SLIDES USED IN MEETINGS WITH PROSPECTIVE INVESTORS AND STOCKHOLDERS OF DENDREON CORPORATION AND OF CORVAS INTERNATIONAL, INC., BEGINNING JUNE 5, 2003
Filed by Dendreon Corporation
Pursuant to Rule 165 and Rule 425
under the Securities Act of 1933 and
deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: Corvas International, Inc.
Form S-4 File No. 333-104167
This filing relates to the proposed acquisition (“Acquisition”) by Dendreon Corporation (“Dendreon”) of Corvas International, Inc. (“Corvas”) pursuant to the terms of an Agreement and Plan of Merger, dated February 24, 2003 (the “Merger Agreement”), by and among Dendreon, Seahawk Acquisition, Inc., Charger Project LLC and Corvas. The Merger Agreement is on file with the Securities and Exchange Commission (“SEC”) as an exhibit to the Current Report on Form 8-K filed by Dendreon on February 25, 2003, and is incorporated by reference into this filing.
Additional Information About the Acquisition and Where to Find It
Dendreon and Corvas have filed with the SEC a Registration Statement on Form S-4 (the “Registration Statement”), which contains a preliminary joint proxy statement/prospectus with respect to the Acquisition and other relevant materials. The Registration Statement has not been declared effective by the SEC. INVESTORS AND SECURITY HOLDERS OF DENDREON AND CORVAS ARE URGED TO READ THE COMPANIES’ RELEVANT FILINGS WITH THE SEC, INCLUDING THE DEFINITIVE JOINT PROXY STATEMENT/PROSPECTUS WHEN IT BECOMES AVAILABLE, BECAUSE THEY CONTAIN IMPORTANT INFORMATION ABOUT DENDREON, CORVAS AND THE ACQUISITION. The preliminary joint proxy statement/prospectus and other relevant materials, and any other documents filed by Dendreon or Corvas with the SEC, may be obtained free of charge at the SEC’s web site at www.sec.gov.
In addition, investors and security holders may obtain free copies of the documents filed with the SEC by Dendreon by directing a request to: Dendreon Corporation, 3005 First Avenue, Seattle, WA 98121, Attn: Investor Relations. Investors and security holders may obtain free copies of the documents filed with the SEC by Corvas by contacting Corvas Investor Relations at 3030 Science Park Road, San Diego CA 92121.
Dendreon, Corvas and their respective executive officers and directors may be deemed to be participants in the solicitation of proxies from the stockholders of Dendreon and Corvas in favor of the Acquisition. Information about the executive officers and directors of Dendreon and their ownership of Dendreon common stock is set forth in the preliminary joint proxy statement/prospectus, which is included in the Registration Statement. Information about the executive officers and directors of Corvas and their ownership of Corvas common stock is set forth in Corvas’ Annual Report on Form 10-K, which was filed with the SEC on March 14,
2003, and in the preliminary joint proxy statement/prospectus included in the Registration Statement. Certain directors and executive officers of Corvas may have direct or indirect interests in the Acquisition due to securities holdings, pre-existing or future indemnification arrangements, vesting of options, or rights to severance payments if their employment is terminated following the Acquisition. Additional information regarding Dendreon, Corvas, and the interests of their respective executive officers and directors in the Acquisition is contained in the preliminary joint proxy statement/prospectus.
Investors and security holders are urged to read the definitive joint proxy statement/prospectus and the other relevant materials when they become available, and any other documents filed with the SEC by Dendreon and Corvas, before making any voting or investment decision with respect to the Acquisition.
Forward-looking Statements
Except for historical information contained herein, the slides contain forward-looking statements, including statements about the future product pipeline of the combined company. These statements are based on management’s current expectations and beliefs and are subject to a number of risks and uncertainties, particularly those risks and uncertainties inherent in any acquisition transaction and the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, that could cause actual results to differ materially from those described in the forward-looking statements.
Risks and uncertainties include the possibility that the market for the sale of certain products may not develop as expected; that development of the companies’ products, including potential cardiovascular and cancer products, may not proceed as planned; risks associated with completing ongoing clinical trials, including the rNAPc2 clinical trail for the treatment of patients with unstable angina and non-ST-segment elevation myocardial infarction; the risk that the results of one clinical trial will not be repeated in another clinical trial; the risk that results in preclinical studies may not be confirmed in clinical trials or that other preclinical studies will reveal adverse characteristics that preclude further development of a preclinical product candidate; the risk that the results of a clinical trial, including Phase III trials of Provenge, will not support applying for or approval of a biologics license by the FDA; the risk that the Acquisition does not close or that the companies may be required to modify aspects of the transaction to achieve regulatory approval; that prior to the closing of the Acquisition, the businesses of the companies, including the retention of key employees, suffer due to uncertainty; that the parties are unable to successfully execute their integration strategies or achieve planned synergies; risks related to Dendreon’s limited operating history; the risk that the companies may not secure or maintain relationships with collaborators; the companies’ dependence on intellectual property; and other risks and uncertainties that are described in the reports filed by Dendreon and Corvas with the SEC. Additional information on the risks and uncertainties that could affect the companies’ business, financial condition and results of operations are contained in their respective filings with the SEC, which are available atwww.sec.gov.
The following are slides from presentations made to prospective investors and stockholders of Dendreon and of Corvas beginning June 5, 2003.

Forward-Looking Statement Disclaimer
This presentation includes forward-looking statements, including statements about Dendreon’s proposed acquisition of Corvas, post-closing business synergies and product opportunities, and product development and commercialization. Actual results may differ materially from those projected in the forward-looking statements. Factors that could cause actual results to differ materially from those in the forward-looking statements are contained in Dendreon’s filings with the SEC, including its Registration Statement on Form S-4/A (Reg. No. 333-104167), filed on March 8, 2003, under the caption “Risk Factors.”
DENDREON
CORPORATION

Momentum Continues in 2003
Significant Scientific Progress
| • | | Alternative reading frames (antigen engineering) |
Corvas Acquisition
| • | | Stock for stock transaction |
| • | | Valued at $73 M at the time of announcement, an 83% premium to Corvas’ stock price as of 2/24/03 |
| • | | Value creation for both companies |
DENDREON
CORPORATION
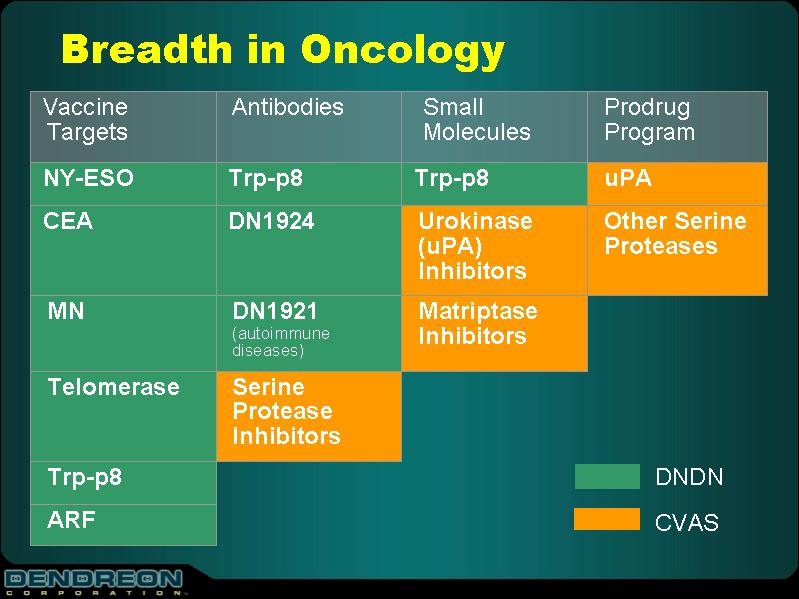
Breadth in Oncology
Vaccine Targets | | Antibodies | | Small Molecules | | Prodrug Program |
NY-ESO | | Trp-p8 | | Trp-p8 | | uPA |
| | | | | | | |
CEA | | DN1924 | | Urokinase (uPA) Inhibitors | | Other Serine Proteases |
| | | | | | | |
MN | | DN1921 (autoimmune diseases) | | Matriptase Inhibitors | | |
| | | | | | | |
Telomerase | | Serine Protease Inhibitors | | | | |
| | | | | | | |
Trp-p8 | | | | | | |
| | | | | | | |
ARF | | | | | | |
| | | | | | | |
DVDN | | | | | | |
CVAS | | | | | | |
DENDREON
CORPORATION
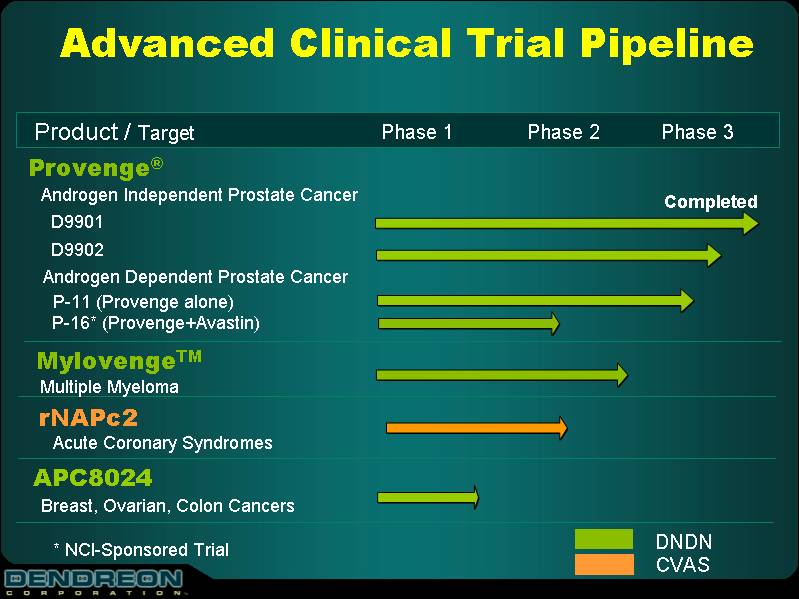
Advanced Clinical Trial Pipeline
| Product/Target | | Phase 1 | | Phase 2 | | Phase 3 |
| | | | | | | |
Provenge® | | | | | | |
Androgen Independent Prostate Cancer | | | | Completed |
D9901 D9902 | | | | | | |
Androgen Dependent Prostate Cancer | | | | |
P-11 (Provenge alone) P-16* (Provenge+Avastin) | | | | |
| | | | | | | |
Mylovenge™ | | | | | | |
Multiple Myeloma | | | | | | |
| | | | | | | |
rNAPc2 | | | | | | |
Acute Coronary Syndromes | | | | |
| | | | | | | |
APC8024 | | | | | | |
Breast, Ovarian, Colon Cancers | | | | |
| | | | | | | |
* NCI-sponsored trial | | | | | | |
| | | | | | | |
DNDN | | | | | | |
CVAS | | | | | | |
DENDREON
CORPORATION
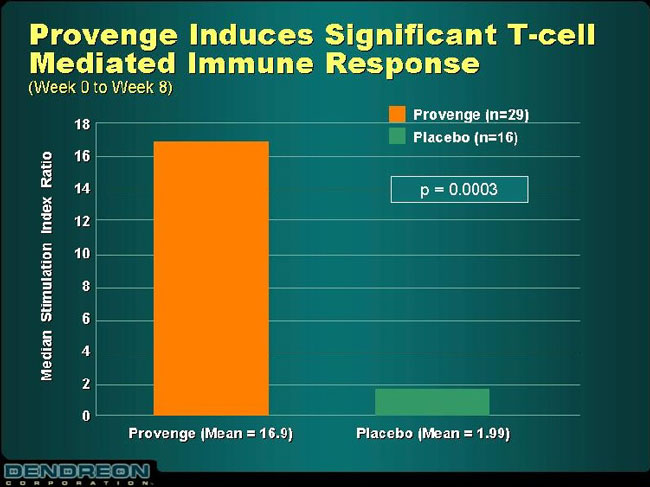
Provenge Induces Significant T-cell Mediated Immune Response
(Week 0 to Week 8)
[GRAPHIC]
DENDREON
CORPORATION
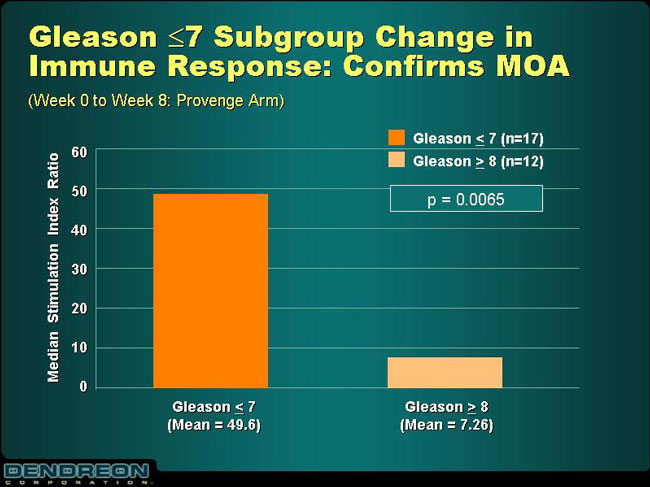
Gleason£7 Subgroup Change in Immune Response: Confirms MOA
(Week 0 to Week 8: Provenge Arm)
[GRAPHIC]
DENDREON
CORPORATION
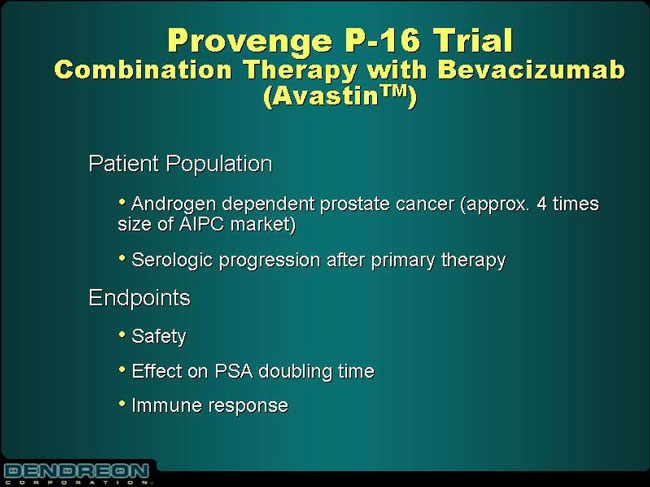
Provenge P-16 Trial
Combination Therapy with Bevacizumab
(Avastin™)
Patient Population
| • | | Androgen dependent prostate cancer (approx. 4 times size of AIPC market) |
| • | | Serologic progression after primary therapy |
Endpoints
| • | | Effect on PSA doubling time |
DENDREON
CORPORATION

P-16 Trial Results to Date
PSA Doubling Time | | Pre-Treatment | | On-Treatment |
Median | | 8.2 months | | 21.4 months |
| | | | | |
PSA Changes | | | | |
Decrease in PSA | | 3 patients (12%, 33%, 64%) | | |
DENDREON
CORPORATION
10

Provenge Collaboration Discussions
| | • | | Discussions progressing as planned |
| | • | | Large market, strong data and regulatory plan have driven interest |
| | • | | Will be structured as co-development and co-promotion agreement |
| | • | | Global agreement minus Asia and Oceania |
DENDREON
CORPORATION

APC8024 Phase 1 Trials
Patient Population
| | • | | 18 patients enrolled; 16 evaluable |
| | • | | Advanced, metastatic breast cancer patients |
| | • | | Failed multiple courses of chemotherapy including 15/16 who failed Herceptin |
| | • | | Historical median time to progression 3-6 months |
Endpoints
DENDREON
CORPORATION
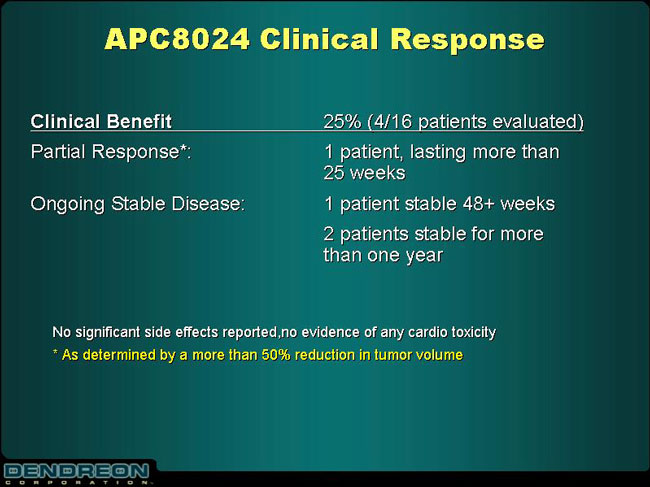
APC8024 Clinical Response
Clinical Benefit
| | 25% (4/16 patients evaluated)
|
Partial Response*: | | 1 patient, lasting more than 25 weeks |
Ongoing Stable Disease: | | 1 patient stable 48+ weeks |
| | | 2 patients stable for more than one year |
| | | |
No significant side effects reported, no evidence of any cardio toxicity
| * | | As determined by a more than 50% reduction in tumor volume |
DENDREON
CORPORATION

Key Messages: American Society of Clinical Oncology 2003 Meeting
| • | | “Immunotherapy may be one of the only ways left to deal with cancer… We’ve gone as far as we can with chemotherapy.” - Dr. Herman Kattlove, American Cancer Society |
| • | | Cellular therapies will be an important approach in fighting the war on cancer |
| • | | Provenge is the most advanced product in this category with promising Phase 3 placebo-controlled data |
| • | | Data presented by Dr. Eric Small, UCSF, confirms product’s mechanism of action in patients who benefit most from Provenge treatment |
DENDREON
CORPORATION
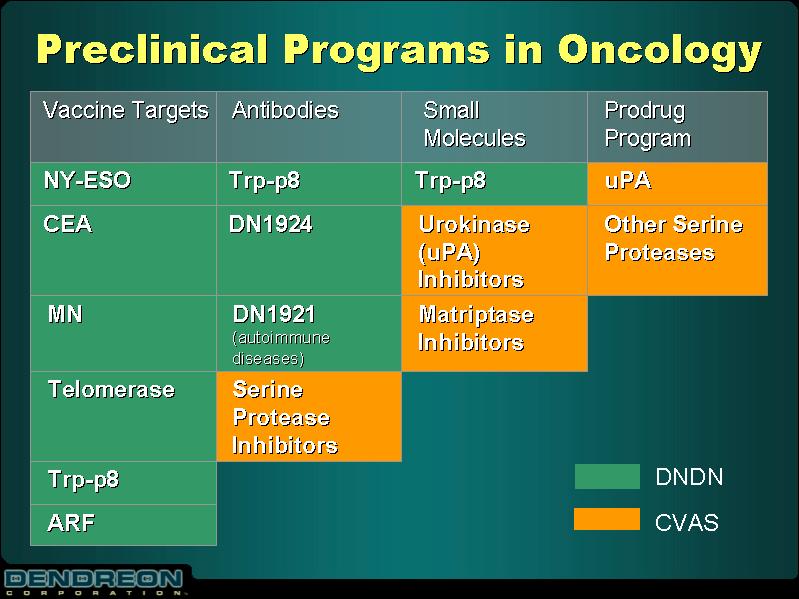
Preclinical Programs in Oncology
Vaccine Targets | | Antibodies | | Small Molecules | | Prodrug Program |
NY-ESO | | Trp-p8 | | Trp-p8 | | uPA |
CEA | | DN1924 | | Urokinase (uPA) Inhibitors | | Other Serine Proteases |
MN | | DN1921 (autoimmune diseases) | | Matriptase Inhibitors | | |
Telomerase | | Serine Protease Inhibitors | | | | |
Trp-p8 | | | | | | |
ARF | | | | | | |
DVDN CVAS | | | | | | |
DENDREON
CORPORATION

Combining Strengths
| Technology | |
| | | |
| Broad oncology portfolio covering 4 platforms | |
| | | |
| 5 ongoing clinical programs | |
| | | |
| Enhanced intellectual property estate, 87 issued U.S. patents | |
| | | |
| Small molecule, medicinal chemistry expertise | |
| | | |
| Operations | |
| | | |
| Experienced management team | |
| | | |
| Research and development expertise | |
| | | |
| Collaborations with industry leaders | |
| | | |
| Financial | |
| | | |
| Combined balance sheet of approximately $110M at 6/30/03 | |
| | | |
| Resources to fuel Provenge development | |
| | | |
| Resources to advance other clinical and preclinical programs | |
| | | |
| | | |













