SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2010
Commission File Number 1-5620
Safeguard Scientifics, Inc.
(Exact name of Registrant as specified in its charter)
| | | |
Pennsylvania | | 23-1609753 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer ID No.) |
| | | |
435 Devon Park Drive
Building 800
Wayne, PA | | 19087
(Zip Code) |
| (Address of principal executive offices) | | |
(610) 293-0600
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | |
Title of Each Class | | Name of Each Exchange on Which Registered |
| |
| Common Stock ($.10 par value) | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No þ
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 ofRegulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” inRule 12b-2 of the Exchange Act. (Check one):
| | | | | | | |
Large accelerated filer o | | Accelerated filer þ | | Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the Registrant is a shell company (as defined inRule 12b-2 of the Exchange Act). Yes o No þ
As of June 30, 2010, the aggregate market value of the Registrant’s common stock held by non-affiliates of the registrant was $213,153,252 based on the closing sale price as reported on the New York Stock Exchange.
The number of shares outstanding of the Registrant’s Common Stock, as of March 3, 2011 was 20,644,164.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive proxy statement (the “Definitive Proxy Statement”) to be filed with the Securities and Exchange Commission for the Company’s 2011 Annual Meeting of Shareholders are incorporated by reference into Part III of this report.
SAFEGUARD SCIENTIFICS, INC.
FORM 10-K
DECEMBER 31, 2010
2
PART I
Cautionary Note concerning Forward-Looking Statements
Except for the historical information and discussions contained herein, statements contained in this Annual Report onForm 10-K may constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Our forward-looking statements are subject to risks and uncertainties. The risks and uncertainties that could cause actual results to differ materially, include, among others, managing rapidly changing technologies, limited access to capital, competition, the ability to attract and retain qualified employees, the ability to execute our strategy, the uncertainty of the future performance of our companies, acquisitions and dispositions of companies, the inability to manage growth, compliance with government regulations and legal liabilities, additional financing requirements, the effect of economic conditions in the business sectors in which our companies operate, and other uncertainties described in the Company’s filings with the Securities and Exchange Commission. Many of these factors are beyond our ability to predict or control. In addition, as a result of these and other factors, our past financial performance should not be relied on as an indication of future performance. The Company does not assume any obligation to update any forward-looking statements or other information contained in this news release.
Business Overview
Safeguard’s charter is to build value in growing businesses by providing capital and strategic, operational and management resources. Safeguard participates in expansion financings, corporate spin-outs, management buyouts, recapitalizations, industry consolidations, and early-stage financings. Our vision is to be the preferred catalyst to build great companies across diverse capital platforms. Throughout this document, we use the term “partner company” to generally refer to those companies that we have an economic interest in and that we are actively involved in influencing the development of, through board representation and management support in addition to our equity ownership stake. From time to time, in addition to our partner companies, we also hold relatively small economic interests in other enterprises that we are not actively involved in the management of.
We strive to create long-term value for our shareholders by helping partner companies increase their market penetration, grow revenue and improve cash flow. We focus principally on companies in which we anticipate deploying up to $25 million and that operate in two sectors:
Life Sciences — including companies focused on molecular andpoint-of-care diagnostics, medical devices, regenerative medicine, specialty pharmaceuticals and selected healthcare services; and
Technology — including companies focused on internet/new media, financial services IT, healthcare IT and selected business services that have transaction-enabling applications with a recurring revenue stream.
As we continue to develop and grow, we will consider partner companies in additional sectors; participating in different capital structures; other types of partner company relationships; and extensions to our business model which leverage our core capabilities.
In 2010, our management team executed against the following objectives, to:
| | |
| | • | Deploy capital in companies within our strategic focus; |
| |
| | • | Build value in partner companies by developing strong management teams, growing the companies organically and through acquisitions, and positioning the companies for liquidity at premium valuations; |
| |
| | • | Realize the value of partner companies through selective, well-timed exits to maximize risk-adjusted value; and |
| |
| | • | Provide the tools needed for investors to fully recognize the shareholder value that has been created by our efforts. |
3
To meet our strategic objectives during 2011, Safeguard will focus on:
| | |
| | • | finding opportunities to deploy our capital in additional partner companies and, possibly, extensions of our business model; |
| |
| | • | helping partner companies to achieve additional market penetration, revenue growth, cash flow improvement and growth in long-term value; and |
| |
| | • | realizing value in our partner companies if and when we believe doing so will maximize value for our shareholders. |
We incorporated in the Commonwealth of Pennsylvania in 1953. Our corporate headquarters are located at 435 Devon Park Drive, Building 800, Wayne, Pennsylvania 19087.
Significant 2010 Highlights
Here are our key developments from 2010:
| | |
| | • | During 2010, we deployed $23.9 million in additional capital to support the growth of the partner companies which we already had an interest in at December 31, 2009. |
| |
| | • | In March 2010, Safeguard entered into privately negotiated agreements with certain institutional holders of an aggregate of $46.9 million in face value of our 2.625% senior convertible debentures due 2024 to exchange the debentures held by such holders for the same face amount of newly issued 10.125% senior convertible debentures, due 2014. The remaining $31.3 million outstanding face amount of the 2024 debentures remain outstanding. |
| |
| | • | In September 2010, Safeguard deployed $6.8 million in Good Start Genetics, Inc., co-leading an $18.0 million financing. Good Start Genetics, a diagnostic company developing a clinical diagnostic DNA sequencing platform, is using proceeds from the financing to complete the development and launch of the company’s pre-pregnancy genetic test, which utilizes an advanced DNA sequencing technology to screen for a panel of genetic disorders, including those recommended by the American Congress of Obstetricians and Gynecologists and the American College of Medical Genetics. In addition, proceeds will be used for additional product development, sales and marketing, and other general operation and working capital needs. |
| |
| | • | In December 2010, Safeguard received net proceeds from the successful completion of GE Healthcare’s acquisition of partner company Clarient, Inc. (NASDAQ: CLRT) for $146.5 million. The taxable gain on the transaction will be offset by tax loss carryforwards. |
| |
| | • | In December 2010, Safeguard received $32.3 million in initial sale proceeds from the successful completion of Eli Lilly and Company’s (NYSE: LLY) acquisition of partner company Avid Radiopharmaceuticals, Inc. (“Avid”). Under terms of the Lilly definitive agreement, Lilly acquired all outstanding shares of Avid for an upfront payment of $300 million, adjusted based on existing cash on hand at closing, and subject to a portion of such amounts being held in escrow. Safeguard expects to receive an additional $3.4 million currently held in escrow and could receive up to an additional $60.0 million based upon contingent consideration payable upon the achievement of future regulatory and commercial milestones. It is difficult to assess the likelihood of receiving some or all of such amounts, or the timing thereof. |
| |
| | • | Subsequent to the end of 2010, NuPathe Inc.’s (Nasdaq: PATH) New Drug Application (“NDA”) for Zelrix was accepted for filing by the U.S. Food and Drug Administration (“FDA”). NuPathe submitted the Zelrix NDA on October 29, 2010. NuPathe received a Prescription Drug User Fee Act date of August 29, 2011. Zelrix is the first ever submission to the FDA of a transdermal patch for the treatment of migraine. In August, NuPathe raised $43 million in net proceeds from its initial public offering of public stock. The company continues to prepare for commercial launch. |
4
Our Strategy
We currently focus principally on companies that address the strategic challenges facing businesses today and the opportunities they present. We believe these challenges have five general themes:
| | |
| | • | Maturity — Many existing technologies, solutions and therapies are reaching the end of their designed lives or patent protection; the population of the U.S. is aging; IT infrastructure is maturing and the sectors are consolidating; and many businesses based on once-novel technologies are now facing consolidation and other competitive pressures. |
| |
| | • | Migration — Many technology platforms are migrating to newer technologies with changing cost structures; many medical treatments are moving toward earlier stage intervention or generics; there is a migration from generalized treatments to personalized medicine; many business models are migrating towards different revenue-generation models, integrating technologies and services; and traditional media such as newspapers and advertising are migrating online. |
| |
| | • | Convergence — Many technology and life sciences businesses are intersecting in fields like medical devices and diagnostics for targeted therapies. Within life sciences itself, devices, diagnostics and therapeutics are converging. |
| |
| | • | Compliance — Regulatory compliance is driving buying behavior in technology and life sciences. HIPPA, Sarbanes-Oxley, the FDA and the SEC are all telling businesses how to spend money. |
| |
| | • | Cost containment — The importance of cost containment grows as healthcare costs and IT infrastructure maintenance costs grow. |
These strategic themes tend to drive growth and attract entrepreneurs who need capital, operational support and strategic guidance. Safeguard deploys capital, combined with management expertise, process excellence and marketplace insight, to provide tangible benefits to our partner companies.
Our corporate staff (28 employees at December 31, 2010) is dedicated to creating long-term value for our shareholders by helping our partner companies build value and by finding additional acquisition opportunities.
Identifying Partner Company Opportunities
Safeguard’sgo-to-market strategy, marketing and sourcing activities within our sectors of focus (Life Sciences and Technology) are designed to generate a large volume of high-quality opportunities to acquire majority or primary shareholder stakes in partner companies. Our principal focus is to acquire stakes in growth-stage companies with attractive growth prospects in the technology and life sciences sectors. Generally, we prefer to deploy capital into companies:
| | |
| | • | operating in largeand/or growing markets; |
| |
| | • | with barriers to entry by competitors, such as proprietary technology and intellectual property, or other competitive advantages; |
| |
| | • | with capital requirements of up to $25 million; and |
| |
| | • | with a compelling growth strategy. |
Our sourcing efforts are targeted primarily in the eastern U.S. However, in-bound deal leads generate opportunities throughout the U.S. and Canada. Leads come from a variety of sources, including investment bankers, syndication partners, existing partner companies and advisory board members.
Our Life Sciences Group currently targets companies with Product or Technology-Enabled Service business models that represent lesser regulatory risk and companies in the molecular andpoint-of-care diagnostics, medical device, regenerative medicine, specialty pharmaceuticals and selected healthcare services vertical markets.
Our Technology Group currently targets companies with recurring revenue, transaction-enabled applications or software as a service business models and companies in the internet/new media, financial services IT, healthcare IT and selected business services vertical markets.
5
We believe there are many opportunities within these business models and vertical markets, and our sourcing activities are focused on finding candidate companies and evaluating how well they align with our criteria. However, we recognize we may have difficulty identifying candidate companies and completing transactions on terms we believe appropriate. As a result, we cannot be certain how frequently we will enter into transactions with new or existing partner companies.
Competition. We face intense competition from other companies that acquire, or provide capital to life sciences and technology businesses. Competitors include venture capital and, occasionally, private equity investors, as well as companies seeking to make strategic acquisitions. Many providers of growth capital also offer strategic guidance, networking access for recruiting and general advice. Nonetheless, we believe we are an attractive capital provider to potential partner companies because our strategy and capabilities offer:
| | |
| | • | responsive operational assistance, including strategy design and execution, business development, corporate development, sales, marketing, finance, risk management, human resources and legal support; |
| |
| | • | the flexibility to structure minority or majority transactions with or without debt; |
| |
| | • | occasional liquidity opportunities for founders and existing investors; |
| |
| | • | a focus on maximizingrisk-adjustedvalue growth, rather thanabsolutevalue growth within a narrow or predetermined time frame; |
| |
| | • | interim C-level management support, as needed; |
| |
| | • | opportunities to leverage Safeguard’s balance sheet for borrowing and stability; and |
| |
| | • | a record of building value in our partner companies. |
Helping Our Partner Companies Build Value
We offer operational and management support to each of our partner companies through our experienced professionals. Our employees have expertise in business and technology strategy, sales and marketing, operations, finance, legal and transactional support. We provide hands-on assistance to the management teams of our partner companies to support their growth. We believe our strengths include:
| | |
| | • | applying our expertise to support a company’s introduction of new products and services; |
| |
| | • | leveraging our market knowledge to generate additional growth opportunities; |
| |
| | • | leveraging our business contacts and relationships; and |
| |
| | • | identifying and evaluating potential acquisitions and providing capital to pursue potential acquisitions to accelerate growth. |
Strategic Support. By helping our partner companies’ management teams remain focused on critical objectives through the provision of human, financial and strategic resources, we believe we are able to accelerate their development and success. We play an active role in developing the strategic direction of our partner companies, including:
| | |
| | • | defining short and long-term strategic goals; |
| |
| | • | identifying and planning for the critical success factors to reach these goals; |
| |
| | • | identifying and addressing the challenges and operational improvements required to achieve the critical success factors and, ultimately, the strategic goals; |
| |
| | • | identifying and implementing the business measurements that we and others will apply to measure a company’s success; and |
| |
| | • | providing capital to drive growth. |
Management and Operational Support. We provide management and operational support, as well as ongoing planning and development assessment. Our executives and Advisory Board members provide mentoring,
6
advice and guidance to develop partner company management. Our executives serve on the boards of directors of partner companies, working with them to develop and implement strategic and operating plans. We measure and monitor achievement of these plans through regular operational and financial performance measurements. We believe these services provide partner companies with significant competitive advantages within their respective markets.
Realizing Value
In general, we will hold our stake in a partner company as long as we believe the risk-adjusted value of that stake is maximized by our continued ownership and effort. From time to time, we engage in discussions with other companies interested in our partner companies, either in response to inquiries or as part of a process we initiate. To the extent we believe that a partner company’s further growth and development can best be supported by a different ownership structure or if we otherwise believe it is in our shareholders’ best interests, we may sell some or all of our stake in the partner company. These sales may take the form of privately negotiated sales of stock or assets, public offerings of the partner company’s securities and, in the case of publicly traded partner companies, sales of their securities in the open market. In the past, we have taken partner companies public through rights offerings and directed share subscription programs. We will continue to consider these (or similar) programs to maximize partner company value for our shareholders. We expect to use proceeds from these sales (and sales of other assets) primarily to pursue opportunities to create new partner company relationships or for other working capital purposes, either with existing partner companies or at Safeguard.
Our Partner Companies
An understanding of our partner companies is important to understanding Safeguard and its value-building strategy. Following are descriptions of certain of our partner companies in which we owned a stake at December 31, 2010. The indicated ownership percentage is presented as of December 31, 2010 and reflects the percentage of the vote we are entitled to cast based on issued and outstanding voting securities (on a common stock equivalent basis), excluding the effect of options, warrants and convertible debt (primary ownership).
LIFE SCIENCES PARTNER COMPANIES
| |
| Advanced BioHealing, Inc. | (Safeguard Ownership: 28.1%) |
Headquartered in Westport, Connecticut, Advanced BioHealing develops and commercializes living cell therapies that repair damaged human tissue and enable the body to heal itself. Its lead product, Dermagraft®, is a bio-engineered skin substitute that assists in restoring damaged tissue and supports the body’s natural healing process. It is FDA approved to treat diabetic foot ulcers and is the focus of an ongoing pivotal trial in subjects with venous leg ulcers (VLUs) to assess the product’s safety and efficacy in the promotion of healing VLUs. According to Advanced BioHealing, to date, more than 250,000 applications of Dermagraft have been administered in over 1,000 wound care centers and outpatient clinics nationwide.www.advancedbiohealing.com
| |
| Alverix, Inc. | (Safeguard Ownership: 49.6%) |
Headquartered in San Jose, California, Alverix provides next-generation instrument and connectivity platforms for diagnosticPoint-of-Care testing. Building upon a30-year legacy within Hewlett Packard and Agilent, Alverix was spun off as a privately held company in 2007. Alverix’s systems enable laboratory class performance in a mobile, inexpensive format, extending testing beyond high volume sites to physician office labs, retail clinics, emerging markets and the home. Alverix’s target markets include infectious disease, drugs of abuse, cardiac markers and cholesterol/lipids, among others. With strength in product design, product development and high volume manufacturing, Alverix is leading the transition of diagnostic testing from the laboratory to all locations where immediate results are critical to patient care.www.alverix.com
Alverix’s business is based on patented optical sensing technology and proprietary imaging enhancement algorithms developed at Hewlett-Packard and refined at Agilent and Avago Technologies. Usingoff-the-shelf parts, the company has created a low-cost, next-generation meter platform that overcomes several limitations of current
7
bench-top instrumentation. By partnering with assay companies as an OEM provider, Alverix can extend diagnostic testing beyond high volume labs into lower volume sites.
| |
| Good Start Genetics, Inc. | (Safeguard Ownership: 26.3%) |
Headquartered in Boston, Massachusetts, Good Start Genetics is a diagnostic company that is developing a more accurate and comprehensive pre-pregnancy genetic test based on proprietary gene sequencing technology, designed to replace single-disorder-only tests currently on the market. The Company’s affordable pre-pregnancy test utilizes an advanced DNA sequencing technology to screen for a panel of genetic disorders including those recommended by the American Congress of Obstetricians and Gynecologists and the American College of Medical Genetics. Good Start Genetics’ offering will allow improved identification of carriers of heritable genetic disorders, enabling physicians to help prospective parents make more knowledgeable medical decisions before conception. Good Start Genetics’ platform also can be used in oncology, cardiovascular and adult genetic disorders.www.goodstartgenetics.com
Good Start Genetics’ next-generation sequencing technology is designed to deliver a higher detection rate and improved clinical performance compared to currently available screening methods, such as genotyping and single nucleotide polymorphism analysis. Higher detection rates provide physicians with more accurate results and more clinically relevant genetic information. The Company expects to offer its test exclusively through board certified physicians in the U.S. starting in 2011.
| |
| Molecular Biometrics, Inc. | (Safeguard Ownership: 35.0%) |
Headquartered in Norwood, Massachusetts, Molecular Biometrics, Inc. is developing novel clinical diagnostic tools for applications in personalized medicine. Its first product, ViaMetrics-Etm is intended to be a rapid, non-invasive procedure designed to enhance in vitro fertilization (IVF) outcomes. Ultimately, the goal of ViaMetrics-E is to reduce the number of embryos transferred during an IVF cycle, as well as the complications and healthcare costs that accompany multiple births.www.molecularbiometrics.com
To offset low pregnancy success rates, couples often request the implantation of multiple embryos to increase their chances, resulting in multiple birth pregnancies for one-third of couples. Molecular Biometrics seeks to improve the likelihood of selecting a viable embryo, increase the success rate within IVF and reduce the number of needed cycles for couples. The patented technology may also be applicable to other areas of reproductive medicine, and neurodegenerative and other diseases.
| |
| NuPathe, Inc. (Nasdaq: PATH) | (Safeguard Ownership: 18.1%) |
Headquartered in Conshohocken, Pennsylvania, NuPathe is a specialty pharmaceutical company focused on the development and commercialization of branded therapeutics for diseases of the central nervous system, including neurological and psychiatric disorders. NuPathe’s most advanced product candidate, Zelrixtm, is a single-use, transdermal sumatriptan patch being developed for the treatment of migraine headaches. In addition to Zelrix, NuPathe has two proprietary product candidates in preclinical development: NP201 for the continuous symptomatic treatment of Parkinson’s disease and NP202 for the long-term treatment of schizophrenia and bipolar disorder.www.nupathe.com
Migraine-related GI disturbances, including nausea and vomiting, reduce (or even prevent) the effectiveness of oral treatments. In addition, many patients on triptan therapy experience recurring migraines despite their medication. The lack of adequate therapies addressing these needs has created an opportunity for the SmartRelieftm patch.
| |
| Tengion, Inc. (Nasdaq: TNGN) | (Safeguard Ownership: 4.8%) |
Headquartered in East Norriton, Pennsylvania, Tengion is a clinical-stage, organ-regeneration company with programs for urologic, renal and gastrointestinal regeneration. It has pioneered the Autologous Organ Regeneration Platformtm that enables Tengion to create proprietary product candidates that are intended to harness the intrinsic regenerative pathways of the body to produce a range of native-like organs and tissues. Tengion’s product
8
candidates seek to eliminate the need to: utilize other tissues of the body for a purpose to which they are poorly suited; procure donor organs; or administer anti-rejection medications.www.tengion.com
Patients suffering from severe bladder disease often opt for radical cystectomy, bladder augmentation or removal. Bowel tissue is generally used to replace bladder tissue, although these procedures have a number of significant complications that increase patient morbidity. By incorporating a patient’s own cells into a biodegradable scaffold, the Tengion Neo-Bladder products serve as a template that, once implanted, recruit other cells to develop and regenerate the organ.
Tengion’s products address the shortage of donor organs, the risk of organ rejection and the high cost and toxicity of anti-rejection medications.
TECHNOLOGY PARTNER COMPANIES
| |
| AdvantEdge Healthcare Solutions, Inc. | (Safeguard Ownership: 40.2%) |
Headquartered in Warren, New Jersey, AHS is a provider of physician billing and practice management services and software to hospital-based physician groups, large office-based physician practices, and ambulatory surgery centers throughout the United States. AHS’ technology efficiently collects patient demographic and clinical information and speeds the reimbursement of third-party claims and patient payments, enabling physicians and providers to maximize revenue and decrease their billing and practice management costs, frequently in dramatic ways. AHS is an IBM Business Partner for physician billing solutions.www.ahsrcm.com
AHS is a growing provider with proven technology, operating in a fragmented market for outsourced revenue cycle management comprised of many “mom and pop” companies. The AHS management team has extensive experience in acquiring smaller competitors and in developing effective revenue-cycle technology platforms.
AHS plans to grow both organically and via acquisition, purchasing companies atbelow-to-average industry multiples and then achieving significant margin synergies through proprietary technology.
| |
| Beyond.com, Inc. | (Safeguard Ownership: 38.3%) |
Headquartered in King of Prussia, Pennsylvania, Beyond.com is the world’s largest network of niche career communities, providing access to thousands of top-tier industry and local web sites. Beyond’s career search services and networking tools enable job seekers and employers to create targeted connections across thousands of online communities. Beyond delivers aone-of-a-kind recruitment solution that provides the targeted exposure of a specialized job board, reinforced by the breadth and volume of a larger career network.www.beyond.com
Approximately 90% of job seekers search locally. Beyond.com capitalizes on the fact that most job seekers search locally, monetizing its network of local and niche career websites via job postings, career services, lead generation and online advertising. As the online jobs-search market consolidates, Beyond.com is exploring growth opportunities through acquisition.
Much of the growth in online job recruitment is expected to come from small and medium-sized businesses, posting local ads for jobs, a trend that favors Beyond.com’s business model.
| |
| Bridgevine, Inc. | (Safeguard Ownership: 22.8%) |
Headquartered in Vero Beach, Florida, Bridgevine is an online performance marketing company. Its proprietary AMPtm technology — a seamless and highly automated campaign management, merchandising, fulfillment and business intelligence platform — connects advertising clients with new, high-quality customers. Bridgevine’s broad catalog of brands and unique technology are major reasons why thousands of third-party retail sites, retail outlets, call centers, and equipment manufacturers use the Bridgevine platform to offer complementary services to their own customers. According to Bridgevine, since 2003, the company has facilitated more than 8 million transactions and generated over $1 billion in incremental revenue for a growing list of nationally recognized advertisers.www.bridgevine.com
9
Bridgevine raised capital to develop proprietary software that exploits the explosive growth of broadband, cable, voice and other digital service subscriptions. Bridgevine’s structure and technology generate solid margins, and increasing revenue and market share.
Bridgevine competes at the convergence of two markets: 1) digital service customer acquisition, and 2) online lead generation.
| |
| MediaMath, Inc. | (Safeguard Ownership: 17.3%) |
Headquartered in New York City, MediaMath is a provider of digital media trading technology and services. Its TerminalOne® platform is the first and only enterprise-class demand side platform, providing an unmatched combination of supply, data, analytics, workflow automation and optimization to return focus to marketing strategy rather than media execution. MediaMath drives results for dozens of agencies representing the world’s leading brands — including units within all seven global agency holding companies — on thousands of worldwide campaigns.www.mediamath.com
MediaMath’s combination of algorithmic bidding and data integration provides advertisers with the efficiencies of search and the branding impact of display advertising. Exchange-traded advertising plays an increasingly important role in online advertising.
As consumers spend more time on the Internet, online ad expenditures are growing at the expense of traditional media. The Internet is more measureable and less expensive than traditional media and is the only medium where marketers can reach consumers through the entire buying cycle from initial awareness to purchase to post-sale feedback.
| |
| Portico Systems, Inc. | (Safeguard Ownership: 45.4%) |
Headquartered in Blue Bell, Pennsylvania, Portico Systems’ innovative solutions enable health plans to reduce administrative, medical and IT costs. Portico’s exclusive focus on provider operations and 500+ staff-years of provider experience has allowed Portico to design the only modularend-to-end provider platform. www.porticosys.com
According to a Gartner study, worldwide healthcare IT spending (hardware, software, services and telecommunications) exceeded $77 billion in 2007 and was projected to grow at a compound annual rate of 5% through 2011, with software and services highlighted as key growth areas. The U.S. component of that market was estimated at approximately $34 billion in 2008 by DataMonitor. The approximate 250 Tier 1 and Tier 2 healthcare plans targeted by Portico spend more than $300 million annually on IT infrastructure. Ancillary target markets include pharmaceutical companies.
| |
| SafeCentral, Inc. | (Safeguard Ownership: 20.1%) |
Headquartered in Palm Beach Gardens, Florida, SafeCentral, Inc. is a developer of security software applications and technologies for data loss prevention on the endpoint, the user’s browser. The SafeCentral product is web session security, in one click. SafeCentral is the secure companion to everyday Web browsing, providingend-to-end security against unauthorized network access and data and identity theft by locking out desktop malware and establishing trusted Web connections. It features patent-pending TSX technology to block key loggers, screen scrapers, and other malware agents, even on an already infected PC; SecureDNS to ensure a connection to the actual site, eliminatingman-in-the-middle attacks; automated “launch anywhere” protection for seamless integration into your existing browsing habits; and peace of mind when transferring sensitive information or transacting online.www.safecentral.com
Consumers and businesses will seek next-generation software to protect against growing threats of online identity theft and data piracy. SafeCentral is developing various channels to distribute its software to end customers.
10
| |
| Swap.com | (Safeguard Ownership: 45.6%) |
Headquatered in Boston, Massachusetts, Swap.com enables consumers to swap books, music, movies, video games and fashion, using its website and offline events. www.swap.com
Swap.com is taking advantage of and leveraging several Internet trends including the success ofe-Commerce sites such as eBay and Amazon.com, and the growth of member-centric organizations such as Facebook and MySpace.
Other Initiatives
As we continue to develop and grow, we will consider partner companies in additional sectors; participating in different capital structures; other types of partner company relationships; and extensions to our business model which leverage our core capabilities.
FINANCIAL INFORMATION ABOUT OPERATING SEGMENTS
Information on revenue, operating income (loss), equity income (loss) and net income (loss) from continuing operations for each operating segment of Safeguard’s business for each of the three years in the period ended December 31, 2010 and assets as of December 31, 2010 and 2009 is contained in Note 18 to the Consolidated Financial Statements.
OTHER INFORMATION
The operations of Safeguard and its partner companies are subject to environmental laws and regulations. Safeguard does not believe that expenditures relating to those laws and regulations will have a material adverse effect on the business, financial condition or results of operations of Safeguard.
AVAILABLE INFORMATION
Safeguard is subject to the informational requirements of the Securities Exchange Commission Act of 1934, as amended. Therefore, we file our annual report onForm 10-K, quarterly reports onForm 10-Q, current reports onForm 8-K, proxy statements and other information with, and furnish other reports to, the Securities and Exchange Commission (“SEC”). You can read and copy such documents at the SEC’s public reference facilities in Washington, D.C., New York, New York and Chicago, Illinois. You may obtain information on the operation of the SEC’s public reference facilities by calling the SEC at1-800-SEC-0330. Such material may also be accessed electronically by means of the SEC’s home page on the internet atwww.sec.gov or through Safeguard’s Internet website atwww.safeguard.com.Such documents are available as soon as reasonably practicable after electronic filing of the material with the SEC. Copies of these reports (excluding exhibits) also may be obtained free of charge, upon written request to: Investor Relations, Safeguard Scientifics, Inc., 435 Devon Park Drive, Building 800, Wayne, Pennsylvania 19087.
The internet website addresses for Safeguard and its partner companies are included in this report for identification purposes. The information contained therein or connected thereto are not intended to be incorporated into this Annual Report onForm 10-K.
The following corporate governance documents are available free of charge on Safeguard’s website: the charters of our Audit, Compensation and Nominating & Corporate Governance Committees, our Corporate Governance Guidelines and our Code of Business Conduct and Ethics. We also will post on our website any amendments to or waivers of our Code of Business Conduct and Ethics that relate to our directors and executive officers.
11
You should carefully consider the information set forth below. The following risk factors describe situations in which our business, financial conditionand/or results of operations could be materially harmed, and the value of our securities may be adversely affected. You should also refer to other information included or incorporated by reference in this report.
Our business depends upon our ability to make good decisions regarding the deployment of capital into new or existing partner companies and, ultimately, the performance of our partner companies, which is uncertain.
If we make poor decisions regarding the deployment of capital into new or existing partner companies, our business model will not succeed. Our success as a company ultimately depends on our ability to choose the right partner companies. If our partner companies do not succeed, the value of our assets could be significantly reduced and require substantial impairments or write-offs and our results of operations and the price of our common stock would be adversely affected. The risks relating to our partner companies include:
| | |
| | • | most of our partner companies have a history of operating lossesand/or limited operating history; |
| |
| | • | the intense competition affecting the products and services our partner companies offer could adversely affect their businesses, financial condition, results of operations and prospects for growth; |
| |
| | • | the inability to adapt to changing marketplaces; |
| |
| | • | the inability to manage growth; |
| |
| | • | the need for additional capital to fund their operations, which we may not be able to fund or which may not be available from third parties on acceptable terms, if at all; |
| |
| | • | the inability to protect their proprietary rightsand/or infringing on the proprietary rights of others; |
| |
| | • | that certain of our partner companies could face legal liabilities from claims made against them based upon their operations, products or work; |
| |
| | • | the impact of economic downturns on their operations, results and growth prospects; |
| |
| | • | the inability to attract and retain qualified personnel; and |
| |
| | • | the existence of government regulations and legal uncertainties may place financial burdens on the businesses of our partner companies. |
These and other risks are discussed in detail under the caption “Risks Related to Our Partner Companies” below.
Our partner companies (and the nature of our interests in them) could vary widely from period to period.
As part of our strategy, we continually assess the value to our shareholders of our interests in our partner companies. We also regularly evaluate alternative uses for our capital resources. As a result, depending on market conditions, growth prospects and other key factors, we may at any time:
| | |
| | • | change the individualand/or types of partner companies on which we focus; |
| |
| | • | sell some or all of our interests in any of our partner companies; or |
| |
| | • | otherwise change the nature of our interests in our partner companies. |
Therefore, the nature of our holdings could vary significantly from period to period.
Our consolidated financial results also may vary significantly based upon which, if any of our partner companies are included in our Consolidated Financial Statements. For example: For the period from January 1, 2009 through May 14, 2009 and the year ended December 31, 2008, we consolidated the results of operations of Clarient in continuing operations. On May 14, 2009, we deconsolidated Clarient and between such date and
12
December 2010 (when we sold our remaining interests in Clarient) we accounted for our holdings in Clarient under the fair value option.
Our business model does not rely, or plan, upon the receipt of operating cash flows from our partner companies. Our partner companies generally provide us with no cash flow from their operations. We rely on cash on hand, liquidity events and our ability to generate cash from capital raising activities to finance our operations.
We need capital to develop new partner company relationships and to fund the capital needs of our existing partner companies. We also need cash to service and repay our outstanding debt, finance our corporate overhead and meet our existing funding commitments. As a result, we have substantial cash requirements. Our partner companies generally provide us with no cash flow from their operations. To the extent our partner companies generate any cash from operations; they generally retain the funds to develop their own businesses. As a result, we must rely on cash on hand, partner company liquidity events and new capital raising activities to meet our cash needs. If we are unable to find ways of monetizing our holdings or to raise additional capital on attractive terms, we may face liquidity issues that will require us to curtail our new business efforts, constrain our ability to execute our business strategy and limit our ability to provide financial support to our existing partner companies.
Fluctuations in the price of the common stock of our publicly traded holdings may affect the price of our common stock.
Fluctuations in the market prices of the common stock of our publicly traded holdings may affect the price of our common stock. The market prices of our publicly traded holdings have been highly volatile and subject to fluctuations unrelated or disproportionate to operating performance.
Intense competition from other acquirors of interests in companies could result in lower gains or possibly losses on our partner companies.
We face intense competition from other capital providers as we acquire and develop interests in our partner companies. Some of our competitors have more experience identifying, acquiring and selling companies and have greater financial and management resources, brand name recognition or industry contacts than we have. Despite making most of our acquisitions at a stage when our partner companies are not publicly traded, we may still pay higher prices for those equity interests because of higher valuations of similar public companies and competition from other acquirers and capital providers, which could result in lower gains or possibly losses.
We may be unable to obtain maximum value for our holdings or to sell our holdings on a timely basis.
We hold significant positions in our partner companies. Consequently, if we were to divest all or part of our holdings in a partner company, we may have to sell our interests at a relative discount to a price which may be received by a seller of a smaller portion. For partner companies with publicly traded stock, we may be unable to sell our holdings at then-quoted market prices. For instance, the trading volume and public float in the common stock of NuPathe, one of our two publicly traded partner companies, is small relative to our holdings. As a result, any significant open-market divestiture by us of our holdings in these partner companies, if possible at all, would likely have a material adverse effect on the market price of their common stock and on our proceeds from such a divestiture. Additionally, we may not be able to take our partner companies public as a means of monetizing our position or creating shareholder value.
Registration and other requirements under applicable securities laws may adversely affect our ability to dispose of our holdings on a timely basis.
13
Our success is dependent on our executive management.
Our success is dependent on our executive management team’s ability to execute our strategy. A loss of one or more of the members of our executive management team without adequate replacement could have a material adverse effect on us.
Our business strategy may not be successful if valuations in the market sectors in which our partner companies participate decline.
Our strategy involves creating value for our shareholders by helping our partner companies build value and, if appropriate, accessing the public and private capital markets. Therefore, our success is dependent on the value of our partner companies as determined by the public and private capital markets. Many factors, including reduced market interest, may cause the market value of our publicly traded partner companies to decline. If valuations in the market sectors in which our partner companies participate decline, their access to the public and private capital markets on terms acceptable to them may be limited.
Our partner companies could make business decisions that are not in our best interests or with which we do not agree, which could impair the value of our holdings.
Although we may seek a controlling or influential equity interest and participation in the management of our partner companies, we may not be able to control the significant business decisions of our partner companies. We may have shared control or no control over some of our partner companies. In addition, although we currently own a controlling or influential interest in some of our partner companies, we may not maintain those levels of interest. Acquisitions of interests in partner companies in which we share or have no control, and the dilution of our interests in or loss of control of partner companies, will involve additional risks that could cause the performance of our interests and our operating results to suffer, including:
| | |
| | • | the management of a partner company having economic or business interests or objectives that are different from ours; and |
| |
| | • | the partner companies not taking our advice with respect to the financial or operating issues they may encounter. |
Our inability to control our partner companies also could prevent us from assisting them, financially or otherwise, or could prevent us from liquidating our interests in them at a time or at a price that is favorable to us. Additionally, our partner companies may not act in ways that are consistent with our business strategy. These factors could hamper our ability to maximize returns on our interests and cause us to incur losses on our interests in these partner companies.
We may have to buy, sell or retain assets when we would otherwise not wish to do so in order to avoid registration under the Investment Company Act.
The Investment Company Act of 1940 regulates companies which are engaged primarily in the business of investing, reinvesting, owning, holding or trading in securities. Under the Investment Company Act, a company may be deemed to be an investment company if it owns investment securities with a value exceeding 40% of the value of its total assets (excluding government securities and cash items) on an unconsolidated basis, unless an exemption or safe harbor applies. We refer to this test as the “40% Test.” Securities issued by companies other than consolidated partner companies are generally considered “investment securities” for purpose of the Investment Company Act; unless other circumstances exist which actively involve the company holding such interests in the management of the underlying company. We are a company that partners with growth-stage companies to build value; we are not engaged primarily in the business of investing, reinvesting or trading in securities. We are in compliance with the 40% Test. Consequently, we do not believe that we are an investment company under the Investment Company Act.
We monitor our compliance with the 40% Test and seek to conduct our business activities to comply with this test. It is not feasible for us to be regulated as an investment company because the Investment Company Act rules are inconsistent with our strategy of actively helping our partner companies in their efforts to build value. In order to
14
continue to comply with the 40% Test, we may need to take various actions which we would otherwise not pursue. For example, we may need to retain a controlling interest in a partner company that we no longer consider strategic, we may not be able to acquire an interest in a company unless we are able to obtain a controlling ownership interest in the company, or we may be limited in the manner or timing in which we sell our interests in a partner company. Our ownership levels also may be affected if our partner companies are acquired by third parties or if our partner companies issue stock which dilutes our controlling ownership interest. The actions we may need to take to address these issues while maintaining compliance with the 40% Test could adversely affect our ability to create and realize value at our partner companies.
Economic disruptions and downturns may have negative repercussions for the Company.
Events in the United States and international capital markets, debt markets and economies may negatively impact the Company’s ability to pursue certain tactical and strategic initiatives, such as accessing additional public or private equity or debt financing for itself or for its partner companies and selling the Company’s interests in partner companies on terms acceptable to the Company and in time frames consistent with our expectations.
We have had material weaknesses in our internal controls over financial reporting in the recent past and cannot provide assurance that additional material weaknesses will not be identified in the future. Our failure to effectively maintain our internal control over financial reporting could result in material misstatements in our Consolidated Financial Statements which could require us to restate financial statements, cause us to fail to meet our reporting obligations, cause investors to lose confidence in our reported financial information and/or have a negative effect on our stock price.
We cannot assure that material weaknesses in our internal controls over financial reporting will not be identified in the future. Any failure to maintain or implement required new or improved controls, or any difficulties we encounter in their implementation, could result in additional material weaknesses, or could result in material misstatements in our Consolidated Financial Statements. These misstatements could result in a restatement of financial statements, cause us to fail to meet our reporting obligationsand/or cause investors to lose confidence in our reported financial information, leading to a decline in our stock price.
Risks Related to our Partner Companies
Most of our partner companies have a history of operating losses and/or limited operating history and may never be profitable.
Most of our partner companies have a history of operating losses or limited operating history, have significant historical losses and may never be profitable. Many have incurred substantial costs to develop and market their products, have incurred net losses and cannot fund their cash needs from operations. We expect that the operating expenses of certain of our partner companies will increase substantially in the foreseeable future as they continue to develop products and services, increase sales and marketing efforts, and expand operations.
Our partner companies face intense competition, which could adversely affect their business, financial condition, results of operations and prospects for growth.
There is intense competition in the technology and life sciences marketplaces, and we expect competition to intensify in the future. Our business, financial condition, results of operations and prospects for growth will be materially adversely affected if our partner companies are not able to compete successfully. Many of the present and potential competitors may have greater financial, technical, marketing and other resources than those of our partner companies. This may place our partner companies at a disadvantage in responding to the offerings of their competitors, technological changes or changes in client requirements. Also, our partner companies may be at a competitive disadvantage because many of their competitors have greater name recognition, more extensive client bases and a broader range of product offerings. In addition, our partner companies may compete against one another.
15
The success or failure of many of our partner companies is dependent upon the ultimate effectiveness of newly-created information technologies, medical devices, healthcare diagnostics etc.
Our partner companies’ business strategies are often highly dependent upon the successful launch and commercialization of an innovative information technology, medical device, healthcare diagnostic etc. Despite all of our efforts to understand the research and development underlying the innovation or creation of such technologies, etc. before we deploy capital to a partner company, often times the performance of the technology, device etc. never matches the expectations of us or the partner company. In those situations, it is likely that we will incur a partial or total loss of the capital which we deployed in such partner company.
Our partner companies may fail if they do not adapt to changing marketplaces.
If our partner companies fail to adapt to changes in technology and customer and supplier demands, they may not become or remain profitable. There is no assurance that the products and services of our partner companies will achieve or maintain market penetration or commercial success, or that the businesses of our partner companies will be successful.
The technology and life sciences marketplaces are characterized by:
| | |
| | • | rapidly changing technology; |
| |
| | • | evolving industry standards; |
| |
| | • | frequently introducing new products and services; |
| |
| | • | shifting distribution channels; |
| |
| | • | evolving government regulation; |
| |
| | • | frequently changing intellectual property landscapes; and |
| |
| | • | changing customer demands. |
Our future success will depend on our partner companies’ ability to adapt to these evolving marketplaces. They may not be able to adequately or economically adapt their products and services, develop new products and services or establish and maintain effective distribution channels for their products and services. If our partner companies are unable to offer competitive products and services or maintain effective distribution channels, they will sell fewer products and services and forego potential revenue, possibly causing them to lose money. In addition, we and our partner companies may not be able to respond to the marketplace changes in an economically efficient manner, and our partner companies may become or remain unprofitable.
Our partner companies may grow rapidly and may be unable to manage their growth.
We expect some of our partner companies to grow rapidly. Rapid growth often places considerable operational, managerial and financial strain on a business. To successfully manage rapid growth, our partner companies must, among other things:
| | |
| | • | improve, upgrade and expand their business infrastructures; |
| |
| | • | scale up production operations; |
| |
| | • | develop appropriate financial reporting controls; |
| |
| | • | attract and maintain qualified personnel; and |
| |
| | • | maintain appropriate levels of liquidity. |
If our partner companies are unable to manage their growth successfully, their ability to respond effectively to competition and to achieve or maintain profitability will be adversely affected.
16
Based on our business model, some or all of our partner companies will need to raise additional capital to fund their operations at any given time. We may not be able to fund some or all of such amounts and such amounts may not be available from third parties on acceptable terms, if at all.
We cannot be certain that our partner companies will be able to obtain additional financing on favorable terms, if at all. Because our resources and our ability to raise capital are not unlimited, we may not be able to provide partner companies with sufficient capital resources to enable them to reach a cash-flow positive position, even if we wished to do so. General economic disruptions and downturns may also negatively affect the ability of some of our partner companies to fund their operations from other stockholders and capital sources. We also may fail to accurately project the capital needs of partner companies. If partner companies need to but are not able to raise capital from us or other outside sources, then they may need to cease or scale back operations. In such event, our interest in any such partner company will become less valuable.
Economic disruptions and downturns may negatively affect our partner companies’ plans and their results of operations.
Many of our partner companies are largely dependent upon outside sources of capital to fund their operations. Disruptions in the availability of capital from such sources will negatively affect the ability of such partner companies to pursue their business models and will force such companies to revise their growth and development plans accordingly. Any such changes will, in turn, affect the ability of the Company to realize the value of its capital deployments in such companies.
In addition, downturns in the economy as well as possible governmental responses to such downturnsand/or to specific situations in the economy could affect the business prospects of certain of our partner companies, including, but not limited to, in the following ways: weaknesses in the financial services industries; reduced businessand/or consumer spending;and/or systematic changes in the ways the healthcare system operates in the United States.
Some of our partner companies may be unable to protect their proprietary rights and may infringe on the proprietary rights of others.
Our partner companies assert various forms of intellectual property protection. Intellectual property may constitute an important part of partner company assets and competitive strengths. Federal law, most typically, copyright, patent, trademark and trade secret laws, generally protects intellectual property rights. Although we expect that our partner companies will take reasonable efforts to protect the rights to their intellectual property, third parties may develop similar intellectual property independently. Moreover, the complexity of international trade secret, copyright, trademark and patent law, coupled with the limited resources of our partner companies and the demands of quick delivery of products and services to market, create a risk that partner company efforts to prevent misappropriation of their technology will prove inadequate.
Some of our partner companies also license intellectual property from third parties and it is possible that they could become subject to infringement actions based upon their use of the intellectual property licensed from those third parties. Our partner companies generally obtain representations as to the origin and ownership of such licensed intellectual property. However, this may not adequately protect them. Any claims against our partner companies’ proprietary rights, with or without merit, could subject the companies to costly litigation and divert their technical and management personnel from other business concerns. If our partner companies incur costly litigation and their personnel are not effectively deployed, the expenses and losses incurred by our partner companies will increase and their profits, if any, will decrease.
Third parties have and may assert infringement or other intellectual property claims against our partner companies based on their patents or other intellectual property claims. Even though we believe our partner companies’ products do not infringe any third-party’s patents, they may have to pay substantial damages, possibly including treble damages, if it is ultimately determined that they do. They may have to obtain a license to sell their products if it is determined that their products infringe another person’s intellectual property. Our partner companies might be prohibited from selling their products before they obtain a license, which, if available at all, may require them to pay substantial royalties. Even if infringement claims against our partner companies are without merit,
17
defending these types of lawsuits takes significant time, is expensive and may divert management attention from other business concerns.
Certain of our partner companies could face legal liabilities from claims made against their operations, products or work.
Because manufacture and sale of certain partner company products entail an inherent risk of product liability, certain partner companies maintain product liability insurance. Although none of our current partner companies have experienced any material losses in this regard, there can be no assurance that they will be able to maintain or acquire adequate product liability insurance in the future and any product liability claim could have a material adverse effect on a partner company’s financial stability, revenues and results of operations. In addition, many of the engagements of our partner companies involve projects that are critical to the operation of their clients’ businesses. If our partner companies fail to meet their contractual obligations, they could be subject to legal liability, which could adversely affect their business, operating results and financial condition. Partner company contracts typically include provisions designed to limit their exposure to legal claims relating to their services and products. However, these provisions may not protect our partner companies or may not be enforceable. Also, as consultants, some of our partner companies depend on their relationships with their clients and their reputation for high-quality services and integrity to retain and attract clients. As a result, claims made against our partner companies’ work may damage their reputation, which in turn could impact their ability to compete for new work and negatively impact their revenue and profitability.
Our partner companies’ success depends on their ability to attract and retain qualified personnel.
Our partner companies depend upon their ability to attract and retain senior management and key personnel, including trained technical and marketing personnel. Our partner companies also will need to continue to hire additional personnel as they expand. At present, none of our partner companies have employees represented by labor unions. Although our partner companies have not been the subject of a work stoppage, any future work stoppage could have a material adverse effect on their respective operations. A shortage in the availability of the requisite qualified personnel or work stoppage would limit the ability of our partner companies to grow, to increase sales of their existing products and services, and to launch new products and services.
Government regulations and legal uncertainties may place financial burdens on the businesses of our partner companies.
Failure to comply with applicable requirements of the FDA or comparable regulation in foreign countries can result in fines, recall or seizure of products, total or partial suspension of production, withdrawal of existing product approvals or clearances, refusal to approve or clear new applications or notices and criminal prosecution. Manufacturers of pharmaceuticals and medical diagnostic devices and operators of laboratory facilities are subject to strict federal and state regulation regarding validation and the quality of manufacturing and laboratory facilities. Failure to comply with these quality regulation systems requirements could result in civil or criminal penalties or enforcement proceedings, including the recall of a product or a “cease distribution” order. The enactment of any additional laws or regulations that affect healthcare insurance policy and reimbursement (including Medicare reimbursement) could negatively affect our partner companies. If Medicare or private payors change the rates at which our partner companies or their customers are reimbursed by insurance providers for their products, such changes could adversely impact our partner companies.
Some of our partner companies are subject to significant environmental, health and safety regulation.
Some of our partner companies are subject to licensing and regulation under federal, state and local laws and regulations relating to the protection of the environment and human health and safety, including laws and regulations relating to the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials, as well as to the safety and health of manufacturing and laboratory employees. In addition, the federal Occupational Safety and Health Administration has established extensive requirements relating to workplace safety.
18
| |
| Item 1B. | Unresolved Staff Comments |
None.
Our corporate headquarters and administrative offices in Wayne, Pennsylvania contain approximately 20,000 square feet of office space in one building. We currently lease our corporate headquarters under a lease with approximately 3.5 years remaining.
| |
| Item 3. | Legal Proceedings |
We, as well as our partner companies, are involved in various claims and legal actions arising in the ordinary course of business. While in the current opinion of management, the ultimate disposition of these matters will not have a material adverse effect on our consolidated financial position or results of operations, no assurance can be given as to the outcome of these lawsuits, and one or more adverse rulings could have a material adverse effect on our consolidated financial position and results of operations, or that of our partner companies. See Note 15 included in “Item 8 — Financial Statements and Supplementary Data” in this Annual Report onForm 10-K for a discussion of ongoing claims and legal actions.
ANNEX TO PART I — EXECUTIVE OFFICERS OF THE REGISTRANT
| | | | | | | | | | | |
Name | | Age | | Position | | Executive Officer Since |
| |
| Peter J. Boni | | | 65 | | | President, Chief Executive Officer and Director | | | 2005 | |
| James A. Datin | | | 48 | | | Executive Vice President and Managing Director, Life Sciences | | | 2005 | |
| Kevin L. Kemmerer | | | 42 | | | Executive Vice President and Managing Director, Technology | | | 2008 | |
| Brian J. Sisko | | | 50 | | | Senior Vice President and General Counsel | | | 2007 | |
| Stephen T. Zarrilli | | | 49 | | | Senior Vice President and Chief Financial Officer | | | 2008 | |
Mr. Boni joined Safeguard as President and Chief Executive Officer in August 2005. Prior to joining Safeguard, Mr. Boni was an Operating Partner for Advent International, a global private equity firm with $10 billion under management, from April 2004 to August 2005; Chairman and Chief Executive Officer of Surebridge, Inc., an applications outsourcer serving the mid-market, from March 2002 to April 2004; Managing Principal of Vested Interest LLC, a management consulting firm, from January 2001 to March 2002; and President and Chief Executive Officer of Prime Response, Inc., an enterprise applications software provider, from February 1999 to January 2001.
Mr. Datin joined Safeguard as Executive Vice President and Managing Director, Life Sciences Group in September 2005. Mr. Datin served as Chief Executive Officer of Touchpoint Solutions, Inc., a provider of software that enables customers to develop and deploy applications, content and media on multi-user interactive devices, from December 2004 to June 2005; Group President in 2004, and as Group President, International, from 2001 to 2003, of Dendrite International, a provider of sales, marketing, clinical and compliance solutions and services to global pharmaceutical and other life sciences companies; and Group Director, Corporate Business Strategy and Planning at GlaxoSmithKline, from 1999 to 2001, where he also was a member of the company’s Predictive Medicine Board of Directors that evaluated acquisitions and alliances. His prior experience also includes international assignments with and identifying strategic growth opportunities for E Merck and Baxter.
Mr. Kemmerer joined Safeguard as Principal, Technology Group, in June 2004, became Senior Vice President, Technology in April 2006, Senior Vice President and Managing Director, Technology in April 2008 and Executive Vice President and Managing Director, Technology in September 2008. Mr. Kemmerer served most recently as
19
Director of Kennet Venture Partners, a venture capital firm for which he worked from November 2000 to June 2004 and previously as Principal, Mergers and Acquisitions of Broadview International, for whom he worked from August 1997 to November 2000.
Mr. Sisko joined Safeguard as Senior Vice President and General Counsel in August 2007. Prior to joining Safeguard, Mr. Sisko served as Chief Legal Officer, Senior Vice President and General Counsel of Traffic.com (at the time, a public company), a former partner company of Safeguard that is a leading provider of accurate, real-time traffic information in the United States, from February 2006 until June 2007 (following its acquisition by NAVTEQ Corporation in March 2007); Chief Operating Officer from February 2005 to January 2006 of Halo Technology Holdings, Inc., a public holding company for enterprise software businesses (Halo Technology Holdings filed for bankruptcy protection under Chapter 11 of the United States Bankruptcy Code in August 2007); ran B/T Business and Technology, an advisor and strategic management consultant to a variety of public and private companies, from January 2002 to February 2005; and was a Managing Director from April 2000 to January 2002, of Katalyst, LLC, a venture capital and consulting firm. Mr. Sisko also previously served as Senior Vice President — Corporate Development and General Counsel of National Media Corporation, at the time a New York Stock Exchange-listed multi-media marketing company with operations in 70 countries, and as a partner in the corporate finance, mergers and acquisitions practice group of the Philadelphia-based law firm, Klehr, Harrison, Harvey, Branzburg & Ellers LLP.
Mr. Zarrilli joined Safeguard as Senior Vice President and Chief Financial Officer in June 2008. Prior to joining Safeguard, Mr. Zarrilli co-founded, in 2004, the Penn Valley Group, a middle-market management advisory and private equity firm, and served as a Managing Director until June 2008, and continues to serve as non-executive chairman of the Penn Valley Group. While at the Penn Valley Group, Mr. Zarrilli also served as Acting Senior Vice President, Acting Chief Administrative Officer and Acting Chief Financial Officer of Safeguard from December 2006 to June 2007. Mr. Zarrilli also served as the Chief Financial Officer, from 2001 to 2004, of Fiberlink Communications Corporation, a provider of remote access VPN solutions for large enterprises; as the Chief Executive Officer, from 2000 to 2001, of Concellera Software, Inc., a developer of content management software; as the Chief Executive Officer, from 1999 to 2000, and Chief Financial Officer, from 1994 to 1998, of US Interactive, Inc. (at the time a public company), a provider of internet strategy consulting, marketing and technology services (US Interactive filed for bankruptcy protection under Chapter 11 of the United States Bankruptcy Code in January 2001); and, previously, with Deloitte & Touche from 1983 to 1994. Mr. Zarrilli is a director and Chairman of the Audit Committee of NutriSystem, Inc.
PART II
| |
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Safeguard’s common stock is listed on the New York Stock Exchange (Symbol: SFE). The high and low sale prices reported within each quarter of 2010 and 2009 were as follows:
| | | | | | | | | |
| | | High | | Low |
| |
Fiscal year 2010: | | | | | | | | |
| First quarter | | $ | 13.34 | | | $ | 10.07 | |
| Second quarter | | | 14.35 | | | | 10.02 | |
| Third quarter | | | 13.27 | | | | 10.04 | |
| Fourth quarter | | | 17.44 | | | | 12.25 | |
Fiscal year 2009: | | | | | | | | |
| First quarter | | $ | 5.16 | | | $ | 2.04 | |
| Second quarter | | | 8.40 | | | | 3.48 | |
| Third quarter | | | 11.94 | | | | 6.60 | |
| Fourth quarter | | | 12.47 | | | | 8.60 | |
20
The high and low sale prices reported in the first quarter of 2011 through March 3, 2011 were $21.35 and $16.25, respectively, and the last sale price reported on March 3, 2011, was $20.35. No cash dividends have been declared in any of the years presented, and Safeguard has no present intention to declare cash dividends. Sale prices for periods prior to August 27, 2009 have been restated to reflect aone-for-six reverse split of our common stock which became effective on August 27, 2009.
As of March 3, 2011, there were approximately 26,500 beneficial holders of Safeguard’s common stock.
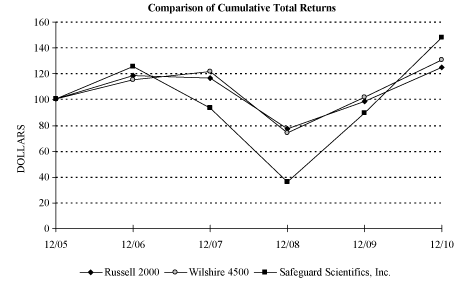
The following graph compares the cumulative total return on $100 invested in our common stock for the period from December 31, 2005 through December 31, 2010 with the cumulative total return on $100 invested for the same period in the Russell 2000 Index and the Wilshire 4500 Index. In light of the diverse nature of Safeguard’s business and based on our assessment of available published industry orline-of-business indices, we determined that no single industry orline-of-business index would provide a meaningful comparison to Safeguard. Further, we did not believe that we could readily identify an appropriate group of industry peer companies for this comparison. Accordingly, under SEC rules, we selected the Wilshire 4500 Index, a published market index in which the median market capitalization of the included companies is similar to our own. Safeguard’s common stock is included as a component of the Russell 2000 and Wilshire 4500 indices.
Comparison of Cumulative Total Returns
| | |
| | • | Assumes reinvestment of dividends. We have not distributed cash dividends during this period. |
| |
| | • | Assumes an investment of $100 on December 31, 2005. |
21
| |
| Item 6. | Selected Consolidated Financial Data |
The following table sets forth our selected consolidated financial data for the five-year period ended December 31, 2010. The selected consolidated financial data presented below should be read in conjunction with Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations and Item 8. Consolidated Financial Statements and Notes thereto included in this report. The historical results presented herein may not be indicative of future results. During the five-year period ended December 31, 2010, certain consolidated partner companies, or components thereof, were sold. These businesses are reflected in discontinued operations through their respective disposal dates: Acsis, Inc., Alliance Consulting Group Associates, Inc. and Laureate Pharma, Inc. (May, 2008), Pacific Title & Art Studio Inc. (March 2007), Clarient’s technology business (March 2007) and Mantas (October 2006). The accounts of Clarient are included in continuing operations through May 14, 2009, the date of its deconsolidation.
| | | | | | | | | | | | | | | | | | | | | |
| | | December 31, |
| | | 2010 | | 2009 | | 2008 | | 2007 | | 2006 |
| | | (In thousands) |
| |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 183,419 | | | $ | 67,347 | | | $ | 75,051 | | | $ | 96,201 | | | $ | 60,381 | |
| Short-term investments | | | 42,411 | | | | 39,066 | | | | 14,701 | | | | 590 | | | | 94,155 | |
| Restricted cash equivalents | | | 16,774 | | | | — | | | | — | | | | — | | | | — | |
| Cash held in escrow | | | 6,434 | | | | 6,910 | | | | 6,934 | | | | 22,686 | | | | 19,398 | |
| Working capital of continuing operations | | | 197,769 | | | | 105,983 | | | | 88,400 | | | | 97,184 | | | | 133,643 | |
| Total assets of continuing operations | | | 337,050 | | | | 282,099 | | | | 232,402 | | | | 258,075 | | | | 277,019 | |
| Convertible senior debentures | | | 75,919 | | | | 78,225 | | | | 86,000 | | | | 129,000 | | | | 129,000 | |
| Long-term debt, net of current portion | | | — | | | | — | | | | 345 | | | | 906 | | | | 1,939 | |
| Other long-term liabilities | | | 5,311 | | | | 5,461 | | | | 9,600 | | | | 9,111 | | | | 9,276 | |
| Total equity | | | 246,936 | | | | 190,507 | | | | 104,710 | | | | 155,831 | | | | 216,698 | |
22
Consolidated Statements of Operations Data
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | (In thousands except per share amounts) | |
| |
| Revenue | | $ | — | | | $ | 34,839 | | | $ | 73,736 | | | $ | 42,995 | | | $ | 27,723 | |
| Operating Expenses: | | | | | | | | | | | | | | | | | | | | |
| Cost of sales | | | — | | | | 13,811 | | | | 33,007 | | | | 26,914 | | | | 19,824 | |
| Selling, general and administrative | | | 20,847 | | | | 37,214 | | | | 60,744 | | | | 50,783 | | | | 44,924 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 20,847 | | | | 51,025 | | | | 93,751 | | | | 77,697 | | | | 64,748 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating loss | | | (20,847 | ) | | | (16,186 | ) | | | (20,015 | ) | | | (34,702 | ) | | | (37,025 | ) |
| Other income (loss), net | | | 74,809 | | | | 108,881 | | | | 10,280 | | | | (5,077 | ) | | | 5,762 | |
| Interest income | | | 718 | | | | 480 | | | | 3,097 | | | | 7,520 | | | | 6,805 | |
| Interest expense | | | (5,737 | ) | | | (3,164 | ) | | | (4,732 | ) | | | (5,489 | ) | | | (5,203 | ) |
| Equity loss | | | (21,829 | ) | | | (23,227 | ) | | | (34,697 | ) | | | (15,178 | ) | | | (3,732 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations before income taxes | | | 27,114 | | | | 66,784 | | | | (46,067 | ) | | | (52,926 | ) | | | (33,393 | ) |
| Income tax benefit | | | — | | | | 14 | | | | 24 | | | | 687 | | | | 1,260 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | | 27,114 | | | | 66,798 | | | | (46,043 | ) | | | (52,239 | ) | | | (32,133 | ) |
| Income (loss) from discontinued operations, net of tax | | | — | | | | 1,975 | | | | (9,620 | ) | | | (17,282 | ) | | | 70,358 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) | | | 27,114 | | | | 68,773 | | | | (55,663 | ) | | | (69,521 | ) | | | 38,225 | |
| Net (income) loss attributable to noncontrolling interest | | | — | | | | (1,163 | ) | | | 3,650 | | | | 3,653 | | | | 7,218 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. | | $ | 27,114 | | | $ | 67,610 | | | $ | (52,013 | ) | | $ | (65,868 | ) | | $ | 45,443 | |
| | | | | | | | | | | | | | | | | | | | | |
| Basic Income (Loss) Per Share: | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.32 | | | $ | 3.26 | | | $ | (2.10 | ) | | $ | (2.28 | ) | | $ | (1.32 | ) |
| Net income (loss) from discontinued operations attributable to Safeguard Scientifics, Inc. common shareholders | | | — | | | | 0.07 | | | | (0.46 | ) | | | (0.96 | ) | | | 3.54 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.32 | | | $ | 3.33 | | | $ | (2.56 | ) | | $ | (3.24 | ) | | $ | 2.22 | |
| | | | | | | | | | | | | | | | | | | | | |
| Shares used in computing basic income (loss) per share | | | 20,535 | | | | 20,308 | | | | 20,326 | | | | 20,328 | | | | 20,246 | |
| | | | | | | | | | | | | | | | | | | | | |
| Diluted Income (Loss) Per Share: | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.26 | | | $ | 3.08 | | | $ | (2.10 | ) | | $ | (2.28 | ) | | $ | (1.32 | ) |
| Net income (loss) from discontinued operations attributable to Safeguard Scientifics, Inc. common shareholders | | | — | | | | 0.06 | | | | (0.46 | ) | | | (0.96 | ) | | | 3.54 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.26 | | | $ | 3.14 | | | $ | (2.56 | ) | | $ | (3.24 | ) | | $ | 2.22 | |
| | | | | | | | | | | | | | | | | | | | | |
| Shares used in computing diluted income (loss) per share | | | 21,507 | | | | 22,383 | | | | 20,326 | | | | 20,328 | | | | 20,246 | |
| | | | | | | | | | | | | | | | | | | | | |
23
| |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Cautionary Note concerning Forward-Looking Statements
This Annual Report onForm 10-K contains forward-looking statements that are based on current expectations, estimates, forecasts and projections about Safeguard Scientifics, Inc. (“Safeguard” or “we”), the industries in which we operate and other matters, as well as management’s beliefs and assumptions and other statements regarding matters that are not historical facts. These statements include, in particular, statements about our plans, strategies and prospects. For example, when we use words such as “projects,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “should,” “would,” “could,” “will,” “opportunity,” “potential” or “may,” variations of such words or other words that convey uncertainty of future events or outcomes, we are making forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Our forward-looking statements are subject to risks and uncertainties. Factors that could cause actual results to differ materially, include, among others, managing rapidly changing technologies, limited access to capital, competition, the ability to attract and retain qualified employees, risks associated with the development and commercialization of innovative technologies, etc, the ability to execute our strategy, the uncertainty of the future performance of our partner companies, acquisitions and dispositions of companies, the inability to manage growth, compliance with government regulation and legal liabilities, additional financing requirements, labor disputes and the effect of economic conditions in the business sectors in which our partner companies operate, all of which are discussed in Item 1A. “Risk Factors.” Many of these factors are beyond our ability to predict or control. In addition, as a result of these and other factors, our past financial performance should not be relied on as an indication of future performance. All forward-looking statements attributable to us, or to persons acting on our behalf, are expressly qualified in their entirety by this cautionary statement. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this report might not occur.
Overview
Safeguard’s charter is to build value in growing businesses by providing partner companies with capital and a range of strategic, operational and management resources. Safeguard may participate in expansion financings, corporate spin-outs, management buy-outs, recapitalizations, industry consolidations and early-stage financings. Our vision is to be the preferred catalyst to build great companies across diverse capital platforms.
We strive to create long-term value for our shareholders by helping our partner companies increase market penetration, grow revenue and improve cash flow. We focus principally on companies in which we anticipate deploying up to $25 million and that operate in two categories:
Life Sciences — including companies focused on molecular andpoint-of-care diagnostics, medical devices, regenerative medicine, specialty pharmaceuticals and selected healthcare services; and
Technology — including companies focused on internet/new media, financial services IT, healthcare IT and selected business services that have transaction-enabling applications with a recurring revenue stream.
Principles of Accounting for Ownership Interests in Partner Companies
We account for our interests in our partner companies and private equity funds using one of the following methods: consolidation, fair value, equity, cost oravailable-for-sale. The accounting method applied is generally determined by the degree of our influence over the entity, primarily determined by our voting interest in the entity.
Consolidation Method. We account for partner companies in which we maintain a controlling financial interest, generally those in which we directly or indirectly own more than 50% of the outstanding voting securities, using the consolidation method of accounting. Upon consolidation of our partner companies, we reflect the portion of equity (net assets) in a subsidiary not attributable, directly or indirectly, to the parent company as a noncontrolling interest in the Consolidated Balance Sheet. The noncontrolling interest is presented within equity, separately from the equity of the parent company. Losses attributable to the parent company and the noncontrolling interest may exceed their interest in the subsidiary’s equity. As a result, the noncontrolling interest shall continue to be attributed
24
its share of losses even if that attribution results in a deficit noncontrolling interest balance as of each balance sheet date. Revenue, expenses, gains, losses, net income or loss are reported in the Consolidated Statements of Operations at the consolidated amounts, which include the amounts attributable to the parent company’s common shareholders and the noncontrolling interest. As of and for the year ended December 31, 2010, we did not hold a controlling interest in any of our partner companies.
Fair Value Method. We accounted for our holdings in Clarient, our publicly traded partner company, under the fair value option following its deconsolidation on May 14, 2009 and through the date of the sale of the remainder of our interests in Clarient in December 2010. Unrealized gains and losses on themark-to-market of our holdings in Clarient and realized gains and losses on the sale of any of our holdings in Clarient were recognized in Income (loss) from continuing operations in the Consolidated Statements of Operations.
Equity Method. We account for partner companies whose results are not consolidated, but over whom we exercise significant influence, using the equity method of accounting. We also account for our interests in some private equity funds under the equity method of accounting, based on our non-controlling general and limited partner interests. Under the equity method of accounting, our share of the income or loss of the company is reflected in Equity loss in the Consolidated Statements of Operations. We report our share of the income or loss of the equity method partner companies on a one quarter lag.
When the carrying value of our holdings in an equity method partner company is reduced to zero, no further losses are recorded in our Consolidated Statements of Operations unless we have outstanding guarantee obligations or have committed additional funding to the equity method partner company. When the equity method partner company subsequently reports income, we will not record our share of such income until it equals the amount of our share of losses not previously recognized.
Cost Method. We account for partner companies which are not consolidated or accounted for under the equity method or fair value method under the cost method of accounting. Under the cost method, our share of the income or losses of such partner companies is not included in the Company’s Consolidated Statements of Operations. The Company includes the carrying value of cost method partner companies in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
Available-for-Sale Securities. We account for our ownership interests in Tengion and NuPathe, our publicly traded partner companies, asavailable-for-sale securities.Available-for-sale securities are carried at fair value, based on quoted market prices, with the unrealized gains and losses, net of tax, reported as a separate component of equity. Unrealized losses are charged against net loss when a decline in the fair value is determined to be other than temporary.
Critical Accounting Policies and Estimates
Accounting policies, methods and estimates are an integral part of the Consolidated Financial Statements prepared by management and are based upon management’s current judgments. These judgments are normally based on knowledge and experience with regard to past and current events and assumptions about future events. Certain accounting policies, methods and estimates are particularly important because of their significance to the financial statements and because of the possibility that future events affecting them may differ from management’s current judgments. While there are a number of accounting policies, methods and estimates affecting our financial statements as described in Note 1 to our Consolidated Financial Statements, areas that are particularly significant include the following:
| | |
| | • | Impairment of ownership interests in and advances to partner companies; |
| |
| | • | Income taxes; |
| |
| | • | Commitments and contingencies; and |
| |
| | • | Stock-based compensation. |
25
Impairment of Ownership Interests In and Advances to Partner Companies
On a periodic basis, but no less frequently than at the end of each quarter, we evaluate the carrying value of our equity and cost method partner companies for possible impairment based on achievement of business plan objectives and milestones, the financial condition and prospects of the company, market conditions and other relevant factors. The business plan objectives and milestones we consider include, among others, those related to financial performance, such as achievement of planned financial results or completion of capital raising activities, and those that are not primarily financial in nature, such as hiring of key employees or the establishment of strategic relationships. We then determine whether there has been an other than temporary decline in the value of our ownership interest in the company. Impairment to be recognized is measured as the amount by which the carrying value of an asset exceeds its fair value. The adjusted carrying value of a partner company is not increased if circumstances suggest the value of the partner company has subsequently recovered.
The fair value of privately held partner companies is generally determined based on the value at which independent third parties have invested or have committed to invest in these companies, or based on other valuation methods including discounted cash flows, valuations of comparable public companies and valuations of acquisitions of comparable companies. The fair value of our ownership interests in private equity funds is generally determined based on the value of our pro rata portion of the funds’ net assets and estimated future proceeds from sales of investments provided by the funds’ managers. The fair value of our ownership interests in our publicly traded partner companies is determined by reference to quoted prices in an active market for the partner company’s publicly traded common stock.
Our partner companies operate in industries which are rapidly evolving and extremely competitive. It is reasonably possible that our accounting estimates with respect to the ultimate recoverability of the carrying value of ownership interests in and advances to partner companies could change in the near term and that the effect of such changes on our Consolidated Financial Statements could be material. While we believe that the current recorded carrying values of our equity and cost method companies are not impaired, there can be no assurance that our future results will confirm this assessment or that a significant write-down or write-off will not be required in the future.
Total impairment charges related to ownership interests in and advances to our equity and cost method partner companies were as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
Accounting Method | | 2010 | | | 2009 | | | 2008 | |
| | | (In millions) | |
| |
| Equity | | $ | 4.8 | | | $ | 4.1 | | | $ | 6.6 | |
| Cost | | | 2.1 | | | | 10.1 | | | | 2.3 | |
| Available-for-sale | | | 1.1 | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Total | | $ | 8.0 | | | $ | 14.2 | | | $ | 8.9 | |
| | | | | | | | | | | | | |
Impairment charges related to equity method partner companies are included in Equity loss in the Consolidated Statements of Operations. Impairment charges related to cost method andavailable-for-sale partner companies are included in Other income (loss), net in the Consolidated Statements of Operations.
Income Taxes
We are required to estimate income taxes in each of the jurisdictions in which we operate. This process involves estimating our actual current tax exposure together with assessing temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our Consolidated Balance Sheets. We must assess the likelihood that the deferred tax assets will be recovered from future taxable income and to the extent that we believe recovery is not likely, we must establish a valuation allowance. To the extent we establish a valuation allowance in a period, we must include an expense within the tax provision in the Consolidated Statements of Operations. We have recorded a valuation allowance to reduce our deferred tax assets to an amount that is more likely than not to be realized in future years. If we determine in the future that it is more likely than not that the net deferred tax assets would be realized, then the previously provided valuation allowance would be reversed.
26
Commitments and Contingencies
From time to time, we are a defendant or plaintiff in various legal actions which arise in the normal course of business. Additionally, we have received distributions as both a general partner and a limited partner from private equity funds. In certain circumstances, we may be required to return a portion or all the distributions we received as a general partner of a fund for a further distribution to such fund’s limited partners (“clawback”). We are also a guarantor of various third-party obligations and commitments and are subject to the possibility of various loss contingencies arising in the ordinary course of business (see Note 15). We are required to assess the likelihood of any adverse outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of provision required for these commitments and contingencies, if any, which would be charged to earnings, is made after careful analysis of each matter. The provision may change in the future due to new developments or changes in circumstances. Changes in the provision could increase or decrease our earnings in the period the changes are made.
Stock-Based Compensation
We measure all employee stock-based compensation awards using a fair value method and record such expense in our Consolidated Statements of Operations.
We estimate the grant date fair value of stock options using the Black-Scholes option-pricing model which requires the input of highly subjective assumptions. These assumptions include estimating the expected term of the award and the estimated volatility of our stock price over the expected term. Changes in these assumptions and in the estimated forfeitures of stock option awards can materially affect the amount of stock-based compensation recognized in the Consolidated Statements of Operations. The requisite service periods for market-based stock option awards are based on our estimate of the dates on which the market conditions will be met as determined using a Monte Carlo simulation model. Changes in the derived requisite service period or achievement of market capitalization targets earlier than estimated can materially affect the amount of stock-based compensation recognized in the Consolidated Statements of Operations. The requisite service periods for performance-based awards are based on our best estimate of when the performance conditions will be met. Compensation expense is recognized for performance-based awards for which the performance condition is considered probable of achievement. Changes in the requisite service period or the estimated probability of achievement of performance conditions can materially affect the amount of stock-based compensation recognized in the Consolidated Statements of Operations.
Results of Operations
Prior to deconsolidating Clarient on May 14, 2009, we presented Clarient as a separate segment. In December 2010 Clarient was acquired by GE Healthcare. From May 14, 2009 through the date of the sale of Clarient, we accounted for our retained interest in Clarient at fair value with unrealized gains and losses on themark-to-market of our Clarient holdings and realized gains and losses on the sale of any of our Clarient holdings included in Other income (loss), net in the Consolidated Statements of Operations. During 2009, we re-evaluated our reportable operating segments and we made the determination that Clarient would no longer be reported as a separate segment since we did not separately evaluate Clarient’s performance based upon its operating results. Themark-to-market activity associated with Clarient and the gain recorded upon its disposition is included in the Life Sciences segment. The results of operations of all of our partner companies are reported in our Life Sciences and Technology segments. The Life Sciences and Technology segments also include the gain or loss on the sale of respective partner companies, except for gains and losses included in discontinued operations.
On May 6, 2008, we consummated a transaction (the “Bundle Transaction’) pursuant to which we sold all of our equity and debt interests in Acsis, Inc. (“Acsis”), Alliance Consulting Group Associates, Inc. (“Alliance Consulting”), Laureate Pharma, Inc. (“Laureate Pharma”), ProModel Corporation (“ProModel”) and Neuronyx, Inc. (“Neuronyx”) (collectively, the “Bundle Companies”). Of the companies included in the Bundle Transaction, Acsis, Alliance Consulting and Laureate Pharma were majority-owned partner companies and Neuronyx and ProModel were minority-owned partner companies. We have presented the results of operations of Acsis, Alliance Consulting and Laureate Pharma as discontinued operations for all periods presented.
27
Our management evaluates the Life Sciences and Technology segments’ performance based on equity income (loss) which is based on the number of partner companies accounted for under the equity method, our voting ownership percentage in these partner companies and the net results of operations of these partner companies and Other income or loss associated with cost method partner companies.
Other items include certain expenses, which are not identifiable to the operations of our operating business segments. Other items primarily consist of general and administrative expenses related to corporate operations, including employee compensation, insurance and professional fees, interest income, interest expense, other income (loss) and equity income (loss) related to private equity holdings. Other Items also includes income taxes, which are reviewed by management independent of segment results.
The following tables reflect our consolidated operating data by reportable segment. Segment results include our share of income or losses for entities accounted for under the equity method, when applicable. Segment results also include impairment charges and gains or losses related to the disposition of partner companies, except for those reported in discontinued operations. All significant inter-segment activity has been eliminated in consolidation. Our operating results, including net income (loss) before income taxes by segment, were as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Life Sciences | | $ | 70,658 | | | $ | 99,289 | | | $ | (26,317 | ) |
| Technology | | | (10,003 | ) | | | (12,742 | ) | | | (12,947 | ) |
| | | | | | | | | | | | | |
| Total segments | | | 60,655 | | | | 86,547 | | | | (39,264 | ) |
| | | | | | | | | | | | | |
| Other items: | | | | | | | | | | | | |
| Corporate operations | | | (33,541 | ) | | | (19,763 | ) | | | (6,803 | ) |
| Income tax benefit | | | — | | | | 14 | | | | 24 | |
| | | | | | | | | | | | | |
| Total other items | | | (33,541 | ) | | | (19,749 | ) | | | (6,779 | ) |
| | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | | 27,114 | | | | 66,798 | | | | (46,043 | ) |
| Income (loss) from discontinued operations, net of tax | | | — | | | | 1,975 | | | | (9,620 | ) |
| | | | | | | | | | | | | |
| Net income (loss) | | | 27,114 | | | | 68,773 | | | | (55,663 | ) |
| Net (income) loss attributable to noncontrolling interest | | | — | | | | (1,163 | ) | | | 3,650 | |
| | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. | | $ | 27,114 | | | $ | 67,610 | | | $ | (52,013 | ) |
| | | | | | | | | | | | | |
There is intense competition in the markets in which our partner companies operate, and we expect competition to intensify in the future. Additionally, the markets in which these companies operate are characterized by rapidly changing technology, evolving industry standards, frequent introduction of new products and services, shifting distribution channels, evolving government regulation, frequently changing intellectual property landscapes and changing customer demands. Their future success depends on each company’s ability to execute its business plan and to adapt to its respective rapidly changing markets.
As previously stated, throughout this document, we use the term “partner company” to generally refer to those companies that we have an economic interest in and that we are actively involved in influencing the development of, usually through board representation in addition to our equity ownership stake.
For purposes of the following listing of our Life Science and Technology partner companies, we omit from the listing companies which we have since sold our interest in or which we no longer consider to be active partner companies because we no longer actively influence the operations of such entities.
28
Life Sciences
The following active partner companies as of December 31, 2010 were included in Life Sciences:
| | | | | | | | | | | | | | | |
| | | Safeguard Primary Ownership as of
| | |
| | | December 31, | | Accounting
|
Partner Company | | 2010 | | 2009 | | 2008 | | Method |
| |
| Advanced BioHealing | | | 28.1 | % | | | 28.3 | % | | | 28.3 | % | | Equity |
| Alverix | | | 49.6 | % | | | 49.6 | % | | | 50.0 | % | | Equity |
| Good Start Genetics | | | 26.3 | % | | | NA | | | | NA | | | Equity |
| Molecular Biometrics | | | 35.0 | % | | | 35.4 | % | | | 37.8 | % | | Equity |
| NuPathe | | | 18.1 | % | | | 22.9 | % | | | 23.5 | % | | Available-for-sale(1) |
| Tengion | | | 4.8 | % | | | 4.5 | % | | | 4.5 | % | | Available-for-sale(2) |
| | |
| (1) | | The Company’s ownership interest in NuPathe is accounted for asavailable-for-sale securities following NuPathe’ s completion of an initial public offering in August 2010. We previously accounted for NuPathe under the equity method. |
| |
| (2) | | The Company’s ownership interest in Tengion is accounted for asavailable-for-sale securities following Tengion’s completion of an initial public offering in April 2010. We previously accounted for Tengion under the cost method. |
Results for the Life Sciences segment were as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Revenue | | $ | — | | | $ | 34,839 | | | $ | 73,736 | |
| Operating Expenses: | | | | | | | | | | | | |
| Cost of sales | | | — | | | | 13,811 | | | | 33,007 | |
| Selling, general and administrative | | | — | | | | 19,407 | | | | 42,329 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | — | | | | 33,218 | | | | 75,336 | |
| | | | | | | | | | | | | |
| Operating income (loss) | | | — | | | | 1,621 | | | | (1,600 | ) |
| Other income (loss), net | | | 82,444 | | | | 114,222 | | | | — | |
| Interest income | | | — | | | | 4 | | | | 21 | |
| Interest expense | | | — | | | | (275 | ) | | | (880 | ) |
| Equity loss | | | (11,786 | ) | | | (16,283 | ) | | | (23,858 | ) |
| | | | | | | | | | | | | |
| Net income (loss) from continuing operations before income taxes | | $ | 70,658 | | | $ | 99,289 | | | $ | (26,317 | ) |
| | | | | | | | | | | | | |
Year ended December 31, 2010 versus year ended December 31, 2009
Results of operations for the year ended December 31, 2009 include the results of operations of Clarient for the 134 days in the period from January 1, 2009 through May 14, 2009 that Clarient was consolidated. Upon the deconsolidation of Clarient on May 14, 2009, we no longer reported revenue, cost of sales, selling, general and administrative expenses interest income and interest expense from Clarient’s continuing operations in our results of operations. Prior to that date, for the periods presented, all of our Life Science segment revenue, cost of sales, selling, general and administrative expenses, interest income and interest expense from continuing operations were attributable to Clarient.
Other Income (Loss), Net. Other Income (Loss), net in 2010 related primarily to a $43.0 million gain on the sale of Clarient to GE Healthcare in December 2010, a $20.3 million gain on the sale of Avid to Eli Lilly and Company in December 2010, unrealized gains of $22.4 million on themark-to-market of our holdings in Clarient
29
prior to its sale, partially offset by $3.2 million in impairment charges associated with our holdings in Tengion, including amounts recognized both when Tengion was classified as a cost method partner company and when Tengion was classified asavailable-for-sale.
On May 14, 2009, we deconsolidated our holdings in Clarient because we ceased to have a controlling financial interest in Clarient and recognized an unrealized gain on deconsolidation of $106.0 million as of that date. In addition, we recognized an unrealized gain of $19.5 million on themark-to-market of our holdings in Clarient through December 31, 2009, which was offset by a $7.3 million realized loss on the sale of 18.4 million shares of common stock of Clarient in the third quarter of 2009 and an impairment charge of $3.9 million related to our holdings in Tengion.
Equity Loss. Equity loss fluctuates with the number of Life Sciences partner companies accounted for under the equity method, our voting ownership percentage in these partner companies and the net results of operations of these partner companies. We recognize our share of losses to the extent we have cost basis in the equity of the partner company or we have outstanding commitments or guarantees. Certain amounts recorded to reflect our share of the income or losses of our partner companies accounted for under the equity method are based on estimates and on unaudited results of operations of those partner companies and may require adjustments in the future when audits of these entities are made final. We report our share of the results of our equity method partner companies on a one quarter lag basis.
Equity loss for Life Sciences decreased $4.5 million for the year ended December 31, 2010 compared to the prior year primarily due to an unrealized gain of $5.8 million on the decrease of our ownership interest in NuPathe upon completion of NuPathe’s initial public offering, partially offset by a $1.8 million impairment charge related to our holdings in Molecular Biometrics.
Year ended December 31, 2009 versus year ended December 31, 2008
Equity Loss. Equity loss for Life Sciences decreased $7.6 million for the year ended December 31, 2009 compared to 2008. Included in equity loss for the year ended December 31, 2009 were impairment charges and equity losses of $4.0 million associated with Rubicor Inc., a former partner company, and $0.8 million in impairment charges associated with Acelerate, Inc. (“Acelerate”, formerly Cellumen, Inc.), also a former partner company. Also included in 2008 Equity loss were expenses of $2.5 and $1.3 million associated with acquired in-process research and development related to our holdings in Molecular Biometrics and NuPathe, respectively.
Technology
The following active partner companies as of December 31, 2010 were included in Technology:
| | | | | | | | | | | | | | | |
| | | Safeguard Primary Ownership as of
| | | |
| | | December 31 | | | Accounting
|
Partner Company | | 2010 | | | 2009 | | | 2008 | | | Method |
| |
| AHS | | | 40.2 | % | | | 39.7 | % | | | 37.7 | % | | Equity |
| SafeCentral | | | 20.1 | % | | | 20.0 | % | | | 20.0 | % | | Equity |
| Beyond.com | | | 38.3 | % | | | 38.3 | % | | | 37.1 | % | | Equity |
| Bridgevine | | | 22.8 | % | | | 23.6 | % | | | 20.8 | % | | Equity |
| MediaMath | | | 17.3 | % | | | 17.5 | % | | | NA | | | Cost |
| Portico Systems | | | 45.4 | % | | | 45.4 | % | | | 46.8 | % | | Equity |
| Swap.com | | | 45.6 | % | | | 29.3 | % | | | 29.3 | % | | Equity |
30
Results for the Technology segment were as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31. | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Other income (loss), net | | $ | 36 | | | $ | (5,846 | ) | | $ | (2,251 | ) |
| Equity loss | | | (10,039 | ) | | | (6,896 | ) | | | (10,696 | ) |
| | | | | | | | | | | | | |
| Net loss from continuing operations before income taxes | | $ | (10,003 | ) | | $ | (12,742 | ) | | $ | (12,947 | ) |
| | | | | | | | | | | | | |
Year ended December 31, 2010 versus year ended December 31, 2009
Other Income (Loss), Net. Other income (loss), net decreased $5.9 million in 2010 compared to the prior year. The 2009 loss was entirely attributable to an impairment related to our holdings in GENBAND, a former partner company.
Equity Loss. Equity loss fluctuates with the number of Technology partner companies accounted for under the equity method, our voting ownership percentage in these partner companies and the net results of operations of these partner companies. We recognize our share of losses to the extent we have cost basis in the equity partner company or we have outstanding commitments or guarantees. Certain amounts recorded to reflect our share of the income or losses of our partner companies accounted for under the equity method are based on estimates and on unaudited results of operations of those partner companies and may require adjustments in the future when audits of these entities are made final. We report our share of the results of our equity method partner companies on a one quarter lag. Equity loss increased $3.1 million in 2010 compared to the prior year. The increase was due primarily to a $1.5 million impairment charge related to our holdings in SafeCentral, Inc. (“SafeCentral,” formerly Authentium, Inc.) as well as larger losses incurred at certain partner companies in 2010.
Year ended December 31, 2009 versus year ended December 31, 2008
Other Income (Loss), Net. Other income (loss), net increased $3.6 million in 2009 compared to the prior year. The 2009 loss was entirely attributable to an impairment related to our holdings in GENBAND, a former partner company. The 2008 loss reflected an impairment related to our holdings in Kadoo Inc., a former partner company.
Equity Loss. Equity loss for Technology decreased $3.8 million for the year ended December 31, 2009 compared to the prior year. The decrease was due principally to smaller losses incurred at certain partner companies and an impairment charge of $2.6 million related to our holdings in SafeCentral recorded in 2008.
Corporate Operations
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| General and administrative | | $ | (16,949 | ) | | $ | (14,695 | ) | | $ | (16,511 | ) |
| Stock-based compensation | | | (3,777 | ) | | | (2,982 | ) | | | (1,738 | ) |
| Depreciation | | | (121 | ) | | | (130 | ) | | | (166 | ) |
| Interest income | | | 718 | | | | 476 | | | | 3,076 | |
| Interest expense | | | (5,737 | ) | | | (2,889 | ) | | | (3,852 | ) |
| Other income (loss), net | | | (7,671 | ) | | | 505 | | | | 12,531 | |
| Equity loss | | | (4 | ) | | | (48 | ) | | | (143 | ) |
| | | | | | | | | | | | | |
| | | $ | (33,541 | ) | | $ | (19,763 | ) | | $ | (6,803 | ) |
| | | | | | | | | | | | | |
Year Ended December 31, 2010 versus year ended December 31, 2009
General and Administrative. Our general and administrative expenses consist primarily of employee compensation, insurance, professional services such as legal, accounting and consulting, and travel-related costs.
31
General and administrative expenses increased $2.3 million in 2010 as compared to the prior year primarily due to a $1.8 million increase in employee costs and a $0.7 million increase in professional fees partially offset by a $0.4 million decrease in insurance costs. General and administrative expenses decreased $1.8 million in 2009 as compared to the prior year, primarily due to a $1.5 million decrease in severance costs, a $0.9 million decrease in professional fees and a $0.9 million decrease in other administrative expenses offset by an increase in employee costs of $1.5 million.
Stock-Based Compensation. Stock-based compensation consists primarily of expense related to grants of stock options, restricted stock and deferred stock units to our employees.
Stock-based compensation increased $0.8 million in 2010 compared to the prior year, primarily due to higher expense related to restricted stock units driven by the acceleration of expense recognized for grants to directors and executives who have reached retirement age and higher expense related to market-based awards due to acceleration of expense based on increases in our stock price. Stock-based compensation increased $1.2 million in 2009 as compared to the prior year, primarily due to stock option forfeitures during 2008. Stock-based compensation expense related to market-based awards was $1.7 million, $1.5 million and $0.4 million in 2010, 2009 and 2008, respectively. Stock-based compensation expense related to service-based awards was $1.2 million, $1.0 million and $1.1 million in 2010, 2009 and 2008, respectively. Stock-based compensation expense related to corporate operations is included in Selling, general and administrative expenses in the Consolidated Statements of Operations.
Interest Income. Interest income includes all interest earned on cash and marketable security balances.
Interest income increased $0.2 million in 2010 compared to the prior year. The increase was due to higher average cash balances in the second half of 2010. Interest income decreased $2.6 million in 2009 compared to the prior year. The decrease was due to declining interest rates and lower average cash balances over 2008.
Interest Expense. Interest expense is primarily related to our 2024 and 2014 Debentures. As discussed below under Liquidity and Capital Resources, we exchanged a portion of our 2024 Debentures effective March 26, 2010. The increase in interest expense of $2.8 million compared to the prior year is related to the higher coupon rate of 10.125% payable on our 2014 Debentures as compared to a 2.625% coupon rate on the 2024 Debentures and accretion of the discount and amortization of debt issuance costs in the amount of $0.5 million associated with our 2014 Debentures.
Interest expense decreased $1.0 million in 2009 as compared to the prior period. The decline was attributable to the repurchase of $7.8 million in face value of the 2024 Debentures in 2009.
Other Income (Loss), net. Other income (loss), net in 2010 included an $8.5 million loss on exchange of $46.9 million in face value of our convertible senior debentures, partially offset by a $0.3 million gain on sales of legacy assets and $0.3 million related to a change in our estimated net clawback liability attributable to a private equity fund.
Other income (loss), net in 2008 included a net gain of $9.0 million on the repurchase of $43 million in face value of the 2024 Debentures, a $2.5 million net gain on the sale of company interests, including the receipt of escrowed funds from a legacy asset and a $1.0 million gain on distributions from private equity funds.
Income Tax (Expense) Benefit
Our consolidated net income tax (expense) benefit for 2010, 2009 and 2008 was $0.0 million in each year. We have recorded a valuation allowance to reduce our net deferred tax asset to an amount that is more likely than not to be realized in future years. Accordingly, the income tax expense that would have been recognized in 2010 and 2009 and the net operating loss benefit that would have been recognized in 2008 was offset by changes in the valuation allowance.
Discontinued Operations
The following are reported in discontinued operations for all periods through their respective sale date.
32
In May 2008, we sold all of our equity and debt interests in Acsis, Alliance Consulting, Laureate Pharma, ProModel and Neuronyx in the Bundle Transaction. The gross proceeds from the Bundle Transaction were $74.5 million, of which $6.4 million was placed in escrow pending expiration of a claims period, plus amounts advanced to certain Bundle Companies during the time between the signing of the Bundle Transaction agreement and its consummation.
In March 2007, we sold Pacific Title & Art Studio for net cash proceeds of approximately $21.9 million, including $2.3 million held in escrow. As a result of the sale, we recorded a pre-tax gain of $2.7 million in 2007. In the first quarter of 2010, we received the final $0.5 million in cash from the escrow account. This amount was recorded as income from discontinued operations in 2009.
In March 2007, Clarient sold its technology business and related intellectual property for an aggregate purchase price of $12.5 million. The $12.5 million consisted of $11.0 million in cash and an additional $1.5 million in contingent consideration, subject to the satisfaction of certain post-closing conditions through March 2009. Clarient received the contingent consideration and recorded the $1.5 million in income from discontinued operations in 2009.
Income from discontinued operations in 2009 of $2.0 million was attributable to the receipt by Clarient of $1.5 million contingent consideration, prior to the deconsolidation of Clarient, and our receipt of $0.5 million from escrow related to the sale of Pacific Title & Art Studio. The loss from discontinued operations in 2008 of $9.2 million was primarily attributable to operating losses incurred by Acsis, Alliance Consulting and Laureate Pharma of $1.6 million, an impairment loss of $3.6 million related to the write down of the aggregate carrying value of the Bundle Companies to the anticipated net proceeds, a $1.4 million pre-tax gain on disposal of the Bundle Companies, a $0.9 million charge to accrue for severance payments due the former CEO of Alliance Consulting and charges totaling $2.7 million related to additional compensation paid to the former CEO of Pacific Title & Art Studio in connection with the March 2007 sale and related legal fees.
Liquidity And Capital Resources
Parent Company
We fund our operations with cash on hand as well as proceeds from sales of and distributions from partner companies, private equity funds and marketable securities. In prior periods, we have also used sales of our equity and the issuance of debt as sources of liquidity and may do so in the future. Our ability to generate liquidity from sales of partner companies, sales of marketable securities and from equity and debt issuances has been adversely affected from time to time by adverse circumstances in the U.S. capital markets and other factors.
As of December 31, 2010, we had $183.4 million of cash and cash equivalents and $42.4 million of marketable securities for a total of $225.8 million. In addition, $6.4 million of cash was held in escrow, including accrued interest, related to the Bundle Transaction and $16.8 million was held in a restricted escrow account to service interest on the 2014 Debentures, as discussed below.
In December 2010, Clarient was acquired by GE Healthcare, a unit of GE, via a public tender offer for all outstanding common and preferred shares of Clarient, followed by a second step merger of Clarient with an indirect subsidiary of GE. In connection with the transactions, we received gross proceeds of $153.4 million and paid retention bonuses to Clarient management in the amount of $6.9 million, resulting in net proceeds of $146.5 million. We held a 27.5% primary ownership stake in Clarient at the time of the sale.
In December 2010, Avid was acquired by Eli Lily and Company resulting in net proceeds to us of $32.3 million. We held a 13% primary ownership interest in Avid at the time of the sale. Depending on the achievement of certain milestones, we could receive additional proceeds of $60.0 million, as well as an additional $3.4 million currently being held in escrow.
In December 2010, we received cash proceeds of $2.6 million on the sale of Quinnova Pharmaceuticals, Inc. Depending on certain milestones, we could receive additional proceeds of $2.2 million.
In connection with the Bundle Transaction, an aggregate of $6.4 million of the gross proceeds from the sale were placed in escrow pending the expiration of a predetermined notification period, subject to possible extension
33
in the event of a claim against the escrowed amounts. On April 25, 2009, the purchaser in the Bundle Transaction notified us of claims being asserted against the entire escrowed amounts. We do not believe that such claims are valid and have instituted legal action to obtain the release of such amounts from escrow. The proceeds being held in escrow will remain there until the dispute over the claims have been settled or determined pursuant to legal process.
In February 2004, we completed the sale of $150 million of 2.625% convertible senior debentures with a stated maturity of March 15, 2024 (the “2024 Debentures”). Through December 31, 2010, we have repurchased a total of $71.8 million in face value of the 2024 Debentures. Interest on the 2024 Debentures is payable semi-annually. At the debentures holders’ option, the 2024 Debentures are convertible into our common stock through March 14, 2024, subject to certain conditions. The adjusted conversion rate of the debentures is $43.3044 of principal amount per share. The closing price of our common stock at December 31, 2010 was $17.08. The 2024 Debentures holders have the right to require us to repurchase the 2024 Debentures on March 21, 2011, March 20, 2014 or March 20, 2019 at a repurchase price equal to 100% of their face amount, plus accrued and unpaid interest. The 2024 Debentures holders also have the right to require repurchase of the 2024 Debentures upon certain events, including sale of all or substantially all of our common stock or assets, liquidation, dissolution, a change in control or the delisting of our common stock from the New York Stock Exchange if we were unable to obtain a listing for our common stock on another national or regional securities exchange. Subject to certain conditions, we have the right to redeem all or some of the 2024 Debentures. We expect the holders of the 2024 Debentures to exercise their rights to require us to repurchase the entire $31.3 million in face value of the outstanding 2024 Debentures on March 21, 2011.
On March 10, 2010, we entered into agreements with institutional holders of an aggregate of $46.9 million in face value of our 2024 Debentures to exchange the 2024 Debentures held by such holders for $46.9 million in face amount of newly issued 10.125% senior convertible debentures, due 2014 (the “2014 Debentures”). The exchange became effective on March 26, 2010. The remaining $31.3 million outstanding face amount of the 2024 Debentures remains outstanding under the original terms and has been classified as a current liability on the Consolidated Balance Sheet as of December 31, 2010 because the first required repurchase date is within one year. We recorded a loss on exchange of $8.5 million determined as the excess of the fair value of the 2014 Debentures at the exchange date over the carrying value of the exchanged 2024 Debentures. This loss is reported in Other income (loss), net in the Consolidated Statements of Operations for the year ended December 31, 2010.
At December 31, 2010, the fair value of the $31.3 million outstanding 2024 Debentures was approximately $30.8 based on quoted market prices as of such date.
As discussed above, in March 2010, we issued $46.9 million in face value of our 2014 Debentures in an exchange transaction. Interest on the 2014 Debentures is payable semi-annually. As required by the terms of the 2014 Debentures, at issuance we placed approximately $19.0 million in a restricted escrow account to service interest associated with the 2014 Debentures through their maturity. At the debentures holders’ option, the 2014 Debentures are convertible into our common stock prior to March 15, 2013 subject to certain conditions, and at anytime after March 15, 2013. The conversion rate of the 2014 Debentures is $16.50 of principal amount per share. The closing price of our common stock at December 31, 2010 was $17.08. The 2014 Debentures holders have the right to require repurchase of the 2014 Debentures upon certain events, including sale of all or substantially all of our common stock or assets, liquidation, dissolution, a change in control or the delisting of our common stock from the New York Stock Exchange if we were unable to obtain a listing for our common stock on another national or regional securities exchange. Subject to certain conditions, we may mandatorily convert all or some of the 2014 Debentures at any time after March 15, 2012. If we elect to mandatorily convert any of the 2014 Debentures, we will be required to pay any interest that would have accrued and become payable on the debentures through their maturity. Upon a conversion of the 2014 Debentures, we have the right to settle the conversion in stock, cash or a combination thereof.
Because the 2014 Debentures may be settled in cash or partially in cash upon conversion, we have separately accounted for the liability and equity components of the 2014 Debentures. The carrying amount of the liability component was determined at the exchange date by measuring the fair value of a similar liability that does not have an associated equity component. The carrying amount of the equity component represented by the embedded conversion option was determined by deducting the fair value of the liability component from the carrying value of the 2014 Debentures as a whole. The carrying value of the 2014 Debentures as a whole was equal to their fair value
34
at the exchange date. We are amortizing the excess of the face value of the 2014 Debentures over their carrying value to interest expense over their term. At December 31, 2010, the fair value of the $46.9 million outstanding 2014 Debentures was approximately $63.4 million based on a convertible bond valuation model.
In May 2008, our Board of Directors authorized us, from time to time and depending on market conditions, to repurchase shares of our outstanding common stock, with up to an aggregate value of $10.0 million, exclusive of fees and commissions. Approximately 4 thousand fractional shares were repurchased in 2009 for $44 thousand in connection with ourone-for-six stock split in August 2009. During the year ended December 31, 2008, we repurchased approximately 163 thousand shares of common stock at a cost of $1.3 million.
We are party to a loan agreement which provides us with a revolving credit facility in the maximum aggregate amount of $50 million in the form of borrowings, guarantees and issuances of letters of credit (subject to a $20 million sublimit). Actual availability under the credit facility is based on the amount of cash maintained at the bank as well as the value of our public and private partner company interests. This credit facility bears interest at the prime rate for outstanding borrowings, subject to an increase in certain circumstances. Other than for limited exceptions, we are required to maintain all of our depository and operating accounts and the lesser of $80 million or 75% of our investment and securities accounts at the bank. The credit facility, as amended December 31, 2010, matures on December 31, 2012. Under the credit facility, we provided a $6.3 million letter of credit expiring on March 19, 2019 to the landlord of CompuCom Systems, Inc.’s Dallas headquarters which has been required in connection with our sale of CompuCom Systems in 2004. Availability under our revolving credit facility at December 31, 2010 was $43.7 million.
We have committed capital of approximately $0.4 million, including conditional commitments to provide partner companies with additional funding and commitments made to various private equity funds in prior years. These commitments are expected to be funded in the next 12 months.
The transactions we enter into in pursuit of our strategy could increase or decrease our liquidity at any point in time. As we seek to acquire interests in technology and life sciences companies, provide additional funding to existing partner companies, or commit capital to other initiatives, we may be required to expend our cash or incur debt, which will decrease our liquidity. Conversely, as we dispose of our interests in partner companies from time to time, we may receive proceeds from such sales, which could increase our liquidity. From time to time, we are engaged in discussions concerning acquisitions and dispositions which, if consummated, could impact our liquidity, perhaps significantly.
In May 2001, we entered into a $26.5 million loan agreement with Warren V. Musser, our former Chairman and Chief Executive Officer. In December 2006, we restructured the obligation to reduce the amount outstanding to $14.8 million, bearing interest at a rate of 5.0% per annum. Since 2001 and through December 31, 2010, we have received a total of $16.8 million in payments on the loan. We received nominal amounts of cash from the sale of collateral in 2009 and no payments in 2010. We received cash from the sale of collateral in early 2011 in the amount of $0.1 million. As a result, the carrying value of the loan at December 31, 2010 was $0.1 million.
We have received distributions as both a general partner and a limited partner from certain private equity funds. Under certain circumstances, we may be required to return a portion or all the distributions we received as a general partner of a fund for further distribution to such fund’s limited partners (“clawback”). The maximum clawback we could be required to return related to our general partner interest is $2.2 million, of which $0.9 million was reflected in accrued expenses and other current liabilities and $1.3 million was reflected in Other long-term liabilities on the Consolidated Balance Sheet at December 31, 2010. We paid $1.1 million of our estimated clawback liabilities in 2010.
Our previous ownership in the general partners of the funds that have potential clawback liabilities ranges from19-30%. The clawback liability is joint and several, such that we may be required to fund the clawback for other general partners should they default. The funds have taken several steps to reduce the potential liabilities should other general partners default, including withholding all general partner distributions and placing them in escrow and adding rights of set-off among certain funds. We believe our potential liability due to the possibility of default by other general partners is remote.
35
For the reasons we presented above, we believe our cash and cash equivalents at December 31, 2010, availability under our revolving credit facility and other internal sources of cash flow will be sufficient to fund our cash requirements for at least the next 12 months, including debt repayments, commitments to our existing companies and funds, possible additional funding of existing partner companies and our general corporate requirements. Our acquisition of new partner company interests is always contingent upon our availability of cash to fund such deployments, and our timing of monetization events directly affects our availability of cash.
Analysis of Parent Company Cash Flows
Given no partner companies were consolidated during the year ended December 31, 2010, only Parent Company Cash Flows for the years ended December 31, 2009 and 2008 are presented below. Cash flow activity was as follows:
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Net cash used in operating activities | | $ | (14,682 | ) | | $ | (14,124 | ) |
| Net cash provided by investing activities | | | 15,861 | | | | 27,327 | |
| Net cash used in financing activities | | | (7,045 | ) | | | (34,675 | ) |
| | | | | | | | | |
| | | $ | (5,866 | ) | | $ | (21,472 | ) |
| | | | | | | | | |
Cash Used In Operating Activities
Year ended December 31, 2009 versus year ended December 31, 2008. Net cash used in operating activities increased $0.6 million in 2009 as compared to 2008. The increase is primarily attributable to $1.2 million in cash used for interest payments on the 2024 Debentures in 2009 for which interest payments were previously made using restricted marketable securities held in escrow, partially offset by lower corporate operating expenditures.
Cash Provided by Investing Activities
Year ended December 31, 2009 versus year ended December 31, 2008. Net cash provided by investing activities decreased $11.5 million in 2009 as compared to 2008. The decrease was attributable to an $84.5 million decrease in proceeds from the sale of discontinued operations, a $10.3 million net increase in purchases of marketable securities and a $5.4 million increase in cash paid to acquire ownership interests in companies and funds, partially offset by a $57.0 million increase in proceeds from sales of and distributions from companies and funds, a $16.5 million decrease in advances to partner companies, a $14.3 million increase in repayment of advances from partner companies and a $0.9 decrease in restricted cash.
Cash Used In Financing Activities
Year ended December 31, 2009 versus year ended December 31, 2008. Net cash used in financing activities decreased $27.6 million. The decrease was primarily attributable to a $26.3 million decrease in cash used to repurchase certain of our 2024 Debentures, as well as a $1.3 million decrease in cash used to repurchase our common stock as compared to the prior year.
Consolidated Working Capital From Continuing Operations
Consolidated working capital from continuing operations increased to $197.8 at December 31, 2010 compared to $106.0 at December 31, 2009. The increase was primarily due to the sale of our interests in Clarient and Avid in December 2010 resulting in net sale proceeds to Safeguard of $146.5 and $32.3 million, respectively.
36
Analysis of Consolidated Cash Flows
Cash flow activity was as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Net cash used in operating activities | | $ | (16,019 | ) | | $ | (19,170 | ) | | $ | (21,514 | ) |
| Net cash provided by investing activities | | | 131,856 | | | | 47 | | | | 32,805 | |
| Net cash provided by (used in) financing activities | | | 235 | | | | 11,419 | | | | (31,386 | ) |
| | | | | | | | | | | | | |
| | | $ | 116,072 | | | $ | (7,704 | ) | | $ | (20,095 | ) |
| | | | | | | | | | | | | |
Cash Used In Operating Activities
Year ended December 31, 2010 versus year ended December 31, 2009. Net cash used in operating activities decreased $3.2 million in 2010 as compared to the prior year. The change was primarily related to cash used in operating activities of Clarient in the prior year period prior to its deconsolidation of $4.5 million, partially offset by cash used for interest payments on the 2024 and 2014 Debentures.
Year ended December 31, 2009 versus year ended December 31, 2008. Net cash used in operating activities decreased $2.3 million in 2009 as compared to the prior year. The decrease was primarily attributable to the deconsolidation of Clarient. The decrease was partially offset by $1.2 million in cash used for interest payments on the 2024 Debentures in 2009 for which interest payments were previously made using restricted marketable securities held in escrow, partially offset by lower corporate operating expenditures.
Cash Provided by Investing Activities
Year ended December 31, 2010 versus year ended December 31, 2009. Net cash provided by investing activities increased $131.8 million in 2010 as compared to the prior year. The increase was primarily related to a $122.5 million increase in proceeds from sales of and distributions from companies and funds, a $21.0 million net decrease in cash paid to acquire marketable securities, a $15.5 million decrease in cash paid to acquire ownership interests in partner companies and funds, a $2.0 million decrease in restricted cash, a $2.1 million decrease in capital expenditures and a $2.7 million decrease in cash related to the deconsolidation of subsidiary cash in the prior year period, partially offset by $18.9 million of cash transferred to escrow to service interest payments on the 2014 Debentures, a $10.4 million increase in advances to partner companies and a $3.7 million decrease in repayment of advances to partner companies.
Year ended December 31, 2009 versus year ended December 31, 2008. Net cash provided by investing activities decreased $32.8 million in 2009 as compared to the prior year. The decrease was attributable to an $82.2 million decrease in proceeds from the sale of discontinued operations, a $10.3 million net increase in purchases of marketable securities, a $5.4 million increase in cash paid to acquire ownership interests in companies and funds, a $2.0 million increase in restricted cash and a $2.7 million decrease in cash resulting from the deconsolidation of Clarient, partially offset by a $57.0 million increase in proceeds from sales of and distributions from companies and funds, a $2.9 million decrease in advances to partner companies, a $5.7 million increase in repayment of advances from partner companies, a $2.8 million decrease in the cash used in discontinued operations, and a $1.4 million decrease in capital expenditures.
Cash Provided by (Used In) Financing Activities
Year ended December 31, 2010 versus year ended December 31, 2009. Net cash provided by financing activities decreased by $11.2 million in 2010 as compared to the prior year. The decrease was primarily related to a $28.1 million decrease in proceeds received from the issuance of subsidiary common stock, partially offset by a $9.5 million reduction in payments on revolving credit facilities, a $7.3 million reduction in the repurchase of convertible senior debentures and a $0.8 million increase related to the issuance of our common stock associated with stock option exercises.
37
Year ended December 31, 2009 versus year ended December 31, 2008. Net cash provided by financing activities increased $42.8 million in 2009 as compared to the prior year. The increase was primarily related to a $27.1 million increase in proceeds from the issuance of subsidiary common stock, a $26.3 million decrease in cash used to repurchase certain of our 2024 Debentures and a $1.3 million decrease in cash used to repurchase our common stock in the prior year period, partially offset by $7.1 million net repayments of outstanding borrowings and a $4.8 million decrease in cash provided from financing activities of discontinued operations.
Contractual Cash Obligations and Other Commercial Commitments
The following table summarizes our contractual obligations and other commercial commitments as of December 31, 2010, by period due or expiration of the commitment.
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
| | | | | | | | | 2012 and
| | | 2014 and
| | | Due after
| |
| | | Total | | | 2011 | | | 2013 | | | 2015 | | | 2015 | |
| | | (In millions) | |
| |
| Contractual Cash Obligations: | | | | | | | | | | | | | | | | | | | | |
| Convertible senior debentures(a) | | $ | 78.2 | | | $ | 31.3 | | | $ | — | | | $ | 46.9 | | | $ | — | |
| Operating leases | | | 1.9 | | | | 0.5 | | | | 1.0 | | | | 0.4 | | | | — | |
| Funding commitments(b) | | | 0.4 | | | | 0.4 | | | | — | | | | — | | | | — | |
| Potential clawback liabilities(c) | | | 2.2 | | | | 0.9 | | | | 1.3 | | | | — | | | | — | |
| Other long-term obligations(d) | | | 4.0 | | | | 0.8 | | | | 1.5 | | | | 1.5 | | | | 0.2 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total Contractual Cash Obligations | | $ | 86.7 | | | $ | 33.9 | | | $ | 3.8 | | | $ | 48.8 | | | $ | 0.2 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | Amount of Commitment Expiration by Period | |
| | | | | | | | | 2012 and
| | | 2014 and
| | | After
| |
| | | Total | | | 2011 | | | 2013 | | | 2015 | | | 2015 | |
| | | | | | | | | (In millions) | | | | |
| |
| Other Commitments: | | | | | | | | | | | | | | | | | | | | |
| Letters of credit(e) | | $ | 6.3 | | | $ | — | | | $ | — | | | $ | — | | | $ | 6.3 | |
| | | | | | | | | | | | | | | | | | | | | |
| | |
| (a) | | In February 2004, we completed the issuance of $150.0 million of our 2024 Debentures with a stated maturity of March 15, 2024. Through December 31, 2010, we have repurchased $71.8 million in face value of the 2024 Debentures. The 2024 Debentures holders have the right to require the Company to repurchase the remaining outstanding 2024 Debentures on March 21, 2011, March 20, 2014 or March 20, 2019 at a repurchase price equal to 100% of their respective face amount, plus accrued and unpaid interest. On March 10, 2010, we entered into agreements with institutional holders of an aggregate of $46.9 million in face value of our 2024 Debentures to exchange the 2024 Debentures held by such holders for $46.9 million in face amount of our 2014 Debentures. The exchange became effective on March 26, 2010. Although contractually due in 2024, the remaining $31.3 million outstanding face amount of the 2024 Debentures has been classified as due in 2011 to reflect the first required repurchase date and conform with the presentation of the 2024 Debentures as a current liability on the Consolidated Balance Sheet at December 31, 2010. |
| |
| (b) | | These amounts include $0.4 million in conditional commitments to provide non-consolidated partner companies with additional funding. Also included are funding commitments to private equity funds which have been included in the respective years based on estimated timing of capital calls provided to us by the funds’ management. |
| |
| (c) | | We have received distributions as both a general partner and a limited partner from certain private equity funds. Under certain circumstances, we may be required to return a portion or all the distributions we received as a general partner of a fund for a further distribution to such fund’s limited partners (“clawback”). The maximum clawback we could be required to return is approximately $2.2 million, of which $0.9 million was reflected in Accrued expenses and other current liabilities and $1.3 million was reflected in Other long-term liabilities on the Consolidated Balance Sheets. |
| |
| (d) | | Reflects the estimated amount payable to our former Chairman and CEO under an ongoing agreement. |
| |
| (e) | | A $6.3 million letter of credit is provided to the landlord of CompuCom’s Dallas headquarters lease as required in connection with our sale of CompuCom in 2004. |
38
We have agreements with certain employees that provide for severance payments to the employee in the event the employee is terminated without cause or if the employee terminates his employment for “good reason.” The maximum aggregate cash exposure under the agreements was approximately $8 million at December 31, 2010.
We remain guarantor of Laureate Pharma’s Princeton, New Jersey facility lease (the “Laureate Lease Guaranty”). Such guarantee may extend through the lease expiration in 2016 under certain circumstances. However, we are entitled to indemnification in connection with the continuation of such guaranty. As of December 31, 2010, scheduled lease payments to be made by Laureate Pharma over the remaining lease term equaled $7.1 million.
As of December 31, 2010, Safeguard had federal net operating loss carryforwards and federal capital loss carryforwards totaling approximately $266.5 million. The net operating loss carryforwards expire in various amounts from 2011 to 2030. The capital loss carryforwards expire in 2013.
We are involved in various claims and legal actions arising in the ordinary course of business. In the opinion of management, the ultimate disposition of these matters will not have a material adverse effect on the consolidated financial position or results of operations.
| |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We are exposed to equity price risks on the marketable portion of our ownership interests in our partner companies. At December 31, 2010, these interests include our equity positions in NuPathe and Tengion, our publicly-traded partner companies, which have experienced significant volatility in their stock prices. Historically, we have not attempted to reduce or eliminate our market exposure related to these types of interests. Based on closing market prices at December 31, 2010, the fair market value of our holdings in NuPathe and Tengion were $24.0 million and $1.5 million, respectively. A 20% decrease in NuPathe and Tengion’s stock price would result in an approximate $5.1 million decrease in the aggregate fair value of our holdings in these companies.
In February 2004, we completed the issuance of $150.0 million of our 2024 Debentures with a stated maturity of March 15, 2024. Through March 31, 2010, we repurchased $71.8 million in face value of the 2024 Debentures. Interest payments are due in March and September of each year. The holders of these 2024 Debentures have the right to require repurchase of the remaining outstanding 2024 Debentures on March 21, 2011, March 20, 2014 or March 20, 2019 at a repurchase price equal to 100% of their face amount plus accrued and unpaid interest. On March 10, 2010, we entered into agreements with institutional holders of an aggregate of $46.9 million in face value of our 2024 Debentures to exchange the 2024 debentures held by such holders for $46.9 million in face amount of our 2014 Debentures. The exchange became effective on March 26, 2010. Although contractually due in 2024, the remaining $31.3 million outstanding face amount of the 2024 Debentures has been classified as due in 2011 to reflect the first required repurchase date and to conform with the presentation of the 2024 Debentures as a current liability on the Consolidated Balance Sheet at December 31, 2010.
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Fair
|
| | | | | | | | | After
| | Value at
|
Liabilities | | 2011 | | 2012 | | 2013 | | 2013 | | December 31, 2010 |
| |
| 2024 Debentures due by year (in millions) | | $ | 31.3 | | | $ | — | | | $ | — | | | $ | — | | | $ | 30.8 | |
| Fixed interest rate | | | 2.625 | % | | | 2.625 | % | | | 2.625 | % | | | 2.625 | % | | | N/A | |
| Interest expense (in millions) | | $ | 0.8 | | | $ | 0.8 | | | $ | 0.8 | | | $ | 8.4 | | | | N/A | |
| 2014 Debentures due by year (in millions) | | $ | — | | | $ | — | | | $ | — | | | $ | 46.9 | | | $ | 63.4 | |
| Fixed interest rate | | | 10.125 | % | | | 10.125 | % | | | 10.125 | % | | | 10.125 | % | | | N/A | |
| Interest expense (in millions) | | $ | 5.5 | | | $ | 5.6 | | | $ | 5.8 | | | $ | 1.2 | | | | N/A | |
We have historically had very low exposure to changes in foreign currency exchange rates, and as such, have not used derivative financial instruments to manage foreign currency fluctuation risk.
We maintain cash and cash equivalents and marketable securities with various financial institutions. The financial institutions are highly rated.
39
| |
| Item 8. | Financial Statements and Supplementary Data |
The following Consolidated Financial Statements, and the related Notes thereto, of Safeguard Scientifics, Inc. and the Reports of Independent Registered Public Accounting Firm are filed as a part of thisForm 10-K.
| | | | | |
| | | Page |
| |
| | | 41 | |
| | | 42 | |
| | | 43 | |
| | | 44 | |
| | | 45 | |
| | | 46 | |
| | | 47 | |
| | | 48 | |
40
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Safeguard Scientifics, Inc.:
We have audited Safeguard Scientifics, Inc.’s (the Company) internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Safeguard Scientifics, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting (Item 9A.(b)). Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Safeguard Scientifics, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Safeguard Scientifics, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, comprehensive income (loss), changes in equity and cash flows for each of the years in the three-year period ended December 31, 2010, and our report dated March 4, 2011 expressed an unqualified opinion on those consolidated financial statements.
Philadelphia, Pennsylvania
March 4, 2011
41
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Safeguard Scientifics, Inc.:
We have audited the accompanying consolidated balance sheets of Safeguard Scientifics, Inc. (the “Company”) and subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of operations, comprehensive income (loss), changes in equity and cash flows for each of the years in the three-year period ended December 31, 2010. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Safeguard Scientifics, Inc. and subsidiaries as of December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Safeguard Scientifics, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 4, 2011 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Philadelphia, Pennsylvania
March 4, 2011
42
| | | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands except per share data) | |
| |
ASSETS |
| Current Assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 183,419 | | | $ | 67,347 | |
| Cash held in escrow | | | 6,434 | | | | 6,910 | |
| Marketable securities | | | 42,411 | | | | 39,066 | |
| Restricted cash equivalents | | | 4,893 | | | | — | |
| Prepaid expenses and other current assets | | | 785 | | | | 566 | |
| | | | | | | | | |
| Total current assets | | | 237,942 | | | | 113,889 | |
| Property and equipment, net | | | 295 | | | | 310 | |
| Ownership interests in and advances to partner companies ($80,483 at fair value at December 31, 2009) | | | 60,761 | | | | 167,387 | |
Available-for-sale securities | | | 25,447 | | | | — | |
| Long-term restricted cash equivalents | | | 11,881 | | | | — | |
| Other | | | 724 | | | | 513 | |
| | | | | | | | | |
| Total Assets | | $ | 337,050 | | | $ | 282,099 | |
| | | | | | | | | |
| |
| LIABILITIES AND EQUITY |
| Current Liabilities: | | | | | | | | |
| Convertible senior debentures — current | | $ | 31,289 | | | $ | — | |
| Accounts payable | | | 493 | | | | 156 | |
| Accrued compensation and benefits | | | 4,168 | | | | 3,425 | |
| Accrued expenses and other current liabilities | | | 4,223 | | | | 4,325 | |
| | | | | | | | | |
| Total current liabilities | | | 40,173 | | | | 7,906 | |
| Other long-term liabilities | | | 5,311 | | | | 5,461 | |
| Convertible senior debentures — non-current | | | 44,630 | | | | 78,225 | |
| Commitments and contingencies | | | | | | | | |
| Equity: | | | | | | | | |
| Preferred stock, $0.10 par value; 1,000 shares authorized | | | — | | | | — | |
| Common stock, $0.10 par value; 83,333 shares authorized; 20,630 and 20,420 shares issued and outstanding in 2010 and 2009, respectively | | | 2,063 | | | | 2,042 | |
| Additional paid-in capital | | | 806,859 | | | | 790,868 | |
| Accumulated deficit | | | (574,802 | ) | | | (601,916 | ) |
| Accumulated other comprehensive income | | | 12,816 | | | | — | |
| Treasury stock, at cost | | | — | | | | (487 | ) |
| | | | | | | | | |
| Total equity | | | 246,936 | | | | 190,507 | |
| | | | | | | | | |
| Total Liabilities and Equity | | $ | 337,050 | | | $ | 282,099 | |
| | | | | | | | | |
See Notes to Consolidated Financial Statements.
43
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands except per share data) | |
| |
| Revenue | | $ | — | | | $ | 34,839 | | | $ | 73,736 | |
| | | | | | | | | | | | | |
| Operating Expenses: | | | | | | | | | | | | |
| Cost of sales | | | — | | | | 13,811 | | | | 33,007 | |
| Selling, general and administrative | | | 20,847 | | | | 37,214 | | | | 60,744 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 20,847 | | | | 51,025 | | | | 93,751 | |
| | | | | | | | | | | | | |
| Operating loss | | | (20,847 | ) | | | (16,186 | ) | | | (20,015 | ) |
| Other income (loss), net | | | 74,809 | | | | 108,881 | | | | 10,280 | |
| Interest income | | | 718 | | | | 480 | | | | 3,097 | |
| Interest expense | | | (5,737 | ) | | | (3,164 | ) | | | (4,732 | ) |
| Equity loss | | | (21,829 | ) | | | (23,227 | ) | | | (34,697 | ) |
| | | | | | | | | | | | | |
| Net income (loss) from continuing operations before income taxes | | | 27,114 | | | | 66,784 | | | | (46,067 | ) |
| Income tax benefit | | | — | | | | 14 | | | | 24 | |
| | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | | 27,114 | | | | 66,798 | | | | (46,043 | ) |
| Income (loss) from discontinued operations, net of tax | | | — | | | | 1,975 | | | | (9,620 | ) |
| | | | | | | | | | | | | |
| Net income (loss) | | | 27,114 | | | | 68,773 | | | | (55,663 | ) |
| Net (income) loss attributable to noncontrolling interest | | | — | | | | (1,163 | ) | | | 3,650 | |
| | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. | | $ | 27,114 | | | $ | 67,610 | | | $ | (52,013 | ) |
| | | | | | | | | | | | | |
| Basic Income (Loss) Per Share: | | | | | | | | | | | | |
| Net income (loss) from continuing operations attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.32 | | | $ | 3.26 | | | $ | (2.10 | ) |
| Net income (loss) from discontinued operations attributable to Safeguard Scientifics, Inc. common shareholders | | | — | | | | 0.07 | | | | (0.46 | ) |
| | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.32 | | | $ | 3.33 | | | $ | (2.56 | ) |
| | | | | | | | | | | | | |
| Diluted Income (Loss) Per Share: | | | | | | | | | | | | |
| Net income (loss) from continuing operations attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.26 | | | $ | 3.08 | | | $ | (2.10 | ) |
| Net income (loss) from discontinued operations attributable to Safeguard Scientifics, Inc. common shareholders | | | — | | | | 0.06 | | | | (0.46 | ) |
| | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.26 | | | $ | 3.14 | | | $ | (2.56 | ) |
| | | | | | | | | | | | | |
| Average shares used in computing income (loss) per share: | | | | | | | | | | | | |
| Basic | | | 20,535 | | | | 20,308 | | | | 20,326 | |
| | | | | | | | | | | | | |
| Diluted | | | 21,507 | | | | 22,383 | | | | 20,326 | |
| | | | | | | | | | | | | |
| Amounts attributable to Safeguard Scientifics, Inc. common shareholders: | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | $ | 27,114 | | | $ | 66,240 | | | $ | (42,777 | ) |
| Net income (loss) from discontinued operations | | | — | | | | 1,370 | | | | (9,236 | ) |
| | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. | | $ | 27,114 | | | $ | 67,610 | | | $ | (52,013 | ) |
| | | | | | | | | | | | | |
See Notes to Consolidated Financial Statements.
44
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Net income (loss) | | $ | 27,114 | | | $ | 68,773 | | | $ | (55,663 | ) |
| Other comprehensive income (loss), before taxes: | | | | | | | | | | | | |
Unrealized net gain onavailable-for-sale securities | | | 11,708 | | | | — | | | | — | |
Reclassification adjustment for other than temporary impairment ofavailable-for-sale securities included in net income (loss) | | | 1,108 | | | | — | | | | — | |
| Foreign currency translation adjustments | | | — | | | | (2 | ) | | | (54 | ) |
| Reclassification adjustment for deconsolidation of subsidiary | | | — | | | | 31 | | | | — | |
| | | | | | | | | | | | | |
| Total comprehensive income (loss) | | | 39,930 | | | | 68,802 | | | | (55,717 | ) |
| Comprehensive (income) loss attributable to the noncontrolling interest | | | — | | | | (1,163 | ) | | | 3,650 | |
| | | | | | | | | | | | | |
| Comprehensive income (loss) attributable to Safeguard Scientifics, Inc. | | $ | 39,930 | | | $ | 67,639 | | | $ | (52,067 | ) |
| | | | | | | | | | | | | |
See Notes to Consolidated Financial Statements.
45
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Accumulated
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Other
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Comprehensive
| | | | | | | | | Additional
| | | | | | | | | | |
| | | | | | Accumulated
| | | Income
| | | Common Stock | | | Paid-In
| | | Treasury Stock | | | Noncontrolling
| |
| | | Total | | | Deficit | | | (Loss) | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Interest | |
| | | | | | | | | | | | | | | | | | (In thousands) | | | | | | | | | | |
| |
Balance — December 31, 2007 | | $ | 155,831 | | | $ | (617,513 | ) | | $ | 25 | | | | 20,187 | | | $ | 2,019 | | | $ | 768,608 | | | | — | | | $ | — | | | $ | 2,692 | |
| Net loss | | | (55,663 | ) | | | (52,013 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,650 | ) |
| Stock options exercised, net | | | 115 | | | | — | | | | — | | | | 4 | | | | — | | | | 33 | | | | (12 | ) | | | 82 | | | | — | |
| Issuance of restricted stock, net | | | 255 | | | | — | | | | — | | | | 74 | | | | 7 | | | | 251 | | | | 5 | | | | (3 | ) | | | — | |
| Stock-based compensation expense— continuing and discontinued operations | | | 3,911 | | | | — | | | | — | | | | — | | | | — | | | | 3,911 | | | | — | | | | — | | | | — | |
| Repurchase of common stock | | | (1,296 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 162 | | | | (1,296 | ) | | | | |
| Gain on change in interest of equity method partner company | | | 653 | | | | | | | | | | | | — | | | | — | | | | 653 | | | | | | | | | | | | | |
| Impact of subsidiary equity transactions | | | 958 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 958 | |
| Other comprehensive loss | | | (54 | ) | | | — | | | | (54 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance — December 31, 2008 | | | 104,710 | | | | (669,526 | ) | | | (29 | ) | | | 20,265 | | | | 2,026 | | | | 773,456 | | | | 155 | | | | (1,217 | ) | | | — | |
| Net income | | | 68,773 | | | | 67,610 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,163 | |
| Stock options exercised, net | | | 270 | | | | — | | | | — | | | | 34 | | | | 3 | | | | 267 | | | | (1 | ) | | | — | | | | — | |
| Issuance of restricted stock, net | | | 225 | | | | — | | | | — | | | | 121 | | | | 13 | | | | (1,038 | ) | | | (157 | ) | | | 1,250 | | | | — | |
| Stock-based compensation expense | | | 3,825 | | | | — | | | | — | | | | — | | | | — | | | | 3,825 | | | | — | | | | — | | | | — | |
| Repurchase of common stock | | | (44 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4 | | | | (44 | ) | | | — | |
| Note receivable repayment in Company common stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | 476 | | | | 43 | | | | (476 | ) | | | — | |
| Impact of subsidiary equity transactions | | | 12,750 | | | | — | | | | 31 | | | | — | | | | — | | | | 13,882 | | | | — | | | | — | | | | (1,163 | ) |
| Other comprehensive loss | | | (2 | ) | | | — | | | | (2 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance — December 31, 2009 | | | 190,507 | | | | (601,916 | ) | | | — | | | | 20,420 | | | | 2,042 | | | | 790,868 | | | | 44 | | | | (487 | ) | | | — | |
| Net income | | | 27,114 | | | | 27,114 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Stock options exercised, net | | | 1,107 | | | | — | | | | — | | | | 102 | | | | 10 | | | | 923 | | | | (18 | ) | | | 174 | | | | — | |
| Issuance of restricted stock, net | | | 142 | | | | — | | | | — | | | | 84 | | | | 9 | | | | 133 | | | | 3 | | | | — | | | | — | |
| Stock-based compensation expense | | | 3,777 | | | | — | | | | — | | | | — | | | | — | | | | 3,777 | | | | — | | | | — | | | | — | |
| Equity component of convertible senior debentures issued, net of issuance costs | | | 10,842 | | | | — | | | | — | | | | — | | | | — | | | | 10,842 | | | | — | | | | — | | | | — | |
| Stock awards | | | 631 | | | | — | | | | — | | | | 24 | | | | 2 | | | | 316 | | | | (29 | ) | | | 313 | | | | — | |
| Other comprehensive income | | | 12,816 | | | | — | | | | 12,816 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance — December 31, 2010 | | $ | 246,936 | | | $ | (574,802 | ) | | $ | 12,816 | | | | 20,630 | | | $ | 2,063 | | | $ | 806,859 | | | | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See Notes to Consolidated Financial Statements.
46
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
Cash Flows from Operating Activities: | | | | | | | | | | | | |
| Net income (loss) | | $ | 27,114 | | | $ | 68,773 | | | $ | (55,663 | ) |
| Adjustments to reconcile to net cash used in operating activities: | | | | | | | | | | | | |
| (Income) loss from discontinued operations | | | — | | | | (1,975 | ) | | | 9,620 | |
| Depreciation and amortization | | | 121 | | | | 1,425 | | | | 3,551 | |
| Equity loss | | | 21,829 | | | | 23,227 | | | | 34,697 | |
| Other (income) loss, net | | | (74,809 | ) | | | (108,881 | ) | | | (10,280 | ) |
| Bad debt expense | | | — | | | | 3,936 | | | | 12,199 | |
| Stock-based compensation expense | | | 3,777 | | | | 3,825 | | | | 3,449 | |
| Changes in assets and liabilities, net of effect of acquisitions and dispositions: | | | | | | | | | | | | |
| Accounts receivable, net | | | (195 | ) | | | (11,467 | ) | | | (20,495 | ) |
| Accounts payable, accrued expenses, deferred revenue and other | | | 6,144 | | | | 1,967 | | | | 4,696 | |
| Cash flows from operating activities of discontinued operations | | | — | | | | — | | | | (3,288 | ) |
| | | | | | | | | | | | | |
| Net cash used in operating activities | | | (16,019 | ) | | | (19,170 | ) | | | (21,514 | ) |
| | | | | | | | | | | | | |
Cash Flows from Investing Activities: | | | | | | | | | | | | |
| Investment in restricted cash equivalents for interest on convertible senior debentures | | | (18,864 | ) | | | — | | | | — | |
| Proceeds from sales of and distributions from partner companies and funds | | | 183,813 | | | | 61,302 | | | | 4,263 | |
| Advances and loans to partner companies | | | (11,710 | ) | | | (1,350 | ) | | | (4,210 | ) |
| Repayment of advances to partner companies | | | 2,009 | | | | 5,679 | | | | — | |
| Acquisitions of ownership interests in partner companies and funds, net of cash acquired | | | (20,418 | ) | | | (35,939 | ) | | | (30,496 | ) |
| Increase in marketable securities | | | (65,201 | ) | | | (73,187 | ) | | | (75,809 | ) |
| Decrease in marketable securities | | | 61,856 | | | | 48,822 | | | | 61,698 | |
| Increase in restricted cash, net | | | — | | | | (1,956 | ) | | | — | |
| Capital expenditures | | | (106 | ) | | | (2,157 | ) | | | (3,530 | ) |
| Deconsolidation of subsidary cash | | | — | | | | (2,667 | ) | | | — | |
| Proceeds from sale of discontinued operations, net | | | 477 | | | | 1,500 | | | | 83,756 | |
| Cash flows from investing activities of discontinued operations | | | — | | | | — | | | | (2,867 | ) |
| | | | | | | | | | | | | |
| Net cash provided by investing activities | | | 131,856 | | | | 47 | | | | 32,805 | |
| | | | | | | | | | | | | |
Cash Flows from Financing Activities: | | | | | | | | | | | | |
| Costs on exchange of convertible senior debentures | | | (872 | ) | | | — | | | | — | |
| Repurchase of convertible senior debentures | | | — | | | | (7,271 | ) | | | (33,494 | ) |
| Borrowings on revolving credit facilities | | | — | | | | 23,726 | | | | 37,633 | |
| Repayments on revolving credit facilities | | | — | | | | (33,237 | ) | | | (37,526 | ) |
| Repayments on term debt | | | — | | | | (107 | ) | | | (2,574 | ) |
| Issuance of Company common stock, net | | | 1,107 | | | | 270 | | | | 115 | |
| Issuance of subsidiary equity, net | | | — | | | | 28,082 | | | | 966 | |
| Repurchase of Company common stock | | | — | | | | (44 | ) | | | (1,296 | ) |
| Cash flows from financing activities of discontinued operations | | | — | | | | — | | | | 4,790 | |
| | | | | | | | | | | | | |
| Net cash provided by (used in) financing activities | | | 235 | | | | 11,419 | | | | (31,386 | ) |
| | | | | | | | | | | | | |
| Net Increase (Decrease) in Cash and Cash Equivalents | | | 116,072 | | | | (7,704 | ) | | | (20,095 | ) |
| Changes in Cash and Cash Equivalents from and Advances to Acsis, Alliance Consulting and Laureate Pharma included in assets of discontinued operations | | | — | | | | — | | | | (1,055 | ) |
| | | | | | | | | | | | | |
| | | | 116,072 | | | | (7,704 | ) | | | (21,150 | ) |
| Cash and Cash Equivalents at beginning of period | | | 67,347 | | | | 75,051 | | | | 96,201 | |
| | | | | | | | | | | | | |
| Cash and Cash Equivalents at end of period | | $ | 183,419 | | | $ | 67,347 | | | $ | 75,051 | |
| | | | | | | | | | | | | |
See Notes to Consolidated Financial Statements.
47
SAFEGUARD SCIENTIFICS, INC.
| |
| 1. | Significant Accounting Policies |
Description of the Company
Safeguard Scientifics, Inc. (“Safeguard” or the “Company”) seeks to build value in growth-stage technology and life sciences businesses by providing partner companies with capital and a range of strategic, operational and management resources. The Company may participate in expansion financings, corporate spin-outs, management buy-outs, recapitalizations, industry consolidations and early-stage financings. The Company’s vision is to be the preferred catalyst for creating great technology and life sciences companies.
The Company strives to create long-term value for its shareholders by helping its partner companies increase market penetration, grow revenue and improve cash flow. Safeguard principally focuses on companies in which it anticipates deploying up to $25 million and that operate in two categories:
Life Sciences — including companies focused on molecular andpoint-of-care diagnostics, medical devices, regenerative medicine, specialty pharmaceuticals and selected healthcare services; and
Technology — including companies focused on internet/new media, financial services IT, healthcare IT and selected business services that have transaction-enabling applications with a recurring revenue stream.
Basis of Presentation
The Company’s Consolidated Financial Statements include the accounts of Clarient Inc. (“Clarient”) in continuing operations through May 14, 2009, the date of its deconsolidation. Clarient was acquired by GE Healthcare in December 2010. The Company had elected to apply the fair value option to account for its retained interest in Clarient upon deconsolidation. Unrealized gains and losses on themark-to-market of its holdings in Clarient and realized gains and losses on the sale of any of its holdings in Clarient were recognized in Other income (loss), net in the Consolidated Statement of Operations for all periods subsequent to the date that Clarient was deconsolidated through the date of its disposition. See Note 3. The Company believes that accounting for its holdings in Clarient at fair value rather than applying the equity method of accounting provided a better measure of the value of its holdings, given the reliable evidence provided by quoted prices in an active market for Clarient’s publicly traded common stock. The Company has not elected the fair value option for its other partner company holdings, which are accounted for under the equity method or cost method, due to less readily determinable evidence of fair value for these privately held companies and due to the potential competitive disadvantage to the Company of such disclosure.
The Company’s ownership interests in Tengion, Inc. (“Tengion”) and NuPathe, Inc. (“NuPathe”) are accounted for asavailable-for-sale securities following Tengion’s and NuPathe’s completion of initial public offerings in April 2010 and August 2010, respectively.Available-for-sale securities are carried at fair value, based on quoted market prices, with the unrealized gains and losses, net of tax, reported as a separate component of equity. Unrealized losses are charged against net income (loss) when a decline in the fair value is determined to be other than temporary.
During 2008, certain consolidated partner companies, or components thereof, were sold and are reported in discontinued operations. See Note 2.
Principles of Accounting for Ownership Interests in Companies
The Company’s ownership interests in its partner companies and private equity funds are accounted for using one of the following methods: consolidation, equity, cost, fair value andavailable-for-sale. The accounting method applied is generally determined by the degree of the Company’s influence over the entity, primarily determined by its voting interest in the entity.
48
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In addition to holding voting and non-voting equity and debt securities, the Company also periodically makes advances to its partner companies in the form of promissory notes which are included in the Ownership interests in and advances to partner companies line item in the Consolidated Balance Sheet.
Consolidation Method. The Company generally accounts for partner companies in which it directly or indirectly owns more than 50% of the outstanding voting securities under the consolidation method of accounting. Under this method, the Company includes the partner companies’ financial statements within the Company’s Consolidated Financial Statements, and all significant intercompany accounts and transactions are eliminated. The Company reflects participation of other stockholders in the net assets and in the income or losses of these consolidated partner companies in Equity in the Consolidated Balance Sheets and in Net (income) loss attributable to noncontrolling interest in the Statements of Operations. Net (income) loss attributable to noncontrolling interest adjusts the Company’s consolidated operating results to reflect only the Company’s share of the earnings or losses of the consolidated partner company. The Company accounts for results of operations and cash flows of a consolidated partner company through the latest date in which it holds a controlling interest. If the Company subsequently relinquishes control but retains an interest in the partner company, the accounting method is adjusted to the equity, cost or fair value method of accounting, as appropriate. As of and for the year ended December 31, 2010, the Company did not hold a controlling interest in any of its partner companies.
Fair Value Method. The Company accounted for its holdings in Clarient, one of the Company’s publicly traded partner companies, under the fair value method of accounting following its deconsolidation on May 14, 2009 and through the date of the sale of the Company’s remaining interest in Clarient in December 2010. Unrealized gains and losses on themark-to-market of the Company’s holdings in Clarient and realized gains and losses on the sale of any holdings in Clarient were recognized in Other income (loss), net in the Consolidated Statements of Operations.
Equity Method. The Company accounts for partner companies whose results are not consolidated, but over which it exercises significant influence, under the equity method of accounting. Whether or not the Company exercises significant influence with respect to a partner company depends on an evaluation of several factors including, among others, representation of the Company on the partner company’s board of directors and the Company’s ownership level, which is generally a 20% to 50% interest in the voting securities of a partner company, including voting rights associated with the Company’s holdings in common, preferred and other convertible instruments in the company. The Company also accounts for its interests in some private equity funds under the equity method of accounting based on its non-controlling general and limited partner interests in such funds. Under the equity method of accounting, the Company does not reflect a partner company’s financial statements within the Company’s Consolidated Financial Statements; however, the Company’s share of the income or loss of such partner company is reflected in Equity loss in the Consolidated Statements of Operations. The Company includes the carrying value of equity method partner companies in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets. The Company reflects its share of the income or loss of the equity method partner companies on a one quarter lag. This reporting lag could result in a delay in recognition of the impact of changes in the business or operations of these partner companies.
When the Company’s carrying value in an equity method partner company is reduced to zero, the Company records no further losses in its Consolidated Statements of Operations unless the Company has an outstanding guarantee obligation or has committed additional funding to such equity method partner company. When such equity method partner company subsequently reports income, the Company will not record its share of such income until it exceeds the amount of the Company’s share of losses not previously recognized.
Cost Method. The Company accounts for partner companies not consolidated or accounted for under the equity method or fair value method under the cost method of accounting. Under the cost method, the Company does not include its share of the income or losses of partner companies in the Company’s Consolidated Statements of Operations. The Company includes the carrying value of cost method partner companies in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
49
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Available-for-Sale Securities. The Company accounts for its ownership interests in Tengion and NuPathe, the Company’s publicly traded partner companies at December 31, 2010, asavailable-for-sale securities.Available-for-sale securities are carried at fair value, based on quoted market prices, with the unrealized gains and losses, net of tax, reported as a separate component of equity. Unrealized losses are charged against net income (loss) when a decline in the fair value is determined to be other than temporary.
Accounting Estimates
The preparation of the Consolidated Financial Statements in accordance with accounting principles generally accepted in the United States of America requires management to make estimates and judgments that affect amounts reported in the financial statements and accompanying notes. Actual results may differ from these estimates. These estimates include the evaluation of the recoverability of the Company’s ownership interests in and advances to partner companies and investments in marketable securities, income taxes, stock-based compensation and commitments and contingencies. Following the deconsolidation of Clarient on May 14, 2009, the Company no longer records goodwill, intangible assets or revenue in its consolidated financial statements. Management evaluates its estimates on an ongoing basis using historical experience and other factors, including the current economic environment, which management believes to be reasonable under the circumstances.
Certain amounts recorded to reflect the Company’s share of income or losses of partner companies accounted for under the equity method are based on unaudited results of operations of those companies and may require adjustments in the future when audits of these entities’ financial statements are completed.
It is reasonably possible that the Company’s accounting estimates with respect to the ultimate recoverability of the carrying value of the Company’s ownership interests in and advances to partner companies could change in the near term and that the effect of such changes on the financial statements could be material. At December 31, 2010, the Company believes the recorded amount of carrying value of the Company’s ownership interests in and advances to partner companies is not impaired, although there can be no assurance that the Company’s future results will confirm this assessment, that a significant write-down or write-off will not be required in the future, or that a significant loss will not be recorded in the future upon the sale of a company.
Cash and Cash Equivalents and Short-Term Marketable Securities
The Company considers all highly liquid instruments with an original maturity of 90 days or less at the time of purchase to be cash equivalents. Cash and cash equivalents consist of deposits that are readily convertible into cash. The Company determines the appropriate classification of marketable securities at the time of purchase and reevaluates such designation as of each balance sheet date.Held-to-maturity securities are carried at amortized cost, which approximates fair value. Short-term marketable securities consist ofheld-to-maturity securities, primarily consisting of commercial paper and certificates of deposits. The Company has not experienced any significant losses on cash equivalents and does not believe it is exposed to any significant credit risk on cash and cash equivalents.
Restricted Cash Equivalents
Restricted cash equivalents consist of certificates of deposit with various maturity dates. Pursuant to the terms of the 10.125% senior convertible debentures, due March 14, 2014, the Company placed funds in a restricted escrow account to make all scheduled interest payments on the 2014 Debentures through their maturity date (See Note 7).
Financial Instruments
The Company’s financial instruments (principally cash and cash equivalents, marketable securities, restricted cash equivalents, accounts receivable, notes receivable, accounts payable and accrued expenses) are carried at cost,
50
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
which approximates fair value due to the short-term maturity of these instruments. The Company’s long-term debt is carried at cost. At December 31, 2010, the market value of the Company’s outstanding 2024 and 2014 Debentures was approximately $30.8 million and $63.4 million, respectively based on quoted market prices as of that date.
Property and Equipment
Property and equipment are stated at cost. Provision for depreciation and amortization is based on the lesser of the estimated useful lives of the assets or the remaining lease term (buildings and leasehold improvements, 5 to 15 years; machinery and equipment, 3 to 15 years) and is computed using the straight-line method.
Impairment of Ownership Interests In and Advances to Partner Companies
On a periodic basis, but no less frequently than quarterly, the Company evaluates the carrying value of its equity and cost method partner companies andavailable-for-sale securities for possible impairment based on achievement of business plan objectives and milestones, the fair value of each partner company relative to its carrying value, the financial condition and prospects of the partner company and other relevant factors. The business plan objectives and milestones the Company considers include, among others, those related to financial performance, such as achievement of planned financial results or completion of capital raising activities, and those that are not primarily financial in nature, such as hiring of key employees or the establishment of strategic relationships. Management then determines whether there has been an other than temporary decline in the value of its ownership interest in the company. Impairment is measured by the amount by which the carrying value of an asset exceeds its fair value.
The fair value of privately held companies is generally determined based on the value at which independent third parties have invested or have committed to invest in these companies or based on other valuation methods, including discounted cash flows, valuation of comparable public companies and the valuation of acquisitions of similar companies. The fair value of the Company’s ownership interests in private equity funds generally is determined based on the value of its pro rata portion of the fair value of the funds’ net assets.
Impairment charges related to equity method partner companies are included in Equity loss in the Consolidated Statements of Operations. Impairment charges related to cost method partner companies are included in Other income (loss), net in the Consolidated Statements of Operations.
The reduced cost basis of a previously impaired partner company is notwritten-up if circumstances suggest the value of the company has subsequently recovered.
Defined Contribution Plans
Defined contribution plans are contributory and cover eligible employees of the Company. The Company’s defined contribution plan allows eligible employees, as defined in the plan, to contribute to the plan up to 75% of their pre-tax compensation, subject to the maximum contributions allowed by the Internal Revenue Code. The Company may make matching contributions under the plan. Expense relating to defined contribution plans was $0.3 million in 2010, $0.4 million in 2009 and $0.3 million in 2008.
Income Taxes
The Company accounts for income taxes under the asset and liability method whereby deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. The Company measures deferred tax assets and liabilities using enacted tax rates in effect for the year in which the temporary differences are expected to be recovered or settled. The Company recognizes the effect on deferred tax assets and liabilities of a change in tax rates in income in the period of the enactment date. The Company provides valuation allowances against the net deferred tax asset for amounts which are not considered more likely than not to be realized.
51
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Net income (loss) per share attributable to Safeguard Scientifics, Inc.
The Company computes net income (loss) per share (EPS) using the weighted average number of common shares outstanding during each year. The Company includes in diluted EPS common stock equivalents (unless anti-dilutive) which would arise from the exercise of stock options and conversion of other convertible securities and is adjusted, if applicable, for the effect on net income (loss) of such transactions. Diluted EPS calculations adjust net income (loss) for the dilutive effect of common stock equivalents and convertible securities issued by the Company’s consolidated or equity method partner companies.
Comprehensive income (loss) attributable to Safeguard Scientifics, Inc.
Comprehensive income (loss) is the change in equity of a business enterprise during a period from non-owner sources. Excluding net income (loss), the Company’s sources of other comprehensive income (loss) are from net unrealized appreciation (depreciation) onavailable-for-sale securities and foreign currency translation adjustments. Reclassification adjustments result from the recognition in net income (loss) of unrealized gains or losses that were included in comprehensive income (loss) in prior periods.
Segment Information
The Company reports segment data based on the management approach which designates the internal reporting used by management for making operating decisions and assessing performance as the source of the Company’s reportable operating segments.
| |
| 2. | Discontinued Operations |
The following are reported in discontinued operations for all periods through their respective sale date.
Acsis, Alliance Consulting and Laureate Pharma
In May 2008, the Company consummated a transaction (the “Bundle Transaction”) pursuant to which it sold all of its equity and debt interests in Acsis, Inc. (“Acsis”), Alliance Consulting Group Associates, Inc. (“Alliance Consulting”), Laureate Pharma, Inc. (“Laureate Pharma”), ProModel Corporation (“ProModel”) and Neuronyx, Inc. (“Neuronyx”) (collectively, the “Bundle Companies”).
Of the companies included in the Bundle Transaction, Acsis, Alliance Consulting and Laureate Pharma were consolidated partner companies and Neuronyx and ProModel were minority-owned partner companies. The Company has presented the results of operations of Acsis, Alliance Consulting and Laureate Pharma as discontinued operations for all periods presented.
During 2008, the Company recognized an impairment loss of $3.6 million to write down the aggregate carrying value of the Bundle Companies to the total anticipated proceeds, less estimated costs to complete the Bundle Transaction. Prior to the completion of the Bundle Transaction, the Company recorded a net loss of $1.6 million in discontinued operations related to the operations of Acsis, Alliance Consulting and Laureate Pharma. The Company recorded a charge of $0.9 million in discontinued operations to accrue for severance payments due to the former CEO of Alliance Consulting in connection with the Bundle Transaction and recorded a pre-tax gain on disposal of $1.4 million which was also recorded in discontinued operations.
The gross proceeds to the Company from the Bundle Transaction were $74.5 million, of which $6.4 million was placed in escrow pending expiration of a claims period (see Note 15), plus amounts advanced to certain of the Bundle Companies during the time between the signing of the Bundle Transaction agreement and its consummation.
52
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Clarient Technology Business
In March 2007, Clarient sold its technology business and related intellectual property to Carl Zeiss MicroImaging, Inc. (“Zeiss”) for an aggregate purchase price of $12.5 million. The $12.5 million consisted of $11.0 million in cash and an additional $1.5 million in contingent purchase price, subject to the satisfaction of certain post-closing conditions through March 2009. Clarient received the contingent consideration and recorded the $1.5 million in income from discontinued operations in 2009.
Pacific Title & Art Studio
In March 2007, the Company sold Pacific Title & Art Studio for net cash proceeds of approximately $21.9 million, including $2.3 million in cash deposited into escrow. During 2008, the Company recorded a loss of $2.7 million, which was included within Income (loss) from discontinued operations in the Consolidated Statements of Operations, related to additional compensation paid to the former CEO of Pacific Title & Art Studio in connection with the March 2007 sale and related legal fees (see Note 15). Pacific Title & Art Studio is reported in discontinued operations for all periods presented. In the first quarter of 2010, the Company received the final $0.5 million in cash from the escrow account. This amount was recorded as income from discontinued operations in 2009.
There was no activity associated with discontinued operations in 2010. Results of discontinued operations for 2009 and 2008 were as follows:
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Revenue | | $ | — | | | $ | 45,712 | |
| Operating expenses | | | — | | | | (49,652 | ) |
| Impairment of carrying value | | | — | | | | (3,634 | ) |
| Other | | | — | | | | (1,547 | ) |
| | | | | | | | | |
| Loss from operations before income taxes | | | — | | | | (9,121 | ) |
| Income tax benefit | | | — | | | | — | |
| | | | | | | | | |
| Loss from operations | | | — | | | | (9,121 | ) |
| Gain (loss) on disposal, net of tax | | | 1,975 | | | | (499 | ) |
| | | | | | | | | |
| Income (loss) from discontinued operations | | $ | 1,975 | | | $ | (9,620 | ) |
| | | | | | | | | |
53
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| |
| 3. | Ownership Interests in and Advances to Partner Companies |
The following summarizes the carrying value of the Company’s ownership interests in and advances to partner companies and private equity funds.
| | | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands) | |
| |
| Fair Value: | | $ | — | | | $ | 80,483 | |
| Equity Method: | | | | | | | | |
| Partner companies | | | 44,412 | | | | 54,597 | |
| Private equity funds | | | 2,265 | | | | 2,224 | |
| | | | | | | | | |
| | | | 46,677 | | | | 56,821 | |
| Cost Method: | | | | | | | | |
| Partner companies | | | 6,654 | | | | 24,887 | |
| Private equity funds | | | 2,908 | | | | 3,096 | |
| | | | | | | | | |
| | | | 9,562 | | | | 27,983 | |
| Advances and loans to partner companies | | | 4,522 | | | | 2,100 | |
| | | | | | | | | |
| | | $ | 60,761 | | | $ | 167,387 | |
| | | | | | | | | |
Available-for-sale securities | | $ | 25,447 | | | $ | — | |
| | | | | | | | | |
The Company recognized a $5.8 million unrealized gain in the third quarter of 2010 which is reflected in Equity loss in the Consolidated Statements of Operations related to the decrease in its percentage ownership interest in NuPathe upon completion of NuPathe’s initial public offering. As discussed in Note 1, following NuPathe’s initial public offering, the Company accounts for its holdings in NuPathe asavailable-for-sale securities. As of December 31, 2010, the Company’s adjusted cost basis inavailable-for-sale securities of NuPathe was $10.8 million. As of December 31, 2010 the Company’s holdings ofavailable-for-sale securities in NuPathe had generated an unrealized gain of $13.2 million which was reflected in Accumulated other comprehensive income, a separate component of equity on the Consolidated Balance Sheet.
The Company recognized an impairment charge of $1.1 million for the year ended December 31, 2010 representing the unrealized loss on themark-to-market of its ownership interest in Tengion, which was previously recorded as a separate component of equity. The Company determined that the decline in the value of its public holdings in Tengion was other than temporary. Following the impairment charge, the Company’s adjusted cost basis in Tengion was $1.8 million. As of December 31, 2010 the Company’s holdings ofavailable-for-sale securities in Tengion had generated an unrealized loss of $0.3 million which was reflected in Accumulated other comprehensive income, a separate component of equity on the Consolidated Balance Sheet.
Impairment charges related to cost method partner companies were $2.1 million, $10.1 million and $2.3 million for the years ended December 31, 2010, 2009 and 2008, respectively. The charge in 2010 related to Tengion, prior to its classification as anavailable-for-sale security. The charges in 2009 included $5.8 million related to GENBAND, a former partner company, $3.9 million related to Tengion and $0.4 million related to a private equity fund. The charge in 2008 related to Kadoo, Inc. (“Kadoo”), a former partner company. Impairment charges related to cost method partner companies are included in Other income (loss), net in the Consolidated Statements of Operations.
Impairment charges related to equity method partner companies were $4.8 million, $4.1 million and $6.6 million for the years ended December 31, 2010, 2009 and 2008 respectively. The impairment charges in 2010 included $1.8 million related to Molecular Biometrics, Inc. (“Molecular Biometrics”), $1.5 million related to
54
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
SafeCentral, Inc. (“SafeCentral” formerly Authentium, Inc.), $1.1 million related to Garnet BioTherapeutics, Inc. (“Garnet”) and $0.4 million related to Acelerate, Inc. (“Acelerate”, formerly Cellumen, Inc.), a former partner company. The impairment charges in 2009 included $3.3 million related to Rubicor Medical, Inc. (“Rubicor”), a former partner company and $0.8 million impairment related to Acelerate. The Company previously recognized a $4.0 million impairment charge related to Rubicor in 2008 based on estimates of fair value provided by an independent valuation firm and a range of values indicated by potential investors in Rubicor. During 2008, the Company also recognized an impairment charge of $2.6 million related to SafeCentral, Inc. Impairment charges associated with equity method partner companies are included in Equity loss in the Consolidated Statements of Operations.
In December 2010, Avid Radiopharmaceuticals, Inc. (“Avid”), formerly a cost method partner company, was acquired by Eli Lily and Company resulting in net sale proceeds to the Company of $32.3 million, excluding cash held in escrow of $3.4 million. The Company recognized a gain on the sale of $20.3 million. Under the terms of the definitive agreement, the Company may realize additional proceeds of up to $60 million based on the achievement by Avid of several regulatory and commercial milestones over a period of eight years.
In December 2010, the Company sold its equity and debt interests in Quinnova Pharmaceuticals, Inc. (“Quinnova”) for $2.6 million, recognizing a loss on sale of $0.9 million. The Company may realize additional proceeds of up to $2.2 million.
In March 2009, Clarient entered into a stock purchase agreement with Oak Investment Partners XII (“Oak”), pursuant to which Clarient agreed to sell up to an aggregate of 6.6 million shares of its Series A Convertible Preferred Stock in two or more tranches for aggregate consideration of up to $50.0 million. Each preferred share was initially convertible, at any time, into four shares of Clarient’s common stock, subject to certain adjustments. The initial closing of the Oak private placement occurred on March 26, 2009, at which time Clarient issued 3.8 million preferred shares for aggregate consideration of $29.1 million. After paying closing fees and legal expenses, Clarient used the proceeds to repay in full and terminate its revolving credit agreement with a bank and repay a portion of the outstanding balance of its credit facility with the Company.
Later during 2009, the Company publicly sold 18.4 million shares of common stock of Clarient for $61.3 million in net proceeds. The Company recognized a loss of $7.3 million on the sale, based on the net proceeds received compared to the fair value at the end of the previous quarter, which is included in Other income (loss), net in the Consolidated Statements of Operations for the year ended December 31, 2009.
In December 2010, Clarient was acquired by GE Healthcare. The Company received gross proceeds of $153.4 million in connection with the transaction and paid retention bonuses to Clarient management of $6.9 million, resulting in net proceeds of $146.5 million. The Company recognized a gain of $43.0 million on the transaction, based on the net proceeds received compared to the fair value at the end of the previous quarter which was included in Other income (loss), net in the Consolidated Statements of Operations.
For the period from January 1, 2010 through September 30, 2010, the Company recognized unrealized gains of $22.4 million on themark-to-market of its holdings in Clarient which were included in Other income (loss), net in the Consolidated Statements of Operations. For the period from May 14, 2009 through December 31, 2009, the Company recognized unrealized gains of $19.5 million on themark-to-market of its holdings in Clarient. At December 31, 2009, the fair value of the Company’s holdings in Clarient of $80.5 million was included in Ownership interests in and advances to partner companies in the Consolidated Balance Sheet.
The following unaudited summarized balance sheet for Clarient at September 30, 2010 and the results of operations for the nine months ended September 30, 2010, have been compiled from the unaudited financial statements of Clarient. The results of Clarient are reported on a one quarter lag.
55
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | | | | |
| | | September 30, 2010 | |
| | | (In thousands)
| |
| | | (Unaudited) | |
| |
Balance Sheet: | | | | |
| Current assets | | $ | 42,758 | |
| Non-current assets | | | 32,392 | |
| | | | | |
| Total Assets | | $ | 75,150 | |
| | | | | |
| Current liabilities | | $ | 14,241 | |
| Non-current liabilities | | | 4,626 | |
| Redeemable preferred stock | | | 38,586 | |
| Shareholders’ equity | | | 17,697 | |
| | | | | |
| Total liabilities and shareholders’ equity | | $ | 75,150 | |
| | | | | |
| | | | | |
| | | Nine Months Ended
| |
| | | September 30, 2010 | |
| | | (In thousands)
| |
| | | (Unaudited) | |
| |
Results of Operations: | | | | |
| Revenue | | $ | 86,803 | |
| | | | | |
| Operating income | | $ | 3,564 | |
| | | | | |
| Net income from continuing operations | | $ | 2,914 | |
| | | | | |
The following unaudited summarized financial information for partner companies and funds accounted for under the equity method at December 31, 2010 and 2009 and for the three years ended December 31, 2010, has been compiled from the unaudited financial statements of our respective partner companies and funds and reflects certain historical adjustments. Results of operations of the partner companies and funds are excluded for periods prior to their acquisition and subsequent to their disposition. The unaudited financial information below does not include information pertaining to Clarient. The Company reports its share of the income or loss of the equity method partner companies on a one quarter lag.
| | | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands) | |
| |
Balance Sheets: | | | | | | | | |
| Current assets | | $ | 115,319 | | | $ | 98,276 | |
| Non-current assets | | | 60,130 | | | | 64,889 | |
| | | | | | | | | |
| Total Assets | | $ | 175,449 | | | $ | 163,165 | |
| | | | | | | | | |
| Current liabilities | | $ | 52,136 | | | $ | 48,460 | |
| Non-current liabilities | | | 29,511 | | | | 28,633 | |
| Shareholders’ equity | | | 93,802 | | | | 86,072 | |
| | | | | | | | | |
| Total liabilities and shareholders’ equity | | $ | 175,449 | | | $ | 163,165 | |
| | | | | | | | | |
As of December 31, 2010, the Company’s carrying value in equity method partner companies, in the aggregate, exceeded the Company’s share of the net assets of such companies by approximately $29.6 million. Of this excess, $21.6 million was allocated to goodwill and $8.0 million was allocated to intangible assets.
56
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
Results of Operations: | | | | | | | | | | | | |
| Revenue | | $ | 226,871 | | | $ | 144,771 | | | $ | 92,366 | |
| | | | | | | | | | | | | |
| Gross profit | | $ | 162,820 | | | $ | 98,626 | | | $ | 52,906 | |
| | | | | | | | | | | | | |
| Net loss | | $ | (20,118 | ) | | $ | (50,436 | ) | | $ | (61,560 | ) |
| | | | | | | | | | | | | |
Included above for the year ended December 31, 2008 are the results of operations of Rubicor. Rubicor ceased operations in 2008. Due to the significance of Rubicor’s results of operations and the related impairment charge to the Company’s net loss from continuing operations before income tax for the year ended December 31, 2008, unaudited summarized financial information for Rubicor for the year ended December 31, 2008 is reported as follows (in thousands): Revenue — $638; Gross loss — $2,540; and Net loss — $12,300. Summarized financial information is not available for Rubicor for periods subsequent to December 31, 2008.
| |
| 4. | Acquisitions of Ownership Interests in Partner Companies |
In December 2010, the Company funded a $5.0 million mezzanine debt financing to Portico Systems, Inc. (“Portico”). The Company previously deployed an aggregate of $9.3 million in cash in Portico from August 2006 through April 2009. Portico offers software and services to health insurance providers that help reduce administrative, medical and IT costs. The Company accounts for its holdings in Portico under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of Portico was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
In December 2010, the Company deployed an additional $1.8 million in Advantedge Healthcare Solutions, Inc. (“AHS”), increasing its ownership interest in AHS from 39.7% to 40.2%. In March 2010, the Company funded a $1.3 million short-term loan to AHS which was repaid in May 2010. The Company previously deployed a total of $13.5 million into AHS. AHS is a provider of physician billing and practice management services and software to hospital-based physician groups, large office-based physician practices, and ambulatory surgery centers. The Company accounts for its holdings in AHS under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of AHS was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
In December, June and January 2010, the Company funded an aggregate of $1.8 million convertible bridge loans to Alverix, Inc. (“Alverix”). The Company previously deployed an aggregate of $4.5 million in Alverix and currently maintains a 49.6% ownership interest. Alverix provides next-generation instrument and connectivity platforms for diagnosticPoint-of-Care (POC) testing. The Company accounts for its holdings in Alverix under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of Alverix was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
In September 2010, the Company exercised a total of $0.6 million of warrants in Clarient, increasing its ownership interest to 27.5% from 27.2%. The Company sold its remaining interest in Clarient in December 2010 for net proceeds of $146.5 million, recognizing a gain on sale of $43.0 million.
In September 2010, the Company acquired a 26.5% ownership interest in Good Start Genetics, Inc. (“Good Start”) for $6.8 million. Good Start is developing a pre-pregnancy genetic test, which utilizes an advanced DNA sequencing technology to screen for a panel of genetic disorders, including those recommended by the American Congress of Obstetricians and Gynecologists and the American College of Medical Genetics. The Company accounts for its interest in Good Start under the equity method. The difference between the Company’s
57
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
cost and its interest in the underlying net assets of Good Start was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
In September and June 2010, the Company funded an aggregate of $0.7 million convertible bridge loans to Quinnova. The Company previously deployed $5.0 million in Quinnova in October 2009 for a 25.7% ownership interest. Quinnova is a specialty pharmaceutical company that develops and markets novel delivery platforms-based prescription dermatology drugs. The Company sold its equity and debt interests in Quinnova in December 2010 for $2.6 million, recognizing a loss on sale of $0.9 million. The Company accounted for its interest in Quinnova under the equity method.
In August 2010, in conjunction with NuPathe’s initial public offering, the Company deployed an additional $3.5 million in NuPathe. In April 2010, the Company funded a $2.7 million convertible bridge loan to NuPathe, which was converted to common shares in conjunction with the initial public offering. The Company previously deployed $12.0 million in NuPathe from August 2006 through August 2009. NuPathe is a specialty pharmaceutical company focused on the development and commercialization of branded therapeutics for diseases of the central nervous system, including neurological and psychiatric disorders. The Company recognized a $1.3 million charge in 2008 in Equity loss in the Consolidated Statements of Operations, related to an in-process research and development charge recorded by NuPathe. Following NuPathe’s initial public offering, the Company accounts for its holdings in NuPathe asavailable-for-sale securities and holds an 18.1% ownership interest.
In April 2010, in conjunction with Tengion’s initial public offering, the Company deployed an additional $1.5 million in Tengion. The Company previously deployed $7.5 million in Tengion in October 2008. Tengion is a clinical-stage biotechnology company. It has pioneered the Organ Regeneration Platformtm that enables the Company to create proprietary product candidates that are intended to harness the intrinsic regenerative pathways of the body to produce a range of native-like organs and tissues. Following Tengion’s initial public offering, the Company accounts for its holdings in Tengion asavailable-for-sale securities and holds a 4.8% ownership interest.
In March 2010, the Company deployed an additional $4.7 million in Swap.com (“Swap.com” formerly Swaptree, Inc.) in connection with a larger round of financing, resulting in an increase in the Company’s ownership interest from 29.3% to 46.6%. The Company had previously acquired an interest in Swap.com in July 2008 for $3.4 million in cash. Swap.com is an internet-based business that enables users to trade books, CDs, DVDs and video games using its proprietary trade matching software. The Company accounts for its holdings in Swap.com under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of Swap.com was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
In December, May and February 2009, the Company deployed an aggregate of $6.5 million in Molecular Biometrics, in conjunction with a larger round of financing resulting in a decrease in the Company’s ownership interest from 37.8% to 35.4%. The Company had previously acquired an interest in Molecular Biometrics in September and December 2008, for $3.5 million in cash, including the conversion into equity interests of $1.9 million previously advanced to the company. Molecular Biometrics is a metabolomics company that applies novel metabolomic technologies to develop accurate, non-invasive clinical tools to increase the probability of pregnancy and decrease multiple births from in vitro fertilization. The Company accounts for its holdings in Molecular Biometrics under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of Molecular Biometrics was allocated to in-process research and development, resulting in $0.4 million and $2.5 million of charges which are reflected in Equity loss in the Consolidated Statements of Operations for 2009 and 2008, respectively.
In October, May and February, 2009 the Company provided additional funding of $0.8 million to Acelerate, Inc., as part of an up to $2.5 million convertible note financing to be funded in five tranches. The Company previously acquired an interest in Acelerate in June 2007, deploying $6.0 million in cash. During 2010, the assets of
58
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Acelerate, Inc. were sold to a third party for cash and future consideration based on sales milestones. The Company received no proceeds from this transaction and does not expect to receive any proceeds related to future milestones. The Company accounted for its interest in Acelerate under the equity method.
In 2009 and 2008, the Company deployed an aggregate of $4.0 million in Garnet. Garnet is a clinical stage regenerative medicine company focused on accelerating healing and reducing scarring in cosmetic, orthopedic and cardiovascular surgical wounds. The Company accounted for its holdings in Garnet under the equity method. In the third quarter of 2010, the Company impaired the carrying value of Garnet to zero.
In July 2009, the Company acquired 17.9% of MediaMath, Inc. (“MediaMath”) for $6.7 million in cash. MediaMath is an online media trading company that enables advertising agencies and their advertisers to optimize their ad spending across various exchanges through its proprietary algorithmic bidding platform and data integration technology. The Company accounts for its holdings in MediaMath under the cost method.
In 2009 and 2007, the Company deployed an aggregate of $12.0 million of cash in Avid for a 13.7% ownership interest. Avid develops molecular imaging agents to detect neurodegenerative diseases. In December 2010, Avid was acquired by Eli Lily and Company resulting in net sale proceeds to the Company of $32.3 million, excluding cash held in escrow of $3.4 million. The Company accounted for its holdings in Avid under the cost method.
In March 2009, the Company deployed an additional $2.0 million in Bridgevine, Inc. (“Bridgevine”). The Company had previously acquired an interest in Bridgevine in August 2007 for $8.0 million. Bridgevine is an internet marketing company that enables online consumers to compare and purchase digital services, including internet, phone, VoIP, TV, wireless, music, and entertainment. The Company accounts for its holdings in Bridgevine under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of Bridgevine was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
In December 2008, the Company purchased additional shares of Rubicor from an existing investor for nominal consideration, increasing its ownership interest in Rubicor to 44.6% from 35.7%. The Company had previously acquired an interest in Rubicor in August 2006 for $20.0 million in cash. In 2009, the Company impaired the carrying value of Rubicor to zero. The Company accounted for its interest in Rubicor under the equity method. Rubicor has ceased operations.
In 2008, the Company provided $1.6 million in funding to NextPoint Networks, Inc. (“NextPoint Networks”). In September 2008, NextPoint Networks was merged with GENBAND, resulting in the Company holding a 2.3% ownership interest in the combined company. GENBAND provides media gateway, IP security and session border gateway technology to telecommunications providers. In 2009, the Company impaired the carrying value of GENBAND to zero. The Company accounted for its holdings in GENBAND under the cost method.
In July 2008, the Company provided additional funding to SafeCentral in the form of $0.8 million convertible notes. In conjunction with this funding, due to anti-dilution provisions contained in an earlier equity funding, the Company’s voting interest in SafeCentral increased from 19.9% to 20.0%, the threshold at which the Company believes it exercises significant influence.
Accordingly, the Company adopted the equity method of accounting for its holdings in SafeCentral. The Company previously deployed an aggregate of $8.5 million in SafeCentral from April 2006 to June 2007. SafeCentral is a developer of security software applications and technologies for data loss prevention on the user’s browser.
In August 2007, the Company acquired 14.0% of Kadoo for $2.2 million in cash. Kadoo was astart-up company established to enable online users to post, manage and securely share large volumes of digital photos, videos and other files. The Company accounted for its holdings in Kadoo under the cost method. In 2008, the Company impaired its entire carrying value in Kadoo and in 2009 sold its equity interest in exchange for a $0.2 million interest in a convertible promissory note, the carrying value of which is zero.
59
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In May 2007, the Company increased its ownership interest in Advanced BioHealing, Inc. (“Advanced BioHealing”) to 28.3% for $2.8 million in cash. The Company previously had acquired a 23.9% interest in Advanced BioHealing in February 2007 for $8.0 million in cash. Advanced BioHealing develops and commercializes living cell therapies that repair damaged human tissue and enable the body to heal itself. The Company accounts for its holdings in Advanced BioHealing under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of Advanced BioHealing was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
In March 2007, the Company acquired 37.1% of Beyond.com, Inc. (“Beyond.com”) for $13.5 million in cash. Beyond.com is an internet-based business that provides career services and technology to job seekers and employers throughout the United States and Canada. The Company accounts for its holdings in Beyond.com under the equity method. The difference between the Company’s cost and its interest in the underlying net assets of Beyond.com was allocated to intangible assets and goodwill as reflected in the carrying value in Ownership interests in and advances to partner companies on the Consolidated Balance Sheets.
| |
| 5. | Fair Value Measurements |
The Company categorizes its financial instruments into a three-level fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). If the inputs used to measure fair value fall within different levels of the hierarchy, the category level is based on the lowest priority level input that is significant to the fair value measurement of the instrument. Financial assets recorded at fair value on the Company’s Consolidated Balance Sheets are categorized as follows:
Level 1 — Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 — Include other inputs that are directly or indirectly observable in the marketplace.
Level 3 — Unobservable inputs which are supported by little or no market activity.
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The following table provides the assets and liabilities measured at fair value on a recurring basis as of December 31, 2010 and 2009:
| | | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurement at
| |
| | | Carrying
| | | December 31, 2010 | |
| | | Value | | | Level 1 | | | Level 2 | | | Level 3 | |
| | | (In thousands) | |
| |
| Cash and cash equivalents | | $ | 183,419 | | | $ | 183,419 | | | $ | — | | | $ | — | |
| Cash held in escrow | | $ | 6,434 | | | $ | 6,434 | | | $ | — | | | $ | — | |
| Restricted cash equivalents | | $ | 16,774 | | | $ | 16,774 | | | $ | — | | | $ | — | |
Available-for-sale securities | | $ | 25,447 | | | $ | 25,447 | | | $ | — | | | $ | — | |
Marketable securities —held-to-maturity: | | | | | | | | | | | | | | | | |
| Commercial paper | | $ | 27,362 | | | $ | 27,362 | | | $ | — | | | $ | — | |
| U.S. Treasury Bills | | | 12,053 | | | | 12,053 | | | | — | | | | — | |
| Government agency bonds | | | 2,996 | | | | 2,996 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| | | $ | 42,411 | | | $ | 42,411 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | |
60
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurement at
| |
| | | Carrying
| | | December 31, 2009 | |
| | | Value | | | Level 1 | | | Level 2 | | | Level 3 | |
| | | (In thousands) | |
| |
| Cash and cash equivalents | | $ | 67,347 | | | $ | 67,347 | | | $ | — | | | $ | — | |
| Cash held in escrow | | $ | 6,910 | | | $ | 6,910 | | | $ | — | | | $ | — | |
| Ownership interest in Clarient | | $ | 80,483 | | | $ | 80,483 | | | $ | — | | | $ | — | |
Marketable securities —held-to-maturity: | | | | | | | | | | | | | | | | |
| Commercial paper | | $ | 10,380 | | | $ | 10,380 | | | $ | — | | | $ | — | |
| U.S. Treasury Bills | | | 4,981 | | | | 4,981 | | | | — | | | | — | |
| Government agency bonds | | | 8,384 | | | | 8,384 | | | | — | | | | — | |
| Certificates of deposit | | | 15,321 | | | | 15,321 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| | | $ | 39,066 | | | $ | 39,066 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | |
As of December 31, 2010, the contractual maturities of the marketable securities were less than one year.Held-to-maturity securities are carried at amortized cost, which, due to the short-term maturity of these instruments, approximates fair value using quoted prices in active markets for identical assets or liabilities, defined as Level 1 inputs under the fair value hierarchy.
Prior to its sale in December 2010, the Company’s holdings in Clarient were measured at fair value using quoted prices for Clarient’s common stock as traded on the NASDAQ Capital Market, which is considered a Level 1 input under the valuation hierarchy.
The Company recorded an impairment charge of $1.8 million related to Molecular Biometrics in 2010 measured as the amount by which Molecular Biometrics’ carrying value exceed its estimated fair value. The fair market value of the Company’s interest in Molecular Biometrics was determined to be $0.2 million based on Level 3 inputs as defined above. The inputs and valuation techniques used include discounted cash flows and valuation of comparable public companies.
The Company recorded an impairment charge of $1.5 million related to SafeCentral in 2010 measured as the amount by which SafeCentral’s carrying value exceed its estimated fair value. The fair market value of the Company’s interest in SafeCentral was determined to be $2.3 million based on Level 3 inputs as defined above. The inputs and valuation techniques used include discounted cash flows and valuation of comparable public companies.
Following NuPathe’s initial public offering, the Company accounts for its holdings in NuPathe asavailable-for-sale securities. Accordingly, the Company recorded an unrealized gain of $13.2 million as a separate component of equity in 2010 measured by reference to quoted prices for NuPathe’s common stock as traded on the NASDAQ Capital Market, which is considered a Level 1 input under the valuation hierarchy.
Following Tengion’s initial public offering, the Company accounts for its holdings in Tengion asavailable-for-sale securities. The Company recognized an unrealized loss of $0.3 million as a separate component of equity in 2010. The Company recognized an impairment charge of $1.1 million in the third quarter of 2010, representing the unrealized loss on themark-to-market of its ownership interest in Tengion which was previously recorded as a separate component of equity. The Company also recognized an impairment charge of $2.1 million related to Tengion in the first quarter of 2010, measured as the amount by which Tengion’s carrying value exceeded its estimated fair value. In each case, the value of the Company’s holdings in Tengion was measured by reference to quoted prices for Tengion’s common stock as traded on the NASDAQ Capital Market, which is considered a Level 1 input under the valuation hierarchy.
The Company recognized an impairment charge of $1.1 million related to Garnet in 2010 measured as the amount by which Garnet’s carrying value exceeded its estimated fair value. The fair market value of the Company’s
61
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
interest in Garnet was determined to be zero based on Level 3 inputs as defined above. The inputs and valuation techniques used include discounted cash flows and valuation of comparable public companies.
The Company recognized an impairment charge of $0.4 million related to Acelerate, a former equity method partner company, in 2010, measured as the amount by which Acelerate’s carrying value exceeded its estimated fair value. The fair market value of the Company’s interest in Acelerate was determined to be zero based on Level 3 inputs as defined above. The inputs and valuation techniques used include discounted cash flows and valuation of comparable public companies.
As described in Note 7, in 2010, the Company recognized a loss on exchange of its convertible senior debentures. The fair value of the newly issued 10.125% convertible senior debentures was determined at the exchange date based on Level 3 inputs using a convertible bond valuation model.
As described in Note 3, the Company recognized impairment charges of $10.1 million related to cost method partner companies and $4.1 million related to equity method partner companies during the year ended December 31, 2009 measured as the amount by which the partner companies’ carrying values exceeded their respective estimated fair values. The fair value measurements of these companies of $6.7 million at December 31, 2009 were based on Level 3 inputs as defined above. The inputs and valuation techniques used include discounted cash flows and valuation of comparable public companies.
| |
| 6. | Property and Equipment |
Property and equipment consisted of the following:
| | | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands) | |
| |
| Building and improvements | | $ | 503 | | | $ | 483 | |
| Machinery and equipment | | | 985 | | | | 1,039 | |
| | | | | | | | | |
| | | | 1,488 | | | | 1,522 | |
| Accumulated depreciation | | | (1,193 | ) | | | (1,212 | ) |
| | | | | | | | | |
| | | $ | 295 | | | $ | 310 | |
| | | | | | | | | |
| |
| 7. | Convertible Debentures and Credit Arrangements |
The carrying values of the Company’s convertible senior debentures were as follows:
| | | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands) | |
| |
| Convertible senior debentures due 2024 | | $ | 31,289 | | | $ | 78,225 | |
| Convertible senior debentures due 2014 | | | 44,630 | | | | — | |
| | | | | | | | | |
| | | | 75,919 | | | | 78,225 | |
| Less: current portion | | | (31,289 | ) | | | — | |
| | | | | | | | | |
| Convertible senior debentures — non-current | | $ | 44,630 | | | $ | 78,225 | |
| | | �� | | | | | | |
Convertible Senior Debentures due 2024
In February 2004, the Company completed the sale of $150 million of 2.625% convertible senior debentures with a stated maturity of March 15, 2024 (the “2024 Debentures”). Interest on the 2024 Debentures is payable semi-annually on March 15 and September 15. At the debentures holders’ option, the 2024 Debentures are convertible
62
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
into the Company’s common stock through March 14, 2024, subject to certain conditions. As adjusted, the conversion price of the debentures is $43.3044 of principal amount per share, equivalent to a conversion rate of 23.0923 shares of Company common stock per $1,000 principal amount of the 2024 Debentures. The closing price of the Company’s common stock at December 31, 2010 was $17.08. The 2024 Debentures holders have the right to require the Company to repurchase the 2024 Debentures on March 21, 2011, March 20, 2014 or March 20, 2019 at a repurchase price equal to 100% of their face amount, plus accrued and unpaid interest. The 2024 Debentures holders also have the right to require repurchase of the 2024 Debentures upon a fundamental change, including sale of all or substantially all of the Company’s common stock or assets, liquidation, dissolution or change in control or the delisting of the Company’s common stock from the New York Stock Exchange if the Company were unable to obtain a listing for its common stock on another national or regional securities exchange. Subject to certain conditions, the Company may redeem all or some of the 2024 Debentures. Through December 31, 2010, the Company has repurchased a total of $71.8 million in face value of the 2024 Debentures.
At December 31, 2010, the fair value of the $31.3 million outstanding 2024 Debentures was approximately $30.8 million based on quoted market prices as of such date.
On March 10, 2010, the Company entered into agreements with institutional holders of an aggregate of $46.9 million in face value of its 2024 Debentures to exchange the debentures held by such holders for $46.9 million in face amount of newly issued 10.125% senior convertible debentures, due March 15, 2014 (the “2014 Debentures”). The exchange became effective on March 26, 2010 and represents a non-cash financing in the year ended December 31, 2010. The remaining $31.3 million outstanding face amount of the 2024 Debentures remains outstanding under the original terms and has been classified as a current liability on the Consolidated Balance Sheet as of December 31, 2010 because the first required repurchase date is within one year. The Company recognized a loss on exchange of $8.5 million in the first quarter of 2010 determined as the excess of the fair value of the 2014 Debentures at the exchange date over the carrying value of the exchanged 2024 Debentures. This loss is reported in Other income (loss), net in the Consolidated Statements of Operations.
Convertible Senior Debentures due 2014
Interest on the 2014 Debentures is payable semi-annually on March 15 and September 15. In the first quarter of 2010, as required under the terms of the 2014 Debentures, the Company placed approximately $19.0 million in a restricted escrow account to make all scheduled interest payments on the 2014 Debentures through their maturity. During 2010, interest payments of $2.2 million were made out of the restricted escrow account and are considered non-cash investing activities. Including accrued interest, a total of $16.8 million was reflected in Restricted cash equivalents on the Consolidated Balance Sheet at December 31, 2010, of which $4.9 million was classified as a current asset.
At the debentures holders’ option, the 2014 Debentures are convertible into the Company’s common stock at anytime after March 15, 2013; and, prior to March 15, 2013, under any of the following conditions:
| | |
| | • | during any fiscal quarter commencing after June 30, 2010 if the closing sale price per share of Company common stock is greater than or equal to 120% of the conversion price for at least 20 trading days during the period of 30 trading days ending on the last day of the preceding fiscal quarter; |
| |
| | • | during the five day period immediately following any 10 consecutive trading day period in which the trading price per $1,000 principal amount of 2014 Debentures for each trading day of such period was less than 100% of the product of the closing sale price per share of Company common stock multiplied by the conversion rate on each such trading day; |
| |
| | • | If a fundamental change (as defined) occurs, including sale of all or substantially all of the Company’s common stock or assets, liquidation, dissolution or a change in control. |
63
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The conversion price is $16.50 of principal amount per share, equivalent to a conversion rate of 60.6061 shares of Company common stock per $1,000 principal amount of the 2014 Debentures. The closing price of the Company’s common stock at December 31, 2010 was $17.08. The 2014 Debentures holders have the right to require repurchase of the 2014 Debentures upon a fundamental change, including sale of all or substantially all of the Company’s common stock or assets, liquidation, dissolution or a change in control or the delisting of the Company’s common stock from the New York Stock Exchange if the Company were unable to obtain a listing for its common stock on another national or regional securities exchange.
The Company may mandatorily convert all or some of the 2014 Debentures at any time after March 15, 2012 if the closing sale price per share of Company common stock exceeds 130% of the conversion price for at least 20 trading days in a period of 30 consecutive trading days. If the Company elects to mandatorily convert any of the 2014 Debentures, the Company will be required to pay any interest that would have accrued and become payable on the debentures through their maturity. Upon a conversion of the 2014 Debentures, the Company has the right to settle the conversion in stock, cash or a combination thereof.
Because the 2014 Debentures may be settled in cash or partially in cash upon conversion, the Company separately accounts for the liability and equity components of the 2014 Debentures. The carrying amount of the liability component was determined at the exchange date by measuring the fair value of a similar liability that does not have an associated equity component. The carrying amount of the equity component represented by the embedded conversion option was determined by deducting the fair value of the liability component from the carrying value of the 2014 Debentures as a whole at the exchange date. The carrying value of the 2014 Debentures as a whole at the exchange date was equal to their fair value of $55.2 million determined using a convertible bond valuation model. At December 31, 2010, the fair value of the $46.9 million outstanding 2014 Debentures was approximately $63.4 million based on quoted market prices as of such date. At December 31, 2010, the carrying amount of the equity component was $10.8 million, the principal amount of the liability component was $46.9 million, the unamortized discount was $2.3 million and the net carrying value of the liability component was $44.6 million. The Company is amortizing the excess of the face value of the 2014 Debentures over their carrying value to interest expense over their term. The effective interest rate on the 2014 Debentures is 12.5%.
Credit Arrangements
The Company is party to a loan agreement which provides it with a revolving credit facility in the maximum aggregate amount of $50 million in the form of borrowings, guarantees and issuances of letters of credit (subject to a $20 million sublimit). Actual availability under the credit facility is based on the amount of cash maintained at the bank as well as the value of the Company’s public and private partner company interests. This credit facility bears interest at the prime rate for outstanding borrowings, subject to an increase in certain circumstances. Other than for limited exceptions, the Company is required to maintain all of its depository and operating accounts and the lesser of $80 million or 75% of its investment and securities accounts at the bank. The credit facility, as amended December 31, 2010, matures on December 31, 2012. Under the credit facility, the Company provided a $6.3 million letter of credit expiring on March 19, 2019 to the landlord of CompuCom Systems, Inc.’s Dallas headquarters which has been required in connection with the sale of CompuCom Systems in 2004. Availability under the Company’s revolving credit facility at December 31, 2010 was $43.7 million.
64
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| |
| 8. | Accrued Expenses and Other Current Liabilities |
Accrued expenses consisted of the following:
| | | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands) | |
| |
| Accrued interest | | $ | 1,640 | | | $ | 600 | |
| Other | | | 2,583 | | | | 3,725 | |
| | | | | | | | | |
| | | $ | 4,223 | | | $ | 4,325 | |
| | | | | | | | | |
Preferred Stock
Shares of preferred stock, par value $0.10 per share, are voting and are issuable in one or more series with rights and preferences as to dividends, redemption, liquidation, sinking funds and conversion determined by the Board of Directors. At December 31, 2010 and 2009, there were one million shares authorized and none outstanding.
Shareholders’ Rights Plan
In February 2000, the Company adopted a shareholders’ rights plan. Under the plan, each shareholder of record on March 24, 2000 received the right to purchase 1/1000 of a share of the Company’s Series A Junior Participating Preferred Stock at the rate of one right for each share of the Company’s common stock then held of record. Each1/1000 of a share of the Company’s Series A Junior Participating Preferred Stock is designed to be equivalent in voting and dividend rights to one share of the Company’s common stock. The rights would have become exercisable only if a person or group acquired beneficial ownership of 15% or more of the Company’s common stock or commenced a tender or exchange offer that would have resulted in such a person or group owning 15% or more of the Company’s common stock. This plan expired on March 1, 2010.
| |
| 10. | Stock-Based Compensation |
Equity Compensation Plans
The Company has three equity compensation plans: the 1999 Equity Compensation Plan, with 1.5 million shares authorized for issuance; the 2001 Associates Equity Compensation Plan with 0.9 million shares authorized for issuance; and the amended and restated 2004 Equity Compensation Plan, with 2.2 million shares authorized for issuance. Employees and consultants are eligible for grants of stock options, restricted stock awards, stock appreciation rights, stock units, performance units and other stock-based awards under each of these plans; directors and executive officers are eligible for grants only under the 1999 and 2004 Equity Compensation Plans. During 2008, the Company issued 250 thousand options outside of existing plans as inducement awards in accordance with New York Stock Exchange rules. The 1999 Equity Compensation Plan expired by its terms on February 10, 2009 and no further grants may be made under that plan.
To the extent allowable, service-based awards are incentive stock options. Options granted under the plans are at prices equal to or greater than the fair market value at the date of grant. Upon exercise of stock options, the Company issues shares first from treasury stock, if available, then from authorized but unissued shares. At December 31, 2010, the Company had reserved 4.5 million shares of common stock for possible future issuance under its equity compensation plans.
65
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Classification of Stock-Based Compensation Expense
Stock-based compensation expense from continuing operations was recognized in the Consolidated Statements of Operations as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Cost of sales | | $ | — | | | $ | 49 | | | $ | 133 | |
| Selling, general and administrative | | | 3,777 | | | | 3,776 | | | | 3,316 | |
| | | | | | | | | | | | | |
| | | $ | 3,777 | | | $ | 3,825 | | | $ | 3,449 | |
| | | | | | | | | | | | | |
At December 31, 2010, the Company had outstanding options that vest based on three different types of vesting schedules:
1) market-based;
2) performance-based; and
3) service-based.
Market-based awards entitle participants to vest in a number of options determined by achievement by the Company of certain target market capitalization increases (measured by reference to stock price increases on a specified number of outstanding shares) over an eight-year period. The requisite service periods for the market-based awards are based on the Company’s estimate of the dates on which the market conditions will be met as determined using a Monte Carlo simulation model. Compensation expense is recognized over the requisite service periods using the straight-line method but is accelerated if market capitalization targets are achieved earlier than estimated. During the years ended December 31, 2010 and 2009, the Company did not issue any market-based option awards to employees. During the year ended December 31, 2008, the Company issued 250 thousand market-based stock option awards to employees. During the years ended December 31, 2010, 2009 and 2008, respectively, 21 thousand, 16 thousand and seven thousand market-based options vested based on achievement of market capitalization targets. During the years ended December 31, 2010, 2009 and 2008, respectively, 10 thousand, 67 thousand and 463 thousand market-based options were cancelled or forfeited. The Company recorded compensation expense related to these awards of $1.7 million, $1.5 million and $0.4 million during the years ended December 31, 2010, 2009 and 2008, respectively. Depending on the Company’s stock performance, the maximum number of unvested shares at December 31, 2010 attainable under these grants was 1.2 million shares.
Performance-based awards entitle participants to vest in a number of awards determined by achievement by the Company of target capital returns based on net cash proceeds received by the Company upon the sale, merger or other exit transaction of certain identified partner companies. Vesting may occur, if at all, once per year. The requisite service periods for the performance-based awards are based on the Company’s estimate of when the performance conditions will be met. Compensation expense is recognized for performance-based awards for which the performance condition is considered probable of achievement. Compensation expense is recognized over the requisite service periods using the straight-line method but is accelerated if capital return targets are achieved earlier than estimated. During the years ended December 31, 2010, 2009 and 2008, respectively, the Company issued 130 thousand, 155 thousand and 341 thousand performance-based option awards to employees. During the years ended December 31, 2010, 2009 and 2008, no options vested based on the achievement of capital returns targets. During the year ended December 31, 2010, six thousand performance-based option awards were canceled or forfeited. The Company recorded compensation expense related to these option awards of $0.1 million, $0.1 million and $0.0 million for the years ended December 31, 2010, 2009 and 2008, respectively.
The maximum number of unvested shares at December 31, 2010 attainable under these grants was 619 thousand shares.
66
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
All other outstanding options are service-based awards that generally vest over four years after the date of grant and expire eight years after the date of grant. Compensation expense is recognized over the requisite service period using the straight-line method. The requisite service period for service-based awards is the period over which the award vests. During the years ended December 31, 2010, 2009 and 2008, respectively, the Company issued 95 thousand, 113 thousand and 288 thousand service-based option awards to employees. During the years ended December 31, 2010, 2009 and 2008, respectively, nine thousand, 231 thousand and 605 thousand service-based options were canceled or forfeited. The Company recorded compensation expense related to these awards of $1.2 million, $1.0 million and $1.1 million during the years ended December 31, 2010, 2009 and 2008, respectively.
The fair value of the Company’s stock-based awards to employees was estimated at the date of grant using the Black-Scholes option-pricing model. The risk-free rate is based on the U.S. Treasury yield curve in effect at the end of the quarter in which the grant occurred. The expected term of stock options granted was estimated using the historical exercise behavior of employees. Expected volatility was based on historical volatility measured using weekly price observations of the Company’s common stock for a period equal to the stock option’s expected term. Assumptions used in the valuation of options granted in each period were as follows:
| | | | | | | |
| | | Year Ended December 31, |
| | | 2010 | | 2009 | | 2008 |
| |
Service-Based Awards | | | | | | |
| Dividend yield | | 0% | | 0% | | 0% |
| Expected volatility | | 58% | | 59% | | 52% |
| Average expected option life | | 5 years | | 5 years | | 5 years |
| Risk-free interest rate | | 2.0% | | 2.7% | | 3.1% |
| | | | | | | |
| | | Year Ended December 31, |
| | | 2010 | | 2009 | | 2008 |
| |
Market-Based Awards | | | | | | |
| Dividend yield | | N/A | | N/A | | 0% |
| Expected volatility | | N/A | | N/A | | 59% |
| Average expected option life | | N/A | | N/A | | 5 - 7 years |
| Risk-free interest rate | | N/A | | N/A | | 3.4% |
| | | | | | | |
| | | Year Ended December 31, |
| | | 2010 | | 2009 | | 2008 |
| |
Performance-Based Awards | | | | | | |
| Dividend yield | | 0% | | 0% | | 0% |
| Expected volatility | | 58% | | 59% | | 50% |
| Average expected option life | | 4.9 years | | 4.9 years | | 4.4 years |
| Risk-free interest rate | | 2.0% | | 2.7% | | 3.0% |
The weighted-average grant date fair value of options issued by the Company during the years ended December 31, 2010, 2009 and 2008 was $7.42, $5.22 and $3.72 per share, respectively.
67
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Option activity of the Company is summarized below:
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Weighted
| | | | |
| | | | | | Weighted
| | | Average
| | | | |
| | | | | | Average
| | | Remaining
| | | Aggregate
| |
| | | | | | Exercise
| | | Contractual
| | | Intrinsic
| |
| | | Shares | | | Price | | | Life | | | Value | |
| | | (In thousands) | | | | | | (In years) | | | (In thousands) | |
| |
| Outstanding at December 31, 2007 | | | 3,570 | | | $ | 12.22 | | | | | | | | | |
| Options granted | | | 880 | | | | 7.47 | | | | | | | | | |
| Options exercised | | | (34 | ) | | | 7.88 | | | | | | | | | |
| Options canceled/forfeited | | | (1,080 | ) | | | 15.18 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding at December 31, 2008 | | | 3,336 | | | | 10.05 | | | | | | | | | |
| Options granted | | | 267 | | | | 9.99 | | | | | | | | | |
| Options exercised | | | (34 | ) | | | 7.77 | | | | | | | | | |
| Options canceled/forfeited | | | (301 | ) | | | 16.21 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding at December 31, 2009 | | | 3,268 | | | | 9.51 | | | | | | | | | |
| Options granted | | | 224 | | | | 14.65 | | | | | | | | | |
| Options exercised | | | (121 | ) | | | 9.16 | | | | | | | | | |
| Options canceled/forfeited | | | (50 | ) | | | 12.13 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding at December 31, 2010 | | | 3,321 | | | | 9.83 | | | | 4.4 | | | $ | 24,144 | |
| | | | | | | | | | | | | | | | | |
| Options exercisable at December 31, 2010 | | | 1,263 | | | | 10.12 | | | | 3.4 | | | | 8,901 | |
| Options vested and expected to vest at December 31, 2010 | | | 2,548 | | | | 9.80 | | | | 4.0 | | | | 18,632 | |
| Shares available for future grant | | | 864 | | | | | | | | | | | | | |
The total intrinsic value of options exercised for the years ended December 31, 2010, 2009 and 2008 was $0.4 million, $0.1 million and $0.0 million, respectively.
At December 31, 2010, total unrecognized compensation cost related to non-vested stock options granted under the plans for service-based awards was $0.8 million. That cost is expected to be recognized over a weighted-average period of 2.2 years.
At December 31, 2010, total unrecognized compensation cost related to non-vested stock options granted under the plans for market-based awards was $1.1 million. That cost is expected to be recognized over a weighted-average period of 1.8 years, but would be accelerated if market capitalization targets are achieved earlier than estimated.
At December 31, 2010, total unrecognized compensation cost related to non-vested stock options granted under the plans for performance-based awards was $1.5 million. That cost is expected to be recognized over a weighted-average period of 3.1 years but would be accelerated if stock price targets are achieved earlier than estimated.
During the years ended December 31, 2010 and 2009, respectively, the Company issued 74 thousand and 103 thousand performance-based stock units to employees which vest based on achievement by the Company of target capital returns based on net cash proceeds received by the Company on the sale, merger or other exit transaction of certain identified partner companies, as described above related to performance-based option awards. Performance-based stock units represent the right to receive shares of the Company’s common stock, on aone-for-one basis. During the years ended December 31, 2010 and 2009, respectively, the Company issued 25 thousand and 197 thousand restricted shares to employees. The restricted shares issued vest 25% on the first
68
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
anniversary of grant and the remaining 75% thereafter in equal monthly installments over the next two or three years, as applicable. During the year ended December 31, 2010, the Company issued 53 thousand unrestricted shares to employees in connection with the 2009 management incentive plan payments earned by certain senior employees.
The Company issued deferred stock units during the years ended December 31, 2010, 2009 and 2008, to all non-employee directors as annual service grants and during the years ended December 31, 2010, 2009 and 2008, to directors who deferred all or a portion of directors’ fees earned. Deferred stock units issued to directors in lieu of directors fees are 100% vested at the grant date; matching deferred stock units equal to 25% of directors’ fees deferred generally vest one year following the grant date. Deferred stock units represent the right to receive shares of the Company’s common stock, on aone-for-one basis, following termination of employment or service, death or permanent disability. During the years ended December 31, 2010, 2009 and 2008, respectively, the Company issued 32 thousand, 70 thousand and 64 thousand deferred stock units to directors.
During the years ended December 31, 2010, 2009 and 2008, respectively, the Company granted two thousand restricted shares, 12 thousand stock options and 11 thousand stock options to members of its advisory boards, which comprise non-employees. Such awards generally vest within one year following grant, are equity-classified and aremarked-to-market each period until vesting.
Total compensation expense for deferred stock units, performance-based stock units and restricted stock was approximately $0.8 million, $0.4 million and $0.2 million for the years ended December 31, 2010, 2009 and 2008, respectively. Unrecognized compensation expense related to deferred stock units, performance stock units and restricted stock at December 31, 2010 was $1.8 million. The total fair value of deferred stock units, performance stock units and restricted stock vested during the years ended December 31, 2010, 2009 and 2008 was $1.8 million, $0.5 million and $0.2 million, respectively.
Deferred stock unit, performance-based stock unit and restricted stock activity is summarized below:
| | | | | | | | | |
| | | | | | Weighted Average
| |
| | | | | | Grant Date Fair
| |
| | | Shares | | | Value | |
| | | (In thousands) | | | | |
| |
| Unvested at December 31, 2008 | | | 32 | | | $ | 7.87 | |
| Granted | | | 370 | | | | 6.02 | |
| Vested | | | (83 | ) | | | 5.71 | |
| Forfeited | | | (2 | ) | | | 15.18 | |
| | | | | | | | | |
| Unvested at December 31, 2009 | | | 317 | | | | 5.95 | |
| Granted | | | 133 | | | | 14.80 | |
| Vested | | | (147 | ) | | | 5.80 | |
| Forfeited | | | (5 | ) | | | 7.73 | |
| | | | | | | | | |
| Unvested at December 31, 2010 | | | 298 | | | | 10.09 | |
| | | | | | | | | |
Stock based compensation expense for Clarient prior to its deconsolidation was included in the Company’s consolidated results of operations. During the period from January 1, 2009 through May 14, 2009 and the year ended December 31, 2008, respectively, the Company recognized stock-based compensation related to Clarient of $0.8 million and $1.8 million.
69
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| |
| 11. | Other Income (Loss), Net |
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Loss on exchange of convertible debentures | | $ | (8,289 | ) | | $ | — | | | $ | — | |
| Gain on repurchase of convertible debentures, net | | | — | | | | 457 | | | | 9,030 | |
| Gain (loss) on sale of companies and funds, net | | | 20,291 | | | | (7,338 | ) | | | 1,737 | |
| Gain on distributions from private equity funds | | | — | | | | 30 | | | | 1,042 | |
| Gain on deconsolidation of Clarient | | | — | | | | 105,991 | | | | — | |
| Gain on sale of Clarient | | | 42,956 | | | | — | | | | — | |
Gain onmark-to-market of holdings in Clarient | | | 22,394 | | | | 19,502 | | | | — | |
| Impairment charges on cost method partner companies | | | (2,146 | ) | | | (10,079 | ) | | | (2,251 | ) |
Other than temporary impairment onavailable-for-sale securities | | | (1,108 | ) | | | — | | | | — | |
| Other | | | 711 | | | | 318 | | | | 722 | |
| | | | | | | | | | | | | |
| | | $ | 74,809 | | | $ | 108,881 | | | $ | 10,280 | |
| | | | | | | | | | | | | |
The provision (benefit) for income taxes was as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Current, primarily state | | $ | — | | | $ | (14 | ) | | $ | (24 | ) |
| Deferred, primarily state | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | |
| | | $ | — | | | $ | (14 | ) | | $ | (24 | ) |
| | | | | | | | | | | | | |
The total income tax provision (benefit) differed from the amounts computed by applying the U.S. federal income tax rate of 35% to net loss from continuing operations before income taxes as a result of the following:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| |
| Statutory tax expense (benefit) | | | 35.0 | % | | | 35.0 | % | | | (35.0 | )% |
| Increase (decrease) in taxes resulting from: | | | | | | | | | | | | |
| State taxes, net of federal tax benefit | | | 0.0 | | | | 0.0 | | | | (0.1 | ) |
| Valuation allowance | | | (36.7 | ) | | | (35.2 | ) | | | 34.1 | |
| Other adjustments | | | 1.7 | | | | 0.2 | | | | 0.9 | |
| | | | | | | | | | | | | |
| | | | 0.0 | % | | | 0.0 | % | | | (0.1 | )% |
| | | | | | | | | | | | | |
70
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The tax effects of temporary differences that gave rise to significant portions of the deferred tax assets and deferred tax liabilities were as follows:
| | | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands) | |
| |
| Deferred tax asset (liability): | | | | | | | | |
| Carrying values of partner companies and other holdings | | $ | 54,821 | | | $ | 43,006 | |
| Tax loss and credit carryforwards | | | 97,161 | | | | 129,923 | |
| Accrued expenses | | | 1,928 | | | | 1,854 | |
| Stock-based compensation | | | 6,405 | | | | 5,088 | |
| Other | | | 1,244 | | | | 1,152 | |
| | | | | | | | | |
| | | | 161,559 | | | | 181,023 | |
| Valuation allowance | | | (161,559 | ) | | | (181,023 | ) |
| | | | | | | | | |
| Net deferred tax liability | | $ | — | | | $ | — | |
| | | | | | | | | |
As of December 31, 2010, the Company and its subsidiaries consolidated for tax purposes had federal net operating loss carryforwards and federal capital loss carryforwards of approximately $230.0 million and $36.5 million, respectively. These carryforwards expire as follows:
| | | | | |
| | | Total | |
| | | (In thousands) | |
| |
| 2011 | | $ | 3,225 | |
| 2012 | | | 173 | |
| 2013 | | | 36,465 | |
| 2014 | | | — | |
| 2015 and thereafter | | | 226,609 | |
| | | | | |
| | | $ | 266,472 | |
| | | | | |
Limitations on utilization of both the net operating loss carryforward and capital loss carryforward may apply.
In assessing the recoverability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The Company has determined that it is more likely than not that certain future tax benefits may not be realized as a result of current and future income. Accordingly, a valuation allowance has been recorded against substantially all of the Company’s deferred tax assets.
The Company recognizes in its Consolidated Financial Statements the impact of a tax position if that position is more likely than not to be sustained upon examination, based on the technical merits of the position. All uncertain tax positions relate to unrecognized tax benefits that would impact the effective tax rate when recognized.
The Company does not expect any material increase or decrease in its income tax expense, in the next twelve months, related to examinations or changes in uncertain tax positions.
71
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Changes in the Company’s uncertain tax positions for the years ended December 31, 2010, 2009 and 2008 were as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Balance at beginning of year | | $ | — | | | $ | 14 | | | $ | 44 | |
| Settlements/lapses in statutes of limitation | | | — | | | | (14 | ) | | | (30 | ) |
| | | | | | | | | | | | | |
| Balance at end of year | | $ | — | | | $ | — | | | $ | 14 | |
| | | | | | | | | | | | | |
The Company files income tax returns in the U.S. federal jurisdiction, and various state jurisdictions. Tax years 2007 and forward remain open for examination for federal tax purposes and tax years 2005 and forward remain open for examination for the Company’s more significant state tax jurisdictions. To the extent utilized in future years’ tax returns, net operating loss and capital loss carryforwards at December 31, 2010 will remain subject to examination until the respective tax year is closed. The Company recognizes penalties and interest accrued related to income tax liabilities in the provision (benefit) for income taxes in its Consolidated Statements of Operations.
72
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| |
| 13. | Net Income (Loss) Per Share |
The calculations of net income (loss) per share were:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands except per share data) | |
| |
| Basic: | | | | | | | | | | | | |
| Amounts attributable to Safeguard Scientifics, Inc. common shareholders: | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | $ | 27,114 | | | $ | 66,240 | | | $ | (42,777 | ) |
| Net income (loss) from discontinued operations | | | — | | | | 1,370 | | | | (9,236 | ) |
| | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. | | $ | 27,114 | | | $ | 67,610 | | | $ | (52,013 | ) |
| | | | | | | | | | | | | |
| Average common shares outstanding | | | 20,535 | | | | 20,308 | | | | 20,326 | |
| | | | | | | | | | | | | |
| Net income (loss) per share from continuing operations attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.32 | | | $ | 3.26 | | | $ | (2.10 | ) |
| Net income (loss) per share from discontinued operations attributable to Safeguard Scientifics, Inc. common shareholders | | | — | | | | 0.07 | | | | (0.46 | ) |
| | | | | | | | | | | | | |
| Net income (loss) per share attributable to Safeguard Scientifics Inc. common shareholds | | $ | 1.32 | | | $ | 3.33 | | | $ | (2.56 | ) |
| | | | | | | | | | | | | |
| Diluted: | | | | | | | | | | | | |
| Amounts attributable to Safeguard Scientifics, Inc. common shareholders: | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | $ | 27,114 | | | $ | 66,240 | | | $ | (42,777 | ) |
| Interest on covertible senior debentures | | | — | | | | 2,616 | | | | — | |
| | | | | | | | | | | | | |
| Net income (loss) from continuing operations for diluted per share computation | | | 27,114 | | | | 68,856 | | | | (42,777 | ) |
| Net income (loss) from discontinued operations | | | — | | | | 1,370 | | | | (9,236 | ) |
| | | | | | | | | | | | | |
| Net income (loss) for diluted per share calculation | | $ | 27,114 | | | $ | 70,226 | | | $ | (52,013 | ) |
| | | | | | | | | | | | | |
| Number of shares used in basic per share computation | | | 20,535 | | | | 20,308 | | | | 20,326 | |
| Effect of dilutive securities: | | | | | | | | | | | | |
| Convertible senior debentures | | | — | | | | 1,956 | | | | — | |
| Unvested restricted stock and DSUs | | | 115 | | | | 111 | | | | — | |
| Employee stock options | | | 857 | | | | 8 | | | | — | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Number of shares used in diluted per share computation | | | 21,507 | | | | 22,383 | | | | 20,326 | |
| | | | | | | | | | | | | |
| Net income (loss) per share from continuing operations attributable to Safeguard Scientifics, Inc. common shareholders | | $ | 1.26 | | | $ | 3.08 | | | $ | (2.10 | ) |
| Net income (loss) per share from discontinued operations attributable to Safeguard Scientifics, Inc. common shareholders | | | — | | | | 0.06 | | | | (0.46 | ) |
| | | | | | | | | | | | | |
| Net income (loss) per share attributable to Safeguard Scientifics Inc. common shareholders | | $ | 1.26 | | | $ | 3.14 | | | $ | (2.56 | ) |
| | | | | | | | | | | | | |
Basic and diluted average common shares outstanding for purposes of computing net income (loss) per share includes outstanding common shares and vested deferred stock units (DSUs).
73
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
If a consolidated or equity method partner company has dilutive stock options, unvested restricted stock, DSUs, warrants or securities outstanding, diluted net income (loss) per share is computed by first deducting from net income (loss) the income attributable to the potential exercise of the dilutive securities of the partner company. This impact is shown as an adjustment to net income (loss) for purposes of calculating diluted net income (loss) per share.
The following potential shares of common stock and their effects on income were excluded from the diluted net loss per share calculation because their effect would be anti-dilutive:
| | |
| | • | At December 31, 2010, 2009 and 2008, options to purchase 0.6 million, 2.7 million and 3.3 million shares of common stock, respectively, at prices ranging from $10.10 to $21.36 per share, $7.50 to $21.36 per share, and $3.90 to $39.42 per share were excluded from the calculation. |
| |
| | • | At December 31, 2010, 2009 and 2008, unvested restricted stock units, performance stock units and DSUs convertible into 2 thousand, 6 thousand and 32 thousand shares of stock, respectively, were excluded from the calculations. |
| |
| | • | At December 31, 2010, 2009 and 2008 a total of 0.7 million, 0.0 million and 2.0 million related to the Company’s 2024 Debentures representing the effect of assumed conversion of the 2024 Debentures were excluded from the calculation. |
| |
| | • | At December 31, 2010, 2.8 million shares related to the Company’s 2014 Debentures representing the effect of assumed conversion of the 2014 Debentures were excluded from the calculations. |
| |
| 14. | Related Party Transactions |
In May 2001, the Company entered into a $26.5 million loan agreement with Warren V. Musser, the Company’s former Chairman and Chief Executive Officer. Through December 31, 2010, the Company recognized impairment charges against the loan of $15.7 million. The Company’s efforts to collect Mr. Musser’s outstanding loan obligation have included the sale of existing collateral, obtaining and selling additional collateral, litigation and negotiated resolution. Since 2001 and through December 31, 2010, the Company has received a total of $16.8 million in payments on the loan. In December 2006, the Company restructured the obligation to reduce the amount outstanding to $14.8 million, bearing interest at a rate of 5.0% per annum, in order to obtain new collateral, which is expected to be the primary source of any repayment. Subsequent to the restructuring of the obligation, the Company received nominal amounts of cash from the sale of collateral in 2009 and 2008, which exceeded the Company’s then carrying value of the loan. The Company received cash from the sale of collateral in early 2011 in the amount of $0.1 million. As a result, the carrying value of the loan at December 31, 2010 was $0.1 million.
In the normal course of business, the Company’s directors, officers and employees hold board positions of companies in which the Company has a direct or indirect ownership interest.
| |
| 15. | Commitments and Contingencies |
The Company and its partner companies are involved in various claims and legal actions arising in the ordinary course of business. While in the current opinion of the Company the ultimate disposition of these matters will not have a material adverse effect on the Company’s consolidated financial position or results of operations, no assurance can be given as to the outcome of these actions, and one or more adverse rulings could have a material adverse effect on the Company’s consolidated financial position and results of operations or that of its partner companies. The Company records costs associated with legal fees as such services are rendered.
The Company leases its corporate headquarters and office equipment under leases expiring at various dates to 2015. Total rental expense under operating leases was $0.5 million, $0.8 million and $1.9 million in 2010, 2009 and 2008, respectively. Rent expense includes amounts attributed to Clarient prior to its deconsolidation. Future minimum lease payments under non-cancelable operating leases with initial or remaining terms of one year or more
74
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
at December 31, 2010, are (in millions): $0.5 — 2011; $0.5 — 2012; $0.5 — 2013; $0.4 — 2014; and $0.0 thereafter.
Not including the Laureate Pharma lease guaranty described below, the Company had outstanding guarantees of $3.8 million at December 31, 2010 related to the Company’s general partner interest in a private equity fund.
The Company has committed capital of approximately $0.4 million, including conditional commitments to provide non-consolidated partner companies with additional funding and commitments made to various private equity funds in prior years. These commitments be funded during the next 12 months.
Under certain circumstances, the Company may be required to return a portion or all of the distributions it received as a general partner of certain private equity funds (“clawback”). The maximum clawback the Company could be required to return due to our general partner interest is approximately $2.2 million, of which $0.9 million was reflected in Accrued expenses and other current liabilities and $1.3 million was reflected in other long-term liabilities on the Consolidated Balance Sheet at December 31, 2010.
The Company’s ownership in the funds which have potential clawback liabilities ranges from19-30%. The clawback liability is joint and several, such that the Company may be required to fund the clawback for other general partners should they default. The funds have taken several steps to reduce the potential liabilities should other general partners default, including withholding all general partner distributions in escrow and adding rights of set-off among certain funds. The Company believes its liability due to the default of other general partners is remote.
As described in Note 2, in connection with the Bundle Transaction, an aggregate of $6.4 million of the gross proceeds of the sale were placed in escrow pending the expiration of a predetermined notification period, subject to possible extension in the event of a claim against the escrowed amounts. On April 25, 2009, the purchaser in the Bundle Transaction notified the Company of claims being asserted against the entire escrowed amounts. The Company does not believe that such claims are valid and has instituted legal action to obtain the release of such amounts from escrow. The proceeds being held in escrow will remain there until the dispute over the claims has been settled or determined pursuant to legal process.
The Company remains guarantor of Laureate Pharma’s Princeton, New Jersey facility lease (the “Laureate Lease Guaranty”). Such guarantee may extend through the lease expiration in 2016 under certain circumstances. However, the Company is entitled to indemnification in connection with the continuation of such guaranty. As of December 31, 2010, scheduled lease payments to be made by Laureate Pharma over the remaining lease term equaled $7.1 million.
The Company provided a $6.3 million letter of credit expiring on March 19, 2019 to the landlord of CompuCom Systems, Inc.’s Dallas headquarters as required in connection with the sale of CompuCom Systems in 2004.
In October 2001, the Company entered into an agreement with its former Chairman and Chief Executive Officer, to provide for annual payments of $650,000 per year and certain health care and other benefits for life. The related current liability of $0.8 million was included in Accrued expenses and the long-term portion of $3.2 million was included in Other long-term liabilities on the Consolidated Balance Sheet at December 31, 2010.
The Company has agreements with certain employees that provide for severance payments to the employee in the event the employee is terminated without cause or an employee terminates his employment for “good reason.” The maximum aggregate exposure under the agreements was approximately $8 million at December 31, 2010.
| |
| 16. | Parent Company Financial Information |
Parent company financial information is provided to present the financial position and results of operations of the Company as if the consolidated partner companies (see Note 1) were accounted for under the equity method of accounting for all periods presented during which the Company owned its interest in these companies. Given no
75
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
partner companies were consolidated during the year ended December 31, 2010 only the Statements of Operations and Cash Flows for the years ended December 31, 2009 and 2008 are presented below.
Parent Company Statements of Operations
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Operating expenses | | $ | (17,807 | ) | | $ | (18,415 | ) |
| Other income (loss), net | | | 108,881 | | | | 10,275 | |
| Recovery — related party | | | — | | | | 5 | |
| Interest income | | | 476 | | | | 3,076 | |
| Interest expense | | | (2,889 | ) | | | (3,852 | ) |
| Equity loss | | | (22,435 | ) | | | (33,896 | ) |
| | | | | | | | | |
| Net income (loss) from continuing operations before income taxes | | | 66,226 | | | | (42,807 | ) |
| Income tax benefit | | | 14 | | | | 30 | |
| Equity income (loss) attributable to discontinued operations | | | 1,370 | | | | (9,236 | ) |
| | | | | | | | | |
| Net income (loss) | | $ | 67,610 | | | $ | (52,013 | ) |
| | | | | | | | | |
76
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Parent Company Statements of Cash Flows
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| |
Cash Flows from Operating Activities: | | | | | | | | |
| Net income (loss) | | $ | 67,610 | | | $ | (52,013 | ) |
| Adjustments to reconcile to net cash used in operating activities: | | | | | | | | |
| Equity (income) loss from discontinued operations | | | (1,370 | ) | | | 9,236 | |
| Depreciation | | | 130 | | | | 166 | |
| Equity loss | | | 22,435 | | | | 33,896 | |
| Non-cash compensation charges | | | 2,982 | | | | 1,738 | |
| Other income (loss), net | | | (108,881 | ) | | | (10,275 | ) |
| Changes in assets and liabilities, net of effect of acquisitions and dispositions | | | 2,412 | | | | 3,128 | |
| | | | | | | | | |
| Net cash used in operating activities | | | (14,682 | ) | | | (14,124 | ) |
| | | | | | | | | |
Cash Flows from Investing Activities | | | | | | | | |
| Proceeds from sales of and distributions from companies and funds | | | 61,302 | | | | 4,263 | |
| Advances to partner companies | | | (7,150 | ) | | | (23,731 | ) |
| Repayments of advances to partner companies | | | 21,179 | | | | 6,913 | |
| Acquisitions of ownership interests in partner companies and funds, net of cash acquired | | | (35,939 | ) | | | (30,496 | ) |
| Increase in marketable securities | | | (73,187 | ) | | | (75,809 | ) |
| Decrease in marketable securities | | | 48,822 | | | | 61,698 | |
| Decrease in restricted cash | | | 861 | | | | — | |
| Capital expenditures | | | (27 | ) | | | (28 | ) |
| Proceeds from sale of discontinued operations | | | — | | | | 84,517 | |
| | | | | | | | | |
| Net cash provided by investing activities | | | 15,861 | | | | 27,327 | |
| | | | | | | | | |
Cash Flows from Financing Activities: | | | | | | | | |
| Repurchase of convertible senior debentures | | | (7,271 | ) | | | (33,494 | ) |
| Issuance of Company common stock, net | | | 270 | | | | 115 | |
| Repurchase of Company common stock | | | (44 | ) | | | (1,296 | ) |
| | | | | | | | | |
| Net cash used in financing activities | | | (7,045 | ) | | | (34,675 | ) |
| | | | | | | | | |
Net Decrease in Cash and Cash Equivalents | | | (5,866 | ) | | | (21,472 | ) |
| Cash and Cash Equivalents at beginning of period | | | 73,213 | | | | 94,685 | |
| | | | | | | | | |
Cash and Cash Equivalents at end of period | | $ | 67,347 | | | $ | 73,213 | |
| | | | | | | | | |
| |
| 17. | Supplemental Cash Flow Information |
During the years ended December 31, 2010, 2009 and 2008, the Company converted $2.7 million, $0.4 million and $2.1 million, respectively, of advances to partner companies into ownership interests in partner companies.
Cash payments for interest in the years ended December 31, 2010, 2009 and 2008 were $1.5 million, $1.4 million and $0.9 million, respectively. In addition, during the year ended December 31, 2010, interest payments of $2.2 million on the 2014 Debentures were made using restricted cash equivalents and during the years ended December 31, 2009 and 2008, interest payments on the 2024 Debentures of $1.1 million and $3.4 million, respectively, were made using restricted marketable securities.
77
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
As discussed in Note 7, during the year ended December 31, 2010, the Company completed a non-cash exchange of $46.9 million in face value of its 2024 Debentures for the same amount in face value of its newly issued 2014 Debentures.
Cash paid for taxes in the years ended December 31, 2010, 2009 and 2008 was $0.0 million in each year.
As of December 31, 2010, the Company held an interest in 13 non-consolidated partner companies. The Company’s reportable operating segments are Life Sciences and Technology.
The Company’s active partner companies as of December 31, 2010 by segment were as follows for the years ended December 31, 2010, 2009 and 2008:
Life Sciences
| | | | | | | | | | | | | | | |
| | | Safeguard Primary Ownership as of December 31 | | Accounting
|
Partner Company | | 2010 | | 2009 | | 2008 | | Method |
| |
| Advanced BioHealing, Inc. | | | 28.1 | % | | | 28.3 | % | | | 28.3 | % | | Equity |
| Alverix, Inc. | | | 49.6 | % | | | 49.6 | % | | | 50.0 | % | | Equity |
| Good Start Genetics, Inc. | | | 26.3 | % | | | NA | | | | NA | | | Equity |
| Molecular Biometrics, Inc. | | | 35.0 | % | | | 35.4 | % | | | 37.8 | % | | Equity |
| NuPathe, Inc. | | | 18.1 | % | | | 22.9 | % | | | 23.5 | % | | Available-for-sale(1) |
| Tengion, Inc. | | | 4.8 | % | | | 4.5 | % | | | 4.5 | % | | Available-for-sale(2) |
| | |
| (1) | | The Company’s ownership interest in NuPathe is accounted for asavailable-for-sale securities following NuPathe’s completion of an initial public offering in August 2010. The Company previously accounted for NuPathe under the equity method. |
| |
| (2) | | The Company’s ownership interest in Tengion is accounted for asavailable-for-sale securities following Tengion’s completion of an initial public offering in April 2010. The Company previously accounted for Tengion under the cost method. |
Technology
| | | | | | | | | | | | | | | |
| | | Safeguard Primary Ownership as of December 31 | | | Accounting
|
Partner Company | | 2010 | | | 2009 | | | 2008 | | | Method |
| |
| Advantedge Healthcare Solutions, Inc. | | | 40.2 | % | | | 39.7 | % | | | 37.7 | % | | Equity |
| SafeCentral, Inc. (formerly | | | | | | | | | | | | | | |
| Authentium, Inc.) | | | 20.1 | % | | | 20.0 | % | | | 20.0 | % | | Equity |
| Beyond.com Inc. | | | 38.3 | % | | | 38.3 | % | | | 37.1 | % | | Equity |
| Bridgevine, Inc. | | | 22.8 | % | | | 23.6 | % | | | 20.8 | % | | Equity |
| MediaMath, Inc. | | | 17.3 | % | | | 17.5 | % | | | NA | | | Cost |
| Portico Systems, Inc. | | | 45.4 | % | | | 45.4 | % | | | 46.8 | % | | Equity |
| Swap.com (formerly Swaptree, Inc.) | | | 45.6 | % | | | 29.3 | % | | | 29.3 | % | | Equity |
Results of the Life Sciences and Technology segments reflect the equity income (loss) of their respective equity method partner companies, other income (loss) associated with cost method partner companies and the gains or losses on the sale of their respective partner companies.
78
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Management evaluates its Life Sciences and Technology segments’ performance based on net loss which is based on the number of partner companies accounted for under the equity method, the Company’s voting ownership percentage in these partner companies and the net results of operations of these partner companies and any impairment charges or gain (loss) on sale of partner companies.
Other Items include certain expenses which are not identifiable to the operations of the Company’s operating business segments. Other Items primarily consist of general and administrative expenses related to corporate operations, including employee compensation, insurance and professional fees, including legal and finance, interest income, interest expense, other income (loss) and equity income (loss) related to private equity fund holdings. Other Items also include income taxes, which are reviewed by management independent of segment results.
Prior to its sale in December 2010, Clarient was included in the Life Sciences segment for all periods presented. As of May 14, 2009 the Company accounted for its interest in Clarient under the fair value method. Prior to May 14, 2009, Clarient was consolidated.
Revenue related entirely to Clarient prior to its deconsolidation and was attributed to geographic areas based on where the services were performed or the customer’s shipped to location. A majority of the Company’s revenue was generated in the United States.
As of December 31, 2010 and 2009, the Company’s assets were located in the United States.
Segment assets in Other items included primarily cash, cash equivalents, cash held in escrow and marketable securities of $232.3 million and $113.3 million at December 31, 2010 and 2009, respectively, excluding discontinued operations.
The following represents segment data from continuing operations:
| | | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, 2010 |
| | | | | | | | | | | Total
|
| | | Life
| | | | Total
| | Other
| | Continuing
|
| | | Sciences | | Technology | | Segments | | Items | | Operations |
| | | (In thousands) |
| |
| Revenue | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Operating income (loss) | | | — | | | | — | | | | — | | | | (20,847 | ) | | | (20,847 | ) |
| Equity loss | | | (11,786 | ) | | | (10,039 | ) | | | (21,825 | ) | | | (4 | ) | | | (21,829 | ) |
| Net income (loss) from continuing operations | | | 70,658 | | | | (10,003 | ) | | | 60,655 | | | | (33,541 | ) | | | 27,114 | |
Segment Assets: | | | | | | | | | | | | | | | | | | | | |
| December 31, 2010 | | | 37,710 | | | | 43,325 | | | | 81,035 | | | | 256,015 | | | | 337,050 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, 2009 |
| | | | | | | | | | | Total
|
| | | Life
| | | | Total
| | Other
| | Continuing
|
| | | Sciences | | Technology | | Segments | | Items | | Operations |
| | | (In thousands) |
| |
| Revenue | | $ | 34,839 | | | $ | — | | | $ | 34,839 | | | $ | — | | | $ | 34,839 | |
| Operating income (loss) | | | 1,621 | | | | — | | | | 1,621 | | | | (17,807 | ) | | | (16,186 | ) |
| Equity loss | | | (16,283 | ) | | | (6,896 | ) | | | (23,179 | ) | | | (48 | ) | | | (23,227 | ) |
| Net income (loss) from continuing operations | | | 99,289 | | | | (12,742 | ) | | | 86,547 | | | | (19,749 | ) | | | 66,798 | |
Segment Assets: | | | | | | | | | | | | | | | | | | | | |
| December 31, 2009 | | | 117,529 | | | | 41,876 | | | | 159,405 | | | | 122,694 | | | | 282,099 | |
79
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, 2008 |
| | | | | | | | | | | Total
|
| | | Life
| | | | Total
| | Other
| | Continuing
|
| | | Sciences | | Technology | | Segments | | Items | | Operations |
| | | (In thousands) |
| |
| Revenue | | $ | 73,736 | | | $ | — | | | $ | 73,736 | | | $ | — | | | $ | 73,736 | |
| Operating loss | | | (1,600 | ) | | | — | | | | (1,600 | ) | | | (18,415 | ) | | | (20,015 | ) |
| Equity loss | | | (23,858 | ) | | | (10,696 | ) | | | (34,554 | ) | | | (143 | ) | | | (34,697 | ) |
| Net loss from continuing operations | | | (26,317 | ) | | | (12,947 | ) | | | (39,264 | ) | | | (6,779 | ) | | | (46,043 | ) |
Segment Assets: | | | | | | | | | | | | | | | | | | | | |
| December 31, 2008 | | | 84,508 | | | | 41,050 | | | | 125,558 | | | | 106,844 | | | | 232,402 | |
Net loss from continuing operations from Other Items was as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
| |
| Corporate operations | | $ | (33,541 | ) | | $ | (19,763 | ) | | $ | (6,803 | ) |
| Income tax benefit | | | — | | | | 14 | | | | 24 | |
| | | | | | | | | | | | | |
| | | $ | (33,541 | ) | | $ | (19,749 | ) | | $ | (6,779 | ) |
| | | | | | | | | | | | | |
80
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| |
| 19. | Selected Quarterly Financial Information (Unaudited) |
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31 | | | June 30 | | | September 30 | | | December 31 | |
| | | (In thousands except per share data) | |
| |
2010: | | | | | | | | | | | | | | | | |
| Revenue | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | |
| Cost of sales | | | — | | | | — | | | | — | | | | — | |
| Selling, general and administrative | | | 4,833 | | | | 4,910 | | | | 4,256 | | | | 6,848 | |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 4,833 | | | | 4,910 | | | | 4,256 | | | | 6,848 | |
| | | | | | | | | | | | | | | | | |
| Operating loss | | | (4,833 | ) | | | (4,910 | ) | | | (4,256 | ) | | | (6,848 | ) |
| Other income (loss), net | | | (11,297 | ) | | | 14,408 | | | | 8,144 | | | | 63,554 | |
| Interest income | | | 97 | | | | 239 | | | | 180 | | | | 202 | |
| Interest expense | | | (730 | ) | | | (1,657 | ) | | | (1,674 | ) | | | (1,676 | ) |
| Equity loss | | | (5,009 | ) | | | (5,155 | ) | | | (1,801 | ) | | | (9,864 | ) |
| | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations before income taxes | | | (21,772 | ) | | | 2,925 | | | | 593 | | | | 45,368 | |
| Income tax benefit | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. | | $ | (21,772 | ) | | $ | 2,925 | | | $ | 593 | | | $ | 45,368 | |
| | | | | | | | | | | | | | | | | |
| Basic income (loss) per share(a) | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | $ | (1.07 | ) | | $ | 0.14 | | | $ | 0.03 | | | $ | 2.20 | |
| | | | | | | | | | | | | | | | | |
| Diluted income (loss) per share(a) | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | $ | (1.07 | ) | | $ | 0.13 | | | $ | 0.03 | | | $ | 1.84 | |
| | | | | | | | | | | | | | | | | |
2009: | | | | | | | | | | | | | | | | |
| Revenue | | $ | 23,192 | | | $ | 11,647 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | |
| Cost of sales | | | 8,966 | | | | 4,845 | | | | — | | | | — | |
| Selling, general and administrative | | | 17,089 | | | | 10,983 | | | | 4,237 | | | | 4,905 | |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 26,055 | | | | 15,828 | | | | 4,237 | | | | 4,905 | |
| | | | | | | | | | | | | | | | | |
| Operating loss | | | (2,863 | ) | | | (4,181 | ) | | | (4,237 | ) | | | (4,905 | ) |
| Other income (loss), net | | | (245 | ) | | | 158,573 | | | | (1,908 | ) | | | (47,539 | ) |
| Interest income | | | 157 | | | | 111 | | | | 111 | | | | 101 | |
| Interest expense | | | (926 | ) | | | (817 | ) | | | (728 | ) | | | (693 | ) |
| Equity loss | | | (5,513 | ) | | | (7,446 | ) | | | (4,827 | ) | | | (5,441 | ) |
| | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations before income taxes | | | (9,390 | ) | | | 146,240 | | | | (11,589 | ) | | | (58,477 | ) |
| Income tax benefit | | | — | | | | 14 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | | (9,390 | ) | | | 146,254 | | | | (11,589 | ) | | | (58,477 | ) |
| Income from discontinued operations, net of tax | | | 1,500 | | | | — | | | | — | | | | 475 | |
| | | | | | | | | | | | | | | | | |
| Net income (loss) | | | (7,890 | ) | | | 146,254 | | | | (11,589 | ) | | | (58,002 | ) |
| Net loss attributable to noncontrolling interest | | | (1,139 | ) | | | (24 | ) | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Net income (loss) attributable to Safeguard Scientifics, Inc. | | $ | (9,029 | ) | | $ | 146,230 | | | $ | (11,589 | ) | | $ | (58,002 | ) |
| | | | | | | | | | | | | | | | | |
| Basic income (loss) per share(a) | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | $ | (0.49 | ) | | $ | 7.21 | | | $ | (0.57 | ) | | $ | (2.88 | ) |
| Net income from discontinued operations | | | 0.04 | | | | — | | | | — | | | | 0.03 | |
| | | | | | | | | | | | | | | | | |
| | | $ | (0.45 | ) | | $ | 7.21 | | | $ | (0.57 | ) | | $ | (2.85 | ) |
| | | | | | | | | | | | | | | | | |
| Diluted income (loss) per share(a) | | | | | | | | | | | | | | | | |
| Net income (loss) from continuing operations | | $ | (0.49 | ) | | $ | 6.56 | | | $ | (0.57 | ) | | $ | (2.88 | ) |
| Net income from discontinued operations | | | 0.04 | | | | — | | | | — | | | | 0.03 | |
| | | | | | | | | | | | | | | | | |
| | | $ | (0.45 | ) | | $ | 6.56 | | | $ | (0.57 | ) | | $ | (2.85 | ) |
| | | | | | | | | | | | | | | | | |
| | |
| (a) | | Per share amounts for the quarters have each been calculated separately. Accordingly, quarterly amounts may not add to the annual amounts because of differences in the average common shares outstanding during each period. Additionally, in regard to diluted per share amounts only, quarterly amounts may not add to the annual amounts because of the inclusion of the effect of potentially dilutive securities only in the periods in which such effect would have been dilutive, and because of the adjustments to net income (loss) for the dilutive effect of partner company common stock equivalents and convertible securities. |
81
SAFEGUARD SCIENTIFICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| |
| 20. | Trade Accounts Receivable |
The Company does not have trade accounts receivable subsequent to the deconsolidation of Clarient on May 14, 2009. The following table summarizes the activity in the allowance for doubtful accounts through that date:
| | | | | |
| | | (In thousands) | |
| |
| Balance, December 31, 2007 | | $ | 3,370 | |
| Charged to costs and expenses | | | 12,199 | |
| Charge-offs | | | (7,524 | ) |
| | | | | |
| Balance, December 31, 2008 | | | 8,045 | |
| Charged to costs and expenses | | | 3,856 | |
| Charge-offs | | | (3,684 | ) |
| Deconsolidation of Clarient | | | (8,217 | ) |
| | | | | |
| Balance, December 31, 2009 | | | — | |
| Charged to costs and expenses | | | — | |
| Charge-offs | | | — | |
| | | | | |
| Balance, December 31, 2010 | | $ | — | |
| | | | | |
82
| |
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
None.
| |
| Item 9A. | Controls and Procedures |
| |
| (a) | Evaluation of Disclosure Controls and Procedures |
We maintain disclosure controls and procedures, as such term is defined inRules 13a-15(e) and15d-15(e) of the Securities Exchange Act of 1934 (“the Exchange Act”), that are designed to provide reasonable assurance that the information required to be disclosed by us in reports filed under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding disclosure. A controls system cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected. Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2010. Based on that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures as of December 31, 2010 are functioning effectively.
Our business strategy involves the acquisition of new businesses on an ongoing basis, most of which are young, growing companies. Typically, these companies historically have not had all of the controls and procedures they would need to comply with the requirements of the Securities Exchange Act of 1934 and the rules promulgated thereunder. These companies also frequently develop new products and services. Following an acquisition, or the launch of a new product or service, we work with the company’s management to implement all necessary controls and procedures.
| |
| (b) | Management’s Report on Internal Control Over Financial Reporting |
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2010. In making this assessment, management used the framework established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). As a result of this assessment and based on the criteria in the COSO framework, management has concluded that, as of December 31, 2010, the Company’s internal control over financial reporting was effective.
Our independent registered public accounting firm, KPMG LLP, has audited the effectiveness of our internal control over financial reporting as of December 31, 2010. Their opinion on the effectiveness of our internal control over financial reporting and their opinion on our Consolidated Financial Statements are included in Item 8 in thisForm 10-K.
83
| |
| (c) | Change in Internal Control over Financial Reporting |
No change in our internal control over financial reporting occurred during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| |
| Item 9B. | Other Information |
None
PART III
| |
| Item 10. | Directors, Executive Officers and Corporate Governance |
Incorporated by reference to the portion of our Definitive Proxy Statement entitled “Election of Directors,” “Corporate Governance and Board Matters” and “Section 16(a) Beneficial Ownership Reporting Compliance.” Information about our Executive Officers is included in Annex to Part I above.
| |
| Item 11. | Executive Compensation |
Incorporated by reference to the portions of our Definitive Proxy Statement entitled “Compensation Discussion and Analysis,” “Compensation Committee Report” and “Executive Compensation.”
| |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Incorporated by reference to the portion of our Definitive Proxy Statement entitled “Stock Ownership of Certain Beneficial Owners, Directors and Officers.”
SECURITIES AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS
Our equity compensation plans provide a broad-based program designed to attract and retain talent while creating alignment with the long-term interests of our shareholders. Employees at all levels participate in our equity compensation plans. In addition, members of our Board of Directors (“Board”) and members of our Technology and Life Sciences Advisory Boards (“Advisory Boards”) receive equity grants for their service on our Board and Advisory Boards, respectively. Members of our Board also receive deferred stock unit awards and are eligible to defer directors’ fees and receive deferred stock units with a value equal to the directors’ fees deferred and matching deferred stock units equal to 25% of the directors’ fees deferred.
The 2001 Associates Equity Compensation Plan (“2001 Plan”) provides for the grant of nonqualified stock options, stock appreciation rights, restricted stock, performance units, and other stock-based awards to employees, consultants or advisors of Safeguard and its subsidiaries, provided that no grants can be made under this plan to executive officers or directors of Safeguard. Under the NYSE rules that were in effect at the time this plan was adopted in 2001, shareholder approval of the plan was not required. Except for the persons eligible to participate in the 2001 Plan and the inability to grant incentive stock options under the 2001 Plan, the terms of the 2001 plan are substantially the same as the other equity compensation plans approved by our shareholders (which have been described in previous filings).
A total of 900,000 shares of our common stock are authorized for issuance under the 2001 Plan. At December 31, 2010, 418,943 shares were subject to outstanding options and performance stock units, 108,326 shares were available for future issuance, and 372,731 shares had been issued under the 2001 Plan. The 2001 Plan expired by its terms on February 21, 2011. Equity grants previously awarded under this plan that have not yet expired or otherwise become unexercisable continue to be administered in accordance with the terms of the grants. Any portions of outstanding equity grants under the 2001 Plan that expire or become unexercisable for any reason shall be cancelled and shall be unavailable for future issuance.
84
During 2005, 2007 and 2008, the Compensation Committee granted “employee inducement” awards to four newly hired executive officers. The awards were granted outside of Safeguard’s existing equity compensation plans in accordance with NYSE rules and consisted of options to purchase up to an aggregate of 1,416,665 shares of Safeguard common stock. All of these “employee inducement” awards were granted with an eight-year term and a per share exercise price equal to the average of the high and low prices of Safeguard common stock on the grant date. Of the shares underlying the “employee inducement” awards that were outstanding at December 31, 2010, 354,165 shares are subject to time-based vesting, with an aggregate of 88,541 shares vesting on the first anniversary of the grant date and 265,624 shares vesting in 36 equal monthly installments thereafter. The remaining shares underlying the “employee inducement” awards that were outstanding at December 31, 2010 vest incrementally based upon the achievement of certain specified levels of increase in Safeguard’s stock price. With the exception of the market-based vesting provisions, the terms and provisions of the employee inducement awards are substantially the same as options previously awarded to other executives under Safeguard’s equity compensation plans.
The following table provides information as of December 31, 2010 about the securities authorized for issuance under our equity compensation plans. The material features of our equity compensation plans are described in Note 10 to the Consolidated Financial Statements filed as part of our Annual Report onForm 10-K for the year ended December 31, 2010.
Equity Compensation Plan Information
| | | | | | | | | | | | | |
| | | | | | | | | Number of Securities
| |
| | | | | | | | | Remaining Available for
| |
| | | Number of Securities to
| | | Weighted-Average
| | | Future Issuance Under
| |
| | | Be Issued Upon Exercise
| | | Exercise Price of
| | | Equity Compensation Plans
| |
| | | of Outstanding Options,
| | | Outstanding Options,
| | | (Excluding Securities
| |
| | | Warrants and Rights(1)
| | | Warrants and Rights(2)
| | | Reflected in Column (a))
| |
Plan Category | | (a) | | | (b) | | | (c) | |
| |
| Equity compensation plans approved by security holders(3) | | | 1,803,243 | | | $ | 10.3345 | | | | 755,195 | |
| Equity compensation plans not approved by security holders(4) | | | 1,835,609 | | | $ | 9.3885 | | | | 108,326 | |
| Total | | | 3,638,852 | | | $ | 9.8277 | | | | 863,521 | |
| | |
| (1) | | Includes a total of 243,762 shares underlying performance stock units and deferred stock units awarded for no consideration and 73,786 shares underlying deferred stock units awarded to directors in lieu of all or a portion of directors’ fees. |
| |
| (2) | | The weighted average exercise price calculation excludes 317,548 shares underlying outstanding deferred stock units and performance stock units included in column (a) which are payable in stock, on aone-for-one basis. |
| |
| (3) | | Represents awards granted under the 1999 Equity Compensation Plan and the 2004 Equity Compensation Plan and shares available for issuance under the 2004 Equity Compensation Plan. |
| |
| (4) | | Includes awards granted and shares available for issuance under the 2001 Plan and 1,416,665 “employee inducement” awards. |
| |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Incorporated by reference to the portions of the Definitive Proxy Statement entitled “Corporate Governance Principles and Board Matters — ‘Board Independence’ and “Review and Approval of Transactions with Related Persons” and “Relationships and Transactions with Management and Others.”
| |
| Item 14. | Principal Accountant Fees and Services |
Incorporated by reference to the portion of the Definitive Proxy Statement entitled “Independent Public Accountant — Audit Fees.”
85
PART IV
| |
| Item 15. | Exhibits and Financial Statement Schedules |
| |
| (a) | Consolidated Financial Statements and Schedules |
Incorporated by reference to Item 8 of this Report onForm 10-K.
The exhibits required to be filed as part of this Report are listed in the exhibit index below.
| |
| (c) | Financial Statement Schedules |
The separate consolidated financial statements of Clarient, Inc. as of December 31, 2009 and for the year ended December 31, 2009 required to be included in this report pursuant toRule 3-09 ofRegulation S-X, are filed as Exhibit 99.1.
Exhibits
The following is a list of exhibits required by Item 601 ofRegulation S-K filed as part of this Report. For exhibits that previously have been filed, the Registrant incorporates those exhibits herein by reference. The exhibit table below includes the Form Type and Filing Date of the previous filing and the location of the exhibit in the previous filing which is being incorporated by reference herein. Documents which are incorporated by reference to filings by parties other than the Registrant are identified in footnotes to this table.
| | | | | | | | | | | |
| | | | | Incorporated Filing Reference |
Exhibit
| | | | Form Type & Filing
| | Original
|
Number | | Description | | Date | | Exhibit Number |
| |
| | 2 | .1.1 | | Purchase Agreement, dated as of February 29, 2008, by and between Safeguard Scientifics, Inc., as Seller, and Saints Capital Dakota, L.P., as Purchaser. | | Form 8-K
3/4/08 | | | 2 | .1 |
| | 2 | .1.2 | | First Amendment to Purchase Agreement, dated May 6, 2008, by and between Safeguard Scientifics, Inc., as Seller, and Saints Capital Dakota, L.P., as Purchaser | | Form 8-K
5/7/08 | | | 2 | .1 |
| | 3 | .1.1 | | Seconded Amended and Restated Articles of Incorporation of Safeguard Scientifics, Inc. | | Form 8-K
10/25/07 | | | 3 | .1 |
| | 3 | .1.2 | | Amendment to Seconded Amended and Restated Articles of Incorporation of Safeguard Scientifics, Inc. | | Form 8-K
8/27/09 | | | 3 | .1 |
| | 3 | .1.3 | | Statement with Respect to Shares | | Registration
Statement on
Form S-4
12/17/10 | | | 3 | .1.3 |
| | 3 | .2 | | Amended and Restated By-laws of Safeguard Scientifics, Inc. | | Form 8-K
10/25/07 | | | 3 | .2 |
| | 4 | .1 | | Indenture, dated as of February 18, 2004, between Safeguard Scientifics, Inc. and Wachovia Bank, National Association, as trustee, including the form of 2.625% Convertible Senior Debentures due 2024 | | Form 10-K
3/15/04 | | | 4 | .10 |
| | 4 | .2 | | Indenture, dated as of March 26, 2010, by and between Safeguard Scientifics, Inc. and U.S. Bank, National Association | | Form 8-K
3/30/10 | | | 4 | .1 |
| | 4 | .3 | | Global Note representing 10.125% Convertible Senior Debentures due March 15, 2014 | | Form 8-K
3/30/10 | | | 4 | .2 |
86
| | | | | | | | | | | |
| | | | | Incorporated Filing Reference |
Exhibit
| | | | Form Type & Filing
| | Original
|
Number | | Description | | Date | | Exhibit Number |
| |
| | 4 | .4 | | Escrow Agreement, dated as of March 26, 2010, by and among Safeguard Scientifics, Inc., U.S. Bank, National Association (as trustee) and U.S. Bank, National Association (in its capacity as escrow agent) | | Form 8-K
3/30/10 | | | 4 | .3 |
| | 10 | .1* | | Safeguard Scientifics, Inc. 1999 Equity Compensation Plan, as amended and restated on October 21, 2008 | | Form 10-Q
11/6/08 | | | 10 | .4 |
| | 10 | .2 | | Safeguard Scientifics, Inc. 2001 Associates Equity Compensation Plan, as amended and restated on October 21, 2008 | | Form 10-Q
11/6/08 | | | 10 | .5 |
| | 10 | .3* | | Safeguard Scientifics, Inc. 2004 Equity Compensation Plan, as amended and restated on July 13, 2009 (attached to the Company’s Definitive Proxy Statement filed on July 23, 2009) | | Form 10-K
3/16/10 | | | 10 | .3 |
| | 10 | .4* | | Safeguard Scientifics, Inc. Executive Deferred Compensation Plan (amended and restated as of January 1, 2009) | | Form 10-K
3/19/09 | | | 10 | .4 |
| | 10 | .5* | | Management Incentive Plan | | Form 8-K
4/25/08 | | | 10 | .1 |
| | 10 | .6* | | Compensation Summary — Non-employee Directors | | Form 10-Q
7/30/10 | | | 10 | .1 |
| | 10 | .7.1* | | Amended and Restated Agreement by and between Safeguard Scientifics, Inc. and Peter J. Boni dated December 5, 2008 | | Form 10-K
3/19/09 | | | 10 | .7 |
| | 10 | .7.2* | | Compensation Agreement by and between Safeguard Scientifics, Inc. and Peter J. Boni dated December 14, 2009 | | Form 10-K
3/16/10 | | | 10 | .7.2 |
| | 10 | .8.1* | | Amended and Restated Agreement by and between Safeguard Scientifics, Inc. and James A. Datin dated December 31, 2008 | | Form 10-K
3/19/09 | | | 10 | .8 |
| | 10 | .8.2* | | Compensation Agreement by and between Safeguard Scientifics, Inc. and James A. Datin dated December 14, 2009 | | Form 10-K
3/16/10 | | | 10 | .8.2 |
| | 10 | .9.1* | | Agreement by and between Safeguard Scientifics, Inc. and Stephen Zarrilli dated as of May 28, 2008 | | Form 8-K
5/29/08 | | | 10 | .1 |
| | 10 | .9.2* | | Letter Amendment dated December 9, 2008, to Agreement by and between Safeguard Scientifics, Inc. and Stephen Zarrilli dated as of May 28, 2008 | | Form 10-K
3/19/09 | | | 10 | .9.2 |
| | 10 | .10.1* | | Agreement by and between Safeguard Scientifics, Inc. and Kevin L. Kemmerer dated December 29, 2008 | | Form 10-K
3/19/09 | | | 10 | .11 |
| | 10 | .10.2* | | Compensation Agreement by and between Safeguard Scientifics, Inc. and Kevin L. Kemmerer dated December 14, 2009 | | Form 10-K
3/16/10 | | | 10 | .10.2 |
| | 10 | .11.1* | | Amended and Restated Letter Agreement by and between Safeguard Scientifics, Inc. and Brian J. Sisko dated December 3, 2008 | | Form 10-K
3/19/09 | | | 10 | .12 |
| | 10 | .11.2* | | Compensation Agreement by and between Safeguard Scientifics, Inc. and Brian J. Sisko dated December 14, 2009 | | Form 10-K
3/16/10 | | | 10 | .11.2 |
| | 10 | .12.1 | | Amended and Restated Loan and Security Agreement dated as of May 27, 2009, by and among Silicon Valley Bank, Safeguard Scientifics, Inc., Safeguard Delaware, Inc. and Safeguard Scientifics (Delaware), Inc. | | Form 8-K
5/28/09 | | | 10 | .1 |
| | 10 | .12.2 | | Joinder and First Loan Modification Agreement dated as of December 31, 2010, by and among Silicon Valley Bank, Safeguard Scientifics, Inc., Safeguard Delaware, Inc., Safeguard Scientifics (Delaware), Inc. and Safeguard Delaware II, Inc. | | Form 8-K
1/4/11 | | | 10 | .1 |
87
| | | | | | | | | | | |
| | | | | Incorporated Filing Reference |
Exhibit
| | | | Form Type & Filing
| | Original
|
Number | | Description | | Date | | Exhibit Number |
| |
| | 10 | .13 | | Purchase and Sale Agreement dated as of December 9, 2005 by and among HarbourVest VII Venture Ltd., Dover Street VI L.P. and several subsidiaries and affiliated limited partnerships of Safeguard Scientifics, Inc. | | Form 10-K
3/13/06 | | | 10 | .36 |
| | 10 | .14 | | Exchange Agreement dated as of March 10, 2010, by and between Safeguard Scientifics, Inc. and First Manhattan Co. | | Form 8-K
3/11/10 | | | 10 | .1 |
| | 10 | .15 | | Exchange Agreement dated as of March 10, 2010, by and between Safeguard Scientifics, Inc. and the holders of the Old Debentures set forth on the schedules thereto | | Form 8-K
3/11/10 | | | 10 | .2 |
| | 10 | .16 | | Exchange Agreement dated as of March 10, 2010, by and between Safeguard Scientifics, Inc. and Prism Partners IV Leveraged Offshore Fund | | Form 8-K
3/11/10 | | | 10 | .3 |
| | 10 | .17 | | Exchange Agreement dated as of March 10, 2010, by and between Safeguard Scientifics, Inc. and Zazove Associates LLC | | Form 8-K
3/11/10 | | | 10 | .4 |
| | 14 | .1† | | Code of Business Conduct and Ethics | | — | | | — | |
| | 21 | .1† | | List of Subsidiaries | | — | | | — | |
| | 23 | .1† | | Consent of Independent Registered Public Accounting Firm — KPMG LLP | | — | | | — | |
| | 23 | .2† | | Consent of Independent Registered Public Accounting Firm — Deloitte & Touche LLP | | — | | | — | |
| | 31 | .1† | | Certification of Peter J. Boni pursuant toRules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934 | | — | | | — | |
| | 31 | .2† | | Certification of Stephen T. Zarrilli pursuant toRules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934 | | — | | | — | |
| | 32 | .1† | | Certification of Peter J. Boni pursuant to 18 U.S.C. Section 1350, as Adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | — | | | — | |
| | 32 | .2† | | Certification of Stephen T. Zarrilli pursuant to 18 U.S.C. Section 1350, as Adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | — | | | — | |
| | 99 | .1† | | Consolidated Financial Statements of Clarient, Inc. | | — | | | — | |
| | |
| † | | Filed herewith |
| |
| * | | These exhibits relate to management contracts or compensatory plans, contracts or arrangements in which directors and/or executive officers of the Registrant may participate. |
88
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Safeguard Scientifics, Inc.
Peter J. Boni
President and Chief Executive Officer
Dated: March 4, 2011
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | | | | |
Signature | | Title | | Date |
| |
| | | | | |
Peter J. Boni
Peter J. Boni | | President and Chief Executive Officer and Director (Principal Executive Officer) | | March 4, 2011 |
| | | | | |
Stephen T. Zarrilli
Stephen T. Zarrilli | | Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | | March 4, 2011 |
| | | | | |
Julie A. Dobson
Julie A. Dobson | | Director | | March 4, 2011 |
| | | | | |
Andrew E. Lietz
Andrew E. Lietz | | Chairman of the Board of Directors | | March 4, 2011 |
| | | | | |
George MacKenzie
George MacKenzie | | Director | | March 4, 2011 |
| | | | | |
George D. McClelland
George D. McClelland | | Director | | March 4, 2011 |
| | | | | |
Jack L. Messman
Jack L. Messman | | Director | | March 4, 2011 |
| | | | | |
John J. Roberts
John J. Roberts | | Director | | March 4, 2011 |
| | | | | |
Robert J. Rosenthal
Robert J. Rosenthal | | Director | | March 4, 2011 |
89