Exhibit 99.1

Analyst Teach - In June 21, 2022 11 am EDT
2 LEGAL DISCLAIMERS About this Presentation This presentation is for informational purposes only to assist interested parties in making their own evaluation with respect to a proposed business combination (the Business Combination) between Avista Public Acquisition Corp. II (APAC) and OmniAb, Inc. (OmniAb), a wholly owned subsidiary of Ligand Pharmaceuticals Incorporated (Liga nd) , and related transactions, and for no other purpose. The information contained herein does not purport to be all inclusive and no representation or warranty, express or implied, is or will be given by APA C, OmniAb or Ligand or any of their respective affiliates, directors, officers, employees or advisers or any other person as to the accuracy, completeness or reliability of the information contained in this presentatio n. Forward - Looking Statements This presentation contains forward - looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expe ct,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “would” and similar expressions may identify forward - looking statements, but the absence of these words does not mean that a sta tement is not forward - looking. All statements other than statements of historical facts contained in this presentation, including statements regarding the expected timing and structure of the proposed transa cti on, the ability of the parties to complete the proposed transaction, the expected benefits of the proposed transaction, the tax consequences of the proposed transaction, the amount of gross proceeds expected to be available to OmniAb after the closing and giving effect to any redemptions by APAC shareholders, OmniAb’s future results of operations and financial position, business strategy and its expectations regar din g the application of, and the rate and degree of market acceptance of, the OmniAb technology platform and other technologies, OmniAb’s expectations regarding the addressable markets for our technologies, inc lud ing the growth rate of the markets in which it operates, the potential for and timing of receipt of milestones and royalties under OmniAb’s license agreements with partners, are forward - looking statements. T hese forward - looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factor s, many of which are outside the control of Ligand, OmniAb and APAC, that could cause actual results or outcomes to differ materially from those discussed in the forward - looking statements. Important factors, among others, that may affect actual results or outcomes include, but are not limited to: the risk that the transactions may not be completed in a timely manner or at all, which may adversely affect the price of Li gand’s or APAC’s securities; the risk that APAC shareholder approval of the proposed transactions is not obtained; the inability to recognize the anticipated benefits of the proposed transactions, which may be aff ected by, among other things, the amount of funds available in APAC’s trust account following any redemptions by APAC’s shareholders; the failure to receive certain governmental and regulatory approvals; the o ccu rrence of any event, change or other circumstance that could give rise to the termination of the merger agreement; changes in general economic conditions, including as a result of the COVID - 19 pandemic or t he conflict between Russia and Ukraine; the outcome of litigation related to or arising out of the proposed transactions, or any adverse developments therein or delays or costs resulting therefrom; the eff ect of the announcement or pendency of the transactions on Ligand’s, OmniAb’s or APAC’s business relationships, operating results, and businesses generally; the ability to continue to meet Nasdaq’s listing sta ndards following the consummation of the proposed transactions; costs related to the proposed transactions; that the price of APAC’s or Ligand’s securities may be volatile due to a variety of factors, including Li gand’s, APAC’s or OmniAb’s inability to implement their business plans or meet or exceed their financial projections and changes in the combined capital structure; the ability to implement business plans, forecasts , a nd other expectations after the completion of the proposed transactions, and identify and realize additional opportunities; and the ability of OmniAb to implement its strategic initiatives. The foregoing list of factors is not exhaustive. You should carefully consider the foregoing factors and the other risks and unc ertainties described in the “Risk Factors” section of APAC’s registration statement on Form S - 1 (File No. 333 - 257177), the registration statement on Form S - 4 (File No. 333 - 264525), the registration statement on Form 10, the proxy/information statement/prospectus and certain other documents filed or that may be filed by APAC, Ligand or OmniAb from time to time with the SEC following the date hereof. These filings identi fy and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward - looking statements. Forward - looking statements speak only a s of the date they are made. Readers are cautioned not to put undue reliance on forward - looking statements, and Ligand, OmniAb and APAC assume no obligation and do not intend to update or revise these forward - looking statements, whether as a result of new information, future events, or otherwise. Neither Ligand, OmniAb, or APAC gives any assurance that Ligand, OmniAb or APAC will achieve their expectations. T his caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Market and Industry Data This presentation also contains estimates and other statistical data made by independent parties and by us relating to market si ze and growth and other data about the antibody industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, project ion s, assumptions, and estimates of OmniAb’s future performance and the future performance of the markets in which OmniAb operates are necessarily subject to a high degree of uncertainty and risk.
3 LEGAL DISCLAIMERS Important Information and Where to Find It In connection with the proposed transaction, OmniAb will file a registration statement on Form 10 registering shares of OmniA b c ommon stock and APAC has filed a registration statement on Form S - 4 (File No. 333 - 264525) with the SEC registering shares of APAC common stock, warrants and certain equity awards. The Form S - 4 to be filed by A PAC will include a proxy statement/prospectus in connection with the APAC shareholder vote required in connection with the proposed transaction. The Form 10 to be filed by OmniAb will include the Form S - 4 registration statement filed by APAC which will serve as an information statement/prospectus in connection with the spin - off of OmniAb. This communication does not contain all the information that sho uld be considered concerning the Business Combination. This communication is not a substitute for the registration statements that OmniAb and APAC will file with the SEC or any other documents that APAC or OmniAb may file with the SEC or that APAC, Ligand or OmniAb may send to stockholders in connection with the Business Combination. It is not intended to form the basis of any investment decision or an y other decision in respect to the Business Combination. APAC's shareholders and Ligand’s stockholders and other interested persons are advised to read, when available, the preliminary and definitive regist rat ion statements, and documents incorporated by reference therein, as these materials will contain important information about APAC, OmniAb and the Business Combination. The proxy statement/prospectus contained in APAC’s registration statement will be mailed to APAC's shareholders as of a record date to be established for voting on the Business Combination. The registration statements, proxy statement/prospectus and other documents (when they are available) will also be available fre e of charge, at the SEC's website at www.sec.gov, or by directing a request to: Avista Healthcare Public Acquisition Corp. II, 65 East 55th Street, 18th Floor, New York, NY 10022. Participants in the Solicitation APAC, Ligand and OmniAb and each of their respective directors, executive officers and other members of their management and emp loyees may be deemed to be participants in the solicitation of proxies from APAC’s shareholders in connection with the Business Combination. Shareholders are urged to carefully read the proxy statement/p rospectus regarding the Business Combination when it becomes available, because it will contain important information. Information regarding the persons who may, under the rules of the SEC, be deemed partici pants in the solicitation of APAC’s shareholders in connection with the Business Combination will be set forth in the registration statement when it is filed with the SEC. Information about APAC's executive o fficers and directors and OmniAb's management and directors also will be set forth in the registration statement relating to the Business Combination when it becomes available. No Solicitation or Offer This presentation and any oral statements made in connection with this presentation shall neither constitute an offer to sell no r the solicitation of an offer to buy any securities, or the solicitation of any proxy, vote, consent or approval in any jurisdiction in connection with the proposed Business Combination, nor shall there be any sale of sec urities in any jurisdiction in which the offer, solicitation or sale would be unlawful prior to any registration or qualification under the securities laws of any such jurisdictions. This communication is restricted by la w; it is not intended for distribution to, or use by any person in, any jurisdiction where such distribution or use would be contrary to local law or regulation. Trademarks This presentation contains trademarks, service marks, and trade names of Ligand, OmniAb, APAC and other companies, which are the property of their respective owners. Solely for convenience, the trademarks, service marks, trade names and copyrights referred to in this presentation may appear without the TM, SM, ® or © symbols, but su ch references are not intended to indicate, in any way, that APAC, Ligand or OmniAb will not assert, to the fullest extent under applicable law, their rights or the right of the applicable licensor to t hes e trademarks, service marks, trade names and copyrights.

Welcome Matt Foehr

5 TODAY’S AGENDA Topic Presenter Slides Intro to OmniAb Matt Foehr 6 - 22 Diverse Antibody Repertoires Bill Harriman, PhD 23 - 35 Screening Technologies and Identification Bob Chen, PhD 36 - 43 Ion Channel Technology and Partnerships Doug Krafte, PhD 44 - 50 Partners and Portfolio Christel Iffland, PhD 51 - 60 Financial Review Kurt Gustafson 61 - 74
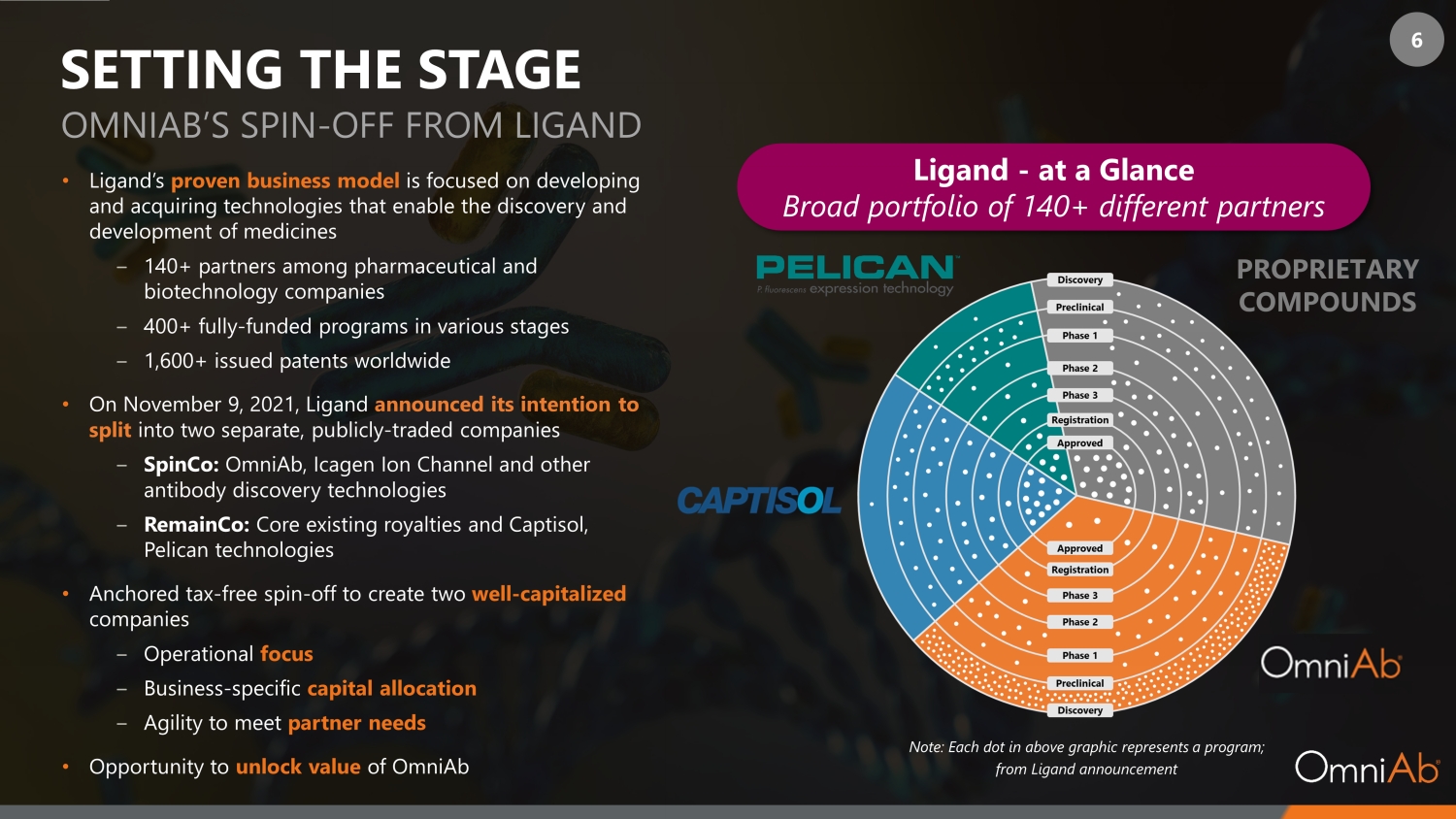
6 SETTING THE STAGE • Ligand’s proven business model is focused on developing and acquiring technologies that enable the discovery and development of medicines ‒ 140+ partners among pharmaceutical and biotechnology companies ‒ 400+ fully - funded programs in various stages ‒ 1,600+ issued patents worldwide • On November 9, 2021, Ligand announced its intention to split into two separate, publicly - traded companies ‒ SpinCo: OmniAb, Icagen Ion Channel and other antibody discovery technologies ‒ RemainCo: Core existing royalties and Captisol, Pelican technologies • Anchored tax - free spin - off to create two well - capitalized companies ‒ Operational focus ‒ Business - specific capital allocation ‒ Agility to meet partner needs • Opportunity to unlock value of OmniAb Ligand - at a Glance Broad portfolio of 140+ different partners PROPRIETARY COMPOUNDS Note: Each dot in above graphic represents a program; from Ligand announcement OMNIAB’S SPIN - OFF FROM LIGAND
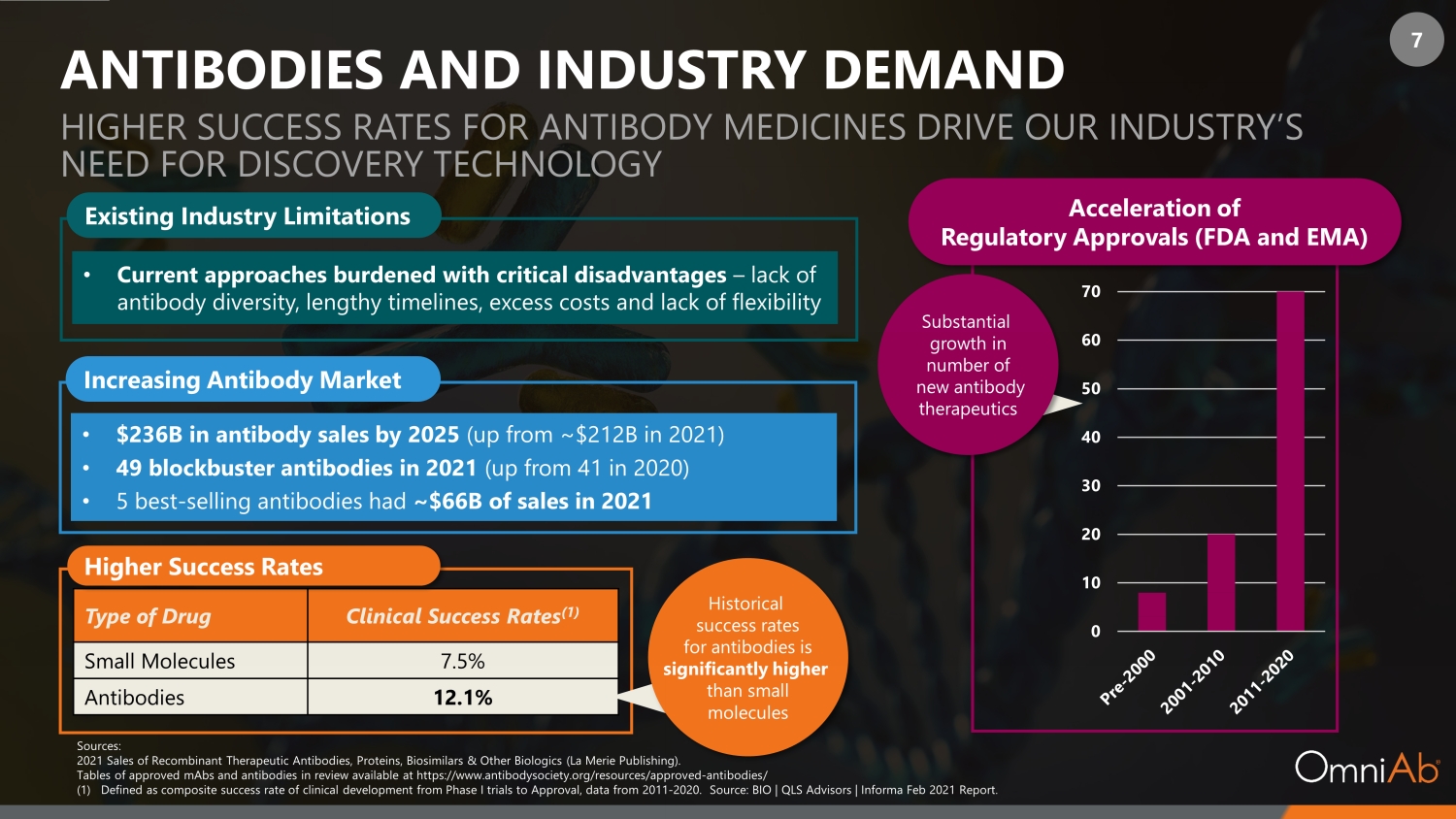
7 0 10 20 30 40 50 60 70 80 Type of Drug Clinical Success Rates (1) Small Molecules 7.5% Antibodies 12.1% Sources: 2021 Sales of Recombinant Therapeutic Antibodies, Proteins, Biosimilars & Other Biologics (La Merie Publishing). Tables of approved mAbs and antibodies in review available at https://www.antibodysociety.org/resources/approved - antibodies/ (1) Defined as composite success rate of clinical development from Phase I trials to Approval, data from 2011 - 2020. Source: BIO | QLS Advisors | Informa Feb 2021 Report . Higher Success Rates Historical success rates for antibodies is significantly higher than small molecules Substantial growth in number of new antibody therapeutics Acceleration of Regulatory Approvals (FDA and EMA) ANTIBODIES AND INDUSTRY DEMAND HIGHER SUCCESS RATES FOR ANTIBODY MEDICINES DRIVE OUR INDUSTRY’S NEED FOR DISCOVERY TECHNOLOGY Increasing Antibody Market • $236B in antibody sales by 2025 (up from ~ $ 212B in 2021 ) • 49 blockbuster antibodies in 2021 (up from 41 in 2020 ) • 5 best - selling antibodies had ~$ 66B of sales in 2021 Existing Industry Limitations • Current approaches burdened with critical disadvantages – lack of antibody diversity, lengthy timelines, excess costs and lack of flexibility

8 The OmniAb discovery platform provides our partners access to the most diverse antibody repertoires and cutting - edge screening technologies to enable discovery of next - generation therapeutics. Value Proposition

9 Mission Our mission is to enable the rapid development of innovative therapeutics by pushing the frontiers of drug discovery technologies . We achieve this by enabling the discovery of high - quality therapeutic candidates and by being the partner of choice for pharma and biotech companies.
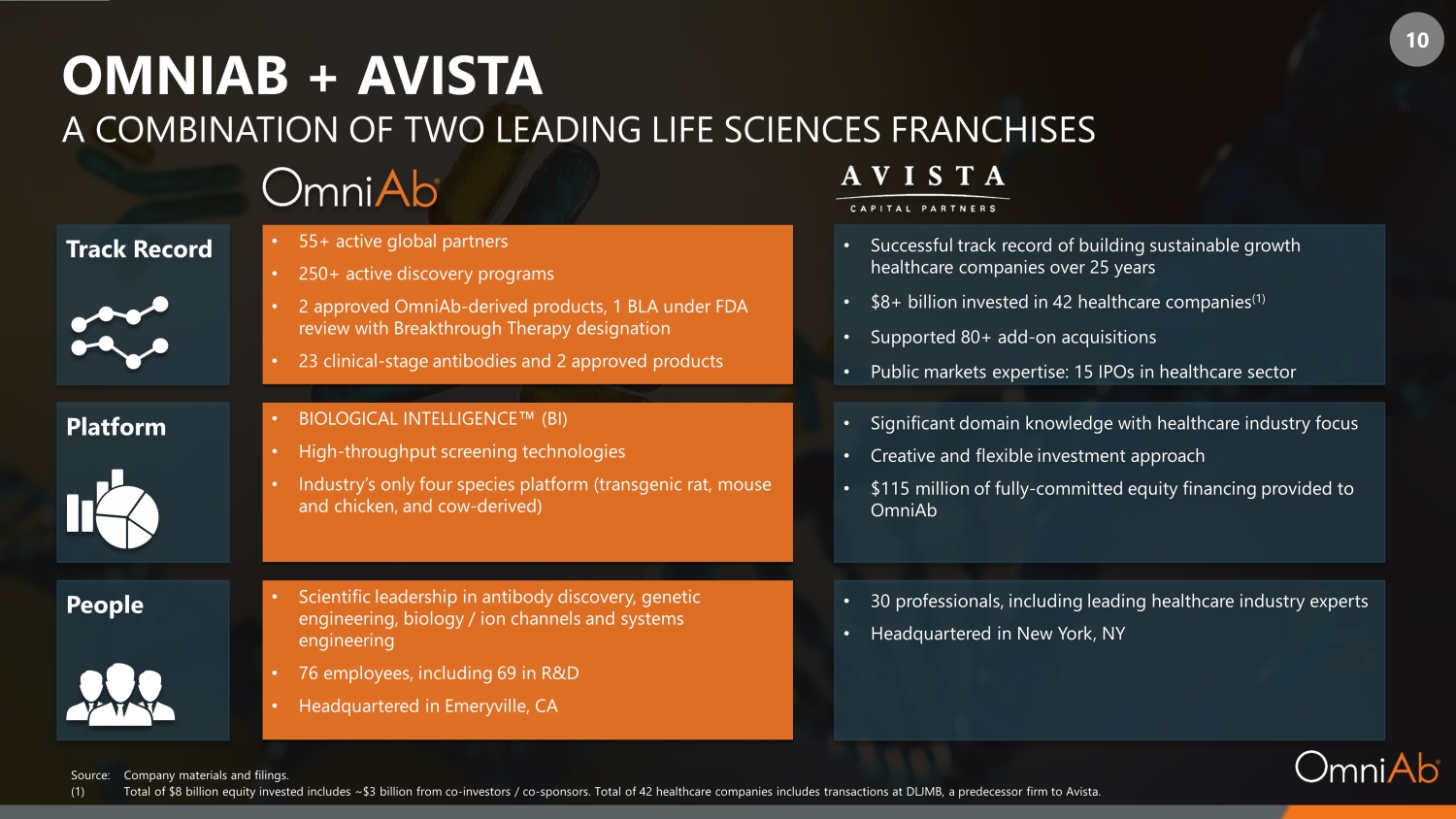
10 OMNIAB + AVISTA A COMBINATION OF TWO LEADING LIFE SCIENCES FRANCHISES Platform Track Record People Source: Company materials and filings. (1) Total of $8 billion equity invested includes ~$3 billion from co - investors / co - sponsors. Total of 42 healthcare companies inclu des transactions at DLJMB, a predecessor firm to Avista. • BIOLOGICAL INTELLIGENCE Œ (BI) • High - throughput screening technologies • Industry’s only four species platform (transgenic rat, mouse and chicken, and cow - derived) • 55+ active global partners • 250+ active discovery programs • 2 approved OmniAb - derived products, 1 BLA under FDA review with Breakthrough Therapy designation • 23 clinical - stage antibodies and 2 approved products • Scientific leadership in antibody discovery, genetic engineering, biology / ion channels and systems engineering • 76 employees, including 69 in R&D • Headquartered in Emeryville, CA • Significant domain knowledge with healthcare industry focus • Creative and flexible investment approach • $115 million of fully - committed equity financing provided to OmniAb • Successful track record of building sustainable growth healthcare companies over 25 years • $8+ billion invested in 42 healthcare companies (1) • Supported 80+ add - on acquisitions • Public markets expertise: 15 IPOs in healthcare sector • 30 professionals, including leading healthcare industry experts • Headquartered in New York, NY
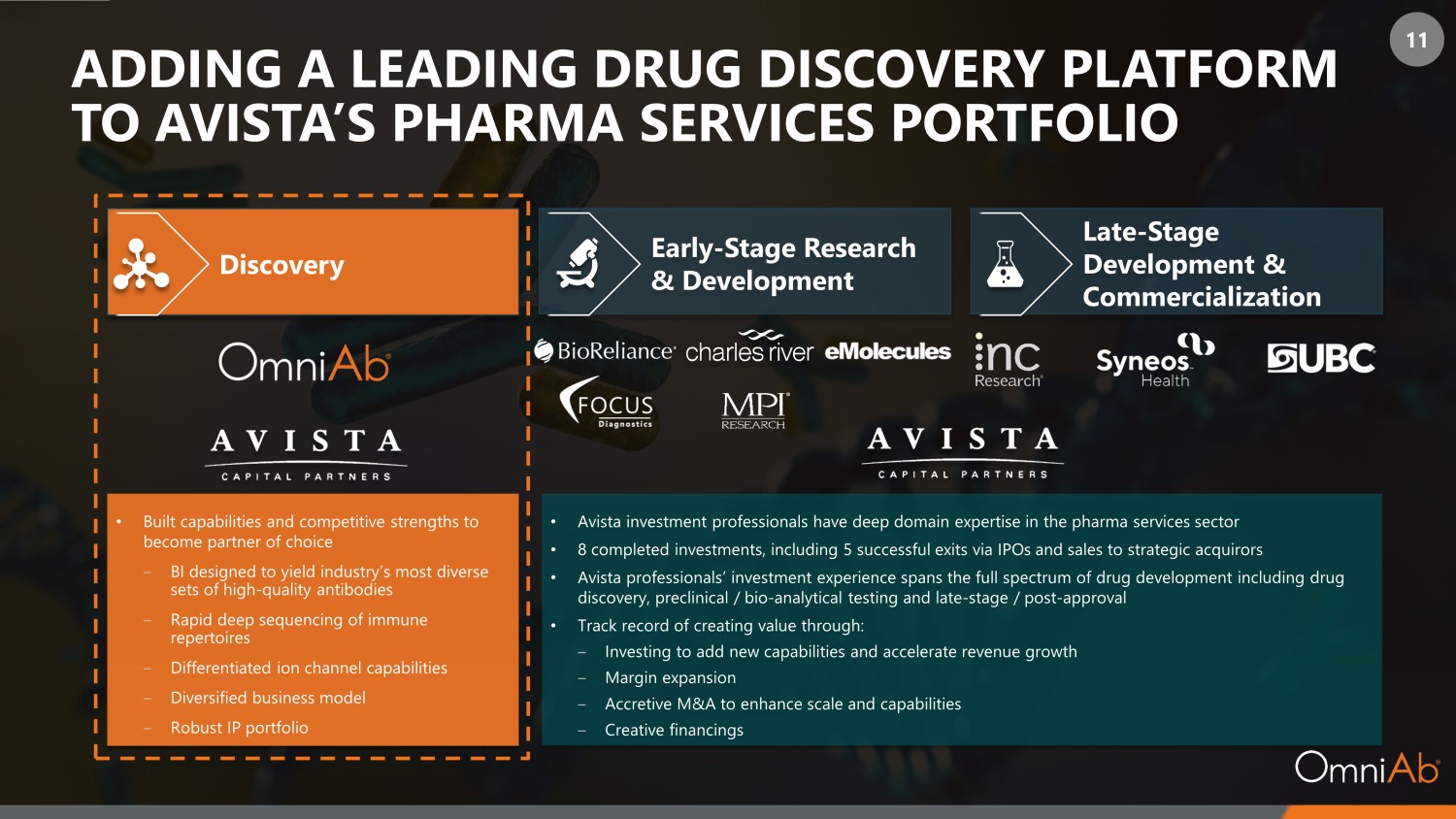
11 ADDING A LEADING DRUG DISCOVERY PLATFORM TO AVISTA’S PHARMA SERVICES PORTFOLIO Discovery Early - Stage Research & Development Late - Stage Development & Commercialization • Built capabilities and competitive strengths to become partner of choice ‒ BI designed to yield industry’s most diverse sets of high - quality antibodies ‒ Rapid deep sequencing of immune repertoires ‒ Differentiated ion channel capabilities ‒ Diversified business model ‒ Robust IP portfolio • Avista investment professionals have deep domain expertise in the pharma services sector • 8 completed investments, including 5 successful exits via IPOs and sales to strategic acquirors • Avista professionals’ investment experience spans the full spectrum of drug development including drug discovery, preclinical / bio - analytical testing and late - stage / post - approval • Track record of creating value through: ‒ Investing to add new capabilities and accelerate revenue growth ‒ Margin expansion ‒ Accretive M&A to enhance scale and capabilities ‒ Creative financings

12 EXPERIENCED LEADERSHIP TEAM & BOARD Carolyn Bertozzi, PhD Board Member Professor of Humanities & Sciences Stanford University Investigator HHMRI Former Board Member Eli Lilly, Advisor to GlaxoSmithKline Founder of multiple companies Sarah Boyce Board Member Chair of HC&C Committee CEO, Avidity Board Member Berkeley Lights Former Akcea Therapeutics, Ionis Pharmaceuticals, Forest Labs Sunil Patel Board Member Chair of Audit Committee Public & Private Biotech Executive Former Abgenix, Gilead, BiPar and OncoMed Jennifer Cochran, PhD Board Member Chair of Nominating Committee Professor of Bioengineering Stanford University Founder of multiple companies John Higgins Board Chair CEO and Board Member, Ligand Former CFO, Connetics Corp Board Member Bio - Techne Matt Foehr Board Member CEO, OmniAb Inc. Board Member Viking Therapeutics Former Exec at Ligand, GlaxoSmithKline, Stiefel Labs, Connetics Kurt Gustafson CFO, OmniAb Inc. Board Member Xencor, Inc Former CFO/Exec at Spectrum Pharmaceuticals, Halozyme Therapeutics, Amgen OmniAb Board Nominees OmniAb Leadership Charles Berkman Chief Legal Officer, OmniAb Inc. General Counsel at Ligand, Baker & McKenzie Lyon & L yon Note: Avista to name additional Board nominee

THE OMNIAB PLATFORM
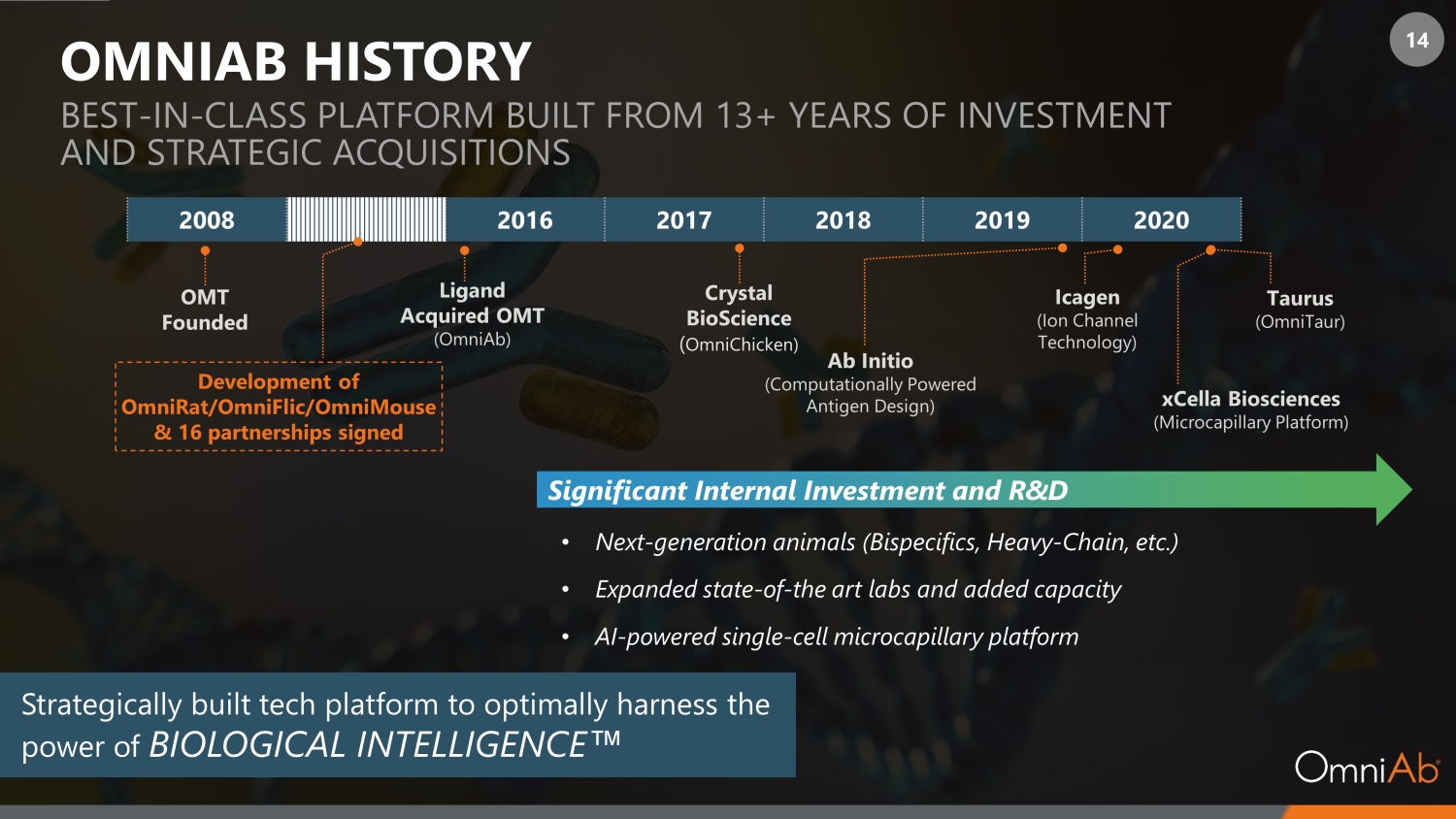
14 OMNIAB HISTORY BEST - IN - CLASS PLATFORM BUILT FROM 13+ YEARS OF INVESTMENT AND STRATEGIC ACQUISITIONS Ligand Acquired OMT (OmniAb) Crystal BioScience ( OmniChicken) 2008 2016 2017 2018 2019 2020 OMT Founded Ab Initio (Computationally Powered Antigen Design) xCella Biosciences (Microcapillary Platform) Taurus (OmniTaur) • Next - generation animals (Bispecifics, Heavy - Chain, etc.) • Expanded state - of - the art labs and added capacity • AI - powered single - cell microcapillary platform Strategically built tech platform to optimally harness the power of BIOLOGICAL INTELLIGENCE Œ Development of OmniRat/OmniFlic/OmniMouse & 16 partnerships signed Icagen (Ion Channel Technology) Significant Internal Investment and R&D
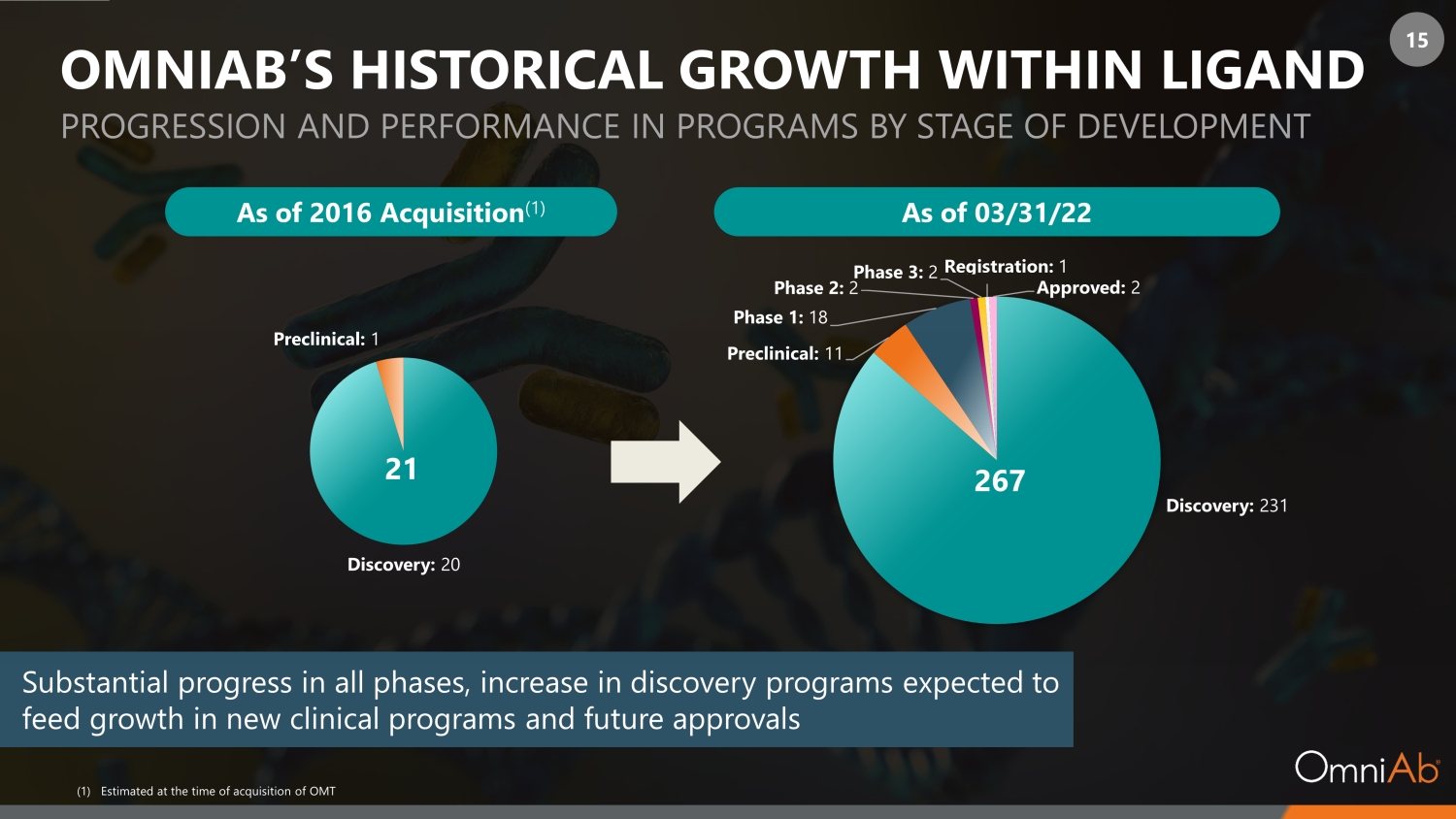
15 Substantial progress in all phases, increase in discovery programs expected to feed growth in new clinical programs and future approvals Discovery: 231 Preclinical: 11 Phase 1: 18 Phase 2: 2 Phase 3: 2 Registration: 1 Approved : 2 Discovery : 20 Preclinical : 1 OMNIAB’S HISTORICAL GROWTH WITHIN LIGAND PROGRESSION AND PERFORMANCE IN PROGRAMS BY STAGE OF DEVELOPMENT As of 2016 Acquisition (1) As of 03/31/22 (1) Estimated at the time of acquisition of OMT 21 267
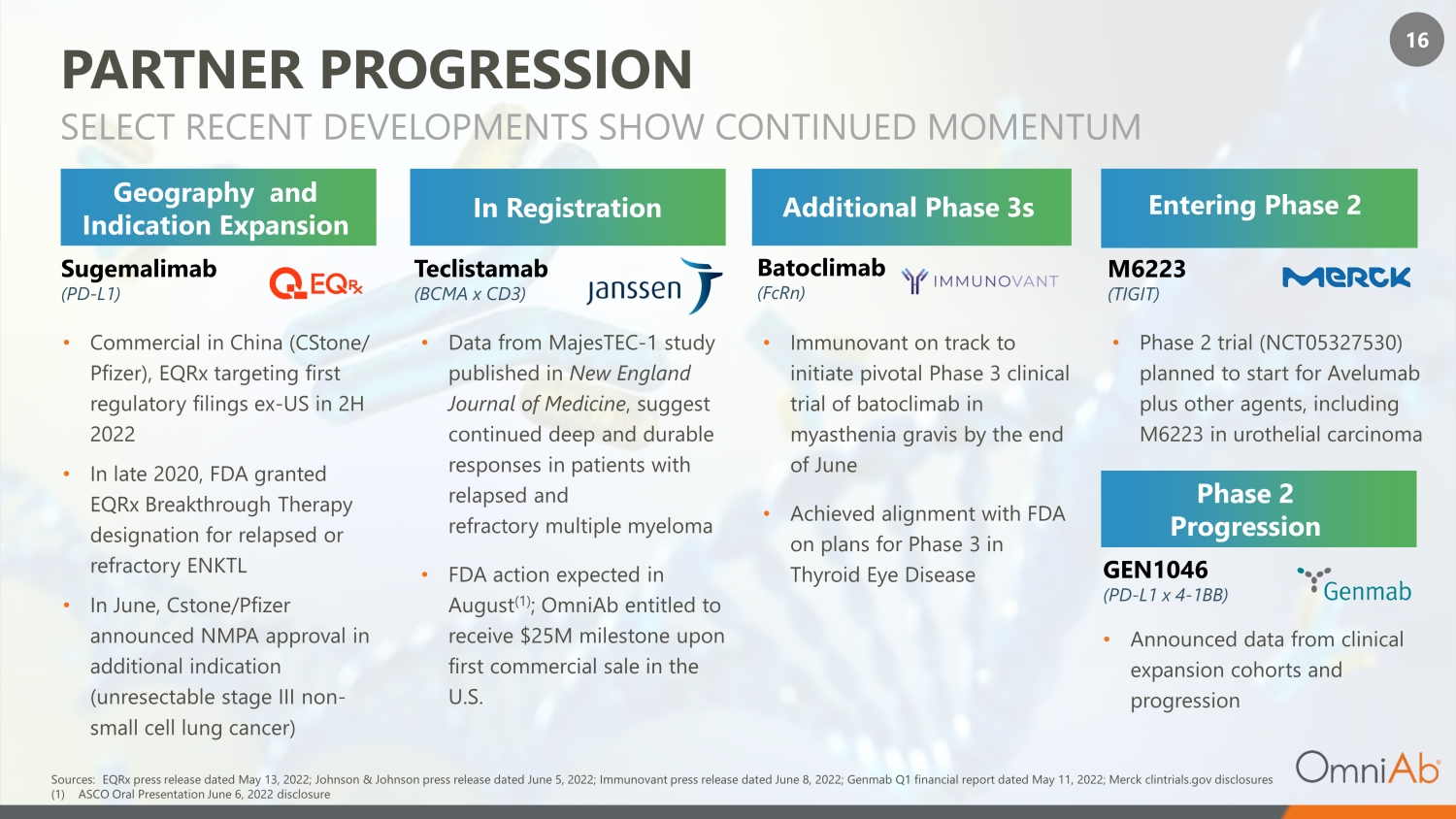
16 PARTNER PROGRESSION SELECT RECENT DEVELOPMENTS SHOW CONTINUED MOMENTUM In Registration Additional Phase 3s Phase 2 Progression Entering Phase 2 • Data from MajesTEC - 1 study published in New England Journal of Medicine , suggest continued deep and durable responses in patients with relapsed and refractory multiple myeloma • FDA action expected in August (1) ; OmniAb entitled to receive $25M milestone upon first commercial sale in the U.S. Teclistamab (BCMA x CD3) Batoclimab (FcRn) GEN1046 (PD - L1 x 4 - 1BB) M6223 (TIGIT) • Immunovant on track to initiate pivotal Phase 3 clinical trial of batoclimab in myasthenia gravis by the end of June • Achieved alignment with FDA on plans for Phase 3 in Thyroid Eye Disease • Announced data from clinical expansion cohorts and progression • Phase 2 trial (NCT05327530) planned to start for Avelumab plus other agents, including M6223 in urothelial carcinoma Geography and Indication Expansion Sugemalimab (PD - L1) • Commercial in China (CStone/ Pfizer), EQRx targeting first regulatory filings ex - US in 2H 2022 • In late 2020, FDA granted EQRx Breakthrough Therapy designation for relapsed or refractory ENKTL • In June, Cstone/Pfizer announced NMPA approval in additional indication (unresectable stage III non - small cell lung cancer) Sources: EQRx press release dated May 13, 2022 ; Johnson & Johnson press release dated June 5, 2022; Immunovant press release dated June 8, 2022; Genmab Q1 financial report d ate d May 11, 2022; Merck clintrials.gov disclosures (1) ASCO Oral Presentation June 6, 2022 disclosure
17 THE OMNIAB PLATFORM Technology offering addresses critical industry needs and is paired with our highly specialized and efficient operations Create Diverse Pools of High - Quality Naturally Optimized Antibodies Screen Millions of Cells to Find Potential Therapeutic Candidates Further Characterize, Select & Optimize the Right Antibody We leverage our proprietary and differentiated technologies rather than commoditized industry services that are widely available from CROs or built into big pharma
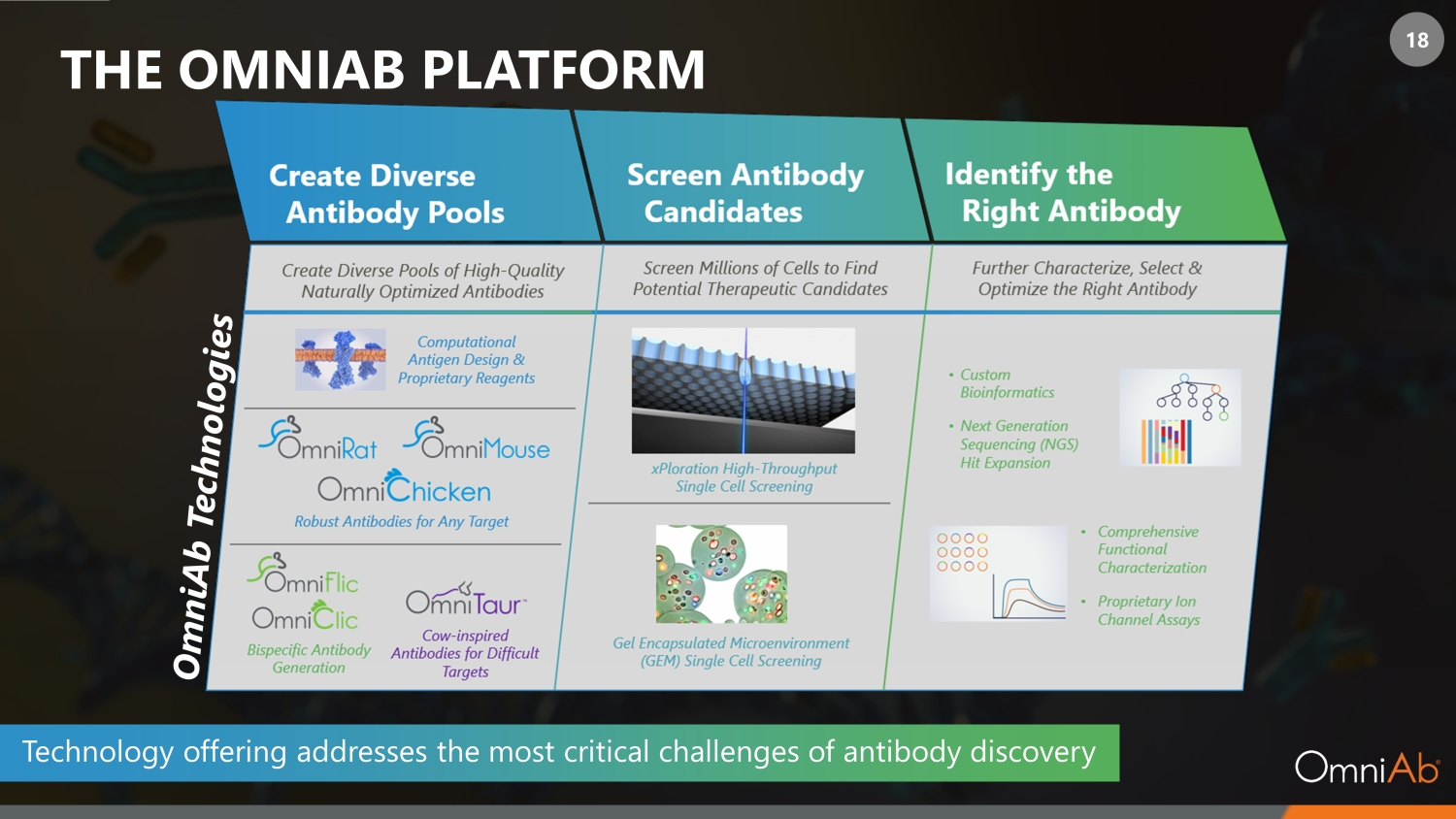
18 THE OMNIAB PLATFORM Technology offering addresses the most critical challenges of antibody discovery
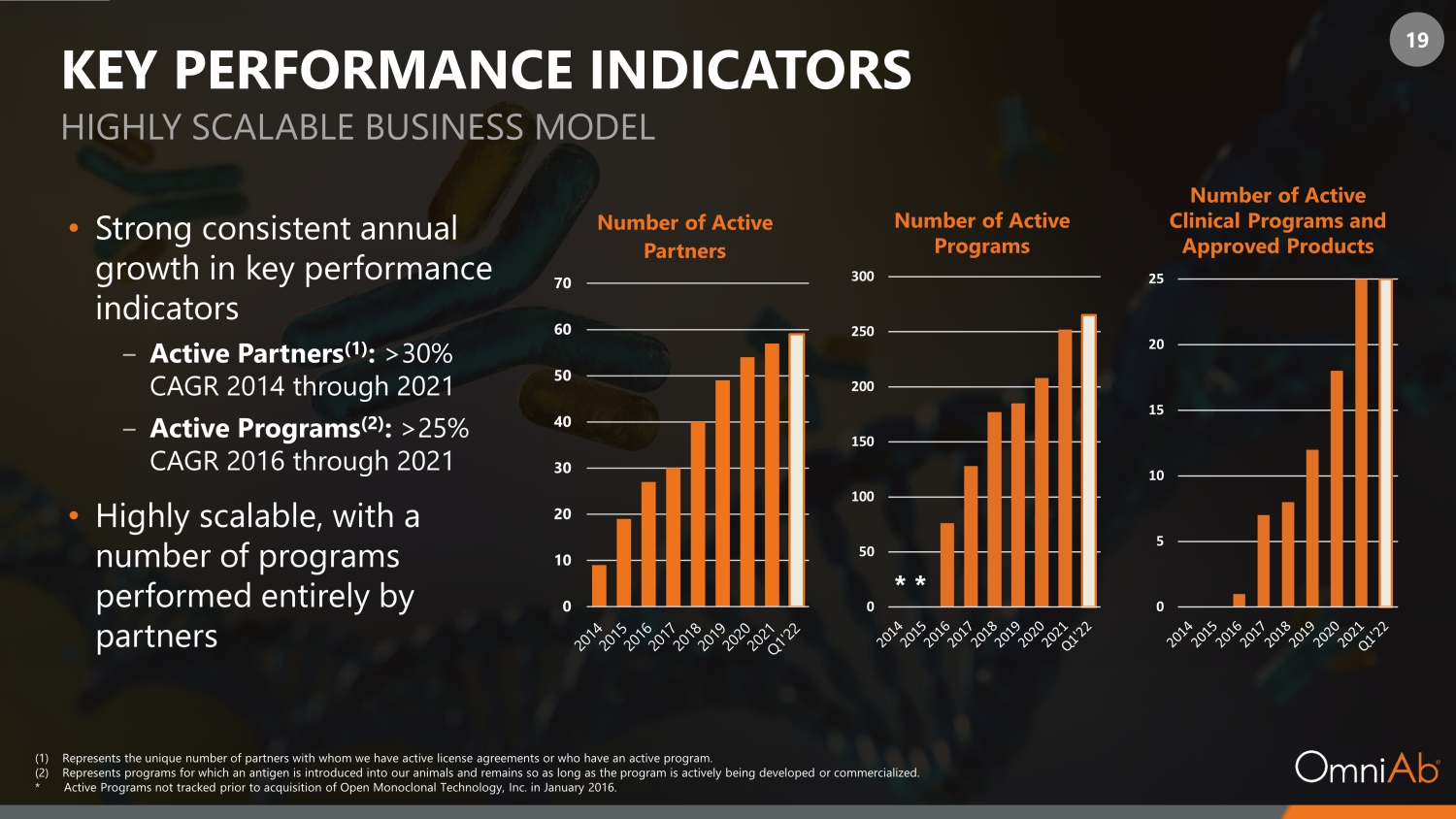
19 KEY PERFORMANCE INDICATORS • Strong consistent annual growth in key performance indicators ‒ Active Partners (1) : >30% CAGR 2014 through 2021 ‒ Active Programs (2) : >25% CAGR 2016 through 2021 • Highly scalable, with a number of programs performed entirely by partners HIGHLY SCALABLE BUSINESS MODEL (1) Represents the unique number of partners with whom we have active license agreements or who have an active program. (2) Represents programs for which an antigen is introduced into our animals and remains so as long as the program is actively bei ng developed or commercialized. * Active Programs not tracked prior to acquisition of Open Monoclonal Technology, Inc. in January 2016. * * 0 10 20 30 40 50 60 70 Number of Active Partners Number of Active Programs Number of Active Clinical Programs and Approved Products 0 50 100 150 200 250 300 0 5 10 15 20 25
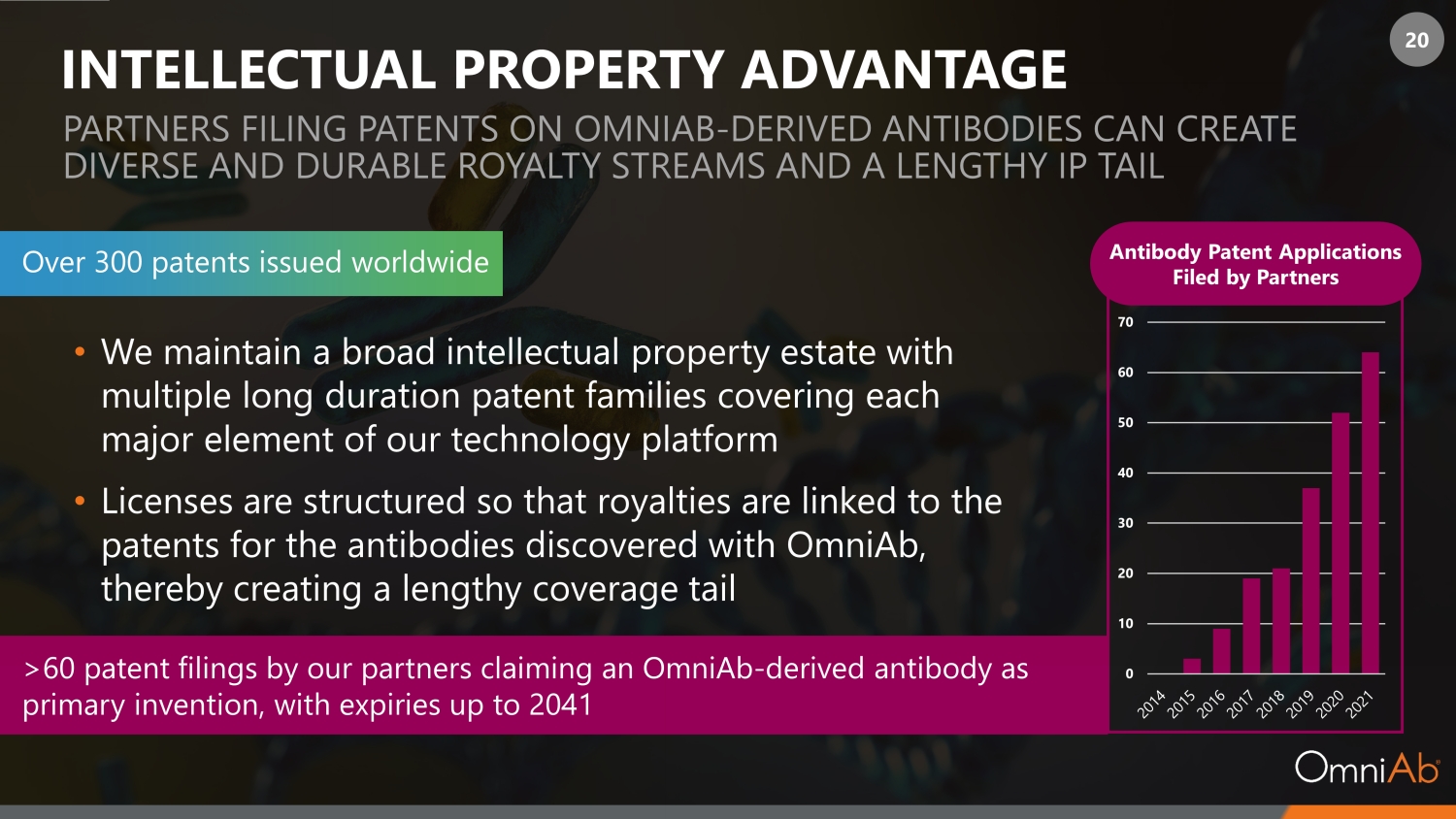
20 INTELLECTUAL PROPERTY ADVANTAGE Antibody Patent Applications Filed by Partners PARTNERS FILING PATENTS ON OMNIAB - DERIVED ANTIBODIES CAN CREATE DIVERSE AND DURABLE ROYALTY STREAMS AND A LENGTHY IP TAIL >60 patent filings by our partners claiming an OmniAb - derived antibody as primary invention, with expiries up to 2041 • We maintain a broad intellectual property estate with multiple long duration patent families covering each major element of our technology platform • Licenses are structured so that royalties are linked to the patents for the antibodies discovered with OmniAb, thereby creating a lengthy coverage tail Over 300 patents issued worldwide 0 10 20 30 40 50 60 70
21 OMNIAB TODAY – KEY AREAS OF FOCUS WE BELIEVE WE ARE WELL - POSITIONED FOR FUTURE GROWTH WHILE WE MAKE AN ENDURING AND SIGNIFICANT IMPACT ON THE INDUSTRY WE LEVERAGE A HIGHLY - SCALABLE BUSINESS WHERE INVESTMENTS IN Partnered Pipeline Development, Expansion and Advancement Expanding the Reach of our Platform Continued Workflow Versatility Initiatives New Technologies (including new transgenic animals) TECHNOLOGIES AND INNOVATION ARE INFORMED BY DISCOVERY RELATIONSHIPS WITH OUR PARTNERS

22 SCIENTIFIC LEADERSHIP TEAM Phil Leighton, PhD Sr. Director, Molecular Biology Genetic Engineering Lead, Crystal Bioscience and Origen Princeton, UCSF Ellen Collarini, PhD Sr. Director, Cell Biology Crystal Bioscience, Trellis, Roche Univ. Michigan, Univ. College - London Shelley Izquierdo, PhD Sr. Director, Antibody Discovery Crystal Bioscience, Trellis UC Berkeley Christel Iffland, PhD VP, Antibody Technologies Former Associate Director at Merck KGaA / EMD Serono, Co - inventor of Avelumab Dana Farber, Albert Einstein College Bob Chen, PhD Sr. Director, Systems Engineering Former Exec at xCella Bio (Co - Founder/CTO) Stanford Bioengineering Marie - Cecile Van De Lavoir, PhD, DVM VP, Operations Co - Founder/COO, Crystal Bioscience Origen Therapeutics, Inventor Germ Cell Technology Fulbright Scholar, UCSF, Utrecht, Guelph, Cornell Bill Harriman, PhD SVP, Antibody Discovery Co - Founder/CSO, Crystal Bioscience Trellis , Roche , Abgenix UCSF - Immunology, Haas MBA Doug Krafte, PhD SVP, Ion Channels Former Exec at Icagen, Pfizer Pain & Sensory Disorders, Boehringer Ingelheim, Aurora Biosciences Univ. Rochester, Vanderbilt

Creation of Diverse Antibody Repertoires Bill Harriman, PhD

24 THE OMNIAB PLATFORM We believe generating large and diverse pools of high - quality antibodies increases the likelihood of discovering the antibody with the most desirable therapeutic characteristics Robust Antibodies for Any Target Bispecific Antibody Generation Cow - inspired Antibodies for Difficult Targets Computational Antigen Design & Proprietary Reagents Antibody Generation Technologies Industry’s only 4 - species platform A heritage of genetic engineering advancements Bispecific and cow - inspired technologies enable next - generation therapeutics 23 clinical - stage and 2 approved antibodies (1) Carefully designed transgenes for robust response (1) In August 2021, zimberelimab, was approved in China for the treatment of recurrent or refractory classical Hodgkin’s lymphoma , a nd in December 2021 sugemalimab, was approved in China for the first - line treatment of metastatic (stage IV) non - small cell lung cancer in combinati on with chemotherapy.
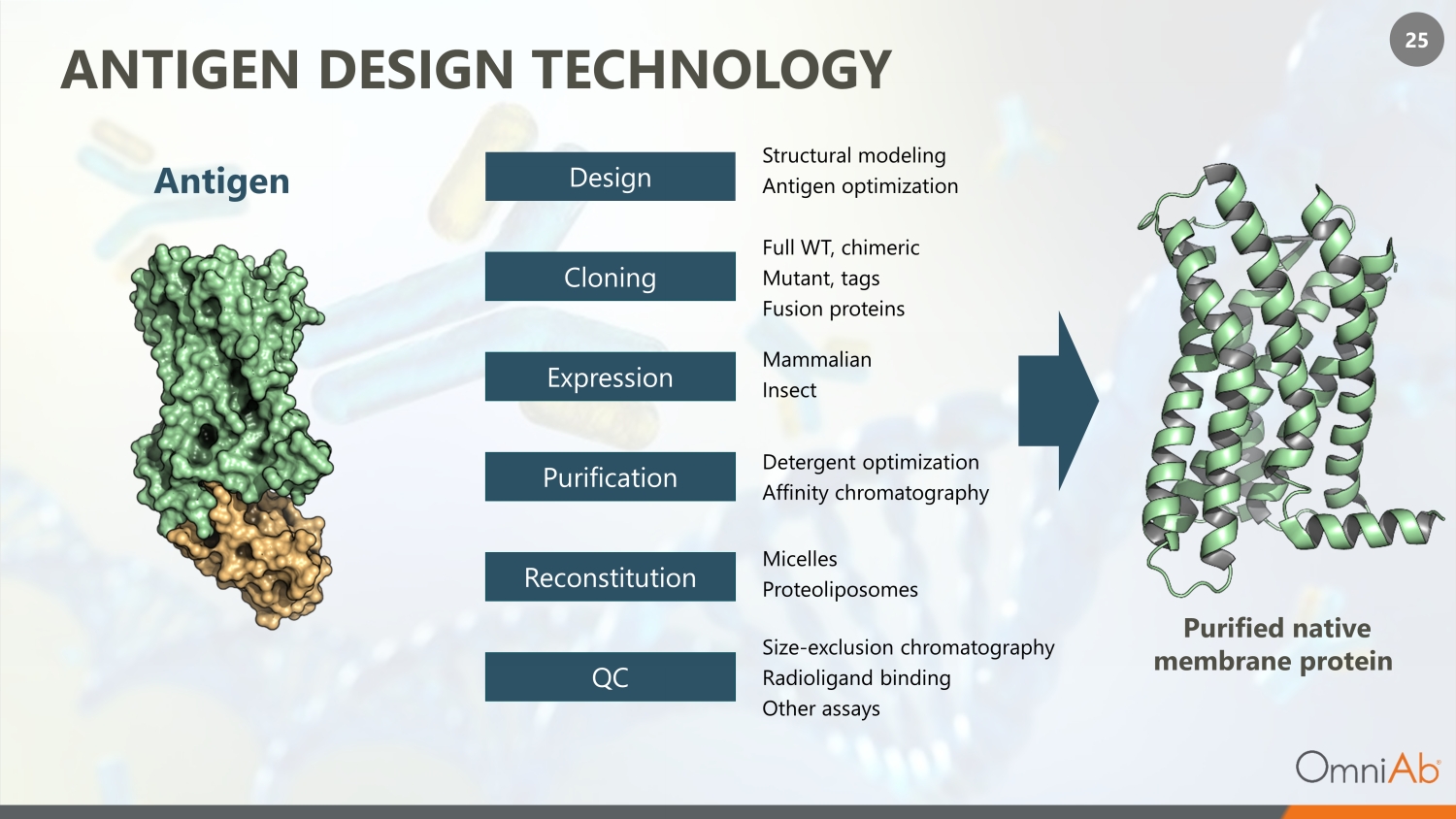
25 ANTIGEN DESIGN TECHNOLOGY Purified native membrane protein Antigen Expression Full WT, chimeric Mutant, tags Fusion proteins Cloning Purification Reconstitution QC Mammalian Insect Detergent optimization Affinity chromatography Micelles Proteoliposomes Size - exclusion chromatography Radioligand binding Other assays Design Structural modeling Antigen optimization
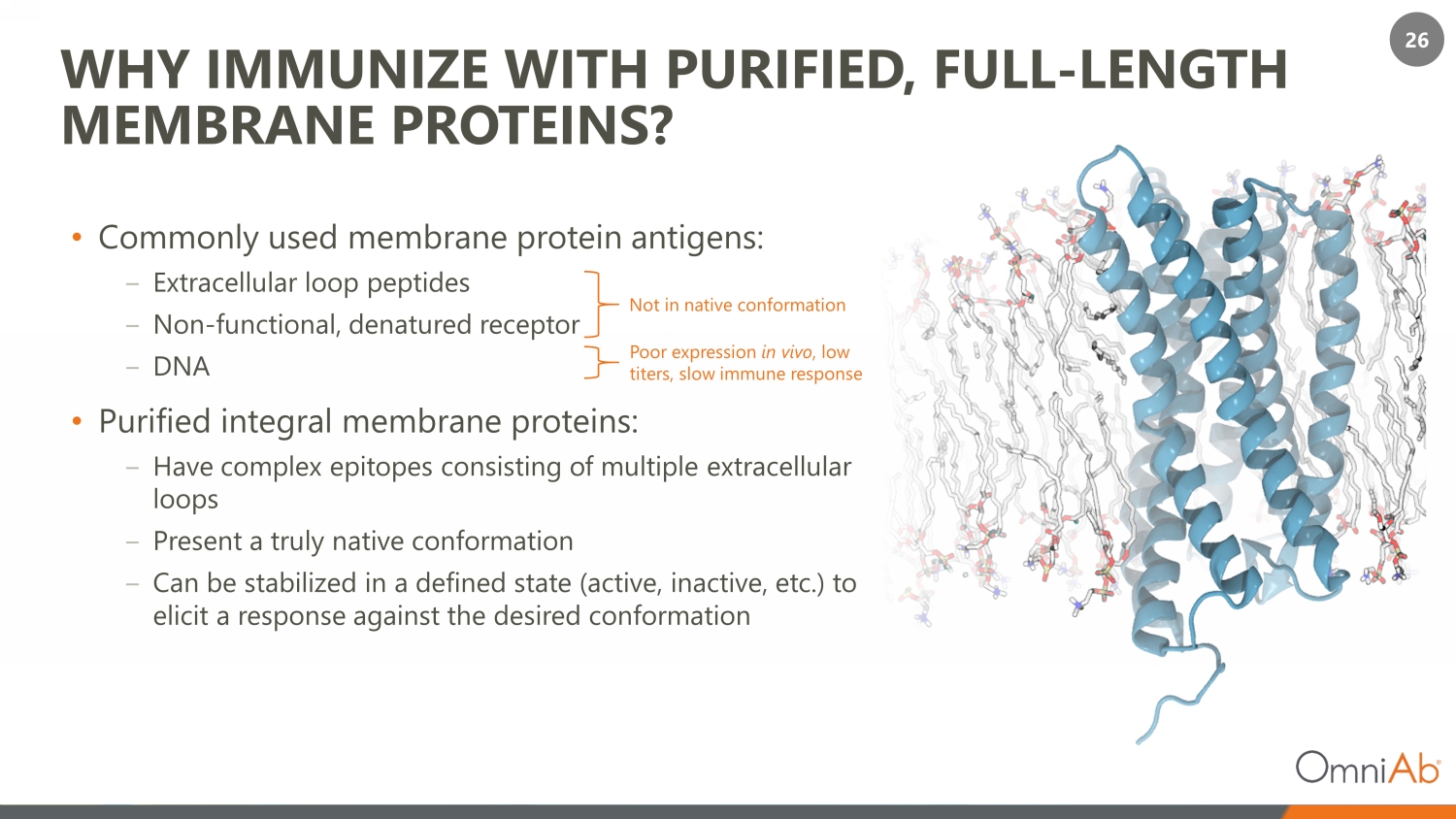
26 WHY IMMUNIZE WITH PURIFIED, FULL - LENGTH MEMBRANE PROTEINS? • Commonly used membrane protein antigens: ‒ Extracellular loop peptides ‒ Non - functional, denatured receptor ‒ DNA • Purified integral membrane proteins: ‒ Have complex epitopes consisting of multiple extracellular loops ‒ Present a truly native conformation ‒ Can be stabilized in a defined state (active, inactive, etc.) to elicit a response against the desired conformation Not in native conformation Poor expression in vivo , low titers, slow immune response
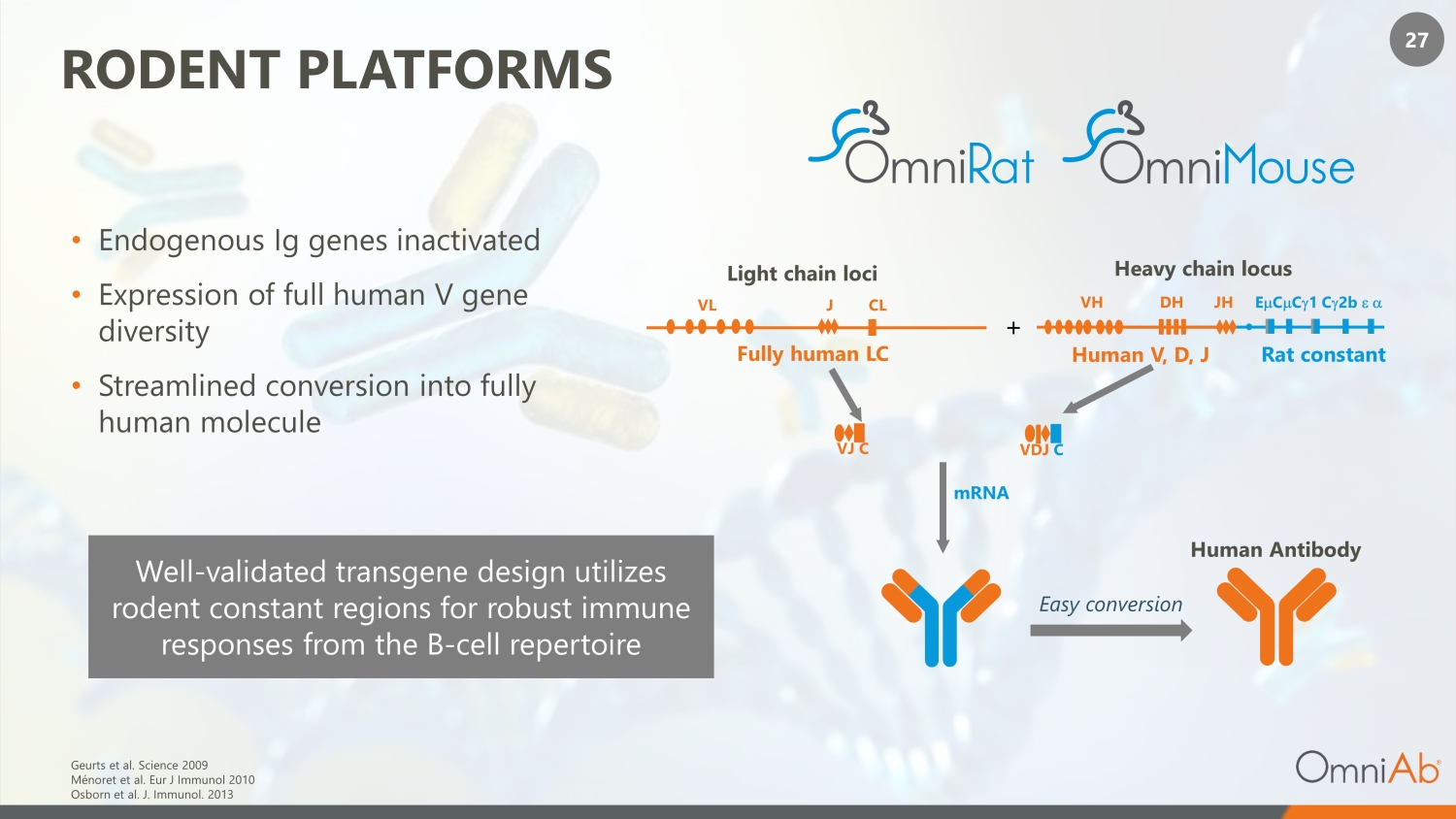
27 RODENT PLATFORMS • Endogenous Ig genes inactivated • Expression of full human V gene diversity • Streamlined conversion into fully human molecule Geurts et al. Science 2009 Ménoret et al. Eur J Immunol 2010 Osborn et al. J. Immunol. 2013 mRNA Human Antibody Light chain loci VL J CL + VJ C VH DH JH E C m C g 1 C 2b e a Easy conversion Heavy chain locus VDJ C Rat constant Human V, D, J Fully human LC Well - validated transgene design utilizes rodent constant regions for robust immune responses from the B - cell repertoire
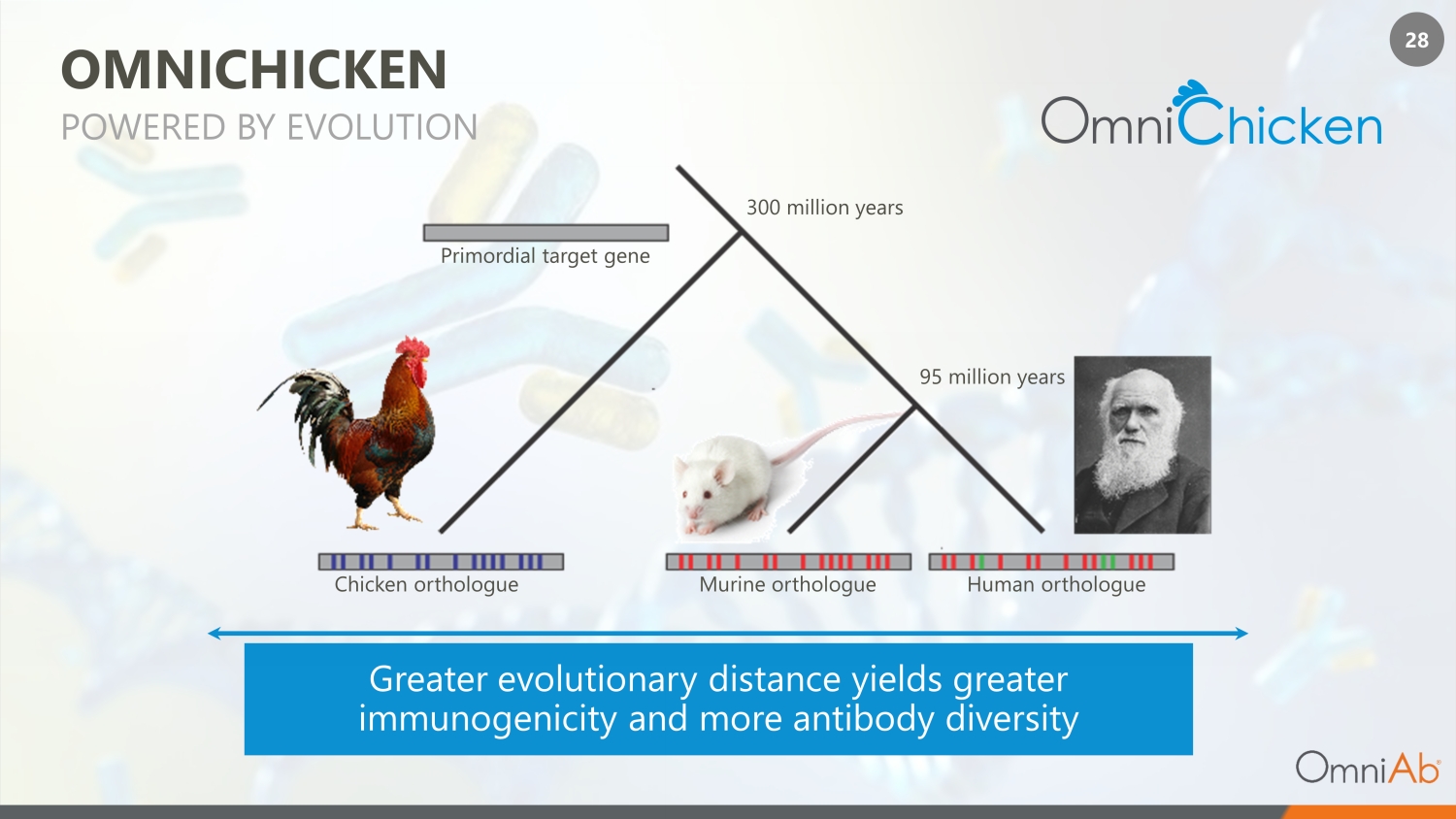
28 P rimordial target gene C hicken orthologue M urine orthologue Human orthologue 300 million years 95 million years OMNICHICKEN Greater evolutionary distance yields greater immunogenicity and more antibody diversity POWERED BY EVOLUTION
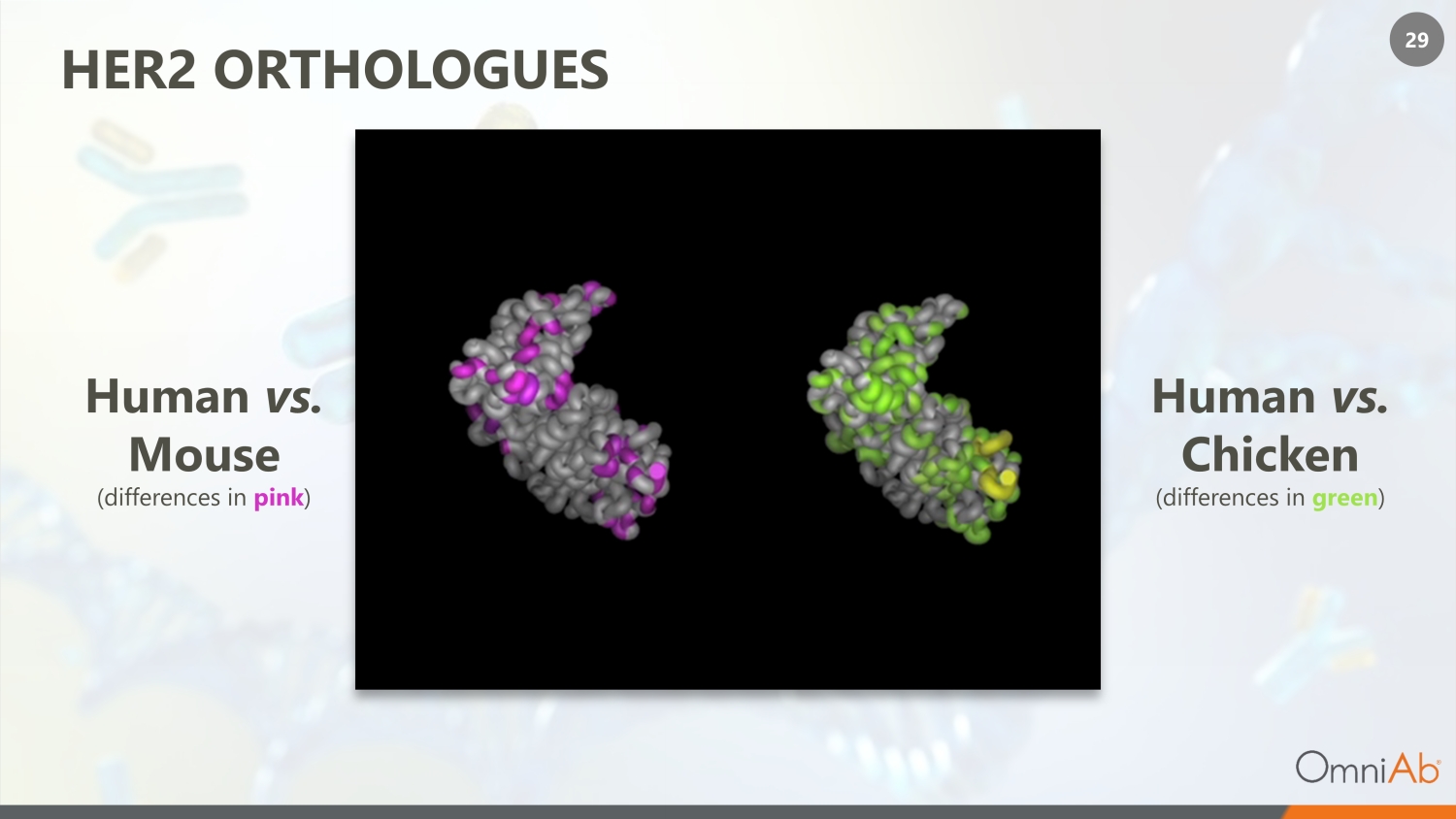
29 HER2 ORTHOLOGUES H uman vs . C hicken (differences in green ) H uman vs . Mouse (differences in pink )
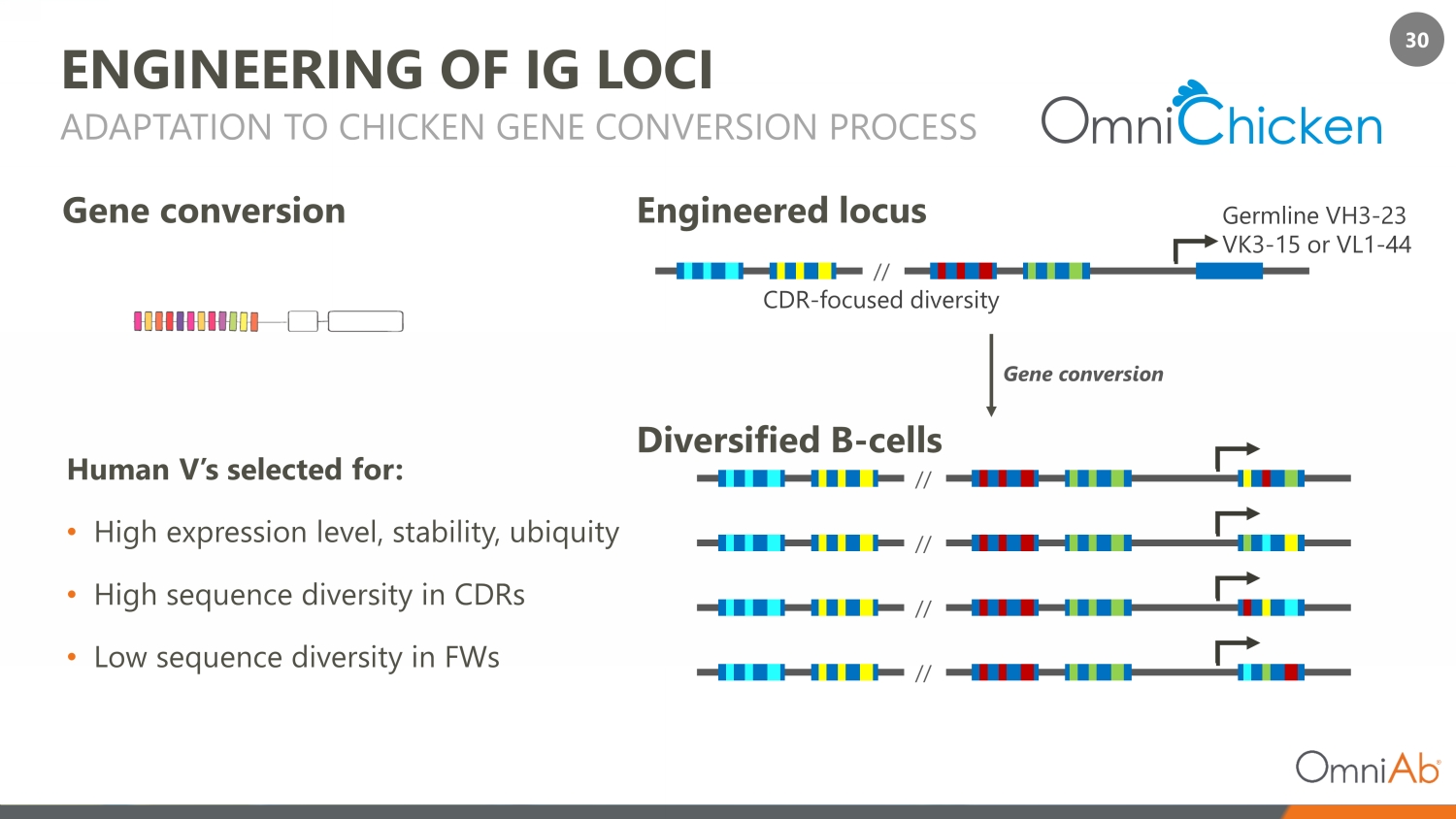
30 ENGINEERING OF IG LOCI Human V’s selected for: • High expression level, stability, ubiquity • High sequence diversity in CDRs • Low sequence diversity in FWs ADAPTATION TO CHICKEN GENE CONVERSION PROCESS CDR - focused diversity G ene conversion Germline VH3 - 23 VK3 - 15 or VL1 - 44 // // // // // Gene conversion Engineered locus Diversified B - cells
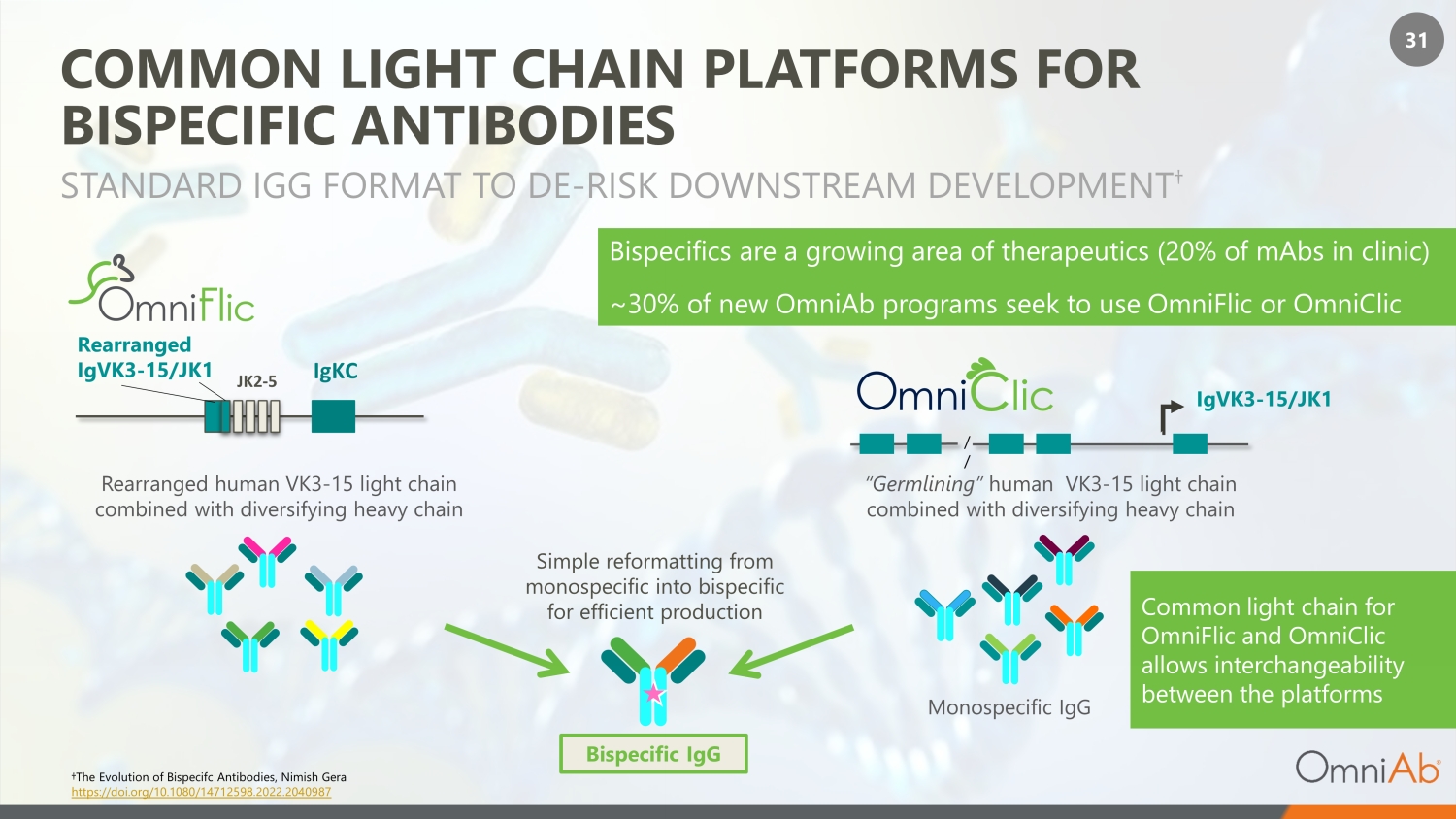
31 COMMON LIGHT CHAIN PLATFORMS FOR BISPECIFIC ANTIBODIES STANDARD IGG FORMAT TO DE - RISK DOWNSTREAM DEVELOPMENT † Monospecific IgG Bispecific IgG Rearranged human VK3 - 15 light chain combined with diversifying heavy chain JK2 - 5 Rearranged IgVK3 - 15/JK1 IgKC IgVK3 - 15/JK1 / / Simple reformatting from monospecific into bispecific for efficient production “Germlining” human VK3 - 15 light chain combined with diversifying heavy chain Common light chain for OmniFlic and OmniClic allows interchangeability between the platforms Bispecifics are a growing area of therapeutics (20% of mAbs in clinic) ~30% of new OmniAb programs seek to use OmniFlic or OmniClic †The Evolution of Bispecifc Antibodies, Nimish Gera https://doi.org/10.1080/14712598.2022.2040987
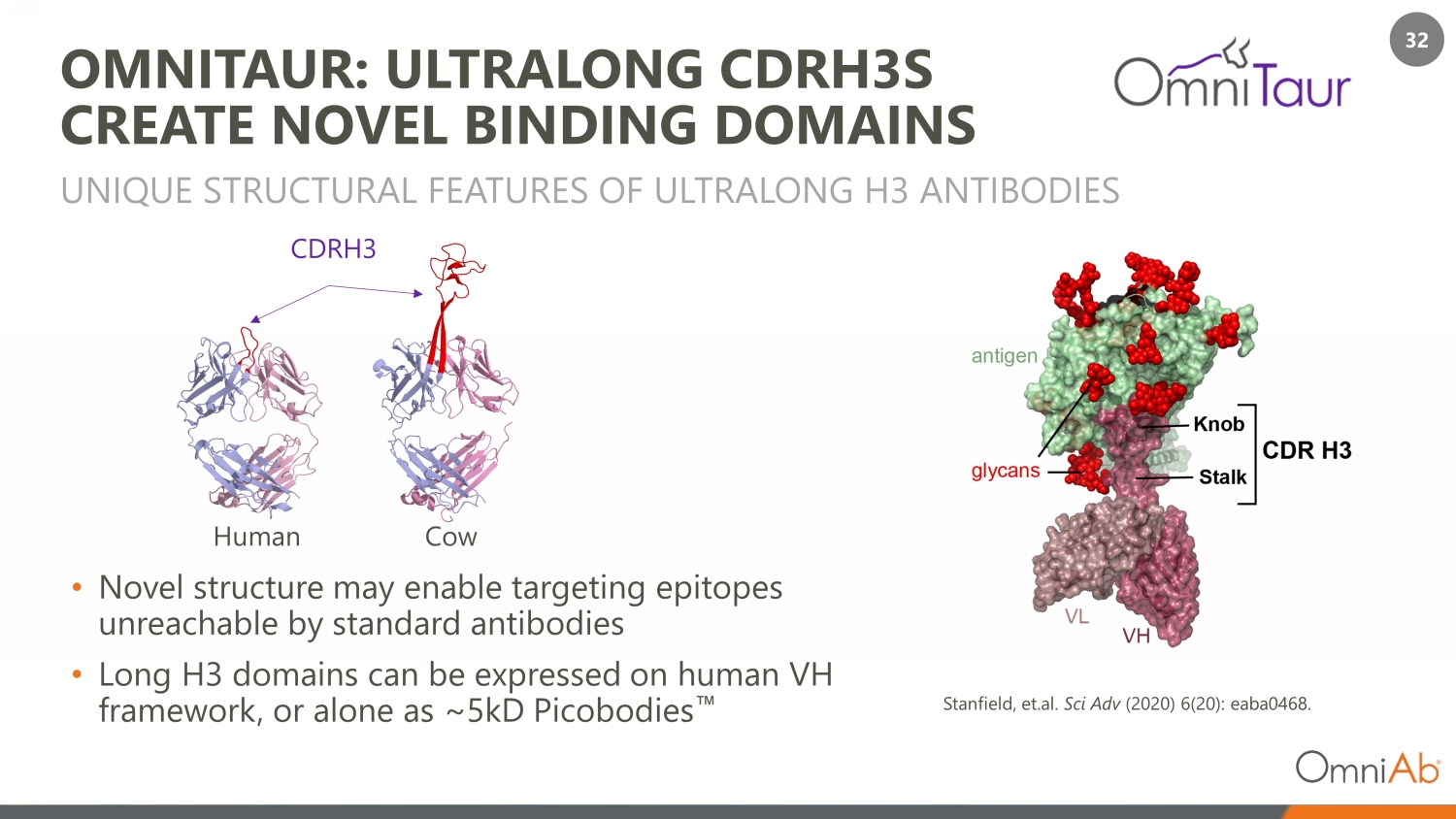
32 OMNITAUR: ULTRALONG CDRH3S CREATE NOVEL BINDING DOMAINS • Novel structure may enable targeting epitopes unreachable by standard antibodies • Long H3 domains can be expressed on human VH framework, or alone as ~5kD Picobodies Œ UNIQUE STRUCTURAL FEATURES OF ULTRALONG H3 ANTIBODIES CDRH3 Human Cow Stanfield, et.al. Sci Adv (2020) 6(20): eaba0468.
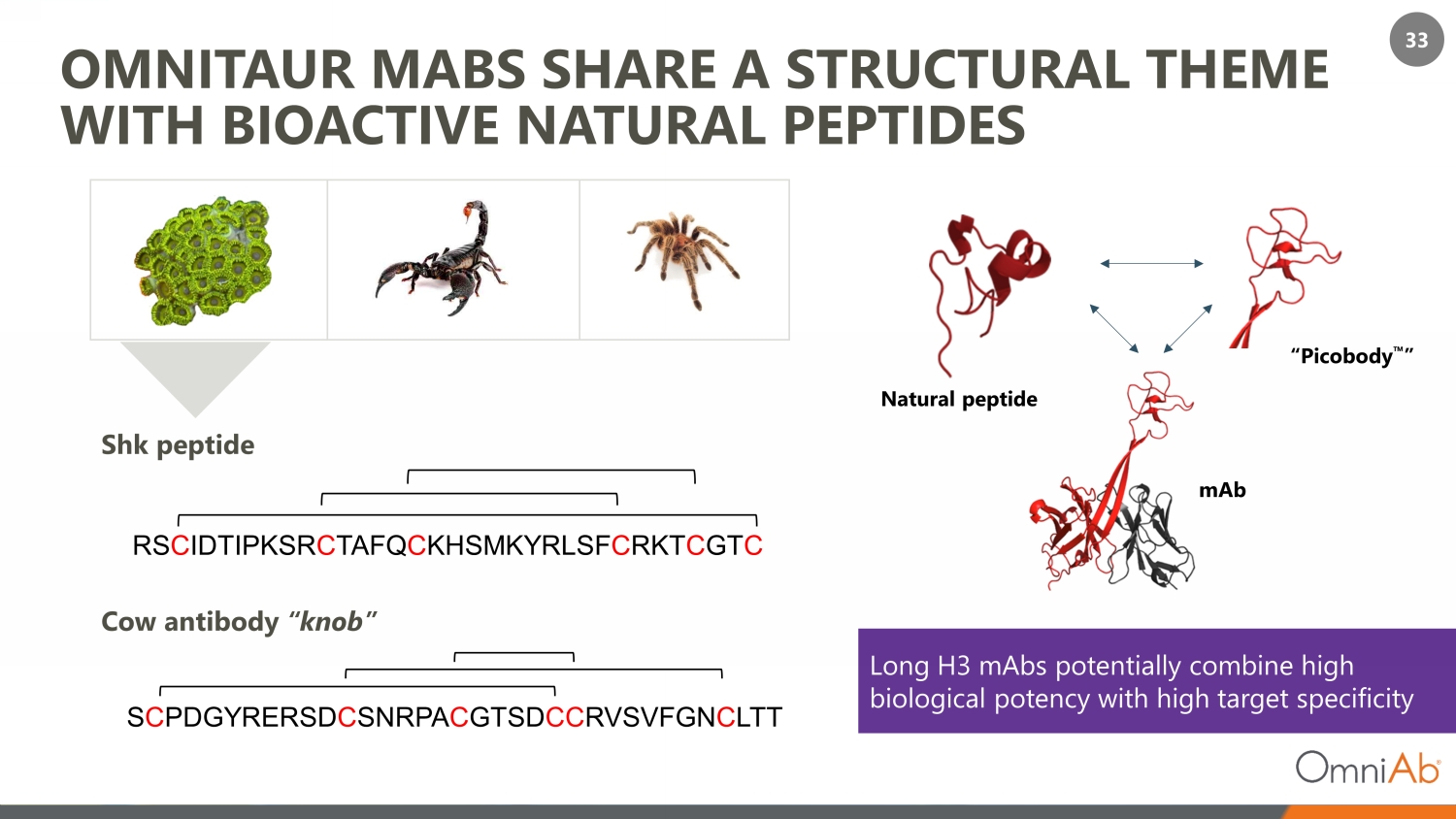
33 OMNITAUR MABS SHARE A STRUCTURAL THEME WITH BIOACTIVE NATURAL PEPTIDES RS C IDTIPKSR C TAFQ C KHSMKYRLSF C RKT C GT C Shk peptide S C PDGYRERSD C SNRPA C GTSD CC RVSVFGN C LTT Cow antibody “knob” Natural peptide “Picobody ” mAb Long H3 mAbs potentially combine high biological potency with high target specificity
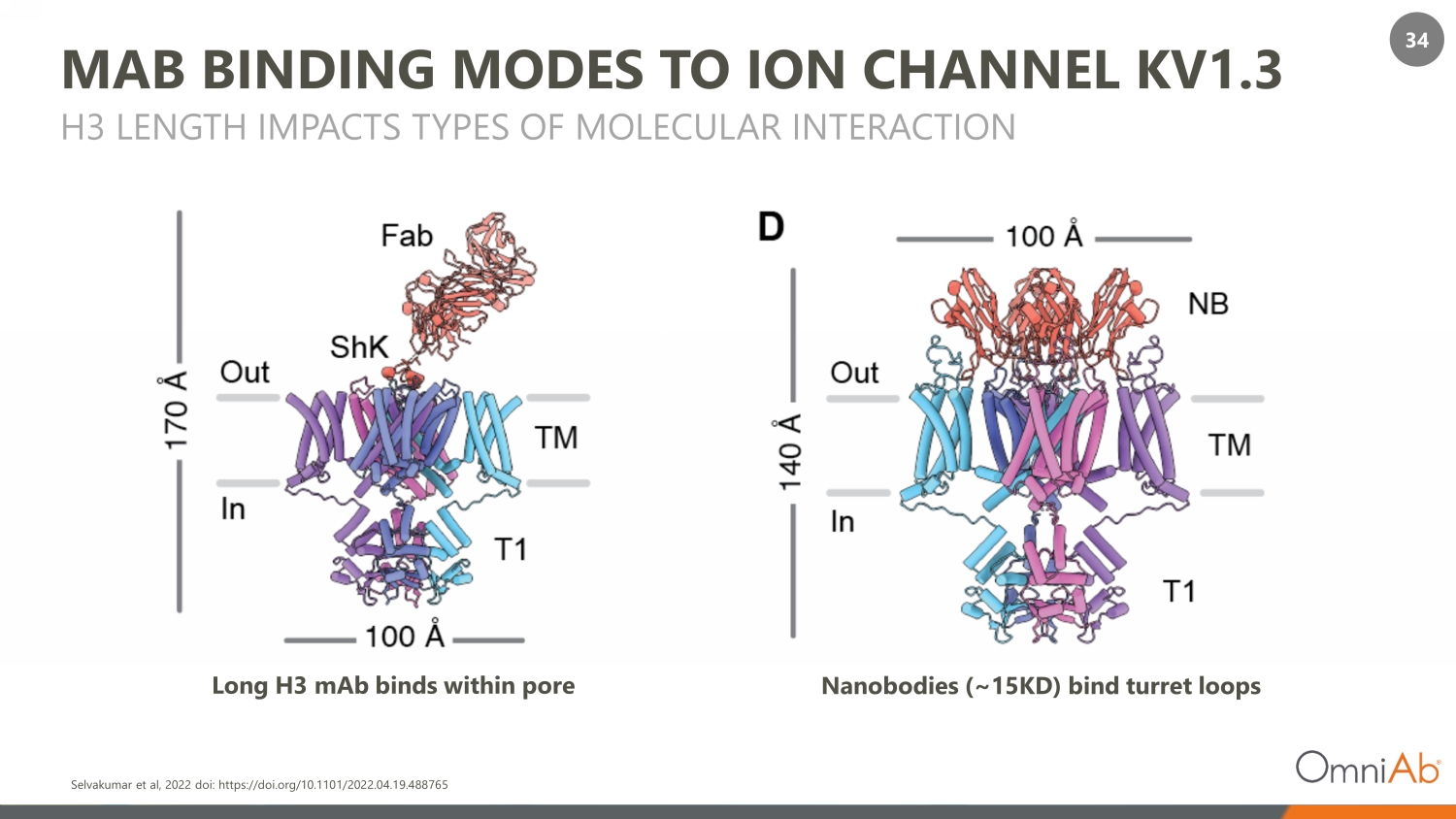
34 MAB BINDING MODES TO ION CHANNEL KV1.3 H3 LENGTH IMPACTS TYPES OF MOLECULAR INTERACTION Long H3 mAb binds within pore Nanobodies (~15KD) bind turret loops Selvakumar et al, 2022 doi: https://doi.org/10.1101/2022.04.19.488765
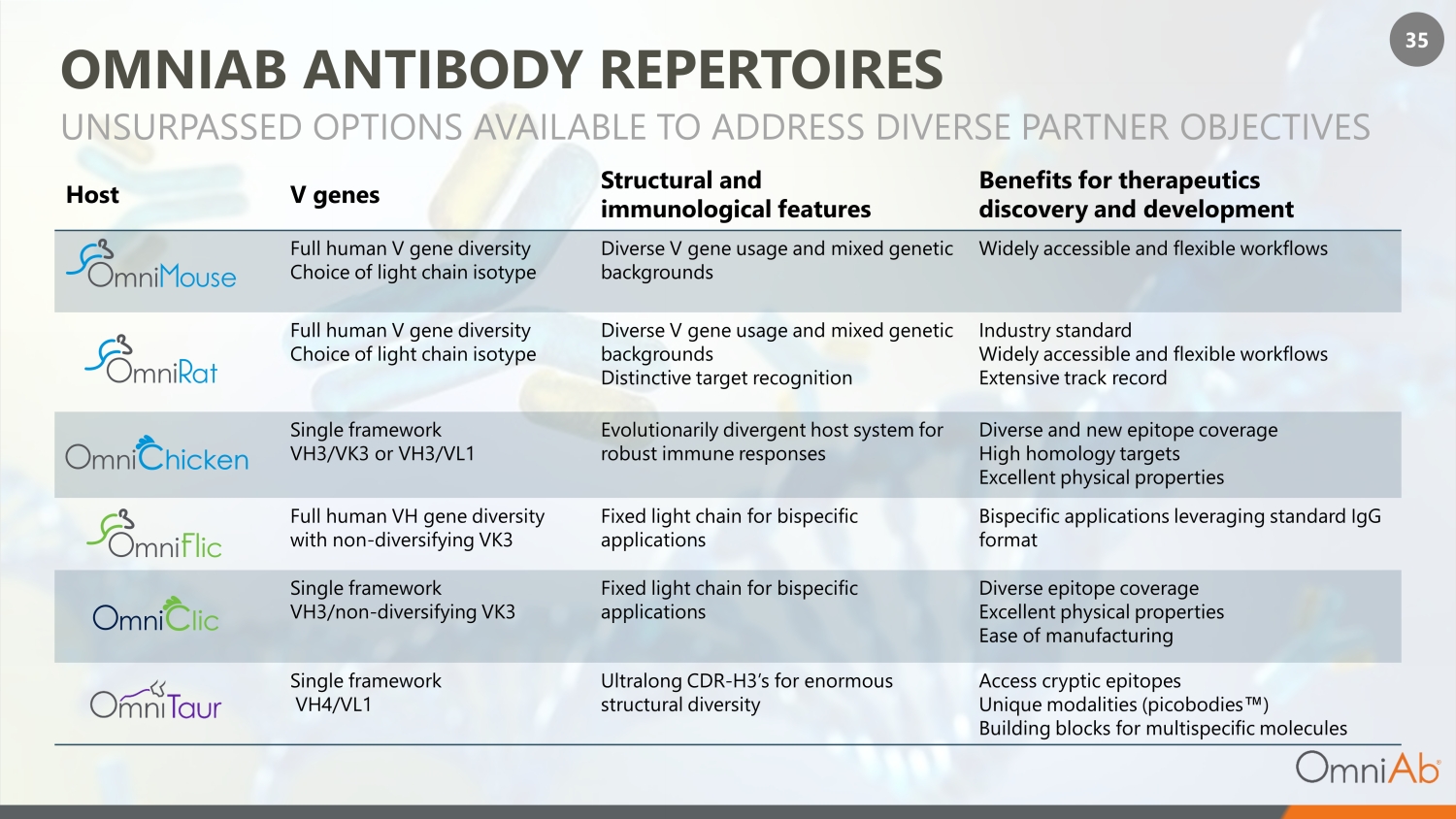
35 OMNIAB ANTIBODY REPERTOIRES Host V genes Structural and immunological features Benefits for therapeutics discovery and development Full human V gene diversity Choice of light chain isotype Diverse V gene usage and mixed genetic backgrounds Widely accessible and flexible workflows Full human V gene diversity Choice of light chain isotype Diverse V gene usage and mixed genetic backgrounds Distinctive target recognition Industry standard Widely accessible and flexible workflows Extensive track record Single framework VH3/VK3 or VH3/VL1 Evolutionarily divergent host system for robust immune responses Diverse and new epitope coverage High homology targets Excellent physical properties Full human VH gene diversity with non - diversifying VK3 Fixed light chain for bispecific applications Bispecific applications leveraging standard IgG format Single framework VH3/non - diversifying VK3 Fixed light chain for bispecific applications Diverse epitope coverage Excellent physical properties Ease of manufacturing Single framework VH4/VL1 Ultralong CDR - H3’s for enormous structural diversity Access cryptic epitopes Unique modalities (picobodies Œ ) Building blocks for multispecific molecules UNSURPASSED OPTIONS AVAILABLE TO ADDRESS DIVERSE PARTNER OBJECTI VES

Screening Technology and Antibody Identification Bob Chen, PhD
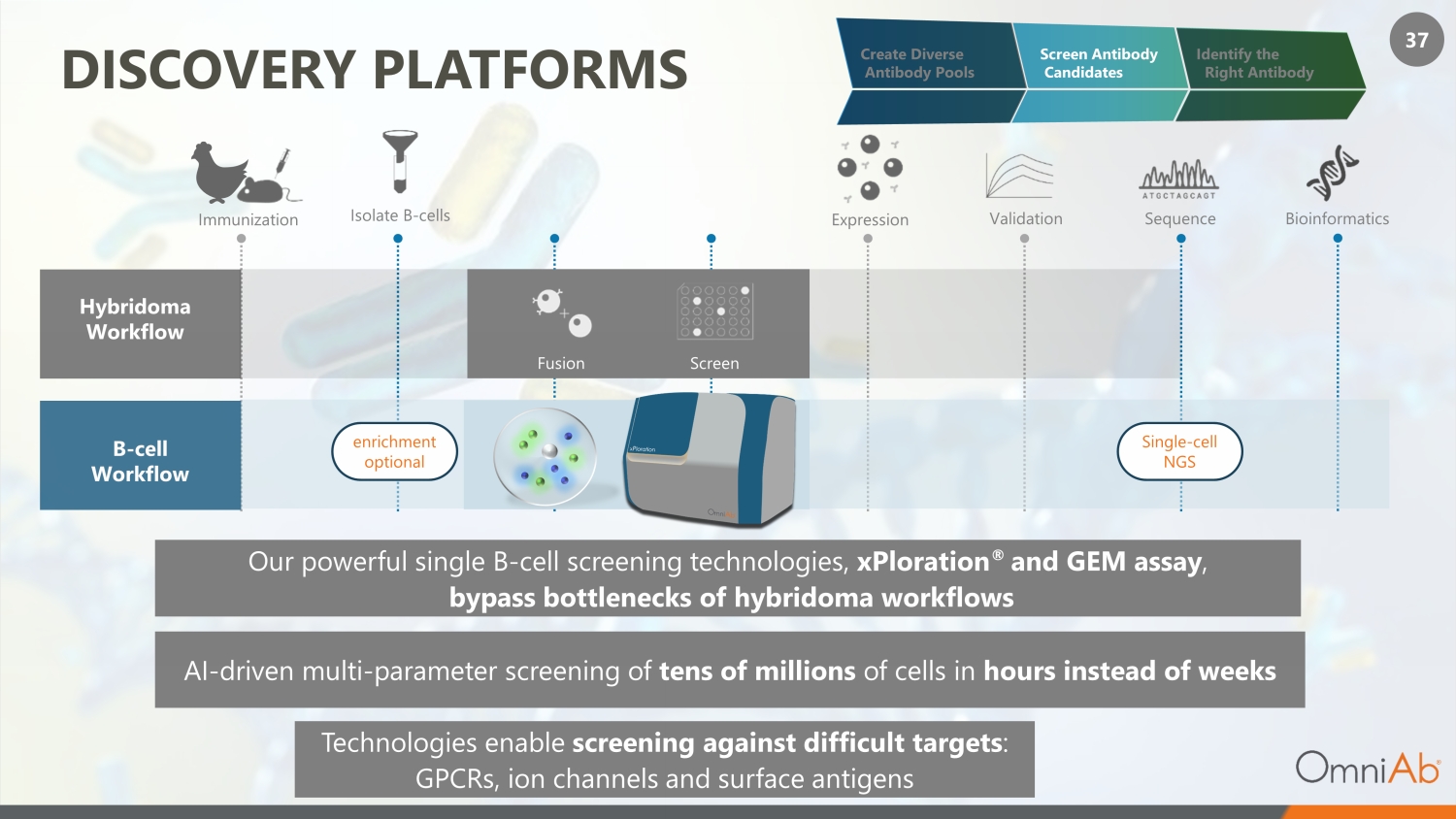
37 DISCOVERY PLATFORMS Hybridoma Workflow B - cell Workflow Immunization Isolate B - cells Sequence Bioinformatics enrichment optional Single - cell NGS Expression Validation Screen Fusion Technologies enable screening against difficult targets : GPCRs, ion channels and surface antigens Our powerful single B - cell screening technologies, xPloration ® and GEM assay , bypass bottlenecks of hybridoma workflows AI - driven multi - parameter screening of tens of millions of cells in hours instead of weeks Create Diverse Antibody Pools Screen Antibody Candidates Identify the Right Antibody
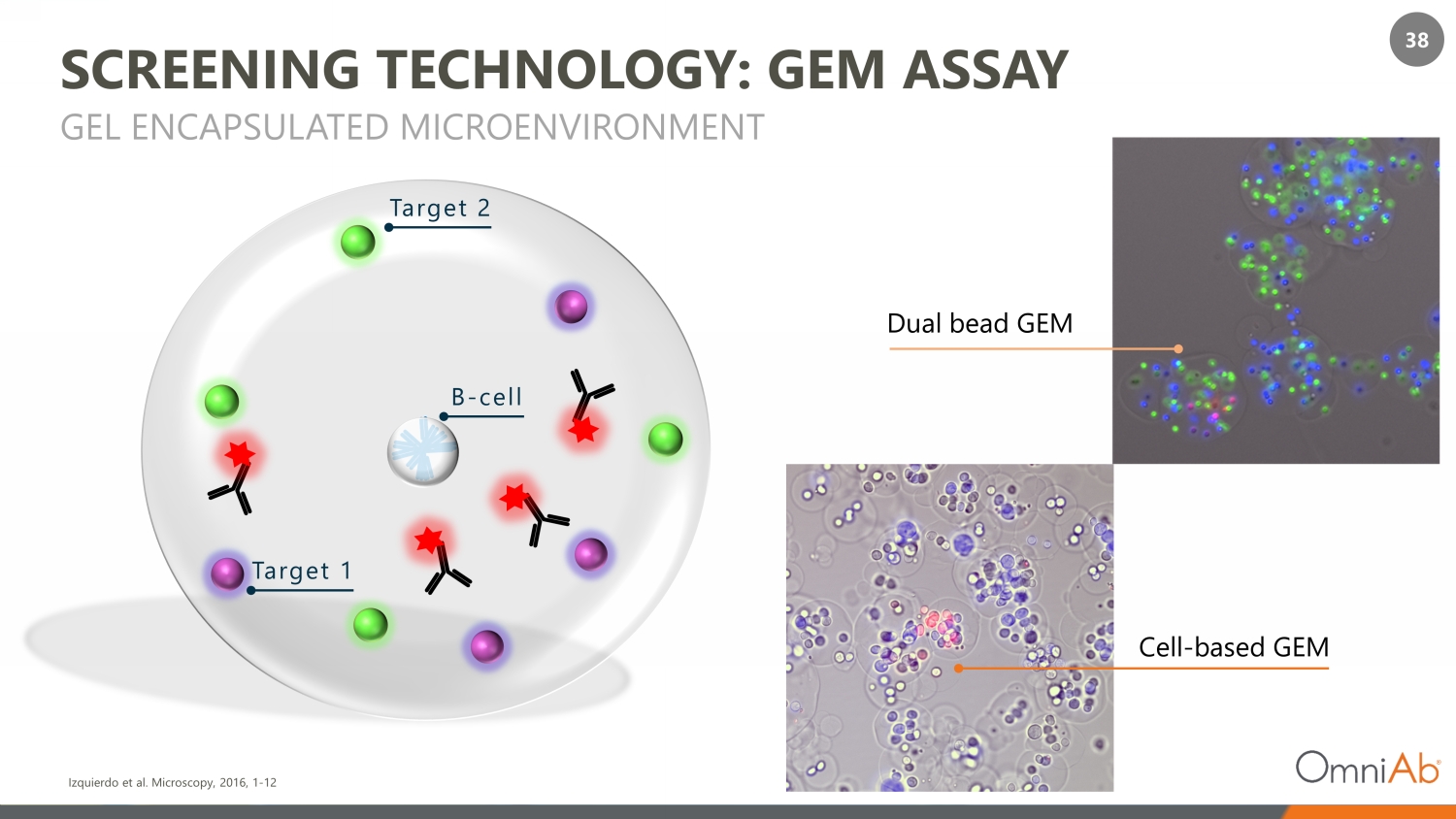
38 SCREENING TECHNOLOGY: GEM ASSAY GEL ENCAPSULATED MICROENVIRONMENT Izquierdo et al. Microscopy, 2016, 1 - 12 B - cell Target 2 Target 1 Dual bead GEM Cell - based GEM
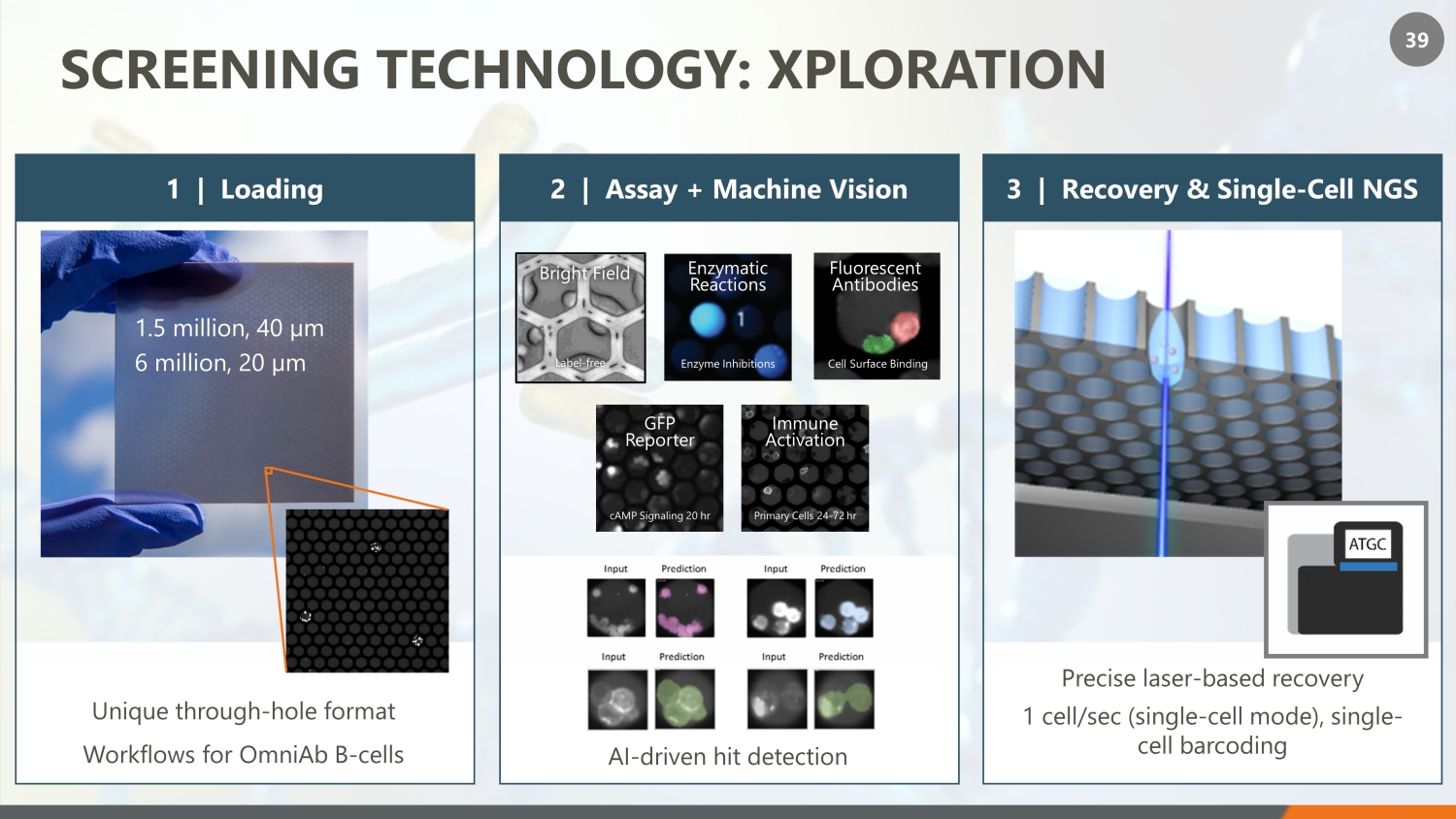
39 SCREENING TECHNOLOGY: XPLORATION 3 | Recovery & Single - Cell NGS Precise laser - based recovery 1 cell/sec (single - cell mode), single - cell barcoding 2 | Assay + Machine Vision Label - free Bright Field Enzyme Inhibitions Enzymatic Reactions cAMP Signaling 20 hr GFP Reporter Primary Cells 24 - 72 hr Immune Activation Cell Surface Binding Fluorescent Antibodies AI - driven hit detection Unique through - hole format Workflows for OmniAb B - cells 1 | Loading 1.5 million, 40 µm 6 million, 20 µm
40 40
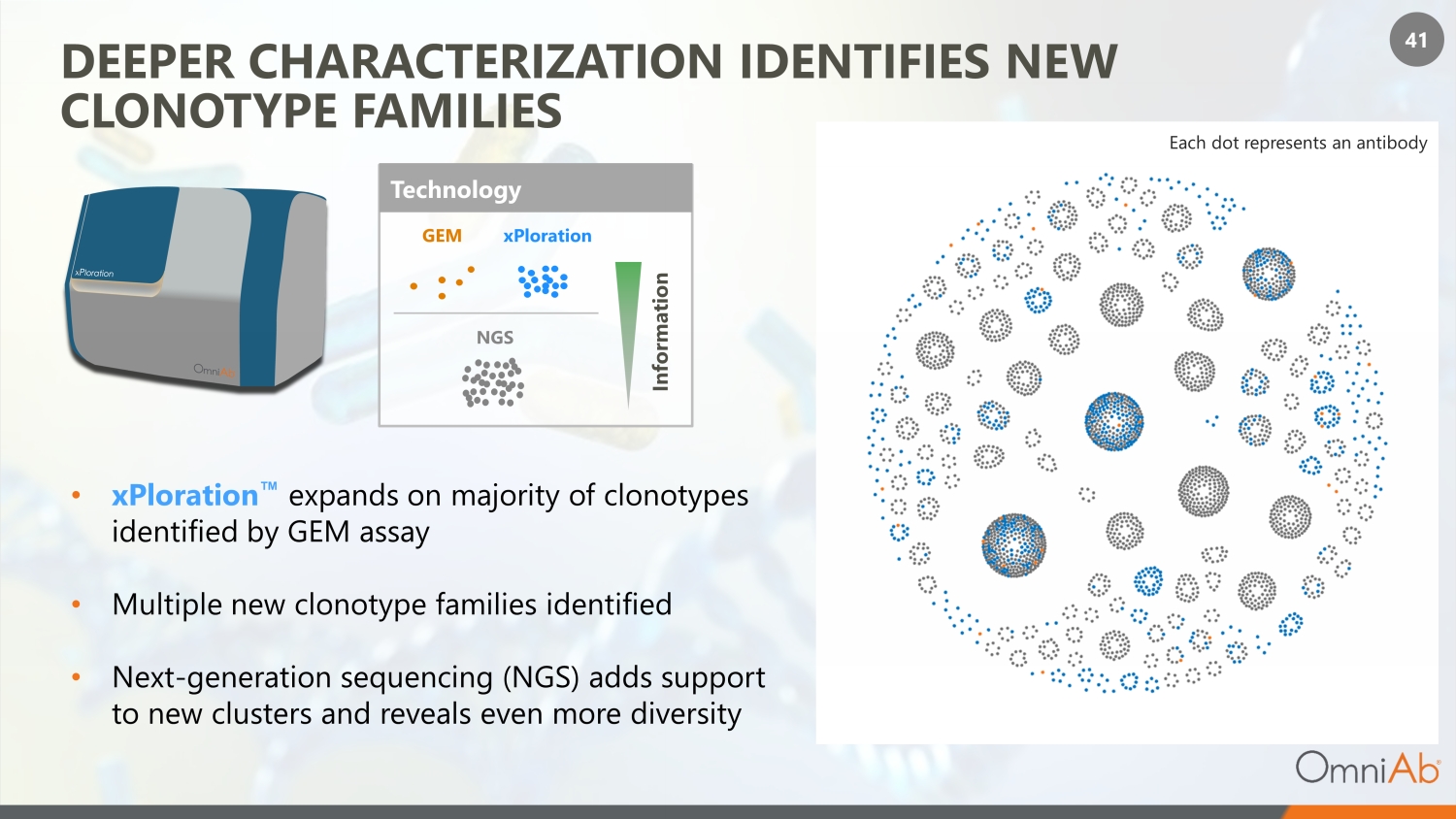
41 DEEPER CHARACTERIZATION IDENTIFIES NEW CLONOTYPE FAMILIES • xPloration Œ expands on majority of clonotypes identified by GEM assay • Multiple new clonotype families identified • Next - generation sequencing (NGS) adds support to new clusters and reveals even more diversity Each dot represents an antibody Technology GEM xPloration NGS Information
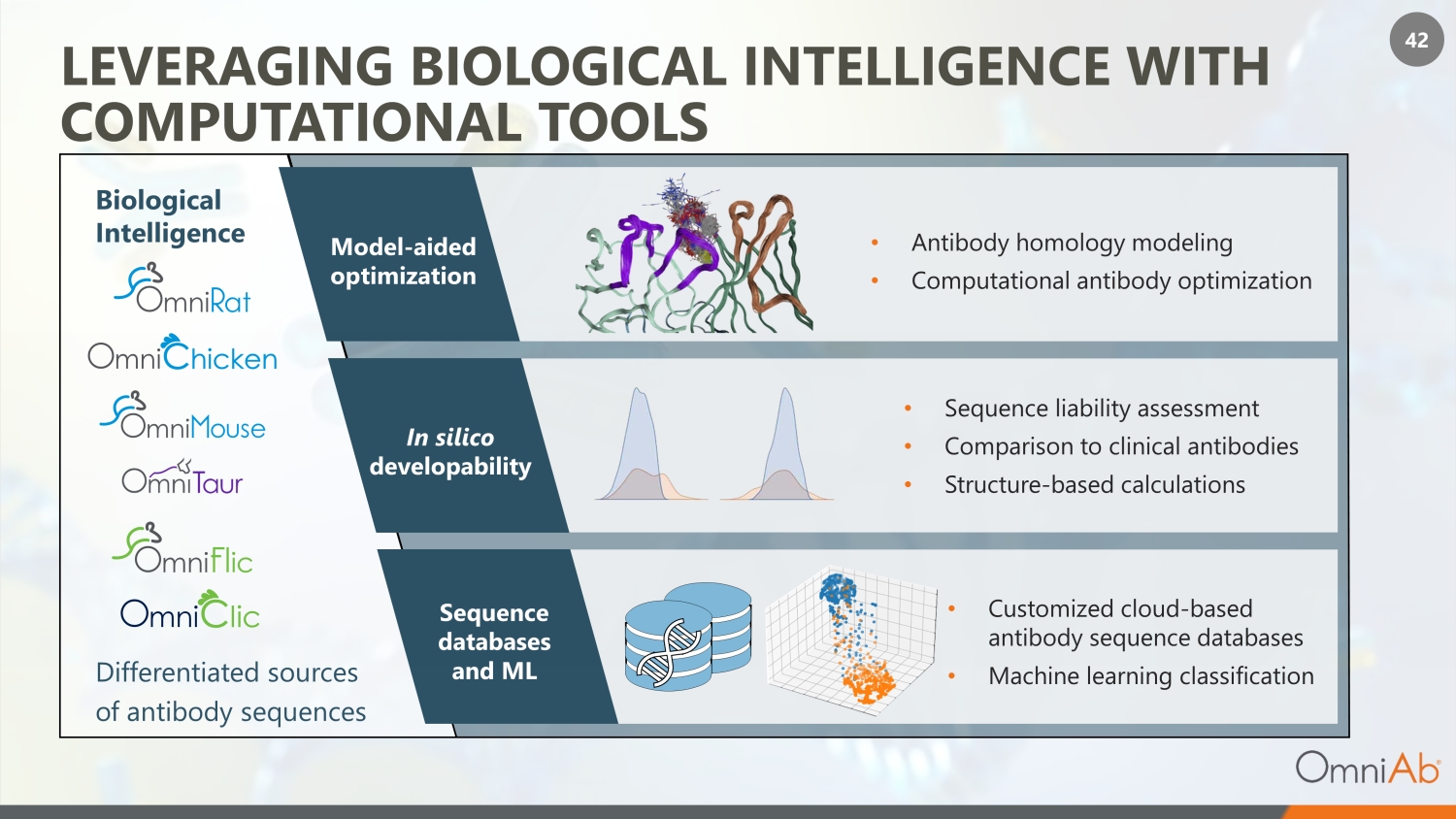
42 LEVERAGING BIOLOGICAL INTELLIGENCE WITH COMPUTATIONAL TOOLS Biological Intelligence Differentiated sources of antibody sequences In silico developability • Sequence liability assessment • Comparison to clinical antibodies • Structure - based calculations Sequence databases and ML • Customized cloud - based antibody sequence databases • Machine learning classification Model - aided optimization • Antibody homology modeling • Computational antibody optimization
43 THE OMNIAB PLATFORM • Data from multi - parameter screening and performance assays used in combination with bioinformatics • NGS h it expansion to identify variant antibodies with improved characteristics • High - throughput epitope binning and kinetics analysis, and target - specific functional assays • Proprietary assays for ion channel and transporter targets Our discovery teams are flexibly positioned to work closely with partners to identify the right antibody

Ion Channel/Transporter Technology Partnerships Doug Krafte, PhD

45 ION CHANNELS & TRANSPORTERS • One of the industry’s most experienced teams of ion channel drug - discovery experts; assembled over decades with a current staff of ~ 40 scientists, including bioinformatics • Continuous growth and development of cutting - edge technologies including high - throughput electrophysiology, structure - based optimization leveraging cryo - EM and molecular dynamics, deep learning models, proprietary X - ray fluorescence, and others • State - of - the - art facilities DEPTH OF EXPERIENCE 1990 1995 2000 2005 2010 2015 2020
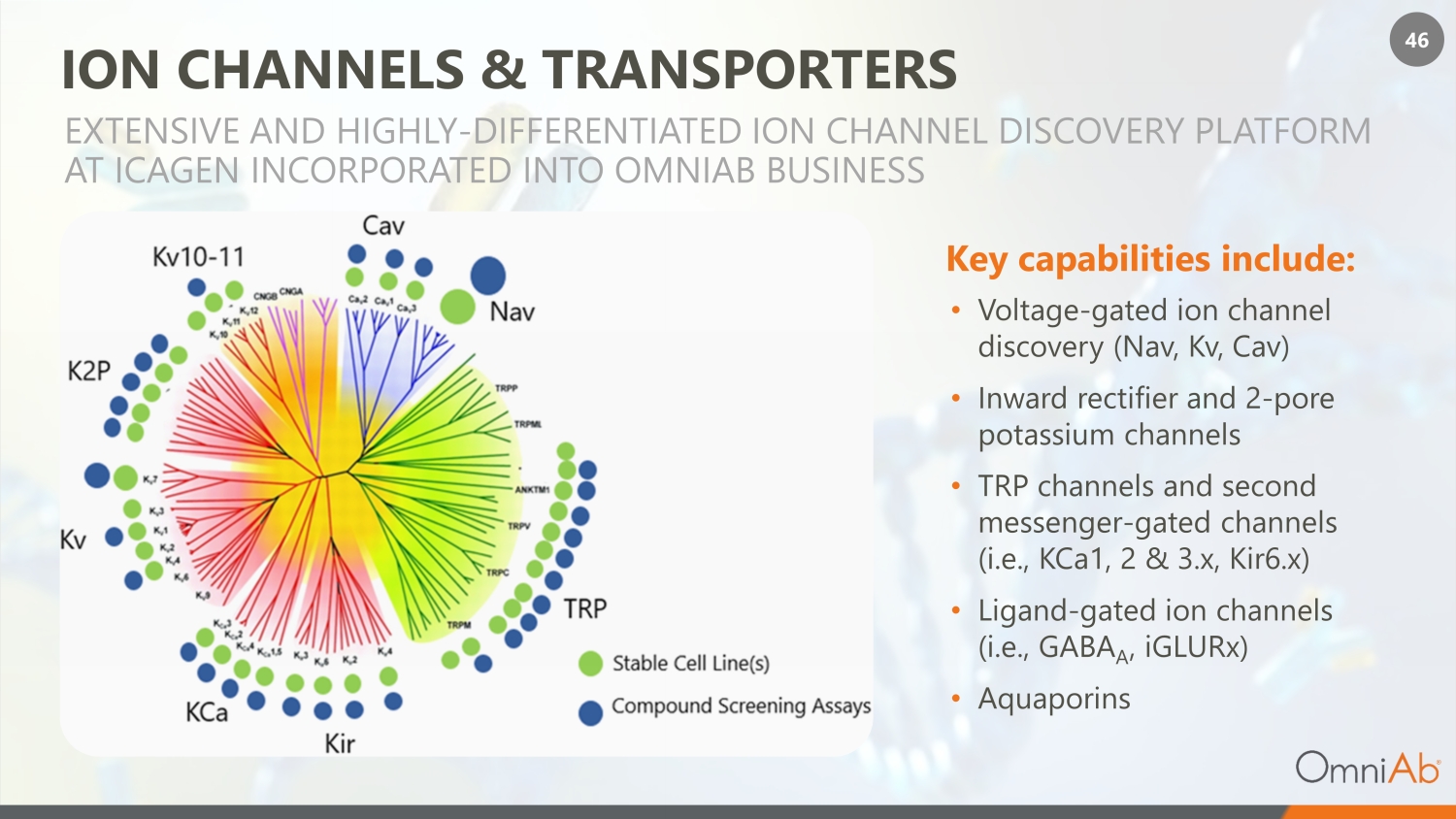
46 ION CHANNELS & TRANSPORTERS • Voltage - gated ion channel discovery (Nav, Kv, Cav) • Inward rectifier and 2 - pore potassium channels • TRP channels and second messenger - gated channels (i.e., KCa1, 2 & 3.x, Kir6.x) • Ligand - gated ion channels (i.e., GABA A , iGLURx) • Aquaporins EXTENSIVE AND HIGHLY - DIFFERENTIATED ION CHANNEL DISCOVERY PLATFORM AT ICAGEN INCORPORATED INTO OMNIAB BUSINESS Key capabilities include:
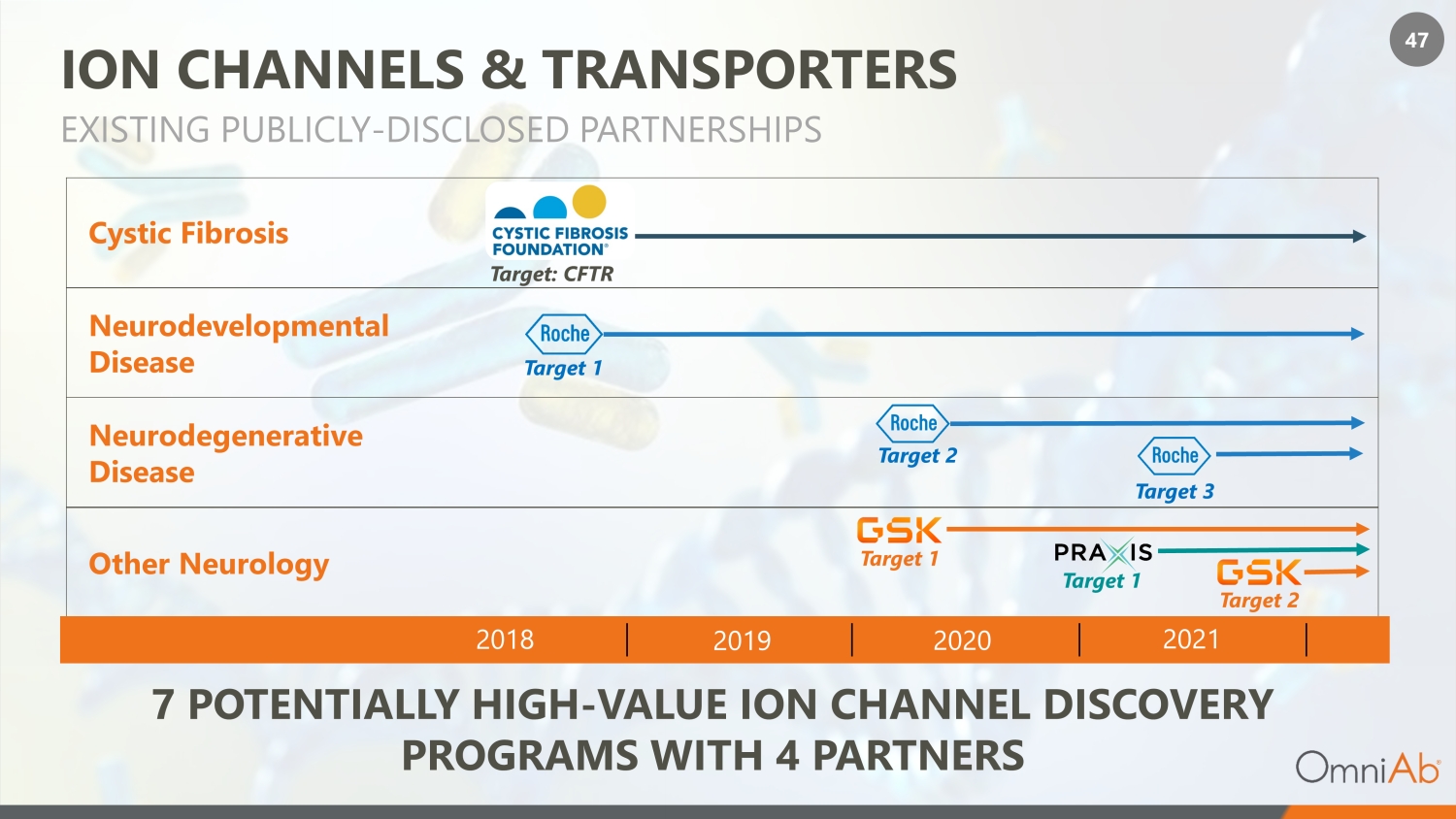
47 ION CHANNELS & TRANSPORTERS EXISTING PUBLICLY - DISCLOSED PARTNERSHIPS 2018 2019 2020 2021 Neurodevelopmental Disease Neurodegenerative Disease Cystic Fibrosis 7 POTENTIALLY HIGH - VALUE ION CHANNEL DISCOVERY PROGRAMS WITH 4 PARTNERS Other Neurology Target 1 Target 2 Target 3 Target 1 Target 2 Target 1 Target: CFTR
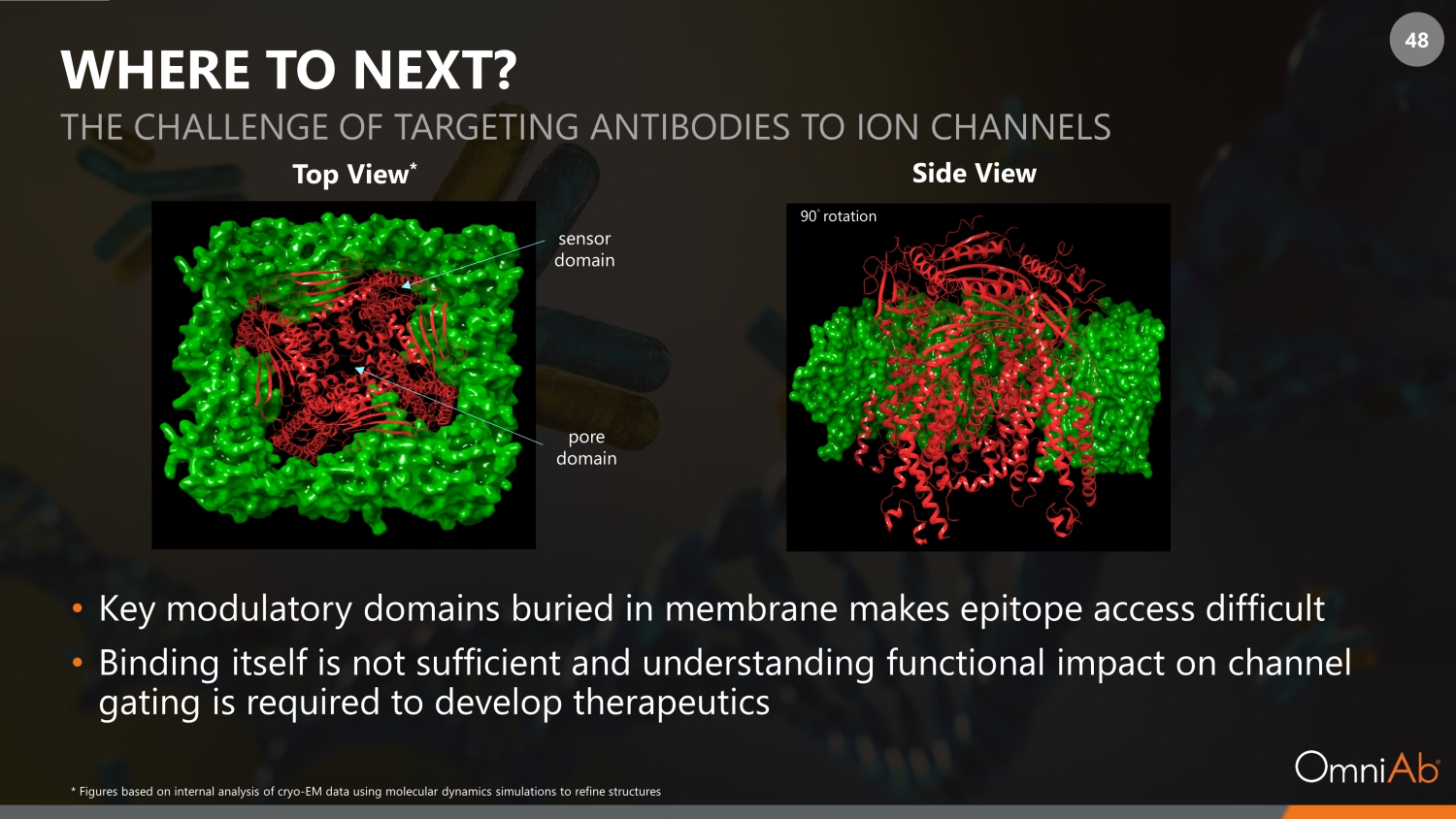
48 WHERE TO NEXT? • Key modulatory domains buried in membrane makes epitope access difficult • Binding itself is not sufficient and understanding functional impact on channel gating is required to develop therapeutics THE CHALLENGE OF TARGETING ANTIBODIES TO ION CHANNELS sensor domain pore domain 90 ◦ rotation Top View * Side View * Figures based on internal analysis of cryo - EM data using molecular dynamics simulations to refine structures

49 NEXT - GEN SOLUTIONS SOLVING THE CHALLENGE OF TARGETING ION CHANNELS OmniChicken Leveraging evolutionary distance xPloration Identifying and selecting very rare antibodies Icagen Complex biophysical selection of therapeutic candidates OmniTaur mABs structurally biased for binding deep clefts Binding PoC Fab fragment with targeting vector for a potassium channel Functional PoC Targeted channel block confirmed biophysically Greater Diversity and High - Volume Selection Mimicking Nature with Unique mAB Structures Our proprietary solutions are designed to greatly increase the probability of identifying clinical candidates
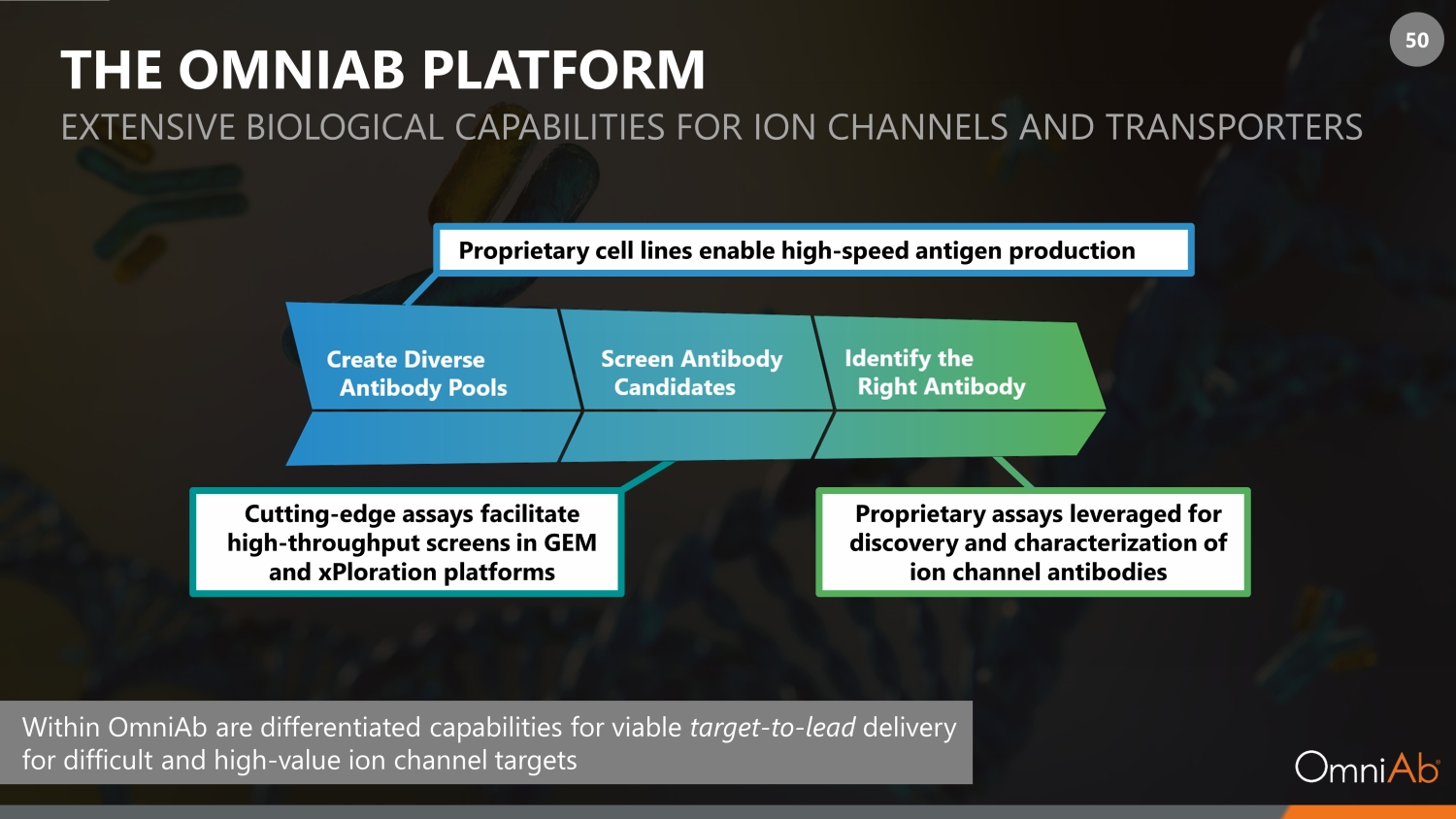
50 THE OMNIAB PLATFORM EXTENSIVE BIOLOGICAL CAPABILITIES FOR ION CHANNELS AND TRANSPORT ERS Proprietary cell lines enable high - speed antigen production Cutting - edge assays facilitate high - throughput screens in GEM and xPloration platforms Proprietary assays leveraged for discovery and characterization of ion channel antibodies Within OmniAb are differentiated capabilities for viable target - to - lead delivery for difficult and high - value ion channel targets

Partnered Portfolio Update Christel Iffland, PhD

52 SELECT OMNIAB PARTNERS >55 COMPANIES CURRENTLY HAVE ACCESS TO OMNIAB ANTIBODIES
53 THE POWER OF OMNIAB - PARTNER CASE STUDIES • All previously - known antibodies to target could only bind to denatured or fixed form, therefore unsuitable for therapeutic use • Our antigen tech was applied to deliver mg - scale quantities of purified receptor in native conformation for immunization and screening • OmniChicken immunization led to discovery of a large and diverse panel of fully - human antibodies capable of binding target on live tissues PARTNER A: EMERGING BIOTECH Novel multi - transmembrane target for triple - negative breast cancer
54 THE POWER OF OMNIAB - PARTNER CASE STUDIES • Human version of target non - immunogenic in other rodents; no titer achieved despite numerous immunization attempts by partner • Genetic knockout of target gene attempted in mice but was lethal • OmniChicken immunization led to robust titers and diverse antibody panels • >90% of sequences recovered had excellent developability profiles based on multi - parameter in silico analysis PARTNER B: BIG PHARMA Growth factor target, highly conserved among mammals

55 THE POWER OF OMNIAB - PARTNER CASE STUDIES • Partner needed a flexible, scalable antibody discovery solution to start dozens of new programs every year • Deep collaboration to develop next - generation rodents , which were tested in parallel with active novel programs • OmniAb wholly owns rights to next - generation animals and can include them in the OmniAb technology offering to other partners PARTNER C: ESTABLISHED BIOTECH Partner has history of success in developing antibodies, with large discovery group and expanding novel biology
56 THE POWER OF OMNIAB - PARTNER CASE STUDIES PARTNER D: GLOBAL PHARMA Asia - based global pharma player that is e stablishing a new and substantial presence in antibody space with large investment and expansion of global antibody team • Partner needed OmniAb’s antibody discovery engine to power their growth • Selected OmniAb as core technology to feed robust discovery and development efforts • Developed three - way collaboration with deep repertoire analysis to rapidly identify best binders for bispecific antibodies
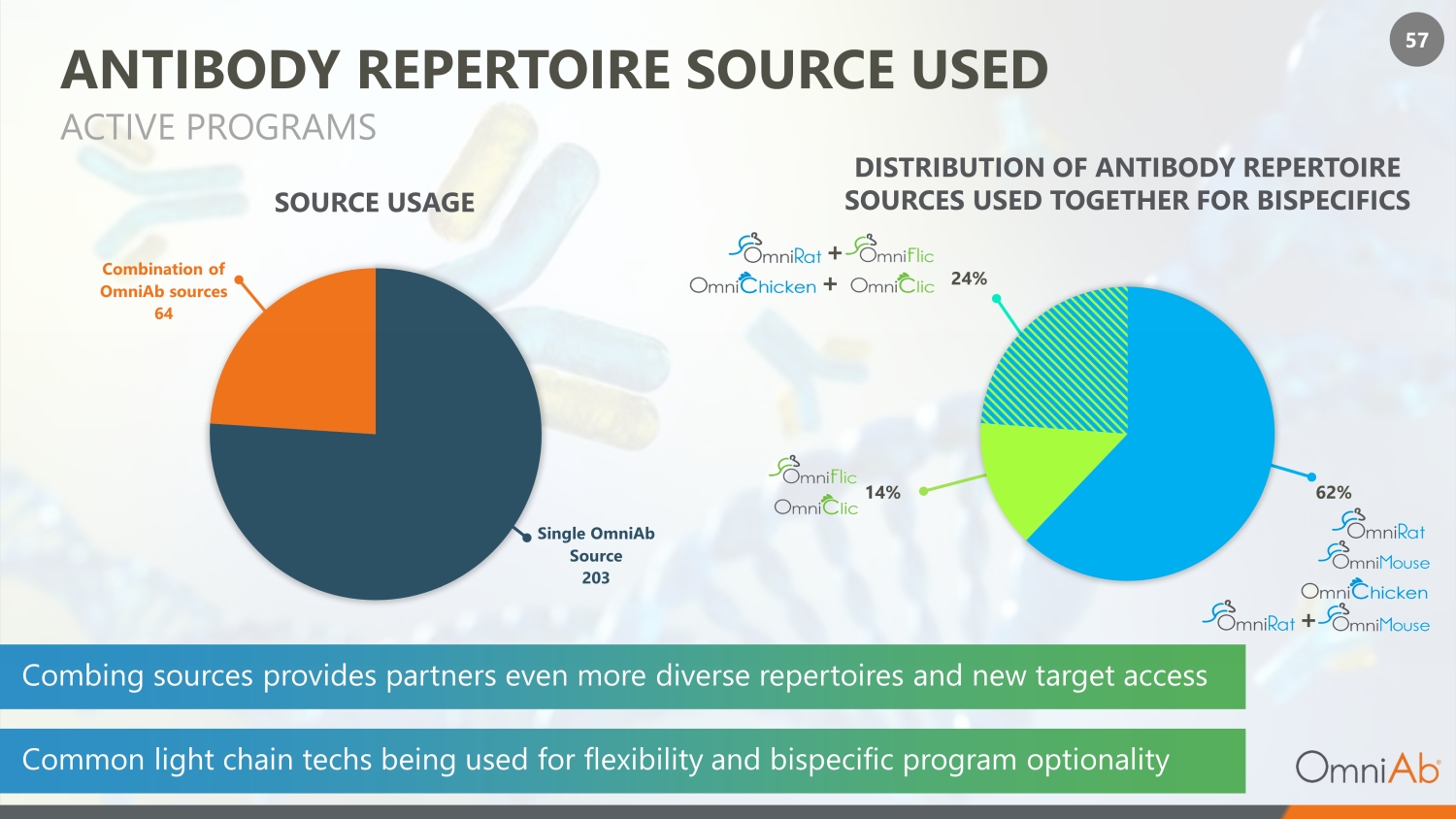
57 ANTIBODY REPERTOIRE SOURCE USED ACTIVE PROGRAMS Single OmniAb Source 203 Combination of OmniAb sources 64 DISTRIBUTION OF ANTIBODY REPERTOIRE SOURCES USED TOGETHER FOR BISPECIFICS SOURCE USAGE 24% 62% 14% + + + Combing sources provides partners even more diverse repertoires and new target access Common light chain techs being used for flexibility and bispecific program optionality
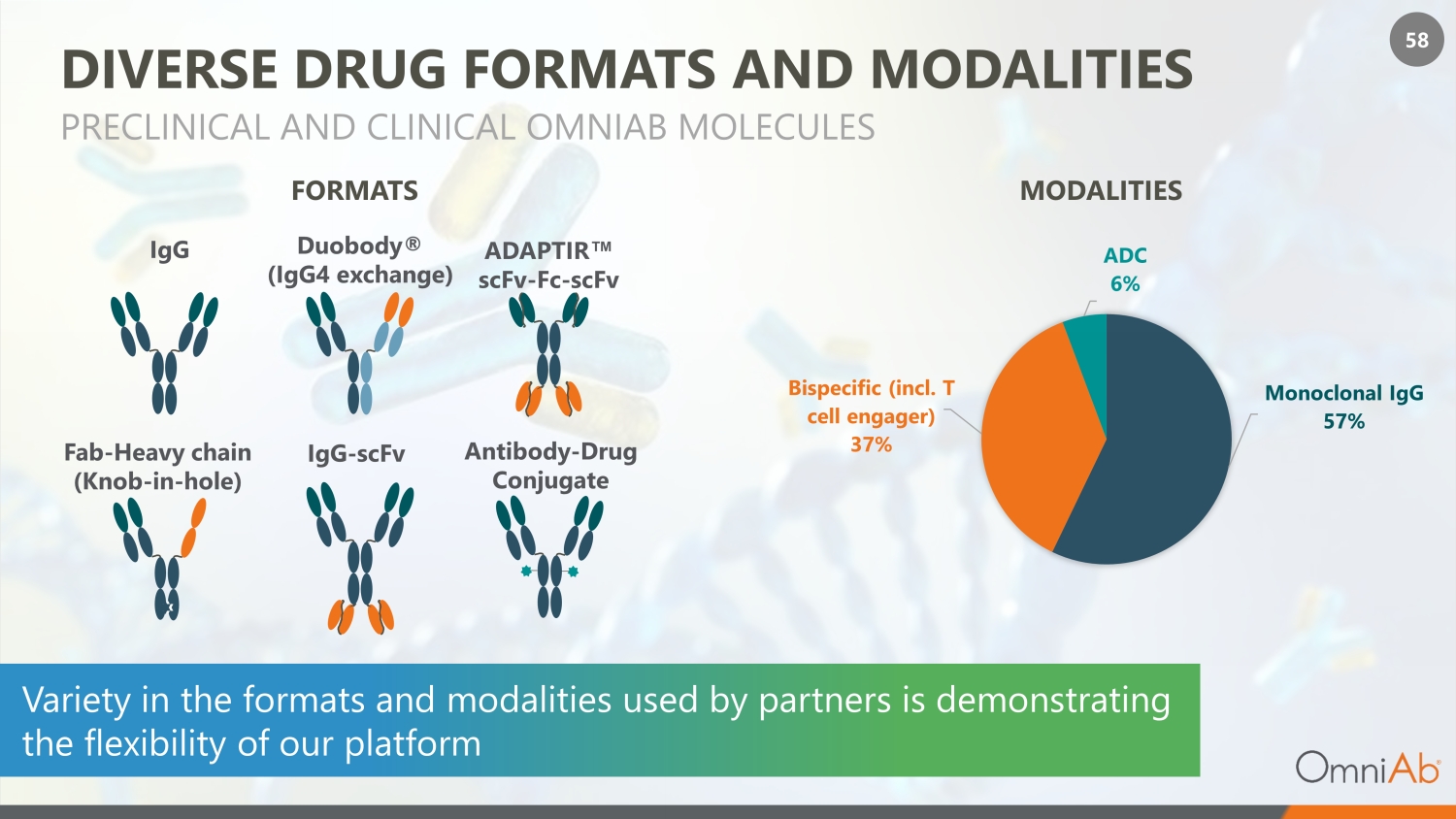
58 Monoclonal IgG 57% Bispecific (incl. T cell engager) 37% ADC 6% DIVERSE DRUG FORMATS AND MODALITIES PRECLINICAL AND CLINICAL OMNIAB MOLECULES Duobody® (IgG4 exchange) ADAPTIR Œ scFv - Fc - scFv IgG IgG - scFv Antibody - Drug Conjugate Fab - Heavy chain (Knob - in - hole) Variety in the formats and modalities used by partners is demonstrating the flexibility of our platform MODALITIES FORMATS
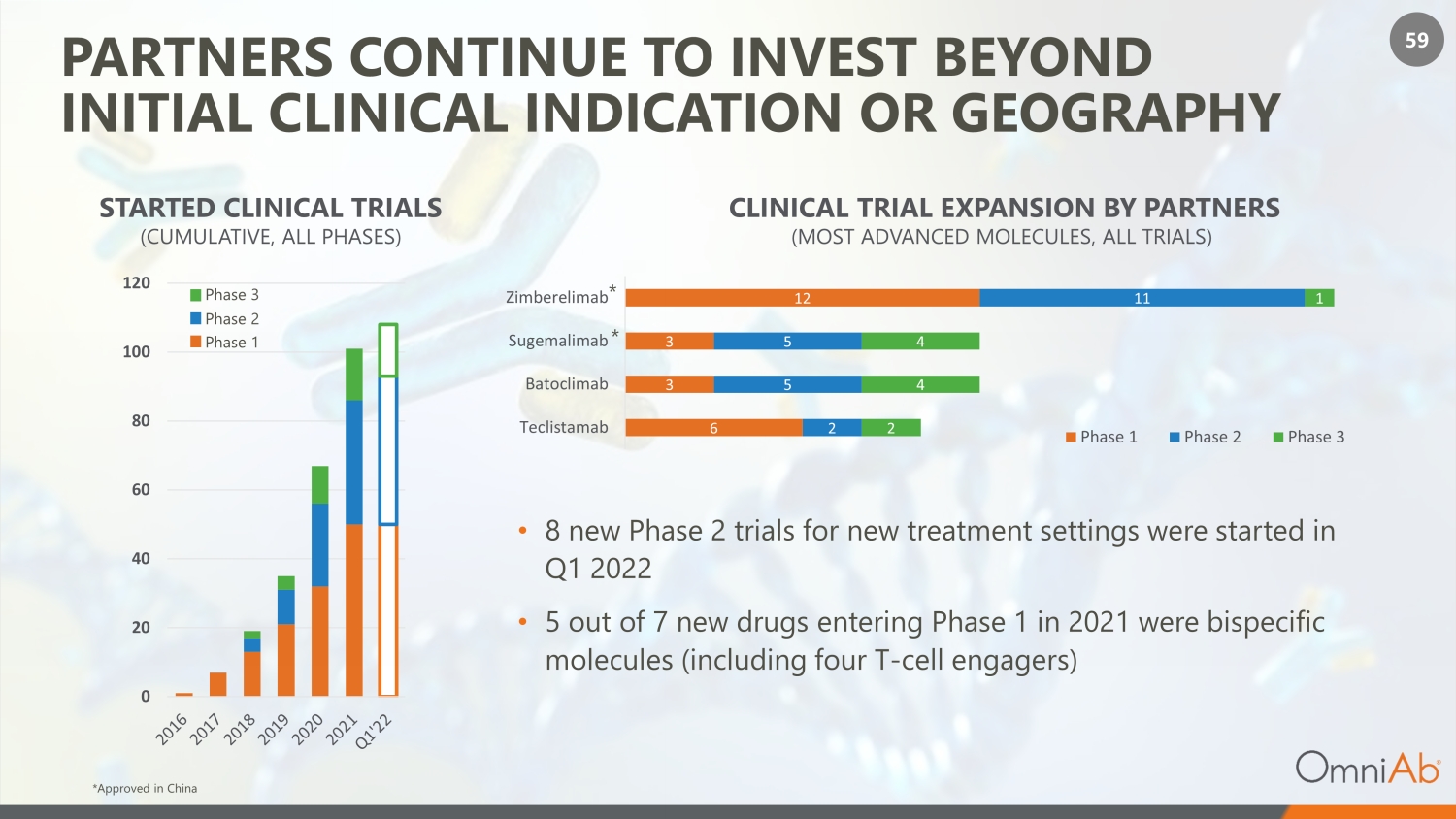
59 PARTNERS CONTINUE TO INVEST BEYOND INITIAL CLINICAL INDICATION OR GEOGRAPHY • 8 new Phase 2 trials for new treatment settings were started in Q1 2022 • 5 out of 7 new drugs entering Phase 1 in 2021 were bispecific molecules (including four T - cell engagers) STARTED CLINICAL TRIALS (CUMULATIVE, ALL PHASES) 0 20 40 60 80 100 120 CLINICAL TRIAL EXPANSION BY PARTNERS (MOST ADVANCED MOLECULES, ALL TRIALS) *Approved in China Phase 1 Phase 2 Phase 3 6 3 3 12 2 5 5 11 2 4 4 1 Teclistamab Batoclimab Sugemalimab Zimberelimab Phase 1 Phase 2 Phase 3 * *
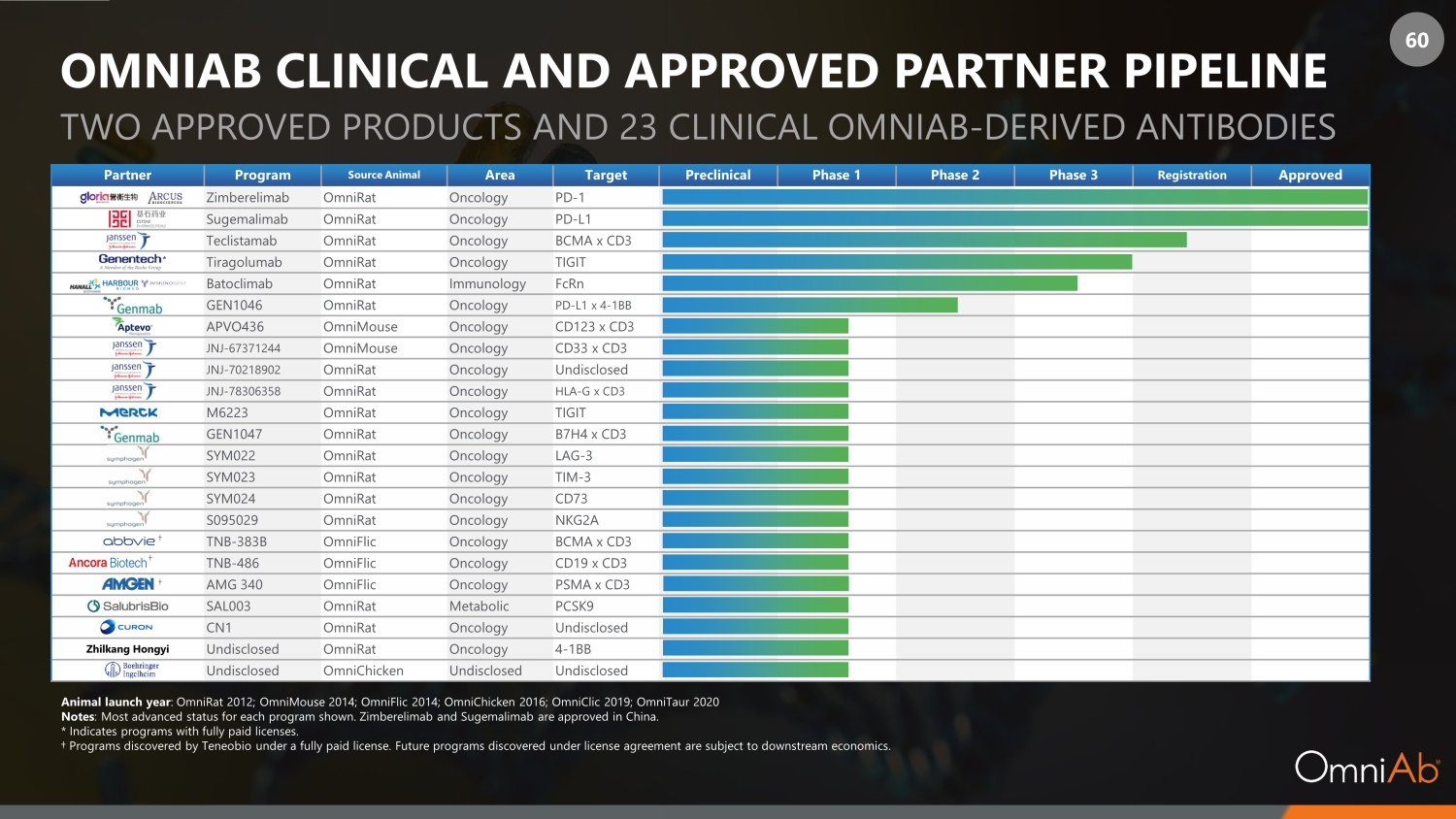
60 OMNIAB CLINICAL AND APPROVED PARTNER PIPELINE TWO APPROVED PRODUCTS AND 23 CLINICAL OMNIAB - DERIVED ANTIBODIES Partner Program Source Animal Area Target Preclinical Phase 1 Phase 2 Phase 3 Registration Approved Zimberelimab OmniRat Oncology PD - 1 Sugemalimab OmniRat Oncology PD - L1 Teclistamab OmniRat Oncology BCMA x CD3 Tiragolumab OmniRat Oncology TIGIT Batoclimab OmniRat Immunology FcRn GEN1046 OmniRat Oncology PD - L1 x 4 - 1BB APVO436 OmniMouse Oncology CD123 x CD3 JNJ - 67371244 OmniMouse Oncology CD33 x CD3 JNJ - 70218902 OmniRat Oncology Undisclosed JNJ - 78306358 OmniRat Oncology HLA - G x CD3 M6223 OmniRat Oncology TIGIT GEN1047 OmniRat Oncology B7H4 x CD3 SYM022 OmniRat Oncology LAG - 3 SYM023 OmniRat Oncology TIM - 3 SYM024 OmniRat Oncology CD73 S095029 OmniRat Oncology NKG2A TNB - 383B OmniFlic Oncology BCMA x CD3 Ancora TNB - 486 OmniFlic Oncology CD19 x CD3 AMG 340 OmniFlic Oncology PSMA x CD3 SAL003 OmniRat Metabolic PCSK9 CN1 OmniRat Oncology Undisclosed Zhilkang Hongyi Undisclosed OmniRat Oncology 4 - 1BB Undisclosed OmniChicken Undisclosed Undisclosed Animal launch year : OmniRat 2012; OmniMouse 2014; OmniFlic 2014; OmniChicken 2016; OmniClic 2019; OmniTaur 2020 Notes : Most advanced status for each program shown. Zimberelimab and Sugemalimab are approved in China. * Indicates programs with fully paid licenses. † Programs discovered by Teneobio under a fully paid license. Future programs discovered under license agreement are subject to downstream economics. † * † † †

Financial Review Kurt Gustafson
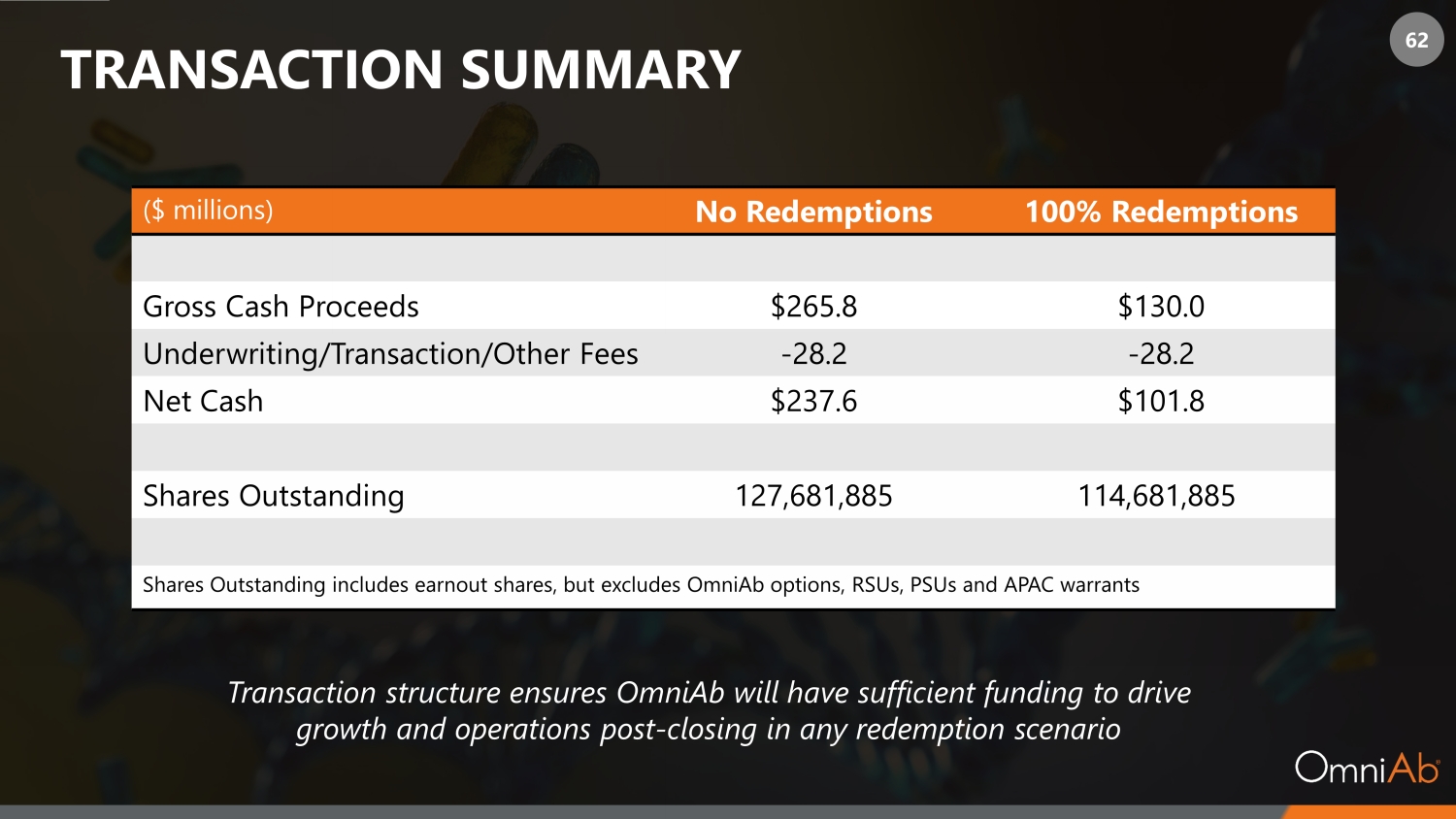
62 TRANSACTION SUMMARY ($ millions) No Redemptions 100% Redemptions Gross Cash Proceeds $265.8 $130.0 Underwriting/Transaction/Other Fees - 28.2 - 28.2 Net Cash $237.6 $101.8 Shares Outstanding 127,681,885 114,681,885 Shares Outstanding includes earnout shares, but excludes OmniAb options, RSUs, PSUs and APAC warrants Transaction structure ensures OmniAb will have sufficient funding to drive growth and operations post - closing in any redemption scenario

63 OMNIAB BUSINESS MODEL OUR AGREEMENTS ARE STRUCTURED TO ALIGN ECONOMIC AND SCIENTIFIC INTERESTS WITH OUR PARTNERS License partnerships designed to include: • Technology access and collaboration/service fees • Milestones • Royalties on commercial sales
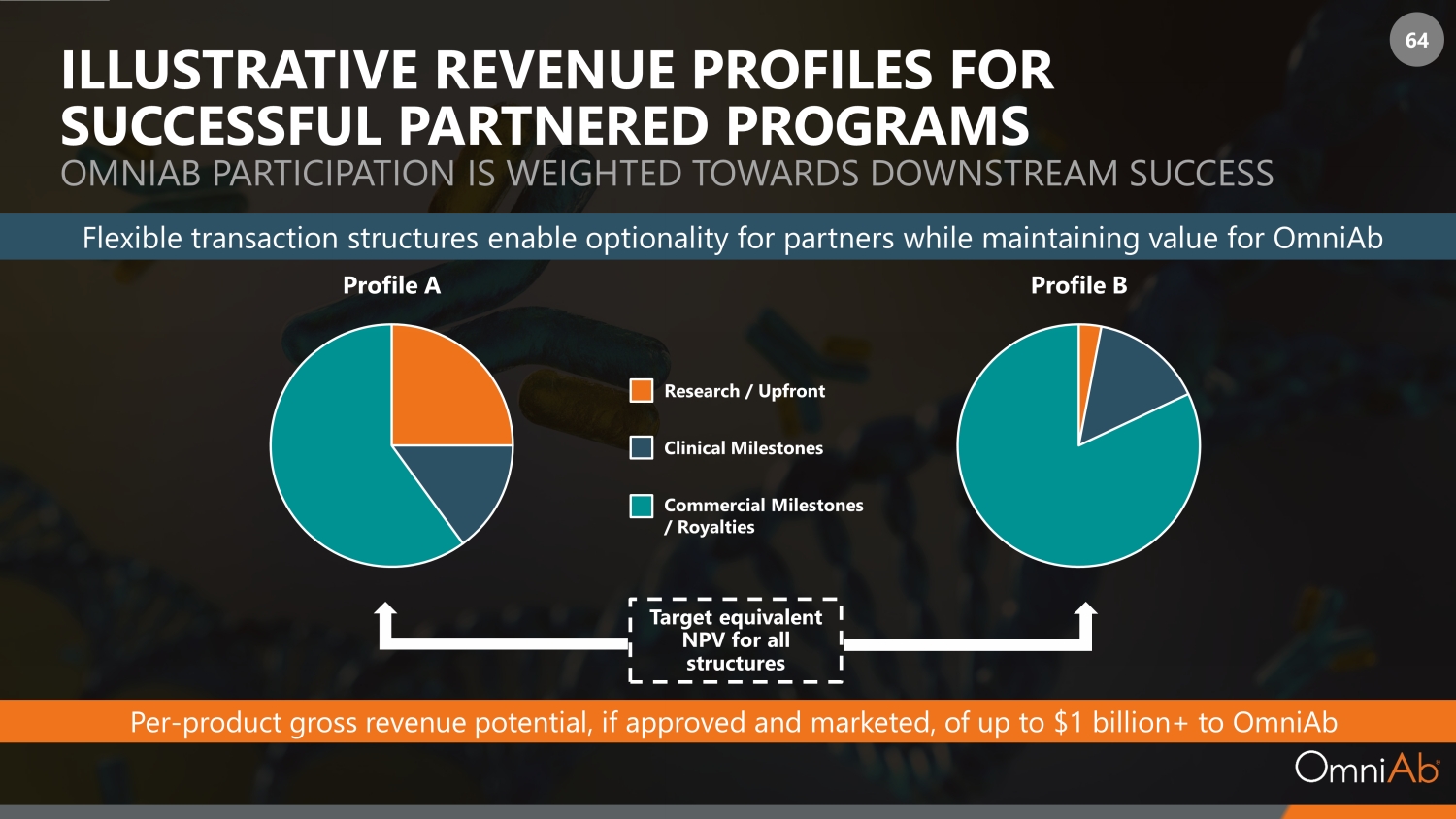
64 ILLUSTRATIVE REVENUE PROFILES FOR SUCCESSFUL PARTNERED PROGRAMS OMNIAB PARTICIPATION IS WEIGHTED TOWARDS DOWNSTREAM SUCCESS Target equivalent NPV for all structures Profile A Profile B Flexible transaction structures enable optionality for partners while maintaining value for OmniAb Per - product gross revenue potential, if approved and marketed, of up to $1 billion+ to OmniAb Research / Upfront Clinical Milestones Commercial Milestones / Royalties
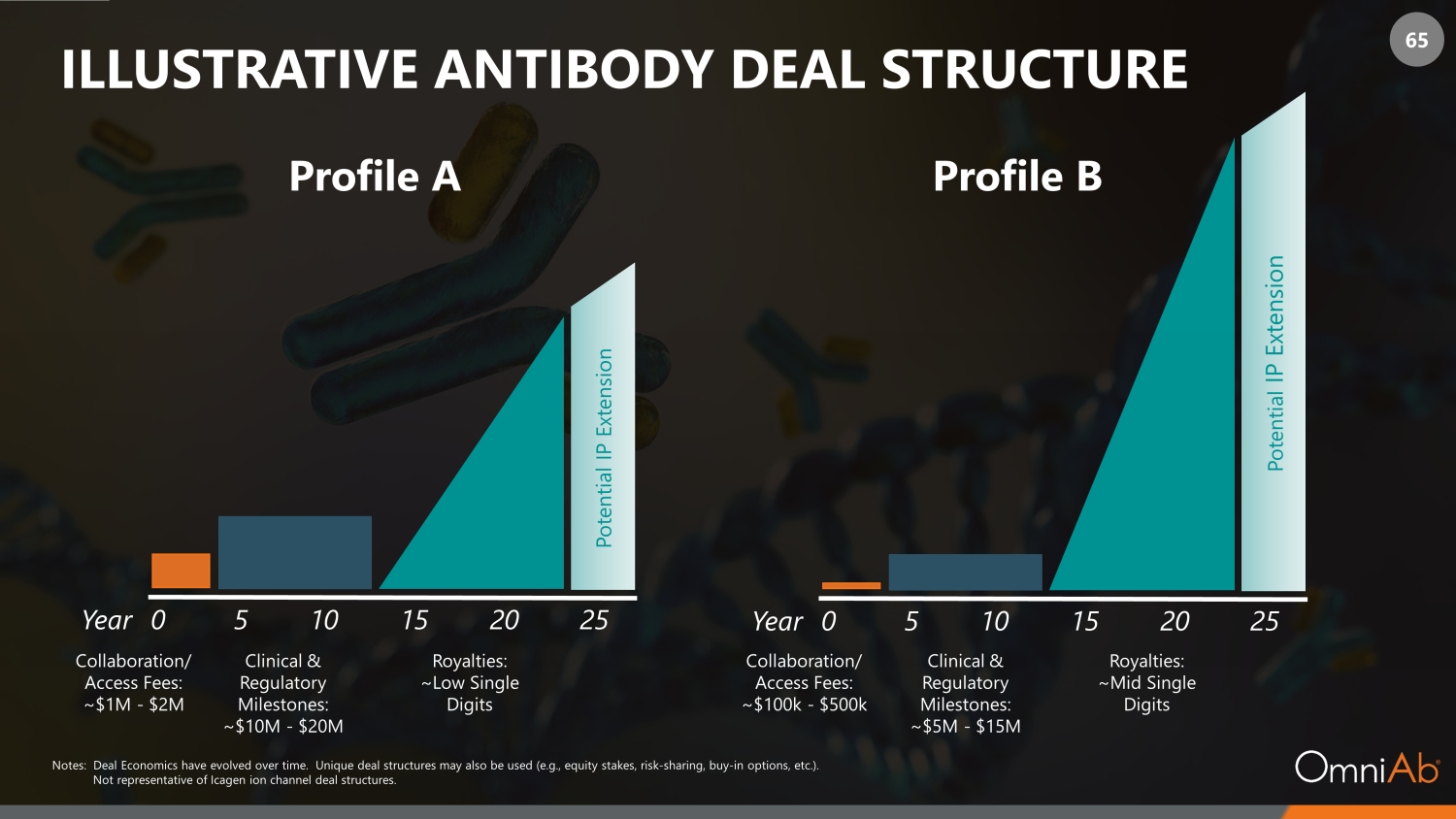
65 ILLUSTRATIVE ANTIBODY DEAL STRUCTURE Year 0 5 10 15 20 25 Collaboration/ Access Fees: ~$1M - $2M Potential IP Extension Notes: Deal Economics have evolved over time. Unique deal structures may also be used (e.g., equity stakes, risk - sharing, buy - in options, etc.). Not representative of Icagen ion channel deal structures. Clinical & Regulatory Milestones: ~$10M - $20M Royalties: ~Low Single Digits Profile A Profile B Year 0 5 10 15 20 25 Collaboration/ Access Fees: ~$100k - $500k Potential IP Extension Clinical & Regulatory Milestones: ~$5M - $15M Royalties: ~Mid Single Digits

Illustrative Valuation Modeling

67 MODELING OMNIAB VALUE • Approved Products & Clinical Pipeline • Existing Discovery/Preclinical Programs • New Programs from New and Existing Partners • Operating Expense 2 Products Approved 23 Clinical - Stage Programs 242 Active Programs in Discovery /Preclinical Development 68 Programs Added in 2021 Scalable Infrastructure
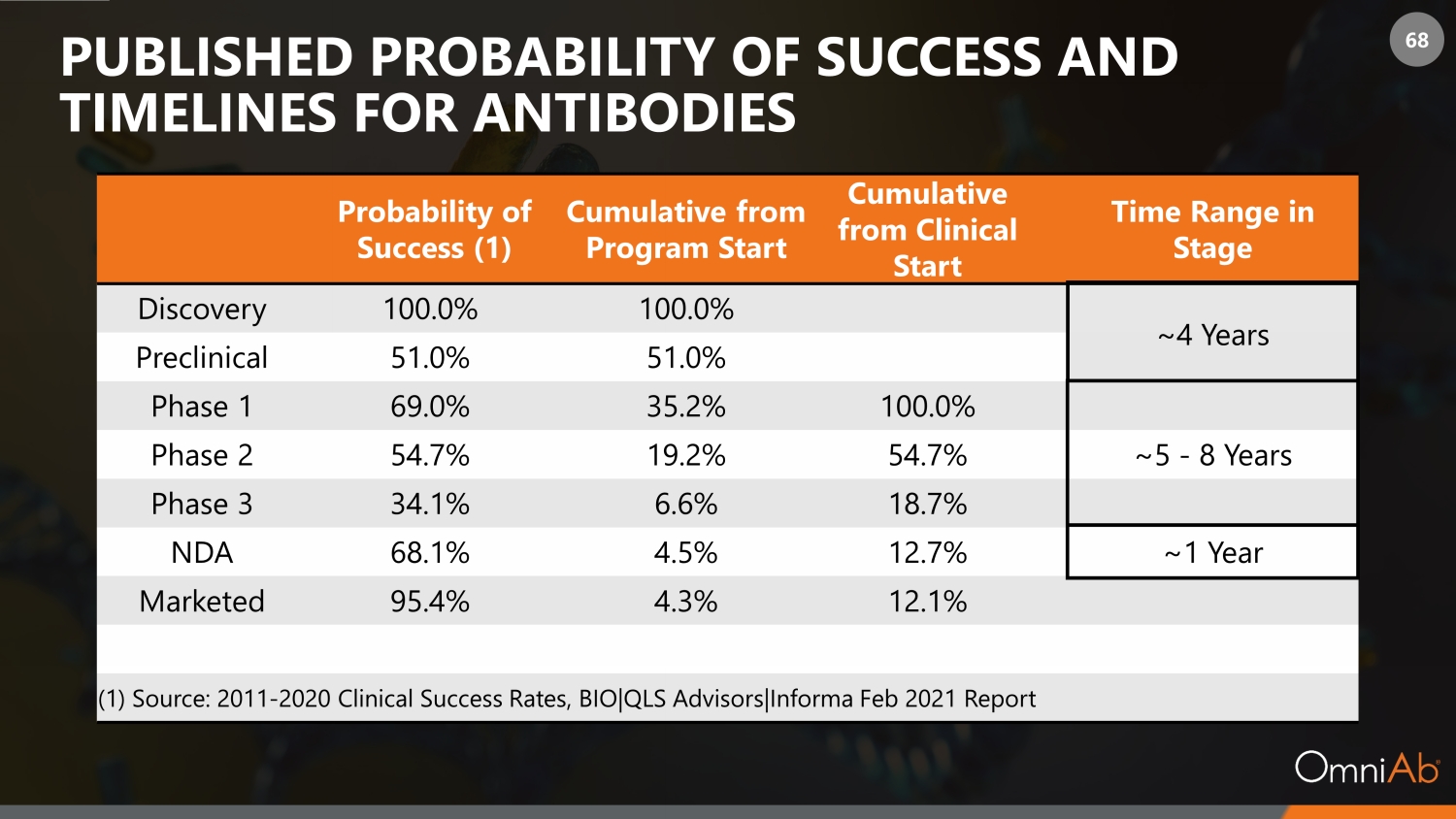
68 PUBLISHED PROBABILITY OF SUCCESS AND TIMELINES FOR ANTIBODIES Probability of Success (1) Cumulative from Program Start Cumulative from Clinical Start Time Range in Stage Discovery 100.0% 100.0% ~4 Years Preclinical 51.0% 51.0% Phase 1 69.0% 35.2% 100.0% Phase 2 54.7% 19.2% 54.7% ~5 - 8 Years Phase 3 34.1% 6.6% 18.7% NDA 68.1% 4.5% 12.7% ~1 Year Marketed 95.4% 4.3% 12.1% (1) Source: 2011 - 2020 Clinical Success Rates, BIO|QLS Advisors|Informa Feb 2021 Report
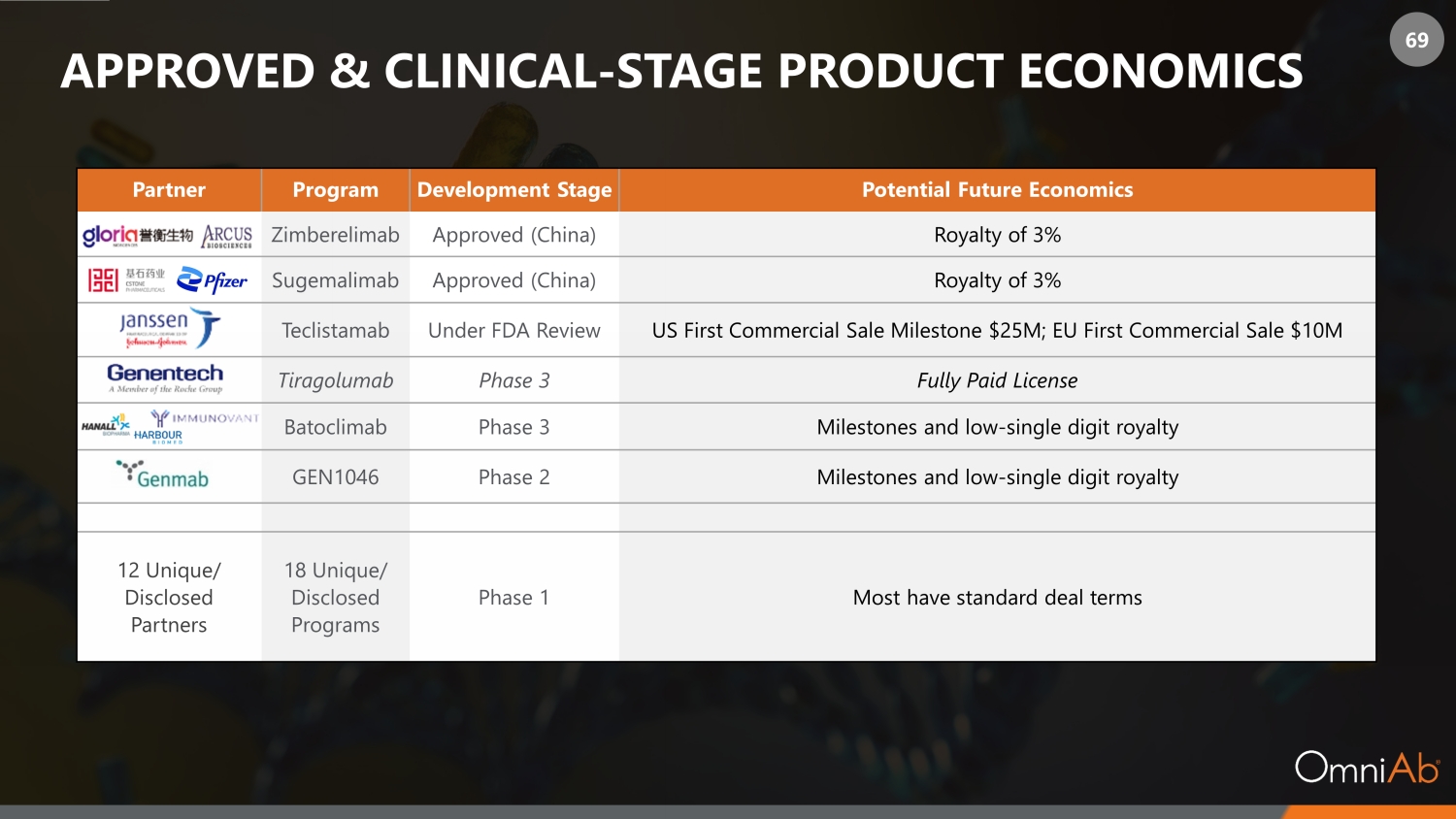
69 APPROVED & CLINICAL - STAGE PRODUCT ECONOMICS Partner Program Development Stage Potential Future Economics Zimberelimab Approved (China) Royalty of 3% Sugemalimab Approved (China) Royalty of 3% Teclistamab Under FDA Review US First Commercial Sale Milestone $25M; EU First Commercial Sale $10M Tiragolumab Phase 3 Fully Paid License Batoclimab Phase 3 Milestones and low - single digit royalty GEN1046 Phase 2 Milestones and low - single digit royalty 12 Unique/ Disclosed Partners 18 Unique/ Disclosed Programs Phase 1 Most have standard deal terms
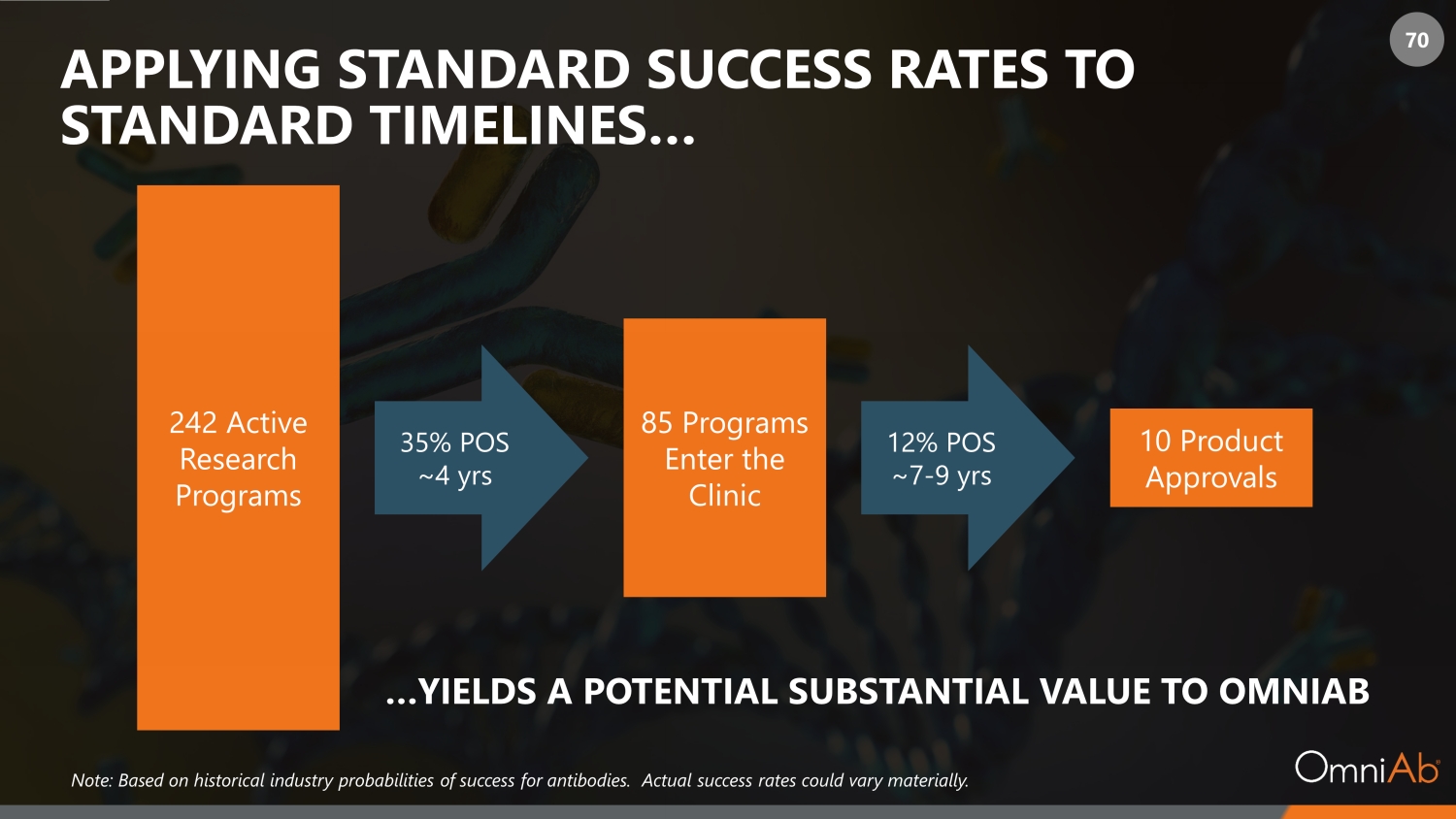
70 APPLYING STANDARD SUCCESS RATES TO STANDARD TIMELINES… 242 Active Research Programs 85 Programs Enter the Clinic 10 Product Approvals 35% POS ~4 yrs 12% POS ~7 - 9 yrs …YIELDS A POTENTIAL SUBSTANTIAL VALUE TO OMNIAB Note: Based on historical industry probabilities of success for antibodies. Actual success rates could vary materially.

71 NEW PROGRAMS CAN DRIVE FUTURE GROWTH Note: Based on historical industry probabilities of success for antibodies. Actual success rates could vary materially. New Program Starts (68 Starts in 2021 ) Programs Enter the Clinic Product Approvals 35% POS ~4 yrs 12% POS ~7 - 9 yrs

72 BUILDING BLOCKS OF VALUE Collaboration Revenue Existing Approved Products/Clinical Pipeline 242 Research - Stage Programs x POS x Standard Deal Terms New Program Starts x POS x Standard Deal Terms (68 in 2021 and growing) Novel Platform Technologies

73 PROFORMA REVENUE & OPEX ('000s) 2020 2021 License and milestone Revenue 11,385$ 14,664$ Collaboration Revenue 11,883 20,084 Total revenues 23,268 34,748 Operating costs and expenses: Research and development 24,796 39,232 Selling, general and administrative 10,225 16,947 Amortization of intangibles 11,800 12,968 Other operating income 2,070 1,210 Total operating costs and expenses 48,891 70,357 * Income from operations (25,623)$ (35,609)$ * Includes ~$30M in Stock Comp, Dep/Amort

74 SUMMARY • Demand for antibody - based approaches drives the industry’s need for cutting - edge discovery technologies • We maintain a highly - scalable business where investments in and extensions of our differentiated technology are informed by a deep portfolio of partnerships • Our partner pipeline is progressing and expanding • Current transaction creates robust financial position that fully funds operations, creates an opportunity to unlock value, and positions the business for future growth

APPENDIX

76 ZIMBERELIMAB THE FIRST APPROVED OMNIRAT - DERIVED ANTIBODY • On August 30, 2021, zimberelimab (GLS - 010), an OmniAb - derived fully human anti - PD - 1 mAb, was approved in China for the treatment of recurrent or refractory classical Hodgkin’s lymphoma ‒ Marked the first approval of an OmniAb - derived mAb • In 2015, GloriaBio contracted with WuXi Biologics to discover and develop zimberelimab in China using OmniRat ‒ Zimberelimab entered clinic in March 2017, and NDA was submitted to China NMPA in February 2020 ‒ GloriaBio is also conducting trials in advanced solid tumors, and was granted Breakthrough Therapy designation for the treatment of patients with recurrent/metastatic cervical cancer • Zimberelimab is being developed by Arcus Biosciences (in collaboration with Gilead) in North America, Europe, Japan and certain other territories through a 2017 agreement ‒ Arcus is conducting Phase 2 and 3 clinical trials investigating zimberelimab as part of combination therapy in lung, prostate, colorectal, pancreatic and GI cancers
77 SUGEMALIMAB THE SECOND APPROVED OMNIRAT - DERIVED ANTIBODY • Fully human, full - length anti - PD - L1 immunoglobulin G4 (IgG4) mAb, which may reduce risk of immunogenicity and toxicity, a unique advantage over similar drugs • Sugemalimab ( Cejemly ® ) was approved in China on June 6, 2022 for unresectable stage III non - small cell lung cancer (NSCLC) following concurrent or sequential chemoradiotherapy ‒ Previously approved in China in December 2021, and launched in January 2022, for first - line treatment of metastatic non - squamous NSCLC in combination with chemotherapy ‒ Becomes first anti - PD - 1/PD - L1 mAb approved for stage III NSCLC, and is also the only one approved for both stage III and stage IV NSCLC • Pfizer responsible for commercializing in China via 2020 strategic collaboration • EQRx licensed exclusive rights to sugemalimab for development / commercialization outside of China, targeting first regulatory filings ex - US in 2H 2022
78 TECLISTAMAB BISPECIFIC ANTIBODY FOR MULTIPLE MYELOMA • Janssen is developing teclistamab, an investigational, fully humanized IgG4, T - cell redirecting, bispecific antibody targeting both BCMA (B - cell maturation antigen) and CD3 ‒ Discovered in part with the OmniAb platform technology (anti - BMCA portion) • In December 2021 and January 2022, Janssen submitted a BLA to the FDA and a MAA to the EMA, respectively, for approval in the treatment of relapsed or refractory multiple myeloma (RRMM) ‒ Granted Orphan Drug Designation and Breakthrough Therapy Designation by the FDA, and Orphan Drug Designation and a PRIority MEdicines (PRIME) designation by EMA • At ASCO 2022, Janssen presented updated efficacy and safety results from the Phase 1/2 MajesTEC - 1 study , demonstrating continued deep and durable responses in the treatment of RRMM; results from the MajesTEC - 1 study were also published in The New England Journal of Medicine ‒ Updated positive results from the Phase 1 TRIMM - 2 study were also presented, evaluating teclistamab in combination with DARZALEX FASPRO®, a subcutaneous CD38 - directed monoclonal antibody

79 ($m) Sources Uses APAC Cash $236 Cash to Balance Sheet $250 Avista Minimum Commitment 15 Estimated Transaction Fees 16 Ligand Contribution 15 Total $266 Total $266 75.0% 20.3% 4.7% Ligand Shareholders APAC Shareholders Avista Shares ▪ OmniAb, Inc., an antibody discovery business wholly owned by Ligand, has entered into a definitive agreement to merge with Avista Public Acquisition Corp. II (APAC) (NASDAQ: AHPA), valuing OmniAb at a fully diluted pre - money equity valuation of $850 million - OmniAb to become an independent publicly traded company - Anchored spin - off transaction is backed with committed capital from Avista and Ligand - Tax - free distribution for existing Ligand shareholders via all SPAC consideration ▪ All - primary transaction will result in gross proceeds of up to $266 million, through a combination of: - APAC’s $236 million cash in trust - Avista to invest $15 million at $10.00 per share and provide up to an additional $100 million to backstop redemptions - $15 million contribution from Ligand - Earn - out payable to existing Ligand shareholders of 7.5mm shares @ $12.50 and 7.5mm shares @$15.00 - 33% of Avista’s sponsor shares (1.9 million) subject to earnout at the same thresholds as Ligand shareholders ▪ Closing Expected 2H 2022 TRANSACTION SUMMARY Note: Assumes no redemptions. Excludes impact of 8.2 million Avista warrants and 7.7 million public warrants, each struck at $11 .50. Excludes impact of 15.0 million Ligand earnout shares and 1.9 million Avista earnout shares. Up to 37.5% of Avista’s earnout shares are immediately vested pro rata to any u til ization of the $100 million backstop facility. (1) Excludes OmniAb’s expenses. (2) Includes 1.5 million co - investment shares and 3.8 million sponsor shares. Transaction Summary Illustrative Sources & Uses Illustrative Pro Forma Ownership Pro Forma Enterprise Value (2) (1)

For more information, please visit www.omniab.com