estimated IDM transaction fees of $4,668,000 which are included as a component of purchase price. |
| |
| | (4) Adjustments to reflect preliminary estimates of the fair market values of Epimmune’s assets and liabilities at March 31, 2005 and related impacts on the statement of operations for the year ended |
112
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL STATEMENTS — (Continued)
| |
| | December 31, 2004 and the three months ended March 31, 2005. These adjustments are calculated as follows (in thousands): |
| | | | | | | | | |
| | | December 31, 2004 | | | March 31, 2005 | |
| | | | | | | |
| Intangibles Other than Goodwill: | | | | | | | | |
| Preliminary Fair Value of Licensing and Milestone Agreements | | | | | | $ | 1,500 | |
| Write-off of the Existing Book Value of Intangibles Other than Goodwill | | | | | | | (3,447 | ) |
| | | | | | | |
| Balance Sheet Adjustment | | | | | | $ | (1,947 | ) |
| | | | | | | |
| Pro Forma Amortization of Licensing and Milestone Agreements | | $ | 300 | | | $ | 75 | |
| Historical Amortization of Intangibles Other than Goodwill | | | 601 | | | | 142 | |
| | | | | | | |
| Statement of Operations Adjustment | | $ | (301 | ) | | $ | (67 | ) |
| | | | | | | |
| Deferred Rent: | | | | | | | | |
| Preliminary Fair Value | | | | | | $ | — | |
| Book Value | | | | | | | 208 | |
| | | | | | | |
| Balance Sheet Adjustment | | | | | | $ | (208 | ) |
| | | | | | | |
| Pro Forma Rent Expense Adjustment due to Deferred Rent | | $ | — | | | $ | — | |
| Historical Rent Expense Adjustment due to Deferred Rent | | | 2 | | | | 2 | |
| | | | | | | |
| Statement of Operations Adjustment | | $ | (2 | ) | | $ | (2 | ) |
| | | | | | | |
| |
| | Intangibles other than goodwill, referred to as other intangibles, were amortized over ten years in the historical financial statements of Epimmune. For pro forma purposes, other intangibles are amortized over five years, which is the preliminary estimate of their useful lives. Preliminary estimates of the fair market values of Epimmune’s assets and liabilities, other than deferred rent and other intangibles, approximate book value. Accordingly, no adjustment has been made to these assets and liabilities. |
| |
| | (5) The final determination of the fair market value of the assets acquired and liabilities assumed, as well as the useful lives of the assets acquired, and the resulting effect on the statement of operations may differ significantly from the pro forma amounts included herein. There can be no assurance that such adjustments will not be material. The preliminary estimate of the fair value of Epimmune IPR&D is $12,900,000. U.S. GAAP requires that IPR&D is immediately expensed. This balance sheet adjustment gives effect to the immediate write-off of Epimmune IPR&D. This adjustment is not considered in the pro forma condensed statements of operations as the write-off will not have a continuing impact on operations. |
113
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL STATEMENTS — (Continued)
| |
| | (6) Adjustment to record goodwill. Goodwill is calculated as the excess of purchase price over the fair value of the assets acquired and liabilities assumed. The components of purchase price and the calculation of goodwill are as follows (in thousands): |
| | | | | | |
| Purchase Price: | | | | |
| | Value of Epimmune Common Stock | | $ | 21,268 | |
| | Value of Epimmune Preferred Stock (as-converted to common stock) | | | 2,588 | |
| | Fair Value of Outstanding Epimmune Options and Warrants | | | 2,831 | |
| Estimated IDM Transaction Costs | | | 4,668 | |
| | | | |
| | | $ | 31,355 | |
| | | | |
| Preliminary Fair Value of Assets Acquired and Liabilities: | | | | |
| Tangible Assets | | $ | 8,433 | |
| Licensing and Milestone Agreements | | | 1,500 | |
| IPR&D | | | 12,900 | |
| Liabilities Assumed | | | (2,571 | ) |
| | | | |
| | | | 20,262 | |
| Goodwill | | | 11,093 | |
| | | | |
| | | $ | 31,355 | |
| | | | |
| |
| | The value of Epimmune common stock and preferred stock, on an as-converted to common stock basis, was calculated by multiplying the number of shares of Epimmune common stock outstanding, immediately preceding the close of the transaction, by $1.33, the average closing stock price of Epimmune common stock for the five-day period beginning March 14, 2005. The fair value of the outstanding Epimmune options and warrants was determined based on the following assumptions: risk-free interest rate of 4%, dividend yield of 0%, stock volatility factor of .947, stock price of $1.33 (5-day average), and weighted average expected life of 2.9 years. |
| |
| | (7) Adjustment to eliminate deferred revenue of $335,000 as of March 31, 2005, revenue of $319,000 for the year ended December 31, 2004, and revenue of $46,000 for the quarter ended March 31, 2005. These amounts were recorded in Epimmune’s financial statements and resulted from transactions between Epimmune and IDM. No amounts related to transactions with Epimmune were recorded on IDM’s March 31, 2005 balance sheet or the statement of operations for the year ended December 31, 2004 or the quarter ended March 31, 2005. |
| |
| | (8) Adjustment to reflect the issuance of 1,949,278 shares of Epimmune common stock, par value $19,000, issued in exchange for 1,409,288 of Epimmune preferred stock, in accordance with the Amended and Restated Preferred Exchange Agreement with G.D. Searle, the holder of the Epimmune preferred stock. |
| |
| | (9) Adjustment to reflect the exercise of the Sanofi-Aventis warrants, as required by the Share Exchange Agreement and to record the fair value of the consideration provided to Sanofi-Aventis for the related license. Sanofi-Aventis holds a total of 14,566 warrants exercisable into a total of 404,600 Class B shares of IDM which will be exchanged in the transaction for 1,526,323 shares of Epimmune common stock based on the exchange ratio of 1 IDM share to 3.771865 shares of Epimmune common stock. No proceeds are expected from the exercise of the Sanofi warrants because the exercise price to be paid by Sanofi-Aventis will be offset by an extended license to use Sanofi-Aventis products. IDM calculated the consideration provided to Sanofi-Aventis which amounts to $2,030,000 as the value of the stock to be issued in the transaction by multiplying the 1,526,323 shares of Epimmune common stock Sanofi will |
114
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL STATEMENTS — (Continued)
| |
| | hold by $1.33, the average closing stock price of Epimmune common stock for the five-day period beginning March 14, 2005. The balance sheet adjustments reflect IDM immediately expensing the value of the consideration as the associated license is related to technology still in development. This adjustment is not considered in the pro forma condensed statement of operations as the write-off will not have a continuing impact on operations. |
| |
| | (10) Adjustment to reflect the issuance of Epimmune common stock in exchange for IDM common stock by eliminating the par value of the historical IDM common stock ($2,035,000) and adding the par value of the Epimmune common stock offered in exchange. The number of shares to be issued, 59,197,332, with par value of $591,973, was calculated by multiplying the number of IDM outstanding shares as of December 31, 2004, 15,694,446, by the exchange ratio of 1 IDM share to 3.771865 shares of Epimmune common stock. The terms of the Share Exchange Agreement offer holders of PEA shares the option to either exchange their PEA shares for shares of Epimmune common stock or enter into a Put/ Call Agreement. The terms of the Put/ Call agreement is attached asAnnex C tothis proxy statement. The pro forma financial information assumes that each holder of PEA shares will exchange their PEA shares for shares of Epimmune common stock. The actual number of holders of PEA shares that choose to exchange their PEA shares for shares of Epimmune common stock may differ from the estimate used in preparing this pro forma financial information. |
| |
| | (11) Adjustment to reflect the net exercise of IDM warrants with exercise prices less than 6.32 Euros. Upon exercise, the warrants will be exchanged for 359,036 Epimmune common shares, with par value of $4,000. |
| |
| | (12) Immediately prior to the consummation of the transaction, the Medarex warrants will be exercised as required by the Share Exchange Agreement. Medarex holds 3,653,282 warrants that are exercisable into 3,653,282 Class B shares of IDM. Upon completion of the Exchange, Medarex will hold 13,779,687 shares of Epimmune common stock, based upon the share exchange ratio of 1 IDM share to 3.771865 shares of Epimmune common stock. The fair value of the warrants was previously accounted for by IDM. Accordingly, the actual exercise of the warrants is reflected as an adjustment to additional paid-in capital and common stock for the par value ($138,000) of the shares of Epimmune stock issued to Medarex. No cash consideration is expected from the exercise of the Medarex warrants. |
| |
| | (13) Pro forma combined basic and diluted net loss per share is based on the weighted average shares outstanding of Epimmune plus the 74.9 million shares of Epimmune common stock issued in the proposed transaction. The pro (13) forma financial information has not been adjusted for the Epimmune reverse stock split that is contemplated in the Share Exchange Agreement. |
| |
| | (14) Adjustment to additional paid-in capital to record the value of the stock component of the purchase price that is attributable to existing Epimmune common stock and common stock issued to holders of Epimmune preferred (14) stock. This amount is calculated as the excess of the stock component of the purchase price less amounts associated with the par value and is shown as follows (in thousands): |
| | | | | |
| Fair Value of Common Stock | | $ | 21,268 | |
| Fair Value of Preferred Stock (As-converted to Common Stock) | | | 2,588 | |
| | | | |
| Total Fair Value | | $ | 23,856 | |
| Common Stock at Par | | $ | 160 | |
| Preferred Stock at Par (As-converted to Common Stock) | | | 19 | |
| | | | |
| Total Par Value | | | 179 | |
| | | | |
| Additional Paid-in Capital | | $ | 23,677 | |
| | | | |
115
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL STATEMENTS — (Continued)
| |
| | (15) Stock awards have been granted under compensation agreements, effective as of the closing of the Exchange, for Dr. Emile Loria, Dr. Mark Newman, and Mr. Robert De Vaere. Under the terms of the compensation agreements, a total of 625,500 stock awards will be granted and will vest over an 18 month period. The value of these stock awards is estimated to be $556,695, and is calculated by multiplying the total number of awards by $.89, the Epimmune closing stock price on May 23, 2005. Amortization is calculated to be $467,072 and $116,768 for the year ended December 31, 2004 and the three months ended March 31, 2005, respectively, based upon the vesting schedule and the Epimmune stock price of $.89. However, this amount is not included as an adjustment to the statement of operations, as the stock awards are not expected to have a continuing impact on operations. The final calculation of deferred compensation and amortization will be based on the Epimmune stock price on the date of closing. Accordingly, the amount of deferred compensation and amortization recorded could differ significantly from this estimate. |
| |
| | (16) Adjustment to reflect the elimination of Epimmune’s historical additional paid-in capital and accumulated deficit, in the amounts of $172,942,000, and ($164,185,000), respectively. |
| |
| | (17) Epimmune’s historical financial statements include deferred compensation and compensation expense related to certain options and warrants. This adjustment reflects the fair value of the outstanding Epimmune options and warrants as a component of purchase price and reflects the reversal of deferred compensation and amortization of deferred compensation expense relating to Epimmune that would not have been incurred if the transaction occurred at the beginning of the period on a pro forma basis. The fair value of the options and warrants was determined to be $2,831,000 and is included as an adjustment to additional paid-in capital. Deferred compensation of ($165,000) was recorded in the March 31, 2005 balance sheet. Compensation expense of $71,000 and $15,000 were recorded in the statement of operations for the year ended December 31, 2004 and the quarter ended March 31, 2005, respectively. No amount of option or warrant value was attributed to the future services of the holders of the options and warrants. Accordingly, pro forma deferred compensation and compensation expense are $0. |
| |
| | (18) Adjustment to reflect an increase in base salary under the terms of compensation agreements, effective as of the closing of the Exchange, for Dr. Emile Loria, Dr. Mark Newman, and Mr. Robert De Vaere. The total increase to base salary for these three individuals is $55,000 per year ($13,750 per quarter). Total retention bonuses are $353,000, are earned over a one year period, and will be paid to Dr. Mark Newman and Mr. Robert De Vaere. An adjustment for retention bonuses has not been made to the statement of operations, as these bonuses are not expected to have a continuing impact on operations. |
Epimmune will incur certain non-recurring expenses in connection with the transaction. These expenses, which are not reflected in the accompanying pro forma condensed financial statements, are currently estimated as follows (in thousands):
| | | | | |
| Investment banking advisory fees | | $ | 1,075 | |
| Accounting and legal fees | | | 1,825 | |
| Printing and distribution costs | | | 290 | |
| Employee bonuses | | | 957 | |
| Other | | | 110 | |
| | | | |
| Subtotal | | | 4,257 | |
| Less amounts expensed through December 31, 2004 | | | (443 | ) |
| | | | |
| Balance | | $ | 3,814 | |
| | | | |
116
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL STATEMENTS — (Continued)
The combined entity will incur certain non-recurring expenses in connection with the transaction. These expenses are reflected in the pro forma balance sheet, but are not reflected in the pro forma statement of operations as they are not expected to have a continuing impact on operations. These amounts are currently estimated as follows (in thousands):
| | | | | | | | | |
| | | December 31, 2004 | | | March 31, 2005 | |
| | | | | | | |
| Write-off of IPR&D (Footnote 5) | | | 12,900 | | | | 12,900 | |
| Stock awards granted under new compensation agreements (Footnote 15) | | | 467 | | | | 117 | |
| Retention bonuses (Footnote 18) | | | 55 | | | | 14 | |
| | | | | | | |
| | | | 13,422 | | | | 13,031 | |
| | | | | | | |
117
CHAPTER THREE — OTHER PROPOSALS FOR THE ANNUAL
MEETING OF EPIMMUNE’S STOCKHOLDERS
PROPOSAL 2 — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND
RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A NAME CHANGE
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to change our corporate name to “IDM, Inc.” In the Share Exchange Agreement, we agreed to take all action necessary to change our corporate name to “IDM, Inc.,” effective as of the closing of the transactions under the Share Exchange Agreement. The Board of Epimmune believes that the name “IDM, Inc.,” which is an abbreviated version of IDM’s name, Immuno-Designed Molecules, will better represent the activities and direction of the combined company, which will focus on development of immunotherapeutic products for cancer and infectious disease indications. The amendment under this proposal would amend Article I of our Amended and Restated Certificate of Incorporation as follows:
| |
| | “The name of the Corporation is IDM, Inc.” |
The full text of the proposed amendments of our Amended and Restated Certificate of Incorporation, which includes the proposed changes described in proposals 3 and 4 for informational purposes, are attached to this proxy statement asAnnex F-1, Annex F-2, Annex F-3, Annex F-4, Annex F-5, Annex F-6andAnnex F-7.
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes. If approved by the stockholders, the proposed amendment of our Amended and Restated Certificate of Incorporation described in this proposal will become effective upon the filing of the amendment with the Secretary of State of the State of Delaware, which will occur as soon as reasonably practicable.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 2.
118
PROPOSAL 3 — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND
RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A REVERSE STOCK SPLIT
Background
Our common stock has been quoted on the Nasdaq National Market under the symbol “EPMN” since the open of business on July 2, 1999. Prior to that time, our common stock was quoted on the Nasdaq National Market under the symbol “CYTL” since the date of our initial public offering of common stock.
As more fully described in the section entitled“The Exchange —Listing of Epimmune Common Stock,” under the Nasdaq Marketplace Rule 4330(f), we expect that the Exchange, if completed, would constitute a “reverse merger.” As a result, we have applied for initial inclusion of our common stock for listing on the Nasdaq National Market following the Exchange. The Nasdaq National Market initial listing requirements include, among other things, a $5.00 per share price minimum bid price for the 90 trading days preceding the application for listing.
In the Share Exchange Agreement, we have agreed to take all action necessary to effect a one-for-four, one-for-five, one-for-six, one-for-seven, one-for-eight, one-for-nine or one-for-ten reverse stock split of all outstanding shares of our common stock immediately prior to the closing of the transactions under the Share Exchange Agreement. In addition, receipt of the requisite stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect the reverse stock split is a condition to the obligations of IDM’s shareholders and Epimmune to consummate the Exchange.
Accordingly, our Board considered and has authorized a series of proposed amendments to our Amended and Restated Certificate of Incorporation to effect a reverse stock split, at the discretion of the Board pursuant to Section 242(c) of the Delaware General Corporation Law, referred to as DGCL, to be implemented for the purpose of increasing the market price of our common stock above the minimum bid requirement for initial listing on the Nasdaq National Market and complying with our obligations under the Share Exchange Agreement.
Our stockholders are now being asked to vote upon the proposed amendments of our Amended and Restated Certificate of Incorporation to effect a reverse stock split in which each four, five, six, seven, eight, nine or ten outstanding shares of our common stock will be combined into one share of our common stock. Upon receiving stockholder approval, the Board will have the sole discretion pursuant to Section 242(c) of the DGCL, to elect, as it determines to be in the best interests of Epimmune and its stockholders, whether or not to effect a reverse stock split, and if so, whether to effect a one-for-four, one-for-five, one-for-six, one-for-seven, one-for-eight, one-for-nine or one-for-ten reverse stock split. Our Board believes that stockholder approval of the proposed amendments granting the Board this discretion, rather than approval of only one specified reverse stock split ratio, provides the Board with maximum flexibility to react to then-current market conditions and, therefore, is in the best interests of Epimmune and its stockholders.
The text of the proposed amendments of our Amended and Restated Certificate of Incorporation, which text includes the proposed changes described in proposals 2 and 4, are attached to this proxy asAnnex F-1,Annex F-2, Annex F-3, Annex F-4, Annex F-5, Annex F-6andAnnex F-7. The text of these amendments is subject to modification, however, to include changes as may be required by the office of the Secretary of State of the State of Delaware and as the Board may deem necessary or advisable to effect a reverse stock split. By approving the proposed amendments detailed in proposal 3-A, proposal 3-B, proposal 3-C, proposal 3-D, proposal 3-E, proposal 3-F and proposal 3-G below, the stockholders will approve the proposed amendments to our Amended and Restated Certificate of Incorporation to effect a reverse stock split, and authorize our Board to file only one such amendment (assuming such amendment was approved by the stockholders), as determined by the Board. If the Board determines to effect one of the reverse stock splits by filing one of these amendments with the Secretary of State of the State of Delaware, all other proposed amendments will be abandoned. The Board may also elect not to effect any reverse stock split.
119
If the Board elects to effect a reverse stock split following stockholder approval, the number of issued and outstanding shares of common stock would be reduced in accordance with the applicable reverse stock split ratio determined by the Board in the manner described above. Except for adjustments that may result from the treatment of fractional shares as described below, each stockholder will hold the same percentage of outstanding common stock of Epimmune immediately following the reverse stock split as that stockholder held immediately prior to the reverse stock split. The par value of our common stock would remain unchanged at $0.01 per share. The reverse stock split would not change the number of authorized shares of our common stock; however, proposal 4 does request approval of an increase in the number of authorized shares of our common stock. The Board does not have any definite plans with regard to the authorized but unissued shares of common stock of Epimmune following the reverse stock split other than as detailed in other proposals in this proxy statement. The authorized but unissued shares of our common stock following the reverse stock split may also be used for fundraising purposes through the sale and issuance of our common stock.
Certain Risks Associated with the Reverse Stock Split
Stockholders should note that the effect of the reverse stock split upon the market prices for our common stock cannot be accurately predicted. In particular, there is no assurance that prices for shares of our common stock after the reverse stock split is implemented will be four, five, six, seven, eight, nine or ten times, as applicable, the prices for shares of the common stock immediately prior to the reverse stock split. In some cases, the total market capitalization of a company following a reverse stock split is lower, and may be substantially lower, than the total market capitalization before such a reverse stock split. Furthermore, the proposed reverse stock splits may not achieve the desired results which have been outlined above, and we cannot offer any assurance that our common stock will meet the Nasdaq National Market initial listing requirements following the reverse stock split. In addition, the fewer number of shares that will be available to trade will possibly cause the trading market of the common stock to become less liquid, which could have an adverse effect on the price of our common stock and any increased price per share of our common stock immediately after the reverse stock split may not be sustained for any prolonged period of time. The market price of our common stock is based on our performance and other factors, some of which may be unrelated to the number of our shares of common stock outstanding.
Reasons for the Reverse Stock Split
Our Board believes that a reverse stock split may be desirable for a number of reasons. First, the Board believes that a reverse stock split will enable us to continue having our common stock listed on the Nasdaq National Market following the Exchange. As more fully described in the section entitled“The Exchange —Listing of Epimmune Common Stock,” Under the Nasdaq Marketplace Rule 4330(f)we expect that the Exchange, if completed, would constitute a “reverse merger.” As a result, we have applied for initial inclusion of our common stock for listing on the Nasdaq National Market following the Exchange. In order to satisfy the Nasdaq initial listing requirements, we must have among other things, a $5.00 per share price minimum bid price for the 90 trading days preceding the application for listing. The Share Exchange Agreement also requires that Nasdaq has approved the common stock to be issued in the Exchange for quotation on the Nasdaq National Market and that we obtain stockholder approval of a reverse stock split of our common stock. Accordingly, our Board has deemed it advisable to amend our Amended and Restated Certificate of Incorporation to effect a reverse stock split. Second, the Board believes that a reverse stock split could improve the marketability and liquidity of Epimmune’s common stock and that a reverse stock split is desirable in order to increase our common stock price in the near term while we continue to progress towards achieving our business objectives.
We have filed a new listing application with Nasdaq in order for Epimmune’s common stock to continue to be quoted on the Nasdaq National Market following the Exchange. Among other things, if the closing bid price of our common stock does not reach $5.00 per share, Nasdaq may delist our common stock from trading on the Nasdaq National Market. If our common stock were to be delisted, Epimmune’s common stock would trade on the Nasdaq SmallCap Market, the OTC Bulletin Board or in the “pink sheets” maintained by the
120
National Quotation Bureau, Inc. Such alternative markets are generally considered to be less efficient than, and not as broad as, the Nasdaq National Market.
Our Board expects that a reverse stock split of our common stock will increase the market price of the common stock so that we can comply with the Nasdaq minimum bid price listing standard. However, the effect of a reverse stock split upon the market price of our common stock cannot be predicted with any certainty, and the history of the effect of a similar reverse stock split for companies in like circumstances is varied. Taking into the expected advantages of the reverse stock split together with the risks associated with the reverse stock split, our Board believes that the proposed reverse stock split, when one of the proposed ratios is implemented, will result in the market price of our common stock rising to the level necessary to satisfy the $5.00 minimum bid price requirement.
The Board also believes that the increased market price of our common stock expected as a result of implementing a reverse stock split will improve the marketability of our common stock and will encourage interest and trading in our common stock. Because of the trading volatility often associated with low-priced stocks, many brokerage houses and institutional investors have internal policies and practices that prohibit them from investing in low-priced stocks or discourage individual brokers from recommending low-priced stocks to their customers. Some of those policies and practices may make trading in low-priced stocks economically unattractive to brokers. Additionally, because brokers’ commissions on low-priced stocks generally represent a higher percentage of the stock price than commissions on higher-priced stocks, the current average price per share of our common stock can result in individual stockholders paying transaction costs representing a higher percentage of their total share value than would be the case if the share price were substantially higher. The proposed reverse stock split may adversely affect the liquidity of our common stock by reducing the number of shares that would be outstanding after the reverse stock split. Our Board is hopeful, however, that the anticipated higher market price will, to some extent, reduce the negative effects on the liquidity and marketability of the common stock inherent in some of the policies and practices of institutional investors and brokerage houses described above.
Board Discretion to Implement the Reverse Stock Split
If the reverse stock split is approved by our stockholders, it will be effected, if at all, only upon a determination by the Board that a reverse stock split (at a ratio determined by the Board as described above) is in the best interests of Epimmune and its stockholders. The Board’s determination as to whether the reverse stock split will be effected and, if so, at what ratio, will be based upon certain factors, including meeting the initial listing requirements for the Nasdaq National Market, existing and expected marketability and liquidity of our common stock, prevailing market conditions and the likely effect on the market price of our common stock. If the Board determines to effect the reverse stock split, the Board will consider various factors in selecting one of the proposed ratios (assuming such proposed ratio was approved by the stockholders), including the overall market conditions at the time and the recent trading history of our common stock.
Even if the stockholders approve the reverse stock split, the Board may, in its sole discretion, abandon all of the proposed amendments and determine prior to the effectiveness of any filing with the Secretary of State of the State of Delaware not to effect the reverse stock split as permitted under Section 242(c) of the DGCL.
Effects of the Reverse Stock Split
After the effective date of the proposed reverse stock split, each holder of our common stock will own a reduced number of shares of common stock. However, the proposed reverse stock split will affect all of our holders of common stock uniformly and will not affect any stockholder’s percentage ownership interest in Epimmune, except to the extent that the reverse stock split results in any stockholder owning a fractional share as described below. The exchange ratio for the Exchange will be adjusted such that each IDM shareholder will receive the same percentage ownership as it would have if the Exchange closed prior to the reverse stock split. Proportionate voting rights and other rights and preferences of the holders of our common stock will not be affected by the proposed reverse stock split (other than as a result of the payment of cash in
121
lieu of fractional shares). For example, a holder of 3% of the voting power of the outstanding shares of common stock immediately prior to the reverse stock split would continue to hold 3% of the voting power of the outstanding shares of common stock immediately after the reverse stock split. The number of stockholders of record will not be affected by the proposed reverse stock split (except to the extent that any stockholder holds only a fractional share interest and receives cash for such interest after the proposed reverse stock split).
Although the proposed reverse stock split will not affect the rights of holders of our common stock or any stockholder’s proportionate equity interest in Epimmune, subject to the treatment of fractional shares, the number of authorized shares of common stock will not be reduced by virtue of the proposed reverse stock split. See, however, the proposed increase in authorized capital stock described in proposal 4.
The following table contains approximate information relating to our common stock under each of the proposed amendments described in subproposals 3-A, 3-B, 3-C, 3-D, 3-E, 3-F and 3-G based on information as of June 29, 2005 (in thousands) after giving effect to the Exchange and the related transactions described in proposal 1:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Pre-Reverse | | | | | | | | | | | | | | | |
| | | Stock Split | | | 1-for-4 | | | 1-for-5 | | | 1-for-6 | | | 1-for-7 | | | 1-for-8 | | | 1-for-9 | | | 1-for-10 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Authorized* | | | 55,000 | | | | 55,000 | | | | 55,000 | | | | 55,000 | | | | 55,000 | | | | 55,000 | | | | 55,000 | | | | 55,000 | |
| Issued and Outstanding | | | 16,024 | | | | 4,006 | | | | 3,205 | | | | 2,671 | | | | 2,289 | | | | 2,003 | | | | 1,780 | | | | 1,602 | |
| Reserved for future issuance pursuant to Epimmune’s equity plans** | | | 3,039 | | | | 760 | | | | 608 | | | | 507 | | | | 434 | | | | 380 | | | | 338 | | | | 304 | |
| Reserved for future issuance upon exchange of outstanding Series S and S-1 preferred stock*** | | | 1,949 | | | | 487 | | | | 390 | | | | 325 | | | | 278 | | | | 244 | | | | 217 | | | | 195 | |
| Reserved for future issuance upon exercise of outstanding warrants | | | 2,275 | | | | 569 | | | | 455 | | | | 379 | | | | 325 | | | | 284 | | | | 253 | | | | 228 | |
| Proposed for issuance pursuant to proposal 1 | | | 74,862 | | | | 18,716 | | | | 14,972 | | | | 12,477 | | | | 10,695 | | | | 9,358 | | | | 8,318 | | | | 7,486 | |
| Authorized and unreserved | | | (43,150 | ) | | | 30,462 | | | | 35,370 | | | | 38,642 | | | | 40,979 | | | | 42,731 | | | | 44,094 | | | | 45,185 | |
| |
| * | Includes the proposed increase in authorized capital stock described in proposal 4. |
| |
| ** | Does not include the proposed share increases to the equity plans described in proposals 5 and 6 or shares reserved under the equity plan described in proposal 7. |
| |
| *** | Reflects the terms of the Amended and Restated Preferred Exchange Agreement, providing for the exchange of all outstanding shares of Series S and S-1 preferred stock for a specified number of shares of common stock. If the Exchange does not close, the Series S and S-1 preferred stock would be convertible into 1,788,000 shares of common stock on a pre-reverse stock split basis, 447,000 shares of common stock in the event of a 1-for-4 reverse stock split, 358,000 shares of common stock in the event of a 1-for-5 reverse stock split, 298,000 shares of common stock in the event of a 1-for-6 reverse stock split, 255,000 shares of common stock in the event of a 1-for-7 reverse stock split, 223,000 shares of common stock in the event of a 1-for-8 reverse stock split, 199,000 shares of common stock in the event of a 1-for-9 reverse stock split, and 179,000 shares of common stock in the event of a 1-for-10 reverse stock split, without giving effect to any potential issuance of additional shares of common stock upon conversion of the Series S preferred stock as a result of antidilution adjustment provisions in the Certificate of Designations of the Series S and Series S-1 preferred stock. |
The proposed reverse stock split will reduce the number of shares of common stock available for issuance under the 2000 Plan and the Purchase Plan in proportion to the reverse stock split ratio selected by the Board in the manner described herein. If the French Purchase Plan described in proposal 7 is approved by the
122
stockholders, the proposed reverse stock split will reduce the number of shares of common stock available for issuance under such plan in proportion to the selected ratio. Epimmune also has outstanding stock options and warrants to purchase shares of Epimmune’s common stock. Under the terms of the outstanding stock options and warrants, the proposed reverse stock split will effect a reduction in the number of shares of common stock issuable upon exercise of those stock options and warrants in proportion to the ratio of the reverse stock split and will effect a proportionate increase in the exercise price of those outstanding stock options and warrants. In connection with the proposed reverse stock split, the number of shares of common stock issuable upon exercise of outstanding warrants will be rounded down to the nearest whole share and a cash payment will be made in an amount reflecting the value of the fractional share. In connection with the proposed reverse stock split, the number of shares of common stock issuable upon exercise of outstanding stock options will be rounded down to the nearest whole share and the exercise price will be rounded up to the nearest whole cent.
If the proposed reverse stock split is implemented, it will increase the number of our stockholders who own “odd lots” of fewer than 100 shares of our common stock. Brokerage commission and other costs of transactions in odd lots are generally higher than the costs of transactions for more than 100 shares of common stock.
Our common stock is currently registered under Section 12(g) of the Exchange Act, and we are subject to the periodic reporting and other requirements of the Exchange Act. The proposed reverse stock split will not affect the registration of the common stock under the Exchange Act. Subject to Nasdaq’s consent, if the proposed reverse stock split is implemented, our common stock will continue to be reported on the Nasdaq National Market. However, our common stock will be reported under the symbol “IDMI” (assuming that proposal 2 is approved) rather than the symbol “EPMN,” although Nasdaq would likely add the letter “D” to the end of the trading symbol for a period of 20 trading days to indicate that the reverse stock split has occurred.
Accounting Consequences
The par value per share of our common stock will remain unchanged at $0.01 per share after the reverse stock split. As a result, on the effective date of the reverse stock split, the stated capital on our balance sheet attributable to the common stock will be reduced proportionally, based on the ratio of the reverse stock split, from its present amount, and the additional paid-in capital account will be credited with the amount by which the stated capital is reduced. The per share common stock net income or loss and net book value will be increased because there will be fewer shares of our common stock outstanding. We do not anticipate that any other accounting consequences would arise as a result of the reverse stock split.
Effective Date
The proposed reverse stock split would become effective as of 5:00 p.m. Eastern Time on the date of filing of the Certificate of Amendment of Epimmune’s Amended and Restated Certificate of Incorporation with the office of the Secretary of State of the State of Delaware. Except as explained below with respect to fractional shares, on the effective date, each four, five, six, seven, eight, nine or ten shares of common stock issued and outstanding immediately prior thereto will be combined, automatically and without any action on the part of the stockholders, into one share of common stock in accordance with the reverse stock split ratio determined by our Board in the manner described above.
Cash Payment in Lieu of Fractional Shares
No fractional shares of common stock will be issued as a result of the proposed reverse stock split. Instead, holders of common stock who otherwise would be entitled to receive fractional shares, upon surrender to the exchange agent of such certificates representing such fractional shares, will be entitled to receive cash, without interest, in an amount equal to the product obtained by multiplying (i) the average of the closing sales prices on the Nasdaq National Market of shares of our common stock during the five consecutive trading days
123
ending on and including the trading day immediately preceding the closing of the Exchange by (ii) the number of shares of our common stock held by such stockholder that would otherwise have been exchanged for such fractional share interest.
Exchange of Stock Certificates
As soon as practicable after the effective date, stockholders will be notified that the reverse stock split has been effected. Our transfer agent will act as exchange agent for purposes of implementing the exchange of stock certificates. Holders of pre-reverse stock split shares will be asked to surrender to the exchange agent certificates representing pre-reverse stock split shares in exchange for certificates representing post-reverse stock split shares in accordance with the procedures to be set forth in a letter of transmittal to be sent by us. No new certificates will be issued to a stockholder until the stockholder has surrendered the stockholder’s outstanding certificate(s) together with the properly completed and executed letter of transmittal to the exchange agent. Stockholders should not destroy any stock certificate and should not submit any certificates until requested to do so.
No Appraisal Rights
Under the DGCL, our stockholders are not entitled to appraisal rights with respect to the proposed amendments to our Amended and Restated Certificate of Incorporation to effect the reverse stock split.
Material Federal U.S. Income Tax Consequences of the Reverse Stock Split
The following is a summary of important tax considerations of the proposed reverse stock split. It addresses only stockholders who hold the pre-reverse stock split shares and post-reverse stock split shares as capital assets. It does not address the tax consequences to IDM shareholders, warrant holders or optionholders who receive post-reserve stock split shares in the Exchange. It does not purport to be complete and does not address stockholders subject to special rules, such as financial institutions, tax-exempt organizations, insurance companies, dealers in securities, mutual funds, non-U.S. stockholders, stockholders who hold the pre-reverse stock split shares as part of a straddle, hedge or conversion transaction or other risk reduction strategy, stockholders who hold the pre-reverse stock split shares as qualified small business stock within the meaning of Section 1202 of the Code, stockholders who are subject to the alternative minimum tax provisions of the Code and stockholders who acquired their pre-reverse stock split shares pursuant to the exercise of employee stock options or otherwise as compensation. This summary is based upon current law, which may change, possibly even retroactively. It does not address tax considerations under state, local, foreign and other laws. Furthermore, we have not obtained a ruling from the Internal Revenue Service or an opinion of legal or tax counsel with respect to the consequences of the reverse stock split. Each stockholder is advised to consult such stockholder’s tax advisor as to such stockholder’s own situation.
The reverse stock split is intended to constitute a reorganization within the meaning of Section 368 of the Code. Assuming the reverse stock split qualifies as a reorganization, a stockholder generally will not recognize gain or loss on the reverse stock split, except (as discussed below) to the extent of cash, if any, received in lieu of a fractional share interest in the post-reverse stock split shares. The aggregate tax basis of the post-reverse stock split shares received will be equal to the aggregate tax basis of the pre-reverse stock split shares exchanged therefore (excluding any portion of the holder’s basis allocated to fractional shares), and the holding period of the post-reverse stock split shares received will include the holding period of the pre-reverse stock split shares exchanged.
A holder of the pre-reverse stock split shares who receives cash in lieu of a fractional share interest in the post-reverse stock split shares will generally recognize gain or loss equal to the difference between the portion of the tax basis of the pre-reverse stock split shares allocated to the fractional share interest and the cash received. Such gain or loss will be a capital gain or loss and will be short term if the pre-reverse stock split shares were held for one year or less and long term if held more than one year. It is assumed for this purpose that cash will be paid in lieu of fractional shares only as a mechanical rounding off of fractions resulting from
124
the reverse stock split rather than separately bargained-for consideration. It is also assumed that the reverse stock split is not being undertaken to increase any stockholder’s proportionate ownership of Epimmune.
We will not recognize any gain or loss as a result of the reverse stock split.
PROPOSAL 3-A — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION TO EFFECT A ONE-FOR-FOUR REVERSE STOCK SPLIT
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect a one-for-four reverse stock split of all outstanding shares of our common stock. The amendment under this proposal would add the following as the first paragraph in Article V of the Amended and Restated Certificate of Incorporation:
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every four shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the “Reverse Split”); provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
The full text of the proposed amendment of our Amended and Restated Certificate of Incorporation, which includes the proposed changes described in proposals 2 and 4 for informational purposes, is attached to this proxy statement asAnnex F-1.
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 3-A.
PROPOSAL 3-B — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A ONE-FOR-FIVE REVERSE STOCK SPLIT
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect a one-for-five reverse stock split of all outstanding shares of our common stock. The amendment under this proposal would add the following as the first paragraph in Article V of the Amended and Restated Certificate of Incorporation:
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every five shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the “Reverse Split”); provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
The full text of the proposed amendment of our Amended and Restated Certificate of Incorporation, which includes the proposed changes described in proposals 2 and 4 for informational purposes, is attached to this proxy statement asAnnex F-2.
125
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 3-B.
PROPOSAL 3-C — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A ONE-FOR-SIX REVERSE STOCK SPLIT
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect a one-for-six reverse stock split of all outstanding shares of our common stock. The amendment under this proposal would add the following as the first paragraph in Article V of the Amended and Restated Certificate of Incorporation:
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every six shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the “Reverse Split”); provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
The full text of the proposed amendment of our Amended and Restated Certificate of Incorporation, which text includes the proposed changes described in proposals 2 and 4 for informational purposes, is attached to this proxy statement asAnnex F-3.
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 3-C.
PROPOSAL 3-D — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A ONE-FOR-SEVEN REVERSE STOCK SPLIT
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect a one-for-seven reverse stock split of all outstanding shares of our common stock. The amendment under this proposal would add the following as the first paragraph in Article V of the Amended and Restated Certificate of Incorporation:
“Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every seven shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the “Reverse Split”); provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.”
The full text of the proposed amendment of our Amended and Restated Certificate of Incorporation, which text includes the proposed changes described in proposals 2 and 4 for informational purposes, is attached to this proxy statement asAnnex F-4.
126
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 3-D.
PROPOSAL 3-E — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A ONE-FOR-EIGHT REVERSE STOCK SPLIT
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect a one-for-eight reverse stock split of all outstanding shares of our common stock. The amendment under this proposal would add the following as the first paragraph in Article V of the Amended and Restated Certificate of Incorporation:
“Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every eight shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the “Reverse Split”); provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.”
The full text of the proposed amendment of our Amended and Restated Certificate of Incorporation, which text includes the proposed changes described in proposals 2 and 4 for informational purposes, is attached to this proxy statement asAnnex F-5.
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 3-E.
PROPOSAL 3-F — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A ONE-FOR-NINE REVERSE STOCK SPLIT
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect a one-for-nine reverse stock split of all outstanding shares of our common stock. The amendment under this proposal would add the following as the first paragraph in Article V of the Amended and Restated Certificate of Incorporation:
“Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every nine shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the “Reverse Split”); provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.”
The full text of the proposed amendment of our Amended and Restated Certificate of Incorporation, which text includes the proposed changes described in proposals 2 and 4 for informational purposes, is attached to this proxy statement asAnnex F-6.
127
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 3-F.
PROPOSAL 3-G — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO EFFECT A ONE-FOR-TEN REVERSE STOCK SPLIT
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to effect a one-for-ten reverse stock split of all outstanding shares of our common stock. The amendment under this proposal would add the following as the first paragraph in Article V of the Amended and Restated Certificate of Incorporation:
“Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every ten shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the “Reverse Split”); provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.”
The full text of the proposed amendment of our Amended and Restated Certificate of Incorporation, which text includes the proposed changes described in proposals 2 and 4 for informational purposes, is attached to this proxy statement asAnnex F-7.
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 3-G.
128
PROPOSAL 4 — APPROVAL OF AMENDMENT OF EPIMMUNE’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO EFFECT AN INCREASE IN AUTHORIZED SHARES
The Board is requesting stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to increase our authorized capital stock to a total of 65,000,000 shares, consisting of 10,000,000 shares of authorized preferred stock and 55,000,000 shares of authorized common stock after giving effect to the reverse stock split further described in proposal 3. More specifically, pursuant to this proposal, the Board is requesting stockholder approval to increase the total number of authorized shares from 50,000,000 to 65,000,000 and to increase the number of authorized common stock from 40,000,000 to 55,000,000.
In the Share Exchange Agreement, we agreed to take all action necessary, immediately prior to the closing of the transactions under the Share Exchange Agreement, to cause our authorized capital stock to be increased to a new total of 65,000,000 shares, consisting of 10,000,000 shares of authorized preferred stock and 55,000,000 shares of authorized common stock. In addition, receipt of the requisite stockholder approval for the amendment of our Amended and Restated Certificate of Incorporation to increase our authorized capital stock is a condition to the obligations of IDM’s shareholders and Epimmune to consummate the Share Exchange.
As of June 29, 2005, we had 16,023,786 shares of common stock outstanding and 1,787,572 shares of preferred stock outstanding, on an as-converted to common stock basis. An additional 2,900,164 shares of common stock were reserved for future issuance under our stock plans, of which 2,390,271 shares were covered by outstanding options (including grants covered in the section entitled“Chapter Four — Other Information for the Annual Meeting of Epimmune’s Stockholders — Compensation of Executive Officers), 509,893 shares were available for future grant or purchase under our 2000 Plan (not including 8,800,000 shares subject to stockholder approval pursuant to proposal 5) and 139,101 shares reserved for issuance under our Purchase Plan (not including 185,000 shares subject to stockholder approval pursuant to proposal 6 or 215,000 shares under the proposed French Purchase Plan subject to stockholder approval pursuant to proposal 7). In addition, 2,275,426 shares of common stock were reserved for issuance upon the exercise of existing stock warrants.
The additional common stock to be authorized by adoption of the amendment would have rights identical to our currently outstanding common stock. Adoption of the proposed amendment and issuance of the common stock would not affect the rights of the holders of our currently outstanding common stock and preferred stock, except for effects incidental to increasing the number of shares of our common stock outstanding, such as the potential future dilution of the earnings per share and voting rights of current holders of common stock. If the amendment is adopted, it will become effective upon filing of a Certificate of Amendment of our Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware.
The Board believes that the authorized common stock remaining available, taking into account the Exchange and related transactions under the Share Exchange Agreement, will not be sufficient to enable us to respond to potential business opportunities and to pursue important objectives that may be anticipated. Accordingly, the Board believes that it is in the best interests of us and our stockholders to increase the number of authorized shares of common stock, and the total authorized shares of capital stock, as described above. Other than as described in the other proposals in this proxy statement, the Board has no current plans to issue common stock. However, the Board believes that the availability of such shares will provide us with the flexibility to issue common stock for proper corporate purposes that may be identified by the Board from time to time, such as financings, acquisitions, strategic business relationships or stock dividends (including stock splits in the form of stock dividends). Further, the Board believes the availability of additional shares of common stock will enable us to attract and retain talented employees through the grant of stock options and other stock-based incentives. The issuance of additional shares of common stock may have a dilutive effect on earnings per share and, for a person who does not purchase additional shares to maintain his or her pro rata interest, on a stockholder’s percentage voting power.
129
The authorized shares of common stock in excess of those issued from time to time will be available for issuance at such times and for such corporate purposes as the Board may deem advisable without further action by our stockholders, except as may be required by applicable laws or the rules of any stock exchange or national securities association trading system on which the securities may be listed or traded. Other than as described in the other proposals in this proxy statement, we do not have any present agreement, understanding, commitment or arrangement which would result in the issuance of the newly authorized common stock sought under this proposal. The Board does not intend to issue any common stock except on terms which the Board deems to be in the best interests of us and our then-existing stockholders.
We could also use the additional shares of common stock that would become available for issuance if the proposal is adopted to oppose a hostile takeover attempt or to delay or prevent changes in control or management of Epimmune. For example, without further stockholder approval, the Board could strategically sell shares of common stock in a private transaction to purchasers who would oppose a takeover or favor the current Board. Although this proposal to increase the authorized common stock has been prompted by business and financial considerations and not by the threat of any hostile takeover attempt (nor is the Board currently aware of any such attempts directed at us), nevertheless, stockholders should be aware that approval of this proposal could facilitate future efforts by us to deter or prevent changes in control of Epimmune, including transactions in which the stockholders might otherwise receive a premium for their shares over then current market prices.
The amendment under this proposal would amend Article V of the Amended and Restated Certificate of Incorporation as follows:
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
The full text of the proposed amendments of Epimmune’s Amended and Restated Certificate of Incorporation, which text includes the proposed changes described in proposals 2 and 3 for informational purposes, are attached to this proxy statement asAnnex F-1, Annex F-2, Annex F-3, Annex F-4, Annex F-5, Annex F-6and Annex F-7.
The affirmative vote of the holders of a majority of our outstanding common stock is required to approve this proposal. As a result, abstentions and broker non-votes will have the same effect as negative votes.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 4.
130
PROPOSAL 5 — APPROVAL OF AMENDMENT OF THE EPIMMUNE 2000 STOCK PLAN TO INCREASE THE NUMBER OF SHARES OF EPIMMUNE’S COMMON STOCK AVAILABLE FOR ISSUANCE UNDER THE PLAN
The share numbers referenced in this proposal do not give effect to the reverse stock split referenced in proposal 3. In April 2000, the Board adopted, and Epimmune’s stockholders subsequently approved, the 2000 Plan. As a result of a series of amendments, as of June 15, 2004 there were 2,600,000 shares of common stock reserved for issuance under the 2000 Plan. On March 15, 2005, the Board approved, subject to stockholder approval, an amendment of the 2000 Plan to increase the number of shares of common stock available for issuance under the 2000 Plan by 8,800,000 shares, which increased the number of shares of common stock available under the 2000 Plan to a total of 11,400,000 shares (the amendment adding the additional shares is referred to as the 2000 Plan Amendment in this proposal). A copy of the 2000 Plan, as amended pursuant to this proposal, is attached to this proxy statement asAnnex GandAnnex H.
As of June 29, 2005, options to purchase 2,064,500 shares were outstanding under the 2000 Plan, 509,893 shares (plus any shares that might in the future be returned to the 2000 Plan as a result of cancellations or expiration of options but excluding the additional shares subject to the 2000 Plan Amendment) remained available for future grant under the 2000 Plan, and 25,607 shares had been issued pursuant to option exercises and direct stock issuances.
The Board believes the 2000 Plan Amendment is necessary to ensure that a sufficient number of shares remain available for issuance under the 2000 Plan to allow Epimmune to continue to use equity incentives to attract and retain the services of key individuals essential to Epimmune’s long-term growth and financial success, including the expanded employee base of the proposed combined company. Both Epimmune and IDM rely significantly on equity incentives in the form of stock option grants and other stock awards to attract and retain key employees, and Epimmune believes that such equity incentives are necessary for the proposed combined company to remain competitive in the marketplace for executive talent and other key employees. Epimmune grants options to newly hired or continuing employees based on both competitive market conditions and individual performance.
Stockholders are requested in this proposal 5 to approve the 2000 Plan Amendment. The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the annual meeting will be required to approve the 2000 Plan Amendment as described in this proposal 5. Abstentions will be counted toward the tabulation of votes cast on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether this proposal has been approved.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 5.
The essential features of the 2000 Plan are outlined below:
General
The 2000 Plan provides for the grant of incentive stock options to employees and the grant of nonstatutory stock options, rights to purchase restricted stock and stock bonuses, referred to as stock awards, to consultants, employees (including officers) and directors. Incentive stock options granted under the 2000 Plan are intended to qualify as “incentive stock options” within the meaning of Section 422 of the Code. Nonstatutory stock options granted under the 2000 Plan are not intended to qualify as incentive stock options under the Code. See the section entitled“Federal Income Tax Information” below for a discussion of the tax treatment of the various stock awards permitted under the 2000 Plan.
Purpose
The 2000 Plan was adopted to provide a means by which selected employees and directors of and consultants and advisors to Epimmune and its affiliates could be given an opportunity to benefit from increases
131
in value of the common stock through the granting of stock awards, assist in retaining the services of persons holding key positions, assist in securing and retaining the services of persons capable of filling such positions, and provide incentives for such persons to exert maximum efforts for the success of Epimmune. All of the approximately 50 employees, directors and consultants of Epimmune and its affiliates are eligible to participate in the 2000 Plan. Upon completion of the proposed Exchange, all employees and consultants of IDM also would become eligible to participate in the 2000 Plan.
Administration
The Board administers the 2000 Plan. Subject to the provisions of the 2000 Plan, the Board has the power to construe and interpret the 2000 Plan and, subject to the provisions of the 2000 Plan, to determine, among other things, the following: the persons to whom stock awards will be granted; when and how stock awards will be granted; the form of stock awards; the provisions of each stock award granted, including the time or times when a person will be permitted to receive stock pursuant to a stock award, the number of shares of common stock subject to each stock award, the time(s) at which shares of common stock subject to each stock award shall vest and/or become exercisable, if applicable, the exercise or purchase price, the type of consideration that may be used as payment for the stock awards and other terms of the stock awards.
The Board has the power to delegate administration of the 2000 Plan to a committee composed of one or more members of the Board. In the discretion of the Board, a committee may consist solely of two or more “non-employee directors” within the meaning of Rule 16b-3 of the Exchange Act, or solely of two or more “outside directors” within the meaning of Section 162(m) of the Code. For this purpose, a “non-employee director” generally is a director who does not receive remuneration from Epimmune other than compensation for service as a director (except for amounts not in excess of specified limits applicable pursuant to Rule 16b-3 of the Exchange Act). An “outside director” generally is a director who is neither a (i) current employee of Epimmune or an affiliate, (ii) former employee of Epimmune or an affiliate receiving compensation for past services (other than benefits under a tax-qualified pension plan), (iii) current and former officers of Epimmune or an affiliate, (iv) director currently receiving direct or indirect remuneration from Epimmune or an affiliate in any capacity (other than as a director), and (v) any other person who is otherwise not considered an “outside director” for purposes of Section 162(m). If administration is delegated to a committee, the committee has the power to delegate administrative powers to a subcommittee. Subject to certain limitations, the Board also has the power to delegate administration of the 2000 Plan to an officer for purposes of determining recipients of stock awards. As used herein with respect to the 2000 Plan, the “Board” refers to any committee the Board appoints or, if applicable, any such subcommittee, as well as to the Board itself. In accordance with the foregoing provisions, the Board has delegated administration of the 2000 Plan to our Compensation Committee.
Eligibility
Incentive stock options may be granted under the 2000 Plan only to employees (including officers) of Epimmune and its affiliates. Stock awards other than incentive stock options may be granted under the 2000 Plan only to employees (including officers) and directors of, and advisors and consultants to, Epimmune or its affiliates. The 2000 Plan specifically provides that stock awards other than incentive stock options may be granted to non-employee directors of Epimmune. For this purpose, a “non-employee director” is defined in the 2000 Plan as a director of Epimmune who is not otherwise an employee of Epimmune or any affiliate. Each of Epimmune’s current directors except Dr. Loria is currently a non-employee director.
No incentive stock option may be granted under the 2000 Plan to any person who, at the time of the grant, owns (or is deemed to own) stock possessing more than 10% of the total combined voting power of Epimmune or any affiliate of Epimmune, unless the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and the term of the option does not exceed five years from the date of grant. In addition, the aggregate fair market value, determined at the time of grant, of the shares of common stock with respect to which incentive stock options are exercisable for the first time by an optionholder during any calendar year (under all plans of Epimmune and its affiliates) may not exceed $100,000.
132
Under the 2000 Plan, no employee may be granted options and restricted stock purchase rights covering, in the aggregate, more than 500,000 shares of common stock during any calendar year, referred to as the Section 162(m) limitation.
Stock Subject to the 2000 Plan
Subject to stockholder approval of this proposal 5, an aggregate of 11,400,000 shares of common stock is reserved for issuance under the 2000 Plan. Stock subject to the 2000 Plan may be unissued shares or reacquired shares, bought on the market or otherwise. If any stock award granted under the 2000 Plan expires or otherwise terminates without being exercised in full, the shares of common stock not acquired pursuant to such stock award shall again become available for issuance under the 2000 Plan.
Terms of Options
The following is a description of the permissible terms of stock options under the 2000 Plan. Individual option grants may be more restrictive as to any or all of the permissible terms described below. In addition, the Board may at any time amend outstanding options, although certain amendments that would impair the rights of the optionholder to whom the option was granted require the optionholder’s consent. The 2000 Plan requires, however, that we obtain stockholder approval before repricing any stock options or engaging in any option cancellation or regrant program providing for the grant of stock awards at a lower price.
Exercise Price; Payment. The per share exercise price of an incentive stock option granted under the 2000 Plan may not be less than 100% of the fair market value of a share of common stock on the date of the option grant, and in some cases may not be less than 110% of such fair market value (see the section entitled“Eligibility” above). The per share exercise price of nonstatutory options granted under the 2000 Plan may not be less than 100% of the fair market value of a share of common stock on the date of the option grant. Any change to this requirement regarding the exercise price of nonstatutory stock options requires stockholder approval.
As of June 29, 2005, the closing price of our common stock as reported on the Nasdaq National Market System was $0.72 per share.
The exercise price of options granted under the 2000 Plan must be paid either in cash at the time the option is exercised or, at the discretion of the Board, by delivery of other common stock, pursuant to a deferred payment arrangement, or in any other form of legal consideration acceptable to the Board.
Transferability. Under the 2000 Plan, an incentive stock option may not be transferred by the employee to whom the option was granted other than by will or by the laws of descent and distribution or pursuant to a domestic relations order. During the lifetime of the employee, an incentive stock option may be exercised only by the employee. A nonstatutory stock option is transferable to the extent provided in a participant’s option grant agreement. If the nonstatutory stock option does not provide for transferability, then the nonstatutory stock option is not transferable other than by will or by the laws of descent and distribution or pursuant to a domestic relations order. An optionholder may also designate a beneficiary who may exercise the option following the optionholder’s death.
Option Exercise. Options granted under the 2000 Plan may vest, or become exercisable in cumulative increments, as determined by the Board. Vesting typically will occur during the optionholder’s continued service with Epimmune or an affiliate, whether such service is performed in the capacity of employee, director or consultant (collectively referred to as service) and regardless of any change in the capacity of such service. Options granted under the 2000 Plan may be subject to different vesting terms. The Board has the power to accelerate the time during which an option may vest or be exercised. In addition, options granted under the 2000 Plan may permit exercise prior to vesting, but in such event the optionholder may be required to enter into an early exercise stock purchase agreement that allows us to repurchase unvested shares, generally at their exercise price, should the optionholder’s service terminate before vesting. Shares subject to repurchase by Epimmune under an early exercise stock purchase agreement also may be subject to such restrictions on transfer that the Board deems appropriate.
133
To the extent provided by the terms of an option, an optionholder may satisfy any federal, state or local tax withholding obligation relating to the exercise of such option by a cash payment upon exercise, by authorizing Epimmune to withhold a portion of the stock otherwise issuable to the optionholder, by delivering already-owned stock of Epimmune or by a combination of these means.
Term. The maximum term of options under the 2000 Plan is 10 years, except that in certain cases the maximum term is five years (see the section entitled“Eligibility”). Unless a shorter term is established by the Board and specifically provided in the option grant agreement, nonstatutory options granted to non-employee directors have a term of 10 years. Options under the 2000 Plan generally terminate three months after the optionholder’s service terminates, unless (i) termination of service is due to the optionholder’s disability (as defined in the 2000 Plan), in which case the option may, but need not, provide that it may be exercised at any time within twelve months of such termination; (ii) the optionholder dies before termination of service, or within not more than three months after termination of service, in which case the option may, but need not, provide that it may be exercised (to the extent the option was exercisable at the time of the optionholder’s death) within 18 months of the optionholder’s death by the person or persons to whom the rights to such option pass by will or by the laws of descent and distribution; or (iii) the option by its terms specifically provides otherwise. With respect to nonstatutory options granted to non-employee directors, the 2000 Plan provides that such options terminate 12 months after the termination of the non-employee director’s service to Epimmune, unless otherwise determined by the Board and provided in the option grant agreement.
The option term may be extended in the event that exercise of the option within these periods is prohibited. A participant’s option agreement also may provide that if the exercise of the option following the termination of the participant’s service would be prohibited because the issuance of stock would violate the registration requirements under the Securities Act, then the option will terminate on the earlier of (i) the expiration of the term of the option or (ii) three months after the termination of the participant’s service during which the exercise of the option would not be in violation of such registration requirements.
Additional Terms of French Qualified Options
On March 15, 2005, the Board approved an amendment of the 2000 Plan, referred to as the French annex, to provide for additional terms of stock options granted under the 2000 Plan to certain persons who are employees, managers or directors of Epimmune subsidiaries organized under the laws of France so that such stock options will qualify for the favorable tax and social security treatment applicable to stock options under French law. Options granted pursuant to the terms of the French annex are referred to as the French qualified options in this proposal 5.
Share Limitation. The total number of shares of common stock underlying French qualified options granted pursuant to the French annex may not exceed one-third of Epimmune’s share capital, as defined under French law, on the date of grant. However, the foregoing limitation does not increase the total number of shares of common stock reserved for issuance under the 2000 Plan, as described above.
Eligibility. Provided that on the date of grant such persons are residents of France and do not hold more than 10% of the share capital of Epimmune (as defined under French law) the following individuals are eligible to receive French qualified options pursuant to the terms of the French annex: the Chairman (Président du Conseil d’administration), the CEO (directeur général ou directeur général délégué), members of the Directorate (Directoire), and managers (Gérant) of a French affiliate, and persons who are employed pursuant to an employment contract by a French affiliate. For eligibility purposes, a French affiliate means an Epimmune affiliate that is also an Epimmune subsidiary organized under the laws of France. For such purposes, an Epimmune subsidiary means, if the shares of Epimmune are not listed on a public stock exchange on the date of grant, a company 10% of the share capital or voting rights of which are held directly or indirectly by Epimmune, and, if the shares of Epimmune are listed on a public stock exchange on the date of grant, a company 10% of the share capital or voting rights of which are held directly or indirectly by Epimmune or a company holding directly or indirectly 10% of the share capital or voting rights of Epimmune or a company at least 50% of the share capital or voting rights of which are held directly or indirectly by a
134
company holding itself directly or indirectly at least 50% of the capital or voting rights of Epimmune, in each case as determined under French law.
Additional Restrictions. The terms of French qualified options will contain additional restrictions that are intended for such options to qualify for the favorable tax and social security treatment applicable to stock options that comply with French law, the material terms of which are described below. Individual option grants may be more restrictive as to any or all of the permissible terms.
Amendments. The Board may at any time amend outstanding French qualified options to the extent permitted by French law, although certain amendments that would impair the rights of the optionholder to whom the option was granted will also require the optionholder’s consent.
Exercise Price. The per share exercise price of a French qualified option may not be less than 100% of the fair market value of a share of common stock on the date of the option grant. Additionally, the per share exercise price of a French qualified option shall not be less than (i) 80% of the average opening share price on the 20 trading days preceding the date of grant, if the common stock is admitted to trading on a regulated stock market, and (ii) as determined by the Board according to objective methods used for the valuation of the shares, taking into account Epimmune’s net accounting situation, profitability and business prospects by means of an appropriate weighting in each case, if the common stock is not admitted to trading on a regulated stock market. These criteria shall be assessed on a consolidated basis if necessary or, failing that, by taking into account the financial statements issued by any significant Epimmune subsidiaries. In the event these methods cannot be used, the share price shall be determined by dividing the total amount of the revalued net assets, calculated according to the most recent balance sheet, by the number of existing shares. Notwithstanding the preceding, with respect to French qualified options to purchase existing shares (as opposed to newly issued shares), and in addition to the above limits, the per share exercise price must be no less than 80% of the average share price of the shares repurchased by Epimmune in order to satisfy the exercise of French qualified options.
Transferability. French qualified options may not be transferred by the individual to whom the option was granted other than by will or by the laws of descent and distribution and during his or her lifetime, may be exercised only by such individual.
Term. The maximum term of French qualified options is 10 years. In the event of the optionholders’ death, the French qualified option will expire upon the earlier of six months following the date of death or the expiration of the term of the French qualified option as set forth in the option agreement.
Lock-Up Period. Generally, any shares acquired upon exercise of a French qualified option may not be sold, assigned or donated until the fourth anniversary of the date of grant. However, such lock-up period shall not apply in the event of the death or disability of the optionholder. Additionally, such lock-up period shall not apply in the event of the retirement or dismissal of the optionholder, provided that the option was exercised at least three months prior to the date of retirement or dismissal.
Adjustment Provisions. Transactions not involving receipt of consideration by Epimmune, such as a merger, consolidation, reorganization, stock dividend, or stock split, may change the outstanding French qualified options as to the class, number of shares and price per share of common stock subject to such options to the extent allowed by French law. Additionally, the Board may make such other similar adjustments to French qualified options in order to comply with French law.
Effect of Certain Corporate Events. The 2000 Plan provisions relating to a “corporate transaction” and other significant corporate events (as described below) that would provide for the assumption, continuation, substitution, termination and/or acceleration of vesting of outstanding options under the terms of the 2000 Plan shall apply to the French qualified options unless otherwise determined by the Board, in its sole discretion.
Duration and Termination. The Board may suspend or terminate the French annex without stockholder approval or ratification at any time or from time to time. Unless sooner terminated, the French annex will terminate on May 15, 2008.
135
Terms of Stock Bonuses and Restricted Stock
The following is a description of the permissible terms of stock bonuses and purchases of restricted stock under the 2000 Plan. Individual stock bonuses or purchases of restricted stock may be more restrictive as to any or all of the permissible terms described below. In addition, the Board may at any time amend outstanding stock bonuses and restricted stock, although certain amendments that would impair the rights of the person to whom the award was granted require the award holder’s consent.
Purchase Price; Payment. The purchase price for restricted stock is determined by the Board, but in no event may it be less than 100% of the fair market value on the date of the grant or at the time the purchase is consummated. This requirement may be modified so that the purchase price for restricted stock may be not less than 85% of the fair market value on the date of grant or at the time the purchase is consummated if stockholder approval of this change is obtained. The Board may determine that eligible participants may be awarded stock pursuant to a stock bonus agreement in consideration for past services rendered to Epimmune.
The purchase price of stock acquired pursuant to a restricted stock purchase agreement must be paid either (i) in cash at the time of purchase; (ii) at the discretion of the Board, according to a deferred payment arrangement, or (iii) in any other form of legal consideration acceptable to the Board.
Repurchase Option. Shares of common stock sold or awarded under the 2000 Plan may, but need not, be subject to a repurchase option in favor of Epimmune in accordance with a vesting schedule to be determined by the Board. In the event a participant’s service terminates before the shares of common stock subject to such participant’s stock award have vested, Epimmune may repurchase or otherwise reacquire any or all of the unvested shares of common stock held by that person on the date of termination, if such repurchase is provided for pursuant to the terms of the stock bonus or restricted stock purchase agreement. The repurchase price may be the lower of the fair market value of the common stock on the relevant date or the original purchase price paid by the participant for the common stock.
Transferability. No rights under a stock bonus or restricted stock purchase agreement may be assigned by any participant under the 2000 Plan, except as expressly authorized by the terms of the applicable stock bonus or restricted stock purchase agreement.
Adjustment Provisions
Transactions not involving receipt of consideration by Epimmune, such as a merger, consolidation, reorganization, stock dividend, or stock split, may change the class and number of shares of common stock subject to the 2000 Plan and outstanding stock awards. In that event, the 2000 Plan will be appropriately adjusted as to the class and the maximum number of shares of common stock subject to the 2000 Plan and the Section 162(m) limitation, and outstanding stock awards will be adjusted as to the class, number of shares and price per share of common stock subject to such stock awards. If the reverse stock split described in proposal 3 is approved and implemented, the 2000 Plan and outstanding stock awards will be appropriately adjusted.
Effect of Certain Corporate Events
The 2000 Plan provides that, in the event of a dissolution or liquidation of Epimmune, then with respect to stock awards held by participants whose service with Epimmune or an affiliate has not terminated, the vesting of such stock awards shall be accelerated in full. Additionally, all outstanding stock awards shall terminate if not exercised (if applicable) prior to such dissolution or liquidation. The 2000 Plan further provides that, in the event of a sale, lease or other disposition of all or substantially all of the assets of Epimmune or certain specified types of mergers (as more fully described in the 2000 Plan) (such events each referred to as a corporate transaction), any surviving corporation may either assume stock awards outstanding under the 2000 Plan or substitute similar awards for those outstanding under the 2000 Plan. If any surviving corporation does not either assume stock awards outstanding under the 2000 Plan, or substitute similar awards, then, with respect to outstanding stock awards held by participants whose service has not terminated, the vesting and, if applicable, the time during which such stock awards may be exercised, will be accelerated in full as of the occurrence of such corporate transaction. Additionally, all outstanding stock awards shall
136
terminate if not exercised (if applicable) at or prior to such corporate transaction. The acceleration of stock awards held by participants whose service has not terminated in the event of an acquisition or similar corporate event may be viewed as an anti-takeover provision, which may have the effect of discouraging a proposal to acquire or otherwise obtain control of Epimmune.
In addition, in the event there occurs a securities acquisition by any person or group within the meaning of Section 13(a) or 14(d) of the Exchange Act of Epimmune securities representing 50% or more of Epimmune’s combined voting power or a change in the Board composition representing 50% or more of the incumbent Board members, other than in a corporate transaction or where the election of new Board members was approved by a vote of at least 50% of the incumbent Board, then, with respect to participants whose service has not terminated, the vesting and, if applicable, exercisability of outstanding stock awards will be accelerated in full. The Exchange and the related transactions do not constitute a corporate transaction giving rise to acceleration of vesting. However, the Board exercised its discretion and accelerated the vesting of certain options granted pursuant to the 2003 reduction in force in connection with the Exchange.
If there occurs any dissolution or liquidation of Epimmune, or any corporate transaction or other event described above occurs, the vesting and exercisability of nonstatutory options held by non-employee directors whose service has not terminated shall be accelerated in full, unless otherwise specifically provided in the applicable option grant agreement.
Duration, Amendment and Termination
The Board may suspend or terminate the 2000 Plan without stockholder approval or ratification at any time or from time to time. Unless sooner terminated, the 2000 Plan will terminate on April 20, 2010.
The Board also may amend the 2000 Plan at any time or from time to time. However, no amendment will be effective unless approved by the stockholders of Epimmune to the extent that stockholder approval is necessary in order to satisfy the requirements of Section 422 of the Code or any Nasdaq or other applicable securities exchange listing requirements. In addition, the provisions of the 2000 Plan regarding the exercise price of nonstatutory stock options, the pricing of restricted stock purchases and the limitations on option repricing and option cancellation and regrant programs may not be amended without stockholder approval. The Board may submit any other amendment of the 2000 Plan for stockholder approval, including, but not limited to, amendments intended to satisfy the requirements of Section 162(m) of the Code regarding the exclusion of performance-based compensation from the limitation on the deductibility of compensation paid to certain employees.
Federal Income Tax Information
Incentive Stock Options. Incentive stock options under the 2000 Plan are intended to be eligible for the favorable federal income tax treatment accorded incentive stock options under the Code.
There generally are no federal income tax consequences to the participant or Epimmune by reason of the grant of an incentive stock option. There generally are also no immediate federal income tax consequences to the participant or Epimmune by reason of the exercise of an incentive stock option. However, the exercise of an incentive stock option may increase the participant’s alternative minimum tax liability, if any.
If a participant holds stock acquired through exercise of an incentive stock option for more than two years from the date on which the option is granted and more than one year from the date on which the shares are transferred to the participant upon exercise of the option, any gain or loss on a disposition of such stock will be a long-term capital gain or loss if the participant held the stock for more than one year.
Generally, if the participant disposes of the stock before the expiration of either of these holding periods (referred to as a disqualifying disposition), then at the time of disposition the participant will realize taxable ordinary income equal to the lesser of (i) the excess of the stock’s fair market value on the date of exercise over the exercise price (or, if later, the excess of the stock’s fair market value on the date of vesting over the exercise price), or (ii) the participant’s actual gain, if any, on the purchase and sale. The participant’s
137
additional gain or any loss upon the disqualifying disposition will be a capital gain or loss, which will be long-term or short-term depending on whether the stock was held for more than one year.
To the extent the participant recognizes ordinary income by reason of a disqualifying disposition, Epimmune will generally be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) of the Code and the satisfaction of a tax reporting obligation) to a corresponding business expense deduction in the tax year in which the disqualifying disposition occurs.
Nonstatutory Stock Options, Restricted Stock Purchase Awards and Stock Bonuses. Nonstatutory stock options, restricted stock purchase awards and stock bonuses granted under the 2000 Plan generally have the following federal income tax consequences.
There are no tax consequences to the participant or Epimmune by reason of the grant. Upon acquisition of the stock, the participant normally will recognize taxable ordinary income equal to the excess, if any, of the stock’s fair market value on the acquisition date over the purchase price. However, to the extent the stock is subject to certain types of vesting restrictions following exercise or purchase, the taxable event will be delayed until the vesting restrictions lapse unless the participant elects to be taxed on receipt of the stock. With respect to employees, Epimmune is generally required to withhold from regular wages or supplemental wage payments an amount based on the ordinary income recognized. Subject to the requirement of reasonableness, the provisions of Section 162(m) of the Code and the satisfaction of a tax reporting obligation, Epimmune will generally be entitled to a business expense deduction equal to the taxable ordinary income realized by the participant.
Upon disposition of the stock, the participant will recognize a capital gain or loss equal to the difference between the selling price and the sum of the amount paid for such stock plus any amount recognized as ordinary income upon acquisition (or vesting) of the stock. Such gain or loss will be long-term or short-term depending on whether the stock was held for more than one year. Slightly different rules may apply to participants who acquire stock subject to certain repurchase options.
Potential Limitation on Company Deductions. Section 162(m) of the Code denies a deduction to any publicly held corporation for compensation paid to certain “covered employees” in a taxable year to the extent that compensation to such covered employee exceeds $1 million. It is possible that compensation attributable to awards, when combined with all other types of compensation received by a covered employee from Epimmune, may cause this limitation to be exceeded in any particular year.
Certain kinds of compensation, including qualified “performance-based compensation,” are disregarded for purposes of the deduction limitation. In accordance with Treasury Regulations issued under Section 162(m), compensation attributable to stock options will qualify as performance-based compensation if the award is granted by a compensation committee comprised solely of “outside directors” and either (i) the plan contains a per-employee limitation on the number of shares for which such awards may be granted during a specified period, the per-employee limitation is approved by the stockholders, and the exercise price of the award is no less than the fair market value of the stock on the date of grant, or (ii) the award is granted (or exercisable) only upon the achievement (as certified in writing by the compensation committee) of an objective performance goal established in writing by the compensation committee while the outcome is substantially uncertain, and the award is approved by stockholders.
Awards to purchase restricted stock and stock bonus awards will qualify as performance-based compensation under the Treasury Regulations only if (i) the award is granted by a compensation committee comprised solely of “outside directors,” (ii) the award is granted (or exercisable) only upon the achievement of an objective performance goal established in writing by the compensation committee while the outcome is substantially uncertain, (iii) the compensation committee certifies in writing prior to the granting (or exercisability) of the award that the performance goal has been satisfied and (iv) prior to the granting (or exercisability) of the award, stockholders have approved the material terms of the award (including the class of employees eligible for such award, the business criteria on which the performance goal is based, and the maximum amount — or formula used to calculate the amount — payable upon attainment of the performance goal).
138
Other Tax Consequences. The foregoing discussion is not intended to be a complete description of the federal income tax aspects of stock awards granted under the 2000 Plan. In addition, administrative and judicial interpretations of the application of the federal income tax laws are subject to change. Furthermore, no information is given with respect to state or local taxes that may be applicable. The tax treatment of French qualified options will be governed by applicable French tax laws.
Option Transactions
The following table presents certain information with respect to options granted under the 2000 Plan as of June 29, 2005 to (i) Epimmune’s Chief Executive Officer and its two other named executive officers whose total salary and bonus at December 31, 2004 exceeded $100,000 (referred to as the named executive officers), (ii) all executive officers as a group, (iii) all non-executive officer employees as a group and (iv) all non-employee directors as a group.
Option Transactions
Epimmune 2000 Stock Plan
| | | | | |
| | | Number of Shares | |
| | | Underlying Options | |
| Name and Position | | Granted | |
| | | | |
| Dr. Emile Loria, President and Chief Executive Officer(1) | | | 1,062,500 | |
| Dr. Mark J. Newman, Vice President, Research and Development and Assistant Secretary(1) | | | 325,000 | |
| Mr. Robert J. De Vaere, Vice President, Finance and Administration, Chief Financial Officer and Secretary(1) | | | 335,000 | |
| All Executive Officers as a Group(1) | | | 1,722,500 | |
| All Non-Executive Officer Employees as a Group(2) | | | 1,196,500 | |
| All Non-Employee Directors as a Group(3) | | | 175,000 | |
| |
| (1) | Includes the following options granted to the named executive officers of the Company, the vesting of which was subject to the achievement of certain corporate goals established by the Board, which goals were not met, which options terminated unexercised, are no longer outstanding, and have again become available for issuance under the 2000 Plan: |
| |
| | Emile Loria, 187,500 terminated unexercised options;
Mark J. Newman, 60,000 terminated unexercised options;
Robert J. De Vaere, 60,000 terminated unexercised options; and
All executive officers as a group, 307,500 terminated unexercised options. |
| |
| (2) | Includes a total of 5,000 options granted to certain non-executive officer employees of the Company, the vesting of which was subject to the achievement of certain corporate goals established by the Board, which goals were not met, which options terminated unexercised, are no longer outstanding, and have again become available for issuance under the 2000 Plan. Also includes an additional total of 669,432 options which terminated unexercised, are no longer outstanding, and have again become available for issuance under the 2000 Plan. Also includes a total of 13,340 options which were exercised. |
| |
| (3) | Includes a total of 18,333 options granted to certain non-employee directors, which options terminated unexercised, are no longer outstanding, and have again become available for issuance under the 2000 Plan. Also includes a total of 11,667 options which were exercised. |
New Plan Benefits
As of June 29, 2005, no options or other stock awards have been granted on the basis of the 8,800,000 share increase for which stockholder approval is sought under this proposal 5. At the annual meeting, each individual who will continue to serve as a non-employee Board member will receive an option grant under the 2000 Plan to purchase 5,000 shares of common stock at an exercise price equal to the fair market value per share of common stock on the grant date.
139
PROPOSAL 6 — APPROVAL OF AMENDMENT OF THE EPIMMUNE 2001
EMPLOYEE STOCK PURCHASE PLAN TO INCREASE THE NUMBER OF SHARES OF
EPIMMUNE’S COMMON STOCK AVAILABLE FOR ISSUANCE UNDER THE PLAN
The share numbers referenced in this proposal do not give effect to the reverse stock split referenced in proposal 3. On March 5, 2001, the Board adopted, and the stockholders subsequently approved, the Purchase Plan. On March 15, 2005, the Board amended the Purchase Plan, subject to stockholder approval, to increase the number of shares of common stock authorized for issuance under the Purchase Plan from a total of 300,000 shares to a total of 485,000 shares. The Board adopted this amendment in order to ensure that we can continue to grant purchase rights at levels determined appropriate by the Board. A copy of the Purchase Plan, as amended pursuant to this proposal, is attached to this proxy statement asAnnex I.
As of June 29, 2005 an aggregate of 160,899 shares of Epimmune’s common stock had been issued under the Purchase Plan. Only 139,101 shares of common stock (plus any shares that might in the future be returned to the Purchase Plan as a result of cancellations or expiration of purchase rights) remained available for future issuance under the Purchase Plan.
Stockholders are requested in this proposal 6 to approve the amendment of the Purchase Plan. The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the annual meeting will be required to approve the amendment of the Purchase Plan. Abstentions will be counted toward the tabulation of votes cast on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether this proposal has been approved.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 6.
The essential features of the Purchase Plan are outlined below:
Purpose
The purpose of the Purchase Plan is to provide a means by which employees of Epimmune (and any parent or subsidiary of Epimmune designated by the Board to participate in the Purchase Plan) may be given an opportunity to purchase common stock of Epimmune through payroll deductions, to assist Epimmune in retaining the services of its employees, to secure and retain the services of new employees, and to provide incentives for such persons to exert maximum efforts for the success of Epimmune. All of Epimmune’s 37 full-time employees are eligible to participate in the Purchase Plan.
The rights to purchase common stock granted under the Purchase Plan are intended to qualify as options issued under an “employee stock purchase plan” as that term is defined in Section 423(b) of the Code.
Administration
The Board administers the Purchase Plan and has the final power to construe and interpret both the Purchase Plan and the rights granted under it. The Board has the power, subject to the provisions of the Purchase Plan, to determine when and how rights to purchase common stock of Epimmune will be granted, the provisions of each offering of such rights (which need not be identical), and whether employees of any parent or subsidiary of Epimmune will be eligible to participate in the Purchase Plan.
The Board has the power to delegate administration of the Purchase Plan to a committee composed of not fewer than one member of the Board. The Board has delegated administration of the Purchase Plan to the Compensation Committee of the Board. As used herein with respect to the Purchase Plan, the “Board” refers to any committee the Board appoints and to the Board.
Offerings
The Purchase Plan is implemented by offerings of rights to all eligible employees from time to time by the Board. Generally, each offering is 24 months long and is divided into four shorter “purchase periods”
140
approximately six months long. If the fair market value of the common stock on any purchase date is less than the fair market value of the common stock on the first day of the offering, then the current offering will automatically end and a new 24-month offering period will begin, based on the lower fair market value.
Eligibility
Under the current offering, the following eligibility requirements apply. Any person who is customarily employed more than 20 hours per week and five months per calendar year by Epimmune (or by any parent or subsidiary of Epimmune incorporated in the United States) on the first day of an offering is eligible to participate in the offering, provided such employee has been continuously employed by Epimmune or the designated affiliate for at least 10 days preceding the first day of the offering. Officers of Epimmune who are “highly compensated” as defined in the Code are eligible to participate in the offering. Employees that reside or are employed in jurisdictions outside the United States are not eligible to participate in the offering if such participation would not be in compliance with the applicable laws of those jurisdictions.
However, no employee is eligible to participate in the Purchase Plan if, immediately after the grant of purchase rights, the employee would own, directly or indirectly, stock possessing 5% or more of the total combined voting power or value of all classes of stock of Epimmune or of any parent or subsidiary of Epimmune (including any stock which such employee may purchase under all outstanding rights and options). In addition, no employee may purchase more than $25,000 worth of common stock (determined at the fair market value of the shares at the time such rights are granted) under all employee stock purchase plans of Epimmune and its affiliates in any calendar year.
In addition to the preceding limitation, under the current offering, no employee may purchase more than 5,000 shares of common stock during the offering.
Participation in the Plan
Eligible employees enroll in the Purchase Plan by delivering to Epimmune, prior to the date selected by the Board as the offering date for the offering, an agreement authorizing payroll deductions of up to 15% of such employees’ total compensation during the offering.
Purchase Price
The purchase price per share at which shares of common stock may be sold in an offering under the Purchase Plan may be at a minimum of the lower of (i) 85% of the fair market value of a share of common stock on first day of the offering or (ii) 85% of the fair market value of a share of common stock on the last day of the purchase period.
As of June 29, 2005, the closing price of Epimmune’s common stock as reported on the Nasdaq National Market System was $0.72 per share.
Payment of Purchase Price; Payroll Deductions
The purchase price of the shares is accumulated by payroll deductions over the offering. A participant may increase or decrease (including to zero) his or her participation level once during a purchase period, excluding only each 10 day period immediately preceding a purchase date (or such shorter period of time as determined by Epimmune). Additionally, a participant may reduce his or her participation level to zero at any time during the course of an offering, excluding only each 10 day period immediately preceding a purchase date (or such shorter period of time as determined by Epimmune). A participant may not begin payroll deductions after the beginning of any purchase period, except, if the Board provides, in the case of an employee who first becomes eligible to participate as of a date specified during the purchase period. All payroll deductions made for a participant are credited to his or her account under the Purchase Plan and deposited with the general funds of Epimmune (except where otherwise required by applicable law). A participant may not make additional payments into such account.
141
Purchase of Stock
By executing an agreement to participate in the Purchase Plan, the employee is entitled to purchase shares under the Purchase Plan. In connection with offerings made under the Purchase Plan, the Board specifies a maximum number of shares of common stock an employee may be granted the right to purchase and the maximum aggregate number of shares of common stock that may be purchased pursuant to such offering by all participants. If the aggregate number of shares to be purchased upon exercise of rights granted in the offering would exceed the maximum aggregate number of shares of common stock available, the Board would make a pro rata allocation of available shares in a uniform and equitable manner. Unless the employee’s participation is discontinued, his or her right to purchase shares is exercised automatically at the end of the purchase period at the applicable price. See the section entitled“Withdrawal” below.
Withdrawal
While each participant in the Purchase Plan is required to sign an agreement authorizing payroll deductions, the participant may withdraw from a given offering by terminating his or her payroll deductions and by delivering to us a notice of withdrawal from the Purchase Plan. Such withdrawal may be elected at any time prior to the end of the offering, excluding only each 10 day period immediately preceding a purchase date.
Upon any withdrawal from an offering by the employee, we will distribute to the employee his or her accumulated payroll deductions without interest, less any accumulated deductions previously applied to the purchase of shares of common stock on the employee’s behalf during such offering, and such employee’s interest in the offering will be automatically terminated. The employee is not entitled to again participate in that offering. However, an employee’s withdrawal from an offering will not have any effect upon such employee’s eligibility to participate in subsequent offerings under the Purchase Plan.
Termination of Employment
Rights granted pursuant to any offering under the Purchase Plan terminate immediately upon cessation of an employee’s employment for any reason, and we will distribute to such employee all of his or her accumulated payroll deductions, without interest.
Restrictions on Transfer
Rights granted under the Purchase Plan are not transferable other than by will or the laws of descent and distribution and may be exercised during the participant’s lifetime only by the participant.
Duration, Amendment and Termination
The Board may suspend or terminate the Purchase Plan at any time. Unless terminated earlier, the Purchase Plan shall terminate at the time that all of the shares of common stock reserved for issuance under the Purchase Plan have been issued under the terms of the Purchase Plan.
The Board may amend the Purchase Plan at any time. Any amendment of the Purchase Plan must also be approved by the stockholders within 12 months of its adoption by the Board if the amendment would (i) increase the number of shares of common stock reserved for issuance under the Purchase Plan, (ii) modify the requirements relating to eligibility for participation in the Purchase Plan, or (iii) modify any other provision of the Purchase Plan in a manner that would materially increase the benefits accruing to participants under the Purchase Plan, if such approval is required in order to comply with the requirements under other applicable laws and regulations.
Rights granted before amendment or termination of the Purchase Plan will not be altered or impaired by any amendment or termination of the Purchase Plan without consent of the employee to whom such rights were granted except as necessary to comply with any laws or government regulations or to maintain favorable accounting treatment for the Purchase Plan.
142
Adjustment Provisions
Transactions not involving receipt of consideration by Epimmune, such as a merger, consolidation, reorganization, stock dividend or stock split, may change the type, class and number of shares of common stock subject to the Purchase Plan and to outstanding purchase rights. In that event, the Purchase Plan will be appropriately adjusted in the type, class and maximum number of shares subject to the Purchase Plan and the outstanding purchase rights granted under the Purchase Plan will be appropriately adjusted in the type, class, number of shares and purchase limits of such purchase rights. If the reverse stock split described in proposal 3 is approved and implemented, the Purchase Plan and outstanding purchase rights will be appropriately adjusted.
Effect of Certain Corporate Events
In the event of a dissolution or liquidation of Epimmune, the surviving corporation either will assume the rights under the Purchase Plan or substitute similar rights, or the exercise date of any ongoing offering will be accelerated such that the outstanding rights may be exercised immediately prior to, or concurrent with, any such event.
In the event of (i) the sale, lease, license or other disposition of all or substantially all of the assets of Epimmune, (ii) the sale or other disposition of at least ninety percent of the outstanding securities of Epimmune, or (iii) certain specified types of merger, consolidation or similar transactions (collectively referred to as the triggering transaction), any surviving or acquiring corporation may continue or assume rights outstanding under the Purchase Plan or may substitute similar rights. If any surviving or acquiring corporation does not assume such rights or substitute similar rights, then the participants’ accumulated payroll deductions will be used to purchase shares of common stock within five business days prior to the triggering transaction under the ongoing offering and the participants’ rights under the ongoing offering will terminate immediately after such purchase
Stock Subject to Purchase Plan
Subject to stockholder approval of this proposal 6, an aggregate of 485,000 shares of common stock is reserved for issuance under the Purchase Plan. If rights granted under the Purchase Plan expire, lapse or otherwise terminate without being exercised, the shares of common stock not purchased under such rights again becomes available for issuance under the Purchase Plan.
Federal Income Tax Information
Rights granted under the Purchase Plan are intended to qualify for favorable federal income tax treatment associated with rights granted under an employee stock purchase plan which qualifies under provisions of Section 423 of the Code.
A participant will be taxed on amounts withheld for the purchase of shares of common stock as if such amounts were actually received. Other than this, no income will be taxable to a participant until disposition of the acquired shares, and the method of taxation will depend upon the holding period of the acquired shares.
If the stock is disposed of more than two years after the beginning of the offering period and more than one year after the stock is transferred to the participant, then the lesser of (i) the excess of the fair market value of the stock at the time of such disposition over the exercise price or (ii) the excess of the fair market value of the stock as of the beginning of the offering period over the exercise price (determined as of the beginning of the offering period) will be treated as ordinary income. Any further gain or any loss will be taxed as a long-term capital gain or loss. At present, such capital gains generally are subject to lower tax rates than ordinary income.
If the stock is sold or disposed of before the expiration of either of the holding periods described above, then the excess of the fair market value of the stock on the exercise date over the exercise price will be treated as ordinary income at the time of such disposition. The balance of any gain will be treated as capital gain. Even if the stock is later disposed of for less than its fair market value on the exercise date, the same amount
143
of ordinary income is attributed to the participant, and a capital loss is recognized equal to the difference between the sales price and the fair market value of the stock on such exercise date. Any capital gain or loss will be short-term or long-term, depending on how long the stock has been held.
There are no federal income tax consequences to us by reason of the grant or exercise of rights under the Purchase Plan. We are entitled to a deduction to the extent amounts are taxed as ordinary income to a participant (subject to the requirement of reasonableness and the satisfaction of tax reporting obligations).
Purchase Plan Transactions
The table below shows, as to each of the named executive officers and the various indicated groups, the number of shares of common stock purchased under the Purchase Plan between the March 5, 2001 effective date of the Purchase Plan and the December 31, 2004 purchase date.
| | | | | |
| | | Number of Shares | |
| Name and Position | | Purchased | |
| | | | |
| Dr. Emile Loria, President and Chief Executive Officer | | | 0 | |
| Dr. Mark J. Newman, Vice President, Research and Development and Assistant Secretary | | | 15,839 | |
| Mr. Robert J. De Vaere, Vice President, Finance and Administration, Chief Financial Officer and Secretary | | | 7,605 | |
| All Executive Officers as a Group | | | 23,444 | |
| All Non-Executive Officer Employees as a Group | | | 137,455 | |
| All Non-Employee Directors as a Group | | | 0 | |
New Plan Benefits
No purchase rights have been granted, and no shares of common stock have been issued, under the Purchase Plan on the basis of the 185,000 share increase for which stockholder approval is sought under this proposal 6.
Equity Compensation Plan Information
The following table sets forth certain information as of December 31, 2004 regarding our equity compensation plans.
| | | | | | | | | | | | | |
| | | | | | | (c) | |
| | | | | | | Number of Securities | |
| | | | | | | Remaining Available | |
| | | (a) | | | (b) | | | for Issuance Under | |
| | | Number of Securities | | | Weighted-Average | | | Equity Compensation | |
| | | to be Issued upon | | | Exercise Price of | | | Plans (Excluding | |
| | | Exercise of Options, | | | Outstanding Options, | | | Securities Reflected in | |
| Name of Plan | | Warrants and Rights | | | Warrants and Rights | | | Column (a)) | |
| | | | | | | | | | |
| Equity compensation plans approved by security holders | | | 2,521,147 | (1) | | $ | 2.59 | | | | 399,393 | |
| Equity compensation plans not approved by security holders | | | — | | | $ | 0.00 | | | | — | |
| Total | | | 2,521,147 | | | $ | 2.59 | | | | 399,393 | |
| |
| (1) | The number does not include the 8,800,000 shares of common stock represented in the amendment of the 2000 Plan; although the Board has approved this amendment, stockholders are being asked in proposal 5 to approve the amendment and therefore, the additional shares are not reflected in the table. Additionally, the number does not include the 185,000 shares of common stock represented in the amendment of the Purchase Plan; although the Board has approved this amendment, stockholders are being asked in proposal 6 to approve the amendment and therefore, the additional shares are not reflected in the table. |
We do not have in effect any equity compensation plans under which our equity securities are authorized for issuance that were adopted without the approval of our security holders.
144
PROPOSAL 7 — APPROVAL OF ADOPTION OF THE EPIMMUNE EMPLOYEE STOCK PURCHASE PLAN FOR IDM EMPLOYEES
The share numbers referenced in this proposal do not give effect to the reverse stock split referenced in proposal 3. On May 31, 2005 the Board adopted the French Purchase Plan, subject to stockholder approval.
Subject to stockholder approval of the French Purchase Plan, there are 215,000 of common stock of Epimmune reserved for issuance under the French Purchase Plan.
Stockholders are requested in this proposal 7 to approve the French Purchase Plan. The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the annual meeting will be required to approve this proposal 7. Abstentions will be counted toward the tabulation of votes cast on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether this proposal has been approved.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 7.
The essential features of the French Purchase Plan are outlined below. The following summary is qualified in its entirety by reference to the French Purchase Plan, a copy of which is attached to this proxy statement asAnnex J.
Purpose
The purpose of the French Purchase Plan is to provide a means by which employees of IDM may be given an opportunity to purchase common stock of Epimmune through cash or check payments or payroll deductions, to assist IDM in retaining the services of its employees, to secure and retain the services of new employees, and to provide incentives for such persons to exert maximum efforts for the success of IDM. Approximately 77 current IDM employees and retired former IDM employees will be eligible to participate in the French Purchase Plan.
The rights to purchase common stock granted under the French Purchase Plan are intended to qualify for favorable tax treatment under applicable French laws. IDM is currently negotiating with its French works council for the creation of aplan d’epargne d’entrepriseor PEE, referred to as a French Savings Plan, that would hold any shares of Epimmune common stock issued under the French Purchase Plan. A PEE is a tax efficient vehicle under French law whereby a holder of securities may receive preferential tax treatment provided that, among other things, the securities are held in a separate account for a certain period of time. Even if the stockholders approve the French Purchase Plan pursuant to this proposal, if the negotiations for the creation of the French Savings Plan are not successful, then the French Purchase Plan will be terminated by the Board prior to establishing any offerings under the French Purchase Plan.
Administration
The Board administers the French Purchase Plan and has the final power to construe and interpret both the French Purchase Plan and the rights granted under it. The Board has the power, subject to the provisions of the French Purchase Plan, to determine when and how rights to purchase common stock of Epimmune will be granted, and the provisions of each offering of such rights (which need not be identical).
The Board has the power to delegate administration of the French Purchase Plan to a committee composed of not fewer than one member of the Board. The Board has delegated administration of the French Purchase Plan to the Compensation Committee of the Board. As used herein with respect to the French Purchase Plan, the “Board” refers to the Compensation Committee to the extent the Board has delegated administration of the French Purchase Plan to the Compensation Committee.
145
Offerings and Purchase dates
The French Purchase Plan will be implemented by offerings of rights to all eligible employees as established from time to time by the Board. The Board shall determine the terms and conditions of offerings including the length of offering periods and the purchase dates applicable to the offerings. The maximum length for an offering period under the French Purchase Plan is six months. Each purchase date for an offering shall occur sometime within the period commencing on the last date of the offering period and ending fifteen days thereafter.
Eligibility
To be eligible to participate in the French Purchase Plan, current IDM employees must have been employed by IDM for such minimum period as the Board may require as set forth in the French Savings Plan. Additionally, retired former employees of IDM are eligible to participate in French Purchase Plan offerings if they have made contributions to the French Savings Plan prior to commencement of the offering period and have not previously requested the liquidation of the assets held on their behalf under the French Savings Plan.
Participation in the French Purchase Plan
For each offering, participants will be granted rights to purchase a whole number of shares based upon the per share purchase price established by the Board for the offering. Such number of shares shall equal the sum of (a) the whole number of shares purchasable with participant contributions made during the offering, without accrued interest, and (b) the whole number of shares purchasable with IDM contributions, if any, made on behalf of the participant following the offering period and prior to the purchase date.
Eligible employees may enroll in the French Purchase Plan by delivering a participation agreement to IDM at any time prior to the last day of the offering period setting forth the number of shares to purchase and the elected method of payment. Participants may elect to pay for the shares, in whole or in part, through payroll deductions, by separate cash or check payments, or such other method of payment as is permitted under the offering. To elect payroll deductions, the participant must provide his or her authorization for monthly payroll deductions in the participation agreement. Such monthly payroll deductions may not exceed 10% of such participant’s net monthly remuneration paid by IDM to such participant during the offering period. The participant may additionally or alternatively make a separate cash or check payment for the shares of common stock, which payment must be delivered prior to the expiration of the offering period. Participants may also make separate cash or check payment contributions so that their total contributions exceed the maximum 10% payroll deduction limitation. However, the maximum amount of contributions that a participant may make during the offering period shall be limited to the lower of (a) 25% of the participant’s gross annual remuneration (as defined under French law) or, (b) the amount necessary to purchase the maximum number of shares, if any, approved by the Board for issuance to each participant in connection with an offering.
Participation agreement elections are generally revocable through the last day of the offering period. Unless otherwise provided under the terms of an offering, participation agreement elections shall become irrevocable on the last day of the offering period at 18:00 hours Paris time.
Purchase Price
The purchase price per share at which shares of Epimmune common stock are sold in an offering under the French Purchase Plan shall be established by the Board for each offering and shall be no greater than 100% or less than 80% of the twenty-day trading average of the common stock preceding the date that the Board determines the terms of the offering. For such purposes, the twenty day trading average means the per share average opening sales price (rounded up where necessary to the nearest whole cent) of the common stock as quoted on the stock exchange where the common stock is listed or on the Nasdaq National Market or the Nasdaq SmallCap Market where the common stock is traded (or the exchange or market with the greatest volume of trading in the common stock) during the consecutive twenty trading days immediately preceding
146
the date the Board determines the terms of the offering, as reported in The Wall Street Journal or such other source as the Board deems reliable.
As of June 29, 2005, the closing price of Epimmune’s common stock as reported on the Nasdaq National Market System was $0.72 per share.
Payment of Purchase Price; Payroll Deductions
The purchase price of the shares can be paid by the eligible employee by separate cash or check payment and/or accumulated payroll deductions during the offering. A participant may increase or reduce his or her level of participation during the offering to the extent that the Board provides that such adjustments may be made under the terms of the offering and in accordance with the administrative procedures established by the Board for the offering. All cash or check payments made by the participant and all payroll deductions will be credited to his or her bookkeeping account under the French Purchase Plan and deposited with the general funds of Epimmune. The participant contributions credited to his or her bookkeeping account shall bear interest at a rate that is the higher of (i) the legal interest rate as provided under Article L.313-2 of the French Financial and Monetary Code and (ii) the prevailing market rate, in each case, as specified by the Board for each offering.
IDM Contributions
For each offering, to the extent provided under the then-existing terms of the French Savings Plan, IDM will make a cash contribution that will be deposited in the participant’s French Savings Plan account subsequent to the last day of the offering period and prior to the purchase date of such offering, and used along with participant contributions transferred from the French Purchase Plan bookkeeping account for the purchase of shares on the purchase date. The determination whether to make such contributions for a particular offering will be made by IDM in accordance with the terms of the French Savings Plan. IDM contributions shall not accrue interest.
Purchase of Stock
By executing an agreement to participate in the French Purchase Plan and making contributions, the participant is entitled to have shares purchased on behalf of the participant under the French Purchase Plan. In connection with offerings made under the French Purchase Plan, in its discretion, the Board may specify a maximum number of shares of common stock each participant may purchase on any applicable purchase date. Additionally, the Board may specify the maximum aggregate number of shares of common stock that may be purchased on such purchase date by all participants. If the aggregate number of shares to be purchased upon exercise of rights granted in the offering would exceed the maximum aggregate number of shares of common stock available to all participants, the Board would make a pro rata allocation of available shares in a uniform and equitable manner. Unless the employee’s participation is discontinued, his or her right to purchase shares will exercised automatically on the purchase date following the end of the offering period at the applicable purchase price. See the section entitled“Withdrawal” below.
Both the participant contributions, without accrued interest, and discretionary IDM contributions for the participant, if any, shall be deposited in the participant’s French Savings Plan account following the end of the offering period and prior to the purchase date and used to purchase shares on the applicable purchase date. Any accrued interest applicable to the participant’s contributions that is credited to the participant’s French Purchase Plan bookkeeping account along with any remaining amount of contributions in the participant’s French Savings Plan account that is more than the amount required to purchase one or more whole shares of common stock on the applicable purchase date shall be distributed to the participant as soon as administratively practicable following the purchase date. Any remaining contributions that are less than the amount required to purchase one share of common stock shall be deposited with the general funds of Epimmune and credited to the participant’s French Purchase Plan bookkeeping account for the purchase of shares under the next offering under the French Purchase Plan, unless the participant withdraws from the next offering or is not eligible to participate in the next offering, in which case the remaining amount will be distributed to the
147
participant along with the applicable accrued interest credited to the participant’s French Purchase Plan bookkeeping account.
Withdrawal
While each participant in the French Purchase Plan is required to sign an agreement for participation and authorizing payroll deductions, providing for a cash or check payment, or authorizing a combination of the foregoing as a method of payment for the shares, except as otherwise provided under the terms of the offering, the participant may withdraw from a given offering by terminating his or her payroll deductions and requesting a refund of prior cash or check payment contributions and/or payroll deductions by delivering to IDM a notice of withdrawal from the French Purchase Plan. Such withdrawal may be elected at any time prior to the end of the applicable offering period.
Upon any withdrawal from an offering by the participant, IDM will distribute to the participant his or her accumulated payroll deductions and any other separate cash or check payments made by the participant with accrued interest, and his or her participation in the offering will be automatically terminated. The employee is not entitled to again participate in that offering. However, an employee’s withdrawal from an offering will not have any effect upon such employee’s eligibility to participate in subsequent offerings under the French Purchase Plan.
Termination of Eligibility
Rights granted pursuant to any offering under the French Purchase Plan terminate immediately upon cessation of an employee’s eligibility for any reason, and IDM will distribute to such employee all of his or her accumulated separate cash or check payment contributions and payroll deductions, with accrued interest as soon as administratively practicable.
Restrictions on Transfer
Rights granted under the French Purchase Plan are not transferable other than by will or the laws of descent and distribution, or a beneficiary designation (and in all such cases of transfer only as allowed under the French Savings Plan), and may be exercised during the employee’s lifetime only by the employee to whom the rights were granted.
Duration, Amendment and Termination
The Board may suspend or terminate the French Purchase Plan at any time. Unless terminated earlier, the French Purchase Plan will terminate once all the shares of common stock reserved for issuance under the French Purchase Plan have been issued under the terms of the French Purchase Plan
The Board may amend the French Purchase Plan at any time. Any amendment of the French Purchase Plan must be approved by the stockholders if the amendment is necessary for the French Purchase Plan to satisfy the requirements of applicable laws and regulations.
Rights granted before amendment or termination of the French Purchase Plan will not be altered or impaired by any amendment or termination of the French Purchase Plan without consent of the employee to whom such rights were granted or as necessary to comply with applicable laws and regulations or to maintain favorable accounting treatment under the French Purchase Plan.
Adjustment Provisions
Transactions not involving receipt of consideration by Epimmune, such as a merger, consolidation, reorganization, recapitalization, reincorporation stock dividend, dividend in property other than cash, stock split, liquidating dividend, combination of shares, exchange of shares, or change in corporate structure, may change the type(s), class(es) and number of shares of common stock subject to the French Purchase Plan and to outstanding purchase rights. In that event, the French Purchase Plan will be appropriately adjusted in the type(s), class(es) and maximum number of shares subject to the French Purchase Plan and the outstanding
148
purchase rights granted under the French Purchase Plan will be appropriately adjusted in the type(s), class(es), number of shares and purchase limits of such purchase rights. If the reverse stock split described in proposal 3 is approved and implemented, the number of shares reserved for issuance under the French Purchase Plan will be appropriately adjusted.
Effect of Certain Corporate Transactions
To the extent permitted under French law applicable to the French Savings Plan, the following provisions shall govern the treatment of purchase rights in the event of certain corporate transactions.
In the event of a dissolution or liquidation of IDM or Epimmune, subject to Epimmune’s discretionary approval, the surviving corporation either will assume the rights under the French Purchase Plan or substitute similar rights, or the exercise date of any ongoing offering will be accelerated such that the outstanding rights may be exercised within five business days prior to, or concurrent with, any such event.
In the event of (i) the sale, lease, license or other disposition of all or substantially all of the assets of IDM or Epimmune, (ii) the sale or other disposition of at least ninety percent of the outstanding securities of IDM or Epimmune, (iii) certain specified types of merger, consolidation or similar transactions involving IDM or Epimmune, or (iv) any similar transaction in which IDM ceases to be a majority owned subsidiary of Epimmune (collectively, “corporate transaction”), subject to the Board’s discretionary approval, any surviving or acquiring corporation may continue or assume rights outstanding under the French Purchase Plan or may substitute similar rights. If any surviving or acquiring corporation will not assume such rights or substitute similar rights in connection with a corporate transaction, then, to the extent the Board then determines in its sole discretion, upon at least 15 calendar days’ prior notice to the participants, the participants’ accumulated payroll deductions shall be used to purchase shares of common stock within five business days prior to the corporate transaction under the ongoing offering and the participants’ rights under the ongoing offering will terminate immediately after such purchase.
Stock Subject to French Purchase Plan
Subject to stockholder approval of this proposal 7, an aggregate of two hundred fifteen thousand shares of common stock is reserved for issuance under the French Purchase Plan. If rights granted under the French Purchase Plan expire, lapse or otherwise terminate without being exercised, the shares of common stock not purchased under such rights again become available for issuance under the French Purchase Plan. Only shares of Epimmune common stock that have not been previously issued shall be available for issuance under the French Purchase Plan.
Tax Information
If the shares received pursuant to the French Purchase Plan are lodged in the French Savings Plan, (Plan d’Epargne d’Entreprise) when such plan will be approved, the French, tax regime for Participants who are French tax resident can be summarized as follows (rules applicable as of today):
| | |
| | • | Save the case of certain limited cases of early withdrawal, the French Savings Plan regime entails a five-year unavailability period; |
| |
| | • | The contribution paid by a Participant and credited to the French Savings Plan is not deductible from the taxable income of the Participant; |
| |
| | • | The Company Contribution, if any, referred to in § 9 (a) would be exempt from French income tax and social charges, however, 97% of the Company Contribution would be subject to French social contributions (CSG, CRDS) at the rate of 8%; |
| |
| | • | The discount, if any, provided by §7 (d) of the French Purchase Plan (i.e. maximum 20% of the Purchase Price) would be exempt from income tax and from French social charges; |
| |
| | • | Dividends derived from the shares acquired in the French Savings Plan are exempt from income tax if such dividends are reinvested in the French Savings Plan and the shares acquired with the dividends |
149
| | |
| | | fall under the 5-year period of unavailability applicable to initial shares held in the French Savings Plan. The dividends will however be subject to French social contributions at the rate of 11% at the time the shares are sold (cf. below); |
| |
| | • | Capital gains derived from the sale of such shares after the five year holding period (or upon a case of early withdrawal) are exempt from income tax. Moreover, such shares should have a nominative form (or, ear- marked), and it should be clearly stated that such shares are acquired in the context of legal mechanisms of employees incentives. They are however subject to French social contributions at the rate of 11%; |
| |
| | • | Also, any interest paid by Epimmune on the Bookkeeping Account will be subject to French income tax and social contributions at the rate of 11%. |
IDM will be entitled to deduct from its French corporate income tax its contribution to the French Savings Plan.
New Plan Benefits
Officers, directors and non-officer employees of Epimmune are not eligible to participate in the French Purchase Plan. No purchase rights have been granted, and no shares of common stock have been issued, under the French Purchase Plan for which stockholder approval is sought under this proposal 7.
150
PROPOSAL 8 — ELECTION OF DIRECTORS
Our Amended and Restated Certificate of Incorporation permits the Board to fix the number of members comprising the Board. Currently, the number of members is fixed at six. The Board is currently comprised of six members, and there are six nominees for director at the annual meeting. Votes may not be made for a greater number of persons than the number of nominees named herein. Each nominee listed below is currently a director of the Company. All of these directors were elected by the stockholders.
It is our policy to encourage nominees for director to attend the annual meeting. Four of the six directors attended the 2004 annual meeting of stockholders.
Directors are elected by a plurality of the votes properly cast in person or by proxy. The six nominees receiving the highest number of affirmative votes will be elected. Shares represented by executed proxies will be voted, if authority to do so is not withheld, for the election of the six nominees named below. If any nominee becomes unavailable for election as a result of an unexpected occurrence, your shares will be voted for the election of a substitute nominee proposed by our management. Each person nominated for election has agreed to serve if elected, subject to the proposed resignation of Howard E. (“Ted”) Greene, Jr., William Comer and George Hibon if the Exchange is approved as described below in the section entitled“The Exchange — Directors and Management of Epimmune Following the Exchange”. If the Exchange is not approved, we expect that all six nominees would continue to serve as our directors. Our management has no reason to believe that any nominee will be unable to serve.
Information Regarding Nominees
The names of the nominees and certain information about them are set forth below:
| | | | | | | |
| Name | | Age | | | Position Held with the Company; Principal Occupation |
| | | | | | |
| Howard E. (“Ted”) Greene, Jr. | | | 62 | | | Chairman of the Board of Directors; Retired CEO of Amylin Pharmaceuticals, Inc. |
| William T. Comer, Ph.D. | | | 69 | | | Director; Director of TorreyPines Therapeutics, Inc. |
| Michael G. Grey | | | 52 | | | Director; Chief Executive Officer and Director of Structural GenomiX, Inc. |
| Georges Hibon | | | 67 | | | Director; Advisor to pharmaceutical and biotechnology companies |
| Emile Loria, M.D. | | | 55 | | | President, Chief Executive Officer and Director |
| John P. McKearn, Ph.D. | | | 51 | | | Director; Chief Executive Officer, President and Director of Kalypsys, Inc. |
Mr. Greene,a founder of Epimmune, has served as a director since our inception. He was elected Chairman of the Board in January 1989 and served as President from July 1987 to January 1989. Mr. Greene is a director and founder of Amylin Pharmaceuticals, Inc., a biotechnology company involved in research and development of medicines for treating diabetes and served as Chairman of the Board from 1987 to 1998. He was a general partner of Biovest Partners, a seed venture capital firm specializing in medical technology companies from 1986 until 1993. Prior to Biovest, he was Chief Executive Officer of Hybritech Incorporated, a biotechnology company acquired by Eli Lilly & Company in 1986. Mr. Greene is a director of Amylin and Biosite Incorporated.
Dr. Comerhas served as a director since January 1994. Since April 2000, he has been a director of TorreyPines Therapeutics, Inc., a privately held biopharmaceutical company, where he also served as Chairman of the Board from May 2000 through December 2004 and as Interim Chief Executive Officer from March 2000 through March 2002. Dr. Comer served as President and Chief Executive Officer and a member of the board of directors of SIBIA Neurosciences, Inc., a biotechnology company, from April 1991 to November 1999. SIBIA was acquired by Merck & Co., Inc. in November 1999. Dr. Comer resigned in November 1999, but continued to serve as a consultant to Merck from December 1999 until August 2000. Dr. Comer previously served in various roles with Bristol-Myers Squibb, a pharmaceutical company,
151
culminating in his position as Senior Vice President of Strategic Management, Pharmaceuticals and Nutritionals. He served as Chairman of Prescient Neuropharma, Inc. until December 17, 2002 and is currently a director of Innapharma, Inc.
Mr. Grey —Please see the section entitled“The Exchange — Directors and Management of Epimmune Following the Exchange” for biographical information.
Mr. Hibonhas served as a director since August 2001. He currently serves as an advisor and has served since 1998 to several companies and organizations in Europe and North America. From 1990 to 1998, he was with Pasteur Merieux Connaught, now Aventis Pasteur, a pharmaceutical company, most recently as Chairman and Chief Executive Officer of PMC North America, a vaccine focused business. From 1986 to 1989, he was with Gillette group as President Director General of ST Dupont, a luxury goods distributor. He was with Merck & Co., a pharmaceutical company, from 1968 to 1986 during which time he held various executive positions in their European and international operations. He currently serves on the Boards of Directors of Cerep, Aphton Corporation and Care France.
Dr. Loria — Please see the section entitled“The Exchange — Directors and Management of Epimmune Following the Exchange” for biographical information.
Dr. McKearn — Please see the section entitled“The Exchange — Directors and Management of Epimmune Following the Exchange”for biographical information.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF EACH NAMED NOMINEE.
Appointment of Directors Following the Exchange
The Share Exchange Agreement provides that our Board will take all actions necessary so that, as of the closing of the Exchange, our Board will be expanded to nine members, three directors of Epimmune are expected to resign and the Board has agreed to name Jean-Loup Romet-Lemonne, M.D., Donald Drakeman, David Haselkorn, Jean Deleage, Ph.D., Sylvie Grégoire and Robert Beck to fill vacancies on the Board created by the resignations and the increase in the size of the Board. All of these persons have agreed to be named to the Board and consented to being identified in this proxy statement. For further details regarding the persons who are expected to be appointed to our Board upon the closing of the Exchange, see the section entitled“The Exchange —Directors and Management of Epimmune Following the Exchange.”
Independence of the Board of Directors
As required under Nasdaq listing standards, a majority of the members of the Board must qualify as “independent,” as affirmatively determined by the Board. The Board consults with our counsel to ensure that the Board’s determinations are consistent with all relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent listing standards of Nasdaq, as in effect from time to time.
Consistent with these considerations, after review of all relevant transactions or relationships between each director, or any of his family members, and the Company, its senior management and its independent auditors, the Board has affirmatively determined that all of our directors are independent directors within the meaning of Rule 4200(a)(15) of the applicable Nasdaq listing standards, except for Dr. Loria, our Chief Executive Officer.
As required under the Nasdaq listing standards, the Board has adopted a policy of holding executive sessions, at which only independent directors are present, in conjunction with all regularly scheduled board meetings.
152
Board Committees and Meetings
During the fiscal year ended December 31, 2004, the Board held 10 meetings. The Board currently has an Audit Committee, a Compensation Committee and a Nominating Committee. The following table provides membership information for 2004 for each of the Board committees:
| | | | | | | | | | | | | |
| Name | | Audit | | | Compensation | | | Nominating | |
| | | | | | | | | | |
| Howard E. (“Ted”) Greene, Jr. | | | X* | | | | X* | | | | | |
| William T. Comer, Ph.D. | | | X | | | | X | | | | | |
| Michael G. Grey | | | X | | | | | | | | X* | |
| John P. McKearn, Ph.D. | | | | | | | X | | | | X | |
Below is a description of each committee of the Board and information regarding committee meetings held in 2004. The Board has determined that each member of each committee meets the applicable rules and regulations regarding “independence” and that each member is free of any relationship that would interfere with his individual exercise of independent judgment with regard to the Company.
Audit Committee. The Audit Committee of the Board oversees our corporate accounting and financial reporting process. The Board has adopted an Audit Committee Charter, which among other responsibilities, requires that this committee monitor our financial reporting process and internal control systems, review audit and management reports and review and approve the engagement of the independent auditors. Our Audit Committee Charter can be found on our corporate website atwww.epimmune.com. The Audit Committee met a total of five times in 2004. The Audit Committee met two times prior to March 30, 2004 to plan for and discuss the 2003 annual audit with our independent auditors. The Audit Committee met three times after March 30, 2004, to review and discuss our first, second and third quarter financial results and financial statements to be included in our Form 10-Q filings. The Audit Committee met one time following the 2004 fiscal year end to discuss the 2004 annual audit with our independent auditors. The Audit Committee recommends the independent auditors to the Board and provides a direct line of communication between the auditors and the Board. The independent auditors separately meet with the Audit Committee, with and without our management present, to review and discuss various matters, including our financial statements, the report of the independent auditors on the results, scope and terms of their work and their recommendations concerning the Company’s financial practices and procedures. The Board annually reviews the Nasdaq listing standards definition of independence for Audit Committee members and has determined that all members of our Audit Committee are independent, as independence is currently defined in Rule 4350(d)(2)(A)(i) and (ii) of the Nasdaq listing standards. In addition, the Board has determined that Mr. Greene qualifies as an audit committee financial expert as defined in the applicable SEC rules. The Board made a qualitative assessment of Mr. Greene’s level of knowledge and experience based on a number of factors, including his formal education and experience as a chief executive officer for public reporting companies.
Compensation Committee. The Compensation Committee of the Board reviews and approves our overall compensation strategy and policies. The Board has adopted a Compensation Committee Charter, which can be found on our corporate website atwww.epimmune.com. The Compensation Committee administers our stock option plans, stock purchase plan and 401(k) plan, approves (or recommends to the Board for approval) salaries, bonuses and other compensation arrangements for our officers, including our Chief Executive Officer, and performs such other functions regarding compensation as the Board may delegate. The Compensation Committee held three meetings and acted by unanimous written consent two times during 2004. All members of the Compensation Committee are independent, as defined in Rule 4200(a)(15) of the Nasdaq listing standards.
Nominating Committee. The Nominating Committee is responsible for interviewing, evaluating, nominating and recommending individuals for membership on the Board and committees thereof and nominating specific individuals to be elected as our officers by the Board. Our Nominating Committee Charter can be
153
found on our corporate website atwww.epimmune.com. All members of the Nominating Committee are independent, as independence is currently defined in Rule 4200(a)(15) of the Nasdaq listing standards.
The Nominating Committee believes that candidates for director should possess certain minimum qualifications, including high personal integrity and ethics and the ability to understand basic financial statements. The Nominating Committee also considers factors such as relevant expertise upon which to be able to offer advice and guidance to management, sufficient time to devote to the affairs of the Company, demonstrated excellence in his or her field, experience in the markets the Company serves, and the ability to exercise sound business judgment. However, the Nominating Committee retains the right to modify these factors from time to time. Candidates for director are reviewed in the context of the current composition of the Board, the operating requirements of the Company, and the long-term interests of stockholders. In the case of incumbent directors whose terms of office are set to expire, the Nominating Committee reviews such directors’ overall service to the Company during their term, including the number of meetings attended, level of participation, quality of performance, and any other relationships and transactions that might impair such directors’ independence.
The Nominating Committee does not consider director candidates recommended by stockholders at this time. The Nominating Committee believes that it is in the best position to identify, review, evaluate, and select qualified director candidates based upon its comprehensive criteria for membership on the Board.
The Nominating Committee did not hold any meetings during 2004. The Nominating Committee acted by unanimous written consent in March 2004 when it recommended the candidates for election to our Board.
Attendance at Board and Committee Meetings. All of our directors attended or participated in 75% or more of the aggregate of (i) the total number of meetings of the Board and (ii) the total number of meetings held by all committees of the Board on which such director served during the year.
Stockholder Communications with the Board of Directors
Historically, we have not adopted a formal process for stockholder communications with the Board. Nevertheless, every effort has been made to ensure that the views of stockholders are heard by the Board or individual directors, as applicable, and that appropriate responses are provided to stockholders in a timely manner. We believe our responsiveness to stockholder communications to the Board has been excellent, and to date, we have not considered it necessary to adopt a formal process. Nevertheless, during the upcoming year the Board will continue to monitor whether it would be appropriate to adopt a formal process for stockholder communications with the Board.
Code of Ethics
On December 9, 2003 we adopted the Code of Business Conduct and Ethics that applies to all officers, directors and employees. The Code of Business Conduct and Ethics is available on our corporate website atwww.epimmune.com. If we make any substantive amendments to the Code of Business Conduct and Ethics or grant any waiver from a provision of the Code to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website atwww.epimmune.com.
Report of the Audit Committee of the Board of Directors1
Management is responsible for the Company’s internal controls and the financial reporting process. Ernst & Young LLP, Independent Registered Public Accounting Firm, as the independent accountants is responsible for performing an independent audit of the Company’s consolidated financial statements in accordance with auditing standards generally accepted in the United States and to issue a report thereon. The
1 The material in this report is not “soliciting material,” is not deemed “filed” with the SEC, and is not to be incorporated by reference into any filing of the Company under the Securities Act or the Exchange Act, each as amended, whether made before or after the date hereof and irrespective of any general incorporation language contained in such filing.
154
Audit Committee is responsible for monitoring and overseeing these processes, as well as recommending to the Board the selection of the Company’s independent accountants.
In this context, the Audit Committee has met and held discussions with management and the independent accountants. Management represented to the Audit Committee that the Company’s consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States, and the Audit Committee has reviewed and discussed the audited financial statements with management and the independent accountants. The Audit Committee discussed with the independent accountants matters required to be discussed by Statement on Auditing Standards No. 61 (Codification of Statements on Auditing Standards, AV §380).
The Company’s independent accountants also provided to the Audit Committee the written disclosures and the letter required by Independence Standards Board Standard No. 1 (Independence Standards Board Standard No. 2, Independence Discussions with Audit Committees), and the Audit Committee discussed with the independent accountants that firm’s independence.
Based on the Audit Committee’s discussion with management and the independent accountants a well as the Audit Committee’s review of the representation of management and the report of the independent accountants to the Audit Committee, the Audit Committee recommended that the Board include the audited consolidated financial statements in the Company’s Annual Report on Form 10-K/A for the fiscal year ended December 31, 2004 filed with the SEC.
From the members of the Audit Committee of Epimmune Inc.:
| |
| | Howard E. (“Ted”) Greene, Jr. |
| | Michael G. Grey |
| | William T. Comer, Ph.D. |
155
PROPOSAL 9 — RATIFICATION OF THE SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Audit Committee of the Board has selected Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2005 and has further directed that management submit the selection of an independent registered public accounting firm for ratification by the stockholders at the annual meeting. Ernst & Young LLP has audited our financial statements since our inception in 1987. Representatives of Ernst & Young LLP are expected to be present at the annual meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither our Bylaws nor other governing documents or law require stockholder ratification of the selection of Ernst & Young LLP as our independent auditors. However, the Board is submitting the selection of Ernst & Young LLP to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Board will reconsider whether or not to retain that firm. Even if the selection is ratified, the Board in its discretion may direct the appointment of different independent auditors at any time during the year if they determine that such a change would be in the best interests of us and our stockholders.
The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the annual meeting will be required to ratify the selection of Ernst & Young LLP. Abstentions will be counted toward the tabulation of votes cast on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether this matter has been approved.
Auditors’ Fees
Audit Fees. Total audit fees billed by Ernst & Young LLP, including fees for professional services and expenses relating to the audit of our consolidated financial statements for the fiscal years ended December 31, 2004 and 2003, as well as fees related to the review of our consolidated financial statements included in our Form 10-Qs for 2004, accounting consultations and review of documents filed with the SEC, totaled $143,000 and $124,000, respectively.
Audit-Related Fees. Total audit-related fees billed by Ernst & Young LLP for the fiscal years ended December 31, 2004 and 2003, primarily including due diligence associated with a proposed business combination, totaled $110,000 and $10,000, respectively.
Tax Fees. Tax fees billed by Ernst & Young LLP for the years ended December 31, 2004 and 2003 totaled $34,000 and $27,000, respectively and consisted of the services related to tax compliance, tax advice and tax planning.
All Other Fees. There were no fees billed by Ernst & Young LLP for the years ended December 31, 2004 and 2003 that qualified as “all other fees.”
All fees described above were approved in advance by our Audit Committee.
During the fiscal year ended December 31, 2004, none of the total hours expended on our financial audit by Ernst & Young LLP were provided by persons other than full-time permanent employees of Ernst & Young LLP.
Pre-Approval Policies and Procedures
The Audit Committee has adopted a policy and procedures for the pre-approval of audit and non-audit services rendered by our independent auditor, Ernst & Young LLP. The policy generally pre-approves specified services in the defined categories of audit services, audit-related services and tax services up to specified amounts. Pre-approval may also be given as part of our Audit Committee’s approval of the scope of the engagement of the independent auditor or on an individual explicit case-by-case basis before the independent auditor is engaged to provide each service. The pre-approval of services may be delegated to one or more of our Audit Committee members, but the decision must be reported to the full Audit Committee at its next scheduled meeting.
The Audit Committee has determined that the rendering of the services, other than the audit services by Ernst & Young LLP, is compatible with maintaining the principal accountant’s independence.
MANAGEMENT AND THE BOARD OF DIRECTORS
RECOMMEND A VOTE IN FAVOR OF PROPOSAL 9.
156
CHAPTER FOUR — OTHER INFORMATION FOR THE ANNUAL MEETING OF
EPIMMUNE’S STOCKHOLDERS
Executive Officers
The following table sets forth information regarding our current executive officers:
| | | | | | | |
| Name | | Age | | | Position |
| | | | | | |
| Emile Loria, M.D. | | | 55 | | | Director, President and Chief Executive Officer |
| Robert J. De Vaere | | | 47 | | | Vice President, Finance and Administration, and Chief Financial Officer |
| Mark J. Newman, Ph.D | | | 50 | | | Vice President, Research and Development |
Dr. Loria — Please see the section entitled“The Exchange — Directors and Management of Epimmune Following the Exchange”for biographical information.
Mr. De Vaerehas served as our Vice President, Finance and Chief Financial Officer since May 2000 and became our Vice President, Finance and Administration in December 2001. Prior to joining us in May 2000, Mr. De Vaere was with Vista Medical Technologies, Inc., a medical device company, since January 1996 where he served as Vice President of Finance and Administration and Chief Financial Officer. Prior to his employment with Vista, he was Director of Finance and Business Management for Kaiser Electro-Optics from April 1993 to January 1996 and Controller for Kaiser Rollmet, an aerospace company, from January 1991 to April 1993.
Dr. Newmanhas served as our Vice President, Infectious Disease Program since March 1999 and became our Vice President, Research and Development in September 2003. Prior to joining Epimmune, Dr. Newman served as Vice President of Research and Development of Vaxcel, Inc., a vaccine delivery/adjuvant company, from January 1995 to March 1999. Prior to joining Vaxcel, he was Associate Vice President, Research and Development for Apollon, Inc., a DNA vaccine company. He also previously held the position of Senior Director at Cambridge Biotech Corporation.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding the ownership of our common stock as of February 1, 2005 by (i) each director and nominee; (ii) each of the named executive officers; (iii) all executive officers and directors as a group; and (iv) all those known by us to be beneficial owners of more than five percent of our common stock:
| | | | | | | | | | |
| | | Beneficial Ownership(1) | |
| | | | |
| | | Number of | | | Percent of | |
| Beneficial Owner | | Shares | | | Shares | |
| | | | | | | |
| G.D. Searle LLC(2) | | | 2,105,032 | | | | 11.8 | % |
| | 235 East 42nd Street | | | | | | | | |
| | New York, NY 10017 | | | | | | | | |
| Genencor International, Inc. | | | 1,342,324 | | | | 8.4 | % |
| | 200 Meridian Centre Blvd. | | | | | | | | |
| | Rochester, NY 14618 | | | | | | | | |
| International Biotechnology Trust plc | | | 1,279,659 | | | | 8.0 | % |
| | 71 Kingsway | | | | | | | | |
| | London, WC2B 6ST, England | | | | | | | | |
| Mr. Peter Allard(3) | | | 1,204,716 | | | | 7.5 | % |
| | Seaview, Chancery Lane | | | | | | | | |
| | Christ Church, Barbados, West Indies | | | | | | | | |
157
| | | | | | | | | | |
| | | Beneficial Ownership(1) | |
| | | | |
| | | Number of | | | Percent of | |
| Beneficial Owner | | Shares | | | Shares | |
| | | | | | | |
| The Animi Master Fund Ltd.(4) | | | 1,016,949 | | | | 6.2 | % |
| | c/o Archeus Capital Management Ltd. | | | | | | | | |
| | 360 Madison Avenue, 10thFloor | | | | | | | | |
| | New York, NY 10014 | | | | | | | | |
| Dr. Emile Loria(5) | | | 688,133 | | | | 4.1 | % |
| Dr. Mark J. Newman(5) | | | 289,649 | | | | 1.8 | % |
| Mr. Robert J. De Vaere(5) | | | 286,926 | | | | 1.8 | % |
| Mr. Howard E. (“Ted”) Greene, Jr.(5)(6)(7) | | | 246,431 | | | | 1.5 | % |
| Dr. William T. Comer(5) | | | 48,948 | | | | * | |
| Mr. Michael Grey(5) | | | 43,750 | | | | * | |
| Mr. Georges Hibon(5) | | | 31,667 | | | | * | |
| Dr. John P. McKearn(5) | | | 30,000 | | | | * | |
| All executive officers and directors as a group (8 persons)(8) | | | 1,665,504 | | | | 9.6 | % |
| |
| (1) | This table is based upon information supplied by officers, directors and principal stockholders and on any Schedules 13D or 13G filed with the SEC. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, each stockholder named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentage ownership is based on 16,014,569 shares of common stock outstanding on February 1, 2005, as adjusted by the rules promulgated by the SEC. |
| |
| (2) | Includes 1,787,572 shares of common stock issuable upon conversion of shares of Series S preferred stock and Series S-1 preferred stock held by G.D. Searle. G.D. Searle owns 100% of the outstanding shares of our Series S preferred stock and Series S-1 preferred stock. The Series S preferred stock and Series S-1 preferred stock may be converted into common stock at any time by G.D. Searle. |
| |
| (3) | Includes 141,943 shares of common stock underlying currently exercisable warrants. |
| |
| (4) | Includes 338,983 shares of common stock underlying currently exercisable warrants. |
| |
| (5) | Includes shares, which certain executive officers and directors of the Company have the right to acquire within 60 days after February 1, 2005 pursuant to outstanding options, as follows: |
| |
| | Dr. William T. Comer, 48,034 shares; |
| | Mr. Robert J. De Vaere, 279,221 shares; |
| | Mr. Howard E. (“Ted”) Greene, Jr., 46,606 shares; |
| | Mr. Michael G. Grey, 43,750 shares; |
| | Mr. Georges Hibon, 31,667 shares; |
| | Dr. Emile Loria, 595,472 shares; |
| | Dr. John P. McKearn, 30,000 shares; |
| | Dr. Mark J. Newman, 273,710 shares; |
| | All executive officers and directors as a group, 1,371,058 shares. |
| |
| (6) | Includes 174,942 shares held in trust for the benefit of Mr. Greene and his wife and 2,285 shares held in trust for the benefit of Mr. Greene’s children. Mr. Greene is a trustee of both trusts. Mr. Greene acting as trustee has voting and investment power with respect to such shares and may be deemed to be the beneficial owner of such shares. |
| |
| (7) | Includes 22,598 shares of common stock underlying currently exercisable warrants. |
| |
| (8) | Includes shares described in notes (5) through (7) above. |
158
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act, requires our directors and executive officers, and persons who own more than 10% of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports in changes in ownership of our common stock and other of our equity securities. Specific due dates for these reports have been established, and we are required to disclose any failure to file by these dates during 2004. Our officers, directors and greater than 10% stockholders are required by the SEC regulations to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us and written representations that no other reports were required, during the fiscal year ended December 31, 2004, all Section 16(a) filing requirements applicable to its officers, directors and greater than 10% beneficial owners were complied with.
Compensation of Directors
Non-employee directors are paid $2,000 per meeting attended in person and $500 per meeting attended by phone as compensation for their service on the Board. Directors are not compensated for actions taken by written consent. The members of the Board are eligible for reimbursement of expenses incurred in connection with their service on the Board. Under the Directors’ Deferred Compensation Plan, participating directors may elect on an annual basis to defer all of their cash compensation in a deferred compensation account pursuant to which the deferred fees are credited in the form of share units having a value equal to shares of our common stock share units, based on the market price of the stock at the time the deferred fees are earned. We will continue to credit share units to the participants’ deferred compensation accounts on a quarterly basis. When a participant ceases serving as a director, the participant shall be entitled to receive the value of his or her account either in a single lump-sum payment or in equal annual installments, as determined by us, in our sole discretion. No participant entitled to receive a payment of benefits shall receive payment in the form of our common stock. Effective as of the closing of the transactions under the Share Exchange Agreement, each of Dr. Comer and Messrs. Greene and Hibon will resign as a member of our Board and Dr. Comer and Mr. Greene, who are participants in the Directors’ Deferred Compensation Plan, will be entitled to receive the value of their accounts in a single lump-sum payment.
Directors are currently eligible to receive option grants under the 2000 Plan in accordance with the policy regarding non-employee director compensation adopted by the Board in 1999. This policy calls for each non-employee director to be granted annual options to purchase 5,000 shares of our common stock as of the date of each annual meeting of our stockholders. The shares subject to such option are to vest monthly over a 12-month period, provided the director remains a director upon the date of his re-election to our Board. Newly appointed or elected non-employee directors are eligible for a 20,000-share option grant under this policy with monthly vesting over a 48-month period. On June 15, 2004, the Board granted annual options to purchase 5,000 shares of our common stock in connection with the annual meeting of our stockholders to the following non-employee directors: Mr. Greene, Dr. Comer, Mr. Grey, Mr. Hibon and Dr. McKearn at an exercise price of $1.92 per share.
In connection with the approval of the proposed combination with IDM, our Board approved the amendment, effective as of the closing of the transactions under the Share Exchange Agreement, of certain options to purchase shares of our common stock granted to Dr. Comer and Messrs. Greene and Hibon, in light of their resignation from the Board as of the closing of the transactions under the Share Exchange Agreement, to provide that their outstanding options shall remain exercisable until the date that the option would have originally expired but for the resignation of the optionholder from service as our director, except that, with respect to any options that have an exercise price less than the fair market value of our common stock as of the date the resolutions were adopted, such options shall remain exercisable until the earlier of (i) the date that the options would have originally expired but for the resignation of the optionholder from service as our director or (ii) the latest date on which the option can expire without the option being treated as deferred compensation under Section 409A of the Code, and the treasury regulations thereunder and subject to the
159
additional tax under Section 409A of the Code (which under current guidance would be March 15, 2006 but could be extended).
Compensation of Executive Officers
The following table shows for the fiscal years ended December 31, 2004, 2003 and 2002, compensation awarded or paid to, or earned by the named executive officers. During the last three fiscal years, none of the named executive officers received any restricted stock awards or long-term incentive payouts; provided, however, Dr. Loria purchased stock from us in 2001 that was subject to vesting.
Summary Compensation Table
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Long-Term Compensation | | | |
| | | | | Awards | | | |
| | | Annual Compensation(1) | | | | | | |
| | | | | | Restricted | | | Securities | | | All Other | |
| | | | | Salary | | | Bonus | | | Stock Awards | | | Underlying | | | Compensation | |
| Name and Principal Position | | Year | | | ($) | | | ($)(2) | | | ($) | | | Options (#) | | | ($)(3) | |
| | | | | | | | | | | | | | | | | | | |
| Dr. Emile Loria(4)(5)(6)(9) | | | 2004 | | | | 350,000 | | | | 50,000 | | | | 0 | | | | 500,000 | | | | 2,408 | |
| | President, Chief Executive Officer | | | 2003 | | | | 350,000 | | | | 25,000 | | | | 0 | | | | 500,000 | | | | 1,387 | |
| | | | | 2002 | | | | 300,000 | | | | 100,098 | | | | 0 | | | | 0 | | | | 1,058 | |
| Dr. Mark J. Newman(7)(9) | | | 2004 | | | | 225,000 | | | | 50,000 | | | | 0 | | | | 160,000 | | | | 828 | |
| | Vice President, Research and | | | 2003 | | | | 195,833 | | | | 25,000 | | | | 0 | | | | 50,000 | | | | 724 | |
| | Development & Asst. Secretary | | | 2002 | | | | 185,000 | | | | 98 | | | | 0 | | | | 0 | | | | 597 | |
| Mr. Robert J. De Vaere(8)(9) | | | 2004 | | | | 215,000 | | | | 50,000 | | | | 0 | | | | 160,000 | | | | 789 | |
| | Vice President, Finance and | | | 2003 | | | | 195,000 | | | | 25,000 | | | | 0 | | | | 50,000 | | | | 724 | |
| | Administration, Chief Financial | | | 2002 | | | | 185,000 | | | | 98 | | | | 0 | | | | 0 | | | | 398 | |
| | Officer and Secretary | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| (1) | As permitted by rules promulgated by the SEC, no amounts are shown with respect to certain “perquisites,” where such amounts do not exceed the lesser of 10% of bonus plus salary or $50,000, in the column “Other Annual Compensation.” Accordingly, because no amounts would be included in this column, we have excluded this column from the above table. |
| |
| (2) | All officers of the Company were granted a stock bonus award during 2002 of 100 shares of our common stock in exchange for the termination of their participation in the 2002 Management Bonus Plan. The fair market value of our common stock on December 16, 2002, the issuance date, was $0.98 per share, or $98 for each award. |
| |
| (3) | All other compensation consists of life insurance premiums paid by us unless otherwise noted. |
| |
| (4) | Dr. Loria joined as our President and Chief Executive Officer in June 2001 at an annual salary of $300,000. Dr. Loria received a signing bonus of $125,000 and was eligible to earn a performance bonus equal to two percent of any proceeds received by us from any public or private equity financing or other transaction pursuant to which we received funding (other than research funding) that was completed by us between January 16, 2001 and January 16, 2002. During the period from January 16, 2001 and January 16, 2002, we completed transactions in which we received total funding of $16,379,581 making Dr. Loria eligible for bonus payments of $327,592 under the provisions of this agreement. Dr. Loria was paid a bonus of $227,592 in 2001 and the remaining accrued balance of $100,000 was paid in January 2002. |
| |
| (5) | Dr. Loria joined as our President and Chief Executive Officer in June 2001. In connection with his employment offer letter and joining our Board in January 2001, and as an inducement to accept the offer, we sold Dr. Loria 1,056,301 shares of our common stock at a purchase price of $2.50 per share, the closing price of our common stock on the Nasdaq National Market on the date of purchase. The shares were subject to vesting in equal daily installments during the four-year period following the date of purchase, and we had a right to purchase any unvested shares at the purchase price paid by Dr. Loria in the event of termination of Dr. Loria’s service to Epimmune. Dr. Loria issued us a promissory note for $2,641,000, the aggregate purchase price of the shares, which is secured by a pledge of the shares. In |
160
| |
| September 2003, Dr. Loria surrendered an aggregate of 963,740 shares of our common stock at the fair market value of $3.17 per share, in exchange for the prepayment of the outstanding principal and interest under the promissory note. |
| |
| (6) | Of the 500,000 options granted to Dr. Loria in 2004, 187,500 were contingent upon the achievement of certain performance milestones by specific dates. These performance milestones were not met and the option grants associated with them subsequently terminated. |
| |
| (7) | Of the 160,000 options granted to Dr. Newman in 2004, 60,000 were contingent upon the achievement of certain performance milestones by specific dates. These performance milestones were not met and the option grants associated with them subsequently terminated. |
| |
| (8) | Of the 160,000 options granted to Mr. De Vaere in 2004, 60,000 were contingent upon the achievement of certain performance milestones by specific dates. These performance milestones were not met and the option grants associated with them subsequently terminated. |
| |
| (9) | The performance milestones associated with the contingent option grants included: completion of a licensing transaction with a third party to assist in the development of any cancer or HIV vaccine candidate; completion of an equity financing of at least $10 million; and enrollment (injection) of the first patient in any Phase II clinical trial. |
Our Board approved salaries for our executive officers for 2005, which will be effective January 1, 2005 but only if the closing of the proposed transactions under the Share Exchange Agreement occur, as set forth in the following table. Our Board also approved the payment of bonuses, to be made only if the closing of the proposed transactions under the Share Exchange Agreement occur, to all of our employees who are employed at the time of the closing, including the executive officers set forth in the following table.
| | | | | | | | | | | |
| | | 2005 Salary | | | | | |
| Executive Officer | | ($)(1) | | | | | Amount of Bonus ($)(1) | |
| | | | | | | | | |
| Emile Loria, President and Chief Executive Officer | | | 375,000 | | | 375,000 | | | (12 months of then current salary) | |
| Mark Newman, Vice President, Research and Development | | | 235,000 | | | 117,500 | | | (six months of then current salary) | |
| Robert De Vaere, Vice President, Finance, and Chief Financial Officer | | | 235,000 | | | 117,500 | | | (six months of then current salary) | |
| |
| (1) | Effective only upon the closing of the proposed combination with IDM. |
Stock Option Grants and Exercises
We currently grant options to our executive officers under the 2000 Plan and have previously granted options under our 1997 Stock Plan and our 1989 Stock Option Plan, which terminated in 1999. As of December 31, 2004, options to purchase a total of 119,209 shares were outstanding under the 1997 Stock Plan, options to purchase a total of 219,798 shares were outstanding under the 1989 Stock Option Plan, options to purchase a total of 7,140 shares were outstanding under the 1994 Non-Employee Directors’ Stock Option Plan and options to purchase a total of 2,175,000 shares were outstanding under the 2000 Plan. On December 16, 2002 we granted stock bonus awards of 600 shares to our executive officers from the 2000 Plan. There are no options available for grant under the 1997 Stock Plan, the 1989 Stock Option Plan or the 1994 Non-Employee Directors’ Stock Option Plan. The Board approved an amendment of the 2000 Plan to include a 8,800,000 increase in the number of shares reserved for issuance under the plan, which is subject to stockholder approval pursuant to proposal 5. As of December 31, 2004, 399,393 shares of common stock were available for future grant under the 2000 Plan.
Options granted under the 1989 Stock Option Plan prior to 1996 generally vested 20% at the end of the first year of the optionee’s employment and thereafter daily at the rate of 20% per year during such period of employment. Options granted under the 1989 Stock Option Plan after November 1996 and options granted under the 2000 Plan generally vest 25% at the end of the first year of the optionee’s employment and thereafter daily at the rate of 25% per year during such period of employment. In September 2003, in light of the
161
reduction in force completed by the Company and as part of a program to retain our remaining employees and provide them with an incentive for future performance, the Compensation Committee granted options under the 2000 Plan subject to daily vesting over two years following the date of grant, except that the vesting of these options will accelerate so that the options will become immediately exercisable for all of the shares subject to the options in the event of a change of control of the Company. Options granted under our 1997 Plan which we assumed from a subsidiary, generally vest 25% at the end of the first year of the optionee’s employment and thereafter monthly at the rate of 25% per year during such period of employment.
The following tables show for the fiscal year ended December 31, 2004, certain information regarding options granted to, exercised by, and held at year-end by the named executive officers:
Options Granted in Last Fiscal Year
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Individual Grants | | | |
| | | | | | Potential Realizable | |
| | | | | % Total | | | | | Value at Assumed | |
| | | Number of | | | Options | | | | | Annual Rates of Stock | |
| | | Securities | | | Granted to | | | | | Price Appreciation for | |
| | | Underlying | | | Employees | | | Exercise or | | | | | Option Term(2) | |
| | | Options | | | in Fiscal | | | Base Price | | | Expiration | | | | |
| | | Granted | | | Year(1) | | | ($/Sh) | | | Date | | | 5% ($) | | | 10% ($) | |
| | | | | | | | | | | | | | | | | | | |
| Dr. Emile Loria(3)(6) | | | 250,000 | | | | 21.80% | | | | 1.53000 | | | | 12/26/13 | | | | 240,552 | | | | 609,606 | |
| | | | 62,500 | | | | 5.45% | | | | 1.92000 | | | | 06/15/14 | | | | 75,467 | | | | 191,249 | |
| | | | 62,500 | | | | 5.45% | | | | 1.53000 | | | | 12/26/13 | | | | 60,138 | | | | 152,502 | |
| | | | 62,500 | | | | 5.45% | | | | 1.53000 | | | | 12/26/13 | | | | 60,138 | | | | 152,502 | |
| | | | 62,500 | | | | 5.45% | | | | 1.53000 | | | | 12/26/13 | | | | 60,138 | | | | 152,502 | |
| Dr. Mark J. Newman(4)(6) | | | 80,000 | | | | 6.97% | | | | 1.53000 | | | | 12/26/13 | | | | 76,977 | | | | 195,074 | |
| | | | 20,000 | | | | 1.74% | | | | 1.92000 | | | | 06/15/14 | | | | 24,150 | | | | 61,200 | |
| | | | 20,000 | | | | 1.74% | | | | 1.53000 | | | | 12/26/13 | | | | 19,244 | | | | 48,769 | |
| | | | 20,000 | | | | 1.74% | | | | 1.53000 | | | | 12/26/13 | | | | 19,244 | | | | 48,769 | |
| | | | 20,000 | | | | 1.74% | | | | 1.53000 | | | | 12/26/13 | | | | 19,244 | | | | 48,769 | |
| Mr. Robert J. De Vaere(5)(6) | | | 80,000 | | | | 6.97% | | | | 1.53000 | | | | 12/26/13 | | | | 76,977 | | | | 195,074 | |
| | | | 20,000 | | | | 1.74% | | | | 1.92000 | | | | 06/15/14 | | | | 24,150 | | | | 61,200 | |
| | | | 20,000 | | | | 1.74% | | | | 1.53000 | | | | 12/26/13 | | | | 19,244 | | | | 48,769 | |
| | | | 20,000 | | | | 1.74% | | | | 1.53000 | | | | 12/26/13 | | | | 19,244 | | | | 48,769 | |
| | | | 20,000 | | | | 1.74% | | | | 1.53000 | | | | 12/26/13 | | | | 19,244 | | | | 48,769 | |
| |
| (1) | Based on 1,147,000 options granted in 2004 under the 2000 Plan, including grants to executive officers. |
| |
| (2) | The potential realizable value is calculated based on the terms of the option at its time of grant (10 years in the case of all options). It is calculated by assuming that the stock price on the date of grant appreciates at the indicated annual rate, compounded annually for the entire term of the option and that the option is exercised and sold on the last day of its term for the appreciated stock price. These amounts represent certain assumed rates of appreciation, in accordance with rules of the SEC, and do not reflect our estimate or projection of future stock price performance. Actual gains, if any, are dependent on the actual future performance of our common stock, and no gain to the optionee is possible unless the stock price increases over the option term, which will benefit all stockholders. |
| |
| (3) | Of the 500,000 options granted to Dr. Loria in 2004, 187,500 were contingent upon the achievement of certain performance milestones by specific dates. These performance milestones were not met and the option grants associated with them subsequently terminated. |
| |
| (4) | Of the 160,000 options granted to Dr. Newman in 2004, 60,000 were contingent upon the achievement of certain performance milestones by specific dates. These performance milestones were not met and the option grants associated with them subsequently terminated. |
| |
| (5) | Of the 160,000 options granted to Mr. De Vaere in 2004, 60,000 were contingent upon the achievement of certain performance milestones by specific dates. These performance milestones were not met and the option grants associated with them subsequently terminated. |
162
| |
| (6) | The performance milestones associated with the contingent option grants included: completion of a licensing transaction with a third party to assist in the development of any cancer or HIV vaccine candidate; completion of an equity financing of at least $10 million; and enrollment (injection) of the first patient in any Phase II clinical trial. |
Aggregated Option Exercises in Last Fiscal Year and Fiscal Year-end Option Values
The following table sets forth summary information with respect to exercisable and unexercisable stock options held as of December 31, 2004 by each of the named executive officers. None of the named executive officers exercised options in the fiscal year ended December 31, 2004. The value of the stock options is calculated using the market value of our common stock on December 31, 2004 ($1.66 per share) minus the exercise price of the options.
| | | | | | | | | | | | | | | | | |
| | | | | | | Number of Securities | | | |
| | | | | | | Underlying Unexercised | | | Value of Unexercised | |
| | | | | | | Options at | | | In-the-Money Options at | |
| | | | | | | December 31, 2004 | | | December 31, 2004 | |
| | | Shares Acquired | | | Value | | | | | | | |
| Name | | on Exercise | | | Realized | | | Exercisable/Unexercisable | | | Exercisable/Unexercisable | |
| | | | | | | | | | | | | |
| Dr. Emile Loria | | | — | | | | — | | | | 454,301/483,199 | | | $ | 47,651/$61,099 | |
| Dr. Mark J. Newman | | | — | | | | — | | | | 241,996/125,470 | | | $ | 79,092/$14,401 | |
| Mr. Robert J. De Vaere | | | — | | | | — | | | | 245,387/124,613 | | | $ | 6,581/$15,019 | |
Employment, Change of Control and Severance Agreements
Current Agreements. In May 2000, we entered into severance benefits agreements with Dr. Newman, Vice President, Research and Development, and Mr. Robert De Vaere, Vice President, Finance and Administration and Chief Financial Officer. In March 2001, the severance agreements with Dr. Newman and Mr. De Vaere were amended. Under the agreements, as amended, in the event Dr. Newman or Mr. De Vaere is terminated without cause within one year following a change of control of us, he shall receive a lump-sum payment equal to twelve months of his annual base salary, and all of his unvested stock options shall immediately vest and become exercisable.
In January 2001, we entered into an employment agreement with Dr. Emile Loria, one of our directors, for the position of President and Chief Executive Officer, contingent upon obtaining satisfactory approval to work in the United States. Dr. Loria subsequently obtained such approval in June 2001. The agreement provided an annual salary of $300,000 for Dr. Loria. In addition, Dr. Loria was eligible to earn a performance bonus equal to two percent of any proceeds received by us from any public or private equity financing or other transaction pursuant to which we received funding (other than research funding) that was completed by us between January 16, 2001 and January 16, 2002. We also agreed to pay Dr. Loria a signing bonus of $125,000, certain of his relocation expenses, including the costs of moving household goods to San Diego, temporary furnished living accommodations in San Diego for six months, automobile rental costs in San Diego for up to six months and cost of up to three trips for him and his family to and from France (such expenses were approximately $200,000), and agreed to pay him $60,000 to assist him in his relocation.
In addition, in January 2001, we sold Dr. Loria 1,056,301 shares of our common stock at the closing price of such common stock as reported by the Nasdaq National Market on the date of purchase, which was $2.50 per share. These shares vest in equal daily installments over the four-year period following the purchase date and we have a right to purchase any unvested shares at the purchase price paid by Dr. Loria in the event of termination of Dr. Loria’s service to us. Dr. Loria purchased the shares with a promissory note in the principal amount of $2,641,000, which is secured by a pledge of the shares. The note bears interest at the rate of 5.61% per year, compounded annually. In September 2003, Dr. Loria surrendered an aggregate of 963,740 shares of our common stock at the fair market value of $3.17 per share, in exchange for the prepayment of the outstanding principal and interest under the promissory note, a total of $3,055,000.
Under the terms of the employment agreement, Dr. Loria is entitled to continued salary payments for twelve months in the event he is terminated without cause or voluntarily resigns for good reason. In addition, if
163
Dr. Loria is terminated without cause or voluntarily resigns for good reason following a change in control of Epimmune, then Dr. Loria is entitled to receive a lump sum payment equal to one year of his base salary and all of the unvested shares he initially purchased from us will become fully vested.
In February 2004, we entered into an accelerated benefits agreement with Dr. Loria. Under the terms of the agreement, if Dr. Loria is terminated without cause or voluntarily resigns for good reason within one year following a change of control of Epimmune, then any stock options granted to him after December 9, 2003, which are unvested shall immediately vest and become exercisable.
New Agreements Effective upon Closing of Transactions under Share Exchange Agreement. On March 16, 2005, we entered into employment agreements with Drs. Loria and Newman and Mr. De Vaere, our current President and Chief Executive Officer, Vice President, Research and Development, and Chief Financial Officer and Vice President, Finance and Administration and Secretary, respectively. The employment agreements will become effective upon the closing of the proposed combination with IDM, will supercede the prior employment agreements between us and these individuals, and will provide that Dr. Loria will become our President and Chief Business Officer, Dr. Newman will become our Vice President, Infectious Diseases, and Mr. De Vaere will be our Chief Financial Officer and Vice President following the closing. The employment agreements provide for a minimum annual salary of $375,000 for Dr. Loria and $235,000 for each of Mr. De Vaere and Dr. Newman and the grant to each employee of the right to receive a restricted stock grant. Pursuant to the terms of the restricted stock grants, Drs. Loria and Newman, and Mr. De Vaere are eligible to receive up to 370,700 shares, 128,300 shares, and 127,200 shares, respectively. The restricted stock grants are subject to the following terms:
| | |
| | • | the restricted stock vests in one or more installments, subject to continuous employment with us through the applicable installment date; |
| |
| | • | the restricted stock is subject to accelerated vesting upon the closing of a transaction providing a specified level of financing to us, or the closing of a transaction providing a specified level of funding to our infectious disease business, or both, depending on the employee; and |
| |
| | • | shares subject to the restricted stock grant that become vested will be issued to the employee on the earlier of (i) the employee’s termination, or (ii) 36 months from the date of the agreement. |
Each agreement provides for continued exercisability of outstanding options granted to the employee prior to the effective date of the agreement, to the extent the options were not in the money on the effective date of the agreement, generally until the later of (i) three months after employee’s termination, or (ii) December 31, 2007.
The agreements with Dr. Newman and Mr. De Vaere provide for the grant of retention bonuses, as follows:
| | |
| | • | Dr. Newman will be eligible for up to two retention bonuses at six and 12 months after the date of his agreement equal, in total, to 50% of his annual salary if he has been employed by us through the applicable bonus date; upon closing of a transaction providing a specified level of funding for our infectious disease business, any such retention bonuses not previously earned will be paid immediately; and |
| |
| | • | Mr. De Vaere will be eligible for up to three retention bonuses at six, nine, and 12 months after the date of his agreement, equal, in total, to 100% of his annual salary if he has been employed by us through the applicable bonus date. |
In case of a termination of the employee’s employment due to death or disability during the term of his agreement, the employee will be entitled to full acceleration of vesting and exercisability of any outstanding options granted before the effective date of the agreement. In the event that we terminate an employee’s employment without cause (as defined in the agreement), or the employee terminates his employment with good reason (as defined in the agreement), in each case during the term of his agreement, or upon the
164
expiration of the term of his agreement, the employee will be entitled to, subject to the execution by the employee of an effective waiver and release of claims against the combined company:
| | |
| | • | severance payments, consisting of the employee’s base salary in effect at the time of termination, paid for a period of 12 months (or, at employee’s option, payment in a lump sum of the employee’s base salary) in the case of termination without cause, and, in the case of termination by the employee with good reason or upon the expiration of the agreement, such severance shall be paid for a period of the shorter of 12 months or until the date the employee begins full time employment with another entity; |
| |
| | • | reimbursement for a portion of COBRA health insurance premiums for a period of up to 12 months; |
| |
| | • | full acceleration, as of the date of termination, of vesting and exercisability of any outstanding options granted before the effective date of the agreement; and |
| |
| | • | full acceleration of vesting and exercisability of any unvested restricted stock granted pursuant the agreement. |
On March 15, 2005, our Board interpreted the terms of options to purchase our common stock, which were previously granted to all of our employees in September 2003, including options to purchase 500,000 shares of common stock held by Dr. Loria, options to purchase 35,000 shares of common stock held by Dr. Newman and options to purchase 50,000 shares of common stock held by Mr. De Vaere. Under their original terms these options would vest in full upon a change in control of our Company and the Board clarified that the proposed combination with IDM would constitute a change in control so that those options that remain unvested will accelerate and vest in full as of the closing of the proposed combination.
Report of the Compensation Committee of the Board of Directors on Executive Compensation1
The Compensation Committee is comprised of Mr. Greene, Dr. Comer and Dr. McKearn, none of whom is an employee of the Company. The Compensation Committee is responsible for setting and administering our policies governing annual executive salaries, bonuses (if any) and stock ownership programs. The Compensation Committee evaluates the performance of management and determines the compensation of the Chief Executive Officer, or the CEO, and our other executives based upon the accomplishment of defined objectives in the Company’s research and product development programs and achievement of financial targets. The full Board of Directors reviews the Compensation Committee’s recommendations regarding the compensation of the CEO and the other executive officers.
Executive Officer Compensation Program. Our executive officer compensation program consists of base salary, annual incentives in the form of cash bonuses and long-term compensation in the form of stock awards. Our executive officer compensation program is designed to achieve the following objectives:
| | |
| | • | Attract, retain and motivate quality executives who possess the necessary leadership and management skills. |
| |
| | • | Provide an incentive to advance the research and clinical development of our therapeutic products. |
| |
| | • | Emphasize stock-based compensation to provide longer-term motivation by aligning the executives’ interests with those of the Company and its stockholders. |
Compensation is based on the level of job responsibility and the level of the individual’s performance as well as the Company’s performance. The Compensation Committee endeavors to set executive compensation within a range which the Compensation Committee believes is comparable to the average range of compensation set by companies of comparable size and stage of development in the biotechnology industry.
1 The material in this report is not “soliciting material,” is not deemed “filed” with the SEC, and is not to be incorporated by reference into any filing of the Company under the Securities Act or the Exchange Act each as amended, whether made before or after the date hereof and irrespective of any general incorporation language contained in such filing.
165
The group of comparable companies is not necessarily the same as the companies reflected in the market indices included in the performance graph on page 168.
The Compensation Committee believes that the Company’s executive compensation program reflects the principles described above and provides executives strong incentives to maximize Company performance and enhance stockholder value. The Compensation Committee believes that a stock option program to reward performance is an appropriate method of executive compensation, with relatively modest increases in base compensation.
Base Salary. Base salary levels for each of our executive officers are reviewed annually. The Compensation Committee applies various subjective criteria, including performance of the individual and the Company and relative responsibility and experience, to determine appropriate base salaries. In evaluating Company performance, the Compensation Committee considers the meeting of certain product development milestones, achieving certain corporate objectives (related to financings, collaborations and other strategic transactions) and organizational effectiveness. In addition, the Company compares its executives’ base salaries to management salaries at companies in the biotechnology industry, in comparable geographic areas and at similar stages of growth and considers industry surveys regarding executive compensation. The Compensation Committee uses these criteria as a frame of reference for annual salary adjustments although other factors are considered, as appropriate, and no specific weights are ascribed to the factors considered by the Compensation Committee.
Annual Incentives. Annual incentives in the form of cash bonuses are established and awarded by the Compensation Committee based upon its evaluation of the performance of each executive officer and the achievement of the Company’s performance goals during the year. In September 2003, the Board approved retention bonuses for all Epimmune employees as part of a special retention program following a reduction in work force. The retention bonuses were paid in three equal installments in December 2003, June 2004 and December 2004.
In January 2004, the Compensation Committee of our Board of Directors approved a 2004 bonus plan for the officers of the Company under which the officers would be eligible to receive bonuses for achievement of specific performance milestones by a target date. The performance milestones included completion of two equity financings of at least $5 and $10 million respectively, completion of a licensing transaction with a third party to assist in the development of any cancer or HIV vaccine candidate and enrollment (injection) of the first patient in any Phase II clinical trial. An added requirement for the payment of any cash bonuses was that the Company would have at least one year of cash on hand at December 31, 2004. As a result of transaction expenses related to the combination with IDM, the Board determined that this criteria was not met and no cash bonuses were paid to any officers.
Long-Term Incentive Compensation. The Company’s long-term incentive program includes stock options and other awards granted under the 2000 Plan, other stock awards and the Purchase Plan. Stock options are an important part of the Company’s performance-based compensation. Option grants include vesting periods (generally over four years) to encourage key employees to continue in the employ of the Company. In September 2003, the Board approved option grants for all Epimmune employees with two-year vesting as part of a special retention program following a reduction in work force. Through option grants, executives receive significant equity incentives to build long-term stockholder value.
The Compensation Committee believes that providing management a substantial economic interest in the long-term appreciation of the Company’s common stock further aligns the interests of stockholders and management. The size of option grants to an individual is primarily determined by such individual’s position within management of the Company, as well as competitive practices at biotechnology companies of comparable size. The Compensation Committee also considered the size of grants to individuals in previous years and internal relativity.
Section 162(m) of the Code limits the Company to a deduction for federal income tax purposes of no more than $1 million paid to certain named executive officers in a taxable year. Compensation above $1 million may be deducted if it is “performance-based compensation” within the meaning of the Code. The
166
Compensation Committee intends to continue to evaluate the effects of Code Section 162(m) and to comply with the requirements of that statute to the extent consistent with the best interests of the Company. The Compensation Committee believes that at the present time it is unlikely that the compensation paid to any named executive officer in a taxable year that is subject to the deduction limit will exceed $1 million. Therefore, the Compensation Committee has not yet established a policy for determining which forms of incentive compensation awarded to named executive officers will be designed to qualify as performance-based compensation.
CEO Compensation. During 2004, the total compensation program for Dr. Emile Loria, Chief Executive Officer of the Company, was largely based on the same components as for other senior executives of the Company, as described above in more detail. Each year the Compensation Committee reviews the Chief Executive Officer’s existing compensation arrangement, the individual performance for the calendar year under review, as well as the Company’s performance relative to its peers.
Dr. Loria’s base salary was set at $350,000 for 2004 based on the Compensation Committee’s review of his total compensation, the significance of his role at the Company and comparative data for Chief Executive Officer salaries at certain other relevant biotechnology companies. The Compensation Committee determined not to award any performance bonus to Dr. Loria, or any other executive officer of the Company, for 2003 based upon the Committee’s evaluation of performance in 2004 and because specific performance milestones and criteria were not met. In September 2003, the Compensation Committee approved retention bonuses for all of the Company’s employees as part of a special retention program following a reduction in work force. Dr. Loria received a retention bonus of $25,000 in 2003 and $50,000 in 2004.
In connection with his employment offer letter and joining our Board of Directors in January 2001, and as an inducement to accept the offer, we sold Dr. Loria 1,056,301 shares of our common stock at a purchase price of $2.50 per share, the closing price of our common stock on the Nasdaq National Market on the date of purchase. The shares were subject to vesting in equal daily installments during the four-year period following the date of purchase, and we had a right to purchase any unvested shares at the purchase price paid by Dr. Loria in the event of termination of Dr. Loria’s service to Epimmune. Dr. Loria issued us a promissory note for $2,641,000, the aggregate purchase price of the shares, which was secured by a pledge of the shares. In September 2003, Dr. Loria surrendered an aggregate of 963,740 shares of our common stock that he purchased in 2001, which shares had a fair market value of $3.17 per share upon the date of surrender, in exchange for the prepayment in full of the outstanding principal and interest under the promissory note.
In September 2003, in light of the reduction in force completed by the Company and as part of a program to retain the Company’s remaining employees and provide them with an incentive for future performance, the Compensation Committee granted Dr. Loria an option to purchase 500,000 shares of common stock of the Company under the 2000 Plan at an exercise price of $1.54, the fair market value on the date of grant, as an incentive to Dr. Loria for future performance. The option is subject to daily vesting over two years following the date of grant, except that the vesting of this option will accelerate so that the option will become immediately exercisable for all of the shares subject to the option in the event of a change of control of the Company.
From the members of the Compensation Committee of Epimmune Inc.:
| |
| | Howard E. (“Ted”) Greene, Jr. |
| | William T. Comer, Ph.D. |
| | John P. McKearn, Ph.D. |
Compensation Committee Interlocks and Insider Participation
Mr. Greene, a member of the Compensation Committee, is Chairman of our Board and was our President from July 1987 to January 1989.
167
Performance Measurement Comparison(1)
The following chart shows total shareholder return of the Nasdaq CRSP Total Return Index, or Nasdaq Broad Index, for the Nasdaq Stock Market (US Companies) and the Nasdaq CRSP Pharmaceutical Index, or Nasdaq Pharmaceutical Index (2), and for the Company as of the end of each year since December 31, 1999.
Effective July 1, 1999, the Company changed its name from Cytel Corporation to Epimmune Inc. and changed its Nasdaq ticker symbol to EPMN.
Comparison of Total Cumulative Return on Investment(3)
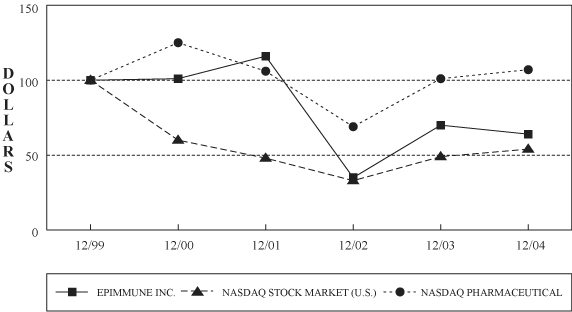
| |
| (1) | This Section is not “soliciting material,” is not deemed filed with the SEC, and is not to be incorporated by reference into any filing of the Company under the Securities Act or the Exchange Act, each as amended, whether made before or after the date hereof and irrespective of any general incorporation language contained in such filing. |
| |
| (2) | The Nasdaq Pharmaceutical Index is made up of all companies with the Standard Industrial Classification (SIC) code 283 (category description “Drugs”). Information regarding the companies comprising this index is available upon written request to Secretary, Epimmune Inc., 5820 Nancy Ridge Drive, San Diego, California 92121. |
| |
| (3) | The total return on investment (including investment of dividends) assumes $100 invested on December 31, 1999 in the Company’s common stock, the Nasdaq CRSP Total Return Index for the Nasdaq Stock (U.S. Companies) Index and the Nasdaq CRSP Pharmaceutical Index. The return shown is not necessarily indicative of future performance, and the Company will not make or endorse any predictions as to future stockholder returns. |
CERTAIN TRANSACTIONS
Our Bylaws provide that we will indemnify our directors and executive officers and may indemnify our other officers, employees and other agents to the fullest extent permitted by Delaware law. We are also empowered under our Bylaws to enter into indemnification contracts with our directors and officers and to purchase insurance on behalf of any person whom it is required or permitted to indemnify. Pursuant to this provision, we have entered into Indemnity Agreements with each of our directors and executive officers.
168
In addition, our Amended and Restated Certificate of Incorporation provides that to the fullest extent permitted by Delaware law, our directors will not be liable for monetary damages for breach of the directors’ fiduciary duty of care to us and our stockholders. This provision in the Certificate of Incorporation does not eliminate the duty of care, and in appropriate circumstances equitable remedies such as an injunction or other forms of non-monetary relief would remain available under Delaware law. Each director will continue to be subject to liability for breach of the director’s duty of loyalty to us, for acts or omissions not in good faith or involving intentional misconduct or knowing violations of law, for acts or omissions that the director believes to be contrary to our best interests or our stockholders, for any transaction from which the director derived an improper personal benefit, for acts or omissions involving a reckless disregard for the director’s duty to us or our stockholders when the director was aware or should have been aware of a risk of serious injury to us or our stockholders, for acts or omissions that constitute an unexcused pattern of inattention that amounts to an abdication of the director’s duty to us or our stockholders, for improper transactions between the director and us, and for improper distributions to stockholders and loans to directors and officers. This provision also does not affect a director’s responsibilities under any other laws, such as the federal securities laws or state or federal environmental laws.
On March 15, 2005, we entered into a Preferred Exchange Agreement with G.D. Searle, the holder of all of the outstanding shares of our preferred stock. On April 12, 2005, we entered into an Amended and Restated Preferred Exchange Agreement with G.D. Searle. Pursuant to this agreement, effective immediately prior to the closing of the Share Exchange Agreement, 859,666 shares of our Series S preferred stock and 549,622 shares of our Series S-1 preferred stock will be exchanged for an aggregate of 1,949,278 shares of our common stock.
On March 15, 2005, we entered into a Voting Agreement with our directors and executive officers pursuant to which they agreed, among other things, to vote the shares of our common stock that they hold in favor of the Exchange with the shareholders of IDM and other transactions contemplated by the Share Exchange Agreement that will be submitted for approval by our stockholders.
We have entered into certain additional transactions with our directors and officers, as described in the sections entitled“Chapter Four — Other Information for the Annual Meeting of Epimmune’s Stockholders — Compensation of Directors,” “Chapter Four — Other Information for the Annual Meeting of Epimmune’s Stockholders — Compensation of Executive Officers” and“Chapter Four — Other Information for the Annual Meeting of Epimmune’s Stockholders — Employment, Change of Control and Severance Agreements.”
HOUSEHOLDING OF PROXY MATERIALS
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for proxy statements and annual reports with respect to two or more stockholders sharing the same address by delivering a single proxy statement addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
This year, a number of brokers with account holders who are Epimmune’s stockholders will be “householding” our proxy materials. A single proxy statement will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate proxy statement and annual report, please notify your broker, direct your written request to Robert J. De Vaere, Vice President of Finance and Administration, Epimmune Inc., 5820 Nancy Ridge Drive, San Diego, California 92121 or contact Robert J. De Vaere at (858) 860-2500. Stockholders who currently receive multiple copies of the proxy statement at their address and would like to request “householding” of their communications should contact their broker.
169
CHAPTER FIVE — ADDITIONAL INFORMATION
WHERE YOU CAN FIND MORE INFORMATION
This document incorporates by reference the Annual Report on Form 10-K/A, for the year ended December 31, 2004, as well as the Quarterly Report on Form 10-Q/A for the quarter ended March 31, 2005 of Epimmune and the current Report on Form 8-K filed with the SEC on May 23, 2005. Copies of the Epimmune Annual Report on Form 10-K/A and the Quarterly Report on Form 10-Q/A are delivered with this proxy statement to our stockholders.
In addition, we file reports, proxy statements and other information with the SEC. Our stockholders may read and copy any reports, proxy statements or other information filed by Epimmune at the SEC’s Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at (800) SEC-0330.
Copies of these materials can also be obtained by mail at prescribed rates from the Public Reference Section of the Securities and Exchange Commission, 450 Fifth Street, N.W., Washington, D.C. 20549 or by calling the SEC at (800) SEC-0330. The SEC maintains a website that contains reports, proxy statements and other information regarding Epimmune. The address of the SEC website is www.sec.gov.
Reports, proxy statements and other information regarding Epimmune may also be inspected at The National Association of Securities Dealers, Inc., 1735 K Street, N.W., Washington, D.C. 20006.
Epimmune has supplied all information contained or incorporated by reference in this proxy statement relating to Epimmune.
If you are an Epimmune stockholder, you may have previously received some of the documents incorporated by reference in this proxy statement, but you can obtain any of them through Epimmune, the SEC or the SEC’s Internet website as described above. Documents incorporated by reference are available from Epimmune without charge, excluding all exhibits, unless Epimmune has specifically incorporated by reference an exhibit in this proxy statement. You may obtain documents incorporated by reference in this proxy statement by requesting them in writing or by telephone from Epimmune at the following address:
Epimmune Inc.
5820 Nancy Ridge Drive
San Diego, California 92121
Attn: Corporate Secretary
Telephone: (858) 860-2500
Any statement contained herein or in a document incorporated or deemed to be incorporated by reference into this document will be deemed to be modified or superseded for purposes of the document to the extent that a statement contained in this document modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this document.
If you are an Epimmune stockholder, you should rely only on the information contained or incorporated by reference in this proxy statement to vote on the proposals described in this proxy statement. We have not authorized anyone to provide you with information that is different from what is contained in this proxy statement. This proxy statement is dated June 30, 2005. You should not assume that the information contained in this proxy statement is accurate as of any date other than June 30, 2005.
Information on Epimmune’s Website
Information on Epimmune’s Internet website is not part of this document and you should not rely on that information in deciding whether to approve the proposals described in the proxy statement, unless that information is also in this document or in a document that is incorporated by reference in this proxy statement.
170
Information on IDM’s Website
Information on IDM’s Internet website is not part of this document and you should not rely on that information in deciding whether to approve the proposals described in the proxy statement, unless that information is also in this document or in a document that is incorporated by reference in this proxy statement.
OTHER MATTERS
The Board knows of no other matters that will be presented for consideration at the annual meeting. If any other matters are properly brought before the meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
| |
| | By Order of the Board of Directors |
| |
| |  |
| |
| | Robert J. De Vaere |
| | Vice President, Finance and Administration, |
| | Chief Financial Officer and Secretary |
June 30, 2005
A copy of Epimmune’s Annual Report to the SEC on Form 10-K/A for the fiscal year ended December 31, 2004 is available without charge upon written request to: Corporate Secretary, Epimmune Inc., 5820 Nancy Ridge Drive, San Diego, California 92121.
171
INDEX TO FINANCIAL STATEMENTS OF IDM
Audited Consolidated Financial Statements of IDM, S.A. and Subsidiaries for the Years Ended December 31, 2002, 2003 and 2004
Unaudited Interim Consolidated Financial Statements of IDM, SA and Subsidiaries for the Three Months Ended March 31, 2005
| | | | | |
| Condensed Interim Consolidated Balance Sheets | | | F-24 | |
| Condensed Interim Consolidated Statements of Operations and Comprehensive Loss | | | F-25 | |
| Condensed Interim Consolidated Statement of Cash Flows | | | F-26 | |
| Notes to the Condensed Interim Consolidated Financial Statements | | | F-27 | |
F-1
Report of Independent Registered Accounting Firm
The Board of Directors
Immuno-Designed Molecules SA
We have audited the accompanying consolidated balance sheets of Immuno-Designed Molecules, S.A. (IDM) as of December 31, 2003 and 2004, and the related consolidated statements of operations and comprehensive loss, shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2004. These consolidated financial statements are the responsibility of IDM’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit includes consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Immuno-Designed Molecules, S.A. at December 31, 2003, and 2004, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2004, in conformity with accounting principles generally accepted in the United States.
| |
| | The Independent Auditors |
| |
| | ERNST & YOUNG Audit |
| |
| | /s/Jean-Yves Jégourel |
| | |
| | ERNST & YOUNG Audit represented by |
| | Jean-Yves Jégourel, Partner |
Paris-La Défense,
March 7, 2005
F-2
IDM S.A.
Consolidated Balance Sheets
| | | | | | | | | | |
| | | Year Ended December 31, | |
| | | | |
| | | 2003 | | | 2004 | |
| | | | | | | |
| | | € | | | € | |
| | | (In thousands, except share | |
| | | and per share data) | |
| ASSETS |
| Current assets: | | | | | | | | |
| | Cash and cash equivalents | | | 33,326 | | | | 30,859 | |
| | Related party accounts receivable (Sanofi-Aventis) | | | 2,001 | | | | 1,465 | |
| | Research and development tax credit, current portion | | | 526 | | | | 531 | |
| | Prepaid expenses and other current assets | | | 1,611 | | | | 955 | |
| | | | | | | |
| Total current assets | | | 37,464 | | | | 33,810 | |
| Fixed assets - net | | | 2,264 | | | | 2,173 | |
| Intangible assets - net: | | | | | | | | |
| | Patents, trade marks and other licenses | | | 4,907 | | | | 3,916 | |
| | Medarex Licenses | | | 6,512 | | | | — | |
| | | | | | | |
| Total Intangible assets - net | | | 11,419 | | | | 3,916 | |
| Research and development tax credit, less current portion | | | 1,060 | | | | 878 | |
| Other long-term assets | | | 64 | | | | 63 | |
| | | | | | | |
Total assets | | | 52,271 | | | | 40,840 | |
| | | | | | | |
| |
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
| Current Liabilities: | | | | | | | | |
| | Accounts payable & accrued expenses | | | 2,850 | | | | 3,767 | |
| | Accrued payroll | | | 1,188 | | | | 1,419 | |
| | Related party deferred revenues (Sanofi-Aventis), current portion | | | 552 | | | | 552 | |
| | Other liabilities | | | 380 | | | | 617 | |
| | | | | | | |
| Total current liabilities | | | 4,970 | | | | 6,355 | |
| Long-term debt, less current portion | | | 109 | | | | 234 | |
| | Related party deferred revenues (Sanofi-Aventis), less current portion | | | 3,313 | | | | 2,761 | |
| Other long term liabilities | | | 134 | | | | 96 | |
| Shareholders’ equity | | | | | | | | |
| | Common stock: 13,603,674 and 15,694,446 shares of€0.1 par value authorized, issued and outstanding as of December 31, 2003 and 2004, respectively | | | 1,360 | | | | 1,569 | |
| | Additional paid-in capital | | | 129,206 | | | | 142,169 | |
| | Deferred compensation | | | (107 | ) | | | (28 | ) |
| | Accumulated other comprehensive loss | | | (160 | ) | | | (246 | ) |
| | Accumulated deficit | | | (86,554 | ) | | | (112,070 | ) |
| | | | | | | |
| Total shareholders’ equity | | | 43,745 | | | | 31,394 | |
| | | | | | | |
Total liabilities and shareholders’ equity | | | 52,271 | | | | 40,840 | |
| | | | | | | |
See notes to consolidated financial statements
F-3
IDM S.A.
Consolidated Statements of Operations and comprehensive loss
| | | | | | | | | | | | | | |
| | | Year Ending December 31, | |
| | | | |
| | | 2002 | | | 2003 | | | 2004 | |
| | | | | | | | | | |
| | | € | | | € | | | € | |
| | | (In thousands, except share and per share data) | |
| Related party research and development revenues | | | 3,571 | | | | 5,325 | | | | 4,652 | |
| Research and development expenses | | | (12,301 | ) | | | (15,616 | ) | | | (16,087 | ) |
| Impairment of patents & licenses | | | — | | | | (985 | ) | | | (6,287 | ) |
| Selling and marketing expenses | | | (1,602 | ) | | | (1,456 | ) | | | (944 | ) |
| General and administrative expenses | | | (4,796 | ) | | | (4,686 | ) | | | (5,280 | ) |
| Other operating expenses | | | — | | | | — | | | | (2,395 | ) |
| | | | | | | | | | |
| | Total operating expenses | | | (18,699 | ) | | | (22,743 | ) | | | (30,993 | ) |
| | | | | | | | | | |
| | Loss from operations | | | (15,128 | ) | | | (17,418 | ) | | | (26,341 | ) |
| Interest income | | | 1,284 | | | | 961 | | | | 561 | |
| Other income | | | 241 | | | | — | | | | — | |
| Foreign exchange gain (loss) | | | 16 | | | | 29 | | | | (17 | ) |
| | | | | | | | | | |
| Loss before income tax benefit | | | (13,587 | ) | | | (16,428 | ) | | | (25,797 | ) |
| Income tax benefit | | | 640 | | | | 208 | | | | 281 | |
| | | | | | | | | | |
| | Net loss | | | (12,947 | ) | | | (16,220 | ) | | | (25,516 | ) |
| | | | | | | | | | |
| Weighted average number of shares outstanding | | | 12,063,474 | | | | 13,516,211 | | | | 13,649,499 | |
| Basic and diluted loss per share | | | (1.07 | ) | | | (1.20 | ) | | | (1.87 | ) |
| Comprehensive Loss: | | | | | | | | | | | | |
| Net Loss | | | (12,947 | ) | | | (16,220 | ) | | | (25,516 | ) |
| Other comprehensive Loss | | | (17 | ) | | | (115 | ) | | | (86 | ) |
| | | | | | | | | | |
| Comprehensive Loss | | | (12,964 | ) | | | (16,335 | ) | | | (25,602 | ) |
| | | | | | | | | | |
See notes to consolidated financial statements
F-4
IDM S.A.
Consolidated Statements of Shareholders’ Equity
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Number of Shares | | | | | | | | | Accumulated | | | | | |
| | | Authorized, Issued and Outstanding | | | | | Additional | | | | | Other | | | | | |
| | | | | | Capital | | | Paid-In | | | Deferred | | | Comprehensive | | | Accumulated | | | Shareholders’ | |
| | | Class A | | | Class B | | | Total | | | Amount | | | Capital | | | Compensation | | | Loss | | | Deficit | | | Equity | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | € | | | € | | | € | | | € | | | € | | | € | |
| | | (In thousands, except share and per share data) | |
| At January 1, 2002 | | | 1,674,020 | | | | 10,108,369 | | | | 11,782,389 | | | | 1,178 | | | | 106,085 | | | | (232 | ) | | | (28 | ) | | | (57,387 | ) | | | 49,616 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of shares at€12.866 per share | | | — | | | | 1,554,485 | | | | 1,554,485 | | | | 156 | | | | 19,840 | | | | — | | | | — | | | | — | | | | 19,996 | |
| Conversion of Class B to Class A shares | | | 1,935,113 | | | | (1,935,113 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Deferred compensation | | | — | | | | — | | | | — | | | | — | | | | 213 | | | | (213 | ) | | | — | | | | — | | | | — | |
| Amortization of deferred compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | 157 | | | | — | | | | — | | | | 157 | |
| Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (17 | ) | | | — | | | | (17 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (12,947 | ) | | | (12,947 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| At December 31, 2002 | | | 3,609,133 | | | | 9,727,741 | | | | 13,336,874 | | | | 1,334 | | | | 126,138 | | | | (288 | ) | | | (45 | ) | | | (70,334 | ) | | | 56,805 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of shares at€12.866 per share | | | — | | | | 225,400 | | | | 225,400 | | | | 22 | | | | 2,878 | | | | — | | | | — | | | | — | | | | 2,900 | |
Exercise of warrants at€0.1 per share | | | — | | | | 41,400 | | | | 41,400 | | | | 4 | | | | 217 | | | | — | | | | — | | | | — | | | | 221 | |
| Issuance of warrants | | | — | | | | — | | | | — | | | | — | | | | 11 | | | | — | | | | — | | | | — | | | | 11 | |
| Conversion of Class B to Class A shares | | | 8,101 | | | | (8,101 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Deferred compensation | | | — | | | | — | | | | — | | | | — | | | | (38 | ) | | | 38 | | | | — | | | | — | | | | — | |
| Amortization of deferred compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | 143 | | | | — | | | | — | | | | 143 | |
| Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (115 | ) | | | — | | | | (115 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (16,220 | ) | | | (16,220 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| At December 31, 2003 | | | 3,617,234 | | | | 9,986,440 | | | | 13,603,674 | | | | 1,360 | | | | 129,206 | | | | (107 | ) | | | (160 | ) | | | (86,554 | ) | | | 43,745 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of shares at€6.32 per share | | | 2,090,772 | | | | — | | | | 2,090,772 | | | | 209 | | | | 12,982 | | | | — | | | | — | | | | — | | | | 13,191 | |
| Conversion of Class B to Class A shares | | | 243,115 | | | | (243,115 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Deferred compensation | | | — | | | | — | | | | — | | | | — | | | | (19 | ) | | | 19 | | | | — | | | | — | | | | — | |
| Amortization of deferred compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | 60 | | | | — | | | | — | | | | 60 | |
| Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (86 | ) | | | — | | | | (86 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (25,516 | ) | | | (25,516 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| At December 31, 2004 | | | 5,951,121 | | | | 9,743,325 | | | | 15,694,446 | | | | 1,569 | | | | 142,169 | | | | (28 | ) | | | (246 | ) | | | (112,070 | ) | | | 31,394 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-5
IDM S.A.
Consolidated Statements of Cash Flows
| | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | | |
| | | 2002 | | | 2003 | | | 2004 | |
| | | | | | | | | | |
| | | € | | | € | | | € | |
| | | (In thousands) | |
| Cash flows from operating activities: | | | | | | | | | | | | |
| | Net loss | | | (12,947 | ) | | | (16,220 | ) | | | (25,516 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
| | Amortization of deferred compensation | | | 157 | | | | 143 | | | | 60 | |
| | Depreciation and amortization | | | 2,551 | | | | 2,323 | | | | 2,199 | |
| | Gain related to the forgiveness of Anvar loans | | | (241 | ) | | | — | | | | — | |
| | Impairment of licenses | | | — | | | | 985 | | | | 6,287 | |
| | Foreign exchange (gain)/loss | | | (16 | ) | | | (29 | ) | | | 17 | |
| Increase (decrease) in cash from: | | | | | | | | | | | | |
| | Related party accounts receivable (Sanofi-Aventis) | | | 1,600 | | | | (1,209 | ) | | | 536 | |
| | Prepaid expenses and other current assets | | | 158 | | | | (1,054 | ) | | | 656 | |
| | Research and development tax credit receivable | | | (69 | ) | | | 94 | | | | 177 | |
| | Other long-term assets | | | 2 | | | | 1 | | | | 1 | |
| | Accounts payable and accrued expenses | | | (205 | ) | | | 1,055 | | | | 1,617 | |
| | Accrued payroll | | | 279 | | | | 206 | | | | 231 | |
| | Related party deferred revenues (Sanofi-Aventis) | | | 2,434 | | | | (552 | ) | | | (552 | ) |
| | Other liabilities | | | (232 | ) | | | 212 | | | | 199 | |
| | | | | | | | | | |
| Net cash used in operating activities | | | (6,529 | ) | | | (14,045 | ) | | | (14,088 | ) |
| Cash flow from investing activities: | | | | | | | | | | | | |
| | Purchase of fixed assets | | | (1,183 | ) | | | (348 | ) | | | (406 | ) |
| | Patent costs and acquisition of other intangibles | | | (1,113 | ) | | | (1,397 | ) | | | (486 | ) |
| | | | | | | | | | |
| Net cash used in investing activities | | | (2,296 | ) | | | (1,745 | ) | | | (892 | ) |
| Cash flow from financing activities: | | | | | | | | | | | | |
| | Proceeds from loans | | | 109 | | | | — | | | | 125 | |
| | Repayment of loans | | | (229 | ) | | | (320 | ) | | | — | |
| | Proceeds from issuance of shares | | | 19,996 | | | | — | | | | 12,491 | |
| | Proceeds from warrants | | | — | | | | 232 | | | | — | |
| | | | | | | | | | |
| Net cash provided by (used in) financing activities | | | 19,876 | | | | (88 | ) | | | 12,616 | |
| Effect of exchange rate changes on cash and cash equivalents | | | (1 | ) | | | (86 | ) | | | (103 | ) |
| | | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | 11,050 | | | | (15,964 | ) | | | (2,467 | ) |
| | | | | | | | | | |
| Cash and cash equivalents, beginning of year | | | | | | | | | | | | |
| Cash and cash equivalents, end of year | | | 38,240 | | | | 49,290 | | | | 33,326 | |
| | | | | | | | | | |
| | | | 49,290 | | | | 33,326 | | | | 30,859 | |
| | | | | | | | | | |
| Supplemental Cashflow Information: | | | | | | | | | | | | |
| Non-cash investing activities: | | | | | | | | | | | | |
| | Acquisition of Jenner Licenses | | | — | | | | 2,900 | | | | — | |
| | Settlement of accounts payable to consultants by means of the issue of 110,759 class A shares | | | — | | | | — | | | | 700 | |
F-6
IDM S.A.
Notes to Consolidated Financial Statements
(All amounts stated in thousands of euros except share and per share data)
| |
| 1. | NATURE OF BUSINESS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Immuno-Designed Molecules, S.A. (for the purposes of these notes, referred to as “IDM” or the “Company”) was incorporated as a“Société Anonyme”in 1993. IDM develops products that are designed to stimulate the immune system, destroy tumor cells after surgery or chemotherapy, and immunize patients to prevent tumor recurrence.
The Company’s lead potential product has completed a Phase III clinical trial, for the treatment of bone cancer. The Company has also several cellular therapy products in Phase II/ III, II and I/ II clinical trials for a variety of cancers including bladder and colorectal cancers. Two products are in the late stage of clinical development.
In July 2001, IDM entered into a significant collaboration with Sanofi-Aventis, a leading global pharmaceutical company, from which it has generated revenues in 2002, 2003 and 2004 (see note 2.2). The Company expects its principal sources of revenues over the next several years to be milestone payments and reimbursement of research and development expenses from its collaboration with Sanofi-Aventis. The Company is also seeking to enter into other collaborative agreements for certain products with other partners, which may provide additional sources of revenues. Accordingly, the accompanying financial statements have been prepared on the basis of the Company being an operating company.
The Company’s business is subject to significant risks, which is consistent with biotechnology companies that are conducting research in cellular therapy. These risks include, but are not limited to, uncertainties regarding research and development, access to capital, obtaining and enforcing patents, receiving regulatory approval, and competition with other biotechnology and pharmaceutical companies. The Company plans to continue to finance its operations with a combination of equity and debt financing, revenues from research and development programs and, in the longer term, product sales, if any. However, there can be no assurance that the Company will successfully develop any marketable products or, if it does, that the revenues generated from the sales of these products will be sufficient to sustain the Company’s operations.
| |
| Basis of presentation and principles of consolidation |
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries in the United States of America and Canada. IDM Inc. (Irvine, California, USA) commenced operations in 1996. IDM Biotech Ltd was created in Montreal (Quebec, Canada) in February 2000.
All intercompany accounts and transactions have been eliminated in the consolidation.
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
| |
| Foreign currency translation |
The reporting currency of the Company and its subsidiaries is the euro.
The euro is the functional currency for all IDM businesses except for its subsidiaries in the United States and Canada, for which the functional currencies are the U.S. dollar and the Canadian dollar, respectively. Foreign currency-denominated assets and liabilities for these units are translated into euros based on exchange
F-7
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
rates prevailing at the end of the period; revenues and expenses are translated at average exchange rates during the period, and shareholders’ equity accounts are translated at historical exchange rates. The effects of foreign exchange gains and losses arising from the translation of assets and liabilities of those entities where the functional currency is not the euro are included as a component of other comprehensive income.
Gains and losses resulting from foreign currency transactions are reflected in net loss. The Company does not undertake hedging transactions to cover its currency exposure.
The Company generates revenues from a collaborative agreement with Sanofi-Aventis, which consists of up-front fees, milestone payments, and ongoing research and development funding.
Non-refundable up-front payments that the Company receives in connection with collaborative research and development agreements are deferred and recognized on a straight-line basis over the research term. When the research term cannot be specifically identified from the agreement, the Company estimates it based upon its current development plan for the product. These estimates are continually reviewed and could result in a change in the deferral period, such as, for example, when the estimated development period for a product changes. As a result, the timing and amount of revenue recognized may change.
Revenues from milestone payments for products selected by collaborative partners, are recognized in full upon achievement of the relevant milestone when a fair value can be ascribed. During the development phase of a collaborative research and development agreement, the Company considers that no fair value can be ascribed to the upfront fee and any milestone payments, given the inherent uncertainty of the technological outcome at this stage of the research and development process, which does not enable the Company to make a reliable, verifiable and objective determination of the fair value of each payment. As no fair value can reasonably be ascribed, such payments are recorded as deferred revenue and are recognized over the remaining development term on a straight-line basis.
Reimbursement of ongoing research and development expenses for products selected by collaborative partners, are recognized as revenues once a product has been selected and the services have been performed and the payment is assured.
Reimbursement of research and development expenses incurred prior to selection of a product by a collaborative partner are considered as additional up-front payments and are recorded as deferred revenue and are recognized on a straight-line basis over the research term.
The Company believes that the value assigned to the funding of research and development costs incurred prior to the selection of a product by a collaborative partner is not representative of the fair value of the corresponding research and development costs incurred prior to such product selection by such collaborative partner, and as a result should not be singled out.
| |
| Research and development (“R&D”) expenses and related income tax credit |
Research and development expenses consist primarily of costs associated with the clinical trials of the Company’s products, compensation and other expenses for research and development personnel, supplies and development materials, costs for consultants and related contract research, facility costs and amortization and depreciation of patents and licenses. Expenses related to research and development are expensed as incurred.
In France, such expenses form the basis for an income tax credit, which is recorded as a current income tax benefit in the period in which the expenses are incurred. If the credit is not used to offset income taxes payable in the four years following its generation, the credit is recoverable from the government in cash.
F-8
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
In Quebec, subject to advance approval from governmental authorities, R&D expenses related to approved projects form a basis for an income tax credit, which is recorded as a current income tax benefit in the period in which the expenses are incurred. If the credit is not used to offset income taxes payable in the year following its generation, the credit is recoverable from the government in cash.
| |
| Cash and cash equivalents |
The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. Cash equivalents primarily include money market funds and short-term certificates of deposits.
| |
| Major customer and concentration of credit risk |
Accounts receivable from Sanofi-Aventis as well as cash and cash equivalents are the main sources that potentially subject the Company to a significant concentration of risk. The Company’s deposits, which are mainly kept in euro, are maintained in major French institutions. The Company does not require collateral to hedge its credit risk as the Company believes it is not exposed to a significant risk due to the financial position of Sanofi-Aventis and these financial institutions.
| |
| Prepaid expenses and other current assets. |
As of December 31, 2003 and 2004, other current assets included principally the total Value Added Tax (VAT) owed by the French Government to IDM.
For the year ended December 31, 2003, prepaid expenses consisted primarily of legal and other professional services fees incurred while preparing a capital increase contemplated to occur in the first half of 2004. There were no similar prepaid expenses capitalized as at December 31, 2004.
| |
| Fair value of financial instruments |
At December 31, 2002, 2003 and 2004, the carrying values of financial instruments such as cash and cash equivalents, trade receivables and payables, other receivables and accrued liabilities and the current portion of long-term debt approximated their market values, based on the short-term maturities of these instruments.
Intangible assets principally include patent registration costs and acquisition of licenses. Costs associated with licenses acquired to use pharmaceutical products from third parties prior to receipt of regulatory approval to market the products are capitalized if:
| | |
| | • | the licenses are to be used in the scope of an R&D program which is in Phase III of clinical development at the time the license is acquired. At this stage the absence of toxicity has been assessed and the Company reasonably expects to achieve market approval for the program, and |
| |
| | • | the licenses can be used in other specifically identified R&D programs to be commenced after the date they were acquired. |
These costs are capitalized and amortized on a straight-line basis over the useful life of the patent or license, which is estimated to be 10 years and corresponds to the expected technology life. For the Medarex licenses, management estimated the economic useful lives using the starting date of cash flow projections in the licenses valuation process and the estimated period during which the technology will generate substantial revenues, but without considering any extension that might result from life cycle management.
F-9
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
Fixed assets — net are stated at cost less accumulated depreciation and are depreciated on a straight-line basis over their estimated useful lives as follows:
| | | | | |
| Laboratory Equipment: | | | 5 years | |
| Computer Equipment: | | | 3 years | |
| Furniture & Office Equipment: | | | 8 years | |
Leasehold improvements are amortized over the shorter of their estimated useful lives or the remaining term of the related lease, being nine years.
| |
| Impairment of long lived assets |
Long-lived assets including intangible assets, other than indefinite lived intangibles, to be held and used are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values.
The Company calculates earnings per share in accordance with Statement of Financial Accounting Standards No. 128“Earnings Per Share” (“SFAS 128”). Basic earnings per share are computed by dividing net income by the weighted average number of shares outstanding during the year. Diluted earnings per share are computed by adjusting weighted average outstanding shares, assuming conversion of all potentially dilutive securities and awards. As net losses have been reported in all periods presented, the dilutive effects of stock options and warrants have been excluded from the calculation of net loss per share, as they are antidilutive.
The liability method is used in accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is recorded if it is more likely than not that some portion or all of the deferred tax assets will not be realized.
The Company operates in one segment, immunotherapy research. The majority of the Company’s assets are located in France. In addition, operating losses are primarily generated from the French operations.
| |
| Warrants and stock options granted to employees, directors and consultants |
IDM accounts for stock options and warrants granted to employees in accordance with the provisions of Accounting Principles Board Opinion No. 25 (“APB 25”),“Accounting for Stock Issued to Employees”. Under APB 25, no compensation expense is recognized for stock options and warrants issued to employees with an exercise price equal to or greater than the fair value of the underlying shares. Options and warrants issued with an exercise price less than the fair value result in deferred compensation which is recorded within shareholder’s equity and amortized to expense over the vesting period.
Statement of Financial Accounting Standards No. 123 (“SFAS 123”),“Accounting for Stock-Based Compensation”provides an alternative to APB 25 in accounting for stock-based compensation issued to
F-10
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
employees. SFAS 123 provides for a fair-value-based method of accounting for employee stock options and similar equity instruments. Companies that elect to continue to account for stock-based compensation arrangements under APB 25 are required by SFAS 123 to disclose the pro forma effect on net income and net income per share as if the fair-value-based method proposed by SFAS 123 had been adopted.
Pro forma information regarding net loss is required by SFAS 123 as if the Company had accounted for its employee stock options under the fair value method. The fair value of the Company’s options was estimated at the date of grant using the minimum value method, with the following assumptions for 2002, 2003 and 2004:
| | | | | | | | | | | | | |
| Weighted Average Information | | 2002 | | | 2003 | | | 2004 | |
| | | | | | | | | | |
| Risk-free interest rate | | | 5.02 | % | | | 4.33 | % | | | 4.39 | % |
| Dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
| Expected option life | | | 7 | | | | 7 | | | | 7 | |
Because the determination of the fair value of the Company’s options is based on assumptions described above, and because additional option grants are expected to be made in future periods, this pro forma information is not likely to be representative of the pro forma effects on reported net income or loss for future periods.
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the options’ vesting period. The following table illustrates the net loss that would have occurred had the Company accounted for its stock options under the provisions of SFAS 123.
Had compensation cost for the company’s stock option plan been determined on the fair value method set forth in SFAS 123, the Company’s net loss and basic and diluted net loss per share would have been changed to the pro forma amounts indicated below:
| | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | | |
| | | 2002 | | | 2003 | | | 2004 | |
| | | | | | | | | | |
| | | € | | | € | | | € | |
| Net loss as reported | | | (12,947 | ) | | | (16,220 | ) | | | (25,516 | ) |
| | Add: stock based employee compensation expense included in reported net loss | | | 157 | | | | 143 | | | | 60 | |
| | Deduct: total stock-based employee compensation expense determined under fair value based method for all awards | | | (951 | ) | | | (623 | ) | | | (843 | ) |
| | | | | | | | | | |
| Pro forma net loss | | | (13,741 | ) | | | (16,700 | ) | | | (26,299 | ) |
| | | | | | | | | | |
| Net loss per share: | | | | | | | | | | | | |
| | Basic and diluted - as reported | | | (1.07 | ) | | | (1.20 | ) | | | (1.87 | ) |
| | | | | | | | | | |
| | Basic and diluted - pro forma | | | (1.14 | ) | | | (1.24 | ) | | | (1.93 | ) |
| | | | | | | | | | |
Comprehensive loss is comprised of net loss and other comprehensive loss (or OCL). OCL includes certain changes in stockholders’ equity that are excluded from net loss such as foreign currency translation adjustment and unrealized gains and losses on available-for-sale securities. Comprehensive loss for the years ended December 31, 2002, 2003 and 2004, has been reflected in the consolidated statements of operations. The components of accumulated other comprehensive loss consists solely of foreign currency translation adjustments.
F-11
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
On December 16, 2004, the Financial Accounting Standards Board (FASB) issued FASB Statement No. 123 (revised 2004), Share-Based Payment, which is a revision of FASB Statement No. 123, Accounting for Stock-Based Compensation. Statement 123(R) supersedes APB Opinion No. 25, Accounting for Stock Issued to Employees, and amends FASB Statement No. 95, Statement of Cash Flows. Generally, the approach in Statement 123(R) is similar to the approach described in Statement 123. However, Statement 123(R) requires all share-based payments to employees, including grants of employee stock options and warrants, to be recognized in the income statement based on their fair values. Pro forma disclosure is no longer an alternative.
Statement 123(R) is effective for nonpublic companies in the fiscal years beginning after December 15, 2005 Currently, as permitted by Statement 123, the Company accounts for share-based payments to employees using Opinion 25’s intrinsic value method and, as such, no compensation expense is recognized for stock options and warrants with an exercise price equal to or greater than the fair value of the underlying shares.
In Note 1 to our consolidated financial statements, we described the impact on the pro forma net loss and loss per share using the fair values at the date of grant based on the fair value method, excluding the impact of stock volatility.
Under statement 123R, non-public entities that used the minimum value method to measure compensation costs for pro forma disclosure purposes, as in our case, will be required to use the prospective method. Under the prospective method, non-public entities would continue to account for non-vested awards outstanding at the date of the adoption of Statement 123R in the same manner as they had been accounted for prior to adoption for financial recognition purposes. All awards granted, modified or settled after the date of adoption will be accounted for using the measurement, recognition, and attribution provisions of Statement 123R.
| |
| 2. | RESEARCH AND DEVELOPMENT AGREEMENTS |
In March 2003, The Company entered into an asset purchase agreement with Jenner Biotherapies, Inc. (“Jenner”), a biotechnology company devoted to the development of cancer vaccines and macrophage activators. Pursuant to the terms of the agreement, the Company purchased Jenner’s lead product candidate, Mepact,. This asset was acquired by issuing 225,400 shares of the Company’s Class B ordinary shares, per value€0.10 each. The asset purchase was consummated in April 2003.
In accordance with EITF 96-18 “Accounting For Equity Instruments That Are Issued to Other Than Employees For Acquiring, or in conjunction with Selling, Goods or Services,” the Mepact license was valued at fair value for an amount of€2,900 on the date the shares were issued to Jenner by the Company. The fair value of the shares was recorded as common stock and additional paid-in capital, and represented the basis for the total valuation of the license acquired. Total consideration was allocated to the Mepact license based on its fair value at the date of issuance, which was also valued by the Company, based on estimated future cash flows and an expected rate of success, as determined by IDM’s management. The fair values allocated to the license with alternate future use amounted to€2,900 and is reflected in intangible assets.
The license capitalized from Jenner is being amortized over 10 years, which is management’s estimate of the expected life of future products developed from the use of the license.
IDM’s direct research and development expenses related to Mepact amounted to approximately€2.0 million and€0.4 million in 2004 and 2003, respectively.
F-12
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
| |
| 2.2. Agreement with Sanofi-Aventis (Related Party) |
In July 1999, the Company entered into an agreement with Sanofi-Aventis (the “1999 Agreement”) under which the Company has the right to use certain proprietary molecules and certain patents, licenses and intellectual property of Sanofi-Aventis in exchange for 145,660 Class B IDM shares with attached warrants. Each warrant gave Sanofi-Aventis the right to buy two of the Company’s shares at€5.34 per share (see note 6.3).
The assets provided by Sanofi-Aventis were valued at€778 and expensed during the year ended December 31, 2000, because of the absence of any alternative future use, as well as uncertainty of future cash flows.
In July 2001, the Company signed a ten-year agreement (the “July 2001 agreement”) with Sanofi-Aventis to cooperate in cellular immunotherapy research for the development and marketing of immunologic treatments for cancers. Under this agreement, Sanofi-Aventis has the right to select up to twenty cell-drug development programs (individually “an option”) from IDM’s line of research and development activities. The Company will undertake preclinical development, and if Sanofi-Aventis exercises its option, Sanofi-Aventis will finance the clinical development and have exclusive worldwide marketing rights for the selected drugs, if the clinical trials are successful.
For each exercised option, Sanofi-Aventis will pay an initial nonrefundable upfront payment, followed by milestone payments upon the completion of Phase I, and Phase II clinical trials, and a fee upon Sanofi-Aventis exercising an exclusive license option. In addition, Sanofi-Aventis will also reimburse all corresponding research and development expenses for each program that is selected. Under certain circumstances, Sanofi-Aventis can exercise two of the twenty potential options under which several of the required payments will be waived. Sanofi-Aventis can terminate its involvement in any program at any time without penalty. If this occurs, the Company’s obligations with respect to that program will be waived, and the Company will be able to proceed with the development program and commercialize the product on its own. None of the proceeds are refundable to Sanfofi-Aventis in the event of termination.
At all times, the Company retains the intellectual property rights attached to the immunological treatments developed in programs subject to the July 2001 agreement and will grant Sanofi-Aventis an option for an exclusive worldwide license for the commercialization of each treatment. If Sanofi-Aventis exercises the commercialization option, a nonrefundable fee will be due to IDM upon exercise, followed by milestone payments, based on the potential market size for the treatment. During the commercialization phase, IDM will manufacture the treatment.
On November 30, 2001, the Company entered into a new agreement with Sanofi-Aventis, which replaces and supercedes the 1999 Agreement and adds new clauses to the July 2001 Agreement.
On December 21, 2001, Sanofi-Aventis exercised its first option on the ongoing melanoma development program Uvidem (Uvidem option). Pursuant to the terms of the agreement, IDM received: (i) up-front fees of€2,000; (ii) a completion of Phase I milestone payment of€2,000 since the program was already in Phase II and (iii) reimbursement of all research and development costs incurred from 1999 through December 2001, which approximated€1,900. Repayment received for past R&D expenses incurred by IDM prior to the selection of an option by Sanofi-Aventis are considered as an additional upfront fee. IDM believes that no specific fair value can be ascribed to each of these individual payments, given that the technological outcome of the development program has not been assured and as such cannot be accounted for separately. The revenue from these three payments has been recognized over the remaining program development period, which is estimated to be nine years. The estimated development period for Uvidem was increased in 2003 from 3.5 years to 9 years to reflect IDM and Sanofi-Aventis’ decision to enhance the clinical development program.
F-13
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
Revenue recognized for the years ending December 31, 2002, 2003 and 2004, under the Sanofi-Aventis agreement, by source, is as follows:
| | | | | | | | | | | | | |
| | | 2002 | | | 2003 | | | 2004 | |
| | | | | | | | | | |
| Amortization of up-front fees | | | 571 | | | | 176 | | | | 176 | |
| Amortization of Phase I milestone payments | | | 334 | | | | 208 | | | | 208 | |
| Amortization of initial R&D expenses from 1999 to 2001 | | | 536 | | | | 167 | | | | 167 | |
| Reimbursment of current R&D expenses | | | 2,130 | | | | 4,774 | | | | 4,101 | |
| | | | | | | | | | |
| Total Revenue | | | 3,571 | | | | 5,325 | | | | 4,652 | |
| | | | | | | | | | |
In October of 2002, the Company issued 1,554,485 new shares at a price of€12.866 per share to Sanofi-Aventis for€20,000 in total.
In December of 2004, the Company issued 1,582,279 new shares at a price of€6.32 per share to Sanofi-Aventis for€10,000 in total. As a result of this investment, as of December 31, 2004, Sanofi-Aventis owns 20.91% of the Company’s outstanding common shares.
IDM’s direct research and development expenses related to Uvidem amounted to approximately€2.5 million,€2.7 million and€1.7 million in 2004, 2003 and 2002, respectively.
| |
| 2.3. Agreements with Medarex |
In December 1993, the Company entered into a research, development and commercialization agreement with Medarex. This agreement was subsequently amended and restated on July 21, 2000.
In July 2000, the Company consummated several interrelated agreements with Medarex (collectively, the “Arrangement”). Under the Arrangement, Medarex paid to the Company $2,000 (€2,300) in cash, released the Company from obligations under the 1993 research, development and commercialization agreement, and granted exclusive and non-exclusive worldwide licenses for the use, manufacturing and commercialization of four specific antibodies developed by Medarex. In return, the Company issued to Medarex 7,528 Class B shares and 192,278 units (discussed further below) that consist of Class B shares with warrant instruments attached that are ultimately convertible or redeemable only into Class B common shares of the Company. Each of the instruments issued, Class B shares and the units, were independently valued at $10.01 (€11.46) per share or per unit, respectively. In addition, the Company agreed to expend, through 2003, $2,000 (equal to€2,300 in July 2000) towards the further research and development of products incorporating certain of the specific antibodies licensed from Medarex.
The units are a specific form of equity instrument under French law known as “Actions à Bons de Soucription d’Obligations Convertibles ou Remboursables en Actions — ABSOCRA.” These instruments are, in substance, IDM common shares with warrants attached that are exercisable into intermediate instruments that are, in turn, only convertible or redeemable into Class B common shares of IDM. Each unit comprises one Class B share and includes 19 warrants, each convertible into one class B share of IDM at a price of $10.01 (€9.55) beginning September 11, 2002.
The warrants will be convertible at one time and will be convertible or redeemable into Class B shares six months after the exercise date and will expire on September 11, 2010. Upon exercise of the warrants and conversion or redemption of the resulting bonds through the issuance of shares, Medarex would own an additional 3,653,282 Class B shares of IDM and would therefore own 23.09% of IDM capital on a fully diluted basis. The exercise price is to be made through the offset of royalties owed to Medarex by IDM for the development of future products from the use of Medarex licenses. Accordingly, no cash consideration is expected from the exercise and ultimate conversion of the warrants into common shares.
F-14
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
In accordance with EITF 96-18 “Accounting For Equity Instruments That Are Issued to Other Than Employees For Acquiring, or in conjunction with Selling, Goods or Services,” the ABSOCRA were valued at fair value for an amount of€41,246 on the date of their issuance. The fair value of the ABSOCRA was recorded as common stock and additional paid in capital, and represents the basis for the total valuation of the licenses acquired. Total consideration was allocated to each license and to the repurchase of a commercialization option initially granted by IDM to Medarex, based on their respective fair values based on estimated future cash flows and an expected rate of success, as determined in accordance with IDM’s management. The fair values allocated to licenses with alternative future use amounted to€14,354 and were reflected in intangible assets. The amounts pertaining to the cancellation of the original commercialization agreement of€6,310 and to additional licenses with no alternative future use in the amount of€20,582 were charged directly to operating results.
The licenses acquired from Medarex and capitalized are being amortized over 10 years, which is management’s estimate of the expected life of future products developed from the use of the respective licenses. The Company reviews intangible assets for impairment whenever impairment indicators are present. During the year ended December 31, 2004,€5,540 were recorded as an impairment charge.
In July 2001 and December 2002, the Agreement was further amended in order to extend the expiration date of an option that enables the Company to negotiate licenses on other antibodies with Medarex.
IDM’s direct research and development expenses related to Osidem amounted to approximately€0.1 million,€0.6 million and€1.4 million in 2004, 2003 and 2002, respectively.
| |
| 3. | INTANGIBLE ASSETS — NET |
The Company’s intangible assets-net consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | As of December 31, 2004 | |
| | | As of December 31, 2003 | | | | |
| | | | | | | | Accumulated | | | |
| | | | | Accumulated | | | | | | | Amortization and | | | |
| | | Original Cost | | | Amortization | | | Net | | | Original Cost | | | Impairment | | | Net | |
| | | | | | | | | | | | | | | | | | | |
| | | € | | | € | | | € | | | € | | | € | | | € | |
| Patents | | | 1,952 | | | | (585 | ) | | | 1,367 | | | | 2,330 | | | | (1,440 | ) | | | 890 | |
| Trade marks | | | 399 | | | | (189 | ) | | | 210 | | | | 440 | | | | (212 | ) | | | 228 | |
| Other licenses(1) | | | 4,177 | | | | (847 | ) | | | 3,330 | | | | 3,694 | | | | (896 | ) | | | 2,798 | |
| Medarex licenses(1) | | | 14,354 | | | | (7,842 | ) | | | 6,512 | | | | 14,354 | | | | (14,354 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | |
| Total | | | 20,882 | | | | (9,463 | ) | | | 11,419 | | | | 20,818 | | | | (16,902 | ) | | | 3,916 | |
| | | | | | | | | | | | | | | | | | | |
| |
| (1) | In 2000, the Company acquired licenses from Medarex, which were capitalized for an amount of€14,354 (see note 2.3). In 2002, 2003 and 2004, these licenses were reviewed for impairment. |
| |
| | In 2004, the Company recorded an impairment charge of€5,542 relating to the remaining carrying value of the Medarex licenses. This impairment charge was related to IDM’s decision not to further pursue a development program in connection with the MDX-210 antibody, an antibody used in the Company’s Osidem-2 product candidate. |
| |
| | Following a successful Phase I/ II clinical trial of Osidem, the Company had initiated Phase III clinical trials of the product in May 2000 in Europe and Australia. It also received approval for a Phase II clinical trial in the United States in April 2002. This approval required that the product be manufactured in a frozen form in compliance with Good Manufacturing Practice (“GMP”). At that time, all of the Company’s products, with the exception of Osidem, were frozen and manufactured according to the FDA’s GMP standards. The Company therefore decided to stop the clinical trials underway in Europe |
F-15
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
| |
| | and Australia in order to begin work immediately on a frozen version of Osidem, known as Osidem-2, to be manufactured in compliance with the FDA’s requirements. |
| |
| | In September 2003, upon successful preclinical testing of Osidem-2, the Company terminated the Phase III studies of Osidem in order to start a new clinical development program for Osidem-2. The Company intended to either pursue the development of Osidem-2 on its own, subject to appropriate financing, or seek a strategic partnership to explore the potential of Osidem-2 as a first-line treatment for advanced ovarian cancer. |
| |
| | In September 2004, without new financing, the Company decided not to pursue its Osidem-2 development program. In the absence of other available collaborations or strategic partnerships to continue the development of the product candidate, the Company considered that no commercially viable alternative future use existed and accordingly, the fair value of the license was deemed to be zero. The Company impaired the remaining value of the corresponding license for€5.5 million, as of September 30, 2004. |
| |
| | In 2003 and 2004, following a review of other patents and licenses, the Company recorded additional impairment charges of€985 and€745, respectively. |
| |
| | The Company recorded amortization expense of€1,717,€1,576 and€1,702 in 2002, 2003 and 2004, respectively. The estimated amortization expense for each of the succeeding 5 years are as follows: 2005:€515; 2006:€504; 2007:€495; 2008:€487; and 2009:€469. These amounts may vary as acquisitions and disposals occur in the future. |
| |
| | During 2003, the main acquisition of the Company was the Jenner licenses for an amount of€2,900. The amortization period of these licenses is 10 years. (See note 2.1) |
The Company’s fixed assets consisted of the following:
| | | | | | | | | | |
| | | As of December 31, | |
| | | | |
| | | 2003 | | | 2004 | |
| | | | | | | |
| | | € | | | € | |
| Laboratory equipment | | | 1,929 | | | | 2,011 | |
| Computer equipment | | | 1,131 | | | | 1,313 | |
| Furniture and other equipment | | | 740 | | | | 787 | |
| Leasehold improvements | | | 1,471 | | | | 1,566 | |
| | | | | | | |
| | Total fixed assets | | | 5,271 | | | | 5,677 | |
| Less accumulated depreciation and amortization | | | (3,007 | ) | | | (3,504 | ) |
| | | | | | | |
| | Fixed assets - net | | | 2,264 | | | | 2,173 | |
| | | | | | | |
Depreciation and amortization expense for the years ended December 31, 2002, 2003 and 2004 approximated,€835,€745 and€497, respectively.
F-16
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
| |
| 5. | CASH AND CASH EQUIVALENTS |
The Company’s cash and cash equivalents consisted of the following:
| | | | | | | | | |
| | | As of December 31, | |
| | | | |
| | | 2003 | | | 2004 | |
| | | | | | | |
| | | € | | | € | |
| Money market funds | | | 119 | | | | 345 | |
| Cash, including certificates of deposit | | | 33,207 | | | | 30,514 | |
| | | | | | | |
| Total cash and cash equivalents | | | 33,326 | | | | 30,859 | |
| | | | | | | |
As of December 31, 2004, the authorized, issued and outstanding share capital of the Company consisted of 15,694,446 ordinary shares with a nominal value of€0.1 per share, of which 5,951,121 were Class A shares and 9,743,325 were Class B shares. Both categories have the same rights except that Class B shares have certain additional rights relating to board representation and rights to communication of Company financial information. These rights are in effect until the number of Class B shares represent less than 20% of the non-diluted number of shares of the Company. Class B shares are automatically converted into Class A shares in case of transfer of ownership.
In December 2004, the Company issued 2,090,772 Class A shares at a price of€6.32 per share, which were acquired by Sanofi-Aventis (see note 2) and existing shareholders, resulting in net proceeds of€12,491.
In December 2004, the Company issued 110,759 Class A shares at a price of€6.32 in order to settle accounts payable to legal consultants.
In August 2003, following the exercise of 2,070 warrants, 41,400 Class B shares were issued at a price of€5.34 per share resulting in proceeds of€221.
In April 2003, the Company issued 225,400 Class B shares at the price of€12.866 for the acquisition of an asset from Jenner Biotherapies Inc (see note 2).
In October 2002, the Company issued 1,554,485 Class B shares at a price of€12.866 per share, which were acquired exclusively by Sanofi-Aventis (see note 2), resulting in net proceeds of€19,996.
| |
| 6.2. Preemptive subscription rights |
Shareholders have preemptive rights to subscribe for additional shares issued by the Company for cash on apro ratabasis. Shareholders may waive such preemptive subscription rights at an extraordinary general meeting of shareholders under certain circumstances. Preemptive subscription rights, if not previously waived, are transferable during the subscription period relating to a particular offer of shares.
| |
| 6.3. Warrants granted to investors |
In June 2003, the Company issued 10,000 Class B warrants to a member of the Scientific Advisory Board at a subscription price of€1.10 per warrant. These warrants were totally subscribed in June 2003, vested immediately and expire in June 2008. Each warrant allows the holder to purchase one Class B share at an exercise price of€12.866 per share. As at December 31, 2004, none of these warrants were exercised.
Pursuant to the Agreement signed in July 2000 with Medarex (see Note 2.3), the Company issued in September 2000 to Medarex 192,278 units (see note 6.1). Each unit comprises one Class B share and 19
F-17
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
warrants that expire in September 2010. Upon exercise, the warrants are ultimately redeemed in Class B shares for no additional cash consideration. The units including the Class B shares and warrants were valued at€11.29 per share and were issue in exchange for various licenses (Note 2). As of December 31, 2003, none of these warrants have been exercised.
Pursuant to the agreement concluded with Sanofi-Aventis in July 1999 (see Note 2.2.), the Company’s shareholders authorized on January 7, 2000, the issuance of 145,660 Class B shares with two categories of warrants to Sanofi-Aventis at a price of€5.34, which are exercisable respectively upon first Phase III clinical trial being undertaken by the Company and upon of regulatory approval. These warrants expire in January 2010 and will be cancelled if Sanofi-Aventis decides not to exercise the corresponding options attached to each warrant.
In January 1998, the Company issued 8,745 Class A warrants at a price of€0.21 per warrant. During 1998, certain directors and external consultants, respectively, subscribed 7,500 and 1,245 warrants. These warrants vested immediately and expire in January 2006. Each warrant allows the holder to purchase 20 Class A share at an exercise price of€2.88 per share. One of these warrants was exercised in September 2001.
A summary of the activity for warrants granted to external consultants is presented below:
| | | | | | | | | | |
| | | Shares Underlying | | | Weighted Average | |
| | | Warrants Subscribed | | | Exercise Price | |
| | | not yet Exercised | | | in€ | |
| | | | | | | |
| Balance as of January 1, 2002 | | | 218,598 | | | | 3.62 | |
| | | | | | | |
| | Issued | | | — | | | | — | |
| | Subscribed | | | — | | | | — | |
| | Exercised | | | — | | | | — | |
| | Cancelled | | | (7,542 | ) | | | 11.29 | |
| | | | | | | |
| Balance as of December 31, 2002 | | | 211,056 | | | | 3.35 | |
| | | | | | | |
| | Issued | | | — | | | | — | |
| | Subscribed | | | 10,000 | | | | 12.87 | |
| | Exercised | | | — | | | | — | |
| | Cancelled | | | (41,400 | ) | | | 5.34 | |
| | | | | | | |
| Balance as of December 31, 2003 | | | 179,656 | | | | 3.42 | |
| | | | | | | |
| | Issued | | | — | | | | — | |
| | Subscribed | | | — | | | | — | |
| | Exercised | | | — | | | | — | |
| | Cancelled | | | — | | | | — | |
| | | | | | | |
| Balance as of December 31, 2004 | | | 179,656 | | | | 3.42 | |
| | | | | | | |
Warrants granted to external consultants which are exercisable as of December 31, 2004, are as follows:
| | | | | | | | | |
| Number of Shares | | Exercise Price in | | | |
| | | € | | | Expiration Date | |
| | | | | | | |
| 169,656 Class A shares | | | 2.88 | | | | January 2006 | |
| 10,000 Class B shares | | | 12.87 | | | | June 2008 | |
F-18
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
| |
| 6.4. Stock options and warrant granted to employees |
In August 1998, the Company’s shareholders approved the 1998 IDM Stock Option Plan (the “1998 IDM Stock Option Plan”) and authorized the board of directors to grant through August 2003 stock options to purchase a number of Class A shares such that the total stock options granted to employees could not exceed 5% of the fully diluted number of shares of the Company. These stock options expire ten years after the grant date, and vest ratably over five years after the grant date subject to continued employment. Upon exercise, the resale of the corresponding share is restricted until 5 years after the grant date. This Stock Option Plan was cancelled in October 2000 and replaced by the IDM 2000 Stock Option Plan.
In October 2000, the shareholders approved the 2000 IDM Stock Option Plan and authorized the board of directors to grant through October 2005 employee stock options to purchase a maximum of 1,000,000 Class A shares, to be issued or to be repurchased by the Company. The options expire ten years after the grant date, and vest ratably over four years after grant date subject to continued employment. Each stock option allows the holder to purchase one share. Upon exercise, the resale of the corresponding share is restricted until 4 years after the exercise date.
A summary of stock options and warrant activity under all plans is as follows:
| | | | | | | | | | | |
| | | | | | | Weighted | |
| | | | | | | Average | |
| | | | | | | Exercise | |
| | | | | Number of | | | Price in | |
| | | | | Shares | | | € | |
| | | | | | | | | |
| Balance at January 1, 2002 | | | | | 530,618 | | | | 6.85 | |
| | | | | | | | | |
2002 Activity: | | | | | | | | | | |
| | | Granted | | | 525,200 | | | | 11.11 | |
| | | Exercised | | | — | | | | — | |
| | | Cancelled | | | (7,800 | ) | | | 7.56 | |
| | | | | | | | | |
| Balance at December 31, 2002 | | | | | 1,048,018 | | | | 8.97 | |
| | | | | | | | | |
2003 Activity: | | | | | | | | | | |
| | | Granted | | | 363,900 | | | | 12.87 | |
| | | Exercised | | | — | | | | — | �� |
| | | Cancelled | | | (250,897 | ) | | | 10.05 | |
| | | | | | | | | |
| Balance at December 31, 2003 | | | | | 1,161,021 | | | | 9.22 | |
| | | | | | | | | |
2004 Activity: | | | | | | | | | | |
| | | Granted | | | 71,000 | | | | 10.32 | |
| | | Exercised | | | — | | | | — | |
| | | Cancelled | | | (69,800 | ) | | | 12.29 | |
| | | | | | | | | |
| Balance at December 31, 2004 | | | | | 1,162,221 | | | | 9.13 | |
| | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, | |
| | | | |
| | | 2004 | | | 2003 | | | 2002 | |
| | | | | | | | | | |
| | | | | Weighted | | | | | Weighted | | | | | Weighted | |
| | | | | Average | | | | | Average | | | | | Average | |
| | | Shares | | | Exercise | | | | | Exercise | | | | | Exercise | |
| | | Underlying | | | Price in | | | Shares | | | Price in | | | Shares | | | Price in | |
| | | | | | € | | | Underlying | | | € | | | Underlying | | | € | |
| | | | | | | | | | | | | | | | | | |
| Exercisable | | | 670,971 | | | | 8.78 | | | | 458,746 | | | | 7.8 | | | | 251,422 | | | | 5.34 | |
| Available for future grants | | | 134,603 | | | | — | | | | 136,603 | | | | — | | | | 331,206 | | | | — | |
F-19
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
The following table summarizes information about stock options and warrants outstanding as of December 31, 2004:
| | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding | | | Options Exercisable | |
| | | | | | | |
| | | | | Weighted | | | | | | | Weighted | |
| | | | | Average | | | Weighted | | | | | Average | |
| | | Number | | | Remaining | | | Average | | | Number | | | Exercise | |
| | | Outstanding | | | Contractual | | | Exercise | | | Exercisable | | | Price in | |
| Exercise Prices | | As of 12/31/04 | | | Life (In Years) | | | Price in€ | | | As of 12/31/04 | | | € | |
| | | | | | | | | | | | | | | | |
| 5.34 | | | 296,824 | | | | 4.79 | | | | 5.34 | | | | 296,824 | | | | 5.34 | |
| 11.1134 | | | 491,997 | | | | 7.19 | | | | 11.11 | | | | 297,422 | | | | 11.11 | |
| 12.866 | | | 302,400 | | | | 8.75 | | | | 12.87 | | | | 76,725 | | | | 12.87 | |
| 10.5 | | | 68,000 | | | | 9.46 | | | | 10.5 | | | | — | | | | 10.5 | |
| 6.32 | | | 3,000 | | | | 9.73 | | | | 6.32 | | | | — | | | | 6.32 | |
| | | | | | | | | | | | | | | | |
Total | | | 1,162,221 | | | | 7.13 | | | | — | | | | 670,971 | | | | — | |
| | | | | | | | | | | | | | | | |
| |
| 7. | LONG-TERM DEBT, LESS CURRENT PORTION |
The Company’s long-term debt consist of an interest-free, loan from a French government agency, Anvar, of€109 at December 31, 2003, and€234 at December 31, 2004. The loan matures in 2007.
| | | | | | | | | |
| | | As of | |
| | | December 31, | |
| | | | |
| | | 2003 | | | 2004 | |
| | | | | | | |
| | | € | | | € | |
| | | (In thousands) | |
| Interest-free loan from governmental agencies | | | 109 | | | | 234 | |
| | | | | | | |
In November 2000, the Company received an interest-free conditionally reimbursable loan from Anvar for an amount of€305, in connection with the Company’s planned initial public offering. In accordance with the terms of the agreement, the Company repaid€64 of the principal balance in 2001. After receiving approval from Anvar in 2002, the outstanding obligation of€241 was forgiven and was recognized as other income in 2002.
| |
| 8. | INCOME TAXES AND INCOME TAX CREDITS |
For financial reporting purposes, loss before income tax benefit includes the following components:
| | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | | |
| | | 2002 | | | 2003 | | | 2004 | |
| | | | | | | | | | |
| | | € | | | € | | | € | |
| France | | | (10,218 | ) | | | (10,434 | ) | | | (20,670 | ) |
| Canada | | | (864 | ) | | | (558 | ) | | | (132 | ) |
| United States | | | (2,505 | ) | | | (5,436 | ) | | | (4,995 | ) |
| | | | | | | | | | |
| | Total | | | (13,587 | ) | | | (16,428 | ) | | | (25,797 | ) |
| | | | | | | | | | |
Income tax benefits for the years 2002, 2003 and 2004, comprises solely of research and development credits.
F-20
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
The Company has no deferred tax liabilities. Significant components of the Company’s deferred tax assets consist of the following:
| | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | | |
| | | 2002 | | | 2003 | | | 2004 | |
| | | | | | | | | | |
| | | € | | | € | | | € | |
| Deferred tax assets: | | | | | | | | | | | | |
| | Net operating loss carryforwards | | | 14,325 | | | | 20,136 | | | | 30,734 | |
| | Research and development costs capitalized and amortized for tax purposes | | | 190 | | | | 63 | | | | — | |
| | | | | | | | | | |
| Total deferred tax assets | | | 14,515 | | | | 20,199 | | | | 30,734 | |
| Valuation allowances | | | (14,515 | ) | | | (20,199 | ) | | | (30,734 | ) |
| | | | | | | | | | |
| | Net deferred tax assets | | | — | | | | — | | | | — | |
| | | | | | | | | | |
Due to its history of losses, the Company does not believe that sufficient objective, positive evidence exists to conclude that recoverability of its net deferred tax assets is more likely than not. Consequently, the Company has provided valuation allowances covering 100% of its deferred tax assets.
As of December 31, 2004, the Company has French net operating loss carryforwards of approximately€71,738 which have no expiration date.
The Company has U.S. net operating loss carryforwards of approximately€11 158 ($15 219) which expire in the years 2016 through 2024 for federal tax purposes, and€10 084 ($13 755) which expire in the years 2013 through 2014 for state tax purposes.
The Company also has Canadian net operating loss carryforwards of approximately€2,030 (CND 3,335),€1,208 (CND 1,985) which expire in the years 2007 through 2011 and€822 (CND 1,350) which have no expiration date for federal tax purposes and, for provincial tax purposes,€2,300 (CND 3,778),€1,143 (CND 1,878) which expire in the years 2007 through 2011, and€1,157 (CND 1,900) which have no expiration date.
The utilization of these net operating loss carryforwards is limited to the future operations of the Company in the tax jurisdictions in which such net operating losses arose.
The Company has a French and Canadian research income tax receivable of€281 (€279 for IDM SA and€2 for IDM-Biotech Ltd.) for the year ended December 31, 2004. The Canadian research income tax receivable is recoverable in cash if not used to offset taxes payable in the year following its generation. The French research income tax credit receivable is recoverable in cash if not used to offset taxes payable in the fourth year following its generation.
| | | | | | |
| | | As of December 31, | |
| | | | |
| | | € | |
| Year of expiration: | | | | |
| | 2005 | | | 531 | |
| | 2006 | | | 429 | |
| | 2007 | | | 152 | |
| | 2008 | | | 297 | |
| | | | |
| | Total | | | 1,409 | |
| | | | |
F-21
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
The Company contributes to state-sponsored pension plan for personnel in France in accordance with French law. Contributions are based on salaries to the relevant state-sponsored organizations. The Company has no further liability in connection with these plans. Expense recognized associated with the plans was€33,€40, and€42 in 2002, 2003 and 2004, respectively.
French law also requires payment of a lump sum retirement indemnity to employees based upon years of service and compensation at retirement. Benefits do not vest prior to retirement. The Company’s accrued obligation at December 31, 2002, 2003 and 2004 was€100,€134 and€96 respectively.
| |
| 10. | OTHER OPERATIONAL EXPENSES |
Other operating expenses incurred in 2004 consisted of professional and legal costs related to the attempted initial public offering during that year.
| |
| 11.1 Operating lease commitments |
In France, the Company leases certain equipment under operating leases, which expired in 2003 and have been renewed for three years. Office space is also leased under operating leases that expire in March 2005.
In June 2002, the Company’s U.S. subsidiary, IDM Inc., located in Irvine, California, entered into a new lease agreement. IDM Inc. leases office and laboratory space under an operating lease, which expired in November 2004 has been renewed for 3 new years (until November 2007).
As of December 31, 2004, the future minimum lease payments under non-cancellable operating leases are as follows:
| | | | | |
| | | € | |
| Due within 1 year | | | 729 | |
| Due between 1 and 3 years | | | 1,390 | |
| Due between 3 and 5 years | | | 955 | |
| | | | |
| Total | | | 3,074 | |
| | | | |
Rental expense for the years ended December 31, 2002, 2003, and 2004, was approximately€584,€846 and€861, respectively.
| |
| 11.2 Commitment with Epimmune |
Following an agreement signed in October 2002 with Epimmune, a San Diego-based biotech company, to acquire a license to develop and use several antigenic peptides, IDM agreed to pay Epimmune a non-refundable upfront license fee of $150 (€154), which was expensed as incurred
In 2003, after pre-clinical evaluation, IDM and Epimmune chose to proceed with the clinical trials and IDM paid an additional license fee of $350 (€328) in February 2003 and a milestone payment of $350 (€304) upon a successful investigational new drug filing in June 2003. These amounts were expensed as incurred.
Other milestone payments for which IDM may be liable to Epimmune in the future, but only upon the occurrence of future events, being the initiation of Phase III trials through to market approval, total $2,750 (€2,016). The Company can however terminate the license agreement subject to giving Epimmune 30 days prior notice before such milestone events occur.
F-22
IDM S.A.
Notes to Consolidated Financial Statements — (Continued)
| |
| 11.3 Commitment with Biotecnol |
On March 8, 2001 the Company entered into a Prototype Production Contract with Biotecnol SA, a Portugese Company. The purpose of this collaboration is to enable IDM to obtain a preliminary process for the production of a certain molecule. The Company has been pursuing development in collaboration with Biotecnol since April 1, 2003, based on a Letter of Intent executed by the Company and Biotecnol. On December 2003, the Company and Biotecnol entered into the Development and Manufacturing Agreement, which aims to expand upon the Prototype Production Contract. As of December 31, 2003 and 2004, and under the terms of the agreement, the Company recorded invoices for€880 and€805 following the successful completion of studies performed by Biotecnol. The Company may be obligated to pay additional amount up to€675 until 2005.
| |
| 11.4 Commitment with Accovion |
On December 28, 2004 the Company entered into an agreement with Accovion Gmbh (ex Covidence), a German Clinical Research Organization, in relation with its Phase II/ III clinical trial of Bexidem. This agreement which expires in September 2007 covers patient recruitment and monitoring of clinical centers in several European countries. The Company has agreed to pay a total of€1,311 spread over the life of the trial and reimburse specific pass-through costs. As at December 31, 2004 no invoice had been received but the Company has provisioned€229 corresponding to the prorated amount spent in 2004.
| |
| 12. | RELATED PARTY TRANSACTIONS |
IDM reimbursed expenses incurred by members of the board of directors, members of Scientific Advisory Board or advisors within their mandate during fiscal years 2002, 2003 and 2004.
In June 2003 and in January 1998, the Company issued warrants to directors. These warrants are described under paragraph 6.3.
In July 1999 and 2001, the Company entered into an agreement with Sanofi-Aventis. These agreements are described under footnote 2.2. The Company has recognized€3,571,€5,327 and€4,652 of revenues from Sanofi-Aventis for the years ended December 31, 2002, 2003 and 2004, respectively. Sanofi-Aventis has been a shareholder of IDM since January 2000 and as of December 31, 2004, owns 20.91% of the Company’s class A and B shares (17.48% on a fully diluted basis).
In 1993, 2000 and 2001, the Company entered into agreements with Medarex. These agreements are described under paragraph 2.3. Medarex has been a shareholder of the Company since 1991 and has had a representative on the Company’s board of directors since June 2000. As of December 31, 2004, Medarex owns 7.75% of the Company’s class A and B shares (23.09% on a fully diluted basis). The Company has recorded expenses of€696,€665 and€259 related to certain Medarex agreements for the years ended December 31, 2002, 2003 and 2004, respectively.
Following the postponement of the Company’s IPO project in June 2004, several options have been evaluated in order to meet the Company’s future financial needs. One such option, a merger and acquisition (M&A) opportunity, is currently being examined with the agreement of the board of directors of the Company and the assistance of financial and legal counsels.
Consequently, the Company signed an engagement letter with UBS bank whereby the Company has agreed to pay a commission, upon successful completion of an M&A transaction. The commission is equal to 1% of the transaction’s value with a minimum of€1.3 million.
F-23
IDM S.A.
Condensed Interim Consolidated Balance Sheets
| | | | | | | | | | |
| | | Twelve Months | | | Three Months | |
| | | Ended | | | Ended | |
| | | December 31, 2004 | | | March 31, 2005 | |
| | | | | | | |
| | | | | € | |
| | | € | | | (Unaudited) | |
| | | (In thousands, except share and | |
| | | per share data) | |
| ASSETS |
| Current assets: | | | | | | | | |
| | Cash and cash equivalents | | | 30,859 | | | | 26,765 | |
| | Related party accounts receivable (Sanofi-Aventis) | | | 1,465 | | | | 1,269 | |
| | Research and development tax credit, current portion | | | 531 | | | | 536 | |
| | Prepaid expenses and other current assets | | | 955 | | | | 1,246 | |
| | | | | | | |
| Total current assets | | | 33,810 | | | | 29,816 | |
| Fixed assets — net | | | 2,173 | | | | 2,124 | |
| Intangible assets — net: | | | | | | | | |
| | Patents, trade marks and other licenses | | | 3,916 | | | | 3,618 | |
| Research and development tax credit, less current portion | | | 878 | | | | 1,217 | |
| Other long-term assets | | | 63 | | | | 65 | |
| | | | | | | |
Total assets | | | 40,840 | | | | 36,840 | |
| | | | | | | |
| |
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
| Current Liabilities: | | | | | | | | |
| | Accounts payable & accrued expenses | | | 3,767 | | | | 4,739 | |
| | Accrued payroll | | | 1,419 | | | | 1,203 | |
| | Related party deferred revenues (Sanofi-Aventis), current portion | | | 552 | | | | 552 | |
| | Other liabilities | | | 617 | | | | 616 | |
| | | | | | | |
| Total current liabilities | | | 6,355 | | | | 7,110 | |
| Long-term debt less current portion | | | 234 | | | | 234 | |
| Related party deferred revenues (Sanofi-Aventis), less current portion | | | 2,761 | | | | 2,623 | |
| Other long term liabilities | | | 96 | | | | 92 | |
| Shareholders’ equity | | | | | | | | |
| | Common stock: 15,694,446 shares of€0.1 par value authorized, issued and outstanding as of December 31, 2004 and March 31, 2005 | | | 1,569 | | | | 1,569 | |
| | Additional paid-in capital | | | 142,169 | | | | 142,165 | |
| | Deferred compensation | | | (28 | ) | | | (19 | ) |
| | Accumulated other comprehensive loss | | | (246 | ) | | | (221 | ) |
| | Accumulated deficit | | | (112,070 | ) | | | (116,713 | ) |
| | | | | | | |
| Total shareholders’ equity | | | 31,394 | | | | 26,781 | |
| | | | | | | |
Total liabilities and shareholders’ equity | | | 40,840 | | | | 36,840 | |
| | | | | | | |
See notes to condensed interim consolidated financial statements
F-24
IDM S.A.
Condensed Interim Consolidated Statements of Operations and Comprehensive Loss
| | | | | | | | | | |
| | | Three Months | | | Three Months | |
| | | Ended | | | Ended | |
| | | March 31, 2004 | | | March 31, 2005 | |
| | | | | | | |
| | | € | | | € | |
| | | (Unaudited) | | | (Unaudited) | |
| | | (In thousands, except share and | |
| | | per share data) | |
| Related party research and development revenues | | | 1,213 | | | | 1,200 | |
| Research and development expenses | | | (3,851 | ) | | | (3,696 | ) |
| Impairment of patents & licenses | | | — | | | | (206 | ) |
| Selling and marketing expenses | | | (256 | ) | | | (197 | ) |
| General and administrative expenses | | | (1,046 | ) | | | (1,068 | ) |
| Other operating expenses | | | | | | | (1,157 | ) |
| | | | | | | |
| Total operating expenses | | | (5,153 | ) | | | (6,324 | ) |
| | | | | | | |
| | Loss from operations | | | (3,940 | ) | | | (5,124 | ) |
| Interest income | | | 169 | | | | 142 | |
| Foreign exchange gain (loss) | | | 20 | | | | 3 | |
| | | | | | | |
| Loss before income tax benefit | | | (3,751 | ) | | | (4,979 | ) |
| Income tax benefit | | | 6 | | | | 336 | |
| | | | | | | |
| | Net loss | | | (3,745 | ) | | | (4,643 | ) |
| | | | | | | |
| Weighted average number of shares outstanding | | | 13,603,674 | | | | 15,694,446 | |
| Basic and diluted loss per share | | | (0.28 | ) | | | (0.30 | ) |
| Comprehensive Loss: | | | | | | | | |
| Net Loss | | | (3,745 | ) | | | (4,643 | ) |
| Other comprehensive income (loss) | | | (312 | ) | | | 25 | |
| | | | | | | |
| Comprehensive Loss | | | (4,057 | ) | | | (4,618 | ) |
| | | | | | | |
See notes to condensed interim consolidated financial statements
F-25
IDM S.A.
Condensed Interim Consolidated Statements of Cash Flows
| | | | | | | | | | |
| | | Three Months | | | Three Months | |
| | | Ended | | | Ended | |
| | | March 31, 2004 | | | March 31, 2005 | |
| | | | | | | |
| | | € | | | € | |
| | | (Unaudited) | | | (Unaudited) | |
| | | (In thousands) | |
Cash flows from operating activities: | | | | | | | | |
| | Net loss | | | (3,745 | ) | | | (4,643 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| | Amortization of deferred compensation | | | 21 | | | | 5 | |
| | Depreciation and amortization | | | 626 | | | | 316 | |
| | Impairment of licenses | | | — | | | | 206 | |
Increase (decrease) in cash from: | | | | | | | | |
| | Related party accounts receivable (Sanofi-Aventis) | | | 714 | | | | 196 | |
| | Prepaid expenses and other current assets | | | (637 | ) | | | (291 | ) |
| | Research and development tax credit receivable | | | (11 | ) | | | (344 | ) |
| | Other long-term assets | | | 2 | | | | (2 | ) |
| | Accounts payable and accrued expenses | | | 939 | | | | 972 | |
| | Accrued payroll | | | (84 | ) | | | (216 | ) |
| | Related party deferred revenues (Sanofi-Aventis) | | | (138 | ) | | | (138 | ) |
| | Other liabilities | | | 80 | | | | (1 | ) |
| | | | | | | |
Net cash used in operating activities | | | (2,233 | ) | | | (3,940 | ) |
Cash flow from investing activities: | | | | | | | | |
| | Purchase of fixed assets | | | (255 | ) | | | (140 | ) |
| | Patent costs and acquisition of other intangibles | | | (193 | ) | | | (39 | ) |
| | | | | | | |
Net cash used in investing activities | | | (448 | ) | | | (179 | ) |
| Effect of exchange rate changes on cash and cash equivalents | | | (312 | ) | | | 25 | |
Net (decrease) in cash and cash equivalents | | | (2,993 | ) | | | (4,094 | ) |
| | | | | | | |
| Cash and cash equivalents, beginning of year | | | 33,326 | | | | 30,859 | |
| | | | | | | |
| Cash and cash equivalents, end of period | | | 30,333 | | | | 26,765 | |
| | | | | | | |
See notes to condensed interim consolidated financial statements
F-26
IDM S.A.
Notes to Condensed Interim Consolidated Financial Statements
(All amounts stated in thousands of euros except share and per share data)
| |
| 1. | Nature of business and summary of significant accounting policies |
Immuno-Designed Molecules, S.A. (“IDM” or the “Company”) was incorporated as a “Société Anonyme” in 1993. IDM develops products that are designed to stimulate the immune system, destroy tumor cells after surgery or chemotherapy, and immunize patients to prevent tumor recurrence.
The Company’s lead potential product has completed a Phase III clinical trial, for the treatment of bone cancer. The Company has also several cellular therapy products in Phase II/III, II and I/II clinical trials for a variety of cancers including bladder and colorectal cancers. Two products are in the late stage of clinical development.
In July 2001, IDM entered into a significant collaboration with Sanofi-Aventis, a leading global pharmaceutical company, from which it has generated revenues in 2002, 2003 and 2004 (see note 2.2 of the annual consolidated statements ended 31 December 2004). The Company expects its principal sources of revenues over the next several years to be milestone payments and reimbursement of research and development expenses from its collaboration with Sanofi-Aventis. The Company is also seeking to enter into other collaborative agreements for certain products with other partners, which may provide additional sources of revenues. Accordingly, the accompanying financial statements have been prepared on the basis of the Company being an operating company.
The Company’s business is subject to significant risks, which is consistent with biotechnology companies that are conducting research in cellular therapy. These risks include, but are not limited to, uncertainties regarding research and development, access to capital, obtaining and enforcing patents, receiving regulatory approval, and competition with other biotechnology and pharmaceutical companies. The Company plans to continue to finance its operations with a combination of equity and debt financing, revenues from research and development programs and, in the longer term, product sales, if any. However, there can be no assurance that the Company will successfully develop any marketable products or, if it does, that the revenues generated from the sales of these products will be sufficient to sustain the Company’s operations.
| |
| Basis of Presentation: Interim Financial Statements |
The interim unaudited condensed financial statements contained herein have been prepared in accordance with U.S. generally accepted accounting principles for interim financial information. Accordingly, they do not include all of the information and disclosures required by U.S. generally accepted accounting principles for complete financial statements. In management’s opinion, the unaudited information includes all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the financial position, results of operations and cash flows for the periods presented. Interim results are not necessarily indicative of results to be expected for the full year. The financial statements should be read in conjunction with the audited consolidated financial statements, for the year ended December 31, 2004.
| |
| Impairment of long lived assets |
Long-lived assets including intangible assets, other than indefinite lived intangibles, to be held and used are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values.
F-27
IDM S.A.
Notes to Condensed Interim Consolidated Financial Statements — (Continued)
| |
| Warrants and stock options granted to employees, directors and consultants |
IDM accounts for stock options and warrants granted to employees in accordance with the provisions of Accounting Principles Board Opinion No. 25 (“APB 25”),“Accounting for Stock Issued to Employees”. Under APB 25, no compensation expense is recognized for stock options and warrants issued to employees with an exercise price equal to or greater than the fair value of the underlying shares. Options and warrants issued with an exercise price less than the fair value result in deferred compensation which is recorded within shareholder’s equity and amortized to expense over the vesting period.
Statement of Financial Accounting Standards No. 123 (“SFAS 123”),“Accounting for Stock-Based Compensation”provides an alternative to APB 25 in accounting for stock-based compensation issued to employees. SFAS 123 provides for a fair-value based method of accounting for employee stock options and similar equity instruments. Companies that elect to continue to account for stock-based compensation arrangements under APB 25 are required by SFAS 123 to disclose the pro forma effect on net income and net income per share as if the fair-value based method proposed by SFAS 123 had been adopted.
Pro forma information regarding net loss is required by SFAS 123 as if the Company had accounted for its employee stock options under the fair value method. The fair value of the Company’s options was estimated at the date of grant using the minimum value method, with the following assumptions for the year ended December 31, 2004 and the three-month period ended March 31, 2005:
| | | | | | | | | |
| | | | | Three Months | |
| | | Year Ended | | | Ended | |
| | | December 31, 2004 | | | March 31, 2005 | |
| | | | | | | |
| | | | | (Unaudited) | |
Weighted average information | | | | | | | | |
| Risk-free interest rate | | | 4.39 | % | | | 3.68 | % |
| Dividend yield | | | 0 | % | | | 0 | % |
| Expected option life | | | 7 | | | | 7 | |
Because the determination of the fair value of the Company’s options is based on assumptions described above, and because additional option grants are expected to be made in future periods, this pro forma information is not likely to be representative of the pro forma effects on reported net income or loss for future periods.
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the options’ vesting period. The following table illustrates the net loss that would have occurred had the Company accounted for its stock options under the provisions of SFAS 123.
F-28
IDM S.A.
Notes to Condensed Interim Consolidated Financial Statements — (Continued)
Had compensation cost for the company’s stock option plan been determined on the fair value method set forth in SFAS 123, the Company’s net loss and basic and diluted net loss per share would have been changed to the pro forma amounts indicated below:
| | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31, | |
| | | | |
| | | 2004 | | | 2005 | |
| | | | | | | |
| | | € | | | € | |
| | | (Unaudited) | | | (Unaudited) | |
| Net loss as reported | | | (3,745 | ) | | | (4,643 | ) |
| Add: stock based employee compensation expense included in reported net loss | | | 21 | | | | 6 | |
| Deduct: Total stock-based employee compensation expense determined under fair value based method for all awards | | | (253 | ) | | | (133 | ) |
| Proforma net loss | | | (3,977 | ) | | | (4,770 | ) |
| | | | | | | |
| Net loss per share: | | | | | | | | |
| | Basic and diluted — as reported | | | (0.28 | ) | | | (0.30 | ) |
| | | | | | | |
| | Basic and diluted — pro forma | | | (0.28 | ) | | | (0.30 | ) |
| | | | | | | |
| |
| 2. | Significant events of the quarter |
On March 15, 2005, the Company executed a Share Exchange Agreement and Amendment No. 1 to the Share Exchange Agreement, with the US publicly listed company Epimmune Inc, whereby the Company’s shareholders representing more than 85% of its fully-diluted capital have agreed to exchange each of their Company shares for 3,771,865 newly issued shares of Epimmune Inc. (the “Combination”). On March 16, 2005, the Company and Epimmune Inc. issued a joint press release announcing the execution of the Share Exchange Agreement and held a conference call to discuss the transaction. Completion of the Combination is subject to the approval of the shareholders of Epimmune Inc.
For the first three-month period ended March 31, 2005, the Company recorded a total of€1,157 corresponding to invoices and provisions related to legal and professional fees incurred in relation with the Combination.
| |
| 3. | Research and Development agreements |
In March 2003, The Company entered into an asset purchase agreement with Jenner Biotherapies, Inc. (“Jenner”), a biotechnology company devoted to the development of cancer vaccines and macrophage activators. Pursuant to the terms of the agreement, the Company purchased Jenner’s lead product candidate, Mepact,. This asset was acquired by issuing 225,400 shares of the Company’s Class B ordinary shares, per value€0.10 each. The asset purchase was consummated in April 2003.
In accordance with EITF 96-18 “Accounting For Equity Instruments That Are Issued to Other Than Employees For Acquiring, or in conjunction with Selling, Goods or Services”, the Mepact license was valued at fair value for an amount of€2,900 on the date the shares were issued to Jenner by the Company. The fair value of the shares was recorded as common stock and additional paid-in capital, and represented the basis for the total valuation of the license acquired. Total consideration was allocated to the Mepact license based on its fair value at the date of issuance, which was also valued by the Company, based on estimated future cash flows and an expected rate of success, as determined by IDM’s management. The fair values allocated to the license with alternate future use amounted to€2,900 and is reflected in intangible assets.
F-29
IDM S.A.
Notes to Condensed Interim Consolidated Financial Statements — (Continued)
The license capitalized from Jenner is being amortized over 10 years, which is management’s estimate of the expected life of future products developed from the use of the license.
IDM’s direct research and development expenses related to Mepact amounted to approximately€536 and€177 for the first three months of 2005 and 2004, respectively.
| |
| 3.2 | Agreement with Sanofi-Aventis (Related Party) |
Pursuant to the agreement signed with IDM in July 2001, Sanofi-Aventis exercised in December 2001 its first option on the Company’s ongoing melanoma development program Uvidem. Consequently, IDM received€5,900 corresponding to: (i) an up-front payment, (ii) a completion of Phase I milestone payment since the program was already in Phase II and (iii) reimbursement of all research and development costs incurred from 1999 through December 2001, which approximated€1,900. Repayment received for past R&D expenses incurred by IDM prior to the selection of an option by Sanofi-Aventis are considered as a complementary upfront fee. Thus, IDM is recognizing these three payments over the remaining program development period, which is estimated to be 9 years. Upfront and milestone revenues recognized amounted to€138 for the three-month period ending March 31, 2004 and the three-month period ending March 31, 2005.
In addition, the Company recorded €1,075 and €1,062 in revenues from Sanofi-Aventis for reimbursement of current research and development expenses related to the development of Uvidem, for the three-month period ended March 31, 2004 and March 31, 2005, respectively.
IDM’s direct research and development expenses related to Uvidem amounted to approximately€723 and€682 for the first three months of 2005 and 2004, respectively.
3.3 Agreement with Medarex
IDM’s direct research and development expenses related to Osidem amounted to approximately€3 and€96 for the first three months of 2005 and 2004, respectively.
4.1 Share capital
As at March 31, 2005, the authorized, issued and outstanding share capital of the Company consisted of 15,694,446 ordinary shares with a nominal value of€0.1 per share, of which 5,951,121 were Class A shares and 9,743,325 were Class B shares. Both categories have the same rights except that Class B shares have certain additional rights relating to Board representation and rights to communication of Company financial information. These rights are in effect until the number of Class B shares represent less than 20% of the non-diluted number of shares of the Company. Class B shares are automatically converted into Class A shares in case of transfer of ownership.
4.2 Amendment of warrants exercise conditions
At the Company’s annual shareholders meeting held on March 30, 2005, amendments of exercise conditions for the following series of warrants were approved.
4.2.1 Warrants held by Sanofi-Aventis
Exercise conditions of the following warrants issued to Sanofi (currently Sanofi-Aventis) as part of an agreement related to the IL-13 molecule, were amended:
| | |
| | • | 7,283 warrants called “BSA 99 n°1” issued on January 7, 2000 in favor of Sanofi and amended by the shareholders general extraordinary meeting held on December 20, 2001. |
F-30
IDM S.A.
Notes to Condensed Interim Consolidated Financial Statements — (Continued)
| | |
| | • | 7,283 warrants called “BSA 99 n°2” issued on January 7, 2000 in favor of Sanofi and amended by the shareholders general extraordinary meeting held on December 20, 2001. |
It was initially agreed that these warrants could be exercised by anticipation in case of a change of control of IDM. Such a change of control should take place within the combination between the Company and Epimmune Inc. and all these warrants would become exercisable immediately after closing the transaction. In order to simplify the Combination process, and in agreement with Sanofi-Aventis, the anticipated exercise of these warrants is now authorized in the days preceding the completion of the combination.
4.2.2 Warrants held by Medarex
Exercise conditions of the 3,653,282 warrants to subscribe bonds or notes convertible or redeemable into ordinary shares called “BSOCRA” issued to Medarex Inc. on September 11, 2000 were amended. These warrants, which are already exercisable, allow Medarex to subscribe for bonds or notes which can be converted or redeemed into shares 6 months after issuance. In order to simplify the Combination process, conversion or redemption of the bonds into shares can now be done immediately following issuance, thereby canceling the 6-month waiting period.
5.2 Commitment with Biotecnol
On March 8, 2001 the Company entered into a Prototype Production Contract with Biotecnol SA, a Portugese company. The purpose of this collaboration is to enable IDM to obtain a preliminary process for the production of a certain molecule. The company has been pursuing development in collaboration with Biotecnol since April 1, 2003, based on a Letter of Intent executed by the Company and Biotecnol. On December 2003, the Company and Biotecnol entered into the Development and Manufacturing Agreement, which aims to expand upon the Prototype Production Contract. For the three-month period ended March 31, 2004 and 2005, and under the terms of the agreement, the Company recorded invoices for €95 and €120, respectively following the successful completion of studies performed by Biotecnol. The Company may be obligated to pay additional amount up to €555 until the end of 2005.
5.3 Commitment with Accovion
On December 28, 2004 the Company entered into an agreement with Accovion Gmbh, a German Clinical Research Organization, in relation with its Phase II/ III clinical trial of Bexidem. This agreement which expires in September 2007 covers patient recruitment and monitoring of clinical centers in several European countries. The Company has agreed to pay a total of €1,311 spread over the life of the trial and reimburse specific pass-through costs. As at March 31, 2005 the Company recorded a total of €146 corresponding to both invoices and provisions.
On April 22, 2005, Epimmune Inc. filed information related to the Combination with the SEC (Securities and Exchange Commission). Following approval of this information statement by the SEC, Epimmune Inc. will call a shareholders meeting in order to seek approval of the Combination.
The Company signed an engagement letter with UBS bank whereby the Company has agreed to pay a commission, upon successful completion of an M&A transaction, amounting to 1% of the transaction’s value with a minimum of €1.3 million. The Company anticipates that upon completion of the Combination, UBS would receive a fee of €1.3 million.
F-31
INDEX TO ANNEXES
| | | | | |
| ANNEX A — SHARE EXCHANGE AGREEMENT, AMENDMENT NO. 1 TO THE SHARE EXCHANGE AGREEMENT, AMENDMENT NO. 2 TO THE SHARE EXCHANGE AGREEMENT, AMENDMENT NO. 3 TO THE SHARE EXCHANGE AGREEMENT AND AMENDMENT NO. 4 TO THE SHARE EXCHANGE AGREEMENT | | | | |
| ANNEX B — OPINION OF EPIMMUNE’S FINANCIAL ADVISOR | | | | |
| ANNEX C — FORM OF PUT/ CALL AGREEMENT | | | | |
| ANNEX D — FORM OF OPTION LIQUIDITY AGREEMENT | | | | |
| ANNEX E — AMENDED AND RESTATED PREFERRED EXCHANGE AGREEMENT | | | | |
| ANNEX F-1 — CERTIFICATE OF AMENDMENT OF THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF EPIMMUNE | | | | |
| ANNEX F-2 — CERTIFICATE OF AMENDMENT OF THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF EPIMMUNE | | | | |
| ANNEX F-3 — CERTIFICATE OF AMENDMENT OF THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF EPIMMUNE | | | | |
| ANNEX F-4 — CERTIFICATE OF AMENDMENT OF THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF EPIMMUNE | | | | |
| ANNEX F-5 — CERTIFICATE OF AMENDMENT OF THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF EPIMMUNE | | | | |
| ANNEX F-6 — CERTIFICATE OF AMENDMENT OF THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF EPIMMUNE | | | | |
| ANNEX F-7 — CERTIFICATE OF AMENDMENT OF THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF EPIMMUNE | | | | |
| ANNEX G — EPIMMUNE 2000 STOCK PLAN | | | | |
| ANNEX H — FRENCH ANNEX TO EPIMMUNE 2000 STOCK PLAN | | | | |
| ANNEX I — EPIMMUNE 2001 EMPLOYEE STOCK PURCHASE PLAN | | | | |
| ANNEX J — EPIMMUNE EMPLOYEE STOCK PURCHASE PLAN FOR IDM EMPLOYEES | | | | |
ANNEX A
SHARE EXCHANGE AGREEMENT
by and among
EPIMMUNE INC.,
A Corporation Organized Under the Laws of the State of Delaware, United States of America;
and
CERTAIN SHAREHOLDERS OF IDM S.A.,
Listed onExhibit A Attached Hereto
Dated as of March 15, 2005
TABLE OF CONTENTS
| | | | | | | |
| | | | | Page | |
| | | | | | |
ARTICLE I
EXCHANGE OF SHARES |
SECTION 1.01. | | Exchange of Shares | | | 2 | |
SECTION 1.02. | | Exchange Ratio | | | 2 | |
SECTION 1.03. | | The Closing | | | 3 | |
SECTION 1.04. | | Closing Deliveries by the Principal Company Shareholders | | | 3 | |
SECTION 1.05. | | Closing Deliveries by the Issuer | | | 3 | |
SECTION 1.06. | | Escrow Shares | | | 4 | |
SECTION 1.07. | | Treatment of Company Stock Options | | | 4 | |
SECTION 1.08. | | Treatment of Company Warrants | | | 5 | |
SECTION 1.09. | | Treatment of Company Securities Held in an Individual Shares Savings Plan | | | 6 | |
SECTION 1.10. | | Notices to Other Shareholders | | | 7 | |
| |
ARTICLE II
REPRESENTATIONS AND WARRANTIES REGARDING THE COMPANY |
SECTION 2.01. | | Corporate Organization | | | 7 | |
SECTION 2.02. | | Corporate Books and Records | | | 8 | |
SECTION 2.03. | | Capitalization | | | 8 | |
SECTION 2.04. | | Authority Relative to This Agreement | | | 9 | |
SECTION 2.05. | | No Conflict; Required Filings and Consents | | | 9 | |
SECTION 2.06. | | Permits; Compliance | | | 10 | |
SECTION 2.07. | | Financial Statements | | | 11 | |
SECTION 2.08. | | Absence of Certain Changes or Events | | | 12 | |
SECTION 2.09. | | Absence of Litigation | | | 12 | |
SECTION 2.10. | | Employee Benefit Plans | | | 13 | |
SECTION 2.11. | | Labor and Employment Matters | | | 14 | |
SECTION 2.12. | | Property and Leases | | | 16 | |
SECTION 2.13. | | Intellectual Property | | | 17 | |
SECTION 2.14. | | Taxes | | | 19 | |
SECTION 2.15. | | Environmental Matters | | | 20 | |
SECTION 2.16. | | Material Contracts | | | 20 | |
SECTION 2.17. | | Insurance | | | 22 | |
SECTION 2.18. | | Board Approval | | | 22 | |
SECTION 2.19. | | Certain Business Practices | | | 22 | |
SECTION 2.20. | | Interested Party Transactions | | | 22 | |
SECTION 2.21. | | Brokers | | | 23 | |
| |
ARTICLE III
REPRESENTATIONS AND WARRANTIES REGARDING THE PRINCIPAL
COMPANY SHAREHOLDERS |
SECTION 3.01. | | Organization; Qualification | | | 23 | |
SECTION 3.02. | | Authority Relative to This Agreement | | | 23 | |
SECTION 3.03. | | No Conflict | | | 23 | |
SECTION 3.04. | | Title to the Shares | | | 24 | |
SECTION 3.05. | | Investment Experience and Status | | | 24 | |
| | | | | | | |
| | | | | Page | |
| | | | | | |
| |
ARTICLE IV
REPRESENTATIONS AND WARRANTIES REGARDING THE ISSUER |
SECTION 4.01. | | Corporate Organization | | | 25 | |
SECTION 4.02. | | Corporate Books and Records | | | 25 | |
SECTION 4.03. | | Capitalization | | | 26 | |
SECTION 4.04. | | Authority Relative to This Agreement | | | 27 | |
SECTION 4.05. | | No Conflict; Required Filings and Consents | | | 27 | |
SECTION 4.06. | | Permits; Compliance | | | 28 | |
SECTION 4.07. | | SEC Filings; Financial Statements; Nasdaq | | | 29 | |
SECTION 4.08. | | Absence of Certain Changes or Events | | | 30 | |
SECTION 4.09. | | Absence of Litigation | | | 30 | |
SECTION 4.10. | | Employee Benefit Plans | | | 30 | |
SECTION 4.11. | | Labor and Employment Matters | | | 32 | |
SECTION 4.12. | | Property and Leases | | | 33 | |
SECTION 4.13. | | Intellectual Property | | | 34 | |
SECTION 4.14. | | Taxes | | | 35 | |
SECTION 4.15. | | Environmental Matters | | | 36 | |
SECTION 4.16. | | Material Contracts | | | 36 | |
SECTION 4.17. | | Insurance | | | 38 | |
SECTION 4.18. | | Board Approval; Vote Required | | | 38 | |
SECTION 4.19. | | Certain Business Practices | | | 38 | |
SECTION 4.20. | | Interested Party Transactions | | | 38 | |
SECTION 4.21. | | Opinion of Financial Advisor | | | 39 | |
SECTION 4.22. | | Brokers | | | 39 | |
SECTION 4.23. | | Termination of Anosys Agreement | | | 39 | |
| |
ARTICLE V
CONDUCT OF BUSINESS PENDING THE CLOSING |
SECTION 5.01. | | Conduct of Business of the Company Pending the Closing | | | 39 | |
SECTION 5.02. | | Conduct of Business of the Issuer Pending the Closing | | | 41 | |
SECTION 5.03. | | Control of Other Party’s Business | | | 43 | |
| | | | | | | |
| | | | | Page | |
| | | | | | |
| |
ARTICLE VI
ADDITIONAL AGREEMENTS |
SECTION 6.01. | | Proxy Statement | | | 43 | |
SECTION 6.02. | | Issuer Stockholders’ Meeting | | | 44 | |
SECTION 6.03. | | Access to Information; Confidentiality | | | 44 | |
SECTION 6.04. | | No Solicitation of Transactions | | | 45 | |
SECTION 6.05. | | Directors’ and Officers’ Indemnification and Insurance | | | 46 | |
SECTION 6.06. | | Notification of Certain Matters | | | 47 | |
SECTION 6.07. | | Resale Registration Statement | | | 47 | |
SECTION 6.08. | | Further Action; Reasonable Best Efforts | | | 49 | |
SECTION 6.09. | | Exchange of Issuer Preferred Stock | | | 50 | |
SECTION 6.10. | | Public Announcements | | | 50 | |
SECTION 6.11. | | Corporate Matters | | | 50 | |
SECTION 6.12. | | Covenants of the Principal Company Shareholders | | | 51 | |
SECTION 6.13. | | Nasdaq Listing Application | | | 51 | |
SECTION 6.14. | | Compensation Matters | | | 52 | |
SECTION 6.15. | | Company Shareholders Agreement | | | 52 | |
SECTION 6.16. | | Company Shareholders’ Meeting | | | 52 | |
| |
ARTICLE VII
CONDITIONS TO THE SHARE EXCHANGE |
SECTION 7.01. | | Conditions to the Obligations of Each Party | | | 53 | |
SECTION 7.02. | | Conditions to the Obligations of the Issuer | | | 54 | |
SECTION 7.03. | | Conditions to the Obligations of the Principal Company Shareholders | | | 54 | |
| |
ARTICLE VIII
TERMINATION, AMENDMENT AND WAIVER |
SECTION 8.01. | | Termination | | | 55 | |
SECTION 8.02. | | Effect of Termination | | | 56 | |
SECTION 8.03. | | Fees and Expenses | | | 56 | |
SECTION 8.04. | | Amendment | | | 57 | |
SECTION 8.05. | | Waiver | | | 57 | |
| |
ARTICLE IX
INDEMNIFICATION |
SECTION 9.01. | | Survival of Representations and Warranties | | | 58 | |
SECTION 9.02. | | Indemnification by the Principal Company Shareholders | | | 58 | |
SECTION 9.03. | | Notice of Loss; Third Party Claims | | | 59 | |
SECTION 9.04. | | Shareholder Representative | | | 59 | |
| | | | | | | |
| | | | | Page | |
| | | | | | |
| |
ARTICLE X
GENERAL PROVISIONS |
SECTION 10.01. | | Non-Survival of Representations, Warranties and Agreements | | | 61 | |
SECTION 10.02. | | Notices | | | 61 | |
SECTION 10.03. | | Severability | | | 62 | |
SECTION 10.04. | | Entire Agreement; Assignment | | | 62 | |
SECTION 10.05. | | Parties in Interest | | | 62 | |
SECTION 10.06. | | Specific Performance | | | 62 | |
SECTION 10.07. | | Governing Law; Arbitration | | | 63 | |
SECTION 10.08. | | Construction | | | 63 | |
SECTION 10.09. | | Waiver of Jury Trial | | | 63 | |
SECTION 10.10. | | Headings | | | 63 | |
SECTION 10.11. | | Counterparts | | | 63 | |
SECTION 10.12. | | Language | | | 63 | |
SECTION 10.13. | | Waiver of Conflicts | | | 63 | |
SECTION 10.14. | | Effectiveness of Agreement | | | 64 | |
| |
| EXHIBIT A | | PRINCIPAL COMPANY SHAREHOLDERS | | | | |
| EXHIBIT B | | DEFINED TERMS | | | | |
| EXHIBIT C | | FORM OF ISSUER FRENCH STOCK OPTION PLAN | | | | |
| EXHIBIT D-1 | | INDIVIDUALS ENTERING INTO EMPLOYMENT AGREEMENTS WITH THE ISSUER | | | | |
| EXHIBIT D-2 | | INDIVIDUALS NEGOTIATING EMPLOYMENT AGREEMENTS WITH THE ISSUER | | | | |
| EXHIBIT E | | FORM OF INDEMNITY ESCROW AGREEMENT | | | | |
| EXHIBIT F | | FORM OF EXPENSE ESCROW AGREEMENT | | | | |
| EXHIBIT G | | FORM OF OPTION LIQUIDITY AGREEMENT | | | | |
| EXHIBIT H | | FORM OF PUT/CALL AGREEMENT | | | | |
| EXHIBIT I | | FORM OF OFFER NOTIFICATION | | | | |
| EXHIBIT J | | FORM OF JOINDER AGREEMENT | | | | |
| EXHIBIT K | | FORM OF PREFERRED EXCHANGE AGREEMENT | | | | |
| EXHIBIT L | | EXECUTIVE OFFICERS OF THE ISSUER AFTER THE CLOSING DATE | | | | |
| EXHIBIT M | | FORM OF POWER OF ATTORNEY | | | | |
This SHARE EXCHANGE AGREEMENT (this “Agreement”) is entered into as of March 15, 2005 (the “Effective Date”), by and among EPIMMUNE INC., a Delaware corporation (the “Issuer”), and the shareholders of IDM S.A., asociété anonymeorganized under the laws of France (the “Company”), listed onExhibit A attached hereto (the “Principal Company Shareholders”). Certain capitalized terms used herein are defined onExhibit B attached hereto.
Recitals:
A. The Board of Directors of the Issuer (the “Issuer Board”) has determined that it is in the best interests of its stockholders for the Issuer to acquire the Company upon the terms and subject to the conditions set forth in this Agreement;
B. In furtherance of such acquisition, the Issuer agrees to acquire all of the issued and outstanding class A ordinary shares, nominal value€0.10 per share, of the Company (“Company A Shares”) and all of the issued and outstanding class B ordinary shares, nominal value€0.10 per share, of the Company (“Company B Shares” and, together with the Company A Shares, the “Company Shares”) in exchange for shares of common stock, par value $0.01 per share, of the Issuer (“Issuer Common Stock”), upon the terms and subject to the conditions of this Agreement;
C. The Issuer Board has unanimously (i) approved this Agreement and the transactions contemplated by this Agreement and (ii) resolved to recommend that the stockholders of the Issuer vote to approve: (A) the issuance of shares of Issuer Common Stock to the shareholders and/or warrantholders of the Company (1) in exchange for Company Shares in accordance with Section 1.02 or 1.09 and (2) in exchange for Company Warrants in accordance with Section 1.08(a), in each case, pursuant to the terms of this Agreement (the “Share Exchange”); (B) the change of the corporate name of the Issuer in accordance with Section 6.11(c) of this Agreement (the “Issuer Name Change”); (C) the amendment of the Amended and Restated Certificate of Incorporation of the Issuer (the “Issuer Certificate of Incorporation”) to increase the authorized capital stock of the Issuer (the “Issuer Capital Stock Increase”) and effect a reverse split of the Issuer Common Stock and the Issuer Preferred Stock (the “Issuer Reverse Stock Split”) in accordance with Section 6.11(d) of this Agreement; (D) the increase in the shares available for issuance under the Issuer’s 2000 Stock Plan in accordance with Section 6.14 of this Agreement (the “Issuer Stock Option Plan Amendment”); (E) the adoption of the Epimmune Option Liquidity Plan and the issuance of shares of Issuer Common Stock to the holders of Company Stock Options pursuant to such Option Liquidity Plan (the “Option Liquidity Share Issuance”); (F) the increase in the shares available for issuance under the Issuer’s 2001 Employee Stock Purchase Plan in accordance with Section 6.14 of this Agreement (the “Issuer ESPP Amendment”); and (G) the adoption of the Issuer French Employee Stock Option Sub Plan (the “Issuer French Stock Option Plan”), substantially in the form ofExhibit C attached hereto, which shall be in the form of a sub plan to the Issuer’s 2000 Stock Plan (the transactions specified in clauses (A) through (G) above are sometimes referred to herein collectively as the “Issuer Transactions”);
D. The Board of Directors of the Company (the “Company Board”) has unanimously approved the transactions contemplated by this Agreement;
E. The Principal Company Shareholders, who own, in the aggregate, Company Shares representing at least 85% of the shares of the capital stock and voting rights of the Company as calculated in accordance with Section 6.1 of the Shareholders Agreement, dated as of December 20, 1996, as amended through the date hereof (the “Company Shareholders Agreement”), among the Company and the shareholders of the Company listed on Annex I thereto, have agreed, among other things, (i) to exchange their Company Shares for shares of Issuer Common Stock upon the terms and subject to the conditions of this Agreement; (ii) to appoint the Shareholder Representative to act on behalf of such Principal Company Shareholders for certain purposes related to this Agreement and the transactions contemplated hereby in accordance with Section 9.04 of this Agreement; (iii) to waive their respective rights granted pursuant to Article 4 of the Company Shareholder Agreement in connection with the Share Exchange; and (iv) to take any other actions reasonably requested by the Issuer to require all the Other Shareholders to exchange such shareholder’s Company Shares
for shares of Issuer Common Stock, upon the terms and subject to the conditions of this Agreement, in accordance with Section 6 of the Company Shareholders Agreement;
F. The Issuer, the Shareholder Representative and the directors and executive officers of the Issuer have entered into Voting Agreements, dated as of the date hereof (the “Voting Agreements”), pursuant to which such directors and executive officers have agreed, among other things, to vote their shares of Issuer Common Stock in favor of the Issuer Transactions;
G. The Issuer and the individuals listed onExhibit D-1 attached hereto have entered into Employment Agreements, dated as of the date hereof, with respect to the employment of such individuals with the Issuer effective upon the completion of the transactions contemplated by this Agreement, and the Issuer is negotiating, in good faith, with the individuals listed onExhibit D-2 attached hereto to enter into Employment Agreements with respect to the employment of such individuals with the Issuer effective upon the completion of the Transaction contemplated by this Agreement (all such Employment Agreement entered into or being negotiated with the Issuer being collectively referred to herein as the “Employment Agreements”);
I. The Company has consulted with its workers’ committee (comité d’enterprise) regarding the transactions contemplated by this Agreement; and
J. The parties intend that this Agreement shall become effective upon the receipt of signatures of the Principal Company Shareholders owning the requisite number of Company Shares in accordance with Section 10.14.
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants and agreements herein contained, and intending to be legally bound hereby, the Issuer and the Principal Company Shareholders hereby agree as follows:
ARTICLE I
EXCHANGE OF SHARES
Section 1.01. Exchange of Shares. (a) Upon the terms and subject to the conditions contained in this Agreement, except as otherwise provided in Section 1.09, at the Closing each Principal Company Shareholder shall sell, assign, transfer, convey and deliver to the Issuer, and the Issuer shall purchase, acquire and accept for delivery from such Principal Company Shareholder, the number of Company Shares set forth opposite the name of such Principal Company Shareholders onExhibit A attached hereto.
Section 1.02. Exchange Ratio. (a) Subject to Sections 1.02(d) and 1.02(e) and any adjustment made pursuant to Sections 1.02(c), 1.05 and 1.06 and the Escrow Agreements, each Company Share shall be exchanged for the number of shares of Issuer Common Stock equal to the Exchange Ratio (as defined below) (such number of shares of Issuer Common Stock, or such number as adjusted pursuant to Section 1.02(c), being the “Exchange Consideration”).
(b) For purposes of this Agreement, the “Exchange Ratio” means 3.771865, subject to adjustment pursuant to Section 1.02(c).
(c) Adjustments to Capital Stock. The Exchange Ratio shall be adjusted to reflect appropriately the effect of the Issuer Reverse Stock Split and any other stock split, reverse stock split, stock dividend (including any dividend or distribution of securities convertible into Issuer Common Stock or Company Shares), extraordinary cash dividends, reorganization, recapitalization, reclassification, combination, exchange of shares or other like change with respect to the Issuer Common Stock or Company Shares occurring on or after the date hereof and prior to the Closing.
(d) Fractional Shares. No certificates or scrip representing fractional shares of Issuer Common Stock shall be issued pursuant to this Section 1.02 and such fractional share interests will not entitle the owner thereof to vote or to any other rights of a stockholder of Issuer. Each holder of a fractional share interest shall be paid an amount in cash (without interest and subject to the amount of any withholding taxes as contemplated in Section 1.02(e)) equal to the product obtained by multiplying (i) such fractional share
2
interest to which such holder (after taking into account all fractional share interests then held by such holder) would otherwise be entitled by (ii) the Average Closing Price as of the Closing Date. The Issuer shall pay the amount due to each holder of a fractional share interest pursuant to this Section 1.02(d) (and shall be deemed to have delivered such amount due to each such holder) by the payment, by wire transfer in immediately available funds, to Gestor Finance of an amount in cash equal to the aggregate fractional share cash payments due to such holders within five days following the Closing. For purposes of this Agreement, “Average Closing Price” means, for any specified date, the average of the per share closing (at 4:00 p.m., Eastern Time) prices on the Nasdaq National Market (“Nasdaq”) of shares of Issuer Common Stock during the five consecutive trading days ending on (and including) the trading day immediately preceding such specified date.
(e) Withholding Rights. The Issuer shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any holder of Company Shares such amounts as it is required to deduct and withhold with respect to the making of such payment under the Code (as defined below), or any provision of state, local or foreign tax Law (as defined below). To the extent that amounts are so withheld by the Issuer, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the holder of the Company Shares in respect of which such deduction and withholding was made by the Issuer.
(f) Restricted Securities. The shares of Issuer Common Stock to be issued pursuant to this Agreement have not been, and will not be, registered under the U.S. Securities Act of 1933, as amended (the “Securities Act”), and will be issued in a transaction that is exempt from the registration requirements of the Securities Act. Such shares of Issuer Common Stock will be “restricted securities” under the U.S. federal securities laws and cannot be offered or resold except pursuant to registration under the Securities Act or an available exemption from registration. All certificates representing such shares of Issuer Common Stock shall bear, in addition to any other legends required under applicable securities laws, the following legend:
| |
| | “The shares represented by this certificate have not been registered under the U.S. Securities Act of 1933, as amended (the “Securities Act”), and may not be transferred except pursuant to registration under the Securities Act or pursuant to an available exemption from registration.” |
Section 1.03. The Closing. Subject to the terms and conditions of this Agreement, the Share Exchange contemplated by this Agreement shall take place at a closing (the “Closing”) to be held at the offices of Shearman & Sterling LLP, 114, avenue des Champs-Elysées, 75008, Paris, France, at 7:00 P.M., local time, on the second Business Day following the satisfaction or waiver of all conditions to the obligations of the parties set forth in Article VII (other than conditions to be satisfied at the Closing), or at such other place or at such other time or on such other date as the Company and the Issuer may mutually agree (the day on which the Closing takes place being the “Closing Date”).
Section 1.04. Closing Deliveries by the Principal Company Shareholders. (a) At the Closing, the Principal Company Shareholders or the Shareholder Representative, as appropriate, shall deliver, or cause to be delivered to the Issuer:
| |
| | (i) share transfer orders (ordres de mouvement) for all of the Company Shares held by the Principal Company Shareholders, completed pursuant to the terms hereof, a true and complete copy of theregistre des mouvements de titres, thecomptes d’actionnairesof the Company and any other documents necessary for the transfer of good and marketable title to the Shares; and |
| |
| | (ii) the certificates and other documents required to be delivered pursuant to Section 7.03. |
(b) At the Closing, the Shareholder Representative and the Issuer shall cause appropriate entries to be made in theregistre des mouvements de titres and thecomptes d’actionnairesof the Company to register the consummation of the Share Exchange.
Section 1.05. Closing Deliveries by the Issuer At the Closing, the Issuer shall deliver, or cause to be delivered, to the Shareholder Representative, on behalf of the Principal Company Shareholders:
| |
| | (a) stock certificates in the name of each Principal Company Shareholder evidencing the number of shares of Issuer Common Stock equal to (i) the whole number of shares of Issuer Common Stock |
3
| |
| | (excluding any fractional interest in shares of Issuer Common Stock) issuable to each such person in exchange for such person’s Company Shares in accordance with Section 1.02,less (ii) the sum of (A) 10% of such number of shares (representing such person’s Indemnity Escrow Shares) and (B) 1.2% of such number of shares (representing such person’s Expense Escrow Shares) (in each case, rounded down to the nearest whole number); and |
| |
| | (b) the certificates and other documents required to be delivered pursuant to Section 7.02. |
Section 1.06. Escrow Shares. (a) At or prior to the Closing, the Issuer and the Shareholder Representative shall enter into an escrow agreement (the “Indemnity Escrow Agreement”) with an escrow agent reasonably acceptable to the Issuer and the Shareholder Representative (the “Indemnity Escrow Agent”), substantially in the form ofExhibit E attached hereto. At the Closing, the Issuer shall issue and deliver to the Indemnity Escrow Agent to hold pursuant to the terms of the Indemnity Escrow Agreement stock certificates issued in the name of each Principal Company Shareholder representing the number of shares of Issuer Common Stock (the “Indemnity Escrow Shares”) otherwise issuable to such Principal Company Shareholder in the Share Exchange, but not delivered to such Principal Company Shareholder at the Closing pursuant to Section 1.05(a)(ii)(A). In connection with such deposit of the Indemnity Escrow Shares with the Indemnity Escrow Agent, each Principal Company Shareholder will be deemed to have received and deposited with the Indemnity Escrow Agent such number of shares of Issuer Common Stock.
(b) At or prior to the Closing, the Principal Company Shareholders and the Shareholder Representative shall enter into an escrow agreement (the “Expense Escrow Agreement”; and together with the Indemnity Escrow Agreement, the “Escrow Agreements”) with an escrow agent reasonably acceptable to the Principal Company Shareholders and the Shareholder Representative (the “Expense Escrow Agent”), substantially in the form ofExhibit F attached hereto. At the Closing, the Issuer shall issue and deliver to the Expense Escrow Agent to hold pursuant to the terms of the Expense Escrow Agreement stock certificates issued in the name of each Principal Company Shareholder representing the number of shares of Issuer Common Stock (the “Expense Escrow Shares”) otherwise issuable to such Principal Company Shareholder in the Share Exchange, but not delivered to such Principal Company Shareholder at the Closing pursuant to Section 1.05(a)(ii)(B). In connection with such deposit of the Expense Escrow Shares with the Expense Escrow Agent, each Principal Company Shareholder will be deemed to have received and deposited with the Expense Escrow Agent such number of shares of Issuer Common Stock.
Section 1.07. Treatment of Company Stock Options. (a) All options to purchase Company Shares (“Company Stock Options”) outstanding on the Closing Date, whether or not exercisable and whether or not vested, granted under the IDM S.A. 1998 Stock Option Plan or the IDM S.A. 2000 Stock Option Plan (collectively, the “Company Stock Option Plans”), shall remain outstanding in accordance with their existing terms following the Closing Date, subject to the provisions of this Section 1.07.
(b) Prior to the Closing, but subject to and effective as of the Closing, the Issuer shall offer to each holder of a Company Stock Option residing in France (a “French Company Optionholder”) the right to enter into, and the Issuer shall enter into with each French Company Optionholder who accepts the Issuer’s offer, an Option Liquidity Agreement, in substantially the form ofExhibit G attached hereto (an “Option Liquidity Agreement”). Each Option Liquidity Agreement shall provide, among other provisions, that in the event that the French Company Optionholder exercises such holder’s Company Stock Option, such optionholder shall transfer to the Issuer, and the Issuer shall acquire from such optionholder, each Company Share acquired by such optionholder pursuant to the exercise of a Company Stock Option in exchange for such number of shares of Issuer Common Stock equal to the Exchange Ratio, in accordance with the terms and conditions set forth in the Option Liquidity Agreement. Each Principal Company Shareholder who is also a French Company Optionholder hereby agrees to accept the Issuer’s offer made pursuant to the provisions of this Section 1.07(b) to enter into an Option Liquidity Agreement.
(c) Prior to the Closing, but subject to and effective as of the Closing, the Issuer shall offer to each holder of a Company Stock Option residing outside of France (a “Non-French Company Optionholder”), the right to waive such holder’s rights in such holder’s Company Stock Options in exchange for Substitute Options issued under, and subject to, the Issuer’s 2000 Stock Plan;provided,however, that the Substitute Option shall
4
be vested to the same extent the Company Stock Option for which the Substitute Option was exchanged was vested as of the date the Substitute Option is granted (without giving effect to any acceleration of vesting or exercisability as a result of the Transactions) and shall otherwise continue to vest in accordance with the same vesting schedule applicable to the Company Stock Option for which the Substitute Option was exchanged, and the Substitute Option shall have a term of exercisability equal to the remaining period in the term of exercisability applicable to the Company Stock Option for which the Substitute Option was exchanged (subject to earlier termination upon cessation of service of such holder in accordance with the applicable provisions of the Issuer’s 2000 Stock Plan). Notwithstanding the foregoing, in the event that the Issuer Stock Option Plan Amendment is not approved by the requisite affirmative vote of the stockholders of the Issuer, the Issuer shall not be required to offer to exchange such Non-French Company Optionholder’s waiver of rights in such optionholder’s Company Stock Options for Substitute Options, but instead the Issuer shall offer to each Non-French Company Optionholder the right to enter into, and the Issuer shall enter into with each Non-French Company Optionholder who accepts the Issuer’s offer, an Option Liquidity Agreement. Each option of the Issuer granted pursuant to this Section 1.07(c) (each, a “Substitute Option”), shall entitle its holder to acquire (i) a number of shares of Issuer Common Stock equal to the product (rounded down to the nearest whole number of shares of Issuer Common Stock) of (A) the number of Company Shares that were issuable upon exercise of the related Company Stock Option immediately prior to the Closing multiplied by (B) the Exchange Ratio; and (ii) the per share exercise price of each Substitute Option shall be equal to the quotient (rounded up to the nearest cent) arrived at by dividing (A) the per share exercise price for each related Company Stock Option (as converted from Euros to U.S. dollars as determined by using the daily 12 noon buying rate in New York, as certified by the New York Federal Reserve Bank for customs purposes and published on the website www.ny.frb.org/markets/fxrates/noon.cfm, to exchange Euros for U.S. dollars on the Closing Date) by (B) the Exchange Ratio. Each Principal Company Shareholder who is also a Non-French Company Optionholder hereby agrees to accept the Issuer’s offer made pursuant to the provisions of this Section 1.07(a) to waive such shareholder’s rights in such shareholder’s Company Stock Options in exchange for Substitute Options or to enter into an Option Liquidity Agreement, as the case may be.
(d) As soon as practicable after the Closing Date, the shares of Issuer Common Stock that may be issued under the Option Liquidity Agreements shall be registered under an effective registration statement on Form S-8 (or any successor form) or another appropriate form, and the Issuer shall use its reasonable best efforts to maintain the effectiveness of such registration statement or registration statements for as long as the Option Liquidity Agreements are in effect. In addition, the Issuer shall use its reasonable best efforts to cause the shares of Issuer Common Stock that are issuable under the Option Liquidity Agreements to be listed on Nasdaq.
(e) Prior to the Closing Date, the Issuer shall (to the extent it may do so under applicable Law) take all actions reasonably necessary to cause the transactions contemplated by this Agreement and any other acquisitions of equity securities of the Issuer (including derivative securities) in connection with this Agreement, the Option Liquidity Agreements or the Substitute Options by each individual who (i) is a director or officer of the Issuer or (ii) as of the Closing, will become a director or officer of the Issuer, to be exempt under Rule 16b-3 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Section 1.08. Treatment of Company Warrants. (a) Except with respect to the Medarex Warrants and the Sanofi Warrants or as otherwise provided in Section 1.09, each holder of warrants to purchase Company Shares (the “Company Warrants”) and the Issuer hereby agree that, at the Closing, (i) each Company Warrant outstanding immediately prior to the Closing with an exercise price of less than€6.32 shall be exchanged for the number of shares of Issuer Common Stock (rounded to the nearest whole number of shares of Issuer Common Stock) equal to the product of (A) the difference between (1) the number of Company Shares that were issuable upon exercise of the related Company Warrant immediately prior to the Closing and (2) the result of the aggregate exercise price that was payable upon the exercise of the related Company Warrant divided by€6.32 multiplied by (B) the Exchange Ratio and (ii) such holder shall irrevocably waive all of its rights in such holder’s Company Warrants outstanding immediately prior to the Closing with an exercise price of at least€6.32, effective as of the Closing.
5
(b) Medarex, Inc., a New Jersey corporation (“Medarex”) hereby agrees to exercise in full, as of immediately prior to the Closing and subject to the Closing, the warrants issued by the Company to Medarex on September 11, 2000 (the “Medarex Warrants”) to subscribe for convertible redeemable bonds (the “Convertible Redeemable Bonds”), the terms of which are set forth in the minutes of the Company extraordinary general shareholder meeting held on September 11, 2000 (which references the Unit Purchase Agreement, dated as of July 21, 2000, between the Company and Medarex), in accordance with the terms thereof, and, immediately thereafter, to convert such Convertible Redeemable Bonds received upon exercise of the Medarex Warrants into Company Shares in accordance with the terms of such Convertible Redeemable Bonds, subject to the approval of the shareholders of the Company to amend such warrants as provided in Section 6.16(a).
(c) Sanofi-Aventis S.A., asociété anonymeorganized under the laws of France (“Sanofi”), hereby agrees to exercise in full, as of immediately prior to the Closing and subject to the Closing, the warrants to purchase Company Shares issued by the Company to Sanofi on January 7, 2000 (the “Sanofi Warrants”), subject to the approval of the shareholders of the Company to amend the such warrants as provided in Section 6.16(a).
Section 1.09. Treatment of Company Securities Held in an Individual Shares Savings Plan. (a) Each shareholder of the Company who holds Company Shares in aplan d’épargne en actions(a “PEA”) as of the date of this Agreement shall have the option to either (i) exchange their Company Shares for shares of Issuer Common Stock in accordance with the terms and subject to the conditions of the Share Exchange at the Closing or (ii) enter into a Put/ Call Agreement, substantially in the form ofExhibit H attached hereto (the “Put/ Call Agreement”), with the Issuer, pursuant to which, among other things, such shareholder shall have the right to require the Issuer to purchase, and the Issuer shall have the right to require such shareholder to sell to the Issuer, the Company Shares held in the PEA, at any time for a period of 30 days after the closing of the First Equity Financing, for an aggregate purchase price, payable in cash, equal to the product of (A) the product of the number of Company Shares held in the PEA multiplied by the Exchange Ratio, multiplied by (B) the price per share of Issuer Common Stock sold in the First Equity Financing, less the underwriters’ discounts or commissions;provided,however, that, in the event that the First Equity Financing is not consummated within two years after the Closing, each Company Share subject to a Put/ Call Agreement shall be exchanged for the number of shares of Issuer Common Stock equal to the Exchange Ratio. Such shareholder shall inform the Issuer, in writing, at least five business days prior to the Closing of the option such shareholder has elected in accordance with this Section 1.09(a);provided,however, that, in the event that such shareholder shall fail to inform the Issuer of its election in accordance with this Section 1.09(a), such shareholder shall be deemed to have elected to exchange their Company Shares for shares of Issuer Common Stock in accordance with the terms and subject to the conditions of the Share Exchange. “First Equity Financing” shall mean the first offering of equity securities of the Issuer with aggregate proceeds (net of any underwriters’ discounts or commission and expenses related to the financing) of at least ten times the aggregate U.S. dollar amount which would be payable by the Issuer for the Company Shares held in a PEA in accordance with all of the Put/ Call Agreements entered into by the Issuer in accordance with this Section 1.09 completed by the Issuer after the Closing, but excluding any issuance of equity securities of the Issuer in a strategic partnering, licensing, merger or acquisition transaction.
(b) Each Company Warrant held in a PEA immediately prior to the Closing and with an exercise price of less than€6.32 immediately prior to Closing (a “PEA Warrant”) shall be exchanged, immediately after the Closing, by the Issuer for the number of Company Shares issuable to such holder upon exercise thereof less the result of the aggregate exercise price payable upon the exercise of such Company Warrant divided by 6.32. Each such holder shall have the option to either (i) exchange the Company Shares received upon exchange for the PEA Warrants for shares of Issuer Common Stock in accordance with the terms of the Share Exchange immediately after the Closing, or (ii) enter into a Put/ Call Agreement with the Issuer with respect to the Company Shares received upon exchange for the PEA Warrants. Such holder shall inform the Issuer, in writing, at least five business days prior to the Closing of the Option such holder has elected in accordance with this Section 1.09(b);provided,however, that, in the event that such holder shall fail to so inform the
6
Issuer, such holder shall be deemed to have elected to exchange its Company Shares for shares of Issuer Common Stock in accordance with the terms of the Share Exchange.
Section 1.10. Notices to Other Shareholders. (a) Promptly after the execution and delivery of this Agreement, and in any event within four days after the execution and delivery of this Agreement, the Shareholder Representative, on behalf of the Principal Company Shareholders, shall provide notice (the “Offer Notification”), substantially in the form ofExhibit I attached hereto, to the shareholders of the Company as of the date of this Agreement other than the Principal Company Shareholders (the “Other Shareholders”) pursuant to Section 6 of the Company Shareholder Agreement, together with the documents contemplated by such notices. Any Offer Notification shall be made in accordance with all applicable Laws and the parties hereto acknowledge and agree that, notwithstanding any provision to the contrary contained herein except as provided in Section 6.12(c), the Principal Company Shareholders shall not be prohibited from accepting a valid Counterbid (for purposes of this Agreement, such term shall have the meaning ascribed to it in the Company Shareholders Agreement) in accordance with the Company Shareholders Agreement. The Shareholder Representative, on behalf of the Principal Company Shareholders, shall take any other actions, reasonably requested by the Issuer or reasonably deemed necessary by the Shareholder Representative, to require all the Other Shareholders to exchange such shareholder’s Company Shares for shares of Issuer Common Stock, upon the terms and subject to the conditions of this Agreement, in accordance with Section 6 of the Company Shareholders Agreement.
(b) After delivery of the Offer Notification in accordance with Section 1.10(a) above, each Other Shareholder may submit to the Issuer a signed agreement substantially in the form ofExhibit J attached hereto (a “Joinder Agreement”). Upon execution of the Joinder Agreement by an Other Shareholder, the parties hereto acknowledge and agree that such Other Shareholder shall become a party to this Agreement and shall be deemed to be a “Principal Company Shareholder” for all purposes under this Agreement, with all of the rights and obligations of, and subject to all of the terms and conditions applicable to, a Principal Company Shareholder. The Issuer agrees to purchase Company Shares from each Other Shareholder who submits a signed Joinder Agreement to the Issuer on the terms and subject to the conditions of this Agreement.
ARTICLE II
REPRESENTATIONS AND WARRANTIES REGARDING THE COMPANY
Solely for purposes of Section 7.02(a) and Article IX of this Agreement, except as set forth in the Company Disclosure Schedule that has been delivered to the Issuer in connection with the execution and delivery of this Agreement (the “Company Disclosure Schedule”) (which Company Disclosure Schedule shall be arranged in sections corresponding to the numbered and lettered sections of this Article II, and any information disclosed in any such section of the Company Disclosure Schedule shall be deemed to be disclosed only for purposes of the corresponding section of this Article II, unless it is readily apparent that the disclosure contained in such section of the Company Disclosure Schedule contains enough information regarding the subject matter of other representations and warranties contained in this Article II as to clearly qualify or otherwise clearly apply to such other representations and warranties):
Section 2.01. Corporate Organization. (a) The Company is asociété anonymeduly organized and validly existing under the laws of France. Each subsidiary of the Company (each, a “Company Subsidiary”) is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation. Each of the Company and each Company Subsidiary has the requisite corporate power and authority and all necessary Governmental Authorizations to own, lease and operate its properties and to carry on its business as it is now being conducted, except where the failure to be so organized, existing or in good standing or to have such power, authority and governmental approvals would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of the Share Exchange or any of the other transactions contemplated by this Agreement (collectively, the “Transactions”) or prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. Each of the
7
Company and each Company Subsidiary is duly qualified or licensed as a foreign corporation to do business, and is in good standing, in each jurisdiction where the character of the properties owned, leased or operated by it or the nature of its business makes such qualification or licensing necessary, except for such failures to be so qualified or licensed and in good standing that would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
(b) A true and complete list of all the Company Subsidiaries, together with the jurisdiction of incorporation of each Company Subsidiary and the percentage of the outstanding capital stock of each Company Subsidiary owned by the Company and each other Company Subsidiary, is set forth in Section 2.01(b) of the Company Disclosure Schedule. Except for the Company Subsidiaries, the Company has not owned since January 1, 2000, and does not own, directly or indirectly, any equity or similar interest in, or any interest convertible into or exchangeable or exercisable for any equity or similar interest in, any corporation, partnership, joint venture or other business association or entity.
Section 2.02. Corporate Books and Records. (a) The Company has heretofore made available to the Issuer a complete and correct copy of its organizational documents (including bylaws, articles of association or incorporation and any equivalent governing or charter document (“Organizational Documents”)), each as amended to date, of the Company and each Company Subsidiary. Such Organizational Documents are in full force and effect. Neither the Company nor any Company Subsidiary has violated, or is in violation of, any of the provisions of its Organizational Documents, except for such violations that are not material. No event has occurred and no condition or circumstance exists that would reasonably be expected (with or without notice or lapse of time) to constitute or result, directly or indirectly, in a material violation of the provisions of the Organizational Documents of the Company or any Company Subsidiary.
(b) The attendance register of the Company’sConseil d’Administration,the minutes of theConseil d’Administrationand the shareholders meetings and the share registers (theregistre des mouvements de titresand thecomptes d’actionnaires) required by the FrenchCode de Commerceare up-to-date, have been fully maintained and contain true and accurate records in all material respects. There have been no meetings or proceedings of the Company’sConseil d’Administrationor the shareholders of the Company, other than those reflected in the Company’s records. Complete and accurate copies of all such records and of the stock register of the Company have been made available by the Company to the Issuer.
Section 2.03. Capitalization. (a) As of the date of this Agreement, (i) 5,951,121 Company A Shares and 9,743,325 Company B Shares are issued and outstanding, validly issued, fully paid and not subject to calls for future contributions, (ii) no Company A Shares or Company B Shares are held in the treasury of the Company, or held by subsidiaries of the Company; (iii) 1,167,747 Company A Shares may be issued pursuant to outstanding Company Stock Options granted pursuant to the Company Stock Option Plans, and (iv) 4,242,822 Company Shares may be issued pursuant to the outstanding Company Warrants, all of which are listed in Section 2.03(a) of the Company Disclosure Schedule. Except as set forth in this Section 2.03, there are no options, warrants, convertible securities or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued capital stock of the Company or any Company Subsidiary or obligating the Company or any Company Subsidiary to issue or sell any shares of capital stock of, or other equity interests in, the Company or any Company Subsidiary. Section 2.03(a) of the Company Disclosure Schedule sets forth the following information with respect to each Company Stock Option as of the date of this Agreement, (i) the name of the Company Stock Option recipient; (ii) the particular plan pursuant to which such Company Stock Option was granted; (iii) the number of Company Shares subject to such Company Stock Option; (iv) the exercise or purchase price of such Company Stock Option; (v) the date on which such Company Stock Option was granted; (vi) the applicable vesting schedule; (vii) the date on which such Company Stock Option expires and (viii) whether the exercisability of or right to repurchase of such Company Stock Option will be accelerated in any way by the transactions contemplated by this Agreement, and indicates the extent of acceleration. The Company has made available to the Issuer accurate and complete copies of all Company Stock Option Plans pursuant to which the Company has granted such Company Stock Options that are currently outstanding and the form of all stock award agreements evidencing
8
such Company Stock Options. All Company Shares subject to issuance as aforesaid, upon issuance on the terms and conditions specified in the instruments pursuant to which they are issuable, will be duly authorized, validly issued, fully paid and not subject to calls for future contributions. Except as set forth in Section 2.03(a) of the Company Disclosure Schedule, there are no outstanding contractual obligations of the Company or any Company Subsidiary to repurchase, redeem or otherwise acquire any Company Shares or any capital stock of any Company Subsidiary or to provide funds to, or make any investment (in the form of a loan, capital contribution or otherwise) in, any Company Subsidiary or any other person. Except as set forth in Section 2.03(a) of the Company Disclosure Schedule, there are no commitments or agreements of any character to which the Company is bound obligating the Company to accelerate the vesting of any Company Stock Option as a result of the Transactions. All outstanding Company Shares, all outstanding Company Stock Options, and all outstanding shares of capital stock of each subsidiary of the Company have been issued and granted in compliance with (i) all applicable securities Laws and other applicable Laws and (ii) all requirements set forth in applicable contracts.
(b) Each outstanding share of capital stock of each Company Subsidiary is duly authorized, validly issued, fully paid and nonassessable, and each such share is owned by the Company or another Company Subsidiary free and clear of all security interests, liens, claims, pledges, options, rights of first refusal, agreements, limitations on the Company’s or any Company Subsidiary’s voting rights, charges and other encumbrances of any nature whatsoever.
Section 2.04. Authority Relative to This Agreement. No corporate proceedings on the part of the Company are necessary to authorize this Agreement or to consummate the Transactions (other than the vote of the shareholders of the Company set forth in Section 2.18(b) at the Company Shareholders’ Meeting).
Section 2.05. No Conflict; Required Filings and Consents. (a) The consummation of the Share Exchange and the other Transactions will not (i) conflict with or violate the Organizational Documents of the Company or any Company Subsidiary, (ii) assuming that all consents, approvals, authorizations and other actions described in Section 2.05(b) have been obtained and all filings and obligations described in Section 2.05(b) have been made, conflict with or violate any Law applicable to the Company or any Company Subsidiary or by which any property or asset of the Company or any Company Subsidiary is bound or affected, (iii) cause the Company, any Company Subsidiary, the Issuer or any of the Issuer’s affiliates to become subject to, or liable for the payment of, any Tax (except as set forth in Section 2.05(a) of the Company Disclosure Schedule), (iv) cause any of the assets owned or used by the Company or any Company Subsidiary to be reassessed or revalued by any taxing authority, or (v) result in any breach of or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any right of termination, amendment, acceleration or cancellation of, or result in the creation of a lien or other encumbrance on any property or asset of the Company or any Company Subsidiary pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which the Company or any Company Subsidiary is a party or by which the Company or any Company Subsidiary or any of their assets or properties is bound or affected, except, with respect to clauses (ii) and (iii), for any such conflicts, violations, breaches, defaults or other occurrences which would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
(b) The consummation of the Share Exchange will not require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority, except (i) for applicable requirements, if any, of the Exchange Act, state securities or “blue sky” laws (“Blue Sky Laws”) and state takeover laws; (ii) the pre-merger notification and waiting period requirements of the Hart-Scott-Rodino Improvements Act of 1976, as amended, and the rules and regulations promulgated thereunder (the “HSR Act”), applicable with respect to any Principal Company Shareholder that would acquire shares of Issuer Common Stock valued (in accordance with the HSR Act) at $50 million or more in connection with the Share Exchange; and (iii) where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not reasonably be expected, individually or in the aggregate, to prevent or materially delay
9
consummation of any of the Transactions, or otherwise prevent or materially delay the Company from performing its obligations under this Agreement, and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
Section 2.06. Permits; Compliance. (a) Each of the Company and the Company Subsidiaries is in possession of all franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority (other than the Drug Regulatory Agencies (as defined below)) necessary for each of the Company or the Company Subsidiaries to own, lease and operate its properties or to carry on its business as it is now being conducted (the “Company Permits”), except where the failure to have, or the suspension or cancellation of, any of the Company Permits would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. Each material Company Permit is listed in Section 2.06(a) of the Company Disclosure Schedule. The Company has made available to the Issuer complete and accurate copies of each Company Permit, including all amendments and renewals thereto, listed in Section 2.06(a) of the Company Disclosure Schedule. As of the date of this Agreement, each Company Permit is valid and in full force and effect and no suspension or cancellation of any of the Company Permits is pending or, to the Knowledge of the Company, threatened, except where the failure to have, or the suspension or cancellation of, any of the Company Permits would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. The Company and the Company Subsidiaries are, and have at all times been, in full compliance with all of the terms and requirements of each Company Permit, except where the failure to be in compliance would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. No event has occurred and no condition or circumstance exists that would reasonably be expected (with or without notice or lapse of time) to constitute or result, directly or indirectly, in a violation of or a failure to comply with any term or requirement of any Company Permit or result, directly or indirectly, in the revocation, withdrawal, suspension, cancellation, termination or modification of any Company Permit, except where the violation, failure to comply, revocation, withdrawal, suspension, cancellation, termination or modification would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. Since January 1, 2002, neither the Company nor any of the Company Subsidiaries has received any written communication regarding (A) any actual, alleged, possible or potential violation of, or failure to comply with, any term or requirement of any material Company Permit or (B) any actual, proposed, possible or potential revocation, withdrawal, suspension, cancellation, termination or adverse modification of any material Company Permit. All applications required to have been filed for the renewal of each Company Permit have been duly filed on a timely basis, an d each other notice or filing required to have been given or made with respect to such Company Permit has been duly given or made on a timely basis, except where the failure to make such filing would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. Neither the Company nor any Company Subsidiary is in conflict with, or in default, breach or violation of, (i) any Law applicable to the Company or any Company Subsidiary or by which any property or asset of the Company or any Company Subsidiary is bound or affected, or (ii) any note, bond, mortgage, indenture, contract, agreement, lease, license, Company Permit, franchise or other instrument or obligation to which the Company or any Company Subsidiary is a party or by which the Company or any Company Subsidiary or any property or asset of the Company or any Company Subsidiary is bound, except for any such conflicts, defaults, breaches
10
or violations that would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
(b) Each of the Company and the Company Subsidiaries holds all new drug applications, abbreviated new drug applications, product license applications, investigational new drug applications, product export applications and other approvals issuable by the U.S. Food and Drug Administration (the “FDA”), the European Agency for the Evaluation of Medical Products (the “EMEA”) or theAgence Francaise de Securite Sanitaire des Produits de Sante(the “Agence Francaise”, and together with the FDA and EMEA, the “Drug Regulatory Agencies”) (the “Company Regulatory Permits”) necessary for the conduct of the business of the Company and the Company Subsidiaries as currently conducted and the development, clinical testing, manufacturing, sale, marketing, distribution and importation or exportation, as currently conducted, of any of their products or product candidates, including Mepact, Bexidem, Jenact, Uvidem, Colidem, Dc Ova and Liposomal KSA (the “Company Product Candidates”) and no such Company Regulatory Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner, other than immaterial adverse modifications. The Company is in compliance in all material respects with the Company Regulatory Permits and has not received any written notice or other written communication from any Drug Regulatory Agency regarding (i) any actual or possible violation of or failure to comply with any term or requirement of any Company Regulatory Permit or (ii) any actual or possible revocation, withdrawal, suspension, cancellation, termination or modification of any Company Regulatory Permit. Except for the information and files identified in Section 2.06(b) of the Company Disclosure Schedule, the Company has made available to the Issuer all information in its possession or control relating to the Company Product Candidates and the development, clinical testing, manufacture and sale of the Company Product Candidates, including complete and correct copies of the following (to the extent there are any): (i) adverse event reports; clinical study reports and material study data; and inspection reports, notices of adverse findings, warning letters, filings and letters and other correspondence with any Drug Regulatory Agency; and (ii) similar reports, study data, notices, letters, filings and correspondence with any other Governmental Authority.
(c) All clinical, pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, the Company or in which the Company or its current products or product candidates, including the Company Product Candidates, have participated were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance with the applicable regulations of the Drug Regulatory Agencies and other applicable Laws. All quality control, record keeping, notification, reporting and manufacturing systems used for the Company’s commercial products and for the manufacturing of clinical supplies conforms to the applicable regulations of the Drug Regulatory Agencies and good manufacturing practices.
Section 2.07. Financial Statements. (a) True and complete copies of the audited consolidated balance sheet of the Company for each of the three fiscal years ended as of December 31, 2001, 2002 and 2003, and the related audited consolidated statements of income, retained earnings, shareholders’ equity and changes in financial position of the Company, together with all related notes and schedules thereto, accompanied by the reports thereon of the independent accountants of the Company (collectively, referred to herein as, the “Company Financial Statements”) and the unaudited consolidated balance sheet of the Company as of September 30, 2004, and the related consolidated statements of income, retained earnings, stockholders’ equity and changes in financial position of the Company, together with all related notes and schedules thereto (collectively referred to herein as the “Company Interim Financial Statements”) have been delivered by the Company to the Issuer. The Company Financial Statements and the Company Interim Financial Statement (i) were prepared in accordance with the books of account and other financial records of the Company and the Company Subsidiaries, (ii) give a true and fair view (image sincère et fidèle) of the consolidated financial condition and results of operations of the Company and the Company Subsidiaries as of the dates thereof or for the periods covered thereby, (iii) have been prepared in accordance with French generally accepted accounting principles (“French GAAP”) applied on a basis consistent with the past practices of the Company and the Company Subsidiaries and (iv) include all adjustments (consisting only of
11
normal recurring accruals) that are necessary for a fair presentation of the consolidated financial condition of the Company and the Company Subsidiaries and the results of operations of the Company and the Company Subsidiaries as of the dates thereof or for the periods covered thereby.
(b) True and correct copies of a reconciliation to U.S. GAAP of the consolidated statements of income and shareholders’ equity of the Company for the fiscal year ended December 31, 2003 and consolidated statements of income and shareholders’ equity of the Company for the nine months ended September 30, 2004 and a balance sheet as of September 30, 2004 have been delivered by the Company to the Issuer.
(c) The books of account and other financial records of the Company and the Company Subsidiaries: (i) reflect all items of income and expense and all assets and Liabilities required to be reflected therein in accordance with French GAAP applied on a basis consistent with the past practices of the Company and the Company Subsidiaries, respectively, (ii) are in all material respects complete and correct, and do not contain or reflect any material inaccuracies or discrepancies and (iii) have been maintained in accordance with good business and accounting practices.
(d) Except as and to the extent set forth on the consolidated balance sheet of the Company and the consolidated Company Subsidiaries as at September 30, 2004, including the notes thereto, neither the Company nor any Company Subsidiary has any liability of any nature (whether accrued, absolute, contingent or otherwise) that would be required to be reflected on a balance sheet prepared in accordance with French GAAP, except for Liabilities, (i) incurred pursuant to this Agreement and the Transactions; or (ii) incurred in the ordinary course of business consistent with past practice since September 30, 2004, which, individually or in the aggregate, would not reasonably be expected to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
(e) The Company has in place internal controls over financial reporting that are designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with French GAAP and include policies and procedures that provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with French GAAP, and that receipts and expenditures of the Company are being made only in accordance with authorization of management and the advisors of the Company.
(f) Since January 1, 2002, there have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer or general counsel of the Company, the Company Board or any committee thereof, other than ordinary course audits or reviews of accounting policies and practices or internal controls required by applicable Law.
Section 2.08. Absence of Certain Changes or Events. Since September 30, 2004, except as expressly contemplated by this Agreement, (a) the Company and the Company Subsidiaries have conducted their businesses only in the ordinary course and in a manner consistent with past practice; (b) there has not been any Company Material Adverse Effect or any event, condition or circumstance that would reasonably be expected to have a Company Material Adverse Effect; and (c) none of the Company or any Company Subsidiary has taken any action that, if taken after the date of this Agreement, would constitute a breach of any of the covenants set forth in Section 5.01.
Section 2.09. Absence of Litigation. There is no litigation, suit, claim, action, proceeding or investigation (an “Action”) pending or, to the Knowledge of the Company, threatened against the Company or any Company Subsidiary, or any property or asset of the Company or any Company Subsidiary, before any Governmental Authority that (a) individually or in the aggregate, has had, or would reasonably be expected to have, a Company Material Adverse Effect or (b) seeks to, directly or indirectly, delay, prevent, make illegal or otherwise interfere with the consummation of any of the Transactions. Neither the Company nor any Company Subsidiary nor any property or asset of the Company or any Company Subsidiary is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with, or, to the
12
Knowledge of the Company, continuing investigation by, any Governmental Authority, or any effective or, to the Knowledge of the Company, proposed order, writ, judgment, injunction, decree, determination or award of any Governmental Authority, that would reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement or would reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
Section 2.10. Employee Benefit Plans. (a) Section 2.10(a) of the Company Disclosure Schedule lists: (i) all employee benefit plans (as defined in, and whether or not subject to, Section 3(3) of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”)) and all bonus, stock option, stock purchase, restricted stock, incentive, deferred compensation, retiree medical or life insurance, supplemental retirement, severance or other benefit plans, programs or arrangements, and all employment, termination, severance or other contracts or agreements, to which the Company or any Company Subsidiary is a party, with respect to which the Company or any Company Subsidiary has any obligation or which are maintained, contributed to or sponsored by the Company or any Company Subsidiary for the benefit of any current or former employee, officer or director of the Company or any Company Subsidiary, (ii) each employee benefit plan for which the Company or any Company Subsidiary could incur liability under Section 4069 of ERISA in the event such plan has been or were to be terminated, (iii) any plan in respect of which the Company or any Company Subsidiary could incur liability under Section 4212(c) of ERISA, and (iv) any contracts, arrangements or understandings between the Company or any Company Subsidiary and any employee of the Company or any Company Subsidiary including, without limitation, any contracts, arrangements or understandings relating in any way to a sale of the Company or any Company Subsidiary or the consummation of any Transaction (collectively, the “Company Plans”). The Company has made available to the Issuer a true and complete copy of (i) such Company Plans, (ii) the most recently filed Internal Revenue Service (“IRS”) Form 5500, if any, (iii) the most recent summary plan description for each Company Plan for which a summary plan description is required by applicable law, (iv) the most recently received IRS determination letter, if any, issued by the IRS with respect to any Company Plan that is intended to qualify under Section 401(a) of the Code, and (v) the most recently prepared actuarial report or financial statement, if any, relating to a Company Plan.
(b) None of the Company Plans is a multiemployer plan (within the meaning of Section 3(37) or 4001(a)(3) of ERISA) (a “Multiemployer Plan”) or a single employer pension plan (within the meaning of Section 4001(a)(15) of ERISA) for which the Company or any Company Subsidiary could incur liability under Section 4063 or 4064 of ERISA (a “Multiple Employer Plan”). Except as set forth in Section 2.10(b) of the Company Disclosure Schedule or in accordance with applicable Law, none of the Company Plans (i) provides for the payment of separation, severance, termination or similar-type benefits to any person, (ii) obligates the Company or any Company Subsidiary to pay separation, severance, termination or similar-type benefits solely or partially as a result of any transaction contemplated by this Agreement, or (iii) obligates the Company or any Company Subsidiary to make any payment or provide any benefit as a result of a “change in control”, within the meaning of such term under Section 280G of the Code. Except as set forth in Section 2.10(b) of the Company Disclosure Schedule or in accordance with applicable Law, none of the Company Plans provides for or promises retiree medical, disability or life insurance benefits to any current or former employee, officer or director of the Company or any Company Subsidiary.
(c) Each Company Plan is now and always has been operated in all material respects in accordance with its terms and the requirements of all applicable Laws, including, without limitation, ERISA and the Code. The Company and the Company Subsidiaries have performed all material obligations required to be performed by them under, are not in any material respect in default under or in violation of, and to the Knowledge of the Company, there have not been any defaults or violations by any party to, any Company Plan. No Action is pending or, to the Knowledge of the Company, threatened with respect to any Company Plan (other than claims for benefits in the ordinary course) that would reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect and, to the Knowledge of the Company, no fact or event exists that would reasonably be expected to give rise to any such Action.
13
(d) Each Company Plan that is intended to be qualified under Section 401(a) of the Code or Section 401(k) of the Code has timely received a favorable determination letter from the IRS covering all of the provisions applicable to the Company Plan for which determination letters are currently available that the Company Plan is so qualified and each trust established in connection with any Company Plan which is intended to be exempt from federal income taxation under Section 501(a) of the Code has received a determination letter from the IRS that it is so exempt, and no fact or event has occurred since the date of such determination letter or letters from the IRS to adversely affect the qualified status of any such Company Plan or the exempt status of any such trust.
(e) There has not been any prohibited transaction (within the meaning of Section 406 of ERISA or Section 4975 of the Code) with respect to any Company Plan. Neither the Company nor any Company Subsidiary has incurred any liability under, arising out of or by operation of Title IV of ERISA (other than liability for premiums to the Pension Benefit Guaranty Corporation arising in the ordinary course), including, without limitation, any liability in connection with (i) the termination or reorganization of any employee benefit plan subject to Title IV of ERISA, or (ii) the withdrawal from any Multiemployer Plan or Multiple Employer Plan, and no fact or event exists which would reasonably be expected to give rise to any such liability.
(f) All material contributions, premiums or payments required to be made with respect to any Company Plan have been made on or before their due dates. All such contributions have been fully deducted for income tax purposes, to the extent applicable, and no such deduction has been challenged or disallowed by any Governmental Authority and no fact or event exists which would reasonably be expected to give rise to any such challenge or disallowance.
(g) In addition to the foregoing, with respect to each Company Plan that is not subject to United States Law (a “Non-U.S. Company Plan”):
| |
| | (i) all employer and employee contributions to each Non-U.S. Company Plan required by Law or by the terms of such Non-U.S. Company Plan have been made, or, if applicable, accrued in accordance with normal accounting practices, and a pro rata contribution for the period prior to and including the date of this Agreement has been made or accrued; |
| |
| | (ii) the fair market value of the assets of each funded Non-U.S. Company Plan, the liability of each insurer for any Non-U.S. Company Plan funded through insurance or the book reserve established for any Non-U.S. Company Plan, together with any accrued contributions, is sufficient to procure or provide for the benefits determined on any ongoing basis (actual or contingent) accrued to the date of this Agreement with respect to all current and former participants under such Non-U.S. Company Plan according to the actuarial assumptions and valuations most recently used to determine employer contributions to such Non-U.S. Company Plan, and no Transaction shall cause such assets or insurance obligations to be less than such benefit obligations; and |
| |
| | (iii) each Non-U.S. Company Plan required to be registered has been registered and has been maintained in good standing with applicable regulatory authorities. Each Non-U.S. Company Plan is now and always has been operated, in all material respects, in compliance with all applicable non-United States Laws. |
Section 2.11. Labor and Employment Matters. (a) Except as set forth in Section 2.11(a) of the Company Disclosure Schedule:
| |
| | (i) there are no controversies pending or, to the Knowledge of the Company, threatened between the Company or any Company Subsidiary and any of their respective employees; |
| |
| | (ii) neither the Company nor any Company Subsidiary is a party to any collective bargaining agreement or other labor union contract applicable to persons employed by the Company or any Company Subsidiary, nor, to the Knowledge of the Company, are there any activities or proceedings of any labor union to organize any such employees; |
14
| |
| | (iii) neither the Company nor any Company Subsidiary has breached or otherwise failed to comply with any provision of any such agreement or contract, and there are no grievances outstanding against the Company or any Company Subsidiary under any such agreement or contract, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect; |
| |
| | (iv) there are no unfair labor practice complaints pending against the Company or any Company Subsidiary before the National Labor Relations Board or any other Governmental Authority, nor any current union representation questions involving employees of the Company or any Company Subsidiary; and |
| |
| | (v) there is no strike, slowdown, work stoppage, labor dispute or lockout, or, to the Knowledge of the Company, threat thereof, by or with respect to any employees of the Company or any Company Subsidiary. To the Knowledge of the Company, no event has occurred and no condition or circumstance exists that would reasonably be expected to, directly or indirectly, give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, labor dispute or lockout, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. |
(b) No consent is required pursuant to any collective bargaining agreement set forth in Section 2.11(a) of the Company Disclosure Schedule to consummate the Transactions.
(c) Except as set forth in Section 2.11(c) of the Company Disclosure Schedule:
| |
| | (i) the Company and the Company Subsidiaries are in compliance with all applicable laws relating to the employment of labor, including those related to wages, hours, overtime, collective bargaining and the payment and withholding of taxes and other sums as required by the appropriate Governmental Authority and have withheld and paid to the appropriate Governmental Authority or are holding for payment not yet due to such Governmental Authority all amounts required to be withheld from employees of the Company or any Company Subsidiary and are not liable for any arrears of wages, taxes, penalties or other sums for failure to comply with any of the foregoing, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect; |
| |
| | (ii) the Company and the Company Subsidiaries have paid in full to all employees or adequately accrued for in accordance with French GAAP consistently applied all wages, salaries, commissions, bonuses, benefits and other compensation due to or on behalf of such employees and there is no claim with respect to payment of wages, salary or overtime pay that has been asserted or is now pending or threatened before any Governmental Authority with respect to any persons currently or formerly employed by the Company or any Company Subsidiary, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect; |
| |
| | (iii) neither the Company nor any Company Subsidiary is a party to, or otherwise bound by, any consent decree with, or citation by, any Governmental Authority relating to employees or employment practices; |
15
| |
| | (iv) there is no charge or proceeding with respect to a violation of any occupational safety or health standards that has been asserted or is now pending or, to the Knowledge of the Company, threatened with respect to the Company; and |
| |
| | (v) there is no charge of discrimination in employment or employment practices, for any reason, including, without limitation, age, gender, race, religion or other legally protected category, which has been asserted or is now pending or, to the Knowledge of the Company, threatened before the United States Equal Employment Opportunity Commission, or any other Governmental Authority in any jurisdiction in which the Company or any Company Subsidiary has employed or employ any person. |
(d) All officers, management employees, and technical and professional employees of the Company and the Company Subsidiaries are under written obligation to the Company and the Company Subsidiaries to maintain in confidence all confidential or proprietary information acquired by them in the course of their employment and to assign to the Company and the Company Subsidiaries all inventions made by them within the scope of their employment during such employment and for a reasonable period thereafter and copies of the forms of all such written obligations have been made available by the Company to the Issuer.
(e) Section 2.11(e) of the Company Disclosure Schedule accurately identifies each former employee of the Company or any Company Subsidiary who is receiving or is scheduled to receive (or whose spouse or other dependent is receiving or it scheduled to receive) any benefits relating to such former employee’s employment with the Company or any Company Subsidiary and accurately describes such benefits.
(f) To the Knowledge of the Company, no officer of the Company or any Company Subsidiary: (i) intends to terminate his employment with the Company or Company Subsidiary; or (ii) is a party to or is bound by any confidentiality agreement, noncompetition agreement or other contract that may have an adverse effect on: (A) the performance by such employee of any of his duties or responsibilities as an employee of the Company or Company Subsidiary, or (B) the Company’s business or operations.
(g) Section 2.11(g) of the Company Disclosure Schedule accurately sets forth, with respect to each person that was an independent contractor of the Company or Company Subsidiary as of the Effective Date: (i) the name of such independent contractor and the date as of which such independent contractor was originally hired, (ii) a description of such independent contractor duties and responsibilities, (iii) the aggregate dollar amount of the compensation (including all payments or benefits of any type) received by such independent contractor from the Company or any Company Subsidiary with respect to services performed in 2004, (iv) the terms of compensation of such independent contractor, and (v) any permit, authorization, franchise or other right that is held by such independent contractor and that relates to or is useful in connection with the Company’s business.
(h) None of the current or former independent contractors of the Company or any Company Subsidiary should be reclassified as an employee under applicable Law.
Section 2.12. Property and Leases. (a) Section 2.12(a) of the Company Disclosure Schedule lists each parcel of real property currently or formerly owned by the Company or any Company Subsidiary. Each parcel of real property owned by the Company or any Company Subsidiary (i) is owned free and clear of all mortgages, pledges, liens, security interests, conditional and installment sale agreements, encumbrances, charges or other claims of third parties of any kind, including, without limitation, any easement, right of way or other encumbrance to title, or any option, right of first refusal, or right of first offer (collectively, “Liens”), other than Permitted Liens, and (ii) is neither subject to any Governmental Order to be sold nor is being condemned, expropriated or otherwise taken by any public authority with or without payment of compensation therefor, nor, to the Knowledge of the Company, has any such condemnation, expropriation or taking been proposed.
(b) Section 2.12(b) of the Company Disclosure Schedule lists each parcel of real property currently leased or subleased by the Company or any Company Subsidiary, with the name of the lessor and the date of the lease, sublease, assignment of the lease, any guaranty given or leasing commissions payable by the Company or any Company Subsidiary in connection therewith and each amendment to any of the foregoing (collectively, the “Company Lease Documents”). True, correct and complete copies of all Company Lease
16
Documents have been made available to the Issuer. All such current leases and subleases are in full force and effect, are valid and effective in accordance with their respective terms, and there is not, under any of such leases, any existing material default or event of default (or event which, with notice or lapse of time, or both, would constitute a default) by the Company or any Company Subsidiary or, to the Company’s Knowledge, by the other party to such lease or sublease, or person in the chain of title to such leased premises.
(c) Except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect: (i) there are no contractual or legal restrictions that preclude or restrict the ability to use any real property owned or leased by the Company or any Company Subsidiary for the purposes for which it is currently being used; and (ii) there are no material latent defects or material adverse physical conditions affecting the real property, and improvements thereon, owned or leased by the Company or any Company Subsidiary.
(d) Each of the Company and the Company Subsidiaries has good and valid title to, or, in the case of leased properties and assets, valid leasehold or subleasehold interests in, all of its properties and assets, tangible and intangible, real, personal and mixed, used or held for use in its business, free and clear of any Liens, except for such imperfections of title, if any, that do not materially interfere with the present value of the subject property.
Section 2.13. Intellectual Property. (a) (i) Section 2.13(a)(i) of the Company Disclosure Schedule lists (A) each item of Intellectual Property owned (in whole or in part) by the Company or a Company Subsidiary and which is the subject of a registration (“Company Registered Intellectual Property”) or an application for registration, (B) the jurisdiction(s) in which such item of Intellectual Property has been filed or registered, if any, and the applicable registration or serial number, and (C) any other person that has an ownership interest in such item of Intellectual Property, and (ii) Section 2.13(a)(ii) of the Company Disclosure Schedule lists each material item of Intellectual Property licensed to the Company or a Company Subsidiary (“Company Licensed Intellectual Property”).
(b) Except as set forth in Section 2.13(b) of the Company Disclosure Schedule:
| |
| | (i) to the Knowledge of the Company, the conduct of the business of the Company and the Company Subsidiaries as currently conducted does not infringe upon or misappropriate the Intellectual Property rights of any third party; |
| |
| | (ii) the Company and the Company Subsidiaries own or are licensed to use all Intellectual Property used in or, to the Knowledge of the Company, necessary for the conduct of their businesses as currently conducted; |
| |
| | (iii) there are no claims pending or, to the Knowledge of the Company, threatened, against the Company or any Company Subsidiary (A) alleging that the conduct of the business of the Company and the Company Subsidiaries as currently conducted infringes upon or may infringe upon or misappropriates the Intellectual Property rights of any third party or (B) challenging the ownership, use, validity or enforceability of any Company Owned Intellectual Property or Company Licensed Intellectual Property; |
| |
| | (iv) with respect to each item of Company Owned Intellectual Property, the Company or a Company Subsidiary is the exclusive owner of the entire right, title and interest in and to such Company Owned Intellectual Property free and clear of all liens, encumbrances and other restrictions, and is entitled to use such Company Owned Intellectual Property in the continued operation of its respective business; |
| |
| | (v) there are no settlements, forbearances to sue, consents, judgments, orders or similar obligations that (A) restrict the business of the Company and the Company Subsidiaries in or under any Intellectual Property rights of any third party or (B) with respect to Company Owned Intellectual Property that is exclusively owned by the Company or a Company Subsidiary, permit any third party to use such Company Owned Intellectual Property; |
17
| |
| | (vi) with respect to each item of Company Licensed Intellectual Property, the Company or a Company Subsidiary has the right to use such Company Licensed Intellectual Property in the continued operation of its respective business in accordance with the terms of the license agreement governing such Company Licensed Intellectual Property; |
| |
| | (vii) the Company Registered Intellectual Property has not been adjudged invalid or unenforceable in whole or in part and, to the Knowledge of the Company, is valid and enforceable. Without limiting the foregoing: |
| |
| | (A) each U.S. patent application and U.S. patent in which the Company has or purports to have an ownership interest and which is relevant to the Company Product Candidates was filed within one year of the first printed publication, public use or offer for sale of each invention described in such U.S. patent application or U.S. patent; |
| |
| | (B) each non-U.S. patent application and non-U.S. patent in which the Company has or purports to have an ownership interest and which is relevant to the Company Product Candidates was filed or claims priority to a patent application filed, before the time at which each invention described in such non-U.S. patent application or non-U.S. patent was first made available to the public; |
| |
| | (C) to the Knowledge of the Company, no trademark (whether registered or unregistered) or trade name used by the Company or any Company Subsidiary infringes any trademark (whether registered or unregistered) or trade name of any third party; |
| |
| | (D) the Company has not incurred an impairment charge to goodwill associated with or inherent in any trademark (whether registered or unregistered) in which the Company has or purports to have an ownership interest; and |
| |
| | (E) each item of Company Registered Intellectual Property is, and at all times has been, in compliance in all material respects with all requirements under applicable Laws, and all filings, payments and other actions required to be made or taken to maintain such item of Company Registered Intellectual Property in full force and effect have been made by the applicable deadline, except as would not have a Company Material Adverse Effect; |
| |
| | (viii) the Company has made available to the Issuer complete and accurate copies of all applications, correspondence and other material documents related to each item of Company Registered Intellectual Property, subject to any such applications, correspondence and other documents withheld for reasons related to attorney-client privilege; |
| |
| | (ix) no interference, opposition, reissue, reexamination or other proceeding of any nature is or has been pending or, to the Knowledge of the Company, threatened, in which the scope, validity or enforceability of any Company Registered Intellectual Property is being, has been or could reasonably be expected to be contested or challenged; |
| |
| | (x) to the Knowledge of the Company, there is no legitimate basis for a claim that any Company Owned Intellectual Property is invalid or unenforceable; |
| |
| | (xi) to the Knowledge of the Company, no person is engaging, or has engaged, in any activity that materially infringes upon the Company Owned Intellectual Property; |
| |
| | (xii) to the Knowledge of the Company, each license of the Company Licensed Intellectual Property is valid and enforceable, is binding on all parties to such license, and is in full force and effect; |
| |
| | (xiii) to the Knowledge of the Company, no party to any license of the Company Licensed Intellectual Property is in breach thereof or default thereunder; |
| |
| | (xiv) neither the execution of this Agreement nor the consummation of any Transaction will, with or without notice or the lapse of time, result in or give any third party the right or option to cause or declare: (A) any loss or, or encumbrance on, any Company Owned Intellectual Property, (B) the release, |
18
| |
| | disclosure or delivery of any Company Owned Intellectual Property by or to any escrow agent or other third party, or (C) an adverse affect on any of the Company’s material rights with respect to the Company Owned Intellectual Property or the Company Licensed Intellectual Property; and |
| |
| | (xv) the Company has made available to the Issuer an accurate copy of each of its standard form of (A) end-user license agreement; (B) employee or independent contract agreement containing any assignment or license of Intellectual Property or any confidentiality provision; and (C) confidentiality or non-disclosure agreement. |
Section 2.14. Taxes. (a) The Company and the Company Subsidiaries prepared in compliance with all applicable Laws and have filed all French, federal, local and non-French Tax returns and reports required to be filed by them and have paid and discharged all Taxes required to be paid or discharged, other than such payments as are being contested in good faith by appropriate proceedings and with respect to which adequate reserves for payment have been established on the Company Interim Financial Statements. All such Tax returns are true, accurate and complete in all material respects. No such Tax returns have ever been examined or audited by any Governmental Authority.
(b) Neither the French nor any non-French taxing authority or agency is now asserting or, to the Knowledge of the Company, threatening to assert, against the Company or any Company Subsidiary any material deficiency or claim for any Taxes or interest thereon or penalties in connection therewith. No claim or Action is pending or, to the Knowledge of the Company, threatened against or with respect to any of the Company or the Company Subsidiaries in respect of any Tax.
(c) Neither the Company nor any Company Subsidiary has granted any waiver of any statute of limitations with respect to, or any extension of a period for the assessment of, any Tax.
(d) The accruals and reserves for Taxes reflected in the Company Interim Financial Statements are adequate to cover all Taxes accruable through September 30, 2004 (including interest and penalties, if any, thereon) in accordance with French GAAP. On or before the Closing Date, the Company will establish, in the ordinary course of business and consistent with past practices, reserves adequate for the payment of all Taxes for the period from the date of the Company Interim Financial Statements through the Closing Date.
(e) There are no Tax liens upon any property or assets of the Company or any of the Company Subsidiaries except liens for current Taxes not yet due.
(f) There is no agreement, plan, arrangement or other contract covering, benefiting or relating to any employee of the Company or the Company Subsidiaries that, considered individually or considered collectively with any other such contracts, could reasonably be expected to give rise directly or indirectly to the payment of any amount that would not be deductible pursuant to Section 280G or Section 162 of the Code (or any comparable provision of French federal, local and non French Tax laws). None of the Company or the Company Subsidiaries is, or has ever been, a party to or bound by any tax indemnity agreement, tax sharing agreement, tax allocation agreement or similar arrangement. None of the Company or the Company Subsidiaries is a party to any agreement to compensate any third party for excise taxes payable pursuant to Section 4999 of the Code.
(g) No claim has ever been made by any Governmental Authority in a jurisdiction where the Company or a Company Subsidiary does not file a Tax return that such Company or Company Subsidiary is or may be subject to taxation by that jurisdiction. None of the Company or the Company Subsidiaries: (i) is liable for Taxes of any other person, or is currently under any contractual obligation to indemnify any person with respect to any portion of such person’s Taxes (except for customary agreements to indemnify lenders or security holders in respect of Taxes); or (ii) is a party to or bound by any agreement or obligation providing for payments by the Company or Company Subsidiaries with respect to any amount of Taxes of any other person.
(h) The Company and the Company Subsidiaries will have no federal, state, local, or foreign tax or other Liability arising out of the Transactions. No Tax liability or restriction, reduction or limitation on or of favorable Tax attributes (such as losses or credits, loss or credit carryovers, basis or deduction) will arise directly or indirectly as a result of the Transactions. Section 2.14(h) of the Company Disclosure Schedule sets
19
forth the amount of available net operating loss and credit carryovers to the Company (including the Company Subsidiaries) as of December 31, 2003. Prior to the Closing Date, none of the Company, any Company Subsidiary or any predecessor has been subject to any provision limiting the carryover of the available net operating losses and credit carryovers set forth in the Company Disclosure Schedule has otherwise been restricted, reduced or limited.
(i) The Company has made available to Issuer accurate and complete copies of all Tax Returns of the Company and the Company Subsidiaries.
Section 2.15. Environmental Matters. Except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect:
| |
| | (i) none of the Company or any Company Subsidiary has violated or is in violation of any Environmental Law; |
| |
| | (ii) none of the properties currently or formerly owned, leased or operated by the Company or any Company Subsidiary (including, without limitation, soils and surface and ground waters) are contaminated with any Hazardous Substance; |
| |
| | (iii) none of the Company or any Company Subsidiary is actually, potentially or allegedly liable for any off-site contamination by Hazardous Substances; |
| |
| | (iv) none of the Company or any Company Subsidiary is actually, potentially or allegedly liable under any Environmental Law; |
| |
| | (v) the Company and the Company Subsidiaries have all permits, licenses and other authorizations required under any Environmental Law and the Company and the Company Subsidiaries are in compliance in all material respects with such permits, licenses and authorizations; and |
| |
| | (vi) neither the execution of this Agreement nor the consummation of the Transactions will require any investigation, remediation or other action with respect to Hazardous Substances, or any notice to or consent of Governmental Authorities or third parties, pursuant to any applicable Environmental Law. |
Section 2.16. Material Contracts. (a) Subsections (i) through (xi) of Section 2.16(a) of the Company Disclosure Schedule contain a list of the following types of contracts and agreements to which the Company or any Company Subsidiary is a party (such contracts and agreements are required to be set forth in Section 2.16(a) of the Company Disclosure Schedule being the “Material Company Contracts”):
| |
| | (i) each contract and agreement which is likely to involve consideration of more than $250,000, in the aggregate, over the remaining term of such contract or agreement; |
| |
| | (ii) all contracts and agreements evidencing outstanding indebtedness in a principal amount of $250,000 or more; |
| |
| | (iii) all leases of real property leased for the use or benefit of the Company or any Company Subsidiary; |
| |
| | (iv) all material contracts and agreements with any Governmental Authority to which the Company or any Company Subsidiary is a party; |
| |
| | (v) all contracts and agreements that limit, or purport to limit, the ability of the Company or any Company Subsidiary to compete in any line of business or with any person or entity or in any geographic area or during any period of time; |
| |
| | (vi) all contracts and agreements providing for benefits under any Company Plan; |
| |
| | (vii) all contracts for employment required to be listed in Section 2.10 of the Company Disclosure Schedule; |
20
| |
| | (viii) all contracts and agreements governing the use by the Company (or any Company Subsidiary) of Company Licensed Intellectual Property; |
| |
| | (ix) all material broker, distributor, dealer, manufacturer’s representative, franchise, agency, sales promotion, market research, marketing consulting and advertising contracts and agreements to which the Company or any Company Subsidiary is a party; |
| |
| | (x) all management contracts (excluding contracts for employment) and contracts with other consultants, including any contracts involving the payment of royalties or other amounts calculated based upon the revenues or income of the Company or any Company Subsidiary or income or revenues related to any product of the Company or any Company Subsidiary to which the Company or any Company Subsidiary is a party; and |
| |
| | (xi) all other contracts and agreements that are material to the Company and the Company Subsidiaries, taken as a whole, or the absence of which would reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement or would reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. |
(b) Except as set forth in Section 2.16(b) of the Company Disclosure Schedule:
| |
| | (i) each Material Company Contract is a legal, valid and binding agreement, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect; |
| |
| | (ii) none of the Company or any Company Subsidiary has received any claim of default under any Material Company Contract and none of the Company or any Company Subsidiary is in breach or violation of, or default under, any Material Company Contract; |
| |
| | (iii) no event has occurred and no condition or circumstance exists that would reasonably be expected (with or without notice or lapse of time): (A) result in a violation or breach of any of the provisions of any Material Company Contract, (B) give any third party the right to declare a default or exercise a remedy under any Material Company Contract, (C) give any third party the right to accelerate the maturity or performance of any Material Company Contract, or (D) give any person the right to terminate, cancel or modify any Material Company Contract, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect; |
| |
| | (iv) to the Knowledge of the Company, no other party is in breach or violation of, or default under, any Material Company Contract; |
| |
| | (v) the Company has not explicitly waived any of its rights under any Material Company Contract, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Company from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect; and |
| |
| | (vi) neither the execution of this Agreement nor the consummation of any Transaction shall constitute a default under, give rise to cancellation rights under, or otherwise adversely affect any of the material rights of the Company or any Company Subsidiary under any Material Company Contract. |
The Company has furnished or made available to the Issuer true and complete copies of all Material Company Contracts, including any amendments thereto.
21
(c) Except as set forth in Section 2.16(c) of the Company Disclosure Schedule:
| |
| | (i) none of the Company or any Company Subsidiary is a party to any agreement pursuant to which the Company or any Company Subsidiary guarantees or otherwise has agreed to cause, insure or become liable for, or pledged any of its assets to secure, the performance or payment of any Liability of any third party; |
| |
| | (ii) none of the Company or any Company Subsidiary is a party to or bound by (A) any joint venture agreement, partnership, partnership agreement, profit sharing agreement, cost sharing agreement, loss sharing agreement or similar contract, or (B) any contract that creates or grants to any third party, or provides for the creation or grant of, any stock appreciation right, phantom stock right or similar right or interest; |
| |
| | (iii) the performance of the Material Company Contracts will not result in any violation of or failure to comply with any Law; and |
| |
| | (iv) no third party is renegotiating any material amount paid or payable to the Company or any Company Subsidiary under any term or provision of a Material Company Contract. |
Section 2.17. Insurance. The Company and the Company Subsidiaries maintain insurance coverage with reputable insurers in such amounts and covering such risks as are in accordance with normal industry practice for companies engaged in businesses similar to that of the Company and the Company Subsidiaries (taking into account the cost and availability of such insurance). Since January 1, 2000, none of the Company or any Company Subsidiary has received any notice or other written communication from any of its insurance carriers regarding any actual or threatened cancellation or invalidation of, or any actual or threatened denial of coverage or rejection of any claim under, any such insurance policy of the Company.
Section 2.18. Board Approval. (a) The Company Board, by resolutions duly adopted by unanimous vote of those voting at a meeting duly called and held and not subsequently rescinded or modified in any way, has approved the Transactions and resolved to recommend that the holders of Company Shares enter into this Agreement and approve the Warrant Amendments.
(b) The only vote of the holders of any class or series of capital stock of the Company necessary in connection with the Transactions to which the Company is a party is (i) with respect to the approval of the 2004 Company Financial Statements, the approval thereof by the holders of a majority of the Company Shares, present or represented at the Company Shareholders’ Meeting, voting as a single class at the Company Shareholders’ Meeting and (ii) with respect to the approval of each Warrant Amendment, the approval thereof by the holders of two-thirds of the Company Shares, present or represented at the Company Shareholders’ Meeting, voting as a single class at the Company Shareholders’ Meeting.
Section 2.19. Certain Business Practices. None of the Company, any Company Subsidiary or, to the Knowledge of the Company, any directors or officers, agents or employees of the Company or any Company Subsidiary, has (i) used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to political activity; (ii) made any unlawful payment to foreign or domestic government officials or employees or to foreign or domestic political parties or campaigns or violated any provision of the Foreign Corrupt Practices Act of 1977, as amended; or (iii) made any payment in the nature of criminal bribery.
Section 2.20. Interested Party Transactions. No director, Company Executive Officer or other affiliate of the Company or any Company Subsidiary has or has had, directly or indirectly, (i) an economic interest in any person that has furnished or sold, or furnishes or sells, services or products that the Company or any Company Subsidiary furnishes or sells, or proposes to furnish or sell; (ii) an economic interest in any person that purchases from or sells or furnishes to, the Company or any Company Subsidiary, any goods or services; (iii) a beneficial interest in any contract or agreement disclosed in Section 2.16 of the Company Disclosure Schedule; or (iv) any contractual or other arrangement with the Company or any Company Subsidiary;provided,however, that ownership of no more than one percent (1%) of the outstanding voting stock of a publicly traded corporation shall not be deemed an “economic interest in any person” for purposes of this Section 2.20. The Company, since January 1, 2002, has not, directly or indirectly, including through a
22
Company Subsidiary (i) extended or maintained credit, arranged for the extension of credit or renewed an extension of credit in the form of a personal loan to or for any director or executive officer (or equivalent thereof) of the Company, or (ii) materially modified any term of any such extension or maintenance of credit.
Section 2.21. Brokers. No broker, finder or investment banker (other than UBS Securities LLC) is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of the Company. The Company has heretofore furnished to the Issuer a complete and correct copy of all agreements between the Company and UBS Securities LLC pursuant to which such firm would be entitled to any payment relating to the Transactions.
ARTICLE III
REPRESENTATIONS AND WARRANTIES REGARDING THE PRINCIPAL
COMPANY SHAREHOLDERS
Except as set forth in the Company Disclosure Schedule, each Principal Company Shareholder hereby represents and warrants to the Issuer that:
Section 3.01. Organization; Qualification. (a) Such Principal Company Shareholder, if it is an individual, has all legal capacity to enter into this Agreement and to carry out his or her obligations hereunder and to consummate the transactions contemplated hereby.
(b) Such Principal Company Shareholder, if it is a corporation or other legal entity, is duly organized, validly existing and, if applicable, in good standing under the Laws of the jurisdiction of its incorporation or formation.
Section 3.02. Authority Relative to This Agreement. Such Principal Company Shareholder has all necessary power and authority to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the Transactions. This Agreement has been duly and validly executed and delivered by such Principal Company Shareholder and, assuming the due authorization, execution and delivery by the Issuer, constitutes a legal, valid and binding obligation of such Principal Company Shareholder, enforceable against such Principal Company Shareholder in accordance with its terms, subject to the effect of any applicable bankruptcy, insolvency (including, without limitation, all laws relating to fraudulent transfers), reorganization, moratorium or similar laws affecting creditors’ rights generally and subject to the effect of general principles of equity (regardless of whether considered in a proceeding at law or in equity).
Section 3.03. No Conflict. (a) The execution and delivery of this Agreement by such Principal Company Shareholder do not, and the performance of this Agreement by such Principal Company Shareholder will not, (i) conflict with or violate the Organizational Documents of such Principal Company Shareholder (if such Principal Company Shareholder is a corporation or other legal entity), (ii) assuming that all consents, approvals, authorizations and other actions described in Section 2.05(b) have been obtained and all filings and obligations described in Section 2.05(b) have been made, conflict with or violate any Law applicable to such Principal Company Shareholder or by which the Company Shares owned by such Principal Company Shareholder are bound or affected or (iii) result in any breach of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any right of termination, amendment, acceleration or cancellation of, or result in the creation of a lien or encumbrance on any of the Company Shares owned by such Principal Company Shareholder pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which such Principal Company Shareholder is a party or by which such Principal Company Shareholder or the Company Shares owned by such Principal Company Shareholder are bound or affected, except for any such conflicts, violations, breaches, defaults or other occurrences which would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay such Principal Company Shareholder from performing its obligations under this Agreement.
23
(b) The execution and delivery of this Agreement by such Principal Company Shareholder do not, and the performance of this Agreement by such Principal Company Shareholder will not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority on the part of such Principal Company Shareholder, except (i) for applicable requirements, if any, of the Exchange Act, Blue Sky Laws and state takeover Laws; (ii) the pre-merger notification and waiting period requirements of the HSR Act applicable with respect to any Principal Company Shareholder that would acquire shares of Issuer Common Stock valued (in accordance with the HSR Act) at $50 million or more in connection with the Share Exchange; and (iii) where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent such Principal Company Shareholder from performing its obligations under this Agreement.
Section 3.04. Title to the Shares. As of the date hereof, such Principal Company Shareholder is the record and beneficial owner of the number of Company Shares, Company Stock Options and Company Warrants set forth opposite such Principal Company Shareholder’s name inExhibit A attached hereto. Except as set forth onExhibit A, such Company Shares are all the securities of the Company owned, now and, at all times during the term hereof will be, either of record or beneficially, by such Principal Company Shareholder. The Company Shares owned by such Principal Company Shareholder are now and, at all times prior to the Closing will be, owned free and clear of all Liens, other than any Liens created by this Agreement.
Section 3.05. Investment Experience and Status. (a) Such Principal Company Shareholder has such knowledge and experience in financial and business matters that such Principal Company Shareholder is capable of evaluating the merits and risks of such Principal Company Shareholder’s investment in shares of Issuer Common Stock pursuant to this Agreement, such Principal Company Shareholder is experienced in evaluating and investing in companies and such Principal Company Shareholder can bear the economic risk of its investment in shares of Issuer Common Stock pursuant to this Agreement for an indefinite period of time.
(b) Either: (i) such Principal Company Shareholder is outside the United States as of the date of this Agreement, such Principal Company Shareholder does not reside in the United States and such Principal Company Shareholder is not a corporation, partnership, trust or other entity organized or incorporated under the laws of the United States; or (b) such Principal Company Shareholder is an “accredited investor”, as that term is defined in Rule 501 of Regulation D promulgated under the Securities Act, which means, among other things, that he, she, or it is a person, corporation or other entity with a substantial net worth and significant experience with similar investments.
(c) Such Principal Company Shareholder is acquiring its shares of Issuer Common Stock pursuant to this Agreement as an investment for such person’s own account, not as a nominee or agent, and not with a view to the resale or distribution of any part thereof, and such Principal Company Shareholder has no present intention of selling, granting any participation in, or otherwise distributing its shares of Issuer Common Stock to be acquired pursuant to this Agreement. Such Principal Company Shareholder does not have any contract, undertaking, agreement or arrangement with any person to sell, transfer or grant participations to such person or to any third person, with respect to any of its shares of Issuer Common Stock to be acquired pursuant to this Agreement.
(d) Such Principal Company Shareholder understands that neither the shares of Issuer Common Stock to be issued pursuant to this Agreement nor the issuance and exchange of shares of Issuer Common Stock pursuant to this Agreement have been registered under the Securities Act and that the shares of Issuer Common Stock to be issued pursuant to this Agreement may not be sold, transferred, or otherwise disposed of without registration under the Securities Act or an exemption from the registration requirements of the Securities Act, and that in the absence of an effective registration statement covering such shares of Issuer Common Stock or an available exemption from registration under the Securities Act, such shares of Issuer Common Stock must be held indefinitely. Such Principal Company Shareholder understands that neither the shares of Issuer Common Stock to be issued pursuant to this Agreement nor the issuance and exchange of shares of Issuer Common Stock pursuant to this Agreement are being registered under the Securities Act on the ground that the sale provided for in this Agreement and the issuance of shares hereunder is exempt from
24
registration under the Securities Act pursuant to Regulation S under the Section Act or Section 4(2) of the Securities Act or another exemption under the Securities Act, and that the Issuer’s reliance on such exemption is predicated on the representations of such Principal Company Shareholder set forth in this Section 3.05.
(e) Such Principal Company Shareholder believes that it has received all the information such person considers necessary or appropriate for deciding whether to agree to acquire shares of Issuer Common Stock pursuant to this Agreement. Such Principal Company Shareholder has had an opportunity to ask questions and receive answers from the Issuer regarding the terms and conditions of the issuance and exchange of shares of Issuer Common Stock pursuant to this Agreement and the business, properties, prospects, and financial condition of the Issuer.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES REGARDING THE ISSUER
Solely for purposes of Section 7.03(a) and Article IX of this Agreement, except as set forth in the Issuer Disclosure Schedule that has been delivered to the Company and the Shareholder Representative in connection with the execution and delivery of this Agreement (the “Issuer Disclosure Schedule”) (which Issuer Disclosure Schedule shall be arranged in sections corresponding to the numbered and lettered sections of this Article IV, and any information disclosed in any such section of the Issuer Disclosure Schedule shall be deemed to be disclosed only for purposes of the corresponding section of this Article IV, unless it is readily apparent that the disclosure contained in such section of the Issuer Disclosure Schedule contains enough information regarding the subject matter of other representations and warranties contained in this Article IV as to clearly qualify or otherwise clearly apply to such other representations and warranties):
Section 4.01. Corporate Organization. (a) The Issuer is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware and has the requisite corporate power and authority and all necessary Governmental Authorizations to own, lease and operate its properties and to carry on its business as it is now being conducted, except where the failure to be so organized, existing or in good standing or to have such power, authority and governmental approvals would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. The Issuer is duly qualified or licensed as a foreign corporation to do business, and is in good standing, in each jurisdiction where the character of the properties owned, leased or operated by it or the nature of its business makes such qualification or licensing necessary, except for such failures to be so qualified or licensed and in good standing that would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect.
(b) The Issuer has not had since January 1, 2000, and does not have, any subsidiaries and has not owned since January 1, 2000, and does not own, directly or indirectly, any equity or similar interest in, or any interest convertible into or exchangeable or exercisable for any equity or similar interest in, any corporation, partnership, joint venture or other business association or entity.
Section 4.02. Corporate Books and Records. (a) The Issuer has heretofore made available to the Company a complete and correct copy of the Certificate of Incorporation and the Bylaws or equivalent Organizational Documents, each as amended to date, of the Issuer. Such Certificates of Incorporation, Bylaws or equivalent Organizational Documents are in full force and effect. The Issuer has not violated, or is not in violation of, any of the provisions of its Certificate of Incorporation, Bylaws or equivalent Organizational Documents, except for such violations that are not material. No event has occurred and no condition or circumstance exists that would reasonably be expected (with or without notice or lapse of time) to constitute
25
or result, directly or indirectly, in a material violation of the provisions of the Organizational Documents of the Issuer.
(b) The minute books of the Issuer contain accurate records of all meetings and accurately reflect all other actions taken by the stockholders, the Issuer Board, and all the committees of the Issuer Board. There have been no meetings or proceedings of the Issuer Board (or any committee thereof) or the stockholders of the Issuer, other than those reflected in the Issuer’s records. Complete and accurate copies of all such minute books and of the stock register of the Issuer have been made available by the Issuer to the Company.
Section 4.03. Capitalization. (a) The authorized capital stock of the Issuer consists of (i) 40,000,000 shares of Issuer Common Stock and (ii) 10,000,000 shares of preferred stock, par value $0.01 per share (“Issuer Preferred Stock”), of which 859,666 shares have been designated Series S Preferred Stock (the “Series S Preferred Stock”) and 549,622 shares have been designated Series S-1 Preferred Stock (the “Series S-1 Preferred Stock”). As of the date of this Agreement, (i) 16,014,569 shares of Issuer Common Stock are issued and outstanding, all of which are validly issued, fully paid and non-assessable, (ii) 859,666 shares of Series S Preferred Stock and 549,622 shares of Series S-1 Preferred Stock are issued and outstanding, all of which are validly issued, fully paid and non-assessable, (iii) no shares of Issuer Common Stock are held in the treasury of the Issuer, (iv) 505,665 shares of Issuer Common Stock are reserved for future issuance pursuant to future grants of Issuer Stock Awards under the Issuer’s 2000 Stock Plan, (v) 139,101 shares of Issuer Common Stock are reserved for future issuance pursuant to the Issuer’s 2001 Employee Stock Purchase Plan, (vi) 2,410,533 shares of Issuer Common Stock are reserved for future issuance pursuant to outstanding employee stock options (“Issuer Stock Options”) and other purchase rights (together with Issuer Stock Options, the “Issuer Stock Awards”) granted pursuant to the Issuer’s 2000 Stock Plan, 1997 Stock Plan, 1994 Non-Employee Directors Stock Option Plan and 1989 Stock Plan (the “Issuer Stock Option Plans”) and (vii) 2,275,426 shares of Issuer Common Stock are reserved for issuance pursuant to outstanding warrant or other rights to purchase shares of Issuer Common Stock. Except as set forth in this Section 4.03 or the Voting Agreements, there are no options, warrants, convertible securities or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued capital stock of the Issuer or obligating the Issuer to issue or sell any shares of capital stock of, or other equity interests in, the Issuer. Section 4.03(a) of the Issuer Disclosure Schedule sets forth the following information with respect to each Issuer Stock Award outstanding as of the date of this Agreement: (i) the name of the Issuer Stock Award recipient; (ii) the particular plan pursuant to which such Issuer Stock Award was granted; (iii) the number of shares of Issuer Common Stock subject to such Issuer Stock Award; (iv) the exercise or purchase price of such Issuer Stock Award; (v) the date on which such Issuer Stock Award was granted; (vi) the applicable vesting schedule; (vii) the date on which such Issuer Stock Award expires; and (viii) whether the exercisability or right of repurchase of such Issuer Stock Award will be accelerated in any way by the transactions contemplated by this Agreement, and indicates the extent of acceleration. The Issuer has made available to the Company accurate and complete copies of all Issuer Stock Option Plans pursuant to which the Issuer has granted such Issuer Stock Awards that are currently outstanding and the form of all stock award agreements evidencing such Issuer Stock Awards. All shares of Issuer Common Stock subject to issuance as aforesaid, upon issuance on the terms and conditions specified in the instruments pursuant to which they are issuable, will be duly authorized, validly issued, fully paid and non-assessable. There are no outstanding contractual obligations of the Issuer to repurchase, redeem or otherwise acquire any shares of Issuer Common Stock or to provide funds to, or make any investment (in the form of a loan, capital contribution or otherwise) in, any person. There are no commitments or agreements of any character to which the Issuer is bound obligating the Issuer to accelerate the vesting of any Issuer Stoc k Option as a result of the Share Exchange or any of the Transactions. All outstanding shares of Issuer Common Stock and all outstanding Issuer Stock Options have been issued and granted in compliance with (i) all applicable securities Laws and other applicable Laws and (ii) all requirements set forth in applicable contracts.
(b) Section 4.03(b) of the Issuer Disclosure Schedule specifies the currently applicable conversion price for each of the Series S Preferred Stock and the Series S-1 Preferred Stock. Except as set forth in Section 4.03(b) of the Issuer Disclosure Schedule, the conversion prices applicable to the Series S Preferred Stock and the Series S-1 Preferred Stock will not be subject to adjustment as a result of the Transactions.
26
(c) The shares of Issuer Common Stock to be issued in the Share Exchange in accordance with Section 1.02, 1.08(a) or 1.09 will be duly authorized, validly issued, fully paid and non-assessable and not subject to preemptive rights created by statute, the Issuer’s Certificate of Incorporation or Bylaws or any agreement to which the Issuer is a party or is bound and (ii) will, when issued and assuming the accuracy of the representations and warranties of the Principal Company Shareholders set forth in Section 3.05, be exempt from the registration requirements under the Securities Act and Exchange Act, and registered or exempt from registration under applicable Blue Sky Laws.
(d) The Rights Agreement, dated as of March 19, 1999, and as amended on June 29, 1999, February 15, 2000, July 9, 2001 and December 18, 2001 (the “Issuer Rights Agreement”), between the Issuer and the American Stock Transfer & Trust Company, has expired in accordance with its terms and the Issuer has not adopted and is not a party to any other shareholder rights agreement or similar agreement.
Section 4.04. Authority Relative to This Agreement. The Issuer has all necessary corporate power and authority to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the Transactions. The execution and delivery of this Agreement by the Issuer and the consummation by the Issuer of the Transactions have been duly and validly authorized by all necessary corporate action, and no other corporate proceedings on the part of the Issuer is necessary to authorize this Agreement or to consummate the Transactions (other than the vote of the stockholders of the Issuer set forth in Section 4.18(b) at the Issuer Stockholders’ Meeting). This Agreement has been duly and validly executed and delivered by the Issuer and, assuming due authorization, execution and delivery by the Principal Company Shareholders, constitutes a legal, valid and binding obligation of the Issuer, enforceable against the Issuer in accordance with its terms, subject to the effect of any applicable bankruptcy, insolvency (including, without limitation, all laws relating to fraudulent transfers), reorganization, moratorium or similar laws affecting creditors’ rights generally and subject to the effect of general principles of equity (regardless of whether considered in a proceeding at law or in equity).
Section 4.05. No Conflict; Required Filings and Consents. (a) The execution and delivery of this Agreement by the Issuer does not, and the performance of this Agreement by the Issuer will not, (i) conflict with or violate the Certificate of Incorporation or Bylaws or other Organizational Documents of the Issuer, (ii) assuming that all consents, approvals, authorizations and other actions described in Section 4.05(b) have been obtained and all filings and obligations described in Section 4.05(b) have been made, conflict with or violate any Law applicable to the Issuer or by which any property or asset of the Issuer is bound or affected, (iii) cause the Company, any Company Subsidiary, the Issuer or any of Issuer’s affiliates to become subject to, or liable for the payment of, any Tax, (iv) cause any of the assets owned or used by the Issuer to be reassessed or revalued by any taxing authority, or (v) result in any breach of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a lien or other encumbrance on any property or asset of the Issuer pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which the Issuer is a party or by which the Issuer or any of its properties or assets is bound or affected, except, with respect to clauses (ii) and (iii), for any such conflicts, violations, breaches, defaults or other occurrences which would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect.
(b) The execution and delivery of this Agreement by the Issuer does not, and the performance of this Agreement by the Issuer will not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority, except (i) for applicable requirements, if any, of the Exchange Act, Blue Sky Laws and state takeover laws; (ii) the pre-merger notification and waiting period requirements of the HSR Act applicable with respect to any Principal Company Shareholder that would acquire shares of Issuer Common Stock valued (in accordance with the HSR Act) at $50 million or more in connection with the Share Exchange; and (iii) where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not reasonably be expected, individually or in the aggregate, to
27
prevent or materially delay consummation of any of the Transactions, or otherwise prevent the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect.
Section 4.06. Permits; Compliance. (a) The Issuer is in possession of all franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority (other than the Drug Regulatory Agencies) necessary for the Issuer to own, lease and operate its properties or to carry on its business as it is now being conducted (the “Issuer Permits”), except where the failure to have, or the suspension or cancellation of, any of the Issuer Permits would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. Each material Issuer Permit is listed in Section 4.06(a) of the Issuer Disclosure Schedule. The Issuer has made available to the Company complete and accurate copies of each Issuer Permit, including all amendments and renewals thereto, listed in Section 4.06(a) of the Issuer Disclosure Schedule. As of the date of this Agreement, each Issuer Permit is valid and in full force and effect and no suspension or cancellation of any of the Issuer Permits is pending or, to the Knowledge of the Issuer, threatened, except where the failure to have, or the suspension or cancellation of, any of the Issuer Permits would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. The Issuer is, and has at all times been, in full compliance with all of the terms and requirements of each Issuer Permit, except where the failure to be in compliance would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. No event has occurred and no condition or circumstance exists that would reasonably be expected (with or without notice or lapse of time) to constitute or result, directly or indirectly, in a violation of or a failure to comply with any term or requirement of any Issuer Permit or result, directly or indirectly, in the revocation, withdrawal, suspension, cancellation, termination or modification of any Issuer Permit, except where the violation, failure to comply, revocation, withdrawal, suspension, cancellation, termination or modification would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. Since January 1, 2002, the Issuer has not received any written communication regarding (A) any actual, alleged, possible or potential violation of, or failure to comply with, any term or requirement of any material Issuer Permit or (B) any actual, proposed, possible or potential revocation, withdrawal, suspension, cancellation, termination or adverse modification of any material Issuer Permit. All applications required to have been filed for the renewal of each Issuer Permit have been duly filed on a timely basis, and each other notice or filing required to have been given or made with respect to such Issuer Permit has been duly given or made on a timely basis, except where the failure to make such filing would not reasonably be expected, individually or in the aggregate, to prevent or materially delay the consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. The Issuer is not in conflict with, or in default, breach or violation of, (a) any Law applicable to the Issuer or by which any property or asset of the Issuer is bound or affected, or (b) any note, bond, mortgage, indenture, contract, agreement, lease, license, Issuer Permit, franchise or other instrument or obligation to which the Issuer is a party or by which the Issuer or any property or asset of the Issuer is bound, except for any such conflicts, defaults, breaches or violations that would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect.
28
(b) The Issuer holds all new drug applications, abbreviated new drug applications, product license applications, investigational new drug applications, product export applications and other approvals issuable by the Drug Regulatory Agencies (the “Issuer Regulatory Permits”) necessary for the conduct of the business of the Issuer as currently conducted and development, clinical testing, manufacturing, sale, marketing, distribution and importation or exportation, as currently conducted, of any of its products or product candidates, including EP HIV-1090, EP-1010, EP-1043, EP-1233, EP-2101, and MVA-BN32 (the “Issuer Product Candidates”) and no such Issuer Regulatory Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner, other than immaterial adverse modifications. The Issuer is in compliance in all material respects with the Issuer Regulatory Permits and has not received any written notice or other written communication from any Drug Regulatory Agency regarding (i) any actual or possible violation of or failure to comply with any term or requirement of any Issuer Regulatory Permit or (ii) any actual or possible revocation, withdrawal, suspension, cancellation, termination or modification of any Issuer Regulatory Permit. Except for the information and files identified in Section 4.06(b) of the Issuer Disclosure Schedule, the Issuer has made available to the Company all information in its possession or control relating to the Issuer Product Candidates and the development, manufacture and sale of the Issuer Product Candidates, including without limitation, complete and correct copies of the following (to the extent there are any): (i) adverse event reports; clinical study reports and material study data; and inspection reports, notices of adverse findings, warning letters, filings and letters and other correspondence with any Drug Regulatory Agency; and (ii) similar reports, study data, notices, letters, filings and correspondence with any other Governmental Authority.
(c) All clinical, pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, the Issuer or in which the Issuer or its current products or product candidates, including the Issuer Product Candidates, have participated were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance with the applicable regulations of the Drug Regulatory Agencies and other applicable Laws. All quality control, record keeping, notification, reporting and manufacturing systems used for the Issuer’s commercial products and for the manufacturing of clinical supplies conforms to the applicable regulations of the Drug Regulatory Agencies and good manufacturing practices.
Section 4.07. SEC Filings; Financial Statements; Nasdaq. (a) The Issuer has filed all forms, reports and documents required to be filed by it with the U.S. Securities and Exchange Commission (the “SEC”) since January 1, 2002 (collectively, the “Issuer SEC Reports”). The Issuer SEC Reports (i) were prepared in all material respects in accordance with either the requirements of the Securities Act or the Exchange Act, as the case may be, and the rules and regulations promulgated thereunder, and (ii) did not, at the time they were filed, or, if amended or supplemented, as of the date of the last such amendment or supplement, contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading.
(b) Each of the financial statements (including, in each case, any notes thereto) contained in the Issuer SEC Reports was prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto or, in the case of unaudited statements, as permitted by Form 10-Q of the SEC) and each fairly presents, in all material respects, the financial position, results of operations and cash flows of the Issuer as at the respective dates thereof and for the respective periods indicated therein, except as otherwise noted therein (subject, in the case of unaudited statements, to normal and recurring year-end adjustments which, individually or in the aggregate, have not had, and would not reasonably be expected to have an Issuer Material Adverse Effect).
(c) Except as and to the extent set forth on the balance sheet of the Issuer as at September 30, 2004, including the notes thereto, the Issuer does not have any Liability of any nature (whether accrued, absolute, contingent or otherwise) that would be required to be reflected on a balance sheet prepared in accordance with U.S. GAAP, except for Liabilities: (i) incurred pursuant to this Agreement and the Transactions; or (ii) incurred in the ordinary course of business consistent with past practice since September 30, 2004, which,
29
individually or in the aggregate, would not reasonably be expected to prevent or materially delay consummation of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect.
(d) From January 1, 2002 through the date hereof, the Issuer has not received any comment letter from the SEC or the staff thereof. The Issuer has made available to the Company or its counsel all comment letters and other correspondence from or to Nasdaq dated on or after January 1, 2002 relating to the delisting or maintenance of listing of the Issuer Common Stock on the Nasdaq.
(e) The Issuer has timely filed all certifications and statements required by (x) Rule 13a-14 or Rule 15d-14 under the Exchange Act or (y) 18 U.S.C. Section 1350 (Section 906 of the Sarbanes-Oxley Act of 2002) with respect to any Issuer SEC Report. The Issuer maintains disclosure controls and procedures required by Rule 13a-15 or Rule 15d-15 under the Exchange Act; such controls and procedures are designed to ensure that material information concerning the Issuer is made known on a timely basis to the individuals responsible for the preparation of the Issuer’s SEC filings and other public disclosure documents.
(f) The Issuer has in place internal controls over financial reporting that are designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP and include policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Issuer; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures of the Issuer are being made only in accordance with authorization of management and the advisors of the Issuer; and (iii) provide reasonable assurance regarding prevention or timely detection of the unauthorized acquisition, use or disposition of the assets of the Issuer that could have a material effect on the financial statements.
(g) Since January 1, 2002, there have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer or general counsel of the Issuer, the Issuer Board or any committee thereof, other than ordinary course audits or reviews of accounting policies and practices or internal controls required by the Sarbanes-Oxley Act of 2002.
Section 4.08. Absence of Certain Changes or Events. Since September 30, 2004, except as expressly contemplated by this Agreement, (a) the Issuer has conducted its business only in the ordinary course and in a manner consistent with past practice; (b) there has not been any Issuer Material Adverse Effect or any event, condition or circumstance that would reasonably be expected to have an Issuer Material Adverse Effect; and (c) the Issuer has not taken any action that, if taken after the date of this Agreement, would constitute a breach of any of the covenants set forth in Section 5.02.
Section 4.09. Absence of Litigation. There is no Action pending or, to the Knowledge of the Issuer, threatened against the Issuer or any property or asset of the Issuer before any Governmental Authority that (a) individually or in the aggregate, has had, or would reasonably be expected to have an Issuer Material Adverse Effect or (b) seeks to, directly or indirectly, delay, prevent, make illegal or otherwise interfere with the consummation of any of the Transactions. Neither the Issuer nor any property or asset of the Issuer is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with, or, to the Knowledge of the Issuer, continuing investigation by, any Governmental Authority, or any effective or, to the Knowledge of the Issuer, proposed, order, writ, judgment, injunction, decree, determination or award of Governmental Authority, that would reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement or would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect.
Section 4.10. Employee Benefit Plans. (a) Section 4.10(a) of the Issuer Disclosure Schedule lists: (i) all employee benefit plans (as defined in, and whether or not subject to, Section 3(3) of ERISA) and all
30
bonus, stock option, stock purchase, restricted stock, incentive, deferred compensation, retiree medical or life insurance, supplemental retirement, severance or other benefit plans, programs or arrangements, and all employment, termination, severance or other contracts or agreements, to which the Issuer is a party, with respect to which the Issuer has any obligation or which are maintained, contributed to or sponsored by the Issuer for the benefit of any current or former employee, officer or director of the Issuer, (ii) each employee benefit plan for which the Issuer could incur liability under Section 4069 of ERISA in the event such plan has been or were to be terminated, (iii) any plan in respect of which the Issuer could incur liability under Section 4212(c) of ERISA, and (iv) any contracts, arrangements or understandings between the Issuer and any employee of the Issuer including, without limitation, any contracts, arrangements or understandings relating in any way to a sale of the Issuer or the consummation of any Transaction (collectively, the “Issuer Plans”). The Issuer has made available to the Company a true and complete copy of (i) such Issuer Plans, (ii) the most recently filed IRS Form 5500, if any, (iii) the most recent summary plan description for each Issuer Plan for which a summary plan description is required by applicable law, (iv) the most recently received IRS determination letter, if any, issued by the IRS with respect to any Issuer Plan that is intended to qualify under Section 401(a) of the Code, and (v) the most recently prepared actuarial report or financial statement, if any, relating to an Issuer Plan.
(b) None of the Issuer Plans is a Multiemployer Plan or a Multiple Employer Plan. None of the Issuer Plans (i) provides for the payment of separation, severance, termination or similar-type benefits to any person, (ii) obligates the Issuer to pay separation, severance, termination or similar-type benefits solely or partially as a result of any transaction contemplated by this Agreement, or (iii) obligates the Issuer to make any payment or provide any benefit as a result of a “change in control”, within the meaning of such term under Section 280G of the Code. None of the Issuer Plans provides for or promises retiree medical, disability or life insurance benefits to any current or former employee, officer or director of the Issuer. Each of the Issuer Plans is subject only to the Laws of the United States or a political subdivision thereof.
(c) Each Issuer Plan is now and always has been operated in all material respects in accordance with its terms and the requirements of all applicable Laws, including, without limitation, ERISA and the Code. The Issuer has performed all material obligations required to be performed by them under, are not in any material respect in default under or in violation of, and, to the Knowledge of the Issuer, there have not been any defaults or violations by any party to, any Issuer Plan. No Action is pending or, to the Knowledge of the Issuer, threatened with respect to any Issuer Plan (other than claims for benefits in the ordinary course) that would reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect and, to the Knowledge of the Issuer, no fact or event exists that would reasonably be expected to give rise to any such Action.
(d) Each Issuer Plan that is intended to be qualified under Section 401(a) of the Code or Section 401(k) of the Code has timely received a favorable determination letter from the IRS covering all of the provisions applicable to the Issuer Plan for which determination letters are currently available that the Issuer Plan is so qualified and each trust established in connection with any Issuer Plan which is intended to be exempt from federal income taxation under Section 501(a) of the Code has received a determination letter from the IRS that it is so exempt, and no fact or event has occurred since the date of such determination letter or letters from the IRS to adversely affect the qualified status of any such Issuer Plan or the exempt status of any such trust.
(e) There has not been any prohibited transaction (within the meaning of Section 406 of ERISA or Section 4975 of the Code) with respect to any Issuer Plan. The Issuer has not incurred any liability under, arising out of or by operation of Title IV of ERISA (other than liability for premiums to the Pension Benefit Guaranty Corporation arising in the ordinary course), including, without limitation, any liability in connection with (i) the termination or reorganization of any employee benefit plan subject to Title IV of ERISA, or (ii) the withdrawal from any Multiemployer Plan or Multiple Employer Plan, and no fact or event exists which would reasonably be expected to give rise to any such liability.
(f) All material contributions, premiums or payments required to be made with respect to any Issuer Plan have been made on or before their due dates. All such contributions have been fully deducted for income
31
tax purposes, to the extent applicable, and no such deduction has been challenged or disallowed by any Governmental Authority and no fact or event exists which would reasonably be expected to give rise to any such challenge or disallowance.
Section 4.11. Labor and Employment Matters. (a) Except as set forth in Section 4.11(a) of the Issuer Disclosure Schedule:
| |
| | (i) there are no controversies pending or, to the Knowledge of the Issuer, threatened between the Issuer and any of its employees; |
| |
| | (ii) the Issuer is not a party to any collective bargaining agreement or other labor union contract applicable to persons employed by the Issuer, nor, to the Knowledge of the Issuer, are there any activities or proceedings of any labor union to organize any such employees; |
| |
| | (iii) the Issuer has not breached or otherwise failed to comply with any provision of any such agreement or contract, and there are no grievances outstanding against the Issuer under any such agreement or contract, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect; |
| |
| | (iv) there are no unfair labor practice complaints pending against the Issuer before the National Labor Relations Board or any other Governmental Authority, nor any current union representation questions involving employees of the Issuer; and |
| |
| | (v) there is no strike, slowdown, work stoppage, labor dispute or lockout, or, to the Knowledge of the Issuer, threat thereof, by or with respect to any employees of the Issuer. To the Knowledge of the Issuer, no event has occurred and no condition or circumstance exists that would reasonably be expected to, directly or indirectly, give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, labor dispute or lockout, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. |
(b) Except as set forth in Section 4.11(b) of the Issuer Disclosure Schedule:
| |
| | (i) the Issuer is in compliance with all applicable laws relating to the employment of labor, including those related to wages, hours, overtime, collective bargaining and the payment and withholding of taxes and other sums as required by the appropriate Governmental Authority and have withheld and paid to the appropriate Governmental Authority or are holding for payment not yet due to such Governmental Authority all amounts required to be withheld from employees of the Issuer and are not liable for any arrears of wages, taxes, penalties or other sums for failure to comply with any of the foregoing, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect; |
| |
| | (ii) the Issuer has paid in full to all employees or adequately accrued for in accordance with U.S. GAAP consistently applied all wages, salaries, commissions, bonuses, benefits and other compensation due to or on behalf of such employees and there is no claim with respect to payment of wages, salary or overtime pay that has been asserted or is now pending or threatened before any Governmental Authority with respect to any persons currently or formerly employed by the Issuer, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect; |
32
| |
| | (iii) the Issuer is not a party to, or otherwise bound by, any consent decree with, or citation by, any Governmental Authority relating to employees or employment practices; |
| |
| | (iv) there is no charge or proceeding with respect to a violation of any occupational safety or health standards that has been asserted or is now pending or, to the Knowledge of the Issuer, threatened with respect to the Issuer; and |
| |
| | (v) there is no charge of discrimination in employment or employment practices, for any reason, including, without limitation, age, gender, race, religion or other legally protected category, which has been asserted or is now pending or, to the Knowledge of the Issuer, threatened before the United States Equal Employment Opportunity Commission, or any other Governmental Authority in any jurisdiction in which the Issuer has employed or employs any person. |
(c) All officers, management employees, and technical and professional employees of the Issuer are under written obligation to the Issuer to maintain in confidence all confidential or proprietary information acquired by them in the course of their employment and to assign to the Issuer all inventions made by them within the scope of their employment during such employment and for a reasonable period thereafter and copies of the forms of all such written obligations have been made available by the Issuer to the Company.
(d) Section 4.11(d) of the Issuer Disclosure Schedule accurately identifies each former employee of the Issuer who is receiving or is scheduled to receive (or whose spouse or other dependent is receiving or is scheduled to receive) any benefits relating to such former employee’s employment with the Issuer and accurately describes such benefits.
(e) To the Knowledge of the Issuer, no officer of the Issuer: (i) intends to terminate his employment with the Issuer; or (ii) is a party to or is bound by any confidentiality agreement, noncompetition agreement or other contract that may have an adverse effect on: (A) the performance by such employee of any of his duties or responsibilities as an employee of the Issuer, or (B) the Issuer’s business or operations.
(f) Section 4.11(f) of the Issuer Disclosure Schedule accurately sets forth, with respect to each person that was an independent contractor of the Issuer as of the Effective Date: (i) the name of such independent contractor and the date as of which such independent contractor was originally hired, (ii) a description of such independent contractor duties and responsibilities, (iii) the aggregate dollar amount of the compensation (including all payments or benefits of any type) received by such independent contractor from the Issuer with respect to services performed in 2004, (iv) the terms of compensation of such independent contractor, and (v) any permit, authorization, franchise or other right that is held by such independent contractor and that relates to or is useful in connection with the Issuer’s business.
(g) None of the current or former independent contractors of the Issuer should be reclassified as an employee under applicable Law.
Section 4.12. Property and Leases. (a) Section 4.12(a) of the Issuer Disclosure Schedule lists each parcel of real property currently or formerly owned by the Issuer or any subsidiary of the Issuer. Each parcel of real property owned by the Issuer (i) is owned free and clear of all Liens, other than Permitted Liens, and (ii) is neither subject to any Governmental Order to be sold nor is being condemned, expropriated or otherwise taken by any public authority with or without payment of compensation therefor, nor, to the Knowledge of the Issuer, has any such condemnation, expropriation or taking been proposed.
(b) Section 4.12(b) of the Issuer Disclosure Schedule lists each parcel of real property currently leased or subleased by the Issuer, with the name of the lessor and the date of the lease, sublease, assignment of the lease, any guaranty given or leasing commissions payable by the Issuer in connection therewith and each amendment to any of the foregoing (collectively, the “Issuer Lease Documents”). True, correct and complete copies of all Issuer Lease Documents have been made available to the Company. All such current leases and subleases are in full force and effect, are valid and effective in accordance with their respective terms, and there is not, under any of such leases, any existing material default or event of default (or event which, with notice or lapse of time, or both, would constitute a default) by the Issuer or, to the Issuer’s Knowledge, by the other party to such lease or sublease, or person in the chain of title to such leased premises.
33
(c) Except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect: (i) there are no contractual or legal restrictions that preclude or restrict the ability to use any real property owned or leased by the Issuer for the purposes for which it is currently being used; and (ii) there are no material latent defects or material adverse physical conditions affecting the real property, and improvements thereon, owned or leased by the Issuer.
(d) The Issuer has good and valid title to, or, in the case of leased properties and assets, valid leasehold or subleasehold interests in, all of its properties and assets, tangible and intangible, real, personal and mixed, used or held for use in its business, free and clear of any Liens, except for such imperfections of title, if any, that do not materially interfere with the present value of the subject property.
Section 4.13. Intellectual Property. (a) (i) Section 4.13(a)(i) of the Issuer Disclosure Schedule lists (A) each item of Intellectual Property owned (in whole or in part) by the Issuer and which is the subject of a registration (“Issuer Registered Intellectual Property”) or an application for registration, (B) the jurisdiction(s) in which any such item of Intellectual Property has been filed or registered, if any, and the applicable registration or serial number, and (C) any other person that has an ownership interest in such item of Intellectual Property, and (ii) Section 4.13(a)(ii) of the Issuer Disclosure Schedule lists each material item of Intellectual Property licensed to the Issuer (“Issuer Licensed Intellectual Property”).
(b) Except as set forth in Section 4.13 of the Issuer Disclosure Schedule:
| |
| | (i) to the Knowledge of the Issuer, the conduct of the business of the Issuer as currently conducted does not infringe upon or misappropriate the Intellectual Property rights of any third party; |
| |
| | (ii) the Issuer owns or is licensed to use all Intellectual Property used in or, to the Knowledge of the Issuer, necessary for the conduct of its business as currently conducted; |
| |
| | (iii) there are no claims pending or, to the Knowledge of the Issuer, threatened, against the Issuer (A) alleging that the conduct of the business of the Issuer as currently conducted infringes upon or may infringe upon or misappropriates the Intellectual Property rights of any third party or (B) challenging the ownership, use, validity or enforceability of any Issuer Owned Intellectual Property or any Issuer Licensed Intellectual Property; |
| |
| | (iv) with respect to each item of Issuer Owned Intellectual Property, the Issuer is the owner of the entire right, title and interest in and to such the Issuer Owned Intellectual Property free and clear of all liens, encumbrances and other restrictions, and is entitled to use such the Issuer Owned Intellectual Property in the continued operation of its respective business; |
| |
| | (v) there are no settlements, forbearances to sue, consents, judgments, orders or similar obligations that (A) restrict the business of the Issuer in or under any Intellectual Property rights of any third party or (B) with respect to Issuer Owned Intellectual Property that is exclusively owned by the Issuer, permit any third party to use any Issuer Owned Intellectual Property; |
| |
| | (vi) with respect to each item of Issuer Licensed Intellectual Property, the Issuer has the right to use such Issuer Licensed Intellectual Property in the continued operation of its respective business in accordance with the terms of the license agreement governing such the Issuer Licensed Intellectual Property; |
| |
| | (vii) the Issuer Registered Intellectual Property has not been adjudged invalid or unenforceable in whole or in part and, to the Knowledge of the Issuer, is valid and enforceable. Without limiting the foregoing: |
| |
| | (A) each U.S. patent application and U.S. patent in which the Issuer has or purports to have an ownership interest and which is relevant to the Issuer Product Candidates or PADRE was filed within one year of the first printed publication, public use or offer for sale of each invention described in such U.S. patent application or U.S. patent; |
34
| |
| | (B) each non-U.S. patent application and non-U.S. patent in which the Issuer has or purports to have an ownership interest and which is relevant to the Issuer Product Candidates or PADRE was filed or claims priority to a patent application filed, before the time at which each invention described in such non-U.S. patent application or non-U.S. patent was first made available to the public; |
| |
| | (C) to the Knowledge of the Issuer, no trademark (whether registered or unregistered) or trade name used by the Issuer infringes any trademark (whether registered or unregistered) or trade name of any third party; |
| |
| | (D) the Issuer has not incurred an impairment charge to goodwill associated with or inherent in any trademark (whether registered or unregistered) in which the Issuer has or purports to have an ownership interest; and |
| |
| | (E) each item of Issuer Registered Intellectual Property is and at all times has been in compliance in all material respects with all requirements under applicable Laws, and all filings, payments and other actions required to be made or taken to maintain such item of Issuer Registered Intellectual Property in full force and effect have been made by the applicable deadline, except as would not have an Issuer Material Adverse Effect; |
| |
| | (viii) the Issuer has made available to the Company complete and accurate copies of all applications, correspondence and other material documents related to each item of Issuer Registered Intellectual Property, subject to any such applications, correspondence and other documents withheld for reasons related to attorney-client privilege; |
| |
| | (ix) no interference, opposition, reissue, reexamination or other proceeding of any nature is or has been pending or, to the Knowledge of the Issuer, threatened, in which the scope, validity or enforceability of any Issuer Registered Intellectual Property is being, has been or could reasonably be expected to be contested or challenged; |
| |
| | (x) to the Knowledge of the Issuer, there is no legitimate basis for a claim that any Issuer Owned Intellectual Property is invalid or unenforceable; |
| |
| | (xi) to the Knowledge of the Issuer, no person is engaging or has engaged, in any activity that materially infringes upon the Issuer Owned Intellectual Property; |
| |
| | (xii) to the Knowledge of the Issuer, each license of the Issuer Licensed Intellectual Property is valid and enforceable, is binding on all parties to such license, and is in full force and effect; |
| |
| | (xiii) to the Knowledge of the Issuer, no party to any license of the Issuer Licensed Intellectual Property is in breach thereof or default thereunder; |
| |
| | (xiv) neither the execution of this Agreement nor the consummation of any Transaction will, with or without notice or the lapse of time, result in or give any third party the right or option to cause or declare: (A) any loss or, or encumbrance on, any Issuer Owned Intellectual Property, (B) the release, disclosure or delivery of any Issuer Owned Intellectual Property by or to any escrow agent or other third party, or (C) an adverse affect on any of the Issuer’s material rights with respect to the Issuer Owned Intellectual Property or the Issuer Licensed Intellectual Property; and |
| |
| | (xv) the Issuer has made available to the Company an accurate copy of each standard form (A) end-user license agreement; (B) employee or independent contractor agreements containing any assignment or license or Intellectual Property or any confidentiality provision; and (C) confidentiality or nondisclosure agreements. |
Section 4.14. Taxes. The Issuer has filed all United States federal, state, local and non-United States Tax returns and reports required to be filed by them and have paid and discharged all Taxes required to be paid or discharged, other than such payments as are being contested in good faith by appropriate proceedings. All such Tax returns are true, accurate and complete in all material respects. Neither the IRS nor any other United States or non-United States taxing authority or agency is now asserting or, to the Knowledge of the Issuer, threatening to assert, against the Issuer any material deficiency or claim for any
35
Taxes or interest thereon or penalties in connection therewith. The Issuer has not granted any waiver of any statute of limitations with respect to, or any extension of a period for the assessment of, any Tax. The accruals and reserves for Taxes reflected in the consolidated balance sheet of the Issuer and the consolidated Issuer Subsidiaries as at September 30, 2004 are adequate to cover all Taxes accruable through such date (including interest and penalties, if any, thereon) in accordance with U.S. GAAP. There are no Tax liens upon any property or assets of the Issuer except liens for current Taxes not yet due. The Issuer has not been required to include in income any adjustment pursuant to Section 481 of the Code by reason of a voluntary change in accounting method initiated by the Issuer, and the IRS has not initiated or proposed any such adjustment or change in accounting method, in either case which adjustment or change would reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. The Issuer has not been a “distributing corporation” or a “controlled corporation” within the meaning of Section 355(a)(1)(A) of the Code in a distribution intended to qualify under Section 355(e) of the Code within the past five years.
Section 4.15. Environmental Matters. Except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect:
| |
| | (i) the Issuer has not violated and is not in violation of any Environmental Law; |
| |
| | (ii) none of the properties currently or formerly owned, leased or operated by the Issuer or any subsidiary of the Issuer (including, without limitation, soils and surface and ground waters) are contaminated with any Hazardous Substance; |
| |
| | (iii) the Issuer is not actually, potentially or allegedly liable for any off-site contamination by Hazardous Substances; |
| |
| | (iv) the Issuer is not actually, potentially or allegedly liable under any Environmental Law; |
| |
| | (v) the Issuer has all permits, licenses and other authorizations required under any Environmental Law and the Issuer is in compliance in all material respects with such permits, licenses and other authorizations; and |
| |
| | (vi) neither the execution of this Agreement nor the consummation of the Transactions will require any investigation, remediation or other action with respect to Hazardous Substances, or any notice to or consent of Governmental Authorities or third parties, pursuant to any applicable Environmental Law. |
Section 4.16. Material Contracts. (a) Subsections (i) through (xii) of Section 4.16(a) of the Issuer Disclosure Schedule contain a list of the following types of contracts and agreements to which the Issuer is a party (such contracts and agreements as are required to be set forth in Section 4.16(a) of the Issuer Disclosure Schedule being “Material Issuer Contracts”):
| |
| | (i) each “material contract” (as such term is defined in Item 610(b)(10) of Regulation S-K of the SEC) with respect to the Issuer; |
| |
| | (ii) each contract and agreement which is likely to involve consideration of more than $250,000, in the aggregate, over the remaining term of such contract or agreement; |
| |
| | (iii) all contracts and agreements evidencing outstanding indebtedness in a principal amount of $250,000 or more; |
| |
| | (iv) all leases of real property leased for the use or benefit of the Issuer; |
| |
| | (v) all material contracts and agreements with any Governmental Authority to which the Issuer is a party; |
| |
| | (vi) all contracts and agreements that limit, or purport to limit, the ability of the Issuer to compete in any line of business or with any person or entity or in any geographic area or during any period of time; |
| |
| | (vii) all contracts and agreements providing for benefits under any Issuer Plan; |
36
| |
| | (viii) all contracts and agreements governing the use by the Issuer of Issuer Licensed Intellectual Property; |
| |
| | (ix) all contracts for employment required to be listed in Section 4.10 of the Issuer Disclosure Schedule; |
| |
| | (x) all material broker, distributor, dealer, manufacturer’s representative, franchise, agency, sales promotion, market research, marketing consulting and advertising contracts and agreements to which the Issuer is a party; |
| |
| | (xi) all management contracts (excluding contracts for employment) and contracts with other consultants, including any contracts involving the payment of royalties or other amounts calculated based upon the revenues or income of the Issuer or income or revenues related to any product of the Issuer to which the Issuer is a party; and |
| |
| | (xii) all other contracts and agreements that are material to the Issuer or the absence of which would reasonably be expected to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement or would reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect. |
(b) Except as set forth in Section 4.16(b) of the Issuer Disclosure Schedule:
| |
| | (i) each Material Issuer Contract is a legal, valid and binding agreement, except would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect; |
| |
| | (ii) the Issuer has not received any claim of default under any Material Issuer Contract and the Issuer is not in breach or violation of, or default under, any Material Issuer Contract; |
| |
| | (iii) no event has occurred and no condition or circumstance exists that could reasonably be expected (with or without notice or lapse of time): (A) result in a violation or breach of any of the provisions of any Material Issuer Contract, (B) give any third party the right to declare a default or exercise a remedy under any Material Issuer Contract, or (C) give any third party the right to accelerate the maturity or performance of any Material Issuer Contract, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect; |
| |
| | (iv) to the Knowledge of the Issuer, no other party is in breach or violation of, or default under, any Material Issuer Contract; |
| |
| | (v) the Issuer has not waived any of its rights under any Material Issuer Contract, except as would not reasonably be expected, individually or in the aggregate, to prevent or materially delay consummation of any of the Transactions or otherwise prevent or materially delay the Issuer from performing its obligations under this Agreement and would not reasonably be expected, individually or in the aggregate, to have an Issuer Material Adverse Effect; and |
| |
| | (vi) neither the execution of this Agreement nor the consummation of any Transaction shall constitute a default under, give rise to cancellation rights under, or otherwise adversely affect any of the material rights of the Issuer under any Material Issuer Contract. |
The Issuer has furnished or made available to the Company true and complete copies of all Material Issuer Contracts, including any amendments thereto.
37
(c) Except as set forth in Section 4.16(c) of the Issuer Disclosure Schedule:
| |
| | (i) the Issuer is not a party to any agreement pursuant to which the Issuer guarantees or otherwise has agreed to cause, insure or become liable for, or pledged any of its assets to secure, the performance or payment of any Liability of any third party; |
| |
| | (ii) the Issuer is not a party to or bound by (A) any joint venture agreement, partnership agreement, profit sharing agreement, cost sharing agreement, loss sharing agreement or similar contract, or (B) any contract that creates or grants to any third party, or provides for the creation or grant of, any stock appreciation right, phantom stock right or similar right or interest; |
| |
| | (iii) the performance of the Material Issuer Contracts will not result in any violation of or failure to comply with any Law; and |
| |
| | (iv) no third party is renegotiating any material amount paid or payable to the Issuer under any term or provision of a Material Issuer Contract. |
Section 4.17. Insurance. The Issuer maintains insurance coverage with reputable insurers in such amounts and covering such risks as are in accordance with normal industry practice for companies engaged in businesses similar to that of the Issuer (taking into account the cost and availability of such insurance). Since January 1, 2000, the Issuer has not received any notice or other written communication from any of its insurance carriers regarding any actual or threatened cancellation or invalidation of, or any actual or threatened denial of coverage or rejection of any claim under, any such insurance policy of the Issuer.
Section 4.18. Board Approval; Vote Required. (a) The Issuer Board, by resolutions duly adopted by unanimous vote of those voting at a meeting duly called and held and not subsequently rescinded or modified in any way, has duly (i) determined that this Agreement, the Voting Agreements, the Issuer Transactions and the other Transactions are fair to, and in the best interest of, the Issuer and the stockholders of the Issuer, (ii) approved, adopted and declared advisable this Agreement, the Voting Agreements and the Transactions and (iii) resolved to recommend that the stockholders of the Issuer approve the Issuer Transactions and directed that the Issuer Transactions be submitted for consideration by the Issuer’s stockholders at the Issuer Stockholders’ Meeting.
(b) The only vote of the holders of any class or series of capital stock of the Issuer necessary to approve this Agreement, any of the Issuer Transactions or any other Transaction to which the Issuer is a party is (i) with respect to the Share Exchange, the Issuer Stock Option Plan Amendment, the Option Liquidity Share Issuance, the Issuer ESPP Amendment, the adoption of the Issuer French Stock Option Plan and the adoption of the Issuer French ESPP (as defined below), the approval of each such transaction by the affirmative vote of the holders of a majority of the then outstanding shares of Issuer Common Stock and Issuer Preferred Stock (on an as-converted basis and voting as a single class with the Issuer Common Stock) present in person or represented by proxy and entitled to vote at the Issuer Stockholders’ Meeting, and, with respect to the Issuer Name Change, the Issuer Capital Stock Increase and the Issuer Reverse Stock Split, the approval of each such transaction by the affirmative vote of the holders of a majority of the then outstanding shares of Issuer Common Stock and Issuer Preferred Stock (on an as-converted basis and voting as a single class with the Issuer Common Stock) entitled to vote at the Issuer Stockholders’ Meeting.
Section 4.19. Certain Business Practices. None of the Issuer or, to the Knowledge of the Issuer, any directors or officers, agents or employees of the Issuer, has (i) used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to political activity; (ii) made any unlawful payment to foreign or domestic government officials or employees or to foreign or domestic political parties or campaigns or violated any provision of the Foreign Corrupt Practices Act of 1977, as amended; or (iii) made any payment in the nature of criminal bribery.
Section 4.20. Interested Party Transactions. No director, Issuer Executive Officer or other affiliate of the Issuer has or has had, directly or indirectly, (i) an economic interest in any person that has furnished or sold, or furnishes or sells, services or products that the Issuer furnishes or sells, or proposes to furnish or sell; (ii) an economic interest in any person that purchases from or sells or furnishes to the Issuer any goods or
38
services; (iii) a beneficial interest in any contract or agreement disclosed in Section 4.16 of the Issuer Disclosure Schedule; or (iv) any contractual or other arrangement with the Issuer;provided,however, that ownership of no more than one percent (1%) of the outstanding voting stock of a publicly traded corporation shall not be deemed an “economic interest in any person” for purposes of this Section 4.20. The Issuer has not, since January 1, 2002, directly or indirectly, (i) extended or maintained credit, arranged for the extension of credit or renewed an extension of credit in the form of a personal loan to or for any director or executive officer (or equivalent thereof) of the Issuer, or (ii) materially modified any term of any such extension or maintenance of credit.
Section 4.21. Opinion of Financial Advisor. The Issuer has received the written opinion of Jefferies & Company, dated on or about the date of this Agreement, to the effect that, as of the date of this Agreement, the Exchange Ratio is fair, from a financial point of view, to the Issuer, a copy of which opinion will be delivered to the Issuer promptly after the date of this Agreement.
Section 4.22. Brokers. No broker, finder or investment banker (other than Jefferies & Company) is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of the Issuer. The Issuer has heretofore furnished to the Company a complete and correct copy of all agreements between the Issuer and Jefferies & Company pursuant to which such firm would be entitled to any payment relating to the Transactions.
Section 4.23. Termination of Anosys Agreement. The agreement and plan of merger, dated as of May 9, 2003 (the “Anosys Agreement”), between the Issuer and Anosys, Inc., a California corporation, was terminated in accordance with its terms and the Company does not have any continuing obligations thereunder. The execution and delivery of this Agreement by the Issuer does not, and the performance of this Agreement by the Issuer will not, (i) cause the Company, any Company Subsidiary, the Issuer or any affiliate of the Issuer to become subject to, or liable for, the payment of any amounts to any party pursuant to the terms of the Anosys Agreement and (ii) result in any breach of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default under), or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a lien or other encumbrance on any property or asset of the Company, any Company Subsidiary or the Issuer.
ARTICLE V
CONDUCT OF BUSINESS PENDING THE CLOSING
Section 5.01. Conduct of Business of the Company Pending the Closing. (a) Solely for purposes of Sections 2.08 and 7.02(b) of this Agreement, it is agreed that between the date of this Agreement and the Closing (the “Pre-Closing Period”), except as set forth in Section 5.01 of the Company Disclosure Schedule or as expressly contemplated by any other provision of this Agreement, unless the Issuer shall otherwise consent in writing (which consent shall not be unreasonably withheld or delayed):
| |
| | (i) the businesses of the Company and the Company Subsidiaries shall be conducted only in, and the Company and the Company Subsidiaries shall not take any action except in, the ordinary course of business and in a manner consistent with past practice and the Company’s 2005 budget, a copy of which has been delivered to the Issuer; and |
| |
| | (ii) the Company shall use its reasonable best efforts to preserve substantially intact the business organization of the Company and the Company Subsidiaries, to keep available the services of the current officers, employees and consultants of the Company and the Company Subsidiaries and to preserve the current relationships of the Company and the Company Subsidiaries with customers, suppliers and other persons with which the Company or any Company Subsidiary has significant business relations. |
(b) Solely for purposes of Sections 2.08 and 7.02(b) of this Agreement, by way of amplification and without limiting the provisions in Section 5.01(a), except as expressly contemplated by any other provision of this Agreement or as set forth in Section 5.01 of the Company Disclosure Schedule, during the Pre-Closing Period it is agreed that neither the Company nor any Company Subsidiary shall, between the date of this
39
Agreement and the Closing, directly or indirectly, do, or propose to do, any of the following without the prior written consent of the Issuer (which consent shall not be unreasonably withheld or delayed):
| |
| | (i) amend, otherwise change or propose to amend or otherwise change its Organizational Documents; |
| |
| | (ii) issue, sell, pledge, dispose of, grant or encumber, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of, (A) any shares of any class of capital stock of the Company or any Company Subsidiary, or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock, or any other ownership interest (including, without limitation, any phantom interest), of the Company or any Company Subsidiary (except for (1) the issuance of Company Shares issuable pursuant to Company Stock Options or Company Warrants outstanding on the date hereof and (2) the issuance of employee stock options to purchase up to 100,000 Company Shares to directors, employees and consultants of the Company or any Company Subsidiary, in the ordinary course of business and consistent with past practice) or (B) any assets of the Company or any Company Subsidiary, except in the ordinary course of business and in a manner consistent with past practice; |
| |
| | (iii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock, except for dividends by any direct or indirect wholly owned Company Subsidiary; |
| |
| | (iv) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of its capital stock; |
| |
| | (v) (A) acquire (including, without limitation, by merger, consolidation, or acquisition of stock or assets or any other business combination) any corporation, partnership, other business organization or any division thereof or any material amount of assets; (B) except for borrowings under existing credit facilities not to exceed $100,000, incur any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any person, or make any loans or advances, or grant any security interest in any of its assets except in the ordinary course of business and consistent with past practice; (C) enter into any contract or agreement other than in the ordinary course of business and consistent with past practice; (D) authorize, or make any commitment with respect to, any single capital expenditure which is in excess of $100,000 or capital expenditures which are, in the aggregate, in excess of $500,000 for the Company and the Company Subsidiaries taken as a whole; or (E) enter into or amend any contract, agreement, commitment or arrangement with respect to any matter set forth in this Section 5.01(b)(v); |
| |
| | (vi) hire any additional employees or increase the compensation payable or to become payable or the benefits provided to its directors, officers or employees, except for increases in the ordinary course of business and consistent with past practice in salaries or wages of employees of the Company or any Company Subsidiary who are not directors or officers of the Company or any Company Subsidiary, or grant any severance or termination pay to, or enter into any employment or severance agreement with, any director, officer or other employee of the Company or of any Company Subsidiary, or establish, adopt, enter into or amend any collective bargaining, bonus, profit-sharing, thrift, compensation, stock option, restricted stock, pension, retirement, deferred compensation, employment, termination, severance or other plan, agreement, trust, fund, policy or arrangement for the benefit of any director, officer or employee; |
| |
| | (vii) exercise its discretion with respect to or otherwise voluntarily accelerate the vesting of any Company Stock Option as a result of any Transaction, any other change of control of the Company (as defined in the Company Stock Option Plans) or otherwise; |
| |
| | (viii) change any accounting principles used by it, unless required by French GAAP or applicable Law; |
| |
| | (ix) make any tax election or settle or compromise any material French or U.S. federal, state, local or non-French tax liability; |
40
| |
| | (x) pay, discharge or satisfy any claim, Liability or obligation (absolute, accrued, asserted or unasserted, contingent or otherwise), other than the payment, discharge or satisfaction, in the ordinary course of business and consistent with past practice, of liabilities reflected or reserved against in the consolidated balance sheet of the Company and the consolidated Company Subsidiaries as at September 30, 2004, or subsequently incurred in the ordinary course of business and consistent with past practice; |
| |
| | (xi) amend, modify or consent to the termination of any Material Company Contract, or amend, waive, modify or consent to the termination of the Company’s or any Company Subsidiary’s material rights thereunder; |
| |
| | (xii) commence or settle any Action; |
| |
| | (xiii) permit any item of Company Owned Intellectual Property (including Company Registered Intellectual Property) to lapse or to be abandoned, dedicated or disclaimed, by failing to perform or make any applicable filings, recordings or other similar actions or filings, or by failing to pay all required fees and taxes required or advisable to maintain and protect its interest in each and every item of Company Owned Intellectual Property, except where the failure to make such filings and payments results from the exercise of reasonable business judgment; |
| |
| | (xiv) sell, assign or license any of the Company Owned Intellectual Property, except for licensing of Company Owned Intellectual Property in the ordinary course of business consistent with past practice; or |
| |
| | (xv) announce an intention, enter into any formal or informal agreement or otherwise make a commitment, to do any of the foregoing. |
Section 5.02. Conduct of Business of the Issuer Pending the Closing. (a) Solely for purposes of Sections 4.08 and 7.03(b) of this Agreement, it is agreed that during the Pre-Closing Period, except as set forth in Section 5.02(a) of the Issuer Disclosure Schedule or as expressly contemplated by any other provision of this Agreement, unless the Company shall otherwise consent in writing (which consent shall not be unreasonably withheld or delayed):
| |
| | (i) the businesses of the Issuer and the Issuer Subsidiaries shall be conducted only in, and the Issuer and the Issuer Subsidiaries shall not take any action except in, the ordinary course of business and in a manner consistent with past practice and the Issuer’s 2005 budget, a copy of which has been delivered to the Company; and |
| |
| | (ii) the Issuer shall use its reasonable best efforts to preserve substantially intact the business organization of the Issuer and the Issuer Subsidiaries, to keep available the services of the current officers, employees and consultants of the Issuer and the Issuer Subsidiaries and to preserve the current relationships of the Issuer and the Issuer Subsidiaries with customers, suppliers and other persons with which the Issuer or any Subsidiary has significant business relations. |
(b) Solely for purposes of Sections 4.08 and 7.03(b) of this Agreement, by way of amplification and without limiting the provisions in Section 5.02(c), except as expressly contemplated by any other provision of this Agreement or as set forth in Section 5.02 of the Issuer Disclosure Schedule, during the Pre-Closing Period it is agreed that the Issuer shall not, between the date of this Agreement and the Closing, directly or indirectly, do, or propose to do, any of the following without the prior written consent of the Company (which consent shall not be unreasonably withheld or delayed):
| |
| | (i) amend, otherwise change or propose to amend or otherwise change its Certificate of Incorporation or Bylaws or equivalent Organizational Documents; |
| |
| | (ii) issue, sell, pledge, dispose of, grant or encumber, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of, (A) any shares of any class of capital stock of the Issuer, or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock, or any other ownership interest (including, without limitation, any phantom interest), of the Issuer (except for (1) the issuance of shares of Issuer Common Stock issuable pursuant to employee stock |
41
| |
| | options and other Issuer convertible securities outstanding on the date hereof and (2) the issuance of employee stock options to purchase up to 100,000 shares of Issuer Common Stock to directors, employees and consultants of the Issuer in the ordinary course of business and consistent with past practice) or (B) any assets of the Issuer, except in the ordinary course of business and in a manner consistent with past practice; |
| |
| | (iii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock; |
| |
| | (iv) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of its capital stock; |
| |
| | (v) (A) acquire (including, without limitation, by merger, consolidation, or acquisition of stock or assets or any other business combination) any corporation, partnership, other business organization or any division thereof or any material amount of assets; (B) except for borrowings under existing credit facilities not to exceed $100,000, incur any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any person, or make any loans or advances, or grant any security interest in any of its assets except in the ordinary course of business and consistent with past practice; (C) enter into any contract or agreement other than in the ordinary course of business and consistent with past practice; (D) authorize, or make any commitment with respect to, any single capital expenditure which is in excess of $100,000 or capital expenditures which are, in the aggregate, in excess of $500,000; or (E) enter into or amend any contract, agreement, commitment or arrangement with respect to any matter set forth in this Section 5.02(b)(v); |
| |
| | (vi) hire any additional employees or increase the compensation payable or to become payable or the benefits provided to its directors, officers or employees, except for increases in the ordinary course of business and consistent with past practice in salaries or wages of employees of the Issuer who are not directors or officers of the Issuer, or grant any severance or termination pay to, or enter into any employment or severance agreement with, any director, officer or other employee of the Issuer, or establish, adopt, enter into or amend any collective bargaining, bonus, profit-sharing, thrift, compensation, stock option, restricted stock, pension, retirement, deferred compensation, employment, termination, severance or other plan, agreement, trust, fund, policy or arrangement for the benefit of any director, officer or employee; |
| |
| | (vii) exercise its discretion with respect to or otherwise voluntarily accelerate the vesting of any Issuer Stock Award as a result of any Transaction, any other change of control of the Issuer (as defined in the Issuer Stock Option Plans) or otherwise; |
| |
| | (viii) change any accounting principles used by it, unless required by U.S. GAAP or applicable Law; |
| |
| | (ix) make any tax election or settle or compromise any material U.S. or French federal, state, local or non-U.S. income tax liability; |
| |
| | (x) pay, discharge or satisfy any claim, Liability or obligation (absolute, accrued, asserted or unasserted, contingent or otherwise), other than the payment, discharge or satisfaction, in the ordinary course of business and consistent with past practice, of Liabilities reflected or reserved against in the consolidated balance sheet of the Issuer as at September 30, 2004 or subsequently incurred in the ordinary course of business and consistent with past practice; |
| |
| | (xi) amend, modify or consent to the termination of any Material Issuer Contract, or amend, waive, modify or consent to the termination of the Issuer’s material rights thereunder; |
| |
| | (xii) commence or settle any Action; |
| |
| | (xiii) permit any item of Issuer Owned Intellectual Property (including Issuer Registered Intellectual Property) to lapse or to be abandoned, dedicated or disclaimed, by failing to perform or make any applicable filings, recordings or other similar actions or filings, or by failing to pay all required fees and |
42
| |
| | taxes required or advisable to maintain and protect its interest in each and every item of Issuer Owned Intellectual Property, except where the failure to make such filings and payments results from the exercise of reasonable business judgment; |
| |
| | (xiv) sell, assign or license any of the Issuer Owned Intellectual Property, except for licensing of Issuer Owned Intellectual Property in the ordinary course of business consistent with past practice; |
| |
| | (xv) fail to make in a timely manner any filings with the SEC required under the Securities Act or the Exchange Act or the rules and regulations promulgated thereunder; or |
| |
| | (xvi) announce an intention, enter into any formal or informal agreement or otherwise make a commitment, to do any of the foregoing. |
Section 5.03. Control of Other Party’s Business. Nothing contained in this Agreement shall give the Company or the Principal Company Shareholders, directly or indirectly, the right to control or direct the Issuer’s operations prior to the Closing Date. Nothing contained in this Agreement shall give the Issuer or the Principal Company Shareholders, directly or indirectly, the right to control or direct the Company’s operations prior to the Closing Date. Prior to the Closing Date, each of the Company and the Issuer shall exercise, consistent with the terms and conditions of this Agreement, control and supervision over their respective operations.
ARTICLE VI
ADDITIONAL AGREEMENTS
Section 6.01. Proxy Statement. (a) As promptly as practicable after the execution of this Agreement, the Issuer, with the timely cooperation and assistance of the Company, shall prepare and file with the SEC the proxy statement to be sent to the stockholders of the Issuer relating to the meeting of the Issuer’s stockholders (the “Issuer Stockholders’ Meeting”) to be held to consider approval of the Issuer Transactions or any information statement to be sent to such stockholders, as appropriate (such proxy statement or information statement, as amended or supplemented, being referred to herein as the “Proxy Statement”). The Principal Company Shareholders shall use their reasonable best efforts to cause the Company to provide financial statements and other information regarding the Company as may reasonably be required for inclusion in the Proxy Statement. As promptly as practicable after the definitive Proxy Statement shall have been filed with and approved by the SEC, the Issuer shall mail the Proxy Statement to its stockholders. The Issuer shall also prepare an information statement in connection with the Share Exchange to be delivered to shareholders of the Company who are not accredited investors (as such term is defined in Rule 501 of Regulation D promulgated under the Securities Act)
(b) If, at any time prior to the Issuer Stockholders’ Meeting, any event or circumstance relating to the Issuer, the Company or its respective affiliates, stockholders, officers or directors should be discovered by the Issuer, the Company or the Shareholder Representative, as the case may be, which should be set forth in an amendment or supplement to the Proxy Statement so that the Proxy Statement would not include any untrue statement of a material fact or fail to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading, the Issuer or the Shareholder Representative, as the case may be, shall promptly inform the other parties. Subject to Section 6.01(d), as promptly as practicable after discovering such event or circumstance or being so informed, the Issuer, with the timely cooperation and assistance of the Company, shall prepare and file with the SEC an amendment or supplement to the Proxy Statement containing a description of such event or circumstance and disseminate such amendment or supplement to the Proxy Statement to the stockholders of the Issuer.
(c) The Issuer covenants that none of the Issuer Board or any committee thereof shall withdraw or modify, or propose to withdraw or modify, in a manner adverse to the Principal Company Shareholders, the approval or recommendation by the Issuer Board or any committee thereof of this Agreement, any Issuer Transaction or any other Transaction and the Proxy Statement shall include the recommendation of the Issuer
43
Board to the stockholders of the Issuer to approve each of the Issuer Transactions;provided,however, that the Issuer Board may, at any time prior to the Closing, withdraw or modify any such recommendation to the extent that the Issuer Board determines, in its good faith judgment after consultation with outside legal counsel, that the failure to so withdraw or modify its recommendation would cause the Issuer Board to breach its fiduciary duties to the Issuer and its stockholders under applicable Law.
(d) The Issuer covenants that no amendment or supplement to the Proxy Statement will be made by the Issuer without the approval of the Shareholder Representative (such approval not to be unreasonably withheld or delayed). The Issuer will advise the Shareholder Representative, as promptly as practicable after it receives notice thereof, of any request by the SEC for amendment of the Proxy Statement or comments thereon and responses thereto or requests by the SEC for additional information.
(e) The information supplied by the Issuer for inclusion in the Proxy Statement shall not, at (i) the time the Proxy Statement (or any amendment thereof or supplement thereto) is first mailed to the stockholders of the Issuer, (ii) the time of the Issuer Stockholders’ Meeting and (iii) the Closing, contain any untrue statement of a material fact or fail to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. All documents that the Issuer is responsible for filing with the SEC in connection with the Issuer Stockholders’ Meeting, the Share Exchange or the other Transactions will comply as to form and substance in all material aspects with the applicable requirements of the Securities Act and the rules and regulations thereunder and the Exchange Act and the rules and regulations thereunder.
(f) The information supplied by the Company or the Principal Company Shareholders for inclusion in the Proxy Statement shall not, at (i) the time the Proxy Statement (or any amendment thereof or supplement thereto) is first mailed to the stockholders of the Issuer, (ii) the time of the Issuer Stockholders’ Meeting and (iii) the Closing, contain any untrue statement of a material fact or fail to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading.
Section 6.02. Issuer Stockholders’ Meeting. The Issuer shall call and hold the Issuer Stockholders’ Meeting as promptly as practicable for the purpose of voting upon the approval of each of the Issuer Transactions. The Issuer shall use its reasonable best efforts to hold the Issuer Stockholders’ Meeting as soon as practicable after the date on which the Proxy Statement is mailed to the stockholders of the Issuer. The Issuer shall use its reasonable best efforts to solicit from its stockholders proxies in favor of the approval of each of the Issuer Transactions, and shall use its reasonable best efforts to secure the required vote or consent of its stockholders, except in the event and to the extent that the Issuer Board, in accordance with Section 6.01(c), withdraws or modifies its recommendations to its stockholders in favor of the approval of the Issuer Transactions, in which event the Issuer shall not be obligated to comply with this Section 6.02.
Section 6.03. Access to Information; Confidentiality. (a) Except as required pursuant to any confidentiality agreement or similar agreement or arrangement to which the Issuer is a party or pursuant to applicable Law, during the Pre-Closing Period, the Issuer shall: (i) provide to the Company (and the Company’s officers, directors, employees, accountants, consultants, legal counsel, agents and other representatives, collectively, “Representatives”) access at reasonable times during normal business hours upon prior notice to the officers, employees, agents, properties, offices and other facilities of such party and its subsidiaries and to the books and records thereof; and (ii) furnish promptly to the Company such information concerning the business, properties, contracts, assets, liabilities, personnel and other aspects of the Issuer as the Company or its Representatives may reasonably request.
(b) Except as required pursuant to any confidentiality agreement or similar agreement or arrangement to which a Principal Company Shareholder is a party or pursuant to applicable Law, during the Pre-Closing Period, the Principal Company Shareholders shall use their reasonable best efforts to cause the Company to: (i) provide to the Issuer and its Representatives access at reasonable times during normal business hours upon prior notice to the officers, employees, agents, properties, offices and other facilities of the Company and the Company Subsidiaries and to the books and records thereof; and (ii) furnish promptly to the Issuer such
44
information concerning the business, properties, contracts, assets, liabilities, personnel and other aspects of the Company and the Company Subsidiaries as the Issuer or its Representatives may reasonably request.
(c) All information obtained by the parties pursuant to this Section 6.03 shall be kept confidential in accordance with the Mutual Nondisclosure Agreement, dated as of September 28, 2004 (the “Confidentiality Agreement”), between the Issuer and the Company.
(d) No investigation pursuant to this Section 6.03 or made prior to the signing of this Agreement shall affect any representation or warranty in this Agreement of any party hereto or any condition to the obligations of the parties hereto.
Section 6.04. No Solicitation of Transactions. (a) Each of the Issuer, on the one hand, and the Principal Company Shareholders, on the other hand, agrees that neither it nor any of its directors, officers or employees will, and that it will not authorize or permit any of its agents, advisors or other representatives (including, without limitation, any investment banker, financial advisor, attorney or accountant retained by it or any of its subsidiaries) or, in the case of the Principal Company Shareholders, it will not authorize and will use its reasonable best efforts not to permit any of the Company or its directors, officers, employees, agents, advisors or other representatives (including, without limitation, any investment banker, financial advisor, attorney or accountant retained by the Company), to, directly or indirectly, (i) solicit, initiate or knowingly facilitate or encourage (including by way of furnishing nonpublic information), or take any other action knowingly to facilitate, any inquiries or the making of any proposal or offer (including, without limitation, any proposal or offer to its stockholders) that constitutes, or may reasonably be expected to lead to, any Competing Transaction (as defined below), (ii) other than informing persons of the existence of the provisions contained in this Section 6.04, enter into or maintain or continue discussions or negotiations with any person or entity in furtherance of such inquiries or to obtain a Competing Transaction, or (iii) agree to, approve, endorse or recommend any Competing Transaction or enter into any letter of intent or other contract, agreement or commitment contemplating or otherwise relating to any Competing Transaction;provided,however, that, notwithstanding anything to the contrary contained in this Section 6.04, the Principal Company Shareholders may take all such actions necessary to comply with the provisions of Section 6 of the Company Shareholder Agreement, including providing the Offer Notification to the Other Shareholders. Each of the Issuer, on the one hand, and the Shareholder Representative on behalf of the Principal Company Shareholders, on the other hand, shall notify the other party as promptly as practicable (and in any event within 48 hours after such party attains knowledge thereof), orally and in writing, if any proposal or offer, or any inquiry or contact with any person with respect thereto, regarding a Competing Transaction is made, specifying the material terms and conditions thereof and the identity of the party making such proposal or offer or inquiry or contact (including material amendments or proposed material amendments). Each of the Issuer, on the one hand, and the Principal Company Shareholders, on the other hand, shall provide the other party with 48 hours prior notice (or such lesser prior notice as is provided to the members of the Board of Directors of such party) of any meeting of the Board of Directors of such party at which such Board of Directors is reasonably expected to consider any Competing Transaction. Each of the Issuer, on the one hand, and the Principal Company Shareholders, on the other hand, immediately shall cease and cause to be terminated all existing discussions or negotiations with any parties conducted heretofore with respect to a Competing Transaction. Each of the Issuer, on the one hand, and the Principal Company Shareholders, on the other hand, shall not release any third party from, or waive any provision of, any confidentiality or standstill agreement to which it is a party and each such party also agrees to promptly request each person that has heretofore executed a confidentiality agreement in connection with its consideration of acquiring (whether by merger, acquisition of stock or assets or otherwise) such party or any of its subsidiaries, if any, to return (or if permitted by the applicable confidentiality agreement, destroy) all confidential information heretofore furnished to such person by or on behalf of such party and, if requested by the other party, to enforce such person’s obligation to do so.
(b) Notwithstanding anything to the contrary in this Section 6.04, the Issuer Board may furnish information to, and enter into discussions with, a person who has made an unsolicited, written, bona fide proposal or offer regarding a Competing Transaction, and such Issuer Board has (i) determined, in its good faith judgment (after having received the advice of its current financial advisor (or other financial advisor of internationally recognized reputation) and its outside counsel), that such proposal or offer constitutes, or
45
would reasonably be expected to lead to, a Superior Proposal (as defined below), (ii) determined, in its good faith judgment after consultation with its outside counsel, that, in light of such Superior Proposal, failing to furnish such information or entering into discussions would reasonably be expected to result in a breach by the Issuer Board of its fiduciary duties to the Issuer’s stockholders under applicable Law, (iii) provided written notice to the Company and the Shareholder Representative of its intent to furnish information or enter into discussions with such person at least three business days prior to taking any such action, and (iv) obtained from such person an executed confidentiality agreement on terms no less favorable to the other party than those contained in the Confidentiality Agreement (it being understood that such confidentiality agreement and any related agreements shall not include any provision calling for any exclusive right to negotiate with such party or having the effect of prohibiting such party from satisfying its obligations under this Agreement).
(c) A “Competing Transaction” means any of the following (other than the Transactions): (i) any merger, consolidation, share exchange, business combination, recapitalization, liquidation, dissolution or other similar transaction involving the Company or the Issuer, as the case may be; (ii) any sale, lease, exchange, transfer or other disposition of all or a substantial part of the assets of the Company or of the Issuer, as the case may be; (iii) any sale, exchange, transfer or other disposition of securities of the Company representing 15% or more of the voting power of or equity interest in the Company or of the Issuer, as the case may be; (iv) any tender offer or exchange offer that, if consummated, would result in any person beneficially owning securities of the Company representing 15% or more of the voting power of or equity interest in the Company or of the Issuer, as the case may be; (v) in the case of the Issuer, any solicitation in opposition to approval of any of the Issuer Transactions by the Issuer’s stockholders; or (vi) any other transaction the consummation of which would reasonably be expected to impede, interfere with, prevent or materially delay any of the Transactions.
(d) A “Superior Proposal” means an unsolicited written bona fide offer made by a third party to consummate any of the following transactions: (i) a merger, consolidation, share exchange, business combination or other similar transaction involving the Issuer pursuant to which the stockholders of such party immediately preceding such transaction would hold less than 50% of the equity interest in the surviving or resulting entity of such transaction or (ii) the acquisition by any person or group (including by means of a tender offer or an exchange offer or a two-step transaction involving a tender offer followed with reasonable promptness by a cash-out merger involving the Issuer), directly or indirectly, of ownership of 100% of the then outstanding shares of stock of the Issuer, on terms (including conditions to consummation of the contemplated transaction) that the Issuer Board determines, in its good faith judgment (after having received the advice of its current financial advisor (or other financial advisor of internationally recognized reputation) and its outside counsel) after taking into account, among other things, the terms and conditions of the offer and this Agreement (as may be proposed to be amended by the Shareholder Representative), including price, form of consideration, value of any non-cash consideration, closing conditions, the ability to fully finance the transaction and such other aspects of the offer as the Issuer Board in good faith deems relevant, to be more favorable, from a financial point of view, to the stockholders of the Issuer than the Share Exchange and is reasonably likely to be completed on the terms proposed.
Section 6.05. Directors’ and Officers’ Indemnification and Insurance. (a) The provisions contained in the Organizational Documents of the Company relating to indemnification of the directors and officers of the Company shall not be amended, repealed or otherwise modified for a period of six years from the Closing in any manner that would affect adversely the rights thereunder of individuals who, at or prior to the Closing, were directors, officers, employees, fiduciaries or agents of the Company, unless such modification shall be required by Law.
(b) The provisions contained in the Organizational Documents of the Issuer relating to indemnification of the directors and officers of the Issuer shall not be amended, repealed or otherwise modified for a period of six years from the Closing in any manner that would affect adversely the rights thereunder of individuals who, at or prior to the Closing, were directors, officers, employees, fiduciaries or agents of the Issuer, unless such modification shall be required by Law.
46
(c) The Issuer shall purchase an insurance policy, with an effective date as of the Closing, which maintains in effect for six years from the Closing the current directors’ and officers’ liability insurance policies maintained by the Company (provided that the Issuer may substitute therefor policies of at least the same coverage containing terms and conditions that are not materially less favorable) with respect to matters occurring prior to the Closing;provided,however, that in no event shall the Issuer be required to expend pursuant to this Section 6.05(c) more than an amount equal to 200% of current annual premiums paid by the Company for such insurance.
(d) The Issuer shall purchase an insurance policy, with an effective date as of the Closing, which maintains in effect for six years from the Closing the current directors’ and officers’ liability insurance policies maintained by the Issuer (provided that the Issuer may substitute therefor policies of at least the same coverage containing terms and conditions that are not materially less favorable) with respect to matters occurring prior to the Closing;provided,however, that in no event shall the Issuer be required to expend pursuant to this Section 6.05(d) more than an amount equal to 200% of current annual premiums paid by the Issuer for such insurance.
(e) The Issuer shall purchase directors’ and officers’ liability insurance policies, with an effective date as of the Closing, on commercially available terms and conditions and with coverage limits customary for U.S. public companies similarly situated to the Issuer.
(f) In the event the Issuer or any of its respective successors or assigns (i) consolidates with or merges into any other person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or (ii) transfers all or substantially all of its properties and assets to any person, then, and in each such case, proper provision shall be made so that the successors and assigns of the Issuer, shall assume the obligations set forth in this Section 6.05.
Section 6.06. Notification of Certain Matters. The Shareholder Representative shall give prompt notice to the Issuer, and the Issuer shall give prompt notice to the Shareholder Representative, of (a) the occurrence, or non-occurrence, of any event the occurrence, or non-occurrence, of which would reasonably be expected to cause any representation or warranty of such party contained in this Agreement to be untrue or inaccurate in any material respect and (b) any failure of the Principal Company Shareholders or the Issuer, as the case may be, to comply with or satisfy any covenant or agreement to be complied with or satisfied by it hereunder;provided,however, that the delivery of any notice pursuant to this Section 6.06 shall not limit or otherwise affect the remedies available hereunder to the party receiving such notice.
Section 6.07. Resale Registration Statement. (a) As promptly as practicable after the Closing, and in any event within 60 days after the Closing, the Issuer shall prepare and file with the SEC a registration statement (including the prospectus contained therein and any amendments and supplements, including post-effective supplements, to such registration statement, the “Resale Registration Statement”) providing for an offering to be made on a continuous basis pursuant to Rule 415 of the Securities Act covering all of the shares of Issuer Common Stock issued pursuant to this Agreement. After such Resale Registration Statement is filed with the SEC, the Issuer shall use its best efforts to cause the Resale Registration Statement to be declared effective as soon as practicable and to keep the Resale Registration Statement continuously effective under the Securities Act until the date which is one year after the date the Resale Registration Statement becomes effective, or such earlier date when all shares of Issuer Common Stock covered by the Resale Registration Statement have been sold.
(b) Notwithstanding the provisions of paragraph (a) of this Section 6.07, the Issuer shall be entitled to postpone or suspend, for a reasonable period of time (a “Blackout Period”), the filing, effectiveness or use of the Resale Registration Statement if the Issuer shall determine that any such filing or the offering of any shares of Issuer Common Stock would:
| |
| | (i) in the good faith judgment of the Issuer Board, materially impede, delay or interfere with any material pending or proposed financing, acquisition, corporate reorganization or other similar transaction involving the Issuer for which the Issuer Board has authorized negotiations; |
47
| |
| | (ii) based upon advice from Issuer’s investment banker, materially adversely impair the ability to consummate any pending or proposed material offering or sale of any class of securities by the Issuer; or |
| |
| | (iii) in the good faith judgment of the Issuer Board, require disclosure of material nonpublic information which, if disclosed at such time, would be seriously detrimental to the interests of the Issuer and its stockholders; |
provided,however, that the Issuer may not exercise its rights under this Section 6.07(b) to the extent that the aggregate duration of all Blackout Periods during any 12-month period would exceed 60 days. The Issuer shall use its reasonable best efforts to minimize the duration of any Blackout Period and the Issuer shall make appropriate public disclosure as soon as practicable consistent with the foregoing. Each Blackout Period shall terminate upon the earliest of completion or abandonment of the applicable transaction, public disclosure of the proposal to enter into such merger, acquisition or financing when public disclosure would no longer be seriously detrimental to the Issuer, and the 60th day of the aggregate Blackout Periods in any 12 month period. At the expiration of any Blackout Period and without any further request from the Principal Company Shareholders, the Issuer shall effect its obligations pursuant to Section 6.07(a).
(c) In connection with the Resale Registration Statement, the Issuer shall, as soon as reasonably practicable (and, in any event, subject to the terms of this Agreement, at or before the time required by applicable laws and regulations), subject to any Blackout Period:
| |
| | (i) promptly prepare and file with the SEC such amendments and supplements to the Resale Registration Statement and the prospectus used in connection therewith as may be necessary to comply with the provisions of the Securities Act with respect to the disposition of all securities covered by the Resale Registration Statement; |
| |
| | (ii) furnish to each Principal Company Shareholder such numbers of copies of the Resale Registration Statement and the prospectus included therein (including each preliminary prospectus and any amendments or supplements thereto), in conformity with the requirements of the Securities Act and such other documents and information as it may reasonably request; |
| |
| | (iii) use its reasonable best efforts to register or qualify the securities covered by the Resale Registration Statement under such other securities or blue sky laws of such jurisdiction within the United States as shall be reasonably appropriate for the distribution of the securities covered by the Resale Registration Statement;provided,however, that the Issuer shall not be required in connection therewith or as a condition thereto to qualify to do business in or to file a general consent to service of process in any jurisdiction wherein it would not but for the requirements of this paragraph (iii) be obligated to do so; andprovidedfurther that the Issuer shall not be required to qualify such securities in any jurisdiction in which the securities regulatory authority requires that a Principal Company Shareholder submit any of its securities to the terms, provisions and restrictions of any escrow, lockup or similar agreement(s) for consent to sell securities in such jurisdiction unless such Principal Company Shareholder agrees to do so; |
| |
| | (iv) promptly notify each Principal Company Shareholder, at any time when a prospectus relating to the securities covered by the Resale Registration Statement is required to be delivered under the Securities Act, of the happening of any event as a result of which the prospectus included in the Resale Registration Statement, as then in effect, includes an untrue statement of a material fact or omits to state any material fact required to be stated therein or necessary to make the statements therein not misleading in light of the circumstances under which they were made, and at the request of a Principal Company Shareholder promptly prepare and furnish (subject to the Issuer’s rights in connection with a Blackout Period) to each Principal Company Shareholder a reasonable number of copies of a supplement to or an amendment of such prospectus as may be necessary so that, as thereafter delivered to the purchasers of such securities, such prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein not misleading in light of the circumstances under which they were made; and |
48
| |
| | (v) take such other actions as are reasonably required in order to expedite or facilitate the disposition of the securities included in the Resale Registration Statement (subject to Section 6.12 of this Agreement). |
(d) All expenses incurred in connection with the Resale Registration Statement, excluding underwriters’ discounts and commissions and any stamp or transfer tax or duty, including without limitation, all registration, filing and qualification fees, word processing, duplicating, printers’ and accounting fees (including the expenses of any special audits or “cold comfort” letters required by or incident to such performance and compliance), fees of the National Association of Securities Dealers, Inc. or listing fees, messenger and delivery expenses, all fees and expenses of complying with state securities or blue sky laws, fees and disbursements of one counsel chosen by the Shareholder Representative on behalf of the Principal Company Shareholders (up to a maximum of $15,000) and fees and disbursements of counsel for the Issuer incurred in connection with the Resale Registration Statement shall be paid by the Issuer. Each Principal Company Shareholder shall bear and pay the underwriting commissions and discounts and any stamp or transfer tax or duty and the fees and disbursements of such counsel for the Principal Company Shareholders other than the one counsel referred to above incurred in connection with the Resale Registration Statement.
(e) In connection with the Resale Registration Statement, the Issuer shall, and hereby agrees to, indemnify and hold harmless each Principal Company Shareholder and such Principal Company Shareholder’s affiliates against any Loss to which such Principal Company Shareholder or such Principal Company Shareholder’s affiliates may become subject under the Securities Act or otherwise, insofar as such Loss arises out of or is based upon an untrue statement or alleged untrue statement or a material fact contained in the Resale Registration Statement filed by the Issuer, or any omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein not misleading, and the Issuer shall, and it hereby agrees to, reimburse such Principal Company Shareholder or such Principal Company Shareholder’s affiliates for any legal or other out-of-pocket expenses reasonably incurred by them in connection with investigating or defending any such action, proceeding or claim;provided,however, that the Issuer shall not be liable to any person in any case to the extent that any such Loss or expense arises out of or is based upon an untrue statement or alleged untrue statement or omission or alleged omission made in such Resale Registration Statement contained therein, in reliance upon information furnished to the Issuer by the Company or any of the Principal Company Shareholders or any such other parties acting for the Principal Company Shareholders for use therein. The Issuer shall have the right to assume the defense of any action or claim for which the Principal Company Shareholders seek indemnification pursuant to this Section 6.07(e), including the employment of counsel reasonably satisfactory to the Shareholder Representative.
(f) Each Principal Company Shareholder shall, severally but not jointly, indemnify and hold harmless the Issuer and each of its affiliates against any Loss to which the Issuer may become subject under the Securities Act or otherwise, insofar as such Loss arises out of or is based upon the omission or alleged omission to state therein a required material fact in each case to the extent, but only to the extent, that such untrue statement or alleged untrue statement was made in reliance upon and in conformity with information furnished by or on behalf of such Principal Company Shareholder or by a failure to furnish the Issuer, upon written request specifically identifying the information sought, with the information that is the subject of the untrue statement or omission. Each Principal Company Shareholder shall reimburse any legal or other expenses reasonably incurred by the Issuer or its affiliate in connection with investigating or defending any such Loss or Action. The Principal Company Shareholders shall have the right to assume the defense of any action or claim for which the Issuer seeks indemnification pursuant to this Section 6.07(f), including the employment of counsel reasonably satisfactory to the Issuer.
Section 6.08. Further Action; Reasonable Best Efforts. Upon the terms and subject to the conditions of this Agreement, each of the Issuer, on the one hand, and the Principal Company Shareholders, on the other hand, shall (i) make (or cause to be made) promptly its respective filings, and thereafter make any other required submissions, under applicable antitrust, competition or fair trade Laws with respect to the Transactions and (ii) use its reasonable best efforts to take, or cause to be taken, all appropriate action, and to do, or cause to be done, all things necessary, proper or advisable under applicable Laws or otherwise to consummate and make effective the Transactions, including using its reasonable best efforts to obtain all
49
permits, consents, approvals, authorizations, qualifications and orders of Governmental Authorities and parties to contracts with the Company, the Issuer or their respective subsidiaries as are necessary for the consummation of the Transactions to fulfill the conditions to the Share Exchange;provided that none of the Issuer, the Principal Company Shareholders or the Company will be required by this Section 6.08 to take any action, including entering into any consent decree, hold separate orders or other arrangements, that (A) requires, before or after the Closing, the divestiture of any of its material assets or of any of the material assets of any of its subsidiaries or (B) limits, before or after the Closing, its freedom of action with respect to, or its ability to retain, any of its assets or businesses or any of the assets or businesses of its subsidiaries. In case, at any time after the Closing, any further action is necessary or desirable to carry out the purposes of this Agreement, the proper officers and directors of each party to this Agreement shall use their reasonable best efforts to take all such action.
Section 6.09. Exchange of Issuer Preferred Stock. Concurrently with the execution of this Agreement, the Issuer shall enter into an agreement, in the form ofExhibit K attached hereto (the “Preferred Exchange Agreement”), with the holder (the “Preferred Holder”) of all of the outstanding shares of Series S Preferred Stock and Series S-1 Preferred Stock pursuant to which the Preferred Holder agrees to exchange all of the outstanding shares of Series S Preferred Stock and Series S-1 Preferred Stock into no more than 1,949,278 shares of Issuer Common Stock, subject to the terms and conditions of the Preferred Exchange Agreement, immediately prior to the Closing.
Section 6.10. Public Announcements. The initial press release relating to this Agreement shall be a joint press release the text of which has been agreed to by each of the Issuer, the Company and the Shareholder Representative. Thereafter, unless otherwise required by applicable Law or the requirements of the Nasdaq, each of the Issuer, the Company and the Shareholder Representative shall use its reasonable best efforts to consult with the others before issuing any press release or otherwise making any public statements with respect to this Agreement, the Share Exchange or any of the other Transactions.
Section 6.11. Corporate Matters. (a) The Issuer shall take all such action as may be necessary (i) to cause the number of directors comprising the Issuer Board as of the Closing to be increased to nine, (ii) to cause Howard E. Greene, Jr., William T. Comer and Georges Hibon to resign as members of the Issuer Board as of the Closing, (iii) to cause Jean-Loup Romet-Lemonne, Donald Drakeman, David Haselkorn, Jean Deleage, Sylvie Grégoire and Robert Beck (collectively, the “Company Designated Directors”) to be appointed to the Issuer Board as of the Closing to serve until the next annual election of directors of the Issuer, and (iv) to cause Jean-Loup Romet-Lemonne to be appointed the Chairman of the Issuer Board as of the Closing Date, to serve as Chairman until the next annual election of directors of the Issuer.
(b) The corporate headquarters of the Issuer, effective as of the Closing, will be the Issuer’s current corporate headquarters in San Diego, California.
(c) Subject to the receipt of the requisite stockholder approval at the Issuer Stockholders’ Meeting, the Issuer shall take all action as may be necessary to cause the corporate name of the Issuer to be renamed, effective as of the Closing, to “IDM, Inc.”, or such other name as the Issuer and the Company may agree prior to the mailing of the Proxy Statement, subject to receipt of the requisite approval of the stockholders of the Issuer at the Issuer Stockholders’ Meeting.
(d) Subject to the receipt of the requisite stockholder approval at the Issuer Stockholders’ Meeting, the Issuer shall take all action as may be necessary immediately prior to the Closing to (i) effect a reverse stock split of the Issuer Common Stock and the Issuer Preferred Stock pursuant to which four shares of Issuer Common Stock will be consolidated into one share of Issuer Common Stock and four shares of Issuer Preferred Stock will be consolidated into one share of Issuer Preferred Stock and (ii) cause the authorized capital stock of the Issuer after giving effect to the reverse stock split prescribed in clause (i) above to be increased to a total of 65,000,000 shares, consisting of 55,000,000 shares of Issuer Common Stock and 10,000,000 shares of Issuer Preferred Stock.
(e) The Issuer shall take all such action as may be necessary to cause each person specified onExhibit L attached hereto to be appointed as an executive officer of the Issuer effective as of, and contingent upon, the
50
Closing with the title listed opposite the name of such person onExhibit L attached hereto in accordance with the terms of such person’s Employment Agreement.
Section 6.12. Covenants of the Principal Company Shareholders. (a) No Disposition of Company Shares Prior to the Closing. Each Principal Company Shareholder hereby agrees that, except as contemplated by this Agreement, such Principal Company Shareholder shall not sell, transfer, tender, assign, pledge, encumber, contribute to the capital of any entity, hypothecate, give or otherwise dispose of, grant a proxy or power of attorney with respect to, deposit into any voting trust or enter into a voting arrangement or agreement, or create or permit to exist any Liens of any nature whatsoever (a “Transfer”) with respect to any of such person’s Company Shares (or agree or consent to, or offer to do, any of the foregoing);provided,however, that the restrictions set forth in this Section 6.12(a) shall not prohibit or restrict Transfers of Company Shares to family members or affiliates of such Principal Company Shareholder (or to entities or trusts formed by or for the benefit of such Principal Company Shareholder or family members or affiliates of such Principal Company Shareholder) if the transferee agrees in writing to be bound by this Agreement.
(b) Restrictions on Sales of Issuer Common Stock after the Closing. Each Principal Company Shareholder and its permitted assigns pursuant to Section 6.12(a) hereby agree that (i) during the period between the Closing and the date six months after the Closing, such Principal Company Shareholder (and any permitted assigns) shall not Transfer any of the shares of Issuer Common Stock received by such person pursuant to this Agreement and (ii) during the period between the date six months after the Closing and the date one year after the Closing, (A) such Principal Company Shareholder (and any permitted assigns) shall not Transfer, in the aggregate, more than fifty percent (50%) of the shares of Issuer Common Stock received by such person pursuant to this Agreement and (B) such Principal Company Shareholder agrees not to sell during any single trading day more than the number of shares of Issuer Common Stock equal to fifteen percent (15%) of the average daily trading volume of shares of Issuer Common Stock, as reported by Nasdaq, during the five trading days prior to such date;provided,however, that the restrictions set forth in this Section 6.12(b) shall not prohibit or restrict Transfers of shares of Issuer Common Stock to family members or affiliates of such Principal Company Shareholder (or to entities or trusts formed by or for the benefit of such Principal Company Shareholder or family members or affiliates of such Principal Company Shareholder) that are not made in open market transactions, if the transferee agrees in writing to be bound by the restrictions of this Section 6.12(b) or if the Issuer consents to such Transfer. Notwithstanding the foregoing, if the Principal Company Shareholder is an employee of the Issuer or the Company upon the consummation of the Transactions, and (i) such Principal Company Shareholder is or will be a party to an Employment Agreement, upon the subsequent termination of such Principal Company Shareholder’s employment by the Issuer or the Company, as the case may be, without cause, such Principal Company Shareholder’s resignation for good reason (as “cause” and “good reason” are defined in such Principal Company Shareholder’s Employment Agreement) or the death of such Principal Company Shareholder, or (ii) such Principal Company Shareholder is not a party to an Employment Agreement, upon the subsequent termination of such Principal Company Shareholder’s employment by the Issuer or the Company, such Principal Company Shareholder’s resignation from such employment or the death of such Principal Company Shareholder, in either such case, the provisions of this Section 6.12(b) shall cease to apply to such Principal Company Shareholder effective as of the date of such Principal Company Shareholder’s employment termination, resignation or death, as applicable.
(c) Agreement Regarding Counterbid. Each Principal Company Shareholder hereby agrees that it will not vote in favor of or accept a Counterbid unless the Principal Company Shareholder determines, in its good faith judgment after taking into account, among other things, the terms and conditions of the Counterbid and this Agreement (as may be amended in accordance with its terms prior to the time of such determination), including price, form of consideration, value of any non-cash consideration, closing conditions, the ability to fully finance the transaction and such aspects of the Counterbid as such Principal Company Shareholder in good faith deems relevant, that the Counterbid is more favorable to such Principal Company Shareholder than the Share Exchange.
Section 6.13. Nasdaq Listing Application. As promptly as practicable after the date of this Agreement, the Issuer shall prepare and submit to the Nasdaq a listing application covering the shares of the
51
Issuer Common Stock to be issued pursuant to this Agreement, and shall use its reasonable efforts to obtain, prior to the Closing, approval for the quotation of such shares of Issuer Common Stock.
Section 6.14. Compensation Matters. (a) The Issuer shall take all actions necessary, including, without limitation, seeking stockholder approval for such actions at the Issuer Stockholders’ Meeting, (i) to increase the number of shares of Issuer Common Stock reserved for issuance under the Issuer’s 2000 Stock Plan (including the Issuer French Stock Option Plan) by 8,800,000 shares of Issuer Common Stock, (ii) to increase the number of shares of Issuer Common Stock reserved for issuance under the Issuer’s 2001 Employee Stock Purchase Plan, (iii) to adopt the Issuer French Stock Option Plan, and (iv) to adopt the Issuer French Employee Stock Purchase Plan (the “Issuer French ESPP”), which shall be based on the Issuer’s 2001 Employee Stock Purchase Plan, with such amendments as may be reasonably necessary or desirable under French law, and shall be in form and substance reasonably satisfactory to the Company and the Issuer, and reserve for issuance thereunder 215,000 shares of Issuer Common Stock.
(b) As soon as practicable after the receipt of the requisite approval of the stockholders of the Company of the Issuer Stock Option Plan Amendment and of the adoption of the Issuer French ESPP, the Issuer shall cause the additional shares of Issuer Common Stock reserved for issuance under the Issuer’s 2000 Stock Plan and the shares of Issuer Common Stock reserved for issuance under the Issuer French ESPP to be registered under an effective registration on Form S-8 (or any successor form) or another appropriate form, and the Issuer shall use its reasonable best efforts to maintain the effectiveness of such registration statement or registration statements for as long as shares of Issuer Common Stock remain outstanding under such plans. In addition, the Issuer shall use its reasonable best efforts to cause the shares of Issuer Common Stock that are issuable under such plans to be listed on Nasdaq.
Section 6.15. Company Shareholders Agreement. (a) Each of the Principal Company Shareholders hereby (i) agrees that, notwithstanding anything to the contrary contained in Section 6.2 of the Company Shareholders Agreement, such Principal Company Shareholder shall not require that (A) the Share Exchange remains valid for a period of 70 days after the delivery of the Offer Notification or (B) any other Principal Company Shareholder not commit to accept the Share Exchange for a period of 60 days after the date hereof and (ii) waives its rights under Article 4 and the first sentence of Section 6.5 of the Company Shareholders Agreement in connection with this Agreement and the Transactions.
(b) Effective as of the Closing, each Principal Company Shareholder hereby agrees not to enforce the terms of the Company Shareholders Agreement against any other Principal Company Shareholder. Each Principal Company Shareholder agrees that, effective immediately upon the later of the Closing and the acquisition of all of the capital stock of the Company (including all securities convertible into, and all other rights to acquire, capital stock of the Company, but excluding Company Stock Options) held by parties to the Company Shareholders Agreement that are not Principal Company Shareholders by the Issuer, the Company Shareholders Agreement shall be terminated and shall have no further force or effect.
Section 6.16. Company Shareholders’ Meeting. (a) The Principal Company Shareholders shall cause Company to call and hold a meeting of the shareholders of the Company (the “Company Shareholders’ Meeting”) as promptly as practicable for the purpose of voting upon the approval of each of (i) the audited consolidated accounts of the Company for the fiscal year ended December 31, 2004 (the “2004 Company Financial Statements”); (ii) the amendment of the Medarex Warrants (the “Medarex Amendment”) to provide that, effective immediately prior to the Closing and subject to the Closing, the Medarex Warrants shall be immediately exercisable for Convertible Redeemable Bonds and that such Convertible Redeemable Bonds shall be immediately convertible into Company Shares; and (iii) the amendment of the Sanofi Warrants (the “Sanofi Amendment”, and together with the Medarex Amendment, the “Warrant Amendments”) to provide that, effective immediately prior to the Closing and subject to the Closing, the Sanofi Warrants shall be immediately exercisable for Company Shares. The Principal Company Shareholders shall cause the Company to take all actions reasonably necessary or advisable to secure the required vote of its shareholders.
52
(b) Medarex and Sanofi each hereby agrees to and approves the Medarex Amendment and the Sanofi Amendment, respectively, and shall take any action reasonably requested by the Issuer to approve such Medarex Amendment and Sanofi Amendment, respectively.
(c) Each Principal Company Shareholder hereby agrees that, at every meeting of the shareholders of the Company, including the Company Shareholders’ Meeting and at every adjournment thereof, each Principal Company Shareholder shall vote such shareholders’ Company Shares in favor of the Warrant Amendments. The provisions of this Section 6.16(b) shall not limit or otherwise restrict any Principal Company Shareholder with respect to any act or omission that such shareholder may undertake or authorize in such shareholder’s capacity as a director or officer of the Company.
ARTICLE VII
CONDITIONS TO THE SHARE EXCHANGE
Section 7.01. Conditions to the Obligations of Each Party. The obligations of the Principal Company Shareholders and the Issuer to consummate the Share Exchange are subject to the satisfaction or waiver (where permissible) of the following conditions:
| |
| | (a) Issuer Stockholder Approval. Each of the Share Exchange, the Issuer Capital Stock Increase, the Issuer Reverse Stock Split and the Option Liquidity Share Issuance shall have been approved by the requisite affirmative vote of the stockholders of the Issuer in accordance with the rules and regulations of Nasdaq, the DGCL (as defined below) and the Issuer Certificate of Incorporation. |
| |
| | (b) No Order. No Governmental Authority shall have enacted, issued, promulgated, enforced or entered any law, rule, regulation, judgment, decree, executive order or award (an “Order”) which is then in effect and has the effect of making the Share Exchange illegal or otherwise prohibiting consummation of the Share Exchange. |
| |
| | (c) Required Regulatory Approvals. Any waiting period (and any extension thereof) applicable to the consummation of the Share Exchange under applicable antitrust, competition or fair trade laws shall have expired or been terminated and all approvals of Governmental Authorities required in respect of the transactions contemplated by this Agreement under applicable antitrust, competition or fair trade laws shall have been obtained. |
| |
| | (d) Nasdaq Approval. Nasdaq shall have approved for quotation on the Nasdaq National Market the shares of Issuer Common Stock to be issued in the Share Exchange, subject to notice of issuance. |
| |
| | (e) Exchange of Issuer Preferred Stock. All of the outstanding shares of Series S Preferred Stock and Series S-1 Preferred Stock shall have been exchanged for shares of Issuer Common Stock in accordance with the terms of the Preferred Exchange Agreement. |
| |
| | (f) Company Shareholder Agreement. Either (i) all of the Other Shareholders shall have waived their rights under Section 6 of the Company Shareholders Agreement or (ii) the Principal Company Shareholders shall have complied with the requirements of Section 6 of the Company Shareholders Agreement and shall be permitted thereunder to require the Other Shareholders who have not executed a Joinder Agreement to exchange their Company Shares for shares of Issuer Common Stock upon the terms and subject to the conditions of this Agreement. |
| |
| | (g) Completion of Issuer Capital Stock Increase and Issuer Reverse Stock Split. The Issuer Capital Stock Increase and the Issuer Reverse Stock Split shall have been completed in accordance with this Agreement. |
| |
| | (h) Ownership of Company Shares. The Principal Company Shareholders (including any Other Shareholder who became a Principal Company Shareholder upon executing a Joinder Agreement pursuant to Section 1.09(b)), in the aggregate, shall own, of record and beneficially, the number of Company Shares representing at least 95% of the outstanding Company Shares as of the Closing (and including the Company Shares issuable upon exercise of the Company Warrants outstanding as of the |
53
| |
| | Closing) and no Other Shareholder (or group (as such term is defined for purposes of Section 13(d) of the Exchange Act) of Other Shareholders) who has not signed a Joinder Agreement shall own, of record or beneficially, the number of Company Shares representing 5% or more of the outstanding Company Shares as of the Closing. |
Section 7.02. Conditions to the Obligations of the Issuer. The obligations of the Issuer to consummate the Share Exchange are subject to the satisfaction or waiver (where permissible) of the following additional conditions:
| |
| | (a) Representations and Warranties. The representations and warranties regarding the Company contained in Article II of this Agreement and the representations and warranties of the Principal Company Shareholders contained in Article III of this Agreement shall be true and correct (without giving effect to any limitation as to materiality or Company Material Adverse Effect set forth therein) as of the Closing, as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case as of such earlier date), except where the failure of such representations regarding the Company or the Principal Company Shareholders to be so true and correct (without giving effect to any limitation as to materiality or Company Material Adverse Effect set forth therein) would not, individually or in the aggregate, have a Company Material Adverse Effect. |
| |
| | (b) Agreements and Covenants. The Principal Company Shareholders shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by them on or prior to the Closing and the Company shall not have taken action, or failed to take any action, which constitutes a breach of Section 5.01 of this Agreement. |
| |
| | (c) Officer Certificate. The Shareholder Representative shall have delivered to the Issuer a certificate, dated the date of the Closing, signed by the President or any Vice President of the Company, certifying as to the satisfaction of the conditions specified in Sections 7.02(a) and 7.02(b). |
| |
| | (d) Consents. The consents, approvals or authorizations listed on Section 7.02(d) of the Company Disclosure Schedule shall have been obtained. |
| |
| | (e) Material Adverse Effect. No Company Material Adverse Effect shall have occurred since the date of this Agreement. |
| |
| | (f) Indemnity Escrow Agreement. The Shareholder Representative shall have entered into the Indemnity Escrow Agreement and the Indemnity Escrow Agreement shall be in full force and effect at the Closing. |
Section 7.03. Conditions to the Obligations of the Principal Company Shareholders. The obligations of the Principal Company Shareholders to consummate the Share Exchange are subject to the satisfaction or waiver (where permissible) of the following additional conditions:
| |
| | (a) Representations and Warranties. The representations and warranties regarding the Issuer contained in Article IV of this Agreement shall be true and correct (without giving effect to any limitation as to materiality or Issuer Material Adverse Effect set forth therein) as of the Closing, as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case as of such earlier date), except where the failure of such representations to be so true and correct (without giving effect to any limitation as to materiality or Issuer Material Adverse Effect set forth therein) would not, individually or in the aggregate, have an Issuer Material Adverse Effect. |
| |
| | (b) Agreements and Covenants. The Issuer shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Closing and the Issuer shall not have taken any action, or failed to take any action, which constitutes a breach of Section 5.02 of this Agreement. |
| |
| | (c) Officer Certificate. The Issuer shall have delivered to the Shareholder Representative a certificate, dated the date of the Closing, signed by the President or any Vice President of the Issuer, certifying as to the satisfaction of the conditions specified in Sections 7.03(a) and 7.03(b). |
54
| |
| | (d) Consents. The consents, approvals or authorizations listed on Section 7.03(d) of the Issuer Disclosure Schedule shall have been obtained. |
| |
| | (e) Material Adverse Effect. No Issuer Material Adverse Effect shall have occurred since the date of this Agreement. |
| |
| | (f) Indemnity Escrow Agreement. The Issuer shall have entered into the Indemnity Escrow Agreement and the Indemnity Escrow Agreement shall be in full force and effect at the Closing. |
| |
| | (g) Option Liquidity Agreements. The Issuer shall have complied with its obligations under Section 1.07 of this Agreement and shall have entered into Option Liquidity Agreements with each holder of Company Stock Options who has tendered a signed Option Liquidity Agreement to the Issuer. |
| |
| | (h) Put/ Call Agreements. The Issuer shall have complied with its obligations under Section 1.09 of this Agreement and shall have entered into Put/ Call Agreements with each holder of Company Shares in a PEA who has tendered a signed Put/ Call Agreement to the Issuer. |
ARTICLE VIII
TERMINATION, AMENDMENT AND WAIVER
Section 8.01. Termination. This Agreement may be terminated and the Share Exchange and the other Transactions may be abandoned at any time prior to the Closing, notwithstanding any requisite approval and adoption of this Agreement and the Transactions by the stockholders of the Issuer, as follows:
| |
| | (a) by mutual written consent of the Issuer and the Shareholder Representative on behalf of the Principal Company Shareholders; |
| |
| | (b) by either the Issuer or the Shareholder Representative on behalf of the Principal Company Shareholders if the Closing shall not have occurred on or before June 30, 2005 (the “End Date”);provided,however, that the End Date shall be July 31, 2005 in the event that the Issuer or its counsel shall receive a comment letter on the Proxy Statement from the SEC; andprovidedfurther that the right to terminate this Agreement under this Section 8.01(b) shall not be available to any party whose failure to fulfill any obligation under this Agreement has been the cause of, or resulted in, the failure of the Closing to occur on or before such date; |
| |
| | (c) by either the Issuer or the Shareholder Representative on behalf of the Principal Company Shareholders if any Governmental Authority shall have enacted, issued, promulgated, enforced or entered any injunction, order, decree or ruling (whether temporary, preliminary or permanent) which has become final and nonappealable and has the effect of making consummation of the Share Exchange illegal or otherwise preventing or prohibiting consummation of the Share Exchange; |
| |
| | (d) by the Shareholder Representative on behalf of Principal Company Shareholders (at any time prior to the approval of the Issuer Transactions by the required vote of the stockholders of the Issuer) if a Triggering Event with respect to the Issuer shall have occurred; |
| |
| | (e) by either the Issuer or the Shareholder Representative on behalf of the Principal Company Shareholders if any of the Share Exchange, the Issuer Capital Stock Increase, the Issuer Reverse Stock Split or the Option Liquidity Share Issuance shall fail to receive the requisite vote for approval at the Issuer Stockholders’ Meeting; |
| |
| | (f) by the Issuer upon a breach of any representation, warranty, covenant or agreement regarding or on the part of the Company or the Principal Company Shareholders set forth in this Agreement, or if any representation or warranty regarding or on the part of the Company or the Principal Company Shareholders shall have become untrue, in either case such that the conditions set forth in Sections 7.02(a) and 7.02(b) would not be satisfied (“Terminating Company Breach”);provided,however, that, if such Terminating Company Breach is curable by the Company or the Principal Company Shareholders, the Issuer may not terminate this Agreement under this Section 8.01(f) for so |
55
| |
| | long as the Company or the Principal Company Shareholders continue to exercise their reasonable best efforts to cure such breach, unless such breach is not cured within 30 days after notice of such breach is provided by the Issuer to the Shareholder Representative; |
| |
| | (g) by the Shareholder Representative on behalf of the Principal Company Shareholder upon a breach of any representation, warranty, covenant or agreement regarding or on the part of the Issuer set forth in this Agreement, or if any representation or warranty regarding or on the part of the Issuer shall have become untrue, in either case such that the conditions set forth in Sections 7.03(a) and 7.03(b) would not be satisfied (“Terminating Issuer Breach”);provided,however, that, if such Terminating Issuer Breach is curable by the Issuer, the Shareholder Representative may not terminate this Agreement under this Section 8.01(g) for so long as the Issuer continues to exercise its reasonable best efforts to cure such breach, unless such breach is not cured within 30 days after notice of such breach is provided by the Shareholder Representative to the Issuer; or |
| |
| | (h) by the Issuer, if, prior to the receipt of the requisite vote for approval at the Issuer Stockholders’ Meeting of the Share Exchange, the Issuer Capital Stock Increase, the Issuer Reverse Stock Split and the Option Liquidity Share Issuance, the Issuer receives a Superior Proposal, the Issuer Board resolves to accept such Superior Proposal (after it has determined that such acceptance is required to comply with its fiduciary duties to the Issuer’s stockholders under applicable Law) and the Issuer shall have given the Shareholder Representative four business days prior written notice of its intention to terminate this Agreement pursuant to this Section 8.01(h);provided,however, that the Issuer may not terminate this Agreement under this Section 8.01(h) if it has breached, directly or indirectly, any of its obligations set forth in Section 6.04. |
For purposes of this Agreement, a “Triggering Event” with respect to the Issuer hereto shall be deemed to have occurred if: (i) the Issuer Board or any committee thereof withdraws, modifies or changes its recommendation of this Agreement, the Share Exchange, the Issuer Capital Stock Increase, the Issuer Reverse Stock Split or the Option Liquidity Share Issuance in a manner adverse to the Principal Company Shareholders or shall have resolved to do so; (ii) the Issuer Board shall have recommended to the stockholders of Issuer a Competing Transaction or shall have resolved to do so or shall have entered into any letter of intent or similar document or any agreement, contract or commitment accepting any Competing Transaction; (iii) the Issuer shall have failed to include in the Proxy Statement the recommendation of the Issuer Board to approve all of the Issuer Transactions; or (iv) a tender offer or exchange offer for 15% or more of the outstanding shares of capital stock of the Issuer is commenced, and the Issuer Board recommends in favor of such tender offer or exchange offer, consents to the acquisition of shares of Issuer Common Stock pursuant to such tender offer or exchange offer or otherwise effectively waives Section 203 of the DGCL with respect to such tender offer or exchange offer.
Section 8.02. Effect of Termination. In the event of the termination of this Agreement pursuant to Section 8.01, this Agreement shall forthwith become void, and there shall be no liability under this Agreement on the part of any party hereto, except (a) as set forth in Section 8.03 and (b) that nothing herein shall relieve any party from liability for any breach of any of its representations, warranties, covenants or agreements set forth in this Agreement prior to such termination;provided,however, that the Confidentiality Agreement shall survive any termination of this Agreement.
Section 8.03. Fees and Expenses. (a) All Expenses (as defined below) incurred in connection with this Agreement and the transactions contemplated by this Agreement shall be paid by the party incurring such expenses, whether or not the Share Exchange or any other transaction is consummated. “Expenses”, as used in this Agreement, shall include all reasonable out-of-pocket expenses (including, without limitation, all fees and expenses of counsel, accountants, investment bankers, experts and consultants to a party hereto and its affiliates) incurred by a party or on its behalf in connection with or related to the authorization, preparation (including the conduct of due diligence), negotiation, execution and performance of this Agreement, the preparation, printing, filing and mailing of the Proxy Statement, the solicitation of stockholder approvals, the filing of any required notices under applicable antitrust, competition or fair trade laws or other similar
56
regulations and all other matters related to the closing of the Share Exchange and the other transactions contemplated by this Agreement.
(b) The Issuer agrees that:
| |
| | (i) if the Shareholder Representative shall terminate this Agreement pursuant to Section 8.01(d); |
| |
| | (ii) if (A) the Issuer or the Shareholder Representative shall terminate this Agreement pursuant to Section 8.01(b) or 8.01(e), (B) prior to the time of such termination a Competing Transaction shall have been publicly announced with respect to the Issuer, and (C) the Issuer enters into an agreement providing for a Third Party Acquisition (as defined below) within nine months after the date of such termination or a Third Party Acquisition is consummated within nine months after the date of such termination; or |
| |
| | (iii) if the Issuer shall terminate this Agreement pursuant to Section 8.01(h); |
then the Issuer shall pay to the Company promptly (but in any event no later than one business day after the first of such events shall have occurred) a fee of $1,334,600 (the “Fee”), which amount shall be payable in immediately available funds.
(c) Each of the Issuer and the Principal Company Shareholders acknowledges that the agreements contained in this Section 8.03 are an integral part of the transactions contemplated by this Agreement. In the event that the Issuer shall fail to pay the Fee when due, the Issuer shall also pay the costs and expenses actually incurred or accrued by the Company (including, without limitation, fees and expenses of counsel) in connection with the collection under and enforcement of this Section 8.03, together with interest on such unpaid Fee, commencing on the date that the Fee became due, at a rate equal to the rate of interest publicly announced by Citibank, N.A., from time to time, in The City of New York, as such bank’s Prime Rate plus 1.00%. Payment of the fees and expenses described in this Section 8.03 shall not be in lieu of any damages incurred in the event of willful or intentional breach of this Agreement.
(d) “Third Party Acquisition” means any of the following transactions (other than the transactions contemplated by this Agreement): (i) a merger, consolidation, business combination, recapitalization, liquidation, dissolution or similar transaction involving the Issuer pursuant to which the stockholders of such party immediately preceding such transaction hold less than fifty percent (50%) of the aggregate equity interests in the surviving or resulting entity of such transaction or of any direct or indirect parent thereof; (ii) a sale or other disposition by the Issuer of assets representing in excess of fifty percent (50%) of the aggregate fair market value of the business of such party immediately prior to such sale or other disposition; (iii) an acquisition by any person or group (including by way of a tender offer or an exchange offer) or an issuance of capital stock by the Issuer, directly or indirectly, of beneficial ownership of fifty percent (50%) or more of the voting power of the then outstanding shares of capital stock of the Issuer; (iv) the adoption by the Issuer of a plan of liquidation or the declaration or payment of an extraordinary dividend; or (v) the repurchase by the Issuer or any of its subsidiaries of 50% or more of the outstanding shares of capital stock of such party.
Section 8.04. Amendment. This Agreement may be amended only by mutual agreement of the Issuer and the holders of a majority of the outstanding Company Shares held by the Principal Company Shareholders as of the Effective Date.
Section 8.05. Waiver. At any time prior to the Closing, the Shareholder Representative on behalf of the Principal Company Shareholders, on the one hand, or the Issuer, on the other hand, may (a) extend the time for the performance of any obligation or other act of the other party hereto, (b) waive any inaccuracy in the representations and warranties of the other party contained herein or in any document delivered pursuant hereto and (c) waive compliance with any agreement of the other party or any condition to its own obligations contained herein. Any such extension or waiver shall be valid if set forth in an instrument in writing signed by the party or parties to be bound thereby.
57
ARTICLE IX
INDEMNIFICATION
Section 9.01. Survival of Representations and Warranties. The statements regarding the Company contained in Article II of this Agreement and the representations and warranties of the Principal Company Shareholders contained in Article III of this Agreement shall survive the Closing until 11:59 p.m. California Time, six months following the Closing. If written notice of a claim has been given prior to the expiration of the applicable representations and warranties by a party hereto to another party hereto, then the relevant representations and warranties shall survive as to such claim until such claim has been finally resolved. The representations and warranties of the Issuer contained in Article IV of the Agreement shall not survive the Closing.
Section 9.02. Indemnification by the Principal Company Shareholders. (a) Subject to Section 9.02(b) below, from and after the Closing, the Principal Company Shareholders, severally and not jointly in the case of Losses arising out of or resulting from the matters set forth in clause (i)(y) below, and, jointly and severally in the case of Losses arising out of or resulting from the other matters set forth below, shall indemnify and hold harmless the Issuer and its affiliates, officers, directors, employees, agents, successors and assigns (each an “Issuer Indemnified Party”) from and against any and all Liabilities, losses, damages, injury, claims, costs and expenses, interest, awards, judgments, fine and penalties (including reasonable attorneys’ fees and expenses, external costs of investigation, expert fees, accounting fees and advisory fees) actually suffered or incurred by any of them (including any Action brought or otherwise initiated by any of them or by a third party) (hereinafter a “Loss”) (provided that, solely for purposes of determining the amount of any Loss, but not whether there has been a breach of any representation, warranty or covenant, any materiality or Material Adverse Effect qualifier to any representation or warranty shall be ignored), arising out of or resulting from:
| |
| | (i) (x) the breach of any statement regarding the Company contained in Article II of this Agreement or (y) the breach of any representation or warranty made by such Principal Company Shareholder contained in Article III of this Agreement; and |
| |
| | (ii) the breach of any covenant or agreement regarding the Company or by the Principal Company Shareholders contained in Article V or Article VI of this Agreement. |
(b) Notwithstanding anything to the contrary contained in this Agreement:
| |
| | (i) the Indemnity Escrow Shares shall be the sole and exclusive remedy for any Losses arising out of any and all claims relating to the subject matter of this Agreement, and the maximum amount that may be recovered from any Principal Company Shareholder shall be limited to such person’s pro rata share of the Indemnity Escrow Shares; |
| |
| | (ii) no Issuer Indemnified Party shall be held harmless pursuant to Section 9.02 unless and until the aggregate amount of such party’s Losses equals or exceeds $500,000, after which the Principal Company Shareholders shall be liable only for those Losses in excess of $500,000; |
| |
| | (iii) in no event may the Issuer or any other party seek recourse against the Company, either before or after the Closing, for any breach of any representation, warranty, covenant or other agreement of or regarding the Company or the Principal Company Shareholders contained in this Agreement or any other matter related to this Agreement; and |
| |
| | (iv) in no event shall any Principal Company Shareholder be liable to indemnify or hold harmless any Issuer Indemnified Party pursuant to this Section 9.02 for any Losses to the extent arising out of or resulting from a breach of a representation or warranty made by any other Principal Company Shareholder contained in Article III of this Agreement. |
(c) The indemnification liability of the Principal Company Shareholders with respect to the representations, warranties, covenants and obligations of the Principal Company Shareholders or the representations and
58
warranties regarding the Company set forth in Article II shall not be reduced by any investigation made by the Issuer or any Representative of the Issuer.
Section 9.03. Notice of Loss; Third Party Claims. (a) For purposes of this Section 9.03, a party against which an indemnification claim may be sought is referred to as the “Indemnifying Party” and the party that may be entitled to be indemnified is referred to as the “Indemnified Party”. For purposes of the procedures in Sections 9.03(b) and (c), Indemnifying Party shall mean the Shareholder Representative if an indemnification claim is sought pursuant to Section 9.02.
(b) An Indemnified Party shall give the Indemnifying Party notice of any matter which an Indemnified Party has determined has given or could give rise to a right of indemnification or to be held harmless under this Agreement, within 60 days of such determination, stating the amount of the Loss, if known, and method of computation thereof and containing a reference to the provisions of this Agreement in respect of which such right of indemnification or to be held harmless is claimed or arises.
(c) If an Indemnified Party shall receive notice of any Action, audit, demand or assessment (each, a “Third Party Claim”) against it or which may give rise to a claim for Loss under this Article IX, within 30 days of the receipt of such notice, the Indemnified Party shall give the Indemnifying Party notice of such Third Party Claim;provided,however, that the failure to provide such notice shall not release the Principal Company Shareholders, except to the extent that the Principal Company Shareholders are materially prejudiced by such failure and shall not relieve the Principal Company Shareholders, from any other obligation or Liability that it may have to any Indemnified Party otherwise than under this Article IX. The Indemnifying Party shall be entitled to assume and control the defense of such Third Party Claim at the expense of the Principal Company Shareholders, and through counsel of its choice if it gives notice of its intention to do so to the Indemnified Party within five days of the receipt of such notice from the Indemnified Party;provided,however, that, if there exists or is reasonably likely to exist a conflict of interest that would make it inappropriate in the reasonable judgment of the Indemnified Party for the same counsel to represent both the Indemnified Party and the Indemnifying Party, then the Indemnified Party shall be entitled to retain its own counsel in each jurisdiction for which the Indemnified Party reasonably determines counsel is required, at the expense of the Principal Company Shareholders. In the event that the Indemnifying Party exercises the right to undertake any such defense against any such Third Party Claim as provided above, the Indemnified Party shall cooperate with the Indemnifying Party in such defense and make available to the Indemnifying Party, at the expense of the Principal Company Shareholders, all witnesses, pertinent records, materials and information relating thereto in the possession of or under the Indemnified Party’s control as is reasonably required by the Indemnifying Party. Similarly, in the event the Indemnified Party is, directly or indirectly, conducting the defense against any such Third Party Claim, the Principal Company Shareholders shall cooperate with the Indemnified Party in such defense and make available to the Indemnified Party, at the expense of the Principal Company Shareholders, all such witnesses, records, materials and information relating thereto in the possession of or under the control of the Principal Company Shareholders, as is reasonably required by the Indemnified Party. No such Third Party Claim may be settled by the Indemnifying Party without the prior written consent of the Indemnified Party, which shall not be unreasonably withheld.
Section 9.04. Shareholder Representative. (a) Hélène Ploix (such person and any successor or successors being the “Shareholder Representative”) shall act as the representative of the Principal Company Shareholders, and shall be authorized to act on behalf of the Principal Company Shareholders and to take any and all actions required or permitted to be taken by the Shareholder Representative under this Agreement or the Indemnity Escrow Agreement, with respect to any claims (including the settlement thereof) made by an Issuer Indemnified Party for indemnification or to be held harmless pursuant to this Article IX of the Agreement and with respect to any actions to be taken by the Shareholder Representative pursuant to the terms of the Indemnity Escrow Agreement. The Principal Company Shareholders shall be bound by all actions taken by the Shareholder Representative in its capacity thereof.
(b) The Shareholder Representative shall at all times act in his or her capacity as Shareholder Representative in a manner that the Shareholder Representative believes in good faith to be in the best interest of the Principal Company Shareholders. Neither the Shareholder Representative nor any of its
59
directors, officers, agents or employees shall be liable to any person for any error of judgment, or any action taken, suffered or omitted to be taken, under this Agreement or the Indemnity Escrow Agreement, except in the case of its gross negligence, bad faith or willful misconduct. The Shareholder Representative may consult with legal counsel, independent public accountants and other experts selected by it and shall not be liable for any action taken or omitted to be taken in good faith by it in accordance with the advice of such counsel, accountants or experts. The Shareholder Representative shall not have any duty to ascertain or to inquire as to the performance or observance of any of the terms, covenants or conditions of this Agreement or the Indemnity Escrow Agreement. As to any matters not expressly provided for in this Agreement or the Indemnity Escrow Agreement, the Shareholder Representative shall not be required to exercise any discretion or take any action.
(c) Each Principal Company Shareholder severally shall indemnify and hold harmless and reimburse the Shareholder Representative from and against such Principal Company Shareholder’s ratable share of any and all Losses suffered or incurred by the Shareholder Representative arising out of or resulting from any action taken or omitted to be taken by the Shareholder Representative under this Agreement or the Indemnity Escrow Agreement, other than such Losses arising out of or resulting from the Shareholder Representative’s gross negligence, bad faith or willful misconduct. Each Principal Company Shareholder agrees that the Shareholder Representative may make claims first, against the Expense Escrow Shares, and second, against the Indemnity Escrow Shares, but solely to the extent any Indemnity Escrow Shares are available for transfer to the Shareholder Representative pursuant to Section 1.07(g) or (i) of the Indemnity Escrow Agreement, for any and all Losses against which the Shareholder Representative is to be indemnified, held harmless and reimbursed pursuant to this Section 9.04(c). In the event that the Expense Escrow Shares and, if available pursuant to Section 1.07(g) or (i) of the Indemnity Escrow Agreement, the Indemnity Escrow Shares are not, or the Shareholder Representative reasonably believes will not be, sufficient to indemnify, hold harmless and reimburse the Shareholder Representative pursuant to this Section 9.04(c), the Shareholder Representative may request (an “Additional Funding Request”) that additional funds (the “Additional Funds”) be deposited by the Principal Company Shareholders with the Expense Escrow Agent to be held pursuant to the terms of the Expense Escrow Agreement by delivery to the Principal Company Shareholders of a written notice (a “Funding Request Notice”) making an Additional Funding Request. Each Funding Request Notice shall:
| |
| | (i) state the amount of Additional Funds required, or estimated in the good faith judgment of the Shareholder Representative to be required, to hold harmless the Shareholder Representative pursuant to this Section 9.04(c) and/or to reimburse the Shareholder Representative for any expenses incurred in connection with the performance of its duties pursuant to Section 9.04; |
| |
| | (ii) state the pro rata share (which will be based on each Principal Company Shareholder’s initial interest in the fund established pursuant to the Expense Escrow Agreement (the “Expense Escrow Fund”)) of the Additional Funds (the “Pro Rata Amount”) that each Principal Company Shareholder shall be required to deliver to the Expense Escrow Agent in the event that the Additional Fund Request is approved by the Principal Company Shareholders in accordance with this Section 9.04(c); and |
| |
| | (iii) specify in reasonable detail the nature and amount of the Losses incurred prior to the date of the Funding Request Notice and the intended use of the Additional Funds. |
In the event that the holders of a majority of the outstanding Company Shares held by the Principal Company Shareholders as of the Effective Date agree to the Additional Funding Request, all of the Principal Company Shareholders shall be deemed to have agreed to the Additional Funding Request and shall deliver to the Expense Escrow Agent for deposit into the Expense Escrow Fund either (i) cash in an amount equal to such Principal Company Shareholder’s Pro Rata Amount or (ii) the number of shares of Issuer Common Stock equal to such Principal Company Shareholder’s Pro Rata Amount divided by the Average Closing Price as of the earlier of (i) the date of such delivery to the Expense Escrow Agent and (ii) the date such Principal Company Shareholder mails or instructs its broker to deliver, to the Expense Escrow Agent such shares of Issuer Common Stock. Notwithstanding anything to the contrary contained herein, the Shareholder Representative shall not be liable to any Principal Company Shareholder for taking or omitting to take any action in
60
the event that funds are not, or the Shareholder Representative reasonably believes that funds will not be, available to indemnify, hold harmless or reimburse the Shareholder Representative in accordance with this Section 9.04(c).
(d) Notwithstanding anything to the contrary herein or in the Indemnity Escrow Agreement, the Shareholder Representative shall not in any manner exercise, or seek to exercise, any voting power whatsoever with respect to shares of capital stock of the Company or the Issuer now or hereafter owned of record or beneficially by any Principal Company Shareholder unless the Shareholder Representative is expressly authorized to do so in a writing signed by such Principal Company Shareholder. In all matters relating to this Article IX, the Shareholder Representative shall be the only party entitled to assert the rights of the Principal Company Shareholders, and the Shareholder Representative shall perform all of the obligations of the Principal Company Shareholders hereunder. The Issuer shall be entitled to rely on all statements, representations and decisions of the Shareholder Representative.
(e) Each Principal Company Shareholder shall deliver to the Shareholder Representative a power of attorney, substantially in the form ofExhibit M attached hereto, to appoint the Shareholder Representative as such Principal Company Shareholder’s attorney in fact to perform any act required under this Agreement and the Indemnity Escrow Agreement, subject to the terms hereof and thereof. Each Principal Company Shareholder hereby acknowledges and agrees that the Shareholder Representative may execute and deliver on such Principal Company Shareholder’s behalf the Expense Escrow Agreement and, upon such execution and delivery, the Expense Escrow Agreement shall be a binding obligation on such Principal Company Shareholder.
ARTICLE X
GENERAL PROVISIONS
Section 10.01. Non-Survival of Representations, Warranties and Agreements. The representations, warranties and agreements in this Agreement shall terminate upon the termination of this Agreement pursuant to Section 8.01. The agreements contained in this Agreement shall terminate at the Closing, except that the agreements set forth in Articles I and IX and Sections 6.05, 6.07, 6.12(b) and 8.03 and this Article X shall survive the Closing.
Section 10.02. Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given upon receipt) by delivery in person, by telecopy or email or by registered or certified mail (postage prepaid, return receipt requested) to the respective parties at the following addresses (or at such other address for a party as shall be specified in a notice given in accordance with this Section 10.02):
if to the Issuer:
| |
| | Epimmune Inc. |
| | 5820 Nancy Ridge Drive |
| | San Diego, CA 92121 |
| | Facsimile: (858) 860-2600 |
| | Attention: Chief Financial Office |
| | Email: bdevaere@epimmune.com |
with a copy to:
| |
| | Cooley Godward LLP |
| | 4401 Eastgate Mall |
| | San Diego, CA 92121 |
| | Facsimile: (858) 550-6420 |
| | Attention: Kay Chandler |
| | Email: kchandler@cooley.com |
61
if to the Shareholder Representative or the Company:
| |
| | Hélène Ploix |
| | 71 Boulevard Arago |
| | 75013 Paris |
| | France |
| | Facsimile: +33-1-56-59-7956 |
| | Email: hploix@pechel-industries.com |
| |
| | and |
| |
| | IDM S.A. |
| | 172 Rue de Charonne |
| | 75545 Paris |
| | Cedex 11 France |
| | Facsimile: +33-1-40-09-0425 |
| | Attention: Chief Financial Officer |
| | Email: hdl@idm-biotech.com |
with a copy to:
| |
| | Shearman & Sterling LLP |
| | 114, avenue des Champs-Élysées |
| | 75008 Paris |
| | France |
| | Facsimile: +33-1-53-89-7070 |
| | Attention: Manuel A. Orillac |
| | Email: morillac@shearman.com |
Section 10.03. Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of Law, or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated to the fullest extent possible.
Section 10.04. Entire Agreement; Assignment. This Agreement and the Voting Agreements constitute the entire agreement among the parties with respect to the subject matter hereof and supersedes, except as set forth in Section 6.03(c), all prior agreements and undertakings, both written and oral, among the parties, or any of them, with respect to the subject matter hereof. This Agreement shall not be assigned (whether pursuant to a merger, by operation of Law or otherwise);provided,however, that, after the Closing, the Issuer may assign this Agreement, without the consent of the other parties hereto, in connection with a consolidation or merger of the Issuer or the Company with another corporation, or the sale of all or substantially all of the assets or stock of the Issuer or the Company to any other person.
Section 10.05. Parties in Interest. This Agreement shall be binding upon and inure solely to the benefit of each party hereto, and nothing in this Agreement, express or implied, is intended to or shall confer upon any other person any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement, other than the provisions of Sections 1.09, 6.05, 8.03(b) and 9.02(c) (which are intended to be for the benefit of the persons covered thereby and may be enforced by such persons).
Section 10.06. Specific Performance. The parties hereto agree that irreparable damage would occur in the event any provision of this Agreement were not performed in accordance with the terms hereof and that the parties shall be entitled to specific performance of the terms hereof in addition to any other remedy at law or equity.
62
Section 10.07. Governing Law; Arbitration. (a) This Agreement shall be governed by, and construed in accordance with, the laws of the State of Delaware applicable to contracts executed in and to be performed in that jurisdiction (other than those provisions set forth herein that are required to be governed by the laws of the Republic of France), excluding (to the greatest extent a Delaware court would permit) any rule of law that would cause the application of the laws of any jurisdiction other than the State of Delaware.
(b) The parties irrevocably agree that any dispute, controversy or claim arising out of or relating to this Agreement or the transactions contemplated thereby, or the breach, termination or invalidity thereof, shall be settled by arbitration in accordance with the Rules of Conciliation and Arbitration of the International Chamber of Commerce as at present in force. The place of arbitration shall be Orange County, California and the number of arbitrators shall be three. Each of (i) the Shareholder Representative, on behalf of the Principal Company Shareholders, on the one hand, and (ii) the Issuer, on the other hand, shall designate one arbitrator and the two so designated arbitrators shall jointly designate the third arbitrator. If such designation is not made within fifteen (15) days of the designation of the second party designated arbitrator, the Secretary General of the International Court of Arbitration of the International Chamber of Commerce shall designate the third arbitrator. The language of the arbitral proceedings shall be English, but all submissions and written evidence may be in French or English.
Section 10.08. Construction. (a) For purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include the masculine and feminine genders.
(b) The parties hereto agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting party shall not be applied in the construction or interpretation of this Agreement.
(c) As used in this Agreement, the words “include” and “including”, and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation”.
(d) Except as otherwise indicated, all references in this Agreement to “Sections” and “Exhibits” are intended to refer to Sections of this Agreement and Exhibits to this Agreement.
Section 10.09. Waiver of Jury Trial. Each of the parties hereto hereby waives to the fullest extent permitted by applicable law any right it may have to a trial by jury with respect to any litigation directly or indirectly arising out of, under or in connection with this Agreement or the Transactions. Each of the parties hereto (a) certifies that no representative, agent or attorney of any other party has represented, expressly or otherwise, that such other party would not, in the event of litigation, seek to enforce that foregoing waiver and (b) acknowledges that it and the other parties hereto have been induced to enter into this Agreement and the Transactions, as applicable, by, among other things, the mutual waivers and certifications in this Section 10.09.
Section 10.10. Headings. The descriptive headings contained in this Agreement are included for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement.
Section 10.11. Counterparts. This Agreement may be executed and delivered (including by facsimile transmission) in one or more counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
Section 10.12. Language. The parties hereto confirm that it is their wish that this Agreement, as well as all other documents related hereto, including legal notices, have been and shall be drawn up in the English language only and that such documents will be construed only in the English language.Les parties confirment leur désir que cet accord ainsi que tous les documents, y compris toutes les notifications qui s’y rattachent, soient rédigés en langue anglaise.
Section 10.13. Waiver of Conflicts. (a) Each party to this Agreement acknowledges that Cooley Godward LLP (“Cooley Godward”), outside general counsel to the Issuer, has in the past performed and is or may now or in the future represent one or more Principal Company Shareholders or their affiliates in matters
63
unrelated to the Transactions. The applicable rules of professional conduct require that Cooley Godward inform the parties hereunder of this representation and obtain their consent. Cooley Godward has served as outside general counsel to the Issuer and has negotiated the terms of the Transactions solely on behalf of the Issuer. The Issuer and each Principal Company Shareholder hereby (i) acknowledge that they have had an opportunity to ask for and have obtained information relevant to such representation, including disclosure of the reasonably foreseeable adverse consequences of such representation; (ii) acknowledge that with respect to the Transactions, Cooley Godward has represented solely the Issuer, and not any Principal Company Shareholder or Other Shareholder or any stockholder, director or employee of the Company or any Principal Company Shareholder or Other Shareholder; and (iii) gives its informed consent to Cooley Godward’s representation of the Issuer in the Transactions.
(b) Each party to this Agreement acknowledges that Shearman & Sterling LLP (“Shearman & Sterling”), outside general counsel to the Company, has in the past performed and is or may now or in the future represent one or more Principal Company Shareholders or their affiliates in matters unrelated to the Transactions. The applicable rules of professional conduct require that Shearman & Sterling inform the parties hereunder of this representation and obtain their consent. Shearman & Sterling has served as outside general counsel to the Company and has negotiated the terms of the Transactions solely on behalf of the Company. The Issuer and each Principal Company Shareholder hereby (i) acknowledge that they have had an opportunity to ask for and have obtained information relevant to such representation, including disclosure of the reasonably foreseeable adverse consequences of such representation; (ii) acknowledge that with respect to the Transactions, Shearman & Sterling has represented solely the Company, and not the Issuer, any Principal Company Shareholder, any Other Shareholder or any stockholder, director or employee of the Company, the Issuer, any Principal Company Shareholder or any Other Shareholder; and (iii) gives its informed consent to Shearman & Sterling’s representation of the Company in the Transactions.
Section 10.14. Effectiveness of Agreement. This Agreement shall become effective only upon the execution hereof by Principal Company Shareholders who own, of record and beneficially, in the aggregate, the number of Company Shares representing at least 85% of the shares of the capital stock and voting rights of the Company as calculated in accordance with Section 6.1 of the Company Shareholders Agreement as of the Effective Date.
64
IN WITNESS WHEREOF, the parties hereto have executed, or have caused to be executed by their respective officers thereunto duly authorized, this Agreement as of the date first written above.
| | |
| | Title: | President and Chief Executive Officer |
| |
| | PRINCIPAL COMPANY SHAREHOLDERS: |
| |
| | AA INNOVATION 2002 |
| | By: XAnge Private Equity |
| | |
| | Title: | Chief Executive Officer |
| |
| | ALTA BIOPHARMA PARTNERS, L.P. |
| | By: Alta BioPharma Management, LLC |
| |
| | ALTA EMBARCADERO BIOPHARMA |
| | PARTNERS, LLC |
| |
| | |
| | Name: Hilary Strain, Attorney-in-Fact |
65
| |
| | ALTAMIR & CO. |
| | By: Apax Partners |
| |
| | APAX FRANCE IV |
| | By: Apax Partners |
| |
| | APAX PARTNERS CLUB |
| | By: Apax Partners |
| |
| | ATLAS VENTURE ENTREPRENEURS’ FUND III, L.P. |
| | By: Atlas Venture Associates III, L.P. |
| | |
| | By: | /s/ Jeanne Larkin Henry |
| |
| | |
| | Name: Jeanne Larkin Henry |
66
| |
| | ATLAS VENTURE FUND III, L.P. |
| | By: Atlas Venture Associates III, L.P. |
| | |
| | By: | /s/ Jeanne Larkin Henry |
| |
| | |
| | Name: Jeanne Larkin Henry |
| |
| | BIOTECH TURNAROUND FUND B.V. |
| | |
| | Title: | Chief Executive Officer |
| |
| | BNP PARIBAS LONDON BRANCH |
| | |
| | By: | /s/ Pierre-Henri Francois |
| |
| | |
| | Name: Pierre-Henri Francois, Authorized Signatory |
| |
| | |
| | Name: Anthony Don, Authorized Signatory |
67
| | |
| | By: | /s/ Christian Cuenoud |
| |
| | CLAL BIOTECHNOLOGY INDUSTRIES |
| | |
| | Title: | Chief Technology Officer |
| | |
| | By: | /s/ Jean-Marie Paluel-Marmon |
| |
| | |
| | Name: Jean-Marie Paluel-Marmon |
| | |
| | Title: | President — Director General |
68
| |
| | CRÉDIT LYONNAIS FONDS SECONDAIRE 1 |
| | By: Crédit Agricole Private Equity |
| |
| | CROISSANCE DISCOVERY FCPR |
| | By: Edmund de Rothschild Investment Partners |
| | |
| | By: | /s/ Raphael Wisniewski |
| |
| | FRANCE INNOVATION 4 |
| | By: XAnge Private Equity |
| | |
| | Title: | Chief Executive Officer |
69
| |
| | HERVÉ DUCHESNE DE LAMOTTE |
| | |
| | By: | /s/ Hervé Duchesne de Lamotte |
| |
| | |
| | Name: Hervé Duchesne de Lamotte |
| |
| | IDM CHASE PARTNERS (ALTA BIO), LLC |
| | By: Alta/ Chase BioPharma Management, LLC |
| |
| | IMH HANNOVER VENTURE CAPITAL GMBH & CO KG |
| | |
| | By: | /s/ Clemens von Berger |
| |
| | INVESTISSEMENT INNOVATION 2002 |
| | By: XAnge Private Equity |
| | |
| | Title: | Chief Executive Officer |
| | |
| | By: | /s/ Jean-Loup Romet-Lemonne |
| |
| | |
| | Name: Jean-Loup Romet-Lemonne |
70
| | |
| | By: | /s/ Christian S. Schade |
| |
| | |
| | Name: Christian S. Schade |
| | |
| | Title: | Chief Financial Officer |
| |
| | MERCURE DISCOVERY II FCPR |
| | By: Edmund de Rothschild Investment Partners |
| | |
| | By: | /s/ Raphael Wisniewski |
| |
| | PRIVATE EQUITY CO-FINANCE |
71
| | |
| | By: | /s/ Jean-Claude Leroy |
| | |
| | Title: | Chief Financial Officer |
| |
| | SOFINNOVA CAPITAL II FCPR |
72
AMENDMENT No. 1 (this “Amendment”), dated as of March 15, 2005, to the Share Exchange Agreement (the “Agreement”), dated as of March 15, 2005 by and among EPIMMUNE INC., a Delaware corporation (the “Issuer”), and the shareholders of IDM S.A., asociété anonymeorganized under the laws of France (the “Company”), listed onExhibit A attached thereto (the “Principal Company Shareholders”). Capitalized terms used but not defined herein shall have the meanings assigned to such terms in the Agreement.
RECITALS:
A. The Issuer and the Principal Company Shareholders have entered into the Agreement;
B. The Principal Company Shareholders have appointed Hélène Ploix, as Shareholder Representative, and have empowered her to execute amendments to the Agreement which may be necessary or desirable to complete the Agreement; and
C. The Issuer and the Shareholder Representative, on behalf of the Principal Company Shareholders, have agreed to enter into this Amendment No. 1 to the Agreement.
NOW, THEREFORE, in consideration of the foregoing and the rights and obligations contained herein, and intending to be legally bound hereby, the Issuer and the Shareholder Representative hereby agree as follows:
Section 1. Conditions to the Obligations of Each Party. The following shall be added to Section 7.01 of the Agreement:
| |
| | (i) Warrant Amendments. The Warrant Amendments set out in Section 6.16 have in fact been validly approved by the Company Shareholders’ Meeting. |
Section 2. Shareholder Representative. The following shall be added to Section 9.04 of the Agreement:
| |
| | (f) In the event of the death or permanent disability of the Shareholder Representative, or his resignation as the Shareholder Representative, a successor Shareholder Representative shall be elected by a vote of the Principal Company Shareholders holding at least a majority of the Expense Escrow Shares (or, if no Expense Escrow Shares remain, a majority of the Indemnity Escrow Shares). The Principal Company Shareholders shall cause to be delivered to the Issuer prompt written notice of such election of a successor Shareholder Representative. After the Shareholder Representative’s death, disability or resignation (as the case may be) and pending the election of a successor Shareholder Representative, the Principal Company Shareholder holding the largest number of outstanding shares of the Company immediately prior to the Closing Date shall act as the interim Shareholder Representative. Each interim and successor Shareholder Representative shall have all the power, authority, rights, obligations, duties and privileges conferred by this Agreement upon the original Shareholder Representative and the term “Shareholder Representative” as used herein will be deemed to include an interim or successor Shareholder Representative. |
Section 3. Form of Indemnity Escrow Agreement. The following shall be added as the penultimate paragraph of Section 1.07(a) of the form of Indemnity Escrow Agreement:
| |
| | Promptly upon receipt of any Claim Certificate or any amendment or supplement thereto, the Shareholder Representative shall notify each of the Principal Company Shareholders of the submission of such Claim Certificate and will as soon as reasonably practicable distribute to each Principal Company Shareholder a copy of such Claim Certificate. |
Section 4. Entire Agreement. The Agreement, as amended by this Amendment constitutes the entire agreement of the parties with respect to the subject matter hereof and supersedes all prior agreements and undertakings, both written and oral, between the parties and the Principal Company Shareholders with respect to the subject matter thereof and hereof. Except as amended by this Amendment, the Agreement shall continue in full force and effect in accordance with its terms.
Section 5. Severability. If any term or other provision of this Amendment is invalid, illegal or incapable of being enforced by any rule of Law, or public policy, all other conditions and provisions of this Amendment shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions contemplated by this Amendment is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Amendment so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated by the Agreement as amended by this Amendment to the fullest extent possible.
Section 6. Counterparts. This Amendment may be executed and delivered (including by facsimile transmission) in one or more counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
Section 7. Governing Law. (a) This Amendment shall be governed by, and construed in accordance with, the laws of the State of Delaware applicable to contracts executed in and to be performed in that jurisdiction, excluding (to the greatest extent a Delaware court would permit) any rule of law that would cause the application of the laws of any jurisdiction other than the State of Delaware.
(b) The parties irrevocably agree that any dispute, controversy or claim arising out of or relating to this Amendment or the transactions contemplated thereby, or the breach, termination or invalidity thereof, shall be settled by arbitration in accordance with the Rules of Conciliation and Arbitration of the International Chamber of Commerce as at present in force. The place of arbitration shall be Orange County, California and the number of arbitrators shall be three. Each of (i) the Shareholder Representative, on behalf of the Principal Company Shareholders, on the one hand, and (ii) the Issuer, on the other hand, shall designate one arbitrator and the two so designated arbitrators shall jointly designate the third arbitrator. If such designation is not made within fifteen (15) days of the designation of the second party designated arbitrator, the Secretary General of the International Court of Arbitration of the International Chamber of Commerce shall designate the third arbitrator. The language of the arbitral proceedings shall be English, but all submissions and written evidence may be in French or English.
2
IN WITNESS WHEREOF, the Issuer and the Principal Company Shareholders, represented by the Shareholder Representative have executed, or have caused to be executed by their respective officers thereunto duly authorized, this Amendment as of the date first written above.
| | |
| | Title: | President, Chief Executive Officer and Director |
| |
| | PRINCIPAL COMPANY SHAREHOLDERS, |
| | Represented by the Shareholder Representative |
3
AMENDMENT No. 2 (this “Amendment”), dated as of April 21, 2005, to the Share Exchange Agreement dated as of March 15, 2005 and as amended by Amendment No. 1 dated as of March 15, 2005 (the “Agreement”), by and among EPIMMUNE INC., a Delaware corporation (the “Issuer”), and the shareholders of IDM S.A., asociété anonymeorganized under the laws of France (the “Company”), listed onExhibit A attached thereto (the “Principal Company Shareholders”). Capitalized terms used but not defined herein shall have the meanings assigned to such terms in the Agreement.
RECITALS:
A. The Issuer and the Principal Company Shareholders have entered into the Agreement;
B. The Principal Company Shareholders have appointed Hélène Ploix, as Shareholder Representative, and have empowered her to execute amendments to the Agreement which may be necessary or desirable to complete the Agreement; and
C. The Issuer and the Shareholder Representative, on behalf of the Principal Company Shareholders, have agreed to enter into this Amendment No. 2 to the Agreement.
NOW, THEREFORE, in consideration of the foregoing and the rights and obligations contained herein, and intending to be legally bound hereby, the Issuer and the Shareholder Representative hereby agree as follows:
Section 1. Issuer Reverse Stock Split. Section 6.11(d) of the Agreement shall be deleted and replaced in its entirety by the following paragraph:
| |
| | (d) Subject to the receipt of the requisite stockholder approval at the Issuer Stockholders’ Meeting, the Issuer shall take all action as may be necessary immediately prior to the Closing to (i) effect a reverse stock split of the Issuer Common Stock pursuant to which four, five or six shares of Issuer Common Stock will be consolidated into one share of Issuer Common Stock (it being understood that the final determination of the number of shares of Issuer Common Stock to be consolidated under this Section 6.11(d) shall be made by the Issuer’s board of directors in consultation with the Company, following the approval by the requisite affirmative vote of the stockholders of the Issuer of each proposed ratio for such reverse stock split) and (ii) cause the authorized capital stock of the Issuer after giving effect to such reverse stock split prescribed in clause (i) above to be increased to a total of 65,000,000 shares, consisting of 55,000,000 shares of Issuer Common Stock and 10,000,000 shares of Issuer Preferred Stock. |
Section 2. Compensation Matters. Section 6.14 of the Agreement shall be deleted and replaced in its entirety by the following:
Section 6.14 Compensation Matters. (a) The Issuer shall take all actions necessary, including, without limitation, seeking stockholder approval for such actions at the Issuer Stockholders’ Meeting, (i) to increase the number of shares of Issuer Common Stock reserved for issuance under the Issuer’s 2000 Stock Plan (including the Issuer French Stock Option Plan) by 8,800,000 shares of Issuer Common Stock, (ii) to increase the number of shares of Issuer Common Stock reserved for issuance under the Issuer’s 2001 Employee Stock Purchase Plan, (iii) to adopt the Issuer French Stock Option Plan, and (iv) to adopt the Issuer French Employee Stock Purchase Plan (the “Issuer French ESPP”) if mutually agreed by the Company and the Issuer and reserve for issuance thereunder 215,000 shares of Issuer Common Stock; provided that the Issuer shall not withhold its agreement if the Company has provided the Issuer a draft of the Issuer French ESPP at least five business days in advance of the filing of the first amendment to the Proxy Statement, if any, to allow for inclusion into the first amendment to the Proxy Statement, if any. The Issuer French ESPP shall provide for similar rights and benefits as provided under the Issuer’s 2001 Employee Stock Purchase Plan, enabling participants to purchase shares of Issuer Common Stock, and shall be in such form and substance as may be reasonably necessary or desirable to receive favorable treatment under French law, and as reasonably satisfactory to the Company and the Issuer.
(b) As soon as practicable after the receipt of the requisite approval of the stockholders of the Company of the Issuer Stock Option Plan Amendment and, if applicable, of the adoption of the Issuer French ESPP, the Issuer shall cause the additional shares of Issuer Common Stock reserved for issuance under the Issuer’s 2000 Stock Plan and, if applicable, the shares of Issuer Common Stock reserved for issuance under the Issuer French ESPP to be registered under an effective registration on Form S-8 (or any successor form) or another appropriate form, and the Issuer shall use its reasonable best efforts to maintain the effectiveness of such registration statement or registration statements for as long as shares of Issuer Common Stock remain outstanding under such plans. In addition, the Issuer shall use its reasonable best efforts to cause the shares of Issuer Common Stock that are issuable under such plans to be listed on Nasdaq.
Section 3. Entire Agreement. The Agreement, as amended by this Amendment constitutes the entire agreement of the parties with respect to the subject matter hereof and supersedes all prior agreements and undertakings, both written and oral, between the parties and the Principal Company Shareholders with respect to the subject matter thereof and hereof. Except as amended by this Amendment, the Agreement shall continue in full force and effect in accordance with its terms.
Section 4. Severability. If any term or other provision of this Amendment is invalid, illegal or incapable of being enforced by any rule of Law, or public policy, all other conditions and provisions of this Amendment shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions contemplated by this Amendment is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Amendment so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated by the Agreement as amended by this Amendment to the fullest extent possible.
Section 5. Counterparts. This Amendment may be executed and delivered (including by facsimile transmission) in one or more counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
Section 6. Governing Law. (a) This Amendment shall be governed by, and construed in accordance with, the laws of the State of Delaware applicable to contracts executed in and to be performed in that jurisdiction, excluding (to the greatest extent a Delaware court would permit) any rule of law that would cause the application of the laws of any jurisdiction other than the State of Delaware.
(b) The parties irrevocably agree that any dispute, controversy or claim arising out of or relating to this Amendment or the transactions contemplated thereby, or the breach, termination or invalidity thereof, shall be settled by arbitration in accordance with the Rules of Conciliation and Arbitration of the International Chamber of Commerce as at present in force. The place of arbitration shall be Orange County, California and the number of arbitrators shall be three. Each of (i) the Shareholder Representative, on behalf of the Principal Company Shareholders, on the one hand, and (ii) the Issuer, on the other hand, shall designate one arbitrator and the two so designated arbitrators shall jointly designate the third arbitrator. If such designation is not made within fifteen (15) days of the designation of the second party designated arbitrator, the Secretary General of the International Court of Arbitration of the International Chamber of Commerce shall designate the third arbitrator. The language of the arbitral proceedings shall be English, but all submissions and written evidence may be in French or English.
2
IN WITNESS WHEREOF, the Issuer and the Principal Company Shareholders, represented by the Shareholder Representative have executed, or have caused to be executed by their respective officers thereunto duly authorized, this Amendment as of the date first written above.
| | |
| | Title: | President, Chief Executive |
| |
| | Officer and Director |
| |
| | PRINCIPAL COMPANY SHAREHOLDERS, |
| | Represented by the Shareholder Representative |
3
AMENDMENT No. 3 (this “Amendment”), dated as of May 31, 2005, to the Share Exchange Agreement dated as of March 15, 2005 and as amended by Amendment No. 1 dated as of March 15, 2005 and Amendment No. 2 dated as of April 21, 2005 (the “Agreement”), by and among EPIMMUNE INC., a Delaware corporation (the “Issuer”), and the shareholders of IDM S.A., asociété anonymeorganized under the laws of France (the “Company”), listed onExhibit A attached thereto (the “Principal Company Shareholders”). Capitalized terms used but not defined herein shall have the meanings assigned to such terms in the Agreement.
RECITALS:
A. The Issuer and the Principal Company Shareholders have entered into the Agreement;
B. The Principal Company Shareholders have appointed Hélène Ploix, as Shareholder Representative, and have empowered her to execute amendments to the Agreement which may be necessary or desirable to complete the Agreement; and
C. The Issuer and the Shareholder Representative, on behalf of the Principal Company Shareholders, have agreed to enter into this Amendment No. 3 to the Agreement.
NOW, THEREFORE, in consideration of the foregoing and the rights and obligations contained herein, and intending to be legally bound hereby, the Issuer and the Shareholder Representative hereby agree as follows:
Section 1. Issuer Reverse Stock Split. Section 6.11(d) of the Agreement shall be deleted and replaced in its entirety by the following paragraph:
| |
| | (d) Subject to the receipt of the requisite stockholder approval at the Issuer Stockholders’ Meeting, the Issuer shall take all action as may be necessary immediately prior to the Closing to (i) effect a reverse stock split of the Issuer Common Stock pursuant to which four, five, six, seven, eight, nine or ten shares of Issuer Common Stock will be consolidated into one share of Issuer Common Stock (it being understood that the final determination of the number of shares of Issuer Common Stock to be consolidated under this Section 6.11(d) shall be made by the Issuer’s board of directors in consultation with the Company, following the approval by the requisite affirmative vote of the stockholders of the Issuer of each proposed ratio for such reverse stock split) and (ii) cause the authorized capital stock of the Issuer after giving effect to such reverse stock split prescribed in clause (i) above to be increased to a total of 65,000,000 shares, consisting of 55,000,000 shares of Issuer Common Stock and 10,000,000 shares of Issuer Preferred Stock. |
Section 2. Entire Agreement. The Agreement, as amended by this Amendment constitutes the entire agreement of the parties with respect to the subject matter hereof and supersedes all prior agreements and undertakings, both written and oral, between the parties and the Principal Company Shareholders with respect to the subject matter thereof and hereof. Except as amended by this Amendment, the Agreement shall continue in full force and effect in accordance with its terms.
Section 3. Severability. If any term or other provision of this Amendment is invalid, illegal or incapable of being enforced by any rule of Law, or public policy, all other conditions and provisions of this Amendment shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions contemplated by this Amendment is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Amendment so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated by the Agreement as amended by this Amendment to the fullest extent possible.
Section 4. Counterparts. This Amendment may be executed and delivered (including by facsimile transmission) in one or more counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
Section 5. Governing Law. (a) This Amendment shall be governed by, and construed in accordance with, the laws of the State of Delaware applicable to contracts executed in and to be performed in that jurisdiction, excluding (to the greatest extent a Delaware court would permit) any rule of law that would cause the application of the laws of any jurisdiction other than the State of Delaware.
(b) The parties irrevocably agree that any dispute, controversy or claim arising out of or relating to this Amendment or the transactions contemplated thereby, or the breach, termination or invalidity thereof, shall be settled by arbitration in accordance with the Rules of Conciliation and Arbitration of the International Chamber of Commerce as at present in force. The place of arbitration shall be Orange County, California and the number of arbitrators shall be three. Each of (i) the Shareholder Representative, on behalf of the Principal Company Shareholders, on the one hand, and (ii) the Issuer, on the other hand, shall designate one arbitrator and the two so designated arbitrators shall jointly designate the third arbitrator. If such designation is not made within fifteen (15) days of the designation of the second party designated arbitrator, the Secretary General of the International Court of Arbitration of the International Chamber of Commerce shall designate the third arbitrator. The language of the arbitral proceedings shall be English, but all submissions and written evidence may be in French or English.
2
IN WITNESS WHEREOF, the Issuer and the Principal Company Shareholders, represented by the Shareholder Representative have executed, or have caused to be executed by their respective officers thereunto duly authorized, this Amendment as of the date first written above.
| | |
| | Title: | Vice President, Finance and
Administration, Chief Financial Officer
and Secretary |
| |
| | PRINCIPAL COMPANY SHAREHOLDERS, |
| | Represented by the Shareholder Representative |
| | |
| | Title: | Shareholder Representative |
3
AMENDMENT No. 4 (this “Amendment”), dated as of June 30, 2005, to the Share Exchange Agreement dated as of March 15, 2005 and as amended by Amendment No. 1 dated as of March 15, 2005, Amendment No. 2 dated as of April 21, 2005 and Amendment No. 3 dated as of May 31, 2005 (the “Agreement”), by and among EPIMMUNE INC., a Delaware corporation (the “Issuer”), and the shareholders of IDM S.A., asociété anonymeorganized under the laws of France (the “Company”), listed on Exhibit A attached thereto (the “Principal Company Shareholders”). Capitalized terms used but not defined herein shall have the meanings assigned to such terms in the Agreement.
RECITALS:
A. The Issuer and the Principal Company Shareholders have entered into the Agreement;
B. The Principal Company Shareholders have appointed Hélène Ploix, as Shareholder Representative, and have empowered her to execute amendments to the Agreement which may be necessary or desirable to complete the Agreement; and
C. The Issuer and the Shareholder Representative, on behalf of the Principal Company Shareholders, have agreed to enter into this Amendment No. 4 to the Agreement.
NOW, THEREFORE, in consideration of the foregoing and the rights and obligations contained herein, and intending to be legally bound hereby, the Issuer and the Shareholder Representative hereby agree as follows:
Section 1. Termination. Section 8.01(b) of the Agreement shall be deleted and replaced in its entirety by the following paragraph:
| |
| | (b) by either the Issuer or the Shareholder Representative on behalf of the Principal Company Shareholders if the Closing shall not have occurred on or before June 30, 2005 (the “End Date”); provided, however, that the End Date shall be August 26, 2005 in the event that the Issuer or its counsel shall receive a comment letter on the Proxy Statement from the SEC; and provided further that the right to terminate this Agreement under this Section 8.01(b) shall not be available to any party whose failure to fulfill any obligation under this Agreement has been the cause of, or resulted in, the failure of the Closing to occur on or before such date; |
Section 2. Entire Agreement. The Agreement, as amended by this Amendment constitutes the entire agreement of the parties with respect to the subject matter hereof and supersedes all prior agreements and undertakings, both written and oral, between the parties and the Principal Company Shareholders with respect to the subject matter thereof and hereof. Except as amended by this Amendment, the Agreement shall continue in full force and effect in accordance with its terms.
Section 3. Severability. If any term or other provision of this Amendment is invalid, illegal or incapable of being enforced by any rule of Law, or public policy, all other conditions and provisions of this Amendment shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions contemplated by this Amendment is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Amendment so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated by the Agreement as amended by this Amendment to the fullest extent possible.
Section 4. Counterparts. This Amendment may be executed and delivered (including by facsimile transmission) in one or more counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
Section 5. Governing Law. (a) This Amendment shall be governed by, and construed in accordance with, the laws of the State of Delaware applicable to contracts executed in and to be performed in that
jurisdiction, excluding (to the greatest extent a Delaware court would permit) any rule of law that would cause the application of the laws of any jurisdiction other than the State of Delaware.
(b) The parties irrevocably agree that any dispute, controversy or claim arising out of or relating to this Amendment or the transactions contemplated thereby, or the breach, termination or invalidity thereof, shall be settled by arbitration in accordance with the Rules of Conciliation and Arbitration of the International Chamber of Commerce as at present in force. The place of arbitration shall be Orange County, California and the number of arbitrators shall be three. Each of (i) the Shareholder Representative, on behalf of the Principal Company Shareholders, on the one hand, and (ii) the Issuer, on the other hand, shall designate one arbitrator and the two so designated arbitrators shall jointly designate the third arbitrator. If such designation is not made within fifteen (15) days of the designation of the second party designated arbitrator, the Secretary General of the International Court of Arbitration of the International Chamber of Commerce shall designate the third arbitrator. The language of the arbitral proceedings shall be English, but all submissions and written evidence may be in French or English.
IN WITNESS WHEREOF, the Issuer and the Principal Company Shareholders, represented by the Shareholder Representative have executed, or have caused to be executed by their respective officers thereunto duly authorized, this Amendment as of the date first written above.
| | |
| | Title: | Vice President, Finance and
Administration, Chief Financial Officer
and Secretary |
| |
|
| | PRINCIPAL COMPANY SHAREHOLDERS, |
|
|
| | Represented by the Shareholder Representative |
|
| | |
| | Title: | Shareholder Representative |
2
ANNEX B
| | | |
| | | Jefferies & Company, Inc.
Investment Banking
520 Madison Avenue
10thFloor
New York, NY 1002
tel 212-284-2550
fax 212-284-1716 |
March 15, 2005
The Board of Directors
Epimmune Inc.
5820 Nancy Ridge Drive
San Diego, CA 92121
Members of the Board of Directors:
We understand that Epimmune Inc. (the “Company”) and certain shareholders of Immuno-Designed Molecules. (“IDM”) propose to enter into a Share Exchange Agreement circulated on March 14, 2005, as amended by the accompanying amendment also circulated on such date (the “Agreement”) and related agreements pursuant to which the Company will acquire (the “Transaction”) each issued and outstanding class A ordinary share, nominal value€0.10 per share, of IDM and each issued and outstanding class B ordinary share, nominal value€0.10 per share, of IDM in exchange for newly issued shares of common stock, par value $0.01 per share, of the Company at an exchange rate of 3.771865 shares of Company common stock per share of IDM class A ordinary shares and IDM class B ordinary shares upon the terms and subject to the conditions of the Agreement and the related agreements. Notwithstanding certain terms of the Agreement to the contrary, for purposes of this opinion, we are assuming that (i) the aggregate number of shares of the Company being issued in connection with the Transaction is equal to 79,226,275 (the “Consideration”), (ii) upon the closing of the Transaction, the IDM shareholders (including option holders and warrant holders) will own 77.8% of the outstanding shares of Company, on a fully diluted basis, (iii) upon the closing of the Transaction, the Company will own 100% of the issued and outstanding stock of IDM (including without limitation, any shares held in aplan d’épargne en actionsthat are subject to a Put/ Call Agreement (as defined in the Agreement) and any shares that are held by IDM shareholders who do not become Principal Company Shareholders (as defined in the Agreement)) and will acquire such shares simultaneously and (iv) no warrants or options or similar agreements to purchase shares of IDM stock remain outstanding or allow for the exercise into or purchase of shares of the Company after the closing of the Transaction, including without limitation, those held in aplan d’épargne en actionsafter the closing of the Transaction and those that remain outstanding under French option plans and are subject to Option Liquidity Agreements, and that all such options and warrants were exchanged for Consideration as if exercised on the date of the closing of the Transaction. We understand that prior to the closing of the Transaction, all shares of the Company’s Series S preferred stock and Series S-1 preferred stock held by G.D. Searle LLC will be exchanged for shares of common stock of the Company pursuant to that certain Preferred Exchange Agreement dated as of March 14, 2005 between the Company and G.D. Searle LLC (the “Preferred Share Exchange”). It is agreed that the Preferred Share Exchange shall not be considered part of the Transaction and we express no opinion with respect thereto.
You have requested our opinion as to whether the Consideration is fair, from a financial point of view, to the Company.
Jefferies & Company, Inc. (“Jefferies”), as part of our investment banking business, is regularly engaged in the evaluation of capital structures, valuation of businesses and their securities in connection with mergers and acquisitions, negotiated underwritings, competitive biddings, secondary distributions of listed and unlisted
securities, private placements, financial restructuring and other financial services. The Company retained us pursuant to an engagement agreement dated November 30, 2004 (the “Engagement Letter”) to act as the financial advisor to the Company to assist the Board of Directors (the “Board”) in evaluating an acquisition of IDM. We will receive fees from the Company in connection with the advisory services we have provided pursuant to the Engagement Letter, including fees that are contingent upon the completion of the Transaction. We will receive a separate fee from the Company for rendering this opinion that is not contingent upon the completion of the Transaction, but that is credited against the fees to be received upon completion of the Transaction. In addition, the Company has agreed to reimburse our expenses and to indemnify us for certain liabilities arising out of our engagement.
We have in the past provided investment banking services to and received compensation from the Company on matters unrelated to the Transaction. In the ordinary course of our business, we and our affiliates may publish research reports on the securities of the Company, IDM or their respective affiliates, may trade or hold such securities for our own accounts and for the accounts of our customers and, accordingly, may at any time hold long or short positions in those securities.
In connection with this opinion, we have, among other things:
| |
| | (i) Reviewed a draft of the Share Exchange Agreement circulated on March 14, 2005, as amended by the accompanying amendment also circulated on such date, which, for the purposes of this opinion we have assumed, with your permission, to be identical in all material respects to the agreement to be executed; |
| |
| | (ii) Reviewed the Company’s Annual Reports on Form 10-K and related publicly-available financial information for the three fiscal years ended December 31, 2001, 2002, 2003 and the Company’s Form 10-Q and the related unaudited financial information for the nine months ended September 30, 2004; |
| |
| | (iii) Reviewed certain financial projections prepared by the Company and IDM management; |
| |
| | (iv) Conducted discussions with members of senior management of the Company and IDM concerning their respective operations, financial conditions and prospects; |
| |
| | (v) Reviewed the historical market prices and trading activity for the shares of the common stock of the Company and compared them with those of certain publicly-traded companies which Jefferies deemed to be reasonably similar to the Company and IDM; |
| |
| | (vi) Analyzed the industry, the Company’s key competitors and trends in the industry in which the Company and IDM operate; |
| |
| | (vii) Analyzed financial information of key competitors, similar publicly-traded companies and/or similar precedent transactions to determine appropriate valuation multiples or enterprise values; |
| |
| | (viii) Analyzed the performance and market position of IDM relative to its key competitors and/or similar traded companies; |
| |
| | (ix) Performed a relative revenue contribution analysis with respect to the Transaction; |
| |
| | (x) Compared the proposed financial terms to the Company of the Transaction with the financial terms of certain other mergers and acquisitions which it deemed to be relevant; and |
| |
| | (xi) Reviewed such other financial studies, performance such other analyses and investigations and took into account such other matters as Jefferies deemed appropriate. |
In addition to the foregoing, we performed such other studies, analyses and investigations and considered such other financial, economic and market criteria as we considered appropriate in arriving at our opinion. Our analyses must be considered as a whole. Considering any portion of such analyses or the factors considered, without considering all analyses and factors, could create a misleading or incomplete view of the process underlying the conclusions expressed herein.
2
In rendering this opinion, we have, with your permission, assumed and relied upon the accuracy and completeness of all of the financial information, forecasts and other information provided to or reviewed for us by the Company or IDM or that was publicly available to us, and we have not assumed any responsibility for independent verification of any such information. This opinion is expressly conditioned upon such information (whether written or oral) being complete, accurate and fair in all respects. Our analyses were based, among other things, on the financial projections of the Company and IDM (the “Financial Projections”). With respect to the Financial Projections, which were furnished to us, discussed with us or reviewed for us by Company management, we note that projecting future results of any company is inherently subject to uncertainty. We express no opinion as to the Financial Projections or the assumptions upon which they are based. In addition, in rendering this opinion, we have assumed that such projections and any assumptions derived therefrom have been reasonably prepared on bases reflecting the best currently available estimates and good faith judgments of the future competitive, operating and regulatory environments and related financial performances of IDM and the Company.
We have assumed that there have been no material changes in the Company’s or IDM’s respective assets, financial condition, results of operations, business or prospects since the most recent financial statements made available to us. In addition, we have not conducted a physical inspection of the properties and facilities of the Company or IDM and have not made or obtained an independent valuation or appraisal of the assets or liabilities (contingent or otherwise) of the Company or IDM, nor have we evaluated any reports of inspection, valuations or appraisals of the Company or IDM, nor do we assume any responsibility to obtain any such inspections, valuations or appraisals of the Company or IDM. In addition, in preparing our opinion, we have not taken into account any federal, state, local or other tax consequences of the Transaction to either the Company or IDM.
We have made no independent investigation of any legal or accounting matters affecting the Company or IDM, and we have assumed the correctness of all legal and accounting advice given to the Company and the Board of Directors, including, without limitation, advice as to the legal, accounting and tax consequences of the terms of, and transactions contemplated by, the Agreement to the Company. We have assumed that the Transaction will be consummated in a manner that complies in all respects with the applicable provisions of the federal securities law and all other applicable federal, state and foreign statutes, rules and regulations. We have further assumed, with your permission, (i) that in the course of obtaining the necessary regulatory, third party and IDM shareholder approvals, consents and releases for the Transaction, no modification, delay, limitation, restriction or condition will be imposed that will have a material adverse effect on the Company or IDM and that the Transaction will be consummated in accordance with applicable laws and regulations and the terms of the Agreement, without waiver, amendment or modification of any material term, condition or agreement; (ii) that there is not now, and there will not as a result of the consummation of the Transaction contemplated by the Agreement be, any default, or event of default, under any indenture, credit agreement or other material agreement or instrument to which the Company, IDM or any of their respective subsidiaries or affiliates is a party that are not excluded as a condition to closing the Transaction; (iii) that, notwithstanding anything to the contrary in the Agreement, neither the Company, IDM nor any of their respective affiliates will be required to divest any material assets or become subject to any material agreement or other restriction in connection with obtaining any governmental consent in connection with the Transaction; (iv) that all material assets and liabilities (contingent or otherwise, known or unknown) of the Company and IDM are as set forth in the respective consolidated financial statements reviewed by us regarding the Company and IDM and (v) all IDM shareholders will sign all consents and documentation necessary to consummate the Transaction.
This opinion is solely for the information of the Board of Directors of the Company in connection with its consideration of the Transaction and may not be reproduced, disseminated, quoted or referred to at any time, in any manner or for any purpose including, without limitation, in connection with obtaining any third party financing or consent necessary in connection with the consummation of the Transaction without our prior written consent, which may be withheld in our sole discretion, except that this letter may be disclosed in connection with any proxy statement or registration statement used in connection with the proposed Transaction so long as this letter is quoted in full in such proxy statement or registration statement and except
3
as may otherwise be required by law or by a court of competent jurisdiction. It is understood that any description of or reference to Jefferies or our opinion will be in a form acceptable to us and our counsel in our sole discretion. This opinion does not address the merits of the decision of the Board of Directors or the Company to enter into the Agreement as compared to any alternative business transaction that might be available to the Company, nor does it address the underlying business decision of the Board of Directors or the Company to engage in the Transaction or to approve the terms of the Agreement. Further, this opinion addresses only the fairness, from a financial point of view, to the Company of the Consideration to be paid in the Transaction as of the date hereof and does not address any other aspect of the Transaction. This opinion does not constitute a recommendation to any person as to how such person should vote on the Transaction or act on any matter related to the Transaction. We have not been engaged to prepare, and have not prepared, a valuation of the Company or IDM and our opinion should not be construed as such. This opinion is necessarily based on the economic, market, and other conditions as they exist and as evaluated on the date hereof, and we disclaim any undertaking or obligation to advise any person of any change in any fact or matter affecting this opinion after the date hereof. We express no opinion as to the price at which the Company’s common stock will trade at any future time, including upon announcement and prior to closing of the Transaction.
Based upon and subject to the foregoing, we are of the opinion that, as of the date hereof, the Consideration to be paid in the Transaction is fair, from a financial point of view, to the Company.
| |
| | Very truly yours, |
| |
| | /s/ Jefferies & Company, Inc.
JEFFERIES & COMPANY, INC. |
4
ANNEX C
FORM OF PUT/ CALL AGREEMENT
This PUT/ CALL AGREEMENT (this “Agreement”), dated as of , 2005, between EPIMMUNE INC., a Delaware corporation (the “Issuer”), and [name of shareholder] (the “PEA Shareholder”), a shareholder of IDM, S.A., asociété anonymeorganized under the laws of France (the “Company”).
WITNESSETH:
WHEREAS, the Issuer has entered into a Share Exchange Agreement, dated as of March 15, 2005, as may be amended in accordance with its terms (the “Share Exchange Agreement”; capitalized terms used and not otherwise defined herein shall have the respective meanings assigned to them in the Share Exchange Agreement), with certain shareholders of the Company (the “Principal Company Shareholders”), pursuant to which the Issuer has agreed to acquire from the Principal Company Shareholders, and the Principal Company Shareholders have agreed to sell to the Issuer, certain Company Shares in exchange for shares of Issuer Common Stock;
WHEREAS, as of the date hereof, the PEA Shareholder is the record and beneficial owner of the number of Company Shares set forth below such PEA Shareholder’s name on the signature page hereto, which Company Shares (the “PEA Shares”) are held in aplan d’épargne en actions(a “PEA”);
WHEREAS, as the date hereof, and in accordance with the terms of the Share Exchange Agreement, the PEA Shareholder has elected not to exchange its PEA Shares for shares of Issuer Common Stock in accordance with the terms of the Share Exchange and has instead elected to enter into this Agreement; and
WHEREAS, in connection with the execution and delivery of the Share Exchange Agreement and as an inducement to the parties thereto incurring the obligations set forth therein, the parties hereto have agreed to enter into this Agreement.
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants and agreements contained herein, and intending to be legally bound hereby, the parties hereby agree as follows:
ARTICLE I
GRANT OF PUT AND CALL OPTIONS
Section 1.01 Put Option. Subject to the terms and conditions of this Agreement, the Issuer hereby grants to the PEA Shareholder an irrevocable right and option (the “Put Option”) to require the Issuer to purchase from the PEA Shareholder all (and not less than all) of the PEA Shares, during the period (the “Option Exercise Period”) beginning on the date of the closing of the First Equity Financing (the “FEF Closing Date”) and ending 30 days thereafter, for an aggregate purchase price (the “Purchase Price”), equal to the product of (A) the product of the number of PEA Shares multiplied by the PEA Exchange Ratio (as defined below), multiplied by (B) the price per share of Issuer Common Stock sold in the First Equity Financing, less any underwriters’ discounts or commissions. For purposes of this Agreement, the “PEA Exchange Ratio” shall mean the Exchange Ratio as adjusted to reflect appropriately the effect of any stock split, reverse stock split, stock dividend (including any dividend or distribution of securities convertible into Issuer Common Stock or Company Shares), extraordinary cash dividends, reorganization, recapitalization, reclassification, combination, exchange of shares or other like change with respect to the Issuer Common Stock or Company Shares occurring on or after the date hereof and prior to the FEF Closing Date or, if the FEF Closing Date does not occur by the date that is two years after the Closing Date, the Exchange Closing (as such term is defined below), as the case may be (provided that in the event an adjustment to the Exchange Ratio is made pursuant to Section 1.02(c) of the Share Exchange Agreement with respect to a
given event, no additional adjustment to the PEA Exchange Ratio shall be made hereunder with respect to such event).
Section 1.02 Call Option. Subject to the terms and conditions of this Agreement, the PEA Shareholder hereby grants to the Issuer an irrevocable right and option (the “Call Option”) to require the PEA Shareholder to sell to the Issuer all (and not less than all) of the PEA Shares, at any time during the Option Exercise Period, for the Purchase Price.
Section 1.03 Exercise of Option; Option Closing. (a) As promptly as practicable (but in no event later than five business days) after the FEF Closing Date, the Issuer shall mail, or shall cause to be mailed, to the PEA Shareholder a written notice (the “Financing Notice”) stating that the FEF Closing Date has occurred and the date thereof.
(b) The PEA Shareholder or the Issuer may exercise the Put Option or the Call Option, as the case may be, by delivery to the other party of a written exercise notice (an “Exercise Notice”), within 30 days after the FEF Closing Date, stating that the PEA Shareholder or the Issuer, as the case may be, is exercising the Put Option or Call Option, as the case may be, and the date and time for the closing (the “Option Closing”) for such exercise, which date shall not be more than 10 business days after the date of the Exercise Notice.
(c) Subject to the terms and conditions of this Agreement, the Option Closing will be held at the offices of the Issuer, 5820 Nancy Ridge Drive, San Diego, California, on the date and at the time set forth in the Exercise Notice, or at such other place or at such other time or on such other date as the Issuer and the PEA Shareholder may mutually agree. At the Option Closing, (i) the PEA Shareholder shall deliver, or cause to be delivered to the Issuer, share transfer orders (ordres de mouvement) for all of the PEA Shares, completed pursuant to the terms hereof and any other documents necessary for the transfer to the Issuer of good and marketable title to the PEA Shares and hereby delivers an irrevocable power of attorney to Issuer to complete, date and sign all such documents on his behalf, and (ii) the Issuer shall deliver, or cause to be delivered, to the PEA Shareholder an amount in cash, by check or wire transfer in immediately available funds to an account specified by the PEA Shareholder in writing to the Issuer at least two business days prior to the Option Closing, equal to the Purchase Price.
Section 1.04 Exchange of PEA Shares for shares of Issuer Common Stock. (a) Notwithstanding anything to the contrary contained herein, in the event that the FEF Closing Date does not occur by the date that is two years after the Closing Date (the “Automatic Exchange Date”), the PEA Shares shall automatically be exchanged (the “Automatic Exchange”) for the number of shares of Issuer Common Stock equal to the product of (i) the number of PEA Shares multiplied by (ii) the PEA Exchange Ratio.
(b) No certificates or scrip representing fractional shares of Issuer Common Stock shall be issued pursuant to this Section 1.04 and such fractional share interests will not entitle the owner thereof to vote or to any other rights of a stockholder of Issuer. Each holder of a fractional share interest shall be paid an amount in cash (without interest and subject to the amount of any withholding taxes) equal to the product obtained by multiplying (i) such fractional share interest to which such holder (after taking into account all fractional share interests then held by such holder) would otherwise be entitled by (ii) the Average Closing Price as of the Automatic Exchange Date.
(c) As promptly as practicable after the Automatic Exchange Date, the Issuer shall mail, or shall cause to be mailed, to the PEA Shareholder a written notice (the “Exchange Notice”) stating that a First Equity Financing has not been consummated prior to the Automatic Exchange Date and the date and time for the closing (the “Exchange Closing”) for the Automatic Exchange, which date shall not be more than 10 business days after the date of the Exchange Notice.
(d) Subject to the terms and conditions of this Agreement, the Exchange Closing of the Automatic Exchange contemplated by this Agreement shall take place at the offices of the Issuer, 5820 Nancy Ridge Drive, San Diego, California, or at such other place, date, or time as the Issuer and the PEA Shareholder may mutually agree. At the Exchange Closing, (i) the PEA Shareholder shall deliver, or cause to be delivered to the Issuer, (A) share transfer orders (ordres de mouvement) for all of the PEA Shares, completed pursuant to the terms hereof and any other documents necessary for the transfer to the Issuer of good and marketable title
2
to the PEA Shares and hereby delivers an irrevocable power of attorney to Issuer to complete, date and sign all such documents on his behalf, and (B) an investor letter, substantially in the form attached hereto asExhibit A (the “Investor Letter”) and (ii) the Issuer shall deliver, or cause to be delivered, to the PEA Shareholder (A) a stock certificate in the name of the PEA Shareholder evidencing the number of shares of Issuer Common Stock equal to the whole number of shares of Issuer Common Stock (excluding any fractional interest in shares of Issuer Common Stock) issuable to the PEA Shareholder in exchange for such shareholder’s PEA Shares in accordance with Section 1.04(a) and (B) any fractional share cash payments due to the PEA Shareholder pursuant to Section 1.04(b), by check or wire transfer in immediately available funds.
(e) Restricted Securities. The shares of Issuer Common Stock to be issued pursuant to this Section 1.04 have not been, and will not be, registered under the U.S. Securities Act of 1933, as amended (the “Securities Act”), and will be issued in a transaction that is exempt from the registration requirements of the Securities Act. Such shares of Issuer Common Stock will be “restricted securities” under the U.S. federal securities laws and cannot be offered or resold except pursuant to registration under the Securities Act or an available exemption from registration. All certificates representing such shares of Issuer Common Stock shall bear, in addition to any other legends required under applicable securities laws, the following legend:
| |
| | “The shares represented by this certificate have not been registered under the U.S. Securities Act of 1933, as amended (the “Securities Act”), and may not be transferred except pursuant to registration under the Securities Act or pursuant to an available exemption from registration.” |
ARTICLE II
REPRESENTATIONS AND WARRANTIES OF THE ISSUER
The Issuer hereby represents and warrants to each PEA Shareholder as follows:
Section 2.01 Organization and Authority. (a) The Issuer is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware and has all necessary corporate power and authority to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the transactions set forth herein. The execution and delivery of this Agreement by the Issuer and the consummation by the Issuer of these transactions have been duly and validly authorized by all necessary corporate action, and no other corporate proceedings on the part of the Issuer is necessary to authorize this Agreement or to consummate such transactions. This Agreement has been duly and validly executed and delivered by the Issuer and, assuming due authorization, execution and delivery by the PEA Shareholder, constitutes a legal, valid and binding obligation of the Issuer, enforceable against the Issuer in accordance with its terms, subject to the effect of any applicable bankruptcy, insolvency (including, without limitation, all laws relating to fraudulent transfers), reorganization, moratorium or similar laws affecting creditors’ rights generally and subject to the effect of general principles of equity (regardless of whether considered in a proceeding at law or in equity).
Section 2.02 Capitalization. The shares of Issuer Common Stock to be issued in the Automatic Exchange in accordance with Section 1.04 will be (i) duly authorized, validly issued, fully paid and non-assessable and not subject to preemptive rights created by statute, the Issuer’s Certificate of Incorporation or Bylaws or any agreement to which the Issuer is a party or is bound and (ii) will, when issued and assuming the accuracy of the representations and warranties of the PEA Shareholder set forth in the Investor Letter when delivered to the Issuer, be exempt from the registration requirements under the Securities Act and Exchange Act, and registered or exempt from registration under applicable Blue Sky Laws.
3
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF THE PEA SHAREHOLDER
The PEA Shareholder represents and warrants to the Issuer as follows:
Section 3.01 Qualification. The PEA Shareholder has all legal capacity to enter into this Agreement, to carry out his or her obligations hereunder and to consummate the transactions contemplated hereby.
Section 3.02 Authority Relative to this Agreement. The PEA Shareholder has all necessary power and authority to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated hereby. This Agreement has been duly and validly executed and delivered by the PEA Shareholder and assuming due authorization, execution and delivery by the Issuer, constitutes a legal, valid and binding obligation of such PEA Shareholder, enforceable against such PEA Shareholder in accordance with its terms, subject to the effect of any applicable bankruptcy, insolvency (including, without limitation, all laws relating to fraudulent transfers), reorganization, moratorium or similar laws affecting creditors’ rights generally and subject to the effect of general principles of equity (regardless of whether considered in a proceeding at law or in equity).
Section 3.03 Title to the PEA Shares. As of the date hereof, the PEA Shareholder is the record and beneficial owner of the number of PEA Shares set forth below the PEA Shareholder’s name on the signature page hereto. The PEA Shares are all the securities of the Company owned, now and, at all times during the term hereof will be, either of record or beneficially, by such PEA Shareholder and held in a PEA. The PEA Shares are now and, at all times during the term hereof will be, owned free and clear of all Liens. The PEA Shareholder has not appointed or granted any proxy, which appointment or grant is still effective, with respect to the PEA Shares.
ARTICLE IV
COVENANTS OF THE PEA SHAREHOLDER
Section 4.01 Restrictions on Transfers of Company Shares. The PEA Shareholder hereby agrees that, during the period from the date of this Agreement through its termination, the PEA Shareholder shall not sell, transfer, tender, assign, pledge, encumber, contribute to the capital of any entity, hypothecate, give or otherwise dispose of, grant a proxy or power of attorney with respect to, deposit into any voting trust or enter into a voting arrangement or agreement, or create or permit to exist any Liens of any nature whatsoever with respect to, any of such PEA Shareholder’s PEA Shares (or agree or consent to, or offer to do, any of the foregoing), (ii) take any action that would make any representation or warranty of the PEA Shareholder herein untrue or incorrect in any material respect or have the effect of preventing or adversely affecting the PEA Shareholder from performing the PEA Shareholder’s obligations hereunder or (iii) initiate, solicit or encourage any person to take actions that could reasonably be expected to lead to the occurrence of any of the foregoing.
ARTICLE V
MISCELLANEOUS
Section 5.01 Amendment. This Agreement may be amended only by mutual agreement of the parties hereto.
Section 5.02 Waiver. At any time prior to the termination of this Agreement, the Issuer or the PEA Shareholder may (a) extend the time for the performance of any obligation or other act of the other party hereto, (b) waive any inaccuracy in the representations and warranties of the other party contained herein and (c) waive compliance with any agreement of the other party or any condition to its own obligations contained herein. Any such extension or waiver shall be valid if set forth in an instrument in writing signed by the party or parties to be bound thereby.
4
Section 5.03 Non-Survival of Representations, Warranties and Agreements. The representations, warranties and agreements in this Agreement shall terminate upon the termination of this Agreement pursuant to Section 5.03. This Agreement shall terminate upon the earlier of the Option Closing or the Exchange Closing.
Section 5.04 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given upon receipt) by delivery in person, by telecopy or email or by registered or certified mail (postage prepaid, return receipt requested) to the respective parties at the following addresses (or at such other address for a party as shall be specified in a notice given in accordance with this Section 5.04):
(a) if to the Issuer:
| |
| | Epimmune Inc. |
| | 5820 Nancy Ridge Drive |
| | San Diego, CA 92121 |
| | Facsimile: (858) 860-2600 |
| | Attention: Chief Financial Officer |
| | Email: bdevaere@Epimmune. com |
With a copy to:
| |
| | Cooley Godward LLP |
| | 4401 Eastgate Mall |
| | San Diego, CA 92121 |
| | Facsimile: (858) 550-6420 |
| | Attention: Kay Chandler |
| | Email: kchandler@cooley.com |
(b) If to the PEA Shareholder:
| |
| | |
| |
| | |
| |
| | |
| | Facsimile: |
| | Attention: |
| | Email: |
Section 5.05 Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of Law or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the transactions contemplated hereby is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in an mutually acceptable manner in order that the transactions be consummated as originally contemplated to the fullest extent possible.
Section 5.06 Entire Agreement; Assignability. This Agreement constitutes the entire agreement among the parties with respect to the subject matter hereof and supersedes all prior agreements and undertakings, both written and oral, among the parties, with respect to the subject matter hereof. This Agreement shall not be assigned (whether pursuant to a merger, by operation of Law or otherwise);provided,however, that upon the death of the PEA Shareholder, the PEA Shareholder’s rights under this Agreement shall be transferred to the person(s) who are entitled to receive the PEA Shares under the laws of descent and distribution; andprovidedfurther, that, after the Closing, the Issuer may assign this Agreement, without the consent of the other parties hereto, in connection with a consolidation or merger of the Issuer or the Company with another corporation, or the sale of all or substantially all of the assets of the Issuer or the Company to any other person.
5
Section 5.07 Parties in Interest. This Agreement shall be binding upon and inure solely to the benefit of each party hereto, and nothing in this Agreement, express or implied, is intended to or shall confer upon any other person any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
Section 5.08 Specific Performance. The parties hereto agree that irreparable damage would occur in the event any provision of this Agreement were not performed in accordance with the terms hereof and that the parties shall be entitled to specific performance of the terms hereof, in addition to any other remedy at law or equity.
Section 5.09 Governing Law; Arbitration. (a) This Agreement shall be governed by, and construed in accordance with, the laws of the State of Delaware applicable to contracts executed in and to be performed in that jurisdiction (other than those provisions set forth herein that are required to be governed by the laws of the Republic of France), excluding (to the greatest extent a Delaware court would permit) any rule of law that would cause the application of the laws of any jurisdiction other than the State of Delaware.
(b) The parties irrevocably agree that any dispute, controversy or claim arising out of or relating to this Agreement, or the breach, termination or invalidity thereof, shall be settled by arbitration in accordance with the Rules of Conciliation and Arbitration of the International Chamber of Commerce as at present in force. The place of arbitration shall be Orange County, California and the number of arbitrators shall be three. Each of (i) the PEA Shareholder, on the one hand, and (ii) the Issuer, on the other hand, shall designate one arbitrator and the two so designated arbitrators shall jointly designate the third arbitrator. If such designation is not made within fifteen (15) days of the designation of the second party designated arbitrator, the Secretary General of the International Court of Arbitration of the International Chamber of Commerce shall designate the third arbitrator. The language of the arbitral proceedings shall be English, but all submissions and written evidence may be in French or English.
Section 5.10 Headings. The descriptive headings contained in this Agreement are for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement.
Section 5.11 Counterparts. This Agreement may be executed and delivered (including by facsimile transmission) in one or more counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
Section 5.12 Language. The parties hereto confirm that it is their wish that this Agreement, as well as all other documents related hereto, including legal notices, have been and shall be drawn up in the English language only and that such documents will be construed only in the English language.Les parties confirment leur désir que cet accord ainsi que tous les documents, y compris toutes les notifications qui s’y rattachent, soient rédigés en langue anglaise.
6
IN WITNESS WHEREOF, the parties hereto have executed or have caused this Agreement to be executed by its respective officers thereunto duly authorized as of the date first above written.
| |
| | |
| | Name: |
| | Title: |
| |
| | PEA SHAREHOLDER |
| |
| | |
| | Name: |
| | Number of Company Shares Owned: |
7
ANNEX D
FORM OF OPTION LIQUIDITY AGREEMENT
BETWEEN:
1. Epimmune Inc., 5820 Nancy Ridge Drive, San Diego, CA 92121 a Delaware corporation.
| |
| | Hereafter referred to as “Epimmune”. |
2. Mr./ Ms [Beneficiary’s name, address], as holder of Beneficiary Options, and his heirs.
| |
| | Hereafter referred to as the “Beneficiary”. |
Epimmune and the Beneficiary are hereafter together referred to as the “Parties”.
PREAMBLE
A — It is recalled that:
1. Epimmune and certain shareholders (the “Principal Company Shareholders”) of IDM S.A. (“IDM”) have entered into a share exchange agreement, dated as of March 15, 2005 (the “Exchange Agreement”).
2. Pursuant to the Exchange Agreement, Epimmune has agreed to acquire the issued and outstanding class A ordinary shares, nominal value€0.10 per share, of IDM (“IDM A Shares”) and class B ordinary shares, nominal value€0.10 per share, of IDM (“IDM B Shares” and, together with the Company A Shares, the “IDM Shares”), in exchange for shares of Epimmune common stock, par value $0.01 per share (“Epimmune Shares”), upon the terms and subject to the conditions set forth in the Exchange Agreement (the “Exchange”).
3. The Beneficiary holds a certain number of options granted to him under the IDM 1998 stock option plan and/or the IDM 2000 stock option plan (together, the “Beneficiary Options”) giving him the right to receive IDM Shares (the “Beneficiary Shares”) upon exercise of his Beneficiary Options.
4. Under the terms of the applicable Beneficiary Options, the Beneficiary Shares may not be sold, assigned, donated, converted into bearer form or otherwise transferred or disposed of during a specified period (the “Lock-Up Period”). The Lock-Up Period may be decreased in accordance with applicable regulations and the applicable terms of the Beneficiary Options upon the occurrence of certain events, including death, disability, or retirement of the Beneficiary (the “Early Exit Events”).
5. Under currently applicable French tax laws, IDM Shares issued pursuant to the exercise of Beneficiary Options granted after April 27, 2000 (the “2000 Beneficiary Options”) are eligible for taxation at reduced capital gain rates if they are held for a two-year period beginning on the later to occur of (i) the date of exercise or (ii) the expiration of the Lock-Up Period (the “Retention Period”).
B — In connection with the Exchange, and subject to the terms and conditions hereof:
1. Epimmune wishes to offer the Beneficiary the possibility to enter into this option liquidity agreement (this “Agreement”) with respect to the Beneficiary Shares issued upon exercise of the Beneficiary Options.
2. Epimmune and the Beneficiary wish all Beneficiary Shares issued upon exercise of the Beneficiary Options to be transferred to Epimmune without delay upon exercise of the Beneficiary Options, but in any event, to the extent applicable, (i) no earlier than the expiration of the Lock-Up Period (as may be decreased following an Early Exit Event), and (ii) in the case of 2000 Beneficiary Options, upon election of the Beneficiary, after the end of the Retention Period.
3. The Beneficiary wishes to enter into this Agreement in order to exchange the Beneficiary Shares issued upon exercise of the Beneficiary Options for the consideration set forth in Article 2 below.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual agreements and covenants hereinafter set forth, the Parties hereby agree as follows:
ARTICLE 1 — TRANSFER UNDERTAKINGS
(a) Subject to all terms and conditions of this Agreement, Epimmune and the Beneficiary agree that, if the Beneficiary decides to exercise all or part of his Beneficiary Options after the date hereof, Epimmune shall acquire from the Beneficiary, and the Beneficiary shall transfer to Epimmune, all of the Beneficiary Shares issued upon the exercise of such Beneficiary Options, in exchange for the number of Epimmune Shares as determined in accordance with Article 2 below.
Any exchange of Beneficiary Shares for Epimmune Shares pursuant to this Article 1 shall be hereinafter referred to as a “Transfer”.
(b) The Beneficiary undertakes, warrants and represents that all Beneficiary Shares transferred to Epimmune pursuant to a Transfer shall be transferred to Epimmune with full title guarantee, free of all Encumbrances (as defined below) and with all rights attached thereto.
(c) Epimmune undertakes, warrants and represents that (i) all Epimmune Shares to be issued to the Beneficiary pursuant to this Agreement shall be duly authorized, validly issued, fully paid and non-assessable and not subject to preemptive rights created by statute, Epimmune’s Certificate of Incorporation or Bylaws or any agreement to which Epimmune is a party or by which Epimmune is bound and (ii) the issuance of the Epimmune Shares to be issued to the Beneficiary pursuant to this Agreement shall be registered on Form S-8 under the U.S. Securities Act of 1933, as amended, and registered or exempt from registration under state securities or “blue sky” laws and Epimmune Shares shall be registered under the Securities Exchange Act of 1934, as amended.
(d) Both Parties hereby agree that any Transfer will be suspended for any period during which such Transfer is prohibited by virtue of any applicable laws or regulations.
(e) “Encumbrance” shall mean any claims, charge, pledge, security, lien, option, or other third party rights, retention of title, right of pre-emption, right of first refusal or security interest of any kind.
ARTICLE 2 — CONSIDERATION
(a) The Beneficiary shall receive, as consideration for the Beneficiary Shares he transfers pursuant to any Transfer, a number of Epimmune Shares equal to (x) the number of Beneficiary Shares transferred pursuant to such Transfer, multiplied by (y) the Exchange Ratio (as defined below).
(b) The term “Exchange Ratio” as used herein initially means 3.771865. In accordance with the terms set forth in Annex A attached hereto, the Exchange Ratio shall be adjusted from time to time by Epimmune to reflect appropriately the effect of certain changes with respect to the Epimmune Shares occurring between the Closing Date (as defined in the Exchange Agreement) and the Trigger Date (as defined in Article 3 below).
The Exchange Ratio, as adjusted, shall be rounded to the sixth decimal place (with 0.0000005 being rounded upwards to 0.000001).
In the event the Exchange Ratio is so adjusted, the Exchange Ratio shall thereafter mean the Exchange Ratio as adjusted and any subsequent adjustments will be carried out on the basis of such adjusted Exchange Ratio.
(c) The number of Epimmune Shares delivered to the Beneficiary pursuant to this Article 2 shall be a whole number, which shall be arrived at by rounding down to the nearest whole number of Epimmune Shares, and any fractional share interests shall be paid in cash (without interest and subject to any applicable withholding taxes), in an amount equal to the Closing Price (as defined below) multiplied by the number equal to such fractional share interest the Beneficiary would otherwise be entitled.
2
“Closing Price” shall mean the average of the per share closing prices on the Nasdaq National Market of Epimmune Shares (or, if the Epimmune Shares are not quoted on the Nasdaq National Market, then the then current principal stock exchange on which the Epimmune Shares are listed or the principal automated securities price quotation system on which closing or sales prices of Epimmune Shares are reported) during the five consecutive trading days ending on (and including) the trading day immediately preceding the date on which the Beneficiary Shares are issued upon exercise of Beneficiary Options.
ARTICLE 3 — CLOSING OF THE TRANSFERS
(a) Each Transfer shall take place as follows:
| |
| | i. if the Beneficiary Shares are not subject to a Lock-Up Period or a Retention Period, on the date determined by Epimmune, within six Business Days (as defined below) from the date on which the Beneficiary Shares are registered in the name of the Beneficiary following their issuance; |
| |
| | ii. subject to (iii) below, if the Beneficiary Shares are subject to a Lock-Up Period on a date determined by Epimmune that is within six Business Days of the expiration of the Lock-Up Period (as may be decreased following an Early Exit Event); |
| |
| | iii. if the Beneficiary elects to benefit from a Retention Period, on a date determined by Epimmune that is within six Business Days of the expiration of the Retention Period. |
| |
| | (each such transfer date, a “Transfer Date”). |
Notwithstanding the foregoing, Epimmune shall be entitled at any time to suspend or delay Transfers for a maximum period of 90 days in the event of changes in the share capital of Epimmune. In the event of any such change, the Beneficiary shall be informed by any means chosen by Epimmune at least two Business Days prior to such suspension (the “Information Date”). Such suspension may be extended or renewed by Epimmune, to the extent necessary, but in no event for an aggregate period of 90 additional days. Such suspension shall not apply to any Beneficiary Option exercised by the Beneficiary prior to the Information Date.
“Business Day” shall mean a day on which banks are open for the transaction of normal banking business in the State of California (excluding Saturdays, Sundays and public holidays).
(b) The Beneficiary hereby promises to complete, date, sign and send to Epimmune, upon Epimmune’s request, any transfer order(“ordre de mouvement”) or any other appropriate documentation to effect the Transfer of the Beneficiary Shares on each Transfer Date pursuant to this Agreement, and hereby delivers an irrevocable power of attorney to Epimmune to complete, date and sign all such documents on his behalf. All the transactions described in this paragraph may be effected by electronic means, subject to the prior agreement of Epimmune.
ARTICLE 4 — NEGATIVE COVENANT
The Beneficiary agrees not to sell, assign, donate, transfer, or otherwise dispose of, mortgage, or pledge to any third party or encumber with any Encumbrance, nor to promise to do any of the foregoing, with respect to the Beneficiary Shares, except as provided herein.
ARTICLE 5 — COOPERATION
(a) The Parties hereby undertake to make every effort to ensure that all measures necessary or useful for the completion of the transactions provided for in this Agreement are taken in a timely manner.
(b) The Beneficiary accepts to negotiate in good faith all proposed amendment to this Agreement which would facilitate its implementation or which would be required by the given circumstances.
3
ARTICLE 6 — NOTIFICATIONS
(a) Any notification shall be sent by mail:
| |
| | i. In case of Epimmune, at the following address and addressee: |
Epimmune Inc.
5820 Nancy Ridge Drive
San Diego, CA 92121
| |
| | ii. In case of the Beneficiary, at the name and address set forth on the first page of this Agreement, or at the option of Epimmune, at the business address of the Beneficiary. |
(b) In case of change of address, each party shall notify the other party of such party’s new address, which shall be substituted for the address set forth in Section 6(a) hereof.
(c) Any notification sent by mail shall be deemed received by the Beneficiary two Business Days after it has been sent by Epimmune or on its behalf, or four Business Days after it has been sent by Epimmune or on its behalf if the address of the Beneficiary is located in a country different from the country from which the letter has been sent.
ARTICLE 7 — MISCELLANEOUS
Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any law or public policy, all other terms and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the transactions contemplated hereby is not affected in any manner materially adverse to any Party.
No third party beneficiaries. This Agreement shall be binding upon and inure solely to the benefit of the Parties hereto and their permitted assigns or heirs and nothing herein, express or implied, is intended to or shall confer upon any other person any legal or equitable right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
No Waiver. The waiver of any provision of this Agreement shall be made in writing. Failure to request the application or implementation of any provision of this Agreement shall by no means constitute a waiver to such provision.
Assignability. This Agreement and the rights and privileges conferred hereby shall not be sold, pledged or otherwise transferred (whether by operation of law or otherwise) by the Beneficiary in any manner otherwise than by will or by the laws of descent or distribution and shall not be subject to sale under execution, attachment, levy or similar process.
Singular; plural. Any reference in this Agreement to the singular includes the plural and vice versa.
Amendment. This Agreement may not be amended or modified except (x) by an instrument in writing signed by, or on behalf of, the Parties or (y) by waiver accepted in writing by either Party at the request of the other Party.
Termination. Epimmune may terminate this Agreement, without notice nor indemnity, in the event the Beneficiary is in breach of his obligations under this Agreement, ten days after such breach has been notified to the Beneficiary and not rectified.
Costs. Each Party shall bear its own costs, in particular tax and social security costs, in connection with the implementation of this Agreement and the transactions contemplated herein, and shall hold harmless the other Party in this respect.
Governing law. This Agreement shall be governed by, and construed in accordance with, the laws of the State of Delaware, without reference to its conflict of law rules.
Jurisdiction. Any dispute, controversy or claim arising out of or relating to this Agreement shall be submitted to the sole jurisdiction of the federal and state courts located in the County of Orange, State of California (United States).
4
In witness whereof, each of the Parties hereto has executed, or has caused to be executed by their duly authorized representative, this Agreement.
| |
| | Executed in two (2) originals. |
| |
| | Epimmune Inc. |
| |
| | Executed in __________, on |
| |
| | Signature: |
| | Name: |
| | Title: |
| |
| | Beneficiary |
| |
| | Executed in (place), on (date) |
| |
| | Signature: |
ANNEX E
AMENDED AND RESTATED
PREFERRED EXCHANGE AGREEMENT
This Amended and Restated Preferred Exchange Agreement(this“Agreement”) is entered into effective the 12th day of April, 2005 by and among Epimmune Inc., a Delaware corporation (the“Company”), and G.D. Searle LLC, a Delaware limited liability company(“Holder”).
Recitals
Whereas,Holder is the beneficial owner of 859,666 shares of the Company’s Series S preferred stock and 549,622 shares of the Company’s Series S-1 preferred stock (collectively, the“Preferred Shares”), which Preferred Shares represent all of the outstanding shares of preferred stock of the Company;
Whereas,the Company has entered into that certain Share Exchange Agreement, dated March 15, 2005, by and among the Company and certain shareholders of Immuno-Designed Molecules, S.A.(“IDM”) (the“Share Exchange Agreement”);
Whereas,it is a condition to the closing of the transactions under the Share Exchange Agreement (the“Closing”) that the Preferred Shares be exchanged (the“Exchange”) for shares of common stock of the Company (the“Common Shares”);
Whereas,the Company and Holder have entered into that certain Preferred Exchange Agreement, dated March 15, 2005 (the“Prior Agreement”);
Whereas,the Company and Holder desire to amend and restate the Prior Agreement in its entirety as set forth herein; and
Whereas,Holder desires to effect the Exchange on the terms and subject to the conditions set forth herein.
Agreement
Now, Therefore,in consideration of foregoing and the mutual covenants and promises contained in this Agreement, and for other valuable consideration receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
1. Terms of Exchange.
| |
| | 1.1 Exchange of Preferred Shares. Subject to the terms and conditions herein, immediately prior to the Closing, all of the Preferred Shares shall be exchanged for a total of 1,949,278 Common Shares (as adjusted to reflect the effect of any stock split, reverse stock split, stock dividend, reorganization, recapitalization, reclassification, consolidation, combination or like change with respect to the Common Shares occurring on or after the date of this Agreement and prior to the Closing). |
| |
| | 1.2 Review of Share Exchange Agreement. The Company has provided Holder with a substantially final draft of the Share Exchange Agreement, which is marked “DRAFT 2/15/05” (the“Form Share Exchange Agreement”). |
| |
| | 1.3 Waiver and Termination of Right of First Refusal. Holder hereby waives any and all rights under Section 4 of that certain Investor Rights Agreement, dated July 1, 1999, between the parties hereto (the“Investor Rights Agreement”) and agrees that Section 4 of the Investor Rights Agreement is hereby terminated and of no further force and effect. |
| |
| | 1.4 Delivery of Certificates. Within 10 days following the date hereof, Holder shall deliver to the Company the certificates representing the Preferred Shares, each duly endorsed for transfer and free and |
| |
| | clear of any liens, security interests, encumbrances or adverse claims, to be held by the Company pending the Closing. As soon as reasonably practicable, and in any event within 10 days following the Closing, the Company shall issue to Holder one or more stock certificates representing the Common Shares issuable to the Holder pursuant to Section 1.1 hereof. |
| |
| | 1.5 Conditions to Exchange. The obligations of the Company and Holder hereunder to complete the exchange of Preferred Shares for Common Shares, shall be subject to satisfaction of the following conditions: |
| |
| | (a) the delivery by Holder to the Company of stock certificates representing the Preferred Shares in accordance with Section 1.4 above; |
| |
| | (b) the common stock of the Company is listed for trading on the Nasdaq National Market (the“Nasdaq”); and |
| |
| | (c) all of the conditions (other than the condition relating to the consummation of the Exchange) to the consummation of the transactions contemplated by the Share Exchange Agreement (substantially in accordance with the terms set forth in the Form Share Exchange Agreement, as may be amended from time to time in accordance with Section 4.1 hereof) shall have been satisfied or waived and the Closing shall occur immediately following the Exchange. |
2. Representations and Warranties of the Company. The Company hereby represents and warrants to Holder as follows:
| |
| | 2.1 Corporate Power. The Company has all requisite legal and corporate power to execute and deliver this Agreement, to issue the Company Shares in exchange for the Preferred Shares and to carry out and perform its obligations under the terms of this Agreement. |
| |
| | 2.2 Authorization. The Common Shares to be issued by the Company upon exchange of the Preferred Shares, when issued in compliance with the provisions of this Agreement, will be validly issued and will be fully paid and nonassessable. |
| |
| | 2.3 Valid Obligation; No Conflicts. This Agreement has been duly executed and delivered by the Company, and constitutes a valid and legally binding obligation of the Company, enforceable in accordance with its terms. The execution and performance by the Company of this Agreement does not contravene, violate or conflict with or result in a breach of any contractual obligation of the Company or any judgment, injunction or order of any nature binding upon the Company. |
3. Representations and Warranties of the Holder. Holder hereby represents and warrants to the Company as follows:
| |
| | 3.1 Corporate Power. Holder has all requisite legal and corporate power to execute and deliver this Agreement and to deliver the Preferred Shares, free and clear of all liens, encumbrances, equities, claims, restrictions, security interests, voting trusts or other defects of title, in exchange for the Common Shares and to carry out and perform its obligations under the terms of this Agreement. |
| |
| | 3.2 Authorization; Title. All corporate action on the part of Holder necessary for the exchange of Preferred Shares for Common Shares has been taken. Holder has good and marketable title to the Preferred Shares to be exchanged pursuant to this Agreement, free and clear of all liens, encumbrances, equities, claims, restrictions, security interests, voting trusts or other defects of title. Upon delivery to the Company of the stock certificates representing the Preferred Shares, the Company shall acquire good and valid title to Preferred Shares free and clear of all liens, security interests, encumbrances and adverse claims other than those created by the Company. |
| |
| | 3.3 Valid Obligation; No Conflicts. This Agreement has been duly executed and delivered by Holder, and constitutes a valid and legally binding obligation of Holder, enforceable in accordance with its terms. The execution and performance by Holder of this Agreement does not contravene, violate or conflict with or result in a breach of any contractual obligation of Holder or any judgment, injunction or order of any nature binding upon Holder. |
2
| |
| | 3.4 Purchase Entirely for Own Account. Holder is acquiring the Common Shares pursuant to this Agreement for investment for Holder’s own account, not as a nominee or agent, and not with a view to the resale or distribution of any part thereof, and Holder has no present intention of selling, granting any participation in, or otherwise distributing the same. Holder further represents that Holder does not have any contract, undertaking, agreement or arrangement with any person to sell, transfer or grant participation to such person or to any third person, with respect to any of the Common Shares. |
| |
| | 3.5 Disclosure of Information. Holder has received all the information that it has requested and that it considers necessary or appropriate for deciding whether to enter into this Agreement and to acquire the Common Shares. Holder further represents that it has had an opportunity to ask questions and receive answers from the Company regarding such information. |
| |
| | 3.6 Investment Experience. Holder is an investor in securities of companies in the development stage and acknowledges that it is able to fend for itself, can bear the economic risk of its investment and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment in the Common Shares. Holder also represents it has not been organized solely for the purpose of acquiring the Common Shares. |
| |
| | 3.7 Accredited Investor. Holder is an “accredited investor” as such term is defined in Rule 501 of the General Rules and Regulations prescribed by the SEC pursuant to the Securities Act of 1933, as amended (the“Securities Act”). |
| |
| | 3.8 Restricted Securities. Holder understands that (a) the Common Shares have not been registered under the Securities Act by reason of a specific exemption therefrom, that such securities must be held by it indefinitely and that Holder must, therefore, bear the economic risk of such investment indefinitely, unless in each case a subsequent disposition thereof is registered under the Securities Act or is exempt from such registration; (b) each certificate representing the Common Shares will be endorsed with the legend set forth in Section 5.2 below; and (c) the Company will instruct any transfer agent not to register the transfer of the Common Shares (or any portion thereof) unless the conditions specified in the foregoing legends are satisfied, until such time as a transfer is made, pursuant to the terms of this Agreement, and in compliance with Rule 144 or pursuant to a registration statement or, if the opinion of counsel referred to above is to the further effect that such legend is not required any longer in order to establish or enforce compliance with any provisions of the Securities Act or this Agreement. |
4. Covenants.
| |
| | 4.1 Material Amendments to the Share Exchange Agreement. Prior to the Closing, Holder shall have the right to review, and the Company shall deliver to Holder a copy of, any material change to the Form Share Exchange Agreement provided under Section 1.2 made prior to execution of the Share Exchange Agreement and any material amendment to the Share Exchange Agreement made after it is signed. Holder shall have five business days after delivery of such change to Holder in accordance with this Section 4.1 to object in writing to any material change to the Form Share Exchange Agreement or amendment to the Share Exchange Agreement. In the event that Holder fails to deliver such written objection to the Company within the specified time limit, Holder shall be deemed to have accepted such change or amendment and the Form Share Exchange Agreement shall be deemed to include such change or amendment. |
| |
| | 4.2 Use of Certain Funds. The Company shall not use any cash raised in the financing completed by IDM on December 23, 2004 to make or pay any cash distribution or cash dividend to: (a) the stockholders of the Company; (b) Medarex, Inc.; (c) Sanofi-Aventis, S.A.; or (d) the shareholders, investors, officers, directors or employees of IDM; provided, however, that nothing in this Section 4.2 shall prevent the Company from paying: (x) any amounts due to Medarex, Inc. or Sanofi-Aventis, S.A. in connection with any commercial arrangements between either Medarex, Inc. or Sanofi-Aventis, S.A., on the one hand, and IDM, on the other hand, or (y) payment of salaries and bonuses to employees of the Company in the ordinary course of business. |
3
| |
| | 4.3 Registration Rights. The Company shall register the Common Shares for resale under the Resale Registration Statement (as defined in the Share Exchange Agreement), on the same terms and subject to the same conditions as set forth in Section 6.07 of the Share Exchange Agreement. For purposes of such Section 6.07 only, Holder shall be deemed a “Principal Company Shareholder” and the Common Shares shall be deemed “Issuer Common Stock” issued pursuant to the Share Exchange Agreement. The rights under this Section 4.3 shall be in lieu of any rights under Section 2 of the Investor Rights Agreement and Holder agrees that upon the date that the Resale Registration Statement becomes effective, Section 2 of the Investor Rights Agreement shall terminate and be of no further force and effect. |
| |
| | 4.4 Nasdaq Listing. The Company shall use commercially reasonable efforts to: (a) maintain trading of its shares of common stock on the Nasdaq; and (b) meet the Nasdaq minimum listing standards in effect from time to time. |
5. Miscellaneous.
| |
| | 5.1 Governing Law; Jurisdiction. This Agreement shall be governed by and construed in accordance with the laws of the State of California, without reference to conflicts of laws principles. |
| |
| | 5.2 Legends. Each certificate representing Common Shares issued under this Agreement shall be endorsed with the following legends (in addition to any legend required under applicable state securities laws): |
| |
| | THESE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED. THEY MAY NOT BE OFFERED FOR SALE, PLEDGED OR HYPOTHECATED IN THE ABSENCE OF AN EFFECTIVE REGISTRATION STATEMENT AS TO THE SECURITIES UNDER SAID ACT OR UNLESS THE COMPANY HAS RECEIVED AN OPINION OF COUNSEL SATISFACTORY TO THE COMPANY THAT SUCH REGISTRATION IS NOT REQUIRED. |
| |
| | 5.3 Termination. This Agreement shall terminate in its entirety and be of no further force and effect upon the earlier of: (i) the date that Holder no longer beneficially owns any of the Common Shares issued in the Exchange; or (ii) the two year anniversary of this Agreement. |
| |
| | 5.4 Successors and Assigns. Except as otherwise provided herein, the provisions hereof shall inure to the benefit of, and be binding upon, the successors, assigns, heirs, executors and administrators of the parties hereto. |
| |
| | 5.5 Entire Agreement. This Agreement constitutes the full and entire understanding and agreement among the parties with regard to the subjects hereof. |
| |
| | 5.6 Counterparts. This Agreement may be executed in counterparts, each of which shall be enforceable against the parties actually executing such counterparts, and all of which together shall constitute one instrument. |
| |
| | 5.7 Severability. In the case any provision of this Agreement shall be invalid, illegal or unenforceable, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby. |
| |
| | 5.8 Further Assurances. Each party to this Agreement covenants and agrees to execute and deliver such other agreements and writings and to perform such other acts as may be necessary for the consummation of the matters contemplated by this Agreement. |
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
4
In Witness Whereof,thisAmended and Restated Preferred Exchange Agreement has been executed and delivered as of the date first written above.
| | | |
Epimmune Inc. | | G.D. Searle LLC |
| |
By: /s/Robert De Vaere | | By: /s/Beth Levine |
| | | |
| |
| Name: Robert De Vaere | | Name: Beth Levine |
| |
| Title: VP, Finance and Administration and Chief Financial Officer | | Title: President and Chief Executive Officer |
5
ANNEX F-1
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
EPIMMUNE INC.
Epimmune Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the“Corporation”), does hereby certify as follows:
| |
| | First: The name of the Corporation is Epimmune Inc. |
| |
| | Second: The date on which the Corporation’s Certificate of Incorporation was originally filed with the Secretary of State of the State of Delaware is July 10, 1987. |
| |
| | Third: The Board of Directors of the Corporation, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware, adopted resolutions amending its Certificate of Incorporation as follows: |
| |
| | Article I shall be amended and restated to read in its entirety as follows: |
“I.
The name of the Corporation is IDM, Inc.”
| |
| | The first paragraph of Article V shall be amended and restated to read in its entirety as follows: |
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
| |
| | Article V shall be amended to add the following paragraph immediately after the first paragraph of such Article: |
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every four shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the“Reverse Split”);provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
| |
| | Fourth: The foregoing amendments were submitted to the stockholders of the Corporation for their approval and were duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
In Witness Whereof, Epimmune Inc. has caused this Certificate of Amendment to be signed by its Chief Financial Officer and Secretary this day of , 2005.
| |
| | |
| | Robert J. De Vaere |
| | Chief Financial Officer and Secretary |
ANNEX F-2
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
EPIMMUNE INC.
Epimmune Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the“Corporation”), does hereby certify as follows:
| |
| | First: The name of the Corporation is Epimmune Inc. |
| |
| | Second: The date on which the Corporation’s Certificate of Incorporation was originally filed with the Secretary of State of the State of Delaware is July 10, 1987. |
| |
| | Third: The Board of Directors of the Corporation, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware, adopted resolutions amending its Certificate of Incorporation as follows: |
| |
| | Article I shall be amended and restated to read in its entirety as follows: |
“I.
The name of the Corporation is IDM, Inc.”
| |
| | The first paragraph of Article V shall be amended and restated to read in its entirety as follows: |
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
| |
| | Article V shall be amended to add the following paragraph immediately after the first paragraph of such Article: |
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every five shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the“Reverse Split”);provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
| |
| | Fourth: The foregoing amendments were submitted to the stockholders of the Corporation for their approval and were duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
In Witness Whereof, Epimmune Inc. has caused this Certificate of Amendment to be signed by its Chief Financial Officer and Secretary this day of , 2005.
| |
| | |
| | Robert J. De Vaere |
| | Chief Financial Officer and Secretary |
ANNEX F-3
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
EPIMMUNE INC.
Epimmune Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the“Corporation”), does hereby certify as follows:
| |
| | First: The name of the Corporation is Epimmune Inc. |
| |
| | Second: The date on which the Corporation’s Certificate of Incorporation was originally filed with the Secretary of State of the State of Delaware is July 10, 1987. |
| |
| | Third: The Board of Directors of the Corporation, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware, adopted resolutions amending its Certificate of Incorporation as follows: |
| |
| | Article I shall be amended and restated to read in its entirety as follows: |
“I.
The name of the Corporation is IDM, Inc.”
| |
| | The first paragraph of Article V shall be amended and restated to read in its entirety as follows: |
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
| |
| | Article V shall be amended to add the following paragraph immediately after the first paragraph of such Article: |
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every six shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the“Reverse Split”);provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
| |
| | Fourth: The foregoing amendments were submitted to the stockholders of the Corporation for their approval and were duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
In Witness Whereof, Epimmune Inc. has caused this Certificate of Amendment to be signed by its Chief Financial Officer and Secretary this day of , 2005.
| |
| | |
| | Robert J. De Vaere |
| | Chief Financial Officer and Secretary |
ANNEX F-4
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
EPIMMUNE INC.
Epimmune Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the“Corporation”), does hereby certify as follows:
| |
| | First: The name of the Corporation is Epimmune Inc. |
| |
| | Second: The date on which the Corporation’s Certificate of Incorporation was originally filed with the Secretary of State of the State of Delaware is July 10, 1987. |
| |
| | Third: The Board of Directors of the Corporation, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware, adopted resolutions amending its Certificate of Incorporation as follows: |
| |
| | Article I shall be amended and restated to read in its entirety as follows: |
“I.
The name of the Corporation is IDM, Inc.”
| |
| | The first paragraph of Article V shall be amended and restated to read in its entirety as follows: |
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
| |
| | Article V shall be amended to add the following paragraph immediately after the first paragraph of such Article: |
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every seven shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the“Reverse Split”);provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
| |
| | Fourth: The foregoing amendments were submitted to the stockholders of the Corporation for their approval and were duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
In Witness Whereof, Epimmune Inc. has caused this Certificate of Amendment to be signed by its Chief Financial Officer and Secretary this day of , 2005.
| |
| | |
| | Robert J. De Vaere |
| | Chief Financial Officer and Secretary |
ANNEX F-5
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
EPIMMUNE INC.
Epimmune Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the“Corporation”), does hereby certify as follows:
| |
| | First: The name of the Corporation is Epimmune Inc. |
| |
| | Second: The date on which the Corporation’s Certificate of Incorporation was originally filed with the Secretary of State of the State of Delaware is July 10, 1987. |
| |
| | Third: The Board of Directors of the Corporation, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware, adopted resolutions amending its Certificate of Incorporation as follows: |
| |
| | Article I shall be amended and restated to read in its entirety as follows: |
“I.
The name of the Corporation is IDM, Inc.”
| |
| | The first paragraph of Article V shall be amended and restated to read in its entirety as follows: |
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
| |
| | Article V shall be amended to add the following paragraph immediately after the first paragraph of such Article: |
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every eight shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the“Reverse Split”);provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
| |
| | Fourth: The foregoing amendments were submitted to the stockholders of the Corporation for their approval and were duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
In Witness Whereof, Epimmune Inc. has caused this Certificate of Amendment to be signed by its Chief Financial Officer and Secretary this day of , 2005.
| |
| | |
| | Robert J. De Vaere |
| | Chief Financial Officer and Secretary |
ANNEX F-6
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
EPIMMUNE INC.
Epimmune Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the“Corporation”), does hereby certify as follows:
| |
| | First: The name of the Corporation is Epimmune Inc. |
| |
| | Second: The date on which the Corporation’s Certificate of Incorporation was originally filed with the Secretary of State of the State of Delaware is July 10, 1987. |
| |
| | Third: The Board of Directors of the Corporation, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware, adopted resolutions amending its Certificate of Incorporation as follows: |
| |
| | Article I shall be amended and restated to read in its entirety as follows: |
“I.
The name of the Corporation is IDM, Inc.”
| |
| | The first paragraph of Article V shall be amended and restated to read in its entirety as follows: |
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
| |
| | Article V shall be amended to add the following paragraph immediately after the first paragraph of such Article: |
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every nine shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the“Reverse Split”);provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
| |
| | Fourth:The foregoing amendments were submitted to the stockholders of the Corporation for their approval and were duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
In Witness Whereof, Epimmune Inc. has caused this Certificate of Amendment to be signed by its Chief Financial Officer and Secretary this day of , 2005.
| |
| | |
| | Robert J. De Vaere |
| | Chief Financial Officer and Secretary |
ANNEX F-7
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
EPIMMUNE INC.
Epimmune Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the“Corporation”), does hereby certify as follows:
| |
| | First: The name of the Corporation is Epimmune Inc. |
| |
| | Second: The date on which the Corporation’s Certificate of Incorporation was originally filed with the Secretary of State of the State of Delaware is July 10, 1987. |
| |
| | Third: The Board of Directors of the Corporation, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware, adopted resolutions amending its Certificate of Incorporation as follows: |
| |
| | Article I shall be amended and restated to read in its entirety as follows: |
“I.
The name of the Corporation is IDM, Inc.”
| |
| | The first paragraph of Article V shall be amended and restated to read in its entirety as follows: |
| |
| | “The Corporation is authorized to issue two classes of shares designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock which the Corporation has authority to issue is 65,000,000 shares, consisting of 55,000,000 shares of Common Stock, each having a par value of $.01, and 10,000,000 shares of Preferred Stock, each having a par value of $.01.” |
| |
| | Article V shall be amended to add the following paragraph immediately after the first paragraph of such Article: |
| |
| | “Effective at the time of filing of this Certificate of Amendment with the Secretary of State of the State of Delaware, every ten shares of Common Stock issued and outstanding shall, automatically and without any action on the part of the holders thereof, be combined and converted into one share of Common Stock (the“Reverse Split”);provided, however, that this Corporation shall issue no fractional shares of Common Stock as a result of the Reverse Split, but shall instead pay to any stockholder who would be entitled to receive a fractional share as a result of the actions set forth herein a sum in cash equal to the fair market value of the shares constituting such fractional share as determined by the Board.” |
| |
| | Fourth: The foregoing amendments were submitted to the stockholders of the Corporation for their approval and were duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
In Witness Whereof, Epimmune Inc. has caused this Certificate of Amendment to be signed by its Chief Financial Officer and Secretary this day of , 2005.
| |
| | |
| | Robert J. De Vaere |
| | Chief Financial Officer and Secretary |
ANNEX G
EPIMMUNE INC.
2000 STOCK PLAN
Adopted: April 21, 2000
Approved by Stockholders: June 9, 2000
Amended by the Board: December 4, 2001
Amended by the Board: January 14, 2002
Amendment Approved by Stockholders: June 18, 2002
Amended by the Board: June 4, 2003
Amendment Approved by Stockholders: July 15, 2003
Amended by the Board: March 3, 2004
Amendment Approved by Stockholders: June 15, 2004
Amended by the Board: March 15, 2005
Termination Date: April 20, 2010
(a) Eligible Stock Award Recipients. The persons eligible to receive Stock Awards are the Employees, Directors and Consultants of the Company and its Affiliates.
(b) Available Stock Awards. The purpose of the Plan is to provide a means by which eligible recipients of Stock Awards may be given an opportunity to benefit from increases in value of the Common Stock through the granting of the following Stock Awards: (i) Incentive Stock Options, (ii) Nonstatutory Stock Options, (iii) stock bonuses and (iv) rights to acquire restricted stock.
(c) General Purpose. The Company, by means of the Plan, seeks to retain the services of the group of persons eligible to receive Stock Awards, to secure and retain the services of new members of this group and to provide incentives for such persons to exert maximum efforts for the success of the Company and its Affiliates.
(a) “Affiliate” means any parent corporation or subsidiary corporation of the Company, whether now or hereafter existing, as those terms are defined in Sections 424(e) and (f), respectively, of the Code.
(b) “Board” means the Board of Directors of the Company.
(c) “Code” means the Internal Revenue Code of 1986, as amended.
(d) “Committee” means a committee of one or more members of the Board appointed by the Board in accordance with subsection 3(c).
(e) “Common Stock” means the common stock of the Company.
(f) “Company” means Epimmune Inc., a Delaware corporation.
(g) “Consultant” means any person, including an advisor, (i) engaged by the Company or an Affiliate to render consulting or advisory services and who is compensated for such services or (ii) who is a member of the Board of Directors of an Affiliate. However, the term “Consultant” shall not include either Directors who are not compensated by the Company for their services as Directors or Directors who are merely paid a director’s fee by the Company for their services as Directors.
(h) “Continuous Service” means that the Participant’s service with the Company or an Affiliate, whether as an Employee, Director or Consultant, is not interrupted or terminated. The Participant’s Continuous Service shall not be deemed to have terminated merely because of a change in the capacity in which the Participant renders service to the Company or an Affiliate as an Employee, Consultant or Director or a change
1
in the entity for which the Participant renders such service, provided that there is no interruption or termination of the Participant’s Continuous Service. For example, a change in status from an Employee of the Company to a Consultant of an Affiliate or a Director will not constitute an interruption of Continuous Service. To the extent permitted by law, the Board or the chief executive officer of the Company, in that party’s sole discretion, may determine whether Continuous Service shall be considered interrupted in the case of any leave of absence approved by that party, including sick leave, military leave or any other personal leave. Notwithstanding the foregoing, a leave of absence shall be treated as Continuous Service for purposes of vesting in a Stock Award only to such extent as may be provided in the Company’s leave of absence policy or in the written terms of the Participant’s leave of absence agreement, to the extent permitted by law.
(i) “Covered Employee” means the chief executive officer and the four (4) other highest compensated officers of the Company for whom total compensation is required to be reported to stockholders under the Exchange Act, as determined for purposes of Section 162(m) of the Code.
(j) “Director” means a member of the Board of Directors of the Company.
(k) “Disability” means the permanent and total disability of a person within the meaning of Section 22(e)(3) of the Code.
(l) “Employee” means any person, including Officers and Directors, employed by the Company or an Affiliate. Mere service as a Director or payment of a director’s fee by the Company or an Affiliate shall not be sufficient to constitute “employment” by the Company or an Affiliate.
(m) “Exchange Act” means the Securities Exchange Act of 1934, as amended.
(n) “Fair Market Value” means, as of any date, the value of the Common Stock determined as follows:
| |
| | (i) If the Common Stock is listed on any established stock exchange or traded on the Nasdaq National Market or the Nasdaq SmallCap Market, the Fair Market Value of a share of Common Stock shall be the closing sales price for such stock (or the closing bid, if no sales were reported) as quoted on such exchange or market (or the exchange or market with the greatest volume of trading in the Common Stock) on the day of determination, as reported inThe Wall Street Journalor such other source as the Board deems reliable;provided, however, that if the day of determination is not a market trading day, then the Fair Market Value of a share of Common Stock shall be the closing sales price for such stock (or the closing bid, if no sales were reported) as quoted on such exchange or market (or the exchange or market with the greatest volume of trading in the Common Stock) on the last market trading day prior to the day of determination, as reported inThe Wall Street Journalor such other source as the Board deems reliable. |
| |
| | (ii) In the absence of such markets for the Common Stock, the Fair Market Value shall be determined in good faith by the Board. |
(o) “Incentive Stock Option” means an Option intended to qualify as an incentive stock option within the meaning of Section 422 of the Code and the regulations promulgated thereunder.
(p) “Non-Employee Director” means a Director who either (i) is not a current Employee or Officer of the Company or its parent or a subsidiary, does not receive compensation (directly or indirectly) from the Company or its parent or a subsidiary for services rendered as a consultant or in any capacity other than as a Director (except for an amount as to which disclosure would not be required under Item 404(a) of Regulation S-K promulgated pursuant to the Securities Act (“Regulation S-K”)), does not possess an interest in any other transaction as to which disclosure would be required under Item 404(a) of Regulation S-K and is not engaged in a business relationship as to which disclosure would be required under Item 404(b) of Regulation S-K; or (ii) is otherwise considered a “non-employee director” for purposes of Rule 16b-3. In addition, for purposes of Section 8 only, “Non-Employee Director” also shall include any Director who is not an Employee of the Company or an Affiliate at the time an Option is granted to such Director.
(q) “Nonstatutory Stock Option” means an Option not intended to qualify as an Incentive Stock Option.
2
(r) “Officer” means a person who is an officer of the Company within the meaning of Section 16 of the Exchange Act and the rules and regulations promulgated thereunder.
(s) “Option” means an Incentive Stock Option or a Nonstatutory Stock Option granted pursuant to the Plan.
(t) “Option Agreement” means a written agreement between the Company and an Optionholder evidencing the terms and conditions of an individual Option grant. Each Option Agreement shall be subject to the terms and conditions of the Plan.
(u) “Optionholder” means a person to whom an Option is granted pursuant to the Plan or, if applicable, such other person who holds an outstanding Option.
(v) “Outside Director” means a Director who either (i) is not a current employee of the Company or an “affiliated corporation” (within the meaning of Treasury Regulations promulgated under Section 162(m) of the Code), is not a former employee of the Company or an “affiliated corporation” receiving compensation for prior services (other than benefits under a tax qualified pension plan), was not an officer of the Company or an “affiliated corporation” at any time and is not currently receiving direct or indirect remuneration from the Company or an “affiliated corporation” for services in any capacity other than as a Director or (ii) is otherwise considered an “outside director” for purposes of Section 162(m) of the Code.
(w) “Participant” means a person to whom a Stock Award is granted pursuant to the Plan or, if applicable, such other person who holds an outstanding Stock Award.
(x) “Plan” means this Epimmune Inc. 2000 Stock Plan.
(y) “Rule 16b-3” means Rule 16b-3 promulgated under the Exchange Act or any successor to Rule 16b-3, as in effect from time to time.
(z) “Securities Act” means the Securities Act of 1933, as amended.
(aa) “Stock Award” means any right granted under the Plan, including an Option, a stock bonus and a right to acquire restricted stock.
(bb) “Stock Award Agreement” means a written agreement between the Company and a holder of a Stock Award evidencing the terms and conditions of an individual Stock Award grant. Each Stock Award Agreement shall be subject to the terms and conditions of the Plan.
(cc) “Ten Percent Stockholder” means a person who owns (or is deemed to own pursuant to Section 424(d) of the Code) stock possessing more than ten percent (10%) of the total combined voting power of all classes of stock of the Company or of any of its Affiliates.
(a) Administration by Board. The Board shall administer the Plan unless and until the Board delegates administration to a Committee, as provided in subsection 3(c). Any interpretation of the Plan by the Board and any decision by the Board under the Plan shall be final and binding on all persons.
(b) Powers of Board. The Board shall have the power, subject to, and within the limitations of, the express provisions of the Plan:
| |
| | (i) To determine from time to time which of the persons eligible under the Plan shall be granted Stock Awards; when and how each Stock Award shall be granted; what type or combination of types of Stock Award shall be granted; the provisions of each Stock Award granted (which need not be identical), including the time or times when a person shall be permitted to receive Common Stock pursuant to a Stock Award; and the number of shares of Common Stock with respect to which a Stock Award shall be granted to each such person. |
| |
| | (ii) To construe and interpret the Plan and Stock Awards granted under it, and to establish, amend and revoke rules and regulations for its administration. The Board, in the exercise of this power, may |
3
| |
| | correct any defect, omission or inconsistency in the Plan or in any Stock Award Agreement, in a manner and to the extent it shall deem necessary or expedient to make the Plan fully effective. |
| |
| | (iii) To amend the Plan or a Stock Award as provided in Section 13. |
| |
| | (iv) To terminate or suspend the Plan as provided in Section 14. |
| |
| | (v) Generally, to exercise such powers and to perform such acts as the Board deems necessary or expedient to promote the best interests of the Company which are not in conflict with the provisions of the Plan. |
| |
| | (vi) To adopt such procedures and sub-plans as are necessary or appropriate to permit participation in the Plan by Employees and Consultants who are foreign nationals or employed or providing services outside the United States. |
(c) Delegation to Committee.
| |
| | (i) General. The Board may delegate administration of the Plan to a Committee or Committees of one (1) or more members of the Board, and the term “Committee” shall apply to any person or persons to whom such authority has been delegated. If administration is delegated to a Committee, the Committee shall have, in connection with the administration of the Plan, the powers theretofore possessed by the Board, including the power to delegate to a subcommittee any of the administrative powers the Committee is authorized to exercise (and references in this Plan to the Board shall thereafter be to the Committee or subcommittee), subject, however, to such resolutions, not inconsistent with the provisions of the Plan, as may be adopted from time to time by the Board. The Board may abolish the Committee at any time and revest in the Board the administration of the Plan. |
| |
| | (ii) Committee Composition when Common Stock is Publicly Traded. At times when the Common Stock is publicly traded, in the discretion of the Board, a Committee may consist solely of two or more Outside Directors, in accordance with Section 162(m) of the Code, and/or solely of two or more Non-Employee Directors, in accordance with Rule 16b-3. Within the scope of such authority, the Board or the Committee may (1) delegate to a committee of one or more members of the Board who are not Outside Directors, the authority to grant Stock Awards to eligible persons who are either (a) not then Covered Employees and are not expected to be Covered Employees at the time of recognition of income resulting from such Stock Award or (b) not persons with respect to whom the Company wishes to comply with Section 162(m) of the Code and/or (2) delegate to a committee of one or more members of the Board who are not Non-Employee Directors the authority to grant Stock Awards to eligible persons who are not then subject to Section 16 of the Exchange Act. |
(d) Delegation to an Officer. The Board may delegate to one or more Officers of the Company the authority to do one or both of the following (i) designate Officers and Employees of the Company or any of its Subsidiaries to be recipients of Stock Awards and the terms thereof, and (ii) determine the number of shares of Common Stock to be subject to such Stock Awards granted to such Officers and Employees of the Company; provided, however, that the Board resolutions regarding such delegation shall specify the total number of shares of Common Stock that may be subject to the Stock Awards granted by such Officer and that such Officer may not grant a Stock Award to himself or herself. Notwithstanding anything to the contrary in this Section 3(d), the Board may not delegate to an Officer authority to determine the Fair Market Value of the Common Stock pursuant to Section 2(n)(ii) above.
| |
| 4. | Shares Subject to the Plan. |
(a) Share Reserve. Subject to the provisions of Section 12 relating to adjustments upon changes in Common Stock, the Common Stock that may be issued pursuant to Stock Awards shall not exceed in the aggregate eleven million four hundred thousand (11,400,000) shares of Common Stock.
(b) Reversion of Shares to the Share Reserve. If any Stock Award shall for any reason expire or otherwise terminate, in whole or in part, without having been exercised in full, the shares of Common Stock not acquired under such Stock Award shall revert to and again become available for issuance under the Plan.
4
(c) Source of Shares. The shares of Common Stock subject to the Plan may be unissued shares or reacquired shares, bought on the market or otherwise.
(a) Eligibility for Specific Stock Awards. Incentive Stock Options may be granted only to Employees. Stock Awards other than Incentive Stock Options may be granted to Employees, Directors and Consultants.
(b) Ten Percent Stockholders. A Ten Percent Stockholder shall not be granted an Incentive Stock Option unless the exercise price of such Option is at least one hundred ten percent (110%) of the Fair Market Value of the Common Stock at the date of grant and the Option is not exercisable after the expiration of five (5) years from the date of grant.
(c) Section 162(m) Limitation. Subject to the provisions of Section 12 relating to adjustments upon changes in the shares of Common Stock, no Employee shall be eligible to be granted Options subject to Section 6 and restricted stock purchase rights subject to subsection 7(b) covering, in the aggregate, more than five hundred thousand (500,000) shares of the Common Stock during any calendar year.
(d) Consultants.
| |
| | (i) A Consultant shall not be eligible for the grant of a Stock Award if, at the time of grant, a Form S-8 Registration Statement under the Securities Act (“Form S-8”) is not available to register either the offer or the sale of the Company’s securities to such Consultant because of the nature of the services that the Consultant is providing to the Company, or because the Consultant is not a natural person, or as otherwise provided by the rules governing the use of Form S-8, unless the Company determines both (i) that such grant (A) shall be registered in another manner under the Securities Act (e.g.,on a Form S-3 Registration Statement) or (B) does not require registration under the Securities Act in order to comply with the requirements of the Securities Act, if applicable, and (ii) that such grant complies with the securities laws of all other relevant jurisdictions. |
| |
| | (ii) Form S-8 generally is available to consultants and advisors only if (i) they are natural persons; (ii) they provide bona fide services to the issuer, its parents, its majority-owned subsidiaries or majority-owned subsidiaries of the issuer’s parent; and (iii) the services are not in connection with the offer or sale of securities in a capital-raising transaction, and do not promote or maintain, directly or indirectly, a market for the issuer’s securities. |
Each Option shall be in such form and shall contain such terms and conditions as the Board shall deem appropriate. All Options shall be separately designated Incentive Stock Options or Nonstatutory Stock Options at the time of grant, and, if certificates are issued, a separate certificate or certificates will be issued for shares of Common Stock purchased on exercise of each type of Option. The provisions of separate Options need not be identical, but each Option shall include (through incorporation of provisions hereof by reference in the Option or otherwise) the substance of each of the following provisions:
| |
| | (a) Term. Subject to the provisions of subsection 5(b) regarding Ten Percent Stockholders, no Incentive Stock Option shall be exercisable after the expiration of ten (10) years from the date it was granted. |
| |
| | (b) Exercise Price of an Incentive Stock Option. Subject to the provisions of subsection 5(b) regarding Ten Percent Stockholders, the exercise price of each Incentive Stock Option shall be not less than one hundred percent (100%) of the Fair Market Value of the Common Stock subject to the Option on the date the Option is granted. Notwithstanding the foregoing, an Incentive Stock Option may be granted with an exercise price lower than that set forth in the preceding sentence if such Option is granted pursuant to an assumption or substitution for another option in a manner satisfying the provisions of Section 424(a) of the Code. |
5
| |
| | (c) Exercise Price of a Nonstatutory Stock Option. The exercise price of each Nonstatutory Stock Option shall be not less than one hundred percent (100%) of the Fair Market Value of the Common Stock subject to the Option on the date the Option is granted. Notwithstanding the foregoing, a Nonstatutory Stock Option may be granted with an exercise price lower than that set forth in the preceding sentence if such Option is granted pursuant to an assumption or substitution for another option in a manner satisfying the provisions of Section 424(a) of the Code. This Section 6(c) may not be amended without the affirmative vote of the holders of a majority of the shares present and represented and entitled to vote at a duly convened meeting of stockholders of the Company. |
| |
| | (d) Consideration. The purchase price of Common Stock acquired pursuant to an Option shall be paid, to the extent permitted by applicable statutes and regulations, either (i) in cash at the time the Option is exercised or (ii) at the discretion of the Board (such Board discretion may be exercised either at the time of the grant of the Option or at any time following the grant of the Option to permit the following payment alternatives) (1) by delivery to the Company of other Common Stock, (2) according to a deferred payment or other similar arrangement with the Optionholder or (3) in any other form of legal consideration that may be acceptable to the Board; provided, however, that at any time that the Company is incorporated in Delaware, payment of the Common Stock’s “par value,” as defined in the Delaware General Corporation Law, shall not be made by deferred payment. |
| |
| | In the case of any deferred payment arrangement, interest shall compound at least annually and shall be charged at the minimum rate of interest necessary to avoid (i) the imputation of interest income to the Company and compensation income to the Optionholder under any applicable provisions of the Code, and (ii) adverse financial accounting treatment of the Option. |
| |
| | (e) Transferability of an Incentive Stock Option. An Incentive Stock Option shall not be transferable except by will or by the laws of descent and distribution and shall be exercisable during the lifetime of the Optionholder only by the Optionholder. Notwithstanding the foregoing, (i) the Optionholder may, by delivering written notice to the Company, in a form satisfactory to the Company, designate a third party who, in the event of the death of the Optionholder, shall thereafter be entitled to exercise the Incentive Stock Option, and (ii) the Incentive Stock Option may be transferred pursuant to a domestic relations order. |
| |
| | (f) Transferability of a Nonstatutory Stock Option. A Nonstatutory Stock Option shall be transferable to the extent provided in the Option Agreement. If the Nonstatutory Stock Option does not provide for transferability, then the Nonstatutory Stock Option shall not be transferable except by will or by the laws of descent and distribution and shall be exercisable during the lifetime of the Optionholder only by the Optionholder. Notwithstanding the foregoing, (i) the Optionholder may, by delivering written notice to the Company, in a form satisfactory to the Company, designate a third party who, in the event of the death of the Optionholder, shall thereafter be entitled to exercise the Nonstatutory Stock Option, and (ii) the Nonstatutory Stock Option may be transferred pursuant to a domestic relations order. |
| |
| | (g) Vesting Generally. The total number of shares of Common Stock subject to an Option may, but need not, vest and therefore become exercisable in periodic installments that may, but need not, be equal. The Option may be subject to such other terms and conditions on the time or times when it may be exercised (which may be based on performance or other criteria) as the Board may deem appropriate. The vesting provisions of individual Options may vary. The provisions of this subsection 6(g) are subject to any Option provisions governing the minimum number of shares of Common Stock as to which an Option may be exercised. |
| |
| | (h) Termination of Continuous Service. In the event an Optionholder’s Continuous Service terminates (other than upon the Optionholder’s death or Disability), the Optionholder may exercise his or her Option (to the extent that the Optionholder was entitled to exercise such Option as of the date of termination) but only within such period of time ending on the earlier of (i) the date three (3) months following the termination of the Optionholder’s Continuous Service (or such longer or shorter period specified in the Option Agreement), or (ii) the expiration of the term of the Option as set forth in the |
6
| |
| | Option Agreement. If, after termination, the Optionholder does not exercise his or her Option within the time specified, the Option shall terminate. |
| |
| | (i) Extension of Termination Date. An Optionholder’s Option Agreement may also provide that if the exercise of the Option following the termination of the Optionholder’s Continuous Service (other than upon the Optionholder’s death or Disability) would be prohibited at any time solely because the issuance of shares of Common Stock would violate the registration requirements under the Securities Act, then the Option shall terminate on the earlier of (i) the expiration of the term of the Option set forth in subsection 6(a) or (ii) the expiration of a period of three (3) months after the termination of the Optionholder’s Continuous Service during which the exercise of the Option would not be in violation of such registration requirements. |
| |
| | (j) Disability of Optionholder. In the event that an Optionholder’s Continuous Service terminates as a result of the Optionholder’s Disability, the Optionholder may exercise his or her Option (to the extent that the Optionholder was entitled to exercise such Option as of the date of termination), but only within such period of time ending on the earlier of (i) the date twelve (12) months following such termination (or such longer or shorter period specified in the Option Agreement) or (ii) the expiration of the term of the Option as set forth in the Option Agreement. If, after termination, the Optionholder does not exercise his or her Option within the time specified, the Option shall terminate. |
| |
| | (k) Death of Optionholder. In the event (i) an Optionholder’s Continuous Service terminates as a result of the Optionholder’s death or (ii) the Optionholder dies within the period (if any) specified in the Option Agreement after the termination of the Optionholder’s Continuous Service for a reason other than death, then the Option may be exercised (to the extent the Optionholder was entitled to exercise such Option as of the date of death) by the Optionholder’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by a person designated to exercise the option upon the Optionholder’s death pursuant to subsection 6(e) or 6(f), but only within the period ending on the earlier of (1) the date eighteen (18) months following the date of death (or such longer or shorter period specified in the Option Agreement) or (2) the expiration of the term of such Option as set forth in the Option Agreement. If, after death, the Option is not exercised within the time specified, the Option shall terminate. |
| |
| | (l) Early Exercise. The Option may, but need not, include a provision whereby the Optionholder may elect at any time before the Optionholder’s Continuous Service terminates to exercise the Option as to any part or all of the shares of Common Stock subject to the Option prior to the full vesting of the Option. Any unvested shares of Common Stock so purchased may be subject to a repurchase option in favor of the Company or to any other restriction the Board determines to be appropriate. The price paid for all shares of Common Stock so repurchased by the Company may be at the lesser of: (i) the Fair Market Value on the relevant date, or (ii) the Participant’s original cost for such shares. The Company shall not be required to exercise its repurchase option until at least six (6) months (or such longer or shorter period of time necessary to avoid a charge to earnings for financial accounting purposes) have elapsed following exercise of the Option unless the Board otherwise specifically provides in the Option. |
| |
| | (m) Re-Load Options. Without in any way limiting the authority of the Board to make or not to make grants of Options hereunder, the Board shall have the authority (but not an obligation) to include as part of any Option Agreement a provision entitling the Optionholder to a further Option (a “Re-Load Option”) in the event the Optionholder exercises the Option evidenced by the Option Agreement, in whole or in part, by surrendering other shares of Common Stock in accordance with this Plan and the terms and conditions of the Option Agreement. Any such Re-Load Option shall (i) except as provided in this subsection 6(m) below, be exercisable for a number of shares of Common Stock equal to the number of shares of Common Stock surrendered as part or all of the exercise price of such Option; (ii) have an expiration date which is the same as the expiration date of the Option the exercise of which gave rise to such Re-Load Option; and (iii) have an exercise price which is equal to one hundred percent (100%) of the Fair Market Value of the Common Stock subject to the Re-Load Option on the date of exercise of |
7
| |
| | the original Option. Notwithstanding the foregoing, a Re-Load Option shall be subject to the same exercise price and term provisions heretofore described for Options under the Plan. |
| |
| | Any such Re-Load Option may be an Incentive Stock Option or a Nonstatutory Stock Option, as the Board may designate at the time of the grant of the original Option; provided, however, that the designation of any Re-Load Option as an Incentive Stock Option shall be subject to the one hundred thousand dollar ($100,000) annual limitation on the exercisability of Incentive Stock Options described in subsection 11(d) and in Section 422(d) of the Code. There shall be no Re-Load Options on a Re-Load Option. Any such Re-Load Option shall be subject to the availability of sufficient shares of Common Stock under subsection 4(a) and the “Section 162(m) Limitation” on the grants of Options under subsection 5(c) and shall be subject to such other terms and conditions as the Board may determine which are not inconsistent with the express provisions of the Plan regarding the terms of Options. |
| |
| 7. | Provisions of Stock Awards other than Options. |
(a) Stock Bonus Awards. Each stock bonus agreement shall be in such form and shall contain such terms and conditions as the Board shall deem appropriate. The terms and conditions of stock bonus agreements may change from time to time, and the terms and conditions of separate stock bonus agreements need not be identical, but each stock bonus agreement shall include (through incorporation of provisions hereof by reference in the agreement or otherwise) the substance of each of the following provisions:
| |
| | (i) Consideration. A stock bonus may be awarded in consideration for past services actually rendered to the Company or an Affiliate for its benefit. |
| |
| | (ii) Vesting. Shares of Common Stock awarded under the stock bonus agreement may, but need not, be subject to a share repurchase option in favor of the Company in accordance with a vesting schedule to be determined by the Board. |
| |
| | (iii) Termination of Participant’s Continuous Service. In the event a Participant’s Continuous Service terminates, the Company may reacquire any or all of the shares of Common Stock held by the Participant which have not vested as of the date of termination under the terms of the stock bonus agreement. |
| |
| | (iv) Transferability. Rights to acquire shares under the stock bonus agreement shall be transferable by the Participant only upon such terms and conditions as are set forth in the stock bonus agreement, as the Board shall determine in its discretion, so long as Common Stock awarded under the stock bonus agreement remains subject to the terms of the stock bonus agreement. |
(b) Restricted Stock Awards. Each restricted stock purchase agreement shall be in such form and shall contain such terms and conditions as the Board shall deem appropriate. The terms and conditions of restricted stock purchase agreements may change from time to time, and the terms and conditions of separate restricted stock purchase agreements need not be identical, but each restricted stock purchase agreement shall include (through incorporation of provisions hereof by reference in the agreement or otherwise) the substance of each of the following provisions:
| |
| | (i) Purchase Price. The purchase price under each restricted stock purchase agreement shall be such amount as the Board shall determine and designate in such restricted stock purchase agreement. The purchase price shall not be less than one hundred percent (100%) of the Common Stock’s Fair Market Value on the date such award is made or at the time the purchase is consummated; provided, however, that this Section 7(b)(i) may be amended to provide, or any restricted stock purchase agreement granted under the Plan may provide, that the purchase price shall not be less than eighty five percent (85%) of the Common Stock’s Fair Market Value on the date such award is made or at the time the purchase is consummated if such amendment or grant is approved by the holders of a majority of the shares present or represented and entitled to vote at a duly convened meeting of stockholders. This Section 7(b)(i) may not be amended without the affirmative vote of the holders of a majority of the |
8
| |
| | shares present and represented and entitled to vote at a duly convened meeting of stockholders of the Company. |
| |
| | (ii) Consideration. The purchase price of Common Stock acquired pursuant to the restricted stock purchase agreement shall be paid either: (i) in cash at the time of purchase; (ii) at the discretion of the Board, according to a deferred payment or other similar arrangement with the Participant; or (iii) in any other form of legal consideration that may be acceptable to the Board in its discretion; provided, however, that at any time that the Company is incorporated in Delaware, then payment of the Common Stock’s “par value,” as defined in the Delaware General Corporation Law, shall not be made by deferred payment. Additionally, in the case of any deferred payment arrangement, interest shall compound at least annually and shall be charged at the minimum rate of interest necessary to avoid (i) the imputation of interest income to the Company and compensation income to the Participant under any applicable provisions of the Code, and (ii) adverse financial accounting treatment of the restricted stock award. |
| |
| | (iii) Vesting. Shares of Common Stock acquired under the restricted stock purchase agreement may, but need not, be subject to a share repurchase option in favor of the Company in accordance with a vesting schedule to be determined by the Board. |
| |
| | (iv) Termination of Participant’s Continuous Service. In the event a Participant’s Continuous Service terminates, the Company may repurchase or otherwise reacquire any or all of the shares of Common Stock held by the Participant which have not vested as of the date of termination under the terms of the restricted stock purchase agreement. The price paid for all shares of Common Stock so repurchased or reacquired by the Company may be at the lesser of: (i) the Fair Market Value on the relevant date, or (ii) the Participant’s original cost for such shares. The Company shall not be required to exercise its repurchase or reacquisition option until at least six (6) months (or such longer or shorter period of time necessary to avoid a charge to earnings for financial accounting purposes) have elapsed following the Participant’s purchase of the shares of stock acquired pursuant to the restricted stock award unless otherwise determined by the Board or provided in the restricted stock purchase agreement. |
| |
| | (v) Transferability. Rights to acquire shares under the restricted stock purchase agreement shall be transferable by the Participant only upon such terms and conditions as are set forth in the restricted stock purchase agreement, as the Board shall determine in its discretion, so long as Common Stock awarded under the restricted stock purchase agreement remains subject to the terms of the restricted stock purchase agreement. |
| |
| 8. | Non-Employee Directors. |
(a) Non-Employee Directors shall be eligible to receive any form of Stock Award provided for by the Plan, other than Incentive Stock Options, and such Stock Awards shall be subject to all the terms of the Plan, including Sections 6 and 12, except as modified by this Section 8. Unless otherwise specifically provided in the applicable Option Agreement, Nonstatutory Options granted to Non-Employee Directors (“Non-Employee Director Options”) shall be subject to the provisions of this Section 8.
(b) The term of each Non-Employee Director Option shall commence on the date it is granted and expire on the date ten (10) years from the date of grant, unless sooner terminated due to the Non-Employee Director’s termination of Continuous Service. Non-Employee Director Options may be exercised following the Optionholder’s termination of Continuous Service, for whatever reason (to the extent such Optionholder was entitled to exercise such Option on the date of such termination), within the period of time ending on the earlier of (i) the date twelve (12) months following such termination or (ii) the expiration of the Option as set forth in the Option Agreement.
(c) The exercise price of each Nonstatutory Option granted to a Non-Employee Director Option shall be one hundred percent (100%) of the Fair Market Value of the Common Stock subject to the Option on the date of grant.
(d) In the event of a transaction or event described in any of subsections 12(b), 12(c), 12(d) or 12(e), then, with respect to Non-Employee Director Options held by Participants whose Continuous Service has not
9
terminated, the vesting of such Non-Employee Director Options (and, if applicable, the time during which such Non-Employee Director Options may be exercised) shall be accelerated in full.
| |
| 9. | Covenants of the Company. |
(a) Availability of Shares. During the terms of the Stock Awards, the Company shall keep available at all times the number of shares of Common Stock required to satisfy such Stock Awards.
(b) Securities Law Compliance. The Company shall seek to obtain from each regulatory commission or agency having jurisdiction over the Plan such authority as may be required to grant Stock Awards and to issue and sell shares of Common Stock upon exercise of the Stock Awards; provided, however, that this undertaking shall not require the Company to register under the Securities Act the Plan, any Stock Award or any Common Stock issued or issuable pursuant to any such Stock Award. If, after reasonable efforts, the Company is unable to obtain from any such regulatory commission or agency the authority which counsel for the Company deems necessary for the lawful issuance and sale of Common Stock under the Plan, the Company shall be relieved from any liability for failure to issue and sell Common Stock upon exercise of such Stock Awards unless and until such authority is obtained.
| |
| 10. | Use of Proceeds from Stock. |
Proceeds from the sale of Common Stock pursuant to Stock Awards shall constitute general funds of the Company.
(a) Acceleration of Exercisability and Vesting. The Board shall have the power to accelerate the time at which a Stock Award may first be exercised or the time during which a Stock Award or any part thereof will vest in accordance with the Plan, notwithstanding the provisions in the Stock Award stating the time at which it may first be exercised or the time during which it will vest.
(b) Stockholder Rights. No Participant shall be deemed to be the holder of, or to have any of the rights of a holder with respect to, any shares of Common Stock subject to such Stock Award unless and until such Participant has satisfied all requirements for exercise of the Stock Award pursuant to its terms.
(c) No Employment or other Service Rights. Nothing in the Plan or any instrument executed or Stock Award granted pursuant thereto shall confer upon any Participant any right to continue to serve the Company or an Affiliate in the capacity in effect at the time the Stock Award was granted or shall affect the right of the Company or an Affiliate to terminate (i) the employment of an Employee with or without notice and with or without cause, (ii) the service of a Consultant pursuant to the terms of such Consultant’s agreement with the Company or an Affiliate or (iii) the service of a Director pursuant to the Bylaws of the Company or an Affiliate, and any applicable provisions of the corporate law of the state in which the Company or the Affiliate is incorporated, as the case may be.
(d) Incentive Stock Option $100,000 Limitation. To the extent that the aggregate Fair Market Value (determined at the time of grant) of Common Stock with respect to which Incentive Stock Options are exercisable for the first time by any Optionholder during any calendar year (under all plans of the Company and its Affiliates) exceeds one hundred thousand dollars ($100,000), the Options or portions thereof which exceed such limit (according to the order in which they were granted) shall be treated as Nonstatutory Stock Options.
(e) Investment Assurances. The Company may require a Participant, as a condition of exercising or acquiring Common Stock under any Stock Award, (i) to give written assurances satisfactory to the Company as to the Participant’s knowledge and experience in financial and business matters and/or to employ a purchaser representative reasonably satisfactory to the Company who is knowledgeable and experienced in financial and business matters and that he or she is capable of evaluating, alone or together with the purchaser representative, the merits and risks of exercising the Stock Award; and (ii) to give written assurances satisfactory to the Company stating that the Participant is acquiring Common Stock subject to the Stock
10
Award for the Participant’s own account and not with any present intention of selling or otherwise distributing the Common Stock. The foregoing requirements, and any assurances given pursuant to such requirements, shall be inoperative if (1) the issuance of the shares of Common Stock upon the exercise or acquisition of Common Stock under the Stock Award has been registered under a then currently effective registration statement under the Securities Act or (2) as to any particular requirement, a determination is made by counsel for the Company that such requirement need not be met in the circumstances under the then applicable securities laws. The Company may, upon advice of counsel to the Company, place legends on stock certificates issued under the Plan as such counsel deems necessary or appropriate in order to comply with applicable securities laws, including, but not limited to, legends restricting the transfer of the Common Stock.
(f) Withholding Obligations. To the extent provided by the terms of a Stock Award Agreement, the Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise or acquisition of Common Stock under a Stock Award by any of the following means (in addition to the Company’s right to withhold from any compensation paid to the Participant by the Company) or by a combination of such means: (i) tendering a cash payment; (ii) authorizing the Company to withhold shares of Common Stock from the shares of Common Stock otherwise issuable to the Participant as a result of the exercise or acquisition of Common Stock under the Stock Award; provided, however, that no shares are withheld with a value exceeding the minimum amount of tax required to withheld by law; or (iii) delivering to the Company owned and unencumbered shares of the Common Stock.
(g) Cancellation and Re-Grant of Options. The Board shall not have the authority, at any time, without obtaining the approval of a majority of the shares present or represented and entitled to vote at a duly convened meeting of stockholders, to (1) reduce the exercise price of any Options under the Plan that are either currently outstanding or will be granted in the future; (2) cancel any outstanding Options under the Plan and grant in substitution therefor new Options under the Plan at a lower exercise price (including entering into any “6 month and 1 day” cancellation and re-grant scheme), regardless of whether or not the cancelled Options revert to and again become available for issuance under the Plan; (3) replace Options having an exercise price higher than the then current Fair Market Value with rights to acquire restricted stock and/or stock bonus awards in an exchange, buy-back or other scheme; or (4) replace any outstanding Options under the Plan with new Options under the Plan having a lower exercise price or accelerated vesting schedule in an exchange, buy-back or other scheme. This Section 11(g) may not be amended without the affirmative vote of the holders of a majority of the shares present or represented and entitled to vote at a duly convened meeting of the stockholders of the Company.
| |
| 12. | Adjustments upon Changes in Stock. |
(a) Capitalization Adjustments. If any change is made in the Common Stock subject to the Plan, or subject to any Stock Award, without the receipt of consideration by the Company (through merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or other transaction not involving the receipt of consideration by the Company), the Plan will be appropriately adjusted in the class(es) and maximum number of securities subject to the Plan pursuant to subsection 4(a) and the maximum number of securities subject to award to any person pursuant to subsection 5(c), and the outstanding Stock Awards will be appropriately adjusted in the class(es) and number of securities and price per share of Common Stock subject to such outstanding Stock Awards. The Board shall make such adjustments, and its determination shall be final, binding and conclusive. (The conversion of any convertible securities of the Company shall not be treated as a transaction “without receipt of consideration” by the Company.)
(b) Dissolution or Liquidation. In the event of a dissolution or liquidation of the Company, then, with respect to Stock Awards held by Participants whose Continuous Service has not terminated, the vesting of such Stock Awards (and, if applicable, the time during which such Stock Awards may be exercised) shall be accelerated in full, and the Stock Awards shall terminate if not exercised (if applicable) at or prior to such event, except to the extent that such Stock Awards are assumed or substituted by a surviving or acquiring
11
corporation pursuant to subsection 12(c). With respect to any other Stock Awards outstanding under the Plan, such Stock Awards shall terminate if not exercised (if applicable) prior to such event.
(c) Asset Sale, Merger, Consolidation or Reverse Merger. In the event of (i) a sale, lease or other disposition of all or substantially all of the assets of the Company, (ii) a merger or consolidation in which the Company is not the surviving corporation or (iii) a reverse merger in which the Company is the surviving corporation but the shares of Common Stock outstanding immediately preceding the merger are converted by virtue of the merger into other property, whether in the form of securities, cash or otherwise, then any surviving corporation or acquiring corporation may assume or continue any Stock Awards outstanding under the Plan or may substitute similar stock awards (including an award to acquire the same consideration paid to the stockholders in the transaction described in this subsection 12(c)) for those outstanding under the Plan. In the event any surviving corporation or acquiring corporation does not assume or continue such Stock Awards or substitute similar stock awards for those outstanding under the Plan, then, with respect to Stock Awards held by Participants whose Continuous Service has not terminated, the vesting of such Stock Awards (and, if applicable, the time during which such Stock Awards may be exercised) shall be accelerated in full, and the Stock Awards shall terminate if not exercised (if applicable) at or prior to such event. With respect to any other Stock Awards outstanding under the Plan, such Stock Awards shall terminate if not exercised (if applicable) prior to such event.
(d) Change in Control — Securities Acquisition. In the event of an acquisition by any person, entity or group within the meaning of Section 13(d) or 14(d) of the Exchange Act, or any comparable successor provisions (excluding any employee benefit plan, or related trust, sponsored or maintained by the Company or an Affiliate) of the beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Exchange Act, or comparable successor rule) of securities of the Company representing at least fifty percent (50%) of the combined voting power entitled to vote in the election of Directors, then with respect to Stock Awards held by Participants whose Continuous Service has not terminated, the vesting of such Stock Awards (and, if applicable, the time during which such Stock Awards may be exercised) shall be accelerated in full; provided, however, that this subsection 12(d) shall not apply if the securities acquisition described in this subsection 12(d) is the result of or also constitutes a transaction described in subsection 12(c) above, in which case the provisions of subsection 12(c) shall apply.
(e) Change in Control — Change in Incumbent Board. In the event that the individuals who, as of the date of the adoption of this Plan, are members of the Board (the “Incumbent Board”), cease for any reason to constitute at least fifty percent (50%) of the Board, then with respect to Stock Awards held by persons whose Continuous Service has not terminated, the vesting of such Stock Awards (and, if applicable, the time during which such Stock Awards may be exercised) shall be accelerated in full; provided, however, that this subsection 12(e) shall not apply if the change in the Incumbent Board described in this subsection 12(e) occurs solely as a result of and/or following a transaction described in subsection 12(c), in which case the provisions of subsection 12(c) shall apply. If the election, or nomination for election, by the Company’s stockholders of any new Director was approved by a vote of at least fifty percent (50%) of the Incumbent Board, such new Director shall be considered as a member of the Incumbent Board.
(f) Individual Agreements — Asset Sale, Merger, Consolidation, Reverse Merger or Change in Control. Notwithstanding the foregoing or any other provision of this Plan, the provisions of this Section 12 shall not apply to Stock Awards if otherwise provided in a written agreement between the Company or any Affiliate and the holder of the Stock Award. A Stock Award may be subject to additional acceleration of vesting and exercisability as may be provided in the Stock Award Agreement or as may be provided in any other written agreement between the Company or any Affiliate and the Participant.
| |
| 13. | Amendment of the Plan and Stock Awards. |
(a) Amendment of Plan. The Board at any time, and from time to time, may amend the Plan. However, except as provided in Section 12 relating to adjustments upon changes in Common Stock, no amendment shall be effective unless approved by the stockholders of the Company to the extent stockholder approval is
12
necessary pursuant to the terms of the Plan or necessary to satisfy the requirements of Section 422 of the Code, Rule 16b-3 or any Nasdaq or securities exchange listing requirements.
(b) Stockholder Approval. The Board may, in its sole discretion, submit any other amendment to the Plan for stockholder approval, including, but not limited to, amendments to the Plan intended to satisfy the requirements of Section 162(m) of the Code and the regulations thereunder regarding the exclusion of performance-based compensation from the limit on corporate deductibility of compensation paid to certain executive officers.
(c) Contemplated Amendments. It is expressly contemplated that the Board may amend the Plan in any respect the Board deems necessary or advisable to provide eligible Employees with the maximum benefits provided or to be provided under the provisions of the Code and the regulations promulgated thereunder relating to Incentive Stock Options and/or to bring the Plan and/or Incentive Stock Options granted under it into compliance therewith.
(d) No Impairment of Rights. Rights under any Stock Award granted before amendment of the Plan shall not be impaired by any amendment of the Plan unless (i) the Company requests the consent of the Participant and (ii) the Participant consents in writing.
(e) Amendment of Stock Awards. Subject to Section 6(c), 7(b)(i) and 11(g), the Board at any time, and from time to time, may amend the terms of any one or more Stock Awards; provided, however, that the rights under any Stock Award shall not be impaired by any such amendment unless (i) the Company requests the consent of the Participant and (ii) the Participant consents in writing. Notwithstanding the foregoing, subject to Section 6(c), 7(b)(i) and 11(g), any action by the Board to (A) reduce the exercise price of outstanding Options previously granted, (B) cancel outstanding Options and replace them with Options with a lower exercise price, or (C) effect an exchange of outstanding Options for new Options with a lower exercise price, shall be effective only if approved by the Company’s stockholders, unless taken pursuant to subsection 12(a), in connection a transaction described in subsection 12(c) or otherwise in a manner that would satisfy the provisions of Section 424(a) of the Code or regulations promulgated thereunder.
| |
| 14. | Termination or Suspension of the Plan. |
(a) Plan Term. The Board may suspend or terminate the Plan at any time. Unless sooner terminated, the Plan shall terminate on the day before the tenth (10th) anniversary of the date the Plan is adopted by the Board or approved by the stockholders of the Company, whichever is earlier. No Stock Awards may be granted under the Plan while the Plan is suspended or after it is terminated.
(b) No Impairment of Rights. Suspension or termination of the Plan shall not impair rights and obligations under any Stock Award granted while the Plan is in effect, except with the written consent of the Participant.
| |
| 15. | Effective Date of Plan. |
The Plan shall become effective on the date the Plan is approved by the stockholders, which approval shall be within twelve (12) months before or after the date the Plan is adopted by the Board.
The law of the State of California shall govern all questions concerning the construction, validity and interpretation of this Plan, without regard to such state’s conflict of laws rules.
13
ANNEX H
FRENCH ANNEX TO
EPIMMUNE INC. 2000 STOCK PLAN
The following additional rules shall apply to Qualified Options:
| |
| | 1. Purpose of the Sub-Plan |
| |
| | a. The Company has adopted this addendum (the “Sub-Plan”) to the Plan, so that options granted to a French Beneficiary shall be provided with the benefits applicable to stock options complying with French law, and to provide incentives for such French Beneficiary to exert maximum efforts for the success of the Company. |
| |
| | b. The Company intends that options granted pursuant to the Sub-Plan shall qualify for the favorable tax and social security treatment applicable to stock options that comply with Articles L 225-177 to L 225-186 of the French Commercial Code, and the relevant public rulings released by the French tax and social authorities. In the event of any conflict between the provisions of the Plan and the provisions of the Sub-Plan, the provisions of the Sub-Plan will apply. |
| |
| | c. Additional terms and conditions provided by this Sub-Plan are specific to a French Beneficiary (and their successors in interest) only and do not affect the rights afforded to any other Employee, Director or Consultant of the Company or any Affiliate who has been or may be granted an option under the Plan. |
| |
| | a. “Qualified Option” means any Option granted to a French Beneficiary, if such Option is intended by the Board or Committee to be eligible for the French tax and social security treatment applicable to stock options granted under article L.225-177 and seq. of the French commercial code (Code de Commerce) and as set forth under the French tax code (Code Général des Impôts) and the French social security code (Code de la Sécurité Sociale). |
| |
| | b. “French Affiliate” means an Affiliate of the Company that is a Subsidiary of the Company organized under the laws of France. |
| |
| | c. “French Director” means the Chairman (Président du Conseil d’administration) or the CEO (directeur général ou directeur général délégué) or a member of the Directorate (Directoire) or a manager (Gérant) of a French Affiliate. |
| |
| | d. “French Employee”means a person who is employed pursuant to an employment contract by a French Affiliate. |
| |
| | e. “French Beneficiary” means any person (i) who is a resident of France on the date of grant for tax and social security purposes, (ii) who does not hold more than 10% of the share capital of the Company on the date of grant and (iii) who is a French Director or French Employee of a French Affiliate of the Company on the date of the grant. |
| |
| | f. “Date of Grant” means the date the Board or Committee approves the grant of a Qualified Option. |
| |
| | g. “Subsidiary” means (i) if the shares of the Company are not listed on a public stock exchange on the date of grant, a company whose 10% of the share capital or voting rights are held directly or indirectly by the Company and (ii) if the shares of the Company are listed on a public stock exchange on the date of grant, a company whose 10% of the share capital or voting rights are held directly or indirectly by the Company or a company holding directly or indirectly 10% of the share capital or voting rights of the Company or a company whose at least 50% of the share capital or voting rights are held directly or indirectly by a company holding itself directly or indirectly at least 50% of the capital or voting rights of the Company. |
| |
| | 3. Amendment. Following the Date of Grant, the terms of a Qualified Option may not be amended except as may be permitted by applicable French law. |
| |
| | 4. Share Limitations. The total number of shares of Common Stock underlying Qualified Options awarded, including outstanding Qualified Options from any previous award by the Company, cannot exceed one-third of the share capital on the Date of Grant. |
| |
| | 5. Term. Unless the Option Agreement provides for a shorter duration, in the case of a Qualified Option, the term will be ten years from the Date of Grant. |
| |
| | 6. Date of Grant. A Qualified Option may not be granted during the time periods specified in Article L 225-177 of the French commercial code. |
| |
| | 7. Nature of Options. Any grant of Qualified Options shall be separately designated as (i) Options to acquire existing shares of Common Stock or (ii) Options to subscribe shares of Common Stock to be issued upon exercise of the Options. |
| |
| | 8. Exercise Price. The per share exercise price of a Qualified Option shall be not less than 100% of the Fair Market Value of the Common Stock subject to the Qualified Option on the date the Qualified Option is granted. Additionally, the per share exercise price of a Qualified Option shall not be less than (a) 80% of the average opening Share price on the 20 trading days preceding the Date of Grant, if the Common Stock is admitted to trading on a regulated stock market, and (b) as determined by the Administrator according to objective methods used for the valuation of the shares, taking into account the Company’s net accounting situation, profitability and business prospects by means of an appropriate weighting in each case, if the Common Stock is not admitted to trading on a regulated stock market. These criteria shall be assessed on a consolidated basis if necessary or, failing that, by taking into account the financial statements issued by any significant subsidiaries. In the event these methods cannot be used, the share price shall be determined by dividing the total amount of the revalued net assets, calculated according to the most recent balance sheet, by the number of existing shares. Notwithstanding the preceding, with respect to Qualified Options to purchase existing shares (as opposed to newly issued shares), and in addition to the above limits, the per share exercise price must be no less than 80% of the average share price of the shares repurchased by the Company in order to satisfy the exercise of Qualified Options. |
| |
| | 9. Transferability. Qualified Options shall not be transferable except by will or by the laws of descent and distributions and shall be exercisable during the lifetime of the Optionholder only by the Optionholder. |
| |
| | 10. Death of Optionholder. If an Optionholder’s Continuous Service terminates due to death, the Option may be exercised following the Optionholder’s death within such period of time as is specified in the Option Agreement to the extent that the Option is vested on the date of death (but in no event may the Option be exercised later than the earlier of (a) six months following the date of death, and (b) the expiration of the term of the Option as set forth in the Option Agreement), by the Optionholder’s designated beneficiary, provided such beneficiary has been designated prior to the Optionholder’s death in a form acceptable to the Company, or, in the absence of such a designation, by the Optionholder’s heirs. |
| |
| | 11. Lock-Up Period. In the event of an exercise of Qualified Options prior to the fourth (4th) anniversary of the Date of Grant(1), any shares of Common Stock received upon exercise of such Qualified Options shall be held in a separate account with a Company approved brokerage firm and may not be sold, assigned, donated or otherwise disposed of until the fourth (4th) anniversary of the Date of Grant1. However, this “Lock-Up Period” shall not apply in the case of death or disability (as defined under in the |
(1) The sale of the shares resulting from the exercise of the options can not be restricted for a period longer than three years as from the date of the exercise of the option. Therefore, in order that the lock-up period is four years from the date of the grant, it should be ensured that the options can not be vested during the first year from the date of the grant.
2
second and third categories of Articles L 341-4 of the French social security code) of the Optionholder. In addition, in the event of retirement or dismissal (as both terms are defined under applicable law) of the Optionholder, the Lock-Up Period shall not apply to any shares of Common Stock received upon exercise of Qualified Options, provided that the relevant Options were exercised at least three months prior to the date of retirement or dismissal, as the case may be. The Board shall have the discretionary authority to extend, accelerate or reduce any Lock-Up Period.
| |
| | 12. Adjustments. Adjustments pursuant to Section 12(a) of the Plan shall apply to Qualified Options, to the extent allowed by French law. Additionally, the Board shall make any adjustment as to the number and/or exercise price of Qualified Options, and/or to the number of shares covered by Qualified Options in the event of changes in the share capital of the Company in compliance with article L.225-181 of the French commercial code (Code de Commerce) and any other French regulations applicable to stock options granted under article L.225-177 and seq. of the French commercial code, including any future amendments of, or additions to, such regulations, if any. |
| |
| | 13. Asset Sale, Merger, Consolidation or Reverse Merger. Sections 12(c) through 12(e) of the Plan shall apply to Qualified Options unless the Board determines otherwise in its sole discretion. In the event that Sections 12(c), 12(d) and/or 12(e) of the Plan are applied to the Qualified Options, the Lock-Up Period provisions set forth in Section 10 of this Sub-Plan shall continue to apply to the Qualified Options unless the Board determines otherwise in its sole discretion. |
| |
| | 14. Sub-Plan Term. The Sub-Plan shall terminate on the day immediately preceding the commencement of the 39th month following the date the Sub-Plan is adopted by the Board or approved by the stockholders of the Company, whichever is earlier. Notwithstanding the foregoing, the Board may suspend or terminate the Sub-Plan at any time. No Qualified Options may be granted under the Sub-Plan while the Sub-Plan is suspended or after it is terminated. However, suspension or termination of the Sub-Plan shall not impair rights and obligations under any Qualified Options granted while the Sub-Plan is in effect, except with the written consent of the French Beneficiary. |
3
ANNEX I
EPIMMUNE INC.
2001 EMPLOYEE STOCK PURCHASE PLAN
Adopted by the Board of Directors March 5, 2001
Approved by Stockholders June 18, 2001
Amended by the Board of Directors March 15, 2005
(a) The purpose of the Plan is to provide a means by which Employees of the Company and certain designated Related Corporations may be given an opportunity to purchase shares of the Common Stock of the Company.
(b) The Company, by means of the Plan, seeks to retain the services of such Employees, to secure and retain the services of new Employees and to provide incentives for such persons to exert maximum efforts for the success of the Company and its Related Corporations.
(c) The Company intends that the Purchase Rights granted under the Plan be considered options issued under an Employee Stock Purchase Plan.
(a) “Board”means the Board of Directors of the Company.
(b) “Code”means the Internal Revenue Code of 1986, as amended.
(c) “Committee”means a committee appointed by the Board in accordance with Section 3(c) of the Plan.
(d) “Common Stock”means the common stock of the Company.
(e) “Company”means Epimmune Inc., a Delaware corporation.
(f) “Corporate Transaction”means the occurrence, in a single transaction or in a series of related transactions, of any one or more of the following events:
| |
| | (i) a sale, lease, license or other disposition of all or substantially all of the consolidated assets of the Company; |
| |
| | (ii) a sale or other disposition of at least ninety percent (90%) of the outstanding securities of the Company; |
| |
| | (iii) a merger, consolidation or similar transaction following which the Company is not the surviving corporation; or |
| |
| | (iv) a merger, consolidation or similar transaction following which the Company is the surviving corporation but the shares of Common Stock outstanding immediately preceding the merger, consolidation or similar transaction are converted or exchanged by virtue of the merger, consolidation or similar transaction into other property, whether in the form of securities, cash or otherwise. |
(g) “Director”means a member of the Board.
(h) “Eligible Employee”means an Employee who meets the requirements set forth in the Offering for eligibility to participate in the Offering, provided that such Employee also meets the requirements for eligibility to participate set forth in the Plan.
(i) “Employee”means any person, including Officers and Directors, who is employed for purposes of Section 423(b)(4) of the Code by the Company or a Related Corporation. Neither service as a Director nor
1.
payment of a director’s fee shall be sufficient to make an individual an Employee of the Company or a Related Corporation.
(j) “Employee Stock Purchase Plan”means a plan that grants Purchase Rights intended to be options issued under an “employee stock purchase plan,” as that term is defined in Section 423(b) of the Code.
(k) “Exchange Act”means the Securities Exchange Act of 1934, as amended.
(l) “Fair Market Value”means the value of a security, as determined in good faith by the Board. If the security is listed on any established stock exchange or traded on the Nasdaq National Market or the Nasdaq SmallCap Market, the Fair Market Value of the security, unless otherwise determined by the Board, shall be the closing sales price (rounded up where necessary to the nearest whole cent) for such security (or the closing bid, if no sales were reported) as quoted on such exchange or market (or the exchange or market with the greatest volume of trading in the relevant security of the Company) on the Trading Day prior to the relevant determination date, as reported inThe Wall Street Journalor such other source as the Board deems reliable.
(m) “Offering”means the grant of Purchase Rights under the Plan to Eligible Employees.
(n) “Offering Date”means a date selected by the Board for an Offering to commence.
(o) “Officer”means a person who is an officer of the Company within the meaning of Section 16 of the Exchange Act and the rules and regulations promulgated thereunder.
(p) “Participant”means an Eligible Employee who holds an outstanding Purchase Right granted pursuant to the Plan.
(q) “Plan”means this Epimmune Inc. 2001 Employee Stock Purchase Plan.
(r) “Purchase Date”means one or more dates during an Offering established by the Board on which Purchase Rights granted under the Plan shall be exercised and as of which purchases of shares of Common Stock shall be carried out in accordance with such Offering.
(s) “Purchase Period”means a period of time specified within an Offering beginning on the Offering Date or on the next day following a Purchase Date within an Offering and ending on a Purchase Date, at the end of which there shall be purchased shares of Common Stock on behalf of Participants. An Offering may consist of one or more Purchase Periods.
(t) “Purchase Right”means an option to purchase shares of Common Stock granted pursuant to the Plan.
(u) “Related Corporation”means, with respect to the Company, any parent corporation or subsidiary corporation, whether now or hereafter existing, as those terms are defined in Sections 424(e) and (f), respectively, of the Code.
(v) “Securities Act”means the Securities Act of 1933, as amended.
(w) “Trading Day”means any day the exchange(s) or market(s) on which shares of Common Stock are listed, whether it be any established stock exchange, the Nasdaq National Market, the Nasdaq SmallCap Market or otherwise, is open for trading.
(a) The Board shall administer the Plan unless and until the Board delegates administration to a Committee, as provided in Section 3(c). Whether or not the Board has delegated administration, the Board shall have the final power to determine all questions of policy and expediency that may arise in the administration of the Plan.
2.
(b) The Board (or the Committee) shall have the power, subject to, and within the limitations of, the express provisions of the Plan:
| |
| | (i) To determine when and how Purchase Rights to purchase shares of Common Stock shall be granted and the provisions of each Offering of such Purchase Rights (which need not be identical). |
| |
| | (ii) To designate from time to time which Related Corporations of the Company shall be eligible to participate in the Plan. |
| |
| | (iii) To construe and interpret the Plan and Purchase Rights granted under the Plan, and to establish, amend and revoke rules and regulations for the administration of the Plan. The Board, in the exercise of this power, may correct any defect, omission or inconsistency in the Plan, in a manner and to the extent it shall deem necessary or expedient to make the Plan fully effective. |
| |
| | (iv) To amend the Plan as provided in Section 15. |
| |
| | (v) Generally, to exercise such powers and to perform such acts as it deems necessary or expedient to promote the best interests of the Company and its Related Corporations and to carry out the intent that the Plan be treated as an Employee Stock Purchase Plan. |
(c) The Board may delegate administration of the Plan to a Committee of the Board composed of one (1) or more members of the Board. If administration is delegated to a Committee, the Committee shall have, in connection with the administration of the Plan, the powers theretofore possessed by the Board, subject, however, to such resolutions, not inconsistent with the provisions of the Plan, as may be adopted from time to time by the Board. The Board may abolish the Committee at any time and revest in the Board the administration of the Plan. If administration is delegated to a Committee, references to the Board in this Plan and in the Offering document shall thereafter be deemed to be to the Board or the Committee, as the case may be.
| |
| 4. | Shares of Common Stock Subject to the Plan. |
(a) Subject to the provisions of Section 14 relating to adjustments upon changes in securities, the shares of Common Stock that may be sold pursuant to Purchase Rights granted under the Plan shall not exceed in the aggregate four hundred eighty-five thousand (485,000) shares of Common Stock. If any Purchase Right granted under the Plan shall for any reason terminate without having been exercised, the shares of Common Stock not purchased under such Purchase Right shall again become available for issuance under the Plan.
(b) The shares of Common Stock subject to the Plan may be unissued shares or shares that have been bought on the open market at prevailing market prices or otherwise.
| |
| 5. | Grant of Purchase Rights; Offering. |
(a) The Board may from time to time grant or provide for the grant of Purchase Rights under the Plan to Eligible Employees in an Offering (consisting of one or more Purchase Periods) on an Offering Date or Offering Dates selected by the Board. Each Offering shall be in such form and shall contain such terms and conditions as the Board shall deem appropriate, which shall comply with the requirement of Section 423(b)(5) of the Code that all Employees granted Purchase Rights under the Plan shall have the same rights and privileges. The terms and conditions of an Offering shall be incorporated by reference into the Plan and treated as part of the Plan. The provisions of separate Offerings need not be identical, but each Offering shall include (through incorporation of the provisions of this Plan by reference in the document comprising the Offering or otherwise) the period during which the Offering shall be effective, which period shall not exceed twenty-seven (27) months beginning with the Offering Date, and the substance of the provisions contained in Sections 6 through 9, inclusive.
(b) If a Participant has more than one Purchase Right outstanding under the Plan, unless he or she otherwise indicates in agreements or notices delivered hereunder: (i) each agreement or notice delivered by that Participant shall be deemed to apply to all of his or her Purchase Rights under the Plan, and (ii) a Purchase Right with a lower exercise price (or an earlier-granted Purchase Right, if different Purchase Rights
3.
have identical exercise prices) shall be exercised to the fullest possible extent before a Purchase Right with a higher exercise price (or a later-granted Purchase Right, if different Purchase Rights have identical exercise prices) shall be exercised.
(a) Purchase Rights may be granted only to Employees of the Company or, as the Board may designate as provided in Section 3(b), to Employees of a Related Corporation. Except as provided in Section 6(b), an Employee shall not be eligible to be granted Purchase Rights under the Plan unless, on the Offering Date, such Employee has been in the employ of the Company or the Related Corporation, as the case may be, for such continuous period preceding such Offering Date as the Board may require, but in no event shall the required period of continuous employment be greater than two (2) years. In addition, the Board may provide that no Employee shall be eligible to be granted Purchase Rights under the Plan unless, on the Offering Date, such Employee’s customary employment with the Company or the Related Corporation is more than twenty (20) hours per week and more than five (5) months per calendar year.
(b) The Board may provide that each person who, during the course of an Offering, first becomes an Eligible Employee shall, on a date or dates specified in the Offering which coincides with the day on which such person becomes an Eligible Employee or which occurs thereafter, receive a Purchase Right under that Offering, which Purchase Right shall thereafter be deemed to be a part of that Offering. Such Purchase Right shall have the same characteristics as any Purchase Rights originally granted under that Offering, as described herein, except that:
| |
| | (i) the date on which such Purchase Right is granted shall be the “Offering Date” of such Purchase Right for all purposes, including determination of the exercise price of such Purchase Right; |
| |
| | (ii) the period of the Offering with respect to such Purchase Right shall begin on its Offering Date and end coincident with the end of such Offering; and |
| |
| | (iii) the Board may provide that if such person first becomes an Eligible Employee within a specified period of time before the end of the Offering, he or she shall not receive any Purchase Right under that Offering. |
(c) No Employee shall be eligible for the grant of any Purchase Rights under the Plan if, immediately after any such Purchase Rights are granted, such Employee owns stock possessing five percent (5%) or more of the total combined voting power or value of all classes of stock of the Company or of any Related Corporation. For purposes of this Section 6(c), the rules of Section 424(d) of the Code shall apply in determining the stock ownership of any Employee, and stock which such Employee may purchase under all outstanding Purchase Rights and options shall be treated as stock owned by such Employee.
(d) As specified by Section 423(b)(8) of the Code, an Eligible Employee may be granted Purchase Rights under the Plan only if such Purchase Rights, together with any other rights granted under all Employee Stock Purchase Plans of the Company and any Related Corporations, do not permit such Eligible Employee’s rights to purchase stock of the Company or any Related Corporation to accrue at a rate which exceeds twenty five thousand dollars ($25,000) of Fair Market Value of such stock (determined at the time such rights are granted, and which, with respect to the Plan, shall be determined as of their respective Offering Dates) for each calendar year in which such rights are outstanding at any time.
(e) Officers of the Company and any designated Related Corporation, if they are otherwise Eligible Employees, shall be eligible to participate in Offerings under the Plan. Notwithstanding the foregoing, the Board may provide in an Offering that Employees who are highly compensated Employees within the meaning of Section 423(b)(4)(D) of the Code shall not be eligible to participate.
| |
| 7. | Purchase Rights; Purchase Price. |
(a) On each Offering Date, each Eligible Employee, pursuant to an Offering made under the Plan, shall be granted a Purchase Right to purchase up to that number of shares of Common Stock purchasable either
4.
with a percentage or with a maximum dollar amount, as designated by the Board, but in either case not exceeding fifteen percent (15%), of such Employee’s Earnings (as defined by the Board in each Offering) during the period that begins on the Offering Date (or such later date as the Board determines for a particular Offering) and ends on the date stated in the Offering, which date shall be no later than the end of the Offering.
(b) The Board shall establish one (1) or more Purchase Dates during an Offering as of which Purchase Rights granted under the Plan and pursuant to that Offering shall be exercised and purchases of shares of Common Stock shall be carried out in accordance with such Offering.
(c) In connection with each Offering made under the Plan, the Board may specify a maximum number of shares of Common Stock that may be purchased by any Participant on any Purchase Date during such Offering. In connection with each Offering made under the Plan, the Board may specify a maximum aggregate number of shares of Common Stock that may be purchased by all Participants pursuant to such Offering. In addition, in connection with each Offering that contains more than one Purchase Date, the Board may specify a maximum aggregate number of shares of Common Stock that may be purchased by all Participants on any given Purchase Date under the Offering. If the aggregate purchase of shares of Common Stock issuable upon exercise of Purchase Rights granted under the Offering would exceed any such maximum aggregate number, then, in the absence of any Board action otherwise, a pro rata allocation of the shares of Common Stock available shall be made in as nearly a uniform manner as shall be practicable and equitable.
(d) The purchase price of shares of Common Stock acquired pursuant to Purchase Rights granted under the Plan shall be not less than the lesser of:
| |
| | (i) an amount equal to eighty-five percent (85%) of the Fair Market Value of the shares of Common Stock on the Offering Date; or |
| |
| | (ii) an amount equal to eighty-five percent (85%) of the Fair Market Value of the shares of Common Stock on the applicable Purchase Date. |
| |
| 8. | Participation; Withdrawal; Termination. |
(a) An Eligible Employee may become a Participant in the Plan pursuant to an Offering by delivering a participation agreement to the Company within the time specified in the Offering, in such form as the Company may provide. Each such agreement shall authorize payroll deductions of up to the maximum percentage specified by the Board of such Participant’s Earnings (as defined in each Offering) during the Offering. The payroll deductions made for each Participant shall be credited to a bookkeeping account for such Participant under the Plan and shall be deposited with the general funds of the Company except where applicable law requires that the payroll deductions be deposited with a third party. To the extent provided in the Offering, a Participant may reduce (including to zero) or increase such payroll deductions. To the extent provided in the Offering, a Participant may begin such payroll deductions after the beginning of the Offering. A Participant may make additional payments into his or her account only if specifically provided for in the Offering and only if the Participant has not already had the maximum permitted amount withheld during the Offering.
(b) At any time during an Offering, a Participant may terminate his or her payroll deductions under the Plan and withdraw from the Offering by delivering to the Company a notice of withdrawal in such form as the Company may provide. Such withdrawal may be elected at any time prior to the end of the Offering, except as provided in the Offering. Upon such withdrawal from the Offering by a Participant, the Company shall distribute to such Participant all of his or her accumulated payroll deductions (reduced to the extent, if any, such deductions have been used to acquire shares of Common Stock for the Participant) under the Offering, without interest (unless otherwise specified in the Offering), and such Participant’s interest in that Offering shall be automatically terminated. A Participant’s withdrawal from an Offering shall have no effect upon such Participant’s eligibility to participate in any other Offerings under the Plan, but such Participant shall be required to deliver a new participation agreement in order to participate in subsequent Offerings under the Plan.
5.
(c) Purchase Rights granted pursuant to any Offering under the Plan shall terminate immediately upon a Participant ceasing to be an Employee for any reason or for no reason (subject to any post-employment participation period required by law) or other lack of eligibility. The Company shall distribute to such terminated or otherwise ineligible Employee all of his or her accumulated payroll deductions (reduced to the extent, if any, such deductions have been used to acquire shares of Common Stock for the terminated or otherwise ineligible Employee) under the Offering, without interest (unless otherwise specified in the Offering).
(d) Purchase Rights granted under the Plan shall not be transferable by a Participant otherwise than by will or the laws of descent and distribution, or by a beneficiary designation as provided in Section 13 and, during a Participant’s lifetime, shall be exercisable only by such Participant.
(a) On each Purchase Date during an Offering, each Participant’s accumulated payroll deductions and other additional payments specifically provided for in the Offering (without any increase for interest) shall be applied to the purchase of shares of Common Stock up to the maximum number of shares of Common Stock permitted pursuant to the terms of the Plan and the applicable Offering, at the purchase price specified in the Offering. No fractional shares shall be issued upon the exercise of Purchase Rights granted under the Plan unless specifically provided for in the Offering.
(b) If any amount of accumulated payroll deductions remains in a Participant’s account after the purchase of shares of Common Stock and such remaining amount is less than the amount required to purchase one share of Common Stock on the final Purchase Date of an Offering, then such remaining amount shall be held in each such Participant’s account for the purchase of shares of Common Stock under the next Offering under the Plan, unless such Participant withdraws from such next Offering, as provided in Section 8(b), or is not eligible to participate in such Offering, as provided in Section 6, in which case such amount shall be distributed to the Participant after said final Purchase Date, without interest (unless otherwise specified in the Offering). If any amount, of accumulated payroll deductions remains in a Participant’s account after the purchase of shares of Common Stock and such remaining amount is equal to the amount required to purchase one (1) or more whole shares of Common Stock on the final Purchase Date of the Offering, then such remaining amount shall be distributed in full to the Participant at the end of the Offering without interest (unless otherwise specified in the Offering).
(c) No Purchase Rights granted under the Plan may be exercised to any extent unless the shares of Common Stock to be issued upon such exercise under the Plan are covered by an effective registration statement pursuant to the Securities Act and the Plan is in material compliance with all applicable federal, state, foreign and other securities and other laws applicable to the Plan. If on a Purchase Date during any Offering hereunder the shares of Common Stock are not so registered or the Plan is not in such compliance, no Purchase Rights granted under the Plan or any Offering shall be exercised on such Purchase Date, and the Purchase Date shall be delayed until the shares of Common Stock are subject to such an effective registration statement and the Plan is in such compliance, except that the Purchase Date shall not be delayed more than twelve (12) months and the Purchase Date shall in no event be more than twenty-seven (27) months from the Offering Date. If, on the Purchase Date under any Offering hereunder, as delayed to the maximum extent permissible, the shares of Common Stock are not registered and the Plan is not in such compliance, no Purchase Rights granted under the Plan or any Offering shall be exercised and all payroll deductions accumulated during the Offering (reduced to the extent, if any, such deductions have been used to acquire shares of Common Stock) shall be distributed to the Participants, without interest (unless otherwise specified in the Offering).
| |
| 10. | Covenants of the Company. |
(a) During the terms of the Purchase Rights granted under the Plan, the Company shall ensure that the amount of shares of Common Stock required to satisfy such Purchase Rights are available.
6.
(b) The Company shall seek to obtain from each federal, state, foreign or other regulatory commission or agency having jurisdiction over the Plan such authority as may be required to issue and sell shares of Common Stock upon exercise of the Purchase Rights granted under the Plan. If, after reasonable efforts, the Company is unable to obtain from any such regulatory commission or agency the authority that counsel for the Company deems necessary for the lawful issuance and sale of shares of Common Stock under the Plan, the Company shall be relieved from any liability for failure to issue and sell shares of Common Stock upon exercise of such Purchase Rights unless and until such authority is obtained.
| |
| 11. | Use of Proceeds from Shares of Common Stock. |
Proceeds from the sale of shares of Common Stock pursuant to Purchase Rights granted under the Plan shall constitute general funds of the Company.
| |
| 12. | Rights as a Stockholder. |
A Participant shall not be deemed to be the holder of, or to have any of the rights of a holder with respect to, shares of Common Stock subject to Purchase Rights granted under the Plan unless and until the Participant’s shares of Common Stock acquired upon exercise of Purchase Rights granted under the Plan are recorded in the books of the Company (or its transfer agent).
| |
| 13. | Designation of Beneficiary. |
(a) A Participant may file a written designation of a beneficiary who is to receive any shares of Common Stock and/or cash, if any, from the Participant’s account under the Plan in the event of such Participant’s death subsequent to the end of an Offering but prior to delivery to the Participant of such shares of Common Stock or cash. In addition, a Participant may file a written designation of a beneficiary who is to receive any cash from the Participant’s account under the Plan in the event of such Participant’s death during an Offering.
(b) The Participant may change such designation of beneficiary at any time by written notice. In the event of the death of a Participant and in the absence of a beneficiary validly designated under the Plan who is living at the time of such Participant’s death, the Company shall deliver such shares of Common Stock and/or cash to the executor or administrator of the estate of the Participant, or if no such executor or administrator has been appointed (to the knowledge of the Company), the Company, in its sole discretion, may deliver such shares of Common Stock and/or cash to the spouse or to any one or more dependents or relatives of the Participant, or if no spouse, dependent or relative is known to the Company, then to such other person as the Company may designate.
| |
| 14. | Adjustments upon Changes in Securities; Corporate Transactions. |
(a) If any change is made in the shares of Common Stock, subject to the Plan, or subject to any Purchase Right, without the receipt of consideration by the Company (through merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or other transaction not involving the receipt of consideration by the Company), the Plan shall be appropriately adjusted in the type(s), class(es) and maximum number of shares of Common Stock subject to the Plan pursuant to Section 4(a), and the outstanding Purchase Rights granted under the Plan shall be appropriately adjusted in the type(s), class(es), number of shares and purchase limits of such outstanding Purchase Rights. The Board shall make such adjustments, and its determination shall be final, binding and conclusive. (The conversion of any convertible securities of the Company shall not be treated as a “transaction not involving the receipt of consideration by the Company.”)
(b) In the event of a Corporate Transaction, then: (i) any surviving or acquiring corporation may continue or assume Purchase Rights outstanding under the Plan or may substitute similar rights (including a right to acquire the same consideration paid to stockholders in the Corporate Transaction) for those outstanding under the Plan, or (ii) if any surviving or acquiring corporation does not assume such Purchase Rights or does not substitute similar rights for Purchase Rights outstanding under the Plan, then, the
7.
Participants’ accumulated payroll deductions (exclusive of any accumulated interest that cannot be applied toward the purchase of shares of Common Stock under the terms of the Offering) shall be used to purchase shares of Common Stock within five (5) business days prior to the Corporate Transaction under the ongoing Offering, and the Participants’ Purchase Rights under the ongoing Offering shall terminate immediately after such purchase.
| |
| 15. | Amendment of the Plan. |
(a) The Board at any time, and from time to time, may amend the Plan. However, except as provided in Section 14 relating to adjustments upon changes in securities and except as to amendments solely to benefit the administration of the Plan, to take account of a change in legislation or to obtain or maintain favorable tax, financial accounting, exchange control or regulatory treatment for Participants or the Company or any Related Corporation, no amendment shall be effective unless approved by the stockholders of the Company to the extent stockholder approval is necessary for the Plan to satisfy the requirements of Section 423 of the Code or other applicable laws or regulations.
(b) It is expressly contemplated that the Board may amend the Plan in any respect the Board deems necessary or advisable to provide Employees with the maximum benefits provided or to be provided under the provisions of the Code and the regulations promulgated thereunder relating to Employee Stock Purchase Plans and/or to bring the Plan and/or Purchase Rights granted under the Plan into compliance therewith.
(c) The rights and obligations under any Purchase Rights granted before amendment of the Plan shall not be impaired by any amendment of the Plan except: (i) with the consent of the person to whom such Purchase Rights were granted, (ii) as necessary to comply with any laws or governmental regulations (including, without limitation, the provisions of the Code and the regulations promulgated thereunder relating to Employee Stock Purchase Plans), or (iii) as necessary to obtain or maintain favorable financial accounting treatment with respect to such Purchase Rights.
| |
| 16. | Termination or Suspension of the Plan. |
(a) The Board in its discretion may suspend or terminate the Plan at any time. Unless sooner terminated, the Plan shall terminate at the time that all of the shares of Common Stock reserved for issuance under the Plan, as increased and/or adjusted from time to time, have been issued under the terms of the Plan. No Purchase Rights may be granted under the Plan while the Plan is suspended or after it is terminated.
(b) Any benefits, privileges, entitlements and obligations under any Purchase Rights granted under the Plan while the Plan is in effect shall not be impaired by suspension or termination of the Plan except (i) as expressly provided in the Plan or with the consent of the person to whom such Purchase Rights were granted, (ii) as necessary to comply with any laws, regulations, or listing requirements, or (iii) as necessary to ensure that the Plan and/or Purchase Rights granted under the Plan comply with the requirements of Section 423 of the Code.
| |
| 17. | Effective Date of Plan. |
The Plan shall become effective as determined by the Board, but no Purchase Rights granted under the Plan shall be exercised unless and until the Plan has been approved by the stockholders of the Company within twelve (12) months before or after the date the Plan is adopted by the Board.
| |
| 18. | Miscellaneous Provisions. |
(a) The Plan and Offering do not constitute an employment contract. Nothing in the Plan or in the Offering shall in any way alter the at will nature of a Participant’s employment or be deemed to create in any way whatsoever any obligation on the part of any Participant to continue in the employ of the Company or a Related Corporation, or on the part of the Company or a Related Corporation to continue the employment of a Participant.
(b) The provisions of the Plan shall be governed by the laws of the State of California without resort to that state’s conflicts of laws rules.
8.
ANNEX J
EPIMMUNE INC.
EMPLOYEE STOCK PURCHASE PLAN FOR EMPLOYEES OF IDM S.A.
ADOPTED BY THE BOARD OF DIRECTORS May 31, 2005
APPROVED BY STOCKHOLDERS
(a) The purpose of the Plan is to provide a means by which Employees of the Company may be given an opportunity to purchase shares of the Common Stock of Epimmune.
(b) Epimmune, by means of the Plan, seeks to retain the services of the Employees of the Company, to secure and retain the services of new Employees and to provide incentives for such persons to exert maximum efforts for the success of the Company and Epimmune.
(a) “BOARD” means the Board of Directors of Epimmune.
(b) “BOOKKEEPING ACCOUNT” means a bookkeeping account established in connection with each Offering for each Participant for purposes of recording the Contributions that have been made by the Participant during the Offering and the amount of interest accrued to the Contributions in accordance with Section 8(a) hereof.
(c) “COMMITTEE” means a committee appointed by the Board in accordance with Section 3(c) hereof.
(d) “COMMON STOCK” means the common stock, par value $0.01, of Epimmune.
(e) “COMPANY” means IDM S.A., asociété anonymeorganized under the laws of France, which will become a wholly owned subsidiary of Epimmune as of the Closing Date (as this term is defined in the Share Exchange Agreement), and any successor to IDM S.A.
(f) “COMPANY CONTRIBUTION” means the amount, if any, that is contributed, or to be contributed, by the Company, in accordance with the terms of the French Savings Plan, with respect to the Participant’s Contribution under an Offering, subsequent to the last day of the Offering Period applicable to the Offering and prior to the Purchase Date of such Offering, to be used for the purchase of shares of Common Stock under the Plan. The decision to make a Company Contribution and the determination of the amount of a Company Contribution shall be made in accordance with the French Savings Plan.
(g) “CONTRIBUTION” means the amount that is contributed, or to be contributed, by payroll deductions, cash or check payment, or such other contribution method as permitted under an Offering, by a Participant pursuant to the Participant’s Participation Agreement, to be used for the purchase of shares of Common Stock under the Plan.
(h) “CORPORATE TRANSACTION” means the occurrence, in a single transaction or in a series of related transactions, of any one or more of the following events:
| |
| | (i) a sale, lease, license or other disposition of all or substantially all of the consolidated assets of the Company or Epimmune; |
| |
| | (ii) a sale or other disposition of at least ninety percent (90%) of the outstanding securities of the Company or Epimmune; |
| |
| | (iii) a merger, consolidation or similar transaction following which the Company or Epimmune is not the surviving corporation; |
| |
| | (iv) a merger, consolidation or similar transaction following which the Company or Epimmune is the surviving corporation but the shares of Common Stock outstanding immediately preceding the merger, consolidation or similar transaction are converted or exchanged by virtue of the merger, consolidation or similar transaction into other property, whether in the form of securities, cash or otherwise; or |
| |
| | (v) any similar transaction pursuant to which the Company ceases to be a majority owned subsidiary of Epimmune. |
(i) “ELIGIBLE EMPLOYEE” means an Employee who meets the requirements set forth in an Offering for eligibility to participate in the Offering, provided that such Employee also meets the requirements for eligibility to participate set forth in the Plan.
(j) “ELIGIBLE EARNINGS” means, with respect to an Employee, the gross annual remuneration (as this concept is defined under French law) paid by the Company to such Employee.
(k) “EMPLOYEE” means any person who is employed by the Company or a Retiree. Neither service as a director nor payment of a director’s fee shall be sufficient to make an individual an Employee of the Company.
(l) “EPIMMUNE” means Epimmune Inc., a Delaware corporation.
(m) “FRENCH SAVINGS PLAN” means the savings plan (Plan d’épargne d’entreprise) to be adopted by the Company for the benefit of its Employees.
(n) “MAXIMUM AVAILABLE SHARES” means, for each Offering, the maximum number of shares of Common Stock approved by the Board for issuance to each Participant in connection with the Offering.
(o) “MAXIMUM PARTICIPANT CONTRIBUTION” means the maximum amount of Contributions that a Participant shall be permitted to make in connection with an Offering, which maximum amount shall be equal to 25% of the Participant’s Eligible Earnings; provided, however, that if the Board establishes a number of Maximum Available Shares in connection with an Offering, the Maximum Participant Contribution shall be equal to the lower of (i) 25% the Participant’s Eligible Earnings or (ii) an amount equal to the product of the per share purchase price of the Common Stock established by the Board in connection with the Offering, multiplied by the number of Maximum Available Shares.
(p) “OFFERING” means the grant of Purchase Rights under the Plan to Eligible Employees.
(q) “OFFERING DATE” means the date on which the Board determines the date on which the Offering commences, the length of the Offering Period and the Purchase Price.
(r) “OFFERING PERIOD” means the period of time specified by the Board on the Offering Date during which Eligible Employees will be able to participate in an Offering.
(s) “PARTICIPANT” means an Eligible Employee who has subscribed to purchase Common Stock under an Offering pursuant to the terms of the Plan.
(t) “PARTICIPATION AGREEMENT” means an agreement (either on paper or in electronic form) applicable to an Offering, providing for the Participant’s participation in an Offering and subscription for shares of Common Stock, that is in a form acceptable to the Board, as determined from time to time, and completed and filed by an Eligible Employee in accordance with such terms and conditions as the Board may establish for that purpose.
(u) “PLAN” means this Epimmune Inc. Employee Stock Purchase Plan for Employees of IDM S.A., as the same may be amended from time to time.
(v) “PURCHASE DATE” means the date established by the Board for the automatic exercise of the Participants’ Purchase Rights for the purchase of that number of shares of Common Stock subscribed for under the Participation Agreement, using the amount in the Participant’s Savings Account on behalf of Participants who have not revoked their Participation Agreements in accordance with Section 9 hereof.
2
Except as provided in Section 9(c) hereof, the Purchase Date shall be a date that is within the period commencing on the last day of the Offering Period and ending 15 days thereafter.
(w) “PURCHASE RIGHT” means a right to purchase shares of Common Stock granted pursuant to the Plan.
(x) “RETIREE” means an individual who was retired from employment with the Company who has contributed to the French Savings Plan prior to the commencement of an Offering and has not requested the liquidation of the assets held on behalf of the Retiree under the French Savings Plan.
(y) “SAVINGS ACCOUNT” means an account established for each Participant under the French Savings Plan that records the aggregate amount of accumulated Contributions that have been transferred from the Participant’s Bookkeeping Account to, and Company Contributions, if any, that have been made and deposited on behalf of the Participant with, the Savings Plan, following the expiration of the Offering Period and prior to the Purchase Date, for purposes of purchasing shares of Common Stock in accordance with the terms under the Offering and the Plan.
(z) “SECURITIES ACT” means the United States Securities Act of 1933, as amended.
(aa) “SHARE EXCHANGE AGREEMENT” means that Share Exchange Agreement by and among Epimmune and certain shareholders of the Company, dated as of March 15, 2005.
(bb) “SPECIFIED INTEREST RATE” means the interest rate that is the higher of (i) the legal interest rate as provided under Article L.313-2 of the French Financial and Monetary Code and (ii) the prevailing market rate, in each case, as specified by the Board for each Offering on the Offering Date.
(cc) “TRADING DAY” means any day the exchange(s) or market(s) on which shares of Common Stock are listed, whether it be any established stock exchange, the Nasdaq National Market, the Nasdaq SmallCap Market or otherwise, is open for trading.
(dd) “TWENTY-DAY TRADING AVERAGE” means the per share average opening sales price (rounded up where necessary to the nearest whole cent) of the Common Stock as quoted on the stock exchange where the Common Stock is listed or on the Nasdaq National Market or the Nasdaq SmallCap Market where the Common Stock is traded (or the exchange or market with the greatest volume of trading in the Common Stock) during the consecutive twenty Trading Days immediately preceding the Offering Date, as reported inThe Wall Street Journalor such other source as the Board deems reliable.
(a) The Board shall administer the Plan unless and until the Board delegates administration to a Committee, as provided in Section 3(c) hereof. Whether or not the Board has delegated administration, the Board shall have the final power to determine all questions of policy and expediency that may arise in the administration of the Plan.
(b) The Board (or the Committee to the extent administration has been delegated to it pursuant to Section 3(c) hereof) shall have the power, subject to, and within the limitations of, the express provisions of the Plan:
| |
| | (i) To determine when and how Purchase Rights to purchase shares of Common Stock shall be granted and the provisions of each Offering of such Purchase Rights (which need not be identical). |
| |
| | (ii) To construe and interpret the Plan and Purchase Rights granted under the Plan, and to establish, amend and revoke rules and regulations for the administration of the Plan. The Board, in the exercise of this power, may correct any defect, omission or inconsistency in the Plan, in a manner and to the extent it shall deem necessary or expedient to make the Plan fully effective. |
| |
| | (iii) To amend the Plan as provided in Section 15 hereof. |
3
| |
| | (iv) Generally, to exercise such powers and to perform such acts as it deems necessary or expedient to promote the best interests of Epimmune, the Company, its Employees or any combination of the foregoing. |
(c) The Board may delegate administration of the Plan to a Committee of the Board composed of one (1) or more members of the Board. If administration is delegated to a Committee, the Committee shall have, in connection with the administration of the Plan, the powers theretofore possessed by the Board, subject, however, to such resolutions, not inconsistent with the provisions of the Plan, as may be adopted from time to time by the Board. The Board may abolish the Committee at any time and revest in the Board the administration of the Plan. If administration is delegated to a Committee, references to the Board in this Plan and in the Offering document shall thereafter be deemed to be to the Board or the Committee, as the case may be.
| |
| 4. | SHARES OF COMMON STOCK SUBJECT TO THE PLAN. |
(a) Subject to the provisions of Section 14 hereof relating to adjustments upon changes in securities, the shares of Common Stock that may be sold pursuant to Purchase Rights granted under the Plan shall not exceed in the aggregate two hundred-fifteen thousand (215,000) shares of Common Stock. If any Purchase Right granted under the Plan shall for any reason terminate without having been exercised, the shares of Common Stock not purchased under such Purchase Right shall again become available for issuance under the Plan.
(b) The shares of Common Stock subject to the Plan shall be shares that have not been previously issued.
| |
| 5. | GRANT OF PURCHASE RIGHTS; OFFERING. |
The Board may from time to time grant or provide for the grant of Purchase Rights under the Plan to all Eligible Employees in an Offering during an Offering Period selected by the Board. Each Offering shall be in such form and shall contain such terms and conditions as the Board shall deem appropriate, provided that all Eligible Employees granted Purchase Rights under the Plan shall have the same rights and privileges. The terms and conditions of an Offering shall be incorporated by reference into the Plan and treated as part of the Plan. The provisions of separate Offerings need not be identical, but each Offering shall include (through incorporation of the provisions of this Plan by reference in the document comprising the Offering or otherwise) the period during which the Offering shall be effective, which period shall not exceed six (6) months and the substance of the provisions contained in Sections 6 through 9 hereof, inclusive.
(a) The Board shall grant Purchase Rights to all Eligible Employees. Except as provided in Section 6(b) hereof, an Employee who is not a Retiree shall not be eligible to be granted Purchase Rights under the Plan unless, on the Offering Date, such Employee has been in the employ of the Company for such period (whether or not continuous) preceding the last day of the Offering Period as the Board may require as set forth in the French Savings Plan. A Retiree shall be eligible to be granted Purchase Rights under the Plan.
(b) Each person who, during the course of an Offering Period, first becomes an Eligible Employee shall, on a date or dates specified in the Offering which coincides with the day on which such person becomes an Eligible Employee, be granted a Purchase Right under that Offering, which Purchase Right shall thereafter be deemed to be a part of that Offering. Such Purchase Right shall have the same characteristics as any Purchase Rights originally granted under that Offering, as described herein, except that:
| |
| | (i) the date on which such Purchase Right is deemed to be granted shall be the Offering Date of such Purchase Right for all purposes, including determination of the purchase price of such Purchase Right; |
| |
| | (ii) the Offering Period with respect to such Purchase Right shall begin on the day the Employee becomes an Eligible Employee and end coincident with the end of such Offering Period. |
4
| |
| 7. | PURCHASE RIGHTS; PURCHASE PRICE. |
(a) On each Offering Date, each Eligible Employee, pursuant to an Offering made under the Plan, shall be granted a Purchase Right to purchase up to a number of whole shares of Common Stock equal to the sum of (i) a whole number equal the quotient arrived at by dividing the Maximum Participant Contribution by the per share purchase price of the Common Stock established by the Board in connection with the Offering and (ii) a whole number equal to the quotient arrived at by dividing the Company Contributions, if any, made on behalf of the Participant, by the per share purchase price of the Common Stock established by the Board in connection with the Offering.
(b) During the Offering Period, the Board shall establish the Purchase Date for each Offering as of which Purchase Rights granted under the Plan and pursuant to which Offering shall be exercised in accordance with Section 9 hereof and purchases of shares of Common Stock shall be carried out in accordance with such Offering. Each Participant shall be provided with notification of the Purchase Date of each Offering in which the Participant has subscribed to purchase shares of Common Stock.
(c) In connection with each Offering made under the Plan, the Board may specify the Maximum Available Shares, and may specify a maximum aggregate number of shares of Common Stock that may be purchased by all Participants pursuant to such Offering or both of the foregoing. If the aggregate number of shares of Common Stock issuable upon exercise of Purchase Rights granted under the Offering would exceed any such maximum aggregate number, then, in the absence of any Board action otherwise, a pro rata allocation of the shares of Common Stock available per Participant shall be made in as nearly a uniform manner as shall be practicable and equitable.
(d) The per share purchase price of Common Stock acquired pursuant to Purchase Rights granted under the Plan shall be an amount that is no greater than one hundred percent (100%), and no less than eighty percent (80%), of the Twenty-Day Trading Average. The per share purchase price shall be established by the Board for each Offering on the Offering Date.
| |
| 8. | PARTICIPATION; WITHDRAWAL; TERMINATION. |
(a) An Eligible Employee may become a Participant in the Plan pursuant to an Offering by delivering a properly completed revocable Participation Agreement to the Company at any time prior to the last day of the Offering Period, in such form as the Company or Epimmune may provide, specifying the number of shares of Common Stock to be purchased by the Eligible Employee in connection with the Offering (either by paper copy or in electronic form), together with payment in full of the Participant’s Contribution payable thereunder, or, to the extent the Participant has elected to make Contributions by monthly payroll deductions, completion of the section in the Participation Agreement indicating that the Contributions shall be made, in whole or in part, by monthly payroll deductions. Except as provided otherwise under the terms of an Offering, Contributions may be made by the Participant in cash, by check or by monthly payroll deductions. Once the properly completed Participation Agreement, together with payment for the full amount of the Participant’s Contribution, if applicable, has been filed by an Eligible Employee and accepted by the Company, it shall be revocable by the Eligible Employee through the last day of the Offering Period. Except as provided in Section 8(c) hereof, on the last day of the Offering Period, at [18:00] hour [Paris] time, all Participation Agreements that have not been revoked in accordance with Section 8(c) hereof shall become irrevocable. Each Participation Agreement shall specify the form of consideration to be used in payment of the Contribution elected by the Participant, and, to the extent so elected, shall authorize monthly payroll deductions of up to 10% of the Participant’s net monthly remuneration (in accordance with the procedures prescribed under French law) during the Offering Period. The monthly payroll deductions made for each Participant and any other Contributions made by the Participant by other means shall be credited to the Participant’s Bookkeeping Account and shall be deposited with the general funds of Epimmune. The Participant’s Contributions that have been credited to the Participant’s Bookkeeping Account shall bear interest at the Specified Interest Rate, commencing on the date the Contribution is made through the last day of the Offering Period, except as provided otherwise below in the case of revocation by the Participant or the Participant ceasing to be an Eligible Employee.
5
(b) To the extent provided in the Offering, a Participant may reduce (including to zero) or increase the monthly payroll deductions or amount of Contributions made through other means that the Participant elected under the Participation Agreement. To the extent provided in the Offering, a Participant may begin monthly payroll deductions, or Contributions made otherwise, after the beginning of the Offering Period. After a Participant’s Participation Agreement has been accepted by the Company, the Participant may make additional Contributions into his or her Bookkeeping Account until the last day of the Offering Period only if specifically provided for in the Offering and only if the Participant has not already contributed the maximum amount permitted during the Offering. In order for the Participant to purchase a number of shares of Common Stock that is greater than subscribed for in his or her Participation Agreement, the Participant must revoke the Participation Agreement in accordance with Section 8(c) hereof and deliver a new Participation Agreement in accordance with the procedures prescribed under the Plan.
(c) Except as provided otherwise under the terms of an Offering, at any time prior to the expiration of an Offering Period, or, to the extent the Purchase Date is a date after the date that is 15 days immediately following the last day of the Offering Period, at any time prior to the date that is at least 15 days preceding the Purchase Date, a Participant may terminate his or her monthly payroll deductions under the Plan and withdraw from the Offering by delivering to the Company a notice of revocation in such form as the Company or Epimmune may provide. The withdrawal by a Participant shall not become effective until the revocation form has been received by the Company. Upon a withdrawal from the Offering by a Participant, unless the revocation form is accompanied by a new Participation Agreement, the Company shall distribute to the Participant all of his or her accumulated Contributions under the Offering, as reflected in the Participant’s Bookkeeping Account, with the interest accrued thereon (at the Specified Interest Rate) from the date the Participant’s Contribution was made or the date the monthly payroll deduction was made, as applicable, up through the date of receipt of the revocation form by the Company, and such Participant’s participation in that Offering shall be automatically terminated. Upon withdrawal from an Offering, a Participant shall not be permitted to participate again the same offering. A Participant’s withdrawal from an Offering shall have no effect upon the Participant’s eligibility to participate in any other Offerings under the Plan.
(d) Purchase Rights granted pursuant to any Offering under the Plan shall terminate immediately upon a Participant ceasing to be an Eligible Employee for any reason (subject to any post-employment participation period required by law) during the Offering Period. The Company shall distribute to such terminated or otherwise ineligible Employee all of his or her accumulated Contributions under the Offering, as are reflected in the Participant’s Bookkeeping Account, with the interest accrued thereon (at the Specified Interest Rate) from the date the Participant’s Contribution was made or the date the monthly payroll deduction was made, as applicable, up through the date that the Participant ceases to be an Eligible Employee.
(e) Purchase Rights granted under the Plan shall not be transferable by an Eligible Employee or a Participant other than by will or the laws of descent and distribution, or by a beneficiary designation as provided in Section 13 hereof, and in each of the foregoing cases, only to the extent permitted under the terms of French Savings Plan. During a Participant’s lifetime, Purchase rights granted under the Plan shall be exercisable only by such Participant.
(a) On the Purchase Date of an Offering, the amount of accumulated Contributions reflected in each Participant’s Bookkeeping Account (less any increase in the amount of the Contributions resulting from any interest accrued thereon, which accrued interest will be separately distributed directly to the Participant subsequent to the last day of the applicable Offering Period within a reasonable period and in no event exceeding the period specified in the Offering) shall be transferred to, and Company Contributions, if any, shall be deposited with, the Participant’s Savings Account and immediately thereafter shall be applied to the purchase of shares of Common Stock subscribed for under the Participation Agreement, subject to the maximum number of shares of Common Stock permitted pursuant to the terms of the Plan and the applicable Offering, at the purchase price specified in the Offering and the Plan. No fractional shares shall be issued upon the exercise of Purchase Rights granted under the Plan unless specifically provided for in the Offering.
6
(b) If any amount of accumulated Contributions remains in a Participant’s Bookkeeping Account after the purchase of shares of Common Stock and such remaining amount is less than the amount required to purchase one share of Common Stock on the Purchase Date of an Offering, then such remaining amount shall be held in each such Participant’s Bookkeeping Account for the purchase of shares of Common Stock under the next Offering under the Plan, unless such Participant withdraws from such next Offering, as provided in Section 8(c) hereof, or is not eligible to participate in such Offering, as provided in Section 6 hereof, in which case such amount shall be distributed to the Participant after his withdrawal or after he ceases to be eligible for the Offering, with the accrued interest (at the Specified Interest Rate) credited to the Bookkeeping Account. If any amount of accumulated Contributions remains in a Participant’s Bookkeeping Account after the purchase of shares of Common Stock and such remaining amount is equal to the amount required to purchase one (1) or more whole shares of Common Stock on the Purchase Date of the Offering, then such remaining amount shall be distributed in full to the Participant as soon as administratively possible after the Purchase Date with the accrued interest (at the Specified Interest Rate) credited to the Bookkeeping Account.
(c) No Purchase Rights granted under the Plan shall be exercised to any extent unless the shares of Common Stock to be issued upon such exercise under the Plan are covered by an effective registration statement pursuant to the Securities Act and the Plan is in material compliance with all applicable U.S. federal, state, non-U.S. jurisdiction and other securities and other laws applicable to the Plan. If on a Purchase Date during any Offering hereunder the shares of Common Stock are not so registered or the Plan is not in such compliance, no Purchase Rights granted under the Plan or any Offering shall be exercised on such Purchase Date, and the Purchase Date shall be delayed until the shares of Common Stock are subject to such an effective registration statement and the Plan is in such compliance, except that the Purchase Date shall not be delayed more than two (2) months, and the Purchase Date shall in no event be more than twelve (12) months from the Offering Date. If, on the Purchase Date under any Offering hereunder, as delayed to the maximum extent permissible, the shares of Common Stock are not registered and the Plan is not in such compliance, no Purchase Rights granted under the Plan or any Offering shall be exercised and all Contributions accumulated during the Offering shall be distributed to the Participants, with the accrued interest (at the Specified Interest Rate).
| |
| 10. | COVENANTS OF EPIMMUNE. |
(a) During the terms of the Purchase Rights granted under the Plan, Epimmune shall ensure that the number of shares of Common Stock required to satisfy such Purchase Rights is available, or, if in accordance with Section 7(c) hereof, the Board has specified a maximum aggregate number of shares of Common Stock that may be purchased by all Participants pursuant to an Offering, Epimmune shall ensure that this number of shares is available.
(b) Epimmune shall seek to obtain from each U.S. federal, state, non-U.S. jurisdiction or other regulatory commission or agency having jurisdiction over the Plan such authority as may be required to issue and sell shares of Common Stock upon exercise of the Purchase Rights granted under the Plan. If, after reasonable efforts, Epimmune is unable to obtain from any such regulatory commission or agency the authority that counsel for Epimmune deems necessary for the lawful issuance and sale of shares of Common Stock under the Plan, Epimmune shall be relieved from any liability for failure to issue and sell shares of Common Stock upon exercise of such Purchase Rights unless and until such authority is obtained.
| |
| 11. | USE OF PROCEEDS FROM SHARES OF COMMON STOCK. |
Proceeds from the sale of shares of Common Stock pursuant to Purchase Rights granted under the Plan shall constitute general funds of Epimmune.
| |
| 12. | RIGHTS AS A STOCKHOLDER. |
A Participant shall not be deemed to be the holder of, or to have any of the rights of a holder with respect to, shares of Common Stock subject to Purchase Rights granted under the Plan unless and until the
7
Participant’s shares of Common Stock acquired upon exercise of Purchase Rights granted under the Plan are recorded in the books of Epimmune (or its transfer agent).
| |
| 13. | DESIGNATION OF BENEFICIARY. |
(a) A Participant may file a written designation of a beneficiary who is to become a holder of shares of Common Stock and/or to receive cash, if any, from the Participant’s account under the Plan in the event of such Participant’s death subsequent to the end of an Offering (including during the period commencing on the last day of the Offering Period after the Participant’s Contributions and Company Contribution, if any have been allocated to the Participant’s Savings Account) but prior to delivery with respect to the Participant of such shares of Common Stock, or cash credited to the Bookkeeping Account as the case may be. In addition, a Participant may file a written designation of a beneficiary who is to receive any cash credited to the Participant’s Bookkeeping Account under the Plan in the event of such Participant’s death during an Offering.
(b) The Participant may change his or her designation of beneficiary at any time by written notice. In the event of the death of a Participant and in the absence of a beneficiary validly designated under the Plan who is living at the time of the Participant’s death, Epimmune shall deliver such shares of Common Stock and/or cash to the executor or administrator of the estate of the Participant, or if no such executor or administrator has been appointed (to the knowledge of Epimmune), Epimmune, in its sole discretion, may deliver such shares of Common Stock and/or cash to the spouse or to any one or more dependents or relatives of the Participant, or if no spouse, dependent or relative is known to Epimmune, then to such other person as Epimmune may designate.
| |
| 14. | ADJUSTMENTS UPON CHANGES IN SECURITIES; CORPORATE TRANSACTIONS. |
(a) If any change is made in the shares of Common Stock subject to the Plan or subject to any Purchase Right, without the receipt of consideration by Epimmune (through merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or other transaction not involving the receipt of consideration by Epimmune), the Plan shall be appropriately adjusted in the type(s), class(es) and maximum number of shares of Common Stock subject to the Plan pursuant to Section 4(a) hereof, and the outstanding Purchase Rights granted under the Plan shall be appropriately adjusted in the type(s), class(es), number of shares and purchase limits of such outstanding Purchase Rights. The Board shall make such adjustments, and its determination shall be final, binding and conclusive. (The conversion of any convertible securities of Epimmune shall not be treated as a “transaction not involving the receipt of consideration by Epimmune.”)
(b) To the extent permitted under French law applicable to the French Savings Plan, in the event of a Corporate Transaction, then (i) to the extent the Board then determines in its sole discretion, any surviving or acquiring corporation may continue or assume Purchase Rights outstanding under the Plan or may substitute similar rights (including a right to acquire the same consideration paid to stockholders in the Corporate Transaction) for those outstanding under the Plan, or (ii) if any surviving or acquiring corporation does not assume such Purchase Rights or does not substitute similar rights for Purchase Rights outstanding under the Plan, then, to the extent the Board then determines in its sole discretion, upon at least 15 calendar days’ prior notice to the Participants, the Participants’ Contributions and Company Contributions, if any, shall be used to purchase shares of Common Stock within 5 business days prior to the Corporate Transaction under the ongoing Offering, and the Participants’ Purchase Rights under the ongoing Offering shall terminate immediately after such purchase.
| |
| 15. | AMENDMENT OF THE PLAN. |
(a) The Board at any time, and from time to time, may amend the Plan. However, except as provided in Section 14 hereof relating to adjustments upon changes in securities and except as to amendments solely to benefit the administration of the Plan, to take account of a change in legislation or to obtain or maintain favorable tax, financial accounting, exchange control or regulatory treatment for Participants, the Company, or
8
Epimmune, no amendment shall be effective unless approved by the stockholders of Epimmune to the extent stockholder approval is necessary for the Plan to satisfy the requirements of applicable laws or regulations.
(b) It is expressly contemplated that the Board may amend the Plan in any respect the Board deems necessary or advisable to provide Employees with the maximum benefits provided or to be provided under applicable law and/or to bring the Plan and/or Purchase Rights granted under the Plan into compliance therewith.
(c) The rights and obligations under any Purchase Rights granted before amendment of the Plan shall not be impaired by any amendment of the Plan except (i) with the consent of the person to whom such Purchase Rights were granted, (ii) as necessary to comply with any laws or governmental regulations, or (iii) as necessary to obtain or maintain favorable financial accounting treatment with respect to such Purchase Rights.
| |
| 16. | TERMINATION OR SUSPENSION OF THE PLAN. |
(a) The Board in its discretion may suspend or terminate the Plan at any time. Unless sooner terminated, the Plan shall terminate at the time that all of the shares of Common Stock reserved for issuance under the Plan, as increased and/or adjusted from time to time, have been issued under the terms of the Plan.
(b) No Purchase Rights may be granted under the Plan while the Plan is suspended or after it is terminated. Any benefits, privileges, entitlements and obligations under any Purchase Rights granted under the Plan while the Plan is in effect shall not be impaired by suspension or termination of the Plan except (i) as expressly provided in the Plan or with the consent of the person to whom such Purchase Rights were granted or (ii) as necessary to comply with any laws, regulations, or listing requirements.
| |
| 17. | EFFECTIVE DATE OF PLAN. |
The Plan shall become effective as determined by the Board, but no Purchase Rights shall be granted by the Board unless and until the Plan has been approved by the stockholders of Epimmune within twelve (12) months before or after the date the Plan is adopted by the Board.
| |
| 18. | MISCELLANEOUS PROVISIONS. |
(a) The Plan and Offering do not constitute an employment contract. Nothing in the Plan or in the Offering shall in any way alter the at will nature of a Participant’s employment or be deemed to create in any way whatsoever any obligation on the part of any Participant to continue in the employ of the Company, or on the part of the Company to continue the employment of a Participant.
(b) The provisions of the Plan shall be governed by the laws of the State of California without resort to that state’s conflicts of laws rules.
9
EPIMMUNE INC.
PROXY SOLICITED BY THE BOARD OF DIRECTORS
FOR THE ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON AUGUST 11, 2005
The undersigned hereby appoints Emile Loria, M.D. and Robert J. De Vaere, and each of them, as attorneys and proxies of the undersigned, with full power of substitution, to vote all of the shares of stock of Epimmune Inc. which the undersigned may be entitled to vote at the Annual Meeting of Stockholders of Epimmune Inc. to be held at 5820 Nancy Ridge Drive, San Diego, California, on August 11, 2005 at 11:00 a.m. (local time), and at any and all postponements, continuations and adjournments thereof, with all powers that the undersigned would possess if personally present, upon and in respect of the following matters and in accordance with the following instructions, with discretionary authority as to any and all other matters that may properly come before the meeting.
Unless a contrary direction is indicated, this proxy will be voted for all nominees listed in proposal 8 and for proposals 1, 2, 3-A, 3-B, 3-C, 3-D, 3-E, 3-F, 3-G, 4, 5, 6, 7 and 9 as more specifically described in the proxy statement. If specific instructions are indicated, this proxy will be voted in accordance therewith.
Voting Instructions:
Vote By Mail: Complete, sign, date and promptly return this proxy card in the postage-paid envelope provided.
Vote By Phone-1-800-690-6903: Call toll-free (in the United States), using any touch-tone telephone, to transmit your voting instructions up until 11:59 P.M. Eastern Daylight Time on August 10, 2005. Have the proxy card in hand when you call and then follow the recorded instructions.
Vote By Internet-www.proxyvote.com: Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M. Eastern Daylight Time on August 10, 2005. Have the proxy card in hand when you access the web site and follow the instructions to create an electronic voting instruction form.
DO NOT RETURN THE PROXY CARD IF YOU ARE VOTING BY TELEPHONE
The Board of Directors recommends a vote for proposals 1, 2, 3-A, 3-B, 3-C, 3-D, 3-E, 3-F, 3-G, 4, 5, 6 and 7.
| | | |
| Proposal 1: | | To approve the issuance of shares of Epimmune’s common stock in accordance with the terms of the Share Exchange Agreement, dated March 15, 2005, as amended, between Epimmune and certain shareholders of IDM S.A., the Put/Call Agreements to be entered into between Epimmune and certain shareholders of IDM S.A., the Option Liquidity Agreements to be entered into between Epimmune and certain optionholders of IDM S.A. and the Amended and Restated Preferred Exchange Agreement, dated April 12, 2005, between Epimmune and G.D. Searle LLC. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 2: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to change the corporate name of Epimmune to “IDM, Inc.” |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
Proxy Card
| | | |
| Proposal 3-A: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-four reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 3-B: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-five reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 3-C: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-six reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 3-D: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-seven reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 3-E: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-eight reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 3-F: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-nine reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 3-G: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to effect a one-for-ten reverse stock split of the outstanding shares of Epimmune’s common stock. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 4: | | To approve an amendment of Epimmune’s Amended and Restated Certificate of Incorporation to increase Epimmune’s authorized capital stock to a total of 65,000,000 shares, consisting of 10,000,000 shares of authorized preferred stock and 55,000,000 shares of authorized common stock after giving effect to the reverse stock split. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 5: | | To approve an amendment of the Epimmune 2000 Stock Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 8,800,000 shares (on a pre-reverse stock split basis). |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 6: | | To approve an amendment of the Epimmune 2001 Employee Stock Purchase Plan to increase the number of shares of Epimmune’s common stock available for issuance under the plan by 185,000 shares (on a pre-reverse stock split basis). |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
| | | |
| Proposal 7: | | To approve the adoption of the Epimmune Employee Stock Purchase Plan for IDM S.A. employees with 215,000 shares (on a pre-reverse stock split basis) of Epimmune’s common stock available for issuance under the plan. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
The Board of Directors recommends a vote for the nominees for director listed below.
| | | |
| Proposal 8: | | To elect six directors to serve for the ensuing year or until successors are elected. |
| | | | | | | |
| o | | For the nominees listed below (except as marked to the contrary below). | | o | | Withhold Authority to vote for
the nominee listed below. |
| | | |
| Nominees: | | Howard E. (“Ted”) Greene, Jr.; William T. Comer, Ph.D.; Michael G. Grey; Georges Hibon; Emile Loria, M.D.; and John P. McKearn, Ph.D. |
To withhold authority to vote for any nominee(s), write such nominee(s)’ name(s) below:
The Board of Directors recommends a vote for proposal 9.
| | | |
| Proposal 9: | | To ratify the selection of the Board of Directors of Ernst & Young LLP, the Independent Registered Public Accounting Firm, as the independent auditors of Epimmune for its fiscal year ending December 31, 2005. |
| | | | | | | | | | | |
| o | | For | | o | | Against | | o | | Abstain |
In their discretion, the proxies are authorized to vote upon such other matters as may properly come before the meeting.
SIGNATURE(S)
Please sign exactly as your name appears hereon. If the stock is registered in the names of two or more persons, each should sign. Executors, administrators, trustees, guardians and attorneys-in-fact should add their titles. If signer is a corporation, please give full corporate name and have a duly authorized officer sign, stating title. If signer is a partnership, please sign in partnership name by authorized person.
Please vote, date and promptly return this proxy in the enclosed return envelope which is postage prepaid if mailed in the United States.