| | | OMB APPROVAL |
| | | OMB Number: | 3235-0570 |
| | | Expires: | July 31, 2022 |
| | UNITED STATES | Estimated average burden hours per response. . . . . . . . . . . . . . .20.6 |
| | SECURITIES AND EXCHANGE COMMISSION | |
| | Washington, D.C. 20549 | |
FORM N-CSR
CERTIFIED SHAREHOLDER REPORT OF REGISTERED
MANAGEMENT INVESTMENT COMPANIES
| Investment Company Act file number | 811-06565 |
| |
| Tekla Life Sciences Investors |
| (Exact name of registrant as specified in charter) |
| |
| 100 Federal Street, 19th Floor, Boston, MA | | 02110 |
| (Address of principal executive offices) | | (Zip code) |
| |
| |
| (Name and address of agent for service) |
| |
| Registrant’s telephone number, including area code: | 617-772-8500 | |
| |
| Date of fiscal year end: | September 30 | |
| |
| Date of reporting period: | October 1, 2019 to March 31, 2020 | |
| | | | | | | | | | |
ITEM 1. REPORTS TO STOCKHOLDERS.
TEKLA LIFE SCIENCES INVESTORS



Semiannual Report
March 31, 2020
Beginning on January 1, 2021, as permitted by regulations adopted by the Securities and Exchange Commission, paper copies of the Fund's annual and semi-annual shareholder reports will no longer be sent by mail, unless you specifically request paper copies of the reports. Instead, the reports will be made available on the Fund's website, teklacap.com, and you will be notified by mail each time a report is posted and provided with a website link to access the report.
Beginning on January 1, 2019, you may elect to receive all future reports in paper free of charge. If you invest through a financial intermediary, you can contact your financial intermediary to request that you continue to receive paper copies of your shareholder reports. If you invest directly with the Fund, you can call Computershare at 1-800-426-5523 to inform the Fund that you wish to continue receiving paper copies of your shareholder reports. Your election to receive reports in paper will apply to all funds held in your account if you invest through your financial intermediary or all funds held with the fund complex if you invest directly with the Fund.
TEKLA LIFE SCIENCES INVESTORS
Distribution policy: The Fund has implemented a managed distribution policy (the Policy) that provides for quarterly distributions at a rate set by the Board of Trustees. Under the current Policy, the Fund intends to make quarterly distributions at a rate of 2% of the Fund's net assets to shareholders of record. The Policy would result in a return of capital to shareholders, if the amount of the distribution exceeds the Fund's net investment income and realized capital gains. A return of capital may occur, for example, when some or all of the money that you invested in the Fund is paid back to you. A return of capital distribution does not necessarily reflect the Fund's investment performance and should not be confused with "yield" or "income."
The amounts and sources of distributions reported in the Fund's notices pursuant to Section 19(a) of the Investment Company Act of 1940 are only estimates and are not being provided for tax reporting purposes. The actual amounts and sources of the amounts for tax reporting purposes will depend upon the Fund's investment experience during its fiscal year and may be subject to changes based on tax regulations. The Fund will send you a Form 1099-DIV for the calendar year that tells you how to report distributions for federal income tax purposes.
You should not draw any conclusions about the Fund's investment performance from the amount of distributions pursuant to the Policy or from the terms of the Policy. The Policy has been established by the Trustees and may be changed or terminated by them without shareholder approval. The Trustees regularly review the Policy and the frequency and rate of distributions considering the purpose and effect of the Policy, the financial market environment, and the Fund's income, capital gains and capital available to pay distributions. The suspension or termination of the Policy could have the effect of creating a trading discount or widening an existing trading discount. At this time there are no reasonably foreseeable circumstances that might cause the Trustees to terminate the Policy.
Consider these risks before investing: As with any investment company that invests in equity securities, the Fund is subject to market risk—the possibility that the prices of equity securities will decline over short or extended periods of time. As a result, the value of an investment in the Fund's shares will fluctuate with the market generally and market sectors in particular. You could lose money over short or long periods of time. Political and economic news can influence marketwide trends and can cause disruptions in the U.S. or world financial markets. Other factors may be ignored by the market as a whole but may cause movements in the price of one company's stock or the stock of companies in one or more industries. All of these factors may have a greater impact on initial public offerings and emerging company shares. Different types of equity securities tend to shift into and out of favor with investors, depending on market and economic conditions. The performance of funds that invest in equity securities of Healthcare Companies may at times be better or worse than the performance of funds that focus on other types of securities or that have a broader investment style.
TEKLA LIFE SCIENCES INVESTORS
Dear Shareholders,
At the moment, the healthcare sector is operating within an extremely complicated and volatile macroenvironment. The coronavirus epidemic came on very quickly and has changed the way that America and the world function. This impact may ultimately reverse but, at best, is here for some time.
It would have been hard to imagine, just a few weeks ago, that we would be in the clutches of a worldwide pandemic, sheltering in place, headed toward near-record unemployment and almost certainly in a significant recession. The economic and social situations are dire, people are sick and a significant number are dying.
From a macro perspective, it's true that we have seen and will likely continue to see staggering new jobless claim numbers. We will also almost certainly see an historic swing from robust growth to contraction/recession over just a few weeks' time. That is the bad news. It is surely bad, and I expect it will echo through the U.S. and the world for years to come.
But there is also some good news, generally and specifically for the healthcare sector. From a macro perspective, this tsunami of bad news has been met with a remarkable response locally by the U.S. government and the private sector, as well as globally by a host of other nations.
In the U.S., the Federal Reserve has provided an enormous, stimulatory response that was both rapid and large. In addition, the U.S. executive and legislative branches of government have been no less remarkable. An enormous $2T bailout package was passed by Congress and signed by the U.S. President in just a couple of weeks, remarkably fast given prior experience. There is little doubt that this rapid reaction was necessary to stabilize the economy as well as the equity and bond markets. And while the process wasn't pretty, and may have downstream consequences, the magnitude and timing of the response was up to the considerable task at hand.
One could make a similar analysis of the clinical/medical situation associated with the coronavirus epidemic. The first confirmed U.S. COVID-19 case was thought to have occurred in mid January 2020 at a time when the U.S. was embroiled in a remarkably partisan atmosphere surrounding the impeachment/trial of the U.S. President. Views can and certainly do differ, but approximately six to eight weeks later, the
1
government came together (at least a little bit) and much of the U.S. began sheltering at home. And now, a further six to eight weeks later there is hope that we have or will soon reach peak coronavirus case and death rates and may soon have the worst health crisis in a hundred years in some kind of initial control. If this is true, while we mourn the death of every person we have lost to coronavirus, we may now begin to think about the future in a constructive way.
So where does the healthcare sector fit into the current coronavirus situation? As has been widely reported, the U.S. stock market and nearly all subsectors including healthcare have been wildly volatile over the last eight to ten weeks. In that time frame the biotechnology sector exhibited a sudden and dramatic 24% drop from its most recent high, only to reverse and recover almost all of the earlier drop. Remarkably, the biotechnology sector was only down 1% year to date as of mid April, 2020. This compares favorably to the broad S&P 500® Index* ("SPX") which exhibited a similar path but was down approximately 13% in the same timeframe. There is little doubt that we will have some bad days (and hopefully some good days) ahead, but, given the enormous psychological and physical impact of the coronavirus epidemic, we would not have expected such a limited market drawdown.
We think it is interesting and instructive that healthcare has performed relatively well so far. We think this is due to several factors. It probably helps that the sector has traditionally been thought of as defensive. But more importantly, in a world where commerce has all but shut down, we think that healthcare is positioned to prevail. People will almost certainly continue to take, and pay for, their life saving medicines. Initial observations suggest this is occurring; the economic benefit of this will likely stabilize the healthcare industry in the short-term.
More importantly, it seems to us that healthcare will be a major part of the solution to the current crisis. Sheltering in place will help to "flatten" the services utilization curve. There are and will be many heroes to thank when this crisis is over. But it is the doctors and nurses and other medical professionals working diligently in the hospitals and other facilities that will directly save lives, often in the face of personal health risk. And while this effort is admirable in its own right, the fact of the matter is that much of the healthcare sector is working full time as many other sectors are waiting for the crisis to pass.
It is commonly accepted that, in the short-term, "testing" is what will get the economy back on its feet. And of course, it is the diagnostic healthcare companies that will create these tests, while it is other healthcare sector companies that will distribute and administer the tests.
2
Perhaps most significantly, in the intermediate-term, it is therapeutic medicines created by biotech and pharmaceutical companies that will improve the timing and outcomes for patients diagnosed with COVID-19. And of course, it will be vaccines produced by these same companies that will keep the world well and working long-term. It is amazing to note that upwards of a hundred companies are working to develop therapies or vaccines to address coronavirus just a couple months after the first U.S. case. For example, there is now promising initial data that suggest that Gilead Sciences, Inc.'s drug, remdesivir, may improve outcomes for hospitalized COVID-19 patients.
No one would ever wish that we had gotten to our current situation, but we really think it is the people and companies in the healthcare sector that, along with many others, will play an important role in solving, or at least reducing, the severity of the crisis in which we find ourselves. From firsthand experience I can tell you that this is fulfilling work for the inventors, scientists, physicians, manufacturing, sales and other individuals who do this work, especially at a time like this. But, somewhat sheepishly (due to the gravity of the current situation), I note that it is also a decent place to invest.
Be well,
Daniel R. Omstead
President and Portfolio Manager
3
TEKLA LIFE SCIENCES INVESTORS
Fund Essentials
Objective of the Fund
The Fund's investment objective is to seek long-term capital appreciation by investing primarily in securities of Life Sciences companies.
Description of the Fund
Tekla Life Sciences Investors ("HQL") is a non-diversified closed-end healthcare fund traded on the New York Stock Exchange under the ticker HQL. HQL primarily invests in the life sciences industries and will emphasize the smaller, emerging companies with a maximum of 40% of the Fund's assets in restricted securities of both public and private companies.
Investment Philosophy
Tekla Capital Management LLC, the Investment Adviser to the Fund, believes that:
• Aging demographics and adoption of new medical products and services can provide long-term tailwinds for healthcare companies
• Late stage biotechnology product pipeline could lead to significant increases in biotechnology sales
• Robust M&A activity in healthcare may create additional investment opportunities
Fund Overview and Characteristics as of 3/31/20
Market Price1 | | $14.98 | |
NAV2 | | $17.09 | |
Premium/(Discount) | | -12.35% | |
Average 30 Day Volume | | 169,026 | |
Net Assets | | $401,492,425 | |
Ticker | | HQL | |
NAV Ticker | | XHQLX | |
Commencement of
Operations Date | | 5/8/92 | |
Fiscal Year to Date
Distributions
Per Share | | $0.72 | |
1 The closing price at which the Fund's shares were traded on the exchange.
2 Per-share dollar value of the Fund, calculated by dividing the total value of all the securities in its portfolio, plus any other assets and less liabilities, by the number of Fund shares outstanding.
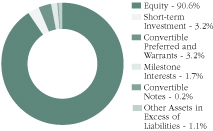
Holdings of the Fund (Data is based on net assets)
Asset Allocation as of 3/31/20
Sector Diversification as of 3/31/20
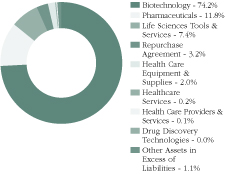
This data is subject to change on a daily basis.
4
TEKLA LIFE SCIENCES INVESTORS
Largest Holdings by Issuer
(Excludes Short-Term Investments)
As of March 31, 2020
(Unaudited)
| Issuer – Sector | | % of Net
Assets | |
| Gilead Sciences, Inc. – Biotechnology | | | 9.7 | % | |
| Vertex Pharmaceuticals, Inc. – Biotechnology | | | 8.0 | % | |
| Biogen, Inc. – Biotechnology | | | 7.7 | % | |
| Amgen, Inc. – Biotechnology | | | 7.6 | % | |
| Regeneron Pharmaceuticals, Inc. – Biotechnology | | | 5.9 | % | |
| Illumina, Inc. – Life Sciences Tools & Services | | | 5.4 | % | |
| Alexion Pharmaceuticals, Inc. – Biotechnology | | | 2.7 | % | |
| Incyte Corp. – Biotechnology | | | 2.4 | % | |
| Seattle Genetics, Inc. – Biotechnology | | | 2.1 | % | |
| BioMarin Pharmaceutical, Inc. – Biotechnology | | | 2.0 | % | |
| Neurocrine Biosciences, Inc. – Biotechnology | | | 1.5 | % | |
| Alnylam Pharmaceuticals, Inc. – Biotechnology | | | 1.4 | % | |
| Sarepta Therapeutics, Inc. – Biotechnology | | | 1.3 | % | |
| Horizon Therapeutics plc – Pharmaceuticals | | | 1.1 | % | |
| Mylan N.V. – Pharmaceuticals | | | 1.1 | % | |
| PRA Health Sciences, Inc. – Life Sciences Tools & Services | | | 0.9 | % | |
| Ionis Pharmaceuticals, Inc. – Biotechnology | | | 0.9 | % | |
| Bio-Techne Corp. – Life Sciences Tools & Services | | | 0.9 | % | |
| Impact Biomedicines Milestone Interest – Pharmaceuticals | | | 0.8 | % | |
| BeiGene Ltd. – Biotechnology | | | 0.8 | % | |
Fund Performance
HQL is a closed-end fund which invests predominantly in life science companies. Subject to regular consideration, the Trustees of HQL have instituted a policy of making quarterly distributions to shareholders. The Fund seeks to make such distributions in the form of long-term capital gains.
The Fund considers investments in companies of all sizes and in all life science subsectors, including but not limited to, biotechnology, pharmaceuticals, healthcare equipment, healthcare supplies, life science tools and services, healthcare distributors, managed healthcare, healthcare technology, and healthcare facilities. The Fund emphasizes innovation, investing both in public and pre-public venture companies. The Fund considers its venture investments to be a differentiating characteristic. Among the various healthcare subsectors, HQL has considered the
5
biotechnology subsector, including both pre-public and public companies, to be a key contributor to the healthcare sector. The Fund holds biotech assets, including both public and pre-public, often representing 65-80% of net assets.
There is no commonly published index which matches the investment strategy of HQL. The S&P Composite 1500® Health Care Index* ("S15HLTH") consists of approximately 170 companies representing most or all of the healthcare subsectors in which HQL typically invests; biotechnology often represents 15-20% of this index. By contrast, the NASDAQ Biotechnology Index®* ("NBI"), which contains approximately 210 constituents, is much more narrowly constructed. The vast majority of this index is comprised of biotechnology, pharmaceutical and life science tools companies. In recent years, biotechnology has often represented 72-82% of the NBI. Neither the S15HLTH nor NBI indices contain any material amount of pre-public company assets.
Given the circumstances, we present both NAV and stock returns for the Fund in comparison to several commonly published indices. One index, the SPX, is a commonly considered broad based index; this index is comprised of companies in many areas of the economy, including, but not limited to healthcare. As described above, the NBI is a healthcare index mostly focused in three healthcare sectors with a uniquely high level of biotechnology comparison. The S15HLTH contains a wider representation of healthcare subsectors, but typically contains a much lower biotechnology composition.
HQL generally invests in a combination of large-cap growth-oriented and earlier stage innovative healthcare companies with a focus on the biotechnology sector. Generally, HQL targets biotechnology exposure below that of the NBI and a higher biotechnology exposure than that of the S15HLTH. We note that in recent periods, biotechnology has been a significant contributor to returns (both positive and negative) associated with those indices. We believe this sector continues to have significant potential for growth in the future.
Fund Performance for the Period Ended March 31, 2020
Period | | HQL NAV | | HQL MKT | | NBI | | S15HLTH | | SPX | |
| 6 month | | | 8.47 | % | | | 4.21 | % | | | 8.77 | % | | | -0.53 | % | | | -12.32 | % | |
| 1 year | | | 0.58 | | | | -3.86 | | | | -2.84 | | | | -1.51 | | | | -6.99 | | |
| 5 year | | | 0.35 | | | | -2.53 | | | | -0.69 | | | | 6.20 | | | | 6.72 | | |
| 10 year | | | 12.98 | | | | 13.65 | | | | 14.24 | | | | 13.12 | | | | 10.52 | | |
6
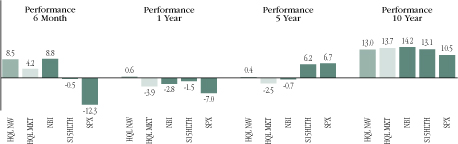
All performance over one-year has been annualized.
Performance data quoted represents past performance, which is no guarantee of future results, and current performance may be lower or higher than the figures shown. The NAV total return takes into account the Fund's total annual expenses and does not reflect transaction charges. If transaction charges were reflected, NAV total return would be reduced. All distributions are assumed to be reinvested either in accordance with the dividend reinvestment plan (DRIP) for market price returns or NAV for NAV returns. Until the DRIP price is available from the Plan Agent, the market price returns reflect the reinvestment at the closing market price on the last business day of the month. Once the DRIP is available around mid-month, the market price returns are updated to reflect reinvestment at the DRIP price.
Portfolio Highlights as of March 31, 2020
Among other investments, Tekla Life Sciences Investors' performance benefitted in the past year by the following:
Avadel Pharmaceuticals plc (AVDL) is a specialty pharmaceutical company domiciled in Ireland developing FT218, the most clinically advanced once-nightly formulation of the sleep promoting drug, sodium oxybate, for the treatment of excessive daytime sleepiness (EDS) and cataplexy in patients suffering from narcolepsy. The market for EDS and cataplexy is well-developed and currently monopolized by a twice-nightly formulation of sodium oxybate (Xyrem; $1.6B sales in 2019) from Jazz Pharmaceuticals, Inc.. Twice-nightly dosing of Xyrem means patients must interrupt the very sleep they are trying to restore to properly take the sleep drug.
Bristol-Myers Squibb Co. (BMY) is a large-cap pharmaceutical company with key franchises in oncology, cardiovascular disease, and immunology. In November 2019, the company completed its acquisition of Celgene Corp., bringing in several assets in the hematology-oncology space. While the combined company faces patent expiries of several of its key blockbusters over the next decade (e.g., Revlimid, Opdivo, and Eliquis), we believe it has a strong pipeline.
Epizyme, Inc. (EPZM) is an oncology-focused biotech company with a recent approval for a small indication in sarcomas for their lead drug Tazverik. This approval raised the chance that the drug would be approved for the much larger follicular lymphoma indication later this summer.
7
Among other examples, Tekla Life Sciences Investors' performance was negatively impacted by the following investments:
Forty Seven, Inc. (FTSV) was an immune-oncology company recently acquired by Gilead for $4.9 billion. We had been fans of the story early on based on their lead agent's mechanism of action but had gotten more skeptical on its future in the treatment of solid tumors and chronic stock weakness until they demonstrated stronger than expected data in certain subtypes of leukemias. The Fund was underweight FTSV during this reporting period.
Moderna, Inc. (MRNA) is a vaccine company based on a new therapeutic modality, messenger RNA. The company has multiple programs in the clinic, most notably a recent vaccine candidate targeting the COVID-19 virus. We have been historically cautious on this name due to valuation and the risk of the platform and consequently missed the recent runup.
Milestone Pharmaceuticals, Inc. (MIST) is a cardiovascular focused biotech company with an inhaled therapy for episodic cardiovascular disorders, in particular PSVT. The stock was weak in the quarter because their lead agent etripamil failed in their pivotal phase III study. We had previously taken advantage of significant pre-study strength to lock in gains on a portion of the Fund's MIST stock.
*The trademarks NASDAQ Biotechnology Index®, S&P Composite 1500® Health Care Index and S&P 500® Index referenced in this report are the property of their respective owners. These trademarks are not owned by or associated with the Fund or its service providers, including Tekla Capital Management LLC.
8
TEKLA LIFE SCIENCES INVESTORS
SCHEDULE OF INVESTMENTS
MARCH 31, 2020
(Unaudited)
SHARES | | CONVERTIBLE PREFERRED AND WARRANTS
(Restricted) (a) (b) - 3.2% of Net Assets | |
VALUE | |
| | | Biotechnology - 2.5% | |
| | 140,000 | | | Amphivena Therapeutics, Inc. Series B, 6.00% | | $ | 2,100,000 | | |
| | 225,416 | | | Amphivena Therapeutics, Inc. Series C, 6.00% | | | 808,848 | | |
| | 343,919 | | | Arkuda Therapeutics, Inc. Series A, 6.00% | | | 818,183 | | |
| | 398,616 | | | Decipher Biosciences, Inc. Series II, 8.00% | | | 1,000,566 | | |
| | 396,284 | | | Decipher Biosciences, Inc. Series III, 8.00% | | | 994,712 | | |
| | 117,704 | | | Oculis SA, Series B2, 6.00% (c) | | | 1,018,185 | | |
| | 1,153,847 | | | Rainier Therapeutics, Inc. Series A, 6.00% | | | 115 | | |
| | 668,449 | | | Rainier Therapeutics, Inc. Series B, 6.00% | | | 67 | | |
| | 2,091,203 | | | Rallybio Holdings, LLC Series B | | | 3,099,999 | | |
| | | | 9,840,675 | | |
| | | Health Care Equipment & Supplies (d) - 0.0% | |
| | 951,000 | | | IlluminOss Medical, Inc. Series AA, 8.00% | | | 95 | | |
| | 895,848 | | | IlluminOss Medical, Inc. Junior Preferred, 8.00% | | | 90 | | |
| | 71,324 | | | IlluminOss Medical, Inc. Warrants (expiration
03/31/27, exercise price $1.00) | | | 0 | | |
| | 59,426 | | | IlluminOss Medical, Inc. Warrants (expiration
09/06/27, exercise price $1.00) | | | 0 | | |
| | 23,771 | | | IlluminOss Medical, Inc. Warrants (expiration
11/20/27, exercise price $1.00) | | | 0 | | |
| | 47,542 | | | IlluminOss Medical, Inc. Warrants (expiration
01/11/28, exercise price $1.00) | | | 0 | | |
| | 47,542 | | | IlluminOss Medical, Inc. Warrants (expiration
02/06/28, exercise price $1.00) | | | 0 | | |
| | 46,462 | | | IlluminOss Medical, Inc. Warrants (expiration
01/29/29, exercise price $1.00) | | | 0 | | |
| | 12,964 | | | IlluminOss Medical, Inc. Warrants (expiration
04/29/29, exercise price $1.00) | | | 0 | | |
| | 20,470 | | | IlluminOss Medical, Inc. Warrants (expiration
05/13/29, exercise price $1.00) | | | 0 | | |
| | 28,301 | | | IlluminOss Medical, Inc. Warrants (expiration
07/02/29, exercise price $1.00) | | | 0 | | |
| | 9,250 | | | IlluminOss Medical, Inc. Warrants (expiration
08/29/29, exercise price $1.00) | | | 0 | | |
| | 53,690 | | | IlluminOss Medical, Inc. Warrants (expiration
09/27/29, exercise price $1.00) | | | 0 | | |
| | 30,834 | | | IlluminOss Medical, Inc. Warrants (expiration
01/08/30, exercise price $1.00) | | | 0 | | |
| | 15,417 | | | IlluminOss Medical, Inc. Warrants (expiration
03/06/30, exercise price $1.00) | | | 0 | | |
| | | | 185 | | |
The accompanying notes are an integral part of these financial statements.
9
TEKLA LIFE SCIENCES INVESTORS
SCHEDULE OF INVESTMENTS
MARCH 31, 2020
(Unaudited, continued)
SHARES | | Pharmaceuticals - 0.7% | | VALUE | |
| | 2,719,854 | | | Curasen Therapeutics, Inc. Series A | | $ | 2,999,999 | | |
| | | | | TOTAL CONVERTIBLE PREFERRED AND
WARRANTS
(Cost $16,862,115) | | | 12,840,859 | | |
PRINCIPAL
AMOUNT | | CONVERTIBLE NOTES (Restricted) (b) - 0.2%
of Net Assets | |
| |
| | | Biotechnology - 0.0% | |
$ | 652,728 | | | Rainier Therapeutics, Inc. Promissory Notes,
8.00% due 12/31/20 | | | 194,493 | | |
| | | Health Care Equipment & Supplies (d) - 0.2% | |
| | 1,867,973 | | | IlluminOss Medical, Inc. Promissory Notes,
8.00% due 12/31/21 | | | 723,094 | | |
| | | | | TOTAL CONVERTIBLE NOTES
(Cost $2,521,488) | | | 917,587 | | |
SHARES | | COMMON STOCKS AND WARRANTS - 90.6%
of Net Assets | |
| |
| | | Biotechnology - 71.4% | |
| | 52,739 | | | AC Immune SA (a) (c) | | | 364,426 | | |
| | 77,207 | | | ACADIA Pharmaceuticals, Inc. (a) | | | 3,261,996 | | |
| | 7,425 | | | Adynxx, Inc. | | | 2,599 | | |
| | 121,312 | | | Alexion Pharmaceuticals, Inc. (a) | | | 10,892,604 | | |
| | 60,092 | | | Alkermes plc (a) | | | 866,527 | | |
| | 22,188 | | | Allakos, Inc. (a) | | | 987,144 | | |
| | 84,929 | | | Allogene Therapeutics, Inc. (a) | | | 1,651,020 | | |
| | 51,404 | | | Alnylam Pharmaceuticals, Inc. (a) | | | 5,595,325 | | |
| | 215,156 | | | Amarin Corp. plc (a) (c) (e) | | | 860,624 | | |
| | 150,081 | | | Amgen, Inc. | | | 30,425,921 | | |
| | 112,529 | | | Amicus Therapeutics, Inc. (a) | | | 1,039,768 | | |
| | 36,239 | | | AnaptysBio, Inc. (a) | | | 512,057 | | |
| | 59,588 | | | Apellis Pharmaceuticals, Inc. (a) | | | 1,596,363 | | |
| | 36,068 | | | ARCA biopharma, Inc. (a) | | | 99,548 | | |
| | 18,027 | | | ARCA biopharma, Inc. Warrants (expiration
07/23/44, exercise price $1.10) (a) (b) | | | 180 | | |
| | 289,336 | | | Ardelyx, Inc. (a) | | | 1,644,875 | | |
| | 13,696 | | | Arena Pharmaceuticals, Inc. (a) | | | 575,232 | | |
| | 15,551 | | | Argenx SE (a) (c) (e) | | | 2,048,533 | | |
| | 33,666 | | | Arrowhead Pharmaceuticals, Inc. (a) | | | 968,571 | | |
| | 26,178 | | | Ascendis Pharma A/S (a) (e) | | | 2,947,905 | | |
| | 48,919 | | | Atreca, Inc. (a) | | | 809,609 | | |
The accompanying notes are an integral part of these financial statements.
10
TEKLA LIFE SCIENCES INVESTORS
SCHEDULE OF INVESTMENTS
MARCH 31, 2020
(Unaudited, continued)
SHARES | | Biotechnology - continued | | VALUE | |
| | 37,142 | | | Avrobio, Inc. (a) | | $ | 577,930 | | |
| | 26,592 | | | BeiGene Ltd. (a) (c) (e) | | | 3,273,741 | | |
| | 6,000 | | | Bellicum Pharmaceuticals, Inc (a) | | | 27,840 | | |
| | 97,312 | | | Biogen, Inc. (a) | | | 30,787,571 | | |
| | 13,910 | | | Biohaven Pharmaceutical Holding Co., Ltd. (a) | | | 473,357 | | |
| | 97,312 | | | BioMarin Pharmaceutical, Inc. (a) | | | 8,222,864 | | |
| | 33,924 | | | bluebird bio, Inc. (a) | | | 1,559,147 | | |
| | 47,578 | | | Blueprint Medicines Corp. (a) | | | 2,782,361 | | |
| | 12,201 | | | Bridgebio Pharma, Inc. (a) | | | 353,829 | | |
| | 47,437 | | | Cellectis S.A. (a) (c) (e) | | | 436,420 | | |
| | 195,256 | | | Cidara Therapeutics, Inc. (a) | | | 484,235 | | |
| | 48,003 | | | Coherus BioSciences, Inc. (a) | | | 778,609 | | |
| | 23,648 | | | CRISPR Therapeutics AG (a) (c) | | | 1,002,912 | | |
| | 32,636 | | | CymaBay Therapeutics, Inc. (a) | | | 48,301 | | |
| | 19,036 | | | Deciphera Pharmaceuticals, Inc. (a) | | | 783,712 | | |
| | 49,733 | | | Editas Medicine, Inc. (a) | | | 986,205 | | |
| | 6,118 | | | Eidos Therapeutics, Inc. (a) | | | 299,721 | | |
| | 71,778 | | | Epizyme, Inc. (a) | | | 1,113,277 | | |
| | 14,732 | | | Esperion Therapeutics, Inc. (a) | | | 464,500 | | |
| | 7,189 | | | Exact Sciences Corp. (a) | | | 416,962 | | |
| | 146,366 | | | Exelixis, Inc. (a) | | | 2,520,423 | | |
| | 59,161 | | | Fate Therapeutics, Inc. (a) | | | 1,313,966 | | |
| | 67,921 | | | FibroGen, Inc. (a) | | | 2,360,255 | | |
| | 58,333 | | | Galera Therapeutics, Inc. (a) | | | 554,163 | | |
| | 87,525 | | | Galera Therapeutics, Inc. (Restricted) (a) (b) | | | 748,341 | | |
| | 5,138 | | | Genmab A/S (a) (c) (e) | | | 108,874 | | |
| | 519,586 | | | Gilead Sciences, Inc. | | | 38,844,249 | | |
| | 40,093 | | | Global Blood Therapeutics, Inc. (a) | | | 2,048,351 | | |
| | 21,774 | | | Halozyme Therapeutics, Inc. (a) | | | 391,714 | | |
| | 132,837 | | | Incyte Corp. (a) | | | 9,727,654 | | |
| | 37,238 | | | Insmed, Inc. (a) | | | 596,925 | | |
| | 14,034 | | | Intellia Therapeutics, Inc. (a) | | | 171,636 | | |
| | 13,082 | | | Intercept Pharmaceuticals, Inc. (a) | | | 823,643 | | |
| | 75,143 | | | Ionis Pharmaceuticals, Inc. (a) | | | 3,552,761 | | |
| | 85,305 | | | Iovance Biotherapeutics, Inc. (a) | | | 2,553,605 | | |
| | 11,225 | | | Ligand Pharmaceuticals, Inc. (a) | | | 816,282 | | |
| | 16,757 | | | Magenta Therapeutics, Inc. (a) | | | 105,234 | | |
| | 6,276 | | | Merus N.V. (a) (c) | | | 75,940 | | |
| | 79,103 | | | Moderna, Inc. (a) | | | 2,369,135 | | |
| | 18,261 | | | Molecular Templates, Inc. (a) | | | 242,689 | | |
| | 7,741 | | | Momenta Pharmaceuticals, Inc. (a) | | | 210,555 | | |
| | 80,164 | | | Nektar Therapeutics (a) | | | 1,430,927 | | |
| | 70,393 | | | Neurocrine Biosciences, Inc. (a) | | | 6,092,514 | | |
The accompanying notes are an integral part of these financial statements.
11
TEKLA LIFE SCIENCES INVESTORS
SCHEDULE OF INVESTMENTS
MARCH 31, 2020
(Unaudited, continued)
SHARES | | Biotechnology - continued | | VALUE | |
| | 44,550 | | | NexGel, Inc. (a) (b) | | $ | 2,383 | | |
| | 17,900 | | | Novavax, Inc. (a) | | | 243,082 | | |
| | 111,820 | | | Ovid Therapeutics, Inc. (a) | | | 333,224 | | |
| | 374,819 | | | Pieris Pharmaceuticals, Inc. (a) | | | 854,587 | | |
| | 23,821 | | | Pieris Pharmaceuticals, Inc., Series A Warrants
(expiration 06/08/21, exercise price $3.00) (a) (b) | | | 13,578 | | |
| | 11,911 | | | Pieris Pharmaceuticals, Inc., Series B Warrants
(expiration 06/08/21, exercise price $2.00) (a) (b) | | | 9,410 | | |
| | 42,810 | | | Portola Pharmaceuticals, Inc. (a) | | | 305,235 | | |
| | 42,892 | | | PTC Therapeutics, Inc. (a) | | | 1,913,412 | | |
| | 23,629 | | | Puma Biotechnology, Inc. (a) | | | 199,429 | | |
| | 20,087 | | | Ra Pharmaceuticals, Inc. (a) | | | 964,377 | | |
| | 48,861 | | | Regeneron Pharmaceuticals, Inc. (a) | | | 23,858,338 | | |
| | 63,455 | | | Rocket Pharmaceuticals, Inc. (a) | | | 885,197 | | |
| | 59,709 | | | Rubius Therapeutics, Inc. (a) | | | 265,705 | | |
| | 33,741 | | | Sage Therapeutics, Inc. (a) | | | 969,042 | | |
| | 45,979 | | | Sangamo Therapeutics, Inc. (a) | | | 292,886 | | |
| | 51,660 | | | Sarepta Therapeutics, Inc. (a) | | | 5,053,381 | | |
| | 74,285 | | | Seattle Genetics, Inc. (a) | | | 8,571,003 | | |
| | 193,471 | | | Sutro Biopharma, Inc. (a) | | | 1,973,404 | | |
| | 131,785 | | | Trillium Therapeutics, Inc. (a) | | | 532,411 | | |
| | 29,770 | | | Ultragenyx Pharmaceutical, Inc. (a) | | | 1,322,681 | | |
| | 19,751 | | | uniQure N.V. (a) (c) | | | 937,185 | | |
| | 20,511 | | | United Therapeutics Corp. (a) | | | 1,944,956 | | |
| | 375,000 | | | Vectivbio Holding AG (Restricted) (a) (b) | | | 296,250 | | |
| | 135,144 | | | Vertex Pharmaceuticals, Inc. (a) | | | 32,157,515 | | |
| | 500 | | | Viela Bio, Inc (a) | | | 19,000 | | |
| | 46,595 | | | Xencor, Inc. (a) | | | 1,392,259 | | |
| | 23,842 | | | Y-mAbs Therapeutics, Inc. (a) | | | 622,276 | | |
| | 11,502 | | | Zai Lab Ltd. (a) (c) (e) | | | 592,123 | | |
| | 15,192 | | | Zymeworks, Inc. (a) (e) | | | 538,860 | | |
| | | | 286,747,346 | | |
| | | Drug Discovery Technologies (a) - 0.0% | |
| | 51,160 | | | ImmunoGen, Inc. | | | 174,456 | | |
| | | Health Care Equipment & Supplies - 1.8% | |
| | 8,700 | | | Axonics Modulation Technologies, Inc. (a) | | | 221,067 | | |
| | 130,000 | | | Cercacor Laboratories, Inc. (Restricted) (a) (b) | | | 856,108 | | |
| | 7,366 | | | Guardant Health, Inc. (a) | | | 512,674 | | |
| | 8,329 | | | IDEXX Laboratories, Inc. (a) | | | 2,017,617 | | |
| | 5,042 | | | Inogen, Inc. (a) | | | 260,470 | | |
| | 39,858 | | | NovoCure Ltd. (a) | | | 2,684,038 | | |
| | 121,721 | | | Quotient Ltd. (a) | | | 480,798 | | |
| | | | 7,032,772 | | |
The accompanying notes are an integral part of these financial statements.
12
TEKLA LIFE SCIENCES INVESTORS
SCHEDULE OF INVESTMENTS
MARCH 31, 2020
(Unaudited, continued)
SHARES | | Health Care Providers & Services - 0.1% | | VALUE | |
| | 148,148 | | | InnovaCare, Inc. Escrow Shares (Restricted) (a) (b) | | $ | 136,504 | | |
| | 5,822 | | | Medpace Holdings, Inc. (a) | | | 427,218 | | |
| | | | 563,722 | | |
| | | Healthcare Services - 0.2% | |
| | 24,811 | | | Syneos Health, Inc. (a) | | | 978,050 | | |
| | | Life Sciences Tools & Services - 7.3% | |
| | 18,419 | | | Bio-Techne Corp. | | | 3,492,611 | | |
| | 79,850 | | | Illumina, Inc. (a) | | | 21,808,632 | | |
| | 151,480 | | | Pacific Biosciences of California, Inc. (a) | | | 463,529 | | |
| | 45,069 | | | PRA Health Sciences, Inc. (a) | | | 3,742,530 | | |
| | | | 29,507,302 | | |
| | | Pharmaceuticals - 9.7% | |
| | 16,387 | | | Acceleron Pharma, Inc. (a) | | | 1,472,700 | | |
| | 20,403 | | | Aerie Pharmaceuticals, Inc. (a) | | | 275,440 | | |
| | 79,542 | | | Aerpio Pharmaceuticals, Inc. (a) | | | 43,732 | | |
| | 63,808 | | | Agios Pharmaceuticals, Inc. (a) | | | 2,263,908 | | |
| | 30,700 | | | Aurinia Pharmaceuticals, Inc. (a) (c) | | | 445,457 | | |
| | 236,018 | | | Avadel Pharmaceuticals plc (a) (e) | | | 1,873,983 | | |
| | 9,784 | | | Axsome Therapeutics, Inc. (a) | | | 575,593 | | |
| | 18,008 | | | Bristol-Myers Squibb Co. | | | 1,003,766 | | |
| | 399,550 | | | Bristol-Myers Squibb Co. CVR 03/31/21 (a) (f) | | | 1,518,290 | | |
| | 165,330 | | | Clearside Biomedical, Inc. (a) | | | 281,061 | | |
| | 29,100 | | | Endo International plc (a) | | | 107,670 | | |
| | 90,238 | | | Foamix Pharmaceuticals Ltd. (a) (b) (c) (g) | | | 54,143 | | |
| | 17,210 | | | GW Pharmaceuticals plc (a) (c) (e) | | | 1,507,080 | | |
| | 40,585 | | | Heron Therapeutics, Inc. (a) | | | 476,468 | | |
| | 150,867 | | | Horizon Therapeutics plc (a) | | | 4,468,680 | | |
| | 152,830 | | | Immunomedics, Inc. (a) | | | 2,060,148 | | |
| | 34,082 | | | Intra-Cellular Therapies, Inc. (a) | | | 523,840 | | |
| | 25,203 | | | Jazz Pharmaceuticals plc (a) | | | 2,513,747 | | |
| | 7,187 | | | Kodiak Sciences, Inc. (a) | | | 342,820 | | |
| | 4,060 | | | Madrigal Pharmaceuticals, Inc. (a) | | | 271,046 | | |
| | 53,456 | | | Menlo Therapeutics, Inc. (a) | | | 143,262 | | |
| | 63,994 | | | Milestone Pharmaceuticals, Inc. (a) | | | 117,749 | | |
| | 27,127 | | | Mirati Therapeutics, Inc. (a) | | | 2,085,252 | | |
| | 294,349 | | | Mylan N.V. (a) | | | 4,388,744 | | |
| | 5,870 | | | MyoKardia, Inc. (a) | | | 275,186 | | |
| | 11,588 | | | Reata Pharmaceuticals, Inc. (a) | | | 1,672,612 | | |
| | 26,600 | | | Revance Therapeutics, Inc. (a) | | | 393,680 | | |
| | 58,272 | | | Sanofi (c) (e) | | | 2,547,652 | | |
| | 34,880 | | | Spectrum Pharmaceuticals, Inc. (a) | | | 81,270 | | |
The accompanying notes are an integral part of these financial statements.
13
TEKLA LIFE SCIENCES INVESTORS
SCHEDULE OF INVESTMENTS
MARCH 31, 2020
(Unaudited, continued)
SHARES | | Pharmaceuticals - continued | | VALUE | |
| | 14,218 | | | Tetraphase Pharmaceuticals, Inc. (a) | | $ | 18,199 | | |
| | 73,516 | | | Theravance Biopharma, Inc. (a) | | | 1,698,955 | | |
| | 1,766 | | | Turning Point Therapeutics, Inc. (a) | | | 78,870 | | |
| | 929,053 | | | Verona Pharma plc (a) (c) | | | 605,838 | | |
| | 106,335 | | | Verona Pharma plc (a) (e) | | | 438,100 | | |
| | 371,622 | | | Verona Pharma plc Warrants (expiration
04/27/22, exercise price $2.07) (a) (b) (c) | | | 15,556 | | |
| | 40,580 | | | WaVe Life Sciences Ltd. (a) | | | 380,235 | | |
| | 71,950 | | | Zogenix, Inc. (a) | | | 1,779,323 | | |
| | | | 38,800,055 | | |
| | | | | TOTAL COMMON STOCKS AND WARRANTS
(Cost $316,462,858) | | | 363,803,703 | | |
PRINCIPAL
AMOUNT | | SHORT-TERM INVESTMENT - 3.1% of Net Assets | |
| |
$ | 12,625,000 | | | Repurchase Agreement, Fixed Income Clearing
Corp., repurchase value $12,625,000, 0.00%,
dated 03/31/20, due 04/01/20 (collateralized
by U.S. Treasury Note 1.38%, due 10/15/22,
market value $12,880,048) | | | 12,625,000 | | |
| | | | | TOTAL SHORT-TERM INVESTMENT
(Cost $12,625,000) | | | 12,625,000 | | |
| | | | | TOTAL INVESTMENTS BEFORE MILESTONE
INTERESTS - 97.2%
(Cost $348,471,461) | | | 390,187,149 | | |
INTERESTS | | MILESTONE INTERESTS (Restricted) (a) (b) - 1.7%
of Net Assets | |
| |
| | | Biotechnology - 0.3% | |
| | 1 | | | Therachon Milestone Interest | | | 1,445,446 | | |
| | | Health Care Equipment & Supplies - 0.0% | |
| | 1 | | | Therox Milestone Interest | | | 4,604 | | |
| | | Pharmaceuticals - 1.4% | |
| | 1 | | | Afferent Milestone Interest | | | 251,261 | | |
| | 1 | | | Ethismos Research Milestone Interest | | | 0 | | |
| | 1 | | | Neurovance Milestone Interest | | | 1,932,961 | | |
| | 1 | | | Impact Biomedicines Milestone Interest | | | 3,301,998 | | |
| | | | 5,486,220 | | |
| | | | | TOTAL MILESTONE INTERESTS
(Cost $4,995,390) | | | 6,936,270 | | |
The accompanying notes are an integral part of these financial statements.
14
TEKLA LIFE SCIENCES INVESTORS
SCHEDULE OF INVESTMENTS
MARCH 31, 2020
(Unaudited, continued)
AMOUNT ($) NUMBER OF
CONTRACTS
(100 SHARES
EACH)/
NOTIONAL | |
CALL OPTION CONTRACTS PURCHASED - 0.0%
of Net Assets | |
VALUE | |
48/1,680,000 | | Biogen, Inc. Jun20 350 Call | | $ | 81,600 | | |
| | | TOTAL CALL OPTION CONTRACTS
PURCHASED
(Premiums paid $247,467) | | | 81,600 | | |
| | | TOTAL INVESTMENTS - 98.9%
(Cost $353,714,318) | | | 397,205,019 | | |
| | | OTHER ASSETS IN EXCESS OF
LIABILITIES - 1.1% | | | 4,287,406 | | |
| | | NET ASSETS - 100% | | $ | 401,492,425 | | |
(a) Non-income producing security.
(b) Security fair valued using significant unobservable inputs. See Investment Valuation and Fair Value Measurements.
(c) Foreign security.
(d) Affiliated issuers in which the Fund holds 5% or more of the voting securities (total market value of $723,279).
(e) American Depository Receipt
(f) Contingent Value Right
(g) Contingent Stock Right
The accompanying notes are an integral part of these financial statements.
15
TEKLA LIFE SCIENCES INVESTORS
STATEMENT OF ASSETS AND LIABILITIES
MARCH 31, 2020
(Unaudited)
ASSETS: | |
Investments in unaffiliated issuers, at value
(cost $344,321,776) | | $ | 389,545,470 | | |
Investments in affiliated issuers, at value
(cost $4,397,152) | | | 723,279 | | |
Milestone interests, at value (cost $4,995,390) | | | 6,936,270 | | |
Total investments | | | 397,205,019 | | |
Cash | | | 803 | | |
Dividends and interest receivable | | | 278,301 | | |
Receivable for investments sold | | | 4,182,344 | | |
Prepaid expenses | | | 34,558 | | |
Other assets (see Note 1) | | | 376,587 | | |
Total assets | | | 402,077,612 | | |
LIABILITIES: | |
Accrued advisory fee | | | 357,630 | | |
Accrued investor support service fees | | | 18,203 | | |
Accrued shareholder reporting fees | | | 57,244 | | |
Accrued other | | | 152,110 | | |
Total liabilities | | | 585,187 | | |
Commitments and Contingencies (see Notes 1 and 5) | | | |
NET ASSETS | | $ | 401,492,425 | | |
SOURCES OF NET ASSETS: | |
Shares of beneficial interest, par value $.01 per share,
unlimited number of shares authorized, amount
paid in on 23,494,847 shares issued and outstanding | | $ | 349,632,625 | | |
Total distributable earnings (loss) | | | 51,859,800 | | |
Total net assets (equivalent to $17.09 per share
based on 23,494,847 shares outstanding) | | $ | 401,492,425 | | |
The accompanying notes are an integral part of these financial statements.
16
TEKLA LIFE SCIENCES INVESTORS
STATEMENT OF OPERATIONS
SIX MONTHS ENDED MARCH 31, 2020
(Unaudited)
INVESTMENT INCOME: | |
Dividend income | | $ | 2,058,098 | | |
Interest and other income from unaffiliated issuers | | | 51,387 | | |
Interest income from affiliated issuers | | | 70,129 | | |
Total investment income | | | 2,179,614 | | |
EXPENSES: | |
Advisory fees | | | 2,119,052 | | |
Investor support service fees | | | 107,355 | | |
Trustees' fees and expenses | | | 62,393 | | |
Custodian fees | | | 59,452 | | |
Shareholder reporting | | | 58,018 | | |
Administration fees | | | 51,568 | | |
Auditing fees | | | 54,403 | | |
Legal fees | | | 36,092 | | |
Transfer agent fees | | | 26,871 | | |
Other (see Note 2) | | | 68,452 | | |
Total expenses | | | 2,643,656 | | |
Net investment loss | | | (464,042 | ) | |
REALIZED AND UNREALIZED GAIN (LOSS): | |
Net realized gain (loss) on: | |
Investments in unaffiliated issuers | | | 20,393,015 | | |
Foreign currency transactions | | | 1,336 | | |
Net realized gain | | | 20,394,351 | | |
Change in unrealized appreciation (depreciation) on: | |
Investments in unaffiliated issuers | | | 12,186,213 | | |
Investments in affiliated issuers | | | (1,937,444 | ) | |
Milestone interests | | | (56,182 | ) | |
Option contracts purchased | | | 68,640 | | |
Change in unrealized appreciation (depreciation) | | | 10,261,227 | | |
Net realized and unrealized gain (loss) | | | 30,655,578 | | |
Net increase in net assets resulting from
operations | | $ | 30,191,536 | | |
The accompanying notes are an integral part of these financial statements.
17
TEKLA LIFE SCIENCES INVESTORS
STATEMENTS OF CHANGES IN NET ASSETS
| | | Six months ended
March 31, 2020
(Unaudited) | | Year ended
September 30,
2019 | |
NET INCREASE (DECREASE) IN NET
ASSETS RESULTING FROM OPERATIONS: | |
Net investment loss | | ($ | 464,042 | ) | | ($ | 2,879,038 | ) | |
Net realized gain | | | 20,394,351 | | | | 35,802,005 | | |
Change in net unrealized appreciation
(depreciation) | | | 10,261,227 | | | | (103,401,585 | ) | |
Net increase (decrease) in net
assets resulting from operations | | | 30,191,536 | | | | (70,478,618 | ) | |
DISTRIBUTIONS TO SHAREHOLDERS
FROM (See Note 1): | |
Distributions | | | (16,689,383 | ) | | | (33,775,050 | ) | |
Total distributions | | | (16,689,383 | ) | | | (33,775,050 | ) | |
CAPITAL SHARE TRANSACTIONS: | |
Reinvestment of distributions
(451,637 and 880,878 shares,
respectively) | | | 6,530,269 | | | | 13,969,734 | | |
Fund shares repurchased
(136,700 and 107,109 shares,
respectively) (see Note 1) | | | (2,191,363 | ) | | | (1,556,624 | ) | |
Total capital share transactions | | | 4,338,906 | | | | 12,413,110 | | |
Net increase (decrease) in
net assets | | | 17,841,059 | | | | (91,840,558 | ) | |
NET ASSETS: | |
Beginning of period | | | 383,651,366 | | | | 475,491,924 | | |
End of period | | $ | 401,492,425 | | | $ | 383,651,366 | | |
The accompanying notes are an integral part of these financial statements.
18
TEKLA LIFE SCIENCES INVESTORS
| | | Six months
ended
March 31, 2020 | | Years ended September 30, | |
| | | (Unaudited) | | 2019 | | 2018 | | 2017 | | 2016 | | 2015 | |
OPERATING PERFORMANCE FOR A SHARE
OUTSTANDING THROUGHOUT EACH PERIOD | |
Net asset value per share,
beginning of period | | $ | 16.55 | | | $ | 21.22 | | | $ | 21.62 | | | $ | 20.00 | | | $ | 23.51 | | | $ | 23.37 | | |
Net investment loss (1) | | | (0.02 | ) | | | (0.13 | ) | | | (0.16 | ) | | | (0.18 | ) | | | (0.19 | ) | | | (0.25 | ) | |
Net realized and unrealized
gain (loss) | | | 1.27 | | | | (3.06 | ) | | | 1.39 | | | | 3.39 | | | | (0.47 | ) | | | 2.48 | | |
Total increase (decrease)
from investment operations | | | 1.25 | | | | (3.19 | ) | | | 1.23 | | | | 3.21 | | | | (0.66 | ) | | | 2.23 | | |
Distributions to shareholders from: | |
Net investment income | | | — | | | | (0.13 | ) | | | (0.38 | )(2) | | | (0.05 | )(2) | | | — | | | | (1.12 | )(2) | |
Net realized capital gains | | | (0.72 | ) | | | (1.36 | ) | | | (1.25 | )(2) | | | (1.54 | )(2) | | | (2.85 | ) | | | (0.97 | )(2) | |
Total distributions | | | (0.72 | ) | | | (1.49 | ) | | | (1.63 | ) | | | (1.59 | ) | | | (2.85 | ) | | | (2.09 | ) | |
Increase resulting from
shares repurchased (1) | | | 0.01 | | | | 0.01 | | | | — | | | | — | (3) | | | — | | | | — | | |
Net asset value per share,
end of period | | $ | 17.09 | | | $ | 16.55 | | | $ | 21.22 | | | $ | 21.62 | | | $ | 20.00 | | | $ | 23.51 | | |
Per share market value,
end of period | | $ | 14.98 | | | $ | 15.10 | | | $ | 20.42 | | | $ | 21.48 | | | $ | 18.73 | | | $ | 22.51 | | |
Total investment return
at market value | | | 4.21 | %* | | | (18.86 | %) | | | 3.31 | % | | | 24.26 | % | | | (4.66 | %) | | | 9.92 | % | |
Total investment return
at net asset value | | | 8.47 | %* | | | (14.38 | %) | | | 6.61 | % | | | 17.12 | % | | | (2.52 | %) | | | 8.56 | % | |
RATIOS | |
Expenses to average net assets | | | 1.24 | %** | | | 1.29 | % | | | 1.25 | % | | | 1.32 | % | | | 1.27 | % | | | 1.21 | % | |
Expenses to average net assets
with waiver | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1.17 | % | |
Net investment loss to average
net assets | | | (0.22 | %)** | | | (0.70 | %) | | | (0.81 | %) | | | (0.92 | %) | | | (0.92 | %) | | | (0.91 | %) | |
SUPPLEMENTAL DATA | |
Net assets at end of period
(in millions) | | $ | 401 | | | $ | 384 | | | $ | 475 | | | $ | 466 | | | $ | 415 | | | $ | 463 | | |
Portfolio turnover rate | | | 20.55 | %* | | | 43.78 | % | | | 37.49 | % | | | 43.08 | % | | | 30.99 | % | | | 45.94 | % | |
* Not annualized.
** Annualized.
(1) Computed using average shares outstanding.
(2) Amount previously presented incorrectly as solely distributions from net realized capital gains has been revised to reflect the proper classification.
(3) Amount represents less than $0.005 per share.
The accompanying notes are an integral part of these financial statements.
19
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(Unaudited)
(1) Organization and Significant Accounting Policies
Tekla Life Sciences Investors (the Fund) is a Massachusetts business trust formed on February 20, 1992, and registered under the Investment Company Act of 1940 as a non-diversified closed-end management investment company. The Fund commenced operations on May 8, 1992. The Fund's investment objective is long-term capital appreciation through investment in U.S. and foreign companies in the life sciences industry (including biotechnology, pharmaceutical, diagnostics, managed healthcare and medical equipment, hospitals, healthcare information technology and services, devices and supplies), agriculture and environmental management. The Fund invests primarily in securities of public and private companies that are believed by the Fund's Investment Adviser, Tekla Capital Management LLC (the Adviser), to have significant potential for above-average growth. The Fund may invest up to 20% of its net assets in securities of foreign issuers, expected to be located primarily in Western Europe, Canada and Japan, and securities of U.S. issuers that are traded primarily in foreign markets.
The preparation of these financial statements requires the use of certain estimates by management in determining the Fund's assets, liabilities, revenues and expenses. Actual results could differ from these estimates and such differences could be material. The following is a summary of significant accounting policies followed by the Fund, which are in conformity with accounting principles generally accepted in the United States of America (GAAP). The Fund is an investment company and follows accounting and reporting guidance in the Financial Accounting Standards Board Accounting Standards Codification 946. Events or transactions occurring after March 31, 2020, through the date that the financial statements were issued, have been evaluated in the preparation of these financial statements.
The impact of COVID-19 outbreak on the financial performance of the Fund's investments will depend on future developments, including the duration and spread of the outbreak and related advisories and restrictions. These developments and the impact of COVID-19 on the financial markets and the overall economy are highly uncertain and cannot be predicted. If the financial markets and/or the overall economy are impacted for an extended period, the Fund's future investment results may be materially adversely affected.
Investment Valuation
Shares of publicly traded companies listed on national securities exchanges or trading in the over-the-counter market are typically valued at the last sale price, as of the close of trading, generally 4 p.m., Eastern Time. The Board of Trustees of the Fund (the Trustees) has established and approved fair valuation policies and procedures with respect to securities for which quoted prices may not be available or which do not reflect fair value. Convertible, corporate and government bonds are valued using a third-party pricing service. Convertible bonds are valued using this pricing service only on days when there is no sale reported. Restricted securities of companies that are publicly traded are typically valued based on the closing market quote on the valuation date adjusted for the impact of the restriction as determined in good faith by the Adviser also using fair valuation policies and procedures approved by the Trustees described below. Non-exchange traded warrants of publicly traded companies are generally valued using the Black-Scholes model, which incorporates both observable and unobservable inputs. Short-term investments with a maturity of 60 days or less are generally valued at amortized cost, which approximates fair value.
20
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
Convertible preferred shares, warrants or convertible note interests in private companies, milestone interests, and other restricted securities, as well as shares of publicly traded companies for which market quotations are not readily available, such as stocks for which trading has been halted or for which there are no current day sales, or which do not reflect fair value, are typically valued in good faith, based upon the recommendations made by the Adviser pursuant to fair valuation policies and procedures approved by the Trustees.
The Adviser has a Valuation Sub-Committee comprised of senior management which reports to the Valuation Committee of the Board at least quarterly. Each fair value determination is based on a consideration of relevant factors, including both observable and unobservable inputs. Observable and unobservable inputs the Adviser considers may include (i) the existence of any contractual restrictions on the disposition of securities; (ii) information obtained from the company, which may include an analysis of the company's financial statements, products, intended markets or technologies; (iii) the price of the same or similar security negotiated at arm's length in an issuer's completed subsequent round of financing; (iv) the price and extent of public trading in similar securities of the issuer or of comparable companies; or (v) a probability and time value adjusted analysis of contractual terms. Where available and appropriate, multiple valuation methodologies are applied to confirm fair value. Significant unobservable inputs identified by the Adviser are often used in the fair value determination. A significant change in any of these inputs may result in a significant change in the fair value measurement. Due to the uncertainty inherent in the valuation process, such estimates of fair value may differ significantly from the values that would have been used had a ready market for the investments existed, and differences could be material. Additionally, changes in the market environment and other events that may occur over the life of the investments may cause the gains or losses ultimately realized on these investments to be different from the valuations used at the date of these financial statements.
Milestone Interests
The Fund holds financial instruments which reflect the current value of future milestone payments the Fund may receive as a result of contractual obligations from other parties. The value of such payments are adjusted to reflect the estimated risk based on the relative uncertainty of both the timing and the achievement of individual milestones. A risk to the Fund is that the milestones will not be achieved and no payment will be received by the Fund. The milestone interests were received as part of the proceeds from the sale of six private companies. Any payments received are treated as a reduction of the cost basis of the milestone interest with payments received in excess of the cost basis treated as a realized gain. The contractual obligations with respect to the milestone interest provide for payments at various stages of the development of Afferent, Ethismos Research, Neurovance, Impact Biomedicines, Therachon and Therox's principal product candidate as of the date of the sale.
The following is a summary of the impact of the milestone interests on the financial statements as of and for the six months ended March 31, 2020:
Statement of Assets and Liabilities, Milestone interests, at value | | $ | 6,936,270 | | |
Statement of Assets and Liabilities, total distributable earnings | | $ | 1,940,880 | | |
Statement of Operations, Change in unrealized appreciation (depreciation)
on Milestone interests | | ($ | 56,182 | ) | |
21
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
Options on Securities
An option contract is a contract in which the writer (seller) of the option grants the buyer of the option, upon payment of a premium, the right to purchase from (call option) or sell to (put option) the writer a designated instrument at a specified price within a specified period of time. Certain options, including options on indices, will require cash settlement by the Fund if the option is exercised. The Fund enters into option contracts in order to hedge against potential adverse price movements in the value of portfolio assets, as a temporary substitute for selling selected investments, to lock in the purchase price of a security or currency which it expects to purchase in the near future, as a temporary substitute for purchasing selected investments, or to enhance potential gain or to gain or hedge exposure to financial market risk.
The Fund's obligation under an exchange traded written option or investment in an exchange traded purchased option is valued at the last sale price or in the absence of a sale, the mean between the closing bid and asked prices. Gain or loss is recognized when the option contract expires, is exercised or is closed.
If the Fund writes a covered call option, the Fund foregoes, in exchange for the premium, the opportunity to profit during the option period from an increase in the market value of the underlying security above the exercise price. If the Fund writes a put option it accepts the risk of a decline in the market value of the underlying security below the exercise price. Over-the-counter options have the risk of the potential inability of counterparties to meet the terms of their contracts. The Fund's maximum exposure to purchased options is limited to the premium initially paid. In addition, certain risks may arise upon entering into option contracts including the risk that an illiquid secondary market will limit the Fund's ability to close out an option contract prior to the expiration date and that a change in the value of the option contract may not correlate exactly with changes in the value of the securities or currencies hedged.
All options on securities and securities indices written by the Fund are required to be covered. When the Fund writes a call option, this means that during the life of the option the Fund may own or have the contractual right to acquire the securities subject to the option or may maintain with the Fund's custodian in a segregated account appropriate liquid securities in an amount at least equal to the market value of the securities underlying the option. When the Fund writes a put option, this means that the Fund will maintain with the Fund's custodian in a segregated account appropriate liquid securities in an amount at least equal to the exercise price of the option.
The average number of outstanding options purchased for the six months ended March 31, 2020 were 48.
Derivatives not accounted
for as hedging instruments
under ASC 815 | | Statement of Assets and
Liabilities Location | | Statement of Operations Location | |
Equity Contracts
| | | | | | Assets: Investments,
at value
| | $ | 81,600 | | | Change in unrealized
appreciation (depreciation)
on option contracts
purchased | | | $68,640 | | |
22
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
Other Assets
Other assets in the Statement of Assets and Liabilities consists of amounts due to the Fund at various times in the future in connection with the sale of investments in five private companies.
Investment Transactions and Income
Investment transactions are recorded on a trade date basis. Gains and losses from sales of investments are recorded using the "identified cost" method. Interest income is recorded on the accrual basis, adjusted for amortization of premiums and accretion of discounts. Dividend income is recorded on the ex-dividend date, less any foreign taxes withheld. Upon notification from issuers, some of the dividend income received may be redesignated as a reduction of cost of the related investment if it represents a return of capital.
The aggregate cost of purchases and proceeds from sales of investment securities (other than short-term investments) for the six months ended March 31, 2020 totaled $88,563,562 and $83,645,105, respectively.
Repurchase Agreements
In managing short-term investments the Fund may from time to time enter into transactions in repurchase agreements. In a repurchase agreement, the Fund's custodian takes possession of the underlying collateral securities from the counterparty, the market value of which is at least equal to the principal, including accrued interest, of the repurchase transaction at all times. In the event of default or bankruptcy by the other party to the agreement, realization and/or retention of the collateral by the Fund may be delayed. The Fund may enter into repurchase transactions with any broker, dealer, registered clearing agency or bank. Repurchase agreement transactions are not counted for purposes of the limitations imposed on the Fund's investment in debt securities.
Distribution Policy
Pursuant to a Securities and Exchange Commission exemptive order, the Fund may make periodic distributions that include capital gains as frequently as 12 times in any one taxable year in respect of its common shares, and the Fund has implemented a managed distribution policy (the Policy) providing for quarterly distributions at a rate set by the Trustees. Under the current Policy, the Fund intends to make quarterly distributions at a rate of 2% of the Fund's net assets to shareholders of record. The Fund intends to use net realized capital gains when making quarterly distributions, if available, but the Policy would result in a return of capital to shareholders if the amount of the distribution exceeds the Fund's net investment income and realized capital gains. If taxable income and net long-term realized gains exceed the amount required to be distributed under the Policy, the Fund will at a minimum make distributions necessary to comply with the requirements of the Internal Revenue Code. The Policy has been established by the Trustees and may be changed by them without shareholder approval. The Trustees regularly review the Policy and the frequency and rate of distribution considering the purpose and effect of the Policy, the financial market environment, and the Fund's income, capital gains and capital available to pay distributions.
The Fund's policy is to declare quarterly distributions in stock. The distributions are automatically paid in newly-issued full shares of the Fund unless otherwise instructed by the shareholder. Fractional shares will generally be settled in cash, except for registered shareholders
23
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
with book entry accounts of the Fund's transfer agent who will have whole and fractional shares added to their accounts. The Fund's transfer agent delivers an election card and instructions to each registered shareholder in connection with each distribution. The number of shares issued will be determined by dividing the dollar amount of the distribution by the lower of net asset value or market price on the pricing date. If a shareholder elects to receive a distribution in cash, rather than in shares, the shareholder's relative ownership in the Fund will be reduced. The shares reinvested will be valued at the lower of the net asset value or market price on the pricing date. Distributions in stock will not relieve shareholders of any federal, state or local income taxes that may be payable on such distributions. Additional distributions, if any, made to satisfy requirements of the Internal Revenue Code may be paid in stock, as described above, or in cash.
Share Repurchase Program
In March 2020, the Trustees approved the renewal of the repurchase program to allow the Fund to repurchase up to 12% of its outstanding shares in the open market for a one year period ending July 14, 2021. Prior to this renewal, in March 2019, the Trustees approved the renewal of the share repurchase program to allow the Fund to repurchase up to 12% of its outstanding shares for a one year period ending July 14, 2020. The share repurchase program is intended to enhance shareholder value and potentially reduce the discount between the market price of the Fund's shares and the Fund's net asset value.
During the six months ended March 31, 2020, the Fund repurchased 136,700 shares at a total cost of $2,191,363. The weighted average discount per share between the cost of repurchase and the net asset value applicable to such shares at the date of repurchase was 11.70%.
During the year ended September 30, 2019, the Fund repurchased 107,109 shares at a total cost of $1,556,624. The weighted average discount per share between the cost of repurchase and the net asset value applicable to such shares at the date of repurchase was 9.94%.
Federal Taxes
It is the Fund's policy to comply with the requirements of the Internal Revenue Code applicable to regulated investment companies and to distribute to its shareholders substantially all of its taxable income and its net realized capital gains, if any. Therefore, no Federal income or excise tax provision is required.
As of March 31, 2020, the Fund had no uncertain tax positions that would require financial statement recognition or disclosure. The Fund's federal tax returns are subject to examination by the Internal Revenue Service for a period of three years.
Distributions
The Fund records all distributions to shareholders on the ex-dividend date. Such distributions are determined in conformity with income tax regulations, which may differ from GAAP. These differences include temporary and permanent differences from losses on wash sale transactions, passive foreign investment companies, installment sale adjustments and ordinary loss netting to reduce short term capital gains. Reclassifications are made to the Fund's capital accounts to reflect income and gains available for distribution under income tax regulations.
24
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
Commitments and Contingencies
Under the Fund's organizational documents, its officers and Trustees may be indemnified against certain liabilities and expenses arising out of the performance of their duties to the Fund. Additionally, in the normal course of business, the Fund enters into agreements with service providers that may contain indemnification clauses. The Fund's maximum exposure under these agreements is unknown as this would involve future claims that may be made against the Fund that have not yet occurred. However, based on experience, the Fund expects the risk of loss to be remote.
Investor Support Services
The Fund has retained Destra Capital Advisors LLC (Destra) to provide investor support services in connection with the ongoing operation of the Fund. The Fund pays Destra a fee in an annual amount equal to 0.05% of the average aggregate daily value of the Fund's Managed Assets pursuant to the investor support services agreement.
(2) Investment Advisory and Other Affiliated Fees
The Fund has entered into an Investment Advisory Agreement (the Advisory Agreement) with the Adviser. Pursuant to the terms of the Advisory Agreement, the Fund pays the Adviser a monthly fee at the rate when annualized of (i) 2.50% of the average net assets for the month of its venture capital and other restricted securities up to 25% of net assets and (ii) for all other net assets, 0.98% of the average net assets up to $250 million, 0.88% of the average net assets for the next $250 million, 0.80% of the average net assets for the next $500 million and 0.70% of the average net assets thereafter. The aggregate fee would not exceed a rate when annualized of 1.36%.
The Fund has entered into a Services Agreement (the Agreement) with the Adviser. Pursuant to the terms of the Agreement, the Fund reimburses the Adviser for certain services related to a portion of the payment of salary and provision of benefits to the Fund's Chief Compliance Officer. During the six months ended March 31, 2020, these payments amounted to $25,395 and are included in the Other category of expenses in the Statement of Operations, together with insurance and other expenses incurred to unaffiliated entities. Expenses incurred pursuant to the Agreement as well as certain expenses paid for by the Adviser are allocated to the Fund in an equitable fashion as approved by the Trustees or officers of the Fund who are also officers of the Adviser.
The Fund pays compensation to Independent Trustees in the form of a retainer, attendance fees, and additional compensation to Board and Committee chairpersons. The Fund does not pay compensation directly to Trustees or officers of the Fund who are also officers of the Adviser.
(3) Other Transactions with Affiliates
An affiliate company is a company in which the Fund holds 5% or more of the voting securities. Transactions involving such companies during the six months ended March 31, 2020 were as follows:
Affiliated Companies | | Beginning
Value as of
September 30,
2019 | | Purchases at
Cost | | Proceeds
from Sales | | Net Realized
Gain/(Loss)
on sale of
Affiliated
Companies | | Change in
Unrealized
Appreciation/
Depreciation | | Ending Value
as of
March 31,
2020 | |
IlluminOss Medical, Inc. | | $ | 2,475,723 | | | $ | 185,000 | | | $ | — | | | $ | — | | | ($ | 1,937,444 | ) | | $ | 723,279 | | |
| | $ | 2,475,723 | | | $ | 185,000 | | | $ | — | | | $ | — | | | ( | $1,937,444 | ) | | $ | 723,279 | | |
25
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
| Affiliated Companies | | Shares as of
March 31,
2020 | | Principal
Amount as of
March 31,
2020 | | Dividend/
Interest
Income from
Affiliated
Companies | | Capital Gain
Distributions
from Affiliated
Companies | |
IlluminOss Medical, Inc. | | | 2,313,841 | | | $ | 1,867,973 | | | $ | 70,129 | | | $ | — | | |
| | | 2,313,841 | | | $ | 1,867,973 | | | $ | 70,129 | | | $ | — | | |
(4) Fair Value Measurements
The Fund uses a three-tier hierarchy to prioritize the assumptions, referred to as inputs, used in valuation techniques to measure fair value. The three-tier hierarchy of inputs is summarized in the three broad levels. Level 1 includes quoted prices in active markets for identical investments. Level 2 includes prices determined using other significant observable inputs (including quoted prices for similar investments, interest rates, credit risk, etc.). The independent pricing vendor may value bank loans and debt securities at an evaluated bid price by employing methodologies that utilize actual market transactions, broker-supplied valuations, and/or other methodologies designed to identify the market value for such securities and such securities are considered Level 2 in the fair value hierarchy. Level 3 includes prices determined using significant unobservable inputs (including the Fund's own assumptions in determining the fair value of investments). These inputs or methodology used for valuing securities are not necessarily an indication of the risk associated with investing in those securities.
For the period ended March 31, 2020, the total amount of transfers between Level 3 and Level 2 was $979,998. The one investment was transferred due to an initial public offering lock-up period and the value is being supported by significant observable inputs. There were no other transfers between levels.
The following is a summary of the levels used as of March 31, 2020 to value the Fund's net assets.
Assets at Value | | Level 1 | | Level 2 | | Level 3 | | Total | |
Convertible Preferred and Warrants | |
Biotechnology | | $ | — | | | $ | — | | | $ | 9,840,675 | | | $ | 9,840,675 | | |
Health Care Equipment & Supplies | | | — | | | | — | | | | 185 | | | | 185 | | |
Pharmaceuticals | | | — | | | | — | | | | 2,999,999 | | | | 2,999,999 | | |
Convertible Notes | |
Biotechnology | | | — | | | | — | | | | 194,493 | | | | 194,493 | | |
Health Care Equipment & Supplies | | | — | | | | — | | | | 723,094 | | | | 723,094 | | |
Common Stocks and Warrants | |
Biotechnology | | | 285,677,204 | | | | 748,341 | | | | 321,801 | | | | 286,747,346 | | |
Drug Discovery Technologies | | | 174,456 | | | | — | | | | — | | | | 174,456 | | |
Health Care Equipment & Supplies | | | 6,176,664 | | | | — | | | | 856,108 | | | | 7,032,772 | | |
Health Care Providers & Services | | | 427,218 | | | | — | | | | 136,504 | | | | 563,722 | | |
Healthcare Services | | | 978,050 | | | | — | | | | — | | | | 978,050 | | |
Life Sciences Tools & Services | | | 29,507,302 | | | | — | | | | — | | | | 29,507,302 | | |
Pharmaceuticals | | | 38,730,356 | | | | — | | | | 69,699 | | | | 38,800,055 | | |
Short-term Investment | | | — | | | | 12,625,000 | | | | — | | | | 12,625,000 | | |
26
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
Assets at Value | | Level 1 | | Level 2 | | Level 3 | | Total | |
Milestone Interests | |
Biotechnology | | $ | — | | | $ | — | | | $ | 1,445,446 | | | $ | 1,445,446 | | |
Health Care Equipment & Supplies | | | — | | | | — | | | | 4,604 | | | | 4,604 | | |
Pharmaceuticals | | | — | | | | — | | | | 5,486,220 | | | | 5,486,220 | | |
Other Assets | | | — | | | | — | | | | 376,587 | | | | 376,587 | | |
Total | | $ | 361,671,250 | | | $ | 13,373,341 | | | $ | 22,455,415 | | | $ | 397,500,006 | | |
Other Financial Instruments | |
Assets | |
Call Option Contracts Purchased | | $ | 81,600 | | | $ | — | | | $ | — | | | $ | 81,600 | | |
The following is a reconciliation of Level 3 assets for which significant unobservable inputs were used to determine fair value.
Investment in
securities | | Balance as of
September 30,
2019 | | Net realized
gain (loss) and
change in
unrealized
appreciation
(depreciation) | | Cost of
purchases
and
conversions | | Proceeds
from
sales and
conversions | | Net
transfers
into
(out of)
Level 3 | | Balance
as of
March 31,
2020 | |
Convertible Preferred and Warrants | |
Biotechnology | | $ | 7,120,498 | | | ($ | 480,031 | ) | | $ | 3,200,208 | | | $ | — | | | $ | — | | | $ | 9,840,675 | | |
Health Care
Equipment &
Supplies | | | 792,748 | | | | (792,563 | ) | | | — | | | | — | | | | — | | | | 185 | | |
Pharmaceuticals | | | 1,499,999 | | | | — | | | | 1,500,000 | | | | — | | | | — | | | | 2,999,999 | | |
Convertible Notes | |
Biotechnology | | | 597,977 | | | | (458,290 | ) | | | 54,806 | | | | — | | �� | | — | | | | 194,493 | | |
Health Care
Equipment &
Supplies | | | 1,682,975 | | | | (1,126,544 | ) | | | 185,000 | | | | (18,337 | ) | | | — | | | | 723,094 | | |
Common Stock and Warrants | |
Biotechnology | | | 1,354,614 | | | | (49,159 | ) | | | — | | | | (3,656 | ) | | | (979,998 | ) | | | 321,801 | | |
Health Care
Equipment &
Supplies | | | 678,730 | | | | 177,378 | | | | — | | | | — | | | | — | | | | 856,108 | | |
Health Care
Providers &
Services | | | 1,359,525 | | | | (743,725 | ) | | | 135,770 | | | | (615,066 | ) | | | — | | | | 136,504 | | |
Pharmaceuticals | | | 822 | | | | 14,734 | | | | — | | | | — | | | | 54,143 | | | | 69,699 | | |
Milestone Interests | |
Biotechnology | | | 1,444,178 | | | | 1,268 | | | | — | | | | — | | | | — | | | | 1,445,446 | | |
Health Care
Equipment &
Supplies | | | 4,567 | | | | 37 | | | | — | | | | — | | | | — | | | | 4,604 | | |
Pharmaceuticals | | | 5,543,707 | | | | (57,487 | ) | | | — | | | | — | | | | — | | | | 5,486,220 | | |
Other Assets | | | 391,097 | | | | — | | | | 3,925 | | | | (18,435 | ) | | | — | | | | 376,587 | | |
| | | $ | 22,471,437 | | | ($ | 3,514,382 | ) | | $ | 5,079,709 | | | ($ | 655,494 | ) | | ($ | 925,855 | ) | | $ | 22,455,415 | | |
Net change in unrealized appreciation (depreciation) from
investments still held as of March 31, 2020 | | ( | $2,788,260 | ) | |
27
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
The following is a quantitative disclosure about significant unobservable inputs used in the determination of the fair value of Level 3 assets.
| | | Fair Value at
March 31,
2020 | | Valuation Technique | | Unobservable Input | | Range
(Weighted Average) | |
Private Companies and
Other Restricted
Securities | | $ | 897,215 | | | Income approach,
Black-Scholes | |
Discount for lack of
marketability | | 20.00%-50.00% (20.14%) | |
| | | | 6,927,032 | | | Probability weighted
expected return model | | Discount rate
Price to sales multiple | | 43.63
4.41x-29.21x (17.64x)%-51.81% (48.16%) | |
| | | 1,995,278 | | | Market approach,
recent transaction | | (a) | | N/A | |
| | | | 5,132,386 | | | Market comparable | | Discount for lack of
marketability
Earning ratio | | 15.00
5.98x (5.98x)% (15.00%) | |
| | | | 7,503,504 | | | Probability adjusted
value | | Probability of events
Timing of events | | 0.00
0.25-17.00 (3.65) years%-100.00% (51.71%) | |
| | | $ | 22,455,415 | | | | | | | | |
(a) The valuation technique used as a basis to approximate fair value of these investments is based upon subsequent financing rounds. There is no quantitative information as these methods of measure are investment specific.
(5) Private Companies and Other Restricted Securities
The Fund may invest in private companies and other restricted securities if these securities would currently comprise 40% or less of net assets. The value of these securities represented 6% of the Fund's net assets at March 31, 2020.
At March 31, 2020, the Fund had a commitment of $3,979,821 relating to additional investments in two private companies.
The following table details the acquisition date, cost, carrying value per unit, and value of the Fund's private companies and other restricted securities at March 31, 2020. The Fund on its own does not have the right to demand that such securities be registered.
Security (#) | | Acquisition
Date | | Cost | | Carrying Value
per Unit | | Value | |
Afferent Milestone Interest | | 07/27/16 | | $ | 161,872 | | | $ | 251,261.00 | | | $ | 251,261 | | |
Amphivena Therapeutics, Inc. | |
Series B Cvt. Pfd | | 07/17/17 | | | 2,101,222 | | | | 15.00 | | | | 2,100,000 | | |
Series C Cvt. Pfd | | 12/10/18 | | | 808,848 | | | | 3.59 | | | | 808,848 | | |
Arkuda Therapeutics, Inc. Series A Cvt. Pfd | | 05/16/19 | | | 818,184 | | | | 2.38 | | | | 818,183 | | |
Cercacor Laoratories, Inc. Common | | 03/31/98† | | | 0 | | | | 6.59 | | | | 856,108 | | |
Curasen Therapeutics, Inc. Series A Cvt. Pfd | | 09/18/18, 01/07/20 | | | 2,999,999 | | | | 1.10 | | | | 2,999,999 | | |
Decipher Biosciences, Inc. | |
Series II Cvt. Pfd | | 03/29/19 | | | 1,846,969 | | | | 2.51 | | | | 1,000,566 | | |
Series III Cvt. Pfd | | 03/29/19 | | | 417,862 | | | | 2.51 | | | | 994,712 | | |
28
TEKLA LIFE SCIENCES INVESTORS
NOTES TO FINANCIAL STATEMENTS
MARCH 31, 2020
(continued)
Security (#) | | Acquisition
Date | | Cost | | Carrying Value
per Unit | | Value | |
Ethismos Research Milestone Interest | | 10/31/17 | | $ | 0 | | | $ | 0.00 | | | $ | 0 | | |
Galera Therapeutics, Inc. Common | | 08/30/18 | | | 980,062 | | | | 8.55 | | | | 748,341 | | |
IlluminOss Medical, Inc. | |
Series AA Cvt. Pfd | | 01/21/16 | | | 960,650 | | | | 0.00 | †† | | | 95 | | |
Junior Preferred | | 01/21/16 | | | 1,566,291 | | | | 0.00 | †† | | | 90 | | |
Cvt. Promissory Note | | 11/21/14 | | | 95,245 | | | | 38.72 | | | | 36,816 | | |
Cvt. Promissory Note | | 03/18/17 | | | 285,837 | | | | 38.72 | | | | 110,466 | | |
Cvt. Promissory Note | | 01/11/18 | | | 190,221 | | | | 38.72 | | | | 73,632 | | |
Cvt. Promissory Note | | 02/06/18 | | | 190,166 | | | | 38.72 | | | | 73,632 | | |
Cvt. Promissory Note | | 09/05/18 | | | 237,708 | | | | 38.72 | | | | 92,040 | | |
Cvt. Promissory Note | | 01/28/19 | | | 185,849 | | | | 38.72 | | | | 71,961 | | |
Cvt. Promissory Note | | 04/10/19 | | | 51,858 | | | | 38.72 | | | | 20,080 | | |
Cvt. Promissory Note | | 05/10/19 | | | 81,882 | | | | 38.72 | | | | 31,705 | | |
Cvt. Promissory Note | | 07/01/19 | | | 113,205 | | | | 38.72 | | | | 43,833 | | |
Cvt. Promissory Note | | 08/29/19 | | | 37,000 | | | | 38.72 | | | | 14,326 | | |
Cvt. Promissory Note | | 09/27/19 | | | 214,763 | | | | 38.72 | | | | 83,156 | | |
Cvt. Promissory Note | | 01/08/20 | | | 123,333 | | | | 38.72 | | | | 47,755 | | |
Cvt. Promissory Note | | 03/05/20 | | | 61,667 | | | | 38.72 | | | | 23,692 | | |
Warrants (expiration 03/31/27) | | 03/28/17 | | | 331 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 09/06/27) | | 09/05/18 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 11/20/27) | | 11/21/17 | | | 87 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 01/11/28) | | 01/11/18 | | | 29 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 02/06/28) | | 02/06/18 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 01/29/29) | | 01/28/19 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 04/29/29) | | 04/10/19 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 05/13/29) | | 05/10/19 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 07/02/29) | | 05/10/19 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 08/29/29) | | 08/29/19 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 09/27/29) | | 09/27/19 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 01/08/30) | | 01/08/20 | | | 0 | | | | 0.00 | | | | 0 | | |
Warrants (expiration 03/06/30) | | 03/05/20 | | | 0 | | | | 0.00 | | | | 0 | | |
Impact Biomedicines Milestone Interest | | 07/20/10 | | | 0 | | | | 3,301,998.00 | | | | 3,301,998 | | |
InnovaCare, Inc. Escrow Shares Common | | 12/21/12† | | | 135,770 | | | | 0.92 | | | | 136,504 | | |
Neurovance Milestone Interest | | 03/20/17 | | | 3,417,500 | | | | 1,932,961.00 | | | | 1,932,961 | | |
Oculis SA, Series B2 Cvt. Pfd | | 01/16/19 | | | 990,902 | | | | 8.65 | | | | 1,018,185 | | |
Rainier Therapeutics, Inc. | |
Series A Cvt. Pfd | | 01/19/16, 10/24/16 | | | 750,652 | | | | 0.00 | †† | | | 115 | | |
Series B Cvt. Pfd | | 03/03/17 | | | 500,091 | | | | 0.00 | †† | | | 67 | | |
Cvt. Promissory Note | | 01/30/19 | | | 189,679 | | | | 0.00 | | | | 0 | | |
Cvt. Promissory Note | | 03/28/19 | | | 189,680 | | | | 0.00 | | | | 0 | | |
Cvt. Promissory Note | | 07/16/19 | | | 218,642 | | | | 71.21 | | | | 155,688 | | |
Cvt. Promissory Note | | 10/07/19 | | | 54,753 | | | | 71.21 | | | | 38,805 | | |
Rallybio Holdings, LLC Series B Cvt. Pfd | | 03/27/20 | | | 3,099,999 | | | | 1.48 | | | | 3,099,999 | | |
Therachon Milestone Interest | | 07/01/19 | | | 1,409,779 | | | | 1,445,446.00 | | | | 1,445,446 | | |
Therox Milestone Interest | | 06/18/19 | | | 6,240 | | | | 4,604.00 | | | | 4,604 | | |
Vectivbio Holding AG Common | | 07/01/19† | | | 292,594 | | | | 0.79 | | | | 296,250 | | |
| | | | | $ | 25,787,421 | | | | | $ | 22,731,919 | | |
(#) See Schedule of Investments and corresponding footnotes for more information on each issuer.
† Interest received as part of a corporate action for a previously owned security.
†† Carrying value per unit is greater that $0.00 but less than $0.01
29
TEKLA LIFE SCIENCES INVESTORS
INVESTMENT ADVISORY AGREEMENT APPROVAL
The Investment Advisory Agreement (the Advisory Agreement) between the Fund and the Adviser continues in effect so long as its continuance is approved at least annually by (i) the Trustees of the Fund and (ii) a majority of the Trustees of the Fund who are not interested persons (the Independent Trustees), by vote cast in person at a meeting called for the purpose of voting on such approval.
After considering the matter in a meeting held on March 19, 2020, the Board, and the Independent Trustees voting separately, determined that the terms of the Advisory Agreement are fair and reasonable and approved the continuance of the Advisory Agreement as being in the best interests of the Fund and its shareholders. In making its determination, the Board considered materials that were specifically prepared by the Adviser and by an independent data provider at the request of the Board and Fund counsel for purposes of the contract review process, including comparisons of (i) the Fund's performance both directly and on a risk adjusted basis to a benchmark, the NASDAQ Biotech Index® (NBI), and to a peer universe of other investment companies, (ii) the Fund's expenses and expense ratios to those of a peer group of other investment companies, and (iii) the Adviser's profitability with respect to its services for the Fund to the profitability of other investment advisers. The Trustees took into account that the Adviser provides investment management services only to Tekla Life Sciences Investors, Tekla Healthcare Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund and does not derive any significant benefit from its relationship with the Fund other than receipt of advisory fees pursuant to the Advisory Agreement, market research and potential marketing exposure for the Adviser. The Board also received and reviewed information throughout the year about the portfolio performance, the investment strategy, the portfolio management team and the fees and expenses of the Fund. In their deliberations, the Independent Trustees had the opportunity to meet privately without representatives of the Adviser present and were represented throughout the process by counsel to the Independent Trustees and the Fund.
In approving the Advisory Agreement, the Board considered, among other things, the nature, extent, and quality of the services to be provided by the Adviser, the investment performance of the Fund and the Adviser, the costs of services provided and profits realized by the Adviser and its affiliates, and whether fee levels reflect any economies of scale for the benefit of Fund shareholders and the extent to which economies of scale would be realized as the Fund grows. The Board reviewed information about the foregoing factors and considered changes, if any, in such information since its previous approval. The Board also evaluated the financial strength of the Adviser and the capability of the personnel of the Adviser, specifically the strength and background of its investment analysts. Fund counsel provided the Board with the statutory and regulatory requirements for approval and disclosure of investment advisory agreements. The Board, including the Independent Trustees, evaluated all of the foregoing and, considering all factors together, determined in the exercise of its business judgment that the continuance of the Advisory Agreement is in the best interests of the Fund and its shareholders. The following provides more detail on certain factors considered by the Trustees and the Board's conclusions with respect to each such factor.
The nature, extent and quality of the services to be provided by the Adviser. On a regular basis the Board considers the roles and responsibilities of the Adviser as a whole, along with specific portfolio management, support and trading functions the Adviser provides to the Fund. The Trustees considered the nature, extent and quality of the services provided by the Adviser to the Fund. The Trustees continue to be satisfied with the quality and value of the investment advisory services provided to the Fund by the Adviser, and, in particular, the management style and discipline followed by the Adviser and the quality of the Adviser's research, trading, portfolio management, compliance and administrative personnel. The Trustees also took into
30
TEKLA LIFE SCIENCES INVESTORS
INVESTMENT ADVISORY AGREEMENT APPROVAL
(continued)
account the Adviser's significant investment in its business through the addition of portfolio management and administrative staff in recent years and the Adviser's commitment to continue to build out its infrastructure as future circumstances require.
The investment performance of the Fund and the Adviser. On a regular basis the Board reviews performance information of the Fund and discusses the Fund's investment strategy with the Adviser. The Trustees reviewed performance information for the Fund and for the past one-, three-, five- and ten-year periods ended December 31, 2019, as compared to its benchmark, the NBI, and a peer universe of other investment companies identified by an independent service provider engaged by the Independent Trustees. The Trustees also reviewed information relating to the performance of the Fund's venture capital portfolio. The Trustees noted that the performance information reviewed reflects a view of the Fund's performance only as of a certain date, and that the results might be significantly different if a different date was selected to generate the performance information. Additionally, the Trustees recognized that longer periods of performance for the Fund may be adversely and disproportionately affected by significant underperformance in one more recent period, and that such underperformance may be caused by a small number of investment decisions or positions.
Unlike many other broader-based healthcare indices, the NBI contains high levels of biotechnology-based companies. Over time, this index has demonstrated higher returns, but has also demonstrated higher price volatility than the broad S&P 500® Index. The Adviser seeks to operate the Fund at a biotechnology exposure level that is higher than many other indices and Funds but at a level that is below that of the NBI. The Adviser also seeks to operate the Fund at lower volatility than that of the NBI. In the current reporting period, the Adviser sought to do so by limiting exposure to biotechnology relative to the NBI and also by maintaining a higher exposure to large biotechnology companies (which exhibit relatively lower volatility) than is present in the NBI and lower exposure to small and mid-capitalization biotechnology companies than is present in the NBI.
The Trustees noted that as of December 31, 2019, the Fund outperformed (on a net asset value basis) the return of the NBI on a direct comparison basis for the one- and five-year periods, but underperformed the NBI for the three- and ten-year periods. The Trustees noted, however, that the Fund exhibited a lower volatility than that of the NBI and outperformed the NBI on a risk adjusted basis for all evaluation periods ended December 31, 2019, except the ten-year period. The Trustees noted that the Fund outperformed the peer universe average on a direct comparison basis for the one- and ten-year periods and underperformed the peer universe average on a direct comparison basis for the three- and five-year periods. The Trustees also noted that the Fund produced returns comparable to the returns of those funds the Adviser believes are most similar to HQL (i.e., high biotech allocation funds).
In considering the Fund's relative performance, the Trustees recognized that the Fund's unique strategy presents challenges when comparing the Fund's performance to a benchmark or group of comparable funds. In particular, the Trustees observed that the Fund's strategy has historically included a lower biotechnology allocation compared to the NBI and a higher biotechnology allocation compared to many other healthcare indices and to many of the other funds in the peer universe. The Trustees noted that, as a result, all other things being equal, in periods when biotechnology performs relatively well, the Fund might be expected to underperform the NBI (and/or the peer universe) and vice versa. Additionally, the Trustees noted that unlike the NBI and most of the peer universe, the Fund often maintains a meaningful allocation to venture and restricted securities. In light of these differences, the Trustees recognized the more limited usefulness of these performance comparisons for the Fund.
The Trustees concluded they continue to be satisfied with the investment performance of the Fund and the Adviser.
31
TEKLA LIFE SCIENCES INVESTORS
INVESTMENT ADVISORY AGREEMENT APPROVAL
(continued)
The costs of services to be provided and profits to be realized by the Adviser from its relationship with the Fund. The Trustees considered the various services provided by the Adviser to the Fund and reviewed comparative information regarding the expenses and expense ratios of the Fund and a peer group of other investment companies identified by an independent service provider engaged by the Independent Trustees. The Trustees noted that the Adviser's fees are within the range of fees presented in the comparative information and noted that the Fund often maintains a meaningful allocation to venture and restricted securities, a portfolio management service that can warrant higher management fees than those charged by the Adviser to the Fund. The Trustees also considered financial information provided by the Adviser, including financial statements of the Adviser and a comparison of the Adviser's profitability with respect to its services for the Fund to the profitability of other investment advisers.
The Trustees noted that the fees charged by the Adviser are within a reasonable range of fees as compared to fees charged by other investment advisers, and the services provided by the Adviser and the amounts paid under the Advisory Agreement are at least comparable to the services rendered and fees charged by others for similar services to warrant a finding that fees to be paid by the Fund are fair. Based on the information provided to and evaluated by the Trustees, the Trustees concluded that the fees charged by the Adviser are fair and reasonable in light of the quality and nature of the services provided by the Adviser and that the profitability of the Adviser's relationship with the Fund has not been excessive.
Whether fee levels reflect economies of scale and the extent to which economies of scale would be realized as the Fund grows. The Trustees considered the advisory fee schedule in the Advisory Agreement and noted that it provides for breakpoints that would reduce the effective fee to the extent the Fund's net assets should increase, allowing the Fund to share in the benefits of any economies of scale that would inure to the Adviser as the Fund's assets increase. Given the closed-end structure of the Fund, its current asset size, and the fact that any economies of scale are modest at current Fund asset levels, the Trustees determined that the Fund's advisory fee schedule is satisfactory and fair.
32
TEKLA LIFE SCIENCES INVESTORS
PRIVACY NOTICE: If you are a registered shareholder of the Fund, the Fund and Tekla Capital Management LLC, the Fund's investment adviser, may receive nonpublic personal information about you from the information collected by the transfer agent from your transactions in Fund shares. Any nonpublic personal information is not disclosed to third parties, except as permitted or required by law. In connection with servicing your account and effecting transactions, the information received may be shared with the investment adviser and non-affiliates, including transfer agents, custodians or other service companies. Access to your nonpublic personal information is restricted to employees who need to know that information to provide products or services to you. To maintain the security of your nonpublic personal information, physical, electronic, and procedural safeguards are in place that comply with federal standards. The policies and practices described above apply to both current and former shareholders.
If your Fund shares are held in "street name" at a bank or brokerage, we do not have access to your personal information and you should refer to your bank's or broker's privacy policies for a statement of the treatment of your personal information.
FOR MORE INFORMATION: A description of the Fund's proxy voting policies and procedures and information on how the Fund voted proxies relating to portfolio securities during the most recent 12-month period ended June 30 is available (i) without charge, upon request by calling 1-800-451-2597; (ii) by writing to Tekla Capital Management LLC at 100 Federal Street, 19th Floor, Boston, MA 02110; (iii) on the Fund's website at www.teklacap.com; and (iv) on the SEC's website at www.sec.gov.
The Fund's complete Schedule of Investments for the first and third quarters of its fiscal year will be filed with the SEC on Form N-PORT. This Schedule of Investments will also be available on the Fund's website at www.teklacap.com or the SEC's website at www.sec.gov.
You can find information regarding the Fund at the Fund's website, www.teklacap.com. The Fund regularly posts information to its website, including information regarding daily share pricing, distributions and press releases, and maintains links to the Fund's SEC filings. The Fund currently publishes and distributes quarterly fact cards, including performance, portfolio holdings and sector information for each fiscal quarter. These fact cards will be available on the Fund's website and by request from the Fund's marketing and investor support services agent, Destra Capital Advisors LLC, at 1-877-855-3434.
DISTRIBUTION POLICY: The Fund has a managed distribution policy as described in the Notes to Financial Statements. For more information contact your financial adviser.
SHARE REPURCHASE PROGRAM: In March 2020, the Trustees reauthorized the share repurchase program to allow the Fund to repurchase up to 12% of its outstanding shares for a one year period ending July 14, 2021.
PORTFOLIO MANAGEMENT: Daniel R. Omstead, Ph.D., Jason C. Akus, M.D./M.B.A., Timothy Gasperoni, M.B.A, Ph.D., Christian M. Richard, M.B.A, M.S., Henry Skinner, Ph.D., Ashton L. Wilson, Christopher Abbott, Robert Benson, Richard Goss, Alan Kwan, M.B.A, M.S., Ph.D., Jack Liu, M.B.A., Ph.D., and Loretta Tse, Ph.D. are members of a team that analyzes investments on behalf of the Fund. Dr. Omstead exercises ultimate decision making authority with respect to investments.
HOUSEHOLDING: A number of banks, brokers and financial advisers have instituted "householding". Under this practice, which has been approved by the SEC, only one copy of shareholder documents may be delivered to multiple shareholders who share the same address and satisfy other conditions. Householding is intended to reduce expenses and eliminate duplicate mailings of shareholder documents. If you do not want the mailing of your shareholder documents to be combined with those of other members of your household, please contact your bank, broker or financial adviser.
33
TEKLA LIFE SCIENCES INVESTORS
New York Stock Exchange Symbol: HQL
NAV Symbol: XHQLX
100 Federal Street, 19th Floor
Boston, Massachusetts 02110
(617) 772-8500
www.teklacap.com
Officers
Daniel R. Omstead, Ph.D., President
Laura Woodward, CPA, Chief Compliance Officer,
Secretary and Treasurer
Trustees
Rakesh K. Jain, Ph.D.
Thomas M. Kent, CPA
Daniel R. Omstead, Ph.D.
Oleg M. Pohotsky, M.B.A., J.D.
William S. Reardon, M.B.A.
Lucinda H. Stebbins, M.B.A., CPA
Investment Adviser
Tekla Capital Management LLC
Administrator & Custodian
State Street Bank and Trust Company
Transfer Agent
Computershare, Inc.
Legal Counsel
Dechert LLP
Shareholders with questions regarding share transfers may call
1-800-426-5523
Daily net asset value may be obtained from
our website (www.teklacap.com) or by calling
617-772-8500
ITEM 2. CODE OF ETHICS.
Not applicable to this semi-annual filing.
ITEM 3. AUDIT COMMITTEE FINANCIAL EXPERT.
Not applicable to this semi-annual filing.
ITEM 4. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Not applicable to this semi-annual filing.
ITEM 5. AUDIT COMMITTEE OF LISTED REGISTRANTS.
Not applicable to this semi-annual filing.
ITEM 6. INVESTMENTS.
The Registrant’s Schedule of Investments is included as part of the Report to Shareholders filed under Item 1 of this form.
ITEM 7. DISCLOSURE OF PROXY VOTING POLICIES AND PROCEDURES FOR CLOSED-END MANAGEMENT INVESTMENT COMPANIES.
Not applicable to this semi-annual filing.
ITEM 8. PORTFOLIO MANAGERS OF CLOSED-END MANAGEMENT INVESTMENT COMPANIES
Not applicable to this semi-annual filing.
ITEM 9. PURCHASES OF EQUITY SECURITIES BY CLOSED-END MANAGEMENT INVESTMENT COMPANY AND AFFILIATED PURCHASERS.
| Period | | (a) Total No.
of Shares
Purchased (1) | | | (b) Average
Price Paid per
Share | | | (c) Total No.
of Shares
Purchased as
Part of
Publicly
Announced Plans
or Programs | | | (d) Maximum No.
of Shares that
May Yet Be
Purchased Under
the Plans or
Programs | |
| Month #1 (Oct. 1, 2019 - Oct. 31, 2019) | | | — | | | | — | | | | — | | | | 2,781,589 | |
| Month #2 (Nov. 1, 2019 — Nov. 30, 2019) | | | 91,683 | | | $ | 16.71 | | | | 91,683 | | | | 2,689,906 | |
| Month #3 (Dec. 1, 2019 — Dec. 31, 2019) | | | — | | | | — | | | | — | | | | 2,689,906 | |
| Month #4 (Jan. 1, 2020 — Jan. 31, 2020) | | | — | | | | — | | | | — | | | | 2,689,906 | |
| Month #5 (Feb. 1, 2020 — Feb. 29, 2020) | | | 20,294 | | | $ | 15.73 | | | | 20,294 | | | | 2,669,612 | |
| Month #6 (Mar. 1, 2020 — Mar. 31, 2020) | | | 33,023 | (2) | | $ | 13.55 | | | | 24,723 | | | | 2,644,889 | |
| Total | | | 145,000 | | | $ | 15.85 | | | | 136,700 | | | | | |
| (1) | On June 30, 2011, the share repurchase program was announced, which has been subsequently reviewed and approved by the Board of Trustees. On March 21, 2019, the share repurchase program was renewed, allowing the Registrant to repurchase up to 12% of its outstanding shares for a one year period ending July 14, 2020. On March 19, 2020, the Trustees approved the renewal of the repurchase program to allow the Registrant to repurchase up to 12% of its outstanding shares in the open market for a one year period ending July 14, 2021. |
| (2) | 8,300 shares were purchased in Month #6 by Daniel R. Omstead in the open market. Dr. Omstead is a director of the Registrant and a controlling person of the Registrant's investment adviser. Accordingly, Dr. Omstead may be deemed an "affiliated purchaser" of the Registrant's shares as such term is defined by Rule 10b-18(a)(3) under the Exchange Act of 1934. |
ITEM 10. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
There have been no material changes to the procedures by which the shareholders may recommend nominees to the Registrant’s Board of Trustees, where those changes were implemented after the Registrant last provided disclosure in response to the requirements of Item 7(d)(2)(ii)(G) of Schedule 14A, or this Item.
ITEM 11. CONTROLS AND PROCEDURES.
| (a) | In the opinion of the principal executive officer and principal financial officer, based on their evaluation which took place within 90 days of this filing, the Registrant’s disclosure controls and procedures are adequately designed and are operating effectively to ensure (i) that material information relating to the Registrant, including its consolidated subsidiaries, is made known to them by others within those entities, particularly during the period in which this report is being prepared; and (ii) that information required to be disclosed by the registrant on Form N-CSR is recorded, processed, summarized and reported within the time period specified in the Securities and Exchange Commission’s rules and forms. |
| (b) | There were no changes in the Registrant’s internal control over financial reporting that occurred during the Registrant’s most recent second fiscal quarter that have materially affected or that are reasonably likely to materially affect the Registrant’s internal control over financial reporting. |
Item 12. Disclosure of Securities Lending Activities for Closed-End Management Investment Companies.
Not applicable.
ITEM 13. EXHIBITS
(a)(1) Code of Ethics -Not applicable to this semi-annual filing.
(a)(2) Separate certifications of the Principal Executive and Financial Officers as required by Rule 30a-2(a) under the 1940Act and Section 302 of the Sarbanes-Oxley Act of 2002 are attached hereto (Exhibit 1 and 2).
(a)(3) Notices to Fund’s shareholders in accordance with Investment Company Act Section 19(a) and Rule 19a-1 (Exhibit 3).
(b) Certifications pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 are attached hereto (Exhibit 4).
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934 and the Investment Company Act of 1940, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| (Registrant) | TEKLA LIFE SCIENCES INVESTORS | |
| | |
| By (Signature and Title)* | /s/ Daniel R. Omstead | |
| | Daniel R. Omstead, President | |
| | |
| Date: | 6/5/20 | |
| | | | | |
Pursuant to the requirements of the Securities Exchange Act of 1934 and the Investment Company Act of 1940, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| By (Signature and Title)* | /s/ Laura Woodward | |
| | Laura Woodward, Treasurer | |
| | |
| Date: | 6/5/20 | |
| | | | |
* Print the name and title of each signing officer under his or her signature.