SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a)
of the Securities Exchange Act of 1934
(Amendment No. __)
Filed by the Registrant [ ]
Filed by a Party other than the Registrant [x]
Check the appropriate box:
| [ ] | Preliminary Proxy Statement |
| [ ] | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| [ ] | Definitive Proxy Statement |
| [ ] | Definitive Additional Materials |
| [X] | Soliciting Material Pursuant to § 240.14a-12 |
Forest Laboratories, Inc.
(Name of Registrant as Specified In Its Charter)
Icahn Partners LP
Icahn Partners Master Fund LP
Icahn Partners Master Fund II L.P.
Icahn Partners Master Fund III L.P.
High River Limited Partnership
Hopper Investments LLC
Barberry Corp.
Icahn Onshore LP
Icahn Offshore LP
Icahn Capital L.P.
IPH GP LLC
Icahn Enterprises Holdings L.P.
Icahn Enterprises G.P. Inc.
Beckton Corp.
Carl C. Icahn
Dr. Eric J. Ende
Pierre Legault
Andrew J. Fromkin
Daniel A. Ninivaggi
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (check the appropriate box):
| [X] | No fee required. |
| [ ] | Fee computed on table below per Exchange Act Rule 14a-6(i)(4) and 0-11. |
1) Title of each class of securities to which transaction applies:
2) Aggregate number of securities to which transaction applies:
3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
4) Proposed maximum aggregate value of transaction:
5) Total fee paid:
[ ] Fee paid previously with preliminary materials.
[ ] Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing.
1) Amount Previously Paid:
2) Form, Schedule or Registration Statement No.:
3) Filing Party:
4) Date Filed:
SECURITY HOLDERS ARE ADVISED TO READ THE PROXY STATEMENT AND OTHER DOCUMENTS RELATED TO THE SOLICITATION OF PROXIES BY CARL C. ICAHN AND HIS AFFILIATES FROM THE STOCKHOLDERS OF FOREST LABORATORIES, INC. FOR USE AT ITS 2012 ANNUAL MEETING WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION, INCLUDING INFORMATION RELATING TO THE PARTICIPANTS IN SUCH PROXY SOLICITATION. WHEN COMPLETED, A DEFINITIVE PROXY STATEMENT AND A FORM OF PROXY WILL BE MAILED TO STOCKHOLDERS OF FOREST LABORATORIES, INC. AND WILL ALSO BE AVAILABLE AT NO CHARGE AT THE SECURITIES AND EXCHANGE COMMISSION’S WEBSITE AT HTTP://WWW.SEC.GOV. INFORMATION RELATING TO THE PARTICIPANTS IN SUCH PROXY SOLICITATION IS CONTAINED IN THE AMENDED PRELIMINARY PROXY STATEMENT FILED BY MR. ICAHN AND HIS AFFILIATES ON JULY 11, 2012 (THE “PRELIMINARY PROXY”). EXCEPT AS OTHERWISE DISCLOSED HEREIN OR IN THE PRELIMINARY PROXY, THE PARTICIPANTS HAVE NO INTEREST IN FOREST LABORATORIES, INC. OTHER THAN THROUGH THE BENEFICIAL OWNERSHIP OF SHARES OF COMMON STOCK, PAR VALUE $0.10 PER SHARE, OF FOREST LABORATORIES, INC., AS DISCLOSED IN THE PRELIMINARY PROXY. THE PRELIMINARY PROXY IS AVAILABLE AT NO CHARGE AT THE SECURITIES AND EXCHANGE COMMISSION’S WEBSITE AT HTTP://WWW.SEC.GOV.

Forest Laboratories
Shareholder Presentation
July 2012

Disclaimer
Special note regarding this presentation
• This presentation includes information based on data found in filings with the SEC, independent industry publications and other
sources. Although we believe that the data is reliable, we do not guarantee the accuracy or completeness of this information and have
not independently verified any such information. We have not sought, nor have we received, permission from any third-party to
include their information in this presentation.
sources. Although we believe that the data is reliable, we do not guarantee the accuracy or completeness of this information and have
not independently verified any such information. We have not sought, nor have we received, permission from any third-party to
include their information in this presentation.
• Many of the statements in this presentation reflect our subjective belief. Although we have reviewed and analyzed the information
that has informed our opinions, we do not guarantee the accuracy of any such beliefs.
that has informed our opinions, we do not guarantee the accuracy of any such beliefs.
• Sections of this presentation refer to our track record of Board representation at Biogen Idec, ImClone Systems Inc., Genzyme
Corporation, and Amylin Pharmaceuticals. We believe our experience at these companies was a success and resulted in an increase in
shareholder value that benefited all shareholders. However, this success at these companies is not necessarily indicative of future
results at Forest Laboratories if our nominees were to be elected to the Forest Laboratories Board of Directors.
Corporation, and Amylin Pharmaceuticals. We believe our experience at these companies was a success and resulted in an increase in
shareholder value that benefited all shareholders. However, this success at these companies is not necessarily indicative of future
results at Forest Laboratories if our nominees were to be elected to the Forest Laboratories Board of Directors.
• SECURITY HOLDERS ARE ADVISED TO READ THE PROXY STATEMENT AND OTHER DOCUMENTS RELATED TO THE SOLICITATION OF
PROXIES BY CARL C. ICAHN AND HIS AFFILIATES FROM THE STOCKHOLDERS OF FOREST LABORATORIES, INC. FOR USE AT ITS 2012
ANNUAL MEETING WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION, INCLUDING
INFORMATION RELATING TO THE PARTICIPANTS IN SUCH PROXY SOLICITATION. WHEN COMPLETED, A DEFINITIVE PROXY STATEMENT
AND A FORM OF PROXY WILL BE MAILED TO STOCKHOLDERS OF FOREST LABORATORIES, INC. AND WILL ALSO BE AVAILABLE AT NO
CHARGE AT THE SECURITIES AND EXCHANGE COMMISSION’S WEBSITE AT HTTP://WWW.SEC.GOV. INFORMATION RELATING TO THE
PARTICIPANTS IN SUCH PROXY SOLICITATION IS CONTAINED IN THE AMENDED PRELIMINARY PROXY STATEMENT FILED BY MR. ICAHN
AND HIS AFFILIATES ON JULY 11, 2012 (THE “PRELIMINARY PROXY”). EXCEPT AS OTHERWISE DISCLOSED HEREIN OR IN THE
PRELIMINARY PROXY, THE PARTICIPANTS HAVE NO INTEREST IN FOREST LABORATORIES, INC. OTHER THAN THROUGH THE BENEFICIAL
OWNERSHIP OF SHARES OF COMMON STOCK, PAR VALUE $0.10 PER SHARE, OF FOREST LABORATORIES, INC., AS DISCLOSED IN THE
PRELIMINARY PROXY. THE PRELIMINARY PROXY IS AVAILABLE AT NO CHARGE AT THE SECURITIES AND EXCHANGE COMMISSION’S
WEBSITE AT HTTP://WWW.SEC.GOV.
PROXIES BY CARL C. ICAHN AND HIS AFFILIATES FROM THE STOCKHOLDERS OF FOREST LABORATORIES, INC. FOR USE AT ITS 2012
ANNUAL MEETING WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION, INCLUDING
INFORMATION RELATING TO THE PARTICIPANTS IN SUCH PROXY SOLICITATION. WHEN COMPLETED, A DEFINITIVE PROXY STATEMENT
AND A FORM OF PROXY WILL BE MAILED TO STOCKHOLDERS OF FOREST LABORATORIES, INC. AND WILL ALSO BE AVAILABLE AT NO
CHARGE AT THE SECURITIES AND EXCHANGE COMMISSION’S WEBSITE AT HTTP://WWW.SEC.GOV. INFORMATION RELATING TO THE
PARTICIPANTS IN SUCH PROXY SOLICITATION IS CONTAINED IN THE AMENDED PRELIMINARY PROXY STATEMENT FILED BY MR. ICAHN
AND HIS AFFILIATES ON JULY 11, 2012 (THE “PRELIMINARY PROXY”). EXCEPT AS OTHERWISE DISCLOSED HEREIN OR IN THE
PRELIMINARY PROXY, THE PARTICIPANTS HAVE NO INTEREST IN FOREST LABORATORIES, INC. OTHER THAN THROUGH THE BENEFICIAL
OWNERSHIP OF SHARES OF COMMON STOCK, PAR VALUE $0.10 PER SHARE, OF FOREST LABORATORIES, INC., AS DISCLOSED IN THE
PRELIMINARY PROXY. THE PRELIMINARY PROXY IS AVAILABLE AT NO CHARGE AT THE SECURITIES AND EXCHANGE COMMISSION’S
WEBSITE AT HTTP://WWW.SEC.GOV.
2

Key Conclusions of Report
3
Key Conclusion | Pages |
Today, We Believe Forest is in Crisis - CEO Solomon Was Wrong in the Past With His Overly Optimistic Predictions Results: Forest’s stock is down 11% in the past 10 years and more than 50% from the peak - The company was completely unprepared for the Lexapro patent cliff since earnings are expected to decline by ~80% in FY13 | 7 - 10 22 - 23 |
We Are Very Concerned Solomon Will Be Wrong Again About His Currently Optimistic View of the Company’s Pipeline Since New Pipeline Drugs Have Missed Guidance 8 out of 11 Times in Past Several Years | 24 |
The Current Strategy Is Not Expected to Offset Lost Revenues From the Lexapro Patent Cliff (~$1 B Shortfall in FY13) and Not Projected to Offset Namenda ($1.4 B in FY12) Patent Cliff in FY16 - We are concerned the current Board will permit Solomon to risk the company’s cash to make up for the projected shortfall | 19 - 23 |
We Believe Strategic Flaws Have Caused Lack Of Focus & Cost Inefficiency | 25 - 28 |
Weak History of Capital Allocation Causes Us to Fear Future Uses for Forest’s $3.2 B of Cash | 29 - 31 |
We Believe at Least 50% (5 of 10) of Board Lacks Independence Including Presiding “Independent” Director | 33 |
Flawed Compensation Policies Have Enriched CEO & Others; Chair of Compensation Committee Still in Role | 34 - 35 |
CEO Has Had Well-Timed Stock Sales Including While Company Repurchased Stock | 36 - 38 |
CEO Solomon’s Son, After Only 5 Years at Forest, Was Promoted and Given Significant Responsibility for Business Development and Strategic Planning; We Believe He Is Significantly Responsible for the Company’s Current Predicament; Despite His Failures, He Has Been Promoted to SVP Business Development and Strategic Planning and He Is Now a Candidate for CEO; How Can a Board that Calls Itself “Strong & Independent” Be Responsible For This? | 39 - 40 |
We Believe Management Has Not Delivered On Its Word | 42 |
If Solomon is Wrong Again as it Appears to Us He Will Be Based On Disappointing Results of Current Pipeline Drugs, It Will Be Devastating for the Company. To Avoid This Outcome, We Believe A Strong & Truly Independent Board Which Will Hold Management Accountable Is Extremely Necessary. | 24 54 - 58 |
Icahn’s Track Record of Board Representation in Biopharma Shows an Impressive Creation of Shareholder Value and Is Well Aligned With All Shareholders | 59 - 60 |

Presentation Summary
• We believe Change is Needed as the Board has overseen:
– significant stock underperformance (p. 7 - 11) and massive destruction of value (p. 12)
– an inadequate and flawed company strategy (p. 15 - 28)
– significant corporate waste and cost structure inefficiency (p. 27 - 28)
– inefficient and ineffective deployment of capital (p. 29 - 31)
– corporate governance failures (p. 32)
• we believe that 50% of Board lacks independence (p.33)
• CEO Solomon’s Son, After Only 5 Years at Forest, Was Promoted and Given Significant Responsibility for Business Development
and Strategic Planning; We Believe He Is Significantly Responsible for the Company’s Current Predicament; Despite His
Failures, He Has Been Promoted to SVP Business Development and Strategic Planning and He Is Now a Candidate for CEO; How
Can a Board that Calls Itself “Strong & Independent” Be Responsible For This? (p. 39 - 40)
and Strategic Planning; We Believe He Is Significantly Responsible for the Company’s Current Predicament; Despite His
Failures, He Has Been Promoted to SVP Business Development and Strategic Planning and He Is Now a Candidate for CEO; How
Can a Board that Calls Itself “Strong & Independent” Be Responsible For This? (p. 39 - 40)
• We believe we Have a Viable Plan for Change (p. 44 - 51)
• We believe we Will Help Generate Change (p. 53 - 58) superior to existing Board based on:
– highly relevant experience in all aspects of biopharmaceuticals and related areas necessary for success in this new era of
reimbursement and cost effectiveness
reimbursement and cost effectiveness
– greater independence
– fresh perspectives from outside of Forest
– better alignment with shareholders; track record of outperformance in biopharma (p. 59 - 60)
– consistent accountability
4

Company Background
• Founded in 1956 - Howard Solomon has been CEO since 1977
• Develops, manufactures and markets drugs with sales derived
primarily from neurology
primarily from neurology
• FY12A total revenues of $4.6 B; FY13E total revenues of $3.3
B; FY16E total revenues of $3.7 B
B; FY16E total revenues of $3.7 B
• Lexapro for depression/anxiety was ~49% of FY12 product
sales; Lost patent protection in FY12
sales; Lost patent protection in FY12
• Namenda for Alzheimer’s was ~32% of FY12 product sales;
Loses patent protection in 2015
Loses patent protection in 2015
• “Next Nine” pipeline drugs represent nine new drugs
launching from 2008 - 2013
launching from 2008 - 2013
Source: Company documents; Analyst Estimates; Forest’s fiscal year (FY) end is March 31st
5

Why We Believe Meaningful &
Sustainable Change is Needed at
Forest Labs
Sustainable Change is Needed at
Forest Labs
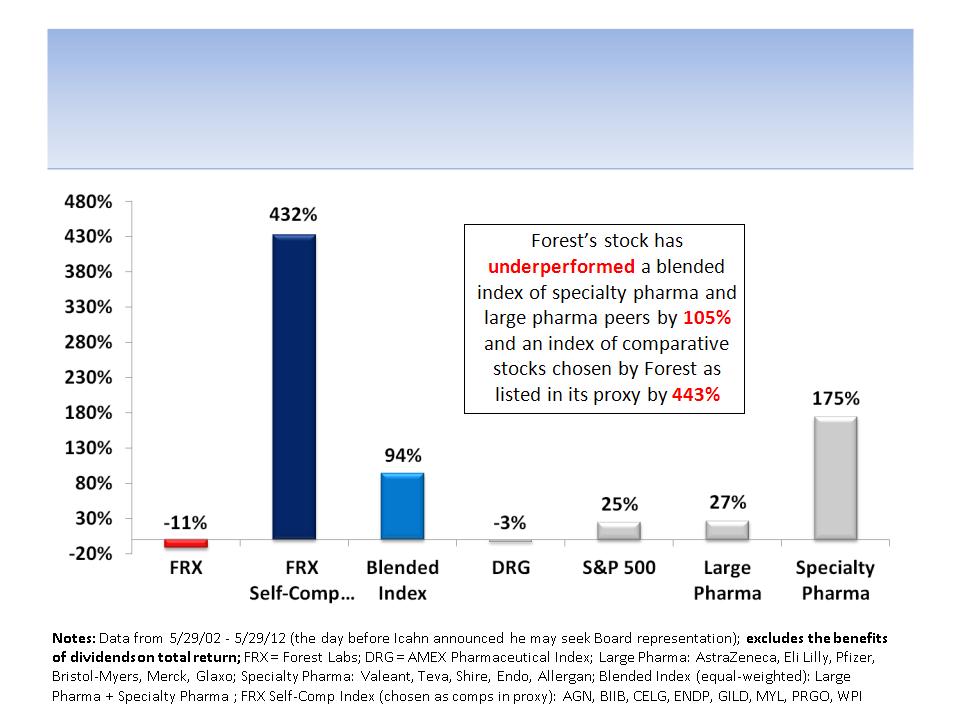
Forest’s Stock Has
Underperformed For 10 Years
Underperformed For 10 Years
7
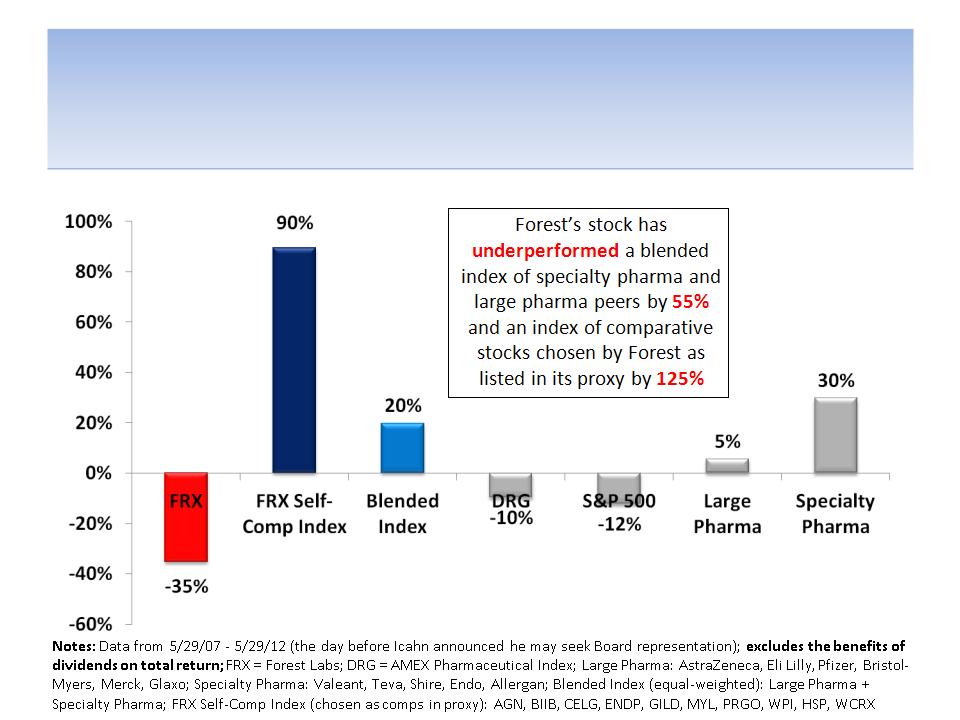
Forest’s Stock Has
Underperformed For 5 Years
Underperformed For 5 Years
8
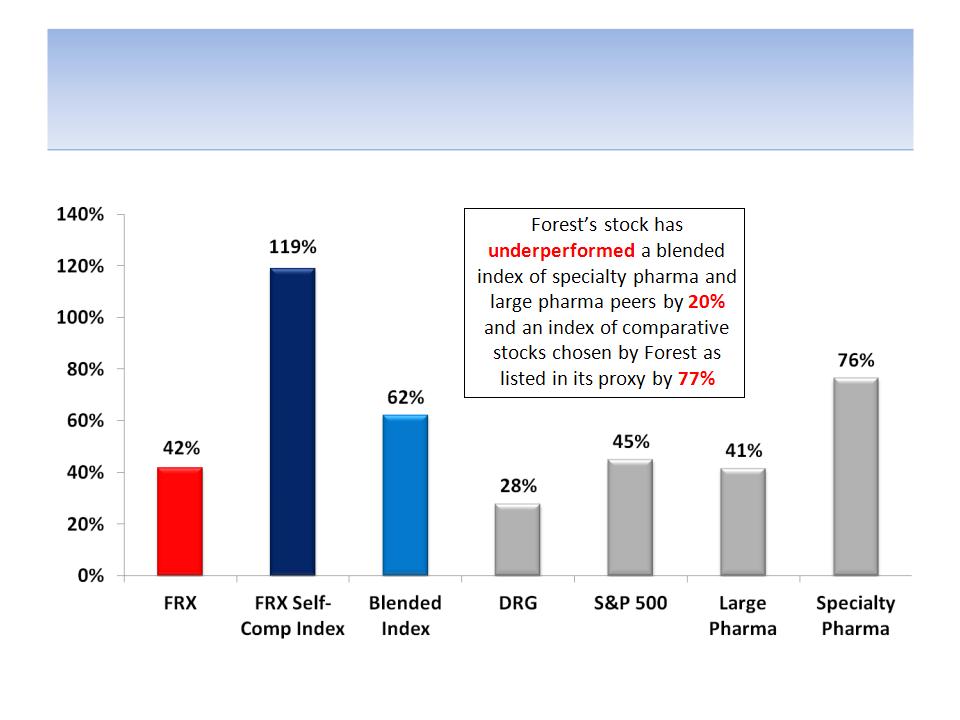
Forest’s Stock Has Underperformed
Against Most Measures For 3 Years
Against Most Measures For 3 Years
Notes: Data from 5/29/09 - 5/29/12 (the day before Icahn announced he may seek Board representation); excludes the benefits of
dividends on total return; FRX = Forest Labs; DRG = AMEX Pharmaceutical Index; Large Pharma: AstraZeneca, Eli Lilly, Pfizer, Bristol-
Myers, Merck, Glaxo; Specialty Pharma: Valeant, Teva, Shire, Endo, Allergan; Blended Index (equal-weighted): Large Pharma +
Specialty Pharma; FRX Self-Comp Index (chosen as comps in proxy): AGN, BIIB, CELG, ENDP, GILD, MYL, PRGO, WPI, HSP, WCRX
dividends on total return; FRX = Forest Labs; DRG = AMEX Pharmaceutical Index; Large Pharma: AstraZeneca, Eli Lilly, Pfizer, Bristol-
Myers, Merck, Glaxo; Specialty Pharma: Valeant, Teva, Shire, Endo, Allergan; Blended Index (equal-weighted): Large Pharma +
Specialty Pharma; FRX Self-Comp Index (chosen as comps in proxy): AGN, BIIB, CELG, ENDP, GILD, MYL, PRGO, WPI, HSP, WCRX
9
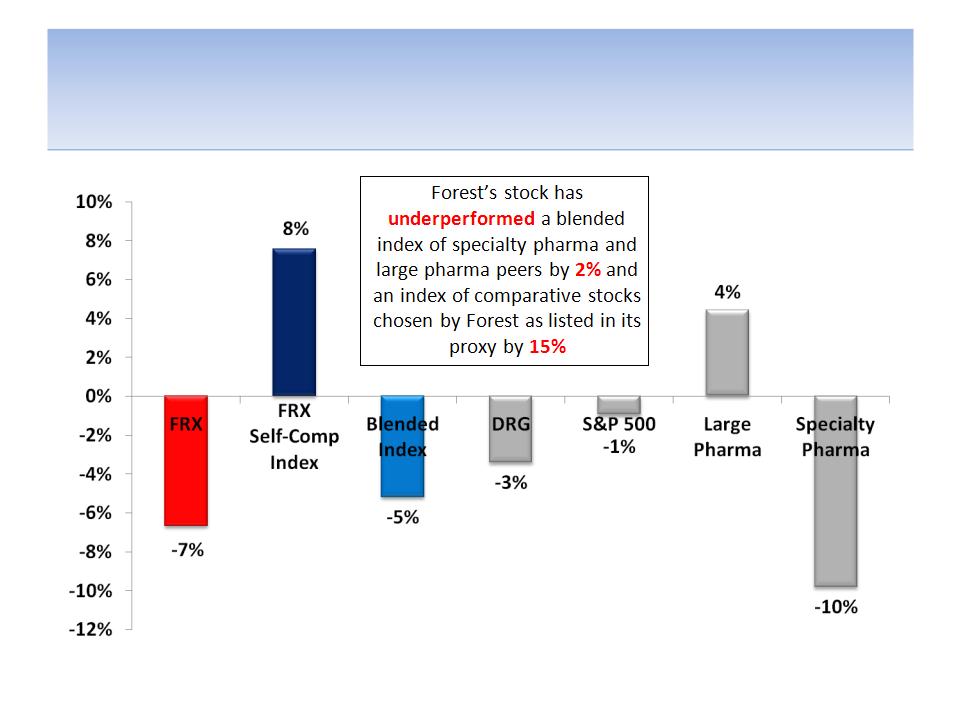
Forest’s Stock Has Underperformed
Against Most Measures For 1 Year
Against Most Measures For 1 Year
Notes: Data from 5/29/11 - 5/29/12 (the day before Icahn announced he may seek Board representation); excludes the benefits of
dividends on total return; FRX = Forest Labs; DRG = AMEX Pharmaceutical Index; Large Pharma: AstraZeneca, Eli Lilly, Pfizer, Bristol
-Myers, Merck, Glaxo; Specialty Pharma: Valeant, Teva, Shire, Endo, Allergan; Blended Index (equal-weighted): Large Pharma +
Specialty Pharma; FRX Self-Comp Index (chosen as comps in proxy): AGN, BIIB, CELG, ENDP, GILD, MYL, PRGO, WPI, HSP, WCRX
dividends on total return; FRX = Forest Labs; DRG = AMEX Pharmaceutical Index; Large Pharma: AstraZeneca, Eli Lilly, Pfizer, Bristol
-Myers, Merck, Glaxo; Specialty Pharma: Valeant, Teva, Shire, Endo, Allergan; Blended Index (equal-weighted): Large Pharma +
Specialty Pharma; FRX Self-Comp Index (chosen as comps in proxy): AGN, BIIB, CELG, ENDP, GILD, MYL, PRGO, WPI, HSP, WCRX
10

…And It Hasn’t Gotten Better Since
Last Year’s Annual Meeting
Last Year’s Annual Meeting
Notes: Data from 8/18/11 - 5/29/12 (the day before Icahn announced he may seek Board representation); excludes the
benefits of dividends on total return; FRX = Forest Labs; DRG = AMEX Pharmaceutical Index; Large Pharma: AstraZeneca, Eli
Lilly, Pfizer, Bristol-Myers, Merck, Glaxo; Specialty Pharma: Valeant, Teva, Shire, Endo, Allergan; Blended Index (equal-
weighted): Large Pharma + Specialty Pharma
benefits of dividends on total return; FRX = Forest Labs; DRG = AMEX Pharmaceutical Index; Large Pharma: AstraZeneca, Eli
Lilly, Pfizer, Bristol-Myers, Merck, Glaxo; Specialty Pharma: Valeant, Teva, Shire, Endo, Allergan; Blended Index (equal-
weighted): Large Pharma + Specialty Pharma
11
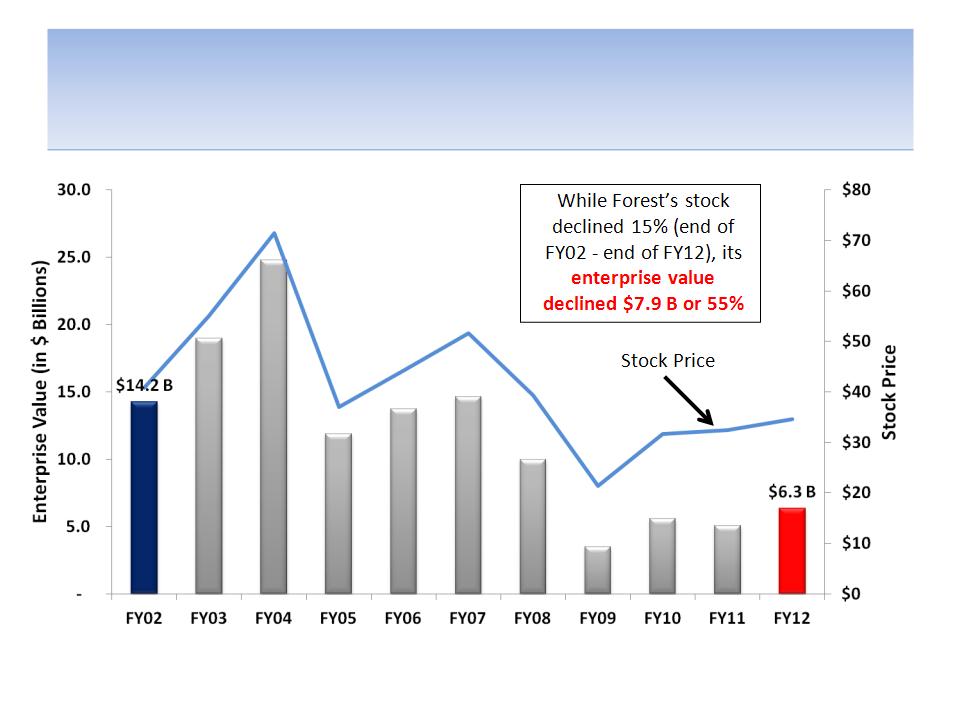
Share Buyback Masked True Extent of
Value Destruction Over 10 Years
Value Destruction Over 10 Years
Source: Company documents; all data measured from fiscal YE02 to fiscal YE12; Enterprise value = Market
Cap + Debt-Cash
Cap + Debt-Cash
12
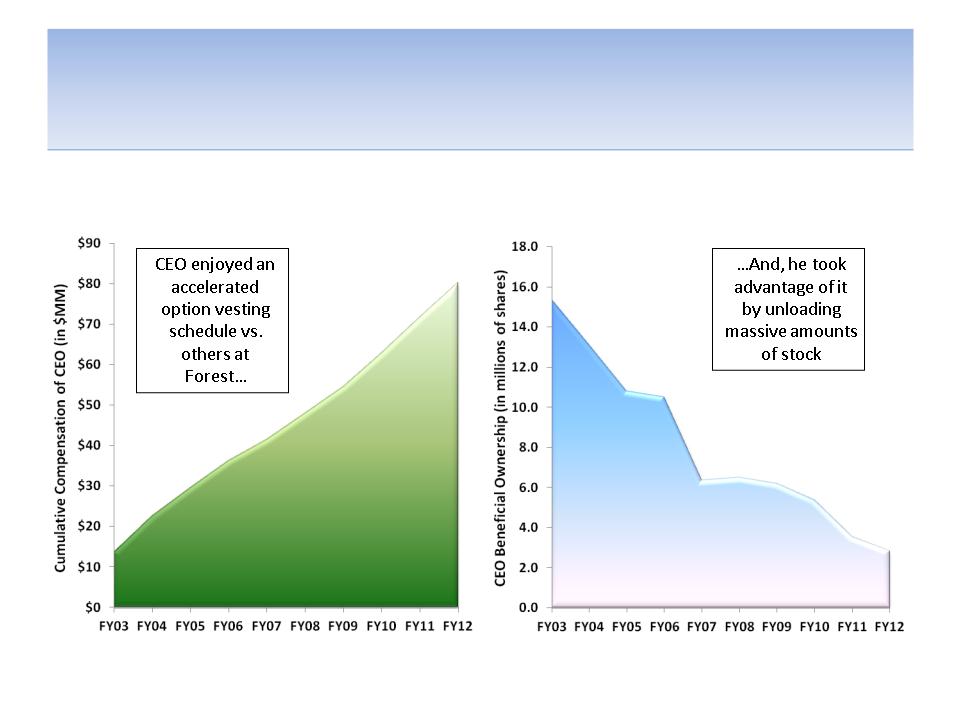
While Shareholders Lost Billions of
Dollars, CEO Solomon Made A Fortune
Dollars, CEO Solomon Made A Fortune
Paid Over $80 MM
Sold Stock Worth $572 MM;
Ownership Reduced by 82%
Ownership Reduced by 82%
Source: Company documents
13

Management Claims it Has Done a Great Job
Then,
(1)Why has the stock underperformed its peer indices
for 1, 3, 5 and 10 years?
for 1, 3, 5 and 10 years?
And,
(2) Why has so much value been destroyed during that
same period of time?
same period of time?
We believe the answer is:
Strategic Failure by the Management & Board
14

We Believe Forest Had an Inadequate & Flawed
Strategy That Destroyed Shareholder Value
Strategy That Destroyed Shareholder Value
• Inadequate Strategy: Management and the Board implemented a strategy that we
believe was inadequate to offset declining revenues and profits due to generic
competition for Lexapro and Namenda
believe was inadequate to offset declining revenues and profits due to generic
competition for Lexapro and Namenda
– The strategy was implemented too late even though there was plenty of time to prepare
– An increasing amount of capital has had to be put at risk for each product
– In spite of all the capital used, a massive amount of value was destroyed
– Pipeline planning to offset lost revenues was insufficient
– Revenues from “Next Nine” drug launches have missed company guidance
– Revenues & profits are expected to remain depressed for the foreseeable future
• Flawed Strategy: We believe the “opportunistic” strategy has caused business
development & Forest’s pipeline to become highly unfocused
development & Forest’s pipeline to become highly unfocused
– Lack of company expertise and critical mass in specific areas
– Sales rep productivity has declined and is below specialty pharma peers
– Loss of cost synergies within sales and marketing, G&A as well as R&D
Source: Company documents; Analyst estimates
15
Management Had Plenty of Time to Prepare
For the Patent Cliff, But Started Too Late
For the Patent Cliff, But Started Too Late
Source: Company documents and analyst estimates
Note: Peer specialty pharmaceutical companies consist of Valeant, Teva, Shire, Endo, Warner Chilcott & Allergan ; Peer
large pharmaceutical companies consist of AstraZeneca, Eli Lilly, Pfizer, Bristol, Merck, Glaxo, Sanofi-Aventis
large pharmaceutical companies consist of AstraZeneca, Eli Lilly, Pfizer, Bristol, Merck, Glaxo, Sanofi-Aventis
Management/Board underinvested in R&D for
several years forcing them to try to “catch up”
later. Depending on the stage of development
that a product is licensed, clinical development of
a single drug can take up to 10 years
several years forcing them to try to “catch up”
later. Depending on the stage of development
that a product is licensed, clinical development of
a single drug can take up to 10 years
Loss of
Lexapro
patent
Lexapro
patent
16

More Capital Had to be Put at Risk to
Obtain Each Additional Product
Obtain Each Additional Product
Source: Company documents
Notes: Capital at-risk is calculated for each product based on up-front payments + acquisition payments; Each year is
calculated based on a cumulative capital at-risk divided by the cumulative number of products
calculated based on a cumulative capital at-risk divided by the cumulative number of products
17

Despite All the Money Spent on Products,
Massive Value Has Been Destroyed
Massive Value Has Been Destroyed
During the last 10 years,
Forest spent $8.3 B on R&D,
licensing/milestone payments
and product/rights
acquisitions. At the same
time, $7.9 B of Enterprise
Value was destroyed
Forest spent $8.3 B on R&D,
licensing/milestone payments
and product/rights
acquisitions. At the same
time, $7.9 B of Enterprise
Value was destroyed
During the last 5 years, Forest
spent more than $6.2 B on
R&D, licensing/rights
payments and product/rights
acquisitions. At the same
time, $8.3 B of Enterprise
Value was destroyed
spent more than $6.2 B on
R&D, licensing/rights
payments and product/rights
acquisitions. At the same
time, $8.3 B of Enterprise
Value was destroyed
Source: Company documents
Notes: Enterprise value is calculated from FY03 through FY12 (10 yrs) and from FY07 through FY12 (5 yrs)
Acquisitions of companies/product rights
Acquisitions of companies/product rights
R&D/licensing & milestones payments
R&D/licensing & milestones payments
18
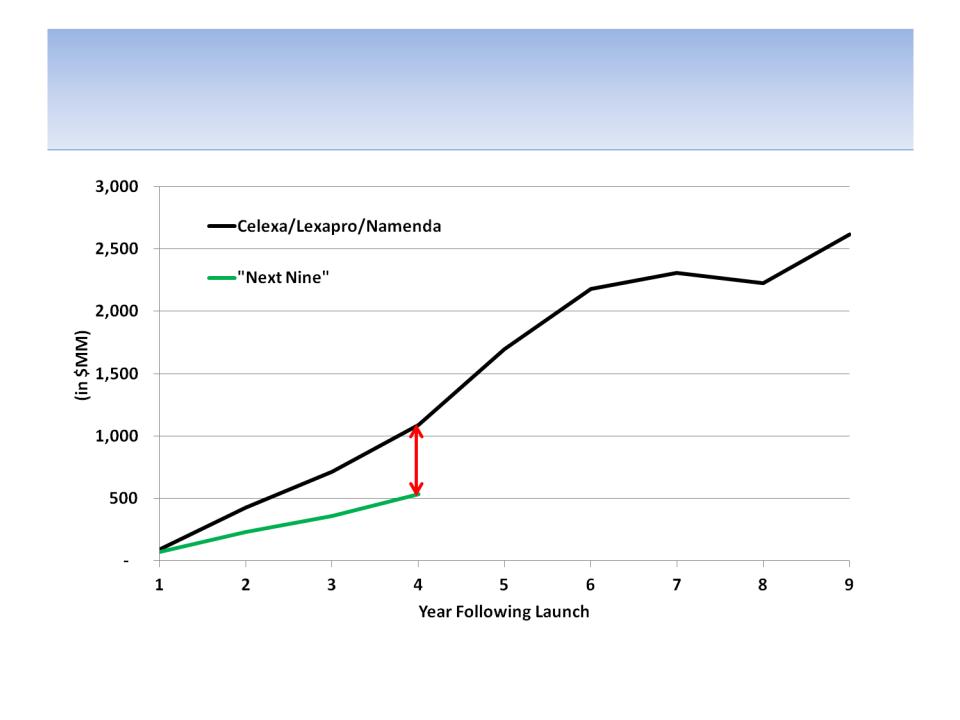
It’s Hard For Us to See How the “Next Nine”
Drugs Will Fill the Revenue Holes
Drugs Will Fill the Revenue Holes
Source: Company documents
Notes: Combined product launch curves are measured for the combined drugs assuming that the 1st year is for the 1st product
launched; additional launches are added as they occur
launched; additional launches are added as they occur
-$554 MM
19
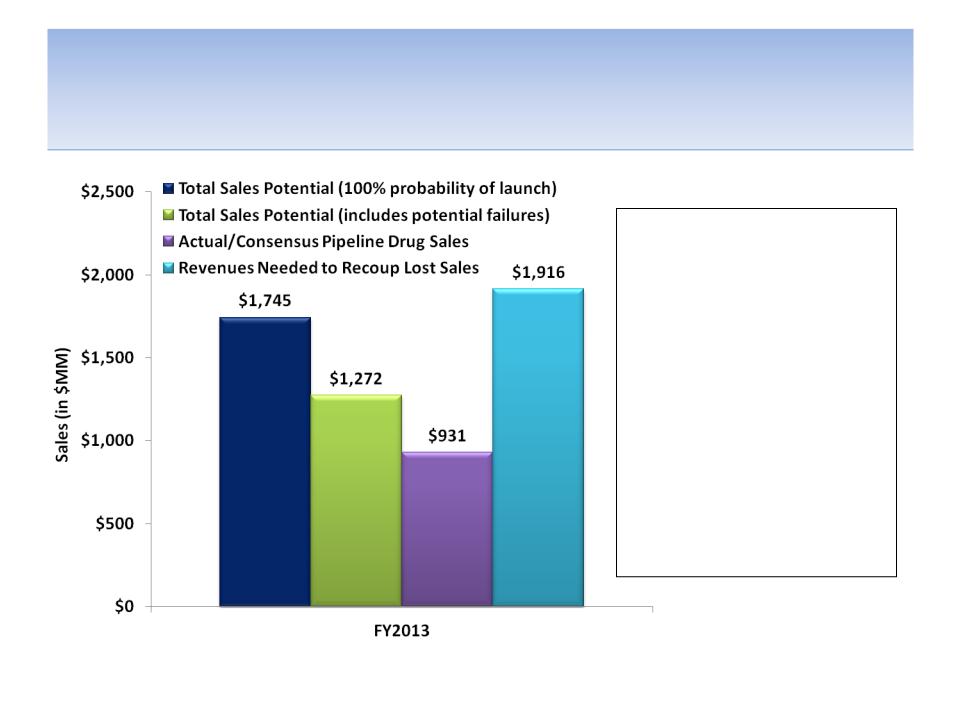
Even If All Pipeline Drugs Were Successful, Sales
Probably Wouldn’t Have Been Enough in FY2013
Probably Wouldn’t Have Been Enough in FY2013
Source: Company documents and analyst estimates; Tufts Center for the Study of Drug Development for average peak drug sales,
average time to peak sales and average clinical development time by therapeutic class; FDAReview.org for probabilities of drug launch
based on stage of development (phase I = 21%; phase II = 28%; phase III = 58% and NDA filed = 90%); SEE APPENDIX C
average time to peak sales and average clinical development time by therapeutic class; FDAReview.org for probabilities of drug launch
based on stage of development (phase I = 21%; phase II = 28%; phase III = 58% and NDA filed = 90%); SEE APPENDIX C
Using industry average peak sales
by therapeutic class, time to peak
sales by therapeutic class and
time in development by
therapeutic class, even
EXCLUDING the likelihood of
failure of some drugs in clinical
development, the sales potential
of drugs licensed by Forest since
2002 were well short of those
needed to offset the loss of the
Lexapro patent. Assuming
industry average drug
development failure rates, FY13
sales potential is about $650 MM
short of what was needed.
by therapeutic class, time to peak
sales by therapeutic class and
time in development by
therapeutic class, even
EXCLUDING the likelihood of
failure of some drugs in clinical
development, the sales potential
of drugs licensed by Forest since
2002 were well short of those
needed to offset the loss of the
Lexapro patent. Assuming
industry average drug
development failure rates, FY13
sales potential is about $650 MM
short of what was needed.
20
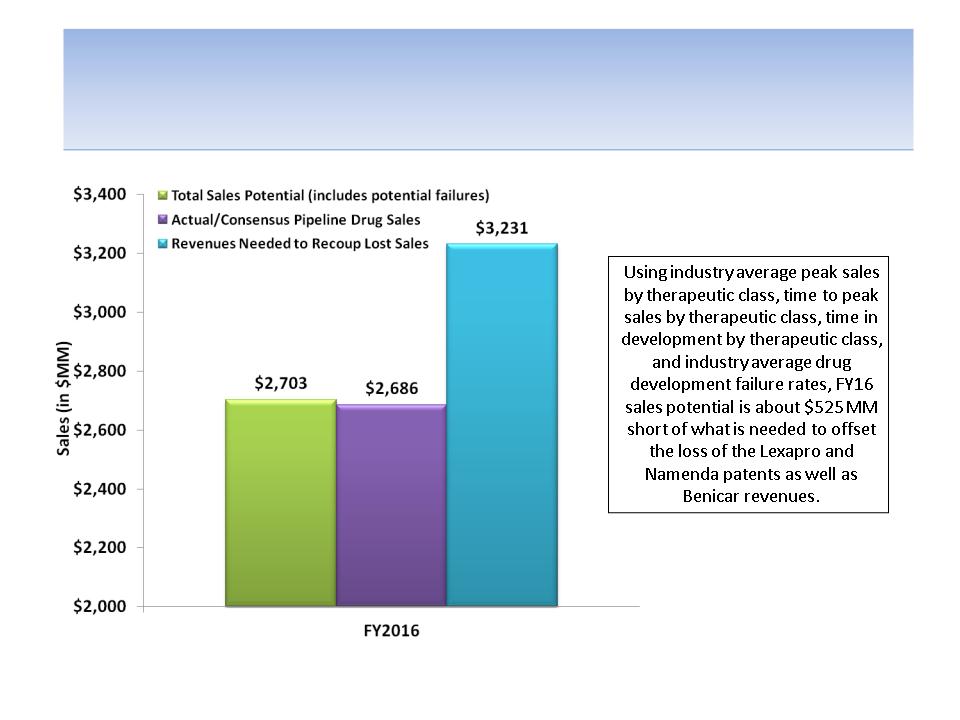
It Doesn’t Appear to Us That Pipeline
Planning for FY2016 Was Much Better…
Planning for FY2016 Was Much Better…
Source: Company documents and analyst estimates; Tufts Center for the Study of Drug Development for average peak drug sales,
average time to peak sales and average clinical development time by therapeutic class; FDAReview.org for probabilities of drug launch
based on stage of development (phase I = 21%; phase II = 28%; phase III = 58% and NDA filed = 90%); SEE APPENDIX C
average time to peak sales and average clinical development time by therapeutic class; FDAReview.org for probabilities of drug launch
based on stage of development (phase I = 21%; phase II = 28%; phase III = 58% and NDA filed = 90%); SEE APPENDIX C
21
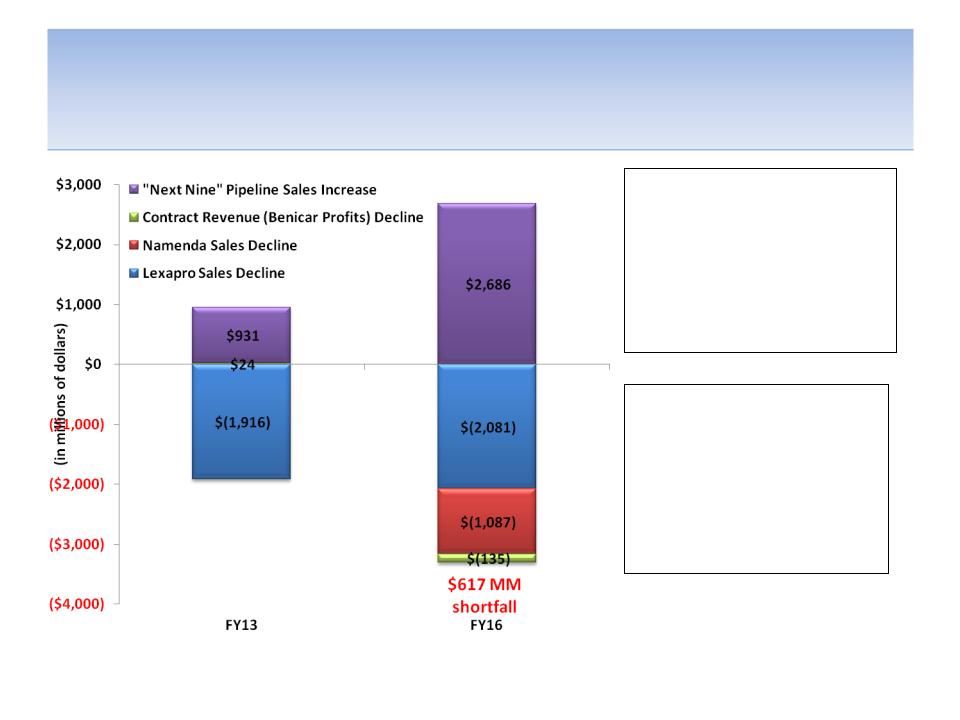
According to Analysts, Sales from “Next Nine” Pipeline
Products Not Projected to Offset Revenue Declines
Products Not Projected to Offset Revenue Declines
Source: Company documents and analyst estimates; Quote from presentation filed by company during 2011 proxy contest
Note: Lexapro sales decline is measured from FY12; Namenda sales decline is measured from FY15; Contract Revenue
decline is measured from FY13; Assumes ALL pipeline products are successfully launched
decline is measured from FY13; Assumes ALL pipeline products are successfully launched
$960 MM
shortfall
“Management efforts over
the last eight years have
built Forest’s pipeline to
offset the Loss of Exclusivity
for these two drugs [Lexapro
& Namenda]…” - company
presentation filed with SEC (2011)
the last eight years have
built Forest’s pipeline to
offset the Loss of Exclusivity
for these two drugs [Lexapro
& Namenda]…” - company
presentation filed with SEC (2011)
Revenues from the “Next
Nine” pipeline drugs are not
expected by analysts to be
enough to offset the effect
of generic competition to
Lexapro/ Namenda and lost
Benicar profits
Nine” pipeline drugs are not
expected by analysts to be
enough to offset the effect
of generic competition to
Lexapro/ Namenda and lost
Benicar profits
22
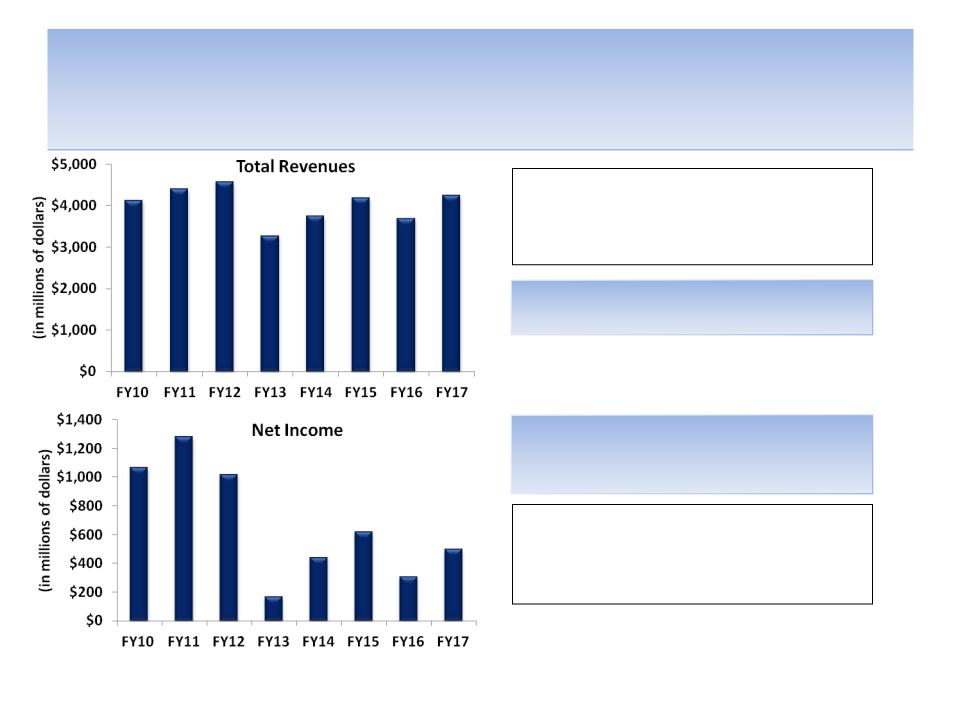
Revenues Are Projected to Decline But Profits
Are Expected to Get Hit Even Harder
Are Expected to Get Hit Even Harder
Source: Company documents and analyst estimates; Frank Perrier quote from Oct. 13, 2011
Total revenues are not expected by analysts
to regain the FY12 peak until after FY17
to regain the FY12 peak until after FY17
Even worse, net income is not expected by
analysts to regain the FY11 peak until well
after FY17
analysts to regain the FY11 peak until well
after FY17
"We think we're in a good place in
managing the next two patent expirations
in Lexapro and Namenda…“ -- Frank Perier,
CFO Forest Labs
managing the next two patent expirations
in Lexapro and Namenda…“ -- Frank Perier,
CFO Forest Labs
We don’t think being “in a good place in
managing the next two patent expirations”
means estimated net income should decline
by 75% from FY11 to FY16
managing the next two patent expirations”
means estimated net income should decline
by 75% from FY11 to FY16
23
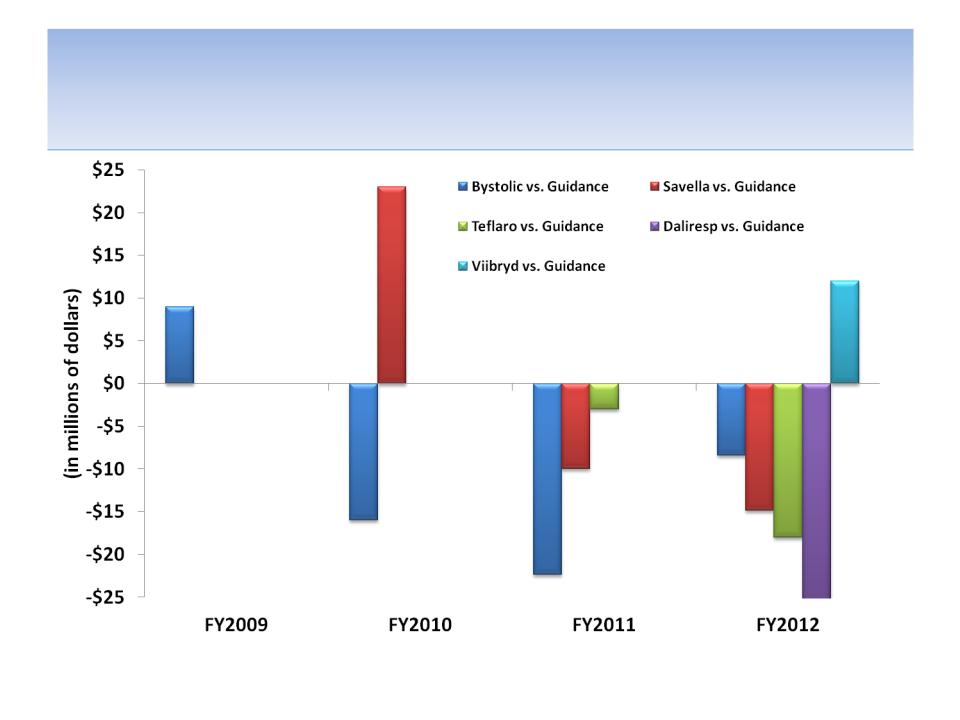
While Forest Has Launched Multiple Products,
They Have Consistently Missed Guidance
They Have Consistently Missed Guidance
Source: Company documents; company press releases for fiscal year end results, which provide next fiscal year product sales
guidance; See APPENDIX A for actual sales guidance for each product
guidance; See APPENDIX A for actual sales guidance for each product
24
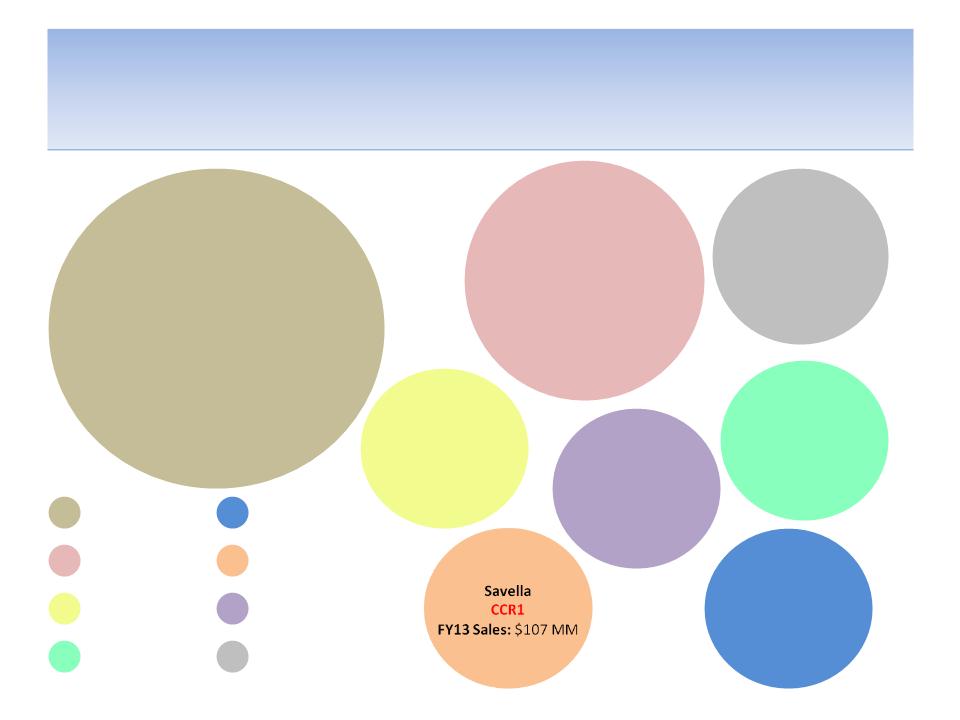
We Believe “Opportunistic” Business
Development Has Led to a Lack of Focus
Development Has Led to a Lack of Focus
TTP399
Dutogliptin
FY13 Sales: $0 MM
Linaclotide
FY13 Sales: $40 MM
GRT6005/6006
RGH-896
FY13 Sales: $0 MM
Teflaro
Avibactam
BC-3781
Faropenam
FY13 Sales: $58 MM
Daliresp
Colostin
Aclidinium
LAS100977
Oglemilast
FY13 Sales: $109 MM
Bystolic
Azimilide
Desmoteplase
FY13 Sales: $438 MM
Lexapro
Namenda
Viibryd
Cariprazine
Levomilnacipran
RGH-618
FY13 Sales: $2.0 B
Neurology
Cardiology
Diabetes
Respiratory
Pain
Other/Inflammation
Antibiotics
Gastrointestinal
Source: Company documents; Analyst estimates; BOLD RED = Failed Projects; BOLD BLACK = LAUNCHED PRODUCTS; Blue = In Development
25
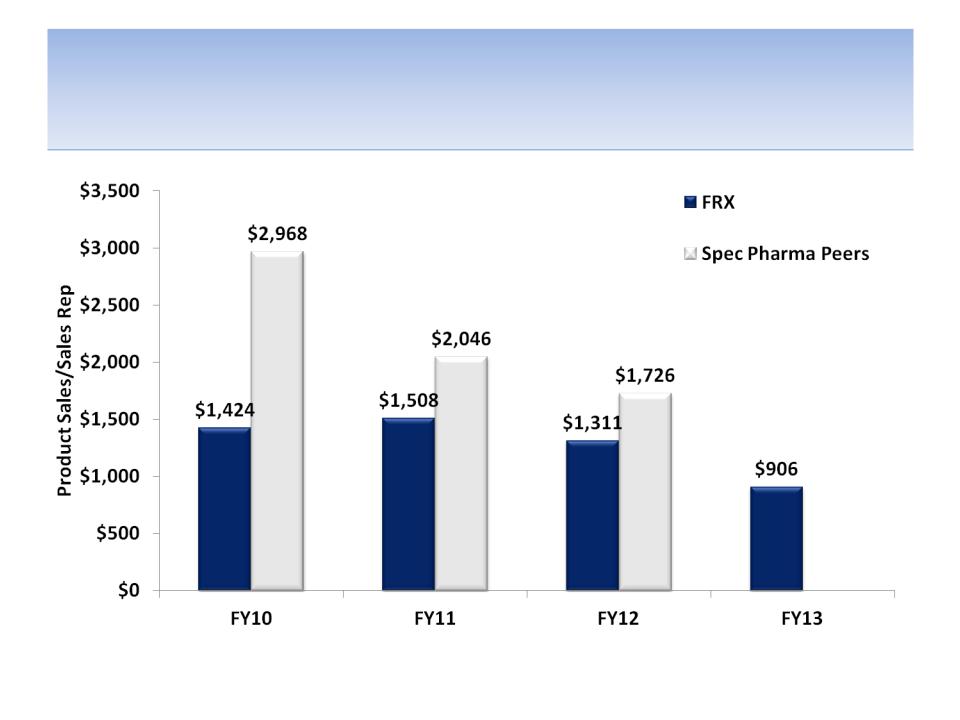
We Believe Lack of Therapeutic Focus
is Hurting Sales Rep Productivity
is Hurting Sales Rep Productivity
Source: Company documents and analyst estimates
Note: Peer specialty pharmaceutical companies include Valeant, Shire, Endo & Allergan; other peers are not available in SEC filings; FRX
operates on a March fiscal year (FY12 ended in March 2012), thus peer data is for most recent fiscal year, i.e. FY12 = FY11 for peers
operates on a March fiscal year (FY12 ended in March 2012), thus peer data is for most recent fiscal year, i.e. FY12 = FY11 for peers
26
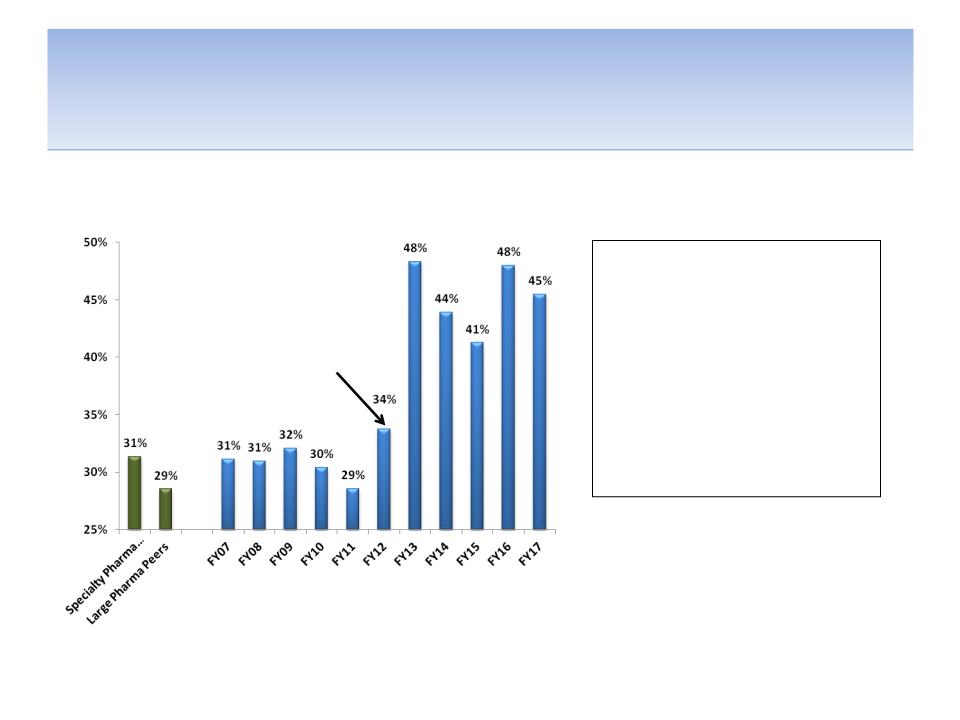
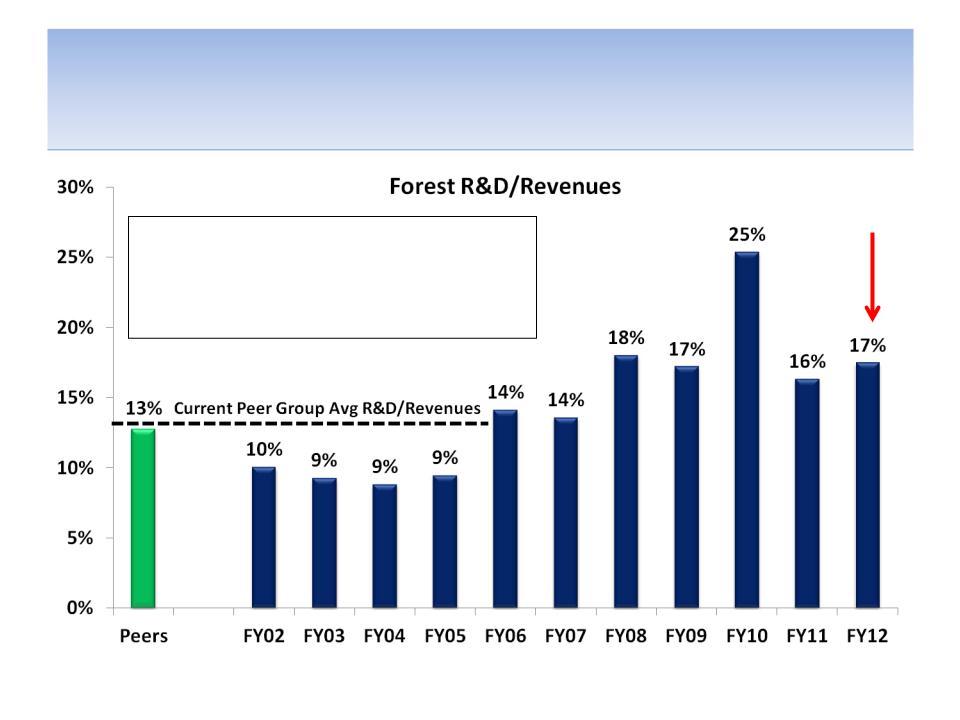
We Believe Loss of Lexapro Sales Has Further Exposed
Massive Corporate Inefficiency & Lack of Cost Synergies
Massive Corporate Inefficiency & Lack of Cost Synergies
SG&A/Revenues (Forest vs. Peers)
Lack of Critical Mass
Source: Company documents and analyst estimates
Note: Peer specialty pharmaceutical companies include Valeant, Teva, Shire, Endo, Warner Chilcott & Allergan ; Peer large
pharmaceutical companies include AstraZeneca, Eli Lilly, Pfizer, Bristol, Merck, Glaxo, Sanofi-Aventis
pharmaceutical companies include AstraZeneca, Eli Lilly, Pfizer, Bristol, Merck, Glaxo, Sanofi-Aventis
Loss of
Lexapro
patent
Lexapro
patent
27
• With the loss of Lexapro sales
due to generic competition, it
has become very clear to us
that SG&A is too high
due to generic competition, it
has become very clear to us
that SG&A is too high
• Without critical mass within
specific therapeutic areas, the
significant fixed costs
associated with the addition
of incremental sales reps
creates cost inefficiency
specific therapeutic areas, the
significant fixed costs
associated with the addition
of incremental sales reps
creates cost inefficiency
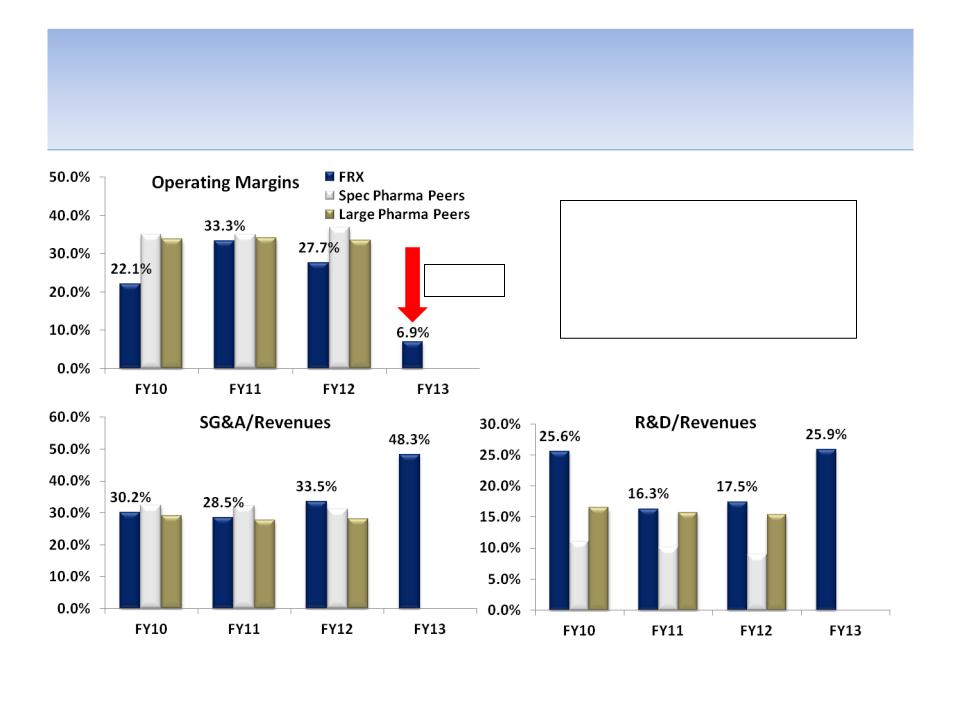
Operating Margins Are Trending in the
Wrong Direction
Wrong Direction
Operating margins have been
and analysts expect them to
continue getting compressed as
SG&A and R&D as a percentage
of revenues increases
and analysts expect them to
continue getting compressed as
SG&A and R&D as a percentage
of revenues increases
Source: Company documents; analyst estimates; FRX operates on a March fiscal year (FY12 ended in March 2012),
thus peer data is for most recent fiscal year, i.e. FY12 = FY11 for peers
thus peer data is for most recent fiscal year, i.e. FY12 = FY11 for peers
-26.4%
28
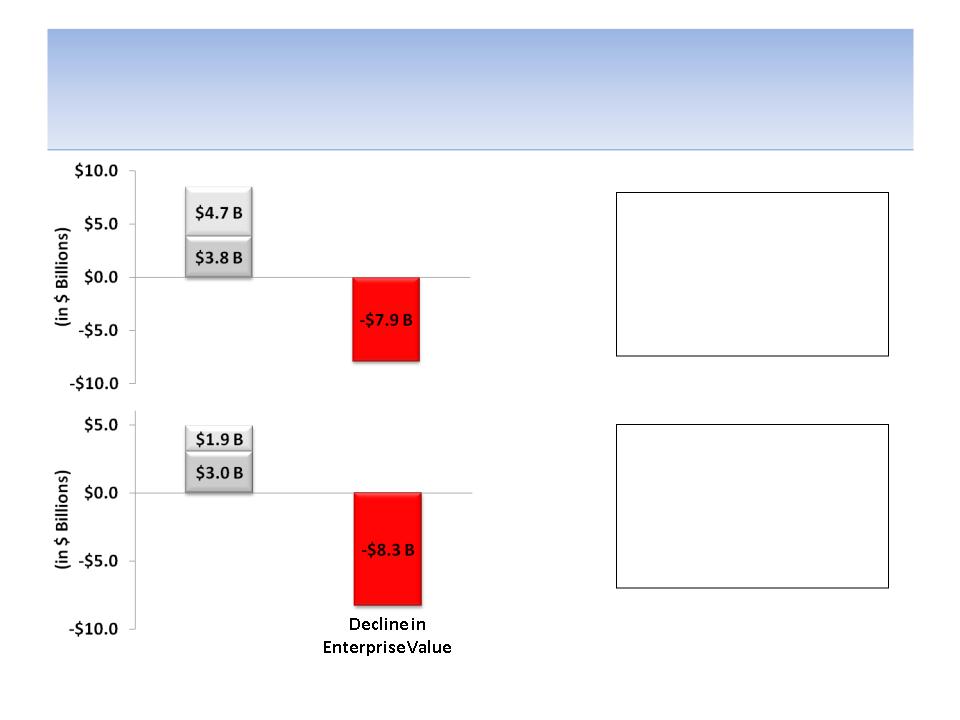
We Believe Management & Board Have a
Poor Track Record of Allocating Capital
Poor Track Record of Allocating Capital
Cash used for share repurchases
Cash used for licensing or acquiring products
Source: Company documents; 10 year values measured from YE 2002 through YE 2012; 5 Year
values measured from YE07 through YE12; Enterprise Value = Market Cap + Debt - Cash
values measured from YE07 through YE12; Enterprise Value = Market Cap + Debt - Cash
Cash used for share repurchases
Cash used for licensing or acquiring products
During the past 10 years as
$8.5 B of capital was
deployed for obtaining
products and repurchasing
shares, there was a decline
in enterprise value of $7.9 B
$8.5 B of capital was
deployed for obtaining
products and repurchasing
shares, there was a decline
in enterprise value of $7.9 B
During the past 5 years as
$4.9 B of capital was
deployed for obtaining
products and repurchasing
shares, there was a decline
in enterprise value of $8.3 B
$4.9 B of capital was
deployed for obtaining
products and repurchasing
shares, there was a decline
in enterprise value of $8.3 B
29
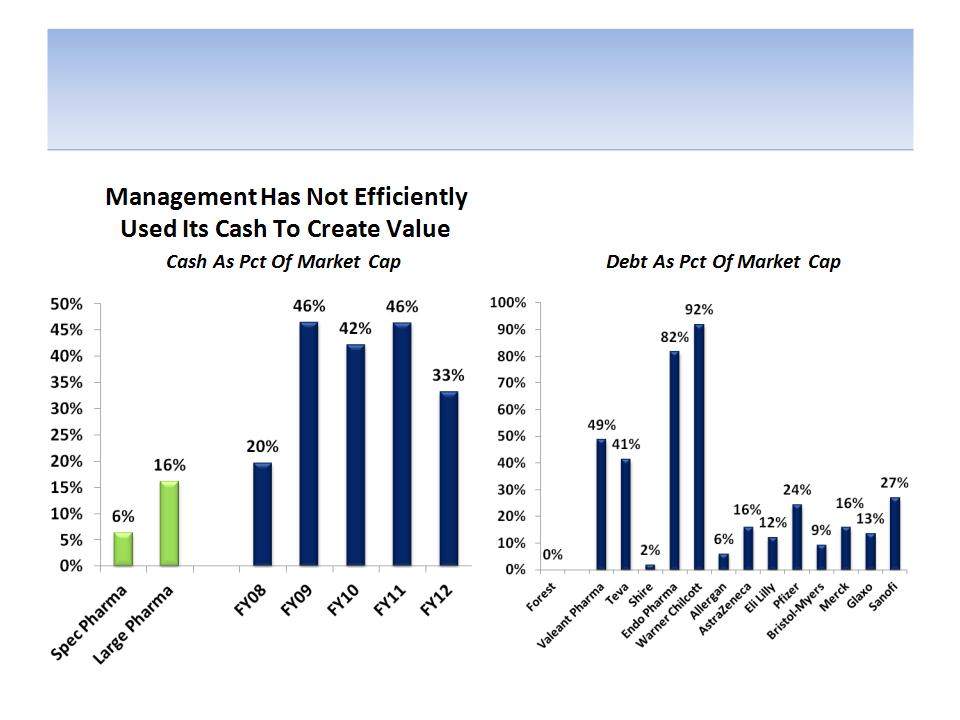
We Believe Management & Board Have Not
Efficiently Used Forest’s Balance Sheet
Efficiently Used Forest’s Balance Sheet
Management Is Not Efficiently
Using Leverage To Create Value
Using Leverage To Create Value
Source: Company documents and analyst estimates
Note: Peer spec pharmaceutical companies include Valeant, Teva, Shire, Endo, Warner Chilcott & Allergan ; Peer large pharmaceutical
companies include AstraZeneca, Eli Lilly, Pfizer, Bristol, Merck, Glaxo, Sanofi-Aventis
companies include AstraZeneca, Eli Lilly, Pfizer, Bristol, Merck, Glaxo, Sanofi-Aventis
30

We Are Concerned About How Management
May Use the Cash Given Its Track Record
May Use the Cash Given Its Track Record
• Given its history of destroying significant value during the last 10
years, we are very concerned about how management may choose
to use the company’s $3.2 B of cash
years, we are very concerned about how management may choose
to use the company’s $3.2 B of cash
• Because of the current & projected revenue shortfalls from generic
competition, we believe management may “swing for the fences”
competition, we believe management may “swing for the fences”
• Analysts do not believe what they have in the pipeline is enough
and therefore may need to do acquisitions:
and therefore may need to do acquisitions:
– “The pipeline, as currently constituted, is not nearly enough to replace
the lost revenue from losing Lexapro and Namenda to generics.” --
David Amsellem of Piper Jaffray
the lost revenue from losing Lexapro and Namenda to generics.” --
David Amsellem of Piper Jaffray
– “I don’t think what they have in their pipeline is enough . At some
point, you’re going to see them use that balance sheet” - Gary
Nachman of Susquehanna
point, you’re going to see them use that balance sheet” - Gary
Nachman of Susquehanna
31
Source: Company documents; SEC filings; quotes from Bloomberg article from July 6, 2012

We Remain Concerned About Corporate
Governance Issues
Governance Issues
• At least 50% of the Board continues to lack true independence, in our
opinion
opinion
– The Presiding “Independent” Director (Kenneth Goodman) lacks true independence
yet he still remains in this important role
yet he still remains in this important role
– The Chair of the Compensation Committee (Dan Goldwasser) who oversaw seriously
flawed compensation policies inexplicably remains in place
flawed compensation policies inexplicably remains in place
• The CEO (Howard Solomon) has had extremely fortunate timing on
large sales of stock
large sales of stock
• The CEO succession plan seems to include the CEO’s son but doesn’t
appear to us to equitably include external candidates
appear to us to equitably include external candidates
• Promotion of CEO’s son to crucial role as head of Strategic Planning
despite relative inexperience in the area
despite relative inexperience in the area
• The Company has had $423 MM of legal settlements including a guilty
plea to a felony charge of obstruction of justice
plea to a felony charge of obstruction of justice
32
Source: Company documents; SEC filings

We Believe At Least 50% of the Board Continues to
Lack True Independence (5 out of 10 Directors)
Lack True Independence (5 out of 10 Directors)
Source: Company documents
33

Compensation Policies Have Been
Flawed But Chairman Remains at Helm
Flawed But Chairman Remains at Helm
• Chair of Compensation Committee (Dan Goldwasser - Board member for 35
years) presided over serious problems related to compensation policy
years) presided over serious problems related to compensation policy
• Policy changes during the last year confirm these problems
– Vesting schedules of equity awards were too short, not linked to performance
and favored the CEO
and favored the CEO
– The Compensation Committee had not previously engaged an independent
compensation consultant
compensation consultant
– The Chair of the Committee chose the peer group for comparison purposes
– The Chair circulated a report of factors he believed were relevant to
determining compensation including a report prepared directly by management
determining compensation including a report prepared directly by management
– Compensation was not linked to pre-determined performance measures
– There were no stock ownership requirements
• Then, why is Dan Goldwasser still Chair of the Compensation
Committee given these problems with compensation policy?
Committee given these problems with compensation policy?
Source: Company documents
34
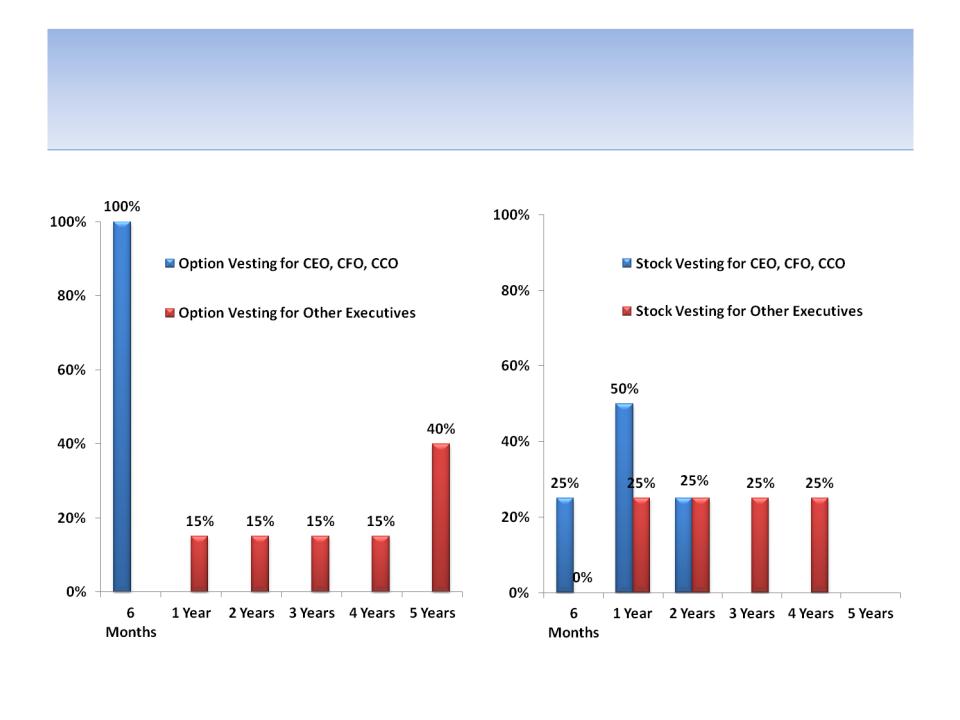
CEO’s Preferential Option & Stock
Vesting Schedule
Vesting Schedule
35
Source: 2011 proxy statement; CEO = Howard Solomon; CFO = Frank Perier; CCO (chief commercial officer) = Elaine Hochberg

Fortunately Timed Stock Sales by CEO
36
Nov ‘04: CEO Solomon sold 2.5
MM shares at $43.26 per share
for proceeds of $108.2 MM
MM shares at $43.26 per share
for proceeds of $108.2 MM
Feb ‘07: CEO Solomon sold 4.3
MM shares at $52.60 per share
for proceeds of $226.2 MM
MM shares at $52.60 per share
for proceeds of $226.2 MM
Source: SEC filings; three largest sales by CEO during period
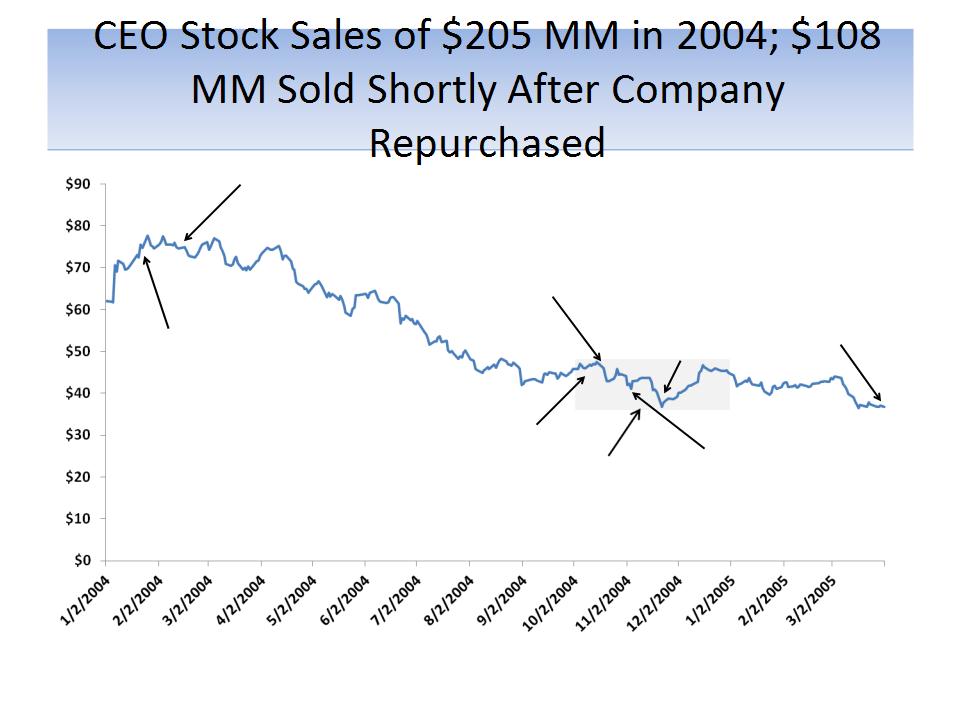
“Our financial performance for
the remainder of the fiscal year
ending March 31, 2004 should
result in earnings per share for
the year at the high end of our
previously issued guidance
[$1.92]. Regarding fiscal 2005
EPS, we continue to project a
range of $2.30 - $2.50.” - Jan 20,
2004
the remainder of the fiscal year
ending March 31, 2004 should
result in earnings per share for
the year at the high end of our
previously issued guidance
[$1.92]. Regarding fiscal 2005
EPS, we continue to project a
range of $2.30 - $2.50.” - Jan 20,
2004
CEO Howard Solomon sells 1.3
MM shares at $74.85 for
proceeds of $97.3 MM from
2/11/04 - 2/17/04
MM shares at $74.85 for
proceeds of $97.3 MM from
2/11/04 - 2/17/04
“Forest Laboratories
to Exceed Fiscal 2005
Second And Third
Quarter Mean
earnings Per Share
Estimates” - Oct. 4,
2004
to Exceed Fiscal 2005
Second And Third
Quarter Mean
earnings Per Share
Estimates” - Oct. 4,
2004
“All of our principal promoted
brands exhibited strong
growth…and we expect this
performance to continue in the
future…Given the underlying
strength…we are increasing our
projected EPS for the fiscal year
ending March 31, 2005 to at least
$2.70.” - Oct 18, 2004
brands exhibited strong
growth…and we expect this
performance to continue in the
future…Given the underlying
strength…we are increasing our
projected EPS for the fiscal year
ending March 31, 2005 to at least
$2.70.” - Oct 18, 2004
“Forest Laboratories …has
revised its guidance for
diluted earnings per share
for the fiscal year ending
March 31, 2005 [to]
approximately $2.50.” - Nov
1, 2004
revised its guidance for
diluted earnings per share
for the fiscal year ending
March 31, 2005 [to]
approximately $2.50.” - Nov
1, 2004
Fiscal Year ending
March 31, 2005 EPS
of $2.25
March 31, 2005 EPS
of $2.25
CEO Howard Solomon sells
2.5 MM shares at $43.26 for
proceeds of $108 MM from
11/8/04 - 11/17/04
2.5 MM shares at $43.26 for
proceeds of $108 MM from
11/8/04 - 11/17/04
Forest repurchases 13.8
MM shares during the 4th
quarter of 2004; it bought
2.3 MM in Nov. at an avg.
price of $37.64
MM shares during the 4th
quarter of 2004; it bought
2.3 MM in Nov. at an avg.
price of $37.64
Source: SEC filings; company press releases; Direct quotes from Howard Solomon
37
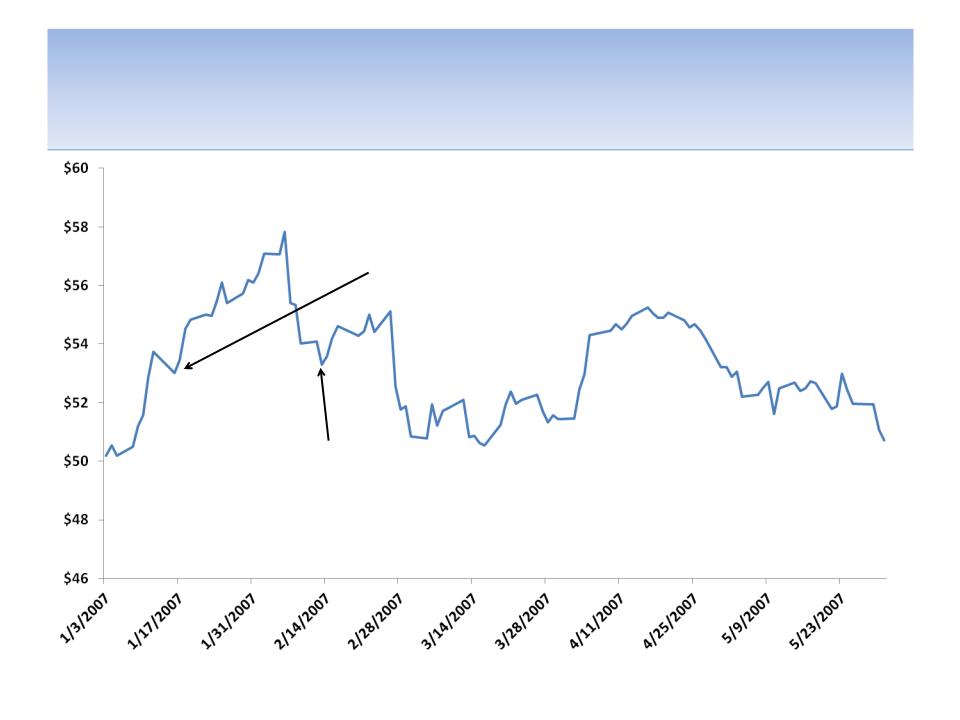
CEO Sells Stock Worth $226 MM All On One Day in
2007 And Says It Was For Estate Planning Purposes
2007 And Says It Was For Estate Planning Purposes
CEO Howard Solomon sells 4.3 MM shares at
$52.60 for proceeds of $226 MM on 2/12/07;
Solomon states, “I have reached an age when it is
necessary for me to further an estate plan... These
share dispositions are being undertaken solely for
those purposes.”
$52.60 for proceeds of $226 MM on 2/12/07;
Solomon states, “I have reached an age when it is
necessary for me to further an estate plan... These
share dispositions are being undertaken solely for
those purposes.”
“We are encouraged by both
the performance of our key
marketed products and by the
opportunities we currently
have in our development
pipeline...“ - CEO Howard
Solomon Jan 16, 2007
the performance of our key
marketed products and by the
opportunities we currently
have in our development
pipeline...“ - CEO Howard
Solomon Jan 16, 2007
Source: SEC filings; company press releases
38

We Believe CEO Succession Is Long Overdue &
Should Equitably Include External Candidates
Should Equitably Include External Candidates
• Howard Solomon is currently 84 years old, has been CEO for 35 years and the
government considered excluding Solomon from government contracts as recently as 1
year ago so succession planning is long overdue
government considered excluding Solomon from government contracts as recently as 1
year ago so succession planning is long overdue
• Instead of preparing for succession by reducing his responsibilities, CEO Solomon took
on a larger role as President after COO Olanoff retired in 2010 and hasn’t yet
relinquished the role
on a larger role as President after COO Olanoff retired in 2010 and hasn’t yet
relinquished the role
• David Solomon, CEO Howard Solomon’s son and still independent movie producer, has
been promoted as an apparent contender for CEO, which we believe represents a
significant conflict and may be setting the stage for the creation of a dynasty
been promoted as an apparent contender for CEO, which we believe represents a
significant conflict and may be setting the stage for the creation of a dynasty
• Other recent promotions of Hochberg, Taglietti, and Perier collectively oversaw the
inadequate execution of the company ‘s flawed strategy, in our opinion
inadequate execution of the company ‘s flawed strategy, in our opinion
• Given potential conflicts, a lack of execution by internal candidates and the need for a
fresh perspective, it is crucial for external candidates to be evaluated as well, in our
opinion
fresh perspective, it is crucial for external candidates to be evaluated as well, in our
opinion
Source: Company documents, company comments, press releases and analyst comments
39

CEOs Son Promoted to Key Role of
Strategic Planning
Strategic Planning
• Business development and strategic planning involve building the
company’s pipeline for future growth
company’s pipeline for future growth
• In Forest’s case, because of the huge revenue holes being generated by
the patent expirations of Lexapro and Namenda, which represent 80% of
revenues, the role has outsized importance
the patent expirations of Lexapro and Namenda, which represent 80% of
revenues, the role has outsized importance
• David Solomon, the CEOs son with a background as a movie producer and
entertainment lawyer, was promoted into this crucial area just 5 years
after joining Forest. And, he was promoted to become the Head of the
division just 4 years later
entertainment lawyer, was promoted into this crucial area just 5 years
after joining Forest. And, he was promoted to become the Head of the
division just 4 years later
• Because the company has not adequately offset the revenue shortfalls
due to the patent cliffs, we believe he has failed in this role
due to the patent cliffs, we believe he has failed in this role
• Therefore, we believe strategic failure of the company rests on his
shoulders and the irresponsible actions of the Board and management
which lead to his promotion
shoulders and the irresponsible actions of the Board and management
which lead to his promotion
40

Poor Risk Management & Compliance Has
Resulted in $423 MM in Legal Settlements
Resulted in $423 MM in Legal Settlements
• We do not see how Lester Salans, a Clinical Professor and physician, as
Chair of the Compliance Committee has any qualifications as a compliance
expert
Chair of the Compliance Committee has any qualifications as a compliance
expert
• These payments may be representative of a lax culture and complacency
at the Board level
at the Board level
• $313 MM related to doctor kickbacks, off-label promotion for children and
obstructing an agency proceeding
obstructing an agency proceeding
• $65 MM related to making false and misleading statements with respect
to anti-depression drugs
to anti-depression drugs
• $25 MM for securities claims against Forest and certain officers
• $20 MM for a patent infringement suit related to Lexapro
• $100 MM gender discrimination class action filed recently
Source: Company documents
41

We Do Not Believe Management Has
Delivered on Its Word
Delivered on Its Word
What They Said … | What Happened … |
Management said, they were “in a good place in managing the next two patent expirations in Lexapro and Namenda…“ -- Frank Perrier, CFO quote from Oct. 13, 2011 | FY13 guidance was for revenues to decline by more that $1 B from FY12 and EPS to decline by almost 80% and almost $3 from FY12 |
“Management efforts over the last eight years have built Forest’s pipeline to offset the Loss of Exclusivity for these two drugs [Lexapro & Namenda]…” - Company presentation filed during 2011 proxy contest | Pipeline product sales have missed initial annual company guidance 8 out of 11 times. Sales from pipeline products will fall ~$1 B short of “offsetting…these two drugs” during FY13. |
Management said that FY13 EPS would not be less than $1.20 - company press release from April 19, 2011 | Management lowered FY13 EPS guidance twice, ultimately to $0.65 - $0.80 |
Management said that they had an “excellent track record of creating shareholder value.” -- Company presentation filed during 2011 proxy contest | During the past 10 years, the company’s equity value has declined by $5.6 B or 37% and its enterprise value by $7.9 B or 55% |
Management claimed to have a long-term incentive plan with stock and options that “vest over time and thus reward sustained performance by executive officers and discourage unnecessary risk.” - proxy statement filed with SEC in 2011 | Three executives including CEO Howard Solomon enjoyed an accelerated vesting schedule for stock and options |
“…therapeutic diversification creates tremendous synergy, as most of these products are to be marketed by our primary care sales forces to a common group of primary care physicians…” -- DEFA14A 8/1/11 | Operating margins have been deteriorating as the direct result of climbing SG&A spending associated with the company’s entrance into multiple unrelated therapeutic areas |
Forest has a longstanding track record of delivering … superior value for shareholders, including share appreciation that has exceeded the S&P 500 and the AMEX Pharmaceutical Index - SEC filing July 29th, 2011 | The stock has underperformed most relevant indices over 1, 3, 5, and 10 years |
Source: Company documents, press releases, presentations
42

Icahn Nominee Plan For Meaningful
Change at Forest Labs
Change at Forest Labs

Icahn Recommendations
How to Get the Forest Growing Again
How to Get the Forest Growing Again
• Independently evaluate current business development strategy, which we
believe is unfocused
believe is unfocused
• Develop a clear strategy focused on creating shareholder value
• Assess potential divestiture of non-core assets
• Evaluate ways to reduce SG&A spending
• Evaluate development programs for potential rationalization
• Identify ways to improve revenue growth with modernized sales &
marketing effort
marketing effort
• Increase efficiency & effectiveness of capital allocation decisions
• Improve corporate governance
• Review current management team as well as culture and implement any
necessary changes
necessary changes
44

Independently Evaluate and Potentially
Change Current Business Development
Strategy
Change Current Business Development
Strategy
• We believe the “Opportunistic” approach by the company has caused
it to lose its focus and has resulted in an inefficient cost structure
it to lose its focus and has resulted in an inefficient cost structure
• Engage an independent consultant to evaluate the company’s business
strategy and its long-term potential
strategy and its long-term potential
• Identify specific therapeutic areas that meet pre-determined criteria
• If a new strategy is focused on specific therapeutic areas then divest
non-core assets and programs
non-core assets and programs
• Focus on a strategy that will better leverage its existing infrastructure
• Only enter into product licensing deals and acquisitions that strictly
adhere to specific return on investment criteria
adhere to specific return on investment criteria
45

Focus on Specific Therapeutic Categories
Likely to Provide The Best Returns
Likely to Provide The Best Returns
Source: company documents; analyst projections; capital at risk = upfront cash payments as part
of licensing deals and cash acquisitions of companies or product rights
of licensing deals and cash acquisitions of companies or product rights
46
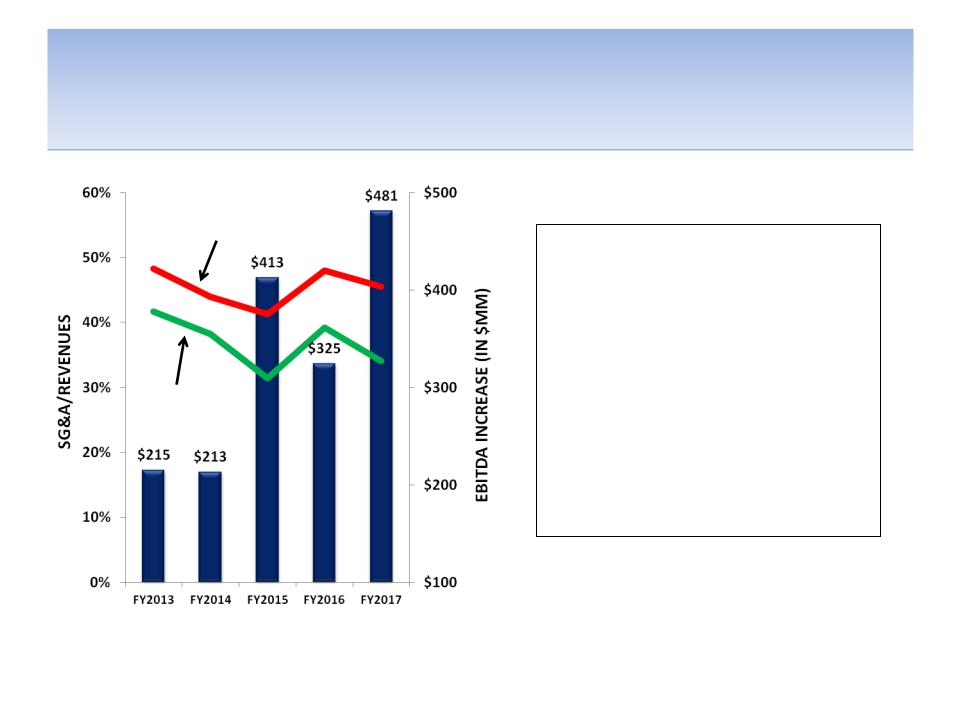
SG&A Efficiencies Could Increase EBITDA
By Almost $500 MM
By Almost $500 MM
• Greater focus within a few key
therapeutic categories should
allow for greater cost synergies
therapeutic categories should
allow for greater cost synergies
• Potential G&A cuts including
procurement efficiencies, head
count reductions and
consolidation of existing
properties
procurement efficiencies, head
count reductions and
consolidation of existing
properties
• Increase efficiency of sales and
marketing effort through
restructuring, retraining and
outsourcing where prudent
marketing effort through
restructuring, retraining and
outsourcing where prudent
Source: Company documents; analyst estimates
Notes: SG&A consists of general & administrative (G&A) and sales & marketing (S&M) costs; Assumes FY12 G&A costs of $350 MM
growing at 3% (long-term inflation rate); S&M costs vary by year depending on new product launches -- associated promotional
spending ($400 - 500 MM per year) and need for reps (3,600 - 3,800) and promotional & rep costs for previously launched drugs
growing at 3% (long-term inflation rate); S&M costs vary by year depending on new product launches -- associated promotional
spending ($400 - 500 MM per year) and need for reps (3,600 - 3,800) and promotional & rep costs for previously launched drugs
Consensus estimates
Post-cost cuts
47

Evaluate Current Pipeline for Potential
Development Program Rationalization
Development Program Rationalization
• Identify existing projects that do not fit within the new
business development strategy or do not achieve pre-
determined return on investment (IRR) criteria
business development strategy or do not achieve pre-
determined return on investment (IRR) criteria
• Reduce R&D spending
– Terminate poorly performing R&D programs
– Evaluate projects for out-licensing
• Consider re-investing R&D expense savings into:
– newly licensed drugs under new business development strategy
– existing programs for which additional resources can be
justified based on strict IRR criteria
justified based on strict IRR criteria
48

Evaluate Capital Allocation Decisions and
Optimize Balance Sheet
Optimize Balance Sheet
• Potentially increase company leverage while
maintaining strong Net Debt/EBITDA and
EBITDA/Interest Expense ratios
maintaining strong Net Debt/EBITDA and
EBITDA/Interest Expense ratios
• Evaluate the best potential uses for excess cash on
the balance sheet that are most likely to enhance
shareholder value
the balance sheet that are most likely to enhance
shareholder value
49

Corporate Governance Changes
• Replace four current Board members with Icahn Nominees who
we believe will continually hold management accountable
we believe will continually hold management accountable
• Split CEO and Chairman roles
• Appoint new Chairs to Compensation and Compliance
Committees
Committees
• Replace the current Presiding “Independent” Director with an
independent Chairman
independent Chairman
• Expand consultations with leading corporate governance experts
and assess need for any appropriate reforms
and assess need for any appropriate reforms
• Monitor CEO succession plan and confirm that viable external
candidates are appropriately included
candidates are appropriately included
50
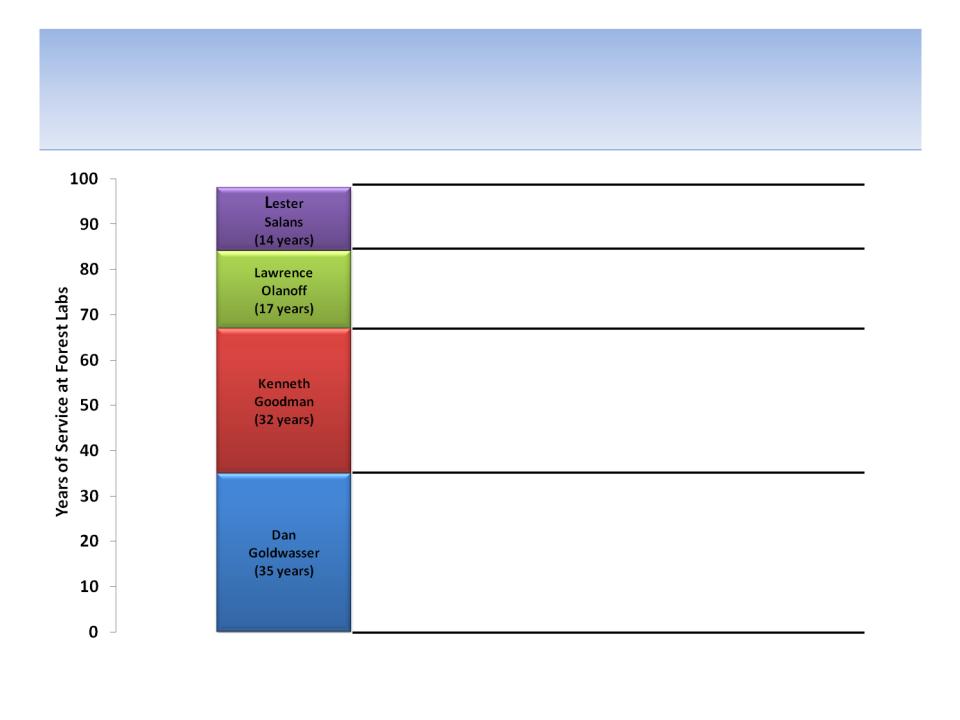
Four Directors That We Believe Have
Trouble “Seeing the FOREST For the Trees”
Trouble “Seeing the FOREST For the Trees”
Source: Company documents
51
• Potential lack of independence due to length of Board tenure
• Need for fresh perspective after 35 years on the Board
• Lack of any biopharma experience outside of Forest
• Oversaw flawed compensation policy as Chair of Compensation Committee
• No other recent public Board experience
• Potential lack of independence due to length of tenure
• Lack of recent relevant operational experience
• No other recent public Board experience
• Lacks independence due to long tenure at Forest and continued compensation as consultant
• One of three former or current Forest executives on Board; Direct report to Solomon
• Need for fresh perspectives from outside of Forest; Employed by Forest for 15 years
• Presided over implementation of, in our belief, inadequate and flawed business strategy
• Potential lack of independence due to long tenure at Forest and direct report to Solomon
• One of three former or current Forest executives on Board
• Need for fresh perspectives from outside of Forest; Employed by Forest for 26 years
• Presided over implementation of, in our belief, inadequate and flawed business strategy
• No other recent public Board experience
98 Years

Icahn Nominees Are Highly Qualified
to Help Produce Positive Change
to Help Produce Positive Change

We Believe the Collective Experience of Icahn
Slate Will Help Create Shareholder Value
Slate Will Help Create Shareholder Value
• A history of helping create shareholder value & stock price appreciation
• Value-enhancing capital allocation & efficient use of balance sheet
• Successful turnarounds of underperforming business operations
• Implemented sales force modernization policies
• Cost structure optimization and right-sizing of organizations
• Public Boards of other biopharmaceutical companies
• Highly efficient clinical development of new drugs with “first-pass” FDA
regulatory approval
regulatory approval
• Multiple successful product launches
• Product licensing focused on creating shareholder value
• Integration of acquisitions and sale of existing businesses
• Effective management of payer relationships and global alliances
53
We Believe the Icahn Slate Will Improve Oversight
and Bring a Fresh & Independent Perspective
and Bring a Fresh & Independent Perspective
Nominee | Background and Experience |
Eric J. Ende, MD | üAs biopharmaceutical analyst for 11 years, he analyzed hundreds of companies with respect to financial statements, cost structure, drug markets, acquisitions & divestitures, clinical data, competitive landscape & product licensing üAs Director of Genzyme on Audit & Risk Management Committees, he worked constructively with existing Board to objectively analyze potential acquisitions, licensing opportunities, divestitures, cost reductions, capital allocation decisions and key business risks while holding the Board and management accountable üMD and MBA degrees enhances understanding of the physician decision-making process from a business perspective, which is highly relevant to commercialization efforts |
Andrew J. Fromkin | üMost recently, as former CEO of Clinical Data, Inc. (acquired by Forest in 2011 for $1.2 B), he gained significant operational experience having managed CLDA’s successful strategic turnaround, highly efficient drug development, first-pass FDA drug approval, product licensing, company acquisitions and divestitures and public Board experience. Significant role in getting FDA approval of Viibryd, one of Forest’s most promising products. üHaving held CEO /President , Corporate Development , COO and other key senior management across healthcare sectors, brings vital, strategic understanding of payer reimbursement, PBM and pharmacy positioning strategies, data strategies for comparative effectiveness, disease and utilization management, provider prescribing requirements and other areas that are necessary for prescription drug adoption and reimbursement. |
Pierre LeGault | üAs President & CEO of Prosidion and CFO of OSI Pharma, he gained valuable experience redefining company strategy, processes, developing drugs, identifying cost savings in addition to divesting and selling both drugs and entire companies üAs Worldwide President of a major division of Sanofi-Aventis, CFO and deputy CEO of several Aventis divisions, CAO of Rite-Aid, Sr. EVP of PJC, and US President of Eckerd, he successfully managed strategic turnarounds, global integrations, product launches, product in-licensings, global alliances, major cost saving efforts, large sales force & marketing groups, FDA product approvals, corporate development strategies and service on public/private Boards |
Dan Ninivaggi | üAs President of Icahn Enterprises and a variety of executive positions at Lear, he gained valuable experience managing strategic turnarounds and cutting costs in dynamic business environments. üAs a director of Icahn Enterprises, CIT Group, Federal-Mogul Corp., XO Holdings, CVR Energy and Motorola Mobility Holdings, he gained valuable experience holding managements accountable and overseeing needed corporate change üAs a lawyer at both a large law firm and in a $17 billion company, he has extensive experience and expertise in corporate governance |
54
We Believe Eric Ende’s Highly Relevant
Experience Will Help Resolve Forest’s Issues
Experience Will Help Resolve Forest’s Issues
Our Issues with Forest Labs | Our Potential Solutions | Relevant Experience |
Long-tenured Directors that lacked independence | Replace long tenured Directors and work constructively with remaining Board | üGenzyme Board contained many long tenured directors üWorked constructively, objectively and independently to gain trust of existing Board üHelped re-focus Board on fiduciary responsibility to all shareholders |
Margin pressure due to corporate inefficiency and a lack of operating leverage in business model | Evaluate company for areas of potential cost reductions | üAs Genzyme audit committee member, oversaw $350 MM of cost reductions and 1,000 person RIF |
Company lacks therapeutic focus | Evaluate each therapeutic area for potential divestiture | üAt Genzyme, evaluated each business unit for potential divestiture, eventually divesting 3 units with cash returned to shareholders |
Business development strategy is not enhancing shareholder value | Evaluate current business development strategy for potential modification | üAs analyst for 11 years, evaluated many company’s strategic direction üAs Genzyme Board member, re-focused strategy towards businesses with high CROIs |
Poor risk management and compliance | Initiate proper execution of comprehensive risk management and compliance program | üAs risk oversight committee member, oversaw initiation and implementation of comprehensive risk management program |
55

We Believe Drew Fromkin’s Highly Relevant
Experience Will Help Resolve Forest’s Issues
Experience Will Help Resolve Forest’s Issues
Our Issues with Forest Labs | Our Potential Solutions | Relevant Experience |
Business development strategy is not enhancing shareholder value | Evaluate current business development strategy for potential modification | üIdentified and executed valuable transactions that enhanced CLDA’s strategic turnaround as CEO and President of Clinical Data (NASDAQ - CLDA) üResponsible for leading numerous M&A, strategic partnerships as VP, Business Development at Medco and during his tenure as CEO, President and other senior roles with healthcare companies. |
Significant stock underperformance | Implement strategy focused on specific therapeutic areas allowing for greater shareholder value creation | üEnacted impressive strategic turnaround at Clinical Data, resulting in stock price rise of 10x during tenure as CEO üCompany was acquired by Forest for $1.2 B and up to an additional $6 per share in CVR’s |
Forest’s operating performance is suboptimal | Drive company growth while maintaining strict cost controls | üTransformed CLDA through organic and inorganic activity while buying, selling, integrating geographically and sector diverse companies. üOperated companies with tight fiscal constraints; understands and managed all aspects of corporate operations and governance. |
Poor commercial uptake of drugs in the market and choices of R&D, drug acquisition | Assess all potential uses of capital utilizing strict criteria to enhance shareholder value | üEnacted aggressive in-licensing and acquisition program to enhance shareholder value as CEO of Clinical Data üStrong strategic understanding of payer reimbursement, PBM and pharmacy positioning strategies, data strategies for comparative effectiveness, disease and utilization management, provider prescribing requirement. |
56
We Believe Pierre Legault’s Highly Relevant
Experience Will Help Resolve Forest’s Issues
Experience Will Help Resolve Forest’s Issues
Our Issues with Forest Labs | Our Potential Solutions | Relevant Experience |
Business development strategy is not enhancing shareholder value | Evaluate current business development strategy for potential modification | üSet successful strategy and negotiated the sales of companies Prosidion to Royalty Pharma & AstraZeneca, OSI Pharma to Astellas and Eckerd to Rite-Aid üSuccessfully turned around global division at Sanofi-Aventis as Worldwide President |
Margin pressure due to corporate inefficiency and a lack of operating leverage in business model | Evaluate company for areas of potential cost reductions | üConsolidated US operations at OSI Pharma resulting in significant cost savings üProduced meaningful cost savings and expense reductions at Aventis üIdentified substantial procurement savings at Rite-Aid |
Sales force productivity is underperforming its peers | Sales force modernization | üLed e-business unit at Aventis with primary focus on sales force modernization (technology & business processes) |
In-licensing and acquisition of drugs has not created value | Set and adhere to specific criteria for product in- licensing and acquisitions | üSuccessfully in-licensed many products as Worldwide President of Sanofi division |
Product launches have lagged expectations | Increase focus and modernize sales force effort | üSuccessfully launched 4 new products as Worldwide President of major Sanofi division |
57

We Believe Dan Ninivaggi’s Highly Relevant
Experience Will Help Resolve Forest’s Issues
Experience Will Help Resolve Forest’s Issues
Our Issues with Forest Labs | Our Potential Solutions | Relevant Experience |
Long-tenured Directors that lacked independence | Replace long tenured Directors and work constructively with remaining Board | üWorked with existing Boards of several companies to help enact productive change, including CIT Group, Inc., Federal−Mogul Corporation, XO Holdings, CVR Energy, Inc., and Motorola Mobility Holdings, Inc. |
Corporate governance needs to be improved | Engage independent Corporate Governance experts to provide industry “best practices” | üWas a partner with the law firm of Winston & Strawn LLP, specializing in corporate finance, mergers and acquisitions, and corporate governance |
Business development strategy is not enhancing shareholder value | Evaluate current business development strategy for potential modification | üMultiple operational roles in a variety of industries in which he helped enact strategic turnarounds |
Margin pressure due to corporate inefficiency and a lack of operating leverage in business model; concerns over misuse of $3.2B of cash | Evaluate company for areas of potential cost reductions; monitor $3.2B of company cash to protect against misuse or inefficient use | üIn multiple operational roles at a variety of companies and as a director of Motorola Mobility, he enacted significant cost reductions focused on operating improvements and G&A spending and oversaw efficient capital allocation |
58
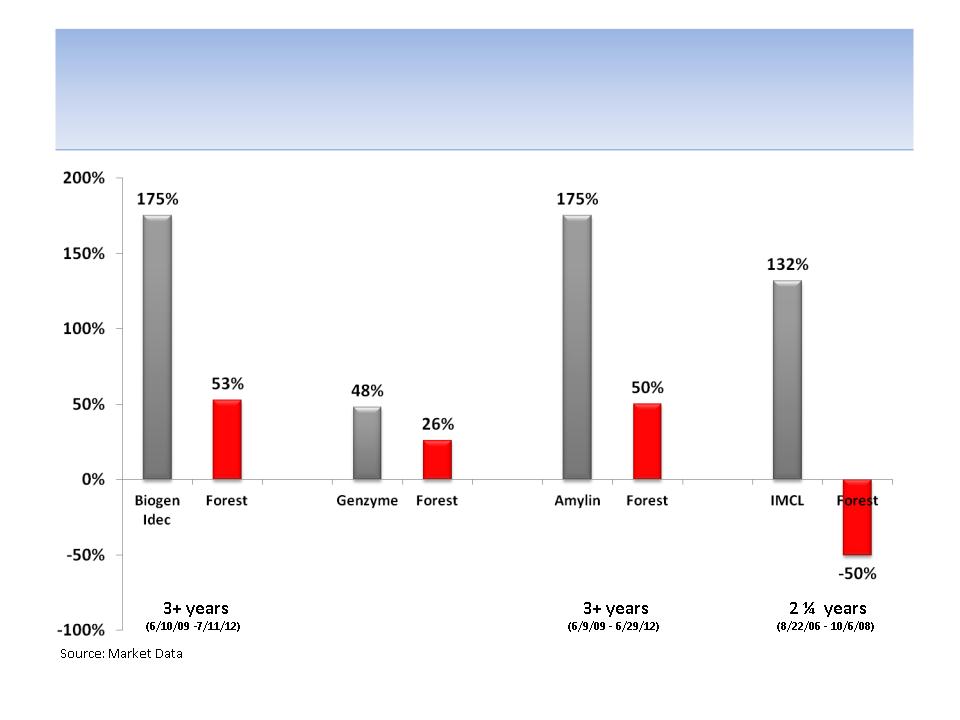
Icahn’s Track Record of Board Representation in
Biopharma Shows Impressive Creation of Shareholder
Value
Biopharma Shows Impressive Creation of Shareholder
Value
Note: All time periods are measured from the day that Icahn gained (or announced gaining) Board representation until the most recent
date of update or when an acquisition price was revealed; This track record represents a sample and does not include all Icahn Board
representation in biopharmaceuticals
date of update or when an acquisition price was revealed; This track record represents a sample and does not include all Icahn Board
representation in biopharmaceuticals
59
8 Months
(6/16/10 - 2/16/11)
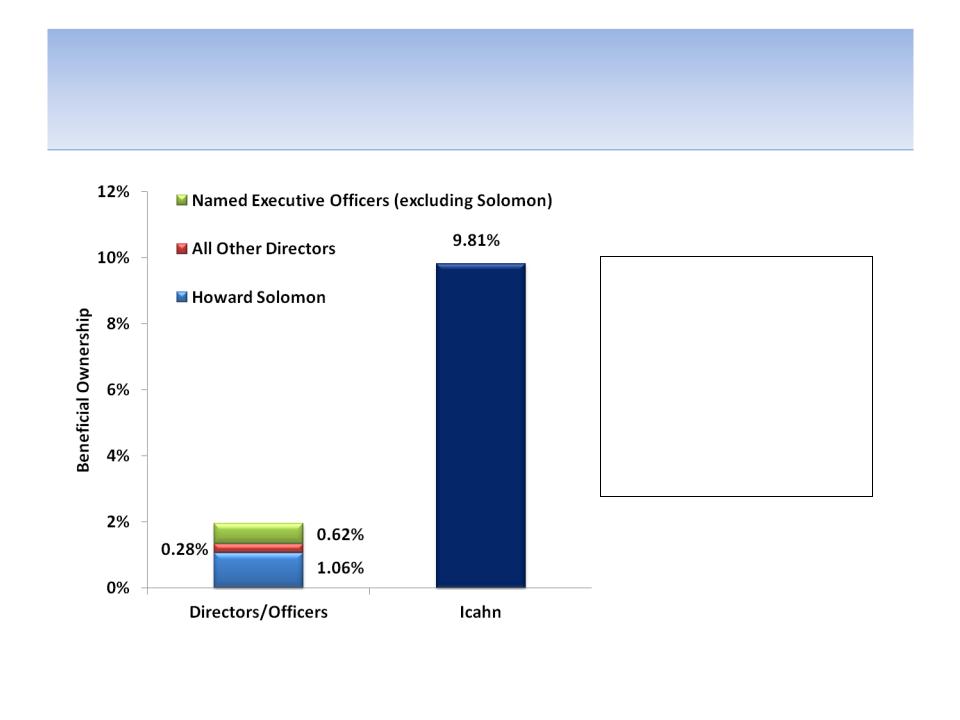
Icahn is Fully Aligned With Shareholders
Despite the Board and
executive officers having a
combined 197 years at the
company, they only own
1.95% of the company
versus Icahn’s 9.81%. Who
do you think is better
aligned with the best
interests of shareholders?
executive officers having a
combined 197 years at the
company, they only own
1.95% of the company
versus Icahn’s 9.81%. Who
do you think is better
aligned with the best
interests of shareholders?
Source: Company documents; proxy statement
Note: Executive officers include Elaine Hochberg, Francis Perier, Marco Taglietti, Howard Solomon and David Solomon
60

We Believe Ende Agreement Fully Aligns
Performance With All Shareholders
Performance With All Shareholders
61
Source: SEC filings; Howard Solomon and Lawrence Olanoff are not considered independent directors by the Company

Appendix A: Pipeline Strategy

New Pipeline Drug Sales vs. Guidance
Source: Company documents and SEC Filings
63
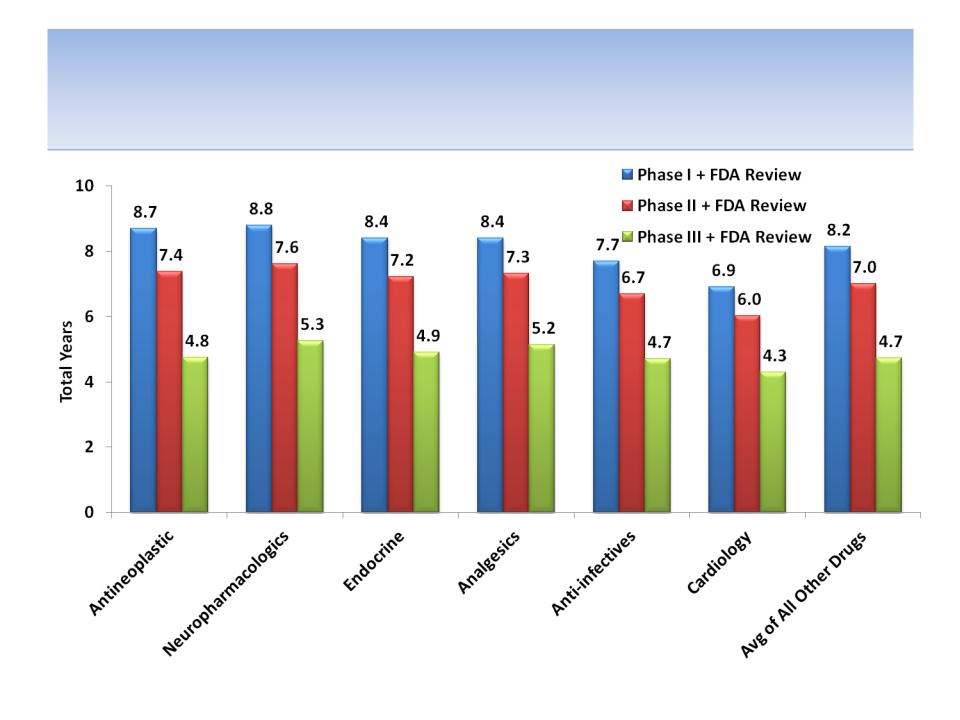
Average Drug Development Timelines
By Therapeutic Class
By Therapeutic Class
Source: Tufts Center for the Study of Drug development
64
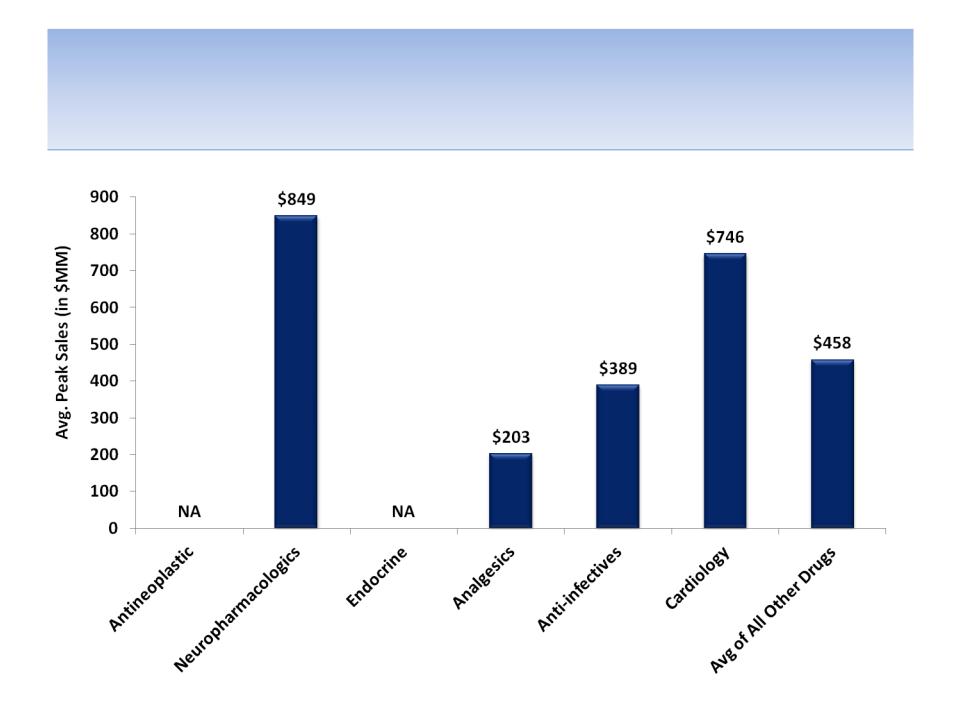
Average Peak Sales by Therapeutic Class
Source: Tufts Center for the Study of Drug development
65
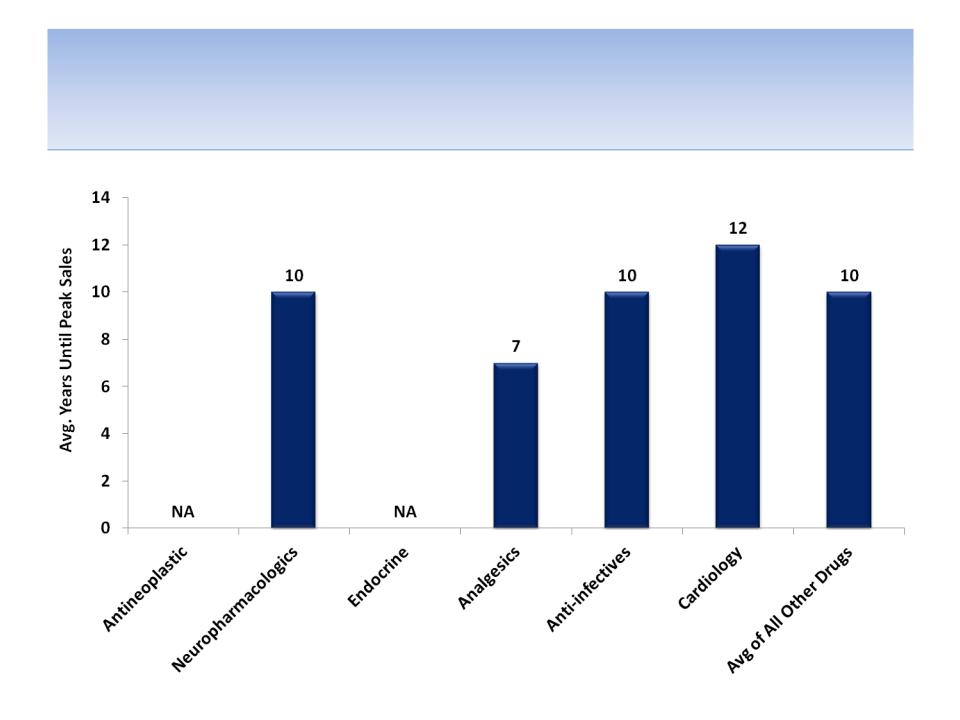
Average Time (years) Until Peak Sales
by Therapeutic Class
by Therapeutic Class
Source: Tufts Center for the Study of Drug development
66
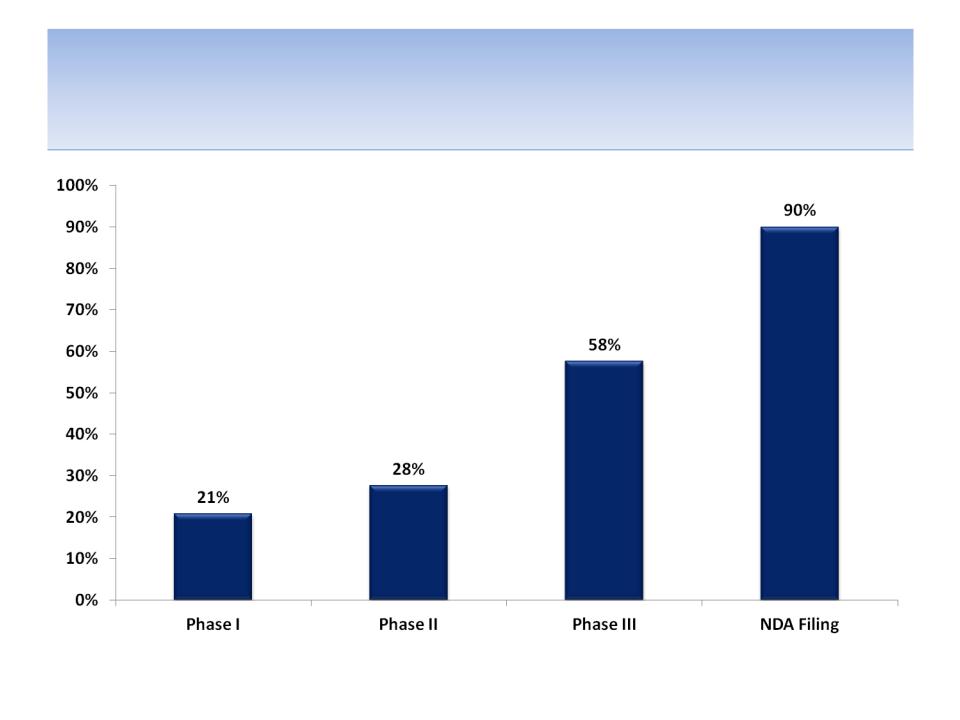
Clinical Development Probabilities
Source: FDAReview.org; odds of product approval assuming entrance into each stage of development
67
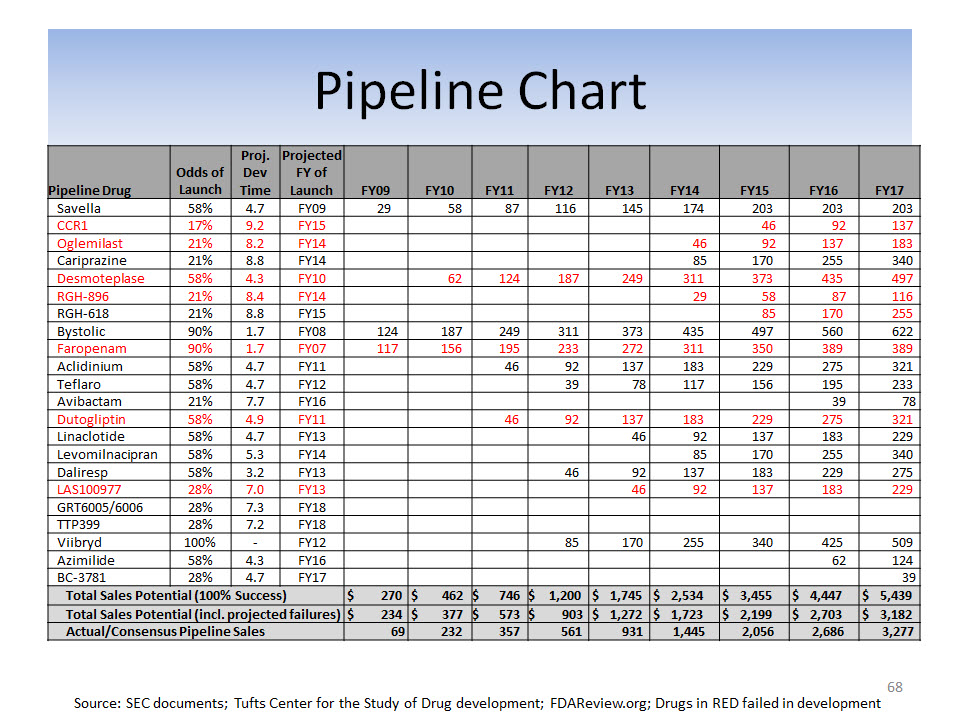

Appendix B: Capital Deployment
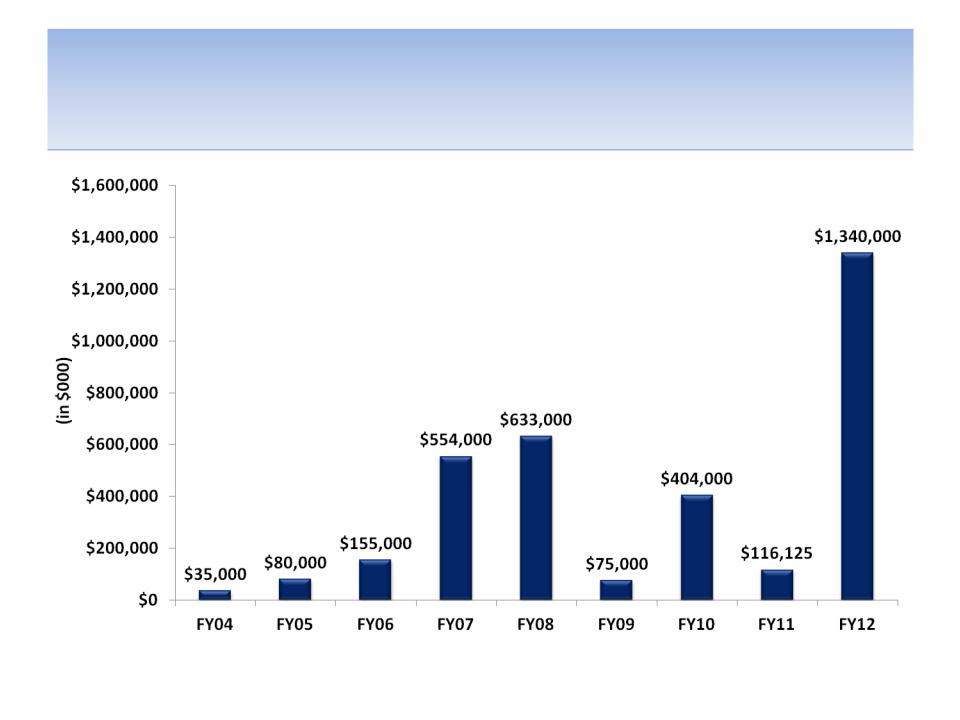
Capital Put At-Risk By Year
Source: SEC filings
Notes: All products licensed FY04 - FY13; Capital At-Risk defined as up-front payments and acquisition of products or product rights
70
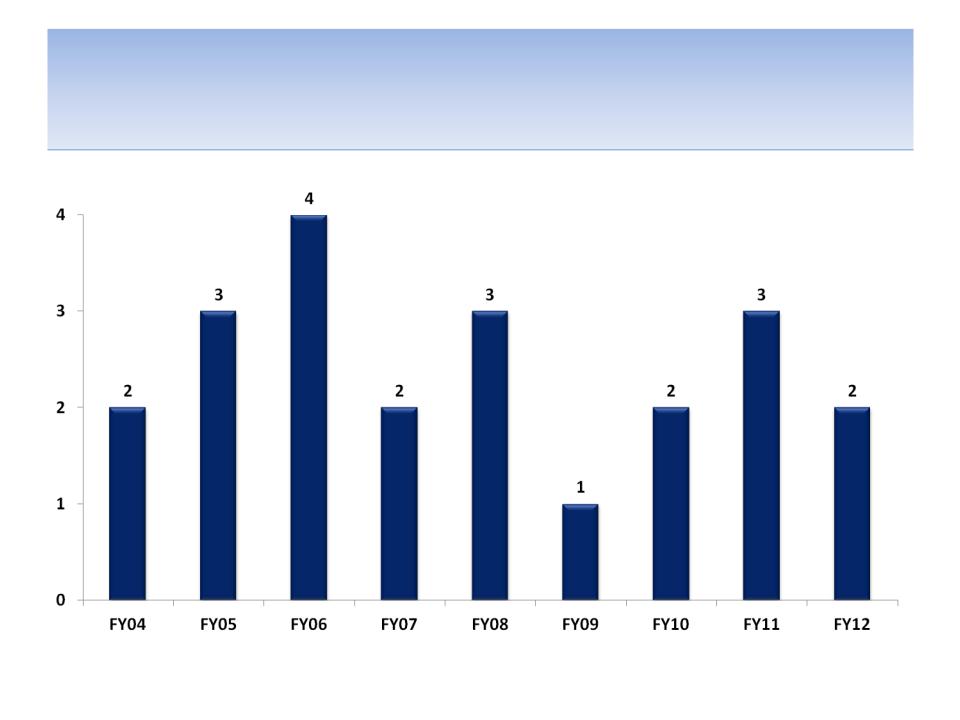
Number of Products Licensed By Year
Notes: All products licensed FY04 - FY13; SEC filing and company documents
71
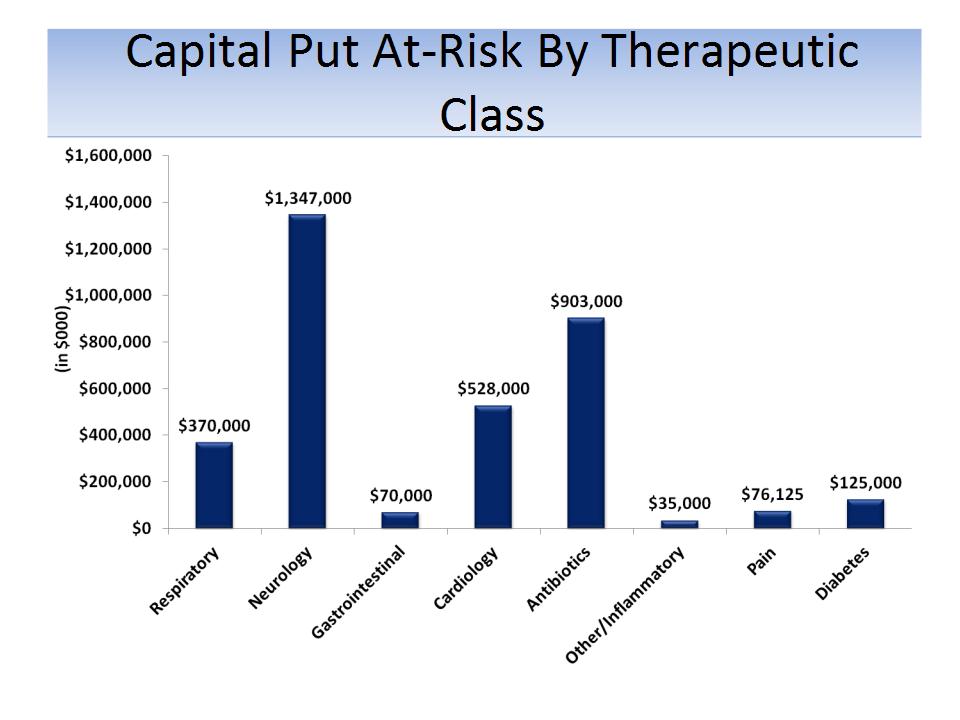
Source: SEC filings
Notes: All products licensed FY04 - FY13 (includes Lexapro and Namenda, which were licensed 1998 and 2000, respectively); Capital
At-Risk defined as up-front payments and acquisition of products or product rights
At-Risk defined as up-front payments and acquisition of products or product rights
72
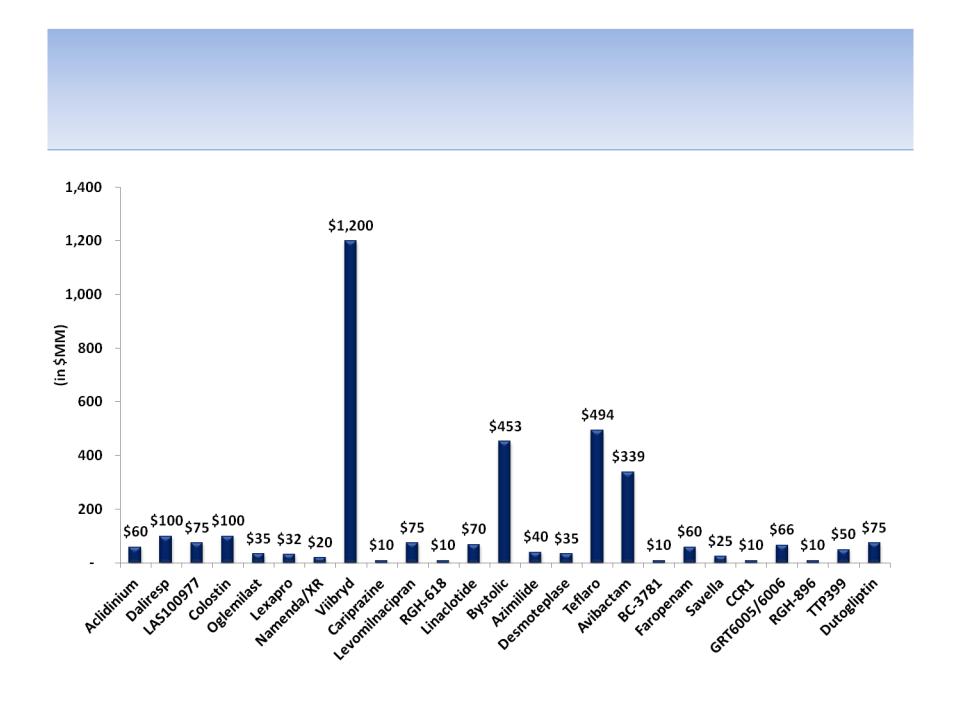
Capital Put At-Risk By Product
Source: SEC filings
Notes: All products licensed FY04 - FY13 (includes Lexapro and Namenda, which were licensed 1998 and 2000, respectively); Capital
At-Risk defined as up-front payments and acquisition of products or product rights
At-Risk defined as up-front payments and acquisition of products or product rights
73

Share Repurchases
Source: SEC filings
74

Appendix C: CEO Compensation
& Stock Sales
& Stock Sales
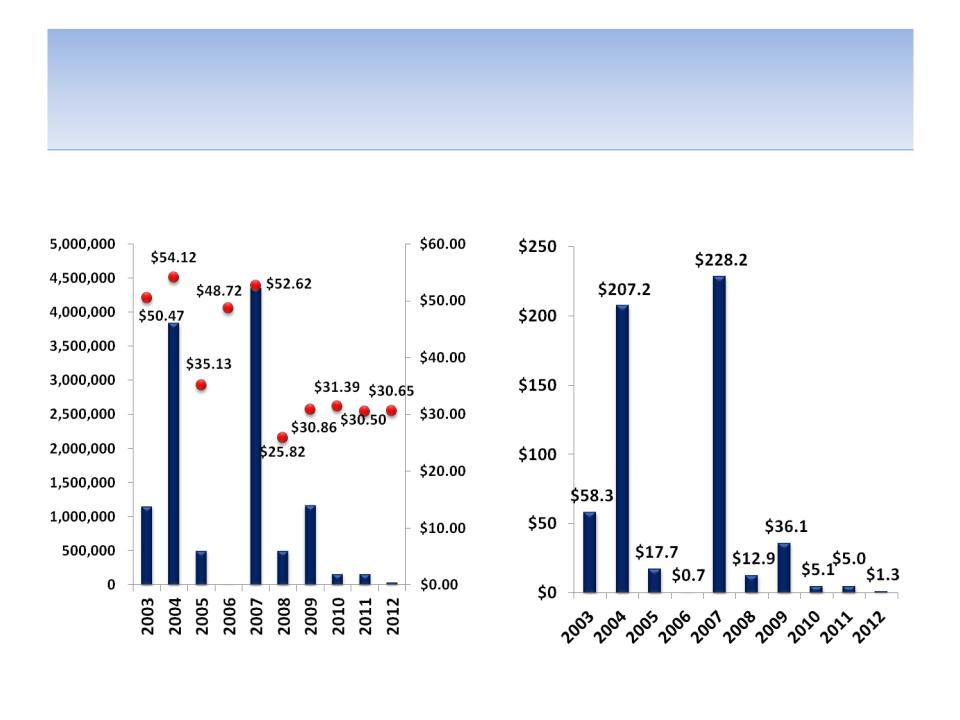
CEO Stock Sales
Shares Sold (& Price) By Year
Total Proceeds ($MM)
Source: SEC filings
77
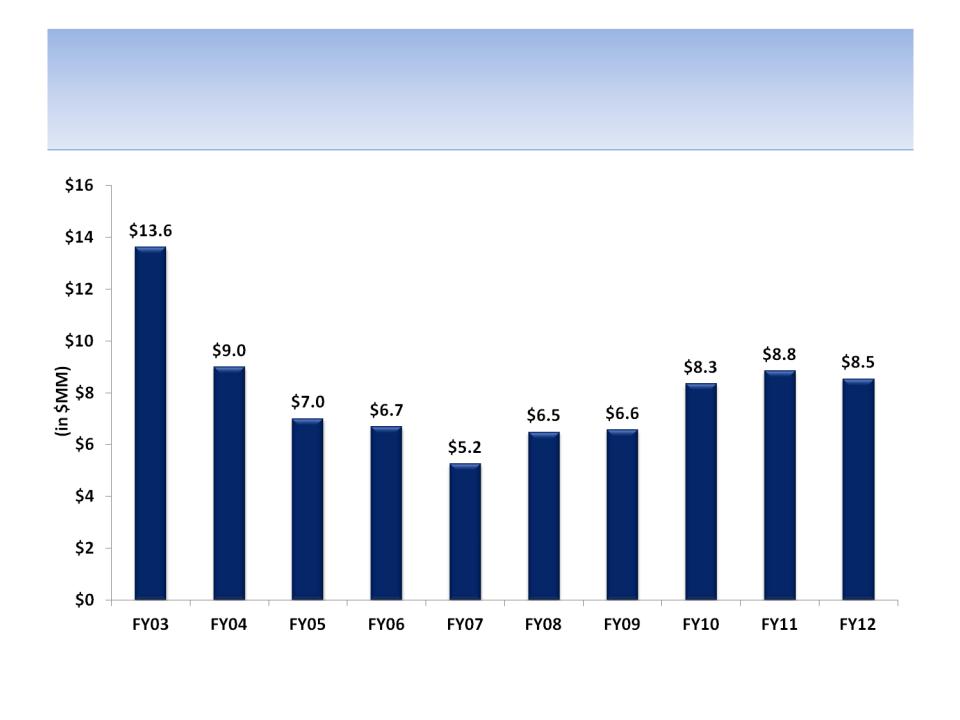
CEO Compensation by Year
Source: SEC filings
78

Appendix D: Comparative
Companies
Companies

SG&A/Revenues
Specialty Pharmaceuticals
Large Pharmaceuticals
Source: SEC filings
80
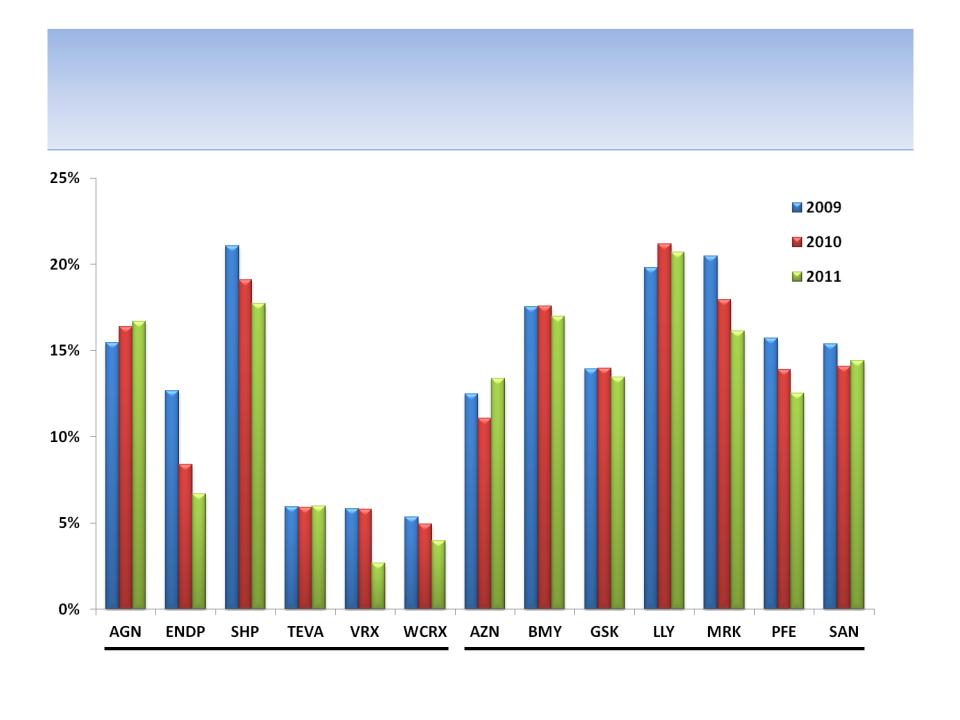
R&D/Revenues
Specialty Pharmaceuticals
Large Pharmaceuticals
Source: SEC filings
81
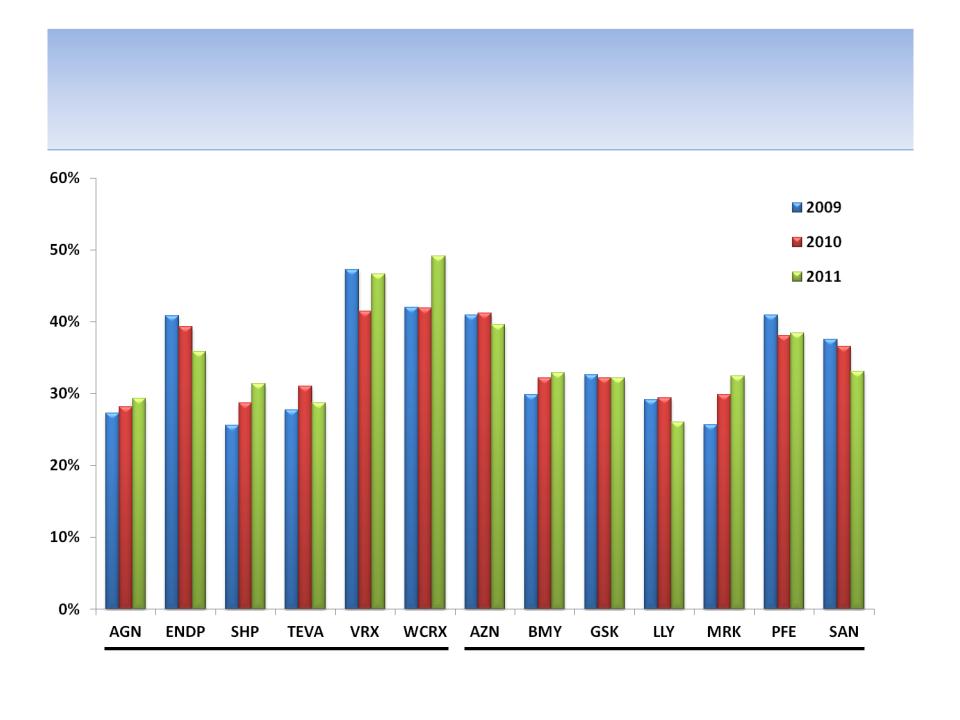
Operating Margins
Specialty Pharmaceuticals
Large Pharmaceuticals
Source: SEC filings
82
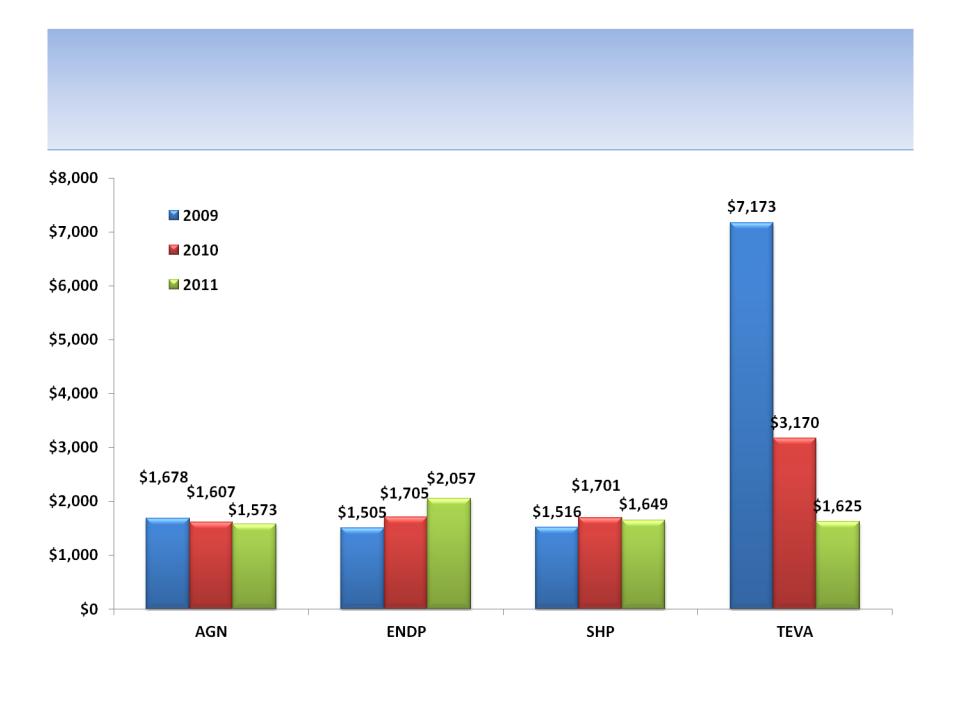
Revenues/Sales Rep
Source: SEC filings
83
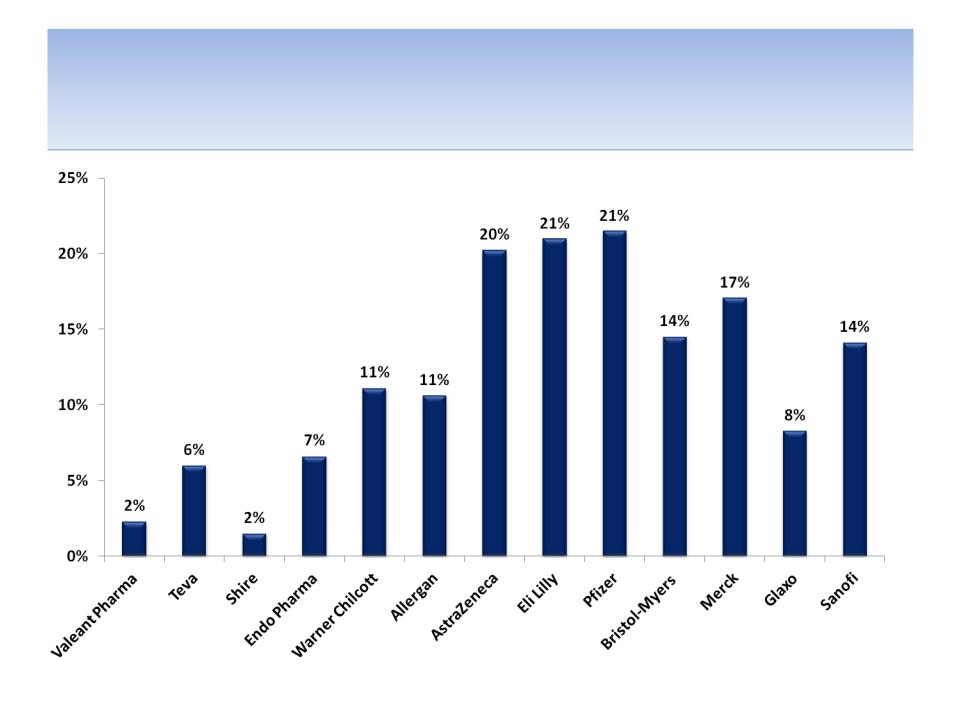
Cash as a Percent of Market Cap
Source: SEC filings
84