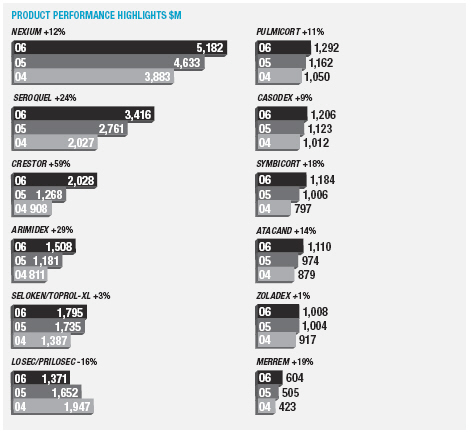
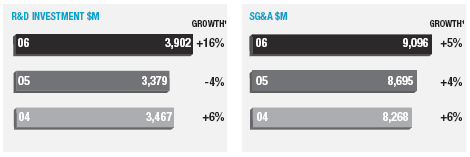
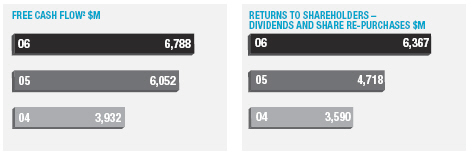
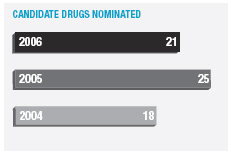
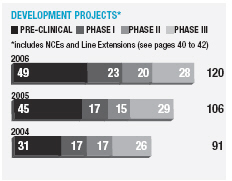
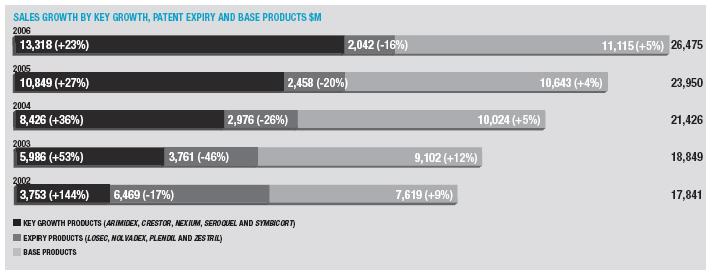
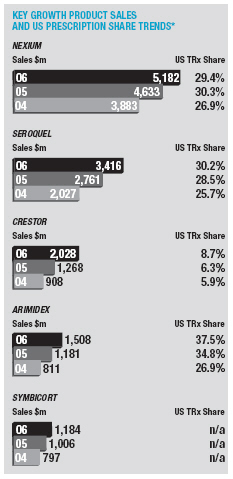
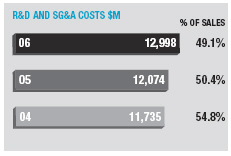
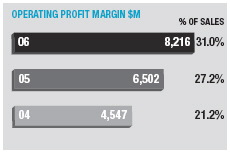
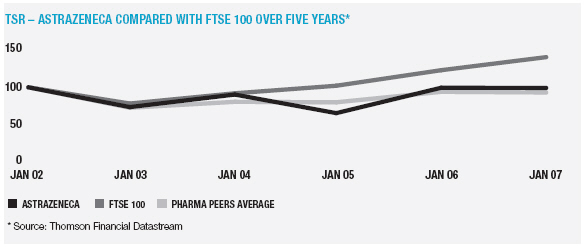
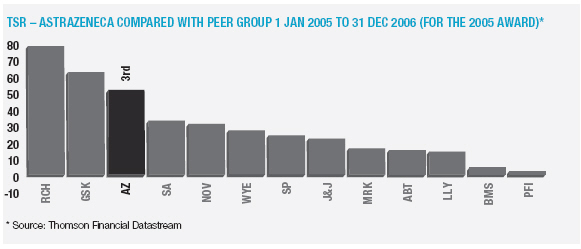
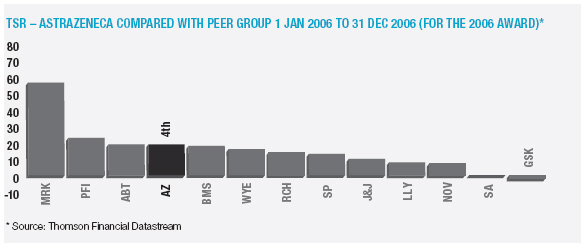
In January 2006, AstraZeneca Canada Inc. was served with a claim in the Federal Court of Canada for payment of an undetermined sum based on damages allegedly suffered by Apotex due to the delay from January 2002 to January 2004 in the issuance to Apotex of a notice of compliance (marketing approval) in Canada for its 20mg omeprazole capsule product. The claim was held in abeyance pending Apotex’s appeal to the Supreme Court of Canada, and following the November 2006 allowance of that appeal Apotex has indicated it will be advancing the damages claim. AstraZeneca believes the claim is without merit and intends to defend it and to pursue its already pending patent infringement actions against Apotex vigorously.
In January 2007, AstraZeneca Canada Inc. discontinued long pending proceedings against Reddy-Cheminor Inc. in respect of patents relating to omeprazole capsules, following Reddy-Cheminor’s withdrawal of its allegations.
The first action was brought in 2004 in the Superior Court of the State of California for the County of Los Angeles by the Afl-CIO, two unincorporated associations and an individual on behalf of themselves, the general public and a class of California consumers, third party payers, cash payers and those making a co-payment. A second action was filed in the same court on behalf of a similar putative class of consumers. Actions making substantially similar allegations were filed in 2004 and 2005 on behalf of putative classes of consumers, third party payers, purchasers and labour management trust funds in the Circuit Court of Searcy County, Arkansas; in the Superior Court of the State of Delaware in and for New Castle County; in the Superior Court of Massachusetts in Boston; in the US District Court for the District of Delaware (three consolidated cases); and in the Circuit Court of the 11th Judicial Court in and for Miami-Dade County, Florida.
In September 2005, the court in California issued a ruling on AstraZeneca’s demurrer and motion to strike in the two California actions. The court granted AstraZeneca’s motion with respect to the associational plaintiffs and denied the motion with respect to the individual plaintiffs, allowing the cases of the individuals to proceed. In October 2005, the court in Massachusetts denied AstraZeneca’s motion to dismiss. Discovery in the California and Massachusetts cases is proceeding, and plaintiffs’ motions for class certification are expected to be filed in mid-2007.
Back to Contents
| 140 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
NOTES TO THE FINANCIAL STATEMENTS CONTINUED
| 26 | COMMITMENTS AND CONTINGENT LIABILITIES CONTINUED |
| In November 2005, the US District Court for the District of Delaware granted AstraZeneca’s motion to dismiss the consolidated class action complaint. The plaintiffs appealed the dismissal to the US Court of Appeals for the Third Circuit. The Delaware state case has been stayed pending the outcome of the Delaware federal cases. |
In May 2006, the Arkansas state court granted AstraZeneca’s motion to dismiss the plaintiffs’ complaint. The plaintiffs filed additional motions and pleadings, including an amended complaint. AstraZeneca filed a motion to dismiss the amended complaint.
In October 2006, the Florida court dismissed the plaintiff’s complaint with prejudice and without leave to amend. The plaintiff has appealed the dismissal, and the opening appeal brief is due in February 2007.
In December 2006 and January 2007, several lawsuits against AstraZeneca entities, including putative class actions, were filed in US District Court for the District of Columbia alleging claims of unlawful monopolisation relating toPrilosecandNexium. Individual actions were filed on 7 December 2006 by Walgreen Co., Eckerd Corporation. Maxi Drug, Inc. d/b/a Brooks Pharmacy, The Kroger Co., New Albertson’s Inc., Safeway, Inc., Hy-Vee, Inc., and American Sales Company, Inc. and on 8 December 2006 by Rite Aid Corporation, and Rite Aid Headquarters Corp. Putative class actions brought on behalf of direct purchasers were filed on 18 December 2006 by Meijer, Inc. and Meijer Distribution, Inc., on 19 December 2006 by Louisiana Wholesale Drug Co., Inc., and on 8 January 2007 by Burlington Drug Co., Inc., Dik Drug Co., Inc, and King Drug Co. of Florence, Inc. The plaintiffs seek treble damages, injunctive relief, and attorney fees. AstraZeneca denies the allegations and intends to vigourously defend each of the actions.
In November 2003, the European Patent Office (EPO) ruled that the European substance patent covering magnesium esomeprazole, the active pharmaceutical ingredient inNexium, was valid. The patent, which expires in May 2014, was challenged by the generic manufacturer ratiopharm. The EPO ruling was appealed by ratiopharm. In December 2006, the Board of Appeals of the EPO ruled that the patent is invalid.
While disappointed with the EPO decision, AstraZeneca has confidence in the intellectual property portfolio protectingNexium. This portfolio includes process, method of use and additional substance patents with expiration dates ranging from 2009 through to 2019. The process patent is under opposition with the EPO and an Opposition Division oral hearing is scheduled for October 2007 (postponed from the original hearing date in March 2007). In addition to these patents,Nexiumhas data exclusivity valid to 2010 in major European markets.
The revocation of the AstraZeneca European substance patent relating toNexiumshould not have any substantive impact on AstraZeneca’s ability to uphold and enforce itsNexiumpatents in the United States. AstraZeneca has several US patents coveringNexium,all of which can be differentiated from the European patent found to be invalid.
In October 2004, AstraZeneca LP filed suit in the US District Court for the District of Delaware seeking declaratory judgment that its ‘Better is Better’ campaign forNexiumwas not false or misleading advertising in violation of section 43(a) of the Lanham Act, a federal statute governing false advertising claims. The action was taken in response to a letter from TAP Pharmaceuticals, Inc. demanding that AstraZeneca immediately withdraw the television commercial and other components of the direct-to-consumer advertising campaign forNexiumon the basis that they allegedly violated the statute. In November 2004, TAP requested expedited consideration of the case by filing a motion for a preliminary injunction, which the court denied in December 2004. In May and June 2006, the court dismissed all of the claims for damages asserted by TAP in its counterclaims and dismissed most of TAP’s claims for injunctive relief. In August 2006, the parties entered into a settlement agreement, and the case has been dismissed in its entirety.
In October 2005, AstraZeneca received a notice from Ranbaxy Pharmaceuticals, Inc. that Ranbaxy Laboratories Limited had submitted an Abbreviated New Drug Application (ANDA) to the US FDA for esomeprazole magnesium delayed-release capsules, 20mg and 40mg. The ANDA contained paragraph IV certifications of invalidity and/or non-infringement in respect of certain AstraZeneca US patents listed in the FDA’s Orange Book with reference toNexium. In November 2005, AstraZeneca commenced wilful infringement patent litigation in the US District Court for the District of New Jersey against Ranbaxy Pharmaceuticals, Inc. and its affiliates in response to Ranbaxy’s paragraph IV certifications regardingNexium.
In January 2006, AstraZeneca received a notice from IVAX Pharmaceuticals Inc. that IVAX Corporation had submitted an ANDA to the US FDA for esomeprazole magnesium delayed-release capsules, 20mg and 40mg. The ANDA contained paragraph IV certifications of invalidity and/or non-infringement in respect of certain AstraZeneca US patents listed in the FDA’s Orange Book with reference toNexium. IVAX also certified in respect of certain other AstraZeneca US patents listed in the Orange Book with reference toNexiumthat IVAX will not launch its product prior to the expiry of those patents, the latter of which expires in October 2007. In March 2006, AstraZeneca commenced wilful patent infringement litigation in the US District Court for the District of New Jersey against IVAX, its parent Teva Pharmaceuticals, and their affiliates. The Ranbaxy and Teva/IVAX matters have been consolidated.
In August 2006, AstraZeneca received a notice from Dr. Reddy’s Laboratories, Ltd. and Dr. Reddy’s Laboratories, Inc. (“Dr. Reddy’s”) that Dr. Reddy’s had submitted an ANDA to the US FDA for esomeprazole magnesium delayed-release capsules, 20mg and 40mg. Dr. Reddy’s was seeking FDA approval to market a generic esomeprazole magnesium product prior to the expiration of some but not all of the patents listed in the FDA Orange Book with reference toNexium.
Back to Contents
| 26 | COMMITMENTS AND CONTINGENT LIABILITIES CONTINUED |
| Dr. Reddy’s notice did not challenge three Orange Book-listed patents claiming esomeprazole magnesium (US Patent Nos. 5,714,504, 5,877,192 and 6,875,872). AstraZeneca’s exclusivity relating to these three patents expires on 3 August 2015, 27 November 2014 and 27 November 2014, respectively. Because AstraZeneca has not received notice from Dr. Reddy’s as to these three US patents, Dr. Reddy’s cannot market generic esomeprazole magnesium until the end of the exclusivity afforded by these patents. As a result, AstraZeneca did not bring a lawsuit at this time. AstraZeneca reserves the right to enforce all patents related to Nexium, including those listed in the FDA Orange Book. |
AstraZeneca continues to have full confidence in and will vigorously defend and enforce its intellectual property protectingNexium.
Nolvadex(tamoxifen)
AstraZeneca is a co-defendant with Barr Laboratories, Inc. in numerous purported class actions filed in federal and state courts throughout the US. All of the state court actions were removed to federal court and have been consolidated, along with all of the cases originally filed in the federal courts, in a federal multi-district litigation proceeding pending in the US District Court for the Eastern District of New York. Some of the cases were filed by plaintiffs representing a putative class of consumers who purchased tamoxifen. The other cases were filed on behalf of a putative class of ‘third party payers’ (including health maintenance organisations, insurers and other managed care providers and health plans) that have reimbursed or otherwise paid for prescriptions of tamoxifen. The plaintiffs allege that they paid ‘supra-competitive and monopolistic prices’ for tamoxifen as a result of the settlement of patent litigation between Zeneca and Barr in 1993. The plaintiffs seek injunctive relief, treble damages under the antitrust laws, disgorgement and restitution. In April 2002, AstraZeneca filed a motion to dismiss the cases for failure to state a cause of action. In May 2003, the US District Court for the Eastern District of New York granted AstraZeneca’s motion to dismiss. The plaintiffs appealed the decision.
In November 2005, the US Court of Appeals for the Second Circuit affirmed the District Court’s decision. The plaintiffs thereafter moved for re-hearing by the original panel of judges in the case and re-hearing by a panel of all of the judges on the US Court of Appeals for the Second Circuit. The plaintiffs’ requests for re-hearing were denied in September 2006. In December 2006, the plaintiffs filed a petition for awrit of certiorarito the US Supreme Court seeking to have the Court hear an appeal of the Second Circuit’s decision.
Pulmicort Respules(budesonide inhalation suspension)
In September 2005, AstraZeneca received a notice from IVAX Pharmaceuticals Inc. that IVAX had submitted an Abbreviated New Drug Application (ANDA) to the US FDA for a budesonide inhalation suspension containing a paragraph IV certification and alleging invalidity and non-infringement in respect of certain of AstraZeneca’s patents relating to budesonide inhalation suspension. In October 2005, AstraZeneca filed a patent infringement action against IVAX in the US District Court for the District of New Jersey. In December 2005, IVAX responded and filed counterclaims alleging non-infringement and invalidity. In January 2006, AstraZeneca filed an amended complaint, withdrawing averments as to the infringement of one of the patents-in-suit. Discovery in the litigation is ongoing.
AstraZeneca continues to have full confidence in and will vigorously defend and enforce its intellectual property protectingPulmicort Respules.
Seroquel(quetiapine fumarate)
In August 2003, Susan Zehel-Miller filed a putative class action against AstraZeneca PLC and AstraZeneca Pharmaceuticals LP on behalf of “all persons in the US who purchased and/or usedSeroquel”. Among other things, the class action alleged that AstraZeneca failed to provide adequate warnings in connection with an alleged association betweenSeroqueland the onset of diabetes. In 2004, the US District Court for the Middle District of Florida denied class certification and the case was ultimately dismissed. Two additional putative class actions raising similar allegations have likewise been dismissed. There are no other US class actions relating toSeroquel; however, four putative class actions raising substantially similar allegations have been filed in Canada.
Additionally, AstraZeneca Pharmaceuticals LP, either alone or in conjunction with one or more affiliates, has been sued in numerous individual personal injury actions involvingSeroquel. In the overwhelming majority of these cases, the nature of the plaintiffs’ alleged injuries is not clear. Although some plaintiffs contend that they developed diabetes or other related injuries as a result of takingSeroqueland/or other atypical anti-psychotic medications, in most instances, little or no factual information regarding the alleged injury has been provided. As of 24 January 2007, AstraZeneca was defending 604 served or answered lawsuits involving approximately 7,450 plaintiff groups. These include a number of recently filed cases that include close to 1,000 plaintiff groups per case. The majority of theSeroquelcases are pending in federal court with clusters of state court activity in Delaware, New Jersey, New York and Missouri. AstraZeneca is also aware of over 600 additional cases that have been filed but not yet served and has not determined how many additional cases, if any, may have been filed. Some of the cases also include claims against other pharmaceutical manufacturers such as Eli Lilly, Janssen Pharmaceutica and/or Bristol-Myers Squibb. AstraZeneca intends to vigorously defend all of the Seroquelcases.
In September 2005, AstraZeneca received a notice from Teva Pharmaceuticals USA that Teva had submitted an Abbreviated New Drug Application (ANDA) for quetiapine fumarate 25mg tablets containing a paragraph IV certification alleging invalidity, unenforceability, or non-infringement respecting AstraZeneca’s US patent listed in the FDA’s Orange Book with reference toSeroquel. In November 2005, AstraZeneca filed a lawsuit directed to Teva’s 25mg tablets ANDA in the US District Court for the District of New Jersey for wilful patent infringement. In February 2006, AstraZeneca received another notice from Teva Pharmaceuticals USA that Teva had amended its previously submitted ANDA for quetiapine fumarate 25mg tablets and added 100, 200 and 300mg tablets to its application to the US FDA. The amended ANDA submission contained a similar paragraph IV certification alleging invalidity, unenforceability, or non-infringement in respect of AstraZeneca’s US patent listed in the FDA’s Orange Book with reference toSeroquel. In March 2006, in response to Teva’s amended ANDA and Teva’s intent to market additional strengths of a generic version ofSeroquelin the US prior to the expiration of AstraZeneca’s patent, AstraZeneca filed an additional lawsuit against Teva in the US District Court for the District of New Jersey for patent infringement.
Back to Contents
| 142 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
NOTES TO THE FINANCIAL STATEMENTS CONTINUED
| 26 | COMMITMENTS AND CONTINGENT LIABILITIES CONTINUED |
| The two lawsuits were consolidated in April 2006. However in March 2006, the US District Court had granted Teva’s motion to strike AstraZeneca’s added allegation of wilfullness in its patent infringement claim in the first complaint directed to Teva’s 25mg tablets. Therefore, in the consolidated action, in response to AstraZeneca’s now-combined allegations of patent infringement directed to Teva’s 25, 100, 200 and 300mg ANDA tablets, Teva alleges non-infringement and patent invalidity. In January 2007, Teva filed a motion seeking leave to amend its pleadings in the consolidated action to add allegations, defences, and counter-claims directed to alleged inequitable conduct in the procurement of AstraZeneca’s patent. Discovery in the consolidated case is proceeding. |
AstraZeneca continues to have full confidence in and will vigorously defend and enforce its intellectual property protectingSeroquel.
Symbicort(budesonide/formoterol)
In March 2005, the European Patent Office ruled that the European patent covering the combination of formoterol and budesonide inSymbicortis valid. The patent, which expires in 2012 (Supplementary Patent Certificate expires 2015), was challenged by the generic manufacturers Yamanouchi Europe BV, Miat SpA, Liconsa, Chiesi Farmaceutici SpA, Zambon Group SpA, Generics (UK) Limited and Norton Healthcare Ltd. In May 2005, the European Patent Office ruled that the European patent forSymbicortin the treatment of chronic obstructive pulmonary disease (COPD) is valid. The patent, which expires in 2018, was challenged by the generic manufacturers Chiesi Farmaceutici SpA, Norton Healthcare Ltd and Generics (UK) Limited.
The European Patent Office rulings relating to both the combination and the COPD European patents forSymbicorthave been appealed by certain of the opponents in the proceedings. It is not anticipated that the appeals will be heard before the latter part of 2007.
In February 2004, IVAX Pharmaceuticals (UK) Limited initiated proceedings against AstraZeneca AB claiming that the UK parts of the two European patents related toSymbicortwere invalid. In May 2004, the court granted AstraZeneca’s application for a stay of the proceedings pending the determination of the parallel opposition proceedings before the European Patent Office, described above. In April 2004, IVAX initiated proceedings against AstraZeneca AB in relation to the Republic of Ireland claiming that the Irish parts of the two European patents related toSymbicortwere invalid. In October 2004, the court granted AstraZeneca’s application for a stay of proceedings pending the final decision of the European Patent Office and its Boards of Appeal in the opposition proceedings.
Toprol-XL(metoprolol succinate)
In May 2003, AstraZeneca filed a patent infringement action against KV Pharmaceutical Company in the US District Court for the Eastern District of Missouri in response to KV’s notification of its intention to market a generic version ofToprol-XLtablets in the 200mg dose prior to the expiration of AstraZeneca’s patents covering the substance and its formulation. In response to later similar notices from KV related to the 25, 50 and 100mg doses, AstraZeneca filed further actions. KV responded in each instance and filed counterclaims alleging non-infringement, invalidity and unenforceability of the listed patents.
In February 2004, AstraZeneca filed a patent infringement action against Andrx Pharmaceuticals LLC in the US District Court for the District of Delaware in response to Andrx’s notification of its intention to market a generic version ofToprol-XLtablets in the 50mg dose prior to the expiration of AstraZeneca’s patents. In response to two later similar notices from Andrx related to the 25,100 and 200mg doses, AstraZeneca filed two additional patent infringement actions in the same court. In each instance, Andrx claimed that each of the listed patents is invalid, not infringed and unenforceable.
In April 2004, AstraZeneca filed a patent infringement action against Eon Labs Manufacturing Inc. in the US District Court for the District of Delaware in response to Eon’s notification of its intention to market generic versions ofToprol-XLtablets in the 25, 50, 100 and 200mg doses prior to the expiration of AstraZeneca’s patents. In its response, Eon alleged that each of the listed patents is invalid, not infringed and unenforceable. Eon also alleged that the filing of the infringement complaints, as well as other actions by AstraZeneca, constitutes anti-competitive conduct in violation of US anti-trust laws. Pursuant to a joint motion of AstraZeneca and Eon these anti-trust counts were severed from the case and stayed, for possible consideration depending on the outcome of the trial of the patent claims.
All of the patent litigation relating toToprol-XLagainst KV, Andrx and Eon was consolidated for pre-trial discovery purposes and motion practice in the US District Court for the Eastern District of Missouri. The defendants filed a motion for summary judgment in December 2004 alleging that theToprol-XLpatents are invalid due to double patenting. A summary judgment motion of unenforceability was filed by the defendants in 2005 and AstraZeneca filed summary judgment motions on infringement and validity in 2005. In January 2006, the US District Court for the Eastern District of Missouri issued a ruling finding that the two patents-in-suit are unenforceable (based on the Company’s inequitable conduct in the prosecution of these patents in the US Patent and Trademark Office) and invalid. AstraZeneca appealed the District Court decision to the US Court of Appeals for the Federal Circuit. The appeal was fully briefed in 2006 and was argued on 8 December 2006. We await the decision of the Court of Appeals.
In August 2006, Sandoz (formerly Eon) received final approval from the US Food and Drug Administration (FDA) on the 25mg dose of metoprolol succinate and tentative approval on the 50, 100 and 200mg doses. On 21 November 2006, Sandoz launched its 25mg metoprolol succinate product, which was followed by Par Pharmaceuticals’ launch of a 25mg generic metoprolol succinate under a distribution agreement by AstraZeneca. There is no longer a stay in effect on the approval of the ANDAs filed by KV and Andrx but neither has received FDA approval.
Back to Contents
| 26 | COMMITMENTS AND CONTINGENT LIABILITIES CONTINUED |
| In the first quarter of 2006, AstraZeneca was served with 14 complaints filed in the US District Courts in Delaware, Massachusetts, and Florida against AstraZeneca Pharmaceuticals LP, AstraZeneca LP, AstraZeneca AB and Aktiebolaget Hässle. The complaints were putative class actions filed on behalf of both direct purchasers and indirect purchasers that allege that the AstraZeneca defendants attempted to illegally maintain monopoly power in the US over Toprol-XL in violation of the Sherman Act through the listing of invalid and unenforceable patents in the FDA’s Orange Book and the enforcement of such patents through litigation against generic manufacturers seeking to market metoprolol succinate. The complaints seek treble damages based on alleged overcharges to the putative classes of plaintiffs. The lawsuit is based upon the finding described above by the US District Court for the Eastern District of Missouri in the consolidated litigation against KV, Andrx and Eon that the AstraZeneca patents relating to Toprol-XL are invalid and unenforceable. As noted above, AstraZeneca is appealing the ruling in the patent litigation. These 14 complaints were consolidated into two amended complaints, one on behalf of direct purchasers, and one on behalf of indirect purchasers. AstraZeneca has filed a motion seeking to dismiss or in the alternative stay the consolidated complaint in both cases. AstraZeneca denies the allegations of the anti-trust complaints and will vigorously defend the lawsuits. |
AstraZeneca continues to maintain that its patents forToprol-XLare valid, enforceable and infringed by the actual and proposed generic products of KV, Andrx and Eon and that its enforcement of its patents did not violate anti-trust laws.
Zestril(lisinopril)
In 1996, two of AstraZeneca’s predecessor companies, Zeneca Limited and Zeneca Pharma Inc. (as licensees), Merck & Co., Inc. and Merck Frosst Canada Inc. commenced a patent infringement action in the Federal Court of Canada against Apotex Inc., alleging infringement of Merck’s lisinopril patent. Apotex sold a generic version of AstraZeneca’sZestriland Merck’s Prinivil™tablets. Apotex admitted infringement but has raised positive defences to infringement, including that it acquired certain quantities of lisinopril prior to issuance of the patent and that certain quantities were licensed under a compulsory licence. Apotex also alleged invalidity of the patent. Following a trial in early 2006, in April 2006 the Federal Court of Canada ruled in favour of AstraZeneca and Merck on the key issues and Apotex stopped selling lisinopril in May 2006. In October 2006, the Federal Court of Appeal in Canada upheld the lower court’s decision and dismissed Apotex’s appeal. In December 2006 Apotex sought leave to appeal to the Supreme Court of Canada and the application remains pending.
AstraZeneca (as licensee) also had a case pending in the Federal Court of Canada against Cobalt Pharmaceuticals Inc., pertaining to the same Merck lisinopril patent, on the basis that Cobalt was seeking a notice of compliance (marketing approval) in Canada based on a comparison with AstraZeneca’sZestril.
However, in 2006, Cobalt withdrew its notice of allegation relating to lisinopril and AstraZeneca discontinued its case against Cobalt.
Zestoretic(lisinopril/hydrochlorothiazide)
AstraZeneca (as licensee) had a case pending in the Federal Court of Canada against Apotex Inc., pertaining to Merck’s lisinopril/hydrochlorothiazide combination patent, on the basis that Apotex was seeking a notice of compliance (marketing approval) in Canada based on a comparison with AstraZeneca’sZestoretic. AstraZeneca is potentially liable for damages in the event that Apotex’s market entry is held to have been improperly delayed.
The case against Apotex was discontinued by AstraZeneca in August 2006. Apotex’s combination product will likely remain off the market until the expiry of a relevant patent in October 2007.
Average wholesale price class action litigation
In January 2002, AstraZeneca was named as a defendant along with 24 other pharmaceutical manufacturers in a class action suit, in Massachusetts, brought on behalf of a putative class of plaintiffs alleged to have overpaid for prescription drugs as a result of inflated wholesale list prices. Following the Massachusetts complaint, nearly identical class action suits were filed against AstraZeneca and various other pharmaceutical manufacturers in four other states. AstraZeneca and other manufacturers have since been sued in similar lawsuits filed by the state Attorneys General of Pennsylvania, Nevada, Montana, Wisconsin, Illinois, Alabama, Kentucky, Arizona, Mississippi, Hawaii, and Alaska, as well as by multiple individual counties in the State of New York. The Attorney General lawsuits seek to recover alleged overpayments under Medicaid and other state-funded healthcare programmes. In several cases, the states are also suing to recover alleged overpayments by state residents. Several of these suits have been consolidated with the Massachusetts action for pre-trial purposes, pursuant to federal multi-district litigation (MDL) procedures.
In January 2006, the District Court in Boston certified three classes of plaintiffs against the “Track 1” manufacturer defendants, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb, Schering-Plough, and Johnson & Johnson. The three certified classes are: (Class1) a nationwide class of consumers who made co-payments for certain physician-administered drugs reimbursed under the Medicare Part B programme (“Part B drugs”); (Class 2) a Massachusetts-only class of third-party payers, including insurance companies, union health and welfare benefit plans, and self-insured employers, who covered consumer co-payments for Part B drugs; and (Class 3) a Massachusetts-only class of third-party payers and consumers who paid for Part B drugs outside of the Medicare programme. For all classes, the only AstraZeneca drug at issue isZoladex(goserelin acetate implant).
Back to Contents
| 144 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
NOTES TO THE FINANCIAL STATEMENTS CONTINUED
| 26 | COMMITMENTS AND CONTINGENT LIABILITIES CONTINUED |
| A bench trial against four of the Track 1 defendants, including AstraZeneca, by Classes 2 and 3 began on 6 November 2006 and concluded on 26 January 2007. The Court has yet to render its decision. A separate jury trial against AstraZeneca only, by Class 1, is scheduled for 30 April 2007. The multiple Attorney General lawsuits filed in state courts are proceeding independently of the Boston MDL proceeding. The first trials that potentially involve AstraZeneca are scheduled for November 2007 in the Alabama and Mississippi Attorney General cases. |
AstraZeneca denies the allegations made in all of the average wholesale price lawsuits and will vigorously defend the actions.
340B Class Action Litigation
In August 2004, AstraZeneca was named as a defendant along with multiple other pharmaceutical manufacturers in a class action suit filed in Alabama Federal Court on behalf of all so-called “disproportionate share” entities. These are the hospitals and clinics that treat a substantial portion of uninsured patients and thus qualify for preferential pricing under the Public Health Service Act drug discount programme (the “340B” Program). According to the complaint, the genesis of the suit was an audit report by the Department of Health and Human Services Office of Inspector General (OIG) in June 2004. The OIG later withdrew the audit report and in 2006, re-issued a revised audit report that substantially modified the previous audit findings. After the issuance of the revised OIG audit report, the named plaintiffs voluntarily dismissed their lawsuit against the defendants.
A similar class action suit was filed in August 2005 by the County of Santa Clara in California state court. The County of Santa Clara sued as a representative of a class of similarly situated counties and cities in California alleged to have overpaid for 340B-covered drugs. The case was removed to the US District Court for the Northern District of California. In 2006, the US District Court dismissed each of the allegations in the County’s complaint. The County appealed the dismissal to the US Court of Appeals for the North Circuit. AstraZeneca denies the allegations in the County’s complaint and intends to continue to defend them vigorously.
Additional government investigations into drug marketing practices
As is true for most, if not all, major prescription pharmaceutical companies operating in the US, AstraZeneca is currently involved in multiple US federal and state criminal and civil investigations into drug marketing and pricing practices. Two of the active investigations are being handled by the US Attorney’s Office in Boston. The first involves a subpoena for documents and information relating to sales and marketing interactions with a leading provider of pharmacy services to long-term care facilities. The second involves an investigation relating to the sale and marketing of products to an individual physician in Worcester, Massachusetts and certain physicians and entities affiliated with that physician. These investigations may be the subject of sealed qui tam lawsuits filed under the False Claims Act.
The US Attorney’s Office in Philadelphia is directing three additional, active investigations. The first two involve requests for documents and information relating to contracting and disease management programmes with two of the leading national Pharmacy Benefits Managers. The third involves a review of sales and marketing practices relating toSeroquel, including allegations that the Company promotedSeroquelfor non-indicated (off-label) uses. AstraZeneca understands that all of these investigations may be the subjects of sealed qui tam lawsuits filed under the False Claims Act.
There are a number of additional active investigations led by state Attorneys General. These include subpoenas received in September 2006 from the Alaska and California Attorney General’s Offices seeking information relating toSeroquelsales and marketing practices. In addition, the Nevada and Delaware Attorney General’s Offices have requested documents and information relating to the development of patient education and practice management materials for physicians.
It is not possible to predict the outcome of any of these investigations, which could include the payment of damages and the imposition of fines, penalties and administrative remedies.
Informal SEC inquiry
In October 2006, AstraZeneca received from the US Securities and Exchange Commission (“SEC”) a letter requesting documents related to its business activities in Italy, Croatia, Russia and Slovakia for the period 1 October 2003 to the present. The SEC’s request generally seeks documents concerning any payments to doctors or government officials and related internal accounting controls. The request also seeks policies, correspondence, audits and other documents concerning compliance with the Foreign Corrupt Practices Act, as well as any allegations or communications with prosecutors’ offices relating to corruption or bribery of doctors or government officials. AstraZeneca is in the process of responding to the SEC’s request. It is not currently possible to predict the outcome of this inquiry.
Drug importation anti-trust litigation
In May 2004, plaintiffs in a purported class action filed complaints in the US District Court for Minnesota and for New Jersey, alleging that AstraZeneca Pharmaceuticals LP and eight other pharmaceutical manufacturer defendants conspired to prevent American consumers from purchasing prescription drugs from Canada, “depriving consumers of the ability to purchase” drugs at competitive prices. The New Jersey case was voluntarily dismissed in July 2004. In August 2005, the Minnesota District Court dismissed with prejudice the plaintiffs’ federal anti-trust claims and declined to exercise supplemental jurisdiction in relation to the state statutory and common law claims, which claims were dismissed without prejudice. The plaintiffs appealed the District Court’s decision to the US Court of Appeals for the Eighth Circuit. In November 2006, the US Court of Appeals for the Eighth Circuit affirmed the District Court’s decision.
Back to Contents
| 26 | COMMITMENTS AND CONTINGENT LIABILITIES CONTINUED |
| In August 2004, Californian retail pharmacy plaintiffs filed an action in the Superior Court of California making similar allegations to the Minnesota action and also alleging a conspiracy by approximately 15 pharmaceutical manufacturer defendants to set the price of drugs sold in California at or above the Canadian sales price for those same drugs. In July 2005, the court overruled in part and sustained in part, without leave to amend, the defendants’ motion to dismiss the plaintiffs’ third amended complaint in these proceedings. The Court overruled the defendants’ motion in respect of conspiracy claims but sustained the motion in respect of the California Unfair Competition Law claims. On 15 December 2006, the court granted the defendants’ motion for summary judgment and the case will be dismissed. In January 2007, plaintiffs filed a Notice of Appeal with the Court of Appeal of the State of California. |
AstraZeneca denies the material allegations of both the Minnesota and California actions and is vigorously defending these matters.
Anti-trust
In July 2006, AstraZeneca Pharmaceuticals LP was named as a defendant, along with a number of other pharmaceutical manufacturers and wholesalers, in a complaint filed by RxUSA Wholesale, Inc. in the US District Court for the Eastern District of New York. The complaint alleges that the defendants violated federal and state anti-trust laws by, among other things, allegedly refusing to deal with RxUSA and other “secondary wholesalers” in the wholesale pharmaceutical industry. The plaintiff alleges a conspiracy among the manufacturers and seeks an injunction and treble damages. AstraZeneca vigorously denies the allegations and in November 2006 filed a motion to dismiss the complaint.
For a description of other anti-trust-related litigation involving AstraZeneca, see the subsections entitled “Losec/Prilosec(omeprazole)”, “Nolvadex(tamoxifen)” and “Toprol-XL(metoprolol succinate)” in this Note 26 to the Financial Statements.
StarLink
AstraZeneca Insurance Company Limited (AZIC) commenced arbitration proceedings in the UK against insurers in respect of amounts paid by Garst Seed Company of the US in settlement of claims arising in the US from Garst’s sale of StarLink, a genetically engineered corn seed. The English High Court ruled, on appeal by reinsurers from a preliminary finding in AZIC’s favour by the arbitration panel, that English law applies to recovery under the reinsurance arrangements. This is contrary to AZIC’s view, which is that recovery should be assessed under Iowa law, and AZIC sought leave to appeal this finding to the Court of Appeal. Leave to appeal was refused and in the circumstances AZIC decided not to proceed further with the case. Taking into account recoveries and a central provision, taken in 2004, this will have no impact on 2006 profits. AstraZeneca’s interest in Garst was through AstraZeneca’s 50% ownership of Advanta BV, the sale of which to Syngenta AG was announced in May 2004 and completed in September 2004.
General
With respect to each of the legal proceedings described above, other than those which have been disposed of, we are unable to make estimates of the possible loss or range of possible losses at this stage, other than where noted in the case of the European Commission fine. We also do not believe that disclosure of the amount sought by plaintiffs, if that is known, would be meaningful with respect to those legal proceedings. This is due to a number of factors including: the stage of the proceedings (in many cases trial dates have not been set) and overall length and extent of legal discovery; the entitlement of the parties to an action to appeal a decision; clarity as to theories of liability; damages and governing law; uncertainties in timing of litigation; and the possible need for further legal proceedings to establish the appropriate amount of damages, if any. However, although there can be no assurance regarding the outcome of any of the legal proceedings or investigations referred to in this Note 26 to the Financial Statements, we do not expect them to have a materially adverse effect on our financial position or profitability.
Taxation
Where tax exposures can be quantified, a provision is made based on best estimates and management’s judgement. Details of the movements in relation to material tax exposures are discussed below.
AstraZeneca faces a number of transfer pricing audits in jurisdictions around the world. The issues under audit are often complex and can require many years to resolve. Accruals for tax contingencies require management to make estimates and judgements with respect to the ultimate outcome of a tax audit, and actual results could vary from these estimates. The total net accrual included in the Financial Statements to cover the worldwide exposure to transfer pricing audits is $995m, an increase of $452m due to a number of new audits, revisions of estimates relating to existing audits, offset by a number of negotiated settlements. For certain of the audits, AstraZeneca estimates the potential for additional losses above and beyond the amount provided to be up to $445m; however, management believes that it is unlikely that these additional losses will arise. Of the remaining tax exposures, the Company does not expect material additional losses. It is not possible to estimate the timing of tax cash flows in relation to each outcome. Included in the provision is an amount of interest of $265m. Interest is accrued as a tax expense.
Back to Contents
| 146 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
NOTES TO THE FINANCIAL STATEMENTS CONTINUED
| 27 | LEASES |
| Total rentals under operating leases charged to the income statement were as follows: |
| | 2006 | | 2005 | | 2004 | |
| | $m | | $m | | $m | |
|
|
|
|
|
| |
| | 197 | | 155 | | 127 | |
|
|
|
|
|
| |
| The future minimum lease payments under operating leases that have initial or remaining terms in excess of one year at 31 December 2006 were as follows: | |
| | | Operating leases | |
| | |
|
|
|
|
| |
| | | 2006 | | 2005 | | 2004 | |
| | | $m | | $m | | $m | |
|
|
|
|
|
|
| |
| Obligations under leases comprise | | | | | | |
| Rentals due within one year | 211 | | 83 | | 112 | |
|
|
|
|
|
|
| |
| Rentals due after more than one year: | | | | | | |
| | After five years | 88 | | 90 | | 69 | |
|
|
|
|
|
|
| |
| | From four to five years | 22 | | 18 | | 28 | |
|
|
|
|
|
|
| |
| | From three to four years | 31 | | 26 | | 35 | |
|
|
|
|
|
|
| |
| | From two to three years | 43 | | 41 | | 45 | |
|
|
|
|
|
|
| |
| | From one to two years | 56 | | 52 | | 63 | |
|
|
|
|
|
|
| |
| | | 240 | | 227 | | 240 | |
|
|
|
|
|
|
| |
| | | 451 | | 310 | | 352 | |
|
|
|
|
|
|
| |
| | | |
| 28 | STATUTORY AND OTHER INFORMATION | |
| | | | | | | | |
| | | 2006 | | 2005 | | 2004 | |
| | | $m | | $m | | $m | |
|
|
|
|
|
|
| |
| Fees payable to KPMG Audit Plc and its associates: | | | | | | |
| | Group audit fee | 3.1 | | 2.5 | | 1.7 | |
|
|
|
|
|
|
| |
| Fees payable to KPMG Audit Plc and its associates for other services: | | | | | | |
| | The audit of subsidiaries pursuant to legislation | 5.4 | | 5.0 | | 4.4 | |
|
|
|
|
|
|
| |
| | Other services pursuant to legislation | 4.1 | | 0.8 | | 1.4 | |
|
|
|
|
|
|
| |
| | Taxation | 1.2 | | 1.0 | | 2.0 | |
|
|
|
|
|
|
| |
| | All other services | 1.0 | | 2.2 | | 1.8 | |
|
|
|
|
|
|
| |
| Fees payable to KPMG Audit Plc in respect of the Company’s pension schemes: | | | | | | |
| | The audit of subsidiaries pension schemes | 0.5 | | 0.5 | | 0.5 | |
|
|
|
|
|
|
| |
| | | 15.3 | | 12.0 | | 11.8 | |
|
|
|
|
|
|
| |
Other services pursuant to legislation includes fees of $3.2m (2005 $nil, 2004 $nil) in respect of Sarbanes-Oxley s404. All other services include $nil (2005 $1.8m, 2004 $1.1m) in respect of Sarbanes-Oxley s404.
Taxation services consist of tax compliance services and tax advice.
Related party transactions
The Group had no material related party transactions which might reasonably be expected to influence decisions made by the users of these Financial Statements.
| Key management personnel compensation | | | | | | | |
| | | 2006 | | 2005 | | 2004 | |
| | | $’000 | | $’000 | | $’000 | |
|
|
|
|
|
|
| |
| Short-term employee benefits | | 21,321 | | 19,334 | | 17,382 | |
|
|
|
|
|
|
| |
| Post-employment benefits | | 3,191 | | 1,731 | | 1,595 | |
|
|
|
|
|
|
| |
| Share-based payments | | 8,417 | | 5,663 | | 6,086 | |
|
|
|
|
|
|
| |
| | | 32,929 | | 26,728 | | 25,063 | |
|
|
|
|
|
|
| |
Total remuneration is included within employee costs (Note 25). The prior periods have been restated.
Subsequent events
Other than the completion of the two collaboration agreements and the acquisition agreement signed in January 2007 (as set out in Note 26) there were no material subsequent events.
Back to Contents
| 29 | SHARE CAPITAL OF PARENT COMPANY |
| | Authorised | | Allotted, called-up and fully paid | |
| |
| |
|
|
|
|
| |
| | 2006 | | 2006 | | 2005 | | 2004 | |
| | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
| |
| Issued Ordinary Shares ($0.25 each) | 383 | | 383 | | 395 | | 411 | |
|
|
|
|
|
|
|
| |
| Unissued Ordinary Shares ($0.25 each) | 217 | | – | | – | | – | |
|
|
|
|
|
|
|
| |
| Redeemable Preference Shares (£1 each – £50,000) | – | | – | | – | | – | |
|
|
|
|
|
|
|
| |
| | 600 | | 383 | | 395 | | 411 | |
|
|
|
|
|
|
|
| |
The total authorised number of Ordinary Shares at 31 December 2006 was 2,400,000,000, of which 1,532,245,608 Ordinary Shares were in issue.
The Redeemable Preference Shares carry limited class voting rights and no dividend rights. This class of shares is capable of redemption at par at the option of the Company on the giving of seven days’ written notice to the registered holder of the shares.
The movements in share capital during the year can be summarised as follows:
| | No. of shares | | | |
| | (million) | | $m | |
|
|
|
| |
| At 1 January 2006 | 1,581 | | 395 | |
|
|
|
| |
| Issues of shares | 23 | | 6 | |
|
|
|
| |
| Re-purchase of shares | (72 | ) | (18 | ) |
|
|
|
| |
| At 31 December 2006 | 1,532 | | 383 | |
|
|
|
| |
Share re-purchase
During the year the Company re-purchased, and subsequently cancelled, 72,205,192 Ordinary Shares at an average price of 3059 pence per share. The total consideration, including expenses, was $4,147m. The excess of the consideration over the nominal value has been charged against retained earnings.
Share schemes
A total of 23,548,800 Ordinary Shares were issued during the year in respect of share schemes. Details of movements in the number of Ordinary Shares under option are shown in Note 25; details of options granted to Directors are shown in the Directors’ Remuneration Report.
Shares held by subsidiaries
No shares in the Company are held by subsidiaries in any year.
Back to Contents
| 148 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
PRINCIPAL SUBSIDIARIES
| | | | Percentage of voting | | | |
| At 31 December 2006 | Country | | share capital held | | Principal activity | |
|
|
|
|
|
| |
| UK | | | | | | |
| AstraZeneca UK Limited | England | | 100 | 1 | Research and development, | |
| | | | | | manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca Reinsurance Limited | England | | 100 | | Insurance and reinsurance underwriting | |
|
|
|
|
|
| |
| AstraZeneca Treasury Limited | England | | 100 | | Treasury | |
|
|
|
|
|
| |
| Continental Europe | | | | | | |
| NV AstraZeneca SA | Belgium | | 100 | | Manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca Dunkerque Production SCS | France | | 100 | | Manufacturing | |
|
|
|
|
|
| |
| AstraZeneca SAS | France | | 100 | | Research, manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca GmbH | Germany | | 100 | | Development, manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca Holding GmbH | Germany | | 100 | | Manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca SpA | Italy | | 100 | | Manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca Farmaceutica Spain SA | Spain | | 100 | | Manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca AB | Sweden | | 100 | | Research and development, | |
| | | | | | manufacturing, marketing | |
|
| |
| |
| |
| AstraZeneca BV | The Netherlands | | 100 | | Marketing | |
|
|
|
|
|
| |
| The Americas | | | | | | |
| AstraZeneca Canada Inc. | Canada | | 100 | | Research, manufacturing, marketing | |
|
|
|
|
|
| |
| IPR Pharmaceuticals Inc. | Puerto Rico | | 100 | | Development, manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca LP | US | | 99 | | Research and development, | |
| | | | | | manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca Pharmaceuticals LP | US | | 100 | | Research and development, | |
| | | | | | manufacturing, marketing | |
|
|
|
|
|
| |
| Zeneca Holdings Inc. | US | | 100 | | Manufacturing, marketing | |
|
|
|
|
|
| |
| Asia, Africa & Australasia | | | | | | |
| AstraZeneca Pty Limited | Australia | | 100 | | Development, manufacturing, marketing | |
|
|
|
|
|
| |
| AstraZeneca KK | Japan | | 80 | | Manufacturing, marketing | |
|
|
|
|
|
| |
The companies and other entities listed above are those whose results or financial position principally affected the figures shown in the Group Financial Statements. A full list of subsidiaries, joint ventures and associates will be annexed to the Company’s next annual return filed with the Registrar of Companies. The country of registration or incorporation is stated alongside each company. The accounting year ends of subsidiaries and associates are 31 December, except for Aptium Oncology, Inc. which, owing to local conditions and to avoid undue delay in the preparation of the Financial Statements, is 30 November. AstraZeneca operates through 240 subsidiaries worldwide. The Group Financial Statements consolidate the Financial Statements of AstraZeneca PLC and its subsidiaries at 31 December 2006. Products are manufactured in 19 countries worldwide and are sold in over 100 countries.
Back to Contents
| ADDITIONAL INFORMATION FOR US INVESTORS | 149 |
ADDITIONAL INFORMATION FOR US INVESTORS
INTRODUCTION
The accompanying consolidated Financial Statements included in this Annual Report are prepared in accordance with adopted IFRSs. There are certain significant differences between adopted IFRS and US GAAP which affect AstraZeneca’s net income and shareholders’ equity and, on pages 149 to 156, additional information under US GAAP is set out as follows:
| > | Summary of differences between adoptedIFRS and US GAAP accounting principles;pages 149 to 150. |
| | |
| > | Net income; page151. |
| | |
| > | US GAAP condensed consolidatedstatement of operations; page 151. |
| | |
| > | US GAAP statement of comprehensiveincome; page 152. |
| | |
| > | Stock-based compensation; page152. |
| | |
| > | Pension and post-retirement benefits;pages152 to 154. |
| | |
| > | Taxation; page 155. |
| | |
| > | Shareholders’ equity; page155. |
| | |
| > | Acquired intangible assets and goodwill;page156. |
DIFFERENCES BETWEEN INTERNATIONAL AND US ACCOUNTING PRINCIPLES
Purchase accounting adjustments
Under adopted IFRS, the merger of Astra and Zeneca is accounted for as a ‘merger of equals’ (pooling-of-interests) as a result of the business combinations exemption permitted by IFRS 1 ‘First-time Adoption of International Financial Reporting Standards’. Under US GAAP, the merger was accounted for as the acquisition of Astra by Zeneca using ‘purchase accounting’. Under purchase accounting, the assets and liabilities of the acquired entity are recorded at fair value. As a result of the fair value exercise, increases in the values of Astra’s property, plant and equipment and inventory were recognised and values attributed to its in-process research and development and existing products, together with appropriate deferred taxation effects. The difference between the cost of investment and the fair value of the assets and liabilities of Astra was recorded as goodwill. The amount allocated to in-process research and development was, as required by US GAAP, expensed immediately in the first reporting period after the business combination. Fair value adjustments to the recorded amount of inventory were expensed in the period the inventory was utilised. Additional amortisation and depreciation have also been recorded in respect of the fair value adjustments to tangible and intangible assets.
Under adopted IFRS, up until 31 December 2002, goodwill was required to be capitalised and amortised. From 1 January 2003, goodwill is tested annually for impairment but not amortised. Under US GAAP, there is an equivalent requirement, but the effective date was 1 January 2002.
Capitalisation of interest
AstraZeneca does not capitalise interest under adopted IFRS. US GAAP requires interest incurred as part of the cost of constructing property, plant and equipment to be capitalised and amortised over the life of the asset.
Deferred taxation
Under adopted IFRS, full provision for deferred taxation is made although there are a number of different bases from US GAAP on which this calculation is made; for example, the elimination of intra-group profit on inventories and share-based payment transactions. Deferred taxation is provided on a full liability basis under US GAAP, which requires deferred tax assets to be recognised without a valuation allowance if their realisation is considered to be more likely than not.
Pension and post-retirement benefits
Adopted IFRS requires that in respect of defined benefit plans, obligations are measured at discounted fair value whilst plan assets are recorded at fair value. The operating and financing costs of such plans are recognised separately in the income statement; service costs are spread systematically over the lives of employees and financing costs are recognised in the periods in which they arise. US GAAP adopts a similar approach. Under adopted IFRS, actuarial gains and losses are permitted to be recognised immediately in the statement of recognised income and expense. Under US GAAP, such actuarial gains and losses are permitted to be amortised on a straight-line basis over the average remaining service period of employees.
The funded status of all post-retirement benefit plans, being the difference between the fair value of the plan assets and its benefit obligation, is now recognised on the Group balance sheet under US GAAP.
Intangible assets
Under adopted IFRS, certain payments to third parties for rights to compounds in development are capitalised and amortised over their economic lives from launch. Under US GAAP, these payments are generally expensed.
In-process research & development (IPR&D)
Under adopted IFRS, IPR&D compounds acquired in a business combination are capitalised as intangible assets and amortised, generally on a straight line basis, over their economic lives from launch. Such intangible assets are subject to impairment testing at each balance sheet date. Deferred tax is provided for IPR&D assets acquired in a business combination. Under US GAAP, such assets are expensed immediately in the first reporting period after the business combination. Consequently no deferred tax is provided, resulting in a reconciling adjustment to deferred tax and goodwill.
Financial instruments and hedging activities
Under adopted IFRS, certain financial assets and certain financial liabilities (including derivatives) are recognised at fair value; movements in the fair value may be recorded in equity or through income, depending upon their designation. Under US GAAP, marketable securities are recognised at fair value, with movements in fair value taken to a separate component of equity. Derivatives are also measured at fair value with movements taken through income. However, financial liabilities are recorded at amortised cost.
Back to Contents
| 150 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
ADDITIONAL INFORMATION FOR US INVESTORSCONTINUED
New accounting standards adopted
In May 2005, the FASB issued SFAS No. 154 ‘Accounting Changes and Error Corrections –a replacement of APB Opinion No. 20 and FASB Statement No. 3’. SFAS No. 154 requires retrospective application of prior periods’ financial statements for changes in accounting principle. SFAS No. 154 applies to accounting periods beginning after 15 December 2005 and has been adopted in the year. There has been no impact upon the results or net assets of AstraZeneca following adoption.
AstraZeneca has adopted the provisions of SFAS No. 158 ‘Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans – an amendment of FASB Statements No. 87, 88, 106, and 132(R)’ in 2006. SFAS No. 158 requires a company that sponsors a post-retirement defined benefit plan to fully recognise, as an asset or liability, the overfunded or underfunded status of its benefit plans on its year end balance sheet. The funded status is measured as the difference between the fair value of the plan’s assets and its projected benefit obligation for pension plans and accumulated post-retirement obligation for other retirement benefit plans. The initial impact of the standard due to unrecognised prior service costs or credit and net actuarial gains or losses as well as subsequent changes in the funded status is recognised as a component of accumulated other comprehensive income. The statement requires application as of the end of fiscal years ending after 15 December 2006 for recognition of the asset or liability related to the funded status of plans. Adoption of SFAS No. 158 has led to the recognition of a liability of $1,890m as at 31 December 2006 in respect of pension and post-retirement plans and a decrease to our accumulated OCI of $1,624m. Statement No. 158 does not change the computation of benefit expense recognised in the income statement, consequently there has been no amendment to this computation in the current year.
New accounting standards not adopted
In September 2006, the FASB issued SFAS No. 157 ‘Fair Value Measurements’ to provide a single definition of fair value, being a market-based measurement, and set out a fair value hierarchy. SFAS No. 157 is effective for fiscal years beginning after 15 November 2007. The adoption of SFAS No. 157 is not expected to have a material effect on the results or net assets of AstraZeneca.
In March 2006, the FASB issued SFAS No. 156 ‘Accounting for Servicing of Financial Assets’ requiring an entity to separately recognise a servicing asset or liability when it undertakes an obligation to service a financial asset in certain conditions. Such assets or liabilities must be initially measured at fair value and can be subsequently measured using the amortisation or fair value measurement methods. SFAS No. 156 is effective for fiscal years beginning after 15 September 2006. The adoption of SFAS No. 156 is not expected to have a material effect on the results or net assets of AstraZeneca.
In February 2006, the FASB issued SFAS No. 155 ‘Accounting for Certain Hybrid Financial Instruments’ to allow an entity to make an irrevocable election, on an instrument-by-instrument basis, to fair value in its entirety a hybrid financial instrument containing an embedded derivative, rather than bifurcate the embedded derivative from its host contract and fair value each component separately in accordance with SFAS No. 133 ‘Accounting for Derivative Instruments and Hedging Activities’. SFAS No. 155 is effective for fiscal years beginning after 15 September 2006. The adoption of SFAS No. 155 is not expected to have a material effect on the results or net assets of AstraZeneca.
In June 2006, the FASB issued FASB Interpretation No. 48 ‘Accounting for Uncertainty in Income Taxes – an interpretation of FASB Statement No. 109’ (FIN 48). The Interpretation establishes a two-step approach for recognising and measuring tax benefits, with tax positions only to be recognised when considered to be more likely than not sustained upon examination by the taxing authority. Explicit disclosures are required at the end of each reporting period about uncertainties in the entity’s tax position. The Company is currently in the process of quantifying the effect of adoption of FIN 48 on the results and net assets of AstraZeneca.
Back to Contents
| ADDITIONAL INFORMATION FOR US INVESTORS | 151 |
NET INCOME
As a result of the significant difference between the adopted IFRS and US GAAP treatment of the combination of Astra and Zeneca in the year of acquisition, and in the results of preceding periods, condensed statements of operations under US GAAP have been prepared for the benefit of US investors.
The following is a summary of the adjustments to net income and shareholders’ equity which would have been required if US GAAP had been applied instead of adopted IFRS.
| | 2006 | | 2005 | | 2004 | |
| For the years ended 31 December | $m | | $m | | $m | |
|
|
|
|
|
| |
| Net income for the period under adopted IFRS | 6,043 | | 4,706 | | 3,664 | |
|
|
|
|
|
| |
| Adjustments to conform to US GAAP | | | | | | |
| Purchase accounting adjustments (including goodwill and intangibles) | | | | | | |
| Deemed acquisition of Astra | | | | | | |
| Amortisation and other acquisition adjustments | (1,017 | ) | (1,019 | ) | (1,014 | ) |
|
|
|
|
|
| |
| In-process research and development | (502 | ) | – | | – | |
|
|
|
|
|
| |
| Capitalisation, less disposals and amortisation of interest | (21 | ) | (13 | ) | (1 | ) |
|
|
|
|
|
| |
| Deferred taxation | | | | | | |
| On fair values of Astra | 283 | | 283 | | 283 | |
|
|
|
|
|
| |
| Others | (101 | ) | 65 | | 55 | |
|
|
|
|
|
| |
| Pension and other post-retirement benefits expense | (128 | ) | (74 | ) | (52 | ) |
|
|
|
|
|
| |
| Financial instruments | 7 | | (35 | ) | 61 | |
|
|
|
|
|
| |
| In-licensed development intangibles | (193 | ) | (29 | ) | (46 | ) |
|
|
|
|
|
| |
| Other | 21 | | – | | 1 | |
|
|
|
|
|
| |
| Net income in accordance with US GAAP | 4,392 | | 3,884 | | 2,951 | |
|
|
|
|
|
| |
US GAAP CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
| 2006 | | 2005 | | 2004 | |
| For the years ended 31 December | $m | | $m | | $m | |
|
|
|
|
|
| |
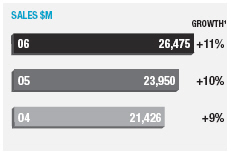
| Sales | 26,475 | | 23,950 | | 21,426 | |
|
|
|
|
|
| |
| Cost of sales | (5,562 | ) | (5,356 | ) | (5,152 | ) |
|
|
|
|
|
| |
| Distribution costs | (226 | ) | (211 | ) | (177 | ) |
|
|
|
|
|
| |
| Research and development | (4,042 | ) | (3,429 | ) | (3,900 | ) |
|
|
|
|
|
| |
| In-process research and development | (502 | ) | – | | – | |
|
|
|
|
|
| |
| Selling, general and administrative expenses | (9,238 | ) | (8,783 | ) | (8,003 | ) |
|
|
|
|
|
| |
| Amortisation of intangibles | (1,007 | ) | (1,009 | ) | (953 | ) |
|
|
|
|
|
|
|
| Other income and expense | 524 | | 193 | | 534 | |
|
|
|
|
|
| |
| Operating income | 6,422 | | 5,355 | | 3,775 | |
|
|
|
|
|
| |
| Net interest income/(expense) | 268 | | 123 | | (1 | ) |
|
|
|
|
|
| |
| Income from continuing operations before taxation | 6,690 | | 5,478 | | 3,774 | |
|
|
|
|
|
| |
| Taxes on income from continuing operations | (2,298 | ) | (1,594 | ) | (823 | ) |
|
|
|
|
|
| |
| Net income from continuing operations | 4,392 | | 3,884 | | 2,951 | |
|
|
|
|
|
| |
| Net income for the year | 4,392 | | 3,884 | | 2,951 | |
|
|
|
|
|
| |
| Weighted average number of $0.25 Ordinary Shares in issue (millions) | 1,564 | | 1,617 | | 1,673 | |
|
|
|
|
|
| |
| Dilutive impact of share options outstanding (millions) | 6 | | 1 | | 2 | |
|
|
|
|
|
| |
| Diluted weighted average number of $0.25 Ordinary Shares (millions) | 1,570 | | 1,618 | | 1,675 | |
|
|
|
|
|
| |
| Net income per $0.25 Ordinary Share and ADS in accordance with US GAAP – basic | $2.81 | | $2.40 | | $1.76 | |
|
|
|
|
|
| |
| Net income per $0.25 Ordinary Share and ADS in accordance with US GAAP – diluted | $2.80 | | $2.40 | | $1.76 | |
|
|
|
|
|
| |
| | | | | | | |
Back to Contents
| 152 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
ADDITIONAL INFORMATION FOR US INVESTORSCONTINUED
US GAAP STATEMENT OF COMPREHENSIVE INCOME
| | 2006 | | 2005 | | 2004 | |
| For the years ended 31 December | $m | | $m | | $m | |
|
|
|
|
|
| |
| Net income for the year | 4,392 | | 3,884 | | 2,951 | |
|
|
|
|
|
| |
| Exchange gains/(losses) net of tax | 2,628 | | (3,279 | ) | 2,106 | |
|
|
|
|
|
| |
| Transitional obligation upon adoption of SFAS No. 158 | (17 | ) | – | | – | |
|
|
|
|
|
| |
| Prior service cost, upon adoption of SFAS No.158 | (89 | ) | – | | – | |
|
|
|
|
|
| |
| Net loss, upon adoption of SFAS No.158 | (1,358 | ) | – | | – | |
|
|
|
|
|
| |
| Recognition of minimum liability | (160 | ) | 181 | | – | |
|
|
|
|
|
| |
| Tax effect of adoption of SFAS No. 158 | 452 | | – | | – | |
|
|
|
|
|
| |
| Other movements, net of tax | (14 | ) | 37 | | 20 | |
|
|
|
|
|
| |
| Total comprehensive income | 5,834 | | 823 | | 5,077 | |
|
|
|
|
|
| |
Tax effects on exchange gains/(losses) were $(77)m and on other movements $44m. The cumulative exchange gains and losses (net of tax) onthe translation of foreign currency financial statements under US GAAP are set out in the following note:
| | | | | | | |
| | 2006 | | 2005 | | 2004 | |
| For the years ended 31 December | $m | | $m | | $m | |
|
|
|
|
|
| |
| Balance at 1 January | 1,063 | | 4,342 | | 2,236 | |
|
|
|
|
|
| |
| Movement in year | 2,628 | | (3,279 | ) | 2,106 | |
|
|
|
|
|
| |
| Balance at 31 December | 3,691 | | 1,063 | | 4,342 | |
|
|
|
|
|
| |
The cumulative total of other movements (net of tax) at 31 December 2006 was a charge of $1,102m (2005 credit of $84m, 2004 charge of $134m).STOCK-BASED COMPENSATION
The Group adopted SFAS No. 123(R) ‘Share-Based Payments’ in the prior year in respect of share options granted and applied its provisions retrospectively. The total compensation cost for non-vested awards not yet recognised at 31 December 2006 was approximately $166m and is expected to be recognised over a weighted average period of 21 months. $985m was received during 2006 from the exercise of share options and similar instruments granted under share-based payment arrangements and $51m tax benefit was realised from share options exercised during the year.
PENSION AND POST-RETIREMENT BENEFITS
For the purposes of US GAAP, the pension in respect of the UK retirement plans and of the retirement plans of the major non-UK subsidiaries has been restated in the following tables in accordance with the requirements of SFAS No. 158 ‘Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans – an amendment of FASB Statements No. 87, 88, 106 and 132(R)’. These plans comprise substantially all of the actuarial liabilities of all AstraZeneca retirement plans. The changes in projected benefit obligations, plan assets and details of the funded status of these retirement plans, together with the changes in the accumulated other post-retirement benefit obligations, under SFAS No. 158 are as follows:
| | Pension benefits | | Other post-retirement benefits | |
| |
| |
| |
| | 2006 | | 2005 | | 2004 | | 2006 | | 2005 | | 2004 | |
| Change in projected benefit obligation | $m | | $m | | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Benefit obligation at beginning of year | 9,047 | | 8,707 | | 7,416 | | 257 | | 249 | | 242 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Service cost | 280 | | 256 | | 229 | | 12 | | 12 | | 11 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Interest cost | 462 | | 419 | | 385 | | 13 | | 14 | | 14 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Participant contributions | 33 | | 31 | | 30 | | – | | 1 | | 1 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Actuarial loss/(gain) | (104 | ) | 764 | | 328 | | 8 | | (1 | ) | (3 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Amendments | 20 | | – | | – | | 56 | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Settlement and curtailment | (290 | ) | – | | 10 | | – | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Benefits paid | (375 | ) | (305 | ) | (281 | ) | (18 | ) | (15 | ) | (18 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Expenses | (10 | ) | – | | – | | 1 | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Exchange | 1,063 | | (825 | ) | 590 | | 6 | | (3 | ) | 2 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Benefit obligation at end of year | 10,126 | | 9,047 | | 8,707 | | 335 | | 257 | | 249 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | | | |
Back to Contents
| ADDITIONAL INFORMATION FOR US INVESTORS | 153 |
PENSION AND POST-RETIREMENT BENEFITS CONTINUED
| | Pension benefits | | Other post-retirement benefits | |
|
| |
| |
| | 2006 | | 2005 | | 2004 | | 2006 | | 2005 | | 2004 | |
| Change in plan assets | $m | | $m | | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Fair value at beginning of year | 7,368 | | 6,972 | | 5,905 | | 230 | | 217 | | 195 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Actual return on plan assets | 284 | | 1,134 | | 565 | | 30 | | 13 | | 22 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Group contribution | 310 | | 165 | | 280 | | 18 | | 13 | | 17 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Participant contributions | 33 | | 31 | | 30 | | – | | 1 | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Settlements | (186 | ) | – | | – | | – | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Benefits paid | (375 | ) | (305 | ) | (281 | ) | (18 | ) | (15 | ) | (17 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Exchange | 887 | | (629 | ) | 473 | | (1 | ) | 1 | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Expenses | (10 | ) | – | | – | | 1 | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Fair value of plan assets at end of year | 8,311 | | 7,368 | | 6,972 | | 260 | | 230 | | 217 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Funded status of plans | (1,815 | ) | (1,679 | ) | (1,735 | ) | (75 | ) | (27 | ) | (32 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Unrecognised net loss | – | | 1,420 | | 1,644 | | – | | 32 | | 29 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Prior service cost not recognised | – | | 25 | | 15 | | – | | (8 | ) | (11 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Unrecognised net obligation on implementation | – | | – | | (1 | ) | – | | 19 | | 25 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| | (1,815 | ) | (234 | ) | (77 | ) | (75 | ) | 16 | | 11 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Adjustments to recognise minimum liability: | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Intangible assets | – | | – | | (36 | ) | – | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Accumulated other comprehensive income | – | | (36 | ) | (217 | ) | – | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Accrued benefit (liability)/asset | (1,815 | ) | (270 | ) | (330 | ) | (75 | ) | 16 | | 11 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| | 2006 – before | | | |
| | adoption of | | | |
| | SFAS No. 158 | | 2005 | |
| Reconciliation of funded status | $m | | $m | |
|
|
|
| |
| Funded status | (1,890 | ) | (1,706 | ) |
|
|
|
| |
| Unrecognised net loss | 1,554 | | 1,452 | |
|
|
|
| |
| Unrecognised prior service cost | 89 | | 17 | |
|
|
|
| |
| Unrecognised transition obligation | 17 | | 19 | |
|
|
|
| |
| Adjustment to recognise minimum liability | (196 | ) | (36 | ) |
|
|
|
| |
| Net amount recognised | (426 | ) | (254 | ) |
|
|
|
| |
| | 2006 | |
| Funded status | $m | |
|
| |
| Projected benefit obligation | (10,461 | ) |
|
| |
| Fair value of plan assets | 8,571 | |
|
| |
| Funded status | (1,890 | ) |
|
| |
| Current liability | – | |
|
| |
| Non-current liabilities | (1,890 | ) |
|
| |
At 31 December 2006, the projected benefit obligation, accumulated benefit obligation and fair value of the plan assets in respect of the pension plans above with accumulated benefit obligations in excess of plan assets were $8,087m, $7,088m and $6,352m (2005 $6,984m, $5,990m and $5,566m), respectively. The total of accumulated benefit obligations for the pension plans was $9,043m (2005 $7,965m). The measurement date for the plan assets and benefit obligations set out above was 31 December 2006. Contributions to the plans in 2007 are estimated to be $266m.
Back to Contents
| 154 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
ADDITIONAL INFORMATION FOR US INVESTORSCONTINUED
PENSION AND POST-RETIREMENT BENEFITS CONTINUED
Assumed discount rates and rates of increase in remuneration used in calculating the projected benefit obligations together with long term rates of return on plan assets vary according to the economic conditions of the country in which the retirement plans are situated. The weighted average rates used for calculation of year end benefit obligations and forecast benefit cost in the retirement plans and other benefit obligations were as follows:
| | | | | | Pension benefits | | Other post-retirement benefits | |
| |
|
|
|
|
| |
|
|
|
|
| |
| | 2006 | | 2005 | | 2004 | | 2006 | | 2005 | | 2004 | |
| | % | | % | | % | | % | | % | | % | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Discount rate | 5.1 | | 4.8 | | 5.2 | | 5.7 | | 5.4 | | 5.7 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Long term rate of increase in remuneration | 4.1 | | 3.8 | | 3.9 | | n/a | | n/a | | n/a | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Expected long term return on assets | 6.7 | | 6.4 | | 6.8 | | 7.5 | | 6.5 | | 7.8 | |
|
|
|
|
|
|
|
|
|
|
|
| |
The Group has assumed a long term rate of increase in healthcare costs of 10%, reducing to 4.9% .
| | | | | | Pension benefits | | | | Other post-retirement benefits | |
| |
|
|
|
|
| |
|
|
| |
| | 2006 | | 2005 | | 2004 | | 2006 | | 2005 | | 2004 | |
| | $m | | $m | | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Net periodic cost | | | | | | | | | | | | |
| Service cost – present value of benefits | | | | | | | | | | | | |
| accruing during the year | 280 | | 256 | | 229 | | 12 | | 12 | | 11 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Interest cost on projected benefit obligations | 462 | | 419 | | 385 | | 13 | | 14 | | 14 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Expected return on assets | (501 | ) | (431 | ) | (406 | ) | (17 | ) | (17 | ) | (15 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Settlements and curtailments | 32 | | – | | – | | – | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Net amortisation and deferral | 118 | | 111 | | 76 | | 6 | | 3 | | 3 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Net periodic cost for the year | 391 | | 355 | | 284 | | 14 | | 12 | | 13 | |
|
|
|
|
|
|
|
|
|
|
|
| |
The weighted average allocation of pension and other post-retirement plan assets was as follows:
| | 2006 | | 2005 | | 2004 | |
| | % | | % | | % | |
|
|
|
|
|
| |
| Equities | 48.6 | | 46.6 | | 48.2 | |
|
|
|
|
|
| |
| Bonds | 33.7 | | 37.5 | | 35.6 | |
|
|
|
|
|
| |
| Other | 17.7 | | 15.9 | | 16.2 | |
|
|
|
|
|
| |
The benefits expected to be paid in the future are as follows:
| | $m | |
|
| |
| 2007 | 370 | |
|
| |
| 2008 | 384 | |
|
| |
| 2009 | 400 | |
|
| |
| 2010 | 414 | |
|
| |
| 2011 | 430 | |
|
| |
| 2012-2016 | 2,431 | |
|
| |
Estimated amount to be amortised from accumulated other comprehensive income into net periodic benefit cost during 2007 are as follows:
| | $m | |
|
| |
| Transition obligation | 1 | |
|
| |
| Prior service cost | 7 | |
|
| |
| Net loss | 120 | |
|
| |
| | | |
Back to Contents
| ADDITIONAL INFORMATION FOR US INVESTORS | 155 |
TAXATION
| | 2006 | | 2005 | | 2004 | |
| For the years ended 31 December | $m | | $m | | $m | |
|
|
|
|
|
| |
| Taxes on income from continuing operations | | | | | | |
| Current tax expense | | | | | | |
| Current year | 2,438 | | 1,747 | | 1,349 | |
|
|
|
|
|
| |
| Adjustment for prior years | 270 | | 112 | | (171 | ) |
|
|
|
|
|
| |
| Deferred tax expense | | | | | | |
| Origination and reversal of temporary differences | (410 | ) | (265 | ) | (355 | ) |
|
|
|
|
|
| |
| Total taxation expense in the income statement | 2,298 | | 1,594 | | 823 | |
|
|
|
|
|
| |
The table below reconciles the UK statutory tax charge with the Group’s actual charge on income from continuing operations.
| | 2006 | | 2005 | | 2004 | |
| For the years ended 31 December | $m | | $m | | $m | |
|
|
|
|
|
| |
| Income from continuing operations | 6,690 | | 5,478 | | 3,774 | |
|
|
|
|
|
| |
| Taxation charge at UK corporation tax rate of 30% for 2006 (30% for 2005, 30% for 2004) | 2,007 | | 1,644 | | 1,132 | |
|
|
|
|
|
| |
| Differences in effective overseas tax rates | (37 | ) | (147 | ) | 2 | |
|
|
|
|
|
| |
| Unrecognised deferred tax asset | (6 | ) | 25 | | 25 | |
|
|
|
|
|
| |
| Items not deductible for tax purposes | 313 | | 136 | | 30 | |
|
|
|
|
|
| |
| Items not chargeable for tax purposes | (109 | ) | (95 | ) | (71 | ) |
|
|
|
|
|
| |
| Adjustments in respect of prior periods | 130 | | 31 | | (171 | ) |
|
|
|
|
|
| |
| Exceptional items | – | | – | | (124 | ) |
|
|
|
|
|
| |
| Tax on income from continuing operations | 2,298 | | 1,594 | | 823 | |
|
|
|
|
|
| |
SHAREHOLDERS’ EQUITY
| | 2006 | | 2005 | | 2004 | |
| | $m | | $m | | $m | |
|
|
|
|
|
| |
| Total shareholders’ equity under adopted IFRS | 15,304 | | 13,597 | | 14,404 | |
|
|
|
|
|
| |
| Adjustments to conform to US GAAP | | | | | | |
| Purchase accounting adjustments (including goodwill and intangibles) | | | | | | |
| Deemed acquisition of Astra | | | | | | |
| Goodwill | 14,765 | | 13,504 | | 15,130 | |
|
|
|
|
|
| |
| Property, plant and equipment and intangible assets | 4,656 | | 5,229 | | 6,988 | |
|
|
|
|
|
| |
| Others | | | | | | |
| Goodwill | (53 | ) | 58 | | 99 | |
|
|
|
|
|
| |
| Property, plant and equipment | (1 | ) | – | | – | |
|
|
|
|
|
| |
| In-process research and development | (605 | ) | – | | – | |
|
|
|
|
|
| |
| Capitalisation, less disposals and amortisation of interest | 220 | | 241 | | 254 | |
|
|
|
|
|
| |
| Deferred taxation | | | | | | |
| On fair value of Astra | (1,485 | ) | (1,629 | ) | (2,134 | ) |
|
|
|
|
|
| |
| On other purchase accounting adjustments | 163 | | – | | – | |
|
|
|
|
|
| |
| Others | (153 | ) | (492 | ) | (618 | ) |
|
|
|
|
|
| |
| In-licensed development intangibles | (309 | ) | (112 | ) | (83 | ) |
|
|
|
|
|
| |
| Pension and other post-retirement benefits | (48 | ) | 1,483 | | 1,418 | |
|
|
|
|
|
| |
| Financial instruments | – | | 18 | | 22 | |
|
|
|
|
|
| |
| Others | 13 | | (3 | ) | (3 | ) |
|
|
|
|
|
| |
| Shareholders’ equity in accordance with US GAAP | 32,467 | | 31,894 | | 35,477 | |
|
|
|
|
|
| |
| | | | | | | |
Back to Contents
| 156 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
ADDITIONAL INFORMATION FOR US INVESTORSCONTINUED
ACQUIRED INTANGIBLE ASSETS AND GOODWILL
Details of the carrying amounts of intangible assets and past and projected amortisation expenses are set out below.
| | | | 2006 | | | | 2005 | | | | 2004 | |
| |
|
|
| |
|
|
| |
|
|
| |
| | Gross carrying | | Accumulated | | Gross carrying | | Accumulated | | Gross carrying | | Accumulated | |
| | amount | | amortisation | | amount | | amortisation | | amount | | amortisation | |
| | $m | | $m | | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Product rights | 14,314 | | (8,846 | ) | 12,961 | | (7,011 | ) | 14,590 | | (6,744 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Marketing and distribution rights | 1,699 | | (1,183 | ) | 1,494 | | (1,043 | ) | 1,729 | | (1,043 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Software | 785 | | (460 | ) | 652 | | (396 | ) | 589 | | (367 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Others | 968 | | (513 | ) | 437 | | (310 | ) | 460 | | (360 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Total | 17,766 | | (11,002 | ) | 15,544 | | (8,760 | ) | 17,368 | | (8,514 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
Aggregate amortisation expense
| | $m | |
|
| |
| For year ended 31 December 2006 | 1,333 | |
|
| |
| For year ended 31 December 2005 | 1,287 | |
|
| |
| For year ended 31 December 2004 | 1,316 | |
|
| |
Estimated amortisation expense
| | $m | |
|
| |
| For year ended 31 December 2007 | 1,234 | |
|
| |
| For year ended 31 December 2008 | 1,234 | |
|
| |
| For year ended 31 December 2009 | 1,234 | |
|
| |
| For year ended 31 December 2010 | 1,234 | |
|
| |
| For year ended 31 December 2011 | 1,234 | |
|
| |
The weighted average amortisation period in respect of each class of intangible asset is as follows:
| Product rights | 13 years | |
| Marketing and distribution rights | 12 years | |
| Software | 4 years | |
| Other | 8 years | |
Goodwill
The changes in the carrying amount of goodwill for the three years ended 31 December 2006 were as follows:
| | $m | |
|
| |
| Balance as at 1 January 2004 | 15,306 | |
|
| |
| Exchange movements | 837 | |
|
| |
| Balance as at 31 December 2004 | 16,143 | |
|
| |
| Exchange movements | (1,737 | ) |
|
| |
| Balance as at 31 December 2005 | 14,406 | |
|
| |
| Exchange movements | 1,281 | |
|
| |
| Balance as at 31 December 2006 | 15,687 | |
|
| |
US GAAP CONDENSED CONSOLIDATED STATEMENT OF CASH FLOWS
There are no significant differences between cash flows under adopted IFRS and US GAAP.
Back to Contents
| GROUP FINANCIAL RECORD | 163 |
GROUP FINANCIAL RECORD – IFRS
| | 2003 | | 2004 | | 2005 | | 2006 | |
| For the year ended 31 December | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
| |
| Turnover and profits | | | | | | | | |
| Sales | 18,849 | | 21,426 | | 23,950 | | 26,475 | |
|
|
|
|
|
|
|
| |
| Cost of sales | (4,463 | ) | (5,193 | ) | (5,356 | ) | (5,559 | ) |
|
|
|
|
|
|
|
| |
| Distribution costs | (162 | ) | (177 | ) | (211 | ) | (226 | ) |
|
|
|
|
|
|
|
| |
| Research and development | (3,012 | ) | (3,467 | ) | (3,379 | ) | (3,902 | ) |
|
|
|
|
|
|
|
| |
| Selling, general and administrative costs | (7,393 | ) | (8,268 | ) | (8,695 | ) | (9,096 | ) |
|
|
|
|
|
|
|
| |
| Other operating income and expense | 188 | | 226 | | 193 | | 524 | |
|
|
|
|
|
|
|
| |
| Operating profit | 4,007 | | 4,547 | | 6,502 | | 8,216 | |
|
|
|
|
|
|
|
| |
| Profit on sale of interest in joint venture | – | | 219 | | – | | – | |
|
|
|
|
|
|
|
| |
| Finance income | 381 | | 532 | | 665 | | 888 | |
|
|
|
|
|
|
|
| |
| Finance expense | (311 | ) | (454 | ) | (500 | ) | (561 | ) |
|
|
|
|
|
|
|
| |
| Profit before tax | 4,077 | | 4,844 | | 6,667 | | 8,543 | |
|
|
|
|
|
|
|
| |
| Taxation | (1,033 | ) | (1,161 | ) | (1,943 | ) | (2,480 | ) |
|
|
|
|
|
|
|
| |
| Profit for the period | 3,044 | | 3,683 | | 4,724 | | 6,063 | |
|
|
|
|
|
|
|
| |
| Attributable to: | | | | | | | | |
| Equity holders of the Company | 3,022 | | 3,664 | | 4,706 | | 6,043 | |
|
|
|
|
|
|
|
| |
| Minority interests | 22 | | 19 | | 18 | | 20 | |
|
|
|
|
|
|
|
| |
| Earnings per share | | | | | | | | |
| Earnings per $0.25 Ordinary Share before exceptional items | $1.77 | | $2.01 | | $2.91 | | $3.86 | |
|
|
|
|
|
|
|
| |
| Earnings per $0.25 Ordinary Share (basic) | $1.77 | | $2.18 | | $2.91 | | $3.86 | |
|
|
|
|
|
|
|
| |
| Earnings per $0.25 Ordinary Share (diluted) | $1.77 | | $2.18 | | $2.91 | | $3.85 | |
|
|
|
|
|
|
|
| |
| Dividends | $0.725 | | $0.835 | | $1.025 | | $1.410 | |
|
|
|
|
|
|
|
| |
| Return on sales | | | | | | | | |
| Operating profit as a percentage of sales | 21.3% | | 21.2% | | 27.2% | | 31.0% | |
|
|
|
|
|
|
|
| |
| Ratio of earnings to fixed charges (IFRS) | 100.4 | | 93.6 | | 85.6 | | 92.7 | |
|
|
|
|
|
|
|
| |
| | | | | | | | | |
| | 2003 | | 2004 | | 2005 | | 2006 | |
| At 31 December | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
| |
| Balance sheet | | | | | | | | |
| Property, plant and equipment and intangible assets | 10,574 | | 11,147 | | 9,697 | | 11,657 | |
|
|
|
|
|
|
|
| |
| Other investments | 133 | | 262 | | 256 | | 119 | |
|
|
|
|
|
|
|
| |
| Deferred tax assets | 1,261 | | 1,218 | | 1,117 | | 1,220 | |
|
|
|
|
|
|
|
| |
| Current assets | 11,593 | | 13,025 | | 13,770 | | 16,936 | |
|
|
|
|
|
|
|
| |
| Total assets | 23,561 | | 25,652 | | 24,840 | | 29,932 | |
|
|
|
|
|
|
|
| |
| Current liabilities | (6,558 | ) | (6,587 | ) | (6,839 | ) | (9,447 | ) |
|
|
|
|
|
|
|
| |
| Non-current liabilities | (3,828 | ) | (4,568 | ) | (4,310 | ) | (5,069 | ) |
|
|
|
|
|
|
|
| |
| Net assets | 13,175 | | 14,497 | | 13,691 | | 15,416 | |
|
|
|
|
|
|
|
| |
| Capital and reserves attributable to equity holders | 13,086 | | 14,404 | | 13,597 | | 15,304 | |
|
|
|
|
|
|
|
| |
| Minority equity interests | 89 | | 93 | | 94 | | 112 | |
|
|
|
|
|
|
|
| |
| Total equity and reserves | 13,175 | | 14,497 | | 13,691 | | 15,416 | |
|
|
|
|
|
|
|
| |
| | | | | | | | | |
| | 2003 | | 2004 | | 2005 | | 2006 | |
| For the year ended 31 December | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
| |
| Cash flows | | | | | | | | |
| Net cash inflow/(outflow) from: | | | | | | | | |
| Operating activities | 3,368 | | 4,817 | | 6,743 | | 7,693 | |
|
|
|
|
|
|
|
| |
| Investing activities | (852 | ) | 970 | | (1,182 | ) | (272 | ) |
|
|
|
|
|
|
|
| |
| Financing activities | (2,674 | ) | (2,761 | ) | (4,572 | ) | (5,366 | ) |
|
|
|
|
|
|
|
| |
| | (158 | ) | 3,026 | | 989 | | 2,055 | |
|
|
|
|
|
|
|
| |
| | | | | | | | | |
Back to Contents
| 164 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
GROUP FINANCIAL RECORD – US GAAP
GROUP FINANCIAL RECORD – US GAAP
The selected financial data set out below, for each of the years in the five year period ended 31 December 2006, have been extracted or derived from the audited Financial Statements.
The selected financial data should be read in conjunction with, and are qualified in their entirety by reference to, the Financial Statements of AstraZeneca and the notes thereto, which are included elsewhere in this document.
| Consolidated income statement data | | | | | | | | | | |
| For the years ended 31 December | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | |
|
|
|
|
|
|
|
|
|
| |
| Net income from operations ($m) | 2,307 | | 2,149 | | 2,951 | | 3,884 | | 4,392 | |
|
|
|
|
|
|
|
|
|
| |
| Net income from operations per $0.25 Ordinary Share | $1.33 | | $1.26 | | $1.76 | | $2.40 | | $2.81 | |
|
|
|
|
|
|
|
|
|
| |
| Diluted income from operations per $0.25 Ordinary Share | $1.33 | | $1.26 | | $1.76 | | $2.40 | | $2.80 | |
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | |
| Ratio of earnings to fixed charges | | | | | | | | | | |
| For the Group, with adjustments to accord with US GAAP | 36.7 | | 77.0 | | 73.5 | | 70.7 | | 73.0 | |
|
|
|
|
|
|
|
|
|
| |
| Consolidated balance sheet data | | | | | | | | | | |
| | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | |
| At 31 December | $m | | $m | | $m | | $m | | $m | |
|
|
|
|
|
|
|
|
|
| |
| Total assets | 42,660 | | 45,483 | | 47,690 | | 43,757 | | 48,600 | |
|
|
|
|
|
|
|
|
|
| |
| Shareholders’ equity | 30,265 | | 33,759 | | 35,477 | | 31,894 | | 32,467 | |
|
|
|
|
|
|
|
|
|
| |
Merger accounting
For the purpose of US GAAP, the merger has been regarded as a purchase accounting acquisition of Astra by Zeneca.
Ratio of earnings to fixed charges (IFRS and US GAAP)
For the purpose of computing these ratios, earnings consist of the income from continuing ordinary activities before taxation of Group companies and income received from companies owned 50% or less, plus fixed charges (excluding capitalised interest). Fixed charges consist of interest (including capitalised interest) on all indebtedness, amortisation of debt discount and expense and that portion of rental expense representative of the interest factor.
Back to Contents
| SHAREHOLDER INFORMATION | 165 |
SHAREHOLDER INFORMATION
| AstraZeneca | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | |
|
|
|
|
|
|
|
|
|
| |
| Ordinary Shares in issue– millions | | | | | | | | | | |
| At year end | 1,719 | | 1,693 | | 1,645 | | 1,581 | | 1,532 | |
|
|
|
|
|
|
|
|
|
| |
| Weighted average for year | 1,733 | | 1,709 | | 1,673 | | 1,617 | | 1,564 | |
|
|
|
|
|
|
|
|
|
| |
| Stock market price – per $0.25 Ordinary Share | | | | | | | | | | |
| Highest (pence) | 3625 | | 2868 | | 2749 | | 2837 | | 3529 | |
|
|
|
|
|
|
|
|
|
| |
| Lowest (pence) | 1799 | | 1820 | | 1863 | | 1861 | | 2574 | |
|
|
|
|
|
|
|
|
|
| |
| At year end (pence) | 2220 | | 2680 | | 1889 | | 2829 | | 2744 | |
|
|
|
|
|
|
|
|
|
| |
| Percentage analysis at 31 December 2006 of issued share capital | | |
| By size of account | 2006 | |
| No. of shares | % | |
|
| |
| 1– 250 | 0.5 | |
|
| |
| 251– 500 | 0.7 | |
|
| |
| 501 – 1,000 | 0.9 | |
|
| |
| 1,001 – 5,000 | 1.3 | |
|
| |
| 5,001 – 10,000 | 0.2 | |
|
| |
| 10,001 – 50,000 | 1.0 | |
|
| |
| 50,001 – 1,000,000 | 12.3 | |
|
| |
| over 1,000,000† | 83.1 | |
|
| |
| Issued share capital | 100.0 | |
|
| |
| | | |
| † Includes VPC and ADR holdings | | |
At 31 December 2006, AstraZeneca PLC had 137,137 registered holders of 1,532,245,608 Ordinary Shares of $0.25 each. At 31 December 2006, there were approximately 100,000 holders of American Depositary Receipts (ADRs) representing 10.48% of the issued share capital and 157,000 holders of shares held under the VPC Services Agreement representing 23.32% of the issued share capital. The ADRs, each of which is equivalent to one Ordinary Share, are issued by JPMorgan Chase Bank.
ASTRAZENECA PLC
Since April 1999, following the AstraZeneca merger, the principal markets for trading in the shares of AstraZeneca PLC are the London, Stockholm and New York Stock Exchanges. The table below sets out, for the four quarters of 2005 and for the first two quarters and last six months of 2006 the reported high and low share prices of AstraZeneca PLC, on the following bases:
| > | For shares listed on the London Stock Exchange (‘LSE’) the reported high and low middle market closing quotations are derived from TheDaily Official List. |
| > | For shares listed on the Stockholm Stock Exchange (‘SSE’) the high and low closing sales prices are as stated in the Official List. |
| > | For American Depositary Shares (‘ADS’) listed on the New York Stock Exchange the reported high and low sales prices are as reported byDow Jones (ADR quotations). |
| | | Ordinary LSE | | ADS | | Ordinary SSE | * |
| | |
|
|
| |
|
|
| |
|
|
| |
| | | High | | Low | | High | | Low | | High | | Low | |
| | | (pence | ) | (pence | ) | (US$ | ) | (US$ | ) | (SEK | ) | (SEK | ) |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| 2005 | – Quarter 1 | 2201 | | 1861 | | 42.12 | | 34.72 | | 288.5 | | 243.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 2 | 2363 | | 2081 | | 45.06 | | 39.29 | | 324.5 | | 279.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 3 | 2668 | | 2311 | | 49.10 | | 40.68 | | 370.5 | | 319.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 4 | 2837 | | 2485 | | 49.50 | | 44.43 | | 392.0 | | 349.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| 2006 | – Quarter 1 | 2975 | | 2574 | | 51.73 | | 45.12 | | 403.5 | | 352.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 2 | 3264 | | 2757 | | 59.82 | | 50.54 | | 434.5 | | 376.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – July | 3320 | | 3101 | | 62.00 | | 56.60 | | 450.5 | | 414.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – August | 3404 | | 3183 | | 65.14 | | 60.13 | | 467.5 | | 432.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – September | 3435 | | 3292 | | 65.43 | | 61.35 | | 477.0 | | 447.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – October | 3529 | | 3098 | | 66.37 | | 58.70 | | 484.0 | | 428.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – November | 3226 | | 2919 | | 61.40 | | 56.24 | | 441.5 | | 388.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – December | 2925 | | 2728 | | 57.78 | | 53.55 | | 394.0 | | 365.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | | | |
| * Principally held in bearer form | | | | | | | | | | | | |
Back to Contents
| 166 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
SHAREHOLDER INFORMATIONCONTINUED
During 2006, AstraZeneca’s share re-purchase programme, which was introduced in 1999, continued with the re-purchase and subsequent cancellation of 72.2 million shares at a total cost of $4,147m, representing 4.7% of the total issued share capital of the Company. The average price paid per share in 2006 was 3059 pence. Between 1999 and 2005, a total of 210.6 million Ordinary Shares were re-purchased, and subsequently cancelled, at an average price of 2568 pence per share for a consideration, including expenses, of $9,172m. The excess of the consideration over the nominal value was charged against the profit and loss account reserve. Shares issued in respect of share schemes totalled 23.5 million.
In 1999, in connection with the merger, AstraZeneca’s share capital was redenominated in US dollars. On 6 April 1999, Zeneca shares were cancelled and US dollar shares issued, credited as fully paid on the basis of one dollar share for each Zeneca share then held. This was achieved by a reduction of capital under section 135 of the Companies Act 1985. Upon the reduction of capital becoming effective, all issued and unissued Zeneca shares were cancelled and the sum arising as a result thereof credited to a special reserve, which was converted into US dollars at the rate of exchange prevailing on the record date. This US dollar reserve was then applied in paying up, at par, newly created US dollar shares.
At the same time as the US dollar shares were issued, the Company issued 50,000 Redeemable Preference Shares with a nominal value of £1.00 each for cash at par. The Redeemable Preference Shares carry limited class voting rights and no dividend rights. This class of shares is also capable of redemption at par at the option of the Company on the giving of seven days’ written notice to the registered holder of the shares.
A total of 826 million AstraZeneca shares were issued to Astra shareholders who accepted the merger offer before the final closing date, 21 May 1999. AstraZeneca received acceptances from Astra shareholders representing 99.6% of Astra’s shares and the remaining 0.4% was acquired in 2000 for cash.
MAJOR SHAREHOLDINGS
At 31 January 2007, the following had disclosed an interest in the issued Ordinary Share capital of the Company in accordance with the requirements of sections 198-208 of the Companies Act 1985:
| | | | Date of | | Percentage | |
| | | | disclosure | | of issued | |
| Shareholder | Number of shares | | to Company | * | share capital | |
|
|
|
|
|
| |
| The Capital Group Companies, Inc. | 179,266,829 | | 15 Dec 2006 | | 11.70% | |
|
|
|
|
|
| |
| Investor AB | 63,465,810 | | 11 Feb 2004 | | 4.14% | |
|
|
|
|
|
| |
| Barclays PLC | 61,721,820 | | 18 Dec 2006 | | 4.03% | |
|
|
|
|
|
| |
| Wellington Management Co., LLP | 60,565,299 | | 30 Oct 2006 | | 3.95% | |
|
|
|
|
|
| |
| Legal & General Investment Management Limited | 52,518,020 | | 13 Jun 2002 | | 3.43% | |
|
|
|
|
|
| |
| * | Since the date of disclosure to the Company, the interest of any person listed above in the Ordinary Shares of the Company may have increased or decreased. No requirement to notify the Company of any increase or decrease would have arisen unless the holding moved up or down through a whole number percentage level. The percentage level may increase (on the cancellation of shares following a re-purchase of shares under the Company’s share re-purchase programme) or decrease (on the issue of new shares under any of the Company’s share plans). |
No other person held a notifiable interest in shares, comprising 3% or more of the issued Ordinary Share capital of the Company, appearing in the register of interests in shares maintained under the provisions of section 211 of the Companies Act 1985.
Changes in the percentage ownership held by major shareholders during the past three years are set out below. Major shareholders do not have different voting rights.
| | Percentage of issued share capital | |
|
|
|
|
|
| |
| Shareholder | 31 Jan 2007 | | 31 Jan 2006 | | 26 Jan 2005 | | 28 Jan 2004 | |
|
|
|
|
|
|
|
| |
| The Capital Group Companies, Inc. | 11.70% | | 12.57% | | 13.39% | | 15.01% | |
|
|
|
|
|
|
|
| |
| Investor AB | 4.14% | | 4.01% | | 3.86% | | 5.41% | |
|
|
|
|
|
|
|
| |
| Barclays PLC | 4.03% | | 3.20% | | 3.08% | | <3.00% | |
|
|
|
|
|
|
|
| |
| Wellington Management Co., LLP | 3.95% | | 4.97% | | 3.25% | | <3.00% | |
|
|
|
|
|
|
|
| |
| Legal & General Investment Management Limited | 3.43% | | 3.32% | | 3.19% | | 3.10% | |
|
|
|
|
|
|
|
| |
AstraZeneca PLC American Depositary Shares (each representing one Ordinary Share) evidenced by American Depositary Receipts issued by JPMorgan Chase Bank, as depositary, are listed on the New York Stock Exchange. At 31 January 2007, the proportion of Ordinary Shares represented by American Depositary Shares was 10.34% of the Ordinary Shares outstanding.
| Number of registered holders of Ordinary Shares at 31 January 2007: |
| > In the US | 816 | |
| > Total | 136,672 | |
| |
| |
| Number of record holders of American Depositary Receipts at 31 January 2007: |
| > In the US | 2,533 | |
| > Total | 2,571 | |
So far as the Company is aware, it is neither directly nor indirectly owned nor controlled by one or more corporations or by any government.
Back to Contents
| SHAREHOLDER INFORMATION | 167 |
At 31 January 2007, the total amount of the Company’s voting securities owned by Directors and Officers of the Company was:
| Title of class | Amount owned | | Percentage of class | |
|
|
|
| |
| Ordinary Shares | 322,601 | | 0.02% | |
|
|
|
| |
The Company does not know of any arrangements, the operation of which might result in a change in the control of the Company.
RELATED PARTY TRANSACTIONS
During the period 1 January 2007 to 31 January 2007, there were no transactions, loans, or proposed transactions between the Company and any related parties which were material to either the Company or the related party, or which were unusual in their nature or conditions (see also Note 28).
| OPTIONS TO PURCHASE SECURITIES FROM REGISTRANT OR SUBSIDIARIES |
| (a) | At 31 January 2007, options outstanding to subscribe for Ordinary Shares of $0.25 of the Company were: |
| | | | |
| Number of shares | Subscription price | Normal expiry date | |
|
|
| |
| 44,547,783 | 1740p-3487p | 2007-2016 | |
|
|
| |
The weighted average subscription price of options outstanding at 31 January 2007 was 2675p. All options were granted under Company employee share schemes.
| (b) | Included in paragraph (a) are options granted to Directors and Officers of AstraZeneca as follows: |
| | | | |
| Number of shares | Subscription price | Normal expiry date | |
|
|
| |
| 2,265,118 | 1740p-3487p | 2007-2016 | |
|
|
| |
| | |
| (c) | Included in paragraph (b) are options granted to individually named Directors. Details of these option holdings at 31 December 2006 are shown in the Directors’ Remuneration Report. |
| |
| | During the period 1 January 2007 to 31 January 2007, no Director exercised any options. |
| |
DIVIDEND PAYMENTS
The record date for the second interim dividend for 2006, payable on 19 March 2007 (in the UK, the US and Sweden), is 9 February 2007. Shares trade ex-dividend on the London and Stockholm Stock Exchanges from 7 February 2007 and ADRs trade ex-dividend on the New York Stock Exchange from the same date. Dividends will normally be paid as follows:
| First interim: | Announced in July and paid in September. |
| Second interim: | Announced in January/February and paid in March. |
The record date for the first interim dividend for 2007, payable on 17 September 2007 (in the UK, the US and Sweden), is 10 August 2007.SHAREVIEW
AstraZeneca’s shareholders with internet access may visit shareview.co.uk and register their details to create a portfolio. Shareview is a free and secure on-line service from the Company’s registrars, Lloyds TSB Registrars,which gives access to shareholdings including balance movements, indicative share prices and information about recent dividends.
SHAREGIFT
AstraZeneca welcomes and values all of its shareholders, no matter how many or how few shares they own. However, shareholders who have only a small number of shares whose value makes it uneconomic to sell them, either now or at some stage in the future, may wish to consider donating them to charity through ShareGift, an independent charity share donation scheme. One feature of the scheme is that there is no gain or loss for UK capital gains tax purposes on gifts of shares through ShareGift, and it may now also be possible to obtain UK income tax relief on the donation. Further information about ShareGift can be found on its website, sharegift.org, or by contacting ShareGift on 020 7337 0501 or at 46 Grosvenor Street, London W1K 3HN. More information about the UK tax position on gifts of shares to ShareGift can be obtained from HM Revenue & Customs, whose website address is hmrc.gov.uk. The share transfer form needed to make a donation may be obtained from the Company’s registrars, Lloyds TSB Registrars, whose address can be found on the back cover of this document. ShareGift is administered by The Orr Mackintosh Foundation, registered charity number 1052686.
Back to Contents
| 168 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
SHAREHOLDER INFORMATION CONTINUED
THE UNCLAIMED ASSETS REGISTER
AstraZeneca supplies unclaimed dividend data to the Unclaimed Assets Register (UAR), which provides investors who have lost track of shareholdings with an opportunity to search the UAR’s database of unclaimed financial assets on payment of a small, fixed fee. The UAR donates part of the search fee to charity. The UAR can be contacted on 0870 2411713 or at 6th Floor, Cardinal Place, 80 Victoria Street, Victoria, London SW1E 5JL.
RESULTS
Unaudited trading results of AstraZeneca in respect of the first three months of 2007 will be published on 26 April 2007 and results in respect of the first six months of 2007 will be published on 26 July 2007.
DOCUMENTS ON DISPLAY
The Memorandum and Articles of Association of the Company and other documents concerning the Company which are referred to in this document may be inspected at the Company’s registered office at 15 Stanhope Gate, London W1K 1LN.
TAXATION FOR US RESIDENTS
The following summary of the material UK and US federal income tax consequences of ownership of Ordinary Shares or ADRs held as capital assets by US resident shareholders is based on current UK and US federal income tax law, including the US/UK double taxation convention relating to income and capital gains, which entered into force on 31 March 2003 (the “Convention”) and practice. This discussion is also based in part on representations of JPMorgan Chase Bank as Depositary for ADRs and assumes that each obligation in the deposit agreement among the Company, the Depositary and the holders from time to time of ADRs and any related agreements will be performed in accordance with its terms. The US Treasury has expressed concerns that parties to whom ADRs are pre-released may be taking actions that are inconsistent with the claiming, by US holders of ADRs, of foreign tax credits for US federal income tax purposes. Such actions would also be inconsistent with the claiming of the reduced tax rate, described below, applicable to dividends received by certain non-corporate US resident shareholders. Accordingly, the availability of the reduced tax rate for dividends received by certain non-corporate US resident shareholders could be affected by actions that may be taken by parties to whom ADRs are pre-released.
This discussion assumes that we are not, and will not become, a passive foreign investment company (PFIC), as discussed below.
UK AND US INCOME TAXATION OF DIVIDENDS
The UK does not currently impose a withholding tax on dividends paid by a UK company, such as the Company.
For US federal income tax purposes, distributions paid by the Company to a US resident shareholder are includible in gross income as foreign source ordinary dividend income to the extent of the Company’s current or accumulated earnings and profits, calculated in accordance with US federal income tax principles. The amount of the dividend will be the US dollar value of the pounds sterling received on the date the dividend is received by the Depositary for US resident holders of ADRs (or in the case of Ordinary Shares, received by the US resident shareholders) regardless of whether the dividend is converted into US dollars. If the dividend is converted into US dollars on the date of receipt, US resident shareholders generally should not be required to recognise foreign currency gain or loss in respect of the dividend income. They may have foreign currency gain or loss if the amount of such dividend is not converted into US dollars on the date of its receipt.
Subject to applicable limitations and the discussion above regarding concerns expressed by the US Treasury, dividends received by certain non-corporate US resident holders of Ordinary Shares or ADRs in taxable years beginning before 1 January 2011 may be subject to US federal income tax at a maximum rate of 15%. US resident shareholders should consult their own tax advisers to determine whether they are subject to any special rules which may limit their ability to be taxed at this favourable rate.
TAXATION ON CAPITAL GAINS
Under the Convention, each contracting state may in general tax capital gains in accordance with the provisions of its domestic law. Under present UK law, individuals who are neither resident nor ordinarily resident in the UK, and companies which are not resident in the UK, will not be liable to UK tax on capital gains made on the disposal of their Ordinary Shares or ADRs, unless such Ordinary Shares or ADRs are held in connection with a trade, profession or vocation carried on in the UK through a branch or agency.
A US resident shareholder will generally recognise US source capital gain or loss for US federal income tax purposes on the sale or exchange of Ordinary Shares or ADRs in an amount equal to the difference between the US dollar amount realised and such holder’s US dollar adjusted tax basis in the Ordinary Shares or ADRs. US resident shareholders should consult their own tax advisers about the treatment of capital gains, which may be taxed at lower rates than ordinary income for non-corporate US resident shareholders and capital losses, the deductibility of which may be limited.
Back to Contents
| SHAREHOLDER INFORMATION | 169 |
PASSIVE FOREIGN INVESTMENT COMPANY RULES
We believe that we were not a passive foreign investment company (PFIC) for US federal income tax purposes for the year ended 31 December 2006, and do not expect to be a PFIC in the foreseeable future. However, since PFIC status depends on the composition of our income and assets and the market value of our assets (including, among others, less than 25%-owned equity investments) from time to time, there can be no assurance that we will not be considered a PFIC for any taxable year. If we were treated as a PFIC for any taxable year during which you held Ordinary Shares or ADRs, certain adverse tax consequences could apply to US resident shareholders.
UK INHERITANCE TAX
Under the current Double Taxation (Estates) Convention (the “Estate Tax Convention”) between the US and the UK, Ordinary Shares or ADRs held by an individual shareholder who is domiciled for the purposes of the Estate Tax Convention in the US, and is not for the purposes of the Estate Tax Convention a national of the UK, will generally not be subject to UK inheritance tax on the individual’s death or on a chargeable gift of the Ordinary Shares or ADRs during the individual’s lifetime, provided that any applicable US federal gift or estate tax liability is paid, unless the Ordinary Shares or ADRs are part of the business property of a permanent establishment of the individual in the UK or, in the case of a shareholder who performs independent personal services, pertain to a fixed base situated in the UK. Where the Ordinary Shares or ADRs have been placed in trust by a settlor who, at the time of settlement, was a US resident shareholder, the Ordinary Shares or ADRs will generally not be subject to UK inheritance tax unless the settlor, at the time of settlement, was not domiciled in the US and was a UK national. In the exceptional case where the Ordinary Shares or ADRs are subject to both UK inheritance tax and US federal gift or estate tax, the Estate Tax Convention generally provides for double taxation to be relieved by means of credit relief.
UK STAMP DUTY RESERVE TAX AND STAMP DUTY
A 1.5% stamp duty reserve tax is payable upon the deposit of Ordinary Shares in connection with the creation of, but not subsequent dealing in, ADRs. A 0.5% stamp duty is payable on all purchases of Ordinary Shares.
EXCHANGE CONTROLS AND OTHER LIMITATIONS AFFECTING SECURITY HOLDERS
There are no governmental laws, decrees or regulations in the UK restricting the import or export of capital or affecting the remittance of dividends, interest or other payments to non-resident holders of Ordinary Shares or ADRs.
There are no limitations under English law or the Company’s Memorandum and Articles of Association on the right of non-resident or foreign owners to be the registered holders of and to vote Ordinary Shares or ADRs or to be registered holders of notes or debentures of Zeneca Wilmington Inc. or AstraZeneca PLC.
Back to Contents
| 170 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
SHAREHOLDER INFORMATIONCONTINUED
EXCHANGE RATES
For the periods up to April 1999, Astra accounted for and reported its results in Swedish kronor, whereas Zeneca accounted for and reported its results in sterling. Consistent with AstraZeneca’s decision to publish its Financial Statements in US dollars, the financial information in this document has been translated from kronor and sterling into US dollars at the following applicable exchange rates:
| | SEK/USD | | USD/GBP | |
|
|
|
| |
| Average rates (profit and loss account, cash flow) | | | | |
| 1995 | 7.1100 | | 1.5796 | |
|
|
|
| |
| 1996 | 6.7000 | | 1.5525 | |
|
|
|
| |
| 1997 | 7.6225 | | 1.6386 | |
|
|
|
| |
| 1998 | 7.9384 | | 1.6603 | |
|
|
|
| |
| 1999 | 8.2189 | | 1.6247 | |
|
|
|
| |
| | | | | |
| End of year spot rates (balance sheet) | | | | |
| 1995 | 6.6500 | | 1.5500 | |
|
|
|
| |
| 1996 | 6.8400 | | 1.6900 | |
|
|
|
| |
| 1997 | 7.8500 | | 1.6600 | |
|
|
|
| |
| 1998 | 8.0400 | | 1.6600 | |
|
|
|
| |
| 1999 | 8.5130 | | 1.6185 | |
|
|
|
| |
The following information relating to average and spot exchange rates used by AstraZeneca is provided for convenience:
| | SEK/USD | | USD/GBP | |
|
|
|
| |
| Average rates (income statement, cash flow) | | | | |
| 2004 | 7.4613 | | 1.8031 | |
|
|
|
| |
| 2005 | 7.3878 | | 1.8306 | |
|
|
|
| |
| 2006 | 7.4472 | | 1.8265 | |
|
|
|
| |
| | | | | |
| End of year spot rates (balance sheet) | | | | |
| 2004 | 6.6144 | | 1.9264 | |
|
|
|
| |
| 2005 | 7.9464 | | 1.7239 | |
|
|
|
| |
| 2006 | 6.8824 | | 1.9626 | |
|
|
|
| |
Back to Contents
| SHAREHOLDER INFORMATION | 171 |
DEFINITIONS AND INTERPRETATION
Figures in parentheses in tables and in the Financial Statements are used to represent negative numbers.
Except where otherwise indicated, figures included in this report relating to pharmaceutical product market sizes and market shares are obtained from syndicated industry sources, primarily IMS Health (IMS), a market research firm internationally recognised by the pharmaceutical industry. The 2006 market share figures included in this report are based primarily on data obtained from an online IMS database.
IMS data may differ from that compiled by the Group with respect to its own products. Of particular significance in this regard are the following: (1) AstraZeneca publishes its financial results on a financial year and quarterly interim basis, whereas IMS issues its data on a monthly and quarterly basis; (2) the online IMS database is updated quarterly and uses the average exchange rates for the relevant quarter; (3) IMS data from the US is not adjusted for Medicaid and similar state rebates; and (4) IMS sales data are compiled using actual wholesaler data and data from statistically representative panels of retail and hospital pharmacies, which data are then projected by IMS to give figures for national markets.
References to prevalence of disease have been derived from a variety of sources and are not intended to be indicative of the current market or any potential market for AstraZeneca’s pharmaceutical products since, among other things, there may be no correlation between the prevalence of a disease and the number of individuals who are treated for such a disease.
| Terms used in the Annual Report | |
| and Form 20-F Information | US equivalent or brief description |
| Accruals | Accrued expenses |
|
|
| Allotted | Issued |
|
|
| Bank borrowings | Payable to banks |
|
|
| Called-up share capital | Issued share capital |
|
|
| Creditors | Liabilities/payables |
|
|
| Current instalments of loans | Long term debt due within one year |
|
|
| Debtors | Receivables and prepaid expenses |
|
|
| Earnings | Net income |
|
|
| Finance lease | Capital lease |
|
|
| Fixed asset investments | Non-current investments |
|
|
| Freehold | Ownership with absolute rights in perpetuity |
|
|
| Interest receivable | Interest income |
|
|
| Interest payable | Interest expense |
|
|
| Loans | Long term debt |
|
|
| Prepayments | Prepaid expenses |
|
|
| Profit | Income |
|
|
| Profit and loss account | Income statement/consolidated statement of income |
|
|
| Reserves | Retained earnings |
|
|
| Short term investments | Redeemable securities and short term deposits |
|
|
| Share premium account | Premiums paid in excess of par value of Ordinary Shares |
|
|
| Statement of recognised | |
| income and expense | Statement of comprehensive income |
|
|
Back to Contents
| 172 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
RISK FACTORS
RISKS ASSOCIATED WITH
FORWARD-LOOKING STATEMENTS
This report contains certain forward-looking statements about AstraZeneca. Although we believe our expectations are based on reasonable assumptions, any forward-looking statements may be influenced by factors that could cause actual outcomes and results to be materially different from those predicted. Forward-looking statements are identified in this report by using the words ‘anticipates’, ‘believes’, ‘expects’, ‘intends’ and similar expressions. These forward-looking statements are subject to numerous risks and uncertainties. Important factors that could cause actual results to differ materially from those in forward-looking statements, certain of which are beyond our control, include, among other things: the loss or expiration of patents, marketing exclusivity or trade marks; the risk of substantial adverse litigation/government investigation claims and insufficient insurance coverage; exchange rate fluctuations; the risk that R&D will not yield new products that achieve commercial success; the risk that strategic alliances will be unsuccessful; the impact of competition, price controls and price reductions; taxation risks; the risk of substantial product liability claims; the impact of any failure by third parties to supply materials or services; the risk of failure to manage a crisis; the risk of delay to new product launches; the difficulties of obtaining and maintaining regulatory approvals for products; the risk of failure to observe ongoing regulatory oversight; the risk that new products do not perform as we expect; the risk of environmental liabilities; the risks associated with conducting business in emerging markets; the risk of reputational damage; and the risk of product counterfeiting.
RISK OF EXPIRATION OF PATENTS, MARKETING EXCLUSIVITY OR TRADE MARKS
Scientific development and technological innovation are crucial if AstraZeneca is to deliver long-term market success. In the pharmaceutical market, a drug, diagnostic or medical device is normally only subject to competition from alternative products, for the same use, during the period of patent protection or other types of marketing exclusivity. Once patent protection or other types of marketing exclusivity have expired the product is generally open to competition from generic copy products. Products under patent protection or other types of marketing exclusivity usually generate significantly higher revenues than those not protected by patents or other types of marketing exclusivity.
For example, during 2004 compared to 2003 and, to a lesser extent, during 2005 compared to 2004, sales in the US ofLosec/Prilosec,Plendil,ZestrilandNolvadexfell significantly following anticipated patent expiries or the end of marketing exclusivity.
We believe that we have robust patent protection for many of our most important products.
Trade mark protection for our products is also an important element of our overall product marketing programmes. Combined with patent protection or other types of marketing exclusivity, products protected by a valid trade mark usually generate higher revenues than those not protected by a trade mark. We believe that we have trade mark protection for many of our most important products. However, trade mark protection may be challenged by third parties.
RISK OF PATENT LITIGATION AND EARLY LOSS OF PATENTS, MARKETING EXCLUSIVITY OR TRADE MARKS
Over the last few years there has been a marked increase in intellectual property litigation. Increasingly, manufacturers of generic pharmaceutical products, whether based in developing countries, such as those in Asia, or elsewhere in the world, seek to challenge our patents or other types of marketing exclusivity in order to gain access to the market for their own generic products. Furthermore, in addition to generic manufacturers, the research-based industry has become more aggressive in recent years in using intellectual property rights offensively as an additional basis for commercial competition between patented products. This has included the use of patent litigation directed at relatively young products and in the case of litigation both by generic manufacturers and other research-based companies, it is to be expected that the greatest challenges will be focused on the most valuable products.
Parts of our technology, techniques and proprietary compounds and potential candidate drugs, including those which are in-licensed, may be found to infringe patents owned by or granted to others. This risk may increase as our focus on biopharmaceuticals increases, as intellectual property questions related to biological medicines can be extremely complex. If we cannot resolve any intellectual property disputes, we may be liable for damages, be required to obtain costly licences or be stopped from manufacturing, using or selling our products. During the
course of our activities, we may become aware of broad patents owned by others relating to some of our intellectual property, and in some instances we may receive notices from the owners of patents claiming that their patents may be infringed by the development, manufacture or sale of some of our products and candidate drugs. In response, we may obtain licences, determine that our products do not infringe the patents or that the patents are not valid, or we may make various modifications that we believe should not infringe the patents and that should permit commercialisation of our products.
There can be no assurance that any of our currently patented products will not be the subject of intellectual property litigation in the future, despite our efforts to establish and defend the most robust patent protection. There can be no assurance that we would prevail in a patent infringement action; will be able to obtain a licence to any third party patent on commercially reasonable terms; successfully develop non-infringing alternatives on a timely basis; license alternative non-infringing technology, if any exists, on commercially reasonable terms; or whether patent protection is available at all. If we are not successful during the patent protection or data exclusivity periods in maintaining exclusive rights to market one or more of our major products, particularly in the US where we have our highest revenue and margins, our revenue and margins would be adversely affected.
For example, we were involved in litigation in the US and elsewhere during 2005 relating to omeprazole, the active ingredient inLosec/ Prilosec, and in the US, relating to metoprolol succinate, the active ingredient inToprol–XL, concerning the infringement of certain patents, including formulation patents, by generic manufacturers. In January 2006, the US District Court for the Eastern District of Missouri issued a decision holding that certain of our US compound and composition patents relating to metoprolol succinate are unenforceable and invalid. We appealed the District Court decision to the US Court of Appeals for the Federal Circuit. Also, during 2005, certain generic manufacturers filed Abbreviated New Drug Applications (ANDAs) with the US Food and Drug Administration containing paragraph IV certifications alleging invalidity and non-infringement in respect of certain of our patents relating toNexium,Pulmicort RespulesandSeroquel. Following filing of the ANDAs, we commenced patent infringement proceedings against such manufacturers.
Back to Contents
The more significant patent litigation relating to our products is described in Note 26 to the Financial Statements.
In addition to challenges to our patented products from manufacturers of generic or other patented pharmaceutical products, there is a risk that some countries, particularly those in the developing world, may seek to impose limitations on the availability of patent protection for pharmaceutical products, or on the extent to which such protection may be obtained, within their jurisdictions.
Limitations on the availability of patent protection in developing countries or the expiration or loss of certain patents, marketing exclusivity or trade marks would have an adverse effect on pricing and sales with respect to these products and, consequently, could result in a material adverse effect on our financial condition and results of operations.
RISK OF SUBSTANTIAL ADVERSE OUTCOMES OF LITIGATION AND GOVERNMENT INVESTIGATIONS AND INSUFFICIENT INSURANCE COVERAGE
See Note 26 of the Financial Statements for a discussion of proceedings in which the Group is currently involved. Unfavourable resolution of these and similar future proceedings, including government investigations and securities class action law suits, may have a material adverse effect on the Group’s financial results, not least because the Group may be required to make significant provisions in its accounts related to legal proceedings and/or governmental investigations, which would reduce earnings. In many cases, the practice of the plaintiff bar is to claim damages – compensatory, punitive and statutory – in amounts that may not bear any relation to the underlying harm. Accordingly, it is difficult to quantify the potential exposure to claims in proceedings of the type referred to in Note 26. Recent insurance loss experience, including pharmaceutical product liability exposures, has increased the cost of, and narrowed the coverage afforded by, pharmaceutical companies’ insurance generally. In order to contain insurance costs in recent years, the Group has continued to adjust its coverage profile, accepting a greater degree of uninsured exposure. In addition, where claims are made under insurance policies, insurers may reserve the right to deny coverage on various grounds. If denial of coverage is ultimately upheld on these claims, this could result in material additional charges to the Group’s earnings.
IMPACT OF FLUCTUATIONS IN EXCHANGE RATES
The results of AstraZeneca’s operations are accounted for in US dollars. Approximately 51% of our 2006 sales were in North America (comprised of the US and Canada) with a significant proportion of that figure being in respect of US sales. The US is, and is expected to remain, our largest market. Sales in certain other countries are also in US dollars, or in currencies whose exchange rates are linked to the US dollar. Major components of our cost base are, however, located in Europe, where an aggregate of approximately 58% of our employees are based. Movements in the exchange rates used to translate foreign currencies into US dollars may therefore have a material adverse effect on AstraZeneca’s financial condition and results of operations.
Certain subsidiaries of AstraZeneca import and export goods and services in currencies other than their own functional currency. The results of such subsidiaries could, therefore, be affected by currency fluctuations arising between the transaction dates and the settlement dates for those transactions. We hedge these exposures through financial instruments. The fair value of financial instruments used to hedge these exposures, principally forward foreign exchange contracts and purchased currency options, at 31 December 2006 was $45m. We have policies that seek to mitigate the effect of exchange rate fluctuations on the value of foreign currency cash flows and in turn their effects on the results of the various subsidiaries, but we do not seek to remove all such risks. See Financial Review – Financial Risk Management Policies – Foreign exchange on page 60. In general, a unilateral strengthening of the US dollar adversely affects our reported results whereas a weakening of the US dollar is generally favourable. We cannot ensure that exchange rate fluctuations will not have a material adverse effect on AstraZeneca’s financial condition and results of operations in the future.
RISK THAT R&D WILL NOT YIELD NEW PRODUCTS THAT ACHIEVE COMMERCIAL SUCCESS
The development of new products involves the commitment of substantial effort, funds and other resources to research and development activities, and also involves a high degree of risk and can take many years. Our product development efforts with respect to any product candidate may fail, and we may ultimately be unable to achieve commercial success for any number of reasons, including:
| > | Difficulty enrolling patients in clinical trials. |
| | |
| > | Our failure to obtain the required regulatoryapprovals for the product candidate or thefacilities in which it is manufactured. |
| | |
| > | Adverse reactions to the product candidateor indications of other safety concerns. |
| | |
| > | Our inability to manufacture sufficientquantities of the product candidate fordevelopment or commercialisation activitiesin a timely and cost-efficient manner. |
| | |
| > | Strategic collaborations that we haveentered into may not be successful. |
As a result of these complexities and uncertainties associated with pharmaceutical research, it cannot be ensured that compounds currently under development will achieve success. For example, in 2006, late-stage development ofGalida(a potential diabetes therapy) and NXY-059 (a potential treatment for stroke) were discontinued due to failure to meet their target product profiles.
STRATEGIC ALLIANCES FORMED AS PART OF OUR EXTERNALISATION STRATEGY MAY BE UNSUCCESSFUL
We may pursue acquisitions of complementary businesses, technology licensing arrangements and strategic alliances to expand our product portfolio and geographic presence as part of our business strategy. Examples of recent such strategic alliances include:
| > | Collaboration with Bristol-Myers SquibbCompany to develop and commercialisetwo investigational compounds beingstudied for the treatment of Type 2 diabetes. |
| | |
| > | Collaboration with Pozen Inc. to co-developfixed dose combinations of naproxen andesomeprazole for chronic pain, utilisingPozen’s proprietary formulation technology. |
| | |
| > | Agreement with AtheroGenics, Inc.to develop and commercialise theiranti-inflammatory cardiovascularproduct candidate for the treatmentof atherosclerosis. |
| | |
| > | Acquisition of Cambridge AntibodyTechnology Group plc and KuDOS
Pharmaceuticals Limited. |
We may not complete these types of transactions in a timely manner, on a cost-effective basis, or at all, and may not realise the expected benefits of any acquisition, licensing arrangement or strategic alliance.
Back to Contents
| 174 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
RISK FACTORS CONTINUED
Other companies may also compete with us for these strategic opportunities. When we are able to complete these transactions, the success of these types of arrangements (whether already existing or to be entered into in the future) is largely dependent on the technology and other intellectual property acquired from a business or contributed from our strategic partners and the resources, efforts and skills of our partners. Disputes and difficulties in such relationships are common, often due to conflicting priorities or conflicts of interest. The benefits of these alliances would be reduced or eliminated should strategic partners: terminate the agreements; fail to devote sufficient financial or other resources to the alliances; or suffer negative outcomes in intellectual property disputes.
If these types of transactions are unsuccessful, our operating results will be negatively impacted. In addition, integration of an acquired business could result in the incurrence of significant debt and unknown or contingent liabilities, as well as the negative effects on our reported results of operations from acquisition-related charges, amortisation of expenses related to intangibles and charges for impairment of long-term assets. These effects, individually or in combination, could cause a deterioration of our credit rating and result in increased borrowing costs and interest expense. We could also experience difficulties in integrating geographically separated organisations, systems and facilities, and personnel with diverse backgrounds. Integration of an acquired business may also require management resources that would otherwise be available for ongoing development of our existing business.
Under many of our strategic alliances we make milestone payments well in advance of commercialisation of products, with no assurance that we will ever recoup those payments, in which case our operating results may be negatively affected.
COMPETITION, PRICE CONTROLS AND PRICE REDUCTIONS
The principal markets for our pharmaceutical products are the Americas, the countries of the European Union (EU), Asia Pacific and Japan. These markets are highly competitive. We compete in all of them, and elsewhere in the world, against major prescription pharmaceutical companies which, in many cases, are able to match or exceed the resources that we have available to us, particularly in the areas of R&D and marketing spend. Industry consolidation has resulted in the formation of a small number of very large companies. Some of our most important
products for future growth, such asCrestor,SeroquelandSymbicort, compete directly with similar products marketed by some of these companies. Increasingly, we also compete directly with biotechnology companies and companies that manufacture generic versions of our products following the expiry or loss of patent protection or other marketing exclusivity. In addition, some of our patented products, includingNexium, are subject to pricing pressure from competition from generic products in the same class.
In most of the principal markets in which we sell our products, there is continued economic, regulatory and political pressure to limit the cost of pharmaceutical products. Certain groups have been involved in exerting price pressure on pharmaceutical companies to ensure medicines are affordable to those who need them.
Currently, there is no direct government control of prices for non-government sales in the US. In 1990, however, federal legislation was enacted which required drug manufacturers to agree to substantial rebates in order for the manufacturer’s drugs to be reimbursed by state Medicaid programmes, and an additional rebate if manufacturer price increases after 1990 exceed the increase in inflation. In addition, certain states have taken action to require further manufacturer rebates on Medicaid drug utilisation and for other state pharmaceutical assistance programmes. For example, some states permit or require the dispensing pharmacist to substitute a less expensive generic drug instead of an original branded drug. Congress has also enacted statutes that place a ceiling on the price manufacturers may charge US government agencies, thereby causing a substantial discount, as well as establishing a minimum discount (comparable to the Medicaid rebate) on manufacturers’ sales to certain clinics and hospitals that serve the poor and other populations with special needs. These government initiatives, together with competitive market pressures, have contributed to restraints on realised prices in the US.
See also pages 33 (Geographic Review) and 50 (Industry Regulation) for a discussion of the impact of Medicare Part D.
In addition, realised prices are being depressed by pressure from managed care organisations and institutional purchasers, who use cost considerations to restrict the sale of preferred drugs that their physicians may prescribe, as well as other competitive activity. Such limited lists or formularies may force manufacturers
either to reduce prices or be excluded from the list, thereby losing all the sales revenue from patients covered by that formulary. In addition, private health insurance companies and employers that self-insure have been raising co-payments required from beneficiaries, particularly for branded pharmaceuticals and biotechnology products, among other reasons, to encourage beneficiaries to utilise generic products. The increased use of strict formularies by institutional customers in response to the current cost-containment environment and increasingly restrictive reimbursement policies could negatively impact our net revenue.
Some governments in Europe, such as Italy and Spain, set price controls having regard to the medical, economic and social impact of the product. In other European countries, primarily Germany, the UK, the Netherlands and, more recently, France, governments have exerted a strong downward pressure on prices by incentives and sanctions to encourage doctors to prescribe cost-effectively. For example, in Germany, jumbo reference price groups are formed around broad drug classes, such as statins and proton pump inhibitors, which include branded as well as generic products, resulting in significant decreases in reimbursed prices for some patented drugs. In other countries, such as Italy and Belgium, clawbacks or price cuts have been imposed to recover budget overruns from the industry and this is a trend that is likely to continue. Efforts by the European Commission to harmonise the disparate national systems have met with little immediate success. The industry is, therefore, exposed to ad hoc national cost-containment measures on prices and the consequent cross-border movement of products from markets with prices depressed by governments into those where higher prices prevail. See also page 51 for further discussion of price regulation in Europe.
The importation of pharmaceutical products from countries where prices are low due to government price controls or other market dynamics (including production of counterfeit products), to countries where prices for those products are higher may increase. The accession of additional countries from Central and Eastern Europe to the EU could result in significant increases in the parallel trading of pharmaceutical products.Movements of pharmaceutical products into the US, in particular from Canada into the US, may increase despite the need to meet current or future safety requirements imposed by regulatory authorities. The effects of any increase in the volume of this cross-border
Back to Contents
movement of products could result in a material adverse effect on AstraZeneca’s financial condition and results of operations.
There is formal central government control of prices in Japan. New product prices are determined primarily by comparison with existing products for the same medical condition. All existing products are subject to a price review at least every two years. Regulations introduced in 2000 included provisions allowing a drug’s price to be set according to the average price of the product in four major countries (the US, the UK, Germany and France).
We expect that pressures on pricing will continue and may increase. Because of these pressures, there can be no certainty that in every instance we will be able to charge prices for a product that, in a particular country or in the aggregate, enable us to earn an adequate return on our investment in that product.
TAXATION
The integrated nature of AstraZeneca’s worldwide operations can produce conflicting claims from revenue authorities as to the profits to be taxed in individual territories. The resolution of these disputes can result in a reallocation of profits between jurisdictions and an increase or decrease in related tax costs. This is a continuing risk for AstraZeneca which is unlikely to change in the foreseeable future.
AstraZeneca operates in many jurisdictions, the majority of which have double tax treaties with other foreign jurisdictions, which enable AstraZeneca’s revenues and capital gains to escape a double tax charge. If any of these double tax treaties should be withdrawn or amended, in a territory where a member of the AstraZeneca Group is involved in a taxation dispute with a tax authority in relation to cross-border transactions, such withdrawal, amendment or a negative outcome of such disputes could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
RISK OF SUBSTANTIAL PRODUCT LIABILITY CLAIMS
Given the widespread impact prescription drugs may have on the health of large patient populations, pharmaceutical and medical device companies have, historically, been subject to large product liability damages claims, settlements and awards for injuries allegedly caused by the use of their products. Product liability claims, regardless of their merits or their outcome, are costly, divert management attention, and may adversely
affect our reputation and demand for our products. In addition, substantial product liability claims that are not covered by insurance could have a material adverse effect on AstraZeneca’s financial condition and results of operations. We are currently subject to extensive product liability litigation, particularly in relation toSeroquel. See Note 26 of the Financial Statements.
RISK OF RELIANCE ON THIRD PARTIES FOR SUPPLIES OF MATERIALS AND SERVICES
Like most, if not all, major prescription pharmaceutical companies, in some of its key business operations, such as the manufacture, formulation and packaging of products, AstraZeneca relies on third parties for the timely supply of specified raw materials, equipment, contract manufacturing, formulation or packaging services and maintenance services. Although we actively manage these third party relationships to ensure continuity of supplies on time and to our required specifications, some events beyond our control could result in the complete or partial failure of supplies or in supplies not being delivered on time. Any such failure could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
RISK OF FAILURE TO MANAGE A CRISIS
AstraZeneca handles toxic materials, runs manufacturing plants and distributes products worldwide. Major disruption to business and damage to reputation may be triggered by an operational incident or actions by third parties. In these circumstances, a well-tried and tested plan for addressing operational and other issues should ensure a timely response and the ability to resume business as usual. Failure to institute proper communication to internal and external stakeholders and mobilise a rapid operational response could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
RISK OF DELAY TO NEW PRODUCT LAUNCHES
AstraZeneca’s continued success depends on the development and successful launch of innovative new drugs. The anticipated launch dates of major new products have a significant impact on a number of areas of our business, including investment in large clinical trials, the manufacture of pre-launch stocks of the products and the timing of anticipated future revenue streams from commercial sales of the products. These launch dates are primarily driven by the development programmes that we run and the demands of the regulatory authorities in the approvals process, as well as pricing negotiation in some countries. Delays in anticipated launch dates can arise
as a result of adverse findings in pre-clinical or clinical studies, regulatory demands, competitor activity and technology transfer. Any delay to the anticipated launch dates may therefore impact AstraZeneca’s business and operations in a number of ways. In 2004 for example, we made provisions of $236 million following setbacks suffered byExantaandIressa. Significant delay to the anticipated launch dates of new products could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
DIFFICULTIES OF OBTAINING AND MAINTAINING REGULATORY APPROVALS FOR NEW PRODUCTS
AstraZeneca is subject to strict controls on the manufacture, labelling, distribution and marketing of pharmaceutical products. The requirement to obtain regulatory approval based on safety, efficacy and quality, before such products may be marketed in a particular country, and to maintain and to comply with licences and other regulations relating to their manufacture, are particularly important. The submission of an application to a regulatory authority does not guarantee that approval to market the products will be granted. The countries that constitute material markets for our pharmaceutical products include the US, the countries of the EU and Japan. Approval of such products is required by the relevant regulatory authority in each country, although a single pan-EU, marketing authorisation approval can be obtained through a centralised mutual recognition procedure. In addition, each jurisdiction has very high standards of regulatory approval and, consequently, in most cases, a lengthy approval process. In recent years, the public and various governments appear to apply more conservative benefit/risk criteria in relation to pharmaceutical products of the type sold by companies such as ours than in the past. This apparent trend could in the future result in even more stringent requirements, including more difficult approval processes for our products. Furthermore, each regulatory authority may impose its own requirements and may refuse to grant, or may require additional data before granting or as a condition to granting, an approval, even though the relevant product has been approved in another country. Post-marketing studies involving our marketed products (whether conducted by us or by others, and whether or not mandated by regulatory agencies), as well as other emerging data about marketed products such as adverse event reports, could lead to a loss of approval, changes in product labelling or concerns about the side effects or efficacy of a product wherever it is marketed. For example, in
Back to Contents
| 176 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
RISK FACTORS CONTINUED
February 2006 we decided to withdrawExantafrom the market and terminate its development as a result of new patient safety data from a clinical trial, which involved the use ofExantafor a longer duration of therapy than was then approved for marketing. In addition although the Japanese regulatory authority granted approval forCrestor, this was conditional on a post-marketing surveillance programme being carried out. New data about our products, or products similar to our products, could negatively impact demand for our products and our net profit due to real or perceived safety or efficacy concerns.
RISK OF FAILURE TO OBSERVE ONGOING REGULATORY OVERSIGHT
AstraZeneca’s products are only licensed following exhaustive regulatory approval processes and only for a specified therapeutic indication or indications. Once a product is licensed, it is subject to ongoing control and regulation, such as the manner of its manufacture, distribution, marketing and safety surveillance. In addition, facilities in which products are produced are subject to ongoing inspections, and minor changes in manufacturing processes may require additional regulatory approvals, either of which could cause us to incur significant additional costs and lose revenue. Regulatory authorities have wide-ranging administrative powers to deal with any failure to comply with their ongoing regulatory oversight (whether such failure is by us or third parties with which we have relationships). These powers include withdrawal of a licence approval previously granted, product recalls, seizure of products and other sanctions for non-compliance. Regulatory sanction, following a failure to comply with such ongoing regulatory oversight, could have a material adverse effect on AstraZeneca’s financial condition and results of operations. In addition, because our products are intended to promote the health of patients, any supply interruption could lead to allegations that the public health has been endangered, and could subject us to lawsuits.
PERFORMANCE OF NEW PRODUCTS
Although we carry out numerous and extensive clinical trials on all our products before they are launched, for a new product it can be difficult, for a period following its launch, to establish from available data a complete assessment of its eventual efficacy and/or safety in broader clinical use on the market. Due to the relatively short time that a product
has been tested and the relatively small number of patients who have taken the product, the available data may be immature. Simple extrapolation of the data may not be accurate and could lead to a misleading interpretation of a new product’s likely future commercial performance. See further discussion of product safety and efficacy in the Managing Risk section of this report.
The successful launch of a new pharmaceutical product involves a substantial investment in sales and marketing costs, launch stocks and other items. If a new product does not succeed as anticipated or its rate of sales growth is slower than anticipated, there is a risk that the costs incurred in launching it could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
ENVIRONMENTAL/OCCUPATIONAL HEALTH & SAFETY LIABILITIES
AstraZeneca has environmental liabilities at some currently or formerly owned, leased and third party sites in the US, as described in more detail on pages 135 and 136. There is no reason for us to believe that associated current and expected expenditure and risks are likely to have a material adverse effect on AstraZeneca’s financial condition and results of operations as a general matter, although they could, to the extent that they exceed applicable provisions, have a material adverse effect on AstraZeneca’s financial condition and results of operations for the relevant period. In addition, a change in circumstances (including a change in applicable laws or regulations) may result in such a material adverse effect. Although we take great care to ensure that we maintain compliance with all applicable environmental, health and safety laws, regulations, licences and permits at each of our operating facilities, a significant non-compliance or incident for which we were responsible could result in AstraZeneca being liable to pay compensation, fines or remediation costs. In some circumstances, such liability could have a material adverse effect on AstraZeneca’s financial condition and results of operations. In addition, our financial provisions for any obligations that we may have relating to environmental liabilities may be insufficient if the assumptions underlying the provisions – including our assumptions regarding the portion of waste at a site for which we are responsible – prove incorrect, or if we are held responsible for additional contamination.
EMERGING MARKETS
Growing our business in emerging markets may be a critical factor in determining our future ability to sustain or increase the level of our global product revenues. Challenges that arise in relation to the development of the business in emerging markets include, but are not limited to, competition from companies that are already present in the market, the need to correctly identify and leverage appropriate opportunities for sales and marketing, poor protection over intellectual property, inadequate protection against crime (including counterfeiting, corruption and fraud), inadvertent breaches of local law/regulation and not being able to recruit sufficient personnel with appropriate skills and experience. The failure to exploit potential opportunities appropriately in emerging markets may have a material adverse effect on AstraZeneca’s financial condition and results of operations.
REPUTATION STRATEGY
There is considerable public sentiment against the pharmaceuticals industry, and the industry is under the close scrutiny of the public, the media and other stakeholders. Rising expectations are especially noteworthy in the areas of improving access to medicines for the underprivileged, both in our established markets and in less-developed nations; business conduct in our supply chain; fair marketing practices; bio-ethical challenges; working conditions; human rights; and animal rights. Whilst we seek to manage these risks through various pro-active measures, there can be no assurance that in the future such risks will not cause our financial condition or results of operations to be materially affected.
PRODUCT COUNTERFEITING
See Managing Risk section on page 46.
Back to Contents
ADDITIONAL INFORMATION
| 177 |
ADDITIONAL INFORMATION
HISTORY AND DEVELOPMENT OF THE COMPANY
AstraZeneca PLC was incorporated in England and Wales on 17 June 1992 under the Companies Act 1985. It is a public limited company domiciled in the UK. The Company’s registered number is 2723534 and its registered office is at 15 Stanhope Gate, London W1K 1LN (telephone + 44 (0)20 7304 5000). From February 1993 until April 1999, the Company was called Zeneca Group PLC. On 6 April 1999, the Company changed its name to AstraZeneca PLC.
The Company was formed when the pharmaceutical, agrochemical and specialty chemical businesses of Imperial Chemical Industries PLC were demerged in 1993. In 1999, the Company sold the specialty chemical business. Also in 1999, the Company merged with Astra AB of Sweden. In 2000, it demerged the agrochemical business and merged it with the similar agribusiness of Novartis AG to form a new company called Syngenta AG.
The Company owns and operates numerous R&D, production and marketing facilities worldwide. Its corporate headquarters are at 15 Stanhope Gate, London W1K 1LN.
MEMORANDUM AND ARTICLES OF ASSOCIATION
Objects
As is typical of companies registered in England and Wales, the Company’s objects, which are detailed in the Memorandum of Association, are broad and wide-ranging and include manufacturing, distributing and trading pharmaceutical products.
Directors
Subject to certain exceptions, Directors do not have power to vote at Board meetings on matters in which they have a material interest.
The quorum for meetings of the Board of Directors is a majority of the full Board, of whom at least four must be Non-Executive Directors. In the absence of a quorum, the Directors do not have power to determine compensation arrangements for themselves or any member of the Board.
The Board of Directors may exercise all the powers of the Company to borrow money. Variation of these borrowing powers would require the passing of a special resolution of the Company’s shareholders.
Directors are not required to retire at a particular age.
Directors are required to beneficially own Ordinary Shares in the Company of an aggregate nominal amount of $125. At present, this means they must own at least 500 shares.
Rights, preferences and restrictions attaching to shares
The share capital of the Company is divided into 2,400,000,000 Ordinary Shares with a nominal value of $0.25 each and 50,000 Redeemable Preference Shares with a nominal value of £1.00 each. The rights and restrictions attaching to the Redeemable Preference Shares differ from those attaching to Ordinary Shares as follows:
| > | The Redeemable Preference Shares carryno rights to receive dividends. |
| | |
| > | The holders of Redeemable PreferenceShares have no rights to receive noticesof, attend or vote at general meetingsexcept in certain limited circumstances.
They have one vote for every 50,000Redeemable Preference Shares held. |
| | |
| > | On a distribution of assets of the Company,on a winding-up or other return of capital(subject to certain exceptions), the holdersof Redeemable Preference Shares havepriority over the holders of OrdinaryShares to receive the capital paid up onthose shares. |
| | |
| > | Subject to the provisions of the CompaniesAct 1985, the Company has the right toredeem the Redeemable PreferenceShares at any time on giving not less thanseven days’ written notice. |
Action necessary to change the rightsof shareholders
In order to vary the rights attached to any classof shares, the consent in writing of the holdersof three quarters in nominal value of the issuedshares of that class or the sanction of anextraordinary resolution passed at a generalmeeting of such holders is required.
Annual general meetings and extraordinary general meetings
Annual general meetings and extraordinary general meetings where a special resolution is to be passed or a Director is to be appointed require 21 clear days’ notice to shareholders. All other extraordinary general meetings require 14 clear days’ notice.
For all general meetings, a quorum of two shareholders present in person or by proxy is required.
Shareholders and their duly appointed proxies and corporate representatives are entitled to be admitted to general meetings.
Limitations on the rights to own shares
There are no limitations on the rights to own shares.
Back to Contents
| 178 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
CROSS-REFERENCE TO FORM 20-F
The information in this document that is referenced on this page is included in AstraZeneca’s Form 20-F for 2006 (2006 Form 20-F) and is filed with the Securities and Exchange Commission (SEC). The 2006 Form 20-F is the only document intended to be incorporated by reference into any filings by AstraZeneca under the Securities Act of 1933, as amended. References to major headings include all information under such major headings, including subheadings. References to subheadings include only the information contained under such subheadings. Graphs are not included unless specifically identified. The 2006 Form 20-F has not been approved or disapproved by the SEC, nor has the SEC passed comment upon the accuracy or adequacy of the 2006 Form 20-F. The 2006 Form 20-F filed with the SEC may contain modified information and may be updated from time to time.
| Item | | | Page |
|
|
|
|
| 3 | Key Information | |
|
|
|
| | A. | Selected financial data | |
| | | Financial Highlights | 6 |
| | | Group Financial Record – IFRS | 163 |
| | | Shareholder Information | 165 |
| | D. | Risk factors | 172 |
| | | |
| 4 | Information on the Company | |
|
|
|
| | A. | History and development of the Company | 177 |
| | | Financial Review – Investments, divestments | |
| | | and capital expenditure | 57, 69 |
| | | Note 7 – Property, plant and equipment | 110 |
| | | Note 22 – Acquisitions of business operations | 121 |
| | | Note 23 – Disposal of business operations | 123 |
| | B. | Business overview | |
| | | Business Review | 8 |
| | C. | Organisational structure | |
| | | Directors’ Report – Governance | 71 |
| | | Principal Subsidiaries | 148 |
| | D. | Property, plant and equipment | |
| | | Business Review – Main Facilities | 49 |
| | | |
| 5 | Operating and Financial Review and Prospects | |
|
|
|
| | A-F. | Business Review | 8 |
| | A-F. | Financial Review | 53 |
| | | Note 15 – Financial instruments | 115 |
| | | |
| 6 | Directors, Senior Management and Employees | |
|
|
|
| | A. | Directors and senior management | |
| | | Board of Directors | 80 |
| | B. | Compensation | |
| | | Directors’ Remuneration Report | 82 |
| | | Note 24 – Post-retirement benefits | 123 |
| | | Note 28 – Statutory and other information | 146 |
| | C. | Board practices | |
| | | Board of Directors | 80 |
| | | Directors’ Remuneration Report | 82 |
| | | Directors’ Report – Governance | 71 |
| | | Audit Committee | 72 |
| | D. | Employees | |
| | | Note 25 – Employee costs and share option | |
| | | plans for employees | 128 |
| | | Directors’ Report – People | 48 |
| | E. | Share ownership | |
| | | Directors’ Remuneration Report – | |
| | | Directors’ Interests in Shares | 91 |
| | | Note 25 – Employee costs and | |
| | | share option plans for employees | 128 |
| | |
| 7 | Major Shareholders and Related Party Transactions |
|
|
| | A. | Major shareholders | |
| | | Shareholder Information – Major shareholdings | 166 |
| | B. | Related party transactions | |
| | | Shareholder Information – Related party | |
| | | transactions | 167 |
| | | Note 28 – Statutory and other information | 146 |
| Item | | | Page |
|
|
|
| 8 | Financial Information | |
|
|
|
| | A. | Consolidated statements and other | |
| | | financial information | |
| | | Financial Statements (excluding Directors’ | |
| | | responsibilities on page 96 and Auditors’ | |
| | | opinion on page 97) | 98 |
| | B. | Significant changes | |
| | | Note 28 – Statutory and other information | 146 |
| | | |
| 9 | The Offer and Listing | |
|
|
|
| | A4. | Price history of listed stock | |
| | | Shareholder Information | 165 |
| | C. | Markets | |
| | | Shareholder Information | 165 |
| | | |
| 10 | Additional Information | |
|
|
|
| | B. | Memorandum and Articles of Association | 177 |
| | C. | Material contracts | n/a |
| | D. | Exchange controls and other limitations | |
| | | affecting security holders | 169 |
| | E. | Taxation | 168 |
| | H. | Documents on display | 168 |
| | I. | Subsidiary information | |
| | | Principal Subsidiaries | 148 |
| | |
| 11 | Quantitative and Qualitative Disclosures about Market Risk |
|
|
| | Financial Review – Financial Risk Management | |
| | Policies – Treasury | 60 |
| | | |
| 12 | Description of Securities other than Equity Securities | n/a |
|
|
|
| | | |
| 13 | Defaults, Dividend Arrearages and Delinquencies | n/a |
|
|
|
| | | |
| 14 | Material Modifications to the Rights of Security | |
| | Holders and Use of Proceeds | n/a |
|
|
|
| | | |
| 15 | Controls and Procedures | |
|
|
|
| | Directors’ Report – Internal controls and | |
| | management of risk | 75 |
| | | |
| 16 | [Reserved] | |
|
|
|
| | A. | Audit Committee financial expert | |
| | | Audit Committee | 72 |
| | B. | Code of ethics | |
| | | Directors’ Report – Code of Conduct | 76 |
| | C. | Principal accountant fees and services | |
| | | Note 28 – Statutory and other information | 146 |
| | D. | Exemptions from the listing standards | |
| | | for audit committees | n/a |
| | E. | Purchases of equity securities by the issuer | |
| | | and affiliated purchasers | |
| | | Note 29 – Share capital of parent company | 147 |
| | | |
| 18 | Financial Statements | |
|
|
|
| | Financial Statements (excluding Directors’ responsibilities | |
| | on page 96 and Auditors’ opinion on page 97) | 98 |
Back to Contents
GLOSSARY
The following abbreviations and expressions have the following meanings when used in this report:
ACE (Inhibitor)– Angiotensin-converting enzyme blocks the production of a hormone called angiotensin II. Angiotensin II narrows blood vessels and thereby raises blood pressure.
ACS– Acute Coronary Syndrome, an umbrella term used to cover any group of clinical symptoms compatible with acute myocardial ischemia.
Adjuvant– Assisting in the prevention, improvement or cure of disease.
ADP– Adenosine diphosphate attaches to receptors on the surface of platelets to form blood clots.
Adverse reaction– An unwanted, negative consequence associated with the use of a medicine.
Agonist– A substance capable of binding to a molecular target to initiate or enhance a physiological reaction.
Anaesthesia– The total or partial loss of sensation, especially in relation to pain.
Analgesia– The inability to feel pain.
Abbreviated New Drug Application (ANDA)–A marketing approval application for a generic drug submitted to the US Food and Drug Administration.
Antagonist– A substance capable of binding to a molecular target to neutralise or counteract a physiological reaction.
Anti-androgen– A drug that blocks the cellular uptake of testosterone by the prostate gland and used in the treatment of prostate cancer.
Anti-psychotic drug– For the treatment of the unrealistic ideas, delusions (false beliefs) and hallucinations (false perceptions) that can appear during depression or mania.
Aromatase inhibitor– A drug that inhibits the enzyme aromatase, which is involved in the production of the female sex hormone, oestrogen.
AstraZeneca or AstraZeneca Group– AstraZeneca PLC and its subsidiaries.
Atherosclerosis– Disease of the arteries linked to the build-up of lipids (fats) in the walls and the formation of atheromatous plaque, which contracts the lumen of these vessels.
Atrial fibrillation (AF)– Abnormal irregular heart rhythm with chaotic generation of electrical signals in the atria of the heart.
Atypical anti-psychotic drugs– Second generation drugs to treat psychosis with reduced likelihood to cause movement disorders.
Biomarker– A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention.
Biopharmaceuticals/Biologics– A new class of systemic therapies that contain proteins (usually produced naturally by living organisms in response to disease, for example antibodies), as opposed to traditional pharmaceutical drugs that are made up of non-living chemicals.
Bipolar disorder– Any of several mood disorders characterised usually by alternating episodes of depression and mania or by episodes of depression alternating with mild non-psychotic excitement.
Bronchodilator– A drug that causes the widening of the bronchi (major air passages of the lungs).
Cardiovascular (CV)– Relating to the heart and blood vessels.
CAT– Cambridge Antibody Technology Group plc.
Candidate Drug (CD)– A drug to be taken into clinical concept testing phase.
CEE– Central and Eastern Europe.
CHF– Congestive Heart Failure. A condition in which the heart’s function as a pump (to circulate blood throughout the body) is inadequate to meet the body’s needs and leads to a poor blood supply, which may cause the body’s organ systems to fail.
Chronic Obstructive Pulmonary Disease (COPD)–Any disorder that persistently obstructs bronchial airflow, eg bronchitis.
Cognitive disorders– The class of disorders consisting of significant impairment of cognition or memory that represents a marked deterioration from a previous level of functioning.
CR– Corporate Responsibility.
Corticosteroid– Any of the steroid hormones made by the cortex (outer layer) of the adrenal gland.
CRC– Colo-rectal cancer.
Crohn’s disease– A chronic inflammatory disorder of the bowels.
Directors– The Directors of the Company.
Diabetes– A metabolic disorder characterised by hyperglycaemia (high glucose blood sugar), among other signs, or a variable disorder of carbohydrate metabolism usually characterised by inadequate secretion or utilisation of insulin.
Diuretic– A drug that causes the increased passing of urine.
Dopamine partial agonists– Mimic the effects of dopamine in the brain by stimulating dopamine receptors.
Double-blind study– A clinical study in which neither the subject, nor the investigator nor the research team interacting with the subject or data during the trial knows what treatment a subject is receiving.
Drug metabolism– The biochemical modification or degradation of drugs, usually through specialised enzymatic systems.
Dyslipidaemia– A condition marked by abnormal concentrations of lipids or lipoproteins in the blood.
EEA– European Economic Area.
Efficacy– The outcomes measured in Phase III clinical trials that indicate that the test drug has the intended benefit.
Epidermal Growth Factor (EGF) receptor– A protein found on the surface of some cells and to which epidermal growth factor binds, causing the cells to divide. It is found at abnormally high levels on the surface of many types of cancer cells, so these cells may divide excessively in the presence of epidermal growth factor.
EFPIA– European Federation for Pharmaceutical Industries and Associations.
EMEA– The European Medicines Agency.
Excipient��� An inactive substance that serves as the vehicle or medium for a drug or other active substance.
Food and Drug Administration (FDA)– Part of the US Department of Health and Human Services Agency responsible for development, approval, manufacture, sale and use of all drugs, biologics, vaccines and medical devices in the US.
First-line therapy– Treatment given to a newly diagnosed patient, who has therefore not yet been treated.
First time in man– The first time that an experimental compound is administered to a human. It implies that the compound has passed ethical review bodies and passed formal regulatory toxicology studies.
Gastrointestinal (GI)– Relating to the stomach and intestines.
Generic– Drugs that are copies of brand-name drugs and have regulatory approval.
Gastro-oesophageal reflux disease (GERD)– A recurrent condition where gastric juices, containing acid, travel back from the stomach into the oesophagus.
Group– The Company and its subsidiaries.
Head-to-head study– A clinical trial in which two different medicines are directly compared with each other with respect to their effect on a marker of the disease or a specific event associated with the disease. (For drugs under development, this is often a comparison with a marketed drug that is seen to be the gold standard.)
Hydrofluoroalkanes (HFAs)– A new propellant for metered-dose inhalers that are more environmentally friendly than the current CFC-based inhalers.
High-density lipoprotein cholesterol (HDL-C)– HDL carries cholesterol in the blood, sometimes referred to as “good” cholesterol.
High-throughput screening– The process of using automated tests to search quickly through large numbers of substances for desired binding or activity characteristics.
Hormone– A chemical “signal” carried in the blood.
HKAPI– Hong Kong Association of the Pharmaceutical Industry.
Hypertension– High blood pressure.
IMS Health Inc.– Provider of pharmaceutical market data globally.
IR– Immediate release.
Ischaemic heart disease– A chronic disease caused by insufficient blood supply to the heart.
Leukotriene receptor antagonist– New type of asthma medication. They are non-steroidal medications, which are taken long term and have been shown to reduce reliever use and may also allow the asthmatic to reduce high doses of inhaled steroids.
Line extension– A new formulation, indication or presentation of a product that is already approved.
Lipid– Another word for “fat”. Lipids are one of the main constituents of plant and animal cells.
Low-density lipoprotein cholesterol (LDL-C)– LDL is the major carrier of cholesterol in the blood, sometimes referred to as “bad” cholesterol.
Luteinising hormone-releasing hormone (LHRH)– A naturally occurring hormone that controls sex hormones in both men and women.
LHRH agonist– A compound that is similar to LHRH in structure and is able to act like it.
Marketing Authorisation Application (MAA)– An application for authorisation to place medicinal products on the market. This is a specific term for the EU/EEA markets.
Medicaid– A US health insurance programme for individuals and families with low incomes and resources. It is jointly funded by the states and federal government, and is managed by the states.
Medicare– A US health insurance programme for US citizens aged 65 or older, US citizens under age 65 with certain disabilities, and US citizens of all ages with permanent kidney failure requiring dialysis or a kidney transplant. Recently, Medicare began offering prescription drug coverage under Part D of the Medicare Prescription Drug Benefit.
Metabolic syndrome– A combination of medical disorders that increase one’s risk for cardiovascular disease and diabetes.
MHLW– Japanese Ministry of Health, Labour and Welfare.
Monoclonal antibody– An antibody derived from a single clone of cells; all antibodies derived from such a group of cells have the same sequence of DNA.Monotherapy– Treatment where only one agent is given.
Myocardial infarction (MI)– A heart attack.
New Chemical Entity (NCE)– A new, pharmacologically-active chemical substance. The term is used to differentiate from line extensions and existing drug products.
NCI– US National Cancer Institute.
New Drug Application (NDA)– An application to the FDA for approval to market a drug product in the US.
Neuroscience– Sciences that deal with the structure or function of the nervous system and brain.
Normotensive– Indicating a normal arterial blood pressure.
NSAID– Non-steroidal anti-inflammatory drug.
NSCLC– Non-small cell lung cancer.
OA– Osteoarthritis.
Oncology– The study of diseases that cause cancer.
Odontology– A science dealing with the teeth, their structure and development, and their diseases.
Outcomes study– A large clinical trial in which the effect of a drug in preventing or delaying a specific and important medical event related to that disease area (eg the occurrence of a heart attack) is measured, rather than the effect on a marker of the disease (eg blood levels of certain enzymes).
Back to Contents
| 180 | ASTRAZENECA ANNUAL REPORT AND FORM 20-F INFORMATION 2006 |
GLOSSARY CONTINUED
Over the counter (OTC)– A term used for medicines that can be purchased without a prescription.
Palliative– Treatment that has no curative intent but is given to maintain quality of life and to relieve suffering in a terminally-ill patient.
Parenteral– Administered by any way other than through the mouth.
Phage– The abbreviation for bacteriophage, a virus that infects bacteria.
Pharmacology– The study of how drugs affect a living organism.
Pharmacogenomics– A biotechnological science that combines the techniques of medicine, pharmacology and genomics and is concerned with developing drug therapies to compensate for genetic differences in patients which cause varied responses to a single therapeutic regimen.
Pharmacokinetics– The study of what the body does to a drug.
Phase I– The phase of clinical study where researchers test a new drug or treatment in a small group (20-80) of people for the first time to evaluate its safety, determine a safe dosage range, and identify side effects.
Phase II– This phase of clinical study includes the controlled clinical activities conducted to evaluate the effectiveness of the drug for a particular indication or indications in patients with the disease or condition under study and to determine the common short-term side effects and risks associated with the drug. Phase II studies are typically conducted in a relatively small number of patients (usually no more than several hundred).
Phase III– This phase of clinical study is performed after preliminary evidence suggesting effectiveness of the drug has been obtained and is intended to gather additional information about effectiveness and safety that is needed to evaluate the overall benefit/risk profile of the drug and to provide an adequate basis for physician labelling. Phase III studies usually include between several hundred and several thousand subjects.
Phase IV– Post-marketing studies to delineate additional information about the drug’s risks, benefits and optimal use, including those that may be requested by regulatory authorities in conjunction with marketing approval.
PhRMA– Pharmaceutical Research andManufacturers of America, the US pharmaceutical industry association.
Placebo– In clinical trials, an inert substance identical in appearance to the substance being tested, also known as a sugar pill.
Pharmaceutical and Medical Devices Agency (PMDA)– Japanese regulatory authority, part of the MHLW.
pMDI– Pressurised metered-dose inhaler.
Post-marketing surveillance (PMS)– The systematic detection and evaluation of adverse reactions occurring in association with pharmaceutical products under customary conditions of use in ordinary clinical practice.
Poly-ADP-ribose polymerase (PARP)– An enzyme critical to the repair of damaged cells and maintenance of cellular energy.
Positron Emission Tomography (PET)– A highly specialised imaging technique that uses short-lived radioactive substances to produce three-dimensional coloured images of those substances functioning within the body. These images are called PET scans.
Proton Pump Inhibitor (PPI)– A medicine that reduces the production of acid in the stomach.
Pre-clinical (PC) studies– Studies conducted before a drug is tested in human subjects, and which support and help establish boundaries for safe use of the drug in subsequent Phase I studies.
Primary care– The medical care the patient receives upon first contact with the healthcare system, before referral elsewhere within the system.
Prolactin– The hormone that stimulates milk production after childbirth.
Proof of concept– Proof of concept provides clinical confirmation that an investigational product possesses a desired pharmacological effect in patients with the disease of interest. This can be achieved after a positive placebo-controlled study or dose-response study using a validated surrogate variable or the final clinical outcome variable. Proof of concept also includes establishing a limited dose range to be used in the subsequent confirmatory studies.
Proof of principle– Proof of principle is achieved when an intended pharmacological effect results in an expected change in a relevant biomarker in a dose range, which does not cause any major unwanted effects. Proof of principle therefore provides the first measurable evidence that an investigational product might work in humans. Proof of principle is normally demonstrated in a limited number of subjects with the disease of interest or in healthy volunteers when a relevant model exists.
Prophylaxis or Prophylactic therapy– A therapy or measure used to prevent disease.
RA– Rheumatoid arthritis.
RAG– Risk Advisory Group.
R&D– Research and Development.
Respiratory– Relating to or affecting breathing or the organs used to breathe.
RET-kinase– A receptor-tyrosine kinase which is normally involved in maturation of a variety of tissues, including the nervous system and kidney. It is sometimes mutated and has an abnormal function in certain types of thyroid cancer.
Ribosome– A large complex molecule that synthesises protein.
RoW– Rest of world.
Qui tamaction (in the US)– An action brought under a statute that allows a private person to sue for a penalty, part of which the government or some specified public institution will receive.
Second-line therapy– Treatment administered after the failure of, or in addition to, first-line therapy.
SEK, kronor, krona– References to Swedish currency.
Sepsis– A life-threatening condition resulting from uncontrolled severe infections.
Senior Executive Team (SET)– a cross-functional, cross-territorial group, established and led by the Chief Executive Officer.
SHE– Safety, Health and the Environment.
SCLC– Small cell lung cancer.
Specialist care– The medical care the patient receives after being referred within the system.
SR– Sustained-release.
Statin– A class of drugs that alter cholesterol levels in the blood.
Sterling, £, GBP, pence or p– References to UK currency.
Thrombosis– The formation of blood clots at sites where they are not required to prevent blood loss.
Target Product Profile (TPP)– Statement of the essential attributes of a clinically and commercially successful product, which can form the basis for commercial evaluation and guide Discovery and Development activities.
Triglycerides– The major form of fat that comes from the food we eat as well as from being produced by the body.
UK– United Kingdom of Great Britain and Northern Ireland.
US dollar, US$, USD or $– References to US currency.
US– United States of America.
World Health Organization (WHO)– The United Nations’ specialised agency for health.
Zollinger Ellison Syndrome– A rare gastric acid disorder.
FINANCIAL TERMS
ADR– American Depositary Receipt evidencing title to an ADS.
ADS– American Depositary Share representing one underlying Ordinary Share.
CER– Constant Exchange Rates.
Cost growth rates– Percentage growth of a particular cost category over the comparable cost category for the previous year.
Depositary– JPMorgan Chase Bank, as depositary under the deposit agreement pursuant to which the ADRs are issued.
Earnings per Share (EPS)– Profit for the year after tax and minority interests, divided by the weighted average number of Ordinary Shares in issue during the year.
Exceptional items– Significantly large items that are distinct in nature from items normally occurring during ordinary business activities.
Finance income and expense– Includes interest earned and payable, and similar items.
Free cash flow– Represents net cash flows before financing activities, and is calculated as: net cash inflow before financing activities, adjusted for acquisitions of businesses, movements in short term investments and fixed deposits and disposal of intangible assets.
Gross margin– The margin as a percentage by which sales exceed cost of sales, calculated by dividing the difference between the two by the sales figure.
IAS– International Accounting Standards.
IFRS– International Financial Reporting Standards.
LSE– London Stock Exchange.
Minority interests– Share of profits that belong to non-AstraZeneca shareholders in partially-owned subsidiaries.
NYSE– New York Stock Exchange.
Operating costs– Distribution costs; Research and Development (R&D) costs; and Selling, General and Administrative (SG&A) costs.
Operating profit– Sales, less cost of sales, less operating costs, plus operating income.
Ordinary Shares– Ordinary Shares of $0.25 each in the capital of the Company.
Profit before tax– Operating profit, plus finance income, less finance expense.
SSE– Stockholm Stock Exchange.
TSR– Total Shareholder Returns.
Back to Contents
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | Trade marks
Trade marks of the AstraZeneca group of companies appear throughout this document in italics. AstraZeneca, the AstraZeneca logotype and the AstraZeneca symbol are all trade marks of the AstraZeneca group of companies. Trade marks of companies other than AstraZeneca appear with a®or™ sign and include: Abraxane®, a registered trade mark of Abraxis BioScience, Inc.; Avastin™, a trade mark of Genentech, Inc.; Cubicin™, a trade mark of Cubist Pharmaceuticals, Inc.; CytoFab™, a trade mark of Protherics, Inc.; Herceptin™, a trade mark of Genentech, Inc.; Humira™, a trade mark of Abbott Laboratories, Inc.; Prinivil™, a trade mark of Merck & Co., Inc.; Taxotere™, a trade mark of Aventis Pharma S.A.; TriCor™, a trade mark of Fournier Industrie et Santé and Zocor™, a trade mark of Merck & Co., Inc. Use of terms
In this Annual Report and Form 20-F Information, unless the context otherwise requires, ‘AstraZeneca’, ‘the Group’, ‘the Company’, ‘we’, ‘us’ and ‘our’ refer to AstraZeneca PLC and its consolidated entities. | | Statements of competitive position
Except as otherwise stated, market information in this Annual Report and Form 20-F Information regarding the position of our business or products relative to its or their competition is based upon published statistical data for the 12 months ended 30 September 2006, obtained from IMS Health, a leading supplier of statistical data to the pharmaceutical industry. Except as otherwise stated, these market share and industry data from IMS Health have been derived by comparing our sales revenue to competitors’ and total market sales revenues for that period. For the purposes of this Annual Report and Form 20-F Information, references to the world pharmaceutical market or similar phrases are to 52 countries contained in IMS Health’s MIDAS Quantum database, which amount to approximately 95% (in value) of the countries audited by IMS Health. | | Statements of growth rates, sales and market data Except as otherwise stated, growth rates and sales in this Annual Report and Form 20-F Information are given at constant exchange rates (CER) to show underlying performance by excluding the effects of exchange rate movements. Market data are given in actual US dollars. Statements of dates
Except as otherwise stated, references to days and/or months in this Annual Report and Form 20-F Information are references to days and/or months in 2006. AstraZeneca websites
Information on or accessible through our websites, including astrazeneca.com, astrazenecaclinicaltrials.com, rosuvastatininformation.com and cambridgeantibody.com, does not form part of this document. | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Back to Contents
| | | |
| | |  |
| | | |
| | | |
| | | |
| | | CONTACT INFORMATION |
| | | |
| | | REGISTERED OFFICE
AND CORPORATE HEADQUARTERS ADDRESS
AstraZeneca PLC
15 Stanhope Gate
London W1K 1LN
UK
Tel: +44 (0)20 7304 5000
Fax: +44 (0)20 7304 5151 |
| | | |
| | | INVESTOR RELATIONS CONTACTS
UK: as above or e-mail
IR@astrazeneca.com
Sweden:
AstraZeneca AB
SE-151 85 Södertälje
Sweden
Tel: +46 (0)8 553 260 00
Fax: +46 (0)8 553 290 00
or e-mail
IR@astrazeneca.com
US:
Investor Relations
AstraZeneca Pharmaceuticals LP
1800 Concord Pike
PO Box 15438
Wilmington
DE 19850-5438
US
Tel: +1 (302) 886 3000
Fax: +1 (302) 886 2972 |
| | | |
| | | REGISTRAR AND TRANSFER OFFICE
Lloyds TSB Registrars
The Causeway
Worthing
West Sussex
BN99 6DA
UK
Tel (freephone in the UK): 0800 389 1580
Tel (outside the UK): +44 121 415 7033 |
| | | |
| | | SWEDISH SECURITIES REGISTRATION CENTRE
VPC AB
PO Box 7822
SE-103 97 Stockholm
Sweden
Tel: +46 (0)8 402 9000 |
| | | |
| The paper used in this report is made using pulp from sawmill residues, forest thinnings and wood from PEFC certified sustainable forests. All mill broke is recycled and accounts for up to 25% of the total fibre content. Pulps are Elemental Chlorine Free (ECF) and the manufacturing mill holds ISO 14001 and EMAS environmental management accreditations. | | US DEPOSITARY
JPMorgan Chase Bank
JPMorgan Service Center
PO Box 3408
South Hackensack
NJ 07606-3408
US
Tel (toll free in the US): 888 697 8018
Tel (outside the US): +1 (201) 680 6630 |
| | |
| Designed by Addison Corporate Marketing Ltd. | | ASTRAZENECA.COM |
| | | |
| | | |
| | | |
| | | |