
July 2015 LJPC-501 Market Opportunity NASDAQ: LJPC

Forward-Looking Statements These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking terminology such as "anticipate", "believe", "continue", "could", "estimate", "expect", "intend", "may", "might", "plan", "potential", "predict", "should" or "will" and include statements regarding La Jolla Pharmaceutical’s product candidates and clinical trial progress and results. These forward-looking statements are based on our current expectations and beliefs, speak only as of the date of this presentation and involve risks and uncertainties, many of which are outside of our control, that can cause actual results to differ materially from those anticipated in the forward-looking statements. Potential risks and uncertainties include, but are not limited to, our ability to commence and complete clinical trials within projected time periods, anticipated regulatory and patent exclusivity periods, estimated market sizes and anticipated pricing levels for drug candidates, anticipated rates of physician adoption, if our drug candidates are approved, and the ability to successfully develop our product candidates, including the results of ongoing and future clinical trials (including product safety issues and efficacy results). Further information is included in La Jolla Pharmaceutical’s periodic reports filed with the U.S. Securities and Exchange Commission at www.sec.gov. We disclaim any intent to update any forward-looking statements to reflect actual events that occur after the date of this presentation. 2

LJPC-501 Market Opportunity 3 • LJPC-501, La Jolla’s lead product candidate for catecholamine-resistant hypotension (CRH), is actively enrolling in a Phase 3 trial • Large market opportunity More than 125,000 patients suffer from CRH each year in US Attractive pricing potential • Positive feedback from in-depth, physician market research High unmet medical need for LJPC-501, as physicians are not satisfied with currently available treatment options Strong adoption in CRH predicted by physicians, including P&T committee members, based on evidence in primary endpoint alone Indication of interest in earlier-stage use (e.g., first-line) of LJPC-501 for treatment of severe hypotension Sources: IMS Health survey, 2015; DRG physician interviews, 2014-2015

LJPC-501: Overview 4 • LJPC-501 is a proprietary formulation of angiotensin II, a naturally occurring regulator of blood pressure • CRH is an acute, life-threatening condition in which blood pressure drops to dangerously low levels and is unresponsive to current treatments • LJPC-501 has been shown to raise blood pressure in a pilot RPC clinical trial in CRH, as well as animal models of hypotension • Special Protocol Assessment (SPA) agreement reached with FDA Agreement reached that blood pressure can be the primary endpoint for approval • Phase 3 trial actively enrolling • Multiple points of potential proprietary protection Potential regulatory exclusivity Proprietary formulation developed, LJPC patent applications filed, patent applications licensed from George Washington University
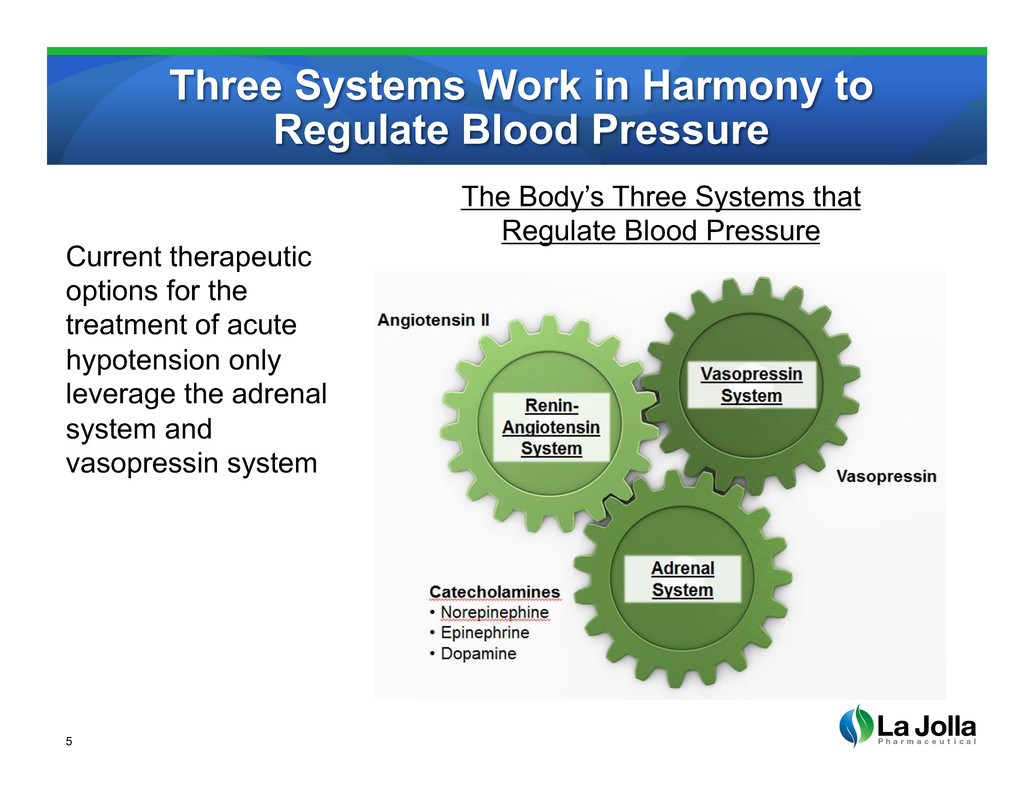
Three Systems Work in Harmony to Regulate Blood Pressure 5 Current therapeutic options for the treatment of acute hypotension only leverage the adrenal system and vasopressin system The Body’s Three Systems that Regulate Blood Pressure

High Doses of Catecholamines Increase Mortality 6 1Sviri et al, J. of Crit Care, 29;157-160, 2014 • Catecholamines (i.e., norepinephrine, epinephrine and dopamine) cause cardiac toxicity, digital necrosis and metabolic complications leading to higher mortality1 0 10 20 30 40 50 60 70 80 90 Low Dose Hi Dose Alive Dead P erc en ta ge Norepinephrine Dose • Blocking the cardiac toxicity of norepinephrine improves outcome2 High Dose 2Morelli et al, JAMA Oct23/30, 310:1683-1691, 2013 Mortality Rates in ICU Based on Norepinephrine Dose Size1

Norepinephrine Dose Decreases with Angiotensin II Surrogate Effect on Blood Pressure 7 Source: Chawla et al, Critical Care, 18:534, 2014 0 5 10 15 20 25 30 35 40 45 50 Pre2 Pre1 Hr0 Hr1 Hr2 Hr3 Hr4 Hr5 Hr6 Post1 Post2 N or ep in ep hr in e D os e (m cg /m in ) Placebo AT-II Arm Angio dose placebo group p<0.05 angiotensin II group angiotensin II dose Catecholamine Resistance • Randomized, placebo-controlled, double-blind pilot trial • Primary efficacy endpoint: Catecholamine dose sparing; surrogate for BP effect • Published October 2014 in Critical Care • Strong proof-of- concept that angiotensin II increases blood pressure in CRH 100% of Angiotensin II-Treated Patients Experienced an Increase in BP

LJPC-501: Phase 3 Trial in CRH SPA Agreement with FDA Reached, Trial Enrolling 8 • ATHOS (Angiotensin II for the Treatment of High-Output Shock) 3 trial initiated in March 2015 • Randomized, placebo-controlled, double-blind Phase 3 trial • Patient population: catecholamine-resistant, based on amount of catecholamine required • Primary endpoint: blood pressure at 3 hours • Secondary endpoint: change in CV SOFA* score • Size: 300 patients, 25-35 sites • Projected results: end of 2016 *Cardiovascular Sequential Organ Failure Assessment

9 • La Jolla recently engaged Decision Resources Group (DRG) and IMS Health (IMS) to conduct market research on the commercial opportunity of LJPC-501 • More than 125,000 patients suffer CRH, LJPC-501’s target market • Attractive pricing potential for LJPC-501 • Physicians, including P&T committee members, show strong interest in LJPC-501 based on evidence in primary endpoint alone ~87% indicated an urgent need for a rescue vasopressor >80% thought that a drug with LJPC-501’s profile could be a “Game Changer” Indication of interest in earlier-stage use (e.g., first-line) of LJPC-501 for treatment of severe hypotension LJPC-501: Market Opportunity Overview Sources: IMS Health data; DRG physician interviews, 2014-2015

10 LJPC-501: Market Opportunity Large Target Population Total Annual Hospitalized U.S. Population with Severe Hypotension Applicable Severe Hypotension: 65% Catecholamine-Treated Patients: 92% Catecholamine-Resistant* Patients: 38% LJPC-501 Target Market 555,479 361,684 332,189 126,232 *Catecholamine-resistant hypotension is defined as requiring a norepinephrine-equivalent dose >20mg/day Sources: IMS Health data; DRG physician interviews, 2014-2015; AHA hospital statistics, 2014; Angus et al, 2001; Kumar et al, 2011; Annane et al, 2002

LJPC-501: Market Opportunity Attractive Pricing Potential 11 Prices of Hospital-Based Drugs Reimbursed under DRGs Source: Wolters Kluwer PriceRx Pro, product package inserts, IMS Health inpatient/outpatient hospital charge master Catecholamine-Resistant* Catecholamine-Responsive Average total cost per patient $60,668 $53,555 Difference $7,113 Difference in Hospital Costs for ICU Hypotensive Patients *Catecholamine-resistant hypotension is defined as requiring a norepinephrine-equivalent dose >20mg/day Drug Name Indication Cost per Treatment Course Panhematin Acute intermittent porphyria $20,356 Cubicin Complicated blood infection $11,917 Kalbitor Hereditary angioedema attack $11,910 Xigris Severe sepsis $11,188 Firazyr Hereditary angioedema attack $9,896 Berinert Hereditary angioedema attack $8,055 Activase Heart attack or pulmonary embolism $7,610 Activase Stroke $4,794 Cubicin Uncomplicated blood infection $4,086 Cubicin Skin infection $2,384 Precedex Sedation $1,481

12 LJPC-501: Market Opportunity Physicians Not Satisfied with Current Treatment Options New Mechanisms of Action Needed Lack of Effective Treatment Options • There is a need for treatments that treat the underlying causes of severe hypotension, especially for heart failure patients Complications with Catecholamines • Physicians most frequently highlighted the current ineffective treatment options for CRH, as ~50% of patients are unresponsive to the first-line treatment for severe hypotension “[Catecholamines] are a necessary evil. They’re not great but are the only things we have to support patients through low cardiac output and the septic stage.” – Surgical Intensivist “Catecholamines are necessary and helpful when used as indicated, but they can have a lot of downsides. Some catecholamines cause arrhythmia,… some of them cause skin or digit[al] necrosis so tips don’t get enough blood and die.” – Surgical Intensivist “[Catecholamines] don’t work that well. It’s not very good medicine, but that’s the only thing available to us at the present time.” – Medical Intensivist • Catecholamines can cause tachycardia, hypoperfusion, necrosis, ischemia and arrhythmia Source: DRG physician interviews, 2014-2015 *Catecholamine-resistant hypotension is defined as requiring a norepinephrine-equivalent dose >20mg/day

13 LJPC-501: Market Opportunity Physicians See High Unmet Medical Need 67% 20% 9% 6% 5% 0% 20% 40% 60% 80% 100% Tachycardia Hypoperfusion Necrosis Ischemia Arrhythmia % P at ie nt s Commonly Reported Side Effects with Catecholamines Increasing Need High Need = 10 Low Need = 1 60% of physicians reported a need between 8-10 27% of physicians reported a need between 5-7 13% of physicians reported a need between 1-4 Physicians expressing need for new CRH agent Source: DRG physician interviews, 2014-2015 87%

14 • Physicians anticipate strong adoption of LJPC-501 in their practices Strong willingness to use in CRH based on evidence in primary endpoint alone Strong adoption predicted by P&T committee decision makers Earlier-stage use (e.g., first-line) for treatment of severe hypotension predicted by some physicians LJPC-501: Market Opportunity LJPC-501 May Be a “Game Changer” Based on evidence in primary endpoint alone, more than 80% of physicians thought LJPC-501 has the potential to be a “game changer” in the treatment of CRH >80% Source: DRG physician interviews, 2014-2015

LJPC-501: Market Opportunity Strong Interest Even among P&T Decision Makers 15 Robust (N=100) survey of physicians, 50% of which are P&T decision makers, shows strong interest based on evidence in primary endpoint alone Source: IMS Health survey, 2015 Endpoints Achieved Scenario 1 Scenario 2 Scenario 3 Improvement in MAP X X X Improvement in urinary output X X Improvement in mortality X Physician Type Estimated Prescription Share of LJPC-501 All (n=100) 48% 48% 65% P&T committee members (n=50) 47% 47% 62% Non-P&T committee members (n=50) 48% 49% 67%

16 • LJPC-501, La Jolla’s lead product candidate for catecholamine-resistant hypotension (CRH), is actively enrolling in a Phase 3 trial • Large market opportunity More than 125,000 patients suffer from CRH each year in US Attractive pricing potential • Positive feedback from in-depth, physician market research High unmet medical need for LJPC-501, as physicians are not satisfied with currently available treatment options Strong adoption in CRH predicted by physicians, including P&T committee members, based on evidence in primary endpoint alone Indication of interest in earlier-stage use (e.g., first-line) of LJPC-501 for treatment of severe hypotension Sources: IMS Health survey, 2015; DRG physician interviews, 2014-2015 LJPC-501: Market Opportunity Summary

Thank You
















