UNITED STATES | |||
SECURITIES AND EXCHANGE COMMISSION | |||
Washington, D.C. 20549 | |||
| |||
SCHEDULE 14A | |||
| |||
Proxy Statement Pursuant to Section 14(a) of | |||
| |||
Filed by the Registrant x | |||
| |||
Filed by a Party other than the Registrant o | |||
| |||
Check the appropriate box: | |||
o | Preliminary Proxy Statement | ||
o | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | ||
o | Definitive Proxy Statement | ||
x | Definitive Additional Materials | ||
o | Soliciting Material Pursuant to §240.14a-12 | ||
| |||
AMYLIN PHARMACEUTICALS, INC. | |||
(Name of Registrant as Specified In Its Charter) | |||
| |||
N.A. | |||
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) | |||
| |||
Payment of Filing Fee (Check the appropriate box): | |||
x | No fee required. | ||
o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | ||
| (1) | Title of each class of securities to which transaction applies: | |
|
|
| |
| (2) | Aggregate number of securities to which transaction applies: | |
|
|
| |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | |
|
|
| |
| (4) | Proposed maximum aggregate value of transaction: | |
|
|
| |
| (5) | Total fee paid: | |
|
|
| |
o | Fee paid previously with preliminary materials. | ||
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | ||
| (1) | Amount Previously Paid: | |
|
|
| |
| (2) | Form, Schedule or Registration Statement No.: | |
|
|
| |
| (3) | Filing Party: | |
|
|
| |
| (4) | Date Filed: | |
|
|
| |

FOR IMMEDIATE RELEASE
AMYLIN SENDS LETTER TO SHAREHOLDERS
Urges Shareholders to Support the Board’s Strategy and Vote the BLUE Proxy Card Today
to Elect Amylin’s Highly-Qualified Nominees
San Diego, CA — May 11, 2009 — Amylin Pharmaceuticals, Inc. (Nasdaq: AMLN) today announced that it is sending the following letter to shareholders in connection with the Company’s Annual Meeting of Stockholders on May 27, 2009. Amylin urges all shareholders to vote FOR Amylin’s Directors on the BLUE proxy card today and reject the nominees of both Carl Icahn and Eastbourne Capital Management, L.L.C.
The text of the letter from James N. Wilson, Lead Independent Director, is below:
Dear Fellow Amylin Shareholder:
Amylin’s Annual Meeting is nearly two weeks away—on May 27th, you will face a clear choice regarding the future of the Company and the value of your investment. We urge you to recognize the importance of your vote: make the right choice by voting FOR your Board’s highly-qualified nominees on the BLUE proxy card TODAY.
Amylin now has the opportunity to transform the treatment of diabetes with exenatide once weekly. Your Company has been carefully executing a comprehensive plan to ensure that this important treatment benefits diabetes patients and subsequently delivers substantial value to Amylin’s shareholders.
Carl Icahn and Eastbourne Capital Management, L.L.C., on the other hand, offer a different approach for the future of the Company. Despite their protestations to the contrary, their often stated agenda is the sale of Amylin, and they have selected a slate of nominees that reflects this intent. Your Board strongly believes that a premature sale of the Company—before Amylin’s shareholders fully realize the value of exenatide once weekly—is simply not the right or responsible strategy.
We urge you to vote to elect Amylin’s Director nominees and protect the value of your investment. Vote the BLUE proxy card TODAY by telephone or Internet, or by signing, dating and returning the BLUE proxy card in the postage paid envelope provided.
Please discard any Gold or White proxy cards you receive from Carl Icahn or Eastbourne Capital Management, L.L.C., respectively, and vote the BLUE proxy card today.
UNDER YOUR BOARD’S LEADERSHIP AMYLIN IS SUCCESSFULLY EXECUTING
ITS STATED STRATEGY AND ACHIEVING KEY MILESTONES
In the fall of 2008, Amylin clearly articulated its strategy for creating value in 2009 and beyond. Your Board and management team have already delivered on several key strategic initiatives, including:
ü May 5, 2009: Early submission of New Drug Application (NDA) for exenatide once weekly to U.S. Food and Drug Administration (FDA).
ü May 4, 2009: New specialty sales force approach to more effectively target the diabetes market — plan will reduce sales force by 35% and save $45 million annually beginning in 2010.
ü April 15, 2009: Launch of ExenatideOne to optimize cost structure and drive efficiency with partner Eli Lilly and Company.
We note that Mr. Icahn and Eastbourne ignore the achievement of these milestones because they are inconsistent with their “sell now” agenda.
THE EXENATIDE ONCE WEEKLY OPPORTUNITY
Within the next two decades, cases of diabetes are estimated to grow by 55% globally. Last year there were over 23 million people with diabetes in the United States alone. Exenatide once weekly will be the first once-a-week treatment for type 2 diabetes and has the potential to transform the treatment of the disease. Industry experts have recognized the significance and commercial potential of exenatide once weekly:
· “Many analysts believe that if [exenatide once weekly] makes it through [the FDA approval process it] may be a blockbuster, maybe even a mega blockbuster drug.”
- Mike Huckman, CNBC, Pharmaceutical Reporter, May 5, 2009
· “We believe exenatide [once weekly], once approved as the first once-weekly agent from the GLP-1 class, should help drive the company toward sustainable profitability.”
- Matthew Osborne, Senior Biotechnology Analyst, Lazard, April 30, 2009
ICAHN AND EASTBOURNE SUFFER FROM A FUNDAMENTAL
MISUNDERSTANDING OF AMYLIN’S BUSINESS
In pursuit of their agenda, Mr. Icahn and Eastbourne have launched a number of ill-informed attacks and comparisons that we believe reflect a lack of basic understanding of our business.
1. Attacking the Relationship with our Partner Eli Lilly and Company
An essential element of Amylin’s growth has been the successful partnership of Amylin with Lilly. We entered into a partnership with Lilly that was extremely beneficial to our shareholders and was well-received by the financial community. Amylin maintained control of the exenatide molecule and obtained the financial and operational support of a powerful leader in the diabetes sector.
This combination enabled us to grow BYETTA® (exenatide) injection, a first-in-class alternative, into a source of nearly $700 million in annual revenues in 2008 over a period of only three and a half years. We built a platform and an overall framework for growth of our next generation, exenatide once weekly, by:
· Establishing a leading franchise with our current injectable product BYETTA.
· Completing extensive clinical studies establishing the superiority of exenatide once weekly to competitive agents.
· Learning what high-prescribing physicians and patients want from our medicines.
As we near the completion of the development phase of exenatide once weekly, we have taken a number of decisive steps to maximize the value of Amylin. The results:
· The ExenatideOne structure — the Amylin and Lilly integrated medical, development and marketing team based in San Diego that will be focused solely on maximizing the value of the exenatide molecule.
· A streamlined sales force focused on high-prescribing physicians and positioned specifically for the exenatide once weekly opportunity.
· Substantial financial support from Lilly as we enter into the next stage of our growth.
· Improved operating results for Amylin shareholders.
The attempts of Mr. Icahn and Eastbourne to undermine this partnership are troubling, given the importance and clear benefits of Amylin working with Lilly at this critical point.
2. Criticizing the Sales Force Strategy
Initially, our sales strategy was focused on the broad and deep market penetration of BYETTA. Through these substantial selling efforts, we developed relationships with key physicians, learned about their prescribing habits and achieved critical mass for BYETTA.
As recently announced, we are optimizing our sales force to realize our objective of maximizing the number of physicians prescribing BYETTA and achieving maximum synergy with the Lilly sales force.
With the level of penetration established through the BYETTA rollout, we believe that this optimization will support the successful launch of exenatide once weekly.
These initiatives support our previously announced objective to be cash flow positive by the end of 2010. The Company’s new sales force approach is expected to result in an annualized benefit of approximately $45 million to GAAP operating results in 2010. Contrast this plan to the observations by Mr. Icahn, who suggested slashing 30% of the costs (over and above the cost savings initiatives adopted last fall) by focusing on areas such as insurance and logistics. We believe this “cookie-cutter” approach demonstrates a fundamental misunderstanding of Amylin’s business and would significantly impair our ability to maximize the value of Amylin through the commercialization of exenatide once weekly.
3. Citing Previous “Success”
Mr. Icahn has frequently referred to his experience at ImClone as a principal reason to vote for his slate. Let’s examine some of the assertions made by Mr. Icahn:
· Tighter cost controls by Icahn nominees led to higher free cash flow.
- ImClone’s costs actually increased by $113 million from 2005 to 2007 when his nominees were on the Board, reducing profitability from otherwise high margin royalties.
- The increase in free cash flow while his nominees were on the Board was largely driven by Merck KGaA nearly tripling sales from $265 million in 2005 to $656 million in 2007 and ImClone completing construction of their $334 million BB50 manufacturing facility during the fourth quarter of 2005. This led to major reduction in CAPEX, not tight cost controls from Icahn.
· Icahn’s nominees helped deliver results.
- During the Icahn representatives’ brief two-year tenure, analysts reduced revenue estimates on four separate occasions and ImClone’s earnings twice fell short of consensus expectations.
· ImClone shares dramatically outperformed.
- Absent the sale to Lilly, during Dr. Alexander Denner’s tenure on the Board, ImClone’s stock price actually underperformed the comparable companies that Icahn himself identified in his proxy presentation, underscoring his only path to value creation is a sale. (Dr. Denner is one of Mr. Icahn’s Director nominees.)
[For additional information, please see the attached slides.]
Mr. Icahn has publicly touted his disdain for corporate boards. Of the nine companies where Mr. Icahn has obtained Board representation since 2000, ImClone is the only one with positive price performance. The other eight are down an average of 63% since his representatives joined the Board:
Company |
| Value “Creation” |
| Situation |
| Industry |
Adventrx Pharmaceuticals |
| - 94 | % | Other |
| Biotechnology |
Blockbuster |
| - 93 | % | Proxy Fight |
| Retail/Services |
BKF Capital |
| - 85 | % | Other |
| Financial |
Federal Mogul(2) |
| - 63 | % | Other |
| Auto Parts |
Motorola(1) |
| - 42 | % | Proxy Fight |
| Technology/Telecom |
ImClone Systems(4) |
| 126 | % | Proxy Fight / Company sold |
| Biotechnology |
WCI Communities(1) |
| - 100 | % | Proxy Fight |
| Construction |
XO Holdings(3) |
| - 12 | % | Other |
| Technology/Telecom |
Yahoo!(1) |
| - 30 | % | Proxy Fight |
| Internet |
Source: CapIQ, Shark Repellant, public sources as of 27-Apr-2009
Note: Companies listed are situations where Icahn and /or his affiliates obtained board representation since 2000. Performance is measured from the day Icahn or an affiliate was placed on the board to 27-Apr-2009
(1) Company agreed to nominate dissident(s) at the annual meeting of shareholders. The board joining date is the date of the annual meeting where the dissident(s) were first up for election.
(2) Federal-Mogul performance is measured from 6-Dec-2007, the date it emerged from bankruptcy.
(3) XO Holdings performance is measured from 23-Jan-2003, the date it emerged from bankruptcy.
(4) ImClone agreed to be acquired by Eli Lilly on 6-Oct-2008. Final sale price is the $70 cash consideration offered by Eli Lilly.
4. Asserting that their Nominees Are Better Qualified than Your Board’s Nominees
In recent years, Amylin has developed and commercialized two first-in-class products, positioning the Company to deliver a potentially transformational drug—exenatide once weekly. Your Board’s slate has extensive experience in biopharmaceuticals and diabetes as well as valuable sales and marketing expertise. In addition to the ten current Directors standing for re-election, we are further strengthening your Board by proposing two new independent Director nominees: Paul N. Clark, former Chairman, Chief Executive Officer and President of Icos Corporation, and Paulo F. Costa, former President and Chief Executive Officer of Novartis U.S. Corporation. Both bring additional commercial and operational expertise in the biopharmaceutical industry, which are key attributes requested by our shareholders.
AMYLIN’S SLATE IS BEST QUALIFIED TO UNLOCK LONG-TERM VALUE
Your Board has the knowledge and expertise to continue to successfully execute Amylin’s strategy, with:
ü A proven track record — successfully overseeing development and commercialization of two first-in-class products and positioning Amylin to deliver a potentially transformational drug.
ü Director nominees who have extensive diabetes and biopharmaceutical experience as well as valuable sales and marketing expertise.
In contrast, the Icahn and Eastbourne nominees:
C Lack diabetes knowledge.
C Lack understanding of the Company and industry expertise.
C Have predominantly financial backgrounds that reflect their short-term “sell now” agenda — one of them would need to retire within one year given the Company’s mandatory retirement age.
The relevant experience of the Icahn and Eastbourne nominees pales in comparison to the qualifications of the five nominees they are seeking to replace.
While Mr. Icahn and Eastbourne have both recently publicly stated their intention to maximize the exenatide once weekly opportunity, their five nominees lack the relevant diabetes knowledge and industry expertise to best serve Amylin and its shareholders at this critical time. Given their nominees, Icahn and Eastbourne clearly appear to be focused on a sale of Amylin before you can realize the substantial value inherent in the exenatide once weekly opportunity.
5. Mischaracterizing our Interactions
It is important for shareholders to know that Amylin’s Board has acted in good faith in its interactions with Mr. Icahn and Eastbourne. We respect their level of stock ownership and have worked with both parties in an effort to resolve the proxy contest and reach an agreement that is in the best interests of all shareholders. Amylin has:
· Offered Board representation to both Mr. Icahn and Eastbourne.
· Facilitated three-way discussions.
· Permitted them to engage in discussions regarding a potential settlement without triggering Amylin’s shareholder rights agreement.
· Worked out, with no up-front cost, a favorable waiver of the “change in control provision” in our credit agreement.
Despite our best efforts, Mr. Icahn and Eastbourne continue to pursue a fundamental change in the composition of your Board and a dramatic shift in strategy with a slate whose only agenda is a premature sale of the Company. We believe their presence on the Board could jeopardize the important actions we are taking to maximize Amylin’s future growth and success.
SECURE AMYLIN’S FUTURE AND POTENTIAL OF EXENATIDE ONCE WEEKLY—
VOTE FOR YOUR COMPANY’S NOMINEES ON THE BLUE PROXY CARD TODAY
The election of any of the nominees put forward by Mr. Icahn and Eastbourne, who reject the Company’s strategy, will jeopardize Amylin’s ability to maximize value for all shareholders and significantly impair the Company’s ability to fulfill the unmet needs of millions of diabetes patients
We urge you to act now to maximize the value of your investment by voting your BLUE proxy card TODAY. Please discard any Gold or White proxy cards you might receive from Mr. Icahn or Eastbourne, respectively.
[HYPERLINK TO SLIDES]
On behalf of your Board of Directors, we thank you for your continued support.
Sincerely,
| /s./ James N. Wilson |
|
| Lead Independent Director |
|
|
|
|
Your Vote Is Important, No Matter How Many Or How Few Shares You Own
If you have questions about how to vote your shares, or need additional assistance, please contact the firm assisting us in the solicitation of proxies:
INNISFREE M&A INCORPORATED
Stockholders Call Toll-Free: (877) 717-3926
Banks and Brokers Call Collect: (212) 750-5833
IMPORTANT
We urge you NOT to vote using any White or Gold proxy card sent to you by Icahn or Eastbourne, as doing so will revoke your vote on the BLUE proxy card. If you have already done so, you have every legal right to change your vote by using the enclosed BLUE proxy card to vote today—by telephone, by Internet, or by signing, dating and returning the BLUE proxy card in the postage-paid envelope provided.
|
|
|
About Amylin
Amylin Pharmaceuticals is a biopharmaceutical company committed to improving lives through the discovery, development and commercialization of innovative medicines. Amylin has developed and gained approval for two first-in-class medicines for diabetes, SYMLIN® (pramlintide acetate) injection and BYETTA® (exenatide) injection. Amylin’s research and development activities leverage the Company’s expertise in metabolism to develop potential therapies to treat diabetes and obesity. Amylin is headquartered in San Diego, California. Further information on Amylin Pharmaceuticals is available at www.amylin.com.
Forward Looking Statements
This press release contains forward-looking statements about Amylin, which involve risks and uncertainties. Our actual results could differ materially from those discussed herein due to a number of risks and uncertainties, including risks that BYETTA, SYMLIN or exenatide once weekly may be affected by competition, unexpected new data, safety and technical issues, or manufacturing and supply issues; risks that our financial results may fluctuate significantly from period to period and may not meet market expectations; risks that any financial guidance we provide may not be accurate; risks that our clinical trials will not be completed when planned, may not replicate previous results or achieve desired end-points; risks that our preclinical studies may not be predictive; risks that our NDAs for product candidates or sNDAs for label expansion requests, such as the exenatide once weekly NDA mentioned in this press release, may not be submitted timely or receive FDA approval; risks that our expense reductions will not be as large as we expect; risks that the restructured operations for exenatide will not produce the results we expect; and other risks inherent in the drug development and commercialization process. Commercial and government reimbursement and pricing decisions and the pace of market acceptance may also affect the potential for BYETTA, SYMLIN or exenatide once weekly. These and additional risks and uncertainties are described more fully in the Company’s most recently filed Form 10-K and Form 10-Q. Amylin disclaims any obligation to update these forward-looking statements.
Additional Information and Where To Find It
This press release may be deemed to be solicitation material in respect of the matters to be considered at the 2009 Annual Meeting of Stockholders. Amylin has filed the definitive proxy statement with the Securities and Exchange Commission (“SEC”) on April 20, 2009. INVESTORS AND SECURITYHOLDERS ARE URGED TO READ THE PROXY STATEMENT, THE BLUE PROXY CARD AND ANY OTHER RELEVANT DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC BECAUSE THEY CONTAIN IMPORTANT INFORMATION. Investors and securityholders may obtain the proxy statement and other relevant documents free of charge at the SEC’s Web site, www.sec.gov or from Amylin Investor Relations at 9360 Towne Centre Drive, San Diego, California 92121.
Participants in Solicitation
Amylin and its directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the matters to be considered at the 2009 Annual Meeting of Stockholders. Information regarding the interests of Amylin’s directors and executive officers in the proxy contest is included in Amylin’s definitive proxy statement.
CONTACTS:
Alice Izzo
Executive Director, Corporate Affairs
Amylin Pharmaceuticals, Inc.
(858) 642-7272
alice.izzo@amylin.com
or
Joele Frank / Annabelle Rinehart
Joele Frank, Wilkinson Brimmer Katcher
(212) 355-4449
| Icahn Influence on Biotechnology Boards: Setting the Record Straight Spring 2009 |
| Legal Information FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements about Amylin. Our actual results could differ materially from those discussed due to a number of factors, including: BYETTA, SYMLIN or exenatide once weekly being affected by competition, safety or other issues; clinical trials not being completed in a timely manner, not confirming previous results or achieving the intended clinical endpoints; NDAs or sNDAs not being submitted in a timely manner or receiving regulatory approval; expense reductions not being as large as we expect; restructure operations for exenatide not producing the results we expect; financial guidance we provide may not be accurate; our label update not being finalized in a timely manner; or manufacturing and supply issues. The pace of market acceptance and rate of patient adherence may also affect the potential for BYETTA, SYMLIN or exenatide once weekly. These and other risks and uncertainties are described more fully in Amylin’s most recently filed Form 10-K and Form 10-Q. Amylin disclaims any obligation to update these forward-looking statements. ADDITIONAL INFORMATION AND WHERE TO FIND IT This presentation may be deemed to be solicitation material in respect of the matters to be considered at the 2009 annual meeting of shareholders. Amylin has filed the definitive proxy statement with the Securities and Exchange Commission (“SEC”) on April 20, 2009. INVESTORS AND SECURITYHOLDERS ARE URGED TO READ THE PROXY STATEMENT, THE BLUE PROXY CARD AND ANY OTHER RELEVANT DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC BECAUSE THEY CONTAIN IMPORTANT INFORMATION. Investors and securityholders may obtain the proxy statement and other relevant documents free of charge at the SEC’s web site, www.sec.gov, or from Amylin Investor Relations at 9360 Towne Centre Drive, San Diego, California 92121. PARTICIPANTS IN SOLICITATION Amylin and its directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the matters to be considered at the 2009 annual meeting of shareholders. Information regarding the interests of Amylin’s directors and executive officers in the proxy contest are included in Amylin’s definitive proxy statement. |
| Debunking Icahn’s Assertions Critical that investors understand what actually happens when Icahn gains representation on biotechnology Boards Operational disappointments Lagging share price performance Focus on sale Prior to the sale of the company, ImClone was not the success story that Icahn claims Icahn and Eastbourne nominees lack the expertise needed to launch a game-changing diabetes therapy Amylin’s slate delivers the operational excellence critical to commercializing exenatide once weekly and maximizing shareholder value |
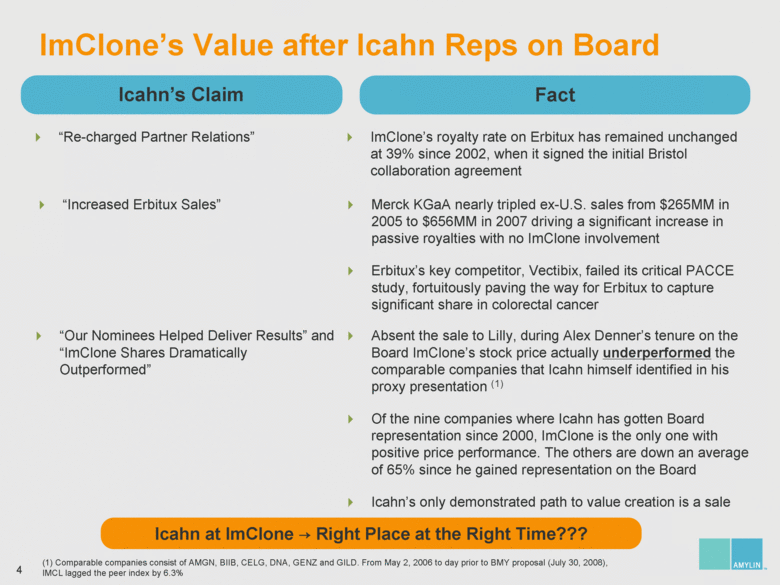
| ImClone’s Value after Icahn Reps on Board ImClone’s royalty rate on Erbitux has remained unchanged at 39% since 2002, when it signed the initial Bristol collaboration agreement Icahn’s Claim Fact “Re-charged Partner Relations” “Increased Erbitux Sales” Merck KGaA nearly tripled ex-U.S. sales from $265MM in 2005 to $656MM in 2007 driving a significant increase in passive royalties with no ImClone involvement Erbitux’s key competitor, Vectibix, failed its critical PACCE study, fortuitously paving the way for Erbitux to capture significant share in colorectal cancer Icahn at ImClone Right Place at the Right Time??? Absent the sale to Lilly, during Alex Denner’s tenure on the Board ImClone’s stock price actually underperformed the comparable companies that Icahn himself identified in his proxy presentation (1) Of the nine companies where Icahn has gotten Board representation since 2000, ImClone is the only one with positive price performance. The others are down an average of 65% since he gained representation on the Board Icahn’s only demonstrated path to value creation is a sale “Our Nominees Helped Deliver Results” and “ImClone Shares Dramatically Outperformed” (1) Comparable companies consist of AMGN, BIIB, CELG, DNA, GENZ and GILD. From May 2, 2006 to day prior to BMY proposal (July 30, 2008), IMCL lagged the peer index by 6.3% |
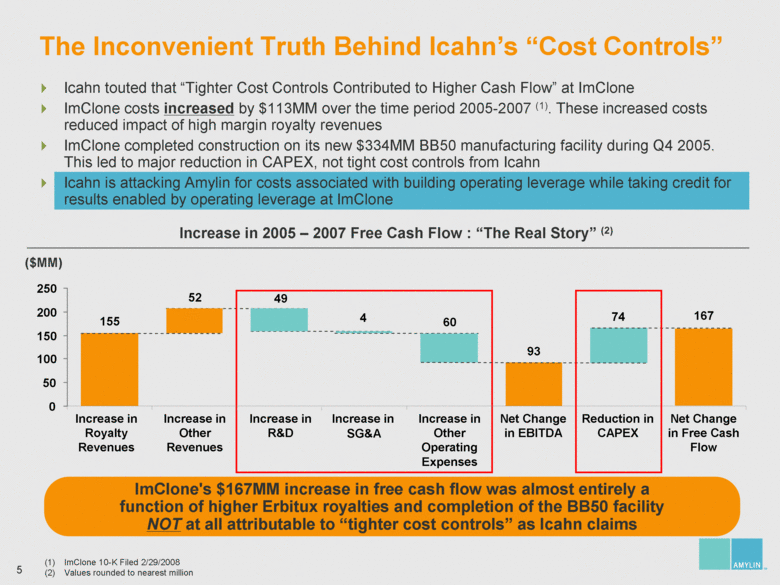
| Increase in Royalty Revenues Increase in Other Revenues Increase in SG&A Increase in Other Operating Expenses Net Change in EBITDA Increase in R&D Reduction in CAPEX Net Change in Free Cash Flow Icahn touted that “Tighter Cost Controls Contributed to Higher Cash Flow” at ImClone ImClone costs increased by $113MM over the time period 2005-2007 (1). These increased costs reduced impact of high margin royalty revenues ImClone completed construction on its new $334MM BB50 manufacturing facility during Q4 2005. This led to major reduction in CAPEX, not tight cost controls from Icahn Icahn is attacking Amylin for costs associated with building operating leverage while taking credit for results enabled by operating leverage at ImClone The Inconvenient Truth Behind Icahn’s “Cost Controls” ImClone 10-K Filed 2/29/2008 Values rounded to nearest million ($MM) Increase in 2005 – 2007 Free Cash Flow : “The Real Story” (2) 93 60 49 52 155 4 0 50 100 150 200 250 ImClone's $167MM increase in free cash flow was almost entirely a function of higher Erbitux royalties and completion of the BB50 facility NOT at all attributable to “tighter cost controls” as Icahn claims 74 167 |
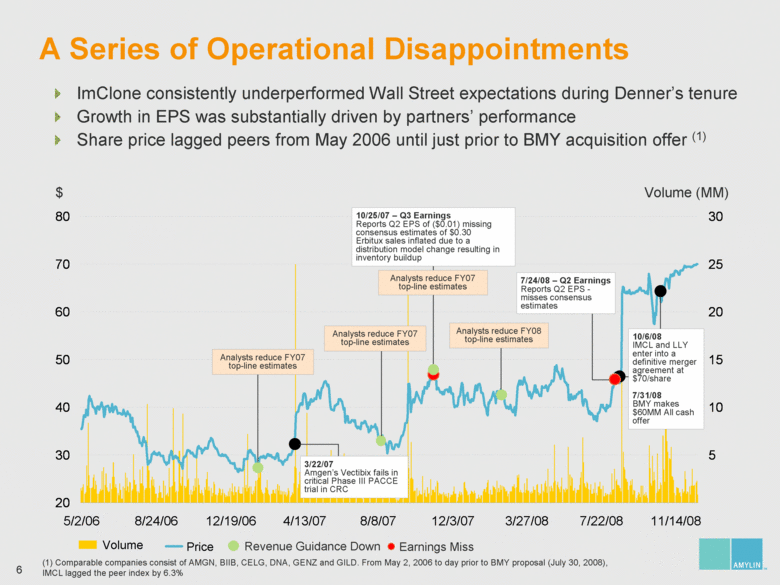
| A Series of Operational Disappointments $ Volume (MM) 5/2/06 8/24/06 12/19/06 4/13/07 8/8/07 12/3/07 3/27/08 7/22/08 11/14/08 Volume Earnings Miss Price Revenue Guidance Down ImClone consistently underperformed Wall Street expectations during Denner’s tenure Growth in EPS was substantially driven by partners’ performance Share price lagged peers from May 2006 until just prior to BMY acquisition offer (1) Analysts reduce FY07 top-line estimates Analysts reduce FY08 top-line estimates 10/25/07 – Q3 Earnings Reports Q2 EPS of ($0.01) missing consensus estimates of $0.30 Erbitux sales inflated due to a distribution model change resulting in inventory buildup Analysts reduce FY07 top-line estimates 3/22/07 Amgen’s Vectibix fails in critical Phase III PACCE trial in CRC 10/6/08 IMCL and LLY enter into a definitive merger agreement at $70/share 7/31/08 BMY makes $60MM All cash offer 7/24/08 – Q2 Earnings Reports Q2 EPS - misses consensus estimates Analysts reduce FY07 top-line estimates (1) Comparable companies consist of AMGN, BIIB, CELG, DNA, GENZ and GILD. From May 2, 2006 to day prior to BMY proposal (July 30, 2008), IMCL lagged the peer index by 6.3% 20 30 40 50 60 70 80 5 10 15 20 25 30 |
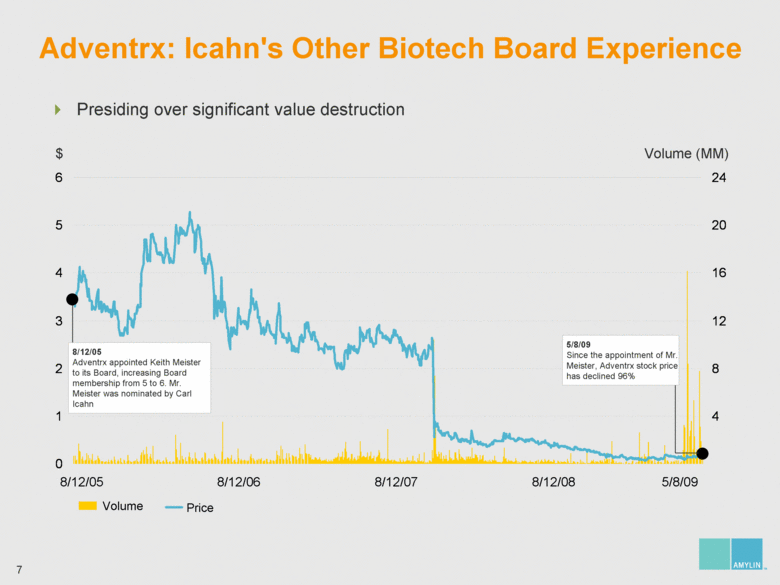
| Adventrx: Icahn's Other Biotech Board Experience $ Volume (MM) 8/12/05 8/12/06 8/12/07 8/12/08 5/8/09 Volume Price Presiding over significant value destruction 8/12/05 Adventrx appointed Keith Meister to its Board, increasing Board membership from 5 to 6. Mr. Meister was nominated by Carl Icahn 5/8/09 Since the appointment of Mr. Meister, Adventrx stock price has declined 96% 0 1 2 3 4 5 6 4 8 12 16 20 24 |