UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
July 25, 2006
(Date of earliest event reported)
LABORATORY CORPORATION OF
AMERICA HOLDINGS
(Exact Name of Registrant as Specified in its Charter)
| | | | | |
| DELAWARE | | 1-11353 | | 13-3757370 |
| |
| |
|
(State or other jurisdiction
of Incorporation) | | (Commission
File Number) | | (I.R.S. Employer
Identification No.) |
| | | | | |
358 SOUTH MAIN STREET,
BURLINGTON, NORTH CAROLINA | | 27215 | | 336-229-1127 |
| |
| |
|
| (Address of principal executive offices) | | (Zip Code)
| | (Registrant's telephone number including area code) |
ITEM 7.01. Regulation FD Disclosure
Summary information of the Company dated July 25, 2006.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | |
| | Laboratory Corporation of America Holdings
(Registrant)
| |
| Date: July 25, 2006 | By: | /s/Bradford T. Smith | |
| | | Bradford T. Smith, Executive Vice President
and Secretary | |
| | | | |
| |

8-K Filed July 25, 2006

This slide presentation contains forward-looking
statements which are subject to change based on
various important factors, including without
limitation, competitive actions in the marketplace and
adverse actions of governmental and other third-party
payors. Actual results could differ materially from
those suggested by these forward-looking statements.
Further information on potential factors that could
affect the Company’s financial results is included in
the Company’s Form 10-K for the year ended
December 31, 2005, and subsequent filings.
2

The Clinical Laboratory Testing
Market - $40 billion Annually
Independent clinical lab share
is $16 billion
Represents 2% to 3% of all
health care spending
Influences /directs
approximately 80% of health
care spending
Rapidly evolving technology,
emphasis on preventative
medicine and aging of
population are all driving
growth
Has grown at a CAGR of
between 5% and 6%
Source: Company estimates, industry reports and 2005 revenue for LabCorp.
3

Profile of LabCorp
A leader in the esoteric and genomic testing
market and second-largest clinical laboratory
company in North America
Offers a broad range of routine and
esoteric/genomic tests
Conducts approximately 1.1 million tests daily on
more than 370,000 specimens
Provides lab services to physicians and other
health care providers
Approximately 24,000 employees nationwide
4

Primary Testing Locations
Primary LabCorp Testing Locations
Corporate Headquarters
Burlington, NC
5
LabCorp’s Strategy
To lead the industry in achieving long-
term growth and profitability by
strengthening our nationwide core
testing business and expanding our
higher-growth, higher-value esoteric and
genomic businesses.
6

Strategic Focus Areas
Scientific
Leadership
Managed
Care
Customer
Retention
-Licensing/partnerships
-Cancer
-Specimen tracking
-Call center consolidation
-Report improvement
-Acquisitions
-Appropriate prices
-Reduce leakage
-Value of new lab tests
-Customer connectivity
7

LabCorp’s Investment and
Performance Fundamentals
History of Strong Financial
Performance
Significant Cash Generator
Industry leading EBITDA margins
Strong Balance Sheet
Investment Grade Credit Ratings
8

Net Sales (in millions)
9

EBITDA Margin
(1) Excluding the impact in 2005 of restructuring and other special charges, and a non-recurring investment loss.
(1)
10

EPS
(1) Excluding the $0.09 per diluted share impact in 2005 of restructuring and other special charges, and a non-recurring investment loss.
(1)
11

Operating Cash Flow (in millions)
(1) Includes approximately $50 million of benefit from one-time tax credits recorded in 2003.
(1)
12
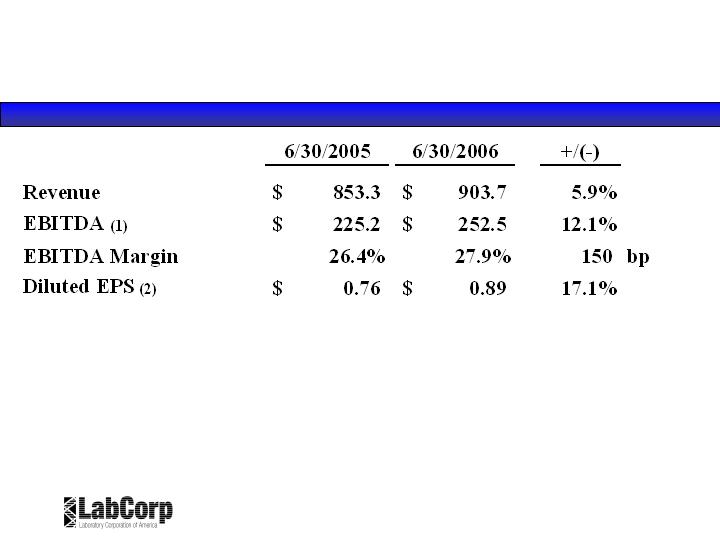
Second Quarter Results (in millions, except per share data)
(1) Excludes a $3.1 million non-recurring investment loss recorded by the Company in the second quarter of 2005, and $5.4 million of
stock compensation expense recorded by the Company for the three months ended June 30, 2006 from the adoption of SFAS 123(R).
(2) Excluding the $0.02 per diluted share impact of the non-recurring investment loss in 2005, and the $0.02 per diluted share impact of
the required change in accounting for stock based compensation adopted in 2006.
13

Six-Month Results (in millions, except per share data)
(1) Excludes a $3.1 million non-recurring investment loss recorded by the Company in the second quarter of 2005, and $11.3 million of
stock compensation expense recorded by the Company for the three months ended June 30, 2006 from the adoption of SFAS 123(R).
(2) Excluding the $0.02 per diluted share impact of the non-recurring investment loss in 2005, and the $0.05 per diluted share impact of
the required change in accounting for stock based compensation adopted in 2006.
14
2006 Six-Month Financial
Achievements
Diluted EPS of $1.67 (1)
EBITDA margin of 27.1% of sales (2)
Operating cash flow of $301.1 million
Increased revenues 7.9% (3.2% volume; 4.7% price)
Repurchased approximately $185 million of
LabCorp stock
(1)
Excluding the $0.05 per diluted share impact of the required change in accounting for stock based compensation.
(2)
Based on EBITDA of $482.2 million, excluding $11.3 million impact of change in accounting for stock based
compensation.
15

Financial Performance
Price & Volumes: Trends by Payer Type
16

Financial Performance
Revenue Analysis by Business Area
% Accns
Accns
17

Free Cash Flow Investment Strategy
Acquisitions
Stock repurchase program
Retain flexibility in utilizing remaining
cash
18

2006 Financial Guidance
Excluding the impact of the required change in accounting for stock based
compensation, any share repurchase activity after June 30, 2006, and any
accounting impact related to the previously announced retirement of the Chief
Executive Officer of the Company, guidance for 2006 is as follows:
Revenue growth of approximately 6.5% to 7.2% compared to 2005.
EBITDA margins of 26.5 to 27.0% of revenues.
Diluted EPS in the range of $3.28 to $3.33.
Operating cash flow of between $610 and $630 million.
Capital expenditures of between $95 and $110 million.
Net interest expense of between $43 and $45 million.
Bad debt rate of approximately 4.8% of sales for the remainder of the year.
We estimate that the implementation of the required change in accounting for
stock based compensation will have an EBITDA impact of approximately $22
million to $23 million and a diluted EPS impact of approximately $0.10.
19
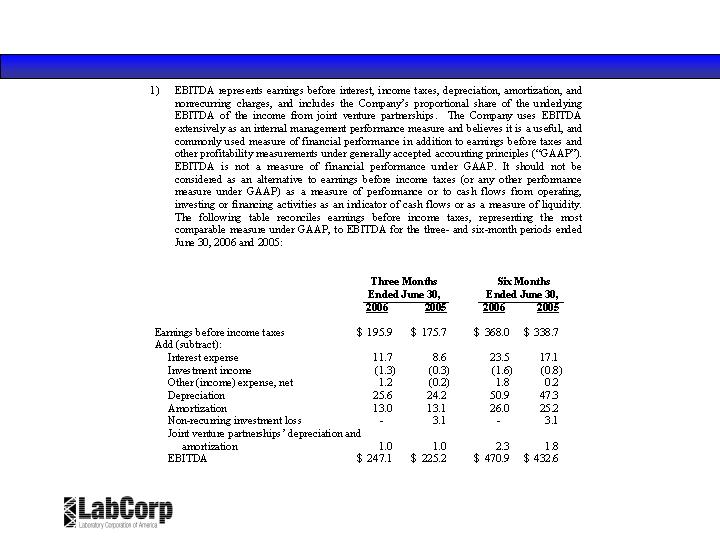
Reconciliation of Non-GAAP Financial Measures ($ in millions)
20

Supplemental Financial Information
21





















