UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: September 26, 2009
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 0-18281
Hologic, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| | |
| Delaware | | 04-2902449 |
| (State or Other Jurisdiction of Incorporation or Organization) | | (IRS Employer Identification No.) |
35 Crosby Drive, Bedford, Massachusetts 01730
(Address of Principal Executive Offices) (Zip Code)
Registrant’s Telephone Number, Including Area Code (781) 999-7300
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of Each Class | | Name of Each Exchange on which Registered |
| Common Stock, $.01 par value | | Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: Rights to Purchase Preferred Stock
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by checkmark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | |
Large accelerated filer | | x | | | | | Accelerated filer | | ¨ | |
| | | | |
Non-accelerated filer | | ¨ | | | (Do not check if a smaller reporting company) | | Smaller reporting company | | ¨ | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of the registrant’s Common Stock held by non-affiliates of the registrant as of March 27, 2009 was $3,448,132,346 based on the price of the last reported sale on the Nasdaq Global Select Market on that date.
As of November 18, 2009, there were 257,963,830 shares of the registrant’s Common Stock, $.01 par value, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for the registrant’s annual meeting of stockholders to be filed within 120 days of the end of its fiscal year ended September 26, 2009 are incorporated into Part III (Items 10, 11, 12, 13 and 14) of this Annual Report on Form 10-K where indicated.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements contained in this report are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements involve known and unknown risks, uncertainties and other factors which may cause our or our industry’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding:
| | • | | the effect of the worldwide macroeconomic downturn on our business and results of operation; |
| | • | | the coverage and reimbursement decisions of third party payors relating to the use of our products and treatments; |
| | • | | the uncertainty of the impact of healthcare reform proposals, including a potential excise tax on medical device companies in the U.S., on our business and results of operation; |
| | • | | the impact and anticipated benefits of recently completed acquisitions and acquisitions we may complete in the future; |
| | • | | our goal of expanding our market positions; |
| | • | | the development of new competitive technologies and products; |
| | • | | regulatory approval and clearances for our products; |
| | • | | production schedules for our products; |
| | • | | the anticipated development of our markets and the success of our products in these markets; |
| | • | | the anticipated performance and benefits of our products; |
| | • | | dependence on significant or sole source suppliers; |
| | • | | the impact and costs and expenses of any litigation we may be subject to now or in the future; |
| | • | | compliance with covenants contained in our credit facility and long-term leases; |
| | • | | anticipated trends relating to our financial condition or results of operations; and |
| | • | | our capital resources and the adequacy thereof. |
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by such forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this report. Except as otherwise required by law, we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statement contained in this report to reflect any change in our expectations or any change in events, conditions or circumstances on which any of our forward-looking statements are based. Factors that could cause or contribute to differences in our future financial results include those discussed in the Risk Factors set forth in Part I Item 1A below as well as those discussed elsewhere in this report. We qualify all of our forward-looking statements by these cautionary statements.
2
PART I
Overview
We are a developer, manufacturer and supplier of medical imaging systems and diagnostic and surgical products focused on the healthcare needs of women. Our core business segments are focused on breast health, diagnostics, GYN surgical and skeletal health. We have historically focused our resources on developing systems and subsystems offering superior image quality and diagnostic accuracy, which has enabled us to capture significant market share and customer loyalty, despite the presence of large competitors.
Our breast health products include a broad portfolio of breast imaging and related products and accessories, including digital and film-based mammography systems, computer-aided detection (“CAD”), minimally invasive breast biopsy and tissue extraction devices, breast biopsy guidance systems, breast imaging comfort pads, and breast brachytherapy products. We have also developed a new breast imaging platform, “Dimensions”, which utilizes a new technology, tomosynthesis, to produce three dimensional (“3D”) images, as well as conventional two dimensional (“2D”) full field digital mammography (FFDM) images. In the United States, our Dimensions product has been approved by the Food and Drug Administration (“FDA”) for providing conventional 2D images, and we are conducting further clinical trials to support our pre-market approval (“PMA”) application for the 3D configuration. Our Dimensions platform has received CE mark approval in Europe in fiscal 2008 and Canadian registration in March 2009, both for 2D and 3D modes of imaging. Within our breast brachytherapy related products is our MammoSite system, which provides accelerated partial breast irradiation technology. We received FDA clearance for our MammoSite ML radiation therapy system, a multi-lumen device that provides the oncologist with additional flexibility in specifically targeting radiation in the tissue with cancer cells, on August 27, 2009.
Our diagnostics products include the ThinPrep System (“ThinPrep”), which is primarily used in cytology applications, such as cervical cancer screening, and the Rapid Fetal Fibronectin Test, which assists physicians in assessing the risk of pre-term birth. In July 2008, we acquired Third Wave Technologies, Inc. (“Third Wave”), a company that develops and markets molecular diagnostic reagents for a wide variety of DNA and RNA analysis applications based on its proprietary Invader chemistry. Our current clinical diagnostic offerings based upon this Invader chemistry include products to assist in the diagnosis of human papillomavirus (“HPV”), Cystic Fibrosis, cardiovascular risk and other diseases. We received FDA approval of Cervista HPV High Risk (“HR”) and Cervista HPV 16/18 tests in March 2009 as well as CE mark approval in Europe in January 2009 for Cervista HPV HR and in May 2009 for Cervista HPV 16/18.
Our GYN surgical products include the NovaSure Impedance Controlled RF Ablation System (“NovaSure System”) and the Adiana Permanent Contraception System (“Adiana System”). The NovaSure System enables physicians to treat women suffering from excessive menstrual bleeding in a minimally invasive manner. The Adiana System is a form of permanent female contraception intended as an alternative to tubal ligation. We received FDA approval of the Adiana System in July 2009 and CE mark approval for the system in Europe in December 2008.
Our skeletal health products include dual-energy X-ray bone densitometry systems, an ultrasound-based osteoporosis assessment product, our Fluoroscan mini C-arm imaging products, and our Esaote line of extremity Magnetic Resonance Imaging (“MRI”) systems that were manufactured by an original equipment manufacturer.
We were incorporated in Massachusetts in October 1985 and reincorporated in Delaware in March 1990. Unless the context otherwise requires, references to us, Hologic or our company refer to Hologic, Inc. and each of its consolidated subsidiaries.
3
Trademark Notice
Hologic is a trademark of Hologic, Inc. Other trademarks, logos, and slogans registered or used by Hologic and its divisions and subsidiaries in the United States and other countries include, but are not limited to, the following:
Adiana, AEG, ATEC, BioLucent, Celero, Cervista, Cytyc, Dimensions, DirectRay, Eviva, FAST Paddle, Fluoroscan, Gestiva, HTC, Invader, LORAD, MammoPad, MammoSite, M-IV, MultiCare, NovaSure, PreservCyt, QDR, R2, Rapid FFN, Sahara, SecurView, Selenia, StereoLoc, Suros, TechMate, ThinPrep, Third Wave, TLI IQ.
Available Information
Our Internet website address is http://www.hologic.com. Through our website, we make available, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (“SEC”). These SEC reports can be accessed through the investor relations section of our website. The information found on our website is not part of this or any other report we file with or furnish to the SEC.
You may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy and information statements, and other information regarding the Company and other issuers that file electronically with the SEC. The SEC’s Internet website address is http://www.sec.gov.
Products
We view our operations and manage our business in four principal reporting segments: Breast Health, Diagnostics, GYN Surgical and Skeletal Health. Financial information concerning these segments is provided in Note 13 of the Notes to our Consolidated Financial Statements included in this report.
Breast Health Products
Our breast health products include a broad portfolio of breast imaging and related products and accessories, including digital and film-based mammography systems, computer-aided detection (“CAD”), minimally invasive breast biopsy and tissue extraction devices, breast biopsy guidance systems, breast imaging comfort pads, and breast brachytherapy products. We have also developed a new breast imaging platform, Dimensions, which utilizes a new technology, tomosynthesis, to produce 3D images. In fiscal 2009, our breast health segment also included the sale of digital detectors to an original equipment manufacturer, and our organic photoconductor coating business acquired in connection with the acquisition of our selenium coating capabilities for our mammography digital detectors. Commencing in fiscal 2010, we do not expect to generate revenues from these two sources, which contributed approximately $17.6 million of revenue in fiscal 2009.
Selenia Full Field Digital Mammography System
The Selenia full field digital mammography system is based on our proprietary, amorphous selenium DirectRay digital detector, which preserves image quality by using amorphous selenium to directly convert x-rays to electronic signals. Many other digital technologies employ an indirect two-step process by first converting x-ray energy into light and then converting the light energy into electrical signals. We believe that digital x-ray imaging technologies that require light conversion may compromise image resolution, lessening detection capability. Our DirectRay flat panel detector technology employs an amorphous selenium (“a-Se”) photoconductor to directly convert x-ray photons into an electrical signal. No intensifying screens or additional processes are required to capture and convert the x-ray energy, enabling high imaging resolution and contrast sensitivity.
4
The Selenia product family has a number of other features designed to improve image quality and patient throughput. The open architecture of the system’s design provides for full integration with existing enterprise Picture Archiving and Communications Systems (“PACS”) and Radiology Information Systems (“RIS”). The Selenia product family includes the Selenia base configuration, the Selenia S configuration (a screening-only configuration), the Selenia Value (a lower cost alternative to the Selenia base configuration) and the Selenia Encore (refurbished units), each of which offer customers varying performance capabilities and product costs.
Breast Tomosynthesis
Our Dimensions platform includes a mammography gantry capable of performing both 2D and 3D image acquisition and display. When operating in 3D mode, the system acquires a series of low dose x-ray images taken in a scanning motion at various angles. The images are mathematically processed into a series of small slices, revealing breast tissue from a 3D perspective. We believe that by allowing the clinician to review breast tissue in three dimensional space, the more subtle architecture of various types of suspicious lesions may be able to be better interpreted, which may ultimately increase cancer detection and reduce unnecessary patient callbacks. In the United States, our Dimensions product has been approved by the Food and Drug Administration (“FDA”) for providing conventional 2D images, and we are conducting further clinical trials to support our pre-market approval (“PMA”) application for the 3D configuration. Our Dimensions 3D configuration received CE mark approval in Europe in fiscal 2008 and Canadian registration in March 2009.
Screen-Film Mammography Systems
Our screen-film mammography systems include our LORAD M-IV and M-IV Platinum systems. The M-IV Platinum system incorporates our Fully Automatic Self-adjusting Tilt (“FAST”) Paddle, and our High Transmission Cellular (“HTC”) Grid.
SecurView Workstation
The images captured by digital mammography systems are typically transmitted electronically for review by a radiologist at a work station. Early product development activities focused on improving digital workflow in the breast-imaging suite due to limited PACS mammography functionality. To this end, we developed the SecurViewDX breast imaging softcopy workstation, approved for interpretation of digital mammograms from most vendors as well as images from other diagnostic breast modalities. To complement this product, we also developed the SecurViewRT workstation, a technologist workstation enabling bi-directional exchange of electronic communications between the reviewer and the technologist. An additional configuration was added to the Selenia acquisition workstation to allow incorporation of a second monitor and computer, providing all functionalities of the SecurViewRT workstation within the exam room. This configuration is called the “Selenia with TechMate” digital mammography system.
CAD Systems
We have developed CAD software tools for a variety of imaging modalities and disease states. CAD is used by an increasing number of radiologists as “a second pair of eyes” when reading a woman’s mammogram. Use of this technology provides reviewers with the potential to detect findings that might otherwise be overlooked during the review process, thus increasing cancer detection. We have integrated our mammography applications CAD software tools into our line of multi-modality breast imaging workstations.
Stereotactic Breast Biopsy Systems
We provide clinicians with the flexibility of choosing upright or prone systems for breast biopsy by offering two minimally invasive stereotactic breast biopsy guidance systems, the MultiCare Platinum dedicated, prone breast biopsy table and the StereoLoc II upright attachment. The StereoLoc II attachment is used in conjunction
5
with our M-IV series of screen-film mammography systems and our Selenia full field digital mammography system. These systems provide an alternative to open surgical biopsy, and can be performed as an outpatient procedure under local anesthesia, allowing shorter recovery times.
Breast Biopsy Products
We offer minimally invasive interventional products for breast biopsy, tissue removal and biopsy site marking. The biopsy technology, which includes a patented fluid management system, allows the removal of tissue or biopsy samples using stereotactic x-ray, ultrasound and MRI guidance systems. Our Automated Tissue Excision and Collection (“ATEC”) product line includes percutaneous, automatic vacuum-assisted breast biopsy collection systems, a disposable device used to collect samples, and biopsy site markers. The ATEC line of products is designed to accommodate a broad range of clinical and patient presentations. In 2007, we began offering the Celero vacuum-assisted, spring-loaded, large-core biopsy device designed for use under ultrasound guidance to access hard-to-reach lesions in the axilla, near the chest wall, near implants or behind the nipple. In November 2008, we began offering the Eviva stereotactic vacuum-assisted breast biopsy device. This premium device offers several new features, including improved compatibility with the MultiCare Platinum prone breast biopsy table, an integrated firing mechanism, and an integrated, end-ploy site marking system.
MammoSite Radiation Therapy System
The MammoSite Radiation Therapy System accelerated partial breast irradiation (APBI) is comprised of an inflatable balloon catheter in which a radioactive source is introduced for therapy delivery. The inflatable balloon is inserted into the surgical cavity remaining after a lumpectomy. The catheter portion of the system allows the radioactive source to be added or withdrawn over the course of the therapy. This local placement of the balloon provides for therapeutic delivery of a 5-day course of radiation to the tissue most likely to contain residual cancerous cells following surgery, while reducing radiation exposure to adjacent healthy tissue. We recently introduced the MammoSite ML radiation therapy system, for which we received FDA clearance on August 27, 2009. The MammoSite ML system is a multi-lumen device that provides radiation oncologists the ability to shape the radiation dose for typical cases and treat patients who are otherwise not appropriate candidates for traditional brachytherapy. The MammoSite ML device has a central lumen, similar to the original MammoSite device, and three offset lumens parallel to the central lumen. In addition to allowing greater flexibility in radiation treatment planning, the use of a multiple-lumen device typically results in a higher reimbursement rate.
MammoPad Breast Cushion
Our mammography related products include a proprietary MammoPad breast cushion. The MammoPad cushion is designed to reduce the discomfort women often experience during mammography. The cushion’s grip-like surface also holds breast tissue in place to improve breast positioning. The radiolucent cushion does not interfere with image quality and can be used with both digital and analog mammography.
Photoconductor Coatings
Our AEG Elektrofotografie GmbH (“AEG”) subsidiary is our sole supplier of the amorphous selenium photoconductor coatings employed in our Selenia and Dimensions full-field digital mammography detectors. AEG also develops, manufactures, and sells non-medical selenium and organic photoconductor materials for use in a variety of other electro photographic applications, including copying and printing. During the fourth quarter of fiscal 2009, we closed our organic photoconductor drum coatings manufacturing operations in Shanghai, China.
6
Diagnostic Products
Our diagnostic product offerings include the ThinPrep System used primarily for cytology applications, such as cervical cancer screening, and the Rapid Fetal Fibronectin Test for pre-term birth risk assessment. Our Third Wave acquisition enables us to develop and market molecular diagnostic reagents for a wide variety of DNA and RNA analysis applications based on Third Wave’s proprietary Invader chemistry, including our two recently approved HPV tests.
ThinPrep System
The ThinPrep System is the most widely used method for cervical cancer screening in the United States. If detected in the pre-cancerous stage, most cervical cancer cases are preventable. The ThinPrep System consists of any one or more of the following: the ThinPrep 2000 Processor, ThinPrep 3000 Processor, ThinPrep Imaging System, and related reagents, filters and other supplies, such as the ThinPrep Pap Test and our proprietary ThinPrep PreservCyt Solution.
The ThinPrep Process.The ThinPrep process begins with the patient’s cervical sample being taken by the physician using a cervical sampling device that, rather than being smeared on a microscope slide as in a conventional Pap smear, is rinsed in a vial filled with our proprietary PreservCyt Solution. This enables most of the patient’s cell sample to be preserved before the cells can be damaged by air drying. The ThinPrep specimen vial is then labeled and sent to a laboratory equipped with a ThinPrep Processor for slide preparation. At the laboratory, the ThinPrep specimen vial is inserted into a ThinPrep Processor, a proprietary sample preparation device which automates the process of preparing cervical slides for staining and microscopic examination.
In the case of manual screening, the cytotechnologist screens each Pap test slide with a microscope to first determine the adequacy of the slide and to then examine the entire slide to differentiate diseased or abnormal cells from normal cells. With the ThinPrep Imaging System, the screening process has been automated to combine the power of computer imaging technology and human interpretive skills. Prior to human review, the ThinPrep Imaging System rapidly scans and locates areas of interest for review. By directing the cytotechnologist to areas of interest on a slide, the system may increase a cytology laboratory’s screening productivity and diagnostic accuracy.
In September 2009, we announced CE marking approval for our ThinPrep Integrated Imager. The Integrated Imager represents the latest innovation in cervical screening by combining proven ThinPrep imaging technology and slide review into a single, convenient stand-alone device. This new product offering allows laboratories of all sizes to benefit from clinical advantages of ThinPrep imaging for cervical screening. CE marking approval allows Hologic to market the ThinPrep integrated imager in the 27 countries of the European Union (EU) and three of the four member states of the European Free Trade Association (EFTA). Marketing of this product in the U.S. will require FDA approval.
Additional Applications. In addition to serving as a replacement for the conventional Pap smear, the ThinPrep System can also be used for non-gynecological cytology screening applications. Non-gynecological cytology applications include fine-needle aspiration specimens (e.g., breast, thyroid, lung or liver), lavage specimens (e.g., breast, gastrointestinal), body fluids (e.g., urine, pleural fluid, ascitic fluid, pericardial fluid), respiratory specimens (e.g., sputum, brushing of respiratory tracts) and ancillary testing (e.g., cell blocks, immunocytochemistry, special stains).
Rapid Fetal Fibronectin Test
The Rapid Fetal Fibronectin Test is a patented single-use disposable test used to determine a woman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, in vaginal secretions during pregnancy. This test is approved by the FDA for use in assessing the risk of preterm birth. The test utilizes a single-use, disposable cassette and is analyzed on our patented instrument, the TLiIQ System.
7
Human Papillomavirus (HPV) Offering and InVitro Diagnostics
HPV is the most common sexually transmitted disease in the U.S. and is recognized as the cause of most cervical cancer. In March 2009, we received FDA approval for both our Cervista HPV HR and Cervista HPV 16/18 tests for the following intended uses:
The Cervista HPV HR test has been approved for two uses:
| | • | | To screen patients with atypical squamous cells of undetermined significance (ASC-US) cervical cytology results to determine the need for referral to colposcopy, and |
| | • | | Used adjunctively with cervical cytology to screen women 30 years and older to assess the presence or absence of high-risk HPV types. |
The Cervista HPV 16/18 test has been approved for two uses:
| | • | | In women 30 years and older, the test may be used adjunctively with the Cervista HPV HR test in combination with cervical cytology to assess the presence or absence of specific high-risk HPV types, and |
| | • | | Used adjunctively with the Cervista HPV HR test in patients with ASC-US cervical cytology results to assess the presence or absence of specific high-risk HPV types. The results of this test are not intended to prevent women from proceeding to colposcopy. |
These tests employ Third Wave’s Invader technology and are performed out of the ThinPrep PreservCyt collection vial. The Cervista HPV HR test is an in vitro diagnostic test for the quantitative detection of DNA from the fourteen high-risk HPV types responsible for most cervical disease. The Cervista HPV 16/18 test is an in vitro diagnostic test for the qualitative detection of DNA from HPV types 16 and 18, the types that cause approximately 70% of cervical disease.
The Invader UGT1A1 Molecular Assay, a molecular diagnostic acquired as part of our recent acquisition of Third Wave, is cleared for use to identify patients who may be at increased risk of adverse reaction to the chemotherapy drug Camptosar (irinotecan) by detecting and identifying specific mutations in the UGT1A1 gene that have been associated with that risk. Camptosar, marketed in the U.S. by Pfizer, Inc., is used to treat colorectal cancer and was relabeled in 2005 to include dosing recommendations based on a patient’s genetic profile.
Our other current clinical diagnostic offerings based upon our Invader chemistry include products to assist in the diagnosis of Cystic Fibrosis, cardiovascular risk and other diseases.
GYN Surgical Products
Our surgical product offerings include the NovaSure System, and the Adiana System.
NovaSure System
The NovaSure System is a minimally-invasive procedure that allows physicians to treat women suffering from excessive menstrual bleeding. The system consists of a disposable device and a controller that delivers radio frequency, or RF, energy to ablate the endometrial lining of the uterus. The NovaSure disposable device is a hand-held, single-use device that incorporates a flexible gold-plated mesh electrode used to deliver the RF energy during the NovaSure procedure. The NovaSure RF Controller generates and delivers the RF energy customized for each patient, monitors several critical treatment and safety parameters, and automatically controls other aspects of the procedure.
8
The NovaSure System is a “second generation” endometrial ablation therapy approved by the FDA to be performed without drug or surgical pre-treatment. Pre-treatment can be time-consuming, expensive and inconvenient for both patients and physicians and can result in uncomfortable or painful side effects and complications. In contrast, the NovaSure procedure is typically performed as an outpatient procedure in the hospital, ambulatory surgery center or physician’s office and often does not require the use of general anesthesia.
Adiana System
The Adiana System is a minimally invasive procedure that requires no incisions and can be performed in the comfort of the doctor’s office using local anesthesia. Patients are often able to return to work or resume their daily activities within one day. In contrast, tubal ligation, a traditional method of female permanent contraception, requires more invasive surgical procedures, is usually conducted in a hospital under general anesthesia and typically requires several days of recovery.
During the Adiana System procedure, a slender, flexible instrument is passed through the body’s natural openings to deliver a low level of radiofrequency (RF) energy to a small section of each fallopian tube. A tiny, soft insert, about the size of a grain of rice, is then placed in each fallopian tube in the location where the energy was applied. During the three months following the procedure, the patient continues to use temporary birth control while new tissue grows in and around the Adiana System inserts, eventually blocking the fallopian tubes. At three months, a special x-ray test (called a hysterosalpingogram or HSG) is performed to confirm the fallopian tubes are completely blocked and the patient may begin relying on the Adiana System for permanent contraception. Because the Adiana insert is fully contained within the fallopian tube and does not use metal, the procedure leaves nothing in the uterus that could interfere with future intra-uterine procedures such as endometrial ablation.
Skeletal Health Products
Our skeletal health products include a family of QDR dual energy x-ray bone densitometers and the Sahara Clinical Bone Sonometer, our mini C-arm imaging products and our Esaote line of extremity MRI systems, which are manufactured by an original equipment manufacturer.
QDR X-Ray Bone Densitometers
Bone densitometry is the measurement of bone density to assist in the diagnosis and monitoring of osteoporosis and other metabolic bone diseases that can lead to debilitating bone fractures. Osteoporosis is a disease that is most prevalent in post-menopausal women. Our proprietary QDR x-ray bone densitometers incorporate dual-energy x-ray technology to precisely assess bone density of the most important fracture sites, the spine and hip. Since our commercial introduction of the first bone densitometer employing dual-energy x-ray technology in 1987, we have continually improved upon our technology, and the use of dual-energy x-ray technology has become and remains a leading bone densitometry assessment tool. We offer a range of bone densitometers with various features and options to address the requirements of our diverse customer base.
Sahara Clinical Bone Sonometers
We have developed and sell a relatively low-cost, lightweight, portable ultrasound bone analyzer, called Sahara, that assesses the bone density of the heel that can assist in initial screening for osteoporosis.
Mini C-arm Imaging
We manufacture and distribute Fluoroscan mini C-arm imaging systems. Mini C-arms provide low intensity, real-time x-ray imaging, with high-resolution images at radiation levels and at a cost below those of conventional x-ray and fluoroscopic equipment. Mini C-arm systems are used primarily by orthopedic surgeons to perform minimally invasive surgical procedures on a patient’s extremities, such as the hand, wrist, knee, foot and ankle.
9
Extremity MRI
We distribute extremity MRI systems manufactured by Esaote. The target markets for these products are rheumatology, with specific emphasis on the early detection of rheumatoid arthritis and orthopedics, with an emphasis on orthopedic interventions and surgical planning.
Marketing, Sales and Service
We sell and service our products through a combination of direct sales and service forces and a network of independent distributors and sales representatives. In fiscal 2009, 2008 and 2007, no customer accounted for more than 10% of our consolidated revenues.
As of October 25, 2009, our direct sales and service force consisted of approximately 1,527 people.
During fiscal year 2009, our U.S. Breast Health and Skeletal Health sales force was comprised of full line modality account managers selling mammography and bone densitometry products, assisted by women’s health product specialists. The radiology sales specialists worked together with modality account managers leveraging our strong market presence in women’s health, calling on radiologists and breast surgeons, and focused on the sale of breast biopsy devices. Our breast surgery sales specialists focused on breast surgeons and radiation oncologists to sell our MammoSite technology and breast biopsy products. Our U.S. sales efforts also included the use of national account managers focused on obtaining purchasing contracts from large purchasing entities, such as managed care organizations, integrated delivery networks (“IDNs”) and government healthcare facilities. In addition, in certain regions of the U.S., we use a limited number of independent dealers or distributors to sell and service our product. These relationships enable us to sell into accounts where we might not otherwise have access.
During fiscal year 2009, we sold our breast health and skeletal health products in international markets through a network of independent distributors and sales representatives, as well as a direct sales and service force in Belgium (and Germany for AEG products). We offer our products in Europe, Latin America, including Argentina, Brazil, Chile and Mexico and into Pacific Rim countries, including Japan, Australia, South Korea, Thailand and Taiwan, by working with local sales representatives and distributors or entering into strategic marketing alliances in those territories. In fiscal 2009, 2008 and 2007 foreign sales accounted for approximately 20%, 20% and 25% of our product sales, respectively. See Note 13 in our Consolidated Financial Statements contained in Item 15 of this Annual Report for geographical information concerning those sales.
Our worldwide Diagnostics and GYN Surgical sales force consists of more than 335 persons focused on healthcare providers, clinical laboratories and third-party payors. A critical element of our strategy in the United States has been to utilize the results of our clinical trials and expanded FDA labeling to demonstrate safety, efficacy and productivity improvements to our target customers. Our Diagnotics’ sales force focuses on selling to clinical laboratories and OB/GYN offices, while our GYN Surgical sales force targets GYN surgeons in both hospital and office settings.
Our Diagnostics and GYN Surgical products are marketed outside of the United States by maintaining a presence in Canada, Europe, Australia and Hong Kong. We established these operations to manage sales, service, training and distribution in the Canadian, European and Asia/Pacific markets. We have also utilized a network of third-party distributors in various other countries throughout the world, including Japan and China. We believe that in order to effectively market our current products and any other new products and applications on a worldwide basis, we will need to continue to increase our international marketing, sales, and service capabilities.
Our service organization is responsible for installing our products, providing warranty and repair services, applications training and biomedical training. Products sold by our direct sales force typically carry limited warranties covering parts and labor for twelve months. Products sold through dealers also carry limited
10
warranties that typically last for twelve months and cover only parts or components. We also offer service contracts to our customers that generally last one to five years after the original warranty period. We provide both repair services and routine maintenance services under these arrangements, and also offer repair and maintenance services on a time and materials basis to customers that do not have service contracts. Internationally, we primarily use distributors, sales representatives and third parties to provide maintenance service for our products.
As of October 25, 2009, we employed approximately 627 people as field service engineers, internal technical support personnel and related administrative personnel.
Competition
The healthcare industry, in general, and the markets in which our products compete are highly competitive and characterized by continual change and improvement in technology. A number of companies have developed, or are expected to develop products that compete or will compete with our products. Many of these competitors offer a range of products in areas other than those in which we compete, which may make these competitors more attractive to hospitals, radiology clients, group purchasing organizations, laboratories, physicians and other potential customers. In addition, many of our competitors and potential competitors are larger and have greater financial resources than we do and offer a range of competitive products broader than our products. Some of the companies with whom we compete have or may have more extensive research, sales, marketing and manufacturing capabilities and significantly greater technical and personnel resources than we do, and may be better positioned to continue to improve their technology in order to compete in an evolving industry. Competitors may develop superior products or products of similar quality for sale at the same or lower prices. Moreover, our products could be rendered obsolete by new industry standards or changing technology. We cannot assure that we will be able to compete successfully with existing or new competitors.
Our mammography and related products and subsystems compete on a worldwide basis with products offered by a number of competitors, including GE, Siemens, Philips, PlanMed, Agfa, Carestream Health, Fuji, IMS Giotto, Sectra and Toshiba. Our Selenia full field digital mammography system competes with products such as GE’s and Siemens’ full field digital mammography systems, as well as Fuji’s Computed Radiography (“CR”) mammography system, a lower-priced alternative to digital mammography. Agfa, Carestream Health, Cedara and Sectra have introduced mammography workstations and are marketing these in competition with our line of radiologist review stations. Other companies are marketing digital mammography systems or technologies in Europe and other international markets and have or are expected to apply for FDA clearance in the U.S. The FDA has announced its intent to reclassify full field digital mammography systems from Class III to Class II devices.A FDA panel meeting was held on November 17, 2009 to review public comments on the proposed FFDM guidance. As a result, these systems are expected to become eligible for clearance for commercialization through the FDA’s 510(k) process rather than the more rigorous pre-market approval process, which may increase the number of competitors entering the United States market. We anticipate that competition in the digital mammography market will intensify.
While we offer a broad product line of breast imaging and related products, we compete most effectively in the high-end segment of the mammography market. We believe that our continued success will depend upon the continued success of our Selenia full field digital mammography system, as well as our ability to maintain our technology leadership through product enhancements and the development of new products and technologies, such as our Dimensions breast tomosynthesis (3D) product, which we recently introduced in Europe and Canada. We have also introduced this product for 2D applications in the United States. We are continuing our clinical trials to support our PMA application to market the 3D configuration of this product in the United States.
The primary competitor for our biopsy and tissue extraction product line is Ethicon, a Johnson & Johnson company. While there are many companies in the biopsy device market, other principal competitors include SenoRx, Bard and Cardinal. In addition, emerging companies like Sanarus and Intact Medical all share some
11
smaller portion of the biopsy device market. We believe that competition for our biopsy and tissue extraction product line is based largely on tissue sampling quality, product features, ease of use, product reliability and price.
Our MammoSite system faces competition from more commonly-known alternatives, such as treatments using external beam whole breast radiation, which has longer-term data on patient outcomes, and recent market entrants such as SenoRx, Inc. and Cianna Medical, which offer multi-lumen products that may support higher reimbursement rates for radiation oncology users. We believe that we will be able to compete more effectively against these multi-lumen products with the introduction of our multi-lumen MammoSite ML product. Internationally, our MammoSite product faces competition from traditional mastectomy, whole breast radiation therapy after lumpectomy, and a more radical breast-conserving procedure called a quadrantectomy. Additional radiation therapy methods, such as intraoperative radiation therapy, are being used in Europe by some institutions; however, such alternative methods have not yet achieved widespread commercial use.
Our ThinPrep liquid-based slide preparation faces direct competition in the United States primarily from Becton, Dickinson and Company, which manufactures liquid-based slide preparation systems and slide imaging systems. We also compete with the conventional Pap smear and other alternative methods for detecting cervical cancer and/or its precursors. Our products compete on the basis of a number of factors, including clinical performance, product quality, marketing and sales capabilities, manufacturing efficiency, price and customer service and support. Internationally, our ThinPrep product competes with a variety of companies and other “off-market” (non-FDA-approved) tests, since fewer regulatory barriers exist in Europe as compared to the United States.
With our Rapid Fetal Fibronectin Test, we are currently the only provider of a molecular test for predicting the risk of preterm birth. However, this product could experience competition from companies that manufacture and market pregnancy-related diagnostic products and services. In addition, healthcare providers use diagnostic techniques such as clinical examination and ultrasound to diagnose the likelihood of preterm birth. Healthcare providers may choose to continue using these techniques to assess their patients, rather than use the Rapid Fetal Fibronectin Test. They may also choose to use these techniques in conjunction with our Rapid Test to predict preterm birth.
In the molecular diagnostics market, our Invader products compete with many companies in the U.S. and abroad engaged in the development, commercialization and distribution of similar products intended for clinical molecular diagnostic applications. These companies may have or develop products competitive with the products offered by us. Clinical laboratories also may offer testing services that are competitive with our products. Clinical laboratories may use reagents purchased from us or others to develop their own diagnostic tests. Such laboratory-developed tests may not be subject to the same requirements for clinical trials and FDA submission requirements that may apply to our products.
In the clinical market, we compete with several companies offering alternative technologies to the Invader chemistry including Abbott Laboratories, Siemens, Becton, Dickinson and Company, Qiagen, Roche Diagnostics Corporation, Gen-Probe, Applera Corporation, Applied Biosystems, Celera, Innogenetics, Inc., and Luminex Corporation. Our Cervista HPV tests, which were approved by the FDA in March 2009, compete with a test marketed by Qiagen, which received FDA approval in 1999. We believe the primary competitive factors of our Invader products are their performance, reliability, cost and ease of use, standardization and our market position. However, we believe the successful completion of our initiative of automating our laboratory products, including our Cervista HPV tests, will be essential to securing the higher volume laboratories as customers.
Our NovaSure System currently faces direct competition from Johnson & Johnson, Boston Scientific, and American Medical Systems, Inc., each of which currently markets an FDA-approved “second generation” endometrial ablation device for the treatment of excessive menstrual bleeding. In addition to these devices, there exist alternative treatments to our NovaSure System, such as drug therapy, hysterectomy, dilation and curettage
12
and rollerball ablation. Internationally our products compete with drug therapy, as well as other endometrial ablation devices, including Johnson & Johnson’s Thermachoice, Boston Scientific’s HTA, and two other relatively small companies that market products that are not FDA approved. Because drug therapy is an alternative to our NovaSure procedure, NovaSure’s competitors also include many major pharmaceutical companies that manufacture hormonal drugs for women. We believe that the initial success of our NovaSure product has been primarily based upon its efficacy, ease of use, including limited patient pre-treatment requirements, and patient recovery.
Our Adiana System is the only non-incisional, non-hormonal permanent contraception method available that does not leave metal in the uterus that might limit future options for gynecological tests or procedures. Our Adiana product will compete directly with Conceptus, Inc.’s Essure product, which is the only other option for hysteroscopic sterilization on the market. We believe the Adiana System has significant advantages in safety and ease of use. In addition, the Adiana System will compete with traditional permanent contraception methods, such as tubal ligation and vasectomy, as well as with other products used for temporary birth control methods, such as diaphragms, condoms, spermicides, birth control pills and IUDs.
GE is the primary competitor for our skeletal health products. We believe that competition in our skeletal health markets is based upon product versatility and features, price, precision, speed of measurement, reputation, cost and ease of operation, product reliability and quality of service.
Manufacturing
We have historically purchased many of the components and raw materials used in our products from numerous suppliers worldwide. For reasons of quality assurance, sole source availability or cost effectiveness, certain components and raw materials used in the manufacture of our products are available only from a sole supplier. We have worked closely with our suppliers to develop contingency plans to assure continuity of supply while maintaining high quality and reliability, and in some cases, we have established long-term supply contracts with our suppliers. In certain instances, we have developed the in-house capability necessary to offset potential shortages caused by sole source suppliers. Due to FDA requirements applicable to the manufacturing of our products, we may not be able to quickly establish additional or replacement sources for certain components or materials. In the event that we are unable to obtain sufficient quantities of raw materials or components on commercially reasonable terms or in a timely manner, our ability to manufacture our products on a timely and cost-competitive basis may be compromised, which may have a material adverse effect on our business, financial condition and results of operations.
We manufacture our direct radiography detectors at our manufacturing facilities in Newark, Delaware and Warstein, Germany. We manufacture substantially all of our mammography and certain of our breast biopsy systems at our manufacturing facilities in Danbury, Connecticut. We manufacture our CAD line of products, the SecurView Work Stations, our osteoporosis assessment and our mini C-arm imaging systems at our headquarters in Bedford, Massachusetts. We continue to develop our software for our CAD products at our Santa Clara, California facility. The MammoPad breast cushion is manufactured by third parties and drop-shipped from our suppliers directly to our customers. Our breast biopsy disposable products are manufactured in Indianapolis, Indiana. Our ATEC control consoles for breast biopsy are manufactured by a third party, with quality control performed by our employees.
Our ThinPrep Processors and ThinPrep Imaging Systems are assembled at our facility in Marlborough, Massachusetts. Our ThinPrep PreservCyt vials are filled at our facility in Londonderry, New Hampshire. Our ThinPrep System filters are manufactured at both our Marlborough and Londonderry facilities. The manufacture of our NovaSure disposable devices has been transferred to our new facility in Coyol, Costa Rica. We also transferred production of the RF Controller component of our NovaSure System from an electronics contract manufacturer to our Marlborough facility. We contract with several third-parties to manufacture certain components of our MammoSite System, and we complete the manufacturing process at our Costa Rica and/or
13
Marlborough locations, depending on the configuration. We recently transferred the manufacturing of our Adiana System to our new manufacturing facility in Coyol, Costa Rica, and we are in the process of ramping up production to support the full scale launch of this product. We could incur delays and unanticipated costs in connection with our transfer and ramping up these manufacturing operations to our new facility that could delay our full scale launch of the Adiana System.
We manufacture our molecular diagnostics products at our facility in Madison, Wisconsin and source certain components from various contract manufacturers.
Backlog
Our backlog as of November 7, 2009 totaled $304.8 million and as of November 9, 2008 totaled $356.7 million. Backlog consists of customer orders for which a delivery schedule within the next twelve months has been specified. Orders included in backlog may be canceled or rescheduled by customers without significant penalty. Backlog as of any particular date should not be relied upon as indicative of our net revenues for any future period.
Research and Development
Our research and development efforts are focused on the further development and improvement of our existing products as well as the engineering and design of new innovative medical diagnostic and interventional devices, therapeutic applications and end use systems focused on women’s health. During fiscal 2009, our development projects included the ongoing development, clinical trials and other support for the FDA clearance or approval process for our Dimensions, Adiana System, Cervista HPV HR and Cervista HPV 16/18 and MammoSite ML products as well as the development of improvements to, or next generation products, for our other product lines. We anticipate continuing research and development to support these ongoing efforts.
In addition to product development, our research and development personnel play an active role in the review of product specifications, clinical protocols and FDA submissions, as well as ensuring that certain of our products conform to European health, safety and environmental requirements (CE marking). Our research and development expenses were $94.3 million in fiscal 2009, $81.4 million in fiscal 2008 and $44.4 million in fiscal 2007.
Patents and Proprietary Rights
We rely primarily on a combination of trade secrets, patents, copyright and trademark laws, and confidentiality procedures to protect our technology. Due to the rapid technological changes that characterize the markets we operate in, we believe that the improvement of existing products, reliance upon trade secrets and unpatented proprietary know-how and the development of new products are generally as important as patent protection in establishing and maintaining a competitive advantage. Nevertheless, we have obtained patents and will continue to make efforts to obtain patents, when available, in connection with our product development program.
We own numerous U.S. patents and have applied for numerous additional U.S. patents relating to our technologies. We also own or have applied for corresponding patents in selected foreign countries. These patents relate to various aspects of most of our products. We do not know if current or future patent applications will be issued with the scope of the claims sought, if at all, or whether any patents issued will be challenged or invalidated. There is a risk that our patent applications will not be granted or that the patent or patent application will not provide significant protection for our products and technology. Unauthorized third parties may infringe our intellectual property rights, copy or reverse engineer portions of our technology. Our competitors may independently develop similar technology that our patents do not cover. In addition, because patent applications in the U.S. are not generally publicly disclosed until eighteen months after the application is filed, applications may have been filed by third parties that relate to our technology. Moreover, there is a risk that foreign
14
intellectual property laws will not protect our intellectual property rights to the same extent as intellectual property laws in the U.S. The rights provided by a patent are finite in time. Over the coming years, certain patents relating to current products will expire in the U.S. and abroad thus allowing third parties to utilize certain of our technologies. In the absence of significant patent protection, we may be vulnerable to competitors who attempt to copy our products, processes or technology.
In addition to the patents we have been issued or we have acquired, we license patents from others on a variety of terms and conditions.
We are engaged in intellectual property litigation as described in Item 3, Legal Proceedings, and may be notified in the future of claims that we may be infringing intellectual property rights possessed by other third parties. In connection with any such litigation or if any claims are asserted against us or our products, we may seek to enter into settlement and/or licensing arrangements. There is a risk in these situations that no license will be available or that a license will not be available on reasonable terms. Alternatively, we may decide to litigate such claims or to design around the patented technology. These actions could be costly and would divert the efforts and attention of our management and technical personnel. As a result, any infringement claims by third parties or other claims for indemnification by customers resulting from infringement claims, whether or not proven to be true, may harm our business and prospects.
Regulation
The manufacture, sale, lease and service of medical diagnostic and surgical devices and pharmaceutical products intended for commercial use are subject to extensive governmental regulation by the FDA in the United States and by a variety of regulatory agencies in other countries. Under the Federal Food, Drug and Cosmetic Act, known as the FD&C Act, manufacturers of medical products and devices must comply with certain regulations governing the design, testing, manufacturing, packaging, servicing and marketing of medical products. Some of our products are also subject to the Radiation Control for Health and Safety Act, administered by the FDA, which imposes performance standards and record keeping, reporting, product testing and product labeling requirements for devices that emit radiation, such as x-rays.
The FDA generally must clear the commercial sale of new medical devices. Commercial sales of our medical devices within the United States must be preceded by either a pre-market notification filing pursuant to Section 510(k) of the FD&C Act or the granting of a pre-market approval (“PMA”). A 510(k) pre-market notification filing must contain information establishing that the device to be sold is substantially equivalent to a device commercially distributed prior to May 28, 1976.
The PMA procedure involves a complex and lengthy testing and review process by the FDA and may require several years to obtain. We may need to first obtain an investigational device exemption, known as an IDE, in order to conduct extensive clinical testing of the device to obtain the necessary clinical data for submission to the FDA. The FDA will grant a PMA only if after evaluating clinical data it finds that the safety and effectiveness of the product has been sufficiently demonstrated. This approval may restrict the number of devices distributed or require additional patient follow-up for an indefinite period of time. In fiscal 2009, we received PMA’s for Cervista HPV HR, Cervista HPV 16/18, and the Adiana System, and we received 510(k) clearance for MammoSite ML. In addition, we are conducting additional clinical trials to support our PMA application for our Dimensions 3D system configuration for tomosynthesis. We cannot assure when we will complete these additional clinical trails, whether the results of the clinical trials will be viewed as sufficient by the FDA to support our application, whether or when the FDA may approve our PMA, if at all, or the conditions for use that may be imposed by the FDA if and when the application is approved.
15
Sales of medical devices outside of the United States are subject to foreign regulatory requirements that vary widely from country to country. The time required to obtain approval from a foreign country to market and sell our products may be longer or shorter than that required for FDA approval and the requirements may differ. In addition, we may be required to meet the FDA’s export requirements or receive FDA export approval for export of our products to foreign countries. Moreover, some of our technology is governed by the International Traffic in Arms Regulations of the United States Department of State. As a result, the export of some of our systems to some countries may be limited or prohibited.
On May 23, 2006, the FDA Radiological Devices Panel recommended the reclassification of full field digital mammography systems from Class III to Class II devices. The reclassification would result in these systems being cleared for commercialization through the 510(k) process. This may result in more competitors entering the United States market. The FDA has issued a guidance document on full field digital mammography for public comment. It is not possible to predict if and when the reclassification will occur. A FDA panel meeting was held on November 17, 2009 to review public comments on the proposed FFDM guidance.
Our manufacturing processes and facilities are subject to continuing review by the FDA and foreign governments or their representatives. Adverse findings could result in various actions against us, including withdrawal of approvals and product recall.
The laboratories that purchase our ThinPrep System, ThinPrep Imaging System and the Rapid Fetal Fibronectin Test are subject to extensive regulation under the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”), which requires laboratories to meet specified standards in the areas of personnel qualifications, administration, participation in proficiency testing, patient test management, quality control, quality assurance and inspections. We believe that the ThinPrep System (including the ThinPrep Imaging System) and the Rapid Test operate in a manner that will allow laboratories purchasing these products to comply with CLIA requirements. However, we cannot assure that adverse interpretations of current CLIA regulations or future changes in CLIA regulations would not have an adverse effect on sales of the ThinPrep System, ThinPrep Imaging System and the Rapid Fetal Fibronectin Test.
The majority of the current clinical diagnostic products we acquired as part of the Third Wave acquisition were sold as Analyte Specific Reagents, known as ASRs. The FDA restricts the sale of these products to clinical laboratories certified under CLIA to perform high complexity testing and also restricts the types of products that can be sold as ASRs. In 2006, followed by additional clarification in 2007, the FDA issued guidance concerning acceptable examples of reagents that meet the threshold of the ASR regulations. In this guidance, the FDA outlined examples of products and marketing practices that go beyond the scope of the ASR regulations making the reagent part of a test system potentially subject to premarket review. These examples include combining, or promoting for use, a single ASR with another product such as other ASRs, general purpose reagents, controls, laboratory equipment, software, etc., or promoting an ASR with specific analytical or performance claims, instructions for use in a particular test, or instructions for validation of a specific test using the ASR. As a result of this recent guidance we have taken steps to comply with the guidance. This resulted in discontinuing certain Third Wave products that were previously sold as ASRs. We have applied for investigational device exemptions for the remaining products which will permit continued commercialization. There is no assurance that the FDA will grant such exemptions, in which case we may be required to discontinue sales of the product if it does not otherwise qualify for use as an ASR.
We cannot assure that the FDA or foreign regulatory agencies will give the requisite approvals or clearances for any of our medical devices under development on a timely basis, if at all. Moreover, after clearance is given, these agencies can later withdraw the clearance or require us to change the device or its manufacturing process or labeling, to supply additional proof of its safety and effectiveness, or to recall, repair, replace or refund the cost of the medical device, if it is shown to be hazardous or defective. The process of obtaining clearance to market products is costly and time-consuming and can delay the marketing and sale of our products.
16
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including federal and state anti-kickback laws, as well as the Foreign Corrupt Practices Act. Anti-kickback laws make it illegal for an entity to solicit, offer, receive, or pay remuneration or anything of value in exchange for, or to induce, the referral of business or the purchasing, leasing, ordering, or arranging for or recommending the purchase, lease or order of any item or service paid for by Medicare, Medicaid or certain other federal healthcare programs. The statute has been broadly interpreted to cover a wide array of practices. Some states have passed similar laws. The federal government has published regulations that identify “safe harbors,” which if applicable will assure that certain arrangements will not be found to violate the federal anti-kickback statutes. Our activities relating to the sale and marketing of our products may be subject to scrutiny under these laws. While we make every effort to comply with the regulations, it is possible that our practices might be challenged under federal anti-kickback or similar laws due to the breadth of the statutory provisions and the absence of extensive guidance regarding compliance. Violations of these laws may be punishable by criminal and/or civil sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid). If the government were to raise questions about our behavior or find that we have violated these laws, there could be a material adverse effect on our business. Our activities could be subject to challenge for the reasons discussed above, due to the broad scope of these laws and the increasing attention being given to them by law enforcement authorities.
We are also subject to numerous federal, state and local laws relating to safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances, among others. We may be required to incur significant costs to comply with these laws and regulations in the future, and complying with these laws may result in a material adverse effect upon our business, financial condition and results of operations.
Federal, state and foreign regulations regarding the manufacture and sale of medical devices and pharmaceuticals are subject to future change. We cannot predict what impact, if any, such changes might have on our business.
Reimbursement
In the U.S., the Centers for Medicare & Medicaid Services, known as CMS, establishes policies for the coverage and reimbursement of Medicare and Medicaid beneficiaries. Under current CMS policies, varying reimbursement levels have been established for bone density assessment, endometrial ablations, mammography and other imaging, diagnostic tests and surgical procedures performed using our products. Coverage policies for Medicare patients may vary by regional Medicare carrier in the absence of a National Coverage Decision and reimbursement rates for procedures will vary based on the geographic price index. Coverage and reimbursement for patients with private insurance is dependent on the individual private payer’s decisions and may not follow the policies and rates established by CMS for Medicare. Moreover, private insurance carriers may choose not to follow the CMS reimbursement policies. The use of our products outside the U.S. is similarly affected by reimbursement policies adopted by foreign regulatory and insurance carriers.
In October 2009, CMS announced 2010 reimbursement rates for physician, hospital and ambulatory surgical center payments. CMS also implemented provisions of the Deficit Reduction Act of 2005 related to certain medical imaging procedures. For 2010, the downward adjustments that affect our products include an approximate 11% decline in reimbursement for CAD, and an approximate 14% reduction in bone density assessments for osteoporosis rates. The CMS reductions that would affect the reimbursement for the use of our products also include a general reduction of 21% in the Sustainable Growth Rate (“SGR”) factor. This factor is used by CMS in a formula to determine doctor reimbursements. Congress has, from time to time, overridden some or all of the proposed reductions in reimbursement, and there is pending legislation to freeze the SGR for 2010 which would eliminate this potential decrease. However, we cannot assure that Congress will override any part of the recent proposed reductions. Significant reductions in reimbursement rates proposed or implemented for the use of any our products has had and may continue to have a material adverse affect on the sales of those products.
17
Political, economic and regulatory influences may subject the healthcare industry to fundamental changes. We anticipate that the current administration, Congress and certain state legislatures will continue to review and assess alternative healthcare delivery systems and payment methods with an objective of ultimately reducing healthcare costs and expanding access. The uncertainties regarding the ultimate features of reform initiatives and their enactment and implementation may have an adverse effect on our customers’ purchasing decisions regarding our products. At this time, we cannot predict which, if any, healthcare reform proposals will be adopted, when they may be adopted or what impact they may have on our business.
Employees
As of October 25, 2009, we had approximately 3,959 full-time employees, including 1,473 in manufacturing operations, 390 in research and development, 1,631 in marketing, sales and support services, and 465 in finance and administration. The non-management employees of our subsidiary, AEG, are represented by a union. AEG’s approximate 236 non-management German employees were subject to collective bargaining agreements negotiated on a national and regional basis between Unternehmens-Verband Südöstliches Westfalen e.V., the Employers Association of North Rhine-Westphalia, and the German Metal Workers Union, IndustrieGewerkschaft Metall. In addition, AEG’s German employees are represented by a works council, a Betriebsrat, with respect to various shop agreements for social matters and working conditions. By Chinese law, all labor contracts of the non-management employees of AEG’s Chinese subsidiary are registered at the labor department of the local authorities, but are currently not members of the labor union. During the fourth quarter of fiscal 2009, the majority of these employees were terminated in connection with the closure of our manufacturing facility in China. We believe that our relationship with our employees is good. Except as described herein, none of our other employees are represented by a union.
This report contains forward-looking information that involves risks and uncertainties, including statements regarding our plans, objectives, expectations and intentions. Such statements made in this report should be read as applicable to all forward-looking statements wherever they appear in this report. Our actual results could differ materially from those discussed herein. Factors that could cause or contribute to such differences include those discussed below, as well as those discussed elsewhere in this report.
Risks Related to our Business
The worldwide macroeconomic downturn may adversely affect our business and prospects.
Market acceptance of our medical products in the United States and other countries is dependent upon the medical equipment purchasing and procurement practices of our customers, patient demand for our products and procedures and the reimbursement of patient’s medical expenses by government healthcare programs and third-party payors. Since the end of calendar 2008, the uncertainty surrounding world financial markets and deteriorating worldwide macroeconomic conditions have caused and may continue to cause the purchasers of medical equipment to decrease their medical equipment purchasing and procurement activities. Additionally, constrictions in world credit markets have caused and may continue to cause our customers to experience increased difficulty securing the financing necessary to purchase our products. Economic uncertainty has and may result in cost-conscious consumers making fewer elective trips to their physicians and specialists, which in turn would adversely affect demand for our products and procedures. Furthermore, governments and other third party payors around the world facing tightening budgets could move to further reduce the reimbursement rates or the scope of coverage offered, which could adversely affect sales of our products. If the current adverse macroeconomic conditions continue, our business and prospects may be adversely affected.
18
Sales and market acceptance of our products is dependent upon the coverage and reimbursement decisions made by third party payors. The failure of third party payors to provide appropriate levels of coverage and reimbursement for the use of our products and treatments facilitated by our products could harm our business and prospects.
Sales and market acceptance of our medical products and the treatments facilitated by our products in the United States and other countries is dependent upon the coverage decisions and reimbursement policies established by government healthcare programs and private health insurers. The costs of our products and treatments to customers are substantial, and market acceptance of our products and treatments will continue to depend upon our customers’ ability to obtain an appropriate level of coverage for, and reimbursement from third-party payors for, these products and treatments. In the U.S., the Centers for Medicare & Medicaid Services, known as CMS, establish coverage and reimbursement policies for healthcare providers treating Medicare and Medicaid beneficiaries. Under current CMS policies, varying reimbursement levels have been established for our products and treatments. Coverage policies for Medicare patients may vary by regional Medicare carriers in the absence of a National Coverage Decision and reimbursement rates for treatments may vary based on the geographic price index. Coverage and reimbursement policies and rates applicable to patients with private insurance are dependent upon individual private payor decisions which may not follow the policies and rates established by CMS. The use of our products and treatments outside the United States is similarly affected by coverage and reimbursement policies adopted by foreign governments and private insurance carriers.
In October 2009, CMS announced 2010 reimbursement rates for physician, hospital and ambulatory surgical center payments. CMS also implemented provisions of the Deficit Reduction Act of 2005 related to certain medical imaging procedures. For 2010, the downward adjustments that affect our products include an approximate 11% decline in reimbursement for CAD, and an approximate 14% reduction in bone density assessments for osteoporosis rates. The CMS reductions that would affect the reimbursement for the use of our products also include a general reduction of 21% in the Sustainable Growth Rate (“SGR”) factor. This factor is used by CMS in a formula to determine doctor reimbursements. Congress has, from time to time, overridden some or all of the proposed reductions in reimbursement, and there is pending legislation to freeze the SGR for 2010 which would eliminate this potential decrease. However, we cannot assure that Congress will override any part of the recent proposed reductions. Significant reductions in reimbursement rates proposed or implemented for the use of any our products has had and may continue to have a material adverse affect on the sales of those products.
These reductions in CMS reimbursement rates, any further reductions in reimbursement rates established by CMS or other third party payors, and any decision to cease providing coverage for any of our products or treatments by CMS or other third party payors could adversely affect our business and prospects.
The uncertainty of healthcare reform could harm our business and prospects.
In recent years, the healthcare industry has undergone significant change driven by various efforts to reduce costs, including efforts at national healthcare reform, trends toward managed care, cuts in Medicare, consolidation of healthcare distribution companies and collective purchasing arrangements by office-based healthcare practitioners. We anticipate that the current administration, Congress and certain state legislatures will continue to review and assess alternative healthcare delivery systems and payment methods with an objective of ultimately reducing healthcare costs and expanding access. Public debate of these issues will likely continue in the future. At this time, we cannot predict which, if any, healthcare reform proposals will be adopted, when they may be adopted or what impact they may have on our business. Healthcare reform proposals and medical cost containment measures in the United States and in many foreign countries could:
| | • | | limit the use of our products and treatments; |
| | • | | reduce reimbursement available for such use; or |
| | • | | adversely affect the use of new therapies for which our products may be targeted. |
19
These reforms or cost containment measures, including the uncertainty in the medical community regarding their nature and effect, could have an adverse effect on our customers’ purchasing decisions regarding our products and treatments and could harm our business and prospects.
Changes in laws affecting the healthcare industry could adversely affect our revenues and profitability.
We operate in a highly regulated industry. As a result, governmental actions may adversely affect our business, operations or financial condition, including:
| | • | | new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, method of delivery and payment for health care products and services; |
| | • | | changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and treatments and result in lost market opportunity; |
| | • | | changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products and treatments to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products or treatments, or otherwise adversely affect the market for our products and treatments; |
| | • | | new laws, regulations and judicial decisions affecting pricing or marketing practices; and |
| | • | | changes in the tax laws relating to our operations, such as the tax proposal included in the health-care reform bill recently approved the Finance Committee of the U.S. Senate which would assess an annual tax on the revenue of medical device manufacturers based upon market share. |
Guidelines, recommendations and studies published by various organizations can reduce the use of our products.
Professional societies, practice management groups, private health/science foundations, and organizations involved in healthcare issues may publish guidelines, recommendations or studies to the healthcare and patient communities from time to time. Recommendations of government agencies or these other groups/organizations may relate to such matters as usage, cost-effectiveness, and use of related therapies. Organizations like these have in the past made recommendations about our products and those of our competitors. Recommendations, guidelines or studies that are followed by patients and healthcare providers could result in decreased use of our products. For example, recently, the American College of Obstetricians and Gynecologists changed their recommendations for pap smear screening, and the United States Preventive Services Task Force changed their recommendations for mammography screening. These new recommendations, if implemented, could significantly reduce the amount of screening using our ThinPrep, mammography and related products and adversely affect the sale of those products. Moreover, the perception by the investment community or stockholders that recommendations, guidelines or studies will result in decreased use of our products could adversely affect prevailing market price for our common stock.
Our long-term success will depend upon our ability to successfully develop and commercialize new products and treatments and enhance our existing products and treatments.
We are expending significant resources on our continuing research and development programs which are designed to develop new products and treatments and to enhance and improve our existing products and treatments. The successful development of our products and product enhancements is subject to numerous risks, both known and unknown, including:
| | • | | unanticipated delays in development or the FDA’s approval or clearance process; |
20
| | • | | third party intellectual property; |
| | • | | technical problems; and |
| | • | | other difficulties that could result in the abandonment or substantial change in the design, development and commercialization of these new products, including, for example, changes requested by the FDA in connection with pre-market approval applications for products or 510(k) clearance. |
Given the uncertainties inherent with product development, introduction, and enhancement our efforts may not be completed on a timely basis or within budget, if at all. Our failure to develop new products and product enhancements, such as our digital mammography tomosynthesis product, on a timely basis or within budget, if at all, could harm our business and prospects.
If we fail to achieve and maintain the high manufacturing standards that our products require, we may not be successful in developing and marketing those products.
The manufacture of many of our products is highly complex and requires precise high quality manufacturing that is difficult to achieve. We have in the past and may in the future experience difficulties in manufacturing our products in sufficient quantities. These difficulties have primarily related to delays and difficulties associated with ramping up production of newly introduced products. Our difficulties may lead to increased delivery lead-times and increased costs of manufacturing these products. Our failure, including the failure of our contract manufacturers, to achieve and maintain the required high manufacturing standards could result in further delays or failures in product testing or delivery, cost overruns, product recalls or withdrawals, increased warranty costs or other problems that could harm our business and prospects.
Problems with manufacturing could result in our inability to deliver products, inventory shortages, product recalls and increased costs.
We manufacture our own commercial requirements for all of our products except the Mammopad. Certain of our products are difficult to manufacture and problems in our manufacturing processes can occur, resulting in product defects or contamination, shipment delays and recalls. These events could result in lower revenues and loss of market share as well as result in inventory write-offs and impair our ability to expand into new markets or supply products in existing markets.
Our delay or inability to obtain any necessary United States or foreign regulatory clearances or approvals for our newly developed products and treatments or product enhancements could harm our business and prospects.
Our products and treatments are subject to a high level of regulatory oversight. Our delay or inability to obtain any necessary United States or foreign regulatory clearances or approvals for our newly developed products or product enhancements, such as our Dimensions digital mammography tomosynthesis product, could harm our business and prospects. The process of obtaining clearances and approvals can be costly and time-consuming. Recently we have encountered delays in the FDA approval process relating to the 3D configuration of our Dimensions digital mammography tomosynthesis product and are conducting additional clinical trials in support of our application to for a PMA for that product. We cannot assure that the additional clinical trials will be successful, that we will be able to obtain FDA approval to market the product, or the scope of any such approval, if and when obtained. Additionally, there is a risk that any approvals or clearances, once obtained, may be withdrawn or modified.
Medical devices cannot be marketed in the United States without clearance or approval by the FDA. Any modifications to a device that has received a pre-market approval that affect its safety or effectiveness require a
21
pre-market approval supplement or possibly a separate pre-market approval, either of which is likely to be time-consuming, expensive and uncertain to obtain. If the FDA requires us to seek one or more pre-market approval supplements or new pre-market approvals for any modification to a previously approved device, we may be required to cease marketing or to recall the modified device until we obtain approval, and we may be subject to significant criminal and/or civic sanctions, including but not limited to, regulatory fines or penalties.
Medical devices sold in the United States must also be manufactured in compliance with FDA Good Manufacturing Practices, which regulate the design, manufacture, packing, storage and installation of medical devices. Moreover, medical devices are required to comply with FDA regulations relating to investigational research and labeling. States may also regulate the manufacture, sale and use of medical devices, particularly those that employ x-ray technology. Our products are also subject to approval and regulation by foreign regulatory and safety agencies.
The markets for our newly developed products and treatments and newly introduced enhancements to our existing products and treatments may not develop as expected.
During fiscal 2009, we received FDA approval for our Cervista HPV HR and 16/18 tests, our Adiana System, and FDA clearance of our MammoSite ML radiation therapy system. The successful commercialization of our newly developed products and treatments and newly introduced enhancements to our existing products and treatments are subject to numerous risks, both known and unknown, including:
| | • | | uncertainty of the development of a market for such product or treatment; |
| | • | | trends relating to, or the introduction or existence of, competing products, technologies or alternative treatments or therapies that may be more effective, safer or easier to use than our products, technologies, treatments or therapies; |
| | • | | perceptions of our products or treatments as compared to other products and treatments; |
| | • | | recommendation and support for the use of our products or treatments by influential customers, such as hospitals, radiological practices, breast surgeons and radiation oncologists and treatment centers; |
| | • | | the availability and extent of data demonstrating the clinical efficacy of our products or treatments; |
| | • | | competition, including the presence of competing products sold by companies with longer operating histories, more recognizable names and more established distribution networks; and |
| | • | | other technological developments. |
Often, the development of a significant market for a product or treatment will depend upon the establishment of a reimbursement code or an advantageous reimbursement level for use of the product or treatment. Moreover, even if addressed, such reimbursement codes or levels frequently are not addressed until after a product or treatment is developed and commercially introduced, which can delay the successful commercialization of a product or treatment.
If we are unable to successfully commercialize and create a significant market for our products and treatments, such as those noted above-, as well as our Dimensions digital mammography tomosynthesis product, due to, among other things, the lack of reimbursement codes or disadvantageous reimbursement levels for such products or treatments, our sales growth, business and prospects could be harmed.
Our business may be harmed by recently completed acquisitions or acquisitions we may complete in the future.
We have acquired a number of businesses, technologies, product lines, and products in our recent past, including Third Wave and Cytyc in fiscal 2008, and may make additional acquisitions in the future. The long-term success of our recently completed acquisitions and any additional acquisitions we may complete in the
22
future will depend upon our ability to realize the anticipated benefits from combining the acquired businesses with our business. We may fail to realize anticipated benefits for a number of reasons, including the following:
| | • | | problems may arise with our ability to successfully integrate the acquired businesses, which may result in us not operating as effectively and efficiently as expected, and may include: |
| | • | | diversion of management time, as well as a shift of focus from operating the businesses to issues related to integration and administration or inadequate management resources available for integration activity and oversight; |
| | • | | failure to retain and motivate key employees; |
| | • | | failure to successfully obtain FDA approval or clearance for products under development; |
| | • | | failure to successfully manage relationships with customers, distributors and suppliers; |
| | • | | failure of customers to accept new products; |
| | • | | failure to effectively coordinate sales and marketing efforts; |
| | • | | failure to combine product offerings and product lines quickly and effectively; |
| | • | | failure to effectively enhance acquired technology and products or develop new products relating to the acquired businesses; |
| | • | | potential difficulties and inefficiencies in managing and operating businesses in multiple locations or operating businesses in which we have either limited or no direct experience; |
| | • | | potential difficulties integrating financial reporting systems; |
| | • | | potential difficulties in the timely filing of required reports with the SEC; and |
| | • | | potential difficulties in implementing controls, procedures and policies, including disclosure controls and procedures and internal controls over financial reporting, appropriate for a larger public company at companies that, prior to the acquisition of such companies, had lacked such controls, procedures and policies, which may result in ineffective disclosure controls and procedures or material weaknesses in internal controls over financial reporting; |
| | • | | we may not be able to achieve the expected synergies from an acquisition or it may take longer than expected to achieve those synergies; |
| | • | | an acquisition may result in future impairment charges related to diminished fair value of businesses acquired as compared to the price we paid for them; |
| | • | | an acquisition may involve restructuring operations or reductions in workforce which may result in substantial charges to our operations; |
| | • | | an acquisition may involve unexpected costs or liabilities, or the effects of purchase accounting may be different from our expectations; and |
| | • | | the acquired businesses may be adversely affected by future legislative, regulatory, or tax decisions and/or changes as well as other economic, business and/or competitive factors. |
Our failure to realize the anticipated benefits from combining acquired businesses could harm our business and prospects and adversely affect the market price of our common stock.
If we are successful in pursuing future acquisitions, we will be required to expend significant funds, incur additional debt or issue additional securities, which may negatively affect our results of operations and be dilutive to our stockholders. If we spend significant funds or incur additional debt, our ability to obtain financing for working capital or other purposes could decline, and we may be more vulnerable to economic downturns and competitive pressures. We cannot guarantee that we will be able to finance additional acquisitions or that we will realize any anticipated benefits from acquisitions that we complete.
23
The historical levels of sales growth experienced by our newly introduced products may not be indicative of future growth as the markets for our products mature.
Historically, the demand for our products and treatments is greatest upon their initial introduction. However, once markets mature, growth in the market may abruptly stop or significantly slow or demand may decline. During fiscal 2009, the demand for certain of our products, such as our direct-to-digital full-field mammography products that had previously experienced rapid growth, declined. We often cannot predict when, or at what rate, the demand for any of our products may decline. Slackening demand or reduced growth rates-for our products could adversely affect our operating results and profitability.
We are dependent upon a relatively small number of large clinical laboratory customers in the United States for a significant portion of our sales of the ThinPrep System and our molecular diagnostic products.
We are dependent upon a relatively small number of large clinical laboratory customers in the United States for a significant portion of our sales of the ThinPrep System and our molecular diagnostic products that were added with the Third Wave acquisition. Due in part to a trend toward consolidation of clinical laboratories in recent years and the relative size of the largest United States laboratories, it is likely that a significant portion of these sales will continue to be concentrated among a relatively small number of large clinical laboratories. Our business and prospects may be harmed if we are unable to increase sales to, or maintain pricing levels with our existing customers and establish new customers both within and outside the United States.
Our business could be harmed if we infringe upon the intellectual property rights of others.
There has been substantial litigation regarding patent and other intellectual property rights in the medical device and related industries. We have been involved in patent litigation, and may in the future be subject to claims of infringement of intellectual property rights possessed by third parties.
As examples, we are currently defending ourselves against infringement complaints filed by Ethicon Endo-Surgery, Inc., a Johnson & Johnson operating company and Conceptus, Inc. For further information regarding these complaints, please refer to Item 3. Legal Proceedings.
In connection with claims of patent infringement, we may seek to enter into settlement and/or licensing arrangements. There is a risk in these situations that no license will be available or that a license will not be available on reasonable terms. Alternatively, we may decide to litigate such claims or to design around the patented technology. These actions could be costly and would divert the efforts and attention of our management and technical personnel. As a result, any infringement claims by third parties or claims for indemnification by customers resulting from infringement claims, whether or not proven to be true, may harm our business and prospects.
Our business could be harmed if we are unable to protect our proprietary technology.
We have relied primarily on a combination of trade secrets, patents, copyright and trademark laws and confidentiality procedures to protect our products and technology. Despite these precautions, unauthorized third parties may infringe our intellectual property, copy or reverse engineer portions of our technology. We do not know if current or future patent applications will be issued with the scope of the claims sought, if at all, or whether any patents issued will be challenged or invalidated. In addition, we have obtained or applied for corresponding patents and patent applications in several foreign countries for some of our patents and patent applications. There is a risk that these patent applications will not be granted or that the patent or patent application will not provide significant protection for our products and technology. The rights provided by a patent are finite in time. Over the coming years, certain patents relating to current products will expire in the U.S. and abroad thus allowing third parties to utilize certain of our technologies. Our competitors may independently develop similar technology that our patents do not cover. In addition, because patent applications in the United
24
States are not generally publicly disclosed until eighteen months after the application is filed, applications may have been filed by third parties that relate to our technology. Moreover, there is a risk that foreign intellectual property laws will not protect our intellectual property rights to the same extent as intellectual property laws in the U.S. Even if we believed our proprietary information is protected by patents or otherwise, the initiation of actions to protect our proprietary information could be costly and divert the efforts and attention of our management and technical personnel, and the outcome of such litigation is often uncertain. As a result of these uncertainties, we could also elect to forego such litigation or settle such litigation without fully enforcing our proprietary rights. In the absence of significant patent protection, we may be vulnerable to competitors who attempt to copy our products, processes or technology.
Our international operations expose us to additional operational challenges that we might not otherwise face.
We are subject to a number of additional risks and expenses due to our international operations. Any of these risks or expenses could have a material adverse effect on our operating results. These risks and expenses include:
| | • | | difficulties in staffing and managing operations in multiple locations as a result of, among other things, distance, language and cultural differences; |
| | • | | protectionist laws and business practices that favor local companies; |
| | • | | greater difficulties in trade accounts receivable collection; |
| | • | | difficulties and expenses related to implementing internal controls over financial reporting and disclosure controls and procedures; |
| | • | | expenses associated with customizing products for clients in foreign countries; |
| | • | | possible adverse tax consequences; |
| | • | | the inability to obtain favorable third-party reimbursements; |
| | • | | the inability to obtain required regulatory approvals; |
| | • | | governmental currency controls; |
| | • | | multiple, conflicting and changing government laws and regulations (including, among other things, antitrust and tax requirements, international trade regulations and the Foreign Corrupt Practices Act); |
| | • | | reduced protection for intellectual property rights in some countries; |
| | • | | political and economic changes and disruptions; |
| | • | | clone or “knock off” products; |
| | • | | the inability to effectively obtain or enforce intellectual property rights; |
| | • | | export/import controls; and |
We utilize distributors for a portion of our sales, the loss of which could harm our revenues in the territory serviced by these distributors.
We have strategic relationships with a number of key distributors for sales and service of our products, principally in foreign countries. If these strategic relationships are terminated and not replaced, our revenues and/or ability to service our products in the territories serviced by these distributors could be adversely affected.
25
Fluctuations in the exchange rates of European currencies and the other foreign currencies in which we conduct our business, in relation to the U.S. dollar, could harm our business and prospects.
We maintain sales and service offices outside the United States, have manufacturing facilities in Germany and Costa Rica and conduct business worldwide. The expenses of our international offices are denominated in local currencies, except at our Costa Rica subsidiary, where the majority of business is conducted in U.S. dollars. Our foreign sales may be denominated in local currencies, the Euro or U.S. dollar, with a majority of our sales to international dealers denominated in U.S. dollars.
Fluctuations in foreign currency exchange rates could affect our revenues, cost of goods and operating margins and could result in exchange losses. In addition, currency devaluation can result in a loss if we hold deposits of that currency. We have historically hedged, and may in the future hedge, our foreign currency exposure by borrowing funds in local European currencies to pay the expenses of our foreign offices. There is a risk that any hedging activities will not be successful in mitigating our foreign exchange risk exposure.
Interruptions, delays, shutdowns or damage at our manufacturing facilities could harm our business.
We manufacture our products at a number of different facilities located throughout the world. An interruption in manufacturing capabilities at any of these facilities, as a result of equipment failure or other reasons, could reduce, delay or prevent the production of our products. Our manufacturing facilities are subject to the risk of catastrophic loss due to unanticipated events, such as fires, earthquakes, explosions, floods or weather conditions. Our manufacturing facilities may experience plant shutdowns, strikes or other labor disruptions, or periods of reduced production as a result of equipment failures, loss of power, gray outs, delays in deliveries or extensive damage to any of our facilities, which could harm our business and prospects. Because some of our manufacturing operations are located in Germany and Costa Rica, those manufacturing operations are also subject to additional challenges and risks associated with international operations described below.
Our business could be harmed if products contain undetected errors or defects or do not meet customer specifications.
We are continuously developing new products and improving our existing products. Our existing and newly introduced products can contain undetected errors or defects. In addition, these products may not meet their performance specifications under all conditions or for all applications. If, despite internal testing and testing by customers, any of our products contain errors or defects or fail to meet customer specifications, then we may be required to enhance or improve those products or technologies. We may not be able to do so on a timely basis, if at all, and may only be able to do so at considerable expense. In addition, any significant reliability problems could result in adverse customer reaction, negative publicity, mandatory or voluntary recall or legal claims and could harm our business and prospects.
We rely on one or only a limited number of suppliers for some key components or subassemblies for our products. This reliance could harm our business and prospects.
We rely on one or only a limited number of suppliers for some key components or subassemblies for our products. Obtaining alternative sources of supply of these components could involve significant delays and other costs and regulatory challenges, and may not be available to us on reasonable terms, if at all. The failure of a component supplier or contract assembler to provide sufficient quantities, acceptable quality and timely components or assembly service at an acceptable price, or an interruption of supplies from such a supplier could harm our business and prospects. Any disruption of supplies of key components could delay or reduce shipments, which could result in lost or deferred sales.
26
Our success depends on our ability to manage growth effectively.
Our failure to manage the growth of our business and scope of our operations effectively could harm our business and prospects. Such growth may significantly strain our managerial, operational and financial resources and systems. To manage such growth effectively, it is expected that we will continue to implement and improve additional management and financial systems and controls, and to effectively retain, expand, train and manage our employee base.
We face intense competition from other companies and may not be able to compete successfully.
A number of companies have developed, or are expected to develop, products that compete or will compete with our products. Some of our competitors are large companies that may enjoy significant competitive advantages over us, including:
| | • | | significantly greater name recognition; |
| | • | | established distribution networks; |
| | • | | additional lines of products, and the ability to offer rebates or bundle products to offer discounts or incentives to gain competitive advantage; |
| | • | | more extensive research, development, sales, marketing, manufacturing and financial capabilities; and |
| | • | | greater financial resources allowing them to continue to improve their technology in order to compete in an evolving industry. |
The markets in which we sell our products are intensely competitive, subject to rapid change and may be significantly affected by new product introductions and other market activities of industry participants. Other companies may develop products that are superior to or less expensive, or both, than our products. Improvements in existing competitive products or the introductions of new competitive products may reduce our ability to compete for sales, particularly if those competitive products demonstrate better safety or effectiveness, clinical results, ease of use or lower costs.
If we are unable to compete effectively against existing and future competitors and existing and future alternative treatments, our business and prospects could be harmed.
Our success depends upon our ability to adapt to rapid changes in technology and customer requirements.
The markets for our products have been characterized by rapid technological change, frequent product introductions and evolving customer requirements. These trends will likely continue into the foreseeable future. Our success depends, in part, upon our ability to enhance our existing products, successfully develop new products that meet increasing customer requirements and gain market acceptance. If we fail to do so our products may be rendered obsolete or uncompetitive by new industry standards or changing technology.
Our results of operations are subject to significant quarterly variation and seasonal fluctuation.
Our results of operations have been and may continue to be subject to significant quarterly variation. Our results for a particular quarter may also vary due to a number of factors, including:
| | • | | the overall state of healthcare and cost containment efforts; |
| | • | | the timing and level of reimbursement for our products domestically and internationally; |
| | • | | the development status and demand for our products; |
| | • | | the development status and demand for therapies to treat the health concerns addressed by our products and treatments; |
27
| | • | | economic conditions in our markets; |
| | • | | foreign exchange rates; |
| | • | | the timing of expenditures in anticipation of future sales; |
| | • | | the mix of products we sell; |
| | • | | regulatory approval of products; |
| | • | | the introduction of new products and product enhancements by us or our competitors; |
| | • | | pricing and other competitive conditions; |
| | • | | unanticipated expenses; and |
| | • | | complex revenue recognition rules pursuant to U.S. generally accepted accounting principles (“U.S. GAAP”). |
Customers may also cancel or reschedule shipments. Production difficulties could also delay shipments. Any of these factors also could harm our business and prospects.
Recent proposed changes to reclassify full-field digital mammography to permit 510(k) clearance could increase competition for our digital mammography products.
On May 23, 2006, the FDA Radiological Devices Panel recommended the reclassification of full-field digital mammography systems from Class III to Class II devices. The FDA has issued guidance on full field digital mammography for public comment during 2008. If the FDA implements the panel’s recommendation, the reclassification would allow full-field digital mammography systems to be cleared for commercialization through the 510(k) process, which is less rigorous than the present pre-market approval process. If and when implemented, the reclassification for full-field digital mammography systems from Class III to Class II devices may lower barriers of entry into the digital mammography market, may result in more competitors entering the United States market and could harm sales of our digital mammography systems. A FDA panel meeting was held on November 17, 2009 to review public comments on the proposed FFDM guidance.
Our products may be subject to recalls even after receiving FDA clearance or approval, which could harm our business and prospects.
The FDA and similar governmental bodies in other countries have the authority to require the recall of medical products in the event of material deficiencies or defects in design or manufacture. A government mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling. Any recall could harm the reputation of our products and adversely affect our business and prospects.
Some of our activities may subject us to risks under federal and state laws prohibiting “kickbacks” and false or fraudulent claims.
We are subject to the provisions of a federal law commonly known as the Medicare/Medicaid anti-kickback law, and several similar state laws, which prohibit payments intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of healthcare products or services. While the federal law applies only to referrals, products or services for which payment may be made by a federal healthcare program, state laws often apply regardless of whether federal funds may be involved. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices by limiting the kinds of financial arrangements, including sales programs, with hospitals, physicians, laboratories and other
28
potential purchasers of medical devices. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, or are for items or services that were not provided as claimed. Anti-kickback and false claims laws prescribe civil and criminal penalties (including fines) for noncompliance that can be substantial. Similarly, we are subject to the provisions of the U.S. Foreign Corrupt Practices Act, which prohibits payments to foreign officials. While we continually strive to comply with these complex requirements, interpretations of the applicability of these laws to marketing practices is ever evolving and even an unsuccessful challenge could cause adverse publicity and be costly to respond to, and thus could harm our business and prospects.
We are subject to the risk of product liability claims relating to our products.
Our business involves the risk of product liability and other claims inherent to the medical device business. If even one of our products is found to have caused or contributed to injuries or deaths, we could be held liable for substantial damages. We maintain product liability insurance subject to deductibles and exclusions. There is a risk that the insurance coverage will not be sufficient to protect us from product and other liability claims, or that product liability insurance will not be available to us at a reasonable cost, if at all. An under-insured or uninsured claim could harm our business and prospects. In addition, claims could adversely affect the reputation of the related product, which could damage that product’s competitive position in the market.
The sale and use of one of our diagnostic products could also lead to the filing of product liability claims if someone were to allege that one of our products contained a design or manufacturing defect that resulted in the failure to detect a disorder for which it was being used to screen, inaccurate test results or caused injuries to a patient. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or the inability to secure additional coverage in the future. Also, even a meritless or unsuccessful product liability claim could be time consuming and expensive to defend, which could result in a diversion of management’s attention from our business and could adversely affect the perceived safety and efficacy of our products, and could harm our business and prospects.
We use hazardous materials and products.
Our research and development and manufacturing processes involve the controlled use of hazardous materials, such as toxic and carcinogenic chemicals and various radioactive compounds. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by federal, state and local regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of this type of accident, we could be held liable for any resulting damages, and any such liability could be extensive. We are also subject to substantial regulation relating to occupational health and safety, environmental protection, hazardous substance control, and waste management and disposal. The failure to comply with such regulations could subject us to, among other things, fines and criminal liability.
Our future success depends on the continued services of key personnel.
The loss of any of our key personnel, particularly key research and development personnel, could harm our business and prospects and could impede the achievement of our research and development, operational or strategic objectives. Our success also depends upon our ability to attract and retain other qualified managerial and technical personnel. Competition for such personnel, particularly software engineers and other technical personnel, is intense. We may not be able to attract and retain personnel necessary for the development of our business.
29
Our failure to manage current or future alliances or joint ventures effectively may harm our business and prospects.
We have entered into alliances, joint ventures or other business relationships. Alliances with certain partners or companies could make it more difficult for us to enter into advantageous business transactions or relationships with others. Moreover, we may not be able to:
| | • | | identify appropriate candidates for alliances or joint ventures; |
| | • | | assure that any alliance or joint venture candidate will provide us with the support anticipated; |
| | • | | successfully negotiate an alliance or joint venture on terms that are advantageous to us; or |
| | • | | successfully manage any alliance or joint venture. |
Furthermore, any alliance or joint venture may divert management time and resources. Entering into a disadvantageous alliance or joint venture, failing to manage an alliance or joint venture effectively, or failing to comply with obligations in connection therewith, could harm our business and prospects.
An adverse change in the projected cash flows from our acquired businesses or the business climate in which they operate, including the continuation of the current financial and economic downturn, could require us to incur an impairment charge which would have an adverse impact on our operating results.
We periodically review the carrying value of our goodwill and other long-lived assets to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment of the value of these assets. Conditions that would indicate impairment and necessitate a revaluation of these assets include, but are not limited to, a significant adverse change in the business climate or the legal or regulatory environment within which we operate. In addition, the deterioration of a company’s market capitalization significantly below its net book value is an indicator of impairment. If the carrying value of an asset is determined to be impaired we will write-down the carrying value of the asset or asset group to its fair value in the period identified. We generally calculate fair value as the present value of estimated future cash flows to be generated by the asset or asset group using a risk-adjusted discount rate.
During the first quarter of fiscal 2009, based upon a combination of factors, including the deteriorating macro-economic environment, declines in the stock market and the decline of our market capitalization significantly below the book value of our net assets, we concluded that potential goodwill impairment indicators existed as of December 27, 2008. As a result, we performed an interim goodwill impairment analysis as of December 27, 2008. The Step 1 impairment analysis indicated that the carrying value of the net assets of three of the Company’s reporting units, acquired in connection with the Cytyc acquisition, exceeded the estimated fair value of those reporting units. As a result, the Company was required to perform Step 2 of the goodwill impairment test to determine the amount, if any, of goodwill impairment charges for each of the applicable reporting units. Due to the complexities and time involved in preparing the Step 1 analysis, the Company had not commenced the Step 2 analysis as of February 5, 2009, the date it filed its Form 10-Q for the quarter ended December 27, 2008. As a result of the fact that the Company had not commenced the Step 2 analysis and the complexity of the analysis required to complete the Step 2 analysis, the Company was unable to determine that an impairment loss, in accordance with ASC 450 (formerly SFAS No. 5,Accounting for Contingencies), was both probable and reasonably estimable at December 27, 2008. The Company completed the Step 2 analysis during its second quarter of fiscal 2009, which resulted in an aggregate goodwill impairment charge of $2.34 billion. This impairment charge is comprised of $1.17 billion for GYN Surgical, $908.3 million for Diagnostics, and $265.9 million for Breast Health. For further information of these charges, please refer to Note 2 in our Consolidated Financial Statements contained in Item 15 of this Annual Report.
30
During the fourth quarter of fiscal 2009, we performed our annual impairment test of goodwill, and no additional impairment charges were required. It is possible that the continuation of the current global financial and economic turmoil could negatively affect our anticipated cash flows, or the discount rates used to value the cash flows for each reporting unit, to such an extent that we could be required to perform an interim impairment test in fiscal 2010. Such a requirement could result in a material impairment charge that would have an adverse impact on our operating results.
Our effective tax rate may fluctuate and we may incur obligations in tax jurisdictions in excess of amounts that have been accrued.
As a global medical devices company, we are subject to taxation in numerous countries, states and other jurisdictions. As a result, our effective tax rate is derived from a combination of applicable tax rates in the various countries, states and other jurisdictions in which we operate. In preparing our financial statements, we estimate the amount of tax that will become payable in each of the countries, states and other jurisdictions in which we operate. Our effective tax rate, however, may be lower or higher than experienced in the past due to numerous factors, including a change in the mix of our profitability from country to country, changes in accounting for income taxes and changes in tax laws. Any of these factors could cause us to experience an effective tax rate significantly different from previous periods or our current expectations, which could have an adverse effect on our business and results of operations. In addition, unfavorable results of audits of our tax filings, our inability to secure or sustain arrangements with tax authorities, and previously enacted and future changes in tax laws in jurisdictions in which we operate, among other things, may cause us to be obligated to accrue for future tax payments in excess of amounts accrued in our financial statements.
Several proposals to reform U.S. tax rules are being considered by U.S. law makers, including proposals that may reduce or eliminate the deferral of U.S. income tax on our unrepatriated earnings, potentially requiring those earnings to be taxed at the U.S. federal income tax rate, reduce or eliminate our ability to claim foreign tax credits, and eliminate various tax deductions until foreign earnings are repatriated to the U.S. Our future reported financial results may be adversely affected by tax rule changes which restrict or eliminate our ability to claim foreign tax credits or deduct expenses attributable to foreign earnings, or otherwise affect the treatment of our unrepatriated earnings.
We are exposed to potential risks and will continue to incur significant costs as a result of the internal control testing and evaluation process mandated by Section 404 of the Sarbanes-Oxley Act.
We assessed the effectiveness of our internal control over financial reporting as of September 26, 2009 and assessed all deficiencies on both an individual basis and in combination to determine if, when aggregated, they constitute a material weakness. As a result of this evaluation, no material weaknesses were identified.
We expect to continue to incur significant costs to maintain compliance with Section 404 of the Sarbanes-Oxley Act. We continue to monitor controls for any weaknesses or deficiencies. No evaluation can provide complete assurance that our internal controls will detect or uncover all failures of persons within the company to disclose material information otherwise required to be reported. The effectiveness of our controls and procedures could also be limited by simple errors or faulty judgments. In addition, as we continue to expand globally, the challenges involved in implementing appropriate internal controls will increase and will require that we continue to improve our internal controls over financial reporting.
In the future, we or our independent registered public accounting firm may identify material weaknesses in internal controls over financial reporting which may result in a loss of public confidence in our internal controls and adversely impact the market price of our common stock. In addition, any failure to implement required, new or improved controls, or difficulties encountered in their implementation, could harm our operating results or cause us to fail to meet our reporting obligations.
31
Risks Related to our Indebtedness
We have incurred significant indebtedness that limits our operating flexibility, and could adversely affect our operations and financial results and prevent us from fulfilling our obligations.
On December 10, 2007, we issued $1.725 billion of 2.0% convertible notes due 2037 (the “Convertible Notes”), which are unsecured and subordinated to our secured indebtedness. These notes may be put to us at par on December 13, 2013 and each fifth anniversary thereafter beginning December 15, 2017. In addition, at September 26, 2009, we had $174.2 million outstanding under our senior secured credit facility, which bears interest at variable rates. Additionally, certain other of our indebtedness may remain outstanding. Our level of indebtedness may:
| | • | | make it more difficult for us to satisfy our obligations with respect to our outstanding indebtedness; |
| | • | | increase our vulnerability to general adverse economic and industry conditions, including increases in interest rates; |
| | • | | require us to dedicate a substantial portion of our cash flow from operations to interest and principal payments on our indebtedness, which would reduce the availability of our cash flow to fund working capital, capital expenditures, expansion efforts and other general corporate purposes; |
| | • | | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| | • | | place us at a competitive disadvantage compared to our competitors that have less debt; and |
| | • | | limit our ability to borrow additional funds for working capital, capital expenditures, general corporate purposes or acquisitions. |
In addition, the terms of our credit facility contains covenants that restrict our ability, and that of our subsidiaries, to engage in certain transactions and may impair our ability to respond to changing business and economic conditions, including, among other things, limitations on the ability to:
| | • | | incur additional indebtedness and additional liens on our assets; |
| | • | | engage in mergers or acquisitions or dispose of assets; |
| | • | | enter into sale-leaseback transactions; |
| | • | | pay dividends or make other distributions; |
| | • | | voluntarily prepay other indebtedness; |
| | • | | enter into transactions with affiliated persons; |
| | • | | change the nature of our businesses. |
Our credit facility also requires us to satisfy certain financial covenants.
Our ability to comply with these provisions may be affected by general economic conditions, political decisions, industry conditions and other events beyond our control. Our failure to comply with the covenants contained in our credit facilities, including financial covenants, could result in an event of default, which could materially and adversely affect our results of operation and financial condition.
If there were an event of default under one of our debt instruments or a change of control, the holders of the defaulted debt may be permitted to cause all amounts outstanding with respect to that debt to be due and payable immediately and may be cross-defaulted to other debt. Our assets or cash flow may not be sufficient to fully repay borrowings under our outstanding debt instruments if accelerated upon an event of default, and there is no guarantee that we would be able to repay, refinance or restructure the payments on those debt securities.
32
We may not be able to generate sufficient cash flow to service all of our obligations, including our obligations under our credit facilities.
Our ability to make payments on and to refinance the indebtedness under our credit facilities and the Convertible Notes or any other of our obligations or indebtedness, and to fund planned capital expenditures, strategic transactions and expansion efforts will depend on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control.
Our business may not be able to generate sufficient cash flow from operations, and we cannot assure that future borrowings will be available to us in amounts sufficient to enable us to pay our indebtedness as such indebtedness matures and to fund our other liquidity needs. If this is the case, we will need to refinance all or a portion of our indebtedness on or before maturity, and there can be no assurance that we will be able to refinance any of our indebtedness on commercially reasonable terms, or at all. We may need to adopt one or more alternatives, such as reducing or delaying planned expenses and capital expenditures, selling assets, restructuring debt, or obtaining additional equity or debt financing. These financing strategies may not be affected on satisfactory terms, if at all. Our ability to refinance our indebtedness or obtain additional financing, or to do so on commercially reasonable terms, will depend on, among other things, our financial condition at the time, restrictions in agreements governing our indebtedness, and other factors, including the condition of the financial markets and the markets in which we compete.
If we do not generate sufficient cash flow from operations, and additional borrowings, refinancings or proceeds of asset sales are not available to us, we may not have sufficient cash to enable us to meet all of our obligations.
The accounting for convertible debt securities such as our Convertible Notes is subject to change that will result in a significant increase in the accrual of interest expense under those notes.
The accounting for convertible debt securities such as our Convertible Notes is subject to frequent scrutiny by the accounting regulatory bodies and is subject to change. In May 2008, the Financial Accounting Standards Board (“FASB”) issued FASB Staff Position (“FSP”) No. APB 14-1,Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) (codified within Accounting Standards Codification (“ASC”) 470,Debt). This accounting guidance applies to convertible debt instruments (such as our Convertible Notes) that, by their stated terms, may be settled in cash (or other assets) upon conversion, including partial cash settlement, unless the embedded conversion option is required to be separately accounted for as a derivative under ASC 815,Derivatives and Hedging (formerly SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities). As a result of the issuance of this FSP, the liability and equity components of our Convertible Notes must be separately accounted for in a manner that will reflect our nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. The excess of the principal amount of the debt over the amount ultimately allocated to the liability component is required to be amortized to interest expense using the interest method. This FSP is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. As a result, we will adopt this standard at the beginning of fiscal 2010. This FSP must be applied retrospectively to all periods presented. The retrospective adoption of this FSP will increase our historical reported interest expense from December 10, 2007 (issuance date of the Convertible Notes) forward. Upon adoption, we expect to revise prior periods by reclassifying approximately $470.0 million of our Convertible Notes to additional paid-in capital, resulting in a debt discount. As a result, our fiscal 2009 and fiscal 2008 non-cash interest expense will increase by approximately $65.5 million and $48.1 million, respectively, resulting in a restated diluted net loss per share of approximately $(8.64) and $(1.69), respectively. Future periods would be similarly affected by amortization of the debt discount as an interest expense. In fiscal 2010, we expect to record approximately an additional $71.1 million of non-cash interest expense.
33
Risks Related to our Common Stock and Convertible Notes
Future issuances of common stock and hedging activities may depress the trading price of our common stock and our Convertible Notes.
Any future issuance of equity securities, including the issuance of shares upon conversion of our Convertible Notes, could dilute the interests of our existing stockholders, including holders who have received shares upon conversion of our Convertible Notes, and could substantially decrease the trading price of our common stock and our Convertible Notes. We may issue equity securities in the future for a number of reasons, including to finance our operations and business strategy (including in connection with acquisitions, strategic collaborations or other transactions), to adjust our ratio of debt to equity, to satisfy our obligations upon the exercise of outstanding warrants or options or for other reasons.
In addition, the price of our common stock could also be affected by possible sales of our common stock by investors who view our Convertible Notes as a more attractive means of equity participation in our company and by hedging or arbitrage trading activity that we expect to develop involving our common stock. The hedging or arbitrage could, in turn, affect the trading price of our Convertible Notes, or any common stock that note holders receive upon conversion of their notes.
Future sales of our common stock in the public market or the issuance of securities senior to our common stock could adversely affect the trading price of our common stock and the value of our Convertible Notes and our ability to raise funds in new securities offerings.
Future sales of our common stock, the perception that such sales could occur or the availability for future sales of shares of our common stock or securities convertible into or exercisable for our common stock could adversely affect the market prices of our common stock and the value of our Convertible Notes prevailing from time to time and could impair our ability to raise capital through future offerings of equity or equity-related securities. In addition, we may issue common stock or equity securities senior to our common stock in the future for a number of reasons, including to finance our operations and business strategy, to adjust our ratio of debt to equity, satisfy our obligations upon the exercise of options or for other reasons.
Provisions in our charter and bylaws and our stockholder rights plan may have the effect of discouraging advantageous offers for our business or common stock and limit the price that investors might be willing to pay in the future for shares of our common stock.
Our charter, bylaws and the provisions of the Delaware General Corporation Law include provisions that may have the effect of discouraging or preventing a change in control. In addition, we have a stockholder rights plan that may have the effect of discouraging or preventing a change in control. These provisions could limit the price that our stockholders might receive in the future for shares of our common stock.
Our stock price is volatile.
The market price of our common stock has been, and may continue to be, highly volatile. We believe that a variety of factors could cause the price of our common stock to fluctuate, perhaps substantially, including:
| | • | | new, or changes in, recommendations, guidelines or studies that could affect the use of our products; |
| | • | | announcements and rumors of developments related to our business, including changes in reimbursement rates or regulatory requirements, proposed and completed acquisitions, or the industry in which we compete; |
| | • | | published studies and reports relating to the comparative efficacy of products and markets in which we participate; |
| | • | | quarterly fluctuations in our actual or anticipated operating results and order levels; |
34
| | • | | general conditions in the worldwide economy; |
| | • | | announcements of technological innovations; |
| | • | | new products or product enhancements by us or our competitors; |
| | • | | developments in patents or other intellectual property rights and litigation; |
| | • | | developments in relationships with our customers and suppliers; and |
| | • | | pending healthcare reform and proposed legislation. |
The price of our common stock also may be adversely affected by the amount of common stock issuable upon conversion of our Convertible Notes. In addition, in recent years the stock market in general and the markets for shares of “high-tech” companies, have experienced extreme price fluctuations which have often been unrelated to the operating performance of affected companies. Any such fluctuations in the future could adversely affect the market price of our common stock, and the market price of our common stock may decline.
Conversion of our Convertible Notes will dilute the ownership interest of existing stockholders, including holders who had previously converted their notes.
To the extent we issue any shares of our common stock upon conversion of our Convertible Notes, the conversion of some or all of our Convertible Notes will dilute the ownership interests of existing stockholders, including holders who have received shares of our common stock upon prior conversion of our Convertible Notes. Any sales in the public market of the common stock issuable upon such conversion could adversely affect prevailing market prices of our common stock. In addition, the existence of our Convertible Notes may encourage short selling by market participants because the conversion of our Convertible Notes could depress the price of our common stock.
| Item 1B. | Unresolved Staff Comments |
None
We own and lease the real property identified below. We believe that we have adequate space for our anticipated needs and that suitable additional space will be available at commercially reasonable prices as needed. See the Business and Manufacturing sections above for a description of the products manufactured at the facilities described below.
Owned Real Property
We own an approximately 164,000 square foot research and development, manufacturing and administrative site in Newark, Delaware at which we conduct our DirectRay digital detector research and development and plate manufacture. We currently occupy approximately 59,000 square feet of this building, which houses our plate manufacturing facility, including both a Class 1 and a Class 2 clean room. We lease approximately 105,000 square feet of the facility to Siemens under a lease which expires in April 2015. Our AEG subsidiary owns an approximately 201,000 square foot facility in Warstein, Germany which is used for its headquarters, manufacturing and research and development. Cytyc also owns approximately 2.7 acres of land and approximately 47,000 square feet of facilities housing additional manufacturing operations in Londonderry, New Hampshire.
35
Leased Real Property
In September 2002, we completed a sale/leaseback transaction for our approximately 207,000 square foot headquarters and manufacturing facility located in Bedford, Massachusetts and our approximately 62,000 square foot Lorad manufacturing facility in Danbury, Connecticut. The lease for these facilities, including the associated land, has a term of 20 years, with four-five year renewal options. In January 2004, Cytyc leased approximately 216,000 square feet in Marlborough, Massachusetts for its administrative, research, manufacturing and distribution operations for a term of 15 years with two (2) five-year options to extend the term upon written notice to the landlord. In July 2006, Cytyc entered into a 12-year lease agreement for a building with approximately 146,000 square feet also located in Marlborough, Massachusetts, which is principally used as an additional manufacturing facility. We also lease approximately 60,000 square feet of office and manufacturing space in Danbury, Connecticut near our Lorad manufacturing facility. This lease expires in December 2012. In April 2007, Cytyc entered into a ten year lease for a building with approximately 164,000 square feet located in Alajuela, Costa Rica. We moved certain of our manufacturing operations to this newly constructed facility in fiscal 2009. We lease approximately 62,000 square feet of office and manufacturing space in Madison, Wisconsin. This lease expires in September 2014.
We lease other facilities utilized for office space and manufacturing and distribution operations across the United States, Europe, Canada and Hong Kong. We also lease several sales and service offices throughout the world.
In connection with our secured credit facility, we entered into mortgages for our Newark, Delaware and Londonderry, New Hampshire properties and leasehold mortgages for our interests in our Danbury, Connecticut, Bedford, Massachusetts and Indianapolis, Indiana facilities.
On October 5, 2007, Ethicon Endo-Surgery, Inc., a Johnson & Johnson operating company, filed a complaint against the Company and its wholly-owned subsidiary Suros in the United States District Court for the Southern District of Ohio, Western Division. The complaint alleges that certain of the ATEC biopsy systems manufactured and sold by Suros infringe four Ethicon patents. An amended complaint filed January 11, 2008 additionally asserts claims of unfair competition. The complaint seeks to enjoin Hologic and Suros from conducting acts of unfair competition and infringing the patents as well as the recovery of unspecified damages and costs. A Markman hearing was held on January 8, 2009, and the Court issued its ruling on April 3, 2009. A court ordered settlement conference occurred on August 11, 2009 without any resolution. This suit is currently scheduled to go to trial on February 1, 2010. The Company is unable to reasonably estimate the ultimate outcome of this case.
On May 22, 2009, Conceptus, Inc. filed suit in the United States District Court for the Northern District of California seeking a declaration by the Court that Hologic’s planned importation, use, sale or offer to sell of its forthcoming Adiana Permanent Contraception System, would infringe five Conceptus patents. On July 9, 2009, Conceptus filed an amended complaint alleging infringement of the same five patents by the Adiana Permanent Contraception System. The complaint seeks preliminary and permanent injunctive relief and unspecified monetary damages. In addition to the amended complaint, Conceptus also filed a motion for preliminary injunction seeking to preliminarily enjoin sales of the Adiana System based on alleged infringement of certain claims of three of the five patents. A hearing on Conceptus’ preliminary injunction motion was held on November 4, 2009, and on November 6, 2009, the judge issued an order denying the motion. A hearing on claim construction is scheduled for March 10, 2010. A trial date has not been set. Based on the early stage of this litigation, the Company is unable to reasonably estimate the ultimate outcome of this case.
On August 6, 2009, Ethicon Endo-Surgery, Inc., a Johnson & Johnson operating company, filed a complaint against the Company and its wholly-owned subsidiary Suros in the United States District Court for the District of Delaware. The complaint alleges that certain of the Eviva biopsy systems manufactured and sold by Suros
36
infringe four Ethicon patents. The complaint seeks to enjoin Hologic and Suros from infringing the patents as well as recovery of damages and costs resulting from the alleged infringement. Based on the early stage of this litigation, the Company is unable to reasonably estimate the ultimate outcome of this case.
The Company is a party to various other legal proceedings and claims arising out of the ordinary course of its business. The Company believes that except for those described above there are no other proceedings or claims pending against it the ultimate resolution of which would have a material adverse effect on its financial condition or results of operations.
| Item 4. | Submission of Matters to a Vote of Security Holders. |
None.
37
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Market Information. Our common stock is traded on the Nasdaq Global Select Market under the symbol “HOLX.” The following table sets forth the high and low sales prices per share of our common stock, as reported by the Nasdaq Global Select Market. This stock price information has been adjusted to give effect for the stock split effected on April 2, 2008.
| | | | | | |
Fiscal Year Ended September 26, 2009 | | High | | Low |
First Quarter | | $ | 19.95 | | $ | 10.54 |
Second Quarter | | | 14.52 | | | 9.31 |
Third Quarter | | | 15.91 | | | 11.36 |
Fourth Quarter | | | 17.83 | | | 12.52 |
| | |
Fiscal Year Ended September 27, 2008 | | High | | Low |
First Quarter | | $ | 35.79 | | $ | 29.40 |
Second Quarter | | | 36.44 | | | 25.73 |
Third Quarter | | | 30.99 | | | 20.15 |
Fourth Quarter | | | 24.22 | | | 17.83 |
Number of Holders. As of November 18, 2009, there were approximately 1,653 holders of record of our common stock, including multiple beneficial holders at depositaries, banks and brokers listed as a single holder in the street name of each respective depositary, bank or broker.
Dividend Policy. We have never declared or paid cash dividends on our capital stock and do not plan to pay any cash dividends in the foreseeable future. Our current policy is to retain all of our earnings to finance future growth. In addition, our amended credit facility prohibits us from declaring or paying any cash dividends.
Recent Sales of Unregistered Securities. We did not sell unregistered securities during the fourth quarter of fiscal 2009.
Issuer’s Purchases of Equity Securities. For the majority of restricted stock units granted, the number of shares issued on the date that the restricted stock units vest is net of the minimum statutory tax withholding requirements that we pay in cash to the appropriate taxing authorities on behalf of our employees. The following table sets forth information about repurchases of our common stock to cover employee income tax withholding obligations in connection with the vesting of restricted stock units under our equity incentive plans for the three months ended September 26, 2009:
| | | | | | | |
Period of Repurchase | | Total Number of
Shares Purchased | | Average Price
Paid Per Share | | Total Number of
Shares
Purchased As
Part of Publicly
Announced
Program |
June 28, 2009—July 25, 2009 | | 68 | | $ | 14.73 | | — |
July 26, 2009—August 22, 2009 | | — | | | — | | — |
August 23, 2009—September 26, 2009 | | 96 | | | 16.01 | | — |
| | | | | | | |
Total | | 164 | | $ | 15.48 | | — |
| | | | | | | |
38
Stock Performance Graph
The following graph compares cumulative total shareholder return on our common stock since September 25, 2004 with the cumulative total return of the Russell 1000 Index and the Standard & Poor’s Health Care Supplies Index. This graph assumes the investment of $100 on September 25, 2004 in our common stock, the Russell 1000 Index and the S&P Health Care Supplies Index. Measurement points are the last trading day of each respective fiscal year.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Hologic, Inc., The Russell 1000 Index
And The S&P Health Care Supplies Index
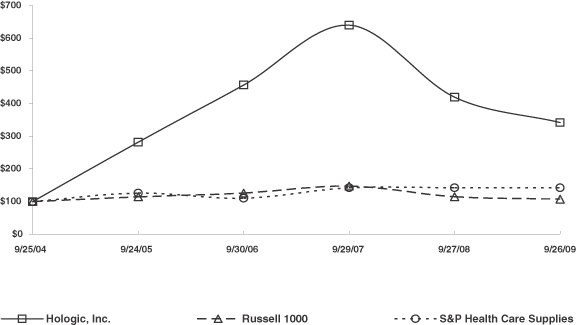
| * | $100 invested on 9/25/04 in stock or on 9/30/04 in index, including reinvestment of dividends. Indexes calculated on month-end basis. |
Copyright© 2009 S&P, a division of The McGraw-Hill Companies Inc. All rights reserved.
39
| Item 6. | Selected Financial Data. |
The following selected financial data should be read in conjunction with our consolidated financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K, beginning on page F-1. In the first and fourth quarters of fiscal 2008, we acquired Cytyc Corporation (“Cytyc”) and Third Wave Technologies, Inc. (“Third Wave”), respectively. In the fourth quarter of fiscal 2007, we acquired BioLucent, Inc. (“BioLucent). In fiscal 2006, we acquired AEG Elektrofotografie (“AEG”), R2 Technology, Inc. (“R2”) and Suros Surgical, Inc. (“Suros”), and we also acquired the intellectual property relating to Fischer Imaging Corporation’s mammography business. Results of operations for each of these businesses are included in our consolidated financial statements from the date of acquisition. Effective in fiscal 2006, we began to record stock-based compensation expense associated with the fair value of stock options in accordance with U.S .generally accepted accounting principles.
| | | | | | | | | | | | | | | | | | | | |
| | | Fiscal Years Ended | |
| | | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | | | September 30,
2006 | | | September 24,
2005 | |
| | | (In thousands, except per share data) | |
Consolidated Statement of Operations Data | | | | | | | | | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | | | | | | | | | |
Product sales | | $ | 1,426,986 | | | $ | 1,502,447 | | | $ | 628,854 | | | $ | 388,111 | | | $ | 229,075 | |
Service and other revenues | | | 210,148 | | | | 172,052 | | | | 109,514 | | | | 74,569 | | | | 58,609 | |
| | | | | | | | | | | | | | | | | | | | |
| | | 1,637,134 | | | | 1,674,499 | | | | 738,368 | | | | 462,680 | | | | 287,684 | |
| | | | | | | | | | | | | | | | | | | | |
Costs and Expenses: | | | | | | | | | | | | | | | | | | | | |
Cost of product sales | | | 470,295 | | | | 535,082 | | | | 267,470 | | | | 188,443 | | | | 116,478 | |
Cost of product sales—amortization of intangible assets | | | 155,519 | | | | 95,310 | | | | 11,262 | | | | 5,011 | | | | 1,153 | |
Cost of product sales—impairment of intangibles | | | 4,065 | | | | — | | | | — | | | | — | | | | — | |
Cost of service and other revenues | | | 149,769 | | | | 151,589 | | | | 114,307 | | | | 75,921 | | | | 58,181 | |
Research and development | | | 94,328 | | | | 81,421 | | | | 44,381 | | | | 28,113 | | | | 18,508 | |
Selling and marketing | | | 238,977 | | | | 261,524 | | | | 85,520 | | | | 56,239 | | | | 34,200 | |
General and administrative | | | 148,825 | | | | 147,405 | | | | 62,092 | | | | 42,176 | | | | 26,533 | |
Amortization of intangible assets | | | 51,210 | | | | 25,227 | | | | 5,584 | | | | 1,631 | | | | — | |
Impairment of goodwill | | | 2,340,023 | | | | — | | | | — | | | | — | | | | — | |
Impairment of intangible assets | | | — | | | | 2,900 | | | | — | | | | — | | | | — | |
Net gain on sale of intellectual property | | | — | | | | — | | | | — | | | | (5,093 | ) | | | — | |
Acquired in-process research and development | | | — | | | | 565,200 | | | | — | | | | 19,900 | | | | — | |
Restructuring charges | | | 797 | | | | 6,383 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
| | | 3,653,808 | | | | 1,872,041 | | | | 590,616 | | | | 412,341 | | | | 255,053 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income from operations | | | (2,016,674 | ) | | | (197,542 | ) | | | 147,752 | | | | 50,339 | | | | 32,631 | |
Interest income | | | 1,161 | | | | 4,528 | | | | 2,815 | | | | 4,082 | | | | 2,219 | |
Interest expense | | | (69,502 | ) | | | (84,912 | ) | | | (2,511 | ) | | | (1,230 | ) | | | (376 | ) |
Other (expense) income, net | | | (3,660 | ) | | | (1,215 | ) | | | 433 | | | | 32 | | | | 221 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income before income taxes | | | (2,088,675 | ) | | | (279,141 | ) | | | 148,489 | | | | 53,223 | | | | 34,695 | |
Provision for income taxes | | | 87,562 | | | | 106,476 | | | | 53,911 | | | | 25,800 | | | | 6,439 | |
| | | | | | | | | | | | | | | | | | | | |
Net (loss) income | | $ | (2,176,237 | ) | | $ | (385,617 | ) | | $ | 94,578 | | | $ | 27,423 | | | $ | 28,256 | |
| | | | | | | | | | | | | | | | | | | | |
40
| | | | | | | | | | | | | | | | | |
| | | Fiscal Years Ended |
| | | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | | September 30,
2006 | | September 24,
2005 |
| | | (In thousands, except per share data) |
Basic net (loss) income per common share (1) | | $ | (8.48 | ) | | $ | (1.57 | ) | | $ | 0.88 | | $ | 0.29 | | $ | 0.33 |
| | | | | | | | | | | | | | | | | |
Diluted net (loss) income per common share (1) | | $ | (8.48 | ) | | $ | (1.57 | ) | | $ | 0.86 | | $ | 0.28 | | $ | 0.31 |
| | | | | | | | | | | | | | | | | |
Weighted average number of common shares outstanding (1): | | | | | | | | | | | | | | | | | |
Basic | | | 256,545 | | | | 245,968 | | | | 106,873 | | | 93,025 | | | 85,648 |
| | | | | | | | | | | | | | | | | |
Diluted | | | 256,545 | | | | 245,968 | | | | 109,669 | | | 97,240 | | | 90,252 |
| | | | | | | | | | | | | | | | | |
Consolidated Balance Sheet Data | | | | | | | | | | | | | | | | | |
Working capital | | $ | 492,226 | | | $ | 352,703 | | | $ | 220,568 | | $ | 123,493 | | $ | 172,615 |
Total assets | | | 5,689,828 | | | | 8,134,632 | | | | 1,066,349 | | | 856,205 | | | 279,839 |
Line of credit | | | — | | | | — | | | | — | | | 55,000 | | | — |
Long-term debt | | | 1,864,955 | | | | 2,162,420 | | | | 9,222 | | | 6,163 | | | — |
Total stockholders’ equity | | | 2,512,715 | | | | 4,642,269 | | | | 805,723 | | | 605,750 | | | 217,834 |
| (1) | All share and per share data have been retroactively restated to reflect the 2-for-1 stock splits effected on November 30, 2005 and April 2, 2008. |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
The following discussion and analysis should be read in conjunction with the Consolidated Financial Statements and the information described under the caption “Risk Factors” included elsewhere in this report.
OVERVIEW
We are a developer, manufacturer and supplier of medical imaging systems and diagnostic and surgical products focused on the healthcare needs of women. Our core business segments are focused on breast health, diagnostics, GYN surgical and skeletal health.
Historically, we have developed, manufactured and marketed products focused on mammography, breast care and osteoporosis assessment. We have historically focused our resources on developing systems and subsystems offering superior image quality and diagnostic accuracy, which has enabled us to capture significant market share and customer loyalty, despite the presence of large competitors. Our merger with Cytyc in the first quarter of fiscal 2008 enabled us to benefit from Cytyc’s strengths in the fields of obstetrics, gynecology, radiation oncology and minimally invasive surgery.
Our breast health products include a broad portfolio of breast imaging and related products and accessories, including digital and film-based mammography systems, computer-aided detection (“CAD”), minimally invasive breast biopsy and tissue extraction devices, breast biopsy guidance systems, breast imaging comfort pads, and breast brachytherapy products. We have also developed a new breast imaging platform, “Dimensions”, which utilizes a new technology, tomosynthesis, to produce three dimensional (“3D”) images, as well as conventional two dimensional (“2D”) full field digital mammography (FFDM) images. In the U.S., our Dimensions product has been approved by the FDA for providing conventional 2D images, and we are conducting further clinical trials to support our PMA application for the 3D configuration. Our Dimensions platform received CE mark approval in Europe in fiscal 2008 and Canadian registration in March 2009, both for 2D and 3D modes of imaging. Currently, we cannot determine the timing of FDA approval for our 3D configuration, if at all. We also sell breast biopsy products, and within our breast brachytherapy products is our MammoSite System, which
41
provides accelerated partial breast irradiation technology. We received FDA clearance for our MammoSite ML radiation therapy system, which is a multi-lumen device that provides the oncologist with additional flexibility in specifically targeting radiation in the tissue where cancer is most likely to recur, on August 27, 2009.
Our diagnostic products include the ThinPrep System, which is primarily used in cytology testing applications, such as cervical cancer screening, and the Rapid Fetal Fibronectin Test, which assists physicians in assessing risk of pre-term birth. In the fourth quarter of fiscal 2008, we acquired Third Wave Technologies, Inc. (“Third Wave”) a company that develops and markets molecular diagnostic reagents for a wide variety of DNA and RNA analysis applications based on its proprietary Invader chemistry. Our current clinical diagnostic offerings based upon this Invader chemistry include products to assist in the diagnosis of human papillomavirus (“HPV”), cystic fibrosis, cardiovascular risk and other diseases. We received FDA approval of Cervista HPV High Risk (“HR”) and Cervista HPV 16/18 tests in March 2009 as well as CE mark approval in Europe in January 2009 for Cervista HPV HR and in May 2009 for Cervista HPV 16/18.
Our GYN surgical products are made up of the NovaSure System and the Adiana Permanent Contraception System (“Adiana System”). The Novasure System enables physicians to treat women suffering from excessive menstrual bleeding in a minimally invasive manner in order to eliminate or reduce their bleeding. The Adiana System is a form of permanent female contraception intended as an alternative to tubal ligation. We received FDA approval of the Adiana System in July 2009 and CE mark approval in Europe in 2008. Our revenues from the Adiana System have been modest to date as both the U.S. and international market launches were limited.
Our skeletal health products primarily consist of dual-energy X-ray bone densitometry systems, an ultrasound-based osteoporosis assessment product, our Fluoroscan mini C-arm imaging product and our Esaote line of extremity Magnetic Resonance Imaging (“MRI”) systems that were manufactured by an original equipment manufacturer.
RECENT DEVELOPMENTS
Market acceptance of our medical products in the United States and other countries is dependent upon the medical equipment purchasing and procurement practices of our customers, patient demand for our products and procedures and the reimbursement of patient’s medical expenses by government healthcare programs, private insurers or other healthcare payors. Since the end of calendar 2008, the uncertainty surrounding world financial markets and deteriorating worldwide macroeconomic conditions have caused and may continue to cause the purchasers of medical equipment to decrease their medical equipment purchasing and procurement activities. Additionally, constrictions in world credit markets have caused and continue to cause our customers to experience increased difficulty securing the financing necessary to purchase our products. Economic uncertainty has and may continue to result in cost-conscious consumers focusing on acute care rather than wellness, which could adversely affect demand for our products and procedures. Furthermore, governments and other third party payors around the world facing tightening budgets could move to further reduce the reimbursement rates or the scope of coverage offered, which could adversely effect sales of our products. If the current adverse economic conditions continue, our business and prospects may be negatively impacted.
In recent years, the healthcare industry has undergone significant change driven by various efforts to reduce costs, including efforts at national healthcare reform, trends toward managed care, cuts in Medicare, consolidation of healthcare distribution companies and collective purchasing arrangements by office-based healthcare practitioners. We anticipate that the current administration, Congress and certain state legislatures will continue to review and assess alternative healthcare delivery systems and payment methods with an objective of ultimately reducing healthcare costs and expanding access. Public debate of these issues will likely continue in the future. At this time, we cannot predict which, if any, healthcare reform proposals will be adopted, when they may be adopted or what impact they may have on our business. Healthcare reform proposals and medical cost containment measures in the United States and in many foreign countries could:
| | • | | limit the use of our products and treatments; |
42
| | • | | reduce reimbursement available for such use; or |
| | • | | adversely affect the use of new therapies for which our products may be targeted. |
These reforms or cost containment measures, including the uncertainty in the medical community regarding their nature and effect, could have an adverse effect on our customers’ purchasing decisions regarding our products and treatments and could harm our business and prospects.
As we operate in a highly regulated industry, other governmental actions may adversely affect our business, operations or financial condition, including, without limitation: new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, method of delivery and payment for health care products and services; changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and treatments and result in lost market opportunity; changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products and treatments to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products or treatments, or otherwise adversely affect the market for our products and treatments; new laws, regulations and judicial decisions affecting pricing or marketing practices; and changes in the tax laws relating to our operations, including those associated with proposed health care reform, such as the tax proposal included in the health-care reform bill recently approved by the Finance Committee of the U.S. Senate that would assess an annual tax on the revenue of medical device manufacturers based upon market share, could have a material adverse impact on our results of operations.
Professional societies, practice management groups, private health/science foundations, and organizations involved in healthcare issues may publish guidelines, recommendations or studies to the healthcare and patient communities from time to time. Recommendations of government agencies or these other groups/organizations may relate to such matters as usage, cost-effectiveness, and use of related therapies. Organizations like these have in the past made recommendations about our products and those of our competitors. Recommendations, guidelines or studies that are followed by patients and healthcare providers could result in decreased use of our products. For example, recently, the American College of Obstetricians and Gynecologists changed their recommendations for pap smear screening, and the United States Preventive Services Task Force changed their recommendations for mammography screening. These new recommendations, if implemented, could significantly reduce the amount of screening using our ThinPrep, mammography and related products and adversely affect the sale of those products.
During the first quarter of fiscal 2009, the value of the U.S. dollar strengthened against the value of many foreign currencies and remained at this strengthened position throughout our second quarter of fiscal 2009. However, in our fiscal third quarter, the dollar weakened slightly and continued to weaken in the fourth quarter. A majority of our sales to international dealers are denominated in U.S. dollars. The ongoing fluctuations of the value of the U.S. dollar may cause our products to be less competitive in international markets and may impact sales and margins over time. In addition, we have international sales, principally in our Diagnostics segment, that are denominated in foreign currencies. The value of these sales is also impacted by fluctuations in the value of the U.S. dollar. Given the uncertainty in the worldwide financial markets, foreign currency fluctuations may be significant in the future, and if the U.S. dollar strengthens, we may experience a material adverse effect on our international sales and margins.
43
ACQUISITIONS
Fiscal 2008 Acquisitions:
Third Wave Technologies, Inc.
On July 24, 2008, we completed our acquisition of Third Wave pursuant to a definitive agreement dated June 8, 2008. We paid $11.25 per share of Third Wave, for an estimated aggregate purchase price of $591.1 million, including $8.1 million for the estimated fair value of fully vested stock-based awards and $7.6 million in acquisition-related expenses. We concluded that the acquisition of Third Wave did not represent a material business combination and therefore no pro forma financial information has been provided herein. Our results of operations include the results of Third Wave since the acquisition date, as a component of our Diagnostics reporting segment.
Our acquisition of Third Wave was accounted for using the purchase method of accounting, and the total purchase price was allocated to the assets acquired and liabilities assumed based on our estimate of their fair values as of the date of the acquisition. The excess of purchase price over those fair values was recorded as goodwill. As a result of this acquisition, we recorded a $195.2 million charge for acquired in-process research and development in the fourth quarter of fiscal 2008, and we have recorded additional amortization expense for the acquired intangible assets and additional interest expense on the funds we borrowed to complete the acquisition in both fiscal 2009 and 2008.
The allocation of the purchase price was based upon preliminary estimates of the fair value of assets acquired and liabilities assumed as of July 24, 2008. We finalized the allocation of the purchase price in fiscal 2009 once we had all necessary information to complete our estimates. The purchase price in excess of net tangible assets acquired was allocated to identifiable intangible assets, including in-process research and development, based upon a detailed valuation that relies on information and assumptions further described below. The excess of the purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed was allocated to goodwill.
As part of the preliminary purchase price allocation, $195.2 million of the purchase price was allocated to acquired in-process research and development projects. The amounts allocated to acquired in-process research and development represents programs for which some research and development has been completed, but technological feasibility has not been determined or FDA approval is pending. The amount allocated to acquired in-process research and development represents the estimated fair value based on risk-adjusted cash flows related to these projects using a discount rate of 20%. The primary basis for determining the technological feasibility of these projects was obtaining regulatory approval to market the underlying products. The fair value attributable to these in-process projects was expensed at the time of the acquisition. If the projects are not successful or completed in a timely manner, we may not realize the financial benefits expected for these projects or for the transaction as a whole.
The most significant acquired in-process technology related to the Cervista HPV HR screening, for which we estimated a value of $151.2 million. At the time of, and subsequent to the acquisition, we sold HPV reagents that detect certain high risk HPV types as Analyte Specific Reagents (“ASRs”). In 2006, Third Wave began clinical trials for PMA submissions to the FDA for Cervista HPV HR. Third Wave submitted the PMAs in April 2008 and received FDA approval in the second quarter of fiscal 2009. Since receiving FDA approval, we have begun to transition to only selling HPV IVDs and expect to complete this transition by the end of fiscal 2010. The HPV in-process research and development related only to the HPV IVDs and the HPV ASRs were valued as developed technology.
The estimated cost to complete Third Wave’s remaining in-process research and development projects as of September 26, 2009 in the aggregate was $4.0 million.
44
On July 17, 2008, we entered into an amended and restated credit agreement with Goldman Sachs Credit Partners L.P. and certain other lenders and borrowed $540.0 million under that facility to finance our acquisition of Third Wave.
Cytyc Corporation
On October 22, 2007, we completed our merger with Cytyc, pursuant to which Cytyc became our wholly-owned subsidiary. Under the terms of the merger agreement, Cytyc shareholders received 1.04 shares of our common stock and $16.50 in cash for each share of Cytyc common stock held by them. The aggregate consideration we paid for Cytyc, including liabilities assumed in connection with the transaction, was $6.2 billion comprised as follows:
| | • | | merger consideration paid to the former Cytyc stockholders of $5.8 billion, consisting of approximately $2.1 billion in cash and approximately 132.0 million shares of our common stock with an estimated fair value of approximately $3.7 billion; |
| | • | | 16.5 million of fully vested stock options issued upon conversion of Cytyc stock options with an estimated fair value of approximately $241.4 million; |
| | • | | the assumption of obligations of Cytyc under its 2.25% Senior Convertible Notes due 2024 with a principal amount outstanding as of October 22, 2007 of approximately $73.0 million and an estimated fair value of approximately $125.0 million; and |
| | • | | direct acquisition costs of $24.2 million. |
In connection with the merger, we entered into a credit agreement relating to a senior secured credit facility with Goldman Sachs Credit Partners L.P. and certain other lenders, in which the lenders committed to provide, in the aggregate, senior secured financing of up to approximately $2.55 billion to pay for the cash portion of the merger consideration, for repayment of existing debt of Cytyc, for expenses relating to the merger and for working capital following the completion of the merger. As of the closing of the merger, we borrowed $2.35 billion under the credit facility. In December 2007, we refinanced a substantial portion of this credit facility through the issuance of 2.00% Convertible Senior Notes due 2037 in the principal amount of $1.725 billion. On July 17, 2008, after having paid off all outstanding term loans under the credit facility, we amended and restated the credit facility to finance our acquisition of Third Wave.
Our merger with Cytyc was accounted for using the purchase method of accounting, and we were considered to be the acquirer of Cytyc for accounting purposes. Our results of operations after completion of the merger include the operations of Cytyc. As a result of the acquisition, we recorded an in-process research and development of $370 million in the first quarter of fiscal 2008, and we have recorded increased amortization expense for the acquired intangible assets and additional interest on the funds we borrowed to complete the merger in both fiscal 2009 and 2008.
We have allocated the purchase price for our merger with Cytyc to the assets acquired and liabilities assumed based on our estimate of their estimated fair values. We then allocated the purchase price in excess of net tangible assets acquired to identifiable intangible assets, including in-process research and development, based upon a detailed valuation that relies on information and assumptions further described below. The excess of the purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed was allocated to goodwill.
Identifiable Intangible Assets
As part of the purchase price allocation, we determined that Cytyc’s identifiable intangible assets include existing technology, customer relationships and trade names. Cytyc’s existing technology relates to patents, patent applications and know-how with respect to the technologies embedded in its currently marketed products.
45
In determining the allocation of the purchase price to existing technology, consideration was only given to patent and patent applications that relate to products that have been approved by the FDA. Cytyc’s customer relationship assets relate to relationships that Cytyc’s sales force has developed with OB/GYNS, breast surgeons, clinical laboratories and other physicians. The trade names relate to both the Cytyc name as well as key product names.
We used the income approach to value the existing technology and marketing based intangibles. This approach calculates fair value by discounting the after-tax cash flows back to a present value. The baseline data for this analysis was the cash flow estimates used to price the transaction. Cash flows were forecasted for each intangible asset, and then discounted based on an appropriate discount rate. The discount rates applied were benchmarked with reference to the implied rate of return from the transaction model as well as Cytyc’s weighted average cost of capital based on the capital asset pricing model.
In estimating the useful life of the acquired assets, we considered ASC 350,Intangible—Goodwill and Other, Subsection 30-35-3 (formerly paragraph 11 of SFAS No. 142,Goodwill and Other Intangible Assets), which lists the pertinent factors to be considered when estimating the useful life of an intangible asset. These factors included a review of the expected use by the combined company of the assets acquired, the expected useful life of another asset (or group of assets) related to the acquired assets, legal, regulatory or other contractual provisions that may limit the useful life of an acquired asset or may enable the extension of the useful life of an acquired asset without substantial cost, the effects of obsolescence, demand, competition and other economic factors, and the level of maintenance expenditures required to obtain the expected future cash flows from the asset. We are amortizing these intangible assets over their estimated useful lives either using a method that is based on estimated future cash flows as we believe this will approximate the pattern in which the economic benefits of the assets will be utilized, or on a straightline basis if those cash flows are not reliably determinable.
Acquired In-Process Research and Development
As part of the purchase price allocation for our merger with Cytyc, we allocated approximately $370 million of the purchase price to acquired in-process research and development projects. The amount allocated to acquired in-process research and development represented the estimated fair value based on risk-adjusted cash flows related to in-process projects that had not yet reached technological feasibility and had no alternative future uses as of the date of the merger. The primary basis for determining the technological feasibility of these projects was obtaining regulatory approval to market the underlying products. The fair value attributable to these in-process projects was expensed at the time of the merger. If the projects are not successful or completed in a timely manner, we may not realize the financial benefits expected for these projects or for the transaction as a whole.
The fair value assigned to acquired in-process research and development was determined by estimating the costs to develop the acquired technology into commercially viable products, estimating the resulting net cash flows from the projects, and discounting the net cash flows to their present value. The revenue projections used to value the acquired in-process research and development was based on estimates of relevant market sizes and growth factors, expected trends in technology, and the nature and expected timing of new product introductions by us and our competitors. The resulting net cash flows from such projects were based on our estimates of cost of sales, operating expenses, and income taxes from such projects.
The rates utilized to discount the net cash flows to their present value were based on estimated cost of capital calculations and the implied rate of return from the transaction model plus a risk premium. Due to the nature of the forecasts and the risks associated with the developmental projects, appropriate risk-adjusted discount rates were used for the in-process research and development projects. The discount rates are based on the stage of completion and uncertainties surrounding the successful development of the purchased in-process technology projects.
46
The acquired in-process research and development of Cytyc related to the following research and development projects: Adiana System and expanded labeling of the NovaSure System, Gestiva, the ThinPrep Imaging System, the ThinPrep Processor and Helica.
The most significant acquired in-process technology related to the Adiana System for which we estimated a value of approximately $220.0 million. The system is an incisionless trans-cervical permanent sterilization device intended to be used during an office or hospital based procedure. In January 2008, the FDA requested an additional year of clinical trial data for the product, and in July 2009, we received FDA approval for the product.
On January 16, 2008, we entered into a definitive agreement to sell our rights to Gestiva, a drug being developed to be used in the prevention of preterm birth in pregnant women with a history of spontaneous preterm birth, to K-V Pharmaceutical Company for a total purchase price of $82.0 million. A portion of the purchase price is to be paid upon final approval by the FDA of a Gestiva New Drug Application (“NDA”) on or before February 19, 2010 and the production of a quantity of Gestiva suitable to enable the commercial launch of the product. Either party has the right to terminate the agreement if FDA approval is not obtained by February 19, 2010. In fiscal 2008, we received $9.5 million of the purchase price, and the balance is due upon the satisfaction of the above conditions. We have agreed to continue our efforts to obtain FDA approval of the NDA for Gestiva as part of this arrangement, for which we are being reimbursed by K-V Pharmaceutical. All costs incurred in these efforts are to be reimbursed by K-V Pharmaceutical and are being recorded as a credit against research and development expenses. We have recorded the $9.5 million as a deferred gain within current liabilities of our Consolidated Balance Sheet. We expect the gain will be recognized upon the closing of the transaction following final FDA approval of the Gestiva NDA or if the agreement is terminated. We had allocated $53.4 million to acquired in-process research for this product as part of the initial purchase price allocation. We cannot assure that we will be able to obtain the requisite FDA approval, that the transaction will be completed or that we will receive the balance of the purchase price. Moreover, if K-V Pharmaceutical terminates the agreement as a result of our breach of a material representation, warranty, covenant or agreement, we will be required to return the funds previously received by us as well as expenses reimbursed to us by K-V.
Subsequent to the merger with Cytyc, we decided to discontinue the development of Cytyc’s Helica Thermal Coagulator System product. We will not incur any further costs or realize any future cash flows from this product. Our intangible asset valuation for Cytyc included $2.9 million related to customer relationships for Helica. As a result of the Helica product discontinuation, we recorded an impairment charge of $2.9 million during the first quarter of fiscal 2008.
The other in-process research and development projects we acquired in our merger with Cytyc were at different stages of development, ranging from the early stages of development to Phase IIb prototype building, ongoing clinical trials and submission to the FDA of PMA and drug applications. FDA approval or clearance had not been granted for any of the products classified as in-process research and development, nor had Cytyc received any foreign approvals or clearances for any of these products. All products classified as in-process research and development require various levels of in-house and external testing, clinical trials and approvals from the FDA before these future products can be marketed. The estimated cash requirements to complete the remaining products as of September 26, 2009 were expected to be approximately $3.8 million.
The successful development of new products and product enhancements is subject to numerous risks and uncertainties, both known and unknown, including, unanticipated delays, access to capital, budget overruns, technical problems and other difficulties that could result in the abandonment or substantial change in the design, development and commercialization of these new products and enhancements, including, for example changes requested by the FDA in connection with PMA or NDA applications for products or 510(k) notification. Given the uncertainties inherent with product development and introduction, we cannot provide assurance that any of our product development efforts will be successful on a timely basis or within budget, if at all. Our failure to develop new products and product enhancements on a timely basis or within budget could harm our results of operations and financial condition.
47
Goodwill
The purchase price allocation for Cytyc initially resulted in goodwill of approximately $3.8 billion. The factors contributing to the recognition of this amount of goodwill were based upon several strategic and synergistic benefits that were expected to be realized from the combination. These benefits include the expectation that our complementary products and technologies will create a leading women’s healthcare company with an enhanced presence in hospitals, private practices and healthcare organizations. We also expected to realize substantial synergies through the use of Cytyc’s OB/GYN and breast surgeon sales channel to cross-sell our existing and future products. Our merger with Cytyc provided us broader channel coverage within the United States and expanded geographic reach internationally, as well as increased scale and scope for further expanding operations through product development and complementary strategic transactions.
As a result of the Company’s interim impairment analysis of goodwill as of December 27, 2008, the Company recorded an impairment charge of $2.34 billion related to the goodwill from the merger with Cytyc. See the Critical Accounting Policies below for additional information pertaining to the interim impairment analysis of the Company’s goodwill.
Fiscal 2007 Acquisition:
BioLucent, Inc.
On September 18, 2007, we completed the acquisition of BioLucent, Inc. (“BioLucent”) pursuant to a definitive agreement dated June 20, 2007. We have concluded that the acquisition of BioLucent does not represent a material business combination and therefore no pro forma financial information has been provided herein. BioLucent, previously located in Aliso Viejo, California, develops, markets and sells MammoPad breast cushions to decrease the discomfort associated with mammography. Prior to the acquisition, BioLucent’s primary research and development efforts were directed at its brachytherapy business, which was focused on breast cancer therapy. Prior to the acquisition, BioLucent spun-off its brachytherapy technology and business to the holders of BioLucent’s outstanding shares of capital stock. As a result, we only acquired BioLucent’s MammoPad cushion business and related assets. We invested $1 million directly in the spun-off brachytherapy business in exchange for shares of preferred stock issued by the new business.
The aggregate purchase price for BioLucent was $73.2 million, consisting of $6.8 million in cash and issuance of 2.3 million shares of our common stock valued at $63.2 million, debt assumed and paid off of $1.6 million and $1.6 million for acquisition related fees and expenses. The acquisition also provided for up to two annual earn-out payments not to exceed $15.0 million in the aggregate based on BioLucent’s achievement of certain revenue targets. We considered the provision of EITF Issue No. 95-8,Accounting for Contingent Consideration Paid to the Shareholders of an Acquired Enterprise in a Purchase Business Combination, and concluded that this contingent consideration will represent additional purchase price. As a result, goodwill will be increased by the amount of the additional consideration, if any, when it becomes due and payable. As of September 26, 2009, we have not recorded any amounts for these potential earn-outs.
48
RESULTS OF OPERATIONS
The following table sets forth, for the periods indicated, the percentage of total revenues represented by items as shown in our Consolidated Statements of Operations. All dollar amounts in tables are presented in thousands.
| | | | | | | | | |
| | | Fiscal Years Ended | |
| | | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Revenues: | | | | | | | | | |
Product sales | | 87.2 | % | | 89.7 | % | | 85.2 | % |
Service and other revenues | | 12.8 | | | 10.3 | | | 14.8 | |
| | | | | | | | | |
| | 100.0 | | | 100.0 | | | 100.0 | |
| | | | | | | | | |
Costs and expenses: | | | | | | | | | |
Cost of product sales | | 28.7 | | | 32.0 | | | 36.2 | |
Cost of product sales—amortization of intangible assets | | 9.5 | | | 5.7 | | | 1.5 | |
Cost of product sales—impairment of intangible assets | | 0.2 | | | — | | | — | |
Cost of service and other revenues | | 9.2 | | | 9.0 | | | 15.5 | |
Research and development | | 5.8 | | | 4.9 | | | 6.0 | |
Selling and marketing | | 14.6 | | | 15.6 | | | 11.6 | |
General and administrative | | 9.2 | | | 8.8 | | | 8.4 | |
Amortization of intangible assets | | 3.1 | | | 1.5 | | | 0.8 | |
Impairment of goodwill | | 142.9 | | | — | | | — | |
Impairment of intangible assets | | — | | | 0.2 | | | — | |
Acquired in-process research and development | | — | | | 33.7 | | | — | |
Restructuring charges | | — | | | 0.4 | | | — | |
| | | | | | | | | |
| | 223.2 | | | 111.8 | | | 80.0 | |
| | | | | | | | | |
(Loss) income from operations | | (123.2 | ) | | (11.8 | ) | | 20.0 | |
Interest income | | 0.1 | | | 0.3 | | | 0.3 | |
Interest expense | | (4.2 | ) | | (5.1 | ) | | (0.3 | ) |
Other (expense) income, net | | (0.2 | ) | | (0.1 | ) | | 0.1 | |
| | | | | | | | | |
(Loss) income before income taxes | | (127.5 | ) | | (16.7 | ) | | 20.1 | |
Provision for income taxes | | 5.4 | | | 6.3 | | | 7.3 | |
| | | | | | | | | |
Net (loss) income | | (132.9 | )% | | (23.0 | )% | | 12.8 | % |
| | | | | | | | | |
Fiscal Year Ended September 26, 2009 Compared to Fiscal Year Ended September 27, 2008
Product Sales.
| | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | % of Total
Revenue | | | Amount | | % of Total
Revenue | | | Amount | | | % | |
Product Sales | | | | | | | | | | | | | | | | | | | |
Breast Health | | $ | 553,065 | | 34 | % | | $ | 731,267 | | 44 | % | | $ | (178,202 | ) | | (24 | )% |
Diagnostics | | | 544,143 | | 33 | % | | | 474,633 | | 28 | % | | | 69,510 | | | 15 | % |
GYN Surgical | | | 263,187 | | 16 | % | | | 219,305 | | 13 | % | | | 43,882 | | | 20 | % |
Skeletal Health | | | 66,591 | | 4 | % | | | 77,242 | | 5 | % | | | (10,651 | ) | | (14 | )% |
| | | | | | | | | | | | | | | | | | | |
| | $ | 1,426,986 | | 87 | % | | $ | 1,502,447 | | 90 | % | | $ | (75,461 | ) | | (5 | )% |
| | | | | | | | | | | | | | | | | | | |
49
In fiscal 2009, our product sales decreased 5% compared to fiscal 2008, primarily due to a $178.2 million decrease in revenues from our Breast Health products and, to a lesser extent, a $10.7 million decrease in revenues from our Skeletal Health products, partially offset by increased revenues in our Diagnostics and GYN Surgical segments of $69.5 million and $43.9 million, respectively. These increases were due in part to a full year of revenue for these segments in fiscal 2009 compared to the inclusion of only 49 weeks of operating results for fiscal 2008 as we acquired these segments with the Cytyc merger on October 22, 2007. Included in the increased Diagnostics’ revenue is additional revenue from Third Wave of $31.2 million. We acquired Third Wave in the fourth quarter of fiscal 2008 and as such had a full year of revenue in fiscal 2009 compared to 11 weeks in fiscal 2008.
Breast Health product sales decreased 24% in fiscal 2009 compared to fiscal 2008, primarily due to a $150.0 million decrease in digital mammography systems sales caused primarily by a reduction in the number of Selenia full field mammography systems and related components, including our CAD software, sold domestically, and to a lesser extent, internationally. In addition, we have seen a slight deterioration of average selling prices, both domestically and internationally, driven by the current economic environment for capital purchases, and less expensive configurations of the units being sold. Also contributing to the decrease was a $15.6 million decrease in multicare stereotactic table sales primarily attributable to a decrease in the number of systems sold, principally in the U.S. We attribute the decline in sales of breast health capital equipment and related products primarily to the more difficult economic and capital spending environment. We also experienced a decline in our MammoSite single-lumen products of $10.2 million due to increased competition as a result of lower reimbursement rates compared to multi-lumen products. We received FDA clearance for our multi-lumen product on August 27, 2009, which we expect will improve our competitive position. Partially offsetting the declines in sales referenced above was an $18.2 million increase in revenues from our breast biopsy products.
Diagnostics product sales, which include ThinPrep, Rapid Fetal Fibronectin Test and our Third Wave products, increased 15% in fiscal 2009 compared to fiscal 2008. This increase was primarily due to the addition of Third Wave revenues of $37.1 million in fiscal 2009 compared to $5.9 million in fiscal 2008 and, to a lesser extent, an increase in the number of ThinPrep Pap Tests. The increase in fiscal 2009 is also due to the inclusion of Cytyc’s results for the full fiscal year versus 49 weeks in fiscal 2008. While we received FDA approval of the Cervista HPV HR and Cervista HPV 16/18 tests in March 2009, the revenue contribution has been modest in fiscal 2009.
GYN Surgical product sales, which include our NovaSure System and Adiana System, increased 20% in fiscal 2009 compared to fiscal 2008. This increase was primarily due to a significant increase in the number of NovaSure systems sold. The increase is also due to the inclusion of GYN Surgical’s revenue for the full fiscal year versus 49 weeks in fiscal 2008. Revenues from the Adiana System, which we received FDA approval on July 6, 2009, have been modest as the US and international market launches have been limited. We recently transferred the manufacturing of our Adiana System to our new manufacturing facility in Coyol, Costa Rica, and we are in the process of ramping up production to support the full scale launch of this product. We could incur delays and unanticipated costs in connection with our transfer and ramping up these manufacturing operations in our new facility that could delay our full scale launch of the Adiana System.
Skeletal Health product sales decreased 14% in fiscal 2009 compared to fiscal 2008, primarily due to a $5.8 million decrease in osteoporosis assessment product sales caused primarily by a decrease in the number of bone densitometry systems sold worldwide and lower average selling prices. This product line continues to face a difficult capital equipment environment in the U.S. and the ongoing effects of the reduction in reimbursement for osteoporosis assessment exams in the U.S. In addition, we experienced a reduction in revenues of $2.8 million in mini-C arm sales and a decrease in extremity MRI sales of $2.1 million. The decrease in mini-C arm and extremity MRI sales was due to a decrease in the number of systems sold.
In fiscal 2009 and 2008, approximately 80% of product sales were generated in the U.S., 12% in Europe, 4% in Asia, and 4% in other international markets.
50
Service and Other Revenues.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | % of Total
Revenue | | | Amount | | % of Total
Revenue | | | Amount | | % | |
Service and Other Revenues | | $ | 210,148 | | 13 | % | | $ | 172,052 | | 10 | % | | $ | 38,096 | | 22 | % |
| | | | | | | | | | | | | | | | | | |
Service and other revenues is primarily comprised of revenue generated from our field service organization to provide ongoing service, installation and repair of our products. Service and other revenues increased 22% in fiscal 2009 compared to fiscal 2008, primarily in our Breast Health segment, due to an increase in the number of service contracts executed driven by an increase in the installed base of our full field digital mammography systems and detectors.
Cost of Product Sales.
| | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | % of Product
Sales | | | Amount | | % of Product
Sales | | | Amount | | | % | |
Cost of Product Sales | | $ | 470,295 | | 33 | % | | $ | 535,082 | | 36 | % | | $ | (64,787 | ) | | (12 | )% |
Cost of Product Sales—Amortization of Intangible Assets | | | 155,519 | | 11 | % | | | 95,310 | | 6 | % | | | 60,209 | | | 63 | % |
Cost of Product Sales—Impairment of Intangible Assets | | | 4,065 | | 0 | % | | | — | | — | | | | 4,065 | | | 100 | % |
| | | | | | | | | | | | | | | | | | | |
| | $ | 629,879 | | 44 | % | | $ | 630,392 | | 42 | % | | $ | (513 | ) | | (0 | )% |
| | | | | | | | | | | | | | | | | | | |
Product sales gross margin decreased to 56% in fiscal 2009 compared to 58% in fiscal 2008 primarily due to the significant increase in intangible asset amortization expense of $60.2 million, partially offset by the increase in sales of our higher gross margin disposable products in our Diagnostics and GYN Surgical segments.
Cost of Product Sales. The cost of product sales as a percentage of product sales in fiscal 2009 was 33% compared to 36% in fiscal 2008. This improvement was primarily attributable to the increase in sales of our Diagnostics and GYN Surgical segments as a percentage of our total product sales as these products have a lower product cost as a percent of revenue compared to our Breast Health and Skeletal Health products. In addition, our cost of product sales in fiscal 2008 included additional costs associated with the write-up of acquired inventory to fair value in purchase accounting of $42.4 million related to the merger with Cytyc and $3.9 million related to the Third Wave acquisition. In fiscal 2009, the impact of these costs was only $1.2 million related to the Third Wave inventory write-up. Our margins in fiscal 2009 were also positively impacted by our cost reduction initiatives implemented in the first half of 2009, which included securing lower material costs from our vendors. Partially offsetting these improvements, was a decrease in gross margin in our Breast Health segment, primarily attributable to lower absorption of manufacturing costs due to lower volumes and to a lesser extent, a slight deterioration of average selling prices, driven by the current economic environment for capital purchases, and less expensive configurations of the units being sold.
Fiscal 2009 and 2008 cost of product sales included charges of $0.7 million and $4.5 million, respectively, for impairment of MRI inventory and a related purchase obligation, which was fulfilled in fiscal 2009.
Cost of Product Sales—Amortization of Intangible Assets.Amortization of intangible assets relates to acquired developed technology, which are generally being amortized over their estimated useful lives of between 8.5 and 15 years using a straight-line method or, if reliably determinable, based on the pattern in which the
51
economic benefits of the assets are expected to be consumed utilizing expected undiscounted future cash flows. The increase in amortization expense is due partly to the method of recognition based on expected economic benefits of the underlying assets, primarily related to the intangible assets acquired in the merger with Cytyc, which increased to $133.2 million from $80.2 million in fiscal 2008, and a full year of Third Wave related amortization of $7.8 million compared to $1.1 million in fiscal 2008.
Cost of Product Sales—Impairment of Intangible Assets.During the second quarter of fiscal 2009, we decided to discontinue selling a certain product acquired in the Third Wave acquisition as a result of communications from the FDA in the second quarter of 2009 regarding the approval process. This decision was an indicator of impairment, and we performed an impairment test, which indicated the undiscounted cash flows the asset group would generate over its remaining estimated useful life would not be sufficient to recover the carrying value of the asset group. Due to the insufficient cash flows to be generated, the Company determined that the related asset group’s fair value was de minimus and recorded an impairment charge of $4.1 million comprised of developed technology of $2.6 million and capitalized license fees of $1.5 million.
Cost of Service and Other Revenues.
| | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | % of Service
and Other
Revenues | | | Amount | | % of Service
and Other
Revenues | | | Amount | | | % | |
Cost of Service and Other Revenues | | $ | 149,769 | | 71 | % | | $ | 151,589 | | 88 | % | | $ | (1,820 | ) | | (1 | )% |
| | | | | | | | | | | | | | | | | | | |
Service and other revenues gross margin has improved to 29% in fiscal 2009 from 12% in fiscal 2008 due in part to the improved absorption of fixed service costs and the continued growth of service contract revenue, primarily in the Breast Health segment. We have increased the number of service contracts due to our increased installed base of our full field digital mammography systems and detectors. In addition, warranty costs have decreased due to lower failure rates in our products.
Operating Expenses.
| | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | September 27, 2008 | | Change | |
| | | Amount | | % of Total
Revenue | | Amount | | % of Total
Revenue | | Amount | | | % | |
Operating Expenses | | | | | | | | | | | | | | | | | |
Research and Development | | $ | 94,328 | | 6% | | $ | 81,421 | | 5% | | $ | 12,907 | | | 16 | % |
Selling and Marketing | | | 238,977 | | 15% | | | 261,524 | | 16% | | | (22,547 | ) | | (9 | )% |
General and Administrative | | | 148,824 | | 9% | | | 147,405 | | 9% | | | 1,419 | | | 1 | % |
Amortization of Intangibles | | | 51,210 | | 3% | | | 25,227 | | 1% | | | 25,983 | | | 103 | % |
Impairment of Goodwill | | | 2,340,023 | | 143% | | | — | | — | | | 2,340,023 | | | 100 | % |
Impairment of Intangibles | | | — | | — | | | 2,900 | | — | | | (2,900 | ) | | (100 | )% |
Acquired In-Process Research and Development | | | — | | — | | | 565,200 | | 34% | | | (565,200 | ) | | (100 | )% |
Restructuring Charges | | | 797 | | — | | | 6,383 | | — | | | (5,586 | ) | | (88 | )% |
| | | | | | | | | | | | | | | | | |
| | $ | 2,874,159 | | 176% | | $ | 1,090,060 | | 65% | | $ | 1,784,099 | | | 164 | % |
| | | | | | | | | | | | | | | | | |
Research and Development Expenses.Research and development expenses increased 16% in fiscal 2009 compared to fiscal 2008. These increases were primarily due to a full year of expenses of $20.7 million related to Third Wave compared to $3.7 million in fiscal 2008, and to a lesser extent a full year of operations from Cytyc in
52
fiscal 2009 compared to 49 weeks in fiscal 2008. In addition, there was an increase in expenses related to the anticipated launch of the Adiana System and additional clinical spending for a number of projects. These increases were partially offset by a decrease in related headcount, bonus and other discretionary areas resulting from a number of cost reduction initiatives implemented in the first half of 2009. In fiscal 2008, we recorded a $1.8 million charge related to a change in control payment associated with the merger with Cytyc. We expect research and development expenses to increase in fiscal 2010 as we conduct additional clinical trials and research to obtain FDA approval for our tomosynthesis product (Dimensions 3D) and continue our efforts to improve our existing products and develop next generation products.
Selling and Marketing Expenses.Selling and marketing expenses decreased 9% in fiscal 2009 compared to fiscal 2008 primarily due to lower commission expenses as a result of lower product revenues, lower bonuses driven by the Company’s operating results, reduced advertising and trade show expenditures, and other cost reductions including lower employee headcount resulting from our cost reduction initiatives implemented in the first half of 2009. These decreases were partially offset by an increase of $6.8 million related to the inclusion of a full year of operations of Third Wave, as well as a full year of operations from Cytyc in fiscal 2009 compared to 49 weeks in fiscal 2008.
General and Administrative Expenses.General and administrative expenses increased slightly in fiscal 2009 compared to fiscal 2008. The increase in the current year is primarily due to an increase of $7.4 million related to the inclusion of a full year of operations of Third Wave, as well as a full year of operations from Cytyc in fiscal 2009 compared to 49 weeks in fiscal 2008. In addition, stock-based compensation expense was higher by $5.2 million in fiscal 2009. Partially offsetting these increases was a decrease in bonus, headcount and related compensation, and other expenses as a result of our cost reduction initiatives implemented in the first half of 2009.
Amortization of Intangible Assets.Amortization of intangible assets results from customer relationships and trade names related to our acquisitions. These intangible assets are being amortized over their estimated useful lives of between 8.5 and 30 years using a straight-line method or, if reliably determinable, based on the pattern in which the economic benefits of the assets are expected to be consumed utilizing expected undiscounted future cash flows. The increases in these costs primarily relate to additional Cytyc-related amortization based on the pattern of economic benefit.
Impairment of Goodwill.Based upon a combination of factors, including the deteriorating macro-economic environment, declines in the stock market and the decline of our market capitalization significantly below the book value of our net assets, we concluded that potential goodwill impairment indicators existed as of December 27, 2008. As a result, we performed an interim goodwill impairment analysis as of December 27, 2008. Step 1 of the impairment analysis indicated that the carrying value of the net assets of certain of our reporting units, acquired in connection with the Cytyc acquisition, exceeded the estimated fair value of those reporting units. As a result, we were required to complete Step 2 of the impairment analysis to determine the amount, if any, of goodwill impairment charges. We completed Step 2 of this analysis during the second quarter of fiscal 2009 and recorded a goodwill impairment charge of $2.34 billion in the second quarter of fiscal 2009. Refer to Note 2 in our Consolidated Financial Statements contained in Item 15 of this Annual Report for more information. We completed our fiscal 2009 annual goodwill impairment analysis as of the first day of our fourth quarter and no additional impairments were recorded.
Impairment of Intangible Assets.Subsequent to the merger with Cytyc, we discontinued the development of Cytyc’s Helica Thermal Coagulator System product, used for the treatment of endometriosis. We will not realize any future cash flows from this product. Our intangible asset valuation for Cytyc included approximately $2.9 million related to customer relationships for Helica. As a result of the Helica product discontinuation, we recorded an impairment charge of $2.9 million in fiscal 2008.
53
Acquired In-Process Research and Development Expenses.The $565.2 million charge for in-process research and development expense is comprised of a $370.0 million charge recorded in connection with our merger with Cytyc and a $195.2 million charge recorded in connection with the Third Wave acquisition. Both of these charges are described in further detail above under the respective acquisitions.
Restructuring Charges.During the fourth quarter of 2009, we closed our manufacturing facility in Shanghai, China due to Chinese government requirements to move the facility. This facility, which manufactured organic photoconductor drum coatings, was acquired in connection with the AEG acquisition in 2006, and contributed approximately $9.7 million of revenue to our Breast Health business in fiscal 2009. In connection with this action, we recorded severance benefits and other costs of $0.8 million. The majority of employees were terminated and all termination benefits were paid as of September 26, 2009. Additional clean-up and closure costs will be recorded in fiscal 2010, however, we do not expect these costs to be material to our consolidated financial statements. Other costs were recorded in connection with this closure of $1.9 million primarily related to the impairment of manufacturing equipment, accelerated depreciation expense, and the write-off of inventory, all of which was recorded in cost of product sales. During the third quarter of fiscal 2008, we recorded $6.4 million in compensation charges, including $1.9 million in stock-based compensation, related to the resignation of our former Executive Chairman, which was effective May 20, 2008. The cash payments were made during fiscal 2008.
Interest Income.
| | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | September 27, 2008 | | Change | |
| | | Amount | | Amount | | Amount | | | % | |
Interest Income | | $ | 1,161 | | $ | 4,528 | | $ | (3,367 | ) | | (74 | )% |
| | | | | | | | | | | | | |
Interest income decreased in fiscal 2009 compared to fiscal 2008 primarily due to a decline in interest rates.
Interest Expense.
| | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | | Amount | | | Amount | | % | |
Interest Expense | | $ | (69,502 | ) | | $ | (84,912 | ) | | $ | 15,410 | | 18 | % |
| | | | | | | | | | | | | | |
Interest expense consists primarily of the interest costs and the related amortization of deferred financing costs for both our senior secured credit agreement entered into on October 22, 2007 in connection with the merger with Cytyc and amended on July 17, 2008 in connection with the Third Wave acquisition and our 2.0% Convertible Notes that were issued in December 2007 to pay down a portion of the term loans, which had higher interest rates. The decrease in interest expense in fiscal 2009 compared to fiscal 2008 was primarily due to lower term loan balances as we pay them down quarterly and lower interest rates on those balances. In addition to the required principal payments, we have made voluntary payments throughout the year resulting in a total decrease of $290.8 million in the principal of our term loans during fiscal 2009. Additionally, we had the benefit of the lower interest rates from our Convertible Notes for all of fiscal 2009 compared to approximately nine months in fiscal 2008 as prior to the issuance of the Convertible Notes we were paying a higher interest rate on the term loans, which had higher outstanding balances. In fiscal 2010, we will implement accounting guidance that requires us to allocate a portion of our Convertible Notes to equity based on the relative fair value of the embedded conversion feature in our Convertible Notes. This component is amortized to interest expense. As a result, we expect non-cash interest to increase approximately $71.1 million in fiscal 2010 from the adoption of this accounting guidance. See discussion under Recent Accounting Pronouncements below for additional information.
54
Other (Expense) Income, net.
| | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Other (Expense) Income, net | | $ | (3,660 | ) | | $ | (1,215 | ) | | $ | (2,445 | ) | | 201 | % |
| | | | | | | | | | | | | | | |
In fiscal 2009, other (expense) income, net primarily includes other-than-temporary impairment charges of cost-method investments of $2.2 million and foreign currency transaction losses of $2.3 million, offset by an increase of $0.7 million in the cash surrender value of life insurance contracts related to our Supplemental Executive Retirement Plan (“SERP”). Included in the foreign currency transaction losses is a gain of $0.7 million related to the elimination of the cumulative translation adjustment related to our manufacturing facility in Shanghai, China due to its closure. In fiscal 2008, these balances were primarily related to a decrease in the cash surrender value of life insurance contracts related to our SERP of $1.4 million and foreign currency transaction losses of $0.7 million. The increase in foreign currency losses is due to the significant volatility of exchange rates during fiscal 2009, primarily the Euro. To the extent that foreign currency exchange rates fluctuate in the future, we may be exposed to continued financial risk.
Provision for Income Taxes.
| | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | September 27, 2008 | | Change | |
| | | Amount | | Amount | | Amount | | | % | |
Provision for Income Taxes | | $ | 87,562 | | $ | 106,476 | | $ | (18,914 | ) | | (18 | )% |
| | | | | | | | | | | | | |
Our effective tax rate for fiscal 2009 was 4.2% of the pre-tax loss compared to 38.1% of the pre-tax loss in fiscal 2008. Our effective tax rate for fiscal 2009 was significantly impacted by the $2.34 billion goodwill impairment charge recorded in the second quarter of fiscal 2009, substantially all of which is not deductible for tax purposes. The effective tax rate for fiscal 2008 was significantly impacted by the acquired in-process research and development charge related to the merger with Cytyc and Third Wave acquisition, which is not tax deductible.
We anticipate an effective tax rate of approximately 36% of pre-tax earnings in fiscal 2010.
Segment Results of Operations
We report our business as four segments: Breast Health, Diagnostics, GYN Surgical and Skeletal Health. The accounting policies of the segments are the same as those described in the footnotes to the accompanying consolidated financial statements. We measure segment performance based on total revenues and operating income or loss. Revenues from product sales of each of these segments are described in further detail above. The discussion that follows is a summary analysis of total revenues and the primary changes in operating income or loss by segment.
Breast Health.
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | | % of Total
Segment
Revenue | | | Amount | | % of Total
Segment
Revenue | | | Amount | | | % | |
Total Revenues | | $ | 728,884 | | | 100 | % | | $ | 860,848 | | 100 | % | | $ | (131,964 | ) | | (15 | )% |
| | | | | | | | | | | | | | | | | | | | |
Operating (Loss) Income | | $ | (122,559 | ) | | (17 | )% | | $ | 211,704 | | 25 | % | | $ | (334,263 | ) | | (158 | )% |
| | | | | | | | | | | | | | | | | | | | |
Breast Health revenues decreased in fiscal 2009 compared to fiscal 2008 primarily due to the $178.2 million decrease in product sales discussed above, partially offset by an increase of $46.2 million in service revenues that
55
is substantially related to additional service contracts for the increased number of Selenia systems in our installed base.
This segment incurred an operating loss in fiscal 2009 compared to operating income in fiscal 2008 primarily due to a $265.9 million goodwill impairment charge recorded in the second quarter related to our MammoSite reporting unit in addition to the reduction of revenue discussed above and lower gross margins, partially offset by a reduction of operating expenses from our cost reduction initiatives implemented in the first half of fiscal 2009. Our gross margin in this business segment was 47% in fiscal 2009 compared to 51% in fiscal 2008. The decrease in gross margin was primarily attributable to lower absorption of manufacturing costs due to lower volumes, an increase of $8.8 million in amortization expense of intangible assets, and to a lesser extent, a slight deterioration of average selling prices driven by the current economic environment for capital purchases, and less expensive configurations of the units being sold. In addition, included in cost of product sales was approximately $1.9 million of costs related to the closure of our manufacturing facility in Shanghai. This segment incurred charges of $3.3 million in fiscal 2008 related to sales of acquired MammoSite inventory that was written up to fair value for purchase accounting purposes.
Diagnostics.
| | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | | % of Total
Segment
Revenue | | | Amount | | | % of Total
Segment
Revenue | | | Amount | | | % | |
Total Revenues | | $ | 547,892 | | | 100 | % | | $ | 485,004 | | | 100 | % | | $ | 62,888 | | | 13 | % |
| | | | | | | | | | | | | | | | | | | | | |
Operating Loss | | $ | (809,640 | ) | | (148 | )% | | $ | (172,538 | ) | | (36 | )% | | $ | (637,102 | ) | | 369 | % |
| | | | | | | | | | | | | | | | | | | | | |
Diagnostics revenues increased in fiscal 2009 compared to fiscal 2008 primarily due to the increase in product sales discussed above.
The operating loss in this segment in fiscal 2009 included a $908.3 million goodwill impairment charge recorded in the second quarter, intangible asset amortization of $119.2 million and a full year of operating costs related to Third Wave compared to 11 weeks in fiscal 2008. Partially offsetting these additional charges in fiscal 2009 were reduced operating expenses resulting from our cost reduction initiatives implemented in the first half of fiscal 2009. The operating loss in fiscal 2008 included a $195.2 million charge for in-process research and development related to the Third Wave acquisition, an $85.2 million charge for in-process research and development related to the merger with Cytyc, intangible asset amortization of $68.7 million, and a $3.6 million restructuring charge in the third quarter related to the resignation of our former Executive Chairman in May 2008. Gross margin in fiscal 2009 was 55% compared to 56% in fiscal 2008. The reduction in gross margin was primarily due to an increase in amortization expense due to an increase of $27.6 million and $6.7 million in the amortization of Cytyc and Third Wave related intangible assets, respectively. In addition, gross margin in fiscal 2009 included the write-off of intangible assets of $4.1 million and $1.2 million of charges for the write-up to fair value of acquired inventory sold by Third Wave in fiscal 2009. Gross margin in fiscal 2008 included charges of $26.6 million and $3.9 million, respectively, for the write-up to fair value of acquired Cytyc and Third Wave inventory sold during fiscal 2008.
GYN Surgical.
| | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | | % of Total
Segment
Revenue | | | Amount | | | % of Total
Segment
Revenue | | | Amount | | | % | |
Total Revenues | | $ | 264,900 | | | 100 | % | | $ | 221,069 | | | 100 | % | | $ | 43,831 | | | 20 | % |
| | | | | | | | | | | | | | | | | | | | | |
Operating Loss | | $ | (1,097,685 | ) | | (414 | )% | | $ | (241,450 | ) | | (109 | )% | | $ | (856,235 | ) | | 355 | % |
| | | | | | | | | | | | | | | | | | | | | |
56
GYN Surgical revenues increased in fiscal 2009 compared to fiscal 2008 due to the increase in product sales discussed above. The operating loss in this segment in fiscal 2009 included a $1.17 billion goodwill impairment charge recorded in the second quarter and additional amortization expense of $26.5 million. Partially offsetting these charges in fiscal 2009 was the increase in revenue discussed above as well as a decrease in operating expenses as a result of cost reduction initiatives implemented in the first half of this year. The operating loss in fiscal 2008 included a $284.8 million charge for in-process research and development related to the merger with Cytyc, a $2.9 million impairment charge for the Helica Thermal Coagulator System intangibles and a $2.4 million restructuring charge in the third quarter related to the resignation of our former Executive Chairman in May 2008. Our gross margin in this business segment was 67% in both fiscal 2009 and 2008. Gross margin in fiscal 2009 and 2008 included amortization expense from intangible assets of $38.2 million and $21.0 million, respectively, and fiscal 2008 included a $12.4 million charge for the write-up to fair value of Cytyc inventory that was sold during the first quarter of fiscal 2008.
Skeletal Health.
| | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 26, 2009 | | | September 27, 2008 | | | Change | |
| | | Amount | | % of Total
Segment
Revenue | | | Amount | | % of Total
Segment
Revenue | | | Amount | | | % | |
Total Revenues | | $ | 95,458 | | 100 | % | | $ | 107,578 | | 100 | % | | $ | (12,120 | ) | | (11 | )% |
| | | | | | | | | | | | | | | | | | | |
Operating Income | | $ | 13,210 | | 14 | % | | $ | 4,742 | | 4 | % | | $ | 8,468 | | | 179 | % |
| | | | | | | | | | | | | | | | | | | |
Skeletal Health revenues decreased in fiscal 2009 compared to fiscal 2008 primarily due to the decline in product sales discussed above. Our gross margin in this business segment was 41% compared to 34% in fiscal 2008. The improvement was primarily due to reductions in material costs and in manufacturing spending. Operating income for this segment improved due to the improved gross margin and from cost reduction initiatives implemented in the first half of 2009. The operating income and gross margin in fiscal 2009 included a $0.7 million charge associated with MRI inventory and purchase obligations compared to $4.5 million in fiscal 2008.
Fiscal Year Ended September 27, 2008 Compared to Fiscal Year Ended September 29, 2007
Product Sales.
| | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | Change | |
| | | Amount | | % of Total
Revenue | | | Amount | | % of Total
Revenue | | Amount | | % | |
Product Sales | | | | | | | | | | | | | | | | | |
Breast Health | | $ | 731,267 | | 44 | % | | $ | 559,092 | | 76% | | $ | 172,175 | | 31 | % |
Diagnostics | | | 474,633 | | 28 | % | | | — | | — | | | 474,633 | | 100 | % |
GYN Surgical | | | 219,305 | | 13 | % | | | — | | — | | | 219,305 | | 100 | % |
Skeletal Health | | | 77,242 | | 5 | % | | | 69,762 | | 9% | | | 7,480 | | 11 | % |
| | | | | | | | | | | | | | | | | |
| | $ | 1,502,447 | | 90 | % | | $ | 628,854 | | 85% | | $ | 873,593 | | 139 | % |
| | | | | | | | | | | | | | | | | |
In fiscal 2008, our product sales increased 139% compared to fiscal 2007, primarily due to the revenues from the addition of the Diagnostics segment, of approximately $474.6 million, and the GYN Surgical segment, of approximately $219.3 million, that we acquired in connection with our merger with Cytyc, and an increase in revenues from our Breast Health products of approximately $172.2 million.
Breast Health product sales increased 31% in fiscal 2008 compared to fiscal 2007, primarily due to a $97.0 million increase in worldwide digital mammography system sales, the addition of $33.9 million of product sales
57
of the MammoSite Radiation Therapy System, a $23.6 million increase in breast biopsy device sales and an increase of $21.5 million in product sales of the MammoPad breast cushion. Partially offsetting these increases was a decrease of $6.4 million in digital array sales to an OEM as we phase out of selling these arrays to third parties. The MammoSite system was acquired in connection with our merger with Cytyc in October 2007 and the MammoPad breast cushion was acquired in connection with our BioLucent acquisition in September 2007. The increase in our digital mammography product sales was primarily attributable to an increase in the number of Selenia systems and related components sold, including our CAD software. In fiscal 2008, we sold 1,678 digital mammography systems compared to 1,189 systems in fiscal 2007. This revenue was partially offset by a decrease in average selling prices primarily attributable to increased competition, higher dealer sales, changes in product configuration and increased multi-system sales. We attribute the increase in digital mammography system sales primarily to the growing acceptance of our Selenia mammography system and of digital mammography in general.
Diagnostics product sales were $474.6 million in fiscal 2008, due to the inclusion of Cytyc results for 49 of the 52 weeks in the current year as well as 9 weeks of Third Wave revenues of approximately $5.9 million. Cytyc Diagnostic sales include our ThinPrep and Rapid products.
GYN Surgical product sales were $219.3 million in fiscal 2008, due to the inclusion of Cytyc results for 49 of the 52 weeks in the current year. These sales include our NovaSure system.
Skeletal Health product sales increased 11% in fiscal 2008 compared to fiscal 2007, primarily due to a $10.8 million increase in mini C-arm sales worldwide, partially offset by a $2.0 million decrease in extremity MRI sales and a $1.2 million decrease in bone densitometry product sales. The increase in mini C-arm sales was primarily due to an increase in the number of units sold and, to a lesser extent, an increase in the average selling prices related to the commercialization of a new and enhanced product version. The decrease in extremity MRI sales was due to a decrease in the number of systems sold. The decrease in bone densitometry sales was primarily due to a decrease in the number of used bone densitometry systems and upgrades sold and a decrease in the average selling prices of our bone densitometry systems in the United States, partially offset by an increase in the number of bone densitometry systems sold internationally. We believe the decrease in our domestic osteoporosis assessment average selling prices reflected a decline in market conditions due in part to a reduction in reimbursement for osteoporosis assessment exams.
In fiscal 2008, approximately 80% of product sales were generated in the United States, 12% in Europe, 4% in Asia, and 4% in other international markets. In fiscal 2007, approximately 75% of product sales were generated in the United States, 15% in Europe, 5% in Asia, and 5% in other international markets. The increase in the percentage of product sales generated in the United States in fiscal 2008 is primarily due to the additional product sales from Cytyc, which had a higher percentage of its product sales from the United States than our historical businesses.
Service and Other Revenues.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | | Change | |
| | | Amount | | % of Total
Revenue | | | Amount | | % of Total
Revenue | | | Amount | | % | |
Service and Other Revenues | | $ | 172,052 | | 10 | % | | $ | 109,514 | | 15 | % | | $ | 62,538 | | 57 | % |
| | | | | | | | | | | | | | | | | | |
Service and other revenues increased 57% in fiscal 2008 compared to fiscal 2007. This increase was primarily due to an increase in service and other revenues of $49.8 million in our Breast Health segment, primarily due to an increase in service contract revenues, and the inclusion of service and other revenues of $10.3 million from the Diagnostics segment as a result of the inclusion of Cytyc results for 49 of the 52 weeks in fiscal 2008. We believe that the increase in our Breast Health service and other revenues reflected the growth in our installed base of systems and detectors.
58
Cost of Product Sales.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | | Change | |
| | | Amount | | % of Product
Sales | | | Amount | | % of Product
Sales | | | Amount | | % | |
Cost of Product Sales | | $ | 535,082 | | 36 | % | | $ | 267,470 | | 43 | % | | $ | 267,612 | | 100 | % |
Cost of Product Sales—Amortization of Intangible Assets | | | 95,310 | | 6 | % | | | 11,262 | | 2 | % | | | 84,048 | | 746 | % |
| | | | | | | | | | | | | | | | | | |
| | $ | 630,392 | | 42 | % | | $ | 278,732 | | 44 | % | | $ | 351,660 | | 126 | % |
| | | | | | | | | | | | | | | | | | |
Our gross margin increased in fiscal 2008 to 58% from 56% in fiscal 2007 due to the inclusion of Cytyc’s products, which have higher gross margins than our Breast Health and Skeletal Health products offset by the additional amortization expense almost entirely related to the merger with Cytyc.
Cost of Product Sales.The cost of product sales increased 100% in fiscal 2008 compared to fiscal 2007 primarily due to the addition of $196.0 million of cost of product sales from the Cytyc products included in our results since October 22, 2007 and, to a lesser extent, increased product sales of our historical products discussed above. Included in the additional Cytyc cost of product sales is approximately $42.3 million of additional costs related to sales of acquired Cytyc inventory that was written up to fair value for purchase accounting purposes as of the date of acquisition.
The cost of product sales as a percentage of product revenue in fiscal 2008 was 36% as compared to 43% in the prior year. These costs as a percentage of product sales decreased primarily due to the higher gross margins earned on Cytyc product sales compared to our historical products, partially offset by the additional charges for the write-up to fair value for the Cytyc inventory sold as noted above. Also contributing to the decrease in cost of product sales as a percentage of product revenue was increased revenues and improved profitability associated with the shift in mammography product sales to our Selenia full field digital mammography systems. Our higher Selenia system sales resulted in an improved absorption of fixed manufacturing costs. Partially offsetting the decreases in costs as a percentage of product sales were charges associated with a MRI inventory impairment charge and related purchase obligations totaling $4.5 million, and $3.9 million related to sales of acquired Third Wave inventory that was written up to fair value in connection with purchase accounting in fiscal 2008.
We identified certain costs recorded within “Cost of Service and Other Revenues” in our Consolidated Statement of Operations during the first three quarters of fiscal 2008 that more appropriately should be classified as “Cost of Product Sales”. We determined that the reclassification was not material to our consolidated financial statements and corrected the classification in the fourth quarter of fiscal 2008. We also reclassified these costs related to prior periods to the current presentation, which resulted in an increase in “Cost of Product Sales” and a corresponding decrease in “Cost of Service and Other Revenues” of $9.3 million in the three months ended December 29, 2007; $12.3 million in the three months ended March 29, 2008; and $13.3 million in the three months ended June 28, 2008.
Cost of Product Sales—Amortization of Intangible Assets.Cost of product sales—amortization of intangible assets increased primarily due to $80.2 million of amortization of intangible assets obtained as part of the merger with Cytyc in the first quarter of fiscal 2008. The underlying intangible assets substantially relate to acquired developed technology and know-how. These intangible assets are generally being amortized over their estimated useful lives of between 8.5 and 15 years.
59
Cost of Service and Other Revenues.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | | Change | |
| | | Amount | | % of Total
Segment
Revenue | | | Amount | | % of Total
Segment
Revenue | | | Amount | | % | |
Cost of Service and Other Revenues | | $ | 151,589 | | 88 | % | | $ | 114,307 | | 104 | % | | $ | 37,282 | | 33 | % |
| | | | | | | | | | | | | | | | | | |
Cost of service and other revenues increased in absolute dollars primarily related to additional costs from the merger with Cytyc of approximately $14.7 million in fiscal 2008. The remainder of the increase was primarily due to personnel and other costs to expand our service capabilities for breast health, especially in the United States, to support our growing installed base of our breast health products as a result of the increased service and other revenues. Please see “Cost of Product Sales” above for discussion of reclassification between cost of product sales and cost of service and other revenues during fiscal 2008.
Operating Expenses.
| | | | | | | | | | | | | | | |
| | | Years Ended |
| | | September 27, 2008 | | September 29, 2007 | | Change |
| | | Amount | | % of Total
Revenue | | Amount | | % of Total
Revenue | | Amount | | % |
Operating Expenses | | | | | | | | | | | | | | | |
Research and Development | | $ | 81,421 | | 5% | | $ | 44,381 | | 6% | | $ | 37,040 | | 83% |
Selling and Marketing | | | 261,524 | | 16% | | | 85,520 | | 12% | | | 176,004 | | 206% |
General and Administrative | | | 147,405 | | 9% | | | 62,092 | | 8% | | | 85,313 | | 137% |
Amortization of Intangibles | | | 25,227 | | 1% | | | 5,584 | | 1% | | | 19,643 | | 352% |
Restructuring Charges | | | 6,383 | | — | | | — | | — | | | 6,383 | | 100% |
Impairment of Intangibles | | | 2,900 | | — | | | — | | — | | | 2,900 | | 100% |
Acquired In-Process Research and Development | | | 565,200 | | 34% | | | — | | — | | | 565,200 | | 100% |
| | | | | | | | | | | | | | | |
| | $ | 1,090,060 | | 65% | | $ | 197,577 | | 27% | | $ | 892,483 | | 452% |
| | | | | | | | | | | | | | | |
Research and Development Expenses.Research and development expenses increased 83% in fiscal 2008 as compared to fiscal 2007. These increases were primarily due to the inclusion of $31.5 million and $3.7 million of expenses in the current year associated with Cytyc-related and Third Wave-related activity, respectively, since the close of the merger with Cytyc and the acquisition of Third Wave. Also contributing to the increase was an increase in mammography related expenses of $2.6 million in fiscal 2008 primarily related to our tomosynthesis project and a $1.8 million charge for a change in control payment related to the merger with Cytyc recorded in the first quarter.
Selling and Marketing Expenses.Selling and marketing expenses increased 206% in fiscal 2008 as compared to fiscal 2007. These increases were primarily due to the inclusion of $160.3 million of expenses associated with Cytyc-related activity since the close of the merger and approximately $5.1 million related to increased compensation and related expenses from the additional sales representatives added from the BioLucent acquisition in the fourth quarter of fiscal 2007. Also contributing to the increase was approximately $3.3 million increased commission expense due to the increased product sales.
General and Administrative Expenses. General and administrative expenses increased 137% in fiscal 2008 as compared to fiscal 2007 primarily due to $77.5 million in expenses associated with Cytyc-related activity since the close of the merger and an increase of $11.2 million due to incremental stock-based compensation.
60
Amortization of Intangible Assets.Amortization expense of intangible assets increased 352% in fiscal 2008 as compared to fiscal 2007, primarily due to $15.9 million of amortization of intangible assets obtained as part of the merger with Cytyc in the first quarter of fiscal 2008. Fiscal years 2008 and 2007 also include the amortization of intangible assets acquired from AEG, R2, and Suros in the third and fourth quarters of fiscal 2006. The underlying intangible assets substantially relate to acquired customer relationships and trade names. The intangible assets acquired in the merger with Cytyc are being amortized over their estimated useful lives of between 8.5 and 30 years.
Restructuring Charges.During fiscal 2008, we recorded $6.4 million in compensation charges, including $1.9 million in stock-based compensation, related to the resignation of our Executive Chairman, which was effective May 20, 2008.
Impairment of Intangible Assets.Subsequent to the merger with Cytyc, we discontinued the development of Cytyc’s Helica Thermal Coagulator System product, used for the treatment of endometriosis. We will not realize any future cash flows from this product. Our intangible asset valuation for Cytyc included approximately $2.9 million related to customer relationships for Helica. As a result of the Helica product discontinuation, we recorded an impairment charge of $2.9 million during the first quarter of fiscal 2008.
Acquired In-Process Research and Development Expenses.Included in this charge in fiscal 2008 is $370.0 million for in-process research and development incurred in connection with our merger with Cytyc as described in further detail above under “Fiscal 2008 Acquisitions—Cytyc Corporation.” Also included is $195.2 million for in-process research and development incurred in connection with our acquisition of Third Wave as described in further detail above under “Fiscal 2008 Acquisitions—Third Wave Technologies, Inc.”
Interest Income.
| | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | September 29, 2007 | | Change | |
| | | Amount | | Amount | | Amount | | % | |
Interest Income | | $ | 4,528 | | $ | 2,815 | | $ | 1,713 | | 61 | % |
| | | | | | | | | | | | |
Interest income increased in fiscal 2008 compared to fiscal 2007 primarily due an increase in our investment balances, partially offset by a decrease in the interest rate earned during the current year compared to fiscal 2007.
Interest Expense.
| | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Interest Expense | | $ | (84,912 | ) | | $ | (2,511 | ) | | $ | (82,401 | ) | | 3,282 | % |
| | | | | | | | | | | | | | | |
In fiscal 2008, these expenses consisted primarily of the interest costs and the related amortization of deferred financing costs related to both the senior secured credit agreement entered into on October 22, 2007 in connection with the merger with Cytyc and our subsequent 2.0% Convertible Note Offering. In fiscal 2007, these expenses consisted primarily of the interest costs and fees on the unsecured revolving line of credit entered into on July 24, 2006 (and amended on September 25, 2006) of $1.5 million as well as interest costs on notes payable assumed with the acquisition of AEG in the amount of $1.0 million. We incurred additional interest expense in the fourth quarter of fiscal 2008 in connection with our borrowing of $540.0 million on July 17, 2008 to fund a portion of the purchase price for the acquisition of Third Wave.
61
Other (Expense) Income, net.
| | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | Change | |
| | | Amount | | | Amount | | Amount | | | % | |
Other (Expense) Income, net | | $ | (1,215 | ) | | $ | 433 | | $ | (1,648 | ) | | 381 | % |
| | | | | | | | | | | | | | |
In fiscal 2008, these balances were primarily related to foreign currency transaction losses of approximately $0.7 million and a decrease in the cash surrender value of life insurance contracts related to our SERP of approximately $1.4 million. In fiscal 2007, other income related primarily to the increase in the cash surrender value of life insurance contracts related to our SERP.
Provision for Income Taxes.
| | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | September 29, 2007 | | Change | |
| | | Amount | | Amount | | Amount | | % | |
Provision for Income Taxes | | $ | 106,476 | | $ | 53,911 | | $ | 52,565 | | 98 | % |
| | | | | | | | | | | | |
Our effective tax rate for fiscal 2008 was 38.1% of the pre-tax loss. For fiscal 2008, our effective tax rate was affected by the in-process research and development and intangible asset impairment charges we incurred in connection with our merger with Cytyc and the in-process research and development that we incurred in connection with the acquisition of Third Wave Technologies. Absent the in-process research and development and intangible asset impairment charges, our effective tax rate would have been approximately 36.9% for fiscal 2008. Our effective tax rate for fiscal 2007 was 36.3% of pre-tax earnings. This represented our normalized rate of approximately 37% reduced by certain tax credits.
Our net deferred tax liability increased approximately $842.0 million in fiscal 2008 primarily due to the increase of intangible assets as a result of the merger with Cytyc, for which the related amortization is not deductible for tax purposes.
Segment Results of Operations
As a result of our merger with Cytyc we began reporting our business as four segments: Breast Health, Diagnostics, GYN Surgical and Skeletal Health. Fiscal 2007 was restated to conform to this presentation. The accounting policies of the segments are the same as those described in the footnotes to the accompanying consolidated financial statements. We measure segment performance based on total revenues and operating income or loss. Revenues from product sales of each of these segments are described in further detail above. The discussion that follows is a summary analysis of total revenues and the primary changes in operating income or loss by segment.
Breast Health.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | | Change | |
| | | Amount | | % of Total
Segment
Revenue | | | Amount | | % of Total
Segment
Revenue | | | Amount | | % | |
Total Revenues | | $ | 860,848 | | 100 | % | | $ | 638,898 | | 100 | % | | $ | 221,950 | | 35 | % |
| | | | | | | | | | | | | | | | | | |
Operating Income | | $ | 211,704 | | 25 | % | | $ | 146,907 | | 23 | % | | $ | 64,797 | | 44 | % |
| | | | | | | | | | | | | | | | | | |
62
Breast Health revenues for fiscal 2008 increased primarily due to the $172.2 million increase in product sales discussed above and due to a $49.8 million increase in service revenues that was primarily related to the increased number of service contracts for the increased number of Selenia systems in our installed base. Operating income for this business segment increased primarily due to the increased revenues. Our gross margin in this business segment was 51% in fiscal 2008 as compared to 49% in fiscal 2007. In fiscal 2008 our gross margins improved from the increase in product revenues of our more profitable Selenia systems versus our analog mammography systems and, to a lesser extent, higher margins realized on our MammoSite product, acquired as part of the merger with Cytyc. In addition, higher total revenues including higher Selenia system sales have allowed for the greater absorption of manufacturing costs. Partially offsetting these improvements was a charge of $3.3 million for the write-up to fair value of MammoSite RTS inventory sold, primarily during the first quarter of fiscal 2008. Operating expenses for this business segment increased 34% in fiscal 2008, primarily due to the addition of $28.4 million of operating expenses from the MammoSite business as well as from increased operating expenses in support of our growing Selenia business. Also contributing to the increases during the year was an increase in stock-based compensation of $7.1 million, as well as a $0.4 million restructuring charge in the third quarter related to the resignation of our Executive Chairman in May 2008.
Diagnostics.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended |
| | | September 27, 2008 | | | September 29, 2007 | | Change |
| | | Amount | | | % of Total
Segment
Revenue | | | Amount | | % of Total
Segment
Revenue | | Amount | | | % |
Total Revenues | | $ | 485,004 | | | 100 | % | | $ | — | | — | | $ | 485,004 | | | — |
| | | | | | | | | | | | | | | | | | |
Operating Loss | | $ | (172,538 | ) | | (36 | %) | | $ | — | | — | | $ | (172,538 | ) | | — |
| | | | | | | | | | | | | | | | | | |
Diagnostics revenues, which include our ThinPrep, Rapid Fetal Fibronectin Test and Third Wave products, totaled $485.0 million in fiscal 2008. Our gross margin in this business segment was 57%, including charges of $26.6 million and $3.9 million, respectively, for the write-up to fair value of the Cytyc inventory sold during the first quarter and the Third Wave inventory sold during the fourth quarter of fiscal 2008. The operating loss also included an $85.2 million charge for in-process research and development as a result of the merger with Cytyc in the first quarter, a $195.2 million charge for in-process research and development as a result of the Third Wave acquisition in the fourth quarter, stock-based compensation of $7.6 million and a $3.6 million restructuring charge in the third quarter related to the resignation of our Executive Chairman in May 2008.
GYN Surgical.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended |
| | | September 27, 2008 | | | September 29, 2007 | | Change |
| | | Amount | | | % of Total
Segment
Revenue | | | Amount | | % of Total
Segment
Revenue | | Amount | | | % |
Total Revenues | | $ | 221,069 | | | 100 | % | | $ | — | | — | | $ | 221,069 | | | — |
| | | | | | | | | | | | | | | | | | |
Operating Loss | | $ | (241,450 | ) | | (109 | %) | | $ | — | | — | | $ | (241,450 | ) | | — |
| | | | | | | | | | | | | | | | | | |
GYN Surgical revenues, which include our NovaSure products and our Adiana System under development in fiscal 2008, totaled $221.1 million in fiscal 2008. In the second and third quarters, we believe that sales of the NovaSure System were adversely affected by a modest softening in sales to the hospital-based market, as well as lower than expected customer inventory utilization. Our sales to both the hospital market and office based market generally have been based upon current order bookings for immediate shipment with little or no backlog. Over the last three quarters, we refocused our sales efforts and programs to increasing longer term customer
63
commitments. However, while we could not assure that we would be successful, our goal was to have an increase in NovaSure system backlog, followed by an increase in sequential growth of revenue during fiscal 2009 with sales to the physician office-based market playing a role in that increase. Our gross margin in this business segment was 67% during this period and includes a charge of $12.4 million for the write-up to fair value of the Cytyc inventory sold during the first fiscal quarter of 2008. The operating loss for fiscal 2008 also includes a $284.8 million charge for in-process research and development as a result of the merger with Cytyc and a $2.9 million impairment charge of the Helica Thermal Coagulator System intangibles. This segment included stock-based compensation of $3.8 million in fiscal 2008, as well as a $2.4 million restructuring charge in the third quarter related to the resignation of our Executive Chairman in May 2008.
Skeletal Health.
| | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 27, 2008 | | | September 29, 2007 | | | Change | |
| | | Amount | | % of Total
Segment
Revenue | | | Amount | | % of Total
Segment
Revenue | | | Amount | | % | |
Total Revenues | | $ | 107,578 | | 100 | % | | $ | 99,470 | | 100 | % | | $ | 8,108 | | 8 | % |
| | | | | | | | | | | | | | | | | | |
Operating Income | | $ | 4,742 | | 4 | % | | $ | 845 | | 1 | % | | $ | 3,897 | | 461 | % |
| | | | | | | | | | | | | | | | | | |
Skeletal Health revenues increased in fiscal 2008 compared to the corresponding period in the prior year primarily due to the $7.5 million increase in product sales discussed above. Our gross margin in this business segment was 34% in fiscal 2008 compared to 32% in fiscal 2007. Operating income and gross margin for the Skeletal Health segment increased in fiscal 2008 over fiscal 2007 primarily due to the increased revenues and due to improved absorption as a result of manufacturing additional products in the facility where the Skeletal Health products are produced, partially offset by charges associated with MRI inventory and purchase obligations recorded in fiscal 2008 totaling $4.5 million compared to $2.0 million in fiscal 2007. Skeletal Health costs and expenses included stock compensation of $2.2 million and $1.1 million in fiscal 2008 and fiscal 2007, respectively.
Liquidity and Capital Resources
At September 26, 2009, we had $492.2 million of working capital, and our cash and cash equivalents totaled $293.2 million. Our cash and cash equivalents balance increased by $197.5 million during fiscal 2009, primarily from cash generated from our operations. This cash source was partially offset by our financing activities relating to our repayment of amounts outstanding under our credit agreement and certain other notes payable and to a lesser extent cash used in our investing activities primarily for purchases of property and equipment and placement of equipment under customer usage agreements.
Our operating activities generated $546.4 million of cash, which included a net loss of $2.18 billion reduced primarily by non-cash charges for goodwill and intangible asset impairments of $2.34 billion, depreciation and amortization expense of $273.9 million, stock-based compensation expense of $32.9 million and non-cash interest expense of $17.7 million from the amortization of debt issuance costs. Cash provided by operations due to changes in our operating assets and liabilities included a decrease in accounts receivable of $57.6 million, an increase in deferred revenue of $19.6 million and a decrease in prepaid income taxes of $17.9 million. The decrease in accounts receivable was primarily due to the decline in sales volume in the current quarter as compared to the fourth quarter of fiscal 2008 as well as improved collections. The increase in deferred revenue was primarily due to an increase in the number of service contracts as our installed base of our Breast Health products continues to grow. The decrease in prepaid income taxes was due to the utilization of amounts to offset current taxable income. Cash provided by operations was offset by an increase in inventories of $15.1 million and a decrease in accounts payable and accrued expenses and other liabilities of $12.9 million and $10.6 million, respectively. The increase in inventories was primarily related to the increase in components on hand as a result
64
of the decline in sales volume. The decrease in accounts payable was primarily due to the timing of payments and our overall efforts to reduce operating expenses. The decrease in accrued expenses and other liabilities was primarily due to lower compensation and bonuses as a result of a lower headcount and operating results below the operating plan, and overall lower operating expenses excluding amortization of intangible assets due to our cost reduction initiatives.
During fiscal 2009, we used $61.5 million of cash in investing activities. This use of cash was primarily attributable to $31.4 million for purchases of property and equipment, which consisted primarily of manufacturing, demonstration and test equipment and computer software and hardware. We also invested $22.8 million in equipment under customer usage agreements and purchased certain intellectual property totaling $6.2 million. In addition, we purchased of $5.3 million of life insurance contracts to fund future payments under our SERP.
During fiscal 2009, we utilized $288.6 million of cash in financing activities, substantially for repayments of the term loans under our credit agreement of $290.8 million and the payment of $10.1 million of notes payable, primarily our AEG loans. Offsetting these payments was proceeds of $10.9 million from the exercise of stock options and the purchase of common shares under the employee stock purchase plan.
Debt
We had total debt of $1.9 billion at September 26, 2009. The majority of our debt, our convertible notes and term loans under our credit agreement, was obtained to fund our Third Wave acquisition and merger with Cytyc in fiscal 2008. The debt maturity schedule for the components of our debt obligations as of September 26, 2009 is as follows:
| | | | | | | | | | | | | | | | | | |
| | | 2010 | | 2011 | | 2012 | | 2013 | | 2014 | | Total |
Term Loan A | | $ | 28,789 | | $ | 18,227 | | $ | 18,227 | | $ | 59,475 | | $ | — | | $ | 124,718 |
Term Loan B | | | 6,785 | | | 628 | | | 628 | | | 41,408 | | | — | | | 49,449 |
AEG debt | | | 1,500 | | | — | | | — | | | — | | | — | | | 1,500 |
Other | | | 1,299 | | | 1,362 | | | — | | | — | | | — | | | 2,661 |
Convertible Notes (1) | | | — | | | — | | | — | | | — | | | 1,725,000 | | | 1,725,000 |
| | | | | | | | | | | | | | | | | | |
| | $ | 38,373 | | $ | 20,217 | | $ | 18,855 | | $ | 100,883 | | $ | 1,725,000 | | $ | 1,903,328 |
| | | | | | | | | | | | | | | | | | |
| (1) | Our Convertible Notes can first be put to us on December 13, 2013 and as such, we have assumed they will be paid off in fiscal 2014. |
Credit Agreement.On October 22, 2007, we entered into a $2.55 billion senior secured credit agreement (the “Credit Agreement”) with Goldman Sachs Credit Partners L.P. and certain other lenders (collectively, the “Lenders”). As of the closing of the merger with Cytyc we borrowed $2.35 billion under the credit facilities, all of which had variable interest rates. We applied the net proceeds from our convertible note offering described below to repay amounts outstanding under the Credit Agreement. During the year ended September 27, 2008, we also made voluntary prepayments of principal to fully repay the terms loans under the Credit Agreement.
On July 17, 2008, in connection with our acquisition of Third Wave, we entered into an amended and restated credit agreement with certain of the Lenders (the “Amended Credit Agreement”). The Amended Credit Agreement amended and restated our existing Credit Agreement with Goldman Sachs Credit Partners L.P. and the lenders named therein, dated as of October 22, 2007.
In order to consummate the purchase of all issued and outstanding stock of Third Wave, we borrowed $540.0 million of term loans under the credit facilities on July 17, 2008 of which $400.0 million was designated Term Loan A and $140.0 million designated Term Loan B. In addition, we have a $200.0 million revolving credit facility under the Amended Credit Agreement (the “Revolving Facility”). Our obligations under the
65
Amended Credit Agreement are secured by substantially all of our assets. As of September 26, 2009, we had an aggregate of $174.2 million of principal outstanding under the Amended Credit Agreement, consisting of $124.7 million under Term Loan A and $49.5 million under Term Loan B. Subsequent to September 26, 2009, we paid an additional $24.6 million of principal of which $21.0 million was a voluntary payment. This voluntary payment of $21.0 million has been reclassified to current portion of long term debt on our Consolidated Balance Sheet at September 26, 2009. As of that date we had no amounts outstanding under the Revolving Facility, and therefore, had full availability. The final maturity dates for the credit facility are September 30, 2012 for the Term Loan A and Revolving Facility and March 31, 2013 for the Term Loan B.
All amounts outstanding under the Amended Credit Agreement bear interest, at Hologic’s option, as follows:
| | With | respect to loans made under the revolving facility and the Term Loan A facility: |
| | (i) | at the Base Rate plus 1.25% per annum, which was reduced from 1.50% in May 2009; or |
| | (ii) | at the reserve adjusted Eurodollar Rate plus 2.25% per annum, which was reduced from 2.50% in May 2009; and |
| | With | respect to loans made under the Term Loan B facility: |
| | (i) | at the Base Rate plus 2.25% per annum; or |
| | (ii) | at the reserve adjusted Eurodollar Rate plus 3.25% per annum. |
The credit facilities contain affirmative and negative covenants customarily applicable to senior secured credit facilities, including covenants restricting the ability of the Hologic loan parties, subject to negotiated exceptions, to: incur additional indebtedness and additional liens on their assets; engage in mergers or acquisitions or dispose of assets, enter into sale-leaseback transactions, pay dividends or make other distributions, voluntarily prepay other indebtedness, enter into transactions with affiliated persons, make investments, and change the nature of their businesses. The credit facilities require the Hologic loan parties to maintain certain maximum leverage and minimum interest coverage ratios as of the last day of each fiscal quarter, as defined in the Amended Credit Agreement. We were in compliance with the financial covenants as of September 26, 2009.
Convertible Notes.On December 10, 2007, we issued and sold $1.725 billion, at par, of our 2.00% Convertible Senior Notes due 2037. The net proceeds from the offering was approximately $1.69 billion, after deducting the underwriters’ discounts and estimated offering expenses of approximately $1.5 million payable by us, and was used to repay a portion of our then outstanding senior secured indebtedness under our Credit Agreement.
Holders may require us to repurchase the notes on December 13 of 2013, and on each of December 15, 2017, 2022, 2027 and 2032 at a repurchase price equal to 100% of their accreted principal amount, plus accrued and unpaid interest. We may redeem any of the notes beginning December 18, 2013, by giving holders at least 30 days’ notice. We may redeem the notes either in whole or in part at a redemption price equal to 100% of their principal amount, plus accrued and unpaid interest, including contingent interest and liquidated damages, if any, to, but excluding, the redemption date.
The notes bear interest at a rate of 2.00% per year on the principal amount, payable semi-annually in arrears in cash on June 15 and December 15 of each year, beginning June 15, 2008, and ending on December 15, 2013 and will accrete principal from December 15, 2013 at a rate that provides holders with an aggregate annual yield to maturity of 2.00% per year. Beginning with the six month interest period commencing December 15, 2013, we will pay contingent interest during any six month interest period to the holders of notes if the “trading price”, as defined, of the notes for each of the five trading days ending on the second trading day immediately preceding the first day of the applicable six month interest period equals or exceeds 120% of the accreted principal amount
66
of the notes. The holders of the notes may convert the notes into shares of our common stock at a conversion price of approximately $38.60 per share, subject to adjustment, prior to the close of business on September 15, 2037, subject to prior redemption or repurchase of the notes, under any of the following circumstances: (1) during any calendar quarter after the calendar quarter ending December 31, 2007 if the last reported sale price of our common stock exceeds 130% of the conversion price for at least 20 trading days in the 30 consecutive trading days ending on the last trading day of the preceding calendar quarter; (2) during the five business day period after any five consecutive trading day period in which the trading price per note for each day of such period was less than 98% of the product of the last reported sale price of our common stock and the conversion rate on each such day; (3) if the notes have been called for redemption; or (4) upon the occurrence of specified corporate events.
In lieu of delivery of shares of our common stock in satisfaction of our obligation upon conversion of the notes, we may elect to deliver cash or a combination of cash and shares of our common stock. If we elect to satisfy our conversion obligation solely in cash, we will deliver cash in an amount as provided in the indenture for the notes. If we elect to satisfy our conversion obligation in a combination of cash and shares of our common stock, we will deliver up to a specified dollar amount of cash per $1,000 original principal amount of notes, and will settle the remainder of our conversion obligation in shares of our common stock, in each case based on the daily conversion value calculated as provided in the indenture for the notes. In addition, at any time on or prior to the 35th scheduled trading day prior to the maturity date of the notes, we may make an irrevocable election to settle conversions of the notes either solely in cash or in a combination of cash and shares of our common stock with a specified cash amount at least equal to the accreted principal amount of the notes. This net share settlement election is in our sole discretion and does not require the consent of holders of the notes. It is our current intent and policy to settle any conversion of the notes as if we had elected to make the net share settlement election.
The notes are our senior unsecured obligations and rank equally with all of our existing and future senior unsecured debt and prior to all future subordinated debt. The notes are effectively subordinated to any future secured indebtedness to the extent of the collateral securing such indebtedness, and structurally subordinated to all indebtedness and other liabilities (including trade payables) of our subsidiaries.
Contingent Earn-Out Payments.As a result of the merger with Cytyc, we assumed the obligation to the former Adiana stockholders to make contingent earn-out payments tied to the achievement of milestones. The milestone payments include potential contingent payments of up to $155 million based on worldwide sales of the Adiana System in the first year following FDA approval and on annual incremental sales growth thereafter through December 31, 2012. We received FDA approval of the Adiana System on July 6, 2009, and the Company began accruing contingent consideration in the fourth quarter of fiscal 2009 based on the defined percentage of worldwide sales of the product. The total accrued contingent consideration net at September 26, 2009 is $1.5 million. These amounts are being recorded as additional purchase price, and under the terms of the agreement the first payment is not due to the Adiana shareholders until October 2010. The agreement includes an indemnification provision that provides for the reimbursement of qualifying legal expenses in defense of the Adiana intellectual property, and we have the right to offset contingent consideration payments to the Adiana shareholders with these qualifying legal costs. Legal costs have not been material to date.
Since it is not possible to estimate the amount, and the timing of such amounts, of contingent consideration we will pay in the future as it is based on future revenues, the maximum amount or any other amount of contingent consideration payments has not been included in the contractual obligations table below.
67
Contractual Obligations.The following table summarizes our contractual obligations and commitments as of September 26, 2009:
| | | | | | | | | | | | | | | |
| | | Payments Due by Period |
Contractual Obligations | | Less than
1 year | | 1-3 years | | 3-5 years | | More than
5 years | | Total |
Long-Term Debt Obligations (1) | | $ | 38,373 | | $ | 39,072 | | $ | 1,825,883 | | $ | — | | $ | 1,903,328 |
Interest on Long-Term Debt Obligations | | | 39,017 | | | 75,862 | | | 52,567 | | | — | | | 167,446 |
Operating Leases | | | 17,823 | | | 30,538 | | | 22,426 | | | 45,326 | | | 116,113 |
Purchase Obligations | | | 27,940 | | | 11,631 | | | 2,059 | | | 554 | | | 42,184 |
Financing Leases (2) | | | 2,523 | | | 5,148 | | | 5,585 | | | 12,282 | | | 25,538 |
Long-Term Supply Contracts (3) | | | 3,035 | | | 6,000 | | | — | | | — | | | 9,035 |
Pension Obligations (4) | | | 332 | | | 727 | | | 815 | | | 4,862 | | | 6,736 |
Private Equity Investment (5) | | | 1,324 | | | — | | | — | | | — | | | 1,324 |
| | | | | | | | | | | | | | | |
Total Contractual Obligations | | $ | 130,367 | | $ | 168,978 | | $ | 1,909,335 | | $ | 63,024 | | $ | 2,271,704 |
| | | | | | | | | | | | | | | |
| (1) | Our Convertible Notes can first be put to us on December 13, 2013 and we have assumed for purpose of the above table that they will be paid off in fiscal 2014. |
| (2) | We acquired the financing leases in connection with our acquisition of Cytyc in fiscal 2008. Cytyc had executed two leases for an office building and for a manufacturing facility, which were required to be recorded on our balance sheet under US GAAP. See Note 12 in our Consolidated Financial Statements contained in Item 15 of this Annual Report for more information. |
| (3) | This represents certain non-cancelable supply contracts. For reasons of quality assurance, sole source availability or cost effectiveness, certain key components and raw materials are available only from a sole supplier. To assure continuity of supply while maintaining high quality and reliability, long-term supply contracts have been executed with these suppliers. In certain of these contracts, a minimum purchase commitment has been established. |
| (4) | Pension obligations do not include our obligation under the Supplemental Executive Retirement Plan, which is recorded as a current liability of $11.2 million. These benefits are generally paid out at retirement or termination of employment. |
| (5) | This represents a private equity investment commitment with a limited liability partnership, which could be paid over the succeeding two years. |
The above table does not reflect our long-term liabilities associated with uncertain tax positions recorded under FIN 48 (codified primarily in ASC 740,Income Taxes) totaling $14.7 million. Due to the complexity associated with tax uncertainties, we cannot reasonably make a reliable estimate of the period in which we expect to settle these non-current liabilities. See Note 8 in our Consolidated Financial Statements contained in Item 15 of this Annual Report for more information on our unrecognized tax benefits. In addition, certain of our cost method equity investments give us the option to acquire the company in the future. Since it is not possible to estimate when, or even if, we will exercise our option to acquire these companies, we have not included these future potential payments in the table above.
We expect to continue to review and evaluate potential acquisitions of businesses, products or technologies, and strategic alliances that we believe will complement our current or future business. Subject to the Risk Factors set forth in Part I, Item 1A of this report and the general disclaimers set forth in our Special Note Regarding Forward-Looking Statements at the outset of this Report, we believe that cash flow from operations and cash available from our Amended Credit Agreement will provide us with sufficient funds in order to fund our expected operations over the next twelve months. Our longer-term liquidity is contingent upon future operating performance and our ability to continue to meet financial covenants under our Amended Credit Agreement. We
68
may also require additional capital in the future to fund capital expenditures, acquisitions or other investments, or to repay our convertible notes. The holders of the Convertible Notes may require us to repurchase the notes on December 13 of 2013, and on each of December 15, 2017, 2022, 2027 and 2032 at a repurchase price equal to 100% of their accreted principal amount. These capital requirements could be substantial. Our operating performance may also be affected by matters discussed under the above-referenced Risk Factors as elsewhere in this report. These risks, trends and uncertainties may also adversely affect our long-term liquidity.
Legal Contingencies
We are currently involved in certain legal proceedings and claims. In connection with these legal proceedings and claims, management periodically reviews estimates of potential costs to be incurred by us in connection with the adjudication or settlement, if any, of these proceedings. These estimates are developed in consultation with outside counsel and are based on an analysis of potential litigation outcomes and settlement strategies. In accordance with ASC 450,Contingencies(formerly SFAS No. 5,Accounting for Contingencies), loss contingencies are accrued if, in the opinion of management, an adverse outcome is probable and such outcome can be reasonably estimated. It is possible that future results for any particular quarter or annual period may be materially affected by changes in our assumptions or the effectiveness of our strategies relating to these proceedings.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to revenue recognition for multiple element arrangements, allowance for doubtful accounts, reserves for excess and obsolete inventories, valuations and purchase price allocations related to business combinations, expected future cash flows including growth rates, discount rates, terminal values and other assumptions and estimates used to evaluate the recoverability of long-lived assets and goodwill, estimated fair values of intangible assets and goodwill, amortization methods and periods, warranty reserves, certain accrued expenses, restructuring and other related charges, stock-based compensation, contingent liabilities, tax reserves and recoverability of our net deferred tax assets and related valuation allowance. We base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances. Actual results could differ from these estimates if past experience or other assumptions do not turn out to be substantially accurate. Any differences may have a material impact on our financial condition and results of operations.
The following is a discussion of what we believe to be the more significant critical accounting policies and estimates used in the preparation of our consolidated financial statements.
Inventory
Our inventories include material, labor and overhead, and are stated at the lower of cost (first-in, first-out) or market. As a designer and manufacturer of high technology medical equipment, we may be exposed to a number of economic and industry factors that could result in portions of our inventory becoming either obsolete or in excess of anticipated usage. These factors include, but are not limited to, technological changes in our markets, our ability to meet changing customer requirements, competitive pressures on products and prices, reliability and replacement of and the availability of key components from our suppliers. Our policy is to establish inventory reserves when conditions exist that suggest that our inventory may be in excess of anticipated demand or is obsolete based upon our assumptions about future demand for our products and market conditions. We regularly evaluate our ability to realize the value of our inventory based on a combination of factors including the following: historical usage rates, forecasted sales or usage, product end of life dates, estimated current and future
69
market values and new product introductions. Assumptions used in determining our estimates of future product demand may prove to be incorrect, in which case the provision required for excess and obsolete inventory would have to be adjusted in the future. If inventory is determined to be overvalued, we would be required to recognize such costs as cost of goods sold at the time of such determination. Although every effort is made to ensure the accuracy of our forecasts of future product demand, any significant unanticipated changes in demand could have a significant negative impact on the value of our inventory and our reported operating results. Additionally, purchasing requirements and alternative usage avenues are explored within these processes to mitigate inventory exposure. When recorded, our reserves are intended to reduce the carrying value of our inventory to its net realizable value.
Provisions for excess or obsolete inventory are primarily based on our estimates of forecasted net sales and service usage levels. A significant change in the timing or level of demand for our products as compared to forecasted amounts may result in recording additional provisions for excess or expired inventory in the future. We record provisions for excess or obsolete inventory as cost of sales.
Accounts Receivable Reserves
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We regularly evaluate the collectibility of our trade receivables based on a combination of factors, including a dialogue with the customer to determine the cause of non-payment, and evaluation of the customer’s current financial situation. In the event it is determined that the customer may not be able to meet its full obligation to us, we record a specific allowance to reduce the receivable to the amount that we expect to recover given all information present. We perform ongoing credit evaluations of our customers and adjust credit limits based upon payment history and our assessment of the customer’s current credit worthiness. We continuously monitor collections from our customers and maintain a provision for estimated credit losses based upon our historical experience and any specific customer collection issues that we have identified. While such credit losses have historically been within our expectations and the provisions established, we cannot guarantee that we will continue to experience the same credit loss rates in the future. If the financial condition of our customers were to deteriorate, for example as a result of the recent financial and economic turmoil or otherwise, resulting in an impairment of their ability to make payments, additional allowances may be required.
We also record a provision for estimated sales returns and allowances on product and service related sales in the same period as the related revenues are recorded. These estimates are based on the specific facts and circumstances of particular orders, analysis of credit memo data and other known factors. If the data we use to calculate these estimates do not properly reflect reserve requirements, then a change in the allowances would be made in the period in which such a determination is made and revenues in that period could be adversely affected.
Valuation of Business Combinations
We record tangible and intangible assets acquired and liabilities assumed in business combinations under the purchase method of accounting. Amounts paid for each acquisition are allocated to the assets acquired and liabilities assumed based on their fair values at the dates of acquisition. The fair value of identifiable intangible assets is based on detailed valuations that use information and assumptions provided by management. We allocate any excess purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed to goodwill. The valuation of purchased research and development represents the estimated fair value at the date of acquisition related to in-process projects. Our purchased research and development represents the value of in-process projects that have not yet reached technological feasibility and have no alternative future uses as of the date of acquisition. We expense the value attributable to these in-process projects at the time of the acquisition. If the projects are not successful or completed in a timely manner, we may not realize the financial benefits expected for these projects or for the acquisitions as a whole.
70
We use the income approach to determine the fair values of our purchased research and development. This approach determines fair value by estimating the after-tax cash flows attributable to an in-process project over its useful life and then discounting these after-tax cash flows back to a present value. We base our revenue assumptions on estimates of relevant market sizes, expected market growth rates, expected trends in technology and expected product introductions by competitors. In arriving at the value of the in-process projects, we consider, among other factors, the in-process projects’ stage of completion, the complexity of the work completed as of the acquisition date, the costs already incurred, the projected costs to complete, the contribution of core technologies and other acquired assets, the expected introduction date and the estimated useful life of the technology. We base the discount rate used to arrive at a present value as of the date of acquisition on the time value of money and medical technology investment risk factors. For the in-process projects we acquired in connection with our fiscal 2008 acquisitions, we used risk-adjusted discount rates to discount our projected cash flows, ranging from 12.5% to 20%. We believe that the estimated purchased research and development amounts so determined represent the fair value at the date of acquisition and do not exceed the amount a third party would pay for the projects.
We have also used the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including developed technology, customer relationships and trade names. Developed technology represents patented and unpatented technology and know-how. Customer relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Tradenames represent acquired product names that we intend to continue to utilize.
Intangible Assets and Goodwill
Intangible Assets
We amortize our intangible assets that have finite lives using either the straight-line method or, if reliably determinable, based on the pattern in which the economic benefit of the asset is expected to be consumed utilizing expected undiscounted future cash flows. Amortization is recorded over the estimated useful lives ranging from 2 to 30 years. We review our intangible assets subject to amortization to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment or a change in the remaining useful life. If the carrying value of an asset exceeds its undiscounted cash flows, we will write-down the carrying value of the intangible asset to its fair value in the period identified. In assessing recoverability, we must make assumptions regarding estimated future cash flows and discount rates. If these estimates or related assumptions change in the future, we may be required to record impairment charges. We generally calculate fair value as the present value of estimated future cash flows to be generated by the asset using a risk-adjusted discount rate. If the estimate of an intangible asset’s remaining useful life is changed, we will amortize the remaining carrying value of the intangible asset prospectively over the revised remaining useful life.
Goodwill
In accordance with ASC 350,Intangibles—Goodwill and Other (formerly FASB Statement of Financial Accounting Standard No. 142 (“SFAS 142”),Goodwill and Other Intangible Assets), we test goodwill at the reporting unit level for impairment on an annual basis and between annual tests if events and circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business, and an adverse action or assessment by a regulator. Our annual impairment test date is the first day of our fiscal fourth quarter.
In performing the test, we utilize the two-step approach prescribed under ASC 350. The first step requires a comparison of the carrying value of the reporting units, as defined, to the fair value of these units. We consider a number of factors to determine the fair value of a reporting unit, including an independent valuation to conduct
71
this test. The valuation is based upon expected future discounted operating cash flows of the reporting unit as well as analysis of recent sales or offerings of similar companies. We base the discount rate used to arrive at a present value as the date of the impairment test on our weighted average cost of capital. If the carrying value of a reporting unit exceeds its fair value, we will perform the second step of the goodwill impairment test to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of a reporting unit’s goodwill to its carrying value.
During the first quarter of fiscal 2009, based upon a combination of factors, including the deteriorating macro-economic environment, declines in the stock market and the decline of our market capitalization significantly below the book value of our net assets, we concluded that potential goodwill impairment indicators existed as of December 27, 2008. As a result, we performed an interim goodwill impairment analysis as of December 27, 2008. Step 1 of the impairment analysis indicated that the carrying value of the net assets of certain reporting units, acquired in connection with the Cytyc acquisition, exceeded the estimated fair value of those reporting units. As a result, we were required to perform Step 2 of the goodwill impairment test to determine the amount, if any, of goodwill impairment charges for each of the applicable reporting units. The Step 2 analysis required us to perform a hypothetical purchase price allocation for each of these reporting units to determine the implied fair value of goodwill and to compare the implied fair value of goodwill to the recorded amount of goodwill by reporting unit. Due to the complexities and time involved in preparing the Step 1 analysis, we had not commenced the Step 2 analysis as of February 5, 2009, the date we filed our Form 10-Q for the quarter ended December 27, 2008. As a result of the fact that we had not commenced the Step 2 analysis and the complexity of the analysis required to complete the Step 2 analysis, we were unable to determine that an impairment loss, in accordance with ASC 450 (formerly SFAS No. 5,Accounting for Contingencies), was both probable and reasonably estimable at December 27, 2008. We completed the Step 2 analysis during our second quarter of fiscal 2009, which resulted in an aggregate goodwill impairment charge of $2.34 billion. This impairment charge is comprised of $1.17 billion for GYN Surgical, $908.3 million for Diagnostics, and $265.9 million for Breast Health related to our MammoSite reporting unit acquired from Cytyc. We believe that our procedures and related assumptions for estimating the reporting units’ fair value are reasonable and consistent with the market conditions that existed at the time of the impairment test.
For illustrative purposes, had the fair values of each reporting unit for which we recorded goodwill impairment charges in the second quarter of fiscal 2009 been lower by 10% as of December 27, 2008, we would have recorded an additional impairment charge of $435.5 million. Based on the Company’s estimates as of December 27, 2008, the impact of reducing our fair value estimates for our other reporting units, for which we did not record any goodwill impairment charges, by 10% would have had no impact on the our goodwill assessment for those reporting units.
We conducted our annual impairment test as of the first day of the fourth quarter of fiscal 2009. In order to complete the annual impairment test, we updated our interim impairment test results and performed detailed analyses estimating the fair value of most of our reporting units utilizing our fiscal 2010 forecast with updated long-term growth assumptions. For one reporting unit, we utilized the results of our interim impairment test. Pursuant to ASC 350-20-35-29 (formerly paragraph 27 of SFAS 142), we concluded that it met the required criteria to use the estimated fair value determined from its interim impairment analysis for this reporting unit because 1) the composition of the assets and liabilities of this reporting unit had not changed significantly since the most recent fair value determination, 2) the most recent fair value determination resulted in a fair value that exceeded the carrying value of the reporting unit by a substantial margin, and 3) management concluded, based on an analysis of current events that had occurred and circumstances that had changed since the most recent fair value determination, that it is remote that the current fair value of the reporting unit would not exceed their carrying amounts.
As a result of completing Step 1, all of our reporting units, except one, had a fair value exceeding their carrying value, and as such Step 2 of the impairment test was not required for these reporting units. For the reporting unit that failed Step 1, we completed Step 2 and determined that an impairment charge was not required
72
due to the fair value of the implied goodwill exceeding the carrying value of the reporting unit’s goodwill. For illustrative purposes, had the fair value of this reporting unit at June 28, 2009 been lower by 10%, the Company still would not have recorded any impairment charge. If the fair value of our other reporting units had been lower by 10%, two reporting units would have failed Step 1 requiring a Step 2 analysis. These reporting units, one in the Diagnostics reportable segment and one in the Skeletal Health reportable segment, had fair values at this date that exceeded their carrying values by 9% and 2%, respectively, and goodwill of $236.0 million and $8.2 million, respectively. The fair value of these reporting units is determined by use of a discounted cash flow analysis (“DCF”) under the income approach. The key assumptions that drive the fair value in this model are the discount rates (i.e., weighted average cost of capital, “WACC”), terminal values, growth rates, and the amount and timing of expected future cash flows. If the current worldwide financial markets and economic environment were to deteriorate, this would likely result in a higher WACC because market participants would require a higher rate of return. In the DCF as the WACC increases, the fair value decreases. The other significant factor in the DCF is our projected financial information (i.e., amount and timing of expected future cash flows and growth rates) and if our assumptions were to be adversely impacted, this could result in a reduction of the fair values of these reporting units. For our other reporting units with goodwill aggregating $1.77 billion, we believe that these reporting units are not at risk of failing Step 1 of the goodwill impairment test.
The estimate of fair value requires significant judgment. Any loss resulting from an impairment test would be reflected in operating income (loss) in our Consolidated Statements of Operations. The annual impairment testing process is subjective and requires judgment at many points throughout the analysis. If these estimates or their related assumptions change in the future, we may be required to record impairment charges for these assets not previously recorded.
Revenue Recognition
We recognize product revenue upon shipment provided that there is persuasive evidence of an arrangement, there are no uncertainties regarding acceptance, the sales price is fixed or determinable, no rights of return exist and collection of the resulting receivable is reasonably assured. Generally, our product arrangements are multiple element arrangements, including services such as installation and training. We account for these arrangements in accordance with ASC 605-25,Multiple Element Arrangements(formerly Emerging Issues Task Force (“EITF”) No. 00-21,Accounting for Revenue Arrangements with Multiple Deliverables). Based on the terms and conditions of the product arrangements, we have concluded that these services and undelivered products can be accounted for separately from the delivered product element as our delivered product has value to our customers on a stand-alone basis and we have objective and reliable evidence of the fair value of such services and undelivered products. Accordingly, service revenue representing the fair value of services not yet performed at the time of product shipment is deferred and recognized as such services are performed. The fair value of the undelivered products is also deferred at the time of product shipment and recognized when these products are delivered. The residual revenue under the product arrangement will be recognized as product revenue upon shipment. There are no customer rights of return in our sales agreements.
We recognize product revenue upon the completion of installation for products whose installation is essential to its functionality, primarily related to our digital imaging systems. A provision is made at that time for estimated warranty costs to be incurred.
Service revenues primarily consist of amounts recorded under service and maintenance contracts and repairs not covered under warranty, installation and training revenues and shipping and handling costs billed to customers. Service and maintenance contract revenues are recognized ratably over the term of the contract. Other service revenues are recorded when the services are delivered.
Although certain of our products contain operating and application software, we have determined that except for our CAD (computer aided detection) products obtained with the acquisition of R2 Technology, Inc. and the newly released Dimensions 2D/3D full field digital mammography product (“Dimensions”), the software element is incidental in accordance with the software revenue recognition rules.
73
We have determined that the provisions of ASC 985-605,Software—Revenue Recognition(formerly AICPA Statement of Position No. 97-2,Software Revenue Recognition)apply to revenue transactions for our CAD systems and the Dimensions product. ASC 985-605 generally requires revenue earned on software arrangements involving multiple elements to be allocated to each element based on the relative fair values of the elements. Revenue recognized from multi-element arrangements is allocated to each element of the arrangement using the residual method based on the fair value of the undelivered elements. Our determination of fair value of the undelivered elements in multi-element arrangements is based on vendor-specific objective evidence (“VSOE”). We limit our assessment of VSOE for each element to either the price charged when the same element is sold separately or the price established by management, having the relevant authority to do so, for an element not yet sold separately. We recognize revenue on CAD systems and Dimensions product sales upon completion of installation, at which time the only remaining undelivered element is Post Contract Support (“PCS”).
Upon its release, we completed an evaluation of the software component of our Dimensions product in accordance with the software revenue recognition rules. We noted the following in our evaluation of the software component of our new Dimensions product:
| | • | | Dimensions is offered in different configurations offering different levels of functionality (2D vs. 3D). Customers who purchase the 2D configuration will be to able to upgrade the product to a 3D version and such upgrade will solely represent a software upgrade that will be marketed and sold separately. This differentiation from our existing 2D digital mammography product is expected to be highlighted in our marketing literature. |
| | • | | As part of the initial warranty of the Dimensions product, customers will receive not only bug fixes related to the software but will also receive any updates and enhancements to the software that are released. Therefore, we concluded that this represents PCS as defined in ASC 985-605. |
As a result, we have determined that the Dimensions product contains software that is more than incidental to the product as a whole and thus, will be accounted for under ASC 985-605. Therefore, we recognize revenue upon installation and acceptance, if required, and defer revenue based on the VSOE of fair value of the initial bundled PCS. We have determined that VSOE of fair value of the initial bundled PCS exists based on the establishment of the price for which this element will be sold separately by management having the relevant authority and that it is probable that this price will not change prior to when this service is sold separately. We have specified the renewal rates at which service can be purchased separately upon expiration of the initial PCS period and those rates have been consistent.
For multi-element arrangements where VSOE of fair value of PCS has been established, we recognize revenue using the residual method at the time all other revenue recognition criteria have been met. Amounts attributable to post contract support are recorded as deferred revenue and recognized ratably over the contractual term of the support.
Under customer usage agreements, we install certain equipment (for example, a ThinPrep Processor or a ThinPrep Imaging System) at customer sites and customers commit to purchasing minimum quantities of disposable supplies at a stated price (generally including a usage fee for the equipment) over a defined contract term, which is typically between three and five years. Revenue is recognized over the term of the customer usage agreement as disposable supplies are delivered. We also rent certain equipment to customers. Revenues from rental agreements are recorded over the terms of the rental agreements.
Product Warranties
Products sold are generally covered by a warranty for a period of one year. We accrue a warranty reserve at the time of revenue recognition for estimated costs to provide warranty services. Our estimate of costs to service our warranty obligations is based on historical experience and expectation of future conditions. To the extent we experience increased or decreased warranty claim activity or increased or decreased costs associated with
74
servicing those claims, our warranty accrual will increase or decrease, respectively, resulting in decreased or increased gross profit. Our warranty accrual was approximately $5.6 million, $9.1 million and $12.1 million in fiscal 2009, 2008 and 2007, respectively. The decrease in the warranty accrual in both fiscal 2009 and fiscal 2008 is primarily attributable to a decrease in warranty claim activity primarily related to our digital mammography systems.
Stock-Based Compensation
The adoption of SFAS No. 123(R),Share-Based Payment (codified in ASC 718,Stock Compensation) in the first quarter of fiscal 2006 required that stock-based compensation expense associated with stock options and related awards be recognized in the statement of income, rather than being disclosed in a pro forma footnote to the consolidated financial statements. Determining the amount of stock-based compensation to be recorded requires us to develop estimates to be used in calculating the grant-date fair value of stock options. Prior to the adoption of SFAS 123(R), we determined the fair value of our stock options using the Black-Scholes Option Pricing Model. In connection with the adoption of SFAS 123(R), we elected to use a binomial lattice model to determine the fair value of our stock options. We consider a number of factors to determine the fair value of stock options including the advice of an outside valuation advisor and the advisor’s model. The model requires us to make estimates of the following assumptions:
Expected volatility—We are responsible for estimating volatility and have considered a number of factors, including third-party estimates, when estimating volatility. We currently use a combination of historical and implied volatility, which is weighted based on a number of factors.
Expected term—We use historical employee exercise and option expiration data to estimate the expected term assumption. We believe that this historical data is currently the best estimate of the expected term of a new option, and that generally, all of our employees exhibit similar exercise behavior.
Risk-free interest rate—The yield on zero-coupon U.S. Treasury securities for a period that is commensurate with the expected term assumption is used as the risk-free interest rate.
The amount of stock-based compensation expense recognized during a period is based on the value of the portion of the awards that are ultimately expected to vest. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the unvested portion of the surrendered option. Based on an analysis of historical forfeitures, the Company has determined a specific forfeiture rate for certain employee groups and has applied forfeiture rates ranging from 0% to 6% as of September 26, 2009 depending on the specific employee group. This analysis is re-evaluated quarterly and the forfeiture rate is adjusted as necessary. Ultimately, the actual expense recognized over the vesting period will only be for those awards that vest.
We recognized $32.9 million, $25.7 million and $6.1 million of stock-based compensation expense for employee equity awards in fiscal years 2009, 2008 and 2007, respectively. As of September 26, 2009, there was $32.0 million of unrecognized compensation expense related to stock option awards that we expect to recognize over a weighted-average period of 3.5 years. As of September 26, 2009, there was $33.0 million of unrecognized compensation expense related to restricted stock units that we expect to recognize over a weighted average period of 2.3 years.
Income Taxes
We use the asset and liability method for accounting for income taxes. Under this method we determine deferred tax assets and liabilities based on the difference between financial reporting and taxes bases of our assets and liabilities. We measure deferred tax assets and liabilities using enacted tax rates and laws that will be in effect when we expect the differences to reverse.
75
We recognized net deferred tax liabilities of $860.8 million at September 26, 2009 and $867.2 million at September 27, 2008. The liabilities primarily relate to deferred taxes associated with our acquisitions and the original issuance discount on our Convertible Notes. The tax assets relate primarily net operating loss carryforwards, accruals and reserves, stock-based compensation and research credits. We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we have considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of the net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax asset in the future, an adjustment to the deferred tax asset would be charged to income in the period such determination was made.
On September 30, 2007, we adopted Financial Accounting Standards Board (“FASB”) Interpretation (“FIN”) No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109(codified primarily in ASC 740,Income Taxes), which clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements. FIN No. 48 prescribes a recognition threshold and measurement criteria for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN No. 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition and defines the criteria that must be met for the benefits of a tax position to be recognized. As a result of our adoption of FIN No. 48, we recorded the cumulative effect of the change in accounting principle of $0.5 million as a decrease to opening retained earnings.
We had gross unrecognized tax benefits, including interest, of approximately $29.2 million as of September 26, 2009 and $20.2 million as of September 27, 2008. At September 26, 2009, $29.2 million represents the amount of unrecognized tax benefits that, if recognized, would result in a reduction of the Company’s effective tax rate. In the next twelve months it is reasonably possible that we will reduce our unrecognized tax benefits by $2.2 million due to expiration of statute of limitations and settlements with taxing authorities, which will reduce the Company’s effective tax rate.
In the ordinary course of global business, there are many transactions and calculations where the ultimate tax outcome is uncertain. Judgment is required in determining our worldwide income tax provision. In our opinion, we have made adequate provisions for income taxes for all years subject to audit. Although we believe our estimates are reasonable, no assurance can be given that the final tax outcome of these matters will not be different than that which is reflected in our historical income tax provisions and accruals. In the event our assumptions are incorrect, the differences could have a material impact on our income tax provision and operating results in the period in which such determination is made.
Recent Accounting Pronouncements
In September 2009, the FASB ratified ASC Update No. 2009-13,Multiple-Deliverable Revenue Arrangements, or ASU 2009-13. ASU 2009-13, amends existing revenue recognition accounting pronouncements that are currently within the scope of FASB Accounting Standards Codification, or ASC, Subtopic 605-25 (previously included within EITF Issue No. 00-21, Revenue Arrangements with Multiple Deliverables, or EITF 00-21). This consensus provides for two significant changes to the existing multiple element revenue recognition guidance. First, this guidance deletes the requirement to have objective and reliable evidence of fair value for undelivered elements in an arrangement and will result in more deliverables being treated as separate units of accounting. The second change modifies the manner in which the transaction consideration is allocated across the separately identified deliverables. These changes may result in entities recognizing more revenue up-front, and entities will no longer be able to apply the residual method and defer the fair value of undelivered elements. Upon adoption of these new rules, each separate unit of accounting must have a selling price, which can be based on management’s estimate when there is no other means to determine the fair value of that undelivered item, and the arrangement consideration is allocated based on the elements’ relative
76
selling price. This accounting guidance is effective no later than fiscal years beginning on or after June 15, 2010 but may be early adopted as of the first quarter of an entity’s fiscal year. Entities may elect to adopt this accounting guidance either through prospective application to all revenue arrangements entered into or materially modified after the date of adoption or through a retrospective application to all revenue arrangements for all periods presented in the financial statements. We are currently evaluating the impact of this revised accounting guidance, which we can adopt as early as the first quarter of fiscal 2010.
In September 2009, the FASB ratified ASU No. 2009-14,Applicability of SOP 97-2 to Certain Arrangements that Include Software Elements(formerly EITF Issue No. 09-3,Certain Revenue Arrangements that Include Software Elements), which amends the existing accounting guidance for how entities account for arrangements that include both hardware and software, which typically resulted in the sale of hardware being accounted for under the software revenue recognition rules. This accounting guidance changes revenue recognition for tangible products containing software elements and non-software elements. The tangible element of the product is always outside of the scope of the software revenue recognition rules, and the software elements of tangible products when the software element and non-software elements function together to deliver the product’s essential functionality are outside of the scope of the software rules. As a result, both the hardware and qualifying related software elements are excluded from the scope of the software revenue guidance and accounted for under the revised multiple-element revenue recognition guidance. This accounting guidance is effective for all fiscal years beginning on or after June 15, 2010 with early adoption permitted. Entities must adopt ASU 2009-14 and ASU 2009-13 in the same manner and at the same time. We are currently evaluating the impact of this revised accounting guidance, which we can adopt as early as the first quarter of fiscal 2010.
In April 2009, the FASB issued FASB Staff Positions (“FSP”) FAS 115-2 and FAS 124-2,Recognition and Presentation of Other-Than-Temporary Impairment(FSP 115-2/124-2) (codified within ASC 320,Investments—Debt and Equity Securities). FSP 115-2/124-2 amends the requirements for the recognition and measurement of other-than-temporary impairments for debt securities by modifying the pre-existing “intent and ability” indicator. Under this FSP, an other-than-temporary impairment is triggered when there is an intent to sell the security, it is more likely than not that the security will be required to be sold before recovery, or the security is not expected to recover the entire amortized cost basis of the security. Additionally, this FSP changes the presentation of an other-than-temporary impairment in the income statement for those impairments involving credit losses. The credit loss component will be recognized in earnings and the remainder of the impairment will be recorded in other comprehensive income. FSP 115-2/124-2 was effective for us beginning with the third quarter of fiscal 2009. The adoption of this FSP did not have a significant impact on our consolidated financial statements.
In December 2007, the FASB issued ASC 805,Business Combinations(formerly SFAS No. 141 (Revised 2007),Business Combinations(“SFAS 141(R)”). This Statement retains the fundamental requirements in SFAS 141 that the acquisition method of accounting (which SFAS 141 called the purchase method) be used for all business combinations and for an acquirer to be identified for each business combination. ASC 805 requires an acquirer to recognize the assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions specified in the Statement. ASC 805 replaces SFAS 141’s cost-allocation process, which required the cost of an acquisition to be allocated to the individual assets acquired and liabilities assumed based on their estimated fair values. The Statement retains the guidance in SFAS 141 for identifying and recognizing intangible assets separately from goodwill. ASC 805 will now require acquisition costs to be expensed as incurred, and changes in deferred tax asset valuation allowances and income tax uncertainties after the acquisition date generally to affect income tax expense. ASC 805 applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008, which is our 2010 fiscal year. Early adoption is prohibited. We are currently evaluating the impact that the adoption of ASC 805 will have on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements—An amendment of ARB No. 51(“SFAS 160”) (codified within ASC 810,Consolidation). SFAS 160 amends Accounting Research Bulletin (“ARB”) No. 51 to establish accounting and reporting standards for the
77
noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. The amount of net income attributable to the noncontrolling interest will be included in consolidated net income on the face of the income statement. SFAS 160 clarifies that changes in a parent’s ownership interest in a subsidiary that do not result in deconsolidation are equity transactions if the parent retains its controlling financial interest. In addition, this Statement requires that a parent recognize a gain or loss in net income when a subsidiary is deconsolidated. SFAS 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008, which is our 2010 fiscal year. Early adoption is prohibited. We do not expect the adoption of this standard to have an impact on our financial position or results of operations.
In April 2008, the FASB issued FASB FSP No. 142-3,Determination of the Useful Life of Intangible Assets (codified within ASC 350,Intangibles—Goodwill and Other), which amends the factors that must be considered in developing renewal or extension assumptions used to determine the useful life over which to amortize the cost of a recognized intangible asset under ASC 350. The objective of this FSP is to improve the consistency between the useful life of a recognized intangible asset under ASC 350 and the period of expected cash flows used to measure the fair value of the asset under ASC 805. The FSP is effective for financial statements for fiscal years beginning after December 15, 2008, which will be the beginning of fiscal 2010 for us. We are currently evaluating the impact that the adoption of this FSP will have on our consolidated financial statements. Early adoption is prohibited.
In May 2008, the FASB issued FSP No. APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) (codified within ASC 470,Debt).This FSP applies to convertible debt instruments that, by their stated terms, may be settled in cash (or other assets) upon conversion, including partial cash settlement, unless the embedded conversion option is required to be separately accounted for as a derivative under ASC 815,Derivatives and Hedging(formerly SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities). The liability and equity components of convertible debt instruments within the scope of this FSP must be separately accounted for in a manner that will reflect the entity’s nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. The excess of the principal amount of the debt over the amount ultimately allocated to the liability component is required to be amortized to interest expense using the effective interest method. This FSP is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. As a result, we will adopt this standard at the beginning of fiscal 2010. This FSP must be applied retrospectively to all periods presented. The retrospective adoption of this FSP will increase our historical reported interest expense from December 10, 2007 (issuance date of the Convertible Notes—See Note 5) forward.
The adoption of FSP APB 14-1 will have no impact on our actual past or future cash flows. However, upon adoption in fiscal 2010, we will restate prior periods by reclassifying approximately $470,000 of our Convertible Notes to additional paid-in capital, resulting in a debt discount. It is estimated that our non-cash interest expense will increase by approximately $65.5 million and $48.1 for the years ended September 26, 2009 and September 27, 2008, respectively, resulting in a restated diluted net loss per share of approximately $(8.64) and $(1.69) for the years ended September 26, 2009 and September 27, 2008, respectively. We expect to record approximately an additional $71.1 million of non-cash interest expense in fiscal 2010.
In June 2008, the FASB ratified the consensus reached on EITF Issue No. 07-5,Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock(“EITF 07-5”) (codified within ASC 815). This accounting guidance clarifies the determination of whether an instrument (or an embedded feature) is indexed to an entity’s own stock, which would qualify as a scope exception under ASC 815, and it is effective for financial statements issued for fiscal years beginning after December 15, 2008. Early adoption for an existing instrument is not permitted. We have concluded that upon the adoption of this standard, the embedded derivative
78
option in our Convertible Notes (See Note 5) will continue to be considered indexed to our own stock. As a result, the adoption of this standard is not expected to have a material impact on our financial condition or results of operations.
In June 2009, the FASB issued SFAS No. 168,The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles (codified within ASC 105,Generally Accepted Accounting Principles), which establishes the FASB Accounting Standards Codification as the single source of authoritative U.S. GAAP. The Codification will supersede all existing non-SEC accounting and reporting standards. As a result, upon adoption, all references to accounting literature in our SEC filings will conform to the appropriate reference within the Codification. The adoption of this standard did not have any impact on our financial position or results of operations.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. |
Financial Instruments, Other Financial Instruments, and Derivative Commodity Instruments. ASC 825,Financial Instruments(formerly SFAS No. 107,Disclosure of Fair Value of Financial Instruments), requires disclosure about fair value of financial instruments. Financial instruments consist of cash equivalents, accounts receivable, and debt obligations. Except for our outstanding convertible note, the fair value of these financial instruments approximates their carrying amount. As of September 26, 2009 we have $1.725 billion of Convertible Notes outstanding. The fair value of our Convertible Notes was approximately $1.4 billion as of September 26, 2009 based on the trading price as of that date.
Primary Market Risk Exposures. Our primary market risk exposures are in the areas of interest rate risk and foreign currency exchange rate risk. We incur interest expense on borrowings outstanding under our Amended Credit Agreement and to a much lesser extent the debt assumed in our acquisition of AEG, which as of September 26, 2009 is $1.5 million. Borrowings under the Amended Credit Agreement bear interest at a rate per annum equal to, at our option, with respect to the borrowings under the Term Loan A of either (1) the Base Rate (the greater of the prime rate as quoted inThe Wall Street Journal and the Federal Funds Effective Rate) plus 1.25%, which was reduced from 1.50% in May 2009 or (2) the Eurodollar Rate, plus 2.25%, which was reduced from 2.50% in May 2009, and with respect to the Term Loan B of either (1) the Base Rate (the greater of the prime rate as quoted inThe Wall Street Journal and the Federal Funds Effective Rate) plus 2.25% or (2) the Eurodollar Rate, plus 3.25%.
As of September 26, 2009, there was approximately $174.2 million outstanding under the Amended Credit Agreement, including $124.7 million under the Term Loan A facility which matures on September 30, 2012 and $49.5 million under the Term Loan B facility which matures on March 31, 2013. Subsequent to September 26, 2009, we paid an additional $24.6 million of principal resulting in an outstanding balance of $149.6 million at the time of filing this Form 10-K.
These debt obligations are variable rate instruments and our interest expense associated with these instruments is, therefore, subject to changes in market interest rates. A 10% adverse movement (increase in LIBOR) would increase annual interest expense by approximately $1.3 million.
The return from cash and cash equivalents will vary as short-term interest rates change. A hypothetical 10% increase or decrease in interest rates, however, would not have a material adverse effect on our financial condition. Interest income on our cash and cash equivalents is recorded as a component of Other (Expense) Income, net in our accompanying Consolidated Statements of Operations.
Foreign Currency Exchange Risk.Our international business is subject to risks, including, but not limited to: unique economic conditions, changes in political climate, differing tax structures, other regulations and restrictions, and foreign exchange rate volatility. Accordingly, our future results could be materially adversely impacted by changes in these or other factors.
79
We maintain sales and service offices outside the United States, have manufacturing facilities in Germany and Costa Rica and conduct business worldwide. The expenses of our international offices are denominated in local currencies, except at our Costa Rica subsidiary, where the majority of the business is conducted in U.S. dollars. Our foreign sales are denominated in local currencies, the Euro or U.S. dollars. Fluctuations in the foreign currency rates could affect our sales, cost of goods and operating margins and could result in exchange losses. In addition, currency devaluations can result in a loss if we hold deposits of that currency.
We believe that the operating expenses of our international subsidiaries that are incurred in local currencies will not have a material adverse effect on our business, results of operations or financial condition. Our operating results and certain assets and liabilities that are denominated in the Euro are affected by changes in the relative strength of the U.S. dollar against the Euro. Our expenses denominated in Euros are positively affected when the United States dollar strengthens against the Euro and adversely affected when the United States dollar weakens. However, we believe that the foreign currency exchange risk is not significant. A hypothetical 10% increase or decrease in foreign currencies that we transact in would not have a material adverse effect on our financial condition or results of operations. During fiscal 2009, 2008 and 2007, we incurred foreign exchange losses of $2.3 million, $0.7 million and $0.4 million, respectively.
| Item 8. | Financial Statements and Supplementary Data. |
Our Consolidated Financial Statements and Supplementary Data are listed under Part IV, Item 15, in this report.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
| Item 9A. | Controls and Procedures. |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Securities Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, as ours are designed to do, and management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of September 26, 2009, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective.
Report of Management on Internal Control over Financial Reporting
We are responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of our principal executive and
80
principal financial officers and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| | • | | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and disposition of our assets; |
| | • | | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and |
| | • | | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
We have assessed the effectiveness of our internal control over financial reporting as of September 26, 2009. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework.
Based on our assessment, we believe that, as of September 26, 2009, our internal control over financial reporting is effective at a reasonable assurance level based on these criteria.
Ernst & Young LLP, an independent registered public accounting firm, has issued an attestation report on the effectiveness of our internal control over financial reporting. This report in which they expressed an unqualified opinion is included below.
81
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Hologic, Inc.:
We have audited Hologic Inc.’s (the “Company”) internal control over financial reporting as of September 26, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Report of Management on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Hologic, Inc. maintained, in all material respects, effective internal control over financial reporting as of September 26, 2009, based on the COSO criteria.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Hologic, Inc. as of September 26, 2009 and September 27, 2008 and the related consolidated statements of operations, stockholders’ equity and other comprehensive income (loss) and cash flows for each of the three years in the period ended September 26, 2009 of Hologic, Inc. and our report dated November 24, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
November 24, 2009
82
Changes in Internal Control over Financial Reporting
During the quarter ended September 26, 2009, there have been no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information. |
None.
83
PART III
| Item 10. | Directors, and Executive Officers and Corporate Governance. |
Pursuant to Section 406 of the Sarbanes-Oxley Act of 2002, we have adopted a Code of Ethics for Senior Financial Officers that applies to our principal executive officer and principal financial officer, principal accounting officer and controller, and other persons performing similar functions. Our Code of Ethics for Senior Financial Officers is publicly available on our website atwww.hologic.comunder Investor Relations. We intend to satisfy the disclosure requirement under Item 5.05 of Current Report on Form 8-K regarding an amendment to, or waiver from, a provision of this code by posting such information on our website, at the address specified above.
The additional information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 11. | Executive Compensation. |
The information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
We maintain a number of equity compensation plans for employees, officers, directors and others whose efforts contribute to our success. The table below sets forth certain information as of the end of our fiscal year ended September 26, 2009 regarding the shares of our common stock available for grant or granted under stock option plans and equity incentives that (i) were approved by our stockholders, and (ii) were not approved by our stockholders.
Equity Compensation Plan Information
| | | | | | | |
Plan Category | | Number of
securities to be
issued upon
exercise of
outstanding
options, warrants
and rights
(a) | | Weighted-average
exercise price of
outstanding
options,
warrants and rights
(b) | | Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a))
(c) |
Equity compensation plans approved by security holders | | 16,814,080 | | $ | 14.09 | | 15,017,869 |
Equity compensation plans not approved by security holders (1) | | 509,263 | | $ | 3.83 | | — |
| | | | | | | |
Total | | 17,323,343 | | $ | 13.79 | | 15,017,869 |
| | | | | | | |
| (1) | Includes the following plans: 1997 Employee Equity Incentive Plan and 2000 Acquisition Equity Incentive Plan. A description of each of these plans is as follows: |
1997 Employee Equity Incentive Plan. The purposes of the 1997 Employee Equity Incentive Plan (the “1997 Plan”), adopted by the Board of Directors in May 1997, were to attract and retain key employees, consultants and advisors, to provide an incentive for them to assist us in achieving long-range performance goals, and to enable such person to participate in our long-term growth. In general, under the 1997 Plan, all employees,
84
consultants, and advisors who were not executive officers or directors were eligible to participate in the 1997 Plan. The 1997 Plan is administered by our Compensation Committee. Participants in the 1997 Plan are eligible to receive non-qualified stock options, stock appreciation rights, restricted stock and performance shares. A total of 4,400,000 shares of our common stock were reserved for issuance under the 1997 Plan. Of the shares reserved for issuance under the 1997 Plan, options to purchase 297,165 shares are outstanding as of September 26, 2009. In September 2005, our Compensation Committee determined that no further awards would be made under this plan and cancelled all remaining 332,168 shares available for issuance under the 1997 Plan that were not subject to outstanding stock option awards.
2000 Acquisition Incentive Plan. The purpose of the 2000 Acquisition Equity Incentive Plan (the “2000 Plan”), adopted by the Board of Directors in April 2001, was to attract and retain (a) employees, consultants and advisors, of newly acquired businesses who have been or were being hired as employees, consultants or advisors of our company or any of our consolidated subsidiaries, and (b) employees, consultants and advisors, of our company who have or were anticipated to provide significant assistance in connection with the acquisition of a newly acquired business or its integration with our company, and to provide such persons an incentive for them to achieve long-range performance goals, and to enable them to participate in our long-term growth. In general, under the 2000 Plan, only employees, consultants and advisors who were not officers or directors of our company were eligible to participate in the 2000 Plan. The 2000 Plan was administered by our Compensation Committee. Participants in the 2000 Plan were eligible to receive non-qualified stock options, stock appreciation rights, restricted stock and performance shares. A total of 3,200,000 shares of our common stock were reserved for issuance under the 2000 Plan. Of the shares reserved for issuance under the 2000 Plan, options to purchase 212,098 shares were outstanding as of September 26, 2009. In September 2005, our Compensation Committee determined that no further awards would be made under this plan and cancelled all remaining 835,408 shares, available for issuance under the 2000 Plan that were not subject to outstanding stock option awards.
The additional information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 13. | Certain Relationships and Related Transactions. |
The information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 14. | Principal Accountant Fees and Services. |
The information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
85
PART IV
| Item 15. | Exhibits, Financial Statement Schedules. |
(a) The following documents are filed as part of this report:
(1) Financial Statements
Report of Independent Registered Public Accounting Firm on Consolidated Financial Statements
Consolidated Balance Sheets as of September 26, 2009 and September 27, 2008
Consolidated Statements of Operations for the years ended September 26, 2009, September 27, 2008 and September 29, 2007
Consolidated Statements of Stockholders’ Equity and Comprehensive Income (Loss) for the years ended September 26, 2009, September 27, 2008 and September 29, 2007
Consolidated Statements of Cash Flows for the years ended September 26, 2009, September 27, 2008 and September 29, 2007
Notes to Consolidated Financial Statements
(2) Financial Statement Schedules
All schedules have been omitted because they are not required or because the required information is given in the Consolidated Financial Statements or Notes thereto.
(b) Listing of Exhibits
| | | | |
Exhibit
Number | | | | Reference |
| | |
| 2.01 | | Agreement and Plan of Merger between Hologic, Nor’easter Corp. and Cytyc dated May 20, 2007 | | K-2.1 |
| | |
| 2.02 | | Agreement and Plan of Merger and Reorganization, dated February 26, 2007, by and among Adiana, Inc., Cytyc, Admiral Acquisition Corp. and the Stockholder Representative Committee | | N-2.1 |
| | |
| 2.03 | | Agreement and Plan of Merger, dated as of June 8, 2008, by and among Hologic, Thunder Tech Corp. and Third Wave Technologies, Inc. | | R-2.1 |
| | |
| 3.01 | | Certificate of Incorporation of Hologic | | A-3.01 |
| | |
| 3.02 | | Amendment to Certificate of Incorporation of Hologic | | C-3.03 |
| | |
| 3.03 | | Certificate of Amendment to Certificate of Incorporation of Hologic | | I-3.03 |
| | |
| 3.04 | | Certificate of Amendment to Certificate of Incorporation of Hologic | | FF-3.1 |
| | |
| 3.05 | | Certificate of Amendment to Certificate of Incorporation of Hologic | | S-3.1 |
| | |
| 3.06 | | Second Amended and Restated By-laws of Hologic, as amended | | DD-3.1 |
| | |
| 3.07 | | Amended and Restated Certificate of Designations of Series A Junior Participating Preferred Stock of Hologic | | T-3.6 |
| | |
| 4.01 | | Specimen Certificate for Shares of Hologic’s Common Stock | | B-1 |
| | |
| 4.02 | | Description of Capital Stock (Contained in the Certificate of Incorporation of Hologic, as Amended, Filed as Exhibits 3.01, 3.02, 3.03, 3.04 and 3.05) | | A-3.01; C-3.03; I-3.03; FF-3.1 and S-3.1 |
86
| | | | |
Exhibit
Number | | | | Reference |
| | |
| 4.03 | | Rights Agreement dated September 17, 2002 | | G-4 |
| | |
| 4.04 | | Amended and Restated Rights Agreement dated April 2, 2008 | | T-4.1 |
| | |
| 4.05 | | Form of Rights Certificate | | Q-4 |
| | |
| 4.06 | | Indenture, dated as of December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic | | U-4.1 |
| | |
| 4.07 | | First Supplemental Indenture, dated December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic | | U-4.2 |
| | |
| 10.01 | | Second Amended and Restated 1990 Non-Employee Director Stock Option Plan | | C-10.26* |
| | |
| 10.02 | | 1995 Combination Stock Option Plan | | C-10.25* |
| | |
| 10.03 | | Second Amended and Restated 1999 Equity Incentive Plan | | I-10.3* |
| | |
| 10.04 | | Amendment No. 1 to Second Amended and Restated 1999 Equity Incentive Plan | | L-10.2* |
| | |
| 10.05 | | Amendment No. 2 to Second Amended and Restated 1999 Equity Incentive Plan | | FF-10.17* |
| | |
| 10.06 | | Amendment No. 3 to Second Amended and Restated 1999 Equity Incentive Plan | | HH-10.3* |
| | |
| 10.07 | | 1997 Employee Equity Incentive Plan | | D-99 |
| | |
| 10.08 | | 2000 Acquisition Equity Incentive Plan | | F-10.05 |
| | |
| 10.09 | | Hologic 2008 Equity Incentive Plan | | S-10.1* |
| | |
| 10.10 | | Form of Employee Stock Option Award Agreement under 2008 Equity Incentive Plan | | V-10.1* |
| | |
| 10.11 | | Form of Employee Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan | | V-10.2* |
| | |
| 10.12 | | Form of Independent Director Stock Option Award Agreement under 2008 Equity Incentive Plan | | HH-10.1* |
| | |
| 10.13 | | Form of Independent Director Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan | | HH-10.2* |
| | |
| 10.14 | | Amended and Restated 2008 Employee Stock Purchase Plan | | filed herewith* |
| | |
| 10.15 | | Hologic 2009 Short-Term Incentive Plan | | V-10.3* |
| | |
| 10.16 | | Hologic 2010 Short-Term Incentive Plan | | EE-10.1* |
| | |
| 10.17 | | Cytyc Corporation 1995 Stock Plan | | L-10.4* |
| | |
| 10.18 | | Cytyc Corporation 1995 Non-Employee Director Stock Option Plan | | L-10.5* |
| | |
| 10.19 | | Cytyc Corporation 1998 Stock Plan of Pro Duct Health, Inc. | | L-10.6* |
| | |
| 10.20 | | Cytyc Corporation 2001 Non-Employee Director Stock Plan | | L-10.7* |
| | |
| 10.21 | | Cytyc Corporation 2004 Omnibus Stock Plan | | L-10.8* |
| | |
| 10.22 | | Form of Indemnification Agreement (as executed with each director of Hologic) | | CC-10.1* |
| | |
| 10.23 | | Executive Bonus Plan Description | | X-10.1* |
| | |
| 10.24 | | Hologic Supplemental Executive Retirement Plan (SERP) | | BB-10.4* |
| | |
| 10.25 | | Form of SERP Rabbi Trust Agreement | | J-10.11* |
87
| | | | |
Exhibit
Number | | | | Reference |
| | |
| 10.26 | | Form of Officer Severance Agreement including list of officers to whom provided | | GG-10.7* |
| | |
| 10.27 | | Retention and Severance Agreement dated May 3, 2006, by and between Hologic and John W. Cumming | | GG-10.4* |
| | |
| 10.28 | | Retention and Severance Agreement dated May 3, 2006, by and between Hologic and Robert A. Cascella | | GG-10.5* |
| | |
| 10.29 | | Retention and Severance Agreement dated May 3, 2006, by and between Hologic and Glenn P. Muir | | GG-10.6* |
| | |
| 10.30 | | Form of Restricted Stock Unit Award under the Retention and Severance Agreement filed as exhibit 10.27, 10.28 and 10.29 | | GG-10.9* |
| | |
| 10.31 | | Transition Agreement dated November 5, 2009, by and between Hologic and John W. Cumming | | DD-10.1* |
| | |
| 10.32 | | Form of Senior Vice President Change of Control Agreement including list of officers to whom provided | | BB-10.5* |
| | |
| 10.33 | | Form of Senior Executive Officer Change of Control Agreement including list of officers to whom provided | | EE-10.2* |
| | |
| 10.34 | | John W. Cumming Waiver Letter Dated As Of May 20, 2007 | | K-10.1* |
| | |
| 10.35 | | Robert A. Cascella Waiver Letter Dated As Of May 20, 2007 | | K-10.2* |
| | |
| 10.36 | | Glenn P. Muir Waiver Letter Dated As Of May 20, 2007 | | K-10.3* |
| | |
| 10.37 | | Second Retention Agreement with Robert A. Cascella dated as of October 22, 2007 | | FF-10.10* |
| | |
| 10.38 | | Restricted Stock Grant Agreement with Robert A. Cascella dated as of October 22, 2007 | | FF-10.18* |
| | |
| 10.39 | | Executive Financial Services Program | | AA-10.46* |
| | |
| 10.40 | | Facility Lease (Danbury) dated as of December 30, 1995 by and among Melvin J. Powers and Mary P. Powers D/B/A M&N Realty and Lorad | | E-10.14 |
| | |
| 10.41 | | Lease Agreement (Danbury and Bedford) by and between BONE (DE) QRS 15-12, INC., and Hologic dated as of August 28, 2002 as amended | | H-10.27; Z-10.41 |
| | |
| 10.42 | | Office Lease dated December 31, 2003 between Cytyc and Marlborough Campus Limited Partnership | | O-10.17 |
| | |
| 10.43 | | Lease Agreement by and between Zona Franca Coyol S.A. and Cytyc Surgical Products Costa Rica S.A. dated April 23, 2007 | | Z-10.45 |
| | |
| 10.44 | | Lease Agreement by and between 445 Simarano Drive, Marlborough LLC and Cytyc dated July 11, 2006 | | Z-10.46 |
| | |
| 10.45 | | Supply Agreement between Cytyc, Whatman, Inc. and Whatman SA dated as of December 31, 2000, as amended, October 16, 2001 and May 2, 2002 | | P-10.13 |
| | |
| 10.46 | | Credit and Guaranty Agreement dated as of October 22, 2007 among Hologic, the Guarantors party thereto and defined below, the Secured Parties party thereto, and the Agent, Banc of America Securities LLC, Bank of America, N.A., Citicorp North America, Inc., JPMorgan Chase Bank, N.A., RBS Citizens, National Association and Fifth Third Bank | | M-10.1 |
88
| | | | |
Exhibit
Number | | | | Reference |
| | |
| 10.47 | | Waiver and First Amendment to Credit and Guaranty Agreement and Pledge and Security Agreement dated as of April 14, 2008 by and among Hologic and its domestic subsidiaries, excluding the subsidiaries which are Massachusetts securities corporations and Goldman Sachs Credit Partners L.P. | | W-10.1 |
| | |
| 10.48 | | Amended and Restated Credit and Guaranty Agreement dated as of July 17, 2008 among Hologic, Goldman Sachs Credit Partners L.P., as Sole Lead Arranger and Sole Lead Bookrunner, Goldman Sachs Credit Partners L.P., JPMorgan Chase Bank, N.A. and RBS Citizens, National Association, as Co-Syndication Agents, Goldman Sachs Credit Partners L.P., as Administrative Agent and Collateral Agent and Royal Bank of Canada, as Documentation Agent and each lender from time to time party thereto | | Y-10.1 |
| | |
| 10.49 | | Pledge and Security Agreement among Hologic, Goldman Sachs Credit Partners L.P., as Collateral Agent thereunder and the other parties therein named dated as of October 22, 2007 | | FF-10.2 |
| | |
| 10.50 | | Amended and Restated Pledge and Security Agreement dated as of July 17, 2008 among Hologic, Goldman Sachs Credit Partners L.P., as Collateral Agent thereunder and the others parties named therein | | Y-10.2 |
| | |
| 10.51 | | Open End Mortgage Deed, Security Agreement, Assignment of Rents and Leases and Fixture Filing for 36 Apple Ridge Road, Danbury, Connecticut dated as of October 22, 2007 | | FF-10.3 |
| | |
| 10.52 | | Open End Mortgage Deed, Security Agreement, Assignment of Rents and Leases and Fixture Filing for 37 Apple Ridge Road, Danbury, Connecticut dated as of October 22, 2007 | | FF-10.4 |
| | |
| 10.53 | | Mortgage, Security Agreement, Assignment of Rents and Leases and Fixture Filing for 35 Crosby Drive, Bedford, Massachusetts dated as of October 22, 2007 | | FF-10.5 |
| | |
| 10.54 | | Lease Guaranty dated October 22, 2007 between Bel Marlborough I LLC and Hologic, as guarantor thereunder | | FF-10.7 |
| | |
| 14.1 | | Code of Ethics for Senior Financial Officers | | FF-14.1 |
| | |
| 21.01 | | Subsidiaries of Hologic | | filed herewith |
| | |
| 23.01 | | Consent of Ernst & Young LLP | | filed herewith |
| | |
| 31.1 | | Certification of Hologic’s CEO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 31.2 | | Certification of Hologic’s CFO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 32.1 | | Certification of Hologic’s CEO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 32.2 | | Certification of Hologic’s CFO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 101.INS | | XBRL Instance Document | | filed herewith |
| | |
| 101.SCH | | XBRL Taxonomy Extension Schema Document | | filed herewith |
| | |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document | | filed herewith |
| | |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document | | filed herewith |
| | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document | | filed herewith |
| * | Management compensation plan or arrangement |
89
| A | We previously filed this exhibit on January 24, 1990 with the referenced exhibit number as an exhibit to our Registration Statement on Form S-1 (Registration No. 33-33128) and the previously filed exhibit is incorporated herein by reference. |
| B | We previously filed this exhibit on January 31, 1990 with the referenced exhibit number as an exhibit to our Registration Statement on Form 8-A, and the previously filed exhibit is incorporated herein by reference. |
| C | We previously filed this exhibit on May 14, 1996, with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the quarter ended March 30, 1996, and the previously filed exhibit is incorporated herein by reference. |
| D | We previously filed this exhibit on August 20, 1997 with the referenced exhibit number as an exhibit to our Registration Statement on Form S-8 (SEC File No. 333-34003) and the previously filed exhibit is incorporated herein by reference. |
| E | Trex Medical Corporation previously filed this exhibit with the referenced exhibit number as an exhibit to its Registration Statement on Form S-1 (Reg. No. 333-2926) and the previously filed exhibit is incorporated by reference. |
| F | We previously filed this exhibit on December 12, 2001 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 29, 2001, and the previously filed exhibit is incorporated by reference. |
| G | We previously filed this exhibit on December 4, 2002 with the referenced exhibit number as an exhibit to our registration statement on Form 8-A (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| H | We previously filed this exhibit on December 24, 2002 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 28, 2002, and the previously filed exhibit is incorporated herein by reference. |
| I | We previously filed this exhibit on December 6, 2005 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 24, 2005, and the previously filed exhibit is incorporated herein by reference. |
| J | We previously filed this exhibit on December 14, 2006 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 30, 2006, and the previously filed exhibit is incorporated herein by reference. |
| K | We previously filed this exhibit on May 21, 2007 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| L | We previously filed this exhibit on October 23, 2007 with the referenced exhibit number to our Registration Statement on Form S-8 (SEC File No. 333-146887) and the previously filed exhibit is incorporated herein by reference. |
| M | We previously filed this exhibit on October 23, 2007 with the referenced exhibit number to our Current Report on Form 8-K/A (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| N | Cytyc Corporation previously filed this exhibit on February 28, 2007 with the referenced exhibit number as an Exhibit to its Current Report on Form 8-K (SEC File No. 000-27558) and the previously filed exhibit is incorporated by reference. |
| O | Cytyc Corporation previously filed this exhibit on January 30, 2004 with the referenced exhibit number as an exhibit to its Annual Report on Form 10-K (SEC File No. 000-27558) and the previously filed exhibit is incorporated by reference. |
90
| P | Cytyc Corporation previously filed this exhibit on March 24, 2003 with the referenced exhibit number as an exhibit to its Annual Report on Form 10-K (SEC File No. 000-27558) and the previously filed exhibit is incorporated by reference. |
| Q | We previously filed this exhibit on September 26, 2002 with the referenced exhibit number as an exhibit to our Registration Statement on Form 8-K (SEC File No. 000-18218) and the previously filed exhibit is incorporated herein by reference. |
| R | We previously filed this exhibit on June 9, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| S | We previously filed this exhibit on March 11, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| T | We previously filed this exhibit on April 3, 2008 with the referenced exhibit number as an exhibit to our Registration Statement on Form 8-A (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| U | We previously filed this exhibit on December 10, 2007 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| V | We previously filed this exhibit on November 17, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| W | We previously filed this exhibit on May 8, 2008 with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the fiscal quarter ended March 29, 2008, and the previously filed exhibit is incorporated herein by reference. |
| X | We previously filed this exhibit on January 17, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| Y | We previously filed this exhibit on July 17, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| Z | We previously filed this exhibit on November 27, 2007 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 29, 2007, and the previously filed exhibit is incorporated herein by reference. |
| AA | We previously filed this exhibit on November 26, 2008 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 27, 2008, and the previously filed exhibit is incorporated herein by reference. |
| BB | We previously filed this exhibit on February 5, 2009, with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the quarter ended December 27, 2008, and the previously filed exhibit is incorporated herein by reference. |
| CC | We previously filed this exhibit on March 6, 2009 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| DD | We previously filed this exhibit on November 9, 2009 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| EE | We previously filed this exhibit on November 17, 2009 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
91
| FF | We previously filed this exhibit on October 22, 2007 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| GG | We previously filed this exhibit on May 4, 2006 with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the fiscal quarter ended March 25, 2006, and the previously filed exhibit is incorporated herein by reference. |
| HH | We previously filed this exhibit on December 12, 2008 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
92
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| HOLOGIC, INC. |
| |
| By: | | /s/ ROBERT A. CASCELLA |
| | Robert A. Cascella |
| | Chief Executive Officer |
Date: November 24, 2009
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/s/ ROBERT A. CASCELLA ROBERT A. CASCELLA | | Director, President and Chief Executive Officer (Principal Executive Officer) | | November 24, 2009 |
| | |
/s/ GLENN P. MUIR GLENN P. MUIR | | Director, Executive Vice President, Finance and Administration and Chief Financial Officer, (Principal Financial Officer) | | November 24, 2009 |
| | |
/s/ ROBERT H. LAVALLEE ROBERT H. LAVALLEE | | Senior Vice President, Chief Accounting Officer (Principal Accounting Officer) | | November 24, 2009 |
| | |
/s/ JOHN W. CUMMING JOHN W. CUMMING | | Chairman, Director and Executive Officer | | November 24, 2009 |
| | |
/s/ SALLY W. CRAWFORD SALLY W. CRAWFORD | | Director | | November 24, 2009 |
| | |
/s/ DAVID R. LAVANCE, JR. DAVID R. LAVANCE, JR. | | Lead Independent Director | | November 24, 2009 |
| | |
/s/ NANCY L. LEAMING NANCY L. LEAMING | | Director | | November 24, 2009 |
| | |
/s/ LAWRENCE M. LEVY LAWRENCE M. LEVY | | Director | | November 24, 2009 |
| | |
/s/ ELAINE S. ULLIAN ELAINE S. ULLIAN | | Director | | November 24, 2009 |
| | |
/s/ WAYNE WILSON WAYNE WILSON | | Director | | November 24, 2009 |
93
Hologic, Inc.
Consolidated Financial Statements
Years ended September 26, 2009, September 27, 2008 and September 29, 2007
Contents
Report of Independent Registered Public Accounting Firm
on Consolidated Financial Statements
The Board of Directors and Stockholders of Hologic, Inc.:
We have audited the accompanying consolidated balance sheets of Hologic, Inc. and subsidiaries as of September 26, 2009 and September 27, 2008, and the related consolidated statements of operations, stockholders’ equity and other comprehensive income (loss), and cash flows for each of the three years in the period ended September 26, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Hologic, Inc. and subsidiaries at September 26, 2009 and September 27, 2008, and the consolidated results of their operations and their cash flows for each of the three years in the period ended September 26, 2009, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 7 in the notes to the consolidated financial statements, effective September 29, 2007, the Company adopted the recognition and disclosure requirements of Statement of Financial Accounting Standard (SFAS) No 158Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans – an amendment of FASB Statements No. 87, 88, 106 and 132(R)(codified primarily in FASB ASC Topic 715-20Defined Benefit Plans-General).
As discussed in Note 8 in the notes of consolidated financial statements, effective September 30, 2007, the Company adopted Financial Accounting Standard Board (FASB) Interpretation No. 48 Accounting for Uncertainty in Income Taxes, an interpretation of FASB Statement No. 109 (codified primarily in FASB ASC Topic 740 Income Taxes).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Hologic, Inc.’s internal control over financial reporting as of September 26, 2009, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated November 24, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
November 24, 2009
F-2
Hologic, Inc.
Consolidated Balance Sheets
(In thousands, except per share data)
| | | | | | | | |
| | | September 26,
2009 | | | September 27,
2008 | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 293,186 | | | $ | 95,661 | |
Restricted cash | | | 916 | | | | 3,629 | |
Accounts receivable, less reserves of $7,279 and $6,326 respectively | | | 263,231 | | | | 321,299 | |
Inventories | | | 182,780 | | | | 174,667 | |
Deferred income tax assets | | | 52,165 | | | | 53,660 | |
Prepaid income taxes | | | 172 | | | | 17,797 | |
Prepaid expenses and other current assets | | | 29,066 | | | | 26,865 | |
| | | | | | | | |
Total current assets | | | 821,516 | | | | 693,578 | |
| | | | | | | | |
Property and equipment, at cost: | | | | | | | | |
Land | | | 8,983 | | | | 8,978 | |
Buildings and improvements | | | 57,214 | | | | 55,743 | |
Equipment and software | | | 187,961 | | | | 172,789 | |
Equipment under customer usage agreements | | | 125,635 | | | | 100,316 | |
Furniture and fixtures | | | 11,112 | | | | 11,083 | |
Leasehold improvements | | | 39,701 | | | | 38,620 | |
| | | | | | | | |
| | | 430,606 | | | | 387,529 | |
Less—accumulated depreciation and amortization | | | 158,978 | | | | 103,554 | |
| | | | | | | | |
| | | 271,628 | | | | 283,975 | |
| | | | | | | | |
Intangible assets, net (Note 2) | | | 2,424,812 | | | | 2,629,651 | |
Goodwill | | | 2,108,963 | | | | 4,450,496 | |
Other assets | | | 62,909 | | | | 76,932 | |
| | | | | | | | |
Total assets | | $ | 5,689,828 | | | $ | 8,134,632 | |
| | | | | | | | |
Liabilities and Stockholders’ Equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Current portion of long-term debt | | $ | 38,373 | | | $ | 38,480 | |
Accounts payable | | | 46,589 | | | | 59,590 | |
Accrued expenses (Note 14) | | | 137,284 | | | | 154,746 | |
Deferred revenue | | | 97,544 | | | | 78,559 | |
Deferred gain(Note 4) | | | 9,500 | | | | 9,500 | |
| | | | | | | | |
Total current liabilities | | | 329,290 | | | | 340,875 | |
| | | | | | | | |
Long-term debt, net of current portion(Note 5) | | | 139,955 | | | | 437,420 | |
Convertible notes(Note 5) | | | 1,725,000 | | | | 1,725,000 | |
Deferred income tax liabilities | | | 912,970 | | | | 920,838 | |
Deferred service obligations—long-term | | | 11,364 | | | | 10,777 | |
Other long-term liabilities(Note 14) | | | 58,534 | | | | 57,453 | |
Commitments and contingencies (Notes 12 and 15) | | | | | | | | |
| | |
Stockholders’ equity | | | | | | | | |
Preferred stock, $0.01 par value–1,623 shares authorized; 0 shares issued | | | — | | | | — | |
Common stock, $0.01 par value–750,000 shares authorized; 257,938 and 256,373 shares issued, respectively | | | 2,579 | | | | 2,564 | |
Capital in excess of par value | | | 4,898,422 | | | | 4,853,837 | |
Accumulated deficit | | | (2,393,881 | ) | | | (217,644 | ) |
Accumulated other comprehensive income | | | 7,028 | | | | 4,945 | |
Treasury stock, at cost—214 shares | | | (1,433 | ) | | | (1,433 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 2,512,715 | | | | 4,642,269 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 5,689,828 | | | $ | 8,134,632 | |
| | | | | | | | |
See accompanying notes.
F-3
Hologic, Inc.
Consolidated Statements of Operations
(In thousands, except per share data)
| | | | | | | | | | | | |
| | | Years ended | |
| | | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Revenues: | | | | | | | | | | | | |
Product sales | | $ | 1,426,986 | | | $ | 1,502,447 | | | $ | 628,854 | |
Service and other revenues | | | 210,148 | | | | 172,052 | | | | 109,514 | |
| | | | | | | | | | | | |
| | | 1,637,134 | | | | 1,674,499 | | | | 738,368 | |
| | | | | | | | | | | | |
Costs and expenses: | | | | | | | | | | | | |
Cost of product sales | | | 470,295 | | | | 535,082 | | | | 267,470 | |
Cost of product sales—amortization of intangible assets | | | 155,519 | | | | 95,310 | | | | 11,262 | |
Cost of product sales—impairment of intangible assets | | | 4,065 | | | | — | | | | — | |
Cost of service and other revenues | | | 149,769 | | | | 151,589 | | | | 114,307 | |
Research and development | | | 94,328 | | | | 81,421 | | | | 44,381 | |
Selling and marketing | | | 238,977 | | | | 261,524 | | | | 85,520 | |
General and administrative | | | 148,825 | | | | 147,405 | | | | 62,092 | |
Amortization of intangible assets | | | 51,210 | | | | 25,227 | | | | 5,584 | |
Impairment of goodwill(Note 2) | | | 2,340,023 | | | | — | | | | — | |
Impairment of intangible assets(Note 2) | | | — | | | | 2,900 | | | | — | |
Acquired in-process research and development(Note 3) | | | — | | | | 565,200 | | | | — | |
Restructuring charges (Note 2) | | | 797 | | | | 6,383 | | | | — | |
| | | | | | | | | | | | |
| | | 3,653,808 | | | | 1,872,041 | | | | 590,616 | |
| | | | | | | | | | | | |
(Loss) income from operations | | | (2,016,674 | ) | | | (197,542 | ) | | | 147,752 | |
| | | |
Interest income | | | 1,161 | | | | 4,528 | | | | 2,815 | |
Interest expense | | | (69,502 | ) | | | (84,912 | ) | | | (2,511 | ) |
Other (expense) income, net | | | (3,660 | ) | | | (1,215 | ) | | | 433 | |
| | | | | | | | | | | | |
(Loss) income before income taxes | | | (2,088,675 | ) | | | (279,141 | ) | | | 148,489 | |
Provision for income taxes | | | 87,562 | | | | 106,476 | | | | 53,911 | |
| | | | | | | | | | | | |
Net (loss) income | | $ | (2,176,237 | ) | | $ | (385,617 | ) | | $ | 94,578 | |
| | | | | | | | | | | | |
Basic net (loss) income per common share | | $ | (8.48 | ) | | $ | (1.57 | ) | | $ | 0.88 | |
| | | | | | | | | | | | |
Diluted net (loss) income per common share | | $ | (8.48 | ) | | $ | (1.57 | ) | | $ | 0.86 | |
| | | | | | | | | | | | |
Weighted average number of common shares outstanding: | | | | | | | | | | | | |
Basic | | | 256,545 | | | | 245,968 | | | | 106,873 | |
| | | | | | | | | | | | |
Diluted | | | 256,545 | | | | 245,968 | | | | 109,669 | |
| | | | | | | | | | | | |
See accompanying notes.
F-4
Hologic, Inc.
Consolidated Statements of Stockholders’ Equity and Comprehensive Income (Loss)
(In thousands, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Capital in
Excess of
Par Value | | | Accumulated
Deficit | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Treasury Stock | | | Total
Stockholders’
Equity | | | Comprehensive
Income (Loss) | |
| | Number of
Shares | | Par Value | | | | | Number of
Shares | | Amount | | | |
Balance at September 30, 2006 | | 105,290 | | $ | 1,053 | | $ | 531,728 | | | $ | 73,875 | | | $ | (442 | ) | | 180 | | $ | (464 | ) | | $ | 605,750 | | | | | |
Issuance of common stock related to acquisitions | | 2,315 | | | 23 | | | 63,155 | | | | — | | | | — | | | — | | | — | | | | 63,178 | | | | | |
Exercise of stock options | | 2,695 | | | 27 | | | 10,564 | | | | — | | | | — | | | — | | | — | | | | 10,591 | | | | | |
Stock-based compensation expense | | — | | | — | | | 6,104 | | | | — | | | | — | | | — | | | — | | | | 6,104 | | | | | |
Purchase of treasury shares to settle minimum withholding taxes | | — | | | — | | | — | | | | — | | | | — | | | 34 | | | (969 | ) | | | (969 | ) | | | | |
Tax benefit related to exercise of stock options | | — | | | — | | | 21,926 | | | | — | | | | — | | | — | | | — | | | | 21,926 | | | | | |
Net income | | — | | | — | | | — | | | | 94,578 | | | | — | | | — | | | — | | | | 94,578 | | | $ | 94,578 | |
Translation adjustments | | — | | | — | | | — | | | | — | | | | 2,353 | | | — | | | — | | | | 2,353 | | | | 2,353 | |
Adjustment to minimum pension liability, net | | — | | | — | | | — | | | | — | | | | 2,212 | | | — | | | — | | | | 2,212 | | | | 2,212 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 99,143 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 29, 2007 | | 110,300 | | | 1,103 | | | 633,477 | | | | 168,453 | | | | 4,123 | | | 214 | | | (1,433 | ) | | | 805,723 | | | | | |
Issuance of common stock related to acquisitions | | 132,060 | | | 1,321 | | | 3,670,818 | | | | — | | | | — | | | — | | | — | | | | 3,672,139 | | | | | |
Exercise of stock options | | 11,398 | | | 114 | | | 170,995 | | | | — | | | | — | | | — | | | — | | | | 171,109 | | | | | |
Fair value of common stock issued in connection with conversion of Cytyc convertible debt | | 2,557 | | | 25 | | | 84,176 | | | | — | | | | — | | | — | | | — | | | | 84,201 | | | | | |
Fair value of vested options exchanged related to acquisitions | | — | | | — | | | 256,941 | | | | — | | | | — | | | — | | | — | | | | 256,941 | | | | | |
Issuance of common stock to employees upon vesting of restricted stock units, net of minimum tax withholdings | | 58 | | | 1 | | | (1,343 | ) | | | — | | | | — | | | — | | | — | | | | (1,342 | ) | | | | |
Stock-based compensation expense | | — | | | — | | | 25,664 | | | | — | | | | — | | | — | | | — | | | | 25,664 | | | | | |
Tax benefit related to exercise of stock options | | — | | | — | | | 13,109 | | | | — | | | | — | | | — | | | — | | | | 13,109 | | | | | |
Cumulative effect of a change in accounting principle—FIN 48 | | — | | | — | | | — | | | | (480 | ) | | | — | | | — | | | — | | | | (480 | ) | | | | |
Net loss | | — | | | — | | | — | | | | (385,617 | ) | | | — | | | — | | | — | | | | (385,617 | ) | | $ | (385,617 | ) |
Translation adjustments | | — | | | — | | | — | | | | — | | | | 1,092 | | | — | | | — | | | | 1,092 | | | | 1,092 | |
Adjustment to minimum pension liability, net | | — | | | — | | | — | | | | — | | | | (270 | ) | | — | | | — | | | | (270 | ) | | | (270 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | (384,795 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 27, 2008 | | 256,373 | | | 2,564 | | | 4,853,837 | | | | (217,644 | ) | | | 4,945 | | | 214 | | | (1,433 | ) | | | 4,642,269 | | | | | |
Exercise of stock options | | 1,306 | | | 13 | | | 9,379 | | | | — | | | | — | | | — | | | — | | | | 9,392 | | | | | |
Issuance of common stock to employees upon vesting of restricted stock units, net of minimum tax withholdings | | 138 | | | 1 | | | (882 | ) | | | | | | | | | | | | | | | | | (881 | ) | | | | |
Issuance of common shares under the employee stock purchase plan | | 121 | | | 1 | | | 1,541 | | | | — | | | | — | | | — | | | — | | | | 1,542 | | | | | |
Stock-based compensation expense | | — | | | — | | | 32,939 | | | | — | | | | — | | | — | | | — | | | | 32,939 | | | | | |
Tax benefit related to exercise of stock options | | — | | | — | | | 1,608 | | | | — | | | | — | | | — | | | — | | | | 1,608 | | | | | |
Net loss | | — | | | — | | | — | | | | (2,176,237 | ) | | | — | | | — | | | — | | | | (2,176,237 | ) | | $ | (2,176,237 | ) |
Translation adjustments | | — | | | — | | | — | | | | — | | | | 1,666 | | | — | | | — | | | | 1,666 | | | | 1,666 | |
Adjustment to minimum pension liability, net | | — | | | — | | | — | | | | — | | | | 417 | | | — | | | — | | | | 417 | | | | 417 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | (2,174,154 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 26, 2009 | | 257,938 | | $ | 2,579 | | $ | 4,898,422 | | | $ | (2,393,881 | ) | | $ | 7,028 | | | 214 | | $ | (1,433 | ) | | $ | 2,512,715 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
F-5
Hologic, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| | | | | | | | | | | | |
| | | Years ended | |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Operating activities | | | | | | | | | | | | |
Net (loss) income | | $ | (2,176,237 | ) | | $ | (385,617 | ) | | $ | 94,578 | |
Adjustments to reconcile net (loss) income to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation | | | 67,195 | | | | 52,413 | | | | 14,291 | |
Amortization | | | 206,729 | | | | 120,537 | | | | 16,871 | |
Fair value write-up of Cytyc and Third Wave inventory sold | | | 1,167 | | | | 46,258 | | | | — | |
Non-cash interest expense | | | 17,742 | | | | 20,541 | | | | 181 | |
Goodwill impairment charge | | | 2,340,023 | | | | — | | | | — | |
Acquired in-process research and development | | | — | | | | 565,200 | | | | — | |
Impairment charges of intangibles | | | 4,065 | | | | 2,900 | | | | — | |
Stock-based compensation expense | | | 32,939 | | | | 25,664 | | | | 6,104 | |
Excess tax benefit related to exercise of non-qualified stock options | | | (2,978 | ) | | | (62,740 | ) | | | (21,926 | ) |
Deferred income taxes | | | (1,941 | ) | | | (10,365 | ) | | | 5,873 | |
Impairment of cost-method investments | | | 2,243 | | | | — | | | | — | |
Loss on disposal and impairment of property and equipment | | | 4,399 | | | | 1,740 | | | | 734 | |
Other non-cash activity | | | (1,660 | ) | | | 2,510 | | | | (368 | ) |
Changes in operating assets and liabilities, excluding the effect of acquisitions: | | | | | | | | | | | | |
Accounts receivable | | | 57,581 | | | | (58,801 | ) | | | (45,367 | ) |
Inventories | | | (15,142 | ) | | | (29,595 | ) | | | (7,997 | ) |
Prepaid income taxes | | | 17,925 | | | | 74,408 | | | | — | |
Prepaid expenses and other assets | | | (3,831 | ) | | | (5,692 | ) | | | 269 | |
Accounts payable | | | (12,881 | ) | | | (10,189 | ) | | | 14,265 | |
Accrued expenses and other liabilities | | | (10,613 | ) | | | (14,596 | ) | | | 59,758 | |
Deferred revenue | | | 19,640 | | | | 27,022 | | | | 16,661 | |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 546,365 | | | | 361,598 | | | | 153,927 | |
| | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | |
Acquisition of businesses, net of cash acquired | | | — | | | | (2,584,947 | ) | | | (9,793 | ) |
Payment of additional acquisition consideration | | | (229 | ) | | | (24,394 | ) | | | (19,033 | ) |
Purchase of property and equipment | | | (31,357 | ) | | | (53,536 | ) | | | (22,840 | ) |
Increase in equipment under customer usage agreements | | | (22,786 | ) | | | (24,731 | ) | | | — | |
Purchase of licensed technology and other intangible assets | | | (6,238 | ) | | | — | | | | — | |
Proceeds from sale of intellectual property | | | 2,250 | | | | 3,000 | | | | — | |
Proceeds from sale of building | | | — | | | | — | | | | 1,427 | |
Purchase of insurance contracts | | | (5,322 | ) | | | (3,322 | ) | | | (3,322 | ) |
Proceeds from sale of cost method investment | | | — | | | | 936 | | | | 2,150 | |
Purchase of cost method investment | | | (550 | ) | | | — | | | | (1,000 | ) |
Purchases of investment securities | | | — | | | | (263 | ) | | | — | |
Proceeds from sales and maturities of investment securities | | | — | | | | 2,638 | | | | — | |
Acquisition of non-controlling interest | | | — | | | | — | | | | (1,100 | ) |
Deferred acquisition costs | | | — | | | | — | | | | (6,393 | ) |
Decrease (increase) in restricted cash | | | 2,713 | | | | (1,332 | ) | | | — | |
Deferred gain | | | — | | | | 9,500 | | | | — | |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (61,519 | ) | | | (2,676,451 | ) | | | (59,904 | ) |
| | | | | | | | | | | | |
F-6
Hologic, Inc.
Consolidated Statements of Cash Flows (continued)
(In thousands)
| | | | | | | | | | | | |
| | | Years ended | |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Financing activities | | | | | | | | | | | | |
Proceeds from issuance of convertible notes, net of issuance costs | | | — | | | | 1,688,974 | | | | — | |
Payments upon conversion of Cytyc convertible notes | | | (298 | ) | | | (40,574 | ) | | | — | |
Proceeds under credit agreements, net of issuance costs | | | — | | | | 2,855,609 | | | | — | |
Repayments under credit agreements | | | (290,833 | ) | | | (2,425,000 | ) | | | (55,000 | ) |
Proceeds from note payable | | | — | | | | 2,062 | | | | 6,889 | |
Repayments of notes payable | | | (10,127 | ) | | | (2,895 | ) | | | (5,884 | ) |
Excess tax benefit related to exercise of non-qualified stock options | | | 2,978 | | | | 62,740 | | | | 21,926 | |
Net proceeds from issuance of common stock pursuant to employee stock plans | | | 10,887 | | | | 171,014 | | | | 10,578 | |
Financing costs on credit agreement | | | (350 | ) | | | — | | | | — | |
Payments of employee restricted stock tax withholdings | | | (881 | ) | | | (851 | ) | | | — | |
Purchase of treasury shares to settle minimum withholding taxes | | | — | | | | — | | | | (969 | ) |
| | | | | | | | | | | | |
Net cash (used in) provided by financing activities | | | (288,624 | ) | | | 2,311,079 | | | | (22,460 | ) |
| | | | | | | | | | | | |
Effect of exchange rate changes on cash and cash equivalents | | | 1,303 | | | | (968 | ) | | | (1,083 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 197,525 | | | | (4,742 | ) | | | 70,480 | |
Cash and cash equivalents, beginning of year | | | 95,661 | | | | 100,403 | | | | 29,923 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of year | | $ | 293,186 | | | $ | 95,661 | | | $ | 100,403 | |
| | | | | | | | | | | | |
See accompanying notes.
F-7
Hologic, Inc.
Notes to Consolidated Financial Statements
(In thousands, except per share data)
Hologic, Inc. (the “Company” or “Hologic”) develops, manufactures and distributes medical imaging systems and diagnostic and surgical products focused on the healthcare needs of women.
In October 2007 (the first quarter of fiscal 2008), the Company completed its merger with Cytyc Corporation (“Cytyc”), a company that develops, manufactures and markets complementary products covering a range of cancer and women’s health applications, including cervical cancer screening, treatment of excessive menstrual bleeding and radiation treatment of early-stage breast cancer. As a result of the Company’s merger with Cytyc, as more fully described in Note 3, the Company has become one of the largest companies in the world focused on women’s health.
| 2. | Summary of Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation. The Company’s fiscal year ends on the last Saturday in September. Fiscal 2009, 2008 and 2007 ended on September 26, 2009, September 27, 2008, and September 29, 2007, respectively, and each fiscal year presented included 52 weeks.
Reclassifications
The Company reclassified other receivable amounts of $5,902 from “Accounts receivable” to “Prepaid expenses and other current assets” for fiscal 2008 to conform to the current period presentation. The Company also reclassified certain amounts in the Consolidated Statement of Cash Flows for fiscal 2008 and 2007 to conform to the current period presentation. As a result, net cash provided by operations decreased to $361,598 from $364,596 for fiscal 2008 primarily due to reclassifying changes in certain other assets and other liabilities to cash flows provided by operating activities from investing activities aggregating $3,849 offset by reclassifying $851 of payments to tax authorities for tax withholdings on the vesting of restricted stock units issued to employees as a cash outflow in the financing section. As result of these changes cash used in investing activities for fiscal 2008 decreased to $2,676,451 from $2,680,300 and cash provided by financing activities decreased to $2,311,079 from $2,311,930. In addition, there were insignificant reclassifications of certain amounts within the line items of the investing activities section. For fiscal 2007, the Company reclassified changes in certain other assets and other liabilities to cash flows provided by operating activities from investing activities resulting in cash flows from operations increasing to $153,927 from $153,250 and cash used in investing activities increasing to $59,904 from $59,226.
Subsequent Events Consideration
The Company considers events or transactions that occur after the balance sheet date but prior to the issuance of the financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. Subsequent events have been evaluated through November 24, 2009, the date these financial statements are considered issued, and the financial statements reflect those material items that arose after the balance sheet date but prior to this date that would be considered recognized subsequent events. Subsequent to September 26, 2009, the Company made voluntary payments of $21,000 on its term notes, and as such, the Company reclassified this amount to short-term debt from long-term debt, which is reflected in the Consolidated Balance Sheet. There were no other material recognized subsequent events recorded in the September 26, 2009 consolidated financial statements.
F-8
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Stock Split
On April 2, 2008, the Company effected a two-for-one stock split in the form of a stock dividend. The stock split is retroactively reflected in the accompanying consolidated financial statements and notes for all periods presented.
Management’s Estimates and Uncertainties
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make significant estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Significant estimates and assumptions by management affect the Company’s revenue recognition for multiple element arrangements, allowance for doubtful accounts, the net realizable value of inventory, estimated fair value of cost method investments, valuations and purchase price allocations related to business combinations, expected future cash flows including growth rates, discount rates, terminal values and other assumptions and estimates used to evaluate the recoverability of long-lived assets and goodwill, estimated fair values of intangible assets and goodwill, amortization methods and periods, warranty reserves, certain accrued expenses, restructuring and other related charges, stock-based compensation, contingent liabilities, tax reserves and recoverability of the Company’s net deferred tax assets and related valuation allowance.
Although the Company regularly assesses these estimates, actual results could differ materially from these estimates. Changes in estimates are recorded in the period in which they become known. The Company bases its estimates on historical experience and various other assumptions that it believes to be reasonable under the circumstances.
The Company is subject to a number of risks similar to those of other companies of similar size in its industry, including, dependence on third party reimbursements to support the markets of the Company’s products, early stage of development of certain products, rapid technological changes, recoverability of long-lived assets, including intangible assets and goodwill, competition, stability of world financial markets, ability to obtain regulatory approvals, limited number of suppliers, customer concentration, integration of acquisitions, substantial indebtedness, government regulations, future sales or issuances of our common stock, management of international activities, protection of proprietary rights, patent and other litigation and dependence on key individuals.
Cash Equivalents
Cash equivalents are highly liquid investments with insignificant interest rate risk and maturities of three months or less at the time of acquisition. At September 26, 2009, the Company’s cash equivalents consisted of money market accounts, and at September 27, 2008 cash equivalents consisted of money market accounts and certificates of deposit.
Restricted Cash
Restricted cash at September 26, 2009 is primarily comprised of various deposits for operating leases and duty taxes.
F-9
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Concentrations of Credit Risk
Financial instruments that subject the Company to credit risk primarily consist of cash and cash equivalents, cost-method investments, and trade accounts receivable. The Company invests its cash and cash equivalents with financial institutions with highly rated credit.
The Company’s customers are principally located in the United States, Europe and Asia. The Company performs ongoing credit evaluations of the financial condition of its customers and generally does not require collateral. Although the Company is directly affected by the overall financial condition of the healthcare industry, as well as global economic conditions, management does not believe significant credit risk exists as of September 26, 2009. The Company generally has not experienced any material losses related to receivables from individual customers or groups of customers in the health care industry. The Company maintains an allowance for doubtful accounts based on accounts past due and historical collection experience. The Company’s losses related to collection of trade receivables have consistently been within management’s expectations. Due to these factors, no additional credit risk beyond amounts provided for collection losses, is believed by management to be probable.
There were no customers with balances greater than 10% of accounts receivable as of September 26, 2009 and September 27, 2008, nor customers that represented greater than 10% of total revenues for fiscal years 2009, 2008 and 2007.
Disclosure of Fair Value of Financial Instruments
The Company’s financial instruments mainly consist of cash and cash equivalents, accounts receivable, cost-method investments, accounts payable and debt obligations. The carrying amounts of the Company’s cash equivalents, accounts receivable and accounts payable approximate their fair value due to the short-term nature of these instruments. Amounts outstanding under the Company’s Amended Credit Agreement (See Note 5) of $174,167 and $465,000 at September 26, 2009 and September 27, 2008, respectively, are subject to variable rates of interest based on current market rates; as such, the Company believes the carrying amounts of this obligation approximate its fair value.
The Company’s AEG subsidiary also has several notes payable outstanding (See Note 5). These notes payable are denominated in either the Euro or US dollar and have variable rates of interest. As of September 26, 2009 and September 27, 2008, amounts outstanding of $1,500 and $10,602 under these notes payable approximated their fair value based on comparable market terms and conditions.
The Company has $1,725,000 of Convertible Notes outstanding (See Note 5) as of September 26, 2009 and September 27, 2008. The fair value of these Convertible Notes was approximately $1,424,000 and $1,300,000 as of September 26, 2009 and September 27, 2008, respectively, based on the trading prices as of those dates.
F-10
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Supplemental Cash Flow Statement Information
| | | | | | | | | | | |
| | | Years ended | |
| | September 26,
2009 | | September 27,
2008 | | | September 29,
2007 | |
Cash paid during the period for income taxes | | $ | 59,077 | | $ | 40,971 | | | $ | 8,344 | |
| | | | | | | | | | | |
Cash paid during the period for interest | | $ | 52,001 | | $ | 53,125 | | | $ | 2,246 | |
| | | | �� | | | | | | | |
Non-Cash Investing Activities: | | | | | | | | | | | |
Additional acquisition contingent consideration accrued | | $ | 1,854 | | $ | 73 | | | $ | — | |
| | | | | | | | | | | |
Non-Cash Financing Activities: | | | | | | | | | | | |
Issuance of common stock upon conversion of Cytyc convertible notes | | $ | — | | $ | 84,201 | | | $ | — | |
| | | | | | | | | | | |
Issuance of note payable related to purchase of licensed technology | | $ | 3,900 | | $ | — | | | $ | — | |
| | | | | | | | | | | |
Business Acquisitions, Net of Cash Acquired: | | | | | | | | | | | |
Fair value of tangible assets acquired | | $ | — | | $ | 695,113 | | | $ | 5,148 | |
Liabilities assumed | | | — | | | (301,441 | ) | | | (11,798 | ) |
Fair value of options exchanged | | | — | | | (249,460 | ) | | | — | |
Fair value of stock issued | | | — | | | (3,671,513 | ) | | | (63,178 | ) |
Cost in excess of fair value of assets acquired (Goodwill) | | | — | | | 4,071,767 | | | | 47,774 | |
Acquired identifiable intangible assets | | | — | | | 2,579,500 | | | | 32,100 | |
Deferred tax liability | | | — | | | (982,630 | ) | | | — | |
In-process research and development | | | — | | | 565,200 | | | | — | |
| | | | | | | | | | | |
| | | — | | | 2,706,536 | | | | 10,046 | |
Less acquisition costs paid prior to September 29, 2007 | | | — | | | 6,400 | | | | — | |
Less cash and cash equivalents acquired | | | — | | | 115,189 | | | | 253 | |
| | | | | | | | | | | |
Net cash paid for business acquisition | | $ | — | | $ | 2,584,947 | | | $ | 9,793 | |
| | | | | | | | | | | |
Inventories
Inventories are valued at the lower of cost or market on a first in, first out basis. Work-in-process and finished goods inventories consist of materials, labor and manufacturing overhead. The valuation of inventory requires management to estimate excess and obsolete inventory. The Company employs a variety of methodologies to determine the net realizable value of its inventory. Provisions for excess and obsolete inventory are primarily based on management’s estimates of forecasted net sales and service usage levels. A significant change in the timing or level of demand for the Company’s products as compared to forecasted amounts may result in recording additional provisions for excess and obsolete inventory in the future. The Company records provisions for excess and obsolete inventory as cost of product sales.
Inventories at September 26, 2009 and September 27, 2008 consisted of the following:
| | | | | | |
| | | 2009 | | 2008 |
Raw materials and work-in-process | | $ | 116,983 | | $ | 111,217 |
Finished goods | | | 65,797 | | | 63,450 |
| | | | | | |
| | $ | 182,780 | | $ | 174,667 |
| | | | | | |
F-11
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Property and Equipment
Property and equipment is recorded at cost less allowances for depreciation. The straight-line method of depreciation is used for all property and equipment. Repair and maintenance costs are expensed as incurred. Property and equipment are depreciated over the following estimated useful lives:
| | |
Asset Classification | | Estimated Useful Life |
Building and improvements | | 35 to 40 years |
Equipment and software | | 3–10 years |
Equipment under customer usage agreements | | 3–8 years |
Furniture and fixtures | | 5–7 years |
Leasehold improvements | | Shorter of the Original Term of Lease or Estimated Useful Life |
The asset on the Company’s balance sheet entitled equipment under customer usage agreements consists of diagnostic and medical imaging equipment located at customer sites but owned by the Company. Generally, the customer has the right to use it for a period of time provided they meet certain agreed to conditions.
The Company applies the provisions of Accounting Standard Codification (“ASC”) ASC 350-40,Internal-Use Software(formerly American Institute of Certified Public Accountants (“AICPA”) Statement of Position (“SOP”) 98-1,Software Developed or Obtained for Internal Use).This accounting guidance requires computer software costs associated with internal use software to be expensed as incurred until certain capitalization criteria are met, and it also defines which types of costs should be capitalized and which should be expensed. The Company capitalized $1,589, $3,215, and $341 during fiscal 2009, 2008 and 2007, respectively, related to a company wide Enterprise Resource Planning (“ERP”) system implementation project, as well as upgrades and enhancements that added significant functionality to the system and has included these amounts in equipment and software in the accompanying consolidated balance sheets. The Company amortizes such costs when the ERP system and new functionality become operational. The initial system costs are being amortized over an estimated useful life of ten years and new functionality is amortized over the remaining useful life of the related system.
As a result of the merger with Cytyc, the Company assumed two leases under which Cytyc or the Company disbursed cash for property and equipment to build out and equip these leased facilities. Pursuant to the provisions of ASC 840,Leases,Subsection 40-15-5 (formerly included within EITF Issue No. 97-10 (“EITF”) 97-10,The Effect of Lessee Involvement in Asset Construction), the Company was deemed to be the owner of the facility during the construction periods and after completion of the construction periods. As a result, these leases are not classified as operating leases but have been recorded by the Company at fair market value within property and equipment on its Consolidated Balance Sheets, with an offsetting increase to accrued expenses and other long-term liabilities. Please refer to Note 12, “Commitments and Contingencies”, for further discussion regarding the Company’s obligations under these lease agreements.
Long-Lived Assets
The Company reviews it long-lived assets, which includes property and equipment and identifiable intangible assets (see below for discussion of intangible assets), for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable in accordance with ASC 360-10-35-15,Impairment or Disposal of Long-Lived Assets (formerly included within SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets). Recoverability of these assets is evaluated by
F-12
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
comparing the carrying value of the assets to the undiscounted cash flows estimated to be generated by those assets over their remaining economic life. If the undiscounted cash flows are not sufficient to recover the carrying value of the assets, the assets are considered impaired. The impairment loss is measured by comparing the fair value of the assets to their carrying value. Fair value is determined by either a quoted market price, if any, or a value determined by a discounted cash flow technique. There were no material impairment charges related to property and equipment in fiscal 2009, 2008 and 2007. See below for discussion of impairment of intangible assets.
Valuation of Business Combinations and Acquisition of Intangible Assets
The Company records tangible and intangible assets acquired in business combinations and acquisitions of intangible assets under the purchase method of accounting. The Company accounts for acquisitions in accordance with FASB Statement No. 141,Business Combinations. Amounts paid for each acquisition are allocated to the assets acquired and liabilities assumed based on their fair values at the dates of acquisition. The Company then allocates the purchase price in excess of the fair value of the net tangible assets acquired to identifiable intangible assets, including purchased research and development, based on detailed valuations that use information and assumptions provided by management. The Company allocates any excess purchase price over the fair value of the net tangible and intangible assets acquired to goodwill. The use of alternative valuation assumptions, including estimated cash flows and discount rates, and alternative useful life assumptions could result in different purchase price allocations, acquired research and development charges, and intangible asset amortization expense in current and future periods.
The valuation of purchased research and development represents the estimated fair value at the dates of acquisition related to in-process projects. The Company’s purchased research and development represents the value of in-process projects that have not yet reached technological feasibility and have no alternative future uses as of the date of acquisition. The Company expenses the value attributable to these in-process projects at the time of the acquisition. If the projects are not successful or completed in a timely manner, the Company may not realize the financial benefits expected for these projects or for the acquisitions as a whole.
The Company uses the income approach to determine the fair values of its purchased research and development. This approach determines fair value by estimating the after-tax cash flows attributable to an in-process project over its useful life and then discounting these after-tax cash flows back to a present value. The Company bases its revenue assumptions on estimates of relevant market sizes, expected market growth rates, expected trends in technology and expected product introductions by competitors. In arriving at the value of the in-process projects, the Company considers, among other factors, the in-process projects’ stage of completion, the complexity of the work completed as of the acquisition date, the costs already incurred, the projected costs to complete, the contribution of core technologies and other acquired assets, the expected introduction date and the estimated useful life of the technology. The Company bases the discount rate used to arrive at a present value as of the date of acquisition on the time value of money and medical technology investment risk factors. Please see Note 3 for a discussion of the risk-adjusted discount rates used to discount projected cash flows for the in-process projects the Company acquired in connection with its 2008 acquisitions. The Company did not acquire any such projects during fiscal 2007. The Company believes that the estimated purchased research and development amounts so determined represent the fair value at the date of acquisition and do not exceed the amount a third party would pay for the projects.
The Company also uses the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including developed technology, customer relationships and trade names. Developed technology represents patented and unpatented technology and know-how. Customer
F-13
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Tradenames represent acquired product names that the Company intends to continue to utilize.
Intangible Assets and Goodwill
Intangible Assets
The majority of the Company’s intangible assets arose in connection with its business combinations. These intangible assets were recorded at fair value and are stated net of accumulated amortization and impairments. The Company amortizes its intangible assets that have finite lives using either the straight-line method, or if reliably determinable, based on the pattern in which the economic benefit of the asset is expected to be consumed utilizing expected undiscounted future cash flows. Amortization is recorded over the estimated useful lives ranging from 2 to 30 years. The Company evaluates the realizability of its definite lived intangible assets, whenever events or changes in circumstances or business conditions indicate that the carrying value of these assets may not be recoverable based on expectations of future undiscounted cash flows for each asset group. If the carrying value of an asset exceeds its undiscounted cash flows, the Company will write-down the carrying value of the intangible asset to its fair value in the period identified. The Company generally calculates fair value as the present value of estimated future cash flows to be generated by the asset using a risk-adjusted discount rate. If the estimate of an intangible asset’s remaining useful life is changed, the Company will amortize the remaining carrying value of the intangible asset prospectively over the revised useful life.
As a result of the Company’s conclusion that an interim impairment test of goodwill was required as of December 27, 2008 (as discussed below), the Company performed an impairment test of certain long-lived assets as of December 27, 2008. The impairment evaluation was based on expectations of future undiscounted cash flows compared to the carrying value of the long-lived asset groups. The Company’s cash flow estimates were based upon historical cash flows, as well as future projected cash flows derived from the Company-wide annual planning process and updated interim forecasting process. The Company believes that its procedures for estimating future cash flows were reasonable and consistent with market conditions at the time of estimation. The results of the Company’s interim impairment testing indicated that there was no impairment of its long-lived assets as of December 27, 2008. In those instances where indicators of impairment were identified, the Company performed an impairment test consistent with the method described above.
Subsequent to the merger with Cytyc, the Company decided to discontinue the development of Cytyc’s Helica product and determined it would not realize any future cash flows from this product. The Company’s intangible asset valuation for Cytyc included approximately $2,900 related to customer relationships for Helica. As a result of the Helica product discontinuation, the Company recorded an impairment charge, as a component of its GYN Surgical segment, of $2,900 in the first quarter of fiscal 2008.
During the second quarter of fiscal 2009, the Company decided to discontinue selling a certain product within the Diagnostic reporting segment as a result of communications from the FDA regarding the approval process. The Company believes that its decision was an indicator of impairment, and therefore, the Company performed an impairment test in accordance with ASC 360-10-35-15. The Company determined that the undiscounted cash flows to be generated by the asset group over its remaining estimated useful life would not be sufficient to recover the carrying value of the asset group. Due to the insufficient cash flows to be generated, the Company determined that the asset group’s fair value was de minimus and recorded an impairment charge of $4,065 comprised of developed technology of $2,594 and capitalized license fees of $1,471. This charge is reflected in cost of product sales in the Consolidated Statement of Operations for fiscal 2009.
F-14
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
During the third quarter of fiscal 2009, the Company acquired certain developed technology of approximately $5,400.
On April 4, 2008, the Company sold its CT CAD technology, acquired as part of its acquisition of R2 Technology in fiscal 2006. As a result of this sale, the Company reduced the net book value of its developed technology in the Breast Health reporting segment by $11,000 during the year ended September 27, 2008.
Intangible assets consist of the following:
| | | | | | | | | | | | |
Description | | As of September 26, 2009 | | As of September 27, 2008 |
| | Gross
Carrying
Value | | Accumulated
Amortization | | Gross
Carrying
Value | | Accumulated
Amortization |
Developed Technology | | $ | 2,137,711 | | $ | 267,259 | | $ | 2,135,688 | | $ | 112,568 |
Customer Relationships | | | 484,993 | | | 63,494 | | | 484,136 | | | 22,509 |
Trade Names | | | 146,965 | | | 20,094 | | | 146,963 | | | 9,950 |
Patents | | | 11,513 | | | 7,771 | | | 11,183 | | | 7,544 |
Capitalized License Fees | | | 2,766 | | | 518 | | | 4,364 | | | 112 |
| | | | | | | | | | | | |
Totals | | $ | 2,783,948 | | $ | 359,136 | | $ | 2,782,334 | | $ | 152,683 |
| | | | | | | | | | | | |
Amortization expense related to developed technology, capitalized license fees and patents is classified as a component of cost of product sales—amortization of intangible assets in the Consolidated Statements of Operations. Amortization expense related to customer relationship and trade name is classified as a component of amortization of intangible assets in the Consolidated Statements of Operations.
The estimated remaining amortization expense at September 26, 2009 for each of the five succeeding fiscal years:
| | | |
Fiscal 2010 | �� | $ | 228,467 |
Fiscal 2011 | | | 232,795 |
Fiscal 2012 | | | 234,148 |
Fiscal 2013 | | | 224,465 |
Fiscal 2014 | | | 215,119 |
Goodwill
In accordance with ASC 350,Intangibles—Goodwill and Other(formerly SFAS No. 142,Goodwill and Other Intangible Assets), the Company tests goodwill at the reporting unit level for impairment on an annual basis and between annual tests if events and circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business, and an adverse action or assessment by a regulator.
In performing the impairment test, the Company utilizes the two-step approach prescribed under ASC 350. The first step requires a comparison of the carrying value of each reporting unit to its estimated fair value. To
F-15
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
estimate the fair value of its reporting units for Step 1, the Company primarily utilizes the income approach. The income approach is based on a discounted cash flow analysis (“DCF”) and calculates the fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting the after-tax cash flows to a present value using a risk-adjusted discount rate. Assumptions used in the DCF require the exercise of significant judgment, including judgment about appropriate discount rates and terminal values, growth rates, and the amount and timing of expected future cash flows. The forecasted cash flows are based on the Company’s most recent budget and for years beyond the budget, the Company’s estimates are based on assumed growth rates. The Company believes its assumptions are consistent with the plans and estimates used to manage the underlying businesses. The discount rates, which are intended to reflect the risks inherent in future cash flow projections, used in the DCF are based on estimates of the weighted-average cost of capital (“WACC”) of market participants relative to each respective reporting unit. The market approach considers comparable market data based on multiples of revenue or earnings before taxes, depreciation and amortization (“EBITDA”). The Company believes its assumptions used to determine the fair value of its respective reporting units are reasonable. If different assumptions were used, particularly with respect to forecasted cash flows, WACCs, or market multiples, different estimates of fair value may result and there could be the potential that an impairment charge could result. Actual operating results and the related cash flows of the reporting units could differ from the estimated operating results and related cash flows.
If the carrying value of a reporting unit exceeds its estimated fair value, the Company is required to perform the second step of the goodwill impairment test to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of a reporting unit’s goodwill to its carrying value. The implied fair value of goodwill is derived by performing a hypothetical purchase price allocation for each reporting unit as of the measurement date, allocating the reporting unit’s estimated fair value to its assets and liabilities. The residual amount from performing this allocation represents the implied fair value of goodwill. To the extent this amount is below the carrying value of goodwill, an impairment charge is recorded.
The Company conducted its annual impairment test for its reporting units as of the first day of the fourth quarter of fiscal 2009. In order to complete the annual impairment test, the Company updated its interim impairment test results (see below) and performed detailed analysis estimating the fair value of its reporting units utilizing its fiscal 2010 forecast with updated long-term growth assumptions. For one reporting unit, the Company utilized the results of its interim impairment test. Pursuant to ASC 350-20-35-29 (formerly paragraph 27 of SFAS 142), the Company concluded that it met the required criteria to use the estimated fair value determined from its interim impairment analysis for this reporting unit because 1) the composition of the assets and liabilities of this reporting unit had not changed significantly since the most recent fair value determination, 2) the most recent fair value determination resulted in a fair value that exceeded the carrying value of the reporting unit by a substantial margin after consideration of the interim goodwill charge, and 3) management concluded, based on an analysis of current events that have occurred and circumstances that have changed since the most recent fair value determination, that it was remote that the current fair value of the reporting unit would not exceed its carrying amount.
As a result of completing Step 1, all of the Company’s reporting units, except one, had a fair value exceeding their carrying value, and as such, Step 2 of the impairment test was not required for these reporting units. For the reporting unit that failed Step 1, the Company completed Step 2, consistent with the procedures described above, and determined that an impairment charge was not required due to the fair value of the implied goodwill exceeding the carrying value of the reporting unit’s goodwill. If the fair value of this reporting unit at June 28, 2009 had been lower by 10%, the Company still would not have recorded an impairment charge. If the fair value of the Company’s other reporting units had been lower by 10%, two reporting units would have failed
F-16
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Step 1 requiring a Step 2 analysis. These reporting units, one in the Diagnostics reportable segment and one in the Skeletal Health reportable segment, had fair values at this date that exceeded their carrying values by 9% and 2%, respectively, and goodwill of $236.0 million and $8.2 million, respectively. The fair value of these reporting units is determined by use of the DCF. As noted above, the key assumptions that drive the fair value in this model are the WACC, terminal values, growth rates, and the amount and timing of expected future cash flows. If the current worldwide financial markets and economic environment were to deteriorate, this would likely result in a higher WACC because market participants would require a higher rate of return. In the DCF as the WACC increases, the fair value decreases. The other significant factor in the DCF is our projected financial information (i.e., amount and timing of expected future cash flows and growth rates) and if these assumptions were to be adversely impacted, this could result in a reduction of the fair values of these reporting units. For the Company’s other reporting units with goodwill aggregating $1.77 billion, the Company believes that these reporting units are not at risk of failing Step 1 of the goodwill impairment test.
During the first quarter of fiscal 2009, based upon a combination of factors, including the deteriorating macro-economic environment, declines in the stock market and the decline of the Company’s market capitalization significantly below the book value of the Company’s net assets, the Company concluded that potential goodwill impairment indicators existed as of December 27, 2008. As a result, the Company performed an interim goodwill impairment analysis as of December 27, 2008 in accordance with ASC 350. As noted above, the Company utilized DCF and market approaches to estimate the fair value of its reporting units as of December 27, 2008 and believes it has used reasonable estimates and assumptions about future revenue, cost projections, cash flows and market multiples. In addition, using a DCF requires the use of a risk-adjusted discount rate for which the Company based its rate on the WACC of market participants. The Company performed a peer company analysis and considered the industry weighted average return on debt and equity from a market participant perspective for its reporting units. Given the disruptions in the credit and equity markets, the WACCs for each reporting unit increased between the Company’s annual test performed on the first day of its fourth quarter of fiscal 2008 and the interim test performed as of December 27, 2008. The long-term growth rates are largely consistent with those applied in the fiscal 2008 annual test, except for MammoSite, which is a reporting unit in Breast Health, in which the long-term growth rate declined due to current competitive pressures on the reporting unit’s products, as well as recent regulatory and reimbursement changes. The Step 1 impairment analysis indicated that the carrying value of the net assets of three of the Company’s reporting units, acquired in connection with the Cytyc acquisition, exceeded the estimated fair value of those reporting units. As a result, the Company was required to perform Step 2 of the goodwill impairment test to determine the amount, if any, of goodwill impairment charges for each of the applicable reporting units. Due to the complexities and time involved in preparing the Step 1 analysis, the Company had not commenced the Step 2 analysis as of February 5, 2009, the date it filed its Form 10-Q for the quarter ended December 27, 2008. As a result of the fact that the Company had not commenced the Step 2 analysis and the complexity of the analysis required to complete the Step 2 analysis, the Company was unable to determine that an impairment loss, in accordance with ASC 450,Contingencies(formerly SFAS No. 5,Accounting for Contingencies), was both probable and reasonably estimable at December 27, 2008.
The Company completed the Step 2 analysis during its second quarter of fiscal 2009, which resulted in an aggregate goodwill impairment charge of $2,340,023. This impairment charge is comprised of $1,165,804 for GYN Surgical, $908,349 for Diagnostics, and $265,870 for Breast Health. The impairment charges for GYN Surgical and Diagnostics are primarily attributable to the assumption of higher discount rates compared to those used in the annual impairment test performed as of the first day of the fourth quarter of fiscal 2008 (the July 2008 valuation) and the assumption that the reporting units would be purchased or sold in a taxable transaction in accordance with EITF Issue No. 02-13,Deferred Income Tax Considerations in Applying the GoodwillImpairment Test in FASB Statement No. 142 (codified within ASC 350,Intangibles-Goodwill and Other). The
F-17
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
impairment charge for MammoSite is a result of a combination of a higher discount rate and lower projected future cash flows compared to those used in the July 2008 valuation. The higher discount rates for the three reporting units, which range from 10% to 13.5% compared to 9% to 10% used in the July 2008 valuation, reflected an increase in the risks inherent in the estimated future cash flows and the higher rate of return a market participant would require based on the macro-economic environment at the measurement date. The reduction in forecasted cash flows for the MammoSite reporting unit is due to current competitive pressure on the reporting unit’s products as well as recent regulatory and reimbursement changes.
The Company also evaluated the aggregate fair value of its reporting units compared to its market capitalization noting an implied control premium of approximately 16% at December 27, 2008. The Company used an average of its market capitalization over the 30 calendar days preceding the impairment testing date as being more reflective of its market value than a single day, point-in-time market price. The Company concluded that its implied control premium was reasonable when compared to industry specific information.
For illustrative purposes, had the fair values of each reporting unit for which the Company has recorded goodwill impairment charges in the second quarter of fiscal 2009 been lower by 10% as of December 27, 2008, the Company would have recorded an additional impairment charge of $435,480. Based on the Company’s estimates as of December 27, 2008, the impact of reducing the Company’s fair value estimates for its other reporting units, for which the Company did not record any goodwill impairment charges, by 10% would have no impact on the Company’s goodwill assessment for those reporting units.
The Company believes that the procedures performed and the estimates and assumptions used in the Step 1 and Step 2 analyses for each reporting unit are reasonable and in accordance with the guidelines for acquisition accounting under U.S. generally accepted accounting principles.
The estimate of fair value requires significant judgment. Any loss resulting from the goodwill impairment analysis is reflected in operating (loss) income in the Company’s Consolidated Statements of Operations. The impairment testing process is subjective and requires judgment at many points throughout the analysis. If these estimates or their related assumptions change in the future, the Company may be required to record impairment charges for these assets not previously recorded. Impairment charges related to goodwill have no impact on the Company’s cash balances or compliance with financial covenants under its Amended and Restated Credit Agreement.
In prior years, the Company conducted its annual impairment test of goodwill for certain of its reporting units (its historical reporting units prior to the merger with Cytyc) as of the last day of the second quarter. In the fourth quarter of fiscal 2008, the Company changed the measurement date from the last day of its second quarter to the first day of its fourth quarter, in order to provide additional time to determine the fair value of its reporting units and to evaluate the results of the impairment testing. This change did not delay, accelerate or avoid an impairment charge. In addition, this change did not have any effect on the Company’s financial performance or results of operations, nor was there any impact on prior periods’ financial statements under the requirements of ASC 250,Accounting Changes and Error Corrections(formerly SFAS No. 154,Accounting Changes and Error Corrections). The retrospective application as required under this accounting guidance was not necessary as no impairment charges had been recorded in any previously recorded financial statements nor did the change in measurement date cause any impairments.
As a result of the change in the measurement date for the Company’s annual goodwill impairment test for its historical reporting units from the last day of the second quarter to the first day of the fourth quarter, the Company evaluated, in accordance with ASC 350-20-35-9, whether the detailed determination of fair value of its
F-18
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
historical reporting units as of March 29, 2008 could be carried forward to the first day of its fiscal fourth quarter of 2008 or if a new test of goodwill impairment was required to be performed for these historical reporting units. In its evaluation, the Company noted that the assets and liabilities of the reporting units had not changed significantly, there was sufficient margin between the carrying amount and fair value determination for each reporting unit and no events or circumstances related to these reporting units would suggest that a current fair value determination of reporting units would result in a valuation lower than the carrying amount of the reporting units. Based on this evaluation, the Company believed it sufficiently met the requirements to carry forward its estimate of fair value for these reporting units.
The Company conducted its fiscal 2008 annual impairment test of goodwill for its new reporting units as a result of the Company’s acquisition of Cytyc Corporation as of the first day of the fourth quarter of fiscal 2008. The fair value of each reporting unit was determined to be in excess of each reporting unit’s carrying value and as a result the second step of the impairment test was not required.
A rollforward of goodwill activity from September 29, 2007 to September 26, 2009 is as follows:
| | | | |
Balance as of September 29, 2007 | | $ | 407,528 | |
Merger with Cytyc. | | | 3,844,100 | |
Acquisition of Third Wave. | | | 241,785 | |
Contingent consideration related to Suros acquisition | | | 24,467 | |
Estimated tax benefit of vested converted options exercised after acquisition. | | | (49,630 | ) |
Cytyc purchase price adjustments | | | (14,227 | ) |
Other purchase price adjustments and foreign currency translation impact | | | (3,527 | ) |
| | | | |
Balance as of September 27, 2008 | | | 4,450,496 | |
Impairment of goodwill | | | (2,340,023 | ) |
Cytyc purchase price adjustments | | | (2,930 | ) |
Third Wave purchase price adjustments | | | 1,450 | |
Contingent consideration related to Adiana | | | 1,854 | |
Other purchase price adjustments and foreign currency translation adjustment | | | (1,884 | ) |
| | | | |
Balance as of September 26, 2009 | | $ | 2,108,963 | |
| | | | |
The other purchase price adjustments in fiscal 2009 and 2008 substantially relate to the adjustment of R2 and Suros tax liabilities. R2 and Suros were acquired in fiscal 2006.
During fiscal 2008, as a result of the merger with Cytyc, the Company reallocated its segment allocation of goodwill to reflect expected revenue synergies in its historical reporting segments. Accordingly, the Company recorded an increase in goodwill allocated to its Breast Health and Skeletal Health segments in the amount of $502,800 and $7,600, respectively in fiscal 2008. The allocation of goodwill by reporting segment consists of the following:
| | | | | | |
Reporting Segment | | Balance as of
September 26, 2009 | | Balance as of
September 27, 2008 |
Breast Health | | $ | 662,735 | | $ | 930,672 |
Diagnostics | | | 578,290 | | | 1,486,988 |
GYN Surgical | | | 859,739 | | | 2,024,639 |
Skeletal Health | | | 8,199 | | | 8,197 |
| | | | | | |
| | $ | 2,108,963 | | $ | 4,450,496 |
| | | | | | |
F-19
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Other Assets
As of September 26, 2009 and September 27, 2008, other assets were comprised primarily of deferred financing costs, cost-method investments and Company owned life insurance contracts.
As of September 26, 2009 and September 27, 2008, other assets included $34,622 and $52,055, respectively, of deferred financing costs related to the Company’s Convertible Notes and the Company’s Amended Credit Agreement (See Note 5). The Company was initially amortizing deferred financing costs related to the Amended Credit Agreement, which was executed in 2008, to interest expense over a five year period; however, as the Company has repaid principal early, it has accelerated amortization of the deferred financing costs. Interest expense related to the amortization of deferred financing costs for the Amended Credit Agreement was $11,738 and $3,532 for fiscal 2009 and 2008, respectively. The Company is amortizing amounts related to the Convertible Notes on a straight-line basis over the period of earliest redemption, which is a six year period. As a result, the Company recorded interest expense related to the amortization of deferred financing costs of $6,004 and $4,775 for fiscal 2009 and 2008, respectively. In connection with the Convertible Notes offering and other voluntary repayments, the Company’s term loans under the original Credit Agreement were repaid, and the Company accelerated the amortization of the related deferred financing costs resulting in total interest expense of $11,516 relating to these term loans in fiscal 2008. Additionally, the Company recorded $718 of interest expense related to its unamortized deferred financing costs upon the termination of its credit facility with Bank of America during fiscal 2008.
Other assets also include certain other cost-method investments in non-publicly traded equity securities aggregating $7,585 and $9,278 for fiscal 2009 and 2008, respectively. These investments are generally carried at cost as the Company owns less than 20% of the voting equity and does not have the ability to exercise significant influence over these companies. The Company regularly evaluates the carrying value of its cost-method investments for impairment and whether any events or circumstances are identified that would significantly harm the fair value of the investment. The indicators the Company utilizes to identify these events and circumstances include (1) the investee’s revenue or earnings trends compared to budgets and pre-defined milestones, (2) the technological feasibility of the investee’s products and technologies, (3) general market conditions in the investee’s industry including adverse regulatory or economic changes, (4) factors related to the investee’s ability to remain in business, such as the investee’s liquidity and rate of cash use, and (5) the investee’s ability to secure additional funding and the value of that additional funding. In the event a decline in fair value is judged to be other-than-temporary, the Company will record an other-than-temporary impairment charge in Other income (expense), net in the Consolidated Statements of Operations. During fiscal 2009, the Company recorded other-than-temporary impairment charges totaling $2,243 related to certain of its cost method investments to adjust their carrying amounts to fair value.
The Company owned life insurance contracts included in other assets primarily include contracts that were purchased in connection with the Company’s Supplemental Executive Retirement Plan (“SERP”) and were valued at $11,602 as of September 26, 2009 and $5,575 as of September 27, 2008 (See Note 11 for further discussion).
Research and Software Development Costs
Costs incurred in the research and development of the Company’s products are expensed as incurred. Nonrefundable advance payments for goods or services to be received in the future by the Company for use in research and development activities are deferred and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed. If the Company’s expectations change such that it does not expect it will need the goods to be delivered or the services to be rendered, capitalized nonrefundable advance payments are charged to expense in that period.
F-20
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The Company accounts for the development costs of software embedded in the Company’s products for which revenues are recognized pursuant to ASC 985-605,Software Revenue Recognition(formerly AICPA SOP 97-2,Software Revenue Recognition), in accordance with ASC 985,Software (formerlySFAS No. 86,Accounting for the Costs of Computer Software to be Sold, Leased or Otherwise Marketed. Costs incurred in the research, design and development of software embedded in products to be sold to customers are charged to expense until technological feasibility of the ultimate product to be sold is established. Software development costs incurred after the establishment of technological feasibility and until the product is available for general release are capitalized, provided recoverability is reasonably assured. Software development costs eligible for capitalization have not been significant to date.
Foreign Currency Translation
The financial statements of the Company’s foreign subsidiaries are translated in accordance with ASC 830,Foreign Currency Matters(formerly SFAS No. 52,Foreign Currency Translation).The reporting currency for the Company is the U.S. dollar. With the exception of its Costa Rica subsidiary, whose functional currency is the U.S. dollar, the functional currency of the Company’s subsidiaries is their local currency. Accordingly, the assets and liabilities of these subsidiaries are translated into U.S. dollars using the exchange rate in effect at each balance sheet date. Before translation, the Company re-measures foreign currency denominated assets and liabilities, including inter-company accounts receivable and payable, into the functional currency of the respective entity, resulting in unrealized gains or losses recorded in other (expense) income, net in the Consolidated Statement of Operations. Revenues and expenses are translated using average exchange rates during the respective period. Foreign currency translation adjustments are accumulated as a component of other comprehensive income as a separate component of stockholders’ equity. Gains and losses arising from transactions denominated in foreign currencies are included in other (expense) income, net on the Consolidated Statements of Operations and to date have not been material.
Comprehensive (Loss) Income
ASC 220,Comprehensive Income(formerly SFAS No. 130, Reporting Comprehensive Income), requires the financial statements to include the reporting of comprehensive (loss) income, which includes net (loss) income and certain transactions that have generally been reported in the statement of shareholders’ equity. Comprehensive (loss) income is disclosed in the Consolidated Statements of Stockholders’ Equity and Comprehensive (Loss) Income.
Accumulated other comprehensive income, net of tax, consists of the following as of September 26, 2009 and September 27, 2008:
| | | | | | |
| | | 2009 | | 2008 |
Foreign currency translation adjustment | | $ | 4,669 | | $ | 3,003 |
Minimum pension liability, net of tax of $1,011 and $832, respectively | | | 2,359 | | | 1,942 |
| | | | | | |
| | $ | 7,028 | | $ | 4,945 |
| | | | | | |
F-21
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Revenue Recognition
The Company generates revenue from the sale of its products, primarily capital equipment and disposable supplies, and related services.
The Company recognizes product revenue upon shipment provided that there is persuasive evidence of an arrangement, there are no uncertainties regarding acceptance, the sales price is fixed or determinable, no right of return exists and collection of the resulting receivable is probable. Generally, the Company’s product arrangements for capital equipment sales, primarily in Breast Health and Skeletal Health, are multiple-element arrangements, including services, such as installation and training and multiple products. In accordance with ASC 605-25,Multiple Element Arrangements(formerly Emerging Issues Task Force (“EITF”) Issue No. 00-21,Accounting for Revenue Arrangements with Multiple Deliverables), based on the terms and conditions of the product arrangements, the Company believes that these services and undelivered products can be accounted for separately from the delivered product element as the Company’s delivered product has value to its customers on a stand-alone basis and the Company has objective and reliable evidence of the fair value of such services and undelivered products. Accordingly, service revenue representing the fair value of services not yet performed at the time of product shipment is deferred and recognized as such services are performed. The fair value of the undelivered products is also deferred at the time of product shipment and recognized when these products are delivered. The residual revenue under the product arrangement is recognized as product revenue upon shipment or installation as discussed. There is no customer right of return in the Company’s sales agreements.
The Company recognizes product revenue upon the completion of installation for products whose installation is essential to its functionality, primarily related to its digital imaging systems. A provision is made at that time for estimated warranty costs to be incurred.
Service revenues primarily consist of amounts recorded under service and maintenance contracts and repairs not covered under warranty, installation and training revenues and shipping and handling costs billed to customers. Service and maintenance contract revenues are recognized ratably over the term of the contract. Other service revenues are recorded when the services are delivered.
Although certain of the Company’s products contain operating and application software, the Company has determined that except for its CAD (computer aided detection) products obtained with the acquisition of R2 Technology, Inc. and the newly released Dimensions 2D/3D full field digital mammography product (“Dimensions”), the software element is incidental in accordance with the software revenue recognition rules.
The Company has determined ASC 985-605,Software—Revenue Recognition(formerly SOP 97-2)applies to revenue transactions for its CAD systems and Dimensions product (see below), which are included in Breast Health. ASC 985-605 generally requires revenue earned on software arrangements involving multiple elements to be allocated to each element based on the relative fair values of the elements. Revenue recognized from multi-element arrangements is allocated to each element of the arrangement using the residual method based on the fair value of the undelivered elements. The Company’s determination of fair value of the undelivered elements in the multi-element arrangements is based on vendor-specific objective evidence (“VSOE”). The Company limits its assessment of VSOE for each element to either the price charged when the same element is sold separately or the price established by management, having the relevant authority to do so for an element not yet sold separately. The Company recognizes revenue on CAD systems and Dimensions product sales upon completion of installation at which time the only remaining undelivered element is Post Contract Support (“PCS”).
F-22
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Upon its release, the Company completed an evaluation of the software component of its Dimensions product in accordance with the software revenue recognition rules. The Company noted the following in its evaluation of the software component of its new Dimensions product:
| | • | | Dimensions is offered in different configurations providing different levels of functionality (2D vs. 3D). Customers who purchase the 2D configuration will be to able to upgrade the product to a 3D version and such upgrade will solely represent a software upgrade that will be marketed and sold separately. This differentiation from the Company’s existing 2D digital mammography product is expected to be highlighted in the Company’s marketing literature. |
| | • | | As part of the initial warranty of the Dimensions product, customers will receive not only bug fixes related to the software but also will receive any updates and enhancements to the software that are released. Therefore, the Company concluded that this represents PCS as defined in the software revenue recognition rules. |
As a result, the Company has determined that the Dimensions product contains software that is more than incidental to the product as a whole and should be accounted for under the software revenue recognition rules. The Company recognizes revenue upon installation and acceptance, if required, and defers the VSOE of fair value of the initial bundled PCS. The Company has determined that VSOE of fair value of the initial bundled PCS exists based on the establishment of a price for which this element will be sold separately by management having the relevant authority and that it is probable that this price will not change prior to when this service is sold separately. The Company has specified the renewal rates at which the PCS service can be purchased separately for upon expiration of the initial PCS period and those rates are consistent among its customers.
For multiple-element arrangements where VSOE of fair value of PCS has been established, the Company recognizes revenue using the residual method at the time all other revenue recognition criteria have been met. Amounts attributable to PCS are recorded as deferred revenue and recognized ratably over the contractual term of PCS.
Under customer usage agreements, the Company installs certain equipment (for example, a ThinPrep Processor or a ThinPrep Imaging System) at customer sites and customers commit to purchasing minimum quantities of disposable supplies at a stated price (generally including a usage fee for the equipment) over a defined contract term, which is typically between three and five years. Revenue is recognized over the term of the customer usage agreement as disposable supplies are delivered. The Company also rents certain equipment to customers. Revenues from rental agreements are recorded over the terms of the rental agreements.
Accounts Receivable and Reserves
The Company records reserves for doubtful accounts based upon a specific review of all outstanding invoices, known collection issues and historical experience. The Company regularly evaluates the collectability of its trade accounts receivables and performs ongoing credit evaluations of its customers and adjusts credit limits based upon payment history and its assessment of the customer’s current credit worthiness. These estimates are based on specific facts and circumstances of particular orders, analysis of credit memo data and other known factors.
F-23
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Accounts receivable reserve activity for the years ended September 26, 2009, September 27, 2008 and September 29, 2007 is as follows:
| | | | | | | | | | | | | | | | | |
| | | Balance at
Beginning
of Period | | Acquisition
and Other
Adjustments | | | Charged to
Costs and
Expenses | | Write-
offs and
Payments | | | Balance at
End of
Period |
Period Ended: | | | | | | | | | | | | | | | | | |
September 26, 2009 | | $ | 6,326 | | $ | — | | | $ | 2,334 | | $ | (1,381 | ) | | $ | 7,279 |
September 27, 2008 | | $ | 4,598 | | $ | (206 | ) | | $ | 2,109 | | $ | (175 | ) | | $ | 6,326 |
September 29, 2007 | | $ | 3,712 | | $ | (20 | ) | | $ | 947 | | $ | (41 | ) | | $ | 4,598 |
Cost of Service and Other Revenues
Cost of service and other revenues primarily represents payroll and related costs associated with the Company’s professional services’ employees, consultants, infrastructure costs and overhead allocations, including depreciation and rent and materials consumed in providing the service.
Stock-Based Compensation
The Company accounts for share-based payments in accordance with ASC 718,Stock Compensation(formerly SFAS 123(R)Share-Based Payments). As such, all share-based payments to employees, including grants of stock options and restricted stock units, are recognized in the statement of operations based on their fair values as the date of grant. The Company adopted SFAS 123(R) using the “modified prospective” transition method in which compensation cost is recognized beginning with the effective date (a) based on the requirements of SFAS 123(R) for all share-based payments granted after the effective date and (b) based on the requirements of SFAS 123 for all awards granted to employees prior to the effective date of SFAS 123(R) that remain unvested on the effective date. As a result, the Company is recognizing compensation for the fair value of the unvested portion of option grants issued prior to the adoption of SFAS 123(R), whose fair value was calculated utilizing a Black-Scholes Option Pricing Model. In accordance with the modified-prospective transition method of SFAS 123(R), results for prior periods have not been restated.
Net (Loss) Income Per Share
Basic net (loss) income per share is computed by dividing net (loss) income by the weighted average number of common shares outstanding. Diluted net (loss) income per share is computed by dividing net (loss) income by the weighted average number of common shares and potential common shares from outstanding stock options, restricted stock units and convertible debt determined by applying the treasury stock method. In accordance with ASC 718 the assumed proceeds under the treasury stock method include the average unrecognized compensation expense of stock options that are in-the-money and restricted stock units.
The Company applies the provisions of ASC 260,Earnings Per Share,Subsection 10-45-44 (formerly EITF No. 04-8,The Effect of Contingently Convertible Instruments on Diluted Earnings per Share),to determine diluted weighted average shares outstanding as it relates to its outstanding Convertible Notes, and due to the type of debt instrument issued, the dilutive impact of the Company’s Convertible Notes is based on the difference between the Company’s current stock price and the conversion price of the Convertible Notes, provided there is a premium. Pursuant to this accounting guidance, there is no dilution from the accreted principal of the Convertible Notes. Accordingly, the Company uses the treasury stock method to determine dilutive weighted average shares related to its Convertible Notes and not the if-converted method.
F-24
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
A reconciliation of basic and diluted share amounts for fiscal years 2009, 2008, and 2007 are as follows:
| | | | | | | | | | | |
| | | September 26,
2009 | | | September 27,
2008 | | | September 20,
2007 |
Numerator: | | | | | | | | | | | |
Net (loss) income | | $ | (2,176,237 | ) | | $ | (385,617 | ) | | $ | 94,578 |
| | | | | | | | | | | |
Denominator: | | | | | | | | | | | |
Basic weighted average common shares outstanding | | | 256,545 | | | | 245,968 | | | | 106,873 |
Weighted average common stock equivalents from assumed exercise of stock options and restricted stock units | | | — | | | | — | | | | 2,796 |
| | | | | | | | | | | |
Diluted weighted average common shares outstanding | | | 256,545 | | | | 245,968 | | | | 109,669 |
| | | | | | | | | | | |
Basic net (loss) income per common share | | $ | (8.48 | ) | | $ | (1.57 | ) | | $ | 0.88 |
| | | | | | | | | | | |
Diluted net (loss) income per common share | | $ | (8.48 | ) | | $ | (1.57 | ) | | $ | 0.86 |
| | | | | | | | | | | |
Weighted-average anti-dilutive shares related to: | | | | | | | | | | | |
Outstanding stock options | | | 13,489 | | | | 7,303 | | | | 1,316 |
Restricted stock units | | | 1,575 | | | | 132 | | | | 168 |
Diluted weighted average shares outstanding do not include any effect resulting from the conversion of the Company’s Convertible Notes issued in December 2007 as their impact would be anti-dilutive for all periods presented. In those reporting periods in which the Company has reported net income, anti-dilutive shares comprise those common stock equivalents that have either an exercise price above the average stock price for the period or the common stock equivalents related average unrecognized stock compensation expense is sufficient to “buy back” the entire amount of shares. In those reporting periods in which the Company has a net loss, anti-dilutive shares comprise the impact of those number of shares that would have been dilutive had the Company had net income plus the number of common stock equivalents that would be anti-dilutive had the company had net income.
Product Warranties
The Company generally offers a one-year warranty for its products. The Company provides for the estimated cost of product warranties at the time product revenue is recognized. Factors that affect the Company’s warranty reserves include the number of units sold, historical and anticipated rates of warranty repairs and the cost per repair. The Company periodically assesses the adequacy of the warranty reserve and adjusts the amount as necessary.
Product warranty activity for the years ended September 26, 2009 and September 27, 2008 is as follows:
| | | | | | | | | | | | | | | | |
| | | Balance at
Beginning of
Period | | Charged to
Costs and
Expenses | | Acquired
Reserves | | Cost
Incurred | | | Balance at End
of Period |
Period end: | | | | | | | | | | | | | | | | |
September 26, 2009 | | $ | 9,109 | | $ | 4,937 | | $ | | | $ | (8,444 | ) | | $ | 5,602 |
September 27, 2008 | | $ | 12,087 | | $ | 5,223 | | $ | 591 | | $ | (8,792 | ) | | $ | 9,109 |
F-25
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Restructuring Charges and Accrual
In the fourth quarter of fiscal 2009, the Company closed its manufacturing facility in Shanghai, China. This facility, which manufactured organic photoconductor drum coatings, was acquired in connection with the AEG acquisition in 2006. The Company recorded, as restructuring charges, severance benefits of $420 and other costs of $377. The severance benefits were paid to the employees as of September 26, 2009. In connection with this action, the Company ceased production during the fourth quarter of 2009 and recorded impairment charges of $661 in cost of product sales for manufacturing equipment that has no further utility. The Company expects to incur additional compensation and other clean-up expenses to complete the closure of fully close the facility, which is expected to be completed during fiscal 2010. These costs are not expected to be material. In addition, since this subsidiary is deemed to be substantially liquidated pursuant to the foreign currency translation accounting rules, the Company has eliminated its cumulative translation adjustment related to this subsidiary resulting in other income of $726, which has been recorded as a component of Other (expense) income, net in the Consolidated Statements of Operations.
In fiscal 2008, the Company recorded a restructuring charge of $6,383 related to the resignation of its former Chairman of the Board of Directors, which is not included in the table below. On May 20, 2008, the Company entered into a Separation and Release Agreement (the “Separation Agreement”) with Patrick J. Sullivan, Chairman of the Board of Directors of the Company. The Separation Agreement required the Company to pay Mr. Sullivan a total of $4,442 and continue to pay Mr. Sullivan’s premiums for COBRA continuation coverage under the Company’s group medical plan for eighteen months following the effective date of the separation. In addition, the Separation Agreement provided that Mr. Sullivan’s 46 restricted stock units granted on October 22, 2007 would become fully vested, and the time period to exercise all of his outstanding stock options, all of which were fully vested, would be extended so as to remain exercisable until August 31, 2009. The acceleration of the restricted stock units and modification of stock options resulted in a stock-based compensation charge of $1,941.
In fiscal 2008, as a result of the merger with Cytyc, the Company assumed previous Cytyc management approved restructuring plans designed to reduce future operating expenses by consolidating its Mountain View, California operations into its existing operations in Costa Rica and Massachusetts as well as restructuring plans relating to Cytyc’s historical acquisitions completed in March 2007. In connection with these plans, the Company assumed a total liability of approximately $4,658. The Company assumed an arrangement in which Cytyc had sub-leasing all of its Mountain View facility to a third party for a term of approximately five years, a period of time equivalent to the remainder of the Company’s lease of this facility. The sub-lease commenced on July 1, 2007, and the sub-lease income under this arrangement exceeded the related lease obligation. The Company did not incur any additional restructuring costs related to these plans, and these costs were paid in full during fiscal 2009.
The Company also recorded a liability related to the merger with Cytyc in accordance with EITF Issue No. 95-3 (“EITF 95-3”),Recognition of Liabilities in Connection with a Purchase Business Combination, primarily related to the termination of certain employees, minimum inventory purchase commitments, and other contractual obligations for which business activities had been discontinued.
In fiscal 2008 as a result of the Third Wave acquisition, the Company assumed previous Third Wave management approved restructuring plans designed to reduce future operating expenses. In connection with these plans, the Company assumed a total liability related to termination benefits of approximately $7,509. The Company did not incur any additional restructuring costs related to retention costs for these employees.
F-26
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Changes in the restructuring accrual are as follows:
| | | | | | | | |
| | | Other | | | Termination
Benefits | |
Balance at September 29, 2007 | | $ | — | | | $ | 105 | |
Cytyc balance acquired, October 22, 2007 | | | — | | | | 4,658 | |
Third Wave balance acquired, July 24, 2008 | | | 261 | | | | 7,029 | |
Provided for under EITF No. 95-3 | | | 1,820 | | | | 1,020 | |
Adjustments | | | (382 | ) | | | (270 | ) |
Payments | | | (817 | ) | | | (11,233 | ) |
| | | | | | | | |
Balance at September 27, 2008 | | | 882 | | | | 1,309 | |
Current period charges | | | 377 | | | | 420 | |
Adjustments | | | (754 | ) | | | (479 | ) |
Payments | | | (130 | ) | | | (1,202 | ) |
| | | | | | | | |
Balance at September 26, 2009 | | $ | 375 | | | $ | 48 | |
| | | | | | | | |
Advertising Costs
Advertising costs are charged to operations as incurred. The Company does not have any direct-response advertising. Advertising costs, which include trade shows and conventions, were approximately $12,385, $15,281 and $6,683 for fiscal 2009, 2008 and 2007, respectively, and were included in selling and marketing expense in the Consolidated Statements of Operations.
Recently Issued Accounting Pronouncements
In September 2009, the FASB ratified ASC Update (“ASU”) No. 2009-13,Multiple-Deliverable Revenue Arrangements (formerly EITF 08-1), or ASU 2009-13. ASU 2009-13, amends existing revenue recognition accounting pronouncements that are currently within the scope of FASB Accounting Standards Codification, or ASC, Subtopic 605-25 (previously included within EITF Issue No. 00-21, Revenue Arrangements with Multiple Deliverables, or EITF 00-21). ASU 2009-13 provides for two significant changes to the existing multiple element revenue recognition guidance. First, this guidance deletes the requirement to have objective and reliable evidence of fair value for undelivered elements in an arrangement and will result in more deliverables being treated as separate units of accounting. The second change modifies the manner in which the transaction consideration is allocated across the separately identified deliverables. These changes may result in entities recognizing more revenue up-front, and entities will no longer be able to apply the residual method and defer the fair value of undelivered elements. Upon adoption of these new rules, each separate unit of accounting must have a selling price, which can be based on management’s estimate when there is no other means to determine the fair value of that undelivered item, and the arrangement consideration is allocated based on the elements’ relative selling price. This accounting guidance is effective no later than fiscal years beginning on or after June 15, 2010 but may be early adopted as of the first quarter of an entity’s fiscal year. Entities may elect to adopt this accounting guidance either through prospective application to all revenue arrangements entered into or materially modified after the date of adoption or through a retrospective application to all revenue arrangements for all periods presented in the financial statements. The Company is currently evaluating the impact of this revised accounting guidance, which it can adopt as early as the first quarter of fiscal 2010.
In September 2009, the FASB ratified ASU No. 2009-14,Applicability of SOP 97-2 to Certain Arrangements that Include Software Elements(formerly EITF Issue No. 09-3,Certain Revenue Arrangements
F-27
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
that Include Software Elements), which amends the existing accounting guidance for how entities account for arrangements that include both hardware and software, which typically resulted in the sale of hardware being accounted for under the software revenue recognition rules. This accounting guidance changes revenue recognition for tangible products containing software elements and non-software elements. The tangible element of the product is always outside of the scope of the software rules, and the software elements of tangible products when the software element and non-software elements function together to deliver the product’s essential functionality are outside of the scope of the software rules. As a result, both the hardware and qualifying related software elements are excluded from the scope of the software revenue guidance and accounted for under the revised multiple-element revenue recognition guidance. ASU 2009-14 is effective for all fiscal years beginning on or after June 15, 2010 with early adoption permitted. Entities must adopt ASU 2009-14 and ASU 2009-13 in the same manner and at the same time. The Company is currently evaluating the impact of this revised accounting guidance, which it can adopt as early as the first quarter of fiscal 2010.
In April 2009, the FASB issued FSP FAS 115-2 and FAS 124-2,The Meaning of Other-Than-Temporary Impairment and Its Applications to Certain Investments (codified within ASC 320Investments-Debt and Equity Securities) FSP 115-2/124-2 amends the requirements for the recognition and measurement of other-than-temporary impairments for debt securities by modifying the pre-existing “intent and ability” indicator. Under this FSP an other-than-temporary impairment is triggered when there is an intent to sell the security, it is more likely than not that the security will be required to be sold before recovery, or the security is not expected to recover the entire amortized cost basis of the security. Additionally, this FSP changes the presentation of an other-than-temporary impairment in the income statement for those impairments involving credit losses. The credit loss component will be recognized in earnings and the remainder of the impairment will be recorded in other comprehensive income. This FSP was effective for the Company beginning with the third quarter of fiscal 2009. The adoption of this FSP did not have a significant impact on the Company’s consolidated financial statements.
In December 2007, the FASB issued ASC 805,Business Combinations(formerly SFAS No. 141 (Revised 2007),Business Combinations). This Statement retains the fundamental requirements in SFAS 141 that the acquisition method of accounting (which SFAS 141 called the purchase method) be used for all business combinations and for an acquirer to be identified for each business combination. ASC 805 requires an acquirer to recognize the assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions specified in the Statement. ASC 805 replaces SFAS 141’s cost-allocation process, which required the cost of an acquisition to be allocated to the individual assets acquired and liabilities assumed based on their estimated fair values. The Statement retains the guidance in SFAS 141 for identifying and recognizing intangible assets separately from goodwill. ASC 805 will now require acquisition costs to be expensed as incurred, and changes in deferred tax asset valuation allowances and income tax uncertainties after the acquisition date generally to affect income tax expense. ASC 805 applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008, which is the Company’s 2010 fiscal year. Early adoption is prohibited. The Company is currently evaluating the impact that the adoption of ASC 805 will have on its consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements—An amendment of ARB No. 51(codified within ASC 810,Consolidation). SFAS 160 amends Accounting Research Bulletin (“ARB”) No. 51 to establish accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. The amount of net income attributable to the noncontrolling
F-28
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
interest will be included in consolidated net income on the face of the income statement. This accounting guidance clarifies that changes in a parent’s ownership interest in a subsidiary that do not result in deconsolidation are equity transactions if the parent retains its controlling financial interest. In addition, this Statement requires that a parent recognize a gain or loss in net income when a subsidiary is deconsolidated. This accounting guidance is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008, which is the Company’s 2010 fiscal year. Early adoption is prohibited. The Company does not expect the adoption of this standard to have a material impact on our financial position or results of operations.
In April 2008, the FASB issued FASB Staff Position (“FSP”) No. 142-3,Determination of the Useful Life of Intangible Assets(codified within ASC 350,Intangibles—Goodwill and Other), which amends the factors that must be considered in developing renewal or extension assumptions used to determine the useful life over which to amortize the cost of a recognized intangible asset under ASC 350. The objective of this FSP is to improve the consistency between the useful life of a recognized intangible asset under ASC 350 and the period of expected cash flows used to measure the fair value of the asset under ASC 805. The FSP is effective for financial statements for fiscal years beginning after December 15, 2008, which will be the beginning of fiscal 2010 for the Company. The Company is currently evaluating the impact that the adoption of this FSP will have on its consolidated financial statements. Early adoption is prohibited.
In May 2008, the FASB issued FSP No. APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) (codified within ASC 470,Debt).This FSP applies to convertible debt instruments that, by their stated terms, may be settled in cash (or other assets) upon conversion, including partial cash settlement, unless the embedded conversion option is required to be separately accounted for as a derivative under ASC 815,Derivatives and Hedging (formerly SFAS No. 133, Accounting for Derivative Instruments and Heading Activities). The liability and equity components of convertible debt instruments within the scope of this FSP must be separately accounted for in a manner that will reflect the entity’s nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. The excess of the principal amount of the debt over the amount ultimately allocated to the liability component is required to be amortized to interest expense using the effective interest method. This FSP is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. As a result, the Company will adopt this standard at the beginning of fiscal 2010. This FSP must be applied retrospectively to all periods presented. The retrospective adoption of this FSP will increase the Company’s historical reported interest expense from December 10, 2007 (issuance date of the Convertible Notes—See Note 5) forward.
The adoption of FSP APB 14-1 will have no impact on the Company’s actual past or future cash flows. However, upon adoption in fiscal 2010, the Company will restate prior periods by reclassifying approximately $470,000 of its Convertible Notes to additional paid-in capital, resulting in a debt discount. It is estimated that the Company’s non-cash interest expense will increase by approximately $65,500 and $48,100 for the years ended September 26, 2009 and September 27, 2008, respectively, resulting in a restated diluted net loss per share of approximately $(8.64) and $(1.69) for the years ended September 26, 2009 and September 27, 2008, respectively.
In June 2008, the FASB ratified the consensus reached on EITF Issue No. 07-5,Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock(“EITF 07-5”) (codified within ASC 815). This accounting guidance clarifies the determination of whether an instrument (or an embedded feature) is indexed to an entity’s own stock, which would qualify as a scope exception under ASC 815, and it is effective for
F-29
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
financial statements issued for fiscal years beginning after December 15, 2008. Early adoption for an existing instrument is not permitted. The Company has concluded that upon the adoption of this standard, the embedded derivative option in the Company’s Convertible Notes (See Note 5) will continue to be considered indexed to the Company’s own stock. As a result, the adoption of this standard is not expected to have a material impact on the Company’s financial condition or results of operations.
In June 2009, the FASB issued SFAS No. 168,The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles (codified within ASC 105,Generally Accepted Accounting Principles), which establishes the FASB Accounting Standards Codification as the single source of authoritative U.S. GAAP. The Codification will supersede all existing non-SEC accounting and reporting standards. As a result, upon adoption, all references to accounting literature in our SEC filings will conform to the appropriate reference within the Codification. The adoption of this standard did not have any impact on the Company’s financial position or results of operations.
Fiscal 2008 Acquisitions:
Acquisition of Third Wave Technologies, Inc.
On July 24, 2008, the Company completed its acquisition of Third Wave Technologies, Inc. (“Third Wave”) pursuant to a definitive agreement dated June 8, 2008. The Company concluded that the acquisition of Third Wave did not represent a material business combination and therefore no pro forma financial information has been provided herein. Subsequent to the acquisition date, the Company’s results of operations include the results of Third Wave, which is a component of the Company’s Diagnostics reporting segment.
Third Wave, located in Madison, Wisconsin, develops and markets molecular diagnostic reagents for a wide variety of DNA and RNA analysis applications based on its proprietary Invader chemistry. Third Wave’s current clinical diagnostic offerings consist of products for conditions such as Cystic Fibrosis, cardiovascular risk and other diseases. In March 2009, Third Wave received approval from the U.S. Food and Drug Administration (“FDA”) for two human papillomavirus (“HPV”) tests; Cervista HPV High Risk (“HR”) and Cervista HPV 16/18.
The Company paid $11.25 per share of Third Wave, for an aggregate purchase price of approximately $591,100 (subject to adjustment) consisting of approximately $575,400 in cash in exchange for stock and warrants; approximately 668 of fully vested stock options granted to Third Wave employees in exchange for their vested Third Wave stock options, with an estimated fair value of approximately $8,100; and approximately $7,600 for acquisition related fees and expenses. There are no potential contingent consideration arrangements payable to the former shareholders in connection with this transaction. Additionally, the Company granted approximately 315 unvested stock options in exchange for unvested Third Wave stock options, with an estimated fair value of approximately $5,100, which is being recognized as compensation expense over the vesting period.
The Company determined the fair value of the options issued in connection with the acquisition in accordance with EITF Issue No. 99-12,Determination of the Measurement Date for the Market Price of Acquirer Securities Issued in a Purchase Business Combination”).The Company determined the measurement date to be July 24, 2008, the date the transaction was completed, as the number of shares to be issued according to the exchange ratio was not fixed until this date. The Company valued the securities based on the average market price for two days before the measurement date and the measurement date itself. The weighted average stock price was determined to be $23.54.
F-30
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The purchase price is as follows:
| | | |
Cash portion of consideration | | $ | 575,400 |
Fair value of vested options exchanged | | | 8,100 |
Direct acquisition costs | | | 7,600 |
| | | |
Total purchase price | | $ | 591,100 |
| | | |
The fair value of vested Hologic common stock options exchanged for vested Third Wave options was included in the purchase price as such options were fully vested. The Company estimated the fair value of these stock options using the Binomial Option Pricing Model assuming no expected dividends and the following weighted-average assumptions:
| | | | |
Expected life | | | 1.48 years | |
Expected volatility | | | 42.16 | % |
Risk free interest rate | | | 2.33 | % |
Fair value per share determined in accordance with EITF 99-12 | | $ | 23.54 | |
The allocation of the purchase price was based on estimates of the fair value of assets acquired and liabilities assumed as of July 24, 2008. The components and allocation of the purchase price consists of the following approximate amounts:
| | | | |
Net tangible assets acquired as of July 24, 2008 | | $ | 87,300 | |
Increase in inventory to fair value | | | 5,100 | |
Increase in property and equipment to fair value | | | 800 | |
In-process research and development | | | 195,200 | |
Developed technology | | | 92,300 | |
Deferred taxes | | | (33,100 | ) |
Goodwill | | | 243,500 | |
| | | | |
Estimated Purchase Price | | $ | 591,100 | |
| | | | |
The preliminary purchase price allocation resulted in goodwill of approximately $241,800 as of July 24, 2008. During fiscal 2009, the Company increased goodwill approximately $1,500 primarily related to a $3,600 decrease in the estimated net operating loss offset by net increases of $1,700 in the estimate of other tax attributes. At September 26, 2009, goodwill related to the Third Wave acquisition is approximately $242,900.
Subsequent to the close of the Third Wave acquisition through September 26, 2009, stock options originally issued by Third Wave and converted into options to purchase Hologic common stock were exercised. The Company recorded the estimated tax benefit of $121 and $368 related to the exercise of these options as a reduction to goodwill during fiscal 2009 and 2008, respectively.
Identifiable Intangible Assets
As part of the purchase price allocation, the Company determined that the only separately identifiable intangible asset was developed technology. The fair value of the developed technology intangible assets was determined through the application of the income approach. Developed technology represented currently marketable purchased products that the Company continues to sell as well as utilize to enhance and incorporate into the Company’s existing products.
F-31
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Acquired In-Process Research and Development
As part of the purchase price allocation, approximately $195,200 of the purchase price was allocated to acquired in-process research and development projects. The amount allocated to acquired in-process research and development represented the estimated fair value of in-process projects based on risk-adjusted cash flows utilizing a discount rate of 20%. These in-process projects had not yet reached technological feasibility and had no future alternative uses as of the date of the merger. The primary basis for determining the technological feasibility of these projects was obtaining regulatory approval to market the underlying products. The fair value attributable to these in-process projects was expensed at the time of the acquisition. If the projects are not successful or completed in a timely manner, the Company may not realize the financial benefits expected for these projects or for the transaction as a whole.
The most significant acquired in-process technology related to Cervista HPV HR, for which the Company estimated a value of approximately $151,200. At the time of, and subsequent to the acquisition, the Company sold HPV reagents that detect certain HPV HR types as Analyte Specific Reagents (“ASRs”). In 2006, Third Wave began clinical trials for PMA submissions to the FDA for Cervista HR and submitted the PMAs in April 2008. During March 2009, the FDA approved the Company’s PMAs for both the Cervista HPV HR and Cervista HPV 16/18 tests. Since receiving FDA approval, the Company has begun to transition to only selling HPV In Vitro Diagnostics (“IVDs”) and expects to complete this transition by the end of fiscal 2010. The HPV in-process research and development related only to the HPV IVDs, and the HPV ASRs were valued as developed technology.
The estimated cost to complete Third Wave’s remaining in-process research and development projects in the aggregate as of September 26, 2009 is approximately $4,000.
The net deferred income taxes primarily relates to the tax effect of acquired identifiable intangible assets and fair value adjustments to acquired inventory and property and equipment, as such amounts are not deductible for tax purposes.
Cytyc Corporation Merger
On October 22, 2007, the Company completed its merger with Cytyc Corporation (“Cytyc”) pursuant to the Agreement and Plan of Merger (“Merger Agreement”) executed on May 20, 2007. Under the terms and conditions of the Merger Agreement, at the effective time of the merger, Cytyc became a wholly-owned subsidiary of the Company and each share of common stock of Cytyc, issued and outstanding immediately prior to the closing, was cancelled and converted into the right to receive (i)1.04 shares of common stock of the Company (as adjusted for the stock split effected on April 2, 2008) and (ii)$16.50 in cash. In accordance with Statement of Financial Accounting Standards (“SFAS”) No. 141,Business Combinations,and based on the terms of the merger, the Company is the accounting acquirer. This conclusion was based on the facts that Hologic board members and senior management control and represent a majority of the board of directors and senior management of the combined company, as well as the terms of the merger consideration, pursuant to which the Cytyc stockholders received a premium over the fair market value of their shares on such date and cash of $16.50 per share (or approximately 35% of the merger consideration). There were no preexisting relationships between the two companies.
F-32
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Cytyc, headquartered in Marlborough, Massachusetts, is a diversified diagnostic and medical device company that designs, develops, manufactures, and markets innovative and clinically effective diagnostics and surgical products. Cytyc products cover a range of cancer and women’s health applications, including cervical cancer screening, prenatal diagnostics, treatment of excessive menstrual bleeding and radiation treatment of early-stage breast cancer.
Upon the close of the merger, Cytyc shareholders received an aggregate of 132,038 shares of Hologic common stock and approximately $2,094,800 in cash. In connection with the close of the merger, the Company entered into a credit agreement relating to a senior secured credit facility (the “Credit Agreement”) with Goldman Sachs Credit Partners L.P. and certain other lenders, in which the lenders committed to provide, in the aggregate, senior secured financing of up to approximately $2,550,000 to pay for the cash portion of the merger consideration, repayment of existing debt of Cytyc, expenses relating to the merger and working capital following the completion of the merger. As of the closing of the merger, the Company borrowed $2,350,000 under this Credit Agreement. See Note 5 for further discussion.
The aggregate purchase price of approximately $6,156,900 included $2,094,800 in cash; 132,038 shares of Hologic common stock at an estimated fair value of $3,671,500; 16,465 of fully vested stock options granted to Cytyc employees in exchange for their vested Cytyc stock options, with an estimated fair value of approximately $241,400; the fair value of Cytyc’s outstanding convertible notes assumed in the merger of approximately $125,000; and approximately $24,200 of direct acquisition costs. There are no potential contingent consideration arrangements payable to the former Cytyc shareholders in connection with this transaction.
The Company measured the fair value of the 132,038 shares of the Company common stock issued as consideration in connection with the merger under EITF 99-12.The Company determined the measurement date to be May 20, 2007, the date the transaction was announced, as the number of shares to be issued according to the exchange ratio was fixed without subsequent revision. The Company valued the securities based on the average market price a few days before and after the measurement date. The weighted average stock price was determined to be $27.81.
(i) Purchase price
The purchase price is as follows:
| | | |
Cash portion of consideration | | $ | 2,094,800 |
Fair value of securities issued | | | 3,671,500 |
Fair value of vested options exchanged | | | 241,400 |
Fair value of Cytyc’s outstanding convertible notes | | | 125,000 |
Direct acquisition costs | | | 24,200 |
| | | |
Total estimated purchase price | | $ | 6,156,900 |
| | | |
F-33
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The fair value of vested Hologic common stock options exchanged for vested Cytyc options was included in the purchase price as such options were fully vested. The Company estimated the fair value of these stock options using the Binomial Option Pricing Model assuming no expected dividends and the following weighted-average assumptions:
| | | | |
Expected life | | | 2.50 years | |
Expected volatility | | | 35.10 | % |
Risk free interest rate | | | 4.82 | % |
Fair value per share determined in accordance with EITF 99-12 | | $ | 27.81 | |
(ii) Purchase Price Allocation
The allocation of the purchase price is based upon estimates of the fair value of assets acquired and liabilities assumed as of October 22, 2007. As a result of the merger, the Company assumed Cytyc’s obligation to the former stockholders of Adiana, Inc. to make contingent earn-out payments based on the achievement of certain milestones. The Company considered the provision of EITF Issue No. 95-8,Accounting for Contingent Consideration Paid to the Shareholders of an Acquired Enterprise in a Purchase Business Combination, and concluded that this contingent consideration represents additional purchase price. As a result, goodwill will be increased by the amount of additional consideration as it is earned. The milestone to begin accrual of the additional consideration was achieved in the fourth quarter of fiscal 2009, and the Company recorded $1,854 as additional goodwill. See Note 12 for additional discussion.
The Company had formulated and undertook a plan to restructure certain of Cytyc’s activities. The Company recorded a liability of approximately $2,800 in accordance with EITF 95-3 primarily related to the termination of certain employees, minimum inventory purchase commitments and other contractual obligations for which the related business activities have been discontinued.
The components and allocation of the purchase price consist of the following approximate amounts:
| | | | |
Book value of net assets acquired as of October 22, 2007 | | $ | 1,158,600 | |
Less: write-off of existing deferred financing costs, goodwill and intangible assets, including related deferred taxes | | | (787,900 | ) |
| | | | |
Adjusted book value of assets acquired | | | 370,700 | |
Remaining allocation: | | | | |
Increase inventory to fair value | | | 42,300 | |
Increase property and equipment to fair value | | | 5,100 | |
Increase in liabilities recorded in accordance with EITF 95-3 | | | (2,800 | ) |
Decrease deferred revenue to fair value | | | 400 | |
Identifiable intangible assets at fair value | | | 2,486,600 | |
Acquired in-process research and development | | | 370,000 | |
Deferred taxes | | | (943,400 | ) |
Goodwill | | | 3,828,000 | |
| | | | |
Total purchase price | | $ | 6,156,900 | |
| | | | |
F-34
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
(iii) Valuation of Intangible Assets and Goodwill
The purchase price for the merger with Cytyc was allocated to assets acquired and liabilities assumed based on management’s estimate of their fair values. Management determined the identifiable intangible assets, including in-process research and development, based upon a detailed valuation that relies on information and assumptions further described below. The excess purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed was allocated to goodwill.
Identifiable Intangible Assets
As part of the purchase price allocation, the Company determined that Cytyc’s identifiable intangible assets included existing technology, customer relationships and trade names. Cytyc’s existing technology relates to patents, patent applications and know-how with respect to the technologies embedded in its currently marketed products. In determining the allocation of the purchase price to existing technology, consideration was only given to patent and patent applications that relate to products that had been approved by the FDA. Cytyc’s customer relationship assets relate to relationships that Cytyc’s sales force had developed with obstetricians/gynecologists and gynecological surgeons, breast surgeons, radiation oncologists, clinical laboratories and other physicians. The trade names related to both the Cytyc name as well as key product names.
The Company used the income approach to value the existing technology and marketing based intangibles. This approach calculates fair value by discounting the after-tax cash flows back to a present value. The baseline data for this analysis was the cash flow estimates used to price the transaction. Cash flows were forecasted for each intangible asset, then discounted based on an appropriate discount rate. The discount rates applied, which ranged between 10.5% and 13.5%, were benchmarked with reference to the implied rate of return from the transaction model as well as Cytyc’s weighted average cost of capital based on the capital asset pricing model.
In estimating the useful life of the acquired assets, the Company considered ASC 350-30-35 (formerly paragraph 11 of SFAS No. 142,Goodwill and Other Intangible Assets), which lists the pertinent factors to be considered when estimating the useful life of an intangible asset. These factors included a review of the expected use by the combined company of the assets acquired, the expected useful life of another asset (or group of assets) related to the acquired assets, legal, regulatory or other contractual provisions that may limit the useful life of an acquired asset or may enable the extension of the useful life of an acquired asset without substantial cost, the effects of obsolescence, demand, competition and other economic factors, and the level of maintenance expenditures required to obtain the expected future cash flows from the asset. The Company is amortizing these intangible assets over their estimated useful lives using a method that is based on estimated future cash flows as the Company believes this will approximate the pattern in which the economic benefits of the assets will be utilized or on a straight-line basis if it was deemed that the cash flows were not reliably determinable.
Acquired In-Process Research and Development
As part of the purchase price allocation, approximately $370,000 of the purchase price was allocated to acquired in-process research and development projects. The amount allocated to acquired in-process research and development represented the estimated fair value, based on risk-adjusted cash flows, related to in-process projects that had not yet reached technological feasibility and had no future alternative uses as of the date of the merger. The primary basis for determining the technological feasibility of these projects was obtaining regulatory approval to market the underlying products. The fair value attributable to these in-process projects was expensed at the time of the merger. If the projects are not successful or completed in a timely manner, the Company may not realize the financial benefits expected for these projects or for the transaction as a whole.
F-35
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The fair value assigned to acquired in-process research and development was determined by estimating the costs to develop the acquired technology into commercially viable products, estimating the resulting net cash flows from the projects, and discounting the net cash flows to their present value. The revenue projections used to value the acquired in-process research and development were based on estimates of relevant market sizes and growth factors, expected trends in technology, and the nature and expected timing of new product introductions by the Company and its competitors. The resulting net cash flows from such projects were based on management’s estimates of cost of sales, operating expenses, and income taxes from such projects.
The rates utilized to discount the net cash flows to their present value of 12.5% to 13.5% were based on estimated cost of capital calculations and the implied rate of return from the transaction model plus a risk premium. Due to the nature of the forecasts and the risks associated with the developmental projects, appropriate risk-adjusted discount rates were used for the in-process research and development projects. The discount rates are based on the stage of completion and uncertainties surrounding the successful development of the purchased in-process technology projects.
The acquired in-process research and development related to the following research and development projects: Adiana Complete TransCervical Sterilization (“TCS”) System, which the Company subsequently renamed the Adiana Permanent Contraception System, and expanded labeling of the NovaSure System, Gestiva, the ThinPrep Imaging System, the ThinPrep Processor and the Helica Thermal Coagulator System (“Helica”).
The most significant acquired in-process technology related to the Adiana Permanent Contraception System for which the Company had estimated a value of approximately $220,000. The Adiana Permanent Contraception System includes an incisionless trans-cervical permanent sterilization device intended to be used during an office or hospital based procedure. The system consists of three different parts: a disposable applicator, an implantable polymer matrix and a radio frequency controller. The Company completed this in-process project during the third quarter of fiscal 2009 and received FDA approval on July 6, 2009.
Cytyc’s other in-process research and development projects were at different stages of development, ranging from the early stages of development to Phase IIb prototype building, ongoing clinical trials and submission to the FDA of Pre-Market Approval (“PMA”) and drug applications. FDA approval or clearance had not been granted for any of the products classified as in-process research and development, nor had Cytyc received any foreign approvals or clearances for any of these products. All products classified as in-process research and development require various levels of in-house and external testing, clinical trials and approvals from the FDA before these future products could be marketed. As of September 26, 2009, the estimated cash requirements in the aggregate to complete these remaining products were expected to be approximately $3,800. Certain of these projects that have been discontinued or delayed are not included in this estimate as their cost to complete and timing of completion are unknown at this time. Certain of the projects included in this estimated cash requirement have been delayed to fiscal 2010 and the estimated costs for these projects have been increased accordingly.
The successful development of new products and product enhancements is subject to numerous risks and uncertainties, both known and unknown, including, unanticipated delays, access to capital, budget overruns, technical problems and other difficulties that could result in the abandonment or substantial change in the design, development and commercialization of these new products and enhancements, including, for example changes requested by the FDA in connection with PMA applications for products or 510(k) notification. Given the uncertainties inherent with product development and introduction, there can be no assurance that any of the Company’s product development efforts will be successful on a timely basis or within budget, if at all. The failure of the Company to develop new products and product enhancements on a timely basis or within budget could harm the Company’s results of operations and financial condition.
F-36
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Goodwill
The preliminary purchase price allocation resulted in goodwill of approximately $3,844,100 as of October 22, 2007, the date of the merger. During the first quarter of fiscal 2009, the Company reduced goodwill in the purchase price allocation by approximately $1,900 primarily due to a decrease in the valuation allowance related to certain tax assets acquired where the Company has determined that it is more likely than not that these assets will be realized. Subsequent to the one year allocation period from the date of acquisition, the Company recorded additional adjustments to goodwill of approximately $1,000 for revisions to certain tax assets. The Company had previously reduced this goodwill in the amount of approximately $14,200 from the date of acquisition through September 27, 2008. The reduction was primarily related to a $16,800 increase in the preliminary valuation of assets acquired (primarily related to deferred tax assets acquired), an $1,845 increase in the preliminary valuation of certain tangible assets and a $1,700 increase in the preliminary valuation of certain intangible assets which were partially offset by a $5,900 increase in the preliminary estimate of liabilities assumed (primarily related to current tax liabilities) and a $200 increase in the preliminary estimate of acquisition costs and expenses.
The factors contributing to the recognition of this amount of goodwill were based upon several strategic and synergistic benefits that were expected to be realized from the combination. These benefits included the expectation that the Company’s complementary products and technologies would create a leading women’s healthcare company with an enhanced presence in hospitals, private practices and healthcare organizations. The Company also expected to realize substantial synergies through the use of Cytyc’s OB/GYN and breast surgeon sales channel to cross-sell the Company’s existing and future products. The merger provided the Company broader channel coverage within the United States and expanded geographic reach internationally, as well as increased scale and scope for further expanding operations through product development and complementary strategic transactions.
Subsequent to the close of the merger with Cytyc, vested stock options originally issued by Cytyc and converted into options to purchase Hologic common stock were exercised. The Company recorded the estimated excess tax benefit of approximately $49,300 related to the exercise of these options as a reduction to goodwill in fiscal 2008 and $988 in fiscal 2009.
Supplemental Pro-forma Information
The following unaudited pro-forma information presents the consolidated results of operations of the Company and Cytyc as if the transaction had occurred at the beginning of fiscal 2007, with pro-forma adjustments to give effect to amortization of intangible assets, an increase in interest expense on acquisition financing, subsequent refinancing and certain other adjustments together with related tax effects:
| | | | | | |
(approximate amounts in thousands except per share data) | | Year ended |
| | September 27,
2008 | | September 29,
2007 |
Revenue | | $ | 1,711,405 | | $ | 1,472,400 |
Net income | | $ | 658,678 | | $ | 62,600 |
Net income per common share: | | | | | | |
Basic | | $ | 0.85 | | $ | 0.26 |
| | | | | | |
Diluted | | $ | 0.83 | | $ | 0.25 |
| | | | | | |
F-37
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The $370,000 charge for acquired in-process research and development, the fair value of the inventory step-up of $42,300, stock-based compensation of approximately $60,000, direct acquisition fees and expenses of approximately $28,000 and change of control payments of approximately $18,600 that were a direct result of the transaction are excluded from the unaudited pro forma information above. The unaudited pro forma results are not necessarily indicative of the results that the Company would have attained had the merger with Cytyc occurred at the beginning of the periods presented. The $195,200 charge for acquired in-process research and development and the fair value of the inventory step-up of $3,933 that were a direct result of the acquisition of Third Wave have been excluded from the unaudited pro forma information above. The Company has not reflected any other pro forma adjustments related to Third Wave as it was not considered a material acquisition.
Prior to the close of the merger, the Board of Directors of Cytyc approved a modification to certain outstanding equity awards for Cytyc employees, which was consented to by Hologic. The modification provided for the acceleration of vesting upon the close of the merger for those awards that did not provide for acceleration upon a change of control as part of the original terms of the award. This modification was consented to by the Company so that the Company would not incur stock-based compensation charges that it otherwise would have if the awards had continued to vest under their original terms.
Fiscal 2007 Acquisition:
Acquisition of BioLucent, Inc.
On September 18, 2007, the Company completed the acquisition of BioLucent, Inc. (“BioLucent”) pursuant to a definitive agreement dated June 20, 2007. The results of operations for BioLucent have been included in the Company’s consolidated financial statements from the date of acquisition as part of its Breast Health segment.
BioLucent, previously located in Aliso Viejo, California, develops, markets and sells MammoPad breast cushions to decrease the discomfort associated with mammography. Prior to the acquisition, BioLucent’s primary research and development efforts were directed at its brachytherapy business which was focused on breast cancer therapy. Prior to the acquisition, BioLucent spun-off its brachytherapy technology and business to the holders of BioLucent’s outstanding shares of capital stock. As a result, the Company only acquired BioLucent’s MammoPad cushion business and related assets. The Company invested $1,000 directly in the spun-off brachytherapy business in exchange for shares of preferred stock issued by the new business.
The aggregate purchase price for BioLucent was approximately $73,200, consisting of approximately $6,800 in cash and 2,314 shares of Hologic common stock valued at approximately $63,200, debt assumed and paid off of approximately $1,600 and approximately $1,600 for acquisition related fees and expenses. The Company determined the fair value of the shares issued in connection with the acquisition in accordance with EITF 99-12.
F-38
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The acquisition also provides for up to two annual earn-out payments not to exceed $15,000 in the aggregate based on BioLucent’s achievement of certain revenue targets. The Company considered the provision of EITF 95-8, and concluded that this contingent consideration represents additional purchase price. As a result, goodwill will be increased by the amount of the additional consideration, if any, as it is earned. As of September 26, 2009, the Company has not recorded any amounts for these potential earn-outs. The allocation of the purchase price was based upon estimates of the fair value of assets acquired and liabilities assumed as of September 18, 2007. The components and allocation of the purchase price consisted of the following approximate amounts:
| | | | |
Net tangible assets acquired as of September 18, 2007 | | $ | 2,800 | |
Developed technology and know how | | | 12,300 | |
Customer relationship | | | 17,000 | |
Trade name | | | 2,800 | |
Deferred income tax liabilities, net | | | (9,500 | ) |
Goodwill | | | 47,800 | |
| | | | |
Final purchase price | | $ | 73,200 | |
| | | | |
As part of the purchase price allocation, all intangible assets that were a part of the acquisition were identified and valued. It was determined that only customer relationship, trade name and developed technology had separately identifiable values. The fair value of these intangible assets was determined through the application of the income approach. Customer relationship represented a large customer base that was expected to purchase the disposable MammoPad product on a regular basis. Trade name represented the BioLucent product name that the Company intended to continue to use. Developed technology represented currently marketable purchased products that the Company continues to sell as well as utilize to enhance and incorporate into the Company’s existing products.
The deferred income tax liability relates to the tax effect of acquired identifiable intangible assets and fair value adjustments to acquired inventory, as such amounts are not deductible for tax purposes, partially offset by acquired net operating loss carryforwards of approximately $2,400.
On January 16, 2008, the Company entered into a definitive agreement pursuant to which it agreed to sell full U.S. and world-wide rights to Gestiva to K-V Pharmaceutical Company upon approval of the pending Gestiva new drug application (the “Gestiva NDA”) by the FDA for a purchase price of $82,000. The Company received $9,500 of the purchase price in fiscal 2008, and the balance is due upon final approval of the Gestiva NDA by the FDA on or before February 19, 2010 and the production of a quantity of Gestiva suitable to enable the commercial launch of the product. Either party has the right to terminate the agreement if FDA approval is not obtained by February 19, 2010. The Company agreed to continue its efforts to obtain FDA approval of the NDA for Gestiva as part of this arrangement. All costs incurred in these efforts will be reimbursed by K-V Pharmaceutical and are being recorded as a credit against research and development expenses. During fiscal 2009 and 2008, these reimbursed costs were not material. The Company recorded the $9,500 as a deferred gain within current liabilities in the Consolidated Balance Sheet. The Company expects that the gain will be recognized upon the closing of the transaction following final FDA approval of the Gestiva NDA or if the agreement is terminated. The Company cannot assure that it will be able to obtain the requisite FDA approval, that the transaction will be completed or that it will receive the balance of the purchase price. Moreover, if K-V Pharmaceutical terminates the agreement as a result of a breach by the Company of a material representation, warranty, covenant or agreement, the Company will be required to return the funds previously received as well as expenses reimbursed by K-V.
F-39
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The development of Gestiva, a drug that, if approved by the FDA, could be used in the prevention of preterm birth in pregnant women with a history of at least one spontaneous preterm birth, was originally begun by Adeza Biomedical Corporation, which was acquired by Cytyc on April 2, 2007. On October 22, 2007, the Company completed its merger with Cytyc and as a result acquired all rights to Gestiva. The Company allocated $53,400 to acquired in-process research and development as part of the initial purchase price allocation.
| 5. | Borrowings and Credit Arrangements |
The Company had total debt of $1,903,328 at September 26, 2009 and $2,200,900 at September 27, 2008. The Company’s borrowings consisted of the following at September 26, 2009 and September 27, 2008:
| | | | | | |
| | | 2009 | | 2008 |
Current debt obligations: | | | | | | |
Term Loan A | | $ | 28,789 | | $ | 34,444 |
Term Loan B | | | 6,785 | | | 1,723 |
AEG debt | | | 1,500 | | | 2,015 |
Cytyc notes | | | — | | | 298 |
Other | | | 1,299 | | | — |
| | | | | | |
Total current debt obligations | | | 38,373 | | | 38,480 |
| | |
Long-term debt obligations: | | | | | | |
Term Loan A | | | 95,929 | | | 310,000 |
Term Loan B | | | 42,664 | | | 118,833 |
AEG debt | | | — | | | 8,587 |
Other | | | 1,362 | | | — |
| | | | | | |
| | | 139,955 | | | 437,420 |
Convertible notes | | | 1,725,000 | | | 1,725,000 |
| | | | | | |
Total long-term debt obligations | | | 1,864,955 | | | 2,162,420 |
| | | | | | |
Total debt obligations | | $ | 1,903,328 | | $ | 2,200,900 |
| | | | | | |
As of September 26, 2009, the debt maturity schedule for the Company’s term loans as well as other components of its debt obligations is as follows for each fiscal year:
| | | | | | | | | | | | | | | | | | |
| | | 2010 | | 2011 | | 2012 | | 2013 | | 2014 | | Total |
Term Loan A | | $ | 28,789 | | $ | 18,227 | | $ | 18,227 | | $ | 59,475 | | $ | — | | $ | 124,718 |
Term Loan B | | | 6,785 | | | 628 | | | 628 | | | 41,408 | | | — | | | 49,449 |
AEG debt | | | 1,500 | | | — | | | — | | | — | | | — | | | 1,500 |
Other | | | 1,299 | | | 1,362 | | | — | | | — | | | — | | | 2,661 |
Convertible Notes (1) | | | — | | | — | | | — | | | — | | | 1,725,000 | | | 1,725,000 |
| | | | | | | | | | | | | | | | | | |
| | $ | 38,373 | | $ | 20,217 | | $ | 18,855 | | $ | 100,883 | | $ | 1,725,000 | | $ | 1,903,328 |
| | | | | | | | | | | | | | | | | | |
| (1) | The earliest date of redemption is December 13, 2013, which is in fiscal 2014 |
F-40
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Credit Agreement
On October 22, 2007, the Company and certain of its domestic subsidiaries entered into a senior secured credit agreement (the “Credit Agreement”) with Goldman Sachs Credit Partners L.P. and certain other lenders, (collectively, the “Lenders”). Pursuant to the terms and conditions of the Credit Agreement, the Lenders committed to provide senior secured financing in an aggregate amount of up to $2,550,000. As of the closing of the merger with Cytyc, the Company borrowed $2,350,000 under the credit facilities. The Company used the proceeds from the credit facilities to pay the cash consideration of the merger with Cytyc, and to pay fees, commissions and expenses incurred by the Company in connection with the merger with Cytyc and the Credit Agreement. In addition, the Company used the proceeds of the credit facilities, together with the Company’s available cash, to pay the cash due upon conversion of Cytyc’s 2.25% Senior Convertible Notes due 2024 that were outstanding after the closing of the merger with Cytyc.
The credit facilities under the Credit Agreement consisted of:
| | • | | $600,000 senior secured Term Loan A (the “Term Loan A facility”) with a final maturity date of September 30, 2012; |
| | • | | $250,000 senior secured Term Loan B-1 and $250,000 senior secured Term Loan B-2 (collectively, the “Term Loan B facility”) with a final maturity date of March 31, 2013; |
| | • | | $1,250,000 senior secured capital markets term loan (the “Term Loan X facility”) with a final maturity date of April 22, 2009; |
| | • | | $200,000 senior secured revolving credit facility (the “revolving facility”) with a final maturity date of October 22, 2012. |
The Company applied the net proceeds from its Convertible Notes offering described below to repay amounts outstanding under the Credit Agreement, including all of the remaining amounts outstanding under Term Loan X of $1,100,000 and Term Loan B-2 of $250,000. The Company also repaid a pro rata portion of the Company’s Term Loan A in the amount of $251,000 and Term Loan B-1 in the amount of $104,000. During fiscal 2008, the Company also made voluntary prepayments of the remaining principal under its Term Loan X, Term Loan A and Term Loan B-1 of $150,000, $349,000 and $146,000, respectively. There were no amounts outstanding under these term notes as of September 27, 2008 or September 26, 2009.
All amounts outstanding under the credit facilities accrued interest, at the Company’s option, initially, with respect to all loans made under the revolving facility and the Term Loan A facility: (i) at the Base Rate plus 1.25% per annum; or (ii) at the reserve adjusted Eurodollar Rate plus 2.25% per annum. With respect to loans made under the Term Loan B facility: (i) at the Base Rate plus 1.5% per annum; or (ii) at the reserve adjusted Eurodollar Rate plus 2.50%; and with respect to loans made under the Term Loan X facility: (i) at the Base Rate plus 0.75% per annum; or (ii) at the reserve adjusted Eurodollar Rate plus 1.75% per annum.
Borrowings outstanding under the credit agreement from initial drawdown through final repayment in June 2008 had a weighted average interest rate of 6.72%. Interest expense under these credit facilities totaled $40,200 during fiscal 2008, which included non-cash interest expense of approximately $12,300 related to the amortization of the capitalized deferred financing costs related to the Amended Credit Agreement. As of September 27, 2008, all of the deferred financing costs had been amortized, with the exception of $3,464 of remaining deferred financing costs allocated to the revolving credit facility, as all Term Loan borrowings had been fully repaid.
F-41
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The Credit Agreement contained affirmative and negative covenants customarily applicable to senior secured credit facilities, including requirements to maintain maximum leverage and minimum interest coverage ratios, as of the last day of each fiscal quarter, as defined within the Credit Agreement. The Company had been in compliance with all covenants through the term of the agreement.
In connection with the acquisition of Third Wave, on July 17, 2008, the Company entered into an amended and restated credit agreement with certain of the Lenders (the “Amended Credit Agreement”). The Amended Credit Agreement amended and restated the Company’s existing credit agreement with Goldman Sachs Credit Partners L.P. and the lenders named therein, dated as of October 22, 2007. Pursuant to the terms and conditions of the Amended Credit Agreement, the Lenders committed to provide senior secured financing in an aggregate amount of up to $800,000. The credit facilities under the Amended Credit Agreement consist of $400,000 senior secured tranche A term loan (“Term Loan A”); $200,000 senior secured tranche B term loan (“Term Loan B”); $200,000 senior secured revolving credit facility (the “revolving facility”).
In order to complete the acquisition of Third Wave, the Company borrowed $540,000 under the credit facilities on July 17, 2008, consisting of $400,000 under the Term Loan A and $140,000 under the Term Loan B. As of September 26, 2009 and September 27, 2008, the Company had an aggregate of $174,167 and $465,000, respectively, of principal outstanding under this credit facility of which $124,718 and $344,444, respectively, was under the Term Loan A and $49,449 and $120,556, respectively, was under the Term Loan B, as of September 26, 2009 and September 27, 2008. The Company has been making voluntary prepayments throughout the term of the loan, and subsequent to September 26, 2009, the Company paid down approximately $24,600 of the outstanding principal of which $21,000 was a voluntary payment. The Company has reclassified this voluntary payment of $21,000 to current portion of long-term debt in its Consolidated Balance Sheet at September 26, 2009. No such voluntary prepayments occurred subsequent to September 27, 2008 but prior to the issuance of the Company’s 2008 Form 10-K. The Company had no amounts outstanding under its Revolving Facility, and therefore, had full availability of the $200,000 Revolving Facility as of September 26, 2009. The final maturity dates for the credit facility are September 30, 2012 for the Term Loan A and Revolving Facility and March 31, 2013 for the Term Loan B.
The domestic subsidiaries of the Company which are party to the Amended Credit Agreement (including Third Wave, which joined as a party to the Amended Credit Agreement on July 24, 2008) have guaranteed the Company’s obligations under the credit facilities and the credit facilities are secured by first-priority liens on, and first-priority security interests in, substantially all of the assets of the Company and all subsidiaries party to the Amended Credit Agreement, a first priority security interest in 100% of the capital stock issued by each guarantor, 65% of the capital stock issued by certain first-tier foreign subsidiaries of the Company and all intercompany debt. The security interests are evidenced by an Amended and Restated Pledge and Security Agreement by and among Goldman Sachs Credit Partners L.P., as collateral agent, Hologic and the other parties therein named (the “Amended Pledge and Security Agreement”). The Amended Pledge and Security Agreement amended and restated Hologic’s existing Pledge and Security Agreement by and among Goldman Sachs Credit Partners L.P., as collateral agent, Hologic and the other parties therein named, dated as of October 22, 2007.
The Company was initially required to make scheduled principal payments under the Term Loan A facility in increasing amounts ranging from $10,000 per quarter commencing with the quarter ending September 30, 2008 to $15,000 per quarter commencing with the quarter ending September 30, 2010, and under the Term Loan B facility, in equal quarterly installments of $500 beginning on the quarter ending September 30, 2008, with the remaining balance of each term loan facility due at the maturity of the applicable term loan facility. The Company is required to make principal repayments first, pro rata among the term loan facilities, and second to
F-42
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
the revolving credit facility from specified excess cash flows from operations and from the net proceeds of specified types of asset sales, debt issuances, insurance recoveries and equity offerings. These minimum scheduled principal payments are reduced on a prorata basis as the Company makes voluntary repayments against the outstanding principal amounts. Since the Company has made voluntary payments, the required scheduled principal payments have been reduced, and the current annual amounts due are reflected in the five year payment schedule above. Amounts borrowed under the revolving credit facility are due at maturity. The Company may voluntarily prepay any of the credit facilities without premium or penalty (other than applicable breakage costs related to interest on Eurodollar loans).
All amounts outstanding under the Amended Credit Agreement bear interest, at Hologic’s option, as follows:
| | With | respect to loans made under the revolving facility and the Term Loan A facility: |
| | (i) | at the Base Rate plus 1.25% per annum, which was reduced from 1.50% in May 2009; or |
| | (ii) | at the reserve adjusted Eurodollar Rate plus 2.25% per annum, which was reduced from 2.50% in May 2009; and |
| | With | respect to loans made under the Term Loan B facility: |
| | (i) | at the Base Rate plus 2.25% per annum; or |
| | (ii) | at the reserve adjusted Eurodollar Rate plus 3.25% per annum. |
The margin applicable to loans under the revolving credit facility and the Term Loan A facility is subject to specified changes based on certain changes in the leverage ratio as specified in the Amended Credit Agreement.
Interest accruing at the base rate generally is payable by the Company on a quarterly basis. Interest accruing at the Eurodollar Rate is payable on the last day of selected interest periods (which shall be one, two, three and six months and in certain circumstances, nine or twelve months) unless the interest period exceeds three months, in which case, interest will be due at the end of every three months.
Borrowings outstanding under the Amended Credit Agreement in fiscal 2009 and 2008 had a weighted average interest rate of 3.81% and 5.24%, respectively. The interest rates on the outstanding Term Loan A and Term Loan B borrowings at September 26, 2009 ranged from 2.5% to 4.5% and 3.5% to 5.5%, respectively and at September 27, 2008 were 5.25% and 6.0%, respectively. Interest expense under the Amended Credit Agreement totaled $23,929 and $8,148, respectively, during fiscal 2009 and 2008, which includes non-cash interest expense of $10,757 and $2,718, respectively, related to the amortization of the deferred financing costs related to the Amended Credit Agreement. As of September 26, 2009, there was $6,441 in deferred financing costs related to the Term Loans classified as Other Assets in the Consolidated Balance Sheet.
Interest expense under the Amended Credit Agreement for the Revolving Facility totaled $1,889 and $1,759 in fiscal 2009 and fiscal 2008, respectively, consisting of commitment fees on the unused portion of this facility and non-cash interest expense of $981 and $814, respectively, related to the amortization of deferred financing costs. As of September 26, 2009, there was $2,974 in deferred financing costs related to the Revolving Facility classified as Other Assets on the Company’s Consolidated Balance Sheets. The Company pays a quarterly commitment fee, at a per annum rate of 0.375%, which was reduced from 0.50% in May 2009, on the undrawn commitments available under the Revolving Facility, which per annum rate is subject to reduction based on a leverage ratio as specified in the Amended Credit Agreement.
F-43
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The credit facilities contain affirmative and negative covenants customarily applicable to senior secured credit facilities, including covenants restricting the ability of the Hologic loan parties, subject to negotiated exceptions, to: incur additional indebtedness and additional liens on their assets; engage in mergers or acquisitions or dispose of assets, enter into sale-leaseback transactions, pay dividends or make other distributions, voluntarily prepay other indebtedness, enter into transactions with affiliated persons, make investments, and change the nature of their businesses. The credit facilities require the Hologic loan parties to maintain certain maximum leverage and minimum interest coverage ratios as of the last day of each fiscal quarter, as defined in the Amended Credit Agreement. The Company was in compliance with its financial covenants as of September 26, 2009.
Convertible Notes
On December 10, 2007, the Company issued and sold $1,725,000 aggregate original principal of 2.00% Convertible Senior Notes due 2037 (the “Convertible Notes”). The net proceeds from the offering of approximately $1,689,000, after deducting the underwriters’ discounts of $34,500 and estimated offering expenses of approximately $1,500, were used to repay the Company’s outstanding senior secured indebtedness under its Credit Agreement, including all of the Company’s Term Loan X and Term Loan B-2, and a pro rata portion of the Company’s Term Loan A and Term Loan B-1 as discussed above. The Convertible Notes are the Company’s senior unsecured obligations and rank equally with all of the Company’s existing and future senior unsecured debt and prior to all future subordinated debt. The Convertible Notes are effectively subordinated to any future secured indebtedness to the extent of the collateral securing such indebtedness, and structurally subordinated to all indebtedness and other liabilities (including trade payables) of the Company’s subsidiaries.
Holders may require the Company to repurchase the Convertible Notes on December 13 of 2013, and each of December 15, 2017, 2022, 2027 and 2032 at a repurchase price equal to 100% of their accreted principal amount, plus accrued and unpaid interest. The Company may redeem any of the Convertible Notes beginning December 18, 2013, by giving holders at least 30 days’ notice. The Company may redeem the Convertible Notes either in whole or in part at a redemption price equal to 100% of their principal amount, plus accrued and unpaid interest, including contingent interest and liquidated damages, if any, to, but excluding, the redemption date.
The Convertible Notes bear interest at a rate of 2.00% per year on the principal amount, payable semi-annually in arrears in cash on June 15 and December 15 of each year, beginning June 15, 2008 and ending on December 15, 2013. The Convertible Notes will accrete principal from December 15, 2013 at a rate that provides holders with an aggregate annual yield to maturity of 2.00% per year. Beginning with the six month interest period commencing December 15, 2013, the Company will pay contingent interest during any six month interest period to the holders of Convertible Notes if the “trading price”, as defined, of the Convertible Notes for each of the five trading days ending on the second trading day immediately preceding the first day of the applicable six month interest period equals or exceeds 120% of the accreted principal amount of the Convertible Notes. Interest expense under the Convertible Notes totaled $40,273 and $27,696, respectively, during fiscal 2009 and 2008, which included non-cash interest expense of $6,004 and $4,775, respectively, related to the amortization of deferred financing costs.
The holders of the Convertible Notes may convert the notes into shares of the Company’s common stock at a conversion price of approximately $38.60 per share, subject to adjustment, prior to the close of business on September 15, 2037 under any of the following circumstances: (1) during any calendar quarter if the last reported sale price of the Company’s common stock exceeds 130% of the conversion price for at least 20 trading days in the 30 consecutive trading days ending on the last trading day of the preceding calendar quarter; (2) during the
F-44
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
five business day period after any five consecutive trading day period in which the trading price per note for each day of such period was less than 98% of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such day; (3) if the notes have been called for redemption; or (4) upon the occurrence of specified corporate events. None of these triggering events had occurred as of September 26, 2009.
In lieu of delivery of shares of the Company’s common stock in satisfaction of the Company’s obligation upon conversion of the Convertible Notes, the Company may elect to deliver cash or a combination of cash and shares of the Company’s common stock. If the Company elects to satisfy its conversion obligation in a combination of cash and shares of the Company’s common stock, the Company is required to deliver up to a specified dollar amount of cash per $1,000 original principal amount of Convertible Notes, and will settle the remainder of its conversion obligation in shares of its common stock. It is the Company’s current intent and policy to settle any conversion of the Convertible Notes as if the Company had elected to make the net share settlement election.
If an event of default, as defined, relates to the Company’s failure to comply with the reporting obligations in the Convertible Notes, if the Company so elects, the sole remedy of the holders of the Convertible Notes for the first 90 days following such event of default consists exclusively of the right to receive an extension fee on the notes in an amount equal to 0.25% of the accreted principal amount of the Convertible Notes.
Based on the Company’s evaluation of the Convertible Notes in accordance with ASC 815,Derivatives and Hedging, Subsection 40,Contracts in Entity’s Own Equity (formerly EITF Issue No. 00-19,Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock, and EITF Issue 07-5,Determining Whether an Instrument (or Embedded Feature) IS Indexed to an Entity’s Own Stock),the Company determined that the Convertible Notes contained a single embedded derivative, comprising both the contingent interest feature and the filing failure penalty payment requiring bifurcation as the features were not clearly and closely related to the host instrument. The Company has determined that the value of this embedded derivative was nominal as of September 26, 2009 and September 27, 2008.
As of September 26, 2009, upon conversion, including the potential premium that could be payable on a fundamental change (as defined), the Company would issue a maximum of approximately 56,000 common shares to the Convertible Note holders.
Please See Note 2, “Summary of Significant Accounting Policies—Recently Issued Accounting Pronouncements” for a discussion related to the impact of the adoption of FSP APB 14-1,Accounting for Convertible Debt Instruments that May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)(codified within ASC 470,Debt) in fiscal 2010.
AEG Debt
The Company’s AEG subsidiary has $1,500 and $10,602, respectively, outstanding at September 26, 2009 and September 27, 2008, under certain debt agreements. During fiscal 2009, the Company paid down certain of these loans, and the remaining loans are currently due on demand. Outstanding borrowings had weighted average interest rates of 3.3%, 6.26% and 6.34% during the years ended September 26, 2009, September 27, 2008 and September 29, 2007, respectively. Interest expense incurred under these debt agreements totaled $347, $755 and $1,208 in fiscal 2009, 2008 and 2007, respectively.
F-45
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Cytyc Convertible Notes
In connection with the merger with Cytyc, the Company assumed the obligations under Cytyc’s 2.25% Senior Convertible Notes due 2024 (the “Cytyc Notes”). As a result of the merger, the Cytyc Notes ceased to be convertible into shares of Cytyc common stock but rather into the kind and amount of shares of stock and cash which a holder of shares of Cytyc common stock would have been entitled to receive upon the merger had the Cytyc Notes been converted into shares of Hologic common stock immediately prior to the merger, such that each $1,000 principal face amount of Cytyc Notes may be converted at any time and from time to time into $556.12 in cash and 35.06 shares of Hologic common stock. The Company offered to repurchase all of the outstanding Cytyc Notes in exchange for the principal face amount of such Cytyc Notes plus accrued but unpaid interest thereon. Under the agreement between the parties, at any time after March 20, 2009 the Cytyc Notes could be redeemed by the Company at a cash redemption price equal to the principal amount of the Cytyc Notes, plus accrued and unpaid interest.
As of the close of the merger with Cytyc, the Company assumed the outstanding principal amount under the Cytyc Notes of $73,258. Subsequent to the close of the merger through September 27, 2008, Cytyc Notes in the principal amount of $72,960 were submitted for conversion upon which the Company issued 2,557 shares of its common stock and made cash payments in the amount of $40,574. No holder of a Cytyc Note accepted the Company’s offer to repurchase the Cytyc Notes. During fiscal 2009, the remaining Cytyc Notes with an aggregate principal amount of $298 were paid and no amounts remain outstanding at September 26, 2009.
| 6. | Fair Value Measurements |
Effective September 28, 2008, the Company adopted ASC 820,Fair Value Measurements and Disclosures (formerly SFAS No. 157, Fair Value Measurement), for its financial assets and financial liabilities that are re-measured and reported at fair value at each reporting period and its nonfinancial assets and nonfinancial liabilities that are re-measured and reported at fair value at least annually. As permitted, the Company has elected to defer implementation of ASC 820 as it relates to its nonfinancial assets and nonfinancial liabilities that are recognized and disclosed at fair value in the financial statements on a non-recurring basis until September 27, 2009. The Company is evaluating the impact, if any, ASC 820 will have on its nonfinancial assets and nonfinancial liabilities.
The adoption of ASC 820 for financial assets and financial liabilities that are re-measured and reported at fair value on a recurring basis did not have an impact on the Company’s financial results.
ASC 820 establishes a three-level valuation hierarchy for disclosure of fair value measurements. Financial assets and financial liabilities are categorized within the valuation hierarchy based upon the lowest level of input that is significant to the measurement of fair value. The three levels of the hierarchy are defined as follows:
| | • | | Level 1—Inputs to the valuation methodology are quoted market prices for identical assets or liabilities. |
| | • | | Level 2—Inputs to the valuation methodology are other observable inputs, including quoted market prices for similar assets or liabilities and market-corroborated inputs. |
| | • | | Level 3—Inputs to the valuation methodology are unobservable inputs based on management’s best estimate of inputs market participants would use in pricing the asset or liability at the measurement date, including assumptions about risk. |
F-46
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
As of September 26, 2009, the Company’s financial assets that are re-measured at fair value on a recurring basis consisted of $313 in money market mutual funds that are classified as cash and cash equivalents in the Consolidated Balance Sheets. As there are no withdrawal restrictions, they are classified within Level 1 of the fair value hierarchy and are valued using quoted market prices for identical assets.
The Company holds certain minority cost-method equity investments in non-publicly traded securities aggregating $7,585 and $9,278 at September 26, 2009 and September 27, 2008, respectively, which are included in other long-term assets on the Company’s Consolidated Balance Sheets. These investments are generally carried at cost. As the inputs utilized for the Company’s periodic impairment assessment are not based on observable market data, these cost method investments are classified within Level 3 of the fair value hierarchy on a non-recurring basis. To determine the fair value of these investments, the Company uses all available financial information related to the entities, including information based on recent or pending third-party equity investments in these entities. In certain instances, a cost method investment’s fair value is not estimated as there are no identified events or changes in circumstances that may have a significant adverse effect on the fair value of the investment and to do so would be impractical. During fiscal 2009, the Company recorded other-than-temporary impairment charges totaling $2,243 related to two of its cost method investments to adjust their carrying amounts to fair value.
| 7. | Pension and Other Employee Benefits |
The Company has certain defined benefit pension plans covering the employees of its AEG German subsidiary (the “Pension Benefits”). As of September 29, 2007, the Company adopted SFAS No. 158,Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans, an amendment of FASB Statements No. 87, 88, 106 and 132(R)(codified primarily in ASC 715,Defined Benefit Plans) using a prospective approach. The adoption of this Standard did not impact the Company’s compliance with its debt covenants under its credit agreements, cash position or results of operations.
The following table summarizes the incremental effect of adopting this Standard on individual line items in the Consolidated Balance Sheet as of September 29, 2007:
| | | | | | | | | |
| | | Before
Adoption of
SFAS No. 158 | | Adjustments
(In thousands) | | After
Adoption of
SFAS No. 158 |
Accumulated other comprehensive income | | $ | — | | $ | 2,212 | | $ | 2,212 |
Total stockholders’ equity | | $ | 803,511 | | $ | 2,212 | | $ | 805,723 |
As of September 26, 2009 and September 27, 2008, the Company’s pension liability is $6,736 and $7,323, respectively, which is primarily recorded as a component of long-term liabilities in the Consolidated Balance Sheets. Under German law, there are no rules governing investment or statutory supervision of the pension plan. As such, there is no minimum funding requirement imposed on employers. Pension benefits are safeguarded by the Pension Guaranty Fund, a form of compulsory reinsurance that guarantees an employee will receive vested pension benefits in the event of insolvency.
F-47
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The tables below provide a reconciliation of benefit obligations, plan assets, funded status, and related actuarial assumptions of the Company’s German Pension Benefits.
| | | | | | | | | | | | |
Change in Benefit Obligation | | Pension Benefits | |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Benefit obligation at beginning of year | | $ | (7,323 | ) | | $ | (7,627 | ) | | $ | (8,005 | ) |
Service cost | | | — | | | | — | | | | — | |
Interest cost | | | (469 | ) | | | (424 | ) | | | (397 | ) |
Plan participants’ contributions | | | — | | | | — | | | | — | |
Actuarial gain | | | 764 | | | | 665 | | | | 1,455 | |
Foreign exchange | | | (28 | ) | | | (229 | ) | | | (947 | ) |
Benefits paid | | | 320 | | | | 292 | | | | 267 | |
| | | | | | | | | | | | |
Benefit obligation at end of year | | | (6,736 | ) | | | (7,323 | ) | | | (7,627 | ) |
Plan assets | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Funded status | | $ | (6,736 | ) | | $ | (7,323 | ) | | $ | (7,627 | ) |
| | | | | | | | | | | | |
The tables below outline the components of the net periodic benefit cost and related actuarial assumptions of the Company’s German Pension Benefits plan.
| | | | | | | | | | | | |
Components of Net Periodic Benefit Cost | | Pension Benefits | |
| | 2009 | | | 2008 | | | 2007 | |
Service cost | | $ | — | | | $ | — | | | $ | — | |
Interest cost | | | 469 | | | | 424 | | | | 397 | |
Expected return on plan assets | | | — | | | | — | | | | — | |
Amortization of prior service cost | | | — | | | | — | | | | — | |
Recognized net actuarial gain | | | (169 | ) | | | (93 | ) | | | (91 | ) |
| | | | | | | | | | | | |
Net periodic benefit cost | | $ | 300 | | | $ | 331 | | | $ | 306 | |
| | | | | | | | | | | | |
| | |
| | | Pension Benefits | | | | |
Weighted-Average Net Periodic Benefit Cost Assumptions | | 2009 | | | 2008 | | | 2007 | |
Discount rate | | | 6.6 | % | | | 6.5 | % | | | 5.5 | % |
Expected return on plan assets | | | 0 | % | | | 0 | % | | | 0 | % |
Rate of compensation increase | | | 0 | % | | | 0 | % | | | 0 | % |
The projected benefit obligation for the German Pension Benefits plans with projected benefit obligations in excess of plan assets was $6,736 and $7,323 at September 26, 2009 and September 27, 2008, respectively, and the accumulated benefit obligation for the German Pension Benefits plans was $6,736 and $7,323 at September 26, 2009 and September 27, 2008, respectively.
The Company is also obligated to pay long-term service award benefits. The projected benefit obligation for long-term service awards was $631 and $601 at September 26, 2009 and September 27, 2008, respectively.
F-48
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The table below reflects the total Pension Benefits expected to be paid as of September 26, 2009 from the plans.
| | | |
| | | Pension
Benefits |
2010 | | $ | 332 |
2011 | | | 354 |
2012 | | | 373 |
2013 | | | 395 |
2014 | | | 420 |
2015 to 2019 | | | 2,316 |
The Company also maintains additional contractual pension benefits for its top German executive officers in the form of a defined contribution plan. Contributions in fiscal 2009, 2008 and 2007 were $62, $179 and $175, respectively.
The Company accounts for income taxes using the liability method as required by ASC 740,Income Taxes(formerly SFAS No. 109,Accounting for Income Taxes). Under this method, deferred income taxes are recognized for the future tax consequences of differences between the tax and financial accounting bases of assets and liabilities at the end of each reporting period. Deferred income taxes are based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. A valuation allowance is established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The provision for income taxes consisted of the following:
| | | | | | | | | | | | |
| | | Years ended | |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Federal: | | | | | | | | | | | | |
Current | | $ | 74,311 | | | $ | 102,212 | | | $ | 39,096 | |
Deferred | | | 4,447 | | | | (10,835 | ) | | | 6,053 | |
| | | | | | | | | | | | |
| | | 78,758 | | | | 91,377 | | | | 45,149 | |
| | | | | | | | | | | | |
State: | | | | | | | | | | | | |
Current | | | 9,804 | | | | 10,411 | | | | 6,735 | |
Deferred | | | (6,210 | ) | | | 265 | | | | (2,101 | ) |
| | | | | | | | | | | | |
| | | 3,594 | | | | 10,676 | | | | 4,634 | |
| | | | | | | | | | | | |
Foreign: | | | | | | | | | | | | |
Current | | | 5,388 | | | | 4,218 | | | | 6,167 | |
Deferred | | | (178 | ) | | | 205 | | | | (2,039 | ) |
| | | | | | | | | | | | |
| | | 5,210 | | | | 4,423 | | | | 4,128 | |
| | | | | | | | | | | | |
| | $ | 87,562 | | | $ | 106,476 | | | $ | 53,911 | |
| | | | | | | | | | | | |
F-49
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
A reconciliation of income taxes at the U.S. federal statutory rate to the Company’s effective tax rate is as follows:
| | | | | | | | | |
| | | Years ended | |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Income tax provision at federal statutory rate | | (35.0 | )% | | (35.0 | )% | | 35.0 | % |
Increase (decrease) in tax resulting from: | | | | | | | | | |
Goodwill impairment | | 39.1 | | | — | | | — | |
Section 199 manufacturing deduction | | (0.3 | ) | | (2.2 | ) | | — | |
State income taxes, net of federal benefit | | 0.3 | | | 2.5 | | | 3.3 | |
In-process research and development | | — | | | 71.2 | | | — | |
State law change | | (0.1 | ) | | 0.8 | | | — | |
Tax credits | | (0.2 | ) | | (0.6 | ) | | (1.4 | ) |
Permanent differences | | 0.2 | | | 1.0 | | | (0.7 | ) |
Change in valuation allowance | | 0.1 | | | — | | | (0.4 | ) |
Other | | 0.1 | | | 0 .4 | | | 0.5 | |
| | | | | | | | | |
| | 4.2 | % | | 38.1 | % | | 36.3 | % |
| | | | | | | | | |
The Company’s (loss) income before income taxes consisted of the following:
| | | | | | | | | | | |
| | | Years ended |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 |
Domestic | | $ | (2,095,809 | ) | | $ | (275,091 | ) | | $ | 137,659 |
Foreign | | | 7,134 | | | | (4,050 | ) | | | 10,830 |
| | | | | | | | | | | |
| | $ | (2,088,675 | ) | | $ | (279,141 | ) | | $ | 148,489 |
| | | | | | | | | | | |
Significant components of the Company’s deferred tax assets and liabilities are as follows:
| | | | | | | | |
| | | September 26,
2009 | | | September 27,
2008 | |
Deferred tax assets | | | | | | | | |
Net operating loss carryforwards | | $ | 96,170 | | | $ | 123,377 | |
Nondeductible accruals | | | 18,533 | | | | 19,038 | |
Nondeductible reserves | | | 9,773 | | | | 7,068 | |
Stock-based compensation | | | 18,926 | | | | 10,373 | |
Other temporary differences | | | 5,421 | | | | 6,486 | |
Research and other credits | | | 10,550 | | | | 8,698 | |
| | | | | | | | |
| | | 159,373 | | | | 175,040 | |
Less: valuation allowance | | | (24,424 | ) | | | (17,710 | ) |
| | | | | | | | |
| | $ | 134,949 | | | $ | 157,330 | |
Deferred tax liabilities | | | | | | | | |
Depreciation and amortization | | $ | (903,017 | ) | | $ | (985,955 | ) |
Original issue discount | | | (89,628 | ) | | | (36,904 | ) |
Investment in subsidiary | | | (3,109 | ) | | | (1,649 | ) |
| | | | | | | | |
| | $ | (995,754 | ) | | $ | (1,024,508 | ) |
| | | | | | | | |
| | $ | (860,805 | ) | | $ | (867,178 | ) |
| | | | | | | | |
F-50
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The effective tax rate for fiscal 2009 was significantly impacted by the goodwill impairment charge recorded in the second quarter of fiscal 2009, substantially all of which is not tax deductible for tax purposes. In addition, the tax provision for fiscal 2009 includes a reversal for a charge recorded in fiscal 2008 for approximately $2,300 related to a clarification in Massachusetts tax law on apportionment for affiliates of manufacturing companies. The Company also recorded an additional $1,328 as a result of losing its tax holiday status due to the closure of its manufacturing facility in Shanghai, China. The effective tax rate for fiscal 2008 was significantly impacted by the acquired in-process research and development charge related to the merger with Cytyc and Third Wave acquisition, which is not tax deductible. The tax provision for fiscal 2008 also included a net charge of $1,498 primarily related to the Company’s U.S. based deferred tax assets and liabilities resulting from newly enacted State legislation. In accordance with ASC 740, the adjustment for the effect of the change in state tax law is included in the tax provision for the period that includes the enactment date.
Under ASC 740, the Company can only recognize a deferred tax asset for the future benefit of its tax loss carryforward to the extent that it is “more likely than not” that these assets will be realized. The Company has established a valuation allowance against a portion of its remaining potential deferred tax assets after consideration of all positive and negative evidence that it is more likely than not a portion of its tax loss carryforward will not be realized. In determining the realizability of these assets, the Company considers numerous factors, including historical profitability, estimated future taxable income and the industry in which it operates. The valuation allowance increased $6,714 in fiscal 2009 from fiscal 2008 primarily due to the deferred taxes acquired in the Third Wave acquisition, and the Company’s belief that it is more likely than not that these state tax assets will expire unutilized.
During fiscal 2009, the Company recorded a $1,608 increase to capital in excess of par and a $1,112 decrease to goodwill related to the excess tax benefit of stock options exercised in fiscal 2009. During fiscal 2008, the Company recorded a $13,109 increase to capital in excess of par and a $49,630 decrease to goodwill related to the excess tax benefit of stock options exercised in fiscal 2008.
As of September 26, 2009, the Company had total net operating loss and credit carryforwards of $220,391 and $10,550, respectively, that it believes are more likely than not that they will be realized. The $220,391 excludes $359,521 of federal, state and international net operating losses, which if tax effected would be $23,362, that the Company believes will expire unutilized. These net operating losses primarily relate to Third Wave, which was acquired in fiscal 2008. The following table summarizes the expiration periods of the net operating loss and credit carryforwards:
| | | | | | | | | | | | | | | | | | |
| | | Period of Expiration |
| | 2010-2015 | | 2016-2020 | | 2021-2025 | | 2026-2030 | | No expiration | | Total |
Net operating loss | | $ | 682 | | $ | 21,289 | | $ | 102,543 | | $ | 95,877 | | $ | — | | $ | 220,391 |
R&D credit | | $ | 90 | | $ | 2,426 | | $ | 3,593 | | $ | 24 | | $ | — | | $ | 6,133 |
CA Credits | | $ | — | | $ | — | | $ | — | | $ | — | | $ | 1,273 | | $ | 1,273 |
CT credit | | $ | — | | $ | 823 | | $ | 424 | | $ | — | | $ | — | | $ | 1,247 |
MA credits | | $ | — | | $ | 719 | | $ | 805 | | $ | — | | $ | — | | $ | 1,524 |
WI credits | | $ | — | | $ | — | | $ | 221 | | $ | — | | $ | — | | $ | 221 |
IN credits | | $ | — | | $ | — | | $ | — | | $ | — | | $ | 152 | | $ | 152 |
On September 30, 2007, the Company adopted Financial Accounting Standards Board (“FASB”) Interpretation (“FIN”) No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109 (codified within ASC 740-10), and recorded the cumulative effect of the change in accounting principle of $480 as a decrease to opening retained earnings.
F-51
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The Company has gross unrecognized tax benefits, including interest, of $29,227 as of September 26, 2009 and $20,154 as of September 27, 2008. At September 26, 2009, $29,227 represents the amount of unrecognized tax benefits that, if recognized, would result in a reduction of the Company’s effective tax rate. The increase in unrecognized tax benefits during fiscal 2009 is primarily due to the finalization of the purchase accounting of the Third Wave acquisition. In the next twelve months it is reasonably possible that the Company will reduce the balance of its unrecognized tax benefits by $2,221 due to expiration of statute of limitations and settlements with taxing authorities, which will reduce the Company’s effective tax rate.
The Company’s unrecognized income tax benefits are as follows:
| | | | | | | | |
| | | September 26,
2009 | | | September 27,
2008 | |
Balance at beginning of fiscal year | | $ | 19,447 | | | $ | 6,200 | |
Tax positions related to current year: | | | | | | | | |
Additions | | | 2,532 | | | | 1,173 | |
Reductions | | | — | | | | — | |
Tax positions related to prior years: | | | | | | | | |
Additions related to change in estimate | | | 1,391 | | | | 363 | |
Reductions | | | — | | | | — | |
Settlements | | | (405 | ) | | | — | |
Lapses in statutes of limitations | | | (492 | ) | | | (545 | ) |
Acquired tax positions: | | | | | | | | |
Additions related to reserves acquired from Cytyc | | | | | | | 12,256 | |
Additions related to reserves acquired from Third Wave | | | 5,600 | | | | | |
| | | | | | | | |
Balance as of the end of the fiscal year | | $ | 28,073 | | | $ | 19,447 | |
| | | | | | | | |
The Company’s policy is to recognize accrued interest and penalties related to unrecognized tax benefits and income tax liabilities, when applicable, as part of income tax expense in its Consolidated Statements of Operations. As of September 26, 2009 and September 27, 2008, accrued interest was $1,154 and $707, respectively, net of federal benefit. As of September 26, 2009, no penalties have been accrued.
The Company and its subsidiaries are subject to United States federal income tax, as well as income tax of multiple state income and foreign jurisdictions. The current tax returns are open for audit through fiscal 2013.
The Company currently has a tax holiday in Costa Rica that is scheduled to expire in 2015. This tax holiday did not materially reduce the Company’s income tax provision for fiscal 2009 or 2008.
The Company intends to reinvest, indefinitely, approximately $18,659 of unremitted earnings. It is not practical to estimate the amount of additional taxes that might be payable upon repatriation of foreign earnings.
| 9. | Stockholders’ Equity and Stock-Based Compensation |
Common Shares Authorization
On October 22, 2007, the Company’s certificate of incorporation was amended to increase the number of authorized shares of the Company’s common stock thereunder from 180,000 to 600,000. At the Company’s March 11, 2008 Annual Meeting of Stockholders, an increase in the number of authorized shares of common stock from 600,000 to 750,000 was approved.
F-52
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Rights Agreement
On April 2, 2008, the Company entered into an Amended and Restated Rights Agreement (the “Amended and Restated Rights Agreement”) between the Company and American Stock Transfer & Trust Company as Rights Agent (the “Rights Agent”). The Amended and Restated Rights Agreement amends and restates the Company’s rights agreement, dated as of September 17, 2002, as amended on May 21, 2007, between the Company and the Rights Agent.
On April 2, 2008, the Company effected a two-for-one stock split in the form of a stock dividend to stockholders as of March 21, 2008. Pursuant to the Amended and Restated Rights Agreement, the Company amended the terms of the rights issued and issuable under the agreement (“Rights”), effective as of April 3, 2008 (after the stock dividend), to reset the Rights such that each share of Common Stock is entitled to receive one Right, to retain the purchase price of each Right at $60 per Right, and to provide that each Right will entitle the holder to purchase one twenty-five thousandth of a share of Series A Junior Participating Preferred Stock (the “Series A Preferred Stock”). Conforming changes have also been made to the Company’s certificate of designation for the Series A Preferred Stock to provide that each share of Series A Preferred Stock carries 25,000 times the dividend, liquidation and voting rights of the Company’s Common Stock. Other modifications have also been made in the Amended and Restated Rights Agreement to update the agreement for certain developments, including the recent amendments to the Company’s by-laws permitting stockholders to hold and transfer shares of the Company’s capital stock in book entry form. The expiration date of the Rights has remained unchanged at January 1, 2013.
Stock-Based Compensation
Equity Compensation Plans
The Company has one share-based compensation plan pursuant to which awards are currently being made—the 2008 Equity Incentive Plan. The Company has four share-based compensation plans pursuant to which outstanding awards have been made, but from which no further awards can or will be made—i) the 1995 Combination Stock Option Plan; ii) the 1997 Employee Equity Incentive Plan; iii) the 1999 Equity Incentive Plan; and iv) the 2000 Acquisition Equity Incentive Plan.
At the Company’s March 11, 2008 Annual Meeting of Stockholders, the Company’s 2008 Equity Incentive Plan (the “2008 Equity Plan”) was approved. In connection with this approval, the Company’s 1999 Second Amended and Restated Equity Incentive Plan was terminated. The purpose of the 2008 Equity Plan is to provide stock options, stock issuances and other equity interests in the Company to employees, officers, directors, consultants and advisors of the Company and its parents and subsidiaries, and any other person who is determined by the Board of Directors to have made (or is expected to make) contributions to the Company. The 2008 Equity Plan is administered by the Board of Directors of the Company, and a total of 20,000 shares were reserved for issuance under this Plan. As of September 26, 2009, the Company had 15,018 shares available for future grant under this plan.
The Company has certain other plans that were assumed by the Company in fiscal 2008 upon merger with Cytyc and Third Wave, and no shares are available for future grant under these plans.
F-53
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Grant-Date Fair Value
Effective with the adoption of SFAS 123(R) (codified in ASC 718), the Company elected to use a binomial lattice model to determine the fair value of its stock options. The Company considers a number of factors to determine the fair value of options including the advice of an outside valuation advisor and the advisor’s model. Information pertaining to stock options granted during fiscal 2009, 2008 and 2007 and related assumptions are noted in the following table:
| | | | | | | | | | | |
| | | Years ended | |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Options granted | | | 3,007 | | | 3,224 | | | | 8,330 | |
Weighted-average exercise price | | $ | 14.43 | | | $32.84 | | | $ | 25.68 | |
Weighted-average grant date fair value | | $ | 5.40 | | | $10.61 | | | $ | 13.19 | |
Assumptions: | | | | | | | | | | | |
Risk-free interest rates | | | 2.0 | % | | 2.7% to 4.0 | % | | | 5.0 | % |
Expected life (in years) | | | 4.0 | | | 3.8 to 4.6 | | | | 5.0 | |
Expected volatility | | | 46 | % | | 36% to 38 | % | | | 55.0 | % |
Dividend yield | | | — | | | — | | | | — | |
The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected life of the stock options. In projecting expected stock price volatility, the Company uses a combination of historical stock price volatility and implied volatility from observable market prices of similar equity instruments. The Company estimated the expected life of stock options based on historical experience using employee exercise and option expiration data.
The reduction in the assumption used for the expected life of the options from 5 years to 3.8 years beginning in the third quarter of fiscal 2008 is due to a change in the contractual life of the options granted beginning in the second quarter of 2008 from 10 years to 7 years.
Stock-Based Compensation Expense
The Company uses the straight-line attribution method to recognize stock-based compensation expense for stock options and restricted stock units (RSU). The vesting term of stock options is generally five years with annual vesting of 20% per year on the anniversary of the grant date, and RSUs either cliff vest at the end of three years or vest over four years with annual vesting at 25% per year on the anniversary of the grant date. The amount of stock-based compensation recognized during a period is based on the value of the portion of the awards that are ultimately expected to vest. ASC 718 requires forfeitures to be estimated at the time granted and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the unvested portion of the surrendered stock option or RSU. Based on an analysis of historical forfeitures, the Company has determined a specific forfeiture rate for certain employee groups and has applied forfeiture rates ranging from 0% to 6% as of September 26, 2009 depending on the specific employee group. This analysis is re-evaluated quarterly and the forfeiture rate will be adjusted as necessary. Ultimately, the actual stock-based compensation expense recognized will only be for those stock options and RSUs that vest.
F-54
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Stock-based compensation expense from the issuance of stock options and RSUs in fiscal 2009, 2008 and 2007 is as follows:
| | | | | | | | | |
| | |
2009 | | 2008 | |
2007 |
Cost of revenues | | $ | 3,522 | | $ | 2,293 | | $ | 695 |
Research and development | | | 3,960 | | | 2,806 | | | 828 |
Selling and marketing | | | 5,161 | | | 3,487 | | | 602 |
General and administrative | | | 20,296 | | | 15,137 | | | 3,979 |
Restructuring charge | | | — | | | 1,941 | | | — |
| | | | | | | | | |
| | $ | 32,939 | | $ | 25,664 | | $ | 6,104 |
| | | | | | | | | |
Stock-based compensation expense related to stock options was $13,829, $13,968, and $4,725. Stock compensation expense related to the fair value of RSUs was $19,110, $11,696, and 1,379 in fiscal years 2009, 2008 and 2007, respectively. The related tax benefit recorded in the consolidated statements of operations was $9,755, $8,365 and $1,715 in fiscal years 2009, 2008 and 2007, respectively. At September 26, 2009, there was $31,966 and $33,026 of unrecognized compensation expense related to stock options and RSUs, respectively, to be recognized over a weighted average period of 3.5 years and 2.3 years, respectively.
Included in stock-based compensation expense for fiscal 2008 was $2,662 as a result of the acceleration of vesting for certain outstanding Hologic stock options upon the close of the merger with Cytyc. The original terms of these employee stock options provided for acceleration of vesting upon a change of control. In addition, stock-based compensation expense includes a total of $3,512 related to option modifications during fiscal 2008. During this period, the Company recorded $768 related to a modification of certain options to extend the period of time to exercise upon termination from 90 days to August 31, 2009 upon termination of the Company’s Chairman of the Board of Directors (See Note 2). The Company also recorded $2,264 of stock-based compensation as a result of a modification of certain Hologic stock options in connection with the merger with Cytyc Agreement in May 2007. The modification provided for acceleration of vesting of the unvested options upon a termination as a result of a change of control, as well as an extension of the period to exercise vested options from 90 days to December 31, 2009, which occurred upon the close of the merger with Cytyc. Additionally, stock-based compensation expense included $480 related to certain former Third Wave executives who were terminated.
In fiscal 2008, stock-based compensation included $570 as a result of the acceleration of vesting for certain outstanding Hologic restricted stock units upon the close of the merger with Cytyc. The original terms of these restricted stock units provided for acceleration of vesting upon a change of control. Fiscal 2008 stock-based compensation also included $1,174 related to the acceleration of certain restricted stock units related to a separation agreement with the Company’s Chairman of the Board of Directors.
Prior to the close of the merger the Board of Directors of both Hologic and Cytyc approved a modification to certain outstanding equity awards for Cytyc employees. The modification provided for the acceleration of vesting upon the close of Merger for those awards that did not provide for acceleration upon a change of control as part of the original terms of the award. This modification was made so that the Company would not incur stock based compensation charges that it otherwise would have if the awards had continued to vest under their original terms.
F-55
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Option Exchange Program
On December 22, 2008, the Board of Directors approved, subject to stockholder approval, a stock option exchange program (the “Option Exchange Program”). The Option Exchange Program was approved at the Annual Meeting of Stockholders held on March 4, 2009. The Option Exchange Program permitted eligible employees to exchange their outstanding options issued on January 16, 2008 at an exercise price per share of $33.31 for a lesser number of new options (“New Options”), with such number of New Options issuable upon exchange calculated pursuant to an exchange ratio based on the original exercise price of the surrendered option. The exchange offer expired on April 5, 2009. Pursuant to the Option Exchange Program, the New Options have an exercise price of $14.87, which is 110% of the last reported closing sales price of the Company’s common stock as of the date of the new grant, which was April 5, 2009. The total number of stock options eligible to be exchanged of 784 was exchanged for 406 New Options.
On the date of exchange, the estimated fair value of the New Options approximated the estimated fair value of the exchanged stock options calculated immediately prior to the exchange. As such, there is no incremental fair value of the New Options, and the Company will not record additional compensation expense related to the exchange. The Company will continue to recognize the remaining compensation expense related to the exchanged options over the remaining vesting period of the original options. The New Options become exercisable over a period of four years, with 25% vesting on the first anniversary of the date the New Options were granted and 25% vesting on each anniversary thereafter, so long as the option holder continues to be employed by the Company.
Share Based Payment Activity
The following table summarizes all stock option activity under the Company’s stock option plans for the year ended September 26, 2009:
| | | | | | | | | | | |
| | | Number
of Shares | | | Weighted-
Average
Exercise Price | | Weighted-
Average
Remaining
Contractual Life
in Year | | Aggregate
Intrinsic
Value |
Options outstanding at September 27, 2008 | | 14,493 | | | | | | | | | |
Granted | | 3,007 | | | $ | 14.43 | | | | | |
Cancelled/forfeited | | (1,263 | ) | | | 19.51 | | | | | |
Net effect of option cancellation/regrant | | (378 | ) | | | 33.31 | | | | | |
Exercised | | (1,306 | ) | | | 7.19 | | | | $ | 10,525 |
| | | | | | | | | | | |
Options outstanding at September 26, 2009 | | 14,553 | | | $ | 16.42 | | 4.91 | | $ | 47,630 |
| | | | | | | | | | | |
Options exercisable at September 26, 2009 | | 9,531 | | | $ | 14.60 | | 4.23 | | $ | 41,310 |
| | | | | | | | | | | |
Options vested and expected to vest at September 26, 2009 (1) | | 14,068 | | | $ | 16.36 | | 4.87 | | $ | 46,946 |
| | | | | | | | | | | |
| (1) | This represents the number of vested stock options as of September 26, 2009 plus the unvested outstanding options at September 26, 2009 expected to vest in the future, adjusted for estimated forfeitures. |
During fiscal 2008 and 2007, the total intrinsic value of options exercised (i.e., the difference between the market price on the date of exercise and the price paid by the employee to exercise the options) was $196,960 and $63,477, respectively.
F-56
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
A summary of the Company’s Restricted Stock Units activity during the year September 26, 2009 is presented below:
| | | | | | |
Non-vested Shares | | Number of
Shares | | | Weighted-Average
Grant-Date Fair
Value |
Non-vested at September 27, 2008 | | 1,461 | | | $ | 31.23 |
Granted. | | 1,669 | | | | 14.46 |
Vested | | (210 | ) | | | 23.87 |
Forfeited | | (150 | ) | | | 23.44 |
| | | | | | |
Non-vested at September 26, 2009 | | 2,770 | | | $ | 21.96 |
| | | | | | |
The number of restricted stock units vested includes shares withheld on behalf of employees to satisfy minimum statutory tax withholding requirements. During fiscal 2009, 2008 and 2007 the total fair value of RSUs vested was $5,014, $2,009 and $0, respectively.
Employee Stock Purchase Plan
At the Company’s March 11, 2008 Annual Meeting of Stockholders, the Company’s 2008 Employee Stock Purchase Plan (the “ESPP”) was approved. The plan meets the criteria set forth in ASC 718’s definition of a non-compensatory plan and does not give rise to stock-based compensation expense. Employees who have completed three consecutive months, or two years, whether or not consecutive, of employment with the Company or any of its participating subsidiaries are eligible to participate in the ESPP. The ESPP plan period is semi-annual and allows participants to purchase the Company’s common stock at 95% of the closing price of the stock on the last day of the plan period. A total of 400 shares may be issued under the ESPP. During fiscal 2009, the Company issued 121 shares under the ESPP.
| 10. | Profit Sharing 401(k) Plan |
The Company has a qualified profit sharing plan covering substantially all of its employees. Contributions to the plan are at the discretion of the Company’s Board of Directors. The Company made contributions of $5,725, $5,305 and $1,572 for fiscal years 2009, 2008 and 2007, respectively.
| 11. | Supplemental Executive Retirement Plan |
Effective March 15, 2006, the Company adopted a SERP to provide non-qualified retirement benefits to a select group of executive officers, senior management and highly compensated employees of the Company. Eligible employees may elect to contribute up to 75% of their annual base salary and 100% of their annual bonus to the SERP and such employee contributions are 100% vested. In addition, the Company may elect to make annual discretionary contributions on behalf of participants in the SERP. Each Company contribution is subject to a three year vesting schedule, such that each contribution vests one third annually. Employee contributions are recorded within accrued expenses in the Consolidated Balance Sheets.
Upon enrollment into the SERP, employees make investment elections for both their voluntary contributions and discretionary contributions, if any, made by the Company. Earnings and losses on contributions based on these investment elections are recorded as a component of compensation expense in the period earned.
F-57
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
On October 30, 2006 and October 22, 2007, the Compensation Committee of the Board of Directors approved a $1,500 discretionary cash contribution to the SERP for each year. In November 2008, the Compensation Committee of the Board of Directors approved a $2,825 discretionary contribution to the SERP for fiscal 2008. Discretionary contributions by the Company to the SERP are held in a Rabbi Trust. The Company is recording compensation expense for the SERP discretionary contribution ratably over the three-year vesting period, which totaled $1,815, $876 and $442 in fiscal years 2009, 2008 and 2007, respectively. The full amount of the discretionary contribution, net of forfeitures, is recorded within accrued expenses in the Consolidated Balance Sheets.
The Company has purchased Company-owned group life insurance contracts, in which both voluntary and discretionary Company SERP contributions are invested, to fund payment of the Company’s obligation to the SERP participants. The total amount invested at September 26, 2009 and September 27, 2008 was $11,602 and $5,575, respectively, which approximated the total of employee voluntary contributions into the plan and the Company’s cash portion of its discretionary contribution. The values of these life insurance contracts are recorded with other long-term assets in the Consolidated Balance Sheets. Changes in the cash surrender value of life insurance contracts, which were immaterial in fiscal 2009, 2008 and 2007, are recorded as a component of (expense) other income, net in the Consolidated Statement of Operations.
| 12. | Commitments and Contingencies |
Contingent Earn-Out Payments
As a result of the merger with Cytyc, the Company assumed the obligation to the former Adiana stockholders to make contingent earn-out payments tied to the achievement of milestones. The milestone payments include potential contingent payments of up to $155,000 based on worldwide sales of the Adiana Permanent Contraception System in the first year following FDA approval and on annual incremental sales growth thereafter through December 31, 2012. FDA approval of the Adiana Permanent Contraception System occurred on July 6, 2009, and the Company began accruing contingent consideration in the fourth quarter of fiscal 2009 based on the defined percentage of worldwide sales of the product. The total accrued contingent consideration, net at September 26, 2009 is $1,454. These amounts are being recorded as additional purchase price, and under the terms of the agreement the first payment is not due to the Adiana shareholders until October 2010. The agreement includes an indemnification provision that provides for the reimbursement of qualifying legal expenses in defense of the Adiana intellectual property, and the Company has the right to offset contingent consideration payments to the Adiana shareholders with these qualifying legal costs (see Note 15). Legal costs have not been material to date.
The Company satisfied its obligation for a second and final earn-out to the former Suros Surgical stockholders related to Suros’ incremental revenue growth for revenues earned through July 31, 2008. The Company accrued an amount of approximately $24,500 for this second annual earn-out in the fourth quarter of 2008, with an increase to goodwill, of which $24,400 was paid as of September 27, 2008 and the remainder was paid as of December 27, 2008. The Company had also made a payment of approximately $19,000 to the former Suros stockholders in the fourth quarter of fiscal 2007 for the first year earn-out.
Finance Lease Obligations
As a result of the merger with Cytyc, the Company assumed the obligation to a non-cancelable lease agreement for a building with approximately 164,000 square feet located in Alajuela, Costa Rica, to be used as a manufacturing and office facility to replace its existing Costa Rica facility. The Company moved into this new
F-58
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
location during fiscal 2009. The Company was responsible for a significant portion of the construction costs and therefore was deemed, for accounting purposes, to be the owner of the building during the construction period, in accordance with ASC 840,Leases,Subsection 40-15-5.During the year ended September 27, 2008, the Company recorded an additional $4,400 in fair market value of the building, which was completed in fiscal 2008. This is in addition to the $3,000 fair market value of the land and the $7,700 fair market value related to the building constructed that Cytyc had recorded as of October 22, 2007. The Company has recorded such fair market value within property and equipment on its Consolidated Balance Sheets. At September 26, 2009, the Company has recorded $1,508 in accrued expenses and $16,329 in other long-term liabilities related to this obligation in the Consolidated Balance Sheet. The term of the lease is for a period of approximately ten years with the option to extend for two consecutive five-year terms. The lease term commenced in May 2008, at which time the Company began transferring the Company’s Costa Rican operations to this facility. It is expected that this process will be complete by February 2009.
At the completion of the construction period, the Company reviewed the lease for potential sale-leaseback treatment in accordance with ASC 840, Subsection 40,Sale-Leaseback Transactions(formerly SFAS No. 98 (“SFAS 98”),Accounting for Leases: Sale-Leaseback Transactions Involving Real Estate, Sales-Type Leases of Real Estate, Definition of the Lease Term, and Initial Direct Costs of Direct Financing Leases—an amendment of Financial Accounting Standards Board (“FASB”) Statements No. 13, 66, and 91 and a rescission of FASB Statement No. 26 and Technical Bulletin No. 79-11). Based on its analysis, the Company determined that the lease did not qualify for sale-leaseback treatment. Therefore, the building, leasehold improvements and associated liabilities will remain on the Company’s financial statements throughout the lease term, and the building and leasehold improvements will be depreciated on a straight line basis over their estimated useful lives of 35 years.
Future minimum lease payments, including principal and interest, under this lease were as follows at September 26, 2009:
| | | | |
| | | Amount | |
Fiscal 2010 | | $ | 1,508 | |
Fiscal 2011 | | | 1,561 | |
Fiscal 2012 | | | 1,616 | |
Fiscal 2013 | | | 1,672 | |
Fiscal 2014 | | | 1,731 | |
Thereafter | | | 7,288 | |
| | | | |
Total minimum payments | | | 15,376 | |
Less-amount representing interest | | | (6,094 | ) |
| | | | |
Total | | $ | 9,282 | |
| | | | |
In addition, as a result of the merger with Cytyc, the Company assumed the obligation to a non-cancelable lease agreement for a building with approximately 146,000 square feet located in Marlborough, Massachusetts, to be principally used as an additional manufacturing facility. In 2011, the Company will have an option to lease an additional 30,000 square feet. As part of the lease agreement, the lessor agreed to allow the Company to make significant renovations to the facility to prepare the facility for the Company’s manufacturing needs. The Company was responsible for a significant amount of the construction costs and therefore was deemed, for accounting purposes, to be the owner of the building during the construction period in accordance with ASC 840-40-15-5. The $13,200 fair market value of the facility is included within property and equipment, net on the Consolidated Balance Sheet. At September 26, 2009, the Company has recorded $982 in accrued expenses and
F-59
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
$15,314 in other long-term liabilities related to this obligation in the Consolidated Balance Sheet. The term of the lease is for a period of approximately 12 years commencing on November 14, 2006 with the option to extend for two consecutive 5-year terms. Based on its ASC 840-40 analysis, the Company determined that the lease did not qualify for sale-leaseback treatment. Therefore, the improvements and associated liabilities will remain on the Company’s financial statements throughout the lease term, and the leasehold improvements will be depreciated on a straight line basis over their estimated useful lives of up to 35 years.
Future minimum lease payments, including principal and interest, under this lease were as follows at September 26, 2009:
| | | | |
| | | Amount | |
Fiscal 2010 | | $ | 982 | |
Fiscal 2011 | | | 982 | |
Fiscal 2012 | | | 982 | |
Fiscal 2013 | | | 1,091 | |
Fiscal 2014 | | | 1,091 | |
Thereafter | | | 4,995 | |
| | | | |
Total minimum payments | | | 10,123 | |
Less-amount representing interest | | | (3,636 | ) |
| | | | |
Total | | $ | 6,487 | |
| | | | |
Long-Term Supply Contract
As a result of the merger with Cytyc, the Company assumed certain non-cancelable supply contracts. For reasons of quality assurance, sole source availability or cost effectiveness, certain key components and raw materials are available only from a sole supplier and Cytyc had entered into certain long-term supply contracts to assure continuity of supply. Some of these contracts have minimum purchase commitments.
Future supply commitments under these long-term supply contracts are as follows as of September 26, 2009:
| | | |
| | | Amount |
Fiscal 2010 | | $ | 3,000 |
Fiscal 2011 | | | 3,000 |
Fiscal 2012 | | | 3,000 |
| | | |
| | $ | 9,000 |
| | | |
Concentration of Suppliers
The Company purchases certain components of the Company’s products from a single or small number of suppliers. A change in or loss of these suppliers could cause a delay in filling customer orders and a possible loss of sales, which could adversely affect results of operations; however, management believes that suitable replacement suppliers could be obtained in such an event.
F-60
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
Operating Leases
The Company conducts its operations in leased facilities under operating lease agreements that expire through fiscal 2022. The Company leases certain equipment under operating lease agreements that expire through fiscal 2015. Substantially all of the Company’s lease agreements require the Company to maintain the facilities during the term of the lease and to pay all taxes, insurance, utilities and other costs associated with those facilities. The Company makes customary representations and warranties and agrees to certain financial covenants and indemnities. In the event the Company defaults on a lease, typically the landlord may terminate the lease, accelerate payments and collect liquidated damages. As of September 26, 2009, the Company was not in default of any covenants contained in the lease. Certain of the Company’s lease agreements provide for renewal options. Such renewal options are at rates similar to the current rates under the agreements.
Future minimum lease payments under all of the Company’s operating leases at September 26, 2009 are as follows:
| | | |
| | | Amount |
Fiscal 2010 | | $ | 17,823 |
Fiscal 2011 | | | 16,400 |
Fiscal 2012 | | | 14,138 |
Fiscal 2013 | | | 11,646 |
Fiscal 2014 | | | 10,780 |
Thereafter | | | 45,326 |
| | | |
Total (not reduced by minimum sublease rentals of $4,300) | | $ | 116,113 |
| | | |
Rent expense, net of sublease income, was $17,140, $13,890, and $7,355 for fiscal years 2009, 2008 and 2007, respectively.
The Company subleased a portion of its Bedford and Santa Clara facilities and has received rental income of $390, $247 and $158 for fiscal years 2009, 2008 and 2007, respectively, which has been recorded as an offset to rent expense. As a result of the merger with Cytyc, the Company assumed an arrangement in which the Company is sub-leasing all of its Mountain View facility to a third party for a term of approximately five years, a period of time equivalent to the remainder of the Company’s lease of this facility. The sub-lease commenced on July 1, 2007. The Company received rental income of $1,062 and $739 for fiscal years 2009 and 2008, respectively, which has been recorded as an offset to rent expense.
The Company subleases a portion of its Newark, DE facility and received rental income of $1,550, $1,531 and $1,551 for fiscal years 2009, 2008 and 2007, respectively, which has been recorded as an offset to rent expense. The future minimum annual rental income payments under these sublease agreements at September 26, 2009 are as follows:
| | | |
| | | Amount |
Fiscal 2010 | | $ | 1,550 |
Fiscal 2011 | | | 1,550 |
Fiscal 2012 | | | 1,543 |
Fiscal 2013 | | | 1,531 |
Fiscal 2014 | | | 1,531 |
Thereafter | | | 893 |
| | | |
Total | | $ | 8,598 |
| | | |
F-61
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
The majority of this sublease income is from one tenant, and this income is being accounted for on a straight-line basis.
Workforce Subject to Collective Bargaining Agreements
Approximately 200 of AEG’s German employees are represented by a Works Council and are subject to collective bargaining agreements. None of the Company’s other employees are subject to a collective bargaining agreement.
| 13. | Business Segments and Geographic Information |
The Company reports segment information in accordance with ASC 280,Segment Reporting(formerly SFAS No. 131,Disclosures about Segments of an Enterprise and Related Information). Operating segments are identified as components of an enterprise about which separate, discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. The Company’s chief operating decision maker is the president, and the Company’s reportable segments have been identified based on the end markets to which its product are sold into. Each reportable segment generates revenue from either the sale of medical equipment and related services and/or sale of disposable supplies, primarily used in providing diagnostic tests and surgical procedures. As of September 26, 2009, the Company has four reportable segments. Certain reportable segments represent an aggregation of operating units within each segment. The Company measures and evaluates its reportable segments based on segment revenues and operating income (loss).
In fiscal 2008, as a result of the merger with Cytyc, the Company reassessed its segment reporting based on the operating and reporting structure of the combined company. Beginning in fiscal 2008, the Company combined its previously reported Other Business segment with its Breast Health (formerly Mammography/Breast Care) and Skeletal Health (formerly Osteoporosis) segments, to better reflect how the Company views its operations and manages its business. The Company’s Other Business segment previously included AEG, mini C-arm, extremity MRI, conventional general radiography service and digital general radiography systems businesses. The AEG business is now part of Breast Health while the remaining reporting units are part of Skeletal Health. In addition, the Company began reporting two new operating segments: Diagnostics and GYN Surgical. Diagnostics includes the ThinPrep Products and the Rapid Fetal Fibronectin test, as well as the Company’s proprietary Invader chemistry and two Cervista HPV tests obtained in the Third Wave acquisition, which received FDA approval in fiscal 2009. GYN Surgical includes the NovaSure system and the Adiana Permanent Contraception System, which received FDA approval in fiscal 2009. The MammoSite Radiation Therapy system, previously part of Cytyc’s surgical reporting segment, which is a single-use device for the treatment of early-stage breast cancer, is part of the Company’s Breast Health segment. The Company received FDA approval for its multi-lumen product, MammoSite ML, in August 2009.The Diagnostic segment also includes the results of Third Wave, which was acquired in the fourth quarter of fiscal 2008.
F-62
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
As a result of these changes in fiscal 2008, the Company reports its business as four segments: Breast Health, Diagnostics, GYN Surgical and Skeletal Health. Identifiable assets for the four principal operating segments consist of inventories, intangible assets, and property and equipment. The Company fully allocates depreciation expense to its four reportable segments; however, certain depreciable assets have not been allocated to these segments. The Company presents all other identifiable assets as corporate assets. Intersegment sales and transfers are not significant. Segment information for fiscal years 2009, 2008 and 2007 is as follows:
| | | | | | | | | | | |
| | | Years ended |
| | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 |
Total revenues: | | | | | | | | | | | |
Breast Health | | $ | 728,884 | | | $ | 860,848 | | | $ | 638,898 |
Diagnostics | | | 547,892 | | | | 485,004 | | | | — |
GYN Surgical | | | 264,900 | | | | 221,069 | | | | — |
Skeletal Health | | | 95,458 | | | | 107,578 | | | | 99,470 |
| | | | | | | | | | | |
| | $ | 1,637,134 | | | $ | 1,674,499 | | | $ | 738,368 |
| | | | | | | | | | | |
Operating (loss) income: | | | | | | | | | | | |
Breast Health | | $ | (122,559 | ) | | $ | 211,704 | | | $ | 146,907 |
Diagnostics | | | (809,640 | ) | | | (172,538 | ) | | | — |
GYN Surgical | | | (1,097,685 | ) | | | (241,450 | ) | | | — |
Skeletal Health | | | 13,210 | | | | 4,742 | | | | 845 |
| | | | | | | | | | | |
| | $ | (2,016,674 | ) | | $ | (197,542 | ) | | $ | 147,752 |
| | | | | | | | | | | |
Depreciation and amortization: | | | | | | | | | | | |
Breast Health | | $ | 50,764 | | | $ | 38,990 | | | $ | 26,891 |
Diagnostics | | | 157,562 | | | | 97,282 | | | | — |
GYN Surgical | | | 56,341 | | | | 30,702 | | | | — |
Skeletal Health | | | 9,257 | | | | 5,976 | | | | 4,271 |
| | | | | | | | | | | |
| | $ | 273,924 | | | $ | 172,950 | | | $ | 31,162 |
| | | | | | | | | | | |
Capital expenditures: | | | | | | | | | | | |
Breast Health | | $ | 10,966 | | | $ | 17,493 | | | $ | 15,570 |
Diagnostics | | | 7,779 | | | | 10,585 | | | | — |
GYN Surgical | | | 5,856 | | | | 14,119 | | | | — |
Skeletal Health | | | 6,756 | | | | 11,339 | | | | 7,270 |
| | | | | | | | | | | |
| | $ | 31,357 | | | $ | 53,536 | | | $ | 22,840 |
| | | | | | | | | | | |
Identifiable assets: | | | | | | | | | | | |
Breast Health | | $ | 1,133,714 | | | $ | 1,435,674 | | | $ | 718,155 |
Diagnostics | | | 1,942,494 | | | | 2,976,854 | | | | — |
GYN Surgical | | | 1,860,834 | | | | 3,080,365 | | | | — |
Skeletal Health | | | 30,937 | | | | 25,151 | | | | 29,531 |
Corporate | | | 721,849 | | | | 616,588 | | | | 318,663 |
| | | | | | | | | | | |
| | $ | 5,689,828 | | | $ | 8,134,632 | | | $ | 1,066,349 |
| | | | | | | | | | | |
F-63
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
As a result of the Company’s interim impairment analysis of goodwill as of December 27, 2008, the Company recorded a goodwill impairment charge of $2,340,023 during the three months ended March 28, 2009 comprised of $1,165,804 for GYN Surgical, $908,349 for Diagnostics, and $265,870 for Breast Health. These charges are reflected in each reportable segments’ operating loss for fiscal 2009. In connection with its acquisitions in fiscal 2008, the Company recorded in-process research and development charges of $280,400 in Diagnostics and $284,800 in GYN Surgical.
Product export sales from the United States to unaffiliated customers, primarily in Europe, Asia and Latin America, during fiscal 2009, 2008 and 2007 totaled approximately $291,356, $297,287 and $158,827, respectively.
Products sold by the Company internationally are manufactured at domestic and international manufacturing locations such as Costa Rica where much of the GYN Surgical products are currently being manufactured.
Transfers between the Company and its subsidiaries are generally recorded at amounts similar to the prices paid by unaffiliated foreign dealers. All intercompany profit is eliminated in consolidation.
Export product sales as a percentage of total product sales are as follows:
| | | | | | | | | |
| | | Years ended | |
| | | September 26,
2009 | | | September 27,
2008 | | | September 29,
2007 | |
Europe | | 12 | % | | 12 | % | | 15 | % |
Asia | | 4 | | | 4 | | | 5 | |
All others | | 4 | | | 4 | | | 5 | |
| | | | | | | | | |
| | 20 | % | | 20 | % | | 25 | % |
| | | | | | | | | |
The Company’s property and equipment are geographically located as follows:
| | | | | | |
| | | Years ended |
| | | September 26,
2009 | | September 27,
2008 |
United States | | $ | 199,881 | | $ | 213,482 |
Costa Rica | | | 35,886 | | | 34,786 |
Europe | | | 32,328 | | | 34,085 |
All other countries | | | 3,533 | | | 1,622 |
| | | | | | |
| | $ | 271,628 | | $ | 283,975 |
| | | | | | |
F-64
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
| 14. | Accrued Expenses and Other Long-Term Liabilities |
Accrued expenses and other long-term liabilities consist of the following:
| | | | | | |
| | | September 26,
2009 | | September 27,
2008 |
Accrued Expenses | | | | | | |
Accrued compensation and employee benefits | | $ | 51,727 | | $ | 68,204 |
Accrued commissions | | | 14,325 | | | 15,768 |
Accrued income and other taxes | | | 18,056 | | | 11,744 |
Accrued interest | | | 10,184 | | | 11,284 |
Accrued warranty, current portion | | | 5,602 | | | 9,080 |
Other accrued expenses | | | 37,390 | | | 38,666 |
| | | | | | |
| | $ | 137,284 | | $ | 154,746 |
| | | | | | |
| | | | | | |
| | | September 26,
2009 | | September 27,
2008 |
Other Long-Term Liabilities | | | | | | |
Accrued lease obligation—long-term | | $ | 31,650 | | $ | 31,164 |
Reserve for income tax uncertainties | | | 14,728 | | | 12,307 |
Pension liabilities—long-term | | | 6,404 | | | 6,995 |
Other | | | 5,752 | | | 6,987 |
| | | | | | |
| | $ | 58,534 | | $ | 57,453 |
| | | | | | |
| 15. | Litigation and Other Matters |
On October 5, 2007, Ethicon Endo-Surgery, Inc., a Johnson & Johnson operating company, filed a complaint against the Company and its wholly-owned subsidiary Suros in the United States District Court for the Southern District of Ohio, Western Division. The complaint alleges that certain of the ATEC biopsy systems manufactured and sold by Suros infringe four Ethicon patents. An amended complaint filed January 11, 2008 additionally asserts claims of unfair competition. The complaint seeks to enjoin Hologic and Suros from conducting acts of unfair competition and infringing the patents as well as the recovery of unspecified damages and costs. A Markman hearing was held on January 8, 2009, and the Court issued its ruling on April 3, 2009. A court ordered settlement conference occurred on August 11, 2009 without any resolution. This suit is currently scheduled to go to trial on February 1, 2010. The Company is unable to reasonably estimate the ultimate outcome of this case.
On May 22, 2009, Conceptus, Inc. filed suit in the United States District Court for the Northern District of California seeking a declaration by the Court that Hologic’s planned importation, use, sale or offer to sell of its forthcoming Adiana Permanent Contraception System, would infringe five Conceptus patents. On July 9, 2009, Conceptus filed an amended complaint alleging infringement of the same five patents by the Adiana Permanent Contraception System. The complaint seeks preliminary and permanent injunctive relief and unspecified monetary damages. In addition to the amended complaint, Conceptus also filed a motion for preliminary injunction seeking to preliminarily enjoin sales of the Adiana System based on alleged infringement of certain claims of three of the five patents. A hearing on Conceptus’ preliminary injunction motion was held on November 4, 2009, and on November 6, 2009, the judge issues an order denying the motion. A hearing on claim construction is scheduled for March 10, 2010. A trial date has not been set. Based on the early stage of this litigation, the Company is unable to reasonably estimate the ultimate outcome of this case.
F-65
Hologic, Inc.
Notes to Consolidated Financial Statements (continued)
(In thousands, except per share data)
On August 6, 2009, Ethicon Endo-Surgery, Inc., a Johnson & Johnson operating company, filed a complaint against the Company and its wholly-owned subsidiary Suros in the United States District Court for the District of Delaware. The complaint alleges that certain of the Eviva biopsy systems manufactured and sold by Suros infringe four Ethicon patents. The complaint seeks to enjoin Hologic and Suros from infringing the patents as well as recovery of damages and costs resulting from the alleged infringement. Based on the early stage of this litigation, the Company is unable to reasonably estimate the ultimate outcome of this case.
As of September 26, 2009, the Company does not believe a loss is probable in any of the matters disclosed above. The Company is a party to various other legal proceedings and claims arising out of the ordinary course of its business. The Company believes that except for those described above there are no other proceedings or claims pending against it the ultimate resolution of which would have a material adverse effect on its financial condition or results of operations.
| 16. | Quarterly Statement of Operations Information (Unaudited) |
The following table presents a summary of quarterly results of operations for fiscal 2009 and 2008:
| | | | | | | | | | | | | |
| | | 2009 |
| | First
Quarter | | Second
Quarter | | | Third
Quarter | | Fourth
Quarter |
Total revenue | | $ | 429,233 | | $ | 402,014 | | | $ | 403,120 | | $ | 402,767 |
Gross profit (1) | | | 229,959 | | | 209,574 | | | | 211,145 | | | 206,808 |
Net income (loss) (2) | | | 47,993 | | | (2,300,170 | ) | | | 41,000 | | | 34,940 |
Diluted net income (loss) per common share | | $ | 0.19 | | $ | (8.97 | ) | | $ | 0.16 | | $ | 0.13 |
| | | | | | | | | | | | | | |
| | | 2008 | |
| | First
Quarter | | | Second
Quarter | | Third
Quarter | | Fourth
Quarter | |
Total revenue (3) | | $ | 371,445 | | | $ | 431,048 | | $ | 429,492 | | $ | 442,513 | |
Gross profit | | | 167,835 | | | | 239,638 | | | 244,763 | | | 240,281 | |
Net income (loss) (2) | | | (358,608 | ) | | | 55,986 | | | 61,379 | | | (144,374 | ) |
Diluted net income(loss) per common share | | $ | (1.65 | ) | | $ | 0.22 | | $ | 0.24 | | $ | (0.56 | ) |
| (1) | During the third quarter of fiscal 2009, the Company determined that certain amounts previously classified as a component of selling and marketing expenses should be reclassified to cost of product sales. As a result, gross profit was reduced by $704 in the first quarter and $689 in the second quarter of fiscal 2009. |
| (2) | See Note 2 for further discussion of goodwill impairment charges recorded in the second quarter of fiscal 2009, and see Note 3 for further discussion of in-process research and development expenses incurred in the first and fourth quarters of fiscal 2008 related to the merger with Cytyc and Third Wave acquisition. |
| (3) | The sum of the quarterly total revenue does not agree with the Consolidated Statements of Operations due to rounding. |
On November 5, 2009, the Company’s Board of Directors appointed Robert Cascella, its President and Chief Operating Officer, to the position of President and Chief Executive Officer. John Cumming, our former Chairman and Chief Executive Officer, will remain with the Company as Chairman and an executive officer. In connection with this change in responsibilities, the Company made a payment of $1,750 to the Chairman and provided that he remains employed by the Company on the first and second anniversary of the effective date of the change, he will receive additional payments of $1,725 for each period.
F-66
Exhibit Index
| | | | |
Exhibit
Number | | | | Reference |
| 2.01 | | Agreement and Plan of Merger between Hologic, Nor’easter Corp. and Cytyc dated May 20, 2007 | | K-2.1 |
| | |
| 2.02 | | Agreement and Plan of Merger and Reorganization, dated February 26, 2007, by and among Adiana, Inc., Cytyc, Admiral Acquisition Corp. and the Stockholder Representative Committee | | N-2.1 |
| | |
| 2.03 | | Agreement and Plan of Merger, dated as of June 8, 2008, by and among Hologic, Thunder Tech Corp. and Third Wave Technologies, Inc. | | R-2.1 |
| | |
| 3.01 | | Certificate of Incorporation of Hologic | | A-3.01 |
| | |
| 3.02 | | Amendment to Certificate of Incorporation of Hologic | | C-3.03 |
| | |
| 3.03 | | Certificate of Amendment to Certificate of Incorporation of Hologic | | I-3.03 |
| | |
| 3.04 | | Certificate of Amendment to Certificate of Incorporation of Hologic | | FF-3.1 |
| | |
| 3.05 | | Certificate of Amendment to Certificate of Incorporation of Hologic | | S-3.1 |
| | |
| 3.06 | | Second Amended and Restated By-laws of Hologic, as amended | | DD-3.1 |
| | |
| 3.07 | | Amended and Restated Certificate of Designations of Series A Junior Participating Preferred Stock of Hologic | | T-3.6 |
| | |
| 4.01 | | Specimen Certificate for Shares of Hologic’s Common Stock | | B-1 |
| | |
| 4.02 | | Description of Capital Stock (Contained in the Certificate of Incorporation of Hologic, as Amended, Filed as Exhibits 3.01, 3.02, 3.03, 3.04 and 3.05) | | A-3.01; C-3.03;
I-3.03; FF-3.1
and S-3.1 |
| | |
| 4.03 | | Rights Agreement dated September 17, 2002 | | G-4 |
| | |
| 4.04 | | Amended and Restated Rights Agreement dated April 2, 2008 | | T-4.1 |
| | |
| 4.05 | | Form of Rights Certificate | | Q-4 |
| | |
| 4.06 | | Indenture, dated as of December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic | | U-4.1 |
| | |
| 4.07 | | First Supplemental Indenture, dated December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic | | U-4.2 |
| | |
| 10.01 | | Second Amended and Restated 1990 Non-Employee Director Stock Option Plan | | C-10.26* |
| | |
| 10.02 | | 1995 Combination Stock Option Plan | | C-10.25* |
| | |
| 10.03 | | Second Amended and Restated 1999 Equity Incentive Plan | | I-10.3* |
| | |
| 10.04 | | Amendment No. 1 to Second Amended and Restated 1999 Equity Incentive Plan | | L-10.2* |
| | |
| 10.05 | | Amendment No. 2 to Second Amended and Restated 1999 Equity Incentive Plan | | FF-10.17* |
| | |
| 10.06 | | Amendment No. 3 to Second Amended and Restated 1999 Equity Incentive Plan | | HH-10.3* |
| | |
| 10.07 | | 1997 Employee Equity Incentive Plan | | D-99 |
| | |
| 10.08 | | 2000 Acquisition Equity Incentive Plan | | F-10.05 |
| | |
| 10.09 | | Hologic 2008 Equity Incentive Plan | | S-10.1* |
| | |
| 10.10 | | Form of Employee Stock Option Award Agreement under 2008 Equity Incentive Plan | | V-10.1* |
| | | | |
Exhibit
Number | | | | Reference |
| | |
| 10.11 | | Form of Employee Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan | | V-10.2* |
| | |
| 10.12 | | Form of Independent Director Stock Option Award Agreement under 2008 Equity Incentive Plan | | HH-10.1* |
| | |
| 10.13 | | Form of Independent Director Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan | | HH-10.2* |
| | |
| 10.14 | | Amended and Restated 2008 Employee Stock Purchase Plan | | filed herewith* |
| | |
| 10.15 | | Hologic 2009 Short-Term Incentive Plan | | V-10.3* |
| | |
| 10.16 | | Hologic 2010 Short-Term Incentive Plan | | EE-10.1* |
| | |
| 10.17 | | Cytyc Corporation 1995 Stock Plan | | L-10.4* |
| | |
| 10.18 | | Cytyc Corporation 1995 Non-Employee Director Stock Option Plan | | L-10.5* |
| | |
| 10.19 | | Cytyc Corporation 1998 Stock Plan of Pro Duct Health, Inc. | | L-10.6* |
| | |
| 10.20 | | Cytyc Corporation 2001 Non-Employee Director Stock Plan | | L-10.7* |
| | |
| 10.21 | | Cytyc Corporation 2004 Omnibus Stock Plan | | L-10.8* |
| | |
| 10.22 | | Form of Indemnification Agreement (as executed with each director of Hologic) | | CC-10.1* |
| | |
| 10.23 | | Executive Bonus Plan Description | | X-10.1* |
| | |
| 10.24 | | Hologic Supplemental Executive Retirement Plan (SERP) | | BB-10.4* |
| | |
| 10.25 | | Form of SERP Rabbi Trust Agreement | | J-10.11* |
| | |
| 10.26 | | Form of Officer Severance Agreement including list of officers to whom provided | | GG-10.7* |
| | |
| 10.27 | | Retention and Severance Agreement dated May 3, 2006, by and between Hologic and John W. Cumming | | GG-10.4* |
| | |
| 10.28 | | Retention and Severance Agreement dated May 3, 2006, by and between Hologic and Robert A. Cascella | | GG-10.5* |
| | |
| 10.29 | | Retention and Severance Agreement dated May 3, 2006, by and between Hologic and Glenn P. Muir | | GG-10.6* |
| | |
| 10.30 | | Form of Restricted Stock Unit Award under the Retention and Severance Agreement filed as exhibit 10.27, 10.28 and 10.29 | | GG-10.9* |
| | |
| 10.31 | | Transition Agreement dated November 5, 2009, by and between Hologic and John W. Cumming | | DD-10.1* |
| | |
| 10.32 | | Form of Senior Vice President Change of Control Agreement including list of officers to whom provided | | BB-10.5* |
| | |
| 10.33 | | Form of Senior Executive Officer Change of Control Agreement including list of officers to whom provided | | EE-10.2* |
| | |
| 10.34 | | John W. Cumming Waiver Letter Dated As Of May 20, 2007 | | K-10.1* |
| | |
| 10.35 | | Robert A. Cascella Waiver Letter Dated As Of May 20, 2007 | | K-10.2* |
| | |
| 10.36 | | Glenn P. Muir Waiver Letter Dated As Of May 20, 2007 | | K-10.3* |
| | |
| 10.37 | | Second Retention Agreement with Robert A. Cascella dated as of October 22, 2007 | | FF-10.10* |
| | | | |
Exhibit
Number | | | | Reference |
| | |
| 10.38 | | Restricted Stock Grant Agreement with Robert A. Cascella dated as of October 22, 2007 | | FF-10.18* |
| | |
| 10.39 | | Executive Financial Services Program | | AA-10.46* |
| | |
| 10.40 | | Facility Lease (Danbury) dated as of December 30, 1995 by and among Melvin J. Powers and Mary P. Powers D/B/A M&N Realty and Lorad | | E-10.14 |
| | |
| 10.41 | | Lease Agreement (Danbury and Bedford) by and between BONE (DE) QRS 15-12, INC., and Hologic dated as of August 28, 2002 as amended | | H-10.27;
Z-10.41 |
| | |
| 10.42 | | Office Lease dated December 31, 2003 between Cytyc and Marlborough Campus Limited Partnership | | O-10.17 |
| | |
| 10.43 | | Lease Agreement by and between Zona Franca Coyol S.A. and Cytyc Surgical Products Costa Rica S.A. dated April 23, 2007 | | Z-10.45 |
| | |
| 10.44 | | Lease Agreement by and between 445 Simarano Drive, Marlborough LLC and Cytyc dated July 11, 2006 | | Z-10.46 |
| | |
| 10.45 | | Supply Agreement between Cytyc, Whatman, Inc. and Whatman SA dated as of December 31, 2000, as amended, October 16, 2001 and May 2, 2002 | | P-10.13 |
| | |
| 10.46 | | Credit and Guaranty Agreement dated as of October 22, 2007 among Hologic, the Guarantors party thereto and defined below, the Secured Parties party thereto, and the Agent, Banc of America Securities LLC, Bank of America, N.A., Citicorp North America, Inc., JPMorgan Chase Bank, N.A., RBS Citizens, National Association and Fifth Third Bank | | M-10.1 |
| | |
| 10.47 | | Waiver and First Amendment to Credit and Guaranty Agreement and Pledge and Security Agreement dated as of April 14, 2008 by and among Hologic and its domestic subsidiaries, excluding the subsidiaries which are Massachusetts securities corporations and Goldman Sachs Credit Partners L.P. | | W-10.1 |
| | |
| 10.48 | | Amended and Restated Credit and Guaranty Agreement dated as of July 17, 2008 among Hologic, Goldman Sachs Credit Partners L.P., as Sole Lead Arranger and Sole Lead Bookrunner, Goldman Sachs Credit Partners L.P., JPMorgan Chase Bank, N.A. and RBS Citizens, National Association, as Co-Syndication Agents, Goldman Sachs Credit Partners L.P., as Administrative Agent and Collateral Agent and Royal Bank of Canada, as Documentation Agent and each lender from time to time party thereto | | Y-10.1 |
| | |
| 10.49 | | Pledge and Security Agreement among Hologic, Goldman Sachs Credit Partners L.P., as Collateral Agent thereunder and the other parties therein named dated as of October 22, 2007 | | FF-10.2 |
| | |
| 10.50 | | Amended and Restated Pledge and Security Agreement dated as of July 17, 2008 among Hologic, Goldman Sachs Credit Partners L.P., as Collateral Agent thereunder and the others parties named therein | | Y-10.2 |
| | |
| 10.51 | | Open End Mortgage Deed, Security Agreement, Assignment of Rents and Leases and Fixture Filing for 36 Apple Ridge Road, Danbury, Connecticut dated as of October 22, 2007 | | FF-10.3 |
| | |
| 10.52 | | Open End Mortgage Deed, Security Agreement, Assignment of Rents and Leases and Fixture Filing for 37 Apple Ridge Road, Danbury, Connecticut dated as of October 22, 2007 | | FF-10.4 |
| | | | |
Exhibit
Number | | | | Reference |
| | |
| 10.53 | | Mortgage, Security Agreement, Assignment of Rents and Leases and Fixture Filing for 35 Crosby Drive, Bedford, Massachusetts dated as of October 22, 2007 | | FF-10.5 |
| | |
| 10.54 | | Lease Guaranty dated October 22, 2007 between Bel Marlborough I LLC and Hologic, as guarantor thereunder | | FF-10.7 |
| | |
| 14.1 | | Code of Ethics for Senior Financial Officers | | FF-14.1 |
| | |
| 21.01 | | Subsidiaries of Hologic | | filed herewith |
| | |
| 23.01 | | Consent of Ernst & Young LLP | | filed herewith |
| | |
| 31.1 | | Certification of Hologic’s CEO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 31.2 | | Certification of Hologic’s CFO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 32.1 | | Certification of Hologic’s CEO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 32.2 | | Certification of Hologic’s CFO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | filed herewith |
| | |
| 101.INS | | XBRL Instance Document | | filed herewith |
| | |
| 101.SCH | | XBRL Taxonomy Extension Schema Document | | filed herewith |
| | |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document | | filed herewith |
| | |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document | | filed herewith |
| | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document | | filed herewith |
| * | Management compensation plan or arrangement |
| A | We previously filed this exhibit on January 24, 1990 with the referenced exhibit number as an exhibit to our Registration Statement on Form S-1 (Registration No. 33-33128) and the previously filed exhibit is incorporated herein by reference. |
| B | We previously filed this exhibit on January 31, 1990 with the referenced exhibit number as an exhibit to our Registration Statement on Form 8-A, and the previously filed exhibit is incorporated herein by reference. |
| C | We previously filed this exhibit on May 14, 1996, with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the quarter ended March 30, 1996, and the previously filed exhibit is incorporated herein by reference. |
| D | We previously filed this exhibit on August 20, 1997 with the referenced exhibit number as an exhibit to our Registration Statement on Form S-8 (SEC File No. 333-34003) and the previously filed exhibit is incorporated herein by reference. |
| E | Trex Medical Corporation previously filed this exhibit with the referenced exhibit number as an exhibit to its Registration Statement on Form S-1 (Reg. No. 333-2926) and the previously filed exhibit is incorporated by reference. |
| F | We previously filed this exhibit on December 12, 2001 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 29, 2001, and the previously filed exhibit is incorporated by reference. |
| G | We previously filed this exhibit on December 4, 2002 with the referenced exhibit number as an exhibit to our registration statement on Form 8-A (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| H | We previously filed this exhibit on December 24, 2002 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 28, 2002, and the previously filed exhibit is incorporated herein by reference. |
| I | We previously filed this exhibit on December 6, 2005 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 24, 2005, and the previously filed exhibit is incorporated herein by reference. |
| J | We previously filed this exhibit on December 14, 2006 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 30, 2006, and the previously filed exhibit is incorporated herein by reference. |
| K | We previously filed this exhibit on May 21, 2007 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| L | We previously filed this exhibit on October 23, 2007 with the referenced exhibit number to our Registration Statement on Form S-8 (SEC File No. 333-146887) and the previously filed exhibit is incorporated herein by reference. |
| M | We previously filed this exhibit on October 23, 2007 with the referenced exhibit number to our Current Report on Form 8-K/A (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| N | Cytyc Corporation previously filed this exhibit on February 28, 2007 with the referenced exhibit number as an Exhibit to its Current Report on Form 8-K (SEC File No. 000-27558) and the previously filed exhibit is incorporated by reference. |
| O | Cytyc Corporation previously filed this exhibit on January 30, 2004 with the referenced exhibit number as an exhibit to its Annual Report on Form 10-K (SEC File No. 000-27558) and the previously filed exhibit is incorporated by reference. |
| P | Cytyc Corporation previously filed this exhibit on March 24, 2003 with the referenced exhibit number as an exhibit to its Annual Report on Form 10-K (SEC File No. 000-27558) and the previously filed exhibit is incorporated by reference. |
| Q | We previously filed this exhibit on September 26, 2002 with the referenced exhibit number as an exhibit to our Registration Statement on Form 8-K (SEC File No. 000-18218) and the previously filed exhibit is incorporated herein by reference. |
| R | We previously filed this exhibit on June 9, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| S | We previously filed this exhibit on March 11, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| T | We previously filed this exhibit on April 3, 2008 with the referenced exhibit number as an exhibit to our Registration Statement on Form 8-A (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| U | We previously filed this exhibit on December 10, 2007 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| V | We previously filed this exhibit on November 17, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| W | We previously filed this exhibit on May 8, 2008 with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the fiscal quarter ended March 29, 2008, and the previously filed exhibit is incorporated herein by reference. |
| X | We previously filed this exhibit on January 17, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| Y | We previously filed this exhibit on July 17, 2008 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| Z | We previously filed this exhibit on November 27, 2007 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 29, 2007, and the previously filed exhibit is incorporated herein by reference. |
| AA | We previously filed this exhibit on November 26, 2008 with the referenced exhibit number as an exhibit to our Annual Report on Form 10-K (SEC File No. 000-18281) for the fiscal year ended September 27, 2008, and the previously filed exhibit is incorporated herein by reference. |
| BB | We previously filed this exhibit on February 5, 2009, with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the quarter ended December 27, 2008, and the previously filed exhibit is incorporated herein by reference. |
| CC | We previously filed this exhibit on March 6, 2009 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| DD | We previously filed this exhibit on November 9, 2009 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| EE | We previously filed this exhibit on November 17, 2009 with the referenced exhibit number as an exhibit to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| FF | We previously filed this exhibit on October 22, 2007 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
| GG | We previously filed this exhibit on May 4, 2006 with the referenced exhibit number as an exhibit to our Quarterly Report on Form 10-Q (SEC File No. 000-18281) for the fiscal quarter ended March 25, 2006, and the previously filed exhibit is incorporated herein by reference. |
| HH | We previously filed this exhibit on December 12, 2008 with the referenced exhibit number to our Current Report on Form 8-K (SEC File No. 000-18281) and the previously filed exhibit is incorporated herein by reference. |
