UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: September 29, 2012
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 0-18281
Hologic, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| | |
| Delaware | | 04-2902449 |
(State or Other Jurisdiction of Incorporation or Organization) | | (IRS Employer Identification No.) |
35 Crosby Drive, Bedford, Massachusetts 01730
(Address of Principal Executive Offices) (Zip Code)
Registrant’s Telephone Number, Including Area Code (781) 999-7300
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of Each Class | | Name of Each Exchange on which Registered |
| Common Stock, $.01 par value | | Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: Rights to Purchase Preferred Stock
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one).
| | | | | | |
| Large accelerated filer | | x | | Accelerated filer | | ¨ |
| | | |
| Non-accelerated filer | | ¨ (Do not check if a smaller reporting company) | | Smaller reporting company | | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of the registrant’s Common Stock held by non-affiliates of the registrant as of March 24, 2012 was $5,549,871,525 based on the price of the last reported sale on the Nasdaq Global Select Market on that date.
As of November 20, 2012, there were 266,770,196 shares of the registrant’s Common Stock, $.01 par value, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for the registrant’s annual meeting of stockholders to be filed within 120 days of the end of its fiscal year ended September 29, 2012 are incorporated into Part III (Items 10, 11, 12, 13 and 14) of this Annual Report on Form 10-K where indicated.
HOLOGIC, INC.
ANNUAL REPORT ON FORM 10-K
For the Fiscal Year Ended September 29, 2012
TABLE OF CONTENTS
2
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements contained in this report are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements involve known and unknown risks, uncertainties and other factors which may cause our or our industry’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding:
| | • | | the effect of the continuing worldwide macroeconomic uncertainty on our business and results of operation; |
| | • | | the coverage and reimbursement decisions of third-party payors relating to the use of our products and treatments; |
| | • | | the uncertainty of the impact of cost containment efforts and federal healthcare reform legislation on our business and results of operation; |
| | • | | the anticipated impact of the U.S. excise tax on the sale of most medical devices, currently scheduled to become effective on January 1, 2013, on our business and results of operation; |
| | • | | the impact and anticipated benefits of the acquisition of Gen-Probe and the challenges associated with successfully integrating and operating the Gen-Probe business; |
| | • | | the impact and anticipated benefits of other recently completed acquisitions and acquisitions we may complete in the future; |
| | • | | our ability to consolidate certain of our manufacturing operations on a timely basis without disrupting our business and to achieve anticipated cost synergies in connection therewith; |
| | • | | our goal of expanding our market positions; |
| | • | | the development of new competitive technologies and products; |
| | • | | regulatory approval and clearances for our products; |
| | • | | production schedules for our products; |
| | • | | the anticipated development of our markets and the success of our products in these markets; |
| | • | | the anticipated performance and benefits of our products; |
| | • | | estimated asset and liability values; |
| | • | | the impact and costs and expenses of any litigation we may be subject to now or in the future; |
| | • | | our compliance with covenants contained in our indebtedness; |
| | • | | anticipated trends relating to our financial condition or results of operations; and |
| | • | | our capital resources and the adequacy thereof. |
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by such forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this report. Except as otherwise required by law, we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statement contained in this report to reflect any change in our expectations or any change in events, conditions or circumstances on which any of our forward-looking statements are based. Factors that could cause or contribute to differences in our future financial results include the cautionary statements set forth herein and in our other filings with the Securities and Exchange Commission, or SEC, including those set forth under “Risk Factors” set forth in Part I, Item 1A of this annual report on Form 10-K. We qualify all of our forward-looking statements by these cautionary statements.
TRADEMARK NOTICE
Hologic is a trademark of Hologic, Inc. Other trademarks, logos, and slogans registered or used by Hologic and its divisions and subsidiaries in the United States and other countries include, but are not limited to, the following: Adiana, Affirm, APTIMA, APTIMA COMBO 2, Aquilex, ATEC, Celero, Cervista, C-View, Dimensions, Eviva, Fluoroscan, Gen-Probe, Healthcome, Interlace, Invader, LIFECODES, LORAD, MammoPad, MammoSite, MultiCare, MyoSure, NovaSure, PANTHER, PROCLEIX, PreservCyt, QDR, Rapid fFN, Sahara, SecurView, Selenia, Sentinelle, Serenity, StereoLoc, Suresound, TCT, ThinPrep, THA, THS, TIGRIS, TLI IQ, and Trident.
3
PART I
Overview
We are a leading developer, manufacturer and supplier of premium diagnostics products, medical imaging systems and surgical products dedicated to serving the healthcare needs of women. Our core business units are focused on breast health, diagnostics, GYN surgical, and skeletal health. We sell and service our products through a combination of direct sales and service forces and a network of independent distributors and sales representatives.
Our breast health products include a broad portfolio of breast imaging and related products and accessories, including digital and film-based mammography systems, magnetic resonance imaging, or MRI, breast coils, computer-aided detection, or CAD, for mammography and MRI, minimally invasive breast biopsy devices, breast biopsy site markers, breast biopsy guidance systems, breast imaging comfort pads, and breast brachytherapy products. Our most advanced breast imaging platform, Dimensions, utilizes a new technology called tomosynthesis to produce three dimensional, or 3D, images, as well as conventional two dimensional, or 2D, full field digital mammography images. In the U.S., our Dimensions product was approved in December 2008 by the Food and Drug Administration, or FDA, for providing conventional 2D images. In February 2011, we received approval from the FDA to enable the 3D tomosynthesis capability of our Dimensions system.
We offer a wide range of diagnostic products which are used primarily to aid in the diagnosis of human diseases and screen donated human blood. Our molecular diagnostics products include our APTIMA family of assays, our proprietary Invader chemistry and advanced instrumentation (PANTHER, TIGRIS and HTA). The APTIMA family of assays is used to detect the common sexually transmitted diseases, or STDs, chlamydia and gonorrhea, certain high-risk strains of the human papillomavirus, or HPV, and Trichomonas vaginalis, the parasite that causes trichomoniasis. Our Invader chemistry comprises molecular diagnostic reagents used for a variety of DNA and RNA analysis applications, including Cervista HPV high risk, or HR, and Cervista HPV 16/18 products to assist in the diagnosis of HPV, as well as other products to diagnose cystic fibrosis, cardiovascular risk and other diseases. Our diagnostics products also include the ThinPrep System, which is primarily used in cytology applications such as cervical cancer screening, and the Rapid Fetal Fibronectin Test, which assists physicians in assessing the risk of pre-term birth. In blood screening, we develop and manufacture the PROCLEIX family of assays, which are used to detect the human immunodeficiency virus, or HIV, the hepatitis C virus, or HCV, the hepatitis B virus, or HBV, and the West Nile virus, or WNV, in donated human blood. These blood screening products are marketed worldwide by our blood screening collaborator, Novartis Vaccines and Diagnostics, Inc., or Novartis, under Novartis’ trademarks.
Our GYN surgical products include the NovaSure Endometrial Ablation System, or NovaSure, and the MyoSure Hysteroscopic Tissue Removal System, or MyoSure. The NovaSure system involves a minimally invasive procedure for the treatment of heavy menstrual bleeding. The MyoSure system is a tissue removal device that is designed to provide transcervical or incision-less removal of fibroids and polyps within the uterus.
Our skeletal health products include dual-energy X-ray bone densitometry systems, an ultrasound-based osteoporosis assessment product, and our Fluoroscan mini C-arm imaging products.
We were incorporated in Massachusetts in October 1985 and reincorporated in Delaware in March 1990.
Recent Events
Acquisition of Gen-Probe Incorporated
On August 1, 2012, pursuant to the terms of the Agreement and Plan of Merger, dated April 29, 2012, referred to as the merger agreement, we completed our acquisition of Gen-Probe. Under the terms and conditions of the merger agreement, at the effective time and as a result of the acquisition, each share of common stock of Gen-Probe issued and outstanding immediately prior to the effective time of the acquisition was cancelled and converted into the right to receive $82.75 in cash. In addition, all outstanding restricted shares, restricted stock units, and performance shares and all stock options granted prior to February 8, 2012 were cancelled and converted into the merger consideration based upon an $82.75 per share price. Stock options granted after February 8, 2012 were cancelled and converted into stock options to acquire shares of Hologic common stock determined by a conversion formula defined in the merger agreement. The total purchase price was approximately $3.97 billion, which was funded through available cash and financing consisting of senior secured credit facilities and Senior Notes discussed below.
Concurrent with the closing of the Gen-Probe acquisition, on August 1, 2012, we and certain domestic subsidiaries entered into a credit and guaranty agreement with Goldman Sachs Bank USA in its capacity as administrative and collateral agent, and the lenders party thereto, pursuant to which we obtained senior secured financing totaling $2.8 billion, consisting of term loans in the aggregate principal amount of $2.5 billion and an undrawn $300 million revolving credit facility. Also on August 1, 2012, we completed a private placement of $1.0 billion aggregate principal amount of our 6.25% Senior Notes due August 1, 2020.
4
Gen-Probe, headquartered in San Diego, California, is a leader in molecular diagnostics products and services that are used primarily to diagnose human diseases, screen donated human blood and test transplant compatibility. Gen-Probe’s results of operations are reported within our diagnostics segment from the date of acquisition.
Available Information
Our Internet website address is http://www.hologic.com. Through our website, we make available, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or SEC. These SEC reports can be accessed through the investor relations section of our website. The information found on our website is not part of this or any other report we file with or furnish to the SEC.
You may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy and information statements, and other information regarding Hologic and other issuers that file electronically with the SEC. The SEC’s Internet website address is http://www.sec.gov.
Products
We view our operations and manage our business in four principal reporting segments: Breast Health, Diagnostics, GYN Surgical and Skeletal Health. Financial information concerning these segments is provided in Note 15 to our audited consolidated financial statements contained in Item 15 of this Annual Report.
Breast Health Products
Full Field Digital Mammography System
Our full field digital mammography systems are based on our proprietary DirectRay digital detector, which employs an amorphous selenium photoconductor to directly convert x-ray photons into an electrical signal. No intensifying screens or additional processes are required to capture and convert the x-ray energy, enabling high imaging resolution and contrast sensitivity. Other digital technologies employ an indirect two-step process by first converting x-ray energy into light and then converting the light energy into electrical signals. We believe that digital x-ray imaging technologies that require light conversion may compromise image resolution, lessening detection capability.
Dimensions: Breast Tomosynthesis
Our Dimensions platform includes a mammography gantry incorporating our DirectRay digital detector capable of performing both 2D and 3D image acquisition and display. When operating in 3D mode, the system acquires a series of low dose x-ray images taken in a scanning motion at various angles. The images are mathematically processed into a series of small slices, revealing breast tissue from a 3D perspective. We believe that by allowing the clinician to review breast tissue in three dimensional space, the more subtle architecture of various types of suspicious lesions may be able to be better interpreted, which may ultimately increase cancer detection and reduce unnecessary patient callbacks. In the U.S., our Dimensions product had previously been approved by the FDA for providing conventional 2D images. In February 2011, we received approval from the FDA to enable the 3D tomosynthesis capability of our Dimensions system. Our clinical results for the approval demonstrated that conventional 2D digital mammography with the addition of 3D tomosynthesis is superior to 2D digital mammography alone for both screening and diagnostics.
5
C-View System
In November 2011, we announced the commercial release of our C-View product, which is a 2D image that is mathematically synthesized from the data within a 3D tomosynthesis exam. Our current recommended clinical practice involves what we refer to as a “Combo” exam involving a tomosynthesis exam and a conventional digital 2D exam, but performed under the same breast compression at a slightly longer compression time than a conventional mammogram. In order to further reduce breast compression time from the current combo exam, the C-View product allows for the mathematical construction of a 2D image from the 3D data, without the need for an actual 2D exposure. Elimination of the 2D exposure reduces the compression time and patient dose. Our C-View software is approved for sale throughout the European Economic Area and in other countries recognizing the CE Mark. During the third quarter of fiscal 2012, we submitted a pre-market supplement application to the FDA for approval to sell this product in the United States. On October 24, 2012, the Radiological Devices Panel of the FDA voted that the expanded indications for use of our Dimensions 3D system to allow our C-View synthesized 2D images in place of traditional 2D images in breast cancer screening is safe, effective and the benefits outweigh the risks. Sale of the 3D version of this system in the United States remains subject to FDA approval.
Selenia
The Selenia product family, our original full field digital mammography platform, has a number of additional features designed to improve image quality and patient throughput. The open architecture of the system’s design provides for full integration with existing enterprise Picture Archiving and Communications Systems, or PACS, and Radiology Information Systems, or RIS. The Selenia product family includes the Selenia base configuration, the Selenia S configuration (a screening-only configuration), the Selenia Performance (a lower cost alternative to the Selenia base configuration) and the Selenia Encore (refurbished units), each of which offer customers varying performance capabilities and product costs.
Healthcome Mammography Products
In July 2011, we completed our acquisition of Beijing Healthcome Technology Company, Ltd., a privately-held manufacturer of medical equipment located in Beijing, China. Healthcome manufactures analog mammography products targeted to lower tier hospital segments in China. Additionally, Healthcome had been collaborating with our research and development team to integrate our selenium digital detector with the Healthcome mammography system. On December 21, 2011, we received State Food and Drug Administration, or SFDA, approval in China for our Serenity digital mammography system. We began selling this product in China in the second quarter of fiscal 2011, and intend to commercialize it throughout Asia and potentially other emerging markets in the future.
Screen-Film Mammography Systems
Our screen-film mammography systems include our LORAD M-IV system. These systems are less expensive than our digital systems and further offer customers varying performance capabilities and product costs.
SecurView Workstation
The images captured by digital mammography systems are typically transmitted electronically for review by a radiologist at a work station. To this end, we developed the SecurViewDX breast imaging softcopy workstation, approved for interpretation of digital mammograms from most vendors as well as images from other diagnostic breast modalities. To complement this product, we also developed the SecurViewRT workstation, a technologist workstation enabling bi-directional exchange of electronic communications between the reviewer and the technologist.
CAD (Computer Aided Detection) Systems
We have developed CAD software tools for our mammography and MRI products. Mammography CAD is used by radiologists as “a second pair of eyes” when reading a woman’s mammogram. Use of this technology provides reviewers with the potential to detect findings that might otherwise be overlooked during the review process, thus potentially increasing cancer detection. We also market an MRI CAD product, which manages the data set from an MRI procedure, designed to improve data workflow for the physician and provide analytical tools to aid in the identification and evaluation of the extent of disease.
Stereotactic Breast Biopsy Systems
We provide clinicians with the flexibility of choosing upright or prone systems for breast biopsy by offering three minimally invasive stereotactic breast biopsy guidance systems, the MultiCare Platinum dedicated, prone breast biopsy table, the StereoLoc II upright attachment, and the Affirm upright attachment. The StereoLoc II attachment is used in conjunction with our M-IV series of screen-film mammography systems and our Selenia full field digital mammography systems. The Affirm upright attachment is employed with our Dimensions systems. These breast biopsy systems provide an alternative to open surgical biopsy, and can be performed as an outpatient procedure under local anesthesia, allowing shorter recovery times.
6
Breast Biopsy Products
We offer a wide range of minimally invasive products for breast biopsy and biopsy site marking. Our breast biopsy portfolio includes two types of tethered breast biopsy products, the Automated Tissue Excision Collection, or ATEC, and Eviva devices. Each tethered device is a disposable biopsy tool that is powered by a console and utilizes our patented fluid management system. The ATEC vacuum-assisted breast biopsy device can be used under all standard imaging guidance modalities (stereotactic x-ray, ultrasound, MRI and molecular breast imaging) whereas our Eviva vacuum-assisted breast biopsy device is used exclusively under stereotactic x-ray guidance. In addition to ATEC and Eviva products, we also offer the Celero device, a non-tethered (no separate console), vacuum-assisted, spring-loaded, disposable core biopsy device which is used exclusively under ultrasound-guidance. All of our breast biopsy devices have been designed to accommodate a broad spectrum of patients as well as hard-to-reach lesions in the axilla, near the chest wall, near implants or behind the nipple.
Breast Brachytherapy Products
The MammoSite Radiation Therapy System is a breast brachytherapy technology that offers accelerated partial breast irradiation, or APBI, therapy to treat breast cancer. A MammoSite balloon, which is inserted into the surgical cavity remaining after a lumpectomy, delivers a 5-day course of concentrated radiation to the tissue most likely to contain residual cancerous cells following surgery, while reducing radiation exposure to adjacent healthy tissue. The MammoSite ML system allows radiation oncologists to shape the radiation dose for typical cases and treat patients who are otherwise not appropriate candidates for traditional brachytherapy. The MammoSite ML device has a central lumen, similar to the original MammoSite device, and three offset lumens parallel to the central lumen. In addition to allowing greater flexibility in radiation treatment planning, the use of a multiple-lumen device typically results in a higher reimbursement rate.
MammoPad Breast Cushion
Our mammography related products include a proprietary MammoPad breast cushion. The MammoPad cushion is designed to reduce the discomfort women often experience during mammography. The cushion’s grip-like surface also holds breast tissue in place to improve breast positioning. The radiolucent cushion does not interfere with image quality and can be used with all of our mammography systems.
Photoconductor Coatings
Our Hologic Hitec-Imaging GmbH subsidiary is our sole supplier of the amorphous selenium photoconductor coatings employed in our Selenia and Dimensions full-field digital mammography detectors. Hitec-Imaging also develops, manufactures, and sells non-medical selenium and organic photoconductor materials for use in a variety of other electro photographic applications, including copying and printing. In the third quarter of fiscal 2012, we finalized our decision to move our selenium panel coating production line to our facility in Newark, Delaware. We expect this transfer to be completed in the second half of fiscal 2013.
Sentinelle Medical MRI Coils and Workstation
Our Sentinelle Medical subsidiary develops, manufactures and markets a suite of high performance breast MRI coils. MRI coils are antenna receivers that are used to collect radio-frequency information emitted from a patient during an MRI procedure. These signals are fed into the MRI magnet system which produces a 3D image from the information. The coils are tuned to specific frequencies and positioned in calculated geometries to provide high quality signal to noise performance of the MRI system. The coils are integrated into various MRI scanning systems, and employ a unique variable coil geometry to obtain improved image quality by positioning the coils in close proximity to the tissue. The coil is not fixed and allows the healthcare provider to adjust positioning to each patient’s unique anatomy. This close positioning results in higher signal to noise ratio and improved image resolution. The improved resolution also enhances guidance for biopsy targeting. We are also developing coils for other indications, and in the fourth quarter of fiscal 2011, we received FDA 510(k) clearance for our new prostate coil, the Sentinelle Endo Coil Array for pelvic imaging including the prostate, cervix, colon and the surrounding tissues in the pelvis. With a similar profile to a transrectal ultrasound probe, this two-channel endo coil array is designed to acquire images in a manner that should help align radiologists and urologists in the diagnosis and treatment of prostate cancer. Commercialization of this product commenced in the first quarter of fiscal 2012. In addition, we sell an MRI CAD workstation designed to simplify workflow and improve diagnostic capabilities.
Trident Specimen Radiography System
In August 2011, we received FDA 510(k) clearance for our new Trident specimen radiography system. The Trident specimen radiography system is a cabinet x-ray system used to provide digital images of surgical and core biopsy specimens to verify that the correct tissue has been excised during surgery or a breast biopsy procedure. The Trident system incorporates our amorphous selenium based detector technology. It is a compact and portable unit designed to be used in the same room or close to where breast surgery or biopsy procedures take place. Performing verification in the same room as the procedure or nearby can improve workflow and reduce the time the patient needs to be undergoing a procedure or surgery. Commercialization of this product commenced in the first quarter of fiscal 2012.
7
Diagnostic Products
APTIMA Family of Assays for Women’s Health
As a result of our recent acquisition of Gen-Probe, we now offer our APTIMA family of assays that includes the APTIMA Combo 2 assay for the simultaneous detection of chlamydia and gonorrhea, the standalone APTIMA CT and APTIMA GC assays for the detection of Chlamydia and gonorrhea, respectively, the APTIMA HPV assay for the detection of 14 sub-types of high-risk HPV associated with cervical cancer, and the APTIMA Trichomonas assay for the detection ofTrichomonas vaginalis, the parasite that causes trichomoniasis. Our APTIMA products integrate our patented transcription-mediated amplification, or TMA, technology, target capture technology, and our patented hybridization protection assay, or HPA, and dual kinetic assay, or DKA, technologies, to produce highly refined amplification assays that increase assay performance, improve laboratory efficiency and reduce laboratory costs. Each of these technologies is described in greater detail below.
Target Capture/Nucleic Acid Extraction Technology. Detection of target organisms that are present in small numbers in a large-volume clinical sample requires that target organisms be concentrated to a detectable level. One way to accomplish this is to isolate the particular nucleic acid of interest by binding it to a solid support. This support, with the target bound to it, can then be separated from the original sample. We refer to such techniques as “target capture.” We have developed target capture techniques to immobilize nucleic acids on magnetic beads by the use of a “capture probe” that attaches to the bead and to the target nucleic acid. We use a magnetic separation device to concentrate the target by drawing the magnetic beads to the sides of the sample tube, while the remainder of the sample is washed away and removed. When used in conjunction with our patented amplification methods, target capture techniques concentrate the nucleic acid target(s) and also remove materials in the sample that might otherwise interfere with amplification.
Transcription-Mediated Amplification (TMA) Technology. The goal of amplification technologies is to produce millions of copies of the target nucleic acid sequences that are present in samples in small numbers. These copies can then be detected using DNA probes. Amplification technologies can yield results in only a few hours versus the several days or weeks required for traditional culture methods. Our patented TMA technology is designed to overcome problems faced by other target amplification methods. TMA is a transcription-based amplification system that uses two different enzymes to drive the process. The first enzyme is a reverse transcriptase that creates a double-stranded DNA copy from an RNA or DNA template. The second enzyme, an RNA polymerase, makes thousands of copies of the complementary RNA sequence, known as the “RNA amplicon,” from the double-stranded DNA template. Each RNA amplicon serves as a new target for the reverse transcriptase and the process repeats automatically, resulting in an exponential amplification of the original target that can produce over a billion copies of amplicon in less than 30 minutes.
Hybridization Protection Assay (HPA) and Dual Kinetic Assay (DKA) Technologies. With our patented HPA technology, we have simplified testing, further increased test sensitivity and specificity, and increased convenience. In the HPA process, the acridinium ester, or AE, molecule is protected within the double-stranded helix that is formed when the probe binds to its specific target. Prior to activating the AE molecule, known as “lighting off,” a chemical is added that destroys the AE molecule on any unhybridized probes, leaving the label on the hybridized probes largely unaffected. When the “light off” or detection reagent is added to the specimen, only the label attached to the hybridized probe is left to produce a signal indicating that the target organism’s DNA or RNA is present. All of these steps occur in a single tube and without any wash steps, which were required as part of conventional probe tests. Our DKA technology uses two types of AE molecules—one that “flashes” and another one that “glows.” By using DKA technology, we have created nucleic acid test, or NAT, assays that can detect two separate targets simultaneously.
Instrumentation, including for the APTIMA Family of Assays. We have developed and continue to develop instrumentation and software designed specifically for performing certain of our diagnostic assays, including the APTIMA family of assays and the PROCLEIX family of assays in the blood screening market. We also provide technical support and instrument service to maintain these instrument systems in the field. By placing our proprietary instrumentation in laboratories and hospitals, we can establish a platform for future sales of our diagnostic assays.
Our instrumentation includes the TIGRIS system, an integrated, fully-automated testing instrument for high-volume laboratories which is approved for use with a number of our APTIMA and PROCLEIX assays, the PANTHER instrument system, an integrated, fully automated testing instrument for low- to mid-volume laboratories, and our semi-automated direct tube sampling (DTS) instruments which are used to run a number of infectious disease and blood screening assays. The PANTHER instrument was CE-marked and launched in Europe for diagnostic use in the fourth quarter of 2010. In August 2011, Health Canada granted us a medical device license to use the PANTHER system to run our APTIMA Combo 2 assay in Canada. In addition, in May 2012 the FDA cleared the PANTHER system to run our APTIMA Combo 2 assay for the detection of chlamydia and gonorrhea in the United States. We are also developing the PANTHER system for use in the blood screening market as part of our blood screening collaboration with Novartis and the PANTHER system and the Ultrio Elite blood screening assay were CE marked in June 2012. We also sell PANTHER systems to Roka Bioscience, Inc. for use in certain industrial markets. In addition, we have recently initiated development programs to add real-time PCR capabilities to a new instrument system that also incorporates the capabilities of our first-generation PANTHER system and to develop a new, standalone instrument to further automate molecular testing from liquid-based cytology specimens.
8
In October 2012, we received FDA approval of our APTIMA HPV 16 18/45 Genotype Assay for use on our TIGRIS system. We expect to begin commercialization during the first quarter of fiscal 2013.
Invader Chemistry Platform
Our Invader chemistry platform is a DNA probe-based system for highly sensitive detection of specific nucleic acid sequences. It is an accurate and specific method for detecting single-base pair changes, insertions, deletions, gene copy number, infectious agents, and gene expression. Invader reactions can be performed using genomic DNA, amplified RNA, polymerase chain reaction (PCR) or real-time PCR products.
Cervista HPV Tests. HPV is the most common sexually transmitted disease in the U.S. and is recognized as the cause of most cervical cancer. We offer two HPV tests using the Invader chemistry: the Cervista HPV HR and the Cervista HPV 16/18. These tests employ our proprietary Invader technology and are performed out of the ThinPrep PreservCyt collection vial. Cervista HPV HR is a qualitative test used for the detection of DNA from fourteen high-risk HPV types responsible for most cervical disease. The Cervista HPV 16/18 test is a qualitative test used for the detection of DNA from HPV types 16 and 18, the types that cause approximately 70% of cervical cancer.
Both our APTIMA and Cervista HPV HR tests have been approved for triaging women with undetermined cervical cytology and co-testing with cervical cytology for women 30 years and older. Our Genotype assays have been approved to be used adjunctively with the APTIMA and Cervista HPV HR tests in combination with cervical cytology to assess the presence of high risk HPV types, as well as to triage women with undetermined cervical cytology results along with our HPV tests. Our APTIMA and Cervista HPV tests are targeted to meet a broad spectrum of customer needs across both centralized and decentralized segments of the clinical laboratory markets.
In December 2011, we announced that the FDA approved our Cervista High Throughput Automation System, which we refer to as the HTA system, for use with our Cervista HPV HR test. The Cervista HTA system automates the DNA extraction and detection steps of the Cervista HPV HR test and allows for significantly less manual time during processing. This product was launched in January 2012.
Other Invader Products. Other current clinical diagnostic offerings based upon our Invader chemistry include the following:
| | • | | A molecular assay to identify patients who may be at increased risk of adverse reaction to the chemotherapy drug Camptosar (irinotecan) by detecting and identifying specific mutations in the UGT1A1 gene that have been associated with that risk. |
| | • | | Products to assist in the diagnosis of cystic fibrosis, cardiovascular risk and other diseases. |
In addition, we sell products to the Agricultural Biotechnology market. We also have an active out-licensing and partner program in areas outside of our core business that allows us to further realize the value of our Invader chemistry platform.
ThinPrep System
The ThinPrep System is the most widely used method for cervical cancer screening in the United States. If detected in the pre-cancerous stage, most cervical cancer cases are preventable. The ThinPrep System consists of any one or more of the following: the ThinPrep 2000 Processor, ThinPrep 3000 Processor, ThinPrep 5000 Processor, ThinPrep Imaging System, and related reagents, filters and other supplies, such as the ThinPrep Pap Test and our proprietary ThinPrep PreservCyt Solution. Our ThinPrep 5000 Processor has been launched for full use, as described below, outside of the U.S. but is limited to non-gynecological screening samples in the U.S.
The ThinPrep Process. The ThinPrep process begins with the patient’s cervical sample being obtained by the physician using a cervical sampling device that, rather than being smeared on a microscope slide as in a conventional Pap smear, is inserted into a vial filled with our proprietary PreservCyt Solution. This enables most of the patient’s cell samples to be preserved before the cells can be damaged by air drying. The ThinPrep specimen vial is then labeled and sent to a laboratory equipped with a ThinPrep Processor for slide preparation. At the laboratory, the ThinPrep specimen vial is inserted into a ThinPrep Processor, a proprietary sample preparation device, which automates the process of preparing cervical slides for staining and microscopic examination.
In the case of manual screening, the cytotechnologist screens each Pap test slide with a microscope to first determine the adequacy of the slide and then to examine the entire slide to differentiate diseased or abnormal cells from normal cells. With the ThinPrep Imaging System, the screening process has been automated to combine the power of computer imaging technology and human interpretive skills. Prior to human review, the ThinPrep Imaging System rapidly scans and locates areas of interest for review. By directing the cytotechnologist to areas of interest on a slide, the system may increase a cytology laboratory’s screening productivity and diagnostic accuracy. In Europe, where laboratories tend to be smaller, processing fewer tests, we also offer a lower throughput imaging device, which we introduced in September 2009 to assist in the detection of cervical cancer.
Additional Applications. In addition to serving as a replacement for the conventional Pap smear, the ThinPrep System can also be used for non-gynecological cytology screening applications including fine-needle aspiration specimens (e.g., breast, thyroid, lung or liver), body fluids (e.g., urine, pleural fluid, ascitic fluid or pericardial fluid), respiratory specimens (e.g., sputum or brushing of respiratory tracts) and ancillary testing (e.g., cell blocks, immunocytochemistry or special stains).
9
Rapid Fetal Fibronectin Test
The Rapid Fetal Fibronectin Test is a patented single-use disposable test used to determine a woman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, in vaginal secretions during pregnancy. This test is approved by the FDA for use in assessing the risk of preterm birth. The test utilizes a single-use, disposable cassette and is analyzed on our patented instrument, the TLI IQ System.
Procleix Family of Assays for Blood Screening
As a result of our acquisition of Gen-Probe, we develop and manufacture the PROCLEIX family of assays, which are marketed and sold worldwide by Novartis, our blood screening collaborator, under Novartis’ trademarks. The PROCLEIX family of assays includes the HIV-1/HCV assay which simultaneously detects HIV type-1, or HIV-1, and HCV in donated blood, plasma, organs and tissues, the Ultrio and Ultrio Plus assays which simultaneously detect HIV-1, HCV and HBV in donated blood, plasma, organs and tissues, the Ultrio Elite assay which simultaneously detects HIV-1, HIV type-2, or HIV-2, HBV and HCV in donated blood, plasma, organs and tissues, and the WNV assay which detects West Nile Virus in donated blood, plasma, organs and tissues.
In June 2012, our Ultrio Elite blood screening assay for the detection of HIV-1, HIV-2, HBV and HCV received a CE mark, which authorizes the sale and marketing of the Ultrio Elite assay in the European Union. The Ultrio Elite assay runs on our PANTHER instrument system.
Infectious Disease and Virology Products
As a result of our acquisition of Gen-Probe, we now offer a number of products in the infectious disease space, including a number of assays for the detection of certain respiratory and gastrointestinal diseases as a result of our acquisition of Prodesse, Inc. in October 2009. Our infectious disease products include multiplex real-time PCR assays to detect and differentiate various influenza types and viruses, a real-time PCR assay to detect the Tuberculosis pathogen, and a rapid assay for the direct detection ofStreptococcus pyogenesin one hour from a throat swab.
In virology, nucleic acid test assays can be used to detect viral DNA or RNA in a patient sample. These tests can be qualitative, meaning that the tests simply provide a “yes-no” answer for the presence or absence of the virus, or quantitative, meaning that the test determines the quantity of virus in the patient sample. We offer APTIMA assays for the qualitative detection of HIV-1 and HCV. In addition, we sell analyte specific reagents for quantitative HCV testing in the United States through our collaboration with Siemens Healthcare Diagnostics, Inc., or Siemens. We are developing quantitative viral assays to run on our PANTHER instrument system.
Prostate Oncology
The field of NAT-based cancer diagnostics is an emerging market as new markers that correlate to the presence of cancer continue to be discovered. According to the Prostate Cancer Foundation, prostate cancer is the most common non-skin cancer in the United States, affecting an estimated one in six men. Through our acquisition of Gen-Probe, we acquired exclusive worldwide diagnostic rights to the PCA3 gene from DiagnoCure, Inc., or DiagnoCure, in November 2003. In addition, in April 2006, we entered into a license agreement with the University of Michigan for exclusive worldwide rights to develop diagnostic tests for genetic translocations that have been shown in preliminary studies to be highly specific for prostate cancer tissue.
In November 2006, we CE-marked our PROGENSA PCA3 assay, allowing it to be marketed in Europe. This gene-based test is designed to detect the over-expression of PCA3 mRNA in urine. Studies have shown that, in greater than 90% of prostate cancer cases, PCA3 is highly over-expressed (65-fold on average) in prostate cancer cells compared to normal cells, indicating that PCA3 may be a useful biomarker for prostate cancer. In February 2012, the FDA approved our PROGENSA PCA3 assay for sale and marketing in the United States. The PROGENSA PCA3 assay is to be used, in conjunction with other patient information, to help guide repeat biopsy decisions for men who have had one or more prior negative biopsies. The test has been approved for use on our semi-automated DTS instrument systems.
10
Transplant Diagnostics
As a result of our acquisition of Gen-Probe, our transplant diagnostics business, which we refer to as our LIFECODES business, now comprises our human leukocyte antigen, or HLA, products and related assays. HLA testing, also known as HLA typing or tissue typing, identifies antigens on white blood cells that determine tissue compatibility for organ transplantation (that is, histocompatibility testing). HLA typing is used to provide evidence of tissue compatibility. The antigens expressed on the surface of the lymphocytes of the recipient are matched against those from various donors. HLA typing is performed for kidney, bone marrow, liver, pancreas, and heart transplants. HLA testing is also performed to reduce the probability of transplant rejection and for the ongoing management of transplant recipients.
We currently offer a range of multiplexed assays in the field of transplant diagnostics pursuant to our development and supply agreement with Luminex Corporation. We also offer a range of HLA antibody detection products under our LIFECODES brand, as well as a number of other HLA-related testing products, including serological typing trays, enzyme immunoassays, and a range of molecular typing products for donor-recipient matching and patient monitoring.
GYN Surgical Products
NovaSure
The NovaSure system involves a minimally-invasive procedure that allows physicians to treat women suffering from excessive menstrual bleeding. The system consists of a disposable device and a controller that delivers radio frequency, or RF, energy to ablate the endometrial lining of the uterus in order to eliminate or reduce the patient’s bleeding. The NovaSure disposable device is a hand-held, single-use device that incorporates a flexible gold-plated mesh electrode used to deliver the RF energy during the NovaSure procedure. The NovaSure RF Controller generates and delivers the RF energy customized for each patient, monitors several critical treatment and safety parameters, and automatically controls other aspects of the procedure.
The NovaSure system is a “second generation” endometrial ablation therapy approved by the FDA to be performed without drug or surgical pre-treatment. Pre-treatment can be time-consuming, expensive and inconvenient for both patients and physicians and can result in uncomfortable or painful side effects and complications. In contrast, the NovaSure procedure is typically performed as an outpatient procedure in the hospital, ambulatory surgery center or physician’s office and often does not require the use of general anesthesia.
MyoSure
The MyoSure system is designed to provide efficient and effective hysteroscopic removal of fibroids located just below the lining of the uterus as well as uterine polyps. Removal of fibroids can provide effective relief of heavy menstrual bleeding commonly attributed to such pathology. Unlike other methods of tissue removal, the excavated tissue samples remain intact, which allows them to be tested for abnormalities. Also, minimal tissue destruction makes the MyoSure system a good choice for women seeking to preserve uterine form and function.
The MyoSure system consists of a tissue removal device, control unit, and hysteroscope. The tissue removal device is single-use and features simultaneous tissue cutting and removal. The device incorporates a rapidly rotating cutting blade designed to remove a 3 cm fibroid in less than 10 minutes. During the procedure, the tissue removal device is inserted through the MyoSure hysteroscope. This tissue removal device is powered by a control unit, which features a simple user interface and is foot pedal activated.
Towerfree Hysteroscopy System
The Towerfree Hysteroscopy System, or THS, is a hysteroscopy system that allows for visualization and inspection of the uterine cavity. The THS instrumentation was designed specifically for the gynecologist’s office, providing a compact and simple platform for uterine diagnosis and minor intrauterine operative procedures. The system consists of a video platform and hysteroscope instrumentation. The components of the THS system (including a light source, camera, monitor and image capture system) have been integrated into a compact and portable unit. This is different from traditional hysteroscope systems, which are generally offered as separate units and require a large cart and significant footprint within the exam room.
The THS instrumentation provides versatility in performing minor operative procedures intended to expand the utilization of the system and support the ‘see and treat’ benefit of hysteroscopy. The operative hysteroscope has a continuous flow, single-piece design for simple assembly and operation. It has been designed to enhance procedural conditions for the physician as well as patient comfort. The instrument channel accommodates instruments that may be used to grasp or remove tissue. For those customers who want to perform diagnostic hysteroscopy, THS offers a small diameter single flow sheath, which reduces the need for cervical dilation and provides a tool for quick and simple visualization of the uterine cavity.
11
Aquilex Fluid Control System
The Aquilex fluid control system is a product that measures the inflow and outflow of fluid from the patient during hysteroscopic procedures and is designed to reduce procedure and anesthesia time associated with hysteroscopic procedures while providing high quality visualization to the surgeon.
Adiana Permanent Contraception System
The Adiana system is a non-invasive procedure for permanent female contraception that requires no incisions and can be performed in the doctor’s office using local anesthesia. In the second quarter of fiscal 2012, we decided to cease manufacturing, marketing and selling our Adiana system. We determined that the product was not financially viable and would not become so in the foreseeable future.
Skeletal Health Products
QDR X-Ray Bone Densitometers
Bone densitometry is the measurement of bone density to assist in the diagnosis and monitoring of osteoporosis and other metabolic bone diseases that can lead to debilitating bone fractures. Osteoporosis is a disease that is most prevalent in post-menopausal women. Our proprietary QDR x-ray bone densitometers incorporate dual-energy x-ray technology to precisely assess bone density of the most important fracture sites, the spine and hip. Since our commercial introduction of the first bone densitometer employing dual-energy x-ray technology in 1987, we have continually improved upon our technology, and the use of dual-energy x-ray technology has become and remains a leading bone densitometry assessment tool. We offer a range of bone densitometers with various features and options to address the requirements of our diverse customer base.
Sahara Clinical Bone Sonometers
We have developed and sell a relatively low-cost, lightweight, portable ultrasound bone analyzer, which assesses the bone density of the heel that can assist in initial screening for osteoporosis.
Mini C-arm Imaging
We manufacture and distribute Fluoroscan mini C-arm imaging systems. Mini C-arms provide low intensity, real-time x-ray imaging, with high-resolution images at radiation levels and at a cost below those of conventional x-ray and fluoroscopic equipment. Mini C-arm systems are used primarily by orthopedic surgeons to perform minimally invasive surgical procedures on a patient’s extremities, such as the hand, wrist, knee, foot and ankle.
Marketing, Sales and Service
We sell and service our products through a combination of direct sales and service forces and a network of independent distributors and sales representatives. In fiscal 2012, 2011 and 2010, no customer accounted for more than 10% of our consolidated revenues. With respect to our Diagnostics’ segment, as a result of our acquisition of Gen-Probe, we expect that one customer, Novartis, will account for more than 10% of annual revenues of that segment in the future. In fiscal 2012, 2011 and 2010, foreign sales accounted for approximately 27%, 24% and 21% of our product sales, respectively. See Note 15 to our consolidated financial statements contained in Item 15 of this Annual Report for geographical information concerning those sales.
U.S. Marketing and Sales
Our U.S. Breast Health and Skeletal Health sales force is comprised of full line modality account managers selling mammography and bone densitometry products, assisted by women’s health product specialists and osteoporosis sales specialists. Our biopsy and MRI sales specialists, who often work together with account managers, sell breast biopsy devices and breast biopsy site markers to radiologists and breast surgeons, as well as custom MRI coils and patient positioning systems to radiologists. Our territory sales specialists sell both our MammoSite and breast biopsy and site marker products and target breast surgeons and radiation oncologists. In addition to our MRI sales specialists, our Sentinelle Medical MRI business also supports the original equipment manufacturers, or OEM, channel with product specialists and sales support. Our U.S. sales efforts also include the use of national account managers focused on obtaining purchasing contracts from large purchasing entities, such as managed care organizations, integrated delivery networks, or IDNs, and government healthcare facilities. In addition, in certain regions of the U.S., we use a limited number of independent dealers or distributors to sell and service our products.
12
Our U.S. Diagnostics and GYN Surgical sales forces focus on clinical laboratories, healthcare providers, and third-party payors. A critical element of our strategy in the United States has been to utilize the results of our clinical trials and expanded FDA labeling to demonstrate safety, efficacy and productivity improvements to our target customers. Our Diagnostics’ sales force includes both cytology and molecular specialists focusing on selling to a broad range of laboratories. In addition, our Diagnostics sales specialists call exclusively on OB/GYN offices, while our GYN Surgical sales force targets GYN surgeons in both hospital and office settings.
International Marketing and Sales
We sell our breast health and skeletal health products in international markets through a network of independent distributors and sales representatives, as well as a direct sales and service force in Belgium, the UK, Australia and most recently in China for our Healthcome products. We offer our products in Europe, the Middle East, Africa, South Asia, Latin America, and Pacific Rim countries, including China, Japan, Australia, South Korea, Thailand and Taiwan, through local sales representatives in select countries and through distributors in those territories.
Our Diagnostics and GYN Surgical products are marketed outside of the United States by direct operations in Canada, Europe, Australia, China and Hong Kong. We established these operations to manage sales, service, training and distribution in the Canadian, European and Asia/Pacific markets. We also utilize a network of third-party distributors in various other countries throughout the world. We believe that in order to effectively market our current products and any other new products and applications on a worldwide basis, we will need to continue to increase our international marketing, sales, and service capabilities.
Service
Our service organization is responsible for installing our products and providing warranty and repair services, applications training and biomedical training. Products sold by our direct sales force typically carry limited warranties covering parts and labor for twelve months. Products sold through dealers also carry limited warranties that typically last for twelve months and cover only parts or components. We also offer service contracts to our customers that generally last one to five years after the original warranty period. We provide both repair services and routine maintenance services under these arrangements, and also offer repair and maintenance services on a time and materials basis to customers that do not have service contracts. Internationally, we primarily use distributors, sales representatives and third parties to provide maintenance service for our products.
Competition
The healthcare industry is highly competitive and characterized by continual change and improvements in technology. This is particularly the case in the market segments in which we operate. A number of companies have developed, or are expected to develop products that compete or will compete with our products. Many of these competitors offer a broader product portfolio and have greater brand recognition than we do, which may make these competitors more attractive to hospitals, radiology clients, group purchasing organizations, laboratories, physicians and other potential customers. In addition, many of our competitors and potential competitors are larger and have greater financial resources than we do. Some of the companies with whom we compete have or may have more extensive research, sales, marketing and manufacturing capabilities and significantly greater technical resources than we do, and may be better positioned to continue to improve their technology in order to compete in an evolving industry. Competitors may develop superior products or products of similar quality for sale at the same or lower prices. Moreover, our products could be rendered obsolete by new industry standards or changing technology. We can give no assurance that we will be able to compete successfully with existing or new competitors.
In the current environment of managed care, economically-motivated buyers, consolidation among healthcare providers, increased competition and declining reimbursement rates, we have been increasingly required to compete on the basis of price, value, reliability and efficiency. We believe the current global economic conditions and healthcare reform measures could put additional competitive pressure on us, including on our average selling prices, overall procedure rates and market sizes.
We believe that the success of our products depends on our ability to differentiate ourselves and to demonstrate that our products deliver the attributes that are most important and cost-effective to customers. This includes, but is not limited to, superiority in efficacy, ease of use, reliability, accuracy, quality and cost. We believe our continued success depends in large part upon our ability to invest in product enhancements and technologies that will help us distinguish ourselves from our competitors.
Breast Health. Our mammography and related products and subsystems compete on a worldwide basis with products offered by a number of competitors, including GE, Siemens, Philips (who recently acquired Sectra), Planmed, Agfa, Carestream Health, Fuji, IMS Giotto, and Toshiba. In the U.S., our full field digital mammography systems compete with digital mammography systems from GE, Siemens, Fuji, Giotto, Philips and Planmed. Our digital mammography systems also compete with Fuji’s and Carestream Health’s Computed Radiography, or CR mammography systems, and other lower-priced alternatives to 2D digital mammography and analog mammography systems. Our 3D tomosynthesis systems compete in certain countries outside of the U.S. with 3D tomosynthesis systems developed by Siemens, Giotto, and Philips. We also understand that GE is developing a 3D tomosynthesis system. Although
13
we understand that certain of our competitors, including GE and Siemens, are developing 3D tomosynthesis systems for commercial use in the U.S., there are no 3D tomosynthesis systems, other than our Dimensions system, that have been approved for use in the U.S. by the FDA. Any such use will require pre-market approval, or PMA, by the FDA. As a result, in the U.S. our 3D tomosynthesis systems currently compete primarily with lower cost 2D digital mammography systems.
Our Sentinelle Medical MRI breast coils compete primarily with products sold by Invivo, which was acquired by Philips in 2006, to end users and original equipment manufacturers, as well as other smaller third-party coil designers and the original equipment manufacturers themselves.
The primary competitors for our breast biopsy product line are Devicor Medical Products, which acquired the Mammotome product line from Ethicon, and C.R. Bard, which recently acquired SenoRx. In addition, other competitors include CareFusion, Sanarus and Intact Medical.
Our MammoSite systems face competition from companies also selling accelerated partial breast irradiation products, including C.R. Bard and Cianna Medical, as well as from other technologies, such as treatments using external beam, whole breast radiation, which has longer-term data on patient outcomes. Alternative radiation therapy methods, such as intraoperative radiation therapy, are being used by some institutions; however, such alternative methods have not yet achieved widespread commercial use. We believe that the breast brachytherapy market has and will continue to experience challenges including downward pressure on procedure volumes due to the continuing adverse economic environment and current trends in breast cancer management, as well as competitive pricing pressures and competition from existing and alternative new technologies.
Diagnostics. Our ThinPrep liquid-based cytology product faces direct competition in the United States primarily from Becton, Dickinson and Company, which manufactures a competitive offering. We also compete with the conventional Pap smear and other alternative methods for detecting cervical cancer and/or its precursors. Internationally, our ThinPrep product competes with a variety of companies and other “off-market” (non-FDA approved) tests, since fewer regulatory barriers exist in most international markets as compared to the United States.
We believe that our Rapid Fetal Fibronectin Test is currently the only approved in vitro diagnostic test for predicting the risk of pre-term birth in the United States. Internationally, our Rapid Fetal Fibronectin Test competes with Actum Partus manufactured by Alere. However, this product could experience competition from companies that manufacture and market pregnancy-related diagnostic products and services. In addition, healthcare providers use diagnostic techniques such as clinical examination and ultrasound to diagnose the likelihood of pre-term birth and may choose these techniques rather than use the Rapid Fetal Fibronectin Test.
In the molecular diagnostics market, our products compete with many companies in the U.S. and abroad engaged in the development, commercialization and distribution of similar products intended for clinical molecular diagnostic applications. These companies may have or develop products competitive with those offered by us. Clinical laboratories also may offer testing services that are competitive with our products and may use reagents purchased from us or others to develop their own diagnostic tests. Such laboratory-developed tests may not be subject to the same clinical trial and FDA submission requirements as our products.
In the global clinical diagnostics market, we compete with several companies offering alternative technologies to our diagnostic products including Abbott Laboratories, Siemens, Becton, Dickinson and Company, bioMérieux, Cepheid, Life Technologies Corporation, Luminex Corporation, Qiagen, Roche Diagnostics Corporation, and Siemens. Specifically, in the U.S. our APTIMA Combo 2 tests compete against Becton, Dickinson and Roche, and our APTIMA HPV tests compete with tests marketed by Qiagen, which received FDA approval in 1999, and Roche Diagnostics, which received PMA approval for a high risk HPV test and 16/18 test in 2011.
In the market for blood screening products, our primary competitor is Roche, which received FDA approval of its first PCR-based nucleic acid tests for blood screening in December 2002. We also compete with assays developed internally by blood screening centers and laboratories based on PCR technology. In the future, our blood screening products may compete with viral inactivation or reduction technologies and blood substitutes.
Novartis retains certain rights to grant licenses of the patents related to HCV and HIV to third parties in blood screening using nucleic acid testing. Prior to its acquisition by Novartis, Chiron Corporation, or Chiron, granted HIV and HCV licenses to Roche in the blood screening and clinical diagnostics fields. Chiron also granted HIV and HCV licenses in the clinical diagnostics field to Bayer Healthcare LLC (now Siemens), together with the right to grant certain additional HIV and HCV sublicenses in the field to third parties. If Novartis or Siemens grant additional licenses, further competition will be created for sales of HCV and HIV assays and these licenses could affect the prices that can be charged for certain of our products.
GYN Surgical. Our NovaSure system currently faces direct competition from Johnson & Johnson, Boston Scientific and CooperSurgical, each of which currently markets an FDA approved “second generation” endometrial ablation device for the treatment of excessive menstrual bleeding. In addition to these devices, we also compete with alternative treatments to our NovaSure system, such as drug therapy, IUDs, hysterectomy, dilation and curettage and rollerball ablation. Internationally our products compete with drug therapy and first generation rollerball technology, as well as other endometrial ablation devices, including Johnson &
14
Johnson’s Thermachoice, Boston Scientific’s HTA, and two other relatively small companies that market products that are not FDA approved. Because drug therapy is an alternative to our NovaSure procedure, NovaSure’s competitors also include many major pharmaceutical companies that manufacture hormonal drugs for women.
Our MyoSure product competes directly with hysteroscopic loop resection and Smith & Nephew’s TruClear tissue morcellator. The MyoSure product also competes with alternative therapeutic techniques such as hysteroscopic resection with a monopolar or bipolar loop, which is currently the most common technique for removing intrauterine fibroids and polyps.
Our THS system competes with a number of endoscopy companies including Richard Wolf, Stryker, ACMI/Olympus, and Karl Storz.
Skeletal Health. GE is our primary competitor in the bone densitometry market, and we also compete with Orthoscan in the mini-C arm market.
Manufacturing
We have historically purchased many of the components and raw materials used in our products from numerous suppliers worldwide. For reasons of quality assurance, scarcity or cost effectiveness, certain components and raw materials used in the manufacture of our products are available only from one or a limited number of suppliers. We have worked closely with our suppliers to develop contingency plans to assure continuity of supply while maintaining high quality and reliability, and in some cases, we have established long-term supply contracts with our suppliers. In certain instances, we have developed in-house capability to offset potential shortages caused by sole source suppliers. Due to the high standards and FDA requirements applicable to the manufacturing of our products, we may not be able to quickly establish additional or replacement sources for certain components or materials. In the event that we are unable to obtain sufficient quantities of raw materials or components on commercially reasonable terms or in a timely manner, our ability to manufacture our products on a timely and cost-competitive basis may be compromised, which may have a material adverse effect on our business, financial condition and results of operations.
We manufacture our direct radiography detectors at our manufacturing facilities in Newark, Delaware and Warstein, Germany. We manufacture substantially all of our mammography equipment at our manufacturing facilities in Danbury, Connecticut. We recently acquired a small mammography equipment business in Beijing, China. We manufacture our CAD line of products, the SecurView workstations, our osteoporosis screening equipment and our mini C-arm imaging systems at our headquarters in Bedford, Massachusetts. We continue to develop our software for our CAD products at our Santa Clara, California facility. The MammoPad breast cushion is manufactured by third-parties and drop-shipped from our suppliers directly to our customers. Our breast biopsy disposable products are manufactured in Indianapolis, Indiana, as well as in our Costa Rica facility. Our ATEC control consoles for breast biopsy are manufactured by a third-party, with quality control performed by our employees. Our Sentinelle Medical MRI breast coils are manufactured at our Toronto, Canada location. We contract with several third-parties to manufacture certain components of our MammoSite system, and we complete the manufacturing process at our Costa Rica and/or Marlborough locations, depending on the configuration.
Our ThinPrep Processors and ThinPrep Imaging Systems are assembled at our facility in Marlborough, Massachusetts. Our ThinPrep PreservCyt vials are filled at our facility in Londonderry, New Hampshire. Our ThinPrep system filters are manufactured at both our Marlborough and Londonderry facilities. We also have a small facility in Sunnyvale, CA that manufactures our Rapid Fetal Fibronectin products. As a result of our acquisition of Gen-Probe, we also have two manufacturing facilities in San Diego, California, which are used to manufacture certain of our diagnostics products, including our blood screening products. Our blood screening facility meets the strict standards set by CBER for the production of biologic products. In addition, we maintain a manufacturing facility in Manchester, England, which is also used to manufacture certain of our diagnostics products. We also manufacture certain of our molecular diagnostics products at our facility in Madison, Wisconsin.
The manufacture of our NovaSure disposable devices occurs at our facility in Alajuela, Costa Rica. The production of the RF Controller component of our NovaSure system takes place at our Marlborough facility. Our MyoSure products are assembled at our Costa Rica facility.
We continually review our operations and facilities in an effort to reduce costs and increase efficiencies and currently plan to consolidate several of our operations and facilities, including the consolidation of our selenium panel coating production line, currently located in Germany, into our digital detector manufacturing facility in Newark, Delaware, the consolidation of our breast biopsy operations, including manufacturing, research and development and sales support, currently located in Indianapolis, Indiana, into our Costa Rica manufacturing facility and our headquarters facilities in Massachusetts, and the consolidation of our Madison, Wisconsin molecular diagnostics operations into our Gen-Probe facilities in San Diego, California. We may experience unexpected problems and expenses associated with our planned consolidation of operations and facilities that could materially harm our business and prospects.
15
We have one third-party manufacturer for each of our molecular diagnostics instrument product lines. KMC Systems, Inc., or KMC Systems, is the only manufacturer of the TIGRIS instrument system; Stratec Biomedical Systems AG, or Stratec, is the only manufacturer of the PANTHER instrument system; and Tecan Group Ltd., or Tecan, is the only manufacturer of the Cervista High Throughput Automation System. We are dependent on these third-party manufacturers, and this dependence exposes us to increased risks associated with production delays, delivery schedules, manufacturing capability, quality control, quality assurance and costs.
As noted above, we manufacture our products at a number of different facilities located throughout the world. An interruption in manufacturing capabilities at any of these facilities, as a result of equipment failure or other reasons, could reduce, delay or prevent the production of our products. Our manufacturing facilities are subject to the risk of catastrophic loss due to unanticipated events, such as fires, earthquakes, explosions, floods or weather conditions. Our manufacturing facilities may experience plant shutdowns, strikes or other labor disruptions, or periods of reduced production as a result of equipment failures, loss of power, gray outs, delays in deliveries or extensive damage to any of our facilities, which could harm our business and prospects. Because some of our manufacturing operations are located in China, Costa Rica, England and Germany, those manufacturing operations are also subject to additional challenges and risks associated with international operations described below.
Backlog
Our backlog as of November 4, 2012 and November 6, 2011 totaled $284.2 million and $254.6 million, respectively. Backlog consists of customer orders for which a delivery schedule within the next twelve months has been specified. Orders included in backlog may be canceled or rescheduled by customers without significant penalty. Backlog as of any particular date should not be relied upon as indicative of our net revenues for any future period.
Research and Development
The markets in which we participate are characterized by rapid technological change, frequent product introductions and evolving customer requirements. Investment in research and development is critical to driving our future growth. Our research and development efforts are focused on the further development and improvement of our existing products, the design and development of innovative medical technologies and regulatory compliance. During fiscal 2012, our development projects included the ongoing development, clinical trials and other support for the FDA clearance or approval process for our 3D Dimensions product, as well as the development of improvements to next generation laboratory automation and GYN surgical products. In addition, we have recently initiated development programs to add real-time PCR capabilities for the next-generation PANTHER instrument system and to develop a new, standalone instrument to further automate molecular testing from liquid-based cytology specimens. We anticipate continuing research and development to support these ongoing efforts.
In addition to product development, our research and development personnel play an active role in the review of product specifications, clinical protocols and FDA submissions, as well as ensuring that certain of our products conform to European health, safety and environmental requirements, or CE marking. Our research and development expenses were $131.0 million, $116.7 million and $104.3 million in fiscal 2012, 2011 and 2010, respectively. These expenses do not include acquired in-process research and development expenses of $4.5 million and $2.0 million in fiscal 2012 and 2010, respectively.
Patents and Proprietary Rights
We rely primarily on a combination of trade secrets, patents, copyrights and confidentiality procedures to protect our technology. Due to the rapid technological changes that characterize the markets we operate in, we believe that the enhancement of existing products, reliance upon trade secrets and unpatented proprietary know-how and the development of new products are generally as important as patent protection in establishing and maintaining a competitive advantage. Nevertheless, we have obtained patents and will continue to make efforts to obtain patents, when available, in connection with our product development program.
We own numerous U.S. patents and have applied for numerous additional U.S. patents relating to our technologies. We also own or have applied for corresponding patents in selected foreign countries. These patents relate to various aspects of most of our products. We do not know if current or future patent applications will issue with the full scope of the claims sought, if at all, or whether any patents issued will be challenged or invalidated. There is a risk that our patent applications will not result in granted patents or that granted patents will not provide significant protection for our products and technology. Unauthorized third parties may infringe our intellectual property rights, or copy or reverse engineer portions of our technology. Our competitors may independently develop similar technology that our patents do not cover. In addition, because patent applications in the U.S. are not generally publicly disclosed until eighteen months after the application is filed, applications may have been filed by third parties that relate to our technology. Moreover, there is a risk that foreign intellectual property laws will not protect our intellectual property rights to the same extent as intellectual property laws in the U.S. The rights provided by a patent are finite in time. Over the coming years, certain patents relating to current products will expire in the U.S. and abroad thus allowing third parties to utilize certain of our technologies. In the absence of significant patent protection, we may be vulnerable to competitors who attempt to copy our products, processes or technology.
16
In addition to the patents we have been issued or we have acquired, we license patents from others on a variety of terms and conditions.
We are engaged in intellectual property litigation as described in “Item 3. Legal Proceedings” below and may be notified in the future of claims that we may be infringing intellectual property rights possessed by third-parties. In connection with any such litigation or if any claims are asserted against us or our products, we may seek to enter into settlement and/or licensing arrangements. There is a risk in these situations that no license will be available or that a license will not be available on reasonable terms. Alternatively, we may decide or be required to litigate such claims. A successful claim by a third-party may require us to remove the accused product from the market or to design around the patented technology, potentially resulting in a less acceptable product.
Regulatory and Reimbursement
Regulatory
The manufacture, sale, lease and service of medical diagnostic and surgical devices intended for commercial use are subject to extensive governmental regulation by the FDA in the United States and by a variety of regulatory agencies in other countries. Under the Federal Food, Drug and Cosmetic Act, known as the FD&C Act, manufacturers of medical products and devices must comply with certain regulations governing the design, testing, manufacturing, packaging, servicing and marketing of medical products. Some of our products are also subject to the Radiation Control for Health and Safety Act, administered by the FDA, which imposes performance standards and record keeping, reporting, product testing and product labeling requirements for devices that emit radiation, such as x-rays.
The FDA generally must clear the commercial sale of new medical devices. Commercial sales of our medical devices within the United States must be preceded by either a pre-market notification filing pursuant to Section 510(k) of the FD&C Act or the granting of a PMA. A 510(k) pre-market notification filing must contain information establishing that the device to be sold is substantially equivalent to a device commercially distributed prior to May 28, 1976.
The PMA procedure involves a complex and lengthy testing and review process by the FDA and may require several years to obtain. We may need to first obtain an investigational device exemption, known as an IDE, in order to conduct extensive clinical testing of the device to obtain the necessary clinical data for submission to the FDA. The FDA will grant a PMA only if after evaluating clinical data it finds that the safety and effectiveness of the product has been sufficiently demonstrated. This approval may restrict the number of devices distributed or require additional patient follow-up for an indefinite period of time.
Our manufacturing processes and facilities are subject to continuing review by the FDA and foreign governments or their representatives. Adverse findings could result in various actions against us, including withdrawal of approvals and product recall.
The laboratories that purchase certain of our products, including the ThinPrep System, ThinPrep Imaging System, Rapid Fetal Fibronectin Test, APTIMA Combo 2, APTIMA HPV and Cervista HPV tests are subject to extensive regulation under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, which requires laboratories to meet specified standards in the areas of personnel qualifications, administration, participation in proficiency testing, patient test management, quality control, quality assurance and inspections. We believe that the ThinPrep System (including the ThinPrep Imaging System), Rapid Fetal Fibronectin Test, Cervista HPV tests and other affected products operate in a manner that will allow laboratories purchasing these products to comply with CLIA requirements. However, we cannot assure that adverse interpretations of current CLIA regulations or future changes in CLIA regulations would not have an adverse effect on sales of any such products.
Our blood screening products are subject to extensive pre- and post-market regulation as biologics by the FDA, including regulations that govern the testing, manufacturing, safety, efficacy, labeling, storage, record keeping, advertising and promotion of the products under the FD&C Act and the Public Health Service Act, and by comparable agencies in most foreign countries. The process required by the FDA before a biologic may be marketed in the United States generally involves the completion of pre-clinical testing; the submission of an investigational new drug application which must become effective before clinical trials may begin; and the performance of adequate and well controlled human clinical trials to establish the safety and effectiveness of the biologic’s proposed intended use.
The FDA requires approval of a biologics license application before a licensed biologic may be legally marketed in the United States. Product approvals may be withdrawn or suspended if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
Certain analyte specific reagents, referred to as ASR products, may be sold without 510(k) clearance or PMA approval. However, ASR products are subject to significant restrictions. The manufacturer may not make clinical or analytical performance claims for the ASR product, may not promote their use with additional laboratory equipment and may only sell the ASR product to clinical laboratories that are qualified to run high complexity tests under CLIA. Each laboratory must validate the ASR product for use in diagnostic procedures as a laboratory developed test.
17
In September 2007, the FDA published guidance for ASRs that define the types of products that can be sold as ASRs. Under the terms of this guidance and the “ASR Manufacturer Letter” issued in June 2008 by the Office of In Vitro Diagnostic Device Evaluation and Safety at the FDA, it may be more challenging for us to market some of our ASR products and we may be required to terminate those ASR product sales, conduct clinical studies and make submissions of the affected products to the FDA for clearance or approval.
Outside the United States, our ability to market our products is contingent upon maintaining our International Standards Organization, or ISO, certification, complying with European directives and in some cases receiving specific marketing authorization from the appropriate foreign regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorizations, pricing and reimbursement vary widely from country to country. Foreign registration is an ongoing process as we register additional products and/or product modifications.
We can give no assurance that the FDA or foreign regulatory agencies will give us requisite approvals, clearances or certifications for any of our product or product enhancements under development on a timely basis, if at all. Moreover, after clearance is given, these agencies can later withdraw the clearance or require us to change the device or its manufacturing process or labeling, to supply additional proof of its safety and effectiveness, or to recall, repair, replace or refund the cost of the medical device, if it is shown to be hazardous or defective. The process of obtaining clearance to market products is costly and time-consuming and can delay the marketing and sale of our products.
In August, 2012, the SEC adopted a new rule requiring disclosures of specified minerals, known as conflict minerals, that are necessary to the functionality or production of products manufactured or contracted to be manufactured by public companies. The new rule will require companies to diligence, disclose and report whether or not such minerals originate from the Democratic Republic of Congo, or DRC, or an adjoining country. If the minerals originated in the DRC, or if a company is not able to establish where the minerals originated, extensive disclosure regarding the sources of the minerals, and in some instances an independent audit of the supply chain, will be required. There may be material costs associated with these disclosure and audit requirements, including compliance costs and costs related to the sourcing and availability of certain minerals used in the manufacture of our products.
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including federal and state anti-kickback laws, as well as the U.S. Foreign Corrupt Practices Act, or FCPA. Anti-kickback laws make it illegal for an entity to solicit, offer, receive, or pay remuneration or anything of value in exchange for, or to induce, the referral of business or the purchasing, leasing, ordering, or arranging for or recommending the purchase, lease or order of any item or service paid for by Medicare, Medicaid or certain other federal and state healthcare programs. The statute has been broadly interpreted to cover a wide array of practices. Some states have passed similar laws and also regulate interactions with health care providers, or HCPs, as well as the requirement to disclose payments to HCPs. The federal government has published regulations that identify “safe harbors,” which if applicable will assure that certain arrangements will not be found to violate the federal anti-kickback statutes. Similarly, our international operations are subject to the provisions of the FCPA, which prohibits U.S. companies and their representatives from offering, promising, authorizing, or making payments to foreign officials for the purpose of influencing any act or decision of such official in his or her official capacity, inducing the official to do any act in violation of his or her lawful duty, or to secure any improper advantage in obtaining or retaining business. In many countries, the healthcare professionals we regularly interact with may meet the definition of a foreign official for purposes of the FCPA. While we make every effort to comply with applicable law and regulations, it is possible that our practices might be challenged under federal or state anti-kickback, FCPA or similar laws due to the breadth of the statutory provisions and the absence of extensive guidance regarding compliance. Violations of these laws may be punishable by criminal and/or civil sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid). If the government were to raise questions about our behavior or find that we have violated these laws, there could be a material adverse effect on our business. Our activities could be subject to challenge for the reasons discussed above, due to the broad scope of these laws and the increasing attention being given to them by law enforcement authorities.
The Patient Protection and Affordable Care Act and Health Care and Education Affordability Reconciliation Act of 2010, enacted into law in the U.S. in March 2010, includes new regulatory mandates and other measures designed to constrain medical costs, as well as stringent new reporting requirements of financial relationships between device manufacturers and physicians and hospitals. These reporting provisions preempt state laws that require reporting of the same information, but not those that require reports of different or additional information. We expect compliance with the new healthcare legislation to impose significant additional administrative and financial burdens on us.
Sales of medical devices outside of the United States are subject to foreign regulatory requirements that vary widely from country to country. The time required to obtain approval from a foreign country to market and sell our products may be longer or shorter than that required for FDA approval and the requirements may differ. In addition, we may be required to meet the FDA’s export requirements or receive FDA export approval for export of our products to foreign countries.
We are further subject to numerous federal, state and local laws relating to safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances, among others. We may be required to incur significant costs to comply with these laws and regulations in the future, and complying with these laws may result in a material adverse effect upon our business, financial condition and results of operations.
18
In August 2010, the FDA issued two reports outlining potential changes to the 510(k) regulatory process. In addition, in January 2011, the FDA issued an implementation plan containing 25 specific actions to be implemented in 2011 relating to the 510(k) regulatory process and associated administrative matters. The FDA also deferred action on several other initiatives, including the creation of a new class of devices that would be subject to heightened review processes, until the Institute of Medicine released a related report on the 510(k) regulatory process in July 2011. The FDA is reviewing the Institute of Medicine’s report as well as public input to determine what, if any, recommendations the FDA will adopt with respect to the 510(k) regulatory process. Many of the actions proposed by the FDA could result in significant changes to the 510(k) regulatory process, which would likely complicate the process of obtaining clearance for products by the FDA.
In September 2012, the European Commission proposed new regulations for medical devices. The proposed new regulations cover in one regulation devices that are currently the subject of two separate directives, the Active Implantable Medical Devices Directive and the Medical Devices Directive. The adoption of these regulations may impact our international operations through a broadened scope of medical device oversight and/or regulatory reach. Compliance with the new European Commission regulations, if and when adopted, may impose additional administrative and financial burdens on us.
Federal, state and foreign regulations regarding the manufacture and sale of medical devices and pharmaceuticals are subject to future change. We cannot predict what impact, if any, such changes might have on our business. See “Our delay or inability to obtain any necessary United States or foreign regulatory clearances or approvals for our newly developed products and treatments or product enhancements could harm our business and prospects” under “Item 1A. Risk Factors” below.
Reimbursement
In the U.S., the Centers for Medicare & Medicaid Services, known as CMS, establishes policies for the coverage and reimbursement of Medicare and Medicaid beneficiaries. Under current CMS policies, varying reimbursement levels have been established for bone density assessment, endometrial ablations, mammography and other imaging, diagnostic tests and surgical procedures performed using our products. Coverage policies for Medicare patients may vary by regional Medicare carrier in the absence of a national coverage determination and reimbursement rates for procedures will vary based on the geographic price index. Coverage and reimbursement for patients with private insurance is dependent on the individual private payer’s decisions and may not follow the policies and rates established by CMS for Medicare. Moreover, private insurance carriers may choose not to follow the CMS reimbursement policies. The use of our products outside the U.S. is similarly affected by reimbursement policies adopted by foreign regulatory authorities and insurance carriers.
Significant reductions in reimbursement rates proposed or implemented for the use of any our products have had and may continue to have a material adverse effect on the sales of those products. On an annual basis, CMS publishes reimbursement rates for laboratory services, physician, hospital and ambulatory surgical center payments. CMS published final 2013 rates on November 1, 2012 The CMS reimbursement rates for 2013 included a general reduction of 27% in the Sustainable Growth Rate, or SGR, factor. This factor is used by CMS in a formula to determine doctor reimbursements and, if implemented, would correspondingly affect the reimbursement for the use of our products. This reduction will go into effect January 1, 2013 unless legislation is passed by Congress.
Currently, there is not an established current procedural terminology, or CPT, code, reimbursement rate or official coverage for the use of 3D mammography (breast tomosynthesis) as it was only approved by the FDA in February 2011 in connection with our PMA application for our Dimensions system. We are working with governmental authorities, professional societies, healthcare providers, insurance companies and other third-party payors in efforts to secure reimbursement for the use of 3D tomosynthesis. However, we can give no assurance that these efforts will be successful. Failure to obtain, or delays in obtaining, adequate reimbursement for the use of 3D mammography would adversely affect sales of our Dimensions 3D systems.
Political, economic and regulatory influences, including those envisioned by the adoption in March 2010 of U.S. healthcare reform, may subject the healthcare industry to fundamental changes. We anticipate that the federal government and certain state legislatures will continue to review and assess alternative healthcare delivery systems and payment methods with the ultimate objective of reducing healthcare costs and expanding access. Healthcare reform proposals and medical cost containment measures in the United States and in many foreign countries could, among other things, limit the use of our products and treatments and further reduce reimbursement available for such use. These reforms or cost containment measures, including the uncertainty in the medical community regarding their nature and effect, could have an adverse effect on our customers’ purchasing decisions regarding our products and treatments and could harm our business, result of operations, financial condition and prospects.
Employees
As of September 29, 2012, we had approximately 6,157 full-time employees, including 1,850 in manufacturing operations, 857 in research and development, 2,750 in marketing, sales and support services, and 700 in finance and administration. The non-management employees of our Hitec-Imaging subsidiary are represented by a union. Hitec-Imaging’s 191 non-management German employees were subject to collective bargaining agreements negotiated on a national and regional basis between Unternehmens-Verband Südôstliches Westfalen e.V., the Employers Association of North Rhine-Westphalia, and the German Metal Workers Union, IndustrieGewerkschaft Metall. In addition, Hitec-Imaging’s German employees are represented by a works council, a Betriebsrat, with respect to various shop agreements for social matters and working conditions. We believe that our relationship with our employees is good. Except as described herein, none of our other employees are represented by a union.
19
Seasonality
Worldwide sales, including U.S. sales, do not reflect any significant degree of seasonality; however, customer purchases of our GYN Surgical products have been historically less in the second fiscal quarter of the year as compared to other quarters. We expect continuing fluctuations in our manufacture and shipment of blood screening products and instruments to Novartis, which vary each period based on Novartis’ inventory levels and supply chain needs. Our respiratory infectious disease product line is also subject to significant seasonal and year-over-year fluctuations. In addition, the summer months, which occur during our fiscal fourth quarter, typically have had lower order rates internationally for most of our products.
This report contains forward-looking information that involves risks and uncertainties, including statements regarding our plans, objectives, expectations and intentions. Our actual results could differ materially from those discussed herein. Other risks and uncertainties not presently known to us or that we currently deem immaterial may also materially adversely affect us. Factors that could cause or contribute to such differences include those discussed below, as well as those discussed elsewhere in this report.The cautionary statements made under the heading “Special Note Regarding Forward-Looking Statements” and elsewhere in this report are intended to be applicable to all related forward-looking statements wherever they may appear in this report. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this report.
Risks Relating to our Business
The continuing worldwide macroeconomic uncertainty may adversely affect our business and prospects.
Market acceptance of our medical products in the United States and other countries is dependent upon the medical equipment purchasing and procurement practices of our customers, patient demand for our products and procedures and the reimbursement of patients’ medical expenses by government healthcare programs and third-party payors. The continuing uncertainty surrounding world financial markets and continuing weak worldwide macroeconomic conditions, including as a result of actual or potential debt default by certain European countries, have caused and may continue to cause the purchasers of medical equipment to decrease their medical equipment purchasing and procurement activities. Additionally, constrictions in world credit markets have caused and may continue to cause our customers to experience increased difficulty securing the financing necessary to purchase our products. Economic uncertainty as well as increasing health insurance premiums and co-payments may continue to result in cost-conscious consumers making fewer elective trips to their physicians and specialists, which in turn would adversely affect demand for our products and procedures. Furthermore, governments and other third-party payors around the world facing tightening budgets could move to further reduce the reimbursement rates or the scope of coverage offered, which could adversely affect sales of our products. If the current adverse macroeconomic conditions continue, our business and prospects may be negatively impacted.
Sales and market acceptance of our products is dependent upon the coverage and reimbursement decisions made by third-party payors. The failure of third-party payors to provide appropriate levels of coverage and reimbursement for the use of our products and treatments facilitated by our products could harm our business and prospects.
Sales and market acceptance of our medical products and the treatments facilitated by our products in the United States and other countries is dependent upon the coverage decisions and reimbursement policies established by government healthcare programs and private health insurers. Market acceptance of our products and treatments has and will continue to depend upon our customers’ ability to obtain an appropriate level of coverage for, and reimbursement from third-party payors for, these products and treatments. In the U.S., CMS establishes coverage and reimbursement policies for healthcare providers treating Medicare and Medicaid beneficiaries. Under current CMS policies, varying reimbursement levels have been established for our products and treatments. Coverage policies for Medicare patients may vary by regional Medicare carriers in the absence of a national coverage determination and reimbursement rates for treatments may vary based on the geographic price index. Coverage and reimbursement policies and rates applicable to patients with private insurance are dependent upon individual private payor decisions which may not follow the policies and rates established by CMS. The use of our products and treatments outside the United States is similarly affected by coverage and reimbursement policies adopted by foreign governments and private insurance carriers.
Significant reductions in reimbursement rates proposed or implemented for the use of any our products have had and may continue to have a material adverse effect on the sales of those products. On an annual basis, CMS publishes reimbursement rates for laboratory services, physician, hospital and ambulatory surgical center payments. CMS published final 2013 rates on November 1, 2012 The CMS reimbursement rates for 2013 included a general reduction of 27% in the SGR factor. This factor is used by CMS in a formula to determine doctor reimbursements and, if implemented, would correspondingly affect the reimbursement for the use of our products. This reduction will go into effect in January 1, 2013 unless legislation is passed by Congress.
Currently, there is not an established CPT code, reimbursement rate or official coverage for the use of 3D mammography (breast tomosynthesis) as it was only approved by the FDA in February 2011 in connection with our PMA application for our
20
Dimensions system. We are working with governmental authorities, professional societies, healthcare providers, insurance companies and other third-party payors in efforts to secure reimbursement for the use of 3D mammography. However, we can give no assurance that these efforts will be successful. Failure to obtain, or delays in obtaining, adequate reimbursement for the use of 3D tomosynthesis would adversely affect sales of our Dimensions 3D systems.
The adoption of healthcare reform in the United States and the uncertainty surrounding the implementation of these reforms could harm our business and prospects.
The healthcare industry has undergone significant change driven by various efforts to reduce costs, trends toward managed care, cuts in Medicare, consolidation of healthcare distribution companies and collective purchasing arrangements by office-based healthcare practitioners. The effect of the implementation of the Patient Protection and Affordable Care Act and Health Care and Education Affordability Reconciliation Act of 2010, enacted into law in the U.S. in March 2010, on our business is uncertain. Among other things, the law requires the medical device industry to subsidize healthcare reform in the form of a 2.3% excise tax on U.S. sales of certain medical devices beginning January 1, 2013. We expect that this excise tax will apply to the majority, if not all, of our products sold in the U.S. U.S. net product sales represent, and will likely continue to represent a substantial majority of our net revenues. Our U.S. product sales represented 73% and 76% of our net product sales for the years ended September 29, 2012 and September 24, 2011, respectively. The law also includes new regulatory mandates and other measures designed to constrain medical costs, as well as stringent new reporting requirements of financial relationships between device manufactures and physicians and hospitals. We expect compliance with the new healthcare legislation, including with these new reporting requirements and the new excise tax, to impose significant additional administrative and financial burdens on us. Various healthcare reform proposals have also emerged at the state level. The healthcare reform legislation and these proposals could reduce medical procedure volumes and impact the demand for our products or the prices at which we sell our products. In addition, the excise tax will increase our costs of doing business. The impact of this healthcare reform legislation and these proposals could harm our business and prospects, results of operations and/or financial condition. Healthcare reform proposals and medical cost containment measures in the United States and in many foreign countries could:
| | • | | limit the use of our products and treatments; |
| | • | | reduce reimbursement available for such use; |
| | • | | further tax the sale or use of our products; |
| | • | | adversely affect the use of new therapies for which our products may be targeted; and |
| | • | | further increase the administrative and financial burden of compliance. |
These reforms, cost containment measures and new taxes, including the uncertainty in the medical community regarding their nature and effect, could also have an adverse effect on our customers’ purchasing decisions regarding our products and treatments and could harm our business, result of operations, financial condition and prospects.
Changes in laws affecting the healthcare industry could adversely affect our revenues and profitability.
We operate in a highly regulated industry. As a result, governmental actions may adversely affect our business, operations or financial condition, including:
| | • | | new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, method of delivery and payment for health care products and services; |
| | • | | changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and treatments and result in lost market opportunity; |
| | • | | changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products and treatments to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products or treatments, or otherwise adversely affect the market for our products and treatments; and |
| | • | | new laws, regulations and judicial decisions affecting pricing or marketing practices. |
We anticipate that governmental authorities will continue to scrutinize our industry closely and that additional regulation by governmental authorities may cause increased compliance costs, exposure to litigation and other adverse effects to our operations.
Guidelines, recommendations and studies published by various organizations can reduce the use of our products.
Professional societies, government agencies, practice management groups, private health/science foundations, and organizations involved in healthcare issues may publish guidelines, recommendations or studies to the healthcare and patient communities. Recommendations of government agencies or these other groups/organizations may relate to such matters as usage, cost-effectiveness, and use of related therapies. Organizations like these have in the past made recommendations about our products and those of our competitors. Recommendations, guidelines or studies that are followed by healthcare providers and insurers could result in decreased use of our products. For example, in November 2012, the American Congress of Obstetrics and Gynecologists, known as the ACOG, released updates in which they have recommended less frequent cervical cancer screening similar to guidelines released by ACOG in November 2009 and guidelines released in March 2012 by the U.S. Preventative Services Task Force, known as the USPSTF, and the American Cancer Society.
21
Our long-term success will depend upon our ability to successfully develop and commercialize new products and treatments and enhance our existing products and treatments.
We are devoting significant resources to our continuing research and development programs which are designed to develop new products and treatments and to enhance and improve our existing products and treatments. The successful development of our products and product enhancements is subject to numerous risks, both known and unknown, including:
| | • | | unanticipated delays in development, clinical trials or the approval or clearance process by the FDA or other applicable regulatory authority; |
| | • | | third-party intellectual property; |
| | • | | technical problems; and |
| | • | | other difficulties that could result in the abandonment or substantial change in the design, development and commercialization of these new products, including, for example, changes requested by the FDA in connection with pre-market approval applications for products or 510(k) clearance. |
Given the uncertainties inherent with product development, introduction, and enhancement our efforts may not be completed on a timely basis or within budget, if at all. Our failure to develop new products and product enhancements on a timely basis or within budget, if at all, could harm our business and prospects.
If we cannot maintain our current corporate collaborations and enter into new corporate collaborations, our product development could be delayed. In particular, any failure by us to maintain our blood screening collaboration with Novartis could have a material adverse effect on our business.
Gen-Probe has relied, to a significant extent, on corporate collaborators for funding the development of and marketing for certain of its products. In addition, we expect to rely on our corporate collaborators for the commercialization of certain products. If any of our corporate collaborators were to breach or terminate its agreement with us or otherwise fail to conduct its collaborative activities successfully and in a timely manner, the development or commercialization and subsequent marketing of the products contemplated by the collaboration could be delayed or terminated. We cannot control the amount and timing of resources our corporate collaborators devote to our programs or potential products.
The continuation of any of these collaboration agreements depends upon their periodic renewal by us and our collaborators. For example, in January 2009 Gen-Probe extended the term of its blood screening collaboration with Novartis to June 30, 2025, subject to earlier termination under certain limited circumstances specified in the collaboration agreement. The collaboration was previously scheduled to expire by its terms in 2013.
If any of our current collaboration agreements are terminated, or if we are unable to renew those collaborations on acceptable terms, we may be required to devote additional internal resources to product development or marketing or to terminate some development programs or seek alternative corporate collaborations. We may not be able to negotiate additional corporate collaborations on acceptable terms, if at all, and these collaborations may not be successful. In addition, in the event of a dispute under our current or any future collaboration agreements, such as our agreements with Novartis, court or arbitrator may not rule in our favor and our rights or obligations under an agreement subject to a dispute may be adversely affected, which may have an adverse effect on our business or operating results.
If we or our contract manufacturers are unable to manufacture our products in sufficient quantities, on a timely basis, at acceptable costs and in compliance with regulatory and quality requirements, our ability to sell our products will be harmed.
The manufacture of many of our products is highly complex and requires precise high quality manufacturing that is difficult to achieve. We have in the past and may in the future experience difficulties in manufacturing our products on a timely basis and in sufficient quantities. These difficulties have primarily related to delays and difficulties associated with ramping up production of newly introduced products and may result in increased delivery lead-times and increased costs of manufacturing these products. In addition, production of these newer products may require the development of new manufacturing technologies and expertise, which we may be unable to develop. Our failure, including the failure of our contract manufacturers, to achieve and maintain the required high manufacturing standards could result in further delays or failures in product testing or delivery, cost overruns, product recalls or withdrawals, increased warranty costs or other problems that could harm our business and prospects.
22
In determining the required quantities of our products and the manufacturing schedule, we must make significant judgments and estimates based on historical experience, inventory levels, current market trends and other related factors. Because of the inherent nature of estimates, there could be significant differences between our estimates and the actual amounts of products we and our distributors require, which could harm our business and results of operations.
Blood screening and clinical diagnostic products are regulated by the FDA as well as other foreign medical regulatory bodies. In some cases, such as in the United States and the EU, certain products may also require individual lot release testing. Maintaining compliance with multiple regulators, and multiple centers within the FDA, adds complexity and cost to our manufacturing processes. In addition, our manufacturing facilities and those of our contract manufacturers are subject to periodic regulatory inspections by the FDA and other regulatory agencies, and these facilities are subject to FDA requirements relating to the Quality System Regulation. We or our contractors may fail to satisfy these regulatory requirements in the future, and any failure to do so may prevent us from selling our products.
Our business could be harmed if our products contain undetected errors or defects or do not meet applicable specifications.
We are continuously developing new products and improving our existing products. Our existing and newly introduced products can contain undetected errors or defects. In addition, these products may not meet their performance specifications under all conditions or for all applications. If, despite internal testing and testing by customers, any of our products contain errors or defects or fail to meet applicable specifications, then we may be required to enhance or improve those products or technologies. We may not be able to do so on a timely basis, if at all, and may only be able to do so at considerable expense. In addition, any significant reliability problems could result in adverse customer reaction, negative publicity, mandatory or voluntary recalls or legal claims and could harm our business and prospects.
Our products may be subject to recalls even after receiving FDA clearance or approval, which could harm our business and prospects.
The FDA and similar governmental bodies in other countries have the authority to require the recall of medical products in the event of material deficiencies or defects in design or manufacture. A government mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling. Any recall could harm the reputation of our products and adversely affect our business and prospects. In the past, Gen-Probe voluntarily recalled products, which, in each case, required it to identify a problem and correct it. In May 2011, Gen-Probe voluntarily recalled certain Elucigene test kits for the detection of genetic mutations associated with cystic fibrosis because of issues Gen-Probe identified during quality control stability testing. All affected customers and appropriate regulatory authorities were advised of the voluntary recall and Gen-Probe made a substitute product available. The affected product is CE marked, but is not cleared by the FDA and is not available for sale in the United States. In addition, in May 2011 Gen-Probe initiated a second voluntary recall of certain Elucigene branded tests in Canada upon determination that such products were not properly registered with Health Canada. In April 2012, Gen-Probe voluntarily recalled certain lots of LIFECODES PAK (platelet antibody) products after determining that the negative controls in the assays were increasing signals over time, leading to the potential for decreased product performance.
Our products may be subject to a future government-mandated recall or further voluntary recalls, and any such recalls could divert managerial and financial resources, be more difficult and costly to correct, result in the suspension of sales of certain of our products and/or harm our reputation and financial results.
Interruptions, delays, shutdowns or damage at our manufacturing facilities could harm our business.
We and our contract manufacturers manufacture our products at a relatively limited number of different facilities located throughout the world. An interruption in manufacturing capabilities at any of these facilities, as a result of equipment failure or other reasons, could reduce, delay or prevent the production of our products. Our manufacturing facilities are subject to the risk of catastrophic loss due to unanticipated events, such as fires, earthquakes, explosions, floods or weather conditions. Our manufacturing facilities may experience plant shutdowns, strikes or other labor disruptions, or periods of reduced production as a result of equipment failures, loss of power, gray outs, delays in deliveries or extensive damage to any of our facilities, which could harm our business and prospects. Because some of our manufacturing operations are located outside the United States, including in Germany, Canada, Costa Rica, the United Kingdom and China, those manufacturing operations are also subject to additional challenges and risks associated with international operations described below.
Our delay or inability to obtain any necessary United States or foreign regulatory clearances or approvals for our newly developed products and treatments or product enhancements could harm our business and prospects.
Our products and treatments are subject to a high level of regulatory oversight. Our delay or inability to obtain any necessary United States or foreign regulatory clearances or approvals for our newly developed products or product enhancements could harm our business and prospects. The process of obtaining clearances and approvals can be costly and time-consuming. In addition, there is a risk that any approvals or clearances, once obtained, may be withdrawn or modified.
23
Medical devices cannot be marketed in the United States without 510(k) clearance or premarket approval by the FDA. Any modifications to a device that has received a pre-market approval that affect the safety or effectiveness of the device require a pre-market approval supplement or possibly a separate pre-market approval, either of which is likely to be time-consuming, expensive and uncertain to obtain. If the FDA requires us to seek one or more pre-market approval supplements or new pre-market approvals for any modification to a previously approved device, we may be required to cease marketing or to recall the modified device until we obtain approval, and we may be subject to significant criminal and/or civil sanctions, including but not limited to, regulatory fines or penalties.
Medical devices sold in the United States must also be manufactured in compliance with FDA Good Manufacturing Practices, which regulate the design, manufacture, packing, storage and installation of medical devices. Moreover, medical devices are required to comply with FDA regulations relating to investigational research and labeling. States may also regulate the manufacture, sale and use of medical devices, particularly those that employ x-ray technology. Our products are also subject to approval and regulation by foreign regulatory and safety agencies.
Delays in receipt of, or failure to obtain, clearances or approvals for future products could delay or preclude realization of product revenues from new products or result in substantial additional costs which could decrease our profitability. In August 2010, the FDA issued two reports outlining potential changes to the 510(k) regulatory process. In addition, in January 2011, the FDA issued an implementation plan containing 25 specific actions to be implemented in 2011 relating to the 510(k) regulatory process and associated administrative matters. The FDA also deferred action on several other initiatives, including the creation of a new class of devices that would be subject to heightened review processes, until the Institute of Medicine released a related report on the 510(k) regulatory process in July 2011. The FDA is reviewing the Institute of Medicine’s report as well as public input to determine what, if any, recommendations the FDA will adopt with respect to the 510(k) regulatory process. Many of the actions proposed by the FDA could result in significant changes to the 510(k) regulatory process, which would likely complicate the process of obtaining clearance for products by the FDA. In September 2012, the European Commission proposed new regulations for medical devices. The proposed new regulations cover in one regulation devices that are currently the subject of two separate directives, the Active Implantable Medical Devices Directive and the Medical Devices Directive. The adoption of these regulations may impact our international operations through a broadened scope of medical device oversight and/or regulatory reach. Compliance with the new European Commission regulations, if and when adopted, may impose additional administrative and financial burdens on us.
The markets for our newly developed products and treatments and newly introduced enhancements to our existing products and treatments may not develop as expected.
The successful commercialization of our newly developed products and treatments and newly introduced enhancements to our existing products and treatments are subject to numerous risks, both known and unknown, including:
| | • | | uncertainty of the development of a market for such product or treatment; |
| | • | | trends relating to, or the introduction or existence of, competing products, technologies or alternative treatments or therapies that may be more effective, safer or easier to use than our products, technologies, treatments or therapies; |
| | • | | the perceptions of our products or treatments as compared to other products and treatments; |
| | • | | recommendation and support for the use of our products or treatments by influential customers, such as hospitals, radiological practices, breast surgeons and radiation oncologists and treatment centers; |
| | • | | the availability and extent of data demonstrating the clinical efficacy of our products or treatments; |
| | • | | competition, including the presence of competing products sold by companies with longer operating histories, more recognizable names and more established distribution networks; and |
| | • | | other technological developments. |
Often, the development of a significant market for a product or treatment will depend upon the establishment of a reimbursement code or an advantageous reimbursement level for use of the product or treatment. Moreover, even if addressed, such reimbursement codes or levels frequently are not established until after a product or treatment is developed and commercially introduced, which can delay the successful commercialization of a product or treatment.
If we are unable to successfully commercialize and create a significant market for our newly developed products and treatments and newly introduced enhancements to our existing products and treatments our business and prospects could be harmed.
The markets for our Dimensions 3D tomosynthesis system may not develop as expected.
The markets for our Dimensions 3D tomosynthesis system and related products may not continue to develop as expected. There is a significant installed base of conventional digital and screen-film mammography products in hospitals and radiological practices. The use of our Dimensions 3D tomosynthesis system in many cases would require these potential customers to either modify or replace their existing x-ray imaging equipment. As our Dimensions 3D tomosynthesis systems are generally more
24
expensive than conventional mammography products, we believe that a major factor in the market’s acceptance of Dimensions 3D tomosynthesis systems has been and will continue to be based upon the benefits of tomosynthesis as compared to less expensive technologies. Moreover, as a new technology, there is currently limited, if any, reimbursement for the use of 3D tomosynthesis. We believe that our ability to continue to gain market acceptance of the Dimensions 3D tomosynthesis system and follow-on products depends on our ability to demonstrate the clinical efficacy and cost-effectiveness of the Dimensions 3D tomosynthesis system and to secure reimbursement to support the use of 3D tomosynthesis. We are seeking to work with healthcare providers, insurance companies and other third-party payors in connection with our efforts to promote, and to secure reimbursement for, the use of 3D tomosynthesis. However, we can give no assurance that these efforts will be successful. The markets for our Dimensions 3D tomosynthesis system and related products have and will continue to be affected by published studies and reports relating to the comparative efficacy of tomosynthesis, as well as decisions relating to the reimbursement of healthcare providers for the use of the system. The publication of an adverse study, or an adverse decision relating to the reimbursement of the use of tomosynthesis, would likely significantly impair the adoption of this technology and harm our business. Sales of our Dimensions 3D tomosynthesis system may also be adversely affected by increased competition. Several companies, including Siemens, Giotto, Philips and Planmed, have recently introduced 3D tomosynthesis systems in certain foreign countries. We also are aware that other companies, several of which have substantially greater resources than we have, such as GE and Siemens, are developing 3D tomosynthesis systems for approval in the U.S. Because the markets for our Dimensions 3D tomosynthesis system and related products are relatively new, it is likely that our evaluation of the potential markets for these products will materially vary with time.
Our business may be harmed by the acquisition of Gen-Probe, our other prior acquisitions or acquisitions we may complete in the future.
We have acquired a number of businesses, technologies, product lines and products, and may make additional acquisitions in the future. Promising acquisitions are difficult to identify and complete for a number of reasons, including competition among prospective buyers and the need for regulatory, including antitrust, approvals. We may not be able to identify and successfully complete acquisition transactions. Any acquisition we may complete may be made at a substantial premium over the fair value of the net assets of the acquired company. Further, the long-term success of our acquisitions and any additional acquisitions we may complete in the future will depend upon our ability to realize the anticipated benefits from combining the acquired businesses with our business. We may fail to realize anticipated benefits for a number of reasons, including the following:
| | • | | problems may arise with our ability to successfully integrate the acquired businesses, which may result in us not operating as effectively and efficiently as expected, and may include: |
| | • | | diversion of management time, as well as a shift of focus from operating the businesses to issues related to integration and administration or inadequate management resources available for integration activity and oversight; |
| | • | | failure to retain and motivate key employees; |
| | • | | failure to successfully oversee international sales efforts and inability to prevent FCPA violations; |
| | • | | failure to successfully obtain appropriate regulatory approval or clearance for products under development; |
| | • | | failure to successfully manage relationships with customers, distributors and suppliers; |
| | • | | failure of customers to accept new products; |
| | • | | failure to effectively coordinate sales and marketing efforts; |
| | • | | failure to combine product offerings and product lines quickly and effectively; |
| | • | | failure to effectively enhance acquired technology and products or develop new products relating to the acquired businesses; |
| | • | | potential difficulties and inefficiencies in managing and operating businesses in multiple locations or operating businesses in which we have either limited or no direct experience; |
| | • | | potential difficulties integrating financial reporting systems; |
| | • | | potential difficulties in the timely filing of required reports with the SEC; and |
| | • | | potential difficulties in implementing controls, procedures and policies, including disclosure controls and procedures and internal controls over financial reporting, appropriate for a larger public company at companies that, prior to the acquisition of such companies, had lacked such controls, procedures and policies, which may result in ineffective disclosure controls and procedures or material weaknesses in internal controls over financial reporting; |
| | • | | we may not be able to achieve the expected synergies from an acquisition or it may take longer than expected to achieve those synergies; |
25
| | • | | an acquisition may result in future impairment charges related to a decline in the fair value of the acquired business as compared to the price we paid for such acquisition; |
| | • | | an acquisition may involve restructuring operations or reductions in workforce which may result in substantial charges to our operations; |
| | • | | our current and prospective customers and suppliers may experience uncertainty associated with an acquisition, including with respect to current or future business relationships with us and may attempt to negotiate changes in existing business; |
| | • | | an acquisition may involve unexpected costs or liabilities, including as a result of pending and future shareholder lawsuits relating to acquisitions or exercise by shareholders of their statutory appraisal rights, or the effects of purchase accounting may be different from our expectations; |
| | • | | an acquisition may involve significant deferred or contingent payments that may adversely affect our future liquidity or capital resources; and |
| | • | | the acquired businesses may be adversely affected by future legislative, regulatory, or tax decisions and/or changes as well as other economic, business and/or competitive factors. |
Our failure to realize the anticipated benefits from combining acquired businesses could harm our business and prospects.
If we are successful in pursuing future acquisitions, we may be required to expend significant funds, incur additional debt or other obligations, or issue additional securities, which may negatively affect our operating results and financial condition. If we spend significant funds or incur additional debt or other obligations, our ability to obtain financing for working capital or other purposes could decline, and we may be more vulnerable to economic downturns and competitive pressures. We cannot guarantee that we will be able to finance additional acquisitions or that we will realize any anticipated benefits from acquisitions that we complete.
We will incur significant acquisition-related costs in connection with the acquisition of Gen-Probe.
We have incurred and expect to incur additional significant costs associated with our acquisition of Gen-Probe and combining the operations of the two companies. The substantial majority of the expenses resulting from the acquisition were comprised of transaction costs related to investment banker fees and other professional services as well as systems consolidation costs and business integration and employment-related costs, including costs for severance, retention and other restructuring activities. Additional unanticipated costs may be incurred in the integration of the two companies’ businesses. Although we expect that the elimination of duplicative costs, as well as the realization of other efficiencies related to the integration of the businesses, should allow us to offset incremental transaction and acquisition-related costs over time, this net benefit may not be achieved in the near term, or at all.
Our business may be harmed by the contingent earn out obligations we incurred in connection with our acquisitions or acquisitions we may complete in the future.
In connection with certain of our acquisitions, we have incurred the obligation to make contingent earn out payments tied to performance criteria, principally revenue growth of the acquired businesses over a specified period. We also expect that acquisitions we may complete in the future may contain contingent earn out payments, and these payments could be significant. In certain circumstances, such as a change of control, a portion of these obligations may be accelerated. In addition, contractual provisions relating to these contingent earn out obligations may include covenants to operate the acquired businesses in a manner that may not otherwise be most advantageous to us. These provisions may also result in the risk of litigation relating to the calculation of the amount due or our operation of the acquired business. Such litigation could be expensive and divert management attention and resources. Our obligation to make contingent payments may also result in significant operating expenses. Depending upon the particular facts and circumstances giving rise to the payment and our previous estimates, all or a portion of these payments may be required to be expensed by us when accrued. For example, our contingent earn out obligations payable in connection with the TCT and Healthcome acquisitions will be fully expensed as accrued because our obligation to make these payments is conditioned on the continued employment of certain key employees of TCT and Healthcome. We can give no assurance that we will have sufficient funds to pay our contingent obligations when due, or that such obligations, including the associated covenants relating to the operation of the acquired business, will not otherwise adversely affect our business, liquidity, capital resources or results of operations.
It may be difficult for us to implement our strategies for improving growth.
Some of the markets in which we compete have been flat or declining over the past several years. To address this issue, we are pursuing a number of strategies to improve our growth, including:
| | • | | expanding our product offerings; |
| | • | | allocating research and development funding to products with higher growth prospects; |
| | • | | developing new applications for our technologies; |
26
| | • | | strengthening our presence in selected geographic markets; |
| | • | | acquiring technologies and businesses that complement or augment our existing products and services; |
| | • | | implementing targeted customer initiatives; and |
| | • | | supporting cross-selling opportunities of products and services to take advantage of the breadth of our product offerings. |
We may not be able to successfully implement these strategies, and these strategies may not result in the growth of our business.
Consolidation in the healthcare industry could lead to increased demands for price concessions or the exclusion of some suppliers from certain of our significant market segments, which could harm our business and prospects.
The cost of healthcare has risen significantly over the past decade and numerous initiatives and reforms by legislators, regulators and third-party payors to curb these costs have resulted in a consolidation trend in the healthcare industry, including hospitals and clinical laboratories. This consolidation has resulted in greater pricing pressures, decreased average selling prices, and the exclusion of certain suppliers from important market segments as group purchasing organizations, independent delivery networks and large single accounts continue to consolidate purchasing decisions for some of our hospital customers. We expect that market demand, government regulation, third-party reimbursement policies, government contracting requirements, and societal pressures will continue to change the worldwide healthcare industry, resulting in further business consolidations and alliances among our customers and competitors, which may reduce competition and continue to exert further downward pressure on the prices of our products and adversely impact our business, financial condition or results of operations. In particular, we are dependent upon a relatively small number of large clinical laboratory customers in the United States for a significant portion of our sales of diagnostics products. Due in part to a trend toward consolidation of clinical laboratories in recent years and the relative size of the largest United States laboratories, it is likely that a significant portion of these sales will continue to be concentrated among a relatively small number of large clinical laboratories.
Our business is dependent on technologies we license, and if we fail to maintain these licenses or license new technologies and rights to particular nucleic acid sequences for targeted diseases in the future, we may be limited in our ability to develop new products.
Our business is dependent on licenses from third parties for some of our key technologies. For example, our patented TMA technology is based on technology we licensed from Stanford University. In addition, we have acquired exclusive worldwide diagnostic rights to the PCA3 gene from DiagnoCure, Inc. We anticipate that we will enter into new licensing arrangements in the ordinary course of business to expand our product portfolio and access new technologies to enhance our products and develop new products. Many of these licenses will provide us with exclusive rights to the subject technology or disease marker. If our license with respect to any of these technologies or markers is terminated for any reason, we may not be able to sell products that incorporate the technology. Similarly, we may lose competitive advantages if we fail to maintain exclusivity under an exclusive license.
Our ability to develop additional diagnostic tests for diseases may depend on the ability of third parties to discover particular sequences or markers and correlate them with disease, as well as the rate at which such discoveries are made. Our ability to design products that target these diseases may depend on our ability to obtain the necessary rights from the third parties that make any of these discoveries. In addition, there are a finite number of diseases and conditions for which our NAT diagnostic assays may be economically viable. If we are unable to access new technologies or the rights to particular sequences or markers necessary for additional diagnostic products on commercially reasonable terms, we may be limited in our ability to develop new diagnostic products.
Our products and manufacturing processes will require access to technologies and materials that may be subject to patents or other intellectual property rights held by third parties. We may need to obtain additional intellectual property rights in order to commercialize our products. We may be unable to obtain such rights on commercially reasonable terms or at all, which could adversely affect our ability to grow our business.
Our business could be harmed if we are unable to protect our proprietary technology.
We have relied primarily on a combination of trade secrets, patents, and copyrights to protect our products and technology. Despite these precautions, unauthorized third parties may infringe our intellectual property, or copy or reverse engineer portions of our technology. The pursuit and assertion of a patent right, particularly in areas like nucleic acid diagnostics and biotechnology, involve complex determinations and, therefore, are characterized by substantial uncertainty. We do not know if current or future patent applications will be issued with the full scope of the claims sought, if at all, or whether any patents that do issue will be challenged or invalidated. The patents that we own or license could also be subject to interference proceedings or similar disputes over the priority of the inventions, and an unfavorable outcome could require us to cease using the related technology or to attempt to license rights to the technology from the prevailing party. In addition, the laws governing patentability and the scope of patent coverage continue to evolve, particularly in biotechnology. As a result, patents might not issue from certain of our patent applications or from applications licensed to us.
27
We have obtained or applied for corresponding patents and patent applications in several foreign countries for some of our patents and patent applications. There is a risk that these patent applications will not be granted or that the patent or patent application will not provide significant protection for our products and technology.
The rights provided by a patent are finite in time. Over the coming years, certain patents relating to current products will expire in the U.S. and abroad thus allowing third parties to utilize certain of our technologies.
Our competitors may independently develop similar technology that our patents do not cover. In addition, because patent applications in the United States are not generally publicly disclosed until eighteen months after the application is filed, applications may have been filed by third parties that relate to our technology. Moreover, there is a risk that foreign intellectual property laws will not protect our intellectual property rights to the same extent as intellectual property laws in the U.S. Even if our proprietary information is protected by patents or otherwise, the initiation of actions to protect our proprietary information could be costly and divert the efforts and attention of our management and technical personnel, and the outcome of such litigation is often uncertain. As a result of these uncertainties, we could also elect to forego such litigation or settle such litigation without fully enforcing our proprietary rights. In the absence of significant patent protection, we may be vulnerable to competitors who attempt to copy our products, processes or technology.
Our business could be harmed if we infringe upon the intellectual property rights of others.
There has been substantial litigation regarding patent and other intellectual property rights in the medical device, diagnostic products and related industries. We are and have been involved in patent litigation, and may in the future be subject to further claims of infringement of intellectual property rights possessed by third parties.
In connection with claims of patent infringement, we may seek to enter into settlement and/or licensing arrangements. There is a risk in these situations that no license will be available or that a license will not be available on reasonable terms. Alternatively, we may decide to litigate such claims or to design around the patented technology. These actions could be costly and would divert the efforts and attention of our management and technical personnel. As a result, any infringement claims by third parties or claims for indemnification by customers resulting from infringement claims, whether or not proven to be true, may harm our business and prospects.
Our international operations and foreign acquisitions expose us to additional operational challenges that we might not otherwise face.
We are subject to a number of additional risks and expenses due to our international operations, including our operations in China. Any of these risks or expenses could harm our operating results. These risks and expenses include:
| | • | | difficulties in staffing and managing operations in multiple locations as a result of, among other things, distance, language and cultural differences; |
| | • | | protectionist laws and business practices that favor local companies; |
| | • | | difficulties in the collection of trade accounts receivable; |
| | • | | difficulties and expenses related to implementing internal controls over financial reporting and disclosure controls and procedures; |
| | • | | expenses associated with customizing products for clients in foreign countries; |
| | • | | possible adverse tax consequences; |
| | • | | the inability to obtain favorable third-party reimbursements; |
| | • | | the inability to obtain required regulatory approvals; |
| | • | | governmental currency controls; |
| | • | | multiple, conflicting and changing government laws and regulations (including, among other things, antitrust and tax requirements, international trade regulations and the FCPA); |
| | • | | reduced protection for intellectual property rights in some countries; |
| | • | | political and economic changes and disruptions, export/import controls and tariff regulations; |
| | • | | the inability to effectively obtain or enforce intellectual property rights and otherwise protect against clone or “knock off” products; and |
| | • | | the lack of ability to enforce non-compete agreements with former owners of acquired businesses competing with us in China and other foreign countries. |
28
We utilize distributors for a portion of our sales, the loss of which could harm our revenues in the territory serviced by these distributors.
We rely on strategic relationships with a number of key distributors for sales and service of our products. For example, in our diagnostics business we are dependent on Novartis to distribute the blood screening products we manufacture. Commercial blood screening product sales to Novartis accounted for 36% of Gen-Probe’s total product sales of $298.0 million for the first six months of Gen-Probe’s fiscal 2012 and 35% of Gen-Probe’s total product sales of $562.6 million for Gen-Probe’s fiscal 2011. In January 2009, Gen-Probe extended the term of its blood screening collaboration with Novartis to June 30, 2025, subject to earlier termination under certain limited circumstances specified in the collaboration agreement. If our relationship with Novartis or any of our other strategic relationships are terminated and not replaced, our revenues and/or ability to service our products in the territories serviced by these distributors could be adversely affected. If any of our distribution or marketing agreements are terminated, particularly our collaboration agreement with Novartis, or if we elect to distribute new products directly, we will have to invest in additional sales and marketing resources, including additional field sales personnel, which would significantly increase future selling, general and administrative expenses. We may not be able to enter into new distribution or marketing agreements on satisfactory terms, or at all. If we fail to enter into acceptable distribution or marketing agreements or fail to successfully market our products, our product sales will decrease. We may also be exposed to risks as a result of transitioning a territory from a distributor sales model to a direct sales model, such as difficulties maintaining relationships with specific customers, hiring appropriately trained personnel or ensuring compliance with local product registration requirements, any of which could result in lower revenues than previously received from the distributor in that territory.
Fluctuations in the exchange rates of European currencies and the other foreign currencies in which we conduct our business, in relation to the U.S. dollar, could harm our business and prospects.
We maintain sales and service offices outside the United States, have manufacturing facilities outside the United States in Canada, China, Costa Rica, England and Germany, and conduct business worldwide. The expenses of our international offices are denominated in local currencies, except at our Costa Rica subsidiary, where the majority of business is conducted in U.S. dollars. Our foreign sales may be denominated in local currencies, the Euro or U.S. dollar. Historically, a majority of our sales of capital equipment to international dealers have been denominated in U.S. dollars; however, in the second half of fiscal 2010 we began to invoice more of our European sales in the Euro.
Fluctuations in foreign currency exchange rates could affect our revenues, cost of goods and operating margins and could result in exchange losses. In addition, currency devaluation can result in a loss if we hold deposits of that currency. In the last few years we have not hedged foreign currency exposures, but we may in the future hedge foreign currency denominated sales. There is a risk that any hedging activities will not be successful in mitigating our foreign exchange risk exposure and may adversely impact our financial condition and results of operations.
We rely on one or only a limited number of suppliers for some key components or subassemblies for our products. This reliance could harm our business and prospects.
We rely on one or only a limited number of suppliers for some key raw materials, components or subassemblies for our products. Obtaining alternative sources of supply of these components could involve significant delays and other costs and regulatory challenges, and may not be available to us on reasonable terms, if at all. The failure of a component supplier or contract assembler to provide sufficient quantities, acceptable quality and timely components or assembly service at an acceptable price, or an interruption of supplies from such a supplier could harm our business and prospects. Any disruption of supplies of key components could delay or reduce shipments, which could result in lost or deferred sales.
Our current supplier of certain key raw materials for certain of our amplified NAT diagnostic assays, pursuant to a fixed-price contract, is Roche Molecular Biochemicals. We have a supply and purchase agreement for oligonucleotides for HPV with Roche Molecular Systems. Each of these entities is an affiliate of Roche Diagnostics GmbH, which is one of our primary competitors in molecular diagnostics.
We have only one third-party manufacturer for each of our molecular diagnostics instrument product lines, which exposes us to increased risks associated with production delays, delivery schedules, manufacturing capability, quality control, quality assurance and costs.
We have one third-party manufacturer for each of our molecular diagnostics instrument product lines. KMC Systems, Inc., or KMC Systems, is the only manufacturer of the TIGRIS instrument; Stratec Biomedical Systems AG, or Stratec, is the only manufacturer of the PANTHER instrument; and Tecan Group Ltd., or Tecan, is the only manufacturer of the Cervista High Throughput Automation System. We are dependent on these third-party manufacturers, and this dependence exposes us to increased risks associated with production delays, delivery schedules, manufacturing capability, quality control, quality assurance and costs.
29
We have no firm long-term commitments from KMC Systems, Stratec, Tecan or any of our other contract manufacturers to supply products to us for any specific period, or in any specific quantity, except as may be provided in a particular purchase order. If KMC Systems, Stratec, Tecan or any of our other third-party manufacturers experiences delays, disruptions, capacity constraints or quality control problems in its development or manufacturing operations or becomes insolvent or otherwise fails to supply us with products in sufficient quantities, then instrument shipments to our customers could be delayed, which would decrease our revenues and harm our competitive position and reputation. Further, because we place orders with our manufacturers based on forecasts of expected demand for our instruments, if we inaccurately forecast demand we may be unable to obtain adequate manufacturing capacity or adequate quantities of components to meet our customers’ delivery requirements, or we may accumulate excess inventories.
We may in the future need to find new contract manufacturers to replace existing suppliers, increase our volumes or reduce our costs. We may not be able to find contract manufacturers that meet our needs, and even if we do, qualifying a new contract manufacturer and commencing volume production is expensive and time consuming. If we are required or elect to change contract manufacturers, we may lose revenues and our customer relationships may suffer.
We may experience unexpected problems and expenses associated with our planned consolidation of operations and facilities that could materially harm our business and prospects.
We continually review our operations and facilities in an effort to reduce costs and increase efficiencies and currently plan to consolidate several of our operations and facilities, including:
| | • | | the consolidation of our selenium panel coating production line, currently located in Germany, into our digital detector manufacturing facility in Newark, Delaware; |
| | • | | the consolidation of our breast biopsy operations, including manufacturing, research and development and sales support, currently located in Indianapolis, Indiana, into our Costa Rica manufacturing facility and our headquarters facilities in Massachusetts; and |
| | • | | the consolidation of our Madison, Wisconsin molecular diagnostics operations into our Gen-Probe facilities in San Diego, California. |
We expect these consolidations to be completed over various periods of time through calendar 2014.
Uncertainty is inherent within the consolidation process, and unforeseen circumstances, costs and expenses could offset the anticipated benefits, disrupt operations, including the timely delivery of products and service to customers, and impact product quality. In addition, we may fail to retain key employees who possess specific knowledge or expertise and who we are depending upon for the timely and successful transition, we may not be able to attract a sufficient number of skilled workers at the new locations to handle the additional production and other demands, and the relocation may absorb significant management and key employee attention and resources. If any of these risks materialize, our business, result of operations, financial condition and prospects may be adversely affected.
We face intense competition from other companies and may not be able to compete successfully.
A number of companies have developed, or are expected to develop, products that compete or will compete with our products. Some of our competitors are large companies that may enjoy significant competitive advantages over us, including:
| | • | | significantly greater name recognition; |
| | • | | larger or more established distribution networks; |
| | • | | additional product lines, and the ability to offer rebates or bundle products to offer discounts or incentives to gain a competitive advantage; |
| | • | | higher levels of automation and more substantial installed bases of such equipment; |
| | • | | more extensive research, development, sales, marketing, manufacturing and financial capabilities; and |
| | • | | greater financial resources allowing them to continue to improve their technology in order to compete in an evolving industry. |
The markets in which we sell our products are intensely competitive, subject to rapid technological change and may be significantly affected by new product introductions and other market activities of industry participants, and these competitive pressures may reduce our gross margins. Other companies may develop products that are superior to or less expensive, or both, than our products. Improvements in existing competitive products or the introductions of new competitive products may reduce our ability to compete for sales, particularly if those competitive products demonstrate better safety or effectiveness, clinical results, ease of use or lower costs.
30
The current environment of managed care, economically-motivated buyers, consolidation among healthcare providers, increased competition and declining reimbursement rates, together with current global economic conditions and healthcare reform measures, may put additional competitive pressure on us, including on our average selling prices, overall procedure rates and market sizes.
If we are unable to compete effectively against existing and future competitors and existing and future alternative treatments, our business and prospects could be harmed.
Because Gen-Probe has historically depended on a small number of customers for a significant portion of its product sales, the loss of any of these customers or any cancellation or delay of a large purchase by any of these customers could significantly reduce our revenues.
Historically, a limited number of customers have accounted for a significant portion of Gen-Probe’s product sales, and Gen-Probe does not have any long-term commitments with these customers, other than pursuant to its collaboration agreement with Novartis. Product sales from Gen-Probe’s blood screening collaboration with Novartis accounted for 36% of its total product sales of $298.0 million for the first six months of Gen-Probe’s fiscal 2012 and 35% of its total product sales of $562.6 million for Gen-Probe’s fiscal 2011. Gen-Probe’s blood screening collaboration with Novartis is largely dependent on two significant customers in the United States, The American Red Cross and Creative Testing Solutions, although Gen-Probe does not receive any revenues directly from those entities. Novartis was Gen-Probe’s only customer that accounted for greater than 10% of its total revenues during the first six months of its fiscal 2012 and 2011. We anticipate that our operating results will continue to depend, to a significant extent, upon revenues from a small number of customers. The loss of any of our key customers, or a significant reduction in sales volume or pricing to those customers, could significantly reduce our revenues.
Our success depends upon our ability to adapt to rapid changes in technology and customer requirements.
The markets for our products have been characterized by rapid technological change, frequent product introductions and evolving customer requirements. These trends will likely continue into the foreseeable future. Our success depends, in part, upon our ability to enhance our existing products, successfully develop new products that meet increasingly challenging customer requirements and gain market acceptance. If we fail to do so our products may be rendered obsolete or uncompetitive by new industry standards or changing technology.
We will likely continue to incur significant research and development expenses, which may reduce our profitability.
Historically, we have incurred significant costs in connection with the development and improvement of our products and technologies. We expect that research and development expenditures will remain high as we seek to expand our product offerings and continue to develop and improve products and technologies. As a result, we will need to continue to generate significant revenues to maintain current levels of profitability. We may not be able to generate sufficient revenues to maintain current levels of profitability in the future.
Our results of operations are subject to significant quarterly variation.
Our results of operations have been and may continue to be subject to significant quarterly variation. Our results for a particular quarter may also vary due to a number of factors, including:
| | • | | the overall state of healthcare and cost containment efforts; |
| | • | | the timing and level of reimbursement for our products domestically and internationally; |
| | • | | the development status and demand for our products; |
| | • | | the development status and demand for therapies to treat the health concerns addressed by our products and treatments; |
| | • | | economic conditions in our markets; |
| | • | | foreign exchange rates; |
| | • | | the timing of expenditures in anticipation of future sales; |
| | • | | the mix of products we sell and markets we serve; |
| | • | | regulatory approval of products; |
31
| | • | | the introduction of new products and product enhancements by us or our competitors; |
| | • | | pricing and other competitive conditions; |
| | • | | unanticipated expenses; |
| | • | | complex revenue recognition rules pursuant to U.S. generally accepted accounting principles, which we refer to as U.S. GAAP; |
| | • | | contingent consideration charges; |
| | • | | restructuring and consolidation charges; and |
| | • | | seasonality of sales of certain of our products. |
Customers may also cancel or reschedule shipments. Production difficulties could also delay shipments. Any of these factors also could harm our business and prospects.
Recent changes to reclassify full-field digital mammography to permit 510(k) clearance could increase competition for our digital mammography products.
The FDA has changed the classification of 2D digital mammography systems from Class III to Class II. As a result, these 2D digital mammography systems will require a 510(k) submission rather than a PMA, which will make it easier for other mammography vendors to gain approval of such systems in the United States. As a result, we anticipate that competition in the digital mammography market will intensify as more companies and products enter this market.
Some of our activities may subject us to risks under federal and state laws prohibiting “kickbacks” and false or fraudulent claims.
We are subject to the provisions of a federal law commonly known as the Medicare/Medicaid anti-kickback law, and several similar state laws, which prohibit payments intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of healthcare products or services. While the federal law applies only to referrals, products or services for which payment may be made by a federal healthcare program, state laws often apply regardless of whether federal funds may be involved. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices by limiting the kinds of financial arrangements, including sales programs, that may be used with hospitals, physicians, laboratories and other potential purchasers of medical devices. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, or are for items or services that were not provided as claimed. Anti-kickback and false claims laws prescribe civil and criminal penalties (including fines) for noncompliance that can be substantial. Similarly, our international operations are subject to the provisions of the FCPA, which prohibits U.S. companies and their representatives from offering, promising, authorizing, or making payments to foreign officials for the purpose of influencing any act or decision of such official in his or her official capacity, inducing the official to do any act in violation of his or her lawful duty, or to secure any improper advantage in obtaining or retaining business. In many countries, the healthcare professionals we regularly interact with may meet the definition of a foreign official for purposes of the FCPA. While we continually strive to comply with these complex requirements, interpretations of the applicability of these laws to marketing practices is constantly evolving and even an unsuccessful challenge could cause adverse publicity and be costly to respond to, and thus could harm our business and prospects. Moreover, our failure to comply with domestic or foreign laws could result in various adverse consequences, including possible delay in approval or refusal to approve a product, recalls, seizures, withdrawal of an approved product from the market, and the imposition of civil or criminal sanctions.
New regulations related to “conflict minerals” may cause us to incur additional expenses and could limit the supply and increase the cost of certain metals used in manufacturing our products.
On August 22, 2012, the SEC adopted a new rule requiring disclosures of specified minerals, known as conflict minerals, that are necessary to the functionality or production of products manufactured or contracted to be manufactured by public companies. The new rule, which is effective for calendar 2013 and requires a disclosure report to be filed by May 31, 2014, will require companies to diligence, disclose and report whether or not such minerals originate from the Democratic Republic of Congo or an adjoining country. The new rule could affect sourcing at competitive prices and availability in sufficient quantities of certain minerals used in the manufacture of our products, including tantalum, tin, gold and tungsten. The number of suppliers who provide conflict-free minerals may be limited. In addition, there may be material costs associated with complying with the disclosure requirements, such as costs related to determining the source of certain minerals used in our products, as well as costs of possible changes to products, processes, or sources of supply as a consequence of such verification activities. Since our supply chain is complex, we may not be able to sufficiently verify the origins of the relevant minerals used in our products through the due diligence procedures that we implement, which may harm our reputation. In addition, we may encounter challenges to satisfy those customers who require that all of the components of our products be certified as conflict-free, which could place us at a competitive disadvantage if we are unable to do so.
32
Security breaches and other disruptions could compromise our information, expose us to liability and harm our reputation and business.
In the ordinary course of our business we collect and store sensitive data, including intellectual property, personal information, our proprietary business information and that of our customers, suppliers and business partners, and personally identifiable information of our customers and employees in our data centers and on our networks. The secure maintenance and transmission of this information is critical to our operations and business strategy. We rely on commercially available systems, software, tools and monitoring to provide security for processing, transmission and storage of confidential information. Computer hackers may attempt to penetrate our computer systems and, if successful, misappropriate personal or confidential business information. In addition, an associate, contractor, or other third-party with whom we do business may attempt to circumvent our security measures in order to obtain such information, and may purposefully or inadvertently cause a breach involving such information. Any such compromise of our data security and access, public disclosure, or loss of personal or confidential business information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, and regulatory penalties, disrupt our operations, damage our reputation and customers’ willingness to transact business with us, and subject us to additional costs and liabilities which could adversely affect our business.
We are subject to the risk of product liability claims relating to our products.
Our business involves the risk of product liability and other claims inherent to the medical device business. If even one of our products is found to have caused or contributed to injuries or deaths, we could be held liable for substantial damages. We maintain product liability insurance subject to deductibles and exclusions. There is a risk that the insurance coverage will not be sufficient to protect us from product and other liability claims, or that product liability insurance will not be available to us at a reasonable cost, if at all. An under-insured or uninsured claim could harm our business and prospects. In addition, claims could adversely affect the reputation of the related product, which could damage that product’s competitive position in the market.
The sale and use of our diagnostic products could also lead to the filing of product liability claims if someone were to allege that one of our products contained a design or manufacturing defect that resulted in the failure to detect a disorder for which it was being used to screen, inaccurate test results or caused injuries to a patient. Any product liability claim brought against us, with or without merit, could result in an increase in our product liability insurance rates or the inability to secure additional coverage in the future. Also, even a meritless or unsuccessful product liability claim could be time consuming and expensive to defend, which could result in a diversion of management’s attention from our business and could adversely affect the perceived safety and efficacy of our products, and could harm our business and prospects.
We use hazardous materials and products.
Our research and development and manufacturing processes involve the controlled use of hazardous materials, such as toxic and carcinogenic chemicals and various radioactive compounds. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by federal, state and local regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of this type of accident, we could be held liable for any resulting damages, and any such liability could be extensive. We are also subject to substantial regulation relating to occupational health and safety, environmental protection, hazardous substance control, and waste management and disposal. The failure to comply with such regulations could subject us to, among other things, fines and criminal liability.
We may incur losses in excess of our insurance coverage.
Our insurance coverage includes product liability, property, fire, terrorism and business interruption policies. Our insurance coverage contains policy limits, specifications and exclusions. We believe that our insurance coverage is consistent with general practices within our industry. Nonetheless, we may incur losses of a type for which we are not covered by insurance or which exceed the limits of liability of our insurance policies. In that event, we could experience a significant loss which could have a material adverse impact on our financial condition.
Our future success depends on the continued services of key personnel.
The loss of any of our key personnel, particularly key research and development personnel, could harm our business and prospects and could impede the achievement of our research and development, operational or strategic objectives. Our success also depends upon our ability to attract and retain other qualified managerial and technical personnel. Competition for such personnel, particularly software engineers and other technical personnel is intense. We may not be able to attract and retain personnel necessary for the development of our business.
33
Our failure to manage current or future alliances or joint ventures effectively may harm our business and prospects.
We have entered into alliances, joint ventures or other business relationships. Alliances with certain partners or companies could make it more difficult for us to enter into advantageous business transactions or relationships with others. Moreover, we may not be able to:
| | • | | identify appropriate candidates for alliances or joint ventures; |
| | • | | assure that any alliance or joint venture candidate will provide us with the support we anticipated; |
| | • | | successfully negotiate an alliance or joint venture on terms that are advantageous to us; or |
| | • | | successfully manage any alliance or joint venture. |
Furthermore, any alliance or joint venture may divert management time and resources. Entering into a disadvantageous alliance or joint venture, failing to manage an alliance or joint venture effectively, or failing to comply with the obligations associated with an alliance or joint venture, could harm our business and prospects.
An adverse change in the projected cash flows from our business units or the business climate in which they operate, including the continuation of the current financial and economic uncertainty, could require us to record an impairment charge, which could have an adverse impact on our operating results.
At least annually, we review the carrying value of our goodwill, and for other long-lived assets when indicators of impairment are present, to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment of the value of these assets. Conditions that could indicate impairment and necessitate an evaluation of these assets include, but are not limited to, a significant adverse change in the business climate or the legal or regulatory environment within which we operate. In addition, the deterioration of a company’s market capitalization significantly below its net book value is an indicator of impairment. We assess goodwill for impairment at the reporting unit level and in evaluating the potential impairment of goodwill, we make assumptions regarding the amount and timing of future cash flows, terminal value growth rates and appropriate discount rates. As a result of this assessment, we recorded significant impairment charges for goodwill and intangible assets in fiscal 2009 and 2010.
During the fourth quarter of fiscal 2012, we performed our annual impairment test of goodwill for our reporting units, and recorded a $5.8 million goodwill impairment charge for our MammoSite reporting unit. All other reporting units passed step 1 of the goodwill impairment test. Although we use reasonable methodologies for developing assumptions and estimates underlying the fair value calculations used in our impairment tests, these estimates are uncertain by nature and can vary from actual results. It is possible that the continuation of the current global financial and economic uncertainty could negatively affect our anticipated future cash flows, or the discount rates used to value the cash flows for each reporting unit, to such an extent that we could be required to perform an interim impairment test in fiscal 2013.
The acquisition of Gen-Probe is expected to have a dilutive effect on our earnings per share calculated in accordance with U.S. GAAP, which may adversely affect the market price of our common stock following the acquisition.
The acquisition of Gen-Probe is expected to have a dilutive effect on our earnings per share calculated in accordance with U.S. GAAP primarily due to the amortization of the intangible assets in connection with the acquisition. These expectations are based on preliminary estimates, which may materially change as a result of the completion of the allocation of the purchase price for the acquisition. We could also encounter additional transaction and integration-related costs or other factors such as the failure to realize all of the benefits anticipated in the acquisition. Any of these factors could cause further dilution to our earnings per share or cause a decrease in the price of our common stock.
Charges to earnings resulting from the application of the purchase method of accounting may adversely affect the market value of our common stock following the acquisition of Gen-Probe.
In accordance with U.S. GAAP, we have accounted for the acquisition using the purchase method of accounting, resulting in charges to our earnings that could adversely affect the market value of our common stock. Under the purchase method of accounting, we allocated the total purchase price to the assets acquired and liabilities assumed from Gen-Probe based on their estimated fair values as of the acquisition date, and recorded any excess of the purchase price over those fair values as goodwill. For certain tangible and intangible assets, recording their fair values as of the acquisition date results in incurring significant additional depreciation and/or amortization expense that exceeds the combined amounts recorded by us and Gen-Probe prior to the acquisition. This increased expense is recorded over the estimated useful lives of the underlying assets. In addition, to the extent the carrying value of goodwill or intangible assets post-acquisition were to become impaired, we may be required to incur charges relating to the impairment of those assets.
34
Our effective tax rate may fluctuate and we may incur obligations in tax jurisdictions in excess of amounts that have been accrued.
We are subject to income taxes and non-income based taxes in both the United States and various foreign jurisdictions. We take certain income tax return positions for which we provide additional taxes if it is more-likely-than-not that they will not withstand a tax authority’s challenge. We are subject to ongoing tax audits in various jurisdictions, and tax authorities may disagree with certain positions we have taken and assess additional taxes. We regularly evaluate the audits likely outcomes in order to determine the appropriateness of our tax provision and tax reserves. However, we cannot give assurance that we will accurately predict the audits’ outcomes, which could have a material impact on our operating results and financial condition.
Our effective tax rate may be lower or higher than prior years due to numerous factors, including a change in our geographic profitability mix and changes in tax laws. Any of these factors could cause us to experience an effective tax rate significantly different from previous periods or our current expectations, which could have a material impact on our business and operating results.
Changes in tax laws or tax rulings could materially impact our effective tax rate. U.S. law makers are considering several U.S. corporate tax reform proposals including those that may reduce or eliminate U.S. income tax deferral on unrepatriated foreign earnings and eliminate tax incentives such as the domestic manufacturing deduction in exchange for a lower U.S. statutory tax rate.
Risks Relating to our Indebtedness
We incurred significant indebtedness in order to finance the acquisition of Gen-Probe, which will limit our operating flexibility, and could adversely affect our operations and financial results and prevent us from fulfilling our obligations.
As of September 29, 2012, following the acquisition of Gen-Probe, we had approximately $5.2 billion aggregate principal of indebtedness. We also have other contractual obligations and deferred tax liabilities. This significant level of indebtedness and our other obligations may:
| | • | | make it more difficult for us to satisfy our obligations with respect to our outstanding indebtedness; |
| | • | | increase our vulnerability to general adverse economic and industry conditions, including increases in interest rates; |
| | • | | require us to dedicate a substantial portion of our cash flow from operations to interest and principal payments on our indebtedness, which will reduce the availability of our cash flow to fund working capital, capital expenditures, expansion efforts and other general corporate purposes; |
| | • | | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| | • | | place us at a competitive disadvantage compared to our competitors that have less debt; and |
| | • | | limit our ability to borrow additional funds for working capital, capital expenditures, general corporate purposes or acquisitions. |
In addition, the terms of our financing obligations require us to meet certain financial covenants that are customary with these types of credit facilities, which are described in Note 5 “Borrowings and Credit Arrangements” in the accompanying notes to the consolidated financial statements included in Item 15 of this Annual Report. If we are unable to comply with these covenants, we could default under the credit facilities, which could cause us to be unable to borrow additional amounts under the credit facilities and may result in the acceleration of the maturity of our outstanding indebtedness under the facilities. If the maturities were accelerated, we may not have sufficient funds available for repayment, and if we were unable to borrow further under the facilities, we may not be able to make investments in our business to support our strategy or we may end up in bankruptcy proceedings, or other processes, in which our business would be negatively impacted. In addition, our shareholders could be adversely impacted as shareholder value could decrease to a point of limited return. Each scenario would result in significant negative implications to our liquidity and results of operations.
In addition, the terms of our financing obligations contain covenants that restrict our ability, and that of our subsidiaries, to engage in certain transactions and may impair our ability to respond to changing business and economic conditions, including, among other things, limitations on our ability to:
| | • | | incur indebtedness or issue certain preferred equity; |
| | • | | pay dividends, redeem stock or make other distributions or restricted payments; |
| | • | | make certain investments; |
| | • | | agree to payment restrictions affecting the restricted subsidiaries; |
| | • | | sell or otherwise transfer or dispose of assets, including equity interests of our subsidiaries; |
| | • | | enter into transactions with our affiliates; |
35
| | • | | designate our subsidiaries as unrestricted subsidiaries; |
| | • | | consolidate, merge or sell substantially all of our assets; and |
| | • | | use the proceeds of permitted sales of our assets. |
Our new senior secured credit facilities also require us to satisfy certain financial covenants. Our ability to comply with these provisions may be affected by general economic conditions, political decisions, industry conditions and other events beyond our control. Our failure to comply with the covenants contained in the new credit facilities, including financial covenants, could result in an event of default, which could materially and adversely affect our results of operation and financial condition.
If there were an event of default under one of our debt instruments or a change of control, the holders of the debt could cause all amounts outstanding with respect to that debt to be due and payable immediately and may be cross-defaulted to other debt, including the Senior Notes. Our assets or cash flow may not be sufficient to fully repay borrowings under our outstanding debt instruments if accelerated upon an event of default or a change of control, and there is no guarantee that we would be able to repay, refinance or restructure the payments on such debt. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources.”
We may not be able to generate sufficient cash flow to service all of our indebtedness and other obligations.
Our ability to make payments on and to refinance our indebtedness and to fund planned capital expenditures, strategic transactions and expansion efforts will depend on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control.
Our business may not be able to generate sufficient cash flow from operations, and we can give no assurance that future borrowings will be available to us in amounts sufficient to enable us to pay our indebtedness as such indebtedness matures and to fund our other liquidity needs. If this occurs, we will need to refinance all or a portion of our indebtedness on or before maturity, and there can be no assurance that we will be able to refinance any of our indebtedness on commercially reasonable terms, or at all. We may need to adopt one or more alternatives, such as reducing or delaying planned expenses and capital expenditures, selling assets, restructuring debt, or obtaining additional equity or debt financing. These financing strategies may not be affected on satisfactory terms, if at all. Our ability to refinance our indebtedness or obtain additional financing, or to do so on commercially reasonable terms, will depend on, among other things, our financial condition at the time, restrictions in agreements governing our indebtedness, and other factors, including the condition of the financial markets and the markets in which we compete.
If we do not generate sufficient cash flow from operations, and additional borrowings, refinancings or proceeds from asset sales are not available to us, we may not have sufficient cash to enable us to meet all of our obligations.
A significant portion of our indebtedness is subject to floating interest rates, which may expose us to higher interest payments.
A significant portion of our indebtedness is subject to floating interest rates, which makes us more vulnerable in the event of adverse economic conditions, increases in prevailing interest rates, or a downturn in our business. As of September 29, 2012, approximately $2.5 billion aggregate principal of our indebtedness, which represents the outstanding principal under our tranche A term loan facility and our tranche B term loan facility, was subject to floating interest rates. We currently have no hedging arrangements in place to mitigate the impact of higher interest rates.
Risks Relating to our Common Stock
Future issuances of common stock and hedging activities may depress the trading price of our common stock and our convertible notes.
Any future issuance of equity securities, including the issuance of shares upon conversion of our convertible notes, could dilute the interests of our existing stockholders, including holders who have received shares upon conversion of our convertible notes, and could substantially decrease the trading price of our common stock and our convertible notes. We may issue equity securities in the future for a number of reasons, including to finance our operations and business strategy (including in connection with acquisitions, strategic collaborations or other transactions), to adjust our ratio of debt to equity, to satisfy our obligations upon the exercise of outstanding warrants or options or for other reasons.
In addition, the price of our common stock could also be affected by possible sales of our common stock by investors who view our convertible notes as a more attractive means of equity participation in our company and by hedging or arbitrage trading activity that we expect to develop involving our common stock. The hedging or arbitrage could, in turn, affect the trading price of our convertible notes, or any common stock that note holders receive upon conversion of their notes.
36
Future sales of our common stock in the public market or the issuance of securities senior to our common stock could adversely affect the trading price of our common stock and the value of our convertible notes and our ability to raise funds in new securities offerings.
Future sales of our common stock, the perception that such sales could occur or the availability of shares of our common stock or securities convertible into or exercisable for our common stock for future sale could adversely affect the market prices of our common stock and the value of our convertible notes prevailing from time to time and could impair our ability to raise capital through future offerings of equity or equity-related securities. In addition, we may issue common stock or equity securities senior to our common stock in the future for a number of reasons, including to finance our operations and business strategy, to adjust our ratio of debt to equity, to satisfy our obligations upon the exercise of options or for other reasons.
Provisions in our charter, bylaws, indebtedness and stockholder rights plan may have the effect of discouraging advantageous offers for our business or common stock and limit the price that investors might be willing to pay in the future for shares of our common stock.
Our charter, bylaws, and the provisions of the Delaware General Corporation Law include provisions that may have the effect of discouraging or preventing a change of control of us. Our indebtedness also contains provisions which either accelerate or require us to offer to repurchase the indebtedness at a premium upon a change of control. In addition, we have a stockholder rights plan that may have the effect of discouraging or preventing a change of control. These provisions could limit the price that our stockholders might receive in the future for shares of our common stock.
Our stock price is volatile.
The market price of our common stock has been, and may continue to be, highly volatile. We believe that a variety of factors could cause the price of our common stock to fluctuate, perhaps substantially, including:
| | • | | new, or changes in, recommendations, guidelines or studies that could affect the use of our products; |
| | • | | announcements and rumors of developments related to our business, including changes in reimbursement rates or regulatory requirements, proposed and completed acquisitions, or the industry in which we compete; |
| | • | | published studies and reports relating to the comparative efficacy of products and markets in which we participate; |
| | • | | quarterly fluctuations in our actual or anticipated operating results and order levels; |
| | • | | general conditions in the worldwide economy; |
| | • | | announcements of technological innovations; |
| | • | | new products or product enhancements by us or our competitors; |
| | • | | developments in patents or other intellectual property rights and litigation; |
| | • | | developments in relationships with our customers and suppliers; |
| | • | | the implementation of healthcare reform legislation and the adoption of additional reform legislation in the future, and; |
| | • | | the success or lack of success of integrating our acquisitions. |
The price of our common stock also may be adversely affected by the amount of common stock issuable upon conversion of our convertible notes. In addition, in recent years the stock market in general and the markets for shares of “high-tech” companies, have experienced extreme price fluctuations which have often been unrelated to the operating performance of affected companies. Any such fluctuations in the future could adversely affect the market price of our common stock, and the market price of our common stock may decline.
| Item 1B. | Unresolved Staff Comments |
None.
37
We own and lease the real property identified below. We believe that we have adequate space for our anticipated needs and that suitable additional space will be available at commercially reasonable prices as needed.
| | | | |
Principal Properties Owned: | | Primary Use (a) | | Floor Space |
| Newark, DE (b)(c) | | DirectRay digital detector research and development and plate manufacturing operations | | 164,000 sq. ft. |
| Warstein, Germany | | Hitec-Imaging’s manufacturing operations, research and development and administrative functions | | 201,000 sq. ft. |
| Londonderry, NH | | Manufacturing operations | | 2.7 acres of land and 47,000 sq. ft. |
| San Diego, CA (c) | | Diagnostics headquarters, including research and development, administrative and manufacturing operations | | 14.6 acres of land and 262,000 sq. ft. |
| San Diego, CA (c)(d) | | Diagnostics headquarters, including research and development, administrative and manufacturing operations | | 16 acres of land and 290,000 sq. ft. |
| San Diego, CA (c) | | Manufacturing operations for blood screening products | | 94,000 sq. ft. |
| | | | | | | | |
Principal Properties Leased: | | Primary Use (a) | | Floor Space | | Lease
Expiration
(fiscal year) | | Renewals |
| Bedford, MA | | Headquarters, including research and development, administrative and manufacturing operations | | 207,000 sq. ft. | | 2022 | | 4, five-yr. periods |
| Danbury, CT | | Manufacturing facility | | 62,000 sq. ft. | | 2022 | | 4, five-yr. periods |
| Marlborough, MA | | Administrative, research and development, manufacturing and distribution operations | | 216,000 sq. ft. | | 2019 | | 2, five-yr. periods |
| Marlborough, MA | | Manufacturing operations | | 146,000 sq. ft. | | 2019 | | 2, five-yr. periods |
| Danbury, CT | | Manufacturing operations and research and development | | 60,000 sq. ft. | | 2013 | | 2, five-yr. periods |
| Alajuela, Costa Rica | | Manufacturing facility | | 164,000 sq. ft. | | 2018 | | 2, five-yr. periods |
| Madison, WI | | Manufacturing operations and research and development | | 62,000 sq. ft. | | 2014 | | None |
| Manchester, England | | Manufacturing operations and research and development | | 66,000 sq. ft. | | 2035 | | None |
| Stamford, CT | | Manufacturing operations and research and development | | 37,000 sq. ft | | 2015 | | None |
| Waukesha, WI | | Manufacturing operations and research and development | | 60,000 sq. ft | | 2025 | | None |
| (a) | See the section entitled “Manufacturing” in Item 1 of this Annual Report for additional information regarding the products manufactured at the facilities listed. |
| (b) | We currently occupy approximately 59,000 square feet of this building, which houses our plate manufacturing facility, including both a Class 1 and a Class 2 clean room. We lease approximately 105,000 square feet of the facility to Siemens under a lease which expires in April 2015. |
| (c) | Subject to a mortgage to secure obligations under our senior secured credit facilities. |
| (d) | We currently occupy approximately 221,000 square feet of this building, with the remaining space available to accommodate future growth. |
We lease other facilities utilized for office space and manufacturing and distribution operations across the United States, Europe, Canada, China and Hong Kong. We also lease several sales and service offices throughout the world.
On May 22, 2009, Conceptus, Inc. filed suit in the United States District Court for the Northern District of California seeking a declaration by the Court that our planned importation, use, sale or offer to sell of our forthcoming Adiana Permanent Contraception System would infringe five Conceptus patents. On July 9, 2009, Conceptus filed an amended complaint alleging infringement of the same five patents by the Adiana system. The complaint sought preliminary and permanent injunctive relief and unspecified monetary damages. In addition to the amended complaint, Conceptus also filed a motion for preliminary injunction seeking to preliminarily enjoin sales of the Adiana System based on alleged infringement of certain claims of three of the five patents which was subsequently denied by the Court. A trial was held from October 3, 2011 through October 14, 2011 related to the asserted claims. On October 17, 2011, the jury returned a verdict in favor of Conceptus and awarded damages to Conceptus in the amount of $18.8 million. On April 29, 2012, we entered into a license and settlement agreement with Conceptus in which Conceptus agreed to forgo the $18.8 million jury award in consideration of our agreeing to a permanent injunction against the manufacture, sale and distribution of the Adiana product. We also granted Conceptus a royalty bearing license to our intellectual property related to the Adiana product.
On June 9, 2010, Smith & Nephew, Inc. filed suit against Interlace, which we acquired on January 6, 2011, in the United States District Court for the District of Massachusetts. In the complaint, it is alleged that the Interlace MyoSure hysteroscopic tissue removal
38
device infringes U.S. patent 7,226,459. The complaint seeks permanent injunctive relief and unspecified damages. A Markman hearing on claim construction was held on November 9, 2010, and a ruling was issued on April 21, 2011. On November 22, 2011, Smith & Nephew, Inc. filed suit against us in the United States District Court for the District of Massachusetts. In the complaint, it is alleged that use of the MyoSure hysteroscopic tissue removal system infringes U.S. patent 8,061,359. The complaint seeks preliminary and permanent injunctive relief and unspecified damages. On January 17, 2012, at a hearing on Smith & Nephew’s motion for preliminary injunction with respect to the suit filed on November 22, 2011, the judge did not issue an injunction, consolidated the two matters for a single trial and scheduled a trial on the merits for both claims for June 25, 2012. A case management conference held on February 14, 2012 resulted in the trial being rescheduled to begin on August 20, 2012. On March 15, 2012, the Court heard summary judgment arguments related to the ‘459 patent and claim construction arguments related to the ‘359 patent. On June 5, 2012, the Court denied Smith & Nephew’s request for summary judgment of infringement, denied Smith & Nephew’s request for preliminary injunction, and denied our requests for summary judgment of non-infringement and invalidity. On September 4, 2012, following a two week trial, a jury returned a verdict of infringement of both the ‘459 and ‘359 patents and assessed damages of $4 million. The court has not yet entered judgment adopting the jury’s finding. Based in part on the fact the United States Patent and Trademark Office, or USPTO, had taken up a re-examination of both the ‘359 and ‘459 patents rejecting all previously issued claims, including all claims asserted against the MyoSure product, we have filed post trial motions seeking to reverse the jury’s rulings. A bench trial regarding our assertion of inequitable conduct on the part of Smith & Nephew with regard to the ‘359 originally scheduled for October 29, 2012 was postponed. A new date has not yet been set. The Court has indicated that until ruling on the inequitable conduct issue, it will not consider either party’s post trial motions. The purchase and sale agreement associated with the acquisition of Interlace includes an indemnification provision that provides for the reimbursement of a portion of legal expenses in defense of the Interlace intellectual property. We have the right to collect certain amounts set aside in escrow and, as applicable, offset contingent consideration payments of qualifying legal costs. We are recording legal fees incurred for this suit pursuant to the indemnification provision net within accrued expenses.
On February 10, 2012, C.R. Bard (as acquirer of SenoRx, Inc. “SenoRx”) filed suit against us in the United States District Court for the District of Delaware. In the complaint, it is alleged that our MammoSite product infringes SenoRx’s U.S. Patents 8,079,946 and 8,075,469. The complaint seeks permanent injunctive relief and unspecified damages. On September 4, 2012 and October 16, 2012 the USPTO took up a re-examination of the ‘946 and ‘469 patents respectively. With respect to the ‘469 patent, all previously issued claims were rejected and with respect to the ‘846 patent all but four claims were rejected. Based on the actions of the USPTO, we have filed a motion seeking to stay all litigation proceedings pending the outcome of the USPTO’s re-examination of both patents in suit.
On March 6, 2012, Enzo Life Sciences, Inc. (“Enzo”) filed suit against us in the United States District Court for the District of Delaware. In the complaint, it is alleged that certain of our molecular diagnostics products, including without limitation products based on our proprietary Invader chemistry such as Cervista HPV high risk and Cervista HPV 16/18, infringe Enzo’s U.S. Patent 6,992,180. The complaint seeks permanent injunctive relief and unspecified damages. We were formally served with the complaint on July 3, 2012, but no hearing has been scheduled. In January 2012, Enzo filed suit against Gen-Probe in the United States District Court for the District of Delaware. In that complaint, it is alleged that certain of Gen-Probe’s diagnostics products, including products that incorporate Gen-Probe’s patented HPA technology such as the APTIMA Combo 2 and APTIMA HPV assays, infringe Enzo’s U.S. Patent 6,992,180. The complaint seeks permanent injunctive relief and unspecified damages.
In October 2009, Gen-Probe filed a patent infringement action against Becton Dickinson (“BD��) in the United States District Court for the Southern District of California. The complaint alleges that BD’s Viper™ XTR™ testing system infringes five of Gen-Probe’s U.S. patents covering automated processes for preparing, amplifying and detecting nucleic acid targets. The complaint also alleges that BD’s ProbeTec™ Female Endocervical and Male Urethral Specimen Collection Kits for Amplified Chlamydia trachomatis/Neisseria gonorrhea (CT/GC) DNA assays used with the Viper XTR testing system infringe two of Gen-Probe’s U.S. patents covering penetrable caps for specimen collection tubes. The complaint seeks monetary damages and injunctive relief. In March 2010, Gen-Probe filed a second complaint for patent infringement against BD in the United States District Court for the Southern District of California alleging that BD’s BD MAX System™ (formerly known as the HandyLab Jaguar system) infringes four of Gen-Probe’s U.S. patents covering automated processes for preparing, amplifying and detecting nucleic acid targets. The second complaint also seeks monetary damages and injunctive relief. In June 2010, these two actions were consolidated into a single legal proceeding. On September 28, 2012, the Court issued rulings on each party’s motions for summary judgment. As part of those rulings the Court found that BD’s accused products infringe nineteen claims of four of the asserted patents. The remaining issues, including BD’s invalidity contentions are scheduled to be heard in a jury trial beginning on December 4, 2012.
A number of lawsuits have been filed against us, Gen-Probe, and Gen-Probe’s board of directors. These include: (1) Teamsters Local Union No. 727 Pension Fund v. Gen-Probe Incorporated, et al. (Superior Court of the State of California for the County of San Diego); (2) Timothy Coyne v. Gen-Probe Incorporated, et al. (Delaware Court of Chancery); and (3) Douglas R. Klein v. John W. Brown, et al. (Delaware Chancery Court). The two Delaware actions have been consolidated into a single action titled: In re: Gen-Probe Shareholders Litigation. The suits were filed after the announcement of our acquisition of Gen-Probe on April 30, 2012 as putative stockholder class actions. Each of the actions assert similar claims alleging that Gen-Probe’s board of directors failed to adequately discharge its fiduciary duties to shareholders by failing to adequately value Gen-Probe’s shares and ensure that Gen-Probe’s
39
shareholders received adequate consideration in our acquisition of Gen-Probe, that the acquisition was the product of a flawed sales process, and that we aided and abetted the alleged breach of fiduciary duty. The plaintiffs demand, among other things, a preliminary and permanent injunction enjoining our acquisition of Gen-Probe and rescinding the transaction or any part thereof that has been implemented. On May 24, 2012, the plaintiffs in the Delaware action filed an amended complaint, adding allegations that the disclosures in Gen-Probe’s preliminary proxy statement were inadequate. The defendants in the Delaware action answered the complaint on June 4, 2012. On July 18, 2012, the parties in the Delaware action entered into a memorandum of understanding regarding a proposed settlement of the litigation. The proposed settlement is conditioned upon, among other things, the execution of an appropriate stipulation of settlement, consummation of the merger, and final approval of the proposed settlement by the Delaware Court of Chancery. On July 9, 2012, the plaintiffs in the California action filed a motion for voluntary dismissal without prejudice. On July 12, 2012, the California Superior Court entered an order dismissing the California complaint without prejudice.
| Item 4. | Mine Safety Disclosures |
Not Applicable.
40
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information. Our common stock is traded on the Nasdaq Global Select Market under the symbol “HOLX.” The following table sets forth the high and low sales prices per share of our common stock, as reported by the Nasdaq Global Select Market.
| | | | | | | | |
Fiscal Year Ended September 29, 2012 | | High | | | Low | |
First Quarter | | $ | 17.84 | | | $ | 14.23 | |
Second Quarter | | | 21.60 | | | | 17.24 | |
Third Quarter | | | 21.93 | | | | 16.32 | |
Fourth Quarter | | | 21.05 | | | | 17.39 | |
| | |
Fiscal Year Ended September 24, 2011 | | High | | | Low | |
First Quarter | | $ | 19.00 | | | $ | 15.80 | |
Second Quarter | | | 21.95 | | | | 18.76 | |
Third Quarter | | | 22.69 | | | | 19.63 | |
Fourth Quarter | | | 20.82 | | | | 15.15 | |
Number of Holders. As of November 20, 2012, there were approximately 1,437 holders of record of our common stock, including multiple beneficial holders at depositaries, banks and brokers listed as a single holder in the street name of each respective depositary, bank or broker.
Dividend Policy. We have never declared or paid cash dividends on our capital stock, and we have no plans to do so. Our current policy is to retain all of our earnings to finance future growth.
Recent Sales of Unregistered Securities. We did not sell unregistered equity securities during the fourth quarter of fiscal 2012.
Issuer’s Purchases of Equity Securities. For the majority of restricted stock units granted, the number of shares issued on the date that the restricted stock units vest is net of the minimum statutory tax withholding requirements that we pay in cash to the appropriate taxing authorities on behalf of our employees. The following table sets forth information about repurchases of our common stock to cover employee income tax withholding obligations in connection with the vesting of restricted stock units under our equity incentive plans for the three months ended September 29, 2012 (shares in thousands):
| | | | | | | | | | | | |
Period of Repurchase | | Total Number of
Shares Purchased | | | Average Price
Paid Per Share | | | Total Number of
Shares
Purchased As
Part of Publicly
Announced
Plans or
Program | |
June 24, 2012—July 21, 2012 | | | — | | | $ | — | | | | — | |
July 22, 2012—August 25, 2012 | | | 149 | | | | 19.75 | | | | — | |
August 26, 2012—September 29, 2012 | | | 31 | | | | 19.63 | | | | — | |
| | | | | | | | | | | | |
Total | | | 180 | | | $ | 19.73 | | | | — | |
| | | | | | | | | | | | |
41
Stock Performance Graph
The following graph compares cumulative total shareholder return on our common stock since September 29, 2007 with the cumulative total return of the Russell 1000 Index and the Standard & Poor’s Health Care Supplies Index. This graph assumes the investment of $100 on September 29, 2007 in our common stock, the Russell 1000 Index and the S&P Health Care Supplies Index. Measurement points are the last trading day of each respective fiscal year.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Hologic, Inc., the Russell 1000 Index
and the S&P Health Care Supplies Index
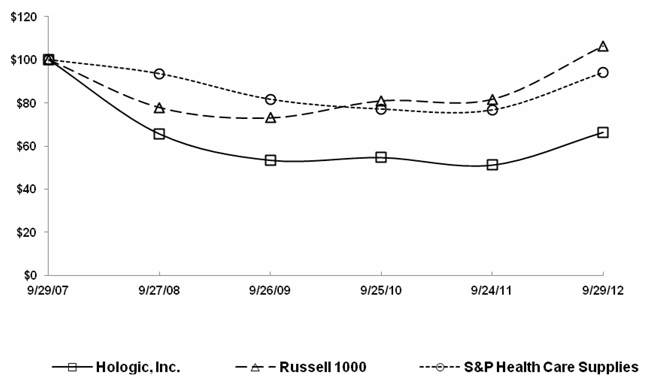
| * | $100 invested on 9/29/07 in stock or index, including reinvestment of dividends. |
Fiscal year ended September 29.
42
| Item 6. | Selected Financial Data |
The following selected financial data should be read in conjunction with our consolidated financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K, beginning on page F-1. In the fourth quarter of fiscal 2012, we acquired Gen-Probe. In the second, third and fourth quarters of fiscal 2011, we acquired Interlace, TCT and Healthcome, respectively. In the fourth quarter of fiscal 2010, we acquired Sentinelle Medical. In the first and fourth quarters of fiscal 2008, we acquired Cytyc Corporation and Third Wave Technologies, Inc., respectively. Results of operations for each of these businesses are included in our consolidated financial statements from the date of acquisition.
| | | | | | | | | | | | | | | | | | | | |
| | | Fiscal Years Ended | |
| | | September 29,
2012 (6) | | | September 24,
2011 (5) | | | September 25,
2010 (3) | | | September 26,
2009 (2) | | | September 27,
2008 (1) | |
| | | (In thousands, except per share data) | |
Consolidated Statement of Operations Data | | | | | | | | | | | | | | | | | | | | |
Total revenues | | $ | 2,002,652 | | | $ | 1,789,349 | | | $ | 1,679,552 | | | $ | 1,637,134 | | | $ | 1,674,499 | |
Total costs and expenses | | $ | 1,888,935 | | | $ | 1,414,904 | | | $ | 1,609,615 | | | $ | 3,653,808 | | | $ | 1,872,041 | |
Net (loss) income | | $ | (73,634 | ) | | $ | 157,150 | | | $ | (62,813 | ) | | $ | (2,216,642 | ) | | $ | (415,588 | ) |
Basic net (loss) income per common share | | $ | (0.28 | ) | | $ | 0.60 | | | $ | (0.24 | ) | | $ | (8.64 | ) | | $ | (1.69 | ) |
Diluted net (loss) income per common share | | $ | (0.28 | ) | | $ | 0.59 | | | $ | (0.24 | ) | | $ | (8.64 | ) | | $ | (1.69 | ) |
| | | | | |
Consolidated Balance Sheet Data | | | | | | | | | | | | | | | | | | | | |
Working capital | | $ | 901,665 | | | $ | 833,450 | | | $ | 656,969 | | | $ | 489,335 | | | $ | 352,703 | |
Total assets | | $ | 10,477,108 | | | $ | 6,008,780 | | | $ | 5,625,834 | | | $ | 5,684,226 | | | $ | 8,126,812 | |
Long-term debt obligations, less current portion (4) | | $ | 4,986,345 | | | $ | 1,506,448 | | | $ | 1,467,519 | | | $ | 1,536,887 | | | $ | 1,769,005 | |
Total stockholders’ equity | | $ | 2,961,031 | | | $ | 2,936,895 | | | $ | 2,698,549 | | | $ | 2,725,977 | | | $ | 4,895,936 | |
| (1) | Included in total costs and expenses in fiscal 2008 were charges of $370.0 million and $195.2 million for in-process research and development from the Cytyc and Third Wave acquisitions, respectively. |
| (2) | Included in total costs and expenses in fiscal 2009 was an aggregate goodwill impairment charge of $2.34 billion comprised of $1.17 billion for GYN Surgical, $908.3 million for Diagnostics and $265.9 million for Breast Health. |
| (3) | Included in total costs and expenses in fiscal 2010 were impairment charges of $143.5 million for intangible assets and $76.7 million for goodwill, both of which related to our MammoSite reporting unit within our Breast Health reportable segment. Also included in total costs and expenses was $11.4 million of net charges for litigation-related settlements. |
| (4) | Long-term obligations are net of unamortized debt discounts of $188.8 million, $236.4 million, $277.9 million and $351.1 million for fiscal years 2012, 2011, 2010 and 2009, respectively. |
| (5) | Included in total costs and expenses in fiscal 2011 was a net gain on the sale of intellectual property of $84.5 million, and included in net income in fiscal 2011 was a debt extinguishment loss of $29.9 million. |
| (6) | Included in total costs and expenses in fiscal 2012 were aggregate charges for contingent consideration of $119.5 million related to certain of our acquisitions, aggregate restructuring and divestiture charges of $36.6 million and acquisition transaction costs related to the Gen-Probe acquisition of $34.3 million. Included in net loss was a debt extinguishment loss of $42.3 million. |
43
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion and analysis should be read in conjunction with the Consolidated Financial Statements and the information described under the caption “Risk Factors” in Part I, Item 1A of this report.
OVERVIEW
We are a leading developer, manufacturer and supplier of premium diagnostics products, medical imaging systems and surgical products dedicated to serving the healthcare needs of women. Our core business units are focused on breast health, diagnostics, GYN surgical, and skeletal health. On August 1, 2012, we completed our acquisition of Gen-Probe. Gen-Probe is a leader in molecular diagnostics products and services that are used primarily to diagnose human diseases, screen donated human blood, and test transplant compatibility. Gen-Probe is part of our Diagnostics business segment.
Our breast health products include a broad portfolio of breast imaging and related products and accessories, including digital and film-based mammography systems, MRI, breast coils, CAD, for mammography and MRI, minimally invasive breast biopsy devices, breast biopsy site markers, breast biopsy guidance systems, breast imaging comfort pads, and breast brachytherapy products. Our most advanced breast imaging platform, Dimensions, utilizes a new technology called tomosynthesis to produce 3D images, as well as conventional 2D full field digital mammography images. In the U.S., our Dimensions product was approved in December 2008 by the FDA for providing conventional 2D images. In February 2011, we received approval from the FDA to enable the 3D tomosynthesis capability of our Dimensions system. In November 2011, we announced the commercial release of our C-View synthesized 2D image reconstruction algorithm that eliminates the need for a conventional 2D mammogram as a component of a 3D mammography exam. C-View software is approved for sale throughout the European Economic Area and in other countries recognizing the CE Mark. During the third quarter of fiscal 2012, we submitted a pre-market approval application to the FDA for this capability.
We offer a wide range of diagnostic products which are used primarily to aid in the diagnosis of human diseases and screen donated human blood. Our molecular diagnostics products include our APTIMA family of assays our Proprietary Invader Chemistry and advanced instrumentation (PANTHER, TIGRIS and HTA). The APTIMA family of assays is used to detect the common STDs chlamydia and gonorrhea, certain high-risk strains of HPV, and Trichomonas vaginalis, the parasite that causes trichomoniasis. Our Invader chemistry comprises molecular diagnostic reagents used for a variety of DNA and RNA analysis applications, including Cervista HPV HR, and Cervista HPV 16/18 products to assist in the diagnosis of HPV, as well as other products to diagnose cystic fibrosis, cardiovascular risk and other diseases. In December 2011, we announced the FDA approved our Cervista HTA system for use with our Cervista HPV HR test. The Cervista HTA system automates the DNA extraction and detection steps of the Cervista HPV HR test. This product was launched in January 2012. Our diagnostics products also include the ThinPrep System, which is primarily used in cytology applications such as cervical cancer screening, and the Rapid Fetal Fibronectin Test, which assists physicians in assessing the risk of pre-term birth. In blood screening, we develop and manufacture the PROCLEIX family of assays, which are used to detect HIV, HCV, HBV, and WNV in donated human blood. These blood screening products are marketed worldwide by our blood screening collaborator, Novartis, under Novartis’ trademarks.
Our GYN surgical products include our NovaSure system, and MyoSure system. The NovaSure system involves a minimally invasive procedure for the treatment of heavy menstrual bleeding. The MyoSure system is a tissue removal device that is designed to provide incision-less removal of fibroids and polyps within the uterus. At the end of the second quarter of fiscal 2012, we decided to cease manufacturing, marketing and selling our Adiana system determining that the product was not financially viable and would not become so in the foreseeable future.
Our skeletal health products include dual-energy X-ray bone densitometry systems, an ultrasound-based osteoporosis assessment product, and our Fluoroscan mini C-arm imaging products.
Unless the context otherwise requires, references to we, us, Hologic or our company refer to Hologic, Inc. and each of its consolidated subsidiaries.
RECENT DEVELOPMENTS
On August 1, 2012, we completed our acquisition of Gen-Probe. Such acquisition, and the significant indebtedness we incurred to fund that acquisition, subject us to risks and uncertainties are described herein, including those set forth under the caption “Risk Factors” in Part I, Item 1A of this report.
Market acceptance of our medical products in the United States and other countries is dependent upon the purchasing and procurement practices of our customers, patient demand for our products and procedures and the reimbursement of patients’ medical expenses by government healthcare programs, private insurers or other healthcare payors. In the United States, the Centers for Medicare & Medicaid Services, known as CMS, establish coverage and reimbursement policies for healthcare providers treating Medicare and Medicaid beneficiaries. Under current CMS policies, varying reimbursement levels have been established for our
44
products and treatments. Coverage and reimbursement policies and rates applicable to patients with private insurance are dependent upon individual private payor decisions which may not follow the policies and rates established by CMS. The use of our products and treatments outside the United States is similarly affected by coverage and reimbursement policies adopted by foreign governments and private insurance carriers. CMS has not adopted a reimbursement rate for the use of 3D tomosynthesis, as tomosynthesis was only recently approved by the FDA in February 2011 in connection with our pre-market approval, or PMA, application for our Dimensions system. We are working with governmental authorities, healthcare providers, insurance companies and other third-party payors in our efforts to secure reimbursement for the use of 3D tomosynthesis. However, we cannot assure that these efforts will be successful. Failure to obtain, or delays in obtaining, adequate reimbursement for the use of 3D tomosynthesis would adversely affect sales of our Dimensions 3D systems.
The continuing uncertainty surrounding worldwide financial markets and macroeconomic conditions has caused and may continue to cause the purchasers of medical equipment to decrease or delay their medical equipment purchasing and procurement activities. Additionally, volatility in world credit markets has caused and continues to cause our customers to experience difficulty securing the financing necessary to purchase our products. Economic uncertainty and unemployment have and may continue to result in cost-conscious consumers focusing on acute care rather than wellness, which has and may continue to adversely affect demand for our products and procedures. Furthermore, governments and other third-party payors around the world facing tightening budgets could move to further reduce the reimbursement rates or the scope of coverage offered, which could adversely affect sales of our products. If the current adverse macroeconomic conditions continue, our business and prospects may be negatively impacted.
In March 2010, significant reforms to the healthcare system were adopted as law in the United States. The law includes provisions that, among other things, reduce and/or limit Medicare reimbursement, require all individuals to have health insurance (with limited exceptions) and imposes new and/or increased taxes. Specifically, the law requires the medical device industry to subsidize healthcare reform in the form of a 2.3% excise tax on United States sales of certain medical devices effective January 1, 2013. We expect that all of our products will fall under the government classification requiring the excise tax. Product sales in the United States represented 73% and 76% of our worldwide net product sales for the years ended September 29, 2012 and September 24, 2011, respectively.
We operate in a highly regulated industry and other governmental actions may adversely affect our business, operations or financial condition, including, without limitation: new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to healthcare availability, methods of delivery and payment for health care products and services; changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity; changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products, or otherwise adversely affect the market for our products and treatments; new laws, regulations and judicial decisions affecting pricing or marketing practices; and changes in the tax laws relating to our operations, including those associated with the recently adopted healthcare reform law discussed above.
Professional societies, government agencies, practice management groups, private health/science foundations, and organizations involved in healthcare issues may publish guidelines, recommendations or studies to the healthcare and patient communities from time to time. Recommendations of government agencies or these other groups/organizations may relate to such matters as usage, cost-effectiveness, and use of related preventative services and treatments/therapies. Recommendations, guidelines or studies that are followed by patients and healthcare providers could result in decreased use of our products. For example, in November 2012, the American Congress of Obstetrics and Gynecologists, known as the ACOG, released updates in which they have recommended less frequent cervical cancer screening similar to guidelines released by ACOG in November 2009 and guidelines released in March 2012 by the U.S. Preventative Services Task Force, known as the USPSTF, and the American Cancer Society. However, the USPSTF recommendations now also include HPV co-testing for certain patient populations, an update from their draft guidelines in October 2011.
Over the last few years, there have been periodic significant fluctuations in foreign currencies relative to the U.S. dollar. The ongoing fluctuations of the value of the U.S. dollar, including the recent strengthening of the U.S. dollar against the Euro, may cause our products to be less competitive in international markets and may impact sales and profitability over time. Historically, a majority of our capital equipment sales to international dealers were denominated in U.S. dollars. However, more sales are now denominated in the Euro compared to the U.S. dollar for our Euro zone dealers. In addition, we have international sales, principally in our Diagnostics segment, that are denominated in foreign currencies. The value of these sales is also impacted by fluctuations in the value of the U.S. dollar. Given the uncertainty in the worldwide financial markets, foreign currency fluctuations may be significant in the future, and if the U.S. dollar continues to strengthen, we may experience a material adverse effect on our international revenues and operating results.
45
ACQUISITIONS
Fiscal 2012 Acquisitions:
Gen-Probe Incorporated
On August 1, 2012, we completed the acquisition of Gen-Probe and acquired all of the outstanding shares of Gen-Probe. Pursuant to the merger agreement, each share of common stock outstanding immediately prior to the effective time of the acquisition was cancelled and converted into the right to receive $82.75 in cash. In addition, all outstanding restricted shares, restricted stock units, performance shares, and those stock options granted prior to February 8, 2012 were cancelled and converted into the right to receive $82.75 per share in cash less the applicable exercise price, as applicable. Stock options granted after February 8, 2012 were cancelled and converted into stock options to acquire shares of Hologic common stock determined by a conversion formula defined in the merger agreement. The total purchase price was $3.97 billion, which was funded through available cash and financing consisting of senior secured credit facilities and senior notes resulting in aggregate proceeds of $3.48 billion, net of discounts.
Gen-Probe, headquartered in San Diego, California, is a leader in molecular diagnostics products and services that are used primarily to diagnose human diseases, screen donated human blood, and test transplant compatibility. We expect this acquisition to enhance our molecular diagnostics franchise and to complement our existing portfolio of diagnostics products. Gen-Probe’s results of operations are reported within our Diagnostics reporting segment from the date of acquisition.
The preliminary allocation of the purchase price is based on estimates of the fair value of assets acquired and liabilities assumed as of August 1, 2012. We are continuing to obtain information to complete our valuation of intangible assets, as well as to determine the acquired assets and liabilities, including tax assets and liabilities. The purchase price has been allocated to the acquired assets and assumed liabilities based on management’s estimate of their fair values. Certain of Gen-Probe’s assets have been designated as assets held-for-sale and recorded at fair value less the estimated cost to sell such assets. These represent non-core assets to the Company’s business plan and are expected to be sold within one year of the acquisition.
As part of the purchase price allocation, the Company has determined the identifiable intangible assets are developed technology of $1.57 billion, in-process research and development of $227.0 million, customer contracts of $585.0 million, and trade names of $97.0 million.The fair value of the intangible assets has been estimated using the income approach, specifically the excess earning method and relief from royalty method, and the cash flow projections were discounted using rates ranging from 10% to 12%. The cash flows are based on estimates used to price the transaction, and the discount rates applied were benchmarked with reference to the implied rate of return from the transaction model and the weighted average cost of capital.
The developed technology assets comprise know-how, patents and technologies embedded in Gen-Probe’s products and relate to currently marketed products and related instrument automation. In valuing the developed technology assets consideration was only given to products that have received regulatory approval. In-process research and development projects relate to in-process projects that have not reached technological feasibility as of the acquisition date and have no alternative future use. The primary basis for determining technological feasibility of these projects is obtaining regulatory approval to market the underlying product, which primarily pertains to receiving approval to perform certain diagnostic testing on Gen-Probe’s instrumentation, such as the PANTHER and TIGRIS systems. The Company recorded $227.0 million of in-process research and development projects related to six projects. One project, valued at $7.0 million, received FDA approval in October 2012. The other projects are expected to be completed within the next 3 months to 45 months with a total estimated cost of approximately $54.2 million to complete such projects. Given the uncertainties inherent with product development and introduction, we cannot assure that any of our product development efforts will be successful, completed on a timely basis or within budget, if at all. All of the in-process research and development assets were valued using the multiple-period excess earnings method approach using a discount rate of 12.0%.
The excess of the purchase price over the estimated fair value of the tangible net assets and intangible assets acquired of $1.65 billion was recorded to goodwill. The factors contributing to the recognition of the amount of goodwill are based on several strategic and synergistic benefits that are expected to be realized from the Gen-Probe acquisition. These benefits include the expectation that the combined company’s complementary products in the molecular diagnostics market with Gen-Probe’s fully automated product franchise will significantly broaden the Company’s offering in women’s health and diagnostics. The combined company should benefit from a broader global presence and with Hologic’s direct sales force and marketing in Europe and its investment in China distribution, the growth prospects of Gen-Probe’s products are expected to be enhanced significantly. The combined company anticipates significant cross-selling opportunities within the diagnostics market through Hologic’s larger channel coverage and physician sales team. None of the goodwill is expected to be deductible for income tax purposes.
46
Gen-Probe’s revenue and pre-tax loss from continuing operations for the period from the acquisition date to September 29, 2012 were $89.5 million and $47.7 million, respectively.
Fiscal 2011 Acquisitions:
TCT International Co., Ltd.
On June 1, 2011, we acquired TCT International Co., Ltd. (“TCT”), a privately-held distributor of medical products, including our ThinPrep Pap Test, related instruments and other diagnostic and surgical products. TCT’s operating subsidiaries are located in Beijing, China. Our acquisition of TCT enabled us to obtain an established nationwide sales organization and customer support infrastructure in China as we execute on our strategy to expand internationally. The purchase price was $148.4 million. In addition, the majority of the former shareholders of TCT may receive two annual contingent earn-out payments (subject to adjustment) not to exceed $200.0 million less a deferred payment of $35.0 million, which was made in the fourth quarter of fiscal 2012. Subsequent to the acquisition date, our results of operations include the results of TCT, which are primarily reported within our Diagnostics reporting segment and to a lesser extent within our GYN Surgical reporting segment.
The allocation of the purchase price was based upon estimates of the fair value of assets acquired and liabilities assumed as of June 1, 2011. The purchase price in excess of net tangible assets acquired was allocated to identifiable intangible assets comprised of customer relationships of $45.8 million, business licenses of $2.5 million and trade names of $2.1 million, based upon a detailed valuation that relies on projections and assumptions. The excess of the purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed of $75.2 million was allocated to goodwill.
The contingent earn-out payments are based on a multiple of incremental revenue growth for the one year periods beginning January 1, 2011 and January 1, 2012 as compared to the respective prior year periods, and are payable after the first and second anniversaries from the date of acquisition, respectively. Since these payments are contingent on future employment, they are being recognized as compensation expense ratably over the required service periods, the first and second year anniversaries from the date of acquisition. Based on actual and projected revenues for the TCT business, we recorded compensation expense of $75.5 million and $17.6 million in fiscal 2012 and 2011, respectively. The first earn-out period was completed in the third quarter of fiscal 2012 and we paid $54 million in the fourth quarter of fiscal 2012. As of September 29, 2012, we had accrued $39.1 million for the second contingent earn-out payment.
Interlace Medical, Inc.
On January 6, 2011, we acquired Interlace Medical, Inc. (“Interlace”), a privately-held company located in Framingham, Massachusetts. Interlace is the developer, manufacturer and supplier of MyoSure. The purchase price was comprised of $126.8 million in cash, which was net of certain adjustments, plus two annual contingent payments up to a maximum of an additional $225.0 million in cash. Subsequent to the acquisition date, our results of operations include the results of Interlace, which has been integrated within our GYN Surgical reporting segment.
The allocation of the purchase price was based upon estimates of the fair value of assets acquired and liabilities assumed as of January 6, 2011. The purchase price in excess of net tangible assets acquired was allocated to identifiable intangible assets comprised of developed technology of $158.7 million and trade names of $1.8 million, based upon a detailed valuation that relies on projections and assumptions. The excess of the purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed of $88.1 million was allocated to goodwill.
The agreement includes an indemnification provision that provides for the reimbursement of a portion of legal expenses in defense of the Interlace intellectual property. We have the right to collect certain amounts set aside in escrow from the initial consideration and, as applicable, offset contingent consideration payments of qualifying legal costs.
The contingent payments are based on a multiple of incremental revenue growth during a two-year period following the completion of the acquisition. Pursuant to ASC 805,Business Combinations, we recorded this liability at its estimated fair value based on future revenue projections of the Interlace business under various potential scenarios and weighted probability assumptions of these outcomes. As of the date of acquisition, these cash flow projections were discounted using a rate of 15.6%, based on the weighted-average cost of capital of the acquired business plus a credit risk premium for non-performance risk related to the liability pursuant to ASC 820. This analysis resulted in an initial contingent consideration liability of $86.6 million, which is being adjusted periodically as a component of operating expenses based on changes in the fair value of the liability due to the accretion of the liability for the time value of money and changes in the assumptions pertaining to the achievement of the defined revenue growth milestones. This fair
47
value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined in ASC 820. During the second quarter of fiscal 2012, the first measurement period lapsed resulting in a total contingent consideration amount recorded for this period of $51.8 million, which was disbursed to the former shareholders of Interlace net of amounts withheld for certain legal indemnification purposes. We recorded charges of $41.8 million and $6.3 million in fiscal 2012 and 2011, respectively, for changes in the fair value of the contingent consideration liability. As of September 29, 2012, we had accrued $83.0 million for the second measurement period contingent payment.
Beijing Healthcome Technology Company, Ltd.
On July 19, 2011, we completed our acquisition of Beijing Healthcome Technology Company, Ltd. (“Healthcome”), a privately-held manufacturer of medical equipment, including mammography equipment, located in Beijing, China. Healthcome develops and manufactures analog mammography products targeted to lower tier hospital segments in China. Subsequent to the acquisition, we worked to integrate our selenium detector technology into the Healthcome mammography system, and on December 21, 2011, we received SFDA approval in China for our Serenity digital mammography system. We began selling this product in China in the second quarter of fiscal 2012, and intend to commercialize it throughout Asia and potentially other emerging markets in the future.
The purchase price was $8.8 million in cash. In addition, we were obligated to make future payments to the shareholders, who remain employed, up to an additional $7.1 million over three years. Since these payments were contingent on future employment, they were being recognized as compensation expense ratably over the respective service periods. In the fourth quarter of fiscal 2012, we and the former shareholders agreed that the former shareholders would terminate their employment. We agreed to pay the majority of the contingent consideration in accordance with the original payment terms. As a result, we accelerated the unearned compensation in the fourth quarter of fiscal 2012 and recorded compensation expense of $5.6 million in fiscal 2012 compared to $0.3 million in fiscal 2011.
Fiscal 2010 Acquisitions:
Sentinelle Medical Inc.
On August 5, 2010, we acquired Sentinelle Medical Inc. (“Sentinelle Medical”), a privately held company located in Toronto, Canada. The purchase price was comprised of an $84.8 million cash payment, which was net of certain adjustments, plus three contingent payments over a two-year period up to a maximum of an additional $250.0 million in cash. Sentinelle Medical results are reported within our Breast Health reporting segment.
The allocation of the purchase price was based upon estimates of the fair value of assets acquired and liabilities assumed as of August 5, 2010. The purchase price in excess of net tangible assets acquired was allocated to identifiable intangible assets aggregating $67.6 million, primarily comprised of developed technology of $60.9 million and in-process research and development projects of $4.8 million, based upon a detailed valuation that relies on projections and assumptions. The excess of the purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed of $48.6 million was allocated to goodwill.
The amount allocated to acquired in-process research and development represented the estimated fair value of in-process projects based on risk-adjusted cash flows utilizing a discount rate of 17%. These in-process projects had not yet reached technological feasibility as of the date of acquisition and had no future alternative uses. The primary basis for determining the technological feasibility of these projects was obtaining regulatory approval to market the underlying products. We received FDA approval for both projects in fiscal 2011.
The contingent payments were based on a multiple of incremental revenue growth during the two-year period following the completion of the acquisition. Pursuant to ASC 805, we recorded an estimate of the fair value of the contingent consideration liability based on future revenue projections of the Sentinelle Medical business under various potential scenarios and weighted probability assumptions of these outcomes. This analysis resulted in an initial contingent consideration liability of $29.5 million, which was adjusted periodically as a component of operating expenses based on changes in fair value of the liability driven by the accretion of the liability for the time value of money and changes in the assumptions pertaining to the achievement of the defined revenue growth milestones. This fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined in ASC 820. Quarterly, we re-evaluated our assumptions and updated the revenue and probability assumptions for future earn-out periods and lowered our projections. As a result of these adjustments, we recorded a reversal of expense of $3.4 million and $14.3 million in fiscal 2012 and 2011, respectively, to record the contingent consideration liability at fair value. The first two earn-out periods were completed and paid prior to the fourth quarter of fiscal 2012, and the third earn-out period ended in the fourth quarter of fiscal 2012. We have accrued payment of $3.4 million for this final payment as of September 29, 2012.
48
RESULTS OF OPERATIONS
The following table sets forth, for the periods indicated, the percentage of total revenues represented by items as shown in our Consolidated Statements of Operations. All dollar amounts in tables are presented in thousands.
| | | | | | | | | | | | |
| | | Fiscal Years Ended | |
| | | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Revenues: | | | | | | | | | | | | |
Product sales | | | 82.8 | % | | | 82.6 | % | | | 84.2 | % |
Service and other revenues | | | 17.2 | | | | 17.4 | | | | 15.8 | |
| | | | | | | | | | | | |
| | | 100.0 | | | | 100.0 | | | | 100.0 | |
| | | | | | | | | | | | |
| | | |
Costs and expenses: | | | | | | | | | | | | |
Cost of product sales | | | 30.8 | | | | 29.1 | | | | 29.0 | |
Cost of product sales—amortization of intangible assets | | | 10.1 | | | | 9.9 | | | | 10.2 | |
Cost of product sales—impairment of intangible assets | | | — | | | | — | | | | 7.3 | |
Cost of service and other revenues | | | 9.5 | | | | 9.4 | | | | 9.6 | |
Research and development | | | 6.5 | | | | 6.5 | | | | 6.2 | |
Selling and marketing | | | 16.1 | | | | 16.0 | | | | 14.7 | |
General and administrative | | | 11.0 | | | | 8.9 | | | | 8.8 | |
Amortization of intangible assets | | | 3.6 | | | | 3.3 | | | | 3.3 | |
Contingent consideration—compensation expense | | | 4.0 | | | | 1.1 | | | | — | |
Contingent consideration—fair value adjustments | | | 1.9 | | | | (0.4 | ) | | | — | |
Impairment of goodwill | | | 0.3 | | | | — | | | | 4.6 | |
Impairment of intangible assets | | | — | | | | — | | | | 1.2 | |
Gain on sale of intellectual property, net | | | (0.6 | ) | | | (4.7 | ) | | | — | |
Litigation-related settlement charges, net | | | 0.0 | | | | — | | | | 0.7 | |
Acquired in-process research and development | | | 0.2 | | | | — | | | | 0.1 | |
Restructuring and divestiture charges, net | | | 0.9 | | | | — | | | | 0.1 | |
| | | | | | | | | | | | |
| | | 94.3 | | | | 79.1 | | | | 95.8 | |
| | | | | | | | | | | | |
Income from operations | | | 5.7 | | | | 20.9 | | | | 4.2 | |
Interest income | | | 0.1 | | | | 0.1 | | | | 0.1 | |
Interest expense | | | (7.0 | ) | | | (6.4 | ) | | | (7.6 | ) |
Debt extinguishment loss | | | (2.1 | ) | | | (1.7 | ) | | | — | |
Other income (expense), net | | | 0.2 | | | | (0.2 | ) | | | 0.1 | |
| | | | | | | | | | | | |
(Loss) income before income taxes | | | (3.1 | ) | | | 12.7 | | | | (3.2 | ) |
Provision for income taxes | | | 0.6 | | | | 3.9 | | | | 0.5 | |
| | | | | | | | | | | | |
Net (loss) income | | | (3.7 | )% | | | 8.8 | % | | | (3.7 | )% |
| | | | | | | | | | | | |
Fiscal Year Ended September 29, 2012 Compared to Fiscal Year Ended September 24, 2011
Product Sales.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | % of Total
Revenue | | | Amount | | | % of Total
Revenue | | | Amount | | | % | |
Product Sales | | | | | | | | | | | | | | | | | | | | | | | | |
Breast Health | | $ | 572,485 | | | | 29 | % | | $ | 550,112 | | | | 31 | % | | $ | 22,373 | | | | 4 | % |
Diagnostics | | | 707,529 | | | | 35 | % | | | 566,349 | | | | 32 | % | | | 141,180 | | | | 25 | % |
GYN Surgical | | | 311,643 | | | | 16 | % | | | 299,120 | | | | 17 | % | | | 12,523 | | | | 4 | % |
Skeletal Health | | | 66,071 | | | | 3 | % | | | 62,759 | | | | 3 | % | | | 3,312 | | | | 5 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 1,657,728 | | | | 83 | % | | $ | 1,478,340 | | | | 83 | % | | $ | 179,388 | | | | 12 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
In fiscal 2012, Breast Health product revenues increased 4% compared to fiscal 2011 primarily due to the increase in our breast biopsy products revenue of $18.5 million as a result of an increase in the number of Eviva and Celero biopsy devices sold in the United States. In addition, our digital mammography systems revenue increased $1.4 million in fiscal 2012 compared to fiscal 2011 primarily attributable to an increase in the number of units sold of both of our 2D and 3D Dimensions products worldwide, partially offset by slightly lower average selling prices internationally in the current year. Partially offsetting the increase in revenues from the
49
Dimensions systems was a decrease in the number of Selenia systems sold worldwide, and to a lesser extent, a slight deterioration of average selling prices, and a continued shift in Selenia product mix and configuration differences. We are selling more Selenia Performance models, which have fewer features than our base Selenia model, which is a full-featured model, and carry lower average selling prices. In addition, we sold more Selenia systems internationally as a percentage of total Selenia systems, and average selling prices are lower in our international markets compared to the domestic market. We expect the shift in sales from our Selenia products to our Dimensions products to continue. We also had an additional $5.0 million in revenue in fiscal 2012 from our new Trident product.
Diagnostics product sales increased 25% in fiscal 2012 compared to fiscal 2011 primarily due to the inclusion of Gen-Probe, which contributed $86.7 million in revenue, and an increase of $29.7 million in ThinPrep pap tests revenue. The ThinPrep increase was principally from an increase in the sales price of ThinPrep in China as well as higher volumes from the inclusion of revenues of TCT (our former distributor in that country acquired in the third quarter of fiscal 2011), and to a lesser extent, an increase in the number of ThinPrep pap tests sold in other international markets, partially offset by a decline in domestic units sold. The inclusion of Gen-Probe’s results is partially impacted by the Novartis collaboration. Pursuant to the collaboration, a portion of Gen-Probe’s revenue is contingent on donations testing revenue earned by Novartis, however, Gen-Probe recognizes the full product cost upon shipment. As a result, amounts to be received for this contingent revenue related to inventory on hand and not yet utilized by Novartis’ customers as of the acquisition date were recorded as unbilled accounts receivable on the balance sheet in purchase accounting and are not recorded as revenue in our results of operations. In fiscal 2012, this contingent revenue of $11.6 million was not recognized in our results of operations. We also experienced growth in our legacy molecular diagnostics products, which contributed a revenue increase of $13.5 million in fiscal 2012 as we continue to gain new customer accounts and unit sales to existing customers and increase our international sales through TCT. In addition, in fiscal 2012, we sold more diagnostics instruments internationally, increasing revenue by $4.8 million. Revenues also increased slightly due to the extra week in fiscal 2012 compared to fiscal 2011. Fiscal 2012 was a 53-week year compared to fiscal 2011, which was a 52-week year.
GYN surgical product sales increased 4% in fiscal 2012 compared to fiscal 2011 primarily due to the inclusion of the MyoSure system, which contributed an increase of $29.7 million of revenue in fiscal 2012, partially offset by a decrease in NovaSure devices revenue of $9.0 million in fiscal 2012. In addition, revenues also increased slightly due to the extra week in fiscal 2012 compared to fiscal 2011. While we experienced an increase in the number of NovaSure devices sold internationally, these increases were more than offset by a decline in the number of NovaSure devices sold domestically, and lower average selling prices driven by product mix and more international sales. We believe the decline in the number of NovaSure devices sold in the United States is primarily attributable to the continuing effect of unemployment and economic uncertainty, which has resulted in patients delaying surgery or opting for lower cost and generally less effective alternatives. In addition, Adiana system revenues declined $10.9 million in fiscal 2012 compared to fiscal 2011. The reduction in Adiana system revenues was due to our decision to cease manufacturing, marketing and selling the product as of the end of the second quarter of fiscal 2012, determining it was not financially viable and would not become so in the foreseeable future.
Skeletal Health product sales increased 5% in fiscal 2012 compared to fiscal 2011 primarily due to an increase of $2.6 million of our mini C-arm systems as a result of our new Insight system introduced in 2012, and an increase of $1.0 million in osteoporosis assessment product sales internationally.
In fiscal 2012, 73% of product sales were generated in the United States, 12% in Europe, 9% in Asia-Pacific, and 6% in other international markets. In fiscal 2011, 76% of product sales were generated in the United States, 13% in Europe, 6% in Asia-Pacific, and 5% in other international markets.
Service and Other Revenues.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | % of Total
Revenue | | | Amount | | | % of Total
Revenue | | | Amount | | | % | |
Service and Other Revenues | | $ | 344,924 | | | | 17 | % | | $ | 311,009 | | | | 17 | % | | $ | 33,915 | | | | 11 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Service and other revenues are primarily comprised of revenue generated from our field service organization to provide ongoing service, installation and repair of our products. Service and other revenues increased 11% in fiscal 2012 compared to fiscal 2011 primarily in our Breast Health business due to an increase in the number of service contracts driven by an increase in our installed base of our digital mammography systems, which are no longer under warranty, and an extra week in fiscal 2012 compared to fiscal 2011.
50
Cost of Product Sales.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | % of Product
Sales | | | Amount | | | % of Product
Sales | | | Amount | | | % | |
Cost of Product Sales | | $ | 616,839 | | | | 37 | % | | $ | 521,189 | | | | 35 | % | | $ | 95,650 | | | | 18 | % |
Cost of Product Sales—Amortization of Intangible Assets | | | 201,864 | | | | 12 | % | | | 177,456 | | | | 12 | % | | | 24,408 | | | | 14 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 818,703 | | | | 49 | % | | $ | 698,645 | | | | 47 | % | | $ | 120,058 | | | | 17 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Product sales gross margin decreased to 51% in fiscal 2012 compared to 53% in fiscal 2011 as discussed below.
Cost of Product Sales.The cost of product sales as a percentage of product sales was 37% in fiscal 2012 compared to 35% in fiscal 2011. In fiscal 2012 cost of product sales as a percentage of product revenues increased in GYN Surgical and Diagnostics, declined slightly in Breast Health and remained relatively flat in Skeletal Health compared to fiscal 2011 resulting in an overall lower gross margin rate in fiscal 2012 compared to fiscal 2011.
The GYN Surgical gross margin rate in fiscal 2012 declined primarily due to charges of $19.1 million related to our decision to shut down our Adiana product line. On April 30, 2012, we announced that we were ceasing to manufacture, market and sell the Adiana system, determining that the product was not financially viable and would not become so in the foreseeable future. The charge related to the write-off of inventory, manufacturing equipment and equipment at customer sites, and contractual purchase orders for which there was no expected future use of the materials and components. In addition, lower sales of the NovaSure system resulted in a lower gross margin rate, which was partially offset by the shift of sales to our MyoSure system compared to lower margin Adiana system sales. For additional information pertaining to the Adiana product line discontinuance charges, please refer to Note 4 to the consolidated financial statements contained in Item 15 of this Annual Report.
The Diagnostics’ gross margin rate in fiscal 2012 declined compared to fiscal 2011 primarily due to the inclusion of Gen-Probe results, which included $19.9 million of additional costs related to the sale of acquired inventory written up to fair value in purchase accounting, higher service costs and depreciation of equipment at customer sites, and distribution costs. Gen-Probe’s gross margin since acquisition was lower than its historical gross margin rate due to the purchase accounting effect on our collaboration agreement with Novartis in our blood screening business. Based on the Novartis collaboration terms, a portion of Gen-Probe’s revenue is contingent on donations testing revenue earned by Novartis, however, Gen-Probe recognizes the full product cost upon shipment. As a result, amounts to be received for this contingent revenue related to inventory on hand and not yet utilized by Novartis’ customers as of the acquisition date were recorded as unbilled accounts receivable on the balance sheet in purchase accounting and are not recorded as revenue in our results of operations. In fiscal 2012, this contingent revenue of $11.6 million was not recognized in our results of operations. Partially offsetting these cost increases were higher ThinPrep pap test volumes resulting in lower fixed overhead costs per unit, favorable manufacturing variances, and the increase in sales price in China attributable to our acquisition of TCT.
In fiscal 2012, Breast Health’s gross margin rate increased slightly compared to fiscal 2011. The gross margin rate for our digital mammography systems increased due to higher sales of our 3D Dimensions systems, which have higher average selling prices and gross margins than our Selenia systems, and an increase in 3D tomosynthesis software upgrades. Partially offsetting the improvement was an increase in Selenia Performance systems sales as a percent of total Selenia system sales compared to the prior year. Our Selenia Performance systems have lower gross margins than our full-featured Selenia systems. We also sold more Selenia systems internationally as a percentage of total Selenia systems where average selling prices are lower compared to the domestic market. Also offsetting the overall increase in Breast Health’s gross margin was the sales mix within our breast biopsy products as we sold more Eviva disposables and less ATEC disposables as a percentage of revenue compared to the prior year. Eviva disposables have a lower gross margin than our ATEC disposables because they have a higher manufacturing cost and carry additional royalty charges.
Cost of Product Sales—Amortization of Intangible Assets. Amortization of intangible assets relates to acquired developed technology and patents. These intangible assets are generally amortized over their estimated useful lives of between 8.5 and 20 years using a straight-line method or, if reliably determinable, based on the pattern in which the economic benefits of the assets are expected to be consumed. The economic pattern is based on undiscounted future cash flows. The increase in amortization expense in fiscal 2012 compared to fiscal 2011 is primarily due to the inclusion of technology assets acquired in our Gen-Probe acquisition in the fourth quarter of fiscal 2012 and from our Interlace acquisition in the second quarter of fiscal 2011. In addition, there was an increase in amortization expense due to the method of recognition based on the expected economic benefits of the underlying assets, primarily related to the intangible assets acquired in the Cytyc merger in the first quarter of fiscal 2008. Amortization was also higher due to the extra week in fiscal 2012 compared to fiscal 2011.
51
Cost of Service and Other Revenues.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | % of Service
and Other
Revenues | | | Amount | | | % of Service
and Other
Revenues | | | Amount | | | % | |
Cost of Service and Other Revenues | | $ | 189,512 | | | | 55 | % | | $ | 167,523 | | | | 54 | % | | $ | 21,989 | | | | 13 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Service and other revenues gross margin was 45% in fiscal 2012 compared to 46% in fiscal 2011. Gross margin decreased due to additional expenses incurred related to international expansion, which has resulted in the hiring of additional service personnel, increasing compensation and travel costs worldwide. In addition, service costs have increased in our Diagnostics segment due to an increase in our installed base of ThinPrep processors and imagers.
Operating Expenses.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | % of Total
Revenue | | | Amount | | | % of Total
Revenue | | | Amount | | | % | |
Operating Expenses | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | $ | 130,962 | | | | 7 | % | | $ | 116,696 | | | | 7 | % | | $ | 14,266 | | | | 12 | % |
Selling and marketing | | | 322,314 | | | | 16 | % | | | 286,730 | | | | 16 | % | | | 35,584 | | | | 12 | % |
General and administrative | | | 220,042 | | | | 11 | % | | | 158,793 | | | | 9 | % | | | 61,249 | | | | 39 | % |
Amortization of intangible assets | | | 72,036 | | | | 4 | % | | | 58,334 | | | | 3 | % | | | 13,702 | | | | 23 | % |
Contingent consideration—compensation expense | | | 81,031 | | | | 4 | % | | | 20,002 | | | | 1 | % | | | 61,029 | | | | 305 | % |
Contingent consideration—fair value adjustments | | | 38,466 | | | | 2 | % | | | (8,016 | ) | | | 0 | % | | | 46,482 | | | | (580 | )% |
Impairment of goodwill | | | 5,826 | | | | 0 | % | | | — | | | | — | | | | 5,826 | | | | 100 | % |
Gain on sale of intellectual property, net | | | (12,424 | ) | | | (1 | )% | | | (84,502 | ) | | | (5 | )% | | | 72,078 | | | | (85 | )% |
Litigation settlement charges, net | | | 452 | | | | 0 | % | | | 770 | | | | 0 | % | | | (318 | ) | | | (41 | )% |
Acquired in-process research and development | �� | | 4,500 | | | | 0 | % | | | — | | | | — | | | | 4,500 | | | | 100 | % |
Restructuring and divestiture charges, net | | | 17,515 | | | | 1 | % | | | (71 | ) | | | 0 | % | | | 17,586 | | | | (24,769 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 880,720 | | | | 44 | % | | $ | 548,736 | | | | 31 | % | | $ | 331,984 | | | | 61 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Research and Development Expenses.Research and development expenses increased 12% in fiscal 2012 compared to fiscal 2011 primarily due to the inclusion of Gen-Probe, which accounted for $17.1 million of additional expenses. Partially offsetting this increase was a decrease in compensation and benefits due to lower bonus expenses, and a reduction in clinical trials and regulatory costs, which was primarily a result of the status and timing of projects. Research and development primarily reflects spending on new product development programs, regulatory compliance and clinical research and trials. At any point in time, we have a number of different research projects and clinical trials being conducted and the timing of these projects and related costs can vary period to period.
Selling and Marketing Expenses.Selling and marketing expenses increased 12% in fiscal 2012 compared to fiscal 2011. These increases were primarily due to additional expenses from the inclusion of Gen-Probe, which accounted for $8.4 million of additional expense, and the inclusion of TCT for a full year, an increase in the number of sales personnel in the GYN Surgical business segment, an increase in compensation and benefits and higher commissions, continuing product launch activities related to our 3D Dimensions product, and higher travel expenses. These expenses were also higher due to the extra week in fiscal 2012 compared to fiscal 2011. The increase in fiscal 2012 was partially offset by lower expenditures for our direct-to-consumer advertising campaign for NovaSure.
General and Administrative Expenses.General and administrative expenses increased 39% in fiscal 2012 compared to fiscal 2011 primarily due to higher acquisition related transaction costs associated with the Gen-Probe acquisition of $34.3 million, additional expenses, including retention costs, from the inclusion of Gen-Probe of $12.8 million, integration and consulting costs related to the Gen-Probe acquisition, an increase in bad debt expense internationally, charges for an ongoing state sales tax audit, an increase in the Nonqualified Deferred Compensation Plan, or DCP, liability, which is driven by the underlying market changes of hypothetical investments, and higher invoice collection fees from higher credit card payments. These expenses were also higher due to
52
the extra week in fiscal 2012 compared to fiscal 2011. These increases were partially offset by an overall decrease in compensation and benefits due to lower bonus expense and lower stock compensation expense as higher valued restricted stock units fully vested in fiscal 2011 and lower depreciation expense.
Amortization of Intangible Assets.Amortization of intangible assets results from customer relationships, trade names, business licenses and non-compete agreements related to our acquisitions. These intangible assets are generally amortized over their estimated useful lives of between 2 and 30 years using a straight-line method or, if reliably determinable, based on the pattern in which the economic benefits of the assets are expected to be consumed. The increase in fiscal 2012 compared to fiscal 2011 was primarily due to the addition of intangible assets from the Gen-Probe acquisition, a full year of amortization from the Interlace and TCT acquisitions, and an increase in amortization due to the method of recognition based on the expected economic benefits of the underlying assets, primarily related to the intangible assets acquired in the Cytyc merger in fiscal 2008. Amortization was also higher due to the extra week in fiscal 2012 compared to fiscal 2011.
Contingent Consideration—Compensation Expense. In connection with our recent acquisitions, excluding Gen-Probe, we are obligated to make contingent earn-out payments. Amounts recorded in this financial statement line item are those contingent payments that are contingent on future employment. These payments are also generally based on achieving certain performance milestones, typically incremental revenue growth, as is the case for TCT. The amounts recorded in fiscal 2012 relate to TCT and, to a lesser extent, Healthcome. Amounts recorded in fiscal 2011 primarily relate to TCT and Interlace. We recorded charges of $81.0 million and $20.0 million in fiscal 2012 and 2011, respectively. For additional information, refer to the prior section titled “Acquisitions” and to Note 3 to the consolidated financial statements contained in Item 15 of this Annual Report.
Contingent Consideration—Fair Value Adjustments. In connection with our acquisitions of Sentinelle Medical and Interlace, we may be required to pay future consideration that is contingent on achieving certain revenue based milestones. As of each respective acquisition date, we recorded contingent consideration liabilities for the estimated fair value of the amount we expected to pay to the former shareholders of the acquired business. This liability is not contingent on future employment and is based on future revenue projections of the respective businesses under various potential scenarios and weighted probability assumptions of these outcomes. At each reporting period, we re-measure the fair value of these liabilities and record the changes in fair value through a separate line item within our Consolidated Statements of Operations. Increases or decreases in the fair value of contingent consideration liabilities can result from accretion of the liability for the passage of time, changes in discount rates, and changes in the timing, probabilities and amount of revenue estimates. In fiscal 2012, we recorded a net charge of $38.5 million reflecting an increase in the fair value of the Interlace liability of $41.8 million due to higher revenue estimates, partially offset by a benefit of $3.4 million related to Sentinelle Medical due to actual revenues being less than those estimated. We recorded a net benefit of $8.0 million in fiscal 2011 reflecting a net decrease in the fair values of these liabilities comprised of a reduction in the fair value of the Sentinelle Medical liability of $14.3 million due primarily to changes in revenue assumptions offset by a charge of $6.3 million related to Interlace based primarily on the accretion of the liability to the expected payment amount. For additional information, refer to the prior section titled “Acquisitions” and to Note 3 to the consolidated financial statements contained in Item 15 of this Annual Report.
Impairment of Goodwill. During the fourth quarter of fiscal 2012, we recorded an impairment charge of $5.8 million related to our MammoSite reporting unit, which is included in our Breast Health segment. The fair value of this reporting unit declined from fiscal 2011 primarily due to our reassessment in the fourth quarter of fiscal 2012 of the overall market size of breast brachytherapy and long-term growth projections. No other reporting units were deemed to be impaired in fiscal 2012. For additional information, refer to Note 2—“Intangible Assets and Goodwill” to the consolidated financial statements contained in Item 15 of this Annual Report.
Gain on Sale of Intellectual Property, Net.During the second quarter of fiscal 2012, we received a scheduled payment of $12.5 million from K-V Pharmaceutical Company (“KV”) pursuant to our amended agreement, which was recorded net of amounts owed to the original inventor of Makena. During the second quarter of fiscal 2011, we received FDA approval of Makena, and all rights to Makena were transferred to KV. Upon transfer, we received $12.5 million, and including the $79.5 million received in prior periods, we recorded a gain on the sale of intellectual property, net of the write-off of certain assets, of $84.5 million in the second quarter of fiscal 2011. For additional information on this arrangement and amounts we may receive in the future, refer to Note 7 to the consolidated financial statements contained in Item 15 of this Annual Report.
Acquired In-Process Research and Development. During the fourth quarter of fiscal 2012, we acquired certain research and development assets that were determined to have no future alternative use and recorded a $4.5 million charge within our GYN Surgical segment.
Restructuring and Divestiture Charges, Net.In fiscal 2012, we recorded aggregate restructuring and divestiture charges of $36.6 million of which $17.5 million was recorded in this line item, and $19.1 million was recorded in cost of product sales. These charges are related to a number of actions in fiscal 2012 as described below. For additional information pertaining to restructuring and divesture charges, please refer to Note 4 to the consolidated financial statements contained in Item 15 of this Annual Report.
53
Abandonment of Adiana Product Line
At the end of the second quarter of fiscal 2012, we decided to cease manufacturing, marketing and selling our Adiana system, which was a product line within our GYN Surgical reporting segment. We determined that the product was not financially viable and would not become so in the foreseeable future. In addition, we settled the intellectual property litigation regarding the Adiana system with Conceptus as discussed below under “Contingent Earn-Out Payments.” As a result, in the second quarter of fiscal 2012, we recorded a charge of $18.3 million and recorded additional adjustments in fiscal 2012 resulting in an aggregate charge of $19.5 million. Of the total charge, $19.1 million was recorded within cost of product sales and $0.4 million was recorded in restructuring. The amount recorded in cost of product sales comprised of impairment charges of $9.9 million to record inventory at its net realizable value, $6.5 million to write down certain manufacturing equipment and equipment placed at customer sites to its fair value that had no further utility, and $2.7 million for outstanding contractual purchase orders of raw materials and components that will not be utilized and other contractual obligations. In connection with this action, we terminated certain manufacturing and other personnel primarily at our Costa Rica location, resulting in severance charges of $0.1 million, and incurred other contractual charges of $0.3 million. All identified employees were terminated and paid as of September 29, 2012.
Consolidation of Diagnostics Operations
In connection with our acquisition of Gen-Probe, we implemented restructuring actions to consolidate our Diagnostics business, such as streamlining product development initiatives, reducing overlapping functional areas such as sales, marketing and general and administrative functions, and consolidation of manufacturing resources, field services and support. As a result, we terminated certain employees from Gen-Probe and our legacy diagnostics business in research and development, sales, marketing and general and administrative functions. We recorded severance and benefit charges of $13.3 million related to this action pursuant to ASC 420,Exit or Disposal Cost Obligations. The majority of these employees ceased working in the fourth quarter of fiscal 2012 and their full severance charge was recorded in the fourth quarter of fiscal 2012. In addition, certain of the terminated Gen-Probe employees had unvested stock options and their vesting terms were accelerated as a result of termination. As such, the severance charges include $3.5 million of stock-based compensation expense. We expect to record an additional $1.1 million for severance and benefits in fiscal 2013.
In addition, we will move our legacy molecular diagnostics operations from Madison, Wisconsin to San Diego, California. This transfer is expected to be finalized by the end of calendar 2014 and the majority of employees in Madison will be terminated in fiscal 2013 and 2014. We are recording severance and benefit charges pursuant to ASC 420 and estimate the total severance and benefits charge to be approximately $6.4 million, which will be recorded ratably over the estimated service period of the affected employees. We recorded $1.5 million in fiscal 2012 related to this action, which was comprised of $0.9 million for severance benefits and a $0.6 million non-cash charge as a result of exiting certain research projects. Additional charges will be recorded as the manufacturing operation is transferred and the facility is closed down. These charges will be recorded as they are incurred.
Closure of Indianapolis Facility
In the fourth quarter of fiscal 2012, we finalized our decision to transfer production of our interventional breast products from our Indianapolis facility to our facility in Costa Rica. The transfer is expected to be completed in the first half of calendar 2014 and all employees at that location will be terminated. We are recording severance and benefit charges pursuant to ASC 420 and estimate the total severance and benefits charge to be approximately $7.0 million, which will be recorded ratably over the estimated service period of the affected employees. We recorded $1.8 million in fiscal 2012 related to this action comprised of $0.9 million of severance benefits and $0.9 million for amounts owed to the state of Indiana for employment credits. Additional charges will be recorded as the manufacturing operation is transferred and the facility is closed down. These charges will be recorded as they are incurred.
Interest Income.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Investment and Interest Income | | $ | 2,340 | | | $ | 1,860 | | | $ | 480 | | | | 26 | % |
| | | | | | | | | | | | | | | | |
Interest income increased in fiscal 2012 compared to fiscal 2011 primarily due to a higher average cash balance in fiscal 2012, prior to the Gen-Probe acquisition.
54
Interest Expense.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Interest Expense | | $ | (140,287 | ) | | $ | (114,846 | ) | | $ | 25,441 | | | | 22 | % |
| | | | | | | | | | | | | | | | |
In fiscal 2012, interest expense increased due to the financing to fund the Gen-Probe acquisition on August 1, 2012. We borrowed an aggregate of $3.5 billion in principal comprised of term loans and Senior Notes. Interest expense also includes the interest costs and amortization of the debt discount of our Convertible Notes and amortization of deferred financing costs. As a result of our additional borrowings totaling $3.5 billion to fund a portion of the purchase price for Gen-Probe, we expect interest expense to be significantly higher in fiscal 2013.
Debt Extinguishment Loss.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Debt Extinguishment Loss | | $ | (42,347 | ) | | $ | (29,891 | ) | | $ | 12,456 | | | | 42 | % |
| | | | | | | | | | | | | | | | |
In the second quarter of fiscal 2012, pursuant to separate, privately-negotiated exchange agreements, we retired $500.0 million in aggregate principal of our 2.00% Convertible Senior Notes due 2037 (“2007 Notes”) for $500.0 million in aggregate principal of new 2.00% Convertible Notes due 2042 (“2012 Notes”). This exchange enabled us to extend the first put date out approximately four and a quarter years to March 1, 2018 as well as the subsequent put dates as disclosed in the Liquidity and Capital Resources section of this Management’s Discussion and Analysis. In consideration, the equity conversion price of the notes was reduced to approximately $31.18 from $38.59, and we must pay the cash coupon for four and a quarter more years, consistent with extending the first put date, instead of accreting the coupon to the principal as required under the original terms. In connection with this transaction, we recorded a debt extinguishment loss of $42.3 million, which includes the write-off of the pro-rata allocation of deferred financing costs.
In the first quarter of fiscal 2011, pursuant to separate, privately-negotiated exchange agreements, we retired $450.0 million in aggregate principal of our 2007 Notes for $450.0 million in aggregate principal of new 2.00% Convertible Exchange Senior Notes due 2037 (“2010 Notes”). This exchange enabled us to extend the first put date out three years to December 15, 2016 from December 13, 2013 as well as the subsequent put dates as disclosed in the Liquidity and Capital Resources section of this Management’s Discussion and Analysis. In consideration, the equity conversion price of the notes was reduced to $23.03 from $38.59, and we must pay the cash coupon for three more years, consistent with extending the first put date, instead of accreting the coupon to the principal as required under the original terms. In connection with this transaction, we recorded a debt extinguishment loss of $29.9 million, which includes the write-off of the pro-rata allocation of deferred financing costs. For additional information, refer to Note 5 to the consolidated financial statements contained in Item 15 of this Annual Report.
Other Income (Expense), net.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Other Income (Expense), net | | $ | 4,916 | | | $ | (4,182 | ) | | $ | 9,098 | | | | 218 | % |
| | | | | | | | | | | | | | | | |
In fiscal 2012, this account was primarily comprised of gains on the cash surrender value of life insurance contracts related to our DCP of $3.2 million, net foreign currency transaction gains of $0.8 million, and other miscellaneous gains.
In fiscal 2011, this account was primarily comprised of impairment charges of $2.4 million for cost-method investments, net foreign currency transaction losses of $1.1 million and a loss on cash surrender value of life insurance contracts related to our DCP of $0.8 million.
Provision for Income Taxes.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Provision for Income Taxes | | $ | 11,973 | | | $ | 70,236 | | | $ | (58,263 | ) | | | (83 | )% |
| | | | | | | | | | | | | | | | |
55
Our effective tax rate for fiscal 2012 was 19.4% of pre-tax losses compared to 30.9% of pre-tax earnings in fiscal 2011. Our effective tax rate in fiscal 2012 was significantly impacted by non-deductible contingent consideration compensation expense, nondeductible acquisition costs, a nondeductible goodwill impairment charge, and a net increase in income tax reserves and valuation allowances on certain foreign losses. The unfavorable tax impact of these items was partially offset by the domestic manufacturing benefit and a loss claimed related to the discontinuance of the Adiana product line. The effect of these permanent items to the effective tax rate was magnified by the current year pre-tax loss. Our effective tax rate for fiscal 2011 was less than the statutory rate primarily due to reversing income tax reserves, the domestic manufacturing benefit and U.S. and Canadian research and development tax credits. The $9.1 million income tax reserve reversal was due to us favorably settling our U.S. federal income tax audit for fiscal years 2007 through 2009 and statutes of limitations expiring in several state and foreign jurisdictions.
Segment Results of Operations
We report our business as four segments: Breast Health, Diagnostics, GYN Surgical and Skeletal Health. The accounting policies of the segments are the same as those described in the footnotes to the accompanying consolidated financial statements contained in Item 15 of this Annual Report. We measure segment performance based on total revenues and operating income or loss. Revenues from product sales of each of these segments are described in further detail above. The discussion that follows is a summary analysis of total revenues and the primary changes in operating income or loss by segment.
Breast Health.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 875,771 | | | $ | 825,551 | | | $ | 50,220 | | | | 6 | % |
| | | | | | | | | | | | | | | | |
Operating Income | | $ | 186,106 | | | $ | 187,970 | | | $ | (1,864 | ) | | | (1 | )% |
| | | | | | | | | | | | | | | | |
Operating Income as a % of Segment Revenue | | | 21 | % | | | 23 | % | | | | | | | | |
| | | | | | | | | | | | | | | | |
Breast Health revenues increased in fiscal 2012 compared to fiscal 2011 due to the increase in product revenue of $22.4 million discussed above and a $27.8 million increase in service revenues.
Operating income for this business segment decreased in fiscal 2012 compared to fiscal 2011 primarily due to an increase in operating expenses partially offset by an increase in gross margin on an absolute dollar basis as a result of increased revenues discussed above.
In fiscal 2012, the overall gross margin rate increased to 49.4% from 49.0% in fiscal 2011 due primarily to improvements in product gross margin discussed above. The product gross margin rate increased to 49.9% in fiscal 2012 compared to 48.7% in fiscal 2011. Operating expenses were higher in fiscal 2012 compared to fiscal 2011 primarily due to the net benefit of $14.3 million recorded in the prior year compared to the $3.4 million benefit in the current year to adjust the Sentinelle Medical contingent consideration liability to fair value, higher contingent compensation expense of $5.6 million related to Healthcome in fiscal 2012, the Mammosite goodwill impairment charge of $5.8 million, and restructuring charges of $2.3 million primarily related to our decision to close our facility in Indianapolis. In addition, expenses were higher in the current year due to higher compensation costs related to hiring additional personnel, continuing product launch activities related to our 3D Dimensions product, medical education, travel, and litigation settlements and related expenses, partially offset by lower clinical trial expenses.
Diagnostics.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 718,064 | | | $ | 571,263 | | | $ | 146,801 | | | | 26 | % |
| | | | | | | | | | | | | | | | |
Operating (Loss) Income | | $ | (32,787 | ) | | $ | 170,693 | | | $ | (203,480 | ) | | | (119 | )% |
| | | | | | | | | | | | | | | | |
Operating (Loss) Income as a % of Segment Revenue | | | (5 | )% | | | 30 | % | | | | | | | | |
| | | | | | | | | | | | | | | | |
Diagnostics revenues increased in fiscal 2012 compared to fiscal 2011 primarily due to the increase in product sales discussed above.
Operating income for this business segment decreased in fiscal 2012 compared to fiscal 2011 primarily due to the net gain of $84.5 million on the sale of Makena intellectual property to KV in the second quarter of fiscal 2011 compared to a net gain of $12.4 million recorded in the second quarter of fiscal 2012 for a scheduled payment received under the amended agreement. The balance of the decrease in fiscal 2012 of $131.4 million was due to higher operating expenses partially offset by an increase in gross margin in
56
absolute dollars. While gross margin in absolute dollars increased, the gross margin rate declined to 50.5% in fiscal 2012 compared to 53.9% in fiscal 2011 as discussed above. Operating expenses increased in fiscal 2012 primarily due to the inclusion of TCT for a full year, which included an increase in contingent consideration compensation expense of $58.0 million, the inclusion of $42.4 million of operating expenses for Gen-Probe (excluding restructuring charges and integration and retention costs), acquisition transaction and integration costs of approximately $42.0 million related to the Gen-Probe transaction, and restructuring charges of $14.8 million related to the consolidation of our Diagnostics business as a result of the Gen-Probe acquisition. We also experienced higher expenses related to trade shows, higher compensation costs related to hiring additional personnel and annual salary increases, travel, and higher bad debt charges, primarily related to one international customer.
GYN Surgical.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 313,089 | | | $ | 300,538 | | | $ | 12,551 | | | | 4 | % |
| | | | | | | | | | | | | | | | |
Operating (Loss) Income | | $ | (51,892 | ) | | $ | 3,623 | | | $ | (55,515 | ) | | | (1,532 | )% |
| | | | | | | | | | | | | | | | |
Operating (Loss) Income as a % of Segment Revenue | | | (17 | )% | | | 1 | % | | | | | | | | |
| | | | | | | | | | | | | | | | |
GYN Surgical revenues increased in fiscal 2012 compared to fiscal 2011 due to the increase in product sales discussed above.
This business segment incurred an operating loss in fiscal 2012 compared to income in fiscal 2011 primarily attributable to the inclusion of Interlace’s operations (acquired in the second quarter of fiscal 2011), which included a charge of $41.8 million in fiscal 2012 to adjust the contingent consideration liability to fair value compared to aggregate contingent consideration charges of $8.4 million in fiscal 2011, and the aggregate charge of $19.5 million related to the Adiana product line discontinuance. Overall, gross margin in absolute dollars decreased in fiscal 2012 compared to fiscal 2011 primarily due to the Adiana product line charge recorded in cost of product sales of $19.1 million, and higher intangible asset amortization expense of $9.2 million, partially offset by the impact of higher sales as discussed above. This resulted in the segment’s gross margin rate declining to 50.3% in fiscal 2012 from 56.8% in fiscal 2011.
In addition, this segment incurred higher operating expenses in fiscal 2012 compared to fiscal 2011 primarily in sales and marketing related to higher compensation and benefits for additional sales personnel, annual salary increases and commissions, and higher travel, an in-process research and development charge of $4.5 million, higher intangible asset amortization expenses, additional expenditures for ongoing research and development projects and charges related to an ongoing state sales tax audit. These increases were partially offset by a reduction in advertising expenditures for our NovaSure system’s direct-to-consumer advertising campaign and lower marketing and medical education expenses related to the discontinuance of the Adiana product line.
Skeletal Health.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 29, 2012 | | | September 24, 2011 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 95,728 | | | $ | 91,997 | | | $ | 3,731 | | | | 4 | % |
| | | | | | | | | | | | | | | | |
Operating Income | | $ | 12,290 | | | $ | 12,159 | | | $ | 131 | | | | 1 | % |
| | | | | | | | | | | | | | | | |
Operating Income as a % of Segment Revenue | | | 13 | % | | | 13 | % | | | | | | | | |
| | | | | | | | | | | | | | | | |
Skeletal Health revenues increased in fiscal 2012 compared to fiscal 2011 primarily due to the increase in product sales discussed above.
Operating income increased slightly in fiscal 2012 compared to fiscal 2011 primarily due to the increase in revenues and improvement in gross margin rates to 44.1% in fiscal 2012 from 43.5% in fiscal 2011. The improvement in gross margin dollars was partially offset by an increase in operating expenses primarily due to higher compensation and benefits and travel expenses.
57
Fiscal Year Ended September 24, 2011 Compared to Fiscal Year Ended September 25, 2010
Product Sales.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | % of Total
Revenue | | | Amount | | | % of Total
Revenue | | | Amount | | | % | |
Product Sales | | | | | | | | | | | | | | | | | | | | | | | | |
Breast Health | | $ | 550,112 | | | | 31 | % | | $ | 525,622 | | | | 31 | % | | $ | 24,490 | | | | 5 | % |
Diagnostics | | | 566,349 | | | | 32 | % | | | 548,832 | | | | 33 | % | | | 17,517 | | | | 3 | % |
GYN Surgical | | | 299,120 | | | | 17 | % | | | 281,364 | | | | 17 | % | | | 17,756 | | | | 6 | % |
Skeletal Health | | | 62,759 | | | | 3 | % | | | 59,082 | | | | 3 | % | | | 3,677 | | | | 6 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 1,478,340 | | | | 83 | % | | $ | 1,414,900 | | | | 84 | % | | $ | 63,440 | | | | 4 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
In fiscal 2011, our product sales increased $63.4 million, or 4%, compared to fiscal 2010 due to an increase in revenues of $24.5 million from Breast Health, $17.8 million from GYN Surgical, $17.5 million from Diagnostics, and $3.7 million from Skeletal Health.
Breast Health product sales increased 5% in fiscal 2011 compared to fiscal 2010 primarily due to growth in our breast biopsy products of $16.8 million, the inclusion of a full year of revenues from Sentinelle Medical (acquired in the fourth quarter of fiscal 2010), which increased $12.9 million, and a favorable foreign currency impact of $3.4 million. The increase in breast biopsy products revenue was primarily attributable to an increase in the number of Eviva biopsy devices sold with the largest increase in the U.S. Overall, our digital mammography systems revenues decreased $2.9 million in fiscal 2011 compared to fiscal 2010. We experienced a decrease in the number of Selenia systems sold worldwide, and to a lesser extent, Selenia product mix and configuration differences resulted in lower revenues in fiscal 2011 compared to fiscal 2010. We sold a greater number of our Selenia value-related models and a lesser amount of our full featured Selenia models in fiscal 2011. Our value-related models have lower average selling prices than our full featured Selenia models. In addition, we sold more Selenia systems internationally as a percentage of total Selenia systems and average selling prices are lower in our international markets compared to the domestic market. Mostly offsetting these decreases was an increase in the number of units sold of our new 2D/3D Dimensions products as these systems continue to gain traction. These systems also have higher average selling prices than our Selenia systems.
Diagnostics product sales increased 3% in fiscal 2011 compared to fiscal 2010 primarily due to an increase in revenues from our Cervista HPV tests, the inclusion of TCT (acquired in the third quarter of fiscal 2011) resulting in incremental revenues of approximately $10 million, a favorable foreign currency impact of $5.2 million and to a lesser extent an increase in the number of ThinPrep pap tests sold internationally, partially offset by a reduction in domestic sales. Cervista HPV revenues have increased as we continue to gain new customer accounts and unit sales to existing customers increase. We believe the decline in the number of ThinPrep pap tests sold domestically was due to the decline in patient visits year over year attributable to the lagging effects of unemployment, continuing economic uncertainty, recent changes in cervical cancer screening guidelines to extend the recommended intervals between such screenings, and to a lesser extent laboratory consolidation.
GYN Surgical product sales increased 6% in fiscal 2011 compared to fiscal 2010 due to an increase in the number of Adiana systems sold and the inclusion of MyoSure system sales (acquired in the second quarter of fiscal 2011). NovaSure system sales were essentially flat year over year. While we experienced an increase in the number of NovaSure devices sold internationally and to a lesser extent a slight increase in average selling prices, these increases were offset by a decline in the number of NovaSure devices sold domestically. We believe the decline in units sold domestically was primarily due to the lagging effects of unemployment and continuing economic uncertainty, which has resulted in patients delaying surgery and opting for lower cost and generally less effective alternatives.
Skeletal Health product sales increased 6% in fiscal 2011 compared to fiscal 2010 primarily due to a $6.0 million increase in osteoporosis assessment product sales worldwide. Offsetting this increase in fiscal 2011 was a reduction in mini C-arm revenues of $2.1 million due to a decrease in the number of units sold domestically and slightly lower average selling prices in fiscal 2011 compared to fiscal 2010.
In fiscal 2011, 76% of product sales were generated in the United States, 13% in Europe, 6% in Asia-Pacific, and 5% in other international markets. In fiscal 2010, 79% of product sales were generated in the United States, 12% in Europe, 5% in Asia-Pacific, and 4% in other international markets.
Service and Other Revenues.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | % of Total
Revenue | | | Amount | | | % of Total
Revenue | | | Amount | | | % | |
Service and Other Revenues | | $ | 311,009 | | | | 17 | % | | $ | 264,652 | | | | 16 | % | | $ | 46,357 | | | | 18 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Service and other revenues are primarily comprised of revenue generated from our field service organization to provide ongoing service, installation and repair of our products. Service and other revenues increased 18% in fiscal 2011 compared to fiscal 2010 primarily in our Breast Health business due to an increase in the number of service contracts driven by an increase in our installed base of our digital mammography systems, which were no longer under warranty.
58
Cost of Product Sales.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | % of Product
Sales | | | Amount | | | % of Product
Sales | | | Amount | | | % | |
Cost of Product Sales | | $ | 521,189 | | | | 35 | % | | $ | 487,057 | | | | 34 | % | | $ | 34,132 | | | | 7 | % |
Cost of Product Sales—Amortization of Intangible Assets | | | 177,456 | | | | 12 | % | | | 171,447 | | | | 12 | % | | | 6,009 | | | | 4 | % |
Cost of Product Sales—Impairment of Intangible Assets | | | — | | | | — | | | | 123,350 | | | | 9 | % | | | (123,350 | ) | | | (100 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 698,645 | | | | 47 | % | | $ | 781,854 | | | | 55 | % | | $ | (83,209 | ) | | | (11 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | |
Product sales gross margin increased to 53% in fiscal 2011 compared to 45% in fiscal 2010 primarily due to the significant intangible asset charge of $123.4 million recorded in fiscal 2010 related to MammoSite.
Cost of Product Sales. The cost of product sales as a percentage of product sales was 35% in fiscal 2011 compared to 34% in fiscal 2010. In fiscal 2011, cost of product sales as a percentage of product revenues increased in Breast Health compared to fiscal 2010 and was relatively flat in Diagnostics, GYN Surgical and Skeletal Health compared to fiscal 2010. The decline in Breast Health gross margin in fiscal 2011 was primarily due to $2.7 million of additional costs related to the sale of acquired Sentinelle Medical inventory written up to fair value in purchase accounting, unfavorable absorption and higher production spend principally in the digital mammography product lines, and a mix shift in sales of our Selenia product line. Our Selenia value-related models have lower average selling prices and gross margins than our full featured Selenia models. In addition, we sold more Selenia systems internationally as a percentage of total Selenia systems, where our average selling prices are lower. Partially offsetting these decreases were higher sales of Dimensions 3D and 3D tomosynthesis software upgrades. The Dimensions 3D product has a higher average selling price and gross margin compared to our full-featured Selenia models. Within our breast biopsy products, the mix of products sold resulted in lower gross margins as we sold more Eviva disposables and less ATEC disposables as a percentage of revenue compared to fiscal 2010. Eviva disposables have a lower gross margin than our ATEC disposables because they have a higher manufacturing cost and carry additional royalty charges.
Cost of Product Sales—Amortization of Intangible Assets. Amortization of intangible assets relates to acquired developed technology. These intangible assets are generally amortized over their estimated useful lives of between 8.5 and 20 years using a straight-line method or, if reliably determinable, based on the pattern in which the economic benefits of the assets are expected to be consumed. The economic pattern is based on undiscounted future cash flows. The increase in amortization expense in fiscal 2011 compared to fiscal 2010 was primarily due to the inclusion of additional amortization expense related to the technology assets acquired from the Sentinelle Medical and Interlace acquisitions in the fourth quarter of fiscal 2010 and second quarter of fiscal 2011, respectively. In addition, there was an increase in amortization expense in fiscal 2011 due to the method of recognition based on the expected economic benefits of the underlying assets, primarily related to the intangible assets acquired in the Cytyc merger in the first quarter of fiscal 2008. Offsetting this increase was a decline in amortization expense in fiscal 2011 related to our MammoSite reporting unit. We recorded an impairment charge for the MammoSite developed technology in the fourth quarter of fiscal 2010, as discussed below, which resulted in a reduced asset value and lower future amortization expense.
Cost of Product Sales—Impairment of Intangible Assets. During the fourth quarter of fiscal 2010 in connection with our Company-wide annual budgeting and strategic planning process, we identified indicators of impairment in our MammoSite reporting unit due to changing market conditions for the breast brachytherapy market, including downward pressure on procedure volumes from the continuing adverse macroeconomic environment and current trends in breast cancer management, as well as competitive pricing pressures and competition from existing and alternative new technologies. These factors resulted in lowering our financial projections for MammoSite. We performed the first step in the long-lived assets impairment test and compared MammoSite’s forecasted undiscounted cash flows to the carrying value of its net assets, which indicated that these cash flows were insufficient to recover MammoSite’s carrying value. Therefore, we determined the fair value of MammoSite’s long-lived assets, which are primarily intangible assets, using a discounted cash flow technique. Based on the fair value of the long-lived assets, we recorded an impairment charge of $123.4 million to developed technology in the fourth quarter of fiscal 2010. For additional information, refer to Note 2 to the consolidated financial statements contained in Item 15 of this Annual Report.
59
Cost of Service and Other Revenues.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | % of Service
and Other
Revenues | | | Amount | | | % of Service
and Other
Revenues | | | Amount | | | % | |
Cost of Service and Other Revenues | | $ | 167,523 | | | | 54 | % | | $ | 161,060 | | | | 61 | % | | $ | 6,463 | | | | 4 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Service and other revenues gross margin improved to 46% in fiscal 2011 compared to 39% in fiscal 2010 primarily due to the improved absorption of fixed service costs and the continued growth of service contract revenue, primarily in the Breast Health business. We were able to convert a high percentage of our domestic installed base of digital mammography systems to service contracts upon the expiration of the warranty period. In addition, warranty costs decreased due to lower failure rates in certain of our products.
Operating Expenses.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | % of Total
Revenue | | | Amount | | | % of Total
Revenue | | | Amount | | | % | |
Operating Expenses | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | $ | 116,696 | | | | 7 | % | | $ | 104,305 | | | | 6 | % | | $ | 12,391 | | | | 12 | % |
Selling and marketing | | | 286,730 | | | | 16 | % | | | 247,374 | | | | 15 | % | | | 39,356 | | | | 16 | % |
General and administrative | | | 158,793 | | | | 9 | % | | | 148,340 | | | | 9 | % | | | 10,453 | | | | 7 | % |
Amortization of intangible assets | | | 58,334 | | | | 3 | % | | | 54,858 | | | | 3 | % | | | 3,476 | | | | 6 | % |
Contingent consideration—compensation expense | | | 20,002 | | | | 1 | % | | | — | | | | — | | | | 20,002 | | | | 100 | % |
Contingent consideration—fair value adjustments | | | (8,016 | ) | | | 0 | % | | | — | | | | — | | | | (8,016 | ) | | | (100 | )% |
Impairment of goodwill | | | — | | | | — | | | | 76,723 | | | | 5 | % | | | (76,723 | ) | | | (100 | )% |
Impairment of intangible assets | | | — | | | | — | | | | 20,117 | | | | 1 | % | | | (20,117 | ) | | | (100 | )% |
Gain on sale of intellectual property, net | | | (84,502 | ) | | | (5 | )% | | | — | | | | — | | | | (84,502 | ) | | | (100 | )% |
Litigation settlement charges, net | | | 770 | | | | 0 | % | | | 11,403 | | | | 1 | % | | | (10,633 | ) | | | (93 | )% |
Acquired in-process research and development | | | — | | | | — | | | | 2,000 | | | | 0 | % | | | (2,000 | ) | | | (100 | )% |
Restructuring and divestiture charges, net | | | (71 | ) | | | 0 | % | | | 1,581 | | | | 0 | % | | | (1,652 | ) | | | (104 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 548,736 | | | | 31 | % | | $ | 666,701 | | | | 40 | % | | $ | (117,965 | ) | | | (18 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | |
Research and Development Expenses. Research and development expenses increased 12% in fiscal 2011 compared to fiscal 2010. The increase was primarily due to the inclusion of additional expenses from Sentinelle Medical (acquired in the fourth quarter of fiscal 2010) and Interlace (acquired in the second quarter of fiscal 2011). In fiscal 2011, compensation and benefits increased due to an increase in headcount, annual salary increases, and higher bonuses based on improved company performance compared to the prior year. In addition, there was an increase in clinical trials and research projects spend primarily attributable to next generation NovaSure and Adiana products, partially offset by a reduction in clinical trial and project spend on the Dimensions 3D tomosynthesis product, which was approved by the FDA in February 2011. Research and development primarily reflects spending on new product development programs, regulatory compliance and clinical research and trials.
Selling and Marketing Expenses.Selling and marketing expenses increased 16% in fiscal 2011 compared to fiscal 2010. This increase was primarily due to additional expenses from the inclusion of Sentinelle Medical, Interlace and TCT, expenditures for our direct-to-consumer advertising campaign for the NovaSure system, higher spending for other marketing and advertising initiatives including the Dimensions 3D product launch, increased compensation and benefits related to an increase in headcount, annual salary increases and higher bonuses based on improved company performance compared to the prior year, and medical education. Partially offsetting these increases were lower distributor and third-party commissions.
General and Administrative Expenses. General and administrative expenses increased 7% in fiscal 2011 compared to fiscal 2010 primarily due to additional expenses from the inclusion of Sentinelle Medical, Interlace and TCT, an increase in compensation and benefits primarily due to an increase in headcount, annual salary increases, higher bonuses due to improved company performance compared to the prior year, and additional expenses related to consummating our acquisitions and integrating them into our operations, including accounting and tax services. In fiscal 2011, these increases were partially offset by a decrease in litigation costs due to lower activity compared to fiscal 2010, in which a number of matters were resolved, primarily the Ethicon lawsuit.
60
Amortization of Intangible Assets. Amortization of intangible assets results from customer relationships, trade names, business licenses and non-compete agreements related to our acquisitions. These intangible assets are generally amortized over their estimated useful lives of between 2 and 30 years using a straight-line method or, if reliably determinable, based on the pattern in which the economic benefits of the assets are expected to be consumed. The increase in fiscal 2011 compared to fiscal 2010 was due to the addition of intangible assets from the Sentinelle Medical, Interlace and TCT acquisitions, and an increase in amortization due to the method of recognition based on the expected economic benefits of the underlying assets, primarily related to the intangible assets acquired in the Cytyc merger in the first quarter of fiscal 2008.
Contingent Consideration—Compensation Expense. In connection with our recent acquisitions, we are obligated to make contingent earn-out payments. Amounts recorded in this financial statement line item are those contingent payments that are contingent on future employment. These payments are also generally based on achieving certain performance milestones, typically incremental revenue growth, as is the case for TCT. The amounts recorded related to Interlace and Healthcome are solely related to continuing employment. In fiscal 2011, we recorded an aggregate charge of $20.0 million comprised of $17.6 million, $2.1 million and $0.3 million related to TCT, Interlace and Healthcome, respectively. For additional information, refer to Note 3 to the consolidated financial statements contained in Item 15 of this Annual Report.
Contingent Consideration—Fair Value Adjustments. In connection with the purchase price allocation for our acquisitions of Sentinelle Medical and Interlace, we recorded an estimate of the fair value of the contingent consideration liability for each acquisition as required by U.S. generally accepted accounting principles. This liability is not contingent on future employment and is based on future revenue projections of the respective businesses under various potential scenarios and weighted probability assumptions of these outcomes. This analysis is updated quarterly and changes to the fair value of this liability are recorded in the statements of operations. As a result, we recorded a net benefit of $8.0 million in fiscal 2011 reflecting a net decrease in the fair values of these liabilities comprised of a reduction in the fair value of the Sentinelle Medical liability of $14.3 million due primarily to changes in revenue assumptions offset by a charge of $6.3 million related to Interlace based primarily on the accretion of the liability to the expected payment amount. For additional information, refer to Note 3 to the consolidated financial statements contained in Item 15 of this Annual Report.
Impairment of Goodwill. During the fourth quarter of fiscal 2010, in connection with performing our company-wide annual budgeting and forecasting process, we determined that there were indicators of impairment related to our MammoSite reporting unit, and we recorded intangible asset impairment charges discussed above and below. The fair value of this reporting unit declined from fiscal 2009 primarily due to our reassessment of the overall market size of breast brachytherapy and a reduction in long-term growth projections. After determining the fair values of MammoSite’s long-lived assets, other than goodwill, and writing these assets down to their fair values, we performed the 2-step goodwill impairment test for MammoSite. As a result of this analysis, we recorded a $76.7 million goodwill impairment charge. No other reporting units were deemed to be impaired in fiscal 2010. For additional information, refer to Note 2—“Intangible Assets and Goodwill” to the consolidated financial statements contained in Item 15 of this Annual Report.
Impairment of Intangible Assets. As noted above under Cost of Product Sales—Impairment of Intangible Assets, in fiscal 2010, we determined that the long-lived assets in the MammoSite reporting unit were impaired. As a result of this analysis, we recorded a $20.1 million charge in fiscal 2010 to write down customer relationships and trade name intangible assets to their fair values. For additional information, refer to Note 2—“Intangible Assets and Goodwill” to the consolidated financial statements contained in Item 15 of this Annual Report.
Gain on Sale of Intellectual Property, Net.During the second quarter of fiscal 2011, we received FDA approval of Makena, formerly known as Gestiva, and all rights to Makena were transferred to KV pursuant to our agreement in which we sold the exclusive worldwide rights of Makena to KV. Upon transfer, we received $12.5 million, and including the $79.5 million received in prior years, we recorded a gain on the sale of intellectual property, net of the write-off of certain assets, of $84.5 million in the second quarter of fiscal 2011. For additional information on this arrangement and amounts we may receive in the future, refer to Note 7 to the consolidated financial statements contained in Item 15 of this Annual Report.
Litigation Settlement Charges, Net. In fiscal 2011, we incurred a charge of $0.8 million relating to the settlement of insignificant cases. In fiscal 2010, we incurred litigation settlement charges of $11.4 million primarily relating to our litigation with Ethicon Endo-Surgery, Inc. (“Ethicon”). We had been engaged in litigation in which Ethicon had alleged patent infringement by our ATEC biopsy system of certain of their patents, and Ethicon had made similar claims with respect to our Eviva biopsy system. On February 17, 2010, we entered into a settlement agreement with Ethicon, and all outstanding litigation between the parties was dismissed. In connection with the settlement agreement, we agreed to make a one-time payment to Ethicon of $12.5 million and ongoing royalties for sales of our ATEC and Eviva products, and Ethicon agreed to pay us ongoing royalties for sales of its Mammotome magnetic resonance imaging product.
61
Acquired In-Process Research and Development. During the fourth quarter of fiscal 2010, we acquired certain assets that were determined to have no future alternative use and recorded a $2.0 million charge within our Diagnostics segment.
Restructuring and Divestiture Charges, Net. During the fourth quarter of fiscal 2011, we terminated the employment of certain employees and recorded a severance benefits charge of $0.3 million, which was offset by a gain of $0.4 million related to the sale of an insignificant product line. During the fourth quarter of fiscal 2010, we terminated the employment of certain employees in connection with completing the Sentinelle Medical acquisition and recorded severance and related benefit costs of $0.9 million. During the second quarter of fiscal 2010, we completed the sale of the capital stock of our organic photoconductor drum coating manufacturing operation in Shanghai, China for a net sales price of $3.8 million resulting in a loss on disposal of $0.3 million. As a result of previously closing this facility, we incurred additional net charges of $0.4 million in fiscal 2010.
Interest Income.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Interest Income | | $ | 1,860 | | | $ | 1,278 | | | $ | 582 | | | | 46 | % |
| | | | | | | | | | | | | | | | |
Interest income increased in fiscal 2011 compared to fiscal 2010 primarily due to an increase in cash and cash equivalents.
Interest Expense.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Interest Expense | | $ | (114,846 | ) | | $ | (127,107 | ) | | $ | (12,261 | ) | | | (10 | )% |
| | | | | | | | | | | | | | | | |
In fiscal 2011, interest expense consisted primarily of the interest costs and the related amortization of the debt discount of our Convertible Notes as well as the amortization of deferred financing costs. In fiscal 2010, in addition to the interest expense related to our Convertible Notes, we incurred interest costs and the amortization of deferred financing costs related to our senior secured credit agreement. The amounts outstanding under our senior secured credit agreement were paid off in the third quarter of fiscal 2010, and we terminated the agreement.
Debt Extinguishment Loss.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Debt Extinguishment Loss | | $ | (29,891 | ) | | $ | — | | | $ | (29,891 | ) | | | (100 | )% |
| | | | | | | | | | | | | | | | |
In the first quarter of fiscal 2011, pursuant to separate, privately-negotiated exchange agreements, we retired $450.0 million in aggregate principal of our Convertible Notes for $450.0 million in aggregate principal of new 2.00% Convertible Exchange Senior Notes due 2037. This exchange enabled us to extend the first put date three years to December 15, 2016 from December 13, 2013 as well as the subsequent put dates as disclosed in the Liquidity and Capital Resources section of this Management’s Discussion and Analysis. In consideration, the equity conversion price of the notes was reduced to $23.03 from $38.60, and we must pay the cash coupon for three more years, consistent with extending the first put date, instead of accreting the coupon to the principal as required under the original terms. In connection with this transaction, we recorded a debt extinguishment loss of $29.9 million, which includes the write-off of the pro-rata allocation of deferred financing costs. For additional information, refer to Note 5 to the consolidated financial statements contained in Item 15 of this Annual Report.
Other (Expense) Income, net.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Other (Expense) Income, net | | $ | (4,182 | ) | | $ | 901 | | | $ | (5,083 | ) | | | (564 | )% |
| | | | | | | | | | | | | | | | |
In fiscal 2011, this account was primarily comprised of impairment charges of $2.4 million for cost-method investments, net foreign currency transaction losses of $1.1 million and a loss on cash surrender value of life insurance contracts related to our Nonqualified Deferred Compensation Plan of $0.8 million, which is driven by the underlying changes in the stock market. In fiscal
62
2010, this account was primarily comprised of an increase in the cash surrender value of life insurance contracts related to our Nonqualified Deferred Compensation Plan of $1.3 million and an increase related to non-income tax related government credits of $0.8 million partially offset by an impairment charge of $1.1 million for a cost-method investment.
Provision for Income Taxes.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Provision for Income Taxes | | $ | 70,236 | | | $ | 7,822 | | | $ | 62,414 | | | | 798 | % |
| | | | | | | | | | | | | | | | |
Our effective tax rate for fiscal 2011 was 30.9% of pre-tax earnings compared to 14.2% of the pre-tax loss in fiscal 2010. The effective tax rate in fiscal 2011 was less than the statutory rate primarily due to the reversal of income tax reserves, the domestic manufacturing benefit, and U.S. and Canadian research and development tax credits. The $9.1 million income tax reserve reversal was due to us favorably settling our U.S. federal income tax audit for fiscal years 2007 through 2009 and the statute of limitations expiring in various state and foreign jurisdictions. Our effective tax rate for fiscal 2010 was significantly impacted by the $76.7 million goodwill impairment charge recorded in the fourth quarter, substantially all of which was not deductible for tax purposes.
Segment Results of Operations
Breast Health.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 825,551 | | | $ | 755,542 | | | $ | 70,009 | | | | 9 | % |
| | | | | | | | | | | | | | | | |
Operating Income (Loss) | | $ | 187,970 | | | $ | (93,623 | ) | | $ | 281,593 | | | | 301 | % |
| | | | | | | | | | | | | | | | |
Operating Income (Loss) as a % of Segment Revenue | | | 23 | % | | | (12 | )% | | | | | | | | |
| | | | | | | | | | | | | | | | |
Breast Health revenues increased in fiscal 2011 compared to fiscal 2010 primarily due to a $45.5 million increase in service revenues that was substantially related to additional service contracts for the increased number of digital mammography systems in our installed base and the increase in product revenues of $24.5 million discussed above.
Operating income for this business segment increased $281.6 million in fiscal 2011 compared to fiscal 2010 primarily due to a $220.2 million intangible asset and goodwill impairment charge related to our MammoSite reporting unit recorded in the fourth quarter of fiscal 2010 discussed above. The balance of the increase in fiscal 2011, $61.4 million, was primarily due to increased gross margin on an absolute dollar basis as a result of higher product and service revenues as discussed above and lower operating expenses. Overall the gross margin rate as a percentage of revenues increased to 49.0% in fiscal 2011 compared to 30.7% in fiscal 2010 primarily due to the $123.4 million impairment charge to MammoSite’s developed technology included in fiscal 2010. In addition, in fiscal 2011, there were improvements in service gross margins and lower intangible asset amortization expense resulting from the write-off of MammoSite intangibles in the fourth quarter of fiscal 2010. In fiscal 2010, overall gross margin, excluding the impact of the MammoSite impairment charge, was 47.0%.
Operating expenses for this business segment decreased $108.8 million in fiscal 2011 compared to fiscal 2010 primarily due to the impact of the goodwill and intangible asset charge of $96.8 million recorded in operating expenses in fiscal 2010. Other operating expense decreases in fiscal 2011 included a net benefit of $14.3 million related to adjusting the Sentinelle Medical contingent consideration to fair value, lower clinical trials spending related to our Dimensions 3D tomosynthesis system, lower third-party commissions, lower intangible asset amortization from the write-off of MammoSite intangibles in the fourth quarter of fiscal 2010, and a decrease in litigation costs. In the second quarter of fiscal 2010, we recorded a litigation settlement charge of $12.5 million as discussed above. Partially offsetting these operating expense decreases was the inclusion of a full year of expenses for Sentinelle Medical (acquired in the fourth quarter of fiscal 2010), and higher compensation costs related to hiring additional personnel, annual salary increases, higher bonuses due to improved company performance and higher sales commissions for our breast biopsy business.
Diagnostics.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 571,263 | | | $ | 552,501 | | | $ | 18,762 | | | | 3 | % |
| | | | | | | | | | | | | | | | |
Operating Income | | $ | 170,693 | | | $ | 100,469 | | | $ | 70,224 | | | | 70 | % |
| | | | | | | | | | | | | | | | |
Operating Income as a % of Segment Revenue | | | 30 | % | | | 18 | % | | | | | | | | |
| | | | | | | | | | | | | | | | |
63
Diagnostics revenues increased in fiscal 2011 compared to fiscal 2010 primarily due to the increase in product sales discussed above.
Operating income increased in fiscal 2011 compared to fiscal 2010 primarily due to the net gain of $84.5 million on the sale of the Makena intellectual property to KV in the second quarter of fiscal 2011 discussed above and an increase in gross margin in absolute dollars due to higher revenues. Gross margin rates were 53.9% and 53.4% in fiscal 2011 and 2010, respectively. These increases were partially offset by higher operating expenses attributable to the inclusion of TCT (acquired in the third quarter of fiscal 2011) and related integration costs and contingent consideration compensation expense of $17.6 million, and higher compensation costs related to hiring additional personnel, annual salary increases, and higher bonuses due to improved company performance.
GYN Surgical.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 300,538 | | | $ | 283,142 | | | $ | 17,396 | | | | 6 | % |
| | | | | | | | | | | | | | | | |
Operating Income | | $ | 3,623 | | | $ | 53,071 | | | $ | (49,448 | ) | | | (93 | )% |
| | | | | | | | | | | | | | | | |
Operating Income as a % of Segment Revenue | | | 1 | % | | | 19 | % | | | | | | | | |
| | | | | | | | | | | | | | | | |
GYN Surgical revenues increased in fiscal 2011 compared to fiscal 2010 primarily due to the increase in product sales discussed above.
Operating income decreased in fiscal 2011 compared to fiscal 2010 primarily due to the inclusion of Interlace’s operations (acquired in the second quarter of fiscal 2011), including intangible asset amortization expense, a charge of $6.3 million to adjust the Interlace contingent consideration liability to fair value and a charge of $2.1 million to record Interlace contingent consideration compensation expense as payments were tied to continuing employment. Overall, gross margin in absolute dollars decreased slightly in fiscal 2011 compared to fiscal 2010. This segment’s gross margin rates declined to 56.8% compared to 61.0% in fiscal 2010, primarily due to higher intangible asset amortization expense. In addition, this segment incurred higher operating expenses, principally in sales and marketing for increased advertising of the NovaSure system, including expenditures related to our direct-to-consumer advertising campaign, an increase in compensation and benefits related to hiring additional personnel, annual salary increases, and higher bonuses due to improved company performance, increased amortization expense from intangible assets, and higher project expense on the development of next generation products. In addition, the first quarter of fiscal 2010 included the reversal of stock compensation due to the departure of a senior executive.
Skeletal Health.
| | | | | | | | | | | | | | | | |
| | | Years Ended | |
| | | September 24, 2011 | | | September 25, 2010 | | | Change | |
| | | Amount | | | Amount | | | Amount | | | % | |
Total Revenues | | $ | 91,997 | | | $ | 88,367 | | | $ | 3,630 | | | | 4 | % |
| | | | | | | | | | | | | | | | |
Operating Income | | $ | 12,159 | | | $ | 10,020 | | | $ | 2,139 | | | | 21 | % |
| | | | | | | | | | | | | | | | |
Operating Income as a % of Segment Revenue | | | 13 | % | | | 11 | % | | | | | | | | |
| | | | | | | | | | | | | | | | |
Skeletal Health revenues increased in fiscal 2011 compared to fiscal 2010 primarily due to the increase in product sales discussed above.
Operating income increased in fiscal 2011 compared to fiscal 2010 primarily due to the increase in revenues and improvement in gross margin rates to 43.5% compared to 42.4% in the prior year. Operating expenses remained relatively flat.
LIQUIDITY AND CAPITAL RESOURCES
At September 29, 2012, we had $901.7 million of working capital, and our cash and cash equivalents totaled $560.4 million. Our cash and cash equivalents balance decreased by $151.9 million during fiscal 2012 due to funds used for the Gen-Probe acquisition
64
and other investing activities primarily related to payments for contingent consideration, capital expenditures and placement of equipment under customer usage agreements. During fiscal 2012, our sources of funds included our borrowings of $3.48 billion to fund a portion of the purchase price for Gen-Probe, cash generated from operations and net proceeds from stock option exercises.
In fiscal 2012, our operating activities provided us with $370.2 million of cash, which included a net loss of $73.6 million offset by non-cash charges for depreciation and amortization aggregating $345.8 million, non-cash interest expense of $75.0 million related to our outstanding debt, a debt extinguishment loss of $42.3 million, stock-based compensation expense of $40.6 million, fair value adjustments of $38.5 million for our contingent consideration liabilities related to our Interlace and Sentinelle Medical acquisitions, fair value adjustments related to Gen-Probe acquired inventory sold of $19.9 million, non-cash restructuring charges of $16.9 million primarily related to our decision to discontinue selling our Adiana product, and a goodwill impairment charge of $5.8 million related to our MammoSite reporting unit. These adjustments to net loss were partially offset by a decrease in net deferred tax liabilities of $155.2 million, primarily the result of recapturing taxes from the debt extinguishment and amortization of intangible assets, and the gain on sale of intellectual property of $12.4 million. Cash provided by operations included a net cash inflow of $28.2 million from changes in our operating assets and liabilities. Changes in our operating assets and liabilities were driven primarily by a reduction of prepaid expenses and other current assets of $69.8 million principally due to receiving tax withholding amounts recorded in Gen-Probe’s opening balance sheet in purchase accounting related to the pay-off of restricted stock units and shares and acceleration of stock options in connection with the Gen-Probe acquisition. In addition, deferred revenue increased $12.7 million as we continue to convert our increasing installed base of digital mammography systems to service contracts upon expiration of the warranty period, and we received net cash of $6.1 million from an income tax receivable acquired from Gen-Probe. Partially offsetting these cash inflows was a decrease in accrued expenses of $41.0 million, primarily due to payments of taxes withheld from equity awards in connection with the Gen-Probe acquisition, income taxes, and contingent consideration recorded as compensation expense, partially offset by compensation related accruals and interest on our debt. In addition, inventory increased $12.2 million primarily due to an increase in components on hand to support higher sales volume, the introduction of new products and last-time buys, and accounts receivable increased $11.0 million primarily due to higher revenues in the fourth quarter of fiscal 2012 compared to the fourth quarter of fiscal 2011.
In fiscal 2012, we used $3.85 billion of cash for investing activities. This use of cash was primarily attributable to our purchase of Gen-Probe utilizing $3.76 billion, net of cash acquired. Cash used in investing also related to $78.8 million for purchases of property and equipment, which consisted primarily of the placement of equipment and instruments under customer usage agreements and manufacturing equipment and computer hardware, the payment of contingent consideration to the former shareholders of Adiana of $8.8 million, an increase in restricted cash of $5.2 million related to an international customer deposit, and the acquisition of in-process research and development assets of $4.5 million. Partially offsetting these uses of cash was the receipt of $12.5 million for a scheduled payment under our amended agreement in which we sold the rights to our Makena intellectual property to KV.
In fiscal 2012, our financing activities provided us net cash of $3.33 billion, primarily reflecting our borrowings of $3.48 billion to fund our Gen-Probe acquisition, which was comprised of $2.48 billion of net proceeds under our Credit Agreement and $1.0 billion under our senior notes, which are described below, and cash proceeds from employee stock plans of $28.6 million. These financing proceeds were partially offset by payments for debt issuance costs of $81.4 million related to the Credit Agreement, Senior Notes and 2012 Notes, contingent consideration of $51.7 million related to our Interlace and Sentinelle acquisitions, deferred payments to the TCT shareholders of $44.2 million, and $5.7 million for employee-related taxes withheld for the net share settlement of vested restricted stock units. Under ASC 805, the payment of contingent consideration recorded at fair value in purchase accounting as of the acquisition date is treated as a financing activity.
Debt
We had total recorded debt outstanding of $5.04 billion at September 29, 2012, which is comprised of our amounts outstanding under our Credit Agreement of $2.48 billion (principal $2.5 billion), Senior Notes of $1.0 billion and Convertible Notes of $1.56 billion (principal $1.725 billion).
Concurrent with closing the Gen-Probe acquisition, on August 1, 2012, we and certain domestic subsidiaries (the “Guarantors”) entered into a credit and guaranty agreement (the “Credit Agreement”) with Goldman Sachs Bank USA, in its capacity as administrative and collateral agent, and the lenders party thereto.
The credit facilities under the Credit Agreement consist of:
| | • | | $1.0 billion senior secured tranche A term loan (“Term Loan A”) with a final maturity date of August 1, 2017; |
| | • | | $1.5 billion secured tranche B term loan (“Term Loan B”) with a final maturity date of August 1, 2019; and |
| | • | | $300.0 million secured revolving credit facility (“Revolving Facility”) with a final maturity date of August 1, 2017. |
Pursuant to the terms and conditions of the Credit Agreement, the lenders have committed to provide senior secured financing in an aggregate amount of up to $2.8 billion. On August 1, 2012 concurrently with the closing of the Gen-Probe acquisition, we borrowed $2.5 billion aggregate principal under the Credit Agreement.
65
The Guarantors have guaranteed our obligations under the credit facilities, and the credit facilities are secured by first-priority liens on, and a first-priority security interest in, substantially all of our assets and the assets of the Guarantors, including all of the capital stock of substantially all of the U.S. subsidiaries owned by us and the Guarantors, 65% of the capital stock of certain of our first-tier foreign subsidiaries and all intercompany debt. The security interests are evidenced by a pledge and security agreement by and among Goldman Sachs Bank USA, as collateral agent, us and the Guarantors and other related agreements, including certain intellectual property security agreements and mortgages.
We are required to make scheduled principal payments under Term Loan A in increasing amounts ranging from $12.5 million per three month period beginning October 31, 2012 to $50.0 million per three month period commencing October 31, 2015, and under Term Loan B in equal installments of $3.75 million per three month period beginning on October 31, 2012 and for 27 three month periods thereafter. The remaining balance for each term loan is due at maturity. Any amounts outstanding under the Revolving Facility are due at maturity. We are required to make principal repayments first, pro rata among the term loan facilities, and second to the Revolving Facility from specified excess cash flows from operations and from the net proceeds of specified types of asset sales, debt issuances, insurance recoveries and equity offerings. Subject to certain limitations, we may voluntarily prepay any of the credit facilities without premium or penalty.
All amounts outstanding under the Credit Agreement will bear interest, at our option, initially, with respect to all loans made under Term Loan A and the Revolving Facility: (i) at the Base Rate plus 2.00% per annum, or (ii) at the Adjusted Eurodollar Rate (i.e., the Libor rate) plus 3.00%, and with respect to loans made under Term Loan B: (i) at the Base Rate, with a floor of 2.00%, plus 2.50%, or (ii) at the Adjusted Eurodollar Rate, with a floor of 1.00% plus 3.50%. The applicable margin to the Base Rate or Eurodollar Rate on Term Loan A and the Revolving Facility are subject to specified changes depending on the total net leverage ratio as defined in the Credit Agreement. We are required to pay a quarterly commitment fee at an annual rate of 0.50% on the undrawn committed amount available under the Revolving Facility (which rate is subject to reduction depending on the total net leverage ratio as defined in the Credit Agreement).
The Credit Agreement contains affirmative and negative covenants customarily applicable to senior secured credit facilities, including covenants restricting the ability of the Company and the guarantors, subject to negotiated exceptions, to: incur additional indebtedness and additional liens on their assets, engage in mergers or acquisitions or dispose of assets, enter into sale-leaseback transactions, pay dividends or make other distributions, voluntarily prepay other indebtedness, enter into transactions with affiliated persons, make investments, and change the nature of their businesses.
The credit facilities contain total net leverage ratio and interest coverage ratio financial covenants measured as of the last day of each fiscal quarter, beginning with our first quarter of fiscal 2013. The total net leverage ratio is 7.00:1.00 beginning on our fiscal quarter ending December 29, 2012, and then decreases over time to 4.00:1.00 for the quarter ending September 30, 2017 and each fiscal quarter thereafter. The interest coverage ratio is 3.25:1.00 beginning on our fiscal quarter ending December 29, 2012, and then increases over time to 3.75:1.00 for the fiscal quarter ending September 30, 2017 and each quarter thereafter. The total net leverage ratio is defined as the ratio of our consolidated net debt as of the quarter end to our consolidated adjusted EBITDA for the four-fiscal quarter period ending on the measurement date. The interest coverage ratio is defined as the ratio of our consolidated adjusted EBITDA for the prior four-fiscal quarter period ending on the measurement date to adjusted consolidated cash interest expense for the same measurement period. These terms, and the calculation thereof, are defined in further detail in the Credit Agreement.
On August 1, 2012, we completed a private placement of $1.0 billion aggregate principal amount of our Senior Notes at an offering price of 100% of the aggregate principal amount of the Senior Notes. The Senior Notes were not registered under the Securities Act or any state securities laws, and were offered only to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the United States in accordance with Regulation S under the Securities Act. The Senior Notes are our general senior unsecured obligations and are guaranteed on a senior unsecured basis by the Guarantors.
On August 1, 2012, we, together with the Guarantors, entered into an indenture with Wells Fargo Bank, National Association, as trustee, relating to the Senior Notes. The Senior Notes mature on August 1, 2020 and bear interest at the rate of 6.25% per year, payable semi-annually on February 1 and August 1 of each year, commencing on February 1, 2013.
The indenture contains covenants which limit, among other things, our and certain of our subsidiaries’ ability to incur additional indebtedness, pay dividends or repurchase or redeem capital stock, make certain investments, incur liens, enter into certain types of transactions with our affiliates, and sell assets or consolidate or merge with or into other companies. These covenants are subject to a number of exceptions and qualifications.
We may redeem up to 35% of the aggregate principal amount of the Senior Notes with the net cash proceeds of certain equity offerings at any time and from time to time before August 1, 2015, at a redemption price equal to 106.250% of the aggregate principal amount so redeemed, plus accrued and unpaid interest, if any, to the redemption date. We also have the option to redeem the Senior Notes on or after: August 1, 2015 through July 31, 2016 at 103.125% of par; August 1, 2016 through July 31, 2017 at 102.083% of par; August 1, 2017 through July 31, 2018 at 101.042% of par; and August 1, 2018 and thereafter at 100% of par. In addition, if we undergo a change of control, as provided in the indenture, we will be required to make an offer to purchase each holder’s Senior Notes at a price equal to 101% of the aggregate principal amount of the Senior Notes, plus accrued and unpaid interest, if any, to the repurchase date.
66
On August 1, 2012, in connection with the issuance of the Senior Notes, we, together with the Guarantors, entered into an exchange and registration rights agreement with the initial purchasers of the Senior Notes. Pursuant to the terms of the registration rights agreement, we, together with the Guarantors, agreed to (i) file a registration statement covering an offer to exchange the Senior Notes for a new issue of identical exchange notes registered under the Securities Act on or before 180 days from August 1, 2012, (ii) use commercially reasonable efforts to cause such registration statement to become effective, and (iii) use commercially reasonable efforts to complete the exchange prior to 270 days after August 1, 2012. Under certain circumstances, we, together with the Guarantors, may be required to provide a shelf registration statement to cover resales of the Senior Notes.
Convertible Notes.
At September 29, 2012, our convertible notes, in the aggregate principal amount of $1.725 billion, are recorded at $1.56 billion, which is net of the unamortized debt discount attributed to the embedded conversion feature of the convertible notes. These notes consist of:
| | • | | $775 million of our 2.00% Convertible Senior Notes due 2037 issued in December 2007 (the “2007 Notes”); |
| | • | | $450 million of our 2.00% Convertible Exchange Senior Notes due 2037 issued in November 2010 (the “2010 Notes”); and |
| | • | | $500 million of our 2.00% Convertible Senior Notes due 2042 issued in March 2012 (the “2012 Notes”). |
The 2012 Notes were issued on March 5, 2012 pursuant to agreements entered into on February 29, 2012 in exchange for an equal principal amount of the 2007 Notes.
Holders may require us to repurchase the 2007 Notes on December 13, 2013, and on each of December 15, 2017, 2022, 2027 and 2032, or upon a fundamental change, as provided in the indenture for the 2007 Notes, at a repurchase price equal to 100% of their accreted principal amount, plus accrued and unpaid interest.
Holders may require us to repurchase the 2010 Notes on each of December 15, 2016, 2020, 2025, on December 13, 2030 and on December 14, 2035 or upon a fundamental change, as provided in the indenture for the 2010 Notes, at a repurchase price equal to 100% of their accreted principal amount, plus accrued and unpaid interest.
Holders may require us to repurchase the 2012 Notes on each of March 1, 2018, 2022, 2027 and 2032, and on March 2, 2037 or upon a fundamental change, as provided in the indenture for the 2012 Notes, at a repurchase price equal to 100% of their accreted principal amount, plus accrued and unpaid interest.
We may redeem any of the 2007 Notes, 2010 Notes and 2012 Notes beginning December 13, 2013, December 19, 2016, and March 6, 2018, respectively. We may redeem all or a portion of the 2007 Notes, 2010 Notes, and 2012 Notes (i.e., in cash or a combination of cash and shares of our common stock) at a redemption price equal to 100% of their principal amount, plus accrued and unpaid interest to, but excluding, the redemption date.
We have recorded deferred tax liabilities related to the convertible notes original issuance discount, representing the spread between the cash coupon rate and the higher interest rate deductible for tax purposes. When our convertible notes are extinguished, we are required to recapture the original issuance discount previously deducted for tax purposes. The 2007 Notes first put date is December 13, 2013 and the estimated tax due if the 2007 Notes are put to us on this date is approximately $144 million.
For additional information on the terms of our indebtedness, see Note 5 to the consolidated financial statements contained in Item 15 of this Annual Report.
Contingent Earn-Out Payments.
In connection with certain of our acquisitions, we have incurred the obligation to make contingent earn-out payments tied to performance criteria, principally revenue growth of the acquired businesses over a specified period. In certain circumstances, such as a change of control, a portion of these obligations may be accelerated. In addition, contractual provisions relating to these contingent earn-out obligations may include covenants to operate the acquired businesses in a manner that may not otherwise be most advantageous to us. These provisions may also result in the risk of litigation relating to the calculation of the amount due or our operation of the acquired business. Such litigation could be expensive and divert management attention and resources. Our obligation to make contingent payments may also result in significant operating expenses. Depending upon the particular facts and circumstances giving rise to the payment and our previous estimates, all or a portion of these payments may be required to be expensed by us when accrued. For example, our contingent earn-out obligations payable in connection with the TCT acquisition will be fully expensed as accrued because our obligation to make these payments is conditioned on the continued employment of certain key employees of TCT.
67
Our contingent consideration arrangements are recorded as either additional purchase price or compensation expense if continuing employment is required to receive such payments. Pursuant to ASC 805, contingent consideration that is deemed to be part of the purchase price is recorded as a liability based on the estimated fair value of the consideration we expect to pay to the former shareholders of the acquired business as of the acquisition date. This liability is re-measured each reporting period with the change in fair value recorded through a separate line item within our Consolidated Statements of Operations. Increases or decreases in the fair value of contingent consideration liabilities can result from accretion of the liability for the passage of time, changes in discount rates, and changes in the timing, probabilities and amount of revenue estimates. Contingent consideration arrangements from acquisitions completed prior to the adoption of ASC 805 (effective in fiscal 2010 for us) that are deemed to be part of the purchase price of the acquisition are not subject to the fair value measurement requirements of ASC 805 and are recorded as additional purchase price to goodwill.
In connection with the acquisition of Adiana, Inc., we have an obligation to the former Adiana shareholders to make contingent payments tied to the achievement of milestones. The milestone payments include potential contingent payments of up to $155.0 million based on worldwide sales of the Adiana Permanent Contraception System in the first year following FDA approval and on annual incremental sales growth thereafter through December 31, 2012. FDA approval of the Adiana system occurred on July 6, 2009, and we began accruing contingent consideration in the fourth quarter of fiscal 2009 based on the defined percentage of worldwide sales of the product. Since this contingent consideration obligation arose from an acquisition prior to the adoption of ASC 805, the amounts accrued are recorded as additional purchase price to goodwill. The purchase agreement includes an indemnification provision that provides for the reimbursement of qualifying legal expenses and liabilities associated with legal claims against the Adiana products and intellectual property, and we have the right to offset contingent consideration payments to the Adiana shareholders with these qualifying legal costs. We made payments of $8.8 million and $19.7 million in fiscal 2012 and 2011, respectively, to the former Adiana shareholders, net of amounts withheld for the legal indemnification provision. We had been in litigation with Conceptus regarding certain intellectual property matters related to the Adiana system, and to the extent available, we have been recording legal fees related to the Conceptus litigation matter as a reduction to the accrued contingent consideration payments. No contingent consideration was earned and recorded in fiscal 2012, and as of the end of the second quarter of fiscal 2012 the Company decided to discontinue the manufacture, marketing and sales of the Adiana system. On October 17, 2011, the jury returned a verdict in the Conceptus litigation matter (see below) in favor of Conceptus awarding damages in the amount of $18.8 million. On April 29, 2012, the Company entered into a license and settlement agreement with Conceptus in which Conceptus agreed to forgo the $18.8 million jury award in consideration of the Company agreeing to a permanent injunction against the manufacture, sale and distribution of the Adiana product. At September 29, 2012, the Company has accrued $16.8 million for the payment of contingent consideration to the former Adiana shareholders, which is net of amounts withheld for qualifying legal costs. As a result of our decision to discontinue sales of the Adiana product in the second quarter of fiscal 2012, on account of our determination that the product was not financially viable and would not become so in the foreseeable future, we do not expect to incur any further contingent payment obligations to the former Adiana shareholders.
We also have contingent consideration obligations related to our Sentinelle Medical, Interlace, TCT and Healthcome acquisitions. Pursuant to ASC 805, contingent consideration pertaining to Sentinelle Medical and Interlace is required to be recorded as a liability at fair value and the adjustments to fair value are recorded in the Consolidated Statement of Operations. Contingent consideration pertaining to TCT and Healthcome is contingent upon future employment and is being recorded as compensation expense as it is earned over the respective service periods. For additional information pertaining to the acquisitions, contingent consideration terms and the assumptions used to fair value contingent consideration, refer to Note 3 to the consolidated financial statements contained in Item 15 of this Annual Report.
A summary of amounts recorded to the Consolidated Statement of Operations in fiscal 2012 and fiscal 2011 are as follows:
| | | | | | | | | | | | | | | | | | | | |
Statement of Operations Line Item – September 29, 2012 | | Sentinelle
Medical | | | Interlace | | | TCT | | | Healthcome | | | Total | |
Contingent consideration—compensation expense | | $ | — | | | $ | — | | | $ | 75,459 | | | $ | 5,572 | | | $ | 81,031 | |
Contingent consideration—fair value adjustments | | | (3,364 | ) | | | 41,830 | | | | — | | | | — | | | | 38,466 | |
| | | | | | | | | | | | | | | | | | | | |
| | $ | (3,364 | ) | | $ | 41,830 | | | $ | 75,459 | | | $ | 5,572 | | | $ | 119,497 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Statement of Operations Line Item – September 24, 2011 | | Sentinelle
Medical | | | Interlace | | | TCT | | | Healthcome | | | Total | |
Contingent consideration—compensation expense | | $ | — | | | $ | 2,102 | | | $ | 17,581 | | | $ | 319 | | | $ | 20,002 | |
Contingent consideration—fair value adjustments | | | (14,328 | ) | | | 6,312 | | | | — | | | | — | | | | (8,016 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | $ | (14,328 | ) | | $ | 8,414 | | | $ | 17,581 | | | $ | 319 | | | $ | 11,986 | |
| | | | | | | | | | | | | | | | | | | | |
68
In connection with our acquisition of Sentinelle Medical, we have an obligation to the former stockholders to make contingent payments over a two-year period of up to a maximum of $250.0 million based on a multiple of incremental revenue growth during the two-year period following the completion of the acquisition. We made payments of $4.1 million and $4.3 million in the first quarter of fiscal 2012 and the third quarter of fiscal 2011, respectively. At September 29, 2012, this liability was recorded at $3.4 million.
In connection with our acquisition of Interlace, we have an obligation to the former stockholders to make contingent payments over a two-year period up to a maximum payout of $225.0 million based on a multiple of incremental revenue growth during the two-year period following the completion of the acquisition. During the second quarter of fiscal 2012, the first measurement period lapsed resulting in a total contingent amount recorded for this period of $51.8 million, which was disbursed to the former shareholders of Interlace, net of amounts withheld for certain legal indemnification purposes. At September 29, 2012, this liability was recorded at $83.0 million.
Under the sale and purchase agreement for TCT, $35.0 million of the purchase price has been deferred for one year from the date of the acquisition. This amount plus a portion of the working capital adjustment of $8.5 million were paid in the fourth quarter of fiscal 2012. An additional $4.7 million working capital adjustment payment is due upon the completion of fiscal 2013. In addition, we have an obligation to certain of the former shareholders, based on future employment, to make contingent payments over a two year period not to exceed $200.0 million less the deferred payment of $35.0 million. The first earn-out payment of $54.0 million was made in the fourth quarter of fiscal 2012. At September 29, 2012, we have accrued $39.1 million for the second contingent earn-out payment.
In connection with our acquisition of Healthcome, we have an obligation to the former shareholders to make contingent payments of $5.0 million over the next two fiscal years. At September 29, 2012, we have accrued $5.0 million for these contingent payments.
Contractual Obligations
The following table summarizes our contractual obligations and commitments as of September 29, 2012:
| | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
Contractual Obligations | | Less than
1 year | | | 1-3 years | | | 3-5 years | | | More than
5 years | | | Total | |
Long-Term Debt Obligations (1) | | $ | 65,000 | | | $ | 955,000 | | | $ | 1,280,000 | | | $ | 2,925,000 | | | $ | 5,225,000 | |
Interest on Long-Term Debt Obligations | | | 195,574 | | | | 360,151 | | | | 324,129 | | | | 298,465 | | | | 1,178,319 | |
Operating Leases | | | 21,415 | | | | 31,459 | | | | 21,474 | | | | 42,871 | | | | 117,219 | |
Financing Leases (2) | | | 2,764 | | | | 5,705 | | | | 6,173 | | | | 3,225 | | | | 17,867 | |
Accrued Contingent Consideration (3) | | | 143,881 | | | | 3,322 | | | | — | | | | — | | | | 147,203 | |
Deferred acquisition payments | | | 1,655 | | | | 4,677 | | | | — | | | | — | | | | 6,332 | |
Purchase Obligations (4) | | | 63,654 | | | | 10,403 | | | | 642 | | | | — | | | | 74,699 | |
Collaborative commitments (5) | | | 450 | | | | 814 | | | | 659 | | | | 10 | | | | 1,933 | |
Minimum royalty commitments (6) | | | 1,985 | | | | 4,540 | | | | 1,570 | | | | 6,020 | | | | 14,115 | |
Pension Obligations (7) | | | 347 | | | | 751 | | | | 809 | | | | 7,837 | | | | 9,744 | |
| | | | | | | | | | | | | | | | | | | | |
Total Contractual Obligations | | $ | 496,725 | | | $ | 1,376,822 | | | $ | 1,635,456 | | | $ | 3,283,428 | | | $ | 6,792,431 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Included within long-term debt obligations, we have three issuances (2007 Notes, 2010 Notes and 2012 Notes) of Convertible Notes which can first be put to us on December 13, 2013 ($775 million principal), December 15, 2006 ($450 million principal) and March 1, 2018 ($500 million principal), and we have assumed for purpose of the above table that the principal amounts for each issuance will be paid off when they first can be put to us, which is in fiscal 2014, fiscal 2017 and fiscal 2018, respectively. The amounts in the table do not include deferred tax liabilities for the recapture of the original issuance discount. See Convertible Notes above. |
| (2) | The financing leases represent two leases for an office building and a manufacturing facility, which were required to be recorded on our balance sheet under US GAAP. See Note 12 to our consolidated financial statements contained in Item 15 of this Annual Report for more information. |
| (3) | Amounts represent those recorded in accrued expenses and other long-term liabilities on our consolidated balance sheet. See Contingent Earn-Out Payments for a more complete description of our contingent earn-out obligations. |
69
| (4) | Purchase obligations primarily represent minimum purchase commitments for inventory and instruments, and to a lesser extent, other operating expense commitments. |
| (5) | In addition to the minimum payments due under our collaborative agreements included above, we may be required to pay up to $4.8 million in milestone payments, plus royalties on net sales of any products using specified technology. |
| (6) | Amounts represent minimum royalties due on net sales of products incorporating licensed technology and subject to a minimum annual royalty payment. |
| (7) | Pension obligations do not include our obligation under our deferred compensation plans of $32.1 million, which is recorded as a current liability. Deferred compensation plan benefits are generally paid out at retirement or termination of employment. |
The above table does not reflect our long-term liabilities associated with uncertain tax positions recorded under FIN 48 (codified primarily in ASC 740,Income Taxes) totaling $38.9 million. Due to the complexity associated with tax uncertainties, we cannot reasonably make a reliable estimate of the period in which we expect to settle these non-current liabilities. See Note 8 to our consolidated financial statements contained in Item 15 of this Annual Report for more information on our unrecognized tax benefits. In addition, certain of our cost method equity investments give us the option to acquire the company in the future. Since it is not possible to estimate when, or even if, we will exercise our option to acquire these companies, we have not included these future potential payments in the table above.
Future Liquidity Considerations
We expect to continue to review and evaluate potential acquisitions of businesses, products or technologies, and strategic alliances that we believe will complement our current or future business. Subject to the Risk Factors set forth in Part I, Item 1A of this Annual Report and the general disclaimers set forth in our Special Note Regarding Forward-Looking Statements at the outset of this Annual Report, we believe that cash flow from operations and the cash available under our Revolving Facility will provide us with sufficient funds in order to fund our expected normal operations, debt payments, including interest, and contingent consideration obligations over the next twelve months. Our longer-term liquidity is contingent upon future operating performance. We may also require additional capital in the future to fund capital expenditures, repayment of debt, contingent consideration obligations, acquisitions or other investments, or to repay our convertible notes and related deferred tax liabilities. As described above, we have significant indebtedness outstanding under our Credit Agreement, Senior Notes and convertible notes. These capital requirements could be substantial. Our operating performance may also be affected by matters discussed under the above-referenced Risk Factors elsewhere in this report. These risks, trends and uncertainties may also adversely affect our long-term liquidity.
Legal Contingencies
We are currently involved in certain legal proceedings and claims. In connection with these legal proceedings and claims, management periodically reviews estimates of potential costs to be incurred by us in connection with the adjudication or settlement, if any, of these proceedings. These estimates are developed in consultation with outside counsel and are based on an analysis of potential litigation outcomes and settlement strategies. In accordance with ASC 450,Contingencies, loss contingencies are accrued if, in the opinion of management, an adverse outcome is probable and such outcome can be reasonably estimated. It is possible that future results for any particular quarter or annual period may be materially affected by changes in our assumptions or the effectiveness of our strategies relating to these proceedings.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to revenue recognition for multiple element arrangements, allowance for doubtful accounts, reserves for excess and obsolete inventories, valuations, purchase price allocations and contingent consideration related to business combinations, expected future cash flows including growth rates, discount rates, terminal values and other assumptions used to evaluate the recoverability of long-lived assets and goodwill, estimated fair values of intangible assets and goodwill, amortization methods and periods, warranty reserves, certain accrued expenses, restructuring and other related charges, stock-based compensation, contingent liabilities, tax reserves and recoverability of our net deferred tax assets and related valuation allowances. We base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances. Actual results could differ from these estimates if past experience or other assumptions do not turn out to be substantially accurate. Any differences may have a material impact on our financial condition and results of operations.
The following is a discussion of what we believe to be the more significant critical accounting policies and estimates used in the preparation of our consolidated financial statements.
70
Inventory
Our inventories include material, labor and overhead, and are stated at the lower of cost (first-in, first-out) or market. As a developer and manufacturer of high technology medical equipment, we may be exposed to a number of economic and industry factors that could result in portions of our inventory becoming either obsolete or in excess of anticipated usage. These factors include, but are not limited to, technological changes in our markets, our ability to meet changing customer requirements, competitive pressures on products and prices, reliability and replacement of and the availability of key components from our suppliers. Our policy is to establish inventory reserves when conditions exist that suggest that our inventory may be in excess of anticipated demand or is obsolete based upon our assumptions about future demand for our products and market conditions. We regularly evaluate our ability to realize the value of our inventory based on a combination of factors including the following: historical usage rates, forecasted sales or usage, product expiration or end of life dates, estimated current and future market values and new product introductions. Assumptions used in determining our estimates of future product demand may prove to be incorrect, in which case the provision required for excess and obsolete inventory would have to be adjusted in the future. If inventory is determined to be overvalued, excess or obsolete, we would be required to record impairment charges within cost of goods sold at the time of such determination. Although considerable effort is made to ensure the accuracy of our forecasts of future product demand, any significant unanticipated changes in demand or expected usage could have a significant negative impact on the value of our inventory and our operating results. When recorded, our reserves are intended to reduce the carrying value of our inventory to its net realizable value.
Accounts Receivable Reserves
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We regularly evaluate the collectability of our trade receivables based on a combination of factors, including a dialogue with the customer to determine the cause of non-payment, and evaluation of the customer’s current financial situation. In the event it is determined that the customer may not be able to meet its full obligation to us, we record a specific allowance to reduce the receivable to the amount that we expect to recover given all information present. We perform ongoing credit evaluations of our customers and adjust credit limits based upon payment history and our assessment of the customer’s current credit worthiness. We continuously monitor collections from our customers and maintain a provision for estimated credit losses based upon our historical experience and any specific customer collection issues that we have identified. While such credit losses have historically been within our expectations and the provisions established, we cannot guarantee that we will continue to experience the same credit loss rates in the future. If the financial condition of our customers were to deteriorate, for example as a result of the ongoing financial and economic uncertainty or otherwise, resulting in an impairment of their ability to make payments, additional allowances may be required.
We also record a provision for estimated sales returns and allowances on product and service related sales in the same period as the related revenues are recorded. These estimates are based on the specific facts and circumstances of particular orders, analysis of credit memo data and other known factors. If the data we use to calculate these estimates do not properly reflect reserve requirements, then a change in the allowances would be made in the period in which such a determination is made and revenues in that period could be adversely affected.
Business Combinations
We record tangible and intangible assets acquired and liabilities assumed in business combinations under the purchase method of accounting. Amounts paid for each acquisition are allocated to the assets acquired and liabilities assumed based on their fair values at the dates of acquisition. As a result of our adoption of ASC 805 in fiscal 2010, contingent consideration, which is not deemed to be linked to continuing employment, is recorded at fair value as measured on the date of acquisition. The value recorded is based on estimates of future financial projections under various potential scenarios, which are generally probability weighted as to the outcome of each scenario. These cash flow projections are discounted with an appropriate risk adjusted rate. Quarterly until such contingent amounts are earned, the fair value of the liability is reassessed at each reporting period and adjusted as a component of operating expenses based on changes to the underlying assumptions. The estimates used to determine the fair value of the contingent consideration liability are subject to significant judgment and actual results are likely to differ from the amounts originally recorded.
The fair value of identifiable intangible assets is based on detailed valuations that use information and assumptions provided by management, which consider management’s best estimates of inputs and assumptions that a market participant would use. We allocate any excess purchase price over the fair value of the net tangible and intangible assets acquired and liabilities assumed to goodwill. The valuation of purchased research and development represents the estimated fair value at the date of acquisition related to in-process projects. Our purchased research and development represents the value of in-process projects that have not yet reached technological feasibility and have no alternative future uses as of the date of acquisition. As a result of our adoption of ASC 805, we capitalize these assets and record them in our consolidated balance sheet. Under ASC 805, in-process research and development assets are evaluated for impairment similar to goodwill and once the project is complete, if at all, the asset is amortized over its remaining useful life. Prior to the adoption of ASC 805, we expensed the value attributable to these in-process projects at the time of acquisition. If the projects are not successful or completed in a timely manner, we may not realize the financial benefits expected for these projects or for the acquisitions as a whole and impairments may result.
71
We use the income approach to determine the fair values of our purchased research and development. This approach determines fair value by estimating the after-tax cash flows attributable to an in-process project over its useful life and then discounting these after-tax cash flows back to a present value. We base our revenue assumptions on estimates of relevant market sizes, expected market growth rates, expected trends in technology and expected product introductions by competitors. In arriving at the value of the in-process projects, we consider, among other factors, the in-process projects’ stage of completion, the complexity of the work completed as of the acquisition date, the costs already incurred, the projected costs to complete, the contribution of core technologies and other acquired assets, the expected introduction date and the estimated useful life of the technology. We base the discount rate used to arrive at a present value as of the date of acquisition on the time value of money and medical technology investment risk factors. We believe that the estimated purchased research and development amounts so determined represent the fair value at the date of acquisition and do not exceed the amount a third-party would pay for the projects.
We have also used the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including developed technology, customer relationships and contracts, and trade names. Developed technology represents patented and unpatented technology and know-how. Customer relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Trade names represent acquired company and product names. For business licenses, we use a combination of the lost profits method and replacement cost method to value such assets. Business licenses allow us to conduct business in a certain country, namely China.
With respect to property, plant and equipment, we estimate the fair value of these assets using a combination of the cost and market approaches, depending on the component. Generally, we apply the cost approach as the primary method in estimating the fair value of land and buildings as the market approach is less reliable based on potential significant differences between the property being valued and the potentially comparable sales of similar properties.
Intangible Assets and Goodwill
Intangible Assets
We amortize our intangible assets that have finite lives using either the straight-line method or, if reliably determinable, based on the pattern in which the economic benefit of the asset is expected to be consumed. The economic pattern is based on undiscounted future cash flows. Amortization is recorded over the estimated useful lives ranging from 2 to 30 years. We review our intangible assets subject to amortization to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment or a change in the remaining useful life. If the carrying value of an asset exceeds its undiscounted cash flows, we will write-down the carrying value of the intangible asset to its fair value in the period identified. In assessing fair value, we must make assumptions regarding estimated future cash flows and discount rates. If these estimates or related assumptions change in the future, we may be required to record impairment charges. We generally calculate fair value as the present value of estimated future cash flows to be generated by the asset using a risk-adjusted discount rate. If the estimate of an intangible asset’s remaining useful life is changed, we will amortize the remaining carrying value of the intangible asset prospectively over the revised remaining useful life.
During the fourth quarter of fiscal 2012 in connection with the company-wide annual budgeting and strategic planning process, we determined that indicators of impairment existed in our MammoSite reporting unit, which is included in the Breast Health reportable segment. The impairment indicators were due to a reduction in our revenue projections and long-term growth rates as a result of the continuing deterioration of the brachytherapy market and competition from existing technologies. Our cash flow estimates were based upon historical cash flows, as well as future projected cash flows derived from the company-wide annual planning process. The analysis indicated that MammoSite’s long-lived assets were recoverable based on the undiscounted cash flows over the remaining life of the predominant long-lived asset. We believe that our procedures for estimating future cash flows were reasonable and consistent with market conditions at the measurement date.
During the fourth quarter of fiscal 2010, in connection with our company-wide annual budgeting and strategic planning process, we determined that indicators of impairment existed in our MammoSite reporting unit, due to changing market conditions for the brachytherapy market, including downward pressure on procedure volumes due to the continuing adverse economic environment and current trends in breast cancer management, as well as competitive pricing pressures and competition from existing and alternative new technologies. These factors resulted in us lowering the financial projections for MammoSite. As a result, we performed the first step in the long-lived assets impairment test pursuant to ASC 360 and compared MammoSite’s forecasted undiscounted cash flows to the carrying value of its net assets. These undiscounted cash flows were insufficient to recover MammoSite’s carrying value. Therefore, we determined the fair value of MammoSite’s long-lived assets, which are primarily intangible assets, using a discounted cash flow technique. The expected future cash flows are Level 3 inputs under ASC 820 and are those expected to be generated by the market participants, discounted at an appropriate risk-adjusted rate. Based on the fair value of the long-lived assets, we recorded an aggregate impairment charge of $143.5 million to write these intangible assets down to their fair value. The charge was comprised of
72
$123.4 million related to developed technology, which was recorded in cost of product sales, $11.8 million related to customer relationships and $8.3 million related to trade names, which were recorded in impairment of intangible assets. In addition, the Company recorded a goodwill impairment charge of $76.7 million (see below for further discussion).
Goodwill
We test goodwill at the reporting unit level for impairment on an annual basis and between annual tests if events and circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business, and an adverse action or assessment by a regulator. Our annual impairment test date is the first day of our fiscal fourth quarter.
In performing the test, we utilize the two-step approach prescribed under ASC 350. The first step requires a comparison of the reporting unit’s carrying value to its fair value. We consider a number of factors to determine the fair value of a reporting unit, including an independent valuation to conduct this test. The valuation is based upon expected future discounted operating cash flows of the reporting unit as well as analysis of recent sales and ratio comparisons of similar companies. We base the discount rate on the weighted average cost of capital, or WACC, of market participants. If the carrying value of a reporting unit exceeds its fair value, we will perform the second step of the goodwill impairment test to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of a reporting unit’s goodwill to its carrying value. The second step requires us to perform a hypothetical purchase allocation as of the measurement date and estimate the fair value of net tangible and intangible assets. The fair value of intangible assets is determined as described above and is subject to significant judgment.
We conducted our fiscal 2012 annual impairment test on the first day of the fourth quarter. We utilized discounted cash flow, or DCF, and market approaches to estimate the fair value of our reporting units as of June 24, 2012, and ultimately used the fair value determined by the DCF in making our impairment test conclusions. We believe we have used reasonable estimates and assumptions about future revenue, cost projections, cash flows, market multiples and discount rates as of the measurement date. As a result of completing Step 1, all of our reporting units, except MammoSite, had fair values exceeding their carrying values, and as such, Step 2 of the impairment test was not required for these reporting units. MammoSite’s fair value has declined from fiscal 2011 primarily due to a reduction in our revenue projections and long-term growth rates. The changes in MammoSite’s financial projections were a result of the continuing deterioration of the brachytherapy market, and competition from existing technologies. We performed the Step 2 analysis for MammoSite, consistent with the procedures described above, and recorded a $5.8 million goodwill impairment charge, resulting in no remaining goodwill for this reporting unit.
For our other reporting units, if their respective fair values had been lower by 10%, each reporting unit would have still passed Step 1 of the goodwill impairment test. Since, the fair value of our reporting units was determined by use of the DCF, and the key assumptions that drive the fair value in this model are the WACC, terminal values, growth rates, and the amount and timing of expected future cash flows, significant judgment is applied in determining fair value. If the current economic environment were to deteriorate, this would likely result in a higher WACC because market participants would require a higher rate of return. In the DCF as the WACC increases, the fair value decreases. The other significant factor in the DCF is our projected financial information (i.e., amount and timing of expected future cash flows and growth rates) and if these assumptions were to be adversely impacted, this could result in a reduction of the fair value of this reporting unit.
We conducted our fiscal 2011 annual impairment test on the first day of the fourth quarter. We utilized DCF and market approaches to estimate the fair value of our reporting units as of June 26, 2011, and ultimately used the fair value determined by the DCF in making our impairment test conclusions. We believe we used reasonable estimates and assumptions about future revenue, cost projections, cash flows, market multiples and discount rates as of the measurement date. As a result of completing Step 1, all of the reporting units had fair values exceeding their carrying values, and as such, Step 2 of the impairment test was not required. For illustrative purposes, had the fair value of each reporting unit been lower by 10%, each reporting unit would have still passed Step 1 of the goodwill impairment test.
We conducted our fiscal 2010 annual impairment test on the first day of the fourth quarter. We utilized DCF and market approaches to estimate the fair value of our reporting units as of June 27, 2010, and believe we used reasonable estimates and assumptions about future revenue, cost projections, cash flows and market multiples as of the measurement date. As a result of completing Step 1, all of the Company’s reporting units, except MammoSite, had fair values exceeding their carrying values, and as such, Step 2 of the impairment test was not required for these reporting units. MammoSite’s fair value has declined from fiscal 2009 primarily due to a reduction in its long-term growth rates. The changes in MammoSite’s financial projections were a result of changing market conditions for the brachytherapy market, including downward pressure on procedure volumes due to the continuing adverse economic environment and current trends in breast cancer management, as well as competitive pricing pressures and competition from existing and alternative new technologies. The DCF calculation of fair value was positively impacted by a reduction in the discount rate to 11.0% from 12.5% used in the fiscal 2009 annual impairment test.
73
We performed the Step 2 analysis for MammoSite, consistent with the procedures described above, and recorded a $76.7 million goodwill impairment charge. For illustrative purposes had the fair value of MammoSite been 10% lower, the charge would have been higher by $2.5 million. If the fair value of the Company’s other reporting units had been lower by 10%, one reporting unit would have failed Step 1 requiring a Step 2 analysis. This reporting unit is in the Breast Health reportable segment and had a fair value at the annual impairment measurement date that exceeded its carrying value by 4% with goodwill of $256.5 million. The fair value of the reporting unit was determined by use of the DCF. At September 25, 2010, for our other reporting units with goodwill aggregating $1.85 billion as of September 25, 2010, we believed that these reporting units were not at risk of failing Step 1 of the goodwill impairment test.
The estimate of fair value requires significant judgment. Any loss resulting from an impairment test would be reflected in operating income (loss) in our Consolidated Statements of Operations. The annual impairment testing process is subjective and requires judgment at many points throughout the analysis. If these estimates or their related assumptions change in the future, we may be required to record impairment charges for these assets not previously recorded.
Revenue Recognition
We generate revenue from the sale of products, primarily medical imaging systems and diagnostic and surgical disposable products, and related services, which are primarily support and maintenance services on our medical imaging systems.
We recognize product revenue upon shipment provided that there is persuasive evidence of an arrangement, there are no uncertainties regarding acceptance, the sales price is fixed or determinable, no right of return exists and collection of the resulting receivable is reasonably assured. Generally, our product arrangements for capital equipment sales, primarily in our Breast Health and Skeletal Health reporting segments, are multiple-element arrangements, including services, such as installation and training, and multiple products. In accordance with ASC 605-25, based on the terms and conditions of the product arrangements, we believe that these services and undelivered products can be accounted for separately from the delivered product element as our delivered products have value to our customers on a stand-alone basis. Accordingly, revenue for services not yet performed at the time of product shipment are deferred and recognized as such services are performed. The relative selling price of any undelivered products is also deferred at the time of shipment and recognized as revenue when these products are delivered. There is no customer right of return in our sales agreements.
Service revenues primarily consist of amounts recorded under service and maintenance contracts and repairs not covered under warranty, installation and training, and shipping and handling costs billed to customers. Service and maintenance contract revenues are recognized ratably over the term of the contract. Other service revenues are recognized as the services are performed.
For revenue arrangements with multiple deliverables, we record revenue as separate units of accounting if the delivered items have value to the customer on a stand-alone basis, and if the arrangement includes a general right of return relative to the delivered items, the delivery or performance of the undelivered items is considered probable and substantially within our control. Some of our products have both software and non-software components that function together to deliver the product’s essential functionality. We determined that except for our CAD products, the software element in our other products is incidental in accordance with the software revenue recognition rules and are not within the scope of the software revenue recognition rules, ASC 985-605,Software—Revenue Recognition. We determined that given the significance of the software component’s functionality to our CAD systems, which are sold by our Breast Health segment, these products are within the scope of the software revenue recognition rules. We evaluated the appropriate revenue recognition treatment of our other hardware products, including our Dimensions digital mammography systems, which have both software and non-software components that function together to deliver the products’ essential functionality (i.e., it is a tangible product), and determined they are not within the scope of ASC 985-605.
We are required to allocate revenue to multiple element arrangements based on the relative fair value of each element’s selling price. We typically determine the selling price of our products based on our best estimate of selling price, referred to as ESP, and services based on vendor-specific objective evidence of selling price, referred to as VSOE. We determine VSOE based on our normal pricing and discounting practices for the specific product or service when sold on a stand-alone basis. In determining VSOE, our policy requires a substantial majority of selling prices for a product or service to be within a reasonably narrow range. We also consider the class of customer, method of distribution, and the geographies into which our products and services are sold when determining VSOE. We typically have had VSOE for our products and services. If VSOE cannot be established, which may occur in instances when a product or service has not been sold separately, stand-alone sales are too infrequent, or product pricing is not within a narrow range, we attempt to establish the selling price based on third-party evidence of selling price, referred to as TPE. TPE is determined based on competitor prices for similar deliverables when sold separately. When we cannot determine VSOE or TPE, we use ESP in our allocation of arrangement consideration. The objective of ESP is to determine the price at which we would typically transact a stand-alone sale of the product or service. ESP is determined by considering a number of factors including our pricing policies, internal costs and gross margin objectives, method of distribution, information gathered from experience in customer negotiations, market research and information, recent technological trends, competitive landscape and geographies.
74
For those arrangements accounted for under the software revenue recognition rules, ASC 985-605 generally requires revenue earned on software arrangements involving multiple elements to be allocated to each element based on their relative VSOE of fair value. If VSOE does not exist for a delivered element, the residual method is applied in which the arrangement consideration is allocated to the undelivered elements based on their VSOE with the remaining consideration recognized as revenue for the delivered elements. For multiple-element software arrangements where VSOE of fair value of Post-Contract Customer Support, referred to as PCS, has been established, we recognize revenue using the residual method at the time all other revenue recognition criteria have been met.
As part of our Diagnostics reporting segment and as a result of the Gen-Probe acquisition, we manufacture blood screening products according to demand schedules provided by our collaboration partner, Novartis. Our agreement provides that we share a portion of Novartis’s revenue from screening blood donations. Upon shipment to Novartis, we recognize blood screening product sales at an agreed upon fixed transfer price, which is not refundable, and record the related cost of products sold. Based on the terms of our collaboration agreement with Novartis, our ultimate share of the net revenue from sales to the end user in excess of the transfer price revenues recognized is not known until it is reported to us by Novartis. On a monthly basis, Novartis reports net revenue generated during the prior month and remits an additional corresponding net payment to us which we record as revenue at that time. This payment combined with the transfer price revenues previously recognized represents our ultimate share of net revenue under the agreement.
Within our Diagnostics business, and to a lesser extent, our Surgical business, we provide our instrumentation (for example, the ThinPrep Processor, ThinPrep Imaging System, PANTHER and TIGRIS) and certain other hardware to customers without requiring them to purchase the equipment or enter into a lease. Instead, we recover the cost of providing the instrumentation and equipment in the amount it charges for its diagnostic tests and assays and other disposables. Customers enter into a customer usage agreement, and we install the equipment at customer sites and customers commit to purchasing minimum quantities of disposable products at a stated price over a defined contract term, which is typically between three and five years. Revenue is recognized over the term of the customer usage agreement as tests, assays and other disposable products are shipped. The depreciation costs associated with an instrument are charged to cost of product sales on a straight-line basis over the estimated life of the instrument. The costs to maintain these instruments in the field are charged to cost of product sales as incurred.
We sell our instruments to Novartis for use in blood screening and record these instrument sales upon delivery since Novartis is responsible for the placement, maintenance and repair of the units with its customers. We also sell instruments to our clinical diagnostics customers and record sales of these instruments upon delivery and customer acceptance. For certain customers with non-standard payment terms, instrument sales are recorded based upon expected cash collection. Prior to delivery, each instrument is tested to meet the Company’s specifications and the specifications of the FDA, and is shipped fully assembled. Customer acceptance of the Company’s clinical diagnostic instrument systems requires installation and training by our technical service personnel. Installation is a standard process consisting principally of uncrating, calibrating and testing the instrumentation.
Stock-Based Compensation
We recognize stock-based compensation expense associated with the fair value of stock options and restricted stock units issued to our employees. Determining the amount of stock-based compensation to be recorded requires us to develop estimates to be used in calculating the grant-date fair value of stock options. We use a binomial lattice model to determine the fair value of our stock options. We consider a number of factors to determine the fair value of stock options including the advice of an outside valuation advisor and the advisor’s model. The model requires us to make estimates of the following assumptions:
Expected volatility—We are responsible for estimating volatility and have considered a number of factors, including third-party estimates, when estimating volatility. We currently use a combination of historical and implied volatility, which is weighted based on a number of factors.
Expected term—We use historical employee exercise and option expiration data to estimate the expected term assumption. We believe that this historical data is currently the best estimate of the expected term of a new option, and that generally, all of our employees exhibit similar exercise behavior.
Risk-free interest rate—The yield on zero-coupon U.S. Treasury securities for a period that is commensurate with the expected term assumption is used as the risk-free interest rate.
The amount of stock-based compensation expense recognized during a period is based on the value of the portion of the awards that are ultimately expected to vest. ASC 718,Stock Compensation,requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Based on an analysis of historical forfeitures, we have determined a specific forfeiture rate for certain employee groups and have applied forfeiture rates ranging from 0% to 7% as of September 29, 2012 depending on the specific employee group. This analysis is re-evaluated periodically and the forfeiture rate is adjusted as necessary. Ultimately, the actual expense recognized over the vesting period will only be for those awards that vest.
75
We recognized $40.6 million, $35.5 million and $34.2 million of stock-based compensation expense for employee equity awards in fiscal years 2012, 2011 and 2010, respectively. The increase in fiscal 2012 was due to the addition of assumed stock options in the Gen-Probe acquisition, which included the acceleration of vesting for certain terminated employees, resulting in $3.5 million of additional stock-based compensation expense. As of September 29, 2012, there was $44.9 million and $36.6 million of unrecognized compensation expense related to stock options and restricted stock units, respectively, that we expect to recognize over a weighted-average period of 3.2 years and 2.4 years, respectively.
Income Taxes
We use the asset and liability method for accounting for income taxes. Under this method, we determine deferred tax assets and liabilities based on the difference between financial reporting and taxes bases of our assets and liabilities. We measure deferred tax assets and liabilities using enacted tax rates and laws that will be in effect when we expect the differences to reverse.
We have recognized net deferred tax liabilities of $1.76 billion at September 29, 2012 and $917.8 million at September 24, 2011. The liabilities primarily relate to deferred taxes associated with our acquisitions and the debt discount and original issuance discount on our Convertible Notes. The tax assets relate primarily to net operating loss carryforwards, accruals and reserves, stock-based compensation, and research credits. We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we have considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of the net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax asset in the future, an adjustment to the deferred tax asset would be charged to income in the period such determination was made.
We had gross unrecognized tax benefits, excluding interest, of $53.1 million as of September 29, 2012 and $31.0 million as of September 24, 2011. At September 29, 2012, $53.1 million represents the amount of unrecognized tax benefits that, if recognized, would result in a reduction of the Company’s effective tax rate. In the next twelve months, it is reasonably possible that we will reduce our unrecognized tax benefits by $1.0 to $2.0 million due to expiration of statute of limitations and favorable settlements with taxing authorities, all of which would reduce our effective tax rate.
In the ordinary course of global business, there are many transactions and calculations where the ultimate tax outcome is uncertain. Judgment is required in determining our worldwide income tax provision. In our opinion, we have made adequate provisions for income taxes for all years subject to audit. Although we believe our estimates are reasonable, no assurance can be given that the final tax outcome of these matters will not be different than that which is reflected in our historical income tax provisions and accruals. In the event our assumptions are incorrect, the differences could have a material impact on our income tax provision and operating results in the period in which such determination is made.
Recent Accounting Pronouncements
Disclosures about Offsetting Assets and Liabilities
In December 2011, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2011-11,Disclosures about Offsetting Assets and Liabilities. ASU 2011-11 amended ASC 210,Balance Sheet,to converge the presentation of offsetting assets and liabilities between U.S. GAAP and IFRS. ASU 2011-11 requires that entities disclose both gross information and net information about both instruments and transactions eligible for offset in the statement of financial position and instruments and transactions subject to an agreement similar to a master netting arrangement. ASU 2011-11 is effective for fiscal years, and interim periods within those years, beginning after January 1, 2013, which is our fiscal year 2014. We are currently evaluating the impact of the adoption of ASU 2011-11 on our consolidated financial statements.
Presentation of Comprehensive Income
In June 2011, the FASB issued ASU No. 2011-05,Comprehensive Income (Topic 220): Presentation of Comprehensive Income, which requires an entity to present total comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. ASU 2011-05 does not change any of the components of comprehensive income, but it eliminates the option to present the components of other comprehensive income as part of the statement of stockholders equity. ASU 2011-05 is effective for us in our first quarter of fiscal 2013 and should be applied retrospectively. We have elected to early-adopt ASU 2011-05 in fiscal 2012 and have provided a separate statement of comprehensive income (loss) in our consolidated financial statements.
Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements
In May 2011, the FASB issued ASU No. 2011-04—Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs, to provide a consistent definition of fair value and
76
ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and International Financial Reporting Standards. ASU 2011-04 changes certain fair value measurement principles and enhances the disclosure requirements particularly for level 3 fair value measurements. ASU 2011-04 is effective for us in our second quarter of fiscal 2012 and should be applied prospectively. The adoption of ASU 2011-04 did not have a material impact on our consolidated financial statements.
Business Combinations
In December 2010, the FASB issued ASU No. 2010-29,Business Combinations (Topic 805)—Disclosure of Supplementary Pro Forma Information for Business Combinations. ASU 2010-29 requires a public entity to disclose revenue and earnings of the combined entity as though the business combination that occurred during the current year had occurred as of the beginning of the prior year. It also requires a description of the nature and amount of material, nonrecurring adjustments directly attributable to the business combination included in the reported revenue and earnings. The new disclosure was effective for our first quarter of fiscal 2012 and did not have a material impact on our consolidated financial statements.
Intangibles—Goodwill and Other
In December 2010, the FASB issued ASU No. 2010-28,Intangibles—Goodwill and Other (Topic 350). ASU 2010-28 modifies Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that a goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that an impairment may exist. ASU 2010-28 is effective for us in fiscal 2012. The adoption of ASU 2010-28 did not have a material impact on our consolidated financial statements.
In September 2011, the FASB issued ASU No. 2011-08,Intangibles—Goodwill and Other (Topic 350): Testing Goodwill for Impairment. ASU 2011-08 allows entities to first assess qualitatively whether it is necessary to perform the two-step goodwill impairment test. If an entity believes, as a result of its qualitative assessment, that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, the quantitative two-step impairment test is required; otherwise, no further testing is required. ASU 2011-08 is effective for us beginning in fiscal 2013, although early adoption is permitted. We do not believe that ASU 2011-08 will have a material impact on our consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Financial Instruments, Other Financial Instruments, and Derivative Commodity Instruments. Financial instruments consist of cash equivalents, accounts receivable, cost-method equity investments, insurance contracts and related Nonqualified Deferred Compensation Plan liability, accounts payable and debt obligations. Except for our outstanding convertible notes, the fair value of these financial instruments approximates their carrying amount. As of September 29, 2012, we have $1.725 billion of principal of convertible notes outstanding, which are comprised of our 2007 Notes with a principal of $775.0 million, our 2010 Notes with a principal of $450.0 million, and our 2012 Notes with a principal of $500.0 million. The convertible notes are recorded net of the unamortized discount on our consolidated balance sheets. The fair value of our 2007 Notes, 2010 Notes and 2012 Notes as of September 29, 2012 was approximately $771.6 million, $505.6 million and $490.7 million, respectively. Amounts outstanding under our Credit Agreement aggregating $2.5 billion aggregate principal are subject to variable rates of interest based on current market rates, and as such, we believe the carrying amount of these obligations approximates fair value. In addition, based on the recent issuance of our Senior Notes, we believe their carrying amount approximates fair value.
Primary Market Risk Exposures. Our primary market risk exposure is in the areas of interest rate risk and foreign currency exchange rate risk. We incur interest expense on borrowing outstanding under our Convertible Notes, Senior Notes and Credit Agreement. The Convertible Notes and Senior Notes have fixed interest rates. Borrowings under our Credit Agreement bear interest at a rate per annum, at our option, initially, with respect to all loans made under Term Loan A (i) at the Base Rate plus 2.00% per annum, or (ii) at the Adjusted Eurodollar Rate (i.e., the Libor rate) plus 3.00%, and with respect to loans made under Term Loan B: (i) at the Base Rate, with a floor of 2.00%, plus 2.50%, or (ii) at the Adjusted Eurodollar Rate, with a floor of 1.00% plus 3.50%.
As of September 29, 2012, there was $2.5 billion of aggregate principal outstanding under the Credit Agreement comprised of $1.0 billion under Term Loan A and $1.5 billion under Term Loan B. Since these debt obligations are variable rate instruments, our interest expense associated with these instruments is subject to change. A 10% adverse movement (increase in Libor rate) would increase annual interest expense by less than $1.0 million due to the low current interest rate environment and the floor on our Term Loan B.
The return from cash and cash equivalents will vary as short-term interest rates change. A hypothetical 10% increase or decrease in interest rates, however, would not have a material adverse effect on our financial condition.
77
Foreign Currency Exchange Risk.Our international business is subject to risks, including, but not limited to: unique economic conditions, changes in political climate, differing tax structures, other regulations and restrictions, and foreign exchange rate volatility. Accordingly, our future results could be materially adversely impacted by changes in these or other factors.
We conduct business worldwide and maintain sales and service offices outside the United States as well as manufacturing facilities in Costa Rica, Germany, Canada and China. The expenses of our international offices are denominated in local currencies, except at our Costa Rica subsidiary, where the majority of the business is conducted in U.S. dollars. Our international sales are denominated in a number of currencies, primarily the Euro and U.S. dollar. Fluctuations in the foreign currency rates could affect our sales, cost of goods and operating margins and could result in exchange losses. In addition, currency devaluations can result in a loss if we hold deposits of that currency.
We believe that the operating expenses of our international subsidiaries that are incurred in local currencies will not have a material adverse effect on our business, results of operations or financial condition. Our operating results and certain assets and liabilities that are denominated in the Euro are affected by changes in the relative strength of the U.S. dollar against the Euro. Our expenses, denominated in Euros, are positively affected when the U.S. dollar strengthens against the Euro and adversely affected when the U.S. dollar weakens. However, we believe that the foreign currency exchange risk is not significant. A hypothetical 10% increase or decrease in foreign currencies that we transact in would not have a material adverse impact on our financial condition or results of operations. During fiscal 2012, 2011 and 2010, we incurred net foreign exchange gains (losses) of $0.8 million, $(0.7) million and $(1.1) million, respectively.
| Item 8. | Financial Statements and Supplementary Data |
Our Consolidated Financial Statements and Supplementary Data are listed under Part IV, Item 15, in this report.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Securities Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, as ours are designed to do, and management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of September 29, 2012, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective.
Report of Management on Internal Control over Financial Reporting
We are responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of our principal executive and principal financial officers and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| | • | | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and disposition of our assets; |
| | • | | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and |
| | • | | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
78
Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management has assessed the effectiveness of our internal control over financial reporting as of September 29, 2012. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework.
Management has excluded from our assessment of and conclusion on the effectiveness of internal control over financial reporting the internal controls of Gen-Probe Incorporated acquired August 1, 2012, which is included in the consolidated financial statements of Hologic, Inc. as of and for the year ended September 29, 2012 constituting $5.0 billion and $3.9 billion of total and net assets, respectively, as of September 29, 2012, and $89.5 million and $29.7 million of revenues and net loss, respectively, for the year then ended.
Subject to the foregoing, based on management’s assessment, we believe that, as of September 29, 2012, our internal control over financial reporting is effective at a reasonable assurance level based on these criteria.
Ernst & Young LLP, an independent registered public accounting firm, has issued an attestation report on the effectiveness of our internal control over financial reporting. This report in which they expressed an unqualified opinion is included below.
79
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Hologic, Inc.:
We have audited Hologic Inc.’s (the “Company”) internal control over financial reporting as of September 29, 2012, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Report of Management on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As indicated in the accompanying Form 10-K, management’s assessment of and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of Gen-Probe Incorporated acquired on August 1, 2012, which is included in the September 29, 2012 consolidated financial statements of Hologic, Inc. and constituted $5.0 billion and $3.9 billion of total and net assets for Gen-Probe Incorporated, respectively, as of September 29, 2012 and $89.5 million and $29.7 million of revenues and net loss, respectively, for the year then ended. Our audit of internal control over financial reporting of Hologic, Inc. also did not include an evaluation of the internal control over financial reporting of Gen-Probe Incorporated.
In our opinion, Hologic, Inc. maintained, in all material respects, effective internal control over financial reporting as of September 29, 2012, based on the COSO criteria.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Hologic, Inc. as of September 29, 2012 and September 24, 2011 and the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended September 29, 2012 of Hologic, Inc. and our report dated November 28, 2012 expressed an unqualified opinion thereon.
|
| Boston, Massachusetts |
| November 28, 2012 |
80
Changes in Internal Control over Financial Reporting
During the quarter ended September 29, 2012, there have been no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information. |
None.
81
PART III
| Item 10. | Directors, and Executive Officers and Corporate Governance |
Pursuant to Section 406 of the Sarbanes-Oxley Act of 2002, we have adopted a Code of Ethics for Senior Financial Officers that applies to our principal executive officer and principal financial officer, principal accounting officer and controller, and other persons performing similar functions. Our Code of Ethics for Senior Financial Officers is publicly available on our website atinvestors.hologic.comas Appendix A to our Code of Conduct. We intend to satisfy the disclosure requirement under Item 5.05 of Current Report on Form 8-K regarding an amendment to, or waiver from, a provision of this code by posting such information on our website, at the address specified above.
The additional information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 11. | Executive Compensation |
The information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
We maintain a number of equity compensation plans for employees, officers, directors and others whose efforts contribute to our success. The table below sets forth certain information as of the end of our fiscal year ended September 29, 2012 regarding the shares of our common stock available for grant or granted under stock option plans and equity incentives that (i) were approved by our stockholders, and (ii) were not approved by our stockholders.
Equity Compensation Plan Information
| | | | | | | | | | | | |
Plan Category | | Number of
securities to be
issued upon
exercise of
outstanding
options, warrants
and rights
(a) | | | Weighted-average
exercise price of
outstanding
options,
warrants and rights
(b) (2) | | | Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a))
(c) | |
Equity compensation plans approved by security holders (1) | | | 21,479,028 | | | $ | 17.49 | | | | 5,350,158 | |
Equity compensation plans not approved by security holders (3) | | | 139,335 | | | $ | 6.30 | | | | — | |
| | | | | | | | | | | | |
Total | | | 21,618,363 | | | $ | 17.40 | | | | 5,350,158 | |
| | | | | | | | | | | | |
| (1) | Includes 3,579,681 shares that are issuable upon RSUs vesting. The remaining balance consists of outstanding stock option grants. |
| (2) | The weighted average exercise price does not take into account the shares issuable upon outstanding RSUs vesting, which have no exercise price. |
| (3) | Includes the following plans: 1997 Employee Equity Incentive Plan and 2000 Acquisition Equity Incentive Plan. A description of each of these plans is as follows: |
1997 Employee Equity Incentive Plan. The purposes of the 1997 Employee Equity Incentive Plan (the “1997 Plan”), adopted by the Board of Directors in May 1997, were to attract and retain key employees, consultants and advisors, to provide an incentive for them to assist us in achieving long-range performance goals, and to enable such person to participate in our long-term growth. In general, under the 1997 Plan, all employees, consultants, and advisors who were not executive officers or directors were eligible to participate in the 1997 Plan. The 1997 Plan is administered by our Compensation Committee. Participants in the 1997 Plan are eligible to receive non-qualified stock options, stock appreciation rights, restricted stock and performance shares. A total of 4,400,000 shares of our common stock were reserved for issuance under the 1997 Plan. Of the shares reserved for issuance under the 1997 Plan, options to purchase 60,760 shares are outstanding as of September 29, 2012. In September 2005, our Compensation Committee determined that no further awards would be made under this plan and cancelled all remaining 332,168 shares available for issuance under the 1997 Plan that were not subject to outstanding stock option awards.
82
2000 Acquisition Incentive Plan. The purpose of the 2000 Acquisition Equity Incentive Plan (the “2000 Plan”), adopted by the Board of Directors in April 2001, was to attract and retain (a) employees, consultants and advisors, of newly acquired businesses who have been or were being hired as employees, consultants or advisors of our company or any of our consolidated subsidiaries, and (b) employees, consultants and advisors, of our company who have or were anticipated to provide significant assistance in connection with the acquisition of a newly acquired business or its integration with our company, and to provide such persons an incentive for them to achieve long-range performance goals, and to enable them to participate in our long-term growth. In general, under the 2000 Plan, only employees, consultants and advisors who were not officers or directors of our company were eligible to participate in the 2000 Plan. The 2000 Plan was administered by our Compensation Committee. Participants in the 2000 Plan were eligible to receive non-qualified stock options, stock appreciation rights, restricted stock and performance shares. A total of 3,200,000 shares of our common stock were reserved for issuance under the 2000 Plan. Of the shares reserved for issuance under the 2000 Plan, options to purchase 78,575 shares were outstanding as of September 29, 2012. In September 2005, our Compensation Committee determined that no further awards would be made under this plan and cancelled all remaining 835,408 shares available for issuance under the 2000 Plan that were not subject to outstanding stock option awards.
The additional information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 13. | Certain Relationships and Related Transactions |
The information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
| Item 14. | Principal Accountant Fees and Services |
The information required by this item is incorporated by reference to our Definitive Proxy Statement for our annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of our fiscal year.
83
PART IV
| Item 15. | Exhibits, Financial Statement Schedules |
(a) The following documents are filed as part of this report:
(1) Financial Statements
Report of Independent Registered Public Accounting Firm on Consolidated Financial Statements
Consolidated Statements of Operations for the years ended September 29, 2012, September 24, 2011 and September 25, 2010
Consolidated Statements of Comprehensive Income (Loss) for the years ended September 29, 2012, September 24, 2011 and September 25, 2010
Consolidated Balance Sheets as of September 29, 2012 and September 24, 2011
Consolidated Statements of Stockholders’ Equity for the years ended September 29, 2012, September 24, 2011 and September 25, 2010
Consolidated Statements of Cash Flows for the years ended September 29, 2012, September 24, 2011 and September 25, 2010
Notes to Consolidated Financial Statements
(2) Financial Statement Schedules
All schedules have been omitted because they are not required or because the required information is given in the Consolidated Financial Statements or Notes thereto.
(b) Listing of Exhibits
| | | | | | |
| | | | | Incorporated by Reference |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date |
| | | |
| 2.1 | | Agreement and Plan of Merger, dated as of April 29, 2012, by and among Hologic, Gold Acquisition Corp. and Gen-Probe Incorporated. | | 8-K | | 05/01/2012 |
| | | |
| 3.1 | | Certificate of Incorporation of Hologic. | | S-1 | | 01/24/1990 |
| | | |
| 3.2 | | Amendment to Certificate of Incorporation of Hologic. | | 10-Q | | 03/30/1996 |
| | | |
| 3.3 | | Certificate of Amendment to Certificate of Incorporation of Hologic. | | 10-K | | 09/24/2005 |
| | | |
| 3.4 | | Certificate of Amendment to Certificate of Incorporation of Hologic. | | 8-K | | 10/22/2007 |
| | | |
| 3.5 | | Certificate of Amendment to Certificate of Incorporation of Hologic. | | 8-K | | 03/11/2008 |
| | | |
| 3.6 | | Fourth Amended and Restated By-laws of Hologic. | | 8-K | | 03/08/2012 |
| | | |
| 3.7 | | Amended and Restated Certificate of Designations of Series A Junior Participating Preferred Stock of Hologic. | | 8-A | | 04/03/2008 |
| | | |
| 4.1 | | Specimen Certificate for Shares of Hologic’s Common Stock. | | 8-A | | 01/31/1990 |
| | | |
| 4.2 | | Description of Capital Stock (Contained in Hologic’s Certificate of Incorporation, as amended, filed as Exhibits 3.1, 3.2, 3.3, 3.4 and 3.5 hereto). | | | | |
| | | |
| 4.3 | | Amended and Restated Rights Agreement dated April 2, 2008. | | 8-A | | 04/03/2008 |
| | | |
| 4.4 | | Form of Rights Certificate. | | 8-K | | 09/26/2002 |
| | | |
| 4.5 | | Indenture, dated as of December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 8-K | | 12/10/2007 |
84
| | | | | | |
| | | | | Incorporated by Reference |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date |
| | | |
| 4.6 | | First Supplemental Indenture, dated December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 8-K | | 12/10/2007 |
| | | |
| 4.7 | | Form of 2.00% Convertible Senior Note due 2037 (included in Exhibit 4.6). | | 8-K | | 12/10/2007 |
| | | |
| 4.8 | | Second Supplemental Indenture, dated November 23, 2010, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 10-K | | 09/25/2010 |
| | | |
| 4.9 | | Form of 2.00% Convertible Exchange Senior Note due 2037 (included in Exhibit 4.8). | | 10-K | | 09/25/2010 |
| | | |
| 4.10 | | Third Supplemental Indenture, dated March 5, 2012, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 8-K | | 03/08/2012 |
| | | |
| 4.11 | | Form of 2.00% Convertible Senior Note due 2042 (included in Exhibit 4.10). | | 8-K | | 03/08/2012 |
| | | |
| 4.12 | | Indenture, dated as of August 1, 2012, by and among Wells Fargo Bank, National Association, as Trustee, Hologic and certain subsidiaries of Hologic party thereto. | | 8-K | | 08/01/2012 |
| | | |
| 4.13 | | Forms of 6.25% Senior Note due 2020 (included in Exhibit 4.12). | | 8-K | | 08/01/2012 |
| | | |
| 4.14 | | Exchange and Registration Rights Agreement, dated as of August 1, 2012, by and among Goldman Sachs & Co., Hologic and certain subsidiaries of Hologic party thereto. | | 8-K | | 08/01/2012 |
| | | |
| 10.1* | | Second Amended and Restated 1999 Equity Incentive Plan. | | 10-Q | | 03/25/2006 |
| | | |
| 10.2* | | Amendment No. 1 to Second Amended and Restated 1999 Equity Incentive Plan. | | S-8 | | 10/23/2007 |
| | | |
| 10.3* | | Amendment No. 2 to Second Amended and Restated 1999 Equity Incentive Plan. | | 8-K | | 10/22/2007 |
| | | |
| 10.4* | | Amendment No. 3 to Second Amended and Restated 1999 Equity Incentive Plan. | | 8-K | | 12/12/2008 |
| | | |
| 10.5 | | 2000 Acquisition Equity Incentive Plan. | | 10-K | | 09/29/2001 |
| | | |
| 10.6* | | 2008 Equity Incentive Plan. | | 8-K | | 03/11/2008 |
| | | |
| 10.7* | | Form of Employee Stock Option Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | 11/17/2008 |
| | | |
| 10.8* | | Form of Employee Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | 11/17/2008 |
| | | |
| 10.9* | | Form of Special Retention Employee Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan. | | 10-Q | | 06/26/2010 |
| | | |
| 10.10* | | Form of Independent Director Stock Option Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | 12/12/2008 |
| | | |
| 10.11* | | Form of Independent Director Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | 12/12/2008 |
| | | |
| 10.12* | | Form of Market Stock Unit Award Agreement. | | 8-K | | 11/13/2012 |
| | | |
| 10.13* | | Hologic, Inc. 2012 Employee Stock Purchase Plan. | | 8-K | | 03/08/2012 |
| | | |
| 10.14* | | Hologic, Inc. 2012 Short-Term Incentive Plan. | | 8-K | | 11/07/2011 |
| | | |
| 10.15* | | Hologic, Inc. 2013 Short-Term Incentive Plan. | | 8-K | | 11/13/2012 |
| | | |
| 10.16* | | Hologic, Inc. 2013 Synergy Bonus Plan. | | 8-K | | 11/13/2012 |
| | | |
| 10.17* | | Cytyc Corporation 1995 Stock Plan. | | S-8 | | 10/23/2007 |
| | | |
| 10.18* | | Cytyc Corporation 1995 Non-Employee Director Stock Option Plan. | | S-8 | | 10/23/2007 |
85
| | | | | | |
| | | | | Incorporated by Reference |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date |
| | | |
| 10.19* | | Cytyc Corporation 2004 Omnibus Stock Plan. | | S-8 | | 10/23/2007 |
| | | |
| 10.20* | | The 2003 Incentive Award Plan of Gen-Probe Incorporated, as amended and restated. | | S-8 | | 08/02/2012 |
| | | |
| 10.21* | | Transition Agreement dated November 5, 2009, by and between Hologic and John W. Cumming | | 8-K | | 11/09/2009 |
| | | |
| 10.22* | | Transition Acknowledgement dated July 28, 2011, by and between Hologic and John W. Cumming | | 8-K | | 08/01/2011 |
| | | |
| 10.23* | | Form of Indemnification Agreement (as executed with each director of Hologic). | | 8-K | | 03/06/2009 |
| | | |
| 10.24*** | | Amended and Restated Deferred Compensation Program. | | | | |
| | | |
| 10.25* | | Rabbi Trust Agreement. | | 10-K | | 09/30/2006 |
| | | |
| 10.26* | | Form of Officer Severance Agreement including list of officers to whom provided. | | 10-Q | | 03/25/2006 |
| | | |
| 10.27* | | Form of Senior Vice President Change of Control Agreement including list of officers to whom provided. | | 10-Q 10-K | | 12/27/2008
09/25/2010 |
| | | |
| 10.28* | | Form of Senior Executive Officer Change of Control Agreement including list of officers to whom provided. | | 8-K | | 11/17/2009 |
| | | |
| 10.29* | | Retention and Severance Agreement by and between Hologic and Carl W. Hull dated as of July 10, 2012. | | 8-K | | 07/12/2012 |
| | | |
| 10.30* | | Change of Control Agreement by and between Hologic and Carl W. Hull dated as of July 10, 2012. | | 8-K | | 07/12/2012 |
| | | |
| 10.31* | | Restricted Stock Unit Award Agreement by and between Hologic and Carl W. Hull dated as of August 1, 2012. | | 8-K | | 08/01/2012 |
| | | |
| 10.32 | | Facility Lease (Danbury) dated as of December 30, 1995 by and among Melvin J. Powers and Mary P. Powers D/B/A M&N Realty and Lorad. | | Trex Medical Corporation S-1 | | 03/29/1996 |
| | | |
| 10.33 | | Lease Agreement (Danbury and Bedford) by and between BONE (DE) QRS 15-12, INC., and Hologic dated as of August 28, 2002. | | 10-K | | 09/28/2002 |
| | | |
| 10.34 | | First Amendment to Lease Agreement (Danbury and Bedford) by and between BONE (DE) QRS 15-12, INC., and Hologic dated as of October 29, 2007. | | 10-K | | 09/29/2007 |
| | | |
| 10.35 | | Office Lease dated December 31, 2003 between Cytyc and Marlborough Campus Limited Partnership. | | Cytyc Corporation 10-K | | 12/31/2003 |
| | | |
| 10.36 | | Lease Agreement by and between Zona Franca Coyol S.A. and Cytyc Surgical Products Costa Rica S.A. dated April 23, 2007. | | 10-K | | 09/29/2007 |
| | | |
| 10.37 | | Lease Agreement by and between 445 Simarano Drive, Marlborough LLC and Cytyc dated July 11, 2006. | | 10-K | | 09/29/2007 |
| | | |
| 10.38 | | Lease Guaranty dated October 22, 2007 between Bel Marlborough I LLC and Hologic, as guarantor thereunder. | | 8-K | | 10/22/2007 |
86
| | | | | | |
| | | | | Incorporated by Reference |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date |
| | | |
| 10.39 | | Supply Agreement between Cytyc, Whatman, Inc. and Whatman SA dated as of December 31, 2000, as amended, October 16, 2001 and May 2, 2002. | | Cytyc Corporation 10-K | | 12/31/2002 |
| | | |
| 10.40 | | Form of Exchange Agreement. | | 8-K | | 02/29/2012 |
| | | |
| 10.41 | | Credit and Guaranty Agreement, dated as of August 1, 2012, by and among Hologic, the guarantors party thereto, Goldman Sachs Bank USA, as Administrative Agent and Collateral Agent, and the lenders party thereto. † | | 8-K/A | | 10/15/2012 |
| | | |
| 10.42 | | Pledge and Security Agreement, dated as of August 1, 2012, by and among the grantors party thereto and Goldman Sachs Bank USA, as Collateral Agent. | | 8-K/A | | 10/15/2012 |
| | | |
| 10.43 | | Purchase Agreement, dated July 19, 2012, by and among Hologic, Inc., the guarantors party thereto, Goldman, Sachs & Co. | | 8-K | | 07/19/2012 |
| | | |
| 10.44 | | Restated Agreement dated as of July 24, 2009 by and between Gen-Probe Incorporated and Novartis Vaccines and Diagnostics, Inc. ‡ | | Gen-Probe 10-Q/A | | 09/30/2009 |
| | | |
| 10.45 | | Supply Agreement for Panther Instrument System effective November 22, 2006 between Gen-Probe Incorporated and STRATEC Biomedical Systems AG. ‡ | | Gen-Probe 10-Q | | 09/30/2007 |
| | | |
| 21.1** | | Subsidiaries of Hologic. | | | | |
| | | |
| 23.1** | | Consent of Independent Registered Public Accounting Firm. | | | | |
| | | |
| 31.1** | | Certification of Hologic’s CEO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | | | |
| | | |
| 31.2** | | Certification of Hologic’s CFO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | | | |
| | | |
| 32.1*** | | Certification of Hologic’s CEO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | | | |
| | | |
| 32.2*** | | Certification of Hologic’s CFO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | | | |
| | | |
| 101.INS** | | XBRL Instance Document. | | | | |
| | | |
| 101.SCH** | | XBRL Taxonomy Extension Schema Document. | | | | |
| | | |
| 101.CAL** | | XBRL Taxonomy Extension Calculation Linkbase Document. | | | | |
| | | |
| 101.DEF** | | XBRL Taxonomy Extension Definition Linkbase Document. | | | | |
| | | |
| 101.LAB** | | XBRL Taxonomy Extension Label Linkbase Document. | | | | |
| | | |
| 101.PRE** | | XBRL Taxonomy Extension Presentation Linkbase Document. | | | | |
| * | Indicates management contract or compensatory plan or arrangement. |
| † | Confidential treatment has been requested for certain portions of this exhibit pursuant to Exchange Act Rule 24b-2. A complete version of this exhibit has been filed separately with the U.S. Securities and Exchange Commission. |
| ‡ | Confidential treatment has been granted with respect to certain portions of this exhibit. A complete version of this exhibit has been filed separately with the U.S. Securities and Exchange Commission. |
87
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| HOLOGIC, INC. |
| |
| By: | | /S/ ROBERT A. CASCELLA |
| | Robert A. Cascella |
| | Chief Executive Officer |
Date: November 28, 2012
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/S/ ROBERT A. CASCELLA | | Director, President, and Chief Executive Officer (Principal Executive Officer) | | November 28, 2012 |
| ROBERT A. CASCELLA | | |
| | |
/S/ GLENN P. MUIR | | Director, Executive Vice President, Finance and Administration, and Chief Financial Officer (Principal Financial Officer) | | November 28, 2012 |
| GLENN P. MUIR | | |
| | | |
| | |
/S/ ROBERT H. LAVALLEE | | Senior Vice President and Chief Accounting Officer (Principal Accounting Officer) | | November 28, 2012 |
| ROBERT H. LAVALLEE | | |
| | |
/S/ DAVID R. LAVANCE, JR. | | Chairman of the Board | | November 28, 2012 |
| DAVID R. LAVANCE, JR. | | |
| | |
/S/ SALLY W. CRAWFORD | | Director | | November 28, 2012 |
| SALLY W. CRAWFORD | | |
| | |
/S/ NANCY L. LEAMING | | Director | | November 28, 2012 |
| NANCY L. LEAMING | | |
| | |
/S/ LAWRENCE M. LEVY | | Director | | November 28, 2012 |
| LAWRENCE M. LEVY | | |
| | |
/S/ CHRISTIANA STAMOULIS | | Director | | November 28, 2012 |
| CHRISTIANA STAMOULIS | | |
| | |
/S/ ELAINE S. ULLIAN | | Director | | November 28, 2012 |
| ELAINE S. ULLIAN | | |
| | |
/S/ WAYNE WILSON | | Director | | November 28, 2012 |
| WAYNE WILSON | | |
Hologic, Inc.
Consolidated Financial Statements
Years ended September 29, 2012, September 24, 2011 and September 25, 2010
Contents
Report of Independent Registered Public Accounting Firm
on Consolidated Financial Statements
The Board of Directors and Stockholders of Hologic, Inc.:
We have audited the accompanying consolidated balance sheets of Hologic, Inc. as of September 29, 2012 and September 24, 2011 and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity and cash flows for each of the three years in the period ended September 29, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Hologic, Inc. at September 29, 2012 and September 24, 2011, and the consolidated results of its operations and cash flows for each of the three years in the period ended September 29, 2012, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Hologic, Inc.’s internal control over financial reporting as of September 29, 2012, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated November 28, 2012 expressed an unqualified opinion thereon.
|
| Boston, Massachusetts |
| November 28, 2012 |
F-2
Hologic, Inc.
Consolidated Statements of Operations
(In thousands, except per share data)
| | | | | | | | | | | | |
| | | Years ended | |
| | | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Revenues: | | | | | | | | | | | | |
Product sales | | $ | 1,657,728 | | | $ | 1,478,340 | | | $ | 1,414,900 | |
Service and other revenues | | | 344,924 | | | | 311,009 | | | | 264,652 | |
| | | | | | | | | | | | |
| | | 2,002,652 | | | | 1,789,349 | | | | 1,679,552 | |
| | | | | | | | | | | | |
Costs and expenses: | | | | | | | | | | | | |
Cost of product sales | | | 616,839 | | | | 521,189 | | | | 487,057 | |
Cost of product sales—amortization of intangible assets | | | 201,864 | | | | 177,456 | | | | 171,447 | |
Cost of product sales—impairment of intangible assets | | | — | | | | — | | | | 123,350 | |
Cost of service and other revenues | | | 189,512 | | | | 167,523 | | | | 161,060 | |
Research and development | | | 130,962 | | | | 116,696 | | | | 104,305 | |
Selling and marketing | | | 322,314 | | | | 286,730 | | | | 247,374 | |
General and administrative | | | 220,042 | | | | 158,793 | | | | 148,340 | |
Amortization of intangible assets | | | 72,036 | | | | 58,334 | | | | 54,858 | |
Contingent consideration—compensation expense | | | 81,031 | | | | 20,002 | | | | — | |
Contingent consideration—fair value adjustments | | | 38,466 | | | | (8,016 | ) | | | — | |
Impairment of goodwill | | | 5,826 | | | | — | | | | 76,723 | |
Impairment of intangible assets | | | — | | | | — | | | | 20,117 | |
Gain on sale of intellectual property, net | | | (12,424 | ) | | | (84,502 | ) | | | — | |
Litigation settlement charges, net | | | 452 | | | | 770 | | | | 11,403 | |
Acquired in-process research and development | | | 4,500 | | | | — | | | | 2,000 | |
Restructuring and divestiture charges, net | | | 17,515 | | | | (71 | ) | | | 1,581 | |
| | | | | | | | | | | | |
| | | 1,888,935 | | | | 1,414,904 | | | | 1,609,615 | |
| | | | | | | | | | | | |
Income from operations | | | 113,717 | | | | 374,445 | | | | 69,937 | |
Interest income | | | 2,340 | | | | 1,860 | | | | 1,278 | |
Interest expense | | | (140,287 | ) | | | (114,846 | ) | | | (127,107 | ) |
Debt extinguishment loss | | | (42,347 | ) | | | (29,891 | ) | | | — | |
Other income (expense), net | | | 4,916 | | | | (4,182 | ) | | | 901 | |
| | | | | | | | | | | | |
(Loss) income before income taxes | | | (61,661 | ) | | | 227,386 | | | | (54,991 | ) |
Provision for income taxes | | | 11,973 | | | | 70,236 | | | | 7,822 | |
| | | | | | | | | | | | |
Net (loss) income | | $ | (73,634 | ) | | $ | 157,150 | | | $ | (62,813 | ) |
| | | | | | | | | | | | |
Basic net (loss) income per common share | | $ | (0.28 | ) | | $ | 0.60 | | | $ | (0.24 | ) |
| | | | | | | | | | | | |
Diluted net (loss) income per common share | | $ | (0.28 | ) | | $ | 0.59 | | | $ | (0.24 | ) |
| | | | | | | | | | | | |
Weighted average number of common shares outstanding: | | | | | | | | | | | | |
Basic | | | 264,041 | | | | 261,099 | | | | 258,743 | |
| | | | | | | | | | | | |
Diluted | | | 264,041 | | | | 264,305 | | | | 258,743 | |
| | | | | | | | | | | | |
See accompanying notes.
F-3
Hologic, Inc.
Consolidated Statements of Comprehensive Income (Loss)
(In thousands)
| | | | | | | | | | | | |
| | | Years ended | |
| | | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Net (loss) income | | $ | (73,634 | ) | | $ | 157,150 | | | $ | (62,813 | ) |
Foreign currency translation adjustment | | | 6,217 | | | | 1,088 | | | | (4,763 | ) |
Adjustment to minimum pension liability (net of taxes of $636 in 2012, $327 in 2011 and $909 in 2010) | | | (1,484 | ) | | | 764 | | | | (2,122 | ) |
Unrealized gain on available-for-sale security (net of taxes of $36) | | | 62 | | | | — | | | | — | |
| | | | | | | | | | | | |
Other comprehensive income (loss) | | | 4,795 | | | | 1,852 | | | | (6,885 | ) |
| | | | | | | | | | | | |
Comprehensive (loss) income | | $ | (68,839 | ) | | $ | 159,002 | | | $ | (69,698 | ) |
| | | | | | | | | | | | |
See accompanying notes.
F-4
Hologic, Inc.
Consolidated Balance Sheets
(In thousands, except per share data)
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 560,430 | | | $ | 712,332 | |
Restricted cash | | | 5,696 | | | | 537 | |
Accounts receivable, less reserves of $6,396 and $6,516 respectively | | | 409,333 | | | | 318,712 | |
Inventories | | | 367,191 | | | | 230,544 | |
Deferred income tax assets | | | 11,715 | | | | 39,607 | |
Prepaid income taxes | | | 69,845 | | | | 10,098 | |
Prepaid expenses and other current assets | | | 44,301 | | | | 31,070 | |
Other current assets—assets held-for-sale | | | 94,503 | | | | — | |
| | | | | | | | |
Total current assets | | | 1,563,014 | | | | 1,342,900 | |
| | | | | | | | |
Property and equipment, at cost: | | | | | | | | |
Land | | | 51,430 | | | | 8,883 | |
Buildings and improvements | | | 156,665 | | | | 58,937 | |
Equipment and software | | | 296,776 | | | | 223,403 | |
Equipment under customer usage agreements | | | 249,692 | | | | 172,614 | |
Furniture and fixtures | | | 21,495 | | | | 12,401 | |
Leasehold improvements | | | 71,943 | | | | 43,554 | |
| | | | | | | | |
| | | 848,001 | | | | 519,792 | |
Less accumulated depreciation and amortization | | | (340,003 | ) | | | (281,126 | ) |
| | | | | | | | |
| | | 507,998 | | | | 238,666 | |
| | | | | | | | |
Intangible assets, net | | | 4,301,250 | | | | 2,090,807 | |
Goodwill | | | 3,942,779 | | | | 2,290,330 | |
Other assets | | | 162,067 | | | | 46,077 | |
| | | | | | | | |
Total assets | | $ | 10,477,108 | | | $ | 6,008,780 | |
| | | | | | | | |
Liabilities and Stockholders’ Equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 87,223 | | | $ | 63,467 | |
Accrued expenses | | | 372,381 | | | | 325,327 | |
Deferred revenue | | | 129,688 | | | | 120,656 | |
Current portion of long-term debt | | | 64,435 | | | | — | |
Other current liabilities—assets held-for-sale | | | 7,622 | | | | — | |
| | | | | | | | |
Total current liabilities | | | 661,349 | | | | 509,450 | |
| | | | | | | | |
Long-term debt, net of current portion | | | 4,971,179 | | | | 1,488,580 | |
Deferred income tax liabilities | | | 1,771,585 | | | | 957,426 | |
Deferred service obligations—long-term | | | 13,714 | | | | 9,467 | |
Other long-term liabilities | | | 98,250 | | | | 106,962 | |
Commitments and contingencies (Notes 12 and 13) | | | | | | | | |
Stockholders’ equity | | | | | | | | |
Preferred stock, $0.01 par value—1,623 shares authorized; 0 shares issued | | | — | | | | — | |
Common stock, $0.01 par value—750,000 shares authorized; 265,635 and 262,459 shares issued, respectively | | | 2,656 | | | | 2,625 | |
Additional paid-in-capital | | | 5,396,657 | | | | 5,303,713 | |
Accumulated deficit | | | (2,443,554 | ) | | | (2,369,920 | ) |
Accumulated other comprehensive income | | | 6,790 | | | | 1,995 | |
Treasury stock, at cost—219 shares | | | (1,518 | ) | | | (1,518 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 2,961,031 | | | | 2,936,895 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 10,477,108 | | | $ | 6,008,780 | |
| | | | | | | | |
See accompanying notes.
F-5
Hologic, Inc.
Consolidated Statements of Stockholders’ Equity
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Additional
Paid-in-
Capital | | | Accumulated
Deficit | | | Accumulated
Other
Comprehensive
Income | | | Treasury Stock | | | Total
Stockholders’
Equity | |
| | | Number of
Shares | | | Par Value | | | | | | Number of
Shares | | | Amount | | |
Balance at September 26, 2009 | | | 257,938 | | | $ | 2,579 | | | $ | 5,182,060 | | | $ | (2,464,257 | ) | | $ | 7,028 | | | | 214 | | | $ | (1,433 | ) | | $ | 2,725,977 | |
Exercise of stock options | | | 1,123 | | | | 12 | | | | 11,112 | | | | | | | | | | | | | | | | | | | | 11,124 | |
Issuance of common stock to employees upon vesting of restricted stock units, net of shares withheld for employee taxes | | | 331 | | | | 3 | | | | (2,442 | ) | | | — | | | | — | | | | 5 | | | | (85 | ) | | | (2,524 | ) |
Issuance of common shares under the employee stock purchase plan | | | 96 | | | | 1 | | | | 1,436 | | | | — | | | | — | | | | — | | | | — | | | | 1,437 | |
Stock-based compensation expense | | | — | | | | — | | | | 34,160 | | | | — | | | | — | | | | — | | | | — | | | | 34,160 | |
Excess tax benefit from employee equity awards | | | — | | | | — | | | | 757 | | | | — | | | | — | | | | — | | | | — | | | | 757 | |
Purchase of non-controlling interest | | | — | | | | — | | | | (2,684 | ) | | | — | | | | — | | | | — | | | | — | | | | (2,684 | ) |
Net loss | | | — | | | | — | | | | — | | | | (62,813 | ) | | | — | | | | — | | | | — | | | | (62,813 | ) |
Foreign currency translation adjustment | | | ��� | | | | — | | | | — | | | | — | | | | (4,763 | ) | | | — | | | | — | | | | (4,763 | ) |
Adjustment to minimum pension liability, net | | | — | | | | — | | | | — | | | | — | | | | (2,122 | ) | | | — | | | | — | | | | (2,122 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 25, 2010 | | | 259,488 | | | | 2,595 | | | | 5,224,399 | | | | (2,527,070 | ) | | | 143 | | | | 219 | | | | (1,518 | ) | | | 2,698,549 | |
Exercise of stock options | | | 1,779 | | | | 18 | | | | 23,876 | | | | — | | | | — | | | | — | | | | — | | | | 23,894 | |
Issuance of common stock to employees upon vesting of restricted stock units, net of shares withheld for employee taxes | | | 1,104 | | | | 11 | | | | (10,410 | ) | | | — | | | | — | | | | — | | | | — | | | | (10,399 | ) |
Issuance of common shares under the employee stock purchase plan | | | 88 | | | | 1 | | | | 1,509 | | | | — | | | | — | | | | — | | | | — | | | | 1,510 | |
Stock-based compensation expense | | | — | | | | — | | | | 35,472 | | | | — | | | | — | | | | — | | | | — | | | | 35,472 | |
Reduction in excess tax benefit from employee equity awards | | | — | | | | — | | | | (5,832 | ) | | | — | | | | — | | | | — | | | | — | | | | (5,832 | ) |
Allocation of equity component related to convertible notes exchange, net of taxes | | | — | | | | — | | | | 34,699 | | | | — | | | | — | | | | — | | | | — | | | | 34,699 | |
Net income | | | — | | | | — | | | | — | | | | 157,150 | | | | — | | | | — | | | | — | | | | 157,150 | |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 1,088 | | | | — | | | | — | | | | 1,088 | |
Adjustment to minimum pension liability, net | | | — | | | | — | | | | — | | | | — | | | | 764 | | | | — | | | | — | | | | 764 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 24, 2011 | | | 262,459 | | | | 2,625 | | | | 5,303,713 | | | | (2,369,920 | ) | | | 1,995 | | | | 219 | | | | (1,518 | ) | | | 2,936,895 | |
Exercise of stock options | | | 2,457 | | | | 24 | | | | 27,663 | | | | — | | | | — | | | | — | | | | — | | | | 27,687 | |
Issuance of common stock to employees upon vesting of restricted stock units, net of shares withheld for employee taxes | | | 673 | | | | 7 | | | | (5,717 | ) | | | — | | | | — | | | | — | | | | — | | | | (5,710 | ) |
Issuance of common shares under the employee stock purchase plan | | | 46 | | | | — | | | | 907 | | | | — | | | | — | | | | — | | | | — | | | | 907 | |
Stock-based compensation expense | | | — | | | | — | | | | 40,011 | | | | — | | | | — | | | | — | | | | — | | | | 40,011 | |
Excess tax benefit from employee equity awards | | | — | | | | — | | | | 4,413 | | | | — | | | | — | | | | — | | | | — | | | | 4,413 | |
Fair value of options exchanged in a business combination | | | — | | | | — | | | | 2,655 | | | | — | | | | — | | | | — | | | | — | | | | 2,655 | |
Allocation of equity component related to convertible notes exchange, net of taxes | | | — | | | | — | | | | 23,012 | | | | — | | | | — | | | | — | | | | — | | | | 23,012 | |
Net loss | | | — | | | | — | | | | — | | | | (73,634 | ) | | | — | | | | — | | | | — | | | | (73,634 | ) |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 6,217 | | | | — | | | | — | | | | 6,217 | |
Adjustment to minimum pension liability, net | | | — | | | | — | | | | — | | | | — | | | | (1,484 | ) | | | — | | | | — | | | | (1,484 | ) |
Unrealized gain on marketable security | | | — | | | | — | | | | — | | | | — | | | | 62 | | | | — | | | | — | | | | 62 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 29, 2012 | | | 265,635 | | | $ | 2,656 | | | $ | 5,396,657 | | | $ | (2,443,554 | ) | | $ | 6,790 | | | | 219 | | | $ | (1,518 | ) | | $ | 2,961,031 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
F-6
Hologic, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Operating activities | | | | | | | | | | | | |
Net (loss) income | | $ | (73,634 | ) | | $ | 157,150 | | | $ | (62,813 | ) |
Adjustments to reconcile net (loss) income to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation | | | 71,851 | | | | 68,946 | | | | 68,463 | |
Amortization | | | 273,900 | | | | 235,790 | | | | 226,305 | |
Non-cash interest expense—amortization of debt discount and deferred financing costs | | | 74,974 | | | | 76,814 | | | | 86,638 | |
Impairment of goodwill | | | 5,826 | | | | — | | | | 76,723 | |
Impairment of intangible assets | | | — | | | | — | | | | 143,467 | |
Stock-based compensation expense | | | 40,572 | | | | 35,472 | | | | 34,160 | |
Excess tax benefit related to equity awards | | | (6,206 | ) | | | (3,652 | ) | | | (2,043 | ) |
Deferred income taxes | | | (155,192 | ) | | | (48,107 | ) | | | (121,726 | ) |
Gain on sale of intellectual property, net | | | (12,424 | ) | | | (84,502 | ) | | | — | |
Debt extinguishment loss | | | 42,347 | | | | 29,891 | | | | — | |
Fair value adjustments to contingent consideration | | | 38,466 | | | | (8,016 | ) | | | — | |
Fair value write-up of inventory sold | | | 19,918 | | | | 3,298 | | | | 732 | |
Non-cash restructuring charges | | | 16,901 | | | | — | | | | — | |
Acquired in-process research and development | | | 4,500 | | | | — | | | | 2,000 | |
Impairment of cost-method investments | | | — | | | | 2,445 | | | | 1,100 | |
Loss on disposal of property and equipment | | | 3,809 | | | | 2,639 | | | | 3,765 | |
(Gain) loss on divestiture | | | — | | | | (354 | ) | | | 341 | |
Other non-cash activity | | | (3,568 | ) | | | 1,447 | | | | 1,008 | |
Changes in operating assets and liabilities, excluding the effect of acquisitions: | | | | | | | | | | | | |
Accounts receivable | | | (11,005 | ) | | | (17,131 | ) | | | (20,211 | ) |
Inventories | | | (12,174 | ) | | | (32,158 | ) | | | (5,247 | ) |
Prepaid income taxes | | | 6,071 | | | | (6,154 | ) | | | (3,772 | ) |
Prepaid expenses and other assets | | | 69,806 | | | | (471 | ) | | | (254 | ) |
Accounts payable | | | 3,768 | | | | 2,589 | | | | 7,151 | |
Accrued expenses and other liabilities | | | (41,017 | ) | | | 40,569 | | | | (348 | ) |
Deferred revenue | | | 12,733 | | | | (481 | ) | | | 21,273 | |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 370,222 | | | | 456,024 | | | | 456,712 | |
| | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | |
Acquisition of businesses, net of cash acquired | | | (3,762,403 | ) | | | (198,744 | ) | | | (84,322 | ) |
Payment of additional acquisition consideration | | | (9,784 | ) | | | (19,660 | ) | | | — | |
Divestiture activities, net of cash transferred | | | — | | | | 2,267 | | | | (1,035 | ) |
Proceeds from sale of intellectual property | | | 12,500 | | | | 13,250 | | | | 73,000 | |
Purchase of property and equipment | | | (33,149 | ) | | | (27,785 | ) | | | (28,010 | ) |
Increase in equipment under customer usage agreements | | | (45,624 | ) | | | (27,878 | ) | | | (18,648 | ) |
Purchase of licensed technology and other intangible assets | | | — | | | | (3,021 | ) | | | (500 | ) |
Purchase of insurance contracts | | | — | | | | (5,322 | ) | | | (5,322 | ) |
Acquisition of in-process research and development assets | | | (4,500 | ) | | | — | | | | (2,000 | ) |
Proceeds from sale of cost method investment | | | — | | | | — | | | | 678 | |
Purchases of cost method investments | | | (250 | ) | | | (99 | ) | | | (795 | ) |
(Increase) decrease in restricted cash | | | (5,159 | ) | | | 405 | | | | (26 | ) |
Increase in other assets | | | (2,415 | ) | | | — | | | | — | |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (3,850,784 | ) | | | (266,587 | ) | | | (66,980 | ) |
| | | | | | | | | | | | |
F-7
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Financing activities | | | | | | | | | | | | |
Proceeds from long-term debt | | | 3,476,320 | | | | — | | | | — | |
Repayment of long-term debt and notes payable | | | — | | | | (1,362 | ) | | | (177,004 | ) |
Payment of debt issuance costs | | | (81,408 | ) | | | (5,327 | ) | | | — | |
Payment of contingent consideration | | | (51,680 | ) | | | (4,294 | ) | | | — | |
Payment of deferred acquisition consideration | | | (44,223 | ) | | | — | | | | — | |
Purchase of non-controlling interest | | | — | | | | — | | | | (2,684 | ) |
Net proceeds from issuance of common stock pursuant to employee stock plans | | | 28,594 | | | | 25,404 | | | | 12,594 | |
Excess tax benefit related to equity awards | | | 6,206 | | | | 3,652 | | | | 2,043 | |
Payment of employee restricted stock minimum tax withholdings | | | (5,710 | ) | | | (10,399 | ) | | | (2,524 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 3,328,099 | | | | 7,674 | | | | (167,575 | ) |
| | | | | | | | | | | | |
Effect of exchange rate changes on cash and cash equivalents | | | 561 | | | | (404 | ) | | | 282 | |
| | | | | | | | | | | | |
Net increase in cash and cash equivalents | | | (151,902 | ) | | | 196,707 | | | | 222,439 | |
Cash and cash equivalents, beginning of year | | | 712,332 | | | | 515,625 | | | | 293,186 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of year | | $ | 560,430 | | | $ | 712,332 | | | $ | 515,625 | |
| | | | | | | | | | | | |
See accompanying notes.
F-8
Hologic, Inc.
Notes to Consolidated Financial Statements
(all tabular amounts in thousands except per share data)
Hologic, Inc. (the “Company” or “Hologic”) develops, manufactures and distributes premium diagnostics, medical imaging systems and surgical products dedicated to serving the healthcare needs of women. The Company’s core business segments are focused on breast health, diagnostics, GYN surgical and skeletal health.
On August 1, 2012, the Company completed the acquisition of Gen-Probe Incorporated (“Gen-Probe”), which resulted in the Company significantly expanding its suite of molecular diagnostic products. Gen-Probe develops, manufactures and markets rapid, accurate and cost effective molecular diagnostics products and services that are used primarily to diagnose human diseases, screen donated blood, and test transplant compatibility. Gen-Probe’s results of operations are reported within the Company’s diagnostics reportable segment. The Company’s acquisition of Gen-Probe is more fully described in Note 3. In connection with the acquisition, the Company borrowed $3.5 billion in aggregate principal to fund a portion of the purchase price, which is described in Note 5.
| 2. | Summary of Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation. The Company’s fiscal year ends on the last Saturday in September. Fiscal 2012, 2011 and 2010 ended on September 29, 2012, September 24, 2011 and September 25, 2010, respectively. Fiscal 2012 was a 53 week fiscal period and fiscal 2011 and 2010 were 52 week fiscal periods.
Management’s Estimates and Uncertainties
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make significant estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Significant estimates and assumptions by management affect the Company’s revenue recognition for multiple element arrangements, allowance for doubtful accounts, the net realizable value of inventory, estimated fair value of cost-method equity investments, valuations, purchase price allocations and contingent consideration related to business combinations, expected future cash flows including growth rates, discount rates, terminal values and other assumptions and estimates used to evaluate the recoverability of long-lived assets and goodwill, estimated fair values of intangible assets and goodwill, amortization methods and periods, warranty reserves, certain accrued expenses, restructuring and other related charges, stock-based compensation, contingent liabilities, tax reserves and recoverability of the Company’s net deferred tax assets and related valuation allowance.
Although the Company regularly assesses these estimates, actual results could differ materially from these estimates. Changes in estimates are recorded in the period in which they become known. The Company bases its estimates on historical experience and various other assumptions that it believes to be reasonable under the circumstances.
The Company is subject to a number of risks similar to those of other companies of similar size in its industry, including, dependence on third-party reimbursements to support the markets of the Company’s products, early stage of development of certain products, rapid technological changes, recoverability of long-lived assets (including intangible assets and goodwill), competition, stability of world financial markets, ability to obtain regulatory approvals, changes in the regulatory environment, limited number of suppliers, customer concentration, integration of acquisitions, substantial indebtedness, government regulations, future sales or issuances of its common stock, management of international activities, protection of proprietary rights, patent and other litigation and dependence on key individuals.
Cash Equivalents
Cash equivalents are highly liquid investments with insignificant interest rate risk and maturities of three months or less at the time of acquisition. At September 29, 2012 and September 24, 2011, the Company’s cash equivalents consisted of money market accounts.
Marketable Securities
As a result of its acquisition of Gen-Probe, the Company assumed certain marketable securities, which were comprised of an equity security and mutual funds. The equity security is an investment in the common stock of a publicly traded company, and the
F-9
mutual funds are to fund the Gen-Probe deferred compensation plan. The equity security is classified as available-for-sale and is recorded at fair value with the unrealized gains or losses, net of tax, within accumulated other comprehensive income (loss), which is a component of stockholders’ equity. The mutual funds are classified as trading and are recorded at fair value with unrealized gains and losses recorded in interest income in the Consolidated Statements of Operations.
The Company periodically reviews its marketable equity securities classified as available-for-sale for other-than-temporary declines in fair value below cost basis, or whenever events or circumstances indicate that the carrying amount of an asset may not be recoverable. The determination that a decline is other-than-temporary is, in part, subjective and influenced by many factors. When assessing marketable equity securities for other-than-temporary declines in value, the Company considers factors including: the significance of the decline in value compared to the cost basis; the underlying factors contributing to a decline in the prices of the security; how long the market value of the investment has been less than its cost basis; any market conditions that impact liquidity; the views of external investment analysts; the financial condition and near-term prospects of the investee; any news or financial information that has been released specific to the investee; and the outlook for the overall industry in which the investee operates. No such impairment appeared to exist at September 29, 2012.
The Company has one investment in a publicly traded security and the following reconciles its cost basis to its fair market value as of September 29, 2012. There were no marketable securities at September 24, 2011.
| | | | | | | | | | | | | | | | |
| | | Cost | | | Gross Unrealized
Gains | | | Gross Unrealized
Losses | | | Fair Value | |
| | | | |
Equity security | | $ | 5,931 | | | $ | 98 | | | $ | — | | | $ | 6,029 | |
Concentrations of Credit Risk
Financial instruments that subject the Company to credit risk primarily consist of cash and cash equivalents, cost-method equity investments, and trade accounts receivable. The Company invests its cash and cash equivalents with high credit quality financial institutions.
The Company’s customers are principally located in the United States, Europe and Asia. The Company performs ongoing credit evaluations of the financial condition of its customers and generally does not require collateral. Although the Company is directly affected by the overall financial condition of the healthcare industry, as well as global economic conditions, management does not believe significant credit risk exists as of September 29, 2012. The Company generally has not experienced any material losses related to receivables from individual customers or groups of customers in the health care industry. The Company maintains an allowance for doubtful accounts based on accounts past due and historical collection experience.
There were no customers with balances greater than 10% of accounts receivable as of September 29, 2012 and September 24, 2011, nor customers that represented greater than 10% of total revenues for fiscal years 2012, 2011 and 2010.
Supplemental Cash Flow Statement Information
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Cash paid during the period for income taxes | | $ | 166,565 | | | $ | 118,850 | | | $ | 130,486 | |
| | | | | | | | | | | | |
Cash paid during the period for interest | | $ | 55,045 | | | $ | 36,268 | | | $ | 39,382 | |
| | | | | | | | | | | | |
Non-Cash Investing Activities: | | | | | | | | | | | | |
Fair value of stock options assumed in the Gen-Probe acquisition | | $ | 2,655 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
Additional acquisition contingent consideration accrued | | $ | — | | | $ | 18,924 | | | $ | 32,489 | |
| | | | | | | | | | | | |
Non-Cash Financing Activities: | | | | | | | | | | | | |
Fair value of contingent consideration at acquisition | | $ | — | | | $ | 86,600 | | | $ | 29,500 | |
| | | | | | | | | | | | |
Deferred payments for acquisitions | | $ | 1,655 | | | $ | 47,258 | | | $ | — | |
| | | | | | | | | | | | |
Inventories
Inventories are valued at the lower of cost or market on a first in, first out basis. Work-in-process and finished goods inventories consist of materials, labor and manufacturing overhead. The valuation of inventory requires management to estimate excess and obsolete inventory. The Company employs a variety of methodologies to determine the net realizable value of its inventory.
F-10
Provisions for excess and obsolete inventory are primarily based on management’s estimates of forecasted net sales and service usage levels. A significant change in the timing or level of demand for the Company’s products as compared to forecasted amounts may result in recording additional provisions for excess and obsolete inventory in the future. The Company records provisions for excess and obsolete inventory as cost of product sales.
Inventories consisted of the following:
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Raw materials | | $ | 134,983 | | | $ | 119,991 | |
Work-in-process | | | 93,218 | | | | 23,908 | |
Finished goods | | | 138,990 | | | | 86,645 | |
| | | | | | | | |
| | $ | 367,191 | | | $ | 230,544 | |
| | | | | | | | |
Property and Equipment
Property and equipment is recorded at cost less allowances for depreciation. The straight-line method of depreciation is used for all property and equipment. Repair and maintenance costs are expensed as incurred. Property and equipment are depreciated over the following estimated useful lives:
| | |
Asset Classification | | Estimated Useful Life |
| Building and improvements | | 35–40 years |
| Equipment and software | | 3–10 years |
| Equipment under customer usage agreements | | 3–8 years |
| Furniture and fixtures | | 5–7 years |
| Leasehold improvements | | Shorter of the Original Term of Lease or Estimated Useful Life |
Equipment under customer usage agreements primarily consists of diagnostic instrumentation and medical imaging equipment located at customer sites but owned by the Company. Generally, the customer has the right to use it for a period of time provided they meet certain agreed to conditions. The Company recovers the cost of providing the equipment from the sale of disposables. The depreciation costs associated with equipment under customer usage agreements are charged to cost of product sales over the estimated useful life of the equipment. The costs to maintain the equipment in the field are charged to cost of product sales as incurred.
Long-Lived Assets
The Company reviews its long-lived assets, which includes property and equipment and identifiable intangible assets (see below for discussion of intangible assets), for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable in accordance with ASC 360-10-35-15,Property, Plant and Equipment—Impairment or Disposal of Long-Lived Assets (ASC 360). Recoverability of these assets is evaluated by comparing the carrying value of the assets to the undiscounted cash flows estimated to be generated by those assets over their remaining economic life. If the undiscounted cash flows are not sufficient to recover the carrying value of the assets, the assets are considered impaired. The impairment loss is measured by comparing the fair value of the assets to their carrying value. Fair value is determined by either a quoted market price, if any, or a value determined by a discounted cash flow technique. At the end of the second quarter of fiscal 2012, the Company decided to cease manufacturing, marketing and selling its Adiana system, which was a product line within the Company’s GYN Surgical reporting segment, determining that the product was not financially viable and would not become so in the foreseeable future. As a result, in fiscal 2012, the Company recorded charges of $19.5 million of which $6.5 million was recorded within cost of product sales to write down certain manufacturing equipment and equipment placed at customer sites to its fair value that had no further utility. There were no material impairment charges related to property and equipment in fiscal 2011 and 2010.
Business Combinations and Acquisition of Intangible Assets
The Company records tangible and intangible assets acquired in business combinations under the purchase method of accounting. The Company accounts for acquisitions in accordance with ASC 805,Business Combinations. Amounts paid for each acquisition are allocated to the assets acquired and liabilities assumed based on their fair values at the dates of acquisition. The Company allocates the purchase price in excess of the fair value of the net tangible assets acquired to identifiable intangible assets, including purchased research and development, based on detailed valuations that use certain information and assumptions provided by management. The Company allocates any excess purchase price over the fair value of the net tangible and intangible assets acquired to goodwill. The use of alternative valuation assumptions, including estimated cash flows and discount rates, and alternative useful life assumptions could result in different purchase price allocations and intangible asset amortization expense in current and future periods.
F-11
The valuation of purchased research and development as part of a business combination represents the estimated fair value at the dates of acquisition related to in-process projects. The Company’s purchased research and development represents the value of in-process projects that have not yet reached technological feasibility and have no alternative future uses as of the date of acquisition. As required by ASC 805, the Company capitalizes the value attributable to these in-process projects at the time of the acquisition pursuant to ASC 805. Subsequent to acquisition, in-process research and development is evaluated as an indefinite-lived intangible asset, consistent with the accounting treatment of goodwill. No additional amounts are capitalized and once the project is completed the asset is amortized over its estimated useful life. If the projects are not successful or completed in a timely manner, the Company may not realize the financial benefits expected for these projects or for the acquisitions as a whole and impairments may result.
The Company uses the income approach to determine the fair value of its purchased research and development acquired in a business combination. This approach determines fair value by estimating the after-tax cash flows attributable to an in-process project over its useful life and then discounting these after-tax cash flows back to a present value. The Company bases its revenue assumptions on estimates of relevant market sizes, expected market growth rates, expected trends in technology and expected product introductions by competitors. In arriving at the value of the in-process projects, the Company considers, among other factors, the in-process projects’ stage of completion, the complexity of the work completed as of the acquisition date, the costs already incurred, the projected costs to complete, the contribution of core technologies and other acquired assets, the expected introduction date and the estimated useful life of the technology. The Company bases the discount rate used to arrive at a present value as of the date of acquisition on the time value of money and medical technology investment risk factors. The Company believes that the estimated purchased research and development amounts so determined represent the fair value at the date of acquisition and do not exceed the amount a third-party would pay for the projects.
The Company also uses the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including developed technology, customer relationships, trade names and business licenses. Developed technology represents patented and unpatented technology and know-how. Customer relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Trade names represent acquired company and product names.
Intangible Assets and Goodwill
Intangible Assets
Intangible assets are initially recorded at fair value and stated net of accumulated amortization and impairments. The Company amortizes its intangible assets that have finite lives using either the straight-line method, or if reliably determinable, based on the pattern in which the economic benefit of the asset is expected to be utilized. Amortization is recorded over the estimated useful lives ranging from 2 to 30 years. The Company evaluates the realizability of its definite lived intangible assets whenever events or changes in circumstances or business conditions indicate that the carrying value of these assets may not be recoverable based on expectations of future undiscounted cash flows for each asset group. If the carrying value of an asset or asset group exceeds its undiscounted cash flows, the Company estimates the fair of the assets, generally utilizing a discounted cash flow analysis based on the present value of estimated future cash flows to be generated by the assets using a risk-adjusted discount rate. To estimate the fair value of the assets, the Company uses market participant assumptions pursuant to ASC 820,Fair Value Measurements.
During the fourth quarter of fiscal 2012 in connection with the company-wide annual budgeting and strategic planning process, the Company determined that indicators of impairment existed in its MammoSite reporting unit, which is included in the Breast Health reportable segment. The impairment indicators were due to a reduction in the Company’s revenue projections and long-term growth rates as a result of the continuing deterioration of the brachytherapy market and competition from existing technologies. The Company’s cash flow estimates were based upon historical cash flows, as well as future projected cash flows derived from the company-wide annual planning process. The analysis indicated that MammoSite’s long-lived assets were recoverable based on the undiscounted cash flows over the remaining life of the predominant long-lived asset. The Company believes that its procedures for estimating future cash flows were reasonable and consistent with market conditions at the measurement date.
During the fourth quarter of fiscal 2010 in connection with the company-wide annual budgeting and strategic planning process, the Company determined that indicators of impairment existed in its MammoSite reporting unit. The impairment indicators were due to changing market conditions for the breast brachytherapy market, including downward pressure on procedure volumes due to the continuing adverse economic environment and current trends in breast cancer management, as well as competitive pricing pressures and competition from existing and alternative new technologies. These factors resulted in the Company lowering its financial projections for MammoSite. As a result, the Company performed the first step in the long-lived assets impairment test pursuant to ASC 360 and compared MammoSite’s forecasted undiscounted cash flows to the carrying value of its net assets. These cash flows were insufficient to recover MammoSite’s carrying value. Therefore, the Company determined the fair value of MammoSite’s long-lived assets, which are primarily intangible assets, using a discounted cash flow technique. The expected future cash flows are Level 3 inputs under ASC 820 and are those expected to be generated by market participants. Based on the estimated fair value of the long-lived
F-12
assets, the Company recorded an aggregate impairment charge of $143.5 million to write down these intangible assets to their fair value. The charge was comprised of $123.4 million related to developed technology, which was recorded in cost of product sales in the Consolidated Statement of Operations, $11.8 million related to customer relationships and $8.3 million related to trade names, which were recorded in impairment of intangible assets in the Consolidated Statements of Operations. In addition, the Company recorded a goodwill impairment charge of $76.7 million (see below for further discussion).
During the fourth quarter of fiscal 2012 and 2010, the Company acquired certain in-process research and development assets that were not part of a business acquisition. Since these assets had no alternative future use, the Company recorded in-process research and development charges of $4.5 million and $2.0 million in fiscal 2012 and 2010, respectively.
Intangible assets consist of the following:
| | | | | | | | | | | | | | | | |
| | | As of September 29, 2012 | | | As of September 24, 2011 | |
Description | | Gross
Carrying
Value | | | Accumulated
Amortization | | | Gross
Carrying
Value | | | Accumulated
Amortization | |
Developed technology | | $ | 3,784,689 | | | $ | 788,274 | | | $ | 2,215,323 | | | $ | 586,647 | |
In-process research and development | | | 227,000 | | | | — | | | | 840 | | | | — | |
Customer relationships and contracts | | | 1,097,842 | | | | 205,612 | | | | 507,974 | | | | 150,039 | |
Trade names | | | 240,092 | | | | 60,318 | | | | 142,799 | | | | 44,267 | |
Patents | | | 11,417 | | | | 7,906 | | | | 9,937 | | | | 7,752 | |
Business licenses | | | 2,577 | | | | 344 | | | | 2,535 | | | | 81 | |
Non-compete agreements | | | 310 | | | | 223 | | | | 297 | | | | 112 | |
| | | | | | | | | | | | | | | | |
| | $ | 5,363,927 | | | $ | 1,062,677 | | | $ | 2,879,705 | | | $ | 788,898 | |
| | | | | | | | | | | | | | | | |
During 2012, the in-process research and development project from the Healthcome acquisition was completed and transferred to developed technology. In October 2012, one of the in-process research and development projects from the Gen-Probe acquisition, valued at $7.0 million, was completed.
Amortization expense related to developed technology and patents is classified as a component of cost of product sales—amortization of intangible assets in the Consolidated Statements of Operations. Amortization expense related to customer relationships and contracts, trade names, business licenses and non-competes is classified as a component of amortization of intangible assets in the Consolidated Statements of Operations.
The estimated amortization expense at September 29, 2012 for each of the five succeeding fiscal years is as follows:
| | | | |
Fiscal 2013 | | $ | 413,310 | |
Fiscal 2014 | | | 398,747 | |
Fiscal 2015 | | | 383,914 | |
Fiscal 2016 | | | 370,106 | |
Fiscal 2017 | | | 361,061 | |
Goodwill
In accordance with ASC 350,Intangibles—Goodwill and Other(ASC 350),the Company tests goodwill at the reporting unit level for impairment on an annual basis and between annual tests if events and circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to, current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business, and an adverse action or assessment by a regulator.
In performing the impairment test, the Company utilizes the two-step approach prescribed under ASC 350. The first step requires a comparison of the carrying value of each reporting unit to its estimated fair value. To estimate the fair value of its reporting units for Step 1, the Company primarily utilizes the income approach. The income approach is based on a discounted cash flow analysis (“DCF”) and calculates the fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting the after-tax cash flows to a present value using a risk-adjusted discount rate. Assumptions used in the DCF require significant judgment, including judgment about appropriate discount rates and terminal values, growth rates, and the amount and timing of expected future cash flows. The forecasted cash flows are based on the Company’s most recent budget and for years beyond the budget, the Company’s estimates are based on assumed growth rates. The Company believes its assumptions are consistent with the plans and estimates used to manage the underlying businesses. The discount rates, which are intended to reflect the risks inherent in future cash flow projections, used in the DCF are based on estimates of the weighted-average cost of capital (“WACC”) of market participants relative to each respective reporting unit. The market approach considers comparable market data based on multiples of revenue or earnings before interest, taxes, depreciation and amortization (“EBITDA”) and is primarily used as a corroborative analysis to the results of the DCF. The Company believes its assumptions used to determine the fair value of its respective reporting units are
F-13
reasonable. If different assumptions were used, particularly with respect to forecasted cash flows, terminal values, WACCs, or market multiples, different estimates of fair value may result and there could be the potential that an impairment charge could result. Actual operating results and the related cash flows of the reporting units could differ from the estimated operating results and related cash flows.
If the carrying value of a reporting unit exceeds its estimated fair value, the Company is required to perform the second step of the goodwill impairment test to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of a reporting unit’s goodwill to its carrying value. The implied fair value of goodwill is derived by performing a hypothetical purchase price allocation for each reporting unit as of the measurement date and allocating the reporting unit’s estimated fair value to its assets and liabilities. The residual amount from performing this allocation represents the implied fair value of goodwill. To the extent this amount is below the carrying value of goodwill, an impairment charge is recorded.
The Company conducted its fiscal 2012 annual impairment test on the first day of the fourth quarter. The Company utilized DCF and market approaches to estimate the fair value of its reporting units as of June 24, 2012, and ultimately used the fair value determined by the DCF in making its impairment test conclusions. The Company believes it has used reasonable estimates and assumptions about future revenue, cost projections, cash flows and market multiples. In addition, using a DCF requires the use of a risk-adjusted discount rate for which the Company based its rate on the WACC of market participants. As a result of completing Step 1, all of the Company’s reporting units, except MammoSite, had fair values exceeding their carrying values, and as such, Step 2 of the impairment test was not required. MammoSite’s fair value has declined from fiscal 2011 primarily due to a reduction in the Company’s revenue projections and long-term growth rates. The changes in MammoSite’s financial projections were a result of the continuing deterioration of the brachytherapy market, and competition from existing technologies. The Company performed the Step 2 analysis for MammoSite, consistent with the procedures described above, and recorded a $5.8 million goodwill impairment charge, resulting in no remaining goodwill for this reporting unit.
For the Company’s other reporting units, if their respective fair values had been lower by 10%, each reporting unit would have still passed Step 1 of the goodwill impairment test. Since, the fair value of the reporting units was determined by use of the DCF, and the key assumptions that drive the fair value in this model are the WACC, terminal values, growth rates, and the amount and timing of expected future cash flows, significant judgment is applied in determining fair value. If the current economic environment were to deteriorate, this would likely result in a higher WACC because market participants would require a higher rate of return. In the DCF as the WACC increases, the fair value decreases. The other significant factor in the DCF is our projected financial information (i.e., amount and timing of expected future cash flows and growth rates) and if these assumptions were to be adversely impacted, this could result in a reduction of the fair values of these reporting units.
The Company previously had ongoing litigation with Conceptus regarding potential patent infringement of a Conceptus patent by the Company’s Adiana system. In the first quarter of fiscal 2012, the jury returned a verdict in favor of Conceptus and awarded Conceptus $18.8 million in damages. Post trial motions were filed, and Conceptus sought to enjoin the Company from further sales of the Adiana system. At the time, the Company was appealing the jury verdict. The jury verdict in the first quarter of fiscal 2012 and related subsequent litigation status was an indicator of impairment for the Company’s GYN Surgical reporting unit, and a reduction in the anticipated future cash flows of the GYN Surgical reporting unit could result in a material impairment charge. Accordingly, the Company performed an interim goodwill impairment analysis of the GYN Surgical reporting unit as of December 24, 2011, updating its cash flow projections and related assumptions from its fiscal 2011 annual impairment test, including the WACC, under various potential scenarios. The Company applied the weighted average probability approach to these scenarios to estimate the fair value of the GYN Surgical reporting unit. As a result of completing Step 1, GYN Surgical’s fair value exceeded its carrying value. Therefore, Step 2 of the impairment test was not required as of December 24, 2011. The Company believed it used reasonable estimates and assumptions about future revenue, cost projections, cash flows, probabilities of cash flow scenarios, and market multiples as of that measurement date.
In connection with the Company’s decision to discontinue the Adiana product line in the second quarter of fiscal 2012 and the Company’s updated lower forecast for the GYN Surgical reporting unit, the Company concluded that potential goodwill impairment indicators existed as of March 24, 2012. As such, the Company performed another interim goodwill impairment test of the GYN Surgical reporting unit as of March 24, 2012, updating its cash flow projections and related assumptions from the analysis performed as of December 24, 2011. As a result of completing Step 1, GYN Surgical’s fair value exceeded its carrying value. Therefore, Step 2 of the impairment test was not required as of March 24, 2012. The Company believes it used reasonable estimates and assumptions about future revenue, cost projections, cash flows, probabilities of cash flow scenarios, and market multiples as of that measurement date.
The Company conducted its fiscal 2011 annual impairment test on the first day of the fourth quarter, and as noted above used DCF and market approaches to estimate the fair value of its reporting units as of June 26, 2011, and ultimately used the fair value determined by the DCF in making its impairment test conclusions. The Company believes it used reasonable estimates and assumptions about future revenue, cost projections, cash flows and market multiples as of the measurement date. As a result of completing Step 1, all of the Company’s reporting units had fair values exceeding their carrying values, and as such, Step 2 of the impairment test was not required. For illustrative purposes, had the fair value of each reporting unit been lower by 10%, each reporting unit would have still passed Step 1 of the goodwill impairment test.
F-14
The Company conducted its fiscal 2010 annual impairment test on the first day of the fourth quarter. The Company utilized DCF and market approaches to estimate the fair value of its reporting units as of June 27, 2010, and ultimately used the fair value determined by the DCF in making its impairment test conclusions. The Company believes it used reasonable estimates and assumptions about future revenue, cost projections, cash flows and market multiples as of the measurement date. As a result of completing Step 1, all of the Company’s reporting units, except MammoSite, had fair values exceeding their carrying values, and as such, Step 2 of the impairment test was not required for these reporting units. MammoSite’s fair value declined from fiscal 2009 primarily due to a reduction in its long-term growth rates. The changes in MammoSite’s financial projections were a result of changing market conditions for the brachytherapy market, including downward pressure on procedure volumes due to the continuing adverse economic environment and current trends in breast cancer management, as well as competitive pricing pressures and competition from existing and alternative new technologies. The DCF calculation of fair value was positively impacted by a reduction in the discount rate to 11.0% from 12.5% used in the fiscal 2009 annual impairment test due to slight overall improvements in economic conditions and changes in the financial projections.
The Company performed the Step 2 analysis for MammoSite and recorded a $76.7 million impairment charge. For illustrative purposes had the fair value of MammoSite been 10% lower, the charge would have been higher by $2.5 million. If the fair value of the Company’s other reporting units had been lower by 10%, one reporting unit would have failed Step 1 requiring a Step 2 analysis. This reporting unit is in the Breast Health reportable segment and had a fair value at the annual impairment measurement date that exceeded its carrying value by 4% with goodwill of $256.5 million. The fair value of the reporting unit was determined by use of the DCF, and the key assumptions that drive the fair value in this model are the WACC, terminal values, growth rates, and the amount and timing of expected future cash flows. At September 25, 2010, for the Company’s other reporting units with goodwill aggregating $1.85 billion, the Company believed that these reporting units were not at risk of failing Step 1 of the goodwill impairment test.
The Company believes that the procedures performed and the estimates and assumptions used in the Step 1 and Step 2 analyses for each reporting unit are reasonable and in accordance with U.S. generally accepted accounting principles. The estimate of fair value requires significant judgment. The impairment testing process is subjective and requires judgment at many points throughout the analysis. If these estimates or their related assumptions change in the future, the Company may be required to record impairment charges for these assets not previously recorded.
A rollforward of goodwill activity by reportable segment from September 24, 2011 to September 29, 2012 is as follows:
| | | | | | | | | | | | | | | | | | | | |
| | | Breast Health | | | Diagnostics | | | GYN Surgical | | | Skeletal Health | | | Total | |
Balance at September 24, 2011 | | $ | 638,887 | | | $ | 633,319 | | | $ | 1,009,973 | | | $ | 8,151 | | | $ | 2,290,330 | |
Gen-Probe acquisition | | | — | | | | 1,652,546 | | | | — | | | | — | | | | 1,652,546 | |
Impairment charge | | | (5,826 | ) | | | — | | | | — | | | | — | | | | (5,826 | ) |
Tax adjustments | | | — | | | | (1,315 | ) | | | 6,212 | | | | — | | | | 4,897 | |
Foreign currency | | | 2,082 | | | | 907 | | | | 325 | | | | (26 | ) | | | 3,288 | |
Other adjustments | | | 598 | | | | (2,010 | ) | | | (1,044 | ) | | | — | | | | (2,456 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at September 29, 2012 | | $ | 635,741 | | | $ | 2,283,447 | | | $ | 1,015,466 | | | $ | 8,125 | | | $ | 3,942,779 | |
| | | | | | | | | | | | | | | | | | | | |
A rollforward of accumulated goodwill impairment losses by reportable segment from September 24, 2011 to September 29, 2012 is as follows:
| | | | | | | | | | | | | | | | |
| | | Breast Health | | | Diagnostics | | | GYN Surgical | | | Total | |
Balance at September 24, 2011 | | $ | 342,593 | | | $ | 908,349 | | | $ | 1,165,804 | | | $ | 2,416,746 | |
Impairment charge | | | 5,826 | | | | — | | | | — | | | | 5,826 | |
| | | | | | | | | | | | | | | | |
Balance at September 29, 2012 | | $ | 348,419 | | | $ | 908,349 | | | $ | 1,165,804 | | | $ | 2,422,572 | |
| | | | | | | | | | | | | | | | |
F-15
Other Assets
Other assets consist of the following:
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Other Assets | | | | | | | | |
Deferred financing costs | | $ | 82,760 | | | $ | 11,918 | |
Life insurance contracts | | | 25,978 | | | | 22,736 | |
Mutual funds | | | 6,995 | | | | — | |
Marketable security | | | 6,029 | | | | — | |
Manufacturing access fees | | | 18,323 | | | | — | |
Cost-method equity investments | | | 15,976 | | | | 4,608 | |
Other | | | 6,006 | | | | 6,815 | |
| | | | | | | | |
| | $ | 162,067 | | | $ | 46,077 | |
| | | | | | | | |
Deferred financing costs are related to the Company’s Convertible Notes, Credit Agreement and Senior Notes (see Note 5 for further discussion). The Company is amortizing amounts related to each debt issuance using the effective interest rate method over the period of earliest redemption or the term of such debt. Life insurance contracts were purchased in connection with the Company’s Nonqualified Deferred Compensation Plan (“DCP”) and are recorded at their cash surrender value (see Note 11 for further discussion). The marketable security represents a publicly traded equity security, and the mutual funds are the underlying investments related to the deferred compensation liabilities the Company assumed in connection with the Gen-Probe acquisition. The manufacturing access fees are related to a manufacturing supply and purchase agreement for our HPV products acquired in the Gen-Probe acquisition, and these fees are being amortized over the term of the agreement.
The Company’s cost-method equity investments are generally carried at cost as the Company owns less than 20% of the voting equity and does not have the ability to exercise significant influence over these companies. The Company regularly evaluates the carrying value of its cost-method equity investments for impairment and whether any events or circumstances are identified that would significantly harm the fair value of the investment. The primary indicators the Company utilizes to identify these events and circumstances are the investee’s ability to remain in business, such as the investee’s liquidity and rate of cash use, and the investee’s ability to secure additional funding and the value of that additional funding. In the event a decline in fair value is judged to be other-than-temporary, the Company will record an other-than-temporary impairment charge in other income (expense), net in the Consolidated Statements of Operations. During fiscal 2011 and 2010, the Company recorded other-than-temporary impairment charges of $2.4 million and $1.1 million, respectively, related to certain of its cost-method equity investments to adjust their carrying amounts to fair value. No such charges were recorded in fiscal 2012.
Research and Software Development Costs
Costs incurred for the research and development of the Company’s products are expensed as incurred. Nonrefundable advance payments for goods or services to be received in the future by the Company for use in research and development activities are deferred and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed. If the Company’s expectations change such that it does not expect it will need the goods to be delivered or the services to be rendered, capitalized nonrefundable advance payments are recorded to expense in that period.
The Company accounts for the development costs of software embedded in the Company’s products in accordance with ASC 985,Software.Costs incurred in the research, design and development of software embedded in products to be sold to customers are charged to expense until technological feasibility of the ultimate product to be sold is established. The Company’s policy is that technological feasibility is achieved when a working model, with the key features and functions of the product, is available for customer testing. Software development costs incurred after the establishment of technological feasibility and until the product is available for general release are capitalized, provided recoverability is reasonably assured. Software development costs eligible for capitalization have not been significant to date.
Foreign Currency Translation
The financial statements of the Company’s foreign subsidiaries are translated in accordance with ASC 830,Foreign Currency Matters.The reporting currency for the Company is the U.S. dollar. With the exception of its Costa Rica subsidiary, whose functional currency is the U.S. dollar, the functional currency of the Company’s foreign subsidiaries is their local currency. Accordingly, assets and liabilities of these subsidiaries are translated at the exchange rate in effect at each balance sheet date. Before translation, the Company re-measures foreign currency denominated assets and liabilities, including inter-company accounts receivable and payable, into the functional currency of the respective entity, resulting in unrealized gains or losses recorded in other income (expense), net in the Consolidated Statement of Operations. Revenues and expenses are translated using average exchange rates during the respective period. Foreign currency translation adjustments are accumulated as a component of other comprehensive income (loss) as a separate component of stockholders’ equity. Gains and losses arising from transactions denominated in foreign currencies are included in other income (expense), net in the Consolidated Statements of Operations and to date have not been material.
F-16
Accumulated Other Comprehensive Income
Othercomprehensive income (loss) includes certain transactions that have generally been reported in the statement of stockholders’ equity. The components of accumulated other comprehensive income consisted of the following:
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Foreign currency translation adjustment | | $ | 7,211 | | | $ | 994 | |
Unrealized gains on available-for-sale securities, net of tax of $36 | | | 62 | | | | — | |
Minimum pension liability, net of tax of $207 and $300, respectively | | | (483 | ) | | | 1,001 | |
| | | | | | | | |
| | $ | 6,790 | | | $ | 1,995 | |
| | | | | | | | |
Revenue Recognition
The Company generates revenue from the sale of its products, primarily medical imaging systems and diagnostic and surgical disposable products, and related services, which are primarily support and maintenance services on its medical imaging systems.
The Company recognizes product revenue upon shipment provided that there is persuasive evidence of an arrangement, there are no uncertainties regarding acceptance, the sales price is fixed or determinable, no right of return exists and collection of the resulting receivable is reasonably assured. Generally, the Company’s product arrangements for capital equipment sales, primarily in its Breast Health and Skeletal Health reporting segments, are multiple-element arrangements, including services, such as installation and training, and multiple products. Based on the terms and conditions of the product arrangements, the Company believes that these services and undelivered products can be accounted for separately from the delivered product element as the Company’s delivered products have value to its customers on a stand-alone basis. Accordingly, revenue for services not yet performed at the time of product shipment are deferred and recognized as such services are performed. The relative selling price of any undelivered products is also deferred at the time of shipment and recognized as revenue when these products are delivered. There is no customer right of return in the Company’s sales agreements.
Service revenues primarily consist of amounts recorded under service and maintenance contracts and repairs not covered under warranty, installation and training, and shipping and handling costs billed to customers. Service and maintenance contract revenues are recognized ratably over the term of the contract. Other service revenues are recognized as the services are performed.
For revenue arrangements with multiple deliverables, the Company records revenue as separate units of accounting if the delivered items have value to the customer on a stand-alone basis, and if the arrangement includes a general right of return relative to the delivered items, the delivery or performance of the undelivered items is considered probable and substantially within the Company’s control. Some of the Company’s products have both software and non-software components that function together to deliver the product’s essential functionality. The Company determined that except for its computer-aided detection (“CAD”) products, the software element in its other products is incidental in accordance with the software revenue recognition rules and are not within the scope of the software revenue recognition rules, ASC 985-605,Software—Revenue Recognition. The Company determined that given the significance of the software component’s functionality to its CAD systems, which are sold by its Breast Health segment, these products are within the scope of the software revenue recognition rules. The Company evaluated the appropriate revenue recognition treatment of its other hardware products, including its Dimensions digital mammography systems, which have both software and non-software components that function together to deliver the products’ essential functionality (i.e., it is a tangible product), and determined they are not within the scope of ASC 985-605.
The Company is required to allocate revenue to its multiple element arrangements based on the relative fair value of each element’s selling price. The Company typically determines the selling price of its products based on its best estimate of selling prices (“ESP”) and services based on vendor-specific objective evidence of selling price (“VSOE”). The Company determines VSOE based on its normal pricing and discounting practices for the specific product or service when sold on a stand-alone basis. In determining VSOE, the Company’s policy requires a substantial majority of selling prices for a product or service to be within a reasonably narrow range. The Company also considers the class of customer, method of distribution, and the geographies into which its products and services are sold when determining VSOE. The Company typically has had VSOE for its products and services. If VSOE cannot be established, which may occur in instances when a product or service has not been sold separately, stand-alone sales are too infrequent, or product pricing is not within a narrow range, the Company attempts to establish the selling price based on third-party evidence of selling price (“TPE”). TPE is determined based on competitor prices for similar deliverables when sold separately. When the Company cannot determine VSOE or TPE, it uses ESP in its allocation of arrangement consideration. The objective of ESP is to determine the price at which the Company would typically transact a stand-alone sale of the product or service. ESP is determined by considering a number of factors including Company pricing policies, internal costs and gross margin objectives, method of distribution, information gathered from experience in customer negotiations, market research and information, recent technological trends, competitive landscape and geographies.
F-17
For those arrangements accounted for under the software revenue recognition rules, ASC 985-605 generally requires revenue earned on software arrangements involving multiple elements to be allocated to each element based on their relative VSOE of fair value. If VSOE does not exist for a delivered element, the residual method is applied in which the arrangement consideration is allocated to the undelivered elements based on their VSOE with the remaining consideration recognized as revenue for the delivered elements. For multiple-element software arrangements where VSOE of fair value of Post-Contract Customer Support (“PCS”) has been established, the Company recognizes revenue using the residual method at the time all other revenue recognition criteria have been met.
As part of the Diagnostics reporting segment and as a result of the Gen-Probe, acquisition, the Company manufactures blood screening products according to demand schedules provided by its collaboration partner, Novartis Vaccines and Diagnostics, Inc. (“Novartis”). The Company’s agreement provides that it shares a portion of Novartis’s revenue from screening blood donations. Upon shipment to Novartis, the Company recognizes blood screening product sales at an agreed upon fixed transfer price, which is not refundable, and records the related cost of products sold. Based on the terms of the Company’s collaboration agreement with Novartis, the Company’s ultimate share of the net revenue from sales to the end user in excess of the transfer price revenues recognized is not known until it is reported to the Company by Novartis. On a monthly basis, Novartis reports net revenue generated during the prior month and remits an additional corresponding net payment to the Company, which is recorded as revenue at that time. This payment combined with the transfer price revenues previously recognized represents the Company’s ultimate share of net revenue under the agreement.
Within its Diagnostics business, and to a lesser extent, its GYN Surgical business, the Company provides its instrumentation (for example, the ThinPrep Processor, ThinPrep Imaging System, PANTHER and TIGRIS systems) and certain other hardware to customers without requiring them to purchase the equipment or enter into a lease. Instead, the Company recovers the cost of providing the instrumentation and equipment in the amount it charges for its diagnostic tests and assays and other disposables. Customers enter into a customer usage agreement, and the Company installs the equipment at customer sites and customers commit to purchasing minimum quantities of disposable products at a stated price over a defined contract term, which is typically between three and five years. Revenue is recognized over the term of the customer usage agreement as tests, assays and other disposable products are shipped. The depreciation costs associated with an instrument are charged to cost of product sales on a straight-line basis over the estimated life of the instrument. The costs to maintain these instruments in the field are charged to cost of product sales as incurred.
The Company sells its instruments to Novartis for use in blood screening and records these instrument sales upon delivery since Novartis is responsible for the placement, maintenance and repair of the units with its customers. The Company also sells instruments to its clinical diagnostics customers and records sales of these instruments upon delivery and customer acceptance. For certain customers with non-standard payment terms, instrument sales are recorded based upon expected cash collection. Prior to delivery, each instrument is tested to meet the Company’s specifications and the specifications of the United States Food and Drug Administration (“FDA”), and is shipped fully assembled. Customer acceptance of the Company’s clinical diagnostic instrument systems requires installation and training by the Company’s technical service personnel. Installation is a standard process consisting principally of uncrating, calibrating and testing the instrumentation.
Accounts Receivable and Reserves
The Company records reserves for doubtful accounts based upon a specific review of all outstanding invoices, known collection issues and historical experience. The Company regularly evaluates the collectability of its trade accounts receivables and performs ongoing credit evaluations of its customers and adjusts credit limits based upon payment history and its assessment of the customer’s current credit worthiness. These estimates are based on specific facts and circumstances of particular orders, analysis of credit memo data and other known factors.
Accounts receivable reserve activity for fiscal 2012, 2011 and 2010 is as follows:
| | | | | | | | | | | | | | | | |
| | | Balance at
Beginning
of Period | | | Charged to
Costs and
Expenses | | | Write-
offs and
Payments | | | Balance at
End of
Period | |
Period Ended: | | | | | | | | | | | | | | | | |
September 29, 2012 | | $ | 6,516 | | | $ | 3,270 | | | $ | (3,390 | ) | | $ | 6,396 | |
September 24, 2011 | | $ | 7,769 | | | $ | 1,614 | | | $ | (2,867 | ) | | $ | 6,516 | |
September 25, 2010 | | $ | 7,279 | | | $ | 1,895 | | | $ | (1,405 | ) | | $ | 7,769 | |
Cost of Service and Other Revenues
Cost of service and other revenues primarily represents payroll and related costs associated with the Company’s professional services’ employees, consultants, infrastructure costs and overhead allocations, including depreciation and rent and materials consumed in providing the service.
F-18
Stock-Based Compensation
The Company accounts for share-based payments in accordance with ASC 718,Stock Compensation. As such, all share-based payments to employees, including grants of stock options and restricted stock units and shares issued under the Company’s employee stock purchase plan, are recognized in the Consolidated Statements of Operations based on their fair values on the date of grant.
Net Income (Loss) Per Share
Basic net income (loss) per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding. Diluted net income (loss) per share is computed by dividing net income (loss) by the weighted average number of common shares and the dilutive effect of potential future issuances of common stock from outstanding stock options, restricted stock units and convertible debt determined by applying the treasury stock method. In accordance with ASC 718, the assumed proceeds under the treasury stock method include the average unrecognized compensation expense of in-the-money stock options and restricted stock units. This results in the assumed buyback of additional shares, thereby reducing the dilutive impact of stock options.
The Company applies the provisions of ASC 260,Earnings Per Share,Subsection 10-45-44, to determine the diluted weighted average shares outstanding as it relates to its Convertible Notes, and due to the type of debt instrument issued, the Company applies the treasury stock method and not the if-converted method. The dilutive impact of the Company’s Convertible Notes is based on the difference between the Company’s current period average stock price and the conversion price of the Convertible Notes, provided there is a premium. Pursuant to this accounting standard, there is no dilution from the accreted principal of the Convertible Notes.
A calculation of net income (loss) per share and a reconciliation of basic and diluted share amounts for fiscal 2012, 2011, and 2010 is as follows:
| | | | | | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Numerator: | | | | | | | | | | | | |
Net (loss) income | | $ | (73,634 | ) | | $ | 157,150 | | | $ | (62,813 | ) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Basic weighted average common shares outstanding | | | 264,041 | | | | 261,099 | | | | 258,743 | |
Weighted average common stock equivalents from assumed exercise of stock options and restricted stock units | | | — | | | | 3,206 | | | | — | |
| | | | | | | | | | | | |
Diluted weighted average common shares outstanding | | | 264,041 | | | | 264,305 | | | | 258,743 | |
| | | | | | | | | | | | |
Basic net (loss) income per common share | | $ | (0.28 | ) | | $ | 0.60 | | | $ | (0.24 | ) |
| | | | | | | | | | | | |
Diluted net (loss) income per common share | | $ | (0.28 | ) | | $ | 0.59 | | | $ | (0.24 | ) |
| | | | | | | | | | | | |
Weighted-average anti-dilutive shares related to: | | | | | | | | | | | | |
Outstanding stock options | | | 10,491 | | | | 7,747 | | | | 13,260 | |
Restricted stock units | | | 1,378 | | | | — | | | | 1,427 | |
In those reporting periods in which the Company has reported net income, anti-dilutive shares generally are comprised of those stock options that either have an exercise price above the average stock price for the period or the stock options’ combined exercise price, average unrecognized stock compensation expense and assumed tax benefits upon exercise is greater than the average stock price for the period. In those reporting periods in which the Company has a net loss, anti-dilutive shares are comprised of the impact of those number of shares that would have been dilutive had the Company had net income plus the number of common stock equivalents that would be anti-dilutive had the company had net income.
Product Warranties
The Company generally offers a one-year warranty for its products. The Company provides for the estimated cost of product warranties at the time product revenue is recognized. Factors that affect the Company’s warranty reserves include the number of units sold, historical and anticipated rates of warranty repairs and the cost per repair. The Company periodically assesses the adequacy of the warranty reserve and adjusts the amount as necessary.
F-19
Product warranty activity for fiscal 2012 and 2011 is as follows:
| | | | | | | | | | | | | | | | | | | | |
| | | Balance at
Beginning of
Period | | | Provisions | | | Acquired | | | Settlements/
adjustments | | | Balance at End
of Period | |
Period ended: | | | | | | | | | | | | | | | | | | | | |
September 29, 2012 | | $ | 4,448 | | | $ | 9,535 | | | $ | 230 | | | $ | (8,034 | ) | | $ | 6,179 | |
September 24, 2011 | | $ | 2,830 | | | $ | 5,535 | | | $ | 657 | | | $ | (4,574 | ) | | $ | 4,448 | |
Advertising Costs
Advertising costs are charged to operations as incurred. The Company does not have any direct-response advertising. Advertising costs, which include trade shows and conventions, were approximately $29.8 million, $29.0 million and $15.3 million for fiscal 2012, 2011 and 2010, respectively, and were included in selling and marketing expense in the Consolidated Statements of Operations.
Recently Issued Accounting Pronouncements
Disclosures about Offsetting Assets and Liabilities
In December 2011, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2011-11,Disclosures about Offsetting Assets and Liabilities. ASU 2011-11 amended ASC 210,Balance Sheet,to converge the presentation of offsetting assets and liabilities between U.S. GAAP and IFRS. ASU 2011-11 requires that entities disclose both gross information and net information about both instruments and transactions eligible for offset in the statement of financial position and instruments and transactions subject to an agreement similar to a master netting arrangement. ASU 2011-11 is effective for fiscal years, and interim periods within those years, beginning after January 1, 2013, which is the Company’s fiscal year 2014. The Company is currently evaluating the impact of the adoption of ASU 2011-11 on its consolidated financial statements.
Presentation of Comprehensive Income
In June 2011, the FASB issued ASU No. 2011-05,Comprehensive Income (Topic 220): Presentation of Comprehensive Income, which requires an entity to present total comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. ASU 2011-05 does not change any of the components of comprehensive income, but it eliminates the option to present the components of other comprehensive income as part of the statement of stockholders equity. ASU 2011-05 is effective for the Company in the first quarter of fiscal 2013 and should be applied retrospectively. The Company elected to early-adopt ASU 2011-05 in fiscal 2012 and has provided a separate statement of comprehensive income (loss) in its consolidated financial statements.
In December 2011, the FASB issued ASU 2011-12, deferring certain provisions of ASU 2011-05. One of the provisions of ASU 2011-05 required entities to present reclassification adjustments out of accumulated other comprehensive income (loss) by component in both the statement in which net income is presented and the statement in which other comprehensive income (loss) is presented (for both interim and annual financial statements). This requirement is indefinitely deferred by ASU 2011-12 and will be further deliberated by the FASB at a future date. The effective date of ASU 2011-12 is the same as that for the unaffected provisions of ASU 2011-05.
Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements
In May 2011, the FASB issued ASU No. 2011-04—Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs, to provide a consistent definition of fair value and ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and International Financial Reporting Standards. ASU 2011-04 changes certain fair value measurement principles and enhances the disclosure requirements particularly for level 3 fair value measurements. ASU 2011-04 was effective for the Company in its second quarter of fiscal 2012 and should be applied prospectively. The adoption of ASU 2011-04 did not have a material impact on the Company’s consolidated financial statements.
Business Combinations
In December 2010, the FASB issued ASU No. 2010-29,Business Combinations (Topic 805)—Disclosure of Supplementary Pro Forma Information for Business Combinations. ASU 2010-29 requires a public entity to disclose revenue and earnings of the combined entity as though the business combination that occurred during the current year had occurred as of the beginning of the prior year. It also requires a description of the nature and amount of material, nonrecurring adjustments directly attributable to the business combination included in the reported revenue and earnings. The new disclosure was effective for the Company’s first quarter of fiscal 2012 and did not have a material impact on the Company’s consolidated financial statements.
F-20
Intangibles—Goodwill and Other
In December 2010, the FASB issued ASU 2010-28,Intangibles—Goodwill and Other (Topic 350). ASU 2010-28 modifies Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that a goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that an impairment may exist. ASU 2010-28 is effective for the Company in fiscal 2012. The adoption of ASU 2010-28 is not expected to have a material impact on the Company’s consolidated financial statements.
In September 2011, the FASB issued ASU No. 2011-08,Intangibles—Goodwill and Other (Topic 350): Testing Goodwill for Impairment. ASU 2011-08 allows entities to first assess qualitatively whether it is necessary to perform the two-step goodwill impairment test. If an entity believes, as a result of its qualitative assessment, that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, the quantitative two-step impairment test is required; otherwise, no further testing is required. ASU 2011-08 is effective for the Company beginning in fiscal 2013, although early adoption is permitted. The Company does not believe that ASU 2011-08 will have a material impact on its consolidated financial statements.
Fiscal 2012 Acquisition:
Gen-Probe, Inc.
On August 1, 2012, the Company completed the acquisition of Gen-Probe and acquired all of the outstanding shares of Gen-Probe. Pursuant to the merger agreement, each share of common stock outstanding immediately prior to the effective time of the acquisition was cancelled and converted into the right to receive $82.75 in cash. In addition, all outstanding restricted shares, restricted stock units, performance shares, and those stock options granted prior to February 8, 2012 were cancelled and converted into the right to receive $82.75 per share in cash less the applicable exercise price, as applicable. Stock options granted after February 8, 2012 were converted into stock options to acquire shares of Hologic common stock determined by a conversion formula defined in the merger agreement. The Company paid the Gen-Probe shareholders $3.8 billion and $169.0 million to equity award holders. The Company funded the acquisition using available cash and financing consisting of senior secured credit facilities and Senior Notes (see Note 5 for further discussion) resulting in aggregate proceeds of $3.48 billion, excluding financing fees to the underwriters. The Company incurred approximately $34.3 million of direct transaction costs recorded within general and administrative expenses.
Gen-Probe, headquartered in San Diego, California, is a leader in molecular diagnostics products and services that are used primarily to diagnose human diseases, screen donated human blood, and test transplant compatibility. The Company expects this acquisition to enhance its molecular diagnostics franchise and to complement its existing portfolio of diagnostics products. Gen-Probe’s results of operations are reported within the Company’s Diagnostics reportable segment from the date of acquisition.
The purchase price consideration was as follows:
| | | | |
Cash paid | | $ | 3,967,866 | |
Deferred payment | | | 1,655 | |
Fair value of stock options exchanged | | | 2,655 | |
| | | | |
Total purchase price | | $ | 3,972,176 | |
| | | | |
The fair value of stock options exchanged recorded as purchase price represents the fair value of the Gen-Probe options converted into the Company’s stock options attributable to pre-combination services pursuant to ASC 805,Business Combinations. The remainder of the fair value of these options of $23.2 million will be recognized as stock-based compensation expense over the remaining vesting period, which is approximately 3.5 years. The Company estimated the fair value of the stock options using a binomial valuation model with the following weighted average assumptions: risk free rate of 0.41%, expected volatility of 39.9%, expected life of 3.6 years and dividend of 0.0%. The weighted average fair value of stock options granted is $7.07 per share.
F-21
The preliminary allocation of the purchase price is based on estimates of the fair value of assets acquired and liabilities assumed as of August 1, 2012. The Company is continuing to obtain information to complete its valuation of intangible assets, as well as to determine the acquired assets and liabilities, including tax assets and liabilities. The components of the preliminary purchase price allocation are as follows:
| | | | |
Cash | | $ | 205,463 | |
Accounts receivable | | | 80,301 | |
Inventory | | | 153,416 | |
Property, plant and equipment | | | 274,095 | |
Other assets | | | 191,868 | |
Assets held-for-sale, net | | | 87,465 | |
Accounts payable | | | (19,671 | ) |
Accrued expenses | | | (131,102 | ) |
Other liabilities | | | (19,255 | ) |
Identifiable intangible assets: | | | | |
Developed technology | | | 1,565,000 | |
In-process research and development | | | 227,000 | |
Customer contract | | | 585,000 | |
Trade names | | | 97,000 | |
Deferred income taxes, net | | | (976,950 | ) |
Goodwill | | | 1,652,546 | |
| | | | |
Purchase Price | | $ | 3,972,176 | |
| | | | |
The purchase price has been allocated to the acquired assets and liabilities based on management’s estimate of their fair values. Certain of Gen-Probe’s assets have been designated as assets held-for-sale and have been recorded at fair value less the estimated cost to sell such assets. These represent non-core assets to the Company’s business plan and are expected to be sold within one year of the acquisition. Assets and liabilities held for sale are reflected separately in the Company’s Consolidated Balance Sheet. The following represents the components of the asset groups classified as held-for-sale as of September 29, 2012:
| | | | |
Assets: | | | | |
Cash | | $ | 2,563 | |
Accounts receivable | | | 8,520 | |
Inventory | | | 15,680 | |
Property, plant and equipment | | | 13,259 | |
Other assets | | | 3,083 | |
Intangible assets and goodwill | | | 51,398 | |
| | | | |
Total assets held-for-sale | | $ | 94,503 | |
| | | | |
Liabilities: | | | | |
Accrued liabilities | | | (7,622 | ) |
| | | | |
Net assets held-for-sale | | $ | 86,881 | |
| | | | |
As part of the preliminary purchase price allocation, the Company has determined the identifiable intangible assets are developed technology, in-process research and development (“IPR&D”), customer contracts, and trade names.The fair value of the intangible assets has been estimated using the income approach and the cash flow projections were discounted using rates ranging from 10% to 12%. The cash flows are based on estimates used to price the transaction, and the discount rates applied were benchmarked with reference to the implied rate of return from the transaction model and the weighted average cost of capital.
The developed technology assets are comprised of know-how, patents and technologies embedded in Gen-Probe’s products and relate to currently marketed products and related instrument automation. In valuing the developed technology assets consideration was only given to products that have received regulatory approval. The developed technology assets primarily comprise the significant product families used in diagnostic testing, and the majority of fair value relates to the APTIMA family of assays for testing of certain sexually transmitted diseases and microbial infectious diseases and the PROCLEIX family of assays for blood screening. The Company applied the Excess Earnings Method under the income approach to fair value the developed technology assets excluding the PROCLEIX technology asset. The Company applied the Relief-from-Royalty Method to fair value this asset.
IPR&D projects relate to in-process projects that have not reached technological feasibility as of the acquisition date and have no alternative future use. The primary basis for determining technological feasibility of these projects is obtaining regulatory approval
F-22
to market the underlying product, which primarily pertains to receiving approval to perform certain diagnostic testing on Gen-Probe’s instrumentation, such as the PANTHER and TIGRIS systems. The Company recorded $227.0 million of IPR&D related to 6 projects. One project, valued at $7.0 million received FDA approval in October 2012, and amortization over the estimated useful life will begin in the first quarter of fiscal 2013. The other projects are expected to be completed within the next 3 months to 45 months with a total cost of approximately $54.2 million to complete such projects. Given the uncertainties inherent with product development and introduction, there can be no assurance that any of the Company’s product development efforts will be successful, completed on a timely basis or within budget, if at all. All of the IPR&D assets were valued using the multiple-period excess earnings method approach using a discount rate of 12.0%.
The customer contract intangible asset pertains to Gen-Probe’s relationship with Novartis, and the Company used the Excess Earnings Method to estimate the fair value of this asset. Trade names relate to the Gen-Probe corporate name and the primary product names, and the Company used the Relief-from-Royalty Method to estimate the fair value of this asset.
Developed technology, customer contract and trade names are being amortized on a straight-line basis over a weighted average period of 12.5 years, 13.0 years and 11.0 years, respectively.
The Company estimated the fair value of property, plant and equipment using a combination of the cost and market approaches, depending on the component. The Company applied the cost approach as the primary method in estimating the fair value of land and buildings. In total, the fair value adjustment to increase the carrying amount of property, plant and equipment was $107.9 million, of which $70.6 million related to land and buildings.
The excess of the purchase price over the estimated fair value of the tangible net assets and intangible assets acquired was recorded to goodwill. The factors contributing to the recognition of the amount of goodwill are based on several strategic and synergistic benefits that are expected to be realized from the Gen-Probe acquisition. These benefits include the expectation that the combined company’s complementary products in the molecular diagnostics market with Gen-Probe’s fully automated product franchise will significantly broaden the Company’s offering in women’s health and diagnostics. The combined company should benefit from a broader global presence and with Hologic’s direct sales force and marketing in Europe and its investment in China distribution, the growth prospects of Gen-Probe’s products are expected to be enhanced significantly. The combined company anticipates significant cross-selling opportunities within the diagnostics market through Hologic’s larger channel coverage and physician sales team. None of the goodwill is expected to be deductible for income tax purposes.
Gen-Probe’s revenue and pre-tax loss for the period from the acquisition date to September 29, 2012 were $89.5 million and $47.7 million, respectively. The following unaudited pro forma information presents the combined financial results for the Company and Gen-Probe as if the acquisition of Gen-Probe had been completed at the beginning of the prior fiscal year, September 26, 2010:
| | | | | | | | |
| | | Year Ended
September 29, 2012 | | | Year Ended
September 24, 2011 | |
| | |
Revenue | | $ | 2,526,336 | | | $ | 2,310,384 | |
Net loss | | $ | (164,539 | ) | | $ | (127,240 | ) |
Basic and diluted net loss per common share | | $ | (0.62 | ) | | $ | (0.49 | ) |
The unaudited pro forma information for fiscal 2012 and 2011 was calculated after applying the Company’s accounting policies and the impact of acquisition date fair value adjustments. Fiscal 2012 unaudited pro forma net loss was adjusted to exclude acquisition-related transaction costs and restructuring costs solely related to the consolidation of the Diagnostics business. These expenses have been added to fiscal 2011 unaudited pro forma net loss. In addition, the fiscal year 2012 unaudited pro forma net loss was adjusted to exclude nonrecurring expenses related to the fair value adjustments associated with the acquisition of Gen-Probe that were recorded by the Company. The fiscal year 2011 pro forma net loss was adjusted to include these acquisition-related transaction costs and expenses related to the fair value adjustments. These pro forma condensed consolidated financial results have been prepared for comparative purposes only and include certain adjustments to reflect pro forma results of operations as if the acquisition occurred on September 26, 2010, such as fair value adjustment to inventory, accounts receivable, and property, plant and equipment, increased expenses for restructuring charges and retention costs, increased interest expense on debt obtained to finance the transaction, lower investment income and increased amortization for the fair value of acquired intangible assets. The pro forma information does not reflect the effect of costs, other than restructuring and retention, or synergies that would have been expected to result from the integration of the acquisition. The pro forma information does not purport to be indicative of the results of operations that actually would have resulted had the combination occurred at the beginning of each period presented, or of future results of the consolidated entities.
F-23
Fiscal 2011 Acquisitions:
TCT International Co., Ltd.
On June 1, 2011, the Company completed the acquisition of 100% of the equity interest in TCT International Co., Ltd. (“TCT”) and subsidiaries, a privately-held distributor of medical products, including the Company’s ThinPrep Pap Test, related instruments and other diagnostic and surgical products. TCT’s operating subsidiaries are located in Beijing, China. The Company’s acquisition of TCT has enabled it to obtain an established nationwide sales organization and customer support infrastructure in China, which is consistent with the Company’s international expansion strategy. TCT has been integrated within the Company’s international operations, and its results are primarily reported within the Company’s Diagnostics reporting segment and to a lesser extent within the Company’s GYN Surgical reporting segment from the date of acquisition. The Company concluded that the acquisition of TCT did not represent a material business combination, and therefore, no pro forma financial information has been provided herein.
The purchase price of $148.4 million was comprised of $135.0 million in cash, of which $100.0 million was paid up-front and $35.0 million plus a working capital adjustment $13.2 million, was deferred for one year. In addition, $0.9 million was paid in the first quarter of fiscal 2012 for additional assets acquired. The deferred payment was recorded on a present value basis of $47.5 million in purchase accounting to reflect fair value, and such payment was being accreted through interest expense over the one year deferral period. The $35.0 million and a portion of the working capital adjustment of $8.5 million were paid in the fourth quarter of fiscal 2012. As agreed to by the parties, the remainder is due after the completion of fiscal 2013. In addition, the majority of the former shareholders of TCT may receive two annual contingent earn-out payments (subject to adjustment) not to exceed $200.0 million less the deferred payment. The contingent earn-out payments are based on a multiple of incremental revenue growth for the one year periods beginning January 1, 2011 and January 1, 2012 as compared to the respective prior year periods, and are payable after the first and second anniversaries from the date of acquisition, respectively. Since these payments are contingent on future employment, they are being recognized as compensation expense ratably over the required service periods, the first and second year anniversaries from the date of acquisition. Based on actual and projected revenues for the TCT business, the Company recorded compensation expense of $75.5 million and $17.6 million in fiscal 2012 and 2011, respectively. In the third quarter of fiscal 2012, the first measurement period was completed, and the Company paid the earned contingent consideration of $54.0 million in the fourth quarter of fiscal 2012. As of September 29, 2012, the Company has accrued $39.1 million for the second contingent earn-out payment.
The Company did not issue any equity awards in connection with this acquisition, and third-party transaction costs were not significant.
The allocation of the purchase price was based on estimates of the fair value of assets acquired and liabilities assumed as of June 1, 2011. The components of the purchase price allocation consisted of the following:
| | | | |
Cash | | $ | 27,961 | |
Accounts receivable | | | 17,811 | |
Inventory | | | 5,301 | |
Property and equipment | | | 4,710 | |
Other tangible assets | | | 1,082 | |
Accrued taxes | | | (14,874 | ) |
Accounts payable and accrued expenses | | | (6,641 | ) |
Customer relationships | | | 45,780 | |
Business licenses | | | 2,500 | |
Trade names | | | 2,110 | |
Deferred taxes, net | | | (12,473 | ) |
Goodwill | | | 75,161 | |
| | �� | | |
Purchase Price | | $ | 148,428 | |
| | | | |
In connection with the purchase price allocation, the Company determined that the separately identifiable intangible assets were customer relationships, business licenses, and trade names related to the TCT company name. The fair value of the intangible assets was determined through the application of the income approach, and the cash flow projections were discounted at 12.5%. Customer relationships relate to relationships that TCT’s founders and sales force have developed with obstetricians, gynecologists, hospitals, and clinical laboratories. Customer relationships, business licenses and trade names are being amortized over a weighted average period of 12.7 years, 10 years and 12 years, respectively. The excess of the purchase price over the fair value of the tangible net assets and intangible assets acquired was recorded to goodwill. The goodwill recognized is attributable to the established sales and distribution network of TCT and expected synergies that the Company will realize from this acquisition. None of the goodwill is expected to be deductible for income tax purposes.
F-24
Interlace Medical, Inc.
On January 6, 2011, the Company consummated the acquisition of 100% of the equity interest in Interlace, a privately-held company located in Framingham, Massachusetts. Interlace is the developer, manufacturer and supplier of the MyoSure hysteroscopic tissue removal system (“MyoSure”). The MyoSure system is a tissue removal device that is designed to provide incision-less removal of fibroids and polyps within the uterus. Interlace’s operations are reported within the Company’s GYN Surgical reporting segment from the date of acquisition. The Company believes that MyoSure is a complementary product to its existing surgical product portfolio. The Company concluded that the acquisition of Interlace did not represent a material business combination, and therefore, no pro forma financial information has been provided herein.
The purchase price was comprised of $126.8 million in cash (“Initial Consideration”), which was net of certain adjustments, plus two annual contingent payments up to a maximum of an additional $225.0 million in cash. In addition to the Initial Consideration, $2.1 million was paid to certain employees upon the completion of three and six months of service from the date of acquisition. Since these payments were contingent on future employment, they were recognized as compensation expense in fiscal 2011. The purchase agreement includes an indemnification provision that provides for the reimbursement of a portion of legal expenses in defense of the Interlace intellectual property. The Company has the right to collect certain amounts set aside in escrow from the Initial Consideration and, as applicable, offset contingent consideration payments of qualifying legal costs.
The contingent payments are based on a multiple of incremental revenue growth during a two-year period following the completion of the acquisition. Pursuant to ASC 805,Business Combinations, the Company recorded its estimate of the fair value of the contingent consideration liability based on future revenue projections of the Interlace business under various potential scenarios and weighted probability assumptions of these outcomes. As of the date of acquisition, these cash flow projections were discounted using a rate of 15.6%. The discount rate is based on the weighted-average cost of capital of the acquired business plus a credit risk premium for non-performance risk related to the liability pursuant to ASC 820. This analysis resulted in an initial contingent consideration liability of $86.6 million, which is adjusted periodically as a component of operating expenses based on changes in fair value of the liability due to the accretion of the liability for the time value of money and changes in the assumptions pertaining to the achievement of the defined revenue growth milestones. This fair value measurement was based on significant inputs not observable in the market and thus represented a Level 3 measurement as defined in ASC 820,Fair Value Measurements and Disclosures. This fair value measurement is directly impacted by the Company’s estimate of future incremental revenue growth of the business. Accordingly, if actual revenue growth is higher or lower than the estimates within the fair value measurement, the Company would record additional charges or benefits, respectively, as appropriate. The Company recorded charges of $41.8 million in fiscal 2012 due to an increase in revenue estimates for Interlace and $6.3 million in fiscal 2011 for accretion to record the contingent consideration liability at fair value. The fair value of the contingent consideration for the first measurement period was $51.8 million. This payment was disbursed during the second quarter of fiscal 2012 of which $47.6 million is reflected in the Consolidated Statements of Cash Flows as cash used in financing activities, representing the liability recognized at fair value for the first measurement period as of the acquisition date. The remainder, which is related to changes in the fair value of the liability, is reflected within cash provided by operating activities. As of September 29, 2012, the Company has accrued $83.0 million for the second measurement period contingent payment.
The Company did not issue any equity awards in connection with this acquisition, and third-party transaction costs were not significant.
The purchase price consideration was as follows:
| | | | |
Cash | | $ | 126,798 | |
Contingent consideration | | | 86,600 | |
| | | | |
Total purchase price | | $ | 213,398 | |
| | | | |
The allocation of the purchase price was based on estimates of the fair value of assets acquired and liabilities assumed as of January 6, 2011. The components of the purchase price allocation consisted of the following:
| | | | |
Cash | | $ | 9,070 | |
Inventory, including fair value adjustments | | | 1,795 | |
Other tangible assets | | | 1,291 | |
Accounts payable and accrued expenses | | | (1,988 | ) |
Developed technology | | | 158,741 | |
Trade names | | | 1,750 | |
Deferred taxes, net | | | (45,342 | ) |
Goodwill | | | 88,081 | |
| | | | |
Purchase Price | | $ | 213,398 | |
| | | | |
F-25
As part of the purchase price allocation, the Company determined that the separately identifiable intangible assets were developed technology and trade names related to the MyoSure product name. The fair value of the intangible assets was determined through the application of the income approach, and the cash flow projections were discounted at 12.7%. Developed technology represented currently marketable Interlace products that the Company will continue to sell and utilize to enhance and incorporate into the Company’s existing products. In determining the fair value of developed technology, consideration was only given to products that had been approved by the FDA. Based on the early stage of other projects and an insignificant allocation of resources to those projects, the Company concluded that there were no in-process projects of a material nature. Developed technology and trade names are being amortized over 15 years and 13 years, respectively. The excess of the purchase price over the fair value of the tangible net assets and intangible assets acquired was recorded to goodwill. The goodwill recognized is attributable to expected synergies that the Company will realize from this acquisition. None of the goodwill is expected to be deductible for income tax purposes.
Beijing Healthcome Technology Company, Ltd.
On July 19, 2011, the Company completed its acquisition of 100% of the equity in Beijing Healthcome Technology Company, Ltd. (“Healthcome”), a privately-held manufacturer of medical equipment, including mammography equipment, located in Beijing, China. Healthcome manufactured analog mammography products targeted to lower tier hospital segments in China. Additionally, Healthcome had been collaborating with the Company’s research and development team to integrate its selenium detector technology into the Healthcome mammography platform. On December 21, 2011, the Company received SFDA approval in China for its Serenity digital mammography system. This acquisition provides the Company with manufacturing capability in China and additional access to the Chinese markets. The purchase price was $8.8 million in cash, which is net of a working capital adjustment. The Company concluded that the acquisition of Healthcome did not represent a material business combination, and therefore, no pro forma financial information has been provided herein. The Company was obligated to make future payments to the shareholders, who remain employed, up to an additional $7.1 million over three years. Since these payments were contingent on future employment, they were being recognized as compensation expense ratably over the respective service periods. In the fourth quarter of fiscal 2012, the Company and former shareholders agreed that the former shareholders would terminate their employment. The Company agreed to pay the majority of the contingent consideration in accordance with the original payment terms. As a result, the Company accelerated the unearned compensation in the fourth quarter of fiscal 2012. The Company recorded compensation expense of $5.6 million and $0.3 million in fiscal 2012 and 2011, respectively. Healthcome’s operations are reported within the Company’s Breast Health reporting segment from the date of acquisition.
As part of the purchase price allocation, the Company determined that the separately identifiable intangible assets were developed technology of $3.3 million, in-process research and development of $0.9 million, and trade names of $0.2 million. The in-process research and development project was completed in the first quarter of fiscal 2012. The Company is continuing to obtain information pertaining to certain acquired assets and liabilities, including tax assets and liabilities. The fair value of the intangible assets was determined through the application of the income approach, and the cash flow projections were discounted using rates ranging from 27% to 30%. Developed technology and trade names are being amortized over their useful lives of 13 and 7 years, respectively. The excess of the purchase price over the fair value of the tangible net assets and intangible assets acquired of $6.4 million was recorded to goodwill. The goodwill recognized is attributable to expected synergies that the Company will realize from this acquisition. None of the goodwill is expected to be deductible for income tax purposes.
Fiscal 2010 Acquisition:
Sentinelle Medical Inc.
On August 5, 2010, the Company completed its acquisition of 100% of the equity interests in Sentinelle Medical Inc. (“Sentinelle Medical”), a privately-held company located in Toronto, Canada, pursuant to a definitive agreement dated July 6, 2010. Sentinelle Medical develops, manufactures and markets magnetic resonance imaging (“MRI”) breast coils, tables and visualization software. Sentinelle Medical is dedicated to developing advanced imaging technologies used in high-field strength MRI systems. Sentinelle Medical’s products enhanced and broadened the Company’s portfolio of product offerings in the areas of breast cancer detection and intervention. Sentinelle Medical’s operations are reported within the Company’s Breast Health reporting segment from the date of acquisition. The Company concluded that the acquisition of Sentinelle Medical did not represent a material business combination, and therefore, no pro forma financial information has been provided herein.
The purchase price was comprised of an $84.8 million cash payment, which was net of certain adjustments, plus three contingent payments up to a maximum of an additional $250.0 million in cash. The contingent payments are based on a multiple of incremental revenue growth during the two-year period following the completion of the acquisition as follows: six months after acquisition, 12 months after acquisition, and 24 months after acquisition. Pursuant to ASC 805, the Company recorded its estimate of the fair value of the contingent consideration liability based on future revenue projections of the Sentinelle Medical business under various potential scenarios and weighted probability assumptions of these outcomes. As of the date of acquisition, these cash flow projections were discounted using a rate of 16.5%. The discount rate is based on the weighted-average cost of capital of the acquired
F-26
business plus a credit risk premium for non-performance risk related to the liability pursuant to ASC 820. This analysis resulted in an initial contingent consideration liability of $29.5 million, which is adjusted periodically as a component of operating expenses based on changes in fair value of the liability due to the accretion of the liability for the time value of money and changes in the assumptions pertaining to the achievement of the defined revenue growth milestones. This fair value measurement was based on significant inputs not observable in the market and thus represented a Level 3 measurement as defined in ASC 820. This fair value measurement is directly impacted by the Company’s estimate of future incremental revenue growth of the business. Accordingly, if actual revenue growth is higher or lower than the estimates within the fair value measurement, the Company would record additional charges or benefits, respectively, as appropriate.
Each quarter, the Company re-evaluates its assumptions, including the revenue and probability assumptions for future earn-out periods, which has resulted in lower revenue projections. As a result of these adjustments, which were partially offset by the accretion of the liability, and using a current discount rate of approximately 17.0%, the Company recorded a reversal of expense of $14.3 million in fiscal 2011 to record the contingent consideration liability at fair value. The first two earn-out periods have lapsed, and the Company made payments of $4.1 million and $4.3 million in fiscal 2012 and 2011, respectively. In fiscal 2012, as a result of lower revenues than initially projected for the remaining measurement period, the Company recorded a net benefit of $3.4 million. At September 29, 2012, the Company has accrued $3.4 million for the last measurement period contingent payment.
The Company did not issue any equity awards in connection with this acquisition and third-party transaction costs were not significant.
The purchase price was as follows:
| | | | |
Cash | | $ | 84,751 | |
Contingent consideration | | | 29,500 | |
| | | | |
Total purchase price | | $ | 114,251 | |
| | | | |
The allocation of the purchase price was based on estimates of the fair value of assets acquired and liabilities assumed as of August 5, 2010. The components and allocation of the purchase price consisted of the following:
| | | | |
Cash | | $ | 429 | |
Inventory, including fair value adjustments | | | 9,899 | |
Other tangible assets | | | 7,247 | |
Accounts payable and accrued expenses | | | (6,304 | ) |
Deferred revenue, including fair value adjustments | | | (2,056 | ) |
Developed technology | | | 60,900 | |
In-process research and development | | | 4,800 | |
Trade names | | | 1,600 | |
Non-compete agreements | | | 300 | |
Deferred taxes, net | | | (11,181 | ) |
Goodwill | | | 48,617 | |
| | | | |
Purchase Price | | $ | 114,251 | |
| | | | |
As part of the purchase price allocation, the Company determined that the separately identifiable intangible assets were developed technology, in-process research and development, trade names and non-compete agreements. The fair value of the intangible assets was determined through the application of the income approach, and the cash flow projections were discounted using rates of 15.0% to 16.0%. Developed technology represented currently marketable purchased products that the Company will continue to sell as well as utilize to enhance and incorporate into the Company’s existing products. In determining the allocation of the purchase price to existing technology, consideration was only given to products that had been approved by the FDA. The trade names related to both the Sentinelle Medical name and certain product names.
The amount allocated to acquired in-process research and development represented the estimated fair value of in-process projects based on risk-adjusted cash flows utilizing a discount rate of 17.0%. These in-process projects had not yet reached technological feasibility and had no future alternative uses as of the date of the acquisition. The primary basis for determining the technological feasibility of these projects was obtaining regulatory approval to market the underlying products. The Company received FDA approval for both projects during fiscal 2011 and began to amortize them over their estimated useful lives.
The developed technology assets are being amortized over a weighted average life of approximately 19 years, and trade names are being amortized over a weighted average life of approximately 9 years. Non-compete agreements are being amortized over 3 years.
F-27
The excess of the purchase price over the fair value of the tangible net assets and intangible assets acquired was recorded to goodwill. The goodwill recognized is attributable to expected synergies that the Company will realize from this acquisition. None of the goodwill is expected to be deductible for income tax purposes.
| 4. | Restructuring and Divestiture Charges |
In addition to monitoring the global macro-economic environment and impact on its businesses and products, the Company also evaluates its operations for opportunities to improve operational effectiveness and efficiency and to better align expenses with revenues. As a result of these assessments, the Company has undertaken various restructuring actions. These actions are described below. The following table displays charges taken related to restructuring actions in fiscal 2012 and a rollforward of the charges to the accrued balances at September 29, 2012. Such initiatives were not significant in fiscal 2011 and 2010.
| | | | | | | | | | | | | | | | | | | | |
Statement of Operations | | Abandonment of
Adiana Product
Line | | | Consolidation of
Diagnostics
Operations | | | Closure of
Indianapolis
Facility | | | Other
Operating
Cost
Reductions | | | Total | |
| | | | | |
Non-cash impairment charge | | $ | 16,316 | | | $ | 585 | | | $ | — | | | $ | — | | | $ | 16,901 | |
Purchase orders and other contractual obligations | | | 3,099 | | | | — | | | | — | | | | — | | | | 3,099 | |
Workforce reductions | | | 128 | | | | 14,202 | | | | 879 | | | | 40 | | | | 15,249 | |
Facility closure costs | | | — | | | | — | | | | — | | | | 430 | | | | 430 | |
Other | | | — | | | | — | | | | 900 | | | | — | | | | 900 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Total charges | | $ | 19,543 | | | $ | 14,787 | | | $ | 1,779 | | | $ | 470 | | | $ | 36,579 | |
| | | | | | | | | | | | | | | | | | | | |
Recorded to cost of product sales | | $ | 19,064 | | | $ | — �� | | | $ | — | | | $ | — | | | $ | 19,064 | |
| | | | | | | | | | | | | | | | | | | | |
Recorded to restructuring | | $ | 479 | | | $ | 14,787 | | | $ | 1,779 | | | $ | 470 | | | $ | 17,515 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Rollforward of Accrued Restructuring | | | | | | | | | | | | | | | |
| | | | | |
Total charges | | $ | 19,543 | | | $ | 14,787 | | | $ | 1,779 | | | $ | 470 | | | $ | 36,579 | |
Non-cash impairment charges | | | (16,316 | ) | | | (585 | ) | | | — | | | | — | | | | (16,901 | ) |
Stock compensation | | | — | | | | (3,500 | ) | | | — | | | | — | | | | (3,500 | ) |
Severance payments | | | (128 | ) | | | (2,423 | ) | | | — | | | | (78 | ) | | | (2,629 | ) |
Purchase orders and other contractual obligations payments | | | (2,572 | ) | | | — | | | | — | | | | — | | | | (2,572 | ) |
Other payments | | | — | | | | — | | | | — | | | | (430 | ) | | | (430 | ) |
Acquired | | | — | | | | 83 | | | | — | | | | — | | | | 83 | |
Foreign exchange and other adjustments | | | — | | | | 22 | | | | — | | | | 91 | | | | 113 | |
| | | | | | | | | | | | | | | | | | | | |
Balance at September 29, 2012 | | $ | 527 | | | $ | 8,384 | | | $ | 1,779 | | | $ | 53 | | | $ | 10,743 | |
| | | | | | | | | | | | | | | | | | | | |
F-28
Abandonment of Adiana Product Line
At the end of the second quarter of fiscal 2012, the Company decided to cease manufacturing, marketing and selling its Adiana system, which was a product line within the Company’s GYN Surgical reporting segment. Management determined that the product was not financially viable and would not become so in the foreseeable future. In addition, the Company settled its intellectual property litigation regarding the Adiana system with Conceptus as discussed in Note 13. As a result, in the second quarter of fiscal 2012, the Company recorded a charge of $18.3 million and recorded additional adjustments in fiscal 2012 resulting in an aggregate charge of $19.5 million. Of the total charge, $19.1 million was recorded within cost of product sales and $0.4 million was recorded in restructuring. The amount recorded in cost of product sales comprised impairment charges of $9.9 million to record inventory at its net realizable value, $6.5 million to write down certain manufacturing equipment and equipment placed at customer sites to its fair value that had no further utility, and $2.7 million for outstanding contractual purchase orders of raw materials and components that will not be utilized and other contractual obligations. In connection with this action, the Company terminated certain manufacturing and other personnel primarily at its Costa Rica location, resulting in severance charges of $0.1 million, and incurred other contractual charges of $0.3 million. All identified employees were terminated and paid as of September 29, 2012.
Consolidation of Diagnostics Operations
In connection with its acquisition of Gen-Probe, the Company implemented restructuring actions to consolidate its Diagnostics business, such as streamlining product development initiatives, reducing overlapping functional areas such as sales, marketing and general and administrative functions, and consolidation of manufacturing resources, field services and support. As a result, the Company terminated certain employees from Gen-Probe and its legacy diagnostics business in research and development, sales, marketing, and general and administrative functions. The Company recorded severance and benefit charges of $13.3 million related to this action pursuant to ASC 420,Exit or Disposal Cost Obligations. The majority of these employees ceased working in the fourth quarter of fiscal 2012 and their full severance charge was recorded in the fourth quarter of fiscal 2012. In addition, certain of the terminated Gen-Probe employees had unvested stock options and their vesting terms were accelerated as a result of termination. As such, the severance charges include $3.5 million of stock-based compensation expense. The Company expects to record an additional $1.1 million for severance in fiscal 2013.
In addition, the Company will move its legacy molecular diagnostics operations from Madison, Wisconsin to San Diego, California. This transfer is expected to be finalized by the end of calendar 2014 and the majority of employees in Madison will be terminated in fiscal 2013 and 2014. The Company is recording severance and benefit charges pursuant to ASC 420 and estimates the total severance and benefits charge to be approximately $6.4 million, which will be recorded ratably over the estimated service period of the affected employees. The Company recorded $0.9 million in fiscal 2012. The Company has also recorded non-cash charges of $0.6 million as a result of exiting certain research projects. Additional charges, which are not expected to be significant, will be recorded as the manufacturing operation is transferred and the facility is closed down. These charges will be recorded as they are incurred.
Closure of Indianapolis Facility
In the fourth quarter of fiscal 2012, the Company finalized its decision to transfer production of its interventional breast products, which are included within the Breast Health reporting segment, from its Indianapolis facility to its facility in Costa Rica. The transfer is expected to be completed in the first half of calendar 2014 and all employees at that location will be terminated. The Company is recording severance and benefit charges pursuant to ASC 420 and estimates the total severance and benefits charge to be approximately $7.0 million, which will be recorded ratably over the estimated service period of the affected employees. The Company recorded $0.9 million of severance benefits in fiscal 2012. In addition, the Company recorded $0.9 million for amounts owed to the state of Indiana for employment credits. Additional charges, which are not expected to be significant, will be recorded as the manufacturing operation is transferred and the facility is closed down. These charges will be recorded as they are incurred.
Consolidation of Selenium Panel Coating Production
During the third quarter of fiscal 2012, the Company finalized its decision to consolidate its Selenium panel coating process and transfer the production line to its Newark, Delaware facility from its Hitec-Imaging German subsidiary. This production line is included within the Breast Health segment. The transfer is expected to be completed in the second half of fiscal 2013. In connection with this consolidation plan, the Company expects to terminate certain employees, primarily manufacturing personnel. Severance charges will be recorded pursuant to ASC 420 because the severance benefits qualify as one-time employee termination benefits, which are currently being negotiated between the Company and the local works council on behalf of the employees. Since the benefit amount is not determinable nor has any severance benefit been communicated to the affected employees, no charge has been recorded as of September 29, 2012. Employees must continue to be employed by the Company until their employment is involuntarily terminated in order to receive the severance benefit. As such, the severance benefit will be recognized ratably over the required service period once the individual severance benefits are known and communicated to the employees. The Company expects to incur between $1.2 million and $1.4 million for severance charges.
F-29
Other Operating Cost Reductions
During the second quarter of fiscal 2012, the Company abandoned certain lease space and recorded charges of $0.4 million to terminate the leases and write-off related leasehold improvements that have no further utility. The obligation to the landlord was paid in the third quarter of fiscal 2012. During the fourth quarter of fiscal 2012, the Company terminated the employment of certain individuals and recorded a severance and benefits charge of $0.1 million, which was partially offset by the reversal of severance charges recorded in fiscal 2011.
Fiscal 2011 and 2010 Charges
In the fourth quarter of fiscal 2011, the Company terminated the employment of certain individuals and recorded a severance and benefits charge of $0.3 million, all of which was unpaid as of September 24, 2011. In addition, in the fourth quarter of fiscal 2011, the Company sold a minor non-core product line for $1.1 million resulting in a net gain of $0.4 million.
In the fourth quarter of fiscal 2010, the Company terminated the employment of certain employees in connection with completing the Sentinelle Medical acquisition. As a result, the Company recorded a severance and benefits charge of $0.9 million. In addition, during fiscal 2010, the Company recorded additional charges of $0.7 million in connection with closing and sale of its organic photoconductor drum coatings manufacturing facility in Shanghai, China.
| 5. | Borrowings and Credit Arrangements |
The Company had total debt with a carrying value of $5.04 billion and $1.49 billion at September 29, 2012 and September 24, 2011, respectively. The Company’s borrowings consisted of the following at September 29, 2012 and September 24, 2011:
| | | | | | | | |
| | | 2012 | | | 2011 | |
Current debt obligations, net of debt discount: | | | | | | | | |
Term Loan A | | $ | 49,582 | | | $ | — | |
Term Loan B | | | 14,853 | | | | — | |
| | | | | | | | |
Total current debt obligations | | | 64,435 | | | | — | |
| | |
Long-term debt obligations, net of debt discount: | | | | | | | | |
Term Loan A | | | 942,065 | | | | — | |
Term Loan B | | | 1,470,454 | | | | — | |
Senior Notes | | | 1,000,000 | | | | — | |
| | | | | | | | |
| | | 3,412,519 | | | | — | |
Convertible Notes (principal of $1,725,000) | | | 1,558,660 | | | | 1,488,580 | |
| | | | | | | | |
Total long-term debt obligations | | | 4,971,179 | | | | 1,488,580 | |
| | | | | | | | |
Total debt obligations | | $ | 5,035,614 | | | $ | 1,488,580 | |
| | | | | | | | |
The debt maturity schedule for the components of the Company’s obligations as of September 29, 2012 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2013 | | | 2014 | | | 2015 | | | 2016 | | | 2017 | | | 2018 and
Thereafter | | | Total | |
| | | | | | | |
Term Loan A | | $ | 50,000 | | | $ | 50,000 | | | $ | 100,000 | | | $ | 200,000 | | | $ | 600,000 | | | $ | — | | | $ | 1,000,000 | |
Term Loan B | | | 15,000 | | | | 15,000 | | | | 15,000 | | | | 15,000 | | | | 15,000 | | | | 1,425,000 | | | | 1,500,000 | |
Senior Notes | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,000,000 | | | | 1,000,000 | |
Convertible Notes (1) | | | — | | | | 775,000 | | | | — | | | | — | | | | 450,000 | | | | 500,000 | | | | 1,725,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 65,000 | | | $ | 840,000 | | | $ | 115,000 | | | $ | 215,000 | | | $ | 1,065,000 | | | $ | 2,925,000 | | | $ | 5,225,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | Classified based on the earliest date of redemption for each respective issuance. |
F-30
Credit Agreement
On August 1, 2012, the Company and certain domestic subsidiaries (the “Guarantors”) entered into a credit and guaranty agreement (the “Credit Agreement”) with Goldman Sachs Bank USA, in its capacity as administrative and collateral agent, and the lenders party thereto (collectively, the “Lenders”).
The credit facilities under the Credit Agreement consist of:
| | • | | $1.0 billion senior secured tranche A term loan (“Term Loan A”) with a final maturity date of August 1, 2017; |
| | • | | $1.5 billion secured tranche B term loan (“Term Loan B”) with a final maturity date of August 1, 2019; and |
| | • | | $300.0 million secured revolving credit facility (“Revolving Facility”) with a final maturity date of August 1, 2017. |
Pursuant to the terms and conditions of the Credit Agreement, the Lenders committed to provide senior secured financing in an aggregate amount of up to $2.8 billion. As of the closing of the Gen-Probe acquisition, the Company borrowed $2.5 billion aggregate principal under the term loans of the Credit Agreement. Net proceeds to the Company were $2.41 billion, after issuing the term loans at a discount and deducting associated fees and expenses, all of which will be amortized to interest expense over the respective maturity dates of the debt. The proceeds were used to fund a portion of the purchase price for the Gen-Probe acquisition.
The Guarantors have guaranteed the Company’s obligations under the credit facilities, and the credit facilities are secured by first-priority liens on, and a first-priority security interest in, substantially all of the assets of the Company and the Guarantors, including all of the capital stock of substantially all of the U.S. subsidiaries owned by the Company and the Guarantors, 65% of the capital stock of certain of the Company’s first-tier foreign subsidiaries and all intercompany debt. The security interests are evidenced by a pledge and security agreement by and among Goldman Sachs Bank USA, as collateral agent, the Company and the Guarantors and other related agreements, including certain intellectual property security agreements and mortgages.
The Company is required to make scheduled principal payments under Term Loan A in increasing amounts ranging from $12.5 million per three month period beginning October 31, 2012 to $50.0 million per three month period commencing October 31, 2015, and under Term Loan B in equal installments of $3.75 million per three month period beginning on October 31, 2012 and for 27 three month periods thereafter. The remaining balance for each term loan is due at maturity. Any amounts outstanding under the Revolving Facility are due at maturity. The Company is required to make principal repayments first, pro rata among the term loan facilities, and second to the Revolving Facility from specified excess cash flows from operations and from the net proceeds of specified types of asset sales, debt issuances, insurance recoveries and equity offerings. Subject to certain limitations, the Company may voluntarily prepay any of the credit facilities without premium or penalty.
All amounts outstanding under the Credit Agreement bear interest, at the Company’s option, initially, with respect to all loans made under Term Loan A and the Revolving Facility: (i) at the Base Rate plus 2.00% per annum, or (ii) at the Adjusted Eurodollar Rate (i.e., the Libor rate) plus 3.00%, and with respect to loans made under Term Loan B: (i) at the Base Rate, with a floor of 2.00%, plus 2.50%, or (ii) at the Adjusted Eurodollar Rate, with a floor of 1.00% plus 3.50%. The applicable margin to the Base Rate or Eurodollar Rate on Term Loan A and the Revolving Facility are subject to specified changes depending on the total net leverage ratio as defined in the Credit Agreement. Interest accruing at the Base Rate generally is payable by the Company on a quarterly basis. Interest accruing at the Eurodollar Rate generally is payable on the last day of selected interest periods (which can be one, two, three and six months and in certain circumstances nine or twelve months) unless the interest period exceeds three months, in which case, interest is due at the end of every three month period. The Company is required to pay a quarterly commitment fee at an annual rate of 0.50% on the undrawn committed amount available under the Revolving Facility (which rate is subject to reduction depending on the total net leverage ratio as defined in the Credit Agreement).
Borrowings outstanding under the Credit Agreement in fiscal 2012 had a weighted average interest rate of 4.0%. The interest rates on the outstanding Term Loan A and Term Loan B borrowings at September 29, 2012 ranged from 3.23% to 4.5%. Interest expense under the Credit Agreement totaled $18.4 million during fiscal 2012, which includes non-cash interest expense of $2.4 million related to the amortization of the deferred financing costs and accretion of the debt discount.
The Credit Agreement contains affirmative and negative covenants customarily applicable to senior secured credit facilities, including covenants restricting the ability of the Company and the guarantors, subject to negotiated exceptions, to: incur additional indebtedness and additional liens on their assets, engage in mergers or acquisitions or dispose of assets; enter into sale-leaseback transactions; pay dividends or make other distributions; voluntarily prepay other indebtedness; enter into transactions with affiliated persons; make investments; and change the nature of their businesses.
The credit facilities contain total net leverage ratio and interest coverage ratio financial covenants measured as of the last day of each fiscal quarter, which are effective in our first quarter of fiscal 2013. The total net leverage ratio is 7.00:1.00 beginning on our fiscal quarter ending December 29, 2012, and then decreases over time to 4.00:1.00 for the quarter ending September 30, 2017 and each fiscal quarter thereafter. The interest coverage ratio is 3.25:1.00 beginning on our fiscal quarter ending December 29, 2012, and then increases over time to 3.75:1.00 for the fiscal quarter ending September 30, 2017 and each quarter thereafter. The total net leverage ratio is defined as the ratio of our consolidated net debt as of the quarter end to our consolidated adjusted EBITDA for the four-fiscal
F-31
quarter period ending on the measurement date. The interest coverage ratio is defined as the ratio of our consolidated adjusted EBITDA for the prior four-fiscal quarter period ending on the measurement date to adjusted consolidated cash interest expense for the same measurement period. These terms, and the calculation thereof, are defined in further detail in the Credit Agreement.
The Company has evaluated the Credit Agreement for derivatives pursuant to ASC 815,Derivatives and Hedging, and identified embedded derivatives that require bifurcation as the features are not clearly and closely related to the host instrument. The embedded derivatives are a default provision, which could require additional interest payments, and provision requiring contingent payments to compensate the lenders for changes in tax deductions. The Company has determined that the fair value of these embedded derivatives was nominal as of September 29, 2012.
Senior Notes
On August 1, 2012, the Company completed a private placement of $1.0 billion aggregate principal amount of its 6.25% senior notes due 2020 (the “Senior Notes”) at an offering price of 100% of the aggregate principal amount of the Senior Notes. Net proceeds to the Company were $987.4 million after deducting underwriting fees and offering expenses, which will be amortized to interest expense over the term of the Senior Notes. The Senior Notes were not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws, and were offered only to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the United States in accordance with Regulation S under the Securities Act. The Senior Notes are general senior unsecured obligations of the Company and are guaranteed on a senior unsecured basis by the Guarantors. The proceeds were used to fund a portion of the Gen-Probe acquisition.
On August 1, 2012, the Company and the Guarantors entered into an indenture with Wells Fargo Bank, National Association, as trustee, relating to the Senior Notes. The Senior Notes mature on August 1, 2020 and bear interest at the rate of 6.25% per year, payable semi-annually on February 1 and August 1 of each year, commencing on February 1, 2013. The Company recorded interest expense of $10.7 million in fiscal 2012, which includes non-cash interest expense of $0.3 million related to the amortization of the deferred financing costs related to the Senior Notes.
The indenture contains customarily applicable affirmative and negative covenants, including covenants restricting the ability of the Company and certain of its subsidiaries’, subject to negotiated exceptions and qualifications to: incur additional indebtedness; pay dividends or repurchase or redeem capital stock; make certain investments; incur liens; enter into certain types of transactions with the Company’s affiliates; and sell assets or consolidate or merge with or into other companies. The Company is not required to maintain any financial covenants with respect to the Senior Notes.
The Company may redeem up to 35% of the aggregate principal amount of the Senior Notes with the net cash proceeds of certain equity offerings at any time and from time to time before August 1, 2015, at a redemption price equal to 106.250% of the aggregate principal amount so redeemed, plus accrued and unpaid interest, if any, to the redemption date. The Company also has the option to redeem the Senior Notes on or after: August 1, 2015 through July 31, 2016 at 103.125% of par; August 1, 2016 through July 31, 2017 at 102.083% of par; August 1, 2017 through July 31, 2018 at 101.042% of par; and August 1, 2018 and thereafter at 100% of par. In addition, if the Company undergoes a change of control, as provided in the indenture, the Company will be required to make an offer to purchase each holder’s Senior Notes at a price equal to 101% of the aggregate principal amount of the Senior Notes, plus accrued and unpaid interest, if any, to the repurchase date.
The Company has evaluated the Senior Notes for derivatives pursuant to ASC 815 and did not identify any embedded derivatives that require bifurcation. All features were deemed to be clearly and closely related to the host instrument.
On August 1, 2012, in connection with the issuance of the Senior Notes, the Company and the Guarantors entered into an exchange and registration rights agreement with the initial purchasers of the Senior Notes. Pursuant to the terms of the registration rights agreement, the Company and the Guarantors agreed to (i) file a registration statement covering an offer to exchange the Senior Notes for a new issue of identical exchange notes registered under the Securities Act on or before 180 days from August 1, 2012, (ii) use commercially reasonable efforts to cause such registration statement to become effective, and (iii) use commercially reasonable efforts to complete the exchange prior to 270 days after August 1, 2012. Under certain circumstances, the Company and the Guarantors may be required to provide a shelf registration statement to cover resales of the Senior Notes.
Convertible Notes
On December 10, 2007, the Company issued and sold $1.725 billion, at par, of 2.00% Convertible Senior Notes due December 15, 2037 (“2007 Notes”). Net proceeds from the offering were $1.69 billion, after deducting the underwriters’ discounts and offering expenses. On November 18, 2010, the Company entered into separate, privately-negotiated exchange agreements under which it retired $450.0 million in aggregate principal of its 2007 Notes for $450.0 million in aggregate principal of new 2.00% Convertible Exchange Senior Notes due December 15, 2037 (“2010 Notes”). In connection with this exchange transaction, the Company recorded a debt extinguishment loss of $29.9 million in its Consolidated Statements of Operations in the first quarter of fiscal 2011. On February 29, 2012, the Company entered into separate, privately-negotiated exchange agreements under which it retired $500.0
F-32
million in aggregate principal of the 2007 Notes for $500.0 million in aggregate principal of new 2.00% Convertible Senior Notes due March 1, 2042 (“2012 Notes”). In connection with this exchange transaction, the Company recorded a debt extinguishment loss of $42.3 million in the second quarter of fiscal 2012. Following this transaction, $775.0 million in principal amount of the 2007 Notes remain outstanding. The 2007 Notes, 2010 Notes and 2012 Notes are collectively referred to herein as the “Convertible Notes”.
Holders may require the Company to repurchase the Convertible Notes prior to maturity on the dates set forth below:
| | • | | the 2007 Notes on December 13, 2013, and each of December 15, 2017, 2022, 2027 and 2032; |
| | • | | the 2010 Notes on each of December 15, 2016, 2020 and 2025, December 13, 2030 and December 14, 2035; and |
| | • | | the 2012 Notes on each of March 1, 2018, 2022, 2027 and 2032 and March 2, 2037. |
Holders may also require the Company to repurchase the Convertible Notes upon a fundamental change, as defined in each of the applicable indentures. The Company may redeem all or a portion of the 2007 Notes at any time on or after December 18, 2013, all or a portion of the 2010 Notes at any time on or after December 19, 2016 and all or a portion of the 2012 Notes at any time on or after March 6, 2018. If, prior to maturity, a holder requires the Company to repurchase the Convertible Notes or the Company elects to redeem the Convertible Notes, the repurchase or redemption price of each Convertible Note will equal 100% of its principal amount, plus accrued and unpaid interest to, but excluding, the redemption or repurchase date, as applicable.
The 2007 Notes bear interest at a rate of 2.00% per year on the principal amount, payable semi-annually in arrears in cash on June 15 and December 15 of each year, ending on December 15, 2013. The 2007 Notes will accrete principal from December 15, 2013 at a rate that provides holders with an aggregate annual yield to maturity of 2.00% per year. Beginning with the six month interest period commencing December 15, 2013, the Company will pay contingent interest during any six month interest period to the holders of 2007 Notes if the “trading price”, as defined, of the 2007 Notes for each of the five trading days ending on the second trading day immediately preceding the first day of the applicable six month interest period equals or exceeds 120% of the accreted principal amount of the 2007 Notes. The holders of the 2007 Notes may convert the notes into shares of the Company’s common stock at a conversion price of approximately $38.59 per share, subject to adjustment, prior to the close of business on September 15, 2037 under any of the following circumstances: (1) during any calendar quarter if the last reported sale price of the Company’s common stock exceeds 130% of the conversion price for at least 20 trading days in the 30 consecutive trading days ending on the last trading day of the preceding calendar quarter; (2) during the five business day period after any five consecutive trading day period in which the trading price per note for each day of such period was less than 98% of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such day; (3) if the notes have been called for redemption; or (4) upon the occurrence of specified corporate events. None of these triggering events had occurred as of September 29, 2012.
The 2010 Notes bear interest at a rate of 2.00% per year on the principal amount, payable semi-annually in arrears in cash on June 15 and December 15 of each year ending on December 15, 2016 and will accrete principal from December 15, 2016 at a rate that provides holders with an aggregate annual yield to maturity of 2.00% per year. Beginning with the six month interest period commencing December 15, 2016, the Company will pay contingent interest during any six month interest period to the holders of 2010 Notes if the “trading price”, as defined, of the 2010 Notes for each of the five trading days ending on the second trading day immediately preceding the first day of the applicable six month interest period equals or exceeds 120% of the accreted principal amount of the 2010 Notes. The holders of the 2010 Notes may convert the 2010 Notes into shares of the Company’s common stock at a conversion price of approximately $23.03 per share, subject to adjustment, prior to the close of business on September 15, 2037 under any of the following circumstances: (1) during any calendar quarter if the last reported sale price of the Company’s common stock exceeds 130% of the conversion price for at least 20 trading days in the 30 consecutive trading days ending on the last trading day of the preceding calendar quarter; (2) during the five business day period after any five consecutive trading day period in which the trading price per note for each day of such period was less than 98% of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such day; (3) if the 2010 Notes have been called for redemption; or (4) upon the occurrence of specified corporate events. None of these triggering events had occurred as of September 29, 2012.
The 2012 Notes bear interest at a rate of 2.00% per year on the principal amount, payable semi-annually in arrears in cash on March 1 and September 1 of each year, beginning September 1, 2012 and ending on March 1, 2018 and will accrete principal from March 1, 2018 at a rate that provides holders with an aggregate annual yield to maturity of 2.00% per year. Beginning with the six month interest period commencing March 1, 2018, the Company will pay contingent interest during any six month interest period to the holders of 2012 Notes if the “trading price”, as defined, of the 2012 Notes for each of the five trading days ending on the second trading day immediately preceding the first day of the applicable six month interest period equals or exceeds 120% of the accreted principal amount of the 2012 Notes. The holders of the 2012 Notes may convert the 2012 Notes into shares of the Company’s common stock at a conversion price of $31.175 per share, subject to adjustment, prior to the close of business on March 1, 2042, subject to prior redemption or repurchase of the 2012 Notes, under any of the following circumstances: (1) during any calendar quarter if the last reported sale price of the Company’s common stock exceeds 130% of the conversion price for at least 20 trading days in the 30 consecutive trading days ending on the last trading day of the preceding calendar quarter; (2) during the five business day period after any five consecutive trading day period in which the trading price per note for each day of such period was less than 98% of the
F-33
product of the last reported sale price of the Company’s common stock and the conversion rate on each such day; (3) if the 2012 Notes have been called for redemption; or (4) upon the occurrence of specified corporate events. None of these triggering events had occurred as of September 29, 2012.
In lieu of delivery of shares of the Company’s common stock in satisfaction of the Company’s obligation upon conversion of the Convertible Notes, the Company may elect to deliver cash or a combination of cash and shares of its common stock. If the Company elects to satisfy its conversion obligation in a combination of cash and shares of the Company’s common stock, the Company is required to deliver up to a specified dollar amount of cash per $1,000 original principal amount of Convertible Notes, and will settle the remainder of its conversion obligation in shares of its common stock, in each case based on the daily conversion value calculated as provided in the respective indentures for the Convertible Notes. This net share settlement election is in the Company’s sole discretion and does not require the consent of holders of the Convertible Notes. It is the Company’s current intent and policy to settle any conversion of the Convertible Notes as if the Company had elected to make the net share settlement election.
The Convertible Notes are the Company’s senior unsecured obligations and rank equally with all of its existing and future senior unsecured debt and prior to all future subordinated debt. The Convertible Notes are effectively subordinated to any future secured indebtedness to the extent of the collateral securing such indebtedness, and structurally subordinated to all indebtedness and other liabilities (including trade payables) of the Company’s subsidiaries.
Accounting for the Convertible Notes
The Convertible Notes have been recorded pursuant to FASB Staff Position (“FSP”) APB 14-1,Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) (FSP APB 14-1) (codified within ASC 470,Debt) since they can be settled in cash, or partially in cash, upon conversion. FSP APB 14-1 requires the liability and equity components of the convertible debt instrument to be separately accounted for in a manner that reflects the entity’s nonconvertible debt borrowing rate when interest expense is subsequently recognized. The excess of the debt’s principal amount over the amount allocated to the liability component is recognized as the value of the embedded conversion feature (“equity component”) within additional-paid-in capital in stockholders’ equity and amortized to interest expense using the effective interest method. The liability component is initially recorded at its fair value, which is calculated using a discounted cash flow technique. Key inputs used to estimate the fair value of the liability component included the Company’s estimated nonconvertible debt borrowing rate as of the measurement date (i.e. the date the Convertible Notes are issued), the amount and timing of cash flows, and the expected life of the Convertible Notes. In addition, third-party transaction costs are required to be allocated to the liability and equity components based on their relative values.
On September 27, 2009 (the first day of fiscal 2010), as required, the Company adopted this accounting standard, which was applicable to the original issuance of its Convertible Notes at which time there was one issue, the 2007 Notes. The Company estimated the fair value of the 2007 Notes without the conversion feature as of the date of issuance (“liability component”). The estimated fair value of the liability component of $1.256 billion was determined using a discounted cash flow technique. Key inputs used to estimate the fair value of the liability component included the Company’s estimated nonconvertible debt borrowing rate as of December 10, 2007 (the date the 2007 Notes were issued), the amount and timing of cash flows, and the expected life of the 2007 Notes. The estimated effective interest rate of 7.62% was estimated by comparing other companies’ debt issuances that had features similar to the Company’s debt excluding the conversion feature and who had similar credit ratings during the same annual period as the Company.
The excess of the gross proceeds received over the estimated fair value of the liability component totaling $468.9 million was allocated to the conversion feature (“equity component”) as an increase to additional paid-in-capital with a corresponding offset recognized as a discount to reduce the net carrying value of the 2007 Notes. The discount, after adjustment for the exchange of convertible notes as discussed below, is being amortized to interest expense over a six-year period ending December 18, 2013 (the expected life of the liability component) using the effective interest method. In addition, a portion of the deferred financing costs were allocated to the equity component and recorded as a reduction to additional paid-in-capital.
The Company accounted for both 2007 Notes retirements in fiscal 2012 and 2011, discussed above, under the derecognition provisions of subtopic ASC 470-20-40, which requires the allocation of the fair value of the consideration transferred (i.e., the 2010 Notes and 2012 Notes, respectively) between the liability and equity components of the original instrument to determine the gain or loss on the transaction. In connection with the 2010 Notes and 2012 Notes transactions, the Company recorded a loss on debt extinguishment of $29.9 million and $42.3 million in fiscal 2011 and 2012, respectively. The 2010 Notes exchange loss is comprised of the loss on the debt itself of $26.0 million and the write-off of the pro-rata amount of debt issuance costs of $3.9 million allocated to the notes retired. The 2012 Notes exchange loss is comprised of the loss on the debt itself of $39.7 million and the write-off of the pro-rata amount of debt issuance costs of $2.6 million allocated to the notes retired. The loss on the debt itself is calculated as the difference between the fair value of the liability component of the 2007 Notes amount retired immediately before the respective exchanges and its related carrying value immediately before the exchanges. The fair value of the liability component in each transaction was calculated similar to the description above for initially recording the 2007 Notes under FSP APB 14-1, and the Company used an effective interest rate of 5.46% and 2.89% for the 2010 Notes and 2012 Notes, respectively, representing the estimated nonconvertible debt borrowing rate with a maturity as of the measurement date consistent with the 2007 Notes first put date
F-34
of December 2013. In addition, under this accounting standard, a portion of the fair value of the consideration transferred is allocated to the reacquisition of the equity component, which is the difference between the fair value of the consideration transferred and the fair value of the liability component immediately before the exchange. As a result, on a gross basis in the 2010 Notes and 2012 Notes transactions, $39.9 million and $41.6 million, respectively, were allocated to the reacquisition of the equity component of the original instrument, which was recorded net of deferred taxes within capital in excess of par value.
Since the 2010 Notes and 2012 Notes have the same characteristics as the 2007 Notes and can be settled in cash or a combination of cash and shares of common stock (i.e., partial settlement), the Company is required to account for the liability and equity components of its 2010 Notes and 2012 Notes separately to reflect its nonconvertible debt borrowing rate. The Company estimated the fair value of the liability component of 2010 Notes and 2012 Notes to be $349.0 million and $454.2 million, respectively, using a discounted cash flow technique with an estimated effective interest rate of 6.52% and 3.72%, respectively. The rates represent the estimated nonconvertible debt borrowing rate with a maturity as of the measurement date consistent with the 2010 Notes and 2012 Notes first put dates of December 2017 and March 2018, respectively.
The excess of the fair value of the consideration transferred, which was estimated using a binomial lattice model, over the estimated fair value of the liability component of $97.3 million and $79.7 million for the 2010 Notes and 2012 Notes, respectively, was allocated to the embedded conversion feature as an increase to additional paid-in-capital with a corresponding offset recognized as a discount to reduce the net carrying value of the respective notes. As a result of the fair value of the 2010 Notes being lower than the 2010 Notes principal value, there is an additional discount on the 2010 Notes of $3.7 million at the measurement date. The total discount on the 2010 Notes is being amortized to interest expense over a six-year period ending December 15, 2016 (the expected life of the liability component) using the effective interest method. The net debt discount of the 2012 Notes is being amortized to interest expense over a six-year period ending March 1, 2018 (the expected life of the liability component) using the effective interest method. In addition, third-party transaction costs in each transaction have been allocated to the liability and equity components based on the relative values of these components.
As of September 29, 2012 and September 24, 2011, the Convertible Notes and related equity components (recorded in additional paid-in-capital, net of deferred taxes) consisted of the following:
| | | | | | | | |
| | | 2012 | | | 2011 | |
2007 Notes principal amount | | $ | 775,000 | | | $ | 1,275,000 | |
Unamortized discount | | | (50,591 | ) | | | (147,287 | ) |
| | | | | | | | |
Net carrying amount | | $ | 724,409 | | | $ | 1,127,713 | |
| | | | | | | | |
| | |
Equity component, net of taxes | | $ | 233,353 | | | $ | 259,000 | |
| | | | | | | | |
| | |
2010 Notes principal amount | | $ | 450,000 | | | $ | 450,000 | |
Unamortized discount | | | (74,062 | ) | | | (89,133 | ) |
| | | | | | | | |
Net carrying amount | | $ | 375,938 | | | $ | 360,867 | |
| | | | | | | | |
| | |
Equity component, net of taxes | | $ | 60,054 | | | $ | 60,054 | |
| | | | | | | | |
| | |
2012 Notes principal amount | | $ | 500,000 | | | $ | — | |
Unamortized discount | | | (41,687 | ) | | | — | |
| | | | | | | | |
Net carrying amount | | $ | 458,313 | | | $ | — | |
| | | | | | | | |
| | |
Equity component, net of taxes | | $ | 49,195 | | | $ | — | |
| | | | | | | | |
Interest expense under the Convertible Notes is as follows:
| | | | | | | | | | | | |
| | | Years ended | |
| | | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Amortization of debt discount | | $ | 68,532 | | | $ | 72,908 | | | $ | 73,130 | |
Amortization of deferred financing costs | | | 3,828 | | | | 3,906 | | | | 4,092 | |
| | | | | | | | | | | | |
Non-cash interest expense | | | 72,360 | | | | 76,814 | | | | 77,222 | |
| | | | | | | | | | | | |
2.00% accrued interest | | | 34,898 | | | | 34,427 | | | | 34,500 | |
| | | | | | | | | | | | |
| | $ | 107,258 | | | $ | 111,241 | | | $ | 111,722 | |
| | | | | | | | | | | | |
F-35
If the Company fails to comply with the reporting obligations contained in the Convertible Notes agreements, the sole remedy of the holders of the Convertible Notes for the first 90 days following such event of default consists exclusively of the right to receive an extension fee in an amount equal to 0.25% of the accreted principal amount of the Convertible Notes. Based on the its evaluation of the Convertible Notes in accordance with ASC 815,the Company determined that the Convertible Notes contain a single embedded derivative, comprising both the contingent interest feature and the filing failure penalty payment, requiring bifurcation as the features are not clearly and closely related to the host instrument. The Company has determined that the value of this embedded derivative was nominal as of September 29, 2012 and September 24, 2011.
As of September 29, 2012, upon conversion, including the potential premium that could be payable on a fundamental change (as defined), the Company would issue a maximum of approximately 75.6 million common shares to the Convertible Note holders.
| 6. | Fair Value Measurements |
The Company applies the provisions of ASC 820 for its financial assets and liabilities that are re-measured and reported at fair value each reporting period and its nonfinancial assets and liabilities that are re-measured and reported at fair value on a non-recurring basis. Fair value is the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining fair value, the Company considers the principal or most advantageous market in which it would transact and considers assumptions that market participants would use when pricing the asset or liability.
Fair Value Hierarchy
ASC 820 establishes a three-level valuation hierarchy for disclosure of fair value measurements. Financial assets and liabilities are categorized within the valuation hierarchy based upon the lowest level of input that is significant to the measurement of fair value. The three levels of the hierarchy are defined as follows:
| | • | | Level 1—Inputs to the valuation methodology are quoted market prices for identical assets or liabilities. |
| | • | | Level 2—Inputs to the valuation methodology are other observable inputs, including quoted market prices for similar assets or liabilities and market-corroborated inputs. |
| | • | | Level 3—Inputs to the valuation methodology are unobservable inputs based on management’s best estimate of inputs market participants would use in pricing the asset or liability at the measurement date, including assumptions about risk. |
Assets/Liabilities Measured and Recorded at Fair Value on a Recurring Basis
As of September 29, 2012 and September 24, 2011, the Company’s financial assets that are re-measured at fair value on a recurring basis included $0.3 million in money market mutual funds in both periods that are classified as cash and cash equivalents in the Consolidated Balance Sheets. Money market mutual funds are classified within Level 1 of the fair value hierarchy and are valued using quoted market prices for identical assets. As a result of its Gen-Probe acquisition, the Company has an equity investment in a publicly-traded company and mutual funds, both of which are valued using quoted market prices, representing Level 1 assets. The Company has a payment obligation under its DCP to the participants of the DCP and the deferred compensation plan assumed in the Gen-Probe acquisition. This aggregate liability is recorded at fair value based on the underlying value of certain hypothetical investments DCP and actual investments for the Gen-Probe plan as designated by each participant for their benefit. Since the value of the DCP obligation is based on market prices, the liability is classified within Level 1. In addition, the Company has contingent consideration liabilities related to its acquisitions that it records at fair value. The fair values of these liabilities are based on Level 3 inputs and are discussed in Note 3.
F-36
Assets and liabilities measured at fair value on a recurring basis consisted of the following:
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at September 29, 2012 | |
| | | Carrying Value | | | Quoted Prices in
Active Market for
Identical Assets
(Level 1) | | | Significant
Other
Observable
Inputs (Level 2) | | | Significant
Unobservable
Inputs (Level 3) | |
Assets: | | | | | | | | | | | | | | | | |
Money market funds | | $ | 315 | | | $ | 315 | | | $ | — | | | $ | — | |
Marketable securities: | | | | | | | | | | | | | | | | |
Equity security | | | 6,029 | | | | 6,029 | | | | — | | | | — | |
Mutual funds | | | 6,995 | | | | 6,995 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Total | | $ | 13,339 | | | $ | 13,339 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | | | | | |
Deferred compensation liabilities | | $ | 32,082 | | | $ | 32,082 | | | $ | — | | | $ | — | |
Contingent consideration | | | 86,368 | | | | — | | | | — | | | | 86,368 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 118,450 | | | $ | 32,082 | | | $ | — | | | $ | 86,368 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at September 24, 2011 | |
| | | Carrying Value | | | Quoted Prices in
Active Market for
Identical Assets
(Level 1) | | | Significant
Other
Observable
Inputs (Level 2) | | | Significant
Unobservable
Inputs (Level 3) | |
Assets: | | | | | | | | | | | | | | | | |
Money market funds | | $ | 314 | | | $ | 314 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Total | | $ | 314 | | | $ | 314 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | | | | | |
Deferred compensation liabilities | | $ | 17,168 | | | $ | 17,168 | | | $ | — | | | $ | — | |
Contingent consideration | | | 103,790 | | | | — | | | | — | | | | 103,790 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 120,958 | | | $ | 17,168 | | | $ | — | | | $ | 103,790 | |
| | | | | | | | | | | | | | | | |
Changes in the fair value of recurring fair value measurements, which solely consisted of contingent consideration liabilities, using significant unobservable inputs (Level 3) during the years ended September 29, 2012 and September 24, 2011 were as follows:
| | | | | | | | |
| | | 2012 | | | 2011 | |
Beginning balance | | $ | 103,790 | | | $ | 29,500 | |
Contingent consideration liabilities recorded at fair value at acquisition | | | — | | | | 86,600 | |
Fair value adjustments | | | 38,466 | | | | (8,016 | ) |
Payments made | | | (55,888 | ) | | | (4,294 | ) |
| | | | | | | | |
Ending balance | | $ | 86,368 | | | $ | 103,790 | |
| | | | | | | | |
Refer to Note 3 for a description of the valuation of contingent consideration and related sensitivities of the estimates.
Assets Measured and Recorded at Fair Value on a Nonrecurring Basis
The Company remeasures the fair value of certain assets and liabilities upon the occurrence of certain events. Such assets are comprised of cost-method equity investments and long-lived assets, including property and equipment, intangible assets and goodwill. During fiscal 2012, the Company recorded a goodwill impairment charge of $5.8 million related to its MammoSite reporting unit. During fiscal 2010, the Company recorded impairment charges of $143.5 million and $76.7 million to adjust intangible assets and goodwill, respectively, related to its MammoSite reporting unit to their estimated fair values. These adjustments fall within Level 3 of the fair value hierarchy due to the use of significant unobservable inputs to determine fair value. The fair value measurements using a discounted cash flow technique, and the amount and timing of future cash flows within the analysis were based on the Company’s most recent operational budgets, long-range strategic plans and other estimates at the time such remeasurement was made.
The Company holds certain cost-method equity investments in non-publicly traded securities aggregating $16.0 million and $4.6 million at September 29, 2012 and September 24, 2011, respectively, which are included in other long-term assets on the Company’s Consolidated Balance Sheets. The increase in these investments from fiscal 2011 was due to those acquired in the Gen-Probe acquisition. These investments are generally carried at cost, which for the investments acquired from Gen-Probe was the estimated fair value on the date of acquisition. As the inputs utilized for the Company’s periodic impairment assessment are not based on observable
F-37
market data, these cost method investments are classified within Level 3 of the fair value hierarchy. To determine the fair value of these investments, the Company uses all available financial information related to the entities, including information based on recent or pending third-party equity investments in these entities. In certain instances, a cost method investment’s fair value is not estimated as there are no identified events or changes in circumstances that may have a significant adverse effect on the fair value of the investment and to do so would be impractical. During fiscal 2011 and 2010, the Company recorded other-than-temporary impairment charges of $2.4 million and $1.1 million, respectively, related to certain of its cost-method equity investments to adjust their carrying amounts to fair value.
The following chart depicts the level of inputs within the fair value hierarchy used to estimate the fair value of equipment, intangible assets, goodwill and cost-method equity investments measured on a nonrecurring basis for which the Company recorded impairment charges:
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements Using | | | | |
| | | Fair Value | | | Quoted Prices in
Active Market for
Identical Assets
(Level 1) | | Significant
Other
Observable
Inputs (Level 2) | | Significant
Unobservable
Inputs (Level 3) | | | Total Gains
(Losses) | |
Fiscal 2012: | | | | | | | | | | | | | | | | |
Equipment | | $ | — | | | | | | | $ | — | | | $ | (6,452 | ) |
Goodwill | | | — | | | | | | | | — | | | | (5,826 | ) |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | $ | (12,278 | ) |
| | | | | | | | | | | | | | | | |
Fiscal 2011: | | | | | | | | | | | | | | | | |
Cost-method equity investments | | $ | 345 | | | | | | | $ | 345 | | | $ | (2,445 | ) |
| | | | | | | | | | | | | | | | |
Fiscal 2010: | | | | | | | | | | | | | | | | |
Intangible assets | | $ | 24,290 | | | | | | | $ | 24,290 | | | $ | (143,467 | ) |
Goodwill | | | 5,826 | | | | | | | | 5,826 | | | | (76,723 | ) |
Cost-method equity investment | | | — | | | | | | | | — | | | | (1,100 | ) |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | $ | (221,290 | ) |
| | | | | | | | | | | | | | | | |
The above fair value amounts represent only those individual assets remeasured and not the consolidated balances. Refer to Note 5 for disclosure of the nonrecurring fair value measurement related to the loss on extinguishment of debt recorded in the second quarter of fiscal 2012 and the first quarter of fiscal 2011.
Disclosure of Fair Value of Financial Instruments
The Company’s financial instruments mainly consist of cash and cash equivalents, accounts receivable, marketable securities, cost-method equity investments, insurance contracts, DCP liability, accounts payable and debt obligations. The carrying amounts of the Company’s cash equivalents, accounts receivable and accounts payable approximate their fair value due to the short-term nature of these instruments. The carrying amount of the insurance contracts are recorded at the cash surrender value, as required by U.S. generally accepted accounting principles, which approximates fair value, and the related DCP liability is recorded at fair value. The Company believes the carrying amounts of its cost-method investments approximate fair value.
Amounts outstanding under our Credit Agreement aggregating $2.5 billion aggregate principal are subject to variable rates of interest based on current market rates, and as such, we believe the carrying amount of these obligations approximates fair value. In addition, based on the recent issuance of our Senior Notes, we believe their carrying amount approximates fair value. The fair value of the Company’s Convertible Notes is based on the trading prices of the respective notes at the dates noted and represent Level 1 measurements. Refer to Note 5 for the various components of the Company’s debt respective carrying amounts.
The estimated fair values of the Company’s Convertible Notes as of September 29, 2012 and September 24, 2011 are as follows:
| | | | | | | | |
| | | 2012 | | | 2011 | |
2007 Notes | | $ | 771,600 | | | $ | 1,200,000 | |
2010 Notes | | | 505,600 | | | | 468,700 | |
2012 Notes | | | 490,700 | | | | — | |
| | | | | | | | |
| | $ | 1,767,900 | | | $ | 1,668,700 | |
| | | | | | | | |
F-38
On January 16, 2008, the Company entered into an agreement to sell the full world-wide rights of its Makena (formerly Gestiva) pharmaceutical product to K-V Pharmaceutical Company (“KV”) upon FDA approval of the then pending Makena new drug application for $82.0 million. The Company executed certain amendments to this agreement resulting in an increase of the total sales price to $199.5 million and changing the timing of when payments are due to the Company. Gains attributable to payments in the amount of $79.5 million received from KV prior to FDA approval were deferred.
On February 3, 2011, the Company received FDA approval of Makena, and subject to a security interest and a right of reversion for failure to make future payments, all rights to Makena were transferred to KV. Upon FDA approval, the Company received $12.5 million, and including the $79.5 million previously received, the Company recorded a gain on the sale of intellectual property, net of the write-off of certain assets, of $84.5 million in the second quarter of fiscal 2011. Pursuant to the amended agreement, the Company received $12.5 million in the second quarter of fiscal 2012, which was recorded net of amounts due to the inventor of Makena. Currently, the remaining $95.0 million of the sales price is due over a period of 18 to 30 months from FDA approval (subject to further deferral elections) depending on which one of two payment options KV selects. KV will also owe the Company a 5% royalty on sales for certain time periods determined based upon the payment option or deferral elections selected by KV. On August 4, 2012, KV and certain of its subsidiaries filed voluntary petitions for reorganization under Chapter 11 of Title 11 of the United States Code in the United States Bankruptcy Court for the Southern District of New York. The Company is pursuing its claims against KV in these proceedings for amounts due to the Company under its agreement with KV.
Due to uncertainty regarding collection, any amounts to be received in the future from KV have not been recorded in the Company’s consolidated financial statements, and as the Company receives the amounts owed, the payments will be recorded as a gain within operating expenses in the Consolidated Statement of Operations in the period received.
The Company’s (loss) income before income taxes consisted of the following:
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Domestic | | $ | (46,018 | ) | | $ | 235,204 | | | $ | (70,750 | ) |
Foreign | | | (15,643 | ) | | | (7,818 | ) | | | 15,759 | |
| | | | | | | | | | | | |
| | $ | (61,661 | ) | | $ | 227,386 | | | $ | (54,991 | ) |
| | | | | | | | | | | | |
The provision for income taxes consisted of the following:
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Federal: | | | | | | | | | | | | |
Current | | $ | 146,164 | | | $ | 97,834 | | | $ | 105,664 | |
Deferred | | | (143,582 | ) | | | (33,808 | ) | | | (108,002 | ) |
| | | | | | | | | | | | |
| | | 2,582 | | | | 64,026 | | | | (2,338 | ) |
| | | | | | | | | | | | |
State: | | | | | | | | | | | | |
Current | | | 15,348 | | | | 15,739 | | | | 18,334 | |
Deferred | | | (10,186 | ) | | | (5,909 | ) | | | (11,501 | ) |
| | | | | | | | | | | | |
| | | 5,162 | | | | 9,830 | | | | 6,833 | |
| | | | | | | | | | | | |
Foreign: | | | | | | | | | | | | |
Current | | | 5,653 | | | | 4,770 | | | | 5,550 | |
Deferred | | | (1,424 | ) | | | (8,390 | ) | | | (2,223 | ) |
| | | | | | | | | | | | |
| | | 4,229 | | | | (3,620 | ) | | | 3,327 | |
| | | | | | | | | | | | |
| | $ | 11,973 | | | $ | 70,236 | | | $ | 7,822 | |
| | | | | | | | | | | | |
F-39
A reconciliation of income taxes at the U.S. federal statutory rate to the Company’s effective tax rate is as follows:
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Income tax provision at federal statutory rate | | | (35.0 | )% | | | 35.0 | % | | | (35.0 | )% |
Increase (decrease) in tax resulting from: | | | | | | | | | | | | |
Goodwill impairment | | | 3.3 | | | | — | | | | 48.7 | |
Section 199 manufacturing deduction | | | (20.3 | ) | | | (4.1 | ) | | | (11.6 | ) |
State income taxes, net of federal benefit | | | 5.3 | | | | 3.6 | | | | 2.7 | |
Research and investment tax credits | | | (1.6 | ) | | | (3.2 | ) | | | (6.9 | ) |
Unrecognized tax benefits | | | 13.5 | | | | (3.3 | ) | | | 7.2 | |
Contingent consideration | | | 59.8 | | | | 1.5 | | | | — | |
Nondeductible transaction expenses | | | 7.5 | | | | — | | | | — | |
Cessation of Adiana | | | (28.6 | ) | | | — | | | | — | |
Executive compensation | | | 2.3 | | | | (1.1 | ) | | | 6.1 | |
Foreign rate differential | | | 3.1 | | | | 0.8 | | | | (1.0 | ) |
Change in valuation allowance | | | 5.4 | | | | — | | | | (0.6 | ) |
Other | | | 4.7 | | | | 1.7 | | | | 4.6 | |
| | | | | | | | | | | | |
| | | 19.4 | % | | | 30.9 | % | | | 14.2 | % |
| | | | | | | | | | | | |
The Company’s effective tax rate in fiscal 2012 was significantly impacted by non-deductible contingent consideration compensation expense, nondeductible acquisition costs, a nondeductible goodwill impairment charge, and a net increase in income tax reserves and valuation allowances on certain foreign losses. The unfavorable tax impact of these items was partially offset by the domestic manufacturing benefit and a loss claimed related to the discontinuance of the Adiana product line. The effect of these permanent items to the effective tax rate was magnified by the current year pre-tax loss.
The Company’s effective tax rate for fiscal 2011 was less than the statutory rate primarily due to reversing income tax reserves, the domestic manufacturing benefit and both U.S. and Canadian research and development tax credits. The $9.1 million income tax reserve reversal was due to the Company favorably settling its U.S. federal income tax audit for fiscal years 2007 through 2009 and statutes of limitations expiring in several state and foreign jurisdictions.
The effective tax rate for fiscal 2010 was significantly impacted by the goodwill impairment charge recorded in the fourth quarter of fiscal 2010, substantially all of which was not deductible for tax purposes. In addition, the Company recorded provision to return adjustments and additional reserve needs partially offset by reversing reserves no longer required related to selling the Company’s manufacturing operation in Shanghai, China in the second quarter of fiscal 2010, and statutes of limitations expiring in several jurisdictions.
The Company accounts for income taxes using the liability method in accordance with ASC 740,Income Taxes. Under this method, deferred income taxes are recognized for the future tax consequences of differences between the tax and financial accounting bases of assets and liabilities at the end of each reporting period. Deferred income taxes are based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. A valuation allowance is established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company’s significant deferred tax assets and liabilities are as follows:
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Deferred tax assets | | | | | | | | |
Net operating loss carryforwards | | $ | 47,472 | | | $ | 56,641 | |
Capital losses | | | 46,750 | | | | — | |
Nondeductible accruals | | | 22,198 | | | | 17,767 | |
Nondeductible reserves | | | 10,346 | | | | 8,400 | |
Stock-based compensation | | | 31,437 | | | | 23,826 | |
Research and other credits | | | 11,392 | | | | 13,207 | |
Convertible notes issuance costs | | | 811 | | | | 1,283 | |
Nonqualified deferred compensation plan | | | 12,007 | | | | 7,324 | |
Other temporary differences | | | 3,000 | | | | 4,267 | |
| | | | | | | | |
| | | 185,413 | | | | 132,715 | |
Less: valuation allowance | | | (64,337 | ) | | | (13,930 | ) |
| | | | | | | | |
| | $ | 121,076 | | | $ | 118,785 | |
| | | | | | | | |
F-40
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Deferred tax liabilities | | | | | | | | |
Depreciation and amortization | | $ | (1,635,043 | ) | | $ | (771,504 | ) |
Debt discounts and deferrals | | | (209,011 | ) | | | (258,593 | ) |
Fair value adjustments to current assets and liabilities | | | (28,413 | ) | | | — | |
Investment in subsidiary | | | (8,479 | ) | | | (6,507 | ) |
| | | | | | | | |
| | $ | (1,880,946 | ) | | $ | (1,036,604 | ) |
| | | | | | | | |
| | $ | (1,759,870 | ) | | $ | (917,819 | ) |
| | | | | | | | |
Under ASC 740, the Company can only recognize a deferred tax asset for the future benefit attributable to its tax losses and credit carryforwards to the extent that it is “more likely than not” that these assets will be realized. After considering all available positive and negative evidence, the Company has established a valuation allowance against a portion of its remaining deferred tax assets because it is more likely than not that some of its tax losses and credit carryforwards will not be realized. In determining these assets realizability, the Company considered numerous factors including historical profitability, the character and amount of estimated future taxable income, and the industry in which it operates. The valuation allowance increased $50.4 million in fiscal 2012 from fiscal 2011 primarily due to fully reserved tax assets acquired in the Gen-Probe acquisition.
As of September 29, 2012, the Company had $30.7 million, $99.0 million and $108.7 million in gross federal, state and foreign net operating losses respectively, and $0.9 million, $12.6 million and $2.3 million in federal, state and foreign credit carryforwards respectively, that are more likely than not to be realized. These losses and credits expire between 2013 and 2031, except for $53.0 million of losses and $8.7 million of credits that have unlimited carryforward periods. The federal and state net operating losses exclude $1.0 million and $74.0 million, respectively, of net operating losses, which the Company expects will expire unutilized.
The Company had gross unrecognized tax benefits, excluding interest, of $53.1 million as of September 29, 2012 and $31.0 million as of September 24, 2011. At September 29, 2012, $53.1 million represents the unrecognized tax benefits that, if recognized, would reduce the Company’s effective tax rate. In the next twelve months it is reasonably possible that the Company will reduce its unrecognized tax benefits by $1.0 to $2.0 million due to statutes of limitations expiring and favorable settlements with taxing authorities, which would reduce the Company’s effective tax rate.
Activity of the Company’s unrecognized income tax benefits for fiscal 2012 and 2011 are as follows:
| | | | | | | | |
| | | 2012 | | | 2011 | |
Balance at beginning of fiscal year | | $ | 31,026 | | | $ | 31,790 | |
Tax positions related to current year: | | | | | | | | |
Additions | | | 11,673 | | | | 2,014 | |
Reductions | | | — | | | | — | |
Tax positions related to prior years: | | | | | | | | |
Additions related to change in estimate | | | 1,327 | | | | 5,934 | |
Reductions | | | 307 | | | | (700 | ) |
Payments | | | (197 | ) | | | (1,182 | ) |
Lapses in statutes of limitations and settlements | | | (4,144 | ) | | | (9,162 | ) |
Acquired tax positions: | | | | | | | | |
Additions related to reserves acquired from acquisitions | | | 13,156 | | | | 2,332 | |
| | | | | | | | |
Balance as of the end of the fiscal year | | $ | 53,148 | | | $ | 31,026 | |
| | | | | | | | |
The Company’s policy is to include accrued interest and penalties related to unrecognized tax benefits and income tax liabilities, when applicable, in income tax expense in its Consolidated Statements of Operations. As of September 29, 2012 and September 24, 2011, accrued interest was $2.6 million and $1.4 million, respectively. As of September 29, 2012, no penalties have been accrued.
The Company and its subsidiaries are subject to various federal, state, and foreign income taxes. The Company’s U.S. Federal income tax returns are no longer subject to examination for years prior to tax year 2009, and its State income tax returns are generally no longer subject to examination for years prior to tax year 2009. The IRS concluded its examination for fiscal years 2007 through 2009, and the Company paid $7.6 million to settle issues raised, substantially all of which had been previously recorded within deferred tax liabilities. The Company is also undergoing a tax audit in Germany for fiscal years 2008 through 2010. The Company has a tax holiday in Costa Rica that currently does not materially impact its effective tax rate and is scheduled to expire in 2015.
F-41
The Company intends to reinvest, indefinitely, approximately $50.6 million of unremitted foreign earnings. It is not practical to estimate the additional taxes that might be payable upon repatriating these foreign earnings.
| 9. | Stockholders’ Equity and Stock-Based Compensation |
Rights Agreement
On April 2, 2008, the Company entered into an Amended and Restated Rights Agreement (the “Amended and Restated Rights Agreement”) between the Company and American Stock Transfer & Trust Company as Rights Agent (the “Rights Agent”). The Amended and Restated Rights Agreement amends and restates the Company’s rights agreement, dated as of September 17, 2002, as amended on May 21, 2007, between the Company and the Rights Agent.
On April 2, 2008, the Company effected a two-for-one stock split in the form of a stock dividend to stockholders as of March 21, 2008. Pursuant to the Amended and Restated Rights Agreement, the Company amended the terms of the rights issued and issuable under the agreement (“Rights”), effective as of April 3, 2008 (after the stock dividend), to reset the Rights such that each share of Common Stock is entitled to receive one Right, to retain the purchase price of each Right at $60.00 per Right, and to provide that each Right will entitle the holder to purchase one twenty-five thousandth of a share of Series A Junior Participating Preferred Stock (the “Series A Preferred Stock”). Conforming changes have also been made to the Company’s certificate of designation for the Series A Preferred Stock to provide that each share of Series A Preferred Stock carries 25,000 times the dividend, liquidation and voting rights of the Company’s Common Stock. Other modifications have also been made in the Amended and Restated Rights Agreement to update the agreement for certain developments, including the recent amendments to the Company’s by-laws permitting stockholders to hold and transfer shares of the Company’s capital stock in book entry form. The expiration date of the Rights has remained unchanged at January 1, 2013.
Stock-Based Compensation
Equity Compensation Plans
The Company has one share-based compensation plan pursuant to which awards are currently being made—the 2008 Equity Incentive Plan. The Company has four share-based compensation plans pursuant to which outstanding awards have been made, but from which no further awards can or will be made—i) the 1995 Combination Stock Option Plan; ii) the 1997 Employee Equity Incentive Plan; iii) the 1999 Equity Incentive Plan; and iv) the 2000 Acquisition Equity Incentive Plan.
The purpose of the 2008 Equity Plan is to provide stock options, stock issuances and other equity interests in the Company to employees, officers, directors, consultants and advisors of the Company and its parents and subsidiaries, and any other person who is determined by the Board of Directors to have made (or is expected to make) contributions to the Company. The 2008 Equity Plan is administered by the Board of Directors of the Company, and a total of 20 million shares were reserved for issuance under this plan. As of September 29, 2012, the Company had 5.4 million shares available for future grant under the 2008 Equity Plan.
The Company assumed certain other plans in connection with the Gen-Probe, Cytyc and Third Wave acquisitions, and no shares are available for future grant under these plans.
The following presents stock-based compensation expense in the Company’s Consolidated Statement of Operations in fiscal 2012, 2011 and 2010:
| | | | | | | | | | | | |
| | | 2012 | | | 2011 | | | 2010 | |
Cost of revenues | | $ | 5,722 | | | $ | 4,602 | | | $ | 4,332 | |
Research and development | | | 5,328 | | | | 4,852 | | | | 4,011 | |
Selling and marketing | | | 7,355 | | | | 5,954 | | | | 5,313 | |
General and administrative | | | 18,667 | | | | 20,064 | | | | 20,504 | |
Restructuring | | | 3,500 | | | | — | | | | — | |
| | | | | | | | | | | | |
| | $ | 40,572 | | | $ | 35,472 | | | $ | 34,160 | |
| | | | | | | | | | | | |
F-42
Grant-Date Fair Value
The Company uses a binomial lattice model to determine the fair value of its stock options. The Company considers a number of factors to determine the fair value of options including the assistance of an outside valuation advisor. Information pertaining to stock options granted during fiscal 2012, 2011 and 2010 and related assumptions are noted in the following table:
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Options granted | | | 2,259 | | | | 2,249 | | | | 2,858 | |
Weighted-average exercise price | | $ | 17.21 | | | $ | 17.15 | | | $ | 15.65 | |
Weighted-average grant date fair value | | $ | 6.48 | | | $ | 6.16 | | | $ | 5.87 | |
Assumptions: | | | | | | | | | | | | |
Risk-free interest rates | | | 0.7 | % | | | 1.0 | % | | | 1.8 | % |
Expected life (in years) | | | 4.3 | | | | 4.2 | | | | 3.9 | |
Expected volatility | | | 47 | % | | | 45 | % | | | 47 | % |
Dividend yield | | | — | | | | — | | | | — | |
The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected life of the stock options. In projecting expected stock price volatility, the Company uses a combination of historical stock price volatility and implied volatility from observable market prices of similar equity instruments. The Company estimated the expected life of stock options based on historical experience using employee exercise and option expiration data.
Stock-Based Compensation Expense Attribution
The Company uses the straight-line attribution method to recognize stock-based compensation expense for stock options and restricted stock units (“RSU”). The vesting term of stock options is generally five years with annual vesting of 20% per year on the anniversary of the grant date, and RSUs generally vest over four years with annual vesting at 25% per year on the anniversary of the grant date. The amount of stock-based compensation recognized during a period is based on the value of the portion of the awards that are ultimately expected to vest. ASC 718 requires forfeitures to be estimated at the time granted and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Based on an analysis of historical forfeitures, the Company has determined a specific forfeiture rate for certain employee groups and has applied forfeiture rates ranging from 0% to 7% as of September 29, 2012 depending on the specific employee group. This analysis is re-evaluated annually and the forfeiture rate will be adjusted as necessary. Ultimately, the actual stock-based compensation expense recognized will only be for those stock options and RSUs that vest.
Stock-based compensation expense related to stock options was $18.7 million, $15.2 million, and $13.3 million in fiscal 2012, 2011 and 2010, respectively. Stock compensation expense related to RSUs was $21.4 million, $20.3 million, and $20.9 million in fiscal 2012, 2011 and 2010, respectively. The related tax benefit recorded in the Consolidated Statements of Operations was $12.2 million, $14.8 million and $9.9 million in fiscal 2012, 2011 and 2010, respectively. Included within stock-based compensation expense is $3.5 million related to the acceleration of vesting for certain options assumed in the Gen-Probe acquisition related to employees who were terminated in connection with the Company’s restructuring action to consolidate its Diagnostics operations. The original terms of the options provided for acceleration upon a change-in-control and termination within 18 months of the change-in-control. At September 29, 2012, there was $44.9 million and $36.6 million of unrecognized compensation expense related to stock options and RSUs, respectively, to be recognized over a weighted average period of 3.2 years and 2.4 years, respectively.
Share Based Payment Activity
The following table summarizes all stock option activity under the Company’s stock option plans for the year ended September 29, 2012:
| | | | | | | | | | | | | | | | |
| | | Number
of Shares | | | Weighted-
Average
Exercise Price | | | Weighted-
Average
Remaining
Contractual Life
in Years | | | Aggregate
Intrinsic
Value | |
Options outstanding at September 24, 2011 | | | 15,478 | | | $ | 17.01 | | | | 4.11 | | | $ | 30,455 | |
Granted | | | 2,258 | | | | 17.21 | | | | | | | | | |
Assumed from Gen-Probe Acquisition | | | 3,663 | | | | 15.28 | | | | | | | | | |
Cancelled/forfeited | | | (903 | ) | | | 18.28 | | | | | | | | | |
Exercised | | | (2,457 | ) | | | 11.27 | | | | | | | $ | 20,399 | |
| | | | | | | | | | | | | | | | |
Options outstanding at September 29, 2012 | | | 18,039 | | | $ | 17.40 | | | | 4.40 | | | $ | 78,962 | |
| | | | | | | | | | | | | | | | |
Options exercisable at September 29, 2012 | | | 8,141 | | | $ | 18.73 | | | | 3.15 | | | $ | 37,574 | |
| | | | | | | | | | | | | | | | |
Options vested and expected to vest at September 29, 2012 (1) | | | 17,385 | | | $ | 17.47 | | | | 4.33 | | | $ | 75,870 | |
| | | | | | | | | | | | | | | | |
F-43
| (1) | This represents the number of vested stock options as of September 29, 2012 plus the unvested outstanding options at September 29, 2012 expected to vest in the future, adjusted for estimated forfeitures. |
During fiscal 2011 and 2010, the total intrinsic value of options exercised (i.e., the difference between the market price on the date of exercise and the price paid by the employee to exercise the options) was $12.3 million and $7.3 million, respectively.
A summary of the Company’s RSU activity during the year ended September 29, 2012 is presented below:
| | | | | | | | |
Non-vested Shares | | Number of
Shares | | | Weighted-Average
Grant-Date Fair
Value | |
Non-vested at September 24, 2011 | | | 3,112 | | | $ | 15.67 | |
Granted | | | 1,691 | | | | 17.29 | |
Vested | | | (997 | ) | | | 15.71 | |
Forfeited | | | (226 | ) | | | 15.87 | |
| | | | | | | | |
Non-vested at September 29, 2012 | | | 3,580 | | | $ | 16.41 | |
| | | | | | | | |
The number of RSUs vested includes shares withheld on behalf of employees to satisfy minimum statutory tax withholding requirements. The Company pays the minimum statutory tax withholding requirement on behalf of its employees. During fiscal 2012, 2011 and 2010 the total fair value of RSUs vested was $15.7 million, $43.2 million and $7.5 million, respectively.
Employee Stock Purchase Plan
The Company’s 2008 Employee Stock Purchase Plan (the “2008 ESPP”) met the criteria set forth in ASC 718’s definition of a non-compensatory plan and did not give rise to stock-based compensation expense. The ESPP plan period was semi-annual and allowed participants to purchase the Company’s common stock at 95% of the closing price of the stock on the last day of the plan period. A total of 0.4 million shares were authorized for issuance under the 2008 ESPP.
In March 2012, the Company’s stockholders approved the Hologic, Inc. 2012 Employee Stock Purchase Plan (“2012 ESPP”), which provides for the granting of up to 2.5 million shares of the Company’s common stock to eligible employees, and resulted in the termination of the 2008 ESPP. The 2012 ESPP plan period is semi-annual and allows participants to purchase the Company’s common stock at 85% of the lower of (i) the market value per share of the common stock on the first day of the offering period or (ii) the market value per share of the common stock on the purchase date. The first plan period began on July 1, 2012 and no shares have been issued out of the 2012 ESPP as of September 29, 2012. Stock-based compensation expense in fiscal 2012 was $0.4 million.
The Company uses the Black-Scholes model to estimate the fair value of shares to be issued as of the grant date using the following weighted average assumptions:
| | | | |
| | | September 29,
2012 | |
Assumptions: | | | | |
Risk-free interest rates | | | 0.16 | % |
Expected life (in years) | | | 0.5 | |
Expected volatility | | | 35 | % |
Dividend yield | | | — | |
| 10. | Profit Sharing 401(k) Plan |
The Company has a qualified profit sharing plan covering substantially all of its employees. Contributions to the plan are at the discretion of the Company’s Board of Directors. The Company made contributions of $9.4 million, $6.4 million and $5.9 million for fiscal 2012, 2011 and 2010, respectively.
| 11. | Nonqualified Deferred Compensation Plan |
Effective March 15, 2006, the Company adopted its DCP to provide non-qualified retirement benefits to a select group of executive officers, senior management and highly compensated employees of the Company. Eligible employees may elect to contribute up to 75% of their annual base salary and 100% of their annual bonus to the DCP and such employee contributions are 100% vested. In addition, the Company may elect to make annual discretionary contributions on behalf of participants in the DCP. Each Company contribution is subject to a three-year vesting schedule, such that each contribution vests one third annually. Employee contributions are recorded within accrued expenses.
F-44
Upon enrollment into the DCP, employees make investment elections for both their voluntary contributions and discretionary contributions, if any, made by the Company. Earnings and losses on contributions based on these investment elections are recorded as a component of compensation expense in the period earned.
Annually the Compensation Committee of the Board of Directors has approved a discretionary cash contribution to the DCP for each year. Discretionary contributions by the Company to the DCP are held in a Rabbi Trust. The Company is recording compensation expense for the DCP discretionary contributions ratably over the three-year vesting period of each annual contribution, and totaled $2.6 million, $2.7 million and $2.1 million in fiscal 2012, 2011 and 2010, respectively. The full amount of the discretionary contribution, net of forfeitures, along with employee deferrals and the deferred compensation liability assumed from the Gen-Probe acquisition is recorded within accrued expenses and totaled $32.1 million and $17.2 million at September 29, 2012 and September 24, 2011, respectively.
The Company has purchased Company-owned group life insurance contracts, in which both voluntary and discretionary Company DCP contributions are invested, to fund payment of the Company’s obligation to the DCP participants. The total amount invested at September 29, 2012 and September 24, 2011 was $26.0 million and $22.7 million. The values of these life insurance contracts are recorded in other long-term assets. Changes in the cash surrender value of life insurance contracts, which were not significant in fiscal 2012, 2011 and 2010, are recorded within other income (expense), net in the Consolidated Statements of Operations. In addition, the Gen-Probe deferred compensation plan investments of $7.0 million are in mutual funds, which are classified as trading and the gains and losses in these investments are recorded in interest income.
| 12. | Commitments and Contingencies |
Contingent Earn-Out Payments
In connection with its acquisitions, the Company has incurred the obligation to make contingent earn-out payments tied to performance criteria, principally revenue growth of the acquired businesses over a specified period. In certain circumstances, such as a change of control, a portion of these obligations may be accelerated. In addition, contractual provisions relating to these contingent earn-out obligations may include covenants to operate the acquired businesses in a manner that may not otherwise be most advantageous to the Company.
These contingent consideration arrangements are recorded as either additional purchase price or compensation expense if continuing employment is required to receive such payments. Pursuant to ASC 805, contingent consideration that is deemed to be part of the purchase price is recorded as a liability based on the estimated fair value of the consideration the Company expects to pay to the former shareholders of the acquired business as of the acquisition date. This liability is re-measured each reporting period with the changes in fair value recorded through a separate line item within the Company’s Consolidated Statements of Operations. Increases or decreases in the fair value of contingent consideration liabilities can result from accretion of the liability for the passage of time, changes in discount rates, and changes in the timing, probabilities and amount of revenue estimates. Contingent consideration arrangements from acquisitions completed prior to the adoption of ASC 805 (effective in fiscal 2010 for the Company) that are deemed to be part of the purchase price of the acquisition are not subject to the fair value measurement requirements of ASC 805 and are recorded as additional purchase price to goodwill.
In connection with the acquisition of Adiana, Inc., the Company has an obligation to the former Adiana shareholders to make contingent payments tied to the achievement of milestones. The milestone payments include potential contingent payments of up to $155.0 million based on worldwide sales of the Adiana Permanent Contraception System in the first year following FDA approval and on annual incremental sales growth thereafter through December 31, 2012. FDA approval of the Adiana system occurred on July 6, 2009, and the Company began accruing contingent consideration in the fourth quarter of fiscal 2009 based on the defined percentage of worldwide sales of the product. Since this contingent consideration obligation arose from an acquisition prior to the adoption of ASC 805, the amounts accrued are recorded as additional purchase price to goodwill. The purchase agreement includes an indemnification provision that provides for the reimbursement of qualifying legal expenses and liabilities associated with legal claims against the Adiana products and intellectual property, and the Company has the right to offset contingent consideration payments to the Adiana shareholders with these qualifying legal costs. The Company made payments of $8.8 million and $19.7 million in fiscal 2012 and 2011, respectively, to the former Adiana shareholders, net of amounts withheld for the legal indemnification provision. The Company had been in litigation with Conceptus regarding certain intellectual property matters related to the Adiana system, and to the extent available, the Company has been recording legal fees related to the Conceptus litigation matter as a reduction to the accrued contingent consideration payments. On October 17, 2011, the jury returned a verdict in the Conceptus litigation matter (see below) in favor of Conceptus awarding damages in the amount of $18.8 million. On April 29, 2012, the Company entered into a license and settlement agreement with Conceptus in which Conceptus agreed to forgo the $18.8 million jury award in consideration of the Company agreeing to a permanent injunction against the manufacture, sale and distribution of the Adiana product. No contingent consideration was earned and recorded in fiscal 2012, and as of the end of the second quarter of fiscal 2012 the Company decided to discontinue the manufacture, marketing and sales of the Adiana system. At September 29, 2012, the Company has accrued $16.8 million for the payment of contingent consideration to the former Adiana shareholders, which is net of amounts withheld for qualifying legal costs.
F-45
The Company also has contingent consideration obligations related to its Sentinelle Medical, Interlace, TCT and Healthcome acquisitions. Pursuant to ASC 805, contingent consideration pertaining to Sentinelle Medical and Interlace is required to be recorded as a liability at fair value and totaled $3.4 million and $83.0 million, respectively, at September 29, 2012. Contingent consideration pertaining to TCT and Healthcome is contingent upon future employment and is being recorded as compensation expense as it is earned. As of September 29, 2012, the Company had accrued $39.1 million and $5.0 million, respectively, for these obligations. For additional information pertaining to the Sentinelle Medical, Interlace, TCT and Healthcome acquisitions, contingent consideration terms and the assumptions used to fair value contingent consideration, refer to Note 3.
A summary of amounts recorded to the Consolidated Statement of Operations is as follows:
| | | | | | | | | | | | | | | | | | | | |
Statement of Operations Line Item – Fiscal 2012 | | Sentinelle
Medical | | | Interlace | | | TCT | | | Healthcome | | | Total | |
Contingent consideration—compensation expense | | $ | — | | | | — | | | $ | 75,459 | | | $ | 5,572 | | | $ | 81,031 | |
Contingent consideration—fair value adjustments | | | (3,364 | ) | | | 41,830 | | | | — | | | | — | | | | 38,466 | |
| | | | | | | | | | | | | | | | | | | | |
| | $ | (3,364 | ) | | $ | 41,830 | | | $ | 75,459 | | | $ | 5,572 | | | $ | 119,497 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Statement of Operations Line Item – Fiscal 2011 | | Sentinelle
Medical | | | Interlace | | | TCT | | | Healthcome | | | Total | |
Contingent consideration—compensation expense | | $ | — | | | $ | 2,102 | | | $ | 17,581 | | | $ | 319 | | | $ | 20,002 | |
Contingent consideration—fair value adjustments | | | (14,328 | ) | | | 6,312 | | | | — | | | | — | | | | (8,016 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | $ | (14,328 | ) | | $ | 8,414 | | | $ | 17,581 | | | $ | 319 | | | $ | 11,986 | |
| | | | | | | | | | | | | | | | | | | | |
Finance Lease Obligations
The Company has two non-cancelable lease agreements for buildings that are primarily used for manufacturing. The Company was responsible for a significant portion of the construction costs, and in accordance with ASC 840,Leases,Subsection 40-15-5, the Company was deemed to be the owner of the respective buildings during the construction period.The Company has recorded the fair market value of the buildings and land aggregating $28.3 million within property and equipment on its Consolidated Balance Sheets. At September 29, 2012, the Company has recorded $2.8 million in accrued expenses and $33.2 million in other long-term liabilities related to these obligations. The term of the leases is for a period of approximately ten and 12 years, respectively, with the option to extend for two consecutive 5-year terms. At the completion of the construction period, the Company reviewed the lease for potential sale-leaseback treatment in accordance with ASC 840, Subsection 40,Sale-Leaseback Transactions.Based on its analysis, the Company determined that the lease did not qualify for sale-leaseback treatment. Therefore, the building, leasehold improvements and associated liabilities remain on the Company’s financial statements throughout the lease term, and the building and leasehold improvements are being depreciated on a straight line basis over their estimated useful lives of 35 years.
Future minimum lease payments, including principal and interest, under these leases were as follows at September 29, 2012:
| | | | |
Fiscal 2013 | | $ | 2,764 | |
Fiscal 2014 | | | 2,822 | |
Fiscal 2015 | | | 2,883 | |
Fiscal 2016 | | | 3,054 | |
Fiscal 2017 | | | 3,119 | |
Thereafter | | | 3,225 | |
| | | | |
Total minimum payments | | | 17,867 | |
Less-amount representing interest | | | (4,956 | ) |
| | | | |
Total | | $ | 12,911 | |
| | | | |
Non-cancelable Purchase and Royalty Commitments
The Company has certain non-cancelable purchase obligations primarily related to inventory purchases and Diagnostics instruments, primarily the TIGRIS and PANTHER systems, and to a lesser extent other operating expense commitments. These obligations are not recorded in the Consolidated Balance Sheet. For reasons of quality assurance, sole source availability or cost effectiveness, certain key components and raw materials and instruments are available only from a sole supplier and the Company has certain long-term supply contracts to assure continuity of supply. At September 29, 2012, purchase commitments aggregated approximately $74.7 million through fiscal 2017 of which $63.7 million relates to fiscal 2013.
F-46
In addition, as part of its R&D efforts assumed from the Gen-Probe acquisition, the Company has various license agreements with unrelated parties that provide the Company with rights to develop and market products using certain technology and patent rights. Terms of the various license agreements require the Company to pay royalties ranging from 1% up to 35% of future sales on products using the specified technology. Such agreements generally provide for a term that commences upon execution and continues until expiration of the last patent covering the licensed technology. Under certain of these agreements, the Company is required to pay minimum annual royalty payments. At September 29, 2012, minimum royalty commitments aggregated approximately $14.1 million, of which $2.0 million relates to fiscal 2013.
Concentration of Suppliers
The Company purchases certain components of its products from a single or small number of suppliers. A change in or loss of these suppliers could cause a delay in filling customer orders and a possible loss of sales, which could adversely affect results of operations; however, management believes that suitable replacement suppliers could be obtained in such an event.
Operating Leases
The Company conducts its operations in leased facilities under operating lease agreements that expire through fiscal 2035. Substantially all of the Company’s lease agreements require the Company to maintain the facilities during the term of the lease and to pay all taxes, insurance, utilities and other costs associated with those facilities. The Company makes customary representations and warranties and agrees to certain financial covenants and indemnities. In the event the Company defaults on a lease, typically the landlord may terminate the lease, accelerate payments and collect liquidated damages. As of September 29, 2012, the Company was not in default of any covenants contained in its lease agreements. Certain of the Company’s lease agreements provide for renewal options. Such renewal options are at rates similar to the current rates under the agreements.
Future minimum lease payments under all of the Company’s operating leases at September 29, 2012 are as follows:
| | | | |
Fiscal 2013 | | $ | 21,415 | |
Fiscal 2014 | | | 18,228 | |
Fiscal 2015 | | | 13,231 | |
Fiscal 2016 | | | 11,425 | |
Fiscal 2017 | | | 10,049 | |
Thereafter | | | 42,871 | |
| | | | |
Total | | $ | 117,219 | |
| | | | |
Rent expense, net of sublease income, was $18.3 million, $19.3 million, and $17.8 million for fiscal 2012, 2011 and 2010, respectively.
The Company subleases a portion of a building it owns and some of its facilities and has received aggregate rental income of $3.2 million, $3.5 million and $3.5 million in fiscal 2012, 2011 and 2010, respectively, which has been recorded as an offset to rent expense. The future minimum annual rental income payments under these sublease agreements at September 29, 2012 are as follows:
| | | | |
Fiscal 2013 | | $ | 1,574 | |
Fiscal 2014 | | | 1,550 | |
Fiscal 2015 | | | 912 | |
| | | | |
Total | | $ | 4,036 | |
| | | | |
Workforce Subject to Collective Bargaining Agreements
Approximately 191 of Hitec Imaging’s German employees are represented by a Worker’s Council and are subject to collective bargaining agreements. None of the Company’s other employees are subject to a collective bargaining agreement.
| 13. | Litigation and Other Matters |
On May 22, 2009, Conceptus, Inc. filed suit in the United States District Court for the Northern District of California seeking a declaration by the Court that the Company’s planned importation, use, sale or offer to sell of our forthcoming Adiana Permanent Contraception System would infringe five Conceptus patents. On July 9, 2009, Conceptus filed an amended complaint alleging infringement of the same five patents by the Adiana system. The complaint sought preliminary and permanent injunctive relief and unspecified monetary damages. In addition to the amended complaint, Conceptus also filed a motion for preliminary injunction seeking to preliminarily enjoin sales of the Adiana System based on alleged infringement of certain claims of three of the five patents
F-47
which was subsequently denied by the Court. A trial was held from October 3, 2011 through October 14, 2011 related to the asserted claims. On October 17, 2011, the jury returned a verdict in favor of Conceptus and awarded damages to Conceptus in the amount of $18.8 million. On April 29, 2012, the Company entered into a license and settlement agreement with Conceptus in which Conceptus agreed to forgo the $18.8 million jury award in consideration of the Company agreeing to a permanent injunction against the manufacture, sale and distribution of the Adiana product. The Company also granted Conceptus a royalty bearing license to its intellectual property related to the Adiana product.
On June 9, 2010, Smith & Nephew, Inc. filed suit against Interlace, which the Company acquired on January 6, 2011, in the United States District Court for the District of Massachusetts. In the complaint, it is alleged that the Interlace MyoSure hysteroscopic tissue removal device infringes U.S. patent 7,226,459. The complaint seeks permanent injunctive relief and unspecified damages. A Markman hearing on claim construction was held on November 9, 2010, and a ruling was issued on April 21, 2011. On November 22, 2011, Smith & Nephew, Inc. filed suit against the Company in the United States District Court for the District of Massachusetts. In the complaint, it is alleged that use of the MyoSure hysteroscopic tissue removal system infringes U.S. patent 8,061,359. The complaint seeks preliminary and permanent injunctive relief and unspecified damages. On January 17, 2012, at a hearing on Smith & Nephew’s motion for preliminary injunction with respect to the suit filed on November 22, 2011, the judge did not issue an injunction, consolidated the two matters for a single trial and scheduled a trial on the merits for both claims for June 25, 2012. A case management conference held on February 14, 2012 resulted in the trial being rescheduled to begin on August 20, 2012. On March 15, 2012, the Court heard summary judgment arguments related to the ‘459 patent and claim construction arguments related to the ‘359 patent. On June 5, 2012, the Court denied Smith & Nephew’s request for summary judgment of infringement, denied Smith & Nephew’s request for preliminary injunction, and denied the Company’s requests for summary judgment of non-infringement and invalidity. On September 4, 2012, following a two week trial, the jury returned a verdict of infringement of both the ‘459 and ‘359 patents and assessed damages of $4.0 million. The court has not yet entered judgment adopting the jury’s finding. Based in part on the fact the United States Patent and Trademark Office (“USPTO) has taken up a re-examination of both the ‘359 and ‘459 patents rejecting all previously issued claims, including all claims asserted against the MyoSure product, the Company has filed post trial motions seeking to reverse the jury’s rulings. A bench trial regarding the Company’s assertion of inequitable conduct on the part of Smith & Nephew with regard to the ‘359 originally scheduled for October 29, 2012 was postponed. A new date has not yet been set. The Court has indicated that until ruling on the inequitable conduct issue, it will not consider either party’s post trial motions. At this time, based on available information regarding this litigation, the Company does not believe a loss is probable and is unable to reasonably assess the ultimate outcome of this case or determine an estimate, or a range of estimates, of potential losses, beyond the pending jury verdict. The purchase and sale agreement associated with the acquisition of Interlace includes an indemnification provision that provides for the reimbursement of a portion of legal expenses in defense of the Interlace intellectual property. The Company has the right to collect certain amounts set aside in escrow and, as applicable, offset contingent consideration payments of qualifying legal costs. The Company is recording legal fees incurred for this suit pursuant to the indemnification provision net within accrued expenses.
On February 10, 2012, C.R. Bard (as acquirer of SenoRx, Inc. “SenoRx”) filed suit against the Company in the United States District Court for the District of Delaware. In the complaint, it is alleged that the Company’s MammoSite product infringes SenoRx’s U.S. Patents 8,079,946 and 8,075,469. The complaint seeks permanent injunctive relief and unspecified damages. On September 4, 2012 and October 16, 2012 the USPTO took up a re-examination of the ‘946 and ‘469 patents respectively. With respect to the ‘469 patent, all previously issued claims were rejected and for the ‘846 patent all but four claims were rejected. Based on the actions of the USPTO, the Company has filed a motion seeking to stay all litigation proceedings pending the outcome of the USPTO’s re-examination of both patents in suit. At this time, based on available information regarding this litigation, the Company is unable to reasonably assess the ultimate outcome of this case or determine an estimate, or a range of estimates, of potential losses.
On March 6, 2012, Enzo Life Sciences, Inc. (“Enzo”) filed suit against the Company in the United States District Court for the District of Delaware. In the complaint, it is alleged that certain of the Company’s molecular diagnostics products, including without limitation products based on its proprietary Invader chemistry such as Cervista HPV high risk and Cervista HPV 16/18, infringe Enzo’s U.S. Patent 6,992,180. The complaint seeks permanent injunctive relief and unspecified damages. The Company was formally served with the complaint on July 3, 2012, but no hearing has been scheduled. In January 2012, Enzo filed suit against Gen-Probe in the United States District Court for the District of Delaware. In that complaint, it is alleged that certain of Gen-Probe’s diagnostics products, including products that incorporate Gen-Probe’s patented HPA technology such as the APTIMA Combo 2 and APTIMA HPV assays, infringe Enzo’s U.S. Patent 6,992,180. The complaint seeks permanent injunctive relief and unspecified damages. At this time, based on available information regarding this litigation, the Company is unable to reasonably assess the ultimate outcome of this case or determine an estimate, or a range of estimates, of potential losses.
In October 2009, Gen-Probe filed a patent infringement action against Becton Dickinson (“BD”) in the United States District Court for the Southern District of California. The complaint alleges that BD’s Viper™ XTR™ testing system infringes five of Gen-Probe’s U.S. patents covering automated processes for preparing, amplifying and detecting nucleic acid targets. The complaint also alleges that BD’s ProbeTec™ Female Endocervical and Male Urethral Specimen Collection Kits for Amplified Chlamydia trachomatis/Neisseria gonorrhea (CT/GC) DNA assays used with the Viper XTR testing system infringe two of Gen-Probe’s U.S. patents covering penetrable caps for specimen collection tubes. The complaint seeks monetary damages and injunctive relief. In
F-48
March 2010, Gen-Probe filed a second complaint for patent infringement against BD in the United States District Court for the Southern District of California alleging that BD’s BD MAX System™ (formerly known as the HandyLab Jaguar system) infringes four of Gen-Probe’s U.S. patents covering automated processes for preparing, amplifying and detecting nucleic acid targets. The second complaint also seeks monetary damages and injunctive relief. In June 2010, these two actions were consolidated into a single legal proceeding. On September 28, 2012, the Court issued rulings on each party’s motions for summary judgment. As part of those rulings the Court found that BD’s accused products infringe nineteen claims of four of the asserted patents. The remaining issues, including BD’s invalidity contentions are scheduled to be heard in a jury trial beginning on December 4, 2012.
A number of lawsuits have been filed against the Company, Gen-Probe, and Gen-Probe’s board of directors. These include: (1) Teamsters Local Union No. 727 Pension Fund v. Gen-Probe Incorporated, et al. (Superior Court of the State of California for the County of San Diego); (2) Timothy Coyne v. Gen-Probe Incorporated, et al. (Delaware Court of Chancery); and (3) Douglas R. Klein v. John W. Brown, et al. (Delaware Chancery Court). The two Delaware actions have been consolidated into a single action titled: In re: Gen-Probe Shareholders Litigation. The suits were filed after the announcement of our acquisition of Gen-Probe on April 30, 2012 as putative stockholder class actions. Each of the actions assert similar claims alleging that Gen-Probe’s board of directors failed to adequately discharge its fiduciary duties to shareholders by failing to adequately value Gen-Probe’s shares and ensure that Gen-Probe’s shareholders received adequate consideration in our acquisition of Gen-Probe, that the acquisition was the product of a flawed sales process, and that the Company aided and abetted the alleged breach of fiduciary duty. The plaintiffs demand, among other things, a preliminary and permanent injunction enjoining our acquisition of Gen-Probe and rescinding the transaction or any part thereof that has been implemented. On May 24, 2012, the plaintiffs in the Delaware action filed an amended complaint, adding allegations that the disclosures in Gen-Probe’s preliminary proxy statement were inadequate. The defendants in the Delaware action answered the complaint on June 4, 2012. On July 18, 2012, the parties in the Delaware action entered into a memorandum of understanding regarding a proposed settlement of the litigation. The proposed settlement is conditioned upon, among other things, the execution of an appropriate stipulation of settlement, consummation of the merger, and final approval of the proposed settlement by the Delaware Court of Chancery. On July 9, 2012, the plaintiffs in the California action filed a motion for voluntary dismissal without prejudice. On July 12, 2012, the California Superior Court entered an order dismissing the California complaint without prejudice.
The Company is a party to various other legal proceedings and claims arising out of the ordinary course of its business. The Company believes that except for those described above there are no other proceedings or claims pending against it the ultimate resolution of which would have a material adverse effect on its financial condition or results of operations. In all cases, at each reporting period, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under ASC 450,Contingencies. Legal costs are expensed as incurred.
Litigation-related Settlement Charge
On October 5, 2007, Ethicon Endo-Surgery, Inc. (“Ethicon”), a Johnson & Johnson operating company, filed a complaint against Hologic and its wholly-owned subsidiary Suros in the United States District Court for the Southern District of Ohio, Western Division. The complaint alleged that certain of the ATEC biopsy systems manufactured and sold by Suros infringed Ethicon patents, and sought to enjoin Hologic and Suros from conducting acts of unfair competition and infringing the patents as well as the recovery of unspecified damages and costs. On August 6, 2009, Ethicon filed a second complaint against the Company and its wholly-owned subsidiary Suros in the United States District Court for the District of Delaware. The complaint alleged that certain of the Eviva biopsy systems manufactured and sold by Suros infringed Ethicon patents and sought to enjoin Hologic and Suros from infringing the patents as well as recovery of damages and costs resulting from the alleged infringement. On February 17, 2010, the Company entered into a settlement agreement with Ethicon relating to the two lawsuits previously filed by Ethicon, and one previously filed by Hologic against Ethicon. As a result of the settlement agreement, all outstanding litigation between the parties was dismissed, without acknowledgement of liability by either party. While details of the agreement are confidential, under the terms of the settlement agreement, Ethicon agreed to pay Hologic ongoing royalties for sales of its Mammotome magnetic resonance imaging product. In addition, the Company agreed to pay Ethicon a one-time payment of $12.5 million, plus ongoing royalties for sales of its ATEC and EVIVA hand pieces. The Company recorded the $12.5 million charge in the second quarter of fiscal 2010.
In fiscal 2012 and 2011, the Company settled minor litigation cases resulting in charges of $0.5 million and $0.8 million respectively.
| 14. | Novartis Collaboration Agreement |
The Company, through its Gen-Probe acquisition, has a collaboration agreement with Novartis. In July 2009, Gen-Probe entered into an amended and restated collaboration agreement with Novartis, which sets forth the current terms of the parties’ blood screening collaboration. The term of the collaboration agreement runs through June 30, 2025, unless terminated earlier pursuant to its terms under certain specified conditions. Under the collaboration agreement, the Company manufactures blood screening products, while Novartis is responsible for marketing, sales and service of those products, which Novartis sells under its trademarks.
Under the amended agreement, the Company is entitled to recover 50% of its manufacturing costs incurred in connection with the collaboration and will receive a percentage of the blood screening assay revenue generated under the collaboration. The
F-49
Company’s share of revenue from any assay that includes a test for HCV is as follows: 2012-2013, 47%; 2014, 48%; and 2015 through the remainder of the term of the collaboration, 50%. The Company’s share of blood screening assay revenue, from any assay that does not test for HCV is 50%. Novartis is obligated to purchase all of the quantities of assays specified on a 90-day demand forecast, due 90 days prior to the date Novartis intends to take delivery, and certain quantities specified on a rolling 12-month forecast.
Novartis has also agreed to provide certain funding to customize the Company’s PANTHER instrument for use in the blood screening market and to pay the Company a milestone payment upon the earlier of certain regulatory approvals or the first commercial sale of the PANTHER instrument for use in the blood screening field. The parties will share equally in any profit attributable to Novartis’ sale or lease of PANTHER instruments under the collaboration.
The Company recognizes product revenue, and collaborative research and license revenue, which is included within services and other revenues, under this collaboration agreement.
| 15. | Business Segments and Geographic Information |
The Company reports segment information in accordance with ASC 280,Segment Reporting.Operating segments are identified as components of an enterprise about which separate, discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. The Company’s chief operating decision maker is the chief executive officer, and the Company’s reportable segments have been identified based on the types of products manufactured and the end markets to which the product are sold. Each reportable segment generates revenue from either the sale of medical equipment and related services and/or sale of disposable supplies, primarily used for diagnostic testing and surgical procedures. The Company has four reportable segments: Breast Health, Diagnostics, GYN Surgical and Skeletal Health. Certain reportable segments represent an aggregation of operating units within each segment. The Company measures and evaluates its reportable segments based on segment revenues and operating income (loss) adjusted to exclude the effect of non-cash charges, such as intangible asset amortization expense, contingent consideration charges, restructuring and divestiture charges, and other one-time or unusual items, and related tax effects.
Identifiable assets for the four principal operating segments consist of inventories, intangible assets including goodwill, and property and equipment. The Company fully allocates depreciation expense to its four reportable segments. The Company presents all other identifiable assets as corporate assets. There were no intersegment revenues. Segment information for fiscal 2012, 2011 and 2010 is as follows:
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Total revenues: | | | | | | | | | | | | |
Breast Health | | $ | 875,771 | | | $ | 825,551 | | | $ | 755,542 | |
Diagnostics | | | 718,064 | | | | 571,263 | | | | 552,501 | |
GYN Surgical | | | 313,089 | | | | 300,538 | | | | 283,142 | |
Skeletal Health | | | 95,728 | | | | 91,997 | | | | 88,367 | |
| | | | | | | | | | | | |
| | $ | 2,002,652 | | | $ | 1,789,349 | | | $ | 1,679,552 | |
| | | | | | | | | | | | |
Operating income (loss): | | | | | | | | | | | | |
Breast Health | | $ | 186,106 | | | $ | 187,970 | | | $ | (93,623 | ) |
Diagnostics | | | (32,787 | ) | | | 170,693 | | | | 100,469 | |
GYN Surgical | | | (51,892 | ) | | | 3,623 | | | | 53,071 | |
Skeletal Health | | | 12,290 | | | | 12,159 | | | | 10,020 | |
| | | | | | | | | | | | |
| | $ | 113,717 | | | $ | 374,445 | | | $ | 69,937 | |
| | | | | | | | | | | | |
Depreciation and amortization: | | | | | | | | | | | | |
Breast Health | | $ | 42,924 | | | $ | 45,165 | | | $ | 52,660 | |
Diagnostics | | | 197,274 | | | | 165,065 | | | | 169,616 | |
GYN Surgical | | | 103,781 | | | | 92,587 | | | | 70,724 | |
Skeletal Health | | | 1,772 | | | | 1,919 | | | | 1,768 | |
| | | | | | | | | | | | |
| | $ | 345,751 | | | $ | 304,736 | | | $ | 294,768 | |
| | | | | | | | | | | | |
Capital expenditures: | | | | | | | | | | | | |
Breast Health | | $ | 9,821 | | | $ | 12,069 | | | $ | 10,392 | |
Diagnostics | | | 44,939 | | | | 23,128 | | | | 18,317 | |
GYN Surgical | | | 12,233 | | | | 11,467 | | | | 9,575 | |
Skeletal Health | | | 171 | | | | 2,198 | | | | 3,637 | |
Corporate | | | 11,609 | | | | 6,801 | | | | 4,737 | |
| | | | | | | | | | | | |
| | $ | 78,773 | | | $ | 55,663 | | | $ | 46,658 | |
| | | | | | | | | | | | |
F-50
| | | | | | | | | | | | |
| | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Identifiable assets: | | | | | | | | | | | | |
Breast Health | | $ | 956,134 | | | $ | 985,196 | | | $ | 988,041 | |
Diagnostics | | | 6,170,553 | | | | 1,770,107 | | | | 1,802,148 | |
GYN Surgical | | | 1,944,386 | | | | 2,049,682 | | | | 1,834,773 | |
Skeletal Health | | | 32,778 | | | | 31,864 | | | | 30,293 | |
Corporate | | | 1,373,257 | | | | 1,171,931 | | | | 970,579 | |
| | | | | | | | | | | | |
| | $ | 10,477,108 | | | $ | 6,008,780 | | | $ | 5,625,834 | |
| | | | | | | | | | | | |
As a result of the Company’s long-lived assets impairment test and goodwill impairment test related to MammoSite, a reporting unit in Breast Health, performed as of June 27, 2010, the Company recorded intangible asset impairment charges of $143.5 million to write down intangible assets to fair value and $76.7 million to reduce the reporting unit’s goodwill. These charges are reflected in Breast Health’s operating loss for fiscal 2010. In fiscal 2012, the Company recorded a goodwill impairment charge of $5.8 million related to its MammoSite reporting unit.
Products sold by the Company internationally are manufactured at domestic and international locations. Transfers between the Company and its subsidiaries are generally recorded at amounts similar to the prices paid by unaffiliated foreign dealers. All intercompany profit is eliminated in consolidation.
The Company operates in the following major geographic areas as noted in the below chart. Revenue data is based upon customer location, and internationally totaled $510.5 million, $414.4 million and $344.5 million in fiscal 2012, 2011 and 2010, respectively. The Company’s sales in Europe are predominantly derived from Germany, the United Kingdom and the Netherlands. The Company’s sales in Asia-Pacific are predominantly derived from China, Australia and Japan. The “All others” designation includes Canada, Latin America and the Middle East.
Revenues by geography as a percentage of total revenues are as follows:
| | | | | | | | | | | | |
| | | Years ended | |
| | | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
United States | | | 75 | % | | | 77 | % | | | 79 | % |
Europe | | | 12 | % | | | 14 | % | | | 12 | % |
Asia-Pacific | | | 8 | % | | | 6 | % | | | 5 | % |
All others | | | 5 | % | | | 3 | % | | | 4 | % |
| | | | | | | | | | | | |
| | | 100 | % | | | 100 | % | | | 100 | % |
| | | | | | | | | | | | |
The Company’s property and equipment, net are geographically located as follows:
| | | | | | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
United States | | $ | 405,141 | | | $ | 165,177 | | | $ | 183,383 | |
Costa Rica | | | 30,452 | | | | 34,107 | | | | 35,984 | |
Europe | | | 59,927 | | | | 29,591 | | | | 28,060 | |
All other countries | | | 12,478 | | | | 9,791 | | | | 4,271 | |
| | | | | | | | | | | | |
| | $ | 507,998 | | | $ | 238,666 | | | $ | 251,698 | |
| | | | | | | | | | | | |
F-51
| 16. | Accrued Expenses and Other Long-Term Liabilities |
Accrued expenses and other long-term liabilities consist of the following:
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Accrued Expenses | | | | | | | | |
Compensation and employee benefits | | $ | 143,673 | | | $ | 97,747 | |
Contingent consideration | | | 143,881 | | | | 100,255 | |
Deferred payment to TCT | | | — | | | | 47,949 | |
Income taxes and other taxes | | | 12,424 | | | | 33,070 | |
Interest | | | 18,422 | | | | 9,802 | |
Other | | | 53,981 | | | | 36,504 | |
| | | | | | | | |
| | $ | 372,381 | | | $ | 325,327 | |
| | | | | | | | |
| | | | | | | | |
| | | September 29,
2012 | | | September 24,
2011 | |
Other Long-Term Liabilities | | | | | | | | |
Accrued lease obligation—long-term | | $ | 33,256 | | | $ | 32,846 | |
Reserve for income tax uncertainties | | | 38,518 | | | | 11,202 | |
Pension liabilities | | | 9,397 | | | | 7,714 | |
Contingent consideration | | | 3,322 | | | | 48,872 | |
Other | | | 13,757 | | | | 6,328 | |
| | | | | | | | |
| | $ | 98,250 | | | $ | 106,962 | |
| | | | | | | | |
| 17. | Pension and Other Employee Benefits |
The Company has certain defined benefit pension plans covering the employees of its Hitec Imaging German subsidiary (the “Pension Benefits”). As of September 29, 2012 and September 24, 2011, the Company’s pension liability is $9.7 million and $8.1 million, respectively, which is primarily recorded as a component of long-term liabilities in the Consolidated Balance Sheets. Under German law, there are no rules governing investment or statutory supervision of the pension plan. As such, there is no minimum funding requirement imposed on employers. Pension benefits are safeguarded by the Pension Guaranty Fund, a form of compulsory reinsurance that guarantees an employee will receive vested pension benefits in the event of insolvency.
The tables below provide a reconciliation of benefit obligations, plan assets, funded status, and related actuarial assumptions of the Company’s German Pension Benefits.
| | | | | | | | | | | | |
Change in Benefit Obligation | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Benefit obligation at beginning of year | | $ | (8,064 | ) | | $ | (9,093 | ) | | $ | (6,736 | ) |
Service cost | | | — | | | | — | | | | — | |
Interest cost | | | (391 | ) | | | (389 | ) | | | (401 | ) |
Plan participants’ contributions | | | — | | | | — | | | | — | |
Actuarial gain (loss) | | | (2,002 | ) | | | 1,092 | | | | (2,814 | ) |
Foreign exchange | | | 383 | | | | (5 | ) | | | 541 | |
Benefits paid | | | 330 | | | | 331 | | | | 317 | |
| | | | | | | | | | | | |
Benefit obligation at end of year | | | (9,744 | ) | | | (8,064 | ) | | | (9,093 | ) |
Plan assets | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Funded status | | $ | (9,744 | ) | | $ | (8,064 | ) | | $ | (9,093 | ) |
| | | | | | | | | | | | |
The tables below outline the components of the net periodic benefit cost and related actuarial assumptions of the Company’s German Pension Benefits plan.
| | | | | | | | | | | | |
Components of Net Periodic Benefit Cost | | Years ended | |
| | September 29,
2012 | | | September 24,
2011 | | | September 25,
2010 | |
Service cost | | $ | — | | | $ | — | | | $ | — | |
Interest cost | | | 391 | | | | 389 | | | | 401 | |
Expected return on plan assets | | | — | | | | — | | | | — | |
Amortization of prior service cost | | | — | | | | — | | | | — | |
Recognized net actuarial gain | | | (38 | ) | | | — | | | | (217 | ) |
| | | | | | | | | | | | |
Net periodic benefit cost | | $ | 353 | | | $ | 389 | | | $ | 184 | |
| | | | | | | | | | | | |
F-52
| | | | | | | | | | | | |
Weighted-Average Net Periodic Benefit Cost Assumptions | | 2012 | | | 2011 | | | 2010 | |
Discount rate | | | 3.52 | % | | | 5.20 | % | | | 4.35 | % |
Expected return on plan assets | | | 0 | % | | | 0 | % | | | 0 | % |
Rate of compensation increase | | | 0 | % | | | 0 | % | | | 0 | % |
The projected benefit obligation for the German Pension Benefits plans with projected benefit obligations in excess of plan assets was $9.7 million and $8.1 million at September 29, 2012 and September 24, 2011, respectively, and the accumulated benefit obligation for the German Pension Benefits plans was $9.7 million and $8.1 million at September 29, 2012 and September 24, 2011, respectively.
The Company is also obligated to pay long-term service award benefits. The projected benefit obligation for long-term service awards was $0.6 million at September 29, 2012 and September 24, 2011, respectively.
The table below reflects the total Pension Benefits expected to be paid each fiscal year as of September 29, 2012:
| | | | |
2013 | | $ | 347 | |
2014 | | | 367 | |
2015 | | | 384 | |
2016 | | | 399 | |
2017 | | | 410 | |
2018 to 2022 | | | 2,238 | |
The Company also maintains additional contractual pension benefits for its top German executive officers in the form of a defined contribution plan. These contributions were insignificant in fiscal 2012, 2011 and 2010.
| 18. | Quarterly Statement of Operations Information (Unaudited) |
The following table presents a summary of quarterly results of operations for fiscal 2012 and 2011:
| | | | | | | | | | | | | | | | |
| | | 2012 | |
| | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter(2) | |
Total revenue | | $ | 472,711 | | | $ | 471,165 | | | $ | 470,228 | | | $ | 588,548 | |
Gross profit | | | 249,370 | | | | 226,110 | | | | 244,640 | | | | 274,317 | |
Net income (loss) (1) | | | 20,812 | | | | (40,273 | ) | | | 23,594 | | | | (77,767 | ) |
Diluted net income (loss) per common share | | $ | 0.08 | | | $ | (0.15 | ) | | $ | 0.09 | | | $ | (0.29 | ) |
| | | | | | | | | | | | | | | | |
| | | 2011 | |
| | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter | |
Total revenue | | $ | 432,571 | | | $ | 438,651 | | | $ | 451,082 | | | $ | 467,045 | |
Gross profit | | | 224,734 | | | | 220,687 | | | | 234,561 | | | | 243,199 | |
Net income (3) | | | 10,940 | | | | 82,445 | | | | 36,196 | | | | 27,569 | |
Diluted net income per common share | | $ | 0.04 | | | $ | 0.31 | | | $ | 0.14 | | | $ | 0.10 | |
| (1) | Net loss in the second quarter of fiscal 2012 includes a charge for the discontinuance of the Adiana product line of $18.3 million and the loss on debt extinguishment of $42.3 million. See Note 5 for further discussion. Net loss in the fourth quarter of fiscal 2012 includes additional amortization expense from the Gen-Probe acquisition of $29.7 million, direct acquisition transaction costs of $30.7 million, and restructuring charges of $16.7 million, a goodwill impairment charge of $5.8 million and an in-process research and development charge of $4.5 million. |
| (2) | The fourth quarter was a 14-week quarter compared to all other quarters which were 13-week quarters. |
| (3) | Net income in the first quarter of fiscal 2011 includes a loss on debt extinguishment of $29.9 million and the second quarter of fiscal 2011 includes a gain on the sale of intellectual property of $84.5 million. See Notes 5 and 7 for further discussion. |
F-53
Exhibit Index
| | | | | | | | |
| | | | | Incorporated by Reference | |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date | |
| | | |
2.1 | | Agreement and Plan of Merger, dated as of April 29, 2012, by and among Hologic, Gold Acquisition Corp. and Gen-Probe Incorporated. | | 8-K | | | 05/01/2012 | |
| | | |
3.1 | | Certificate of Incorporation of Hologic. | | S-1 | | | 01/24/1990 | |
| | | |
3.2 | | Amendment to Certificate of Incorporation of Hologic. | | 10-Q | | | 03/30/1996 | |
| | | |
3.3 | | Certificate of Amendment to Certificate of Incorporation of Hologic. | | 10-K | | | 09/24/2005 | |
| | | |
3.4 | | Certificate of Amendment to Certificate of Incorporation of Hologic. | | 8-K | | | 10/22/2007 | |
| | | |
3.5 | | Certificate of Amendment to Certificate of Incorporation of Hologic. | | 8-K | | | 03/11/2008 | |
| | | |
3.6 | | Fourth Amended and Restated By-laws of Hologic. | | 8-K | | | 03/08/2012 | |
| | | |
3.7 | | Amended and Restated Certificate of Designations of Series A Junior Participating Preferred Stock of Hologic. | | 8-A | | | 04/03/2008 | |
| | | |
4.1 | | Specimen Certificate for Shares of Hologic’s Common Stock. | | 8-A | | | 01/31/1990 | |
| | | |
4.2 | | Description of Capital Stock (Contained in Hologic’s Certificate of Incorporation, as amended, filed as Exhibits 3.1, 3.2, 3.3, 3.4 and 3.5 hereto). | | | | | | |
| | | |
4.3 | | Amended and Restated Rights Agreement dated April 2, 2008. | | 8-A | | | 04/03/2008 | |
| | | |
4.4 | | Form of Rights Certificate. | | 8-K | | | 09/26/2002 | |
| | | |
4.5 | | Indenture, dated as of December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 8-K | | | 12/10/2007 | |
| | | |
4.6 | | First Supplemental Indenture, dated December 10, 2007, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 8-K | | | 12/10/2007 | |
| | | |
4.7 | | Form of 2.00% Convertible Senior Note due 2037 (included in Exhibit 4.6). | | 8-K | | | 12/10/2007 | |
| | | |
4.8 | | Second Supplemental Indenture, dated November 23, 2010, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 10-K | | | 09/25/2010 | |
| | | |
4.9 | | Form of 2.00% Convertible Exchange Senior Note due 2037 (included in Exhibit 4.8). | | 10-K | | | 09/25/2010 | |
| | | |
4.10 | | Third Supplemental Indenture, dated March 5, 2012, by and between Wilmington Trust Company, as Trustee, and Hologic. | | 8-K | | | 03/08/2012 | |
| | | |
4.11 | | Form of 2.00% Convertible Senior Note due 2042 (included in Exhibit 4.10). | | 8-K | | | 03/08/2012 | |
| | | |
4.12 | | Indenture, dated as of August 1, 2012, by and among Wells Fargo Bank, National Association, as Trustee, Hologic and certain subsidiaries of Hologic party thereto. | | 8-K | | | 08/01/2012 | |
| | | |
4.13 | | Forms of 6.25% Senior Note due 2020 (included in Exhibit 4.12). | | 8-K | | | 08/01/2012 | |
| | | |
4.14 | | Exchange and Registration Rights Agreement, dated as of August 1, 2012, by and among Goldman Sachs & Co., Hologic and certain subsidiaries of Hologic party thereto. | | 8-K | | | 08/01/2012 | |
| | | |
10.1* | | Second Amended and Restated 1999 Equity Incentive Plan. | | 10-Q | | | 03/25/2006 | |
| | | |
10.2* | | Amendment No. 1 to Second Amended and Restated 1999 Equity Incentive Plan. | | S-8 | | | 10/23/2007 | |
| | | |
10.3* | | Amendment No. 2 to Second Amended and Restated 1999 Equity Incentive Plan. | | 8-K | | | 10/22/2007 | |
| | | |
10.4* | | Amendment No. 3 to Second Amended and Restated 1999 Equity Incentive Plan. | | 8-K | | | 12/12/2008 | |
| | | |
10.5 | | 2000 Acquisition Equity Incentive Plan. | | 10-K | | | 09/29/2001 | |
| | | | | | | | |
| | | | | Incorporated by Reference | |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date | |
| | | |
10.6* | | 2008 Equity Incentive Plan. | | 8-K | | | 03/11/2008 | |
| | | |
10.7* | | Form of Employee Stock Option Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | | 11/17/2008 | |
| | | |
10.8* | | Form of Employee Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | | 11/17/2008 | |
| | | |
10.9* | | Form of Special Retention Employee Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan. | | 10-Q | | | 06/26/2010 | |
| | | |
10.10* | | Form of Independent Director Stock Option Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | | 12/12/2008 | |
| | | |
10.11* | | Form of Independent Director Restricted Stock Unit Award Agreement Under 2008 Equity Incentive Plan. | | 8-K | | | 12/12/2008 | |
| | | |
10.12* | | Form of Market Stock Unit Award Agreement. | | 8-K | | | 11/13/2012 | |
| | | |
10.13* | | Hologic, Inc. 2012 Employee Stock Purchase Plan. | | 8-K | | | 03/08/2012 | |
| | | |
10.14* | | Hologic, Inc. 2012 Short-Term Incentive Plan. | | 8-K | | | 11/07/2011 | |
| | | |
10.15* | | Hologic, Inc. 2013 Short-Term Incentive Plan. | | 8-K | | | 11/13/2012 | |
| | | |
10.16* | | Hologic, Inc. 2013 Synergy Bonus Plan. | | 8-K | | | 11/13/2012 | |
| | | |
10.17* | | Cytyc Corporation 1995 Stock Plan. | | S-8 | | | 10/23/2007 | |
| | | |
10.18* | | Cytyc Corporation 1995 Non-Employee Director Stock Option Plan. | | S-8 | | | 10/23/2007 | |
| | | |
10.19* | | Cytyc Corporation 2004 Omnibus Stock Plan. | | S-8 | | | 10/23/2007 | |
| | | |
10.20* | | The 2003 Incentive Award Plan of Gen-Probe Incorporated, as amended and restated. | | S-8 | | | 08/02/2012 | |
| | | |
10.21* | | Transition Agreement dated November 5, 2009, by and between Hologic and John W. Cumming | | 8-K | | | 11/09/2009 | |
| | | |
10.22* | | Transition Acknowledgement dated July 28, 2011, by and between Hologic and John W. Cumming | | 8-K | | | 08/01/2011 | |
| | | |
10.23* | | Form of Indemnification Agreement (as executed with each director of Hologic). | | 8-K | | | 03/06/2009 | |
| | | |
10.24*** | | Amended and Restated Deferred Compensation Program. | | | | | | |
| | | |
10.25* | | Rabbi Trust Agreement. | | 10-K | | | 09/30/2006 | |
| | | |
10.26* | | Form of Officer Severance Agreement including list of officers to whom provided. | | 10-Q | | | 03/25/2006 | |
| | | |
10.27* | | Form of Senior Vice President Change of Control Agreement including list of officers to whom provided. | | 10-Q 10-K | |
| 12/27/2008
09/25/2010 |
|
| | | |
10.28* | | Form of Senior Executive Officer Change of Control Agreement including list of officers to whom provided. | | 8-K | | | 11/17/2009 | |
| | | |
10.29* | | Retention and Severance Agreement by and between Hologic and Carl W. Hull dated as of July 10, 2012. | | 8-K | | | 07/12/2012 | |
| | | | | | | | |
| | | | | Incorporated by Reference | |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date | |
| | | |
10.30* | | Change of Control Agreement by and between Hologic and Carl W. Hull dated as of July 10, 2012. | | 8-K | | | 07/12/2012 | |
| | | |
10.31* | | Restricted Stock Unit Award Agreement by and between Hologic and Carl W. Hull dated as of August 1, 2012. | | 8-K | | | 08/01/2012 | |
| | | |
10.32 | | Facility Lease (Danbury) dated as of December 30, 1995 by and among Melvin J. Powers and Mary P. Powers D/B/A M&N Realty and Lorad. | | Trex Medical Corporation S-1 | | | 03/29/1996 | |
| | | |
10.33 | | Lease Agreement (Danbury and Bedford) by and between BONE (DE) QRS 15-12, INC., and Hologic dated as of August 28, 2002. | | 10-K | | | 09/28/2002 | |
| | | |
10.34 | | First Amendment to Lease Agreement (Danbury and Bedford) by and between BONE (DE) QRS 15-12, INC., and Hologic dated as of October 29, 2007. | | 10-K | | | 09/29/2007 | |
| | | |
10.35 | | Office Lease dated December 31, 2003 between Cytyc and Marlborough Campus Limited Partnership. | | Cytyc Corporation 10-K | | | 12/31/2003 | |
| | | |
10.36 | | Lease Agreement by and between Zona Franca Coyol S.A. and Cytyc Surgical Products Costa Rica S.A. dated April 23, 2007. | | 10-K | | | 09/29/2007 | |
| | | |
10.37 | | Lease Agreement by and between 445 Simarano Drive, Marlborough LLC and Cytyc dated July 11, 2006. | | 10-K | | | 09/29/2007 | |
| | | |
10.38 | | Lease Guaranty dated October 22, 2007 between Bel Marlborough I LLC and Hologic, as guarantor thereunder. | | 8-K | | | 10/22/2007 | |
| | | |
10.39 | | Supply Agreement between Cytyc, Whatman, Inc. and Whatman SA dated as of December 31, 2000, as amended, October 16, 2001 and May 2, 2002. | | Cytyc Corporation 10-K | | | 12/31/2002 | |
| | | |
10.40 | | Form of Exchange Agreement. | | 8-K | | | 02/29/2012 | |
| | | |
10.41 | | Credit and Guaranty Agreement, dated as of August 1, 2012, by and among Hologic, the guarantors party thereto, Goldman Sachs Bank USA, as Administrative Agent and Collateral Agent, and the lenders party thereto. † | | 8-K/A | | | 10/15/2012 | |
| | | |
10.42 | | Pledge and Security Agreement, dated as of August 1, 2012, by and among the grantors party thereto and Goldman Sachs Bank USA, as Collateral Agent. | | 8-K/A | | | 10/15/2012 | |
| | | |
10.43 | | Purchase Agreement, dated July 19, 2012, by and among Hologic, Inc., the guarantors party thereto, Goldman, Sachs & Co. | | 8-K | | | 07/19/2012 | |
| | | |
10.44 | | Restated Agreement dated as of July 24, 2009 by and between Gen-Probe Incorporated and Novartis Vaccines and Diagnostics, Inc. ‡ | | Gen-Probe 10-Q/A | | | 09/30/2009 | |
| | | |
10.45 | | Supply Agreement for Panther Instrument System effective November 22, 2006 between Gen-Probe Incorporated and STRATEC Biomedical Systems AG. ‡ | | Gen-Probe 10-Q | | | 09/30/2007 | |
| | | |
21.1** | | Subsidiaries of Hologic. | | | | | | |
| | | |
23.1** | | Consent of Independent Registered Public Accounting Firm. | | | | | | |
| | | |
31.1** | | Certification of Hologic’s CEO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | | | | | |
| | | |
31.2** | | Certification of Hologic’s CFO pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | | | | | |
| | | | | | |
| | | | | Incorporated by Reference |
Exhibit Number | | Exhibit Description | | Form | | Filing Date/
Period End
Date |
| | | |
32.1*** | | Certification of Hologic’s CEO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | | | |
| | | |
32.2*** | | Certification of Hologic’s CFO pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | | | |
| | | |
101.INS** | | XBRL Instance Document. | | | | |
| | | |
101.SCH** | | XBRL Taxonomy Extension Schema Document. | | | | |
| | | |
101.CAL** | | XBRL Taxonomy Extension Calculation Linkbase Document. | | | | |
| | | |
101.DEF** | | XBRL Taxonomy Extension Definition Linkbase Document. | | | | |
| | | |
101.LAB** | | XBRL Taxonomy Extension Label Linkbase Document. | | | | |
| | | |
101.PRE** | | XBRL Taxonomy Extension Presentation Linkbase Document. | | | | |
| * | Indicates management contract or compensatory plan or arrangement. |
| † | Confidential treatment has been requested for certain portions of this exhibit pursuant to Exchange Act Rule 24b-2. A complete version of this exhibit has been filed separately with the U.S. Securities and Exchange Commission. |
| ‡ | Confidential treatment has been granted with respect to certain portions of this exhibit. A complete version of this exhibit has been filed separately with the U.S. Securities and Exchange Commission. |
