Use these links to rapidly review the document
Cubist Pharmaceuticals, Inc. Annual Report on Form 10-K Table of Contents
ITEM 8. FINANCIAL STATEMENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2007 |
OR |
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 0-21379
CUBIST PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
Delaware
(State or Other Jurisdiction of
Incorporation or Organization) | | 22-3192085
(I.R.S. Employer
Identification No.) |
65 Hayden Avenue, Lexington, MA 02421
(Address of Principal Executive Offices and Zip Code) |
(781) 860-8660
(Registrant's Telephone Number, Including Area Code) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class
| | Name of each exchange on which registered
|
|---|
| Common Stock, $0.001 Par Value | | Nasdaq Global Select MarketSM |
| Series A Junior Participating Preferred Stock Purchase Rights | | Nasdaq Global Select MarketSM |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Each Class)
(Name of Each Exchange on Which Registered)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ý No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No ý
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ý | | Accelerated filer o | | Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's common stock, $0.001 par value per share, held by non-affiliates of the registrant as of June 30, 2007 was approximately $930.0 million, based on 47,186,820 shares held by such non-affiliates at the closing price of a share of common stock of $19.71 as reported on the NASDAQ Global Select MarketSM on such date. The number of outstanding shares of common stock of Cubist on February 25, 2008 was 56,218,625.
DOCUMENTS INCORPORATED BY REFERENCE
PORTIONS OF THE REGISTRANT'S DEFINITIVE PROXY STATEMENT FOR ITS
ANNUAL MEETING OF STOCKHOLDERS TO BE HELD ON JUNE 10, 2008
ARE INCORPORATED BY REFERENCE INTO PART III.
Cubist Pharmaceuticals, Inc.
Annual Report on Form 10-K
Table of Contents
FORWARD-LOOKING STATEMENTS
This document contains and incorporates by reference "forward-looking statements" within the meaning of section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. In some cases, these statements can be identified by the use of forward-looking terminology such as "may," "will," "could," "should," "would," "expect," "anticipate," "continue" or other similar words. These statements discuss future expectations, contain projections of results of operations or of financial condition, or state trends and known uncertainties or other forward-looking information. You are cautioned that forward-looking statements are based on current expectations and are inherently uncertain. Actual performance and results of operations may differ materially from those projected or suggested in the forward-looking statements due to certain risks and uncertainties, including the risks and uncertainties described or discussed in the section entitled "Risk Factors" in this Annual Report. The forward-looking statements contained and incorporated herein represent our judgment as of the date of this Annual Report, and we caution readers not to place undue reliance on such statements. The information contained in this Annual Report is provided by us as of the date of this Annual Report, and we do not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
Forward-looking statements include information concerning possible or assumed future results of our operations, including statements regarding:
- •
- our expectations regarding publishing, clinical trials, development time lines and regulatory authority approval for and oversight of CUBICIN or other drug candidates;
- •
- our expected research and development investment and expenses and gross margins;
- •
- our expectations regarding our personnel needs, including with respect to our sales force;
- •
- our expectations regarding our acquisition and integration of Illumigen Biosciences, Inc., or Illumigen;
- •
- the continuation of our collaborations and our ability to establish and maintain successful manufacturing, sales and marketing, distribution and development collaborations;
- •
- our expectations regarding our needs for CUBICIN active pharmaceutical ingredient, or API;
- •
- our intention not to repurchase our shares in the foreseeable future;
- •
- our expectations regarding the payment of dividends and potential repurchases of our securities;
- •
- the impact of new accounting pronouncements;
- •
- our future capital requirements and our ability to finance our operations;
- •
- our expected efforts to evaluate product candidates and build our pipeline; and
- •
- our business strategy and our expectations regarding general business conditions and growth in the biopharmaceutical industry and the overall economy.
Many factors could affect our actual financial results and could cause these actual results to differ materially from those in these forward-looking statements. These factors include the following:
- •
- the level of acceptance of CUBICIN by physicians, patients, third-party payors and the medical community;
- •
- any changes in the current or anticipated market demand or medical need for CUBICIN;
- •
- any unexpected adverse events related to CUBICIN, particularly as CUBICIN is used in the treatment of a growing number of patients around the world;
2
- •
- the effectiveness of our sales force and our sales force's ability to access targeted physicians;
- •
- whether or not third parties may seek to market generic versions of our products by filing Abbreviated New Drug Applications, or ANDAs, with the FDA, and the results of any litigation that we file to defend and/or assert our patents against such generic companies;
- •
- competition in the markets in which we and our partners market CUBICIN, including marketing approvals for new products that will be competitive with CUBICIN;
- •
- our ability to discover, acquire or in-license drug candidates and develop and achieve commercial success for drug candidates;
- •
- the ability of our third party manufacturers, including our single source provider of API, to manufacture sufficient quantities of CUBICIN in accordance with Good Manufacturing Practices and other requirements of the regulatory approvals for CUBICIN and at an acceptable cost;
- •
- our ability to integrate successfully the operations of any business that we may acquire and the potential impact of any future acquisition on our financial results;
- •
- whether the U.S. Food and Drug Administration, or FDA, accepts proposed clinical trial protocols that may be achieved in a timely manner for additional studies of CUBICIN or any other drug candidate we seek to enter into clinical trials;
- •
- our ability to conduct successful clinical trials in a timely manner;
- •
- the effect that the results of ongoing or future clinical trials of CUBICIN may have on its acceptance in the medical community;
- •
- whether we will receive, and the potential timing of, regulatory approvals or clearances to market CUBICIN in countries where it is not yet approved;
- •
- legislative and policy changes in the United States and other jurisdictions where our products are sold that may affect the ease of getting a new product or a new indication approved;
- •
- changes in government reimbursement for our or our competitors' products;
- •
- our dependence upon collaborations with our partners and our partners' ability to execute on development, regulatory and sales expectations in their territories;
- •
- our ability to finance our operations;
- •
- potential costs resulting from product liability or other third party claims;
- •
- our ability to protect our proprietary technologies; and
- •
- a variety of risks common to our industry, including ongoing regulatory review, public and investment community perception of the industry, legislative or regulatory changes, and our ability to attract and retain talented employees.
3
PART I
ITEM 1. BUSINESS
Cubist Pharmaceuticals, Inc., which we refer to as "Cubist" or the "Company", was incorporated in Delaware in 1992. We completed our initial public offering in 1996, and our shares are listed on the NASDAQ Global Select Market, where our symbol is CBST. Our principal offices are located at 65 Hayden Avenue, Lexington, Massachusetts. Our telephone number is 781-860-8660, and our website address is www.cubist.com.
Corporate Overview and Business Strategy
Cubist is a biopharmaceutical company focused on the research, development and commercialization of pharmaceutical products that address unmet medical needs in the acute care environment. To date, we have concentrated exclusively on developing products for the anti-infective marketplace.
Cubist has one marketed product, an intravenous (IV) antibiotic, CUBICIN® (daptomycin for injection), which was launched in the U.S. in November 2003. CUBICIN is approved in the U.S. for the treatment of complicated skin and skin structure infections, or cSSSI, caused byStaphylococcus aureus (S. aureus) and certain other Gram-positive bacteria, and for blood-stream infections (bacteremia), including right-sided infective endocarditis, caused by methicillin susceptible and methicillin resistantS. aureus (MSSA and MRSA). Since its U.S. launch, CUBICIN also has received similar regulatory approvals in many markets outside the U.S., including the European Union and Canada. Cubist commercializes CUBICIN in the U.S. and has established marketing agreements with other companies for commercialization of CUBICIN in all countries outside the U.S.
We have focused our pipeline building efforts on opportunities that leverage our anti-infective and acute-care discovery, development, regulatory, and commercialization expertise. Currently, we have two anti-infective programs approaching the Investigational New Drug Application, or IND, filing stage preparatory to clinical trials. These programs are described in the Product Pipeline section of this report.
The Markets for Which We Develop and Market Acute Care Therapy Today
Antibacterial Agents for Serious Infections
Antibacterial therapies work by inhibiting specific critical processes in a bacterial pathogen. Such therapies can be either static—inhibiting growth of the pathogen, or cidal—causing the death of the pathogen. Many antibiotics in use today were developed and introduced into the market from the 1950s to the 1980s. Most of these were developed from existing classes of drugs such as semi-synthetic penicillins, cephalosporins, macrolides, quinolones and carbapenems. Only two new antibiotics from new chemical classes have been introduced to the market in the past 35 years—Zyvox, a static agent which is known generically as linezolid and is from the oxazolidinones chemical class, and CUBICIN, a cidal agent known generically as daptomycin, which is a lipopeptide.
The increasing prevalence of drug-resistant bacterial pathogens has led to increased mortality rates, prolonged hospitalizations, and increased healthcare costs. The resistant organisms have emerged from both the Gram-positive and Gram-negative classes of bacteria. Gram-positive bacteria can be differentiated from Gram-negative bacteria by the differences in the structure of the bacterial envelope. Gram-positive bacteria possess a single cellular membrane and a thick cell wall component, whereas Gram-negative bacteria possess a double cellular membrane with a thin cell wall component. These cellular structures greatly affect the ability of an antibiotic to penetrate the bacterium and reach its target site.
4
Examples of drug-resistant Gram-positive bacterial pathogens include:
- •
- MRSA (methicillin-resistantStaphylococcus aureus): S. aureus, often referred to simply as "staph," are bacteria commonly carried on the skin or in the nose of healthy people. In some cases,S. aureus can cause an infection, and these bacteria are among the most common causes of skin infections in the U.S. These infections can be minor (such as pimples or boils) which can be treated in many cases without antibiotics (by draining an abscess for example). However,S. aureus bacteria can also cause more serious infections (such as post-surgical wound infections, pneumonia, and infections of the bloodstream and of the bone and joints). Over the past 50 years, treatment of these infections has become more difficult due to the prevalence of MRSA, that is,S. aureus that have become resistant to various antibiotics, including commonly used penicillin-related antibiotics. As reported by the CDC and others, more than 60% ofS. aureus isolates in the U.S. are methicillin-resistant.
The practical definition of resistance for a pathogen is when the minimum inhibitory concentration, or MIC value, exceeds a pre-specified limit for that specific antibiotic. Vancomycin has been the standard of care for patients who have serious MRSA infections. However, several strains of staphylococci, such as GISA (glycopeptides intermediateStaphylococcus aureus, MIC = 8-16 µg/ml), and VRSA (vancomycin-resistantStaphylococcus aureus, MIC >/= 32 µg/ml), have developed reduced susceptibility or resistance to vancomycin. In addition, recent published reports document a poor clinical success rate for vancomycin therapy against someS. aureus isolates with a vancomycin MIC of 1.0 to 2.0 µg/ml; levels which are still officially designated as within the FDA susceptibility range (</= 4 µg/ml) for vancomycin. In recognition of the issues with vancomycin susceptibility, the Clinical Laboratory Standards Institute, or CLSI has approved lower susceptibility criteria (</=2 mcg/mL as susceptible) for vancomycin againstS. aureus, and the American Society of Microbiology has issued a statement in support of these tighter standards.
While infections caused by MRSA had been associated mostly with hospital and long-term care settings, the incidence of community-acquired MRSA, or CA-MRSA, infections has been increasing rapidly. Of great concern to the infectious disease community and public health authorities, such as the U.S. Centers for Disease Control and Prevention, is the fact that community-acquired MRSA infections show up in otherwise healthy individuals—not fitting the traditional profile for an "at risk" patient such as a frequent user of the health care system who is more likely to be exposed to MRSA infections. As a result, individuals contracting a MRSA infection outside of the healthcare system can be misdiagnosed and receive inappropriate initial therapy. Such patients can get more seriously ill and require hospitalization. Of additional concern to the infectious disease community is the fact that most community-acquired MRSA strains are more virulent than the strains traditionally found in hospitals. The CA-MRSA strains have the ability to defeat the host's immune system, thereby resulting in an infection becoming more severe more quickly.
- •
- GISA or VISA (glycopeptide- or vancomycin-intermediately susceptibleS. aureus): The first reports ofS. aureus infections with decreased susceptibility to vancomycin occurred in 1998. Such bacterial strains have been found in wide geographic areas throughout Japan and North America and have recently emerged in Europe. However, the incidence of these strains remains rare.
- •
- Heteroresistance: Heteroresistance refers to the situation in which a small sub-population of bacteria survives at concentrations of antibiotic that effectively kill the majority of the population (or stop them from growing). Specialized testing techniques are required to detect heteroresistance to vancomycin, which appears to be becoming more common inS. aureus. The clinical impact of heteroresistance is unknown.
5
- •
- VRSA (vancomycin-resistantS. aureus): During 2002, the first isolates of fully vancomycin-resistantS. aureus were discovered in the U.S. Unexpectedly, rather than evolving from a VISA strain, these VRSA emerged from MRSA strains that had acquired the vancomycin-resistance gene from vancomycin-resistant Enterococci, or VRE.
- •
- VRE (vancomycin-resistant Enterococci): The emergence of VRE strains in the 1990s has led to infections for which only limited commercially available therapy exists.
- •
- Clostridium difficile: C.difficile is an opportunistic anaerobic Gram-positive bacterium causing the most commonly diagnosed form of hospital-acquired, or nosocomial, diarrhea—Clostridium difficile associated diarrhea, or CDAD. Recent years have witnessed the emergence of a hypervirulent strain ofC. difficile that produces much higher levels of toxins. This strain also demonstrates high level resistance to fluoroquinolones which may have contributed to its spread through the U.S., Canada, the United Kingdom, the Netherlands and Belgium. Physicians have noted an increase in incidence and mortality rates as well as increases in numbers of patients requiring emergency colectomy (removal of all or part of the colon) or admission to ICUs.
Examples of resistant Gram-negative pathogens are:
- •
- Pan-resistantPseudomonas aeruginosa:P.aeruginosa is a major cause of opportunistic infections among immunocompromised patients. Multi-drug resistance is increasingly observed in clinical isolates reflecting both their innate resistance (limited permeability of theP. aeruginosa outer membrane) along with acquisition of resistance mechanisms. It is now commonplace for a burn patient to develop an infection with a pan-resistant organism—resistant to B-lactams, fluoroquinolones, tetracycline, chloramphenicol, macrolides, trimethoprim/sulfa, and aminoglycosides.
- •
- ESBL positive Gram-negatives: Extended-spectrum B-lactamases (ESBLs) are plasmid-mediated bacterial enzymes that result from genetic mutations of native B-lactamases such that they confer resistance to a broader group of antibiotics including third-generation cephalosporins. Since the first ESBL positive strain was recognized approximately 20 years ago, these ESBL producing pathogens have spread and are now found in every part of the world. Clinical failures have been associated with use of the third generation cephalosporins—most frequently ceftazidime. Proper detection of ESBLs and appropriate treatment strategies are needed to overcome such rising resistance.
The prevalence of resistant organisms creates a growing need for therapies with novel mechanisms of action.
Antiviral therapy for Hepatitis C Virus (HCV) infections
HCV is a virus that primarily targets the liver, currently causing infection in more than 4 million people in the U.S. and 180 million people worldwide. The virus is difficult to eradicate, with infected patients eventually developing chronic liver infection, and, in some cases, liver cancer. HCV infection is the most common reason for liver transplantation in the U.S. and Western Europe and the leading cause of death from liver disease.
No vaccine is currently available to prevent HCV infection. Current HCV therapy combines a pegylated-interferon with ribavirin for up to 48 weeks of treatment. Current therapy has significant problems with both safety (e.g., significant treatment limiting adverse effects and contraindications) and efficacy (e.g., 80% of HCV infections in the U.S. are due to genotype 1 virus for which the efficacy rate of current therapy is approximately 40 to 50%). The HCV market was $2.2 billion in 2005 and is projected to double to $4.4 billion in 2010. This growth will be driven by an increase in the number of patients being treated, uptake of new drugs, and the use of multi-drug treatment regimens.
6
Our Flagship Product: CUBICIN
CUBICIN is the first antibiotic from a class of anti-infectives called lipopeptides. CUBICIN is currently the only marketed once-daily, bactericidal, intravenous (IV) antibiotic with activity against methicillin-resistantS. aureus, or MRSA. CUBICIN is approved in the U.S. for the treatment of complicated skin and skin structure infections, or cSSSI, caused byS. aureus and certain other Gram-positive bacteria, and forS. aureus bloodstream infections (bacteremia), including right-sided infective endocarditis caused by MRSA and MSSA. In the European Union, or EU, CUBICIN is approved for the treatment of complicated skin and soft tissue infections, or cSSTI, where the presence of susceptible Gram-positive bacteria is confirmed or suspected. In September 2007, the Marketing Authorization for the CUBICIN label in the EU was expanded to include right-sided infective endocarditis, or RIE, due toS. aureus bacteremia andS. aureus bacteremia associated with RIE or cSSTI. Other markets where CUBICIN has an approved label for cSSSI caused by certain Gram-positive bacteria and forS. aureus blood stream infections include Argentina, Canada, India, Israel, Korea and Taiwan.
We believe that CUBICIN provides important advantages over existing antibiotic therapies in its approved indications, given its rapid bactericidal properties demonstratedin vitro*, distinct mechanism of action, convenient once-daily dosing regimen, and established safety profile. In addition, CUBICIN's approval in the U.S. for the treatment ofS. aureus bloodstream infections is the first such approval by the FDA in more than 20 years, and is based on results from the only prospective, randomized, and controlled registration trial ofS. aureus bacteremia and endocarditis ever undertaken. CUBICIN's spectrum of activity includes both susceptible strains of Gram-positive pathogens and strains that are resistant to other antibiotic therapies.
- *
- the clinical relevance ofin vitro data has not been established.
In our review of the infectious disease marketplace above, we referenced the increasing prevalence of drug-resistant bacterial pathogens as a concern to the infectious disease community. The need for multiple mutation steps and the small impact of each step on susceptibility substantially decreases the likelihood that daptomycin-susceptible bacteria will become daptomycin-resistant. At year-end, CUBICIN had been on the market for 50 months and has been used in the treatment of an estimated 460,000 patients. The number of reported resistant isolates, compared to the number of patients treated (or the numbers of bacteria being tested for susceptibility) continues to be extremely small, consistent with the findings of the pre-New Drug Application, or NDA, clinical program. Where clinical circumstances are known,S. aureus nonsusceptible isolates are generally associated with antibiotic underdosing or difficult-to-treat infections involving undrained abscesses or retained material.
Clinical Development of CUBICIN
We continue to undertake research which can add to the medical knowledge about CUBICIN. In 2007, we completed enrollment in a Phase 2 clinical study of CUBICIN designed to test the feasibility of treating complicated skin infections with shorter duration therapy at a higher dose of CUBICIN. We enrolled 102 patients in this initial evaluation of safety and efficacy of short duration therapy for cSSSI. In our conference call discussing our results for the fiscal 2007 year-end, we reported that:
- •
- Four days of CUBICIN therapy at 10mg/kg/day resulted in cure rates consistent with what we saw in the cSSSI pivotal studies. This is despite the fact that more patients in this trial had MRSA infections, which are more difficult to treat. The trial was not sized for statistical significance vs. standard of care therapy. There was a slightly higher numerical success rate for the longer—up to two weeks—standard of care therapy.
7
- •
- A dose of 10mg/kg/day for cSSSI was well tolerated, with safety findings consistent with what we have seen in our pivotal skin trials and with CUBICIN use since launch.
- •
- A majority of CUBICIN patients in the study were treated as outpatients at 10 mg/kg/day for 4 days.
We expect to submit these findings for publication or presentation in appropriate peer-reviewed journals or forums. We may consider additional studies regarding shorter duration therapy, but would likely want to make refinements based on the learning from this study. The benefit of higher doses of CUBICIN, such as 10 mg/kg, may be more important in more serious infections, such as bacteremia, and it is likely that we will further investigate the effects of higher doses on such infections.
Higher doses of CUBICIN are being investigated in a number of settings both by Cubist and outside investigators. One of these studies is our comparative dosing prosthetic joint infection, or PJI, Phase 2 trial. Here we are comparing 6 and 8 mg/kg of CUBICIN for 6 weeks against standard therapy (either vancomycin or teicoplanin) for PJI, which is one form of osteomyelitis (infection in the bone). This is a difficult trial in which to enroll candidates because the incidence of infections following knee or hip implants is very low (under 5%) and we are dealing with an older, sicker population who will not always qualify for the study based on the entry criteria. We will continue to monitor enrollment to determine if we need to seek approval from the FDA to loosen the entry criteria. We currently expect enrollment to continue through 2008 with data available from the PJI trial in 2009.
We will begin to enroll patients this year in our Pediatric safety and efficacy trial in complicated skin infections. This is a regulatory commitment and represents an area of growing unmet need, and an opportunity to optimize the utility of CUBICIN in the infectious disease armamentarium. We plan to enroll 225 children, ages 7 to 17, with about 150 receiving CUBICIN therapy. The study should take approximately one year and, accordingly, we expect data to be available in 2009. We will begin a pharmacokinetics, or PK, study in younger children, ages 2 to 6. This should begin enrolling in Q3, but will be slower to enroll, with data expected to be available in 2010.
Other development for CUBICIN this year includes progressing our work with renal-impaired patients. We have a PK study underway, in patients receiving hemodialysis or chronic ambulatory peritoneal dialysis, and following our receipt of these results, we will review them with the FDA and begin to prepare for a Phase 4 safety and efficacy study in renal-impaired patients with complicated skin infections.
An additional post-approval regulatory commitment that we have with the FDA is a study of CUBICIN as part of combination therapy for infective endocarditis. Here we will compare safety and efficacy of CUBICIN at 6 mg/kg with and without gentamicin. We expect this exploratory Phase 2 study should begin enrollment in the first half of 2008.
CUBICIN in the U.S. Market
We generated $285.1 million, $189.5 million and $113.4 million in U.S. net product sales of CUBICIN in 2007, 2006 and 2005, respectively. We market CUBICIN to more than 2,000 institutions (hospitals and outpatient acute care settings) that represent approximately 83% of the total market opportunity for IV antibiotics to treat serious Gram-positive infections. In addition to our in-house marketing team, our acute care sales force as of February 1, 2008 included approximately 157 clinical business managers (CBMs). We have been hiring CBMs over the past few months to achieve our stated goal of increasing the number of CBMs in the U.S. from 135 to 164 by April 1, 2008. In addition, the U.S. acute care sales organization includes small numbers of regional business directors (RBDs), regional access managers (RAMs) who help to facilitate the acquisition of CUBICIN for use in the outpatient infusion market, and Senior Sales Directors (SSDs).
8
Our International Marketing Partners for CUBICIN
In 2007, total international revenue for CUBICIN was $5.3 million. CUBICIN is being introduced to markets outside the U.S. through alliances we have entered into with other companies. Novartis AG, or Novartis, through a subsidiary, is responsible for regulatory filings, sales, marketing and distribution costs in Europe, Australia, New Zealand, India, and certain Central American, South American and Middle Eastern countries. Other international partners for CUBICIN include Medison Pharma, Ltd., for Israel, Oryx Pharmaceuticals, Inc. for Canada, TTY BioPharm for Taiwan, and Kuhnil Pharma Co., Ltd. for Korea. AstraZeneca AB, or AstraZeneca, has licensed rights to CUBICIN in China as well as more than one hundred additional countries. In 2007, Cubist completed global marketing alliances for CUBICIN when it licensed the Japanese rights to CUBICIN to Merck & Co. which will develop and commercialize CUBICIN in Japan through its wholly owned subsidiary, Banyu Pharmaceutical Co., Ltd.
Each partner is responsible for seeking regulatory approvals to market CUBICIN in its territory. Cubist is responsible for manufacturing and supplying CUBICIN to our partners in exchange for a transfer price and, in the case of Novartis, a possible additional royalty.
Our Product Pipeline
Our research and development programs focus on opportunities created by unmet needs in the acute care and anti-infective market and leverage the expertise and experience we have gained through our past and continued development of CUBICIN. We have some promising preclinical programs underway.
For example, in the fourth quarter of 2007, we acquired a pre-clinical hepatitis C therapy candidate, IB657, when we purchased Illumigen. IB657 is a protein therapeutic which is an optimized oligoadenylate synthetase (or OAS). IB657 has potentin vitro antiviral activity against HCV and some other viruses, and has the potential to be a safe and effective mainstay of therapy for Hepatitis C. We have valued the compound as an add-on to current HCV therapy, where it could act as an interferon-sparing agent. This program is moving forward in pre-IND mode. We expect to create good manufacturing practices, or GMP, quality materials and assemble an IND dossier while also beginning to work on contract research organization, or CRO, logistics so we will be prepared to move quickly into the clinic. We have set an objective of an IND filing in the second half of 2008.
As announced in our conference call announcing our results for our 2007 fiscal year, Cubist scientists have been working on an antibiotic agent for the treatment of CDAD. We have identified an interesting lead molecule with preclinical efficacy againstC. difficile. Unlike other marketed agents forC. difficile, our research shows this candidate is rapidly cidal, meaning it kills the bacteria quickly, which could give the compound advantages in treatment of infections caused byC. difficile. We are focusing attention on this antibiotic CDAD program and are moving aggressively towards our goal of an IND filing by the end of this year.
We also are developing compounds which we believe have promise as potential therapy for infections caused by resistant Gram-negative pathogens, where there is a significant unmet medical need. We have made progress in assessing potency, efficacy, PK, and safety of the molecules we are developing, in animal models of infections caused by a variety of Gram-negative pathogens.
In the past, we have referenced two additional preclinical programs: our lipopeptide program for a CUBICIN-like therapy with activity in the lung, and a toxin binder therapy for the treatment of CDAD we were developing in collaboration with Ilypsa. In the fourth quarter, we suspended our lipopeptide pneumonia program due to the difficulty in improving upon daptomycin and adding pulmonary activity, and we terminated our CDAD toxin binder research collaboration with Ilypsa which we began in 2006.
9
Our Research and Development Expenditures
Our research and development expenditures, which include research related to CUBICIN, as well as our drug discovery projects, were $85.2 million, $57.4 million and $51.7 million in 2007, 2006 and 2005, respectively. Based on our ongoing investments in CUBICIN, and the progression of our product pipeline programs, we expect that our investments in research and development will grow in 2008.
Our Significant Customers
Revenues from Cardinal Health, Inc., or Cardinal, accounted for approximately 32%, 33% and 32% of all revenues for the years ended December 31, 2007, 2006 and 2005, respectively. Revenues from Amerisource Bergen Drug Corporation accounted for approximately 30%, 32% and 31% of all revenues for the years ended December 31, 2007, 2006 and 2005, respectively. Revenues from McKesson Corporation accounted for approximately 20%, 21% and 21% of all revenues for the years ended December 31, 2007, 2006 and 2005, respectively.
Our Intellectual Property Portfolio
We seek to protect our novel compounds, cloned targets, expressed proteins, assays, organic synthetic processes, screening technology and other technologies by, among other things, filing, or causing to be filed on our behalf, patent applications.
To date, Cubist and its subsidiaries own or co-own 34 issued U.S. patents, 28 pending U.S. patent applications, 42 issued foreign patents and approximately 189 pending foreign patent applications. We have exclusively licensed technology from Eli Lilly and Company, or Eli Lilly, related to the composition, manufacture, and use of daptomycin, the active ingredient in CUBICIN. The primary composition of matter patent covering daptomycin in the U.S. has expired; however, currently there are five issued U.S. patents owned by Cubist (U.S. Patent Nos. 6,852,689; 6,696,412; 6,468,967; RE39,071; and 4,885,243) that cover the drug product, manufacture, and/or administration or use of daptomycin. In addition, we have also filed a number of patent applications in our name relating to the composition, manufacture, administration and/or use of daptomycin and/or other lipopeptides.
Manufacturing, Distribution and Other Agreements
In September 2001, we entered into a manufacturing and supply agreement with ACS Dobfar SpA, or ACS, pursuant to which ACS agreed to provide scale-up services and to construct a production facility dedicated to the manufacture and sale of API for CUBICIN exclusively to us for commercial purposes. In accordance with this agreement, we purchase API from ACS subject to minimum annual quantity requirements. We also currently engage ACS to manufacture API for our clinical trials of CUBICIN. Upon the termination of our contract with a previous supplier in 2006, ACS became the single source supplier of API for CUBICIN. We expect that ACS's substantial fermentation and purification plant capacity can meet all of our anticipated needs for CUBICIN API.
In April 2000, we entered into an agreement with Hospira, Inc., or Hospira, formerly the core global hospital products business of Abbott Laboratories. Under this agreement, Hospira currently converts API into our finished, vialed formulation of CUBICIN. In September 2003, we entered into a packaging services agreement with Catalent Pharma Solutions, LLC, or Catalent, the successor-in-interest to Cardinal Health PTS, LLC, pursuant to which Catalent packages and labels the finished CUBICIN product. In September 2004, we entered into an additional services agreement with Catalent to provide fill/finish as well as packaging services for the finished CUBICIN product. Hospira and Catalent both continue to provide fill/finish services for CUBICIN, with Catalent also providing packaging services.
10
In June 2003, we entered into a services agreement with Integrated Commercialization Solutions, Inc., or ICS, whereby ICS agreed to exclusively manage our CUBICIN warehousing and inventory program and to distribute finished product to our customers. ICS also provides us with order processing, order fulfillment, shipping, collection and invoicing services in support of the direct ship model we have employed since the launch of CUBICIN in the U.S.
In September 2001, Cubist entered into a services agreement with PPD Development, LLC, or PPD, pursuant to which PPD has agreed to provide various clinical, laboratory, GMP and other research and testing services. In December 2006, Cubist received approval from the FDA to begin release testing of CUBICIN at its Lexington facility. Testing for the U.S. market that was previously performed at PPD is now performed by Cubist.
Competition
CUBICIN is currently approved in the U.S. for the treatment of cSSSI caused by certain Gram-positive bacteria, and for the treatment ofS. aureus bloodstream infections (bacteremia), including right-sided infective endocarditis caused by MRSA and MSSA. There are many currently approved antibiotics used to treat these types of infections. The most commonly prescribed antibiotics for susceptible strains of bacteria are: first-generation cephalosporins, such as cefazolin, and semi-synthetic penicillins, such as oxacillin and nafcillin. For the treatment of resistant organisms, the most commonly prescribed treatments are vancomycin and linezolid. All of these antibiotics, except linezolid, which is marketed as Zyvox®, and tygacycline, a broad spectrum antibiotic which is marketed as Tygacil®, are distributed by generic manufacturers at relatively low drug acquisition cost. In addition, the FDA is reviewing an NDA for the Gram-positive agent telavancin (submitted by Theravance, Inc., whose partner for telavancin is Astellas Pharma US, Inc.). Telavancin received an approvable letter on October 22, 2007. The FDA also is reviewing ceftobiprole, a broad spectrum agent with MRSA activity (NDA submitted in May 2007 by a division of Johnson & Johnson). Another Gram-positive agent under review is dalbavancin from Pfizer, Inc. Pfizer received its first approvable letter for dalbavancin in September 2005, and has received multiple approvable letters from the FDA since then, citing the need for additional data, among other requests. In February 2008, Targanta Therapeutics Corporation announced that it had submitted an NDA to the FDA for oritivancin to be used for the treatment of cSSSI caused by Gram-positive bacteria, including MRSA. In July 2007, Arpida Ltd. reported the completion of the Phase 3 trial for iclaprim, a broad spectrum agent with MRSA activity, in cSSSI. Accordingly, some or all of these agents may be approved and marketed in the near future and could compete with CUBICIN.
Government Regulation
Overview
Our development, manufacture and marketing of pharmaceutical drugs is subject to extensive regulation by numerous governmental agencies within the jurisdictions where we choose to market our products, principally the FDA in the United States, the European Medicines Agency, or EMEA for EU member states, and by various country-specific regulatory bodies in other countries. These regulations not only dictate the form and content of safety and efficacy data regarding a proposed product, but also impose specific requirements regarding manufacture of the product, quality assurance, packaging, storage, documentation and record keeping, labeling, advertising and marketing procedures. All of these regulations and required oversight are intended to ensure the efficacy, safety and consistency of pharmaceuticals. The time and expense involved in meeting the requirements to obtain and maintain regulatory approvals are quite substantial.
11
U.S.—FDA Process
Pre Clinical Testing: Before testing of any compounds with potential therapeutic value in human subjects may begin in the U.S., stringent government requirements for pre-clinical data must be satisfied. Pre-clinical testing includes bothin vitro andin vivo (within a living organism) laboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. Pre-clinical testing results obtained from studies in several animal species, as well as fromin vitro studies, are submitted to the FDA as part of an IND application, and are reviewed by the FDA prior to the commencement of human clinical trials. These pre-clinical data must provide an adequate basis for evaluating both the safety and the scientific rationale for the initial clinical studies in human volunteers. Unless the FDA objects to an IND, the IND becomes effective 30 days following its receipt by the FDA. Once trials have commenced, the FDA may stop the trials by placing them on "clinical hold" because of concerns about, for example, the safety of the product being tested.
Clinical Trials: Clinical trials involve the administration of the drug to healthy human volunteers or to patients under the supervision of a qualified investigator, usually a physician, pursuant to an FDA-reviewed protocol. Human clinical trials are typically conducted in three sequential phases, although the phases may overlap with one another. Clinical trials must be conducted under protocols that detail the objectives of the study, the parameters to be used to monitor safety, and the efficacy criteria, if any, to be evaluated. Each protocol must be submitted to the FDA as part of the IND. Each clinical trial must be conducted under the auspices of an Institutional Review Board that considers, among other things, ethical factors, the safety of human subjects, the possible liability of the institution and the informed consent disclosure, which must be made to participants in the clinical trial.
Phase 1 Clinical Trials: Phase 1 clinical trials represent the initial administration of the investigational drug to a small group of healthy human subjects or, more rarely, to a group of select patients with the targeted disease or disorder. The goal of Phase 1 clinical trials is typically to test for safety, dose tolerance, absorption, bio-distribution, metabolism, excretion and clinical pharmacology and, if possible, to gain early evidence regarding efficacy.
Phase 2 Clinical Trials: Phase 2 clinical trials involve a small sample of the actual intended patient population and seek to assess the efficacy of the drug for specific targeted indications, to determine dose response and the optimal dose range and to gather additional information relating to safety and potential adverse effects.
Phase 3 Clinical Trials: Once an investigational drug is found to have some efficacy and an acceptable safety profile in the targeted patient population, Phase 3 clinical trials are initiated to establish further clinical safety and efficacy of the investigational drug in a broader sample of the general patient population at geographically dispersed study sites in order to determine the overall risk-benefit ratio of the drug and to provide an adequate basis for product labeling. The Phase 3 clinical development program consists of expanded, large-scale studies of patients with the target disease or disorder to obtaindefinitive statistical evidence of the efficacy and safety of the proposed product and dosing regimen.
All of the phases of clinical studies must be conducted in conformance with the FDA's bioresearch monitoring regulations.
New Drug Application: All data obtained from a comprehensive development program including research and product development, manufacturing, pre-clinical and clinical trials and related information are submitted in a New Drug Application, or NDA, to the FDA and in similar regulatory filings with the corresponding agencies in other countries for review and approval. In certain circumstances, this information is submitted in a Biologics License Application, or BLA. In addition to reports of the trials conducted under the IND, the NDA includes information pertaining to the
12
preparation of the new drug, analytical methods, details of the manufacture of finished products and proposed product packaging and labeling. Although the Food Drug and Cosmetic Act requires the FDA to review NDAs within 180 days of their filing, in practice, longer times may be required.
In some cases, the FDA may decide to expedite the review of new drugs that are intended to treat serious or life threatening conditions and demonstrate the potential to address unmet medical needs. Cubist was granted such a Priority Review after the CUBICIN NDA was submitted in 2002; and in 2005 after submission of the supplemental new drug application, or sNDA, for the expansion of the CUBICIN label.
Phase 4 Clinical Trials: Phase 4 clinical trials are studies that are conducted after a product has been approved. These trials can be conducted to collect long-term safety information or to collect additional data about a specific population. As part of a product approval, the FDA may require that certain Phase 4 studies be conducted post-approval, and in these cases these Phase 4 studies are called post-marketing commitments.
Hatch-Waxman Act: Under the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, Congress created an abbreviated FDA review process for generic versions of pioneer (brand name) drug products like CUBICIN. The law also provides incentives by awarding, in certain circumstances, non-patent marketing exclusivities to pioneer drug manufacturers. Newly approved drug products and changes to the conditions of use of approved products may benefit from periods of non-patent marketing exclusivity in addition to any patent protection the drug product may have. The Hatch-Waxman Act provides five years of "new chemical entity", or NCE, marketing exclusivity to the first applicant to gain approval of an NDA for a product that does not contain an active ingredient found in any other approved product. The FDA granted CUBICIN five years of NCE exclusivity, which expires on September 12, 2008. During this five-year period, the FDA is prohibited from accepting any Abbreviated New Drug Application, or ANDA, for a generic drug. The FDA is also prohibited from accepting any NDA during this five-year period where the applicant does not own or have a legal right of reference to all of the data required for approval, otherwise known as a 505(b)(2) application. The five-year exclusivity protects the entire new chemical entity franchise, including all products containing the active ingredient for any use and in any strength or dosage form. This exclusivity will not prevent the submission or approval of a full NDA, as opposed to an ANDA or 505(b)(2) application, for any drug, including, for example, a drug with the same active ingredient, dosage form, route of administration, strength and conditions of use.
The Hatch-Waxman Act also provides three years of exclusivity for applications containing the results of new clinical investigations (other than bioavailability studies) essential to the FDA's approval of new uses of approved products, such as new indications, dosage forms, strengths, or conditions of use. The FDA granted CUBICIN three years of exclusivity, which expires on May 25, 2009, for the additional indication ofStaphylococcus aureus, orS. aureus, bloodstream infections (bacteremia). However, this exclusivity only protects against the approval of ANDAs and 505(b)(2) applications for the protected use and will not prohibit the FDA from accepting or approving ANDAs or 505(b)(2) applications for other products containing the same active ingredient.
The Hatch-Waxman Act requires NDA applicants and NDA holders to provide certain patent information for listing in "Approved Drug Products with Therapeutic Equivalence Evaluations," also known as the "Orange Book". ANDA and 505(b)(2) applicants must then certify regarding each of the patents listed with the FDA for the reference product(s). A certification that each listed patent is invalid or will not be infringed by the marketing of the applicant's product is called a "Paragraph IV certification." If the ANDA or 505(b)(2) applicant provides such a notification of patent invalidity or noninfringement, then the FDA may accept the ANDA or 505(b)2) application four years after approval of the NDA.
13
If a Paragraph IV certification is filed and the ANDA has been accepted as a reviewable filing by the FDA, the ANDA or 505(b)(2) applicant must then within 30 days provide notice to the NDA holder and patent owner stating that the application has been submitted and providing the factual and legal basis for the applicant's opinion that the patent is invalid or not infringed. If the NDA holder or patent owner files suit against the ANDA or 505(b)(2) applicant for patent infringement within 45 days of receiving notice of the Paragraph IV certification, a one-time 30-month stay of the FDA's ability to approve the ANDA or 505(b)(2) application is triggered. The 30 month stay begins at the end of the NDA holder's 5 years of data exclusivity, or, if data exclusivity has expired, on the date that the patent holder is notified. The FDA may approve the proposed product before the expiration of the 30-month stay if a court finds the patent invalid or not infringed, or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.
Pediatric Exclusivity: Section 505(a) of the Food & Drug Act provides for 6 months of exclusivity based on the submission of pediatric data subsequent to a written request from the FDA. This period of exclusivity is added to whatever period of exclusivity covers a drug (e.g., Hatch-Waxman, Orphan, patent). This is not a patent term extension, rather, it extends the period during which the FDA cannot approve an ANDA.
European Union—EMEA Process
In the EU, the EMEA requires approval of a marketing authorization application, or MAA before a pharmaceutical drug is brought to market in EU member states. In many EU countries, pricing negotiations also must take place before the product is sold.
Other International Markets—Drug approval process
In some international markets (e.g., China, Japan) additional clinical trials may be required prior to the filing or approval of marketing applications within the country.
Other Regulatory Processes
We are subject to a variety of financial disclosure and securities trading regulations as a public company in the United States, including the laws relating to the oversight activities of the Securities and Exchange Commission and the regulations of the NASDAQ Stock Market, on which our shares are traded. We are also subject to regulation under other federal laws and regulation under state and local laws, including laws relating to occupational safety, laboratory practices, environmental regulations, and hazardous substance control.
Our Employees
As of February 1, 2008, we had approximately 489 full-time employees, approximately 140 of whom were engaged in research and development and approximately 349 of whom were engaged in management, marketing, sales, administration and finance. We consider our employee relations to be good.
14
Our Executive Officers and Directors
| Michael W. Bonney | | 49 | | President, Chief Executive Officer and Director |
| Robert J. Perez, MBA | | 43 | | Executive Vice President, Chief Operating Officer |
| Lindon M. Fellows | | 56 | | Senior Vice President, Technical Operations |
| Steven C. Gilman, Ph.D. | | 55 | | Senior Vice President, Discovery and Non-clinical Development and Chief Scientific Officer |
| Christopher D.T. Guiffre, J.D., MBA | | 39 | | Senior Vice President, General Counsel and Secretary |
| David W.J. McGirr, MBA | | 53 | | Senior Vice President and Chief Financial Officer |
| Kenneth M. Bate, MBA(1) | | 57 | | Lead Director |
| Sylvie Grégoire, Pharm. D.(3)(4) | | 46 | | Director |
| David W. Martin, Jr., M.D.(2)(4)* | | 67 | | Director |
| Walter R. Maupay, Jr., MBA(2)(3)* | | 69 | | Director |
| Martin Rosenberg, Ph.D. (3)(4) | | 62 | | Director |
| J. Matthew Singleton, MBA, CPA(1)* | | 55 | | Director |
| Martin H. Soeters(1)(3) | | 53 | | Director |
| Michael B. Wood, M.D.(2)* | | 64 | | Director |
- (1)
- Member of Audit Committee
- (2)
- Member of Compensation Committee
- (3)
- Member of Corporate Governance and Nominating Committee
- (4)
- Member of Scientific Affairs Committee
- *
- Chair of Committee
Mr. Bonney has served as our President and Chief Executive Officer and as a member of the Board of Directors since June 2003. From January 2002 to June 2003, he served as our President and Chief Operating Officer. From 1995 to 2001, he held various positions of increasing responsibility at Biogen, Inc., a biopharmaceutical company, including Vice President, Sales and Marketing from 1999 to 2001. While at Biogen, Mr. Bonney built the commercial infrastructure for the launch of Avonex. Prior to that, Mr. Bonney held various positions of increasing responsibility in sales, marketing and strategic planning at Zeneca Pharmaceuticals, ending his eleven-year career there serving as National Business Director. Mr. Bonney received a B.A. in Economics from Bates College. Mr. Bonney is a director of NPS Pharmaceuticals, Inc., a biopharmaceutical company, and serves on the Boards of Trustees of the Beth Israel Deaconess Medical Center and Bates College. Mr. Bonney is also a member of the Biotechnology Industry Organization, or BIO, Health Section Governing Body.
Mr. Perez has served as our Executive Vice President and Chief Operating Officer since August 2007. Prior to this, he was our Senior Vice President, Commercial Operations since July 2004. From August 2003 to July 2004 he served as our Senior Vice President, Sales and Marketing. Prior to joining Cubist, he served as Vice President of Biogen, Inc.'s CNS Business Unit where he was responsible for leading the U.S. neurology franchise. From 1995 to 2001 he served as a Regional Director, Director of Sales, and Avonex® Commercial Executive at Biogen. From 1987 to 1995, Mr. Perez held various sales and marketing positions at Zeneca Pharmaceuticals, ultimately serving as Regional Business Director, responsible for sales, marketing and national accounts for the Western Regional Business Unit. Mr. Perez is a director of EPIX Pharmaceuticals, Inc., a biopharmaceuticals company. Mr. Perez
15
received a B.S. from California State University, Los Angeles and an M.B.A. from The Anderson School at UCLA.
Mr. Fellows has served as our Senior Vice President, Technical Operations since August 2005. From July 2004 until August 2005 Mr. Fellows was Vice President, Corporate Quality Assurance of Millennium Pharmaceuticals, a biopharmaceutical company, where he was responsible for ensuring product quality and compliance to both U.S. and international requirements. From July 1995 until July 2004, Mr. Fellows held various positions of increasing responsibility at DSM Life Sciences Products, including Managing Director, Director of Quality Compliance, and Vice President of Quality Assurance and Regulatory Affairs with responsibility for anti-infectives, fine chemicals, and food sciences. Mr. Fellows holds a B.S. in Microbiology from Colorado State University.
Dr. Gilman has served as our Senior Vice President, Discovery & Nonclinical Development and Chief Scientific Officer, since February 2008. From April 2007 until February 2008 Dr. Gilman served as Chairman of the board of directors and CEO of ActivBiotics, a biopharmaceutical company. From 2004 to April 2007, he served as President, CEO, and a member of the board of directors of ActivBiotics. Previously Dr. Gilman worked at Millennium Pharmaceuticals, Inc., where he held a number of senior leadership roles including Vice President and General Manager, Inflammation, responsible for all aspects of the Inflammation business from early gene discovery to product commercialization. Prior to Millennium, he was Group Director at Pfizer Global Research and Development, where he was responsible for drug discovery of novel antibacterial agents as well as several other therapeutic areas. Dr. Gilman has also held scientific, business, and academic appointments at Wyeth, Cytogen Corporation, Temple Medical School, and Connecticut College. He currently serves on the boards of directors of the Massachusetts Biotechnology Council and Nextcea, Inc., a private drug discovery company. Dr. Gilman received his Ph.D. and M.S. degrees in microbiology from Pennsylvania State University, his post-doctoral training at Scripps Clinic and Research Foundation, and received a B.A. in microbiology from Miami University of Ohio.
Mr. Guiffre has served as our Senior Vice President, General Counsel and Secretary since January 2004. He served as our Vice President, General Counsel and Secretary from December 2001 to December 2003. From 1997 to 2001, Mr. Guiffre held various positions of increasing responsibility at Renaissance Worldwide, Inc., a provider of information technology consulting services, including Counsel, Corporate Counsel and Director of Legal Affairs, and Vice President, General Counsel and Clerk. Prior to joining Renaissance Worldwide, he was an Associate at Bingham McCutchen LLP, a national law firm. He received a B.S. in Marketing from Babson College, a J.D. from Boston College Law School, and an M.B.A. from Boston College Carroll School of Management. Mr. Guiffre is a member of the Massachusetts Bar.
Mr. McGirr has served as our Senior Vice President and Chief Financial Officer since November 2002. He also served as our Treasurer from November 2002 until January 2003. From 1999 to 2002, Mr. McGirr was the President and Chief Operating Officer of hippo inc, an internet technology, venture-financed company. Mr. McGirr served as a member of hippo's Board of Directors from 1999 to 2003. From 1996 to 1999, he was the President of GAB Robins North America, Inc., a risk management company, serving also as Chief Executive Officer from 1997 to 1999. Mr. McGirr was a private equity investor from 1995 to 1996. From 1978 to 1995, Mr. McGirr served in various positions within the S.G. Warburg Group, ultimately as Chief Financial Officer, Chief Administrative Officer and Managing Director of S.G. Warburg & Co., Inc., a position held from 1992 to 1995. Mr. McGirr is a director of LifeCell Corporation, or LifeCell, a biotechnology company, and also serves as Chairman of the Audit Committee of LifeCell. Mr. McGirr received a B.Sc. in Civil Engineering from the University of Glasgow and received an M.B.A. from The Wharton School at the University of Pennsylvania.
Mr. Bate has served as one of our directors since June 2003 and became our lead director in June 2006. Since January 2007, Mr. Bate has been President and Chief Executive Officer and a director of
16
Nitromed, Inc., a pharmaceutical company. From March 2006 until January 2007, Mr. Bate was Chief Operating Officer and Chief Financial Officer of Nitromed. From 2002 to January 2005, Mr. Bate was head of commercial operations and Chief Financial Officer at Millennium Pharmaceuticals, Inc. In 1999, Mr. Bate co-founded JSB Partners, an investment banking and transaction advisory firm serving the biopharmaceutical industry. He was a partner at JSB Partners through 2002. From 1997 to 1999, Mr. Bate served as Senior Managing Director and Chief Executive Officer of MPM Capital, LP, a venture capital company. He was also an advisor to BB Bioventures, a venture capital fund. Mr. Bate's life sciences industry experience also includes six years at Biogen, Inc.; from 1993 to 1996 as the company's vice president of sales and marketing, and as Chief Financial Officer from 1990 to 1993. Mr. Bate is a director of AVEO Pharmaceuticals, Inc., a biopharmaceutical company. Mr. Bate received his B.A. degree in Chemistry from Williams College, and an MBA from The Wharton School of the University of Pennsylvania.
Dr. Grégoire has served as one of our directors since June 2006. Since 2007, Dr. Grégoire has served as President, Human Genetic Therapies division of Shire Pharmaceuticals Group plc, a pharmaceuticals company. From 2004 to 2005, Dr. Grégoire served as President and Chief Executive Officer of GlycoFi, Inc., a biotherapeutics company. From 2003 to 2004, Dr. Grégoire was a consultant to the biopharmaceuticals industry. From 2001 through 2003, Dr. Grégoire served as Executive Vice President, Technical Operations, of Biogen Idec Inc., a biotechnology company, and from 1995 to 2001, she held various roles of increasing responsibility with Biogen. Prior to Biogen, Dr. Grégoire held clinical research and regulatory roles with Merck & Co., a pharmaceuticals company. She is currently a director of IDM-Pharma, a biopharmaceuticals company. She received her Pharm.D. degree from the State University of New York at Buffalo and her pharmacy graduate degree (Bachalaureat en Pharmacie) from the Université Laval, Quebec City.
Dr. Martin has served as one of our directors since October 1997 and as our lead director from October 2004 until June 2006. Since 2004, he has been the Founder, Chairman, and Chief Executive Officer of AvidBiotics Corporation, a biotechnology company. In 2003, he was Chairman and Chief Executive Officer of GangaGen, Inc., a biotechnology company. From July 1997 until April 2003, Dr. Martin served as President, Chief Executive Officer and a founder of Eos Biotechnology, Inc., a biotechnology company. From 1995 to 1996, Dr. Martin was President and Chief Executive Officer of Lynx Therapeutics, Inc., a biotechnology company. During 1994 and through May 1995, Dr. Martin served as Senior Vice President of Chiron Corporation, a biopharmaceutical company. From 1991 to 1994, Dr. Martin served as Executive Vice President of DuPont Merck Pharmaceutical Company. From 1983 to 1990, Dr. Martin was Vice President and then Senior Vice President of Research and Development at Genentech, Inc., a biopharmaceutical company. Prior to 1983, Dr. Martin was a Professor of Medicine, Professor of Biochemistry and an Investigator of the Howard Hughes Medical Institute at the University of California, San Francisco. Dr. Martin is also Lead Director of Varian Medical Systems, Inc., a medical equipment and software supplier. Dr. Martin received an M.D. from Duke University.
Mr. Maupay has served as one of our directors since June 1999. From January 1995 to June 1995, Mr. Maupay served as Group Executive of Calgon Vestal Laboratories, a division of Bristol-Myers Squibb Corporation. From 1988 to 1995, Mr. Maupay served as President of Calgon Vestal Laboratories, a subsidiary of Merck and Company. From 1984 to 1988, Mr. Maupay served as Vice President, Healthcare at Calgon Vestal Laboratories. Mr. Maupay is a director of SyntheMed, Inc., a biomaterials company. He is also a director and non-executive chair of Kensey Nash Corporation, a medical device company. Mr. Maupay received his B.S. in Pharmacy from Temple University and an M.B.A. from Lehigh University.
Dr. Rosenberg has served as one of our directors since March 2005. Since 2003, Dr. Rosenberg has been the Chief Scientific Officer of Promega Corporation, a biotechnology company. From 2001 to 2003, Dr. Rosenberg served as Vice President, Research and Development of Promega Corporation.
17
From 2000 until 2001, Dr. Rosenberg was Senior Vice President, Anti-Infectives, Drug Discovery at GlaxoSmithKline, a pharmaceutical company. From 1996 until 2000, Dr. Rosenberg was Senior Vice President, Anti-Infectives at SmithKline Beecham Corporation, a predecessor company to GlaxoSmithKline. Prior to 2000, Dr. Rosenberg held a variety of roles of increasing responsibility with SmithKline Beecham Corporation. Before joining SmithKline Beecham, Dr. Rosenberg spent 10 years at the National Institutes of Health and was a Section Chief at the National Cancer Institute. Dr. Rosenberg is a director of Promega Corporation, the Medical College of Wisconsin Research Foundation, and Scarab Genomics, a biotechnology company. He also serves as a member of the Advisory Council for the National Institutes of Allergy & Infectious Diseases at the National Institute of Health. He participates on a variety of academic and industry Scientific Advisory Boards and holds an adjunct Professorship at the University of Wisconsin, Department of Bacteriology, Madison, WI. Dr. Rosenberg is Editor-in-Chief of Current Opinions in Biotechnology, a Senior Editor of the Journal of Bacteriology and a member of several other journal Editorial Boards. Dr. Rosenberg received a B.A. degree from the University of Rochester and a Ph.D. from Purdue University.
Mr. Singleton has served as one of our directors since June 2003. From 2000 to the present, he has served as Executive Vice President and Chief Financial Officer of CitationShares, LLC, a majority-owned subsidiary of Cessna Aircraft Company and Textron Inc. From 1994 to 1997, Mr. Singleton served as a Managing Director, Executive Vice President and Chief Administrative Officer of CIBC World Markets, an investment banking firm. Previous to that, he served in a variety of roles from 1974 until 1994 at Arthur Andersen & Co., a public accounting firm, ending his tenure there as Partner-In-Charge of the Metro New York Audit and Business Advisory Practice. During 1980 and 1981, he served as a Practice Fellow at the Financial Accounting Standards Board. Mr. Singleton served as a director of Salomon Asset Reinvestment Company from 1998 to 2006. He received an A.B. in Economics from Princeton University and an M.B.A. from New York University. Mr. Singleton is a Certified Public Accountant.
Mr. Soeters has served as one of our directors since September 2006. Since 2007, Mr. Soeters has served as Senior Vice President of Novo Nordisk Europe A/S, a healthcare company located in Zurich, Switzerland. From 2000 to 2007, Mr. Soeters served as President and Senior Vice President of Novo Nordisk Inc. in Princeton, NJ. From 1998 to 2000, he served as Senior Vice President International Marketing at Novo Nordisk Denmark, and from 1994 to 1998, he served as Managing Director of Novo Nordisk France. From 1992 to 1995, Mr. Soeters was Managing Director at Novo Nordisk Belgium, and in 1991, he was International Marketing Director at Novo Nordisk Denmark. Prior to that time, he held various sales and marketing positions at Novo Nordisk in the Netherlands between 1980 and 1991. Mr. Soeters is currently a director of Pharmacopeia, Inc., a biopharmaceutical company. He is also a member of the Board of Overseers of the Joslin Diabetes Center. He was a Trustee of the HealthCare Institute of New Jersey, and from 2005 to 2007, a member of the BIO Board of Directors. From 2004-2006, he served on the Board of Directors of the Pharmaceutical Research and Manufacturers of America (PhRMA) in Washington, D.C. Mr. Soeters studied meteorology, as well as sales, product and marketing management in the Netherlands, and he also attended the Stanford Executive Program.
Dr. Wood has served as one of our directors since March 2005. Dr. Wood is currently an Orthopedic Surgeon and retired President-emeritus of the Mayo Foundation and Professor of Orthopedic Surgery, Mayo Clinic School of Medicine. He was previously Chief Executive Officer of the Mayo Foundation from 1999 until 2003. Prior to 1999, Dr. Wood held a variety of roles within the Mayo Clinic. Dr. Wood is a director of Steris Corporation, a medical sterilization company, and Assistive Technology Group, Inc., a rehabilitation and durable medical equipment company. Dr. Wood is also a director of SingHealth, an integrated health system in Singapore and a member of the board of overseers of the Baldrige National Quality Award. Dr. Wood received a B.A. degree from Franklin and Marshall College, an M.D., C.M. degree from McGill University and an M.S. degree from the University of Minnesota.
18
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the information and reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, under which we file periodic reports, proxy and information statements and other information with the United States Securities and Exchange Commission, or SEC. Copies of the reports, proxy statements and other information may be examined without charge at the Public Reference Room of the SEC, 100 F Street, N.E., Room 1580, Washington, D.C. 20549, or on the Internet athttp://www.sec.gov. Copies of all or a portion of such materials can be obtained from the Public Reference Room of the SEC upon payment of prescribed fees. Please call the SEC at 1-800-SEC-0330 for further information about the Public Reference Room.
Financial and other information about Cubist is available on our website(http://www.cubist.com). We make available on our website, free of charge, copies of our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the SEC. Copies are available in print to any Cubist shareholder upon request in writing to "Investor Relations, Cubist Pharmaceuticals, Inc., 65 Hayden Ave., Lexington, MA 02421."
19
ITEM 1A. RISK FACTORS
Investing in our company involves a high degree of risk. You should consider carefully the risks described below, together with the other information in and incorporated by reference into this annual report. If any of the following risks actually occur, our business, operating results or financial condition could be materially adversely affected. This could cause the market price of our common stock to decline, and could cause you to lose all or part of your investment.
Risks Related to Our Business
We depend heavily on the success of our sole marketed product CUBICIN, which may not continue to be widely accepted in the United States by physicians, patients, third-party payors, or the medical community in general for the treatment of cSSSI andS. aureus bacteremia, including right-sided endocarditis, caused by MRSA and MSSA.
We have invested a significant portion of our time and financial resources in the development of CUBICIN. For the foreseeable future, our ability to generate revenues will depend solely on the commercial success of CUBICIN, which depends upon its continued acceptance by the medical community and the future market demand and medical need for CUBICIN. CUBICIN was approved by the FDA in September 2003 for the treatment of complicated skin and skin structure infections, or cSSSI, and launched in the United States in November 2003. On May 25, 2006, the FDA approved CUBICIN for the additional indication ofS. aureus bloodstream infections (bacteremia), including those with right-sided infective endocarditis, caused by methicillin-susceptible and methicillin-resistant isolates.
As of December 31, 2007 we had just over four years experience selling this product, and only 19 months of sales since the approval of theS. aureus bacteremia indication. Although we have been successful in our commercialization of CUBICIN to date, because this is still a relatively new product, we cannot be sure that CUBICIN will continue to be accepted by purchasers in the pharmaceutical market for the treatment of cSSSI andS. aureus bacteremia. Further, CUBICIN currently competes with a number of existing anti-infective drugs manufactured and marketed by major pharmaceutical companies and potentially will compete against new anti-infective drugs whose approval is anticipated to be imminent and others that are in development at other companies.
The degree of continued market acceptance of CUBICIN, and our ability to grow revenues from the sale of CUBICIN, depends on a number of additional factors, including:
- •
- the continued safety and efficacy of CUBICIN;
- •
- the ability of target organisms to develop resistance to CUBICIN;
- •
- risks of any unanticipated adverse reactions to CUBICIN in patients;
- •
- the advantages and disadvantages of CUBICIN compared to alternative therapies with respect to cost, availability of reimbursement, convenience, safety, efficacy and other factors;
- •
- our ability to educate the medical community about the safety and efficacy of CUBICIN in compliance with FDA and other government rules and regulations;
- •
- the reimbursement policies of government and third-party payors;
- •
- the level of access that our sales force has to physicians who are likely to prescribe CUBICIN;
- •
- the market price of CUBICIN as compared to alternative therapies and physicians and third-party payors' attitudes towards the relative value of CUBICIN versus other therapies; and
- •
- our international partners' efforts and their success in selling CUBICIN in their respective territories.
20
Because CUBICIN is the only product that we sell currently, any impediment to the success of this product would have a significant effect on our business and financial results.
We may not be able to obtain, maintain or protect certain proprietary rights necessary for the development and commercialization of CUBICIN, our other drug candidates and our research technologies.
Our commercial success will depend in part on obtaining and maintaining U.S. and foreign patent protection for CUBICIN, our drug candidates, and our research technologies and successfully enforcing and defending these patents against third party challenges. We consider that in the aggregate our unpatented proprietary technology, patent applications, patents and licenses under patents owned by third parties are of material importance to our operations. The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions. The actual protection afforded by a patent can vary from country to country and may depend upon the type of patent, the scope of its coverage and the availability of legal remedies in the country. Legal standards relating to the validity and scope of patents covering pharmaceutical and biotechnological inventions are continually developing, both in the United States and in other important markets outside the United States. Our patent position is highly uncertain and involves complex legal and factual questions, and we cannot predict the scope and breadth of patent claims that may be afforded to our patents or to other companies' patents. We cannot assure you that the patents we obtain or the unpatented proprietary technology we hold will afford us commercial protection.
The primary composition of matter patent covering CUBICIN in the United States has expired. We own or have licensed rights to a limited number of patents directed toward methods of administration and methods of manufacture of CUBICIN. We cannot be sure that patents will be granted with respect to any of our pending patent applications for CUBICIN, our other drug candidates, or our research technologies or with respect to any patent applications filed by us in the future; nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting CUBICIN, our other drug candidates or our other technology.
The degree of future protection for our proprietary rights is uncertain. We cannot be certain that the named applicants or inventors of the subject matter covered by our patent applications or patents, whether directly owned by us or licensed to us, were the first to invent or the first to file patent applications for such inventions. Third parties may challenge, infringe, circumvent or seek to invalidate existing or future patents owned by or licensed to us. Even if we have valid and enforceable patents, these patents still may not provide sufficient protection against competing products or processes.
Of particular concern for a company like ours, having one marketed product, is that third parties may seek to market generic versions of CUBICIN by filing an Abbreviated New Drug Application, or ANDA, with the FDA in which they claim that patents protecting CUBICIN owned or licensed by us and listed with the FDA in what is called "the Orange Book" are invalid, unenforceable and/or not infringed, a so-called Paragraph IV filing. From 1997 through 2002, about one third of all new chemical entities, such as daptomycin, the chemical ingredient in CUBICIN, have been the subject of a Paragraph IV filing. September 2007 was the first opportunity under United States patent law for a generic company to make a Paragraph IV filing. To date, Cubist has not received notice of a Paragraph IV filing. If such a filing is made, we may need to defend and/or assert our patents, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid, not infringed and/or unenforceable. During the period in which such litigation is pending, the uncertainty of its outcome may cause investors to disfavor our stock, and our stock price could decline.
21
In September 2007, we asked the FDA to de-list one of the CUBICIN patents listed in the Orange Book, US Patent No. RE39,071. We sought this de-listing so that we could correct a technical error in the patent, originally issued to Eli Lilly and recently assigned to Cubist. The United States Patent and Trademark Office, or USPTO issued a certificate of correction for this patent. We have asked the FDA to re-list the patent in the Orange Book. The patent would have been re-listed upon our request, and this re-listing should be reflected in the next publication of the Orange Book. While this patent was de-listed, an ANDA filer that is seeking to market generic versions of CUBICIN would not have needed to address that patent as part of its ANDA filing.
If our collaborators or consultants develop inventions or processes independently that may be applicable to our products under development, disputes may arise about ownership of proprietary rights to those inventions and/or processes. Such inventions and/or processes will not necessarily become our property but may remain the property of those persons or their employers. Protracted and costly litigation could be necessary to enforce and determine the scope of our proprietary rights. Moreover, the laws of foreign countries in which we market our drug products may afford little or no effective protection to our intellectual property, thereby easing our competitors' ability to compete with us in such countries.
We have and may in the future engage in collaborations, sponsored research agreements, and other arrangements with academic researchers and institutions that have received and may receive funding from U.S. government agencies. As a result of these arrangements, the U.S. government or certain third parties may have rights in certain inventions developed during the course of the performance of such collaborations and agreements as required by law or by such agreements.
We also rely on trade secrets and other unpatented proprietary information in our product development activities. To the extent that we maintain a competitive advantage by relying on trade secrets and unpatented proprietary information, such competitive advantage may be compromised if others independently develop the same or similar technology, resulting in an adverse effect on our business, financial condition and results of operations. We seek to protect trade secrets and proprietary information in part through confidentiality provisions and invention assignment provisions in agreements with our collaborative partners, employees and consultants. It is possible that these agreements could be breached and we might not have adequate remedies for any such breaches.
Our trademarks, CUBICIN and Cubist, in the aggregate are considered to be material to our business. These trademarks are covered by registrations or pending applications for registration in the USPTO and in other countries. Trademark protection continues in some countries for as long as the mark is used and, in other countries, for as long as it is registered. Registrations generally are for fixed, but renewable, terms. We cannot assure you that the trademark protection that we have pursued or will pursue in the future will afford us commercial protection.
Beyond the specific concerns addressed above, intellectual property laws and regulations are constantly changing, and vary amongst different jurisdictions around the world, in ways that may affect our ability to protect or enforce our rights.
We face significant competition from other biotechnology and pharmaceutical companies, particularly with respect to CUBICIN, and our operating results will suffer if we fail to compete effectively.
The biotechnology and pharmaceutical industries are intensely competitive. We have competitors both in the United States and internationally, including major multinational pharmaceutical and chemical companies, biotechnology companies and universities and other research institutions. Many of our competitors have greater financial and other resources, such as larger research and development staffs and more experienced marketing and manufacturing organizations. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis technologies and drug products that are more effective or less costly than CUBICIN or any drug candidate that we may have or develop, which could
22
render our technology obsolete and noncompetitive. If price competition inhibits the acceptance of CUBICIN, if physicians prefer existing drug products over CUBICIN, or if physicians switch to new drug products or choose to reserve CUBICIN for use in limited circumstances, we will not achieve our business plan. In addition, CUBICIN may face competition from drug candidates currently in clinical development and drug candidates that could receive regulatory approval before CUBICIN in countries where CUBICIN is not yet approved.
The competition in the market for therapeutic products that address serious Gram-positive bacterial infections is intense. CUBICIN faces competition in the United States from commercially available drugs such as vancomycin, marketed generically by Abbott Laboratories, Shionogi & Co., Ltd. and others, Zyvox®, marketed by Pfizer, Inc., Synercid®, marketed by King Pharmaceuticals, Inc., and Tygacil®, marketed by Wyeth. In particular, vancomycin has been a widely used and well known antibiotic for over 40 years and is sold in a relatively inexpensive generic form. In addition, the FDA is reviewing an NDA for the Gram-positive agent telavancin (submitted by Theravance, Inc., whose partner for telavancin is Astellas Pharma US, Inc.). Telavancin received an approvable letter on October 22, 2007. The FDA also is reviewing ceftobiprole, a broad spectrum agent with MRSA activity (NDA submitted in May 2007 by a division of Johnson & Johnson). Another Gram-positive agent under review is dalbavancin from Pfizer, Inc. Pfizer received its first approvable letter for dalbavancin in September 2005, and has received multiple approvable letters from the FDA since, citing the need for additional data, among other requests. In February 2008, Targanta Therapeutics Corporation announced that it had submitted an NDA to the FDA for oritivancin to be used for the treatment of cSSSI caused by Gram-positive bacteria, including MRSA. In July 2007, Arpida Ltd. reported the completion of the Phase 3 trial for iclaprim, a broad-spectrum agent with MRSA activity in cSSSI. Accordingly, some or all of these agents may be approved and marketed in the near future and could compete with CUBICIN. Other antibiotics in clinical development could compete with CUBICIN, if approved by the appropriate regulatory agencies, in future years.
Any inability on our part to compete with existing drug products or subsequently introduced drug products would have a material adverse impact on our operating results.
We are completely dependent on third parties to manufacture CUBICIN, and our commercialization of CUBICIN could be stopped, delayed, or made less profitable if those third parties fail to provide us with sufficient quantities of CUBICIN or fail to do so at acceptable prices.
We do not have the capability to manufacture our own CUBICIN active pharmaceutical ingredient, or API. We have entered into a manufacturing and supply agreement with ACS to manufacture and supply us with CUBICIN API for commercial purposes. ACS is our sole provider of our commercial supply of CUBICIN API. Pursuant to our agreement with ACS, ACS currently stores some CUBICIN API at its facilities in Italy.
In order to offset the risk of a single-source API supplier, we currently hold a safety stock of API in addition to what is held at ACS. Any disaster at the facilities where we hold this safety stock, such as a fire or loss of power, that causes a loss of this safety stock, would heighten the risk that we face from having only one supplier of API.
In addition, we do not have the capability to manufacture our own CUBICIN finished drug product. We have entered into manufacturing and supply agreements with both Hospira and Catalent to manufacture and supply to us finished product.
If Catalent, Hospira, or, in particular, ACS, experiences any significant difficulties in its respective manufacturing processes for CUBICIN API or finished product, we could experience significant interruptions in the supply of CUBICIN. Our inability to coordinate the efforts of our third party manufacturing partners, or the lack of capacity available at our third party manufacturing partners, could impair our ability to supply CUBICIN at required levels. Because of the significant regulatory
23
requirements that we would need to satisfy in order to qualify a new bulk or finished product supplier, we could experience significant interruptions in the supply of CUBICIN if we decided to transfer the manufacture of CUBICIN to one or more other suppliers in an effort to deal with these or other difficulties with our current suppliers.
Because the ACS manufacturing facilities are located in Italy, we must ship CUBICIN API to the United States for finishing, packaging and labeling. Each shipment of our API is of significant value, and while in transit, it could be lost or damaged. Moreover, at any time after shipment to the United States, our API could be lost or damaged as it is stored at our warehouser, Integrated Commercialization Solutions, Inc., or ICS, and moves through our finished product manufacturers. We have taken risk mitigation steps and have purchased insurance to protect against such loss or damage. However, depending on when in this process the API is lost or damaged, we may have limited recourse for recovery against our finished product manufacturers or insurers. As a result, our financial performance could be impacted by any such loss or damage to our API. We are also subject to financial risk from volatile fuel costs due to shipping CUBICIN API to the United States, as well as shipping of finished product within the United States and to our international distribution partners for packaging, labeling and distribution.
We may also experience interruption or significant delay in the supply of CUBICIN API due to natural disasters, acts of war or terrorism, shipping embargoes, labor unrest or political instability. In any such event in Italy, the supply of CUBICIN API stored at ACS could also be impacted.
While we have reduced the cost of producing CUBICIN in recent years, we cannot guarantee that we will be able to continue to reduce the costs of commercial scale manufacturing of CUBICIN over time. In order to continue to reduce costs, we may need to develop and implement process improvements. In order to implement such process improvements, we will need, from time to time, to notify or make submissions with regulatory authorities, and the improvements may be subject to approval by such regulatory authorities. We cannot be sure that such approvals will be granted or granted in a timely fashion. We cannot guarantee that we will be able to enhance and optimize output in our commercial manufacturing process. If we cannot enhance and optimize output, we may not be able to further reduce our costs over time.
If we are unable to maintain satisfactory sales and marketing capabilities, we may not succeed in commercializing CUBICIN.
We cannot guarantee that we will continue to be successful in marketing CUBICIN. Until our launch of CUBICIN in November 2003, we had not previously marketed or sold a drug product. In connection with our launch of CUBICIN, we developed our own sales and marketing capabilities in the United States, and we continue to develop those capabilities. In 2007, we added three new sales representatives to our existing sales force and expect to add 26 more in 2008. We do not yet know whether this sales force expansion will be successful.
In addition, our partner in Europe, Novartis, has significant pharmaceutical sales experience but limited experience marketing and selling CUBICIN. Novartis began its launch of CUBICIN in nine EU countries in 2006 and six more in 2007. To date, sales of CUBICIN by Novartis have not been as strong as anticipated. Other than our partners in Israel, Canada and Macau, none of our other international partners have launched CUBICIN in their respective territories. We cannot guarantee that our partners will be successful in marketing CUBICIN in their markets.
If we are unable to discover, in-license, or acquire drug candidates, we will not be able to implement our current business strategy.
Our approach to drug discovery is unproven. Notwithstanding the investment of significant resources to research and development over the years since Cubist was founded, we have not reached
24
the stage of clinical testing in humans of any drug candidates developed from our drug discovery program. We cannot assure you that we will reach this stage for any internally developed drug candidates or that there will be clinical benefits associated with any drug candidates that we do develop. While we are researching other drug candidates for potential clinical development, most drug candidates never make it to the clinical development stage. Even those that do make it into clinical development have only a small chance of gaining regulatory approval and becoming a drug product. We are making a significant investment in our research and development capabilities by increasing our research and development workforce by 20 employees. However, we cannot guarantee that this expansion will lead to greater success, so this investment may not be a useful expenditure of our limited resources.
Our drug product, CUBICIN, and our other former drug candidates that reached the stage of clinical trials in humans were the result of in-licensing patents and technologies from third parties. These in-licensing activities represent a significant expense for Cubist and would generally require us to pay royalties to other parties on product sales. Unless we are able to use our drug discovery approach to identify suitable drug candidates, acquisition or in-licensing will be our only source of drug candidates. However, there can be no assurance that we will be able to acquire or in-license additional desirable drug candidates on acceptable terms, or at all. In fact, we have faced and will continue to face significant competition for the acquisition or in-licensing of any promising drug candidates from a variety of other companies with interest in the anti-infective and acute care marketplace, many of which have significantly more experience than we have in pharmaceutical development and sales and significantly more financial resources than Cubist. In particular, in recent years, very large pharmaceutical companies with significant resources have refocused their attention on opportunities in the anti-infective marketplace. Because of the rising intensity of the level of competition for such products, the cost of acquiring or in-licensing such candidates has grown dramatically in recent years, and candidates are often priced and sold at levels that we cannot afford or that we believe are not justified by market potential. Such competition and higher prices are most pronounced for late-stage candidates and already-marketed products, which have the lowest risk and would have the most immediate impact on our business.
If we are unable to discover or acquire promising candidates, we will not be able to implement our business strategy. Even if we succeed in discovering or acquiring drug candidates, there can be no assurance that we will be successful in developing them to gain approval for use in humans. For example in February 2004 we discontinued, due to observed adverse events, clinical development of CAB-175, a parenteral cephalosporin antibiotic that we had in-licensed from Sandoz GmbH. Also, in April 2004, we discontinued, as a result of data from human clinical research studies, development of oral formulations of ceftriaxone, a broad-spectrum antibiotic for which we had licensed the underlying technology from International Health Management Associates and the University of Utah. More recently, we decided in 2006 not to make any further investment in the development of HepeX-B and have since terminated our license to the technology underlying HepeX-B. Failure to develop new drug candidates successfully could have a material adverse effect on our business, operating results and financial condition.
If pre-clinical or clinical trials for our drug candidates are unsuccessful or delayed, we will be unable to meet our anticipated development and commercialization timelines, which could harm our business.
Before we receive regulatory approvals for the commercial sale of any of our drug candidates, our drug candidates are subject to extensive pre-clinical testing and clinical trials to demonstrate their safety and efficacy in humans. Conducting pre-clinical testing and clinical trials is a lengthy, time-consuming and expensive process that often takes many years. Furthermore, we cannot be sure that pre-clinical testing or clinical trials of any drug candidates will demonstrate the safety and efficacy of our drug candidates at all or to the extent necessary to obtain regulatory approvals. Companies in
25
the biotechnology and pharmaceutical industries, including companies with greater experience in pre-clinical testing and clinical trials than we have, have suffered significant setbacks in advanced clinical trials, even after demonstrating promising results in earlier trials. In our own case, clinical trials of CUBICIN for the treatment of community acquired pneumonia failed to demonstrate sufficient efficacy despite promising results in pre-clinical and early clinical trials.
Other than CUBICIN, all of the drug candidates that we are seeking to develop commercially are in the pre-clinical stage. In order for a drug candidate to move from this stage to human clinical trials, the FDA must approve an IND. The FDA will approve the IND if it is established that a potential drug candidate will not expose humans to unreasonable risks and that the compound has pharmacological activity that justifies commercial development. It takes significant time and expense to generate the data to support an IND filing. In many cases, companies spend the time and resources only to discover that the data is not sufficient to support a filing or gain IND approval. This has happened to us in the past, and likely will happen again in the future. In fact, most compounds that are discovered never make it into human clinical trials.
Once a drug candidate enters human clinical trials, the trials must be carried out under protocols that are acceptable to regulatory authorities and to the committees responsible for clinical studies at the sites at which the studies are conducted. There may be delays in preparing protocols or receiving approval for them that may delay either or both of the start and finish of the clinical trials. Feedback from regulatory authorities or results from earlier stage clinical studies might require modifications or delays in later stage clinical trials or could cause a termination or suspension of drug development. These types of delays or suspensions can result in increased development costs and delayed regulatory approvals. Our ability to secure clinical trial insurance at a reasonable cost could also cause delays.
Furthermore, there are a number of additional factors that may cause delays in our clinical trials. The rate of completion of our clinical trials is dependent in part on the rate of patient enrollment. There may be limited availability of patients who meet the criteria for certain clinical trials. For example, the limited number of patients each year who receive liver transplants for Hepatitis B, the target population for our former drug candidate HepeX-B, in part led us to discontinue investment in clinical development of HepeX-B. Delays in planned patient enrollment can result in increased development costs and delays in regulatory approvals. For example, our clinical trial to determine the safety and efficacy of using CUBICIN to treat bacteremia with known or suspected endocarditis experienced delays attributable to slow enrollment. In addition, our clinical trials may be delayed by one or more of the following factors:
- •
- inability to manufacture sufficient quantities of acceptable materials for use in clinical trials;
- •
- inability to adequately follow patients after treatment;
- •
- the failure of third-party clinical trial managers to perform their oversight of the trials;
- •
- the failure of our clinical investigational sites and related facilities and records to be in compliance with the FDA's Good Clinical Practices;
- •
- inability to enroll study subjects;
- •
- our inability to reach agreement with the FDA on a trial design that we are able to execute; or
- •
- the FDA placing a trial on "clinical hold" or temporarily or permanently stopping a trial for a variety of reasons, principally for safety concerns.
If clinical trials for our drug candidates are unsuccessful, delayed, or cancelled, we will be unable to meet our anticipated development and commercialization timelines, which could harm our business and cause our stock price to decline.
26
We will need to obtain regulatory approvals for any other drug candidates, and our ability to generate revenues from the commercialization and sale of products resulting from our development efforts will be limited by any failure to obtain these approvals.
The FDA and comparable regulatory agencies in foreign countries impose substantial requirements for the development, production and commercial introduction of drug products. These include lengthy and detailed pre-clinical, laboratory and clinical testing procedures, sampling activities and other costly and time-consuming procedures. Any drug candidate will require governmental approvals for commercialization. To date, we have not obtained government approval in the United States for any drug product other than CUBICIN for the indications of cSSSI andS. aureus bacteremia, including those with right-sided infective endocarditis. Our collaborator, Novartis, has received approval for marketing CUBICIN in the EU and other non-EU European countries for the indications of cSSTI, RIE due toS. aureus bacteremia, andS. aureus bacteremia associated with RIE or cSSTI, in Argentina and Colombia for cSSSI, SAB and RIE, in Switzerland for cSSTI andS. aureus bacteremia and in India for cSSSI andS. aureus bacteremia. Our collaborator, AstraZeneca, received an import license for Macau for CUBICIN for cSSSI, SAB and RIE. Our collaborators Oryx Pharmaceuticals Inc., Kuhnil Pharmaceutical Corp., TTY Biopharm Co. Ltd., and Medison Pharma, Ltd., have received approval for marketing CUBICIN in Canada, South Korea, Taiwan and Israel, respectively, for the same, or very similar, indications for which we have approval in the United States. We and our partners are pursuing approvals for CUBICIN in various other countries. Pre-clinical testing, clinical trials and manufacturing of our drug candidates will be subject to rigorous and extensive regulation by the FDA and corresponding foreign regulatory authorities. In addition, regulation is not static and regulatory authorities, including the FDA, evolve in their staff, interpretations and practices and may impose more stringent requirements than currently in effect, which may adversely affect our planned drug development and/or our sales and marketing efforts. Satisfaction of the requirements of the FDA and of foreign regulators typically takes a significant number of years and can vary substantially based upon the type, complexity and novelty of the drug candidate. The approval procedure and the time required to obtain approval also varies among countries. Regulatory agencies may have varying interpretations of the same data, and approval by one regulatory authority does not ensure approval by regulatory authorities in other jurisdictions.
No product can receive FDA approval unless human clinical trials show both safety and efficacy for each target indication in accordance with FDA standards. The large majority of drug candidates that begin human clinical trials fail to demonstrate the desired safety and efficacy characteristics. Failure to demonstrate the safety and efficacy of any drug candidates for each target indication in clinical trials would prevent us from obtaining required approvals from regulatory authorities, which would prevent us from commercializing those drug candidates. The results of our clinical testing of a drug candidate may cause us to suspend, terminate or redesign our clinical testing program for that drug candidate. We cannot be sure when we, independently or with our collaborators, might be in a position to submit additional drug candidates for regulatory review. Negative or inconclusive results from the clinical trials or adverse medical events during the trials could lead to requirements that trials be repeated or extended, or that a program be terminated, even if other studies or trials relating to the program are successful. In addition, data obtained from clinical trials are susceptible to varying interpretations that could delay, limit or prevent regulatory approval and could even affect the commercial success of a product that is already on the market based on earlier trials, such as CUBICIN. In addition, we cannot be sure that regulatory approval will be granted for drug candidates that we submit for regulatory review. Moreover, if regulatory approval to market a drug product is granted, the approval may impose limitations on the indicated use for which the drug product may be marketed as well as additional post-approval requirements.
Our ability to generate revenues from the commercialization and sale of additional drug products will be limited by any failure to obtain these approvals.
27
The FDA may change its approval requirements or policies for antibiotics, or apply interpretations to its requirements or policies, in a manner that could delay or prevent commercialization of any new antibiotic product candidates or any additional indications for CUBICIN that we may seek in the United States.
Regulatory requirements for the approval of antibiotics in the United States may change in a manner that requires us to conduct additional large-scale clinical trials, which may delay or prevent commercialization of any new antibiotic product candidates or any additional indications for CUBICIN that we may seek. Historically, the FDA has not required placebo-controlled clinical trials for approval of antibiotics but instead has relied on non-inferiority studies. In a non-inferiority study, a drug candidate is compared with an approved antibiotic treatment, and it must be shown that the product candidate is not less effective than the approved treatment by a defined margin.
In 2006, the FDA refused to accept approval studies of successfully completed non-inferiority studies as the basis for approval for certain types of antibiotics. In October 2007, the FDA issued draft guidance on the use of non-inferiority studies to support approval of antibiotics. Under this draft guidance, the FDA recommends that for some antibiotic indications, sponsor companies carefully consider study designs other than non-inferiority, such as placebo-controlled trials demonstrating the superiority of a drug candidate to placebo. Conducting placebo-controlled trials for antibiotics can be time-consuming, expensive, and difficult to complete. Institutional review boards may not grant approval for placebo-controlled trials because of ethical concerns about denying some participating patients access to any antibiotic therapy during the course of the trial. Even if institutional review board approval is obtained, it may be difficult to enroll patients in placebo-controlled trials because certain patients would not receive antibiotic therapy. While the indications called out by the FDA in the draft guidance are not indications currently being pursued by Cubist for CUBICIN, the draft guidance does not articulate clear standards or policies for demonstrating the safety and efficacy of antibiotics generally and reserves until a later date the FDA's guidance on the use of non-inferiority studies in all therapeutic areas. The lack of clear guidance from the FDA creates uncertainties about the standards for the approval of antibiotics in the United States. These factors could delay for several years or ultimately prevent commercialization of any new antibiotic product candidates or any additional indications for CUBICIN in the United States for which the FDA requires placebo-controlled trials. Even if we complete these trials, we may not be able to obtain adequate evidence of safety or efficacy to support approval.
Moreover, recent events, including complications arising from FDA-approved drugs, have raised questions about the safety of marketed drugs and may result in new legislation by the U.S. Congress and increased caution by the FDA and comparable foreign regulatory authorities in reviewing new drugs based on safety, efficacy or other regulatory approvals. In particular, non-inferiority studies have come under scrutiny from Congress, in part because of a congressional investigation as to the safety of Ketek®, an antibiotic approved by the FDA on the basis of non-inferiority studies. Certain key members of Congress have asked the U.S. Government Accountability Office (GAO), an independent, nonpartisan arm of Congress, to investigate the FDA's reliance on non-inferiority studies as a basis for approval. Congress may draft, introduce, and pass legislation that could significantly change the process for approval of antibiotics by the FDA.
The increased scrutiny by Congress and regulatory authorities may significantly delay or prevent regulatory approval, as well as impose more stringent product labeling and post-marketing testing requirements on pharmaceutical products generally and particularly in our areas of focus. Any delay in obtaining, or an inability to obtain, applicable regulatory approvals could prevent us from successfully commercializing any new antibiotic product candidates, receiving any additional indications for CUBICIN, generating revenues, and sustaining profitability.
28
If we are unable to generate revenues from any drug products other than CUBICIN, our ability to create long-term shareholder value may be limited.
Apart from CUBICIN, we have no other drug products that have been approved by the FDA, and our current pipeline does not include any drug candidates that are in clinical development. Because of the long development time of drug candidates, even once they are in clinical development, none of the drug candidates that we are currently developing, even if one were to overcome the significant hurdles of a pre-clinical candidate ever making it to the commercial market, would generate revenues for many years. Unless and until we are able to develop, in-license or acquire other successful drug products, we will continue to rely solely on CUBICIN for our sales revenues. If we are unable to bring any of our current or future drug candidates to market, or to acquire any marketed drug products, our ability to create long-term shareholder value may be limited.
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive biotechnology and pharmaceuticals industries depends in large part upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. Historically, we have been highly dependent on our management and scientific and medical personnel. In order to induce valuable employees to remain at Cubist, we have provided options that vest over time. The value to employees of these options is significantly affected by movements in our stock price that we cannot control and may at any time be insufficient to counteract more lucrative offers from other companies. We have also provided retention letters to a limited number of key employees. Despite our efforts to retain valuable employees, members of our management, scientific and medical teams have in the past and may in the future terminate their employment with us. The loss of the services of any of our executive officers or other key employees could potentially harm our business or financial results if we are unable to effectively compensate for these losses.
Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level, and senior managers as well as junior, mid-level, and senior scientific and medical personnel. Other biotechnology and pharmaceutical companies with which we compete for qualified personnel have greater financial and other resources, different risk profiles, and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than what we have to offer. If we are unable to grow our business according to our business plan, including by developing or acquiring additional drug products, we may become a less attractive place to work for our existing employees and for high quality candidates. If we are unable to continue to attract and retain high quality personnel, the rate and success at which we can discover, develop and commercialize drug candidates will be limited.
We have recently and may in the future undertake strategic acquisitions and we may not realize the benefits of such acquisitions.
We acquired Illumigen in December 2007, which was only the second business acquisition since our inception. Although we have limited experience in acquiring businesses, we may acquire additional businesses that we believe will complement or augment our existing business. Acquisitions involve a number of risks, including: diversion of management's attention from current operations; disruption of our ongoing business; difficulties in integrating and retaining all or part of the acquired business, its customers and its personnel; assumption of disclosed and undisclosed liabilities; dealing with unfamiliar laws, customs and practices in foreign jurisdictions; and the effectiveness of the acquired company's internal controls and procedures. The individual or combined effect of these risks could have a material adverse effect on our business. As well, in paying for an acquisition we may deplete our cash resources
29
or dilute our shareholder base by issuing additional shares. Furthermore, there is the risk that our valuation assumptions and our models for an acquired product or business may turn out to be erroneous or inappropriate due to foreseen or unforeseen circumstances and thereby cause us to have overvalued an acquisition target. There also is the risk that the contemplated benefits of an acquisition may not materialize as planned or may not materialize within the time period or to the extent anticipated. Because our acquisition of Illumigen occurred so recently, many of these risks still exist with respect to this transaction.
If we acquire businesses with promising drug candidates or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to move one or more drug candidates through pre-clinical and/or clinical development to regulatory approval and commercialization. We cannot assure you that, following an acquisition, we will achieve revenues that justify the acquisition or that the acquisition will result in increased earnings, or reduced losses, for the combined company in any future period. Moreover, we may need to raise additional funds through public or private debt or equity financings to acquire any businesses, which would result in dilution for stockholders or the incurrence of indebtedness. We may not be able to operate acquired businesses profitably or otherwise implement our growth strategy successfully.
The investment of our cash is subject to risks which could result in losses.
We invest our cash in a variety of financial instruments; principally securities issued by the U.S. government and its agencies, investment grade corporate bonds and notes, auction rate securities and money market instruments. These investments are subject to credit, liquidity, market and interest rate risk. For example, if the issuers of auction rate securities are unable to successfully close auctions or if the credit ratings of any of our investments decline after initial purchase, we may be required to adjust the carrying value of these investments through an impairment charge. In fact, in August 2007, auctions for $58.1 million of our investments in auction rate securities failed and have failed since then. These auction rate notes consist of private placement, credit swap structured securities which reference synthetic portfolios of corporate bonds. These securities have long-term nominal maturities for which the interest rates are reset through a monthly auction. These auctions have historically provided a liquid market for these securities. All of the auction rate securities that failed are still AAA rated and none are backed by sub-prime mortgages. As a result of the auction failure, we have recorded temporary unrealized losses of $14.7 million in other comprehensive income as a reduction in shareholders' equity. The failure resulted in the interest rate on these investments resetting at the default coupon rate per the terms of the certificate. While we now earn a premium interest rate on the investments, the investments are not liquid. In the event we need to access these funds, we will not be able to until a future auction on these investments is successful, or until they reach their underlying maturity. If the issuers are unable to successfully close future auctions and the credit ratings on the underlying corporate tracking bond portfolios deteriorate significantly, we may be required to adjust the carrying value of these investments through an other-than-temporary impairment charge. The credit and capital markets have continued to deteriorate in 2008 and the bids on the auction rate notes we hold have since declined. If a certain concentration, as defined in the auction rate documents, of the underlying reference portfolios default, or if the issuing bank fails to make the required interest payments or the final principal payment upon the ultimate maturity of the notes, or if the credit ratings on the underlying reference portfolios deteriorate significantly, the company may be required to adjust the carrying value of these investments through an other-than-temporary impairment charge. Such risks, including the failure of future auctions for the auction rate securities, may result in a loss of liquidity, substantial impairment to our investments, realization of substantial future losses, or a complete loss of the investment in the long-term, which may have a material adverse effect on our business, results of operations, liquidity and financial condition.
30
Our ability to grow revenues from the commercialization and sale of CUBICIN will be limited if we or our partners do not obtain approval to market CUBICIN for any additional indications in countries where CUBICIN is approved, or if our partners do not receive approvals to market CUBICIN at all in countries where CUBICIN is not yet approved, or if we fail to fulfill certain post-approval requirements of the FDA relating to CUBICIN.
We may seek regulatory approval for additional indications for CUBICIN. To do so, we must successfully conduct additional clinical trials and then apply for and obtain the appropriate regulatory approvals. Our revenues may not grow as expected and our business and operating results may be harmed if additional indications for CUBICIN are not approved in the United States.
In January 2006, the EMEA granted final approval for marketing CUBICIN in the EU for the treatment of cSSTI, where the presence of susceptible Gram-positive bacteria is confirmed or suspected. In August, 2007, the EMEA granted final approval for marketing CUBICIN in the EU for the additional indications of RIE due toS. aureus bacteremia and forS. aureus bacteremia associated with RIE or cSSTI. CUBICIN is also approved in Canada, Korea, Taiwan, India, Colombia and Israel for the same, or very similar, indications as our U.S. approval, in other non-EU European countries for the same indications as the EU approval, in Switzerland for cSSTI andS. aureus bacteremia and in Argentina for cSSSI, SAB and RIE. An import license for Macau was also received for CUBICIN for cSSSI, SAB and RIE. Our international collaborators have submitted or plan on submitting applications for approvals to market CUBICIN in other territories, however, we cannot be sure that any regulatory authority will approve these or any future submissions on a timely basis or at all.
In connection with our United States marketing approvals for CUBICIN, we have made certain Phase 4 clinical study commitments to the FDA, including for studies of renal-compromised patients, pediatric patients, and those with RIE. We have worked with the FDA to design these studies, which we expect to initiate this year. Our business would be seriously harmed if we do not complete these studies and the FDA, as a result, requires us to change the marketing label for CUBICIN. In addition, adverse medical events that occur during clinical trials or during commercial marketing could result in claims against Cubist and the temporary or permanent withdrawal of CUBICIN from commercial marketing, which could seriously harm our business and cause our stock price to decline. In particular, our planned pediatric trial exposes us to more uncertain and potentially greater risk because of the age of the subjects.
We have collaborative relationships that expose us to a number of risks.
We have entered into, and anticipate continuing to enter into, collaborative arrangements with multiple third parties to discover, test, manufacture and market drug candidates and drug products. In October 2003, we entered into an international license and product supply agreement with a subsidiary of Chiron Corporation, or Chiron, to seek regulatory approvals and commercialize CUBICIN in Europe, Australia, New Zealand, India and certain Central American, South American and Middle Eastern countries. In April 2006, Novartis acquired Chiron. In December 2006, we entered into a license and product supply agreement with AstraZeneca to seek regulatory approvals and commercialize CUBICIN in China and other countries in Asia, Africa and the Middle East. In March 2007, we entered into a license agreement with Merck & Co., Inc., or Merck, for the development and commercialization of CUBICIN in Japan. We also have entered into agreements with partners for the commercialization of CUBICIN in Israel, Taiwan, Canada and South Korea. In addition to commercial collaborations, we collaborate with a variety of other companies for manufacturing, clinical trials, clinical and preclinical testing, and research activities. Collaborations such as these are necessary for us to research, develop, and commercialize drug candidates. We cannot be sure that we will be able to establish any additional collaborative relationships on terms acceptable to us or that we will be able to work successfully with our existing collaborators or their successors.
31
Reliance on collaborative relationships poses a number of risks including the following:
- •
- the focus, direction, amount and timing of resources dedicated by our collaborators to their respective collaborations with us is not under our control, which may result in less successful commercialization of CUBICIN in our partners' territories than if we had control over the CUBICIN franchise in these territories;
- •
- our collaborators may not perform their obligations, including appropriate and timely reporting on CUBICIN adverse events in their territories, as expected;
- •
- some drug candidates discovered in collaboration with us may be viewed by our collaborators as competitive with their own drug candidates or drug products;
- •
- our collaborators may not elect to proceed with the development of drug candidates that we believe to be promising;
- •
- disagreements with collaborators, including disagreements over proprietary rights, contract interpretation, or the preferred course of development or commercialization strategy, might cause delays or termination of the research, development or commercialization of drug candidates, lead to additional responsibilities with respect to drug candidates, or result in litigation or arbitration, any of which would be time-consuming and expensive; and
- •
- some of our collaborators might develop independently, or with others, drug products that compete with ours.
Collaborative arrangements with third parties are a critical part of our business strategy, and any inability on our part to establish collaborations on terms favorable to us or working successfully with our collaborators will have an adverse effect on our operations and financial performance.
A variety of risks associated with our international business relationships could materially adversely affect our business.
We have manufacturing, collaborative and clinical trial relationships outside the United States, and CUBICIN is marketed internationally through licensees and distributors. Consequently, we are, and will continue to be, subject to additional risks related to operating in foreign countries. Associated risks of conducting operations in foreign countries include:
- •
- differing regulatory requirements for drug approvals in foreign countries;
- •
- unexpected CUBICIN adverse events that occur in foreign markets that we have not experienced in the United States;
- •
- foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
- •
- the potential for so-called parallel importing;
- •
- unexpected changes in tariffs, trade barriers and regulatory requirements;
- •
- economic weakness, including inflation, or political instability in particular foreign economies and markets;
- •
- compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
- •
- foreign taxes, including withholding of payroll taxes;
- •
- workforce uncertainty in countries where labor unrest is more common than in the United States;
32
- •
- production shortages resulting from events affecting raw material supply or manufacturing capabilities abroad; and
- •
- business interruptions resulting from geo-political actions, including war and terrorism, and natural disasters in other countries.
These and other risks associated with our international operations may materially adversely affect our ability to maintain profitability.
We depend on third parties in the conduct of our clinical trials for CUBICIN and expect to do so with respect to other drug candidates, and any failure of those parties to fulfill their obligations could adversely affect our development and commercialization plans.
We depend on independent clinical investigators, CROs and other third party service providers in the conduct of our clinical trials for CUBICIN and expect to do so with respect to other drug candidates. We rely heavily on these parties for successful execution of our clinical trials but do not control many aspects of their activities. For example, the investigators are not our employees. However, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Third parties may not complete activities on schedule or may not conduct our clinical trials in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations could delay or prevent the further development, approval and commercialization of CUBICIN and that of future drug candidates.
We have incurred substantial losses in the past and may incur additional losses.
Since we began operations, we incurred substantial net losses in every fiscal period until the third quarter of 2006. We generated income of $48.1 million for the year ended December 31, 2007 and incurred a net loss of $0.4 million for the year ended December 31, 2006. At December 31, 2007, we had an accumulated deficit of $436.0 million. These losses have resulted from costs associated with conducting research and development, conducting clinical trials, commercialization efforts and associated administrative costs.
We cannot be certain that we will not incur future operating losses related to the continued development and commercialization of CUBICIN, the development of our other drug candidates, as well as investments in other product opportunities. As a result, we cannot make specific predictions about our continued profitability. If we fail to maintain profitability, the market price of our common stock may decline.
We may require additional funds and we do not know if additional funds would be available to us at all, or on terms that we find acceptable.
Until the third quarter of 2006, we were not a self-sustaining business, and we cannot guarantee that certain economic and strategic factors will not require us to seek additional funds. We believe that our existing cash, cash equivalents, investments and the anticipated cash flow from revenues will be sufficient to fund our operating expenses, debt obligations and capital requirements under our current business plan for the foreseeable future. We expect capital outlays and operating expenditures to increase over the next several years as we continue our commercialization of CUBICIN, actively seek to acquire or in-license additional products or product candidates, and expand our research and development activities and infrastructure. We may need to spend more money than currently expected because of unforeseen circumstances or circumstances beyond our control. We have no committed sources of capital and do not know whether additional financing will be available when and if needed, or, if available, that the terms will be favorable to our shareholders or us.
33
We may seek additional funding through public or private financing or other arrangements with collaborators. If we raise additional funds by issuing equity securities, further dilution to existing stockholders may result. In addition, as a condition to providing additional funds to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. We cannot be certain, however, that additional financing will be available from any of these sources or, if available, will be on acceptable or affordable terms.
Our annual debt service obligations on our 2.25% subordinated convertible notes due in June 2013 are approximately $6.8 million per year in interest payments. We may add additional lease lines to finance capital expenditures and may obtain additional long-term debt and lines of credit. If we issue other debt securities in the future, our debt service obligations will increase further. If we are unable to generate sufficient cash to meet these obligations and need to use existing cash or liquidate investments in order to fund our debt service obligations or to repay our debt, we may be forced to delay or terminate clinical trials or curtail operations. We may also be forced to obtain funds through collaborative and licensing arrangements that may require us to relinquish commercial rights or potential markets or grant licenses on terms that are not favorable to us. If we fail to obtain additional capital, if needed, we will not be able to execute our current business plan successfully.
Our business may suffer if we fail to manage our growth effectively.
If our potential drug candidates progress in development or we are able to continue expanding the commercialization of CUBICIN, we will need to continue to build our organization and require significant additional investment in personnel, management systems and resources. Our ability to develop and grow the commercialization of our products, achieve our research and development objectives, and satisfy our commitments under our collaboration agreements depends on our ability to respond effectively to these demands and expand our internal organization to accommodate additional anticipated growth. If we are unable to achieve or manage our continued growth effectively, there could be a material adverse effect on our business.
Risks Related to Our Industry
We may become involved in patent litigation or other intellectual property proceedings relating to our products or processes that could result in liability for damage or stop our development and commercialization efforts.
The pharmaceutical industry has been characterized by significant litigation and interference and other proceedings regarding patents, patent applications, trademarks and other intellectual property rights. The types of situations in which we may become parties to such litigation or proceedings include:
- •
- We or our collaborators may initiate litigation or other proceedings against third parties to enforce our patent rights;
- •
- We or our collaborators may initiate litigation or other proceedings against third parties to seek to invalidate the patents held by such third parties or to obtain a judgment that our products or processes do not infringe such third parties' patents;
- •
- If our competitors file patent applications that claim technology also claimed by us, we or our collaborators may participate in interference or opposition proceedings to determine the priority of invention;
- •
- If third parties initiate litigation claiming that our processes or products infringe their patent or other intellectual property rights, we and our collaborators will need to defend against such proceedings;
34
- •
- If third parties initiate litigation claiming that our brand names infringe their trademarks, we and our collaborators will need to defend against such proceedings; and
- •
- If third parties file ANDAs with the FDA seeking to market generic versions of our products prior to expiration of relevant patents owned or licensed by us, we may need to defend our patents, including by filing lawsuits alleging patent infringement.
An adverse outcome in any litigation or other proceeding could subject us to significant liabilities to third parties and require us to cease using the technology that is at issue or to license the technology from third parties. We may not be able to obtain any required licenses on commercially acceptable terms or at all.
The cost of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the cost of such litigation and proceedings more effectively than we can because of their substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
Competitors may develop drug products that make our drug products obsolete, less cost effective or otherwise less attractive to use.
Researchers are continually learning more about diseases, which may lead to new technologies for treatment. Even if we are successful in developing effective drug products, new drug products introduced after we commence marketing of any drug product may be safer, more effective, less expensive or easier to administer than our drug products.
Revenues generated by products we currently market or that we successfully develop and for which we obtain regulatory approval depend on reimbursement from third-party payors such that if reimbursement for our products is reduced or is insufficient, there could be a negative impact on the utilization of our products.
Acceptable levels of reimbursement for costs of developing and manufacturing drug products and treatments related to those drug products by government authorities, private health insurers, and other organizations, such as HMOs, can have an affect on the successful commercialization of, and attracting collaborative partners to invest in the development of, our drug products and drug candidates. In both the United States and in foreign jurisdictions, legislative and regulatory actions can affect health care systems and reimbursement for our products.
For example, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, and its implementing regulations, altered the manner in which Medicare sets payment levels for many prescription drugs, including CUBICIN. Under this legislation, beginning in 2005, Medicare reimbursement for CUBICIN was based on average sales price, or ASP, rather than average wholesale price in both the physician office and hospital outpatient settings. This resulted in lower payment rates in 2005 as compared to 2004. Moreover, under this payment methodology the payment rate for CUBICIN is set on a quarterly basis based upon the ASP for previous quarters, and significant downward fluctuations in such reimbursement rate could negatively affect sales of CUBICIN. In addition, further changes to this methodology are possible.
Another action that may affect reimbursement related to our products involves a statutory requirement, and its implementing regulations, that Medicare may not make a higher payment for inpatient services that are caused by medical conditions arising after a patient is admitted to the hospital. Medicare pays for inpatient hospital services under a prospective payment system in which cases are grouped into Medicare Severity Diagnosis Related Groups, or MS-DRGs, and the amount of
35
the single Medicare payment depends upon the applicable MS-DRG. That can vary based on the condition of the patient. Under the statute, effective October 1, 2008, if a case would be assigned to a higher paying MS-DRG because of a specified condition that arose after admission to the hospital, the Medicare payment would remain at the lower paying MS-DRG that would have applied in the absence of such condition. The Centers for Medicare and Medicaid Services has specified the conditions to which this policy would apply and they include conditions that may be treated with CUBICIN. In addition, other conditions may be added in the future, including MRSA. As a result of this policy, in certain circumstances, hospitals may receive less reimbursement for Medicare patients that obtain a hospital acquired infection, which may be treated with CUBICIN. We cannot be sure what impact this upcoming policy will have on the demand for CUBICIN.
There have been a number of other legislative and regulatory actions affecting health care systems. The current uncertainty and the potential for adoption of additional changes could affect the timing and amount of our product revenue, our ability to raise capital, obtain additional collaborators and market our products. Medicare payments for CUBICIN can influence pricing in the non-Medicare market as third party payors may base their reimbursement on the Medicare rate. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our drug products. Any reduction in demand would adversely affect our business. If reimbursement is not available or is available only at limited levels, we may not be able to obtain collaborators to manufacture and commercialize drug products, and may not be able to obtain a satisfactory financial return on our own manufacture and commercialization of any future drug products.
Third-party payors are increasingly challenging prices charged for medical products and services. Also, the trend toward managed health care in the United States and the concurrent growth of organizations such as HMOs, as well as possible legislative changes to reform health care or reduce government insurance programs, may result in lower prices for pharmaceutical products, including any products that may be offered by us in the future. Cost-cutting measures that health care providers are instituting, and the effect of any health care reform, could materially adversely affect our ability to sell any drug products that are successfully developed by us and approved by regulators. Moreover, we are unable to predict what additional legislation or regulation, if any, relating to the health care industry or third-party coverage and reimbursement may be enacted in the future or what effect such legislation or regulation would have on our business. Outside the United States, certain countries set prices in connection with the regulatory process. We cannot be sure that such prices will be acceptable to us or our collaborators. Such prices may negatively impact our sales revenue in those countries.
Our industry is highly regulated and our products are subject to ongoing regulatory review.
Our company, our drug products, the manufacturing facilities for our drug products and our promotion and marketing materials are subject to continual review and periodic inspection by the FDA and other regulatory agencies for compliance with pre-approval and post-approval regulatory requirements, including good manufacturing practices, or GMP, regulations, adverse event reporting, advertising and product promotion regulations, and other requirements. In addition, if there are any modifications to a drug product that we are developing or commercializing, further regulatory approval will be required.
State laws and regulations may also affect our ability to manufacture, market and ship our product, including legislation in California which, if implemented, would require an electronic "pedigree" on all pharmaceutical products delivered to patients in California. This, or other state law requirements, may be difficult or costly for us to implement, and if any changes to our product or the manufacturing process are required, we may have to seek approval from the FDA or other regulatory agencies in order to comply with the state law.
36
Failure to comply with manufacturing and other post-approval state law, regulations of the FDA and other regulatory agencies can, among other things, result in fines, increased compliance expense, denial or withdrawal of regulatory approvals, product recalls or seizures, forced discontinuance of or changes to important promotion and marketing campaigns, operating restrictions and criminal prosecution. Later discovery of previously unknown problems with a drug product, manufacturer or facility may result in restrictions on the drug product, us or our manufacturing facilities, including withdrawal of the drug product from the market. The cost of compliance with pre- and post-approval regulation may have a negative effect on our operating results and financial condition.
New accounting pronouncements or guidance may require us to change the way in which we account for our operational or business activities.
The Financial Accounting Standards Board, or FASB, the SEC, and other bodies that have jurisdiction over the form and content of our accounts are constantly discussing and interpreting proposals and existing pronouncements designed to ensure that companies best display relevant and transparent information relating to their respective businesses. The pronouncements and interpretations of pronouncements by FASB, the SEC and other bodies may have the effect of requiring us to account for revenues and/or expenses in a different manner than we have done in the past which could have a material adverse impact on our financial results.
Our corporate compliance program cannot ensure that we are in compliance with all applicable "fraud and abuse" laws and regulations and other applicable laws and regulations in the jurisdictions in which we sell CUBICIN, and a failure to comply with such regulations or prevail in litigation related to noncompliance could harm our business.
Our general operations, and the research, development, manufacture, sale and marketing of our products, are subject to extensive laws and regulation, including but not limited to, health care "fraud and abuse" laws, such as the federal false claims act, the federal anti-kickback statute, and other state and federal laws and regulations. While we have developed and implemented a corporate compliance program based upon what we believe are current best practices, we cannot guarantee that this program will protect us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
We could incur substantial costs resulting from product liability claims relating to our pharmaceutical products.
The nature of our business exposes us to potential liability risks inherent in the testing, manufacturing and marketing of pharmaceutical and biotechnology products. Our products and the clinical trials utilizing our products and drug candidates may expose us to product liability claims and possible adverse publicity. Product liability insurance is expensive, is subject to deductibles and coverage limitations, and may not be available in the future. While we currently maintain product liability insurance coverage, we cannot be sure that such coverage will be adequate to cover any incident or all incidents. In addition, we cannot be sure that we will be able to maintain or obtain insurance coverage at acceptable costs or in a sufficient amount, that our insurer will not disclaim coverage as to a future claim or that a product liability claim would not otherwise adversely affect our business, operating results or financial condition.
37
Our use of hazardous materials, chemicals, microorganisms and radioactive compounds exposes us to potential liabilities.
Our research and development efforts involve the controlled use of hazardous materials, chemicals, viruses, bacteria and various radioactive compounds. We are subject to numerous environmental and safety laws and regulations and to periodic inspections for possible violations of these laws and regulations. Any such violation and the cost of compliance with any resulting order or fine could adversely effect our operations. We cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of an accident or a determination of non-compliance, we could be held liable for significant damages or fines.
If we are unable to adequately protect our confidential, electronically stored, transmitted and communicated information, it could significantly harm our business.
In our business, we electronically store large amounts of scientific, technical, employee, customer and other data. The amount of confidential, digital information that we store and that we transmit and communicate to third parties continues to grow as technology continues to evolve. If we have inadequate security to protect this information from a breach and/or if such a breach should occur, crucial confidential information about our research, development, employees, customers and future prospects could be unintentionally disclosed. In addition, our information could be improperly disclosed if we are unable to restrict what third parties with whom we share such information may do with the information, or how long they may access it. If our competitors were able to acquire our confidential information, our business and future prospects could be harmed.
Risks Related to Ownership of Our Common Stock
Our stock price may be volatile, and the value of our stock could decline.
The trading price of our common stock has been, and is likely to continue to be volatile. Our stock price could be subject to downward fluctuations in response to a variety of factors, including the following:
- •
- the investment community's view of the revenue, financial and business projections we provide to the public, and whether we succeed or fail in meeting or exceeding these projections;
- •
- actual or anticipated variations in our quarterly operating results;
- •
- failure of third party reporters of sales data to accurately report our sales figures;
- •
- third parties filing ANDAs with the FDA, and the results of any litigation that we file to defend and/or assert our patents against such third parties;
- •
- adverse results or delays in our clinical trials;
- •
- our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial;
- •
- our inability to obtain adequate product supply for any approved drug product or inability to do so at acceptable prices;
- •
- our termination of a collaboration or our inability to establish additional collaborations;
- •
- regulatory decisions that are adverse to us and/or our products;
- •
- safety concerns related to the use of CUBICIN;
- •
- introduction of new products or services offered by us or our competitors;
38
- •
- the announcements of acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors;
- •
- expectations in the financial markets that Cubist may or may not be the target of potential acquirors;
- •
- our failure to develop or acquire additional drug candidates and commercialize additional drug products;
- •
- our issuance of additional debt or equity securities;
- •
- litigation, including stockholder or patent litigation;
- •
- the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community; and
- •
- other events or factors, many of which are beyond our control.
In addition, the stock market in general, and the NASDAQ Global Select Market and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In the past, following periods of volatility in the market price of a company's securities, securities class action litigation has often been instituted against companies. This type of litigation, if instituted, could result in substantial costs and a diversion of management's attention and resources, which would harm our business.
If our officers, directors and certain stockholders choose to act together, they would be able to influence our management and operations, acting in their best interests and not necessarily those of other stockholders.
Our directors, executive officers and greater than 5% stockholders and their affiliates beneficially own a significant percentage of our issued and outstanding common stock. Accordingly, they collectively would have the ability to influence the election of all of our directors and to influence the outcome of some corporate actions requiring stockholder approval. They may exercise this ability in a manner that advances their best interests and not necessarily those of other stockholders.
We have implemented anti-takeover provisions that could discourage, prevent or delay a takeover, even if the acquisition would be beneficial to our stockholders.
The existence of our stockholder rights plan and provisions of our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it difficult for a third party to acquire us, even if doing so would benefit our stockholders.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our headquarters are located at 65 Hayden Avenue in Lexington, Massachusetts, where we own approximately 88,000 square feet of commercial and laboratory space and twelve acres of land.
Our operating leases consist of approximately 121,000 square feet of office and data center space at 45/55 Hayden Avenue in Lexington, Massachusetts, pursuant to a term lease that expires in April 2016, 24,000 square feet of commercial space at 24 Emily Street in Cambridge, Massachusetts, pursuant to a term lease that expires in September 2008 and 15,000 square feet of commercial space at 148
39
Sidney Street in Cambridge Massachusetts, pursuant to a term lease that expires in December 2010. We have subleased the space located at 24 Emily Street for a term that coincides with the September 2008 lease expiration. We have subleased the space located at 148 Sidney Street through October 2010.
ITEM 3. LEGAL PROCEEDINGS
None.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted to a vote of security holders during the last quarter of the fiscal year ended December 31, 2007.
40
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
The information required to be disclosed by Item 201(d) of Regulation S-K, "Securities Authorized for Issuance Under Equity Compensation Plans," is included under Item 12 of Part III of this Annual Report on Form 10-K.
Market Information
Cubist's common stock is traded on the NASDAQ Global Select MarketSM under the symbol CBST. The following table shows the high and low sales price for Cubist's common stock as reported by the NASDAQ Global Select MarketSM for each quarter in the years ended December 31, 2007 and 2006.
| | Common Stock Price
|
|---|
| | 2007
| | 2006
|
|---|
| | High
| | Low
| | High
| | Low
|
|---|
| First Quarter | | $ | 22.68 | | $ | 16.97 | | $ | 25.30 | | $ | 19.84 |
| Second Quarter | | $ | 23.80 | | $ | 19.52 | | $ | 26.77 | | $ | 18.63 |
| Third Quarter | | $ | 25.72 | | $ | 19.16 | | $ | 25.40 | | $ | 19.56 |
| Fourth Quarter | | $ | 24.75 | | $ | 19.68 | | $ | 23.35 | | $ | 17.82 |
Holders
As of February 25, 2008, Cubist had 198 stockholders of record. This figure does not reflect persons or entities that hold their stock in nominee or "street" name through various brokerage firms.
Dividends
Cubist has never declared or paid cash dividends on its capital stock and does not anticipate paying any dividends in the foreseeable future. The Company intends to retain future earnings, if any, to operate and expand the business. Payment of any future dividends will be at the discretion of the board of directors after taking into account various factors, including the Company's financial condition, operating results, cash needs and growth plans.
Recent Sales of Unregistered Securities
None.
41
Corporate Performance Graph
The following Performance Graph and related information shall not be deemed to be "soliciting material" or to be "filed" with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933 or Securities Exchange Act of 1934, each as amended, except to the extent that the Company specifically incorporates it by reference into such filing.
The following graph compares the performance of Cubist's common stock to the NASDAQ Stock Market (U.S.) and to the NASDAQ Pharmaceutical Index from December 31, 2002 through December 31, 2007. The comparison assumes $100 was invested on December 31, 2002 in our common stock and in each of the foregoing indices and assumes reinvestment of dividends, if any. The points on the graph are as of December 31 of the year indicated.
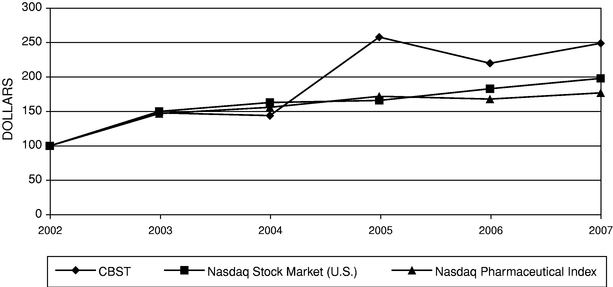
| | CBST
| | Nasdaq
Stock Market
(U.S.)
| | Nasdaq
Pharmaceutical
Index
|
|---|
| 12/31/2002 | | 100 | | 100 | | 100 |
| 12/31/2003 | | 148 | | 150 | | 147 |
| 12/31/2004 | | 144 | | 163 | | 156 |
| 12/30/2005 | | 258 | | 166 | | 172 |
| 12/29/2006 | | 220 | | 183 | | 168 |
| 12/31/2007 | | 249 | | 198 | | 177 |
42
ITEM 6. SELECTED FINANCIAL DATA
The selected financial data presented below for the years ended December 31, 2007, 2006, 2005, 2004, and 2003 are derived from our audited consolidated financial statements.
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| | 2004
| | 2003
| |
|---|
| | (in thousands, except share and per share data)
| |
|---|
| Statement of Operations Data: | | | | | | | | | | | | | | | | |
| U.S. product revenues, net | | $ | 285,059 | | $ | 189,512 | | $ | 113,434 | | $ | 58,559 | | $ | 1,673 | |
| International product revenues | | | 5,347 | | | 808 | | | 80 | | | — | | | — | |
| Other revenues | | | 4,214 | | | 4,428 | | | 7,131 | | | 9,512 | | | 2,043 | |
| | |
| |
| |
| |
| |
| |
| | | Total revenues, net | | | 294,620 | | | 194,748 | | | 120,645 | | | 68,071 | | | 3,716 | |
| Costs and expenses: | | | | | | | | | | | | | | | | |
| | Cost of product revenues | | | 68,860 | | | 48,803 | | | 32,739 | | | 20,249 | | | 816 | |
| | Research and development | | | 85,175 | (1) | | 57,405 | | | 51,673 | | | 57,182 | | | 54,505 | |
| | Sales and marketing | | | 67,662 | | | 56,879 | | | 42,331 | | | 35,019 | | | 21,090 | |
| | General and administrative | | | 31,485 | | | 26,745 | | | 19,335 | | | 20,234 | | | 29,978 | (2) |
| | |
| |
| |
| |
| |
| |
| | | Total costs and expenses | | | 253,182 | | | 189,832 | | | 146,078 | | | 132,684 | | | 106,389 | |
| Interest income | | | 18,036 | | | 10,589 | | | 3,292 | | | 1,767 | | | 2,182 | |
| Interest expense | | | (9,427 | ) | | (15,893 | ) | | (9,836 | ) | | (13,607 | ) | | (13,601 | ) |
| Other income (expense) | | | (20 | ) | | 12 | | | 125 | | | (59 | ) | | (911 | ) |
| Provision for income taxes | | | 1,880 | | | — | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| | | Net income (loss) | | $ | 48,147 | | $ | (376 | ) | $ | (31,852 | ) | $ | (76,512 | ) | $ | (115,003 | ) |
| | |
| |
| |
| |
| |
| |
| Basic net income (loss) per common share | | $ | 0.87 | | $ | (0.01 | ) | $ | (0.60 | ) | $ | (1.86 | ) | $ | (3.61 | ) |
| Diluted net income (loss) per common share | | $ | 0.83 | | $ | (0.01 | ) | $ | (0.60 | ) | $ | (1.86 | ) | $ | (3.61 | ) |
| Shares used in calculating: | | | | | | | | | | | | | | | | |
| Basic net income (loss) per common share | | | 55,591,775 | | | 54,490,376 | | | 53,053,307 | | | 41,228,275 | | | 31,872,555 | |
| Diluted net income (loss) per common share | | | 68,822,996 | | | 54,490,376 | | | 53,053,307 | | | 41,228,275 | | | 31,872,555 | |
- (1)
- In 2007, Cubist recorded an in-process research and development, or IPR&D charge of $14.4 million related to the acquisition of Illumigen.
- (2)
- In 2003, Cubist recorded a lease termination charge of $12.9 million related to the planned closure of its UK facility.
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| | 2004
| | 2003
| |
|---|
| | (in thousands)
| |
|---|
| Balance Sheet Data: | | | | | | | | | | | | | | | | |
| Cash, cash equivalents and investments | | $ | 398,184 | | $ | 309,169 | | $ | 101,748 | | $ | 128,417 | | $ | 142,429 | |
| Working capital | | | 342,496 | | | 303,482 | | | 99,004 | | | 93,703 | | | 90,530 | |
| Total assets | | | 534,515 | | | 439,035 | | | 218,065 | | | 215,908 | | | 222,558 | |
| Total debt | | | 350,000 | | | 350,000 | | | 165,000 | | | 165,000 | | | 197,500 | |
| Long-term obligations | | | 352,698 | | | 351,760 | | | 165,000 | | | 165,078 | | | 195,693 | |
| Stockholders' equity (deficit) | | | 98,702 | | | 40,590 | | | 16,599 | | | 20,846 | | | (18,216 | ) |
| Dividends | | | — | | | — | | | — | | | — | | | — | |
43
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with Cubist's financial statements and related notes appearing elsewhere in this annual report. The following discussion contains forward-looking statements. Actual results may differ significantly from those projected in the forward-looking statements. Factors that might cause future results to differ materially from those projected in the forward-looking statements include, but are not limited to, those discussed in "Risk Factors" and elsewhere in this annual report. See also "Forward-Looking Statements."
Overview
Cubist is a biopharmaceutical company headquartered in Lexington, Massachusetts, focused on the research, development and commercialization of pharmaceutical products that address unmet medical needs in the acute care environment. To date, we have concentrated exclusively on developing products for the anti-infective marketplace. We have one marketed product, CUBICIN, which was launched in the U.S. in November 2003. CUBICIN is currently the only marketed once-daily, bactericidal, intravenous (IV) antibiotic with activity against methicillin-resistantS. aureus, or MRSA. CUBICIN is approved in the U.S. for the treatment of cSSSI, caused byS. aureus and certain other Gram-positive bacteria, and forS. aureus bloodstream infections (bacteremia), including those with right-sided infective endocarditis, caused by methicillin-susceptible and methicillin-resistant isolates. In the EU, CUBICIN is approved for the treatment of complicated skin and soft tissue infections, or cSSTI, where the presence of susceptible Gram-positive bacteria is confirmed or suspected. In September 2007, the Marketing Authorization for the CUBICIN label in the EU was expanded to include right-sided infective endocarditis, or RIE, due toS. aureus bacteremia associated with RIE or cSSTI.
Net product sales of CUBICIN for the twelve months ended December 31, 2007 were $290.4 million, as compared to $190.3 million in the twelve months ended December 31, 2006. Net income for the twelve months ended December 31, 2007 was $48.1 million or $0.87 and $0.83 per basic and diluted share, respectively, as compared to a net loss of $0.4 million or $0.01 per basic and diluted share for the twelve months ended December 31 2006.
In December 2007, Cubist acquired Illumigen pursuant to its October 2007 option agreement. Per the merger agreement, Cubist agreed to pay $9.0 million, plus Illumigen's closing cash and less Illumigen's closing liability balances, in cash to Illumigen shareholders, and Illumigen became a wholly-owned subsidiary of Cubist. Illumigen's lead compound, IB657, is a protein therapeutic in late-stage pre-clinical development as an interferon-sparing agent for the treatment of HCV infections. Cubist has evaluated whether the Illumigen acquisition meets the criteria of a business as outlined in Emerging Issues Task Force, or EITF, 98-3, "Determining Whether a Nonmonetary Transaction Involves Receipt of Productive Assets or of a Business," and has concluded that the entity did not qualify as a business. Accordingly, we accounted for this transaction as an acquisition of assets. The total costs associated with the acquisition were $16.4 million and include the closing cash consideration paid to Illumigen shareholders, the option agreement payment of $4.7 million made in October 2007, transaction costs of $0.8 million, and $0.7 million of costs paid by Cubist during the option period related to an IND enabling study of IB657 and Illumigen's operating costs during the option period. The total consideration was allocated to net tangible assets acquired of $1.3 million, consisting primarily of cash, IPR&D of $14.4 million and research and development expense of $0.7 million. The IPR&D represents the value assigned to the IB657 compound, and is included in research and development expense for the year ended December 31, 2007. We expect to file an IND for IB657 in the second half of 2008. Cubist will make additional payments to the former Illumigen shareholders of up to $75.5 million if certain development and regulatory milestones are achieved during the development of IB657 as a therapy for HCV. In addition, if Cubist develops an Illumigen product for the treatment of viral infections other than HCV, additional development and regulatory milestone payments of up to
44
$117.0 million would apply. If Illumigen product(s) are commercialized, sales milestones of up to $140.0 million, as well as tiered royalties, would apply.
In March 2007, we entered into a license agreement with Merck for the development and commercialization of CUBICIN in Japan, the last country outside the U.S. for which Cubist did not have a partner for the distribution of CUBICIN. Merck will develop and commercialize CUBICIN through its wholly-owned subsidiary, Banyu Pharmaceutical Co., Ltd., or Banyu. In exchange for the development and commercialization rights in Japan, Merck paid us $6.0 million cash upfront. This $6.0 million was recorded as deferred revenue, and will be recognized as revenue over the estimated performance period of the agreement. We may receive up to an additional $39.5 million in total milestone payments if Merck reaches certain regulatory and sales milestones. In addition, Merck will purchase finished but unlabeled vials of CUBICIN from us in exchange for a transfer price.
In July 2007, Kuhnil Pharmaceutical Corp., or Kuhnil, our CUBICIN marketing partner for South Korea, and TTY BioPharm Company, Ltd., our CUBICIN marketing partner for Taiwan, received approvals forS. aureus bacteremia, including RIE, in both South Korea and Taiwan, respectively. The approval in South Korea includes both cSSSI andS. aureus bacteremia, including RIE. CUBICIN was previously approved in Taiwan for cSSSI caused by Gram-positive bacteria. In September 2007, Oryx Pharmaceuticals, Inc., our CUBICIN marketing partner in Canada, received approval for cSSSI andS. aureus bacteremia. Oryx formally launched CUBICIN in Canada in January 2008.
We continue to sell CUBICIN in the U.S. in accordance with our drop-ship program under which orders are processed through wholesalers but shipments are sent directly to our end-users. This provides us with greater visibility into end-user ordering and reordering trends. We outsource many of our supply chain activities, including: (i) manufacturing and supplying CUBICIN API; (ii) converting CUBICIN API into its finished, vialed and packaged formulation; (iii) managing warehousing and distribution of CUBICIN to our customers; and (iv) performing the order processing, order fulfillment, shipping, collection and invoicing services related to our CUBICIN product sales.
We have focused our pipeline building efforts on opportunities that leverage our anti-infective and acute-care discovery, development, regulatory, and commercialization expertise. Currently, we have multiple anti-infective programs approaching the IND filing stage preparatory to clinical trials.
Since our inception, we have incurred net losses in every fiscal period until the third quarter of 2006 principally as a result of research and development efforts, preclinical testing, clinical trials, and administrative costs. As of December 31, 2007, we had an accumulated deficit of $436.0 million.
Results of Operations
Years Ended December 31, 2007 and 2006
Revenues
The following table sets forth revenues for the years ended December 31, 2007 and 2006:
| | December 31,
| |
| |
|---|
| | % Change
| |
|---|
| | 2007
| | 2006
| |
|---|
| | (in millions)
| |
| |
|---|
| U.S. product revenues, net | | $ | 285.1 | | $ | 189.5 | | 50 | % |
| International product revenues | | | 5.3 | | | 0.8 | | 562 | % |
| Other revenues | | | 4.2 | | | 4.4 | | -5 | % |
| | |
| |
| |
| |
| | Total revenues, net | | $ | 294.6 | | $ | 194.7 | | 51 | % |
| | |
| |
| |
| |
45
Product Revenues, net
Net sales of CUBICIN were $290.4 million in 2007 and $190.3 million in 2006. Gross sales of CUBICIN totaled $306.7 million and $199.8 million for the years ended December 31, 2007 and 2006, respectively, and are offset by $16.3 million and $9.5 million of allowances for sales returns, Medicaid and customer rebates, chargebacks, prompt-pay discounts and wholesaler management fees. The increase in product revenues was primarily due to increased U.S. customer volume, as well as a 6.2% price increase in January 2007. International revenues for the years ended December 31, 2007 and 2006 consisted primarily of product sales to Novartis.
We generally do not allow wholesalers to stock CUBICIN. We have a drop-ship program in place through which orders are processed through wholesalers, but shipments are sent directly to our end-users. This results in sales trends closely tracking actual hospital and out-patient administration location purchases of our product. Certain wholesalers seek various fees for data supply and administration services. Net product revenues are reduced by any such fees paid to the wholesalers.
Other Revenues
Other revenues for the year ended December 31, 2007 were $4.2 million as compared to $4.4 million for the year ended December 31, 2006, a decrease of $0.2 million or 5%. Included in other revenues for the year ended December 31, 2007 is revenue related to payments totaling $3.0 million under our license agreement with Novartis. The payments were received as a result of regulatory approvals for an expanded CUBICIN label in the EU. Also included in other revenues for the year ended December 31, 2007 is the amortization of license fees received from AstraZeneca, Merck and TTY. Included in other revenues for the year ended December 31, 2006 is revenue related to payments totaling $4.0 million under our license agreement with Novartis. The payments were received as a result of regulatory and pricing approvals for CUBICIN in Europe. Also included in other revenues for the year ended December 31, 2006 is $0.3 million of Small Business Innovation Research, or SBIR, grant revenue.
Costs and Expenses
The following table sets forth costs and expenses for the years ended December 31, 2007 and 2006:
| | December 31,
| |
| |
|---|
| | % Change
| |
|---|
| | 2007
| | 2006
| |
|---|
| | (in millions)
| |
| |
|---|
| Cost of product revenues | | $ | 68.9 | | $ | 48.8 | | 41 | % |
| Research and development | | | 85.2 | | | 57.4 | | 48 | % |
| Sales and marketing | | | 67.7 | | | 56.9 | | 19 | % |
| General and administrative | | | 31.5 | | | 26.7 | | 18 | % |
| | |
| |
| |
| |
| | Total costs and expenses | | $ | 253.3 | | $ | 189.8 | | 33 | % |
| | |
| |
| |
| |
Cost of Product Revenues
Cost of product revenues were $68.9 million and $48.8 million in the years ended December 31, 2007 and 2006, respectively. Our gross margin for the year ended December 31, 2007, was 76% as compared to 74% for the year ended December 31, 2006. The increase in our gross margin is primarily due to reduced overall pricing from our manufacturing vendors as well as higher volume resulting in lower cost per unit sold. Included in our cost of product revenues are royalties owed to Eli Lilly on net sales of CUBICIN under our license agreement with Eli Lilly. In March of 2005, we issued to Eli Lilly $20.0 million of our common stock in exchange for a 2% reduction in the royalties payable to Eli Lilly. In 2003, we issued to Eli Lilly $8.0 million of our common stock in exchange for a 1% reduction in the
46
royalties payable to Eli Lilly. We also issued 38,922 shares of our common stock valued at $0.5 million in 2003 as a milestone payment to Eli Lilly. These amounts have been capitalized on our balance sheet as intangible assets and are amortized to cost of product revenues over the remaining life of our license agreement with Eli Lilly. Amortization included in cost of product revenues related to these items was $2.5 million for the years ended December 31, 2007 and 2006.
As our production volumes increase, there is the potential for our gross margin to increase as we work to develop manufacturing process improvements. Whether that potential can be realized and the extent to which such potential can be realized are uncertain.
Research and Development Expense
Total research and development expense in the year ended December 31, 2007 was $85.2 million as compared to $57.4 million in the year ended December 31, 2006, an increase of $27.8 million or 48%. The increase in research and development expenses was due primarily to (i) the IPR&D charge of $14.4 million and other related expense of $0.7 million related to the acquisition of Illumigen in December 2007; (ii) an increase of $4.5 million in payroll, benefits, travel and other employee related expenses; (iii) an increase of $4.2 million in clinical and non-clinical study costs; (iv) an increase of $2.3 million in collaboration expense; (v) an increase of $0.7 million in professional services expense; (vi) an increase of $0.6 million in research grant expense; (vii) an increase of $0.4 million in information technology expense; and (vii) an increase of $0.4 million in laboratory supplies and equipment expense.
We expect to continue incurring substantial research and development expenses related to: (i) Phase 2 and Phase 4 clinical trials for CUBICIN; (ii) pre-clinical and clinical testing of other products under development, such as our HCV and CDAD preclinical compounds and our resistant Gram-positive and Gram-negative programs; (iii) regulatory matters; and (iv) medical affairs activities.
Sales and Marketing Expense
Sales and marketing expense in the year ended December 31, 2007 was $67.7 million as compared to $56.9 million in the year ended December 31, 2006, an increase of $10.8 million or 19%. The increase in sales and marketing expense is primarily due to (i) an increase of $5.3 million in payroll, benefits, travel and other employee related expenses; (ii) an increase of $4.7 million in marketing, promotional programs and trade show expense; and (iii) an increase of $0.5 million in information technology expense. Sales and marketing expense are expected to increase in 2008 as we continue our commercialization efforts related to CUBICIN in the U.S. and work towards achieving our goal of increasing the number of CBMs in the U.S. from 135 to 164 by April 1, 2008.
General and Administrative Expense
General and administrative expense in the year ended December 31, 2007, was $31.5 million as compared to $26.7 million in the year ended December 31, 2006, an increase of $4.7 million or 18%. This increase is primarily due to (i) an increase of $1.9 million in payroll, benefits and other employee related expenses; and (ii) an increase of $3.0 million in professional services.
47
Other Income (Expense), net
The following table sets forth other income (expense); net for the years ended December 31, 2007 and 2006:
| | December 31,
| |
| |
|---|
| | % Change
| |
|---|
| | 2007
| | 2006
| |
|---|
| | (in millions)
| |
| |
|---|
| Interest income | | $ | 18.0 | | $ | 10.6 | | 70 | % |
| Interest expense | | | (9.4 | ) | | (15.9 | ) | -41 | % |
| Other income | | | — | | | — | | -267 | % |
| | |
| |
| |
| |
| | Total other income (expense), net | | $ | 8.6 | | $ | (5.3 | ) | -262 | % |
| | |
| |
| |
| |
Interest Income and Expense
Interest income in the year ended December 31, 2007 was $18.0 million as compared to $10.6 million in the year ended December 31, 2006, an increase of $7.4 million or 70%. The increase in interest income is due primarily to a higher average cash balance during 2007 as compared to 2006 as well as higher rates of return on our investments. The higher average cash balance is due to increased cash from operations as well as the net proceeds of $339.1 million resulting from the closing of our $350.0 million aggregate principal amount of 2.25% convertible subordinated notes offering on June 6, 2006, offset by the repayment of the principal and outstanding interest of our $165.0 million aggregate principal amount of 5.5% convertible subordinated notes, plus a prepayment penalty.
Interest expense in the year ended December 31, 2007 was $9.4 million as compared to $15.9 million in the year ended December 31, 2006, a decrease $6.5 million or 41%. The decrease in interest expense is primarily due to the early repayment of our $165.0 million aggregate principal amount of 5.5% convertible subordinated notes due in November 2008 on June 28, 2006. We used a portion of the proceeds from the June 2006 $350.0 million 2.25% convertible subordinated notes offering to repay the principal and outstanding interest of the $165.0 million aggregate principal amount of 5.5% convertible subordinated notes. This early prepayment in 2006 resulted in one time charges to interest expense of the prepayment penalty of $3.9 million as well as the write-off of the remaining unamortized balance of related debt issuance costs of $1.8 million.
Provision for Income Taxes
Cubist's effective tax rate for the years ended December 31, 2007 and 2006 were 3.7% and 0%, respectively. The effective tax rate for the year ended December 31, 2007 relates to federal alternative minimum tax expense and state tax expense. The Company and its subsidiaries file income tax returns with the U.S. federal government and with multiple state and local jurisdictions in the U.S. All of our deferred tax assets have a full valuation allowance recorded against them. We continue to monitor the available information in determining whether there is sufficient positive evidence to consider releasing the valuation allowance on the deferred tax assets. Should we determine the valuation allowance is no longer required, a tax benefit would be recorded in the financial period of the change in determination.
48
Years Ended December 31, 2006 and 2005
Revenues
The following table sets forth revenues for the year ended December 31, 2006 and 2005:
| | December 31,
| |
| |
|---|
| | % Change
| |
|---|
| | 2006
| | 2005
| |
|---|
| | (in millions)
| |
| |
|---|
| U.S. product revenues, net | | $ | 189.5 | | $ | 113.4 | | 67 | % |
| International product revenues | | | 0.8 | | | 0.1 | | 910 | % |
| Other revenues | | | 4.4 | | | 7.1 | | -38 | % |
| | |
| |
| |
| |
| | Total revenues, net | | $ | 194.7 | | $ | 120.6 | | 61 | % |
| | |
| |
| |
| |
Product Revenues, net
Net sales of CUBICIN were $190.3 million and $113.5 million in 2006 and 2005, respectively. Gross sales of CUBICIN totaled $199.8 million and $118.6 million for the years ended December 31, 2006 and 2005, respectively, and are offset by $9.5 million and $5.1 million of allowances for sales returns, Medicaid and customer rebates, chargebacks, prompt-pay discounts and wholesaler management fees. The increase in revenues was primarily due to increased customer volume. Also impacting net product revenues was a 6.6% price increase in October 2005 and an additional 6.5% price increase in May 2006. Included in net product revenues for the year ended December 31, 2006 is $0.8 million of international sales.
We generally do not allow wholesalers to stock CUBICIN. We have a drop-ship program in place through which orders are processed through wholesalers, but shipments are sent directly to our end-users. This results in sales trends closely tracking actual hospital and out-patient administration location purchases of our product. Certain wholesalers seek various fees for data supply and administration services. Net product revenue is reduced by any such fees paid to the wholesalers.
Other Revenues
Other revenues for the year ended December 31, 2006 were $4.4 million as compared to $7.1 million for the year ended December 31, 2005, a decrease of $2.7 million or 38%. The decrease in other revenues is primarily due to a decrease in revenue related to our 2003 license agreement with Novartis. Other revenues under this agreement totaled $4.0 million for the year ended December 31, 2006 compared to $6.5 million for the year ended December 31, 2005. Other revenues under this agreement recognized in the year ended December 31, 2006 consisted of payments received as a result of regulatory and pricing approvals for CUBICIN in Europe which were recognized as revenue upon their receipt. Other revenues under this agreement recognized in the year ended December 31, 2005 consisted of the recognition of $4.3 million of previously deferred upfront payments and $2.2 million of development revenue. The upfront payments totaled $11.3 million, and included a $3.3 million premium paid upon purchasing our common stock. This $11.3 million was recorded as deferred revenue and was amortized to license fee revenues over the estimated development period of the agreement of two years which was completed in September 2005. Also included in other revenues for the year ended December 31, 2006 and 2005 are Small Business Innovation Research, or SBIR, grant revenue of $0.3 million and $0.4 million, respectively.
49
Costs and Expenses
The following table sets forth costs and expenses for the years ended December 31, 2006 and 2005:
| | December 31,
| |
| |
|---|
| | % Change
| |
|---|
| | 2006
| | 2005
| |
|---|
| | (in millions)
| |
| |
|---|
| Cost of product revenues | | $ | 48.8 | | $ | 32.7 | | 49 | % |
| Research and development | | | 57.4 | | | 51.7 | | 11 | % |
| Sales and marketing | | | 56.9 | | | 42.3 | | 34 | % |
| General and administrative | | | 26.7 | | | 19.3 | | 38 | % |
| | |
| |
| |
| |
| | Total costs and expenses | | $ | 189.8 | | $ | 146.0 | | 30 | % |
| | |
| |
| |
| |
Cost of Product Revenues
Cost of product revenues were $48.8 million and $32.7 million in the years ended December 31, 2006 and 2005, respectively. Our gross margin for the year ended December 31, 2006, was 74% as compared to 71% for the year ended December 31, 2005, primarily due to reduced overall pricing from our manufacturing vendors as well as higher volume resulting in lower cost per unit sold. Included in our cost of product revenues are royalties owed to Eli Lilly on net sales of CUBICIN under our license agreement with Eli Lilly. In March of 2005, we issued to Eli Lilly $20.0 million of our common stock in exchange for a 2% reduction in the royalties payable to Eli Lilly. In 2003, we issued to Eli Lilly $8.0 million of our common stock in exchange for a 1% reduction in the royalties payable to Eli Lilly. We also issued 38,922 shares of our common stock valued at $0.5 million in 2003 as a milestone payment to Eli Lilly. These amounts have been capitalized on our balance sheet as intangible assets and are amortized to cost of product revenues over the remaining life of our license agreement with Eli Lilly. Amortization included in cost of product revenues related to these expenses was $2.5 million and $1.9 million for the years ended December 31, 2006 and 2005, respectively.
Research and Development Expense
Total research and development expense in the year ended December 31, 2006 was $57.4 million as compared to $51.7 million in the year ended December 31, 2005, an increase of $5.7 million or 11%. The increase in research and development expenses was due primarily to (i) an increase of $9.4 million in payroll, benefits, travel and other employee related expenses due to increased headcount as well as non-cash stock-based compensation charges associated with the implementation of FAS 123(R); (ii) an increase of $2.1 million in collaborations expense due primarily to costs associated with our Ilypsa collaboration which we entered into in the second quarter of 2006; (iii) an increase of $1.2 million in process development costs associated with the development of our lipopeptide program; (iv) an increase of $0.9 million in research grants; (v) an increase of $1.3 million in lab supplies, equipment, and services; (vi) an increase of $0.7 million in non-clinical studies; and (vii) an increase of $0.7 million in publications expense. These increases were offset by (i) a decrease of $6.2 million in clinical study costs due primarily to our decision to discontinue investment in our HepeX-B program as well as the completion of our clinical trial of CUBICIN in the treatment of bacteremia with known or suspected endocarditis caused byS. aureus; (ii) a $1.6 million decrease in medical education expense; and (iii) a $0.7 million decrease in process development costs related to HepeX-B. Additionally, $1.6 million of manufacturing development costs associated with our license agreement with Novartis, and $0.8 million of costs related to the establishment of a second API manufacturer and a second fill-finish manufacturer for our CUBICIN product were incurred in 2005 and were not repeated in 2006.
50
Sales and Marketing Expense
Sales and marketing expense in the year ended December 31, 2006 was $56.9 million as compared to $42.3 million in the year ended December 31, 2005, an increase of $14.5 million or 34%. The increase in sales and marketing expense is primarily due to an increase of $12.4 million in payroll, benefits, travel and other employee related expenses due to our sales force expansion in the first quarter of 2006 and the non-cash stock-based compensation charges associated with the implementation of FAS 123(R). Also included in sales and marketing expense is an increase of $2.8 million in promotional programs.
General and Administrative Expense
General and administrative expense in the year ended December 31, 2006 was $26.7 million as compared to $19.3 million in the year ended December 31, 2005, an increase of $7.4 million or 38%. This increase is primarily due to (i) an increase of $4.7 million in payroll, benefits and other employee related expenses due to headcount growth and the non-cash stock-based compensation charges associated with the implementation of FAS 123(R); (ii) an increase of $1.6 million in professional services; and (iii) an increase of $0.9 million in rent expense due to additional space we have leased at 55 Hayden Avenue in Lexington, MA.
Other Expense, net
The following table sets forth other expense, net for the years ended December 31, 2006 and 2005:
| | December 31,
| |
| |
|---|
| | % Change
| |
|---|
| | 2006
| | 2005
| |
|---|
| | (in millions)
| |
| |
|---|
| Interest income | | $ | 10.6 | | $ | 3.3 | | 222 | % |
| Interest expense | | | (15.9 | ) | | (9.8 | ) | 62 | % |
| Other income | | | — | | | 0.1 | | -90 | % |
| | |
| |
| |
| |
| | Total other expense, net | | $ | (5.3 | ) | $ | (6.4 | ) | (18 | )% |
| | |
| |
| |
| |
Interest Income and Expense
Interest income in the year ended December 31, 2006 was $10.6 million as compared to $3.3 million in the year ended December 31, 2005, an increase of $7.3 million or 222%. The increase in interest income was due to a higher average cash balance from June to December 2006 compared to the same period in 2005 as well as higher rates of return on our investments. The higher average cash balance is due to the net proceeds of $339.1 million resulting from the closing of our $350.0 million aggregate principal amount of 2.25% convertible subordinated notes offering on June 6, 2006, offset by the repayment of the principal and outstanding interest of our $165.0 million aggregate principal amount of 5.5% convertible subordinated notes, plus a prepayment penalty.
Interest expense in the year ended December 31, 2006 was $15.9 million as compared to $9.8 million in the year ended December 31, 2005, an increase of $6.1 million or 62%. The increase in interest expense is primarily due to the early repayment of our $165.0 million aggregate principal amount of 5.5% convertible subordinated notes due in November 2008 on June 28, 2006. The early repayment of the $165.0 million aggregate principal amount of 5.5% convertible subordinated notes resulted in charges to interest expense of the prepayment penalty of $3.9 million as well as the write-off of the remaining unamortized balance of related debt issuance costs of $1.8 million.
51
Other Income
Other income in the year ended December 31, 2006 was $0 as compared to $0.1 million in the year ended December 31, 2005. Other income for the year ended December 31, 2005 primarily consisted of a gain of $0.1 million due to the merger of Syrrx, Incorporated, or Syrrx, and Takeda Pharmaceutical Company Limited, which resulted in the return of our original investment in Syrrx.
Liquidity and Capital Resources
Currently, we require cash to fund our working capital needs, to purchase capital assets, and to pay our debt service, including principal, interest and capital lease obligations. We fund our cash requirements through the following methods:
- •
- sales of CUBICIN;
- •
- payments from our strategic collaborators including license fees, royalties and milestone payments, sponsored research funding and research grants;
- •
- equity and debt financings; and
- •
- interest earned on invested capital.
Net cash provided by operating activities was $100.8 million in 2007, compared to net cash provided by operating activities of $30.7 million in 2006 and to net cash used in operating activities of $30.6 million in 2005. Net cash provided by operating activities in 2007 includes our net income for the year of $48.1 million increased by non-cash charges of $37.8 million that primarily consists of $14.4 million of acquired IPR&D, $10.6 million of stock-based compensation expenses, $9.7 million of depreciation and amortization expense, $1.6 million of amortization of debt issuance costs and $2.1 million in expense associated with our 401(k) company match that is made in the form of common stock shares. Uses of cash consisted of an increase of $8.0 million in accounts receivable due to increased sales of CUBICIN, and an increase of $2.8 million (net of non-cash amortization expense related to manufacturing assets from our previous API supplier DSM Capua, S.p.A., or DSM) in inventory primarily due to increased purchases from our manufacturing vendors as we build a sufficient supply of CUBICIN to meet projected sales requirements. These uses of cash were offset by a $20.4 million increase in accounts payable and accrued liabilities due primarily to increased royalties paid to Eli Lilly related to increased sales of CUBICIN and a $6.0 million increase in deferred revenue, primarily due to the receipt of a $6.0 million upfront payment from Merck.
Net cash provided by investing activities in 2007 was $226.2 million, compared to $227.8 million used in investing activities in 2006 and $32.9 million provided by investing activities in 2005. Cash provided by investing activities in 2007 represents cash inflows from the maturity of securities, offset by purchases of securities, as well as cash outflows for purchases of property and equipment and for the acquisition of Illumigen, net of cash acquired. Purchases of property and equipment during the year ended December 31, 2007, were $5.1 million compared to $7.4 million and $2.1 million in the years ended December 31, 2006 and 2005, respectively. Property and equipment additions in 2007 consisted primarily of expenses related to building out additional lease space at 45 and 55 Hayden Avenue, lab equipment and computer software. Property and equipment additions in 2006 consisted primarily of lab equipment, expenditures related to building out additional leased space at 55 Hayden Avenue, as well as various IT upgrades. Property and equipment additions in 2005 consisted primarily of computer hardware and software and lab equipment. Net cash used in investing activities may fluctuate significantly from period to period due to the timing of our capital expenditures and other investments. We anticipate that our capital expenditures for 2008 will increase to approximately $24.0 million, primarily driven by the construction of approximately 30,000 square feet of laboratory space at our main building in 65 Hayden Avenue, as well expenses related to building out additional leased space at 55 and 45 Hayden Avenue.
52
Net cash of $11.8 million was provided by financing activities in the year ended December 31, 2007, as compared to $184.0 million and $6.2 million provided by financing activities in the years ended December 31, 2006 and 2005, respectively. Proceeds from financing activities in 2007 consisted primarily of $12.1 million from employees' exercise of stock options and purchases of common stock through our employee stock purchase plan.
Auctions for $58.1 million of our investments in auction rate securities have failed repeatedly since August 2007. These auction rate notes consist of private placement, credit default swap structured securities which reference synthetic portfolios of corporate bonds. These securities have long-term nominal maturities for which the interest rates are reset through a monthly auction. These monthly auctions historically have provided a liquid market for these securities. Consistent with our investment policy guidelines, the auction rate securities held by us all had AAA credit ratings at the time of purchase. As a result of the auction failures, we have recorded temporary unrealized losses of $14.7 million in other comprehensive income as a reduction in shareholders' equity. All of the auction rate securities that failed are still AAA rated and none are backed by sub-prime mortgages. The failure resulted in the interest rate on these investments resetting at the default coupon rate per the terms of the certificate. Historically, given the liquidity created by the auctions, auction rate securities were presented as current assets under marketable securities on our balance sheet. Given the failed auctions, while we now earn a premium interest rate on the investments, the investments are not liquid. In the event that we need to access these funds, we will not be able to do so until a future auction on these investments is successful or until they reach their underlying maturity. Accordingly, the entire amount of these auction rate securities, net of unrealized losses of $14.7 million, has been classified from current to non-current assets on our balance sheet. The credit and capital markets have continued to deteriorate in 2008 and the bids on the auction rate notes we hold have since declined but there have been no sales at these lower bids. If a certain concentration, as defined in the auction rate documents, of the underlying reference portfolios default, or if the issuing bank fails to make the required interest payments or the final principal payment upon the ultimate maturity of the notes, or if the credit ratings on the underlying reference portfolios deteriorate significantly, the Company may be required to adjust the carrying value of these investments through an other-than- temporary impairment charge. Based on our ability to access our cash and other short-term investments, our expected operating cash flows, and other sources of cash, we do not anticipate the lack of liquidity on these investments will affect our ability to execute our current business plan.
In June 2006, we completed the public offering of $350.0 million aggregate principal amount of 2.25% convertible subordinated notes (less financing costs of $10.9 million). The notes are convertible at any time prior to maturity into common stock at an initial conversion rate of 32.4981 shares of common stock per $1,000 principal amount of convertible notes, subject to adjustment upon certain events, which equates to approximately $30.77 per share. Interest is payable on each June 15 and December 15, beginning December 15, 2006. In January 2008, we repurchased $50.0 million of the original principal amount of the 2.25% notes (see Note S.).
In March 2005, we announced that we had entered into an agreement to purchase from Eli Lilly a 2% reduction in the royalty rate payable to Eli Lilly on net sales of CUBICIN. In exchange for this reduction, we issued to Eli Lilly $20.0 million in Cubist common stock with associated registration rights. A total of 1,876,173 shares were issued at a price of $10.66 in March 2005. Our global royalty rate obligation payable to Eli Lilly on CUBICIN sales was reduced by two percentage points upon registration of the common stock on April 22, 2005. In July 2003, we entered into an amendment to the license agreement with Eli Lilly and issued to Eli Lilly 723,619 shares of common stock valued at $8.0 million, in consideration for a 1% reduction in the royalty rate payable to Eli Lilly on net sales of CUBICIN.
In October 2001, we completed the private placement of $165.0 million aggregate principal amount of 51/2% convertible subordinated notes (less financing costs of $5.3 million). The notes were
53
convertible at any time prior to maturity into common stock at a conversion price of $47.20 per share, subject to adjustment upon certain events. Interest was payable on each November 1 and May 1, beginning May 1, 2002. Cubist paid $9.1 million in interest on these notes during 2005 and 2004. We used a portion of the proceeds from the June 2006 $350.0 million 2.25% convertible subordinated notes offering to repay the principal and outstanding interest of our $165.0 million aggregate principal amount of 5.5% convertible subordinated notes, plus a prepayment penalty. This repayment resulted in charges of $5.7 million related to the prepayment penalty and the write-off of debt issuance costs associated with the debt.
From time to time, our board of directors may consider authorizing Cubist to repurchase shares of our common stock or our outstanding convertible subordinated notes in privately negotiated transactions, or publicly announced programs. If and when our board of directors should determine to authorize any such action, it would be on terms and under market conditions the board determines are in the best interest of our company. Any such repurchases could deplete some of our cash resources.
Contractual Obligations
Contractual obligations represent future cash commitments and liabilities under agreements with third parties and exclude contingent liabilities, such as royalties on future sales above the contractual minimums or known accrued royalty balance, for which we cannot reasonably predict future payment. The following summarizes our significant contractual obligations at December 31, 2007, and the effects such obligations are expected to have on our liquidity and cash flows in future periods.
| | Payments due by period
|
|---|
| | 1 year
or less
| | 2-3
Years
| | 4-5
Years
| | More than
5 Years
| | Total
|
|---|
| | (in millions)
|
|---|
| Subordinated convertible notes | | $ | — | | $ | — | | $ | — | | $ | 350.0 | | $ | 350.0 |
| Interest on subordinated convertible notes | | | 7.9 | | | 15.7 | | | 15.7 | | | 3.9 | | | 43.2 |
| Operating leases, net of sublease income | | | 2.7 | | | 6.9 | | | 7.1 | | | 13.0 | | | 29.7 |
| Inventory purchase obligations | | | 17.7 | | | 21.6 | | | 23.3 | | | 28.1 | | | 90.7 |
| Capital purchase obligations | | | 3.0 | | | | | | | | | | | | 3.0 |
| Royalty payments due | | | 23.7 | | | — | | | — | | | — | | | 23.7 |
| Other purchase obligations | | | 5.6 | | | | | | | | | | | | 5.6 |
| | |
| |
| |
| |
| |
|
| | Total contractual cash obligations | | $ | 60.6 | | $ | 44.2 | | $ | 46.1 | | $ | 395.0 | | $ | 545.9 |
| | |
| |
| |
| |
| |
|
The subordinated convertible notes consist of $350.0 million aggregate principal amount of our 2.25% convertible subordinated notes, due in June 2013. These notes require semi-annual interest payments through maturity. In January 2008, we repurchased $50.0 million of the original principal amount of the 2.25% notes (see Note S.).
Our operating leases consist of approximately 121,000 square feet of office and data center space at 45 and 55 Hayden Avenue in Lexington, Massachusetts, pursuant to a term lease that expires in April 2016, 24,000 square feet of commercial space at 24 Emily Street in Cambridge, Massachusetts, pursuant to a term lease that expires in September 2008 and 15,000 square feet of commercial space at 148 Sidney Street in Cambridge Massachusetts, pursuant to a term lease that expires in December 2010. We have subleased the space located at 24 Emily Street for a term that coincides with the September 2008 lease expiration. We have subleased the space located at 148 Sidney Street through October 2010.
The inventory purchase obligations listed above represent minimum volumes that we are required to purchase from our contract manufacturers. The capital purchase obligations listed above represent capital purchase commitments related to building out additional leased space at 55 and 45 Hayden
54
Avenue. The royalty payments listed above represent amounts owed to Eli Lilly on sales of CUBICIN product. The other purchase obligations listed above represent payments related to the development of IB657.
Critical Accounting Policies and Estimates
Cubist prepares its consolidated financial statements in conformity with accounting principles generally accepted in the United States of America. The Company is required to make certain estimates, judgments and assumptions that affect certain reported amounts and disclosures; actual amounts may differ.
We believe that the following critical accounting policies affect the more significant judgments and estimates used in the preparation of our consolidated financial statements:
- •
- Revenue recognition;
- •
- Inventories;
- •
- Accrued clinical research costs;
- •
- Investments;
- •
- Long-lived assets;
- •
- Income taxes; and
- •
- Stock-based compensation.
I. Revenue recognition
We recognize revenue in accordance with SEC Staff Accounting Bulletin No. 101 (SAB 101), as amended by SAB 104, and Emerging Issues Task Force (EITF) Issue No. 00-21. Principal sources of revenue are sales of CUBICIN and license fees and milestone payments that are derived from collaborative agreements with other biopharmaceutical companies and distribution agreements. We have followed the following principles in recognizing revenue:
Product Revenues, net
We recognize revenue from product sales when persuasive evidence of an arrangement exists, title to product and associated risk of loss has passed to the customer, the price is fixed or determinable, collection from the customer is reasonably assured and we have no further performance obligations. All revenues from product sales are recorded net of applicable provisions for returns, chargebacks, discounts, wholesaler management fees and rebates in the same period the related sales are recorded.
Since the launch of CUBICIN in November 2003, we generally have not allowed wholesalers to stock CUBICIN. Instead, we instituted a drop-ship program that we have continued to maintain. Under our drop-ship program, orders are processed through wholesalers, but shipments are sent directly to our end-users, who are generally hospitals and acute care settings. This results in sales trends closely tracking actual hospital and acute care settings purchases of our product, and also prevents unusual purchasing patterns since it closely tracks end-user demand.
We maintain a return policy that allows our customers to return product within a specified period prior to and subsequent to the expiration date. Our estimate of the provision for returns is analyzed quarterly and is based upon many factors, including industry data of product return rates, historical experience of actual returns, analysis of level inventory in the distribution channel, if any, and reorder rates of end-users. If the history of our product returns changes, the reserve will be adjusted appropriately. If we discontinue the drop ship program and allow wholesalers to stock CUBICIN, our
55
net product sales may be impacted by the timing of wholesaler inventory stocking and activity and provisions for returns which will be based on estimated product in the distribution channel that may not sell through to end-users.
We analyze our estimates and assumptions for chargebacks and Medicaid rebate reserves quarterly. Our Medicaid and chargeback reserves have two components: (i) an estimate of outstanding claims for known end-user rebate eligible sales that have occurred, but for which related claim submissions have not been received; and (ii) an estimate of chargebacks and Medicaid rebates based on an analysis of customer sales mix data to determine which sales may flow through to a rebate or chargeback eligible customer. Because the second component is calculated based on the amount of inventory in the distribution channel, if any, our assessment of distribution channel inventory levels impacts our estimated reserve requirements. We accrue for the expected liability at the time we record the sale, however, the time lag between sale and payment of rebate can be lengthy. Due to the time lag, in any particular period our rebate adjustments may incorporate revisions of accruals for several periods.
Reserves for Medicaid rebate programs are included in accrued liabilities and were $601,000 and $652,000 at December 31, 2007 and 2006, respectively. Reserves for returns, discounts, chargebacks, wholesaler management fees and customer rebates are offset against accounts receivable and were $3.9 million and $2.8 million at December 31, 2007 and 2006, respectively. In the year ended December 31, 2007, 2006 and 2005, provisions for sales returns, chargebacks, rebates, wholesaler management fees and prompt-pay discounts that were offset against product revenues totaled $16.3 million, $9.5 million and $5.1 million, respectively.
We believe that the reserves we have established are reasonable and appropriate based upon current facts and circumstances. Applying different judgments to the same facts and circumstances would result in the estimated amounts for sales returns, chargebacks and Medicaid rebate reserves to vary. However, due to the drop-ship model that we currently operate under, and the low level of actual product returns, chargebacks and Medicaid rebate claims experienced to date, we do not expect that the differences would be material.
Multiple Element Arrangements
We analyze our multiple element arrangements to determine whether the elements can be separated and accounted for individually as separate units of accounting in accordance with EITF No. 00-21, "Revenue Arrangements with Multiple Deliverables." We recognize up-front license payments as revenue if the license has standalone value and the fair value of the undelivered items can be determined. If the license is considered to have standalone value but the fair value on any of the undelivered items cannot be determined, the license payments are recognized as revenue over the period of performance for such undelivered items or services. Our assessment of our obligations and related performance periods require significant management judgement.
License Revenues
Non-refundable license fees are recognized depending on the provisions of each agreement. License fees with ongoing involvement or performance obligations are recorded as deferred revenue once received and are generally recognized ratably over the period of such performance obligation only after both the license period has commenced and the technology has been delivered. If an agreement contains product development services, the relevant time period for the product development phase is based on management estimates and could vary depending on the outcome of clinical trials and the regulatory approval process. Such changes could materially impact the revenue recognized and as a result, management reviews the estimates related to the relevant time period of product development quarterly.
56
Milestones
Revenues from milestone payments related to arrangements under which we have continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: the milestone payments are non-refundable; achievement of the milestone was not reasonably assured at the inception of the arrangement; substantive effort is involved in achieving the milestone; and the amount of the milestone is reasonable in relation to the effort expended or the risk associated with the achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as we complete our performance obligations. Contingent payments under license agreements that do not involve substantial effort on the part of the Company are not considered substantive milestones. Such payments are recognized as revenue when the contingency is met only if there are no remaining performance obligations or any remaining performance obligations are priced at fair value. Otherwise, the contingent payment is recognized as the Company completes its performance obligations under the arrangement.
Research services
Revenues from SBIR grants to conduct research and development are recognized as the eligible costs are incurred up to the granted funding limit.
II. Inventories
Inventories are stated at the lower of cost or market with cost determined under the first-in, first-out, or FIFO, basis. Included in the cost of inventories are employee stock-based compensation costs capitalized under SFAS 123(R). On a quarterly basis, we analyze our inventory levels, and write-down inventory that is expected to expire prior to being sold, inventory that has a cost basis in excess of its expected net realizable value, inventory in excess of expected sales requirements, or inventory that fails to meet commercial sale specifications through a charge to cost of product revenues. Expired inventory is disposed of and the related costs are written off to cost of product revenues. Charges for inventory write-downs are not reversed if it is later determined that the product is saleable, therefore, any such inventory would be sold at zero cost. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required.
III. Accrued clinical research costs
We utilize external entities such as contract research organizations, independent clinical investigators, and other third-party service providers to assist us with the execution of our clinical studies. We record costs for clinical study activities based upon the estimated amount of services provided but not yet invoiced for each study, and include these costs in accrued liabilities in our Consolidated Balance Sheets and within research and development expense in our Consolidated Statements of Operations. Contracts and studies vary significantly in length, and are generally composed of a fixed management fee, variable indirect reimbursable costs that have a dollar limit cap, and amounts owed on a per patient enrollment basis. We monitor the activity levels and patient enrollment levels of the studies to the extent possible through communication with the service providers, detailed invoice and task completion review, analysis of actual expenses against budget, pre-approval of any changes in scope, and review of contractual terms. These estimates may or may not match the actual services performed by the service providers as determined by actual patient enrollment levels and other variable activity costs. Clinical trial expenses totaled $5.6 million, $2.6 million and $8.8 million for the years ended December 31, 2007, 2006 and 2005, respectively. The level of clinical study expense may vary from period to period based on the number of studies that are in process, the duration of the study, the required level of patient enrollment, and the number of sites involved in the study. Clinical trials that bear the greatest risk of change in estimates are typically those
57
with a significant number of sites, require a large number of patients, have complex patient screening requirements and that span multiple years. If we receive incomplete or inaccurate information from our third-party service providers, we may under or over estimate activity levels associated with various studies at a given point in time. In this event, we could record adjustments to prior period accruals that increase or reverse research and development expenses in future periods when the actual activity level becomes known.
IV. Investments
It is our intent to hold all investments to their effective maturity in accordance with our investment policy. However, if the circumstances regarding an investment were to change, such as a change in an investment's external credit rating, we would consider a sale of the related security to minimize any losses. The appropriateness of all investment classifications is reviewed at each reporting date. The amount of the unrealized loss on the auction rate notes is determined through a valuation analysis which includes an assessment of the risk related to the underlying corporate bond portfolio as well as the actual sales activity of these auction rate notes at current available market bids.
Included in our investments are auction rate notes, which consist of private placement, credit default swap structured securities which reference synthetic portfolios of corporate bonds. These securities have long-term nominal maturities for which the interest rates are reset through a monthly auction. While the underlying securities of auction rate securities may have contractual maturities of more than ten years, the interest rates on such securities reset at intervals of 7, 28 or 35 days. Historically, auction rate securities have been priced and traded as short-term investments because of this reset feature and have been considered short-term available-for-sale investments. Given the repeated failure of auctions for $58.1 million of our investments in auction rate securities, we do not consider these investments to be liquid and classified them as long-term investments as of December 31, 2007. As a result of the auction failures, we have recorded temporary unrealized losses of $14.7 million in other comprehensive income as a reduction in shareholders' equity. The amount of the unrealized loss on the auction rate notes is determined through a valuation analysis which is based on bids from the broker who is the market maker for these instruments, corroborated through an analysis of the underlying instruments. We consider this loss to be temporary in nature. The credit and capital markets have continued to deteriorate in 2008 and the bids on the auction rate notes we hold have since declined. If a certain concentration, as defined in the auction rate documents, of the underlying reference portfolios default, or if the issuing bank fails to make the required interest payments or the final principal payment upon the ultimate maturity of the notes, or if the credit ratings on the underlying reference portfolios deteriorate significantly, we may be required to adjust the carrying value of these investments through an other-than-temporary impairment charge. Investment classification detail can be found in Note E., "Investments" in the Notes to the Consolidated Financial Statements.
V. Long-lived assets
In the ordinary course of our business, we incur substantial costs to purchase and construct property, plant and equipment. The treatment of costs to purchase or construct these assets depends on the nature of the costs and the stage of construction. We generally depreciate plant and equipment using the straight-line method over the asset's estimated economic life, which ranges from 3 years to 40 years. Determining the economic lives of plant and equipment requires us to make significant judgments that can materially impact our operating results. Property and equipment primarily consists of our corporate headquarters building.
As of December 31, 2007, there were approximately $22.7 million of net other intangible assets on our consolidated balance sheet, which consisted of patents, intellectual property, acquired technology rights, manufacturing rights, and other intangibles. We amortize our intangible assets using the straight-line method over their estimated economic lives, which range from 5 years to 16 years.
58
Determining the economic lives of intangible assets requires us to make significant judgment and estimates, and can materially impact our operating results.
Property and equipment and intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Judgments regarding the existence of impairment indicators are based on historical and projected future operating results, changes in the manner of use of the acquired assets, overall business strategy, and market and economic trends. Future events could cause management to conclude that impairment indicators exist and that certain long-lived assets are impaired.
VI. Income Taxes
We record deferred tax assets and liabilities based on the net tax effects of tax credits, operating loss carry forwards, and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. We then assess the likelihood that deferred tax assets will be recovered from future taxable income and, to the extent that we determine that recovery is not likely, a valuation allowance is established. The valuation allowance is based on estimates of taxable income by jurisdiction in which we operate and the period over which deferred tax assets will be recoverable. Through December 31, 2007, we believe it is more likely than not that all of our deferred tax assets will not be realized and, accordingly, have recorded a valuation allowance against all deferred tax assets. We continue to monitor the available information in determining whether there is sufficient positive evidence to consider releasing the valuation allowance on the deferred tax assets. Should we determine the valuation allowance is no longer required, a tax benefit would be recorded in the financial period of the change in determination.
VI. Stock-Based Compensation
Effective January 1, 2006, our accounting policy related to stock option accounting changed upon our adoption of SFAS 123(R). SFAS 123(R) requires us to expense the fair value of employee stock options and other forms of share-based compensation. Under the fair value recognition provisions of SFAS 123(R), share-based compensation cost is estimated at the grant date based on the value of the award and is recognized as expense ratably over the requisite service period of the award (generally the vesting period of the equity award). Determining the appropriate fair value model and calculating the fair value of share-based awards requires judgment, including estimating the expected life of the share-based award, the expected stock price volatility over the expected life of the share-based award and forfeiture rates.
In order to determine the fair value of share-based awards on the date of grant, we use the Black-Scholes option-pricing model. Inherent in this model are assumptions related to expected stock price volatility, option life, risk-free interest rate and dividend yield. The risk-free interest rate is a less subjective assumption as it is based on factual data derived from public sources. We use a dividend yield of zero as we have never paid cash dividends and have no intention to pay cash dividends in the immediate future. The expected stock price volatility and option life assumptions require a greater level of judgment which makes them critical accounting estimates. Estimating forfeitures also requires significant judgment.
Our expected stock-price volatility assumption is based on both current and historical volatilities of our stock which is obtained from public data sources. The expected life represents the weighted average period of time that share-based awards are expected to be outstanding giving consideration to vesting schedules and our historical exercise patterns. We determine the expected life assumption based on the exercise behavior and post-vesting behavior that has been exhibited historically, adjusted for specific factors that may influence future exercise patterns. We estimate forfeitures based on our historical experience of share-based pre-vesting cancellations. We believe that our estimates are based
59
on outcomes that are reasonably likely to occur. To the extent actual forfeitures differ from our estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. During the years ended December 31, 2007 and 2006 we incurred $10.5 million and $10.6 million of compensation cost under SFAS 123(R), respectively.
Recent Accounting Pronouncements
In February 2008, the FASB issued a FASB Staff Position, or FSP, to defer the effective date of FASB Statement No. 157, "Fair Value Measurements," or SFAS 157, for nonfinancial assets and nonfinancial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis. The FSP defers the effective date of SFAS 157 to fiscal years beginning after November 15, 2008, and interim periods within those fiscal years for items within the scope of the FSP. The delay is intended to provide the Board additional time to consider the effect of certain implementation issues that have arisen from the application of SFAS 157 to these assets and liabilities. SFAS 157 was issued on September 15, 2006, and as issued, was effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. Early application was encouraged. SFAS 157 defines fair value, establishes a framework for measuring fair value in accordance with Generally Accepted Accounting Principles, and expands disclosures about fair value measurements. The Statement codifies the definition of fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The standard clarifies the principle that fair value should be based on the assumptions market participants would use when pricing the asset or liability and establishes a fair value hierarchy that prioritizes the information used to develop those assumptions. The Company is currently evaluating the effect that the adoption of SFAS 157 will have on its results of operations and financial condition.
In December 2007, the FASB issued SFAS No. 141 (revised 2007),"Business Combinations," or SFAS 141(R), which is effective for financial statements issued for fiscal years beginning on or after December 15, 2008. SFAS 141(R) establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree, and the goodwill acquired in the business combination. SFAS 141(R) also establishes disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. SFAS 141(R) will be applied prospectively. The Company is currently evaluating the effect that the adoption of SFAS 141(R) will have on its results of operations and financial condition.
In December 2007, the FASB issued SFAS No. 160,"Noncontrolling Interests in Consolidated Financial Statements, an amendment of ARB No. 51," or SFAS 160. SFAS 160 changes the accounting and reporting for minority interests, which will be recharacterized as noncontrolling interests (NCI) and classified as a component of equity. The Statement also requires that entities provide sufficient disclosures that clearly identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. SFAS 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. SFAS 160 shall be applied prospectively as of the beginning of the fiscal year in which this Statement is initially applied, except for the presentation and disclosure requirements. The presentation and disclosure requirements shall be applied retrospectively for all periods presented. The Company is currently evaluating the effect that the adoption of SFAS 160 will have on its results of operations and financial condition.
In June 2007, the EITF reached a consensus on EITF Issue No. 07-03,"Accounting for Nonrefundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities," or EITF 07-03. EITF 07-03 concludes that non-refundable advance payments for future research and development activities should be deferred and capitalized until the goods have been delivered or the related services have been performed. If an entity does not expect the goods to be
60
delivered or services to be rendered, the capitalized advance payment should be charged to expense. This consensus is effective for fiscal years beginning after December 15, 2007. The initial adjustment to reflect the effect of applying the consensus as a change in accounting principle would be accounted for as a cumulative-effect adjustment to retained earnings as of the beginning of the year of adoption. The Company is currently evaluating the effect that the adoption of EITF 07-03 will have on its results of operations and financial condition.
In February 2007, the FASB issued SFAS No. 159,"The Fair Value Option for Financial Assets and Financial Liabilities," or SFAS 159, which is effective for financial statements issued for fiscal years beginning after November 15, 2007. Early adoption is permitted as of the beginning of a fiscal year that begins on or before November 15, 2007, provided the entity also elects to apply the provisions of FASB Statement No. 157, "Fair Value Measurements." SFAS 159 provides companies with an option to report selected financial assets and liabilities at fair value. The Statement also establishes presentation and disclosure requirements designed to facilitate comparisons between companies that choose different measurement attributes for similar types of assets and liabilities. SFAS 159 requires companies to provide additional information that will help investors and other users of financial statements to more easily understand the effect of the company's choice to use fair value on its earnings. It also requires entities to display the fair value of those assets and liabilities for which the company has chosen to use fair value on the face of the balance sheet. The Company is currently evaluating the effect that the adoption of SFAS 159 will have on its results of operations and financial condition.
The Company adopted FASB Interpretation No. 48, "Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement 109", or FIN 48, on January 1, 2007. FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements in accordance with SFAS No. 109,"Accounting for Income Taxes." This interpretation requires that the Company determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authority. This interpretation also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The adoption of FIN 48 did not have a material impact on the Company's financial statements. See Note P., "Income Taxes," for additional information.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We invest our cash in a variety of financial instruments; principally securities issued by the U.S. government and its agencies, investment grade corporate bonds and notes, auction rate securities, and money market instruments. These investments are denominated in U.S. dollars. All of our interest-bearing securities are subject to interest rate risk, and could decline in value if interest rates fluctuate.
We currently own financial instruments that are sensitive to market risks as part of our investment portfolio. The investment portfolio is used to preserve capital until it is required to fund operations. None of these market-risk sensitive instruments are held for trading purposes. Included in our investments are auction rate notes, which consist of private placement, credit default swap structured securities which reference synthetic portfolios of corporate bonds. These securities have long-term nominal maturities for which the interest rates are reset through a monthly auction. These auctions historically have provided a liquid market for these securities. In August 2007, auctions for $58.1 million of our investments in auction rate securities failed and have failed repeatedly since then. As a result of the auction failures, we have recorded unrealized losses of $14.7 million in other comprehensive income as a reduction in shareholders' equity. All of the auction rate securities that failed are still AAA rated and none are backed by sub-prime mortgages. The failure resulted in the interest rate on these investments resetting at the default coupon rate per the terms of the certificate. Given the failed auctions, while we now earn a premium interest rate on the investments, the investments are not liquid. In the event that we need to access these funds, we will not be able to do so until a future auction on these investments is successful. The credit and capital markets have continued
61
to deteriorate in 2008 and the bids on the auction rate notes the Company holds have since declined. If a certain concentration, as defined in the auction rate documents, of the underlying reference portfolios default, or if the issuing bank fails to make the required interest payments or the final principal payment upon the ultimate maturity of the notes, or if the credit ratings on the underlying reference portfolios deteriorate significantly, the Company may be required to adjust the carrying value of these investments through an other-than-temporary impairment charge. Based on our ability to access our cash and other short-term investments, our expected operating cash flows, and other sources of cash, we do not anticipate the lack of liquidity on these investments will affect our ability to execute our current business plan.
As of December 31, 2007, the fair market value of our 2.25% convertible subordinated notes due in 2013 amounted to $331.0 million. The estimated fair value of long-term debt was determined using quoted market rates. The interest rates on the 2.25% convertible subordinated notes and capital lease obligation are fixed and are therefore not subject to interest rate risk.
62
ITEM 8. FINANCIAL STATEMENTS
Cubist Pharmaceuticals, Inc.
Index to Consolidated Financial Statements and Schedule
63
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Cubist Pharmaceuticals, Inc.:
In our opinion, the consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of Cubist Pharmaceuticals, Inc. and its subsidiaries at December 31, 2007 and 2006, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2007 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note B to the consolidated financial statements, the Company changed the manner in which it accounts for share-based compensation in fiscal 2006.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
64
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| /s/ PRICEWATERHOUSECOOPERS LLP | | |
Boston, Massachusetts
February 29, 2008 |
|
|
65
CUBIST PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| | (in thousands, except share amounts)
| |
|---|
| ASSETS | | | | | | | |
Current assets: |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 354,785 | | $ | 15,979 | |
| | Short-term investments | | | — | | | 278,012 | |
| | Accounts receivable, net | | | 29,075 | | | 21,070 | |
| | Inventory | | | 18,733 | | | 18,111 | |
| | Prepaid expenses and other current assets | | | 6,686 | | | 5,195 | |
| | |
| |
| |
| | | Total current assets | | | 409,279 | | | 338,367 | |
| Property and equipment, net | | | 50,150 | | | 49,584 | |
| Intangible assets, net | | | 22,698 | | | 25,639 | |
| Long-term investments, net | | | 43,399 | | | 15,178 | |
| Other assets | | | 8,989 | | | 10,267 | |
| | |
| |
| |
| | | Total assets | | $ | 534,515 | | $ | 439,035 | |
| | |
| |
| |
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
| | Accounts payable | | $ | 6,564 | | $ | 3,602 | |
| | Accrued liabilities | | | 58,735 | | | 31,038 | |
| | Short-term deferred revenue | | | 1,484 | | | — | |
| | Current portion of capital lease obligations | | | — | | | 245 | |
| | |
| |
| |
| | | Total current liabilities | | | 66,783 | | | 34,885 | |
| Long-term deferred revenue, net of current portion | | | 16,332 | | | 11,800 | |
| Other long-term liabilities | | | 2,698 | | | 1,760 | |
| Long-term debt | | | 350,000 | | | 350,000 | |
| | |
| |
| |
| | | Total liabilities | | | 435,813 | | | 398,445 | |
| Commitments and contingencies (Notes C, D, M, N and P) | | | | | | | |
| Stockholders' equity: | | | | | | | |
| | Preferred stock, non-cumulative; convertible, $.001 par value;
authorized 5,000,000 shares; no shares issued and outstanding | | | — | | | — | |
| | Common stock, $.001 par value; authorized 150,000,000 shares;
56,142,105 and 55,001,058 shares issued and outstanding as of December 31, 2007 and 2006, respectively | | | 56 | | | 55 | |
| Additional paid-in capital | | | 549,391 | | | 524,726 | |
| Accumulated other comprehensive loss | | | (14,701 | ) | | — | |
| Accumulated deficit | | | (436,044 | ) | | (484,191 | ) |
| | |
| |
| |
| | | Total stockholders' equity | | | 98,702 | | | 40,590 | |
| | |
| |
| |
| Total liabilities and stockholders' equity | | $ | 534,515 | | $ | 439,035 | |
| | |
| |
| |
The accompanying notes are an integral part of the consolidated financial statements.
66
CUBIST PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | For the Years Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| | (in thousands except share and per share amounts)
| |
|---|
| Revenues: | | | | | | | | | | |
| | U.S. product revenues, net | | $ | 285,059 | | $ | 189,512 | | $ | 113,434 | |
| | International product revenues | | | 5,347 | | | 808 | | | 80 | |
| | Other revenues | | | 4,214 | | | 4,428 | | | 7,131 | |
| | |
| |
| |
| |
| | | Total revenues, net | | | 294,620 | | | 194,748 | | | 120,645 | |
Costs and expenses: |
|
|
|
|
|
|
|
|
|
|
| | Cost of product revenues | | | 68,860 | | | 48,803 | | | 32,739 | |
| | Research and development | | | 85,175 | | | 57,405 | | | 51,673 | |
| | Sales and marketing | | | 67,662 | | | 56,879 | | | 42,331 | |
| | General and administrative | | | 31,485 | | | 26,745 | | | 19,335 | |
| | |
| |
| |
| |
| | | Total costs and expenses | | | 253,182 | | | 189,832 | | | 146,078 | |
| | |
| |
| |
| |
Operating income (loss) |
|
|
41,438 |
|
|
4,916 |
|
|
(25,433 |
) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
| | Interest income | | | 18,036 | | | 10,589 | | | 3,292 | |
| | Interest expense | | | (9,427 | ) | | (15,893 | ) | | (9,836 | ) |
| | Other income (expense) | | | (20 | ) | | 12 | | | 125 | |
| | |
| |
| |
| |
| | | Total other income (expense), net | | | 8,589 | | | (5,292 | ) | | (6,419 | ) |
| | |
| |
| |
| |
| Income (loss) before income taxes | | | 50,027 | | | (376 | ) | | (31,852 | ) |
Provision for income taxes |
|
|
1,880 |
|
|
— |
|
|
— |
|
| | |
| |
| |
| |
| Net income (loss) | | $ | 48,147 | | $ | (376 | ) | $ | (31,852 | ) |
| | |
| |
| |
| |
Basic net income (loss) per common share |
|
$ |
0.87 |
|
$ |
(0.01 |
) |
$ |
(0.60 |
) |
| Diluted net income (loss) per common share | | $ | 0.83 | | $ | (0.01 | ) | $ | (0.60 | ) |
Shares used in calculating: |
|
|
|
|
|
|
|
|
|
|
| | Basic net income (loss) per common share | | | 55,591,775 | | | 54,490,376 | | | 53,053,307 | |
| | Diluted net income (loss) per common share | | | 68,822,996 | | | 54,490,376 | | | 53,053,307 | |
The accompanying notes are an integral part of the consolidated financial statements.
67
CUBIST PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | For the Years Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| | (in thousands)
| |
|---|
| Cash flows from operating activities: | | | | | | | | | | |
| | Net income (loss) | | $ | 48,147 | | $ | (376 | ) | $ | (31,852 | ) |
| | Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities, net of assets and liabilities acquired: | | | | | | | | | | |
| | Depreciation and amortization | | | 9,669 | | | 9,194 | | | 7,835 | |
| | Amortization and write-off of debt issuance costs | | | 1,551 | | | 3,123 | | | 760 | |
| | Amortization of premium or discount on investments | | | (558 | ) | | (144 | ) | | 251 | |
| | Stock-based compensation | | | 10,605 | | | 11,105 | | | 438 | |
| | Charge for company 401(k) common stock match | | | 2,109 | | | 1,825 | | | 1,394 | |
| | Other non-cash | | | (24 | ) | | 11 | | | 13 | |
| | Acquired IPR&D | | | 14,433 | | | — | | | — | |
| | Changes in assets and liabilities, net of assets and liabilities acquired: | | | | | | | | | | |
| | | Accounts receivable | | | (8,005 | ) | | (6,369 | ) | | (4,847 | ) |
| | | Inventory | | | (2,774 | ) | | (1,447 | ) | | (8,681 | ) |
| | | Prepaid expenses and other current assets | | | (1,468 | ) | | 434 | | | (2,003 | ) |
| | | Other assets | | | (271 | ) | | 273 | | | 162 | |
| | | Accounts payable and accrued liabilities | | | 20,401 | | | 718 | | | 9,639 | |
| | | Deferred revenue | | | 6,016 | | | 10,550 | | | (3,700 | ) |
| | | Other long-term liabilities | | | 938 | | | 1,760 | | | — | |
| | |
| |
| |
| |
| | | | Total adjustments | | | 52,622 | | | 31,033 | | | 1,261 | |
| | |
| |
| |
| |
| | | | Net cash provided by (used in) operating activities | | | 100,769 | | | 30,657 | | | (30,591 | ) |
| | |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | |
| | Acquisition of Illumigen, net of cash acquired | | | (4,350 | ) | | — | | | — | |
| | Purchases of property and equipment | | | (5,133 | ) | | (7,391 | ) | | (2,052 | ) |
| | Purchases of investments | | | (3,407,532 | ) | | (1,714,151 | ) | | (696,142 | ) |
| | Maturities of investments | | | 3,643,180 | | | 1,493,704 | | | 731,136 | |
| | |
| |
| |
| |
| | | | Net cash provided by (used in) investing activities | | | 226,165 | | | (227,838 | ) | | 32,942 | |
| | |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | |
| | Issuance of common stock, net | | | 12,073 | | | 10,010 | | | 6,357 | |
| | Proceeds from sale of convertible subordinated debt | | | — | | | 350,000 | | | — | |
| | Costs associated with sale of convertible subordinated debt | | | — | | | (10,925 | ) | | — | |
| | Repayments of long-term debt and capital lease obligations | | | (245 | ) | | (165,078 | ) | | (117 | ) |
| | |
| |
| |
| |
| | | | Net cash provided by financing activities | | | 11,828 | | | 184,007 | | | 6,240 | |
| | |
| |
| |
| |
| Net increase (decrease) in cash and cash equivalents | | | 338,762 | | | (13,174 | ) | | 8,591 | |
| Effect of changes in foreign exchange rates on cash balances | | | 44 | | | 4 | | | (14 | ) |
| Cash and cash equivalents at beginning of year | | | 15,979 | | | 29,149 | | | 20,572 | |
| | |
| |
| |
| |
| Cash and cash equivalents at end of year | | $ | 354,785 | | $ | 15,979 | | $ | 29,149 | |
| | |
| |
| |
| |
Cash paid during the year for: |
|
|
|
|
|
|
|
|
|
|
| | Interest | | $ | 7,875 | | $ | 8,672 | | $ | 9,075 | |
| | Cash paid for income taxes | | $ | 1,413 | | $ | — | | $ | — | |
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
|
| | Non-cash investing and financing activities: | | | | | | | | | | |
| | | Acquisition obligation payable to former Illumigen shareholders | | $ | 10,191 | | $ | — | | $ | — | |
| | | Issuance of common stock to Eli Lilly | | $ | — | | $ | — | | $ | 20,000 | |
| | | Capital lease obligations incurred | | $ | — | | $ | 245 | | $ | — | |
The accompanying notes are an integral part of the consolidated financial statements.
68
CUBIST PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
| | Number of
Common
Shares
| | Common
Stock
| | Additional
Paid-in
Capital
| | Accumulated
Other
Comprehensive
Loss
| | Accumulated
Deficit
| | Total
Stockholders
Equity
| |
|---|
| | (in thousands, except share data)
| |
|---|
| Balance at December 31, 2004 | | 51,153,827 | | $ | 51 | | $ | 472,758 | | $ | — | | $ | (451,963 | ) | $ | 20,846 | |
| Comprehensive income (loss): | | | | | | | | | | | | | | | | | | |
| | Net loss | | — | | | — | | | — | | | — | | | (31,852 | ) | | (31,852 | ) |
| | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive income (loss) | | — | | | — | | | — | | | — | | | — | | | (31,852 | ) |
| | | | | | | | | | | | | | | | |
| |
| Exercise of stock options | | 680,860 | | | 1 | | | 5,650 | | | — | | | — | | | 5,651 | |
| Shares issued in connection with employee stock purchase plan and 401(k) plan | | 160,724 | | | — | | | 1,737 | | | — | | | — | | | 1,737 | |
| Issuance of common stock related to business agreements | | 1,876,173 | | | 2 | | | 20,003 | | | — | | | — | | | 20,005 | |
| Stock-based compensation to employees and consultants | | 11,997 | | | — | | | 212 | | | — | | | — | | | 212 | |
| | |
| |
| |
| |
| |
| |
| |
Balance at December 31, 2005 |
|
53,883,581 |
|
|
54 |
|
|
500,360 |
|
|
— |
|
|
(483,815 |
) |
|
16,599 |
|
| Comprehensive income (loss): | | | | | | | | | | | | | | | | | | |
| | Net loss | | — | | | — | | | — | | | — | | | (376 | ) | | (376 | ) |
| | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive income (loss) | | — | | | — | | | — | | | — | | | — | | | (376 | ) |
| | | | | | | | | | | | | | | | |
| |
| Exercise of stock options | | 892,790 | | | 1 | | | 8,871 | | | — | | | — | | | 8,872 | |
| Shares issued in connection with employee stock purchase plan and 401(k) plan | | 207,062 | | | — | | | 3,968 | | | — | | | — | | | 3,968 | |
| Stock-based compensation to employees and consultants | | 17,625 | | | — | | | 11,527 | | | — | | | — | | | 11,527 | |
| | |
| |
| |
| |
| |
| |
| |
Balance at December 31, 2006 |
|
55,001,058 |
|
|
55 |
|
|
524,726 |
|
|
— |
|
|
(484,191 |
) |
|
40,590 |
|
| Comprehensive income (loss): | | | | | | | | | | | | | | | | | | |
| | Net income | | — | | | — | | | — | | | — | | | 48,147 | | | 48,147 | |
| | Unrealized loss on investments | | — | | | — | | | — | | | (14,701 | ) | | — | | | (14,701 | ) |
| | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive income | | — | | | — | | | — | | | — | | | — | | | 33,446 | |
| | | | | | | | | | | | | | | | |
| |
| Exercise of stock options | | 965,538 | | | 1 | | | 10,945 | | | — | | | — | | | 10,946 | |
| Shares issued in connection with employee stock purchase plan and 401(k) plan | | 172,509 | | | — | | | 3,108 | | | — | | | — | | | 3,108 | |
| Stock-based compensation to employees and consultants | | 3,000 | | | — | | | 10,612 | | | — | | | — | | | 10,612 | |
| | |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2007 | | 56,142,105 | | $ | 56 | | $ | 549,391 | | $ | (14,701 | ) | $ | (436,044 | ) | $ | 98,702 | |
| | |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of the consolidated financial statements.
69
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
A. NATURE OF BUSINESS
Cubist is a biopharmaceutical company headquartered in Lexington, Massachusetts, focused on the research, development and commercialization of pharmaceutical products that address unmet medical needs in the acute-care environment. To date, Cubist has concentrated exclusively on developing products for the anti-infective marketplace. Cubist has one marketed product, CUBICIN (daptomycin for injection), which was launched in the U.S. in November 2003. CUBICIN is approved in the U.S. for the treatment of cSSSI, caused byS. aureus and certain other Gram-positive bacteria, and forS. aureus bloodstream infections (bacteremia), including those with right-sided infective endocarditis, caused by methicillin-susceptible and methicillin-resistant isolates. Since its U.S. launch, CUBICIN also has received similar regulatory approvals in many countries outside the U.S. The Company has focused its pipeline building efforts on opportunities that leverage its anti-infective and acute-care discovery, development, regulatory, and commercialization expertise. Currently, Cubist has multiple anti-infective programs approaching the IND filing stage preparatory to clinical trials. Cubist is subject to risks common to companies in the pharmaceutical industry including, but not limited to, risks related to the development by Cubist or its competitors of new technological innovations, the ability to market products or services, the Company's dependence on key personnel, the market acceptance of CUBICIN, the Company's dependence on key suppliers, protection of the Company's proprietary technology, the Company's ability to obtain additional financing, and the Company's compliance with governmental and other regulations.
B. ACCOUNTING POLICIES
Basis of Presentation and Consolidation
The accompanying consolidated financial statements include the accounts of Cubist and its wholly owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles, or GAAP, requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and contingent liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The most significant assumptions are employed in estimates used in determining values of inventories, long-lived assets, accrued clinical research costs, income taxes, stock-based compensation, sales rebate and return accruals, legal contingencies, as well as in estimates used in applying the revenue recognition policy. Actual results could differ from estimated results.
Fair Value of Financial Instruments
The carrying amounts of Cubist's cash and cash equivalents, short-term investments, accounts receivable, accounts payable, and accrued expenses approximate their fair value due to the short-term maturities of these instruments. At December 31, 2007, long-term investments had a fair value of $43.4 million and a cost of $58.1 million. At December 31, 2006, long-term investments had a fair value of $15.0 million and a cost of $15.0 million. At December 31, 2006, the carrying amounts of long-term investments approximate their fair value. The fair market value of long-term debt at December 31, 2007, amounted to $331.0 million, and consisted of fixed-rate debt due in 2013. The estimated fair value of long-term debt was determined using quoted market rates.
70
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
In evaluating the fair value information, considerable judgment is required to interpret the market data used to develop the estimates. The use of different market assumptions and/or different valuation techniques may have a material effect on the estimated fair value amounts. Accordingly, the estimates of fair value presented herein may not be indicative of the amounts that could be realized in a current market exchange.
Included in our investments are auction rate securities, which consist of private placement, credit default swap structured securities which reference synthetic portfolios of corporate bonds. These securities have long-term nominal maturities for which the interest rates are reset through a monthly auction While the underlying securities of auction rate securities may have contractual maturities of more than ten years, the interest rates on such securities reset at intervals of 7, 28 or 35 days. Historically, auction rate securities have been priced and traded as short-term investments because of this reset feature and have been considered available-for-sale investments. Given the repeated failure of auctions for $58.1 million of our investments in auction rate securities, we do not consider these investments to be liquid and classified them as long-term investments as of December 31, 2007. As a result of the auction failures, we have recorded temporary unrealized losses of $14.7 million in other comprehensive income as a reduction in shareholders' equity. The amount of the unrealized loss on the auction rate notes was determined through a valuation analysis which is based on bids from the broker who is the market maker for these instruments, corroborated through an analysis of the underlying instruments. The Company considers this loss to be temporary in nature. The credit and capital markets have continued to deteriorate in 2008 and the bids on the auction rate notes the Company holds have since declined.
Cash and Cash Equivalents
Cash and cash equivalents consist of short-term interest-bearing instruments with initial maturities of three months or less at the date of purchase. These instruments are carried at cost, which approximates market value.
Investments
Investments consisted of certificates of deposit, corporate bonds, government bonds and agencies, investment-grade commercial paper and auction rate securities at December 31, 2007 and 2006. Investments which are considered held-to-maturity are stated at amortized cost plus accrued interest, which approximates market value. Investments which are considered available-for-sale are carried at fair market value plus accrued interest. Unrealized gains and losses are included in accumulated other comprehensive income (loss) as a separate component of stockholders' equity. Realized gains and losses, dividends and interest income, including amortization of the premium and discount arising at purchase, are included in interest and investment income. While the underlying securities of auction rate securities may have underlying maturities of more than ten years, the interest rates on such securities reset at intervals of 7, 28 or 35 days. Historically, auction rate securities have been priced and traded as short-term investments because of this rate reset feature and have been considered available-for-sale investments. Given the repeated failure of auctions for $58.1 million of the Company's investments in auction rate securities, these investments are not considered liquid and have been reclassified as of December 31, 2007 as long-term investments. Investment classification detail can be found in Note E., "Investments" in the Notes to the Consolidated Financial Statements.
71
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
Concentration of Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash and cash equivalents, investments and accounts receivable. Cash, cash equivalents, certificates of deposit and investments consist of commercial paper, corporate bonds, U.S. Government securities, money market funds and auction rate securities all held with financial institutions. Approximately 80% and 87% of the accounts receivable balances represent amounts due from three wholesalers at December 31, 2007 and 2006, respectively.
Revenues from Cardinal accounted for approximately 32%, 33% and 32% of all revenues for the years ended December 31, 2007, 2006 and 2005, respectively. Revenues from Amerisource Bergen Drug Corporation accounted for approximately 30%, 32% and 31% of all revenues for the years ended December 31, 2007, 2006 and 2005, respectively. Revenues from McKesson Corporation accounted for approximately 20%, 21% and 21% of all revenues for the years ended December 31, 2007, 2006 and 2005, respectively.
Inventory
Inventories are stated at the lower of cost or market. Cost is computed using standard cost, which approximates actual cost, on a first-in, first-out, or FIFO, basis. The Company analyzes its inventory levels quarterly, and writes-down inventory that has become obsolete, inventory that has a cost basis in excess of its expected net realizable value, inventory in excess of expected sales requirements or inventory that fails to meet commercial sale specifications to cost of product revenues. Expired inventory is disposed of and the related costs are written off to cost of product revenues.
Inventories consisted of the following at December 31:
| | 2007
| | 2006
|
|---|
| | (in thousands)
|
|---|
| Raw materials | | $ | 9,432 | | $ | 8,240 |
| Work in process | | | 2,858 | | | 3,616 |
| Finished goods | | | 6,443 | | | 6,255 |
| | |
| |
|
| | | $ | 18,733 | | $ | 18,111 |
| | |
| |
|
72
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
Property and Equipment
Property and equipment, including leasehold improvements, are recorded at cost and are depreciated when placed into service using the straight-line method, based on their estimated useful lives as follows:
Asset Description
| | Estimated
Useful Life
(Years)
|
|---|
| Building | | 40 |
| Fermentation equipment | | 15 |
| Lab equipment | | 5 |
| Furniture and fixtures | | 5 |
| Computer hardware and software | | 3 |
Leasehold improvements are amortized over the shorter of the estimated useful life of the asset or the lease term. Costs for capital assets not yet placed into service have been capitalized as construction in progress and will be depreciated in accordance with the above guidelines once placed into service. Costs for repairs and maintenance are expensed as incurred, while major betterments are capitalized. When assets are retired or otherwise disposed of, the assets and related allowances for depreciation and amortization are eliminated from the accounts and any resulting gain or loss is reflected in operating costs and expenses.
Capital Leases
Assets acquired under capital lease agreements are recorded at the present value of the future minimum rental payments using interest rates appropriate at the inception of the lease. Property and equipment subject to capital lease agreements are amortized over the shorter of the life of the lease or the estimated useful life of the asset unless the lease transfers ownership or contains a bargain purchase option, in which case the leased asset is amortized over the estimated useful life of such asset. There are no outstanding capital leases at December 31, 2007.
Intangible Assets
Cubist's intangible assets consist of acquired intellectual property, processes, patents and technology rights. These assets are amortized on a straight-line basis over their estimated useful life of four to seventeen years. The fair value of patents obtained through an acquisition transaction are capitalized and amortized over the lesser of the patent's remaining legal life or its useful life. Costs to obtain, maintain and defend the Company's patents are expensed as incurred.
Impairment of Long-Lived Assets
In accordance with SFAS No. 144"Accounting for the Impairment or Disposal of Long-Lived Assets," Cubist reviews long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. Each impairment test is based on a comparison of the undiscounted cash flows to the recorded value of the asset. If impairment is indicated, the asset
73
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
is written down by the amount by which the carrying value of the asset exceeds the related fair value of the asset.
Revenue Recognition
Cubist recognizes revenue in accordance with SEC Staff Accounting Bulletin No. 101 (SAB 101), as amended by SAB 104, and Emerging Issues Task Force (EITF) Issue No. 00-21. Principal sources of revenue are sales of CUBICIN, license fees and milestone payments that are derived from collaborative agreements with other biotechnology companies and distribution agreements. The Company has followed the following principles in recognizing revenue:
Multiple Element Arrangements
Cubist analyzes its multiple element arrangements to determine whether the elements can be separated and accounted for individually as separate units of accounting in accordance with EITF No. 00-21,"Revenue Arrangements with Multiple Deliverables." An element of a contract can be accounted for separately if the delivered elements have stand-alone value and the fair value of any undelivered elements is determinable. If an element is considered to have standalone value but the fair value of any of the undelivered items cannot be determined, all elements of the arrangement are recognized as revenue over the period of performance for such undelivered items or services.
Product Revenues, net
Cubist recognizes revenue from product sales when persuasive evidence of an arrangement exists, title to product and associated risk of loss has passed to the customer, the price is fixed or determinable, collection from the customer is reasonably assured and the Company has no further performance obligations. All revenues from product sales are recorded net of applicable provisions for returns, chargebacks, rebates, wholesaler management fees and discounts in the same period the related sales are recorded.
Certain product sales qualify for rebates or discounts from standard list pricing due to government sponsored programs or other contractual agreements. Reserves for rebate programs are included in accrued liabilities and were $601,000 and $652,000 at December 31, 2007 and 2006, respectively. The Company allows customers to return product within a specified period prior to and subsequent to the expiration date. Reserves for product returns are based upon many factors, including industry data of product return rates, historical experience of actual returns, analysis of the level of inventory in the distribution channel and reorder rates of end-users. Reserves for returns, discounts, chargebacks, customer rebates and wholesaler management fees are offset against accounts receivable and were $3.9 million and $2.8 million at December 31, 2007 and 2006, respectively. In the year ended December 31, 2007, 2006 and 2005, provisions for sales returns, chargebacks, rebates, wholesaler management fees and prompt-pay discounts that were offset against product revenues totaled $16.3 million, $9.5 million and $5.1 million, respectively.
Product Revenues from International Distribution Partners
Under agreements with international distribution partners, Cubist sells its product to international distribution partners based upon a transfer price arrangement. The transfer price is generally
74
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
established annually. Once Cubist's distribution partner sells the product to a third party, Cubist is owed an additional payment based on a percentage of the net selling price to the third party, less the transfer price previously paid on such product. Under no circumstances would the subsequent royalty adjustment result in a refund to the distribution. Cubist recognizes revenue related to product shipped to international distribution partners when persuasive evidence of an arrangement exists, title to product and associated risk of loss has passed to the distribution partner, the price is fixed or determinable, collection from the distribution partner is reasonably assured and the Company has no further performance obligations.
License Revenues
Non-refundable license fees are recognized depending on the provisions of each agreement. License fees with ongoing involvement or performance obligations are recorded as deferred revenue once received and are generally recognized ratably over the period of such performance obligation only after both the license period has commenced and the technology has been delivered.
Research services
Revenues from SBIR grants to conduct research and development are recognized as the eligible costs are incurred up to the granted funding limit.
Milestones
Revenue from milestone payments related to arrangements under which the Company has continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: the milestone payments are non-refundable; achievement of the milestone was not reasonably assured at the inception of the arrangement; substantive effort is involved in achieving the milestone; and the amount of the milestone is reasonable in relation to the effort expended or the risk associated with the achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as the Company completes its performance obligations. Contingent payments under license agreements that do not involve substantial effort on the part of the Company are not considered substantive milestones. Such payments are recognized as revenue when the contingency is met only if there are no remaining performance obligations or any remaining performance obligations are priced at fair value. Otherwise, the contingent payment is recognized as the Company completes its performance obligations under the arrangement.
Research and Development
All research and development costs, including upfront fees and milestones paid to collaborators, are expensed as incurred if no planned alternative future use exists for the technology. When the Company is reimbursed by a collaborative partner for work it performs it records the costs incurred as research and development expenses and the related reimbursement as other revenues in its Consolidated Statement of Operations. Research and development expenses consist of internal labor, clinical and non-clinical studies, materials and supplies, facilities, depreciation, third party costs for contracted services, manufacturing process improvement and testing costs, and other research and development related costs.
75
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
Advertising Costs
Advertising costs are expensed as incurred and are included in sales and marketing expense within the Consolidated Statements of Operations. Advertising costs, which include promotional expenses and trade shows, were approximately $9.6 million, $6.0 million and $6.6 million at December 31, 2007, 2006 and 2005, respectively.
Income Taxes
Cubist accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted rates in effect for the year in which those temporary differences are expected to be recovered or settled. A deferred tax asset is established for the expected future benefit of net operating loss and credit carryforwards. A valuation reserve against net deferred tax assets is required if, based upon available evidence, it is "more likely than not" that some or all of the deferred tax assets will not be realized.
Foreign Currency Translation
The functional currency of Cubist's U.K. subsidiary is the U.S. dollar. Accordingly, the remeasurement method is used to convert the foreign currency balances from the local currency into the U.S. dollar.
Comprehensive Income (Loss)
For the year ended December 31, 2007, comprehensive income is comprised of net income for the year and unrealized losses recognized on available-for-sale marketable securities. Total comprehensive income for the year ended December 31, 2007 was $33.4 million. For the years ended December 31, 2006 and 2005, total comprehensive income (loss) is comprised of only net loss, as there was no other comprehensive income (loss) for the years ended December 31, 2006 and 2005.
Basic and Diluted Net Income (Loss) Per Common Share
Basic net income (loss) per share has been computed by dividing net income (loss) by the weighted average number of shares outstanding during the period. Except where the result would be antidilutive to income from continuing operations, diluted net income (loss) per share has been computed assuming the conversion of convertible obligations and the elimination of the related interest expense, and the exercise of stock options, as well as their related income tax effects.
76
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
The following table sets forth the computation of basic and diluted net income (loss) per share for the years ended December 31, 2007, 2006 and 2005 (amounts in thousands, except share and per share amounts):
| | December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Net income (loss) basic | | $ | 48,147 | | $ | (376 | ) | $ | (31,852 | ) |
Effect of dilutive securities: |
|
|
|
|
|
|
|
|
|
|
| | Interest on 2.25% convertible subordinated notes, net of tax | | | 7,586 | | | — | | | — | |
| | Debt issuance costs, net of tax | | | 1,494 | | | — | | | — | |
| | |
| |
| |
| |
| Net income (loss) diluted | | $ | 57,227 | | $ | (376 | ) | $ | (31,852 | ) |
| | |
| |
| |
| |
Shares used in calculating basic net income (loss) per common share |
|
|
55,591,775 |
|
|
54,490,376 |
|
|
53,053,307 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
|
|
|
| | Options to purchase shares of common stock | | | 1,856,886 | | | — | | | — | |
| | Notes payable convertible into shares of common stock | | | 11,374,335 | | | — | | | — | |
| | |
| |
| |
| |
| Shares used in calculating diluted net income (loss) per common share | | | 68,822,996 | | | 54,490,376 | | | 53,053,307 | |
| | |
| |
| |
| |
| |
Net income (loss) per share, basic |
|
$ |
0.87 |
|
$ |
(0.01 |
) |
$ |
(0.60 |
) |
| | Net income (loss) per share, diluted | | $ | 0.83 | | $ | (0.01 | ) | $ | (0.60 | ) |
77
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
Potential common shares excluded from the calculation of diluted net income (loss) per share as their inclusion would have been antidilutive, were:
| | 2007
| | 2006
| | 2005
|
|---|
| Options to purchase shares of common stock | | 3,183,803 | | 7,271,450 | | 6,836,077 |
Convertible debt and notes payable convertible into shares of common stock |
|
— |
|
11,374,335 |
|
3,495,763 |
Stock Based Compensation
In December 2004, the Financial Accounting Standards Board, or FASB, issued SFAS No. 123 (revised 2004),"Share-Based Payment," or SFAS 123(R), which requires all companies to measure compensation cost for all share-based payments (including employee stock options) at fair value. SFAS 123(R) supersedes APB Opinion No. 25,"Accounting for Stock Issued to Employees" and amends SFAS No. 95, "Statement of Cash Flows." In March 2005, the Securities and Exchange Commission issued Staff Accounting Bulletin No. 107 ("SAB 107") relating to SFAS 123(R). The Company has applied the provisions of SAB 107 in its adoption of SFAS 123(R). SFAS 123(R) requires the determination of the fair value of the share-based compensation at the grant date and the recognition of the related expense over the requisite service period. The Company elected to adopt the modified prospective application method as provided by SFAS 123(R). As a result, the Company recognized compensation expense associated with awards granted after January 1, 2006, and the unvested portion of previously granted awards that remained outstanding as of January 1, 2006. See Note L. for additional information.
Recent Accounting Pronouncements
In February 2008, the FASB issued a FASB Staff Position, or FSP, to defer the effective date of FASB Statement No. 157,"Fair Value Measurements, " or SFAS 157, for nonfinancial assets and nonfinancial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis. The FSP defers the effective date of SFAS 157 to fiscal years beginning after November 15, 2008, and interim periods within those fiscal years for items within the scope of the FSP. The delay is intended to provide the Board additional time to consider the effect of certain implementation issues that have arisen from the application of SFAS 157 to these assets and liabilities. SFAS 157 was issued on September 15, 2006, and as issued, was effective for financial statements issues for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. Early application was encouraged. SFAS 157 defines fair value, establishes a framework for measuring fair value in accordance with Generally Accepted Accounting Principles, and expands disclosures about fair value measurements. The Statement codifies the definition of fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The standard clarifies the principle that fair value should be based on the assumptions market participants would use when pricing the asset or liability and establishes a fair value hierarchy that prioritizes the information used to develop those assumptions. The Company is currently evaluating the effect that the adoption of SFAS 157 will have on its results of operations and financial condition.
78
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
In December 2007, the FASB issued SFAS No. 141 (revised 2007),"Business Combinations," or SFAS 141(R), which is effective for financial statements issued for fiscal years beginning on or after December 15, 2008. SFAS 141(R) establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree, and the goodwill acquired in the business combination. SFAS 141(R) also establishes disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. SFAS 141(R) will be applied prospectively. The Company is currently evaluating the effect that the adoption of SFAS 141(R) will have on its results of operations and financial condition.
In December 2007, the FASB issued SFAS No. 160,"Noncontrolling Interests in Consolidated Financial Statements, an amendment of ARB No. 51," or SFAS 160. SFAS 160 changes the accounting and reporting for minority interests, which will be recharacterized as noncontrolling interests (NCI) and classified as a component of equity. The Statement also requires that entities provide sufficient disclosures that clearly identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. SFAS 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. SFAS 160 shall be applied prospectively as of the beginning of the fiscal year in which this Statement is initially applied, except for the presentation and disclosure requirements. The presentation and disclosure requirements shall be applied retrospectively for all periods presented. The Company is currently evaluating the effect that the adoption of SFAS 160 will have on its results of operations and financial condition.
In June 2007, the EITF reached a consensus on EITF Issue No. 07-03,"Accounting for Nonrefundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities," or EITF 07-03. EITF 07-03 concludes that non-refundable advance payments for future research and development activities should be deferred and capitalized until the goods have been delivered or the related services have been performed. If an entity does not expect the goods to be delivered or services to be rendered, the capitalized advance payment should be charged to expense. This consensus is effective for fiscal years beginning after December 15, 2007. The initial adjustment to reflect the effect of applying the consensus as a change in accounting principle would be accounted for as a cumulative-effect adjustment to retained earnings as of the beginning of the year of adoption. The Company is currently evaluating the effect that the adoption of EITF 07-03 will have on its results of operations and financial condition.
In February 2007, the FASB issued SFAS No. 159,"The Fair Value Option for Financial Assets and Financial Liabilities," or SFAS 159, which is effective for financial statements issued for fiscal years beginning after November 15, 2007. Early adoption is permitted as of the beginning of a fiscal year that begins on or before November 15, 2007, provided the entity also elects to apply the provisions of FASB Statement No. 157,"Fair Value Measurements." SFAS 159 provides companies with an option to report selected financial assets and liabilities at fair value. The Statement also establishes presentation and disclosure requirements designed to facilitate comparisons between companies that choose different measurement attributes for similar types of assets and liabilities. SFAS 159 requires companies to provide additional information that will help investors and other users of financial statements to more easily understand the effect of the company's choice to use fair value on its earnings. It also requires entities to display the fair value of those assets and liabilities for which the company has chosen to use
79
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
B. ACCOUNTING POLICIES (Continued)
fair value on the face of the balance sheet. The Company is currently evaluating the effect that the adoption of SFAS 159 will have on its results of operations and financial condition.
The Company adopted FASB Interpretation No. 48,"Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement 109", or FIN 48, on January 1, 2007. FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements in accordance with SFAS No. 109,"Accounting for Income Taxes." This interpretation requires that the Company determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authority. This interpretation also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The adoption of FIN 48 did not have a material impact on the Company's financial statements. See Note P., "Income Taxes," for additional information.
C. BUSINESS AGREEMENTS
Licensing Agreements
In November 1997, Cubist entered into a license agreement with Eli Lilly that was amended and restated in October 2000, and pursuant to which Cubist acquired exclusive worldwide rights to develop, manufacture and market CUBICIN. In exchange for such license, Cubist paid an upfront license fee in cash and, if certain drug development milestones were achieved, agreed to pay milestone payments by issuing shares of common stock to Eli Lilly. In addition, Cubist is required to pay royalties to Eli Lilly on worldwide sales of CUBICIN. In July 2003, Cubist entered into an amendment to the restated license agreement with Eli Lilly and issued to Eli Lilly 723,619 shares of common stock valued at $8.0 million, in consideration for a 1% reduction in the royalty rates under the original license agreement. The $8.0 million was recorded as an intangible asset within the Consolidated Balance Sheet and is being amortized over approximately 13 years, which was the estimated remaining life of the license agreement with Eli Lilly on the date of the transaction. In September 2003, Cubist issued 38,922 shares of common stock valued at $0.5 million as a milestone payment to Eli Lilly upon Cubist receiving FDA approval for the commercial sale of CUBICIN. The $0.5 million was recorded as an intangible asset within the Consolidated Balance Sheet and is being amortized over approximately 13 years, which was the remaining life of the license agreement with Eli Lilly on the date of the transaction. In March 2005, Cubist entered into a second amendment to the license agreement with Eli Lilly and issued to Eli Lilly 1,876,173 shares of common stock valued at $20.0 million, in consideration for a 2% reduction in the royalty rates under the original license agreement. The $20.0 million was recorded as an intangible asset within the Consolidated Balance Sheet and is being amortized over approximately 11 years, which was the remaining life of the license agreement with Eli Lilly on the date of the transaction. The amortization of these intangibles is included in the cost of product revenues.
Commercialization Agreements
In March 2007, Cubist entered into a license agreement with Merck for the development and commercialization of CUBICIN in Japan, the last country outside the U.S. for which Cubist did not have a partner for the distribution of CUBICIN. Merck will develop and commercialize CUBICIN through its wholly-owned subsidiary, Banyu. In exchange for the development and commercialization rights in Japan, Merck paid Cubist an upfront fee of $6.0 million. This $6.0 million was recorded as deferred revenue and will be recognized over the estimated performance period. Cubist will receive up
80
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
C. BUSINESS AGREEMENTS (Continued)
to $39.5 million in additional payments if Merck reaches certain regulatory and sales milestones. In addition, Merck will purchase finished but unlabeled vials from Cubist in exchange for a transfer price.
In December 2006, Cubist entered into a license agreement with AstraZeneca, for the development and commercialization of CUBICIN in China and certain other countries in Asia, the Middle East and Africa not yet covered by existing CUBICIN international partnering agreements. In exchange for development and commercialization rights, AstraZeneca paid Cubist an up-front fee of $10.3 million. This $10.3 million was recorded as deferred revenue and will be recognized over the estimated performance period. Additionally, Cubist will receive payments upon AstraZeneca reaching regulatory and sales milestones. AstraZeneca will pay Cubist a transfer price for their purchases of CUBICIN. Cubist may receive payments for the achievement of the aforementioned milestones of up to $24.3 million under the agreement.
In July 2005, Cubist entered into a Distribution Agreement with Kuhnil Pharmaceuticals, Inc., or Kuhnil. Under the agreement, Kuhnil will commercialize CUBICIN in the Republic of Korea, or Korea, CUBICIN on an exclusive basis. In exchange for exclusive rights to commercialize CUBICIN in Korea, Kuhnil paid Cubist an up-front fee of $0.6 million, which was recorded as deferred revenue and will be recognized as revenue over the manufacturing and supply period commencing on first shipment of product in Korea. Kuhnil will pay Cubist a transfer price for their purchases of CUBICIN. Cubist may earn milestones of up to $0.9 million under the agreement.
In December 2003, Cubist entered into a Distribution Agreement with TTY Biopharm Company Limited, or TTY. Under the agreement, TTY will commercialize CUBICIN in Taiwan on an exclusive basis. In exchange for exclusive rights to commercialize CUBICIN in Taiwan, TTY paid Cubist an up-front fee of $0.5 million, which was recorded as deferred revenue and will be recognized as revenue over the manufacturing and supply period commencing on first shipment of product in Taiwan. In December 2003, Cubist also received $0.2 million from TTY for the achievement of a milestone under this agreement, which was also recorded to deferred revenue and will be recognized over the manufacturing and supply period commencing on first shipment of product in Taiwan. TTY will pay Cubist a transfer price for their purchases of CUBICIN. Cubist may earn additional milestones of up to $0.8 million under the agreement.
In October 2003, Cubist signed a License Agreement and a Manufacturing and Supply Agreement with a predecessor-in-interest to Novartis for the development and commercialization of CUBICIN in Western and Eastern Europe, Australia, New Zealand, India and certain Central American, South American and Middle Eastern countries. Novartis' predecessor paid Cubist an up-front licensing fee of $8.0 million, which was recorded as deferred revenue and was amortized to revenue over the estimated development period of two years, which completed in September 2005. Per the License Agreement, Cubist is entitled to receive from Novartis additional cash payments of up to $32.0 million upon achievement of certain development and sales milestones. Per the Manufacturing and Supply Agreement, Novartis shall pay Cubist a transfer price for CUBICIN and per the License Agreement, Novartis will owe Cubist royalty payments based on Novartis's sales of CUBICIN.
In October 2003, Cubist also entered into a Stock Purchase Agreement with Novartis' predecessor pursuant to which Novartis' predecessor purchased 529,942 shares of Cubist common stock for $10.0 million, at a 50% premium to the fair value of the Company's common stock. The premium paid
81
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
C. BUSINESS AGREEMENTS (Continued)
was allocated to the License Agreement and was accounted for as part of the up-front payment and amortized over the estimated development period of two years, which completed in September 2005.
D. ACQUISITION OF ILLUMIGEN
In October 2007, Cubist and Illumigen entered into an agreement under which Cubist purchased an exclusive option to acquire Illumigen. In December 2007, Cubist exercised its option and acquired Illumigen pursuant to a definitive agreement and plan of merger. Per the merger agreement, Cubist agreed to pay $9.0 million, plus Illumigen's closing cash and less Illumigen's closing liability balances, in cash to Illumigen shareholders, and Illumigen became a wholly-owned subsidiary of Cubist. Illumigen's lead compound, IB657, is a protein therapeutic in late-stage pre-clinical development as an interferon-sparing agent for the treatment of HCV infections. The results of operation of Illumigen have been included in the Company's financial statement from the acquisition date. The acquisition was accounted for under the purchase method of accounting.
Cubist has evaluated whether the Illumigen acquisition meets the criteria of a business as outlined in EITF 98-3,"Determining Whether a Nonmonetary Transaction Involves Receipt of Productive Assets or of a Business," and has concluded that the entity did not qualify as a business. Accordingly, the Company appropriately accounted for this transaction as an acquisition of assets. The costs associated with the acquisition were $16.4 million and include the closing cash consideration paid to Illumigen shareholders of $10.2 million, the option agreement payment of $4.7 million made in October 2007, transaction costs of $0.8 million and $0.7 million of costs paid by Cubist during the option period related to an IND enabling study of IB657 and Illumigen's operating costs. The total consideration was allocated to net tangible assets acquired of $1.3 million, consisting primarily of cash, and in-process research and development, or IPR&D, of $14.4 million and research and development expense of $0.7 million. The IPR&D represents the value assigned to the IB657 compound. At the date of the acquisition, IB657 had not yet reached technological feasibility and the research and development in progress had no alternative future uses. Accordingly, the full value of the IPR&D of $14.4 million is included in research and development expense for the year ended December 31, 2007. This charge is not deductible for tax purposes.
IB657 is a protein therapeutic in pre-clinical development for the treatment of HCV infections. If the pre-clinical development goes as planned, Cubist expects that an IND for IB657 will be filed in the second half of 2008. Cubist will make payments to Illumigen's former shareholders during the development of IB657 as a therapy for HCV infections of up to $75.5 million upon achieving certain development and regulatory milestones. If Cubist develops Illumigen products for the treatment of viruses other than HCV, development and regulatory milestone payments of up to $117.0 million could apply. Assuming that HCV or other Illumigen antiviral products are commercialized, additional milestone payments of up to $140.0 million, as well as tiered royalties, could apply.
E. INVESTMENTS
Investments classified as held-to-maturity are carried in Cubist's Consolidated Balance Sheets at amortized cost plus interest receivable. Investments classified as available-for-sale are carried at fair value plus interest receivable. Interest receivable related to short-term investments $0.7 million at 2006. Interest receivable related to long-term investments was $0.1 million at December 31, 2007 and 2006, respectively.
82
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
E. INVESTMENTS (Continued)
Included in long-term investments at December 31, 2007 are auction rate securities. Historically, given the liquidity created by the auctions, auction rate securities have been priced and traded as short-term investments and have been considered short-term available-for-sale investments because of this interest rate reset feature. Given the repeated failure of auctions for $58.1 million of the Company's investments in auction rate securities, these investments are not considered liquid and have been classified as of December 31, 2007 as long-term investments. The cost basis, gross unrealized gains and losses and fair value for these securities as of December 31, 2007 and 2006 are as follows:
| | December 31, 2007
| | December 31, 2006
|
|---|
| | Cost
Basis
| | Gross
Unrealized
Gains
| | Gross
Unrealized
Losses
| | Fair Value
| | Carrying
Value
| | Gross
Unrealized
Gains
| | Gross
Unrealized
Losses
| | Fair Value
|
|---|
| | (in thousands)
| | (in thousands)
|
|---|
| Auction rate securities | | $ | 58,100 | | $ | — | | $ | (14,701 | ) | $ | 43,399 | | $ | 256,950 | | $ | — | | $ | — | | $ | 256,950 |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| | Total | | $ | 58,100 | | $ | — | | $ | (14,701 | ) | $ | 43,399 | | $ | 256,950 | | $ | — | | $ | — | | $ | 256,950 |
| | |
| |
| |
| |
| |
| |
| |
| |
|
The amount of the unrealized loss on the auction rate notes was determined through a valuation analysis which is based on bids from the broker who is the market maker for these instruments, corroborated through analysis of the underlying instruments. The Company considers this loss to be temporary in nature. The credit and capital markets have continued to deteriorate in 2008 and the bids on the auction rate notes the Company holds have since declined. If a certain concentration, as defined in the auction rate documents, of the underlying reference portfolios default, or if the issuing bank fails to make the required interest payments or the final principal payment upon the ultimate maturity of the notes, or if the credit ratings on the underlying reference portfolios deteriorate significantly, the Company may be required to adjust the carrying value of these investments through an other-than-temporary impairment charge.
There were no held-to-maturity investments at December 31, 2007. The cost basis, gross unrealized gains and losses and fair value of held-to-maturity securities at December 31, 2006 were as follows:
| | December 31, 2006
|
|---|
| | Amortized
Cost
| | Gross
Unrealized
Gains
| | Gross
Unrealized
Losses
| | Fair Value
|
|---|
| | (in thousands)
|
|---|
| Short-Term: | | | | | | | | | | | | |
| Corporate bonds | | $ | 13,360 | | $ | 2 | | $ | — | | $ | 13,362 |
| Government bonds | | | 7,000 | | | — | | | (16 | ) | | 6,984 |
| | |
| |
| |
| |
|
| | Total | | $ | 20,360 | | $ | 2 | | $ | (16 | ) | $ | 20,346 |
| | |
| |
| |
| |
|
Long-Term: |
|
|
|
|
|
|
|
|
|
|
|
|
| Corporate bonds | | $ | — | | $ | — | | $ | — | | $ | �� |
| Government bonds | | | 15,038 | | | — | | | (32 | ) | | 15,006 |
| | |
| |
| |
| |
|
| | Total | | $ | 15,038 | | $ | — | | $ | (32 | ) | $ | 15,006 |
| | |
| |
| |
| |
|
83
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
E. INVESTMENTS (Continued)
The following is a summary of the cost basis and estimated fair value of investments at December 31, 2007 by contractual maturity:
| | Available-for-Sale
| | Held-to-Maturity
|
|---|
| | Cost
Basis
| | Fair Value
| | Cost
Basis
| | Fair Value
|
|---|
| | (in thousands)
| | (in thousands)
|
|---|
| Due within one year | | $ | — | | $ | — | | $ | — | | $ | — |
| Due after five years through ten years | | | 58,100 | | | 43,399 | | | — | | | — |
| | |
| |
| |
| |
|
| | Total | | $ | 58,100 | | $ | 43,399 | | $ | — | | $ | — |
| | |
| |
| |
| |
|
Included in the table above are auction rate securities, which typically reset to current interest rates every 7 to 35 days, but are included in the table above based on the stated maturities of the underlying investments.
F. ACCOUNTS RECEIVABLE
Cubist's trade receivables in 2007 and 2006 primarily represent amounts due to the Company from wholesalers and distributors of its pharmaceutical product. Cubist performs ongoing credit evaluations of its customers and generally does not require collateral.
G. PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at December 31,
| | 2007
| | 2006
| |
|---|
| | (in thousands)
| |
|---|
| Building | | $ | 43,385 | | $ | 43,069 | |
| Leasehold improvements | | | 10,042 | | | 9,473 | |
| Laboratory equipment | | | 15,251 | | | 12,988 | |
| Furniture and fixtures | | | 1,500 | | | 1,482 | |
| Computer equipment | | | 11,060 | | | 8,812 | |
| Construction in progress | | | 1,194 | | | 1,474 | |
| | |
| |
| |
| | | | 82,432 | | | 77,298 | |
| Less accumulated depreciation and amortization | | | (32,282 | ) | | (27,714 | ) |
| | |
| |
| |
| Property and equipment, net | | $ | 50,150 | | $ | 49,584 | |
| | |
| |
| |
Depreciation and amortization expense was $4.6 million, $4.1 million and $4.0 million in 2007, 2006 and 2005, respectively.
84
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
H. INTANGIBLE ASSETS
Intangible assets consisted of the following at:
| |
| | December 31,
| |
|---|
| |
| | 2007
| | 2006
| |
|---|
| |
| | (in thousands)
| |
|---|
| Patents | | $ | 2,673 | | $ | 2,673 | |
| Manufacturing rights | | | 2,500 | | | 2,500 | |
| Acquired technology rights | | | 28,500 | | | 28,500 | |
| Intellectual property and processes and other intangibles | | | 5,388 | | | 5,388 | |
| | | | |
| |
| |
| | | | | | 39,061 | | | 39,061 | |
| Less: | | accumulated amortization—patents | | | (2,128 | ) | | (2,063 | ) |
| | | | accumulated amortization—manufacturing rights | | | (1,250 | ) | | (833 | ) |
| | | | accumulated amortization—acquired technology rights | | | (7,610 | ) | | (5,152 | ) |
| | | | accumulated amortization—intellectual property | | | (5,375 | ) | | (5,374 | ) |
| | | | |
| |
| |
| Intangible assets, net | | $ | 22,698 | | $ | 25,639 | |
| | | | |
| |
| |
There were no additions to intangible assets during the twelve months ended December 31, 2007 and 2006. Additions to intangible assets during 2005 resulted from $20.0 million of payments made to Eli Lilly, in the form of common stock in exchange for a 2% reduction in the royalty rate payable to Eli Lilly on net sales of CUBICIN pursuant to the Company's license agreement with Eli Lilly. Cubist is amortizing the $20.0 million over approximately eleven years, which was the remaining life of the license agreement with Eli Lilly on the date of the transaction. In 2003, Cubist issued to Eli Lilly $8.0 million of common stock in exchange for a 1% reduction in the royalties payable to Eli Lilly. The Company also issued 38,922 shares of common stock valued at $0.5 million in 2003 as a milestone payment to Eli Lilly. This $8.5 million payment is being amortized over approximately thirteen years, which was the estimated remaining life of the license agreement with Eli Lilly on the dates of the transactions. The amortization of the Eli Lilly intangible assets are included in cost of product revenues.
In November 2005, Cubist announced that it selected ACS as the single source supplier of API for CUBICIN. Cubist provided notice to DSM in accordance with contract agreement terms to terminate its manufacturing and supply agreement with DSM for API. The useful life of the DSM manufacturing rights was adjusted to coincide with the revised termination date of May 2006. As Cubist received no future benefit from the DSM manufacturing rights, their gross asset value and related allowance for amortization expense were eliminated from the manufacturing rights accounts in 2006 with no resulting gain or loss. The remaining balance of these assets was allocated to inventory and was expensed to cost of product revenues as the related inventory lots were sold. The DSM assets have been expensed as of December 31, 2007. The manufacturing rights associated with the ACS agreement are being amortized to inventory over the contractual term of six years and expensed to cost of product revenues as the related inventory lots are sold.
Amortization expense, including amounts relating to the DSM manufacturing rights, was $5.1 million, $4.9 million and $7.0 million in 2007, 2006 and 2005 respectively. The estimated aggregate
85
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
H. INTANGIBLE ASSETS (Continued)
amortization of intangible assets as of December 31, 2007, for each of the five succeeding years is as follows:
| | (in thousands)
|
|---|
| 2008 | | $ | 2,941 |
| 2009 | | | 2,941 |
| 2010 | | | 2,941 |
| 2011 | | | 2,524 |
| 2012 | | | 2,524 |
| 2013 and thereafter | | | 8,827 |
| | |
|
| | | $ | 22,698 |
| | |
|
I. ACCRUED LIABILITIES
Accrued liabilities consisted of the following at:
| | December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| | (in thousands)
|
|---|
| Accrued payroll | | $ | 1,115 | | $ | 590 |
| Accrued incentive compensation | | | 4,424 | | | 2,353 |
| Accrued bonus | | | 5,645 | | | 4,458 |
| Accrued benefit costs | | | 2,056 | | | 1,879 |
| Accrued clinical trials | | | 193 | | | 494 |
| Accrued interest | | | 350 | | | 350 |
| Accrued Illumigen acquisition costs | | | 10,191 | | | — |
| Accrued manufacturing costs | | | 2,672 | | | 1,804 |
| Accrued royalty | | | 23,729 | | | 12,905 |
| Other accrued costs | | | 8,360 | | | 6,205 |
| | |
| |
|
| | Total | | $ | 58,735 | | $ | 31,038 |
| | |
| |
|
J. ACCRUED CLINICAL TRIAL EXPENSE
Accrued clinical trial expenses are comprised of amounts owed to third party contract research organizations, or CROs, for research and development work performed on behalf of Cubist. At each period end, the Company evaluates the accrued clinical trial expense balance based upon information received from each CRO, and ensures that the balance is appropriately stated based upon work performed to date. The accrued clinical trial expense balance of $193,000 and $494,000 at December 31, 2007 and 2006, respectively, represents the Company's best estimate of amounts owed for clinical trial services performed through those periods based on all information available. Such estimates are subject to change as additional information becomes available.
86
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
K. STOCKHOLDERS' EQUITY
Stock Issuances
In March 2005, Cubist announced that it entered into an agreement to purchase from Eli Lilly a 2% reduction in the royalty rate payable to Eli Lilly on net sales of CUBICIN. Cubist issued to Eli Lilly $20.0 million in Cubist common stock with associated registration rights. A total of 1,876,173 shares were issued at a price of $10.66 per share in March 2005. Cubist's global royalty rate obligation to Eli Lilly on CUBICIN sales was reduced by two percentage points upon registration of the common stock on April 22, 2005.
L. EMPLOYEE STOCK BENEFIT PLANS
Summary of Stock Option Plans
Cubist has several stock-based compensation plans. Under the Cubist Amended and Restated 1993 Stock Option Plan, options to purchase 5,837,946 shares of common stock were available for grant to employees, directors, officers or consultants. The options were generally granted at fair market value on the grant date, vested ratably over a four-year period and expired ten years from the grant date. There are no shares available for future grant under this plan as it expired in accordance with its terms in 2003.
Under the Cubist Amended and Restated 2000 Equity Incentive Plan, or the 2000 Equity Incentive Plan, 11,535,764 shares of common stock may be issued to employees, directors, officers or consultants. Options under this plan are generally granted with exercise prices equal to the fair market value on the grant date, vest ratably over a four-year period and expire ten years from the date of grant. At December 31, 2007, there were 4,056,161 shares available for future grant under this plan.
Under the Cubist Amended and Restated 2002 Directors Equity Incentive Plan, 975,000 shares of common stock may be issued to members of the Board of Directors. Options under this plan are granted at fair market value on the grant date, vest ratably over a one-year or a three-year period and expire ten years from the grant date. At December 31, 2007, there were 501,250 shares available for future grant under this plan.
Cubist does not currently hold any treasury shares. Upon share option exercise, the Company issues new shares and delivers them to the participant. In line with its current business plan, Cubist does not intend to repurchase shares in the foreseeable future.
Summary of Employee Stock Purchase Plan
Qualifying employees are eligible to participate in an employee stock purchase plan sponsored by the Company. Under this program, participants may purchase Cubist common stock, after a pre-determined six-month period, at 85% of the lower of the fair market value at the beginning or end of the purchase period. Shares are purchased through payroll deductions of up to 15% of each participating employee's annual compensation, subject to certain limitations. The current plan allows for the issuance of 750,000 shares of common stock to eligible employees. During 2007, 2006 and 2005 Cubist issued 75,303, 79,558 and 73,224 shares of common stock, respectively, pursuant to this plan. At December 31, 2007 there were 342,739 shares available for future issuance under this plan.
87
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
L. EMPLOYEE STOCK BENEFIT PLANS (Continued)
Stock Compensation Expense Prior to the Adoption of SFAS 123(R)
Prior to the adoption of SFAS 123(R), the Company provided the disclosures required under SFAS No. 123,"Accounting for Stock-Based Compensation," as amended by SFAS No. 148,"Accounting for Stock-Based Compensation—Transition and Disclosures." No significant employee stock-based compensation was reflected in net loss for the year ended December 31, 2005 related to employee option grants, as all options granted under those plans had an exercise price equal to the market value of the underlying common stock on the date of grant. The pro-forma information for the year ended December 31, 2005 was as follows (in thousands, except per share data):
| | Year Ended
December 31,
2005
| |
|---|
| Net loss, as reported | | $ | (31,852 | ) |
| Add: Stock-based employee compensation recorded in net loss, as reported | | | 77 | |
| Deducted: Total stock-based employee compensation expense determined under fair value based method for all awards, net of related tax effects | | | (12,962 | ) |
| | |
| |
| | Pro forma net loss | | $ | (44,737 | ) |
| | |
| |
| | Loss per share: | | | | |
| | | Basic and diluted—as reported | | $ | (0.60 | ) |
| | |
| |
| | | Basic and diluted—pro forma | | $ | (0.84 | ) |
| | |
| |
Summary of SFAS 123(R) Expense
The effect of recording stock-based compensation in the Consolidated Statement of Operations for the years ended December 31, 2007 and 2006 was as follows:
| | December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| | (in thousands except per share data)
|
|---|
| Stock-based compensation expense by type of award: | | | | | | |
| Employee stock options | | $ | 10,215 | | $ | 10,214 |
| Employee stock purchase plan | | | 324 | | | 409 |
| | |
| |
|
| | Total stock-based compensation | | $ | 10,539 | | $ | 10,623 |
| | |
| |
|
| Effect on earnings per share: | | | | | | |
| | Basic | | $ | 0.19 | | $ | 0.19 |
| | Diluted | | $ | 0.15 | | $ | 0.19 |
The carrying value of inventory in the Consolidated Balance Sheet for the years ended December 31, 2007 and 2006 includes employee stock-based compensation costs of $0.2 million.
88
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
L. EMPLOYEE STOCK BENEFIT PLANS (Continued)
Valuation Assumptions
The fair value of each share-based award was estimated on the date of grant using the Black-Scholes option-pricing model and expensed under the accelerated method for option grants prior to the first quarter of 2006 and under the straight-line method for option grants commencing in the first quarter of 2006. The following weighted-average assumptions were used:
| | 2007
| | 2006
| | 2005
|
|---|
| Stock option plans: | | | | | | |
| Expected stock price volatility | | 47% | | 52% | | 72% |
| Risk free interest rate | | 4.6% | | 4.7% | | 3.9% |
| Expected annual dividend yield per share | | 0% | | 0% | | 0% |
| Expected life of options | | 4.3 years | | 4.3 years | | 5 years |
Stock purchase plan: |
|
|
|
|
|
|
| Expected stock price volatility | | 30% | | 30% | | — |
| Risk free interest rate | | 4.8% | | 4.8% | | — |
| Expected annual dividend yield per share | | 0% | | 0% | | — |
| Expected life of options | | 6 months | | 6 months | | — |
Cubist's expected stock price volatility assumption is based on both current and historical volatilities of the Company's stock which is obtained from public data sources. The expected stock price volatility is determined based on the instrument's expected term. Since the employee stock purchase plan has a shorter term than the stock option plans, volatility for this plan is estimated over a shorter period. The risk-free interest rate is a less subjective assumption as it is based on factual data derived from public sources. Cubist uses a dividend yield of zero as it has never paid cash dividends and has no intention of paying cash dividends in the foreseeable future. The expected life represents the weighted average period of time that share-based awards are expected to be outstanding giving consideration to vesting schedules and the Company's historical exercise patterns. Cubist determines the expected life assumption based on the exercise behavior and post vesting cancellations that has been exhibited historically, adjusted for specific factors that may influence future exercise patterns. The Company estimates forfeitures based on its historical experience of share-based pre-vesting cancellations. The Company believes that its estimates are based on outcomes that are reasonably likely to occur. To the extent actual forfeitures differ from its estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised.
89
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
L. EMPLOYEE STOCK BENEFIT PLANS (Continued)
General Option Information
A summary of the status of Cubist's stock option plans, as of December 31, 2007 and changes during the year then ended, is presented below:
| | 2007
|
|---|
| | Number
| | Weighted
Average Exercise
Price
|
|---|
| Balance at January 1 | | 7,276,450 | | $ | 16.49 |
| Granted | | 2,012,575 | | $ | 20.88 |
| Exercised | | (965,538 | ) | $ | 11.34 |
| Canceled | | (687,076 | ) | $ | 19.22 |
| | |
| |
|
| Balance at December 31 | | 7,636,411 | | $ | 18.05 |
| | |
| |
|
| Options vested and exercisable as of December 31, | | 4,395,312 | | $ | 17.11 |
The total intrinsic value of options exercised during the years ended December 31, 2007, 2006 and 2005 was $10.5 million, $11.6 million and $5.2 million, respectively. The aggregate intrinsic value of options outstanding as of December 31, 2007 was $18.8 million. These options have a weighted average remaining contractual life of 7.1 years.
As of December 31, 2007, there was $19.6 million of total unrecognized compensation cost related to nonvested options granted under the Plans. That cost is expected to be recognized over the weighted-average period of 1.4 years. The aggregate intrinsic value of options fully vested and exercisable as of December 31, 2007 was $14.9 million. These options have a weighted average remaining contractual life of 6.0 years.
The weighted average grant-date fair value of options granted during the years ended December 31, 2007, 2006 and 2005 was $9.15, $10.40 and $7.20, respectively. The weighted-average grant-date fair value of options vested as of December 31, 2007, 2006 and 2005 was $11.32, $11.61 and $13.13, respectively.
90
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
L. EMPLOYEE STOCK BENEFIT PLANS (Continued)
The following table summarizes information about stock options outstanding at December 31, 2007:
| | Options Outstanding
| | Options Exercisable
|
|---|
Range of
Exercise Prices
| | Number
Outstanding
| | Remaining
Contractual Life
| | Weighted-Average
Exercise Price
| | Number
Exercisable
| | Weighted-Average
Exercise Price
|
|---|
| $0.42 —$6.34 | | 98,149 | | 1.1 | | $ | 3.14 | | 98,149 | | $ | 3.14 |
| $6.35 —$12.68 | | 2,792,249 | | 6.2 | | | 10.27 | | 2,227,265 | | | 10.19 |
| $12.69—$19.01 | | 427,495 | | 6.7 | | | 14.67 | | 313,226 | | | 14.07 |
| $19.02—$25.35 | | 3,553,708 | | 8.8 | | | 21.49 | | 991,862 | | | 21.73 |
| $25.36—$31.69 | | 226,005 | | 3.0 | | | 29.33 | | 226,005 | | | 29.33 |
| $31.70—$38.03 | | 513,051 | | 3.9 | | | 35.14 | | 513,051 | | | 35.14 |
| $50.70—$57.04 | | 1,254 | | 1.3 | | | 53.08 | | 1,254 | | | 53.08 |
| $57.05—$63.38 | | 24,500 | | 2.3 | | | 61.71 | | 24,500 | | | 61.71 |
| | |
| |
| |
| |
| |
|
| | | 7,636,411 | | 7.1 | | $ | 18.05 | | 4,395,312 | | $ | 17.11 |
| | |
| |
| |
| |
| |
|
M. COMMITMENTS AND CONTINGENCIES
Leases
Cubist leases various facilities and equipment under leases that expire at varying dates through 2016. Certain of these leases contain renewal options and provisions that adjust the rent payment based upon changes in the consumer price index and require Cubist to pay operating costs, including property taxes, insurance and maintenance.
At December 31, 2007, future minimum lease payments under all non-cancelable leases net of sublease income are as follows (in thousands):
| | Operating
|
|---|
| 2008 | | $ | 2,711 |
| 2009 | | | 3,338 |
| 2010 | | | 3,511 |
| 2011 | | | 3,514 |
| 2012 | | | 3,631 |
| Thereafter | | | 13,035 |
| | |
|
| | Total minimum lease payments | | $ | 29,740 |
| | |
|
Rental expense for operating leases was $4.1 million, $3.6 million and $2.7 million in the years ended December 31, 2007, 2006 and 2005, respectively. Sublease income, which is recorded as a reduction of rent expense, was $2.6 million, $2.5 million and $2.4 million in the years ended December 31, 2007, 2006 and 2005, respectively.
91
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
M. COMMITMENTS AND CONTINGENCIES (Continued)
Foreign currency
Cubist operates internationally, which gives rise to a risk that earnings and cash flows may be negatively impacted by fluctuations in interest and foreign exchange rates. During 2007, 2006 and 2005, Cubist entered into limited foreign currency transactions between the U.S. dollar, the European Euro and the British pound.
Guarantees and Indemnification Obligations
The Company has vacated some of its leased facilities or sublet them to third parties. When the Company sublets a facility to a third party, it remains the primary obligor under the master lease agreement with the owner of the facility. As a result, if a third party defaults on their payments related to the sublet facility, the Company would be obligated to make lease or other payments under the master lease agreement. The Company believes that the financial risk of default by sublessors is individually and in the aggregate not material to the Company's financial position or results of operations.
Other
We have minimum volume purchase commitments with third party contract manufacturers with scheduled payments over the next five years that total $90.7 million at December 31, 2007.
N. DEBT
Cubist's outstanding debt at December 31, 2007 and 2006 consists of $350.0 million aggregate principal amount of 2.25% convertible subordinated notes due June 2013. Cubist's outstanding debt at December 31, 2005 consisted of $165.0 million aggregate principal amount of 51/2% convertible subordinated notes due November 2008.
In June 2006, Cubist completed the public offering of $350.0 million aggregate principal amount of 2.25% convertible subordinated notes (the "2.25% Notes"). The 2.25% Notes are convertible at any time prior to maturity into common stock at an initial conversion rate of 32.4981 shares of common stock per $1,000 principal amount of convertible notes, subject to adjustment upon certain events, which equates to approximately $30.77 per share of common stock. Cubist may deliver cash or a combination of cash and common stock in lieu of shares of common stock. Interest is payable on each June 15 and December 15, beginning December 15, 2006. The 2.25% Notes mature on June 15, 2013. Cubist retains the right to redeem the 2.25% Notes at 100% of the principal amount to be redeemed plus accrued and unpaid interest commencing in June 2011 if Cubist's common stock closing price exceeded the conversion price for a period of time as defined in the 2.25% Notes agreement. The deferred financing costs associated with the sale of the 2.25% Notes were $10.9 million. These costs are amortized ratably over the life of the note. See Note S. for additional information related to the 2.25% Notes.
In 2001, Cubist completed the private placement of $165.0 million aggregate principal amount of 5.5% convertible subordinated notes (the "5.5% Notes"). The offering was made through initial purchasers to qualified institutional buyers under Rule 144A of the Securities Act. The 5.5% Notes were convertible at any time prior to maturity into common stock at a conversion price of $47.20 per share, subject to adjustment upon certain events. Interest was payable on each November 1 and May 1,
92
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
N. DEBT (Continued)
beginning May 1, 2002. The 5.5% Notes had a maturity date of November 1, 2008. Cubist retained the right to redeem the 5.5% Notes prior to November 2004 if Cubist's common stock closing price exceeded the conversion price for a period of time as defined in the 5.5% Notes agreement. The deferred financing costs associated with the sale of the 5.5% Notes were $5.3 million. In June 2006, Cubist repaid the outstanding principal and accrued interest on the 5.5% Notes, plus a prepayment penalty of $3.9 million that was recorded to interest expense. The remaining unamortized balance of the debt issuance costs, totaling $1.8 million, associated with the 5.5% Notes was written off to interest expense at the time of the repayment.
At December 31, 2007, future payments of principal and interest on existing debt are due as follows:
Fiscal year ending December 31,
| | Principal
| | Interest
| | Total
|
|---|
| | (in thousands)
|
|---|
| 2008 | | $ | — | | $ | 7,875 | | $ | 7,875 |
| 2009 | | | — | | | 7,875 | | | 7,875 |
| 2010 | | | — | | | 7,875 | | | 7,875 |
| 2011 | | | — | | | 7,875 | | | 7,875 |
| 2012 | | | — | | | 7,875 | | | 7,875 |
| 2013 | | | 350,000 | | | 3,938 | | | 353,938 |
| | |
| |
| |
|
| Total payments | | $ | 350,000 | | $ | 43,313 | | $ | 393,313 |
| Less current portion | | | — | | | | | | |
| | |
| | | | | | |
| | Total long term debt | | $ | 350,000 | | | | | | |
| | |
| | | | | | |
O. EMPLOYEE BENEFITS
401(k) Savings Plan
Cubist maintains a 401(k) savings plan in which substantially all of its permanent employees in the U.S. are eligible to participate. Participants may contribute up to 100% of their annual compensation to the plan, subject to certain limitations. Cubist matches each employee's contribution in Cubist common stock up to 4% of a participant's total compensation. Employer common stock matches have immediate vesting. Cubist issued 97,206, 127,504 and 87,500 shares of common stock in 2007, 2006 and 2005, respectively, pursuant to this plan.
P. INCOME TAXES
Effective Tax Rate
For each of the years ended December 31, 2007, 2006 and 2005, Cubist's statutory tax rate was 35%, 34% and 34%, respectively. During 2007, the Company revised its estimate concerning the future reversal of temporary differences and concluded that the reversal is likely to occur when the U.S. federal incremental tax rate is 35% versus the 34% used previously. The effective tax rate for the years ended December 31, 2007, 2006 and 2005 was 3.7%, 0% and 0%, respectively. The effective tax rate
93
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
P. INCOME TAXES (Continued)
for the year ended December 31, 2007 relates to federal alternative minimum tax expense and state tax expense. The Company and its subsidiaries file income tax returns with the U.S. federal government and with multiple state and local jurisdictions in the U.S.
The effective rate differs from the statutory rate of 35% and 34% due to the following:
| | 2007
| | 2006
| | 2005
| |
|---|
| Federal | | 35.0 | % | 34.0 | % | 34.0 | % |
| State | | 6.4 | % | -49.8 | % | 6.5 | % |
| Federal and state credits | | -3.3 | % | 600.9 | % | 8.9 | % |
| Valuation allowance | | -47.2 | % | -345.2 | % | -47.6 | % |
| In-process research & development | | 10.6 | % | 0 | % | 0 | % |
| Other | | 2.2 | % | -239.9 | % | -1.8 | % |
| | |
| |
| |
| |
| Effective tax rate | | 3.7 | % | 0.0 | % | 0.0 | % |
| | |
| |
| |
| |
Changes in the effective tax rates from period to period may be significant as they depend on many factors including, but not limited to, changes in circumstances surrounding the need for a valuation allowance, size of the Company's income or loss, or one time activities occurring during the period.
Deferred Taxes and Valuation Allowance
All of the Company's deferred tax assets have a full valuation allowance recorded against them. Based on management's review of the Company's historical tax position and operational results, realization of Cubist's deferred tax assets does not meet the "more likely than not" criteria under SFAS No. 109. Management will continue to monitor the available information in determining whether there is sufficient positive evidence to consider releasing the valuation allowance on the deferred tax assets. Should management determine the valuation allowance is no longer required, a tax benefit would be recorded in the financial period of the change in determination. The components of the tax affected net deferred tax assets and the related valuation allowance are as follows:
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| | (in thousands)
| |
|---|
| Deferred income tax assets: | | | | | | | |
| Net operating loss carryforwards | | $ | 122,212 | | $ | 142,309 | |
| Research and development costs | | | 23,914 | | | 31,012 | |
| Tax credit carryforwards | | | 17,754 | | | 16,923 | |
| Impairment charge | | | 672 | | | 738 | |
| Deferred revenues | | | 4,208 | | | 490 | |
| Other, net | | | 4,965 | | | 4,960 | |
| | |
| |
| |
| Total deferred tax assets | | | 173,725 | | | 196,432 | |
| Valuation allowance | | | (173,725 | ) | | (196,432 | ) |
| | |
| |
| |
| Net deferred tax assets | | $ | — | | $ | — | |
| | |
| |
| |
94
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
P. INCOME TAXES (Continued)
At December 31, 2007, Cubist has gross federal net operating loss carryforwards of approximately $338.5 million, which begin to expire in 2022, and gross state net operating loss carryforwards of $183.2 million, which begin to expire in 2008. State net operating loss carryforwards of $5.3 million and $27.8 million expired in 2007 and 2006, respectively. Of the $173.7 million valuation allowance at December 31, 2007, $7.9 million relates to the tax benefit of the exercise of stock options. This amount will result in an increase in Additional Paid in Capital upon realization of these losses. Cubist also has federal and state credit carryforwards of $13.6 million and $6.5 million respectively, which begin to expire in 2008 and 2011, respectively.
The Company has excluded the benefit of $7.7 million ($19.7 million pre-tax) of U.S. federal and state net operating loss carryforwards from the deferred tax asset balance at December 31, 2007. This amount represents an "excess tax benefit", as the term is defined in SFAS No. 123(R), which will be recognized as a reduction to the Company's accrued income taxes and an addition to its additional paid-in capital when it is realized in the Company's tax returns.
As stated in Note D., Cubist acquired Illumigen in December 2007. Illumigen had approximately $17.9 million of gross net operating loss carryforwards available, resulting in a net deferred tax asset of $7.1 million, which, consistent with the Company's other deferred tax assets, is fully offset by a valuation allowance. Due to the timing of the acquisition, the Company has not as yet completed a Section 382 study to assess whether past changes in ownership may limit or restrict the Company's ability to utilize these net operating loss carryforwards. Since a full valuation allowance has been provided against these net operating loss carryforwards, any adjustment to the net operating loss carryforward amount required upon completion of a Section 382 study would be offset by a corresponding reduction to the valuation allowance. Thus, there would be no impact to the consolidated balance sheet or statement of operations if an adjustment were required.
Ownership changes resulting from the issuance of capital stock may limit the amount of net operating loss and tax credit carryforwards that can be utilized annually to offset future taxable income. The amount of the annual limitation is determined based on Cubist's value immediately prior to the ownership change. The Company has analyzed its historical changes in ownership and does not believe there are any limitations to the usage of its net operating losses. Subsequent significant changes in ownership could affect the limitation in future years.
FIN 48—Uncertain Tax Positions
On January 1, 2007, the Company adopted the provisions of FIN 48,"Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement 109." There were no adjustments to retained earnings as a result of the implementation of FIN 48. The Company's only adjustment upon adoption of FIN 48 related to a $2.0 million adjustment to research and development tax credit carryforwards, relating to issues that were identified in the context of a recently concluded state tax examination. This adjustment to the credit carryforwards did not impact retained earnings or the statement of operations, as there is a full valuation allowance recorded against the deferred tax asset. The Company is in the process of conducting a study of its research and development credit carryforwards. This study may result in additional changes to the Company's research and development credit carryforwards. Since a full valuation allowance has been provided against these carryforwards, any adjustment to the credit carryforwards upon completion of the R&D Credit study would be offset by a corresponding reduction
95
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
P. INCOME TAXES (Continued)
to the valuation allowance. Thus, there would be no impact to the consolidated balance sheet or statement of operations.
A reconciliation of the Company's changes in uncertain tax positions from January 1, 2007 to December 31, 2007 is as follows (in thousands):
| Uncertain tax positions January 1, 2007 | | $ | 2,000 |
| Additions based on tax positions related to the current year | | | — |
| Additions for tax positions of prior years | | | — |
| Reductions for tax positions of prior years | | | — |
| Settlements | | | — |
| | |
|
| Balance at December 31, 2007 | | $ | 2,000 |
| | |
|
Interest and penalty charges, if any, related to unrecognized tax benefits would be classified as income tax expense in the accompanying consolidated statements of operations. At January 1, 2007 and December 31, 2007 the Company did not have any interest or penalties accrued related to uncertain tax positions.
In many cases the Company's uncertain tax positions are related to tax years that remain subject to examination by relevant tax authorities. Since the Company is in a loss carryforward position, the Company is generally subject to U.S. federal, state and local income tax examinations by tax authorities for all years for which a loss carryforward is available.
Q. BUSINESS SEGMENTS
Cubist operates in one business segment, the research, development and commercialization of pharmaceutical products that address unmet medical needs in the acute care environment. To date, the Company has concentrated exclusively on developing products for the anti-infective marketplace. The Company's entire business is managed by a single management team, which reports to the Chief Executive Officer. Substantially all of the Company's revenues are currently generated within the U.S.
R. SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
The following table contains quarterly financial information for fiscal years 2007 and 2006. Cubist believes that the following information reflects all normal recurring adjustments necessary for a fair
96
CUBIST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
R. SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED) (Continued)
presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
| | First
Quarter
| | Second
Quarter
| | Third
Quarter
| | Fourth
Quarter
| |
|---|
| | (in thousands, except per share data)
| |
|---|
| 2007 | | | | | | | | | | | | | |
| Total revenues, net | | $ | 59,479 | | $ | 69,764 | | $ | 79,796 | | $ | 85,581 | |
| Product revenues, net | | $ | 59,435 | | $ | 69,525 | | $ | 76,326 | | $ | 85,120 | |
| Cost of product revenues | | $ | 16,738 | | $ | 15,834 | | $ | 17,153 | | $ | 19,135 | |
| Net income | | $ | 5,601 | | $ | 14,490 | | $ | 20,023 | | $ | 8,033 | (1) |
| Basic net income per share | | $ | 0.10 | | $ | 0.26 | | $ | 0.36 | | $ | 0.14 | (1) |
| Diluted net income per share | | $ | 0.10 | | $ | 0.24 | | $ | 0.32 | | $ | 0.14 | (1) |
2006 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total revenues, net | | $ | 40,055 | | $ | 47,794 | | $ | 50,419 | | $ | 56,480 | |
| Product revenues, net | | $ | 37,941 | | $ | 45,681 | | $ | 50,318 | | $ | 56,380 | |
| Cost of product revenues | | $ | 10,132 | | $ | 11,790 | | $ | 12,742 | | $ | 14,139 | |
| Net income (loss) | | $ | (5,880 | ) | $ | (5,073 | ) | $ | 5,184 | | $ | 5,393 | |
| Basic net income (loss) per share | | $ | (0.11 | ) | $ | (0.09 | ) | $ | 0.09 | | $ | 0.10 | |
| Diluted net income (loss) per share | | $ | (0.11 | ) | $ | (0.09 | ) | $ | 0.09 | | $ | 0.09 | |
- (1)
- In the fourth quarter of 2007, Cubist recorded an IPR&D charge of $14.4 million related to the acquisition of Illumigen (See Note D.).
S. SUBSEQUENT EVENT
On February 7, 2008, Cubist announced that it repurchased, in privately negotiated transactions, $50.0 million in original principal amount of its 2.25% Notes due June 15, 2013 at an average price of approximately $93.69 per $100 of debt. Following these repurchases, $300.0 million principal amount of the 2.25% Notes remain outstanding. These repurchases will reduce Cubist fully-diluted shares of common stock outstanding by approximately 1,624,905 shares. Cubist repurchased the outstanding principal and accrued interest on the 2.25% Notes of $46.8 million and incurred transaction fees of $0.2 million. Debt issuance costs of $1.2 million were also written off as a non-cash charge to interest expense at the time of the repurchase. The transaction was funded out of the Company's working capital.
97
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the Exchange Act). Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management's Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework inInternal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2007.
PricewaterhouseCoopers LLP, our independent registered public accounting firm which audited our financial statements for the fiscal year ended December 31, 2007 has issued an attestation report on our internal control over financial reporting, as stated in its report which is included herein.
There have not been any changes in the Company's internal control over financial reporting during the quarter ended December 31, 2007 that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
98
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Certain information with respect to our executive officers and directors may be found under the section captioned "Our Executive Officers and Directors" in Part I of this Annual Report on Form 10-K. Other information required by Item 10 of Form 10-K may be found in the definitive Proxy Statement to be delivered to stockholders in connection with the Annual Meeting of Stockholders to be held on June 10, 2008. Such information is incorporated herein by reference.
Our board of directors adopted a Code of Conduct and Ethics applicable to the board of directors, our Chief Executive Officer, Chief Financial Officer, other officers of Cubist and all other employees of Cubist. The Code of Conduct and Ethics is available on our web site, www.cubist.com and in our filings with the SEC.
ITEM 11. EXECUTIVE COMPENSATION
The information required with respect to this item may be found in the definitive Proxy Statement to be delivered to stockholders in connection with the Annual Meeting of Stockholders to be held on June 10, 2008. Such information is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED SHAREHOLDER MATTERS
The information required with respect to this item may be found in the definitive Proxy Statement to be delivered to Stockholders in connection with the Annual Meeting of Stockholders to be held on June 10, 2008. Such information is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required with respect to this item may be found in the definitive Proxy Statement to be delivered to stockholders in connection with the Annual Meeting of Stockholders to be held on June 10, 2008. Such information is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required with respect to this item may be found in the definitive Proxy Statement to be delivered to stockholders in connection with the Annual Meeting of Stockholders to be held on June 10, 2008. Such information is incorporated herein by reference.
99
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
- (A)
- Documents Filed As Part Of Form 10-K:
1. Financial Statements
The following financial statements and supplementary data are included in Part II Item 8 filed as part of this report:
- •
- Report of Independent Registered Public Accounting Firm
- •
- Balance Sheets as of December 31, 2007 and 2006
- •
- Statements of Operations for the years ended December 31, 2007, 2006 and 2005
- •
- Statements of Cash Flows for the years ended December 31, 2007, 2006 and 2005
- •
- Statements of Stockholders' Equity for the years ended December 31, 2007, 2006 and 2005
- •
- Notes to Financial Statements
2. Financial Statement Schedule
The following financial statement schedule is filed as part of this Annual Report on Form 10-K. Schedules not listed below have been omitted because they are not applicable, not required or the information required is shown in the financial statements or the notes thereto.
100
SCHEDULE II
Cubist Pharmaceuticals, Inc.
Valuation and Qualifying Accounts and Reserves
Years Ended December 31, 2007, 2006 and 2005
Description
| | Balance at
Beginning
of Year
| | Additions
| | Deductions
| | Balance at
End of Year
|
|---|
| | (in thousands)
|
|---|
| Sales Returns & Allowances, Chargebacks, Prompt Pay Discounts, Wholesaler Fees and Rebates (1) | | | | | | | | | | |
| Year Ended December 31, 2007 | | $ | 3,418 | | 14,055 | | (12,989 | ) | $ | 4,484 |
| Year Ended December 31, 2006 | | $ | 1,554 | | 9,140 | | (7,276 | ) | $ | 3,418 |
| Year Ended December 31, 2005 | | $ | 775 | | 4,297 | | (3,518 | ) | $ | 1,554 |
- (1)
- Additions to sales returns and allowances, chargebacks, prompt pay discounts, wholesaler fees and rebates are recorded as a reduction of revenue.
101
3. List of Exhibits
| 3.1 | | Amended and Restated Certificate of Incorporation (Exhibit 3.1, Cubist's Quarterly Report on Form 10-Q, filed on August 6, 2004, File No. 000-21379) |
| 3.2 | | Certificate of Amendment to the Amended and Restated Certificate of Incorporation (Exhibit 3.1, Quarterly Report on Form 10-Q, filed on August 3, 2007, File No. 000-21379) |
| 3.3 | | Amended and Restated By-Laws of Cubist, as amended to date (Exhibit 3.1, Current Report on Form 8-K, filed December 26, 2007, File No. 000-21379) |
| 4.1 | | Specimen certificate for shares of Common Stock (Exhibit 4.1, Annual Report on Form 10-K, filed on March 1, 2006, File No. 000-21379) |
| 4.2 | | Rights Agreement, dated as of July 21, 1999, between Cubist and BankBoston, N.A., as Rights Agent (Exhibit 4.1, Current Report on Form 8-K filed on August 5, 2005, File No. 000-21379) |
| 4.3 | | First Amendment, dated as of March 3, 2000, to the Rights Agreement between Cubist and Fleet National Bank (f/k/a BankBoston, N.A.), as Rights Agent, dated as of July 21, 1999 (Exhibit 4.2, Current Report on Form 8-K filed on August, 5, 2005, File No. 000-21379) |
| 4.4 | | Amendment, dated as of March 20, 2002, to the Rights Agreement between Cubist and EquiServe Trust Company, N.A. (f/k/a Fleet National Bank f/k/a BankBoston, N.A.), as Rights Agent, dated as of July 21, 1999 (Exhibit 4.3, Current Report on Form 8-K filed on August 5, 2005, File No. 000-21379) |
| 4.5 | | Third Amendment, dated as of August 2, 2005, to the Rights Agreement between Cubist and EquiServe Trust Company, N.A. (f/k/a Fleet National Bank f/k/a BankBoston, N.A.), as Rights Agent, dated as of July 21, 1999 (Exhibit 4.4, Current Report on Form 8-K filed on August 5, 2005, File No. 000-21379) |
| 4.6 | | Indenture, dated as of June 6, 2006, between Cubist and The Bank of New York Trust Company, N.A., as trustee (Exhibit 4.1, Current Report on Form 8-K filed on June 9, 2006, File No. 000-21379) |
| 4.7 | | Note, dated June 6, 2006 (Exhibit 4.7, Annual Report on Form 10-K filed on March 1, 2007, File No. 000-21379) |
| **10.1 | | Amended and Restated 1993 Stock Option Plan (Exhibit 10.6, Pre-effective Amendment No. 1 to Form S-1 Registration Statement filed on July 31, 1996, File No. 333-6795) |
| **10.2 | | First Amendment to Amended and Restated 1993 Stock Option Plan (Exhibit 10.3, Quarterly Report on Form 10-Q, filed August 12, 1998, File No. 000-21379) |
| **10.3 | | 1997 Employee Stock Purchase Plan (Exhibit 10.4, Quarterly Report on Form 10-Q, filed August 12, 1998, File No. 000-21379) |
| **10.4 | | Second Amendment to Amended and Restated 1993 Stock Option Plan (Exhibit 10.41, Annual Report on Form 10-K, filed March 10, 2000, File No. 000-21379) |
| **10.5 | | Third Amendment to Amended and Restated 1993 Stock Option Plan (Exhibit 10.42, Annual Report on Form 10-K, filed March 10, 2000, File No. 000-21379) |
| †10.6 | | Development and Supply Agreement, dated April 3, 2000, by and between Cubist and Abbott Laboratories (currently known as Hospira Worldwide, Inc., or Hospira) (Exhibit 10.2, Quarterly Report on Form 10-Q, filed August 9, 2006, File No. 000-21379) |
| †10.7 | | Assignment and License Agreement, dated October 6, 2000, by and between Eli Lilly & Company, or Eli Lilly, and Cubist (Exhibit 10.59, Annual Report on Form 10-K, filed April 2, 2001, File No. 000-21379) |
102
| **10.8 | | Fourth Amendment to Amended and Restated 1993 Stock Option Plan (Exhibit 10.73, Annual Report on Form 10-K, filed April 2, 2001, File No. 000-21379) |
| **10.9 | | Fifth Amendment to Amended and Restated 1993 Stock Option Plan (Exhibit 10.74, Annual Report on Form 10-K, filed April 2, 2001, File No. 000-21379) |
| **10.10 | | Sixth Amendment to Amended and Restated 1993 Stock Option Plan (Exhibit 10.75, Annual Report on Form 10-K, filed April 2, 2001, File No. 000-21379) |
| **10.11 | | Seventh Amendment to Amended and Restated 1993 Stock Option Plan (Exhibit 10.62, Annual Report on Form 10-K, filed March 29, 2002, File No. 000-21379) |
| †10.12 | | Manufacturing and Supply Agreement, entered into as of September 30, 2001, by and between ACS Dobfar S.p.A., or ACS, and Cubist (Exhibit 10.63, Annual Report on Form 10-K, filed March 29, 2002, File No. 000-21379) |
| **10.13 | | Amended and Restated 2000 Equity Incentive Plan (Exhibit 10.1, Quarterly Report on Form 10-Q, filed August 8, 2002, File No. 000-21379) |
| †10.14 | | Amendment No. 2, dated as of February 12, 2003, to the Manufacturing and Supply Agreement by and between ACS and Cubist, entered into as of September 30, 2001 (Exhibit 10.67, Annual Report on Form 10-K, filed March 28, 2003, File No. 000-21379) |
| 10.15 | | Form of Employee Confidentiality Agreement (Exhibit 10.69, Annual Report on Form 10-K, filed March 28, 2003, File No. 000-21379) |
| 10.16 | | Amendment No. 1, dated July 1, 2003, to the Assignment and License Agreement between Cubist and Eli Lilly, dated October 6, 2000 (Exhibit 10.2, Quarterly Report on Form 10-Q, filed August 14, 2003, File No. 000-21379) |
| †10.17 | | License Agreement, dated as of October 2, 2003, by and between Cubist, Chiron Healthcare Ireland Ltd. and Chiron Corporation (Exhibit 10.1, Quarterly Report on Form 10-Q, filed May 4, 2007, File No. 000-21379) |
| 10.18 | | Lease, dated January 2004, between the California State Teachers' Retirement System, or CALSTERS, and Cubist regarding 55 Hayden Avenue (Exhibit 10.1, Quarterly Report on Form 10-Q filed on May 7, 2004, File No. 000-21379) |
| †10.19 | | Amendment #1, dated April 1, 2004, to the License Agreement by and between Cubist, Chiron Healthcare Ireland, Ltd. and Chiron Corporation, dated October 2, 2003 (Exhibit 10.2, Quarterly Report on Form 10-Q filed on August 6, 2004, File No. 000-21379) |
| †10.20 | | Processing Services Agreement entered into as of August 11, 2004 by and between Cardinal Health PTS, LLC, or Cardinal, and Cubist (Exhibit 10.3, Quarterly Report on Form 10-Q filed on November 4, 2005, File No. 00021379) |
| | 10.21 | | Amendment No. 2, dated March 31, 2005, to the Assignment and License Agreement between Cubist and Eli Lilly, dated October 6, 2000 (Exhibit 10.1, Quarterly Report on Form 10-Q, filed May 5, 2005, File No. 000-21379) |
| **10.22 | | First Amendment to Amended and Restated 2000 Equity Incentive Plan (Exhibit 10.1, Current Report on Form 8-K filed on August 5, 2005, File No. 000-21379) |
| 10.23 | | First Amendment, dated September 29, 2005, to Lease by and between Cubist and The Realty Associates Fund VI, L.P., or RA, successor-in-interest to CALSTERS, dated January 2004 (Exhibit 10.7, Quarterly Report on Form 10-Q filed on November 4, 2005, File No. 000-21379) |
103
| †10.24 | | Amendment No. 3, dated as of October 20, 2005, to the Manufacturing and Supply Agreement by and between ACS and Cubist, entered into as of September 30, 2001 (Exhibit 10.2, Quarterly Report on Form 10-Q filed on November 4, 2005, File No. 000-21379) |
| 10.25 | | Second Amendment, entered into as of November 18, 2005, to Lease by and between RA and Cubist, dated January 2004 |
| †10.26 | | First Amendment, dated as of June 1, 2006, to Development and Supply Agreement by and between Cubist and Hospira, entered into as of April 3, 2000 (Exhibit 10.1, Quarterly Report on Form 10-Q filed on August 9, 2006, File No. 000-21379) |
| *10.27 | | Amendment No. 4, dated as of September 22, 2006, to the Manufacturing and Supply Agreement by and between ACS and Cubist, entered into as of September 30, 2001 (Exhibit 10.1, Quarterly Report on Form 10-Q filed on November 3, 2006, File No. 000-21379) |
| *10.28 | | Amendment No. 2, dated April 18, 2007, to the Processing Services Agreement by and between Cardinal and Cubist, entered into as of August 11, 2004 (Exhibit 10.3, Quarterly Report on Form 10-Q filed on August 3, 2007, File No. 000-21379) |
| **10.29 | | Amended and Restated 1997 Employee Stock Purchase Plan (Appendix B, Definitive Proxy Statement on Form DEF-14A filed on April 26, 2007, File No. 000-21379) |
| **10.30 | | Amended and Restated 2002 Directors' Equity Incentive Plan (Appendix C, Definitive Proxy Statement on Form DEF-14A filed on April 26, 2007, File No. 000-21379) |
| 10.31 | | Third Amendment, entered into as of June 28, 2007, to Lease by and between RA and Cubist, dated January 2004 (Exhibit 10.4, Quarterly Report on Form 10-Q filed on August 3, 2007, File No. 000-21379) |
| **10.32 | | Retention Letter, dated October 9, 2007, by and between Cubist and Michael J. Bonney (Exhibit 10.1, Quarterly Report on Form 10-Q filed on November 2, 2007, File No. 000-21379) |
| **10.33 | | Form of Retention Letter by and between Cubist and Christopher D. T. Guiffre, David W.J. McGirr, and Robert J. Perez (Exhibit 10.2, Quarterly Report on Form 10-Q filed on November 2, 2007, File No. 000-21379) |
| 10.34 | | Fourth Amendment, entered into as of October 25, 2007, to Lease by and between RA and Cubist, dated January 2004 |
| **10.35 | | Short Term Incentive Plan Terms and Conditions (Exhibit 10.1, Current Report on Form 8-K filed on February 15, 2008, File No. 000-21379) |
| 10.36 | | Fifth Amendment, entered into as of December 18, 2007, to Lease by and between RA and Cubist, dated January 2004 |
| *10.37 | | Agreement and Plan of Merger, entered into as of December 24, 2007, by and between Edison Merger Corp., Illumigen Biosciences, Inc., IB Securityholders, LLC and Cubist |
| 14.1 | | Code of Conduct and Ethics |
| 23.1 | | Consent of PricewaterhouseCoopers LLP |
| 31.1 | | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | | Certification pursuant to 18 U.S.C Section 1305, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
104
| 32.2 | | Certification pursuant to 18 U.S.C Section 1305, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
Any of the above-listed Exhibits containing parenthetical information are incorporated by reference from the Company's filing indicated next to the title of such exhibit. All other above listed exhibits are filed herewith.
- †
- Confidential Treatment granted.
- *
- Confidential Treatment requested.
- **
- Management contract or compensatory plan or arrangement required to be filed as an exhibit to this annual report.
105
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act, the registrant has duly caused this amendment to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | CUBIST PHARMACEUTICALS, INC. |
|
|
By: |
/s/ MICHAEL W. BONNEY
Michael W. Bonney
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act, this report has been signed by the following persons in the capacities and on the dates indicated.
Signature
| | Title
| | Date
|
|---|
|
|
|
|
|
/s/ MICHAEL W. BONNEY
Michael W. Bonney | | President, Chief Executive Officer and Director (Principal Executive Officer) | | February 29, 2008 |
/s/ DAVID W.J. MCGIRR
David W.J. McGirr |
|
Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) |
|
February 29, 2008 |
/s/ KENNETH M. BATE
Kenneth M. Bate |
|
Director |
|
February 29, 2008 |
/s/ SYLVIE GRÉGOIRE
Sylvie Grégoire |
|
Director |
|
February 29, 2008 |
/s/ DAVID W. MARTIN, JR.
David W. Martin, Jr. |
|
Director |
|
February 29, 2008 |
/s/ WALTER R. MAUPAY, JR.
Walter R. Maupay, Jr. |
|
Director |
|
February 29, 2008 |
/s/ MARTIN ROSENBERG
Martin Rosenberg |
|
Director |
|
February 29, 2008 |
/s/ J. MATTHEW SINGLETON
J. Matthew Singleton |
|
Director |
|
February 29, 2008 |
/s/ MARTIN H. SOETERS
Martin H. Soeters |
|
Director |
|
February 29, 2008 |
/s/ MICHAEL B. WOOD
Michael B. Wood |
|
Director |
|
February 29, 2008 |
106
