
July 2015 Developing Innovative Therapies for Patients Suffering from Life-threatening Diseases NASDAQ: LJPC

Forward-Looking Statements These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking terminology such as "anticipate", "believe", "continue", "could", "estimate", "expect", "intend", "may", "might", "plan", "potential", "predict", "should" or "will" and include statements regarding La Jolla Pharmaceutical’s product candidates and clinical trial progress and results. These forward-looking statements are based on our current expectations and beliefs, speak only as of the date of this presentation and involve risks and uncertainties, many of which are outside of our control, that can cause actual results to differ materially from those anticipated in the forward-looking statements. Potential risks and uncertainties include, but are not limited to, our ability to commence and complete clinical trials within projected time periods, anticipated regulatory and patent exclusivity periods, the ability to manufacture clinical or commercial product successfully, the ability to resolve issues arising in the regulatory process, the ability to out-license programs, estimated market sizes and anticipated pricing levels for drug candidates, anticipated rates of physician adoption, if our drug candidates are approved, the ability to successfully develop our product candidates, including the results of ongoing and future clinical trials (including product safety issues and efficacy results) and the expected duration of the Company’s operating runway based on current cash resources. Further information is included in La Jolla Pharmaceutical’s periodic reports filed with the U.S. Securities and Exchange Commission at www.sec.gov. We disclaim any intent to update any forward- looking statements to reflect actual events that occur after the date of this presentation. 2

Overview of LJPC LJPC-501 (Angiotensin II) for CRH LJPC-401 (Hepcidin) for HH LJPC-30Sa/b (Gentamicin Derivatives) for Bacterial Infections and Rare Genetic Disorders Financial Position

Mission Statement La Jolla is dedicated to improving the lives of patients suffering from life- threatening diseases by discovering and developing innovative therapies 4

LJPC Corporate Highlights • Focused on de-risked product opportunities Naturally occurring peptides with well-understood biological functions Derivative components of FDA-approved products • LJPC-501 (angiotensin II) for catecholamine-resistant hypotension (CRH) Phase 3 registration study actively enrolling – SPA agreement with FDA in place – Data expected end of 2016 • LJPC-401 (hepcidin) for hereditary hemochromatosis (HH) Phase 1 data in 2015 • LJPC-30Sa/b (gentamicin derivatives) for bacterial infections & rare genetic disorders Plan to initiate Phase 1 study, following positive pre-IND meeting 5

Product Pipeline LJPC-501 Angiotensin II LJPC-401 Hepcidin Other R&D Indication IND Phase 1 Phase 2 Phase 3 UnderwayCompleted Planned HRS CRH End 2015 Various H2 2016 HH Phase 1/2 Mid- 2015 LJPC-30Sa & LJPC-30Sb Gentamicin Derivatives Bacterial Infections Rare Genetic Disorders Galectin-3 Inhibitor Program Various Out-licensing Q1 2015 Successful Pre-IND Meeting 6

Overview of LJPC LJPC-501 (Angiotensin II) for CRH LJPC-401 (Hepcidin) for HH LJPC-30Sa/b (Gentamicin Derivatives) for Bacterial Infections and Rare Genetic Disorders Financial Position

LJPC-501: Overview • LJPC-501 is a proprietary formulation of angiotensin II, a naturally occurring regulator of blood pressure • Catecholamine-resistant hypotension (CRH) is an acute, life-threatening condition in which blood pressure drops to dangerously low levels and is unresponsive to current treatments • LJPC-501 has been shown to raise blood pressure in a pilot RPC clinical trial in CRH, as well as animal models of hypotension • Special Protocol Assessment (SPA) agreement reached with FDA Agreement reached that blood pressure can be the primary endpoint for approval • Phase 3 trial actively enrolling • Multiple points of potential proprietary protection Potential regulatory exclusivity Proprietary formulation developed, LJPC patent applications filed, patent applications licensed from George Washington University 8

Three Systems Work in Harmony to Regulate Blood Pressure Current therapeutic options for the treatment of acute hypotension only leverage the adrenal system and vasopressin system The Body’s Three Systems that Regulate Blood Pressure 9

High Doses of Catecholamines Increase Mortality 1Sviri et al, J. of Crit Care, 29;157-160, 2014 • Catecholamines (i.e., norepinephrine, epinephrine and dopamine) cause cardiac toxicity, digital necrosis and metabolic complications leading to higher mortality1 0 10 20 30 40 50 60 70 80 90 Low Dose Hi Dose Alive DeadP er ce nt ag e Norepinephrine Dose • Blocking the cardiac toxicity of norepinephrine improves outcome2 High Dose 2Morelli et al, JAMA Oct23/30, 310:1683-1691, 2013 Mortality Rates in ICU Based on Norepinephrine Dose Size1 10

Norepinephrine Dose Decreases with Angiotensin II Surrogate Effect on Blood Pressure Source: Chawla et al, Critical Care, 18:534, 2014 0 5 10 15 20 25 30 35 40 45 50 Pre2 Pre1 Hr0 Hr1 Hr2 Hr3 Hr4 Hr5 Hr6 Post1 Post2 N or ep in ep hr in e D os e (m cg /m in ) Placebo AT-II Arm Angio dose placebo group p<0.05 angiotensin II group angiotensin II dose Catecholamine Resistance • Randomized, placebo-controlled, double-blind pilot trial • Primary efficacy endpoint: Catecholamine dose sparing; surrogate for BP effect • Published October 2014 in Critical Care • Strong proof-of- concept that angiotensin II increases blood pressure in CRH 100% of Angiotensin II-Treated Patients Experienced an Increase in BP 11

LJPC-501: Phase 3 Trial in CRH SPA Agreement with FDA Reached, Trial Enrolling • ATHOS (Angiotensin II for the Treatment of High-Output Shock) 3 trial initiated in March 2015 • Randomized, placebo-controlled, double-blind Phase 3 trial • Patient population: catecholamine-resistant, based on amount of catecholamine required • Primary endpoint: blood pressure at 3 hours • Secondary endpoint: change in CV SOFA* score • Size: 300 patients, 25-35 sites • Projected results: end of 2016 *Cardiovascular Sequential Organ Failure Assessment 12
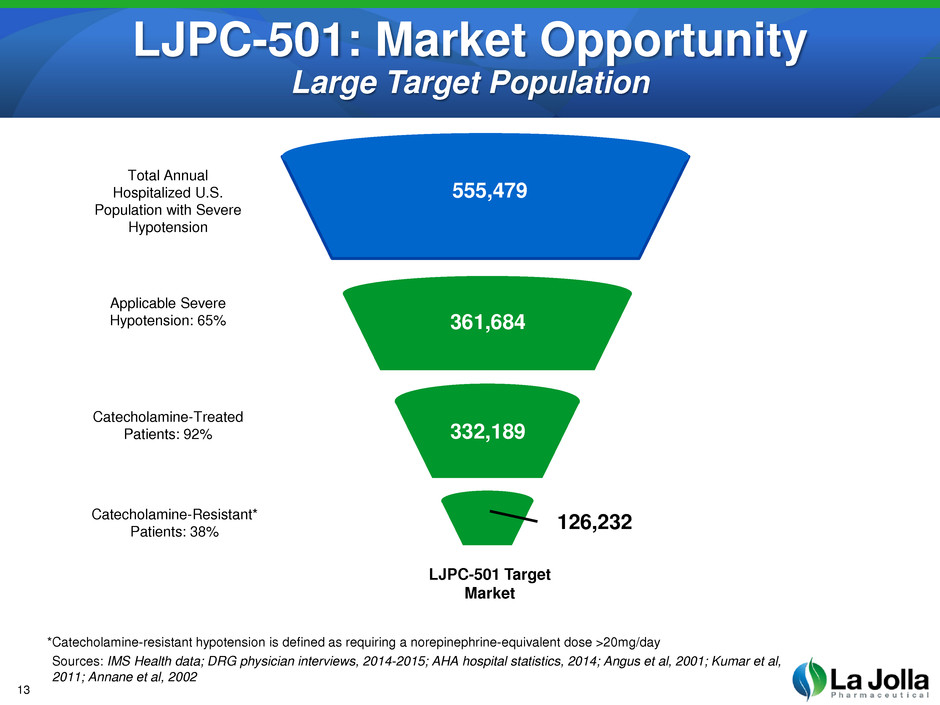
LJPC-501: Market Opportunity Large Target Population Total Annual Hospitalized U.S. Population with Severe Hypotension Applicable Severe Hypotension: 65% Catecholamine-Treated Patients: 92% Catecholamine-Resistant* Patients: 38% LJPC-501 Target Market 555,479 361,684 332,189 126,232 *Catecholamine-resistant hypotension is defined as requiring a norepinephrine-equivalent dose >20mg/day Sources: IMS Health data; DRG physician interviews, 2014-2015; AHA hospital statistics, 2014; Angus et al, 2001; Kumar et al, 2011; Annane et al, 2002 13

LJPC-501: Market Opportunity Attractive Pricing Potential Prices of Hospital-Based Drugs Reimbursed under DRGs Source: Wolters Kluwer PriceRx Pro, product package inserts, IMS Health inpatient/outpatient hospital charge master Catecholamine-Resistant* Catecholamine-Responsive Average total cost per patient $60,668 $53,555 Difference $7,113 Difference in Hospital Costs for ICU Hypotensive Patients *Catecholamine-resistant hypotension is defined as requiring a norepinephrine-equivalent dose >20mg/day Drug Name Indication Cost per Treatment Course Panhematin Acute intermittent porphyria $20,356 Cubicin Complicated blood infection $11,917 Kalbitor Hereditary angioedema attack $11,910 Xigris Severe sepsis $11,188 Firazyr Hereditary angioedema attack $9,896 Berinert Hereditary angioedema attack $8,055 Activase Heart attack or pulmonary embolism $7,610 Activase Stroke $4,794 Cubicin Uncomplicated blood infection $4,086 Cubicin Skin infection $2,384 Precedex Sedation $1,481 14

LJPC-501: Market Opportunity Strong Interest Even among P&T Decision Makers Robust (N=100) survey of physicians, 50% of which are P&T decision makers, shows strong interest based on evidence in primary endpoint alone Source: IMS Health survey, 2015 Endpoints Achieved Scenario 1 Scenario 2 Scenario 3 Improvement in MAP X X X Improvement in urinary output X X Improvement in mortality X Physician Type Estimated Prescription Share of LJPC-501 All (n=100) 48% 48% 65% P&T committee members (n=50) 47% 47% 62% Non-P&T committee members (n=50) 48% 49% 67% 15

Summary of LJPC-501 • LJPC-501 is a proprietary formulation of angiotensin II, a naturally occurring regulator of blood pressure • Catecholamine-resistant hypotension (CRH) is an acute, life-threatening condition in which blood pressure drops to dangerously low levels and is unresponsive to current treatments • LJPC-501 has been shown to raise blood pressure in a pilot RPC clinical trial in CRH, as well as animal models of hypotension • Special Protocol Assessment (SPA) agreement reached with FDA Agreement reached that blood pressure can be the primary endpoint for approval • Phase 3 trial actively enrolling • Multiple points of potential proprietary protection Potential regulatory exclusivity Proprietary formulation developed, LJPC patent applications filed, patent applications licensed from George Washington University 16

Overview of LJPC LJPC-501 (Angiotensin II) for CRH LJPC-401 (Hepcidin) for HH LJPC-30Sa/b (Gentamicin Derivatives) for Bacterial Infections and Rare Genetic Disorders Financial Position

LJPC-401: Overview • LJPC-401 is a novel formulation of hepcidin, a naturally occurring regulator of iron absorption and distribution • Hereditary hemochromatosis (HH) is a disease characterized by a deficiency of hepcidin that results in excessive iron accumulation, which is toxic to vital organs such as the liver and heart Most common genetic disease in Caucasians Causes liver cirrhosis, liver cancer, heart disease and/or failure, dementia and diabetes • LJPC-401 has been shown to be effective at reducing serum iron in preclinical testing • Phase 1 trial to start in mid-2015 Results expected end of 2015, including serum iron results • Multiple points of potential proprietary protection Potential regulatory exclusivity and Orphan Drug Designation IP licensed from INSERM 18

Hepcidin: The Insulin of Iron Metabolism • Hepcidin: the insulin of iron metabolism Regulates iron absorption and disposition in all organs Rapid and sustained lowering of iron levels • Progress SQ formulation developed Completed toxicology • Phase 1 data in 2015 Including serum iron data 0 100 200 300 400 0 hr 4 hr 24 hr 48 hr Day 15 Se ru m Ir on (u g/ dL ) Time Placebo LJPC-401 GLP Toxicology Study in Rats 19

LJPC-401: Phase 1 Development Plan • Key endpoint includes effect on serum iron levels Early insight into LJPC-401’s efficacy using clinically relevant endpoint Study to start in mid-2015 – results will be available by end of 2015 OBJECTIVES: determine safety of escalating doses of LJPC-401 in NHV; evaluate PK; evaluate effect on iron levels • Population: normal healthy volunteers • Iron studies: • serum iron; ferritin; transferrin • TIBC; UIBC Cohorts of escalating doses 3 to 6 subjects each Cohort 4 12 subjects N = 21 to 30 4 Co ho rt s Es ca la ti ng D os es Duration of Study: single dose 20

LJPC-401: Market Overview • Hereditary Hemochromatosis (HH) Most common genetic disease in Caucasians Silent Killer - Iron accumulation can lead to liver cirrhosis, liver cancer, heart disease and/or failure, dementia and diabetes No FDA-approved treatment Current treatments don’t address the underlying disease pathology and/or can have lethal side effects – Iron chelators may cause kidney failure, liver failure or gastrointestinal hemorrhage – Phlebotomy creates heavy patient burden with weekly procedures for >1 year Significantly underdiagnosed despite simple, inexpensive and readily available genetic and serum iron tests ~250,000 people in U.S. have clinically significant iron overload due to HH • Acquired Iron Overload (e.g., beta thalassemia) Attractive treatment alternative for beta thalassemia patient in lieu of iron chelator 21

Overview of LJPC LJPC-501 (Angiotensin II) for CRH LJPC-401 (Hepcidin) for HH LJPC-30Sa/b (Gentamicin Derivatives) for Bacterial Infections and Rare Genetic Disorders Financial Position

LJPC-30Sa/b: Overview • LJPC-30Sa and LJPC-30Sb are purified derivatives of gentamicin, which retain biologic activity but lack traditional kidney toxicity • Gentamicin: FDA-approved, standard-of-care for serious Gram-negative bacterial infections Mixture of several distinct but closely related chemical entities >3 million vials of gentamicin used in the U.S. in 2014 Use is limited due to kidney toxicity, which is believed to be associated only with certain constituent components • Two parallel development paths Bacterial infections: aminoglycosides = $500+ million market in the U.S.* Rare genetic disorders: gentamicin’s mechanism may be leveraged for rare genetic disorders; proof-of-concept data exists in cystic fibrosis • Recent positive FDA feedback on Phase 1 proposal • Multiple points of potential proprietary protection Potential regulatory exclusivity and Orphan Drug Designation Antibiotic exclusivity: 8+ years including Hatch-Waxman + GAIN (QIDP) IP optioned from IU and UAB *Adjusted for branded pricing of comparable hospital antimicrobials 23

Next-generation improved gentamicin derivative 1. Retain activity LJPC-30Sa/b: Potential for Improved Clinical Profile 2. Improve safety 0.0 4.0 8.0 12.0 16.0 placebo next-generation gentamicin derivative gentamicin In hi bi tio n/ ki ll zo ne (m m ) B.subtilus K.pneumonieae (kill) K.pneumonieae (inhib) 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 S er um C re at in in e m g/ dL placebo next- generation gentamicin derivative gentamicin 24

Gentamicin induces errors in the translation of genes into proteins Bacteria LJPC-30Sa/b: Mechanism of Action Can Be Leveraged Down Two Development Paths Alters protein synthesis leading to cell death Alters protein synthesis to allow read through of stop-codon mutations Humans 25

LJPC-30Sa/b: Market Opportunity • Antibiotic opportunity = $500+ million per year in U.S. Market could expand with a safer alternative – Increased duration of therapy, increased penetration, and/or new indications • Other large potential market opportunity in rare genetic disorders, such as cystic fibrosis Source: Source Healthcare Analytics All Aminoglycosides Gentamicin Cubicin U.S. Aminoglycoside Market (2014) - 1,000,000 2,000,000 3,000,000 4,000,000 5,000,000 6,000,000 7,000,000 # of Vials - 500,000 1,000,000 1,500,000 2,000,000 2,500,000 3,000,000 Days of Treatment 26

LJPC-30Sa/b: Clinical Proof-of-Concept in Cystic Fibrosis References: Sermet-Gaudelus et al, BMC Medicine, 5:5, 2007; Wilchanski et al, NEJM, 349:1433-41, 2003; Clancy J.P. et al, Am J Respir Crit Care Med, 163:1683-1692, 2001 • Three, independent studies suggest gentamicin helps read through stop-codon mutations in cystic fibrosis Study of 10 mg/kg IV gentamicin over 15 days in Y122X mutations leads to: Improvement in cystic fibrosis clinical scores (p=0.007) – Improvements seen as early as day 4 Improvement in lung function (FEV1) independent of an antimicrobial effect (p=0.04) Improvement in sweat chloride secretion (p=0.03) and nasal potential difference (p=0.04) • Dose-dependent effect suggests LJPC-30Sa/b could allow chronic dosing with better efficacy and no kidney toxicity Sweat Chloride Secretion Improvement after Gentamicin 27

Overview of LJPC LJPC-501 (Angiotensin II) for CRH LJPC-401 (Hepcidin) for HH LJPC-30Sa/b (Gentamicin Derivatives) for Bacterial Infections and Rare Genetic Disorders Financial Position

Financial Position Cash resources expected to fund Company through 2016 Condensed Balance Sheet Data As of Mar 31, 2015 (in millions) Cash $42.7 Total liabilities $2.6 Total shareholders’ equity $43.0 Fully Diluted, As-Converted Shares Outstanding* 24,019,500 *Includes common stock, preferred stock & outstanding equity awards as of March 31, 2015 29

LJPC Investment Summary • Focused on de-risked product opportunities Naturally occurring peptides with well-understood biological functions Derivative components of FDA-approved products • LJPC-501 (angiotensin II) for catecholamine-resistant hypotension (CRH) Phase 3 registration study actively enrolling – SPA agreement with FDA in place – Data expected end of 2016 • LJPC-401 (hepcidin) for hereditary hemochromatosis (HH) Phase 1 data in 2015 • LJPC-30Sa/b (gentamicin derivatives) for bacterial infections & rare genetic disorders Plan to initiate Phase 1 study, following positive pre-IND meeting 30

Thank You






























