SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2006
OR
o TRANSACTION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM __________ TO __________ .
Commission File Number 0-26068
____________
ACACIA RESEARCH CORPORATION
(Exact name of registrant as specified in its charter)
DELAWARE | 95-4405754 |
| (State or other jurisdiction of | (I.R.S. Employer |
| incorporation organization) | Identification No.) |
| | |
500 NEWPORT CENTER DRIVE, NEWPORT BEACH, CA | 92660 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (949) 480-8300
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Name of Each Exchange on Which Registered |
Acacia Research - Acacia Technologies Common Stock, $0.001 par value Acacia Research - CombiMatrix Common Stock, $0.001 par value | The NASDAQ Stock Market LLC The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
____________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to filing requirements for the past 90 days. Yes þ No £
Indicate by check mark that disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ | | Accelerated filer þ | | Non-accelerated filer ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
The aggregate market value of the registrant’s Acacia Research - Acacia Technologies common stock and Acacia Research - CombiMatrix common stock held by non-affiliates of the registrant, computed by reference to the last sales prices of such stocks reported on The Nasdaq Stock Market, as of June 30, 2006, was approximately $382,094,000 and $64,467,000, respectively. (All executive officers and directors of the registrant are considered affiliates.)
As of March 6, 2007, 28,255,628 shares of Acacia Research-Acacia Technologies common stock were issued and outstanding. As of March 6, 2007, 52,365,810 shares of Acacia Research-CombiMatrix common stock were issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its Annual Meeting of Stockholders to be filed with the Commission within 120 days after the close of its fiscal year are incorporated by reference into Part III.
ACACIA RESEARCH CORPORATION
FORM 10-K ANNUAL REPORT
FISCAL YEAR ENDED DECEMBER 31, 2006
TABLE OF CONTENTS
Item | | Page |
| | PART I | |
| | | |
| 1. | Business | 1 |
| 1A. | Risk Factors | 25 |
| 1B. | Unresolved Staff Comments | 50 |
| 2. | Properties | 50 |
| 3. | Legal Proceedings | 50 |
| 4. | Submission of Matters to a Vote of Security Holders | 50 |
| | | |
| | | |
| | PART II | |
| | | |
| 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer | |
| | Purchases of Equity Securities | 51 |
| 6. | Selected Financial Data | 54 |
| 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 58 |
| 7A. | Quantitative and Qualitative Disclosures About Market Risk | 87 |
| 8. | Financial Statements and Supplementary Data | 87 |
| 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 87 |
| 9A. | Controls and Procedures | 87 |
| | | |
| | | |
| | PART III | |
| | | |
| 10. | Directors, Executive Officers and Corporate Governance | 88 |
| 11. | Executive Compensation | 88 |
| 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 88 |
| 13. | Certain Relationships and Related Transactions, and Director Independence | 88 |
| 14. | Principal Accounting Fees and Services | 88 |
| | | |
| | | |
| | PART IV | |
| | | |
| 15. | Exhibits and Financial Statement Schedules | 89 |
PART I
CAUTIONARY STATEMENT
This report contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Reference is made in particular to the description of our plans and objectives for future operations, assumptions underlying such plans and objectives, and other forward-looking statements included in this report. Such statements may be identified by the use of forward-looking terminology such as “may,” “will,” “expect,” “believe,” “estimate,” “anticipate,” “intend,” “continue,” or similar terms, variations of such terms or the negative of such terms. Such statements are based on management’s current expectations and are subject to a number of factors and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. Such statements address future events and conditions concerning product development, capital expenditures, earnings, litigation, regulatory matters, markets for products and services, liquidity and capital resources and accounting matters. Actual results in each case could differ materially from those anticipated in such statements by reason of factors such as future economic conditions, changes in consumer demand, legislative, regulatory and competitive developments in markets in which we and our subsidiaries operate, and other circumstances affecting anticipated revenues and costs, as more fully disclosed in our discussion of risk factors incorporated by reference in Item 1.A of Part I of this report. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. Additional factors that could cause such results to differ materially from those described in the forward-looking statements are set forth in connection with the forward-looking statements.
As used in this Form 10-K, “we,” “us” and “our” refer to Acacia Research Corporation and its subsidiary companies.
Item 1. BUSINESS
Overview
Acacia Research Corporation is comprised of two operating groups.
Acacia Technologies Group
The Acacia Technologies group, a division of Acacia Research Corporation, develops, acquires, licenses and enforces patented technologies. The Acacia Technologies group is primarily comprised of certain of Acacia Research Corporation’s wholly owned subsidiaries and limited liability companies including:
· Acacia Global Acquisition Corporation · Acacia Media Technologies Corporation · Acacia Patent Acquisition Corporation · Acacia Technologies Services Corporation · AV Technologies LLC · Broadcast Data Retrieval Corporation · Broadcast Innovation LLC · Computer Acceleration Corporation · Computer Cache Coherency Corporation · Computer Docking Station Corporation · Credit Card Fraud Control Corporation · Database Structures Inc. · Data Encryption Corporation · Data Innovation LLC · Diagnostic Systems Corporation · Disc Link Corporation · Financial Systems Innovation LLC · Fluid Dynamics Corporation · High Resolution Optics Corporation · Information Technology Innovation LLC | · InternetAd LLC · IP Innovation LLC · KY Data Systems LLC · Location Based Services Corporation · Micromesh Technology Corporation · Microprocessor Enhancement Corporation · Mobile Traffic Systems Corporation · New Medium LLC · Peer Communications Corporation · Product Activation Corporation · Remote Video Camera Corporation · Resource Scheduling Corporation · Safety Braking Corporation · Screentone Systems Corporation · Soundview Technologies Inc. · Spreadsheet Automation Corporation · TechSearch LLC · Telematics Corporation · VData LLC |
The Acacia Technologies group also includes all corporate assets, liabilities, and related transactions of Acacia Research Corporation attributed to Acacia Research Corporation’s intellectual property licensing and enforcement business.
The Acacia Technologies group currently owns or controls the rights to 62 patent portfolios, covering technologies used in a wide variety of industries, including the following:
· Aligned Wafer Bonding · Audio Communications Fraud Detection · Audio Video Enhancement & Synchronization · Broadcast Data Retrieval · Color Correction For Video Graphics Systems · Compact Disk · Computer Memory Cache Coherency · Computing Device Performance · Continuous TV Viewer Measuring · Credit Card Fraud Protection · Database Management · Data Encryption · Digital Video Production · DMT® · Document Generation · Dynamic Manufacturing Modeling · Electronic Address List Management · Enhanced Internet Navigation · File Locking In Shared Storage Networks · Fluid Flow Control And Monitoring · Hearing Aid ECS · High Quality Image Processing · High Resolution Optics · Image Resolution Enhancement | · Information Monitoring · Interactive Television · Laptop Connectivity · Location Based Services · Medical Image Stabilization · Micromesh Laminate · Micromirror Digital Display · Microprocessor Enhancement · Multi-dimensional Bar Codes · Picture Archiving & Communication Systems · Pointing Device · Pop-Up Advertising · Portable Storage Devices With Links · Product Activation · Remote Video Camera · Resource Scheduling · Software License Management · Spreadsheet Automation · Storage Technology · Telematics · User Activated Internet Advertising · Vehicle Anti-Theft Parking System · Vehicle Magnetic Braking · Web Personalization · Wireless Traffic Information |
CombiMatrix Group
Our life sciences business, referred to as the “CombiMatrix group,” a division of Acacia Research Corporation, is comprised of our wholly owned subsidiary, CombiMatrix Corporation and CombiMatrix Corporation’s wholly owned subsidiary, CombiMatrix Molecular Diagnostics, Inc. and includes all corporate assets, liabilities and transactions related to Acacia Research Corporation’s life sciences business.
The CombiMatrix group is seeking to become a broadly diversified biotechnology business, through the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where its products and services could be utilized. The technologies that the CombiMatrix group has developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. The CombiMatrix group has also developed the capabilities of producing arrays that utilize bacterial artificial chromosomes on its arrays, also enabling genetic analysis. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs.
Through the year ended December 31, 2005, the CombiMatrix group’s life sciences business included two subsidiaries, CombiMatrix Molecular Diagnostics, Inc. and CombiMatrix K.K. CombiMatrix Molecular Diagnostics, Inc., a wholly owned subsidiary located in Irvine, California, is exploring opportunities for the CombiMatrix group’s arrays in the field of molecular diagnostics. CombiMatrix K.K. is a Japanese corporation located in Tokyo, Japan, and has existed for the purposes of exploring opportunities for the CombiMatrix group’s array system with pharmaceutical and biotechnology companies in the Asian market. In January of 2006, the CombiMatrix group sold 67% of its ownership interest in CombiMatrix K.K. to a third party, and continued to retain a 33% ownership interest. Based upon the annual financial statements for the year ended December 31, 2005, this sale did not constitute the sale of a "significant subsidiary" as that term is defined by the Commission in Rule 1-02 of Regulation S-X.
In January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, to become an independent public company. The transaction is expected to be completed no sooner than the second quarter of 2007, subject, however, to completing the required filings with the Securities and Exchange Commission (“SEC”). We have received a private letter ruling from the IRS addressing certain tax implications of the transaction and have received a tax opinion from counsel. CombiMatrix Corporation filed a registration statement on Form S-1 on December 26, 2006, which has not been declared effective. If CombiMatrix Corporation’s registration statement on Form S-1 is declared effective by the SEC, Acacia Research Corporation will redeem all of the issued and outstanding shares of AR-CombiMatrix common stock for all of the common stock of CombiMatrix Corporation, which will register its common stock under the Securities and Exchange Act of 1934. Following the redemption, CombiMatrix Corporation will apply to list its shares for trading on a national exchange.
Capital Structure
Acacia Research Corporation has two classes of common stock called Acacia Research-Acacia Technologies common stock (“AR-Acacia Technologies stock”) and Acacia Research-CombiMatrix common stock (“AR-CombiMatrix stock”). AR-Acacia Technologies stock is intended to reflect separately the performance of Acacia Research Corporation’s Acacia Technologies group. AR-CombiMatrix stock is intended to reflect separately the performance of Acacia Research Corporation’s CombiMatrix group. Although the AR-Acacia Technologies stock and the AR-CombiMatrix stock are intended to reflect the performance of our different business groups, they are both classes of common stock of Acacia Research Corporation and are not stock issued by the respective groups. As a result, holders of Acacia Research-Acacia Technologies stock and Acacia Research-CombiMatrix stock continue to be subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. The assets Acacia Research Corporation attributes to one group could be subject to the liabilities of the other group. Included in Acacia Research Corporation’s operating groups are certain wholly owned subsidiaries that are not material, quantitatively or qualitatively, either individually or in the aggregate, to either group, or to Acacia Research Corporation as a whole.
Other
Acacia Research Corporation, a Delaware corporation, was originally incorporated in California in January 1993 and reincorporated in Delaware in December 1999. Our website address is www.acaciaresearch.com. We make our filings with the SEC, including our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports, available free of charge on our website as soon as reasonably practicable after we file these reports. In addition, we post the following information on our website:
· our corporate code of conduct, our board of directors - code of conduct and our fraud policy;
| | · | charters for our audit committee, nominating and corporate governance committee, disclosure committee and compensation committee; |
The public may read and copy any materials that Acacia Research Corporation files with the SEC at the SEC’s Public Reference Room at 450 Fifth Street N.W., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Also, the SEC maintains an Internet website that contains reports, proxy and information statements, and other information regarding issuers, including the Acacia Research Corporation, that file electronically with the SEC. The public can obtain any documents that Acacia Research Corporation files with the SEC at http://www.sec.gov.
BUSINESS GROUPS
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
Intellectual Property Licensing Business
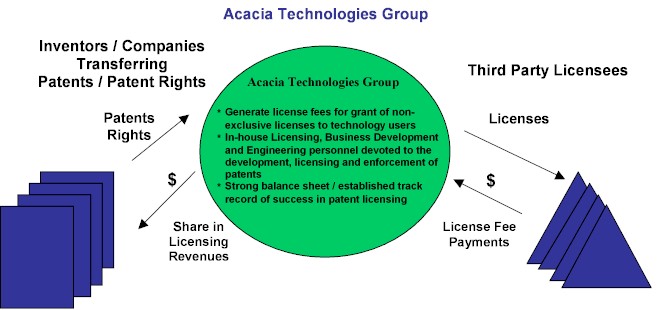
The Acacia Technologies group, a division of Acacia Research Corporation, develops, acquires, licenses and enforces patented technologies. The Acacia Technologies group generates license fee revenues and related cash flows from the granting of licenses for the use of its patented technologies. The Acacia Technologies group assists patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, if necessary, with the enforcement against unauthorized users of their patented technologies. The Acacia Technologies group’s subsidiary companies currently own or control the rights to more than 60 patent portfolios and have established a track record of licensing success with more than 500 license agreements executed to date. Our professional staff includes in-house patent attorneys, licensing executives, engineers and business development executives.
The Acacia Technologies group’s clients are primarily individual inventors and small companies who have limited resources and/or expertise to effectively address the unauthorized use of their patented technologies, and also include large companies seeking to effectively and efficiently monetize their portfolio of patented technologies. In a typical client arrangement, the Acacia Technologies group will acquire the patent portfolio or acquire rights to the patent portfolio, with our client receiving an upfront payment for the purchase of the patent portfolio or patent portfolio rights, or receiving a percentage of our net recoveries from the licensing and enforcement of the patent portfolio, or a combination of the two.
The Acacia Technologies group is primarily comprised of certain of Acacia Research Corporation’s wholly owned subsidiaries and limited liability companies, as described earlier, and also includes all corporate assets, liabilities, and related transactions of Acacia Research Corporation attributed to Acacia Research Corporation’s intellectual property licensing and enforcement business.
On January 28, 2005, Acacia Global Acquisition Corporation acquired substantially all of the assets of Global Patent Holdings, LLC, a privately held patent holding company based in Northbrook, Illinois, which owned 11 patent licensing companies (“GPH Acquisition”). The acquisition provided the Acacia Technologies group 100% ownership of companies that own or control the rights to 27 patent portfolios, which included 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. Refer to Note 8 in the accompanying Acacia Research Corporation consolidated financial statements for additional information regarding the GPH Acquisition.
The Acacia Technologies Group’s Business Model and Strategy
The Acacia Technologies group’s business model is summarized in the following diagram:
The Acacia Technologies group’s business strategy includes the following key elements:
| | · | Identify Emerging Growth Areas where Patented Technologies will Play a Vital Role |
The patent process breeds innovation and invention by granting a limited monopoly to the inventor in exchange for sharing the invention with the public. Certain technologies, including several of the technologies controlled by the Acacia Technologies group described below, become core technologies in the way products and services are manufactured, sold and delivered. The Acacia Technologies group identifies core, patented technologies that have been or are anticipated to be widely adopted by third parties in connection with the manufacture or sale of products and services.
| | · | Contact and Form Alliances with Owners of Core, Patented Technologies |
Often individual inventors and small companies have limited resources and/or expertise and are unable to effectively address the unauthorized use of their patented technologies. Individual inventors and small companies may lack sufficient capital resources and may also lack in-house personnel with patent licensing expertise and/or experience, which may make it difficult to effectively out-license and/or enforce their patented technologies.
For years, many large companies have earned substantial revenue licensing patented technologies to third parties. Other companies that do not have internal licensing resources and expertise have continued to record the estimated value of intellectual property on their financial statements without deriving income from their intellectual property. Securities and financial reporting regulations require these companies to evaluate and potentially reduce or write-off these intellectual property assets if they are unable to substantiate these reported values.
The Acacia Technologies group seeks to enter into business agreements with owners of intellectual property that do not have experience or expertise in the areas of intellectual property licensing and enforcement or that do not possess the in-house resources to devote to licensing and enforcement activities.
| | · | Effectively and Efficiently Evaluate Patented Technologies for Acquisition, Licensing and Enforcement |
Subtleties in the language of a patent, recorded interactions with the patent office, and the evaluation of prior art and literature can make a significant difference in the potential licensing and enforcement revenue derived from a patent or patent portfolio. The Acacia Technologies group’s specialists are trained and skilled in these areas. It is important to identify potential problem areas prior to commercialization and determine whether potential problem areas can be overcome, before launching a licensing program. We have developed processes and procedures for identifying problem areas and evaluating the strength of a patent before the decision is made to allocate resources to a licensing and enforcement effort.
| | · | Purchase or Acquire the Rights to Patented Technologies |
After evaluation, the Acacia Technologies group may elect to purchase the patented technology, or become the exclusive licensing agent for the patented technology in all or in specific fields of use. In either case, the owner of the patent generally retains the rights to a portion of the revenues generated from a patent’s licensing and enforcement program. The Acacia Technologies group generally controls the licensing and enforcement process and utilizes its experienced in-house personnel to reduce outside costs and to ensure that the Acacia Technologies group’s capital is allocated and utilized in an efficient and cost effective manner.
| | · | Successfully License and Enforce Patents with Significant Royalty Potential |
As part of our patent evaluation process, significant consideration is also given to the identification of potential infringers, industries within which the potential infringers exist, longevity of the patented technology, and a variety of other factors that directly impact the magnitude and potential success of a licensing and enforcement program. Acacia Technologies group’s specialists are trained in evaluating potentially infringing technologies and in presenting the claims of our patents and demonstrating how they apply to companies we believe are using our technologies in their products or services. These presentations generally take place in a non-adversarial business setting, but can also occur through the litigation process, if necessary.
Acacia Technologies Group - Patented Technologies
Currently, the Acacia Technologies group owns or controls the rights to 62 patent portfolios, with patent expiration dates ranging from 2007 to 2020, and covering technologies used in a wide variety of industries, including the following:
This patented technology generally relates to the precision alignment and bonding of micromechanical, electrical and optical structures. This technology can be used for the bonding of surface features in the fabrication of Micro Electromechanical Systems (“MEMS”) and semiconductors.
| | · | Audio Communications Fraud Detection |
The patents and applications generally relate to methods for determining and preventing fraud when using telephonic, computer network, or other communication services to complete a sale. The claims cover methods for preventing fraud during the purchase of services for entertainment or technical support, such as psychic readings and computer software support. These methods help protect vendors from credit card charge-backs and help protect customers whose credit card numbers may have been stolen.
| | · | Audio/Video Enhancement and Synchronization |
The Audio/Video Enhancement and Synchronization technologies generally relate to the use of an adaptive noise reduction filtering system for analog and digital video signals, especially those used in video compression systems and for video displays, and for synchronizing video and audio signals utilized in systems where the audio and video components are stored, transmitted or otherwise processed separately.
| | · | Broadcast Data Retrieval |
This patented technology generally relates to a system for broadcasting and receiving programming content together with supplemental data such as the title of a song, artist, or a catalog number, that can be stored and recalled for later viewing. This technology can be used in satellite radio and other broadcasting where data is transmitted along with the content.
| | · | Color Correction For Video Graphics Systems |
This patented technology generally relates to methods for altering the colors of isolated objects in a video graphics system. The technology can be used to efficiently and accurately change colors of objects in a video frame or digital picture without affecting the entire image.
The patent, currently being reexamined by the United States Patent and Trademark Office, covers certain systems for recording and playing compact disks containing compressed audio data utilizing certain data-compression methods. The CD player technology, consisting in part of a CD drive that reads MPEG Layer-3 (“MP3”) compressed digital audio data recorded on a CD, and an integrated circuit chip which decompresses the data and produces a non-compressed audio output, is commonly found in DVD and CD players.
| | · | Computer Memory Cache Coherency |
This patented technology generally relates to interface circuits used by intelligent peripheral devices with cache memory to communicate with the main computer memory. By synchronizing main computer memory and main cache memory, peripheral devices such as graphics processors can operate at much higher speeds, without costs associated with their own memory. This technology can be used in desktop, notebook, and server computer systems.
| | · | Computing Device Performance Technology |
The patents relate to technology to improve the startup and use of desktop and portable computers and other computing devices, including those that access software and information from mass storage devices, such as CD-ROM's and magnetic hard drives. The use of the patented technology results in a significant reduction in the startup time and improvement in the performance of applications running on computing devices.
| | · | Continuous Television Viewer Measuring Technology |
The patented technology, unlike prior systems that take snapshots of tuning to assess viewership, relates to uninterrupted and passive continuous monitoring and measuring of television viewers' actions. Measurements include channel changes, channel surfing, video-on-demand and DVR viewership captured through the set-top box, stored and transmitted to a remote location for aggregation.
| | · | Credit Card Fraud Protection |
This patented technology generally relates to a computerized system for protecting retailers and consumers engaged in credit card, check card, and debit transactions. The system includes an electronic card reader, and the generation and use of a transaction number, which specifically identifies each transaction processed within the system. As a result, the retailer does not necessarily have to print detailed information concerning the cardholder’s identity or account number on the customer’s receipt.
This patented technology generally relates to the improved combination, display, and coordination of certain information from data tables in a relational database software program. The user is able to easily track the impact of a change to one table, on other tables in the program through various tools including a graphical representation.
This patented technology generally relates to the use of an operating system to transparently create encrypted file storage subsystem to fully secure user files from access by anyone other than the user.
| | · | Digital Video Production |
These patented technologies generally relate to features that can be found in production video processing equipment. They cover improved methods of equipment interconnection, aspects of graphical user interface displays, and automation of video processing. These features allow ease of equipment interconnection, clearer information display, and automation of video production tasks previously performed manually. Other aspects of the technologies generally relate to automatic color correction, commonly used when transferring film to video, and certain 3D effects, commonly used in video scene transitions.
| | · | Digital Media Transmission (“DMT®”) Technology |
This patented technology generally relates to the transmission and receipt of digital audio and/or audio video content via a variety of means including the internet, cable, satellite, and local area networks. Elements of the DMT® transmission process include a source material library, identification encoding process, format conversion, sequence encoding, compressed data storage, and transmission. Elements of the DMT® receiving process include a transceiver, format conversion, storage, decompression, and playback. Acacia Technologies group has entered into 315 DMT® licensing agreements to date, including cable TV licenses, licenses for online entertainment, movies, news, sports, e-learning and corporate websites and licenses with companies that provide over 90% of video-on-demand TV entertainment to the hotel industry in the United States.
This patented technology generally relates to storing data in databases such that it could be used to quickly populate multiple document templates. This technology can be used in medical applications such as Electronic Medical Records (“EMR”) and Electronic Health Records (“EHR”), as well as document generation applications in the financial, legal, and insurance industries.
| | · | Dynamic Manufacturing Modeling |
This patented technology generally relates to a modeling and control process used to decrease costs and increase production for factory operations. Such simulation modeling can include a variety of parameters such as products, fabrication sequences, collections of job sets, scheduling rules, and machine reliability standards. This technology can be used for exacting manufacturing processes such as semiconductor fabrication.
| | · | Electronic Address List Management |
This patented technology can allow a user to manage an address list on a computer and transfer the list to an electronic device such as a cell phone.
| | · | Enhanced Internet Navigation |
These patented technologies generally relate to enhanced Internet navigation by retrieving a page from a hyper-linked website for retrieval offline on a personal computer. This enables certain website content to be saved, retrieved, and accessed locally, without the need for Internet connectivity. Other aspects of the technologies generally relate to information distribution and processing via the use of a linking reference to access sets of data. These technologies can be used in email transmissions with links to websites, special offers, and other information.
| | · | File Locking In Shared Storage Networks |
The patent relates to a file locking system for use in shared storage networks such as iSCSI. The use of the patented technology removes a single point of failure for companies migrating existing Storage Area Network (“SAN”) implementations to iSCSI or for those creating new shared storage networks.
| | · | Fluid Flow Control and Monitoring |
The patent relates to systems used in the remote control and monitoring of fluid flow, both gas and liquid. This technology can be used in heating/ventilation/air conditioning (“HVAC”), plumbing and other industrial, commercial and residential fluid flow systems.
This patented technology shields hearing aids from electromagnetic interference produced by portable electronic devices such as cell phones, cordless phones, wireless headphones and headsets, and WIFI and Bluetooth enabled devices.
| | · | High Quality Image Processing |
This patented technology generally relates to methods for improving print image quality through the use of advanced signal processing techniques for transforming continuous toned images into high quality half toned images. This technology can be used in printing products such as high end laser printers and image setters.
These patents generally relate to refractive and diffractive systems and methods for improving imaging capabilities in multi-element optical systems by using fewer elements. The patented systems and techniques have direct applications in military imaging systems such as thermal weapon sites, as well as commercial products like camera lenses and optical printers.
| | · | Image Resolution Enhancement |
This patented technology generally relates to the modification of images to be displayed on a video or printed display to improve the perceived image quality beyond the basic pixel resolution of the display original image.
| | · | Information Monitoring Technology |
This patented technology, which can be incorporated into software products, can be used to monitor infrastructure components such as hardware, servers, networks, and operating systems, and can also be used for information monitoring and other information databases.
These patented technologies generally relate to various aspects of interactive television including receivers such as set-top boxes and certain televisions used in digital satellite and digital cable systems that permit television viewers to access interactive television features supplied by their satellite and cable providers as part of their digital programming packages. Data, which is associated with the interactive television features and is broadcast along with the video signal, is extracted and processed by components within the receivers, and is then made available to viewers who choose to access the interactive television features through their remote control. Examples of such data include sports scores, weather information, stock updates, interactive games, and movie listings. Other aspects of the technologies generally relate to the scrambling or encrypting of broadcast signals whereby the unscrambling or decryption is accomplished through a removable card, commonly known as a “smart card.”
This patented technology is used to connect a laptop or other portable computer to multiple external devices such as a keyboard, monitor, printer, or mouse, through a single connector from the laptop to the docking station. The use of a single connector for multiple devices makes it easier to remove the laptop from the devices when it is used remotely, and to reconnect the laptop to the devices when it is returned to the docking station.
This patented technology generally relates to locating mobile units, such as cell phones and embedded vehicle radios, within a cellular network and using the position information to provide services to the mobile user. It covers various means of accurately locating a mobile unit, including GPS and cell site triangulation. This technology is applicable to wireless emergency services (“E911”), vehicle tracking, vehicle assistance services and many other services that rely on knowing the location of a mobile user.
| | · | Medical Image Stabilization |
This patented technology can be used in stabilizing medical images for interventional procedures such as cardiac catheters and stents, and for diagnostics procedures such as visualization of arterial lesions.
This patented technology can be used in fabrics to maximize moisture transport and increase breathability and is often used in sports apparel.
| | · | Micromirror Digital Display |
This patented technology generally relates to techniques for using micromirrors to display a color image having gray scale gradations and is utilized in large screen televisions and projectors.
| | · | Microprocessor Enhancement |
This patented technology generally relates to an architecture employed in advanced pipeline microprocessors. This architecture allows for conditional execution of microprocessor instructions, and a later determination of whether the instructions executed should be written back to memory. By conditionally executing instructions in this architecture, significant improvements in microprocessor speed can be achieved. Certain pipelined processor manufacturers are adopting this method of processing to improve processor speed.
| | · | Multi-dimensional Bar Codes |
This patented technology generally relates to encoding and reading a data matrix consisting of an array of data cells with a border. The data matrix can contain a variety, amount, and depth of information that would not fit on to an ordinary bar code. This patented technology can have many applications in the manufacturing, distribution, operations, accounting, and security industries such as tracking the movement of products, collection of data, improved production capabilities and anti-counterfeiting.
| | · | Picture Archiving & Communication Systems (“PACS”) |
PACS enable multiple, remote users to simultaneously access image data from remote display terminals over common phone and data networks, such as the Internet. PACS are commonly used by hospitals to acquire, store, archive and transmit patient image data for remote access by their physicians, at their homes, offices or within the hospital at the point of care.
This patented technology generally relates to hand held devices that include pointing devices, such as a joy stick, capable of carrying out multiple user selectable functions.
This patented technology generally relates to user activated displays on a website, most commonly known as Pop-Ups or Pop-Unders. The displays can include advertising, surveys, messages, sound, video, applets and any other graphic or textual content.
| | · | Portable Storage Devices With Links |
This patented technology generally relates to products sold or distributed on CDs or DVDs that include a link to retrieve additional data via the Internet. This technology can be used by companies that distribute their products on CDs or DVDs and provide links for software upgrades, user manuals, audio files, and other purposes.
These patented technologies generally relate to accessing data through the submission of a product identification and computer specific information to a remote station. The remote station sends an encrypted key that is stored on the computer and provides access to the data. This technology can be used by software and other products to help deter the distribution of illegal copies. Other aspects of the technologies generally relate to accessing clear data, and encrypted data via an identification label. Once decrypted, the clear and decrypted data are combined to activate software programs, and other files.
These patents relate to remote control of video cameras and other devices used in areas such as videoconferencing and surveillance systems. The uses of the patented technology include improved remote management of video camera functions such as pan, tilt, and focus, and improved device control in a networked videoconferencing system.
This patented technology generally relates to the creation and maintenance of a schedule through the periodic management and monitoring of interrelated and interdependent resources from a database. These resource management tools can be part of scheduling software used to plan and monitor the use of facilities, the allocation of manpower, and the use and scheduling of other resources.
| | · | Software License Management |
This patented technology generally relates to technology for monitoring and tracking the use of software applications across a network. This technology can be used to provide a system for managing software license compliance in an enterprise environment as well as metering actual usage levels in a Software-as-a-Service (“SaaS”) environment.
This patented technology generally relates to automating the production of worksheet files for use by electronic spreadsheet programs. Specifically, the patented technology permits the efficient retrieval of data from external databases by allowing the user to select specific data from a database and import the specified data into a spreadsheet program through uniquely streamlined spreadsheet commands. The adaptive quality of the technology permits, among other things, the user to retrieve updated information from an external database without creating formatting issues in the user’s spreadsheet program.
This patented technology generally relates to diverse aspects of storage devices and related technology. The patented technologies relate generally to data transfer, fault tolerance, caching, data integrity and error correction.
This patented technology generally relates to technology for displaying mobile vehicle information on a map. This technology can be used in navigation and fleet management systems that combine wireless communication with GPS tracking and map displays.
| | · | User Activated Internet Advertising |
This patented technology generally relates to the display of certain advertising, informational, and branding messages that appear between or outside web pages when the user is conducting a search, by storing the message prior to being displayed. This technology is most commonly used by travel based and other reservation based websites.
| | · | Vehicle Anti-Theft Parking System |
This patented technology generally relates to methods of automatically identifying a vehicle through a characteristic, such as a license plate number, in order to deter vehicle theft. This technology is applicable to airports, hotels, shopping centers and other parking areas that employ access control.
| | · | Vehicle Magnetic Braking |
This patent generally relates to technology for smooth, reliable braking and acceleration of vehicles on parallel rails.
This patented technology generally relates to technology for learning user preferences and automatically personalizing a user's online experience. The technology is applicable to web sites that use categories plus attributes to identify items, and where individual attributes apply to multiple categories.
| | · | Wireless Traffic Information |
This patented technology generally relates to transmitting, receiving and displaying traffic information on portable handheld and mobile displays. It covers a variety of wireless distribution methods, such as FM radio and satellite, as well as the devices used to display the traffic maps. This technology enables users to identify traffic congestion and can be used with in-vehicle navigation displays and portable handheld units such as cell phones and PDA's.
Patent Enforcement Litigation
Companies comprising the Acacia Technologies group are often required to engage in litigation to enforce their patents and patent rights. In the litigation listed below, certain companies comprising the Acacia Technologies group are parties to ongoing litigation alleging infringement of certain of our patented technologies by the companies listed. Current patent enforcement litigation, by related patented technology, is as follows:
Audio/Video Enhancement and Synchronization Technology -
Image Resolution Enhancement Technology
| | · | New Medium Technologies, LLC and AV Technologies, LLC v. Barco NV, Miranda Technologies, LG Philips LCD, Toshiba Corporation, Toshiba America Consumer Products, LLC., and Syntax-Brillian Corporation. United States District Court for the Northern District of Illinois. Filed 9/29/05. Case No. 1:05-cv-05620. |
Computer Memory Cache Coherency Technology
| | · | Computer Cache Coherency Corporation v. VIA Technologies, Inc., Via Technologies, Inc. (USA) and Intel Corporation. United States District Court for the Northern District of California. Filed 12/2/04. Case No. 5:05-cv-01668. |
Credit Card Fraud Protection Technology
| | · | Financial Systems Innovation LLC and Paul N. Ware v. The Kroger Company. United States District Court for the Northern District of Georgia. Filed 3/3/04. Case No. 4:04-cv-00065. |
| | · | Financial Systems Innovation LLC and Paul N. Ware v. Williams-Sonoma, Inc., and Costco Wholesale Corporation. United States District Court for the Northern District of Texas. Filed 6/30/04. Case No. 4:04-cv-00479. |
| | · | Financial Systems Innovation LLC and Paul N. Ware v. Circuit City Stores, Inc., Officemax Incorporated, Staples, Inc., Cracker Barrel Old Country Store, Inc., Fry’s Electronics, Inc., and Rite Aid Corporation. United States District Court for the Northern District of Georgia. Filed 7/19/05. Case No. 4:05-cv-00156. |
| | · | Reinalt-Thomas Corporation, dba Discount Tire Corporation, v. Acacia Research Corporation, Paul N. Ware and Financial Systems Innovation LLC. United States District Court for the District of Arizona. Filed 10/27/05. Case No. 2:05-cv-03459. |
| | · | Financial Systems Innovation LLC and Paul Ware v. Discount Tire Company of Georgia, Inc. and Reinalt-Thomas Corporation, dba Discount Tire Company. United States District Court for the Northern District of Georgia. Filed 11/21/05. Case No. 4:05-cv-00252. |
| | · | Lone Star Steakhouse and Saloon, Inc. v. Acacia Technologies group and Financial Systems Innovation LLC. United States District Court for the District of Kansas. Filed 8/5/05. Case No. 6:05-cv-01249. |
Computing Device Performance Technology
| | · | Computer Acceleration Corporation vs. Microsoft Corporation. United States District Court for the Eastern District of Texas. Filed 7/6/06. Case No. 9:06-cv-0140. |
Data Encryption Technology
| | · | Data Encryption Corporation v. Microsoft Corporation and Dell Computer Corporation. United States District Court for the Central District of California. On appeal to the U.S. Court of Appeals for the Federal Court. Lower Court Case No. 2:05-cv-05531. |
Digital Media Transmission Technology
In accordance with the Transfer Order issued February 24, 2005, by the Judicial Panel on Multidistrict Litigation, all of the following Digital Media Transmission Technology cases have been transferred to the Northern District of California. The lead case number is 5:05-cv-01114.
| | · | Acacia Media Technologies Corporation v. Comcast Cable Communications, LLC, Charter Communications, Inc., The DirectTV Group, Inc., Echostar Communications Corporation, Cox Communications, Inc., Hospitality Network, Inc. (a wholly owned subsidiary of Cox that supplies hotel on-demand TV services), Mediacom, LLC, Armstrong Group, Arvig Communication Systems, Block Communications, Inc., Cable America Corporation, Cable One, Inc., Cannon Valley Communications, Inc., East Cleveland Cable TV and Communications, LLC, Loretel Cablevision, Massillon Cable TV, Inc., Mid-Continent Media, Inc., NPG Cable, Inc., Savage Communications, Inc., Sjoberg's Cablevision, Inc., US Cable Holdings LP, and Wide Open West, LLC, Time Warner Cable, Cablevision Systems Corporation, Insight Communications Company, Cebridge Communications and Bresnan Communications. |
| | · | Acacia Media Technologies Corporation v. New Destiny Internet Group, Inc., Audio Communications Inc., VS Media Inc., Ademia Multimedia, LLC, International Web Innovations, Inc., Offendale Commercial BV, Ltd., Adult Entertainment Broadcast Network, Cybertrend, Inc., Lightspeed Media Corporation, Adult Revenue Services, Innovative Ideas International, AskCS.com, Game Link, Inc., Club Jenna, Inc., Cybernet Ventures, Inc., ACMP, LLC, Global AVS, Inc. d/b/a DrewNet, and National A-1 Advertising. |
High Quality Image Processing
| | · | Lexmark International, Inc. v. Acacia Research Corporation, dba Acacia Technologies Group and Acacia Patent Acquisition Corp. United States District Court for the Eastern District of Kentucky. Filed 2/13/07. Case No. 5:07-cv-00047. |
High Resolution Optics Technology
| | · | Theodore Whitney and High Resolution Optics Corporation v. The United States. United States Court of Federal Claims. Filed 8/23/06. Case No. 1:06-cv-00601. |
Information Monitoring Technology
| | · | Diagnostic Systems Corporation v. Symantec Corporation; CA, Inc., F-Secure, Inc., NetIQ Corporation, Quest Software Inc., NetScout Systems, Inc., and Motive, Inc. United States District Court for the Central District of California. Filed 12/14/06. Case No. 806-cv-01211. |
Interactive Television Technology
| | · | Broadcast Innovation, LLC and IO Research, Ltd. v. Charter Communications, Inc. United States District Court for the District of Colorado. Case No. 1:03-cv-02223. On appeal to the U.S. Court of Appeals for the Federal Court from 9/28/04 to 11/21/05. Remanded to the U. S. District Court for further proceedings on 11/21/05. |
| | · | Broadcast Innovation, LLC v. Echostar Communications Corporation. United States District Court for the District of Colorado. Filed 11/9/01. Case No. 1:01-cv-02201. |
Laptop Connectivity Technology
| | · | Computer Docking Station Corporation v. Dell, Inc., Gateway, Inc., Toshiba America, Inc., and Toshiba America Information Systems, Inc. On appeal to the U.S. Court of Appeals for the Federal Court. Lower Court Case No. 06-c-0032-c. |
Micromesh Technology
| | · | Micromesh Technology Corporation v. American Recreation Productions, Inc. and American Recreation Products, Inc., dba Kelty. United States District Court for the Northern District of California. Filed 9/27/06. Case No. 3:06-cv-06030. |
| | · | Micromesh Technology Corporation v. Columbia Sportswear Company. United States District Court for the Northern District of California. Filed 9/27/06. Case No. 3:06-cv-06031. |
| | · | Micromesh Technology Corporation v. Red Wing Shoe Company and Red Wing Shoe Company, dba Vasque. United States District Court for the Eastern District of Texas. Filed 10/4/06. Case No. 2:06-cv-00421. |
| | · | Micromesh Technology Corporation v. VF Corporation, VF Corporation, dba JanSport, VF Outdoor, Inc., dba The North Face. United States District Court for the Eastern District of Texas. Filed 10/4/06. Case No. 2:06-cv-00422. |
Microprocessor Enhancement Technology
| | · | Microprocessor Enhancement Corporation and Michael H. Branigin v. Texas Instruments, Incorporated. United States District Court for the Central District of California. Filed 4/7/05. Case No. 8:05-cv-00323. Appeal pending. |
| | · | Microprocessor Enhancement Corporation and Michael H. Branigin v. Intel Corporation. United States District Court for the Central District of California. Filed 8/3/05. Case No. 2:05-cv-05667. |
Multi-Dimensional Bar Code Technology
| | · | Cognex Corporation v. VCode Holdings, Inc., VData LLC, Acacia Research Corporation, dba Acacia Technologies Group, and Veritec Inc. United States District Court for the District of Minnesota. Filed 3/13/06. Case No. 0:06-cv-01040. |
| | · | VData LLC and VCode Holdings, Inc. v. Aetna, Inc., PNY Technologies Inc., A144 and Merchant’s Credit Guide Co. United States District Court for the District of Minnesota. Filed 5/8/06. Case No. 0:06-cv-01701. |
Peer to Peer Communications Technology
| | · | Peer Communications Corporation v. Skype Technologies SA, Skype, Inc., and eBay, Inc. United States District Court for the Eastern District of Texas. Filed 8/22/06. Case No. 6:06-cv-00370. |
Portable Storage Devices With Links Technology
| | · | Disc Link Corporation v. H&R Block, Inc., McAfee, Inc., Riverdeep, Inc. and Sage Software SB, Inc. United States District Court for the Eastern District of Texas. Filed 12/27/06. Case No. 5:06-cv-00295. |
Resource Scheduling Technology
| | · | Epic Systems Corporation v. Resource Scheduling Corporation. United States District Court for the District of Delaware. Filed 4/19/06. Case No. 1:06-cv-00255. |
Telematics Technology
| | · | Telematics Corporation v. United Parcel Service Co., @Road, Inc., Motorola Inc., Ryder System, Inc., Sprint Nextel Corporation, Teletrac, Inc., Verizon Communications, Inc., and Xata Corporation. United States District Court for the Northern District of Georgia. Filed 1/16/07. Case No. 1:07-cv-00105. |
User Activated Internet Advertising Technology
| | · | InternetAd Systems, LLC v. Turner Broadcasting System, Inc., Freerealtime.com, Inc., Knight Ridder Digital, Homestore, Inc., Condenet, Inc., Tribune Company. United States District Court for the Northern District of Texas. Filed 6/15/06. Case No. 3:06-cv-01063. |
| | · | InternetAd Systems, LLC v. Opodo Limited, Amadeus Global Travel Distribution S.A., and Amadeus North America, LLC. United States District Court for the Northern District of Texas. Filed 6/19/06. Case No. 3:06-cv-01084. |
Vehicle Magnetic Braking Technology
| | · | Safety Braking Corporation, Magnetar Technologies Corp., and G&T Conveyor Co. v. Six Flags, Inc., Six Flags Theme Parks Inc., SF Partnership, Tierco Maryland, Inc., Busch Entertainment Corp., Cedar Fair LP, Paramount Parks, Inc., NBC Universal, Inc., Universal Studios, Inc., Blackstone Group L.P., The Walt Disney Co., and Walt Disney Parks and Resorts, LLC. United States District Court for the District of Delaware. Filed 3/1/07. Case No. 1:07-cv-00127. |
Competition
The Acacia Technologies group expects to encounter competition in the area of patent acquisition and enforcement as the number of companies entering this market is increasing. This includes competitors seeking to acquire the same or similar patents and technologies that we may seek to acquire. Companies such as British Technology Group, Rembrandt Management Group, and Intellectual Ventures LLC are already in the business of acquiring the rights to patents for the purpose of enforcement, and we expect more companies to enter the market. As new technological advances occur, many of our patented technologies may become obsolete before they are completely monetized. If we are unable to replace obsolete technologies with more technologically advanced patented technologies, then this obsolescence could have a negative effect on our ability to generate future revenues.
The Acacia Technologies group also competes with venture capital firms and various industry leaders for technology licensing opportunities. Many of these competitors may have more financial and human resources than our company. As we become more successful, we may find more companies entering the market for similar technology opportunities, which may reduce our market share in one or more technology industries that we currently rely upon to generate future revenue.
Other companies may develop competing technologies that offer better or less expensive alternatives to our patented technologies that we may acquire or out-license. Many potential competitors may have significantly greater resources. Technological advances or entirely different approaches developed by one or more of its competitors could render Acacia Technologies group’s technologies obsolete or uneconomical.
Employees
As of December 31, 2006, the Acacia Technologies group had 33 full-time employees. None of the companies included in the Acacia Technologies group is a party to any collective bargaining agreement. The Acacia Technologies group considers its employee relations to be good.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
Life Sciences Business
The CombiMatrix group is comprised of our wholly owned subsidiary, CombiMatrix Corporation and CombiMatrix Corporation’s wholly owned subsidiary, CombiMatrix Molecular Diagnostics, Inc. The CombiMatrix group is seeking to become a broadly diversified biotechnology business, through the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where its products and services could be utilized. The technologies that the CombiMatrix group has developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. The CombiMatrix group has also developed the capabilities of producing arrays that utilize bacterial artificial chromosomes, also enabling genetic analysis. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs.
Technologies
· Semiconductor Based Array.
The CombiMatrix group’s semiconductor based array technology enables the rapid, parallel synthesis, immobilization and detection of molecules and materials at discrete electrodes on a semiconductor chip. These chips, also known as microelectrode arrays, are used in multiple applications in the areas described above. The CombiMatrix group’s technology integrates semiconductor micro fabrication, proprietary software, chemistry and hardware into systems that it believes will enable it, its customers and its partners to design and fabricate arrays for biological, diagnostic, material sciences and nanotechnology applications, typically within a few days. The CombiMatrix group’s system should enable researchers to conduct rapid, iterative experiments in each of these fields.
The primary use of this technology is the fabrication of oligonuceotide arrays. Oligonucleotides are short sequences of DNA, which when synthesized in an array format, can be used to perform several types of genetic analysis. The most common types of analyses using these arrays are the evaluation of gene expression or the identification of mutations. Gene expression is the term used to describe the identification of genes in cell that are expressed or not expressed or “active” or “inactive.” By evaluating the expression patterns of normal cells versus suspected diseased cells (for example a growth) one may be able to diagnose that disease and also provide information on how to address that disease. Abnormal expression patterns have been implicated for a number of diseases.
Mutation analysis is the identification of specific changes the sequence of DNA that may be linked to particular diseases or drug responses. By identifying a specific mutation in an individual’s genome, it may be possible to identify a disease, pre-disposition to disease or potential drug response.
· Bacterial Artificial Chromosome Arrays (or “BAC” Arrays)
The CombiMatrix group’s BAC Arrays enable us to perform comparative genomic hybridization (or “CGH”) studies to evaluate gross genetic anomalies in genomes. These arrays incorporate BACs, which are fragments of DNA that have been cloned or manufactured in a bacteria. These fragments can be placed on a substrate, in the CombiMatrix group’s case a chemically modified glass slide. These BACs are either developed by us or obtained through other sources. The CombiMatrix group utilizes these arrays to perform CGH analysis in a diagnostic and research setting to identify genetic imbalances in the form of copy number differences. CGH analysis allows us to compare the genomic DNA of a normal individual with that of an individual who may have a disease. This type of analysis evaluates the genes rather than the expression of those genes. CGH analysis is useful in evaluating gross defects in an individual’s genome, such as copy number differences. Copy number differences are situations where a gene or a large portion of a gene is missing or an extra copy exists.
Utilizing these array technologies, the CombiMatrix group is engaged in three strategic business areas:
| 1. | The development of services and products in the field of molecular diagnostics; |
| 2. | The development, manufacture and sale of research tools and services to life sciences researchers; and |
| 3. | The development, manufacture and sale of biosensor systems and technology for national defense and homeland security. |
· Technologies and Compound Libraries for Oncological Drug Development
Through the CombiMatrix group’s minority ownership of Leuchemix, Inc., we have access to proprietary compounds that have been shown to be cytotoxic towards certain cancers in-vitro and in vivo. Many of these compounds were discovered through combinatorial chemistry, natural product chemistry and certain cellular screening assays. Leuchemix, Inc. has access to state of the art laboratories and equipment, which includes flow cytometry, molecular biology and cell culture facilities. In addition, Leuchemix, Inc. has access to a bank of over 150 primary leukemia specimens and a panel of 15 leukemia and lymphoma cell lines as well as several xenogenic animal model systems. Leuchemix also has licensed proprietary compounds and compound libraries, which are being developed as drugs against a number of oncology indications including hematological disorders as well as solid tumors.
Market Overview
The markets for the CombiMatrix group’s products and services include pharmaceutical and biotechnology markets (also referred to as life sciences), molecular diagnostics, national defense and homeland security applications. In the future, if the CombiMatrix group is successful in developing approved drugs either internally or through its investments in companies such as Leuchemix, Inc., the CombiMatrix group’s market opportunities will expand to include pharmacies, physicians, hospitals, patients and other consumers of therapeutics. In addition, there may be opportunities for the CombiMatrix group’s products and services to address consumer-based genetic analysis as that market develops. At this time, the majority of the CombiMatrix group’s commercial efforts are focused on developing and marketing molecular diagnostics services.
General Overview of Molecular Diagnostics, Life Sciences and Pharmaceutical Industries
The molecular diagnostics market is a sub-segment of the overall diagnostics market. Molecular diagnostics, within the context of this discussion, refers to the use of genetic analysis of individuals to make medical decisions in the diagnosis of disease as well as the management of patients. The sequencing of the human genome and associated research as well as advances in technology are enabling the growth of this market. Most experts believe that over the next few decades, the use of molecular diagnostics will grow rapidly and will have a revolutionary impact on the way medicine is practiced.
Additionally, the pharmaceutical and biotechnology industries continue to face increasing costs and risks in the drug discovery, development and commercialization process. A primary component of the cost is the effort expended on drugs that failed to meet clinical and regulatory requirements due to a poor safety profile, efficacy in a small fraction of the patient population, or other similar reasons. By identifying patients who are more likely to respond favorably to a drug (and excluding those that will either not respond or have an adverse response), the potential market for the drug is decreased but the chance of achieving regulatory approval is increased. Stratification of patient populations can be performed by analysis of blood or tissue of patients for genetic biomarkers or expression patterns that are characteristic of responders and non-responders.
While there are multiple technologies being developed to address the noted molecular diagnostic applications, the CombiMatrix group feels that its technology and products are ideally suited to aid in many of these market segments.
Genes and Proteins
The human body is composed of billions of cells each containing DNA that encodes the basic instructions for cellular function. The complete set of an individual’s DNA is called the genome, and is organized into 23 pairs of chromosomes, which are further divided into smaller regions called genes. Each gene is composed of a strand of four types of nucleotide bases, referred to as A, C, G and T. The bases of one DNA strand bind to the bases of the other strand in a specific fashion to form base pairs: the base A always binds with the base T and the base G always binds with the base C.
The human genome has approximately 3.0 billion nucleotides and their precise order is known as the DNA sequence. When a gene is turned on, or expressed, the genetic information encoded in the DNA is copied to a specific type of RNA, called messenger RNA, or mRNA. The mRNA provides instructions for the synthesis of proteins. Proteins direct cellular function and the development of individual traits and are involved in many diseases. Abnormal variations in the sequence of a gene, in the level of gene expression, or large anomalies (such as deletions, extra genes, etc.) can interfere with the normal physiology of particular cells and lead to a disease, a predisposition to a disease or an adverse response to drugs.
Genes and Molecular Diagnostics
There are a number of types of genetic analysis that can be useful in a diagnostic context. They include (i) gene expression profiling, (ii) comparative genomic hybridization, (iii) and mutation analysis. For many diagnostic applications using the above paradigms, it is only necessary to analyze either only one or a small number of genetic factors. For such situations, there are a number of ubiquitous techniques to perform the analysis. However, when a larger number of genetic factors need to be analyzed to make a medical decision, the most efficient method of analysis is a microarray. The CombiMatrix group feels that its microarrays provide advantages over other microarrays for molecular diagnostic applications.
Gene Expression Profiling
Gene expression profiling is the process of determining which genes are active in a specific cell or group of cells and is accomplished by measuring mRNA, the intermediary between genes and proteins. By comparing gene expression patterns between cells from normal tissue and cells from diseased tissue, researchers may identify specific genes or groups of genes that play a role in the presence of disease. Studies of this type, used in drug discovery, require monitoring thousands, and preferably tens of thousands, of mRNAs in large numbers of samples. As the correlation between gene expression patterns and specific diseases is determined, the CombiMatrix group believes that gene expression profiling will have an increasingly important role as a diagnostic tool. Diagnostic use of expression profiling tools is anticipated to grow rapidly with the combination of the sequencing of various genomes and the availability of more cost-effective technologies.
The CombiMatrix group’s gene expression arrays utilize sequences of genes that can hybridize or bind to their complementary sequences. By analyzing the amount of bound material, the CombiMatrix group is able to identify whether a particular gene has been expressed and what level of expression exists. By exposing a suitably processed genetic sample from a tissue or tissues, one is able to monitor the expression pattern and compare that pattern to previously validated patterns that are characteristic of disease.
Array Comparative Genomic Hybridization (or “CGH”)
CGH is the study at the gene level of gross level anomalies such as copy number polymorphisms. Array CGH is the use of an array that multiplexes the analysis of a large portion of the genome. Unlike, gene expression, which monitors the activity of genes, CGH analysis studies and identifies defects at the gene level that are characteristic of disease, predisposition to disease or response to drugs. The CombiMatrix group’s CGH arrays utilize probes, cloned or manufactured in bacteria, which are referred to as bacterial artificial chromosomes, or BACs. Recent studies have shown that copy number polymorphisms are the cause of many diseases, susceptibility to disease, and response to therapy.
Genetic Mutations
The most common form of genetic variation occurs as a result of a difference in a single nucleotide in the DNA sequence, commonly referred to as a single nucleotide polymorphism, or SNP. The human genome is estimated to contain between three and six million SNPs. SNPs are believed to be associated with a large number of human diseases, although most SNPs are believed to be benign and not to be associated with disease. It is believed that the genetic component of most major diseases is associated with a combination of SNPs.
Array Based Analysis
Traditional technologies for analyzing genetic or protein variation and function generally perform experiments individually, or serially, and often require relatively large sample volumes, adding significantly to the cost of conducting experiments. Arrays were developed to overcome the limitations of traditional technologies and enable the parallel evaluation of large numbers of genetic factors.
An array is a collection of miniaturized test sites arranged in a manner that permits many tests to be performed simultaneously, or in parallel, in order to achieve higher throughput. The average size of test sites in an array and the spacing between them defines the array’s density. Higher density increases parallel processing throughput. In addition to increasing the throughput, higher density reduces the required volume for the sample being tested, and thereby lowers costs. Principal commercially available ways to produce arrays include mechanical deposition, bead immobilization, inkjet printing and photolithography.
While current array technologies have revolutionized drug discovery and development and are poised to enter the molecular diagnostics markets, the CombiMatrix group believes that its advanced array technologies provide characteristics, including flexibility, superior cost metrics, and increased performance, which address certain needs not addressed by conventional arrays.
The CombiMatrix Solution
The CombiMatrix group believes that its microarrays will offer several important advantages over competing arrays. These advantages include flexibility, cost, performance, and other capabilities. Also, the CombiMatrix group is the only company that utilizes both in-situ synthesized oligomer arrays and spotted BAC arrays.
Products and Services
CustomArrayTM
The CombiMatrix group’s primary product for genetic studies is marketed under the trade name CustomArray, which is a highly flexible custom oligonucleotide array that addresses researchers’ specific requirements for high-performance arrays that can interrogate small sets of target genes or whole genomes at a low cost. CustomArrays currently come in two density formats: first are the medium-density CustomArray 12K and the 4 X 2K CustomArray. The CustomArray 12K enables analysis of up to 12,000 genes, whereas the 4 X 2K array enables the analysis of four separate experiments of up to 2,000 genes each. Second is the CustomArray 90K, which enables the analysis of approximately 90,000 genes in one experiment. This enables analysis of the full human genome, or that of other species with redundant probes for better performance and reliability.
CustomArray is an advanced tool used to understand gene expression by measuring mRNA activity within a cell type or groups of cells, enabling users to understand and diagnose disease, predisposition to disease, drug response as well as provide information regarding drug development. CustomArray can also be used as a SNP genotyping tool providing statistics on the effect of a SNP or groups of SNPs, giving rise to data that is important in diagnostic testing. Because of the product’s flexibility, researchers have utilized and are evaluating the use of CustomArrays for other applications such as gene assembly, sequencing, protein translation and others. CustomArrays can also be read on most commercially available scanners, thus enabling many researchers to perform assays without requiring additional capital expenditures for scanning equipment that several competing technologies require.
CatalogArraysTM
The CombiMatrix group has also launched several dozen CatalogArrays, which are pre-designed arrays built using the CombiMatrix group’s platform that can be used for gene expression studies, mutation analysis, and other studies. These arrays include several human genome sets, mouse, rat, dog and several other organisms including plants, animals, bacteria and viruses. These arrays are updated as new genetic or sequence information is published. In addition, similar to CustomArrays, the CombiMatrix group’s CatalogArrays can be read on most commercially available scanners and do not require additional capital investment or start-up fees by the customer.
Micro-RNA Arrays
The CombiMatrix group also offers a series of arrays that can be used to study micro-RNA molecules, which are relatively small strands of RNA molecules in cells that appear to have significant regulatory control over cell function. Until recently, micro-RNA molecules were thought to be oddities and perhaps superfluous genetic material. However, recent research indicates that these molecules play a significant role in the physiology of the cell. The CombiMatrix group offers Micro-RNA arrays for human, mouse, rat and other organisms. These arrays are updated as new information is published. In addition, similar to CustomArrays, the CombiMatrix group’s Micro-RNA Arrays can be read on most commercially available scanners and do not require additional capital investment or start-up fees by the customer.
DNA Array Synthesizer
The CombiMatrix group’s DNA Array Synthesizer is a bench-top instrument that enables researchers to fabricate DNA arrays to their exact specifications with complete control over the content that is synthesized onto the array. The system consists of a synthesizer instrument that is operated by a personal computer that is connected to a cabinet that contains reagents necessary for array synthesis. The system is able to fabricate up to eight, 12K arrays within a 24-hour period, or up to thirty-two, 2K sectored arrays in the same period of time. The synthesizer’s flexibility enables researchers to synthesize multiple designs or the same design in each synthesis run. To operate the synthesizers, researchers must purchase blank microarray slides (i.e., slides on which no DNA synthesis has been performed) from the CombiMatrix group and reagents from either the CombiMatrix group or other vendors.
Stripping Reagents
The CombiMatrix group has created the first commercially available array stripping kit. The kit allows researchers to re-use the CombiMatrix group’s CustomArrays up to four times. The ability to re-use CustomArray reduces the cost per CustomArray to the researcher while eliminating problems associated with chip-to-chip reproducibility.
EC Reader-Electrochemical Scanning Instrument
The EC Reader is a compact scanner for CombiMatrix arrays. The EC Reader was developed to provide the market with a compact, inexpensive and easy to use scanner for performing array experiments. Current arrays, including those manufactured by the CombiMatrix group, are designed to be analyzed using optical scanning instruments. While these scanners are quite functional, they are also relatively expensive, bulky, and can be difficult to use. Due to the electrochemical nature of the CombiMatrix group’s arrays, it is possible to scan them using an electrochemical scanner as well as an optical scanner. The advantages of the electrochemical scanner arise out of the fact that the EC Reader does not have any optical components (such as lasers, lenses and optical detectors). By eliminating these components, the EC Reader is more compact, cost efficient and easier to use than most optical scanners. The EC Reader is designed to read only CombiMatrix arrays.
Comparative Genomic Hybridization Arrays
The CombiMatrix group’s CGH arrays are fabricated using a traditional spotting instrument, and are prepared on glass substrates. These arrays are used for research applications as well as molecular diagnostic applications.
Applications
Pharmaceutical and Life Sciences Research and Development Applications
To date, the CombiMatrix group’s products have been used primarily for research and development applications by academic and industrial researchers. The CombiMatrix group’s products have and can be used for such diverse applications as drug target discovery and validations, genotyping, pathogen detection, agricultural analysis and others. In addition, the CombiMatrix group’s products can be used to synthesize oligonucleotides that are then utilized in various research applications. Due to the flexibility of the CombiMatrix group’s technologies, the CombiMatrix group expects the potential R&D applications of its products to continually expand.
Molecular Diagnostics Applications
In addition to the life science research and development applications of the CombiMatrix group’s products, the CombiMatrix group feels that its proprietary products have utility in the emerging field of molecular diagnostics. The current market for molecular diagnostics in the USA is roughly $2.5 billion annually. The compounded annual growth rate of this market is over 15%, and it is expected that the growth rate will accelerate as more products and technologies are brought to bear on the opportunity.
The CombiMatrix group has formed a wholly owned subsidiary, CombiMatrix Molecular Diagnostics (or “CMDX”) to take advantage of the capabilities of its DNA array technologies to develop molecular diagnostic services and products. The primary focus of CMDX’s efforts will be the development of diagnostic services for the diagnosis of diseases, for the management of patients, as well as the stratification of patients during clinical trials. CMDX plans to be a fully functional molecular diagnostics laboratory and has received federal certification by the Clinical Laboratory Improvement Amendments (or “CLIA”) as well as by other state and local regulatory agencies that are required for analysis of patient samples. As such, CMDX is currently operating as a service organization, providing testing services for patients. Although many of CMDX’s initial services are designed to avoid pre-market approval by the United States Food and Drug Administration (or “FDA”), many of the services CMDX will provide may require different levels of regulatory approval from the FDA.
An area of focus will be pre- and post-natal analysis for the diagnosis of developmental delays. Children who present with developmental delays during early childhood are often not easily diagnosed. An accurate diagnosis is essential to provide good guidance on the developmental expectations of the child. Additionally, pre-natal analysis is dominated by the use of the standard chromosomal analytical technique of karyotyping. Pre-natal analysis is utilized to determine if a fetus has a genetic defect that could lead to problems after birth.
In the third quarter of 2006, the CombiMatrix group introduced its constitutional genetic array test, which can identify over 50 different genetic disorders in one multiplexed analysis. In October of 2006, the US FDA indicated that this test does not require approval under its guidance (IVDMIA-In-Vitro Diagnostics Multivariate Index Analysis). Therefore, this test has been launched under CLIA guidelines and is available for patients and physicians today.
Another area of focus is cancer. In the Unites States alone, the American Cancer Society indicates that 1.4 million individuals are diagnosed with cancer annually, and this rate is expected to grow rapidly as the overall population, including the "baby boomer" generation, ages. At any given time in the United States, there are several million living patients that either have cancer or are cancer survivors that are at high risk for recurrence.
Patients who are newly diagnosed with cancer require significant levels of care, which includes surgery, hospital stays, examinations, drugs and diagnostics. CMDX plans to develop a series of products that, through the genetic analysis of blood, tissue or biopsy samples, will provide information to physicians in managing their patients.
In the last quarter of 2006, the CombiMatrix group launched a test to evaluate skin growths for evidence of malignancy. Periodically, especially as individuals age, skin growths are observed. Most of these are benign, but on occasion some are malignant and need to be addressed aggressively. Conventional methods to identify these skin growths involve histological analysis, which is very subjective. The CombiMatrix group’s molecular diagnostics test is designed to evaluate the gene expression pattern of the skin growth and provide an objective assessment of the growth.
Homeland Security and Defense Applications
Through U.S. government funding, the CombiMatrix group’s array technology is being developed to simultaneously detect toxins, viruses, and bacteria using either genomic analysis or antigen-antibody experiments, or assays. The ability to conduct over 12,000 individual assays simultaneously means that the CombiMatrix group’s array can be configured to detect many biothreat agents of interest to the U.S. Department of Defense and Department of Homeland Security within hours and with a high degree of certainty that surpasses current technologies. The CombiMatrix group’s goal is that these systems will eventually be portable and ultimately be completely automated.
The CombiMatrix group’s technology can simultaneously identify hundreds of different microbes (including viruses), determine their ability to cause disease, and discover their characteristics, such as antibiotic resistance. Working with academia, industry, and government laboratories, the CombiMatrix group is developing assays, arrays and bioinformatics for quickly identifying human, animal, and plant pathogens in a single-assay format. This format and single test eliminates the need for a different test for each disease or threat and eliminates the time lost in developing a new test for each new disease or threat. For disease-control agencies, it simplifies the process, reduces costs, and allows more rapid identification and reaction, all in an environment where increased time can equate to increased illness and loss of lives.
This program is enabled by the characteristic of the CombiMatrix group’s array technology, which allows the binding reactions to be measured through electrochemical means instead of optical methods. Though optical detection has been successful in many applications and the CombiMatrix group’s other products utilize these methods, the CombiMatrix group feels that electrochemical detection techniques have the potential to be far superior. By eliminating the need for light sources, optical components, their corresponding mechanical requirements as well as their power requirements, the CombiMatrix group feels that it will be able to build detection systems that will be less expensive, smaller, lighter and portable. In addition, certain technical characteristics of electrochemical detection on the arrays may enable higher sensitivity, better dynamic range and superior reproducibility in measurements.
Though the initial focus of the CombiMatrix group’s Government-funded development program is a product for military and homeland security markets, the core technology being developed will be applicable to products in the life sciences, molecular diagnostics, and other human healthcare markets as well.
An example of a test that has been developed substantially with government funding is the CombiMatrix group’s Influenza A array. This array is designed to identify specifically and objectively, which strain of Influenza A has infected an individual. This test can be used on animal samples as well as human samples. The array has been validated in collaboration with a World Health Organization laboratory, and has been used in collaboration with the US government in monitoring birds that may be infected by Influenza A, including the H5N1 strain that is known commonly as the Eurasian Bird Flu.
The CombiMatrix Group’s Strategy
The CombiMatrix group’s goal is to provide customers and partners with tools in their discovery efforts as well as to perform discovery ourselves.
Focusing on High-growth Markets
In the year 2007, the CombiMatrix group has made a transition from selling the CombiMatrix group’s products into the academic and industrial research and development market to selling services into the molecular diagnostic market. The CombiMatrix group feels that the molecular diagnostic market provides the greatest opportunity for it to benefit individual patients. The market also offers the greatest opportunity for growth and value creation. The CombiMatrix group will continue to serve its existing customers and distributors in the research and development markets, but will no longer expend significant resources in marketing and selling into this segment.
Partnering to Expand Marketing and Sales Efforts
The CombiMatrix group plans to pursue multiple relationships to facilitate the expansion of the CombiMatrix group’s array services. The CombiMatrix group hopes to enter into relationships and collaborations to gain access to sales marketing and distribution channels.
Expanding the CombiMatrix Group’s Offerings
The CombiMatrix group intends to utilize the flexibility of its array technologies to develop multiple diagnostic services. In addition to providing new sources of revenue, the CombiMatrix group believes these product lines will further its goal of establishing the CombiMatrix group as a leader in the molecular diagnostic market.
Strengthening Technological Leadership
The CombiMatrix group plans to continue advancing its proprietary technologies through its internal research efforts, collaborations with industry leaders and strategic licensing. The CombiMatrix group may also pursue acquisitions of complementary technologies and leverage its technologies into other value-added businesses.
Protecting and Strengthening Intellectual Property
Through the CombiMatrix group’s five patents issued in the United States and three corresponding patents granted in Europe, Australia and Taiwan, the CombiMatrix group’s 87 patent applications pending in the United States, Europe and elsewhere and the CombiMatrix group’s trade secrets, the CombiMatrix group believes that it has suitable intellectual property protection for its proprietary technologies in those markets where it operates and where a market for its products and services exists. The CombiMatrix group plans to build its intellectual property portfolio through internal research efforts, collaborations with industry leaders, strategic licensing and possible acquisitions of complementary technologies. The CombiMatrix group also plans to pursue patent protection for downstream products created using its proprietary products.
Regulatory Matters
The CombiMatrix group sells array products to the pharmaceutical, biotechnology and academic communities for research applications as well as non-life sciences customers. The CombiMatrix group also sells molecular diagnostic services in the US under CLIA guidelines. Therefore, the CombiMatrix group’s initial products do not require approval from, and are not regulated by, the FDA. Supporting this belief, in late October of 2006, the US FDA provided its opinion regarding the regulatory nature of the CombiMatrix group’s first test in a letter to Matt Watson, the former CEO of CMDX. The text of that letter, which can be found on the CombiMatrix group’s corporate website at www.combimatrix.com, indicated that the US FDA did not believe that the CombiMatrix group’s device, as described to the US FDA, met the definition of an In-Vitro Diagnostic Multivariate Index Assay (“IVDMIA”) as defined in the Draft Guidance document on IVDMIAs.
Though the CombiMatrix group’s initial products are not subject to regulations promulgated by the FDA or by foreign regulatory agencies, future products may require regulatory approval. Additionally, current FDA guidelines may change.
Subsidiaries
During the second quarter of 2005, the CombiMatrix group formed a wholly owned subsidiary, CombiMatrix Molecular Diagnostics, Inc. (“CMDX”), in order to exploit the CombiMatrix group’s array technologies in the field of molecular diagnostics. As of December 31, 2005 and 2006, CMDX had 15 and 18 employees, respectively, located in Irvine, California.
Prior to July 11, 2003, CombiMatrix K.K., the CombiMatrix group’s majority-owned subsidiary, was operating under a joint venture agreement with Marubeni Japan (“Marubeni”), one of Japan’s leading trading companies. The primary purpose of the joint venture was to focus on development and licensing opportunities for the CombiMatrix group’s array technology with academic, pharmaceutical and biotechnology organizations in the Japanese market. Marubeni held a 10% minority interests in the joint venture. On July 11, 2003, Acacia Research Corporation purchased the outstanding minority interests in CombiMatrix K.K. from Marubeni. Acacia Research Corporation issued 200,000 shares of its AR-CombiMatrix stock to Marubeni in exchange for Marubeni’s 10% minority interests in CombiMatrix K.K. This increase in ownership interest was attributed to the CombiMatrix group. On January 26, 2006, the CombiMatrix group sold a majority of the CombiMatrix group’s interest in CombiMatrix K.K. to InBio, an Australian distributor of CustomArray products. As a result of this transaction, the CombiMatrix group retained a 33% minority ownership position in CombiMatrix K.K.
Refer to "Management’s Discussion and Analysis of Financial Condition and Results of Operations" for a description of impairment charges incurred in 2005 related to CombiMatrix K.K. and Advanced Material Sciences.
Marketing and Distribution
During 2004, the CombiMatrix group launched its CustomArray™ products and are currently selling these products directly and through distributors to customers in the United States, Europe and Asia. Since that time the CombiMatrix group has executed several non-exclusive distribution agreements with partners such as VWR International to market and sell its products worldwide. Beginning in 2006, the CombiMatrix group executed several manufacturing and distribution agreements to expand its worldwide product reach. These agreements allow for exclusive distribution of various CustomArray products in specific territories and for distribution of locally synthesized CustomArray-brand microarrays, where the manufacturer purchases and uses CustomArray synthesizers and supplies from the CombiMatrix group for use in their manufacturing process. Current manufacturers and distributors include BioInsight Pty. Ltd., Prisma Biotech Corp., Macrogen, Inc., and BioTeltec. Where appropriate, the CombiMatrix group will continue to market and sell its products directly or through distribution arrangements and/or through other strategic alliances. The CombiMatrix group has also executed its CombiCore™ distribution program, which provides for microarray service centers such as academic research laboratories to become authorized providers of CustomArray products and services within their research and development networks.
In July 2001, the CombiMatrix group entered into non-exclusive worldwide license, supply, research and development agreements with Roche. These agreements were amended in September 2002, primarily to grant Roche manufacturing rights with respect to the products under development in return for additional cash consideration under the agreements. The agreements are non-exclusive with respect to the CombiMatrix group’s core technology, meaning that the CombiMatrix group remains free to license its core technology to third parties for applications in the genomics, proteomics and other fields. The agreements contain exclusivity or co-exclusivity provisions only with respect to the specific products being co-developed for, and partially funded by, Roche pursuant to the agreements. Since July 2001, the CombiMatrix group has received approximately $26.6 million in cash payments from Roche from July 2001 through December 31, 2003. The agreements contain provisions that would allow Roche to terminate the agreements. Although Roche has not done so, in March 2004, the agreements were modified to indicate that the CombiMatrix group had completed all phases of its research and development commitments to Roche, and the CombiMatrix group has not received any additional payments from Roche since December 31, 2003.
The CombiMatrix group’s diagnostic services are being run at its CLIA laboratory in Irvine, California. Marketing of these services will be conducted through web-based initiatives, trade-shows, direct sales and marketing.
Manufacturing
The CombiMatrix group has developed automated, computer-directed manufacturing processes for the synthesis of sequences or spotting of DNA, RNA, BACs, peptides or small molecules on its arrays. Certain portions of the CombiMatrix group’s manufacturing are outsourced to subcontractors, while the CombiMatrix group conducts the steps involving synthesis or spotting of biological materials and quality control of its products.
Substantially all of the components and raw materials used in the manufacture of the CombiMatrix group’s products, including semiconductors and reagents, are currently provided from a limited number of sources or in some cases from a single source. Although the CombiMatrix group believes that alternative sources for those components and raw materials are available, any supply interruption in a sole-sourced component or raw material might result in up to a several-month production delay and materially harm its ability to manufacture products until a new source of supply, if any, could be located and qualified. In addition, an uncorrected impurity or supplier’s variation in a raw material, either unknown to the CombiMatrix group or incompatible with the CombiMatrix group’s manufacturing process, could have a material adverse effect on the CombiMatrix group’s ability to manufacture products. The CombiMatrix group may be unable to find a sufficient alternative supply channel in a reasonable time period, or on commercially reasonable terms, if at all. The CombiMatrix group utilizes non-standard semiconductor manufacturing processes to fabricate the electrode array that is a key aspect of the array structure. Although the CombiMatrix group has a supply agreement in place with the semiconductor wafer manufacturer to ensure availability of the raw materials, it does not guarantee a permanent supply. These non-standard processes are not widely available, and it may be difficult or expensive to obtain sufficient quantities of semiconductor wafers if the current manufacturer changes or discontinues the CombiMatrix group’s manufacturing production capability.
Patents and Licenses
The CombiMatrix group continues to build its intellectual property portfolio to protect its product in those markets where it operates and where a market for its products and services exists. In the United States, the CombiMatrix group has been issued five United States patents. Three of the United States patents (U.S. Patent No. 6,093,302 expiration date January 5, 2018; U.S. Patent No. 6,280,595 expiration date January 5, 2018 and U.S. Patent No. 6,444,111 expire on January 5, 2018) are first generation technology relating to methods for electrochemical synthesis of arrays of DNA and other biological materials as well as non-biological materials. These three patents are currently under ex parte reexamination in the United States Patent and Trademark Office, and we expect all claims to remain essentially the same as before the reexamination. . The fourth United States Patent (U.S. Patent No. 6,456,942 expiration date January 25, 2020) describes and claims a network infrastructure for a customized array synthesis and analysis. The fifth United States Patent (U.S. Patent No. 7,075,187, expiring on November 9, 2021) describes and claims a coating material that covers electrodes and is used as a support material for electrochemical synthesis on arrays. Ex parte reexamination has been requested but has not yet been granted for these fourth and fifth patents. Corresponding core patents describing and claiming methods for electrochemical synthesis of arrays have been issued to the CombiMatrix group in Europe (entire EU), Australia and Taiwan and are pending in the remaining major industrialized markets. In total, the CombiMatrix group has 87 patent applications pending in the Unites States, Europe and elsewhere.
The CombiMatrix group seeks to protect its corporate identity with trademarks and service marks. In addition, the CombiMatrix group’s trademark strategy includes protecting the identity and goodwill associated with its biological and chemical array processor products. The CombiMatrix group purchases chemical reagents from suppliers who are licensed under appropriate patent rights. It is the CombiMatrix group’s policy to obtain licenses from patent holders, or as a purchaser from licensed suppliers, if needed, to practice its chemical processes.
The CombiMatrix group’s success will depend, in part, upon its ability to obtain patents and maintain adequate protection of its intellectual property in the United States and other countries. If the CombiMatrix group does not protect its intellectual property adequately, competitors may be able to use its technologies and thereby erode any competitive advantage that it may have. The laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting their proprietary rights abroad. These problems can be caused by the absence of laws, rules and/or methods for defending intellectual property rights. In addition, the laws of foreign jurisdictions, such as the European Union, provide an opportunity for parties to oppose the granting of patents when such claims may be construed as too broad or significantly beyond the scope of the initial teaching or disclosure in a patent filed. Moreover, the laws of the United States provide an opportunity for parties to file for reexamination of issued U.S. Patents based upon prior art patents and publications. Reexamination can result in narrower claims and invalidation of claims. The CombiMatrix group has been active in Europe challenging the rights of competitors who have patent claims extending well beyond the scope of any teachings provided. There is no assurance that the CombiMatrix group will continue to be successful in such oppositions.
The patent positions of companies developing tools and drugs for the biotechnology and pharmaceutical industries, including the CombiMatrix group’s patent position, generally are uncertain and involve complex legal and factual questions. The CombiMatrix group will be able to protect its proprietary rights from unauthorized use by third parties only to the extent that its proprietary technologies are covered by valid and enforceable patents or are effectively maintained as trade secrets. The CombiMatrix group’s existing patent and any future patents it obtains may not be sufficiently broad to prevent others from practicing its specific technologies or from developing competing products. There also is risk that others may independently develop similar or alternative technologies or design around the CombiMatrix group’s patented technologies. In addition, others may challenge or invalidate the CombiMatrix group’s patents, or the CombiMatrix group’s patents may fail to provide the CombiMatrix group with any competitive advantage. Enforcing the CombiMatrix group’s intellectual property rights may be difficult, costly and time consuming, and ultimately may not be successful.
The CombiMatrix group also relies upon trade secret protection for the CombiMatrix group’s confidential and proprietary information. The CombiMatrix group seeks to protect its proprietary information by entering into confidentiality and invention disclosure and transfer agreements with employees, collaborators and consultants. These measures, however, may not provide adequate protection for the CombiMatrix group’s trade secrets or other proprietary information. Employees, collaborators or consultants may still disclose the CombiMatrix group’s proprietary information, and the CombiMatrix group may not be able to meaningfully protect its trade secrets. In addition, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to the CombiMatrix group’s trade secrets. Also, former employees may also knowingly violate such agreements, forcing the CombiMatrix group to enforce its intellectual property rights.
The CombiMatrix group cannot assure you that any of its patent applications will result in the issuance of any additional patents, that its patent applications will have priority of invention or filing date over similar rights of others, or that, if issued, any of its patents will offer protection against its competitors. Additionally, the CombiMatrix group cannot assure you that any patent issued to it will not be challenged, invalidated or circumvented in the future or that the intellectual property rights the CombiMatrix group has created will provide a competitive advantage. Litigation may be necessary to enforce the CombiMatrix group’s intellectual property rights or to determine the enforceability, scope of protection or validity of the intellectual property rights of others.
Competition
The CombiMatrix group expects to encounter competition for business opportunities from other entities having similar business objectives. Many of these potential competitors possess greater financial, technical, human and other resources than the CombiMatrix group possess. The CombiMatrix group anticipates that it will face increased competition in the future as new companies enter the market and advanced technologies become available. Technological advances or entirely different approaches developed by one or more of the CombiMatrix group’s competitors could render its processes obsolete or uneconomical. The existing approaches of competitors or new approaches or technology developed by competitors may be more effective than those developed by the CombiMatrix group.
The CombiMatrix group is aware of other companies or companies with divisions that have, or are developing, technologies for the molecular diagnostic markets. The CombiMatrix group believes that its primary competitors will be Affymetrix, Inc., Agilent Technologies, Inc., Applera Corporation, Ciphergen Biosystems, Inc., Gene Logic Inc., Genomic Health, Inc., Illumina, Inc., Nanogen, Inc., Roche Diagnostics GmbH and Sequenom, Inc. However, the CombiMatrix group’s market is rapidly changing, and the CombiMatrix group expects to face additional competition from new market entrants, new product developments and consolidation of its existing competitors. Many of the CombiMatrix group’s competitors have existing strategic relationships with major pharmaceutical and biotechnology companies, greater commercial experience and substantially greater financial and personnel resources than the CombiMatrix group does. The CombiMatrix group expects new competitors to emerge and the intensity of competition to increase in the future.
Research, Development and Engineering
The CombiMatrix group’s research and development expenses were $9.5 million, $5.8 million and $5.4 million during 2006, 2005 and 2004, respectively. Of these amounts, research and development related non-cash stock compensation charges were $1.1 million, $0 and $91,000 during 2006, 2005 and 2004, respectively. The CombiMatrix group intends to invest in its proprietary technologies through internal development and, to the extent available, licensing of third-party technologies to increase and improve other characteristics of its products. The CombiMatrix group also plans to continue to invest in improving the cost-effectiveness of its products through further automation and improved information technologies. The CombiMatrix group’s future research and development efforts may involve research conducted by the CombiMatrix group, collaborations with other researchers and the acquisition of chemistries and other technologies developed by universities and other academic institutions.
The CombiMatrix group is developing a variety of life sciences and non-life sciences products and services. Potential customers for these products operate in industries characterized by rapid technological development. The CombiMatrix group believes that its future success will depend in large part on its ability to continue to enhance its existing products and services and to develop other products and services, which complement existing ones. In order to respond to rapidly changing competitive and technological conditions, the CombiMatrix group expects to continue to incur significant research and development expenses during the initial development phase of new products and services, as well as on an ongoing basis.
Government Grants and Contracts
Government grants and contracts have allowed the CombiMatrix group to fund certain internal scientific programs and exploratory research. The CombiMatrix group retains ownership of all intellectual property and commercial rights generated during these projects. The United States government, however, retains a non-exclusive, non-transferable, paid-up license to practice the inventions made with federal funds pursuant to applicable statutes and regulations. The CombiMatrix group does not believe that the retained license will have any impact on its ability to market its products. The CombiMatrix group does not need government approval to enter into collaborations or other relationships with third parties.
The CombiMatrix group has been awarded several grants from the federal government in connection with its biological and chemical array processor technology since its inception. In March of 2004, the CombiMatrix group was awarded a two-year, $5.9 million contract with the Department of Defense (“DoD”) to further the development of its array technology for the electrochemical detection of biological and chemical threat agents. Under the terms of the contract, the CombiMatrix group will be reimbursed on a periodic basis for actual costs incurred to perform its obligations, plus a fixed fee, of up to $5.9 million. This project was completed during 2005. In February of 2006, the CombiMatrix group was awarded a one-year, $2.1 million contract with the DoD to further the development of its array technology for the electrochemical detection of biological and chemical threat agents developed under the previous contracts and grants. In August of 2006, the CombiMatrix group executed a two-year, $1.9 million contract with the DoD, focusing on the integration of its electrochemical detection technology currently under development with its microfluidics "lab-on-a-chip" technology to be used for military and homeland security applications.
The CombiMatrix group will continue to pursue grants and contracts that complement its research and development efforts.
Employees
As of December 31, 2007, the CombiMatrix group had 73 full-time employees, 17 of whom hold a Ph.D. or an M.D. degree and 49 of whom are engaged in full-time research and development activities. The CombiMatrix group has no part-time employees. The CombiMatrix group is not a party to any collective bargaining agreement. The CombiMatrix group considers its employee relations to be good.
Environmental Matters
The CombiMatrix group’s operations involve the use, transportation, storage and disposal of hazardous substances, and as a result, the CombiMatrix group is subject to environmental and health and safety laws and regulations. The cost of complying with these and any future environmental regulations could be substantial. In addition, if the CombiMatrix group fails to comply with environmental laws and regulations, or releases any hazardous substance into the environment, the CombiMatrix group could be exposed to substantial liability in the form of fines, penalties, remediation costs and other damages, or could suffer a curtailment or shut down of its operations.
An investment in our stock involves a number of risks. Before making a decision to purchase our securities, you should carefully consider all of the risks described in this annual report. If any of the risks discussed in this annual report actually occur, our business, financial condition and results of operations could be materially adversely affected. If this were to occur, the trading price of our securities could decline significantly and you may lose all or part of your investment.
GENERAL RISKS
WE HAVE A HISTORY OF LOSSES AND WILL PROBABLY INCUR ADDITIONAL LOSSES IN THE FUTURE.
We have sustained substantial losses since our inception resulting in a consolidated net accumulated deficit, as disclosed in the accompanying consolidated financial statements of Acacia Research Corporation included in Part IV. Item 15 of this report. We may never become profitable, or if we do, we may never be able to sustain profitability. We expect to incur significant research and development, marketing, general and administrative and legal expenses. As a result, it is more likely than not that we will incur losses for the foreseeable future.
IF WE, OR OUR SUBSIDIARIES, ENCOUNTER UNFORESEEN DIFFICULTIES AND CANNOT OBTAIN ADDITIONAL FUNDING ON FAVORABLE TERMS, OUR BUSINESS MAY SUFFER.
Acacia Research Corporation's consolidated cash and cash equivalents along with short-term investments totaled $59.3 million at December 31, 2006. The Acacia Technologies group’s cash and cash equivalents and short-term investments totaled $45.0 million at December 31, 2006. The CombiMatrix group’s cash and cash equivalents and short-term investments totaled $14.3 million at December 31, 2006.
To date, the CombiMatrix group has relied primarily upon selling equity securities, as well as payments from strategic partners, to generate the funds needed to finance the implementation of the CombiMatrix group’s business strategies. To date, the Acacia Technologies group has relied primarily upon selling of equity securities and payments from our licensees to generate the funds needed to finance the operations of the Acacia Technologies group.
We cannot assure you that we will not encounter unforeseen difficulties, including the outside influences identified above, that may deplete our capital resources more rapidly than anticipated. As a result, our subsidiary companies may be required to obtain additional financing through bank borrowings, debt or equity financings or otherwise, which would require us to make additional investments or face a dilution of our equity interests. Any efforts to seek additional funds could be made through equity, debt or other external financings. Nevertheless, we cannot assure that additional funding will be available on favorable terms, if at all. If we fail to obtain additional funding when needed for our subsidiary companies and ourselves, we may not be able to execute our business plans and our business may suffer. Refer to “Risks related to the CombiMatrix group” below for additional information.
BECAUSE WE HAVE SUSTAINED LOSSES SINCE OUR INCEPTION, WE CANNOT ASSURE THAT OUR OPERATIONS WILL BE PROFITABLE.
We commenced operations in 1993 and, we have sustained substantial losses since our inception resulting in a net consolidated accumulated deficit, as disclosed in the accompanying consolidated financial statements of Acacia Research Corporation included in Part IV. Item 15 of this report. If we continue to incur operating losses in future periods, we may not have enough money to expand our business and our subsidiary companies’ businesses in the future.
FAILURE TO EFFECTIVELY MANAGE OUR GROWTH COULD PLACE STRAINS ON OUR MANAGERIAL, OPERATIONAL AND FINANCIAL RESOURCES AND COULD ADVERSELY AFFECT OUR BUSINESS AND OPERATING RESULTS.
Our growth has placed, and is expected to continue to place, a strain on our managerial, operational and financial resources. Further, as our subsidiary companies’ businesses grow, we will be required to manage multiple relationships. Any further growth by us or our subsidiary companies or an increase in the number of our strategic relationships will increase this strain on our managerial, operational and financial resources. This strain may inhibit our ability to achieve the rapid execution necessary to successfully implement our business plan.
OUR FUTURE SUCCESS DEPENDS ON OUR ABILITY TO EXPAND OUR ORGANIZATION TO MATCH THE GROWTH OF OUR SUBSIDIARIES.
As our subsidiaries grow, the administrative demands upon Acacia Research Corporation will grow, and our success will depend upon our ability to meet those demands. These demands include increased accounting, management, legal services, staff support, and general office services. We may need to hire additional qualified personnel to meet these demands, the cost and quality of which is dependent in part upon market factors outside of our control. Further, we will need to effectively manage the training and growth of our staff to maintain an efficient and effective workforce, and our failure to do so could adversely affect our business and operating results.
THE AVAILABILITY OF SHARES FOR SALE IN THE FUTURE COULD REDUCE THE MARKET PRICE OF OUR COMMON STOCK.
In the future, we may issue securities to raise cash for acquisitions. We may also pay for interests in additional subsidiary companies by using a combination of cash and our common stock or just our common stock. We may also issue securities convertible into our common stock. Any of these events may dilute your ownership interest in our company and have an adverse impact on the price of our common stock.
In addition, sales of a substantial amount of our common stock in the public market, or the perception that these sales may occur, could reduce the market price of our common stock. This could also impair our ability to raise additional capital through the sale of our securities.
DELAWARE LAW AND OUR CHARTER DOCUMENTS CONTAIN PROVISIONS THAT COULD DISCOURAGE OR PREVENT A POTENTIAL TAKEOVER OF ACACIA RESEARCH CORPORATION THAT MIGHT OTHERWISE RESULT IN OUR STOCKHOLDERS RECEIVING A PREMIUM OVER THE MARKET PRICE OF THEIR SHARES.
Provisions of Delaware law and our certificate of incorporation and bylaws could make the following more difficult: the acquisition of our company by means of a tender offer, proxy contest or otherwise, and the removal of incumbent officers and directors. These provisions include:
| · | section 203 of the Delaware General Corporation Law, which prohibits a merger with a 15%-or-greater stockholder, such as a party that has completed a successful tender offer, until three years after that party became a 15%-or-greater stockholder; |
| | |
| · | amendment of our bylaws by the stockholders requires a two-thirds approval of the outstanding shares; |
| | |
| the authorization in our certificate of incorporation of undesignated preferred stock, which could be issued without stockholder approval in a manner designed to prevent or discourage a takeover; |
| | |
| provisions in our bylaws eliminating stockholders’ rights to call a special meeting of stockholders, which could make it more difficult for stockholders to wage a proxy contest for control of our board of directors or to vote to repeal any of the anti-takeover provisions contained in our certificate of incorporation and bylaws; and |
| | |
| the division of our board of directors into three classes with staggered terms for each class, which could make it more difficult for an outsider to gain control of our board of directors. |
Such potential obstacles to a takeover could adversely affect the ability of our stockholders to receive a premium price for their stock in the event another company wants to acquire us.
WE MAY INCUR INCREASED COSTS AS A RESULT OF RECENTLY ENACTED AND PROPOSED CHANGES IN LAWS AND REGULATIONS RELATING TO CORPORATE GOVERNANCE MATTERS.
Recently enacted and proposed changes in the laws and regulations affecting public companies, including the provisions of the Sarbanes-Oxley Act of 2002 and rules adopted or proposed by the Securities and Exchange Commission and by NASDAQ, will result in increased costs to us as we evaluate the implications of any new rules and respond to their requirements. New rules could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. We cannot predict or estimate the amount of the additional costs we may incur or the timing of such costs to comply with any new rules and regulations.
WE ARE PLANNING TO REDEEM ALL OF THE ISSUED AND OUTSTANDING SHARES OF AR-COMBIMATRIX STOCK FOR ALL OF THE ISSUED AND OUTSTANDING SHARES OF COMBIMATRIX CORPORATION, AND FOLLOWING THE REDEMPTION, THE COMBIMATRIX GROUP WILL CEASE TO BE A PART OF ACACIA RESEARCH CORPORATION.
We are planning to redeem all of the issued and outstanding shares of AR-CombiMatrix stock for all of the common stock of CombiMatrix Corporation pursuant to our Amended and Restated Certificate of Incorporation. Holders of AR-CombiMatrix stock and AR-Acacia Technologies stock will not have an opportunity to vote on the redemption, nor will they have any right to dissent or otherwise be compensated. Following the redemption, holders of AR-CombiMatrix stock will no longer be shareholders of Acacia Research Corporation but will own all of the issued and outstanding stock of CombiMatrix Corporation. Holders of AR-Acacia Technologies stock will be the sole shareholders of Acacia research Corporation. On the redemption date, CombiMatrix Corporation will hold all of the assets of the CombiMatrix group and will thereafter operate as a separate public company with its common stock registered under the Securities and Exchange Act of 1934. The price of shares of either or both companies could decline as a result of the split-off.
AS A RESULT OF THE REDEMPTION OF AR-COMBIMATRIX STOCK FOR THE COMMON STOCK OF COMBIMATRIX CORPORATION, ACACIA RESEARCH CORPORATION AND COMBIMATRIX CORPORATION MAY BE SUBJECT TO CERTAIN TAX LIABILITY UNDER THE INTERNAL REVENUE CODE.
Our distribution of the common stock of CombiMatrix Corporation in the redemption will be tax-free to Acacia Research Corporation and CombiMatrix Corporation if the distribution qualifies under Sections 368 and 355 of the Internal Revenue Code. If the split-off failed to qualify under Section 355 of the Internal Revenue Code, corporate tax would be payable by the consolidated group of which Acacia Research Corporation is the common parent based upon the difference between the aggregate fair market value of the assets of CombiMatrix Corporation’s business and the adjusted tax bases of such business to Acacia Research Corporation prior to the redemption. The corporate level tax would be payable by Acacia Research Corporation. In addition, under the Internal Revenue Code’s consolidated return regulations, each member of the Acacia Research Corporation consolidated group, including Acacia Research Corporation and CombiMatrix Corporation following the redemption, will be severally liable for these tax liabilities. If we are found liable to the IRS, the resulting obligation could materially and adversely affect our financial condition. CombiMatrix Corporation has agreed to indemnify Acacia Research Corporation for this and other tax liabilities if they result from certain actions taken by CombiMatrix Corporation or from the redemption.
Acacia Research Corporation received a private letter ruling from the IRS to the effect that, among other things, the redemption would be tax free to Acacia Research Corporation and the holders of AR-Acacia Technologies stock and AR-CombiMatrix stock under Sections 368 and 355 of the Internal Revenue Code. The private letter ruling, while generally binding upon the IRS, was based upon factual representations and assumptions and commitments on our behalf with respect to future operations made in the ruling request. The IRS could modify or revoke the private letter ruling retroactively if the factual representations and assumptions in the request were materially incomplete or untrue, the facts upon which the private letter ruling was based were materially different from the facts at the time of the redemption, or if we do not meet certain commitments made.
If the split-off failed to qualify under Section 355 of the Internal Revenue Code, corporate tax would be payable by the consolidated group of which Acacia Research Corporation is the common parent based upon the difference between the aggregate fair market value of the assets of our business and the adjusted tax bases of such business to Acacia Research Corporation prior to the split-off. The corporate level tax would be payable by Acacia Research Corporation. CombiMatrix Corporation has agreed however, to indemnify Acacia Research Corporation for this and certain other tax liabilities if they result from actions taken by CombiMatrix Corporation or from the split-off. Notwithstanding CombiMatrix Corporation’s agreement to indemnify us, under the Internal Revenue Code’s consolidated return regulations, each member of the Acacia Research Corporation consolidated group, including our company, will be severally liable for these tax liabilities. If we are found liable to the IRS for these liabilities, the resulting obligation could materially and adversely affect our financial condition, and we may be unable to recover on the indemnity from CombiMatrix Corporation.
FOLLOWING THE REDEMPTION OF AR-COMBIMATRIX STOCK FOR THE COMMON STOCK OF COMBIMATRIX CORPORATION, ACACIA RESEARCH CORPORATION AND COMBIMATRIX CORPORATION MAY BE SUBJECT TO CERTAIN TAX LIABILITY UNDER THE INTERNAL REVENUE CODE FOR ACTIONS TAKEN BY EITHER OF THEM FOLLOWING THE REDEMPTION.
Even if the distribution qualifies under Section 368 and 355 of the Internal Revenue Code, it will be taxable to Acacia if Section 355(e) of the Internal Revenue Code applies to the distribution. Section 355(e) will apply if 50% or more of the AR-Acacia Technologies stock or CombiMatrix Corporation’s common stock, by vote or value, is acquired by one or more persons, other than the holders of AR-CombiMatrix stock who receive the common stock of CombiMatrix Corporation in the redemption, acting pursuant to a plan or a series of related transactions that includes the redemption. Any shares of the AR-Acacia Technologies stock, the AR-CombiMatrix stock or the common stock of CombiMatrix Corporation acquired directly or indirectly within two years before or after the redemption generally are presumed to be part of such a plan unless we can rebut that presumption. To prevent applicability of Section 355(e) or to otherwise prevent the distribution from failing to qualify under Section 355 of the Internal Revenue Code, CombiMatrix Corporation has agreed that, until two years after the redemption, it will not take any of the following actions unless prior to taking such action, it has obtained (and provided to Acacia) a written opinion of tax counsel or a ruling from the Internal Revenue Service to the effect that such action will not cause the redemption to be taxable to Acacia (collectively “Disqualifying Actions”):
| | • | merge or consolidate with another corporation; |
| | • | liquidate or partially liquidate; |
| | • | sell or transfer all or substantially all of its assets; |
| | • | redeem or repurchase its stock (except in certain limited circumstances); or |
| | • | take any other action which could reasonably be expected to cause Section 355(e) to apply to the distribution. |
Many of our competitors are not subject to similar restrictions and may issue their stock to complete acquisitions, expand their product offerings and speed the development of new technology. Therefore, these competitors may have a competitive advantage over us. In addition, substantial uncertainty exists on the scope of Section 355(e), and we have undertaken, contemplate undertaking or may otherwise undertake in the future transactions which may cause Section 355(e) to apply to the redemption. Accordingly, we cannot provide you any assurance that we will not be liable for taxes if Section 355(e) applies to the redemption.
ACACIA RESEARCH CORPORATION AND COMBIMATRIX CORPORATION MAY BE REQUIRED TO INDEMNIFY THE OTHER FOR TAX LIABILITY RESULTING FROM THE REDEMPTION OF AR-COMBIMATRIX STOCK FOR THE COMMON STOCK OF COMBIMATRIX CORPORATION, WHICH MAY INTERFERE WITH BOTH COMPANIES’ ABILITY TO ENGAGE IN DESIRABLE STRATEGIC TRANSACTIONS AND ISSUE THEIR EQUITY SECURITIES.
If Section 355(e) applies to the distribution because of some action or omission by Acacia or by CombiMatrix Corporation after the distribution, then it must indemnify the other for any resulting tax-related liabilities. The CombiMatrix Corporation will have to indemnify Acacia if the redemption becomes taxable to Acacia by failing to qualify under Section 355 of the Internal Revenue Code or from the application of Section 355(e) of the Internal Revenue Code as a result of these or any other transactions that it undertakes after the redemption. In the event that CombiMatrix Corporation were liable for such taxes, the payment would have a substantial and material adverse effect on its business, financial position and results of operations. Further, if the redemption becomes taxable to Acacia by failing to qualify under Section 355 of the Internal Revenue Code or from the application of Section 355(e) of the Internal Revenue Code as a result of these or any other transactions that Acacia undertakes before or after the redemption, then Acacia will be liable for such taxes without recourse against CombiMatrix Corporation. This obligation may discourage, delay or prevent a merger, change of control, or other strategic or capital raising transactions involving our AR-CombiMatrix stock or CombiMatrix Corporation’s future outstanding equity or our issuance of other equity securities. If we cannot engage in equity financing transactions because of these constraints, we may not be able to fund the working capital, capital expenditure and research and development requirements, as well as to make other investments. As a result, our business may be harmed.
RISKS RELATING TO THE ACACIA TECHNOLOGIES GROUP
The risk factors beginning on this page discuss risks relating to the Acacia Technologies group. Because each holder of AR- Acacia Technologies stock is also a holder of the common stock of one company, Acacia Research Corporation, the risks associated with the CombiMatrix group could affect our AR-Acacia Technologies stock. As such, we urge you to read carefully the section “Risks Relating to the CombiMatrix Group” below.
BECAUSE OUR BUSINESS OPERATIONS ARE SUBJECT TO MANY UNCONTROLLABLE OUTSIDE INFLUENCES, WE MAY NOT SUCCEED.
Our Acacia Technologies group’s business operations are subject to numerous risks from outside influences, including the following:
· New legislation, regulations or rules related to obtaining patents or enforcing patents could significantly increase Acacia Technologies group’s operating costs and decrease its revenue.
Our Acacia Technology group acquires patents with enforcement opportunities and is spending a significant amount of resources to enforce those patents. If new legislation, regulations or rules are implemented either by Congress, the United States Patent and Trademark Office, or the courts that impact the patent application process, the patent enforcement process or the rights of patent holders, these changes could negatively affect our expenses and revenue. For example, new rules regarding the burden of proof in patent enforcement actions could significantly increase the cost of our enforcement actions, and new standards or limitations on liability for patent infringement could negatively impact our revenue derived from such enforcement actions. While we are not aware that any such changes are likely to occur in the foreseeable future, we cannot assure you that such changes will not occur.
· Trial judges and juries often find it difficult to understand complex patent enforcement litigation, and as a result, we may need to appeal adverse decisions by lower courts in order to successfully enforce our patents.
It is difficult to predict the outcome of patent enforcement litigation at the trial level. It is often difficult for juries and trial judges to understand complex, patented technologies, and as a result, there is a higher rate of successful appeals in patent enforcement litigation than more standard business litigation. Such appeals are expensive and time consuming, resulting in increased costs and delayed revenue. Although we diligently pursue enforcement litigation, we cannot predict with significant reliability the decisions made by juries and trial courts.
· More patent applications are filed each year resulting in longer delays in getting patents issued by the United States Patent and Trademark Office.
Our Acacia Technology group holds and continues to acquire pending patents. We have identified a trend of increasing patent applications each year, which we believe is resulting in longer delays in obtaining approval of pending patent applications. The delays could cause delays in recognizing revenue from these patents and could cause us to miss opportunities to license patents before other competing technologies are developed or introduced into the market. See the subheading “Competition is intense in the industries in which our subsidiaries do business and as a result, we may not be able to grow or maintain our market share for our technologies and patents,” below.
· Federal courts are becoming more crowded, and as a result, patent enforcement litigation is taking longer.
Our patent enforcement actions are almost exclusively prosecuted in federal court. Federal trial courts that hear our patent enforcement actions also hear criminal cases. Criminal cases always take priority over our actions. As a result, it is difficult to predict the length of time it will take to complete an enforcement action. Moreover, we believe there is a trend in increasing numbers of civil lawsuits and criminal proceedings before federal judges, and as a result, we believe that the risk of delays in our patent enforcement actions will have a greater affect on our business in the future unless this trend changes.
· Any Reductions in the funding of the United States Patent and Trademark Office could have an adverse impact on the cost of processing pending patent applications and the value of those pending patent applications.
The assets of Acacia Technologies group consists of patent portfolios, including pending patent applications before the U.S. Patent and Trademark Office (USPTO). The value of our patent portfolios is dependent upon the issuance of patents in a timely manner, and any reductions in the funding of the USPTO could negatively impact the value of our assets. Further, reductions in funding from Congress could result in higher patent application filing and maintenance fees charged by the USPTO, causing an unexpected increase in our expenses.
· Competition is intense in the industries in which our subsidiaries do business and as a result, we may not be able to grow or maintain our market share for our technologies and patents.
Our Acacia Technologies group expects to encounter competition in the area of patent acquisition and enforcement as the number of companies entering this market is increasing. This includes competitors seeking to acquire the same or similar patents and technologies that we may seek to acquire. Companies such as British Technology Group, Rembrandt Management Group, and Intellectual Ventures LLC are already in the business of acquiring the rights to patents for the purpose of enforcement, and we expect more companies to enter the market. As new technological advances occur, many of our patented technologies may become obsolete before they are completely monetized. If we are unable to replace obsolete technologies with more technologically advanced patented technologies, then this obsolescence could have a negative effect on our ability to generate future revenues.
Our Acacia Technologies group also competes with venture capital firms and various industry leaders for technology licensing opportunities. Many of these competitors may have more financial and human resources than our company. As we become more successful, we may find more companies entering the market for similar technology opportunities, which may reduce our market share in one or more technology industries that we currently rely upon to generate future revenue.
· Our patented technologies face uncertain market value.
Our Acacia Technologies group has acquired patents and technologies that are at early stages of adoption in the commercial and consumer markets. Demand for some of these technologies is untested and is subject to fluctuation based upon the rate at which our licensees will adopt our patents and technologies in their products and services. See the related risk factor below.
· As patent enforcement litigation becomes more prevalent, it may become more difficult for us to voluntarily license our patents.
We believe that the more prevalent patent enforcement actions become, the more difficult it will be for us to voluntarily license our patents. As a result, we may need to increase the number of our patent enforcement actions to cause infringing companies to license the patent or pay damages for lost royalties. This may increase the risks associated with an investment in our company.
· The foregoing outside influences may affect other risk factors described in this annual report.
Any one of the foregoing outside influences may cause our company to need additional financing to meet the challenges presented or to compensate for a loss in revenue, and we may not be able to obtain the needed financing. See the heading “If we, or our subsidiaries, encounter unforeseen difficulties and cannot obtain additional funding on favorable terms, our business may suffer” above.
THE ACACIA TECHNOLOGIES GROUP HAS INCURRED LOSSES IN THE PAST AND EXPECTS TO INCUR ADDITIONAL LOSSES IN THE FUTURE.
The Acacia Technologies group has sustained substantial losses in the past. We expect the Acacia Technologies group to incur significant legal, marketing, general and administrative expenses in future periods. As a result, we expect the Acacia Technologies group to incur losses for the foreseeable future.
THE ACACIA TECHNOLOGIES GROUP MAY FAIL TO MEET MARKET EXPECTATIONS BECAUSE OF FLUCTUATIONS IN ITS QUARTERLY OPERATING RESULTS, WHICH COULD CAUSE THE PRICE OF ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK TO DECLINE.
The Acacia Technologies group’s revenues and operating results have fluctuated in the past and may continue to fluctuate significantly from quarter to quarter in the future. It is possible that in future periods the Acacia Technologies group’s revenues could fall below the expectations of securities analysts or investors, which could cause the market price of our Acacia Research-Acacia Technologies common stock to decline. The following are among the factors that could cause the Acacia Technologies group’s operating results to fluctuate significantly from period to period:
| the performance of our third-party licensees; |
| | |
| costs related to acquisitions, alliances, licenses and other efforts to expand our operations; |
| | |
| the timing of payments under the terms of any customer or license agreements into which the Acacia Technologies group may enter; and |
| | |
| expenses related to, and the results of, patent filings and other enforcement proceedings relating to intellectual property rights, as more fully described in this section. |
THE ACACIA TECHNOLOGIES GROUP’S REVENUES WILL BE UNPREDICTABLE, AND THIS MAY HARM ITS FINANCIAL CONDITION.
Acacia Global Acquisition Corporation's acquisition of the assets of Global Patent Holdings, LLC in 2005, provided the Acacia Technologies group with ownership of companies that control 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts. Rights to additional patent portfolios were acquired subsequent to the acquisition of the assets of Global Patent Holdings, bringing the current total number of patent portfolios controlled by the Acacia Technologies group to approximately 62, covering technologies used in a wide variety of industries. The acquisitions expand and diversify the Acacia Technologies group's revenue generating opportunities. The Acacia Technologies group believes that its cash and cash equivalent balances, anticipated cash flow from operations and other external sources of available credit, will be sufficient to meet its cash requirements through at least March 2008, and for the foreseeable future. However, due to the nature of our licensing business and uncertainties regarding the amount and timing of the receipt of license fees from potential infringers, stemming primarily from uncertainties regarding the outcome of enforcement actions, rates of adoption of our patented technologies, the growth rates of our existing licensees and other factors, we cannot currently predict the amount and timing of the receipt of license fee revenues with a sufficient degree of precision.
As a result, the Acacia Technologies group’s revenues may vary significantly from quarter to quarter, which could make its business difficult to manage and cause its quarterly results to be below market expectations. If this happens, the price of our Acacia Research-Acacia Technologies common stock may decline significantly.
THE ACACIA TECHNOLOGIES GROUP DEPENDS UPON RELATIONSHIPS WITH OTHERS TO PROVIDE TECHNOLOGY-BASED OPPORTUNITIES THAT CAN DEVELOP INTO PROFITABLE ROYALTY-BEARING LICENSES, AND IF IT IS UNABLE TO MAINTAIN AND GENERATE NEW RELATIONSHIPS, THEN IT MAY NOT BE ABLE TO SUSTAIN EXISTING LEVELS OF REVENUE OR INCREASE REVENUE.
The Acacia Technologies group does not invent new technologies or products; it depends on acquiring new patents and inventions through its relationships with inventors, universities, research institutions, and others. If the Acacia Technologies group is unable to maintain those relationship and continue to grow new relationships, then it may not be able to identify new technology-based opportunities for growth and sustainable revenue. Further, because we rely upon acquiring technology from others, we cannot be certain that we will be able to obtain the volume and quality of available new technologies necessary to sustain our current growth. If we are unable to obtain the necessary volume and quality of new technologies, then we may need to reduce operations or revise our business model.
TECHNOLOGY COMPANY STOCK PRICES ARE ESPECIALLY VOLATILE, AND THIS VOLATILITY MAY DEPRESS THE PRICE OF OUR ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK.
The stock market has experienced significant price and volume fluctuations, and the market prices of technology companies have been highly volatile. We believe that various factors may cause the market price of our Acacia Research-Acacia Technologies common stock to fluctuate, perhaps substantially, including, among others, the following:
| announcements of developments in our patent enforcement actions; |
| | |
| developments or disputes concerning our patents; |
| | |
| our or our competitors’ technological innovations; |
| | |
| developments in relationships with licensees; |
| | |
| variations in our quarterly operating results; |
| | |
| our failure to meet or exceed securities analysts’ expectations of our financial results; or |
| | |
| a change in financial estimates or securities analysts’ recommendations; |
| | |
| changes in management’s or securities analysts’ estimates of our financial performance; |
| | |
| changes in market valuations of similar companies; |
| | |
| announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies, or patents; and |
| | |
| failure to complete significant transactions. |
For example, the Nasdaq Computer Index had a range of $846.89 - $1,102.80 during the 52-weeks ended December 31, 2006. Over the same period, our Acacia Research-Acacia Technologies common stock fluctuated within a range of $6.65 - $15.58. We believe fluctuations in our stock price during this period could have been impacted by court rulings in our patent enforcement actions. Court rulings in patent enforcement actions are often difficult to understand, even when favorable or neutral to the value of our patents, and we believe that investors in the market may overreact, causing fluctuations in our stock prices that may not accurately reflect the impact of court rulings on our business operations and assets.
In the past, companies that have experienced volatility in the market price of their stock have been the objects of securities class action litigation. If our Acacia Research-Acacia Technologies common stock was the object of securities class action litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could materially harm the business and financial results of the Acacia Technologies group.
THE MARKETS SERVED BY THE ACACIA TECHNOLOGIES GROUP ARE SUBJECT TO RAPID TECHNOLOGICAL CHANGE, AND IF THE ACACIA TECHNOLOGIES GROUP IS UNABLE TO DEVELOP AND ACQUIRE NEW TECHNOLOGIES AND PATENTS, ITS REVENUES COULD STOP GROWING OR COULD DECLINE.
The markets served by the licensees of Acacia Technologies group frequently undergo transitions in which products rapidly incorporate new features and performance standards on an industry-wide basis. Products for communications applications, high-speed computing applications, as well as other applications covered by the Acacia Technologies group’s intellectual property, are based on continually evolving industry standards. The Acacia Technologies group’s ability to compete in the future will, however, depend on its ability to identify and ensure compliance with evolving industry standards. This will require our continued efforts and success of acquiring new patent portfolios with licensing and enforcement opportunities. However, we expect to have sufficient liquidity and capital resources for the foreseeable future in order to maintain the level of acquisitions we believe we need to keep pace with these technological advances. However, outside influences may cause the need for greater liquidity and capital resources than expected, as described under the caption “Because our business operations are subject to many uncontrollable outside influences, we may not succeed” above.
THE SUCCESS OF OUR ACACIA TECHNOLOGIES GROUP DEPENDS IN PART UPON OUR ABILITY TO RETAIN THE BEST LEGAL COUNSEL TO REPRESENT US IN PATENT ENFORCEMENT LITIGATION.
In addition, the success of the Acacia Technologies group depends upon our ability to retain the best legal counsel to prosecute patent infringement litigation. As our patent enforcement actions increase, it will become more difficult to find the best legal counsel to handle all of our cases because many of the best law firms may have a conflict of interest that prevents its representation of our company.
THE ACACIA TECHNOLOGIES GROUP, IN CERTAIN CIRCUMSTANCES, RELIES ON REPRESENTATIONS, WARRANTIES AND OPINIONS MADE BY THIRD PARTIES, THAT IF DETERMINED TO BE FALSE OR INACCURATE, MAY EXPOSE THE ACACIA TECHNOLOGIES GROUP TO CERTAIN LIABILITIES THAT COULD BE MATERIAL.
From time to time, the Acacia Technologies group may rely upon representations and warranties made by third parties from whom the Acacia Technologies group acquires patents or the exclusive rights to license and enforce patents. We also may rely upon the opinions of purported experts. In certain instances, we may not have the opportunity to independently investigate and verify the facts upon which such representations, warranties, and opinions are made. By relying on these representations, warranties and opinions, companies that are part of the Acacia Technologies group may be exposed to liabilities in connection with the licensing and enforcement of certain patents and patent rights. It is difficult to predict the extent and nature of such liabilities which, in some instances, may be material.
RISKS RELATING TO THE COMBIMATRIX GROUP
The risk factors beginning on this page discuss risks relating to the CombiMatrix group. Because each holder of AR- CombiMatrix stock is also a holder of the common stock of one company, Acacia Research Corporation, the risks associated with the Acacia Technologies group could affect our AR-CombiMatrix stock. As such, we urge you to read carefully the section “Risks Relating to the Acacia Technologies Group” above.
OUR COMBIMATRIX GROUP WILL NOT BE ABLE TO MEET ITS CASH REQUIREMENTS BEYOND THE NEXT 12 MONTHS WITHOUT OBTAINING ADDITIONAL CAPITAL FROM EXTERNAL SOURCES, AND IF IT IS UNABLE TO DO SO, IT MAY NOT BE ABLE TO SUSTAIN OPERATIONS.
As a result of our recent financings with Oppenheimer & Co. and Cornell Capital, the CombiMatrix group’s cash and cash equivalent balances, anticipated cash flows from operations and other external sources of available credit should be sufficient to meet its cash requirements through December 31, 2007. In order for the CombiMatrix group to sustain operations beyond this point, it will be required to obtain capital from external sources. If external financing sources are not available or are inadequate to fund the CombiMatrix group’s operations, it could result in reduced revenues and cash flows from the sales of our CustomArray products and services and/or could jeopardize its ability to launch, market and sell additional products and services necessary to grow and sustain its operations in order to eventually achieve profitability. As a result of the above, the audit opinion on our consolidated financial statements for the CombiMatrix group for the year ended December 31, 2006, includes a qualifying paragraph regarding the CombiMatrix group’s ability to sustain operations, as described in footnote 1 to the consolidated financial statements of the CombiMatrix group included in this report. You should review the additional information about our liquidity and capital resources in the Management's Discussion and Analysis of Financial Condition and Results of Operations section of this Form 10-K.
THE COMBIMATRIX GROUP HAS A HISTORY OF LOSSES AND EXPECT TO INCUR ADDITIONAL LOSSES IN THE FUTURE.
The CombiMatrix group has sustained substantial losses since its inception resulting in consolidated accumulated net losses as of December 31, 2005 and 2006, of $124.6 million and $144.6 million, respectively. The CombiMatrix group may never become profitable, or if it does, it may never be able to sustain profitability. We expect the CombiMatrix group to incur significant research and development, marketing, general and administrative expenses. As a result, we expect the CombiMatrix group to incur losses for the foreseeable future. The CombiMatrix group’s consolidated cash and cash equivalents along with short-term investments totaled $20.3 million and $14.3 million at December 31, 2005 and 2006, respectively.
To date, the CombiMatrix group has relied primarily upon selling equity securities, as well as payments from strategic partners, to generate the funds needed to finance the implementation of its business strategies. We cannot assure you that the CombiMatrix group will not encounter unforeseen difficulties, including the outside influences identified above that may deplete the CombiMatrix group’s capital resources more rapidly than anticipated. As a result, the CombiMatrix group’s subsidiaries may be required to obtain additional financing through bank borrowings, debt or equity financings or otherwise, which would require the CombiMatrix group to make additional investments or face a dilution of its equity interests. Any efforts to seek additional funds could be made through equity, debt or other external financings. Nevertheless, we cannot assure that additional funding will be available on favorable terms, if at all. If we fail to obtain additional funding when needed for the CombiMatrix group, or its subsidiaries, we may not be able to execute our business plans and the business of the CombiMatrix group may suffer.
THE CONTINUED DECLINE IN AR-COMBIMATRIX STOCK PRICE COULD RESULT IN A GOODWILL IMPAIRMENT FOR COMBIMATRIX CORPORATION.
Due to the recent decline in the AR-CombiMatrix stock, the market value of the CombiMatrix group as indicated by the trading of AR-CombiMatrix stock has approximated its book value at times during the fourth quarter of 2006, though currently exceeds its book value by approximately $10 million as of February 26, 2007. Should the AR-CombiMatrix stock continue to decline below its book value and if management concludes that the decline is other than temporary, the CombiMatrix group’s goodwill in the amount of $16.9 million as of December 31, 2006, could be impaired.
WE ARE PLANNING TO REDEEM ALL OF THE ISSUED AND OUTSTANDING SHARES OF AR-COMBIMATRIX STOCK FOR ALL OF THE ISSUED AND OUTSTANDING SHARES OF COMBIMATRIX CORPORATION, AND FOLLOWING THE REDEMPTION, HOLDERS OF AR-COMBIMATRIX STOCK WILL NO LONGER BE STOCKHOLDERS OF ACACIA RESEARCH CORPORATION.
We are planning to redeem all of the issued and outstanding shares of AR-CombiMatrix stock for all of the common stock of CombiMatrix Corporation pursuant to our Amended and Restated Certificate of Incorporation. Holders of AR-CombiMatrix stock will not have an opportunity to vote on the redemption, nor will they have any right to dissent or otherwise be compensated for their shares of AR-CombiMatrix stock. Following the redemption, holders of AR-CombiMatrix stock will no longer be shareholders of Acacia Research Corporation but will own all of the issued and outstanding stock of CombiMatrix Corporation. On the redemption date, CombiMatrix Corporation will hold all of the assets of the CombiMatrix group and will thereafter operate as a separate public company with its common stock registered under the Securities and Exchange Act of 1934.
FOLLOWING THE REDEMPTION OF AR-COMBIMATRIX STOCK FOR THE COMMON STOCK OF COMBIMATRIX CORPORATION, HOLDERS OF THE COMMON STOCK OF COMBIMATRIX CORPORATION WILL BE SUBJECT TO THE FOLLOWING NEW RISKS:
| | · | After the separation, we will be required to raise capital on a stand-alone basis, and we will not have the benefit of Acacia’s consolidated financial strength or size to support our capital needs. |
A substantial portion of the CombiMatrix group’s operations are financed by Acacia’s sales of AR-CombiMatrix stock. After the separation, the CombiMatrix Corporation will be required to raise capital on a stand-alone basis. Although one of the purposes of the separation is to permit the CombiMatrix Corporation to achieve what our management believes is the most appropriate capital structure for our businesses, there can be no assurance that this will be achieved, and the risk therefore exists that the CombiMatrix Corporation may not be able to secure adequate debt or equity financing on desirable terms. If future developments in the capital markets adversely affect the biotechnology industry, the CombiMatrix Corporation will not have the benefit of Acacia’s consolidated financial strength or size to support its capital needs.
| | · | Historical financial information may not be representative of the results of CombiMatrix Corporation as an independent entity, and, therefore, may not be reliable as an indicator of the CombiMatrix Corporation’s historical or future results. |
The historical financial information included in this report regarding the CombiMatrix group may not reflect what the results of operations, financial position and cash flows would have been had the CombiMatrix Corporation been an independent entity for the periods presented. Because the financial information included in this document reflects allocations for services provided to the CombiMatrix group by Acacia, these allocations may not reflect the costs the CombiMatrix Corporation would have incurred for similar or incremental services as an independent entity. In addition, the historical financial information included in this report does not reflect transactions that have occurred since December 31, 2006, or that are expected to occur in connection with the redemption. This historical financial information also may not be reliable as an indicator of future results of the CombiMatrix Corporation following the redemption.
| | · | After the redemption, the common stock of CombiMatrix Corporation may fail to meet the investing guidelines of institutional investors, which may negatively affect the price of its common stock and impair its ability to raise capital through the sale of common stock. |
Some of the holders of AR-CombiMatrix stock are institutional investors bound by various investing guidelines. In some cases companies are selected by institutional investors based on factors such as market capitalization, industry, trading liquidity and financial condition. The separation of CombiMatrix Corporation following the redemption will reduce Acacia’s market capitalization as well as the market capitalization of the CombiMatrix group held by CombiMatrix Corporation. As a result, the common stock that the holders of AR-CombiMatrix stock will receive in the redemption may not meet the investing guidelines of some institutional investors. Consequently, these institutional investors may be required to sell the CombiMatrix Corporation common stock that they receive in the redemption or to sell the AR-CombiMatrix stock prior to the redemption date. A sufficient number of buyers may not be available in the market to absorb these potential sales. Consequently, the stock price of the AR-CombiMatrix stock or the common stock of the CombiMatrix Corporation following the redemption, may fall. Any such decline could impair our ability to raise capital for the CombiMatrix group, or CombiMatrix Corporation’s to raise capital following the redemption, through future sales of common stock.
| | · | Holders of AR-CombiMatrix Stock will incur significant tax liability if the redemption does not qualify for tax free treatment. |
If the redemption does not to qualify as a tax-free split-off under Section 355 of the Internal Revenue Code, then each owner of AR-CombiMatrix common stock that receives shares of common stock of CombiMatrix Corporation in the redemption will be treated as if such stockholder received a taxable distribution in an amount equal to the fair market value of the CombiMatrix common stock received on the date it is received.
| | · | CombiMatrix Corporation will apply to have its common stock traded as a new listing on the Nasdaq Global Market, and if the price of its stock does not meet the minimum requirements for stabilizing above $5.00 per share, the common stock of CombiMatrix Corporation may not be listed on Nasdaq. |
Although AR-CombiMatrix stock is currently traded on Nasdaq, CombiMatrix Corporation will be subject to the new listing requirements of Nasdaq or another national exchange. As a result, CombiMatrix Corporation will be submitting a new listing application for its stock to be traded on the Nasdaq Global Market. There can be no assurance that its stock will be accepted for listing on Nasdaq. Its common stock will be subject to the new listing requirements of Nasdaq that include a requirement that the stock initially trade above $5.00 per share. If the price of its common stock following the redemption does not stabilize at $5.00 or more per share, its stock may be delisted from Nasdaq. Consequently, holders of AR-CombiMatrix stock cannot be assured of continued listing of its stock on a national exchange following the redemption. If CombiMatrix Corporation’s common stock is not accepted for listing on Nasdaq, its stock will likely be traded on the Over-the-Counter Bulletin Board until it is able to meet the listing requirements of Nasdaq or another national exchange. Failure to maintain a market for its stock on Nasdaq or another national exchange will likely have a negative impact upon the trading price of its stock.
BECAUSE OUR COMBIMATRIX GROUP BUSINESS OPERATIONS ARE SUBJECT TO MANY UNCONTROLLABLE OUTSIDE INFLUENCES, IT MAY NOT SUCCEED.
Our CombiMatrix group's business operations are subject to numerous risks from outside influences, including the following:
| · | TECHNOLOGICAL ADVANCES MAY MAKE OUR COMBIMATRIX GROUP SEMICONDUCTOR BASED ARRAY TECHNOLOGY OBSOLETE OR LESS COMPETITIVE, AND AS A RESULT, OUR REVENUE AND THE VALUE OF OUR ASSETS COULD BECOME OBSOLETE OR LESS COMPETITIVE. |
Our CombiMatrix group products and services are dependent upon our semiconductor based array technology. The semiconductor based array technology is an advancement in conventional arrays that are used for the same purpose. Current array technologies have revolutionized drug discovery and development, and we believe that our CombiMatrix group's array technology provides characteristics, including flexibility, superior cost metrics, and performance, which address certain needs of the life sciences market which are not addressed by conventional arrays and offers the latest in technological advances in this area. Our products and services are substantially dependent upon our ability to offer the latest in semiconductor based array technology in the SNP genotyping, gene expression profiling and proteomic markets. We believe technological advances of conventional arrays and semiconductor based arrays are currently being developed by our existing competition and potential new competitors in the market, including Affymetrix, Inc., Agilent Technologies, Inc., Applera Corporation, Becton, Dickinson and Company, Ciphergen Biosystems, Inc., Gene Logic Inc., Illumina, Inc., Johnson & Johnson, Nanogen, Inc., Orchid Biosciences, Inc., Roche Diagnostics GmbH and Sequenom, Inc. We also expect to face additional competition from new market entrants and consolidation of our existing competitors. Many of the CombiMatrix group's competitors have existing strategic relationships with major pharmaceutical and biotechnology companies, greater commercial experience and substantially greater financial and personnel resources than we do. We expect new competitors to emerge and the intensity of competition to increase in the future. If these companies are able to offer technological advances to conventional arrays or semiconductor based arrays, our products may become less valuable or even obsolete. While we continue to invest resources in research and development to enhance the technology of our products and services, we cannot provide any assurance that our competitors or new competitors will not enter the market with the same or similar technological advances before we are able to do so.
| · | NEW ENVIRONMENTAL REGULATION MAY MATERIALLY INCREASE THE NET LOSSES OF OUR COMBIMATRIX GROUP. |
The CombiMatrix group's operations involve the use, transportation, storage and disposal of hazardous substances, and as a result it is subject to environmental and health and safety laws and regulations. Any changes in these laws and regulations could increase the CombiMatrix group's compliance costs, and as a result, could materially increase the net losses of our CombiMatrix group.
| · | OUR TECHNOLOGIES FACE UNCERTAIN MARKET VALUE. |
Our CombiMatrix group includes the following technologies and products that were recently introduced into the market: CustomArrayTM, DNA Microarray, 12K DNA expression array and related products, Design-on-DemandTM Arrays, and NanoArrayTM technology and our Bench-Top DNA Microarray Synthesizer for CustomArrayTM. These technologies and products have not gained widespread market acceptance, and we cannot provide any assurance that the increase, if any, in market acceptance of these technologies and products will meet or exceed our expectations.
Further, our CombiMatrix group is currently developing the following technologies and products, some of which have not yet been introduced into the market: (a) microarray technology for the detection of biological threat agents, (b) molecular diagnostics drug discovery and development using the CustomArrayTM platform, and (c) additional products for the research and development and diagnostics markets including higher density arrays. The level of market acceptance of these technologies and products will have a significant impact upon our results of operations, and we cannot provide any assurance that the increase, if any, in market acceptance of these technologies and products will meet or exceed our expectations.
| · | THE FOREGOING OUTSIDE INFLUENCES MAY AFFECT OTHER RISK FACTORS DESCRIBED IN THIS ANNUAL REPORT. |
Any one of the foregoing outside influences may cause our company to need additional financing to meet the challenges presented or to compensate for a loss in revenue, and we may not be able to obtain the needed financing. See the heading "If we, or our subsidiaries, encounter unforeseen difficulties and cannot obtain additional funding on favorable terms, our business may suffer" above. Further, any one of the foregoing outside influences affecting the CombiMatrix group could make it less likely that our CombiMatrix group will be able to gain acceptance of its array technology by researchers in the pharmaceutical, biotechnology and academic communities. See the heading "If the CombiMatrix group's new and unproven technology is not used by researchers in the pharmaceutical, biotechnology and academic communities, its business will suffer" below.
THE COMBIMATRIX GROUP MAY HAVE TO ENTER INTO NEW STRATEGIC PARTNERSHIPS TO GENERATE REVENUE CONSISTENT WITH ITS OPERATING HISTORY OF WORKING WITH STRATEGIC PARTNERS SUCH AS ROCHE DIAGNOSTICS GmbH.
In March 2004, the CombiMatrix group completed all phases of its research and development agreement with Roche Diagnostics GmbH (“Roche”). As a result of completing all of its obligations under this agreement and in accordance with the CombiMatrix group's revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized all previously deferred Roche related contract revenues totaling $17,302,000 during the first quarter of 2004. To date, the CombiMatrix group has relied primarily upon selling equity securities, as well as payments from strategic partners, to generate the funds needed to finance the implementation of the CombiMatrix group's business strategies. Prior to 2004, the CombiMatrix
group had been dependent on its arrangements with Roche, and has relied upon payments by Roche and other partners for a majority of its working capital. The CombiMatrix group intends to enter into additional strategic partnerships to develop and commercialize future products. The CombiMatrix group is deploying unproven technologies and continues to develop its commercial products. There can be no assurance that the CombiMatrix group will be able to implement its future plans. Failure by management to achieve its plans would have a material adverse effect on the CombiMatrix group's and Acacia Research Corporation's ability to achieve its intended business objectives.
THE COMBIMATRIX GROUP MAY FAIL TO MEET MARKET EXPECTATIONS BECAUSE OF FLUCTUATIONS IN ITS QUARTERLY OPERATING RESULTS, WHICH COULD CAUSE ITS STOCK PRICE TO DECLINE.
The CombiMatrix group's revenues and operating results have fluctuated in the past and may continue to fluctuate significantly from quarter to quarter in the future. It is possible that in future periods the CombiMatrix group's revenues could fall below the expectations of securities analysts or investors, which could cause the market price of our AR-CombiMatrix stock to decline. The following are among the factors that could cause the CombiMatrix group's operating results to fluctuate significantly from period to period:
| · | its unpredictable revenue sources, as described below; |
| | |
| · | the nature, pricing and timing of the CombiMatrix group's and its competitors' products; |
| | |
| · | changes in the CombiMatrix group's and its competitors' research and development budgets; |
| | |
| · | expenses related to, and the CombiMatrix group's ability to comply with, governmental regulations of its products and processes; and |
| | |
| · | expenses related to, and the results of, patent filings and other proceedings relating to intellectual property rights. |
The CombiMatrix group anticipates significant fixed expenses due in part to its need to continue to invest in product development. It may be unable to adjust its expenditures if revenues in a particular period fail to meet its expectations, which would harm its operating results for that period. As a result of these fluctuations, the CombiMatrix group believes that period-to-period comparisons of the CombiMatrix group's financial results will not necessarily be meaningful, and you should not rely on these comparisons as an indication of its future performance.
THE COMBIMATRIX GROUP'S REVENUES WILL BE UNPREDICTABLE, AND THIS MAY HARM ITS FINANCIAL CONDITION.
The amount and timing of revenues that the CombiMatrix group may realize from its business will be unpredictable because:
| · | whether products and services are commercialized and generate revenues depends, in part, on the efforts and timing of its potential customers; and |
| · | its sales cycles may be lengthy. |
As a result, the CombiMatrix group's revenues may vary significantly from quarter to quarter, which could make its business difficult to manage and cause its quarterly results to be below market expectations. If this happens, the price of the CombiMatrix group's common stock may decline significantly.
TECHNOLOGY COMPANY STOCK PRICES ARE ESPECIALLY VOLATILE, AND THIS VOLATILITY MAY DEPRESS THE PRICE OF OUR AR-COMBIMATRIX STOCK.
The stock market has experienced significant price and volume fluctuations, and the market prices of technology companies, particularly biotechnology companies, has been highly volatile. In addition, our stock has historically experienced greater price fluctuations than the biotechnology index of other Nasdaq listed stock. We believe that various factors may cause the market price of our AR-CombiMatrix stock to fluctuate, perhaps substantially, including, among others, announcements of:
| · | its or its competitors' technological innovations; |
| | |
| · | developments or disputes concerning patents or proprietary rights; |
| | |
| · | supply, manufacturing or distribution disruptions or other similar problems; |
| | |
| · | proposed laws regulating participants in the biotechnology industry; |
| | |
| · | developments in relationships with collaborative partners or customers; |
| | |
| · | its failure to meet or exceed securities analysts' expectations of its financial results; or |
| | |
| · | a change in financial estimates or securities analysts' recommendations. |
In the past, companies that have experienced volatility in the market price of their stock have been the objects of securities class action litigation. If our AR-CombiMatrix stock was the object of securities class action litigation, it could result in substantial costs and a diversion of management's attention and resources, which could materially harm the business and financial results of the CombiMatrix group.
THE COMBIMATRIX GROUP IS DEPLOYING NEW AND UNPROVEN TECHNOLOGIES WHICH MAKES EVALUATION OF ITS BUSINESS AND PROSPECTS DIFFICULT, AND IT MAY BE FORCED TO CEASE OPERATIONS IF IT DOES NOT DEVELOP COMMERCIALLY SUCCESSFUL PRODUCTS.
The CombiMatrix group has not proven its ability to commercialize products on a large scale. In order to successfully commercialize products on a large scale, it will have to make significant investments, including investments in research and development and testing, to demonstrate their technical benefits and cost-effectiveness. Problems frequently encountered in connection with the commercialization of products using new and unproven technologies might limit its ability to develop and commercialize its products. For example, the CombiMatrix group's products may be found to be ineffective, unreliable or otherwise unsatisfactory to potential customers. The CombiMatrix group may experience unforeseen technical complications in the processes it uses to develop, manufacture, customize or receive orders for its products. These complications could materially delay or limit the use of products the CombiMatrix group attempts to commercialize, substantially increase the anticipated cost of its products or prevent it from implementing its processes at appropriate quality and scale levels, thereby causing its business to suffer.
THE COMBIMATRIX GROUP MAY NEED TO RAISE ADDITIONAL CAPITAL IN THE FUTURE, AND IF ADDITIONAL CAPITAL IS NOT AVAILABLE ON ACCEPTABLE TERMS, THE COMBIMATRIX GROUP MAY HAVE TO CURTAIL OR CEASE OPERATIONS.
The CombiMatrix group's future capital requirements will be substantial and will depend on many factors including how quickly it commercializes its products, the progress and scope of its collaborative and independent research and development projects, the filing, prosecution, enforcement and defense of patent claims and the need to obtain regulatory approval for certain products in the United States or elsewhere. Changes may occur that would cause the CombiMatrix group's available capital resources to be consumed significantly sooner than it expects.
The CombiMatrix group may be unable to raise sufficient additional capital on favorable terms or at all. If it fails to do so, it may have to curtail or cease operations or enter into agreements requiring it to relinquish rights to certain technologies, products or markets because it will not have the capital necessary to exploit them.
IF THE COMBIMATRIX GROUP DOES NOT ENTER INTO SUCCESSFUL PARTNERSHIPS AND COLLABORATIONS WITH OTHER COMPANIES, IT MAY NOT BE ABLE TO FULLY DEVELOP ITS TECHNOLOGIES OR PRODUCTS, AND ITS BUSINESS WOULD BE HARMED.
Since the CombiMatrix group does not possess all of the resources necessary to develop and commercialize products that may result from its technologies on a mass scale, it will need either to grow its sales, marketing and support group or make appropriate arrangements with strategic partners to market, sell and support its products. The CombiMatrix group believes that it will have to enter into additional strategic partnerships to develop and commercialize future products. If it does not enter into adequate agreements, or if its existing arrangements or future agreements are not successful, its ability to develop and commercialize products will be impacted negatively, and its revenues will be adversely affected.
THE COMBIMATRIX GROUP HAS LIMITED EXPERIENCE COMMERCIALLY MANUFACTURING, MARKETING OR SELLING ANY OF ITS POTENTIAL PRODUCTS, AND UNLESS IT DEVELOPS THESE CAPABILITIES, IT MAY NOT BE SUCCESSFUL.
Even if the CombiMatrix group is able to develop its products for commercial release on a large-scale, it has limited experience in manufacturing its products in the volumes that will be necessary for it to achieve commercial sales and in marketing or selling its products to potential customers. We cannot assure you that the CombiMatrix group will be able to commercially produce its products on a timely basis, in sufficient quantities or on commercially reasonable terms.
THE COMBIMATRIX GROUP FACES INTENSE COMPETITION AND WE CANNOT ASSURE YOU THAT IT WILL BE SUCCESSFUL COMPETING IN THE MARKET.
The CombiMatrix group expects to compete with companies that design, manufacture and market instruments for analysis of genetic variation and function and other applications using established sequential and parallel testing technologies. The CombiMatrix group is also aware of other biotechnology companies that have or are developing testing technologies for the SNP genotyping, gene expression profiling and proteomic markets. The CombiMatrix group anticipates that it will face increased competition in the future as new companies enter the market with new technologies and its competitors improve their current products.
The markets for the CombiMatrix group's products are characterized by rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition and new product introductions. One or more of the CombiMatrix group's competitors may offer technology superior to those of the CombiMatrix group and render its technology obsolete or uneconomical. Many of its competitors have greater financial and personnel resources and more experience in marketing, sales and research and development than it has. Some of its competitors currently offer arrays with greater density than it does and have rights to intellectual property, such as genomic information or proprietary technology, which provides them with a competitive advantage. If the CombiMatrix group were not able to compete successfully, its business and financial condition would be materially harmed.
IF THE COMBIMATRIX GROUP'S NEW AND UNPROVEN TECHNOLOGY IS NOT USED BY RESEARCHERS IN THE PHARMACEUTICAL, BIOTECHNOLOGY AND ACADEMIC COMMUNITIES, ITS BUSINESS WILL SUFFER.
The CombiMatrix group's products may not gain market acceptance. In that event, it is unlikely that its business will succeed. Biotechnology and pharmaceutical companies and academic research centers have historically analyzed genetic variation and function using a variety of technologies, and many of them have made significant capital investments in existing technologies. Compared to existing technologies, the CombiMatrix group's technologies are new and unproven. In order to be successful, its products must meet the commercial requirements of the biotechnology, pharmaceutical and academic communities as tools for the large-scale analysis of genetic variation and function. Market acceptance will depend on many factors, including:
| · | the development of a market for its tools for the analysis of genetic variation and function, the study of proteins and other purposes; |
| · | the benefits and cost-effectiveness of its products relative to others available in the market; |
| · | its ability to manufacture products in sufficient quantities with acceptable quality and reliability and at an acceptable cost; |
| · | its ability to develop and market additional products and enhancements to existing products that are responsive to the changing needs of its customers; |
| · | the willingness and ability of customers to adopt new technologies requiring capital investments or the reluctance of customers to change technologies in which they have made a significant investment; and |
| · | the willingness of customers to transmit test data and permit the CombiMatrix group to transmit test results over the Internet, which will be a necessary component of its product and services packages unless customers purchase or license its equipment for use in their own facilities. |
IF THE MARKET FOR ANALYSIS OF GENOMIC INFORMATION DOES NOT DEVELOP OR IF GENOMIC INFORMATION IS NOT AVAILABLE TO THE COMBIMATRIX GROUP'S POTENTIAL CUSTOMERS, ITS BUSINESS WILL NOT SUCCEED.
The CombiMatrix group is designing its technology primarily for applications in the biotechnology, pharmaceutical and academic communities. The usefulness of the CombiMatrix group's technology depends in part upon the availability of genomic data. The CombiMatrix group is initially focusing on markets for analysis of genetic variation and function, namely gene expression profiling. These markets are new and emerging, and they may not develop as the CombiMatrix group anticipates, or at all. Also, researchers may not seek or be able to convert raw genomic data into medically valuable information through the analysis of genetic variation and function. If genomic data is not available for use by the CombiMatrix group's customers or if its target markets do not emerge in a timely manner, or at all, demand for its products will not develop as it expects, and it may never become profitable.
IF THIRD-PARTY PAYORS, SUCH AS INSURANCE COMPANIES, MANAGED CARE ORGANIZATIONS AND MEDICARE, DO NOT PROVIDE REIMBURSEMENT FOR OUR PRODUCTS, THEIR COMMERCIAL VIABILITY MAY BE LIMITED.
Many of our diagnostic services are new and payors may choose not to reimburse patients for such tests. Each payor makes its own decision as to whether to establish a policy to reimburse for tests. If we are unable to garner broad payment support for our tests, we may have to ask patients to pay for tests themselves. This may reduce the use and ordering of our tests by physicians, and may limit our ability to fully realize the commercial value of our tests.
OUR PRODUCT DEVELOPMENT EFFORTS MAY BE HINDERED SHOULD WE BE UNABLE TO GAIN ACCESS TO PATIENTS’ TISSUE AND BLOOD SAMPLES.
The development of our diagnostic products requires access to tissue and blood samples from patients who have the diseases we are addressing. Our clinical development relies on our ability to secure access to these samples, as well as information pertaining to their associated clinical outcomes. Access to samples can be difficult since it may involve multiple levels of approval, complex usage rights, privacy rights, among other issues.
IF OUR CURRENT LABORATORY FACILITY BECOMES INOPERABLE OR LOSES CERTIFICATION, WE WILL BE UNABLE TO PERFORM OUR TESTS AND OUR BUSINESS WILL BE HARMED.
Our diagnostic tests are operated out of our CLIA certified laboratory in Irvine, California. Currently, we do not have a second certified laboratory. Should our only laboratory be unable to perform tests, for any reason, our business will be harmed.
WE COULD FACE SUBSTANTIAL LIABILITIES IF WE WERE SUED FOR PRODUCT LIABILITY.
Product liability claims could be filed, if someone were to allege that our product failed to perform as claimed. We may also be subject to liability for errors in the performance of our tests. Product liability claims could be substantial. Though we believe we carry sufficient liability insurance, defense of such a claim could be time consuming and could result in damages that are not covered by our insurance.
THE COMBIMATRIX GROUP'S FUTURE SUCCESS DEPENDS ON THE CONTINUED SERVICE OF ITS ENGINEERING, TECHNICAL AND KEY MANAGEMENT PERSONNEL AND ITS ABILITY TO IDENTIFY, HIRE AND RETAIN ADDITIONAL ENGINEERING, TECHNICAL AND KEY MANAGEMENT PERSONNEL.
There is intense competition for qualified personnel in the CombiMatrix group's industry, particularly for engineers and senior level management. Loss of the services of, or failure to recruit, engineers or other technical and key management personnel could be significantly detrimental to the group and could adversely affect its business and operating results. The CombiMatrix group may not be able to continue to attract and retain engineers or other qualified personnel necessary for the development of its products and business or to replace engineers or other qualified personnel who may leave the group in the future. The CombiMatrix group's anticipated growth is expected to place increased demands on its resources and likely will require the addition of new management personnel.
THE EXPANSION OF THE COMBIMATRIX GROUP'S PRODUCT LINES MAY SUBJECT IT TO REGULATION BY THE UNITED STATES FOOD AND DRUG ADMINISTRATION AND FOREIGN REGULATORY AUTHORITIES, WHICH COULD PREVENT OR DELAY ITS INTRODUCTION OF NEW PRODUCTS.
If the CombiMatrix group manufactures, markets or sells any products for any regulated clinical or diagnostic applications, those products will be subject to extensive governmental regulation as medical devices in the United States by the FDA and in other countries by corresponding foreign regulatory authorities. The process of obtaining and maintaining required regulatory clearances and approvals is lengthy, expensive and uncertain. Products that CombiMatrix Corporation manufactures, markets or sells for research purposes only are not subject to governmental regulations as medical devices or as analyte specific reagents to aid in disease diagnosis. We believe that the CombiMatrix group's success will depend upon commercial sales of improved versions of products, certain of which cannot be marketed in the United States and other regulated markets unless and until the CombiMatrix group obtains clearance or approval from the FDA and its foreign counterparts, as the case may be. Delays or failures in receiving these approvals may limit our ability to benefit from new CombiMatrix group products.
AS THE COMBIMATRIX GROUP'S OPERATIONS EXPAND, ITS COSTS TO COMPLY WITH ENVIRONMENTAL LAWS AND REGULATIONS WILL INCREASE, AND FAILURE TO COMPLY WITH THESE LAWS AND REGULATIONS COULD HARM ITS FINANCIAL RESULTS.
The CombiMatrix group's operations involve the use, transportation, storage and disposal of hazardous substances, and as a result it is subject to environmental and health and safety laws and regulations. As the CombiMatrix group expands its operations, its use of hazardous substances will increase and lead to additional and more stringent requirements. The cost to comply with these and any future environmental and health and safety regulations could be substantial. In addition, the CombiMatrix group's failure to comply with laws and regulations, and any releases of hazardous substances into the environment or at its disposal sites, could expose the CombiMatrix group to substantial liability in the form of fines, penalties, remediation costs and other damages, or could lead to a curtailment or shut down of its operations. These types of events, if they occur, would adversely impact the group's financial results.
THE COMBIMATRIX GROUP'S BUSINESS DEPENDS ON ISSUED AND PENDING PATENTS, AND THE LOSS OF ANY PATENTS OR THE GROUP'S FAILURE TO SECURE THE ISSUANCE OF PATENTS COVERING ELEMENTS OF ITS BUSINESS PROCESSES WOULD MATERIALLY HARM ITS BUSINESS AND FINANCIAL CONDITION.
The CombiMatrix group's success depends on its ability to protect and exploit its intellectual property. The CombiMatrix group currently has five patents issued in the United States, three patents issued in Europe and 87 patent applications pending in the United States, Europe and elsewhere. The patents covering the CombiMatrix group's core technology begin to expire January 5, 2018.
The patent application process before the United States Patent and Trademark Office and other similar agencies in other countries is initially confidential in nature. Patent Applications that are filed outside the United States, however, are published approximately eighteen months after filing. Similarly, patent applications that are filed in the United States will be published approximately eighteen months after filing unless the applicant has opted out of publication and will not file any foreign applications on the same invention. Due to the confidential nature of the patent application process, the CombiMatrix group cannot determine in a timely manner whether patent applications covering technology that competes with its technology have been filed in the United States or other foreign countries or which, if any, will ultimately issue or be granted as enforceable patents. Considering the CombiMatrix group's patent applications and those of others, some of the CombiMatrix group's patent applications may claim compositions, methods or uses that may also be claimed in patent applications filed by others. In some or all of these applications, a determination of priority of inventorship may need to be decided in a proceeding before the United States Patent and Trademark Office or a court. In contrast, in foreign jurisdictions, the first to file on the invention will generally prevail on a priority contest. If the CombiMatrix group is unsuccessful in these invention ownership proceedings, it could be blocked from further developing, commercializing or selling products that fall under the scope of the claims of the patents that issue to others. Regardless of the ultimate outcome, this ownership determination process can be time-consuming and expensive.
ANY INABILITY TO ADEQUATELY PROTECT THE COMBIMATRIX GROUP'S PROPRIETARY TECHNOLOGIES COULD MATERIALLY HARM THE COMBIMATRIX GROUP'S COMPETITIVE POSITION AND FINANCIAL RESULTS.
If the CombiMatrix group does not protect its intellectual property adequately, competitors may be able to use its technologies and erode any competitive advantage that it may have. The laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting their proprietary rights abroad. These problems can be caused by the absence of laws, rules and/or methods for defending intellectual property rights.
The patent positions of companies developing tools for the biotechnology, pharmaceutical and academic communities, including the CombiMatrix group's patent position, generally are uncertain and involve complex legal and factual questions. The CombiMatrix group will be able to protect its proprietary rights from unauthorized use by third parties only to the extent that its proprietary technologies are covered by valid and enforceable patents or are effectively maintained as trade secrets. The CombiMatrix group's existing patents and any future issued or granted patents it obtains may not be sufficiently broad in scope to prevent others from practicing its technologies or from developing competing products. There also is a risk that others may independently develop similar or alternative technologies or designs around the CombiMatrix group's patented technologies. In addition, others may cause reexamination of the CombiMatrix group patents in the United States or may oppose the CombiMatrix group’s patents in Europe, either of which may result in narrower patent claims or cancellation of some or all of the patent claims, or invalidate the CombiMatrix group’s patents during enforcement proceedings, or the CombiMatrix group’s patents may fail to provide it with any competitive advantage. Enforcing the CombiMatrix group's intellectual property rights may be difficult, costly and time-consuming and ultimately may not be successful.
The CombiMatrix group also relies upon trade secret protection for its confidential and proprietary information. While it has taken security measures to protect its proprietary information, these measures may not provide adequate protection for its trade secrets or other proprietary information. The CombiMatrix group seeks to protect its proprietary information by entering into confidentiality and invention disclosure and transfer agreements with employees, collaborators and consultants. Nevertheless, employees, collaborators or consultants may still disclose its proprietary information, and the CombiMatrix group may not be able to meaningfully protect its trade secrets. In addition, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to its trade secrets.
ANY LITIGATION TO PROTECT THE COMBIMATRIX GROUP'S INTELLECTUAL PROPERTY, OR ANY THIRD-PARTY CLAIMS OF INFRINGEMENT, COULD DIVERT SUBSTANTIAL TIME AND MONEY FROM THE COMBIMATRIX GROUP'S BUSINESS AND COULD SHUT DOWN SOME OF ITS OPERATIONS.
The CombiMatrix group's commercial success depends in part on its non-infringement of the patents or proprietary rights of third parties. Many companies developing tools for the biotechnology and pharmaceutical industries use litigation aggressively as a strategy to protect and expand the scope of their intellectual property rights. Accordingly, third parties may assert that the CombiMatrix group is employing their proprietary technology without authorization. In addition, third parties may claim that use of the CombiMatrix group's technologies infringes their current or future patents. The CombiMatrix group could incur substantial costs and the attention of its management and technical personnel could be diverted while defending ourselves against any of these claims. The CombiMatrix group may incur the same liabilities in enforcing its patents against others. The CombiMatrix group has not made any provision in its financial plans for potential intellectual property related litigation, and it may not be able to pursue litigation as aggressively as competitors with substantially greater financial resources.
If parties making infringement claims against the CombiMatrix group are successful, they may be able to obtain injunctive or other equitable relief, which effectively could block the CombiMatrix group's ability to further develop, commercialize and sell products, and could result in the award of substantial damages against it. If the CombiMatrix group is unsuccessful in protecting and expanding the scope of its intellectual property rights, its competitors may be able to develop, commercialize and sell products that compete with it using similar technologies or obtain patents that could effectively block its ability to further develop, commercialize and sell its products. In the event of a successful claim of infringement against the CombiMatrix group, we may be required to pay substantial damages and either discontinue those aspects of its business involving the technology upon which it infringed or obtain one or more licenses from third parties. While the CombiMatrix group may license additional technology in the future, it may not be able to obtain these licenses at a reasonable cost, or at all. In that event, it could encounter delays in product introductions while it attempts to develop alternative methods or products, and such attempts may not be successful. Defense of any lawsuit or failure to obtain any of these licenses could prevent it from commercializing available products.
A FORMER VICE PRESIDENT OF COMBIMATRIX CORPORATION HAS FILED A COMPLAINT AGAINST THE COMPANY WITH THE U.S. DEPARTMENT OF LABOR ALLEGING THAT HE WAS WRONGFULLY TERMINATED.
A former Vice President of CombiMatrix Corporation, following his termination of employment, filed a complaint with the U.S. Department of Labor alleging that his employment was terminated out of fear the former employee would report CombiMatrix Corporation’s failure to disclose certain information to be disclosed to the public. See the section titled “Legal Proceedings” on page 50 of this report. This complaint was filed following a letter to the Acacia Research Corporation board of directors containing the same allegations. Following an internal investigation in conjunction with Acacia’s outside counsel, neither Acacia’s Audit Committee nor outside counsel was able to verify any of the allegations made by the former employee. Nonetheless, in an abundance of caution, the Audit Committee engaged an independent counsel to conduct an investigation of the allegations. The independent counsel found no merit to the allegations. Management does not believe the allegations have any merit, nor does management believe the resolution of this matter will have any material effect upon the financial statements or other information included in this report.
BECAUSE WE HAVE A LIMITED OPERATING HISTORY SELLING PRODUCTS AND SERVICES, WE CANNOT ASSURE THAT OUR OPERATIONS WILL BE PROFITABLE.
The CombiMatrix group commenced operations in 1996 and began commercialization of its CustomArray platform in 2004, and accordingly, have a limited operating history generating revenues from products and services. In addition, we are still developing our product and service offerings and you should consider the CombiMatrix group’s prospects in light of the risks, expenses and difficulties frequently encountered by companies with such limited operating histories. Since the CombiMatrix group has a limited operating history, we cannot assure you that its operations will be profitable or that it will generate sufficient revenues to meet its expenditures and support its activities.
The CombiMatrix group has sustained substantial losses since its inception. If the CombiMatrix group continues to incur operating losses in future periods, it may not have enough money to expand its business and its subsidiary companies’ businesses in the future.
FAILURE TO EFFECTIVELY MANAGE OUR GROWTH COULD PLACE STRAINS ON OUR MANAGERIAL, OPERATIONAL AND FINANCIAL RESOURCES AND COULD ADVERSELY AFFECT OUR BUSINESS AND OPERATING RESULTS.
Our CombiMatrix group’s growth has placed, and is expected to continue to place, a strain on its managerial, operational and financial resources. Further, as its subsidiary companies’ businesses grow, the CombiMatrix group will be required to manage multiple relationships. Any further growth by the CombiMatrix group or its subsidiary companies or an increase in the number of our strategic relationships will increase this strain on the CombiMatrix group’s managerial, operational and financial resources. This strain may inhibit the CombiMatrix group’s ability to achieve the rapid execution necessary to successfully implement its business plan.
OUR FUTURE SUCCESS DEPENDS ON OUR ABILITY TO EXPAND OUR ORGANIZATION TO MATCH THE GROWTH OF OUR SUBSIDIARIES.
As the CombiMatrix group subsidiaries grow, the administrative demands upon its management will grow, and its success will depend upon its ability to meet those demands. These demands include increased accounting, management, legal services, staff support for the CombiMatrix group’s board of directors, and general office services. The CombiMatrix group may need to hire additional qualified personnel to meet these demands, the cost and quality of which is dependent in part upon market factors outside of the CombiMatrix group’s control. Further, the CombiMatrix group will need to effectively manage the training and growth of its staff to maintain an efficient and effective workforce, and its failure to do so could adversely affect its business and operating results.
RISKS RELATING TO OUR CAPITAL STRUCTURE
HOLDERS OF BOTH CLASSES OF OUR STOCK ARE STOCKHOLDERS OF ONE COMPANY, AND THE FINANCIAL PERFORMANCE OF ONE GROUP COULD AFFECT THE OTHER, THUS EXPOSING THE HOLDERS OF EACH GROUP’S STOCK TO THE RISKS OF AN INVESTMENT IN THE ENTIRE COMPANY.
Holders of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock are stockholders of a single company. The CombiMatrix group and the Acacia Technologies group are not separate legal entities. As a result, stockholders will continue to be subject to all of the risks of an investment in Acacia Research Corporation and all of our businesses, assets and liabilities. The issuance of our Acacia Research-CombiMatrix common stock and our Acacia Research-Acacia Technologies common stock and the allocation of assets and liabilities and stockholders’ equity between the CombiMatrix group and the Acacia Technologies group did not result in a distribution or spin-off to stockholders of any of our assets or liabilities and did not affect ownership of our assets or responsibility for our liabilities or those of our subsidiaries. The assets we attribute to the Acacia Technologies group could be subject to the liabilities of the CombiMatrix group, whether such liabilities arise from lawsuits, contracts or indebtedness that we attribute to the other group. If we are unable to satisfy one group’s liabilities out of the assets we attribute to it, we may be required to satisfy those liabilities with assets we have attributed to the other group. However, our business is conducted by our operating subsidiaries. Creditors of one subsidiary may not make claims against the assets of another subsidiary, absent a separate guaranty from the other subsidiaries. None of our subsidiaries currently guaranty the obligations of other subsidiaries.
Financial effects from one group that affect our consolidated results of operations or financial condition could, if significant, affect the results of operations or financial condition of the other group and the market price of the common stock relating to the other group. In addition, net losses of either group and dividends or distributions on, or repurchases of, either class of common stock will reduce the funds we can pay as dividends on each class of common stock under Delaware law. For these reasons, you should read our consolidated financial information with the financial information we provide for each group elsewhere in this Form 10-K.
THE MARKET PRICE OF EITHER CLASS OF OUR COMMON STOCK MAY NOT REFLECT THE SEPARATE PERFORMANCE OF THE GROUP RELATED TO THAT CLASS OF COMMON STOCK.
The market price of our Acacia Research-CombiMatrix common stock or Acacia Research-Acacia Technologies common stock may not reflect the separate performance of the business of the group relating to that class of common stock. The market price of either class of common stock could simply reflect the performance of Acacia Research Corporation as a whole, or the market price of either class of common stock could move independently of the performance of the business of either group. Investors may discount the value of either class of common stock because it is part of a common enterprise rather than a stand-alone company.
THE MARKET PRICE OF EITHER CLASS OF OUR COMMON STOCK MAY BE AFFECTED BY FACTORS THAT DO NOT AFFECT TRADITIONAL COMMON STOCK.
| · | The complex nature of the terms of our Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock may adversely affect the market price of either class of common stock. |
The complex nature of the terms of our two classes of common stock, such as the convertibility of Acacia Research-CombiMatrix common stock into Acacia Research-Acacia Technologies common stock, or vice versa, and the potential difficulties investors may have understanding these terms, may adversely affect the market price of either class of common stock.
| · | The market price of our Acacia Research-Acacia Technologies common stock may be adversely affected by the fact that holders have limited legal interests in the group relating to the class of common stock. |
For example, as described in greater detail in the subsequent risk factors, holders of either class of common stock generally do not have separate class voting rights with respect to significant matters affecting either group. In addition, upon our liquidation or dissolution, holders of either class of common stock will not have specific rights to the assets of the group relating to the class of common stock held and will not be entitled to receive proceeds that are proportional to the relative performance of that group. The voting rights of the Acacia Research-Acacia Technologies common stock fluctuates based upon the relative market prices of the Acacia Research-CombiMatrix common stock and the Acacia Research-Acacia Technologies common stock. The record date for our last stockholder meeting was March 27, 2006, and holders of Acacia Research-Acacia Technologies common stock had 4.043 votes per share, and holders of Acacia Research-CombiMatrix common stock had one vote per share.
| · | The market price of our Acacia Research-Acacia Technologies common stock may be adversely affected by events involving the CombiMatrix group or the performance of the Acacia Research-CombiMatrix common stock. |
Events, such as earnings announcements or other developments concerning one group that the market does not view favorably and which thus adversely affect the market price of the class of common stock relating to that group, may adversely affect the market price of the class of common stock relating to the other group. Because both classes of common stock are common stock of Acacia Research Corporation, an adverse market reaction to one class of common stock may, by association, cause an adverse reaction to the other class of common stock. This reaction may occur even if the triggering event was not material to us as a whole.
THE HOLDERS OF ACACIA RESEARCH-COMBIMATRIX COMMON STOCK AND THE HOLDERS OF ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK HAVE ONLY LIMITED SEPARATE STOCKHOLDER RIGHTS.
Holders of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock have the rights customarily held by common stockholders. They also have these specific rights related to their corresponding group:
| · | certain rights with regard to dividends and liquidation; |
| · | requirements for a mandatory dividend, redemption or conversion upon the disposition of all or substantially all of the assets of their corresponding group; |
| · | a right to vote on matters as a separate voting class in the limited circumstances provided under Delaware law, by stock exchange rules or as determined by our board of directors (such as an amendment of our certificate of incorporation that changes the rights, privileges or preferences of the class of stock held by such stockholders); and |
| · | we will not hold separate stockholder meetings for holders of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock. |
THE HOLDERS OF ACACIA RESEARCH-COMBIMATRIX COMMON STOCK AND THE HOLDERS OF ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK WILL HAVE CERTAIN LIMITS ON THEIR RESPECTIVE VOTING POWERS.
| · | Group common stock with a majority of voting power can control voting outcomes. |
The holders of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock will vote together as a single class, except in limited circumstances. If a separate vote on a matter by the holders of either our Acacia Research-CombiMatrix common stock or our Acacia Research-Acacia Technologies common stock is not required under Delaware law or by stock exchange rules, and if our board of directors does not require a separate vote, either class of common stock that is entitled to more than the number of votes required to approve such matter could control the outcome of such vote - even if the matter involves a divergence or conflict of the interests between the holders of our Acacia Research-CombiMatrix common stock and our Acacia Research-Acacia Technologies common stock . In addition, if the holders of common stock having a majority of the voting power of all shares of common stock outstanding approve a merger, the terms of which did not require separate class voting under stock exchange rules, then the merger could be consummated - even if the holders of a majority of either class of common stock were to vote against the merger.
The last time we determined the floating voting power of our Acacia Research-Acacia Technologies common stock was at our last annual meeting on May 16, 2006, and our record date for voting purposes was March 27, 2006. As of March 27, 2006, 27,766,909 shares of Acacia Research-Acacia Technologies common stock were issued and outstanding. As of March 27, 2006, 38,992,402 shares of Acacia Research-CombiMatrix common stock were issued and outstanding. For purposes of the annual meeting, each holder of Acacia Research-Acacia Technologies common stock had 4.043 votes per share, and each holder of Acacia Research-CombiMatrix common stock had one vote per share. Collectively, holders of Acacia Research-Acacia Technologies common stock had a total of 112,261,613 potential votes, or approximately 74% of the total available votes. As the number of issued and outstanding shares of each class of stock increases, and as the market price of each class of stock fluctuates, the relative voting power between the classes of stock could change significantly.
| · | Group common stock with less than majority voting power can block action if a class vote is required. |
If Delaware law, stock exchange rules or our board of directors requires a separate vote on a matter by the holders of either our Acacia Research-CombiMatrix common stock or our Acacia Research-Acacia Technologies common stock, such as a proposal to amend the terms of one class of stock, those holders could prevent approval of the matter, even if the holders of a majority of the total number of votes cast or entitled to be cast, voting together as a class, were to vote in favor of it.
| · | Holders of only one class of common stock cannot ensure that their voting power will be sufficient to protect their interests. |
Since the relative voting power per share of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock will fluctuate based on the market values of the two classes of common stock, the relative voting power of a class of common stock could decrease. As a result, holders of shares of only one of the two classes of common stock cannot ensure that their voting power will be sufficient to protect their interests.
OUR RESTATED CERTIFICATE OF INCORPORATION MAY BE AMENDED TO INCREASE OR DECREASE THE AUTHORIZED SHARES OF EITHER CLASS OF COMMON STOCK WITHOUT THE APPROVAL OF EACH CLASS VOTING SEPARATELY.
Our restated certificate of incorporation provides that an amendment to our restated certificate to increase or decrease the number of authorized shares of either class of common stock will require the approval of the holders of a majority of the voting power of all shares of common stock, voting together as a single class, and will not require the approval of each class of stock voting as a separate class. Accordingly, if the holders of one class of common stock hold a majority of the voting power of all shares of common stock, then that majority could approve an amendment to our restated certificate to increase or decrease the authorized shares of stock of either class without the approval of the holders of the minority class of stock.
STOCKHOLDERS MAY NOT HAVE ANY REMEDIES FOR BREACH OF FIDUCIARY DUTIES IF ANY ACTION BY OUR DIRECTORS OR OFFICERS HAS A DISADVANTAGEOUS EFFECT ON EITHER CLASS OF COMMON STOCK.
Stockholders may not have any remedies if any action or decision of our directors and officers has a disadvantageous effect on either class of common stock compared to the other class of common stock. We are not aware of any legal precedent under Delaware law involving the fiduciary duties of directors and officers of corporations having two classes of common stock, or separate classes or series of capital stock, the rights of which, like our Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock, are defined by reference to separate businesses of the corporation.
Principles of Delaware law established in cases involving differing treatment of two classes of capital stock or two groups of holders of the same class of capital stock provide that a board of directors owes an equal duty to all stockholders regardless of class or series. Under these principles of Delaware law and the related principle known as the “business judgment rule,” absent abuse of discretion, a good faith business decision made by a disinterested and adequately informed board of directors, board of directors’ committee or officer with respect to any matter having different effects on holders of Acacia Research-CombiMatrix common stock and holders of Acacia Research-Acacia Technologies common stock would be a defense to any challenge to such determination made by or on behalf of the holders of either class of common stock.
NUMEROUS POTENTIAL CONFLICTS OF INTERESTS EXIST BETWEEN OUR ACACIA RESEARCH-COMBIMATRIX COMMON STOCK AND OUR ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK WHICH MAY BE DIFFICULT TO RESOLVE BY OUR BOARD OR WHICH MAY BE RESOLVED ADVERSELY TO ONE OF THE CLASSES.
The existence of separate classes of common stock could give rise to occasions when the interests of the holders of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock diverge or conflict. Examples include determinations by our directors or officers to:
| · | pay or omit the payment of dividends on Acacia Research-CombiMatrix common stock or Acacia Research-Acacia Technologies common stock ; |
| | |
| · | allocate consideration to be received by holders of each of the classes of common stock in connection with a merger or consolidation involving Acacia Research Corporation; |
| | |
| · | convert one class of common stock into shares of the other; |
| · | approve certain dispositions of the assets of either group; |
| | |
| · | allocate the proceeds of future issuances of our stock either to the Acacia Technologies group or the CombiMatrix group; |
| | |
| · | allocate corporate opportunities between the groups; |
| | |
| · | make other operational and financial decisions with respect to one group that could be considered detrimental to the other group; and |
| | |
| · | Acacia Technology group may seek to license and enforce its patented technologies against companies that have business relationships or potential business relationships with CombiMatrix group. |
When making decisions with regard to matters that create potential diverging or conflicting interests, our directors and officers will act in accordance with their fiduciary duties, the terms of our restated certificate of incorporation, and, to the extent applicable, our management and allocation policies.
THE PERFORMANCE OF ONE GROUP OR THE DIVIDENDS PAID TO ONE GROUP MAY ADVERSELY AFFECT THE DIVIDENDS AVAILABLE FOR THE OTHER GROUP.
Our board of directors currently has no intention to pay dividends on our Acacia Research-CombiMatrix common stock or our Acacia Research-Acacia Technologies common stock. Determinations as to future dividends on our Acacia Research-CombiMatrix common stock and our Acacia Research-Acacia Technologies common stock will be based primarily on the financial condition, results of operations and business requirements of the relevant group and Acacia Research Corporation as a whole. Subject to the limitations referred to below, our board of directors has the authority to declare and pay dividends on our Acacia Research-CombiMatrix common stock and our Acacia Research-Acacia Technologies common stock in any amount and could, in its sole discretion, declare and pay dividends exclusively on our Acacia Research-CombiMatrix common stock, exclusively on our Acacia Research-Acacia Technologies common stock, or on both, in equal or unequal amounts. Our board of directors will not be required to consider the amount of dividends previously declared on each class, the respective voting or liquidation rights of each class or any other factor.
The performance of one group may cause our board of directors to pay more or less dividends on the common stock relating to the other group than if that other group was a stand-alone company. In addition, Delaware law and our restated certificate of incorporation impose limitations on the amount of dividends which may be paid on each class of common stock.
PROCEEDS OF MERGERS OR CONSOLIDATIONS MAY BE ALLOCATED UNFAVORABLY.
Our restated certificate of incorporation does not contain any provisions governing how consideration to be received by holders of common stock in connection with a merger or consolidation involving Acacia Research Corporation is to be allocated among holders of each class of common stock. Our board of directors will determine the percentage of the consideration to be allocated to holders of each class of common stock in any such transaction. Such percentage may be materially more or less than that which might have been allocated to such holders had our board of directors chosen a different method of allocation.
HOLDERS OF EITHER CLASS OF COMMON STOCK MAY BE ADVERSELY AFFECTED BY A CONVERSION OF GROUP COMMON STOCK.
Our board of directors could, in its sole discretion and without stockholder approval, determine to convert shares of Acacia Research-Acacia Technologies common stock into shares of Acacia Research-CombiMatrix common stock, or vice versa, at a time when either or both classes of common stock may be considered to be overvalued or undervalued. Any such conversion would dilute the interests in Acacia Research Corporation of the holders of the class of common stock being issued in the conversion. It could also give holders of shares of the class of common stock converted a greater or lesser premium than any premium that might be paid by a third-party buyer of all or substantially all of the assets of the group whose stock is converted.
HOLDERS OF EITHER CLASS OF COMMON STOCK COULD BE ADVERSELY AFFECTED BY A DISPOSITION OF THE ASSETS ATTRIBUTED TO THEIR RESPECTIVE GROUPS.
Our board of directors could, in its sole discretion and without stockholder approval, determine to dispose of all or substantially all the assets of a group. If a disposition of group assets occurs at a time when those assets are considered undervalued, then holders of that group’s stock would receive less consideration than they could have received had the assets been disposed of at a time when they had a higher value.
PROCEEDS OF FUTURE ISSUANCES OF OUR STOCK COULD BE ATTRIBUTED UNFAVORABLY.
We may in the future issue a new class of stock, such as a class of preferred stock, or additional shares of Acacia Research-CombiMatrix common stock or Acacia Research-Acacia Technologies common stock. Proceeds from any future issuance of any class of stock would be attributed among the CombiMatrix group or the Acacia Technologies group as determined by our board of directors. There is no requirement that the proceeds from an issuance of Acacia Research-CombiMatrix common stock or Acacia Research-Acacia Technologies common stock be attributed to the corresponding group. Such allocations might be materially more or less for the respective groups than what might have been attributed had our board of directors chosen a different allocation method. Also, any designated preferred class may be designed to reflect the performance of Acacia Research Corporation as a whole, rather than the performance of the CombiMatrix group or the Acacia Technologies group.
ALLOCATION OF CORPORATE OPPORTUNITIES COULD FAVOR ONE GROUP OVER ANOTHER.
Our board of directors may be required to allocate corporate opportunities between the groups. In some cases, our directors could determine that a corporate opportunity, such as a business that we are acquiring, should be shared by the groups. Any such decisions could favor one group at the expense of the other.
OTHER OPERATIONAL AND FINANCIAL DECISIONS WHICH MAY FAVOR ONE GROUP OVER THE OTHER.
Our board of directors or our senior officers will review other operational and financial matters affecting the CombiMatrix group and the Acacia Technologies group, including the allocation of financing resources and capital, technology and know-how and corporate overhead, taxes, debt, interest and other matters. Any decision of our board of directors or our senior officers in these matters could favor one group at the expense of the other.
OUR BOARD OF DIRECTORS MAY CHANGE OUR MANAGEMENT AND ALLOCATION POLICIES WITHOUT STOCKHOLDER APPROVAL TO THE DETRIMENT OF EITHER GROUP.
Our board of directors may modify or rescind our policies with respect to the allocation of corporate overhead, taxes, debt, interest and other matters, or may adopt additional policies, in its sole discretion without stockholder approval. A decision to modify or rescind these policies, or adopt additional policies could have different effects on holders of either class of common stock or could result in a benefit or detriment to one class of stockholders compared to the other class. Our board of directors will make any such decision in accordance with its good faith business judgment that the decision is in the best interests of Acacia Research Corporation and all of our stockholders as a whole.
EITHER GROUP MAY FINANCE THE OTHER GROUP ON TERMS UNFAVORABLE TO ONE OF THE GROUPS.
We may transfer cash and other property between groups to finance their business activities. The group providing the financing will be subject to the risks relating to the group receiving the financing. We will account for those transfers generally as a short-term or long-term loan between groups or as a repayment of a previous borrowing.
THERE ARE LIMITS ON THE CONSIDERATION WHICH MAY BE RECEIVED BY THE STOCKHOLDERS IN THE EVENT OF THE DISPOSITION OF ASSETS OF A GROUP.
Our restated certificate of incorporation provides that if a disposition of all or substantially all of the properties and assets of either group occurs, we must, subject to certain exceptions:
| · | distribute through a dividend or redemption to holders of the class of common stock relating to such group an amount equal to the net proceeds of such disposition; or |
| · | convert at a 10% premium such common stock into shares of the class of common stock relating to the other group. |
If the group subject to the disposition were a separate, independent company and its shares were acquired by another person, certain costs of that disposition, including corporate level taxes, might not be payable in connection with that acquisition. As a result, stockholders of the separate, independent company might receive a greater amount than the net proceeds that would be received by holders of the class of common stock relating to that group if the assets of such group were sold. In addition, we cannot assure you that the net proceeds per share of the common stock relating to that group will be equal to or more than the market value per share of such common stock prior to or after announcement of a disposition.
The term “substantially all of the properties and assets” of a group is subject to potentially conflicting interpretations. Resolution of such a dispute could adversely impact the holders of either the class of common stock related to the assets being disposed or the holders of the other class because the consideration, if any, to be received by the holders of the class related to the disposed assets may depend on whether the disposition involved “substantially all” of the properties and assets of that class.
HOLDERS OF EITHER CLASS OF COMMON STOCK MAY BE ADVERSELY AFFECTED BY A REDEMPTION OF THEIR COMMON STOCK.
We are entitled to redeem the outstanding common stock relating to a group when all or substantially all of that group’s assets are sold. We can redeem the assets for cash, securities, a combination of cash and securities or other property at fair value. A disposition-related redemption could occur when the assets being disposed of are considered undervalued. If that were the case, the holders of our common stock related to that group would receive less consideration for their shares than they may deem reasonable.
We can also redeem on a pro rata basis all of the outstanding shares of a group’s common stock for shares of the common stock of one or more of our wholly owned subsidiaries. If this were to occur, the holders of the redeemed class of common stock would no longer have stockholder voting rights in Acacia Research Corporation or any other benefits to be derived from holding a class of stock in Acacia Research Corporation. In addition, if the outstanding shares of a class of our common stock are redeemed for shares that are not publicly traded, the holders of such redeemed stock will no longer be able to publicly trade their shares and accordingly their investment will be substantially less liquid.
OUR CAPITAL STRUCTURE AND THE VARIABLE VOTE PER SHARE COULD ENABLE A POTENTIAL ACQUIRER TO TAKE CONTROL OF OUR COMPANY THROUGH THE ACQUISITION OF ONLY ONE OF THE CLASSES OF OUR COMMON STOCK.
A potential acquirer could acquire control of Acacia Research Corporation by acquiring shares of common stock having a majority of the voting power of all shares of common stock outstanding. Such a majority could be obtained by acquiring a sufficient number of shares of both classes of common stock or, if one class of common stock has a majority of such voting power, only shares of that class. Currently, our Acacia Research-Acacia Technologies common stock has a majority of the voting power. As a result, currently, it might be possible for an acquirer to obtain control of Acacia Research Corporation by purchasing only shares of Acacia Research-Acacia Technologies common stock.
DECISIONS BY DIRECTORS AND OFFICERS THAT AFFECT DIFFERENTLY ONE CLASS OF OUR COMMON STOCK COMPARED TO THE OTHER COULD ADVERSELY AFFECT THE MARKET VALUE OF EITHER OR BOTH OF THE CLASSES OF OUR COMMON STOCK.
The relative voting power per share of our Acacia Research-CombiMatrix common stock and our Acacia Research-Acacia Technologies common stock and the number of shares of one class of common stock issuable upon the conversion of the other class of common stock will vary depending upon the relative market values of our Acacia Research-CombiMatrix common stock and our Acacia Research-Acacia Technologies common stock. The market value of either or both classes of common stock could be affected by market reaction to decisions by our board of directors or our management that investors perceive to affect differently one class of common stock compared to the other. These decisions could involve changes to our management and allocation policies, allocations of corporate opportunities and financing resources between groups, and changes in dividend policies.
INVESTORS MAY NOT VALUE OUR ACACIA RESEARCH-COMBIMATRIX COMMON STOCK AND OUR ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK BASED ON GROUP FINANCIAL INFORMATION AND POLICIES.
We cannot assure you that investors will value our Acacia Research-CombiMatrix common stock and our Acacia Research-Acacia Technologies common stock based on the reported financial results and prospects of the separate groups or the dividend policies established by our board of directors with respect to those groups. Holders of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock will continue to be common stockholders of Acacia Research Corporation subject to all the risks associated with an investment in Acacia Research Corporation as a whole. Additionally, the separate stockholder rights related to each group are limited and relate to events that may never occur, such as dividend and liquidation rights and the disposition of all or substantially all of the assets of a group. Accordingly, investors may discount the value of Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock because both groups are part of a common enterprise rather than a stand-alone entity and each class of stock has limited separate stockholder rights.
HOLDERS OF ACACIA RESEARCH-COMBIMATRIX COMMON STOCK AND ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK MAY NOT RECEIVE A PREMIUM FROM AN INVESTOR ACQUIRING CONTROL OF THEIR RESPECTIVE CLASSES OF STOCK.
Control of Acacia Research-CombiMatrix common stock or Acacia Research-Acacia Technologies common stock may not provide control of Acacia Research Corporation as a whole. Accordingly, unlike many acquisition transactions, holders of Acacia Research-CombiMatrix common stock and AR-Technologies stock may not receive a controlling interest premium from an investor acquiring control of their respective classes of stock.
THERE ARE CERTAIN PROVISIONS IN OUR TWO-CLASS CAPITAL STRUCTURE THAT COULD HAVE ANTI-TAKEOVER EFFECTS.
The existence of the two classes of common stock could, under certain circumstances, prevent stockholders from profiting from an increase in the market value of their shares as a result of a change in control of Acacia Research Corporation by delaying or preventing such change in control. The existence of two classes of common stock could present complexities and could, in certain circumstances, pose obstacles, financial and otherwise, to an acquiring person. We could, in the sole discretion of our board of directors and without stockholder approval, exercise the right to convert the shares of one class of common stock into shares of the other at a 10% premium over their respective average market values. This conversion could result in additional dilution to persons seeking control of Acacia Research Corporation.
Our board of directors could issue shares of preferred stock or common stock that could be used to create voting or other impediments to discourage persons seeking to gain control of Acacia Research Corporation, and preferred stock could also be privately placed with purchasers favorable to our board of directors in opposing such action.
Item 1B. UNRESOLVED STAFF COMMENTS
None
Item 2. PROPERTIES
Acacia Research Corporation leases approximately 14,883 square feet of office space in Newport Beach, California, under a lease agreement that expires in February 2012.
Prior to February 1, 2007, our wholly owned subsidiary, CombiMatrix Corporation leased office and laboratory space totaling 90,111 square feet located in Mukilteo, Washington, under a lease agreement that expired in October 2008. On February 1, 2007, CombiMatrix Corporation entered into an amendment to its Mukilteo, Washington lease that reduces its office and laboratory space to 30,724 square feet and extends its lease term to October 2010. However, under the terms of the lease amendment, CombiMatrix Corporation is able to terminate the lease as of the original termination date of October 31, 2008, if notice is provided to the landlord by July 31, 2008. CombiMatrix Molecular Diagnostics leases approximately 3,500 square feet in Irvine, California under a lease agreement that expires in August 2007.
Presently, we are not seeking any additional facilities.
Item 3. LEGAL PROCEEDINGS
General. In the ordinary course of business, we are the subject of, or party to, various pending or threatened legal actions, including various counterclaims in connection with our intellectual property enforcement activities. We believe that any liability arising from these actions will not have a material adverse effect on our financial position, results of operations or cash flows.
Acacia Technologies Group. Companies comprising the Acacia Technologies group are often required to engage in litigation to enforce their patents and patent rights. A summary of patent enforcement related litigation is provided at Item 1. “Acacia Technologies group - Patent Enforcement Litigation.”
CombiMatrix Group. On or about December 6, 2006, Mr. Jeffrey Oster filed a complaint against CombiMatrix Corporation, Acacia Research Corporation, Amit Kumar and Brooke Anderson before the U.S. Department of Labor, alleging discriminatory employment practices in violation of Section 806 of the Corporate and Criminal Fraud Accountability Act of 2002, Title VIII of the Sarbanes-Oxley Act of 2002. Mr. Oster alleges that he made complaints to his superiors regarding two separate circumstances in which he felt that Acacia Research Corporation and CombiMatrix Corporation violated federal securities laws. He alleges that within two or three weeks following his complaints that CombiMatrix Corporation installed tracking software on its computer used by Mr. Oster for the purpose of finding cause to terminate him. He also claims his responsibilities were gradually stripped away until he was terminated. He alleges these actions were done in retaliation against his complaints of violations of federal securities laws. Mr. Oster is seeking the following remedies: (a) back pay, (b) front pay or severance pay and benefits in lieu of reinstatement, (c) prejudgment interest, (d) attorneys fees, (e) additional monetary damages to compensate him for adverse tax consequences, and (e) additional relief that may be determined appropriate or just. No specific amount of damages is being sought. The complaint is under review by the Department of Labor. We intend to vigorously defend against this action.
Management does not believe the allegations made by Mr. Oster in the complaint have any merit, nor does management believe the resolution of this matter will have any material affect on our financial position, results of operations or cash flows. This complaint was filed following a letter to the board of directors of Acacia Research Corporation containing the same allegations. Following an internal investigation in conjunction with Acacia Research Corporation’s outside counsel, neither Acacia’s Audit Committee nor outside counsel was able to verify any of the allegations made by the former employee. Nonetheless, in an abundance of caution, the Audit Committee engaged an independent counsel to conduct an investigation of the allegations. The independent counsel found no merit to the allegations.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
None.
PART II
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Recent Market Prices
Acacia Research Corporation’s two classes of common stock, Acacia Research-CombiMatrix common stock and Acacia Research-Acacia Technologies common stock commenced trading on the Nasdaq Stock Market on December 16, 2002. The two classes of common stock were created as a result of Acacia Research Corporation’s recapitalization that was approved by Acacia Research Corporation’s stockholders on December 11, 2002. The two classes of stock replaced Acacia Research Corporation’s common stock formerly traded on the Nasdaq stock market under the symbol ACRI. Acacia Research-Acacia Technologies common stock and Acacia Research-CombiMatrix common stock are listed on the Nasdaq Stock Market LLC under the symbols “ACTG” and “CBMX,” respectively. Acacia Research-Acacia Technologies stock is intended to reflect the performance of Acacia Research Corporation’s Acacia Technologies group, and Acacia Research-CombiMatrix stock is intended to reflect the performance of Acacia Research Corporation’s CombiMatrix group.
Holders of Acacia Research-Acacia Technologies stock and Acacia Research-CombiMatrix stock are stockholders of Acacia Research Corporation. As a result, holders of Acacia Research-Acacia Technologies stock and Acacia Research-CombiMatrix stock continue to be subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. The assets Acacia Research Corporation attributes to one group could be subject to the liabilities of the other group.
The markets for securities such as the two classes of our common stock have historically experienced significant price and volume fluctuations during certain periods. These broad market fluctuations and other factors, such as new product developments and trends in our industry and the investment markets generally, as well as economic conditions and quarterly variations in our results of operations, may adversely affect the market price of our two classes of common stock.
The high and low bid prices for our two classes of common stock as reported by NASDAQ for the periods indicated are as follows. Such prices are inter-dealer prices without retail markups, markdowns or commissions and may not necessarily represent actual transactions.
| | | 2006 | | 2005 | |
| | | Fourth Quarter | | Third Quarter | | Second Quarter | | First Quarter | | Fourth Quarter | | Third Quarter | | Second Quarter | | First Quarter | |
| | | | | | | | | | | | | | | | | | |
| Acacia Research-Acacia Technologies stock: | | | | | | | | | | | | | | | |
| High | | $ | 15.58 | | $ | 14.95 | | $ | 14.65 | | $ | 9.00 | | $ | 7.83 | | $ | 6.25 | | $ | 6.24 | | $ | 6.05 | |
| Low | | $ | 11.05 | | $ | 9.31 | | $ | 8.85 | | $ | 6.65 | | $ | 5.85 | | $ | 4.38 | | $ | 4.45 | | $ | 4.89 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Acacia Research-CombiMatrix stock: | | | | | | | | | | | | | | | | | | | | | | | | | |
| High | | $ | 1.07 | | $ | 1.68 | | $ | 2.75 | | $ | 2.90 | | $ | 2.59 | | $ | 2.60 | | $ | 3.05 | | $ | 4.08 | |
| Low | | $ | 0.70 | | $ | 0.96 | | $ | 1.45 | | $ | 1.34 | | $ | 1.29 | | $ | 1.55 | | $ | 2.15 | | $ | 2.14 | |
STOCK PERFORMANCE GRAPH
This performance graph shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities under that Section and shall not be deemed to be incorporated by reference into any filing of Acacia Research Corporation under the Securities Act of 1933, as amended or the Exchange Act.
The Stock Performance Graph depicted below compares the yearly change in Acacia Research Corporation’s cumulative total stockholder return for the last five fiscal years with the cumulative total return of the Nasdaq Stock Market (U.S.) Index and the Nasdaq Biotech Index.
| | | 2001 | | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | |
| | | | | | | | | | | | | | |
| Acacia Research Corporation | | $ | 100 | | $ | 40 | | $ | 66 | | $ | 68 | | $ | 69 | | $ | 125 | |
| Nasdaq Index | | $ | 100 | | $ | 68 | | $ | 103 | | $ | 112 | | $ | 113 | | $ | 124 | |
| Nasdaq Biotech Index | | $ | 100 | | $ | 55 | | $ | 80 | | $ | 85 | | $ | 87 | | $ | 88 | |
The graph covers the period from December 31, 2001 to December 31, 2006. Cumulative total returns are calculated assuming that $100 was invested on December 31, 2001, in Acacia Research Corporation’s common stock, and in each index, and that all dividends were reinvested. Acacia Research Corporation has not paid or declared any cash dividends on its common stock. On December 13, 2002, each share of Acacia Research Corporation's common stock was converted into one share of AR - Acacia Technologies stock and 0.5582 of a share of AR - CombiMatrix stock. As a result, the graph reflects a composite return for the two classes of Acacia Research Corporation’s common stock for the periods presented. Stockholder returns over the indicated period should not be considered indicative of future stock prices or shareholder returns.
On March 6, 2007, there were approximately 167 owners of record of Acacia Research-Acacia Technologies stock and 134 owners of record of Acacia Research-CombiMatrix stock. The majority of the outstanding shares of Acacia Research-Acacia Technologies stock and Acacia Research-CombiMatrix stock are held by a nominee holder on behalf of an indeterminable number of ultimate beneficial owners.
As described earlier, Acacia Research Corporation’s board of directors approved a plan for our wholly owned subsidiary, CombiMatrix Corporation, to become an independent public company.
Dividend Policy
To date, we have not declared or paid any cash dividends with respect to our capital stock, and the current policy of the board of directors is to retain earnings, if any, to provide for the growth of Acacia Research Corporation. Consequently, we do not expect to pay any cash dividends in the foreseeable future. Further, there can be no assurance that our proposed operations will generate revenues and cash flow needed to declare a cash dividend or that we will have legally available funds to pay dividends.
Equity Compensation Plan Information
The following table provides information with respect to Acacia Research Corporation’s common shares issuable under our equity compensation plans, including subsidiary plans, as of December 31, 2006:
Plan Category | (a) Number of securities to be issued upon exercise of outstanding options | (b) Weighted-Average exercise price of outstanding options | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
Equity compensation plans approved by security holders | | | |
2002 Acacia Technologies Stock Incentive Plan(1) | 5,958,000 | $7.93 | 13,000 |
2002 CombiMatrix Stock Incentive Plan(2) | 8,068,000 | $5.77 | 1,528,000 |
Subtotal(3) | N/A | N/A | N/A |
Equity compensation plans not approved by security holders(4) | | | |
CombiMatrix Molecular Diagnostics 2005 Stock Award Plan (3) | 1,807,000 | $0.31 | 2,193,000 |
Total(4) | N/A | N/A | N/A |
____________________
(1) Our 2002 Acacia Technologies Stock Incentive Plan, as amended, or the Acacia Technologies Plan, allows for the granting of stock options and other awards to eligible individuals, which generally includes directors, officers, employees and consultants. The Acacia Technologies Plan does not segregate the number of securities remaining available for future issuance among stock options and other awards. The shares authorized for future issuance represents the total number of shares available through any combination of stock options or other awards. The share reserve under the Acacia Technologies Plan automatically increases on the first trading day in January each calendar year by an amount equal to three percent (3%) of the total number of shares of our Acacia Research-Acacia Technologies stock outstanding on the last trading day of December in the prior calendar year, but in no event will this annual increase exceed 500,000 shares and in no event will the total number of shares of common stock in the share reserve (as adjusted for all such annual increases) exceed twenty million shares. Column (a) excludes 428,000 in nonvested restricted stock awards outstanding at December 31, 2006. Refer to Note 13 to our consolidated financial statements.
(2) Our 2002 CombiMatrix Stock Incentive Plan, as amended, or the CombiMatrix Plan, allows for the granting of stock options and other awards to eligible individuals, which generally includes directors, officers, employees and consultants. The CombiMatrix Plan does not segregate the number of securities remaining available for future issuance among stock options and other awards. The shares authorized for future issuance represents the total number of shares available through any combination of stock options or other awards. The share reserve under the CombiMatrix Plan automatically increases on the first trading day in January each calendar year by an amount equal to three percent (3%) of the total number of shares of our Acacia Research-CombiMatrix stock outstanding on the last trading day of December in the prior calendar year, but in no event will this annual increase exceed 600,000 shares and in no event will the total number of shares of common stock in the share reserve (as adjusted for all such annual increases) exceed twenty million shares. Refer to Note 13 to our consolidated financial statements.
(3) CombiMatrix Corporation’s wholly owned subsidiary, CMDX, executed the CombiMatrix Molecular Diagnostics 2005 Stock Award Plan with plan provisions and terms similar to that of the CombiMatrix Plan, as described above. Refer to Note 13 to our consolidated financial statements.
(4) Subtotal and total information is not provided because the Acacia Technologies Plan and the CombiMatrix Plan relate to two different classes of our common stock, and common stock issued under the CombiMatrix Molecular Diagnostics 2005 Stock Award Plan relates to stock of our corresponding wholly owned subsidiary.
Item 6. SELECTED FINANCIAL DATA
The consolidating selected balance sheet data as of December 31, 2006 and 2005 and the consolidating selected statement of operations data for the years ended December 31, 2006, 2005 and 2004 set forth below have been derived from our audited consolidated and separate group financial statements included elsewhere herein, and should be read in conjunction with those financial statements (including notes thereto). The consolidating selected balance sheet data as of December 31, 2004, 2003 and 2002 and the consolidating selected statement of operations data for the years ended December 31, 2003 and 2002 have been derived from audited consolidated and separate group financial statements not included herein, but which were previously filed with the SEC.
The AR-Acacia Technologies stock and the AR-CombiMatrix stock are intended to reflect the separate performance of the respective divisions of Acacia Research Corporation, rather than the performance of Acacia Research Corporation as a whole. The chief mechanisms intended to cause the AR-Acacia Technologies stock and the AR-CombiMatrix stock to reflect the financial performance of the respective groups are provisions in our restated certificate of incorporation and common stock policies governing dividends and distributions to each class of stock, which specifically require the allocation of earnings to each class based upon the performance of the two groups determined in accordance with generally accepted accounting principles. Under these provisions, Acacia Research Corporation factors the assets and liabilities and income or losses attributable to the respective groups, determined as described under Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Critical Accounting Policies,” into the determination of the amounts available to pay dividends, if any, on the shares issued for the respective groups and require Acacia Research Corporation to exchange, redeem or distribute a dividend on the stock of a group if all or substantially all of the assets allocated to the respective group are sold to a third party.
The Acacia Technologies group and the CombiMatrix group are not separate legal entities. Holders of AR-Acacia Technologies stock and AR-CombiMatrix stock are stockholders of Acacia Research Corporation. As a result, stockholders continue to be subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. The assets that Acacia Research Corporation attributes to one group could be subject to the liabilities of the other group.
Consolidating Statement of Operations Data(3) |
(In thousands, except share and per share data) |
| | | | | | | | | | | | |
| | | For the Years Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 | |
| | | | | | | | | | | | |
Revenues: | | | | | | | | | | | |
| Acacia Technologies group | | $ | 34,825 | | $ | 19,574 | | $ | 4,284 | | $ | 692 | | $ | 43 | |
| CombiMatrix group | | | 5,740 | | | 8,033 | | | 19,641 | | | 456 | | | 839 | |
Acacia Research Corporation | | $ | 40,565 | | $ | 27,607 | | $ | 23,925 | | $ | 1,148 | | $ | 882 | |
Operating (loss) income | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | (6,980 | ) | $ | (7,246 | ) | $ | (6,055 | ) | $ | (6,013 | ) | $ | (9,865 | ) |
| CombiMatrix group | | | (22,187 | ) | | (13,903 | ) | | 244 | | | (19,349 | ) | | (70,460 | ) |
Acacia Research Corporation | | $ | (29,167 | ) | $ | (21,149 | ) | $ | (5,811 | ) | $ | (25,362 | ) | $ | (80,325 | ) |
Other (expense) income, net: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | 1,524 | | $ | 1,071 | | $ | 471 | | $ | 408 | | $ | (3,503 | ) |
| CombiMatrix group | | | 2,193 | | | 1,335 | | | 330 | | | 214 | | | 392 | |
Acacia Research Corporation | | $ | 3,717 | | $ | 2,406 | | $ | 801 | | $ | 622 | | $ | (3,111 | ) |
(Loss) income from continuing operations before minority interests: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | (5,496 | ) | $ | (6,040 | ) | $ | (5,445 | ) | $ | (5,468 | ) | $ | (12,658 | ) |
| CombiMatrix group | | | (19,960 | ) | | (12,401 | ) | | 710 | | | (18,999 | ) | | (69,921 | ) |
Acacia Research Corporation | | $ | (25,456 | ) | $ | (18,441 | ) | $ | (4,735 | ) | $ | (24,467 | ) | $ | (82,579 | ) |
Minority interests: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | - | | $ | 2 | | $ | 6 | | $ | 17 | | $ | 104 | |
| CombiMatrix group | | | - | | | - | | | - | | | 30 | | | 23,702 | |
Acacia Research Corporation | | $ | - | | $ | 2 | | $ | 6 | | $ | 47 | | $ | 23,806 | |
(Loss) income from continuing operations: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | (5,496 | ) | $ | (6,038 | ) | $ | (5,439 | ) | $ | (5,451 | ) | $ | (12,554 | ) |
| CombiMatrix group | | | (19,960 | ) | | (12,401 | ) | | 710 | | | (18,969 | ) | | (46,219 | ) |
Acacia Research Corporation | | $ | (25,456 | ) | $ | (18,439 | ) | $ | (4,729 | ) | $ | (24,420 | ) | $ | (58,773 | ) |
Loss from discontinued operations: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | - | | $ | (237 | ) | $ | (104 | ) | $ | - | | $ | (200 | ) |
| CombiMatrix group | | | - | | | - | | | - | | | - | | | - | |
Acacia Research Corporation | | $ | - | | $ | (237 | ) | $ | (104 | ) | $ | - | | $ | (200 | ) |
Net (loss) income: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | (5,496 | ) | $ | (6,275 | ) | $ | (5,543 | ) | $ | (5,451 | ) | $ | (12,754 | ) |
| CombiMatrix group | | | (19,960 | ) | | (12,401 | ) | | 710 | | | (18,969 | ) | | (46,219 | ) |
Acacia Research Corporation | | $ | (25,456 | ) | $ | (18,676 | ) | $ | (4,833 | ) | $ | (24,420 | ) | $ | (58,973 | ) |
| | | | | | | | | | | | | | | | | |
Income (loss) per common share - basic and diluted(4): | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | | | | | | | | | | | | | | | | |
| Acacia Research - Acacia Technologies stock | | $ | (0.20 | ) | $ | (0.23 | ) | $ | (0.27 | ) | $ | (0.28 | ) | $ | (0.64 | ) |
| Acacia Research - CombiMatrix stock | | | (0.49 | ) | | (0.37 | ) | | 0.02 | | | (0.76 | ) | | (2.01 | ) |
Loss from discontinued operations | | | | | | | | | | | | | | | | |
| Acacia Research - Acacia Technologies stock | | $ | - | | $ | (0.01 | ) | $ | (0.01 | ) | $ | - | | $ | (0.01 | ) |
| Acacia Research - CombiMatrix stock | | | - | | | - | | | - | | | - | | | - | |
Net income (loss) | | | | | | | | | | | | | | | | |
| Acacia Research - Acacia Technologies stock | | $ | (0.20 | ) | $ | (0.24 | ) | $ | (0.28 | ) | $ | (0.28 | ) | $ | (0.65 | ) |
| Acacia Research - CombiMatrix stock | | | (0.49 | ) | | (0.37 | ) | | 0.02 | | | (0.76 | ) | | (2.01 | ) |
| | | | | | | | | | | | | | | | | |
Weighted average number of common and potential common shares | | | | | | | | | | | | | | | | |
used in computation of income (loss) per common share(1) (4): | | | | | | | | | | | | | | | | |
| Acacia Research - Acacia Technologies stock: | | | | | | | | | | | | | | | | |
| Basic and diluted | | | 27,547,651 | | | 26,630,732 | | | 19,784,883 | | | 19,661,655 | | | 19,640,808 | |
| Acacia Research - CombiMatrix stock: | | | | | | | | | | | | | | | | |
| Basic | | | 40,605,038 | | | 33,678,603 | | | 29,962,596 | | | 24,827,819 | | | 22,950,746 | |
| Diluted | | | 40,605,038 | | | 33,678,603 | | | 30,995,663 | | | 24,827,819 | | | 22,950,746 | |
Consolidating Balance Sheet Data(3) |
(In thousands) &# 160; |
| | | | | | | | | | | | |
| | | At December 31, | |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 | |
| | | | | | | | | | | | |
Total assets: | | | | | | | | | | | |
| Acacia Technologies group | | $ | 65,770 | | $ | 68,893 | | $ | 33,058 | | $ | 39,978 | | $ | 47,212 | |
| CombiMatrix group | | | 44,214 | | | 52,541 | | | 55,388 | | | 50,161 | | | 49,973 | |
| Eliminations | | | (380 | ) | | - | | | (119 | ) | | (99 | ) | | (114 | ) |
Acacia Research Corporation | | $ | 109,604 | | $ | 121,434 | | $ | 88,327 | | $ | 90,040 | | $ | 97,071 | |
Total liabilities(2): | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | 4,276 | | $ | 6,647 | | $ | 3,472 | | $ | 4,188 | | $ | 5,183 | |
| CombiMatrix group | | | 11,399 | | | 7,443 | | | 8,560 | | | 24,424 | | | 13,972 | |
| Eliminations | | | (380 | ) | | - | | | (119 | ) | | (99 | ) | | (114 | ) |
Acacia Research Corporation | | $ | 15,295 | | $ | 14,090 | | $ | 11,913 | | $ | 28,513 | | $ | 19,041 | |
Minority interests: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | - | | $ | 443 | | $ | 778 | | $ | 1,127 | | $ | 1,487 | |
| CombiMatrix group | | | - | | | 4 | | | - | | | - | | | 684 | |
Acacia Research Corporation | | $ | - | | $ | 447 | | $ | 778 | | $ | 1,127 | | $ | 2,171 | |
Redeemable stockholders' equity: | | | | | | | | | | | | | | | | |
| Acacia Technologies group | | $ | 61,494 | | $ | 61,803 | | $ | 28,808 | | $ | 34,663 | | $ | 40,542 | |
| CombiMatrix group | | | 32,815 | | | 45,094 | | | 46,828 | | | 25,737 | | | 35,317 | |
Acacia Research Corporation | | $ | 94,309 | | $ | 106,897 | | $ | 75,636 | | $ | 60,400 | | $ | 75,859 | |
| | | | | | | | | | | | | | | | | |
___________________
(1) Certain potential common shares for the periods shown above have been excluded from the per share calculations because the effect of their inclusion would be anti-dilutive.
(2) Included in total liabilities for 2006, 2005, 2004, 2003 and 2002 are deferred revenues totaling $1,441,000, $1,604,000, $3,959,000, $20,405,000, and $9,172,000 related to the CombiMatrix group, and $360,000, $639,000, $428,000, $1,604,000 and $1,503,000 related to the Acacia Technologies group, respectively.
(3) Refer to Item 7. “Management’s Discussion and Analysis of Financial Condition - Critical Accounting Policies” for a description of allocation policies applied in preparation of the separate group financial statements.
(4) The 2002 share and per share information gives effect to the recapitalization transaction described elsewhere herein as of January 1, 2002. Historical share and per share information for the Acacia Research-Acacia Technologies stock and Acacia Research-CombiMatrix stock is not presented as these classes of securities were not part of Acacia Research Corporation’s capital structure prior to 2002.
Other Factors Affecting Comparability:
| | · | During the year ended December 31, 2000, CombiMatrix Corporation recorded deferred non-cash stock compensation charges aggregating approximately $53.8 million in connection with the granting of stock options. Deferred non-cash stock compensation charges were amortized by the CombiMatrix group over the respective option grant vesting periods, which ranged from one to four years. Amortization of deferred non-cash stock compensation charges totaled $606,000, $1.5 million, and $6.4 million in 2004, 2003, and 2002 respectively. Deferred non-cash stock compensation charges were fully amortized as of December 31, 2004. Effective January 1, 2006, Acacia Research Corporation adopted the provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123R”), which sets forth the accounting requirements for “share-based” compensation payments to employees and non-employee directors and requires that compensation cost relating to share-based payment transactions be recognized in the statement of operations. Refer to Note 2 and Note 13 in the accompanying Acacia Research Corporation consolidated financial statements for additional information, and impact on the 2006 consolidated statement of operations. |
| | · | In June 2006, the Acacia Technologies group recorded a non-cash charge of $297,000, related to the write-off of a patent-related intangible asset. In December 2005, the CombiMatrix group recorded a goodwill impairment charge related to investments in CombiMatrix K.K. and Advanced Material Sciences totaling $565,000. In June 2003 and September 2002, Acacia Research Corporation recorded impairment charges of $207,000 and $2.7 million, respectively, for an other-than-temporary decline in the fair value of a cost method investment, attributed to the Acacia Technologies group. |
| | · | On December 13, 2002, Acacia Research Corporation increased its consolidated ownership interest in CombiMatrix Corporation from 48% to 100%. $17.2 million of the total purchase price of $46.0 million was attributed to acquired in-process research and development, or IPR&D, and was charged to expense in the consolidated statement of operations and comprehensive loss for the year ended December 31, 2002. Amounts allocated to IPR&D were attributed to the CombiMatrix group. |
| | · | On September 30, 2002, CombiMatrix Corporation and Dr. Donald Montgomery, an officer and stockholder of CombiMatrix Corporation, entered into a settlement agreement with Nanogen to settle all pending litigation between the parties. In addition to other terms of the settlement agreement as described elsewhere herein, CombiMatrix Corporation agreed to pay Nanogen $1.0 million and issued 4,016,346 shares, or 17.5% of its outstanding shares post issuance, to Nanogen. The $1.0 million in payments have been expensed in the consolidated statement of operations for the year ended December 31, 2002 under “legal settlement charges.” The issuance of the CombiMatrix Corporation common shares in settlement of the litigation with Nanogen was accounted for as a nonmonetary transaction. Accordingly, included in “legal settlement charges” in the consolidated statements of operations for the year ended December 31, 2002 is a charge in the amount of $17.5 million based on the fair value of the CombiMatrix Corporation common shares issued to Nanogen. Amounts related to the settlement have been attributed to the CombiMatrix group. |
| | · | In March 2004, the CombiMatrix group completed all phases of its research and development agreement with Roche Diagnostics, GmbH (“Roche”). As a result of completing all of its obligations under this agreement and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized all previously deferred Roche related contract revenues totaling $17,302,000 during the first quarter of 2004. |
| | · | As a result of the conclusion of the V-chip patent litigation, the Acacia Technologies group recognized $1,500,000 of V-chip related deferred license fee revenues, $668,000 of V-chip related deferred legal costs, and a non-cash V-chip related goodwill impairment charge of $1.6 million in the third quarter of 2004. The Acacia Technologies group recognized $43,000 and $24.1 million in V-chip license fees in 2002 and 2001, respectively. Refer to Item 1. “Acacia Technologies group - Patent Enforcement Litigation.” |
| | · | On January 28, 2005, Acacia Global Acquisition Corporation consummated the GPH Acquisition. The aggregate purchase consideration was approximately $25.1 million, including $5.0 million of cash, the issuance of 3,938,832 shares of Acacia Research-Acacia Technologies common stock, or AR-Acacia Technologies stock, valued at $19.3 million (net of estimated common stock registration costs of $212,000) and acquisition costs, including registration costs, of $796,000. $25.1 million of the purchase price was allocated to patent related intangible assets acquired, which are being amortized on a straight-line basis over a weighted-average estimated economic useful life of six years. As a result of the GPH Acquisition, and additional patent acquisitions during 2005 and 2006, amortization expense recorded by the Acacia Technologies group was $5.3 million in 2006, as compared to approximately $4.9 million, $501,000, $502,000, and $1.6 million in 2005, 2004, 2003, and 2002, respectively. |
The income statement for the year ended December 31, 2006 and 2005 includes license fee revenues, inventor royalties and contingent legal fees expenses recognized as a result of the licensing activities of certain of the entities acquired in the GPH Acquisition.
| | · | Acacia Research Corporation adopted FASB Staff Position No. 150-5 (“FSP No. 150-5”), effective July 1, 2005, which requires that warrants for shares that are redeemable be classified as liabilities, based on the fair values of the warrants, which are required to be marked to market at each balance sheet date. The warrant liability related to contingently redeemable AR-CombiMatrix stock purchase warrants outstanding at December 31, 2006 and 2005 was $6,732,000 and $1,381,000 respectively. Warrant gains included in 2006 and 2005 consolidated and CombiMatrix group other income totaled $1,754,000 and $812,000, respectively. |
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our financial statements included elsewhere in this Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors including those set forth under item 1A. “Risk Factors” elsewhere herein.
General
Acacia Research Corporation is comprised of two operating groups, the Acacia Technologies group and the CombiMatrix group.
Our intellectual property licensing business, referred to as the “Acacia Technologies group,” develops, acquires, licenses and enforces patented technologies. The Acacia Technologies group generates license fee revenues from the granting of licenses for the use of its patented technologies. The Acacia Technologies group assists patent holders with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, if necessary, with the enforcement against unauthorized users of their patented technologies. The Acacia Technologies group currently owns or controls the rights to 62 patent portfolios, which include U.S. patents, and in certain instances, foreign counterparts, covering technologies used in a wide variety of industries.
Our life sciences business, referred to as the “CombiMatrix group,” is seeking to become a broadly diversified biotechnology business, through the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where its products and services could be utilized. The technologies that the CombiMatrix group has developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. The CombiMatrix group has also developed the capabilities of producing arrays that utilize bacterial artificial chromosomes on its arrays, also enabling genetic analysis. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs.
As described more fully at Part I Item 1. “Business,” of this report, in January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, to become an independent public company (herein referred to as the “CombiMatrix Split-off Transaction”).
The Acacia Technologies group and the CombiMatrix group’s businesses are described more fully in Item 1. “Business,” of this report.
Overview
Acacia Technologies Group
The Acacia Technologies group’s operating activities for 2006 and 2005, were principally focused on the continued development, licensing and enforcement of its patent portfolios, including the continued pursuit of multiple ongoing technology licensing and enforcement programs and the commencement of new technology licensing and enforcement programs. In addition, we continued our focus on business development, including the acquisition of numerous additional patent portfolios and the continued pursuit of opportunities to partner with patent owners and provide Acacia Technologies group’s unique intellectual property licensing, development and enforcement services.
On January 28, 2005, Acacia Global Acquisition Corporation consummated the GPH Acquisition, providing the Acacia Technologies group 100% ownership of companies that controlled 27 patent portfolios, which included 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. The GPH Acquisition, and other patent acquisitions during 2005, 2006 and in future periods, will continue to expand and diversify the Acacia Technologies group’s future revenue generating opportunities, as we continue to build our leadership position in patent licensing.
License fee revenues recognized in 2006 totaled $34.8 million, representing a 78% increase over revenues recognized in 2005 which totaled $19.6 million, and a greater than 100% increase over revenues recognized in 2004, which totaled $4.3 million. The increase in license fee revenues in 2006 and 2005, as compared to 2004, reflects the impact of the increase in patent portfolios controlled by the Acacia Technologies group, including the impact of the GPH Acquisition, and the increase in the number of patent licensing and enforcement programs launched and generating revenues since the end of 2004. Acacia Technologies group management measures and assesses the performance and growth of our patent licensing and enforcement business based on total license fee revenues recognized across all of our technology licensing and enforcement programs on a trailing twelve-month basis. Trailing twelve-month revenues for the Acacia Technologies group totaled $34.8 million as of December 31, 2006, as compared to $35.8 million at September 30, 2006, $34.1 million as of June 30, 2006, $22.4 million as of March 31, 2006, $19.6 million as of December 31, 2005, $12.1 million as of September 30, 2005, $7.6 million as of June 30, 2005 and $5.5 million as of March 31, 2005.
Revenues for 2006 included license fees from 126 new licensing agreements covering 14 of our technology licensing and enforcement programs, as compared to 83 new licensing agreements covering 12 of our technology licensing and enforcement programs in 2005. The Acacia Technologies group generated licensing revenues from 7 new technology licensing and enforcement programs during 2006, and to date, the Acacia Technologies group has generated revenues from 22 of its technology licensing and enforcement programs. License fee revenues for 2006 included fees from the licensing of our DMT® technology, Audio/Video Enhancement and Synchronization technology, Image Resolution Enhancement technology, Credit Card Fraud Control technology, Interstitial Internet Advertising technology, Laptop Connectivity technology, Multi-Dimensional Bar Code technology, Resource Scheduling technology, Dynamic Manufacturing Modeling technology, Product Activation technology, Enhanced Internet Navigation technology, Interactive Data Sharing technology, Pop-Up Advertising technology and Audio Communications Fraud Detection technology.
Operating expenses increased during 2006 and 2005, as compared to 2004, due primarily to the hiring of additional patent licensing, business development and engineering personnel, an increase in patent related legal, research and consulting expenses incurred in connection with the continued growth and expansion of our technology licensing and enforcement business and an increase in corporate, general and administrative costs related to the Acacia Technologies group’s ongoing operations. Inventor royalties expenses and contingent legal fees expenses increased in 2006 and 2005, as compared to 2004, primarily due to the related increase in license fee revenues, as discussed above, and the impact of the varying economic terms related to inventor agreements and contingent legal fee arrangements associated with the revenue generating patent portfolios in each period.
During 2006, the Acacia Technologies group continued to execute its business strategy in the area of patent portfolio acquisitions, including the acquisition of, or the acquisition of the rights to, the following patent portfolios:
| | · | Video Tracking Technology Patent. Acquired rights to a patent relating to improving the performance and user experience of video conferencing technologies. The patent generally relates to technology for automatically tracking and centering the images of videoconference participants. This technology allows webcams and other digital cameras to automatically optimize and align a videoconference participant’s image in each of the other conference participants’ displays. The Video Tracking technology improves desktop videoconferencing and video mail performance. |
| | · | Portable Audio Device Patent. Acquired a patent relating to portable audio recording and playback devices from ESPRO Information Technologies, Ltd., www.espro.com, a provider of electronic audio guiding and interpretation systems. The patented technology relates to products such as certain MP3 players and cell phones using solid state memory that can download compressed audio and record analog audio. |
| | · | Software License Management Patent. Acquired rights to a patent relating to software license management technology. The patent generally relates to technology for monitoring and tracking the use of software applications across a network. This technology can be used to provide a system for managing software license compliance in an enterprise environment as well as metering actual usage levels in a Software-as-a-Service (“SaaS”) environment. |
| | · | Telematics Technology Patents. Acquired patents relating to the rapidly growing field of telematics. Telematics refers to systems used in vehicles that combine wireless communication with GPS tracking and can be used in vehicle navigation systems and mobile fleet management. The patents generally relate to technology for displaying mobile vehicle information on a map. This technology can be used in navigation and fleet management systems that combine wireless communication with GPS tracking and map displays. |
| | · | Micromesh Laminate Patent. Acquired patent relating to technology that can be used in fabrics to maximize moisture transport and increase breathability and is often used in sports apparel. |
| | · | File Locking in Shared Storage Network Patent. Acquired rights to a patent relating to a file locking system for use in shared storage networks such as iSCSI. The use of the patented technology removes a single point of failure for companies migrating existing Storage Area Network (“SAN”) implementations to iSCSI or for those creating new shared storage networks. |
| | · | Remote Video Camera Patents. Acquired patents relating to remote control of video cameras and other devices used in areas such as videoconferencing and surveillance systems. The uses of the patented technology include improved remote management of video camera functions such as pan, tilt, and focus, and improved device control in a networked videoconferencing system. |
| | · | Audio Communications Fraud Detection Patents. Acquired rights to patents relating to the detection of fraud in connection with paid communication services, such as audio communications. The patented technology generally relates to a process for detecting, reducing and preventing fraud in connection with payments for certain communication services, including audio sessions delivered via the telephone, Internet, and other communication networks. |
| | · | Micromirror Digital Display Patents. Acquired a patent portfolio relating to the use of micromirrors to create a digital image in televisions, monitors, and projectors. The patented technology generally relates to techniques for using micromirrors to display a color image having gray scale gradations and is utilized in large screen televisions and projectors. |
| | · | Fluid Flow Control and Monitoring Patent. Acquired rights to patent relating to systems used in the remote control and monitoring of fluid flow, both gas and liquid. This technology can be used in heating/ventilation/air conditioning (“HVAC”), plumbing and other industrial, commercial and residential fluid flow systems. |
| | · | Medical Images Stabilization Patents. This patented technology can be used in stabilizing medical images for interventional procedures such as cardiac catheters and stents, and for diagnostics procedures such as visualization of arterial lesions. |
| | · | Vehicle Magnetic Braking and Motor Technology Patents. These patents generally relate to technology for smooth, reliable braking and acceleration of vehicles on parallel rails. |
| | · | Web Personalization Patents. This patented technology generally relates to technology for learning user preferences and automatically personalizing a user's online experience. The technology is applicable to web sites that use categories plus attributes to identify items, and where individual attributes apply to multiple categories. |
| | · | Wireless Traffic Information Patents. This patented technology generally relates to transmitting, receiving and displaying traffic information on portable handheld and mobile displays. It covers a variety of wireless distribution methods, such as FM radio and satellite, as well as the devices used to display the traffic maps. This technology enables users to identify traffic congestion and can be used with in-vehicle navigation displays and portable handheld units such as cell phones and PDA's. |
| | · | Automated Notification of Tax Return Status Patent. This patented technology generally relates to a system for monitoring the status of a client’s tax return and automatically notifying the client of a change in status. This system can be used by a tax preparation service to monitor the electronic filing of their client’s income tax returns. |
| | · | Aligned Wafer Bonding Patent. This patented technology generally relates to the precision alignment and bonding of micromechanical, electrical and optical structures. This technology can be used for the bonding of surface features in the fabrication of Micro Electromechanical Systems (“MEMS”) and semiconductors. |
| | · | Location-Based Services Patents This patented technology generally relates to locating mobile units, such as cell phones and embedded vehicle radios, within a cellular network and using the position information to provide services to the mobile user. It covers various means of accurately locating a mobile unit, including GPS and cell site triangulation. This technology is applicable to wireless emergency services (“E911”), vehicle tracking, vehicle assistance services and many other services that rely on knowing the location of a mobile user. |
| | · | Electronic Address List Management Patent This patented technology can allow a user to manage an address list on a computer and transfer the list to an electronic device such as a cell phone. |
| | · | Document Generation Technology Patents. This patented technology generally relates to storing data in databases such that it could be used to quickly populate multiple document templates. This technology can be used in medical applications such as Electronic Medical Records (“EMR”) and Electronic Health Records (“EHR”), as well as document generation applications in the financial, legal, and insurance industries. |
| | · | Medical Monitoring Patent. This patented technology detects patient statistics or lab results, such as vital signs or blood tests, which are outside a specified range and then automatically pages the appropriate medical personnel with a critical event message. |
Patent acquisition activity in 2005 included both the consummation of the GPH Acquisition in the first quarter of 2005, as previously described, and the subsequent acquisition of rights to the following patent portfolios:
· Laptop Connectivity Patent
· Hearing Aid ECM Patent
· Digital Ink Jet Printing Patents
· High Resolution Optics Patent
· Picture Archiving & Communication Systems Patents
· Information Monitoring Technology Patents
· Continuous Television Viewer Measuring Technology Patent
· Computing Device Performance Technology Patents
Refer to “Liquidity and Capital Resources” below for information regarding the impact of patent and patent rights acquisitions on the Acacia Technologies group’s financial statements for the periods presented.
As of December 31, 2006, the Acacia Technologies group also had several executed letters of intent with third-party patent portfolio owners regarding the potential acquisition of additional patent portfolios. Future patent portfolio acquisitions will continue to expand and diversify the Acacia Technologies group’s revenue generating opportunities and accelerate the execution of our business strategy, as we continue to build our leadership position in patent licensing.
In 2004, the Acacia Technologies group’s operating activities were principally focused on the continued development and commercialization of its DMT® patent portfolio. License fee revenues totaling $4.3 million were primarily comprised of license fees from our DMT® patent portfolio. The Acacia Technologies group began to recognize DMT® license fee revenues in 2003, significantly increasing the number of DMT® technology licensees and related revenues during 2003 and 2004, while continuing to focus on the expansion of its licensing and enforcement business. To date, the Acacia Technologies group has entered into over 300 DMT® licensing agreements, including cable TV licenses, licenses for online entertainment, movies, news, sports, e-learning and corporate websites and licenses with companies that provide over 90% of video-on-demand TV entertainment to the hotel industry in the United States.
The Acacia Technologies group’s continued development, and commercialization of the DMT® patent portfolio included increased marketing, general and administrative expenses in 2004, related to the hiring of additional patent licensing and business development personnel and an increase in patent related consulting and marketing expenses. Patent related legal expenses, excluding V-chip related legal fees, also increased due to an increase in costs incurred in connection with the Acacia Technologies group’s ongoing DMT® patent commercialization and enforcement programs, including increased legal costs related to new patent claims and the identification of additional potential licensees of our DMT® technology and increased patent enforcement costs related to ongoing DMT® patent related litigation.
CombiMatrix Group
During 2006, the CombiMatrix group’s operating activities were driven by the execution of two new government contracts with the U.S. Department of Defense (“DoD”) totaling $4.0 million, including a new one-year, $2.1 million contract to further the development of the CombiMatrix group’s array technology for the detection of biological and chemical threat agents, both to be recognized through 2008, as well as the execution of several new distribution agreements for our CustomArrayTM products, both nationally and internationally. The CombiMatrix group launched its first 90K, high-density array and array synthesizer in May of 2006 and also launched, in collaboration with Furuno Electric Co., Ltd., its QuadroCASTM CustomArray synthesizer and made available new versions of its Influenza A and miRNA product offerings. The CombiMatrix group’s diagnostics subsidiary, CMDX, received CLIA certification over its diagnostics laboratory and subsequently launched its first molecular diagnostic service using its Constitutional Genetic Array Test (“CGAT”), during the second quarter of 2006. The CombiMatrix group also recently received notice from the U.S. Food and Drug Administration (“FDA”), that CMDX does not require regulation covered by recent FDA guidelines covering certain of its diagnostic assays.
Historically, the CombiMatrix group has relied primarily upon investing and financing activities to fund operating activities. Although the CombiMatrix group’s net proceeds from investing and financing activities in 2006 were consistent with 2005, its cash and cash equivalent balances, anticipated cash flows from operations and other existing sources of credit may not be sufficient to meet its cash flow requirements beyond December 31, 2007. As a result, the CombiMatrix group will be seeking additional sources of capital including the issuance of debt and/or equity securities. Refer to “CombiMatrix group - Liquidity and Capital Resources” below for additional discussion of the CombiMatrix group’s financial position.
During 2005 the CombiMatrix group’s activities included the formation of its wholly owned subsidiary, CombiMatrix Molecular Diagnostics, Inc.(“CMDX”), and the launch of its molecular diagnostics business for the purpose of exploiting the opportunities in the molecular diagnostics market for the CombiMatrix group’s array technology. The CombiMatrix group executed several distribution agreements for its CustomArray™ platform and related products with distributors in the United States, Asia and Australia. The CombiMatrix group also expanded its product offerings by launching a desktop version of its DNA array synthesizer as well as new CustomArray™ catalog arrays, including an influenza H5N1 array, sectored arrays and micro-RNA products. In the area of bio-defense, the CombiMatrix group continued progress on its $5.9 million contract with the Department of Defense, which was completed in December of 2005. The CombiMatrix group also completed all obligations under its collaboration and supply agreement with Toppan Printing, Ltd. (“Toppan”) in the fourth quarter of 2005. As a result of these activities, the CombiMatrix group’s research and development efforts were focused primarily on completing its bio-defense contract, launching its molecular diagnostics business and continuing development of new products and services based on its core array technology as well as making improvements to existing CustomArray™ products launched during 2005 and earlier.
During 2004, the CombiMatrix group’s operating activities included the completion of its research and development agreement with Roche, the execution of a two-year, $5.9 million contract with the Department of Defense to further the development of the CombiMatrix group’s array technology for the detection of biological and chemical threat agents, execution of a multi-year collaboration agreement with Furuno Electric Co. to develop a bench-top DNA array synthesizer and the launch of CustomArrayTM, its first commercially available array platform. As a result of completing its research and development agreement with Roche, the CombiMatrix group’s research and development programs shifted to a number of internally funded programs that support the activities summarized above. With the completion of its obligations under the Roche agreements, research and development expenses continued to decrease in 2004, as compared to 2003, as efforts shifted to internally funded research and development programs. The decrease in research and development expenses was partially offset by an increase in marketing and sales expenses related to the launch of the CombiMatrix group’s CustomArray™ 902 DNA array platform in March 2004 and its CustomArray™ 12K DNA expression array in July 2004.
At December 31, 2006, the CombiMatrix group had cash and cash equivalents and short-term investments of $14.3 million. As a result, management anticipates that the CombiMatrix group’s cash and cash equivalent balances, anticipated cash flows from operations and other sources of funding from the capital markets will be sufficient to meet the CombiMatrix group’s cash requirements through December 31, 2007. In order for the CombiMatrix group to sustain operations beyond this point and ultimately to achieve profitability, the CombiMatrix group will be required to obtain capital from external sources, increase revenues and reduce operating costs. However, there can be no assurance that such capital will be available at times and at terms acceptable to us, or that higher levels of product and service revenues or reductions in operating costs will be achieved. The issuance of additional equity securities will also cause dilution to our shareholders. If external financing sources of financing are not available or are inadequate to fund the CombiMatrix group’s operations, the CombiMatrix group will be required to reduce operating costs including research projects and personnel, which could jeopardize the future strategic initiatives and business plans of the CombiMatrix group. As discussed in Note 1 to the consolidated financial statements included elsewhere herein, the anticipation that the CombiMatrix group will be required to obtain additional financing in the foreseeable future raises substantial doubt about the CombiMatrix group’s ability to sustain operations beyond December 31, 2007. The CombiMatrix group’s plans in regard to these matters are also described later in this section and in Note 1 to Acacia Research Corporation’s consolidated financial statements.
Critical Accounting Policies
Our consolidated financial statements and the separate group financial statements are prepared in conformity with accounting principles generally accepted in the United States of America. In preparing these financial statements, we make assumptions, judgments and estimates that can have a significant impact on amounts reported in our financial statements. We base our assumptions, judgments and estimates on historical experience and various other factors that we believe to be reasonable under the circumstances. Actual results could differ materially from these estimates under different assumptions or conditions. On a regular basis we evaluate our assumptions, judgments and estimates and make changes accordingly.
We believe that, of the significant accounting policies discussed in Note 2 to our consolidated and separate group financial statements, the following accounting policies require our most difficult, subjective or complex judgments:
| | · | stock-based compensation expense; |
| | · | valuation of long-lived and intangible assets and goodwill; and |
| | · | management and allocation policies relating to AR-Acacia Technologies stock and AR-CombiMatrix stock. |
We discuss below the critical accounting assumptions, judgments and estimates associated with these policies. Historically, our assumptions, judgments and estimates relative to our critical accounting policies have not differed materially from actual results. For further information on our critical accounting policies, refer to Note 2 to the consolidated financial statements and separate group statements included herein.
Revenue Recognition
As described below, significant management judgments must be made and used in connection with the revenue recognized in any accounting period. Material differences may result in the amount and timing of revenue recognized or deferred for any period, if management made different judgments.
Revenue is recognized, in accordance with Staff Accounting Bulletin No. 104, “Revenue Recognition,”(“ SAB No. 104”), when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been performed pursuant to the terms of the agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
Acacia Technologies Group
We make estimates and judgments when determining whether the collectibility of license fees receivable from licensees is reasonably assured. The Acacia Technologies group assesses the collectibility of accrued license fees based on a number of factors, including past transaction history with licensees and the credit-worthiness of licensees. If it is determined that collection is not reasonably assured, the fee is recognized when collectibility becomes reasonably assured, assuming all other revenue recognition criteria have been met, which is generally upon receipt of cash. Management estimates regarding collectibility impact the actual revenues recognized each period and the timing of the recognition of revenues. Our assumptions and judgments regarding future collectibility could differ from actual events, thus materially impacting our financial position and results of operations.
The Acacia Technologies group recognizes license fee revenues when earned over the term of the license agreement in exchange for the grant of non-exclusive licenses to use certain technologies for which we own or control patents. We recognize revenue for estimates of license fees earned during the applicable period, based on historical activities of licensees, historical sales or per unit growth rates of licensees and other relevant available information regarding licensee activities that factor into the calculation of periodic license fees due. Revisions are made for actual licensee fees received in the following quarter. Historically, these revisions have not been material to our consolidated financial statements. For those arrangements where royalties cannot be reasonably estimated, we recognize revenue upon the receipt of cash or license fee statements from our licensees, as described at Note 2 to our consolidated financial statements contained elsewhere herein. Our estimates of periodic license fees due could differ from actual events, thus materially impacting our financial position and results of operations.
Certain license agreements provide for the payment of contractually determined paid-up license fees to us in consideration for the grant of a non-exclusive, retroactive and future license to manufacture and/or sell products covered by our patented technologies. The execution of these license agreements may also result in the dismissal of any pending litigation. Pursuant to the terms of these agreements, the Acacia Technologies group has no further obligation with respect to the grant of the non-exclusive retroactive and future license, including no express or implied obligation on the Acacia Technologies group’s part to maintain or upgrade the technology, or provide future support or services. Generally, the agreements provide for the grant of the license upon execution of the agreement. As such, the earnings process is generally complete upon the execution of the agreement, and revenue is recognized upon execution of the agreement, when collectibility is reasonably assured, and all other revenue recognition criteria have been met.
The Acacia Technologies group is responsible for the licensing and enforcement of its patented technologies and pursues third parties that are utilizing its intellectual property without a license or who have under-reported the amount of royalties owed under a license agreement with the Acacia Technologies group. As a result of these activities, from time to time, we may recognize royalty revenues that relate to infringements by our licensees that occurred in prior periods. These royalty recoveries may cause revenues to be higher than expected during a particular reporting period and may not occur in subsequent periods. Differences between amounts initially recognized and amounts subsequently audited or reported as an adjustment to those amounts, will be recognized in the period such adjustment is determined as a change in accounting estimate.
CombiMatrix Group
In general, the CombiMatrix group recognizes revenue in accordance with SAB No. 104, when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been performed pursuant to the terms of the agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
Revenues from government grants and contracts are recognized in accordance with Accounting Research Bulletin (“ARB”) No. 43, "Government Contracts," and related pronouncements, such as Statement of Position 81-1, “Accounting for Performance of Construction-Type and Certain Production-Type Contracts.” Accordingly, revenues are recognized under the percentage-of-completion method of accounting, using the cost-to-cost approach to measure completeness at each reporting period. Under the percentage-of-completion method of accounting, contract revenues and expenses are recognized in the period that work is performed based on the percentage of actual incurred costs to the total contract costs. Actual contract costs include direct charges for labor and materials and indirect charges for labor, overhead and certain general and administrative charges. Contract change orders and claims are included when they can be reliably estimated and are considered probable. For contracts that extend over a reporting period, revisions in contract cost estimates, if they occur, have the effect of adjusting current period earnings applicable to performance in prior periods. Should current contract estimates indicate an overall future loss to be incurred, a provision is made for the total anticipated loss in the current period.
Significant estimates, judgments and assumptions are required primarily in connection with the CombiMatrix group’s accounting for multiple-element arrangements with strategic partners and licensees.
The CombiMatrix group accounts for revenues under multiple-element arrangements in accordance with SAB No. 104 and Emerging Issues Task Force Consensus (“EITF”), Issue 00-21, "Revenue Arrangements with Multiple Deliverables," and related pronouncements. Arrangements with multiple elements or deliverables must be segmented into individual units of accounting based on the separate deliverables only if there is objective and verifiable evidence of fair value to allocate the consideration received to the deliverables. Accordingly, revenues from multiple-element arrangements involving license fees, up-front payments and milestone payments, which are received and/or billable in connection with other rights and services that represent the CombiMatrix group’s continuing obligations are deferred until all of the multiple elements have been delivered or until objective and verifiable evidence of the fair value of the undelivered elements has been established. Upon establishing objective and verifiable evidence of the fair value of the elements in multiple-element arrangements, the fair value is allocated to each element of the arrangement, such as license fees or research and development projects, based on the relative fair values of the elements. The CombiMatrix group determines the fair value of each element in multiple-element arrangements based on objective and verifiable evidence of fair value, which is determined for each element based on the prices charged when the similar elements are sold separately to third parties. If objective and verifiable evidence of fair value of all undelivered elements exists but objective and verifiable evidence of fair value does not exist for one or more delivered elements, then revenue is recognized using the residual method. Under the residual method, the revenues from delivered elements are not recognized until the fair value of the undelivered element or elements have been determined. Significant contract interpretation is sometimes required to determine the appropriate accounting, including whether the deliverables specified in a multiple element arrangement should be treated as separate units of accounting for revenue recognition purposes, and if so, how the price should be allocated among the deliverable elements, when to recognize revenue for each element, and the period over which revenue should be recognized. Changes in the allocation of the sales price between delivered to undelivered elements might impact the timing of revenue recognition, but would not change the total revenue recognized on the contract.
Stock-based Compensation Expense
Effective January 1, 2006, Acacia Research Corporation adopted the provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123R”), which is a revision of SFAS No. 123, “Accounting for Stock-Based Compensation.” SFAS No. 123R supersedes Accounting Principles Board (“APB”) Opinion No. 25, Accounting for Stock Issued to Employees, and amends SFAS No. 95, “Statement of Cash Flows.” SFAS No. 123R sets forth the accounting requirements for “share-based” compensation payments to employees and non-employee directors and requires all share based-payments to be recognized as expense in the statement of operations. In March 2005, the SEC published Staff Accounting Bulletin No. 107 (“SAB 107”), which requires stock-based compensation to be classified in the same expense line items as cash compensation (i.e. marketing, general and administrative and research and development expenses). The compensation cost for all stock-based awards is measured at the grant date, based on the fair value of the award (determined using a Black-Scholes option pricing model), and is recognized as an expense over the employee’s requisite service period (generally the vesting period of the equity award). Determining the fair value of stock-based awards at the grant date requires significant estimates and judgments, including estimating the market price volatility of our classes of common stock and employee stock option exercise behavior.
SFAS No. 123R also requires stock-based compensation expense to be recorded only for those awards expected to vest using an estimated pre-vesting forfeiture rate. As such, SFAS No. 123R requires Acacia Research Corporation to estimate pre-vesting option forfeitures at the time of grant and reflect the impact of estimated pre-vesting option forfeitures on compensation expense recognized. Estimates of pre-vesting forfeitures must be periodically revised in subsequent periods if actual forfeitures differ from those estimates. We consider several factors in connection with our estimate of pre-vesting forfeitures including types of awards, employee class, and historical pre-vesting forfeiture data. The estimation of stock awards that will ultimately vest requires judgment, and to the extent that actual results differ from our estimates, such amounts will be recorded as cumulative adjustments in the period the estimates are revised. If actual results differ significantly from these estimates, stock-based compensation expense and our results of operations could be materially impacted.
Refer to Notes 2 and 13 to the Acacia Research Corporation consolidated financial statements included in Part IV, Item 15 of this report for more information.
Valuation of Long-lived and Intangible Assets and Goodwill
Goodwill is evaluated for impairment using a fair value approach at the reporting unit level annually, or earlier if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. A reporting unit can be an operating segment or a business if discrete financial information is prepared and reviewed by management. As of December 31, 2006, our reporting units were: 1) the Acacia Technologies group and 2) the CombiMatrix group. Under the impairment test, if a reporting unit’s carrying amount exceeds its estimated fair value, goodwill impairment is recognized to the extent that the reporting unit’s carrying amount of goodwill exceeds the implied fair value of the goodwill. The fair value of Acacia Research Corporation’s reporting units are estimated using the fair market value of common stock and other valuation techniques. Significant judgments and estimates are required in determining forecasted cash inflows and outflows, the timing of cash flows and discount rates commensurate with the risks involved.
We review long-lived assets, including patent related intangibles, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Factors we consider important, which could trigger an impairment review include the following:
| · | significant underperformance relative to expected historical or projected future operating results; |
| · | significant changes in the manner of our use of the acquired assets or the strategy for our overall business; |
| · | significant negative industry or economic trends; |
| | · | significant adverse changes in legal factors or in the business climate, including adverse regulatory actions or assessments; and |
| | · | significant decline in our stock price for a sustained period. |
We calculate estimated future undiscounted cash flows, before interest and taxes, resulting from the use of the asset and its estimated value at disposal and compare it to its carrying value in determining whether impairment potentially exists. If a potential impairment exists, a calculation is performed to determine the fair value of the long-lived asset. This calculation is based on a valuation model and discount rate commensurate with the risks involved. Third party appraised values may also be used in determining whether impairment potentially exists.
As described above, in assessing the recoverability of goodwill and other intangible assets, estimates of market values, estimates of the amount and timing of future cash flows, and estimates of other factors are used to determine the fair value of the respective assets. If these estimates or related projections change in future periods, future goodwill and intangible asset impairment tests may result in a charges to earnings.
Refer to Note 7 to the Acacia Research Corporation consolidated financial statements, included elsewhere herein, for information on impairment charges recorded during the periods presented.
Management and Allocation Policies Relating to AR-Acacia Technologies Stock and AR-CombiMatrix Stock
The management and allocation policies applicable to the preparation of the divisional financial statements of the CombiMatrix group and the Acacia Technologies group (collectively, the “groups”) may be modified or rescinded, or additional policies may be adopted, at the sole discretion of the Acacia Research Corporation board of directors at any time without approval of the stockholders. The group divisional financial statements reflect the application of the management and allocation policies adopted by the Acacia Research Corporation board of directors to various corporate activities, as described below. The group’s divisional financial statements should be read in conjunction with Acacia Research Corporation’s consolidated financial statements and related notes.
Corporate General and Administrative Services and Facilities
Acacia Research Corporation allocates the cost of corporate general and administrative services and facilities between the groups generally based upon utilization. Where determinations based on utilization alone are impracticable, Acacia Research Corporation uses other methods and criteria, which require the use of judgments and estimates, that management believes to be equitable and to provide a reasonable estimate of the cost attributable to each group. Except as otherwise determined by management, the allocated costs of providing such services and facilities include, without limitation, all costs and expenses of personnel employed in connection with such services and facilities, including, without limitation, all direct costs of such personnel, such as payroll, payroll taxes and fringe benefit costs (calculated at the appropriate annual composite rate therefore) and all overhead costs and expenses directly related to such personnel and the services or facilities provided by them. The corporate general and administrative services and facilities allocated between the groups include, without limitation, legal services, accounting services (tax and financial), insurance and deductibles payable in connection therewith, employee benefit plans and administration thereof, investor relations, stockholder services and services relating to the Acacia Research Corporation board of directors.
Refer to Note 2 in the consolidated financial statements for details on allocation methodologies used to allocate costs between the two groups.
Acacia Research Corporation Consolidated
Results of Operations
Net Loss (In thousands)
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| Net loss | | $ | (25,456 | ) | $ | (18,676 | ) | $ | (4,833 | ) |
The changes in consolidated net loss were primarily due to operating results and activities, as discussed below.
Revenues and Cost of Revenues (In thousands)
| | | | 2006 | | | 2005 | | | 2004 | |
| | | | | | | | | | | |
| Collaboration agreements | | $ | - | | $ | 2,266 | | $ | 17,302 | |
| License fees | | | 34,825 | | | 19,574 | | | 4,284 | |
| Government contracts | | | 2,074 | | | 3,849 | | | 1,993 | |
| Cost of government contract revenues | | | (1,959 | ) | | (3,683 | ) | | (1,874 | ) |
| Products | | | 3,278 | | | 1,765 | | | 230 | |
| Cost of product sales | | | (1,258 | ) | | (820 | ) | | (173 | ) |
| Service contracts | | | 388 | | | 153 | | | 116 | |
Collaboration Agreements (CombiMatrix group only). During the fourth quarter of 2005, the CombiMatrix group completed all obligations under its collaboration and supply agreement with Toppan. As a result of completing its obligations under this agreement, and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized $2.3 million of previously deferred collaboration agreement revenues during the fourth quarter of 2005. Research and development activities and expenses related to the Toppan agreement were incurred during the two-year term of the agreement, which was originally executed in May 2003.
In March 2004, the CombiMatrix group completed all phases of its research and development agreement with Roche. As a result of completing all obligations under this agreement and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized $17.3 million of collaboration agreement revenues during the first quarter of 2004, all of which were previously deferred. The majority of research and development efforts under the Roche agreement were incurred prior to 2004.
License Fees (Acacia Technologies group only). Revenues for 2006 included license fees from 126 new licensing agreements covering 14 of our technology licensing and enforcement programs, as compared to 83 new licensing agreements covering 12 of our technology licensing and enforcement programs in 2005. The increase in license fee revenues in 2006 and 2005, as compared to 2004, reflects the impact of the increase in patent portfolios controlled by the Acacia Technologies group since 2004, including the impact of the GPH Acquisition, and the related increase in the number of patent licensing and enforcement programs developed, launched and generating revenues since 2004. License fee revenues recognized by the Acacia Technologies group fluctuate from period to period primarily based on the following factors:
| | · | the dollar amount of agreements executed each period, which is primarily driven by the nature and characteristics of the technology being licensed and the magnitude of infringement associated with a specific licensee; |
| | · | the specific terms and conditions of agreements executed each period and the periods of infringement contemplated by the respective payments; |
| · | fluctuations in the total number of agreements executed; |
| | · | fluctuations in the sales results or other royalty per unit activities of our licensees that impact the calculation of license fees due; |
| · | the timing of the receipt of periodic license fee payments and/or reports from licensees; and |
| | · | fluctuations in the net number of active licensees period to period. |
Revenues in 2004 were comprised of $2.8 million in DMT patent portfolio revenues and $1.5 million in previously deferred V-chip license fees (originally received and deferred in 2001) recognized as a result of the conclusion of V-chip patent litigation and related licensing program in August 2004.
Costs incurred in connection with the Acacia Technologies group’s ongoing licensing activities, other than inventor royalties expense, contingent legal fees expense and patent-related legal expenses, are included in marketing, general and administrative expenses.
Government Contracts and Cost of Government Contract Revenues (CombiMatrix group only). Under the terms of its contracts with the Department of Defense, the CombiMatrix group is reimbursed on a periodic basis for actual costs incurred to perform its obligations, plus a fixed fee. Revenues are recognized under the percentage-of-completion method of accounting, using the cost-to-cost approach to measure completeness at the end of each reporting period. Cost of government contract revenues reflect research and development expenses incurred in connection with the CombiMatrix group’s commitments under its DoD contracts.
Revenues and associated costs decreased during 2006, as compared to 2005 due to a higher level of contract related activity leading to the completion in December 2005, of the CombiMatrix group’s commitments under its previous two-year, $5.9 million research and development contract with the DoD to further the development of the CombiMatrix group’s array technology for the electrochemical detection of biological threat agents. In February 2006, the CombiMatrix group executed a new one-year, $2.1 million contract with the DoD to further the development of its electrochemical detection system. In August 2006, the CombiMatrix group executed a new two-year, $1.9 million contract with the DoD to integrate its electrochemical detection technology with its microfluidics “lab on a chip” technology for national defense and homeland security applications. Overall, the activity on these new DoD contracts was lower in 2006 than activity under the CombiMatrix group’s previous $5.9 million contract in 2005, resulting in the overall decrease in government contract revenues and cost of revenues in 2006, as compared to 2005. These revenues and costs increased during 2005, as compared to 2004 due to increased activity on the two-year, $5.9 million contract and due to the fact that only nine months of activity were incurred in 2004, versus a full year of activity in 2005. The $5.9 million contract was completed in 2005 and there are no additional revenues or costs expected to be recognized from this contract in future periods.
Product Revenues and Cost of Product Sales (CombiMatrix group only). Product revenues and costs of product sales relate to domestic and international sales of the CombiMatrix group’s array products. Product revenues in 2006 include the sale of DNA synthesizer instruments and CustomArray 12K DNA expression arrays and related hardware, as compared to lower instrument and 12K DNA expression array sales in 2005. The overall increase in product revenues in 2006 was due primarily to the increased product offerings currently available to the CombiMatrix group’s customers, which includes 12K and 4X2K arrays, DNA synthesizer and electrochemical detection reader instruments and related hardware, as compared to only the 902 and 12K expression arrays and DNA synthesizer instruments in 2005. Product revenues and costs of product sales during 2004 and 2005 relate to domestic and international sales of the CombiMatrix group’s array products, including its CustomArray 902 DNA array platform launched in March 2004, CustomArray 12K DNA expression array launched in July 2004 and its commercial DNA array synthesizer instrument launched in August 2005. The CombiMatrix group’s product revenues increased in 2005, as compared to 2004 due primarily to a full year of array sales recognized in 2005, as compared to only a partial year’s recognition in 2004, as well as the launch of the CombiMatrix group’s DNA array synthesizer instrument in 2005.
Service Contracts (CombiMatrix group only). Prior to 2005, all service contract revenues were recognized by CombiMatrix K.K. from existing array customers in Japan. As of December 31, 2004, the terms of these contracts had expired. Costs incurred in connection with these services were not material. In 2006 and 2005, service contract revenues include maintenance and service contract fees relating to DNA array synthesizers sold during those periods, and have increased in 2006 due to the sale of additional array synthesizers. Such service contracts are typically for twelve months, and the consideration received is recognized ratably over the service period.
Operating Expenses (In thousands)
| | | | 2006 | | | 2005 | | | 2004 | |
| | | | | | | | | | | |
| Research and development expenses | | $ | 9,485 | | $ | 5,783 | | $ | 5,385 | |
Research and Development Expenses (CombiMatrix group only). In 2006, the CombiMatrix group continued internal research and development efforts to improve and expand its technology and product offerings. The increase in internal research and development expenses in 2006, as compared to 2005 was due primarily to costs incurred in connection with the development of higher density array products, as well as an increase in expenses incurred by the CombiMatrix group’s subsidiary, CMDX, which was formed and began research and development activities in the area of diagnostic applications during the second quarter of 2005. In addition, research and development expenses include $1.1 million, $0 and $91,000 in 2006, 2005 and 2004, respectively, of non-cash stock based compensation expense. The increase in non-cash stock compensation included in research and development expense was due to the adoption of SFAS No. 123R effective January 1, 2006, as described under “Critical Accounting Estimates.” The CombiMatrix group’s research and development costs increased in 2005, as compared to 2004 primarily due to the launch of the CustomArray platform and continued launch of related array products, including the CombiMatrix group’s DNA array synthesizer instrument launched in August 2005. Research and development activities at the CombiMatrix group’s wholly owned subsidiary, CMDX, which was formed in April of 2005, also contributed to the overall increase in research and development expenses for 2005.
Future research and development expenses were and will continue to be incurred in connection with the CombiMatrix group’s efforts in the area of genomics, diagnostics, and drug discovery and development. The CombiMatrix group expects its research and development expenses to continue to be volatile and such expenses could increase in future periods as additional contract and/or internal research and development projects are undertaken and/or as new collaborations are executed with strategic partners.
| | | | 2006 | | | 2005 | | | 2004 | |
| | | | | | | | | | | |
| Marketing, general and administrative expenses | | $ | 26,963 | | $ | 17,926 | | $ | 14,951 | |
| Legal expenses - patents | | | 4,780 | | | 2,468 | | | 3,133 | |
| Inventor royalties and contingent legal fees expense - patents | | | 17,159 | | | 11,106 | | | - | |
| Inventor royalties - V-chip | | | - | | | 225 | | | - | |
Marketing, General and Administrative Expenses.
Acacia Technologies Group. The increase in 2006, as compared to 2005 primarily reflects Acacia Research Corporation’s adoption of SFAS No. 123R, effective January 1, 2006, which requires public companies to measure all employee stock-based compensation awards using a fair-value method and record such expense in their consolidated financial statements, as described under “Critical Accounting Estimates.” Non-cash stock compensation charges included in marketing, general and administrative expense in 2006 totaled $3,946,000, as compared to $356,000 in 2005, prior to the adoption of FAS 123R.
Excluding the impact of the adoption of SFAS No. 123(R), the increase in marketing, general and administrative expenses in 2006, as compared to 2005 was due primarily to the addition of licensing, business development and engineering personnel for the Acacia Technologies group, an increase in the Acacia Technologies group’s patent-related research and consulting expenses for new and ongoing licensing and enforcement programs, an increase in accounting and legal fees related to the CombiMatrix Split-off Transaction and an increase in corporate, general and administrative costs related to the continued growth and expansion of Acacia Technologies group’s ongoing operations.
The change in marketing, general and administrative expenses in 2005, as compared to 2004, was due to an increase in personnel costs primarily related to the addition of patent licensing and business development personnel for the Acacia Technologies group, an increase in the Acacia Technologies group’s consulting expenses related to a consulting agreement executed with the former CEO of Global Patent Holdings, LLC in connection with the GPH Acquisition and an increase in the Acacia Technologies group’s patent-related research and consulting expenses for new and ongoing licensing and enforcement programs and other general and administrative expenses, including increases related to certain of the companies acquired in the GPH Acquisition. These increases were partially offset by a reduction in the Acacia Technologies group’s Sarbanes-Oxley compliance costs.
A summary of the main drivers of the change in marketing, general and administrative expenses, excluding the impact of non-cash stock compensation, for the periods presented is as follows (in thousands):
| | | | 2006 vs. 2005 | | | 2005 vs. 2004 | |
| | | | | | | | |
| Acacia Technologies group: | | | | | | | |
| Increase in personnel expenses | | $ | 1,247 | | $ | 1,202 | |
| Increase in GPH Acquisition related consulting expenses | | | 96 | | | 1,009 | |
| Increase (decrease) in accounting and other professional fees | | | 323 | | | (53 | ) |
| Increase in patent development / commercialization and other | | | | | | | |
| and other marketing, general and administrative costs | | | 701 | | | 256 | |
| Increase in CombiMatrix Split-off transaction costs | | | 200 | | | - | |
| Increase in GPH Acquisition related patent development/commercialization | | | | | | | |
| and other general and administrative expenses | | | - | | | 280 | |
CombiMatrix Group. The increase in 2006, as compared to 2005 primarily reflects Acacia Research Corporation’s adoption of SFAS No. 123R, effective January 1, 2006, as described under “Critical Accounting Estimates.” Marketing, general and administrative expenses include non-cash stock compensation of $1.3 million, ($159,000) and $663,000 in 2006, 2005 and 2004, respectively. Excluding the impact of the adoption of SFAS No. 123(R), the increase was primarily due to the impact of a full year of general and administrative expenses incurred by CMDX in 2006, which commenced operations in the second quarter of 2005, as well as increased legal and accounting expenses primarily related to the planned CombiMatrix Split-off Transaction. These increases were partially offset by a decrease in marketing, sales and other expenses.
The change in 2005, as compared to 2004, reflects an increase in marketing and sales costs related to the CombiMatrix group’s CustomArray™ platform, which were driven primarily by increases in the CombiMatrix group’s sales force and expanded marketing and advertising efforts and an increase in marketing, general and administrative expenses in connection with the creation of CombiMatrix Molecular Diagnostics in April 2005. These increases were partially offset by a reduction in the CombiMatrix group’s Sarbanes-Oxley compliance costs in 2005.
A summary of the main drivers of the change in marketing, general and administrative expenses, excluding the impact of non-cash stock compensation, for the periods presented is as follows (in thousands):
| | | | 2006 vs. 2005 | | | 2005 vs. 2004 | |
| | | | | | | | |
| CombiMatrix group: | | | | | | | |
| (Decrease) increase in marketing and sales expenses | | $ | (924 | ) | $ | 478 | |
| Increase in general and administrative expenses related to CMDX | | | 1,683 | | | 598 | |
| Increase (decrease) in legal, accounting and other professional fees | | | 1,169 | | | (250 | ) |
| Decrease in general and administrative expenses | | | (467 | ) | | - | |
Legal Expense - Patents (Acacia Technologies group only). Patent-related legal expenses include patent-related prosecution and enforcement costs, incurred by outside patent attorneys engaged on an hourly basis and the out-of-pocket expenses incurred by law firms engaged on a contingent fee basis. Patent-related legal expenses fluctuate from period to period based on patent enforcement and prosecution activity associated with ongoing licensing and enforcement programs and the timing of the commencement of new licensing and enforcement programs in each period. Patent-related legal expenses include case related costs billed by outside counsel for economic analyses and damages assessments, expert witnesses and other consultants, case related audio/video presentations for the court, and other litigation support and administrative costs.
The increase in patent related legal expenses in 2006, as compared to 2005 is primarily due to a net increase in the number of ongoing patent enforcement litigations in 2006 compared to 2005 and an increase in the number of portfolios where we have engaged outside law firms on an hourly or discounted hourly basis. The increase also reflects significant third party consulting and expert expenses, including costs incurred related to expert witnesses and the preparation of damages reports, incurred in connection with certain of our patent portfolios that are further along in litigation. Refer to Item 1. “Business - Acacia Technologies Group” for a summary of ongoing Acacia Technologies group patent enforcement litigation.
We expect patent-related legal expenses to continue to fluctuate period to period based on the factors summarized above, in connection with the Acacia Technologies group’s current and future patent commercialization and enforcement programs.
As described earlier, patent related legal expenses in 2004 included $668,000 in previously deferred V-chip related legal expenses. Excluding the V-chip related costs recognized, the change in 2005, as compared to 2004 was primarily due to corresponding fluctuations in DMT® patent portfolio related claims prosecution, litigation and enforcement activity in the respective periods. DMT® related legal fees paid to outside attorneys are incurred based on actual time and out-of-pocket expenses incurred by external counsel and fluctuate from period to period based on patent enforcement and prosecution activity in each period. In addition, patent related legal expenses for 2005 included $654,000 in patent related prosecution and enforcement costs incurred by certain of the companies acquired in the GPH Acquisition.
Inventor Royalties and Contingent Legal Fees Expense (Acacia Technologies group only). The Acacia Technologies group incurred inventor royalties expense totaling $9.6 million and $5.5 million in 2006 and 2005, respectively, and contingent legal fees expense totaling $7.5 million and $5.6 million in 2006 and 2005, respectively. Inventor royalties expenses and contingent legal fees expenses were incurred in connection with the recognition of the related license fee revenues summarized above. The majority of the Acacia Technologies group’s patent portfolios are subject to patent and patent rights agreements with inventors containing provisions granting to the original patent owner the right to receive inventor royalties based on future net revenues, as defined in the respective agreements and may also be subject to contingent legal fee arrangements with external law firms engaged on a contingent fee basis. The economic terms of the inventor and contingent arrangements, if any, vary across the Acacia Technologies group’s patent portfolios. As such, inventor royalties and contingent legal fees expenses fluctuate period to period based on the amount of revenues recognized each period and the mix of specific patent portfolios generating revenues each period.
Inventor royalties and contingent legal fees expense increased primarily as a result of the increase in license fee revenues recognized in 2006, as compared to 2005. However, certain of the patent portfolios generating significant revenues in 2006 were not subject to contingent fee arrangements with law firms, as described earlier. As such, the percentage increase in contingent legal fees in 2006, as compared to 2005, was less than the percentage increase in revenues for the same periods.
Inventor Royalties V-chip. Results in 2005 included $225,000 of V-chip related inventor royalties expense recognized as a result of the conclusion of all V-chip related litigation activities in October of 2005. As a result of the conclusion of all V-chip related activities, no additional V-chip related inventor royalties expense will be incurred in future periods.
| | | | 2006 | | | 2005 | | | 2004 | |
| | | | | | | | | | | |
| Goodwill impairment charge | | $ | - | | $ | 565 | | $ | 1,656 | |
| Write-off of patent-related intangible asset | | | 297 | | | - | | | - | |
| Amortization of patents and royalties | | | 6,795 | | | 6,234 | | | 1,735 | |
| Legal settlement charges (gains) | | | - | | | (406 | ) | | 812 | |
| Loss from equity investment | | | 1,036 | | | 352 | | | 17 | |
Amortization of Patent and Royalties. The increase in 2006, as compared to 2005 was due to twelve full months of patent amortization expense resulting from the January 28, 2005 GPH Acquisition in 2006, as compared to 11 months of amortization in 2005. Patent amortization expense related to the GPH Acquisition was $4.7 million and $4.4 million in 2006 and 2005, respectively. In addition, patent amortization expense in 2006 and 2005 includes $207,000 and $65,000 in additional patent amortization charges related to certain of the patent portfolios acquired by the Acacia Technologies group subsequent to the GPH Acquisition. Patent amortization charges will continue to be significant in future periods as the Acacia Technologies group continues to amortize its acquired patent related costs over a weighted-average remaining economic useful life of approximately 4 years.
The increase in 2005, as compared to 2004 was due primarily to the amortization of patent related intangibles acquired in connection with the GPH Acquisition. Approximately $25.1 million of the purchase consideration paid in the GPH Acquisition was allocated to amortizable patents and related patent rights acquired, and are being amortized over an original weighted-average economic useful life of approximately 6 years. Amortization expense related to the patents and patent rights acquired in the GPH Acquisition was $4.4 million in 2005.
Amortization of patents and royalties includes royalty expense of $348,000, $217,000 and $138,000, in 2006, 2005 and 2004, respectively, related to the CombiMatrix group’s September 2002 settlement agreement with Nanogen, Inc., and are equal to 12.5% of payments made to the CombiMatrix group from sales of certain products developed based on the patents that had been in dispute in the litigation with Nanogen, Inc. prior to settlement. The increase in royalties expense for the years presented is due to the corresponding increase in CombiMatrix group product revenue payments received for the period.
Goodwill and Other Impairment Charges. The CombiMatrix group recognized a goodwill impairment charge of $565,000, during the fourth quarter of 2005, related to its Advanced Materials Sciences and CombiMatrix K.K. reporting units. These reporting units were tested for impairment in the fourth quarter of 2005, in connection with the CombiMatrix group’s annual forecasting process. Due to the lack of third-party research and development funding for Advanced Materials Sciences and declining array product sales at CombiMatrix K.K., operating profits and cash flows were lower than expected during the preceding three quarters. Based on these trends, the operating forecasts for 2006 were revised downward, resulting in the goodwill impairment charge.
As a result of the conclusion of the Acacia Technologies group’s V-chip patent licensing program in August 2004, 2004 results included a non-cash impairment charge of $1.6 million associated with the write-off of goodwill related to the V-chip.
Legal Settlement Charges(Gains). Legal settlement charges (gains) related to AR-CombiMatrix stock issuable and/or potentially issuable in connection with certain anti-dilution provisions of the September 2002 settlement agreement between CombiMatrix Corporation, Dr. Donald Montgomery, and Nanogen, Inc. The related liability reflected management’s estimate, as of each balance sheet date, of the fair value of AR-CombiMatrix stock to be issued to Nanogen, Inc. as a result of certain options and warrants exercised during the period, if any, and the fair value of AR-CombiMatrix stock potentially issuable to Nanogen, Inc. as of each balance sheet date, pursuant to the anti-dilution terms of the agreement. The liability was adjusted at each balance sheet date for changes in the market value of the AR-CombiMatrix stock and reflected as long-term until settled in equity. All anti-dilution provisions of the settlement agreement expired as of September 30, 2005, resulting in no further liability subsequent to September 30, 2005, or in any future periods, and a net gain in the statement of operations and comprehensive loss of $406,000 in 2005.
Write-off of Patent-related Intangible Asset. In June 2006, the Acacia Technologies group recorded a non-cash impairment charge of $297,000, related to the write-off of a patent-related intangible asset. During the second quarter of 2006, pursuant to the terms of the respective license agreement, management elected to terminate its rights to exclusively license and enforce the patent, resulting in the write-off of the remaining carrying value of the patent-related intangible asset as of June 30, 2006.
Loss from Equity Investment. The CombiMatrix group owned 33% and 19% as of December 31, 2006 and 2005, respectively, of Leuchemix Inc.(“Leuchemix”), a private drug development firm, which is developing several compounds for the treatment of leukemia and other cancers. The CombiMatrix group’s equity in the losses of Leuchemix increased due to the CombiMatrix group’s increased ownership in Leuchemix as well as an increase in expenses incurred by Leuchemix. The CombiMatrix group was under a contractual commitment to increase its ownership interest to 33% in 2006. As of October 2006, the CombiMatrix group satisfied its contractual commitment to increase its ownership interest in Leuchemix pursuant to the terms of the underlying agreement.
Other
Warrant Charges (Gains). In accordance with SFAS No. 150, “Accounting for Certain Instruments with Characteristics of Both Liabilities and Equity,” (“SFAS No. 150”), and related interpretations, certain AR-CombiMatrix stock purchase warrants outstanding at December 31, 2006, which were issued in connection with equity financings in December 2006, September 2005 and May 2003, have been classified as a long-term liability due to certain redemption provisions associated with the underlying AR-CombiMatrix stock. The warrant liability is marked to market at each balance sheet date. Changes in the fair value of the stock purchase warrant liability are reflected in the consolidated statement of operations and comprehensive loss. Warrant gains totaled $1.8 million and $812,000 in 2006 and 2005, respectively. The increase in warrant gains reflects the decrease in the AR-CBMX stock price during the periods presented, and a $825,000 gain related to the mark to market of the warrants issued in connection with the CombiMatrix group’s December 2006 equity financing, as described below. Refer to Note 10 to the Acacia Research Corporation consolidated financial statements elsewhere herein.
Discontinued Operations. Results for 2005 and 2004 include charges, net of minority interests, of $237,000 and $104,000, respectively, related to estimated additional costs to be incurred in connection with the discontinued operations of Soundbreak.com (originally ceased operations in February 2001), related primarily to certain noncancellable lease obligations and a reduction in estimated amounts recoverable from existing sublease arrangements. The related lease obligations, which were guaranteed by Acacia Research Corporation, expired in August 2005.
Inflation
Inflation has not had a significant impact on Acacia Research Corporation in the current or prior periods.
Liquidity and Capital Resources
Acacia Research Corporation’s consolidated cash and cash equivalents and short-term investments totaled $59.3 million at December 31, 2006, compared to $59.2 million at December 31, 2005. Working capital at December 31, 2006 was $54.6 million, compared to $58.1 million at December 31, 2005.
The net change in cash and cash equivalents and short term investments for 2006, 2005 and 2004 was comprised of the following (in thousands):
| | Year Ended December 31, 2006 | | Year Ended December 31, 2005 | | Year Ended December 31, 2004 | |
| | Acacia | | | | | | Acacia | | | | | | Acacia | | | | | |
| | Technologies | | CombiMatrix | | | | Technologies | | CombiMatrix | | | | Technologies | | CombiMatrix | | | |
| | Group | | Group | | Consolidated | | Group | | Group | | Consolidated | | Group | | Group | | Consolidated | |
| Net cash provided by (used in) continuing operations: | | | | | | | | | | | | | | | | | | |
| Operating activities | $ | 6,095 | | $ | (15,145 | ) | $ | (9,050 | ) | $ | (2,477 | ) | $ | (13,696 | ) | $ | (16,173 | ) | $ | (3,232 | ) | $ | (11,584 | ) | $ | (14,816 | ) |
| Investing activities | | 10,513 | | | 4,981 | | | 15,494 | | | (13,094 | ) | | 3,390 | | | (9,704 | ) | | (321 | ) | | (8,448 | ) | | (8,769 | ) |
| Financing activities | | 1,198 | | | 12,327 | | | 13,525 | | | 19,657 | | | 12,914 | | | 32,571 | | | (305 | ) | | 19,227 | | | 18,922 | |
| Effect of exchange rate on cash | | - | | | - | | | - | | | - | | | 73 | | | 73 | | | - | | | (17 | ) | | (17 | ) |
| Net cash used in discontinued operations | | (89 | ) | | - | | | (89 | ) | | (513 | ) | | - | | | (513 | ) | | (925 | ) | | - | | | (925 | ) |
| Increase (decrease) in cash and cash equivalents | $ | 17,717 | | $ | 2,163 | | $ | 19,880 | | $ | 3,573 | | $ | 2,681 | | $ | 6,254 | | $ | (4,783 | ) | $ | (822 | ) | $ | (5,605 | ) |
Operating Activities. The change to net cash inflows from operations for the Acacia Technologies group in 2006, as compared to net cash outflows from operations in 2005, was primarily due to the increase in license fee payments received from licensees, which totaled $38.6 million in 2006, compared to $15.6 million in 2005, reflecting the increase in license fee revenues recognized in 2006, as compared to 2005, as discussed above. The increase in license fee revenues in 2006 was partially offset by increases in inventor royalties expenses, contingent legal fees expenses, patent-related legal expenses, personnel expenses, and other corporate, general and administrative expenses, as described above, and the impact of the timing of payments to inventors, attorneys and other vendors. Accounts receivable for the Acacia Technologies group decreased to $269,000 at December 31, 2006, compared to $4.4 million at December 31, 2005, due to the collection of license fees receivable at December 31, 2005, during the first quarter of 2006, in accordance with the terms of the related underlying license agreements.
The decrease in net cash outflows from operations for the Acacia Technologies group in 2005, as compared to 2004, was primarily due to the increase in license fee payments received from licensees, which totaled $15.6 million in 2005, compared to $3.1 million in 2004. The increase in license fee revenues recognized was partially offset by an increase in marketing, general and administrative expenses related to the continued expansion of the Acacia Technologies group’s business, including increased inventor royalties, contingent legal fees and consulting expenses related to the GPH Acquisition, as discussed above. The change also reflects the impact of the timing of receipt of license fee payments from licensees and the timing of payments to inventors, contingent law firms and vendors. Accounts receivable for the Acacia Technologies group increased to $4.4 million at December 31, 2005, compared to $193,000 at December 31, 2004, primarily due to the timing of the execution of certain paid-up license agreements with payment terms. The majority of accounts receivable balances at December 31, 2005 were received from the respective licensees in the first quarter of 2006, in accordance with the terms of the respective license agreements.
Cash receipts from customers for the CombiMatrix group for 2006, were $6.3 million, compared to $5.3 million in 2005. The increase was primarily due to increased sales and related cash receipts from CustomArray customers totaling $3.8 million in 2006, as compared to $1.7 million in 2005. This increase was partially offset by decreased cash collections from the CombiMatrix group’s government contract billings, which were $2.5 million in 2006, as compared to $3.6 million in 2005. Cash outflows from operations for the CombiMatrix group for 2006 increased due to an increase in cash operating expenses totaling $21.4 million, as compared to $18.9 million in 2005, due primarily to an increase in research and development, marketing, general and administrative expenses related to CMDX as described above, and the impact of the timing of vendor payments.
The change in net cash outflows from operations for the CombiMatrix group in 2005, as compared to 2004, was due primarily to an increase in operating expenses totaling $18.9 million in 2005, as compared to $14.7 million in 2004. The increase was due primarily to increased research and development and general and administrative costs incurred as discussed above, as well as the net impact of the timing of the receipt of payments from customers and payments to vendors. The increase in cash outflows from operating expenses was partially offset by an increase in cash receipts from customers, which totaled $5.3 million in 2005, as compared to $3.0 million in 2004. The increase was primarily due to increased activity under the CombiMatrix group’s two-year research and development contract with the Department of Defense, resulting in billings and cash payments during 2005 of $3.6 million as compared to $1.7 million in 2004, as well as increased sales and related cash receipts from CustomArray™ customers totaling $1.7 million in 2005, as compared to $113,000 in 2004.
Investing Activities. The change in net cash flows used in investing activities for the periods presented reflects fluctuations in net purchases and sales of available-for-sale investments by the Acacia Technologies group and the CombiMatrix group in connection with ongoing short-term cash management activities. Short term investments represent capital available to fund current operations and fund capital expenditures. Net cash outflows from investing activities for 2005 also included the impact of cash consideration and related acquisition and registration costs, totaling $5.8 million, paid by the Acacia Technologies group in connection with the GPH Acquisition in the first quarter of 2005. In addition, the Acacia Technologies group incurred patent acquisition costs of $1.0 million and $445,000 in 2006 and 2005, respectively, related to the acquisition of additional patent portfolios, as described earlier.
The CombiMatrix group’s cash outflows from investing activities included additional contractual investments in Leuchemix totaling $2.2 million, $1.6 million and $250,000 in 2006, 2005 and 2004, respectively. Fixed asset purchases, primarily related to the CombiMatrix group, totaled $715,000, $1.4 million, and $891,000 in 2006, 2005 and 2004, respectively. In addition, in April 2006, the Acacia Technologies group made a final distribution to Soundbreak.com’s (ceased operations in 2001) minority shareholders totaling $353,000.
Financing Activities. The net cash flows provided by financing activities for the periods presented were comprised of the following (in thousands):
| | 2006 | | | 2005 | | | 2004 | | |
| AR-ACTG stock: | | | | | | | | | | | | |
| Equity financing, net of issuance costs | $ | - | | | $ | 19,532 | (4) | | $ | - | | |
| Proceeds from option exercises | | 1,475 | | | | 304 | | | | 90 | | |
| | | | | | | | | | | | | |
| AR-CBMX stock: | | | | | | | | | | | | |
| Equity financings, net of issuance costs | | 12,050 | (1) | | | 12,724 | (2) | | | 13,715 | (3) | |
| Proceeds from option / warrant exercises | | - | | | | 11 | | | | 5,117 | | |
| | $ | 13,525 | | | $ | 32,571 | | | $ | 18,922 | | |
| | (1) | In June 2006, Acacia Research Corporation entered into a Standby Equity Distribution Agreement (the “SEDA”) with Cornell Capital Partners, LP (“Cornell”), providing up to $50 million of equity financing from Cornell through the sale of up to 13,024,924 shares of AR-CombiMatrix common stock through June 2008. Proceeds from AR-CBMX equity financings in 2006 includes $3,070,000 in net proceeds from the sale of 3,211,345 shares of AR-CombiMatrix stock under the SEDA, which was cancelled in December 2006. Proceeds from equity financings in 2006 also includes the December 2006, registered direct offering with Oppenheimer & Co., Inc. (“Oppenheimer”), as the placement agent, raising net proceeds of $9,266,000 through the issuance of 9,768,313 units. Each unit consisted of one share of AR-CombiMatrix common stock and 1.2 five-year common stock warrants. Refer to Note 9 to the consolidated financial statements included in this report. |
| | (2) | Includes July 2005 Equity Financing - 1,400,444 shares of AR-CombiMatrix stock at $2.25 per share and September 2005 Equity Financing - 6,385,907 shares of AR- CombiMatrix stock and 1,596,478 warrants at $1.65 per unit. |
| (3) | April 2004 Equity Financing - 3,000,000 shares of AR-CombiMatrix stock at $5.00 per share. |
| (4) | February 2005 Equity Financing - 3,500,000 shares of AR-Acacia Technologies stock at $5.60 per share. |
The cash and cash equivalent balances, anticipated cash flow from operations, and other external sources of available credit of the Acacia Technologies group and the CombiMatrix group are discussed separately below. The cash and cash equivalent balances, anticipated cash flow from operations, and other external sources of available credit of one group are not generally available to the other group. Please carefully review the discussion of the sufficiency of these resources under the heading “Liquidity and Capital Resources” within the discussion of each operating group below.
Acacia Research Corporation’s cash and cash equivalent and short term investment balances, cash flows and anticipated cash flows from operations and other sources of external credit, are attributed to the Acacia Technologies group and the CombiMatrix group based on the respective assets of the specific businesses comprising each group. Issuances of AR-Acacia Technologies stock (and the proceeds thereof) are attributed to the Acacia Technologies group and issuances of AR-CombiMatrix stock (and the proceeds thereof) are attributed to the CombiMatrix group. Neither of the groups is obligated to fund the ongoing operations of the other group. Management has no intent to use the cash and cash equivalent balances, anticipated cash flow from operations, and other external sources of available credit of one group to fund the ongoing operations of the other group.
Off-Balance Sheet Arrangements
We have not entered into off-balance sheet financing arrangements, other than operating leases. Other than as set forth below, we have no significant commitments for capital expenditures in 2006. Other than as set forth below, we have no committed lines of credit or other committed funding or long-term debt. The following table lists Acacia Research Corporation’s material known future cash commitments as of December 31, 2006, and any material known commitments arising from events subsequent to year end:
| | | Payments Due by Period (In thousands) | |
| Contractual Obligations | | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | | | 2012 and Thereafter | |
Operating leases(2) | | $ | 1,205 | | $ | 1,084 | | $ | 1,121 | | $ | 1,086 | | $ | 783 | | $ | 131 | |
| Minimum license payments - CombiMatrix group | | | 500 | | | - | | | - | | | - | | | - | | | - | |
Minimum royalty payments - CombiMatrix group (1) | | | 100 | | | 100 | | | 100 | | | 100 | | | 100 | | | 675 | |
| Consulting contract - Acacia Technologies group | | | 99 | | | - | | | - | | | - | | | - | | | - | |
| Total contractual cash obligations | | $ | 1,904 | | $ | 1,184 | | $ | 1,221 | | $ | 1,186 | | $ | 883 | | $ | 806 | |
__________________________________
| | (1) | Refer to Note 14 to the Acacia Research Corporation consolidated financial statements for a description of the September 30, 2002 settlement agreement between CombiMatrix Corporation and Dr. Donald Montgomery and Nanogen. |
| | (2) | On February 1, 2007, the CombiMatrix group executed an amendment to their operating lease for office and laboratory space, the impact of which is included above. Refer to Note 14 to the consolidated financial statements included elsewhere herein. |
Recent Accounting Pronouncements
Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
Quantitative and Qualitative Disclosures About Market Risk
Our exposure to market risk is limited primarily to interest income sensitivity, which is affected by changes in the general level of United States interest rates, particularly because a significant portion of our investments are in short-term debt securities issued by the U.S. government, U.S. corporations, institutional money market funds and other money market instruments. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income received without significantly increasing risk. To minimize risk, we maintain a portfolio of cash, cash equivalents and short-term investments in a variety of investment-grade securities and with a variety of issuers, including corporate notes, commercial paper and money market instruments. Due to the nature of our short-term investments, we believe that we are not subject to any material market risk exposure. We do not have any derivative financial instruments.
ACACIA TECHNOLOGIES GROUP MANAGEMENT’S DISCUSSION AND ANALYSIS
(A Division of Acacia Research Corporation)
You should read this discussion in conjunction with the Acacia Technologies group, a division of Acacia Research Corporation, financial statements and related notes and the Acacia Research Corporation consolidated financial statements and related notes, both included elsewhere herein. Historical results and percentage relationships are not necessarily indicative of operating results for any future periods.
General
Refer to Item 1. “Business,” for a description of the Acacia Technologies group’s business. Although the AR-Acacia Technologies stock is intended to reflect the separate performance of the Acacia Technologies group, rather than the performance of Acacia Research Corporation as a whole, the Acacia Technologies group is not a separate legal entity. Holders of the AR-Acacia Technologies stock are stockholders of Acacia Research Corporation. As a result, they continue to be subject to all of the risks of an investment in Acacia Research Corporation and all of Acacia Research Corporation’s businesses, assets and liabilities. The assets Acacia Research Corporation attributes to the Acacia Technologies group could be subject to the liabilities of the CombiMatrix group.
Acacia Technologies Group
(A Division of Acacia Research Corporation)
Results of Operations
Division Net Loss (In thousands)
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| Division net loss | | $ | (5,496 | ) | $ | (6,275 | ) | $ | (5,543 | ) |
The changes in net loss were primarily due to operating results and activities, as discussed below.
Revenues (In thousands)
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| License fees | | $ | 34,825 | | $ | 19,574 | | $ | 4,284 | |
License Fees. Revenues for 2006 included license fees from 126 new licensing agreements covering 14 of our technology licensing and enforcement programs, as compared to 83 new licensing agreements covering 12 of our technology licensing and enforcement programs in 2005. The increase in license fee revenues in 2006 and 2005, as compared to 2004, reflects the impact of the increase in patent portfolios controlled by the Acacia Technologies group since 2004, including the impact of the GPH Acquisition, and the related increase in the number of patent licensing and enforcement programs developed, launched and generating revenues since 2004. License fee revenues recognized by the Acacia Technologies group fluctuate from period to period primarily based on the following factors:
| | · | the dollar amount of agreements executed each period, which is primarily driven by the nature and characteristics of the technology being licensed and the magnitude of infringement associated with a specific licensee; |
| | · | the specific terms and conditions of agreements executed each period and the periods of infringement contemplated by the respective payments; |
| · | fluctuations in the total number of agreements executed; |
| | · | fluctuations in the sales results or other royalty per unit activities of our licensees that impact the calculation of license fees due; |
| · | the timing of the receipt of periodic license fee payments and/or reports from licensees; and |
| | · | fluctuations in the net number of active licensees period to period. |
Revenues in 2004 were comprised of $2.8 million in DMT patent portfolio revenues and $1.5 million in previously deferred V-chip license fees (originally received and deferred in 2001) recognized as a result of the conclusion of V-chip patent litigation and related licensing program in August 2004.
Costs incurred in connection with the Acacia Technologies group’s ongoing licensing activities, other than inventor royalties expense, contingent legal fees expense and patent-related legal expenses, are included in marketing, general and administrative expenses.
Operating Expenses (In thousands)
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| Marketing, general and administrative expenses | | $ | 14,256 | | $ | 8,099 | | $ | 5,049 | |
| Legal expenses - patents | | | 4,780 | | | 2,468 | | | 3,133 | |
| Inventor royalties and contingent legal fees expense - patents | | | 17,159 | | | 11,106 | | | - | |
| Inventor royalties - V-chip | | | - | | | 225 | | | - | |
| Goodwill and other impairment charges | | | - | | | - | | | 1,656 | |
| Write-off of patent-related intangible asset | | | 297 | | | - | | | - | |
| Amortization of patents | | | 5,313 | | | 4,922 | | | 501 | |
Marketing, General and Administrative Expenses. The increase in 2006, as compared to 2005 primarily reflects Acacia Research Corporation’s adoption of SFAS No. 123R, effective January 1, 2006, which requires public companies to measure all employee stock-based compensation awards using a fair-value method and record such expense in their consolidated financial statements, as described under “Critical Accounting Estimates.” Non-cash stock compensation charges included in marketing, general and administrative expense in 2006 totaled $3,946,000, as compared to $356,000 in 2005, prior to adoption of FAS 123R.
Excluding the impact of the adoption of SFAS No. 123(R), the increase in marketing, general and administrative expenses in 2006, as compared to 2005 was due primarily to the addition of licensing, business development and engineering personnel for the Acacia Technologies group, an increase in the Acacia Technologies group’s patent-related research and consulting expenses for new and ongoing licensing and enforcement programs, an increase in accounting and legal fees related to the CombiMatrix Split-off Transaction and an increase in corporate, general and administrative costs related to the continued growth and expansion of Acacia Technologies group’s ongoing operations.
The change in marketing, general and administrative expenses in 2005, as compared to 2004, was due to an increase in personnel costs primarily related to the addition of patent licensing and business development personnel for the Acacia Technologies group, an increase in the Acacia Technologies group’s consulting expenses related to a consulting agreement executed with the former CEO of Global Patent Holdings, LLC in connection with the GPH Acquisition and an increase in the Acacia Technologies group’s patent-related research and consulting expenses for new and ongoing licensing and enforcement programs and other general and administrative expenses, including increases related to certain of the companies acquired in the GPH Acquisition. These increases were partially offset by a reduction in the Acacia Technologies group’s Sarbanes-Oxley compliance costs.
A summary of the main drivers of the change in marketing, general and administrative expenses, excluding the impact of non-cash stock compensation, for the periods presented is as follows (in thousands):
| | | | 2006 vs. 2005 | | | 2005 vs. 2004 | |
| | | | | | | | |
| Acacia Technologies group: | | | | | | | |
| Increase in personnel expenses | | $ | 1,247 | | $ | 1,202 | |
| Increase in GPH Acquisition related consulting expenses | | | 96 | | | 1,009 | |
| Increase (decrease) in accounting and other professional fees | | | 323 | | | (53 | ) |
| Increase in patent development / commercialization and other | | | | | | | |
| and other marketing, general and administrative costs | | | 701 | | | 256 | |
| Increase in CombiMatrix Split-off transaction costs | | | 200 | | | - | |
| Increase in GPH Acquisition related patent development/commercialization | | | | | | | |
| and other general and administrative expenses | | | - | | | 280 | |
Legal Expense - Patents. Patent-related legal expenses include patent-related prosecution and enforcement costs, incurred by outside patent attorneys engaged on an hourly basis and the out-of-pocket expenses incurred by law firms engaged on a contingent fee basis. Patent-related legal expenses fluctuate from period to period based on patent enforcement and prosecution activity associated with ongoing licensing and enforcement programs and the timing of the commencement of new licensing and enforcement programs in each period. Patent-related legal expenses include case related costs billed by outside counsel for economic analyses and damages assessments, expert witnesses and other consultants, case related audio/video presentations for the court, and other litigation support and administrative costs.
The increase in patent related legal expenses in 2006, as compared to 2005 is primarily due to a net increase in the number of ongoing patent enforcement litigations in 2006 compared to 2005 and an increase in the number of portfolios where we have engaged outside law firms on an hourly or discounted hourly basis. The increase also reflects significant third party consulting and expert expenses, including costs incurred related to expert witnesses and the preparation of damages reports, incurred in connection with certain of our patent portfolios that are further along in litigation. Refer to Item 1. “Business - Acacia Technologies Group” for a summary of ongoing Acacia Technologies group patent enforcement litigation.
We expect patent-related legal expenses to continue to fluctuate period to period based on the factors summarized above, in connection with the Acacia Technologies group’s current and future patent commercialization and enforcement programs.
As described earlier, patent related legal expenses in 2004 included $668,000 in previously deferred V-chip related legal expenses. Excluding the V-chip related costs recognized, the change in 2005, as compared to 2004 was primarily due to corresponding fluctuations in DMT® patent portfolio related claims prosecution, litigation and enforcement activity in the respective periods. DMT® related legal fees paid to outside attorneys are incurred based on actual time and out-of-pocket expenses incurred by external counsel and fluctuate from period to period based on patent enforcement and prosecution activity in each period. In addition, patent related legal expenses for 2005 included $654,000 in patent related prosecution and enforcement costs incurred by certain of the companies acquired in the GPH Acquisition.
Inventor Royalties and Contingent Legal Fees Expense. The Acacia Technologies group incurred inventor royalties expense totaling $9.6 million and $5.5 million in 2006 and 2005, respectively, and contingent legal fees expense totaling $7.5 million and $5.6 million in 2006 and 2005, respectively. Inventor royalties expenses and contingent legal fees expenses were incurred in connection with the recognition of the related license fee revenues summarized above. The majority of the Acacia Technologies group’s patent portfolios are subject to patent and patent rights agreements with inventors containing provisions granting to the original patent owner the right to receive inventor royalties based on future net revenues, as defined in the respective agreements and may also be subject to contingent legal fee arrangements with external law firms engaged on a contingent fee basis. The economic terms of the inventor and contingent arrangements, if any, vary across the Acacia Technologies group’s patent portfolios. As such, inventor royalties and contingent legal fees expenses fluctuate period to period based on the amount of revenues recognized each period and the mix of specific patent portfolios generating revenues each period.
Inventor royalties and contingent legal fees expense increased primarily as a result of the increase in license fee revenues recognized in 2006, as compared to 2005. However, certain of the patent portfolios generating significant revenues in 2006 were not subject to contingent fee arrangements with law firms, as described earlier. As such, the percentage increase in contingent legal fees in 2006, as compared to 2005, was less than the percentage increase in revenues for the same periods.
Inventor Royalties V-chip. Results in 2005 included $225,000 of V-chip related inventor royalties expense recognized as a result of the conclusion of all V-chip related litigation activities in October of 2005. As a result of the conclusion of all V-chip related activities, no additional V-chip related inventor royalties expense will be incurred in future periods.
Goodwill and Other Impairment Charges. As a result of the conclusion of the Acacia Technologies group’s V-chip patent licensing program in August 2004, 2004 results included a non-cash impairment charge of $1.6 million associated with the write-off of goodwill related to the V-chip.
Write-off of Patent-related Intangible Asset. In June 2006, the Acacia Technologies group recorded a non-cash impairment charge of $297,000, related to the write-off of a patent-related intangible asset. During the second quarter of 2006, pursuant to the terms of the respective license agreement, management elected to terminate its rights to exclusively license and enforce the patent, resulting in the write-off of the remaining carrying value of the patent-related intangible asset as of June 30, 2006.
Amortization of Patent. The increase in 2006, as compared to 2005 was due to twelve full months of patent amortization expense resulting from the January 28, 2005 GPH Acquisition in 2006, as compared to 11 months of amortization in 2005. Patent amortization expense related to the GPH Acquisition was $4.7 million and $4.4 million in 2006 and 2005, respectively. In addition, patent amortization expense in 2006 and 2005 includes $207,000 and $65,000 in additional patent amortization charges related to certain of the patent portfolios acquired by the Acacia Technologies group subsequent to the GPH Acquisition. Patent amortization charges will continue to be significant in future periods as the Acacia Technologies group continues to amortize its acquired patent related costs over a weighted-average remaining economic useful life of approximately 4 years.
The increase in 2005, as compared to 2004 was due primarily to the amortization of patent related intangibles acquired in connection with the GPH Acquisition. Approximately $25.1 million of the purchase consideration paid in the GPH Acquisition was allocated to amortizable patents and related patent rights acquired, and are being amortized over an original weighted-average economic useful life of approximately 6 years. Amortization expense related to the patents and patent rights acquired in the GPH Acquisition was $4.4 million in 2005.
Other
Discontinued Operations. Results for 2005 and 2004 include charges, net of minority interests, of $237,000 and $104,000, respectively, related to estimated additional costs to be incurred in connection with the discontinued operations of Soundbreak.com (originally ceased operations in February 2001), related primarily to certain noncancellable lease obligations and a reduction in estimated amounts recoverable from existing sublease arrangements. The related lease obligations, which were guaranteed by Acacia Research Corporation, expired in August 2005.
Inflation
Inflation has not had a significant impact on the Acacia Technologies group in the current or previous periods.
Liquidity and Capital Resources
The Acacia Technologies group’s cash and cash equivalents and short-term investments totaled $45.0 million at December 31, 2006, compared to $39.0 million at December 31, 2005. Working capital at December 31, 2006 was $42.6 million, compared to $38.9 million at December 31, 2005.
The net change in cash and cash equivalents for 2006, 2005 and 2004 was comprised of the following (in thousands):
| | | For the Years Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
| Net cash provided by (used in) continuing operations: | | | | | | | | | | |
| Operating activities | | $ | 6,095 | | $ | (2,477 | ) | $ | (3,232 | ) |
| Investing activities | | | 10,513 | | | (13,094 | ) | | (321 | ) |
| Financing activities | | | 1,198 | | | 19,657 | | | (305 | ) |
| Net cash used in discontinued operations | | | (89 | ) | | (513 | ) | | (925 | ) |
| Increase (decrease) in cash and cash equivalents | | $ | 17,717 | | $ | 3,573 | | $ | (4,783 | ) |
Operating Activities. The change to net cash inflows from operations for the Acacia Technologies group in 2006, as compared to net cash outflows from operations in 2005, was primarily due to the increase in license fee payments received from licensees, which totaled $38.6 million in 2006, compared to $15.6 million in 2005, reflecting the increase in license fee revenues recognized in 2006, as compared to 2005, as discussed above. The increase in license fee revenues in 2006 was partially offset by increases in inventor royalties expenses, contingent legal fees expenses, patent-related legal expenses, personnel expenses, and other corporate, general and administrative expenses, as described above, and the impact of the timing of payments to inventors, attorneys and other vendors. Accounts receivable for the Acacia Technologies group decreased to $269,000 at December 31, 2006, compared to $4.4 million at December 31, 2005, due to the collection of license fees receivable at December 31, 2005, during the first quarter of 2006, in accordance with the terms of the related underlying license agreements.
The decrease in net cash outflows from operations for the Acacia Technologies group in 2005, as compared to 2004, was primarily due to the increase in license fee payments received from licensees, which totaled $15.6 million in 2005, compared to $3.1 million in 2004. The increase was partially offset by an increase in marketing, general and administrative expenses related to the continued expansion of the Acacia Technologies group’s business, including increased inventor royalties, contingent legal fees and consulting expenses related to the GPH Acquisition, as discussed above. The change also reflects the impact of the timing of receipt of license fee payments from licensees and the timing of payments to inventors, contingent law firms and vendors. Accounts receivable for the Acacia Technologies group increased to $4.4 million at December 31, 2005, compared to $193,000 at December 31, 2004, primarily due to the timing of the execution of certain paid-up license agreements with payment terms. The majority of accounts receivable balances at December 31, 2005 were received from the respective licensees in the first quarter of 2006, in accordance with the terms of the respective license agreements.
Investing Activities. The change in net cash flows used in investing activities for the periods presented reflects fluctuations in net purchases and sales of available-for-sale investments in connection with ongoing short-term cash management activities. Short term investments represent capital available to fund current operations and fund capital expenditures. Net cash outflows from investing activities for 2005 also included the impact of cash consideration and related acquisition and registration costs, totaling $5.8 million, paid by the Acacia Technologies group in connection with the GPH Acquisition in the first quarter of 2005. In addition, the Acacia Technologies group incurred patent acquisition costs of $1.0 million and $445,000 in 2006 and 2005, respectively, related to the acquisition of additional patent portfolios, as described earlier.
Financing Activities. Net cash inflows attributed to the Acacia Technologies group from financing activities in 2005 were primarily comprised of net proceeds of approximately $19.5 million, related to the sale of 3.5 million shares of AR-Acacia Technologies stock in February 2005. Net cash inflows attributed to the Acacia Technologies group in 2006, 2005 and 2004 also included AR-Acacia Technologies stock option exercise proceeds of $1.5 million, $304,000 and $90,000, respectively. Corporate costs allocated by Acacia Research Corporation to the CombiMatrix group in 2006, 2005 and 2004 totaled $277,000, $179,000 and $396,000, respectively.
Management believes that the Acacia Technologies group’s cash and cash equivalent balances, anticipated cash flow from operations and other external sources of available credit, will be sufficient to meet its cash requirements through at least March 2008 and for the foreseeable future. The Acacia Technologies group may however encounter unforeseen difficulties that may deplete its capital resources more rapidly than anticipated, including those set forth in the Acacia Technologies group Risk Factors elsewhere herein. Any efforts to seek additional funding could be made through equity, debt or other external financing and there can be no assurance that additional funding will be available on favorable terms, if at all. If the Acacia Technologies group fails to obtain additional funding when needed, it may not be able to execute its business plans and its business may suffer. Refer to the “Liquidity and Risks” discussion included in Note 1 to the Acacia Research Corporation consolidated financial statements included elsewhere herein for additional information.
Off-Balance Sheet Arrangements
The Acacia Technologies group has not entered into off-balance sheet financing arrangements, other than operating leases. The Acacia Technologies group has no significant commitments for capital expenditures in 2006. Other than as set forth below, the Acacia Technologies group has no committed lines of credit or other committed funding or long-term debt. The following table lists the Acacia Technologies group’s material known future cash commitments as of December 31, 2006, and material known commitments arising from events subsequent to year end:
| | | Payments Due by Period (In thousands) | |
Contractual Obligations | | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | | | 2012 and Thereafter | |
Operating leases(1) | | $ | 617 | | $ | 696 | | $ | 724 | | $ | 753 | | $ | 783 | | $ | 131 | |
| Consulting contract | | | 99 | | | - | | | - | | | - | | | - | | | - | |
| Total contractual cash obligations | | $ | 716 | | $ | 696 | | $ | 724 | | $ | 753 | | $ | 783 | | $ | 131 | |
________________
| | (1) | Excludes any allocated rent expense in connection with Acacia Research Corporation’s management allocation policies. |
Recent Accounting Pronouncements
Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
Quantitative and Qualitative Disclosures About Market Risk
The Acacia Technologies group’s exposure to market risk is limited primarily to interest income sensitivity, which is affected by changes in the general level of United States interest rates, particularly because a significant portion of our investments are in short-term debt securities issued by the United States government and United States corporations, auction rate securities, institutional money market funds and other money market instruments. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income received without significantly increasing risk. To minimize risk, we maintain a portfolio of cash, cash equivalents and short-term investments in a variety of investment-grade securities and with a variety of issuers, including U.S. government and corporate notes and bonds, commercial paper, auction rate securities and money market instruments. Due to the nature of our short-term investments, we believe that we are not subject to any material market risk exposure. We do not have any derivative financial instruments.
DISCUSSION OF SEGMENTS’ OPERATIONS, FINANCIAL RESOURCES AND LIQUIDITY
COMBIMATRIX GROUP MANAGEMENT’S DISCUSSION AND ANALYSIS
(A Division of Acacia Research Corporation)
You should read this discussion in conjunction with the CombiMatrix group, a division of Acacia Research Corporation, financial statements and related notes and the Acacia Research Corporation consolidated financial statements and related notes, both included elsewhere herein. Historical results and percentage relationships are not necessarily indicative of operating results for any future periods.
General
Refer to Item 1. “Business,” for a general overview of the CombiMatrix group’s business. Although AR-CombiMatrix stock is intended to reflect the separate performance of the CombiMatrix group, rather than the performance of Acacia Research Corporation as a whole, the CombiMatrix group is not a separate legal entity. Holders of AR-CombiMatrix stock are stockholders of Acacia Research Corporation. As a result, they continue to be subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. The assets Acacia Research Corporation attributes to the CombiMatrix group could be subject to the liabilities of the Acacia Technologies group.
CombiMatrix Group
(A Division of Acacia Research Corporation)
Results of Operations
Division Net Income (Loss) (In thousands)
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| Division net income (loss) | | $ | (19,960 | ) | $ | (12,401 | ) | $ | 710 | |
The changes in net income (loss) were primarily due to operating results and activities, as discussed below.
Revenues and Cost of Revenues (In thousands)
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| Collaboration agreements | | $ | - | | $ | 2,266 | | $ | 17,302 | |
| Government contracts | | | 2,074 | | | 3,849 | | | 1,993 | |
| Cost of government contract revenues | | | (1,959 | ) | | (3,683 | ) | | (1,874 | ) |
| Products | | | 3,278 | | | 1,765 | | | 230 | |
| Cost of product sales | | | (1,258 | ) | | (820 | ) | | (173 | ) |
| Service contracts | | | 388 | | | 153 | | | 116 | |
Collaboration Agreements. During the fourth quarter of 2005, the CombiMatrix group completed all obligations under its collaboration and supply agreement with Toppan. As a result of completing its obligations under this agreement, and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized $2.3 million of previously deferred collaboration agreement revenues during the fourth quarter of 2005. Research and development activities and expenses related to the Toppan agreement were incurred during the two-year term of the agreement, which was originally executed in May 2003.
In March 2004, the CombiMatrix group completed all phases of its research and development agreement with Roche. As a result of completing all obligations under this agreement and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized $17.3 million of collaboration agreement revenues during the first quarter of 2004, all of which were previously deferred. The majority of research and development efforts under the Roche agreement were incurred prior to 2004.
Government Contracts and Cost of Government Contract Revenues. Under the terms of its contracts with the Department of Defense, the CombiMatrix group is reimbursed on a periodic basis for actual costs incurred to perform its obligations, plus a fixed fee. Revenues are recognized under the percentage-of-completion method of accounting, using the cost-to-cost approach to measure completeness at the end of each reporting period. Cost of government contract revenues reflect research and development expenses incurred in connection with the CombiMatrix group’s commitments under its DoD contracts.
Revenues and associated costs decreased during 2006, as compared to 2005 due to a higher level of contract related activity leading to the completion in December 2005, of the CombiMatrix group’s commitments under its previous two-year, $5.9 million research and development contract with the DoD to further the development of the CombiMatrix group’s array technology for the electrochemical detection of biological threat agents. In February 2006, the CombiMatrix group executed a new one-year, $2.1 million contract with the DoD to further the development of its electrochemical detection system. In August 2006, the CombiMatrix group executed a new two-year, $1.9 million contract with the DoD to integrate its electrochemical detection technology with its microfluidics “lab on a chip” technology for national defense and homeland security applications. Overall, the activity on these new DoD contracts was lower in 2006 than activity under the CombiMatrix group’s previous $5.9 million contract in 2005, resulting in the overall decrease in government contract revenues and cost of revenues in 2006, as compared to 2005. These revenues and costs increased during 2005, as compared to 2004 due to increased activity on the two-year, $5.9 million contract and due to the fact that only nine months of activity were incurred in 2004, versus a full year of activity in 2005. The $5.9 million contract was completed in 2005 and there are no additional revenues or costs expected to be recognized from this contract in future periods.
Product Revenues and Cost of Product Sales. Product revenues and costs of product sales relate to domestic and international sales of the CombiMatrix group’s array products. Product revenues in 2006 include the sale of DNA synthesizer instruments and CustomArray 12K DNA expression arrays and related hardware, as compared to lower instrument and 12K DNA expression array sales in 2005. The overall increase in product revenues in 2006 was due primarily to the increased product offerings currently available to the CombiMatrix group’s customers, which includes 12K and 4X2K arrays, DNA synthesizer and electrochemical detection reader instruments and related hardware, as compared to only the 902 and 12K expression arrays and DNA synthesizer instruments in 2005. Product revenues and costs of product sales during 2004 and 2005 relate to domestic and international sales of the CombiMatrix group’s array products, including its CustomArray 902 DNA array platform launched in March 2004, CustomArray 12K DNA expression array launched in July 2004 and its commercial DNA array synthesizer instrument launched in August 2005. The CombiMatrix group’s product revenues increased in 2005, as compared to 2004 due primarily to a full year of array sales recognized in 2005, as compared to only a partial year’s recognition in 2004, as well as the launch of the CombiMatrix group’s DNA array synthesizer instrument in 2005.
Service Contracts. Prior to 2005, all service contract revenues were recognized by CombiMatrix K.K. from existing array customers in Japan. As of December 31, 2004, the terms of these contracts had expired. Costs incurred in connection with these services were not material. In 2006 and 2005, service contract revenues include maintenance and service contract fees relating to DNA array synthesizers sold during those periods, and have increased in 2006 due to the sale of additional array synthesizers. Such service contracts are typically for twelve months, and the consideration received is recognized ratably over the service period.
Operating Expenses (In thousands)
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| Research and development expenses | | $ | 9,485 | | $ | 5,783 | | $ | 5,385 | |
Research and Development Expenses. In 2006, the CombiMatrix group continued internal research and development efforts to improve and expand its technology and product offerings. The increase in internal research and development expenses in 2006, as compared to 2005 was due primarily to costs incurred in connection with the development of higher density array products, as well as an increase in expenses incurred by the CombiMatrix group’s subsidiary, CMDX, which was formed and began research and development activities in the area of diagnostic applications during the second quarter of 2005. In addition, research and development expenses include $1.1 million, $0 and $91,000 in 2006, 2005 and 2004, respectively, of non-cash stock based compensation expense. The increase in non-cash stock compensation included in research and development expense was due to the adoption of SFAS No. 123R effective January 1, 2006, as described under “Critical Accounting Estimates.” The CombiMatrix group’s research and development costs increased in 2005, as compared to 2004 primarily due to the launch of the CustomArray platform and continued launch of related array products, including the CombiMatrix group’s DNA array synthesizer instrument launched in August 2005. Research and development activities at the CombiMatrix group’s wholly owned subsidiary, CMDX, which was formed in April of 2005, also contributed to the overall increase in research and development expenses for 2005.
Future research and development expenses were and will continue to be incurred in connection with the CombiMatrix group’s efforts in the area of genomics, diagnostics, and drug discovery and development. The CombiMatrix group expects its research and development expenses to continue to be volatile and such expenses could increase in future periods as additional contract and/or internal research and development projects are undertaken and/or as new collaborations are executed with strategic partners.
| | | 2006 | | 2005 | | 2004 | |
| | | | | | | | |
| Marketing, general and administrative expenses | | $ | 12,707 | | $ | 9,827 | | $ | 9,902 | |
| Goodwill impairment charge | | | - | | | 565 | | | - | |
| Amortization of patents and royalties | | | 1,482 | | | 1,312 | | | 1,234 | |
| Legal settlement charges (gains) | | | - | | | (406 | ) | | 812 | |
| Loss from equity investment | | | 1,036 | | | 352 | | | 17 | |
Marketing, General and Administrative Expenses. The increase in 2006, as compared to 2005 primarily reflects Acacia Research Corporation’s adoption of SFAS No. 123R, effective January 1, 2006, as described under “Critical Accounting Estimates.” Marketing, general and administrative expenses include non-cash stock compensation of $1.3 million, ($159,000) and $663,000 in 2006, 2005 and 2004, respectively. Excluding the impact of the adoption of SFAS No. 123(R), the increase was primarily due to the impact of a full year of general and administrative expenses incurred by CMDX in 2006, which commenced operations in the second quarter of 2005, as well as increased legal and accounting expenses primarily related to the planned CombiMatrix Split-off Transaction. These increases were partially offset by a decrease in marketing, sales and other expenses.
The change in 2005, as compared to 2004, reflects an increase in marketing and sales costs related to the CombiMatrix group’s CustomArray™ platform, which were driven primarily by increases in the CombiMatrix group’s sales force and expanded marketing and advertising efforts and an increase in marketing, general and administrative expenses in connection with the creation of CombiMatrix Molecular Diagnostics in April 2005. These increases were partially offset by a reduction in the CombiMatrix group’s Sarbanes-Oxley compliance costs in 2005.
A summary of the main drivers of the change in marketing, general and administrative expenses, excluding the impact of non-cash stock compensation, for the periods presented is as follows (in thousands):
| | | | 2006 vs. 2005 | | | 2005 vs. 2004 | |
| | | | | | | | |
| CombiMatrix group: | | | | | | | |
| (Decrease) increase in marketing and sales expenses | | $ | (924 | ) | $ | 478 | |
| Increase in general and administrative expenses related to CMDX | | | 1,683 | | | 598 | |
| Increase (decrease) in legal, accounting and other professional fees | | | 1,169 | | | (250 | ) |
| Decrease in general and administrative expenses | | | (467 | ) | | - | |
Included in marketing, general and administrative expenses are allocated corporate charges of $551,000 in 2006, $498,000 in 2005 and $689,000 in 2004. Refer to “Critical Accounting Policies” for a description of the management allocation policies implemented.
Amortization of Patent and royalties. Amortization of patents and royalties includes royalty expense of $348,000, $217,000 and $138,000, in 2006, 2005 and 2004, respectively, related to the CombiMatrix group’s September 2002 settlement agreement with Nanogen, Inc., and are equal to 12.5% of payments made to the CombiMatrix group from sales of certain products developed based on the patents that had been in dispute in the litigation with Nanogen, Inc. prior to settlement. The increase in royalties expense for the years presented is due to the corresponding increase in CombiMatrix group product revenue payments received for the period.
Goodwill and Other Impairment Charges. The CombiMatrix group recognized a goodwill impairment charge of $565,000, during the fourth quarter of 2005, related to its Advanced Materials Sciences and CombiMatrix K.K. reporting units. These reporting units were tested for impairment in the fourth quarter of 2005, in connection with the CombiMatrix group’s annual forecasting process. Due to the lack of third-party research and development funding for Advanced Materials Sciences and declining array product sales at CombiMatrix K.K., operating profits and cash flows were lower than expected during the preceding three quarters. Based on these trends, the operating forecasts for 2006 were revised downward, resulting in the goodwill impairment charge.
Legal Settlement Charges(Gains). Legal settlement charges (gains) related to AR-CombiMatrix stock issuable and/or potentially issuable in connection with certain anti-dilution provisions of the September 2002 settlement agreement between CombiMatrix Corporation, Dr. Donald Montgomery, and Nanogen, Inc. The related liability reflected management’s estimate, as of each balance sheet date, of the fair value of AR-CombiMatrix stock to be issued to Nanogen, Inc. as a result of certain options and warrants exercised during the period, if any, and the fair value of AR-CombiMatrix stock potentially issuable to Nanogen, Inc. as of each balance sheet date, pursuant to the anti-dilution terms of the agreement. The liability was adjusted at each balance sheet date for changes in the market value of the AR-CombiMatrix stock and reflected as long-term until settled in equity. All anti-dilution provisions of the settlement agreement expired as of September 30, 2005, resulting in no further liability subsequent to September 30, 2005, or in any future periods, and a net gain in the statement of operations and comprehensive loss of $406,000 in 2005.
Loss from Equity Investment. The CombiMatrix group owned 33% and 19% as of December 31, 2006 and 2005, respectively, of Leuchemix, a private drug development firm, which is developing several compounds for the treatment of leukemia and other cancers. The CombiMatrix group’s equity in the losses of Leuchemix increased due to the CombiMatrix group’s increased ownership in Leuchemix as well as an increase in expenses incurred by Leuchemix. The CombiMatrix group was under a contractual commitment to increase its ownership interest to 33% in 2006. As of October 2006, the CombiMatrix group satisfied its contractual commitment to increase its ownership interest in Leuchemix pursuant to the terms of the underlying agreement.
Other
Warrant Charges (Gains). In accordance with SFAS No. 150, and related interpretations, certain AR-CombiMatrix stock purchase warrants outstanding at December 31, 2006, which were issued in connection with equity financings in December 2006, September 2005 and May 2003, have been classified as a long-term liability due to certain redemption provisions associated with the underlying AR-CombiMatrix stock. The warrant liability is marked to market at each balance sheet date. Changes in the fair value of the stock purchase warrant liability are reflected in the consolidated statement of operations and comprehensive loss. Warrant gains totaled $1.8 million and $812,000 in 2006 and 2005, respectively. The increase in warrant gains reflects the decrease in the AR-CBMX stock price during the periods presented, and a $825,000 gain related to the mark to market of the warrants issued in connection with the CombiMatrix group’s December 2006 equity financing, as described below. Refer to Note 10 to the Acacia Research Corporation consolidated financial statements elsewhere herein.
Inflation
Inflation has not had a significant impact on the CombiMatrix group in the current or prior periods.
Liquidity and Capital Resources
At December 31, 2006, cash, cash equivalents and short-term investments totaled $14.3 million, compared to $20.2 million at December 31, 2005. Working capital was $12.0 million at December 31, 2006, compared to $19.2 million at December 31, 2005.
The change in cash and cash equivalents for the years ended December 31, 2006, 2005 and 2004 was comprised of the following (in thousands):
| | | For the Years Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
| Net cash provided by (used in): | | | | | | | | | | |
| Operating activities | | $ | (15,145 | ) | $ | (13,696 | ) | $ | (11,584 | ) |
| Investing activities | | | 4,981 | | | 3,390 | | | (8,448 | ) |
| Financing activities | | | 12,327 | | | 12,914 | | | 19,227 | |
| Effect of exchange rate on cash | | | - | | | 73 | | | (17 | ) |
| Increase (decrease) in cash and cash equivalents | | $ | 2,163 | | $ | 2,681 | | $ | (822 | ) |
Operating Activities. Cash receipts from customers for the CombiMatrix group for 2006, were $6.3 million, compared to $5.3 million in 2005. The increase was primarily due to increased sales and related cash receipts from CustomArray customers totaling $3.8 million in 2006, as compared to $1.7 million in 2005. This increase was partially offset by decreased cash collections from the CombiMatrix group’s government contract billings, which were $2.5 million in 2006, as compared to $3.6 million in 2005. Cash outflows from operations for the CombiMatrix group for 2006 increased due to an increase in cash operating expenses totaling $21.4 million, as compared to $18.9 million in 2005, due primarily to an increase in research and development, marketing, general and administrative expenses related to CMDX as described above, and the impact of the timing of vendor payments.
The change in net cash outflows from operations for the CombiMatrix group in 2005, as compared to 2004, was due primarily to an increase in operating expenses totaling $18.9 million in 2005, as compared to $14.7 million in 2004. The increase was due primarily to increased research and development and general and administrative costs incurred as discussed above, as well as the net impact of the timing of the receipt of payments from customers and payments to vendors. The increase in cash outflows from operating expenses was partially offset by an increase in cash receipts from customers, which totaled $5.3 million in 2005, as compared to $3.0 million in 2004. The increase was primarily due to increased activity under the CombiMatrix group’s two-year research and development contract with the Department of Defense, resulting in billings and cash payments during 2005 of $3.6 million as compared to $1.7 million in 2004, as well as increased sales and related cash receipts from CustomArray™ customers totaling $1.7 million in 2005, as compared to $113,000 in 2004.
Investing Activities. The change in net cash flows used in investing activities for the periods presented reflects fluctuations in net purchases and sales of available-for-sale investments by the CombiMatrix group in connection with ongoing short-term cash management activities. Short term investments represent capital available to fund current operations and fund capital expenditures. Net cash outflows from investing activities included additional contractual investments in Leuchemix totaling $2.2 million, $1.6 million and $250,000 in 2006, 2005 and 2004, respectively. Fixed asset purchases, totaled $536,000, $1.3 million, and $810,000 in 2006, 2005 and 2004, respectively.
Financing Activities. In June 2006, Acacia Research Corporation entered into a Standby Equity Distribution Agreement (the “SEDA”) with Cornell Capital Partners, LP ("Cornell"), providing up to $50 million of equity financing from Cornell through the sale of up to 13,024,924 shares of AR-CombiMatrix common stock through June 2008. Cash inflows attributed to the CombiMatrix group from financing activities in 2006 includes equity financings raising net proceeds of $3,070,000 through the sale of 3,211,345 shares of AR-CombiMatrix stock under the SEDA, which was cancelled in December 2006. Equity financings in 2006 also included the December 2006, registered direct offering with Oppenheimer & Co., Inc. as the placement agent, raising net proceeds of $9,266,000 through the issuance of 9,768,313 units. Each unit consists of one share of AR-CombiMatrix common stock and 1.2 five-year common stock warrants. Refer to Note 9 to the consolidated financial statements included in this report for additional information.
The change in net cash inflows attributed to the CombiMatrix group from financing activities in 2005, compared to 2004, was due to the completion of equity financings which raised net proceeds of approximately $12.7 million through the sale of Acacia Research - CombiMatrix common stock during 2005, compared to equity financing net proceeds of $13.7 million during 2004.
Management believes that the CombiMatrix group’s cash and cash equivalent balances, anticipated cash flows from operations and external sources of funding from the capital markets will be sufficient to meet its cash requirements through December 31, 2007. In order for the CombiMatrix group to sustain operations beyond December 31, 2007, the CombiMatrix group will be required to obtain capital from external sources. However, there can be no assurances that the CombiMatrix group will be able to secure additional sources of financing at times and at terms acceptable to management. The issuance of additional equity securities will also cause dilution to the AR-CombiMatrix shareholders. If external financing sources of financing are not available or are inadequate to fund the CombiMatrix group’s operations, management will be required to reduce our operating costs including research projects and personnel, which could jeopardize its future strategic initiatives and business plans. For example, reductions in research and development activities and/or personnel at the CombiMatrix group’s Mukilteo, Washington facility could result in the inability to invest the resources necessary to continue to develop next-generation products and improve existing product lines in order to remain competitive in the marketplace, resulting in reduced revenues and cash flows from the sales of the CombiMatrix group’s CustomArray products and services. Also, reduction in operating costs at the CombiMatrix group’s diagnostics subsidiary in Irvine, California, (“CMDX”), should they occur, could jeopardize the CombiMatrix group’s ability to launch, market and sell additional products and services necessary to order grow and sustain its operations and eventually achieve profitability. As discussed in Note 1 to the consolidated financial statements included elsewhere herein, the anticipation that the CombiMatrix group will be required to obtain additional financing in the foreseeable future raises substantial doubt about the CombiMatrix group’s ability to sustain operations beyond December 31, 2007. In addition to seeking capital from outside sources, the CombiMatrix group’s plans in regard to these matters included reductions in personnel and in fixed overhead costs (i.e., the CombiMatrix group lease commitment reduction discussed elsewhere herein) made in late 2006 and early 2007. Also, the CombiMatrix group is focusing its sales and product development efforts on its core diagnostic array platform as well as its funded research and development projects for the DoD.
The CombiMatrix group may also encounter unforeseen difficulties that may deplete its capital resources more rapidly than anticipated, including those set forth in the CombiMatrix group Risk Factors included elsewhere herein. Any efforts to seek additional funding could be made through equity, debt or other external financing, and there can be no assurance that additional funding will be available on favorable terms, if at all.
The CombiMatrix group’s long-term capital requirements will be substantial and the adequacy of available funds will depend upon many factors, including:
• the costs of commercialization activities, including sales and marketing, manufacturing and capital equipment;
• our continued progress in research and development programs;
• the costs involved in filing, prosecuting, enforcing and defending any patents claims, should they arise;
• our ability to license technology;
• competing technological developments;
• the creation and formation of strategic partnerships;
• the costs associated with leasing and improving our headquarters in Mukilteo, Washington and in Irvine, California; and
• other factors that may not be within our control.
Off-Balance Sheet Arrangements
The CombiMatrix group has not entered into off-balance sheet financing arrangements, other than operating leases. Other than as set forth below, the CombiMatrix group has no significant commitments for capital expenditures in 2007. Other than as set forth below, the CombiMatrix group has no committed lines of credit or other committed funding or long-term debt. The following table lists the CombiMatrix group’s material known future cash commitments as of December 31, 2006:
| | | Payments Due by Period (In thousands) | |
Contractual Obligations | | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | | | 2012 and Thereafter | |
Operating leases(2) | | $ | 588 | | $ | 388 | | $ | 397 | | $ | 333 | | $ | - | | $ | - | |
| Minimum license payments | | | 500 | | | - | | | - | | | - | | | - | | | - | |
Minimum royalty payments (1) | | | 100 | | | 100 | | | 100 | | | 100 | | | 100 | | | 675 | |
| Total contractual cash obligations | | $ | 1,188 | | $ | 488 | | $ | 497 | | $ | 433 | | $ | 100 | | $ | 675 | |
____________________________________
| (1) | Refer to Note 14 to the Acacia Research Corporation consolidated financial statements for a description of the September 30, 2002 settlement agreement between CombiMatrix Corporation and Dr. Donald Montgomery and Nanogen. |
| (2) | On February 1, 2007, the CombiMatrix group executed an amendment to their operating lease for office and laboratory space, the impact of which is included above. Refer to Note 14 to the consolidated financial statements included elsewhere herein. Excludes any allocated rent expense in connection with Acacia Research Corporation’s management allocation policies. |
Recent Accounting Pronouncements
Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
Quantitative and Qualitative Disclosures About Market Risk
The CombiMatrix group’s exposure to market risk is limited to interest income sensitivity, which is affected by changes in the general level of United States interest rates, particularly because the majority of the group’s investments are in short-term debt securities issued by the U.S. treasury and by U.S. corporations and auction rate securities. The primary objective of the group’s investment activities is to preserve principal while at the same time maximizing the income the CombiMatrix group receives without significantly increasing risk. To minimize risk, the CombiMatrix group maintains its portfolio of cash, cash equivalents and short-term investments in a variety of investment-grade securities and with a variety of issuers, including corporate notes, commercial paper, government securities, auction rate securities and money market funds. Due to the nature of its short-term investments, the CombiMatrix group believes that it is not subject to any material market risk exposure.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Refer to the caption “Quantitative and Qualitative Disclosures About Market Risk” for Acacia Research Corporation, the CombiMatrix group and the Acacia Technologies group under Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
The primary objective of our investment activities is to preserve principal while concurrently maximizing the income we receive from our investments without significantly increasing risk. Some of the securities that we may invest in may be subject to market risk. This means that a change in prevailing interest rates may cause the principal amount of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the current value of the principal amount of our investment will decline. To minimize this risk in the future, we intend to maintain our portfolio of cash equivalents and short-term investments in a variety of securities, including commercial paper, money market funds, high-grade corporate bonds, government and non-government debt securities and certificates of deposit. In general, money market funds are not subject to market risk because the interest paid on such funds fluctuates with the prevailing interest rate. As of December 31, 2006, all of our investments were in money market funds, high-grade corporate bonds, auction rate securities, certificates of deposit and U.S. government debt securities. A hypothetical 100 basis point increase in interest rates would not have a material impact on the fair value of our available-for-sale securities as of December 31, 2006. Refer to Note 3 to the Acacia Research Corporation consolidated financial statements.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and related financial information required to be filed hereunder are indexed under Item 15 of this report and are incorporated herein by reference.
Item 9. CHANGES IN AND DISAGREEMENTS WITH INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
Item 9A. CONTROLS AND PROCEDURES
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended. Based on this evaluation, our principal executive officer and our principal financial officer concluded that, as of the end of the period covered by this annual report, our disclosure controls and procedures were effective to ensure that the information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure, and that such information is recorded, processed, summarized and reported within the time periods prescribed by the SEC.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control - Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2006.
Our management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2006 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included herein.
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Except as provided below, the information required by this Item is incorporated by reference from the information under the captions entitled “Election of Directors-Nominees,” “Executive Officers” and “Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive proxy statement to be filed with the SEC no later than April 30, 2006.
Code of Conduct.
Acacia Research Corporation has adopted a Code of Conduct that applies to all of its employees, including its chief executive officer, chief financial and accounting officer, president and any persons performing similar functions. Our Code of Conduct is provided on our internet website at www.acaciaresearch.com.
Item 11. EXECUTIVE COMPENSATION
The information required by this Item is incorporated by reference from the information under the caption entitled “Executive Officer Compensation and Other Information” in our definitive proxy statement to be filed with the SEC no later than April 30, 2006.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The information required by this Item is incorporated by reference from the information under the caption entitled “Security Ownership of Certain Beneficial Owners and Management” in our definitive proxy statement to be filed with the SEC no later than April 30, 2006.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item is incorporated by reference from the information under the caption entitled “Certain Transactions” in our definitive proxy statement to be filed with the SEC no later than April 30, 2006.
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item is incorporated by reference from the information under the caption entitled “Audit Committee Report” in our definitive proxy statement to be filed with the SEC no later than April 30, 2006.
PART IV
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this report.
(1) Financial Statements
| | Page |
| Acacia Research Corporation Consolidated Financial Statements | |
| | |
| Report of Independent Registered Public Accounting Firm | F-1 |
| Consolidated Balance Sheets as of December 31, 2006 and 2005 | F-2 |
| Consolidated Statements of Operations and Comprehensive Loss for the Years Ended December 31, 2006, 2005 and 2004 | F-3 |
| Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2006, 2005 and 2004 | F-4 |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2006, 2005 and 2004 | F-5 |
| Notes to Consolidated Financial Statements | F-6 |
| | |
| | |
| *Acacia Technologies Group Financial Statements | |
| (A Division of Acacia Research Corporation) | |
| | |
| Report of Independent Registered Public Accounting Firm | F-46 |
| Balance Sheets as of December 31, 2006 and 2005 | F-47 |
| Statements of Operations for the Years Ended December 31, 2006, 2005 and 2004 | F-48 |
| Statements of Allocated Net Worth for the Years Ended December 31, 2006, 2005 and 2004 | F-49 |
| Statements of Cash Flows for the Years Ended December 31, 2006, 2005 and 2004 | F-50 |
| Notes to Financial Statements | F-51 |
| | |
| | |
| *CombiMatrix Group Financial Statements | |
| (A Division of Acacia Research Corporation) | |
| | |
| Report of Independent Registered Public Accounting Firm | F-66 |
| Balance Sheets as of December 31, 2006 and 2005 | F-67 |
| Statements of Operations for the Years Ended December 31, 2006, 2005 and 2004 | F-68 |
| Statements of Allocated Net Worth for the Years Ended December 31, 2006, 2005 and 2004 | F-69 |
| Statements of Cash Flows for the Years Ended December 31, 2006, 2005 and 2004 | F-70 |
| Notes to Financial Statements | F-71 |
*NOTE: We are presenting the Acacia Research Corporation consolidated financial statements and the separate financial statements for the CombiMatrix group and the Acacia Technologies group. The separate financial statements and accompanying notes of the two groups are being provided as additional disclosure regarding the financial performance of the two divisions and to provide investors with information regarding the potential value and operating results of the respective businesses, which may affect the respective share values. The separate financial statements should be reviewed in conjunction with Acacia Research Corporation’s consolidated financial statements and accompanying notes. The presentation of separate financial statements is not intended to indicate that we have changed the title to any of our assets or changed the responsibility for any of our liabilities, nor is it intended to indicate that the rights of our creditors have been changed. Acacia Research Corporation, and not the individual groups, is the issuer of the securities. Holders of the two securities are stockholders of Acacia Research Corporation and do not have a separate and exclusive interest in the respective groups.
(2) Financial Statement Schedules
Financial statement schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or the Notes thereto.
(2) Exhibits
Refer to Item 15(b) below.
(b) Exhibits. The following exhibits are either filed herewith or incorporated herein by reference:
Exhibit Number | Description |
| | |
| 2.1 | Agreement and Plan of Merger of Acacia Research Corporation, a California corporation, and Acacia Research Corporation, a Delaware corporation, dated as of December 23, 1999 (1) |
| 2.2 | Agreement and Plan of Reorganization by and among Acacia Research Corporation, Combi Acquisition Corp. and CombiMatrix Corporation dated as of March 20, 2002 (2) |
| 3.1 | Restated Certificate of Incorporation (3) |
| 3.2 | Amended and Restated Bylaws (4) |
| 10.1* | Acacia Research Corporation 1996 Stock Option Plan, as amended (5) |
| 10.2* | Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (6) |
| 10.3* | CombiMatrix Corporation 1998 Stock Option Plan (7) |
| 10.4* | CombiMatrix Corporation 2000 Stock Awards Plan (7) |
| 10.5* | 2002 CombiMatrix Stock Incentive Plan (8) |
| 10.6* | 2002 Acacia Technologies Stock Incentive Plan (9) |
| 10.7 | Lease Agreement dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (10) |
| 10.8 | Settlement Agreement dated September 30, 2002, by and among Acacia Research Corporation, CombiMatrix Corporation, Donald D. Montgomery, Ph.D. and Nanogen, Inc.(7) |
| 10.9† | Research & Development Agreement dated September 25, 2002, between CombiMatrix Corporation and Roche Diagnostics GmbH(7) |
| 10.10† | License Agreement dated September 25, 2002 between CombiMatrix Corporation and Roche Diagnostics GmbH(7) |
| 10.11 | Form of Indemnification Agreement (11) |
| 10.12 | Series A Preferred Stock Purchase Agreement dated October 1, 2004, by and between Leuchemix, Inc. and CombiMatrix Corporation(12) |
| 10.13 | Investor Rights Agreement dated October 1, 2004, by and among Leuchemix, Inc., the holders of Common Stock set forth on Exhibit A attached thereto, and CombiMatrix Corporation(12) |
| 10.14 | Voting Agreement dated October 1, 2004, by and among Leuchemix, Inc., CombiMatrix Corporation and the holders of the Common Stock set forth on Exhibit A attached thereto(12) |
| 10.15 | Right of First Refusal and Co-Sale Agreement dated October 1, 2004, by and among Leuchemix, Inc., the holders of Common Stock set forth on Exhibit A attached thereto, and CombiMatrix Corporation(11) |
| 10.16 | Letter of Intent dated December 15, 2004 between Acacia Research Corporation and Global Patent Holdings LLC (13) |
| 10.17† | First Addendum to Roche/CBMX Research and Development Agreement dated March 25, 2003 (20) |
| 10.18 | Research & Development Agreement Second Amendment dated March 19, 2004, between Roche Diagnostics GmbH and CombiMatrix Corporation (20) |
| 10.19 | Sublease Guaranty dated as of June 15, 2005 by CombiMatrix Corporation in favor of Accupath Diagnostic Laboratories, Inc. (20) |
| 10.20 | Sublease dated June 15, 2005, by and between Accupath Diagnostic Laboratories, Inc., dba U.S. Labs, and CombiMatrix Molecular Diagnostics, Inc. (20) |
| 10.21 | Lease Agreement dated October 19, 2000 by and between Wiredzone Property, L.P. and CombiMatrix Corporation (20) |
| 10.22 | First Amendment to Lease Agreement dated April 22, 2001 by and between Wiredzone Property, L.P. and CombiMatrix Corporation (20) |
| 10.23 | Form of Subscription Agreement between Acacia Research Corporation and certain investors (14) |
| 10.24 | Third Amendment to lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (15) |
| 10.25 | Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (16) |
| 10.26 | Amendment to Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (17) |
Exhibit Number | Description |
| | |
| 10.27 | Manufacturing and Supply Agreement between Acacia Research Corporation and Furuno Electric Company, Ltd. Effective July 1, 2006 (18) |
| 10.28 | Placement Agency Agreement between Acacia Research Corporation and Oppenheimer & Co., dated December 7, 2006 (19) |
| 10.29 | Form of Subscription Agreement (19) |
| 10.30 | Form of Investors Warrant (19) |
| 21.1 | List of Subsidiaries |
| 23.1 | Consent of PricewaterhouseCoopers LLP (relating to the financial statements of Acacia Research Corporation) |
| 23.2 | Consent of PricewaterhouseCoopers LLP (relating to the financial statements of the CombiMatrix group) |
| 23.3 | Consent of PricewaterhouseCoopers LLP (relating to the financial statements of the Acacia Technologies group) |
| 31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | Certification of the Chief Executive Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 32.2 | Certification of the Chief Financial Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
___________________________
* The referenced exhibit is a management contract, compensatory plan or arrangement.
| † | Portions of this exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission. |
| | |
| (1) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on December 30, 1999 (SEC File No. 000-26068). |
| | |
| (2) | Incorporated by reference as Appendix A to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (3) | Incorporated by reference as Appendix B to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (4) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on August 10, 2001 (SEC File No. 000-26068). |
| | |
| (5) | Incorporated by reference as Appendix A to the Definitive Proxy Statement on Schedule 14A filed on April 10, 2000 (SEC File No. 000-26068). |
| | |
| (6) | Incorporated by reference from Acacia Research Corporation’s Definitive Proxy as Appendix A Statement on Schedule 14A filed on April 26, 1996 (SEC File No. 000-26068). |
| | |
| (7) | Incorporated by reference to Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (8) | Incorporated by reference as Appendix D to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (9) | Incorporated by reference as Appendix E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| (10) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2001 filed on March 27, 2002 (SEC File No. 000-26068). |
| | |
| (11) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2002 filed on March 27, 2003 (SEC File No. 000-26068). |
| | |
| (12) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on November 5, 2004 (SEC File No. 000-26068). |
| | |
| (13) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2004 filed on March 15, 2005 (SEC File No. 000-26068). |
| | |
| (14) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on September 19, 2005 (SEC File No. 000-26068). |
| | |
| (15) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on May 10, 2006 (SEC File No. 000-26068). |
| | |
| (16) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on June 15, 2006 (SEC File No. 000-26068). |
| | |
| (17) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on June 22, 2006 (SEC File No. 000-26068). |
| | |
| (18) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on November 9, 2006 (SEC File No. 000-26068). |
| | |
| (19) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on December 13, 2006 (SEC File No. 000-26068). |
| | |
| (20) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2005 filed on March 16, 2006 (SEC File No. 000-26068). |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: March 14, 2007 ACACIA RESEARCH CORPORATION
/s/ Paul R. Ryan
Paul R. Ryan
Chairman of the Board
and Chief Executive Officer
(Authorized Signatory)
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | | |
/s/ Paul R. Ryan | | Chairman of the Board and Chief Executive Officer | | March 14, 2007 |
| Paul R. Ryan | | (Principal Chief Executive) | | |
| | | | | |
| | | | | |
/s/ Robert L. Harris, II | | Director and President | | March 14, 2007 |
| Robert L. Harris, II | | | | |
| | | | | |
| | | | | |
/s/ Clayton J. Haynes | | Chief Financial Officer and Treasurer | | March 14, 2007 |
| Clayton J. Haynes | | (Principal Financial Officer) | | |
| | | | | |
| | | | | |
/s/ Thomas B. Akin | | Director | | March 14, 2007 |
| Thomas B. Akin | | | | |
| | | | | |
| | | | | |
/s/ Fred A. de Boom | | Director | | March 14, 2007 |
| Fred A. de Boom | | | | |
| | | | | |
| | | | | |
/s/ Edward W. Frykman | | Director | | March 14, 2007 |
| Edward W. Frykman | | | | |
| | | | | |
| | | | | |
/s/ G. Louis Graziadio, III | | Director | | March 14, 2007 |
| G. Louis Graziadio, III | | | | |
| | | | | |
| | | | | |
/s/ Amit Kumar, Ph.D. | | Director | | March 14, 2007 |
| Amit Kumar, Ph.D. | | | | |
| | | | | |
| | | | | |
/s/ Rigdon Currie | | Director | | March 14, 2007 |
| Rigdon Currie | | | | |
Report of Independent Registered Public Accounting Firm
To The Board of Directors and Shareholders
of Acacia Research Corporation:
We have completed integrated audits of Acacia Research Corporation’s consolidated financial statements and of its internal control over financial reporting as of December 31, 2006 in accordance with the standards of the Public Company Accounting Oversight Board (United States). Our opinions, based on our audits, are presented below.
Consolidated financial statements
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a) (1) present fairly, in all material respects, the financial position of Acacia Research Corporation and its subsidiaries at December 31, 2006 and December 31, 2005, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2006 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of Acacia Research Corporation’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit of financial statements includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 1 to the consolidated financial statements, the Company's CombiMatrix group will need to raise additional capital to achieve its intended business objectives.
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for share-based compensation in 2006.
Internal control over financial reporting
Also, in our opinion, management’s assessment, included in Management’s Report on Internal Control over Financial Reporting, appearing under Item 9A, that the Company maintained effective internal control over financial reporting as of December 31, 2006 based on the criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), is fairly stated, in all material respects, based on those criteria. Furthermore, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2006, based on the criteria established in Internal Control - Integrated Framework issued by the COSO. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express opinions on management’s assessment and on the effectiveness of the Company’s internal control over financial reporting based on our audit. We conducted our audit of internal control over financial reporting in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. An audit of internal control over financial reporting includes obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we consider necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Orange County, California
March 12, 2007
ACACIA RESEARCH CORPORATION
CONSOLIDATED BALANCE SHEETS
As of December 31, 2006 and 2005
(In thousands, except share and per share information)
| | | December 31, | | December 31, | |
| | | 2006 | | 2005 | |
| | | | | | |
ASSETS | | | | | | | |
| | | | | | | | |
| Current assets: | | | | | | | |
| Cash and cash equivalents | | $ | 40,044 | | $ | 20,164 | |
| Short-term investments | | | 19,296 | | | 39,009 | |
| Accounts receivable | | | 874 | | | 5,332 | |
| Prepaid expenses, inventory, and other assets | | | 1,792 | | | 2,115 | |
| | | | | | | | |
| Total current assets | | | 62,006 | | | 66,620 | |
| | | | | | | | |
| Property and equipment, net of accumulated depreciation | | | 2,006 | | | 2,484 | |
| Patents and licenses, net of accumulated amortization | | | 25,807 | | | 31,712 | |
| Goodwill | | | 17,039 | | | 18,980 | |
| Other assets | | | 2,746 | | | 1,638 | |
| | | $ | 109,604 | | $ | 121,434 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
| | | | | | | | |
| Current liabilities: | | | | | | | |
| Accounts payable and accrued expenses | | $ | 5,047 | | $ | 3,924 | |
| Royalties and legal fees payable | | | 1,684 | | | 3,758 | |
| Current portion of deferred revenues | | | 725 | | | 804 | |
| Total current liabilities | | | 7,456 | | | 8,486 | |
| | | | | | | | |
| Deferred income taxes | | | - | | | 2,701 | |
| Deferred revenues, net of current portion | | | 1,076 | | | 1,439 | |
| Warrant liability | | | 6,732 | | | 1,381 | |
| Other liabilities | | | 31 | | | 83 | |
| Total liabilities | | | 15,295 | | | 14,090 | |
| Minority interests | | | - | | | 447 | |
| | | | | | | | |
| Commitments and contingencies (Note 14) | | | | | | | |
| | | | | | | | |
| Redeemable stockholders' equity: | | | | | | | |
| Preferred stock | | | | | | | |
| Acacia Research Corporation, par value $0.001 per share; 10,000,000 shares authorized; | | | | | | | |
| no shares issued or outstanding | | | - | | | - | |
| Common stock | | | | | | | |
| Acacia Research - Acacia Technologies stock, par value $0.001 per share; 100,000,000 | | | | | | | |
| shares authorized; 28,231,701 and 27,722,242 shares issued and outstanding as of | | | | | | | |
| December 31, 2006 and December 31, 2005, respectively | | | 28 | | | 28 | |
| Acacia Research - CombiMatrix stock, par value $0.001 per share; 100,000,000 shares | | | | | | | |
| authorized; 50,365,810 and 38,992,402 shares issued and outstanding as of | | | | | | | |
| December 31, 2006 and December 31, 2005, respectively | | | 50 | | | 39 | |
| Additional paid-in capital | | | 326,599 | | | 315,146 | |
| Deferred stock compensation | | | - | | | (1,400 | ) |
| Accumulated comprehensive income | | | 2 | | | (2 | ) |
| Accumulated deficit | | | (232,370 | ) | | (206,914 | ) |
| Total stockholders' equity | | | 94,309 | | | 106,897 | |
| | | $ | 109,604 | | $ | 121,434 | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
For the Years Ended December 31, 2006, 2005 and 2004
(In thousands, except share and per share information)
| | | 2006 | | 2005 | | 2004 | |
| Revenues: | | | | | | | |
| Collaboration agreements | | $ | - | | $ | 2,266 | | $ | 17,302 | |
| License fees | | | 34,825 | | | 19,574 | | | 4,284 | |
| Government contracts | | | 2,074 | | | 3,849 | | | 1,993 | |
| Products | | | 3,278 | | | 1,765 | | | 230 | |
| Service contracts | | | 388 | | | 153 | | | 116 | |
| | | | | | | | | | | |
| Total revenues | | | 40,565 | | | 27,607 | | | 23,925 | |
| | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | |
| Cost of government contract revenues | | | 1,959 | | | 3,683 | | | 1,874 | |
| Cost of product sales | | | 1,258 | | | 820 | | | 173 | |
| Research and development expenses (including non-cash stock compensation | | | | | | | | | | |
| expense of $1,097 for 2006, $0 for 2005 and $91 for 2004) | | | 9,485 | | | 5,783 | | | 5,385 | |
| Marketing, general and administrative expenses (including non-cash stock compensation | | | | | | | | | | |
| expense of $5,206 for 2006, $197 for 2005 and $663 for 2004) | | | 26,963 | | | 17,926 | | | 14,951 | |
| Legal expenses - patents | | | 4,780 | | | 2,468 | | | 3,133 | |
| Inventor royalties and contingent legal fees expense - patents | | | 17,159 | | | 11,106 | | | - | |
| Inventor royalties - V-chip | | | - | | | 225 | | | - | |
| Goodwill impairment charge | | | - | | | 565 | | | 1,656 | |
| Write-off of patent-related intangible asset | | | 297 | | | - | | | - | |
| Amortization of patents and royalties | | | 6,795 | | | 6,234 | | | 1,735 | |
| Legal settlement charges (gains) | | | - | | | (406 | ) | | 812 | |
| Loss from equity investment | | | 1,036 | | | 352 | | | 17 | |
| | | | | | | | | | | |
| Total operating expenses | | | 69,732 | | | 48,756 | | | 29,736 | |
| | | | | | | | | | | |
| Operating loss | | | (29,167 | ) | | (21,149 | ) | | (5,811 | ) |
| | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | |
| Interest and investment income | | | 2,047 | | | 1,594 | | | 801 | |
| Loss on sale of interest in subsidiary | | | (84 | ) | | - | | | - | |
| Warrant gains (charges) | | | 1,754 | | | 812 | | | - | |
| | | | | | | | | | | |
| Total other income | | | 3,717 | | | 2,406 | | | 801 | |
| | | | | | | | | | | |
| Loss from continuing operations before income taxes and minority interests | | | (25,450 | ) | | (18,743 | ) | | (5,010 | ) |
| | | | | | | | | | | |
| (Provision) benefit for income taxes | | | (6 | ) | | 302 | | | 275 | |
| | | | | | | | | | | |
| Loss from continuing operations before minority interests | | | (25,456 | ) | | (18,441 | ) | | (4,735 | ) |
| | | | | | | | | | | |
| Minority interests | | | - | | | 2 | | | 6 | |
| | | | | | | | | | | |
| Loss from continuing operations | | | (25,456 | ) | | (18,439 | ) | | (4,729 | ) |
| | | | | | | | | | | |
| Discontinued operations: | | | | | | | | | | |
| | | | | | | | | | | |
| Estimated loss on disposal of discontinued operations | | | - | | | (237 | ) | | (104 | ) |
| | | | | | | | | | | |
| Net loss | | | (25,456 | ) | | (18,676 | ) | | (4,833 | ) |
| | | | | | | | | | | |
| Unrealized gains (losses) on short-term investments | | | 61 | | | 2 | | | (65 | ) |
| Unrealized gains (losses) on foreign currency translation | | | (57 | ) | | 73 | | | (20 | ) |
| | | | | | | | | | | |
| Comprehensive loss | | $ | (25,452 | ) | $ | (18,601 | ) | $ | (4,918 | ) |
| | | | | | | | | | | |
| Earnings (loss) per common share: | | | | | | | | | | |
| Attributable to the Acacia Technologies group: | | | | | | | | | | |
| Loss from continuing operations | | $ | (5,496 | ) | $ | (6,038 | ) | $ | (5,439 | ) |
| Basic and diluted loss per share | | | (0.20 | ) | | (0.23 | ) | | (0.27 | ) |
| Loss from discontinued operations | | $ | - | | $ | (237 | ) | $ | (104 | ) |
| Basic and diluted loss per share | | | - | | | (0.01 | ) | | (0.01 | ) |
| Net loss | | $ | (5,496 | ) | $ | (6,275 | ) | $ | (5,543 | ) |
| Basic and diluted loss per share | | | (0.20 | ) | | (0.24 | ) | | (0.28 | ) |
| | | | | | | | | | | |
| Attributable to the CombiMatrix group: | | | | | | | | | | |
| Net income (loss) | | $ | (19,960 | ) | $ | (12,401 | ) | $ | 710 | |
| Basic and diluted earnings (loss) per share | | | (0.49 | ) | | (0.37 | ) | | 0.02 | |
| | | | | | | | | | | |
| Weighted average shares: | | | | | | | | | | |
| Acacia Research - Acacia Technologies stock: | | | | | | | | | | |
| Basic and diluted | | | 27,547,651 | | | 26,630,732 | | | 19,784,883 | |
| Acacia Research - CombiMatrix stock: | | | | | | | | | | |
| Basic | | | 40,605,038 | | | 33,678,603 | | | 29,962,596 | |
| Diluted | | | 40,605,038 | | | 33,678,603 | | | 30,995,663 | |
| | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2006, 2005 and 2004
(In thousands, except share information)
| | | AR-Acacia | | AR- | | AR-Acacia | | AR- | | | | | | | | | | | |
| | | Technologies | | CombiMatrix | | Technologies | | CombiMatrix | | | | | | | | | | | |
| | | Redeemable | | Redeemable | | Redeemable | | Redeemable | | Additional | | Deferred | | | | Other | | | |
| | | Common | | Common | | Common | | Common | | Paid-in | | Stock | | Accumulated | | Comprehensive | | | |
| | | Shares | | Shares | | Stock | | Stock | | Capital | | Compensation | | Deficit | | Income (Loss) | | Total | |
| | | | | | | | | | | | | | | | | | | | |
2004 | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2003 | | | 19,739,984 | | | 26,328,122 | | $ | 20 | | $ | 26 | | $ | 244,517 | | $ | (766 | ) | $ | (183,405 | ) | $ | 8 | | $ | 60,400 | |
| Net loss | | | | | | | | | | | | | | | | | | | | | (4,833 | ) | | | | | (4,833 | ) |
| Stock options exercised | | | 71,540 | | | 987,911 | | | | | | 1 | | | 3,113 | | | | | | | | | | | | 3,114 | |
| Warrants exercised | | | | | | 761,205 | | | | | | 1 | | | 2,093 | | | | | | | | | | | | 2,094 | |
| Units issued in direct offering, net offering costs | | | | | | 3,000,000 | | | | | | 3 | | | 13,712 | | | | | | | | | | | | 13,715 | |
Compensation expense relating to stock options | | | | | | | | | | | | | | | 250 | | | 689 | | | | | | | | | 939 | |
| Stock option cancellations | | | | | | | | | | | | | | | (262 | ) | | 77 | | | | | | | | | (185 | ) |
| Unrealized loss on short-term investments | | | | | | | | | | | | | | | | | | | | | | | | (65 | ) | | (65 | ) |
Unrealized gain on foreign currency translation | | | | | | | | | | | | | | | | | | | | | | | | (20 | ) | | (20 | ) |
| Legal settlement (see Note 14) | | | | | | 123,258 | | | | | | | | | 477 | | | | | | | | | | | | 477 | |
| Balance at December 31, 2004 | | | 19,811,524 | | | 31,200,496 | | $ | 20 | | $ | 31 | | $ | 263,900 | | $ | - | | $ | (188,238 | ) | $ | (77 | ) | $ | 75,636 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
2005 | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | (18,676 | ) | | | | | (18,676 | ) |
| Stock options exercised | | | 133,986 | | | 5,555 | | | | | | | | | 315 | | | | | | | | | | | | 315 | |
| Stock issued for the acquisition of Global Patent Holdings, net of registration costs (Note 8) | | | 3,938,832 | | | | | | 4 | | | | | | 19,289 | | | | | | | | | | | | 19,293 | |
| Units issued in direct offering, net offering costs | | | 3,500,000 | | | 7,786,351 | | | 4 | | | 8 | | | 32,244 | | | | | | | | | | | | 32,256 | |
| Warrant liability (see Note 10) | | | | | | | | | | | | | | | (2,194 | ) | | | | | | | | | | | (2,194 | ) |
| Deferred stock compensation | | | 337,900 | | | | | | | | | | | | 1,713 | | | (1,713 | ) | | | | | | | | - | |
Compensation expense relating to stock options | | | | | | | | | | | | | | | (121 | ) | | 313 | | | | | | | | | 192 | |
| Unrealized loss on short-term investments | | | | | | | | | | | | | | | | | | | | | | | | 2 | | | 2 | |
Unrealized gain on foreign currency translation | | | | | | | | | | | | | | | | | | | | | | | | 73 | | | 73 | |
| Balance at December 31, 2005 | | | 27,722,242 | | | 38,992,402 | | $ | 28 | | $ | 39 | | $ | 315,146 | | $ | (1,400 | ) | $ | (206,914 | ) | $ | (2 | ) | $ | 106,897 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
2006 | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | (25,456 | ) | | | | | (25,456 | ) |
| Stock options exercised | | | 389,959 | | | | | | | | | | | | 1,475 | | | | | | | | | | | | 1,475 | |
| Units issued in direct offering, net offering costs | | | | | | 11,323,408 | | | | | | 11 | | | 12,098 | | | | | | | | | | | | 12,109 | |
| Warrant liability (see Note 10) | | | | | | | | | | | | | | | (7,104 | ) | | | | | | | | | | | (7,104 | ) |
Reclassification of deferred stock compensation (see Note 2) | | | | | | | | | | | | | | | (1,400 | ) | | 1,400 | | | | | | | | | - | |
| Stock issued to consultant | | | | | | 50,000 | | | | | | | | | 94 | | | | | | | | | | | | 94 | |
Compensation expense relating to stock options and restricted stock awards | | | 119,500 | | | | | | | | | | | | 6,306 | | | | | | | | | | | | 6,306 | |
| Unrealized loss on short-term investments | | | | | | | | | | | | | | | | | | | | | | | | 61 | | | 61 | |
Unrealized gain on foreign currency translation | | | | | | | | | | | | | | | | | | | | | | | | (57 | ) | | (57 | ) |
| Other | | | | | | - | | | | | | - | | | (16 | ) | | | | | | | | | | | (16 | ) |
| Balance at December 31, 2006 | | | 28,231,701 | | | 50,365,810 | | $ | 28 | | $ | 50 | | $ | 326,599 | | $ | - | | $ | (232,370 | ) | $ | 2 | | $ | 94,309 | |
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Years Ended December 31, 2006, 2005 and 2004
(In thousands)
| | | | | 2006 | | 2005 | | 2004 | |
| Cash flows from operating activities: | | | | | | | | | |
| Net loss | | | | | $ | (25,456 | ) | $ | (18,676 | ) | $ | (4,833 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | |
| Depreciation and amortization | | | | | | 7,417 | | | 7,164 | | | 2,751 | |
| Minority interests | | | | | | - | | | (2 | ) | | - | |
| Non-cash stock compensation | | | | | | 6,303 | | | 197 | | | 754 | |
| Deferred tax benefit | | | | | | (70 | ) | | (280 | ) | | (279 | ) |
| Non-cash warrant charges (gains) | | | | | | (1,754 | ) | | (812 | ) | | - | |
| Non-cash legal settlement charges (gains) | | | | | | - | | | (406 | ) | | 812 | |
| Non-cash impairment charges | | | | | | - | | | 565 | | | 1,656 | |
| Loss on disposal of discontinued operations | | | | | | - | | | 237 | | | 104 | |
| Write-off of patent-related intangible asset | | | | | | 297 | | | - | | | - | |
| Loss from equity investments | | | | | | 1,036 | | | 352 | | | 17 | |
| Loss on sale of interest in subsidiary | | | | | | 84 | | | - | | | - | |
| Stock issued to consultant | | | | | | 94 | | | - | | | - | |
| Other | | | | | | 147 | | | (79 | ) | | 65 | |
| Changes in assets and liabilities, excluding effect of business acquisition: | | | | | | | | | | | | | |
| Accounts receivable | | | | | | 4,440 | | | (4,796 | ) | | (223 | ) |
Prepaid expenses, inventory and other assets | | | | | | (46 | ) | | (942 | ) | | 809 | |
| Accounts payable, accrued expenses and other | | | | | | 922 | | | (309 | ) | | 1,173 | |
| Royalties and legal fees payable | | | | | | (2,074 | ) | | 3,758 | | | - | |
| Deferred revenues | | | | | | (390 | ) | | (2,144 | ) | | (17,622 | ) |
| Net cash used in operating activities from continuing operations | | | | | | (9,050 | ) | | (16,173 | ) | | (14,816 | ) |
| Net cash provided by (used in) operating activities from discontinued operations | | | | | | 264 | | | (513 | ) | | (727 | ) |
| Net cash used in operating activities | | | | | | (8,786 | ) | | (16,686 | ) | | (15,543 | ) |
| | | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | |
| Purchase of property and equipment | | | | | | (715 | ) | | (1,400 | ) | | (891 | ) |
| Purchase of available-for-sale investments | | | | | | (21,946 | ) | | (76,690 | ) | | (59,382 | ) |
| Sale of available-for-sale investments | | | | | | 41,720 | | | 76,227 | | | 51,759 | |
| Business acquisition | | | | | | (16 | ) | | (5,796 | ) | | - | |
| Purchase of additional interests in equity method investee | | | | | | (2,150 | ) | | (1,600 | ) | | (250 | ) |
| Patent acquisition costs | | | | | | (1,030 | ) | | (445 | ) | | - | |
| Sale of interest in subsidiary (net of cash disposed) | | | | | | (369 | ) | | - | | | - | |
| Other | | | | | | - | | | - | | | (5 | ) |
| Net cash provided by (used in) investing activities from continued operations | | | | | | 15,494 | | | (9,704 | ) | | (8,769 | ) |
| Net cash used in investing activities from discontinued operations | | | | | | (353 | ) | | - | | | (198 | ) |
| Net cash provided by (used in) investing activities | | | | | | 15,141 | | | (9,704 | ) | | (8,967 | ) |
| | | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | |
| Proceeds from sale of common stock and warrants, net of issuance costs | | | | | | 12,050 | | | 32,256 | | | 13,715 | |
| Proceeds from the exercise of stock options and warrants | | | | | | 1,475 | | | 315 | | | 5,207 | |
| Net cash provided by financing activities | | | | | | 13,525 | | | 32,571 | | | 18,922 | |
| Effect of exchange rate on cash | | | | | | - | | | 73 | | | (17 | ) |
| Increase (decrease) in cash and cash equivalents | | | | | | 19,880 | | | 6,254 | | | (5,605 | ) |
| | | | | | | | | | | | | | |
| Cash and cash equivalents, beginning | | | | | | 20,164 | | | 13,910 | | | 19,515 | |
| | | | | | | | | | | | | | |
| Cash and cash equivalents, ending | | | | | $ | 40,044 | | $ | 20,164 | | $ | 13,910 | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESSES
Acacia Research Corporation (“we,” “us” and “our”) is comprised of two operating groups.
Acacia Technologies Group
The Acacia Technologies group, a division of Acacia Research Corporation, develops, acquires, licenses and enforces patented technologies. The Acacia Technologies group owns and has rights to patent portfolios covering a wide range of technology areas. The Acacia Technologies group is primarily comprised of certain of Acacia Research Corporation’s direct and or indirect wholly owned subsidiaries and limited liability companies including:
· Acacia Global Acquisition Corporation · Acacia Media Technologies Corporation · Acacia Patent Acquisition Corporation · Acacia Technologies Services Corporation · AV Technologies LLC · Broadcast Data Retrieval Corporation · Broadcast Innovation LLC · Computer Acceleration Corporation · Computer Cache Coherency Corporation · Computer Docking Station Corporation · Credit Card Fraud Control Corporation · Database Structures Inc. · Data Encryption Corporation · Data Innovation LLC · Diagnostic Systems Corporation · Disc Link Corporation · Financial Systems Innovation LLC · Fluid Dynamics Corporation · High Resolution Optics Corporation | · Information Technology Innovation LLC · InternetAd LLC · IP Innovation LLC · KY Data Systems LLC · Location Based Services Corporation · Micromesh Technology Corporation · Microprocessor Enhancement Corporation · New Medium LLC · Peer Communications Corporation · Product Activation Corporation · Remote Video Camera Corporation · Resource Scheduling Corporation · Safety Braking Corporation · Screentone Systems Corporation · Soundview Technologies Inc. · Spreadsheet Automation Corporation · TechSearch LLC · Telematics Corporation · VData LLC |
The Acacia Technologies group also includes all corporate assets, liabilities, and related transactions of Acacia Research Corporation attributed to Acacia Research Corporation’s intellectual property licensing and enforcement business.
Business Acquisition. On January 28, 2005, Acacia Global Acquisition Corporation acquired the assets of Global Patent Holdings, LLC, which owned 11 patent licensing companies (“GPH Acquisition”). The acquisition provided the Acacia Technologies group ownership of companies that own or control the rights to 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. Refer to Note 8 for a description of the acquisition transaction and the related accounting treatment.
CombiMatrix Group
Our life sciences business, referred to as the “CombiMatrix group,” a division of Acacia Research Corporation, is comprised of our wholly owned subsidiary, CombiMatrix Corporation and CombiMatrix Corporation’s wholly owned subsidiary, CombiMatrix Molecular Diagnostics, Inc. (“CMDX”) and includes all corporate assets, liabilities and transactions related to Acacia Research Corporation’s life sciences business.
The CombiMatrix group develops proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where its products and services could be utilized. The technologies that the CombiMatrix group has developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. The CombiMatrix group has also developed the capabilities of producing arrays that utilize bacterial artificial chromosomes on its arrays, also enabling genetic analysis. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
CombiMatrix Molecular Diagnostics, Inc., a wholly owned subsidiary located in Irvine, California, is exploring opportunities for the CombiMatrix group’s arrays in the field of molecular diagnostics. CombiMatrix K.K., a Japanese corporation located in Tokyo, Japan, has existed for the purpose of exploring opportunities for CombiMatrix Corporation’s array system with pharmaceutical and biotechnology companies in the Asian market. In January 2006, CombiMatrix Corporation sold 67% of its ownership interest in CombiMatrix K.K. to a third party. Refer to Note 6.
Other
We were incorporated on January 25, 1993 under the laws of the State of California. In December 1999, we changed our state of incorporation from California to Delaware.
In January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, to become an independent public company. The transaction is expected to be completed no sooner than the second quarter of 2007, subject, however, to completing the required filings with the Securities and Exchange Commission (“SEC”). We have received a private letter ruling from the IRS addressing certain tax implications of the transaction and have received a tax opinion from counsel. CombiMatrix Corporation filed a registration statement on Form S-1 on December 26, 2006, which has not been declared effective. If CombiMatrix Corporation’s registration statement on Form S-1 is declared effective by the SEC, Acacia Research Corporation will redeem all of the issued and outstanding shares of AR-CombiMatrix common stock for all of the common stock of CombiMatrix Corporation, which will register its common stock under the Securities and Exchange Act of 1934. Following the redemption, CombiMatrix Corporation will apply to list its shares for trading on a national exchange.
Capital Structure. On December 11, 2002, our stockholders voted in favor of a recapitalization transaction, which became effective on December 13, 2002, whereby we created two new classes of common stock called Acacia Research-CombiMatrix common stock (“AR-CombiMatrix stock”) and Acacia Research-Acacia Technologies common stock (“AR-Acacia Technologies stock”), and divided our existing Acacia Research Corporation common stock into shares of the two new classes of common stock. AR-CombiMatrix stock is intended to reflect separately the performance of Acacia Research Corporation’s CombiMatrix group. AR-Acacia Technologies stock is intended to reflect separately the performance of Acacia Research Corporation’s Acacia Technologies group. Although the AR-CombiMatrix stock and the AR-Acacia Technologies stock are intended to reflect the performance of our different business groups, they are both classes of common stock of Acacia Research Corporation and are not stock issued by the respective groups.
Liquidity and Risks
General. Management believes that Acacia Research Corporation’s consolidated cash and cash equivalent and short-term investment balances, anticipated cash flow from operations and other external sources of available credit will be sufficient to meet Acacia Research Corporation’s cash requirements, on a consolidated basis, through at least March 2008. To date, we and our subsidiaries have relied primarily upon selling equity securities and payments from our strategic partners and licensees to generate the funds needed to finance the implementation of our plans of operation for our subsidiaries.
Acacia Research Corporation’s cash and cash equivalent and short term investment balances, cash flows and anticipated cash flows from operations and other sources of external credit, are attributed to the Acacia Technologies group and the CombiMatrix group based on the respective assets of the specific businesses comprising each group. Issuances of AR-Acacia Technologies stock (and the proceeds thereof) are attributed to the Acacia Technologies group and issuances of AR-CombiMatrix stock (and the proceeds thereof) are attributed to the CombiMatrix group. The cash and cash equivalent balances, anticipated cash flow from operations, and other external sources of available credit of one group are not generally available to the other group. Neither of the groups is obligated to fund the ongoing operations of the other group. Management has no intent to use the cash and cash equivalent balances, anticipated cash flow from operations, and other external sources of available credit of one group to fund the ongoing operations of the other group.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Acacia Technologies Group. Management believes that the Acacia Technologies group’s cash and cash equivalent and short-term investment balances, anticipated cash flow from operations and other external sources of available credit will be sufficient to meet our cash requirements through at least March 2008, and for the foreseeable future. To date, the Acacia Technologies group has relied upon the receipt of license fee payments from the licensing of the Acacia Technologies group’s patented technologies and the selling of Acacia Research Corporation equity securities to generate the funds needed to finance the operations of the Acacia Technologies group. The Acacia Technologies group concluded its V-chip patent licensing program in August 2004, recognizing a total of $25.7 million in license fees over the life of the program. The Acacia Technologies group has been commercially licensing its DMT® technology portfolio since 2003. The GPH Acquisition provided the Acacia Technologies group with ownership of companies that own or control the rights to 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. Subsequent to the GPH Acquisition, the Acacia Technologies group has acquired or acquired the rights to over 30 additional patent portfolios, covering a wide range of technology areas, which it intends to develop, license and enforce.
There can be no assurance that the Acacia Technologies group will be able to implement its future plans. Failure by management to achieve its plans would have a material adverse effect on the Acacia Technologies group and on Acacia Research Corporation’s ability to achieve its intended business objectives. We may be required to obtain additional financing. There can be no assurance that additional funding will be available on favorable terms, if at all. If we fail to obtain additional funding when needed, we may not be able to execute our business plans and our businesses may suffer.
The timing of the receipt of revenues by the Acacia Technologies group’s business operations are subject to certain risks and uncertainties, including:
| | · | market acceptance of our patented technologies and services; |
| | · | business activities and financial results of our licensees; |
| | · | technological advances that may make our patented technologies obsolete or less competitive; |
| | · | increases in operating costs, including costs for legal services, engineering and research and personnel; |
| | · | the availability and cost of capital; and |
| | · | governmental regulation that may restrict the Acacia Technologies group’s business. |
The Acacia Technologies group’s success also depends on its ability to protect its intellectual property. The Acacia Technologies group relies on its proprietary rights and their protection. Although reasonable efforts will be taken to protect the Acacia Technologies group’s proprietary rights, the complexity of international trade secret, copyright, trademark and patent law, and common law, coupled with limited resources and the demands of quick delivery of technologies to market, create risk that these efforts will prove inadequate. Accordingly, if the Acacia Technologies group is unsuccessful with litigation to protect its intellectual property rights, the future revenues of the Acacia Technologies group could be adversely affected.
The CombiMatrix Group. The CombiMatrix group has a history of incurring net losses and net operating cash flow deficits. The CombiMatrix group is also deploying new and unproven technologies and continues to develop commercial products. The CombiMatrix group has several ongoing long-term development projects that involve experimental technology and may require several years and substantial expenditures to complete. Management believes that the CombiMatrix group’s cash and cash equivalent and short-term investment balances, anticipated cash flows from operations and other external sources of available credit will be sufficient to meet the CombiMatrix group’s cash requirements through December 31, 2007. In order for the CombiMatrix group to sustain operations beyond this point, management will be required to obtain capital from external sources. However, there can be no assurances that additional sources of financing, including the issuance of debt and/or equity securities will be available at times and at terms acceptable to management. The issuance of equity securities will also cause dilution to Acacia Research Corporation’s shareholders. If external financing sources of financing are not available or are inadequate to fund the CombiMatrix group’s operations, management will be required to reduce operating costs including research projects and personnel, which could jeopardize the future strategic initiatives and business plans of the CombiMatrix group. For example, reductions in research and development activities and/or personnel at the CombiMatrix group’s Mukilteo, Washington facility could result in the inability to invest the resources necessary to continue to develop next-generation products and improve existing product lines in order to remain competitive in the marketplace, resulting in reduced revenues and cash flows from the sales of the CombiMatrix group’s CustomArray products and services. Also, reduction in operating costs at the CombiMatrix group’s diagnostics subsidiary, CMDX, in Irvine, California, should they occur, could jeopardize its ability to launch, market and sell additional products and services necessary in order to grow and sustain its operations and eventually achieve profitability.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The CombiMatrix group’s business operations are also subject to certain risks and uncertainties, including:
| | · | market acceptance of products and services; |
| | · | technological advances that may make the CombiMatrix group’s products and services obsolete or less competitive; |
| | · | increases in operating costs, including costs for supplies, personnel and equipment; |
| | · | the availability and cost of capital; |
| | · | general economic conditions; and |
| | · | governmental regulation that may restrict the CombiMatrix group’s business. |
Historically, the CombiMatrix group has been substantially dependent on arrangements with strategic partners and have relied upon payments by the CombiMatrix group’s partners for a significant component of its working capital. The CombiMatrix group intends to enter into additional strategic partnerships to develop and commercialize future products. However, there can be no assurance that the CombiMatrix group will be able to implement its future plans. Failure to achieve its plans would have a material adverse effect on the CombiMatrix group’s ability to achieve its intended business objectives. The CombiMatrix group’s success also depends on its ability to protect its intellectual property, the loss thereof or the failure to secure the issuance of additional patents covering elements of the CombiMatrix group’s business processes could materially harm its business and financial condition. The patents covering the CombiMatrix group’s core technology begin to expire in 2018.
The CombiMatrix group’s products and services are concentrated in a highly competitive market that is characterized by rapid technological advances, frequent changes in customer requirements and evolving regulatory requirements and industry standards. Failure to anticipate or respond adequately to technological advances, changes in customer requirements, changes in regulatory requirements or industry standards, or any significant delays in the development or introduction of planned products or services, could have a material adverse effect on the CombiMatrix group’s business and operating results.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Accounting Principles and Fiscal Year End. The consolidated financial statements and accompanying notes are prepared on the accrual basis of accounting in accordance with generally accepted accounting principles in the United States of America. We have a December 31 year end.
Principles of Consolidation. The accompanying consolidated financial statements include the accounts of Acacia Research Corporation and its wholly owned and majority-owned subsidiaries. We consider the principles of Financial Accounting Standards Board Interpretation No. 46, “Consolidation of Variable Interest Entities” and Accounting Research Bulletin No. 51, “Consolidation of Financial Statements,” when determining whether an entity is subject to consolidation. Investments for which Acacia Research Corporation possesses the ability to direct or cause the direction of the management and policies, either through majority ownership or other means, are accounted for under the consolidation method. Material intercompany transactions and balances have been eliminated in consolidation. Investments in companies in which we maintain an ownership interest of 20% to 50% or exercise significant influence over operating and financial policies are accounted for under the equity method. The cost method is used where we maintain ownership interests of less than 20% and do not exercise significant influence over the investee.
Revenue Recognition. We recognize revenue in accordance with Staff Accounting Bulletin No. 104, “Revenue Recognition” (“SAB No. 104”) and related authoritative pronouncements. Revenues from multiple-element arrangements are accounted for in accordance with Emerging Issues Task Force (“EITF”) Issue 00-21, “Revenue Arrangements with Multiple Deliverables.” Revenue is recognized when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been performed pursuant to the terms of the license agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
Acacia Technologies Group
Under the terms of our license agreements, the Acacia Technologies group grants non-exclusive licenses for the use of its patented technologies. In general, pursuant to the terms of our agreements with our licensees, upon the grant of the licenses, the Acacia Technologies group has no further obligations with respect to the licenses granted. License fees paid to and recognized as revenue by the Acacia Technologies group are non-refundable.
Revenues generated from license agreements are generally accrued and recognized as revenue in the period earned, provided that amounts are fixed or determinable and collectibility is reasonably assured.
Certain license agreements provide for the calculation of license fees based on a licensee’s actual quarterly sales or actual per unit activity, applied to a contractual royalty rate. Licensees that pay license fees on a quarterly basis generally report actual quarterly sales or actual per unit activity information and related quarterly license fees due to the Acacia Technologies group within 30 to 45 days after the end of the quarter in which such sales or activity takes place. The amount of license fees due under these license agreements each quarter cannot be reasonably estimated by management. Consequently, the Acacia Technologies group recognizes revenue from these licensing agreements on a three-month lag basis, in the quarter following the quarter of sales or per unit activity, provided amounts are fixed or determinable and collectibility is reasonably assured. The lag method described above allows for the receipt of licensee royalty reports prior to the recognition of revenue.
Certain license agreements provide for the payment of a minimum upfront annual license fee at the inception of each annual license term. Minimum upfront annual license fees are generally determined based on a licensee’s estimated annual sales or a licensee’s base level of per unit activity. These minimum upfront annual license fee payments are deferred and amortized to revenue on a straight-line basis over the annual license term. To the extent actual annual royalties, determined and reported in accordance with the terms of the respective agreements, exceed the minimum upfront annual license fees paid, the additional royalties are recognized in revenue in the quarter following the quarter in which the base per unit activity was exceeded or the quarter following the annual license term, depending on the terms of the respective agreement, provided that amounts are fixed or determinable and collectibility is reasonably assured. Amounts of additional royalties due under these license agreements cannot be reasonably estimated by management.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Certain license agreements provide for the payment of contractually determined paid-up license fees to us in consideration for the grant of a non-exclusive, retroactive and future license to manufacture and/or sell products covered by our patented technologies. Certain of the agreements also provide for future royalties or additional required payments based on future activities. The execution of these license agreements may also result in the dismissal of any pending litigation. Pursuant to the terms of these agreements, the Acacia Technologies group has no further obligation with respect to the grant of the non-exclusive retroactive and future license, including no express or implied obligation on the Acacia Technologies group’s part to maintain or upgrade the technology, or provide future support or services. Generally, the agreements provide for the grant of the license upon execution of the agreement. As such, the earnings process is complete upon the execution of the agreement, and revenue is recognized upon execution of the agreement, when collectibility is reasonably assured, and all other revenue recognition criteria have been met. Refer to Note 14 for information on inventor royalties and contingent legal fees.
License fee payments received by the Acacia Technologies group that do not meet the revenue recognition criteria described above are deferred until the revenue recognition criteria are met. The Acacia Technologies group assesses the collectibility of accrued license fees based on a number of factors, including past transaction history and credit-worthiness. If it is determined that collection is not reasonably assured, the fee is recognized when collectibility becomes reasonably assured, assuming all other revenue recognition criteria have been met, which is generally upon receipt of cash.
CombiMatrix Group
The CombiMatrix group accounts for revenues under multiple-element arrangements in accordance with SAB No. 104 and Emerging Issues Task Force Consensus (“EITF”), Issue 00-21, "Revenue Arrangements with Multiple Deliverables," and related pronouncements. Arrangements with multiple elements or deliverables must be segmented into individual units of accounting based on the separate deliverables only if there is objective and verifiable evidence of fair value to allocate the consideration received to the deliverables. Accordingly, revenues from multiple-element arrangements involving license fees, up-front payments and milestone payments, which are received and/or billable in connection with other rights and services that represent the CombiMatrix group’s continuing obligations are deferred until all of the multiple elements have been delivered or until objective and verifiable evidence of the fair value of the undelivered elements has been established. Upon establishing objective and verifiable evidence of the fair value of the elements in multiple-element arrangements, the fair value is allocated to each element of the arrangement, such as license fees or research and development projects, based on the relative fair values of the elements. The CombiMatrix group determines the fair value of each element in multiple-element arrangements based on objective and verifiable evidence of fair value, which is determined for each element based on the prices charged when the similar elements are sold separately to third parties. If objective and verifiable evidence of fair value of all undelivered elements exists but objective and verifiable evidence of fair value does not exist for one or more delivered elements, then revenue is recognized using the residual method. Under the residual method, the revenues from delivered elements are not recognized until the fair value of the undelivered element or elements have been determined.
Historically, the CombiMatrix group’s multiple-element arrangements have arisen from executing research and development agreements with various strategic partners including Roche Diagnostics, GmbH (“Roche”), Toppan Printing Ltd. (“Toppan”) and Furuno Electric Co. (“Furuno”). The CombiMatrix group entered into development agreements with these partners to perform certain research and development activities, which provided for payments to the CombiMatrix group as various development milestones were achieved. While these agreements typically included several elements of performance, the agreements have been accounted for as single elements of accounting under EITF Issue No. 00-21 due to the lack of verifiable, objective evidence of fair value for undelivered elements in the agreements at the time that up-front or milestone payments were received by the CombiMatrix group. As a result, payments from the CombiMatrix group’s partners were recorded as deferred revenues and were not recognized as revenues until all of the undelivered elements had been completed.
Revenues from government grants and contracts are recognized in accordance with Accounting Research Bulletin (“ARB”) No. 43, “Government Contracts,” and related pronouncements, such as Statement of Position 81-1, “Accounting for Performance of Construction-Type and Certain Production-Type Contracts.” Accordingly, revenues are recognized under the percentage-of-completion method of accounting, using the cost-to-cost approach to measure completeness at each reporting period. Under the percentage-of-completion method of accounting, contract revenues and expenses are recognized in the period that work is performed based on the percentage of actual incurred costs to total contract costs. Actual contract costs include direct charges for labor and materials and indirect charges for labor, overhead and certain general and administrative charges. Contract change orders and claims are included when they can be reliably estimated and are considered probable. For contracts that extend over a one-year period, revisions in contract cost estimates, if they occur, have the effect of adjusting current period earnings applicable to performance in prior periods. Should current contract estimates indicate an overall future loss to be incurred, a provision is made for the total anticipated loss in the current period.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Revenue from the sale of products and services, including shipping and handling fees, are recognized when delivery has occurred or services have been rendered. The CombiMatrix group sells its products and services directly to customers and also through distributors, and the right to collection is not dependent upon installation or a subsequent sale of the CombiMatrix group products to end users. The CombiMatrix group’s agreements do not provide for credits, returns or exchanges with its customers or distributors. The CombiMatrix group’s distribution agreements include fixed pricing arrangements for its products and after customer acceptance, there is no written or implied right to return or exchange the products.
Deferred revenues arise from payments received in advance of the culmination of the earnings process. Deferred revenues expected to be recognized within the next twelve months are classified within current liabilities. Deferred revenues will be recognized as revenue in future periods when the applicable revenue recognition criteria, as described above, are met.
Cash and Cash Equivalents. We consider all highly liquid, short-term investments with original maturities of three months or less when purchased to be cash equivalents.
Short-term Investments. Our short-term investments are held in a variety of interest bearing instruments including U.S. government debt securities, high-grade corporate bonds, commercial paper, auction rate securities, money market accounts, certificates of deposit and other high-credit quality marketable securities. Investments in securities with original maturities of greater than three months and less than one year and other investments representing amounts that are available for current operations are classified as short-term investments. Investments are classified into categories in accordance with the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 115, “Accounting for Certain Investments in Debt and Equity Securities” (“SFAS No. 115”) and FASB Technical Bulletin 85-4: “Accounting for Purchases of Life Insurance (“FTB 85-4”).” At December 31, 2006 and 2005, all of our investments are classified as available-for-sale, which are reported at fair value with related unrealized gains and losses in the value of such securities recorded as a separate component of comprehensive income (loss) in stockholders’ equity until realized.
The cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income (expense). Interest and dividends on all securities are included in interest income.
At December 31, 2006 and 2005, we held $10,815,000 and $19,104,000, respectively, of short-term investments, which consist of auction rate municipal bonds and variable rate municipal demand notes classified as available-for-sale securities. Our investments in these securities are recorded at cost, which approximates fair market value due to their variable interest rates, which typically reset every 7 to 35 days, and, despite the long-term nature of their stated contractual maturities, we have the ability to quickly liquidate these securities. As a result, we had no cumulative gross unrealized holding gains (losses) or gross realized gains (losses) from these investments. All income generated from these current investments was recorded as interest income.
As of December 31, 2005, we had $1,667,000 of annuity investments classified as current investments as these amounts are available for current operations and are highly liquid, despite the long-term nature of their stated contractual maturities.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Concentration of Credit Risks. Financial instruments that potentially subject Acacia Research Corporation to concentrations of credit risk are cash equivalents and short-term investments. We place our cash equivalents and short-term investments primarily in investment grade, short-term debt instruments. Cash equivalents are also invested in deposits with certain financial institutions and may, at times, exceed federally insured limits. We have not experienced any significant losses on our deposits of cash and cash equivalents.
One, three and two licensee(s) individually accounted for greater than 10% of the Acacia Technologies group’s license fee revenues recognized during the years ended December 31, 2006, 2005 and 2004, respectively, and three and two licensees represented approximately 74% and 95% of the Acacia Technologies group’s accounts receivable at December 31, 2006 and 2005, respectively, as follows:
| | Revenue | | Accounts Receivable |
| | 2006 | | 2005 | | 2004 | | 2006 | | 2005 |
| | | | | | | | | | |
Licensee: | | | | | | | | | |
| A | 14% | | - | | - | | - | | - |
| B | - | | 19% | | - | | - | | 31% |
| C | - | | 15% | | - | | - | | - |
| D | - | | 15% | | - | | - | | 64% |
| E | - | | - | | 11% | | 37% | | - |
| F | - | | - | | 35% | | - | | - |
| G | - | | - | | - | | 24% | | - |
| H | - | | - | | - | | 13% | | - |
Collaboration agreement revenues recognized by the CombiMatrix group for the years ended December 31, 2005 and 2004 relate to its collaborative research and development agreements with Toppan and Roche, respectively. Government contract revenues recognized by the CombiMatrix group for all periods presented relate to the CombiMatrix group’s ongoing contracts with the Department of Defense regarding its electrochemical and microfluidics technologies. At December 31, 2006 and 2005, accounts receivable included $85,000 and $537,000, respectively, due from the Department of Defense. For the years ended December 31, 2005 and 2004, 18% and 45%, respectively, of the CombiMatrix group’s array product and service revenues were recognized by CombiMatrix K.K. Receivables from the Department of Defense totaled 14% of accounts receivable at December 31, 2006, and another customer represented approximately 59% of accounts receivable at December 31, 2006. At December 31, 2005, 59% of accounts receivable was due from the Department of Defense, 24% of our accounts receivable was due from one customer and 10% was due from another customer.
Acacia Research Corporation performs regular credit evaluations of its significant licensees and customers and has not experienced any significant credit losses.
Substantially all of the components and raw materials used in the manufacture of the CombiMatrix group’s products, including semiconductors and reagents, are currently procured from a limited number of sources or in some cases from a single source. Although the CombiMatrix group believes that alternative sources for those components and raw materials are available, any supply interruption in a sole-sourced component or raw material might result in up to a several-month production delay and materially harm the CombiMatrix group’s ability to manufacture products until a new source of supply, if any, could be located and qualified. The CombiMatrix group utilizes non-standard semiconductor manufacturing processes to fabricate the electrode array that is a key aspect of the array structure. Although the CombiMatrix group has a supply agreement in place with a semiconductor wafer manufacturer to ensure availability of the raw materials, the agreement does not guarantee a permanent supply.
Inventory. Inventory, which consists primarily of raw materials to be used in the production of the CombiMatrix group’s array products, is stated at the lower of cost or market using the first-in, first-out method.
Property and Equipment. Property and equipment are recorded at cost. Major additions and improvements that materially extend useful lives of property and equipment are capitalized. Maintenance and repairs are charged against the results of operations as incurred. When these assets are sold or otherwise disposed of, the asset and related depreciation are relieved, and any gain or loss is included in the statement of operations and comprehensive income (loss) for the period of sale or disposal. Depreciation is computed on a straight-line basis over the following estimated useful lives of the assets:
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Machine shop and laboratory equipment | 3 to 5 years |
| Furniture and fixtures | 3 to 7 years |
| Computer hardware and software | 3 to 5 years |
| Leasehold improvements | 2 to 8 years (Lesser of lease term or useful life of improvement) |
Construction in progress includes direct costs incurred related to internally constructed assets which are depreciated once the asset is placed into service. Certain leasehold improvements, furniture and equipment held under capital leases are classified as property and equipment and are amortized over their useful lives using the straight-line method. Capital lease amortization is included in depreciation expense.
Organization Costs. Costs of start-up activities, including organization costs, are expensed as incurred.
Patents and Goodwill. Goodwill is recorded when the consideration paid for acquisitions exceeds the fair value of the net tangible and identifiable intangible assets acquired. Patents, once issued or purchased, are amortized on the straight-line method over their remaining economic useful lives, ranging from two to twenty years. Goodwill is not amortized.
Impairment of Long-lived Assets and Goodwill. We review long-lived assets and intangible assets for potential impairment annually and when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. In the event the sum of the expected undiscounted future cash flows resulting from the use of the asset is less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. If an asset is determined to be impaired, the loss is measured based on quoted market prices in active markets, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including a discounted value of estimated future cash flows.
Goodwill is evaluated for impairment in accordance with SFAS No. 142, “Goodwill and Other Intangible Assets” (“SFAS No. 142”) and is subject to a periodic review for potential impairment at a reporting unit level. Reviews for potential impairment must occur at least annually and may be performed earlier, if circumstances indicate that an impairment may have occurred. Acacia Research Corporation has elected to perform its annual tests for indications of goodwill impairment as of December 31 of each year. Our two reporting units as of December 31, 2006 are: 1) the Acacia Technologies group and 2) the CombiMatrix group. The fair values of our reporting units are estimated by reference to quoted market prices of Acacia Research Corporation’s classes of stock.
SFAS No. 142 requires us to compare the fair value of our reporting units to their carrying amounts on an annual basis to determine if there is potential goodwill impairment. If the fair value of a reporting unit is less than its carrying value, an impairment loss is recorded to the extent that the fair value of the goodwill within the reporting unit is less than its carrying value. In accordance with this policy and as more fully disclosed in Note 7, the CombiMatrix group recognized a goodwill impairment charge of $565,000 for the year ended December 31, 2005. There can be no assurance that future goodwill impairment tests will not result in additional impairment charges in future periods.
Fair Value of Financial Instruments. The carrying value of cash and cash equivalents, accounts receivables, accounts payable and accrued expenses approximate fair value due to their short-term maturity.
Foreign Currency Translation. As result of transactions with licensees located in foreign countries, from time to time, the Acacia Technologies group may have certain receivables denominated in foreign currencies. Assets and liabilities recorded in foreign currencies are translated at the exchange rate on the balance sheet date. Translation adjustments resulting from this process are charged or credited to other comprehensive income. Revenue and expenses are translated at average rates of exchange prevailing during the year. Foreign currency transactions gains and losses were insignificant for the periods presented.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The functional currency of CombiMatrix K.K. is the local currency (Japanese Yen). Foreign currency translation is reported pursuant to SFAS No. 52, “Foreign Currency Translation” (“SFAS No. 52”). Assets and liabilities recorded in foreign currencies are translated at the exchange rate on the balance sheet date. Translation adjustments resulting from this process are charged or credited to other comprehensive income. Revenue and expenses are translated at average rates of exchange prevailing during the year. Foreign currency transactions gains and losses were insignificant for the periods presented.
Stock-Based Compensation. Effective January 1, 2006, Acacia Research Corporation adopted the provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123R”), which sets forth the accounting requirements for “share-based” compensation payments to employees and non-employee directors and requires that compensation cost relating to share-based payment transactions be recognized in the statement of operations. In March 2005, the SEC published Staff Accounting Bulletin No. 107 (“SAB 107”), which requires stock-based compensation to be classified in the same expense line items as cash compensation (i.e. marketing, general and administrative and research and development expenses). The compensation cost for all stock-based awards is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the employee’s requisite service period (generally the vesting period of the equity award).
In addition, SFAS No. 123R requires stock-based compensation expense to be recorded only for those awards expected to vest using an estimated forfeiture rate. As such, SFAS No. 123R requires Acacia Research Corporation to estimate pre-vesting option forfeitures at the time of grant and reflect the impact of estimated pre-vesting option forfeitures on compensation expense recognized. Acacia Research Corporation considers several factors in connection with our estimates of pre-vesting forfeitures including types of awards, employee classification, and historical pre-vesting forfeiture data. Estimates of pre-vesting forfeitures must be periodically revised in subsequent periods if actual forfeitures differ from those estimates. To the extent that actual results differ from our estimates, such amounts will be recorded as cumulative adjustments in the period the estimates are revised. Prior to the adoption of SFAS No. 123R, Acacia Research Corporation accounted for forfeitures as they occurred under the pro forma disclosure provisions of Statement of Financial Accounting Standards No. 123, “Accounting for Stock-Based Compensation.” All references to stock-based compensation expense in these notes, upon adoption of SFAS No. 123R, refers to stock-based compensation net of estimated forfeitures, as required by SFAS No. 123R.
We adopted SFAS No. 123R using the modified prospective transition method. Under this transition method, compensation cost recognized for the year ended December 31, 2006 includes: (i) compensation cost for all stock-based awards granted prior to, but not yet vested as of January 1, 2006 (based on the grant-date fair value estimated in accordance with the original provisions of SFAS No. 123 and previously presented in the pro forma footnote disclosures), and (ii) compensation cost for all stock-based awards granted subsequent to January 1, 2006 (based on the grant-date fair value estimated in accordance with the new provisions of SFAS No. 123R). The cumulative effect of applying an estimated forfeiture percentage to stock-based payments granted prior to, but not yet vested as of January 1, 2006 was not material.
Prior to January 1, 2006, Acacia Research Corporation accounted for share-based compensation to employees in accordance with Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB No. 25”), and related interpretations. Acacia Research Corporation also followed the disclosure requirements of Statement of Financial Accounting Standards No. 123, “Accounting for Stock-Based Compensation” (“SFAS No. 123”), as amended by Statement of Financial Accounting Standards No. 148, “Accounting for Stock-Based Compensation-Transition and Disclosure.” Because Acacia Research Corporation previously adopted only the pro forma disclosure provisions of SFAS No. 123, we will recognize compensation cost relating to the unvested portion of awards granted prior to the date of adoption using the same estimate of the grant-date fair value and the same attribution method used to determine the pro forma disclosures under SFAS No. 123, except that forfeiture rates will be estimated for all awards, as required by SFAS No. 123R. In accordance with the requirements of the modified prospective transition method of adoption of SFAS No. 123R, the financial statement amounts for prior periods presented in this Form 10-K have not been restated to reflect the fair value method of recognizing compensation cost relating to stock-based awards.
The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model that uses the assumptions noted in the table below. Expected volatility is based on the separate historical volatility of the market prices of the AR-Acacia Technologies stock and AR-CombiMatrix stock. Volatilities of peer companies were also considered, when applicable, to address the lack of extensive historical volatility data for Acacia Research Corporation’s classes of common stock. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The expected term assumption was determined in accordance with guidance set forth in SAB 107, which provides a “simplified method” for estimating the expected term for stock options, granted prior to December 31, 2007, that 1) are granted at-the-money, 2) have exercisability conditioned only on completion of a service condition through the vesting date, 3) require that employees who terminate their service prior to vesting must forfeit the options, 4) provide that employees who terminate their service after vesting are granted limited time to exercise their stock options (typically 30-90 days), and 5) are nontransferable and nonhedgeable. The simplified method is based on the vesting period and the contractual term for each grant, or for each vesting-tranche for awards with graded vesting. The mid-point between the vesting commencement date and the expiration date is used as the expected term under this method. For awards with multiple vesting-tranches, the times from grant until these mid-points for each of the tranches were averaged to provide an overall expected term.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The fair value of restricted stock awards is determined by the product of the number of shares granted and the grant date market price of the AR-Acacia Technologies stock or AR-CombiMatrix stock.
The fair value of share-based awards is expensed on a straight-line basis over the requisite service period (generally the vesting period of the award), which is generally two to four years.
Acacia Research Corporation adopted the alternative transition method provided in FASB Staff Position No. FAS 123(R)-3, "Transition Election Related to Accounting for Tax Effects of Share-Based Payment Awards". The alternative transition method includes a simplified method to establish the beginning balance of the additional paid-in capital pool ("APIC pool") related to the tax effects of employee stock-based compensation which is available to absorb tax deficiencies recognized subsequent to the adoption of SFAS 123(R).
The fair value of stock options for the year ended December 31, 2006 was estimated using the Black-Scholes option-pricing model based on the following weighted-average assumptions:
| | | Risk Free Interest Rate | | Term | | Volatility | | Dividends | |
| AR-CombiMatrix stock | | | 5.06% | | | 6 years | | | 82% | | | 0% | |
| AR-Acacia Technologies stock | | | 4.30% | | | 6 years | | | 75% | | | 0% | |
| CMDX stock | | | 5.05% | | | 6.25 years | | | 82% | | | 0% | |
The following table illustrates the impact of share-based compensation expense on reported amounts (in thousands, except for per share data):
| | | For the Year Ended December 31, 2006 | |
| | | | | Impact of Stock | |
| | | As Reported | | Based Compensation | |
| Loss from continuing operations before income taxes and minority interests | | | (25,450 | ) | | (5,083 | ) |
| Net loss | | | (25,456 | ) | | (5,083 | ) |
| | | | | | | | |
| Loss per share: | | | | | | | |
| AR-Acacia Technologies stock: | | | | | | | |
| Stock-based compensation | | | - | | | (2,767 | ) |
| Basic and diluted | | | (0.20 | ) | | (0.10 | ) |
| | | | | | | | |
| AR-CombiMatrix stock: | | | | | | | |
| Stock-based compensation | | | - | | | (2,316 | ) |
| Basic and diluted | | $ | (0.49 | ) | | (0.06 | ) |
Stock-based compensation expense for the periods presented is included in research and development expenses and marketing, general and administrative expenses, as disclosed in the accompanying consolidated statement of operations and comprehensive income (loss).
Option awards granted prior to Acacia Research Corporation’s implementation of SFAS No. 123R were accounted for under the recognition and measurement principles of APB No. 25 and related interpretations. Accordingly, no stock-based employee compensation cost was reflected in the accompanying consolidated statements of operations for the years ended December 31, 2006, 2005 and 2004, because all options granted under Acacia Research Corporation’s plans had exercise prices equal to the market value of the underlying common stock on the date of grant. Stock-based compensation expense reflected in the accompanying consolidated statement of operations for the year ended December 31, 2005 related to restricted stock awards originally granted in 2005.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following table illustrates the pro forma effect on net loss and loss per share, if Acacia Research Corporation had applied the fair value recognition provisions of SFAS No. 123 (in thousands, except per share data):
| | | AR-Acacia Technologies Stock | | | AR-CombiMatrix Stock 2005 | | | | AR-Acacia Technologies Stock 2004 | | | | AR-CombiMatrix Stock 2004 | |
| | | | | | | | | | | | | | | |
| Income (loss) from operations, as reported | $ | (6,275 | ) | $ | (12,401 | ) | | $ | (5,543 | ) | | $ | 710 | |
| Add: Stock-based compensation, intrinsic | | | | | | | | | | | | | | |
value method reported in net loss (2) | | 356 | | | - | | | | - | | | | 606 | (4) |
| Deduct: Pro forma stock-based compensation | | | | | | | | | | | | | | |
fair value method(2) | | (2,103 | ) | | (2,834 | ) (3) | | | (2,137 | ) (3) | | | (6,127 | ) |
| Income (loss) from operations, pro forma | $ | (8,022 | ) | $ | (15,235 | ) | | $ | (7,680 | ) | | $ | (4,811 | ) |
| Basic and diluted earnings (loss) per share from operations, as reported | $ | (0.24 | ) | $ | (0.37 | ) | | $ | (0.28 | ) | | $ | 0.02 | |
| Basic and diluted earnings (loss) per share from operations, pro forma | $ | (0.30 | ) | $ | (0.45 | ) (3) | | $ | (0.39 | ) (3) | | $ | (0.16 | ) |
| | | | | | | | | | | | | | | |
Weighted Average Assumptions used(1): | | | | | | | | | | | | | | |
| Risk free interest rate | | 3.90 | % | | 3.84 | % | | | 3.35 | % | | | 3.18 | % |
| Volatility | | 91 | % | | 88 | % | | | 99 | % | | | 100 | % |
| Expected term | | 5 years | | 5 years | | | 5 years | | | 5 years |
| | | | | | | | | | | | | | | |
___________________________
| (1) | The fair value of stock options was determined using the Black-Scholes option-pricing model. The fair value calculations assume no expected dividends. |
| (2) | The previously reported 2005 and 2004 pro forma income (loss) from operations and related pro forma earnings (loss) per share amounts have been revised for a computational error in the effective tax rate due to the full valuation allowance recoded by Acacia Research Corporation for all periods presented and to exclude stock compensation expense related to non-employees. |
| (3) | Includes the impact of non-cash stock compensation expense related to restricted stock grants. The pro forma impact on net income (loss) and earnings (loss) per share of options outstanding under the CombiMatrix Molecular Diagnostics, Inc. Plan was not material. |
| (4) | During the year ended December 31, 2000, CombiMatrix Corporation recorded deferred non-cash stock compensation charges aggregating approximately $53.8 million in connection with the granting of stock options, which were amortized by the CombiMatrix group over the respective option grant vesting periods, which ranged from one to four years. Deferred non-cash stock compensation charges were fully amortized as of December 31, 2004. |
SFAS No. 123R does not require the recording of deferred stock compensation charges in stockholder’s equity on the grant date of a stock-based award. As such, in accordance with SFAS No. 123R, all deferred stock compensation charges previously recorded under APB No. 25, totaling $1,400,000 at December 31, 2005, related to restricted stock awards, have been reversed upon adoption of SFAS No. 123R, with a corresponding reduction being recorded in consolidated additional paid-in capital.
Research and Development Expenses. Research and development expenses consist of costs incurred for direct and overhead-related research expenses and are expensed as incurred. Costs to acquire technologies, which are utilized in research and development and which have no alternative future use are expensed when incurred. Software developed for use in our products is expensed as incurred until both (i) technological feasibility for the software has been established and (ii) all research and development activities for the other components of the system have been completed. We believe these criteria are met after we have received evaluations from third-party test sites and completed any resulting modifications to the products. Expenditures to date have been classified as research and development expense.
Advertising. Costs associated with the marketing and advertising of the CombiMatrix group’s products and services are expensed as incurred. For the years ended December 31, 2006, 2005 and 2004, marketing and advertising expenses, incurred solely by the CombiMatrix group, were $253,000, $516,000 and $314,000, respectively.
Income Taxes. Income taxes are accounted for using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in Acacia Research Corporation’s financial statements or tax returns. A valuation allowance is established to reduce deferred tax assets if all, or some portion, of such assets will more than likely not be realized.
Comprehensive Income (Loss). Comprehensive income (loss) is the change in equity from transactions and other events and circumstances other than those resulting from investments by owners and distributions to owners.
Segment Reporting. We use the management approach, which designates the internal organization that is used by management for making operating decisions and assessing performance as the basis of Acacia Research Corporation’s reportable segments.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
Earnings (Loss) Per Share. Basic earnings per share for each class of common stock is computed by dividing the income or loss allocated to each class of common stock by the weighted-average number of outstanding shares of that class of common stock. Diluted earnings per share is computed by dividing the income or loss allocated to each class of common stock by the weighted-average number of outstanding shares of that class of common stock including the dilutive effect of common stock equivalents. Potentially dilutive common stock equivalents primarily consist of employee stock options, unvested restricted stock grants and common stock purchase warrants.
The earnings or losses allocated to each class of common stock are determined by Acacia Research Corporation’s board of directors. This determination is generally based on the net income or loss amounts of the corresponding group determined in accordance with accounting principles generally accepted in the United States of America, consistently applied. Acacia Research Corporation believes this method of allocation is systematic and reasonable. The Acacia Research Corporation board of directors can, at its discretion, change the method of allocating earnings or losses to each class of common stock at any time.
The following table presents a reconciliation of basic and diluted income (loss) per share:
| | | For the Year | | For the Year | | For the Year | |
| | | Ended December 31, | | Ended December 31, | | Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
Acacia Research - Acacia Technologies stock | | | | | | | |
| Basic and diluted weighted average number of common shares outstanding | | | 27,547,651 | | | 26,630,732 | | | 19,784,883 | |
| All outstanding stock options and nonvested restricted stock excluded from the computation | | | | | | | | | | |
| of diluted loss per share because the effect of inclusion would have been anti-dilutive | | | 6,385,810 | | | 6,315,000 | | | 5,726,000 | |
| | | | | | | | | | | |
Acacia Research - CombiMatrix stock | | | | | | | | | | |
| Basic weighted average number of common shares outstanding | | | 40,605,038 | | | 33,678,603 | | | 29,962,596 | |
| Dilutive effect of outstanding stock options and warrants | | | - | | | - | | | 1,033,067 | |
| Diluted weighted average number of common and | | | | | | | | | | |
| potential common shares outstanding | | | 40,605,038 | | | 33,678,603 | | | 30,995,663 | |
| Outstanding stock options excluded from the computation of diluted loss per share | | | | | | | | | | |
| because the effect of inclusion would have been anti-dilutive | | | 8,068,139 | | | 6,925,000 | | | 3,966,000 | |
In addition, all common stock purchase warrants outstanding for 2006 and 2005, as disclosed at Note 10, have been excluded from the computation of diluted loss per share because the effect of inclusion would have been anti-dilutive.
Separate Group Presentation. AR-CombiMatrix stock and AR-Acacia Technologies stock are intended to reflect the separate performance of the respective division of Acacia Research Corporation. The CombiMatrix group and the Acacia Technologies group are not separate legal entities. Holders of AR-CombiMatrix stock and AR-Acacia Technologies stock are stockholders of Acacia Research Corporation. As a result, holders of AR-CombiMatrix stock and AR-Acacia Technologies stock continue to be subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. The assets Acacia Research Corporation attributes to one of the groups could be subject to the liabilities of the other group. The group financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America, and taken together, comprise all the accounts included in the corresponding consolidated financial statements of Acacia Research Corporation. The financial statements of the groups reflect the financial condition, results of operations, and cash flows of the businesses included therein. The financial statements of the groups include the accounts or assets of Acacia Research Corporation specifically attributed to the groups and were prepared using amounts included in Acacia Research Corporation’s consolidated financial statements.
Minority interests represent participation of other stockholders in the net equity and in the division earnings and losses of the groups and are reflected in the caption “Minority interests” in the group financial statements. Minority interests adjust group net results of operations to reflect only the group’s share of the division earnings or losses of non-wholly owned investees.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Financial effects arising from one group that affect Acacia Research Corporation’s results of operations or financial condition could, if significant, affect the results of operations or financial condition of the other group and the market price of the class of common stock relating to the other group. Any division net losses of the CombiMatrix group or of the Acacia Technologies group, and dividends or distributions on, or repurchases of, AR-CombiMatrix stock or AR-Acacia Technologies stock, will reduce the assets of Acacia Research Corporation legally available for payment of dividends on AR-CombiMatrix stock or AR-Acacia Technologies stock.
Management Allocation Policies. The management and allocation policies applicable to the preparation of the financial statements of the CombiMatrix group and the Acacia Technologies group may be modified or rescinded, or additional policies may be adopted, at the sole discretion of the Acacia Research Corporation board of directors at any time without approval of the stockholders. The group’s financial statements reflect the application of the management and allocation policies adopted by the Acacia Research Corporation board of directors to various corporate activities, as described below. Management has no plans to change allocation methods or the composition of the groups. The group financial statements should be read in conjunction with the Acacia Research Corporation consolidated financial statements and related notes.
Treasury and Cash Management Policies. Cash and cash equivalents and short-term investments are attributed to the groups based on the respective cash and cash equivalents and short-term investment balances of the entities comprising each group. Acacia Research Corporation’s cash and the cash held by its intellectual property licensing businesses, including all cash raised through Acacia Research Corporation’s previous offerings, have been attributed to the Acacia Technologies group as these funds are intended to support the intellectual property licensing businesses of Acacia Research Corporation. All cash raised by CombiMatrix Corporation and Advanced Material Sciences have been attributed to the CombiMatrix group. Acacia Research Corporation manages most treasury and cash management activities on a decentralized basis, with each group separately managing its own treasury activities. Pursuant to treasury and cash management policies adopted by the Acacia Research Corporation board of directors, the following applies:
| · | Acacia Research Corporation will attribute each future issuance of AR-Acacia Technologies stock (and the proceeds thereof) to the Acacia Technologies group and will attribute each future issuance of AR-CombiMatrix stock (and the proceeds thereof) to the CombiMatrix group; |
| · | Acacia Research Corporation will attribute each future incurrence or issuance of external debt or preferred stock (and the proceeds thereof), if any, between the groups or entirely to one group as determined by the Acacia Research Corporation board of directors, based on the extent to which Acacia Research Corporation incurs or issues the debt or preferred stock for the benefit of the CombiMatrix group or the Acacia Technologies group; |
| · | Dividends, if any, on AR-Acacia Technologies stock will be charged against the Acacia Technologies group, and dividends, if any on AR-CombiMatrix stock will be charged against the CombiMatrix group; |
| · | Repurchases of AR-Acacia Technologies stock will be charged against the Acacia Technologies group and repurchases of AR-CombiMatrix stock will be charged against the CombiMatrix group; |
| · | Acacia Research Corporation accounts for any cash transfers from Acacia Research Corporation to or for the account of a group, from a group to or for the account of Acacia Research Corporation, or from one group to or for the account of the other group (other than transfers in return for assets or services rendered) as short-term loans unless (i) the Acacia Research Corporation board of directors determines that a given transfer (or type of transfer) should be accounted for as a long-term loan, (ii) the Acacia Research Corporation board of directors determines that a given transfer (or type of transfer) should be accounted for as a capital contribution or (iii) the Acacia Research Corporation board of directors determines that a given transfer (or type of transfer) should be accounted for as a return of capital. There are no specific criteria to determine when Acacia Research Corporation will account for a cash transfer as a long-term loan, a capital contribution or a return of capital rather than an inter-group revolving credit advance; provided, however, that cash advances from Acacia Research Corporation to the Acacia Technologies group or to the CombiMatrix group up to $25.0 million on a cumulative basis shall be accounted for as short-term or long-term loans at interest rates at which Acacia Research Corporation could borrow such funds and shall not be accounted for as a capital contribution. The Acacia Research Corporation board of directors will make such a determination in the exercise of its business judgment at the time of such transfer based upon all relevant circumstances. Factors the Acacia Research Corporation board of directors may consider include, without limitation, the current and projected capital structure of each group; the financing needs and objectives of the recipient group; the availability, cost and time associated with alternative financing sources; and prevailing interest rates and general economic conditions; and |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| · | Any cash transfers accounted for as short-term loans will bear interest at the rate at which Acacia Research Corporation could borrow such funds. In addition, any cash transfers accounted for as a long-term loan will have interest rates, amortization, maturity, redemption and other terms that reflect the then-prevailing terms on which Acacia Research Corporation could borrow such funds. |
Assets and Liabilities. Acacia Research Corporation’s assets and liabilities have been attributed to the Acacia Technologies group and the CombiMatrix group based on the respective asset and liabilities of the business comprising each group. Net intangible assets recorded at the Acacia Research Corporation level, primarily consisting of acquired patents and goodwill balances, have been attributed to the respective businesses comprising each group to which the intangibles and goodwill relate.
Corporate General and Administrative Services and Facilities. Acacia Research Corporation allocates the cost of corporate general and administrative services and facilities between the groups generally based upon utilization. Where determinations based on utilization alone are impracticable, Acacia Research Corporation utilizes other methods and criteria that management believes to be equitable and to provide a reasonable estimate of the cost attributable to each group. Except as otherwise determined by management, the allocated costs of providing such services and facilities include, without limitation, all costs and expenses of personnel employed in connection with such services and facilities, including, without limitation, all direct costs of such personnel, such as payroll, payroll taxes and fringe benefit costs (calculated at the appropriate annual composite rate therefore) and all overhead costs and expenses directly related to such personnel and the services or facilities provided by them. In addition, allocated costs include all materials used in connection with such services or facilities, billed at their net cost to the provider of the services or facilities plus all overhead costs and expenses related to such materials. Except as may otherwise be specifically provided pursuant to the terms of any agreements among Acacia Research Corporation and the groups or any resolutions of the Acacia Research Corporation board of directors, the corporate general and administrative services and facilities to be allocated between the groups include, without limitation, legal services, accounting services (tax and financial), insurance and deductibles payable in connection therewith, employee benefit plans and administration thereof, investor relations, stockholder services, and services relating to the board of directors.
Direct salaries, payroll taxes and fringe benefits are allocated to the groups based on the percentage of actual time incurred by specific employees to total annual time available and direct costs including, postage, insurance, legal fees, accounting and tax and other are allocated to the groups based on specific identification of costs incurred on behalf of each group. Other direct costs, including direct depreciation expense, computer costs, general office supplies and rent are allocated to the groups based on the ratio of direct salaries to total salaries. Indirect costs, including indirect salaries and benefits, investor relations, rent, general office supplies and indirect depreciation are allocated to the groups based on the ratio of direct salaries for each group to total direct salaries. Included in marketing, general and administrative expenses of the Acacia Technologies group are allocated corporate charges of $3,124,000, $2,612,000 and $2,158,000 relating to the periods ended December 31, 2006, 2005, and 2004, respectively. Included in marketing, general and administrative expenses of the CombiMatrix group are allocated corporate charges of $551,000, $498,000, and $689,000 relating to the periods ended December 31, 2006, 2005, and 2004, respectively.
Management believes that the methods and criteria used to allocate costs are equitable and provide a reasonable estimate of the cost attributable to the groups. Based on the allocation methods used, Acacia Research Corporation believes that the allocation of expenses as presented in the accompanying consolidating financial information reflects a reasonable estimation of expenses that would be recognized if the groups were separate stand-alone registrants.
Allocation of Federal and State Income Taxes. Acacia Research Corporation determines its federal income taxes and the federal income taxes of its subsidiaries that own assets allocated between the groups on a consolidated basis. Acacia Research Corporation allocates consolidated federal income tax provisions and related tax payments or refunds between the Acacia Technologies’ group and CombiMatrix group based principally on the taxable income and tax credits directly attributable to each group. Such allocations reflect each group’s contribution, whether positive or negative, to Acacia Research Corporation’s consolidated federal taxable income and consolidated federal tax liability and tax credit position. Acacia Research Corporation will credit tax benefits that cannot be used by the group generating those benefits but can be used on a consolidated tax return basis to the group that generated such benefits.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Inter-group transactions are treated as taxed as if each group was a stand-alone company. Depending on the tax laws of the respective jurisdictions, state and local income taxes are calculated on either a consolidated or combined basis between the groups based on their respective contribution to such consolidated or combined state taxable incomes. State and local income tax provisions and related tax payments or refunds which are determined on a separate corporation basis are allocated between the groups in a manner designed to reflect the respective contributions of the groups to Acacia Research Corporation’s separate or local taxable income.
Recent Accounting Pronouncements. In July 2006, the FASB issued Financial Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109” (“FIN 48”), which clarifies the accounting for uncertainty in income taxes recognized in the financial statements in accordance with FASB Statement No. 109, “Accounting for Income Taxes.” FIN 48 specifies how tax benefits for uncertain tax positions are to be recognized, measured, and derecognized in financial statements; requires certain disclosures of uncertain tax matters; specifies how reserves for uncertain tax positions should be classified on the balance sheet; and provides transition and interim period guidance, among other provisions. FIN 48 is effective for fiscal years beginning after December 15, 2006 and as a result, is effective for Acacia Research Corporation in the first quarter of fiscal 2007. We are currently evaluating the impact of FIN 48 on our consolidated and separate group financial position, results of operations and cash flows.
In September 2006, the SEC issued Staff Accounting Bulletin No. 108 (“SAB 108”), “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements.” SAB 108 is effective for fiscal years ending on or after November 15, 2006 and addresses how financial statement errors should be considered from a materiality perspective and corrected. The literature provides interpretive guidance on how the effects of the carryover or reversal of prior year misstatements should be considered in quantifying a current year misstatement. Historically there have been two common approaches used to quantify such errors: (i) the “rollover” approach, which quantifies the error as the amount by which the current year income statement is misstated, and (ii) the “iron curtain” approach, which quantifies the error as the cumulative amount by which the current year balance sheet is misstated. The SEC Staff believes that companies should quantify errors using both approaches and evaluate whether either of these approaches results in quantifying a misstatement that, when all relevant quantitative and qualitative factors are considered, is material. Historically, we have evaluated uncorrected differences utilizing the “rollover” approach. The adoption of the SAB 108 did not have an impact on our consolidated or separate group financial position, results of operations and cash flows.
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“SFAS No. 157”). SFAS No. 157 establishes a common definition for fair value to be applied to US GAAP guidance requiring use of fair value, establishes a framework for measuring fair value, and expands disclosure about such fair value measurements. SFAS No. 157 is effective for fiscal years beginning after November 15, 2007. We are currently assessing the impact, if any, of adopting SFAS No. 157 on our consolidated and separate group financial position, results of operations and cash flows.
In June 2006, the Emerging Issues Task Force issued EITF 06-3, “How Taxes Collected from Customers and Remitted to Governmental Authorities Should Be Presented in the Income Statement (That Is, Gross versus Net Presentation)” (“EITF 06-3”) to clarify diversity in practice on the presentation of different types of taxes in the financial statements. The Task Force concluded that, for taxes within the scope of the issue, a company may adopt a policy of presenting taxes either gross within revenue or net. That is, it may include charges to customers for taxes within revenues and the charge for the taxes from the taxing authority within cost of sales, or, alternatively, it may net the charge to the customer and the charge from the taxing authority. If taxes subject to this Issue are significant, a company is required to disclose its accounting policy for presenting taxes and the amounts of such taxes that are recognized on a gross basis. The guidance in this consensus is effective for the first interim reporting period beginning after December 15, 2006. We are currently assessing the impact, if any, of adopting EITF 06-3 on our consolidated and separate group financial position, results of operations and cash flows.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities” ("SFAS No. 159"), which provides reporting entities an option to report selected financial assets, including investment securities designated as available-for-sale, and liabilities, including most insurance contracts, at fair value. SFAS No. 159 establishes presentation and disclosure requirements designed to facilitate comparisons between companies that choose different measurement attributes for similar types of assets and liabilities. The standard also requires additional information to aid financial statement users' understanding of a reporting entity's choice to use fair value on its earnings and also requires entities to display on the face of the balance sheet the fair value of those assets and liabilities for which the reporting entity has chosen to measure at fair value. SFAS No. 159 is effective as of the beginning of a reporting entity's first fiscal year beginning after November 15, 2007. Early adoption is permitted as of the beginning of the previous fiscal year provided the entity makes that choice in the first 120 days of that fiscal year and also elects to apply the provisions of SFAS No. 157. Because application of the standard is optional, any impacts are limited to those financial assets and liabilities to which SFAS No. 159 would be applied, which has yet to be determined.
3. SHORT-TERM INVESTMENTS
Short-term investments consist of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | Amortized | | Fair | | Amortized | | Fair | |
| | | Cost | | Value | | Cost | | Value | |
| | | | | | | | | | |
| Available-for-sale securities: | | | | | | | | | |
| Corporate and municipal bonds and notes | | $ | 2,965 | | $ | 2,980 | | $ | 4,858 | | $ | 4,846 | |
| Auction rate securities and annuity investments | | | 10,815 | | | 10,815 | | | 20,772 | | | 20,771 | |
| U.S. government securities | | | 5,513 | | | 5,501 | | | 11,437 | | | 11,392 | |
| Certificates of deposit | | | - | | | - | | | 2,000 | | | 2,000 | |
| | | $ | 19,293 | | $ | 19,296 | | $ | 39,067 | | $ | 39,009 | |
| | | | | | | | | | | | | | |
| Due within one year | | $ | 5,992 | | $ | 5,995 | | $ | 17,041 | | $ | 16,987 | |
| Due after one year through two years | | | 2,486 | | | 2,486 | | | 1,254 | | | 1,251 | |
| Auction rate securities and annuity investments, with stated maturities up to 48 years | | | 10,815 | | | 10,815 | | | 20,772 | | | 20,771 | |
| | | $ | 19,293 | | $ | 19,296 | | $ | 39,067 | | $ | 39,009 | |
Gross unrealized gains and losses related to available-for-sale securities were not material for the periods presented. As disclosed in Note 2, auction rate securities are classified as short-term available-for-sale securities due to our ability to quickly liquidate these securities as their variable rates reset, typically every 7 to 63 days. Annuity investments are classified as current as the instruments provide daily liquidity, similar to a money market instrument and all funds are available for current operations. Refer to Note 2 for more information.
4. PROPERTY AND EQUIPMENT
Property and equipment consists of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Machine shop and laboratory equipment | | $ | 4,322 | | $ | 4,931 | |
| Furniture and fixtures | | | 386 | | | 407 | |
| Computer hardware and software | | | 968 | | | 1,242 | |
| Leasehold improvements | | | 1,117 | | | 1,061 | |
| Construction in progress | | | - | | | 17 | |
| | | | 6,793 | | | 7,658 | |
| Less: accumulated depreciation and amortization | | | (4,787 | ) | | (5,174 | ) |
| | | $ | 2,006 | | $ | 2,484 | |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Depreciation and amortization expense was $970,000 $1,147,000 and $1,154,000 for the years ended December 31, 2006, 2005 and 2004, respectively.
5. BALANCE SHEET COMPONENTS
Accounts payable, accrued expenses and other consists of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Accounts payable | | $ | 1,420 | | $ | 936 | |
| Payroll and other employee benefits | | | 395 | | | 486 | |
| Accrued vacation | | | 720 | | | 686 | |
| Accrued liabilities of discontinued operations | | | 12 | | | 136 | |
| Accrued legal expenses | | | 1,131 | | | 464 | |
| Accrued consulting and other professional fees | | | 663 | | | 447 | |
| Deferred rent | | | 269 | | | 315 | |
| Inventor royalties - V-chip | | | - | | | 225 | |
| Other accrued liabilities | | | 437 | | | 229 | |
| | | $ | 5,047 | | $ | 3,924 | |
Deferred revenues consisted of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Milestone and up-front payments | | $ | 1,441 | | $ | 1,604 | |
| License fee payments | | | 360 | | | 639 | |
| | | | 1,801 | | | 2,243 | |
| Less: current portion | | | (725 | ) | | (804 | ) |
| | | $ | 1,076 | | $ | 1,439 | |
In August 2004, the CombiMatrix group received a $1,000,000 upfront payment from Furuno as part of a multi-year collaboration agreement to develop a bench-top array synthesizer for commercial applications, which was included in deferred revenue at December 31, 2005 and 2004. During the third quarter of 2006, the CombiMatrix group entered into a manufacturing agreement and completed its obligations under its collaboration agreement with Furuno. As a result, the CombiMatrix group began amortizing the $1,000,000 upfront payment previously received under the collaboration agreement over the economic life of the manufacturing agreement, which is estimated to be four years. At December 31, 2006, deferred revenue related to the Furuno collaboration agreement totaled $875,000.
In 2003, the CombiMatrix group received upfront and milestone payments from Toppan totaling $2,400,000, pursuant to a multi-year collaboration and supply agreement to develop and manufacture arrays using the CombiMatrix group’s proprietary electrochemical detection approach, which was included in deferred revenues at December 31, 2004. During the fourth quarter of 2005, the CombiMatrix group completed all obligations under its collaboration and supply agreement with Toppan. As a result of completing all of its obligations under this agreement and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized all previously deferred payments received from Toppan, totaling $2,266,000, as collaboration agreement revenues in the accompanying consolidated statement of operations and comprehensive loss.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In March 2004, the CombiMatrix group completed all phases of its research and development agreement with Roche. As a result of completing all of its obligations under this agreement and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized all previously deferred payments received from Roche as collaboration agreement revenues totaling $17,302,000 during the first quarter of 2004.
6. INVESTMENTS
In October 2004 (the “Investment Date”), the CombiMatrix group entered into an agreement to acquire up to a one-third ownership interest in Leuchemix, Inc. (“Leuchemix”), a private drug development firm, which is developing several compounds for the treatment of leukemia and other cancers. In accordance with the terms of the purchase agreement, the CombiMatrix group purchased 3,137,500 shares of Series A Preferred Stock of Leuchemix for a total purchase price of $4,000,000. The ownership interest was acquired and paid for quarterly, beginning with the fourth quarter of 2004 and continuing through the fourth quarter of 2006. As of December 31, 2006, 2005 and 2004, the CombiMatrix group had invested $4,000,000, $1,850,000 and $250,000, representing a 33%, 19% and 3% interest in the total outstanding voting securities of Leuchemix, respectively. In accordance with the terms of the purchase agreement, CombiMatrix Corporation’s CEO was named a director of Leuchemix. The CombiMatrix group’s investment in Leuchemix is being accounted for under the equity method for all periods presented, as the CombiMatrix group has the ability to exercise significant influence over Leuchemix, primarily due to CombiMatrix Corporation’s representation on Leuchemix’s board of directors.
The CombiMatrix group’s equity in the losses of Leuchemix, including its share of the amortization expense related to the excess purchase consideration over the book value of Leuchemix was $963,000, $352,000 and $17,000 for the years ended December 31, 2006, 2005 and 2004, respectively. Summary financial information for Leuchemix was not significant for the periods presented.
In January 2006, the CombiMatrix group expanded its relationship with one of its existing distributors, InBio, for the Asia Pacific region. Major components of the expanded relationship included the transfer of day-to-day operational responsibility and majority ownership of CombiMatrix K.K. to InBio, along with an expanded distribution agreement that encompasses Japan. InBio obtained 67% of the voting interests in CombiMatrix K.K. for a nominal amount of consideration. As a result, InBio assumed all operational and financial responsibilities of CombiMatrix K.K. The net loss on the sale of 67% of the voting interest in CombiMatrix K.K. recorded in the consolidated statement of operations for the year ended December 31, 2006 was $84,000. Subsequent to the sale, our investment in CombiMatrix K.K. was accounted for under the equity method. The deconsolidation of CombiMatrix K.K. did not have a material impact on the consolidated balance sheets as of December 31, 2006.
7. GOODWILL AND INTANGIBLE ASSETS
The Acacia Technologies group had $121,000 of goodwill at December 31, 2006 and 2005.
The CombiMatrix group had $16,918,000 and $18,859,000 of goodwill at December 31, 2006 and 2005, respectively. Prior to December 31, 2005, $565,000 of the total amount of goodwill resulted from the acquisitions of Advanced Materials Sciences, Inc. (“AMS”) and CombiMatrix K.K. during July 2003. These reporting units were tested for impairment in the fourth quarter of 2005 in connection with our annual forecasting process. Due to the lack of third-party research and development funding for AMS and declining array product sales at CombiMatrix K.K., operating profits and cash flows were lower than expected during the preceding three quarters. Based on these trends, the operating forecasts for 2006 were revised downward and as a result, a goodwill impairment loss of $565,000 was recognized in December 2005. The fair values of these reporting units were estimated using the expected present value of their future cash flows. As a result of the impairment charge recorded, the carrying value of these reporting units was reduced to zero. As such, as of December 31, 2006, AMS and CombiMatrix K.K. are no longer considered reporting units.
In August 2004, as a result of the conclusion of the Acacia Technologies group’s V-chip patent infringement litigation and completion of the V-chip licensing program, as described at Note 14, the Acacia Technologies group recorded an impairment charge totaling $1,616,000 in connection with the write-down of 100% of the goodwill related to the V-chip.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Acacia Research Corporation’s only identifiable intangible assets at December 31, 2006 and 2005 are patents and licenses.
For the year ended December 31, 2006, the Acacia Technologies group incurred patent acquisition costs totaling $1,030,000 in connection with the acquisition of the rights to six additional patent portfolios. The patents have estimated economic useful lives ranging from five to seven years and are being amortized over a weighted-average economic useful life of six years. Refer to Note 8 for additions to patent related intangibles in connection with the January 2005 GPH Acquisition.
The gross carrying amounts and accumulated amortization as of December 31, 2006 and 2005 and amortization expense for the periods presented, related to patents and licenses, by segment, are as follows (in thousands):
| | | Acacia Technologies Group | | CombiMatrix Group | | Consolidated | |
| | | 2006 | | 2005 | | 2006 | | 2005 | | 2006 | | 2005 | |
| | | | | | | | | | | | | | |
| Gross carrying amount - patents and licenses | | $ | 30,317 | | $ | 30,392 | | $ | 12,595 | | $ | 12,095 | | $ | 42,912 | | $ | 42,487 | |
| Accumulated amortization | | | (11,802 | ) | | (6,606 | ) | | (5,303 | ) | | (4,169 | ) | | (17,105 | ) | | (10,775 | ) |
| Patents and licenses, net | | $ | 18,515 | | $ | 23,786 | | $ | 7,292 | | $ | 7,926 | | $ | 25,807 | | $ | 31,712 | |
| | | | | | | | | | | | | | | | | | | | |
| | | Acacia Technologies Group | | CombiMatrix Group | |
| | | 2006 | | 2005 | | 2004 | | 2006 | | 2005 | | 2004 | |
| | | | | | | | | | | | | | |
| Patent amortization expense | | $ | 5,313 | | $ | 4,922 | | $ | 501 | | $ | 1,096 | | $ | 1,095 | | $ | 1,096 | |
| | | | | | | | | | | | | | | | | | | | |
The Acacia Technologies group and the CombiMatrix group’s patents have remaining estimated economic useful lives up to 2013 and 2020, respectively. The weighted-average remaining estimated economic useful life of the Acacia Technologies group’s patents is 4 years. Annual aggregate amortization expense for the Acacia Technologies group for each of the five fiscal years through December 31, 2011, is estimated to be $5,237,000 in 2007, $3,914,000 in 2008, $3,463,000 in 2009, $3,272,000 in 2010 and $2,325,000 in 2011 for the Acacia Technologies group. Annual aggregate amortization expense from patents and licenses for the CombiMatrix group for each of the next five years through December 31, 2011 is estimated to be $1,133,000 per year. At December 31, 2006 and December 31, 2005, all of our acquired intangible assets other than goodwill were subject to amortization.
In June 2006, the Acacia Technologies group recorded a non-cash charge of $297,000, related to the write-off of a patent-related intangible asset. We review long-lived assets and intangible assets for potential impairment when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. In the event the sum of the expected undiscounted future cash flows resulting from the use of the asset is less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. During the second quarter of 2006, pursuant to the terms of the respective license agreement, management elected to terminate its rights to exclusively license and enforce the patent, resulting in the write-off of the remaining carrying value of the patent-related intangible asset as of June 30, 2006.
As of March 31, 2006, the Acacia Technologies group reduced its patents and deferred tax liability by $691,000, which were initially recorded in fiscal 2002, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability. As of March 31, 2006, the CombiMatrix group reduced its goodwill and deferred tax liability balances by $1,941,000, which were initially recorded in fiscal 2000, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability.
8. ACQUISITIONS
On January 28, 2005, Acacia Global Acquisition Corporation, a wholly owned subsidiary of Acacia Research Corporation, acquired substantially all of the assets of Global Patent Holdings, LLC, a privately held patent holding company based in Northbrook, Illinois, which owned 11 patent licensing companies. The acquisition provided the Acacia Technologies group with 100% ownership of companies that own or control the rights to 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. As a result of the acquisition, we have expanded and diversified the Acacia Technologies group’s potential revenue generating activities.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The acquisition was accounted for using the purchase method of accounting. Under the purchase method of accounting, the purchase consideration is allocated to the assets acquired, including tangible assets, patents and other identifiable intangibles and liabilities assumed, based on their estimated fair market values at the date of acquisition. The statement of operations includes the results of the acquired companies beginning on January 28, 2005, the date of acquisition. The aggregate purchase consideration was approximately $25,105,000, including $5.0 million of cash, the issuance of 3,938,832 shares of AR-Acacia Technologies stock valued at $19,293,000 (net of estimated common stock registration costs of $228,000) and other acquisition costs, including registration costs, totaling $812,000. The value of the common shares issued was determined based on the average market price of AR-Acacia Technologies stock, as reported on NASDAQ, over the 5-day period (December 13 - December 17, 2004) before and after the terms of the acquisition were agreed to and announced.
The following table summarizes the total purchase consideration and the allocation of the consideration paid to the estimated fair value of the assets acquired and liabilities assumed (in thousands):
| Purchase Consideration: | | | |
| Cash paid | | $ | 5,000 | |
Fair value of AR-Acacia Technologies stock issued(1) | | | 19,293 | |
| Acquisition and registration costs | | | 812 | |
| Total purchase consideration | | $ | 25,105 | |
Purchase Price Allocation: | | | | |
| Fair value of net tangible assets acquired at January 28, 2005 | | $ | (26 | ) |
Intangible assets acquired - patents and patent rights(1) | | | 25,131 | |
| Total | | $ | 25,105 | |
____________________________________________
(1) Reflects non-cash investing activity.
Management was primarily responsible for determining the fair value of the tangible and identifiable intangible assets acquired and liabilities assumed at the date of acquisition. Management considered a number of factors, including reference to an independent valuation. The patents and patent rights acquired were valued using a discounted cash flow model on a patent portfolio by portfolio basis, which estimated the future net cash flows expected to result from the licensing of each portfolio, taking into account potential infringers of the patents, usage of the underlying technologies, estimated license fee revenues, contingent legal fee arrangements, inventor royalties due to former patent holders, other estimated costs, tax implications and other factors. A discount rate consistent with the risks associated with achieving the estimated net cash flows was used to estimate the present value of future estimated net cash flows. Management’s valuation resulted in an estimated fair value of patent related assets acquired of approximately $27,000,000, resulting in approximately $1,900,000 of excess fair value over the cost of net assets acquired, which has been allocated as a pro rata reduction to the amounts that otherwise would have been assigned to the assets acquired, in accordance with the purchase method of accounting.
Amounts attributable to patents and patent rights acquired are amortized using the straight-line method over the estimated economic useful lives of the underlying patents which range from two to seven years. As of the date of acquisition, the estimated weighted-average useful life of amortizable patent related intangibles acquired is approximately 6 years.
In connection with the acquisition described above, Acacia Global Acquisition Corporation entered into a consulting agreement with the former CEO of Global Patent Holdings, LLC who, as a result of the acquisition transaction, became a shareholder of Acacia Research Corporation. The agreement requires the payment of $2,000,000 in consulting fees over a two-year period, and certain reimbursable consulting related expenses, commencing on the date of acquisition. Marketing, general and administrative expenses for the year ended December 31, 2006 and 2005 include $1,087,000 and $1,009,000, respectively in expenses related to the consulting agreement. Consulting services performed consist primarily of consultation on intellectual property matters associated with the patents and patent rights acquired in the transaction. The consulting fees are being expensed in the consolidated statement of operations and comprehensive loss as the consulting services are rendered during the two-year term of the consulting agreement. Acacia Global Acquisition Corporation may terminate the consulting agreement for cause as provided for in the agreement. The consulting agreement also contains certain automatic termination provisions, including; the failure by Acacia Global Acquisition Corporation to make timely consulting payments in accordance with the agreement; a significant decrease in working capital of Acacia Research Corporation, as defined in the agreement; material breach of the agreement by Acacia Global Acquisition Corporation; and the death of the consultant. Any occurrence of these conditions may require the payment of all remaining consulting fees outstanding under the agreement within thirty days of the occurrence of the termination event. Acacia Research Corporation also executed an agreement guaranteeing Acacia Global Acquisition Corporation’s performance of its obligations under the consulting agreement. The consulting agreement expired in January 2007.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The acquisition was treated for tax purposes as a taxable asset acquisition and, as such, there were no book/tax basis differences associated with the acquisition. As such, Acacia Research Corporation did not record any deferred income taxes in connection with the application of the purchase method of accounting.
The following unaudited pro forma combined results of operations for the periods presented are provided for illustrative purposes only and assume the acquisition occurred as of January 1, 2005, and 2004. The unaudited pro forma combined financial results do not purport to be indicative of the results of operations for future periods or the results that actually would have been realized had the entities been a single entity during these periods. The unaudited pro forma combined results are presented in thousands, except share and per share information.
| | | For the Year Ended | |
| | | December 31, 2005 (1) | | December 31, 2004 | |
| | | | | | |
| Total revenues | | $ | 27,607 | | $ | 40,693 | |
| Total operating expenses | | | 49,233 | | | 49,143 | |
| Operating income (loss) | | | (21,626 | ) | | (8,450 | ) |
| Total other income | | | 2,406 | | | 1,099 | |
| Income (loss) from continuing operations before income taxes | | | (19,220 | ) | | (7,351 | ) |
| Benefit for income taxes and minority interests | | | 304 | | | 261 | |
| Estimated loss on discontinued operations | | | (237 | ) | | (104 | ) |
| Net income (loss) | | $ | (19,153 | ) | $ | (7,194 | ) |
| | | | | | | | |
| Pro forma earnings (loss) per common share: | | | | | | | |
| Attributable to the Acacia Technologies group: | | | | | | | |
| Net loss | | $ | (6,752 | ) | $ | (7,904 | ) |
| Basic and diluted loss per share | | | (0.25 | ) | | (0.33 | ) |
Weighted average shares (2): | | | | | | | |
| Acacia Research - Acacia Technologies stock: | | | | | | | |
| Basic and diluted | | | 26,922,097 | | | 23,723,715 | |
| | | | | | | | |
| (1) | Results of operations for the LLCs acquired in the GPH Acquisition were not material for the period January 1, 2005, through January 28, 2005. Pro forma adjustments reflect the impact of the acquisition for the 28-day period from January 1, 2005 to January 28, 2005. |
| (2) | There is no pro forma impact on earnings (loss) per share attributable to the CombiMatrix group for any periods. |
9. STOCKHOLDERS’ EQUITY
Redeemable Capital Stock
The authorized capital stock of Acacia Research Corporation consists of 210,000,000 shares, of which 100,000,000 shares is a class of common stock designated as “AR-CombiMatrix stock,” having a par value of $0.001 per share, 100,000,000 shares is a class of common stock designated as “AR-Acacia Technologies stock,” having a par value of $0.001 per share, and 10,000,000 is a class of preferred stock having a par value of $0.001 per share (the “Preferred Stock”) and issuable in one or more series as determined by the board of directors pursuant to Acacia Research Corporation’s restated certificate of incorporation. Holders of AR-CombiMatrix stock and AR-Acacia Technologies stock vote together as a single class (except in certain limited circumstances). Each share of AR-CombiMatrix stock entitles the holder to one vote. Each share of AR-Acacia Technologies stock entitles the holder, for any particular vote, to a number of votes equal to the average market value of a share of AR-Acacia Technologies stock divided by the average market value of a share of AR-CombiMatrix stock over a specified 20-trading day period ending on the tenth trading day prior to the record date for determining the stockholders entitled to vote.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Holders of each class of common stock are entitled to receive ratably such dividends, if any, as may be declared by the board of directors out of funds legally available therefore.
Under our restated certificate of incorporation, in the event of our dissolution, liquidation or winding up, after payment or provision for payment of the debts and other liabilities and full preferential amounts to which holders of any preferred stock are entitled, regardless of the group to which such shares of preferred stock were attributed, the holders of AR-CombiMatrix stock and AR-Acacia Technologies stock will be entitled to receive our assets remaining for distribution to holders of common stock on a per share basis in proportion to the liquidation units per share of such class. Each share of AR-CombiMatrix stock will have one liquidation unit. Each share of AR-Acacia Technologies stock will have a number of liquidation units equal to the quotient of the average market value of a share of AR-Acacia Technologies stock over the 20-trading day period ending on the 40th trading day after the effective date of the recapitalization, divided by the average market value of a share of AR-CombiMatrix stock over the same period.
Holders of each class of common stock have no preemptive, subscription, redemption or conversion rights. Management, at its discretion may, at any time, convert each share of AR-CombiMatrix stock into a number of shares of AR-Acacia Technologies stock at a 10% premium over the average market price.
Each class of stock is designed to reflect the financial performance of the respective group, rather than the performance of Acacia Research Corporation as a whole. The chief mechanisms intended to cause the AR-CombiMatrix stock and the AR-Acacia Technologies stock to reflect the financial performance of the respective group are provisions in Acacia Research Corporation’s restated certificate of incorporation governing dividends and distributions. Under these provisions, Acacia Research Corporation will:
| | · | factor the assets and liabilities and income or losses attributable to the respective group into the determination of the amount available to pay dividends on the shares issued for the respective group; and |
| | · | require Acacia Research Corporation to exchange, redeem or distribute a dividend on the stock of a group if all or substantially all of the assets allocated to the respective group are sold to a third party. |
Management of Acacia Research Corporation cannot assure the holders of AR-CombiMatrix stock or AR-Acacia Technologies stock that the market values of the two share classes will in fact reflect the separate performance of each class of stock. Holders of AR-CombiMatrix stock and AR-Acacia Technologies stock are stockholders of Acacia Research Corporation and as a result, are subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. Financial effects from one group that affect Acacia Research Corporation’s consolidated results of operations or financial condition could, if significant, affect the results of operations or financial condition of the other group.
Acacia Research Corporation’s board of directors, subject to state laws and limits in our restated certificate of incorporation, including those discussed above, will be able to declare dividends on AR-CombiMatrix stock and AR-Acacia Technologies stock in its discretion. To date, Acacia Research Corporation has never paid or declared cash dividends on shares of our stock, nor do we anticipate paying cash dividends on either of the two classes of stock in the foreseeable future.
The allocation of corporate expenses is generally based on utilization and is in accordance with Acacia Research Corporation’s restated certificate of incorporation, for the purpose of measuring earnings available to stockholders of AR-CombiMatrix stock and AR-Acacia Technologies stock and does not necessarily reflect the financial condition, cash flows and operating results of each division as if it were a stand-alone entity. The management and allocation policies applicable to the determination of the assets and liabilities and income or losses attributable to the respective group may be modified or rescinded, or additional policies may be adopted, at the sole discretion of Acacia Research Corporation’s board of directors at any time without approval of the stockholders. Acacia Research Corporation’s management and board of directors have the ability to: transfer funds between the groups at the discretion of management and the board of directors; allocate financing costs between groups that may not reflect the separate borrowing costs of the groups; and charge a greater or lesser portion of the total corporate tax liability to the groups than that which would have been charged if the groups were stand-alone entities. Acacia Research Corporation’s management and board of directors do not presently intend to modify or rescind the methodologies and assumptions underlying the allocations in the pro forma financial statements. Refer to Note 2 for a description of applicable management allocation policies.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Other
On December 13, 2006, Acacia Research Corporation completed a registered direct offering with Oppenheimer & Co., Inc. (“Oppenheimer”) as the placement agent, raising gross proceeds of $9,964,000 through the issuance of 9,768,313 units. Each unit consists of one share of AR-CombiMatrix common stock and 1.2 five-year common stock warrants, for a total of 9,768,313 shares and warrants to purchase 11,721,975 shares of AR-CombiMatrix common stock, respectively, issued to investors. Each warrant entitles the holder to purchase a share of AR-CombiMatrix stock at a price of $0.87 per share. Acacia Research Corporation issued additional warrants to purchase 488,416 shares of AR-CombiMatrix stock with an exercise price of $1.09 per share to Oppenheimer. Net proceeds raised from the private equity financing of $9,266,000 were attributed to the CombiMatrix group.
On June 14, 2006, Acacia Research Corporation entered into a standby equity distribution agreement (the “SEDA”) with Cornell Capital Partners, LP (“Cornell”). Under the terms of the SEDA, Acacia Research Corporation was able to require Cornell to purchase up to $50.0 million of AR-CombiMatrix common stock, or up to 13,024,924 shares, over a two-year period following the effective date of the SEDA. Such shares were in the form of registered securities drawn from Acacia Research Corporation’s current shelf registration statement. Acacia Research Corporation could request advances under the SEDA in up to $5.0 million increments. At the closing of each advance, Acacia Research Corporation issued to Cornell a number of shares of AR-CombiMatrix common stock equal to the amount of the advance divided by the lowest daily volume weighted-average price (“VWAP”) of AR-CombiMatrix common stock during the five trading days following the advance notice to Cornell, which would purchase the shares at 97.5% of the VWAP. At each closing, Acacia Research Corporation paid an underwriting fee of 4% of the gross amount of each advance on the first $20.0 million and 5% of the gross proceeds of each advance on the remaining $30.0 million of the SEDA to Cornell. A total of 13,024,924 shares of AR-CombiMatrix common stock were authorized to be issued under the SEDA.
Upon closing of the SEDA, the CombiMatrix group paid Cornell a one-time commitment fee of $550,000 and an additional $20,000 in due diligence and other closing-related costs. The $550,000 fee was recorded as a long-term asset and was amortized against future advances as costs of equity issuances. In June 2006, Cornell purchased 343,750 shares of AR-CombiMatrix common stock at $1.60 per share (which was not an advance under the SEDA), based on the fair value of AR-CombiMatrix stock on June 12, 2006. Since executing the SEDA, through December 20, 2006, Acacia Research Corporation has requested five advances from Cornell to purchase a total of 3,211,345 shares of AR-CombiMatrix stock at prices ranging from $1.16 to $0.73 per share, resulting in net proceeds of $3,070,000, which were contributed to the CombiMatrix group. On December 20, 2006, a notice to cancel the SEDA was sent by Acacia Research Corporation to Cornell. The unamortized SEDA costs of $444,000 were charged against the net proceeds of the Oppenheimer financing.
In September 2005, Acacia Research Corporation raised gross proceeds of $10,537,000 through the sale of 6,385,907 shares of AR-CombiMatrix stock and common stock purchase warrants to purchase 1,596,478 shares of AR-CombiMatrix stock, at a price of $1.65 per unit, in a registered direct offering. Each unit consisted of one share of AR-CombiMatrix stock and one-quarter of a five-year AR-CombiMatrix stock purchase warrant. Each full AR-CombiMatrix stock purchase warrant entitles the holder to purchase a share of AR-CombiMatrix stock at a price of $2.40 per share and is exercisable immediately upon issue. Net proceeds raised of approximately $9,609,000, which are net of related issuance costs, were attributed to the CombiMatrix group. Refer to Note 10 regarding classification of the warrants in the accompanying consolidated balance sheet.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In July 2005, Acacia Research Corporation raised gross proceeds of $3,151,000 through the sale of 1,400,444 shares of AR-CombiMatrix stock at a price of $2.25 per share in a registered direct offering. Net proceeds raised of approximately $3,114,000, which are net of related issuance costs, were attributed to the CombiMatrix group.
In February 2005, Acacia Research Corporation raised gross proceeds of $19,600,000 through the sale of 3,500,000 shares of AR-Acacia Technologies stock at a price of $5.60 per share in a registered direct offering. Net proceeds raised of approximately $19,532,000, which are net of related issuance costs, were attributed to the Acacia Technologies group.
In April 2004, Acacia Research Corporation raised gross proceeds of $15,000,000 through the sale of 3,000,000 shares of Acacia Research - CombiMatrix common stock at a price of $5.00 per share in a registered direct offering. Net proceeds raised of approximately $13,715,000, which are net of related issuance costs, were attributed to the CombiMatrix group.
During 2004, proceeds of $2,093,000 were received from the issuance of 761,205 shares of AR-CombiMatrix stock related to the exercise of certain warrants issued in connection with the CombiMatrix group’s May 2003 private equity financing. The proceeds from the warrants exercised were attributed to the CombiMatrix group.
10. COMMON STOCK PURCHASE WARRANT LIABILITY
At December 31, 2006 there were common stock purchase warrants outstanding, issued in connection with the December 2006 equity financing, discussed elsewhere herein, representing rights to purchase 11,722,000 shares of AR-CombiMatrix common stock at a per share exercise price of $0.87 and 488,000 shares of AR-CombiMatrix common stock at a per share price of $1.09, which are exercisable through December 2011.
At December 31, 2006 and 2005, there were common stock purchase warrants outstanding, issued in connection with the September 2005 equity financing, discussed elsewhere herein, representing rights to purchase 1,596,000 shares of AR-CombiMatrix common stock at a per share exercise price of $2.40, which are exercisable through September 2010.
At December 31, 2006 and 2005, there were common stock purchase warrants outstanding, issued in connection with the CombiMatrix group’s May 2003 equity financing, representing rights to purchase 283,000 shares of AR-CombiMatrix common stock at a per share exercise price of $2.75, which are exercisable through May 2008.
Acacia Research Corporation’s classes of common stock are subject to certain redemption provisions in the event that Acacia Research Corporation sells, transfers, assigns or otherwise disposes of, in one transaction or a series of related transactions, all or substantially all of the properties and assets attributed to either group.
Acacia Research Corporation adopted FASB Staff Position No. 150-5 (“FSP No. 150-5”), effective July 1, 2005, which requires that warrants for shares that are redeemable be classified as liabilities, based on the fair values of the warrants, which are required to be marked to market at each balance sheet date. The fair value of contingently redeemable AR-CombiMatrix stock purchase warrants outstanding at December 31, 2006 and December 31, 2005 was $6,732,000 and $1,381,000, respectively. Net warrant gains for the year ended December 31, 2006 and December 31, 2005, reflected in other income (expense), related to changes in the fair value of the warrant liability totaled $1,754,000 and $812,000, respectively.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The fair value of AR-CombiMatrix stock purchase warrants at December 31, 2006 and 2005 was determined using the Black-Scholes option-pricing model, using weighted-average assumptions as follows:
| | | December 31, | |
AR-CombiMatrix Warrants: | | 2006 | | 2005 | |
| | | | | | |
| Risk free interest rate | | | 4.71% | | | 4.35% | |
| Volatility | | | 80% | | | 84% | |
| Expected term | | | 4.7 years | | | 4.4 years | |
11. INCOME TAXES
The benefit for income taxes consists of the following (in thousands):
| | 2006 | | 2005 | | 2004 | |
Current: | | | | | | | |
| U.S. Federal tax | | $ | - | | $ | - | | $ | - | |
| State taxes | | | 76 | | | (23 | ) | | 4 | |
| | | | 76 | | | (23 | ) | | 4 | |
| Deferred: | | | | | | | | | | |
| U.S. Federal tax | | | (70 | ) | | (279 | ) | | (279 | ) |
| State taxes | | | - | | | - | | | - | |
| | | | (70 | ) | | (279 | ) | | (279 | ) |
| | | $ | 6 | | $ | (302 | ) | $ | (275 | ) |
The tax effects of temporary differences and carryforwards that give rise to significant portions of deferred assets and liabilities consist of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | | 2004 | |
| Deferred tax assets: | | | | | | | |
| Basis of investments in affiliates | | $ | 28,808 | | $ | 28,808 | | $ | 28,808 | |
| Depreciation and amortization | | | 2,449 | | | 915 | | | - | |
| Deferred revenue | | | 589 | | | 743 | | | 1,000 | |
| Stock compensation | | | 9,328 | | | 8,319 | | | 8,231 | |
| Accrued liabilities and other | | | 380 | | | 767 | | | 1,022 | |
| Write-off of investments | | | 1,842 | | | 1,842 | | | 1,842 | |
| Net operating loss and capital loss carryforwards and credits | | | 65,490 | | | 59,753 | | | 54,278 | |
| Total deferred tax assets | | | 108,886 | | | 101,147 | | | 95,181 | |
Less: valuation allowance | | | (106,062 | ) | | (100,501 | ) | | (94,118 | ) |
| Deferred tax assets, net of valuation allowance | | | 2,824 | | | 646 | | | 1,063 | |
| Deferred tax liabilities: | | | | | | | | | | |
| Depreciation and amortization | | | - | | | - | | | (197 | ) |
| Intangibles | | | (2,824 | ) | | (3,347 | ) | | (3,847 | ) |
| | | | | | | | | | | |
| Deferred tax liabilities | | | - | | | (3,347 | ) | | (4,044 | ) |
| | | | | | | | | | | |
| Net deferred tax liabilities | | $ | - | | $ | (2,701 | ) | $ | (2,981 | ) |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of March 31, 2006, the Acacia Technologies group reduced its patents and deferred tax liability by $691,000, which were initially recorded in fiscal 2002, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability. As of March 31, 2006, the CombiMatrix group reduced its goodwill and deferred tax liability balances by $1,941,000, which were initially recorded in fiscal 2000, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability.
A reconciliation of the federal statutory income tax rate and the effective income tax rate is as follows:
| | | 2006 | | 2005 | | 2004 | |
| Statutory federal tax rate | | | (34 | %) | | (34 | %) | | (34 | %) |
Stock-based compensation | | | 3 | % | | - | | | - | |
Tax exempt interest | | | - | | | - | | | (1 | %) |
Goodwill impairment | | | - | | | 1 | % | | - | |
Non deductible permanent items | | | (2 | %) | | (2 | %) | | (1 | %) |
Intangibles | | | - | | | - | | | - | |
Tax credits and other | | | (2 | %) | | 2 | % | | (9 | %) |
Other | | | 2 | % | | - | | | - | |
Valuation allowance | | | 33 | % | | 31 | % | | 40 | % |
| | | | (0 | %) | | (2 | %) | | (5 | %) |
At December 31, 2006, Acacia Research Corporation has consolidated net deferred tax assets totaling approximately $106,062,000, which are fully offset by a valuation allowance due to management’s determination that the criteria for recognition have not been met.
At December 31, 2006, consolidated U.S. Federal and state income tax net operating loss carry forwards (“NOLs”), excluding NOLs related to subsidiaries for which we do not file a consolidated tax return, were approximately $170,783,000 and $54,700,000, respectively, expiring between 2010 and 2026, and 2007 and 2016, respectively. In addition, we had consolidated tax credit carryforwards of approximately $4,014,000. The amount of the CombiMatrix Corporation NOLs and tax credits acquired in 2002, totaling approximately $65,064,000 (expiring between 2010 and 2022) and $1,981,000, respectively, that can be utilized annually to offset future taxable income or tax liability has been limited under the Internal Revenue Code due to the ownership change resulting from our December 2002 increase in ownership interest in CombiMatrix Corporation to 100%.
As of December 31, 2006, the aggregate tax NOLs at other subsidiaries for which we do not file a consolidated tax return are approximately $21,164,000 for federal income tax purposes, expiring between 2010 and 2025. However, the use of these NOLs is limited to the separate earnings of the respective subsidiaries. In addition, ownership changes may also restrict the use of NOLs and tax credits.
Had the Acacia Technologies group and the CombiMatrix group each filed separate tax returns, the provision (benefit) for income taxes and division net income (loss) would not have differed from the amounts reported in Acacia Research Corporation’s statement of operations and comprehensive loss for the periods presented.
As of December 31, 2005, approximately $9,081,000 of the valuation allowance related to the tax benefits of stock option deductions included in Acacia Research Corporation’s NOLs. At such time as the valuation allowance is released, the benefit will be credited to additional paid-in capital.
12. DISCONTINUED OPERATIONS
In 2005 and 2004, the Acacia Technologies group accrued an additional $237,000 and $104,000 (net of minority interests), respectively, in estimated costs to be incurred in connection with the discontinued operations of Soundbreak.com (originally ceased operations in February 2001). The additional accruals relate primarily to certain noncancellable lease obligations, the inability to sublease the related office space at rates commensurate with our existing obligations and certain lease termination costs. The related lease obligations, which were guaranteed by Acacia Research Corporation, expired in August 2005.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The assets and liabilities of the discontinued operations at December 31, 2006 and 2005 consist primarily of $38,000 and $741,000 of cash and cash equivalents and lease deposits and $44,000 and $144,000 of accounts payable and accrued expenses, respectively.
13. STOCK-BASED INCENTIVE PLANS
The 2002 Acacia Technologies Stock Incentive Plan (the “AR-Acacia Technologies Group Plan”) and the 2002 CombiMatrix Stock Incentive Plan (the “AR-CombiMatrix Group Plan”) were approved by the stockholders of Acacia Research Corporation in December 2002. The AR-Acacia Technologies Group Plan authorizes grants of stock options, stock awards and performance shares with respect to AR-Acacia Technologies stock. The AR-CombiMatrix Group Plan authorizes grants of stock options, stock awards and performance shares with respect to AR-CombiMatrix stock. Directors and certain officers and key employees with responsibilities involving both the Acacia Technologies group and the CombiMatrix group may be granted awards under both incentive plans in a manner which reflects their responsibilities. The board of directors believes that granting participants awards tied to performance of the group in which the participants work and, in certain cases the other group, is in the best interest of the Acacia Research Corporation and its stockholders. The terms of the AR-Acacia Technologies Group Plan and the AR-CombiMatrix Group Plan are identical except that AR-Acacia Technologies stock may be issued only under the AR-Acacia Technologies Group Plan and AR-CombiMatrix stock may be issued only under the AR-CombiMatrix Group Plan.
Acacia Research Corporation’s compensation committee administers the discretionary option grant and stock issuance programs. This committee determines which eligible individuals are to receive option grants or stock issuances under those programs, the time or times when the grants or issuances are to be made, the number of shares subject to each grant or issuance, the status of any granted option as either an incentive stock option or a non-statutory stock option under the federal tax laws, the vesting schedule to be in effect for the option grant or stock issuance and the maximum term for which any granted option is to remain outstanding. The exercise price of options is generally equal to the fair market value of the AR-CombiMatrix stock or AR-Acacia Technologies stock on the date of grant. Options generally begin to be exercisable six months to one year after grant and generally expire ten years after grant. Stock options generally vest over three to four years and restricted shares generally vest in full after two years (represents the requisite service period under SFAS No. 123R).
Programs
Each of the incentive plans has four separate programs:
| | · | Discretionary Option Grant Program. Under the discretionary option grant program, our compensation committee may grant (1) non-statutory options to purchase shares of AR-Acacia Technologies stock and AR-CombiMatrix stock, as applicable, to eligible individuals in the employ or service of Acacia Research Corporation or our subsidiaries (including employees, non-employee board members and consultants) at an exercise price not less than 85% of the fair market value of those shares on the grant date and (2) incentive stock options to purchase shares of AR-Acacia Technologies stock and AR-CombiMatrix stock, as applicable, to eligible employees at an exercise price not less than 100% of the fair market value of those shares on the grant date (not less than 110% of fair market value if such employee actually or constructively owns more than 10% of our voting stock or the voting stock of any of our subsidiaries). |
| | · | Stock Issuance Program. Under the stock issuance program, eligible individuals may be issued shares of AR-Acacia Technologies stock and AR-CombiMatrix stock, as applicable, directly, upon the attainment of performance milestones or the completion of a specified period of service or as a bonus for past services. Under this program, the purchase price for the shares shall not be less than 100% of the fair market value of the shares on the date of issuance, and payment may be in the form of cash or past services rendered. |
| | · | Automatic Option Grant Program. Under the automatic option grant program, option grants will automatically be made at periodic intervals to eligible non-employee members of our board of directors to purchase shares of AR-Acacia Technologies stock and AR-CombiMatrix stock, as applicable, at an exercise price equal to 100% of the fair market value of those shares on the grant date. Each individual who first becomes a non-employee board member at any time after the date of the adoption of the incentive plans by our board of directors will automatically receive an option to purchase 20,000 shares of AR-Acacia Technologies stock and 20,000 shares of AR-CombiMatrix stock on the date the individual joins the board of directors. In addition, on the first business day in each calendar year following the adoption of the incentive plans by our board of directors, each non-employee board member then in office, including each of our current non-employee board members who is then in office, will automatically be granted an option to purchase 15,000 shares of AR-Acacia Technologies stock and 15,000 shares of AR-CombiMatrix stock, provided that the individual has served on the board of directors for at least six months. |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | · | Director Fee Option Grant Program. If this program is put into effect in the future, it will allow non-employee members of our board of directors the opportunity to apply a portion of any retainer fee otherwise payable to them in cash each year to the acquisition of special below-market option grants. |
The authorized number of shares of common stock subject to the AR-Acacia Technologies Group Plan is 7,208,000 shares. The authorized number of shares of common stock subject to the AR-CombiMatrix Group Plan is 10,910,000 shares. The number of shares of common stock available for issuance under the AR-Acacia Technologies Group Plan and the AR-CombiMatrix Group Plan automatically increases on the first trading day of January each calendar year during the term of the Plan by an amount equal to three percent (3%) of the total number of shares of common stock outstanding on the last trading day in December of the immediately preceding calendar year, but in no event shall any such annual increase exceed 500,000 shares for the AR-Acacia Technologies Group Plan and 600,000 shares for the AR-CombiMatrix Group Plan. The aggregate number of shares of common stock available for issuance under either Plan shall not exceed 20,000,000 shares. At December 31, 2006, shares available for grant are 13,000 and 1,528,000 under the AR-Acacia Technologies Group Plan and the AR-CombiMatrix Group Plan, respectively. The AR-Acacia Technologies Group Plan and the AR-CombiMatrix Group Plan do not segregate the number of securities remaining available for future issuance among stock options and other awards. The shares authorized for future issuance represents the total number of shares available through any combination of stock options or other awards. Upon the exercise of stock options or the granting of restricted stock, it is Acacia Research Corporation’s policy to issue new shares of the respective class of common stock.
Our board of directors may amend or modify the incentive plans at any time, subject to any required stockholder approval. The incentive plans will terminate no later than the tenth anniversary of the approval of the incentive plans by our stockholders. In December 2006, Acacia Research Corporation’s board of directors approved an amendment to the 2002 CombiMatrix Stock Incentive Plan and the CombiMatrix 2000 Stock Awards Plan (the “Plans”) to include the planned split-off of CombiMatrix Corporation, as discussed at Note 1, as a change in control under the terms of the Plans.
The following summarizes stock-based compensation activities under our plans:
AR-Acacia Technologies Stock: | | Options | | Weighted Average Exercise Price | | Weighted-Average Remaining Contractual Term | | Aggregate Intrinsic Value | |
| | | | | | | | | | |
| Balance at December 31, 2003 | | | 5,139,000 | | $ | 8.29 | | | | | | | |
| Granted | | | 913,000 | | $ | 4.63 | | | | | | | |
| Exercised | | | (155,000 | ) | $ | 4.04 | | | | | | | |
| Forfeited | | | (130,000 | ) | $ | 5.55 | | | | | | | |
| Expired | | | (41,000 | ) | $ | 18.86 | | | | | | | |
| Balance at December 31, 2004 | | | 5,726,000 | | $ | 7.81 | | | | | | | |
| Granted | | | 603,000 | | $ | 5.84 | | | | | | | |
| Exercised | | | (134,000 | ) | $ | 2.27 | | | | | | | |
| Forfeited | | | (55,000 | ) | $ | 3.13 | | | | | | | |
| Expired | | | (163,000 | ) | $ | 12.80 | | | | | | | |
Outstanding at December 31, 2005 | | | 5,977,000 | | $ | 7.64 | | | | | | | |
Granted | | | 465,000 | | $ | 7.75 | | | | | | | |
Exercised | | | (390,000 | ) | $ | 3.78 | | | | | | | |
| Forfeited | | | (94,000 | ) | $ | 6.12 | | | | | | | |
Outstanding at December 31, 2006 | | | 5,958,000 | | $ | 7.93 | | | 5.7 years | | $ | 39,408,000 | |
| Vested and Expected to vest at December 31, 2006 | | | 5,932,000 | | $ | 7.94 | | | 5.3 years | | $ | 39,198,000 | |
| Exercisable at December 31, 2006 | | | 4,952,000 | | $ | 8.40 | | | 5.2 years | | $ | 31,588,000 | |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The weighted-average grant date fair value of stock options granted during the years ended December 31, 2006, 2005 and 2004 was $5.35, $4.17 and $3.49, respectively. The total intrinsic value of options exercised during the years ended December 31, 2006, 2005 and 2004 was $3,463,000, $464,000 and $327,000, respectively. The fair value of options vested during the years ended December 31, 2006, 2005 and 2004 was $3,960,000, $1,455,000, and $2,616,000, respectively. As of December 31, 2006, the total unrecognized compensation expense related to nonvested stock option awards was $2,740,000, which is expected to be recognized over a weighted-average term of approximately 1.7 years.
A summary of the status of AR-Acacia Technologies nonvested restricted shares as of December 31, 2006, and changes during the year ended December 31, 2006, is as follows:
AR-Acacia Technologies Stock: | | Nonvested Restricted Shares | | Weighted Average Grant Date Fair Value | |
| | | | | | |
| Nonvested restricted stock at January 1, 2005 | | - | | | |
| Granted | | | 338,000 | | $ | 5.07 | |
| Vested | | | - | | | | |
| Forfeited | | | - | | | | |
| Nonvested restricted stock at December 31, 2005 | | | 338,000 | | $ | 5.07 | |
| Granted | | | 143,000 | | $ | 11.87 | |
| Vested | | | (30,000 | ) | $ | 7.16 | |
| Forfeited | | | (23,000 | ) | $ | 6.75 | |
| Nonvested restricted stock at December 31, 2006 | | | 428,000 | | $ | 7.10 | |
As of December 31, 2006, the total unrecognized compensation expense related to nonvested restricted stock awards was $1,640,000, which is expected to be recognized over a weighted-average period of approximately 8 months. The total fair value of shares vested during the year ended December 31, 2006 was $215,000. There are no restricted share grants outstanding under the AR-CombiMatrix Group Plan.
AR-CombiMatrix Stock: | | Options | | Weighted Average Exercise Price | | Weighted-Average Remaining Contractual Term | | Aggregate Intrinsic Value | |
| | | | | | | | | | |
| Balance at December 31, 2003 | | | 6,617,000 | | $ | 7.28 | | | | | | | |
| Granted | | | 1,173,000 | | $ | 5.79 | | | | | | | |
| Exercised | | | (1,023,000 | ) | $ | 3.19 | | | | | | | |
| Forfeited | | | (316,000 | ) | $ | 5.93 | | | | | | | |
| Expired | | | (219,000 | ) | $ | 15.64 | | | | | | | |
| Balance at December 31, 2004 | | | 6,232,000 | | $ | 7.44 | | | | | | | |
| Granted | | | 1,010,000 | | $ | 2.95 | | | | | | | |
| Exercised | | | (6,000 | ) | $ | 1.95 | | | | | | | |
| Forfeited | | | (101,000 | ) | $ | 3.66 | | | | | | | |
| Expired | | | (210,000 | ) | $ | 8.43 | | | | | | | |
| Outstanding at December 31, 2005 | | | 6,925,000 | | $ | 6.82 | | | | | | | |
| Granted | | | 1,839,000 | | $ | 1.41 | | | | | | | |
| Forfeited | | | (305,000 | ) | $ | 2.36 | | | | | | | |
| Expired | | | (391,000 | ) | $ | 6.53 | | | | | | | |
| Outstanding at December 31, 2006 | | | 8,068,000 | | $ | 5.77 | | | 6.1 years | | | - | |
| Vested and Expected to Vest at December 31, 2006 | | | 7,956,000 | | $ | 5.82 | | | 5 years | | | - | |
| Exercisable at December 31, 2006 | | | 6,044,000 | | $ | 7.09 | | | 5 years | | | - | |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The weighted-average grant date fair value of stock options granted during the years ended December 31, 2006, 2005 and 2004 was $1.03, $2.08 and $4.44, respectively. The total intrinsic value of options exercised during the years ended December 31, 2005 and 2004 was $4,000 and $2,439,000, respectively. The fair value of options vested during the years ended December 31, 2006, 2005 and 2004 was $2,561,000, $3,267,000 and $8,055,000, respectively. As of December 31, 2006, the total unrecognized compensation expense related to nonvested stock option awards was $2,141,000, which is expected to be recognized over a weighted-average term of approximately 9 months.
At December 31, 2006, Acacia Research Corporation and its separate operating groups continue to record a full valuation allowance against net deferred tax assets due to management’s determination that the criteria for recognition have not been met. As such, the implementation and subsequent accounting for stock based awards under SFAS No. 123R did not have an impact on Acacia Research Corporation’s or the separate group’s deferred taxes or related tax provisions for the periods presented.
CombiMatrix Molecular Diagnostics 2005 Stock Award Plan
CombiMatrix Corporation’s wholly owned subsidiary, CMDX, executed the CombiMatrix Molecular Diagnostics 2005 Stock Award Plan (the "CMDX Plan") with plan provisions and terms similar to that of the AR-CombiMatrix Group Plan, as described above. The authorized number of shares of common stock subject to the CMDX Plan is 4,000,000 shares. At December 31, 2006, shares available for grant under the CMDX Plan were 2,193,000. A summary of option activity under CMDX Plan for the year ended December 31, 2006 is as follows:
CMDX Stock: | | Options | | Weighted Average Exercise Price | | Weighted-Average Remaining Contractual Term | | Aggregate Intrinsic Value | |
| | | | | | | | | | |
| Outstanding at January 1, 2005 | | - | | | | | | | |
| Granted | | | 1,692,000 | | $ | 0.10 | | | | | | | |
| Exercised | | | - | | | | | | | | | | |
| Forfeited | | | - | | | | | | | | | | |
| Expired | | | - | | | | | | | | | | |
| Outstanding at December 31, 2005 | | | 1,692,000 | | $ | 0.10 | | | | | | | |
| Granted | | | 943,000 | | $ | 0.50 | | | | | | | |
| Exercised | | | - | | | - | | | | | | | |
| Forfeited | | | (752,000 | ) | $ | 0.10 | | | | | | | |
| Expired | | | (76,000 | ) | $ | 0.10 | | | | | | | |
| Outstanding at December 31, 2006 | | | 1,807,000 | | $ | 0.31 | | | 9 years | | $ | 357,000 | |
| Vested and Expected to Vest at December 31, 2006 | | | 1,602,000 | | $ | 0.31 | | | 8.9 years | | $ | 319,000 | |
| Exercisable at December 31, 2006 | | | 523,000 | | $ | 0.26 | | | 8.9 years | | $ | 129,000 | |
The weighted-average grant date fair value of stock options granted during the years ended December 31, 2006 and 2005 was $0.37 and $.07, respectively. The fair value of options vested during the year ended December 31, 2006 was $99,000. The fair value of options vested during the year ended December 31, 2005 was immaterial. As of December 31, 2006, the total unrecognized compensation expense related to nonvested stock option awards was $233,000, which is expected to be recognized over a weighted-average term of approximately 2.5 years. Total stock compensation expense recognized for the year ended December 31, 2006 was not material.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Other
Stock option expense reflected in the consolidated statement of operations related to stock options issued to the CombiMatrix group’s scientific advisory board members is accounted for under the fair value method required by EITF 96-18: “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services” and related interpretations. The fair value of options granted to scientific advisory board members was determined using the Black-Scholes option-pricing model with weighted-average assumptions consistent with those disclosed at Note 2 under “Stock-Based Compensation.” At December 31, 2006, included in the AR-CombiMatrix stock table above, are 143,000 options outstanding held by non-employees (with a weighed average exercise price of $4.08), of which 125,000 options were vested and exercisable (with a weighted-average exercise price of $4.13). Stock compensation charges (credits) related to non-employee stock awards was not material for the periods presented.
In the third quarter of 2006, the CombiMatrix group issued 50,000 shares of fully vested restricted stock to a third-party consultant in consideration for consulting services performed, resulting in a stock compensation charge of $94,000.
14. COMMITMENTS AND CONTINGENCIES
Operating Leases
We lease certain office furniture and equipment and laboratory and office space under various operating lease agreements expiring over the next 6 years. Minimum annual rental commitments on operating leases having initial or remaining non-cancelable lease terms in excess of one year are as follows (in thousands):
Year | | | |
| 2007 | | $ | 1,205 | |
| 2008 | | | 1,084 | |
| 2009 | | | 1,121 | |
| 2010 | | | 1,086 | |
| 2011 | | | 783 | |
| Thereafter | | | 131 | |
| Total minimum lease payments | | $ | 5,410 | |
Rent expense in 2006, 2005 and 2004 was approximately $2,443,000, $2,408,000 and $2,241,000, respectively. Under the terms of the CombiMatrix group’s lease arrangements, a security deposit in the form of a $1,500,000 letter of credit was issued to the landlord. On February 1, 2007, the CombiMatrix group executed an amendment to its operating lease for office and laboratory space, reducing the space leased to 30,724 square feet from 90,111 square feet, reducing its future annual lease commitment by approximately 80%, and reducing its letter of credit to $1,000,000, which will be reduced by $40,000 per month to a floor amount of $300,000 by October 2008. In addition, the lease amendment extends the CombiMatrix group’s lease term to October 2010, however, under the terms of the lease amendment, the CombiMatrix group is able to terminate the lease as of the original termination date of October 31, 2008, if notice is provided to the landlord by July 31, 2008.
Collaborative and Research Agreements
On February 8, 2006, the CombiMatrix group executed a one-year, $2.1 million contract with the DoD to further the development of the CombiMatrix group's array technology for the electrochemical detection of biological and chemical threat agents. Under the terms of this contract, the CombiMatrix group will perform research and development activities, as described under the contract, and will be reimbursed on a periodic basis for actual costs incurred to perform its obligations, plus a fixed fee, of up to $2.1 million. As of December 31, 2006, the CombiMatrix group had incurred $1.3 million in actual contract costs for the electrochemical detection contract. In March 2004, the CombiMatrix group was awarded a two-year, $5.9 million contract with the DoD to further the development of the CombiMatrix group’s array technology for the detection of biological and chemical threat agents. This contract was completed in December 2005.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On August 9, 2006, the CombiMatrix group executed a two-year, $1.9 million contract with the DoD, focusing on the integration of its electrochemical detection technology currently under development with the CombiMatrix group’s microfluidics “lab-on-a-chip” technology to be used for military and homeland security applications. Under the terms of this contract, the CombiMatrix group will perform research and development activities, as described under the contract, and will be reimbursed on a periodic basis for actual costs incurred to perform its obligations, plus a fixed fee, of up to $1.9 million. As of December 31, 2006, the CombiMatrix group had incurred $190,000 in actual contract costs for the microfluidics contract.
As disclosed in Note 6, the CombiMatrix group entered into an agreement with Leuchemix to purchase a total of $4,000,000 of Series A Preferred Stock of Leuchemix over a two-year period. As of December 31, 2005, future contractual cash investments totaled Leuchemix were $2,150,000, all of which were made as of October 2, 2006. There are no future commitments to purchase Leuchemix capital as of October 2, 2006.
Human Resources
The CombiMatrix group provides certain severance benefits such that if an executive who is a vice president or higher is terminated for other than cause, death or disability, the executive will receive payments equal to three months’ base salary and other medical and dental benefits on a bi-weekly basis over a three-month period. If termination occurs as a result of a change in control transaction, these benefits will be extended by three months.
Inventor Royalties and Contingent Legal Expenses
In connection with the acquisition of certain patents and patent rights, certain companies included in the Acacia Technologies group executed related agreements which grant to the former owners of the respective patents or patent rights, the right to receive inventor royalties based on future net license fee revenues (as defined in the respective agreements) generated by the Acacia Technologies group as a result of licensing the respective patents or patent portfolios. Inventor royalties paid pursuant to the agreements are expensed in the consolidated statement of operations and comprehensive loss in the period that the related license fee revenues are recognized.
In connection with the Acacia Technologies group’s licensing and enforcement activities, the Acacia Technologies group may retain the services of law firms that specialize in intellectual property licensing and enforcement and patent law. These law firms may be retained on a contingent fee basis in which the law firms are paid on a scaled percentage of any negotiated license fees, settlements or judgments awarded based on how and when the license fees, settlements or judgments are obtained by the Acacia Technologies group. In instances where the Acacia Technologies group does not recover license fees from potential infringers, no contingent legal fees are paid; however, the Acacia Technologies group may be liable for certain out of pocket legal costs incurred pursuant to the underlying legal services agreement. Legal fees advanced by contingent law firms that are required to be paid in the event that no license recoveries are obtained by the Acacia Technologies group are expensed as incurred and included in liabilities in the statement of financial condition.
Litigation and Patent Enforcement
Acacia Research Corporation is subject to claims, counterclaims and legal actions that arise in the ordinary course of business. Management believes that the ultimate liability with respect to these claims and legal actions, if any, will not have a material effect on our financial position, results of operations or cash flows. Companies comprising the Acacia Technologies group are often required to engage in litigation to enforce their patents and patent rights.
On November 28, 2000, Nanogen, Inc. (“Nanogen”) filed suit against CombiMatrix Corporation and Dr. Donald Montgomery, a former officer of CombiMatrix Corporation. The Nanogen suit alleged, among other things, that CombiMatrix Corporation’s issued patent and certain pending patent applications, trade secrets and related technologies that were inappropriately obtained by CombiMatrix Corporation and that Nanogen was the legal owner of the patents, trade secrets and related technologies.
On September 30, 2002, CombiMatrix Corporation and Dr. Donald Montgomery entered into a settlement agreement with Nanogen, Inc. to settle all pending litigation between the parties. Pursuant to the terms of the settlement agreement, CombiMatrix Corporation agreed to make quarterly payments to Nanogen equal to 12.5% of total sales of products developed by CombiMatrix Corporation and its affiliates and based on the patents that had been in dispute in the litigation, up to an annual maximum of $1,500,000. The minimum quarterly payments under the settlement agreement were $37,500 per quarter for the period from October 1, 2003 through October 1, 2004, and $25,000 per quarter thereafter until the patents expire in 2018. Also, pursuant to the settlement agreement, CombiMatrix Corporation issued to Nanogen 4,016,346 shares, or 17.5% of its outstanding shares post-issuance, subject to an anti-dilution provision related to the exercise of CombiMatrix Corporation options and warrants that were outstanding on the effective date of the agreement, for a period of up to three years.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Royalties recognized under the settlement agreement for the years ended December 31, 2006, 2005 and 2004 were $348,000, $217,000 and $138,000, respectively, and are included in patent amortization and royalties in the accompanying consolidated statements of operations. Patent amortization and royalties for the CombiMatrix group relate to costs of product sales.
During the years ended December 31, 2005 and 2004, the CombiMatrix group recorded net non-cash charges (credits) totaling ($406,000) and $812,000, respectively, in connection with the anti-dilution provisions of the settlement agreement. The non-cash charges (credits) reflect changes in management’s estimate of the fair value of AR-CombiMatrix stock issued to Nanogen, Inc. as a result of certain options and warrants exercised during 2004 and the fair value of AR-CombiMatrix stock potentially issuable to Nanogen, Inc. as of each balance sheet date. The liability was adjusted at each balance sheet date for changes in the market value of the AR-CombiMatrix stock and was reflected as a long-term liability. The anti-dilution provisions of the settlement agreement expired in September 2005, resulting in a net non-cash credit of $211,000 from the reversal of the related liability as of that date. There are no future stock-based obligations to Nanogen.
V-Chip Technology
In October 2005, all V-chip related litigation activities were concluded with no material effect on the Acacia Technologies group’s financial position, results of operations or cash flows. Results for the year ended December 31, 2005 include $225,000 in final V-chip related inventor royalties expense.
As a result of the conclusion of the Acacia Technologies group’s V-chip litigation in August 2004, the Acacia Technologies group recognized $1,500,000 of V-chip related deferred license fee revenues and $668,000 of V-chip related deferred legal costs in the third quarter of 2004.
Guarantees and Indemnifications
Acacia Research Corporation has made guarantees and indemnities under which it may be required to make payments to a guaranteed or indemnified party, in relation to certain transactions, including revenue transactions in the ordinary course of business. In connection with certain facility leases Acacia Research Corporation has indemnified its lessors for certain claims arising from the facility or the lease. Acacia Research Corporation indemnifies its directors and officers to the maximum extent permitted under the laws of the State of Delaware. However, Acacia Research Corporation has a directors and officers insurance policy that may reduce its exposure in certain circumstances and may enable it to recover a portion of future amounts that may be payable, if any. The duration of the guarantees and indemnities varies and, in many cases is indefinite but subject to statute of limitations. The majority of guarantees and indemnities do not provide any limitations of the maximum potential future payments Acacia Research Corporation could be obligated to make. To date, we have made no payments related to these guarantees and indemnities. Acacia Research Corporation estimates the fair value of its indemnification obligations as insignificant based on this history and has therefore, not recorded any liability for these guarantees and indemnities in the accompanying consolidated balance sheets.
15. RETIREMENT SAVINGS PLANS
The Acacia Technologies group and the CombiMatrix group have separate employee savings and retirement plans under section 401(k) of the Internal Revenue Code (the “Plans”). The Plans are defined contribution plans in which eligible employees may elect to have a percentage of their compensation contributed to the Plans, subject to certain guidelines issued by the Internal Revenue Service. The Acacia Technologies group and the CombiMatrix group may contribute to the Plans at the discretion of Acacia Research Corporation’s board of directors. There were no contributions made by the Acacia Technologies group or by the CombiMatrix group during the years ended December 31, 2006, 2005 and 2004.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
16. CONSOLIDATING SEGMENT INFORMATION
Acacia Research Corporation has adopted the provisions of SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information.” Our chief operating decision maker is considered to be Acacia Research Corporation’s CEO. The CEO reviews and evaluates financial information presented on a group basis as described below. Management evaluates performance based on the profit or loss from continuing operations and financial position of its segments. Acacia Research Corporation has two reportable segments as described in Note 1.
Material intercompany transactions and transfers have been eliminated in consolidation. The accounting policies of the segments are the same as those described in the summary of significant accounting policies.
Presented below is consolidating financial information for our reportable segments reflecting the businesses of the CombiMatrix group and the Acacia Technologies group. Earnings attributable to each group have been determined in accordance with accounting principles generally accepted in the United States.
Consolidating Balance Sheets (In thousands)
| | | At December 31, 2006 | | At December 31, 2005 | |
| | | Acacia | | | | | | | | Acacia | | | | | | | |
| | | Technologies | | CombiMatrix | | | | | | Technologies | | CombiMatrix | | | | | |
| | | Group | | Group | | Eliminations | | Consolidated | | Group | | Group | | Eliminations | | Consolidated | |
| | | | | | | | | | | | | | | | | | |
ASSETS | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 32,215 | | $ | 7,829 | | $ | - | | $ | 40,044 | | $ | 14,498 | | $ | 5,666 | | $ | - | | $ | 20,164 | |
| Short-term investments | | | 12,783 | | | 6,513 | | | - | | | 19,296 | | | 24,462 | | | 14,547 | | | - | | | 39,009 | |
| Accounts receivable | | | 269 | | | 605 | | | - | | | 874 | | | 4,421 | | | 911 | | | - | | | 5,332 | |
| Prepaid expenses, inventory and other assets | | | 1,187 | | | 605 | | | - | | | 1,792 | | | 1,406 | | | 709 | | | - | | | 2,115 | |
| Receivable from CombiMatrix group | | | 380 | | | - | | | (380 | ) | | - | | | - | | | - | | | - | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total current assets | | | 46,834 | | | 15,552 | | | (380 | ) | | 62,006 | | | 44,787 | | | 21,833 | | | - | | | 66,620 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Property and equipment, net of accumulated depreciation | | | 221 | | | 1,785 | | | - | | | 2,006 | | | 121 | | | 2,363 | | | - | | | 2,484 | |
| Patents and licenses, net of accumulated amortization | | | 18,515 | | | 7,292 | | | - | | | 25,807 | | | 23,786 | | | 7,926 | | | - | | | 31,712 | |
| Goodwill | | | 121 | | | 16,918 | | | - | | | 17,039 | | | 121 | | | 18,859 | | | - | | | 18,980 | |
| Other assets | | | 79 | | | 2,667 | | | - | | | 2,746 | | | 78 | | | 1,560 | | | - | | | 1,638 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | $ | 65,770 | | $ | 44,214 | | $ | (380 | ) | $ | 109,604 | | $ | 68,893 | | $ | 52,541 | | $ | - | | $ | 121,434 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Current liabilities: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Accounts payable, accrued expenses and other | | $ | 2,201 | | $ | 2,846 | | $ | - | | $ | 5,047 | | $ | 1,441 | | $ | 2,483 | | $ | - | | $ | 3,924 | |
| Royalties and legal fees payable | | | 1,684 | | | - | | | - | | | 1,684 | | | 3,758 | | | - | | | - | | | 3,758 | |
| Current portion of deferred revenues | | | 360 | | | 365 | | | - | | | 725 | | | 639 | | | 165 | | | - | | | 804 | |
| Payable to Acacia Technologies group | | | - | | | 380 | | | (380 | ) | | - | | | - | | | - | | | - | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total current liabilities | | | 4,245 | | | 3,591 | | | (380 | ) | | 7,456 | | | 5,838 | | | 2,648 | | | - | | | 8,486 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Deferred income taxes | | | - | | | - | | | - | | | - | | | 726 | | | 1,975 | | | - | | | 2,701 | |
| Deferred revenues, net of current portion | | | - | | | 1,076 | | | - | | | 1,076 | | | - | | | 1,439 | | | - | | | 1,439 | |
| Warrant liability | | | - | | | 6,732 | | | - | | | 6,732 | | | - | | | 1,381 | | | - | | | 1,381 | |
| Other liabilities | | | 31 | | | - | | | - | | | 31 | | | 83 | | | - | | | - | | | 83 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total liabilities | | | 4,276 | | | 11,399 | | | (380 | ) | | 15,295 | | | 6,647 | | | 7,443 | | | - | | | 14,090 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Minority interests | | | - | | | - | | | - | | | - | | | 443 | | | 4 | | | - | | | 447 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Redeemable stockholders' equity: | | | | | | | | | | | | | | | | | | | | | | | | | |
| AR - Acacia Technologies stock | | | 61,494 | | | - | | | - | | | 61,494 | | | 61,803 | | | - | | | - | | | 61,803 | |
| AR - CombiMatrix stock | | | - | | | 32,815 | | | - | | | 32,815 | | | - | | | 45,094 | | | - | | | 45,094 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total stockholders' equity | | | 61,494 | | | 32,815 | | | - | | | 94,309 | | | 61,803 | | | 45,094 | | | - | | | 106,897 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | $ | 65,770 | | $ | 44,214 | | $ | (380 | ) | $ | 109,604 | | $ | 68,893 | | $ | 52,541 | | $ | - | | $ | 121,434 | |
NOTE: Segment information for the Acacia Technologies group includes discontinued operations related to Soundbreak.com. Refer to Note 12.
Consolidating Statements of Operations (In thousands)
| | | 2006 | | 2005 | |
| | | Acacia | | | | | | Acacia | | | | | |
| | | Technologies | | CombiMatrix | | | | Technologies | | CombiMatrix | | | |
| | | Group | | Group | | Consolidated | | Group | | Group | | Consolidated | |
| Revenues: | | | | | | | | | | | | | |
| Collaboration agreements and government contracts | | $ | - | | $ | 2,074 | | $ | 2,074 | | $ | - | | $ | 6,115 | | $ | 6,115 | |
| License fees | | | 34,825 | | | - | | | 34,825 | | | 19,574 | | | - | | | 19,574 | |
| Products and service contracts | | | - | | | 3,666 | | | 3,666 | | | - | | | 1,918 | | | 1,918 | |
| Total revenues | | | 34,825 | | | 5,740 | | | 40,565 | | | 19,574 | | | 8,033 | | | 27,607 | |
| | | | | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | |
| Cost of government contract revenues | | | - | | | 1,959 | | | 1,959 | | | - | | | 3,683 | | | 3,683 | |
| Cost of product sales | | | - | | | 1,258 | | | 1,258 | | | - | | | 820 | | | 820 | |
| Research and development expenses (including | | | | | | | | | | | | | | | | | | | |
non-cash stock compensation expense) | | | - | | | 9,485 | | | 9,485 | | | - | | | 5,783 | | | 5,783 | |
| Marketing, general and administrative expenses | | | | | | | | | | | | | | | | | | | |
(including non-cash stock compensation expense) | | | 14,256 | | | 12,707 | | | 26,963 | | | 8,099 | | | 9,827 | | | 17,926 | |
| Legal expenses - patents | | | 4,780 | | | - | | | 4,780 | | | 2,468 | | | - | | | 2,468 | |
| Inventor royalties and contingent legal fees | | | | | | | | | | | | | | | | | | | |
| expense - patents | | | 17,159 | | | - | | | 17,159 | | | 11,106 | | | - | | | 11,106 | |
| Inventor royalties - V-chip | | | - | | | - | | | - | | | 225 | | | - | | | 225 | |
| Goodwill impairment charge | | | - | | | - | | | - | | | - | | | 565 | | | 565 | |
| Write-off of patent-related intangible asset | | | 297 | | | - | | | 297 | | | - | | | - | | | - | |
| Amortization of patents and royalties | | | 5,313 | | | 1,482 | | | 6,795 | | | 4,922 | | | 1,312 | | | 6,234 | |
| Legal settlement charges (gains) | | | - | | | - | | | - | | | - | | | (406 | ) | | (406 | ) |
| Loss from equity investment | | | - | | | 1,036 | | | 1,036 | | | - | | | 352 | | | 352 | |
| Total operating expenses | | | 41,805 | | | 27,927 | | | 69,732 | | | 26,820 | | | 21,936 | | | 48,756 | |
| Operating income (loss) | | | (6,980 | ) | | (22,187 | ) | | (29,167 | ) | | (7,246 | ) | | (13,903 | ) | | (21,149 | ) |
| | | | | | | | | | | | | | | | | | �� | | |
| Other income (expense): | | | | | | | | | | | | | | | | | | | |
| Interest and investment income | | | 1,524 | | | 523 | | | 2,047 | | | 1,071 | | | 523 | | | 1,594 | |
| Loss on sale of interest in subsidiary | | | - | | | (84 | ) | | (84 | ) | | - | | | - | | | - | |
| Warrant (charges) gains | | | - | | | 1,754 | | | 1,754 | | | - | | | 812 | | | 812 | |
| Total other income | | | 1,524 | | | 2,193 | | | 3,717 | | | 1,071 | | | 1,335 | | | 2,406 | |
| | | | | | | | | | | | | | | | | | | | |
| Income (loss) from continuing operations before | | | | | | | | | | | | | | | | | | | |
| income taxes and minority interests | | | (5,456 | ) | | (19,994 | ) | | (25,450 | ) | | (6,175 | ) | | (12,568 | ) | | (18,743 | ) |
| (Provision) benefit for income taxes | | | (40 | ) | | 34 | | | (6 | ) | | 135 | | | 167 | | | 302 | |
| Income (loss) from continuing operations before | | | | | | | | | | | | | | | | | | | |
| income taxes | | | (5,496 | ) | | (19,960 | ) | | (25,456 | ) | | (6,040 | ) | | (12,401 | ) | | (18,441 | ) |
| Minority interests | | | - | | | - | | | - | | | 2 | | | - | | | 2 | |
| Income (loss) from continuing operations | | | (5,496 | ) | | (19,960 | ) | | (25,456 | ) | | (6,038 | ) | | (12,401 | ) | | (18,439 | ) |
| Discontinued operations: | | | | | | | | | | | | | | | | | | | |
| Estimated loss on disposal of discontinued operations | | | - | | | - | | | - | | | (237 | ) | | - | | | (237 | ) |
| Net income (loss) | | $ | (5,496 | ) | $ | (19,960 | ) | $ | (25,456 | ) | $ | (6,275 | ) | $ | (12,401 | ) | $ | (18,676 | ) |
(continued)
| | | 2004 | |
| | | Acacia | | | | | |
| | | Technologies | | CombiMatrix | | | |
| | | Group | | Group | | Consolidated | |
| Revenues: | | | | | | | |
| Collaboration agreements and government contracts | | $ | - | | $ | 19,295 | | $ | 19,295 | |
| License fees | | | 4,284 | | | - | | | 4,284 | |
| Products and service contracts | | | - | | | 346 | | | 346 | |
| Total revenues | | | 4,284 | | | 19,641 | | | 23,925 | |
| | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | |
| Cost of government contract revenues | | | - | | | 1,874 | | | 1,874 | |
| Cost of product sales | | | - | | | 173 | | | 173 | |
| Research and development expenses (including non-cash stock | | | | | | | | | | |
| compensation expense) | | | - | | | 5,385 | | | 5,385 | |
| Marketing, general and administrative expenses (including non-cash | | | | | | | | | | |
| stock compensation expense) | | | 5,049 | | | 9,902 | | | 14,951 | |
| Legal expenses - patents | | | 3,133 | | | - | | | 3,133 | |
| Inventor royalties and contingent legal fees expense - patents | | | - | | | - | | | - | |
| Inventor royalties - V-chip | | | - | | | - | | | - | |
| Goodwill impairment charge | | | 1,656 | | | - | | | 1,656 | |
| Write-off of patent-related intangible asset | | | - | | | - | | | - | |
| Amortization of patents and royalties | | | 501 | | | 1,234 | | | 1,735 | |
| Legal settlement charges (gains) | | | - | | | 812 | | | 812 | |
| Loss from equity investment | | | - | | | 17 | | | 17 | |
| Total operating expenses | | | 10,339 | | | 19,397 | | | 29,736 | |
| Operating income (loss) | | | (6,055 | ) | | 244 | | | (5,811 | ) |
| | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | |
| Interest and investment income | | | 471 | | | 330 | | | 801 | |
| Loss on sale of interest in subsidiary | | | - | | | - | | | - | |
| Warrant (charges) gains | | | - | | | - | | | - | |
| Total other income | | | 471 | | | 330 | | | 801 | |
| | | | | | | | | | | |
| Income (loss) from continuing operations before income taxes | | | | | | | | | | |
| and minority interests | | | (5,584 | ) | | 574 | | | (5,010 | ) |
| (Provision) benefit for income taxes | | | 139 | | | 136 | | | 275 | |
| | | | | | | | | | | |
| Income (loss) from continuing operations before minority interests | | | (5,445 | ) | | 710 | | | (4,735 | ) |
| | | | | | | | | | | |
| Minority interests | | | 6 | | | - | | | 6 | |
| Income (loss) from continuing operations | | | (5,439 | ) | | 710 | | | (4,729 | ) |
| | | | | | | | | | | |
| Discontinued operations: | | | | | | | | | | |
| | | | | | | | | | | |
| Estimated loss on disposal of discontinued operations | | | (104 | ) | | - | | | (104 | ) |
| Net income (loss) | | $ | (5,543 | ) | $ | 710 | | $ | (4,833 | ) |
| | | | | | | | | | | |
Consolidating Statements of Cash Flows (In thousands)
| | | Year Ended December 31, 2006 | | Year Ended December 31, 2005 | |
| | | Acacia | | | | | | | | Acacia | | | | | | | |
| | | Technologies | | CombiMatrix | | | | | | Technologies | | CombiMatrix | | | | | |
| | | Group | | Group | | Eliminations | | Consolidated | | Group | | Group | | Eliminations | | Consolidated | |
| Cash flows from operating activities: | | | | | | | | | | | | | | | | | |
| Net income (loss) | | $ | (5,496 | ) | $ | (19,960 | ) | $ | - | | $ | (25,456 | ) | $ | (6,275 | ) | $ | (12,401 | ) | $ | - | | $ | (18,676 | ) |
| Adjustments to reconcile net income (loss) to net | | | | | | | | | | | | | | | | | | | | | | | | | |
| cash used in operating activities: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | 5,392 | | | 2,025 | | | - | | | 7,417 | | | 4,981 | | | 2,183 | | | - | | | 7,164 | |
| Minority interests | | | - | | | - | | | - | | | - | | | (2 | ) | | - | | | - | | | (2 | ) |
| Non-cash stock compensation | | | 3,946 | | | 2,357 | | | - | | | 6,303 | | | 356 | | | (159 | ) | | - | | | 197 | |
| Deferred income taxes | | | (36 | ) | | (34 | ) | | - | | | (70 | ) | | (143 | ) | | (137 | ) | | - | | | (280 | ) |
| Non-cash warrant charges (gains) | | | - | | | (1,754 | ) | | - | | | (1,754 | ) | | - | | | (812 | ) | | - | | | (812 | ) |
| Non-cash legal settlement charges (gains) | | | - | | | - | | | - | | | - | | | - | | | (406 | ) | | - | | | (406 | ) |
| Non-cash impairment charge | | | - | | | - | | | - | | | - | | | - | | | 565 | | | - | | | 565 | |
| Loss on disposal of discontinued operations | | | - | | | - | | | - | | | - | | | 237 | | | - | | | - | | | 237 | |
| Write-off of patent-related intangible asset | | | 297 | | | - | | | - | | | 297 | | | - | | | - | | | - | | | - | |
| Loss from equity investments | | | - | | | 1,036 | | | - | | | 1,036 | | | - | | | 352 | | | - | | | 352 | |
| Loss on sale of interest in subsidiary | | | - | | | 84 | | | - | | | 84 | | | - | | | - | | | - | | | - | |
| Stock issued to consultant | | | - | | | 94 | | | - | | | 94 | | | - | | | - | | | - | | | - | |
| Other | | | (96 | ) | | 243 | | | - | | | 147 | | | - | | | (79 | ) | | - | | | (79 | ) |
| Changes in assets and liabilities, excluding effect | | | | | | | | | | | | | | | | | | | | | | | | | |
| of business acquisition: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Accounts receivable | | | 4,152 | | | 288 | | | - | | | 4,440 | | | (4,228 | ) | | (568 | ) | | - | | | (4,796 | ) |
| Prepaid expenses, inventory and other assets | | | (530 | ) | | 104 | | | 380 | | | (46 | ) | | (643 | ) | | (180 | ) | | (119 | ) | | (942 | ) |
| Accounts payable, accrued expenses and other | | | 819 | | | 483 | | | (380 | ) | | 922 | | | (729 | ) | | 301 | | | 119 | | | (309 | ) |
| Royalties and legal fees payable | | | (2,074 | ) | | - | | | - | | | (2,074 | ) | | 3,758 | | | - | | | - | | | 3,758 | |
| Deferred revenues | | | (279 | ) | | (111 | ) | | - | | | (390 | ) | | 211 | | | (2,355 | ) | | - | | | (2,144 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash provided by (used in) operating | | | | | | | | | | | | | | | | | | | | | | | | | |
| activities from continuing operations | | | 6,095 | | | (15,145 | ) | | - | | | (9,050 | ) | | (2,477 | ) | | (13,696 | ) | | - | | | (16,173 | ) |
| Net cash provided by (used in) operating | | | | | | | | | | | | | | | | | | | | | | | | | |
| activities from discontinued operations | | | 264 | | | - | | | - | | | 264 | | | (513 | ) | | - | | | - | | | (513 | ) |
| Net cash provided by (used in) operating activities | | | 6,359 | | | (15,145 | ) | | - | | | (8,786 | ) | | (2,990 | ) | | (13,696 | ) | | - | | | (16,686 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Purchase of property and equipment | | | (179 | ) | | (536 | ) | | - | | | (715 | ) | | (75 | ) | | (1,325 | ) | | - | | | (1,400 | ) |
| Purchase of available-for-sale investments | | | (16,409 | ) | | (5,537 | ) | | - | | | (21,946 | ) | | (39,919 | ) | | (36,771 | ) | | - | | | (76,690 | ) |
| Sale of available-for-sale investments | | | 28,147 | | | 13,573 | | | - | | | 41,720 | | | 33,141 | | | 43,086 | | | - | | | 76,227 | |
| Business acquisition | | | (16 | ) | | - | | | - | | | (16 | ) | | (5,796 | ) | | - | | | - | | | (5,796 | ) |
| Purchase of additional interests in equity | | | | | | | | | | | | | | | | | | | | | | | | | |
| method investee | | | - | | | (2,150 | ) | | - | | | (2,150 | ) | | - | | | (1,600 | ) | | - | | | (1,600 | ) |
| Patent acquisition costs | | | (1,030 | ) | | - | | | - | | | (1,030 | ) | | (445 | ) | | - | | | - | | | (445 | ) |
| Sale of interest in subsidiary (net of cash disposed) | | | - | | | (369 | ) | | - | | | (369 | ) | | - | | | - | | | - | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash provided by (used in) investing | | | | | | | | | | | | | | | | | | | | | | | | | |
| activities from continuing operations | | | 10,513 | | | 4,981 | | | - | | | 15,494 | | | (13,094 | ) | | 3,390 | | | - | | | (9,704 | ) |
| Net cash used in investing activities from | | | | | | | | | | | | | | | | | | | | | | | | | |
| discontinued operations | | | (353 | ) | | - | | | - | | | (353 | ) | | - | | | - | | | - | | | - | |
| Net cash provided by (used in) investing activities | | | 10,160 | | | 4,981 | | | - | | | 15,141 | | | (13,094 | ) | | 3,390 | | | - | | | (9,704 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash attributed to the Acacia Technologies group | | | 1,198 | | | - | | | - | | | 1,198 | | | 19,657 | | | - | | | - | | | 19,657 | |
| Net cash attributed to the CombiMatrix group | | | - | | | 12,327 | | | - | | | 12,327 | | | - | | | 12,914 | | | - | | | 12,914 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash provided by (used in) financing activities | | | 1,198 | | | 12,327 | | | - | | | 13,525 | | | 19,657 | | | 12,914 | | | - | | | 32,571 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Effect of exchange rate on cash | | | - | | | - | | | - | | | - | | | - | | | 73 | | | - | | | 73 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Increase (decrease) in cash and cash equivalents | | | 17,717 | | | 2,163 | | | - | | | 19,880 | | | 3,573 | | | 2,681 | | | - | | | 6,254 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents, beginning | | | 14,498 | | | 5,666 | | | - | | | 20,164 | | | 10,925 | | | 2,985 | | | - | | | 13,910 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents, ending | | $ | 32,215 | | $ | 7,829 | | $ | - | | $ | 40,044 | | $ | 14,498 | | $ | 5,666 | | $ | - | | $ | 20,164 | |
(continued)
| | | Year Ended December 31, 2004 | |
| | | Acacia | | | | | | | |
| | | Technologies | | CombiMatrix | | | | | |
| | | Group | | Group | | Eliminations | | Consolidated | |
| Cash flows from operating activities: | | | | | | | | | |
| Net income (loss) | | $ | (5,543 | ) | $ | 710 | | $ | - | | $ | (4,833 | ) |
| Adjustments to reconcile net income (loss) to net cash used in | | | | | | | | | | | | | |
| operating activities: | | | | | | | | | | | | | |
| Depreciation and amortization | | | 551 | | | 2,200 | | | - | | | 2,751 | |
| Minority interests | | | - | | | - | | | - | | | - | |
| Non-cash stock compensation | | | - | | | 754 | | | - | | | 754 | |
| Deferred income taxes | | | (143 | ) | | (136 | ) | | - | | | (279 | ) |
| Non-cash warrant charges (gains) | | | - | | | - | | | - | | | - | |
| Non-cash legal settlement charges (gains) | | | - | | | 812 | | | - | | | 812 | |
| Non-cash impairment charge | | | 1,656 | | | - | | | - | | | 1,656 | |
| Loss on disposal of discontinued operations | | | 104 | | | - | | | - | | | 104 | |
| Write-off of patent-related intangible asset | | | - | | | - | | | - | | | - | |
| Loss from equity investments | | | - | | | 17 | | | - | | | 17 | |
| Loss on sale of interest in subsidiary | | | - | | | - | | | - | | | - | |
| Stock issued to consultant | | | - | | | - | | | - | | | - | |
| Other | | | 22 | | | 43 | | | - | | | 65 | |
| Changes in assets and liabilities, excluding effect of business acquisition: | | | | | | | | | | | | | |
| Accounts receivable | | | (69 | ) | | (154 | ) | | - | | | (223 | ) |
| Prepaid expenses, inventory and other assets | | | 654 | | | 135 | | | 20 | | | 809 | |
| Accounts payable, accrued expenses and other | | | 712 | | | 481 | | | (20 | ) | | 1,173 | |
| Royalties and legal fees payable | | | - | | | - | | | - | | | - | |
| Deferred revenues | | | (1,176 | ) | | (16,446 | ) | | - | | | (17,622 | ) |
| | | | | | | | | | | | | | |
| Net cash provided by (used in) operating activities from continuing operations | | | (3,232 | ) | | (11,584 | ) | | - | | | (14,816 | ) |
| Net cash provided by (used in) operating activities from discontinued operations | | | (727 | ) | | - | | | - | | | (727 | ) |
| Net cash provided by (used in) operating activities | | | (3,959 | ) | | (11,584 | ) | | - | | | (15,543 | ) |
| | | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | |
| Purchase of property and equipment | | | (81 | ) | | (810 | ) | | - | | | (891 | ) |
| Purchase of available-for-sale investments | | | (9,239 | ) | | (50,143 | ) | | - | | | (59,382 | ) |
| Sale of available-for-sale investments | | | 9,004 | | | 42,755 | | | - | | | 51,759 | |
| Business acquisition | | | - | | | - | | | - | | | - | |
| Purchase of additional interests in equity method investee | | | - | | | (250 | ) | | - | | | (250 | ) |
| Patent acquisition costs | | | - | | | - | | | - | | | - | |
| Sale of interest in subsidiary (net of cash disposed) | | | - | | | - | | | - | | | - | |
| Other | | | (5 | ) | | - | | | - | | | (5 | ) |
| | | | | | | | | | | | | | |
| Net cash provided by (used in) investing activities from | | | | | | | | | | | | | |
| continuing operations | | | (321 | ) | | (8,448 | ) | | - | | | (8,769 | ) |
| Net cash used in investing activities from discontinued operations | | | (198 | ) | | - | | | - | | | (198 | ) |
| Net cash provided by (used in) investing activities | | | (519 | ) | | (8,448 | ) | | - | | | (8,967 | ) |
| | | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | |
| Net cash attributed to the Acacia Technologies group | | | (305 | ) | | - | | | - | | | (305 | ) |
| Net cash attributed to the CombiMatrix group | | | - | | | 19,227 | | | - | | | 19,227 | |
| | | | | | | | | | | | | | |
| Net cash provided by (used in) financing activities | | | (305 | ) | | 19,227 | | | - | | | 18,922 | |
| | | | | | | | | | | | | | |
| Effect of exchange rate on cash | | | - | | | (17 | ) | | - | | | (17 | ) |
| | | | | | | | | | | | | | |
| Increase (decrease) in cash and cash equivalents | | | (4,783 | ) | | (822 | ) | | - | | | (5,605 | ) |
| | | | | | | | | | | | | | |
| Cash and cash equivalents, beginning | | | 15,708 | | | 3,807 | | | - | | | 19,515 | |
| | | | | | | | | | | | | | |
| Cash and cash equivalents, ending | | $ | 10,925 | | $ | 2,985 | | $ | - | | $ | 13,910 | |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
17. SUPPLEMENTAL CASH FLOW INFORMATION
Cash paid by Acacia Research Corporation for income taxes was not material for the periods presented. Refer to Note 8 for a summary of the Acacia Technologies group’s non cash investing activities in connection with the GPH Acquisition. Refer to Note 7 for goodwill impairment charges, and other non-cash reductions in intangibles during the periods presented.
18. QUARTERLY FINANCIAL DATA (unaudited)
The following table sets forth unaudited consolidated statement of operations data for the eight quarters in the period ended December 31, 2006. This information has been derived from our unaudited condensed consolidated financial statements that have been prepared on the same basis as the audited consolidated financial statements and, in the opinion of management, include all adjustments, consisting of normal recurring adjustments, necessary for a fair statement of the information when read in conjunction with the audited consolidated financial statements and related notes thereto. Our quarterly results have been in the past and may in the future be subject to significant fluctuations. As a result, we believe that results of operations for interim periods should not be relied upon as any indication of the results to be expected in any future periods.
QUARTERLY FINANCIAL DATA (unaudited)
| | | Quarter Ended | |
| | | Mar. 31, 2006 | | | | | | Dec. 31, 2006 | | Mar. 31, 2005 | | Jun. 30, 2005 | | Sep. 30, 2005 | | | |
| | | (In thousands, except share and per share information) | |
| | | (Unaudited) | |
| | | | |
| Revenues: | | | | | | | | | | | | | | | | | |
| Research and development contracts | | $ | - | | $ | - | | $ | - | | $ | - | | $ | - | | $ | - | | $ | - | | $ | 2,266 | |
| License fees | | | 4,717 | | | 14,371 | | | 8,424 | | | 7,313 | | | 1,863 | | | 2,682 | | | 6,783 | | | 8,246 | |
| Government contracts | | | 264 | | | 574 | | | 725 | | | 511 | | | 731 | | | 1,281 | | | 973 | | | 864 | |
| Service contracts | | | 57 | | | 60 | | | 151 | | | 120 | | | 60 | | | 9 | | | 37 | | | 47 | |
| Products | | | 924 | | | 1,158 | | | 968 | | | 228 | | | 278 | | | 567 | | | 453 | | | 467 | |
| Total revenues | | | 5,962 | | | 16,163 | | | 10,268 | | | 8,172 | | | 2,932 | | | 4,539 | | | 8,246 | | | 11,890 | |
| Operating expenses | | | 14,842 | | | 20,457 | | | 17,056 | | | 17,377 | | | 8,015 | | | 10,403 | | | 14,383 | | | 15,955 | |
| Operating income (loss) | | | (8,880 | ) | | (4,294 | ) | | (6,788 | ) | | (9,205 | ) | | (5,083 | ) | | (5,864 | ) | | (6,137 | ) | | (4,065 | ) |
| Other income (expenses) | | | (1,284 | ) | | 2,021 | | | 1,414 | | | 1,566 | | | 273 | | | 383 | | | 597 | | | 1,153 | |
| Income (loss) from continuing operations before | | | | | | | | | | | | | | | | | | | | | | | | | |
| income taxes and minority interests | | | (10,164 | ) | | (2,273 | ) | | (5,374 | ) | | (7,639 | ) | | (4,810 | ) | | (5,481 | ) | | (5,540 | ) | | (2,912 | ) |
| Benefit (provision) for income taxes | | | 66 | | | (70 | ) | | (2 | ) | | - | | | 70 | | | 64 | | | 98 | | | 70 | |
| Income (loss) from continuing operations before | | | | | | | | | | | | | | | | | | | | | | | | | |
| minority interests | | | (10,098 | ) | | (2,343 | ) | | (5,376 | ) | | (7,639 | ) | | (4,740 | ) | | (5,417 | ) | | (5,442 | ) | | (2,842 | ) |
| Minority interests | | | - | | | - | | | - | | | - | | | - | | | - | | | 1 | | | 1 | |
| Income (loss) from continuing operations | | | (10,098 | ) | | (2,343 | ) | | (5,376 | ) | | (7,639 | ) | | (4,740 | ) | | (5,417 | ) | | (5,441 | ) | | (2,841 | ) |
| Loss from discontinued operations | | | - | | | - | | | - | | | - | | | (210 | ) | | - | | | - | | | (27 | ) |
| Net income (loss) | | $ | (10,098 | ) | $ | (2,343 | ) | $ | (5,376 | ) | $ | (7,639 | ) | $ | (4,950 | ) | $ | (5,417 | ) | $ | (5,441 | ) | $ | (2,868 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Earnings (loss) per common share: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Attributable to the Acacia Technologies group: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Earnings (loss) from continuing operations | | $ | (2,409 | ) | $ | 1,099 | | $ | (1,049 | ) | $ | (3,137 | ) | $ | (1,664 | ) | $ | (1,760 | ) | $ | (1,558 | ) | $ | (1,056 | ) |
| Basic and diluted earnings (loss) per share | | | (0.09 | ) | | 0.04 | | | (0.04 | ) | | (0.11 | ) | | (0.07 | ) | | (0.06 | ) | | (0.06 | ) | | (0.04 | ) |
| Loss from discontinued operations | | $ | - | | $ | - | | $ | - | | $ | - | | $ | (210 | ) | $ | - | | $ | - | | $ | (27 | ) |
| Basic and diluted loss per share | | | - | | | - | | | - | | | - | | | (0.01 | ) | | - | | | - | | | (0.00 | ) |
| Net income (loss) | | $ | (2,409 | ) | $ | 1,099 | | $ | (1,049 | ) | $ | (3,137 | ) | $ | (1,874 | ) | $ | (1,760 | ) | $ | (1,558 | ) | $ | (1,083 | ) |
| Basic and diluted loss per share | | | (0.09 | ) | | 0.04 | | | (0.04 | ) | | (0.11 | ) | | (0.08 | ) | | (0.06 | ) | | (0.06 | ) | | (0.04 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Attributable to the CombiMatrix group: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (7,689 | ) | $ | (3,442 | ) | $ | (4,327 | ) | $ | (4,502 | ) | $ | (3,076 | ) | $ | (3,657 | ) | $ | (3,883 | ) | $ | (1,785 | ) |
| Basic and diluted loss per share | | | (0.20 | ) | | (0.09 | ) | | (0.11 | ) | | (0.10 | ) | | (0.10 | ) | | (0.12 | ) | | (0.12 | ) | | (0.05 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Weighted average shares: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Acacia Research - Acacia Technologies stock: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 27,400,857 | | | 27,507,024 | | | 27,567,848 | | | 27,708,902 | | | 24,558,419 | | | 27,271,416 | | | 27,302,693 | | | 27,352,312 | |
| Diluted | | | 27,400,857 | | | 30,324,732 | | | 27,567,848 | | | 27,708,902 | | | 24,558,419 | | | 27,271,416 | | | 27,302,693 | | | 27,352,312 | |
| Acacia Research - CombiMatrix stock: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted | | | 38,992,402 | | | 39,018,844 | | | 40,209,640 | | | 44,120,736 | | | 31,200,496 | | | 31,200,984 | | | 33,239,726 | | | 38,992,402 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Market price per share - Acacia Technologies stock: | | | | | | | | | | | | | | | | | | | | | | | | | |
| High | | $ | 9.00 | | $ | 14.65 | | $ | 14.95 | | $ | 15.58 | | $ | 6.05 | | $ | 6.24 | | $ | 6.25 | | $ | 7.83 | |
| Low | | $ | 6.65 | | $ | 8.85 | | $ | 9.31 | | $ | 11.05 | | $ | 4.89 | | $ | 4.45 | | $ | 4.38 | | $ | 5.85 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Market price per share - CombiMatrix stock: | | | | | | | | | | | | | | | | | | | | | | | | | |
| High | | $ | 2.90 | | $ | 2.75 | | $ | 1.68 | | $ | 1.07 | | $ | 4.08 | | $ | 3.05 | | $ | 2.60 | | $ | 2.59 | |
| Low | | $ | 1.34 | | $ | 1.45 | | $ | 0.96 | | $ | 0.70 | | $ | 2.14 | | $ | 2.15 | | $ | 1.55 | | $ | 1.29 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
(A Division of Acacia Research Corporation)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Shareholders of Acacia Research Corporation:
In our opinion, the financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of Acacia Technologies Group (a division of Acacia Research Corporation as described in Note 1) at December 31, 2006 and December 31, 2005, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2006, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of Acacia Research Corporation’s management; our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2 to the consolidated financial statements, the Acacia Technologies group changed the manner in which it accounts for share-based compensation in 2006.
As more fully described in Note 1 to the financial statements, Acacia Technologies group is a division of Acacia Research Corporation; accordingly, the financial statements of Acacia Technologies group should be read in conjunction with the consolidated financial statements of Acacia Research Corporation.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Orange County, California
March 12, 2007
ACACIA TECHNOLOGIES GROUP |
(A Division of Acacia Research Corporation) |
BALANCE SHEETS |
(In thousands) |
| | | December 31, | | December 31, | |
| | | 2006 | | 2005 | |
| | | | | | |
ASSETS | | | | | |
| | | | | | |
| Current assets: | | | | | | | |
| Cash and cash equivalents | | $ | 32,215 | | $ | 14,498 | |
| Short-term investments | | | 12,783 | | | 24,462 | |
| Accounts receivable | | | 269 | | | 4,421 | |
| Prepaid expenses and other assets | | | 1,187 | | | 1,406 | |
| Receivable from CombiMatrix group | | | 380 | | | - | |
| | | | | | | | |
| Total current assets | | | 46,834 | | | 44,787 | |
| | | | | | | | |
| Property and equipment, net of accumulated depreciation | | | 221 | | | 121 | |
| Patents, net of accumulated amortization | | | 18,515 | | | 23,786 | |
| Goodwill | | | 121 | | | 121 | |
| Other assets | | | 79 | | | 78 | |
| | | | | | | | |
| | | $ | 65,770 | | $ | 68,893 | |
| | | | | | | | |
LIABILITIES AND ALLOCATED NET WORTH | | | | | | | |
| | | | | | | | |
| Current liabilities: | | | | | | | |
| Accounts payable and accrued expenses | | $ | 2,201 | | $ | 1,441 | |
| Royalties and legal fees payable | | | 1,684 | | | 3,758 | |
| Deferred revenues | | | 360 | | | 639 | |
| | | | | | | | |
| Total current liabilities | | | 4,245 | | | 5,838 | |
| | | | | | | | |
| Deferred income taxes | | | - | | | 726 | |
| Other liabilities | | | 31 | | | 83 | |
| | | | | | | | |
| Total liabilities | | | 4,276 | | | 6,647 | |
| | | | | | | | |
| Minority interests | | | - | | | 443 | |
| | | | | | | | |
| Commitments and contingencies (Note 10) | | | | | | | |
| | | | | | | | |
| Allocated net worth: | | | | | | | |
| Funds allocated by Acacia Research Corporation | | | 149,274 | | | 144,087 | |
| Accumulated net losses | | | (87,780 | ) | | (82,284 | ) |
| | | | | | | | |
| Total allocated net worth | | | 61,494 | | | 61,803 | |
| | | | | | | | |
| | | $ | 65,770 | | $ | 68,893 | |
The accompanying notes are an integral part of these financial statements.
ACACIA TECHNOLOGIES GROUP |
(A Division of Acacia Research Corporation) |
STATEMENTS OF OPERATIONS |
(In thousands) |
| | | 2006 | | 2005 | | 2004 | |
| Revenues: | | | | | | | | | | |
| License fees | | $ | 34,825 | | $ | 19,574 | | $ | 4,284 | |
| | | | | | | | | | | |
| Total revenues | | | 34,825 | | | 19,574 | | | 4,284 | |
| Operating expenses: | | | | | | | | | | |
| Marketing, general and administrative expenses (including non-cash stock | | | | | | | | | | |
| compensation expense of $3,946 for 2006, $356 for 2005 and $0 for 2004) | | | 14,256 | | | 8,099 | | | 5,049 | |
| Legal expenses - patents | | | 4,780 | | | 2,468 | | | 3,133 | |
| Inventor royalties and contingent legal fees expense - patents | | | 17,159 | | | 11,106 | | | - | |
| Inventor royalties - V-chip | | | - | | | 225 | | | - | |
| Goodwill impairment charge | | | - | | | - | | | 1,656 | |
| Write-off of patent-related intangible asset | | | 297 | | | - | | | - | |
| Amortization of patents | | | 5,313 | | | 4,922 | | | 501 | |
| | | | | | | | | | | |
| Total operating expenses | | | 41,805 | | | 26,820 | | | 10,339 | |
| | | | | | | | | | | |
| Operating loss | | | (6,980 | ) | | (7,246 | ) | | (6,055 | ) |
| Other income: | | | | | | | | | | |
| Interest and investment income | | | 1,524 | | | 1,071 | | | 471 | |
| | | | | | | | | | | |
| Total other income | | | 1,524 | | | 1,071 | | | 471 | |
| Loss from continuing operations before income taxes and | | | | | | | | | | |
| minority interests | | | (5,456 | ) | | (6,175 | ) | | (5,584 | ) |
| (Provision) benefit for income taxes | | | (40 | ) | | 135 | | | 139 | |
| | | | | | | | | | | |
| Loss from continuing operations before minority interests | | | (5,496 | ) | | (6,040 | ) | | (5,445 | ) |
| | | | | | | | | | | |
| Minority interests | | | - | | | 2 | | | 6 | |
| | | | | | | | | | | |
| Loss from continuing operations | | | (5,496 | ) | | (6,038 | ) | | (5,439 | ) |
| Discontinued operations: | | | | | | | | | | |
| Estimated loss on disposal of discontinued operations | | | - | | | (237 | ) | | (104 | ) |
| | | | | | | | | | | |
| Division net loss | | $ | (5,496 | ) | $ | (6,275 | ) | $ | (5,543 | ) |
The accompanying notes are an integral part of these financial statements.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
STATEMENTS OF ALLOCATED NET WORTH
(In thousands)
| Balance at December 31, 2003 | | $ | 34,663 | |
Net assets attributed to the Acacia Technologies group | | | (312 | ) |
| Division net loss | | | (5,543 | ) |
Balance at December 31, 2004 | | | 28,808 | |
Net assets attributed to the Acacia Technologies group | | | 39,270 | |
| Division net loss | | | (6,275 | ) |
Balance at December 31, 2005 | | | 61,803 | |
Net assets attributed to the Acacia Technologies group | | | 5,187 | |
| Division net loss | | | (5,496 | ) |
Balance at December 31, 2006 | | $ | 61,494 | |
The accompanying notes are an integral part of these financial statements.
ACACIA TECHNOLOGIES GROUP |
(A Division of Acacia Research Corporation) |
STATEMENTS OF CASH FLOWS |
(In thousands) |
| | | For the Years Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
| Cash flows from operating activities: | | | | | | | | | | |
| Division net loss | | $ | (5,496 | ) | $ | (6,275 | ) | $ | (5,543 | ) |
| Adjustments to reconcile division net loss to net cash provided by (used in) | | | | | | | | | | |
| operating activities: | | | | | | | | | | |
| Depreciation and amortization | | | 5,392 | | | 4,981 | | | 551 | |
| Minority interests | | | - | | | (2 | ) | | - | |
| Non-cash stock compensation | | | 3,946 | | | 356 | | | - | |
| Deferred income taxes | | | (36 | ) | | (143 | ) | | (143 | ) |
| Non-cash impairment charge | | | - | | | - | | | 1,656 | |
| Loss on disposal of discontinued operations | | | - | | | 237 | | | 104 | |
| Write-off of patent-related intangible asset | | | 297 | | | - | | | - | |
| Other | | | (96 | ) | | - | | | 22 | |
| Changes in assets and liabilities, excluding effect of business acquisitions: | | | | | | | | | | |
| Accounts receivable | | | 4,152 | | | (4,228 | ) | | (69 | ) |
| Prepaid expenses and other assets | | | (530 | ) | | (643 | ) | | 654 | |
| Accounts payable and accrued expenses | | | 819 | | | (729 | ) | | 712 | |
| Royalties and legal fees payable | | | (2,074 | ) | | 3,758 | | | - | |
| Deferred revenues | | | (279 | ) | | 211 | | | (1,176 | ) |
| | | | | | | | | | | |
| Net cash provided by (used in) operating activities from continuing operations | | | 6,095 | | | (2,477 | ) | | (3,232 | ) |
| Net cash provided by (used in) operating activities from discontinued operations | | | 264 | | | (513 | ) | | (727 | ) |
| Net cash provided by (used in) operating activities | | | 6,359 | | | (2,990 | ) | | (3,959 | ) |
| | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | |
| Purchase of property and equipment | | | (179 | ) | | (75 | ) | | (81 | ) |
| Purchase of available-for-sale investments | | | (16,409 | ) | | (39,919 | ) | | (9,239 | ) |
| Sale of available-for-sale investments | | | 28,147 | | | 33,141 | | | 9,004 | |
| Business acquisition | | | (16 | ) | | (5,796 | ) | | - | |
| Patent acquisition costs | | | (1,030 | ) | | (445 | ) | | - | |
| Other | | | - | | | - | | | (5 | ) |
| | | | | | | | | | | |
| Net cash provided by (used in) investing activities from continued operations | | | 10,513 | | | (13,094 | ) | | (321 | ) |
| Net cash used in investing activities from discontinued operations | | | (353 | ) | | - | | | (198 | ) |
| Net cash provided by (used in) investing activities | | | 10,160 | | | (13,094 | ) | | (519 | ) |
| | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | |
| Net cash flows attributed to the Acacia Technologies group | | | 1,198 | | | 19,657 | | | (305 | ) |
| | | | | | | | | | | |
| Increase (decrease) in cash and cash equivalents | | | 17,717 | | | 3,573 | | | (4,783 | ) |
| | | | | | | | | | | |
| Cash and cash equivalents, beginning | | | 14,498 | | | 10,925 | | | 15,708 | |
| | | | | | | | | | | |
| Cash and cash equivalents, ending | | $ | 32,215 | | $ | 14,498 | | $ | 10,925 | |
The accompanying notes are an integral part of these financial statements.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
Acacia Research Corporation’s continuing operations are comprised of two separate divisions: the Acacia Technologies group and the CombiMatrix group (the “groups”).
The Acacia Technologies group, a division of Acacia Research Corporation, develops, acquires, licenses and enforces patented technologies. The Acacia Technologies group owns and has rights to patent portfolios covering a wide range of technology areas. The Acacia Technologies group is primarily comprised of certain of Acacia Research Corporation’s direct and or indirect wholly owned subsidiaries and limited liability companies including:
· Acacia Global Acquisition Corporation · Acacia Media Technologies Corporation · Acacia Patent Acquisition Corporation · Acacia Technologies Services Corporation · AV Technologies LLC · Broadcast Data Retrieval Corporation · Broadcast Innovation LLC · Computer Acceleration Corporation · Computer Cache Coherency Corporation · Computer Docking Station Corporation · Credit Card Fraud Control Corporation · Database Structures Inc. · Data Encryption Corporation · Data Innovation LLC · Diagnostic Systems Corporation · Disc Link Corporation · Financial Systems Innovation LLC · Fluid Dynamics Corporation · High Resolution Optics Corporation | · Information Technology Innovation LLC · InternetAd LLC · IP Innovation LLC · KY Data Systems LLC · Location Based Services Corporation · Micromesh Technology Corporation · Microprocessor Enhancement Corporation · New Medium LLC · Peer Communications Corporation · Product Activation Corporation · Remote Video Camera Corporation · Resource Scheduling Corporation · Safety Braking Corporation · Screentone Systems Corporation · Soundview Technologies Inc. · Spreadsheet Automation Corporation · TechSearch LLC · Telematics Corporation · VData LLC |
The Acacia Technologies group also includes all corporate assets, liabilities, and related transactions of Acacia Research Corporation attributed to Acacia Research Corporation’s intellectual property licensing and enforcement business.
Business Acquisition. On January 28, 2005, Acacia Global Acquisition Corporation acquired the assets of Global Patent Holdings, LLC, which owned 11 patent licensing companies (“GPH Acquisition”). The acquisition provided the Acacia Technologies group ownership of companies that own or control the rights to 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. Refer to Note 7 for a description of the acquisition transaction and the related accounting treatment.
Liquidity and Risks
Management believes that the Acacia Technologies group’s cash and cash equivalent and short-term investment balances, anticipated cash flow from operations and other external sources of available credit will be sufficient to meet our cash requirements through at least March 2008, and for the foreseeable future.
Acacia Research Corporation’s cash and cash equivalent and short term investment balances, cash flows and anticipated cash flows from operations and other sources of external credit, are attributed to the Acacia Technologies group and the CombiMatrix group based on the respective assets of the specific businesses comprising each group. Issuances of AR-Acacia Technologies stock (and the proceeds thereof) are attributed to the Acacia Technologies group and issuances of AR-CombiMatrix stock (and the proceeds thereof) are
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
attributed to the CombiMatrix group. The cash and cash equivalent balances, anticipated cash flow from operations, and other external sources of available credit of one group are not generally available to the other group. Neither of the groups is obligated to fund the ongoing operations of the other group. Management has no intent to use the cash and cash equivalent balances, anticipated cash flow from operations, and other external sources of available credit of one group to fund the ongoing operations of the other group.
To date, the Acacia Technologies group has relied upon the receipt of license fee payments from the licensing of the Acacia Technologies group’s patented technologies and the selling of Acacia Research Corporation equity securities to generate the funds needed to finance the operations of the Acacia Technologies group. The Acacia Technologies group concluded its V-chip patent licensing program in August 2004, recognizing a total of $25.7 million in license fees over the life of the program. The Acacia Technologies group has been commercially licensing its DMT® technology portfolio since 2003. The GPH Acquisition provided the Acacia Technologies group with ownership of companies that own or control the rights to 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. Subsequent to the GPH Acquisition, the Acacia Technologies group has acquired or acquired the rights to over 30 additional patent portfolios, covering a wide range of technology areas, which it intends to develop, license and enforce.
There can be no assurance that the Acacia Technologies group will be able to implement its future plans. Failure by management to achieve its plans would have a material adverse effect on the Acacia Technologies group and on Acacia Research Corporation’s ability to achieve its intended business objectives. We may be required to obtain additional financing. There can be no assurance that additional funding will be available on favorable terms, if at all. If we fail to obtain additional funding when needed, we may not be able to execute our business plans and our businesses may suffer.
The timing of the receipt of revenues by the Acacia Technologies group’s business operations are subject to certain risks and uncertainties, including:
| | · | market acceptance of our patented technologies and services; |
| | · | business activities and financial results of our licensees; |
| | · | technological advances that may make our patented technologies obsolete or less competitive; |
| | · | increases in operating costs, including costs for legal services, engineering and research and personnel; |
| | · | the availability and cost of capital; and |
| | · | governmental regulation that may restrict the Acacia Technologies group’s business. |
The Acacia Technologies group’s success also depends on its ability to protect its intellectual property. The Acacia Technologies group relies on its proprietary rights and their protection. Although reasonable efforts will be taken to protect the Acacia Technologies group’s proprietary rights, the complexity of international trade secret, copyright, trademark and patent law, and common law, coupled with limited resources and the demands of quick delivery of technologies to market, create risk that these efforts will prove inadequate. Accordingly, if the Acacia Technologies group is unsuccessful with litigation to protect its intellectual property rights, the future revenues of the Acacia Technologies group could be adversely affected.
Recapitalization Transaction
On December 11, 2002, Acacia Research Corporation’s stockholders voted in favor of a recapitalization transaction, which became effective on December 13, 2002, whereby Acacia Research Corporation created two new classes of common stock called Acacia Research-CombiMatrix common stock (“AR-CombiMatrix stock”) and Acacia Research-Acacia Technologies common stock (“AR-Acacia Technologies stock”), and divided the existing Acacia Research Corporation common stock into shares of the two new classes of common stock. AR-CombiMatrix stock is intended to reflect separately the performance of Acacia Research Corporation’s CombiMatrix group. AR-Acacia Technologies stock is intended to reflect separately the performance of Acacia Research Corporation’s Acacia Technologies group. Although the AR-CombiMatrix stock and the AR-Acacia Technologies stock are intended to reflect the performance of the different business groups, they are both classes of common stock of Acacia Research Corporation and are not stock issued by the respective groups.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation. AR-Acacia Technologies stock is intended to reflect the separate performance of the respective division of Acacia Research Corporation. The Acacia Technologies group is not a separate legal entity. Holders of AR-Acacia Technologies stock are stockholders of Acacia Research Corporation. As a result, holders of AR-Acacia Technologies stock are subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. The assets Acacia Research Corporation attributes to Acacia Technologies could be subject to the liabilities of the CombiMatrix group.
The Acacia Technologies group financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America, and taken together with the CombiMatrix group financial statements, comprise all the accounts included in the corresponding consolidated financial statements of Acacia Research Corporation. The financial statements of Acacia Technologies group reflect the financial condition, results of operations, and cash flows of the businesses included therein. The financial statements of the Acacia Technologies group include the accounts or assets of Acacia Research Corporation specifically attributed to the Acacia Technologies group and were prepared using amounts included in Acacia Research Corporation’s consolidated financial statements.
Minority interests represents participation of other stockholders in the allocated net assets and in the division earnings and losses of the Acacia Technologies group and is reflected in the caption minority interests in the Acacia Technologies group financial statements. Minority interests adjust the Acacia Technologies group’s share of the division’s earnings or loss of non-wholly owned subsidiaries of Acacia Research Corporation that have been attributed to the Acacia Technologies group.
Financial effects arising from one group that affect Acacia Research Corporation’s results of operations or financial condition could, if significant, affect the results of operations or financial condition of the other group and the market price of the class of common stock relating to the other group. Any division net losses of the CombiMatrix group or the Acacia Technologies group and dividends or distributions on, or repurchases of, AR-CombiMatrix stock or AR-Acacia Technologies stock or repurchases of preferred stock of Acacia Research Corporation will reduce the assets of Acacia Research Corporation legally available for payment of dividends on AR-CombiMatrix stock or AR-Acacia Technologies stock.
Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein for the Acacia Research Corporation principles of consolidation, management allocation policies, treasury and cash management policies, asset and liability attribution policies, corporate, general and administrative services and facilities allocation policies and federal and state income tax allocation policies, utilized in the preparation of the separate Acacia Technologies group financial statements.
Revenue Recognition. The Acacia Technologies group recognizes revenue in accordance with Staff Accounting Bulletin No. 104, “Revenue Recognition” (“SAB No. 104”) and related authoritative pronouncements. Revenue is recognized when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been performed pursuant to the terms of the license agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
Under the terms of our license agreements, the Acacia Technologies group grants non-exclusive licenses for the use of its patented technologies. In general, pursuant to the terms of our agreements with our licensees, upon the grant of the licenses, the Acacia Technologies group has no further obligations with respect to the licenses granted. License fees paid to and recognized as revenue by the Acacia Technologies group are non-refundable.
Revenues generated from license agreements are generally accrued and recognized as revenue in the period earned, provided that amounts are fixed or determinable and collectibility is reasonably assured.
Certain license agreements provide for the calculation of license fees based on a licensee’s actual quarterly sales or actual per unit activity, applied to a contractual royalty rate. Licensees that pay license fees on a quarterly basis generally report actual quarterly sales or actual per unit activity information and related quarterly license fees due to the Acacia Technologies group within 30 to 45 days after the end of the quarter in which such sales or activity takes place. In general, the amount of license fees due under these license
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
agreements each quarter cannot be reasonably estimated by management. Consequently, the Acacia Technologies group recognizes revenue from these licensing agreements on a three-month lag basis, in the quarter following the quarter of sales or per unit activity, provided amounts are fixed or determinable and collectibility is reasonably assured. The lag method described above allows for the receipt of licensee royalty reports prior to the recognition of revenue.
Certain license agreements provide for the payment of a minimum upfront annual license fee at the inception of each annual license term. Minimum upfront annual license fees are generally determined based on a licensee’s estimated annual sales or a licensee’s base level of per unit activity. These minimum upfront annual license fee payments are deferred and amortized to revenue on a straight-line basis over the annual license term. To the extent actual annual royalties, determined and reported in accordance with the terms of the respective agreements, exceed the minimum upfront annual license fees paid, the additional royalties are recognized in revenue in the quarter following the quarter in which the base per unit activity was exceeded or the quarter following the annual license term, depending on the terms of the respective agreement, provided that amounts are fixed or determinable and collectibility is reasonably assured. Amounts of additional royalties due under these license agreements cannot be reasonably estimated by management.
Certain license agreements provide for the payment of contractually determined paid-up license fees to us in consideration for the grant of a non-exclusive, retroactive and future license to manufacture and/or sell products covered by our patented technologies. Certain of the agreements also provide for future royalties or additional required payments based on future activities. The execution of these license agreements may also result in the dismissal of any pending litigation. Pursuant to the terms of these agreements, the Acacia Technologies group has no further obligation with respect to the grant of the non-exclusive retroactive and future license, including no express or implied obligation on the Acacia Technologies group’s part to maintain or upgrade the technology, or provide future support or services. Generally, the agreements provide for the grant of the license upon execution of the agreement. As such, the earnings process is complete upon the execution of the agreement, and revenue is recognized upon execution of the agreement, when collectibility is reasonably assured, and all other revenue recognition criteria have been met. Refer to Note 10 for information on inventor royalties and contingent legal fees.
License fee payments received by the Acacia Technologies group that do not meet the revenue recognition criteria described above are deferred until the revenue recognition criteria are met. The Acacia Technologies group assesses the collectibility of accrued license fees based on a number of factors, including past transaction history and credit-worthiness. If it is determined that collection is not reasonably assured, the fee is recognized when collectibility becomes reasonably assured, assuming all other revenue recognition criteria have been met, which is generally upon receipt of cash.
Cash and Cash Equivalents. The Acacia Technologies group considers all highly liquid, short-term investments with original maturities of three months or less when purchased to be cash equivalents.
Short-term Investments. The Acacia Technologies group’s short-term investments are held in a variety of interest bearing instruments including U.S. government debt securities, high-grade corporate bonds, commercial paper, auction rate securities, money market accounts, certificates of deposit and other high-credit quality marketable securities. Investments in securities with original maturities of greater than three months and less than one year and other investments representing amounts that are available for current operations are classified as short-term investments. Investments are classified into categories in accordance with the provisions of Statement of Financial Accounting Standards No. 115, “Accounting for Certain Investments in Debt and Equity Securities” (“SFAS No. 115”) and FASB Technical Bulletin 85-4: “Accounting for Purchases of Life Insurance (“FTB 85-4”).” At December 31, 2006 and 2005, all of the Acacia Technologies group’s investments are classified as available-for-sale, which are reported at fair value with related unrealized gains and losses in the value of such securities recorded as a component of allocated net worth until realized.
The fair value of the Acacia Technologies group’s investments is primarily determined by quoted market prices. Realized and unrealized gains and losses are recorded based on the specific identification method. For investments classified as available-for-sale, unrealized losses that are other than temporary are recognized in division net income (loss). An impairment is deemed other than temporary unless (a) the Acacia Technologies group has the ability and intent to hold an investment for a period of time sufficient for recovery of its carrying amount and (b) positive evidence indicating that the investment's carrying amount is recoverable within a reasonable period of time outweighs any evidence to the contrary.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
All available evidence, both positive and negative, is considered to determine whether, based on the weight of that evidence, the carrying amount of the investment is recoverable within a reasonable period of time. In accordance with FTB 85-4, at each balance sheet date, annuity investments are reported at their stated contract value, which is comprised of total amounts invested and cumulative interest and dividends earned.
The cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income (expense). Interest and dividends on all securities are included in interest income.
At December 31, 2006 and 2005, the Acacia Technologies group held $7,288,000 and $10,625,000, respectively, of short-term investments, which consist of auction rate municipal bonds and variable rate municipal demand notes classified as available-for-sale securities. The Acacia Technologies group’s investments in these securities are recorded at cost, which approximates fair market value due to their variable interest rates, which typically reset every 7 to 35 days, and, despite the long-term nature of their stated contractual maturities, the Acacia Technologies group has the ability to quickly liquidate these securities. As a result, there were no cumulative gross unrealized holding gains (losses) or gross realized gains (losses) from current investments. All income generated from these current investments was recorded as interest income.
As of December 31, 2005, we had $1,667,000 of annuity investments classified as current investments as these amounts are available for current operations and are highly liquid, despite the long-term nature of their stated contractual maturities.
Concentration of Credit Risks. Financial instruments that potentially subject the Acacia Technologies group to concentrations of credit risk are cash equivalents and short-term investments. The Acacia Technologies group places its cash equivalents and short-term investments primarily in investment grade, short-term debt instruments. Cash equivalents are invested in deposits with certain financial institutions and may, at times, exceed federally insured limits. The Acacia Technologies group has not experienced any significant losses on its deposits of cash and cash equivalents.
One, three and two licensee(s) individually accounted for greater than 10% of the Acacia Technologies group’s license fee revenues recognized during the years ended December 31, 2006, 2005 and 2004, respectively, and three and two licensees represented approximately 74% and 95% of the Acacia Technologies group’s accounts receivable at December 31, 2006 and 2005, respectively, as follows:
| | Revenue | | Accounts Receivable |
| | 2006 | | 2005 | | 2004 | | 2006 | | 2005 |
| | | | | | | | | | |
Licensee: | | | | | | | | | |
| A................ | 14% | | - | | - | | - | | - |
| B................ | - | | 19% | | - | | - | | 31% |
| C................ | - | | 15% | | - | | - | | - |
| D................ | - | | 15% | | - | | - | | 64% |
| E................. | - | | - | | 11% | | 37% | | - |
| F................. | - | | - | | 35% | | - | | - |
| G................ | - | | - | | - | | 24% | | - |
| H................ | - | | - | | - | | 13% | | - |
Property and Equipment. Property and equipment are recorded at cost. Major additions and improvements that materially extend useful lives of property and equipment are capitalized. Maintenance and repairs are charged against the results of operations as incurred. When these assets are sold or otherwise disposed of, the asset and related depreciation are relieved, and any gain or loss is included in the statement of operations for the period of sale or disposal. Depreciation is computed on a straight-line basis over the following estimated useful lives of the assets:
| Furniture and fixtures | 3 to 5 years |
| Computer hardware and software | 3 to 5 years |
| Leasehold improvements | 2 to 5 years (Lesser of lease term or useful life of improvement) |
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Organization Costs. Costs of start-up activities, including organization costs, are expensed as incurred.
Patents and Goodwill. Goodwill is recorded when the consideration paid for acquisitions exceeds the fair value of the net tangible and identifiable intangible assets acquired. Patents, once issued or purchased, are amortized on the straight-line method over their remaining economic useful lives, ranging from two to nine years. Goodwill is not amortized.
Impairment of Long-lived Assets Goodwill. Long-lived assets and intangible assets are reviewed for potential impairment at least annually and when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. In the event the sum of the expected undiscounted future cash flows resulting from the use of the asset is less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. If an asset is determined to be impaired, the loss is measured based on quoted market prices in active markets, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including a discounted value of estimated future cash flows.
Goodwill is evaluated for impairment in accordance with SFAS No. 142, “Goodwill and Other Intangible Assets” (“SFAS No. 142”) and is subject to a periodic review for potential impairment at a reporting unit level. Reviews for potential impairment must occur at least annually and may be performed earlier, if circumstances indicate that an impairment may have occurred. The Acacia Technologies group has elected to perform its annual tests for indications of goodwill impairment as of December 31 of each year. As of December 31, 2006, the Acacia Technologies group has one reporting unit. The fair value of the Acacia Technologies group reporting unit is estimated based on reference to quoted market prices of Acacia Research Corporations AR-Acacia Technologies stock.
SFAS No. 142 requires the Acacia Technologies group to compare the fair value of its reporting unit to its carrying amount on an annual basis to determine if there is potential goodwill impairment. If the fair value of the reporting unit is less than its carrying value, an impairment loss is recorded to the extent that the fair value of the goodwill within the reporting unit is less than its carrying value. There can be no assurance that future goodwill impairment tests will not result in a charge to earnings.
Fair Value of Financial Instruments. The carrying value of cash and cash equivalents, accounts receivable, accounts payable and accrued expenses approximate fair value due to their short-term maturity.
Foreign Currency Translation. As result of transactions with licensees located in foreign countries, from time to time, the Acacia Technologies group may have certain receivables denominated in foreign currencies. Assets and liabilities recorded in foreign currencies are translated at the exchange rate on the balance sheet date. Translation adjustments resulting from this process are charged or credited to other comprehensive income. Revenue and expenses are translated at average rates of exchange prevailing during the year. Foreign currency transactions gains and losses were insignificant for the periods presented.
Stock-based Compensation. Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
Stock option and related option plan information is omitted from the Acacia Technologies group footnotes because AR-Acacia Technologies stock is part of the capital structure of Acacia Research Corporation. The Acacia Technologies group is not a separate legal entity. Holders of AR-Acacia Technologies stock continue to be stockholders of Acacia Research Corporation. This presentation reflects the fact that the Acacia Technologies group does not have legally issued common or preferred stock, nor are warrant issuances or employee stock transactions legal transactions of the Acacia Technologies group. Refer to the Acacia Research Corporation consolidated financial statements for disclosures regarding Acacia Research Corporation’s stock option plans.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Income Taxes. Income taxes are accounted for using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Acacia Technologies group’s financial statements or tax returns. A valuation allowance is established to reduce deferred tax assets if all, or some portion, of such assets will more than likely not be realized.
Segments. The Acacia Technologies group follows SFAS No. 131, “Disclosure about Segments of an Enterprise and Related Information,” which establishes annual and interim reporting standards for an enterprise’s operating segments and related disclosures about its products, services, geographic areas and major customers. Management has determined that the Acacia Technologies group operates in one segment.
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
Earnings (Loss) Per Share. Earnings (loss) per share information is omitted from the Acacia Technologies group statements of operations because AR-Acacia Technologies stock is part of the capital structure of Acacia Research Corporation. The Acacia Technologies group is not a separate legal entity. Holders of AR-Acacia Technologies stock continue to be stockholders of Acacia Research Corporation. This presentation reflects the fact that the Acacia Technologies group does not have legally issued common or preferred stock, nor are warrant issuances or employee stock transactions legal transactions of the Acacia Technologies group. Refer to the Acacia Research Corporation consolidated financial statements for earnings (loss) per share information for Acacia Research Corporation’s classes of stock.
Recent Accounting Pronouncements. Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
3. SHORT-TERM INVESTMENTS
Short-term investments consist of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | Amortized | | Fair | | Amortized | | Fair | |
| | | Cost | | Value | | Cost | | Value | |
| | | | | | | | | | |
| Available-for-sale securities: | | | | | | | | | |
| Corporate and municipal bonds and notes | | $ | 975 | | $ | 1,000 | | $ | 1,132 | | $ | 1,129 | |
| Auction rate securities and annuity investments | | | 7,288 | | | 7,288 | | | 12,292 | | | 12,292 | |
| U.S. government securities | | | 4,502 | | | 4,495 | | | 9,079 | | | 9,041 | |
| Certificates of deposit | | | - | | | - | | | 2,000 | | | 2,000 | |
| | | $ | 12,765 | | $ | 12,783 | | $ | 24,503 | | $ | 24,462 | |
Gross unrealized gains and losses related to available-for-sale securities were not material for the periods presented. Except for investments in auction rate securities and annuity investments, all investments classified as available-for-sale at December 31, 2006 and 2005 have contractual maturities of one year or less. For auction rate securities and annuity investments, contractual maturity dates range up to forty eight years, with reset dates every 7 to 63 days for auction rate securities and daily liquidity for all funds invested in annuity investments, similar to money market instruments. Refer to Note 2 for more information.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
4. PROPERTY AND EQUIPMENT
Property and equipment consists of the following at December 31, 2006 and 2005 (in thousands):
| | 2006 | | 2005 | |
| | | | | | |
| Furniture and fixtures | | $ | 256 | | $ | 234 | |
| Computer hardware and software | | | 298 | | | 259 | |
| Leasehold improvements | | | 78 | | | 34 | |
| | | | 632 | | | 527 | |
| Less: accumulated depreciation | | | (411 | ) | | (406 | ) |
| | | $ | 221 | | $ | 121 | |
Depreciation expense was $78,000, $59,000 and $49,000 for the years ended December 31, 2006, 2005 and 2004, respectively.
5. BALANCE SHEET COMPONENTS
Accounts payable, accrued expenses and other consists of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Accounts payable | | $ | 150 | | $ | 81 | |
| Payroll and other employee benefits | | | 223 | | | 92 | |
| Accrued vacation | | | 286 | | | 231 | |
| Accrued liabilities of discontinued operations | | | 12 | | | 136 | |
| Accrued legal expenses | | | 1,131 | | | 464 | |
| Accrued consulting and other professional fees | | | 371 | | | 179 | |
| Inventor royalties V-chip | | | - | | | 225 | |
| Other accrued liabilities | | | 28 | | | 33 | |
| | | $ | 2,201 | | $ | 1,441 | |
6. GOODWILL AND INTANGIBLE ASSETS
At December 31, 2006 and 2005, the Acacia Technologies group had $121,000 of goodwill. In August 2004, as a result of the conclusion of the Acacia Technologies group’s V-chip patent infringement lawsuit described at Note 10, the Acacia Technologies group recorded an impairment charge totaling $1,616,000 in connection with the write-down of 100% of the goodwill related to the V-chip.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
The Acacia Technologies group’s only identifiable intangible assets are patents and patent rights, which have remaining economic useful lives up to 7 years. The gross carrying amounts and accumulated amortization related to acquired intangible assets as of December 31, 2006 and 2005 are as follows (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Gross carrying amount - patents | | $ | 30,317 | | $ | 30,392 | |
| Accumulated amortization | | | (11,802 | ) | | (6,606 | ) |
| Patents, net | | $ | 18,515 | | $ | 23,786 | |
The weighted-average remaining estimated economic useful life of the Acacia Technologies group’s patents is 4 years. Aggregate patent amortization expense was $5,313,000, $4,922,000 and $501,000 in 2006, 2005 and 2004, respectively. Annual aggregate amortization expense for each of the next five years through December 31, 2011 is estimated to be $5,237,000 in 2007, $3,914,000 in 2008, $3,463,000 in 2009, $3,272,000 in 2010 and $2,325,000 in 2011.
For the year ended December 31, 2006, the Acacia Technologies group incurred and capitalized patent acquisition costs totaling $1,030,000 in connection with the acquisition of the rights to several additional patent portfolios. The patents have estimated economic useful lives ranging from five to seven years and are being amortized over a weighted-average economic useful life of six years. Refer to Note 7 for additions to patent related intangibles in connection with the GPH Acquisition.
As of March 31, 2006, the Acacia Technologies group reduced its patents and deferred tax liability by $691,000, which were initially recorded in fiscal 2002, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability.
At December 31, 2006 and 2005, all of the Acacia Technologies group’s acquired intangible assets other than goodwill were subject to amortization.
7. ACQUISITIONS
On January 28, 2005, Acacia Global Acquisition Corporation, a wholly owned subsidiary of Acacia Research Corporation, acquired substantially all of the assets of Global Patent Holdings, LLC, a privately held patent holding company based in Northbrook, Illinois, which owned 11 patent licensing companies. The acquisition provided the Acacia Technologies group with 100% ownership of companies that own or control the rights to 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and cover technologies used in a wide variety of industries. As a result of the acquisition, we have expanded and diversified the Acacia Technologies group’s potential revenue generating activities.
The acquisition was accounted for using the purchase method of accounting. Under the purchase method of accounting, the purchase consideration is allocated to the assets acquired, including tangible assets, patents and other identifiable intangibles and liabilities assumed, based on their estimated fair market values at the date of acquisition. The statement of operations includes the results of the acquired companies beginning on January 28, 2005, the date of acquisition. The aggregate purchase consideration was approximately $25,105,000, including $5.0 million of cash, the issuance of 3,938,832 shares of AR-Acacia Technologies stock valued at $19,293,000 (net of estimated common stock registration costs of $228,000) and other acquisition costs, including registration costs, totaling $812,000. The value of the common shares issued was determined based on the average market price of AR-Acacia Technologies stock, as reported on NASDAQ, over the 5-day period (December 13 - December 17, 2004) before and after the terms of the acquisition were agreed to and announced.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
The following table summarizes the total purchase consideration and the allocation of the consideration paid to the estimated fair value of the assets acquired and liabilities assumed (in thousands):
| Purchase Consideration: | | | |
| Cash paid | | $ | 5,000 | |
Fair value of AR-Acacia Technologies stock issued(1) | | | 19,293 | |
| Acquisition and registration costs | | | 812 | |
| Total purchase consideration | | $ | 25,105 | |
Purchase Price Allocation: | | | | |
| Estimated fair value of net tangible assets acquired at January 28, 2005 | | $ | (26 | ) |
Intangible assets acquired - patents and patent rights(1) | | | 25,131 | |
| Total | | $ | 25,105 | |
____________________________________________
(1) Reflects non-cash investing activity.
Management was primarily responsible for determining the fair value of the tangible and identifiable intangible assets acquired and liabilities assumed at the date of acquisition. Management considered a number of factors, including reference to an independent valuation. The patents and patent rights acquired were valued using a discounted cash flow model on a patent portfolio by portfolio basis, which estimated the future net cash flows expected to result from the licensing of each portfolio, taking into account potential infringers of the patents, usage of the underlying technologies, estimated license fee revenues, contingent legal fee arrangements, inventor royalties due to former patent holders, other estimated costs, tax implications and other factors. A discount rate consistent with the risks associated with achieving the estimated net cash flows was used to estimate the present value of future estimated net cash flows. Management’s valuation resulted in an estimated fair value of patent related assets acquired of approximately $27,000,000, resulting in approximately $1,900,000 of excess fair value over the cost of net assets acquired, which has been allocated as a pro rata reduction to the amounts that otherwise would have been assigned to the assets acquired, in accordance with the purchase method of accounting.
Amounts attributable to patents and patent rights acquired are amortized using the straight-line method over the estimated economic useful lives of the underlying patents which range from two to seven years. As of the date of acquisition, the estimated weighted-average useful life of amortizable patent related intangibles acquired is approximately 6 years.
In connection with the acquisition described above, Acacia Global Acquisition Corporation entered into a consulting agreement (which expired in January 2007) with the former CEO of Global Patent Holdings, LLC who as a result of the acquisition transaction, became a shareholder of Acacia Research Corporation. The agreement requires the payment of $2,000,000 in consulting fees over a two-year period, and certain reimbursable consulting related expenses, commencing on the date of acquisition. Marketing, general and administrative expenses for the year ended December 31, 2006 and 2005 include $1,087,000 and $1,009,000, respectively in expenses related to the consulting agreement. Refer to Note 8 to the Acacia Research Corporation consolidated financial statements for a summary of the significant provisions of the consulting agreement.
The acquisition was treated for tax purposes as a taxable asset acquisition and, as such, there were no book/tax basis differences associated with the acquisition. As such, the Acacia Technologies group did not record any deferred income taxes in connection with the application of the purchase method of accounting.
Refer to Note 8 to the Acacia Research Corporation consolidated financial statements for the unaudited pro forma combined results of operations related to the acquisition for the applicable periods presented.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
8. INCOME TAXES
Acacia Technologies group’s allocated provision (benefit) for income taxes consists of the following (in thousands):
| | | 2006 | | 2005 | | 2004 | |
| Current: | | | | | | | |
| U.S. Federal tax | | $ | - | | $ | - | | $ | - | |
| State taxes | | | 76 | | | 8 | | | 4 | |
| | | | 76 | | | 8 | | | 4 | |
| Deferred: | | | | | | | | | | |
| U.S. Federal tax | | | (36 | ) | | (143 | ) | | (143 | ) |
| State taxes | | | - | | | - | | | - | |
| | | | (36 | ) | | (143 | ) | | (143 | ) |
| | | $ | 40 | | $ | (135 | ) | $ | (139 | ) |
The tax effects of temporary differences and carryforwards that give rise to significant portions of deferred assets and liabilities consist of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Deferred tax assets: | | | | | |
| Basis of investments in affiliates | | $ | 28,808 | | $ | 28,808 | |
| Depreciation and amortization | | | 2,292 | | | 1,085 | |
| Deferred revenue | | | 142 | | | 254 | |
| Stock compensation | | | 1,672 | | | 882 | |
| Accrued liabilities and other | | | 659 | | | 659 | |
| Write-off of investments | | | 1,842 | | | 1,842 | |
| Net operating loss and capital loss carryforwards and credits | | | 22,567 | | | 23,443 | |
| Total deferred tax assets | | | 57,982 | | | 56,973 | |
Less: valuation allowance | | | (57,478 | ) | | (56,327 | ) |
| Net deferred tax assets, net of valuation allowance | | | 504 | | | 646 | |
| Deferred tax liabilities: | | | | | | | |
| Intangibles | | | (504 | ) | | (1,372 | ) |
| Net deferred tax liability | | $ | - | | $ | (726 | ) |
As of March 31, 2006, the Acacia Technologies group reduced its patents and deferred tax liability by $691,000, which were initially recorded in fiscal 2002, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability.
A reconciliation of the federal statutory income tax rate and the effective income tax rate is as follows:
| | | 2006 | | 2005 | | 2004 | |
Statutory federal tax rate | | | (34%) | | | (34%) | | | (34%) | |
State income taxes, net of federal tax effect | | | 1% | | | - | | | - | |
Equity compensation | | | 6% | | | - | | | - | |
Non deductible permanent items | | | - | | | 1% | | | - | |
Valuation allowance | | | 27% | | | 31% | | | 32% | |
| | | | - | | | (2%) | | | (2%) | |
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
At December 31, 2006, the Acacia Technologies group has deferred tax assets totaling approximately $57,478,000, which are fully offset by deferred tax liabilities and a full valuation allowance, due to management’s determination that the criteria for recognition have not been met.
At December 31, 2006, the Acacia Technologies group had U.S. federal and state income tax net operating loss carry forwards (“NOLs”), excluding NOLs related to subsidiaries for which Acacia Research Corporation does not file a consolidated return, were approximately $53,727,000 and $54,700,000, expiring between 2010 and 2026 and 2007 and 2016, respectively. In addition, the Acacia Technologies group had tax credit carryforwards of approximately $40,000.
As of December 31, 2006, the aggregate tax NOLs at subsidiaries not consolidated for federal tax purposes are $21,164,000, expiring between 2010 and 2025. However, the use of these NOLs is limited to the separate earnings of the respective subsidiaries. In addition, ownership changes may also restrict the use of NOLs.
Had the Acacia Technologies group filed separate tax returns, the benefit for income taxes and division net loss would not have differed from the amounts reported in the Acacia Technologies group’s statements of operations for the periods presented.
As of December 31, 2006, approximately $9,081,000 of the valuation allowance related to the tax benefits of stock option deductions included in Acacia Research Corporation’s NOLs. At such time as the valuation allowance is released, the benefit will be credited to additional paid-in capital.
9. DISCONTINUED OPERATIONS
In 2005 and 2004, the Acacia Technologies group accrued an additional $237,000 and $104,000 (net of minority interests), respectively, in estimated costs to be incurred in connection with the discontinued operations of Soundbreak.com (originally ceased operations in February 2001). The additional accruals relate primarily to certain noncancellable lease obligations, the inability to sublease the related office space at rates commensurate with our existing obligations and certain lease termination costs. The related lease obligations, which were guaranteed by Acacia Research Corporation, expired in August 2005.
The assets and liabilities of the discontinued operations at December 31, 2006 and 2005 consist primarily of $38,000 and $741,000 of cash and cash equivalents and lease deposits and $44,000 and $144,000 of accounts payable and accrued expenses, respectively.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
10. COMMITMENTS AND CONTINGENCIES
Operating Leases
Acacia Technologies group leases certain office space under various operating lease agreements expiring in 2012. Minimum annual rental commitments for Acacia Technologies group operating leases of continuing operations having initial or remaining noncancellable lease terms in excess of one year are as follows (in thousands):
Year | | | |
| 2007 | | $ | 617 | |
| 2008 | | | 696 | |
| 2009 | | | 724 | |
| 2010 | | | 753 | |
| 2011 | | | 783 | |
| Thereafter | | | 131 | |
| Total minimum lease payments | | $ | 3,704 | |
Rent expense of continuing operations for the years ended December 31, 2006, 2005 and 2004 approximated $565,000, $453,000 and $308,000, respectively.
Inventor Royalties and Contingent Legal Expenses
In connection with the acquisition of certain patents and patent rights, certain companies included in the Acacia Technologies group executed related agreements which grant to the former owners of the respective patents or patent rights, the right to receive inventor royalties based on future net license fee revenues (as defined in the respective agreements) generated by the Acacia Technologies group as a result of licensing the respective patents or patent portfolios. Inventor royalties paid pursuant to the agreements are expensed in the consolidated statement of operations in the period that the related license fee revenues are recognized.
In connection with the Acacia Technologies group’s licensing and enforcement activities, the Acacia Technologies group may retain the services of law firms that specialize in intellectual property licensing and enforcement and patent law. These law firms may be retained on a contingent fee basis in which the law firms are paid on a scaled percentage of any negotiated license fees, settlements or judgments awarded based on how and when the license fees, settlements or judgments are obtained by the Acacia Technologies group. In instances where the Acacia Technologies group does not recover license fees from potential infringers, no contingent legal fees are paid; however, the Acacia Technologies group may be liable for certain out of pocket legal costs incurred pursuant to the underlying legal services agreement. Legal fees advanced by contingent law firms that are required to be paid in the event that no license recoveries are obtained by the Acacia Technologies group are expensed as incurred and included in liabilities in the statement of financial condition.
Patent Enforcement and Other Litigation
Acacia Technologies group is subject to claims, counterclaims and legal actions that arise in the ordinary course of business. Management believes that the ultimate liability with respect to these claims and legal actions, if any, will not have a material effect on the Acacia Technologies group’s financial position, results of operations or cash flows. However, the Acacia Technologies group could be subject to claims and legal actions relating to the CombiMatrix group. Companies comprising the Acacia Technologies group are often required to engage in litigation to enforce their patents and patent rights.
V-Chip Technology
In October 2005, all V-chip related litigation activities were concluded with no material effect on our financial position, results of operations or cash flows. Results for the year ended December 31, 2005 include $225,000 in final V-chip related inventor royalties expense.
As a result of the conclusion of the Acacia Technologies group’s V-chip litigation in August 2004, the Acacia Technologies group recognized $1,500,000 of V-chip related deferred license fee revenues and $668,000 of V-chip related deferred legal costs in the third quarter of 2004.
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
11. RETIREMENT SAVINGS PLANS
The Acacia Technologies group has an employee savings and retirement plan under section 401(k) of the Internal Revenue Code (the “Plan”). The Plan is a defined contribution plan in which eligible employees may elect to have a percentage of their compensation contributed to the Plan, subject to certain guidelines issued by the Internal Revenue Service. The Acacia Technologies group may contribute to the Plan at the discretion of Acacia Research Corporation’s board of directors. There were no contributions made by the Acacia Technologies group during the years ended December 31, 2006, 2005 and 2004.
12. ALLOCATED NET WORTH
The Acacia Technologies group’s statements of allocated net worth present the equity transactions of Acacia Research Corporation, which are attributed to the Acacia Technologies group as “Net assets attributed to the Acacia Technologies group.” This presentation reflects the fact that the Acacia Technologies group does not have legally issued common or preferred stock, nor are warrant issuances or employee stock transactions legal transactions of the Acacia Technologies group. Presented below is a detail of the equity transactions of Acacia Research Corporation which relate to the businesses of the Acacia Technologies group and which therefore comprise the balances reflected in the group’s net assets attributed to Acacia Technologies group (in thousands):
| | | Acacia Technologies Group | |
| | | | |
2004 | | | |
| Allocated corporate charges | | $ | (396 | ) |
| Stock options exercised | | | 90 | |
| Unrealized loss on short-term investments | | | (6 | ) |
| Net assets attributed to the Acacia Technologies group - 2004 | | $ | (312 | ) |
| | | | | |
2005 | | | | |
| Units issued in direct offerings, net of issuance costs | | $ | 19,532 | |
| Stock issued in connection with the GPH Acquisition, net of acquisition costs | | | 19,293 | |
| Allocated corporate charges | | | (179 | ) |
| Stock options exercised | | | 304 | |
| Compensation expense relating to stock options | | | 356 | |
| Unrealized loss on short-term investments | | | (36 | ) |
| Net assets attributed to the Acacia Technologies group - 2005 | | $ | 39,270 | |
| | | | | |
2006 | | | | |
| Allocated corporate charges | | $ | (277 | ) |
| Stock options exercised | | | 1,475 | |
| Compensation expense relating to stock options | | | 3,946 | |
| Unrealized loss on short-term investments | | | 59 | |
| Other | | | (16 | ) |
| Net assets attributed to the Acacia Technologies group - 2006 | | $ | 5,187 | |
ACACIA TECHNOLOGIES GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
In February 2005, Acacia Research Corporation raised gross proceeds of $19,600,000 through the sale of 3,500,000 shares of AR-Acacia Technologies stock at a price of $5.60 per share in a registered direct offering. Net proceeds raised of approximately $19,532,000, which are net of related issuance costs, were attributed to the Acacia Technologies group.
13. SUPPLEMENTAL CASH FLOW INFORMATION
Cash paid by the Acacia Technologies group for income taxes was not material for the periods presented.
Refer to Note 7 for a summary of the Acacia Technologies group’s non cash investing activities related to the GPH Acquisition. Refer to Note 6 for impairment charges, and other non-cash reductions in intangibles during the periods presented.
CombiMatrix Group
(A Division of Acacia Research Corporation)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Acacia Research Corporation:
In our opinion, the financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of CombiMatrix group (a division of Acacia Research Corporation as described in Note 1) at December 31, 2006 and December 31, 2005, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2006, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of Acacia Research Corporation's management; our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming the CombiMatrix group will continue as a going concern. As discussed in Note 1 to the financial statements, the CombiMatrix group has incurred recurring losses from operations and will require additional financing in the foreseeable future that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As discussed in Note 2 to the financial statements, the CombiMatrix group changed the manner in which it accounts for share-based compensation in 2006.
As more fully described in Note 1 to the financial statements, CombiMatrix group is a division of Acacia Research Corporation; accordingly, the financial statements of CombiMatrix group should be read in conjunction with the consolidated financial statements of Acacia Research Corporation.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Seattle, Washington
March 12, 2007
COMBIMATRIX GROUP | |
(A Division of Acacia Research Corporation) | |
BALANCE SHEETS | |
(In thousands) | |
| | | December 31, | | December 31, | |
| | | 2006 | | 2005 | |
| | | | | | |
ASSETS | | | | | |
| | | | | | |
| Current assets: | | | | | | | |
| Cash and cash equivalents | | $ | 7,829 | | $ | 5,666 | |
| Available-for-sale investments | | | 6,513 | | | 14,547 | |
| Accounts receivable | | | 605 | | | 911 | |
| Inventory, prepaid expenses and other assets | | | 605 | | | 709 | |
| | | | | | | | |
| Total current assets | | | 15,552 | | | 21,833 | |
| | | | | | | | |
| Property and equipment, net of accumulated depreciation | | | 1,785 | | | 2,363 | |
| Patents and licenses, net of accumulated amortization | | | 7,292 | | | 7,926 | |
| Goodwill | | | 16,918 | | | 18,859 | |
| Other assets | | | 2,667 | | | 1,560 | |
| | | | | | | | |
| | | $ | 44,214 | | $ | 52,541 | |
| | | | | | | | |
LIABILITIES AND ALLOCATED NET WORTH | | | | | | | |
| | | | | | | | |
| Current liabilities: | | | | | | | |
| Accounts payable, accrued expenses and other | | $ | 2,846 | | $ | 2,483 | |
| Current portion of deferred revenues | | | 365 | | | 165 | |
| Payable to Acacia Technologies group | | | 380 | | | - | |
| | | | | | | | |
| Total current liabilities | | | 3,591 | | | 2,648 | |
| | | | | | | | |
| Deferred income taxes | | | - | | | 1,975 | |
| Deferred revenues, net of current portion | | | 1,076 | | | 1,439 | |
| Warrant liability | | | 6,732 | | | 1,381 | |
| | | | | | | | |
| Total liabilities | | | 11,399 | | | 7,443 | |
| | | | | | | | |
| Minority interests | | | - | | | 4 | |
| | | | | | | | |
| Commitments and contingencies (Note 9) | | | | | | | |
| | | | | | | | |
| Allocated net worth: | | | | | | | |
| | | | | | | | |
| Funds allocated by Acacia Research Corporation | | | 177,404 | | | 169,723 | |
| | | | | | | | |
| Accumulated net losses | | | (144,589 | ) | | (124,629 | ) |
| | | | | | | | |
| Total allocated net worth | | | 32,815 | | | 45,094 | |
| | | | | | | | |
| | | $ | 44,214 | | $ | 52,541 | |
| | | | | | | | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements. |
COMBIMATRIX GROUP |
(A Division of Acacia Research Corporation) |
STATEMENTS OF OPERATIONS |
(In thousands) |
| | | For the Years Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
| Revenues: | | | | | | | | | | |
| Collaboration agreements | | $ | - | | $ | 2,266 | | $ | 17,302 | |
| Government contracts | | | 2,074 | | | 3,849 | | | 1,993 | |
| Products | | | 3,278 | | | 1,765 | | | 230 | |
| Service contracts | | | 388 | | | 153 | | | 116 | |
| | | | | | | | | | | |
| Total revenues | | | 5,740 | | | 8,033 | | | 19,641 | |
| | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | |
| Cost of government contract revenues | | | 1,959 | | | 3,683 | | | 1,874 | |
| Cost of product sales | | | 1,258 | | | 820 | | | 173 | |
| Research and development expenses (including non-cash stock compensation expense of $1,097 for 2006, $0 for 2005 and $91 for 2004) | | | 9,485 | | | 5,783 | | | 5,385 | |
| Marketing, general and administrative expenses (including non-cash stock compensation expense of $1,260 for 2006, ($159) for 2005 and $663 for 2004) | | | 12,707 | | | 9,827 | | | 9,902 | |
| Goodwill impairment charge | | | - | | | 565 | | | - | |
| Amortization of patents and royalties | | | 1,482 | | | 1,312 | | | 1,234 | |
| Legal settlement charges (credits) | | | - | | | (406 | ) | | 812 | |
| Loss from equity investment | | | 1,036 | | | 352 | | | 17 | |
| | | | | | | | | | | |
| Total operating expenses | | | 27,927 | | | 21,936 | | | 19,397 | |
| | | | | | | | | | | |
| Operating income (loss) | | | (22,187 | ) | | (13,903 | ) | | 244 | |
| | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | |
| Interest income | | | 523 | | | 523 | | | 330 | |
| Loss on sale of interest in subsidiary | | | (84 | ) | | - | | | - | |
| Warrant (charges) credits | | | 1,754 | | | 812 | | | - | |
| | | | | | | | | | | |
| Total other income | | | 2,193 | | | 1,335 | | | 330 | |
| | | | | | | | | | | |
| Income (loss) from operations before income taxes | | | (19,994 | ) | | (12,568 | ) | | 574 | |
| | | | | | | | | | | |
| Benefit for income taxes | | | 34 | | | 167 | | | 136 | |
| | | | | | | | | | | |
| Division net income (loss) | | $ | (19,960 | ) | $ | (12,401 | ) | $ | 710 | |
The accompanying notes are an integral part of these financial statements.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
STATEMENTS OF ALLOCATED NET WORTH
(In thousands)
| Balance at December 31, 2003 | | $ | 25,737 | |
Net assets attributed to the CombiMatrix group | | | 20,381 | |
| Division net loss | | | 710 | |
| | | | | |
| Balance at December 31, 2004 | | | 46,828 | |
Net assets attributed to the CombiMatrix group | | | 10,667 | |
| Division net income | | | (12,401 | ) |
| | | | | |
| Balance at December 31, 2005 | | | 45,094 | |
Net assets attributed to the CombiMatrix group | | | 7,681 | |
| Division net loss | | | (19,960 | ) |
Balance at December 31, 2006 | | $ | 32,815 | |
The accompanying notes are an integral part of these financial statements.
COMBIMATRIX GROUP |
(A Division of Acacia Research Corporation) |
STATEMENTS OF CASH FLOWS |
(In thousands) |
| | | For the Years Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
| Cash flows from operating activities: | | | | | | | | | | |
| Division net income (loss) from operations | | $ | (19,960 | ) | $ | (12,401 | ) | $ | 710 | |
| Adjustments to reconcile division net income (loss) from operations | | | | | | | | | | |
| to net cash used in operating activities: | | | | | | | | | | |
| Depreciation and amortization | | | 2,025 | | | 2,183 | | | 2,200 | |
| Minority interests | | | - | | | - | | | - | |
| Non-cash stock compensation | | | 2,357 | | | (159 | ) | | 754 | |
| Deferred tax benefit | | | (34 | ) | | (137 | ) | | (136 | ) |
| Non-cash warrant charges (credits) | | | (1,754 | ) | | (812 | ) | | - | |
| Non-cash legal settlement charges (credits) | | | - | | | (406 | ) | | 812 | |
| Non-cash impairment charge | | | - | | | 565 | | | - | |
| Loss from equity investments | | | 1,036 | | | 352 | | | 17 | |
| Loss on sale of interest in subsidiary | | | 84 | | | - | | | - | |
| Stock issued to consultant | | | 94 | | | - | | | - | |
| Other | | | 243 | | | (79 | ) | | 43 | |
| Changes in assets and liabilities: | | | | | | | | | | |
| Accounts receivable | | | 288 | | | (568 | ) | | (154 | ) |
| Inventory, prepaid expenses and other assets | | | 104 | | | (180 | ) | | 135 | |
| Accounts payable, accrued expenses and other | | | 483 | | | 301 | | | 481 | |
| Deferred revenues | | | (111 | ) | | (2,355 | ) | | (16,446 | ) |
| | | | | | | | | | | |
| Net cash used in operating activities | | | (15,145 | ) | | (13,696 | ) | | (11,584 | ) |
| | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | |
| Purchase of property and equipment | | | (536 | ) | | (1,325 | ) | | (810 | ) |
| Purchase of available-for-sale investments | | | (5,537 | ) | | (36,771 | ) | | (50,143 | ) |
| Sale of available-for-sale investments | | | 13,573 | | | 43,086 | | | 42,755 | |
| Purchase of additional interests in equity method investee | | | (2,150 | ) | | (1,600 | ) | | (250 | ) |
| Sale of interest in subsidiary (net of cash disposed) | | | (369 | ) | | - | | | - | |
| | | | | | | | | | | |
| Net cash provided by (used in) investing activities | | | 4,981 | | | 3,390 | | | (8,448 | ) |
| | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | |
| Net cash flows attributed to the CombiMatrix group | | | 12,327 | | | 12,914 | | | 19,227 | |
| | | | | | | | | | | |
| Effect of exchange rate on cash | | | - | | | 73 | | | (17 | ) |
| | | | | | | | | | | |
| Increase (decrease) in cash and cash equivalents | | | 2,163 | | | 2,681 | | | (822 | ) |
| | | | | | | | | | | |
| Cash and cash equivalents, beginning | | | 5,666 | | | 2,985 | | | 3,807 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Cash and cash equivalents, ending | | $ | 7,829 | | $ | 5,666 | | $ | 2,985 | |
The accompanying notes are an integral part of these financial statements.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
Acacia Research Corporation is comprised of two separate divisions: the CombiMatrix group and the Acacia Technologies group (the “groups”).
Our life sciences business, referred to as the “CombiMatrix group,” a division of Acacia Research Corporation, is comprised of our wholly owned subsidiary, CombiMatrix Corporation and CombiMatrix Corporation’s wholly owned subsidiary, CombiMatrix Molecular Diagnostics, Inc. (“CMDX”) and includes all corporate assets, liabilities and transactions related to Acacia Research Corporation’s life sciences business.
The CombiMatrix group develops proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where its products and services could be utilized. The technologies that the CombiMatrix group has developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. The CombiMatrix group has also developed the capabilities of producing arrays that utilize bacterial artificial chromosomes on its arrays, also enabling genetic analysis. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs.
CombiMatrix Molecular Diagnostics, Inc., a wholly owned subsidiary located in Irvine, California, is exploring opportunities for the CombiMatrix group’s arrays in the field of molecular diagnostics. CombiMatrix K.K., a Japanese corporation located in Tokyo, Japan, has existed for the purpose of exploring opportunities for CombiMatrix Corporation’s array system with pharmaceutical and biotechnology companies in the Asian market. In January 2006, CombiMatrix Corporation sold 67% of its ownership interest in CombiMatrix K.K. to a third party. Refer to Note 6.
In January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, to become an independent public company. The transaction is expected to be completed no sooner than the second quarter of 2007, subject, however, to completing the required filings with the Securities and Exchange Commission (“SEC”). We have received a private letter ruling from the IRS addressing certain tax implications of the transaction and have received a tax opinion from counsel. CombiMatrix Corporation filed a registration statement on Form S-1 on December 26, 2006, which has not been declared effective. If the registration on Form S-1 is declared effective, Acacia Research Corporation will redeem all of the issued and outstanding shares of AR-CombiMatrix common stock for all of the common stock of CombiMatrix Corporation, which will register its common stock under the Securities and Exchange Act of 1934. Following the redemption, CombiMatrix Corporation will apply to list its shares for trading on a national exchange.
Liquidity and Risks
The CombiMatrix group has a history of incurring net losses and net operating cash flow deficits. The CombiMatrix group is also deploying new and unproven technologies and continues to develop commercial products. The CombiMatrix group has several ongoing long-term development projects that involve experimental technology and may require several years and substantial expenditures to complete. Management believes that the CombiMatrix group’s cash and cash equivalent and short-term investment balances, anticipated cash flows from operations and other external sources of available credit will be sufficient to meet the CombiMatrix group’s cash requirements through December 31, 2007. In order for the CombiMatrix group to continue as a going concern beyond this point, management will be required to obtain capital from external sources. However, there can be no assurances that additional sources of financing, including the issuance of debt and/or equity securities will be available at times and at terms acceptable to management. The issuance of equity securities will also cause dilution to Acacia Research Corporation’s shareholders. If external financing sources of financing are not available or are inadequate to fund the CombiMatrix
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
group’s operations, management will be required to reduce operating costs including research projects and personnel, which could jeopardize the future strategic initiatives and business plans of the CombiMatrix group. For example, reductions in research and development activities and/or personnel at the CombiMatrix group’s Mukilteo, Washington facility could result in the inability to invest the resources necessary to continue to develop next-generation products and improve existing product lines in order to remain competitive in the marketplace, resulting in reduced revenues and cash flows from the sales of the CombiMatrix group’s CustomArray products and services. Also, reduction in operating costs at the CombiMatrix group’s diagnostics subsidiary, CMDX, in Irvine, California, should they occur, could jeopardize its ability to launch, market and sell additional products and services necessary in order to grow and sustain its operations and eventually achieve profitability.
The CombiMatrix group’s business operations are also subject to certain risks and uncertainties, including:
| | · | market acceptance of products and services; |
| | · | technological advances that may make the CombiMatrix group’s products and services obsolete or less competitive; |
| | · | increases in operating costs, including costs for supplies, personnel and equipment; |
| | · | the availability and cost of capital; |
| | · | general economic conditions; and |
| | · | governmental regulation that may restrict the CombiMatrix group’s business. |
Historically, the CombiMatrix group has been substantially dependent on arrangements with strategic partners and have relied upon payments by the CombiMatrix group’s partners for a significant component of its working capital. The CombiMatrix group intends to enter into additional strategic partnerships to develop and commercialize future products. However, there can be no assurance that the CombiMatrix group will be able to implement its future plans. Failure to achieve its plans would have a material adverse effect on the CombiMatrix group’s ability to achieve its intended business objectives. The CombiMatrix group’s success also depends on its ability to protect its intellectual property, the loss thereof or the failure to secure the issuance of additional patents covering elements of the CombiMatrix group’s business processes could materially harm its business and financial condition. The patents covering the CombiMatrix group’s core technology begin to expire in 2018.
The CombiMatrix group’s products and services are concentrated in a highly competitive market that is characterized by rapid technological advances, frequent changes in customer requirements and evolving regulatory requirements and industry standards. Failure to anticipate or respond adequately to technological advances, changes in customer requirements, changes in regulatory requirements or industry standards, or any significant delays in the development or introduction of planned products or services, could have a material adverse effect on the CombiMatrix group’s business and operating results.
The accompanying financial statements have been prepared assuming that the CombiMatrix group continues as a going concern. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the matters discussed herein.
Based on cash and cash equivalents and short-term investment balances held at December 31, 2006, CombiMatrix group management anticipates that the CombiMatrix group’s cash and cash equivalent and short-term investment balances, anticipated cash flows from operations and other sources of funding from the capital markets will be sufficient to meet its cash requirements through December 31, 2007. In order for the CombiMatrix group to continue as a going concern beyond this point and ultimately to achieve profitability, the CombiMatrix group will be required to obtain capital from external sources, increase revenues and reduce operating costs. However, there can be no assurance that such capital will be available at times and at terms acceptable to the CombiMatrix group, or that higher levels of product and service revenues will be achieved. The issuance of additional equity securities will also cause dilution to our shareholders. If external financing sources of financing are not available or are inadequate to fund the CombiMatrix group’s operations, the CombiMatrix group will be required to reduce operating costs including research projects and personnel, which could jeopardize the future strategic initiatives and business plans of the CombiMatrix group. The anticipation that the CombiMatrix group will be required to obtain additional financing in the foreseeable future raises substantial doubt about the CombiMatrix group’s ability to continue as a going concern beyond December 31, 2007. In addition to seeking additional capital from outside sources, the CombiMatrix group’s plans in regard to these matters include reductions in personnel and fixed overhead costs (i.e., see CombiMatrix group lease amendment and related lease commitment reduction discussed at Note 9) made in late 2006 and early 2007. Also, the CombiMatrix group is focusing its sales and product development efforts on its core diagnostic array platform as well as its funded research and development projects for the Department of Defense (“DoD”).
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Recapitalization Transaction
On December 11, 2002, Acacia Research Corporation’s stockholders voted in favor of a recapitalization transaction, which became effective on December 13, 2002, whereby Acacia Research Corporation created two new classes of common stock called Acacia Research-CombiMatrix common stock (“AR-CombiMatrix stock”) and Acacia Research-Acacia Technologies common stock (“AR-Acacia Technologies stock”), and divided its then existing Acacia Research Corporation common stock into shares of the two new classes of common stock. AR-CombiMatrix stock is intended to reflect separately the performance of Acacia Research Corporation’s CombiMatrix group. AR-Acacia Technologies stock is intended to reflect separately the performance of Acacia Research Corporation’s Acacia Technologies group. Although the AR-CombiMatrix stock and the AR-Acacia Technologies stock are intended to reflect the performance of Acacia Research Corporation’s different business groups, they are both classes of common stock of Acacia Research Corporation and are not stock issued by the respective groups.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation. AR-CombiMatrix stock is intended to reflect the separate performance of the respective division of Acacia Research Corporation. The CombiMatrix group is not a separate legal entity. Holders of AR-CombiMatrix stock are stockholders of Acacia Research Corporation. As a result, holders of AR-CombiMatrix stock are subject to all of the risks of an investment in Acacia Research Corporation and all of its businesses, assets and liabilities. The assets Acacia Research Corporation attributes to the CombiMatrix group could be subject to the liabilities of the Acacia Technologies group.
The CombiMatrix group financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America, and taken together with the Acacia Technologies group financial statements, comprise all the accounts included in the corresponding consolidated financial statements of Acacia Research Corporation. The financial statements of CombiMatrix group reflect the financial condition, results of operations, and cash flows of the businesses included therein. The financial statements of the CombiMatrix group include the accounts or assets of Acacia Research Corporation specifically attributed to the CombiMatrix group and were prepared using amounts included in Acacia Research Corporation’s consolidated financial statements.
Minority interests represent participation of other stockholders in the allocated net assets and in the division earnings and losses of the CombiMatrix group and are reflected in the caption minority interests in CombiMatrix group’s financial statements. Minority interests adjust CombiMatrix group’s net results of operations to reflect only CombiMatrix group’s share of the division earnings or losses of non-wholly owned investees of Acacia Research Corporation that have been attributed to the CombiMatrix group.
Financial effects arising from one group that affect Acacia Research Corporation’s results of operations or financial condition could, if significant, affect the results of operations or financial condition of the other group and the market price of the class of common stock relating to the other group. Any division net losses of the CombiMatrix group or the Acacia Technologies group and dividends or distributions on, or repurchases of, AR-CombiMatrix stock or AR-Acacia Technologies stock or repurchases of preferred stock of Acacia Research Corporation will reduce the assets of Acacia Research Corporation legally available for payment of dividends on AR-CombiMatrix stock or AR-Acacia Technologies stock.
Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein for the Acacia Research Corporation principles of consolidation, the management allocation policies, treasury and cash management policies, asset and liability attribution policies, corporate, general and administrative services and facilities allocation policies and federal and state income tax allocation policies, utilized in the preparation of the separate CombiMatrix group financial statements.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Revenue Recognition. The CombiMatrix group recognizes revenue in accordance with Staff Accounting Bulletin No. 104, “Revenue Recognition” (“SAB No. 104”) and related authoritative pronouncements. Revenues from multiple-element arrangements are accounted for in accordance with Emerging Issues Task Force (“EITF”) Issue 00-21, “Revenue Arrangements with Multiple Deliverables.” Revenue is recognized when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been performed pursuant to the terms of the license agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
The CombiMatrix group accounts for revenues under multiple-element arrangements in accordance with SAB No. 104 and Emerging Issues Task Force Consensus (“EITF”), Issue 00-21, "Revenue Arrangements with Multiple Deliverables," and related pronouncements. Arrangements with multiple elements or deliverables must be segmented into individual units of accounting based on the separate deliverables only if there is objective and verifiable evidence of fair value to allocate the consideration received to the deliverables. Accordingly, revenues from multiple-element arrangements involving license fees, up-front payments and milestone payments, which are received and/or billable in connection with other rights and services that represent the CombiMatrix group’s continuing obligations are deferred until all of the multiple elements have been delivered or until objective and verifiable evidence of the fair value of the undelivered elements has been established. Upon establishing objective and verifiable evidence of the fair value of the elements in multiple-element arrangements, the fair value is allocated to each element of the arrangement, such as license fees or research and development projects, based on the relative fair values of the elements. The CombiMatrix group determines the fair value of each element in multiple-element arrangements based on objective and verifiable evidence of fair value, which is determined for each element based on the prices charged when the similar elements are sold separately to third parties. If objective and verifiable evidence of fair value of all undelivered elements exists but objective and verifiable evidence of fair value does not exist for one or more delivered elements, then revenue is recognized using the residual method. Under the residual method, the revenues from delivered elements are not recognized until the fair value of the undelivered element or elements have been determined.
Historically, the CombiMatrix group’s multiple-element arrangements have arisen from executing research and development agreements with various strategic partners including Roche Diagnostics, GmbH (“Roche”), Toppan Printing Ltd. (“Toppan”) and Furuno Electric Co. (“Furuno”). The CombiMatrix group entered into development agreements with these partners to perform certain research and development activities, which provided for payments to the CombiMatrix group as various development milestones were achieved. While these agreements typically included several elements of performance, the agreements have been accounted for as single elements of accounting under EITF Issue No. 00-21 due to the lack of verifiable, objective evidence of fair value for undelivered elements in the agreements at the time that up-front or milestone payments were received by the CombiMatrix group. As a result, payments from the CombiMatrix group’s partners were recorded as deferred revenues and were not recognized as revenues until all of the undelivered elements had been completed.
Revenues from government grants and contracts are recognized in accordance with Accounting Research Bulletin (“ARB”) No. 43, “Government Contracts,” and related pronouncements, such as Statement of Position 81-1, “Accounting for Performance of Construction-Type and Certain Production-Type Contracts.” Accordingly, revenues are recognized under the percentage-of-completion method of accounting, using the cost-to-cost approach to measure completeness at each reporting period. Under the percentage-of-completion method of accounting, contract revenues and expenses are recognized in the period that work is performed based on the percentage of actual incurred costs to total contract costs. Actual contract costs include direct charges for labor and materials and indirect charges for labor, overhead and certain general and administrative charges. Contract change orders and claims are included when they can be reliably estimated and are considered probable. For contracts that extend over a one-year period, revisions in contract cost estimates, if they occur, have the effect of adjusting current period earnings applicable to performance in prior periods. Should current contract estimates indicate an overall future loss to be incurred, a provision is made for the total anticipated loss in the current period.
Revenue from the sale of products and services, including shipping and handling fees, are recognized when delivery has occurred or services have been rendered. The CombiMatrix group sells its products and services directly to customers and also through distributors, and the right to collection is not dependent upon installation or a subsequent sale of the CombiMatrix group products to end users. The CombiMatrix group’s agreements do not provide for credits, returns or exchanges with its customers or distributors. The CombiMatrix group’s distribution agreements include fixed pricing arrangements for its products and after customer acceptance, there is no written or implied right to return or exchange the products.
Deferred revenues arise from payments received in advance of the culmination of the earnings process. Deferred revenues expected to be recognized within the next twelve months are classified within current liabilities. Deferred revenues will be recognized as revenue in future periods when the applicable revenue recognition criteria, as described above, are met.
Cash and Cash Equivalents. The CombiMatrix group considers all highly liquid, short-term investments with original maturities of three months or less when purchased to be cash equivalents.
Short-term Investments. The CombiMatrix group’s short-term investments are held in a variety of interest bearing instruments including high-grade corporate bonds, auction rate securities, money market accounts and other high-credit quality marketable securities. Investments in securities with original maturities of greater than three months and less than one year and other investments representing amounts that are available for current operations are classified as short-term investments. Investments are classified in accordance with
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
the provisions of Statement of Financial Accounting Standards No. 115, “Accounting for Certain Investments in Debt and Equity Securities” (“SFAS No. 115”). Investments are classified as available-for-sale, which are reported at fair value with related unrealized gains and losses in the value of such securities recorded as a component of allocated net worth until realized.
The fair value of the CombiMatrix group’s investments is determined by quoted market prices. Realized and unrealized gains and losses are recorded based on the specific identification method. For investments classified as available-for-sale, unrealized losses that are other than temporary are recognized in division net loss. An impairment is deemed other than temporary unless (a) the CombiMatrix group has the ability and intent to hold an investment for a period of time sufficient for recovery of its carrying amount and (b) positive evidence indicating that the investment's carrying amount is recoverable within a reasonable period of time outweighs any evidence to the contrary. All available evidence, both positive and negative, is considered to determine whether, based on the weight of that evidence, the carrying amount of the investment is recoverable within a reasonable period of time.
The cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income (expense). Interest and dividends on all securities are included in interest income.
At December 31, 2006 and 2005, we held $3,527,000 and $8,479,000, respectively, of short-term investments consisting of auction rate securities classified as available-for-sale. Our investments in these securities are recorded at fair market value. Despite the long-term nature of their stated contractual maturities, we have the ability to quickly liquidate these securities and as a result, we had no cumulative gross unrealized holding gains (losses) or gross realized gains (losses) from these investments. All income generated from these investments was recorded as interest income.
Concentration of Credit Risks. Financial instruments that potentially subject the CombiMatrix group to concentrations of credit risk are cash equivalents and short-term investments. The CombiMatrix group places its cash equivalents and short-term investments primarily in investment grade, short-term debt instruments. Cash equivalents are also invested in deposits with certain financial institutions and may, at times, exceed federally insured limits. The CombiMatrix group has not experienced any significant losses on our deposits of cash and cash equivalents.
Collaboration agreement revenues recognized by the CombiMatrix group for the years ended December 31, 2005 and 2004 relate to its collaborative research and development agreements with Toppan and Roche Diagnostics, respectively. Government contract revenues recognized by the CombiMatrix group for all periods presented relate to our ongoing contracts with the Department of Defense regarding our electrochemical and microfluidics technologies. At December 31, 2006 and 2005, accounts receivable included $85,000 and $537,000, respectively, due from the Department of Defense. For the years ended December 31, 2005 and 2004, 18% and 45%, respectively, of the CombiMatrix group’s array product and service revenues were recognized by CombiMatrix K.K. Receivables from the Department of Defense totaled 14% of accounts receivable at December 31, 2006, and another customer represented approximately 59% of accounts receivable at December 31, 2006. At December 31, 2005, 59% of accounts receivable was due from the Department of Defense, 24% of our accounts receivable was due from one customer and 10% from another customer.
The CombiMatrix group performs regular credit evaluations of its significant licensees and customers and has not experienced any significant credit losses.
Substantially all of the components and raw materials used in the manufacture of the CombiMatrix group’s products, including semiconductors and reagents, are currently procured from a limited number of sources or in some cases from a single source. Although the CombiMatrix group believes that alternative sources for those components and raw materials are available, any supply interruption in a sole-sourced component or raw material might result in up to a several-month production delay and materially harm the CombiMatrix group’s ability to manufacture products until a new source of supply, if any, could be located and qualified. The CombiMatrix group utilizes non-standard semiconductor manufacturing processes to fabricate the electrode array that is a key aspect of the array structure. Although the CombiMatrix group has a supply agreement in place with a semiconductor wafer manufacturer to ensure availability of the raw materials, the agreement does not guarantee a permanent supply.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Inventory. Inventory, which consists primarily of raw materials to be used in the production of the CombiMatrix group’s array products, is stated at the lower of cost or market using the first-in, first-out method.
Property and Equipment. Property and equipment is recorded at cost. Additions and improvements that increase the value or extend the life of an asset are capitalized. Maintenance and repairs are expensed as incurred. Disposals are removed at cost less accumulated depreciation or amortization and any gain or loss from disposition is reflected in the statement of operations in the period of disposition. Depreciation is computed on a straight-line basis over the following estimated useful lives of the assets:
| Machine shop and laboratory equipment | 3 to 5 years |
| Furniture and fixtures | 5 to 7 years |
| Computer hardware and software | 3 years |
| Leasehold improvements | 2 to 8 years (Lesser of lease term or useful life of improvement) |
Construction in progress includes direct costs incurred related to internally constructed assets which are depreciated once the asset is placed into service. Certain leasehold improvements, furniture and equipment held under capital leases are classified as property and equipment and are amortized over their useful lives using the straight-line method. Capital lease amortization is included in depreciation expense.
Organization Costs. Costs of start-up activities, including organization costs, are expensed as incurred.
Patents and Goodwill. Goodwill is recorded when the consideration paid for acquisitions exceeds the fair value of the net tangible assets acquired. Patents, once issued or purchased, are amortized on the straight-line method over their remaining economic useful lives, ranging from seven to twenty years. Goodwill is not amortized.
Impairment of Long-Lived Assets and Goodwill. Long-lived assets and intangible assets are reviewed for potential impairment at least annually and when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. In the event the sum of the expected undiscounted future cash flows resulting from the use of the asset is less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. If an asset is determined to be impaired, the loss is measured based on quoted market prices in active markets, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including a discounted value of estimated future cash flows.
Goodwill is evaluated annually for impairment in accordance with SFAS No. 142, "Goodwill and Other Intangible Assets" (SFAS No. 142) at the reporting unit level, or earlier if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. A reporting unit can be an operating segment or a business if discrete financial information is prepared and reviewed by management. Prior to the fourth quarter of 2005, the CombiMatrix group had recognized goodwill in the amounts of $18,859,000, $172,000 and $393,000 at the group’s three reporting units; CombiMatrix Corporation and two wholly owned subsidiaries, Advanced Materials Sciences (“AMS”) and CombiMatrix K.K., respectively. Under the impairment test, if a reporting unit’s carrying amount exceeds its estimated fair value, goodwill impairment is recognized to the extent that the reporting unit’s carrying amount of goodwill exceeds the impaired fair value of the goodwill. In accordance with this policy and as more fully disclosed in Note 7 below, the CombiMatrix group recognized a goodwill impairment charge of $565,000 for the year ended December 31, 2005, representing all of the goodwill associated with AMS and CombiMatrix K.K. As a result, the CombiMatrix group only has one reporting unit as of December 31, 2006. The fair value of the CombiMatrix Corporation reporting unit for 2006 was determined using existing market prices for AR-CombiMatrix stock and no impairment was indicated at December 31, 2006. There can be no assurance that future goodwill impairment tests will not result in additional impaired charges.
Fair Value of Financial Instruments. The carrying value of cash and cash equivalents, accounts receivables, accounts payable and accrued expenses approximate fair value due to their short-term maturity.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Foreign Currency Translation. The functional currency of CombiMatrix K.K. is the local currency (Japanese Yen). Foreign currency translation is reported pursuant to SFAS No. 52, “Foreign Currency Translation” (“SFAS No. 52”). Assets and liabilities recorded in foreign currencies are translated at the exchange rate on the balance sheet date. Translation adjustments resulting from this process are charged or credited to allocated net worth. Revenue and expenses are translated at average rates of exchange prevailing during the year. Foreign currency transactions gains and losses were insignificant for the periods presented. Refer to Note 12.
Stock-based Compensation. Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
Stock option and related option plan information is omitted from the CombiMatrix group footnotes because AR-CombiMatrix stock is part of the capital structure of Acacia Research Corporation. The CombiMatrix group is not a separate legal entity. Holders of AR-CombiMatrix stock continue to be stockholders of Acacia Research Corporation. This presentation reflects the fact that the CombiMatrix group does not have legally issued common or preferred stock, nor are warrant issuances or employee stock transactions legal transactions of the CombiMatrix group. Refer to the Acacia Research Corporation consolidated financial statements for disclosures regarding Acacia Research Corporation’s stock option plans.
Research and Development Expenses. Research and development expenses consist of costs incurred for direct and overhead-related research expenses and are expensed as incurred. Costs to acquire technologies which are utilized in research and development and which have no alternative future use are expensed when incurred. Software developed for use in the CombiMatrix group’s products is expensed as incurred until both (i) technological feasibility for the software has been established and (ii) all research and development activities for the other components of the system have been completed. Management believes these criteria are met after the CombiMatrix group has received evaluations from third-party test sites and completed any resulting modifications to the products. Expenditures to date have been classified as research and development expense.
Advertising. Costs associated with the marketing and advertising of the CombiMatrix group’s products and services are expensed as incurred. For the years ended December 31, 2006, 2005 and 2004, marketing and advertising expenses incurred by the CombiMatrix group were $253,000, $516,000 and $314,000, respectively.
Income Taxes. Income taxes are accounted for using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the CombiMatrix group’s financial statements or tax returns. A valuation allowance is established to reduce deferred tax assets if all, or some portion, of such assets will more than likely not be realized.
Segments. The CombiMatrix group follows SFAS No. 131, “Disclosure about Segments of an Enterprise and Related Information” (“SFAS No. 131”), which establishes annual and interim reporting standards for an enterprise’s operating segments and related disclosures about its products, services, geographic areas and major customers. Management has determined that the CombiMatrix group operates in one segment.
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
Earnings per Share. Earnings per share information is omitted from the CombiMatrix group statements of operations because AR-CombiMatrix stock is part of the capital structure of Acacia Research Corporation. The CombiMatrix group is not a separate legal entity. Holders of AR-CombiMatrix stock continue to be stockholders of Acacia Research Corporation. This presentation reflects the fact that the CombiMatrix group does not have legally issued common or preferred stock, nor are warrant issuances or employee stock transactions legal transactions of the CombiMatrix group. Refer to the Acacia Research Corporation consolidated financial statements for earnings per share information for Acacia Research Corporation’s classes of stock.
Recent Accounting Pronouncements. Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
3. SHORT-TERM INVESTMENTS
Short-term investments consist of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | Amortized | | Fair | | Amortized | | Fair | |
| | | Cost | | Value | | Cost | | Value | |
| | | | | | | | | | |
| Available-for-sale securities: | | | | | | | | | |
| Corporate bonds and notes | | $ | 1,990 | | $ | 1,980 | | $ | 3,726 | | $ | 3,717 | |
| Auction rate securities | | | 3,527 | | | 3,527 | | | 8,480 | | | 8,479 | |
| U.S. government securities | | | 1,011 | | | 1,006 | | | 2,358 | | | 2,351 | |
| | | $ | 6,528 | | $ | 6,513 | | $ | 14,564 | | $ | 14,547 | |
Gross unrealized gains and losses related to available-for-sale securities were not material for the periods presented. At December 31, 2006, the cost and fair market value of securities with contractual maturities of greater than one year, other than auction market securities, was $2,486,000. At December 31, 2005, the cost and fair market value of securities with contractual maturities of greater than one year, other than auction market securities, was $1,254,000 and $1,251,000, respectively. As disclosed in Note 2, auction rate securities, which have no stated maturities, are classified as short-term available-for-sale securities due to the CombiMatrix group’s ability to quickly liquidate these securities as their variable rates reset, typically every 7 to 35 days.
4. PROPERTY AND EQUIPMENT
Property and equipment consists of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Machine shop and laboratory equipment | | $ | 4,322 | | $ | 4,931 | |
| Furniture and fixtures | | | 130 | | | 173 | |
| Computer hardware and software | | | 670 | | | 983 | |
| Leasehold improvements | | | 1,039 | | | 1,027 | |
| Construction in progress | | | - | | | 17 | |
| | | | 6,161 | | | 7,131 | |
| Less: accumulated depreciation and amortization | | | (4,376 | ) | | (4,768 | ) |
| | | $ | 1,785 | | $ | 2,363 | |
Depreciation and amortization expense was $892,000, $1,088,000 and $1,105,000 for the years ended December 31, 2006, 2005 and 2004. Fully depreciated assets totaling $663,000 were written off in 2004.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
5. BALANCE SHEET COMPONENTS
Accounts payable, accrued expenses and other consists of the following at December 31, 2006 and December 31, 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Accounts payable | | $ | 1,270 | | $ | 855 | |
| Payroll and other employee benefits | | | 172 | | | 394 | |
| Accrued vacation | | | 434 | | | 455 | |
| Accrued consulting and other professional fees | | | 292 | | | 268 | |
| Deferred rent | | | 269 | | | 315 | |
| Other accrued liabilities | | | 409 | | | 196 | |
| | | $ | 2,846 | | $ | 2,483 | |
Deferred revenues consist of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Milestone and up-front payments | | $ | 1,441 | | $ | 1,604 | |
| Less: current portion | | | (365 | ) | | (165 | ) |
| | | $ | 1,076 | | $ | 1,439 | |
In August 2004, the CombiMatrix group received a $1,000,000 upfront payment from Furuno as part of a multi-year collaboration agreement to develop a bench-top array synthesizer for commercial applications, which was included in deferred revenue at December 31, 2005 and 2004. During the third quarter of 2006, the CombiMatrix group entered into a manufacturing agreement and completed its obligations under its collaboration agreement with Furuno. As a result, the CombiMatrix group began amortizing the $1,000,000 upfront payment previously received under the collaboration agreement over the economic life of the manufacturing agreement, which is estimated to be four years. At December 31, 2006, deferred revenue related to the Furuno collaboration agreement totaled $875,000.
In 2003, the CombiMatrix group received upfront and milestone payments from Toppan totaling $2,400,000, pursuant to a multi-year collaboration and supply agreement to develop and manufacture arrays using the CombiMatrix group’s proprietary electrochemical detection approach, which was included in deferred revenues at December 31, 2004. During the fourth quarter of 2005, the CombiMatrix group completed all obligations under its collaboration and supply agreement with Toppan. As a result of completing all of its obligations under this agreement and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized all previously deferred payments received from Toppan, totaling $2,266,000, as collaboration agreement revenues in the accompanying consolidated statement of operations and comprehensive loss.
In March 2004, the CombiMatrix group completed all phases of its research and development agreement with Roche. As a result of completing all of its obligations under this agreement and in accordance with the CombiMatrix group’s revenue recognition policies for multiple-element arrangements, the CombiMatrix group recognized all previously deferred payments received from Roche as collaboration agreement revenues totaling $17,302,000 during the first quarter of 2004.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
6. INVESTMENTS
In October 2004 (the “Investment Date”), the CombiMatrix group entered into an agreement to acquire up to a one-third ownership interest in Leuchemix, Inc. (“Leuchemix”), a private drug development firm, which is developing several compounds for the treatment of leukemia and other cancers. In accordance with the terms of the purchase agreement, the CombiMatrix group purchased 3,137,500 shares of Series A Preferred Stock of Leuchemix for a total purchase price of $4,000,000. The ownership interest was acquired and paid for quarterly, beginning with the fourth quarter of 2004 and continuing through the fourth quarter of 2006. As of December 31, 2006, 2005 and 2004, the CombiMatrix group had invested $4,000,000, $1,850,000 and $250,000, representing a 33%, 19% and 3% interest in the total outstanding voting securities of Leuchemix, respectively. In accordance with the terms of the purchase agreement, CombiMatrix Corporation’s CEO was named a director of Leuchemix. The CombiMatrix group’s investment in Leuchemix is being accounted for under the equity method and is included in other long term assets.
The CombiMatrix group’s equity in the losses of Leuchemix, including its share of the amortization expense related to the excess purchase consideration over the book value of Leuchemix was $963,000, $352,000 and $17,000 for the years ended December 31, 2006, 2005 and 2004, respectively. Summary financial information for Leuchemix was not significant for the periods presented.
In January 2006, the CombiMatrix group expanded its relationship with one of its existing distributors, InBio, for the Asia Pacific region. Major components of the expanded relationship included the transfer of day-to-day operational responsibility and majority ownership of CombiMatrix K.K. to InBio, along with an expanded distribution agreement that encompasses Japan. InBio obtained 67% of the voting interests in CombiMatrix K.K. for a nominal amount of consideration. As a result, InBio assumed all operational and financial responsibilities of CombiMatrix K.K. The net loss on the sale of 67% of the voting interest in CombiMatrix K.K. recorded in the consolidated statement of operations for the year ended December 31, 2006 was $84,000. Subsequent to the sale, our investment in CombiMatrix K.K. was accounted for under the equity method. The deconsolidation of CombiMatrix K.K. did not have a material impact on the consolidated balance sheets as of December 31, 2006.
7. GOODWILL AND INTANGIBLE ASSETS
The CombiMatrix group had $16,918,000 and $18,859,000 of goodwill at December 31, 2006 and 2005, respectively. Prior to December 31, 2005, $565,000 of the total amount of goodwill resulted from step-acquisitions of AMS and CombiMatrix K.K. during July 2003. These reporting units were tested for impairment in the fourth quarter of 2005 in connection with our annual forecasting process. Due to the lack of third-party research and development funding for AMS and declining array product sales at CombiMatrix K.K., operating profits and cash flows were lower than expected during the preceding three quarters. Based on these trends, the operating forecasts for 2006 were revised downward and as a result, a goodwill impairment loss of $565,000 was recognized in December 2005. The fair values of these reporting units were estimated using the expected present value of their future cash flows.
As of March 31, 2006, the CombiMatrix group reduced its goodwill and deferred tax liability balances by $1,941,000, which were initially recorded in fiscal 2000, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability.
The CombiMatrix group’s only identifiable intangible assets are patents and licenses, which are being amortized over an economic useful life ranging from 7 to 20 years.
The gross carrying amounts and accumulated amortization related to acquired intangible assets as of December 31, 2006 and 2005 are as follows (in thousands):
| | | 2006 | | 2005 | |
| | | | | | |
| Gross carrying amount - patents and licenses | | $ | 12,595 | | $ | 12,095 | |
| Accumulated amortization | | | (5,303 | ) | | (4,169 | ) |
| Patents and licenses, net | | $ | 7,292 | | $ | 7,926 | |
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Aggregate patent and license amortization expense was $1,096,000, $1,095,000 and $1,096,000 in 2006, 2005 and 2004, respectively. Annual aggregate amortization expense from patents and licenses for each of the next five years through December 31, 2011 is estimated to be $1,133,000 per year.
8. INCOME TAXES
CombiMatrix group’s allocated benefit for income taxes consists of the following (in thousands):
| | | 2006 | | 2005 | | 2004 | |
| Current: | | | | | | | |
| U.S. Federal tax | | $ | - | | $ | - | | $ | - | |
| State taxes | | | - | | | (31 | ) | | - | |
| | | | - | | | (31 | ) | | - | |
| Deferred: | | | | | | | | | | |
| U.S. Federal tax | | | (34 | ) | | (136 | ) | | (136 | ) |
| State taxes | | | - | | | - | | | - | |
| | | | (34 | ) | | (136 | ) | | (136 | ) |
| | | $ | (34 | ) | $ | (167 | ) | $ | (136 | ) |
The tax effects of temporary differences and carryforwards that give rise to significant portions of deferred assets and liabilities consist of the following at December 31, 2006 and 2005 (in thousands):
| | | 2006 | | 2005 | |
| Deferred tax assets: | | | | | |
| Depreciation and amortization | | $ | 157 | | $ | (170 | ) |
| Deferred revenues | | | 447 | | | 489 | |
| Stock compensation | | | 7,656 | | | 7,437 | |
| Accrued liabilities and other | | | (279 | ) | | 108 | |
| Net operating loss carryforwards and credits | | | 42,923 | | | 36,310 | |
| | | | | | | | |
| Total deferred tax assets | | | 50,904 | | | 44,174 | |
Less: valuation allowance | | | (48,584 | ) | | (44,174 | ) |
| | | | | | | | |
| Deferred tax assets, net of valuation allowance | | | 2,320 | | | - | |
| | | | | | | | |
| Deferred tax liabilities: | | | | | | | |
| Intangibles | | | (2,320 | ) | | (1,975 | ) |
| Net deferred tax liability | | $ | - | | $ | (1,975 | ) |
As of March 31, 2006, the CombiMatrix group reduced its goodwill and deferred tax liability balances by $1,941,000, which were initially recorded in fiscal 2000, to reflect the reduction in its income tax valuation allowance after consideration of the deferred tax liability.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
A reconciliation of the federal statutory income tax rate and the effective income tax rate is as follows:
| | | 2006 | | 2005 | | 2004 | |
Statutory federal tax rate | | | (34%) | | | (34%) | | | (34%) | |
Goodwill impairment | | | - | | | 2% | | | - | |
Tax exempt interest | | | - | | | - | | | 10% | |
Impact of foreign rate difference | | | - | | | 4% | | | 10% | |
Research and development tax credits | | | (2%) | | | (5%) | | | 70% | |
Stock compensation | | | 2% | | | - | | | 4% | |
Non deductible permanent items | | | (2%) | | | 4% | | | 11% | |
Valuation allowance | | | 35% | | | 27% | | | (50%) | |
Other | | | 1% | | | 1% | | | 2% | |
| | | | - | | | (1%) | | | 23% | |
At December 31, 2006, the CombiMatrix group has deferred tax assets totaling approximately $50,904,000, which are fully offset by deferred tax liabilities and a full valuation allowance due to management’s determination that the criteria for asset recognition have not been met.
Acacia Research Corporation files a consolidated federal income tax return that includes the Acacia Technologies group (excluding discontinued operations) and the CombiMatrix group.
At December 31, 2006, the CombiMatrix group had federal net operating loss carryforwards of approximately $117,056,000, which will begin to expire in 2010 through 2026. In addition, the CombiMatrix group has tax credit carryforwards of approximately $3,952,000. Utilization of net operating loss carryforwards and tax credit carryforwards are subject to the “change of ownership” provisions under Section 382 of the Internal Revenue Code. The amount of such limitations has not been determined.
Had the CombiMatrix group filed separate tax returns, the benefit for income taxes and division net loss would not have differed from the amounts reported in the CombiMatrix group’s statements of operations for the periods presented.
9. COMMITMENTS AND CONTINGENCIES
Operating Leases
In October 2000, the CombiMatrix group entered into a non-cancelable operating lease for office space. A security deposit in the form of a $1,500,000 letter of credit was issued to the landlord. On February 1, 2007, the CombiMatrix group executed an amendment to its operating lease for office and laboratory space, which reduced its future annual lease commitment by approximately 80%, and reduced its letter of credit to $1,000,000, which will be reduced by $40,000 per month to a floor amount of $300,000 by October, 2008. Inclusive of this lease amendment, future minimum operating lease payments as of December 31, 2006 are as follows (in thousands):
Year | | | |
| 2007 | | $ | 588 | |
| 2008 | | | 388 | |
| 2009 | | | 397 | |
| 2010 | | | 333 | |
| Thereafter | | | - | |
| Total minimum lease payments | | $ | 1,706 | |
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
The amended lease includes an extension of two years beyond the CombiMatrix group’s original termination date of October 31, 2008 (Termination Date). However, the CombiMatrix group has the right to terminate the lease without penalty as of the Termination Date if we provide notice to the landlord on or before July 31, 2008. Future minimum lease payments disclosed above includes the impact of the February 2007 lease amendment. Rent expense for the years ended December 31, 2006, 2005 and 2004 was $1,878,000, $1,955,000 and $1,933,000, respectively.
Collaborative and Research Agreements
On February 8, 2006, the CombiMatrix group executed a one-year, $2.1 million contract with the DoD to further the development of the CombiMatrix group's array technology for the electrochemical detection of biological and chemical threat agents. Under the terms of this contract, the CombiMatrix group will perform research and development activities, as described under the contract, and will be reimbursed on a periodic basis for actual costs incurred to perform its obligations, plus a fixed fee, of up to $2.1 million. As of December 31, 2006, the CombiMatrix group had incurred $1.3 million in actual contract costs for the electrochemical detection contract. In March 2004, the CombiMatrix group was awarded a two-year, $5.9 million contract with the DoD to further the development of the CombiMatrix group’s array technology for the detection of biological and chemical threat agents. This contract was completed in December 2005.
On August 9, 2006, the CombiMatrix group executed a two-year, $1.9 million contract with the DoD, focusing on the integration of its electrochemical detection technology currently under development with the CombiMatrix group’s microfluidics “lab-on-a-chip” technology to be used for military and homeland security applications. Under the terms of this contract, the CombiMatrix group will perform research and development activities, as described under the contract, and will be reimbursed on a periodic basis for actual costs incurred to perform its obligations, plus a fixed fee, of up to $1.9 million. As of December 31, 2006, the CombiMatrix group had incurred $190,000 in actual contract costs for the microfluidics contract.
As disclosed in Note 6, the CombiMatrix group entered into an agreement with Leuchemix to purchase a total of $4,000,000 of Series A Preferred Stock of Leuchemix over a two-year period. As of December 31, 2005, future contractual cash investments totaled Leuchemix were $2,150,000, all of which were made as of October 2, 2006. There are no future commitments to purchase Leuchemix capital as of October 2, 2006.
Human Resources
The CombiMatrix group provides certain severance benefits such that if an executive who is a vice president or higher is terminated for other than cause, death or disability, the executive will receive payments equal to three months’ base salary and other medical and dental benefits on a bi-weekly basis over a three-month period. If termination occurs as a result of a change in control transaction, these benefits will be extended by three months.
Litigation
On November 28, 2000, Nanogen, Inc. (“Nanogen”) filed suit against CombiMatrix Corporation and Dr. Donald Montgomery, a former officer of CombiMatrix Corporation. The Nanogen suit alleged, among other things, that CombiMatrix Corporation’s issued patent and certain pending patent applications, trade secrets and related technologies that were inappropriately obtained by CombiMatrix Corporation and that Nanogen was the legal owner of the patents, trade secrets and related technologies.
On September 30, 2002, CombiMatrix Corporation and Dr. Donald Montgomery entered into a settlement agreement with Nanogen, Inc. to settle all pending litigation between the parties. Pursuant to the terms of the settlement agreement, CombiMatrix Corporation agreed to make quarterly payments to Nanogen equal to 12.5% of total sales of products developed by CombiMatrix Corporation and its affiliates and based on the patents that had been in dispute in the litigation, up to an annual maximum of $1,500,000. The minimum quarterly payments under the settlement agreement were $37,500 per quarter for the period from October 1, 2003 through October 1, 2004, and $25,000 per quarter thereafter until the patents expire in 2018. Also, pursuant to the settlement agreement, CombiMatrix Corporation issued to Nanogen 4,016,346 shares, or 17.5% of its outstanding shares post-issuance, subject to an anti-dilution provision related to the exercise of CombiMatrix Corporation options and warrants that were outstanding on the effective date of the agreement, for a period of up to three years.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Royalties recognized under the settlement agreement for the years ended December 31, 2006, 2005 and 2004 were $348,000, $217,000 and $138,000, respectively, and are included in patent amortization and royalties in the accompanying consolidated statements of operations. Patent amortization and royalties for the CombiMatrix group relate to costs of product sales.
During the years ended December 31, 2005 and 2004, the CombiMatrix group recorded net non-cash charges (credits) totaling ($406,000) and $812,000, respectively, in connection with the anti-dilution provisions of the settlement agreement. The non-cash charges (credits) reflect changes in management’s estimate of the fair value of AR-CombiMatrix stock issued to Nanogen, Inc. as a result of certain options and warrants exercised during 2004 and the fair value of AR-CombiMatrix stock potentially issuable to Nanogen, Inc. as of each balance sheet date. The liability was adjusted at each balance sheet date for changes in the market value of the AR-CombiMatrix stock and was reflected as a long-term liability. The anti-dilution provisions of the settlement agreement expired in September 2005, resulting in a net non-cash credit of $211,000 from the reversal of the related liability as of that date. There are no future stock-based obligations to Nanogen.
The CombiMatrix group is subject to other claims and legal actions that arise in the ordinary course of business. Management believes that the ultimate liability with respect to these claims and legal actions, if any, will not have a material effect on CombiMatrix group’s financial position, results of operations or cash flows.
10. RETIREMENT SAVINGS PLAN
The CombiMatrix group has an employee savings and retirement plan under section 401(k) of the Internal Revenue Code (the “Plan”). The Plan is a defined contribution plan in which eligible employees may elect to have a percentage of their compensation contributed to the Plan, subject to certain guidelines issued by the Internal Revenue Service. The CombiMatrix group may contribute to the Plan at the discretion of Acacia Research Corporation’s board of directors. There were no contributions made by the CombiMatrix group during the years ended December 31, 2006, 2005 and 2004.
11. ALLOCATED NET WORTH
The CombiMatrix group’s statements of allocated net worth present the equity transactions of Acacia Research Corporation, which are attributed to the CombiMatrix group as “Net assets attributed to the CombiMatrix group.” This presentation reflects the fact that the CombiMatrix group does not have legally issued common or preferred stock, nor are warrant issuances or employee stock option transactions legal transactions of the CombiMatrix group. Presented below is a detail of the equity transactions of Acacia Research Corporation which relate to the businesses of the CombiMatrix group and which therefore comprise the balances reflected in the group’s net assets attributed to CombiMatrix group (in thousands):
| | | CombiMatrix Group | |
| | | | |
2004 | | | |
| Units issued in direct offering, net of issuance costs | | $ | 13,715 | |
| Allocated corporate charges | | | 396 | |
| Stock options and warrants exercised | | | 5,117 | |
| Stock option cancellations | | | (185 | ) |
| Compensation expense relating to stock options and warrants | | | 939 | |
| Unrealized loss on short-term investments | | | (59 | ) |
| Unrealized loss on foreign currency translation | | | (20 | ) |
| Shares issued to Nanogen pursuant to September 2002 settlement agreement (refer to Note 9) | | | 478 | |
| Net assets attributed to the CombiMatrix group - 2004 | | $ | 20,381 | |
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
2005 | | | | |
| Units issued in direct offering, net of issuance costs | | $ | 12,724 | |
| Warrants issued in direct offerings (Refer to Note 12) | | | (2,194 | ) |
| Allocated corporate charges | | | 179 | |
| Stock options exercised | | | 11 | |
| Compensation expense relating to stock options and warrants | | | (164 | ) |
| Unrealized gain on short-term investments | | | 38 | |
| Unrealized gain on foreign currency translation | | | 73 | |
| Net assets attributed to the CombiMatrix group - 2005 | | $ | 10,667 | |
| | | | | |
| | | | | |
2006 | | | | |
| Units issued in direct offering, net of issuance costs | | $ | 12,109 | |
| Warrants issued in direct offerings (Refer to Note 12) | | | (7,104 | ) |
| Allocated corporate charges | | | 277 | |
| Stock issued to consultants | | | 94 | |
| Compensation expense relating to stock options and warrants | | | 2,360 | |
| Unrealized gain on short-term investments | | | 2 | |
| Reclassification of foreign currency translation | | | (57 | ) |
| Net assets attributed to the CombiMatrix group - 2006 | | $ | 7,681 | |
Equity Financings
On December 13, 2006, Acacia Research Corporation completed a registered direct offering with Oppenheimer & Co., Inc. (“Oppenheimer”) as the placement agent, raising gross proceeds of $9,964,000 through the issuance of 9,768,313 units. Each unit consists of one share of AR-CombiMatrix common stock and 1.2 five-year common stock warrants, for a total of 9,768,313 shares and warrants to purchase 11,721,975 shares of AR-CombiMatrix common stock, respectively, issued to investors. Each warrant entitles the holder to purchase a share of AR-CombiMatrix stock at a price of $0.87 per share. Acacia Research Corporation issued additional warrants to purchase 488,416 shares of AR-CombiMatrix stock with an exercise price of $1.09 per share to Oppenheimer. Net proceeds raised from the private equity financing of $9,266,000 were attributed to the CombiMatrix group.
On June 14, 2006, Acacia Research Corporation entered into a standby equity distribution agreement (the “SEDA”) with Cornell Capital Partners, LP (“Cornell”). Under the terms of the SEDA, Acacia Research Corporation was able to require Cornell to purchase up to $50.0 million of AR-CombiMatrix common stock, or up to 13,024,924 shares, over a two-year period following the effective date of the SEDA. Such shares were in the form of registered securities drawn from Acacia Research Corporation’s current shelf registration statement. Acacia Research Corporation could request advances under the SEDA in up to $5.0 million increments. At the closing of each advance, Acacia Research Corporation issued to Cornell a number of shares of AR-CombiMatrix common stock equal to the amount of the advance divided by the lowest daily volume weighted-average price (“VWAP”) of AR-CombiMatrix common stock during the five trading days following the advance notice to Cornell, which would purchase the shares at 97.5% of the VWAP. At each closing, Acacia Research Corporation paid an underwriting fee of 4% of the gross amount of each advance on the first $20.0 million and 5% of the gross proceeds of each advance on the remaining $30.0 million of the SEDA to Cornell. A total of 13,024,924 shares of AR-CombiMatrix common stock were authorized to be issued under the SEDA.
COMBIMATRIX GROUP
(A Division of Acacia Research Corporation)
NOTES TO FINANCIAL STATEMENTS
Upon closing of the SEDA, the CombiMatrix group paid Cornell a one-time commitment fee of $550,000 and an additional $20,000 in due diligence and other closing-related costs. The $550,000 fee was recorded as a long-term asset and was amortized against future advances as costs of equity issuances. In June 2006, Cornell purchased 343,750 shares of AR-CombiMatrix common stock at $1.60 per share (which was not an advance under the SEDA), based on the fair value of AR-CombiMatrix stock on June 12, 2006. Since executing the SEDA, through December 20, 2006, Acacia Research Corporation has requested five advances from Cornell to purchase a total of 3,211,345 shares of AR-CombiMatrix stock at prices ranging from $1.16 to $0.73 per share, resulting in net proceeds of $3,070,000, which were contributed to the CombiMatrix group. On December 20, 2006, a notice to cancel the SEDA was sent by Acacia Research Corporation to Cornell. The unamortized SEDA costs of $444,000 were charged against the net proceeds of the Oppenheimer financing, disclosed above, as canceling the SEDA was a condition of closing the December 2006 Oppenheimer financing.
In September 2005, Acacia Research Corporation raised gross proceeds of $10,537,000 through the sale of 6,385,907 shares of AR-CombiMatrix stock and common stock purchase warrants to purchase 1,596,478 shares AR-CombiMatrix stock, at a price of $1.65 per unit, in a registered direct offering. Each unit consisted of one share of AR-CombiMatrix stock and one-quarter of a five-year AR-CombiMatrix stock purchase warrant. Each full AR-CombiMatrix stock purchase warrant entitles the holder to purchase a share of AR-CombiMatrix stock at a price of $2.40 per share and is exercisable immediately upon issue. Net proceeds raised of approximately $9,609,000, which are net of related issuance costs, were attributed to the CombiMatrix group. Refer to Note 12 regarding classification of the warrants in the accompanying balance sheet.
In July 2005, Acacia Research Corporation raised gross proceeds of $3,151,000 through the sale of 1,400,444 shares of AR-CombiMatrix stock at a price of $2.25 per share in a registered direct offering. Net proceeds raised of approximately $3,114,000, which are net of related issuance costs, were attributed to the CombiMatrix group.
In April 2004, Acacia Research Corporation raised gross proceeds of $15,000,000 through the sale of 3,000,000 shares of Acacia Research - CombiMatrix common stock at a price of $5.00 per share in a registered direct offering. Net proceeds raised of approximately $13,715,000, which are net of related issuance costs, were attributed to the CombiMatrix group.
During 2004, proceeds of $2,093,000 were received from the issuance of 761,205 shares of AR-CombiMatrix stock related to the exercise of certain warrants issued in connection with the CombiMatrix group’s May 2003 private equity financing. The proceeds from the warrants exercised were attributed to the CombiMatrix group.
12. COMMON STOCK PURCHASE WARRANT LIABILITY
Acacia Research Corporation’s classes of common stock are subject to certain redemption provisions in the event that Acacia Research Corporation sells, transfers, assigns or otherwise disposes of, in one transaction or a series of related transactions, all or substantially all of the properties and assets attributed to either group.
Acacia Research Corporation adopted FASB Staff Position No. 150-5 (“FSP No. 150-5”), effective July 1, 2005, which requires that warrants for shares that are redeemable be classified as liabilities, based on the fair values of the warrants, which are required to be marked to market at each balance sheet date. The fair value of contingently redeemable AR-CombiMatrix stock purchase warrants outstanding at December 31, 2006 and December 31, 2005 was $6,732,000 and $1,381,000, respectively. Net warrant gains for the year ended December 31, 2006 and December 31, 2005, reflected in other income (expense), related to changes in the fair value of the warrant liability totaled $1,754,000 and $812,000, respectively.
The fair value of AR-CombiMatrix stock purchase warrants was determined using the Black-Scholes option-pricing model. Refer to Note 10 to the Acacia Research Corporation consolidated financial statements, included elsewhere herein, for a summary of weighted-average assumptions used to determine the fair value of the common stock purchase warrant liability at December 31, 2006 and 2005.
13. SUPPLEMENTAL CASH FLOW INFORMATION
Refer to Note 7 for goodwill impairment charges recognized in 2005.
Exhibit Number | Description |
| | |
| 2.1 | Agreement and Plan of Merger of Acacia Research Corporation, a California corporation, and Acacia Research Corporation, a Delaware corporation, dated as of December 23, 1999 (1) |
| 2.2 | Agreement and Plan of Reorganization by and among Acacia Research Corporation, Combi Acquisition Corp. and CombiMatrix Corporation dated as of March 20, 2002 (2) |
| 3.1 | Restated Certificate of Incorporation (3) |
| 3.2 | Amended and Restated Bylaws (4) |
| 10.1* | Acacia Research Corporation 1996 Stock Option Plan, as amended (5) |
| 10.2* | Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (6) |
| 10.3* | CombiMatrix Corporation 1998 Stock Option Plan (7) |
| 10.4* | CombiMatrix Corporation 2000 Stock Awards Plan (7) |
| 10.5* | 2002 CombiMatrix Stock Incentive Plan (8) |
| 10.6* | 2002 Acacia Technologies Stock Incentive Plan (9) |
| 10.7 | Lease Agreement dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (10) |
| 10.8 | Settlement Agreement dated September 30, 2002, by and among Acacia Research Corporation, CombiMatrix Corporation, Donald D. Montgomery, Ph.D. and Nanogen, Inc.(7) |
| 10.9† | Research & Development Agreement dated September 25, 2002, between CombiMatrix Corporation and Roche Diagnostics GmbH(7) |
| 10.10† | License Agreement dated September 25, 2002 between CombiMatrix Corporation and Roche Diagnostics GmbH(7) |
| 10.11 | Form of Indemnification Agreement (11) |
| 10.12 | Series A Preferred Stock Purchase Agreement dated October 1, 2004, by and between Leuchemix, Inc. and CombiMatrix Corporation(12) |
| 10.13 | Investor Rights Agreement dated October 1, 2004, by and among Leuchemix, Inc., the holders of Common Stock set forth on Exhibit A attached thereto, and CombiMatrix Corporation(12) |
| 10.14 | Voting Agreement dated October 1, 2004, by and among Leuchemix, Inc., CombiMatrix Corporation and the holders of the Common Stock set forth on Exhibit A attached thereto(12) |
| 10.15 | Right of First Refusal and Co-Sale Agreement dated October 1, 2004, by and among Leuchemix, Inc., the holders of Common Stock set forth on Exhibit A attached thereto, and CombiMatrix Corporation(11) |
| 10.16 | Letter of Intent dated December 15, 2004 between Acacia Research Corporation and Global Patent Holdings LLC (13) |
| 10.17† | First Addendum to Roche/CBMX Research and Development Agreement dated March 25, 2003 (20) |
| 10.18 | Research & Development Agreement Second Amendment dated March 19, 2004, between Roche Diagnostics GmbH and CombiMatrix Corporation (20) |
| 10.19 | Sublease Guaranty dated as of June 15, 2005 by CombiMatrix Corporation in favor of Accupath Diagnostic Laboratories, Inc. (20) |
| 10.20 | Sublease dated June 15, 2005, by and between Accupath Diagnostic Laboratories, Inc., dba U.S. Labs, and CombiMatrix Molecular Diagnostics, Inc. (20) |
| 10.21 | Lease Agreement dated October 19, 2000 by and between Wiredzone Property, L.P. and CombiMatrix Corporation (20) |
| 10.22 | First Amendment to Lease Agreement dated April 22, 2001 by and between Wiredzone Property, L.P. and CombiMatrix Corporation (20) |
| 10.23 | Form of Subscription Agreement between Acacia Research Corporation and certain investors (14) |
| 10.24 | Third Amendment to lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (15) |
| 10.25 | Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (16) |
| 10.26 | Amendment to Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (17) |
| 10.27 | Manufacturing and Supply Agreement between Acacia Research Corporation and Furuno Electric Company, Ltd. Effective July 1, 2006 (18) |
| 10.28 | Placement Agency Agreement between Acacia Research Corporation and Oppenheimer & Co., dated December 7, 2006 (19) |
| 10.29 | Form of Subscription Agreement (19) |
Exhibit Number | Description |
| | |
| 10.30 | Form of Investors Warrant (19) |
| 21.1 | List of Subsidiaries |
| 23.1 | Consent of PricewaterhouseCoopers LLP (relating to the financial statements of Acacia Research Corporation) |
| 23.2 | Consent of PricewaterhouseCoopers LLP (relating to the financial statements of the CombiMatrix group) |
| 23.3 | Consent of PricewaterhouseCoopers LLP (relating to the financial statements of the Acacia Technologies group) |
| 31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | Certification of the Chief Executive Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 32.2 | Certification of the Chief Financial Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
___________________________
* The referenced exhibit is a management contract, compensatory plan or arrangement.
| † | Portions of this exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission. |
| | |
| (1) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on December 30, 1999 (SEC File No. 000-26068). |
| | |
| (2) | Incorporated by reference as Appendix A to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (3) | Incorporated by reference as Appendix B to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (4) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on August 10, 2001 (SEC File No. 000-26068). |
| | |
| (5) | Incorporated by reference as Appendix A to the Definitive Proxy Statement on Schedule 14A filed on April 10, 2000 (SEC File No. 000-26068). |
| | |
| (6) | Incorporated by reference from Acacia Research Corporation’s Definitive Proxy as Appendix A Statement on Schedule 14A filed on April 26, 1996 (SEC File No. 000-26068). |
| | |
| (7) | Incorporated by reference to Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (8) | Incorporated by reference as Appendix D to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (9) | Incorporated by reference as Appendix E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| | |
| (10) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2001 filed on March 27, 2002 (SEC File No. 000-26068). |
| | |
| (11) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2002 filed on March 27, 2003 (SEC File No. 000-26068). |
| (12) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on November 5, 2004 (SEC File No. 000-26068). |
| | |
| (13) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2004 filed on March 15, 2005 (SEC File No. 000-26068). |
| | |
| (14) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on September 19, 2005 (SEC File No. 000-26068). |
| | |
| (15) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on May 10, 2006 (SEC File No. 000-26068). |
| | |
| (16) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on June 15, 2006 (SEC File No. 000-26068). |
| | |
| (17) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on June 22, 2006 (SEC File No. 000-26068). |
| | |
| (18) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on November 9, 2006 (SEC File No. 000-26068). |
| | |
| (19) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on December 13, 2006 (SEC File No. 000-26068). |
| | |
| (20) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2005 filed on March 16, 2006 (SEC File No. 000-26068). |