UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2021
OR
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____ to ____
Commission File Number: 1-36282
LA JOLLA PHARMACEUTICAL COMPANY
(Exact name of registrant as specified in its charter)
California | | 33-0361285 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
201 Jones Road, Suite 400, Waltham, MA | | 02451 |
(Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (617) 715-3600
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Common Stock, par value $0.0001 per share | | LJPC | | The Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | | ☐ | | Accelerated filer | | ☐ |
| | | | | | |
Non-accelerated filer | | ☒ | | Smaller reporting company | | ☒ |
| | | | | | |
| | | | Emerging growth company | | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of July 23, 2021, there were 27,488,137 shares of common stock outstanding.
TABLE OF CONTENTS
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
LA JOLLA PHARMACEUTICAL COMPANY
Condensed Consolidated Balance Sheets
(in thousands, except par value and share amounts)
| | June 30, | | | December 31, | |
| | 2021 | | | 2020 | |
| | (Unaudited) | | | | | |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 45,888 | | | $ | 21,221 | |
Accounts receivable, net | | | 8,596 | | | | 5,834 | |
Inventory, net | | | 5,481 | | | | 6,013 | |
Prepaid expenses and other current assets | | | 5,201 | | | | 3,388 | |
Total current assets | | | 65,166 | | | | 36,456 | |
Goodwill | | | 20,123 | | | | 20,123 | |
Intangible assets, net | | | 14,097 | | | | 14,873 | |
Right-of-use lease assets | | | 419 | | | | 536 | |
Property and equipment, net | | | 163 | | | | 215 | |
Restricted cash | | | 40 | | | | 40 | |
Total assets | | $ | 100,008 | | | $ | 72,243 | |
| | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ DEFICIT | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 1,846 | | | $ | 2,762 | |
Accrued expenses | | | 12,175 | | | | 6,494 | |
Accrued payroll and related expenses | | | 1,912 | | | | 2,878 | |
Lease liabilities, current portion | | | 168 | | | | 204 | |
Total current liabilities | | | 16,101 | | | | 12,338 | |
Deferred royalty obligation, net | | | 124,470 | | | | 124,437 | |
Accrued interest expense on deferred royalty obligation, less current portion | | | 22,136 | | | | 19,111 | |
Lease liabilities, less current portion | | | 251 | | | | 332 | |
Other noncurrent liabilities | | | 4,493 | | | | 4,112 | |
Total liabilities | | | 167,451 | | | | 160,330 | |
Commitments and contingencies (Note 6) | | | | | | | | |
Shareholders’ deficit: | | | | | | | | |
Common Stock, $0.0001 par value; 100,000,000 shares authorized, 27,482,231 and 27,402,648 shares issued and outstanding at June 30, 2021 and December 31, 2020, respectively | | | 3 | | | | 3 | |
Series C-12 Convertible Preferred Stock, $0.0001 par value; 11,000 shares authorized, 3,906 shares issued and outstanding at June 30, 2021 and December 31, 2020; and liquidation preference of $3,906 at June 30, 2021 and December 31, 2020 | | | 3,906 | | | | 3,906 | |
Additional paid-in capital | | | 987,249 | | | | 984,756 | |
Accumulated deficit | | | (1,058,601 | ) | | | (1,076,752 | ) |
Total shareholders’ deficit | | | (67,443 | ) | | | (88,087 | ) |
Total liabilities and shareholders’ deficit | | $ | 100,008 | | | $ | 72,243 | |
See accompanying notes to the condensed consolidated financial statements.
1
LA JOLLA PHARMACEUTICAL COMPANY
Condensed Consolidated Statements of Operations
(Unaudited)
(in thousands, except per share amounts)
| | Three Months Ended | | | Six Months Ended | |
| | June 30, | | | June 30, | |
| | 2021 | | | 2020 | | | 2021 | | | 2020 | |
Revenue | | | | | | | | | | | | | | | | |
Net product sales | | $ | 11,059 | | | $ | 5,805 | | | $ | 19,696 | | | $ | 13,396 | |
License revenue | | | 5,000 | | | | - | | | | 30,500 | | | | - | |
Total revenue | | | 16,059 | | | | 5,805 | | | | 50,196 | | | | 13,396 | |
Operating expenses | | | | | | | | | | | | | | | | |
Cost of product sales | | | 2,156 | | | | 808 | | | | 4,887 | | | | 1,524 | |
Cost of license revenue | | | - | | | | - | | | | 3,600 | | | | - | |
Selling, general and administrative | | | 8,996 | | | | 8,677 | | | | 17,751 | | | | 16,829 | |
Research and development | | | 1,114 | | | | 8,781 | | | | 2,672 | | | | 17,964 | |
Total operating expenses | | | 12,266 | | | | 18,266 | | | | 28,910 | | | | 36,317 | |
Income (loss) from operations | | | 3,793 | | | | (12,461 | ) | | | 21,286 | | | | (22,921 | ) |
Other (expense) income | | | | | | | | | | | | | | | | |
Interest expense | | | (2,672 | ) | | | (2,470 | ) | | | (5,281 | ) | | | (4,876 | ) |
Interest income | | | - | | | | 32 | | | | 2 | | | | 222 | |
Other income—related party | | | 2,532 | | | | - | | | | 2,532 | | | | 4,085 | |
Other income (expense) | | | 80 | | | | (693 | ) | | | (370 | ) | | | (693 | ) |
Total other (expense) income, net | | | (60 | ) | | | (3,131 | ) | | | (3,117 | ) | | | (1,262 | ) |
Income (loss) before income taxes | | | 3,733 | | | | (15,592 | ) | | | 18,169 | | | | (24,183 | ) |
Provision for income taxes | | | - | | | | - | | | | 18 | | | | - | |
Net income (loss) | | $ | 3,733 | | | $ | (15,592 | ) | | $ | 18,151 | | | $ | (24,183 | ) |
Earnings (loss) per share | | | | | | | | | | | | | | | | |
Basic | | $ | 0.14 | | | $ | (0.57 | ) | | $ | 0.66 | | | $ | (0.89 | ) |
Diluted | | $ | 0.11 | | | $ | (0.57 | ) | | $ | 0.53 | | | $ | (0.89 | ) |
Shares used in computing earnings (loss) per share | | | | | | | | | | | | | | | | |
Basic | | | 27,461 | | | | 27,326 | | | | 27,444 | | | | 27,282 | |
Diluted | | | 34,201 | | | | 27,326 | | | | 34,192 | | | | 27,282 | |
See accompanying notes to the condensed consolidated financial statements.
2
LA JOLLA PHARMACEUTICAL COMPANY
Condensed Consolidated Statements of Shareholders’ Deficit
(Unaudited)
(in thousands)
| | Series C-12 Convertible Preferred Stock | | | Common Stock | | | Additional Paid-in | | | Accumulated | | | Total Shareholders’ (Deficit) | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Equity | |
Balance at December 31, 2020 | | | 4 | | | $ | 3,906 | | | | 27,403 | | | $ | 3 | | | $ | 984,756 | | | $ | (1,076,752 | ) | | $ | (88,087 | ) |
Share-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 1,116 | | | | - | | | | 1,116 | |
Issuance of common stock under 2013 Equity Plan | | | - | | | | - | | | | 29 | | | | - | | | | 154 | | | | - | | | | 154 | |
Issuance of common stock under ESPP | | | - | | | | - | | | | 17 | | | | - | | | | 81 | | | | - | | | | 81 | |
Net income | | | - | | | | - | | | | - | | | | - | | | | - | | | | 14,418 | | | | 14,418 | |
Balance at March 31, 2021 | | | 4 | | | $ | 3,906 | | | | 27,449 | | | $ | 3 | | | $ | 986,107 | | | $ | (1,062,334 | ) | | $ | (72,318 | ) |
Share-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 1,024 | | | | - | | | | 1,024 | |
Issuance of common stock under 2013 Equity Plan | | | - | | | | - | | | | - | | | | - | | | | 1 | | | | - | | | | 1 | |
Issuance of common stock under ESPP | | | - | | | | - | | | | 33 | | | | - | | | | 117 | | | | - | | | | 117 | |
Net income | | | - | | | | - | | | | - | | | | - | | | | | | | | 3,733 | | | | 3,733 | |
Balance at June 30, 2021 | | | 4 | | | $ | 3,906 | | | | 27,482 | | | $ | 3 | | | $ | 987,249 | | | $ | (1,058,601 | ) | | $ | (67,443 | ) |
| | Series C-12 Convertible Preferred Stock | | | Common Stock | | | Additional Paid-in | | | Accumulated | | | Total Shareholders’ (Deficit) | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Equity | |
Balance at December 31, 2019 | | | 4 | | | $ | 3,906 | | | | 27,195 | | | $ | 3 | | | $ | 977,432 | | | $ | (1,037,331 | ) | | $ | (55,990 | ) |
Share-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 2,407 | | | | - | | | | 2,407 | |
Issuance of common stock under 2013 Equity Plan | | | - | | | | - | | | | 44 | | | | - | | | | 305 | | | | - | | | | 305 | |
Issuance of common stock under ESPP | | | - | | | | - | | | | 38 | | | | - | | | | 200 | | | | - | | | | 200 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (8,591 | ) | | | (8,591 | ) |
Balance at March 31, 2020 | | | 4 | | | $ | 3,906 | | | | 27,277 | | | $ | 3 | | | $ | 980,344 | | | $ | (1,045,922 | ) | | $ | (61,669 | ) |
Share-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 1,590 | | | | - | | | | 1,590 | |
Issuance of common stock under 2013 Equity Plan | | | - | | | | - | | | | 50 | | | | - | | | | 300 | | | | - | | | | 300 | |
Issuance of common stock under ESPP | | | - | | | | - | | | | 32 | | | | - | | | | 159 | | | | - | | | | 159 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (15,592 | ) | | | (15,592 | ) |
Balance at June 30, 2020 | | | 4 | | | $ | 3,906 | | | | 27,359 | | | $ | 3 | | | $ | 982,393 | | | $ | (1,061,514 | ) | | $ | (75,212 | ) |
See accompanying notes to the condensed consolidated financial statements.
3
LA JOLLA PHARMACEUTICAL COMPANY
Condensed Consolidated Statements of Cash Flows
(Unaudited)
(in thousands)
| | Six Months Ended | |
| | June 30, | |
| | 2021 | | | 2020 | |
Operating activities | | | | | | | | |
Net income (loss) | | $ | 18,151 | | | $ | (24,183 | ) |
Adjustments to reconcile net income (loss) to net cash provided by (used for) operating activities: | | | | | | | | |
Share-based compensation expense | | | 2,140 | | | | 3,997 | |
Depreciation expense | | | 56 | | | | 1,798 | |
Non-cash interest expense | | | 3,718 | | | | 3,392 | |
Inventory fair value step-up adjustment included in cost of product sales | | | 850 | | | | - | |
Amortization of intangible assets | | | 776 | | | | - | |
Loss on change in fair value of contingent value rights | | | 370 | | | | - | |
Amortization of right-of-use lease assets | | | 117 | | | | 699 | |
Loss on disposal of property and equipment | | | - | | | | 904 | |
Unrealized gains on short-term investments | | | - | | | | (63 | ) |
Changes in operating assets and liabilities: | | | | | | | | |
Accounts receivable, net | | | (2,762 | ) | | | 1,117 | |
Inventory, net | | | (318 | ) | | | (909 | ) |
Prepaid expenses and other current assets | | | (1,813 | ) | | | 1,675 | |
Accounts payable | | | (920 | ) | | | (1,696 | ) |
Accrued expenses | | | 5,032 | | | | (3,378 | ) |
Accrued payroll and related expenses | | | (966 | ) | | | (2,591 | ) |
Lease liabilities | | | (117 | ) | | | (1,357 | ) |
Net cash provided by (used for) operating activities | | | 24,314 | | | | (20,595 | ) |
Investing activities | | | | | | | | |
Proceeds from the sale of property and equipment | | | - | | | | 2,860 | |
Purchases of short-term investments | | | - | | | | (2,999 | ) |
Net cash used for investing activities | | | - | | | | (139 | ) |
Financing activities | | | | | | | | |
Net proceeds from issuance of common stock under 2013 Equity Plan | | | 155 | | | | 605 | |
Net proceeds from issuance of common stock under ESPP | | | 198 | | | | 359 | |
Net cash provided by financing activities | | | 353 | | | | 964 | |
Net increase (decrease) in cash, cash equivalents and restricted cash | | | 24,667 | | | | (19,770 | ) |
Cash, cash equivalents and restricted cash, beginning of period | | | 21,261 | | | | 88,729 | |
Cash, cash equivalents and restricted cash, end of period | | $ | 45,928 | | | $ | 68,959 | |
Reconciliation of cash, cash equivalents and restricted cash to the condensed consolidated balance sheets | | | | | | | | |
Cash and cash equivalents | | $ | 45,888 | | | $ | 68,353 | |
Restricted cash | | | 40 | | | | 606 | |
Total cash, cash equivalents and restricted cash | | $ | 45,928 | | | $ | 68,959 | |
See accompanying notes to the condensed consolidated financial statements.
4
LA JOLLA PHARMACEUTICAL COMPANY
Notes to the Condensed Consolidated Financial Statements
(Unaudited)
1. Business
La Jolla Pharmaceutical Company (collectively with its wholly owned subsidiaries, “La Jolla” or the “Company”) is dedicated to the commercialization of innovative therapies that improve outcomes in patients suffering from life-threatening diseases. GIAPREZA™ (angiotensin II) injection is approved by the U.S. Food and Drug Administration (“FDA”) as a vasoconstrictor indicated to increase blood pressure in adults with septic or other distributive shock. XERAVA™ (eravacycline) for injection is approved by the FDA as a tetracycline class antibacterial indicated for the treatment of complicated intra-abdominal infections (“cIAI”) in patients 18 years of age and older.
On July 28, 2020, La Jolla completed its acquisition of Tetraphase Pharmaceuticals, Inc. and its subsidiaries (“Tetraphase”), a biopharmaceutical company focused on commercializing XERAVA, for $43 million in upfront cash plus potential future cash payments of up to $16 million. The Company’s consolidated financial results exclude Tetraphase’s financial results prior to the acquisition closing date of July 28, 2020 (see Note 11).
In January 2021, La Jolla and certain of its wholly owned subsidiaries, including La Jolla Pharma, LLC, entered into a license agreement with PAION AG to commercialize GIAPREZA and XERAVA in the European Economic Area, the United Kingdom and Switzerland. Pursuant to the agreement: (i) the Company has received an upfront cash payment of $22.5 million, less a 15% refundable withholding tax; and (ii) the Company is entitled to receive potential commercial milestone payments of up to $109.5 million and royalties on net sales of GIAPREZA and XERAVA.
As of June 30, 2021 and December 31, 2020, the Company had cash and cash equivalents of $45.9 million and $21.2 million, respectively. Based on the Company’s current operating plans and projections, the Company expects that its existing cash and cash equivalents will be sufficient to fund operations for at least one year from the date this Quarterly Report on Form 10-Q is filed with the U.S. Securities and Exchange Commission (the “SEC”). The Company expects to fund future operations with existing cash or cash generated from operations.
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The Company’s condensed consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of SEC Regulation S-X. Accordingly, certain information and disclosures required by GAAP for annual financial statements have been omitted. In the opinion of management, all adjustments, consisting of normal recurring adjustments, considered necessary for a fair presentation have been included. These condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto for the year ended December 31, 2020 included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on March 8, 2021 (the “Form 10-K”). The accompanying condensed consolidated financial statements include the accounts of La Jolla Pharmaceutical Company and its wholly owned subsidiaries. All inter-company transactions and balances have been eliminated in consolidation.
The preparation of the Company’s condensed consolidated financial statements requires management to make estimates and assumptions that impact the reported amounts of assets, liabilities, revenue and expenses and the disclosure of contingent assets and liabilities in the Company’s condensed consolidated financial statements and the accompanying notes. Actual results may differ materially from these estimates. Certain amounts previously reported in the financial statements have been reclassified to conform to the current year presentation. Such reclassifications did not affect net loss, shareholders’ deficit or cash flows. The results of operations for the three and six months ended June 30, 2021 are not necessarily indicative of the results to be expected for the full year or any future interim periods. The accompanying condensed consolidated balance sheet as of December 31, 2020 has been derived from the audited consolidated balance sheet as of December 31, 2020 contained in the Form 10-K.
5
Summary of Significant Accounting Policies
During the six months ended June 30, 2021, other than the license revenue recognition policy described below, there have been no changes to the Company’s significant accounting policies as described in Note 2 of the Form 10-K.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist of cash. The Company maintains its cash in checking and savings accounts at federally insured financial institutions in excess of federally insured limits.
During the six months ended June 30, 2021, 399 hospitals in the U.S. purchased GIAPREZA. During the six months ended June 30, 2021, 644 hospitals and other healthcare organizations in the U.S. purchased XERAVA. Hospitals and other healthcare organizations purchase our products through a network of specialty and wholesale distributors. These specialty and wholesale distributors are considered our customers for accounting purposes. The Company does not believe that the loss of one of these distributors would significantly impact the ability to distribute our products, as the Company expects that sales volume would be absorbed by the remaining distributors. The following table includes the percentage of U.S. net product sales and accounts receivable balances for the Company’s 3 major customers, each of which comprised 10% or more of its U.S. net product sales:
| | U.S. Net Product Sales | | | Accounts Receivable | |
| | Three Months Ended June 30, 2021 | | | Six Months Ended June 30, 2021 | | | As of June 30, 2021 | |
Customer A | | | 34 | % | | | 36 | % | | | 37 | % |
Customer B | | | 35 | % | | | 34 | % | | | 48 | % |
Customer C | | | 27 | % | | | 26 | % | | | 12 | % |
Total | | | 96 | % | | | 96 | % | | | 97 | % |
Business Combinations
The Company accounts for business combinations using the acquisition method pursuant to the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 805. This method requires, among other things, that results of operations of acquired companies are included in La Jolla’s financial results beginning on the respective acquisition dates, and that assets acquired and liabilities assumed are recognized at fair value as of the acquisition date. Intangible assets acquired in a business combination are recorded at fair value using a discounted cash flow model. The discounted cash flow model requires assumptions about the timing and amount of future net cash flows, the cost of capital and terminal values from the perspective of a market participant. Any excess of the fair value of consideration transferred (the “Purchase Price”) over the fair values of the net assets acquired is recognized as goodwill. Contingent consideration liabilities are recognized as part of the Purchase Price at the estimated fair value on the acquisition date. Subsequent changes to the fair value of contingent consideration liabilities will be included in other (expense) income, net in the consolidated statements of operations. The fair value of assets acquired and liabilities assumed in certain cases may be subject to revision based on the final determination of fair value during a period of time not to exceed 12 months from the acquisition date. Legal costs, due diligence costs, business valuation costs and all other acquisition-related costs are expensed when incurred.
Intangible Assets
Intangible assets acquired in a business combination are initially recorded at fair value. Intangible assets with a definite useful life are amortized on a straight-line basis over the estimated useful life of the related assets. Intangible assets with an indefinite useful life are not amortized.
The Company reviews its intangible assets for impairment at least annually or whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. If such circumstances are determined to exist, an estimate of undiscounted future cash flows produced by the asset, including its eventual residual value, is compared to the carrying value to determine whether impairment exists. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values. Fair value is estimated through discounted cash flow models to project cash flows from the asset.
6
The Company recognized 0 impairment charge for the six months ended June 30, 2021.
Goodwill
Goodwill represents the excess of the Purchase Price over the fair value of the net assets acquired as of the acquisition date. Goodwill has an indefinite useful life and is not amortized.
The Company reviews its goodwill for impairment at least annually or whenever events or changes in circumstances indicate that the carrying amount of the Company may exceed its fair value. The Company first assesses qualitative factors to determine whether it is more likely than not that the fair value of the Company is less than its carrying amount, including goodwill. If that is the case, the Company performs a quantitative impairment test, and, if the carrying amount of the Company exceeds its fair value, then the Company will recognize an impairment charge for the amount by which its carrying amount exceeds its fair value, not to exceed the carrying amount of the goodwill.
The Company recognized 0 impairment charge for the six months ended June 30, 2021.
Revenue Recognition
Pursuant to FASB ASC Topic 606—Revenue from Contracts with Customers (“ASC 606”), the Company recognizes revenue when its customers obtain control of the Company’s product, which typically occurs on delivery. Revenue is recognized in an amount that reflects the consideration that the Company expects to receive in exchange for those goods. To determine revenue recognition for contracts with customers within the scope of ASC 606, the Company performs the following 5 steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies the relevant performance obligations.
Product Sales
Revenue from product sales is recorded at the transaction price, net of estimates for variable consideration consisting of chargebacks, discounts, returns, Medicaid rebates and administrative fees. Variable consideration is estimated using the expected-value amount method, which is the sum of probability-weighted amounts in a range of possible consideration amounts. Actual amounts of consideration ultimately received may differ from the Company’s estimates. If actual results vary materially from the Company’s estimates, the Company will adjust these estimates, which will affect revenue from product sales and earnings in the period such estimates are adjusted. These items include:
| • | Chargebacks—Chargebacks are discounts the Company provides to distributors in the event that the sales prices to end users are below the distributors’ acquisition price. This may occur due to a direct contract with a health system, a group purchasing organization (“GPO”) agreement or a sale to a government facility. Chargebacks are estimated based on known chargeback rates and recorded as a reduction of revenue on delivery to the Company’s customers. |
| • | Discounts—The Company offers customers various forms of incentives and consideration, including prompt-pay and other discounts. The Company estimates discounts primarily based on contractual terms. These discounts are recorded as a reduction of revenue on delivery to the Company’s customers. |
| • | Returns—The Company offers customers a limited right of return, generally for damaged or expired product. The Company estimates returns based on an internal analysis, which includes actual experience. The estimates for returns are recorded as a reduction of revenue on delivery to the Company’s customers. |
| • | Medicaid Rebates—We participate in Medicaid rebate programs, which provide assistance to certain low-income patients based on each individual state’s guidelines regarding eligibility and services. Under the Medicaid rebate programs, we pay a rebate to each participating state, generally within three months after the quarter in which product was sold. The estimates for rebates are recorded as a reduction of revenue on delivery to the Company’s customers. |
| • | Administrative Fees—The Company pays administrative fees to GPOs for services and access to data. Additionally, the Company pays an Industrial Funding Fee as part of the U.S. General Services Administration’s Federal Supply Schedules program. These fees are based on contracted terms and are paid after the quarter in which the product was purchased by the applicable GPO or government agency. Administrative fees are recorded as a reduction of revenue on delivery to customers. |
7
The Company will continue to assess its estimates of variable consideration as it accumulates additional historical data and will adjust these estimates accordingly.
License Revenue
We enter into out-license agreements with counterparties to develop and/or commercialize our products in territories outside of the U.S. in exchange for: (i) nonrefundable, upfront license fees; (ii) development and regulatory milestone payments; and/or (iii) sales-based royalties and milestones.
If the license to our intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, we recognize revenue from nonrefundable, upfront fees allocated to the license when the license is transferred to the customer and the customer can benefit from the license. For licenses that are bundled with other performance obligations, management uses judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from nonrefundable, upfront fees. We evaluate the measure of progress each reporting period and, if necessary, adjust the measure of progress and related revenue recognition.
At the inception of each arrangement that include milestone and other payments, other than sales-based milestone payments and nonrefundable, upfront license fees, we evaluate whether achieving each milestone payment or other payment is considered probable and estimate the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the value of the associated milestone is included in the transaction price. Milestone payments that are not within our control, such as approvals from regulators or where attainment of the specified event is dependent on the development activities of a third party, are not considered probable of being achieved until those approvals are received or the specified event occurs.
For arrangements that include sales-based royalties and milestone payments, and the license is deemed to be the predominant item to which the royalties relate, we recognize revenue at the later of: (i) when the related sales occur; or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied or partially satisfied.
We enter into commercial supply agreements with our out-licensees to supply our products in territories outside the U.S. in exchange for: (i) nonrefundable, upfront fees; and/or (ii) the reimbursement of manufacturing costs, plus a margin in certain cases. The Company is considered the principal in these arrangements for accounting purposes as it controls the promised goods before transferring these goods to the out-licensee. The Company recognizes revenue when out-licensees obtain control of the Company’s product, which typically occurs on delivery.
Recent Accounting Pronouncements
The Company has implemented all new accounting pronouncements that are in effect and that may impact its financial statements and does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position and results of operations.
3. Earnings (Loss) per Share
Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted-average number of common shares outstanding during the period, without consideration of potential common shares. Diluted earnings (loss) per share is calculated by dividing net income (loss) by the weighted-average number of common shares outstanding plus potential common shares. Convertible preferred stock and stock options are considered potential common shares and are included in the calculation of diluted earnings (loss) per share using the treasury stock method when their effect is dilutive. Potential common shares are excluded from the calculation of diluted earnings (loss) per share when their effect is anti-dilutive. For the three months ended June 30, 2021, there were 6.7 million potential common shares that were included in the calculation of diluted earnings per share, which consists of: (i) 6.7 million shares of common stock issuable upon conversion of existing convertible preferred stock; and (ii) 5,000 stock options. For the six months ended June 30, 2021, there were 6.7 million potential common shares that were included in the calculation of diluted earnings per share, which consists of: (i) 6.7 million shares of common stock issuable upon conversion of existing convertible preferred stock; and (ii) 13,000 stock options. For the three and six months ended June 30, 2021 and 2020, there were 4.1 million and 10.2 million, respectively, of potential common shares that were excluded from the calculation of diluted loss per share because their effect was anti-dilutive.
8
4. Balance Sheet Details
Restricted Cash
Restricted cash as of June 30, 2021 and December 31, 2020 consisted of a $40,000 security deposit for the Company’s corporate purchasing credit card.
Inventory, Net
Inventory, net consisted of the following (in thousands):
| | June 30, | | | December 31, | |
| | 2021 | | | 2020 | |
Raw materials | | $ | 802 | | | $ | 802 | |
Work-in-process | | | 3,636 | | | | 3,213 | |
Finished goods | | | 1,043 | | | | 1,998 | |
Total inventory, net | | $ | 5,481 | | | $ | 6,013 | |
As of June 30, 2021 and December 31, 2020, inventory, net included 0 and $0.9 million, respectively, of the fair value step-up adjustment to Tetraphase’s inventory recorded in connection with the acquisition of Tetraphase (see Note 11). As of June 30, 2021 and December 31, 2020, total inventory is recorded net of inventory reserves of $1.2 million and $0.9 million, respectively.
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consisted of the following (in thousands):
| | June 30, | | | December 31, | |
| | 2021 | | | 2020 | |
Refundable withholding tax | | $ | 3,375 | | | $ | - | |
Prepaid manufacturing costs | | | 491 | | | | 930 | |
Prepaid clinical costs | | | 265 | | | | 820 | |
Prepaid insurance | | | 263 | | | | 505 | |
Other prepaid expenses and current assets | | | 807 | | | | 1,133 | |
Total prepaid expenses and other current assets | | $ | 5,201 | | | $ | 3,388 | |
Property and Equipment, Net
Property and equipment, net consisted of the following (in thousands):
| | June 30, | | | December 31, | |
| | 2021 | | | 2020 | |
Furniture and fixtures | | $ | 313 | | | $ | 309 | |
Computer hardware | | | 310 | | | | 310 | |
Software | | | 203 | | | | 733 | |
Total property and equipment, gross | | | 826 | | | | 1,352 | |
Accumulated depreciation and amortization | | | (663 | ) | | | (1,137 | ) |
Total property and equipment, net | | $ | 163 | | | $ | 215 | |
9
Intangible Assets, Net
Intangible assets, net consisted of the following (in thousands):
| | Useful Life | | June 30, | | | December 31, | |
| | (years) | | 2021 | | | 2020 | |
Technology | | 10 | | $ | 14,000 | | | $ | 14,000 | |
Trade name | | 10 | | | 1,520 | | | | 1,520 | |
Total intangible assets, gross | | | | | 15,520 | | | | 15,520 | |
Accumulated amortization | | | | | (1,423 | ) | | | (647 | ) |
Total intangible assets, net | | | | $ | 14,097 | | | $ | 14,873 | |
The intangible assets were recorded in connection with the acquisition of Tetraphase (see Note 11). The Company recorded amortization expense of $0.4 million and $0.8 million for the three and six months ended June 30, 2021, respectively. The Company recorded 0 amortization expense for the six months ended June 30, 2020.
Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| | June 30, | | | December 31, | |
| | 2021 | | | 2020 | |
Accrued interest expense on deferred royalty obligation, current portion | | $ | 4,227 | | | $ | 3,567 | |
Deferred revenue | | | 3,037 | | | | 188 | |
Accrued royalties and in-license fees | | | 1,235 | | | | 685 | |
Accrued manufacturing costs | | | 1,469 | | | | 627 | |
Accrued professional fees | | | 942 | | | | 660 | |
Accrued other | | | 1,265 | | | | 767 | |
Total accrued expenses | | $ | 12,175 | | | $ | 6,494 | |
Other Noncurrent Liabilities
Other noncurrent liabilities consisted of the following (in thousands):
| | June 30, | | | December 31, | |
| | 2021 | | | 2020 | |
Paycheck Protection Program loan | | $ | 2,313 | | | $ | 2,302 | |
Fair value of contingent value rights (see Note 11) | | | 2,180 | | | | 1,810 | |
Total other noncurrent liabilities | | $ | 4,493 | | | $ | 4,112 | |
On April 22, 2020, Tetraphase entered into a promissory note for $2.3 million under the Paycheck Protection Program (the “PPP Loan”). The interest rate on the PPP Loan is 1.0% per annum. The PPP Loan is unsecured and guaranteed by the U.S. Small Business Administration. The principal amount of the PPP Loan may be forgiven under the Paycheck Protection Program, subject to certain requirements and to the extent that the PPP Loan proceeds are used to pay permitted expenses, including certain payroll, rent and utility payments. The Company applied for forgiveness of the PPP Loan. The Company will be obligated to make monthly payments of principal and interest with respect to any unforgiven portion of the PPP Loan. The obligation to repay the PPP Loan may be accelerated upon the occurrence of an event of default.
10
5. Deferred Royalty Obligation
In May 2018, the Company closed a $125.0 million royalty financing agreement (the “Royalty Agreement”) with HealthCare Royalty Partners (“HCR”). Under the terms of the Royalty Agreement, the Company received $125.0 million in exchange for tiered royalty payments on worldwide net sales of GIAPREZA. HCR is entitled to receive quarterly royalties on worldwide net sales of GIAPREZA beginning April 1, 2018. Quarterly payments to HCR under the Royalty Agreement start at a maximum royalty rate, with step-downs based on the achievement of annual net product sales thresholds. Through December 31, 2021, the royalty rate will be a maximum of 10%. Starting January 1, 2022, the maximum royalty rate may increase by 4% if an agreed-upon, cumulative net product sales threshold has not been met, and, starting January 1, 2024, the maximum royalty rate may increase by an additional 4% if a different agreed-upon, cumulative net product sales threshold has not been met. The Royalty Agreement is subject to maximum aggregate royalty payments to HCR of $225.0 million. The Royalty Agreement expires upon the first to occur of January 1, 2031 or when the maximum aggregate royalty payments have been made. The Royalty Agreement was entered into by the Company’s wholly owned subsidiary, La Jolla Pharma, LLC, and HCR has no recourse under the Royalty Agreement against La Jolla Pharmaceutical Company or any assets other than GIAPREZA.
On receipt of the $125.0 million payment from HCR, the Company recorded a deferred royalty obligation of $125.0 million, net of issuance costs of $0.7 million. For the three and six months ended June 30, 2021, the Company recognized interest expense, including amortization of the obligation discount, of $2.7 million and $5.3 million, respectively. For the three and six months ended June 30, 2020, the Company recognized interest expense, including amortization of the obligation discount, of $2.5 million and $4.9 million, respectively. The carrying value of the deferred royalty obligation as of June 30, 2021 and December 31, 2020 was $124.5 million and $124.4 million, respectively, net of unamortized obligation discount of $0.5 million and $0.6 million, respectively, and was classified as a noncurrent liability. The related accrued interest expense as of June 30, 2021 and December 31, 2020 was $26.4 million and $22.7 million, respectively, of which $22.1 million and $19.1 million was classified as noncurrent liabilities, respectively. During the three and six months ended June 30, 2021, the Company made royalty payments to HCR of $0.7 million and $1.6 million, respectively. As of June 30, 2021 and December 31, 2020, the Company recorded royalty obligations payable of $0.9 million and $0.9 million, respectively, in accrued expenses. The deferred royalty obligation is classified as Level 3 in the FASB ASC Topic 820-10, three-tier fair value hierarchy, and its carrying value approximates fair value.
Under the terms of the Royalty Agreement, La Jolla Pharma, LLC has certain obligations, including the obligation to use commercially reasonable and diligent efforts to commercialize GIAPREZA. If La Jolla Pharma, LLC is held to not have met these obligations, HCR would have the right to terminate the Royalty Agreement and demand payment from La Jolla Pharma, LLC of either $125.0 million or $225.0 million (depending on which obligation La Jolla Pharma, LLC is held to not have met), minus aggregate royalties already paid to HCR. In the event that La Jolla Pharma, LLC fails to timely pay such amount if and when due, HCR would have the right to foreclose on the GIAPREZA-related assets. The Company concluded that certain of these contract provisions that could result in an acceleration of amounts due under the Royalty Agreement are embedded derivatives that require bifurcation from the deferred royalty obligation and fair value recognition. The Company determined the fair value of each derivative by assessing the probability of each event occurring, as well as the potential repayment amounts and timing of such repayments that would result under various scenarios. As a result of this assessment, the Company determined that the fair value of the embedded derivatives is immaterial as of June 30, 2021 and December 31, 2020. Each reporting period, the Company estimates the fair value of the embedded derivatives until the features lapse and/or the termination of the Royalty Agreement. Any material change in the fair value of the embedded derivatives will be recorded as either a gain or loss on the consolidated statements of operations.
11
6. Commitments and Contingencies
Lease Commitments
Future minimum lease payments, excluding Lease Operating Costs, as of June 30, 2021 are as follows (in thousands):
2021 | | $ | 108 | |
2022 | | | 181 | |
2023 | | | 166 | |
Thereafter | | | - | |
Total future minimum lease payments | | | 455 | |
Less: discount | | | 36 | |
Total lease liabilities | | $ | 419 | |
Lease expense under current leases was approximately $0.1 million and $0.2 million for the three and six months ended June 30, 2021, respectively. Lease expense under former leases was approximately $0.7 million and $1.4 million for the three and six months ended June 30, 2020, respectively. Cash paid for amounts included in the measurement of lease liabilities was $51,000 and $0.1 million for the three and six months ended June 30, 2021, respectively. Cash paid for amounts included in the measurement of lease liabilities was $1.0 and $1.9 million for the three and six months ended June 30, 2020, respectively. As of June 30, 2021, the weighted-average remaining lease term and the weighted-average discount rate for the Company’s only operating lease, the Waltham Sublease, was 2.3 years and 3.5%, respectively.
Waltham Sublease
In December 2020, the Company entered into a sublease agreement for office space in Waltham, Massachusetts (the “Waltham Sublease”). The Waltham Sublease commenced on December 21, 2020 and expires on November 30, 2023. In addition to rent of approximately $15,000 per month, the Waltham Sublease requires the Company to pay certain taxes, insurance and operating costs relating to the leased premises (collectively, “Lease Operating Costs”). The Waltham Sublease contains customary default provisions, representations, warranties and covenants. The Waltham Sublease is classified as an operating lease. The Company recognizes the Waltham Sublease expense in the consolidated statements of operations and records a lease liability and right-of-use asset for this lease.
San Diego Sublease
In September 2020, the Company entered into a sublease agreement for office space in San Diego, California with an entity of which the Chairman of the Company’s board of directors is also the chairman and chief executive officer (the “San Diego Sublease”). The San Diego Sublease term is approximately 7 years, and the rent is approximately $12,000 per month. The San Diego Sublease is cancellable without penalty by either party with 30-days’ written notice. The San Diego Sublease is a short-term lease for accounting purposes. The Company made payments of approximately $28,000 and $67,000 under the San Diego Sublease during the three and six months ended June 30, 2021. The Company recognizes the San Diego Sublease payments in the consolidated statements of operations and does not record a lease liability or right-of-use asset for this lease.
Contingencies
From time to time, the Company may become subject to claims and litigation arising in the ordinary course of business. The Company is not a party to any material legal proceedings, nor is it aware of any material pending or threatened litigation.
12
7. Shareholders’ Equity
Preferred Stock
As of June 30, 2021 and December 31, 2020, 3,906 shares of Series C-12 Convertible Preferred Stock (“Series C-12 Preferred”) were issued, outstanding and convertible into 6,735,378 shares of common stock. As of June 30, 2021 and December 31, 2020, the Series C-12 Preferred liquidation preference was approximately $3.9 million. The Series C-12 Preferred does not pay a dividend. The holders of the Series C-12 Preferred do not have voting rights, other than for general protective rights required by the California General Corporation Law.
8. Equity Incentive Plans
2013 Equity Incentive Plan
A total of 9,600,000 shares of common stock have been reserved for issuance under the La Jolla Pharmaceutical Company 2013 Equity Incentive Plan (the “2013 Equity Plan”). As of June 30, 2021 and December 31, 2020, 5,514,933 shares of common stock and 5,478,334 shares of common stock, respectively, remained available for future grants under the 2013 Equity Plan.
2018 Employee Stock Purchase Plan
A total of 750,000 shares of common stock have been reserved for issuance under the La Jolla Pharmaceutical Company 2018 Employee Stock Purchase Plan (the “ESPP”). As of June 30, 2021 and December 31, 2020, 405,564 shares of common stock and 455,768 shares of common stock, respectively, remained available for future grants under the ESPP.
Equity Awards
The activity related to equity awards, which are comprised of stock options, during the six months ended June 30, 2021 is summarized as follows:
| | Equity Awards | | | Weighted- average Exercise Price per Share | | | Weighted- average Remaining Contractual Term(1) (years) | | | Aggregate Intrinsic Value(2) (millions) | |
Outstanding at December 31, 2020 | | | 4,121,666 | | | $ | 8.67 | | | | | | | | | |
Granted | | | 562,329 | | | $ | 4.96 | | | | | | | | | |
Exercised | | | (29,379 | ) | | $ | 5.23 | | | | | | | | | |
Cancelled/forfeited | | | (569,549 | ) | | $ | 9.21 | | | | | | | | | |
Outstanding at June 30, 2021 | | | 4,085,067 | | | $ | 8.10 | | | | 8.3 | | | $ | 0.2 | |
Exercisable at June 30, 2021 | | | 1,107,878 | | | $ | 16.71 | | | | 5.7 | | | $ | - | |
(1) Represents the weighted-average remaining contractual term of stock options.
(2) Aggregate intrinsic value represents the product of the number of equity awards outstanding or equity awards exercisable multiplied by the difference between the Company’s closing stock price per share on the last trading day of the period, which was $4.28 as of June 30, 2021, and the exercise price.
Share-based Compensation Expense
The classification of share-based compensation expense is summarized as follows (in thousands):
| | Three Months Ended | | | Six Months Ended | |
| | June 30, | | | June 30, | |
| | 2021 | | | 2020 | | | 2021 | | | 2020 | |
Selling, general and administrative | | $ | 977 | | | $ | 626 | | | $ | 1,817 | | | $ | 1,470 | |
Research and development | | | 47 | | | | 964 | | | | 323 | | | | 2,527 | |
Total share-based compensation expense | | $ | 1,024 | | | $ | 1,590 | | | $ | 2,140 | | | $ | 3,997 | |
13
As of June 30, 2021, total unrecognized share-based compensation expense related to unvested equity awards was $8.9 million, which is expected to be recognized over a weighted-average period of 3.0 years. As of June 30, 2021, there was 0 unrecognized share-based compensation expense related to shares of common stock issued under the ESPP.
9. Other Income—Related Party
The Company has a non-voting profits interest in a related party, which provides the Company with the potential to receive a portion of the future distributions of profits, if any. Investment funds affiliated with the Chairman of the Company’s board of directors have a controlling interest in the related party. During the three and six months ended June 30, 2021, the Company received distributions of $2.5 million in connection with this profits interest. During the three and six months ended June 30, 2020, the Company received distributions of 0 and $4.1 million, respectively, in connection with this profits interest.
10. License Agreements
In-license Agreements
George Washington University
In December 2014, the Company entered into a patent license agreement with George Washington University (“GW”), which was subsequently amended and restated (the “GW License”) and assigned to La Jolla Pharma, LLC. Pursuant to the GW License, GW exclusively licensed to the Company certain intellectual property rights relating to GIAPREZA, including the exclusive rights to certain issued patents and patent applications covering GIAPREZA. Under the GW License, La Jolla Pharma, LLC is obligated to use commercially reasonable efforts to develop, commercialize, market and sell GIAPREZA. The Company has paid a one-time license initiation fee, annual maintenance fees, an amendment fee, additional payments following the achievement of certain development and regulatory milestones and royalties. As a result of the European Commission’s approval of GIAPREZA in August 2019, the Company made a milestone payment to GW in the amount of $0.5 million in the first quarter of 2020. The Company is obligated to pay a 6% royalty on net sales of GIAPREZA and 15% on payments received from sublicensees. The obligation to pay royalties under this agreement extends through the last-to-expire patent covering GIAPREZA. During the three and six months ended June 30, 2021, the Company made payments to GW of $0.4 million and $3.2 million, respectively. During the three and six months ended June 30, 2020, the Company made payments to GW of $0.5 million and $0.9 million, respectively.
Harvard University
In August 2006, the Company entered into a license agreement with Harvard University (“Harvard”), which was subsequently amended and restated (the “Harvard License”). Pursuant to the Harvard License, Harvard exclusively licensed to the Company certain intellectual property rights relating to tetracycline-based products, including XERAVA, including the exclusive rights to certain issued patents and patent applications covering such products. Under the Harvard License, the Company is obligated to use commercially reasonable efforts to develop, commercialize, market and sell tetracycline-based products, including XERAVA. For each product covered by the Harvard License, the Company is obligated to make certain payments for the following: (i) up to approximately $15.1 million upon the achievement of certain clinical development and regulatory milestones; (ii) a 5% royalty on direct U.S. net sales of XERAVA; (iii) a single-digit tiered royalty on direct ex-U.S. net sales of XERAVA, starting at a minimum royalty rate of 4.5%, with step-ups to a maximum royalty of 7.5% based on the achievement of annual net product sales thresholds; and (iv) 20% on payments received from sublicensees. The obligation to pay royalties under this agreement extends through the last-to-expire patent covering tetracycline-based products, including XERAVA. During the three and six months ended June 30, 2021, the Company made payments to Harvard of $1.5 million and $1.6 million, respectively, NaN of which related to clinical development and regulatory milestones.
Paratek Pharmaceuticals, Inc.
In March 2019, the Company entered into a license agreement with Paratek Pharmaceuticals, Inc. (“Paratek”), which was subsequently amended and restated (the “Paratek License”). Pursuant to the Paratek License, Paratek non-exclusively licensed to the Company certain intellectual property rights relating to XERAVA, including non-exclusive rights to certain issued patents and patent applications covering XERAVA. The Company is obligated to pay Paratek a 2.25% royalty based on direct U.S. net sales of XERAVA. The Company’s obligation to pay royalties with respect to the licensed product is retroactive to the date of the first commercial sale of XERAVA and shall continue until there are no longer any valid claims of the Paratek patents, which will expire in October 2023. During the three and six months ended June 30, 2021, the Company paid $40,000 and $0.1 million, respectively, of royalties to Paratek.
14
Out-license Agreements
PAION AG
On January 12, 2021, La Jolla Pharmaceutical Company and certain of its wholly owned subsidiaries, including La Jolla Pharma, LLC, entered into an exclusive license agreement (the “PAION License”) with PAION AG and its wholly owned subsidiary (collectively, “PAION”). Pursuant to the PAION License, La Jolla granted PAION an exclusive license to commercialize GIAPREZA and XERAVA in the European Economic Area, the United Kingdom and Switzerland (collectively, the “PAION Territory”). La Jolla has received an upfront cash payment of $22.5 million, less a 15% refundable withholding tax, and is entitled to receive potential commercial milestone payments of up to $109.5 million and double-digit tiered royalty payments. In addition, royalties payable under the PAION License will be subject to reduction on account of generic competition and after patent expiration in a jurisdiction. Pursuant to the PAION License, PAION will be solely responsible for the future development and commercialization of GIAPREZA and XERAVA in the PAION Territory. PAION is required to use commercially reasonable efforts to commercialize GIAPREZA and XERAVA in the PAION Territory. The Company has not received any payments from PAION related to either royalties or commercial milestones.
On July 13, 2021, the Company entered into a commercial supply agreement with PAION whereby the Company will supply PAION a minimum quantity of GIAPREZA and XERAVA through July 13, 2024. The supply agreement will automatically renew until the earlier of July 13, 2027, or until a new supply agreement is executed. During the initial 3-year term of the supply agreement, the Company will be reimbursed for direct and certain indirect manufacturing costs at cost.
Everest Medicines Limited
In February 2018, the Company entered into a license agreement with Everest, which was subsequently amended and restated (the “Everest License”). Pursuant to the Everest License, the Company granted Everest an exclusive license to develop and commercialize XERAVA for the treatment of cIAI and other indications in mainland China, Taiwan, Hong Kong, Macau, South Korea, Singapore, the Malaysian Federation, the Kingdom of Thailand, the Republic of Indonesia, the Socialist Republic of Vietnam and the Republic of the Philippines (collectively, the “Everest Territory”). The Company is eligible to receive an additional $8.0 million regulatory milestone payment and up to an aggregate of $20.0 million in sales milestone payments. The Company is also entitled to receive tiered royalties from Everest at percentages in the low double digits on sales, if any, in the Everest Territory of products containing eravacycline. Royalties are payable with respect to each jurisdiction in the Everest Territory until the latest to occur of: (1) the last-to-expire of specified patent rights in such jurisdiction in the Everest Territory; (2) expiration of marketing or regulatory exclusivity in such jurisdiction in the Everest Territory; or (3) 10 years after the first commercial sale of a product in such jurisdiction in the Everest Territory. In March 2021, the Company received a $3.0 million milestone payment associated with the submission of an NDA with the China National Medical Products Administration (“NMPA”) for XERAVA for the treatment of cIAI in patients in China. Amounts due under the Harvard License for this milestone payment were included as research and development expense on the consolidated statements of operations.
In May 2021, the Company entered into a commercial supply agreement with Everest whereby the Company will supply Everest a minimum quantity of XERAVA through December 31, 2023 and will transfer to Everest certain XERAVA-related manufacturing know-how. Pursuant to the supply agreement: (i) the Company has received $6.8 million of upfront payments comprised of: (1) a $4.0 million upfront technology transfer payment; and (2) a $2.8 million partial prepayment for XERAVA that is expected to be delivered to Everest during 2022; (ii) the Company will receive an additional $1.0 million technology transfer payment by January 30, 2022; and (iii) the Company will be reimbursed for direct and certain indirect manufacturing costs at 110% of costs through December 31, 2023. The Company recognized the $5.0 million of technology transfer-related payments as license revenue during the three and six months ended June 30, 2021 as Everest obtained control of the XERAVA-related manufacturing know-how prior to June 30, 2021. The Company recognized the $2.8 million partial prepayment for XERAVA that is expected to be delivered to Everest during 2022 as deferred revenue as of June 30, 2021 as the performance obligation to deliver XERAVA has not yet been satisfied.
15
11. Acquisition of Tetraphase Pharmaceuticals, Inc.
On June 24, 2020, La Jolla entered into an Agreement and Plan of Merger with Tetraphase, a biopharmaceutical company focused on commercializing its novel tetracycline XERAVA to treat serious and life‑threatening infections, and TTP Merger Sub, Inc., a wholly owned subsidiary of La Jolla. On July 28, 2020, La Jolla completed its acquisition of Tetraphase for $43 million in upfront cash plus potential future cash payments of up to $16 million pursuant to contingent value rights (“CVRs”). The holders of the CVRs are entitled to receive potential future cash payments of up to $16 million in the aggregate upon the achievement of certain net sales of XERAVA in the U.S. as follows: (i) $2.5 million if 2021 XERAVA U.S. net sales are at least $20 million; (ii) $4.5 million if XERAVA U.S. net sales are at least $35 million during any calendar year ending on or prior to December 31, 2024; and (iii) $9 million if XERAVA U.S. net sales are at least $55 million during any calendar year ending on or prior to December 31, 2024. Following the acquisition, Tetraphase became a wholly owned subsidiary of La Jolla.
The acquisition of Tetraphase was accounted for as a business combination using the acquisition method pursuant to FASB ASC Topic 805. As the acquirer for accounting purposes, La Jolla has estimated the Purchase Price, assets acquired and liabilities assumed as of the acquisition date, with the excess of the Purchase Price over the fair value of net assets acquired recognized as goodwill. The estimated fair value of assets acquired and liabilities assumed in certain cases may be subject to revision based on the final determination of fair value.
The Purchase Price is comprised of the upfront cash of $43 million and the estimated fair value of potential future cash payments pursuant to the CVRs. The estimated fair value of assets acquired was $54.7 million, and the estimated fair value of liabilities assumed was $9.1 million.
The Purchase Price allocation as of the acquisition date is presented as follows (in thousands):
| | July 28, | |
| | 2020 | |
Cash | | $ | 42,990 | |
Fair Value of CVRs | | | 2,610 | |
Total Purchase Price | | $ | 45,600 | |
| | | | |
Cash and cash equivalents | | $ | 8,778 | |
Accounts receivable | | | 1,187 | |
Inventory | | | 4,767 | |
Prepaid expenses and other current assets | | | 1,218 | |
Property and equipment | | | 58 | |
Right-of-use lease assets | | | 2,302 | |
Restricted cash | | | 699 | |
Identifiable intangible assets | | | 15,520 | |
Goodwill | | | 20,123 | |
Accounts payable | | | (1,400 | ) |
Accrued expenses | | | (2,979 | ) |
Lease liabilities, current portion | | | (967 | ) |
Lease liabilities, less current portion | | | (1,420 | ) |
Other noncurrent liabilities | | | (2,286 | ) |
Total Purchase Price | | $ | 45,600 | |
The estimated fair value of potential future cash payments pursuant to the CVRs was based on a Monte Carlo simulation and is classified as Level 3 in the ASC Topic 820-10, three-tier fair value hierarchy.
CVRs are measured at fair value on a recurring basis. During the three and six months ended June 30, 2021, the Company recorded a $0.1 million gain and $0.4 million loss, respectively, in other (expense) income in the consolidated statements of operations resulting from the change in fair value of CVRs.
The Company recorded a $3.3 million fair value step-up adjustment to Tetraphase’s inventory as of the acquisition date. Raw material components and active pharmaceutical ingredients were recorded based on estimated replacement cost. Finished drug product was valued at estimated selling cost, adjusted for costs of selling effort and a reasonable profit allowance for such selling effort from the viewpoint of a market participant. This fair value step-up adjustment is recorded as cost of product sales when the inventory is sold to customers, 0 and $0.9 million of which was included in cost of product sales during the three and six months ended June 30, 2021, respectively.
16
Identifiable intangible assets consist of certain technology and trade names acquired from Tetraphase, and include the value of the Harvard, Paratek and Everest Licenses (see Note 10). The acquired intangible assets have definite useful lives and are being amortized on a straight-line basis over an estimated useful life of 10 years.
Goodwill represents the excess of the Purchase Price over the fair value of the net assets acquired as of the acquisition date. Goodwill represents the value of the stronger platform to increase patient access to the Company’s commercial products and the operational synergies of the combined Company. Goodwill has an indefinite useful life and is not amortized. The goodwill is only deductible for tax purposes if the Company makes a U.S. Internal Revenue Code Section 338 (“Section 338”) election. The Company did not make a Section 338 election.
12. Company-wide Realignments
On May 28, 2020, the Board of Directors of the Company approved a restructuring plan (the “2020 Realignment”) to align its organization with the Company’s sole focus on the commercialization of its products. The 2020 Realignment reduced the Company’s headcount. For the year ended December 31, 2020, total expense was comprised of $4.1 million for one-time termination benefits to the affected employees, including severance and health care benefits, offset by a $0.4 million reversal of non-cash, share-based compensation expense related to forfeited, unvested equity awards. As of June 30, 2021, the Company had made substantially all of the payments related to the 2020 Realignment.
In connection with the acquisition of Tetraphase, the Company incurred one-time charges related to a reduction in the combined Company’s headcount. For the year ended December 31, 2020, total expense was comprised of $3.1 million for one-time termination benefits to the affected employees, including severance and health care benefits. As of June 30, 2021, the Company had made substantially all of the payments related to this reduction in headcount.
13. Income Taxes
For the three and six months ended June 30, 2021, the Company recorded a provision for income taxes of 0 and $18,000, respectively. For the three and six months ended June 30, 2020, the Company did 0t record a provision for income taxes. As of June 30, 2021 and December 31, 2020, the Company established a full valuation allowance against its federal and state deferred tax assets due to the uncertainty surrounding the realization of such assets. There were 0 unrecognized tax benefits as of June 30, 2021 and December 31, 2020. The Company does not anticipate there will be a significant change in unrecognized tax benefits within the next 12 months.
17
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our condensed consolidated financial statements and the related notes and other financial information included elsewhere in this Quarterly Report on Form 10-Q and our audited financial statements and the related notes and other financial information included in our Annual Report on Form 10-K for the year ended December 31, 2020 filed with the U.S. Securities and Exchange Commission (the “SEC”) on March 8, 2021 (the “Form 10-K”).
Forward-looking Statements
This Quarterly Report on Form 10-Q contains “forward-looking statements” within the meaning of the federal securities laws, and such statements may involve substantial risks and uncertainties. All statements, other than statements of historical facts included in this Quarterly Report on Form 10-Q, including statements concerning our plans, objectives, goals, strategies, future events, future revenues or performance, future expenses, financing needs, plans or intentions relating to acquisitions, business trends and other information referred to under this section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” are forward-looking statements. Forward-looking statements generally relate to future events or our future financial or operating performance. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan,” “anticipate,” “target,” “forecast” or the negative of these terms and similar expressions intended to identify forward-looking statements. Forward-looking statements are not historical facts and reflect our current views with respect to future events. Forward-looking statements are also based on assumptions and are subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
There are a number of risks, uncertainties and other important factors that could cause our actual results to differ materially from the forward-looking statements contained in this Quarterly Report on Form 10-Q. Such risks, uncertainties and other factors are described under “Risk Factors” in Item 1A of our Form 10-K for the year ended December 31, 2020. We caution you that these risks, uncertainties and other factors may not contain all of the risks, uncertainties and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will affect us or our business in the way expected. All forward-looking statements in this Quarterly Report on Form 10-Q apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this Quarterly Report on Form 10-Q. We undertake no obligation to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances.
Business Overview
La Jolla Pharmaceutical Company is dedicated to the commercialization of innovative therapies that improve outcomes in patients suffering from life-threatening diseases. GIAPREZA™ (angiotensin II) injection is approved by the U.S. Food and Drug Administration (“FDA”) as a vasoconstrictor indicated to increase blood pressure in adults with septic or other distributive shock. XERAVA™ (eravacycline) for injection is approved by the FDA as a tetracycline class antibacterial indicated for the treatment of complicated intra-abdominal infections (“cIAI”) in patients 18 years of age and older.
On July 28, 2020, La Jolla completed its acquisition of Tetraphase Pharmaceuticals, Inc. and its subsidiaries (“Tetraphase”), a biopharmaceutical company focused on commercializing XERAVA, for $43 million in upfront cash plus potential future cash payments of up to $16 million. La Jolla’s consolidated financial results exclude Tetraphase’s financial results prior to the acquisition closing date of July 28, 2020.
In January 2021, La Jolla and certain of its wholly owned subsidiaries, including La Jolla Pharma, LLC, entered into a license agreement with PAION AG to commercialize GIAPREZA and XERAVA in the European Economic Area, the United Kingdom and Switzerland. Pursuant to the agreement: (i) the Company has received an upfront cash payment of $22.5 million, less a 15% refundable withholding tax; and (ii) the Company is entitled to receive potential commercial milestone payments of up to $109.5 million and royalties on net sales of GIAPREZA and XERAVA.
18
Product Portfolio
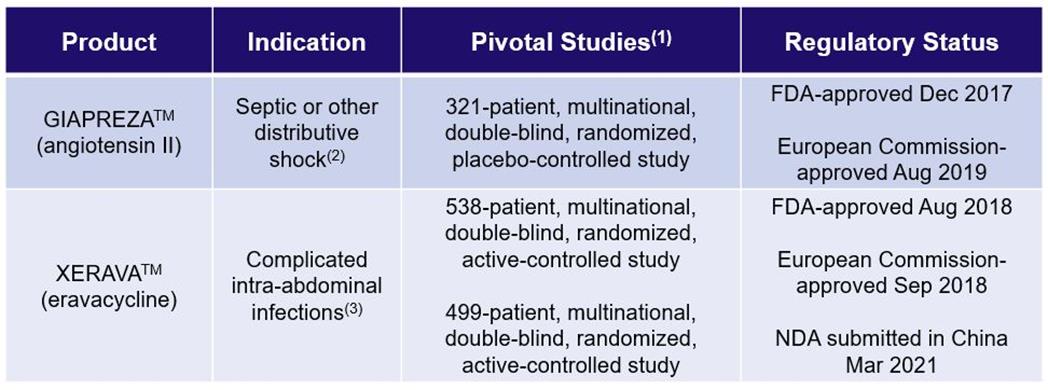
(1) For U.S. and European approval
(2) U.S.: GIAPREZA is a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock
European Union: GIAPREZA is indicated for the treatment of refractory hypotension in adults with septic or other distributive shock who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies
(3) U.S.: XERAVA is a tetracycline class antibacterial indicated for the treatment of cIAIs in patients 18 years of age and older
European Union: XERAVA is indicated for the treatment of cIAI in adults
GIAPREZA™ (angiotensin II)
GIAPREZA™ (angiotensin II) injection is approved by the FDA as a vasoconstrictor indicated to increase blood pressure in adults with septic or other distributive shock. GIAPREZA is approved by the European Commission (“EC”) for the treatment of refractory hypotension in adults with septic or other distributive shock who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies. GIAPREZA mimics the body’s endogenous angiotensin II peptide, which is central to the renin-angiotensin-aldosterone system (“RAAS”), which in turn regulates blood pressure. GIAPREZA is marketed in the U.S. by La Jolla Pharmaceutical Company on behalf of La Jolla Pharma, LLC, its wholly owned subsidiary, and is marketed in Europe by PAION Deutschland GmbH on behalf of La Jolla Pharma, LLC.
XERAVA™ (eravacycline)
XERAVA™ (eravacycline) for injection is approved by the FDA as a tetracycline class antibacterial indicated for the treatment of complicated intra-abdominal infections (“cIAI”) in patients 18 years of age and older. XERAVA is approved by the EC for the treatment of cIAI in adults. XERAVA is marketed in the U.S. by Tetraphase Pharmaceuticals, Inc., a wholly owned subsidiary of La Jolla, and is marketed in Europe by PAION Deutschland GmbH on behalf of Tetraphase. Everest, the Company’s licensee for mainland China, Taiwan, Hong Kong, Macau, South Korea, Singapore, the Malaysian Federation, the Kingdom of Thailand, the Republic of Indonesia, the Socialist Republic of Vietnam and the Republic of the Philippines, recently submitted an NDA in China, which was accepted by the China National Medical Products Administration (“NMPA”) in March 2021.
Product Candidates
In connection with the acquisition of Tetraphase, we acquired the following product candidates that are in early stage clinical development: (1) TP-6076, an IV formulation of a fully synthetic fluorocycline derivative for the treatment of certain multidrug-resistant gram-negative bacteria; (2) TP-271, an IV and oral formulation of a fully synthetic fluorocycline for the treatment of respiratory disease caused by bacterial biothreat and antibiotic-resistant public health pathogens, as well as bacterial pathogens associated with community-acquired bacterial pneumonia; and (3) TP-2846, an IV formulation of a tetracycline for the treatment of acute myeloid leukemia. At this time, there are no active studies nor anticipated future studies for any of these product candidates. We intend to seek out-license opportunities for these product candidates; however, at this time, we are unable to predict the likelihood of successfully out-licensing any of these product candidates.
19
Components of Our Results of Operations
The following table summarizes our results of operations for each of the periods below (in thousands):
| | Three Months Ended | | | Six Months Ended | |
| | June 30, | | | June 30, | |
| | 2021 | | | 2020 | | | Change | | | 2021 | | | 2020 | | | Change | |
Net product sales | | $ | 11,059 | | | $ | 5,805 | | | $ | 5,254 | | | $ | 19,696 | | | $ | 13,396 | | | $ | 6,300 | |
License revenue | | | 5,000 | | | | - | | | | 5,000 | | | | 30,500 | | | | - | | | | 30,500 | |
Cost of product sales | | | 2,156 | | | | 808 | | | | 1,348 | | | | 4,887 | | | | 1,524 | | | | 3,363 | |
Cost of license revenue | | | - | | | | - | | | | - | | | | 3,600 | | | | - | | | | 3,600 | |
Selling, general and administrative expense | | | 8,996 | | | | 8,677 | | | | 319 | | | | 17,751 | | | | 16,829 | | | | 922 | |
Research and development expense | | | 1,114 | | | | 8,781 | | | | (7,667 | ) | | | 2,672 | | | | 17,964 | | | | (15,292 | ) |
Other (expense) income, net | | | (60 | ) | | | (3,131 | ) | | | 3,071 | | | | (3,117 | ) | | | (1,262 | ) | | | (1,855 | ) |
Provision for income taxes | | | - | | | | - | | | | - | | | | 18 | | | | - | | | | 18 | |
Net income (loss) | | $ | 3,733 | | | $ | (15,592 | ) | | $ | 19,325 | | | $ | 18,151 | | | $ | (24,183 | ) | | $ | 42,334 | |
La Jolla’s consolidated financial results for the three and six months ended June 30, 2020 exclude the financial results of Tetraphase. La Jolla acquired Tetraphase, which commercialized XERAVA, on July 28, 2020.
Net Product Sales
Net product sales consist of revenue recognized from sales of GIAPREZA and XERAVA to hospitals and other healthcare organizations in the U.S. through a network of specialty and wholesale distributors. These specialty and wholesale distributors are considered our customers for accounting purposes.
La Jolla’s net product sales were $11.1 million and $19.7 million for the three and six months ended June 30, 2021, respectively, compared to $5.8 million and $13.4 million, respectively, for the same periods in 2020. La Jolla acquired Tetraphase, which commercialized XERAVA, on July 28, 2020. Net product sales excludes XERAVA for the three and six months ended June 30, 2020.
GIAPREZA U.S. net sales were $8.6 million and $15.4 million for the three and six months ended June 30, 2021, respectively, compared to $5.8 million and $13.4 million for the same periods in 2020. GIAPREZA U.S. net sales increased during the three and six months ended June 30, 2021 compared to the same periods in 2020 due to an increase in the number of vials sold to our customers. XERAVA U.S. net sales were $2.5 million and $4.3 million for the three and six months ended June 30, 2021, compared to zero for the same periods in 2020.
License Revenue
License revenue consists of revenue from out-license agreements with counterparties to develop and/or commercialize our products in territories outside of the U.S. in exchange for: (i) nonrefundable, upfront license fees; (ii) development, regulatory or commercial milestone payments; and/or (iii) sales-based royalties. License revenue also consists of revenue from commercial supply agreements with our out-licensees to supply a minimum quantity of our products in territories outside the U.S. in exchange for: (i) nonrefundable, upfront fees; and/or (ii) the reimbursement of manufacturing costs, plus a margin in certain cases.
License revenue was $5.0 million for the three months ended June 30, 2021, which consists of the transfer of certain XERAVA-related manufacturing know-how to Everest in connection with the Everest commercial supply agreement. License revenue was $30.5 million for the six months ended June 30, 2021, which consists of: (i) a $22.5 million upfront cash payment in connection with the PAION License; (ii) a $3.0 million regulatory milestone cash payment in connection with the Everest License; and (iii) $5.0 million for the transfer of certain XERAVA-related manufacturing know-how to Everest in connection with the Everest commercial supply agreement. The Company did not record license revenue during the three and six months ended June 30, 2020.
Cost of Product Sales
Cost of product sales consists primarily of expense associated with: (i) royalties payable to George Washington University, Harvard University and Paratek Pharmaceuticals, Inc.; (ii) manufacturing; (iii) the inventory fair value step-up adjustment recorded in connection with the acquisition of Tetraphase; and (iv) shipping and distribution.
20
Cost of product sales were $2.2 million and $4.9 million for the three and six months ended June 30, 2021, respectively, compared to $0.8 million and $1.5 million, respectively, for the same periods in 2020. Cost of product sales excludes XERAVA for the three and six months ended June 30, 2020. For the three and six months ended June 30, 2021, cost of product sales includes zero and $0.9 million, respectively, of the inventory fair value step-up adjustment recorded in connection with the acquisition of Tetraphase.
Cost of License Revenue
Cost of license revenue consists of amounts due under in-license agreements in connection with license revenue from commercially approved product. Cost of license revenue recognized in connection with product that is not commercially approved is recorded as research and development expense. Cost of license revenue was zero and $3.6 million for the three and six months ended June 30, 2021, respectively, which consists of amounts due under the George Washington University and Harvard University license agreements in connection with the upfront cash payment received from the PAION License. The Company did not record cost of license revenue during the three and six months ended June 30, 2020.
Selling, General and Administrative Expense
Selling, general and administrative expense consists of non-personnel and personnel expenses. Non-personnel-related expense includes expense related to: (i) professional fees for legal, patent, consulting, accounting and audit services; (ii) sales and marketing costs such as speaker programs, advertising and promotion; and (iii) facilities and information technology. Personnel-related expense includes expense related to salaries, benefits and share-based compensation for personnel engaged in sales, finance and administrative functions. We do not expect our selling, general and administrative expense to change significantly in the near term.
The following table summarizes these expenses for each of the periods below (in thousands):
| | Three Months Ended | | | Six Months Ended | |
| | June 30, | | | June 30, | |
| | 2021 | | | 2020 | | | Change | | | 2021 | | | 2020 | | | Change | |
Non-personnel expense: | | | | | | | | | | | | | | | | | | | | | | | | |
Sales and marketing | | $ | 1,315 | | | $ | 588 | | | $ | 727 | | | $ | 2,510 | | | $ | 1,811 | | | $ | 699 | |
Professional fees | | | 1,266 | | | | 1,356 | | | | (90 | ) | | | 2,794 | | | | 2,227 | | | | 567 | |
Facility | | | 105 | | | | 554 | | | | (449 | ) | | | 119 | | | | 1,047 | | | | (928 | ) |
Other | | | 811 | | | | 339 | | | | 472 | | | | 1,603 | | | | 925 | | | | 678 | |
Total non-personnel expense | | | 3,497 | | | | 2,837 | | | | 660 | | | | 7,026 | | | | 6,010 | | | | 1,016 | |
Personnel expense: | | | | | | | | | | | | | | | | | | | | | | | | |
Salaries, bonuses and benefits | | | 4,522 | | | | 3,261 | | | | 1,261 | | | | 8,908 | | | | 7,396 | | | | 1,512 | |
One-time charges for reductions in headcount | | | - | | | | 1,953 | | | | (1,953 | ) | | | - | | | | 1,953 | | | | (1,953 | ) |
Share-based compensation expense | | | 977 | | | | 626 | | | | 351 | | | | 1,817 | | | | 1,470 | | | | 347 | |
Total personnel expense | | | 5,499 | | | | 5,840 | | | | (341 | ) | | | 10,725 | | | | 10,819 | | | | (94 | ) |
Total selling, general and administrative expense | | $ | 8,996 | | | $ | 8,677 | | | $ | 319 | | | $ | 17,751 | | | $ | 16,829 | | | $ | 922 | |
During the three and six months ended June 30, 2021, total selling, general and administrative non-personnel expense increased compared to the same period in 2020 primarily resulting from the inclusion of Tetraphase-related costs, which are excluded from La Jolla’s financial results for the three and six months ended June 30, 2020.
During the three and six months ended June 30, 2021, total selling, general and administrative personnel expense decreased compared to the same periods in 2020 primarily as a result of one-time charges in 2020 resulting from a reduction of headcount from a Company-wide realignment in May 2020; partially offset by an increase in headcount resulting from the acquisition of Tetraphase.
21
Research and Development Expense
Research and development expense consists of non-personnel and personnel expenses. Non-personnel-related expense includes expense related to: (i) amounts due under in-license agreements for drug product that is not commercially approved; (ii) manufacturing development; (iii) facilities and information technology; and (iv) conducting clinical studies. Personnel-related expense includes expense related to salaries, benefits and share-based compensation for personnel engaged in research and development functions. We expect our research and development expense to decrease in the near term.
The following table summarizes these expenses for each of the periods below (in thousands):
| | Three Months Ended | | | Six Months Ended | |
| | June 30, | | | June 30, | |
| | 2021 | | | 2020 | | | Change | | | 2021 | | | 2020 | | | Change | |
Non-personnel expense: | | | | | | | | | | | | | | | | | | | | | | | | |
GIAPREZA | | $ | 304 | | | $ | 1,762 | | | $ | (1,458 | ) | | $ | 479 | | | $ | 2,843 | | | $ | (2,364 | ) |
XERAVA | | | 154 | | | | - | | | | 154 | | | | 416 | | | | - | | | | 416 | |
LJPC-401 | | | 72 | | | | - | | | | 72 | | | | 102 | | | | 1,531 | | | | (1,429 | ) |
LJPC-0118 | | | 11 | | | | 404 | | | | (393 | ) | | | 16 | | | | 917 | | | | (901 | ) |
Facility | | | 2 | | | | 1,124 | | | | (1,122 | ) | | | 5 | | | | 2,560 | | | | (2,555 | ) |
Other | | | 153 | | | | 149 | | | | 4 | | | | 726 | | | | 673 | | | | 53 | |
Total non-personnel expense | | | 696 | | | | 3,439 | | | | (2,743 | ) | | | 1,744 | | | | 8,524 | | | | (6,780 | ) |
Personnel expense: | | | | | | | | | | | | | | | | | | | | | | | | |
Salaries, bonuses and benefits | | | 371 | | | | 1,933 | | | | (1,562 | ) | | | 605 | | | | 4,468 | | | | (3,863 | ) |
One-time charges for reductions in headcount | | | - | | | | 2,445 | | | | (2,445 | ) | | | - | | | | 2,445 | | | | (2,445 | ) |
Share-based compensation expense | | | 47 | | | | 964 | | | | (917 | ) | | | 323 | | | | 2,527 | | | | (2,204 | ) |
Total personnel expense | | | 418 | | | | 5,342 | | | | (4,924 | ) | | | 928 | | | | 9,440 | | | | (8,512 | ) |
Total research and development expense | | $ | 1,114 | | | $ | 8,781 | | | $ | (7,667 | ) | | $ | 2,672 | | | $ | 17,964 | | | $ | (15,292 | ) |
During the three and six months ended June 30, 2021, total research and development non-personnel expense decreased compared to the same periods in 2020 primarily as a result of: (i) decreases in program-related expenses as we de-prioritized our product candidates and focused on the commercialization of GIAPREZA and XERAVA; (ii) decreases in manufacturing development-related expenses for GIAPREZA; and (iii) decreases in facility-related expenses primarily as a result of the termination of our lease for office and laboratory space in San Diego, California effective August 31, 2020; partially offset by increases in manufacturing and clinical development costs related to the acquisition of XERAVA.
During the three and six months ended June 30, 2021, total research and development personnel expense, including share-based compensation expense, decreased compared to the same periods in 2020 as a result of: (i) reduced headcount; (ii) one-time charges in 2020 resulting from a reduction of headcount from a Company-wide realignment in May 2020; and (iii) a decrease in personnel allocations to research and development activities in 2021.
Tetraphase’s research and development expense is excluded from La Jolla’s financial results for the three and six months ended June 30, 2020.
Other (Expense) Income, Net
Other (expense) income, net consists of the following: (i) interest expense accrued for our deferred royalty obligation; (ii) income from distributions received in connection with our non-voting profits interest in a related party; (iii) gain (loss) from changes in the fair value of contingent value rights (“CVRs”); and (iv) interest income generated from cash held in savings accounts.
During the three months ended June 30, 2021, other (expense) income, net was $(0.1) million, compared to $(3.1) million for the same period in 2020. This $3.0 million increase in other (expense) income, net was primarily due to: (i) a $2.5 million increase in the receipt of distributions in connection with the Company’s non-voting profits interest in a related party; and (ii) a $0.9 million decrease in loss on disposal of equipment; partially offset by a $0.2 million increase in interest expense for our deferred royalty obligation.
22
During the six months ended June 30, 2021, other (expense) income, net was $(3.1) million, compared to $(1.3) million for the same period in 2020. This $1.8 million decrease in other (expense) income, net was primarily due to: (i) a $1.6 million decrease in the receipt of distributions in connection with the Company’s non-voting profits interest in a related party; (ii) a $0.4 million decrease in loss from changes in the fair value of CVRs; (iii) a $0.4 million increase in interest expense for our deferred royalty obligation; and (iv) a $0.2 million decrease in interest income generated from cash held in savings accounts; partially offset by a $0.9 million decrease in loss on disposal of equipment.
Liquidity and Capital Resources
As of June 30, 2021 and December 31, 2020, we had cash and cash equivalents of $45.9 million and $21.2 million, respectively. Based on our current operating plans and projections, we believe that our existing cash and cash equivalents will be sufficient to fund operations for at least one year from the date this Quarterly Report on Form 10-Q is filed with the SEC. The Company expects to fund future operations with existing cash or cash generated from operations.
La Jolla’s net cash provided by (used for) operating activities for the three and six months ended June 30, 2021 was $7.1 million and $24.3 million, respectively, compared to $(8.4) million and $(20.6) million, respectively, for the same periods in 2020. Net cash provided by (used for) operating activities for the three and six months ended June 30, 2021, excluding net receipts in connection with out-license agreements, commercial supply agreements and payments related to reductions in headcount, was $2.2 million and $0.4 million, respectively, compared to $(6.7) million and $(16.0) million, respectively, for the same periods in 2020. Net receipts (payments) in connection with out-license agreements were $(1.4) million and $18.4 million for the three and six months ended June 30, 2021,respectively, and zero for the same periods in 2020. Net receipts in connection with commercial supply agreements were $6.8 million for the three and six months ended June 30, 2021, and zero for the same periods in 2020. Payments related to reductions in headcount were $0.5 million and $1.3 million for the three and six months ended June 30, 2021, respectively, and $1.6 million and $4.6 million, respectively, for the same periods in 2020.
The amount and timing of additional future funding needs, if any, will depend on many factors, including the success of our commercialization efforts for GIAPREZA and XERAVA and our ability to control expenses. If necessary, we will raise additional capital through equity or debt financings. We can provide no assurance that additional financing will be available to us on favorable terms, or at all.
Contractual Obligations
HealthCare Royalty Partners Royalty Agreement
In May 2018, we closed a $125.0 million royalty financing agreement (the “Royalty Agreement”) with HealthCare Royalty Partners (“HCR”). Under the terms of the Royalty Agreement, we received $125.0 million in exchange for tiered royalty payments on worldwide net sales of GIAPREZA. HCR is entitled to receive quarterly royalties on worldwide net sales of GIAPREZA beginning April 1, 2018. Quarterly payments to HCR under the Royalty Agreement start at a maximum royalty rate, with step-downs based on the achievement of annual net product sales thresholds. Through December 31, 2021, the royalty rate will be a maximum of 10%. Starting January 1, 2022, the maximum royalty rate may increase by 4% if an agreed-upon, cumulative net product sales threshold has not been met, and, starting January 1, 2024, the maximum royalty rate may increase by an additional 4% if a different agreed-upon, cumulative net product sales threshold has not been met. The Royalty Agreement is subject to maximum aggregate royalty payments to HCR of $225.0 million. The Royalty Agreement expires upon the first to occur of January 1, 2031 or when the maximum aggregate royalty payments have been made. The Royalty Agreement was entered into by our wholly owned subsidiary, La Jolla Pharma, LLC, and HCR has no recourse under the Royalty Agreement against La Jolla Pharmaceutical Company or any assets other than GIAPREZA.
In-license Agreements
George Washington University
In December 2014, the Company entered into a patent license agreement with George Washington University (“GW”), which was subsequently amended and restated (the “GW License”) and assigned to La Jolla Pharma, LLC. Pursuant to the GW License, GW exclusively licensed to the Company certain intellectual property rights relating to GIAPREZA, including the exclusive rights to certain issued patents and patent applications covering GIAPREZA. Under the GW License, La Jolla Pharma, LLC is obligated to use commercially reasonable efforts to develop, commercialize, market and sell GIAPREZA. The Company has paid a one-time license initiation fee, annual maintenance fees, an amendment fee, additional payments following the achievement of certain development and regulatory milestones and royalties. As a result of the European Commission’s approval of GIAPREZA in August
23
2019, the Company made a milestone payment to GW in the amount of $0.5 million in the first quarter of 2020. The Company is obligated to pay a 6% royalty on net sales of GIAPREZA and 15% on payments received from sublicensees. The obligation to pay royalties under this agreement extends through the last-to-expire patent covering GIAPREZA.
Harvard University
In August 2006, the Company entered into a license agreement with Harvard University (“Harvard”), which was subsequently amended and restated (the “Harvard License”). Pursuant to the Harvard License, Harvard exclusively licensed to the Company certain intellectual property rights relating to tetracycline-based products, including XERAVA, including the exclusive rights to certain issued patents and patent applications covering such products. Under the Harvard License, the Company is obligated to use commercially reasonable efforts to develop, commercialize, market and sell tetracycline-based products, including XERAVA. For each product covered by the Harvard License, the Company is obligated to make certain payments for the following: (i) up to approximately $15.1 million upon the achievement of certain clinical development and regulatory milestones; (ii) a 5% royalty on direct U.S. net sales of XERAVA; (iii) a single-digit tiered royalty on direct ex-U.S. net sales of XERAVA, starting at a minimum royalty rate of 4.5%, with step-ups to a maximum royalty of 7.5% based on the achievement of annual net product sales thresholds; and (iv) 20% on payments received from sublicensees. The obligation to pay royalties under this agreement extends through the last-to-expire patent covering tetracycline-based products, including XERAVA.
Paratek Pharmaceuticals, Inc.
In March 2019, the Company entered into a license agreement with Paratek Pharmaceuticals, Inc. (“Paratek”), which was subsequently amended and restated (the “Paratek License”). Pursuant to the Paratek License, Paratek non-exclusively licensed to the Company certain intellectual property rights relating to XERAVA, including non-exclusive rights to certain issued patents and patent applications covering XERAVA. The Company is obligated to pay Paratek a 2.25% royalty based on direct U.S. net sales of XERAVA. The Company’s obligation to pay royalties with respect to the licensed product is retroactive to the date of the first commercial sale of XERAVA and shall continue until there are no longer any valid claims of the Paratek patents, which will expire in October 2023.
Out-license Agreements
PAION AG
On January 12, 2021, La Jolla Pharmaceutical Company and certain of its wholly owned subsidiaries, including La Jolla Pharma, LLC, entered into an exclusive license agreement with PAION AG and its wholly owned subsidiary (collectively, “PAION”) (the “PAION License”). Pursuant to the PAION License, La Jolla granted PAION an exclusive license to commercialize GIAPREZA and XERAVA in the European Economic Area, the United Kingdom and Switzerland (collectively, the “PAION Territory”). La Jolla has received an upfront cash payment of $22.5 million, less a 15% refundable withholding tax, and is entitled to receive potential commercial milestone payments of up to $109.5 million and double-digit tiered royalty payments. In addition, royalties payable under the PAION License will be subject to reduction on account of generic competition and after patent expiration in a jurisdiction. Pursuant to the PAION License, PAION will be solely responsible for the future development and commercialization of GIAPREZA and XERAVA in the PAION Territory. PAION is required to use commercially reasonable efforts to commercialize GIAPREZA and XERAVA in the PAION Territory. The Company has not received any payments from PAION related to either royalties or commercial milestones.
On July 13, 2021, the Company entered into a commercial supply agreement with PAION whereby the Company will supply PAION a minimum quantity of GIAPREZA and XERAVA through July 13, 2024. The supply agreement will automatically renew until the earlier of July 13, 2027, or until a new supply agreement is executed. During the initial 3-year term of the supply agreement, the Company will be reimbursed for direct and certain indirect manufacturing costs at cost.
Everest Medicines Limited
In February 2018, the Company entered into a license agreement with Everest, which was subsequently amended and restated (the “Everest License”). Pursuant to the Everest License, the Company granted Everest an exclusive license to develop and commercialize XERAVA for the treatment of cIAI and other indications in mainland China, Taiwan, Hong Kong, Macau, South Korea, Singapore, the Malaysian Federation, the Kingdom of Thailand, the Republic of Indonesia, the Socialist Republic of Vietnam and the Republic of the Philippines (collectively, the “Everest Territory”). The Company is eligible to receive an additional $8.0 million regulatory milestone payment and up to an aggregate of $20.0 million in sales milestone payments. The Company is also entitled to receive tiered royalties from Everest at percentages in the low double digits on sales, if any, in the Everest Territory of products
24
containing eravacycline. Royalties are payable with respect to each jurisdiction in the Everest Territory until the latest to occur of: (1) the last-to-expire of specified patent rights in such jurisdiction in the Everest Territory; (2) expiration of marketing or regulatory exclusivity in such jurisdiction in the Everest Territory; or (3) 10 years after the first commercial sale of a product in such jurisdiction in the Everest Territory. In March 2021, the Company received a $3.0 million milestone payment associated with the submission of an NDA with the China NMPA for XERAVA for the treatment of cIAI in patients in China.
In May 2021, the Company entered into a commercial supply agreement with Everest whereby the Company will supply Everest a minimum quantity of XERAVA through December 31, 2023 and will transfer to Everest certain XERAVA-related manufacturing know-how. Pursuant to the supply agreement: (i) the Company has received $6.8 million of upfront payments comprised of: (1) a $4.0 million upfront technology transfer payment; and (2) a $2.8 million partial prepayment for XERAVA that is expected to be delivered to Everest during 2022; (ii) the Company will receive an additional $1.0 million technology transfer payment by January 30, 2022; and (iii) the Company will be reimbursed for direct and certain indirect manufacturing costs at 110% of costs through December 31, 2023. The Company recognized the $5.0 million of technology transfer-related payments as license revenue during the three and six months ended June 30, 2021 as Everest obtained control of the XERAVA-related manufacturing know-how prior to June 30, 2021. The Company recognized the $2.8 million partial prepayment for XERAVA that is expected to be delivered to Everest during 2022 as deferred revenue as of June 30, 2021 as the performance obligation to deliver XERAVA has not yet been satisfied.
Off−Balance Sheet Arrangements
We have no off−balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in our financial condition, expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Estimates
We believe the estimates, assumptions and judgments involved in the accounting policies described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 of our Form 10-K for the year ended December 31, 2020 are most critical to understanding and evaluating our reported financial results. During the six months ended June 30, 2021, other than the license revenue recognition policy described in Note 2 to our condensed consolidated financial statements included in Item 1 of this Quarterly Report on Form 10-Q, there have been no material changes to the critical accounting policies and estimates as described in Item 7 of our Form 10-K for the year ended December 31, 2020.
Recent Accounting Pronouncements
See Note 2 to our condensed consolidated financial statements included in Item 1 of this Quarterly Report on Form 10-Q.
25
Item 3. Quantitative and Qualitative Disclosures about Market Risk
We are a smaller reporting company, as defined by Rule 12b-2 under the Securities and Exchange Act of 1934 and in Item 10(f)(1) of Regulation S-K, and are not required to provide the information under this item.
Item 4. Controls and Procedures
Management’s Evaluation of our Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and our principal financial officer, evaluated, as of the end of the period covered by this Quarterly Report on Form 10-Q, the effectiveness of our disclosure controls and procedures. Based on that evaluation of our disclosure controls and procedures as of June 30, 2021, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures as of such date are effective at the reasonable assurance level. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the U.S. Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during our most recent quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
26
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
From time to time, we may become subject to claims and litigation arising in the ordinary course of business. We are not a party to any material legal proceedings, nor are we aware of any material pending or threatened litigation.
Item 1A. Risk Factors
Our business is subject to various risks, including those described in Item 1A of our Annual Report on Form 10-K for the year ended December 31, 2020. There have been no material changes from the risk factors disclosed in Item 1A of our Annual Report on Form 10-K.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
Item 6. Exhibits
27
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | La Jolla Pharmaceutical Company |
| | | |
Date: | August 5, 2021 | By: | /s/ Larry Edwards |
| | | Larry Edwards |
| | | Director, President and Chief Executive Officer |
| | | (principal executive officer) |
| | | |
| | | /s/ Michael Hearne |
| | | Michael Hearne |
| | | Chief Financial Officer |
| | | (principal financial and accounting officer) |
28
