Exhibit 99.(a)(5)(E)

FIRST QUARTER 2024 FINANCIAL RESULTS MAY 6, 2024 ©2024 Vertex Pharmaceuticals Incorporated

SAFE HARBOR STATEMENT & NON-GAAP FINANCIAL MEASURES This presentation contains forward-looking statements that are subject to risks, uncertainties and other factors. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including all statements regarding the intent, belief, or current expectation of Vertex and members of the Vertex senior management team. Forward-looking statements are not purely historical and may be accompanied by words such as “anticipates,” “may,” “forecasts,” “expects,” “intends,” “plans,” “potentially,” “believes,” “seeks,” “estimates,” and other words and terms of similar meaning. Such statements include, without limitation, the information provided regarding and expectations for future financial and operating performance, the section captioned “Reiterate-Full Year 2024 Financial Guidance,” and statements regarding (i) expectations, development plans and timelines for the company’s products and pipeline programs, including expectations for anticipated near-term commercial launch opportunities in CF and acute pain, anticipated benefits of new products and relevant patient populations, and plans to broaden and deepen R&D pipeline across modalities, (ii) expectations, plans, and the anticipated timeline for the acquisition of Alpine Immune Sciences, Inc. (Alpine), including with respect to the therapeutic scope of Alpine and potential benefits of povetacicept, our beliefs regarding povetacicept’s target patient population, and our beliefs regarding the clinical progress and availability of clinical data for Alpine’s pipeline, (iii) expectations for the vanzacaftor triple, including the anticipated benefits and plans to complete various global regulatory submissions in 2024 and preparations for commercial launch in multiple geographies, (iv) expectations regarding VX-522 to reach the >5,000 CF patients who cannot benefit from a CFTR modulator, VX-522 study progress and plans to have VX-522 data in late 2024/early 2025, (v) expectations for our acute pain program, including plans for near-term launch and commercial potential, beliefs regarding the potential benefits of suzetrigine as a non-opioid treatment option, expectations regarding the target profile for suzetrigine, plans to complete the suzetrigine U.S. regulatory submission in the second quarter of 2024, plans to initiate Phase 2 studies of the oral formulation of VX-993 later this year, plans to enroll patients in the Phase 1 study of the intravenous formulation of VX-993, and our expectations, plans, and beliefs regarding the commercial potential of suzetrigine, including as multi-billion dollar opportunity, the treatable patient population, and potential impactful legislation, (vi) expectations for our PNP pain program, including plans to seek a broad PNP label, plans to advance suzetrigine in DPN into Phase 3 pivotal development in the second half of 2024, enrollment and dosing plans for the Phase 2 study of suzetrigine in LSR, plans to advance an oral formulation of VX-993 into a Phase 2 study in PNP in 2024, and plans to advance additional NaV 1.7 and NaV1.8 inhibitors, (vii) expectations for our T1D program, including the potential benefits of VX-880, expectations for completion of dosing in the global VX-880 study, plans for VX-880 data, and beliefs regarding the treatable T1D patient population, (viii) expectations regarding our CF program, (ix) expectations for CASGEVY, including the potential benefits for patients with SCD or TDT, expectations for on-going commercial launch, expectations with respect to access and reimbursement, expectations for additional approvals, and plans for studies in younger age groups. While Vertex believes the forward-looking statements contained in this presentation are accurate, these forward-looking statements represent the company’s beliefs as of the date of this presentation and there are risks and uncertainties that could cause actual events or results to differ materially from those expressed or implied by such forward-looking statements. Those risks and uncertainties include, among other things, that data from clinical trials, especially if based on a limited number of patients, may not to be indicative of final results, the company may not be able to complete, successfully integrate, or profit from the Alpine acquisition, the company’s regulatory submissions may be delayed, actual patient populations eligible for our products may be smaller than anticipated, data from the company’s development programs may not be available on expected timelines, or at all, support registration or further development of its potential medicines due to safety, efficacy or other reasons, and other risks listed under the heading “Risk Factors” in Vertex’s annual report and subsequent quarterly reports filed with the Securities and Exchange Commission at www.sec.gov and available through the company’s website at www.vrtx.com. You should not place any undue reliance on these statements, or the data presented. Vertex disclaims any obligation to update the information contained in this presentation as new information becomes available. In this presentation, Vertex’s financial results and financial guidance are provided in accordance with accounting principles generally accepted in the United States (GAAP) and using certain non-GAAP financial measures. In particular, non-GAAP financial results and guidance exclude from Vertex’s pre-tax income (i) stock-based compensation expense, (ii) intangible asset amortization expense, (iii) gains or losses related to the fair value of the company’s strategic investments, (iv) increases or decreases in the fair value of contingent consideration, (v) acquisition-related costs, and (vi) other adjustments. The company’s non-GAAP financial results also exclude from its provision for income taxes the estimated tax impact related to its non-GAAP adjustments to pre-tax income described above and certain discrete items. These results should not be viewed as a substitute for the company’s GAAP results and are provided as a complement to results provided in accordance with GAAP. Management believes these non-GAAP financial measures help indicate underlying trends in the company’s business, are important in comparing current results with prior period results and provide additional information regarding the company’s financial position that the company believes is helpful to an understanding of its ongoing business. Management also uses these non-GAAP financial measures to establish budgets and operational goals that are communicated internally and externally, to manage the company’s business and to evaluate its performance. The company’s calculation of non-GAAP financial measures likely differs from the calculations used by other companies. The company provides guidance regarding combined R&D, Acquired IPR&D and SG&A expenses and effective tax rate on a non-GAAP basis. Unless otherwise noted, the guidance regarding combined GAAP and non-GAAP R&D, Acquired IPR&D and SG&A expenses does not include estimates associated with any potential future business development transactions, including collaborations, asset acquisitions and/or licensing of third-party intellectual property rights. The company does not provide guidance regarding its GAAP effective tax rate because it is unable to forecast with reasonable certainty the impact of excess tax benefits related to stock-based compensation and the possibility of certain discrete items, which could be material. Non-GAAP financial measures are presented compared to corresponding GAAP measures in the appendix hereto. A reconciliation of the GAAP financial results to non-GAAP financial results is included in the company’s Q1 2024 press release dated May 6, 2024. 2

ACQUISITON OF • Compelling fit with the Vertex strategy of investing in ALPINE IMMUNE SCIENCES: serial innovation to create transformative medicines that $65/SHARE, ~$4.9B CASH target serious diseases with high unmet need in specialty markets • Alpine’s lead asset, povetacicept (“pove”), is a Phase 3-ready, innovative, and potentially transformative approach to IgAN, a serious, progressive, autoimmune kidney disease • Best-in-class potential; Phase 3 trial to begin H2:24 • Povetacicept, given dual BAFF/APRIL inhibition, also offers promise of “pipeline-in-a-product” in multiple other serious diseases, including pMN, LN, and autoimmune cytopenias Announced 4/10/24; expected close Q2:24 • Vertex capabilities expected to accelerate povetacicept development in IgAN and other indications, while Alpine will add protein engineering and immunotherapy expertise to Vertex BAFF: B-cell activating factor; APRIL: a proliferation-inducing ligand; IgAN: immunoglobulin A nephropathy; pMN: primary membranous nephropathy; LN: lupus nephritis 3 ©2024 Vertex Pharmaceuticals Incorporated
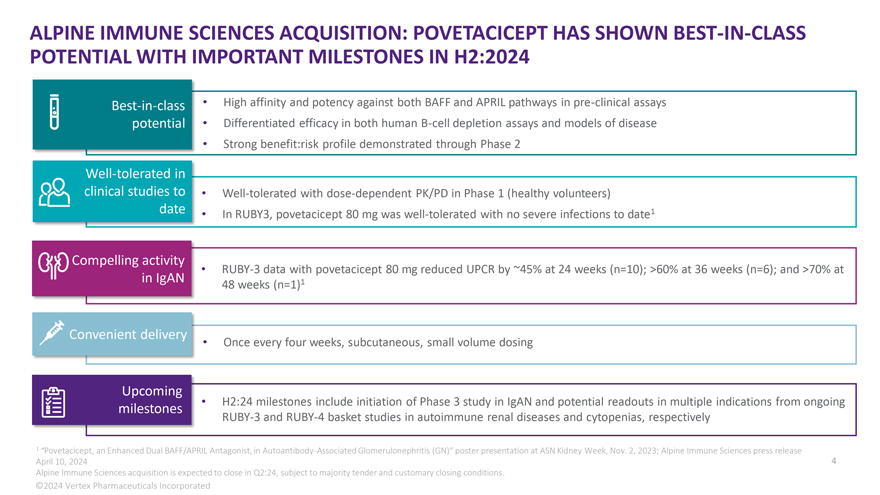
ALPINE IMMUNE SCIENCES ACQUISITION: POVETACICEPT HAS SHOWN BEST-IN-CLASS POTENTIAL WITH IMPORTANT MILESTONES IN H2:2024 Best-in-class • High affinity and potency against both BAFF and APRIL pathways in pre-clinical assays potential • Differentiated efficacy in both human B-cell depletion assays and models of disease • Strong benefit:risk profile demonstrated through Phase 2 Well-tolerated in clinical studies to • Well-tolerated with dose-dependent PK/PD in Phase 1 (healthy volunteers) date • In RUBY3, povetacicept 80 mg was well-tolerated with no severe infections to date1 Compelling activity • RUBY-3 data with povetacicept 80 mg reduced UPCR by ~45% at 24 weeks (n=10); >60% at 36 weeks (n=6); and >70% at in IgAN 1 48 weeks (n=1) Convenient delivery • Once every four weeks, subcutaneous, small volume dosing Upcoming • H2:24 milestones include initiation of Phase 3 study in IgAN and potential readouts in multiple indications from ongoing milestones RUBY-3 and RUBY-4 basket studies in autoimmune renal diseases and cytopenias, respectively 1 “Povetacicept, an Enhanced Dual BAFF/APRIL Antagonist, in Autoantibody-Associated Glomerulonephritis (GN)” poster presentation at ASN Kidney Week, Nov. 2, 2023; Alpine Immune Sciences press release April 10, 2024 4 Alpine Immune Sciences acquisition is expected to close in Q2:24, subject to majority tender and customary closing conditions. ©2024 Vertex Pharmaceuticals Incorporated

ADDITIONAL INFORMATION ABOUT THE ACQUISITION AND WHERE TO FIND IT The tender offer for the outstanding shares of common stock of Alpine Immune Sciences, Inc. referenced in this presentation commenced on April 22, 2024. This presentation is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Alpine Immune Sciences, Inc., nor is it a substitute for any tender offer materials that Vertex or Alpine Immune Sciences, Inc. have filed with the SEC. On April 22, 2024, when the tender offer commenced, Vertex filed with the SEC a Tender Offer Statement on Schedule TO which included an Offer to Purchase, a related Letter of Transmittal and certain other tender offer documents (together, the “Tender Offer Materials”), and Alpine Immune Sciences, Inc. filed with the SEC a Solicitation/Recommendation Statement on Schedule 14D-9 (the “Solicitation/Recommendation Statement”) with respect to the tender offer. ALPINE IMMUNE SCIENCES, INC. SECURITY HOLDERS ARE URGED TO READ THE TENDER OFFER MATERIALS AND THE SOLICITATION/RECOMMENDATION STATEMENT BECAUSE THEY CONTAIN IMPORTANT INFORMATION WHICH SHOULD BE READ CAREFULLY BEFORE ANY DECISION IS MADE WITH RESPECT TO THE TENDER OFFER. The Tender Offer Materials and the Solicitation/Recommendation Statement are available for free at the SEC’s website at www.sec.gov. Additional copies of the Tender Offer Materials can be obtained free of charge under the “Investors” section of Vertex’s website at https://investors.vrtx.com/financial-information/sec-filings or by contacting Vertex by phone at (617) 341-6108, by email at Investorinfo@VRTX.com, or by directing requests for such materials to the information agent for the offer, which is named in the Tender Offer Materials. In addition to the Tender Offer Materials and the Solicitation/Recommendation Statement, Alpine Immune Sciences, Inc. and Vertex file periodic reports and other information with the SEC. Vertex’s and Alpine Immune Sciences, Inc.’s filings with the SEC are also available for free to the public from commercial document-retrieval services, at the website maintained by the SEC at www.sec.gov, and their respective investor relations websites. 5