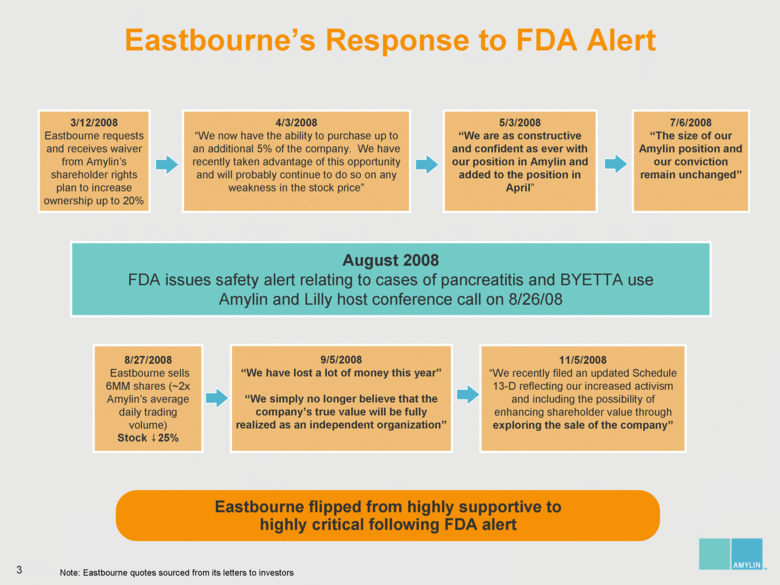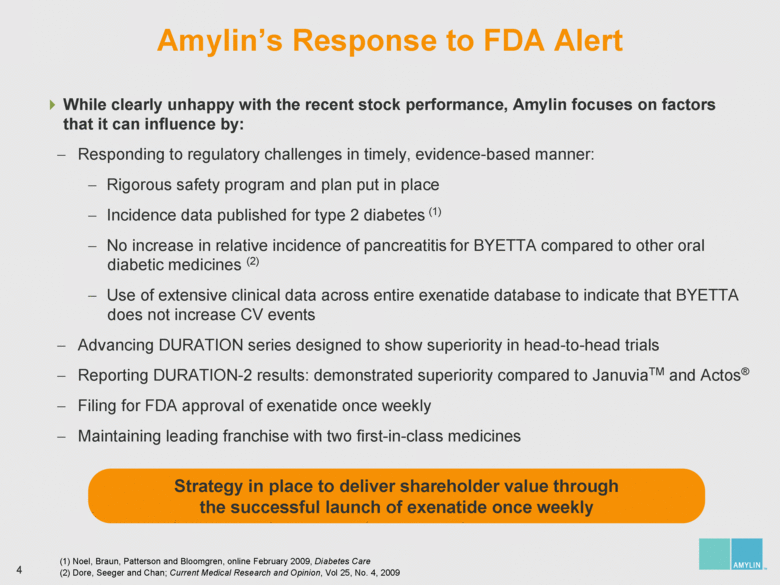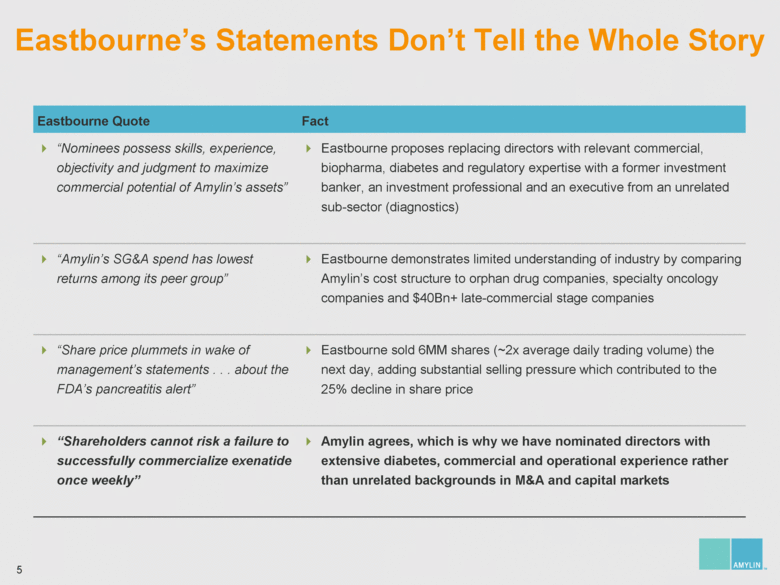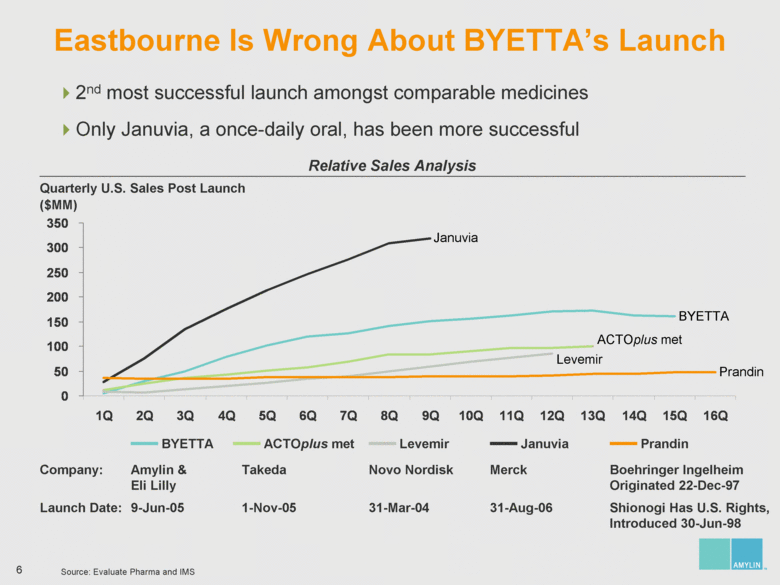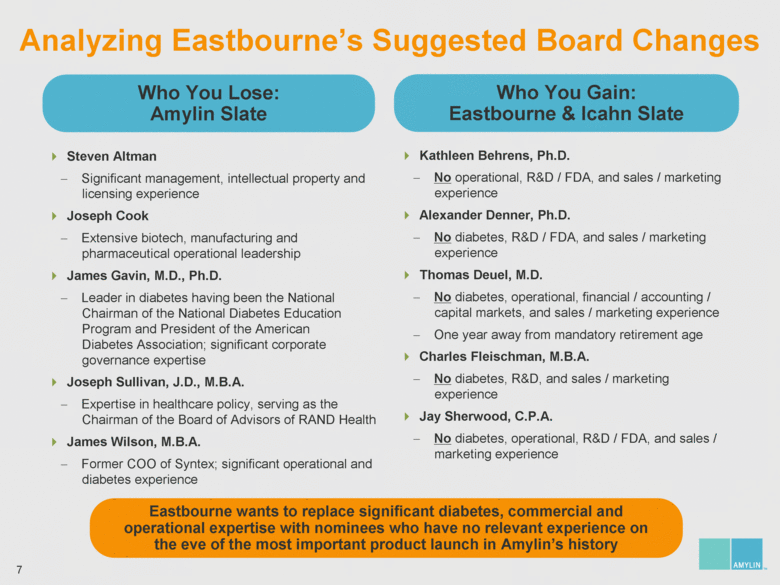
| 7 Analyzing Eastbourne’s Suggested Board Changes Eastbourne wants to replace significant diabetes, commercial and operational expertise with nominees who have no relevant experience on the eve of the most important product launch in Amylin’s history Who You Lose: Amylin Slate Who You Gain: Eastbourne & Icahn Slate Steven Altman Significant management, intellectual property and licensing experience Joseph Cook Extensive biotech, manufacturing and pharmaceutical operational leadership James Gavin, M.D., Ph.D. Leader in diabetes having been the National Chairman of the National Diabetes Education Program and President of the American Diabetes Association; significant corporate governance expertise Joseph Sullivan, J.D., M.B.A. Expertise in healthcare policy, serving as the Chairman of the Board of Advisors of RAND Health James Wilson, M.B.A. Former COO of Syntex; significant operational and diabetes experience Kathleen Behrens, Ph.D. No operational, R&D / FDA, and sales / marketing experience Alexander Denner, Ph.D. No diabetes, R&D / FDA, and sales / marketing experience Thomas Deuel, M.D. No diabetes, operational, financial / accounting / capital markets, and sales / marketing experience One year away from mandatory retirement age Charles Fleischman, M.B.A. No diabetes, R&D, and sales / marketing experience Jay Sherwood, C.P.A. No diabetes, operational, R&D / FDA, and sales / marketing experience |