UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| | x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the fiscal year ended December 31, 2008 |
or
| | ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the transition period from ______________ to ______________ |
Commission file number: 0-19986
CELL GENESYS, INC.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 94-3061375 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. employer identification number) |
| |
400 Oyster Point Boulevard, Suite 525 South San Francisco, CA | | 94080 |
| (Address of principal executive offices) | | (Zip Code) |
(650) 266-3000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12 (b) of the Act:
| | |
Title of each class | | Name of exchange on which registered |
| Common Stock, $.001 Par Value | | The NASDAQ Stock Market LLC |
| Preferred Shares Purchase Rights | | |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check One):
| | | | | | | | | | |
| Large accelerated filer | | ¨ | | | | | Accelerated filer | | x | |
| Non-accelerated filer | | ¨ | | | (Do not check if a smaller reporting company) | | Smaller reporting company | | ¨ | |
Indicate by check mark whether the registrant is a shell company, as defined in Rule 12b-2 of the Exchange Act. Yes ¨ No x
As of June 30, 2008, the last business day of the registrant’s most recently completed second fiscal quarter the approximate aggregate market value of shares held by non-affiliates of the registrant (based on the closing sale price of shares on the NASDAQ Global Market on June 30, 2008) was $221.6 million, which excludes 652,779 shares of common stock held by directors and officers, and any stockholders whose ownership exceeded ten percent of the shares outstanding at June 30, 2008. Exclusion of these shares does not constitute a determination that each such person is an affiliate of the registrant.
As of March 6, 2009, the number of outstanding shares of the registrant’s Common Stock was 86,809,651.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant’s Proxy Statement for the 2009 Annual Meeting of Stockholders, to be filed within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K, are incorporated by reference into Part III of this Annual Report on Form 10-K. Except with respect to information specifically incorporated by reference into this Annual Report on Form 10-K, the Proxy Statement for the 2009 Annual Meeting of Stockholders is not deemed to be filed as part hereof.
TABLE OF CONTENTS
2
PART I
Statements made in this document other than statements of historical fact, including statements about us and our subsidiaries and our respective clinical trials, research programs, product pipelines, current and potential corporate partnerships, licenses and intellectual property, the adequacy of capital reserves and anticipated operating results and cash expenditures, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. As forward-looking statements, they are subject to a number of uncertainties that could cause actual results to differ materially from the statements made, including risks associated with the ability to successfully complete a strategic transaction, the success of research and product development programs, the issuance and validity of patents, the development and protection of proprietary technologies, the ability to raise capital, operating expense levels and other risks. Reference is made to discussions about risks associated with product development programs, intellectual property and other risks that may affect us under Item 1A, “Risk Factors” below. We do not undertake any obligation to update forward-looking statements. The following should be read in conjunction with our consolidated financial statements located elsewhere in this Annual Report on Form 10-K for the year ended December 31, 2008 and in other documents filed by us from time to time with the Securities and Exchange Commission.
In this Annual Report on Form 10-K, “Cell Genesys,” “we,” “us,” “our” and “the Registrant” refer to Cell Genesys, Inc.
Overview
We are a biotechnology company that was focused on the development and commercialization of novel biological therapies for patients with cancer. We were developing cell-based cancer immunotherapies and oncolytic virus therapies to treat different types of cancer. Our clinical stage cancer programs involved cell- or viral-based products that have been modified to impart disease-fighting characteristics that are not found in conventional chemotherapeutic agents. As part of our GVAX(TM) cancer immunotherapy programs, we initiated two Phase 3 clinical trials for GVAX immunotherapy for prostate cancer in July 2004 and June 2005, respectively, each under a Special Protocol Assessment, or SPA, with the United States Food and Drug Administration, or FDA. In March 2008, we entered into a worldwide collaboration agreement with Takeda Pharmaceutical Company Limited, or Takeda, for the development and commercialization of GVAX immunotherapy for prostate cancer. In August 2008, we terminated enrollment and administration of GVAX immunotherapy for prostate cancer in VITAL-2, the second of the two Phase 3 clinical trials in advanced prostate cancer. This action was taken based on the recommendation of the study’s Independent Data Monitoring Committee, or IDMC, which, in a routine safety review meeting of both the VITAL-1 and VITAL-2 trials, observed an imbalance in deaths between the two treatment arms of the VITAL-2 study, with a higher number of deaths in the GVAX immunotherapy in combination with Taxotere® (docetaxel) arm compared to the Taxotere plus prednisone arm. As a result, in September 2008 the FDA placed a partial clinical hold on the GVAX Phase 3 program for prostate cancer. In October 2008, we terminated the VITAL-1 Phase 3 clinical trial based on the results of a previously unplanned futility analysis conducted at our request by the IDMC which indicated that the trial had less than a 30 percent chance of meeting its predefined endpoint of an improvement in survival. In view of the termination of both the VITAL-1 and VITAL-2 trials, we placed on hold further development of GVAX immunotherapy for prostate cancer and implemented a substantial restructuring plan that resulted in the reduction of our staff of 290 by approximately 80 percent as of December 31, 2008, and approximately 90 percent as of the date of this report, with further reductions anticipated in the first half of 2009 as additional activities are phased out, and also resulted in Takeda terminating our collaborative agreement in December 2008.
Cell Genesys, Inc. was incorporated in the State of Delaware in 1988. Our common stock trades on the NASDAQ Global Market under the symbol “CEGE.” In January 2009, we temporarily relocated our principal executive offices to 24590 Clawiter Road, Hayward, CA 94545 from 500 Forbes Boulevard, South San
3
Francisco, California 94080. In February 2009, we relocated our principal executive offices to 400 Oyster Point Boulevard, Suite 525, South San Francisco, CA 94080. Our phone number is (650) 266-3000. Our Internet home page is located athttp://www.cellgenesys.com;however, the information in, or that can be accessed through, our home page is not part of this report. Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to these reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act are available, free of charge, on or through our Internet home page as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or SEC.
In March 2008, we entered into a worldwide collaborative agreement with Takeda for the development and commercialization of GVAX immunotherapy for prostate cancer. Under the terms of the agreement we granted exclusive worldwide commercial rights to GVAX immunotherapy for prostate cancer for the prevention, diagnosis, and treatment of prostate cancer and other urological neoplasms or urological hyperplasias. In exchange for these rights and in consideration for prior costs incurred by us in the development of GVAX immunotherapy for prostate cancer, Takeda made a non-refundable and non-creditable upfront payment of $50 million. We received full payment of the $50 million in April 2008.
Additionally, Takeda agreed to pay for all external development costs associated with the ongoing Phase 3 clinical development of GVAX immunotherapy for prostate cancer, including the cost of product, all internal and external additional development costs and all commercialization costs. As of December 31, 2008, we have received payments from Takeda of $23.9 million for development costs. We also have a receivable of $6.5 million due from Takeda for such costs incurred in the quarter ended December 31, 2008 which we collected in February 2009. Our decision to place on hold further development of GVAX immunotherapy for prostate cancer was agreed to by Takeda and resulted in Takeda terminating our collaborative agreement in December 2008. Further reimbursement revenue from Takeda will end during the first quarter of 2009.
In May 2008, we received gross proceeds of $30.0 million in a registered direct offering, before deducting placement agents’ fees and stock issuance costs of approximately $1.9 million, resulting in net proceeds of approximately $28.1 million, from the sale of 7.1 million shares of our common stock at $4.22 per share and warrants to purchase 8.5 million shares of our common stock at a price of $10.00 per share. The offering was made pursuant to our effective May 2007 shelf registration statement on Form S-3. These warrants became exercisable on November 14, 2008 for a period of seven years thereafter.
In October 2008, we repurchased an aggregate of approximately $26.3 million face value of our 3.125% Convertible Senior Notes due 2011, or Notes, at an overall discount of approximately 60 percent from face value in a series of privately negotiated transactions with institutional holders of the Notes, for aggregate consideration of approximately $10.5 million in cash, plus accrued but unpaid interest. This purchase of debt resulted in a net gain of approximately $15.5 million and a reduction of annualized interest expense by approximately $0.8 million.
In October 2008 we received a Nasdaq Staff Deficiency Letter, or Nasdaq Letter, indicating that we had become non-compliant with the minimum $1.00 bid price requirement for continued listing on The Nasdaq Global Market. The Nasdaq Letter indicated that in light of extraordinary market conditions, Nasdaq had determined to suspend enforcement of the minimum bid price and market value of publicly held shares requirements through January 16, 2009. Accordingly, the Nasdaq Letter stated that we have 180 calendar days from January 20, 2009, or until July 20, 2009, to regain compliance. Subsequently, on December 19, 2008, NASDAQ announced that given the continued extraordinary market conditions, NASDAQ is extending the suspension of the minimum bid price and market value of publicly held shares requirements through April 20, 2009. Accordingly, we now have until October 27, 2009 to regain compliance. Compliance would be achieved if the bid price of our common stock closed at $1.00 per share or more for a minimum of 10 consecutive days during that period.
4
In December 2008, we entered into an agreement to terminate our South San Francisco headquarters facility lease. Under the terms of the agreement, the lease terminated on January 2, 2009. In consideration of the early termination of the lease, we paid the landlord a total fee of $14.7 million in December 2008. Additionally, the landlord gave us an exclusive, irrevocable license to use the facility for general office purposes until January 30, 2009.
In December 2008, we repurchased an aggregate of approximately $47.8 million face value of our Notes, at an overall discount of 60 percent from face value in a tender offer for aggregate consideration of approximately $19.1 million in cash, plus accrued but unpaid interest. This purchase of debt resulted in a net gain of approximately $27.2 million and a further reduction of annualized interest expense by approximately $1.5 million.
In March 2009, we entered into agreements to terminate our Hayward manufacturing facility leases. The termination agreements are subject to certain conditions. Subject to fulfillment or waiver of these conditions, the leases will terminate by March 31, 2009. In consideration of the early termination of the leases, we will pay the landlords an aggregate amount of $3.6 million and issue one million shares of our common stock.
We are exploring strategic alternatives, including merger with or acquisition by another company, further restructuring of our company, allocation of our resources toward other biopharmaceutical product areas, sale of the company’s assets, and liquidation of the company. We have engaged Lazard in connection with the evaluation of strategic alternatives.
We ended 2008 with $86.1 million in cash, cash equivalents and short-term investments, including $2.9 million of restricted cash and investments. We have maintained our financial position through strategic management of our resources including access to debt and equity financing and funding from various corporate collaborations and licensing agreements. Prior to our announcement in October 2008 that we placed on hold the further development of GVAX immunotherapy for prostate cancer, we continued to deploy the majority of our research and development resources to advance GVAX immunotherapy for prostate cancer. Expenses related to GVAX immunotherapy for leukemia, GVAX immunotherapy for pancreatic cancer, the oncolytic virus therapy CG0070 and other potential product candidates in preclinical studies were a minor proportion of our overall spending in research and development activities. During 2008, 2007, and 2006, our research and development expenses were $92.5 million, $106.1 million, and $96.3 million, respectively. We expect total research and development expenses, excluding restructuring costs, to decrease significantly as a result of the announcement that we placed on hold the further development of GVAX immunotherapy for prostate cancer and are implementing a substantial restructuring plan.
Our GVAX™ Cancer Immunotherapy Program
Our GVAX immunotherapies are cancer treatments designed to stimulate the patient’s immune system to effectively fight cancer. GVAX cancer immunotherapies are comprised of tumor cells that are genetically modified to secrete an immune-stimulating cytokine known as granulocyte-macrophage colony-stimulating factor, or GM-CSF, and are then irradiated for safety. Since GVAX cancer immunotherapies consist of whole tumor cells, the cancer patient’s immune system can be activated against multiple tumor cell components, or antigens, potentially resulting in greater clinical benefit than if the immunotherapy consisted of only a single tumor cell component. Additionally, the secretion of GM-CSF by the modified tumor cells can greatly enhance the immune response by recruiting and activating dendritic cells at the injection site, a critical step in the optimal response by the immune system to any immunotherapy product. The antitumor immune response which occurs throughout the body following administration of a GVAX product can potentially result in the destruction of tumor cells that persist or recur following surgery, radiation therapy or chemotherapy treatment.
More than 1,000 patients have received our GVAX cancer immunotherapies in multiple Phase 1, Phase 2 and Phase 3 clinical trials to date, and the immunotherapies have been shown to have a favorable side effect profile that avoids many of the toxicities associated with conventional cancer therapies. GVAX cancer
5
immunotherapies can be conveniently administered in an outpatient setting as an injection into the skin, a site where immune cells, including in particular dendritic cells, can be optimally accessed and activated. Our GVAX cancer immunotherapies were being tested as non patient-specific, or allogeneic, products. We intended to develop these immunotherapies as “off-the-shelf” pharmaceutical products.
GVAX Immunotherapy for Prostate Cancer
Our GVAX immunotherapy for prostate cancer is a non patient-specific product comprised of two genetically-modified prostate cancer cell lines. We intended to develop and manufacture this immunotherapy as an “off-the-shelf” pharmaceutical for use after hormonal therapy for advanced-stage prostate cancer. Prostate cancer is the second leading cause of cancer death in men in the United States, with approximately 30,000 men dying each year from the disease. When a man is diagnosed with early-stage prostate cancer, he is treated with either a prostatectomy, which is the surgical removal of the prostate, and/or radiation therapy. If the patient relapses, he is treated with hormone therapy to suppress testosterone in order to reduce the growth of the tumor. When the hormone therapy fails, the patient may or may not be treated with chemotherapy depending upon whether the disease has spread, or metastasized, to other parts of the body. We had designed our Phase 3 clinical trials to evaluate whether GVAX immunotherapy for prostate cancer was superior to standard chemotherapy in patients who have ceased responding to, or have become refractory to, hormone therapy and have metastatic disease.
We have completed five Phase 1 and Phase 2 clinical trials of our GVAX immunotherapy for prostate cancer in approximately 200 patients with various stages of recurrent prostate cancer, and the immunotherapy has had a favorable safety profile in each trial. These clinical trials included two Phase 2 clinical trials in hormone-refractory prostate cancer, or HRPC, patients with radiologic evidence of metastatic, or spreading disease, which was the target population for our Phase 3 trials. These trials were designed to evaluate the safety and efficacy of the immunotherapy, as well as treatment regimens for Phase 3 clinical trials.
We were conducting two Phase 3 clinical trials of GVAX immunotherapy for prostate cancer in metastatic HRPC. The first Phase 3 clinical trial, referred to as VITAL-1, commenced in July 2004 and compared GVAX immunotherapy for prostate cancer to Taxotere chemotherapy administered with prednisone in metastatic HRPC patients who are asymptomatic with respect to cancer-related pain. The VITAL-1 trial was designed to demonstrate superior survival in the patients receiving GVAX cancer immunotherapy compared to patients receiving Taxotere plus prednisone therapy. VITAL-1 was fully enrolled with a total of 626 patients. In January 2008, we announced that the IDMC for VITAL-1 completed a pre-planned interim analysis in the timeframe originally estimated and recommended that the study continue. As is customary to preserve study blinding, the IDMC provided us no additional information other than the recommendation to continue the trial. The second Phase 3 clinical trial, referred to as VITAL-2, commenced in June 2005 and compared GVAX immunotherapy for prostate cancer plus Taxotere chemotherapy to Taxotere chemotherapy plus prednisone with respect to a survival benefit in metastatic HRPC patients with cancer-related pain.
In August 2008, we terminated the VITAL-2 trial. We ended the trial as recommended by the study’s IDMC which, in a routine safety review meeting of both the VITAL-1 and VITAL-2 trials, observed an imbalance in deaths between the two treatment arms of the VITAL-2 study, with a higher number of deaths in the GVAX immunotherapy in combination with Taxotere arm compared to the Taxotere plus prednisone arm. We conducted an initial analysis of the incomplete clinical trial data set that was reviewed by the IDMC in August 2008. The analysis has revealed no apparent imbalance in patient baseline characteristics with respect to both demographic and disease prognostic factors. In addition, no significant toxicities in the GVAX immunotherapy plus Taxotere combination therapy arm were observed that could explain the imbalance in deaths and in fact, the vast majority of deaths in both treatment arms were reported as due to progression of prostate cancer. In September 2008, the FDA, as expected, placed a partial clinical hold on the GVAX Phase 3 program for prostate cancer as a result of our announcement in August 2008 of the termination of the VITAL-2 trial. In October 2008, we terminated the VITAL-1 Phase 3 clinical trial of GVAX immunotherapy based on the results of a previously unplanned futility
6
analysis conducted at our request by the study’s IDMC which indicated that the trial had less than a 30 percent chance of meeting its predefined endpoint of an improvement in survival. In view of the termination of both the VITAL-1 and VITAL-2 trials, we ended further development of GVAX immunotherapy for prostate cancer.
Government Regulations
FDA and Other Foreign Regulation
Prescription pharmaceutical products and biologics are subject to extensive pre- and post-marketing regulation by the FDA including regulations that govern the testing, manufacturing, safety, efficacy, labeling, storage, record-keeping, advertising and promotion of the products under the Federal Food, Drug and Cosmetic Act and the Public Health Services Act, and by comparable agencies in most foreign countries. The process required by the FDA before a new drug or biologic may be marketed in the U.S. generally involves the following:
| | • | | completion of preclinical laboratory and animal testing; |
| | • | | submission of an Investigational New Drug, or IND application, which must become effective before clinical trials may begin; |
| | • | | performance of adequate and well controlled human clinical trials to establish the safety and efficacy of the proposed drug’s or biologic’s intended use; and |
| | • | | approval by the FDA of a New Drug Application, or NDA, in the case of a drug, or of a Biologics License Application, or BLA, for a biologic. |
Foreign countries have similar requirements.
Preclinical Testing. The activities required before a pharmaceutical agent may be marketed begin with preclinical testing. Preclinical tests include laboratory evaluation of potential products and animal studies to assess the potential safety and efficacy of the product and its formulations. The results of these studies and other information, including chemistry, manufacturing and controls, must be submitted to the FDA or comparable foreign agencies and regulatory bodies as part of an application which must be reviewed and approved before proposed clinical testing can begin. Clinical trials involve the administration of the investigational new drug to healthy volunteers or to patients under the supervision of a qualified principal investigator. Clinical trials are conducted in accordance with Good Clinical Practices under protocols that detail the objectives of the study, the parameters to be used to monitor safety, and the efficacy criteria to be evaluated. Each protocol must be submitted for regulatory approval. Further, each clinical study must be conducted under the auspices of an independent institutional review board at the institution at which the study is conducted. The institutional review board considers, among other things, ethical factors and the safety of human subjects. In addition, certain protocols involving the use of genetically modified products must also be reviewed by the Recombinant DNA Advisory Committee of the National Institutes of Health as well as similar bodies in many European countries.
Clinical Trial Phases. Typically, human clinical trials are conducted in three phases that may overlap. In Phase 1, clinical trials are conducted with a small number of patients to determine the early safety profile and pharmacology of the new therapy. In Phase 2, clinical trials are conducted with groups of patients afflicted with a specific disease in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In Phase 3, large scale, multicenter, comparative clinical trials are conducted with patients afflicted with a target disease in order to provide enough data for the statistical proof of efficacy and safety required by the FDA and other regulatory agencies. In the case of products for life-threatening diseases, the initial human testing is generally done in the target patients rather than in healthy volunteers. Since these patients are already afflicted with the target disease, it is possible that such studies may provide some results traditionally obtained in Phase 2 clinical trials. These trials are frequently referred to as Phase 1/2 clinical trials. Although the preliminary Phase 1/2 and Phase 2 clinical trials of our GVAX cancer immunotherapies and oncolytic virus therapies have shown a
7
generally favorable safety profile to date, there can be no assurance that such therapies or products will be tolerated at higher doses or that the clinical efficacy or safety of such therapy or product will be demonstrated in later stage testing.
Marketing Approvals. The results of the preclinical and clinical testing, together with chemistry, manufacturing and controls information, are submitted to regulatory agencies in order to obtain approval to commence commercial sales. In responding to such an application, regulatory agencies may grant marketing approval, request additional information or further research, or deny the application if they determine that the application does not satisfy their regulatory approval criteria. Approval for a pharmaceutical or biologic product may not be granted on a timely basis, if at all, or if granted may not cover all the clinical indications for which approval is sought, or may contain significant limitations in the form of warnings, precautions or contraindications with respect to conditions of use.
In the United States we had utilized the procedure called a Special Protocol Assessment, or SPA, for GVAX immunotherapy for prostate cancer. Under this procedure, a sponsor may seek the FDA’s agreement on the design and analysis of a clinical trial intended to form the primary basis of an effectiveness claim. If the FDA agrees in writing, its agreement may not be changed after the trial begins except in limited circumstances, such as the FDA determining that a substantial scientific issue essential in determining the safety or effectiveness of the product was identified after the trial had begun. If the outcome of the trial is successful, the sponsor will ordinarily be able to rely on it as the basis for approval with respect to effectiveness. While we had received the FDA’s agreement on a SPA for each of our Phase 3 VITAL-1 and VITAL-2 trials, there could have been no assurance that these trials would have a successful outcome or that we would have ultimately received approval for this product.
Satisfaction of pre-market approval requirements for new drugs and biologics typically takes several years, and the actual time required may vary substantially based upon the type, complexity and novelty of the product or targeted disease. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures upon our activities. Success in early stage clinical trials or with prior versions of products does not assure success in later stage clinical trials. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations that could delay, limit or prevent regulatory approval.
Post-Marketing Regulations. Once approved, regulatory agencies may withdraw the product approval if compliance with pre- and/or post-marketing regulatory standards is not maintained or if problems occur after the product reaches the marketplace. In addition, they may require post-marketing studies, referred to as Phase 4 studies, to monitor the effect of an approved product, and may limit further marketing of the product based on the results of these post-market studies. The FDA and other foreign regulatory agencies have broad post-market regulatory and enforcement powers, including the ability to levy fines and penalties, suspend or delay issuance of approvals, seize or recall products, or withdraw approvals.
Manufacturing. Our manufacturing facilities are subject to periodic inspection by the FDA, the United States Drug Enforcement Administration, or DEA, and other domestic and foreign authorities where applicable, and must comply with cGMP regulations. Manufacturers of biologics also must comply with general biological product standards. Failure to comply with the statutory and regulatory requirements subjects the manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of product, or mandatory or voluntary recall of a product. Adverse experiences with the product must be reported to the FDA and foreign agencies and could result in the imposition of market restrictions through labeling changes or in product removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
Advertising and Promotion. With respect to both pre- and post-market product advertising and promotion, the FDA and similar foreign agencies impose a number of complex regulations on entities that advertise and promote pharmaceuticals and biologics, which include, among other things, standards and regulations relating to
8
direct-to-consumer advertising, off-label promotion, industry sponsored scientific and educational activities, and promotional activities involving the Internet. These agencies have very broad enforcement authority and failure to abide by these regulations can result in penalties including the issuance of a warning letter directing the entity to correct deviations from requisite standards, a requirement that future advertising and promotional materials be pre-cleared by the FDA or relevant foreign agencies, and foreign, state and federal civil and criminal investigations and prosecutions.
Other Government Regulations
We are subject to various laws and regulations regarding laboratory practices, the experimental use of animals, and the use and disposal of hazardous or potentially hazardous substances in connection with our research. In each of these areas, as above, the government has broad regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products, and withdraw approvals, any one or more of which could have a material adverse effect upon us.
In addition to laws and regulations enforced by the FDA, we are also subject to comparable foreign regulations, regulation under National Institutes of Health guidelines, as well as under the Controlled Substances Act, the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential foreign, federal, state or local laws and regulations as our research and development involves the controlled use of hazardous materials, chemicals, viruses and various radioactive compounds.
Manufacturing
Our Hayward, California manufacturing facility, which we operated according to cGMP regulations, consisted of 51,000 square feet of manufacturing space and 50,000 square feet of laboratory and office space. Our Hayward manufacturing facility had the capacity to manufacture products for Phase 3 trials of our prostate cancer immunotherapy. It was designed to produce one or more types of biological products at a scale suitable for Phase 3 trials and potential commercial market launch. It is currently being maintained in a clean idle state.
Corporate Collaborations
Takeda Pharmaceutical Company Limited
We entered into a worldwide collaborative agreement with Takeda for the development and commercialization of GVAX immunotherapy for prostate cancer which has been terminated. Under the terms of the agreement, effective March 31, 2008, we granted exclusive worldwide commercial rights to GVAX immunotherapy for prostate cancer for the prevention, diagnosis, and treatment of prostate cancer and other urological neoplasms or urological hyperplasias. In exchange for these rights and in consideration for prior costs incurred by us in the development of GVAX immunotherapy for prostate cancer, Takeda made a non-refundable and non-creditable upfront payment of $50 million. We received full payment of the $50 million in April 2008.
Additionally, Takeda agreed to pay for all external development costs associated with the ongoing Phase 3 clinical development of GVAX immunotherapy for prostate cancer, including the cost of product, all internal and external additional development costs and all commercialization costs. Our decision to place on hold further development of GVAX immunotherapy for prostate cancer was agreed to by Takeda and resulted in Takeda terminating our collaborative agreement in December 2008. Further reimbursement revenue from Takeda will end during the first quarter of 2009.
Novartis AG
In July 2003, we announced a global alliance between Novartis AG and ourselves for the development and commercialization of oncolytic virus therapies. Under the agreement, we also acquired exclusive worldwide rights to certain oncolytic virus therapy products and related intellectual property of Genetic Therapy, Inc., or
9
GTI, an affiliate of Novartis, as well as certain related intellectual property of Novartis. We also received a payment of $28.5 million from Novartis to be dedicated to the further development of several oncolytic virus therapy products developed by both ourselves and GTI, for which Novartis has certain marketing options. In exchange, we issued to Novartis and GTI 1,999,840 shares of our common stock, with the result that Novartis became the holder of approximately five percent (as of the time of the issuance) of our outstanding common stock. In addition, the agreement provided the basis for the sharing of future additional development costs and potential profits for certain oncolytic virus products on a worldwide basis. Upon the exercise of certain options by Novartis, development costs and profits are to be shared on an approximately equal basis in the United States. Novartis will be responsible for the development costs for markets outside the United States and pay us a royalty on potential future sales outside the United States. Novartis is also required to reimburse us on a cost-plus basis for products that we manufacture for them to sell outside of the United States.
In September 2004, the terms of our agreement with Novartis were amended to include the grant to us of a non-exclusive worldwide perpetual license to all patent rights of Novartis relating to GM-CSF, a component of our GVAX cancer immunotherapies, in the field of gene therapy. This license bears a low single digit royalty. Also included in the agreement was acknowledgment that certain GVAX cancer immunotherapy products, such as our GVAX immunotherapy for prostate cancer, would not require this license and hence would not be subject to future royalty payments to Novartis. In January 2009, we entered into discussions with Novartis to terminate this agreement.
Medarex, Inc.
In May 2003, we entered into a research and development collaboration with Medarex, Inc. to evaluate combination therapy with our GVAX immunotherapy for prostate cancer and Medarex’s anti-CTLA-4 antibody called ipilimumab. Preclinical studies indicate that ipilimumab may enhance the activity of GVAX cancer immunotherapies. We initiated a Phase 1 trial of this combination therapy in September 2004 which was expanded in April 2007 to treat up to a total of approximately 25 to 30 patients. We formally terminated this trial in November 2008 as a result of the decision to end further development of GVAX immunotherapy for prostate cancer.
Patents and Trade Secrets
The patent positions and proprietary rights of pharmaceutical and biotechnology firms, including us, are generally uncertain and involve complex legal and factual questions. We believe there will continue to be significant litigation in the industry regarding patent and other intellectual property rights.
As of December 31, 2008, we had 400 U.S. and non-U.S. patents issued or granted to us or available for use by us based on licensing arrangements and 256 U.S. and non-U.S. applications pending in our name or available for use by us based on licensing arrangements. We are currently prosecuting our patent applications in various patent offices around the world, but we cannot be certain whether any given patent application filed by us or our licensors will result in the issuance of a patent or if any given patent issued to us or our licensors will later be challenged and invalidated. Nor can we be certain whether any given patent that may be issued to us or our licensors will provide any significant proprietary protection to our products and business.
Litigation or other proceedings may also be necessary to enforce or defend our proprietary rights and patents. To determine who was first to make an invention claimed in a United States patent application or patent and thus be entitled to a patent, the United States Patent and Trademark Office, or USPTO, can declare an interference proceeding. In the United States, patents may be revoked or invalidated in court actions or in reexamination proceedings in the USPTO. In Europe, patents can be revoked through opposition or nullity proceedings. Such litigation or proceedings could result in substantial cost or distraction to us, or result in an adverse decision as to our or our licensors’ patent applications and patents. We are not currently involved in any interference proceedings concerning our or our licensors’ patent applications and patents. We may be involved in such proceedings in the future.
10
Competitors may have filed patent applications and obtained patents and may in the future file patent applications and obtain patents relating to our products and technologies. We are aware of competing intellectual property relating to our technologies and products. From time to time we have received communications from third parties claiming to have conflicting rights relating to components of our products and technologies. Regardless of their ultimate merit, any infringement or other intellectual property claims against our products and technologies may be expensive and time-consuming to litigate and may divert management attention. If any such claim were successful, we could be required to obtain licenses to a third party’s technologies, patents or other proprietary rights or to their biological or chemical reagents in order to develop and market our products. Moreover, we may choose to voluntarily seek such a license in order to avoid the expense and uncertainty of fully defending our position. In either event, such a license may not be available to us on acceptable terms or on any terms, and we may have to discontinue that portion of our business, or such third party may seek an injunction to prevent us from practicing their proprietary technology. In addition, to the extent we license our intellectual property to other parties, we may incur expenses as a result of contractual agreements in which we indemnify those licensing our technologies against losses incurred if practicing our intellectual property infringes upon the proprietary rights of others. The failure to license any technologies or biological or chemical reagents required to develop or commercialize our technologies or products at reasonable cost may harm our business, results of operations, financial condition, cash flow and future prospects. We are not currently involved in any litigation concerning our competitors’ patent applications and patents. We may be involved in such litigation in the future.
No assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques, or otherwise gain access to our trade secrets or disclose such technology, or that we can meaningfully protect our rights to our unpatented trade secrets.
We require our employees and consultants to execute confidentiality agreements upon the commencement of employment and consulting relationships with us. These agreements provide that all confidential information developed by or made known to an individual during the course of the employment or consulting relationship generally must be kept confidential. In the case of employees, the agreements provide that all inventions conceived by the individual, while employed by us, relating to our business are our exclusive property. While we have implemented reasonable business measures to protect confidential information, these agreements may not provide meaningful protection for our trade secrets in the event of unauthorized use or disclosure of such information.
Competition
We faced substantial competition in the development of products for cancer and other diseases. This competition from other manufacturers of the same types of products and from manufacturers of different types of products designed for the same uses is expected to continue in both U.S. and international markets. Cancer immunotherapies and oncolytic virus therapies are rapidly evolving areas in the biotechnology industry and are expected to undergo many changes in the coming years as a result of technological advances. We are currently aware of a number of groups that are developing cancer immunotherapies and oncolytic virus therapies including early-stage and established biotechnology companies, pharmaceutical companies, academic institutions, government agencies and research institutions. Examples in the cancer immunotherapy area include Dendreon Corporation, which is undertaking a Phase 3 trial in prostate cancer and has filed a BLA with the FDA, and Onyvax Ltd., which has commenced Phase 2 trials in prostate cancer. Antigenics, Inc., and Oncothyreon Inc. are also developing immunotherapy products for other types of cancers.
11
Human Resources
As of December 31, 2008, we employed 61 people. As of December 31, 2008, all remaining employees were engaged in restructuring and activities related to the evaluation of and implementation of strategic alternatives for the business. Accordingly, as of December 31, 2008, no employees were engaged in research, development and manufacturing operations. Since December 31, 2008, we have further reduced our workforce to 21 people as of the date of this report.
Our ability to successfully complete our restructuring and/or strategic alternative activities and/or further develop our business will depend in large part upon our ability to retain employees. We compete for qualified employees with other companies, research and academic institutions, government entities and other organizations. Our termination of our VITAL-1 and VITAL-2 trials of GVAX immunotherapy for prostate cancer and restructuring plan could make it difficult to retain existing employees.
Executive Officers
Our executive officers and their ages as of February 28, 2009, were as follows:
| | | | |
Name | | Age | | Position |
Stephen A. Sherwin, M.D. | | 60 | | Chairman of the Board and Chief Executive Officer |
Sharon E. Tetlow | | 49 | | Senior Vice President and Chief Financial Officer |
Carol C. Grundfest | | 54 | | Senior Vice President, Regulatory Affairs and Portfolio Management |
Christine B. McKinley (1) | | 55 | | Senior Vice President, Human Resources |
Robert H. Tidwell | | 65 | | Senior Vice President, Corporate Development |
Marc L. Belsky | | 53 | | Vice President, Finance and Chief Accounting Officer |
| (1) | terminated on February 28, 2009 |
Dr. Sherwin, chairman of the board and chief executive officer, joined Cell Genesys in March 1990. Dr. Sherwin has served as chief executive officer since the Company’s inception, and in March 1994 he was elected to the additional position of chairman of the board of directors. From 1983 to 1990, Dr. Sherwin held various positions at Genentech, Inc., a biotechnology company, most recently as vice president of clinical research. Prior to 1983, Dr. Sherwin was on the staff of the National Cancer Institute. Dr. Sherwin currently serves as the chairman of the board of Ceregene, Inc., a former subsidiary of Cell Genesys, which he co-founded in 2001. Dr. Sherwin was also a co-founder of Abgenix, Inc, another former subsidiary of Cell Genesys, which was acquired by Amgen in 2006. He is also a director of Neurocrine Biosciences, Inc. and Rigel Pharmaceuticals, Inc. Dr. Sherwin, who also serves as a board member and a vice chair of the board of the Biotechnology Industry Organization, holds a B.A. in biology from Yale University, an M.D. from Harvard Medical School and is board-certified in internal medicine and medical oncology.
Ms. Tetlow, senior vice president and chief financial officer, joined Cell Genesys in June 2005. Between 2004 and 2005, Ms. Tetlow was a venture partner at Apax Partners, a private equity firm. From 1999 to 2004, Ms. Tetlow was chief financial officer for diaDexus, a pharmacogenomics company. From 1998 to 1999, she was chief financial officer at Reprogen, and prior to that, between 1988 and 1998, she held senior financial management positions in other biotechnology companies including Terrapin Technologies, Inc. (now Telik, Inc.), Synergen (now part of Amgen, Inc.) and Genentech, Inc. Ms. Tetlow received an M.B.A. from the Graduate School of Business, Stanford University, and a Bachelor of Arts and Science from the University of Delaware.
Ms. Grundfest, senior vice president, regulatory affairs and portfolio management, joined Cell Genesys in July 2003. Prior to joining Cell Genesys, Ms. Grundfest served as an independent consultant providing advice, analysis and recommendations regarding the regulation and approval of pharmaceutical products in the United States from 2000 to 2003. From 1998 to 2000, Ms. Grundfest served as executive director of project management and strategic planning at Systemix, Inc. and Genetic Therapy, Inc. (affiliates of Novartis AG). Ms. Grundfest also
12
held senior regulatory positions with Roche Global Development and Syntex from 1990 to 1996, as well as served as assistant vice president, research and development at the Pharmaceutical Research and Manufacturers of America from 1982 to 1990. Ms. Grundfest received an M.H.S. in environmental health sciences from The Johns Hopkins University, School of Public Health and a B.S. in biology from Stanford University.
Ms. McKinley, senior vice president, human resources, joined Cell Genesys in August 1994. From 1985 to 1994, she was with Nellcor Puritan Bennett, Inc., where the last position she held was corporate human resources director. Previously, Ms. McKinley also worked at Genentech, Inc. from 1978 to 1984 in various human resource positions. She received a B.A. in psychology from the University of California, Santa Barbara.
Mr. Tidwell, senior vice president, corporate development, joined Cell Genesys in August 2000. Prior to joining Cell Genesys, Mr. Tidwell was vice president of business development at Calydon, Inc. from 1998 to 2000. Mr. Tidwell has also held various management positions with such companies as Boston Life Sciences, where he served as chief operating officer from 1993 to 1994, Genetics Institute, where he was vice president of marketing and business development from 1988 to 1993, and Eli Lilly and Company, where he held various positions including director of worldwide pharmaceutical licensing, between 1969 and 1985. Mr. Tidwell holds an M.B.A. from The Ohio State Graduate School of Business and a Bachelor of Pharmacy from The Ohio State School of Pharmacy.
Mr. Belsky, vice president, finance and chief accounting officer, joined Cell Genesys in December 2006. Prior to joining Cell Genesys, Mr. Belsky served as vice president, Global Visa Commerce from 2005 to 2006, for Visa International. Mr. Belsky held senior management positions in other companies including chief financial officer at Active Aero Group from 2003 to 2005 and DataWave Systems, Inc. from 2001 to 2003, as well as senior finance and banking positions at Michigan National Corporation from 1986 to 2001. Mr. Belsky started his career as an auditor with Coopers & Lybrand. Mr. Belsky is a certified public accountant, a certified treasury professional, and received an M.B.A. from the University of Michigan and a B.S. in accounting from Wayne State University.
13
Medical Advisory Board
We have established a medical advisory board that includes several prominent leaders in the field of oncology which was discontinued as of December 31, 2008. During 2008, the board consisted of the following individuals:
| | |
Name | | Scientific Position |
Bruce Chabner, M.D. | | Clinical Director Massachusetts General Hospital Cancer Center Professor of Medicine, Harvard Medical School |
| |
Jordan U. Gutterman, M.D. | | Chief of the Section of Cellular and Molecular Growth Regulation Department of Molecular Therapeutics Professor of Medicine University of Texas M.D. Anderson Cancer Center |
| |
I. Craig Henderson, M.D. | | Adjunct Professor of Hematology/Oncology University of California, San Francisco |
| |
Ronald Levy, M.D. | | Robert K. Summy and Helen K. Summy Professor of Medicine Chief of the Division of Oncology Stanford University School of Medicine |
| |
William Nelson, M.D., Ph.D. | | Associate Professor of Oncology, Pathology, Pharmacology and Medicine, and Urology Sidney Kimmel Comprehensive Cancer Center The Johns Hopkins University |
| |
John T. Potts, Jr., M.D. | | Director of Research, Massachusetts General Hospital Physician-in-Chief Emeritus Jackson Distinguished Professor of Clinical Medicine Harvard Medical School |
| |
Joan H. Schiller, M.D. | | Deputy Director of the Cancer Center and Chair of Hematology/Oncology University of Texas Southwestern Medical Center |
Dr. Potts, who is also a member of our board of directors, serves as a liaison between the medical advisory board and the board of directors, making periodic reports on the findings of the medical advisory board to the board of directors.
14
Investors in Cell Genesys, Inc. should carefully consider the risks described below before making an investment decision. The risks described below may not be the only ones relating to our company. This description includes any material changes to and supersedes the description of the risk factors associated with our business previously disclosed in Item 1A of our Quarterly Report on Form 10-Q for the three months ended September 30, 2008. Additional risks that we currently believe are immaterial may also impair our business operations. Our business, results of operation, financial condition, cash flow and future prospects and the trading price of our common stock and our abilities to repay our convertible notes could be harmed as a result of any of these risks, and investors may lose all or part of their investment. In assessing these risks, investors should also refer to the other information contained or incorporated by reference in this Annual Report on Form 10-K, including our consolidated financial statements and related notes, and our other filings from time to time with the Securities and Exchange Commission, or SEC.
The continuation of our business as a going concern may depend on our ability to identify and successfully complete a strategic transaction.
We terminated both the VITAL-1 and VITAL-2 trials and ended further development of GVAX immunotherapy for prostate cancer and have implemented a substantial restructuring plan approved by our Board of Directors. We had previously devoted substantially all of our research, development and clinical efforts and financial resources toward the development of GVAX immunotherapy for prostate cancer, and we have only limited early stage product candidates in clinical or preclinical development. As a result, we expect it to be difficult or impossible to raise the additional financing required to continue to operate our business as currently structured. We accordingly are exploring strategic alternatives, including merger with or acquisition by another company, further restructuring of our company and allocation of our resources toward other biopharmaceutical product areas, and sale of the company’s assets and liquidation of the company. We cannot predict whether we will be able to identify strategic transactions on a timely basis or at all. We also cannot predict whether any such transaction, once identified, would be approved by our stockholders, if approval is required, or consummated on favorable terms. We anticipate that any such transaction would be time-consuming and may require us to incur significant additional costs regardless even if completed. If we are unable to identify and complete an alternate strategic transaction, our business may be liquidated. In any case, our stockholders and noteholders could lose some or all of their investment in our common stock.
We have only limited early stage product candidates in clinical or preclinical development from which we can potentially generate any revenue from product sales.
As a result of terminating both the VITAL-1 and VITAL-2 trials we placed on hold the further development of GVAX immunotherapy for prostate cancer. We only have early stage product candidates in clinical or preclinical development. We do not expect to generate any revenue from product sales for at least the next several years, if ever.
We will need substantial additional funds to continue operations, and our ability to generate funds is extremely limited and depends on many factors beyond our control.
We will need substantial additional funds for lease obligations related to our manufacturing facility in Hayward, California, and for principal and interest payments related to our debt financing obligations.
Our future capital requirements will depend on, and could increase as a result of many factors, including, but not limited to:
| | • | | the progress of implementing our restructuring plan; |
| | • | | whether we acquire other biopharmaceutical products or technologies; |
| | • | | our ability to outlicense our technology and the terms of those outlicensings; |
15
| | • | | the principal amount of our debt that remains outstanding; and |
| | • | | the costs we incur in obtaining, defending and enforcing patent and other proprietary rights or gaining the freedom to operate under the patents of others. |
As mentioned above, we have recently terminated our VITAL-1 and VITAL-2 trials. As a result, it will be difficult or impossible for us to raise additional financing. If adequate funds are not available, we may not be able to pursue other biopharmaceutical product areas, or be required to sell or merge the company or liquidate the company.
We may not be able to maintain our listing on The NASDAQ Global Market.
On October 21, 2008, we received a Nasdaq Staff Deficiency Letter, or Nasdaq Letter, indicating that we had become non-compliant with the minimum $1.00 bid price per share requirement for continued listing on The Nasdaq Global Market as set forth in Nasdaq Marketplace Rule 4450(a)(5) because the price of our stock closed below the minimum bid price of $1.00 per share for a period of 30 consecutive business days. The Nasdaq Letter indicated that in light of extraordinary market conditions, Nasdaq has determined to suspend enforcement of the minimum bid price and market value of publicly held shares requirements through January 16, 2009. Accordingly, the Nasdaq Letter stated that in accordance with Nasdaq Marketplace Rule 4450(e)(2), we have 180 calendar days from January 20, 2009, or until July 20, 2009, to regain compliance. Subsequently, on December 19, 2008, NASDAQ announced that given the continued extraordinary market conditions, NASDAQ is extending the suspension of the minimum bid price and market value of publicly held shares requirements through April 20, 2009. Accordingly we now have until October 27, 2009 to regain compliance. Companies listed on NASDAQ are required to maintain a minimum closing bid price of $1.00 per share. NASDAQ uses the consolidated closing bid price to determine whether a company complies with this requirement. If a company trades for 30 consecutive business days below the $1.00 minimum closing bid price requirement, NASDAQ will send a deficiency notice to the company advising that it has been afforded a “compliance period” of 180 calendar days to regain compliance with the applicable requirements. Thereafter, Capital Market companies can receive an additional 180-day compliance period if they meet all initial inclusion requirements for the Capital Market, except for the bid price requirement. If a Global Select Market or Global Market company is unable to comply with the bid price requirement prior to the expiration of its 180-day compliance period, it may transfer to the NASDAQ Capital Market, so as to take advantage of the additional compliance period offered on that market, provided it meets all requirements for initial listing on the NASDAQ Capital Market, except for the bid price requirement.
Stocks trading on the NASDAQ Capital Market rather than the NASDAQ Global Market tend to be more volatile and less liquid as trading volumes are generally significantly lower on the NASDAQ Capital Market. Furthermore, if we are unable to meet the listing requirements of the NASDAQ Capital Market, we could be delisted from NASDAQ entirely, which would likely result in even less trading volume and liquidity in our common stock.
We may not be able to raise additional funds necessary to continue operating as a going concern on favorable terms, or at all.
Because of our capital requirements, we will need to raise additional funds to continue operations. From time to time, we may seek to access the public or private debt and equity markets. Our announcement that we have terminated the VITAL-1 and VITAL-2 Phase 3 trials of GVAX immunotherapy for prostate cancer has significantly depressed our stock price and severely impaired our ability to raise additional funds. As a result, it will be difficult or impossible for us to raise additional capital. We also may seek to raise additional capital through outlicensing technologies or third-party collaborations. If adequate funds are not available on favorable terms, or at all, we will not be able to pursue certain strategic options, including allocating our resources to other biopharmaceutical product areas.
16
Alternatively, we may need to seek funds through arrangements with collaborative partners or others that require us to relinquish rights to technologies or product candidates that we would otherwise seek to develop or commercialize ourselves. These arrangements could harm our business, results of operations, financial condition, cash flow or future prospects. Currently, we do not have collaborative partners as Takeda terminated our collaborative agreement on December 1, 2008, effective March 1, 2009, upon which time this collaborative partnership will no longer be a source of revenue. We may not be successful in entering into additional collaborative partnerships on favorable terms, if at all. Certain of our oncolytic virus therapy products are currently subject to future commercialization rights belonging to Novartis AG, which at its option may provide further development funding for these products. However, in January 2009, we entered into discussions with Novartis to terminate this agreement. Our efforts to raise capital through such outlicensing activities may fail. Failure to enter into new corporate relationships may harm our business.
Our business, financial condition, results of operations, cash flow and future prospects could suffer as a result of future strategic alternatives.
We may engage in a future merger with or acquisition by another company or investments that could dilute our existing stockholders or cause us to incur contingent liabilities, commitments, debt or significant expense. In the course of pursuing strategic alternatives at this time or in the ordinary course of business in the future, we may evaluate potential acquisitions or investments in related businesses, products or technologies.
Future mergers with or acquisitions by another company could subject us to a number of risks, including, but not limited to:
| | • | | the loss of key personnel and business relationships; |
| | • | | difficulties associated with assimilating and integrating the new personnel, intellectual property and operations of the acquired companies; |
| | • | | the expense associated with maintenance of diverse standards, controls, procedures, employees and clients; and |
| | • | | our inability to generate revenue from acquired technology sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
As a result of such risks, a merger with or an acquisition by another company or investment could harm our business, results of operations, financial condition, cash flow and future prospects.
We expect to continue to incur substantial losses and negative cash flow from operations and may not become profitable in the future.
We have incurred an accumulated deficit since our inception. As of December 31, 2008, our accumulated deficit was $538.1 million. We expect to incur operating losses for the foreseeable future. In the past, these losses were due primarily to the expansion of development programs, clinical trials and manufacturing activities in connection with the development of GVAX immunotherapy for prostate cancer, which we have ended, and, to a lesser extent, general and administrative expenses, at a time when we have yet to realize any product revenues. We have only limited early stage candidates in clinical or preclinical development, and we do not expect to realize any revenues from product sales in the next several years, if ever. We also have substantial lease obligations related to our manufacturing facility in Hayward, California. We expect that losses will fluctuate from quarter to quarter as we implement our substantial restructuring plan and expense restructuring charges. We cannot guarantee that we will ever achieve positive cash flow or profitability.
17
Our substantial indebtedness could harm our financial condition.
We have a significant amount of debt. As of March 9, 2009, we had $68.3 million aggregate principal amount of outstanding convertible notes due in 2011. Our annual interest payment on these notes is approximately $2.1 million. Our substantial indebtedness could harm our business, results of operations, financial condition, cash flow and future prospects. For example, it could:
| | • | | make it more difficult for us to satisfy our obligations with respect to the convertible notes; |
| | • | | increase our vulnerability to general adverse economic and industry conditions; |
| | • | | limit our ability to raise or borrow additional funds for future working capital, capital expenditures, research and development and other general corporate requirements, limit our ability to pursue strategic alternatives, including merger or acquisition transactions, or allocation of resources to new product areas; and |
| | • | | limit our flexibility to react to changes in our business and the industry in which we operate. |
We may not have sufficient funds to pay principal and interest on our outstanding convertible notes due in 2011 when due.
We have substantial indebtedness, but currently no significant source of revenues. As a result, we may seek from time to time to purchase or retire additional amounts of our outstanding convertible debt through cash purchases and/or exchanges for other securities of our company in open market transactions, privately negotiated transactions and/or a tender offer, if we can do so on attractive terms. If we were unable to repurchase these notes on favorable terms, or at all, or obtain additional financing or revenues from other sources, we may not have sufficient cash remaining for operations which could result in bankruptcy. Additionally, if we buy back our outstanding convertible debt through cash purchases it will reduce our cash balance which may impair our ability to execute strategic alternatives, or leave us without sufficient cash remaining for operations, which could harm our financial condition.
If we reallocate our resources to other biopharmaceutical product areas, we may not be successful in developing a new product candidate and we will be subject to all the risks and uncertainties associated with research and development of new biopharmaceutical product candidates.
We may explore the possibility of reallocating our resources towards other biopharmaceutical product areas. We cannot guarantee that any such allocation would result in the identification and successful development of a commercially viable product candidate. The development of biopharmaceutical product candidates is subject to a number of risks and uncertainties, including but not limited to:
| | • | | the time, costs and uncertainty associated with the clinical testing required to demonstrate the safety and effectiveness of our products and obtain regulatory approvals; |
| | • | | the ability to raise sufficient funds to fund the research and development of product candidates; |
| | • | | the ability to find third party collaborators to assist or share in the costs of product development, and potential dependence on strategic partners and third party collaborators, to the extent we rely on them for future sales, marketing or distribution; |
| | • | | the ability to protect the intellectual property rights associated with a product candidate; |
| | • | | ability to comply with ongoing regulatory requirements; |
18
| | • | | government restrictions on the pricing and profitability of prescription pharmaceuticals in the United States and elsewhere; and |
| | • | | the extent to which third-party payers, including government agencies, private health care insurers and other health care payers, such as health maintenance organizations, and self-insured employee plans, will cover and pay for newly approved therapies. |
The commercial value of our patents is uncertain.
As of December 31, 2008, we had 400 U.S. and non-U.S. patents issued or granted to us or available for use by us based on licensing arrangements and 256 U.S. and non-U.S. applications pending in our name or available for use by us based on licensing arrangements. The patent positions of pharmaceutical and biotechnology firms, including ours, are generally uncertain and involve complex legal and factual questions. We believe that there will continue to be significant litigation in the industry regarding patent and other intellectual property rights. We cannot be certain whether any given patent application filed by us or our licensors will result in the issuance of a patent or if any given patent issued to us or our licensors will later be challenged and invalidated. Nor can we be certain whether any given patent that may be issued to us or our licensors will provide any significant proprietary protection to our products and business. Competitors may have filed patent applications or received patents and may file additional patent applications and obtain additional patents that may prevent patents being issued to us or our licensors or limit the scope of those patents. Depending upon their filing date, patent applications in the United States are confidential until the patent applications are published, typically eighteen months after their filing date, or patents are issued. Outside the United States, patent applications are confidential until they are published, typically eighteen months after their first filing date. In addition, publication of discoveries in the scientific or patent literature tends to lag behind actual discoveries by several to many months. Accordingly, we cannot be sure that we or our licensors were the first creator of inventions covered by our or our licensors’ pending patent applications or issued patents or that we or our licensors were the first to file patent applications for these inventions. In addition, because patents have a limited life, subject to potential extensions, patents issued to us or our licensors may have expired prior to or have limited term remaining after the first commercial sale of a related product. As a result, the commercial value of these patents might be limited. The strategic alternatives we are exploring include the sale of our assets, but we may not be able to sell our intellectual property at attractive prices or for any price, or our competitors may challenge our ownership.
We may have to engage in litigation to determine the scope and validity of competitors’ patents and proprietary rights, which, if we do not prevail, could harm our business, results of operations, financial condition, cash flow and future prospects.
Competitors may have filed patent applications and obtained patents and may in the future file patent applications and obtain patents relating to our products and technologies. We are aware of competing intellectual property relating to our technologies and products. From time to time we have received communications from third parties claiming to have conflicting rights relating to components of our products and technologies. Regardless of their ultimate merit, any infringement or other intellectual property claims against our products and technologies may be expensive and time-consuming to litigate and may divert management attention. If any such claim were successful, we could be required to obtain licenses to a third party’s technologies, patents or other proprietary rights or to their biological or chemical reagents in order to develop and market our products. Moreover, we may choose to voluntarily seek such a license in order to avoid the expense and uncertainty of fully defending our position. In either event, such a license may not be available to us on acceptable terms or on any terms, and we may have to discontinue that portion of our business, or such third party may seek an injunction to prevent us from practicing their proprietary technology. In addition, to the extent we license our intellectual property to other parties, we may incur expenses as a result of contractual agreements in which we indemnify those licensing our technologies against losses incurred if practicing our intellectual property infringes upon the proprietary rights of others. The failure to license any technologies or biological or chemical reagents required to develop or commercialize our technologies or products at reasonable cost may harm our business, results of operations, financial condition, cash flow and future prospects.
19
We may have to engage in litigation, which could result in substantial cost or distraction, to enforce or defend our patents and which, if we do not prevail, could harm our business and make us more vulnerable to competition.
In the future, we may have to engage in litigation to enforce or defend our proprietary rights and patents. To determine who was first to make an invention claimed in a U.S. patent application or patent and thus be entitled to a patent, the USPTO can declare an interference proceeding. In the United States, patents may be revoked or invalidated in court actions or in reexamination proceedings in front of the USPTO. In Europe, patents can be revoked through opposition or nullity proceedings. Such litigation or proceedings could result in substantial cost or distraction to us. These proceedings could potentially result in an adverse decision as to our or our licensors’ patent applications and patents.
We cannot predict the outcome of interference, reexamination, opposition or nullity proceedings or patent litigation that we may become involved with in the future. An adverse result in any of these proceedings could have an adverse effect on our intellectual property position in the technologies to which the patent applications or patents involved in the proceedings are directed and on our business in related areas. If we lose in any such proceeding, our patents or patent applications that are the subject matter of the proceeding may be invalidated or may not be issued as patents. We also may be required to obtain a license from the prevailing party.
Our ability to protect and control unpatented trade secrets, know-how and other technological innovation is limited.
We have a limited ability to protect and control unpatented trade secrets, know-how and other technological innovation. Our competitors may independently develop similar or better proprietary information and techniques and disclose them publicly. Also, others may gain access to our trade secrets, and we may not be able to meaningfully protect our rights to our unpatented trade secrets. In addition, confidentiality agreements and other measures may not provide meaningful protection for our trade secrets in the event of unauthorized use or disclosure of such information. Failure to protect and control such trade secrets, know-how and innovation could harm our competitive position.
Inventions or processes discovered by our outside scientific collaborators or consultants may not become our property, which may affect our competitive position.
We have relied on the continued availability of outside scientific collaborators to perform research for us. As these scientific collaborators are not our employees, we have had limited control over their activities. Our arrangements with these collaborators, as well as those with our scientific consultants, provide that any rights we obtain as a result of their research efforts will be subject to the rights of the research institutions for which they work. For these reasons, inventions or processes discovered by our scientific collaborators or consultants may not become our property.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results, maintain investor confidence or prevent fraud.
Effective internal controls are necessary for us to provide reliable financial reports, maintain investor confidence and prevent fraud. As part of our examination of our internal systems in response to Sarbanes-Oxley requirements, we have discovered in the past, and may in the future discover, areas of our internal controls that could be improved. None of these issues have risen to the level that we were unable to attest to the effectiveness of our internal controls when we were required to do so. We cannot be certain that any measures that we take to improve our internal controls will ensure that we implement and maintain adequate controls over our financial processes and reporting in the future. In addition, as a result of our recent restructuring, we have significantly reduced our number of employees and there can be no guarantee that we will be able to retain employees with the requisite expertise to maintain an effective system of internal controls. Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our operating results or cause
20
us to fail to meet our reporting obligations. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
We may in the future be exposed to product liability claims, which could harm our business, results of operations, financial condition and cash flow.
Clinical trials or marketing of any of our potential products, including the recently terminated VITAL-1 and VITAL-2 trials of GVAX immunotherapy for prostate cancer, may expose us to liability claims resulting from the use of our products. These claims might be made by clinical trial participants and associated parties, consumers, health care providers, sellers of our products or others. A claim, particularly resulting from a clinical trial, or a product recall could harm our business, results of operations, financial condition, cash flow and future prospects.
Insurance coverage is increasingly more difficult and costly to obtain or maintain.
We currently maintain a certain amount of insurance with respect to each of our clinical trials as well as insurance to reduce our direct exposure to certain other business risks, but may not be able to maintain insurance or obtain sufficient coverage at reasonable rates in the future. As a result, we may be required to assume more risk in the future or make significant expenditures to maintain sufficient levels of insurance. Any inability to maintain insurance at an acceptable cost, or at all, could also result in a breach of terms of our product license agreements or could prevent or inhibit the clinical testing or commercialization of our products or otherwise affect our business, results of operations, financial condition, cash flow and future prospects. If we are subject to third-party claims or suffer a loss or damage in excess of our insurance coverage, we may be required to pay for claims, losses and damages in excess of our insurance limits. Furthermore, any claims made on our insurance policies may affect our ability to obtain or maintain future insurance coverage at reasonable costs, if at all.
Our operations are vulnerable to interruption by fire, earthquake, power loss, telecommunications failure, terrorist activity and other events beyond our control, which could harm our business.
Our facilities have, in the past, been subject to electrical blackouts as a result of a shortage of available electrical power. Future blackouts could disrupt the operations of our facilities. Our facilities are located in seismically active regions. We have not undertaken a systematic analysis of the potential consequences to our business and financial results from a major earthquake, fire, power loss, terrorist activity or other disasters and do not have a recovery plan for such disasters. We are unable to predict the effects of any such event, but the effects could harm our business, results of operations, financial condition, cash flow and future prospects. In addition, we do not carry sufficient insurance to compensate us for actual losses from interruption of our business that may occur, and any losses or damages incurred by us could harm our business.
We depend on our key management personnel to implement our restructuring plan, and the loss of these personnel could impair our business.
We rely and will continue to rely on our key management personnel. Because all employees are employed at-will, they can leave at any time. The loss of key personnel could harm our business and results of operations. There is intense competition from other companies, research and academic institutions and other organizations for qualified personnel. We may not be able to continue to retain or attract, if required, the qualified personnel necessary for the implementation of our restructuring plan or development of our business. Although we have no current plans to recruit additional personnel, we may, in the event of turnover of key personnel or if we decide to allocate our resources to the development of new product candidates, need to continue to recruit experts in the areas of clinical testing, manufacturing, regulatory, finance, marketing and distribution. If we do not succeed in retaining, or hiring if required, necessary personnel, our business could suffer significantly. In addition, the termination of our VITAL-1 and VITAL-2 trials of GVAX immunotherapy for prostate cancer and the implementation of our restructuring plan could make it difficult to retain existing or attract new employees.
21
The prices of our common stock and convertible senior notes are likely to continue to be volatile in the future.
The stock prices of biopharmaceutical and biotechnology companies, including ours, have historically been highly volatile. Since January 1, 2006, our stock price has fluctuated between a high closing price of $7.98 on March 31, 2006 and a low closing price of $0.10 on October 24, 2008. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. In addition, as our convertible senior notes are convertible into shares of our common stock, volatility or depressed prices of our common stock could have a similar effect on the trading price of the notes. Interest rate fluctuations also can affect the price of our convertible senior notes. The following factors, among others, may affect the prices of our common stock and notes:
| | • | | fluctuations in our financial results; |
| | • | | announcements of potential strategic transactions; |
| | • | | changes in our outstanding indebtedness; |
| | • | | announcements of new collaborative relationships by us or our competitors; |
| | • | | developments in patent or other proprietary rights affecting us or our competitors; |
| | • | | public concern as to the safety of products developed by us or other biotechnology and pharmaceutical companies; |
| | • | | fluctuations in price and volume in the stock market in general, or in the trading of the stock of biopharmaceutical and biotechnology companies in particular, that are unrelated to our operating performance; |
| | • | | issuances of securities in equity, debt or other financings or issuances of common stock upon conversion of our convertible senior notes or exercise of warrants; |
| | • | | sales of common stock by existing stockholders; and |
| | • | | the perception that such issuances by us or sales by existing stockholders could occur. |
Our stockholders may be diluted by the conversion of outstanding convertible senior notes.
As of March 9, 2009, we had $68.3 million aggregate principal amount of convertible notes outstanding that convert into our common stock, at a conversion price of $9.10 per share, equal to a conversion rate of 109.8901 shares per $1,000 principal amount of notes, subject to adjustments for stock dividends, stock splits, and other similar events. The holders of the notes may choose at any time to convert their notes into common stock. The number of shares of common stock issuable upon conversion of the notes, and therefore the dilution of existing common stockholders, could increase as a result of an event triggering the antidilution rights of the notes, including certain acquisitions in which 10% or more of the consideration paid for our common stock in the transaction is in the form of cash or securities that are not freely tradable. Conversion of our convertible senior notes would result in issuance of additional shares of common stock, diluting existing common stockholders.
Our stockholders may be diluted, and the prices of our securities may decrease, by the exercise of outstanding stock options and warrants or by future issuances of securities.
We may issue additional common stock, preferred stock, restricted stock units, or securities convertible into or exchangeable for our common stock. Furthermore, substantially all shares of common stock for which our outstanding stock options or warrants are exercisable are, once they have been purchased, eligible for immediate sale in the public market. The issuance of additional common stock, preferred stock, restricted stock units, or securities convertible into or exchangeable for our common stock or the exercise of stock options or warrants would dilute existing investors and could adversely affect the price of our securities.
22
Our ability to use net operating loss carryforwards could be limited as a result of issuances of equity securities.
To the extent that our taxable income exceeds any current year operating losses, we may use our net operating loss carryforwards to offset income that would otherwise be taxable. However, under the Tax Reform Act of 1986, the amount of benefits from our net operating loss carryforwards may be impaired or limited if we incur a cumulative ownership change of more than 50%, as interpreted by the IRS, over a three year period. As a result, our use of federal net operating loss carryforwards could be limited by the provisions of Section 382 of the Internal Revenue Code, depending upon the timing and amount of additional equity securities that we issue. State net operating loss carryforwards may be similarly limited. Any such disallowances may result in greater tax liabilities than we would incur in the absence of such a limitation and any increased liabilities could adversely affect our business, results of operations, financial condition and cash flow.
We have adopted anti-takeover defenses that could make it difficult for another company to acquire control of us or could limit the price investors might be willing to pay for our stock.
Certain provisions of our certificate of incorporation, bylaws, debt instruments and Delaware law could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions include the adoption of a Stockholder Rights Plan, commonly known as a “poison pill.” Under the Stockholder Rights Plan, we made a dividend distribution of one preferred share purchase right for each share of our common stock outstanding as of August 21, 1995 and each share of our common stock issued after that date. In July 2000, we made certain technical changes to amend the plan and extended the term of such plan until 2010. The rights are exercisable only if a person or group purchases 15% or more of our common stock or announces a tender offer for 15% or more of our common stock . Upon exercise, holders other than the acquirer may purchase our stock at a discount. Our Board of Directors may terminate the rights plan at any time or under certain circumstances redeem the rights. Because the rights may substantially dilute the stock ownership of a person or group attempting to take us over without the approval of our Board of Directors, the plan could make it more difficult for a third party to acquire us (or a significant percentage of our outstanding capital stock) without first negotiating with our Board of Directors regarding such acquisition. These provisions and certain provisions of the Delaware General Corporation Law may have the effect of deterring hostile takeovers or otherwise delaying or preventing changes in our management or in the control of our company, including transactions in which our stockholders might otherwise receive a premium over the fair market value of our common stock.
The Committed Equity Financing Facility that we entered into with Kingsbridge in February 2007, or 2007 CEFF, is not available to us because of the current trading price of our common stock.
The 2007 CEFF entitles us to sell and obligates Kingsbridge to purchase, from time to time over a period of three years, shares of our common stock for cash consideration, subject to certain conditions and restrictions. As of December 31, 2008, there were approximately 4.5 million shares remaining that may be sold under the 2007 CEFF. Kingsbridge will not be obligated to purchase shares under the 2007 CEFF unless certain conditions are met, which include a minimum price of $1.75 for our common stock which our common stock was trading substantially below as of March 9, 2009; the accuracy of representations and warranties made to Kingsbridge; compliance with laws; effectiveness of the registration statement filed by us with the SEC; and the continued listing of our stock on the NASDAQ Global Market. In addition, Kingsbridge is permitted to terminate the 2007 CEFF if it obtains actual knowledge that a material and adverse event has occurred affecting our business, operations, properties or financial condition. If we are unable to access funds through the 2007 CEFF, or if the 2007 CEFF is terminated by Kingsbridge, we may be unable to access capital on favorable terms, or at all.
Accounting pronouncements may impact our reported results of operations and financial position.
U.S. generally accepted accounting principles, or GAAP, and related implementation guidelines and interpretations can be highly complex and involve subjective judgments. Changes in these rules or their interpretation, the adoption of new pronouncements or the application of existing pronouncements to changes in our business could significantly alter our reported financial statements and results of operations.
23
We are subject to federal, state, local and foreign laws and regulations, and complying with these may cause us to incur significant costs.
Research, product development and manufacturing activities have involved the controlled use of hazardous materials, and we may incur significant costs as a result of the need to comply with numerous laws and regulations. We are subject to laws and regulations enforced by the FDA, the DEA, the CDHS, foreign health authorities and other regulatory statutes including the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Food, Drug and Cosmetic Act, the Resource Conservation and Recovery Act, and other current and potential federal, state, local and foreign laws and regulations governing the use, manufacture, storage, handling and disposal of our products, materials used to develop and manufacture our products, and resulting waste products.
We cannot completely eliminate the risk of contamination or injury, by accident or as the result of intentional acts from these materials. In the event of an accident, we could be held liable for any damages that result, and any resulting liability could exceed our resources. We do not carry insurance for potential exposures which could result from these risks. We may also be required to incur significant costs to comply with environmental laws and regulations in the future.
We are also subject to laws generally applicable to businesses, including but not limited to, federal, state and local regulations relating to wage and hour matters, employee classification, mandatory healthcare benefits, unlawful workplace discrimination and whistle-blowing. Any actual or alleged failure to comply with any regulation applicable to our business or any whistle-blowing claim, even if without merit, could result in costly litigation, regulatory action or otherwise harm our business, results of operations, financial condition, cash flow and future prospects.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
We lease all of our facilities. In February 2009, we relocated our corporate offices to 400 Oyster Point Boulevard, Suite 525, South San Francisco, CA 94080. This office space is approximately 4,000 square feet. In January 2009, we temporarily relocated our corporate headquarters to our cGMP manufacturing facility in Hayward, California. Our cGMP manufacturing facility in Hayward, California, consisted of 51,000 square feet of manufacturing space and 50,000 square feet of laboratory and office space. It was designed to produce one or more types of products at a scale suitable for Phase 3 trials and potential commercial market launch. In March 2009, we entered into agreements to terminate our Hayward manufacturing facility leases. The termination agreements are subject to certain conditions. Subject to fulfillment or waiver of these conditions, the leases will terminate by March 31, 2009. Our approximately 12,000 square-foot facility in Memphis, Tennessee is a centrally located facility which we had intended to use in the future as a centralized product distribution center. We do not intend to renew the lease when it expires in April 2009. In January 2009, we vacated and terminated the lease on our former corporate headquarters building in South San Francisco, California, which was a facility consisting of approximately 154,000 square feet of research and development and administrative space.
None.
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
None.
24
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock trades on the NASDAQ Global Market under the symbol “CEGE.” The following table shows, for the periods indicated, the high and low closing prices per share of our common stock as reported by the NASDAQ Global Market. We did not declare or pay any cash dividends with respect to our common stock during any of the periods indicated below and do not expect to pay cash dividends on our common stock in the foreseeable future.
| | | | | | |
Year Ended December 31, 2008 | | High | | Low |
First Quarter | | $ | 2.98 | | $ | 1.81 |
Second Quarter | | | 4.53 | | | 2.60 |
Third Quarter | | | 3.40 | | | 0.59 |
Fourth Quarter | | | 0.61 | | | 0.10 |
| | |
Year Ended December 31, 2007 | | High | | Low |
First Quarter | | $ | 4.20 | | $ | 2.81 |
Second Quarter | | | 6.86 | | | 3.35 |
Third Quarter | | | 4.07 | | | 3.22 |
Fourth Quarter | | | 3.74 | | | 2.17 |
As of January 31, 2009, there were approximately 721 holders of record and approximately 17,175 beneficial holders of our common stock. On March 6, 2009, the last reported sales price on the NASDAQ Global Market for our common stock was $0.20. The market for our common stock is highly volatile.
We did not repurchase any shares of our common stock or declare any dividends during the year ended December 31, 2008. In October 2008, we repurchased an aggregate of approximately $26.3 million face value of our 3.125% Convertible Senior Notes due 2011, or notes, at an overall discount of approximately 60 percent from face value in a series of privately negotiated transactions with institutional holders of the notes, for aggregate consideration of approximately $10.5 million, plus accrued but unpaid interest. In December 2008, we repurchased an aggregate of approximately $47.8 million face value of our notes at an overall discount of 60 percent from face value in a tender offer for aggregate consideration of approximately $19.1 million in cash, plus accrued but unpaid interest. In January 2009, we repurchased an aggregate of $2.6 million face value of our notes, at an overall discount of approximately 60 percent from face value in a series of privately negotiated transactions with institutional holders of the notes, for aggregate consideration of $1.0 million in cash, plus accrued but unpaid interest. There are no restrictions under the indenture for our outstanding convertible senior notes or other agreements that prohibit payment of dividends.
We may seek from time to time to purchase or retire additional amounts of our outstanding convertible notes through cash purchases and/or exchanges for other securities in open market transactions, privately negotiated transactions and/or a tender offer, if we can do so on attractive terms. We will evaluate such transactions, if any, in light of then existing market conditions.
25
STOCKHOLDER RETURN COMPARISON
The following graph shows the total stockholder return of an investment of $100 cash on December 31, 2003 for (i) our common stock, (ii) the NASDAQ Global Market—US Index and (iii) the NASDAQ Biotechnology Index. Pursuant to applicable SEC rules, all values assume reinvestment of the full amount of all dividends, however no dividends have been declared on our common stock to date. The stock price performance shown on the graph is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
Performance Measurement Comparison
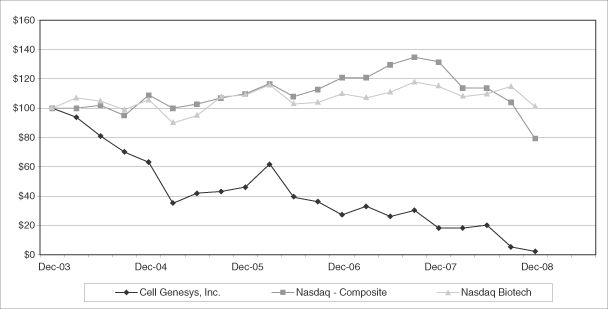
The information provided in the above chart is not “soliciting material,” is not deemed “filed with the SEC” and is not to be incorporated by reference into any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
The information required by this item regarding equity compensation plans is incorporated by reference to the information in Item 12 of this Annual Report on Form 10-K.
26
| ITEM 6. | SELECTED FINANCIAL DATA |
The following selected financial information has been derived from our audited consolidated financial statements. The information below is not necessarily indicative of results of future operations, and should be read together withItem 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations”and the consolidated financial statements and related notes included in Item 8 of this Annual Report on Form 10-K in order to fully understand factors that may affect the comparability of the information presented below.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2004 | |
| | | (In thousands, except per share amounts) | |
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Revenue | | $ | 94,571 | | | $ | 1,380 | | | $ | 1,364 | | | $ | 4,584 | | | $ | 11,458 | |
Total operating expenses | | | 195,613 | * | | | 126,532 | * | | | 114,387 | * | | | 111,097 | | | | 110,061 | |
Gain from purchase of senior notes | | | 42,668 | | | | — | | | | — | | | | — | | | | — | |
Gain from revaluation of warrant liability | | | 11,480 | | | | — | | | | — | | | | — | | | | — | |
Gain on sale of Abgenix, Inc. common stock | | | — | | | | — | | | | 62,677 | | | | 55,123 | | | | 12,160 | |
Net loss | | | (46,975 | )* | | | (99,274 | )* | | | (82,929 | )* | | | (64,939 | ) | | | (97,411 | ) |
Net loss attributed to common stockholders | | | (46,975 | )* | | | (99,274 | )* | | | (82,929 | )* | | | (64,943 | ) | | | (97,511 | ) |
Basic and diluted net loss per common share | | | (0.56 | )* | | | (1.39 | )* | | | (1.67 | )* | | | (1.43 | ) | | | (2.23 | ) |
| |
| | | December 31, | |
| | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2004 | |
| | | (In thousands) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments, including restricted cash and investments | | $ | 86,101 | | | $ | 147,306 | | | $ | 154,074 | | | $ | 129,139 | | | $ | 174,227 | |
Total assets | | | 97,973 | | | | 273,392 | | | | 291,167 | | | | 366,975 | | | | 435,139 | |
Total current liabilities | | | 10,987 | | | | 34,565 | | | | 51,314 | | | | 69,385 | | | | 77,923 | |
Long-term obligations, excluding current portion | | | 4,006 | | | | 56,278 | | | | 51,326 | | | | 52,093 | | | | 51,013 | |
Convertible senior notes | | | 70,867 | | | | 145,000 | | | | 145,000 | | | | 145,000 | | | | 145,000 | |
Redeemable convertible preferred stock | | | — | | | | — | | | | — | | | | — | | | | 1,897 | |
Accumulated deficit | | | (538,090 | ) | | | (491,115 | ) | | | (391,841 | ) | | | (308,912 | ) | | | (243,973 | ) |
Stockholders’ equity | | | 12,113 | | | | 37,549 | | | | 43,527 | | | | 100,497 | | | | 159,306 | |
| * | As discussed inNote 1, “Organization and Summary of Significant Accounting Policies”to the consolidated financial statements included under Item 8 of this Annual Report on Form 10-K, effective January 1, 2006, we changed our method of accounting for stock-based compensation pursuant to Statement of Financial Accounting Standards No. 123(R), “Share-Based Payment”. |
27
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION |
Statements made in this Item other than statements of historical fact, including statements about us and our subsidiaries and our respective clinical trials, research programs, product pipelines, current and potential corporate partnerships, licenses and intellectual property, the adequacy of capital reserves and anticipated operating results and cash expenditures, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. As forward-looking statements, they are subject to a number of uncertainties that could cause actual results to differ materially from the statements made, including risks associated with the ability to successfully complete a strategic transaction, the success of research and product development programs, the issuance and validity of patents, the development and protection of proprietary technologies, the ability to raise capital, operating expense levels and other risks. Reference is made to discussions about risks associated with product development programs, intellectual property and other risks that may affect us underItem 1A, “Risk Factors”above. We do not undertake any obligation to update any forward-looking statement. The following should be read in conjunction with our consolidated financial statements located elsewhere in this Annual Report on Form 10-K for the year ended December 31, 2008 and other documents filed by us from time to time with the SEC.
Overview
We are a biotechnology company that was focused on the development and commercialization of novel biological therapies for patients with cancer. We were developing cell-based cancer immunotherapies and oncolytic virus therapies to treat different types of cancer. Our clinical stage cancer programs involved cell- or viral-based products that have been modified to impart disease-fighting characteristics that are not found in conventional chemotherapeutic agents. As part of our GVAX(TM) cancer immunotherapy programs, we initiated two Phase 3 clinical trials for GVAX immunotherapy for prostate cancer in July 2004 and June 2005, respectively, each under a Special Protocol Assessment, or SPA, with the United States Food and Drug Administration, or FDA. In March 2008, we entered into a worldwide collaboration agreement with Takeda Pharmaceutical Company Limited, or Takeda, for the development and commercialization of GVAX immunotherapy for prostate cancer. In August 2008, we terminated enrollment and administration of GVAX immunotherapy for prostate cancer in VITAL-2, the second of the two Phase 3 clinical trials in advanced prostate cancer. This action was taken based on the recommendation of the study’s Independent Data Monitoring Committee, or IDMC, which, in a routine safety review meeting of both the VITAL-1 and VITAL-2 trials, observed an imbalance in deaths between the two treatment arms of the VITAL-2 study, with a higher number of deaths in the GVAX immunotherapy in combination with Taxotere® (docetaxel) arm compared to the Taxotere plus prednisone arm. As a result, in September 2008 the FDA placed a partial clinical hold on the GVAX Phase 3 program for prostate cancer. In October 2008, we terminated the VITAL-1 Phase 3 clinical trial based on the results of a previously unplanned futility analysis conducted at our request by the IDMC which indicated that the trial had less than a 30 percent chance of meeting its predefined endpoint of an improvement in survival. In view of the termination of both the VITAL-1 and VITAL-2 trials, we placed on hold further development of GVAX immunotherapy for prostate cancer and implemented a substantial restructuring plan that resulted in the reduction of our staff of 290 by approximately 80 percent as of December 31, 2008, and approximately 90 percent as of the date of this report, with further reductions anticipated in the first half of 2009 as additional activities are phased out, and also resulted in Takeda terminating our collaborative agreement in December 2008.
Critical Accounting Policies and the Use of Estimates
The accompanying discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements and related disclosures, which we have prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires management to make estimates, assumptions and judgments that affect the reported amounts in our consolidated financial statements and accompanying notes. These estimates form the basis for making judgments about the
28
carrying values of assets and liabilities. Actual results may differ from these estimates. We consider certain accounting policies related to revenue recognition, income taxes, stock-based compensation, warrant liability, fair value, and asset impairment to be critical accounting policies.
Revenue recognition
Our revenues are derived principally from research and licensing agreements with collaborators. Revenue under such collaboration agreements typically includes up-front payments, cost reimbursements, milestone payments and license fees. We evaluate whether the delivered element under these arrangements has value to our customer on a stand-alone basis and whether objective and reliable evidence of fair value of the undelivered item exists. Deliverables that do not meet these criteria are treated as one unit of accounting for the purposes of revenue recognition.
Up-front payments: Up-front payments from our research collaborations include in part payments for licenses, technology transfer and access rights. Non-refundable up-front license fees and other payments under collaboration agreements where we cannot establish stand-alone value for the delivered license and where we have continued involvement following the execution of the collaboration agreement are deferred and recognized on a straight-line or ratable method over the period of our continuing involvement unless we determine that another methodology is more appropriate. We recognize cost reimbursement revenue under collaborative agreements as the related research and development services are rendered, which approximates when related costs are incurred, as provided for under the terms of these agreements.
Milestones: Payments for milestones that are based on the achievement of substantive and at-risk performance criteria are recognized in full upon achievement of the incentive milestone events in accordance with the terms of the agreement. Incentive milestone payments are triggered either by the results of our research efforts or by events external to us, such as regulatory approval to market a product or the achievement of specified sales levels by a marketing partner. As such, the incentive milestones are substantially at risk at the inception of the agreement, and the amounts of the payments assigned thereto are commensurate with the milestone achieved. Upon the achievement of an incentive milestone event, we have no future performance obligations related to that milestone payment.
License fees: Non-refundable license fees where we have completed all obligations at the execution of the arrangement are recognized as revenue upon execution of the technology licensing agreement when delivery has occurred, collectibility is reasonably assured and the price is fixed and determinable.
Income taxes
On January 1, 2007, we adopted the provisions of FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes,” or FIN 48. FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an entity’s financial statements in accordance with Statement of Financial Accounting Standards No. 109, “Accounting for Income Taxes,” or FAS 109, and prescribes a recognition threshold and measurement attribute for financial statement disclosure of tax positions taken or expected to be taken on a tax return. Additionally, FIN 48 provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
As a result of the implementation of FIN 48, we did not recognize any adjustment in the liability for unrecognized income tax benefits and, therefore, implementation of FIN 48 did not result in a cumulative adjustment to accumulated deficit. As of the adoption date of January 1, 2007, we had $25.0 million of unrecognized tax benefits, all of which would affect our effective tax rate if recognized. As of December 31, 2008, we had zero unrecognized tax benefits and $4.0 million as of December 31, 2007, all of which would affect our effective tax rate if recognized. Our policy is to account for interest and penalties related to income tax matters in the income tax provision in the consolidated statement of operations. Accrued interest and penalties related to income tax matters are included within the related tax liability line in the consolidated balance sheet.
29
On the adoption date of January 1, 2007, we had $10.4 million of accrued interest and zero penalties related to tax contingencies recorded in the consolidated balance sheet. We had zero accrued interest and penalties related to tax contingencies as of December 31, 2008. We had accrued $2.2 million of interest and zero penalties related to tax contingencies as of December 31, 2007.
In July 2005, the IRS issued a Notice of Proposed Adjustment, or NOPA, seeking to disallow $48.7 million of net operating losses that we deducted in our tax return for the year ended December 31, 2000 and seeking a $3.4 million penalty for substantial underpayment of tax in the year ended December 31, 2000. We responded to the NOPA in September 2005, disagreeing with the conclusions reached by the IRS in the NOPA and seeking to resolve this matter in the Appeals Office of the IRS. In May 2007, we reached final settlement regarding this matter with the IRS in the amount of $3.3 million with respect to the fiscal years ended December 31, 2000, 2001 and 2002. This amount was comprised of $2.3 million in federal tax and $1.0 million in related interest. No penalty was assessed.
We file tax returns in the U.S., U.K. and California. In general, our tax returns filed for the years 2005 through 2008 remain open to examination for U.S. and U.K. purposes, and those filed for the years 2001 through 2008 remain open to examination for California purposes.
Income tax benefits previously recorded have been based on a determination of deferred tax assets and liabilities and any valuation allowances that might be required against these deferred tax assets. We record a valuation allowance to reduce deferred tax assets to the amounts that are more likely than not to be realized. We considered anticipated future taxable income, and potential tax planning strategies in assessing the need for valuation allowances. Certain of these determinations require judgment on the part of management. If we determine that we will be able to realize deferred tax assets in the future in excess of the carrying value of our net deferred tax assets, adjustments to the deferred tax assets will increase income by reducing tax expense in the period that such determination is reached. Likewise, if we determine that we will not be able to realize all or part of the carrying value of our net deferred tax assets in the future, adjustments to the deferred tax assets will decrease income by increasing tax expense in the period that such determination is reached. Significant estimates are required in determining our income tax benefits. Various internal and external factors may have favorable or unfavorable effects on our future effective tax rate. These factors include, but are not limited to, changes in tax laws and regulations, our ability to identify and successfully complete a strategic transaction, our future levels of spending for research and development, and changes in our overall level of pre-tax earnings or losses.
Stock-based compensation
Our operating expenses include the cost of stock-based compensation awarded to our employees. We account for employee stock-based compensation in accordance with Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment”, or FAS 123R. Under the provisions of FAS 123R, we estimate the fair value of our employee stock-based awards at the date of grant using the Black-Scholes option valuation model, which requires the use of certain subjective assumptions. When establishing an estimate of the expected term of an award, we consider our historical stock option exercise experience including forfeitures, our post vesting termination pattern and the term of the options outstanding. As required under the accounting rules, we review our valuation assumptions at each grant date and, as a result, we are likely to change our valuation assumptions used to value employee stock-based awards granted in future periods.
FAS 123R requires that employee stock-based compensation costs be recognized over the requisite service period, or the vesting period, in a manner similar to all other forms of compensation paid to employees. Accordingly, for the year ended December 31, 2008, we recognized $4.9 million of stock-based compensation expense in operating expenses with an allocation of $3.6 million to research and development and $1.3 million to general and administrative expenses. For the year ended December 31, 2007, we recognized $6.5 million of stock-based compensation expense in operating expenses with an allocation of $5.0 million to research and development and $1.5 million to general and administrative expenses. The allocation of employee stock-based
30
compensation costs to each operating expense line is estimated based on specific employee headcount information at each grant date and revised, if necessary, in future periods if actual employee headcount information differs materially from those estimates. As a result, the amount of employee stock-based compensation costs we record in future periods in each operating expense line may differ significantly from what we have recorded in the current period. As of December 31, 2008, total stock-based compensation cost related to nonvested stock options not yet recognized was $2.5 million, the majority of which is not expected to be recorded to stock-based compensation expense as we do not expect the majority of these stock options to vest due to our substantial restructuring plan. Total stock-based compensation cost related to nonvested restricted stock units not yet recognized was $0.1 million, which is expected to be fully vested and recorded to stock-based compensation expense over a weighted-average period of six months.
SeeNote 1, “Organization and Summary of Significant Accounting Policies” and Note 9, “Stockholder’s Equity and Stock-Based Compensation” of Notes to Consolidated Financial Statementsincluded under Item 8 of this Annual Report on Form 10-K for further information.
Warrant Liability
We applied the provisions of Statement of Financial Accounting Standards No. 133, “Accounting for Derivative Instruments and Hedging Activities,” or FAS 133, Statement of Financial Accounting Standards No. 150, “Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity,” or FAS 150, and Emerging Issues Task Force Issue 00-19, “Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock,” or EITF 00-19, in accounting for derivative financial instruments.
For a warrant classified as a derivative liability pursuant to FAS 133 and FAS 150, the fair value of the warrant is recorded on the consolidated balance sheet at inception of such classification and adjusted to fair value at each financial reporting date. The changes in fair value of the warrant are recorded in the consolidated statements of operations as a component of other income (expense). The fair value of the warrant is estimated using the Black Scholes option valuation model. The warrant will continue to be reported as a liability until such time as the instrument is exercised or is otherwise modified to remove the provisions which require this treatment, at which time the warrant is adjusted to fair value and reclassified from liabilities to stockholders’ equity. If the warrant is reclassified as permanent equity, the fair value of the warrant would be recorded in stockholders’ equity and no further adjustment would be made in subsequent periods.
Fair Value
In September 2006, the Financial Accounting Standards Board, or FASB, issued Statement of Financial Accounting Standards No. 157, “Fair Value Measurements,” or FAS 157. FAS 157 defines fair value, establishes a framework for measuring fair value and expands the disclosure requirements regarding fair value measurements. FAS 157 is effective for fiscal years beginning after November 15, 2007 for financial assets and liabilities as well as for non-financial assets and liabilities that are recognized or disclosed at fair value on a recurring basis in the financial statements. In accordance with FASB Staff Position No. FAS 157-2, “Effective Date of FASB Statement No. 157,” for all other non-financial assets and liabilities, FAS 157 will be effective for fiscal years beginning after November 15, 2008. In October 2008, the FASB issued FASB Staff Position No. 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active,” or FSP 157-3, that clarifies the application of FAS 157 in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 is effective upon issuance, including prior periods for which the financial statements have not been issued.
31
On January 1, 2008, we adopted the provisions of FAS 157 on a prospective basis for our financial assets and liabilities. FAS 157 requires that we determine the fair value of financial assets and liabilities using the fair value hierarchy established in FAS 157 and describes three levels of inputs that may be used to measure fair value, as follows:
| | • | | Level 1—Quoted prices in active markets for identical assets or liabilities. |
| | • | | Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| | • | | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The adoption of this statement did not have a material impact on our consolidated results of operations and financial condition.
In accordance with FAS 157, the following table represents the fair value hierarchy for our financial assets and liabilities (cash equivalents, investments and warrant liability) measured at fair value on a recurring basis as of December 31, 2008 (in thousands):
| | | | | | | | | | | | |
| | | | | Fair Value Measurements at
December 31, 2008 |
Description | | Total | | Level 1 | | Level 2 | | Level 3 |
Assets: | | | | | | | | | | | | |
Money market funds | | $ | 30,029 | | $ | — | | $ | 30,029 | | $ | — |
Corporate notes | | | 30,498 | | | — | | | 30,498 | | | — |
U.S. government and governmental agency obligations | | | 22,146 | | | — | | | 22,146 | | | — |
| | | | | | | | | | | | |
Total assets | | $ | 82,673 | | $ | — | | $ | 82,673 | | $ | — |
| | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | |
Warrant liability (1) | | $ | 633 | | $ | — | | $ | — | | $ | 633 |
| | | | | | | | | | | | |
| (1) | Refer to theCommon Stock and Warrantsection of Note 8, “Stockholders’ Equity and Stock-Based Compensation” for valuation assumptions. |
The table below includes a roll forward of the balance sheet amounts for the year ended December 31, 2008 (including the change in fair value) for financial instruments classified as Level 3 (the warrant liability). When a determination is made to classify a financial instrument within Level 3, the determination is based upon the significance of the unobservable parameters to the overall fair value measurement. However, Level 3 financial instruments typically include, in addition to the unobservable components, observable components (that is, components that are actively quoted and can be validated to external sources). Accordingly, the gains in the table below include changes in fair value due in part to observable factors that are part of the methodology.
| | | | |
Description | | Year Ended
December 31, 2008 | |
| | | (In thousands) | |
Balance, beginning | | $ | — | |
Purchases, issuances, and settlements | | | 12,113 | |
Total gains included in earnings (1) | | | (11,480 | ) |
| | | | |
Balance, ending | | $ | 633 | |
| | | | |
32
| (1) | Gain as of December 31, 2008 related to the revaluation of the warrant liability from the date of the warrant issuance (May 14, 2008) through December 31, 2008. This gain is reflected in our consolidated statements of operations as a component of other income (expense). |
Asset Impairment
Our policy regarding long-lived assets is to evaluate the recoverability of our assets when the facts and circumstances suggest that the assets may be impaired. This assessment of fair value is performed based on the estimated undiscounted cash flows compared to the carrying value of the assets. If the future cash flows (undiscounted and without interest charges) are less than the carrying value, a write-down would be recorded to reduce the related asset to its estimated fair value.
Recently issued accounting standards
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 160, “Noncontrolling Interest in Consolidated Financial Statements, an amendment of Accounting Research Bulletin No. 51, Consolidated Financial Statements,” or FAS 160. FAS 160 establishes accounting and reporting standards for ownership interests in subsidiaries held by parties other than the parent, the amount of consolidated net income (loss) attributable to the parent and to the noncontrolling interest, changes in a parent’s ownership interest and the valuation of retained noncontrolling equity investments when a subsidiary is deconsolidated. FAS 160 also establishes additional reporting requirements that identify and distinguish between the interest of the parent and the interest of the noncontrolling owners. FAS 160 is effective for fiscal years beginning after December 15, 2008. We are currently evaluating the effect the adoption of FAS 160 will have on our consolidated financial statements.
In December 2007, the FASB ratified the final consensuses in Emerging Issues Task Force Issue No. 07-01, “Accounting for Collaborative Arrangements,” or EITF 07-01, which requires certain income statement presentation of transactions with third parties and of payments between parties to the collaborative arrangement, along with disclosure about the nature and purpose of the arrangement. EITF 07-01 is effective for us beginning January 1, 2009. We have evaluated the impact of adopting EITF 07-01 on our consolidated financial statements and do not expect any impact on our results of operations or financial position.
In May 2008, the FASB issued FASB Staff Position No. APB 14-1, “Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)”, or FSP APB 14-1. FSP APB 14-1 addresses instruments commonly referred to as Instrument C from EITF 90-19, which requires the issuer to settle the principal amount in cash and the conversion spread in cash or net shares at the issuer’s option. FSP APB 14-1 requires that issuers of these instruments account for their liability and equity components separately by bifurcating the conversion option from the debt instrument, classifying the conversion option in equity, and then accreting the resulting discount on the debt as additional interest expense over the expected life of the debt. FSP APB 14-1 is effective for fiscal years beginning after December 15, 2008 and interim periods within those fiscal years, and requires retrospective application to all periods presented. Early application is not permitted. We have evaluated the impact of adopting FSP APB 14-1 on our consolidated financial statements and do not expect any impact on our results of operations or financial position.
33
Results of Operations
Revenue
Revenues were $94.6 million in 2008, compared to $1.4 million in both 2007 and 2006. The following table shows our revenues by source for the periods indicated:
| | | | | | | | | |
| | | 2008 | | 2007 | | 2006 |
| | | (In thousands) |
Takeda | | $ | 80,376 | | $ | — | | $ | — |
GBP IP, LLP | | | 12,000 | | | — | | | — |
sanofi-aventis Group | | | 1,000 | | | 1,000 | | | 1,000 |
Ceregene, Inc. | | | — | | | 13 | | | 83 |
Other | | | 1,195 | | | 367 | | | 281 |
| | | | | | | | | |
| | $ | 94,571 | | $ | 1,380 | | $ | 1,364 |
| | | | | | | | | |
Takeda Development and Commercialization Collaborative Agreement
In March 2008, we entered into a worldwide collaborative agreement with Takeda Pharmaceutical Company Limited, or Takeda, for the development and commercialization of GVAX immunotherapy for prostate cancer. Under the terms of the agreement, effective March 31, 2008, we granted exclusive worldwide commercial rights to GVAX immunotherapy for prostate cancer for the prevention, diagnosis, and treatment of prostate cancer and other urological neoplasms or urological hyperplasias. In exchange for these rights and in consideration for prior costs incurred by us in the development of GVAX immunotherapy for prostate cancer, Takeda made a non-refundable and non-creditable upfront payment of $50 million. We received full payment of the $50 million in April 2008.
We applied EITF Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables”, in evaluating the appropriate accounting for this agreement. In accordance with this guidance, we identified the initial license transfer, certain development services and certain regulatory filing support as deliverables under this agreement and concluded that these deliverables should be accounted for as a single unit of accounting based upon the determination that these deliverables are linked and there is no objective and reliable evidence of the fair value of the undelivered items. Therefore, the elements could not be accounted for separately. Since both our service and the benefit to Takeda were performed and realized consistently throughout the service period and were reflective of a consistent level of effort over the service period, and since benefit was realized consistently throughout, we amortized the upfront payment from Takeda ratably over the estimated term to complete the deliverables.
In October 2008, we terminated both the VITAL-1 and VITAL-2 Phase 3 clinical trials and placed on hold the further development of GVAX immunotherapy for prostate cancer. Our decision to place on hold further development of GVAX immunotherapy for prostate cancer resulted in Takeda terminating our collaborative agreement in December 2008. Due to the termination of our collaborative agreement, all deliverables under this agreement were cancelled. As a result, on December 1, 2008, we recognized as revenue the remaining balance of deferred revenue of $37.9 million of the upfront payment from Takeda.
Additionally, Takeda agreed to pay for all external development costs associated with the ongoing Phase 3 clinical development of GVAX immunotherapy for prostate cancer, including the cost of product, all internal and external additional development costs and all commercialization costs. As of December 31, 2008, we have received payments from Takeda of $23.9 million for development costs. We also have a receivable of $6.5 million, as of December 31, 2008, from Takeda for such costs incurred in the quarter ended December 31, 2008 which we collected in February 2009. Future reimbursement revenue from Takeda will end during the first quarter of 2009 consistent with the wind-down activities associated with this agreement.
34
GBP IP, LLC Technology and Intellectual Property Agreement
In December 2007, we sold for $12.0 million all of our assets, intellectual property and previously established licensing agreements relating to our lentiviral gene delivery technology, commonly referred to as lentiviral vectors, to GBP IP, LLC, an affiliate of GBP Capital, the majority shareholder in privately held Lentigen Corporation. We received full payment of $12.0 million in December 2007. Under the agreement for the transaction, we retained our rights to use the technology for research and development purposes including potential future use with our cancer immunotherapy products. We applied EITF Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables,” in evaluating the appropriate accounting for this agreement. We identified the delivery of biological materials (including certain GMP-compliant materials), intellectual property and previously established licensing agreements related to our lentiviral gene delivery technology as the primary deliverables under this agreement and concluded that these deliverables should be accounted for as a single unit of accounting based upon the determination that the remaining undelivered items did not have reliable and objective evidence of fair value. Therefore, the elements could not be accounted for separately. Recognition of revenue was deferred until we performed all of our obligations under the agreement which occurred during the three months ended March 31, 2008.
Other
For each of the years ended December 31, 2008, 2007 and 2006, we recognized $1.0 million in connection with our gene activation technology license agreement with sanofi-aventis Group for gene activated erythropoietin. In November 2008, sanofi-aventis Group informed us of its intention to terminate this license agreement. We did not recognize any revenue from our previously majority owned subsidiary, Ceregene, Inc., or Ceregene, of which we now own a minority position, for the year ended December 31, 2008. We recorded contract revenue of $13,000 and $0.1 million in 2007 and 2006, respectively, for services provided to Ceregene. In addition, we recognized revenue of $0.8 million from a sublicense of certain patents related to the hemophilia and lysosomal storage disorder technology in 2008, compared to zero for the years ended 2007 and 2006.
Research and development expenses
Research and development expenses were $92.5 million in 2008 compared to $106.1 million in 2007 and $96.3 million in 2006. The decrease in 2008 compared to 2007 was due to the termination of both the VITAL-1 and VITAL-2 Phase 3 clinical trials. The increase in 2007 over 2006 was attributed to the increase in Phase 3 clinical activities for our GVAX immunotherapy program for prostate cancer in Europe, our expansion in clinical trials and other product development activities in both our GVAX cancer immunotherapy and oncolytic virus therapy programs. Prior to our announcement in October 2008 that we placed on hold the further development of GVAX immunotherapy for prostate cancer, we continued to deploy the majority of our research and development resources to advance GVAX immunotherapy for prostate cancer. Expenses related to GVAX immunotherapy for leukemia, GVAX immunotherapy for pancreatic cancer, the oncolytic virus therapy CG0070 and other potential product candidates in preclinical studies were a minor proportion of our overall spending in research and development activities. We expect total research and development expenses, excluding restructuring costs, to decrease significantly as a result of the announcement that we placed on hold the further development of GVAX immunotherapy for prostate cancer.
General and administrative expenses
General and administrative expenses consist primarily of compensation, including non-cash, stock-based compensation, for employees in executive and operational functions, including finance, legal, information technology, human resources and business development. Other significant costs include facilities costs and professional fees for accounting and legal services, including legal services associated with obtaining and maintaining patents. General and administrative expenses were $17.5 million in 2008 compared to $20.4 million in 2007 and $17.9 million in 2006. The decrease in 2008 compared to 2007 was due primarily to implementing a substantial restructuring plan. The increase in 2007 compared to 2006 was primarily due to the continuing
35
increase in infrastructure costs associated with product development and other business activities. Future spending for general and administrative costs, excluding restructuring costs, is expected to decrease significantly as a result of the aforementioned restructuring plan.
Impairment charges
As a result of the termination of both the VITAL-1 and VITAL-2 Phase 3 clinical trials and ending further development of GVAX immunotherapy for prostate cancer, we recorded a $69.5 million impairment charge related to leasehold improvements, equipment and other assets in our former corporate headquarters facility in South San Francisco, Hayward and Memphis facilities that were previously capitalized and deemed not recoverable as we determined that the carrying value exceeded the fair value of the assets. The $69.5 million charge was included in impairment of long-lived assets on the consolidated statement of operations for the year ended December 31, 2008. In addition, we have shortened the estimated life of the remaining fixed assets related to our administrative space in the Hayward facility to February 2009 as we vacated the Hayward facility in February 2009.
In the quarter ended December 31, 2008, we recorded a $2.1 million impairment charge related to certain intangible assets and construction in process that were previously capitalized and deemed not recoverable as we abandoned these projects due to the termination of both the VITAL-1 and VITAL-2 Phase 3 clinical trials and ending further development of GVAX immunotherapy for prostate cancer. In addition, we also recorded $0.1 million of impairment charges during the first six months of the year ended December 31, 2008 due to discontinuation of various capital projects. These charges were included in impairment of long-lived assets on the consolidated statement of operations for the year ended December 31, 2008.
Additionally, we reclassified certain computer equipment to held for sale and ceased the depreciation of these assets as of December 31, 2008. We recorded a $43,000 impairment charge against this equipment as we determined that the carrying value exceeded the fair value of the assets. The $43,000 charge was included in impairment of long-lived assets on the consolidated statement of operations for the year ended December 31, 2008.
Restructuring charges
In October 2008, in view of the termination of both the VITAL-1 and VITAL-2 Phase 3 clinical trials, we placed on hold the further development of GVAX immunotherapy for prostate cancer and following approval by the Board of Directors implemented a substantial restructuring plan that resulted in the reduction of our staff of 290 by approximately 80 percent as of December 31, 2008, and approximately 90 percent as of the date of this report, with further reductions anticipated in the first half of 2009 as additional activities are phased out. In connection with this restructuring, we terminated our lease on our facility in South San Francisco, California, and temporarily relocated our corporate headquarters to Hayward, California. We paid the landlord a lease termination fee of $14.7 million in December 2008. As a result, we recorded a restructuring charge of $13.8 million in the quarter ended December 31, 2008, related to our restructuring, including $14.3 million for workforce reduction costs, $0.1 million of non-cash stock-based compensation expense, and $14.1 million for lease termination costs offset by a $14.7 million gain from the termination of a capital lease. At December 31, 2008, $4.9 million of termination benefits remained unpaid, which were classified under accrued restructuring liabilities on the consolidated balance sheet.
Gain from purchase of convertible senior notes
In October and November 2004, we issued $145.0 million aggregate principal amount of our 3.125% Convertible Senior Notes due in 2011, or Notes. In October and December 2008, we repurchased an aggregate of approximately $74.1 million principal amount of our Notes, at an overall discount of approximately 60 percent from face value for aggregate consideration of approximately $29.6 million in cash, plus accrued but unpaid interest. This purchase of debt resulted in a net gain of approximately $42.7 million after deducting transaction
36
costs of approximately $0.8 million and $1.1 million of unamortized debt issuance costs related to the repurchased Notes. We may seek from time to time to purchase or retire additional amounts of our outstanding Notes through cash purchases and/or exchanges for other of our securities in open market transactions, privately negotiated transactions and/or a tender offer, if we can do so on attractive terms. We will evaluate such transactions, if any, in light of the then-existing market conditions.
Gain from revaluation of warrant liability
The fair value at December 31, 2008 of our warrant liability was $11.5 million lower than its fair value at the date of the issuance of the warrant resulting in a gain of $11.5 million for the year ended December 31, 2008. Potential future increases in our stock price will result in losses being recognized in our consolidated statement of operations in future periods. Conversely, potential future declines in our stock price will result in gains being recognized in our consolidated statement of operations in future periods. Neither of these potential gains or losses will have any impact on our cash balance, liquidity or cash flows from operations.
Gain on sale of Abgenix common stock
During 2006, we sold all of our remaining 3.0 million shares of Abgenix common stock for a gain of $62.7 million.
Interest and other income
Interest and other income was $4.0 million in 2008 compared to $9.0 million in 2007 and $7.5 million in 2006. The decrease in 2008 compared to 2007 is attributed primarily to lower interest rates and, to a lesser extent, lower average cash and short-term investment balances. The increase in 2007 compared to 2006 is primarily attributed to higher average cash and short-term investment balances. In addition, we received $1.0 million from Laboratoire Serono S.A., or Serono, for our agreement to withdraw from a patent dispute between us and Serono relating to gene activation technology in December 2007. The amount of this settlement is included in “Interest and other income” in 2007.
Interest expense
Interest expense was $9.9 million in 2008 compared to $10.3 million in 2007 and $10.5 million in 2006. The decrease in 2008 compared to 2007 was primarily due to the aforementioned repurchase of our Notes. We recorded interest expense related to our Notes, including amortization of related debt issuance costs, of $5.0 million in 2008, compared to $5.3 million in each of 2007 and 2006. The repurchase of our Notes will result in a reduction of annualized interest expense by approximately $2.3 million. In addition, we recorded interest expense associated with our South San Francisco, California capital lease obligation of $4.9 million in 2008, $5.1 million in 2007 and $5.2 million in 2006. The termination of our South San Francisco lease will result in an additional reduction of annualized interest expense of approximately $5.0 million.
Income taxes
In July 2005, the IRS issued a Notice of Proposed Adjustment, or NOPA, seeking to disallow $48.7 million of net operating losses that we deducted for the 2000 fiscal year and seeking a $3.4 million penalty for substantial underpayment of tax in the year ended December 31, 2000. We responded to the IRS in September 2005, disagreeing with the conclusions reached by the IRS in the NOPA and seeking to resolve this matter with the Appeals Office of the IRS. In May 2007, we reached final settlement regarding this matter with the IRS in the amount of $3.3 million with respect to the fiscal years ended December 31, 2000, 2001 and 2002. This amount was comprised of $2.3 million in federal tax and $1.0 million in related interest. No penalty was assessed.
We recorded a tax benefit of $6.2 million in 2008 compared to a tax benefit of $25.9 million in 2007 and a tax provision of $29.6 million in 2006. The tax benefit for the year ended December 31, 2008 is primarily attributed to the reversal of $6.2 million of previously accrued income taxes as a result of closing certain years to
37
examination under relevant statutes of limitation. The tax benefit recorded for the year ended December 31, 2007 is related to the reversal of $26.4 million of previously accrued income taxes as a result of the final settlement with the IRS in May 2007, offset by additional accrued interest for tax contingencies. The tax provision in 2006 relates to a realized gain on the sale of 3.0 million shares of Abgenix common stock and $2.8 million related to additional interest recorded for tax contingencies.
We file tax returns in the U.S., U.K. and California. In general, the years 2005 through 2008, remain open to examination for U.S. and U.K. purposes, and 2001 through 2008 for California purposes. At December 31, 2008, we had federal net operating loss carryforwards of approximately $435 million which will expire in the years beginning 2009 through 2028 if not utilized.
Liquidity and Capital Resources
At December 31, 2008, we had $86.1 million in cash, cash equivalents and short-term investments, of which $2.9 million was classified as restricted and was related to letters of credit for our former corporate headquarters facility in South San Francisco, California and our cGMP manufacturing facility in Hayward, California. Information regarding the classification of these assets is included inNote 4, “Investments” of Notes to Consolidated Financial Statementsincluded under Item 8 of this Annual Report on Form 10-K. We have historically maintained our financial position through strategic management of our resources including accessing debt and equity financing and funding from various corporate collaborations and licensing agreements.
Equity Financing
In February 2003, our shelf registration statement on Form S-3 was declared effective by the SEC under the Securities Act, which allowed us to offer up to $150.0 million of securities on short notice in one or more public offerings registered under the Securities Act. We used this shelf registration in March 2004 to complete a public offering of 4.9 million shares of our common stock, resulting in net proceeds of $57.2 million. We next used this shelf registration in September 2006 to complete an underwritten public offering of 5.8 million shares of our common stock, resulting in net proceeds of $25.0 million. In April 2007, we used the remaining registered amount under this shelf registration to raise net proceeds of $55.4 million in a registered direct offering of 10.8 million shares of our common stock at $5.55 per share and warrants to purchase 2.2 million shares of our common stock at a price of $7.18 per share from selected institutional investors. These warrants are exercisable beginning October 12, 2007 for a period of four and a half years thereafter. The fair value of the warrants, which was recorded in additional paid-in capital, was determined to be $5.3 million on the date of issuance using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 4.66%, (ii) an expected term of 5.0 years, (iii) no dividend yield and (iv) volatility of 57%.
In March 2006, we entered into a Committed Equity Financing Facility, the 2006 CEFF, with Kingsbridge, pursuant to which Kingsbridge committed to purchase, subject to certain conditions, up to the lesser of 8.7 million shares of our common stock or an aggregate of $75.0 million during the three year period following entry into the 2006 CEFF.
In connection with the 2006 CEFF, we issued a warrant to Kingsbridge to purchase 0.4 million shares of our common stock at a price of $9.12 per share exercisable beginning on September 14, 2006 for a period of five years thereafter. The estimated fair value of this warrant was $1.3 million, which was recorded as a contra-equity amount in additional paid-in capital in March 2006. In 2006, we received net proceeds of $27.9 million from the sale of 6.3 million shares of our common stock under the 2006 CEFF. In January 2007, we received net proceeds of $7.1 million from the sale of 2.4 million shares of our common stock under the 2006 CEFF, which concluded the 2006 CEFF. We received cumulative net proceeds of $35.0 million from the sale of 8.7 million shares of our common stock under the 2006 CEFF.
In February 2007, we entered into a new CEFF, the 2007 CEFF, with Kingsbridge, pursuant to which Kingsbridge committed to purchase, subject to certain conditions, up to the lesser of 11.6 million shares of our common stock or an aggregate of $75.0 million during the three year period following entry into the 2007 CEFF.
38
In connection with the 2007 CEFF, we issued a warrant to Kingsbridge to purchase 0.4 million shares of our common stock at a price of $4.68 per share exercisable beginning on September 5, 2007 for a period of five years thereafter. The fair value of the warrant was determined on the date of issuance using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 4.80%, (ii) an expected term of 5.5 years, (iii) no dividend yield and (iv) volatility of 55%. The estimated fair value of this warrant was $0.6 million which was recorded as a contra-equity amount in additional paid-in capital in February 2007. During the year ended December 31, 2007, we received net proceeds of $23.0 million from the sale of 7.1 million shares of our common stock under the 2007 CEFF. There was no drawdown during the year ended December 31, 2008. As of December 31, 2008, there were approximately 4.5 million shares of our common stock remaining that may be sold under the 2007 CEFF. However, Kingsbridge is not obligated to purchase shares at prices below $1.75 per share, which our common stock was trading below as of March 9, 2009, or at a price below 85% of the closing share price of our stock on the trading day immediately preceding the commencement of the drawdown.
In May 2008, we received gross proceeds of $30.0 million in a registered direct offering, before deducting placement agents’ fees and stock issuance costs of approximately $1.9 million, resulting in net proceeds of approximately $28.1 million, from the sale of 7.1 million shares of our common stock at $4.22 per share and warrants to purchase 8.5 million shares of our common stock at a price of $10.00 per share. The offering was made pursuant to our effective May 2007 shelf registration statement on Form S-3. These warrants are exercisable beginning on November 14, 2008 for a period of seven years thereafter. The fair value on the date of issuance of the warrants was determined to be $12.1 million using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 3.6%, (ii) an expected term of 7.5 years, (iii) no dividend yield and (iv) a volatility of 55%. The warrants are classified as a derivative liability pursuant to FAS 133 and FAS 150. Therefore, the fair value of the warrants is recorded on the consolidated balance sheet as a liability and will be adjusted to fair value at each financial reporting date thereafter. As of December 31, 2008, the fair value of the warrants was determined to be $0.6 million using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 1.9%, (ii) an expected term of 6.9 years, (iii) no dividend yield and (iv) a volatility of 101%. For the year ended December 31, 2008, due to the decrease in fair value of the warrants we recorded a gain of $11.5 million to other income.
On May 16, 2007, our new shelf registration statement on Form S-3 was declared effective by the SEC under the Securities Act, which allows us to offer up to $150.0 million of securities on short notice in one or more public offerings under the Securities Act. As of December 31, 2008, $120 million is available for issuance under this shelf registration statement.
Debt Financing
In October and November 2004, we issued and sold a total of $145.0 million aggregate principal amount of 3.125% Convertible Senior Notes due in 2011, or notes, in a private placement. We received approximately $139.9 million in proceeds after deducting the initial purchasers’ discount and estimated offering expenses, which we used, in part, to repay bank debt totaling $95.0 million. Under certain circumstances, we may redeem some or all of the convertible senior notes on or after November 1, 2009 at a redemption price equal to 100% of the principal amount of the notes. Holders of the notes may require us to repurchase some or all of their notes if a fundamental change (as defined in the indenture) occurs, at a repurchase price equal to 100% of the principal amount of the notes, plus accrued and unpaid interest (and additional amounts, if any) to, but not including, the repurchase date. The notes are convertible into our common stock, initially at the conversion price of $9.10 per share, equal to a conversion rate of approximately 109.8901 shares per $1,000 principal amount of notes, subject to adjustments for stock dividends, stock splits and other similar events. Debt issuance costs of $5.3 million incurred in connection with the issuance of the notes were recorded as other noncurrent assets, and were being amortized to interest expense on a straight-line basis over the contractual term of the notes.
In October 2008, we repurchased an aggregate of $26.3 million face value of our notes, at an overall discount of approximately 60 percent from face value in a series of privately negotiated transactions with institutional holders of the notes, for aggregate consideration of $10.5 million in cash, plus accrued but unpaid
39
interest. In November 2008, we commenced a tender offer in which we offered to purchase up to $80 million aggregate principal amount of our outstanding Notes at a price not greater than $400 nor less than $340 per $1,000 principal amount, plus accrued and unpaid interest. In December 2008, as a result of the tender offer, we repurchased an aggregate of $47.8 million face value of the notes, at an overall discount of 60 percent from face value, for aggregate consideration of approximately $19.1 million in cash, plus accrued but unpaid interest. We recorded a net gain of $42.7 million from the purchase of debt comprised of a gross gain of $44.6 million less transaction costs of $0.8 million and $1.1 million of unamortized debt issuance costs related to the repurchased notes.
As of December 31, 2008, there was $70.9 million aggregate principal amount of notes outstanding, and $1.0 million of unamortized debt issuance costs remaining to be amortized as interest expense. In January 2009, we repurchased an aggregate of $2.6 million face value of our notes, at an overall discount of approximately 60 percent from face value in a series of privately negotiated transactions with institutional holders of the notes, for aggregate consideration of $1.0 million in cash, plus accrued but unpaid interest. As a result of the retirement of the repurchased notes, $68.3 million aggregate principal amount remained outstanding as of March 9, 2009.
Net Cash Used in Operating Activities
Net cash used in operating activities was $57.2 million in 2008 compared to $89.7 million in 2007 and $91.7 million in 2006. The decrease in cash used in 2008 compared to 2007 is primarily due to an increase of approximately $62.7 million of receipts from collaborative and license agreements and other income offset by costs related to our restructuring, including a $14.7 million lease termination fee and $11.0 million of employee termination related payments. The decrease in cash used in 2007 compared to 2006 is primarily due to the one time receipt of $12 million from the sale of our intellectual property in December 2007, partially offset by the increased costs associated with Phase 3 clinical activities in Europe. We expect our operating expenses to decrease significantly as a result of the aforementioned restructuring plan and announcement that we have placed on hold the further development of GVAX immunotherapy for prostate cancer.
Net Cash Provided by Investing Activities
Net cash provided by investing activities was $67.5 million in 2008 compared to net cash provided by investing activities of $4.6 million in 2007 and $8.4 million in 2006. Net cash provided in investing activities in 2008 increased by $62.9 million compared to 2007 primarily due to purchasing a lower amount of short-term investments in 2008 as compared to 2007. During the year ended December 31, 2008, we sold three credit card asset-backed debt securities issued by one issuer with a book value of $7.1 million based on possible future credit concerns. These three securities comprised our entire holdings from this issuer. We realized gains of $13,986 from the sale of these securities. Capital expenditures were $1.1 million for the year ended December 31, 2008. Net cash provided by investing activities in 2007 decreased by $3.8 million compared to 2006 primarily due to purchases of short-term investments exceeding maturities of short-term investments in 2007 as compared to 2006. Capital expenditures of $4.5 million in 2007 were partially offset by $2.2 million of proceeds from the sale of property and equipment.
As of December 31, 2008, we did not have any direct exposure to financial assets backed by subprime mortgage loans. We do not invest in asset backed securities backed by subprime mortgages, secured liquidity notes, auction rate securities, structured investments or collateralized debt obligations backed by subprime mortgages.
Net Cash Provided by (Used in) Financing Activities
Net cash used in financing activities was $3.3 million in 2008 compared to net cash provided by financing activities of $85.0 million in 2007 and $52.9 million in 2006. Net cash used in financing activities of $3.3 million for the year ended December 31, 2008, primarily related to the repurchase of an aggregate of $74.1 million face value of our Notes for approximately $30.4 million including transaction costs, offset by net proceeds of
40
approximately $28.1 million in a registered direct offering from the sale of 7.1 million shares of our common stock at $4.22 per share and warrants to purchase 8.5 million shares of our common stock at a price of $10.00 per share. Net cash provided by financing activities increased by $32.1 million in 2007 primarily related to net proceeds of $55.4 million in a registered direct offering from the sale of 10.8 million shares of our common stock at $5.55 per share and warrants to purchase 2.2 million shares of our common stock at a price of $7.18 per share, and $30.1 million from the sale of 9.5 million shares of our common stock to Kingsbridge under our 2006 and 2007 CEFF. Cash flows in 2006 included net proceeds of $27.9 million from the sale of 6.3 million shares of our common stock to Kingsbridge under our 2006 CEFF and net proceeds of $25.0 million from the sale of 5.8 million shares of our common stock under an underwritten public offering.
Capital Leases
Our South San Francisco headquarters facility lease was recorded as a capital lease as a result of certain amendments that required us to fund the costs of certain structural components of the facility. In December 2008, we entered into an agreement to terminate our South San Francisco headquarters facility lease. In consideration of the early termination of the lease, we paid the landlord a total fee of $14.7 million in December 2008.
Operating Leases
We lease certain of our facilities and equipment under non-cancelable operating leases. The Hayward facility lease expires in 2017 and contains options for renewal. In March 2009, we entered into agreements to terminate our Hayward manufacturing facility leases. The termination agreements are subject to certain conditions. Subject to fulfillment or waiver of these conditions, the leases will terminate by March 31, 2009. In consideration of the early termination of the leases, we will pay the landlords an aggregate amount of $3.6 million and issue one million shares of our common stock. The Memphis facility lease expires in April 2009 and we do not intend to renew.
In May 2007, we sold a portion of our leasehold improvements and equipment located in our Memphis facility for $2.2 million in cash. These leasehold improvements and equipment related to manufacturing activities that are no longer being carried out at this facility. Additionally, we amended our existing lease for the Memphis facility with the landlord and the buyer entered into a separate lease with the landlord for a majority portion of the facility. We continued to lease the remaining portion of the facility for our product distribution center. The net book value of the assets sold was $0.8 million, resulting in a gain on sale of $1.4 million.
Our long-term contractual obligations at December 31, 2008 were as follows:
| | | | | | | | | | | | | | | |
| | | | | Payment Due |
| | | Total | | 2009 | | 2010 and 2011 | | 2012 and 2013 | | 2014 and
Thereafter |
| | | (In thousands) |
Convertible senior notes and related interest | | $ | 77,512 | | $ | 2,215 | | $ | 75,297 | | $ | — | | $ | — |
Operating leases | | | 23,740 | | | 1,691 | | | 4,743 | | | 5,774 | | | 11,532 |
| | | | | | | | | | | | | | | |
| | $ | 101,252 | | $ | 3,906 | | $ | 80,040 | | $ | 5,774 | | $ | 11,532 |
| | | | | | | | | | | | | | | |
Under certain circumstances, we may redeem some or all of the convertible senior notes on or after November 1, 2009 at a redemption price equal to 100% of the principal amount of the notes. Holders of the notes may require us to repurchase some or all of their notes if a fundamental change (as defined in the indenture) occurs, at a repurchase price equal to 100% of the principal amount of the notes, plus accrued and unpaid interest (and additional amounts, if any) to, but not including, the repurchase date. The notes are convertible into our common stock, initially at the conversion price of $9.10 per share, equal to a conversion rate of 109.8901 shares per $1,000 principal amount of notes, subject to adjustments for stock dividends, stock splits and other similar events.
41
Our capital requirements depend on numerous factors, including our ability to successfully implement our restructuring plan and whether we determine to pursue other biopharmaceutical product areas. Our announcement that we have terminated VITAL-1 and VITAL-2 has significantly depressed our stock price and severely impaired our ability to raise additional funds. It could be difficult or impossible for us to raise additional capital. Therefore, although we will continue to consider financing alternatives, we are considering strategic alternatives, including merger with or acquisition by another company, further restructuring, other sales of non-core assets, and other potential technology outlicensing or liquidation of the company.
We believe that our current liquidity position will be sufficient to meet our cash needs for at least the next year under our current restructuring plan. However, sources of liquidity available to us are limited, and, if we determine to pursue other biopharmaceutical product areas, we would need to raise significant additional funds, which depend on many factors beyond our control and it may be difficult or impossible to raise additional funds, as discussed above. We may seek from time to time to purchase or retire additional amounts of our outstanding convertible notes through cash purchases and/or exchanges for other of our securities in open market transactions, privately negotiated transactions and/or a tender offer, if we can do so on attractive terms. We will evaluate such transactions, if any, in light of then existing market conditions.
Off-Balance Sheet Arrangements
As of December 31, 2008, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated by the SEC.
42
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rate Risk
We are exposed to interest rate sensitivity on our investments in debt securities and our outstanding fixed rate debt. The objective of our investment activities is to preserve principal, while at the same time maximizing yields without significantly increasing risk. To achieve this objective, we invest in highly liquid, investment grade and government debt securities. Our investments in debt securities are subject to interest rate risk. To minimize the exposure due to an adverse shift in interest rates, we invest in short-term securities and our goal is to maintain an average maturity of less than one year. We have the means and intent to hold these securities to maturity, mitigating any interest rate risk which we have estimated, as of December 31, 2008, to be less than $0.1 million from an immediate one percent increase in interest rates. The following table provides information about our financial instruments that are sensitive to changes in interest rates.
Interest Rate Sensitivity
Principal Amount by Expected Maturity and Average Interest Rate
| | | | | | | | | | | | | | | | | | | | | | | |
As of December 31, 2008 | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | Total | | | Fair Value
December 31,
2008 |
| | | (Dollars in thousands) |
Total Investment Securities Excluding Asset Backed | | $ | 82,427 | | | $ | — | | | $ | — | | | $ | — | | | $ | 82,427 | | | $ | 82,673 |
Average Interest Rate | | | 2.04 | % | | | — | | | | — | | | | — | | | | 2.04 | % | | | |
Fixed Interest Rate Convertible Senior Notes | | $ | 2,215 | | | $ | 2,215 | | | $ | 73,082 | | | $ | — | | | $ | 77,512 | | | $ | 27,329 |
Average Interest Rate | | | 3.125 | % | | | 3.125 | % | | | 3.125 | % | | | — | | | | 3.125 | % | | | |
As of December 31, 2007 | | 2008 | | | 2009 | | | 2010 | | | 2011 | | | Total | | | Fair Value
December 31,
2007 |
| | | (Dollars in thousands) |
Total Investment Securities Excluding Asset Backed | | $ | 114,098 | | | $ | — | | | $ | — | | | $ | — | | | $ | 114,098 | | | $ | 114,394 |
Average Interest Rate | | | 5.04 | % | | | — | | | | — | | | | — | | | | 5.04 | % | | | |
Asset Backed Securities(i) | | | | | | | | | | | | | | | | | | $ | 26,426 | | | $ | 26,452 |
Average Interest Rate | | | | | | | | | | | | | | | | | | | 3.85 | % | | | |
Fixed Interest Rate Convertible Senior Notes | | $ | 4,531 | | | $ | 4,531 | | | $ | 4,531 | | | $ | 149,531 | | | $ | 163,124 | | | $ | 105,828 |
Average Interest Rate | | | 3.125 | % | | | 3.125 | % | | | 3.125 | % | | | 3.125 | % | | | 3.125 | % | | | |
| (i) | Asset backed securities have various contractual maturity dates ranging from 2007 to 2010. The expected maturity dates for these securities range from 2007 to 2008 and differ from the contractual maturity dates because the issuers of these securities have, in some circumstances, the right to prepay the obligations. |
43
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Cell Genesys, Inc.
We have audited Cell Genesys, Inc.’s internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Cell Genesys, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Cell Genesys, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the 2008 consolidated financial statements of Cell Genesys, Inc. and our report dated March 9, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Palo Alto, California
March 9, 2009
44
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Cell Genesys, Inc.
We have audited the accompanying consolidated balance sheets of Cell Genesys, Inc. as of December 31, 2008 and 2007, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cell Genesys, Inc. at December 31, 2008 and 2007, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2008, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cell Genesys, Inc.’s internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 9, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Palo Alto, California
March 9, 2009
45
CELL GENESYS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except par value and share data)
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | |
| ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 30,567 | | | $ | 23,588 | |
Short-term investments | | | 52,644 | | | | 120,828 | |
Short-term restricted cash and investments | | | 1,899 | | | | — | |
Receivable from collaborative partner | | | 6,506 | | | | — | |
Prepaid expenses and other current assets | | | 2,740 | | | | 3,932 | |
| | | | | | | | |
Total current assets | | | 94,356 | | | | 148,348 | |
Restricted cash and investments | | | 991 | | | | 2,890 | |
Property and equipment, net | | | 1,577 | | | | 119,011 | |
Unamortized debt issuance costs and other assets | | | 1,049 | | | | 3,143 | |
| | | | | | | | |
Total assets | | $ | 97,973 | | | $ | 273,392 | |
| | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 2,093 | | | $ | 4,268 | |
Accrued compensation and benefits | | | 819 | | | | 7,730 | |
Accrued restructuring | | | 4,851 | | | | — | |
Other accrued liabilities | | | 2,591 | | | | 8,852 | |
Deferred revenue | | | — | | | | 12,000 | |
Warrant liability | | | 633 | | | | — | |
Current portion of accrued income taxes | | | — | | | | 4 | |
Current portion of capital lease obligation | | | — | | | | 1,711 | |
| | | | | | | | |
Total current liabilities | | | 10,987 | | | | 34,565 | |
Other liabilities | | | 4,006 | | | | 3,451 | |
Non-current portion of accrued income taxes | | | — | | | | 6,192 | |
Non-current portion of capital lease obligation | | | — | | | | 46,635 | |
Convertible senior notes | | | 70,867 | | | | 145,000 | |
| | |
Commitments and contingencies | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Preferred stock, $.001 par value: 5,000,000 shares authorized; none issued and outstanding in 2008 and 2007, respectively | | | — | | | | — | |
Common stock, $.001 par value: 275,000,000 shares authorized; 86,809,651 and 78,473,876 shares issued and outstanding in 2008 and 2007, respectively | | | 87 | | | | 78 | |
Additional paid-in capital | | | 550,280 | | | | 528,674 | |
Accumulated other comprehensive loss | | | (164 | ) | | | (88 | ) |
Accumulated deficit | | | (538,090 | ) | | | (491,115 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 12,113 | | | | 37,549 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 97,973 | | | $ | 273,392 | |
| | | | | | | | |
See accompanying notes
46
CELL GENESYS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (In thousands, except per share data) | |
Revenue | | $ | 94,571 | | | $ | 1,380 | | | $ | 1,364 | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 92,544 | | | | 106,131 | | | | 96,346 | |
General and administrative | | | 17,482 | | | | 20,401 | | | | 17,930 | |
Impairment of long-lived assets | | | 71,789 | | | | — | | | | 193 | |
Restructuring charges | | | 13,798 | | | | — | | | | (82 | ) |
| | | | | | | | | | | | |
Total operating expenses | | | 195,613 | | | | 126,532 | | | | 114,387 | |
| | | | | | | | | | | | |
Loss from operations | | | (101,042 | ) | | | (125,152 | ) | | | (113,023 | ) |
Other income (expense): | | | | | | | | | | | | |
Gain from purchase of convertible senior notes | | | 42,668 | | | | — | | | | — | |
Gain from revaluation of warrant liability | | | 11,480 | | | | — | | | | — | |
Gain on sale of Abgenix, Inc. common stock | | | — | | | | — | | | | 62,677 | |
Gain (loss) on sale of property and equipment | | | (401 | ) | | | 1,306 | | | | (2 | ) |
Interest and other income | | | 4,048 | | | | 9,021 | | | | 7,497 | |
Interest expense | | | (9,923 | ) | | | (10,331 | ) | | | (10,465 | ) |
| | | | | | | | | | | | |
Loss before income tax benefit (provision) | | | (53,170 | ) | | | (125,156 | ) | | | (53,316 | ) |
Income tax benefit (provision) | | | 6,195 | | | | 25,882 | | | | (29,613 | ) |
| | | | | | | | | | | | |
Net loss | | | (46,975 | ) | | | (99,274 | ) | | | (82,929 | ) |
| | | | | | | | | | | | |
Basic and diluted net loss per share | | $ | (0.56 | ) | | $ | (1.39 | ) | | $ | (1.67 | ) |
| | | | | | | | | | | | |
Weighted average shares of common stock outstanding—basic and diluted | | | 83,641 | | | | 71,255 | | | | 49,728 | |
| | | | | | | | | | | | |
See accompanying notes
47
CELL GENESYS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-In
Capital | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Accumulated
Deficit | | | Common
Stock
Subscription
Receivable | | | Total
Stockholders’
Equity | |
| | | Shares | | Amounts | | | | | |
| | | (In thousands, except price per share amounts) | |
Balances at December 31, 2005 | | 45,559 | | $ | 46 | | $ | 375,700 | | | $ | 33,663 | | | $ | (308,912 | ) | | $ | — | | | $ | 100,497 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | — | | | — | | | — | | | | — | | | | (82,929 | ) | | | — | | | | (82,929 | ) |
Change in net unrealized gain on available-for-sale securities, net of taxes | | — | | | — | | | — | | | | (34,041 | ) | | | — | | | | — | | | | (34,041 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | (116,970 | ) |
Issuance of common stock upon exercise of stock options and pursuant to the Employee Stock Purchase Plan | | 256 | | | — | | | 1,196 | | | | — | | | | — | | | | — | | | | 1,196 | |
Issuance of common stock upon drawdown of committed equity financing facility at $3.07-$6.00 per share, net of issuance costs of $0.1 million | | 6,289 | | | 6 | | | 27,845 | | | | — | | | | — | | | | — | | | | 27,851 | |
Issuance of warrant in connection with committed equity financing facility | | — | | | — | | | 1,324 | | | | — | | | | — | | | | — | | | | 1,324 | |
Financing costs related to warrant issued in connection with committed equity financing facility | | — | | | — | | | (1,324 | ) | | | — | | | | — | | | | — | | | | (1,324 | ) |
Issuance of common stock related to public offering, net of issuance costs of $0.3 million | | 5,750 | | | 6 | | | 25,017 | | | | — | | | | — | | | | — | | | | 25,023 | |
Stock-based compensation | | — | | | — | | | 5,930 | | | | — | | | | — | | | | — | | | | 5,930 | |
Common stock subscription receivable | | — | | | — | | | 1,200 | | | | — | | | | — | | | | (1,200 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2006 | | 57,854 | | | 58 | | | 436,888 | | | | (378 | ) | | | (391,841 | ) | | | (1,200 | ) | | | 43,527 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | — | | | — | | | — | | | | — | | | | (99,274 | ) | | | — | | | | (99,274 | ) |
Change in net unrealized loss on available-for-sale securities, net of taxes | | — | | | — | | | — | | | | 284 | | | | — | | | | — | | | | 284 | |
Foreign currency translation adjustment | | — | | | — | | | — | | | | 6 | | | | — | | | | — | | | | 6 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | (98,984 | ) |
Issuance of common stock upon exercise of stock options and pursuant to the Employee Stock Purchase Plan | | 322 | | | — | | | 913 | | | | — | | | | — | | | | — | | | | 913 | |
Issuance of common stock upon drawdown of committed equity financing facility at $2.83-$4.08 per share, net of issuance costs of $0.1 million | | 9,487 | | | 9 | | | 30,133 | | | | — | | | | — | | | | — | | | | 30,142 | |
Issuance of warrant in connection with committed equity financing facility | | — | | | — | | | 623 | | | | — | | | | — | | | | — | | | | 623 | |
Financing costs related to warrant issued in connection with committed equity financing facility | | — | | | — | | | (623 | ) | | | — | | | | — | | | | — | | | | (623 | ) |
Issuance of common stock related to registered direct offering, net of issuance costs of $4.6 million | | 10,811 | | | 11 | | | 50,130 | | | | — | | | | — | | | | — | | | | 50,141 | |
Issuance of warrant in connection with registered direct offering | | — | | | — | | | 5,282 | | | | — | | | | — | | | | — | | | | 5,282 | |
Stock-based compensation | | — | | | — | | | 6,528 | | | | — | | | | — | | | | — | | | | 6,528 | |
Common stock subscription receivable | | — | | | — | | | (1,200 | ) | | | — | | | | — | | | | 1,200 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2007 | | 78,474 | | $ | 78 | | $ | 528,674 | | | $ | (88 | ) | | $ | (491,115 | ) | | $ | — | | | $ | 37,549 | |
See accompanying notes
48
CELL GENESYS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY—(Continued)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-In
Capital | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Accumulated
Deficit | | | Common
Stock
Subscription
Receivable | | Total
Stockholders’
Equity | |
| | | Shares | | Amounts | | | | | |
| | | (In thousands, except price per share amounts) | |
Balances at December 31, 2007 | | 78,474 | | $ | 78 | | $ | 528,674 | | | $ | (88 | ) | | $ | (491,115 | ) | | $ | — | | $ | 37,549 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | — | | | — | | | — | | | | — | | | | (46,975 | ) | | | — | | | (46,975 | ) |
Change in net unrealized loss on available-for-sale securities, net of taxes | | — | | | — | | | — | | | | (77 | ) | | | — | | | | — | | | (77 | ) |
Foreign currency translation adjustment | | — | | | — | | | — | | | | 1 | | | | — | | | | — | | | 1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (47,051 | ) |
Issuance of common stock upon exercise of stock options and pursuant to the Employee Stock Purchase Plan | | 394 | | | 1 | | | 623 | | | | — | | | | — | | | | — | | | 624 | |
Issuance of common stock upon release of restricted stock awards | | 833 | | | 1 | | | 1,586 | | | | — | | | | — | | | | — | | | 1,587 | |
Issuance of common stock and warrant related to registered direct offering, net of issuance costs of $1.9 million | | 7,109 | | | 7 | | | 28,138 | | | | — | | | | — | | | | — | | | 28,145 | |
Less: fair value of the warrant issued in connection with registered direct offering classified as a derivative liability | | — | | | — | | | (12,113 | ) | | | — | | | | — | | | | — | | | (12,113 | ) |
Stock-based compensation | | — | | | — | | | 3,372 | | | | — | | | | — | | | | — | | | 3,372 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2008 | | 86,810 | | $ | 87 | | $ | 550,280 | | | $ | (164 | ) | | $ | (538,090 | ) | | $ | — | | $ | 12,113 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes
49
CELL GENESYS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (In thousands) | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net loss | | $ | (46,975 | ) | | $ | (99,274 | ) | | $ | (82,929 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 14,485 | | | | 14,356 | | | | 14,521 | |
(Gain) loss on disposal of property and equipment | | | 401 | | | | (1,306 | ) | | | 2 | |
Gain on sale of Abgenix, Inc. common stock | | | — | | | | — | | | | (62,677 | ) |
Gain on sale of short-term investments | | | (14 | ) | | | — | | | | — | |
Stock-based compensation expense | | | 4,884 | | | | 6,528 | | | | 5,930 | |
Restructuring charges | | | 75 | | | | — | | | | — | |
Deferred income tax provision | | | — | | | | — | | | | 26,815 | |
Impairment of long-lived assets | | | 71,789 | | | | — | | | | 193 | |
Gain from purchase of convertible senior notes | | | (42,668 | ) | | | — | | | | — | |
Gain from termination of capital lease | | | (14,749 | ) | | | — | | | | — | |
Gain from revaluation of warrant liability | | | (11,480 | ) | | | — | | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Prepaid expenses and other assets | | | 1,909 | | | | 290 | | | | (178 | ) |
Receivable from collaborative partner | | | (6,506 | ) | | | — | | | | — | |
Accounts payable | | | (2,175 | ) | | | 1,908 | | | | 458 | |
Accrued compensation and benefits | | | (6,911 | ) | | | 1,910 | | | | 1,421 | |
Accrued restructuring | | | 4,851 | | | | — | | | | — | |
Deferred revenue | | | (12,000 | ) | | | 12,000 | | | | — | |
Other accrued liabilities | | | (5,908 | ) | | | 3,076 | | | | 1,967 | |
Accrued income taxes | | | (6,212 | ) | | | (29,214 | ) | | | 2,798 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (57,204 | ) | | | (89,726 | ) | | | (91,679 | ) |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchases of short-term investments | | | (216,845 | ) | | | (262,904 | ) | | | (183,511 | ) |
Maturities of short-term investments | | | 277,830 | | | | 269,852 | | | | 103,825 | |
Sales of short-term investments | | | 7,136 | | | | — | | | | 24,521 | |
Conversion of restricted cash and investments | | | — | | | | — | | | | 4 | |
Capital expenditures | | | (1,107 | ) | | | (4,542 | ) | | | (2,046 | ) |
Proceeds from sale of property and equipment | | | 499 | | | | 2,207 | | | | 138 | |
Proceeds from sale of Abgenix, Inc. common stock | | | — | | | | — | | | | 65,425 | |
| | | | | | | | | | | | |
Net cash provided by investing activities | | | 67,513 | | | | 4,613 | | | | 8,356 | |
| | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | |
Repayment of convertible senior notes | | | (30,389 | ) | | | — | | | | — | |
Net proceeds from registered direct offering | | | 28,145 | | | | 55,423 | | | | — | |
Net proceeds from committed equity financing facility | | | — | | | | 30,142 | | | | 27,851 | |
Net proceeds from issuance of public offering | | | — | | | | — | | | | 25,023 | |
Proceeds from exercise of stock options | | | 624 | | | | 913 | | | | 1,196 | |
Payments under capital lease obligation | | | (1,711 | ) | | | (1,475 | ) | | | (1,193 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | (3,331 | ) | | | 85,003 | | | | 52,877 | |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 6,978 | | | | (110 | ) | | | (30,446 | ) |
Effect of foreign exchange rates on cash and cash equivalents | | | 1 | | | | 6 | | | | — | |
Cash and cash equivalents, beginning of the year | | | 23,588 | | | | 23,692 | | | | 54,138 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of the year | | $ | 30,567 | | | $ | 23,588 | | | $ | 23,692 | |
| | | | | | | | | | | | |
Supplemental cash flow information: | | | | | | | | | | | | |
Interest paid | | $ | 9,197 | | | $ | 10,582 | | | $ | 10,158 | |
| | | | | | | | | | | | |
Income tax paid | | $ | 12 | | | $ | 2,338 | | | $ | — | |
| | | | | | | | | | | | |
See accompanying notes
50
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In this Annual Report, “Cell Genesys,” “we,” “us,” “our” and “the Registrant” refer to Cell Genesys, Inc.
1. Organization and Summary of Significant Accounting Policies
Business activity
We are a biotechnology company that was focused on the development and commercialization of novel biological therapies for patients with cancer. We were developing cell-based cancer immunotherapies and oncolytic virus therapies to treat different types of cancer. Following the termination of both the VITAL-1 and VITAL-2 Phase 3 clinical trials of GVAX immunotherapy for prostate cancer, our lead product program, we implemented a substantial restructuring plan in October 2008 and are currently evaluating strategic alternatives for the business.
Deficiency letter from The Nasdaq Global Market
On October 21, 2008, we received a Nasdaq Staff Deficiency Letter, or Nasdaq Letter, indicating that we had become non-compliant with the minimum $1.00 bid price requirement for continued listing on The Nasdaq Global Market as set forth in Nasdaq Marketplace Rule 4450(a)(5) because the price of our stock closed below the minimum bid price of $1.00 per share for a period of 30 consecutive business days.
The Nasdaq Letter indicated that in light of extraordinary market conditions, Nasdaq had determined to suspend enforcement of the minimum bid price and market value of publicly held shares requirements through January 16, 2009. Accordingly, the Nasdaq Letter stated that in accordance with Nasdaq Marketplace Rule 4450(e)(2), we have 180 calendar days from January 20, 2009, or until July 20, 2009, to regain compliance. Subsequently, on December 19, 2008, NASDAQ announced that given the continued extraordinary market conditions, NASDAQ is extending the suspension of the minimum bid price and market value of publicly held shares requirements through April 20, 2009. Accordingly we now have until October 27, 2009 to regain compliance. In the event we do not regain compliance within this period, the Nasdaq Letter provided that we may consider applying to transfer to the Nasdaq Capital Market, which would allow us to take advantage of the further 180 day compliance period provided on the Nasdaq Capital Market, if we meet all requirements for initial listing on the Nasdaq Capital Market, except for the minimum bid price requirement. If compliance is not demonstrated within the compliance period, the Nasdaq Letter indicated that the Nasdaq Staff will provide written notification that our common stock will be delisted, after which we may appeal the Nasdaq Staff determination to the Nasdaq Listing Qualifications Panel. We are currently evaluating our alternatives to resolve the listing deficiency. There can be no assurance that, if we do appeal the Nasdaq Staff’s Determination, that such appeal would be successful.
Principles of consolidation
Our consolidated financial statements include the accounts of Cell Genesys, Inc. and all wholly-owned subsidiaries. Intercompany balances and transactions have been eliminated.
Use of estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make judgments, assumptions and estimates that affect the amounts reported in our consolidated financial statements and accompanying notes. Actual results could differ from those estimates. Management makes estimates when preparing the financial statements including those related to revenue recognition, accrued but unbilled expenses for clinical trials, income taxes, long-term service contracts, stock-based compensation, warrant liability, fair value, asset impairment and contingencies.
51
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Concentrations of risk
We are subject to concentration of risk from our investments. Risk for investments is managed by the purchase of investment grade securities and the diversification of the investment portfolio among issuers and maturities.
Revenue recognition
Our revenues are derived principally from research and licensing agreements with collaborators. Revenue under such collaboration agreements typically includes up-front payments, cost reimbursements, milestone payments and license fees. We evaluate whether the delivered element under these arrangements has value to our customer on a stand-alone basis and whether objective and reliable evidence of fair value of the undelivered item exists. Deliverables that do not meet these criteria are treated as one unit of accounting for the purposes of revenue recognition.
Up-front payments: Up-front payments from our research collaborations include in part payments for licenses, technology transfer and access rights. Non-refundable up-front license fees and other payments under collaboration agreements where we cannot establish stand-alone value for the delivered license and where we have continuing involvement following the execution of the collaboration agreement are deferred and recognized on a straight-line or ratable method over the period of our continuing involvement unless we determine that another methodology is more appropriate. We recognize cost reimbursement revenue under collaborative agreements as the related research and development services are rendered, which approximates when related costs are incurred, as provided for under the terms of these agreements.
Milestones: Payments for milestones that are based on the achievement of substantive and at-risk performance criteria are recognized in full upon achievement of the incentive milestone events in accordance with the terms of the agreement. Incentive milestone payments are triggered either by the results of our research efforts or by events external to us, such as regulatory approval to market a product or the achievement of specified sales levels by a marketing partner. As such, the incentive milestones are substantially at risk at the inception of the agreement, and the amounts of the payments assigned thereto are commensurate with the milestone achieved. Upon the achievement of an incentive milestone event, we have no future performance obligations related to that milestone payment.
License fees: Non-refundable license fees where we have completed all obligations at the execution of the arrangement are recognized as revenue upon execution of the technology licensing agreement when delivery has occurred, collectibility is reasonably assured and the price is fixed and determinable.
Property and equipment
Property and equipment are stated at cost and depreciated using the straight-line method over the estimated useful lives of the assets, generally five to 15 years. Repair and maintenance costs are expensed as incurred. Computer equipment is depreciated over a life of three years. Property and equipment leased under capital leases are amortized over the shorter of the useful lives or the lease term. Amortization of capitalized leased equipment is included in depreciation expense. Leasehold improvements are stated at cost and amortized over the shorter of the useful lives or the lease term. Costs for assets under construction are classified as “construction in process” and such costs are reclassified to an appropriate fixed asset classification and depreciated when the asset is placed into service.
52
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Asset Impairment
Our policy regarding long-lived assets is to evaluate the recoverability of our assets when the facts and circumstances suggest that the assets may be impaired. This assessment of fair value is performed based on the estimated undiscounted cash flows compared to the carrying value of the assets. If the future cash flows (undiscounted and without interest charges) are less than the carrying value, a write-down would be recorded to reduce the related asset to its estimated fair value.
Unamortized debt issuance costs
Unamortized debt issuance costs relate to our convertible senior notes and are amortized over the life of the related debt. Amortization expense totaled $0.7 million in each of the years ended December 31, 2008, 2007 and 2006, respectively, and is reported as interest expense.
Cash, cash equivalents and short-term investments
We invest our excess cash and short-term investments, including restricted cash and investments, with high credit quality United States and foreign financial institutions, and government and corporate issuers. We limit the amount of credit exposure to any one issuer. We consider all highly liquid investments with insignificant interest rate risk with original maturities of less than three months when purchased to be cash equivalents. All investments are denominated in U.S. dollars. We record our investments at fair market value, based on quoted market prices.
Our debt securities are classified as available-for-sale and carried at fair value. Management considers our investments in debt securities to be available for use in current operations. As a result, all investments in debt securities are classified as current assets, even if the remaining maturity of the investment is more than one year beyond the balance sheet date. The cost of securities sold is based on the specific identification method. Realized gains and losses and declines in value on securities classified as available-for-sale that are judged to be other than temporary are included in interest and other income (loss). Unrealized gains and losses on securities classified as available-for-sale are recorded in accumulated other comprehensive income, net of tax. We determine the appropriate classification of debt securities at the time of purchase and re-evaluate such designation as of each balance sheet date.
We regularly review all of our investments for potential “other-than-temporary” declines in fair value. We review the cause of the impairment, the creditworthiness of the security issuers, the number of securities in an unrealized loss position, as well as the severity and duration of the unrealized losses. When we determine that the decline in fair value of an investment below its net carrying value is other-than-temporary, we reduce the carrying value of the securities by recognizing a loss in the amount of such decline. No such reductions have been required during the past three years.
Restricted cash and investments as of December 31, 2008 relate to outstanding letters of credit which secure our former leased corporate headquarters facility in South San Francisco, California, and our leased cGMP manufacturing facility in Hayward, California. On January 2, 2009, we terminated our lease in South San Francisco, California and the landlord agreed to return and have cancelled the related $1.9 million letter of credit on or before March 16, 2009. This letter of credit was reclassified to current assets as of December 31, 2008.
Fair value of financial instruments
The carrying amounts of financial instruments such as cash equivalents, prepaid expenses and other current assets, accounts payable, accrued expenses and other current liabilities approximate fair value because of the short maturities of these instruments. The estimated fair value of our convertible senior notes is determined by using
53
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
available market information and valuation methodologies that correlate fair value with the market price of our common stock which fair value is provided by a third party financial institution. The fair value of our convertible senior notes as of December 31, 2008 and 2007 was approximately $27.3 million and $105.8 million, respectively.
Foreign Currency Translation
Our subsidiary located in the United Kingdom operated using the local currency as the functional currency. Accordingly, all assets and liabilities of this subsidiary were translated using exchange rates in effect at the end of the period, and revenues and expenses were translated using average exchange rates for the period. The resulting translation adjustments are presented as a separate component of accumulated other comprehensive loss.
The principal activity of our subsidiary located in the United Kingdom was to provide management of two Phase 3 clinical trials in prostate cancer in Europe. We terminated all activities of the U.K. subsidiary as of December 31, 2008 as part of our restructuring plan.
Research and Development Expenses
We account for research and development costs in accordance with Statement of Financial Accounting Standards No. 2, “Accounting for Research and Development Costs,” and Emerging Issues Task Force, or EITF, Issue No. 07-03, “Accounting for Advance Payments for Goods or Services to Be Used in Future Research and Development Activities,” or EITF 07-03. For research and development activities in progress at January 1, 2008, costs were expensed as incurred including nonrefundable prepayments for research and development services. On January 1, 2008, we adopted EITF 07-03 and changed our accounting policy. For new contracts entered into as of or subsequent to January 1, 2008, nonrefundable prepayments for research and development services are deferred and recognized as the services are rendered. The adoption of EITF 07-03 did not have a significant impact on our results of operations or our financial position.
Fair Value
In September 2006, the Financial Accounting Standards Board, or FASB, issued Statement of Financial Accounting Standards No. 157, “Fair Value Measurements,” or FAS 157. FAS 157 defines fair value, establishes a framework for measuring fair value and expands the disclosure requirements regarding fair value measurements. FAS 157 is effective for fiscal years beginning after November 15, 2007 for financial assets and liabilities as well as for non-financial assets and liabilities that are recognized or disclosed at fair value on a recurring basis in the financial statements. In February 2008, the FASB issued FASB Staff Position No. FAS 157-2, “Effective Date of FASB Statement No. 157,” which provides a one year deferral of the effective date of FAS 157 for non-financial assets and liabilities, except those that are recognized or disclosed in the financial statements at fair value at least annually. In October 2008, the FASB issued FASB Staff Position No. 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active,” or FSP 157-3, that clarifies the application of FAS 157 in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 is effective upon issuance, including prior periods for which the financial statements have not been issued.
On January 1, 2008, we adopted the provisions of FAS 157 on a prospective basis for our financial assets and liabilities. FAS 157 requires that we determine the fair value of financial assets and liabilities using the fair value hierarchy established in FAS 157 and describes three levels of inputs that may be used to measure fair value, as follows:
| | • | | Level 1—Quoted prices in active markets for identical assets or liabilities. |
54
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| | • | | Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| | • | | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The adoption of this statement did not have a material impact on our consolidated results of operations and financial condition.
In accordance with FAS 157, the following table represents the fair value hierarchy for our financial assets and liabilities (cash equivalents, investments and warrant liability) measured at fair value on a recurring basis as of December 31, 2008 (in thousands):
| | | | | | | | | | | | |
Description | | Total | | Fair Value Measurements at
December 31, 2008 |
| | | Level1 | | Level 2 | | Level3 |
Assets: | | | | | | | | | | | | |
Money market funds | | $ | 30,029 | | $ | — | | $ | 30,029 | | $ | — |
Corporate notes | | | 30,498 | | | — | | | 30,498 | | | — |
U.S. government and governmental agency obligations | | | 22,146 | | | — | | | 22,146 | | | — |
| | | | | | | | | | | | |
Total assets | | $ | 82,673 | | $ | — | | $ | 82,673 | | $ | — |
| | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | |
Warrant liability (1) | | $ | 633 | | $ | — | | $ | — | | $ | 633 |
| | | | | | | | | | | | |
| (1) | Refer to theCommon Stock and Warrantsection of Note 8, “Stockholders’ Equity and Stock-Based Compensation” for valuation assumptions. |
The table below includes a roll forward of the balance sheet amounts for the year ended December 31, 2008 (including the change in fair value), for financial instruments classified as Level 3 (the warrant liability). When a determination is made to classify a financial instrument within Level 3, the determination is based upon the significance of the unobservable parameters to the overall fair value measurement. However, Level 3 financial instruments typically include, in addition to the unobservable components, observable components (that is, components that are actively quoted and can be validated to external sources). Accordingly, the gains in the table below include changes in fair value due in part to observable factors that are part of the methodology (in thousands):
| | | | |
Description | | Year Ended
December 31, 2008 | |
Balance, beginning | | $ | — | |
Purchases, issuances, and settlements | | | 12,113 | |
Total gains included in earnings (1) | | | (11,480 | ) |
| | | | |
Balance, ending | | $ | 633 | |
| | | | |
| (1) | Gain for the year ended December 31, 2008 related to the revaluation of the warrant liability from the date of the warrant issuance (May 14, 2008) through December 31, 2008. This gain is reflected in our consolidated statements of operations as a component of other income (expense). |
55
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Warrant Liability
We applied the provisions of Statement of Financial Accounting Standards No. 133, “Accounting for Derivative Instruments and Hedging Activities,” or FAS 133, Statement of Financial Accounting Standards No. 150, “Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity,” or FAS 150, and Emerging Issues Task Force Issue 00-19, “Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock,” or EITF 00-19, in accounting for derivative financial instruments.
For a warrant classified as a derivative liability pursuant to FAS 133 and FAS 150, the fair value of the warrant is recorded on the consolidated balance sheet at inception of such classification and adjusted to fair value at each financial reporting date. The changes in fair value of the warrant are recorded in the consolidated statements of operations as a component of other income (expense). The fair value of the warrant is estimated using the Black Scholes option-pricing model. The warrant will continue to be reported as a liability until such time as the instrument is exercised or is otherwise modified to remove the provisions which require this treatment, at which time the warrant is adjusted to fair value and reclassified from liabilities to stockholders’ equity. If the warrant is reclassified as permanent equity, the fair value of the warrant would be recorded in stockholders’ equity and no further adjustment would be made in subsequent periods.
Net loss per share
Basic net loss per share is calculated using the weighted average number of shares of common stock outstanding during the period. Diluted net loss per share includes the impact of potentially dilutive securities. As our potentially dilutive securities were anti-dilutive for all years presented, such securities have been excluded from the computation of shares used in computing diluted net loss per share. These outstanding securities consisted of the following (in thousands):
| | | | | | |
| | | December 31, |
| | | 2008 | | 2007 | | 2006 |
Convertible senior notes | | 7,788 | | 15,934 | | 15,934 |
| | | | | | |
Outstanding stock options | | 8,615 | | 9,429 | | 8,368 |
| | | | | | |
Warrants to purchase common stock | | 11,490 | | 2,959 | | 375 |
| | | | | | |
Comprehensive loss
Comprehensive loss is comprised of net loss and other comprehensive income (loss). Other comprehensive income (loss) includes certain changes to our stockholders’ equity that are excluded from net loss, such as unrealized gains or losses on our available-for-sale securities and foreign currency translation adjustments. The following table presents the calculation of comprehensive loss (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Net loss | | $ | (46,975 | ) | | $ | (99,274 | ) | | $ | (82,929 | ) |
Other comprehensive income (loss): | | | | | | | | | | | | |
Net unrealized gain (loss) on investments, net of taxes of zero in both 2008 and 2007 and $0.6 million in 2006 | | | (77 | ) | | | 284 | | | | 1,063 | |
Net change in foreign currency translation gain (loss) | | | 1 | | | | 6 | | | | — | |
Less: reclassification adjustment for gains recognized in net loss, net of related tax of $27.5 million | | | — | | | | — | | | | (35,104 | ) |
| | | | | | | | | | | | |
Comprehensive loss | | $ | (47,051 | ) | | $ | (98,984 | ) | | $ | (116,970 | ) |
| | | | | | | | | | | | |
56
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Income taxes
On January 1, 2007, we adopted the provisions of FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes,” or FIN 48. FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with Statement of Financial Accounting Standards No. 109, “Accounting for Income Taxes,” or FAS 109, and prescribes a recognition threshold and measurement attribute for financial statement disclosure of tax positions taken or expected to be taken on a tax return. Additionally, FIN 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
As a result of the implementation of FIN 48, we did not recognize any adjustment in the liability for unrecognized income tax benefits and, therefore, implementation of FIN 48 did not result in a cumulative adjustment to accumulated deficit. As of the adoption date of January 1, 2007, we had $25.0 million of unrecognized tax benefits, all of which would affect our effective tax rate if recognized. As of December 31, 2008, we had zero unrecognized tax benefits and $4.0 million as of December 31, 2007, all of which would affect our effective tax rate if recognized. Our policy is to account for interest and penalties related to income tax matters in the income tax provision in the consolidated statement of operations. Accrued interest and penalties related to income tax matters are included within the related tax liability line in the consolidated balance sheet. On the adoption date of January 1, 2007, we had $10.4 million of accrued interest and zero penalties related to tax contingencies recorded in the consolidated balance sheet. We had zero accrued interest and penalties related to tax contingencies as of December 31, 2008. We had accrued $2.2 million of interest and zero penalties related to tax contingencies as of December 31, 2007.
Segment reporting
Our operations are treated as one operating segment, as we report profit and loss information only on an aggregate basis to the chief operating decision-makers.
Stock-based compensation
We account for stock-based employee compensation plans under the fair value recognition and measurement provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment”, or FAS 123R. FAS 123R requires the recognition of compensation expense, using a fair-value based method, for costs related to all share-based payments including stock options and stock issued under our employee stock plans. FAS 123R requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service periods in our Consolidated Statements of Operations. We adopted FAS 123R using the modified prospective transition method, which requires that compensation expense be recognized in the financial statements for all awards granted after the date of adoption as well as for existing awards for which the requisite service has not been rendered as of the date of adoption. See Note 9, “Stockholders’ Equity and Stock-Based Compensation” for further information.
Reclassifications
Certain prior year amounts have been reclassified to conform to the current year presentation. We reclassified impairment of long-lived assets from general and administrative expenses and presented it as a separate line item on our consolidated statements of operations. The reclassification had no impact on our total operating expenses or our net loss.
57
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Recent accounting pronouncements
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 160, “Noncontrolling Interest in Consolidated Financial Statements, an amendment of Accounting Research Bulletin No. 51, Consolidated Financial Statements,” or FAS 160. FAS 160 establishes accounting and reporting standards for ownership interests in subsidiaries held by parties other than the parent, the amount of consolidated net income (loss) attributable to the parent and to the noncontrolling interest, changes in a parent’s ownership interest and the valuation of retained noncontrolling equity investments when a subsidiary is deconsolidated. FAS 160 also establishes additional reporting requirements that identify and distinguish between the interest of the parent and the interest of the noncontrolling owners. FAS 160 is effective for fiscal years beginning after December 15, 2008. We are currently evaluating the effect the adoption of FAS 160 will have on our consolidated financial statements.
In December 2007, the FASB ratified the final consensuses in Emerging Issues Task Force Issue No. 07-01, “Accounting for Collaborative Arrangements,” or EITF 07-01, which requires certain income statement presentation of transactions with third parties and of payments between parties to the collaborative arrangement, along with disclosure about the nature and purpose of the arrangement. EITF 07-01 is effective for us beginning January 1, 2009. We have evaluated the impact of adopting EITF 07-01 on our consolidated financial statements and do not expect any impact on our results of operations or financial position.
In May 2008, the FASB issued FASB Staff Position No. APB 14-1, “Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)”, or FSP APB 14-1. FSP APB 14-1 addresses instruments commonly referred to as Instrument C from EITF 90-19, which requires the issuer to settle the principal amount in cash and the conversion spread in cash or net shares at the issuer’s option. FSP APB 14-1 requires that issuers of these instruments account for their liability and equity components separately by bifurcating the conversion option from the debt instrument, classifying the conversion option in equity, and then accreting the resulting discount on the debt as additional interest expense over the expected life of the debt. FSP APB 14-1 is effective for fiscal years beginning after December 15, 2008 and interim periods within those fiscal years, and requires retrospective application to all periods presented. Early application is not permitted. We have evaluated the impact of adopting FSP APB 14-1 on our consolidated financial statements and do not expect any impact on our results of operations or financial position.
2. Restructuring Charges
In October 2008, in view of the termination of both the VITAL-1 and VITAL-2 Phase 3 clinical trials, we placed on hold the further development of GVAX immunotherapy for prostate cancer and following approval by the Board of Directors implemented a substantial restructuring plan that resulted in the reduction of our staff of 290 by approximately 80 percent as of December 31, 2008, and approximately 90 percent as of the date of this report, with further reductions anticipated in the first half of 2009 as additional activities are phased out. In connection with this restructuring, we terminated our lease on our facility in South San Francisco, California, and temporarily relocated our corporate headquarters to Hayward, California. We paid the landlord a lease termination fee of $14.7 million in December 2008. As a result, we recorded a restructuring charge of $13.8 million in the quarter ended December 31, 2008, related to our restructuring, including $14.3 million for workforce reduction costs, $0.1 million of non-cash stock-based compensation expense, and $14.1 million for lease termination costs offset by a $14.7 million gain from the termination of a capital lease. At December 31, 2008, $4.9 million of termination benefits remained unpaid, which were classified under accrued restructuring liabilities on the consolidated balance sheet.
58
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
3. Collaborative and License Agreements
We have derived substantially all of our revenues from collaborative and license agreements, as shown in the following table (in thousands):
| | | | | | | | | |
| | | Year Ended December 31, |
| | | 2008 | | 2007 | | 2006 |
Takeda | | $ | 80,376 | | $ | — | | $ | — |
GBP IP, LLP | | | 12,000 | | | — | | | — |
sanofi-aventis Group | | | 1,000 | | | 1,000 | | | 1,000 |
Ceregene, Inc. | | | — | | | 13 | | | 83 |
Other | | | 1,195 | | | 367 | | | 281 |
| | | | | | | | | |
| | $ | 94,571 | | $ | 1,380 | | $ | 1,364 |
| | | | | | | | | |
Takeda Development and Commercialization Collaborative Agreement
We entered into a worldwide collaborative agreement with Takeda Pharmaceutical Company Limited, or Takeda, for the development and commercialization of GVAX immunotherapy for prostate cancer. Under the terms of the agreement, effective March 31, 2008, we granted exclusive worldwide commercial rights to GVAX immunotherapy for prostate cancer for the prevention, diagnosis, and treatment of prostate cancer and other urological neoplasms or urological hyperplasias. In exchange for these rights and in consideration for prior costs incurred by us in the development of GVAX immunotherapy for prostate cancer, Takeda made a non-refundable and non-creditable upfront payment of $50 million. We received full payment of the $50 million in April 2008.
We applied EITF Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables”, in evaluating the appropriate accounting for this agreement. In accordance with this guidance, we identified the initial license transfer, certain development services and certain regulatory filing support as deliverables under this agreement and concluded that these deliverables should be accounted for as a single unit of accounting based upon the determination that these deliverables are linked and there is no objective and reliable evidence of the fair value of the undelivered items. Therefore, the elements could not be accounted for separately. Since both our service and the benefit to Takeda were performed and realized consistently throughout the service period and were reflective of a consistent level of effort over the service period, and since benefit was realized consistently throughout, we amortized the upfront payment from Takeda ratably over the estimated term to complete the deliverables.
In October 2008, we terminated both the VITAL-1 and VITAL-2 Phase 3 clinical trials and placed on hold the further development of GVAX immunotherapy for prostate cancer. Our decision to place on hold further development of GVAX immunotherapy for prostate cancer resulted in Takeda terminating our collaborative agreement in December 2008. Due to the termination of our collaborative agreement, all deliverables under this agreement were cancelled. As a result, on December 1, 2008, we recognized as revenue the remaining balance of deferred revenue of $37.9 million of the upfront payment from Takeda.
Additionally, Takeda agreed to pay for all external development costs associated with the ongoing Phase 3 clinical development of GVAX immunotherapy for prostate cancer, including the cost of product, all internal and external additional development costs and all commercialization costs. As of December 31, 2008, we have received payments from Takeda of $23.9 million for development costs. We also have a receivable of $6.5 million, as of December 31, 2008, from Takeda for such costs incurred in the quarter ended December 31, 2008 which we collected in February 2009. Future reimbursement revenue from Takeda will end during the first quarter of 2009 consistent with the wind-down activities associated with this agreement.
59
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
GBP IP, LLC Technology and Intellectual Property Agreement
In December 2007, we sold for $12.0 million all of our assets, intellectual property and previously established licensing agreements relating to our lentiviral gene delivery technology, commonly referred to as lentiviral vectors, to GBP IP, LLC, an affiliate of GBP Capital, the majority shareholder in privately held Lentigen Corporation. We received full payment of $12.0 million in December 2007. Under the agreement for the transaction, we retained our rights to use the technology for research and development purposes including potential future use with our cancer immunotherapy products. We applied EITF Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables,” in evaluating the appropriate accounting for this agreement. We identified the delivery of biological materials (including certain GMP-compliant materials), intellectual property and previously established licensing agreements related to our lentiviral gene delivery technology as the primary deliverables under this agreement and concluded that these deliverables should be accounted for as a single unit of accounting based upon the determination that the remaining undelivered items did not have reliable and objective evidence of fair value. Therefore, the elements could not be accounted for separately. Recognition of revenue was deferred until we performed all of our obligations under the agreement which occurred during the three months ended March 31, 2008.
Gene activation technology licenses
In February 1997, we executed a license agreement with Aventis, now sanofi-aventis Group, for gene-activated erythropoietin, or EPO, and a second undisclosed protein. In November 2008, sanofi-aventis Group informed us of its intention to terminate the portion of this license agreement that related to EPO. Previously, in late 2000, sanofi-aventis Group informed us of its intention to terminate the portion of this license agreement that related to the second undisclosed protein. The agreement for gene-activated EPO provided for up to $26.0 million in milestone payments, as well as annual maintenance fees and any royalties on future sales of gene-activated EPO anywhere in the world. As of December 31, 2008, we had received approximately $28.2 million under this license agreement, which included certain milestone payments relating to the development of gene-activated EPO which sanofi-aventis Group is developing in collaboration with Transkaryotic Therapies, Inc., now owned by Shire Pharmaceuticals Group plc. We recognized revenues of $1.0 million in each of the years ended December 31, 2008, 2007 and 2006 pursuant to the agreement.
Other
We also recognized revenue of $0.8 million from a sublicense of certain patents related to the hemophilia and lysosomal storage disorder technology during the year ended December 31, 2008, compared to zero for each of the years ended December 31, 2007 and 2006.
60
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
4. Investments
The following is a summary of our available-for-sale securities at December 31, 2008 and 2007 (in thousands):
| | | | | | | | | | | | | |
December 31, 2008 | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | | Estimated
Fair Value |
Money market funds | | $ | 30,029 | | $ | — | | $ | — | | | $ | 30,029 |
Corporate notes | | | 30,390 | | | 111 | | | (3 | ) | | | 30,498 |
U.S. government agencies | | | 22,008 | | | 138 | | | — | | | | 22,146 |
| | | | | | | | | | | | | |
| | $ | 82,427 | | $ | 249 | | $ | (3 | ) | | $ | 82,673 |
| | | | | | | | | | | | | |
Classified as: | | | | | | | | | | | | | |
Cash equivalents | | | | | | | | | | | | $ | 30,029 |
Short-term investments | | | | | | | | | | | | | 52,644 |
| | | | | | | | | | | | | |
| | | | | | | | | | | | $ | 82,673 |
| | | | | | | | | | | | | |
| | | | |
December 31, 2007 | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | | Estimated
Fair Value |
Money market funds | | $ | 20,018 | | $ | — | | $ | — | | | $ | 20,018 |
Corporate notes | | | 93,475 | | | 314 | | | (19 | ) | | | 93,770 |
Asset backed securities | | | 26,426 | | | 26 | | | — | | | | 26,452 |
U.S. government agencies | | | 605 | | | 1 | | | — | | | | 606 |
| | | | | | | | | | | | | |
| | $ | 140,524 | | $ | 341 | | $ | (19 | ) | | $ | 140,846 |
| | | | | | | | | | | | | |
Classified as: | | | | | | | | | | | | | |
Cash equivalents | | | | | | | | | | | | $ | 20,018 |
Short-term investments | | | | | | | | | | | | | 120,828 |
| | | | | | | | | | | | | |
| | | | | | | | | | | | $ | 140,846 |
| | | | | | | | | | | | | |
As of December 31, 2008, we only had one security that had an unrealized loss and received full value when this corporate note matured on March 4, 2009. The gross unrealized loss in our portfolio of investments represented approximately 0.004% of the total fair value of the portfolio as of December 31, 2008. During the year ended December 31, 2008, we sold three credit card asset-backed debt securities issued by one issuer with a book value of $7.1 million based on possible future credit concerns. These three securities comprised our entire holdings from this issuer. We realized gains of $13,986 from the sale of these securities.
All of our available-for-sale securities have a maturity date in 2009.
61
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
5. Property and Equipment
Property and equipment consists of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | |
Machinery, furniture and equipment | | $ | 19,663 | | | $ | 30,803 | |
Leasehold improvements | | | 35,220 | | | | 101,553 | |
Assets held for sale | | | 735 | | | | — | |
Property and equipment under capital lease obligation | | | — | | | | 52,361 | |
Construction in process | | | — | | | | 3,261 | |
| | | | | | | | |
| | | 55,618 | | | | 187,978 | |
Accumulated depreciation and amortization—assets held for sale | | | (711 | ) | | | — | |
Accumulated depreciation and amortization—assets held in use | | | (53,330 | ) | | | (68,967 | ) |
| | | | | | | | |
| | $ | 1,577 | | | $ | 119,011 | |
| | | | | | | | |
In December 2008, as part of our restructuring, we entered into a lease termination agreement with the landlord to terminate the lease of our South San Francisco, CA, corporate headquarters. We paid the landlord a termination fee of $14.7 million, which included a license to use the facility in January 2009. We classified $0.6 million of the $14.7 million termination fee as prepaid rent, as of December 31, 2008. We recorded a $14.7 million gain from the termination of a capital lease. The gain was recorded as a credit to restructuring charge as of December 31, 2008 and is an offset to the termination fee.
In connection with the lease termination, the landlord took possession and ownership of the leasehold improvements, equipment and furniture in our South San Francisco facility on the lease termination date. Accordingly, we recorded a $23.8 million impairment charge and shortened the estimated life of these assets to December 2008. In addition, we sold a portion of our lab equipment located in our South San Francisco facility for $0.5 million in December 2008. The net book value of the assets sold was $0.8 million, resulting in a loss on sale of $0.3 million.
As a result of the termination of both the VITAL-1 and VITAL-2 Phase 3 clinical trials and ending further development of GVAX immunotherapy for prostate cancer, we recorded a $45.7 million impairment charge related to leasehold improvements, equipment and other assets in our Hayward and Memphis facilities that were previously capitalized and deemed not recoverable as we determined that the carrying value exceeded the fair value of the assets. The $45.7 million charge was included in impairment of long-lived assets on the consolidated statement of operations for the year ended December 31, 2008.
Additionally, we reclassified certain computer equipment to held for sale and ceased the depreciation of these assets as of December 31, 2008. We recorded a $43,000 impairment charge against this equipment as we determined that the carrying value exceeded the fair value of the assets. The $43,000 charge was included in impairment of long-lived assets on the consolidated statement of operations for the year ended December 31, 2008.
In the quarter ended December 31, 2008, we recorded a $2.1 million impairment charge related to certain intangible assets and construction in process that were previously capitalized and deemed not recoverable as we abandoned these projects due to our restructuring. In addition, we also recorded $0.1 million of impairment charges during the first six months of the year ended December 31, 2008 due to discontinuation of various capital projects. These charges were included in impairment of long-lived assets expenses for the year ended December 31, 2008.
62
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In May 2007, we sold a portion of our leasehold improvements and equipment located in our Memphis facility for $2.2 million in cash. Such property was related to manufacturing activities which were no longer being carried out at this facility. The net book value of the assets sold was $0.8 million, resulting in a gain on sale of $1.4 million.
Accumulated amortization related to capital lease assets was zero and $17.0 million as of December 31, 2008 and 2007, respectively.
6. Convertible Senior Notes and Other Debt Financings
In October 2004, we entered into a purchase agreement with initial purchasers relating to the private placement of $110.0 million aggregate principal amount of our 3.125% Convertible Senior Notes due in 2011, or Notes. We granted the initial purchasers a 30-day option to purchase up to an additional $35.0 million principal amount of the notes, which the purchasers elected to exercise in full in November 2004. We received approximately $139.9 million in net proceeds, after deducting the initial purchasers’ discount and estimated offering expenses. We used a portion of the net proceeds to repay bank debt of $60.0 million related to an asset-backed debt financing obligation acquired from Fleet Bank in December 2001 in connection with the construction of our manufacturing facility in Hayward, California, and to repay $35.0 million in term loans acquired in September 2003 from Silicon Valley Bank. We recorded interest expense including the amortization of debt issuance costs related to our convertible senior notes of $5.0 million for the year ended December 31, 2008. We recorded interest expense including the amortization of debt issuance costs related to our convertible senior notes of $5.3 million for the each of the two years ended December 31, 2007 and 2006. Interest on the notes is payable on May 1 and November 1 each year until the notes are converted or redeemed. The notes are due in 2011.
Under certain circumstances, we may redeem some or all of the notes on or after November 1, 2009 at a redemption price equal to 100% of the principal amount of the notes. Holders of the notes may require us to repurchase some or all of their notes if a fundamental change (as defined in the indenture governing the notes) occurs, at a repurchase price equal to 100% of the principal amount of the notes, plus accrued and unpaid interest (and additional amounts, if any) to the repurchase date. The notes are convertible into our common stock, initially at the conversion price of $9.10 per share, equal to a conversion rate of 109.8901 shares per $1,000 principal amount of notes, subject to adjustments for stock dividends, stock splits, and other similar events.
In October 2008, we repurchased an aggregate of $26.3 million face value of our Notes, at an overall discount of approximately 60 percent from face value in a series of privately negotiated transactions with institutional holders of the Notes, for aggregate consideration of $10.5 million in cash, plus accrued but unpaid interest. In November 2008, we commenced a tender offer in which we offered to purchase up to $80 million aggregate principal amount of our outstanding Notes at a price not greater than $400 nor less than $340 per $1,000 principal amount, plus accrued and unpaid interest. In December 2008, as a result of the tender offer, we repurchased an aggregate of $47.8 million face value of the Notes, at an overall discount of 60 percent from face value, for aggregate consideration of approximately $19.1 million in cash, plus accrued but unpaid interest. We recorded a net gain of $42.7 million, or $0.51 per basic and diluted share, from the purchase of debt comprised of a gross gain of $44.6 million less transaction costs of $0.8 million and $1.1 million of unamortized debt issuance costs related to the repurchased Notes.
As of December 31, 2008, there was $70.9 million aggregate principal amount of Notes outstanding, and $1.0 million of unamortized debt issuance costs remaining to be amortized as interest expense.
63
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
7. Leases
Operating leases
We lease certain of our facilities and equipment under non-cancelable operating leases which generally require us to make minimum lease payments as well as to reimburse the lessor for real estate taxes, insurance and maintenance expenses. The Hayward facility lease expires in 2017 and contains options for renewal. In March 2009, we entered into agreements to terminate our Hayward manufacturing facility leases. The termination agreements are subject to certain conditions. Subject to fulfillment or waiver of these conditions, the leases will terminate by March 31, 2009. In consideration of the early termination of the leases, we will pay the landlords an aggregate amount of $3.6 million and issue one million shares of our common stock. The Memphis facility lease expires in April 2009 and we do not intend to renew. Rent expense under operating leases was $3.6 million, $4.4 million, and $3.8 million in 2008, 2007, and 2006, respectively. In May 2007, we sold a portion of our leasehold improvements and equipment located in our Memphis facility. We amended our existing lease for the Memphis facility with the landlord, and the buyer entered into a separate lease with the landlord for a majority portion of the facility.
Future minimum payments under non-cancelable operating lease obligations as of December 31, 2008 are as follows (in thousands):
| | | |
| | | Operating
Leases |
Year ending December 31: | | | |
2009 | | $ | 1,691 |
2010 | | | 2,022 |
2011 | | | 2,721 |
2012 | | | 2,830 |
2013 | | | 2,944 |
2014 and beyond | | | 11,532 |
| | | |
Total minimum payments | | $ | 23,740 |
| | | |
8. Stockholders’ Equity and Stock-Based Compensation
Common stock and warrant
In September 2006, we completed an underwritten public offering and sold 5.8 million shares of our common stock, resulting in net proceeds of $25.0 million. These offerings were pursuant to our shelf registration statement filed in February 2003, which allowed us to offer up to $150.0 million of securities in one or more public offerings. In April 2007, we used the remaining registered amount under this shelf registration statement to raise net proceeds of $55.4 million in a registered direct offering of 10.8 million shares of our common stock at $5.55 per share and warrants to purchase 2.2 million shares of our common stock at a price of $7.18 per share from selected institutional investors.
On May 16, 2007, our new shelf registration statement was declared effective by the Securities and Exchange Commission under the Securities Act of 1933, as amended, which allows us to offer up to $150.0 million of securities on short notice in one or more public offerings under the Securities Act of 1933, as amended. As of December 31, 2008, $120 million is available for issuance under this shelf registration statement.
In May 2008, we received net proceeds of approximately $28.1 million in a registered direct offering, after deducting placement agents’ fees and stock issuance costs of approximately $1.9 million, from the sale of
64
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
7.1 million shares of our common stock at $4.22 per share and warrants to purchase 8.5 million shares of our common stock at a price of $10.00 per share. The offering was made pursuant to our effective May 2007 shelf registration statement on Form S-3. These warrants became exercisable on November 14, 2008 for a period of seven years thereafter. The fair value on the date of issuance of the warrants was determined to be $12.1 million using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 3.6%, (ii) an expected term of 7.5 years, (iii) no dividend yield and (iv) a volatility of 55%. The warrants are classified as a derivative liability pursuant to FAS 133 and FAS 150. Therefore, the fair value of the warrants is recorded on the consolidated balance sheet as a liability and will be adjusted to fair value at each financial reporting date thereafter. As of December 31, 2008, the fair value of warrants was determined to be $0.6 million using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 1.9%, (ii) an expected term of 6.9 years, (iii) no dividend yield and (iv) a volatility of 101%. For the year ended December 31, 2008, due to the decreases in fair value of the warrants we recorded gains of $11.5 million to other income.
Committed Equity Financing Facility
In March 2006, we entered into a Committed Equity Financing Facility, or 2006 CEFF, with Kingsbridge Capital Limited, or Kingsbridge, pursuant to which Kingsbridge committed to purchase, subject to certain conditions, up to the lesser of 8.7 million shares of our common stock or an aggregate of $75.0 million during the three years following the entry into the 2006 CEFF. Under the 2006 CEFF, we were able to draw down in tranches of up to a maximum of 2.5 percent of the closing market value of our common stock on the last trading day prior to the commencement of the drawdown, or $15.0 million, whichever is less, subject to certain conditions. The purchase price of these shares was discounted between 6 to 10 percent from the volume weighted average price of our common stock for each of the eight trading days following the election to sell shares. Kingsbridge was not obligated to purchase shares at prices below $3.00 per share or at a price below 85% of the closing market value of our common stock on the trading day immediately preceding the commencement of the drawdown.
In connection with the 2006 CEFF, we issued to Kingsbridge a warrant to purchase 0.4 million shares of our common stock at a price of $9.12 per share exercisable beginning on September 14, 2006 for a period of five years thereafter. The fair value of the warrant was determined on the date of issuance using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 4.68%, (ii) an expected term of 5.5 years, (iii) no dividend yield and (iv) volatility of 57%. The estimated fair value of this warrant was $1.3 million, which was recorded as a contra-equity amount in additional paid-in capital in March 2006. In 2006, we received net proceeds of $27.9 million from the sale of 6.3 million shares of our common stock under the 2006 CEFF. In January 2007, we received net proceeds of $7.1 million from the sale of 2.4 million shares of our common stock under the 2006 CEFF, which concluded the 2006 CEFF. We received cumulative net proceeds of $35.0 million from the sale of 8.7 million shares of our common stock under the 2006 CEFF.
In February 2007, we entered into a new CEFF, or 2007 CEFF, with Kingsbridge, pursuant to which Kingsbridge committed to purchase, subject to certain conditions, up to the lesser of 11.6 million shares of our common stock or an aggregate of $75.0 million during the three year period following entry into the 2007 CEFF. Under the 2007 CEFF, we are able to draw down in tranches of up to a maximum of 2.5 percent of our closing market value of our common stock on the last trading day prior to the commencement of the drawdown, or $15.0 million, whichever is less, subject to certain conditions. In addition, subject to the $15.0 million limit, we can issue up to 3.5% of our market capitalization once per fiscal quarter. The purchase price of these shares is discounted between 6 to 10 percent from the volume weighted average price of our common stock for each of the eight trading days following the election to sell shares. Kingsbridge is not obligated to purchase shares at prices below $1.75 per share or at a price below 85% of the closing share price of our stock on the trading day immediately preceding the commencement of the drawdown.
65
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In connection with the 2007 CEFF, we issued to Kingsbridge a warrant to purchase 0.4 million shares of our common stock at a price of $4.68 per share exercisable beginning on September 5, 2007 for a period of five years thereafter. The fair value of the warrant was determined on the date of issuance using the Black-Scholes option valuation model applying the following assumptions: (i) a risk-free rate of 4.80%, (ii) an expected term of 5.5 years, (iii) no dividend yield and (iv) volatility of 55%. The estimated fair value of this warrant was $0.6 million which was recorded as a contra-equity amount in additional paid-in capital in February 2007. During the year ended December 31, 2007, we received net proceeds of $23.0 million from the sale of 7.1 million shares of our common stock under the 2007 CEFF. There was no drawdown for the year ended December 31, 2008. Since inception of the 2007 CEFF, we received net proceeds of $23.0 million from the sale of 7.1 million shares of our common stock. As of December 31, 2008, there were approximately 4.5 million shares of our common stock remaining that may be sold under the 2007 CEFF. However, Kingsbridge is not obligated to purchase shares at prices below $1.75 per share, which our common stock was trading below as of December 31, 2008, or at a price below 85% of the closing share price of our stock on the trading day immediately preceding the commencement of the drawdown.
Stock option plans
Prior to May 2005, we had five approved stock option plans: the 1989, 1992, and 1998 Incentive Stock Option Plans, the 2001 Nonstatutory Option Plan and the 2001 Non-Employee Directors Stock Option Plan, or collectively, the Prior Plans. Under the Prior Plans, incentive stock options and non-qualified stock options were granted to eligible employees, directors and consultants to purchase shares of our common stock at no less than the fair market value of the underlying common stock as of the date of grant. Options granted under these plans have a maximum term of ten years and generally vest over four years at the rate of 25 percent one year from the date of grant and 1/48 monthly thereafter. The 1998 Incentive Stock Option Plan replaced the 1989 and 1992 Incentive Stock Option Plans which expired and were retired in 1999 and 2002, respectively.
Amended 2005 Equity Incentive Plan: In May 2005, our stockholders approved the 2005 Equity Incentive Plan, or the 2005 Plan, at which time 1.0 million new shares of common stock were authorized for issuance. The 2005 Plan replaced our 1998 Incentive Stock Option Plan, the 2001 Nonstatutory Option Plan and the 2001 Non-Employee Directors Stock Option Plan. Upon approval of the 2005 Plan, shares in the Prior Plans that had been reserved but not issued were reserved for issuance under the 2005 Plan. Since such approval, shares that would otherwise return to the Prior Plans as a result of option cancellations are rolled into and are reserved for issuance under the 2005 Plan. No additional grants are made under the Prior Plans. On June 19, 2007, our stockholders approved an amendment to the 2005 Plan to increase the number of shares reserved for issuance by 3.5 million.
The 2005 Plan permits the granting of incentive and non-statutory stock options, restricted stock, stock appreciation rights, performance units and performance shares and other stock awards to eligible employees, directors and consultants. We generally grant options to purchase shares of common stock under the 2005 Plan at no less than the fair market value of the underlying common stock as of the date of grant. Options granted under the 2005 Plan have a maximum term of ten years and generally vest over four years at the rate of 25 percent of total shares underlying the option upon the one year anniversary of the date of grant and 1/48 of the total shares monthly thereafter. As of December 31, 2008, there were 4.1 million shares available for grant under the 2005 Plan.
66
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FAS 123R
Stock-based compensation expense recognized during 2008, 2007 and 2006 was calculated based on awards ultimately expected to vest and has been reduced for estimated forfeitures. FAS 123R requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Total stock-based compensation expense recognized under FAS 123R was as follows (in thousands, except per share data):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Research and development | | $ | 3,566 | | | $ | 5,058 | | | $ | 4,640 | |
General and administrative | | | 1,318 | | | | 1,470 | | | | 1,290 | |
| | | | | | | | | | | | |
Total stock-based compensation expense | | $ | 4,884 | | | $ | 6,528 | | | $ | 5,930 | |
| | | | | | | | | | | | |
Effect on earnings per share—basic and diluted | | $ | (0.06 | ) | | $ | (0.09 | ) | | $ | (0.12 | ) |
| | | | | | | | | | | | |
As of December 31, 2008, total compensation cost related to nonvested stock options not yet recognized was $2.5 million, the majority of which is not expected to be recorded to stock-based compensation expense as we do not expect the majority of these stock options to vest due to our substantial restructuring plan. Total compensation cost related to restricted stock units not yet recognized was $0.1 million, which is expected to be allocated to expense over a weighted-average period of six months.
Employee Stock-Based Compensation Valuation Assumptions
The compensation expense related to stock options was determined using the Black-Scholes option valuation model. Option valuation models require the input of subjective assumptions and these assumptions can vary over time. The weighted-average assumptions used were as follows:
| | | | | | | | | | | | | | | | | | |
| | | Options | | | Employee Stock
Purchase Plan | |
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | | | 2008 | | | 2007 | | | 2006 | |
Dividend yield | | 0.00 | % | | 0.00 | % | | 0.00 | % | | 0.00 | % | | 0.00 | % | | 0.00 | % |
Annual risk free rate of return | | 2.56 | % | | 4.52 | % | | 4.82 | % | | 2.53 | % | | 4.86 | % | | 4.37 | % |
Expected volatility | | 0.58 | | | 0.55 | | | 0.57 | | | 0.62 | | | 0.48 | | | 0.48 | |
Expected term (years) | | 5.3 | | | 5.2 | | | 5.1 | | | 1.2 | | | 1.4 | | | 1.0 | |
In estimating the expected term, we considered our historical stock option exercise experience including forfeitures, our post vesting termination pattern and the term of the options outstanding. The expected term of employee stock purchase plan rights is the average of the remaining purchase periods under each offering period. The annual risk free rate of return was based on the U.S. Treasury constant maturity rates with similar terms to the expected term of the stock option awards or of the employee stock purchase plan rights. We based our determination of expected volatility on our historical stock price volatility over the expected term. The fair value of the restricted stock units was estimated based upon the closing sales price of our common stock on the grant date. These restricted stock units will be fully vested and become unforfeitable on the first anniversary of the grant date. No compensation expense will be recognized for restricted stock units that do not vest.
67
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Stock option activity
A summary of the status of our stock option plan as of December 31, 2008, 2007 and 2006 and changes during the periods then ended is presented in the table below:
| | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, |
| | | 2008 | | 2007 | | 2006 |
| | | Shares | | | Weighted
Average
Exercise
Price | | Shares | | | Weighted
Average
Exercise
Price | | Shares | | | Weighted
Average
Exercise
Price |
| | | (In thousands) | | | | | (In thousands) | | | | | (In thousands) | | | |
Outstanding, beginning of year | | 8,563 | | | $ | 8.86 | | 8,368 | | | $ | 10.08 | | 8,087 | | | $ | 11.18 |
Granted | | 2,517 | | | $ | 1.85 | | 1,542 | | | $ | 3.20 | | 1,640 | | | $ | 5.83 |
Exercised | | (7 | ) | | $ | 2.23 | | (7 | ) | | $ | 5.38 | | (74 | ) | | $ | 4.83 |
Forfeited or expired | | (2,490 | ) | | $ | 4.02 | | (1,340 | ) | | $ | 9.95 | | (1,285 | ) | | $ | 11.87 |
| | | | | | | | | | | | | | | | | | |
Outstanding, end of year | | 8,583 | | | $ | 8.22 | | 8,563 | | | $ | 8.86 | | 8,368 | | | $ | 10.08 |
| | | | | | | | | | | | | | | | | | |
Exercisable, end of year | | 7,152 | | | $ | 9.32 | | 6,237 | | | $ | 10.41 | | 6,040 | | | $ | 11.33 |
| | | | | | | | | | | | | | | | | | |
Weighted average grant date fair value | | | | | $ | 0.97 | | | | | $ | 1.70 | | | | | $ | 3.15 |
The total fair value of options that vested during the year ended December 31, 2008 was $3.1 million. The total intrinsic value of options exercised during the year ended December 31, 2008 was $5,000. Cash proceeds from the exercise of stock options were $16,000 for the year ended December 31, 2008. As of December 31, 2008, the aggregate intrinsic value of the stock options outstanding was zero.
The following table summarizes information about stock options outstanding as of December 31, 2008:
| | | | | | | | | | | | |
| | | Options Outstanding | | Options Exercisable |
Range of
Exercise Price | | Number
Outstanding | | Weighted
Average
Remaining
Contractual
Life
(in years) | | Weighted-
Average
Exercise
Price | | Number
Exercisable | | Weighted
Average
Exercise
Price |
| | | (In thousands) | | | | | | (In thousands) | | |
| $ 0.23-$ 0.23 | | 10 | | 0.3 | | $ | 0.23 | | 10 | | $ | 0.23 |
| $ 0.98-$ 1.84 | | 1,466 | | 4.4 | | $ | 1.84 | | 569 | | $ | 1.84 |
| $ 2.33-$ 4.66 | | 1,376 | | 2.8 | | $ | 3.41 | | 1,019 | | $ | 3.46 |
| $ 4.81-$ 6.07 | | 1,462 | | 2.6 | | $ | 5.86 | | 1,298 | | $ | 5.84 |
| $ 6.19-$ 9.14 | | 1,504 | | 1.7 | | $ | 7.53 | | 1,491 | | $ | 7.53 |
| $ 9.50-$14.04 | | 1,452 | | 1.5 | | $ | 12.31 | | 1,452 | | $ | 12.31 |
| $14.07-$42.63 | | 1,313 | | 1.1 | | $ | 19.34 | | 1,313 | | $ | 19.34 |
| | | | | | | | | | | | |
| | 8,583 | | 2.4 | | $ | 8.22 | | 7,152 | | $ | 9.32 |
| | | | | | | | | | | | |
Restricted Stock Units
During 2007, we issued 876,550 restricted stock units under our 2005 Equity Incentive Plan, as amended, at a grant date fair value ranging between $3.35 and $3.51. In June 2008, we issued 32,000 shares of common stock to our outside directors as a result of the vesting of their restricted stock units. In July 2008, we issued 844,550 shares of common stock as a result of the remaining restricted stock units became fully vested and unforfeitable.
68
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In June 2008, we issued 32,000 restricted stock units to our outside directors at a grant date fair value of $2.60. These restricted stock units will be fully vested and become unforfeitable on the first anniversary of the award date. In accordance with FAS 123R, the fair value of the restricted stock units was based upon the closing sales price of our common stock on the grant date.
Information with respect to restricted stock units as of December 31, 2008 was as follows (in thousands, except per share amount):
| | | | | | |
| | | Number of
Shares | | | Weighted Average
Grant-Date Fair
Value |
Outstanding at December 31, 2006 | | — | | | $ | — |
Granted | | 877 | | | $ | 3.50 |
Forfeited | | (11 | ) | | $ | 3.51 |
| | | | | | |
Outstanding at December 31, 2007 | | 866 | | | $ | 3.50 |
Granted | | 32 | | | $ | 2.60 |
Vested | | (834 | ) | | $ | 3.50 |
Forfeited | | (32 | ) | | $ | 3.51 |
| | | | | | |
Outstanding at December 31, 2008 | | 32 | | | $ | 2.60 |
| | | | | | |
Employee stock purchase plan
The 2002 Employee Stock Purchase Plan, or the Purchase Plan, was approved by the stockholders in June 2002. The Purchase Plan allows eligible employees to purchase our common stock at 85 percent of the fair value at certain specified dates. Employee contributions are limited to 10 percent of compensation or $25,000, whichever is less. On June 20, 2006, our stockholders approved an amendment to the Purchase Plan to increase the maximum number of shares of common stock authorized for issuance under the plan automatically on the first day of each year by an amount equal to the lesser of (a) 0.3 million shares, (b) 1/2 percent of the outstanding shares on such date, or (c) an amount determined by the Board of Directors. As of December 31, 2008, a total of 1.4 million shares have been issued pursuant to the Purchase Plan. During 2008, 0.4 million shares were sold under the plan for a total price of $0.6 million. We terminated the Purchase Plan in December 2008. The compensation expense related to the plan during 2008 was $0.2 million.
Non-employee stock-based compensation
We recorded $0.1 million in each of 2008, 2007 and 2006 for non-employee stock-based compensation for grants of stock options to consultants. These amounts were based upon the fair value of the vested portion of the grants. Additional compensation will be recorded in future periods for the remaining unvested portion of these option grants.
Stockholder rights plan
In July 1995, the Board of Directors approved a stockholder rights plan under which stockholders of record on August 21, 1995 received one preferred share purchase right for each outstanding share of our common stock. In July 2000, we made certain technical changes to amend the plan and extend the life of the plan until 2010. The rights are exercisable only if an acquirer purchases 15 percent or more of our common stock or announces a tender offer for 15 percent or more of our common stock. Upon exercise, holders other than the acquirer may purchase our stock at a discount. The Board of Directors may terminate the rights plan at any time or, under certain circumstances, redeem the rights.
69
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Common shares reserved for future issuance
As of December 31, 2008, we had reserved shares of common stock for potential future issuance as follows: 7.8 million shares upon conversion of convertible senior notes, 8.6 million shares for exercises under our stock option plans, 4.5 million shares for future CEFF purchases by Kingsbridge, 0.8 million shares for the warrants issued under the 2006 and 2007 CEFF, and 10.7 million shares for the warrants issued under the 2007 and 2008 registered direct offerings.
9. Income Taxes
In July 2005, the IRS issued a Notice of Proposed Adjustment, or NOPA, seeking to disallow $48.7 million of net operating losses that we deducted for the 2000 fiscal year and seeking a $3.4 million penalty for substantial underpayment of tax in the year ended December 31, 2000. We responded to the IRS in September 2005, disagreeing with the conclusions reached by the IRS in the NOPA and seeking to resolve this matter at the Appeals Office level of the IRS. In May 2007, we reached final settlement regarding this matter with the IRS in the amount of $3.3 million with respect to the fiscal years ended December 31, 2000, 2001 and 2002. This amount was comprised of $2.3 million in federal tax and $1.0 million in related interest. No penalty was assessed.
Our income tax benefit (provision) consists of the following (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Current: | | | | | | | | | | | | |
Federal | | $ | 15 | | | $ | — | | | $ | (2,076 | ) |
State | | | — | | | | (523 | ) | | | (722 | ) |
Foreign | | | (12 | ) | | | (4 | ) | | | — | |
| | | | | | | | | | | | |
| | | 3 | | | | (527 | ) | | | (2,798 | ) |
| | | | | | | | | | | | |
Deferred: | | | | | | | | | | | | |
Federal | | | — | | | | — | | | | (26,815 | ) |
State | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | — | | | | — | | | | (26,815 | ) |
| | | | | | | | | | | | |
Other: | | | | | | | | | | | | |
Federal | | | — | | | | 20,604 | | | | — | |
State | | | 6,192 | | | | 5,805 | | | | — | |
| | | | | | | | | | | | |
| | | 6,192 | | | | 26,409 | | | | — | |
| | | | | | | | | | | | |
Income tax benefit (provision) | | $ | 6,195 | | | $ | 25,882 | | | $ | (29,613 | ) |
| | | | | | | | | | | | |
The tax benefit for the year ended December 31, 2008 is primarily attributed to the reversal of $6.2 million of previously accrued income taxes as a result of closing certain years to examination under relevant statutes of limitation. The tax benefit recorded for the year ended December 31, 2007 is related to the reversal of $26.4 million in May 2007 of previously accrued income taxes as a result of the final settlement with the IRS, offset by additional accrued interest for tax contingencies. The tax provision recorded in 2006 primarily relates to the realized gain on the sale of 3.0 million shares of Abgenix common stock and $2.8 million of additional interest for tax contingencies.
70
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
A reconciliation of our recorded income tax benefit (provision) to the U.S. statutory rate follows (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Tax benefit at U.S. statutory rate | | $ | 18,610 | | | $ | 43,804 | | | $ | 18,661 | |
Change in valuation allowance | | | (24,629 | ) | | | (46,200 | ) | | | (20,969 | ) |
IRS settlement | | | — | | | | 26,409 | | | | — | |
Lapse of the applicable statute of limitations | | | 4,041 | | | | — | | | | — | |
Research and development tax credits | | | 2,889 | | | | 3,188 | | | | 2,865 | |
Tax effect of gain from revaluation of warrant liability | | | 4,018 | | | | — | | | | — | |
Stock-based compensation | | | (882 | ) | | | (912 | ) | | | (531 | ) |
Tax effect of realized and unrealized gains on available-for-sale-securities recorded in other comprehensive income | | | — | | | | — | | | | (26,815 | ) |
Interest on tax contingencies | | | 2,151 | | | | (523 | ) | | | (2,798 | ) |
Other | | | (3 | ) | | | 116 | | | | (26 | ) |
| | | | | | | | | | | | |
Benefit (provision) for income taxes | | $ | 6,195 | | | $ | 25,882 | | | $ | (29,613 | ) |
| | | | | | | | | | | | |
As of December 31, 2008, we had net operating loss carryforwards for federal income tax purposes of approximately $435 million, which will expire on various dates between 2009 and 2028, if not utilized. As of December 31, 2008, we had federal research and development tax credits of approximately $22 million, which will expire on various dates between 2009 and 2028. As of December 31, 2008, we had net operating loss carryforwards for California state income tax purposes of approximately $116 million, which will expire on various dates between 2012 and 2018. As of December 31, 2008, we had California state research and development tax credits of approximately $21 million, which do not expire. We also had Manufacturer Investment Credits of $0.1 million which expire in 2010 and 2011. Utilization of the net operating loss and credit carryforwards may be subject to substantial annual limitation due to ownership change provisions of the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization. To the extent net operating loss carryforwards, when realized, relate to non-qualified stock option deductions, the resulting benefits will be credited to stockholders’ equity.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets and liabilities are as follows (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | |
Deferred tax assets: | | | | | | | | |
Net operating loss carryforwards | | $ | 158,740 | | | $ | 163,767 | |
Research credit carryforwards | | | 35,587 | | | | 33,221 | |
Capitalized research and development, net of amortization | | | 17,692 | | | | 17,343 | |
Basis difference in fixed assets | | | 32,886 | | | | 2,943 | |
Other accruals and reserves | | | 5,234 | | | | 12,892 | |
| | | | | | | | |
Deferred tax assets | | | 250,139 | | | | 230,166 | |
Valuation allowance | | | (250,139 | ) | | | (230,166 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | |
71
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. The valuation allowance increased by $20.0 million, $58.4 million and $52.8 million, in 2008, 2007 and 2006, respectively.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| | | | | | | | |
| | | 2008 | | | 2007 | |
Balance at January 1 | | $ | 4,041 | | | $ | 24,980 | |
Additions for tax positions of current year | | | — | | | | — | |
Additions for tax positions of prior years | | | — | | | | — | |
Reductions for tax positions of prior years | | | — | | | | (18,601 | ) |
Settlement with tax authorities | | | — | | | | (2,338 | ) |
Reductions due to lapse of the applicable statute of limitations | | $ | (4,041 | ) | | | — | |
| | | | | | | | |
Balance at December 31 | | $ | — | | | $ | 4,041 | |
| | | | | | | | |
We file tax returns in the U.S., U.K. and California. In general, the years 2005 through 2008, remain open to examination for U.S. and U.K. purposes, and 2001 through 2008 for California purposes.
The nature of these matters is uncertain and subject to change. As a result, the amount of our liability for certain of these matters could exceed or be less than the amount of our current estimates, depending on the outcome of these matters. An outcome of such matters different than previously estimated could materially impact our financial position or results of operations in the year of resolution.
10. 401(k) Plan
We sponsor a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code covering all full time employees and part-time employees who work at least 20 hours per week, or 401k Plan. Participating employees may contribute up to the annual Internal Revenue Service contribution limit. Our 401k Plan also provides for employer matching contributions up to an annual limit of $3,000 per employee. Our 401k Plan is intended to qualify under Section 401 of the Internal Revenue Code so that contributions by the employees and by us, and income earned on the contributions are not taxable to employees until withdrawn from the plan. Contributions by us are tax deductible when made. At the discretion of each participant, the assets of our 401k Plan are invested in any of seventeen different investment options.
The employer matching contribution is invested in the same investment options selected by the employee for their individual contributions. The employer matching contributions vest ratably over three years. We contributed $0.7 million, $0.6 million and $0.8 million in employer matching contributions in 2008, 2007 and 2006, respectively. The 401k Plan was terminated on December 31, 2008, and all unvested employer matching contributions became fully vested on that date.
11. Related Parties
The accounting policies we apply to our transactions with our related parties are consistent with those applied in transactions with independent third parties.
Ceregene: Ceregene, Inc., or Ceregene, was previously our majority-owned subsidiary. In August 2004, July 2005, and April 2006, Ceregene announced the initial, second and third closings of its Series B preferred stock financing. We participated in these financings through the pro rata conversion of an outstanding
72
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
bridge loan to Ceregene together with related accrued interest into shares of Ceregene’s Series B preferred stock. In January 2007, we participated in Ceregene’s Series C preferred stock financing by acquiring 1.8 million shares of Ceregene’s Series C preferred stock in exchange for an exclusive license to Ceregene of certain of our intellectual property. We did not record a gain on the exchange of the 1.8 million shares because the intangible assets exchanged with Ceregene were nonmonetary and the intangible assets we used as consideration had no carrying value and no determinable fair value. We own approximately 16% of Ceregene capital stock on a fully diluted basis as of December 31, 2008.
We account for our investment in Ceregene under the equity method of accounting for investments because we believe we have the ability to exercise significant influence over Ceregene through our Chairman of the Board of Directors and Chief Executive Officer, or CEO, who is also the Chairman of Ceregene’s Board of Directors. In 2008, we recorded zero revenue from Ceregene. In 2007 and 2006, we recorded revenue from Ceregene of $13,000 and $0.1 million, respectively. We did not recognize losses from Ceregene, nor do we expect to recognize future losses from Ceregene, as the net book value of our investment in Ceregene is zero.
Caliper Life Sciences: The former Chairman and CEO of Xenogen Corporation, or Xenogen, a related party, which was acquired by Caliper Life Sciences, Inc., or Caliper, in August 2006, is now on the Board of Directors of Caliper and is also a member of our Board of Directors. We paid approximately $31,000 for license fees to Caliper during the year ended December 31, 2008. We paid approximately $0.2 million in each of the years ended December 31, 2007 and 2006 for license fees to Xenogen Corporation.
12. Selected Quarterly Financial Information (Unaudited)
| | | | | | | | | | | | | | | | |
| | | Quarter Ended | |
Quarterly Results of Operations | | March 31,
2008 | | | June 30,
2008 | | | September 30,
2008 | | | December 31,
2008 | |
| | | (Unaudited) | |
| | | (In thousands, except per share amounts) | |
Revenue | | $ | 13,418 | | | $ | 16,566 | | | $ | 17,147 | | | $ | 47,440 | |
Research and development expenses | | | 29,265 | | | | 25,289 | | | | 24,602 | | | | 13,388 | |
General and administrative expenses | | | 5,342 | | | | 4,515 | | | | 4,315 | | | | 3,310 | |
Impairment of long-lived assets | | | 41 | | | | 91 | | | | — | | | | 71,657 | |
Restructuring charges | | | — | | | | — | | | | — | | | | 13,798 | |
Gain from revaluation of warrant liability | | | — | | | | 5,716 | | | | 4,936 | | | | 828 | |
Gain from purchase of convertible senior notes | | | — | | | | — | | | | — | | | | 42,668 | |
Net loss | | | (22,552 | ) | | | (2,755 | ) | | | (8,413 | ) | | | (13,255 | ) |
Basic and diluted net loss per share | | | (0.29 | ) | | | (0.03 | ) | | | (0.10 | ) | | | (0.15 | ) |
| |
| | | Quarter Ended | |
Quarterly Results of Operations | | March 31,
2007 | | | June 30,
2007 | | | September 30,
2007 | | | December 31,
2007 | |
| | | (Unaudited) | |
| | | (In thousands, except per share amounts) | |
Revenue | | $ | 1,273 | | | $ | 3 | | | $ | 2 | | | $ | 102 | |
Research and development expenses | | | 24,015 | | | | 25,030 | | | | 28,629 | | | | 28,457 | |
General and administrative expenses | | | 5,245 | | | | 4,782 | | | | 5,114 | | | | 5,260 | |
Net loss | | | (29,449 | ) | | | (1,891 | ) | | | (34,538 | ) | | | (33,396 | ) |
Basic and diluted net loss per share | | | (0.49 | ) | | | (0.03 | ) | | | (0.46 | ) | | | (0.43 | ) |
73
CELL GENESYS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Basic and diluted net income (loss) per share is computed independently for each of the quarters presented. Therefore, the sum of quarterly basic and diluted per share amounts may not equal annual basic and diluted net income (loss) per share amounts.
13. Subsequent Events
In January 2009, we repurchased an aggregate of $2.6 million face value of our Notes, at an overall discount of approximately 60 percent from face value in a series of privately negotiated transactions with institutional holders of the Notes, for aggregate consideration of $1.0 million in cash, plus accrued but unpaid interest. As a result of the retirement of the repurchased Notes, $68.3 million aggregate principal amount remains outstanding. This purchase of debt resulted in a net gain of approximately $1.6 million.
In March 2009, we entered into agreements to terminate our Hayward manufacturing facility leases. The termination agreements are subject to certain conditions. Subject to fulfillment or waiver of these conditions, the leases will terminate by March 31, 2009. In consideration of the early termination of the leases, we will pay the landlords an aggregate amount of $3.6 million and issue one million shares of our common stock.
74
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
We have performed an evaluation under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures, as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Based on that evaluation, our management, including our Chief Executive Officer and Chief Financial Officer, concluded that our disclosure controls and procedures were effective as of December 31, 2008 to provide reasonable assurance that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control system is designed to provide reasonable assurance regarding the preparation and fair presentation of financial statements for external purposes in accordance with generally accepted accounting principles. Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our procedures or our internal controls will prevent or detect all error or fraud. All internal control systems, no matter how well conceived and operated, have inherent limitations and can provide only reasonable, not absolute, assurance that the objectives of the internal control system are met. Because of the inherent limitations in all control systems, no evaluation of our controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our evaluation, we concluded that our internal control over financial reporting was effective as of December 31, 2008.
The effectiveness of our internal control over financial reporting as of December 31, 2008 has been audited by Ernst & Young LLP, our independent registered public accounting firm, as stated in their attestation report, which is included herein.
Changes in Internal Control Over Financial Reporting
There was no change in our internal controls during the fiscal quarter ended December 31, 2008 that materially affected, or is reasonably likely to materially affect, our internal controls over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
In connection with the restructuring and reduction in workforce described in Item 2.05 of the Registrant’s Current Report on Form 8-K dated October 16, 2008, the employment of Carol C. Grundfest, Senior Vice President, Regulatory Affairs and Portfolio Management, will terminate effective March 15, 2009. Ms. Grundfest’s duties will be assumed by Stephen A. Sherwin, M.D., Chairman of the Board and Chief Executive Officer.
75
On March 6, 2009, we agreed with the landlords for our Hayward manufacturing facility leases, to terminate these leases. Under an Agreement for Termination of Lease and Voluntary Surrender of Premises by and between us and BMR-Bridgeview Technology Park II, LLC (“BMR II”), we agreed with BMR II to terminate our lease agreement with BMR II dated as of January 7, 2002 in exchange for a termination fee of $3,310,536 and the issuance of 1,000,000 shares of our common stock pursuant to the terms of a stock purchase agreement to be entered into between us and BioMed Realty, L.P., the sole member of BMR II. The stock has not being registered under the Securities Act of 1933, as amended, and is being sold in reliance on Section 4(2) of the Securities Act and the safe harbor provided by Rule 506 of Regulation D promulgated under the Securities Act. Biomed Realty has represented to us that it is an “accredited investor” within the meaning of Rule 501 of Regulation D under the Securities Act. Under an Agreement for Termination of Lease and Voluntary Surrender of Premises by and between us and BMR-Bridgeview Technology Park, LLC (“BMR”), we agreed with BMR to terminate our lease agreement with BMR (as successor-in-interest to F&S Hayward, LLC) dated as of June 29, 2000, as amended by the First Amendment to Lease dated as of January 2, 2001 for a termination fee of $289,464.
The termination agreements are subject to certain conditions, including BMR and BMR II lender consent. Subject to fulfillment or waiver of these conditions, the leases will terminate pursuant to the termination agreements by March 31, 2009. This description is qualified in its entirety by reference to exhibits 10.29 and 10.30 which are hereby incorporated by reference.
76
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
(a) The information required by this Item concerning our directors is incorporated by reference to the section entitled“Proposal One—Election of Directors” in our Definitive Proxy Statement for the 2009 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the end of our 2008 fiscal year (the “2009 Proxy Statement”).
(b) The information required by this Item concerning our executive officers is set forth in the section entitled“Executive Officers”in Part I of this Form 10-K and is incorporated by reference into this section.
We have adopted a code of ethics that applies to all of our employees, including our principal executive officer, our principal financial officer and our principal accounting officer. This code of ethics, which is part of our Code of Business Conduct and Ethics that applies to all of our employees, is posted on our website and can be accessed from the following link:http://media.corporate-ir.net/media_files/irol/98/98399/corpgov/CodeOfConduct.pdf.
We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding any amendment to, or waiver from, a provision of this code of ethics by posting such information on our website, at the address and location specified above.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this Item is incorporated by reference to the section entitled“Executive Compensation” in our 2009 Proxy Statement.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item regarding security ownership of certain beneficial owners and management, as well as equity compensation plans, is incorporated by reference to the information in the sections “Stock Ownership of Principal Stockholders and Management” and “Equity Compensation Plan Information” in our 2009 Proxy Statement.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this Item is incorporated by reference to the sections entitled“Corporate Governance” and“Other Information” in our 2009 Proxy Statement.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this Item is incorporated by reference to the section entitled“Proposal Two – Ratification of Appointment of the Independent Registered Public Accounting Firm”in our 2009 Proxy Statement.
77
PART IV
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are included as part of this Annual Report on Form 10-K.
1. Index to Financial Statements
2. Financial Statement Schedules
All financial statement schedules are omitted because they are not applicable or not required or because the required information is included in the financial statements or notes thereto.
3. Exhibits
| | | | | |
Number | | Note | | | Description |
| 3.1 | | (1 | ) | | Restated Certificate of Incorporation. |
| | |
| 3.2 | | (2 | ) | | Certificate of Amendment to Restated Certificate of Incorporation. |
| | |
| 3.3 | | (1 | ) | | Certificate of Designation of Rights, Preferences and Privileges of Series A Participating Preferred Stock. |
| | |
| 3.4 | | (1 | ) | | Certificate of Amendment to Certificate of Designation of Rights, Preferences and Privileges of Series A Participating Preferred Stock. |
| | |
| 3.5 | | (1 | ) | | Certificate of Designations, Preferences and Rights of Series B Convertible Preferred Stock. |
| | |
| 3.6 | | (3 | ) | | Amended and Restated Bylaws dated October 17, 2007. |
| | |
| 4.1 | | (4 | ) | | Amended and Restated Preferred Shares Rights Agreement, dated as of July 26, 2000 between the Registrant and Fleet National Bank. |
| | |
| 4.2 | | (5 | ) | | Indenture dated as of October 20, 2004 by and between the Registrant and U.S. Bank National Association. |
| 10.1† | | (6 | ) | | Form of Indemnification Agreement for Directors and Officers. |
| | |
| 10.2 | | (7 | ) | | License Agreement dated August 13, 1990 between the Registrant and the University of North Carolina at Chapel Hill. |
| | |
| 10.3 | | (8 | ) | | License Agreement dated June 28, 1991 between the Registrant and the University of Utah Research Foundation. |
| | |
| 10.4† | | (9 | ) | | Amended Employment Agreement between the Registrant and Stephen A. Sherwin, M.D. |
| | |
| 10.5† | | (10 | ) | | 2001 Nonstatutory Stock Option Plan. |
| | |
| 10.6† | | (11 | ) | | Form of Change of Control Severance Agreement. |
| | |
| 10.7† | | (12 | ) | | Amended and Restated 1998 Incentive Stock Plan. |
| | |
| 10.8* | | (13 | ) | | License Agreement dated June 7, 2002 between Transkaryotic Therapies, Inc. and the Registrant |
78
| | | | | |
Number | | Note | | | Description |
| 10.9* | | (14 | ) | | Patent Assignment and License Agreement dated July 23, 2003 between the Registrant, Novartis AG and Genetic Therapy Inc. |
| | |
| 10.10* | | (15 | ) | | Product Development and Option Agreement dated July 23, 2003 between the Registrant and Novartis Pharma AG. |
| | |
| 10.11 | | (2 | ) | | Lease Agreement dated June 29, 2000 between Lincoln-RECP Industrial OPCO, LLC and the Registrant, First Amendment to Lease Agreement dated January 2, 2001 between F & S Hayward, LLC and the Registrant, and Lease Agreement dated January 7, 2002 between F & S Hayward II, LLC and the Registrant for premises located at 24570 Clawiter Road, Hayward, California. |
| | |
| 10.12 | | (16 | ) | | Standstill and Registration Rights Agreement dated July 23, 2003 between the Registrant, Novartis AG and Genetic Therapy, Inc. |
| | |
| 10.13† | | (17 | ) | | 2005 Equity Incentive Plan, as amended. |
| | |
| 10.14† | | (18 | ) | | 2002 Employee Stock Purchase Plan, as amended. |
| | |
| 10.15 | | (19 | ) | | Common Stock Purchase Agreement dated March 14, 2006 between the Registrant and Kingsbridge Capital Limited. |
| | |
| 10.16 | | (20 | ) | | Registration Rights Agreement dated March 14, 2006 between the Registrant and Kingsbridge Capital Limited. |
| | |
| 10.17 | | (21 | ) | | Warrant Issued to Kingsbridge Capital Limited. |
| | |
| 10.18 | | (22 | ) | | Common Stock Purchase Agreement dated February 5, 2007 between the Registrant and Kingsbridge Capital Limited. |
| | |
| 10.19 | | (23 | ) | | Registration Rights Agreement dated February 5, 2007 between the Registrant and Kingsbridge Capital Limited. |
| | |
| 10.20 | | (24 | ) | | Warrant Issued to Kingsbridge Capital Limited. |
| | |
| 10.21 | | (25 | ) | | Form of Warrant. |
| | |
| 10.22 | | (26 | ) | | Form of Restricted Stock Unit Award Agreement. |
| | |
| 10.23 | | (27 | ) | | Form of Stock Option Award Agreement. |
| | |
| 10.24 | | (28 | ) | | Technology and Intellectual Property Agreement between the Registrant and GBP IP, LLC. |
| | |
| 10.25* | | (29 | ) | | Development And Commercialization Collaboration Agreement between Cell Genesys, Inc. and Takeda Pharmaceutical Company Limited. |
| | |
| 10.26 | | (30 | ) | | Form of Warrant. |
| | |
| 10.27 | | (31 | ) | | Form of Subscription Agreement between the Company and the investors. |
| | |
| 10.28 | | (32 | ) | | Agreement for Termination of Lease and Voluntary Surrender of Premises dated as of December 8, 2008 between the Registrant and ARE-San Francisco No. 41, LLC. |
| | |
| 10.29 | | (32 | ) | | Agreement for Termination of Lease and Voluntary Surrender of Premises dated March 6, 2009 between the Registrant and BMR-Bridgeview Technology Park II, LLC. |
| | |
| 10.30 | | (32 | ) | | Agreement for Termination of Lease and Voluntary Surrender of Premises dated March 6, 2009 between the Registrant and BMR-Bridgeview Technology Park, LLC. |
| | |
| 12.1 | | (32 | ) | | Computation of Ratio of Earnings to Fixed Charges and Ratio of Earnings to Combined Fixed Charges and Preferred Stock Dividend Requirements. |
79
| | | | | |
Number | | Note | | | Description |
| 21.1 | | (32 | ) | | Subsidiaries of the Registrant. |
| | |
| 23.1 | | (32 | ) | | Consent of Independent Registered Public Accounting Firm. |
| | |
| 31.1 | | (32 | ) | | Certification of Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under Sarbanes-Oxley Act of 1934, as amended. |
| | |
| 31.2 | | (32 | ) | | Certification of Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under Sarbanes-Oxley Act of 1934, as amended. |
| | |
| 32.1 | | (33 | ) | | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | |
| 32.2 | | (33 | ) | | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| * | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions of this exhibit have been separately filed with the Commission. |
| † | Management compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15(c) of Form 10-K. |
| (1) | Incorporated by reference to the same numbered exhibit filed with the Registrant’s Registration Statement on Form S-3/A (Reg. No. 333-102122) filed with the SEC on January 30, 2003. |
| (2) | Incorporated by reference to the same numbered exhibit filed with the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2005. |
| (3) | Incorporated by reference to Exhibit 3.1 filed with the Registrant’s Current Report on Form 8-K dated October 23, 2007. |
| (4) | Incorporated by reference to the Registrant’s Form 8-A12G/A dated July 28, 2000. |
| (5) | Incorporated by reference to Exhibit 4.1 filed with the Registrant’s Registration Statement on Form S-3 (Reg. No. 333-121732) filed with the SEC on December 29, 2004. |
| (6) | Incorporated by reference to Exhibit 10.2 filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2007. |
| (7) | Incorporated by reference to Exhibit 10.6 filed with the Registrant’s Registration Statement on Form S-1 (Reg. No. 33-46452) as amended. |
| (8) | Incorporated by reference to Exhibit 10.9 filed with the Registrant’s Registration Statement on Form S-1 (Reg. No. 33-46452) as amended. |
| (9) | Incorporated by reference to Exhibit 10.20 filed with the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1992. |
| (10) | Incorporated by reference to Exhibit 4.2 filed with the Registrant’s Registration Statement on Form S-8 (Reg. No. 333-91796) filed with the SEC on July 2, 2002. |
| (11) | Incorporated by reference to Exhibit 10.3 filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2007. |
| (12) | Incorporated by reference to Exhibit 10.2 filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003. |
| (13) | Incorporated by reference to Exhibit 10.21 filed with the Registrant’s Quarterly Report on Form 10-Q/A for the quarter ended June 30, 2002 (filed July 30, 2003). |
| (14) | Incorporated by reference to Exhibit 10.3 filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003. |
| (15) | Incorporated by reference to Exhibit 10.4 filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003. |
| (16) | Incorporated by reference to Exhibit 10.5 filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003. |
| (17) | Incorporated by reference to Exhibit 10.3 filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2007. |
80
| (18) | Incorporated by reference to Exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K dated June 21, 2006. |
| (19) | Incorporated by reference to Exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K dated March 15, 2006. |
| (20) | Incorporated by reference to Exhibit 10.2 filed with the Registrant’s Current Report on Form 8-K dated March 15, 2006. |
| (21) | Incorporated by reference to Exhibit 10.3 filed with the Registrant’s Current Report on Form 8-K dated March 15, 2006. |
| (22) | Incorporated by reference to Exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K dated February 5, 2007. |
| (23) | Incorporated by reference to Exhibit 10.2 filed with the Registrant’s Current Report on Form 8-K dated February 5, 2007. |
| (24) | Incorporated by reference to Exhibit 10.3 filed with the Registrant’s Current Report on Form 8-K dated February 5, 2007. |
| (25) | Incorporated by reference to Exhibit 4.1 filed with the Registrant’s Current Report on Form 8-K dated April 11, 2007. |
| (26) | Incorporated by reference to Exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K dated August 1, 2007. |
| (27) | Incorporated by reference to Exhibit 4.2 filed with the Registrant’s Registration Statement on Form S-8 (Reg. No. 333-127158) filed with the SEC on August 3, 2005. |
| (28) | Incorporated by reference to Exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K dated December 20, 2007. |
| (29) | Incorporated by reference to Exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K dated March 31, 2008. |
| (30) | Incorporated by reference to Exhibit 1.1 filed with the Registrant’s Current Report on Form 8-K dated May 12, 2008. |
| (31) | Incorporated by reference to Exhibit 4.1 filed with the Registrant’s Current Report on Form 8-K dated May 12, 2008. |
81
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, this 9th day of March 2009.
| | |
| CELL GENESYS, INC. |
| |
| By: | | /s/ SHARON E. TETLOW |
| | Sharon E. Tetlow Senior Vice President and Chief Financial Officer (Principal Financial Officer) |
| |
| By: | | /s/ MARC L. BELSKY |
| | Marc L. Belsky Vice President, Finance and Chief Accounting Officer (Principal Accounting Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/s/ STEPHEN A. SHERWIN, M.D. Stephen A. Sherwin, M.D. | | Chairman of the Board and Chief Executive Officer(Principal Executive Officer) | | March 9, 2009 |
| | |
/s/ SHARON E. TETLOW Sharon E. Tetlow | | Senior Vice President and Chief Financial Officer(Principal Financial Officer) | | March 9, 2009 |
| | |
/s/ MARC L. BELSKY Marc L. Belsky | | Vice President, Finance and Chief Accounting Officer(Principal Accounting Officer) | | March 9, 2009 |
| | |
/s/ DAVID W. CARTER David W. Carter | | Director | | March 9, 2009 |
| | |
/s/ NANCY M. CROWELL Nancy M. Crowell | | Director | | March 9, 2009 |
| | |
/s/ JAMES M. GOWER James M. Gower | | Director | | March 9, 2009 |
| | |
/s/ JOHN T. POTTS, JR., M.D. John T. Potts, Jr., M.D. | | Director | | March 9, 2009 |
| | |
/s/ THOMAS E. SHENK, PH.D. Thomas E. Shenk, Ph.D. | | Director | | March 9, 2009 |
| | |
/s/ EUGENE L. STEP Eugene L. Step | | Director | | March 9, 2009 |
| | |
/s/ INDER M. VERMA, PH.D. Inder M. Verma, Ph.D. | | Director | | March 9, 2009 |
| | |
/s/ DENNIS L. WINGER Dennis L. Winger | | Director | | March 9, 2009 |
82
