SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934
(Amendment No. )
Filed by the Registrant x
Filed by a Party other than the Registrant ¨
Check the appropriate box:
¨ Preliminary Proxy Statement | ¨ Confidential, for Use of the Commission Only |
| | (as Permitted by Rule 14a-6(e)(2)) |
| x | | Definitive Proxy Statement |
| ¨ | | Definitive Additional Materials |
| ¨ | | Soliciting Material Pursuant to §240.14a-11(c) or §240.14a-12 |
Sirna Therapeutics, Inc.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ¨ | | Fee computed on table below per Exchange Act Rules 14a-6(1)(4) and 0-11. |
| | (1) | | Title of each class of securities to which transaction applies: |
| | (2) | | Aggregate number of securities to which transaction applies: |
| | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): |
| | (4) | | Proposed maximum aggregate value of transaction: |
| ¨ | | Fee paid previously with preliminary materials. |
| ¨ | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-1 1(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | (1) | | Amount Previously Paid: |
| | (2) | | Form, Schedule or Registration Statement No.: |
Notes:
SIRNA THERAPEUTICS, INC.
(formerly Ribozyme Pharmaceuticals, Inc.)
2950 Wilderness Place
Boulder, Colorado 80301
To Our Stockholders:
You are cordially invited to attend the Annual Meeting of Stockholders of Sirna Therapeutics, Inc. (the “Company”) to be held on Thursday, the 29th day of May, 2003, at 10:00 a.m., local time, at the principal offices of the Company, 2950 Wilderness Place, Boulder, Colorado, 80301. Your Notice of Annual Meeting, Proxy Statement and Proxy are enclosed, as is the Company’s 2002 Annual Report and Annual Report on Form 10-K for the year ended December 31, 2002, which includes the Company’s financial statements.
At the Annual Meeting, you will be asked to (1) elect two directors to serve for the ensuing three years until the expiration of their terms in 2006 or until their successors are duly elected and qualified; and (2) ratify the appointment of Ernst & Young LLP as the Company’s independent auditors for the fiscal year ending December 31, 2003. The Board of Directors has approved the proposals described in the Proxy Statement and recommends that you vote “FOR” each proposal.
Your vote is important. Whether or not you plan to attend the Annual Meeting in person, we ask that you return your completed Proxy, using the envelope provided, as soon as possible and in any case no later than 3:00 p.m., local time on Wednesday, May 28, 2003.
Thank you for your continued support.
Very truly yours,
SIRNA THERAPEUTICS, INC.
Howard W. Robin
Chief Executive Officer and President
April 30, 2003
SIRNA THERAPEUTICS, INC.
(formerly Ribozyme Pharmaceuticals, Inc.)
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
to be held May 29, 2003
TO OUR STOCKHOLDERS:
The Annual Meeting of Stockholders of Sirna Therapeutics, Inc., a Delaware corporation (the “Company”), will be held on Thursday, May 29, 2003, at 10:00 a.m., local time, at 2950 Wilderness Place, Boulder, Colorado, 80301, for the following purposes:
(1) To elect two (2) directors of the Company to serve for the ensuing three years until the expiration of their terms in 2006 or until their respective successors shall be elected and qualified.
(2) To ratify the selection of Ernst & Young LLP as the Company’s independent auditors for the fiscal year ending December 31, 2003.
(3) To transact such other business as may be properly presented at the meeting.
The names of the Board of Directors’ nominees for directors of the Company and descriptions of the other matters to be voted upon are set forth in the accompanying Proxy Statement. Only stockholders of record at the close of business on April 28, 2003, will be entitled to vote at the meeting. To be sure that your shares are represented at the meeting, you are urged to vote, sign, date and promptly return the enclosed Proxy in the envelope provided. You may revoke your Proxy at any time prior to the time it is voted.
By Order of the Board of Directors
/s/ Bharat M. Chowrira
Bharat M. Chowrira, Secretary
Date: April 30, 2003
Boulder, Colorado
IMPORTANT—PLEASE MAIL YOUR PROXY PROMPTLY. To ensure that your vote is recorded properly, you are urged to sign and return the enclosed Proxy in the envelope provided as soon as possible so that it will be received no later than 3:00 p.m., local time, May 28, 2003.
2
SIRNA THERAPEUTICS, INC.
(formerly Ribozyme Pharmaceuticals, Inc.)
PROXY STATEMENT FOR THE
ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD MAY 29, 2003
INFORMATION CONCERNING SOLICITATION AND VOTING
General
The enclosed proxy is solicited on behalf of the Board of Directors of Sirna Therapeutics, Inc. (the “Company”) for use at the Annual Meeting of Stockholders of the Company to be held on May 29, 2003, at 10:00 a.m. local time, and at any adjournment, continuation or postponement of the meeting. These proxy solicitation materials are first sent or given on or about April 30, 2003 to stockholders entitled to vote at the Annual Meeting.
Record Date and Shares Outstanding
Stockholders who owned shares of our common stock at the close of business on April 28, 2003, referred to in this Proxy Statement as the record date, are entitled to notice of, and to vote at, the Annual Meeting. Each share of common stock outstanding on the record date is entitled to one vote on the matters to be voted on at the Annual Meeting. As of April 28, 2003, 28,165,338 shares of the Company’s common stock were outstanding.
Revoking Your Proxy
You may revoke your proxy at any time before it is exercised; therefore, execution of the proxy will not in any way affect the stockholder’s right to attend the meeting in person. Revocation may be made prior to the meeting by written revocation or duly executed proxy bearing a later date sent to the Company, Attention: Bharat M. Chowrira, Secretary, 2950 Wilderness Place, Boulder, Colorado 80301; or a proxy may be revoked personally at the Annual Meeting by written notice to the Secretary at the Annual Meeting prior to the voting of the Proxy. Any revocation sent to the Company must include the stockholder’s name and must be received prior to the meeting to be effective.
How Your Proxy Will Be Voted
In the absence of specific instructions to the contrary, shares represented by properly executed proxies received by the Company, including unmarked proxies, will be voted to (1) elect the two nominees to the Board described herein; and (2) ratify the selection of Ernst & Young LLP as the Company’s independent auditors for the fiscal year ending December 31, 2003. In addition, if any other matters properly come before the meeting, it is the intention of the persons named in the enclosed proxy to vote the shares they represent as directed by the Board of Directors. We have not received notice of any other matters that may properly be presented at the Annual Meeting.
Quorum
The presence, in person or by proxy, of the holders of a majority of the outstanding shares of the Company’s common stock as of the record date is necessary to constitute a quorum at the Annual Meeting.
Voting
Tabulation
Votes of stockholders entitled to vote who are present at the Annual Meeting in person or by proxy, abstentions and broker non-votes are counted as present or represented at the meeting for purposes of determining whether a quorum exists. Cumulative voting is not permitted. The nominees for election as directors at the Annual Meeting will be elected by a plurality of the votes of the shares entitled to vote and present in person or represented by proxy at the Annual
3
Meeting. For the proposal to ratify the appointment of Ernst & Young LLP as the Company’s independent auditors for the fiscal year ending December 31, 2003, the affirmative vote of a majority of shares of common stock entitled to vote and present or represented by proxy at the Annual Meeting is necessary for approval.
Abstentions, Broker Non-Votes
In the absence of controlling precedent to the contrary, we intend to treat abstentions and broker non-votes in the following manner. A broker “non-vote” occurs when a nominee holding shares for a beneficial owner does not vote on a particular proposal because the nominee does not have the discretionary voting power with respect to that item and has not received instructions from the beneficial owner. Broker “non-votes” and shares as to which proxy authority has been withheld with respect to any matter are not deemed to be entitled to vote for purposes of determining whether stockholder approval of that matter has been obtained. As a result, broker “non-votes” are not included in the tabulation of the voting results on the election of directors or issues requiring the approval of a majority of the shares of common stock present and entitled to vote and, therefore, do not have the effect of votes in opposition for such proposals. With respect to Proposal 1 requiring a plurality vote and Proposal 2 requiring the affirmative vote of a majority of the common stock, present and entitled to vote, broker “non-votes” have no effect. Because abstentions will be included in tabulations of the shares of common stock entitled to vote for purposes of determining whether a proposal has been approved, abstentions have the same effect as a negative vote on Proposal No. 2.
Solicitation of Proxies
This solicitation is being made by mail on behalf of our Board of Directors, but may also be made without additional remuneration by our officers or employees by telephone, telegraph, facsimile transmission, e-mail or personal interview. We will bear the expense of the preparation, printing and mailing of the enclosed form of proxy, notice of annual meeting and this proxy statement and any additional material relating to the meeting that may be furnished to our stockholders by our board subsequent to the furnishing of this proxy statement. We will reimburse banks and brokers who hold shares in their name or custody, or in the name of nominees for others, for their out-of-pocket expenses incurred in forwarding copies of the proxy materials to those persons for whom they hold such shares. To obtain the necessary representation of stockholders at the meeting, supplementary solicitations may be made by mail, telephone or interview by our officers or employees, without additional compensation, or selected securities dealers. We anticipate that the cost of such supplementary solicitations, if any, will not be material.
PROPOSAL NO. 1
ELECTION OF DIRECTORS
Our Amended and Restated Certificate of Incorporation provides for a Board of Directors made up of three classes. The term of office of the Class I Directors expires at the Annual Meeting, the term of office of the Class II Directors expires at the Annual Meeting of Stockholders to be held in 2004, and the term of office of the Class III Directors expires at the Annual Meeting of Stockholders to be held in 2005. Thereafter, the term of each class expires at each third succeeding Annual Meeting of Stockholders after election of the class.
Our Board currently consists of five directors, with two unfilled vacancies. Mr. Howard W. Robin and Mr. Jeremy L. Curnock Cook are the Class I directors and have been nominated by the Board of Directors for election at the Annual Meeting to serve for a three-year term expiring at the Annual Meeting of Stockholders to be held in 2006. Dr. Douglas Fambrough and Dr. Bryan Roberts comprise the Class II directors, along with an additional member to be designated by the Sprout Group in accordance with the terms of the Company’s Amended and Restated Bylaws. Dr. James Niedel and an independent director to be named in the future comprise the Class III directors. The proxies will be voted, unless authority to do so is withheld, in favor of the two Class I nominees recommended by the Board.
If either of the Class I director nominees is unable or declines to serve as a director at the time of the Annual Meeting, the proxies will be voted for any nominee who is designated by the present Board of Directors to fill the vacancy. It is not expected that either nominee will be unable or will decline to serve as a director.
4
Certain information regarding our directors and the director nominees is set forth below.
Name
| | Age
| | Position
|
Howard W. Robin | | 50 | | Chief Executive Officer, President and Director |
Jeremy L. Curnock Cook (2) | | 53 | | Director |
Douglas Fambrough (2)(3) | | 34 | | Director |
James Niedel (1)(3) | | 59 | | Chairman of the Board |
Bryan Roberts (1)(2) | | 36 | | Director |
| (1) | | Member of the Compensation Committee. |
| (2) | | Member of the Audit Committee. |
| (3) | | Member of the Nominating and Corporate Governance Committee. |
Nominees for Election as Class I Directors with Term Expiring in 2006
Howard W. Robin has served as Chief Executive Officer, President and Director since July 2001. From January 2001 to June 2001, Mr. Robin was Chief Operating Officer, President and Director. From 1991 to 2001 Mr. Robin was Corporate Vice President and General Manager at Berlex Laboratories, Inc. and from 1987 to 1991 he served as Vice President of Finance and Business Development and Chief Financial Officer. From 1984 to 1987 Mr. Robin was Director of Business Planning & Development at Berlex. He was a Senior Associate with Arthur Andersen & Company prior to joining Berlex. He received his B.S. in Accounting & Finance from Farleigh Dickinson University in 1974.
Jeremy L. Curnock Cook has served as a director since July 1995. Mr. Cook was a director of Rothschild Asset Management, an investment management company, where he was responsible for the Rothschild Bioscience Unit from 1987 until his retirement in 2000. Mr. Cook founded the International Biochemicals Group in 1975, which he subsequently sold to Royal Dutch Shell in 1985, remaining as Managing Director until 1987. He is Executive Chairman of Bioscience Managers Ltd., a corporate advisory company, and Non-Executive Chairman of Targeted Genetics Inc. He is also Chairman of atugen Gmbh and a director of GlycoDesign, Inc., Valigen, N.V., Inflazyme Pharmaceuticals, Inc. and Biocompatibles International plc. Mr. Cook received an M.A. in Natural Sciences from Trinity College Dublin.
Continuing Class II Directors with Term Expiring in 2004
Douglas Fambroughhas served as a director since April 2003 and is a Principal with Oxford Bioscience Partners, specializing in investments in technology-based discovery companies. Besides Sirna, Dr. Fambrough also represents Oxford on the board of Endogeny Bio Corp. As an observer, Dr. Fambrough represents Oxford to the boards of RibX Pharmaceuticals Inc., Avalon Pharmaceuticals Inc., and Xantos Biomedicine AG. Prior to joining Oxford Bioscience Partners, Dr. Fambrough was a genomics researcher at the Whitehead/MIT Center for Genome Research, where his work in functional genomics led to major publications in the journals Cell and Nature Genetics. He graduated from Cornell University and obtained his Ph.D. in Genetics from the University of California, Berkeley. In addition to his work as a venture capitalist, Dr. Fambrough serves on the board of the Connecticut chapter of the MIT Enterprise Forum.
Bryan Roberts has served as director since April 2003. He joined Venrock in 1997 and is now a General Partner involved with the firm’s activities in healthcare, and is based in Menlo Park, CA. Dr. Roberts is responsible for Venrock’s investments in Illumina and Nanosys, and currently serves on the boards of several private companies including athenahealth, First Genetic Trust, Microbia, Satiety and Xenoport. Prior to joining Venrock, Dr. Roberts worked at Kidder Peabody & Co., Inc. in corporate finance. Dr. Roberts received his Ph.D. in Chemistry and Chemical Biology at Harvard University and graduated from Dartmouth College, where he obtained a B.A. in Chemistry.
5
Continuing Class III Director with Term Expiring 2005
James Niedelhas served as a director since April 2003. He joined Sprout as a Venture Partner on May 1, 2002. Prior to that appointment, Dr. Niedel was Chief Science and Technology Officer for GlaxoSmithKline and from 1995 to 2001 he was a member of the board of directors of Glaxo Wellcome plc with responsibility for Global Research and Development, Information Technology and Product Strategy. From 1988 to 1995 he was VP Research and SVP R&D for the US subsidiary of Glaxo. Before joining the pharmaceutical industry, Dr. Niedel was Professor of Medicine and Chief of the division of Clinical Pharmacology at Duke Medical School, where he had completed an Internal Medicine residency and a Hematology-Oncology fellowship. He received M.D. and Ph.D. (Biochemistry) degrees from the University of Miami, was selected a Searle Scholar, and is a Fellow of the Royal College of Physicians (London).
Pursuant to our Amended and Restated Bylaws, the Sprout Group has the right to designate two persons to be appointed a member of the Company’s Board of Directors. Oxford Bioscience Partners IV and Venrock Associates each have the right to designate one person to be appointed a member of the Board of Directors. In addition, the Sprout Group, Oxford Bioscience Partners IV and Venrock Associates have entered into an agreement whereby each has agreed to vote for the designee appointed by the other parties.
There is no family relationship between any of our directors or executive officers.
Vote Required and Board of Directors’ Recommendation
The two nominees receiving the highest number of affirmative votes of the shares voted at the meeting shall be elected to the Board of Directors.
Our Board of Directors recommends that the stockholders vote “FOR” the director nominees listed above.
Board of Directors and Committee Meetings
In 2002, there were seven meetings of the Board of Directors of the Company. The Board of Directors has a standing audit, compensation and nominating and governance committee. During 2002, all directors attended 75 percent or more of the Company’s Board meetings and meetings of Board committees on which they served.
On April 21, 2003, concurrent with the consummation of the Company’s completion of the private placement of common stock and warrants to the Sprout Group, Venrock Associates, Oxford Bioscience Partners IV L.P. and certain other investors (the “Private Placement”), three members of the Company’s then current Board of Directors resigned. Members of the Company’s Board of Directors prior to the Private Placement included: Mr. Jeremy Curnock Cook (Chairman), Mr. John Groom, Mr. Howard W. Robin, Dr. Samuel Saks and Mr. Anders Wiklund. Messrs. Robin and Cook continue to serve as a director and both stand for re-election as Class I directors at the Annual Meeting.
Compensation Committee
In 2002, the Compensation Committee consisted of Mr. Cook, Mr. Groom (Chairman) and Mr. Wiklund. The current members of the Compensation Committee are Dr. Niedel and Dr. Roberts. The Compensation Committee met four times in 2002. The Compensation Committee:
| | • | | reviews and recommends for Board approval grants of options pursuant to the Company’s Stock Option Plans; |
| | • | | decides salaries and incentive compensation for the Company’s employees and consultants; and |
| | • | | recommends compensation for executive officers for approval by the board. |
Audit Committee
In 2002, the Audit Committee consisted of Mr. Cook, Dr. Saks (Chairman) and Mr. Wiklund. The current members of the Audit Committee are Mr. Cook, Dr. Roberts and Dr. Fambrough. The Audit Committee met five times in 2002. The Audit Committee assists the Board of Directors in its general oversight of our financial reporting, internal controls and audit functions.
6
Additional information regarding the Audit Committee and its members is contained in the “Audit Committee Report” beginning on page 21 of this proxy statement.
Nominating and Governance Committee
In March 2003, the Board of Directors established a Nominating and Governance Committee. The current members of the Nominating and Governance Committee are Dr. Fambrough and Dr. Niedel. The Nominating and Governance Committee was organized to:
| | • | | identify, review and recommend qualified director candidates to the Board, |
| | • | | review qualifications of such candidates, |
| | • | | recommend to the Board for consideration such candidates for membership as a director, and |
| | • | | fill vacancies that may arise from time to time. |
Compensation of Directors
In 2002, all non-employee directors received a $10,000 annual retainer, $1,000 per day for each Board or Committee meeting attended in person and $500 per day for participating telephonically in a Board or Committee meeting. In addition, each non-employee director received an initial stock option grant of 20,000 shares of common stock that vest over four years and an annual grant of stock options for 10,000 shares of common stock that vest after one year of service.
Executive Officers
The officers of the Company serve at the discretion of the Board of Directors and hold office until their successors are appointed by the Board of Directors. Information regarding the Company’s executive officers as of April 30, 2003 is set forth below.
Name
| | Age
| | Position
|
Howard W. Robin (1) | | 50 | | Chief Executive Officer, President and Director |
Marvin Tancer | | 52 | | Chief Financial Officer and Vice President of Operations |
Nassim Usman, Ph.D. | | 43 | | Chief Scientific Officer and Vice President of Research and Development |
| (1) | | Information regarding Howard Robin is discussed under “Nominees for Election as Class I Directors with Term Expiring in 2006.” |
Marvin Tancerhas served as Chief Financial Officer and Vice President of Operations since June 2001. Prior to joining the Company in 2001, Mr. Tancer served as National Sales Director of Oncology and Area Sales Director for the Western United States for Berlex Labs, Inc. from 1999 to 2000. From 1992 to 1998 he held the position of Director of Finance and Administration and Chief Financial Officer at Berlex Biosciences. From 1986 to 1992 he held the position of Corporate Controller for Berlex Labs, Inc. and from 1982 to 1986 was Pharmaceutical Division Controller and Director of Financial Analysis. He earned his BS in Business Administration from the Wharton School of Business.
Nassim Usman, Ph.D., has served as Chief Scientific Officer since February 2002 and Vice President of Research and Development since August 2000. From December 1999 to July 2000, Dr. Usman served as Senior Vice President of Research. From May 1996 to December 1999, Dr. Usman served as Vice President of Research. From
7
April 1994 until May 1996, Dr. Usman served as Director of Chemistry and Biochemistry Research and from September 1992 until April 1994 Dr. Usman served as Senior Scientist in Chemistry and Biochemistry. Prior to joining the Company, Dr. Usman was a Postdoctoral Fellow and Scientist in the Departments of Biology and Chemistry at the Massachusetts Institute of Technology. Dr. Usman received his Ph.D. in chemistry from McGill University.
Compensation of Executive Officers
Summary Compensation Table
The following table summarizes the compensation paid to or earned by the Company’s Chief Executive Officer and the other most highly compensated executive officers whose annual compensation exceeded $100,000 in 2002 (“Named Executive Officers”).
Summary Compensation Table
| | | Annual Compensation
| | | Long-term Compensation
|
Name and Principal Position
| | Year
| | Salary($)
| | Bonus($)
| | Other Annual Comp.($)
| | | Shares Underlying Options Granted(#)*
| | All Other Comp.($)(1)
|
Howard W. Robin Chief Executive Officer and President(2) | | 2002 2001 | | $ | 344,500 309,231 | | $ | 155,025 124,313 | | $ | 181,203 678,273 | (3) (3) | | 62,834 64,167 | | | — — |
|
Marvin Tancer(4) Chief Financial Officer and Vice President of Operations | | 2002 2001 | | $ | 266,000 135,417 | | $
| 79,800
59,500 | | $ | 91,370 480,447 | (5) (5) | | 32,916 32,500 | | $
| 6,000
— |
|
Nassim Usman, Ph.D. Chief Scientific Officer, Vice President of Research and Development | | 2002 2001 2000 | | $ | 254,000 240,000 218,330 | | $
| 76,200
50,907
38,200 | | $ | 23,367 43,958 43,958 | (6) (6) (6) | | 26,984 3,333 5,834 | | $
| 5,500
5,249
5,238 |
|
Lawrence E. Bullock(7) Vice President of Administration and Finance | | 2002 2001 2000 | | $ | 185,400 180,000 166,275 | | $
| 10,000
11,340
29,925 | | $ | 3,000 25,797 25,797 | (7) (7) (7) | | 8,833 1,666 5,000 | | $
| 5,098
5,249
5,238 |
| * | | All option grant amounts reflect a reverse split ratio of one-for-six, effected April 16, 2003. |
| (1) | | The “All Other Compensation” column shows the dollar value of matching contributions in common stock made by the Company under the 401(k) Salary Reduction Plan. |
| (2) | | Mr. Robin joined the Company on January 4, 2001. |
| (3) | | Includes for 2002: (a) reimbursement of relocation expenses of $17,220; (b) $14,876 for taxes related to relocation; (c) $80,000 for partial forgiveness of a loan; and (d) $69,107 for taxes related to the forgiveness of the loan. Includes for 2001: (a) reimbursement of relocation expenses of $323,829; (b) $266,675 for taxes related to relocation; (c) imputed interest of $48,000 on a five year $400,000 forgivable loan; and (d) $39,559 for taxes related to the imputed interest on the loan. |
| (4) | | Mr. Tancer joined the Company on June 18, 2001. |
| (5) | | Includes for 2002: (a) reimbursement of relocation expenses of $9,020; (b) $7,793 for taxes related to relocation; (c) $40,000 for partial forgiveness of a loan; and (d) $34,557 for taxes related to the forgiveness of the loan. Includes for 2001: (a) reimbursement of relocation expenses of $232,485; (b) $191,453 for taxes |
8
| | related to relocation; (c) imputed interest of $30,897 on a five year $200,000 forgivable loan; and (d) $25,463 for taxes related to the imputed interest on the loan. |
| (6) | | Includes (a) $12,000 in 2002 and $27,000 in each of 2001 and 2000, for partial forgiveness of a loan; (b) $8,367 in 2002 and $14,000 in each of 2001 and 2000, for taxes related to partial forgiveness of the loan; and (c) approximately $3,000 in each of 2002, 2001 and 2000 for dependent daycare expenses. |
| (7) | | Includes (a) $15,000 in each of 2001 and 2000 for partial forgiveness of a loan; (b) approximately $7,800 in each of 2001 and 2000 for taxes relating to the loan; and (c) $3,000 in each of 2002, 2001 and 2000 as reimbursements for dependent daycare expenses. Mr. Bullock resigned from the Company in February 2003. |
The foregoing compensation table does not include certain fringe benefits made available on a non-discriminatory basis to all of our employees such as group health insurance, dental insurance, long-term disability insurance, vacation and sick leave. In addition, we may make available certain non-monetary benefits to our executive officers with a view to acquiring and retaining qualified personnel and facilitating job performance. We consider such benefits to be ordinary and incidental business costs and expenses. We also did not include in the table the aggregate value of such benefits in the case of the executive officers, which cannot be precisely ascertained but which is the lesser of either (a) 10% of the salary and bonus paid to each such executive officer or to the group, respectively, or (b) $50,000 or $50,000 times the number of individuals in the group, as the case may be.
Option Grants Table
The following table contains information about stock options granted to each of the Named Executive Officers during 2002 under the Company’s stock option plans. The exercise price of each option was equal to the fair market value of the common stock on the date of the option grant as determined by the Board of Directors. The options are granted for a term of ten years, subject to earlier termination if employment is terminated. In 2002, the Company granted options representing an aggregate of 307,491 shares of the Company’s common stock to employees and directors, including the Named Executive Officers.
Option Grants in Last Fiscal Year
| | | Individual Grants
| | | | Potential Realizable Value at
Annual Rate of Stock
Price Appreciation for Option Term(1)
|
| | | Number of Shares Underlying Options Granted (#)
| | % of Total
Options
Granted
to
Employees
in 2002
| | | Exercise Price
($/Share)
| | | |
| | | | | | Expiration Date
| | 5%($)
| | 10%($)
|
|
Howard W. Robin | | 29,500 | | 9.6 | % | | $ | 4.68 | | 06-04-12 | | 86,825 | | 220,032 |
| | | 16,667 | | 5.4 | % | | | 4.62 | | 08-22-12 | | 48,426 | | 122,721 |
| | | 16,667 | | 5.4 | % | | | 1.86 | | 12-18-12 | | 19,496 | | 49,407 |
| | |
| |
|
| | | | | | |
| |
|
| | | 62,834 | | 20.4 | % | | | | | | | 154,747 | | 392,160 |
|
Marvin Tancer | | 14,583 | | 4.7 | % | | | 4.68 | | 06-04-12 | | 42,921 | | 108,770 |
| | | 12,500 | | 4.1 | % | | | 4.62 | | 08-22-12 | | 36,319 | | 92,039 |
| | | 5,833 | | 1.9 | % | | | 1.86 | | 12-18-12 | | 6,823 | | 17,291 |
| | |
| |
|
| | | | | | |
| |
|
| | | 32,916 | | 10.7 | % | | | | | | | 86,063 | | 218,100 |
|
Nassim Usman | | 8,651 | | 2.8 | % | | | 4.68 | | 06-04-12 | | 25,462 | | 64,525 |
| | | 12,500 | | 4.1 | % | | | 4.62 | | 08-22-12 | | 36,319 | | 92,039 |
| | | 5,833 | | 1.9 | % | | | 1.86 | | 12-18-12 | | 6,823 | | 17,291 |
| | |
| |
|
| | | | | | |
| |
|
| | | 26,984 | | 8.8 | % | | | | | | | 68,604 | | 173,855 |
|
Lawrence E. Bullock | | 7,167 | | 2.3 | % | | | 4.68 | | 06-04-12 | | 21,094 | | 53,457 |
| | | 833 | | 0.3 | % | | | 4.62 | | 08-22-12 | | 2,420 | | 6,133 |
| | | 833 | | 0.3 | % | | | 1.86 | | 12-18-12 | | 974 | | 2,469 |
| | |
| |
|
| | | | | | |
| |
|
| | | 8,833 | | 2.9 | % | | | | | | | 24,488 | | 62,059 |
| (1) | | Amounts reported in these columns show hypothetical gains that may be realized upon exercise of the options, assuming the market price of common stock appreciates at the specified annual rates of appreciation, compounded annually over the term of the options. These numbers are calculated based upon rules |
9
| | promulgated by the SEC. Actual gains, if any, depend on the future performance of common stock and overall market conditions. |
Aggregated Option Exercises and Fiscal Year-End Option Values
The following table contains information about the stock options exercised in 2002 and the number and value of stock options held by each Named Executive Officer as of December 31, 2002. A stock option is “in-the-money” if the closing market price of common stock exceeds the exercise price of the stock option. The value of “in-the-money” unexercised stock options set forth in the table represents the difference between the exercise price of these options and the closing sales price of the common stock on December 31, 2002, as reported by the Nasdaq National Market ($1.44 per share).
Aggregated Option Exercises in Last Fiscal Year and
Fiscal Year-End Option Values
Name
| | Shares Acquired On
Exercise (#)
| | Value
Realized ($)(1)
| | Number of Securities Underlying Unexercised Options at December 31, 2002(#) Exercisable/Unexercisable
| | Value of Unexercised
In-the-Money Options
at December 31, 2002($) Exercisable/Unexercisable
|
Howard W. Robin | | — | | | — | | 20,218/106,787 | | 0/0 |
Marvin Tancer | | — | | | — | | 8,126/57,289 | | 0/0 |
Nassim Usman | | 333 | | $ | 6,633 | | 24,610/36,733 | | 0/0 |
Lawrence E. Bullock | | — | | | — | | 23,301/14,074 | | 0/0 |
| (1) | | Value realized is the difference between the exercise price and the closing market price of the common stock on the day of exercise. |
SECURITY OWNERSHIP OF
CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth the shares of our common stock beneficially owned by (1) each of our directors (including the director nominees), (2) our Chief Executive Officer and each of the Named Executive Officers, (3) all of our directors and executive officers as a group, and (4) all persons known by us to beneficially own more than 5% of our outstanding stock. Except as otherwise indicated in the accompanying footnotes, beneficial ownership is shown as of April 28, 2003.
Name and Address
| | Number of Shares Beneficially Owned(1)
| | Percentage of
Shares Outstanding(2)
| |
Sprout Group(3) | | 13,917,666 | | 45.6 | % |
11 Madison Avenue, 26th Floor | | | | | |
New York, New York 10010 | | | | | |
| | | | | | |
Venrock Associates(4) | | 6,298,506 | | 21.5 | % |
30 Rockefeller Plaza, Suite 5508 | | | | | |
New York, New York 10112 | | | | | |
| | | | | | |
Oxford Bioscience Partners(5) | | 4,774,674 | | 16.5 | % |
222 Berkeley Street, Suite 1650 | | | | | |
Boston, Massachusetts 02116 | | | | | |
10
Techno Venture Management (6) 101 Arch Street, Suite 1950 Boston, Massachusetts 02110 | | 3,047,665 | | 10.6 | % |
James Niedel, M.D., Ph.D. (3) | | 13,917,666 | | 45.6 | % |
Bryan Roberts, Ph.D. (4) | | 6,298,506 | | 21.5 | % |
Douglas Fambrough, Ph.D. (5) | | 4,774,674 | | 16.5 | % |
Jeremy L. Curnock Cook (7) | | 6,666 | | * | |
Lawrence E. Bullock (8) | | 30,544 | | * | |
Howard W. Robin (9) | | 42,541 | | * | |
Marvin Tancer (10) | | 38,551 | | * | |
Nassim Usman, Ph.D. (11) | | 43,929 | | * | |
Executive officers and directors as a group (8 persons)(12) | | 25,153,077 | | 77.2 | % |
| (1) | | Shares are considered beneficially owned, for purposes of this table, only if held by the person indicated, or if such person, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has or shares the power to vote, to direct the voting of and/or to dispose of or to direct the disposition of such security. The business address for each of the Company’s directors and officers listed in the table is 2950 Wilderness Place, Boulder, Colorado 80301. |
| (2) | | Applicable percentages are based on 28,165,338 shares of common stock outstanding on April 28, 2003, adjusted as required by SEC rules. On April 16, 2003, the Company completed a one-for-six reverse stock split for all of its outstanding stock. All share numbers reflected in this Proxy Statement, including this table, give effect to the one-for-six reverse stock split. |
| (3) | | Consists of 10,684,732 shares and 2,210,404 shares of common stock issuable upon the exercise of warrants held by Sprout Capital IX, L.P., a limited partnership; 493,427 shares and 102,077 shares of common stock issuable upon the exercise of warrants held by Sprout IX Plan Investors, L.P., a limited partnership; 42,109 shares and 8,711 shares of common stock issuable upon the exercise of warrants held by Sprout Entrepreneurs Fund, L.P., a limited partnership; and 23,419 shares and 4,845 shares of common stock issuable upon the exercise of warrants held by DLJ Capital Corporation (collectively “Sprout”). In addition, amount includes 288,300 shares and 59,642 shares of common stock issuable upon the exercise of warrants held by Dr. James Niedel, a director of the Company and a Venture Partner of each partnership. Except for shares he holds directly, Dr. Niedel expressly disclaims beneficial ownership of the shares held by Sprout. |
| (4) | | Consists of 4,175,084 shares and 863,721 shares of common stock issuable upon the exercise of warrants held by Venrock Associates III, L.P., a limited partnership; 939,394 shares and 194,337 shares of common stock issuable upon the exercise of warrants held by Venrock Associates, a limited partnership; and 104,377 shares and 21,593 shares of common stock issuable upon the exercise of warrants held by Venrock Entrepreneurs Fund III, L.P., a limited partnership (collectively “Venrock”). Dr. Roberts is a director of the Company and a General Partner of Venrock Associates and a Member of Venrock Management III LLC and VEF Management III LLC, which is the General Partner of Venrock Associates III, L.P. and Venrock Entrepreneurs Fund III, L.P., respectively. He expressly disclaims beneficial ownership of the shares held by Venrock except to the extent of his pecuniary interest therein arising from his general partnership or member interests therein. |
| (5) | | Consists of 3,916,928 shares and 810,315 shares of common stock issuable upon the exercise of warrants held by Oxford Bioscience Partners IV L.P., a limited partnership and 39,301 shares and 8,130 shares of common stock issuable upon the exercise of warrants held by mRNA Fund II L.P., a limited partnership. OBP Management IV L.P. serves as the General Partner of each partnership (collectively “Oxford”). Dr. Fambrough is a director of the Company and a Principal of Oxford and expressly disclaims beneficial ownership of the shares held by Oxford except to the extent of his pecuniary interest therein arising from his general partnership interests therein. |
| (6) | | Consists of 2,525,253 shares and 522,412 shares of common stock issuable upon the exercise of warrants held by TVM V Life Science Ventures GmbH & Co., KG, a limited partnership. |
| (7) | | Includes options to purchase 6,666 shares exercisable within 60 days of April 28, 2003. |
11
| (8) | | Includes options to purchase 23,301 shares exercisable within 60 days of April 28, 2003 and 627 shares held by Mr. Bullock under the Company’s 401(k) Plan. Mr. Bullock resigned from the Company in February 2003. |
| (9) | | Includes options to purchase 40,094 shares held by Mr. Robin exercisable within 60 days of April 28, 2003. |
| (10) | | Includes options to purchase 34,565 shares Mr. Tancer exercisable within 60 days of April 28, 2003. |
| (11) | | Includes options to purchase 42,968 shares exercisable within 60 days of April 28, 2003 and 627 shares held by Dr. Usman under the Company’s 401(k) Plan. |
| (12) | | Includes options to purchase 147,594 shares by all current directors and officers as a group that are exercisable within 60 days of April 28, 2003. |
Employment Agreements
Our Chief Executive Officer and President, Howard W. Robin, entered into an employment agreement with the Company upon the consummation of the Private Placement on April 21, 2003. Pursuant to the employment agreement:
| | • | | he will receive an annual base salary of $345,000 subject to increase at the discretion of the Board of Directors; |
| | • | | he will be eligible for annual bonuses of up to 30% of his then current base salary; |
| | • | | he will be entitled to receive employee benefits, including term life insurance in the amount of $500,000; |
| | • | | the $400,000 interest-free loan he previously received from the Company will be forgiven over a specified period of time (40% of the loan already has been forgiven) and the Company also shall make a “gross-up” payment to Mr. Robin equal to his tax liability associated with such forgiveness; |
| | • | | upon a termination “without cause” or his leaving “for good reason,” 50% of his unvested stock options shall vest, the outstanding balance of the interest-free loan shall be forgiven and the Company shall pay him the “gross-up” payment; he shall receive his base salary plus a bonus for twelve (12) months after termination, subject to (a) increase if there is a change of control of the Company within twelve (12) months of termination, or (b) decrease if he obtains other employment during the severance period; and |
| | • | | upon a change of control of the Company, all of his unvested stock options shall vest one (1) year after the change of control, the interest-free loan will be forgiven and the “gross-up” payment will be made; and, if the change of control results in his termination, he will receive his severance payment as described above and all of his unvested stock options will vest immediately. |
In anticipation of the Private Placement, the Company granted Mr. Robin options to purchase 1,434,166 shares of common stock at an exercise price of $2.10. The options vest 20% upon the one year anniversary of the closing of the Private Placement and monthly thereafter for the next four years.
Our Chief Financial Officer and Vice President of Operations, Marvin Tancer, entered into an employment agreement with the Company upon the consummation of the Private Placement on April 21, 2003. Pursuant to the employment agreement:
| | • | | he will receive an annual base salary of $266,000 subject to increase at the discretion of the Board of Directors; |
| | • | | he will be eligible for annual bonuses of up to 20% of his then current base salary; |
| | • | | he will be entitled to receive employee benefits, including term life insurance in the amount of $500,000; |
12
| | • | | the $200,000 interest-free loan he previously received from the Company will be forgiven over a specified period of time (20% of the loan already has been forgiven) and the Company also shall make a “gross-up” payment to Mr. Tancer equal to his tax liability associated with such forgiveness; |
| | • | | upon a termination “without cause” or his leaving “for good reason,” his unvested stock options shall continue to vest on a monthly basis during an applicable nine (9) or twelve (12) month severance period; the outstanding balance of the interest-free loan shall be forgiven and the Company shall pay him the “gross-up” payment; he shall receive his base salary plus a bonus for nine (9) months after termination, subject to (a) increase if there is a change of control of the Company within twelve (12) months of termination, or (b) decrease if he obtains other employment during the severance period; and the Company shall continue to pay for all costs related to maintaining health care coverage for him and his dependents for a period no longer than eighteen (18) months; and |
| | • | | upon a change of control of the Company, all of his unvested stock options shall vest immediately if he terminates his employment “for good reason” within six (6) months after the change of control or if, under some circumstances, the Company terminates his employment other than “for cause”, the interest-free loan will be forgiven and the “gross-up” payment will be made, and, if the change of control results in his termination, he will receive his severance payment as described above and all of his unvested stock options will vest immediately. |
In anticipation of the Private Placement, the Company granted Mr. Tancer options to purchase 465,000 shares of common stock at an exercise price of $2.10. The options vest monthly for five years.
Our Chief Scientific Officer and Vice President of Research and Development, Nassim Usman, entered into an employment agreement with the Company upon the consummation of the Private Placement on April 21, 2003. Pursuant to the employment agreement:
| | • | | he will receive an annual base salary of $254,000 subject to increase at the discretion of the Board of Directors; |
| | • | | he will be eligible for annual bonuses of up to 20% of his then current base salary; |
| | • | | he will be entitled to receive employee benefits, including term life insurance in the amount of $500,000; |
| | • | | upon a termination “without cause” or his leaving “for good reason,” his unvested stock options shall continue to vest on a monthly basis during an applicable nine (9) or twelve (12) month severance period; he shall receive his base salary plus a bonus for nine (9) months after termination, subject to (a) increase if there is a change of control of the Company within twelve (12) months of termination, or (b) decrease if he obtains other employment during the severance period; and the Company shall continue to pay for all costs related to maintaining health care coverage for him and his dependents for a period no longer than eighteen (18) months; and |
| | • | | upon a change of control of the Company, all of his unvested stock options shall vest immediately if he terminates his employment “for good reason” within six (6) months after the change of control or if, under some circumstances, the Company terminates his employment other than “for cause”, and, if the change of control results in his termination, he will receive his severance payment as described above and all of his unvested stock options will vest immediately. |
In anticipation of the Private Placement, the Company granted Dr. Usman options to purchase 470,833 shares of common stock at an exercise price of $2.10. The options vest monthly for five years.
13
Compensation Pursuant to Plans
Stock Option Plans
The Company has established a Non-Qualified Stock Option Plan and an Incentive Stock Option Plan (collectively, the “Plans,” as amended), under which it authorized stock option grants to purchase shares of the Company’s common stock to eligible employees, consultants, and other individuals, as defined in the Plans. Options to purchase the Company’s common stock are exercisable at a price as determined by the Board of Directors at the time the option is granted, which shall not be less than 100% of the fair market value (110% in the case of 10 percent stockholders) at the date of grant. Vesting rights are determined by the Board of Directors at the time the option is granted and generally options become exercisable at twenty-five percent at the end of each of years one through four.
At a Special Meeting of Stockholders held on April 16, 2003, stockholders approved an amendment to our 1996 Stock Option Plan and to our 2001 Stock Option Plan as a result of which we (a) terminated the 1996 Stock Option Plan and had its outstanding options covered by the 2001 Stock Option Plan, (b) increased the number of shares reserved for issuance pursuant to our 2001 Stock Option Plan by a total of 5,666,667 (after giving effect to a reverse stock split of one-for-six), and (c) provide a maximum limit on options granted to any individual during any calendar year of two million shares (after giving effect to the reverse stock split). Following the amendment to our stock plans and after giving effect to the reverse stock split, as of April 28, 2003, the maximum aggregate number of shares of common stock that are reserved under the 2001 Stock Option Plan is 6,551,502 shares, which includes 384,835 shares that had been reserved and were available for issuance under our 1996 Stock Option Plan, as well as the additional 5,666,667 shares approved by stockholders at the special meeting. The shares reserved under our 2001 Stock Option Plan may be authorized, but unissued, or reacquired common stock. If an option expires or becomes unexercisable without having been exercised in full, the unexercised shares that were subject thereto will become available for future grant or sale under the 2001 Stock Option Plan.
Executive Bonus Plan
The Company adopted an Executive Bonus Plan in March 1999. This Bonus Plan provides the Company’s executive officers with the opportunity to earn an annual bonus contingent upon their fulfillment of annual goals as determined by the Compensation Committee comprised of three independent directors. The Compensation Committee has complete authority to establish the goals for each executive officer, to interpret all provisions of the Bonus Plan and to make all other determinations necessary or advisable for the administration of the Bonus Plan. The Compensation Committee may award each executive officer with an annual bonus comprised of one or more of the following:
| | • | | stock options pursuant to the Company’s stock option plan; or |
| | • | | forgiveness of any portion of the principal of interest-free loans provided to the executive officer. |
Section 401(k) Plan
As part of its effort to attract and maintain high quality staff, the Company adopted a 401(k) Salary Reduction Plan and Trust on June 1, 1992. The Company’s employees may make pre-tax elective contributions of up to 25% of their salary, subject to limitations prescribed by law. All contributions are paid to a trustee who invests for the benefit of members of the 401(k) Plan. In March 1997, the 401(k) Plan was amended to provide that the Company may match the employee’s contributions with common stock. The Company may amend or terminate the 401(k) Plan at any time, subject to legal restrictions.
Employee Stock Purchase Plan
In March 1996, the Company adopted an Employee Stock Purchase Plan (the “Purchase Plan”), which authorizes the issuance of up to 933,333 shares of the Company’s common stock to eligible employees. Generally, each offering may last up to twenty-four months, and purchases are made on each October 31 and April 30 during each offering. For example, the initial offering began on April 11, 1996, and terminated on April 30, 1999. Common stock is purchased for accounts of employees participating in the Purchase Plan at a price per share equal to the lower of:
| | • | | 85% of the fair market value of a share of common stock on the date of commencement of participation in the offering, or |
| | • | | 85% of the fair market value of a share of common stock on the date of purchase. |
Generally, all regular employees, including executive officers, may participate in the Purchase Plan and may authorize payroll deductions of up to 15% of their base compensation for the purchase of common stock under the Purchase Plan. The Company’s Board of Directors has the authority to terminate the Purchase Plan at its discretion. As of April 28, 2003, 95,000 shares had been issued pursuant to the Purchase Plan.
- 14 -
Options Granted in Connection with the Private Placement
In connection with the Private Placement, the Board of Directors and the Compensation Committee considered and approved, subject to stockholder approval, contingent option grants to a number of our executive officers, employees and consultants. All of these options were granted on February 11, 2003, the date on which we entered into the purchase agreement. However, the options were contingent upon the consummation of the Private Placement, which closed on April 21, 2003. All of the options are exercisable at $2.10 per share (after giving effect to the one-for-six reverse stock split). The following table sets forth information regarding these stock options:
Name of Individual and Position
| | Number of Securities Underlying Option(#)
| | Dollar Value($)(1)
|
Howard Robin Chief Executive Officer and President | | 1,434,166 | | $0 |
Marvin Tancer Chief Financial Officer and Vice President of Operations | | 465,000 | | $0 |
Nassim Usman, Ph.D. Chief Scientific Officer and Vice President of Research and Development | | 470,833 | | $0 |
All current officers as a group (3 persons) | | 2,369,999 | | $0 |
All other employees as a group (not including consultants) | | 2,366,669 | | $0 |
| (1) | | Calculated by determining the difference between the fair market value of the securities underlying the options on the grant date, February 11, 2003, and the exercise price of the options, which is $2.10 per share. |
Equity Compensation Plan Information
The following table provides information as of December 31, 2002, the end of our last fiscal year, regarding common stock that may be issued upon the exercise of options, warrants and rights under all of our existing equity compensation plans and individual compensation arrangements, including our 1996 Stock Option Plan, 2001 Stock Option Plan, and 1996 Employee Stock Purchase Plan. All amounts reflect a one-for-six reverse stock split effected April 16, 2003.
Plan Category
| | Number of Securities to be Issued Upon Exercise of
Outstanding Options,
Warrants and Rights
| | Weighted-Average Exercise
Price of Outstanding Options,
Warrants and Rights
| | Number of Securities Remaining Available for Future Issuance Under
Equity
Compensation Plans
(Excluding Securities
Reflected in the First
Column)(1)
|
Equity compensation plans approved by security holders | | 884,835 | | $27.28 | | 889,410 |
Equity compensation plans not approved by security holders | | — | | — | | — |
Total | | 884,835 | | $27.28 | | 889,410 |
| (1) | | This number includes 4,575 shares of common stock available for future issuance under our Employee Stock Purchase Plan. |
- 15 -
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Executive Loans. The Company made interest-free loans to its executive officers. The Company has forgiven all or a portion of the outstanding principal amount of each loan under the terms of each officer’s employment agreement. See “Employment Agreements” above.
Name
| | Loan Amount
| | | Balance as of April 28, 2003
|
Howard W. Robin | | $ | 400,000 | (1) | | $ | 240,000 |
Marvin Tancer | | | 200,000 | (2) | | | 160,000 |
Nassim Usman, Ph.D. | | | 135,000 | (3) | | | 24,000 |
| (1) | | $80,000 forgiven in each of January 2001 and 2002. |
| (2) | | $40,000 forgiven in June 2002. |
| (3) | | $15,000 forgiven in each of June 1997, May 1998, May 1999, May 2000 and May 2001, and $12,000 forgiven in each of October 2000, 2001 and 2002. |
Indemnity Agreements. The Company has entered into indemnity agreements with each of its officers (including the Named Executive Officers) and directors which provide, among other things, that the Company will indemnify such officer or director, under the circumstances and to the extent provided for therein, for expenses, damages, judgements, fines and settlements he may be required to pay in actions or proceedings which he is or may be made a party to by reason of his position as a director, officer or other agent of the Company, and otherwise to the full extent permitted under Delaware law and the Company’s bylaws.
Consulting Agreement. In August 2002, the Company reached an agreement with Jeremy Curnock Cook, the Company’s Chairman of the Board, whereby the Company utilized his consulting firm, Bioscience Management, plc, for strategic planning. During 2002, the Company paid $85,000 and $39,884 to Bioscience Management and Mr. Cook, respectively, in fees for consulting services provided. The agreement was terminated in April 2003.
Schering AG.On April 9, 1997, the Company entered into a research collaboration with Schering AG, Berlin, (Schering AG) focusing on the use of ribozymes for therapeutic target validation, as well as the development of ribozymes as therapeutic agents. The collaboration utilized the special selectivity of ribozymes to validate new molecular therapeutic targets and to discover new therapeutic agents based on those targets. The Company provided its expertise in ribozyme design, synthesis and delivery, and Berlex Laboratories, Inc., a U.S. subsidiary of Schering AG (Berlex) provided candidate targets, cell culture screens, animal models and development and commercialization expertise to the collaboration. Since the formation of atugen in 1998, the Company subcontracted the work related to the collaboration to atugen. Subsequently, all revenues related to the collaboration with Berlex have been transferred to atugen as well.
Schering AG made an equity investment in the Company in May 1997 of $2.5 million in exchange for 212,766 shares of common stock, and made an additional equity investment of $2.5 million for 465,117 shares in April 1998. Separately, Schering AG provided loans of $2.0 million in each of years 1997, 1998, 1999, 2000 and 2001. According to the terms of the Company’s agreement with Schering AG, 50% of borrowings on the line of credit were collateralized by equipment purchases. The loans, which carry an interest rate of 8% per annum, are convertible into equity at the option of Schering AG under certain circumstances. In April 2000, after the completion of a public offering, the Company paid down $6.9 million of the outstanding borrowings from Schering AG. In June 2000, Schering AG converted the remaining balance of $997,205 into 42,435 shares of the Company’s common stock at the market closing price on June 19, 2000 of $23.50. At December 31, 2002, the outstanding borrowings and accrued interest of $3.5 million were convertible into approximately 14.4 million shares of the Company’s common stock. In connection with the Private Placement, Schering AG agreed to restructure the loan. In exchange for warrants and the Company’s agreement to pay accrued interest on the loan on a quarterly basis, Schering AG agreed to extend the due date for the principal amount of the loan from April 2004 to January 2005. Upon the closing of the Private Placement in April 2003, the Company paid $520,000 to Schering AG for interest accrued on the loan through March 2003. Future interest payments to Schering will be paid on a quarterly basis.
16
Elan Corporation.In January 2000, the Company formed a joint venture with Elan Corporation for the development and commercialization of HERZYMETM, the Company’s product to treat breast and other cancers. As part of this arrangement, the Company licensed HERZYME and Elan licensed its MEDIPAD® drug delivery technology to the joint venture, Medizyme Pharmaceuticals, Ltd The MEDIPAD system is a disposable continuous subcutaneous drug delivery system that allows a patient to administer the drug at home.
Initial funding of Medizyme included $12.0 million from the Company and $3.0 million from Elan. The Company’s $12.0 million capital contribution was funded by the sale to Elan of (i) 12,015 shares of the Company’s Series A Convertible Exchangeable Preferred Stock, (ii) a warrant to purchase up to 200,000 shares of the Company’s common stock at an exercise price of $15.00 per share, which expired unexercised in January 2002, and (iii) a warrant to purchase up to 300,000 shares of the Company’s common stock at an exercise price of $20.00 per share with a term of seven years. The Series A Preferred Stock had a stated dividend rate of 6.0% payable semi-annually through the issuance of additional shares of Series A Preferred Stock at a nominal value of $1,000 per share. The Series A Preferred Stock had a liquidation preference of $12,015,000 plus accrued and unpaid dividends. At December 31, 2002, the Company had recorded $2,314,366 as an accreted dividend related to this preferred stock arrangement.
As a result of Medizyme’s initial capitalization, the Company owned 80.1% of the outstanding capital stock of Medizyme and Elan owns 19.9%. Elan had the option of converting its shares of the Company’s Series A Preferred Stock and any related accreted preferred stock dividends into shares of the Company’s common stock at a fixed price. As of December 31, 2002, the Series A Preferred Stock and accreted dividends were convertible into 1,194,114 shares of the Company’s common stock.
Medizyme paid Elan in cash a $15.0 million non-refundable license fee for the MEDIPAD drug delivery technology. As a result of this licensing transaction, Medizyme capitalized the entire license fee and is amortizing the balance over the three-year term of the agreement since Medizyme’s license of the MEDIPAD device was intended for use with any ribozyme drug developed by Medizyme targeting a gene believed responsible for certain breast cancers. The Company estimated that funding for Medizyme would require approximately $15.0 million in additional operating and development costs. In connection with expected funding requirements, Elan agreed to provide the Company with a $12.0 million credit facility to fund the Company’s pro rata portion of Medizyme’s operating costs over the development period, which was 42 months. At the end of December 2002, the Company had utilized the credit facility and had borrowed $9.9 million. The note carried an interest rate of 12% compounded on a semi-annual basis. In June 2002, Elan converted this debt and accrued interest into 9,905 shares of the Company’s Series B Convertible Preferred Stock. The Series B Preferred Stock was ultimately convertible into shares of the Company’s common stock at a 50% premium to the average price of its common stock for the 60 days prior to the time of the applicable draw down on the credit facility. The Series B Preferred Stock had a stated dividend rate of 12%, payable semi-annually through the issuance of additional shares of Series B Preferred Stock at a nominal value of $1,000 per share. If converted at December 31, 2002, the Series B Preferred Stock and the Series B accreted dividends would be convertible into 1,488,106 shares of the Company’s common stock. For the year ended December 31, 2002, the Company recorded $1.4 million in revenues and reimbursements from Medizyme, of the total $6.7 million in research and development expenses recorded by Medizyme. The Company recorded $5.3 million as equity in the net loss of Medizyme.
Also in connection with the Medizyme joint venture, Elan purchased 641,026 shares of the Company’s common stock for a purchase price of $5.0 million in 2000. In addition, Elan purchased 500,500 shares of the Company’s common stock for $5.0 million in May 2001 at a premium to the average closing price for the 45 trading days preceding the purchase. Elan also purchased 750,000 shares of the Company’s common stock and a warrant to purchase 75,000 shares of common stock in December 2001 for $3.0 million.
In connection with the Private Placement, Elan agreed to convert all of the Series A and Series B Preferred Stock into shares of the Company’s common stock immediately prior to the close of the Private Placement.
17
In April 2003, the Company concluded its collaboration with Elan relating to the Medizyme joint venture. Under the terms of the agreement the Company retains full rights to HERZYME and Elan will transfer its 19.9% interest in Medizyme to the Company in exchange for a portion of any future license fee, development revenues and royalties on the commercial sale of HERZYME.
COMPENSATION COMMITTEE REPORT ON EXECUTIVE COMPENSATION
Responsibilities and Objectives.The Committee establishes and administers the general compensation policies and plans for the Company and the specific compensation levels for the executive officers and other key employees. The Committee is responsible for conducting, at a minimum, annual reviews of executive compensation and for taking certain actions regarding the compensation of senior executives of the Company. The Committee determines the salary levels for senior executives, and other key employees, and the types and amounts of cash and other bonuses to be distributed to these individuals in accordance with the Bonus Plan. The Committee also determines grants of stock options pursuant to the stock option plans.
This report is submitted by members of the Committee summarizing their involvement in the compensation decisions and policies adopted by the Company for the Company’s executive officers.
General Policy. The Company’s executive compensation practices are designed to reward and provide an incentive for executives based on the achievements of annual and long-term corporate and individual performance goals. Compensation levels for executives are established after giving consideration to a variety of quantitative measures including, but not limited to, Company financial and operating performance, peer group comparisons and labor market conditions. Before making decisions, the Committee elicits the recommendations and advice of the CEO regarding appropriate or desired levels of compensation for management personnel generally. The Committee has complete access to all necessary Company personnel records, financial reports and other data, and may seek the advice of experts and analysts.
The ultimate purpose of the Company’s compensation structure is to attract and retain executives of the highest caliber and to motivate these executives to put forth maximum effort toward the achievement of Company goals identified through the strategic planning process of the Board and management. Also, the compensation design emphasizes long-term incentives in the form of stock options that will encourage these individuals to maintain their focus on the paramount importance of long-term Stockholder interests.
Components of Compensation. In evaluating executive compensation, the Committee focuses upon several fundamental components: salary, annual bonus and long-term incentive compensation consisting of stock options. The Committee’s recommendations are offered to the full Board of Directors and are ultimately ratified, changed or rejected by the full Board.
Salary levels for senior executives and other officers are reviewed by the Committee annually. The Company has entered into employment agreements with its executive officers, as amended from time to time, which set forth the salary level for each executive. In establishing salary levels, the Committee has relied upon salary survey data and other publicly available information. The Committee also considers the experience of each executive officer as well as his or her past performance and expected future performance.
The annual bonus component of executive compensation has historically been provided to an executive, if and as appropriate, for obtaining pre-determined corporate and individual goals. The Committee typically determines whether annual bonuses will be awarded to executives for attainment of these goals at the end of each year. Pursuant to the Bonus Plan, the Committee may award bonuses comprised of cash, stock options and loan forgiveness based on the executive officer’s fulfillment of annual goals previously established by the Committee. At that time, the Committee also sets the performance goals for the upcoming year.
The third component of the Company’s executive compensation strategy is long-term incentive compensation pursuant to which executives receive stock options pursuant to the Plan which are tied to the long-term appreciation of the value of the Company Stock. The Plan offers executives the possibility of future gains depending upon the
18
executives’ continued employment by, and contributions to, the Company. The Committee believes that a portion of the total compensation of senior executives over a period of years should consist of such long-term incentive awards.
Executive officers of the Company also are permitted to participate in the Purchase Plan which is open to all of the Company’s full-time employees. The Purchase Plan enables the Company’s employees, including executive officers, to acquire Company Stock at a discount to the market price by allocating up to 15 % of their base salary (subject to certain limits) to the acquisition of such stock.
Review of Executive Compensation. The Committee, in making its recommendations and determinations at year end 2002 regarding executive compensation, was influenced by a few primary considerations, namely, that executive management completed the principal goals to (a) develop a new business strategy; (b) reorganize the company accordingly; and (c) reduce the planned 2003 burn rate by approximately 35%. While the principal goals were achieved for 2002, the Committee included a caveat for year-end bonuses to be paid, whereby an agreement had to be reached with respect to a financing that would satisfy the Company’s operational needs for at least the next few years.
In light of the results in 2002, the Committee determined that increases in executive compensation were justifiable, both to reward management for accomplishments to date and to encourage future achievement of both short- and long-term objectives. Accordingly, the Committee approved salary increases and bonuses that it believes reflected appropriate rewards for management’s performance in 2002. Bonuses for the year ended 2002 were paid out upon the signing of the private placement financing transaction in February 2003. The Committee also granted stock options to the executive officers, both at year-end 2002 and upon the signing of the private placement agreement.
Compensation of Chief Executive Officer. In assessing appropriate types and amounts of compensation for the Chief Executive Officer, the Committee evaluates both corporate and individual performance. Corporate factors included in the evaluation are (i) progress and number of products in clinical and preclinical development, (ii) levels and quality of research, and (iii) current and potential funds raised in equity offerings and collaborations. Individual factors include initiation and implementation of successful business strategic partnerships, maintenance of an effective management team and various personal qualities, including leadership, commitment and professional standing.
Other Compensation Considerations. Under Section 162(m) of the Internal Revenue Code the Company may not receive a federal income tax deduction for compensation paid to the Chief Executive Officer or any of the four most highly compensated executive officers to the extent that any of the persons receive more than $1,000,000 in compensation in any one year. However, if the Company pays compensation that is “performance-based” under Section 162(m) it can receive a federal income tax deduction for the compensation paid even if such compensation exceeds $1,000,000 in a single year. To maintain flexibility in compensating executive officers in a manner designed to promote varying corporate goals, the Compensation Committee has not adopted a policy that all compensation must be deductible on the Company’s federal income tax.
Conclusion. For these and other reasons, the Committee recommended an increase in salary for Mr. Robin from $344,000 in 2002 to $365,000 in 2003. In addition, the Committee approved and recommended payment of a $155,025 cash bonus to Mr. Robin after the signing of the private placement agreement which occurred in February 2003. The Committee also granted stock options for 16,667 shares (after giving effect to a one-for-six reverse stock split) of common stock under the Plan to Mr. Robin in 2002. The Committee believes that these compensation amounts and awards reflect appropriate levels given the Company’s performance in 2002 and the individual performance of management. The Committee also believes that these awards evidence the Committee’s philosophy to emphasize long-term incentive rewards tied to the Company’s performance. In connection with the Private Placement, Mr. Robin entered into an employment agreement with the Company as described under the heading “Employment Agreements” on page 12 of this proxy statement.
COMPENSATION COMMITTEE
John Groom, Chairman
Jeremy Curnock Cook
Anders P. Wiklund
19
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
No member of the Compensation Committee was at any time during fiscal 2002 an officer or employee of the Company. In addition, none of our executive officer serves as a member of the Board of Directors or Compensation Committee of any company that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act of 1934 requires our executive officers and directors, and persons who own more than 10% of a registered class of our equity securities, to file reports of ownership and changes in ownership with the Securities and Exchange Commission, the NASD, and the Company.
Based solely on our review of Section 16(a) forms received by us and written representations that no other reports were required, we believe that, during the last fiscal year, all Section 16(a) filing requirements applicable to our executive officers, directors and 10% beneficial owners were complied with.
PERFORMANCE GRAPH
The following line graph presents the cumulative total stockholder return for the Company’s common stock since December 31, 1997, compared with the Nasdaq Composite (US) index and Nasdaq Biotech index. Trade prices are not always disclosed to management. The graph assumes that $100 was invested on December 31, 1997 and that dividends were reinvested.
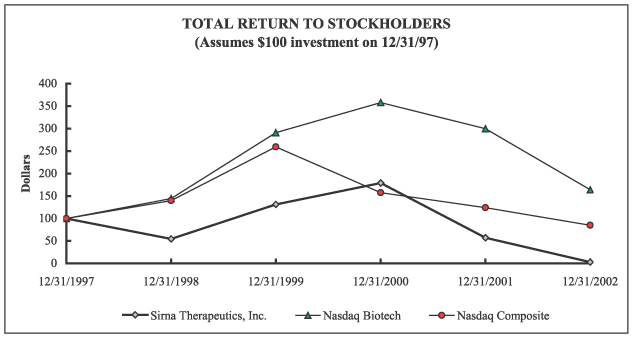
| | | 12/31/97
| | 12/31/98
| | 12/31/99
| | 12/31/00
| | 12/31/01
| | 12/31/02
|
Sirna Therapeutics, Inc., (formerly Ribozyme Pharmaceuticals, Inc.) | | $ | 100.00 | | $ | 54.69 | | $ | 131.25 | | $ | 178.91 | | $ | 57.13 | | $ | 3.00 |
Nasdaq Biotech | | $ | 100.00 | | $ | 144.28 | | $ | 290.92 | | $ | 357.81 | | $ | 299.83 | | $ | 163.92 |
Nasdaq Composite | | $ | 100.00 | | $ | 139.63 | | $ | 259.13 | | $ | 157.32 | | $ | 124.20 | | $ | 85.05 |
20
AUDIT COMMITTEE REPORT
The following is a report of the Audit Committee with respect to our audited financial statements for the fiscal year ended December 31, 2002, which include our balance sheets as of December 31, 2002 and 2001, and the related statements of operations, shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2002, and the notes thereto.
The Company believes that in 2002 two of the members of the Audit Committee were independent within the meaning of the Nasdaq Listing Standards. With respect to the current members of the Audit Committee, Dr. Roberts is a General Partner of Venrock Associates and Dr. Fambrough is a Principal with Oxford Bioscience Partners.
The Audit Committee operates pursuant to a written charter adopted by the Board of Directors, a copy of which was included as Exhibit B to the Proxy Statement for the Annual Meeting of Stockholders held in 2001. In general, the Audit Committee charter sets forth:
| | • | | the scope of the Audit Committee’s responsibilities and the means by which it carries out these responsibilities, |
| | • | | the outside auditor’s accountability to the Board of Directors and the Audit Committee, and |
| | • | | the Audit Committee’s responsibility to monitor the independence of the outside auditor. |
As described more fully in its charter, the purpose of the Audit Committee is to assist the Board of Directors in its general oversight of our financial reporting, internal control and audit functions. Management is responsible for the preparation, presentation and integrity of our financial statements, accounting and financial reporting principles, internal controls and procedures designed to ensure compliance with accounting standards, applicable laws and regulations. Ernst & Young LLP, our independent auditing firm, is responsible for performing an independent audit of the financial statements in accordance with generally accepted auditing standards.
The Audit Committee members are not professional accountants or auditors, and their functions are not intended to duplicate or to certify the activities of management and the independent auditor. The Audit Committee serves in a board-level oversight role, in which it provides advice, counsel and direction to management and the auditors on the basis of the information it receives, discussions with management and the auditors and the experience of the Audit Committee’s members in business, financial and accounting matters.
The Audit Committee members have relied, without independent verification, on management’s representation that the financial statements have been prepared with integrity and objectivity and in conformity with accounting principles generally accepted in the United States of America and on the representations of our independent auditor included in their report on our financial statements. The Audit Committee’s oversight role does not provide it with an independent basis to determine that management has maintained appropriate accounting and financial reporting principles or policies, or appropriate internal controls and procedures designed to assure compliance with accounting standards and applicable laws and regulations. Furthermore, the Audit Committee’s considerations and discussions with management and the independent auditor do not assure that our financial statements are presented in accordance with generally accepted accounting principles, that the audit of our financial statements has been carried out in accordance with generally accepted auditing standards or that our independent accountants are in fact “independent.”
Among other matters, the Audit Committee monitors the activities and performance of our external auditors, including the audit scope, audit fees, auditor independence matters and the extent to which the independent auditor may be retained to perform non-audit services. The Audit Committee is responsible for the appointment, compensation and oversight of our independent auditors. The Audit Committee also reviews the results of the internal and external audit work with regard to the adequacy and appropriateness of our financial, accounting and internal controls. Management and independent auditor presentations to and discussions with the Audit Committee also cover various topics and events that may have significant financial impact or are the subject of discussions between management and the independent auditor.
Review with Management
The Audit Committee has reviewed and discussed our audited financial statements with management. Management represented to the Audit Committee that our financial statements were prepared in accordance with generally accepted accounting principles.
Review and Discussions with Independent Accountants
During 2002, the Audit Committee held meetings with management and the independent auditors to discuss the overall scope and plans for the audit. We also met with the independent auditors, with and without management present, to discuss the results of their examinations and their evaluations of our internal controls. In addition, the Audit Committee has reviewed and discussed the audited financial statements for the year ended December 31, 2002 and held discussions with management and Ernst & Young on the quality, not just the acceptability, of our accounting principles, the reasonableness of significant judgments and the clarity of disclosures in the financial statements.
The Audit Committee has also discussed with Ernst & Young the matters required to be discussed by SAS 61 (Codification of Statements on Accounting Standards) which includes, among other items, matters related to the conduct of the audit of our financial statements.
The Audit Committee has also received written disclosures and the letter from Ernst & Young required by Independence Standards Board Standard No. 1 (which relates to the accountant’s independence from us and our related entities) and has discussed with Ernst & Young their independence from the Company. In addition, the Audit Committee has also considered whether the provision of those services set forth in the table below are compatible with Ernst & Young maintaining its independence from the Company.
In reliance on the reviews and discussions referred to above, the Committee recommended to the Board of Directors (and the Board has approved) that the audited financial statements be included in the Annual Report on Form 10-K for the year ended December 31, 2002 for filing with the Securities and Exchange Commission. The Committee and the Board have also recommended, subject to stockholder approval, the selection of the Company’s independent auditors.
AUDIT COMMITTEE
Samuel R. Saks, Chairman
Jeremy Curnock Cook
Anders P. Wiklund
21
Audit Fees
The following table sets forth the aggregate fees billed by Ernst & Young LLP for the following services during fiscal 2002:
Description of Services
| | Fee Amount
|
Audit Fees(1) | | $ | 122,708 |
Audit Related Services(1) | | $ | 0 |
Financial Information Systems Design and Implementation Fees | | $ | 0 |
All Other Fees(2) | | $ | 53,230 |
| | |
|
|
Total | | $ | 175,938 |
| | |
|
|
| (1) | | Represents the aggregate fees billed for professional services rendered for the audit of the Company’s annual financial statements during fiscal 2002 and for the review of the financial statements included in the Company’s quarterly reports during such period. Fees for the last annual audit were $73,585 and all other audit related fees were $49,123, which includes audit related services for filings with the SEC. Audit related services generally include fees for pension and statutory audits, business acquisitions, accounting consultations, internal audit and SEC registration statements. |
| (2) | | Represents the aggregate fees billed in 2002 for services other than the audit and review of our 2002 financial statements and for 2002 includes fees for tax-related services. |
PROPOSAL NO. 2
RATIFICATION OF SELECTION OF INDEPENDENT AUDITORS
Ernst & Young LLP has served as the Company’s independent auditors since 1992. Our Board of Directors, upon recommendation of the Audit Committee, has selected Ernst & Young LLP as the Company’s independent auditors for the year ending December 31, 2003. At the Annual Meeting, stockholders will be asked to ratify the selection of Ernst & Young LLP as the Company’s independent auditors for 2003.
Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting and will be available to respond to appropriate questions from stockholders. Such representatives of Ernst & Young LLP will also have the opportunity to make a statement if they desire to do so.
Vote Required and Board of Directors’ Recommendation
Although it is not required to do so, the Board of Directors is submitting the Audit Committee’s selection of our independent auditors for ratification by the stockholders at the Annual Meeting in order to ascertain the view of the stockholders regarding such selection. The affirmative vote of the holders of a majority of the shares of our common stock present or represented and voting at the Annual Meeting will be required to approve this proposal. Whether the proposal is approved or defeated, the Audit Committee may reconsider its selection.
Our Board of Directors recommends that the stockholders vote “FOR” the ratification of the appointment of Ernst & Young LLP as our independent auditors for the 2003 fiscal year.
ANNUAL REPORT
A copy of the Company’s 2002 annual report and Annual Report on Form 10-K for the year ended December 31, 2002, are being distributed to the Company’s stockholders along with this Proxy Statement. The Company’s Annual Report on Form 10-K for the year ended December 31, 2002 as filed with the Securities and Exchange Commission may be obtained without charge upon written request directed to Bharat Chowrira, Secretary, 2950 Wilderness Place, Boulder, Colorado 80301.
22
STOCKHOLDERS’ PROPOSALS
In accordance with Rule 14a-8 under the Exchange Act, any stockholder who intends to submit a proposal at our 2004 annual meeting of stockholders and who wishes to have the proposal considered for inclusion in the proxy statement and form of proxy for that meeting must, in addition to complying with the applicable laws and regulations governing submission of such proposals, deliver the proposal to us for consideration no later than January 1, 2004.
SEC rules also establish a different deadline for submission of stockholder proposals that are not intended to be included in the Company’s proxy statement. If a stockholder intends to submit a proposal at the Company’s 2004 annual meeting, which proposal is not intended to be included in the Company’s proxy statement relating to such meeting, the stockholder must give proper notice no later than March 17, 2004. If a stockholder gives notice of such a proposal after the deadline, the proxy holders will be allowed to use their discretionary voting authority to vote against the stockholder proposal when and if the proposal is raised at the Company’s 2004 annual meeting, generally without including any disclosure of the proposal in the proxy statement or on the proxy card.
All notices of proposals, whether or not to be included in the Company’s proxy materials, should be sent to Bharat Chowrira, Secretary, 2950 Wilderness Place, Boulder, Colorado 80301.
HOUSEHOLDING OF PROXY MATERIALS
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for proxy statements and annual reports with respect to two or more stockholders sharing the same address by delivering a single proxy statement addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
This year, a number of brokers with account holders who are stockholders of the Company will be “householding” our proxy materials. A single proxy statement will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate proxy statement and annual report, please notify your broker, direct your written request to Sirna Therapeutics, Inc., Bharat Chowrira, Secretary, 2950 Wilderness Place, Boulder, Colorado 80301 or contact ADP Investor Communication Services at 1-800-542-1061. Stockholders who currently receive multiple copies of the proxy statement at their address and would like to request “householding” of their communications should contact their broker.
By Order of the Board of Directors
/s/ Bharat M. Chowrira
Bharat M. Chowrira, Secretary
Date: April 30, 2003
23
PROXY
SIRNA THERAPEUTICS, INC.
(formerly Ribozyme Pharmaceuticals, Inc.)
This Proxy is Solicited on Behalf of the Board of Directors
for the Annual Meeting of Stockholders to
be Held May 29, 2003
The undersigned stockholder of Sirna Therapeutics, Inc. hereby constitutes and appoints Marvin Tancer and Bharat Chowrira, and each of them, proxies, with full power of substitution, for and on behalf of the undersigned to vote, as designated below, according the number of shares of the Company’s $.01 par value common stock held of record by the undersigned on April 28,2003, and as fully as the undersigned would be entitled to vote if personally present at the Company’s Annual Meeting of Stockholders to be held at the Company’s principal executive offices at 2950 Wilderness Place, Boulder, CO 80301, on May 29, 2003 at 10:00 a.m. local time, and at any and all postponements, continuations and adjournments thereof.
This proxy when properly executed will be voted in the manner directed herein by the undersigned. If properly executed and no direction is made, this proxy will be votedFOR the election of proposed nominees to the Board of Directors of the Company andFOR the proposal to ratify the selection of Ernst & Young LLP as the Company’s independent auditors for the year ending December 31, 2003.
THE BOARD OF DIRECTORS RECOMMENDS THAT YOU VOTE “FOR” PROPOSALS 1 AND 2.
| 1. | | Proposal to elect the following nominees to the Board of Directors: |
| | | | | ¨ | | FOR all nominees listed below (except as marked to the contrary below) | | ¨ | | WITHHOLD AUTHORITY to vote all nominees listed below |
| | | Mr. Howard W. Robin Mr. Jeremy L. Curnock Cook | | |
(INSTRUCTION: To withhold authority to vote for any individual nominee, write that nominee’s name on the space provided below:)
| 2. | | Proposal for ratification of selection of Ernst & Young LLP as the Company’s independent auditors for the year ending December 31, 2003: |
| | | | | ¨ FOR | | ¨ AGAINST | | ¨ ABSTAIN | | | | |
| 3. | | In their discretion the proxies are authorized to vote upon such other business as may properly come before the meeting or any and all postponements, continuations or adjournments thereof. |
The undersigned hereby acknowledges receipt of the Notice of Annual Meeting of Stockholders dated , 2003 and the proxy statement furnished therewith.
Please sign exactly as your name appears hereon. When shares are held by joint tenants, both should sign. Executors, administrators, trustees and other fiduciaries, and persons signing on behalf of corporations or partnerships, should so indicate.
Dated , 2003
Authorized Signature
Title
Authorized Signature
Title
Please mark boxes¨ in ink. Sign, date and return this proxy card promptly using the enclosed envelope.