 2013 Financial Guidance Conference Call January 4, 2013 Exhibit 99.1 |
 1 Forward-looking Statements Forward-looking Statements Certain statements made in this presentation may constitute forward-looking statements, including, but not limited to, statements regarding preliminary results and guidance with respect to expected revenues, non-GAAP cash earnings per share, adjusted cash flows from operations, organic product sales growth, integration-related activities and benefits, synergies, launches and approvals of products, assumptions with respect to 2013 guidance, and the 2013 strategic initiatives of Valeant Pharmaceuticals International, Inc. (the “Company”). Forward-looking statements may be identified by the use of the words “anticipates,” “expects,” “intends,” “plans,” “could,” “should,” “would,” “may,” “will,” “believes,” “estimates,” “potential,” or “continue” and variations or similar expressions. These statements are based upon the current expectations and beliefs of management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, but are not limited to, risks and uncertainties discussed in the company's most recent annual or quarterly report filed with the Securities and Exchange Commission ("SEC") and other risks and uncertainties detailed from time to time in the Company's filings with the SEC and the Canadian Securities Administrators ("CSA"), which factors are incorporated herein by reference. Readers are cautioned not to place undue reliance on any of these forward-looking statements. The Company undertakes no obligation to update any of these forward-looking statements to reflect events or circumstances after the date of this presentation or to reflect actual outcomes. Non-GAAP Information To supplement the financial measures prepared in accordance with generally accepted accounting principles (GAAP), the Company uses non-GAAP financial measures that exclude certain items. Management uses non-GAAP financial measures internally for strategic decision making, forecasting future results and evaluating current performance. By disclosing non-GAAP financial measures, management intends to provide investors with a meaningful, consistent comparison of the Company’s core operating results and trends for the periods presented. Non-GAAP financial measures are not prepared in accordance with GAAP; therefore, the information is not necessarily comparable to other companies and should be considered as a supplement to, not a substitute for, or superior to, the corresponding measures calculated in accordance with GAAP. The Company has provided preliminary results and guidance with respect to cash earnings per share, adjusted cash flows from operations and organic product growth rates, which are non-GAAP financial measures. The Company has not provided a reconciliation of these preliminary and forward-looking non-GAAP financial measures due to the difficulty in forecasting and quantifying the exact amount of the items excluded from the non-GAAP financial measures that will be included in the comparable GAAP financial measures. Reconciliations of historical non-GAAP financials can be found at www.valeant.com. Note 1: The guidance in this presentation is only effective as of the date given, January 4, 2013, and will not be updated or affirmed unless and until the Company publicly announces updated or affirmed guidance. |
 2 Agenda 2012 Review – J. Michael Pearson 2013 Guidance – Howard Schiller New Segment Reporting Other Updates – J. Michael Pearson New 2013 Strategic Objectives Organizational Structure |
 3 2012 Achievements Operations: Revenue growth vs. 2011 of >$1B 1 and >40% 1 Overcame ~$200 M decline related to genericization of Cesamet, Cardizem CD, Ultram ER, Wellbutrin XL Cash EPS growth vs. 2011 of >50% 1 Organic growth of ~8% on a same store basis, ~10% Pro Forma Business Development: Completed over 25 acquisitions Probiotica, Pedinol, OraPharma, Medicis, Gerot Lannach Majority between 2-3 X sales Established new growth platforms Oral Health, Podiatry, Aesthetics , Russia, South East Asia/South Africa Medicis Acquisition Closed in Mid-December 1 Excludes impact of one time items Strengthened Senior Management Team Marcelo Noll Barboza – President, Valeant Brazil Jacques Dessureault - President, Valeant Canada Jason Hanson – Company Group Chairman Andrew Howden – CEO iNova Vince Ippolito – SVP, GM Aesthetics Laizer Kornwasser – Company Group Chairman Pavel Mirovsky – President, Valeant Europe Steve Sembler – SVP, President OraPharma Justin Smith – SVP, GM U.S. Rx Derm |
 4 2012 Achievements (continued) R&D Productivity and Product Launches: Filed multiple New Drug Applications Efinaconazole - Onychomycosis (Valeant) Luliconazole - Tinea Pedis (Medicis) Xerese (in Canada) – Herpes Labialis (Valeant) Launched more than 300 branded generic products in Emerging Markets Launched multiple patented/OTC products Regederm in Brazil Zyclara Pump (Medicis) and Potiga in U.S. Sublinox and Lodalis in Canada >25 OTC line extensions in U.S. and Canada (CeraVe, Bedoyecta) Achieved several regulatory approvals Dysport (Medicis) in Canada Restylane-L (Medicis) in U.S. Balance Sheet Management: Repurchased 5.3 million common shares at average cost of ~$53 per share Raised $4.55 billion in high yield notes and loans |
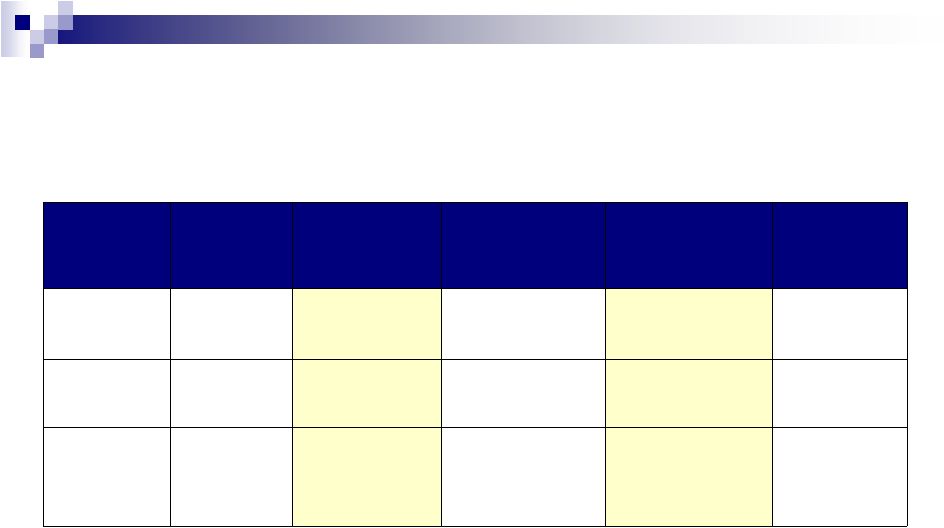 5 2012 Full Year Performance See Note 1 regarding guidance 2011 As Reported 2011 w/o One-time Items 2012 Guidance 2012 Guidance w/o One-time Items Growth Revenue $2.46 billion $2.39 billion $3.4 - $3.6 billion $3.3 - $3.5 billion ~40% - 45% Cash EPS $2.93 $2.63 $4.48 - $4.53 $4.11 - $4.16 ~55% - 60% Adjusted Cash Flow from Operations $925 million $849 million $1.2 - $1.3 billion $1.1 - $1.2 billion ~30% - 40% |
 6 Previous Q4 Guidance Unchanged Fourth Quarter 2012 Guidance Revenue expected to be >$900 million Cash EPS expected to be between $1.18 - $1.23 Adjusted cash flows from operations expected to be between $330 - $430 million Medicis impact expected to be immaterial See Note 1 regarding guidance |
 7 Medicis Integration Update Leadership team in place for nearly one month Nearly all personnel decisions have been made and communicated Sales incentive programs in place to ensure Q1 success Sales force product training scheduled for late January Approximately 350 sales professionals (Dermatology, Aesthetics, Podiatry) in the field Upsides from Medicis R&D Pipeline 2 scheduled product launches Zyclara Pump launched Q3 2012 Dysport Canada to be launched Q1 2013 2 late stage products Luliconazole filed Q4 2012 MetroGel 1.3% Hydrogel - Bacterial Vaginosis (to be filed 1H 2013) Life cycle management opportunities Synergies We now expect synergies to exceed $275M on a run rate basis by end of 2013 Significant amount of synergies will not occur until back half of 2013 (ie. R&D and Legal) Restructuring costs expected to be less than full year run rate synergies with the majority incurred in Q4 2012 |
 8 Agenda 2012 Review – J. Michael Pearson 2013 Guidance – Howard Schiller New Segment Reporting Other Updates – J. Michael Pearson New 2013 Strategic Objectives Organizational Structure |
 9 Financial Guidance for 2013* See Note 1 regarding guidance 2013 % over 2012 Revenue $4.4 - $4.8 billion ~35% Cash EPS $5.45 - ~35% Cash EPS Including Royalty to Meda $5.35 - $5.65 ~33% Adjusted Cash Flow from Operations $1.5 - $1.75 billion ~40% * Excludes potential acquisitions other than Natur Produkt which is expected to close January 2013 |
 10 2013 Guidance Assumptions Exchange rates are based on current spot rates Impact from generics to be >$100M in revenues vs. 2012 Retin-A Micro, BenzaClin, and Cesamet No generic assumption included for Zovirax ~$40-$50M in revenue declines as a result of planned divestitures Solodyn revenues of ~$250M - $275M Includes Natur Produkt No other acquisitions included in guidance Efinaconazole launch to be breakeven in 2013 Cash EPS expected to be 45%/55% 1H vs. 2H Q2 expected to be lowest quarter Q4 expected to be highest quarter Cash tax rate expected <5% Leverage reduced to <4x Pro Forma EBITDA by the end of Q3 |
 11 New Segment Reporting Beginning in 2013, there will be 2 Operating/Reporting Segments Developed Markets Emerging Markets Revenue and Organic Growth (same store and pro forma) to be reported on a more detailed level: Developed Markets U.S. Promoted U.S. Neuro & Other Canada / Australia Emerging Markets Latin America Central/Eastern Europe South East Asia / South Africa |
 12 Agenda 2012 Review – J. Michael Pearson 2013 Guidance – Howard Schiller New Segment Reporting Other Updates – J. Michael Pearson New 2013 Strategic Objectives Organizational Structure |
 13 New 2013 Strategic Initiatives 1) Optimize the balance sheet by reducing leverage to less than 4x and driving improvements in working capital 2) Successfully integrate Medicis and achieve run rate synergies of greater than $275M by year-end 3) Build out key therapeutic areas (Podiatry, Ophthalmology, Oral Health) and geographic platforms (SEA, SA, LA, Russia) through tuck-in acquisitions 4) Receive approval for efinaconazole and luliconazole and launch both in the U.S. 5) Improve gross margins from 2012 to progress towards our goal of 80% 6) Maintain global government reimbursement levels of less than 20% |
 14 New Executive Organization Mike Pearson Chairman & CEO* Howard Schiller EVP, Chief Financial Officer • Finance • Europe • South East Asia • South Africa Jason Hanson EVP, Company Group Chairman • Latin America • Oral Health • Consumer • Ophthal- mology • R&D Robert Chai-Onn EVP, General Counsel • Corporate Secretary • Legal Brian Stolz EVP, Chief Human Capital Officer • HR • Integration Susan Hall SVP, Global R&D • R&D • Medical Affairs • Regulatory Ryan Weldon EVP, Company Group Chairman • U.S. Dermatology • U.S. Aesthetics • Podiatry Laizer Kornwasser EVP, Company Group Chairman • U.S. Neuro & Other • Canada • U.S. Managed Care & Distribution * Chief Medical Officer and Chief Compliance Officer report directly to CEO |
 2013 Financial Guidance Conference Call January 4, 2013 |