The cash payments consisted of: $1.0 million paid at closing on February 28, 2018, $1.0 million at one year from closing, $1.0 million at two years from closing, and $5.0 million at three years from closing (the payments due on the one, two, three year anniversary are collectively the “time-based payment obligation”). The equity payment (the “equity payment” and, together with the time-based payment obligation, the “deferred purchase price arrangement”) was due upon the earlier of the initial public offering of shares of C3J’s common stock pursuant to an effective registration statement under the Securities Act of 1933, as amended, the sale of all or substantially all of C3J’s assets to a third party, or a consolidation or merger into a third party. On December 20, 2018, in contemplation of the Merger (see Note 1), the deferred purchase price arrangement was amended. Under the amended agreement, the purchase consideration consisted of (i) closing consideration of $1.0 million paid on February 28, 2018, (ii) cash payments of $1.0 million on January 31, 2019, $1.0 million on January 31, 2020, and $2.0 million on January 31, 2021, (iii) an issuance of that number of shares of C3J’s common stock equal to ten percent of C3J’s fully-diluted capitalization, excluding options and restricted stock awards, immediately prior to the closing of the Merger, and (iv) potential milestone payments of up to $39.5 million related to the development and relevant regulatory approval of products utilizing bacteriophage from the synthetic phage assets acquired from SGI (the “milestone payment obligation”).
The equity payment was settled on May 9, 2019, the date of the Merger (Note 1).
The present value of the time-based payment obligations was included in the Company’s balance sheet, with interest accreted to the maturity date. The Company paid the last installment of the time-based payment obligation in the amount of $2.0 million during the three months ended March 31, 2021. For three months ended March 31, 2021 and 2020, the Company recognized $60,000 and $159,000 of interest expense related to the time-based payment obligations.
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited consolidated financial statements and related notes included in this Quarterly Report on Form 10-Q, our audited financial statements and notes thereto as of and for the year ended December 31, 2020 included in our Form 10-K filed on March 18, 2021 with the U.S. Securities and Exchange Commission (the “SEC”).
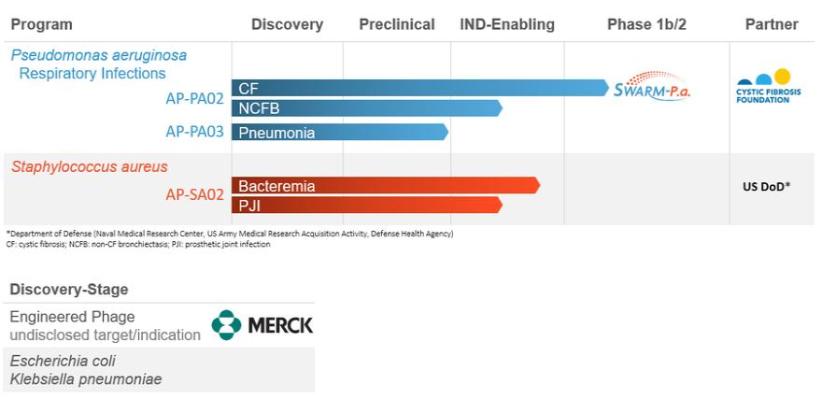
Our predecessor, C3 Jian, Inc., was incorporated under the laws of the State of California on November 4, 2005. On February 26, 2016, as part of a reorganization transaction, C3 Jian, Inc. merged with a wholly owned subsidiary of C3J Therapeutics, Inc. (“C3J”), and as part of this process, C3 Jian, Inc. was converted to a limited liability company organized under the laws of the State of California named C3 Jian, LLC. On May 9, 2019, C3J completed a reverse merger with AmpliPhi Biosciences Corporation, a bacteriophage development stage company (“AmpliPhi”), where Ceres Merger Sub, Inc., a wholly-owned subsidiary of AmpliPhi, merged with and into C3J (the “Merger”). Following the completion of the Merger, and a $10.0 million concurrent private placement financing, the former C3J shareholders owned approximately 76% of our common stock and the former AmpliPhi shareholders owned approximately 24% of our common stock.
Immediately prior to the Merger, AmpliPhi completed a 1-for-14 reverse stock split and changed its name to Armata Pharmaceuticals, Inc. Our common stock is traded on the NYSE American exchange under the symbol “ARMP.” We are headquartered in Marina del Rey, CA, in a 35,000 square-foot research and development facility built for product development with capabilities spanning from bench to clinic. In addition to microbiology, synthetic biology, formulation, chemistry and analytical laboratories, the facility is equipped with two licensed GMP drug manufacturing suites enabling the production, testing and release of clinical material.
Statements contained in this Quarterly Report on Form 10-Q that are not statements of historical fact are forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Such forward-looking statements include, without limitation, statements concerning product development plans, commercialization of our products, the expected market opportunity for our products, the use of bacteriophages and synthetic phages to kill bacterial pathogens, having resources sufficient to fund our operations into the first quarter of 2022, future funding sources, general and administrative expenses, clinical trial and other research and development expenses, costs of